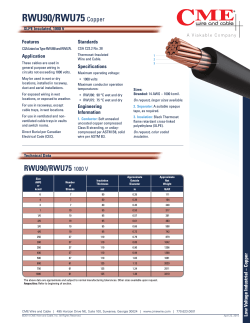
2011 Superbloom Report: Evaluating Effects and Possible Causes
2011 Superbloom Report: Evaluating Effects and Possible Causes with Available Data April 2015 Indian River Lagoon 2011 Consortium Executive summary A major phytoplankton bloom in 2011 prompted the formation of the Indian River Lagoon 2011 Consortium, which comprised at least 26 scientists from 10 organizations. These scientists evaluated available data and observations, with the goal of elucidating the scope of the unprecedented event that was named the “superbloom,” impacts from the bloom, possible causes or controls of the bloom, and important gaps in available data. This report and its appendices document the analyses, results and interpretations generated by the consortium. In 2011, the Indian River Lagoon experienced two phytoplankton blooms. The major bloom of green and blue-green algae was first observed in Banana River in March 2011, and within 1–2 months, it had spread westward through the Barge Canal into the northern portion of the Indian River Lagoon near Cocoa (Figure 2). Eventually, this superbloom expanded northward through Haulover Canal and into southern Mosquito Lagoon to cover 132,500 acres. This bloom was preceded and accompanied by a less intense bloom that began in late 2010 and eventually covered 47,500 acres from southern Banana River Lagoon to just north of Fort Pierce Inlet. The two blooms were composed of different species. The less intense, secondary bloom comprised a mix of cyanobacteria, diatoms and dinoflagellates in the Melbourne reach and was co-dominated by diatoms and dinoflagellates in the Sebastian and Vero Beach reaches. The superbloom was dominated by a mix of extremely small, single-celled algae belonging to two major groups: picoplanktonic blue-green bacteria (picocyanobacteria) and a green microflagellate in the class Pedinophyceae. Both species were found in archived samples, neither alga was known to produce harmful toxins, and neither species had bloomed previously. Although the biology and physiology of the pedinophyte and picocyanobacteria that comprised the 2011 superbloom were not known in detail, related small phytoplankton have been shown to take up nutrients and reproduce more rapidly and efficiently than larger phytoplankton due, in part, to their higher surface area-to-volume ratios. In addition, many similar small phytoplankton utilize nitrogen and phosphorus attached to carbon, which enabled them to 1) outcompete other phytoplankton when dissolved inorganic nutrients were limiting, 2) rapidly recycle organic nutrients to support persistent blooms, and 3) restrict the flow of nutrients and carbon to higher trophic levels by shunting these elements into the “microbial loop” where they were utilized by bacteria. Pedinophytes and picocyanobacteria also survived in low ambient light due to their high chlorophyll a content and in high salinity, which were the prevailing conditions during the superbloom. Unusual and potentially influential conditions preceded and persisted during the superbloom. In particular, higher light attenuation coefficients (Kd; m-1) due to higher chlorophyll a concentrations (µg l-1), higher salinities and lower water temperatures potentially influenced seagrasses, phytoplankton and other organisms. In addition, increased salinity due to evaporation indicated that residence times had increased, which would have promoted accumulation of phytoplankton. Prior to the superbloom, ambient concentrations of dissolved orthophosphate or dissolved Kjeldahl nitrogen did not increase, but increased concentrations of total dissolved phosphorus suggested an increased loading of some compound(s) containing this nutrient. As expected, concentrations of total phosphorus, total nitrogen and total carbon increased as these elements were sequestered in phytoplankton. Furthermore, an absence of diatoms may have led to decreased uptake of dissolved silicon dioxide and a subsequent increase in its ambient concentration. Overall, the superbloom period and location accounted for the longest duration of stress due to reduced light penetration in the period of record. One substantial effect of the superbloom was a loss of seagrasses. Transect lengths (the distance that seagrasses extended offshore) decreased during and following the superbloom, and along some transects, 1 seagrasses disappeared. Mapping of seagrass from aerial photography indicated that seagrass acreage decreased even before the superbloom reached its peak, with the greatest loss in the Banana River Lagoon, where approximately 24,000 acres was reduced by 87% to approximately 3,000 acres. The large losses in seagrass during the superbloom event correlated with changes in concentrations of chlorophyll a and volatile suspended solids, which was expected given the effect of these parameters on turbidity and light attenuation (Kd). In contrast, non-organic suspended particles and color contributed relatively little to the increase in light attenuation. Reduced light penetration represented the primary stressor on seagrasses, but its effects probably were mediated through sulfide stress because seagrasses could not protect their roots and rhizomes by generating oxygen through photosynthesis. Although reduced light penetration was likely the dominant stressor on seagrasses, high salinity and previous low water temperatures may have contributed to the observed losses. Phytoplankton blooms can be driven from the “bottom-up” via increased nutrient inputs, especially nitrogen and phosphorus. Three sources of these macronutrients were examined: external nutrient loads carried in freshwater inputs, internal loads from submarine groundwater and diffusive flux from sediments comprising “muck,” and loads or increased availability associated with changes in the biomass of drift macroalgae. Drier than average conditions preceded the superbloom, which translated into little evidence of increased external loads of nitrogen or phosphorus immediately preceding or during the superbloom. The surficial aquifer was considered the primary source of meteoric groundwater discharge and nutrient loads to the system, and available data suggested that nitrogen and phosphorus loads to the Banana River Lagoon were at least 2× higher than loads to other regions on a consistent basis. Thus, these loads may represent a bottom-up influence that helped initiate and sustain the superbloom, which began in Banana River Lagoon. Muck sediment has a high percentage of fine-grain clays and silts (> 60% dry weight), which inhibits the passage of water and makes diffusion a key process. Ammonium and phosphate diffuse from muck and carry nitrogen and phosphorus into the lagoon. Although surrounded by considerable uncertainty, nutrient loads estimated by multiplying rates of flux multiplied by the aerial coverage of muck approached or exceeded estimated loads from the watershed. Analyses also indicated significant decreases in quantities of drift algae, and nutrients released from decomposing drift algae or made available due to reduced uptake could have promoted the superbloom. “Top-down” control of phytoplankton blooms occurs when animals consume the algae. In an effort to assess top-down control of the superbloom, the phytoplankton assemblage was compared to previous blooms, and abundances of small zooplankton, primarily bacterioplankton and microzooplankton, larger zooplankton, infauna and fish were analyzed to identify significant variation in primary consumption. The small size of Pedinophyceae and picocyanobacteria may have increased the importance of grazing by protozoa and rotifers because these small zooplankton were better equipped to handle small algal cells. In addition, grazing may have been reduced because mucus and toxins produced by picocyanobacteria can deter grazers. High bacterial abundances during the superbloom (after March 2011) suggested that these organisms were responding to the availability of organic compounds, and abundant bacteria would have enhanced cycling of nutrients and prolonged the superbloom. During the superbloom, the assemblage of small zooplankton appeared to shift toward fewer arthropods and higher numbers of protozoa and rotifers. These grazers may have been better equipped to handle the small phytoplankton that comprised the superbloom, but their abundances decreased and became variable during the superbloom, which would have reduced top-down control. Available evidence from a site south of the superbloom region indicated that densities of larger zooplankton were relatively low preceding and during the superbloom for unknown reasons. Reduced grazing pressure would have enhanced accumulation of larger numbers of phytoplankton cells during the superbloom, but the potential impact of larger zooplankton on small phytoplankton species remains uncertain. At sites south of the superbloom region, abundances of 90 out of 187 infaunal taxa decreased prior to the superbloom, but the level of grazing pressure exerted on small phytoplankton by clams and other infauna remains uncertain. Additionally, abundances of fishes, 2 including menhaden (Brevoortia spp.) and other species that can consume phytoplankton, did not decrease significantly immediately before and during the superbloom. Thus, evidence of reduced topdown control remained equivocal. In summary, attempts to explain the initiation and persistence of the 2011 superbloom using available data highlighted some key gaps in our understanding. The following recommendations point to ways to improve our evaluation of the superbloom, our ability to predict future events, and our capacity to develop and assess potential management actions. 1. Garner an improved understanding of the biology and physiology of picocyanobacteria and Pedinophyceae, including their ability to use organic forms of nutrients, their nutrient uptake rates, their reproductive rates and their defenses against grazers. 2. Maintain or expand water quality sampling to ensure spatiotemporal variations are captured adequately, which could include continuous monitoring of various parameters to fill gaps between monthly samples. 3. Develop an improved understanding of the physiological tolerances of drift algae and seagrasses. 4. Maintain or expand surveys of drift algae and seagrasses to improve our capacity to evaluate their role in nutrient cycles. 5. Improve our ability to model bottom-up influences from external and internal nutrient loads, including atmospheric deposition, surface water runoff, groundwater inputs, diffusive flux from muck, and decomposition of drift algae. 6. Enhance surveys of bacterioplankton to improve our understanding of nutrient cycling. 7. Improve surveys of potential zooplanktonic, infaunal, epifaunal and fish grazers to enhance our understanding of spatiotemporal variation in top-down control of phytoplankton blooms. 8. Evaluate grazing pressure exerted by common species to enhance our understanding of top-down control of phytoplankton blooms. 3 Why create this report? A major phytoplankton bloom in 2011 led to the formation of the Indian River Lagoon 2011 Consortium (Table 1). This group of at least 26 scientists from 10 organizations evaluated available data and observations to elucidate the scope of the unprecedented event that was named the “superbloom,” its impacts, possible causes or controls of the bloom, and important gaps in available data. This report summarizes analyses, results and interpretations, with details provided in appendices. Table 1. Members of the Indian River Lagoon 2011 Consortium. Organization Bethune-Cookman University Florida Fish and Wildlife Conservation Commission Florida Institute of Technology Guana-Tolomato-Matanzas National Estuarine Research Reserve Harbor Branch Oceanographic Institute, Florida Atlantic University Nova Southeastern University Seagrass Ecosystems Analysts Smithsonian Marine Station at Fort Pierce University of Florida St. Johns River Water Management District St. Johns River Water Management District (retired) Volunteer Hyun Jung Cho Andrew Kamerosky Paul Carlson Rich Paperno Steve Jachec Kevin Johnson Ashok Pandit John Trefry John Windsor Gary Zarillo Nikki Dix Dennis Hanisak Paul Hargraves Bernhard Riegl Robert Virnstein Bjorn Tunberg Ed Phlips Jon Martin Ron Brockmeyer Robert Chamberlain Lauren Hall Charles Jacoby Margaret Lasi Lori Morris Whitney Green Joel Steward The process of assembling, analyzing and interpreting the information in this report and its appendices provided the scientific foundation for a more intensive investigation of the “superbloom” and its potential causes that was launched by the St. Johns River Water Management District in 2012. The Indian River Lagoon Algal Blooms Investigation is a component of the District’s Indian River Lagoon Protection Initiative. The investigation builds on the information in this report, and it will advance the existing scientific understanding of the lagoon system through monitoring, data collection, field and lab analyses, and model development with the aim of providing knowledge to guide management actions designed to restore and sustain the health of the lagoon. 4 What is the Indian River Lagoon system? Within the jurisdictional boundaries of the St. Johns River Water Management District, the Indian River Lagoon system can be subdivided into five sublagoons, each comprising two or more segments containing at least one water quality station (Figure 1). These delineations are consistent with those used to develop seagrass restoration targets and related limits for nutrient loading (Steward et al. 2005; Steward and Green 2007). The northern portion of the system, comprising the Mosquito Lagoon, Banana River Lagoon, and northern Indian River Lagoon (Figure 1), was affected by the 2011 superbloom. Figure 1. The Indian River Lagoon (IRL) system within the jurisdictional boundaries of the St. Johns River Water Management District. The superbloom region is circled in red, sublagoons are color-coded (ML = Mosquito Lagoon, NIRL = northern Indian River Lagoon, BR = Banana River Lagoon, NCIRL = north central Indian River Lagoon and SCIRL = south central Indian River Lagoon), lagoon segments are separated by black lines and labeled with blue font, and water quality stations are indicated by black dots. 5 What occurred during the 2011 superbloom and associated secondary bloom? In 2011, the Indian River Lagoon experienced two phytoplankton blooms. The major bloom of green and blue-green algae was first observed in Banana River in March 2011, and within 1–2 months, it had spread westward through the Barge Canal into the northern portion of the Indian River Lagoon near Cocoa (Figure 2). Eventually, this superbloom expanded northward through Haulover Canal and into southern Mosquito Lagoon to cover 132,500 acres. This bloom was preceded and accompanied by a less intense secondary bloom that began in late 2010 and eventually covered 47,500 acres from southern Banana River Lagoon to just north of Ft. Pierce Inlet (Figure 2). Apr 1 2011 Sep 14 2011 Dec 8 2011 Mar 18 2012 Relative chlorophyll a concentration Dec 24 2010 Figure 2. Time series of satellite images from the medium resolution imaging spectrometer (MERIS) showing the intensification, spread and waning of the phytoplankton bloom, with higher relative chlorophyll a concentrations indicated by the red tint. Note: narrow, nearshore bands of red represent chlorophyll a in seagrasses rather than phytoplankton. In August and October 2011, concentrations of chlorophyll a in the northern Banana River and Indian River lagoons surpassed 130 µg chlorophyll a L-1 (Figure 3). During the peak of the bloom, chlorophyll a concentrations rivaled levels reported from highly impacted, inland water bodies, such as Lake Apopka. Concentrations of chlorophyll a in the northern Banana River and Indian River lagoons dropped precipitously following the passage of a subtropical depression in early October 2011, and the bloom in southern Mosquito Lagoon finally dissipated in February 2012 (Figure 3). The southern bloom was not as intense, yielding samples with 20–30 µg chlorophyll a L-1, but samples with > 10 µg chlorophyll a L-1 were collected in 57% of the months between October 2008 and March 2012 (Figure 3). 6 140 140 IRLB02 IRLI02 Chlorophyll a (µg L-1) 130.3 µg L-1 120 120 100 100 80 80 60 60 40 40 20 20 0 0 140 140 IRLML02 Chlorophyll a (µg L-1) 120 136.1 µg L-1 IRLI23 120 100 100 86.3 µg L-1 80 80 60 60 40 40 20 20 0 0 Secondary bloom Figure 3. Time series for chlorophyll a concentrations showing the magnitude and waning of the phytoplankton bloom at water quality stations in the Banana River (IRLB02), Indian River (IRLI02 and IRLI23) and Mosquito (IRLML02) lagoons. The two blooms had different species compositions. The secondary bloom comprised a mix of cyanobacteria, diatoms and dinoflagellates in the Melbourne reach and was co-dominated by diatoms and dinoflagellates in the Sebastian and Vero Beach reaches. The 2011 superbloom was dominated by a mix of extremely small, single-celled algae belonging to two major groups: picoplanktonic blue-green bacteria (picocyanobacteria) and a green microflagellate in the class Pedinophyceae. These phytoplankters were 1–4 µm in diameter or about 1/100th the size of a grain of salt (Figure 4). During September and October 2011, densities of the pedinophyte ranged from 700 million to 1 billion cells L-1, comprising more than 50% of the total biomass for these two groups (measured as carbon; Figure 5). Picocyanobacteria are common inhabitants of the IRL, but pedinophytes had never been documented to reach bloom conditions in over 14 years of sampling. Fortunately, neither alga is known to produce harmful toxins, unlike the dinoflagellate Pyrodinium bahamense, which bloomed in the past (Figure 5). In 2002 and 2004, P. bahamense was implicated in more than two dozen cases of human poisoning due to consumption of pufferfish that accumulated saxitoxin (Landsberg et al. 2006). 7 Pedinophyceae Picocyanobacteria Figure 4. Scanning electron micrographs of the pedinophyte and picocyanobacteria found in the 2011 bloom. Phytoplankton carbon (µg ml-1) Phytoplankton carbon (µg ml-1) Central Banana River Titusville 6 6 Pedinophyceae 5 Dino Diatom Cyano Other Dino Diatom Cyano 2011 2012 2010 2009 2008 2007 2006 2005 2004 2003 2002 2011 2012 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 0 2000 0 1999 1 1998 1 2001 2 2000 2 3 1999 3 4 1998 4 1997 Carbon (µg ml-1) Pedinophyceae 1997 Carbon (µg ml-1) 5 Other Figure 5. Time series for biomass (expressed as µg of carbon ml-1) for groups of phytoplankton in the central Banana River Lagoon and the northern Indian River Lagoon (Titusville). Dino = dinoflagellates, Diatom = diatoms, Cyano = cyanobacteria, Other = other phytoplankton, including Pedinophyceae during 2011 Although the biology and physiology of the pedinophyte and picocyanobacteria that comprised the 2011 superbloom have not been studied in detail, general characteristics of small and related phytoplankton can help explain their success. Numerous studies indicate that small phytoplankton take up nutrients more rapidly and efficiently than larger phytoplankton due to their higher surface area-to-volume ratios (Reynolds 2006; Behrenfeld et al. 2008). Small sizes and rapid reproductive rates may allow 8 pedinophytes and picocyanobacteria to escape from primary consumers or reproduce more rapidly than they are consumed (Nugraha et al. 2010; Smayda 2008). In addition, many photosynthetic microflagellates, picocyanobacteria and small pelagophytes utilize organic forms of nutrients (nitrogen and phosphorus attached to carbon; Burkholder et al. 2008; Gobler and Sunda 2012; DeYoe et al. 2007; Sun et al. 2012). This plasticity (known as mixotrophy) enables these organisms to 1) outcompete other phytoplankton when dissolved inorganic nutrients are limiting, 2) rapidly recycle organic nutrients to support persistent blooms, and 3) restrict the flow of nutrients and carbon to higher trophic levels by shunting these elements into the “microbial loop” (Fenchel 2008). Pedinophytes and picocyanobacteria also are equipped to survive in low ambient light and high salinity. Pedinophytes and picocyanobacteria collected during the 2011 bloom yielded higher amounts of chlorophyll a per unit biovolume (3.65 µg chlorophyll a per 109 µm3 and 2.58 µg chlorophyll a per 109 µm3, respectively) than chain-forming, centric diatoms (0.88 µg chlorophyll a per 109 µm3) and Pyrodinium bahamense (1.45 µg chlorophyll a per 109 µm3) from past blooms (Ed Phlips, unpublished data). Concentrated chlorophyll allows more efficient harvesting of light to support photosynthesis under the self-shading conditions typical of blooms (Reynolds 2006). The exceptionally high salinity that prevailed during the superbloom (reaching up to 50 psu during June and July) is evidence that these taxa tolerate extreme conditions. Furthermore, limited laboratory tests have documented survival of pedinophytes and picocyanobacteria at salinities up to 70 psu, with peak growth rates recorded at 35–45 psu (Ed Phlips, unpublished data). Similarly, a wide salinity tolerance (4–38+ psu) has been reported for another estuarine pedinophyte, Marsupiomonas pelliculata (Jones et al. 1994). What environmental conditions potentially influenced the superbloom? Where appropriate, available data were grouped into six periods (Figure 6). Four distal antecedent periods spanned March 1996 through January 2010 (DAP 1–4), one proximal antecedent period (PAP) covered February 2010 through February 2011, and the superbloom event period (SEP) ran from March 2011 to March 2012. In part, these periods were characterized by variation in average annual rainfall relative to the 30-year average, as measured by gauges or radar. Thus, each period was categorized as normal, wet or dry (Figure 6). DAP-1 DAP-2 DAP-3 DAP-4 Mar’96– Aug’99 Sep’99– Jan’03 Feb’03– Jan’06 Feb’06–Jan’10 3 yrs + 6 mos 3 yrs + 5 mos 3 yrs NORMAL WET WET PAP Feb’10– Feb’11 SEP Mar’11– Mar’12 4 yrs 13 mos 13 mos DRY DRY DRY Figure 6. The four distal antecedent periods (DAPs), proximal antecedent period (PAP), and the superbloom event period (SEP) with their beginning and end dates, total durations, and hydrologic conditions. The superbloom was preceded by exceptionally cold water temperatures (5–8°C) in December 2009– January 2010 and December 2010–January 2011 (Figure 7), and by an intense, short-lived diatom bloom in January 2010 (Figure 8). In fact, the winter of 2009–2010 and December 2010 were the coldest air temperatures recorded since records began to be kept in 1937 (Florida Today newspaper, 20 March 2010 and 12 January 2011). 9 Haulover Canal 35 Mean water temperautre (°C) 30 25 20 15 10 8.90°C; 1/5/2001 7.65°C; 12/15/2010 5 4.95°C; 1/10 & 1/11/2010 Jan-13 Jan-12 Jan-11 Jan-10 Jan-09 Jan-08 Jan-07 Jan-06 Jan-05 Jan-04 Jan-03 Jan-02 Jan-01 Jan-00 Jan-99 Jan-98 0 Figure 7. Water temperatures measured at Haulover Canal. Phytoplankton carbon (µg ml-1) Central Banana River 6 Carbon (µg ml-1) 5 Pedinophyceae Diatoms 4 3 2 1 Dino Diatom Cyano 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 0 Other Figure 8. Time series for biomass (expressed as µg of carbon ml-1) showing a bloom of diatoms in the central Banana River Lagoon during 2010–2011, which is prior to initiation of the superbloom and secondary bloom. Dino = dinoflagellates, Diatom = diatoms, Cyano = cyanobacteria, Other = other phytoplankton, including Pedinophyceae during 2011 Was the 2011 bloom actually a superbloom? Placing the phytoplankton or algal bloom of 2011 and the events preceding it in an historical context represents a potentially valuable approach to understanding its causes and effects, with due regard for a relatively short period of record. To achieve that goal, six key characteristics were categorized. Light attenuation directly affected the health of seagrasses, and three water quality parameters primarily determined light attenuation in the Indian River Lagoon, i.e., color, turbidity, and concentrations of chlorophyll a. In addition, unusual salinities and water temperatures potentially contributed to the formation of the superbloom and likely determined the microalgal species that proliferated. 10 Boundaries for categories primarily were derived from statistical metrics applied to “seagrass centric” datasets representing “acceptable” conditions defined as years when seagrasses extended near their depth limits (Steward et al. 2005). The key metrics were the median (50th percentile) and either the 10th, 5th and 1st or 90th, 95th and 99th percentiles (Table 2). Values for parameters of interest that equaled or exceeded the relevant boundaries were tallied using a cumulative approach that paralleled the way water quality is integrated by seagrasses, drift algae and animals. In this approach, months generating moderate stress added to totals for months generating low stress, months generating high stress added to totals for months generating moderate stress, and months generating severe stress added to totals for four categories. In addition, each month was allocated to a single category, and the resulting tallies were compared to expected values derived from the statistical metrics. In total, 4,371 records from 25 stations spanning the Banana River and Indian River lagoons and 102–191 consecutive months were tallied. Table 2. Percentile limits and associated categories of stress for key parameters. Kd = light attenuation coefficient Range if low values are of concern (e.g., water temperature) median ≤ value 10th percentile ≤ value < median 5th percentile ≤ value < 10th percentile 1st percentile ≤ value < 5th percentile value < 1st percentile Range if high values are of concern (e.g., chlorophyll a, Kd, color, turbidity, salinity) value ≤ median median < value ≤ 90th percentile 90th percentile < value ≤ 95th percentile 95th percentile < value ≤ 99th percentile 99th percentile < value Stress Absent Low or weak Moderate High or strong Severe Given an average target depth for the deep edge of seagrasses of 1.4 m, light reaching the bottom drops below the estimated minimum light requirement for seagrasses (20% of ambient light at the water’s surface; Steward et al. 2005) when light attenuation coefficients (Kd) reach 1.15. Cumulative frequency distributions for different combinations of water clarity and duration indicated that seagrasses could have been stressed by low light for up to 29 consecutive months and severely stressed for up to 21 months, with 10 out of 17 periods of severe light stress occurring at stations affected by the 2011 bloom (Table 3). Months with strong and severe light stress were 1.4× and 13.5× more common than expected based on reference distributions. Table 3. Locations, months of occurrence and durations of severe light stress. Yellow highlights indicate locations and times in the superbloom; NIRL = Northern Indian River Lagoon Sublagoon NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL Station IRLI15 IRLI18 IRLI21 IRLI06 IRLI10 IRLI13 IRLI07 IRLI02 IRLI13 IRLI15 IRLI13 IRLI13 IRLI10 IRLI10 IRLI18 IRLI15 IRLI06 Year 2010 2010 2010 2011 2011 2011 2011 2011 2010 2003 1999 1998 2010 2003 2003 2006 2003 Start Month 4 5 7 2 2 2 4 7 5 5 6 3 4 5 7 5 5 Year 2011 2011 2011 2012 2011 2011 2012 2012 2010 2003 2000 1998 2010 2003 2004 2006 2003 End Month 12 11 9 1 12 12 1 2 12 12 1 10 10 11 1 10 10 Span 21 19 15 12 11 11 10 8 8 8 8 8 7 7 7 6 6 Overall, periods of high color did not contribute to the long duration of high Kd values (Table 4). In fact, the most stressful levels of color persisted for more than one month in only nine instances, and only one of those occurred at a station affected by the 2011 bloom. On its own, color was not a key factor in reducing light penetration and shading seagrasses. 11 Table 4. Locations, months of occurrence and durations of high color. Yellow highlights indicate locations and times in the superbloom; NIRL = Northern Indian River Lagoon; NCIRL = North Central Indian River Lagoon Sublagoon NIRL NIRL NIRL NIRL NIRL NCIRL NIRL Station IRLI02 IRLI06 IRLI07 IRLI06 IRLI21 IRLI27 IRLI02 Year 2004 2011 2005 2004 2004 2004 1998 Start Month 9 9 11 9 9 9 3 Year 2004 2011 2005 2004 2004 2004 1998 End Month 12 10 12 10 10 10 4 Span 4 2 2 2 2 2 2 Higher turbidity did contribute to some of the moderately long periods of high light attenuation at stations affected by the 2011 bloom (Table 5). Turbidity that generated severe stress occurred 14.2× more frequently than expected, but it only persisted for only 6 months. Therefore, another factor must have been primarily responsible for low light regimes spanning up to 21 consecutive months. Table 5. Locations, months of occurrence and durations of high turbidity. Yellow highlights indicate locations and times in the superbloom; BR = Banana River Lagoon; NIRL = Northern Indian River Lagoon; NCIRL = North Central Indian River Lagoon; SCIRL = South Central Indian River Lagoon Sublagoon BR NIRL NIRL BR BR BR NIRL NCIRL NIRL NIRL NIRL BR NIRL NIRL NCIRL NIRL NIRL BR SCIRL BR NIRL BR NIRL BR NIRL NIRL Station IRLB02 IRLI02 IRLI07 IRLNFH01 IRLB06 IRLB04 IRLI18 IRLI27 IRLI06 IRLI13 IRLI15 IRLB04 IRLI06 IRLI06 IRLI27 IRLI02 IRLI02 IRLB04 IRLIRJ10 IRLNFH01 IRLI13 IRLB02 IRLI02 IRLB02 IRLI13 IRLI02 Year 2011 2011 2011 2011 2004 2011 2003 2011 2011 2011 2011 2011 2011 2011 2010 2009 2006 2004 2003 2003 2003 2003 2000 1999 1999 1998 Start Month 2 9 8 4 1 5 10 1 3 4 4 9 9 12 7 4 3 1 8 8 10 11 12 3 3 2 Year 2011 2012 2011 2011 2004 2011 2003 2011 2011 2011 2011 2011 2011 2012 2010 2009 2006 2004 2003 2003 2003 2003 2001 1999 1999 1998 End Month 7 1 12 7 4 7 12 2 4 5 5 10 10 1 8 5 4 2 9 9 11 12 1 4 4 3 Span 6 5 5 4 4 3 3 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Chlorophyll a concentrations played a major role in generating periods of high light attenuation spanning up to 21 consecutive months (Table 6). Phytoplankton blooms characterized as severe, strong or moderate persisted for 11–24 months. Strong and severe blooms were 2.5× and 8.0× more common than expected. From February 2010 to March 2012, severe blooms of long duration were observed in the Banana River Lagoon and Northern IRL, with the longest severe blooms (2–11 months) occurring at IRLB04, IRLNFH01, IRLB02, IRLB09, IRLI06, IRLB06, IRLI07, IRLI10, IRLI02, IRLI13, and IRLI15 (Table 6). In addition, weak to strong blooms persisted from May 2010 through January 2012 at IRLI18 and from April 2010 through March 2012 at IRLI21. The data indicated that the algal bloom of 2011 was unusual because of the magnitudes and durations of chlorophyll a concentrations at multiple stations in the Banana River Lagoon and Northern IRL. 12 Table 6. Locations, months of occurrence and durations of high chlorophyll a concentrations. Yellow highlights indicate locations and times in the superbloom; BR = Banana River Lagoon; NIRL = Northern Indian River Lagoon; NCIRL = North Central Indian River Lagoon; SCIRL = South Central Indian River Lagoon Sublagoon BR BR BR BR NIRL BR NIRL NIRL BR NIRL NIRL BR BR BR BR NCIRL BR BR BR SCIRL SCIRL SCIRL SCIRL NIRL BR BR BR NIRL BR BR SCIRL SCIRL BR NIRL SCIRL BR BR NIRL BR BR BR BR NIRL BR BR NIRL NIRL NIRL NIRL NIRL NCIRL NCIRL BR BR BR NCIRL SCIRL NIRL Station IRLB04 IRLNFH01 IRLB02 IRLB09 IRLI06 IRLB06 IRLI07 IRLI10 IRLB09 IRLI02 IRLI13 IRLB09 IRLB06 IRLB09 IRLNFH01 IRLI27 IRLB02 IRLB04 IRLB06 IRLIRJ10 IRLIRJ05 IRLIRJ07 IRLIRJ08 IRLI15 IRLB04 IRLB06 IRLB04 IRLI13 IRLB09 IRLB06 IRLIRJ04 IRLIRJ12 IRLB06 IRLI15 IRLIRJ08 IRLB04 IRLNFH01 IRLI13 IRLB02 IRLB06 IRLB06 IRLB09 IRLI07 IRLB06 IRLB06 IRLI18 IRLI21 IRLI07 IRLI15 IRLI18 IRLI23 IRLI23 IRLB02 IRLB02 IRLB04 IRLI27 IRLIRJ07 IRLI13 Year 2011 2011 2011 2010 2011 2011 2011 2011 2011 2011 2011 2001 2010 2009 2001 2001 2010 2001 2001 2001 2001 2001 2001 2011 2010 2010 2009 2009 2002 2001 2001 2001 2011 2011 2011 2010 2010 2010 2009 2009 2005 2005 2005 2004 2003 2002 2002 2001 2001 2001 2001 2001 2000 1999 1999 1999 1999 1998 Start Month 2 1 3 6 6 4 6 5 6 8 5 7 6 7 8 7 7 9 6 9 9 9 9 5 5 12 11 8 8 11 10 9 12 9 9 9 6 9 8 9 11 8 9 2 8 8 8 8 7 8 8 11 9 3 3 11 9 7 Year 2011 2011 2011 2011 2012 2011 2011 2011 2011 2012 2011 2001 2010 2009 2001 2001 2010 2001 2001 2001 2001 2001 2001 2011 2010 2011 2010 2009 2002 2002 2001 2001 2012 2011 2011 2010 2010 2010 2009 2009 2005 2005 2005 2004 2003 2002 2002 2001 2001 2001 2001 2001 2000 1999 1999 1999 1999 1998 End Month 12 11 12 2 1 10 12 11 11 1 10 12 10 11 12 11 10 12 9 12 12 12 12 7 7 2 1 10 10 1 12 11 1 10 10 10 7 10 9 10 12 9 10 3 9 9 9 9 8 9 9 12 10 4 4 12 10 8 Span 11 11 10 9 8 7 7 7 6 6 6 6 5 5 5 5 4 4 4 4 4 4 4 3 3 3 3 3 3 3 3 3 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 13 Salinities were atypically high for 23 consecutive months three times in the period of record (Table 7). Typical salinities were observed for up to 49 months, and slightly elevated salinities were recorded for up to 75 consecutive months. High salinity characterized 15.7× more months than expected. Approximately 34% of the very high salinities lasted for only 1–2 months, and approximately 21% of the periods with very high salinities spanned a year or longer. From February 2010 to March 2012, very high salinities spanning long durations were observed in the Banana River Lagoon and Northern IRL, with the longest periods (16–23 months) occurring at IRLI06, IRLI07, IRLI02, IRLB02, IRLB04, IRLNFH01, IRLB09, IRLB06, IRLI18, IRLI10, IRLI13, IRLI15 and IRLI21 (Table 7). The data indicated that the algal bloom of 2011 occurred during a period of unusually high salinities in the Banana River Lagoon and Northern IRL, which indicated reduced exchange of water, limited dilution of nutrients and dispersion of phytoplankton, and a probable effect on the species composition of the superbloom. Table 7. Locations, months of occurrence and durations of high salinities lasting at least 12 months. Yellow highlights indicate locations and times associated with the superbloom; BR = Banana River Lagoon; NIRL = Northern Indian River Lagoon Sublagoon NIRL NIRL NIRL NIRL BR BR NIRL BR BR BR NIRL NIRL NIRL NIRL NIRL NIRL NIRL Station IRLI06 IRLI07 IRLI02 IRLI02 IRLB02 IRLB04 IRLI06 IRLNFH01 IRLB09 IRLB06 IRLI18 IRLI07 IRLI10 IRLI13 IRLI15 IRLI21 IRLI02 Year 2010 2010 2006 2010 2010 2010 2006 2010 2010 2010 2010 2007 2010 2010 2010 2010 1997 Start Month 5 5 10 6 7 7 12 8 8 9 8 2 11 11 11 8 1 Year 2012 2012 2008 2012 2012 2012 2008 2012 2012 2012 2012 2008 2012 2012 2012 2011 1997 End Month 3 3 8 3 3 3 8 3 3 3 2 8 3 3 3 11 12 Span 23 23 23 22 21 21 21 20 20 19 19 19 17 17 17 16 12 Water temperatures were atypically low for a maximum of only 4 consecutive months, with this event occurring in the Northern IRL during 1996. Nevertheless, the coldest water temperatures were recorded in 3.0× more months than expected based on data from years when seagrasses were “happy.” During the winters of 2009 and 2010, low water temperatures were observed for 2–3 months in all sublagoons (Table 8). The data indicated that water temperatures were unusually low for two consecutive winters, especially in the Northern IRL, which may have stressed seagrasses and caused mortality of other cold sensitive species, including key primary consumers that could have competed for nutrients that fueled the superbloom. 14 Table 8. Locations, months of occurrence and durations for cold water lasting at least 2 months. Yellow highlights indicate locations and times associated with the superbloom; NIRL = Northern Indian River Lagoon; NCIRL = North Central Indian River Lagoon; Seb = Sebastian; SCIRL = South Central Indian River Lagoon Category Severe stress Strong stress Sublagoon NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NIRL NCIRL NCIRL Seb NIRL NIRL NIRL NIRL NIRL NIRL NCIRL NCIRL NCIRL Seb Seb SCIRL Station IRLI02 IRLI18 IRLI21 IRLI02 IRLI06 IRLI02 IRLI06 IRLI07 IRLI10 IRLI13 IRLI15 IRLI18 IRLI02 IRLI06 IRLI02 IRLI06 IRLI07 IRLI10 IRLI13 IRLI15 IRLI18 IRLI21 IRLI23 IRLI24 IRLI28 IRLI07 IRLI10 IRLI13 IRLI15 IRLI18 IRLI21 IRLI23 IRLI24 IRLI26 IRLI28 IRLIRJ01 IRLIRJ04 Year 2010 2010 2010 2009 2009 2010 2010 2010 2010 2010 2010 2010 2009 2009 2010 2010 2010 2010 2010 2010 2010 2010 2010 2010 2010 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 Start Month 12 12 12 2 2 1 1 1 1 1 1 1 2 2 12 12 12 12 12 12 12 12 12 12 12 2 2 2 2 2 2 2 2 2 2 2 2 Year 2011 2011 2011 2009 2009 2010 2010 2010 2010 2010 2010 2010 2009 2009 2011 2011 2011 2011 2011 2011 2011 2011 2011 2011 2011 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 2009 End Month 1 1 1 3 3 3 3 3 3 3 3 3 4 4 1 1 1 1 1 1 1 1 1 1 1 3 3 3 3 3 3 3 3 3 3 3 3 Span 2 2 2 2 2 3 3 3 3 3 3 3 3 3 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Several conditions preceding and during the period of the superbloom were unusual. In particular, higher light attenuation coefficients (Kd; m-1) due to higher chlorophyll a concentrations (µg l-1), higher salinities and lower water temperatures were important influences on seagrasses, phytoplankton and other organisms. Overall, the superbloom period and location accounted for the longest durations of stress due to reduced light penetration (Table 9), which represents strong evidence that this unusual event deserves its moniker. 15 Table 9. Frequency of occurrence for unique combinations of light attenuation (Kd, m-1) and chlorophyll a concentrations (µg L-1). Yellow highlights indicate locations and times in the superbloom; BR = Banana River Lagoon; NIRL = Northern Indian River Lagoon; NCIRL = North Central Indian River Lagoon; Seb = Sebastian; SCIRL = South Central Indian River Lagoon Sublagoon BR NIRL NCIRL Seb SCIRL Duration (months) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 99th percentiles of data from years when seagrass was “happy” Kd (m-1) Chlorophyll a (µg L-1) > 1.91 > 15.14 > 1.07 > 27.65 > 2.53 > 22.88 > 1.93 > 28.08 > 3.76 > 21.05 Number of unique runs 285 187 108 58 58 32 35 23 21 16 17 12 15 8 12 5 7 4 7 3 6 2 4 3 3 3 2 2 2 2 1 1 Did spatiotemporal variations in water quality exhibit ecologically significant consistency? In order to test for consistent (i.e., statistically significant) variation in water quality parameters, a dataset was created by setting values at or below minimum detection limits to 0.1 times the relevant limit and by replacing missing values in the middle of time series with i) results of regressions between parameters, including between parameters related to each other through the Redfield ratio; ii) calculations from an optical model; iii) results of linear interpolation; and iv) values selected from stations displaying similar time series as determined by non-metric multidimensional scaling (an ordination method), hierarchical agglomerative clustering and a permutation test to identify groups. In total, less than 2% of data were adjusted. The resulting dataset comprised time series for a set of water quality parameters measured at stations within hydrodynamically distinct segments of the lagoon. This dataset contained seventeen (17) parameters representing variables in multivariate analyses, i.e.: • water temperature (°C), • pH (standard units), -1 • conductivity (μmhos cm ), • salinity (psu), -1 • color (platinum-cobalt units), • total suspended solids (mg L ), -1 • turbidity (ntu), • volatile suspended solids (mg L ), -1 • chlorophyll a (µg L-1), • total organic carbon (mg L ), -1 -1 • dissolved silicon dioxide (mg L ), • total phosphorus (mg L ), -1 • total dissolved phosphorus (mg L-1), • dissolved orthophosphate (mg L ), -1 -1 • total Kjeldahl nitrogen (mg L ), • total dissolved Kjeldahl nitrogen (mg L ), and -1 • light attenuation coefficient corrected for sun angle, Kd (m ). 16 The dataset contained values for each parameter across 4011 sampling events comprising: • combinations of five (5) time periods, i.e., distal antecedent period 1 (DAP1; March 1996–August 1999; 3 years and 3 months), distal antecedent period 2 (DAP2; September 1999–January 2003; 3 years and 5 months), distal antecedent period 3 (DAP3; February 2003–January 2006; 3 years), distal antecedent period 4 (DAP4; February 2006–January 2010; 4 years), proximal antecedent period (PAP; February 2010–February 2011; 13 months) and the superbloom event period (SEP; March 2011–March 2012; 13 months); • two (2) seasons nested within each time period, i.e., wet (May, June, July, August, September and October) and dry (November, December, January, February, March and April,); and • five (5) sublagoons with two (2) to seven (7) stations nested within them, i.e., Mosquito Lagoon (ML; IRLV05, IRLV11, IRLV17, and IRLML02); Banana River Lagoon (BR; IRLB02, IRLB04, IRLB06, and IRLB09); Northern Indian River Lagoon (NIRL; IRLI02, IRLI07, IRLI10, IRLI13, IRLI15, IRLI18, and IRLI21); North Central Indian River Lagoon (NCIRL; IRLI23 and IRLI27); and South Central Indian River Lagoon with Sebastian station IRLIRJ01 included to avoid a sublagoon with only one station (SCIRL; IRLIRJ01, IRLIRJ04, IRLIRJ05, and IRLIRJ07). Data were range standardized within a parameter across all sampling events (i.e., [value – minimum across sampling events]/[maximum across sampling events – minimum across sampling events]). This standardization scaled all parameters to values between 0 and 1, which reduced undue influence related to measurements with differing scales, e.g., pH values ranged from 6.18 to 9.55 and conductivities ranged from 14261 to 69400. The permutation analysis of variance indicated that water quality in the sublagoons followed statistically different temporal trajectories among seasons within periods. Overall, the results confirm interpretations from the categorization of water quality, i.e., the superbloom generated unusual and potentially stressful conditions. The light attenuation coefficient (Kd) rose 0.33–0.46 m-1 above historical levels from DAP4 onward in BR and NIRL (Figure 9), which would have reduced light reaching seagrasses. Light attenuation coefficients decreased by 0.11–0.32 m-1 from PAP to SEP in NCIRL and SCIRL, and Kd in ML rose from DAP4 to 0.12 m-1 above the level recorded in DAP1. Overall, the largest and most unusual changes in Kd occurred in BR and NIRL. ML BR NIRL NCIRL SCIRL 2.5 Kd ± SE (m-1) 2.0 1.5 1.0 0.5 0.0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 9. Mean light attenuation coefficients (Kd) ± standard errors (SE). This reduction in light penetration can be attributed to increased turbidity that was due to increased concentrations of volatile suspended solids (organic matter) and chlorophyll a (indicator of phytoplankton biomass), with concentrations of total suspended solids and color exhibiting no corresponding increase 17 (Figure 10). Thus, the increased attenuation of light primarily was due to organic matter in the form of phytoplankton rather than increases in non-organic suspended solids or color. ML BR NIRL NCIRL SCIRL Turbidity ± SE (ntu) 15 10 5 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 ML BR NIRL NCIRL DAP2 DAP3 SEP BR NIRL NCIRL SCIRL Chlorophyll a ± SE (µg L-1) 80 10 5 0 60 40 20 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 ML DAP2 BR DAP3 NIRL DAP4 NCIRL PAP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet SEP DAP1 SCIRL ML DAP2 BR DAP3 NIRL DAP4 NCIRL PAP SEP SCIRL 40 Color ± SE (Pt-Co units) 40 TSS ± SE (mg L-1) PAP ML SCIRL 15 VSS ± SE (mg L-1) DAP4 30 20 10 0 30 20 10 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 10. Mean turbidity and mean concentrations of volatile suspended solids (VSS), chlorophyll a, total suspended solids (TSS) and color ± standard errors (SE). The increase in phytoplankton biomass corresponded with increases in concentrations of total phosphorus, total Kjeldahl nitrogen and total organic carbon, which included nutrients assimilated into the phytoplankton (Figure 11). In contrast, concentrations of total dissolved Kjeldahl nitrogen and dissolved orthophosphate did not increase, which suggested no exceptional loadings of inorganic nutrients (Figure 12). Total dissolved phosphorus and dissolved silicon dioxide concentrations did increase (Figure 13). 18 These data suggested an increased load of phosphorus from some source and an increase in available silicon, potentially related to relatively low abundances of diatoms leading to decreased uptake. BR NIRL NCIRL ML SCIRL BR NIRL NCIRL SCIRL 2.0 TKN ± SE (mg L-1) Total phosphorus ± SE (mg L-1) ML 0.15 0.10 0.05 0.00 1.5 1.0 0.5 0.0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP Total organic carbon ± SE (mg L-1) ML Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet SEP BR DAP1 NIRL NCIRL DAP2 DAP3 DAP4 PAP SEP SCIRL 25 20 15 10 5 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 11. Mean concentrations of total phosphorus, total Kjeldahl nitrogen (TKN) and total organic carbon ± standard errors (SE). ML BR NIRL NCIRL SCIRL ML BR NIRL NCIRL SCIRL 0.15 Dissolved PO4 ± SE (mg L-1) TKND ± SE (mg L-1) 2.0 1.5 0.10 1.0 0.05 0.5 0.0 0.00 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 12. Mean concentrations of total dissolved Kjeldahl nitrogen (TKND) and dissolved orthophosphate (PO4) ± standard errors (SE). 19 ML BR NIRL NCIRL SCIRL ML BR NIRL NCIRL SCIRL 10 Dissolved SiO2 ± SE (mg L-1) TPD ± SE (mg L-1) 0.15 0.10 0.05 0.00 8 6 4 2 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 SEP DAP2 DAP3 DAP4 PAP SEP Figure 13. Mean concentrations of total dissolved phosphorus (TPD) and total dissolved silicon dioxide (SiO2) ± standard errors (SE). Conductivity increased in all segments from DAP3 onward, as did salinity (Figure 14). These increases, especially the 17020–19594 μmhos cm-1 increases in conductivity and 12.4–14.0 psu increases in salinity in BR and NIRL, suggested less inflow of freshwater, more evaporation, less mixing and an increased residence time for water, nutrients and phytoplankton. BR NIRL NCIRL SCIRL ML BR NIRL NCIRL SCIRL 40 50000 Salinity ± SE (psu) Conductivity ± SE (µmhos cm-1) ML 60000 40000 30000 20000 30 20 10 10000 0 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 14. Mean conductivities and salinities ± standard errors (SE). In contrast, there was little to no change or difference in pH among the segments and periods and a 1–2°C decrease in mean water temperature in all segments during PAP (Figure 15). Although relatively minor, a widespread 1–2°C decrease in water temperature over months may represent a significant stress on cold sensitive organisms. 20 ML BR NIRL NCIRL SCIRL ML 8 pH ± SE BR NIRL NCIRL SCIRL 30 Tenmperature ± SE (°C) 10 6 4 2 0 20 10 0 Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet Dry Wet DAP1 DAP2 DAP3 DAP4 PAP SEP Figure 15. Mean pH values and water temperatures ± standard errors (SE). In combination, the results indicated significant and historically unprecedented changes in water quality in BR and NIRL before and during the superbloom. Based on changes in conductivity and salinity, residence times increased. Increased concentrations of chlorophyll a and volatile suspended solids, both related to high phytoplankton abundances, led to an increase in turbidity and light attenuation, which stressed seagrasses. Non-organic suspended particles and color contributed relatively little to the increase in light attenuation. As the biomass of phytoplankton increased, so did the concentrations of phosphorus, nitrogen and carbon sequestered in these biological particles. There was no evidence of increased loadings of dissolved orthophosphate or dissolved Kjeldahl nitrogen, but total dissolved phosphorus concentrations did increase, which suggested an increased loading of some compound(s) containing this nutrient. In addition, the absence of diatoms may have led to decreased uptake of dissolved silicon dioxide and a subsequent increase in its ambient concentration. Differences in pH were unlikely to be biologically significant, but the cold winters of 2010 and 2011 decreased mean water temperatures in all segments by 1–2°C, which could have led to mortality of key plants and animals. Overall, the superbloom lived up to its moniker. What were the effects from the 2011 superbloom? One substantial effect of the superbloom was a loss of seagrasses in the superbloom region (Figure 16). Overall, transect lengths (the distance that seagrasses extended offshore) decreased during and following the superbloom (Figure 17). Seagrasses disappeared from the transect in segment IR8, but they were less affected at transects in segments IR1-3, IR4 and IR5, which may relate to the delayed arrival of the superbloom in these segments (Figure 17). 21 Figure 16. The sublagoons, segments, and locations of seagrass transects within the superbloom area, with green depicting seagrass coverage before the superbloom event and blue representing open water. Mean transect lenghth (m) ± SE BR1-2 600 BR3-5 BR7 IR1-3 IR4 IR5 IR6-7 IR8 400 200 0 DAP-2 DAP-3 DAP-4 PAP SEP PEP Figure 17. Changes in transect lengths (m) within segments of the Indian River Lagoon during the four distal antecedent periods (DAP-2 to DAP-4), the proximal antecedent period (PAP), the superbloom event period (SEP), and the post-event period (PEP). Mapping of seagrass from aerial photography indicates that seagrass coverage in the superbloom area had generally increased during mapping years prior to the superbloom event (Figure 18). In 2009, there were approximately 57,000 acres of seagrass within the superbloom area (Figure 18). Photography for the 2011 map was taken two months before peak bloom conditions, but it still detected loss of seagrass in most segments except IR1-3 and ML3-4 (Figure 19). The greatest loss occurred in the Banana River Lagoon (BRL), where approximately 24,000 acres mapped in 2009 was reduced by 87% to approximately 3,000 acres (Figure 19). 22 70000 Seagrass cover (acres) 60000 50000 40000 30000 20000 10000 0 1992 1994 1996 1999 2003 2005 2007 2009 Figure 18. Changes in seagrass cover (acres) within the superbloom region. Figure 19. Seagrass coverage in 2011 (green) and 2009 (red crosshatch) for segments IR4, IR5, IR6-7, IR8, BR1-2, BR3-5 and BR7. 23 The large losses in seagrass during the superbloom event correlated with changes in chlorophyll a concentrations (Figure 20), which was expected given the effect of chlorophyll a on light attenuation (Kd). Results of sampling at Transect 17 in the southern Banana River Lagoon (BR-7) illustrated the cumulative effect of high chlorophyll a concentrations on mean percentage cover within the median length of the transect, depth at the edge of the seagrass canopy, and transect length. Based on the categorization of chlorophyll a concentrations for Banana River Lagoon, seagrasses along Transect 17 were subjected to strong to severe bloom conditions consistently from July 2010 onward (chlorophyll a > 10.26 µgl-1). During these 15 months, all metrics of seagrass health declined to zero. Transect 17 Figure 20. Three-month average chlorophyll a concentrations from May 1996 to October 2011 compared to mean seagrass cover within the median transect length (top panel), depth at the edge of the seagrass canopy (middle panel), and transect length to the edge of the seagrass canopy (bottom panel) along Transect 17. Green dots represent values prior to 2010, red dots represent values in 2010–2011, blue arrows link consecutive dates in 2010, and red arrows link consecutive dates in 2011 24 Although reduced light penetration caused the expected detrimental effect on seagrasses, high salinity and low water temperature may have contributed to the loss of seagrasses. To address these possibilities, Habitat Suitability Indices (HSIs) were developed to identify responses of seagrasses to a range of salinities, temperatures, and light levels. The approach relied on optimum values for temperatures and salinities, i.e., high and low values represented less suitable conditions, and an increase in suitability with increasing light availability that leveled off at high light levels (Mazzotti et al. 2007). All indices varied from 0 to 1, with lower values being less suitable for seagrasses. For each month from January 2010 to December 2011, Temperature Suitability Indices (TSIs), Salinity Suitability Indices (SSIs), and Light Suitability Indices (LSIs) were calculated from monthly data obtained at relevant water quality stations. In addition, mean monthly indices were calculated from data for all months in 1999–2009. Furthermore, the combined influence of all three parameters was evaluated using relative condition indices (RCIs) calculated as the products of the other three suitability indices (RCI = TSI × SSI × LSI). The duration of stressful RCIs varied among stations, with the most northern station in the Indian River Lagoon (in segment IR1-3) receiving stress only during the last months of 2011. In contrast, water quality near the epicenter of the superbloom in the Banana River Lagoon (IRLB02) translated to persistent stress (RCI < 0.5) at depths > 1.0 m through much of 2010 and 2011 (Figure 21, Table 10). Both the monthly and long-term average TSIs indicated low temperature was a potential source of stress for two to three months during winters (Table 10). In contrast, the monthly SSIs during 2010–2011 ranged from 0.87 to 1.00, which suggested less stressful conditions than those recorded from 1999–2009 (0.64 ≤ SSI ≤ 0.82; Table 10). During wet seasons, low salinity in water shallower than 1.0 m can be a significant stressor, but rainfall was scarce so salinities remained suitable in 2010 and 2011. Overall, light represented the most persistent and critical stress, with many LSIs at 1.0 and 1.5 m being substantially less than their long-term averages (Table 10). Nevertheless, low water temperatures during the winters of 2009–2010 and 2010–2011 may have exacerbated light stress. Banana River Lagoon, segment BR1-2, water quality station IRLB02, near Transect 12 Figure 21. Relative Condition Indices (RCIs) for seagrasses based on temperature suitability indices (TSIs), salinity suitability indices (SSIs) and light suitability indices (LSIs). Suitable conditions exist when the blue dotted line (RCI based on LSI at 1.0 m, TSI and SSI) exceeds 0.5 or the colored band (range of RCI from 0.5 m to 1.5 m based on variation in LSIs and constant TSIs and SSIs) is narrow and exceeds 0.5. 25 Table 10. Habitat Suitability Indices for seagrasses, with mean monthly averages for 1999–2009 in parentheses. Bold numbers indicate the largest value in each cell, red indicates monthly indices < 0.01, orange indicates monthly indices < 0.2, and yellow indicates monthly indices < 0.3 Month–Year Jan 2010 Feb 2010 Mar 2010 Apr 2010 May 2010 June 2010 July 2010 Aug 2010 Sept 2010 Oct 2010 Nov 2010 Dec 2010 TSI 0.03 (0.28) 0.27 (0.31) 0.09 (0.46) 0.68 (0.59) 0.95 (0.87) 1.00 (1.00) 1.00 (0.98) 0.79 (0.88) 1.00 (1.00) 0.84 (0.90) 0.80 (0.58) 0.14 (0.43) Monthly indices and mean indices for 1999–2009 SSI LSI @ 0.5 m LSI @ 1.0 m LSI @ 1.5 m 0.92 (0.64) 0.85 (0.71) 0.68 (0.44) 0.52 (0.27) 0.90 (0.69) 1.00 (0.93) 0.85 (0.62) 0.63 (0.34) 0.90 (0.71) 1.00 (0.99) 1.00 (0.71) 1.00 (0.42) 0.87 (0.76) 1.00 (0.95) 1.00 (0.78) 0.73 (0.62) 0.89 (0.82) 1.00 (1.00) 0.90 (0.93) 0.16 (0.68) 0.91 (0.82) 1.00 (1.00) 0.20 (0.93) 0.00 (0.61) 0.93 (0.79) 1.00 (1.00) 0.44 (0.85) 0.00 (0.42) 0.96 (0.79) 1.00 (1.00) 0.34 (0.87) 0.00 (0.54) 0.95 (0.69) 0.87 (0.96) 0.21 (0.64) 0.00 (0.31) 0.96 (0.68) 1.00 (0.87) 0.43 (0.45) 0.01 (0.19) 0.97 (0.66) 0.62 (0.74) 0.26 (0.45) 0.01 (0.26) 0.97 (0.69) 0.60 (0.56) 0.25 (0.29) 0.00 (0.13) RCI @ 1.0 m 0.02 (0.08) 0.20 (0.14) 0.08 (0.24) 0.59 (0.36) 0.76 (0.66) 0.19 (0.77) 0.41 (0.66) 0.26 (0.60) 0.20 (0.46) 0.35 (0.26) 0.20 (0.17) 0.03 (0.09) Jan 2011 Feb 2011 Mar 2011 Apr 2011 May 2011 June 2011 July 2011 Aug 2011 Sept 2011 Oct 2011 Nov 2011 Dec 2011 0.16 0.40 0.40 0.48 0.93 1.00 1.00 0.68 1.00 0.85 0.73 0.44 0.97 0.97 0.98 0.98 0.99 0.99 1.00 0.99 0.99 0.99 0.96 0.96 0.08 0.05 0.00 0.00 0.07 0.00 0.00 0.00 0.00 0.00 0.09 0.08 (0.28) (0.31) (0.46) (0.59) (0.87) (1.00) (0.98) (0.88) (1.00) (0.90) (0.58) (0.43) (0.64) (0.69) (0.71) (0.76) (0.82) (0.82) (0.79) (0.79) (0.69) (0.68) (0.66) (0.69) 0.75 0.73 0.53 0.51 1.00 0.78 0.76 0.57 0.61 0.40 0.58 0.58 (0.71) (0.93) (0.99) (0.95) (1.00) (1.00) (1.00) (1.00) (0.96) (0.87) (0.74) (0.56) 0.49 0.13 0.00 0.00 0.08 0.00 0.00 0.00 0.00 0.00 0.13 0.19 (0.44) (0.62) (0.71) (0.78) (0.93) (0.93) (0.85) (0.87) (0.64) (0.45) (0.45) (0.29) 0.27 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 (0.27) (0.34) (0.42) (0.62) (0.68) (0.61) (0.42) (0.54) (0.31) (0.19) (0.26) (0.13) (0.08) (0.14) (0.24) (0.36) (0.66) (0.77) (0.66) (0.60) (0.46) (0.26) (0.17) (0.09) In addition to stress associated with temperature, salinity and light, stress in many estuaries subject to eutrophication (increased amounts of organic matter) arises when sulfate reducing bacteria decompose organic matter on or in the sediment and generate sulfide (H2S) as a byproduct of their metabolism (Jorgensen 1982; Holmer et al. 2003). Sulfide can be toxic to seagrasses and other plants (Terrados et al. 1999). When seagrasses are healthy and receiving sufficient light, oxygen produced during photosynthesis diffuses to and through the rhizomes to form a microshield that prevents sulfide intrusion (Calleja et al. 2007). If the rate of sulfide production is too high or the rate of photosynthesis is too low, this oxygen microshield breaks down or disappears, and sulfide diffuses from the porewater into the tissues of seagrasses causing damage to meristems, the sites of growth (Borum et al. 2005). Generally, conditions in the sediments of the IRL effectively preclude high concentrations of sulfide because they contain relatively little organic material, contain moderate to high levels of iron (Fe), and generally remain oxic within and below the seagrass rhizosphere, particularly when seagrasses are healthy and dense. Although fine sediments with moderately high organic matter (i.e., muck) can be found, sediments in the IRL seagrass usually comprise a mixture of quartz sands, siliceous sands and silts, and biogenic calcium carbonate (shell hash), with a very low percentage of organic material that can be metabolized by sulfate reducing bacteria. In fact, it is very common to see sediments with a light coating of seagrass or algal detritus, and less common to see sediments with a heavy layer of fine-grained or flocculent organic detritus except in a few, relatively small embayments that are protected from the wind or hydrodynamically restricted (personal observations of J. Steward). Furthermore, as seagrass disappeared during the 2011 superbloom, it exposed dark-grey sands to bone-white sands and shell hash. Sediments in the IRL also have relatively high concentrations of Fe (J. Trefry, personal communication, 7 December 2013; Moutusi et al. 2010), which mitigates the build-up of H2S by binding the sulfur as 26 insoluble iron sulfide (FeS). In addition, the non-muck sediments throughout the IRL generally exhibit the presence of oxygen to depths of tens of centimeters, which is facilitated by vertical recirculation of water. In such situations, sulfides, including FeS, undergo oxidation to sulfate, and this oxidation can be rapid at the sediment-water interface and around burrows of animals (Jorgensen and Revsbech 1983). Among the seven species of seagrass found in the Indian River Lagoon, Thalassia testudinum may be more sensitive to sulfide because field experiments documented mortality of all T. testudinum, most Syringodium filiforme, and almost no Halodule wrightii (email from P. Carlson, Florida Fish and Wildlife Conservation Commission to J. Steward, L. Morris and R. Chamberlain, 27 June 2012). In another instance, T. testudinum grew or maintained high shoot densities when exposed to 2–10 mM of sulfides (Koch et al. 2007a), but high temperatures (≥ 35oC) and high salinities (55–65 psu) led to a die-back at sulfide concentrations of 6 mM (Koch et al. 2007b). Both S. filiforme and H. wrightii exhibit greater resistance to sulfide toxicity, with sulfide concentrations needing to be much higher than 2 mM to cause mortality (Pulich 1983; Koch et al. 2007b). Given these physiological tolerances, loss of seagrass due to sulfide toxicity in the superbloom region was unlikely because Halodule wrightii is the dominant seagrass in the Indian River Lagoon, with S. filiforme being next most common and T. testudinum found south of Sebastian Inlet and not in the superbloom region. Sulfide toxicity was explored as the cause of seagrass loss in western Turnbull Bay, an embayment in the uppermost reach of the northern IRL (Morris and Virnstein 2004). The sediments became covered with 10–15 cm of organic ooze and flocculent material primarily created from seagrass detritus. Within the year before the summer of 1997, all seagrasses in this bay disappeared (> 100 hectares). However, the seagrass bed had been stressed for years prior to this loss. In the early 1990s, there were “…almost as many dead [leaf blades] as alive.” By the summer of 1994, the bed was very patchy, and by 1995, Ruppia maritima had superceded H. wrightii as the dominant plant, which suggested stress from low salinity because R. maritima is more tolerant of such conditions. Additionally, the seagrasses were not firmly attached to the accumulated organic floc. Sulfide concentrations in the sediment were measured after the die-off, with concentrations < 2.5 mM at a shallow site and just above 1.5 mM at a deeper site. The ability of R. maritima to tolerate such conditions is not known, but H. wrightii to should have survived unless subject to additional stress, like light limitation. It is possible that a storm swept away the weakly attached seagrasses and the organic floc leaving behind a sandy substrate. Overall, sulfide stress was unlikely to be the sole cause of seagrass loss during the 2011 superbloom. P. Carlson surmised that “… sulfide toxicity could [have been] the proximal cause of [seagrass] death, but it [was] not the ecologically-relevant cause of death. In this case [referring to the 2011 superbloom event], sulfide intrusion could result from light stress which alters the redox balance of the roots. While it [may be] true that the final blow to the plant was sulfide intrusion, the cause of mortality was light stress. Furthermore, once roots and rhizomes begin to die, the decomposition of dead belowground tissue can add stress to surviving seagrasses.” (email from P. Carlson, Florida Fish and Wildlife Conservation Commission, to J. Steward, L. Morris and R. Chamberlain, 27 June 2012). Regardless of the causes, the expansive loss of seagrasses could ultimately generate economic effects, especially on fisheries. For example, the harvest of seatrout is influenced by seagrass coverage (Figure 22). In fact, seagrasses are reported to generate $5,000–$10,000 of fishery production acre-1 year-1 (Hazen and Sawyer 2008; SJRWMD unpublished data). Thus, the loss of seagrass in 2011 represented a potential reduction of $150–$320 million in 2011–2012. 27 Figure 22. Relationship between harvest of spotted seatrout (Cynoscion nebulosus) and seagrass coverage. Seatrout data from the Florida Fish and Wildlife Conservation Commission; seagrass data from SJRWMD Did the “bottom-up” influence of nutrient inputs contribute to the 2011 superbloom? Phytoplankton blooms can be driven from the “bottom-up” via increased nutrient inputs. Three sources of nutrients were examined: external nutrient loads carried in freshwater inputs, loads from submarine groundwater and diffusive flux from sediments comprising “muck,” and loads and lack of uptake associated with changes in the biomass of drift macroalgae. External nutrient loads typically reach the lagoon during and after rainfall. Drier than average conditions preceded the superbloom as shown by departures of average monthly rainfall measured by radar from the 30-year average rainfall measured by rain gauges (Figure 23), which meant external loads were less likely to be a major factor driving the bloom. In fact, statistical analysis showed that although external loads of nitrogen did vary significantly among seasons within periods and segments (Figure 24), there was no evidence of increased loading in the proximal antecedent period (PAP) or superbloom event period (SEP). Nitrogen loads were consistently higher in segments BR1-2 and IR8. Another statistical analysis showed that external loads of phosphorus exhibited a more complex pattern, with significant variation among periods that differed among segments (Figure 25). In particular, increases in external phosphorus load during the superbloom event period (SEP) were noted in BR6, BR7, IR4, IR7 and IR8. These increases paralleled increases in total dissolved phosphorus noted in the permutation analysis of variance (Figure 13), and they may have contributed to initiation of the superbloom. 28 Rainfall departure (inches) from 30-year mean Figure 23. The fourth distal antecedent period (DAP4), proximal antecedent period (PAP), and the superbloom event period (SEP) with their beginning and end dates and departures from long-term mean rainfall. 29 0.20 Total nitrogen load (g m-2) ± SE Total nitrogen load (g m-2) ± SE 0.4 0.3 0.2 0.1 0.15 0.10 0.05 0.0 Wet Dry DAP-2 Wet Dry Wet DAP-3 Dry Wet DAP-4 Dry PAP Wet Dry 0.00 BR1-2 BR3-5 BR6 SEP BR7 IR1-3 IR4 IR5 IR6-7 IR8 Figure 24. Variation in external nitrogen loads among seasons within periods and segments. Red bars are means for the superbloom 0.04 0.03 0.02 0.01 0.00 DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP DAP-2 DAP-3 DAP-4 PAP SEP Total phosphorus load (g m-2) ± SE 0.05 BR1-2 BR3-5 BR6 BR7 IR1-3 IR4 IR5 IR6-7 IR8 Figure 25. Variation in external phosphorus loads among seasons within periods and segments. Red bars are means for the superbloom In order to assess nutrient inputs from submarine groundwater and diffusive flux from sediments comprising “muck,” relevant data, literature and other information were collated and synthesized (e.g., Martin et al. 2007; Martin and Cable 2008; Pandit et al. 2009; Trefry et al. 1990; Trefry et al. 1992; Riegl et al. 2009). In general, sparse data supported only rough estimates of nitrogen (N) and phosphorus (P) loads delivered by meteoric groundwater and fluxes generated in sediments, particularly in muck. Water is advected downward and upward through the natural sandy sediments of the lagoon to a depth of ~70 cm (Figure 26). This process is driven by pumping associated with waves and tides, seasonal variations in the elevations of water in the lagoon and the water table, and bio-irrigation by animals that pump water into their burrows (Martin et al. 2004). Water is recirculated at rates of ~10–100 cm d-1, which are 100–1000× greater than rates of movement for meteoric groundwater (Martin et al. 2007). Consequently, the concentrations of nutrients and salts in the porewater within the re-circulation zone are equivalent to or in equilibrium with water in the lagoon. Thus, recirculated lagoon water typically represents a means of rapidly cycling nutrients through the sediments rather than a source of new nutrients. 30 Submarine processes that regulate nutrient loading to the IRL System nearshore seepage face(see Fig. 2 for West strong advection recirculation recirculation recirculation diffusion diffusion Terrestrial SGD strong advection Terrestrial SGD muck ICW East nearshore seepage face Lagoon more detail on this area) weak advection weak advection Meteoric Groundwater Discharge (a.k.a. terrestrial submarine groundwater discharge): produces flow rates of 0.02 – 0.9 m 3/d/m at seepage face (the sediment/water interface near shorelines). The upward advection of MGWD weakens toward center of Lagoon and becomes overwhelmed by Lagoon re-circulation (see below) in top layer (upper 70 cm) of sandy sediments. Re-circulated Lagoon water: exists where natural sandy sediments are present and was measured to ~70 cm sediment depth, may possibly go deeper in some areas. Bio-irrigation is a significant component of this process in the Lagoon. No new internal loading by this process – just recycling (nutrient burial is negligible in re-circulation zones). Diffusion from muck sediment: exists where muck is present; it’s the major process regulating nutrient loading from these fine-grain sediments (intermittent resuspension loading occurs but not measured). Advective loading via MGWD is precluded in muck areas because of muck’s low hydraulic conductivity. Muck appears to be the greatest benthic source of nutrients in many segments. The sources of information that was used to develop this schematic of submarine processes are Martin et al. (2007); Martin and Cable (2008); Martin et al. (2004); Pandit et al. (2009); Trefry et al. (1990); and Trefry et al. (1992). Figure 26. Conceptual model of submarine nutrient inputs to the Indian River Lagoon. Muck sediment has a high percentage of fine-grain clays and silts (> 60% dry weight; Trefry et al. 1990) and, thus, a low hydraulic conductivity, effectively precluding re-circulation of lagoon water and seepage of meteoric groundwater (A. Pandit and J. Martin, personal communication, August 2012). Without these advective processes, diffusion becomes a key process, with N and P released to the water column as ammonium and phosphate 1 (Figure 26). Muck represents a relatively new source of nutrients because it is actually a displaced watershed source comprising eroded terrestrial soils and organic matter, such as grass clippings, carried to the lagoon within the past 50 years (Trefry et al. 1990). Diffusive flux increases during the warm months of June–September to reach 730 kg N km-2 mo-1 and 231 kg P km-2 mo-1, with rates of 484 kg N km-2 mo-1 and 98 kg P km-2 mo-1 recorded during the cool months of December–March (Trefry et al. 1992). These fluxes can be converted to loads by scaling them to the area of muck found in each segment of the lagoon where the superbloom occurred. The areal coverage of muck that is ≥ 5 cm thick was delineated as the union coverage from two complementary surveys (Trefry et al. 1990; Riegl et al. 2009; Figure 27). Although the union coverage represents the best estimate to date, it likely underestimates the total area covered by muck in the superbloom region because the northern, restricted segments (BR1-2) and the extremely shallow BR6 segment were not surveyed. 1 Although intermittent and not measured in the Indian River Lagoon, flux associated with resuspension of muck may be important. 31 A B Figure 27. Muck deposits (tan) in the northern portion of the Indian River Lagoon (A) and in a more southern portion of the Indian River Lagoon and Banana R. Lagoon (B). Segment BR1 was not surveyed, and segment IR11 is outside the superbloom region The surficial aquifer probably represents the primary source of meteoric groundwater discharge (MGWD) and nutrient loads to the IRL system (Martin et al. 2007; Martin and Cable 2008; Pandit et al. 2009; Figure 26), because the deeper Floridan aquifer probably influences only the extreme northern portion of the system where the Hawthorn confining layer is comparatively shallow and thin (Toth 1987). Meteoric groundwater inputs from the western or mainland coast of the IRL are larger than those coming from Merritt Island or the barrier islands on the eastern shore due to a larger watershed and greater potentiometric gradient or hydraulic head (Figure 26; Pandit et al. 2009). For example, discharge rates near Palm Bay ranged from 1.45 to 1.69 m3 d-1 m-1 along the western shoreline and 0.001 to 0.100 m3 d-1 m-1 along the eastern shoreline, and seepage rates along the western and eastern shorelines at Titusville were ~0.40 to 0.42 m3 d-1 m-1 and 0.001 to 0.002 m3 d-1 m-1, respectively (Pandit et al. 2009). In addition, nearshore seepage faces 2 can vary in width among places and times depending on hydraulic conductivity and hydraulic head (Martin et al. 2007). For example, inputs of 0.02–0.90 m-3 d-1 m-1 were recorded from a seepage face that ran 22–25 m out from the shore at Eau Gallie (Martin et al. 2007). Furthermore, seepage faces are virtually nonexistent adjacent to wetlands because subsurface flow is stymied by the wetland’s sluggish hydraulic conductivity and nearly flat hydraulic gradient (A. Pandit, email 2 August 2012; Pandit et al. 2009). Meteoric groundwater does pass under the nearshore seepage faces, with flows 2 A seepage face is defined as the sediment-water interface extending waterward from the lagoon shoreline where meteoric groundwater discharge is measurable. 32 into the lagoon passing upward through sediments with high hydraulic conductivities and flows gradually weakening toward the center of the lagoon (Figure 26). Calculations of nitrogen (N) loads from MGWD originating on the mainland were based on fluxes of 44.8 mg NH4-N m-2 d-1 or 1.036 g NH4-N m-1 d-1 of non-wetland shoreline and a 23-m seepage face (Martin and Cable 2008 and Martin et al. 2007). Results were converted to an annual areal loading of 16,352 kg N km-2 yr-1, which was scaled to the total area of the seepage face in each segment to obtain total mass loading as kg N segment-1 yr-1, with monthly loads estimated as 1/12th of the annual load. Although the width of the seepage face likely varies along the length of the IRL, this variability has not been documented, so a constant width of 23 m was assumed. Phosphorus (P) loads from the mainland were based on a model of groundwater flow and shoreline length (Pandit et al. 2009), with phosphorus concentrations obtained from wells distributed from Titusville to Palm Bay (source file from G. Belaineh, SJRWMD). The resulting annual load per km of mainland shoreline (12.97 kg P km-1 yr-1) was scaled to shoreline length for each segment to yield kg of P load segment-1 yr-1, with monthly loads taken as 1/12th of the annual load. Loads of N and P delivered from MGWD originating on the barrier islands and Merritt Island were derived from concentrations and flows (Pandit et al. 2009). The resulting values, 0.339 kg N km-1 of shoreline yr-1 and 0.034 kg P km-1 of shoreline yr-1, were scaled to shoreline length for each segment to generate kg of N or P segment-1 yr-1, with monthly loads estimated as 1/12th of the annual loads. For all calculations of loads, shoreline lengths or seepage-face areas abutting wetlands were removed before loads were scaled to segments (Pandit et al. 2009). Moreover, loads of N and P in MGWD originating from all shorelines were assumed to be zero during the December–May dry season (Pandit et al. 2009). Based on these calculations, total loads of N and P per segment from MGWD and muck generally increased from north to south in concert with the north-to-south increases in muck (Table 11; Figure 27); high-density, urban land cover; and non-wetland shorelines (Table 11; Figure 27). Nutrient loads from muck range from 84% to nearly 100% of all N loads and 97% to nearly 100% of all P loads (Table 11). For some segments, loads of N and P from muck equal or exceed loads delivered in surface water leaving the watershed. For example, the annual N load from muck in segment IR6-7 (~48,400 kg) nearly equals the nonpoint loading of ~53,000 kg (Gao 2009); and the annual N loads from muck in IR8 and IR9-11 (~21,000 kg and 156,000 kg, respectively) are approximately twice the nonpoint loads (~11,000 kg and ~70,150 kg, respectively; Gao 2009). Table 11. Loads of nitrogen (N) and phosphorus (P) from meteoric groundwater discharge (MGWD) and muck. Segment BR1-2 BR3-5 BR7 IR1-3 IR4 IR5 IR6-7 IR8 IR9-11 Sum MGWD 2 27 3 1 763 4046 8747 4025 9455 27069 N (kg yr-1) Muck 4888 117466 6539 5329 9671 30441 48424 20562 155768 399087 Total 4890 117493 6542 5330 10434 34487 57171 24587 165223 426156 Muck (% of total) 99.95 99.98 99.96 99.98 92.69 88.27 84.70 83.63 94.28 93.65 MGWD 0.2 2.7 0.3 0.1 29.0 155.1 291.9 142.3 336.2 957.9 P (kg yr-1) Muck Total 1280.5 1280.8 30772.4 30775.1 1713.0 1713.3 1396.1 1396.2 2533.4 2562.4 7974.6 8129.6 12685.5 12977.5 5386.5 5528.9 40806.4 41142.6 104548.4 105506.3 Muck (% of total) 99.98 99.99 99.98 99.99 98.87 98.09 97.75 97.43 99.18 99.09 33 Nutrient loads from muck and MGWD are lowest in BR1-2 because vast wetlands lining the segment’s shoreline preclude inputs of MGWD and surveys have revealed comparatively little muck (Table 10; Figure 27). Restricted access obviated surveys in a large portion of the segment, and the presence of dredged areas suggests that muck may be more prevalent than what has been documented. Similarly, undocumented muck may contribute to nutrient loads in segment BR6, because that shallow segment has not been surveyed. Estimated monthly loads of N and P from MGWD and muck indicate that nutrient inputs in the wet, summer months of June–September can be ~2× higher than inputs during the dry, winter months of January–March (~1.5× higher for N and 2.4× higher for P). Unfortunately, the simple models of seasonal and annual loads do not generate estimates that vary among years (Figures 28 and 29); therefore, the estimates are most useful for documenting that N and P loads in Banana River Lagoon are at least 2× higher than loads to other regions. Thus, these loads may represent a bottom-up influence that helped initiate and sustain the superbloom, which began in Banana River Lagoon. Additional data with higher temporal and spatial resolution would support a more robust model of nutrient loads from muck and MGWD. 34 IR8 8,000 6,000 4,000 8,000 6,000 4,000 BR3-5 14,000 BR6 12,000 12,000 10,000 8,000 6,000 4,000 2,000 2,000 0 0 2012 2009 2008 2007 2011 14,000 2011 0 2012 0 2011 2,000 2012 2,000 2010 4,000 2010 6,000 2009 8,000 2010 10,000 2009 12,000 2008 12,000 2007 BR1-2 2008 14,000 2007 14,000 2006 0 2005 0 2006 2,000 2005 2,000 2006 4,000 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 IR1-3 2005 6,000 2004 12,000 2004 IR5 2004 12,000 2003 14,000 2002 14,000 2003 0 2002 0 2003 2,000 2001 2,000 2002 8,000 2000 4,000 2001 6,000 2000 8,000 Nitrogen load (kg month-1) 12,000 2001 10,000 Nitrogen load (kg month-1) 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Nitrogen load (kg month-1) 10,000 2000 10,000 Nitrogen load (kg month-1) 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Nitrogen load (kg month-1) 12,000 2001 10,000 Nitrogen load (kg month-1) 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Nitrogen load (kg month-1) 14,000 2000 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Nitrogen load (kg month-1) 14,000 IR4 10,000 8,000 6,000 4,000 IR6-7 10,000 8,000 6,000 4,000 Figure 28. Estimates of nitrogen loads in groundwater and diffusing from muck for stations in the Indian River (IRL1-3, IRL4, IRL5, IRL6-7 and IRL8) and Banana River (BR1-2, BR3-5 and BR6) lagoons. 35 200 0 0 1,800 BR3-5 1,800 BR6 1,600 1,600 1,200 800 600 400 1,400 1,200 1,000 800 600 200 400 200 0 0 2012 200 2012 400 2011 600 2011 800 2010 1,200 2009 1,600 2010 1,600 2009 1,800 2008 IR8 2007 1,800 2008 2012 2011 2010 2009 2008 2007 0 2007 200 0 2006 200 2006 400 2006 600 2005 800 2004 1,200 2004 IR5 2005 1,600 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 IR1-3 2005 1,600 2004 1,800 2003 1,800 2002 0 2003 0 2002 200 2003 200 2001 400 2001 600 Phosphorus load (kg month-1) 800 2000 2012 1,000 2001 1,000 Phosphorus load (kg 1,400 month-1) 1,200 2000 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 1,600 2002 1,000 Phosphorus load (kg 1,400 month-1) 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2000 2001 Phosphorus load (kg month-1) 1,400 2000 2012 2000 2001 Phosphorus load (kg month-1) 1,600 2001 1,000 Phosphorus load (kg 1,400 month-1) 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Phosphorus load (kg month-1) 1,800 2000 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 Phosphorus load (kg month-1) 1,800 IR4 1,400 1,200 1,000 800 600 400 IR6-7 1,400 1,200 1,000 800 600 400 BR1-2 1,400 1,200 1,000 800 600 400 Figure 29. Estimates of phosphorus loads in groundwater and diffusing from muck for stations in the Indian River (IRL1-3, IRL4, IRL5, IRL6-7 and IRL8) and Banana River (BR1-2, BR3-5 and BR6) lagoons. 36 A third potential source of nutrients arises from changes in the abundance of drift macroalgae. The nitrogen and phosphorus bound in drift algae was calculated using biomass estimates (g m-2) from surveys of seagrass transects, biomass estimates for areas beyond the seagrass canopy derived from hydroacoustic mapping conducted by Nova Southeastern University, and a ratio of those two estimates to calculate biomass outside the canopy from biomass inside the canopy during years when mapping was not undertaken. Biomass was converted to nitrogen and phosphorus content using percentages from the literature (nitrogen content = 1.0% of dry biomass in September–January, 1.5% of dry biomass in February and August, 2.0% of dry biomass in March and July, 2.5% of dry biomass in April and June, and 3.0% of dry biomass in May; phosphorus content = 0.2 % of dry biomass in all months). Analyses of nitrogen and phosphorus in drift algae indicated that the quantities of bound nutrients varied significantly among places and times. Nitrogen bound in drift algae varied among segments of the lagoon and among years, with more nitrogen consistently bound in segments BR1-2, BR3-5 and IR1-3 and less nitrogen bound in 2011 and 2012 (Figure 30). Phosphorus content displayed a different pattern, which was linked more directly to the abundance of drift algae because only one conversion factor was used. Phosphorus content varied among combinations of segments and years, with less phosphorus bound in 2010 and 2011 in all segments where drift algae was common (Figure 31). Thus, nutrients released from decomposing drift algae and increased availability of nutrients due to reduced competition by drift algae could have helped initiate and sustain the superbloom. 120 Mean nitrogen content (g m-2) ± SE Mean nitrogen content (g m-2) ± SE 120 100 80 60 40 20 100 80 60 40 20 2011 2012 2010 2009 2008 2007 2006 2005 2004 2003 IR8 2002 IR6-7 2001 IR5 2000 IR4 1999 IR1-3 1998 BR3-5 1997 BR1-2 1996 1995 0 0 Figure 30. Nitrogen bound in drift algae. 40 30 20 BR1-2 BR3-5 IR1-3 2011 2009 2007 2005 2003 2001 1999 1997 2011 1995 2009 2007 2005 2003 2001 1999 2011 1997 1995 2009 2007 2005 2003 2001 1999 0 1997 10 40 30 20 10 0 1995 1997 1999 2001 2003 2005 2007 2009 2011 1995 1997 1999 2001 2003 2005 2007 2009 2011 1995 1997 1999 2001 2003 2005 2007 2009 2011 1995 1997 1999 2001 2003 2005 2007 2009 2011 Mean phosphorus content (g m-2) ± SE 50 1995 Mean phosphorus content (g m-2 ) ± SE 50 IR4 IR5 IR6-7 IR8 Figure 31. Phosphorus bound in drift algae. Red bars are means for 2010 and 2011 37 Red drift algae (Rhodophyta) were most abundant prior to the superbloom event. To evaluate stresses that could lead to changes in biomass, Habitat Suitability Indices were calculated in a manner similar to those applied to seagrasses (Table 12). Throughout 2009–2011, Salinity Suitability Indices (SSIs) and Temperature Suitability Indices (TSIs) did not appear to fall substantially below their long-term averages (Table 12); therefore, there was little evidence of salinity or temperature stress on drift algae. The analysis of temperature stress was based on monthly records, but continuous monitoring of water temperature in Haulover Canal indicated that extreme temperatures may have been missed, which would lead to an underestimate of temperature stress. Light Suitability Indices (LSIs) varied among segments, but LSIs were consistently below 0.2 from late spring in 2010 through much of 2011 (Table 12). These LSIs often were below long-term averages, which is consistent with the onset of the superbloom. Table 12. Habitat Suitability Indices for drift algae (Rhodophyta), with mean monthly averages for 1999–2009 in parentheses. Bold numbers indicate the largest value in each cell, red indicates monthly indices < 0.01, orange indicates monthly indices < 0.2, and yellow indicates monthly indices < 0.3 Month–Year Monthly indices and mean indices for 1999–2009 LSI @ 1.5 m LSI @ 2.0 m LSI @2.5 m (0.94) 0.82 (0.78) 0.70 (0.66) 0.57 (0.56) (0.96) 0.94 (0.82) 0.89 (0.71) 0.81 (0.58) (0.97) 0.83 (0.84) 0.66 (0.71) 0.48 (0.58) (0.98) 0.87 (0.82) 0.72 (0.73) 0.54 (0.64) (0.95) 0.94 (0.89) 0.88 (0.78) 0.78 (0.66) (0.79) 0.96 (0.88) 0.93 (0.77) 0.86 (0.65) (0.72) 0.96 (0.83) 0.93 (0.69) 0.88 (0.55) (0.56) 0.95 (0.90) 0.88 (0.80) 0.79 (0.67) (0.75) 0.79 (0.81) 0.61 (0.67) 0.42 (0.53) (0.87) 0.48 (0.72) 0.25 (0.56) 0.11 (0.42) (0.93) 0.84 (0.78) 0.74 (0.66) 0.62 (0.54) (0.96) 0.84 (0.69) 0.77 (0.55) 0.68 (0.43) Jan 2009 Feb 2009 Mar 2009 Apr 2009 May 2009 June 2009 July 2009 Aug 2009 Sept 2009 Oct 2009 Nov 2009 Dec 2009 TSI 1.00 1.00 1.00 0.99 0.96 0.82 0.80 0.46 0.69 0.75 0.94 1.00 (0.99) (1.00) (1.00) (1.00) (0.96) (0.80) (0.73) (0.56) (0.80) (0.91) (0.99) (1.00) SSI 0.98 0.99 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 RCI @2.0 m 0.69 (0.62) 0.88 (0.68) 0.66 (0.69) 0.71 (0.72) 0.85 (0.75) 0.76 (0.61) 0.75 (0.50) 0.40 (0.43) 0.42 (0.51) 0.19 (0.49) 0.69 (0.62) 0.77 (0.53) Jan 2010 Feb 2010 Mar 2010 Apr 2010 May 2010 June 2010 July 2010 Aug 2010 Sept 2010 Oct 2010 Nov 2010 Dec 2010 0.95 1.00 1.00 1.00 0.95 0.66 0.84 0.41 0.82 0.97 0.98 1.00 (0.99) (1.00) (1.00) (1.00) (0.96) (0.80) (0.73) (0.56) (0.80) (0.91) (0.99) (1.00) 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 (0.94) (0.96) (0.97) (0.98) (0.95) (0.79) (0.72) (0.56) (0.75) (0.87) (0.93) (0.96) 0.93 0.93 0.97 0.95 0.80 0.50 0.63 0.63 0.55 0.71 0.66 0.65 (0.78) (0.82) (0.84) (0.82) (0.89) (0.88) (0.83) (0.90) (0.81) (0.72) (0.78) (0.69) 0.89 0.89 0.96 0.88 0.59 0.24 0.37 0.38 0.30 0.49 0.47 0.46 (0.66) (0.71) (0.71) (0.73) (0.78) (0.77) (0.69) (0.80) (0.67) (0.56) (0.66) (0.55) 0.83 0.82 0.94 0.78 0.39 0.09 0.19 0.20 0.14 0.30 0.30 0.30 (0.56) (0.58) (0.58) (0.64) (0.66) (0.65) (0.55) (0.67) (0.53) (0.42) (0.54) (0.43) 0.84 0.89 0.96 0.88 0.56 0.16 0.31 0.16 0.25 0.48 0.46 0.46 (0.62) (0.68) (0.69) (0.72) (0.75) (0.61) (0.50) (0.43) (0.51) (0.49) (0.62) (0.53) Jan 2011 Feb 2011 Mar 2011 Apr 2011 May 2011 June 2011 July 2011 Aug 2011 Sept 2011 Oct 2011 Nov 2011 Dec 2011 1.00 1.00 1.00 1.00 0.95 0.81 0.67 0.28 0.76 0.97 0.99 1.00 (0.99) (1.00) (1.00) (1.00) (0.96) (0.80) (0.73) (0.56) (0.80) (0.91) (0.99) (1.00) 1.00 1.00 1.00 1.00 1.00 0.99 0.99 0.99 1.00 1.00 1.00 1.00 (0.94) (0.96) (0.97) (0.98) (0.95) (0.79) (0.72) (0.56) (0.75) (0.87) (0.93) (0.96) 0.84 0.52 0.25 0.18 0.46 0.23 0.24 0.25 0.31 0.22 0.50 0.59 (0.78) (0.82) (0.84) (0.82) (0.89) (0.88) (0.83) (0.90) (0.81) (0.72) (0.78) (0.69) 0.74 0.29 0.08 0.04 0.22 0.06 0.07 0.08 0.11 0.06 0.28 0.38 (0.66) (0.71) (0.71) (0.73) (0.78) (0.77) (0.69) (0.80) (0.67) (0.56) (0.66) (0.55) 0.62 0.14 0.01 0.00 0.08 0.00 0.00 0.01 0.03 0.00 0.13 0.23 (0.56) (0.58) (0.58) (0.64) (0.66) (0.65) (0.55) (0.67) (0.53) (0.42) (0.54) (0.43) 0.74 0.29 0.08 0.04 0.21 0.05 0.04 0.02 0.09 0.06 0.27 0.38 (0.62) (0.68) (0.69) (0.72) (0.75) (0.61) (0.50) (0.43) (0.51) (0.49) (0.62) (0.53) 38 Relative Condition Indices (RCIs) calculated for Segment BR1-2 also indicated that drift algae were stressed between May and September 2010 and in most months between January and September 2011 (Figure 32). These patterns held for drift algae at depths of 1.5–2.5 m. In fact, the duration of such stress may have exceeded the resilience of drift algae, although such considerations could not be factored into these initial assessments. Banana River Lagoon, segment BR1-2, water quality station IRLB02 Figure 32. Relative Condition Indices (RCIs) for drift algae. Suitable conditions exist when the red dashed line (RCI based on LSI at 2.0 m, TSI and SSI) exceeds 0.5 or the colored band (range of RCI from 1.5 m to 2.5 m based on variation in LSIs and constant TSIs and SSIs) is narrow and exceeds 0.5. As a reference, the solid red line represents the RCI at the 2.0 m depth averaged over all months in 1999–2009. Did a lack of “top-down” control by animals foster the 2011 superbloom? “Top-down” control of phytoplankton blooms occurs when animals consume the algae. Changes in phytoplankton assemblages that alter susceptibility to grazing or palatability and changes in populations of primary consumers may alter top-down control. In an effort to assess top-down control of the superbloom, the phytoplankton assemblage was compared to previous blooms, and abundances of zooplankton captured using an integrated tube (primarily bacterioplankton and microzooplankton), zooplankton captured using a net (primarily mesozooplankton and meroplankton), infauna and fish were analyzed to identify significant variation in primary consumption. As part of an ongoing program, phytoplankton were collected with a vertical integrating tube that extended from the surface to 0.1 m above the bottom. Data documenting the phytoplankton assemblage before and during the superbloom of 2010–2011 were derived from samples collected at 9 sites between February 2007 and March 2012 (Figure 33). 39 Figure 33. Locations for samples analyzed in this study. Biomass (expressed as carbon concentrations in pg ml-1) was summed for eleven groups of common phytoplankton. Due to their prominent role in the superbloom, Pedinophyceae and picocyanobacteria (small cyanobacteria) were treated separately from Bacillariophyceae (diatoms), Chlorophyceae (green algae), Chrysophyceae (golden algae), Cryptophyceae (cryptomonads), Cyanophyceae (cyanobacteria), Dinophyceae (dinoflagellates), Euglenophyceae (euglenoids), microflagellates and Raphidiophyceae (raphids). Analysis indicated significant differences in the composition of the phytoplankton assemblage among combinations of sites and times, with diatoms, cyanobacteria, dinoflagellates, Pedinophyceae and small cyanobacteria accounting for 90% of the dissimilarity among replicate samples. A diatom bloom preceded the superbloom, and the superbloom event period was distinguished by relatively high biomasses of cyanobacteria, picocyanobacteria, and Pedinophyceae (Figure 34). Cyanophyceae Picocyanobacteria Pedinophyceae Bacillariophyceae 2.0 1.5 1.0 0.5 0.0 1 1.5 3.5 3 2 4 5 6 8 1 1.5 3.5 3 2 4 5 6 8 1 1.5 3.5 3 2 4 5 6 8 1 1.5 3.5 3 2 4 5 6 8 1 1.5 3.5 3 2 4 5 6 8 Mean carbon concentration (pg l -1) Dinophyceae ML BR IRL Feb 07-Jan 08 ML BR IRL Feb 08-Jan 09 ML BR IRL Feb 09-Jan 10 ML BR IRL Feb 10-Feb 11 ML BR IRL Mar 11-Mar 12 Figure 34. Mean biomass of phytoplankton for combinations of sites and periods. ML =Mosquito Lagoon; BR = Banana River Lagoon; IRL = Indian River Lagoon 40 The diatom bloom that preceded the superbloom likely generated organic compounds containing nitrogen and carbon. Some picocyanobacteria can utilize organic compounds, i.e., dissociate nitrogen and phosphorus from carbon (Scanlan et al. 2009). In addition, many picocyanobacteria can adjust their photosynthetic pigments to adapt to local light regimes. In combination, these ecological adaptations would provide them with a competitive advantage relative to diatoms and, potentially dinoflagellates, as well as an ability to sustain bloom concentrations by adapting to self-shading and cycling nutrients efficiently. Furthermore, replacing the diatoms and dinoflagellates that characterized previous blooms with Pedinophyceae and picocyanobacteria (Phlips et al. 2002) may have generated several important ecological consequences. Effective grazing pressure may have shifted toward protozoa and rotifers because these small zooplankton are better equipped to handle small algal cells (Rassoulzadegan et al. 1988; Buskey et al. 1997; Quinlan et al. 2009). In addition, grazing may have been reduced because picocyanobacteria can produce mucus and toxins that deter grazers (Scanlan et al. 2009; Sorokin and Zakuskina 2010). Finally, the ecological adaptations of picocyanobacteria may have allowed them to adapt to self-shading and increased the pace at which nutrients were cycled (Scanlan et al. 2009). During the superbloom, bacterioplankton could have contributed to cycling of nutrients, and microzooplankton could have exerted “top-down” control on phytoplankton through grazing. As part of an ongoing program, samples of microzooplankton and bacterioplankton were collected with a vertical integrating tube that extends from the surface to 0.1 m above the bottom. Bacteria and zooplankton were enumerated in samples from two sites affected by the 2011 superbloom: site 2 near Titusville in the Indian River Lagoon and site 3 in Banana River Lagoon (Figure 35). Archived samples documented conditions from several months before the superbloom (August 2010) through its decline (November 2011). In addition, samples from other periods characterized bacterioplankton and zooplankton during a non-bloom period (May–September and November 2007 at site 2), during a major bloom of dinoflagellates (August–November 2006 at site 3), during a modest bloom of diatoms and dinoflagellates (May–September and November 2007 at site 3), and during a modest dinoflagellate bloom (August– November 2009 at site 2). Bacterioplankton abundances were expressed as 106 cells ml-1, and zooplankton abundances in four broad taxa were standardized to numbers L-1. 1. Mosquito Lagoon 1.5. S. Mosquito Lagoon (Core 1) 2. Titusville (Core 2) 3.5. N. Banana River (Core 3) 4.1. Cocoa 3. Banana River (Core 5) 4. Eau Gallie 5. Melbourne (Core 6) Sebastian Inlet 6. Sebastian Florida 8. Vero 0 Sampled monthly by UF Sampled monthly by SJRWMD 20km Sampled bi-weekly by UF/SJRWMD Figure 35. Locations for samples analyzed in this study circled in red. 41 Data on the composition of the bacterioplankton assemblage were not collected, but bacterial abundances at site 2 were slightly more variable than abundances at site 3 during all periods (Figure 36). From November 2010 through March 2011, densities at site 3 decreased from ~4 × 106 cells ml-1 to ~2 × 106 cells ml-1. Although bacterial abundances at site 2 were slightly higher in January 2011, densities generally remained below 4 × 106 cells ml-1 from November 2010 through April 2011. At both sites, bacterial abundances were relatively high, typically > 4 × 106 cells ml-1, during the superbloom (after March 2011). (a) (b) Site 2 12 Bacterial density (cells × 106 ml-1) Bacterial density (cells × 106 ml-1) 12 10 8 6 4 2 0 Site 3 10 8 6 4 2 0 Month/Year Month/Year (c) (d) Site 2 12 Bacterial density (cells × 106 ml-1) Bacterial density (cells × 106 ml-1) 12 10 8 6 4 2 0 Site 2 10 8 6 4 2 0 5/07 6/07 7/07 8/07 Month/Year 9/07 11/07 (e) 8/09 9/09 10/09 Month/Year 11/09 (f) 12 Site 3 Bacterial density (cells × 106 ml-1) Bacterial density (cells × 106 ml-1) 12 10 8 6 4 2 0 Site 3 10 8 6 4 2 0 5/07 6/07 7/07 8/07 Month/Year 9/07 11/07 8/06 9/06 10/06 Month/Year 11/06 Figure 36. Density of bacterioplankton during (a) and (b) the superbloom, (c) a non-bloom period, (d) and (e) moderate blooms, and (f) a major bloom. 42 Bacterioplankton were not abundant during the winter of 2010 when water temperatures were low, which suggested a potential limiting influence. High bacterial abundances during the superbloom (after March 2011) suggested that these organisms were responding to the availability of organic compounds (Malone et al. 1991; Wetz and Wheeler 2004; Apple et al. 2008). Abundant bacteria would have enhanced cycling of organically bound nutrients released by dying phytoplankton into inorganic forms that could be used by living cells. Such cycling could have prolonged the superbloom and led to its gradual decline. Immediately preceding and at the beginning of the superbloom, large numbers of protozoa were recorded at both sites, and large numbers of rotifers were found at site 3 (Figure 37). Overall, the assemblage seemed to shift before and during the superbloom, with relatively few arthropods and higher numbers of protozoa and rotifers recorded. Abundances of all zooplankton became variable during the superbloom. (a) Site 2 (b) Site 3 Other Rotifer Arthropod Protozoa Other Rotifer Arthropod Protozoa 7000 Zooplankton density (numbers l-1) Zooplankton density (numbers l-1) 7000 6000 5000 4000 3000 2000 1000 0 6000 5000 4000 3000 2000 1000 0 Month/Year Month/Year (c) Site 2 (d) Site 2 Other Rotifer Arthropod Protozoa Other Rotifer Arthropod Protozoa 7000 Zooplankton density (numbers l-1) Zooplankton density (numbers l-1) 7000 6000 5000 4000 3000 2000 1000 0 6000 5000 4000 3000 2000 1000 0 05/07 06/07 07/07 08/07 Month/Year 09/07 11/07 (e) Site 3 08/09 09/09 10/09 Month/Year 11/09 (f) Site 3 Other Rotifer Arthropod Protozoa Other Zooplankton density (numbers l-1) Zooplankton density (numbers l-1) Rotifer Arthropod Protozoa 7000 7000 6000 5000 4000 3000 2000 1000 0 6000 5000 4000 3000 2000 1000 0 05/07 06/07 07/07 08/07 Month/Year 09/07 11/07 08/06 09/06 10/06 Month/Year 11/06 Figure 37. Density of zooplankton during (a) and (b) the superbloom, (c) a non-bloom period, (d) and (e) moderate blooms, and (f) a major bloom. 43 Data from months were pooled to yield replicates in four categories, i.e., absence of a bloom, presence of a bloom, the period preceding the superbloom and during the superbloom (Table 13). Densities for the periods were significantly different, with the superbloom differing from all other periods and presuperbloom period differing from the bloom period. Means and standard errors showed that the presuperbloom period had high and variable densities of protozoa and the superbloom was characterized by relatively low densities of arthropods (Figure 38). Table 13. Pooling of density data. Category No bloom Bloom Total number of replicate samples 6 14 Pre-superbloom 14 Superbloom 18 Site Months 2 2 3 3 2 3 2 3 May–September & November 2007 August–November 2009 August–November 2006 May–September & November 2007 August–December 2010 & January–February 2011 August–December 2010 & January–February 2011 March–November 2011 March–November 2011 Protozoa Rotifer Arthropod Number of replicate samples 6 4 4 6 7 7 9 9 Other Mean density ± SE (numbers l-1) 2500 2000 1500 1000 500 0 No bloom Bloom Pre-superbloom Superbloom Figure 38. Mean density of zooplankton ± standard error (SE). Similarly, abundances of protozoa and rotifers increased as the superbloom began, which probably reflected a response to increased food availability (Burkhill et al. 1987; Bernard and Rassoulzadegan 1990; Quinlan et al. 2009). Notably, abundances of these grazers decreased and became variable during the superbloom, which may be an effect of increased salinity (Godhantaraman and Uye 2003; Strom et al. 2013) or an effect linked to picocyanobacteria that may be poor food for grazers or produce toxins that deter grazers (Sorokin and Zakuskina 2010). It is possible that the abundances of protozoa and rotifers represented the best estimator of grazing pressure because these grazers were better equipped to handle the small phytoplankton that comprised the superbloom (Rassoulzadegan et al. 1988; Buskey et al. 1997; Quinlan et al. 2009). Larger zooplankton could have exerted “top-down” control on phytoplankton during the superbloom, but data characterizing the net zooplankton assemblage at sites affected by the superbloom was lacking. Therefore, the focus shifted to changes in abundance at a site near Melbourne. In addition to zooplankton sampled with the integrating tube, data for zooplankton sampled with nets was evaluated. Nets were towed at a site in the Indian River Lagoon, between Crane Creek and the Melbourne causeway (Figure 39). The sampling site was positioned in water representative of the Indian River Lagoon by avoiding plumes of low salinity water exiting Crane Creek. 44 Figure 39. General location of sampling. Data from 72 sampling events were pooled to create periods characterized by different water temperatures, i.e., warm periods (March–October) and cold periods (November–February). Data from 2008 were excluded due to lack of sampling during colder months. In general, the data were highly variable, which reflected the spatial and temporal patchiness that characterizes zooplankton assemblages. Densities of gastropod veligers did not vary significantly among the samples. In contrast, densities of barnacle nauplii and trochophores varied significantly among combinations of years and periods, and densities of flatworms, crustacean nauplii, metatrochophores and total zooplankton varied significantly among years. The most robust and reliable data were counts of total zooplankton, and means and confidence limits indicated that densities were relatively high during 2006 and 2007 and relatively low during 2009–2011 (Figure 40). Thus, available evidence indicates that zooplankton abundances may have been reduced preceding and during the superbloom. Mean density (numbers m-3) ± 95% CL 8000 6000 4000 2000 0 2004 2005 2006 2007 2009 2010 2011 Figure 40. Back-transformed mean density of total zooplankton and 95% confidence limits (CL). Available evidence indicated that densities of larger zooplankton were relatively low preceding and during the superbloom. Reduced grazing pressure would have enhanced accumulation of larger numbers of phytoplankton cells during the superbloom, but the potential impact of larger zooplankton on the small 45 (1–4 µm diameter) phytoplankton comprising the superbloom remains uncertain. In experiments with the calanoid copepod Acartia tonsa, densities of < 50 individuals m-3 could remove 20–34% of available phytoplankton that were > 7 μm in diameter (Rhyther and Sanders 1980). In contrast, abundances of smaller phytoplankton increased during grazing experiments (Rhyther and Sanders 1980), and evidence that such copepods can graze efficiently on small particles is inconsistent (Turner 1984). Based on the available evidence, the possibility of reduced grazing pressure immediately preceding and during the superbloom cannot be rejected, but further directed research would help characterize grazing pressure from common zooplankton caught in nets. The cause of a reduction in zooplankton abundance remains uncertain. For example, data from sampling during 1976–1978 indicated that the calanoid copepod Acartia tonsa represented approximately 90% of all copepods in parts of the Indian River Lagoon away from inlets (M. Youngbluth unpub. data). This species has been characterized as being eurythermal and euryhaline, i.e., tolerating a wide range of temperatures and salinities (Conover 1956; Gaudy et al. 2000). Therefore, temperatures of 8°C and salinities of 45 recorded before and during the superbloom, were unlikely to have caused significant mortality, but further directed research would help identify potential stressors, especially high salinity. The fate of dead zooplankton was not followed in detail; therefore, there were no data related to increased deposition of organic matter and nutrients. Filter feeding infauna represented a potential source of grazing pressure and “top-down” control of phytoplankton abundances, but data characterizing the infaunal assemblage at sites affected by the superbloom was lacking. Therefore, the focus shifted to changes in abundance at sites running north from Vero Beach to Eau Gallie. Quantitative sampling of benthic infauna was performed in 17 quarters (4 quarters in 2008, 2009, 2010 and 2011, along with 1 quarter in 2011) at 6 fixed stations (Figure 41). Sediment at the stations differed, with V1, V3, V4 and V6 having fine sand as compared to muddier sediments at V2 and coarser sediment at V5. At each station, three replicate samples were collected quarterly (in January, April, July and October) using a 0.02 m2 ponar grab. Individuals were identified to the lowest possible taxon and enumerated in the laboratory. Figure 41. Stations where infauna was sampled. In total, numbers of individuals per 0.02 m2 were recorded for 187 taxa. Filter feeders represented a primary focus because they graze on phytoplankton, and bivalves represented the most common and abundant filter feeders. Six species found at multiple sites in reasonable numbers illustrated key patterns 46 (Figure 42). Abundances of Macoma tenta, Mulinia lateralis and Tagelus divisus decreased in 2010, which preceded the superbloom. Only M. lateralis increased in abundance in 2012. In contrast, abundances of Nucula proxima, Parastarte triquetra and Anomalocardia auberiana did not decrease during 2009–2010, and abundances of P. triquetra and A. auberiana increased during 2012. (a) Macoma tenta (b) Mulinia lateralis V1 V2 V3 V4 V5 V6 V1 3 2 1 V4 V5 V6 200 100 0 2008 2009 2010 2011 2012 (c) Tagelus divisus 2008 2009 2010 2011 2012 (d) Nucula proxima V1 V2 V3 V4 V5 V1 V6 V2 V3 V4 V5 V6 12 Mean abundance (number 0.02 m-2) ± SE 3 Mean abundance (number 0.02 m-2) ± SE V3 300 0 2 1 9 6 3 0 0 2008 2009 2010 2011 V1 V2 2008 2012 (e) Parastarte triquetra 2009 2010 2011 2012 (f) Anomalocardia auberiania V3 V4 V5 V6 V1 16 V2 V3 V4 V5 V6 25 Mean abundance (numbers 0.02 m-2) ± SE Mean abundance (number 0.02 m-2) ± SE V2 400 Mean abundance (number 0.02 m-2) ± SE Mean abundance (number 0.02 m-2) ± SE 4 12 8 4 0 20 15 10 5 0 2008 2009 2010 2011 2012 2008 2009 2010 2011 2012 Figure 42. Mean abundance ± standard error (SE). 47 If decreases in abundance of three common bivalves (including Mulinia lateralis, the most abundant species) translated to the region affected by the superbloom, then reduced grazing pressure on phytoplankton was likely. In addition to uncertainty regarding spatial heterogeneity, three other taxa did not decrease in abundance during the same period; therefore, the overall level of grazing pressure remains uncertain. In fact, abundances of only 90 taxa out of the 187 recorded during 2008–2012 decreased from 2008 to 2009–2010. Decreases in abundance of some bivalves may have been the consequence of water temperatures or salinities that exceeded their physiological tolerances. Unfortunately, physiological tolerances have not been determined experimentally for these species; therefore, water temperatures and salinities associated with collections provided the best available data (Table 14; Encyclopedia of Life at eol.org). All species survive in full seawater, and available data did not support any conclusion about the effects of salinities above 35. Available data provided some evidence for mortality due to water temperatures that dropped to 7.8°C prior to the superbloom. Macoma tenta, Mulinia lateralis and Tagelus divisus exhibited decreased abundances, and they had not been collected at temperatures that low. In addition, abundances of Nucula proxima did not decrease, and evidence suggested they tolerate lower water temperatures than those recorded in the Indian River Lagoon. Data for Anomalocardia auberiana and Parastarte triquetra did not support any conclusions regarding the effects of low water temperatures. The fate of dead infauna was not followed in detail; therefore, there were no data related to increased deposition of organic matter and nutrients. Table 14. Water temperature and salinity records for common bivalves (from the Encyclopedia of Life, eol.org). – = no available data Species Anomalocardia auberiana Macoma tenta Mulinia lateralis Nucula proxima Parastarte triquetra Tagelus divisus Water temperature (°C) Minimum Maximum 26.6 – 8.4 27.2 9.2 24.7 5.3 26.3 – – 23.6 27.7 Salinity (psu) Minimum Maximum 36.1 – 32.4 36.3 32.4 35.8 32.3 36.3 – – 35.2 35.8 As part of an ongoing fisheries independent monitoring program, fish and macroinvertebrates were collected with a 21.3 m long seine made of 3 mm nylon mesh (Florida Fish and Wildlife Conservation Commission Florida Marine Research Institute 2012). Fish in particular could have interacted with the superbloom in multiple ways. For example, fish feeding on phytoplankton could have exerted “top-down” control on phytoplankton and fish feeding on zooplankton could have released phytoplankton from grazing pressure. Data documenting the fish and macroinvertebrate assemblage before and during the initial months of the superbloom of 2010–2011 were derived from samples collected at randomly selected sites stratified to account for habitat differences in two zones (C and D) between January 2007 and December 2011 (Figure 43). 48 Figure 43. Zones and the universe of potential sites for samples analyzed in this study. Fish and invertebrates were identified to the lowest possible taxonomic level, and size classes were distinguished for key species according to a standardized suite of measures. Counts were treated as catch per unit effort (numbers set-1). The samples from each zone were grouped into three time spans according to their relationship with the superbloom, i.e., distal antecedent period 4 (January 2007–January 2010), proximal antecedent period (February 2010–February 2011), and the superbloom event period (March 2011–December 2011). Analysis indicated significant differences among combinations of zones and periods. Further analysis indicated that 41 combinations of size class and taxon accounted for approximately 90% of the dissimilarity among combinations of zone and period, with all taxa being fish. For each size class and taxon combination in each zone, differences in mean numbers set-1 were calculated for consecutive periods. In terms of filter feeding and zooplanktivorous fish, mean numbers of bay anchovies and silversides were lower during the superbloom event period in both zones, whereas mean numbers of menhaden increased (Figure 44). In general, mean numbers of other fish and macroinvertebrates taken in the seine differed by < 1 individual set-1 (Figure 45). In addition, there were fewer large decreases in mean numbers between the proximal antecedent period and the superbloom event period in both zones (Figure 45). Overall, there was little evidence of a consistent change in the composition of the fish and macroinvertebrate assemblages or the abundances of common fish and macroinvertebrates in zones C and D before and during the initial months of the superbloom. 49 Bay anchovy 0-30 Bay anchovy 31-50 DAP4 - PAP DAP4 - PAP Bay anchovy 0-30 Bay anchovy 31-50 Bay anchovy 51-100 Menhaden 0-30 Silverside 0-30 Silverside 31-50 Menhaden 0-30 Silverside 0-30 Silverside 31-50 Silverside 51-100 Silverside 51-100 Bay anchovy 0-30 Bay anchovy 0-30 Bay anchovy 31-50 PAP - SEP Bay anchovy 31-50 PAP - SEP Bay anchovy 51-100 Bay anchovy 51-100 Menhaden 0-30 Silverside 0-30 Silverside 31-50 Bay anchovy 51-100 Menhaden 0-30 Silverside 0-30 Silverside 31-50 Silverside 51-100 Silverside 51-100 -2 -1 0 1 Difference in mean number per set Zone C 2 -2 -1 0 1 Difference in mean number per set Zone D 2 Figure 44. Difference in mean numbers set-1 between consecutive periods for filter feeding and zooplanktivorous fish from each zone. DAP4 = distal antecedent period 4 (January 2007–January 2010); PAP = proximal antecedent period (February 2010–February 2011); SEP = the superbloom event period (March 2011–December 2011) Atlantic stingray >100 Clown goby 0-30 Clown goby 31-50 Code goby 0-30 Goby 0-30 Goldspotted killifish 0-30 Goldspotted killifish 31-50 Gulf pipefish 51-100 Hardhead catfish >100 Irish pompano 31-50 Mojarra 0-30 Mojarra 31-50 Pinfish 0-30 Pinfish 31-50 Pinfish 51-100 Pinfish >100 Rainwater killifish 0-30 Rainwater killifish 31-50 Redfin needlefish 51-100 Redfin needlefish >100 Sheepshead minnow 0-30 Sheepshead minnow 31-50 Shrimp 0-30 Silver perch 0-30 Silver perch 31-50 Silver perch 51-100 Spot 0-30 Spot 31-50 Spot 51-100 Spotted seatrout 0-30 Spotted seatrout 31-50 Striped mullet 0-30 Tidewater mojarra 31-50 Tidewater mojarra 51-100 Atlantic stingray >100 Clown goby 0-30 Clown goby 31-50 Code goby 0-30 Goby 0-30 Goldspotted killifish 0-30 Goldspotted killifish 31-50 Gulf pipefish 51-100 Hardhead catfish >100 Irish pompano 31-50 Mojarra 0-30 Mojarra 31-50 Pinfish 0-30 Pinfish 31-50 Pinfish 51-100 Pinfish >100 Rainwater killifish 0-30 Rainwater killifish 31-50 Redfin needlefish 51-100 Redfin needlefish >100 Sheepshead minnow 0-30 Sheepshead minnow 31-50 Shrimp 0-30 Silver perch 0-30 Silver perch 31-50 Silver perch 51-100 Spot 0-30 Spot 31-50 Spot 51-100 Spotted seatrout 0-30 Spotted seatrout 31-50 Striped mullet 0-30 Tidewater mojarra 31-50 Tidewater mojarra 51-100 -2 -1 0 1 Difference in mean number per set for Zone C DAP4 - PAP 2 Atlantic stingray >100 Clown goby 0-30 Clown goby 31-50 Code goby 0-30 Goby 0-30 Goldspotted killifish 0-30 Goldspotted killifish 31-50 Gulf pipefish 51-100 Hardhead catfish >100 Irish pompano 31-50 Mojarra 0-30 Mojarra 31-50 Pinfish 0-30 Pinfish 31-50 Pinfish 51-100 Pinfish >100 Rainwater killifish 0-30 Rainwater killifish 31-50 Redfin needlefish 51-100 Redfin needlefish >100 Sheepshead minnow 0-30 Sheepshead minnow 31-50 Shrimp 0-30 Silver perch 0-30 Silver perch 31-50 Silver perch 51-100 Spot 0-30 Spot 31-50 Spot 51-100 Spotted seatrout 0-30 Spotted seatrout 31-50 Striped mullet 0-30 Tidewater mojarra 31-50 Tidewater mojarra 51-100 -2 -1 0 1 Difference in mean number per set for Zone C PAP - SEP 2 -2 -1 0 1 Difference in mean number per set for Zone D PAP - SEP 2 Atlantic stingray >100 Clown goby 0-30 Clown goby 31-50 Code goby 0-30 Goby 0-30 Goldspotted killifish 0-30 Goldspotted killifish 31-50 Gulf pipefish 51-100 Hardhead catfish >100 Irish pompano 31-50 Mojarra 0-30 Mojarra 31-50 Pinfish 0-30 Pinfish 31-50 Pinfish 51-100 Pinfish >100 Rainwater killifish 0-30 Rainwater killifish 31-50 Redfin needlefish 51-100 Redfin needlefish >100 Sheepshead minnow 0-30 Sheepshead minnow 31-50 Shrimp 0-30 Silver perch 0-30 Silver perch 31-50 Silver perch 51-100 Spot 0-30 Spot 31-50 Spot 51-100 Spotted seatrout 0-30 Spotted seatrout 31-50 Striped mullet 0-30 Tidewater mojarra 31-50 Tidewater mojarra 51-100 -2 -1 0 1 Difference in mean number per set for Zone D DAP4 - PAP 2 Figure 45. Difference in mean number set-1 between consecutive periods for fish and macroinvertebrates from each zone. DAP4 = distal antecedent period 4 (January 2007–January 2010); PAP = proximal antecedent period (February 2010–February 2011); SEP = the superbloom event period (March 2011–December 2011) 50 Overall, fish and macroinvertebrates did not appear to respond to events leading up to the superbloom or to the superbloom itself. One exception appeared to be an increase in mean numbers of menhaden, which possibly reflected a response to increased food availability for larvae and young juveniles (Stoecker and Govoni 1984; Friedland et al. 2006; Friedland et al. 2011). An increase in mean numbers of menhaden potentially led to increased grazing on phytoplankton (Stoecker and Govoni 1984; Friedland et al. 2006; Friedland et al. 2011). Otherwise, there was little evidence of a change in trophic dynamics due to a change in the fish and macroinvertebrate assemblage. Again, there was no fish kill reported in association with the superbloom, which did not “crash” and cause a decrease in dissolved oxygen concentrations; therefore, fish mortality was unlikely to contribute to an increase in organic matter and nutrients. Recommendations Attempts to explain the initiation and persistence of the 2011 superbloom using available data highlighted some key gaps in our understanding. The following recommendations point to ways to improve our evaluation of the superbloom, our ability to predict future events, and our capacity to develop and assess potential management actions. 1. Garner an improved understanding of the biology and physiology of picocyanobacteria and Pedinophyceae, including their ability to use organic forms of nutrients, their nutrient uptake rates, their reproductive rates and their defenses against grazers. 2. Maintain or expand water quality sampling to ensure spatiotemporal variations are captured adequately, which could include continuous monitoring of various parameters to fill gaps between monthly samples. 3. Develop an improved understanding of the physiological tolerances of drift algae and seagrasses. 4. Maintain or expand surveys of drift algae and seagrasses to improve our capacity to evaluate their role in nutrient cycles. 5. Improve our ability to model bottom-up influences from external and internal nutrient loads, including atmospheric deposition, surface water runoff, groundwater inputs, diffusive flux from muck, and decomposition of drift algae. 6. Enhance surveys of bacterioplankton to improve our understanding of nutrient cycling. 7. Improve surveys of potential zooplanktonic, infaunal, epifaunal and fish grazers to enhance our understanding of spatiotemporal variation in top-down control of phytoplankton blooms. 8. Evaluate grazing pressure exerted by common species to enhance our understanding of top-down control of phytoplankton blooms. 51 Bibliography Apple, J.K., E.M. Smith and T.J. Boyd. 2008. Temperature, salinity, nutrients, and the covariation of bacterial production and chlorophyll-a in estuarine ecosystems. Journal of Coastal Research 10055: 59–75. Behrenfeld, M.J., K.H. Halsey and A.J. Milligan, A. J. 2008. Evolved physiological responses of phytoplankton to their integrated growth environment. Philosophical Transactions of the Royal Society B 363: 2687–2703. Bernard, C. and F. Rassoulzadegan. 1990. Bacteria or microflagellates as a major food source for marine ciliates: possible implications for the microzooplankton. Marine Ecology Progress Series 64: 147–155. Biber, P.D. 2002. The effects of environmental stressors on the dynamics of three functional groups of algae in Thalassia testudinum habitats of Biscayne Bay, Florida: a modeling approach. Ph.D. Dissertation, University of Miami, Coral Gables, Florida. 367 pp. Biber, P.D., M.A. Harwell and P.C. Wendell, Jr. 2004. Modeling the dynamics of three functional groups of macroalgae in tropical seagrass habitats. Ecological Modeling 175: 25–54. Borum, J., O. Pedersen, T.M. Greve, T.A. Frankovich, J.C. Zieman, J.S. Fourqurean and C.J. Madden. 2005. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology 93: 148–158. Burkhill, P.H., R.F.C. Mantoura, C.A. Llwellyn and N.J.P. Owens. 1987. Microzooplankton grazing and selectivity of phytoplankton in coastal waters. Marine Biology 93: 581–590. Burkholder, J.M., P.M. Glibert and H.M. Skelton. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8: 77–93. Buskey, E.J., P.A. Montagna, A.F. Amos and T.E. Whitledge. 1997. Disruption of grazer populations as a contributing factor to the initiation of the Texas brown tide algal bloom. Limnology and Oceanography 42: 1215–1222. Calleja, M., N. Marbà and C.M. Duarte. 2007. The relationship between seagrass (Posidonia oceanica) decline and sulfide porewater concentration in carbonate sediments. Estuarine Coastal and Shelf Science 73: 583-588. Christian, D. and Y.P. Sheng. 2003. Relative influence of various water quality parameters on light attenuation in the Indian River Lagoon. Estuarine Coastal and Shelf Science 57: 961–971. Conover, R.J. 1956. Oceanography of Long Island Sound, 1952–1954. VI. Biology of Acartia clausi and A. tonsa. Bulletin of the Bingham Oceanographic Collection 15: 156–233. DeYoe, H.R., E.J. Buskey and F.J. Jochem. 2007. Physiological responses of Aureoumbra lagunensis and Synechococcus sp. to nitrogen addition in a mesocosm study. Harmful Algae 6: 48–55. Fenchel, T. 2008. The microbial loop–25 years later. Journal of Experimental Marine Biology and Ecology 366: 99–103. 52 Florida Fish and Wildlife Conservation Commission Florida Marine Research Institute. 2012. Fisheriesindependent monitoring program 2011 annual data summary report. Florida Marine Research Institute, St. Petersburg, Florida. 334 pp. Friedland, K.D., D.W. Ahrenholz, J.W. Smith, M. Manning and J. Ryan. 2006. Sieving functional morphology of the gill raker feeding apparatus of Atlantic menhaden. Journal of Experimental Zoology 305A: 974–985. Friedland, K.D., P.D. Lynch and C.J. Gobler. 2011. Time series mesoscale response of Atlantic menhaden Brevoortia tyrannus to variation in plankton abundances. Journal of Coastal Research 27: 1148–1158. Gao, X. 2009. Nutrient and dissolved oxygen TMDLs for the Indian River Lagoon and Banana River Lagoon. Florida Department of Environmental Protection, Division of Environmental Assessment and Restoration, Tallahassee, Florida. Gaudy, R., G. Cervetto and M. Pagano. 2000. Comparison of the metabolism of Acartia clausi and A. tonsa: influence of temperature and salinity. Journal of Experimental Marine Biology and Ecology 247: 51–65. Gobler, C.J. and W.G. Sunda. 2012. Ecosystem disruptive algal blooms of the brown tide species Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 14: 36–45. Godhantaraman, N. and S. Uye. 2003. Geographical and seasonal variations in taxonomic composition, abundance and biomass of microzooplankton across a brackish-water lagoonal system of Japan. Journal of Plankton Research 25: 465–482. Hanisak, D.M. 1987. Cultivation of Gracilaria and other macroalgae in Florida for energy production. In Bird, K. T. and Benson, P. H. (eds). Seaweed cultivation for renewable resources. Elsevier, New York, 191–218. Hanisak, D.M. 1990. The use of Gracilaria tikvahiae (Gigartinales: Rhodophyta) as a model system to understand the nitrogen nutrition of cultured seaweeds. Hydrobiologia: 204/205: 79–87. Hanisak, D.M. 1993. Nitrogen release from decomposing seaweed: species and temperature effects. Journal of Applied Phycology 5: 175–181. Hazen and Sawyer. 2008. Indian River Lagoon economic assessment and analysis update. Report to the St. Johns River Water Management District, Palm Bay, Florida. 210 pp. Holmer, M., C.M. Duarte and N. Marbà. 2003. Sulfur cycling and seagrass (Posidonia oceanica) status in carbonate sediments. Biogeochemistry 66: 223–239. Indian River Lagoon 2011 Superbloom Plan of Investigation. 2012. Prepared by St. Johns River Water Management District, Bethune-Cookman University, Florida Atlantic University-Harbor Branch Oceanographic Institution, Florida Fish and Wildlife Conservation Commission, Florida Institute of Technology, Nova Southeastern University, Smithsonian Marine Station at Ft. Pierce, University of Florida, and Seagrass Ecosystems Analysts. Copy available at St. Johns River Water Management District, Bureau of Environmental Sciences, Estuaries Section. Palatka, Florida. 53 Jones, H.L.J., B.S.C. Leadbeater and J.C. Green. 1994. An ultrastructural study of Marsupiomonas pelliculata gen. et sp. nov., a new member of the Pedinophyceae. European Journal of Phycology 29: 171–181. Jorgensen, B.B. 1982. The sulfur cycle of a coastal marine sediment (Limfjorden, Denmark). Limnology and Oceanography 22: 814–832. Jorgensen, B.B. and N.P. Revsbech. 1983. Colorless sulfur bacteria, Beggiatoa spp. and Thiovulum spp. in O2 and H2S microgradients. Applied Environmental Microbiology 45: 1261–1270. Koch, M.S., S.A. Schopmeyer, M. Holmer, C.J. Madden, C. Kyhn-Hansen. 2007a. Thalassia testudinum response to the interactive stressors hypersalinity, sulfide and hypoxia. Aquatic Botany 87: 104–110. Koch, M.S., S.A. Schopmeyer, C. Kyhn-Hansen and C.J. Madden. 2007b. Synergistic effects of high temperature and sulfide on tropical seagrasses. Journal of Experimental Marine Biology and Ecology 341: 91–101. Landsberg, J.H., H. Sherwood, J.N. Johannessen, K.D. White, S.M. Conrad, J.P. Abbott, L.J. Flewelling, R.W. Richardson, R.W. Dickey, E.L.E. Jester, S.M. Etheridge, J.R. Deeds, F.M. Van Dolah, T.A. Leighfield, Y. Zou, C.G. Beaudry, R.A. Benner, P.L. Rogers, P.S. Scott, K. Kawabata, J.L. Wolny and K.A. Steidinger. 2006. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environmental Health Perspectives 114: 1502– 1507. Lapointe, B.E. and C.S. Duke. 1984. Biochemical strategies for growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. Journal of Phycology, 20: 488– 495. Lapointe, B.E. and J.H. Rhyther. 1978. Some aspects of the growth and yield of Gracilaria tikvahiae in culture. Aquaculture, 15: 185–193. Lapointe, B.E., K.R. Tenor and C. J. Dawes. 1984. Interaction between light and temperature on the physiological ecology of Gracilaria tikvahiae (Gigartinales: Rhodophyta). Marine Biology 80: 161– 170. Malone, T.C., H.W. Ducklow, E.R. Peele and S.E. Pike. 1991. Picoplankton carbon flux in Chesapeake Bay. Marine Ecology Progress Series 78: 11–22. Martin, J.B. and J. Cable. 2008. Analysis of UF groundwater data from the IRL to quantify nutrient loadings from two sources: fresh groundwater and recirculated lagoon water. Final Report to St. Johns River Water Management District. University of Florida, Dept. of Geology, Gainesville, Florida, and Louisiana State University, Department of Oceanography and Coastal Sciences, Baton Rouge, Louisiana. Martin, J.B., J. Cable, C. Smith, M. Roy and J. Cherrier. 2007. Magnitudes of submarine groundwater discharge from marine and terrestrial sources: Indian River Lagoon, Florida. Water Resources Research 43: W05440, 1–15. Martin, J.B., J. Cable, P. Swarzenski and M. Lindenberg. 2004. Mixing of ground and estuary waters: influences on ground water discharge and contaminant transport. Ground Water 42, 1000–1010. 54 Mazzotti, F.J., L.G. Pearlstine, R. Chamberlain, T. Barnes, K. Chartier and D. DeAngelis. 2007. Stressor response model for the seagrasses, Halodule wrightii and Thalassia testudinum: final report for technical assistance for an ecological evaluation of the Southwest Florida Feasibility Study. 19 pp. Miller-Myers, R. and R. Virnstein. 2000. Development and use of an epiphyte photo-index (EPI) for assessing epiphyte loadings on the seagrass Halodule wrightii. In: Seagrasses: Monitoring, Ecology, Physiology, and Management (S.A. Bortone, ed.). CRC Press, Boca Raton, Florida. Morris, L., L. Hall and R. Virnstein. 2001. Field guide for fixed seagrass transect monitoring in the Indian River Lagoon. St. Johns River Water Management District, Palatka, Florida. Morris, L.J. and R.W. Virnstein. 2004. The demise and recovery of seagrass in the northern Indian River Lagoon, Florida. Estuaries 27: 915–922. Moutusi, R., J.B. Martin, J. Cherrier, J.E. Cable and C.G. Smith. 2010. Influence of sea level rise on iron diagenesis in an east Florida subterranean estuary. Geochimica et Cosmochimica Acta 74: 5560–5573. Nugraha, A., P. Pondaven and P. Tréguer. 2010. Influence of consumer-driven nutrient recycling on primary production and the distribution of N and P in the ocean. Biogeosciences 7: 1285–2010. Pandit, A., H. Heck and N. Ali. 2009. Cross-sectional groundwater modeling in the Indian River Lagoon (2007–08). Draft Final Report to St. Johns River Water Management District. Florida Institute of Technology, Department of Civil Engineering, Melbourne, Florida. Phlips, E.J. and S. Badylak. 2011. Phytoplankton abundance and composition in the Indian River Lagoon: July 2010-July 2011. Annual report submitted to the St. Johns River Water Management District, September 23, 2011. 59 pp. Phlips, E.J. and S. Badylak. 2012. Phytoplankton abundance and composition in the Indian River Lagoon–2011-2012. Annual report submitted to the St. Johns River Water Management District. SP 2013–SP3. Phlips, E.J., S. Badylak and T. Grosskopf. 2002. Factors affecting the abundance of phytoplankton in a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA. Estuarine, Coastal and Shelf Science 55: 385–402. Pulich, W.M. 1983. Growth responses of Halophila engelmannii Ascherson to sulfide, copper, and organic nitrogen in marine sediments. Plant Physiology 71: 975–978. Quinlan, E.L., C.H. Jett and E.J. Phlips. 2009. Microzooplankton grazing and the control of phytoplankton biomass in the Suwannee River estuary, USA. Hydrobiologia 632: 127–137. Rassoulzadegan, F., M. Laval-Peuto and R.W. Sheldon. 1988. Partitioning of the food ration of marine ciliates between pico- and nanoplankton. Hydrobiologia 159: 75–88. Reynolds, C.S. 2006. Ecology of Phytoplankton. Cambridge University Press, Cambridge, United Kingdom. 535 pp. Rhyther, J.H. and J.G. Sanders. 1980. Experimental evidence of zooplankton control of the species composition and size distribution of marine phytoplankton. Marine Ecology Progress Series 3: 279– 283. 55 Riegl, B., G. Foster and K. Foster. 2009. Mapping the distribution and vertical extent of muck in the Indian River Lagoon. Final Report to St. Johns River Water Management District. Nova Southeastern University, Oceanographic Center, Dania Beach, Florida. Riegl, B., R.P. Moyer, L. Morris, R. Virnstein and R.E. Dodge. 2005. Determination of the distribution of shallow-water seagrass and drift algae communities with acoustic seafloor discrimination. International Journal of Tropical Biology 3: 165–174. Scanlan, D.J., M. Ostrowski, S. Mazard, A. Dufresne, L. Garczarek, W.R. Hess, A.F. Post, M. Hagemann, I. Paulsen and F. Partensky. 2009. Ecological genomics of marine picocyanobacteria. Microbiology and Molecular Biology Reviews 73: 249–299. Sigua, G.C., J.S. Steward and W.A. Tweedale. 1996. Indian River Lagoon Water Quality Monitoring Network: Proposed Modifications. SJRWMD Technical Memorandum # 12. Sigua, G.C., J.S. Steward and W.A. Tweedale. 2000. Water-quality monitoring and biological integrity assessment in the Indian River Lagoon, Florida: status, trends, and loadings (1988–1994). Environmental Management 25: 199–209. Sigua, G.C., W.A. Tweedale, J.D. Miller and J.S. Steward. 1996. Inter-agency implementation of a modified water quality monitoring program for the Indian River Lagoon, “methods and QA/QC issues”. St. Johns River Water Management District Technical Memorandum # 19. Smayda, T.J. 2008. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 8: 140–151. Sorokin, Y.I. and O.Y. Zakuskina. 2010. Features of the Comacchio ecosystem transformed during persistent bloom of picocyanobacteria. Journal of Oceanography 66: 373–387. Steward, J.S., R. Brockmeyer, R. Virnstein, P. Gostel, P. Sime and J. VanArman. 2003. Indian River Lagoon Surface Water Improvement and Management (SWIM) Plan, 2002 Update. St. Johns and South Florida Water Management Districts. Palatka and West Palm Beach, Florida. Steward, J.S. and W.C. Green. 2007. Setting load limits for nutrients and suspended solids based upon seagrass depth-limit targets. Estuaries 30: 657–570. Steward, J.S., W. Green and J. Miller. 2010. Using multiple lines of evidence for developing numeric nutrient criteria for Halifax River Estuary, Florida. Final report to Florida Department of Environmental Protection, Tallahassee, Florida. St. Johns River Water Management District, Palatka, Florida. Steward, J.S., R.W. Virnstein, L.J. Morris and E.F. Lowe. 2005. Setting seagrass depth, coverage, and light targets for the Indian River Lagoon system, Florida. Estuaries 28: 923–935. Stoecker, D.K. and J.J. Govoni. 1984. Food selection by young larval gulf menhaden (Brevoortia patronus). Marine Biology 80: 299–306. Strom, S.L., E.L. Harvey, K.A. Fredrickson and S. Menden-Deuer. 2013. Broad salinity tolerance as a refuge from predation in the harmful raphidophyte alga Heterosigma akashiwo (Raphidophyceae). Journal of Phycology 49: 20–31. 56 Sun, M., J. Sun, J. Qiu, H. Jing and H. Liu. 2012. Characterization of the proteomic profiles of the brown tide Aureoumbra lagunensis under phosphate- and nitrogen-limiting conditions and of its phosphate limitation-specific protein with alkaline phosphatase activity. Applied and Environmental Microbiology 78: 2025–2033. Terrados, J., C.M. Duarte, L. Kamp-Nielsen, N.S.R. Agawin, E. Gacia, D. Lacap, M.D. Fortes, J. Borum, M. Lubanski and T. Greve. 1999. Are seagrass growth and survival affected by reducing conditions in the sediment? Aquatic Botany. 65: 175–197. Toth, D. 1987. Chapter 4. Hydrogeology. In: Steward, J. and VanArman, J. (eds.), Indian River Lagoon joint reconnaissance report. St. Johns River Water Management District and South Florida Water Management District, Palatka and West Palm Beach, Florida. Trefry, J., H. Feng, R. Trocine, S. Metz, G. Grguric, R. Vereecke and S. Cleveland. 1992. Concentrations and benthic fluxes of nutrients from sediments in the Indian River Lagoon, Florida (Project MUCK, Phase II). Final Report to St. Johns River Water Management District. Florida Institute of Technology, Department of Oceanography, Ocean Engineering and Environmental Science, Melbourne, Florida. Trefry, J., S. Metz, R. Trocine, N. Iricanin, D. Burnside, N. Chen and B. Webb. 1990. Design and Operation of a Muck Sediment Survey. Final Report to St. Johns River Water Management District. Florida Institute of Technology, Department of Oceanography and Ocean Engineering, Melbourne, Florida. Trefry, J.H., R.P. Trocine and D.W. Woodall. 2007. Composition and sources of suspended matter in the Indian River Lagoon, Florida. Florida Scientist 70: 363–382. Turner, J.T. 1984. The feeding ecology of some zooplankters that are important prey items of larval fish. National Oceanic and Atmospheric Administration Technical Report National Marine Fisheries Service 7. 28 pp. Vlaming, L.N.A. 2013. Comparative ecophysiology of bloom-forming macroalgae in the Indian River Lagoon, Florida: Ulva lactuca (Chlorophyta), Hypnea musciformis, and Gracilaria tikvahiae (Gigartinales: Rhodophyta). M.S. Thesis, Florida Atlantic University, Boca Raton, FL. 71 pp. Wetz, M.S. and P.A. Wheeler. 2004. Response of bacteria to simulated upwelling phytoplankton blooms. Marine Ecology Progress Series 272: 49–57. 57
© Copyright 2026