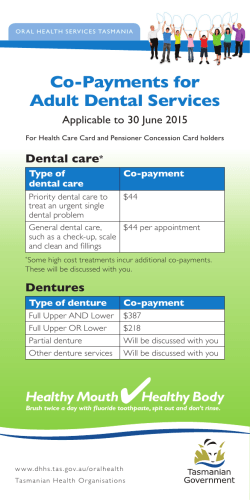
View and
B2 (14) CYTOTOXICITY OF QMix™ ENDODONTIC IRRIGATING SOLUTION ON HUMAN BONE MARROW MESENCHYMAL STEM CELLS & COMPARISON OF DIGITAL AND CONVENTIONAL IMPRESSION TECHNIQUES: EVAUATION OF PATIENTS’ PERCEPTION, TREATMENT COMFORT, EFFECTIVENESS AND CLINICAL OUTCOMES & HOW NOT TO OVERWORK YOUR HANDS & CURRENT FLUORIDE MODALITIES FOR REDUCTION OF DENTAL CARIES Approved for 4 CLINICAL Continuing Educational Units AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 RESEARCH ARTICLE Open Access Cytotoxicity of QMix™ endodontic irrigating solution on human bone marrow mesenchymal stem cells Ahmad AlKahtani1*, Sarah M Alkahtany1, Amer Mahmood2, Mona A Elsafadi2, Abdullah M Aldahmash2 and Sukumaran Anil3 Abstract Background: Debridement and disinfection of the root canal system is a crucial step in endodontic procedures. The effectiveness of irrigation relies on both the mechanical flushing action and the ability of irrigants to dissolve tissue and kill bacteria. The objective of the present study is to evaluate and compare the cytotoxicity of QMix™ root canal irrigating solution on immortalized human bone marrow mesenchymal stem cells (hTERT-MSC-C1) and to compare it with that of sodium hypochlorite (NaOCl). Methods: Immortalized human bone marrow mesenchymal stem cells (hTERT-MSCs) were exposed to QMix™ and NaOCl. Cell viability was assessed by 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and alamarBlue assays. The cell morphology was studied after two hours of exposure to QMix™ and NaOCl. Scanning electron microscopy (SEM) analyses were performed after 2- and 4-hour incubation periods. Finally, ethidium bromide/acridine orange (EB/AO) fluorescent stain was applied to the cells in the 8-chamber slides after they were incubated with the testing agents for 2 hours to detect live and dead cells. The observations were tabulated and analyzed statistically. Results: QMix™ exposure resulted in a significantly higher percentage of cell viability than NaOCl in the MTT and alamarBlue assays at three time points compared to the control. The SEM analysis demonstrated minimal morphological changes associated with cells that were exposed to the QMix™ solution, with little shrinkage and fragmentation of the cell wall. The live/dead analysis showed that the number of live cells after exposure to QMix™ was similar to that of the untreated control. No cell structure could be observed with the NaOCl group, indicating cell lysis. Conclusion: Both the QMix™ and NaOCl solutions were toxic to human bone marrow MSCs. Each solution might have induced cell death in a different way as evidenced in the cell viability, SEM and fluorescent studies. The slower cell death induced by QMix™ might therefore be less aggressive and more acceptable to living tissues. Keywords: Cytotoxicity, Sodium hypochlorite, QMix™, Mesenchymal stem cells, Root canal irrigants Background The success of endodontic therapy depends on the eradication of microbes from the root canal system and the subsequent prevention of reinfection. Root canal irrigation has a key role in the success of endodontic treatment. During and after instrumentation, irrigants facilitate the removal of microorganisms, tissue remnants, and dentin chips from the root canal through a flushing mechanism [1,2]. * Correspondence: [email protected] 1 Department of Restorative Dental Sciences, College of Dentistry, King Saud University, Post Box 60169, Riyadh 11545, Saudi Arabia Full list of author information is available at the end of the article An ideal root canal irrigant solution should be nontoxic, with a broad antimicrobial spectrum and the ability to dissolve necrotic pulp tissue, inactivating endotoxins, and either prevent the formation of a smear layer or dissolve it [3,4]. Currently, no single solution is able to achieve these goals, and the combined, concomitant or sequential use of two or more irrigating solutions is thus required [5]. Sodium hypochlorite (NaOCl) and chlorhexidine digluconate (CHX) are two common antibacterial agents used as root canal irrigants [5-7]. © 2014 AlKahtani et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Currently, sodium hypochlorite (NaOCl) (0.5–6.15%) and EDTA (15–17%) are the two most commonly used intracanal irrigants [5,8]. Although sodium hypochlorite has most of the desirable properties, it also can produce cytotoxicity and severe inflammatory reactions [9,10]. Ethylenediaminetetraacetic acid (EDTA) is effective for removing the inorganic component of the smear layer [11]. However, due to undesirable outcomes, a combination of these irrigants is not advisable [12-15]. Hence, the sequential use of EDTA, CHX and NaOCl has been advocated by many researchers to produce optimal root canal irrigation results [16-18]. It is imperative that the root canal irrigants must not hamper the healing process of the apical region. The biocompatibility of these materials is important, as they come into contact with the peri-radicular tissues. QMix™ is a 2-in-1 solution containing a bisbiguanide antimicrobial agent (2% CHX) and a polyaminocarboxylic acid calcium-chelating agent (17% EDTA) [19]. QMix™ was found to be effective in removing the smear layer and also has substantial antimicrobial properties [19-22]. However, data regarding the cytotoxicity of this agent are not available. Hence, the present study was conducted to evaluate and compare the cytotoxicity of the QMix™ irrigating solution on immortalized human bone marrow mesenchymal stem cells (hTERT-MSC-C1) using cell viability assays, cell morphology evaluation, and fluorescence light microscopy; 5.2% NaOCl was used for comparison. Methods The study was approved by the Departmental review board of College of Dentistry Research Centre, king Saud University, Riyadh. Test solutions QMix™2-in-1 (Dentsply Tulsa Dental, OK, USA) and 5.25% NaOCl (Clorox Co., Oakland, CA, USA) were used in this study. Cell culture Immortalized human bone marrow mesenchymal stem cells (hTERT-MSC-C1), at the 25th passage, were used for all experiments in this study. The protocol of Simbula et al. [23] was used in this study, with some modifications. The human bone marrow mesenchymal stem cells were provided by Professor Moustapha Kassem at the Odense University Hospital, Denmark [24]. The cryopreserved cells were rapidly thawed, transferred into a T-75 flask (BD Falcon™, NJ, USA) and cultured in Dulbecco’s modified Eagle’s medium (Gibco®), which was supplemented with glutamine (Gibco®), 1% penicillin-streptomycin (Gibco®), 10% fetal bovine serum (FBS Gibco®), and 1% non-essential amino acids (HyClone®), in a humidified atmosphere of 5% CO2/95% O2 at 37°C. At 70% confluence, the medium was aspirated, and the cells were washed with phosphate-buffered saline. Page 2 of 9 Three milliliters of pre-warmed 0.05% trypsin/EDTA (Gibco®) was added to the flask, and the cells were incubated for 1 minute. After gentle tapping, cell detachment was checked under an inverted light microscope (Observer A1, Zeiss®, Gottingen, Germany), and 12 ml of culture media was then added to the flask to neutralize the enzymatic effect of trypsin. For cell counting, two samples (10 μl) were taken from the cell suspension after appropriate mixing. The samples were placed in the upper and lower chambers of a Neubauer hemocytometer counting chamber (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany), and the cells were counted manually under an inverted microscope (10X magnification). The cells were seeded on different plates and slides, as discussed below, for each part of the study. All procedures were performed under a class II laminar flow hood (LabGard ES 425 Biological Safety Cabinet, NuAire®). MTT cell viability assay Cells were seeded in 96-well plates (clear, flat bottom, Polystyrene TC-Treated 96-well microplates) at a concentration of 1 × 104 cells/well and were then incubated for 24 hours to allow cell adherence to the bottom of the wells. Culture media were then aspirated from each well and replaced with 150 μl of sterile solution (QMix™ or NaOCl). Eight wells were used as replicates for each group. Each subgroup was incubated for the following periods: 2, 4, or 24 hours. Then, 10 μl of the MTT reagent (Cayman Chemical Company, Ann Arbor, MI, USA) was added to each well. Thereafter, 96-well plates were further incubated for 3 hours at 37°C. Finally, the solution from each well was aspirated, and 150 μl of dissolving agent was added to dissolve the formazan precipitate. The absorbance of each sample was measured with a microplate reader (Epoch Microplate Spectrophotometer, BioTek®) at a wavelength of 570 nm. The data were gathered using Gen5 Data Analysis Software (BioTek®, USA). The experiment was repeated twice for each group in each interval. AlamarBlue (AB) cell viability assay The same groups that were used for the MTT assay (mentioned above) were used for the AB assay with the same intervals. The test agents were added to each well. After 2-, 4-, and 24-hour incubation periods, 10% AB reagent (Serotec®, Oxford, UK) was added to each well. After the plates were further incubated for 4 hours, the fluorescence of each well was measured at wavelengths of 530/25 and 590/35 nm excitation/emission using a fluorescence reader (BioTek®). The data were gathered using the Gen5 Data Analysis Software (BioTek®, USA). The experiment was repeated twice for each group in each interval. AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Cell morphology assessment Cell morphology was evaluated with SEM after 2 and 4 hours of exposure to the test solutions. Briefly, 8 × 104 cells/well were seeded onto glass cover slides (1 × 1.5 cm) in 6-well plates (CellStar®, Carrollton, TX) overnight. The next day, the cells were exposed to the test solutions. Two milliliters of each sterile irrigation solution was added to the slides in each well. Control untreated cells were maintained in culture medium. Immediately after the test solutions were added, the cells were examined under an inverted LM (10x magnification). At the end of the incubation periods (2 and 4 hrs), the solutions in each well were aspirated. The slides were washed with PBS and were then fixed with 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.2) at room temperature. Thereafter, the specimens were washed with 0.1 M sodium cacodylate buffer (pH 7.2). After fixation, the specimens were treated with 1% osmium tetroxide for 1 hour. Then, they were washed with distilled water and dehydrated using graded ethyl alcohol, in concentrations of 50%, 70%, 80%, 90% (5 minutes each), 95% (twice, 15 minutes each), and finally, 100% absolute alcohol (twice, 30 minutes each). The specimens were dried using a critical point dryer with CO2 (SADRI-PVT-3B). Slides were mounted on copper stubs with double adhesive tape and were then gold sputter coated to a thickness of 5–7 μm. The specimens were then observed and photographed using a JSM-6360 LV scanning electron microscope. Two evaluators assessed the photomicrographs. The cell morphology was described according to the following criteria: the shape of the cell (normal or abnormal compared with the control), attachment to the subsurface, attachment to other cells, cytoplasmic surface extensions (blebs or microvilli), and cell wall integrity. Roundness of the cells, the presence of blebs, or detachment of the cells indicated greater cell injury [25,26]. Page 3 of 9 bromide powder that was first dissolved in 1 ml 95% ethanol and then diluted in 49 ml of distilled water (dH2O). The images were evaluated by two evaluators according to previously described criteria. Under the green filter, an intact green nucleus, which is comparable to the control, indicates a viable cell, whereas a green fragmented nucleus indicates early apoptosis. A red nucleus indicates a ruptured cell wall, whereas a red intact nucleus indicates necrosis, and a fragmented red nucleus indicates late apoptosis under the red filter [27,28]. Data and statistical analysis The results of the MTT and AlamarBlue assays were calculated as percentages relative to the control (100% = no toxicity). The data were analyzed using SPSS Pc + version 21.0 statistical software. Descriptive statistics (mean and standard deviation) were used to describe continuous outcome variables. Student’s t-test for independent samples was used to compare the mean values of two groups. A one-way analysis of variance, followed by a multiple comparison Tukey’s test, was used to compare the mean values of the three groups. A p-value of < = 0.05 was considered statistically significant. Results MTT assay The MTT assay was conducted on three groups (the QMix™ group, NaOCl group, and Control group) with cultured hTERT-MSCs in 96-well plates. Each group was incubated for 2, 4 and 24 hours. The cell viability for each group is presented as percentage of the control group at each time point. The percentage cell viability of each solution is illustrated in Figure 1. When the cells were exposed to solutions for 2, 4 and 24 hours, the cell viability decreased with time. The viability of cells exposed NaOCl was significantly lower than that of cells exposed to QMix™ at all time intervals (P < 0.05). Live/dead analysis AlamarBlue assay Two solutions were selected for the live/dead analysis. A modified Eagle’s medium (MEM) without phenol red (Gibco®, Gaithersburg, MD) was used for this experiment. Briefly, cells were seeded in three 8-chamber slides (LabTek®) at a concentration of 1.5 × 104 cells/well and were then incubated for 24 hrs to allow cell adhesion to occur. The culture medium in each well was replaced with 300 μl of each solution and then incubated for 2 hrs. Finally, 10 μl of EB/AO fluorescent dye was added to each chamber, and the fluorescence of the cells was analyzed under a fluorescent inverted microscope (ECLIPSE Ti, Nikon, Tokyo, Japan), with 10X magnification. Images were captured with imaging software (NIS-Elements, Nikon, Tokyo, Japan). The EB/AO fluorescent dye was prepared by mixing 15 mg acridine orange with 50 mg ethidium The AB assay was conducted for the QMix™, NaOCl and Control groups using cultured hTERT-MSCs in 96-well plates. The cell viability for each group is presented as the percentage of the control group. The cell viability percentage of each solution is illustrated in Figure 2. When the cells were incubated for 2 or 4 hrs, the cell viability of the NaOCl group was significantly lower than that of the QMix™ group (P < 0.05). Cell morphology Untreated negative control cells showed variable shapes, including rhomboid, triangular, oval and spindle shapes, as illustrated by light microscopy LM (Figure 3) Cells were attached to each other and to the substrate. The cell wall appeared smooth and intact, with some microvilli and AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Page 4 of 9 Figure 1 The cell viability percentage for NaOCl and QMix solutions at 2, 4 and 24 hours after exposure using MTT assay. surface extensions. According to the LM investigation, the toxic effect of the test solutions was evident immediately after their application to the cultured human bone marrow MSCs, and the normal cell morphology was altered differently in each group (Figure 3). A high concentration (0.5 mg/ml) of NaOCl caused the vacuolization of the cytoplasm (Figure 3B), whereas QMix™ at the same concentration produced few alterations in the cell wall and no vacuolization (Figure 3C). Figure 3 Human bone marrow hTERT-MSCs under inverted LM after adding the testing agents (X10). A- Untreated cells, B - Cells exposed to (0.5) Qmix™ solution (the cell shape was maintained with few alterations), C- Cells exposed to (0.5) NaOCl; vacuolization was evident in the cytoplasm. Figure 2 The cell viability for both NaOcl and QMix™ solutions using alamarBlue assay at 2, 4 and 24 hours of exposure. According to the SEM analysis, the cell number decreased relative to the control after a 2-hour exposure to 0.5 mg/ml NaOCl. The remaining cells were smaller, with a thread-like or round shape (Figure 4). Cells became detached from the subsurface, and cell-to-cell attachments were lost. Lysosomal secretions emerging AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Page 5 of 9 Figure 4 Human bone marrow hTERT-MSCs exposed for 2 hrs to QMix and NaOCl. A - QMix™ (x100), B - QMix™ (x1000), C - NaOCl (x100, note the round or thread-like shape of the remnant cells), D- NaOCl (x1000, the ruptured cell wall and the extruded nucleus). from the cell were observed, and the nucleus was extruded through the ruptured cell wall. After a 2-hour exposure to QMix™, the cell number and cell shape were similar to those of the control, except for the presence of an ill-defined cell outline. However, the cells were still attached to adjacent cells and to the substrate (Figure 4). In some areas, remnants of cell processes were observed. The main dramatic change in the cell morphology was in the cell wall, which had a mesh-like appearance (Figure 4B), indicating disintegration. After 4 hours, the alteration of cell morphology was more significant: the mesh-like appearance of the cells became more intense, with an ill-defined shape and a fading outline, and broken cell-to-cell attachments were observed. However, the attachment to the substrate was maintained by some cells (Figure 5). Live/dead analysis with EB/AO staining Cultured human bone marrow hTERT-MSCs were exposed to 0.5 mg/ml of QMix™ and NaOCl for 2 hrs. The EB/AO stain was applied to the cells, which were then examined under an inverted fluorescence microscope. The images for each group display live cells in green and dead cells in red. The untreated cells had intact, well-defined nuclei, which were green under the green filter, as shown in Figure 6A. Under the red filter, only the cell surface showed red fluorescence, not the nucleus, which indicated an intact cell wall (Figure 6B). When the cells were exposed to QMix™, the cell number was similar to that of the control group, and the nuclei were green, which was similar to the appearance of the control cells. The only difference was that some of these cells had condensed chromatin, which was evident under the red filter (Figure 6C, D). When the cells were exposed to NaOCl, no cell structures, such as the nucleus or cell membrane, were observed; cell lysis was evident, and the remnant material was positive for both green and red fluorescence (Figure 6E, F). Discussion This in vitro study was conducted to assess the cytotoxicity of the QMix™ irrigating solution on human bone marrow MSCs. MSCs have been suggested as a good model for toxicological testing [29]. The MSCs that were used in this study were immortalized by the ectopic expression of human telomerase reverse transcriptase (hTERT), which increased the life span of the cells [24] and maintained their stem-like properties [30]. Earlier studies have reported that immortalized cells can be used as a test model for dental materials [31]. The observations from the study showed that both solutions (QMix™ and NaOCl) are toxic to human bone marrow MSCs and cause cellular damage. This is consistent with the results of previous studies that reported on NaOCl toxicity [23,32,33]. NaOCl toxicity can be attributed to its high pH (hydroxyl ion action), which interferes AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Page 6 of 9 Figure 5 Human bone marrow hTERT-MSCs exposed for 4 hrs to QMix™ and NaOCl. A- QMix™ (x100), B-QMix™ (x1000), C - NaOCl (x100), D- NaOCl (x1000). with cytoplasmic membrane integrity [34]. Furthermore, our results are in agreement with those of a previous in vivo study, which found that QMix™ is toxic and can induce an inflammatory response [35]. CHX is a toxic agent that binds to the cell’s plasma membrane and increases its permeability, allowing the leakage of lysosomal enzymes [36]. EDTA, which is the second QMix™ component, is also known to be cytotoxic, perhaps due to its chelating effect and the accentuated drop in pH that it causes [11]. Cell viability decreased significantly when the cells were exposed to NaOCl for all time periods examined. Cell viability decreased significantly after being exposed to the QMix™ solution for 2 or 4 hours. Moreover, after 24 hours, cell viability was significantly decreased compared to 2 and 4 hours of exposure. These findings show that the toxic effect of an agent gradually increases with time. This observation is in agreement with those of previous studies, confirming that toxicity is time dependent [37]. In contrast, the MTT assay results showed a significant decrease in the cell viability of cells that were exposed to NaOCl at all time periods examined. Compared with QMix™exposed cells, NaOCl decreased viability at 2 and 4 hours . Previous studies have reported that the AB assay is slightly more sensitive than the MTT assay. However, both assays rely on enzymatic metabolism, which may be inhibited or induced by the testing agent, thus producing a false-positive or false-negative result. Therefore, careful interpretation of the results is always recommended [38]. Our observations suggest that the AB assay is a better choice for cell viability testing because it is easy to perform, more consistent than the MTT assay, and recommended by previous studies [38,39]. However, it is always recommended to use more than one assay to assess cytotoxicity. Therefore, previous studies that relied solely on the MTT assay should be re-evaluated and interpreted with caution. Microscopic morphological investigations are necessary to confirm cellular toxicity [40]. This is because a cell can undergo toxic changes, such as detachment, while still continuing to metabolize MTT to formazan, resulting in an over estimation of cell viability in the MTT assay compared with the AB assay [38]. Therefore, cellular morphological characteristics were investigated for both solutions. Cells that were exposed to QMix™ displayed fewer morphological alterations. This observation is in agreement with Faria et al., who reported that higher concentrations of CHX would preserve the shape of the L929 cells [41] due to its cell fixation effect [42]. The SEM images for the QMix™ group showed cytoplasmic shrinkage with partially fragmented but intact cell walls. These characteristics are typical of apoptosis, as reported previously [40]. In addition, the EB/AO staining showed bright green fragmented nuclei, indicating early apoptosis [27]. The cells exposed to NaOCl had cytoplasmic shrinkage or ruptured membranes, which are typical characteristics of necrosis [43]. The EB/AO AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 Page 7 of 9 Figure 6 Live/dead analysis human bone marrow hTERT-MSCs under a fluorescence microscope (X10). 6A, 6B – Untreated cells with the green filter, the nucleus appears intact , which indicates a viable cell. 6B-with the red filter, the nucleus appeared dark, and only the external surface of the cells was red, indicating that the cell wall is intact. 6C, 6D- treated with QMix™ under a fluorescence microscope (X10), 6E,6F- treated with NaOCl under a fluorescence microscope (X10). staining of the NaOCl group displayed red and green, reflecting remnant material that may be the result of cell lysis, and no cell structure could be observed. The overall analysis of these results suggests that the cells in the QMix™ groups are in the early stages of apoptosis but are not yet dead, in contrast to the NaOCl group. Both NaOCl and QMix™ are cytotoxic; however, it seems that their mode of cell killing differs as a result of their different compositions. According to Galluzzi et al. [44], cell death can be classified into four different types based on the morphological characteristics of the dying cells: apoptosis (Type 1), autophagy (Type 2), necrosis (oncosis, Type 3), and mitotic catastrophe. Each mode of cell death has its own function; necrosis induces an inflammatory response when it is needed, whereas apoptotic cells in vivo are rapidly phagocytosed without inducing an inflammatory response, which is considered a mechanism for avoiding immune activation. According to our findings, this mode of cell death could be associated with a high concentration exposure to NaOCl. Apoptosis is an active form of cell death known as programmed cell death, and it is characterized by cell shrinkage and the nuclear chromatin condensation followed by nuclear fragmentation, with the normal morphological appearance of cytoplasmic organelles and the maintenance of an intact plasma membrane [44]. According to our AlKahtani et al. BMC Oral Health 2014, 14:27 http://www.biomedcentral.com/1472-6831/14/27 findings, this mode of cell death could be associated with QMix™ exposure. However further markers such as detection of caspases, cleaved substrates, regulators and inhibitors are necessary to substantiate this mode of cell death speculated with QMix™ [45]. Cell death through apoptosis is known to occur through two primary pathways: an extrinsic pathway that involves death receptors, and an intrinsic pathway that is modulated by members of the Bcl-2 family [46], which consists of outer mitochondrial membrane components [47]. Therefore, mitochondria are considered key organelles in the pathways to cell death in addition to its normal metabolic activity [48,49]. However, these proteins may also function in some normal metabolic pathways. Thus, mitochondrial metabolic activity investigations, such as the MTT assay, must consider these potential overlaps between pre-apoptotic cell activity and normal cell metabolism [40]. This overlap could explain the contradictory results of the AB and MTT assays. In vitro cytotoxicity investigations reported that CHX had a higher toxicity in cell cultures than NaOCl [33,41]. However, in vivo studies suggest that CHX or QMix™ is less aggressive than NaOCl [35,50]. This difference could be attributed to host defense mechanisms that operate in the in vivo environment. Our in vitro study has the following limitations: it was conducted on cultured cells, and the results represent only the response of these cells in isolation, without taking into account the host defense mechanism for detoxification. Furthermore, in a clinical setting, the solutions are always delivered to root canals, which are surrounded by dentine, and are then extruded to the periapical area. Previous studies have reported that the cytotoxic effect of irrigants can be neutralized by dentine [33,51]. We placed the solutions directly onto the cells, and no attempts were made to deliver these solutions through root canals to mimic the clinical scenario. Conclusions Within the limitations of this study, it can be concluded that both the NaOCl and QMix™ solutions are toxic to human bone marrow MSCs. The QMix™ solution, which induces slow cell death, seems to be more biocompatible than the NaOCl solution. Hence, based on these observations, it can be concluded that the QMix™ is a relatively safer root canal irrigant compared to NaOCl. Competing interests The authors declare that they have no competing interests. Authors’ contributions AK, SMA, AM and MAE carried out the study, laboratory procedures and evaluation of the results. SA, AK and AMA involved in the development of the concept, design of the study, revision of the manuscript and statistical analysis. All authors read and approved the final manuscript. Page 8 of 9 Author details 1 Department of Restorative Dental Sciences, College of Dentistry, King Saud University, Post Box 60169, Riyadh 11545, Saudi Arabia. 2Stem Cell Unit, Department of Anatomy College of Medicine, King Saud University, P.O. Box 2925, Riyadh 11461, Saudi Arabia. 3Department of Periodontics and Community Dentistry, College of Dentistry, King Saud University, Riyadh, Post Box 60169 11545, Saudi Arabia. Received: 18 February 2014 Accepted: 25 March 2014 Published: 29 March 2014 References 1. Goldman L, Goldman M, Kronman J, Lin P: The efficacy of several irrigating solutions for endodontics: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol 1981, 52:197–204. 2. Baker NA, Eleazer PD, Averbach RE, Seltzer S: Scanning electron microscopic study of the efficacy of various irrigating solutions. J Endod 1975, 1(4):127–135. 3. Zehnder M: Root canal irrigants. J Endod 2006, 32(5):389–398. 4. Sousa S, Bramante C, Taga E: Biocompatibility of EDTA, EGTA and citric acid. Braz Dent J 2005, 16:3–8. 5. Haapasalo M, Shen Y, Qian W, Gao Y: Irrigation in endodontics. Dent Clin North Am 2010, 54(2):291–312. 6. Jeansonne M, White R: A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod 1994, 20:276–278. 7. Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T: Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002, 94(6):756–762. 8. Zehnder M, Schmidlin P, Sener B, Waltimo T: Chelation in root canal therapy reconsidered. J Endod 2005, 31(11):817–820. 9. Pashley EL, Birdsong NL, Bowman K, Pashley DH: Cytotoxic effects of NaOCl on vital tissue. J Endod 1985, 11(12):525–528. 10. Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M: Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod 1995, 21(10):513–515. 11. Hülsmann M, Heckendorff M, Lennon A: Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J 2003, 36:810–830. 12. Niu W, Yoshioka T, Kobayashi C, Suda H: A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J 2002, 35(11):934–939. 13. Serper A, Calt S, Dogan AL, Guc D, Ozcelik B, Kuraner T: Comparison of the cytotoxic effects and smear layer removing capacity of oxidative potential water, NaOCl and EDTA. J Oral Sci 2001, 43(4):233–238. 14. de Sermeno RF, da Silva LA, Herrera H, Herrera H, Silva RA, Leonardo MR: Tissue damage after sodium hypochlorite extrusion during root canal treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009, 108(1):e46–e49. 15. Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A: Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod 2007, 33(8):966–969. 16. Basrani B, Santos JM, Tjaderhane L, Grad H, Gorduysus O, Huang J, Lawrence HP, Friedman S: Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002, 94(2):240–245. 17. Zamany A, Safavi K, Spangberg L: The effect of chlorhexidine as an endodontic disinfectant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003, 96:578–581. 18. Krishnamurthy S, Sudhakaran S: Evaluation and prevention of the precipitate formed on interaction between sodium hypochlorite and chlorhexidine. J Endod 2010, 36(7):1154–1157. 19. Dai L, Khechen K, Khan S, Gillen B, Loushine BA, Wimmer CE, Gutmann JL, Pashley D, Tay FR: The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod 2011, 37(1):80–84. 20. Eliot C, Hatton JF, Stewart GP, Hildebolt CF, Jane Gillespie M, Gutmann JL: The effect of the irrigant QMix on removal of canal wall smear layer: an ex vivo study. Odontology 2013. 21. Pai S, Thomas MS: The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod 2011, 37(6):741. author reply 741–743. Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 RESEARCH ARTICLE Open Access Comparison of digital and conventional impression techniques: evaluation of patients’ perception, treatment comfort, effectiveness and clinical outcomes Emir Yuzbasioglu*, Hanefi Kurt, Rana Turunc and Halenur Bilir Abstract Background: The purpose of this study was to compare two impression techniques from the perspective of patient preferences and treatment comfort. Methods: Twenty-four (12 male, 12 female) subjects who had no previous experience with either conventional or digital impression participated in this study. Conventional impressions of maxillary and mandibular dental arches were taken with a polyether impression material (Impregum, 3 M ESPE), and bite registrations were made with polysiloxane bite registration material (Futar D, Kettenbach). Two weeks later, digital impressions and bite scans were performed using an intra-oral scanner (CEREC Omnicam, Sirona). Immediately after the impressions were made, the subjects’ attitudes, preferences and perceptions towards impression techniques were evaluated using a standardized questionnaire. The perceived source of stress was evaluated using the State-Trait Anxiety Scale. Processing steps of the impression techniques (tray selection, working time etc.) were recorded in seconds. Statistical analyses were performed with the Wilcoxon Rank test, and p < 0.05 was considered significant. Results: There were significant differences among the groups (p < 0.05) in terms of total working time and processing steps. Patients stated that digital impressions were more comfortable than conventional techniques. Conclusions: Digital impressions resulted in a more time-efficient technique than conventional impressions. Patients preferred the digital impression technique rather than conventional techniques. Keywords: Digital impression, Clinical efficiency, Patient comfort, Patient preference Background The introduction of computer-aided design/computer aided manufacturing (CAD/CAM) technology in dentistry has resulted in more accurate manufacturing of prosthetic frameworks, and greater accuracy of dental restorations, and the technology has improved since the 1980s [1,2]. The development strategy of CAD/CAM techniques included automating the production process and optimizing the quality of restorations by using new biocompatible materials, especially high performance ceramics, such as zirconia and lithium disilicate [3]. Several reports have demonstrated the potential for accurate * Correspondence: [email protected] Department of Prosthodontics, School of Dentistry, Istanbul Medipol University, Istanbul, Turkey and precise restorations using CAD/CAM technology [4-7]. According to the 8th edition of The Glossary of Prosthodontics Terms, “impression” is defined as “a negative likeness or copy in reverse of the surface of an object; an imprint of the teeth and adjacent structures for use in dentistry” [8]. The accuracy of the impression depends on the materials themselves [9-13], impression tray types [14-16], and impression techniques [17-19]. Each step in the process introduces potential human and/or material error [20,21]. There is some variability in impressions and the resulting master casts, depending on the technique and material used by the operator [22]. The accuracy of master casts has been the subject of numerous research projects, © 2014 Yuzbasioglu et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 and is dependent on numerous items, including the water/ powder ratio, vacuum versus hand mixing [23-25], and the type of dental stone and its compatibility with impression materials [26]. Digital impression and scanning systems were introduced in dentistry in the mid 1980s. It was predicted that most of the dentists in the U.S. and Europe would be using digital scanners for taking impressions within the next decade [27]. Digital impressions offer speed, efficiency, ability of storing captured information indefinitely and transferring digital images between the dental office and the laboratory [28]. The advantages of the digital impressions and scanning systems are improving patient acceptance, reducing the distortion of impression materials, 3D pre-visualization of tooth preparations, and potential cost- and time-effectiveness [29]. Several studies on the accuracy of intraoral scanners and digital impressions have been published, testing single-unit restorations [30-33], several teeth in a row [34-36], quadrants [37], and full arch scans [38,39]. A recent report by Lee & Gallucci [40] compared the operator’s preference of digital versus conventional implant impression techniques. In this in vitro study, inexperienced students made impressions on a customized model instead of live patients. The overall perception of the inexperienced students was that they preferred the digital impression technique. Until now there have been no clinical studies comparing the digital and conventional impression techniques. The aim of this clinical trial was to evaluate the effectiveness, clinical outcomes, and patients’ preferences and attitudes towards the digital impression technique compared to the conventional impression technique. The first null hypothesis was that there is no difference in effectiveness and clinical outcomes between the conventional and digital impression techniques. The second null hypothesis was that there is no difference in patients’ preference and treatment comfort between the conventional and digital impression techniques. Methods Study design & patient selection A controlled clinical trial was designed. The study population consisted of first year dental and medical students of the İstanbul Medipol University who had no experience with either conventional or digital impressions. The subjects were informed in detail about the possible risks and benefits, and all signed an informed consent form. The study was performed following the principles outlined in the Declaration of Helsinki on experimentation involving human subjects. The study protocol was reviewed and approved by the Ethical Committee of the Istanbul Medipol University, Istanbul, Turkey, (No:10840098-74). Page 2 of 7 Inclusion/exclusion criteria Twenty-four subjects (12 females, 12 males, aged 21.87 ± 2.76 years) who fulfilled the following inclusion criteria were recruited after an initial examination: no experience with either conventional or digital impressions, good general health, good oral hygiene, no periodontal disease, and good mental health. Prerequisites for exclusion in the study were previous impression experience, fixed or removable prosthetic rehabilitation, orthodontic treatment and preventive appliances, history of use of space maintainers in mixed dentition, moderate to excessive dental anxiety. Clinical scenario A clinical scenario of an “excessive destruction of a mandibular molar and crown fracture of the lateral incisor, which would be restored by post-core and all ceramic crowns” was explained to the subjects during their orientation to the clinical settings of the study. Subjects watched an informational video illustrating the restorative steps of the clinical scenario. The impression phase was excluded from the video. Conventional impressions One operator selected the proper tray for both arches of the subject, and applied the adhesive (Polyether Tray Adhesive, 3 M ESPE, Dental Products, St. Paul, MN, U.S.A.). The conventional impressions of mandibular and maxillary arches were made by polyether impression material (Impregum Penta Soft Quick Step MB, 3 M ESPE, Dental Products, St. Paul, MN, U.S.A.) with stock trays using the monophase impression technique. The interocclusal relationship was recorded with a polysiloxane bite registration material (Futar D, Kettenbach GmbH & Co. KG, Eschenburg, Germany). All materials were used according to the manufacturers’ guidelines and performed by the same operator (E.Y.). The effectiveness and clinical outcomes of the conventional impression technique was evaluated by measuring the total treatment time, including the individual steps (Figure 1): A) tray selection, B) adhesive application, C) upper/lower impression, D) bite registration. The treatment time was measured in seconds and recorded for each step by a second operator (R.T. & H.B.). Immediately after the impressions were made, the attitudes and perceptions of the subjects towards the conventional impression technique were evaluated using a standardized questionnaire. The subjects’ perceived source of stress was also evaluated using the State-Trait Anxiety Scale immediately after the impression technique. Digital impressions A digital impression appointment was scheduled for the same patients 2–3 weeks following the conventional Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 Page 3 of 7 Figure 1 Conventional impression technique. Conventional impression technique. A) Adhesive application, B) Impression tray loading, C) Upper and lower arches impression, D) Bite registration. impressions. The digital impressions were performed with the chairside dental CAD-CAM system (Cerec OMNICAM, Sirona Dental GmBH, Wals Bei Salzburg, Austria). The digital impression electronic data constituents of the virtual models for both arches and bite registration were recorded. All digital scanning procedures were carried out according to the manufacturer’s guidelines and performed by the same operator (EY). The effectiveness and clinical outcomes of the digital impression technique were evaluated by measuring the total treatment time, including the individual steps (Figure 2): A) entering patient information (including name, last name, date of birth, B) laboratory prescription (including shade of restoration, material choice of restoration, form of restoration), C) upper/lower scan, and D) bite scan. Treatment time was measured in seconds and recorded for each step by a second operator (R.T. & H.B.). Immediately after the impressions were made, the attitudes and perceptions of the subjects towards the digital impression technique were evaluated using a standardized questionnaire. The subjects’ perceived source of stress was also evaluated using the State-Trait Anxiety Scale immediately after the impression technique. The subjects were also asked to answer a 9-item comparative questionnaire including the following research questions: Which was the preferred impression technique? Which was the recommended impression technique? Which impression technique was more efficient? Which impression technique would be most comfortable regarding impression techniques? Reliability and validity of questionnaires The questionnaires used in this study were pre-tested, revised, and retested before use. A pilot questionnaire was tested on a representative sample of 10 patients. Test-retest reliability was performed to test the reliability and internal consistency of the questionnaires. The Cronbach Alpha reliability coefficient of the scales were found as 0.921, and 0.982, respectively. The adaptation, reliability and validity of the Turkish version of the State-Trait Anxiety Scale were evaluated by Öner and Le Compte in 1983 [41]. Statistical analysis Statistical analysis by the Wilcoxon Signed-Rank Test, with p = 0.05 as the level for statistical significance, was performed to evaluate the differences in effectiveness and clinical outcomes between conventional and digital impression techniques, using the SPSS 15.0 for Windows statistical software (SPSS Inc., Chicago, IL, USA). The attitudes and perceptions of the subjects on both impression techniques were assessed with a selfadministrated questionnaire using a Visual Analog Scale (VAS) ranging from 0 to 100. The data were analyzed statistical by the Wilcoxon Signed-Rank Test, with p = 0.05 as the level for statistical significance, using the SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA). The subjects’ preferences for the impression techniques were assessed with a 9-item comparative questionnaire, and the distribution of the answers were evaluated by descriptive analysis using the SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA). Results The evaluation of the effectiveness and clinical outcomes for both impression techniques are presented in Table 1. The mean overall treatment times were statistically Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 Page 4 of 7 Figure 2 Digital impression technique. A) Entering patient information, B) Laboratory prescription, C) Upper and lower arches scanning, D) Bite scanning. significantly different (p < 0.001), and comparison of the mean impression times indicated a statistically significant difference (p < 0.001). The mean tray selection time for the conventional impression technique and the mean time for entering patient information for the digital impression technique were not statistically significant (p > 0.05). The mean adhesive application time for the conventional impression technique was statistically significantly different (p < 0.001) from the mean time for entering the laboratory prescription time for the digital impression technique. The difference between the mean bite registration time for the conventional technique and the mean bite scan time for the digital technique was statistically significant (p < 0.001). Outcomes of conventional impressions The mean overall treatment time of the conventional impression technique was 605.38 ± 23.66 s. The mean treatment times of the individual steps of the conventional impression technique was as follows: Mean tray selection time, 18.87 ± 2.42 s; mean adhesive application time, 27.75 ± 3.12 s. The mean conventional impression time of the upper and lower jaws was 240.70 ± 16.38 s and the mean bite registration time was 91.96 ± 10.74 s. Outcomes of digital impressions The mean overall treatment time of the digital impression technique was 248.48 ± 23.48 s. The mean treatment times Table 1 Scores of clinical efficiency outcomes of impression techniques Efficiency Conventional Digital Tray selection/Patient information 18,87 ± 2,42 19,08 ± 3,57 >0.05 Adhesive application/Laboratory prescription 27,75 ± 3,12 13,63 ± 1,98 <0.001* P-value Upper impression/Upper scan 240,70 ± 16,38 102,14 ± 17,77 <0.001* Lower impression/Lower scan 226,10 ± 10,89 98,94 ± 10,56 <0.001* Bite registration/Bite scan 91,96 ± 10,74 14,68 ± 3,82 <0.001* Total treatment time 605,38 ± 23,66 248,48 ± 23,22 <0.001* All data are presented as mean ± SD. Measured time is recorded as seconds. *Statistical significance level p-0.05. Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 Page 5 of 7 Table 2 Participants’ evaluation scores and level of self concerns about impression techniques Evaluation (VAS score) Conventional Digital P-value Overall discomfort of impression 59,00 ± 37,72 90,04 ± 18,37 <0.001* Overall time of impression 65,10 ± 41,55 90,28 ± 18,36 <0.001* Smell/Voice 54,90 ± 39,04 86,52 ± 21,16 <0.001* Taste/Heat 54,20 ± 28,06 88,16 ± 19,76 <0.001* Queasiness 48,20 ± 44,53 91,80 ± 20,37 <0.001* Discomfort during mouth was opened 44,40 ± 36,21 88,04 ± 19,86 <0.001* Discomfort in TMJ 55,90 ± 43,31 88,68 ± 19,83 <0.001* Breathing difficulty 59,90 ± 37,90 87,32 ± 21,02 <0.001* Teeth and Periodontal sensivity 47,10 ± 43,21 85,36 ± 23,70 <0.001* Total evaluation score 507,25 ± 277,34 827,50 ± 171,11 <0.001* 41,33 ± 3,84 43,29 ± 3,89 >0.05 Level of self concern Score of STATI-TX 1 All data are presented as mean ± SD. Visual Analog Scale (VAS). *Statistical significance level p-0.05. of the individual steps of the digital impression technique were as follows: the mean time for entering patient information, 19.08 ± 3.57 s, and the mean time for entering the laboratory prescription time, 13.63 ± 1.98 s. The mean digital impression time for the upper and lower jaws was 98.94 ± 10.56 s and the mean bite scan time was 14.68 ± 3.82 s. Patients’ preferences and self concerns The evaluation scores and the level of concerns of the subjects regarding the impression techniques are presented in Table 2. The mean scores of the subjects’ evaluation criteria regarding the two impression techniques were significantly different (p < 0.001). The subjects’ level of self concern were evaluated by scores of STATI-TX 1. The mean scores were not statistically significant (p > 0.05). All the subjects preferred the digital impression technique (p < 0.001), and patients’ preferences regarding the impression techniques, according to the 9-item comparative questionnaire, are listed in Table 3. Discussion In this clinical trial, according to the clinical scenario, the digital impression technique was more efficient than the conventional impression technique. Thus, the first null hypothesis was rejected. The subjects also preferred the digital impression technique rather than the conventional impression technique because of its comfort. Thus, the second null hypothesis was also rejected. The study population was standardized and homogenized by including subjects who had no experience with conventional or digital impressions in their dental history. To investigate the clinical outcomes of the two impression techniques, homogenizing the study population is an acceptable clinical research method to optimize objectivity and minimize bias. This approach is important to avoid reporting the bias of patients who had previous experience with the dental impression procedure. In this present study, we focused primarily on the efficiency of the two impression techniques and the preference of the patients under controlled clinical conditions. Future investigations should include the assessment of Table 3 Participants’ preferences about impression techniques according to the 9-item questionnaire Preferences Conventional Digital Which impression technique do you prefer in case of one more time for impression procedure? %0 %100 Which impression technique is more comfortable from point of comparison of two impression procedure? %0 %100 Which impression technique do you suggest in case of a friends’ need for impression making? %0 %100 Which impression technique do you prefer from point of time involved with impression procedure? %0 %100 Which impression technique do you prefer from point of feeling taste/smell or voice/heat during impression procedure? %0 %100 Which impression technique do you prefer from point of the size of the intraoral scanner/impression tray used in your mouth during impression procedure? %0 %100 Which impression technique do you prefer from point of having tooth/gingival sensitivity during impression procedure? %0 %100 Which impression technique do you prefer from point of having difficulty in breathing during impression procedure? %0 %100 Which impression technique do you prefer from point of having gagging reflex during impression procedure? %0 %100 Yuzbasioglu et al. BMC Oral Health 2014, 14:10 http://www.biomedcentral.com/1472-6831/14/10 the accuracy of the impressions produced by experienced versus non-experienced operators, comparison of using scanning powders versus non-powder scanning, and comparison of full arch and partial impressions. There are some limitations of this study. The study was designed as a comparative-controlled clinical trial, and the sequence of the evaluation of the two impression techniques was chosen for psychological reasons. There is a 2–3-week interval between the two evaluation appointments. This time period was deemed sufficient to erase from memory an event or a process. The evaluation process focuses on the outcomes of the impression techniques by means of total treatment time in seconds, and the study does not analyze any differences in precision of the two impression techniques. Another limitation of the study was that only one operator performed the impression techniques to avoid the possible inter-operator error, such as the prolonged processing time taken by an inexperienced operator. The main purpose of the study was to focus on the patients’ perceptions and comfort in using different impression techniques. Evaluation by a second operator was not preferred because of main purpose of the study. Further investigations are planned to evaluate the perceptions of patients treated by different dental specialties and operator experience to the digital impression technique. The last limitation of this study is that it ignored the time factors involved in the conventional impression technique, such as pouring and mounting the cast, trimming the dies, painting the die spacer, etc. By eliminating these steps, time for the traditional workflow would be reduced significantly. Furthermore, the digital impression technique and digital workflow are designed as the “digital working model” directly from the intraoral scan, without any additional factors. By virtually eliminating the intermediate processes, error accumulation in treatment and in the manufacturing cycle is no longer an issue. The results of this study have revealed clinical evidence that the digital impression technique can be applied successfully for the impressions of restorative procedures based on clinical outcomes and the patients’ preferences. However, this study was performed in a clinical scenario that excluded the effect of actual treatment conditions, perceived dental anxiety and stress associated with treatment. This is an additional limitation of this study. The major advantage of digital impressions is reducing the chair time. The mean total treatment time (p < 0.001) and the subjects’ evaluation scores (p < 0.001) regarding the impression techniques were significantly different (Tables 1 and 2). Improving the level of the patients’ comfort and treatment acceptance (p < 0.001) were other advantages of the digital impression techniques (Tables 1 Page 6 of 7 and 2). Digital impressions tend to reduce repeat visits and retreatment, while increasing treatment effectiveness [42]. Patients will benefit from more comfort and a pleasant experience in the dentist’s chair. The results of study indicate that the efficiency outcomes of the digital impression technique were higher than that of the conventional impression technique, with respect to treatment time taken up and the perceptions of the subjects. The effectiveness and clinical outcomes of both impression techniques (Table 1) were evaluated by recording the treatment time of each step in seconds, and were significantly different from each other (p < 0.001). The scores of the evaluation criteria regarding the two impression techniques (Table 2) that affect the subjects’ perception differed from one another in a statistically significant manner (p < 0.001). The differences in the level of treatment comfort evaluated by the subjects, including breathing difficulty, queasiness, discomfort in the TMJ, and discomfort while the mouth was kept open were statistically significant (p < 0.001). Thus, the digital impression technique is more patient-friendly than the conventional impression technique. The results of this study present the major reasons why the subjects preferred the digital impression technique instead of the conventional impression technique (Table 3). Conclusions Within the limitations of this study, the following conclusions can be drawn: 1. The digital impression technique was more efficient than the conventional impression technique. The overall treatment time for the conventional impression technique was longer than that for the digital impression technique. Thus, the first null hypothesis was rejected. 2. When compared with the conventional impression technique, the digital impression technique was accepted as the preferred and effective technique, according to the subjects’ perception. Thus, the second null hypothesis was rejected. 3. The treatment comfort of the digital impression technique was higher than that of the conventional impression technique when it was performed by an experienced operator. Competing interests The authors declare that they have no competing interests. Authors’ contributions EY is the designed and carried out the clinical study, collected the data for analysis , performed the statistical analysis and drafted the manuscript. HK participated in the design of the study and interpretation of data. RT and HB were collected the data for analysis. All authors read and approved the final manuscript. Registered Dental Hygienist 05/01/2014 Volume 34, Issue 5 How not to overwork your hands Use a little leverage during periodontal instrumentation By Cynthia Biron Leiseca, RDH, EMT, MA Want to reduce the workload on your hands during instrumentation? This article reviews current basic techniques and presents new techniques that provide the best leverage and require the least effort and workload on your hands. Some very simple changes to your instrumentation techniques could make major improvements in how your hands feel at the end of a working day. As dental hygienists, we have repeatedly heard that incorrect patient-operator positioning while performing periodontal instrumentation often leads to cumulative trauma disorders (CTDs). To compensate for the lack of control from incorrect positioning, clinicians not only strain their necks, backs, shoulders, and arms, they also 1 overwork the hands during instrumentation. Even the greatest hand skills are compromised when the clinician has to compensate for incorrect patient-operator positioning. (Review "patient-operator positioning" in the Nield-Gehrig textbook, "Periodontal Instrumentation and Advanced Root Instrumentation," and self-assess your positioning to make corrections before adding new techniques to your instrumentation methods.) -----------------------------------------------------------------------------See related articles A periodontist's protocols to avoid dental implant complications: Part 2 -- establishing an implant maintenance protocol Creating the Ultimate Patient Experience Through Technology Treating implant gingivitis -----------------------------------------------------------------------------Many dental hygienists have taken great measures to prevent injury. They purchase loupes to improve positioning, new operator stools, perfectly fitted gloves, new instruments, and they even increase their use of 1 ultrasonic instrumentation. Yet, they still experience pain and may be diagnosed with CTDs such as carpal tunnel 2,3,4 syndrome, ulnar nerve entrapment, de Quervain's disease, tenosynovitis, tendinitis, and others. Here is a link to a slideshow of CTDs and physical injuries in dental health-care providers: http://www.slideshare.net/jhpdc/ergonomics-for-dental-hygienists-1178564. The loupes help with positioning, but they can reduce your tactile sense in subgingival instrumentation.5,6 Tactile sensitivity makes you more astute at determining the need for additional instrumentation strokes. Clinicians who lack fine tactile sense end up making excessive strokes, increasing the workload on the hands. During subgingival instrumentation, clinicians need to focus less on seeing and more on feeling the roots. In their mind's 26 eye, they need to visualize the anatomy of the roots of the teeth as they perform the debridement. Ultrasonic instrumentation helps remove tenacious calculus and biofilm, but it presents its own set of problems: hand vibration injuries, hearing impairment, aerosol production, and the fact that it cannot and should not be used on every patient.7,8,9,10 Evidence shows that the best treatment outcome for periodontal patients comes from a combination of hand and ultrasonic instrumentation.11,12 It is also easier on the hands of the clinician to 13, 25,26 alternate their instrumentation methods by using a combination of hand and ultrasonic techniques. Predisposing factors to hand injuries The first place to look when assessing hand or instrumentation problems is at the hands themselves. Physical conditions can be a predisposing factor to hand pain and injury, especially when the hands are placed under the demands of performing periodontal instrumentation. Joint hypermobility -- The most overlooked predisposing condition is benign joint hypermobility syndrome (BJHS), which may occur in one or more joints of the hands and is a predisposing factor to carpal tunnel syndrome.14,15,16 When there is hypermobility of the joints, the muscles of the hand and arm will be overworked to compensate for the laxity of the joints. With BJHS, it is difficult to apply lateral pressure during the 16 working stroke and then to control the stroke and relax the hand between working strokes. Fig. 1 Benign hypermobility joint syndrome Clinicians who do not self-assess their hands might not even know they have BJHS as it is not a pronounced disfigurement. It can be seen when you press the fingertips against a tabletop and notice the fingers are not straight, as under pressure, one or more fingers are bent at the knuckles. BJHS may be the reason why they struggled when learning the techniques and have pain during and after performing periodontal instrumentation (see Figure 1). A physician should evaluate a dental clinician who notices joint weakness or hypermobility. The physician may refer the hygienist to an orthopedic hand specialist. Physical or occupational therapy may be prescribed, as well 16 as joint stabilizing devices that can be worn under surgical gloves. 2 Osteoarthritis or rheumatoid arthritis -- Various types of arthritis can occur, and the condition may require the hygienist to reduce work hours or leave dental hygiene altogether. An orthopedic hand specialist and/or a 17,18,19 rheumatologist can diagnose the type and severity of arthritis and will recommend treatment and exercises. Instrumentation techniques that reduce workload on the hands can make a difference in whether the dental hygienist with arthritis can continue in clinical practice. Hand weakness -- Hand weakness is determined most accurately through testing by an orthopedic hand specialist who use devices such as dynamometers to test hand strength.20 Hand weakness is more common to female clinicians with small hands, but not to male clinicians with small hands.21 Although anyone could have weak hands, women with small hands are more likely to have weak hands that may be contributing to problems 3 they have with pain associated with periodontal instrumentation. Hand strengthening is beneficial for all clinicians 22, 23 but mandatory for those with weak hands. Fingernail length -- Research shows that long fingernails reduce pinch force and hand strength in performing psychomotor tasks. "Long fingernails limit flexion of the finger joints, particularly the metacarpophalangeal joints. Lack of finger flexion will limit excursion of long flexors and extensors in patients. It is recommended that patients 24 cut their fingernails to a length of 0.5 cm to achieve optimal functional outcomes." Leverage is key Leverage is the key to lightening the workload on the hands, and leverage is dependent on proper equipment and specific instrumentation techniques. Using leverage instead of brute strength and force will prevent 25 overworking the hands. Leverage is maximized with appropriate techniques. Proper armamentarium includes an array of periodontal instruments that have large handles (size 10). The instruments must be very sharp to be efficient in latching on to deposits, and this in itself prevents overworking the hands. The hygienist must have a supportive operator stool and a patient chair that goes low enough to allow the clinician to work with the forearms parallel to the floor. A clinician who has to reach up to work on the patient will lose leverage and the ability to control fine hand function in instrumentation.1 Loss of leverage causes: 25,26 Unnecessary tightening of the hands (excessive pinch force) Excessive lateral pressure Pulling instead of lifting deposits off the teeth Unnecessary number of strokes for deposit removal Fatigue, pain, injury Avoid using individual fingers or the thumb to perform instrumentation strokes. Instead use your whole hand as a 1 unit and your arm as a continuum. While reviewing a proper grasp may seem too fundamental to the advanced clinician, it is most often the first aspect of instrumentation to be found in error during an observation of 26 instrumentation of experienced clinicians. 27,28 Thumb pulling during the working stroke results in injury due to overuse of the thumb. It also causes other 25,26 instrumentation errors such as lack of toe third adaptation of the working end of the instrument to the tooth. At Boot Camps for Dental Hygiene Educators,26 a simulation lab is used for teaching instrumentation to students who are from area dental hygiene programs. Educators who are attendees in the "boot camp" take part in educator evaluation activities to calibrate on evaluating student clinical performance of periodontal instrumentation. During the evaluation activities, attendees observed students who were activating the instrumentation working stroke by using a thumb pulling motion. Students using a thumb pulling activation tended 4 to lift the toe third off the tooth, which resulted in their using some of the middle third of the blade during the working stroke. Not using the toe third of the blade was less effective and required the students to apply 25, 26 additional lateral pressure to remove the synthetic calculus deposits from the teeth of typodonts. To remediate "thumb pulling activation," students were reminded to use the whole hand as a unit without independent digital effort. They were given a technique reminder to perform a "roll-check" to make sure the toe third was locked onto the tooth just prior to initiating the working stroke. Roll-check means that, after the exploratory stroke and placement of the instrument blade under the deposit, roll the instrument handle/toe toward 26 the tooth to make sure the toe third is on the tooth before you activate the working stroke. Many experienced clinicians who attended other instrumentation Boot Camps for Dental Hygiene Clinicians (noneducators) were not completely on the toe third of the blade during their working strokes. They too, overworked their hands by compensating with additional lateral pressure. So many clinician attendees were looking for sophisticated techniques to overcome their struggles. The answer was something as simple as not 25,26 really using the toe third of the blade during the working stroke! Protect your wrist The old expression "wrist rock" is one that has left our current teaching vocabulary. Bending and flexing the wrist for each and every stroke often causes injury. The wrist must remain in a neutral position as the hand and arm 1,26,28,29,30 move as a continuum during instrumentation. During instrumentation, the whole hand and arm move 1 just as they do when one is turning a door knob. While extraoral fulcrums are most effective for access to maxillary third molars, they are least effective for leverage and reduced workload on the hand everywhere else in the mouth. This has been clearly demonstrated in a study conducted at the School of Dentistry, University of California at San Francisco by Dr. Hui Dong et al. In the study, muscle activity and thumb pinch force were measured by electromyography and pressure sensor to compare extraoral fulcrums, intraoral single finger rest, and intraoral two-finger rests. Both intraoral fulcrums (finger rests) significantly reduce muscle activity and thumb pinch in comparison to the extraoral fulcrum that had 31 no finger rests. To lift deposits from a root surface, the fulcrum (finger rest) placement should be in a more apical position to help with the leverage of lifting the deposit off the tooth. A more coronal fulcrum (finger rest) placement tends to make clinicians pull to remove deposits, while a more apical fulcrum placement facilitates leverage for lifting deposits off the teeth as opposed to trying to pull deposits off the teeth. Whenever possible, assist with the index finger or thumb of the nondominant hand by pressing against the shank of the instrument to control the instrument tip. A lifting working stroke will place the least effort and workload on 25,26,32 the hands. Whenever the index finger or thumb of the nondominant hand can be used to stabilize the shank of the instrument, there is increased leverage and additional precision for control of the toe third on the tooth (see Figure 2). Having the most deft hand skills in periodontal instrumentation cannot spare the overworked dental hygienist. Too many patients per day and per week with not enough rest periods between patients will overwork the hands. Even the rest periods between working strokes are important. At the end of the working stroke, the hand should be relaxed, evidenced by a grasp with soft "C" of the thumb and index finger. The soft "C" grasp remains during the exploratory stroke and only changes in shape for the working stroke (after the "roll check" at the "lock on toe 1,26 third" step) (see Figure 3). When you are working on patients it is difficult to be modifying your instrumentation technique. Work on only one change per week. Otherwise you will slow yourself down so much you will get behind schedule and become overly stressed. Have someone videotape you often to evaluate your progress in reducing your workload to your hands during instrumentation. You can be your own best teacher. Team up with other hygienists for peer reviews. Be open to feedback so that you can work smarter and be free of hand pain and fatigue. There is no doubt you are a dedicated hygienist, or you would not be reading this article and open to new ideas that could help you last in our profession. Your patients must be pleased with your dedication and excellent care. The techniques described in this article are to help you prevent injury to yourself while being just as effective as you always have been at giving quality care to your patients. 5 Self-assessment of hand pain To determine what is causing your hand pain, start by assessing your hands and joints for arthritis and/or joint mobility. See your physician if you suspect you have physical hand problems. Also, have someone videotape you (even using a cell phone will do) while you are performing instrumentation. Look at the video and run through the following checklist as you assess your performance: Proper patient-operator positioning. "Pen grasp" with all fingers together so hand can function as a unit. Watch to see if you have a soft "C" grasp during the exploratory stroke and working "C" grasp only during the working stroke. Whole hand and arm as a unit during activation of working stroke. No thumb or index finger pulling. Fulcrum (finger rests) in more apical placement whenever possible to get the most leverage to lift rather than pull off subgingival deposits. Relaxed hand during exploratory stroke evidenced by soft "C" thumb and index finger. Cynthia Biron Leiseca, RDH, EMT, MA, is president of DH Methods of Education, Inc. She is the leader of Boot Camps for Dental Hygiene Educators (www.DHmethEd.com) 6 Earn 1 CE credit This course was written for dentists, dental hygienists, and assistants. Current Fluoride Modalities for Reduction of Dental Caries A Peer-Reviewed Publication Written by Heidi Emmerling Muñoz, RDH, PhD, FAADH and Ellen Standley, RDH, BS, MA Abstract The dental profession has long regarded fluoride as a primary element in the prevention of dental caries. Topical and systemic fluorides are regularly incorporated within the community, dental office, and home avenues. Despite the fact there are other preventive modalities, fluoride remains a well-established, evidencebased therapeutic intervention. This article will review the early history; mechanism of action; delivery methods for fluoride in private practice, home, and community; and the clinician’s role in optimizing best practices and safe use of fluoride. Educational Objectives At the completion of this article, the health care provider shall be able to: 1. Discuss the early studies in the United States on fluoride and its relationship to caries. 2. Explain the mechanisms of the preventive actions of fluoride. 3. Describe the basic delivery modalities of systemic and topical fluorides including pertinent information related to their use. 4. Discuss the clinician’s role in optimizing best practices and safe use of fluorides. Author Profiles Heidi Emmerling Muñoz, RDH, PhD, FAADH is a professor of English at Cosumnes River College. Prior to her current role, Dr. Muñoz served as interim director and professor of dental hygiene at Sacramento City College and was a CODA site consultant. Additionally, she is owner of Writing Cures (www.writingcures.com), a writing and editing service, co-author of The Purple Guide: Paper Persona, and creator of the Career Development Center for Friends of Hu-Friedy. She is a frequent contributor to RDH Magazine and has written articles and columns for a variety of publications. Dr. Muñoz can be reached at [email protected] Ellen Standley, RDH, BS, MA, is a recently retired professor of dental hygiene at Sacramento City College where she taught for over 30 years. She is a member of the American Dental Hygienists’ Association, the California Dental Hygiene Educators’ Association and the American Academy of Dental Hygiene. Ms. Standley is a past president of the California Dental Hygienists’ Association and currently serves on the Journal Advisory Board of the Journal of the California Dental Hygienists’ Association. She can be reached at [email protected] Author Disclosure Heidi Emmerling Muñoz and Ellen Standley have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course. Go Green, Go Online to take your course Publication date: Nov. 2012 Expiration date: Oct. 2015 Supplement to PennWell Publications PennWelldesignatesthisactivityfor1ContinuingEducationalCredit. DentalBoardofCalifornia:Provider4527,courseregistrationnumber01-4527-13004 “ThiscoursemeetstheDentalBoardofCalifornia’srequirementsfor1unitofcontinuingeducation.” ThePennWellCorporationisdesignatedasanApprovedPACEProgramProviderbythe AcademyofGeneralDentistry.Theformalcontinuingdentaleducationprogramsofthis programproviderareacceptedbytheAGDforFellowship,Mastershipandmembership maintenancecredit.Approvaldoesnotimplyacceptancebyastateorprovincialboardof dentistryorAGDendorsement.Thecurrenttermofapprovalextendsfrom(11/1/2011)to (10/31/2015) Provider ID# 320452. This educational activity was developed by PennWell’s Dental Group with no commercial support. This course was written for dentists, dental hygienists and assistants, from novice to skilled. Educational Methods: This course is a self-instructional journal and web activity. Provider Disclosure: PennWell does not have a leadership position or a commercial interest in any products or services discussed or shared in this educational activity nor with the commercial supporter. No manufacturer or third party has had any input into the development of course content. Requirements for Successful Completion: To obtain 1 CE credit for this educational activity you must pay the required fee, review the material, complete the course evaluation and obtain a score of at least 70%. CE Planner Disclosure: Heather Hodges, CE Coordinator, does not have a leadership or commercial interest with products or services discussed in this educational activity. Heather can be reached at [email protected]. Educational Disclaimer: Completing a single continuing education course does not provide enough information to result in the participant being an expert in the field related to the course topic. It is a combination of many educational courses and clinical experience that allows the participant to develop skills and expertise. Registration: The cost of this CE course is $20.00 for 1 CE credit. Cancellation/Refund Policy: Any participant who is not 100% satisfied with this course can request a full refund by contacting PennWell in writing. Educational Objectives: At the completion of this article, the health care provider shall be able to: 1. Discuss the early studies in the United States on fluoride and its relationship to caries. 2. Explain the mechanisms of the preventive actions of fluoride. 3. Describe the basic delivery modalities of systemic and topical fluorides including pertinent information related to their use. 4. Discuss the clinician’s role in optimizing best practices and safe use of fluorides. Abstract The dental profession has long regarded fluoride as a primary element in the prevention of dental caries. Topical and systemic fluorides are regularly incorporated within the community, dental office, and home avenues. Despite the fact there are other preventive modalities, fluoride remains a well-established, evidencebased therapeutic intervention. This article will review the early history; mechanism of action; delivery methods for fluoride in private practice, home, and community; and the clinician’s role in optimizing best practices and safe use of fluoride. Early History In 1901, Frederick McKay opened his first dental practice in Colorado where he discovered residents had severe brown stain and mottling that was resistant to decay. McKay researched this aspect of staining, despite the lack of concern by citizens and lack of interest by local dentists. Some early research, funded by a $21 grant posited that excess pork consumption, drinking milk from local cows or even exposure to radium was the cause of “Colorado Brown Stain.”1 G. V. Black, credited with being one of the nation’s most eminent dental researchers, came to Colorado to collaborate with McKay in investigating the prevalence of the Colorado Brown Stain.2 For six years, Black and McKay researched fluoride. First they showed that mottled enamel occurred during tooth development in childhood. Next, they found that the teeth that had the stain had almost no dental decay. Still not knowing the cause, some local residents suggested researching the water. McKay thought this had possibilities while Black was not convinced. In 1923, McKay went to Idaho because he learned that Idaho children also had brown stain, and discovered the stain did not occur until a new water line was installed from a local spring. McKay still did not know what was in this spring and he prudently advised they change water supplies. Several years later, there was no longer mottling or brown stain. Next, McKay traveled to Bauxite, Arkansas, an aluminum company town (ALCOA), because there had been reports of brown stain there too. H.V. Churchill, the chief chemist of ALCOA, hoping to disprove the fear of the dangers of aluminum, decided to conduct his own test of the water. Churchill discovered there were high levels of fluoride in the water and subsequently wrote a letter to McKay in 1931 reporting his findings. McKay collected water samples which confirmed Churchill’s assertion that fluoride, not aluminum, was causing the stain.1-3 64 | rdhmag.com In the 1930s, the United States Public Health Service (USPHS) started investigating fluoride and its relationship to tooth mottling. The head of the Dental Hygiene Unit of the National Institute of Health (NIH) was H. Trendley Dean, who researched the level of fluoride and the degree of fluorosis. Dean devised the fluorosis index and was credited with finding that 1 part per million (ppm) of fluoride in the drinking water was considered the ideal level for prevention of decay along with minimal fluorosis.3 The Health and Human Services (HHS) and the Environmental Protection Agency (EPA) have lowered this level to 0.7 ppm in recent years to further minimize the risk of fluorosis.4-5 In 1945 the USPHS decided to implement a research project by adding fluoride to the water supply in Grand Rapids, Michigan.2-3 Results were compared to a control city with no added fluoride. Thus, Grand Rapids was the first city to have water fluoridation and served as an early model for epidemiological fluoride research.2 After 11 years, the outcomes revealed that the children of Grand Rapids born after the water fluoridation had a 60% reduction in caries.2 This finding was groundbreaking because it proved that dental decay could be prevented. Thanks to the pioneers McKay, Black, Churchill, and Dean, with addition of fluoride to the water and subsequently to dentifrices, rinses, and other dental products, consumers experience significantly less decay.2-3,5-6 Basics of Fluoride Delivery and Application Fluoride is available in many different products and modalities of delivery. Three basic categories of delivery are currently used: 3,6-9 • Systemic: ingested with water supplies or dietary supplementation or food • Topical: home/self application of dentifrices, rinses, or gels. Available in prescriptive higher concentrations or over-thecounter lower concentrations. • Topical: professional application of higher concentration products in the dental office Fluoride delivery methods are either systemic (ingestion) or topical (surfaces of the teeth). It should be noted that systemic fluoride also provides some topical benefits when the ingested fluoride is circulated in the blood and emerges in very low levels in saliva.3,6-9 In early days it was thought that fluorides benefitted mainly children; it is now acknowledged that benefits extend to adults as well as children.3,5-7, 11 The frequent low level concentration of topical delivery is the main source of the decay reduction benefits.3,6,10 Mechanism of Action Fluoride reduces decay via three modes: 1. Incorporation of fluoride into the enamel during tooth development. This results in the formation of fluorhydroxyapatite, which is more resistant to the acid attacks of the decay process and is a systemic benefit.3,7-9 2. Remineralization/demineralization mechanism. A major topical benefit. Fluoride enhances remineralization by combining with the tooth and making the coronal enamel RDH | February 2013 and root surfaces more resistant to decay. The more resistant remineralized enamel in turn serves as a deterrent to the acids, which act to remove minerals from the tooth surface (demineralization).3,6-10 3. Glycolosis inhibition in caries bacteria. Another topical benefit, fluoride interferes with the bacterial metabolism of carbohydrates and reduces acid production, which in turn reduces decay.3,6-10 Delivery Methods and Benefits Systemic Water Fluoridation: Early Grand Rapids studies showed a 60% reduction in decay with water fluoridation. This impressive reduction made for compelling reasons for water fluoridation throughout the country. In 2010, 73.9% of the U.S. population on community water systems received fluoridated water.12 Initially, fluoridated water was the only source of fluoride. Through the years dentifrice and other vehicles for fluoride delivery came on the market. The increased availability of fluoride from a variety of sources produces a dilution of the benefits from any single source. Therefore, decay reduction from water fluoridation alone shows a more modest caries reduction of 2040%.3 Additionally, the diffusion, or halo effect, contributes to this lower number by the fact that residents of nonfluoridated communities may consume food and beverages manufactured in fluoridated areas; individuals may also work in a fluoridated community but still reside in a nonfluoridated community, thereby benefitting from water fluoridation while at work.3,7 Despite other sources of prevention, fluoridation continues to be a safe and effective measure for caries reduction for all members of the community.3-7 In 1999 the Centers for Disease Control and Prevention named water fluoridation as one of the top ten public health measures of the 20th century.3,4,7 Supplements: Besides consuming water, another mechanism for systemic fluoride is through dietary supplements. Supplements are prescribed by physicians or dentists for children when the community does not have optimum water fluoridation. Sodium fluoride supplements are available in drops for infants or tablets/lozenges for young children.3,6-9,11 In 1994 the prescription age/dose schedule for fluoride tablets or drops was lowered to minimize the risk of fluorosis and to account for other available sources such as dentifrices, infant formulas, and foods made with fluoridated water (diffusion and halo effect).3,6Recommendations for supplements are more conservative than in the past. Fluoride supplements are particularly recommended for children who are at high risk for caries and who do not regularly brush with a fluoride dentifrice.3,11,13-14 The American Dental Association chairside guide for fluoride supplements states that children who have low caries risk should not receive supplements.15 Furthermore, the practitioner should carefully evaluate all sources of fluoride and conduct a risk assessment before prescribing supplements. The 2010 Dosage Schedule can be found on the American Dental Association website at http://ebd.ada.org/contentdocs/6327_ Fluoride_Chairside_Tool.pdf.15 When supplements are indicated, they should not be initiated until six months of age.7,9,14-16 FurtherRDH | February 2013 more, to maximize the effect, tablets or lozenges should be chewed or sucked on for at least one to two minutes before being swallowed.6,10,13,16 There has been much discussion on the efficacy of prenatal fluoride. While not contraindicated at this time, the American Academy of Pediatric Dentistry does not support the use of prenatal fluoride supplementsbecauseresearchhasindicatedthatthereisnomeasurable benefit compared to postnatal fluoride alone.6,14-15,17-20 Salt and Milk: Regions that do not have a fluoridated water supply, including places in South America, Latin America, the Caribbean, and Western Europe, add fluoride to their salt. It is a convenient alternative and is met with good public acceptance.3,19-20 Fluoride salt is not used in the United States or Canada, since water fluoridation is fairly common in these regions.19-21 Although not as popular as fluoride salt, the addition of fluoride to milk has been used in a few international communities. However, the evidence supporting fluoride milk to reduce tooth decay is weak.3,19- 20,22 Topical Topical fluorides are considered the main source of postnatal benefits and include both home and professional/office applications.3,6,10,14,19,23 Mechanisms of action and caries reduction benefits for topical fluorides have been the subject of much discussion and ongoing research.3,6-11,14 Recommendations for the use of topical fluorides for children and adults vary with respect to frequency of use (daily, monthly, or yearly), concentration (lower or higher), and level of risk or susceptibility to caries.6-11,13-14,16,20 Home Topical Applications Dentifrices: One major source of topical fluoride is dentifrice. While systemic water fluoridation has impressive benefits, many authorities attribute the use of fluoride dentifrice as the main vehicle for the global reduction in caries because of its ability to reach a wider population.20 Between 1960-1964, fluoride dentifrices were being marketed and recognized as viable therapeutic mechanisms for reduction of decay.7 Dentifrices can contain either sodium fluoride (NaF), stannous fluoride (SnF), or sodium monofluorophosphate (MFP).7-9 The amount of fluoride in the dentifrice is important. Cochrane reviewers found that a minimum of 1,000 ppm of fluoride should be present in dentifrices to be effective in preventing caries in children.24 For the optimal caries reduction benefit, over-the-counter (OTC) fluoride dentifrice levels need to be between 1000-1500 ppm.13,20 Twice-a-day brushing is recommended for over-the-counter fluoride dentifrices.6,11,13,16,19,23 To maximize the effect, individuals should avoid rinsing after brushing so that more fluoride is retained in the saliva.6, 20, 25 Recommendations for the use of daily over-the-counter fluoride dentifrices are targeted for quantity of dentifrice according to age and level of caries risk. Fluoridated dentifrices should be used only by adults and children over two; young children under two with low caries risk should use dentifrice without fluoride or a toothbrush moistened with water.6,11,13,16,19 The American Dental Association and the American Academy of Pediatric Dentistry recommend consulting with a dentist or physician before using a fluoride dentifrice rdhmag.com | 65 on children under age two.3,16 Following advice by a dentist, parents can place a minuscule amount of fluoride dentifrice on a brush for children under two.3,6,19,23 The amount has been described as: smear, film, rice, small pea, tiny touch.3,6-7,19,23 For all slightly older children (aged 3-6 years), the recommendation is to use fluoride dentifrice with a pea or small pea size.6-7,9,11,13-14,19,23 To minimize the risk of fluorosis, experts caution that children must not swallow fluoride dentifrice during or after brushing.6-7,9, 13-14,19,23 Additional safety precautions for the 3- to 6-year-olds include keeping the dentifrice container out of the child’s reach and having adult supervision or assistance while brushing.7,9 Prescription strength fluoride comes in dentifrice and gel formulas for home use. These products are used by the high caries risk patient 6 years of age and older.16,19 NaF and acidulated phosphate fluoride (APF) prescription strength gels contain levels up to 5,000 ppm.7,9,19 An OTC SnF gel that is sometimes recommended by dental professionals is 1,000 ppm.6,19 Mouth rinses: In addition to dentifrices and gels, topical fluorides are marketed in mouth rinses with NaF, SnF, or APF.6-7,8-9,26 Daily OTC sodium-based mouth rinses are 0.02% (NaF), .044% (APF) or, 0.05% (NaF), which is the most common.6-8,19,26 Another daily rinse is .63% SnF, which is indicated for dentinal sensitivity and gingival inflammation as well as caries.8-9,7,9,19 Available by prescription only for weekly use is 0.2% NaF, which is used for highrisk individuals or for public health school rinse programs.7,9,13,20,26 Most sources recognize mouth rinses are not appropriate for children under age 6 or any individual who has difficulty expectorating.6-9,13,19,26 For optimum efficacy, mouthwashes should be used for the prescribed amount of time indicated on the bottle, which is usually one minute.7-9,13 See Table 1 for summary of home-use topical fluoride. Table 1. Home-use topical fluoride 8,13,19,26 PPM Chemical Concentration Formula Dentifrice 1,000- NaF .22%(1,000 1,500 SnF ppm) Na MFP .4% 1,500- NaF 5,000 SnF NaF MFP Gel Up to NaF 5,000 APF 1,000 SnF Mouth rinse 230 NaF 0.05% (most common) NaF 0.02% NaF 0.04% Plus APF 920 NaF 0.2% 200 NaF 0.044% SnF 0.63% 66 | rdhmag.com Prescription OTC Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Professional Topical Application: While some dentifrices, gels, and rinses are patient applied in the home environment, other higher concentration types of fluoride are used in the community and private practice and are applied by dental professionals. The Centers for Disease Control and Prevention, the American Dental Association, and the Academy of Nutrition and Dietetics recognize that professional applications are more beneficial for moderate to high caries risk individuals.6,14,27-28 Furthermore, the ADA’s Council on Scientific Affairs acknowledges that children and adults who are classified as low risk for caries may not receive additional benefits from professional topical fluoride application.27-28 Clinicians are advised to analyze each case and patient preferences before making fluoride recommendations for professional applications.27-28 Varnishes: One of the relatively newer modes of delivery is fluoride varnish. Varnishes are approved by the FDA for desensitization and as a cavity liner.6,19,29 Although the use for caries prevention is off label, it is considered acceptable practice.6,19,20 For example, practitioners in some European countries and Canada have been using varnishes for caries prevention since the 1970s and 1980s.6,19,29-32 Varnishes typically contain NaF 5% with 22,600 ppm or difluorsilane with 1,000 ppm.6,9,13,19 Ease of application, taste acceptance, ability to set on contact with moisture, and the small amount required for use gives varnishes some practical clinical advantages.6,29-30,32 It should be noted that adults and children with a low risk of caries are not likely to receive additional benefits from varnish applications. However, patients with moderate to high risk, such as those with a recent history of incipient or early caries and or other considerations such as xerostomia or dietary factors, can benefit from varnish applilcations.27-28 Varnishes are the best and safest recommendation for professional strength topical fluoride to use for children under age six.9,19 The reason varnishes are safer for younger children is because the quantity of fluoride varnish used is significantly less than other professional strength applications such as gels and rinses, and there is minimal risk of ingestion since varnishes adhere to the teeth.6,19,32 The varnish’s adherence to the teeth is an added advantage to fluoride application because it maximizes and extends delivery.20,30 Varnishes are normally applied two to four times per year, depending upon level of caries risk.14,19,27-28,31-32 They are used for both adults and children in private offices and are commonly incorporated in public health programs for caries in children.20,30-32 Gels and Foams: Professional fluoride applications are primarily indicated for those with a caries risk who do not have access to fluoridated water or do not regularly use a fluoride dentifrice.13,19,23,27-28 Common in-office modalities are gels, which are 1.23% APF (the most widely used) and 2% NaF formulations. The APF gel has a concentration of 12,30012,500 ppm. With a pH of 3.5, APF gels are generally contraindicated for composite or porcelain restorations because APF dissolves some of the filler particles in those materials.6,8 NaF gel has a concentration of 9,000 ppm and, because of its neutral pH, poses no risk to restorations.6,8 However, some RDH | February 2013 Table 2. Professional use topical fluoride 6,7, 13,27 Type PPM Chemical Formulation Concentration Advantages Disadvantages Varnish 22,600 1,000 NaF difluorsilane 5% Decreased risk of ingestion Prolonged delivery Not yet approved for caries prevention Gels 12,30012,500 APF 1.23% Greatest fluoride uptake Pleasant taste Stable shelf life Contraindicated for composite and porcelain restorations 9000 NaF 2% Neutral pH 20,00025,000 SnF 8% Solution of the newer materials are not as sensitive to the effects of the APF.19 Evidence reveals that the NaF and AFP gels are similar in efficacy.27 The foam forms of APF and NaF require a much smaller amount to fill the trays and thereby reduce the risk of ingestion.6,8-9 The recommended time for professional fluoride tray applications is four minutes to obtain maximum benefits.6-9,13,19,27 Patients should be advised not to rinse, eat, or drink for 30 minutes following high concentration fluoride applications to maximize effectiveness.6-9,13,19 Another application that is rarely used, with its high concentration of 20,000-25,000 ppm, is an 8% SnF solution. Since it is SnF, it has a less pleasant taste, and there is a potential problem for staining as well as gingival sloughing. Instability is another disadvantage of this formulation.8 At one time, a two-part fluoride rinse consisting of 0.31% APF and 1.64% SnF was popular. At 1,500-3,000, the ppm was lower than gels and foams. However, the lack of clinical evidence no longer warrants its use.9 See Table 2 for a summary of professional topical solutions. The Clinician’s Role in Optimizing Safe Use of Fluorides Dental hygienists should keep informed on the current use of fluoride modalities, best practices, and safe strategies. Additionally, it is important to inform the patients how to maximize efficacy and minimize potential risks. Caries Risk Assessment: Caries risk assessment is evidence-based and considered the current best practice when clinicians implement and recommend caries prevention strategies.27-28 Each patient is evaluated with consideration for clinical exam, history of recent caries, nutritional and oral hygiene practices, and many other factors. Several risk assessment forms and articles are available and can be downloaded.14-16,27-28 Acute and Chronic Toxicity: Acute fluoride toxicity results from short-term ingestion of higher concentrations of fluoride. Symptoms can range from mild nausea to more severe GI symptoms and, in extremely rare cases, death.7,9 The severity of symptoms is affected by the amount of fluoride ingested, the patient’s weight, and the patient’s health status.7,9 Mild nausea RDH | February 2013 Staining Unpleasant taste Gingival sloughing is the most common form of fluoride toxicity and is experienced when patients swallow too much topical fluoride. The fluoride reacts with the stomach acid, forming hydrogen fluoride and produces symptoms of nausea, cramps, and vomiting, usually within 30 minutes of swallowing.7,9,19 Chronic fluoride toxicity results from long-term ingestion of lower concentrations of fluoride, and is most commonly experienced as dental fluorosis.3,6-7,9 Fluorosis ranges from mild chalky white spots to severe brown mottling.3,6 Fluorosis develops when low doses of fluoride are regularly and inadvertently ingested during enamel development.3,6 Although fluorosis is seen when the fluoride concentration in the water is greater than optimal, some cases of fluorosis result from long-term swallowing of OTC dentifrices, and/or the use of supplements and fluoridated infant formula in addition to consuming water that is already optimally fluoridated.3,6,9,13,17,23 Safe Tray Applications: To avoid the potential of acute toxicity symptoms, common sense dictates that clinicians follow best practices when administering tray applications of fluoride. These include:7,9,19 1. Load a minimal amount of product for tray applications. To minimize the risk of ingestion, load 2 ml per tray for small children and no more than 5 ml per tray for adults. Have the patient sit upright. 2. Apply adequate suctioning throughout the procedure. 3. Directly supervise the patient, especially young children, during the administration of fluoride. Children are at a greater risk of fluoride toxicity since they do not weigh as much as adults and also tend to swallow topical fluoride. Thus, careful supervision of child during fluoride treatments is essential. 4. Instruct the patient to expectorate and not to swallow the product. 5. Advise the patients who have a tendency to swallow product that they can ingest milk or an antacid product (calcium carbonate) to bind with the fluoride, reducing the risk of nausea. Reminders for Patients: It is important that clinicians advise patients of safety precautions for home use of fluoride products. Safety messages to convey include: 7,9,13,14,19,33 rdhmag.com | 67 1. All fluoride products, including OTC dentifrices and rinses, must be stored out of the reach of small children. Further precautions dictate that prescription fluoride should be stored in a secure area. 2. Remind patients that the proper amount of dentifrice for a small child to use is the size of a small pea. 3. No mouth rinses should be used by children under the age of six or by individuals who have limited ability to expectorate. 4. Mouthrinses and dentifrices should not be swallowed. Therefore, parental supervision of young children is essential. 5. Before supplements and/or fluoridated infant formula are used, check the fluoride content of the local water supply. If the fluoride concentration is at the optimal level, supplements and fluoride-enriched formula should not be used. 6. When daily supplements are taken, parents should be advised that if a dose is missed, simply resume the next day with the normal dose. Do not double dose. 7. Remind parents that if fluoride is ingested and nausea is experienced, they should take proper measures by having the child consume milk or an antacid product to bind with the fluoride. If accidental ingestion of a larger amount of fluoride product occurs, the National Hotline for Poison Emergencies is available for 24 hour advice. (1-800-222-1222) 33 Conclusion Because practitioners and consumers have enjoyed the benefits of fluoride for a number of years, fluoride is often taken for granted as one of the most effective tools for caries prevention. Whether in the water supply or in topical agents such as daily dentifrice, fluoride continues to be relied on as a preventive measure. Despite its longstanding history and use, clinicians should have basic knowledge of the products and of the safe use of these products. Communication to the patient is an important adjunct to maximize the benefits and minimize the risks. Timely implementation of caries risk assessments and of appropriate strategies for each patient’s level of risk are essential components of evidence-based practice. From its early history to the present time, fluoride remains an effective, evidencebased modality for caries prevention throughout the lifespan. References 1. “Hall of Fame Inductees Frederick S. McKay” Pierre Fauchard Academy. Web. Accessed 9/2/12 http://www.fauchard.org/awards/ hall_of_fame/frederick_mckay.html. 2. “Story of Water Fluoridation” National Institute of Dental Research, National Institute of Health. Article. 3/11. Web. Accessed 5/23/12. http://www.nidcr.nih.gov/oralhealth/topics/fluoride/ thestoryoffluoridation.htm. 3. Fluoridation Facts. American Dental Association. 5th Ed. 2005. Web. Accessed 9/4/12. http://www.ada.org/sections/newsAndEvents/ pdfs/fluoridation_facts.pdf. 4. Nathe C. “Community Water Fluoridation.” RDH magazine. May 2011. 31(5) Print. 5. “HHS and EPA announce new scientific assessments and actions on fluoride” Health and Human Services. Press Release, January 7, 2011. Web. Accessed 7/3/12. http://www.hhs.gov/news/ press/2011pres/01/20110107a.html. 6. Centers for Disease Control and Prevention. “Recommendations for 68 | rdhmag.com using fluoride to prevent and control dental caries in the United States.” MMWR Recomm Rep. 2001;50(RR-14):1–42. PMID 11521913. Lay summary: CDC, Web. 2007-08-09. Web. Accessed 7/2/12. http:// www.cdc.gov/mmwr/preview/mmwrhtml/rr5014a1.htm. 7. Harris N, Garcia-Godoy F, Nathe C. Primary Preventive Dentistry. 7th Ed. Upper Saddle River: Pearson. 2008. 8. Darby M, Walsh M. Dental Hygiene Theory and Practice. 3rd Ed. St. Louis: Elsevier. 2010. 9. Wilkins E. Ed. Clinical Practice of the Dental Hygienist. 11th Ed. Philadelphia: Lippincott Williams & Wilkins. 2013. 10. Featherstone JD. “Prevention and reversal of dental caries: role of low level fluoride.” Community Dent. Oral Epidemiology, 1999: 27:31-40. Web. Accessed 7/3/12. http://www.ncbi.nlm.nih.gov/ pubmed/10086924. 11. “Using Fluoride to Prevent and Control Tooth Decay” Centers for Disease Control. Fact Sheet. 1/7/11. Web. Accessed 5/23/12. http:// www.cdc.gov/fluoridation/fact_sheets/fl_caries.htm. 12. “2010 Water Fluoridation Statistics.” Centers for Disease Control.7/27/12. Web. Accessed 8/23/12. http://www.cdc.gov/flu oridation/2010waterfluoridationstatistics.html. 13. “Other Fluoride Products.” Centers for Disease Control. Fact Sheet. 1/6/11. Web Accessed 8/23/12. http://www.cdc.gov/fluoridation/ other.htm. 14. Palmer CA, Gilbert JA. “Position of the Academy of Nutrition and Dietetics: The Impact of Fluoride on Health.” Journal of the Academy of Nutrition and Dietetics. 2012: 112: 1443-1453. Web. Accessed 9/3/12. http://www.eatright.org/About/Content.aspx?id=8364. 15. “Dietary Fluoride Supplements: Evidence-based Clinical Recommendations.” ADA chairside guide. Nd. Web. Accessed 7/3/12. http://ebd.ada.org/contentdocs/6327_Fluoride_Chairside_ Tool.pdf. 16. “Guidelines for Fluoride Therapy.” American Academy of Pediatric Dentistry. Oral Health Policy. Revised 2012. Web. Accessed 7/10/12. http://www.aapd.org/media/Policies_Guidelines/G_ FluorideTherapy.pdf. 17. “Findings Suggest Fluoride Supplements During Pregnancy Provide Little or No Benefit.” National Institute of Dental and Craniofacial Research. Science News in Brief. Web. 11/28/05. Accessed 5/23/12. http://www.nidcr.nih.gov/Research/ResearchResults/ScienceBriefs/ Archive/SIB2005/November/SIB11282005.htm. 18.Sa Roriz Fonteleles C, Zero DT, Moss ME, Fu J. “Fluoride Concentrations in Enamel and Dentin of Primary Teeth After Pre- and Postnatal Fluoride Exposure.” Caries Research. 2005 Nov-Dec; 39 (6): 505-508. Web. Accessed 5/23/12. http://www.ncbi.nlm.nih.gov/ pubmed/16251796. 19.Adair S. “Evidence-based Use of Fluoride in Contemporary Pediatric Dental Practice.” Pediatric Dentistry. 2006: 28: 133-142 Web. Accessed 7/10/12. http://health.mo.gov/blogs/wp-content/ uploads/2011/09/Adair-2006.pdf. 20. Burt B, Eklund S. Dentistry, Dental Practice, and the Community. St Louis: Elsevier. 2005. 21. Dais Joyce.“Fluoride Facts.”Dimensions of Dental Hygiene. July 2007: 2830. Web. Accessed 5/23/12. http://www.dimensionsofdentalhygiene. com/ddhnoright.aspx?id=1173&term=2007%20%20Dais%20%20 Fluoride. 22. Yeung A, Hitchings JL, Macfarlane TV, Threlfall A, Tickle M, Flenny A-M, “Fluoridated milk for preventing dental caries.” Cochrane Summaries. 10/8/2008. Web. Accessed 5/23/12. http://summaries. cochrane.org/CD003876/fluoridated-milk-for-preventing-dentalcaries. 23. “Use of Fluorides in Caries Prevention.” Canadian Dental Association. Position Paper. March 2012. Web. Accessed 8/2/12. http://www. fluoridation.com/CalgaryFluoride/CDA_Fluoride-Guidelines.pdf. 24. Walsh T, Worthington HV, Glenny A-M, Appelbe P, Marinho VCC, Shi X. “Comparison between different concentrations of fluoride toothpaste for preventing tooth decay in children and adolescents.” Cochrane Summaries. 2/17/2010. Web. Accessed 9/11/12. http:// summaries.cochrane.org/CD007868/comparison-between-differentconcentrations-of-fluoride-toothpaste-for-preventing-tooth-decay-inchildren-and-adolescents. 25.Duckworth RM, Maguire A, Omid N, Steen IN, McCracken GI, RDH | February 2013 INSTRUCTIONS Read through the article and answer the multiple choice questions provided at the back of the article. Please note that some questions may have more than one answer; in the case of the latter please “tick” every correct answer. When done only fax through your answer sheet to the fax number given on the answer sheet. QUESTIONNAIRE B2 (14) CYTOTOXICITY OF QMix™ ENDODONTIC IRRIGATING SOLUTION ON HUMAN BONE MARROW MESENCHYMAL STEM CELLS Question 1: Which of the following are characteristics of an ideal root irrigant solution? A It must be nontoxic B It must have a broad antimicrobial spectrum C It must have the ability to dissolve pulp tissue D It must be able to inactivate endotoxins E It must prevent the formation of a smear layer or dissolve it Question 2: Is it TRUE or FALSE that there is currently a new irrigant solution that can achieve all the ideal goals? A TRUE B FALSE Question 3: The use of which of the following irrigant solutions has been advocated by many researchers to produce optimal root canal irrigation results? A EDTA and NaOCI combined B EDTA on its own C CHX and NaOCI combined D EDTA, CHX and NaOCI combined E NaOCI on its own Question 4: QMix™ is a 2-in-1 solution containing which of the following agents? A 15% EDTA and 3% CHX B 5% NaOCI and 17% EDTA C 2% CHX and 17% EDTA D 17% CHX and 2% EDTA Question 5: Which of the following statements are TRUE with regard to the observations of the in vitro study? A Both the QMix™ and NaOCI solutions are toxic to human bone marrow MSC’s and cause cellular damage B The NaOCI solution, but not the QMix™ solution, is toxic to human bone marrow MSC’s and cause cellular damage C Both the QMix™ and NaOCI solutions are toxic to human bone marrow MSC’s but do not cause cellular damage D The QMix™ solution, but not the NaOCI solution, is toxic to human bone marrow MSC’s and cause cellular damage Question 6: Is it TRUE or FALSE that Qmix™ was found to be nontoxic and cannot induce an inflammatory response? A TRUE B FALSE Question 7: Which of the following statements are TRUE with regard to cell viability after exposure to irrigant agents? A The toxic effect of an agent gradually increases with time B Cell viability increased significantly when the cells were exposed to NaOCI for all time periods examined C Cell viability decreased significantly after being exposed to the QMix™ solution for 2 or 4 hours D CHX is a toxic agent that binds to the cell’s plasma membrane and increases its permeability 1 Question 8: Which one of the following types of cell death does not induce an inflammatory response, and is characterized by cell shrinkage and the nuclear chromatin condensation followed by nuclear fragmentation? A Autophagy B Apoptosis C Necrosis D Mitotic catastrophe Question 9: Which of the following markers are necessary to substantiate this mode of cell death speculated with QMix™? A Regulators B Cleaved substrates C Inhibitors D Detection of caspases E All the above Question 10: Is it TRUE that in vitro cytotoxicity investigations reported that CHX had a higher toxicity in cell cultures than NaOCI, but that in vivo studies suggest that CHX or QMix™ is less aggressive than NaOCI? A YES B NO COMPARISON OF DIGITAL AND CONVENTIONAL IMPRESSION TECHNIQUES: EVALUATION OF PATIENTS’ PERCEPTION, TREATMENT COMFORT, EFFECTIVENESS AND CLINICAL OUTCOMES Question 11: Which of the following are advantages of digital impressions and scanning systems? A 3D pre-visualization of tooth preparations B Potential cost- and time-effectiveness C Reducing the distortion of impression materials D Improving patient acceptance E All of the above Question 12: With regard to the outcome of this study, which of the following are TRUE? A The conventional impression technique was more efficient than the digital impression technique B Trial subjects preferred the conventional impression technique C The digital impression technique was more efficient than the conventional impression technique D Trial subjects preferred the digital impression technique Question 13: Which one of the following was the major advantage of digital impressions? A Improving the level of patients’ comfort and treatment acceptance B Reducing the chair time C Reducing repeat visits and treatment D Increasing treatment effectiveness Question 14: Which one of the following is the more patientfriendly technique? A The digital impression technique B The conventional impression technique Question 15: The accuracy of the conventional impression technique depends on which of the following? A The materials themselves B Impression tray types C Impression techniques D All of the above 2 HOW NOT TO OVERWORK YOUR HANDS Question 16: Can incorrect patient-operator positioning while performing periodontal instrumentation lead to cumulative trauma disorder? A YES B NO Question 17: Which of the following statements regarding measures to prevent injury are TRUE? A The loupes help with positioning and they increase your tactile sense in subgingival instrumentation strokes B During subgingival instrumentation, clinicians need to focus less on seeing and more on feeling the roots C Ultrasonic instrumentation helps to remove tenacious calculus and biofilm D Ultrasonic instrumentation should and can be used on every patient E According to evidence the best treatment outcome for periodontal patients comes from a combination of handand ultrasonic instrumentation Question 18: Which of the following is FALSE with regard to BJHS? A It is the most overlooked predisposing condition B It may occur in one or more joints of the hands and is a predisposing factor to carpal tunnel syndrome C With BJHS, it is difficult to apply lateral pressure during the working stroke and then to control the stroke and relax the hand between working strokes D Clinicians always know when they have BJHS, even if they do not self-assess their hands E BJHS may be the reason why clinicians have pain during and after performing periodontal instrumentation Question 19: Who is most likely to have hand weakness? A Female clinicians with small hands B Male clinicians with small hands C Female clinicians with large hands D Male clinicians with large hands Question 20: Is it TRUE or FALSE that leverage is key to lightening the workload on the hands? A TRUE B FALSE Question 21: Since leverage is dependent on proper equipment and specific instrumentation techniques, which of the following are TRUE? A The instruments do not have to be sharp to be efficient in latching on to deposits B The patient’s chair must go low enough to allow the clinician to work with the forearms parallel to the floor C Using individual fingers or the thumb to perform instrumentation should be avoided D Thumb pulling during the working stroke does not result in injury due to overuse of the thumb E Thumb pulling causes errors, such as lack of toe third adaptation of the working end of the instrument to the tooth Question 22: To lift deposits from a root surface, the fulcrum placement should be in which position? A The apical position B The coronal position Question 23: Which of the following are CTDs that can be caused by overworking the hands? A B C D E Carpal tunnel syndrome Ulnar nerve entrapment Tendinitis Quervain’s disease Tenosynovitis 3 CURRENT FLUORIDE MODALITIES FOR REDUCTION OF DENTAL CARIES Question 24: Who were the pioneers that collaborated to investigate the prevalence of the Colorado Brown Stain? A Churchill B Dean C McKay D Black Question 25: The Health and Human Services and the Environmental Protection Agency have lowered the fluoride level to which of the following to further minimize the risk of fluorosis? A 0.6 ppm B 0.7 ppm C 0.8 ppm D 0.9 ppm E 1 ppm Question 26: Is it TRUE or FALSE that fluoride benefits both children and adults ? A TRUE B FALSE Question 29: Fluoride supplements are recommended for whom of the following? A Children who have low caries risk B Children who do not regularly brush with a fluoride dentifrice C Children under the age of 6 months D Children who are at high risk from caries Question 30: Which one of the following is attributed by many authorities as the main vehicle for the global reduction in caries because of its ability to reach a wider population? A The use of fluoride dentifrice B Systemic water fluoridation C Varnishes D Mouth rinses Question 31: For optimal caries reduction benefit, what must the over-the-counter fluoride dentifrice levels be? A Below 500 ppm B Between 500 – 800 ppm C Between 800 – 1000 ppm D Never more than 1000 ppm E Between 1000 – 1500 ppm Question 27: Which of the following are systemic methods of fluoride delivery? A Supplements B Water fluoridation C Salt and milk D All of the above Question 28: Early Grand Rapids studies showed what percentage reduction in decay with water fluoridation? A 20% B 40% C 60% D 80% Question 32: Are mouth rinses appropriate for children under age 6? A YES B NO Question 33: Which of the following are FALSE with regard to professional topical application of fluoride? A The CDCP, ADA and AND recognize that professional application of fluoride is less beneficial for moderate to high caries risk individuals B Clinicians should analyze each case, and patient preferences before making fluoride recommendations for professional application C There is no additional benefit for any group of people from professional topical fluoride application D The ADA and Council of Scientific Affairs acknowledge that children and adults who are classified as low risk for caries may not receive additional benefits from professional topical fluoride application 4 Question 34: Which of the following give varnishes some practical clinical advantages? A Taste acceptance B Ease of application C The small amount required D Ability to set on contact with moisture Question 35: Who can benefit from varnish applications? A Adults with low risk of caries B Patients with moderate to high risk of caries C Children with low risk of caries D Patients with xerostomia or other dietary factors E Patients with a recent history of incipient or early caries Question 38: Is it suitable for individuals who have limited ability to expectorate to use mouth rinses? A YES B NO Question 39: Is it TRUE or FALSE that before supplements and/or fluoridated infant formula are used, the fluoride content of the local water supply must be checked and if it is at its optimal level, it should not be used? A TRUE B FALSE Question 40: Which one of the following is TRUE? Question 36: Is it TRUE or FALSE that varnishes are the best and safest recommendation for professional strength topical fluoride to use for children under age six? A TRUE B FALSE A Fluoride is an effective, evidence-based modality for caries prevention for children but not adults B Fluoride remains an effective, evidence-based modality for caries prevention throughout the lifespan Question 37: In which of the following cases can dental fluorosis develop? A From a once-off ingestion of a low dose of fluoride B From a once-off ingestion of high concentrations of fluoride C When low doses of fluoride are regularly and inadvertently ingested during enamel development D From long-term swallowing of OTC dentifrices E When the fluoride concentration in the water is greater than optimal 5 PO Box 71 Wierda Park 0149 Tel: 012 653 2394 Fax: 086 614 4200 Cell: 082 566 6910 Website: www.safocus.co.za ANSWER FORM Postal address Professional Board HPCSA No Surname Initials ID Number FOH Number Time spent on activity E-mail Address Fax Number Contact Number Is this for an audit ______Hour ______Min How would you like to receive your IAR? SMS YES FAX NO EMAIL POST B2 (14) CYTOTOXICITY OF QMix™ ENDODONTIC IRRIGATING SOLUTION ON HUMAN BONE MARROW MESENCHYMAL STEM CELLS;COMPARISON OF DIGITAL AND CONVENTIONAL IMPRESSION TECHNIQUES: EVAUATION OF PATIENTS’ PERCEPTION, TREATMENT COMFORT, EFFECTIVENESS AND CLINICAL OUTCOMES;HOW NOT TO OVERWORK YOUR HANDS;CURRENT FLUORIDE MODALITIES FOR REDUCTION OF DENTAL CARIES A B C D E 1 2 3 4 5 6 7 8 9 10 11 12 13 14 A B C D E A 15 16 17 18 19 20 21 22 23 24 25 26 27 B C D E 28 29 30 31 32 33 34 35 36 37 38 39 40 I hereby declare that the completion of this document is my own effort without any assistance. Signed:_________________________________ Date:_______________________ Please rate the article: POOR 1 FAIR 2 AVERAGE 3 GOOD 4 EXCELLENT 5 FAX TO 0866144200 OR 012 653 2073 AFTER COMPLETION This article is accredited for FOUR Clinical (4 CEU’s) Mark MODERATED BY: /40 PERCENTAGE % (PASS RATE 70%) PASSED FAILED DATE: 6
© Copyright 2026