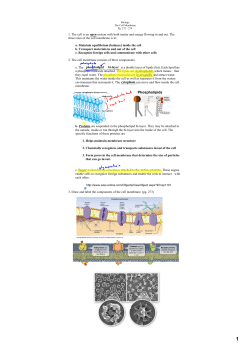
Biological Membranes and Transport
chapter 12 Biological Membranes and Transport The first cell probably came into being when a membrane formed, enclosing a small volume of aqueous solution and separating it from the rest of the universe. Membranes define the external boundaries of cells and regulate the molecular traffic across that boundary; in eukaryotic cells, they divide the internal space into discrete compartments to segregate processes and components (Fig. 12–1). They organize complex reaction sequences and are central to both biological energy conservation and cell-to-cell communication. The biological activities of membranes flow from their remarkable physical properties. Membranes are flexible, self-sealing, and selectively permeable to polar solutes. Their flexibility permits the shape changes that accompany cell growth and movement (such as amoeboid movement). With their ability to break and reseal, two membranes can fuse, as in exocytosis, or a single membrane-enclosed compartment can undergo fission to yield two sealed compartments, as in endocytosis or cell division, without creating gross leaks through cellular surfaces. Because membranes are selectively permeable, they retain certain compounds and ions within cells and within specific cellular compartments, while excluding others. Membranes are not merely passive barriers. They include an array of proteins specialized for promoting or catalyzing various cellular processes. At the cell surface, transporters move specific organic solutes and inorganic ions across the membrane; receptors sense extracellular signals and trigger molecular changes in the cell; adhesion molecules hold neighboring cells together. Within the cell, membranes organize cellular processes such as the synthesis of lipids and certain proteins, and the energy transductions in mitochondria and chloroplasts. Membranes consist of just two layers of molecules and are therefore very thin; they are essentially two-dimensional. Because intermolecular collisions are far more probable in this twodimensional space than in three-dimensional space, the efficiency of enzymecatalyzed processes organized within membranes is vastly increased. In this chapter we first describe the composition of cellular membranes and their chemical architecture—the dynamic molecular structures that underlie their biological functions. We describe several classes of membrane proteins defined by the ways in which they associate with the lipid bilayer. Cell adhesion, endocytosis, and the membrane fusion that occurs with viral infection will illustrate the dynamic role of membrane proteins. We then turn to the protein-mediated passage of solutes across membranes via transporters and ion channels. In later chapters we will discuss the role of membranes in signal transduction (Chapters 13 and 23), energy transduction (Chapter 19), lipid synthesis (Chapter 21), and protein synthesis (Chapter 27). (a) (b) (c) (d) (e) (f) figure 12–1 Biological membranes. Viewed in cross section, all intracellular membranes share a characteristic trilaminar appearance. The protozoan Paramecium contains a variety of specialized membrane-bounded organelles. When a thin section of a Paramecium is stained with osmium tetroxide to highlight membranes, each of the membranes appears as a three-layer structure, 5 to 8 nm (50 to 80 Å) thick. The trilaminar images consist of two electron-dense layers on the inner and outer surfaces separated by a less dense central region. Above are highmagnification views of the membranes of (a) a cell body (plasma and alveolar membranes tightly apposed), (b) a cilium, (c) a mitochondrion, (d) a digestive vacuole, (e) the endoplasmic reticulum, and (f) a secretory vesicle. 389 390 Part II Structure and Catalysis table 12–1 Major Components of Plasma Membranes in Various Organisms Components (% by weight) Protein Phospholipid Sterol 30 45 47 52 56 75 30 27 26 7 40 25 19 25 7 4 4 0 Human myelin sheath Mouse liver Maize leaf Yeast Paramecium (ciliated protist) E. coli Sterol type Other lipids Cholesterol Cholesterol Sitosterol Ergosterol Stigmasterol — Galactolipids, plasmalogens — Galactolipids Triacylglycerols, steryl esters — — The Molecular Constituents of Membranes One approach to understanding membrane function is to study membrane composition—to determine, for example, which components are common to all membranes and which are unique to membranes with specific functions. Before describing membrane structure and function, we therefore consider the molecular components of membranes: proteins and polar lipids, which account for almost all the mass of biological membranes, and carbohydrate present as part of glycoproteins and glycolipids. Rat hepatocyte membrane type Plasma Inner mitochondrial Outer mitochondrial Lysosomal Each Type of Membrane Has Characteristic Lipids and Proteins Nuclear Rough ER Smooth ER Golgi 0 20 40 60 80 100 Percent membrane lipid Cholesterol Sphingolipids Cardiolipin Phosphatidylcholine Minor lipids Phosphatidylethanolamine figure 12–2 Lipid composition of the plasma membrane and organelle membranes of a rat hepatocyte. The functional specialization of each membrane type is reflected in its unique lipid composition. Cholesterol (orange) is prominent in plasma membranes but barely detectable in mitochondrial membranes. Cardiolipin (blue) is a major component of the inner mitochondrial membrane but not of the plasma membrane. Phosphatidylserine, phosphatidylinositol, and phosphatidylglycerol (yellow) are relatively minor components of most membranes but may serve critical functions. Phosphatidylinositol and its derivatives, for example, are important in signal transductions triggered by hormones. Sphingolipids (red), phosphatidylcholine (purple), and phosphatidylethanolamine (green) are present in most membranes, but in varying proportions. Glycolipids, which are major components of the chloroplast membranes of plants, are virtually absent from animal cells. The relative proportions of protein and lipid vary with the type of membrane (Table 12–1), reflecting the diversity of biological roles. For example, certain neurons have a myelin sheath, an extended plasma membrane that wraps around the cell many times and acts as a passive electrical insulator. The myelin sheath consists primarily of lipids, whereas the plasma membranes of bacteria and the membranes of mitochondria and chloroplasts, in which many enzyme-catalyzed processes take place, contain more protein than lipid. For studies of membrane composition, the first task is to isolate a selected membrane. When eukaryotic cells are subjected to mechanical shear, their plasma membranes are torn and fragmented, releasing cytosolic components and membrane-bounded organelles such as mitochondria, chloroplasts, lysosomes, and nuclei. Plasma membrane fragments and intact organelles can be isolated by centrifugal techniques described in Chapter 2 (see Fig. 2–20). Chemical analysis of membranes isolated from various sources reveals certain common properties. Each kingdom, each species, each tissue or cell type, and the organelles of each cell type have a characteristic set of membrane lipids. The plasma membranes of hepatocytes, for example, are enriched in cholesterol and contain no detectable cardiolipin (Fig. 12–2); in the inner mitochondrial membrane of the hepatocytes, this distribution is reversed. Cells clearly have mechanisms to control the kinds and amounts of membrane lipids they synthesize and to target specific lipids to particular organelles. In many cases, we can surmise the adaptive advantages of distinct combinations of membrane lipids; in other cases, the functional significance of these combinations remains to be discovered. The protein composition of membranes from different sources varies even more widely than their lipid composition, reflecting functional specialization. In a rod cell of the vertebrate retina, one portion of the cell is highly specialized for the reception of light; more than 90% of the plasma membrane protein in this region is the light-absorbing glycoprotein rhodopsin. The less specialized plasma membrane of the erythrocyte has about 20 Chapter 12 Biological Membranes and Transport 391 prominent types of proteins as well as dozens of minor ones; many of these are transporters, each moving a specific solute across the membrane. The plasma membrane of Escherichia coli contains hundreds of different proteins, including transporters and many enzymes involved in energyconserving metabolism, lipid synthesis, protein export, and cell division. The outer membrane of E. coli has a different function (protection) and a different set of proteins. Some membrane proteins are covalently linked to complex arrays of carbohydrate. For example, in glycophorin, a glycoprotein of the erythrocyte plasma membrane, 60% of the mass consists of complex oligosaccharide units covalently attached to specific amino acid residues. Ser, Thr, and Asn residues are the most common points of attachment (see Fig. 9–25). At the other end of the scale is rhodopsin of the rod cell plasma membrane, which contains a single hexasaccharide. The sugar moieties of surface glycoproteins influence the folding of the protein, as well as its stability and intracellular destination, and they play a significant role in the specific binding of ligands to glycoprotein surface receptors (see Fig. 9–29). Unlike plasma membranes, intracellular membranes such as those of mitochondria and chloroplasts rarely contain covalently bound carbohydrates. Some membrane proteins are covalently attached to one or more lipids, which serve as hydrophobic anchors that hold the proteins to the membrane, as we shall see. figure 12–3 Fluid mosaic model for membrane structure. The fatty acyl chains in the interior of the membrane form a fluid, hydrophobic region. Integral proteins float in this sea of lipid, held by hydrophobic interactions with their nonpolar amino acid side chains. Both proteins and lipids are free to move laterally in the plane of the bilayer, but movement of either from one face of the bilayer to the other is restricted. The carbohydrate moieties attached to some proteins and lipids of the plasma membrane are exposed on the extracellular face of the membrane. The Supramolecular Architecture of Membranes All biological membranes share certain fundamental properties. They are impermeable to most polar or charged solutes, but permeable to nonpolar compounds; they are 5 to 8 nm (50 to 80 Å) thick and appear trilaminar when viewed in cross section with the electron microscope (Fig. 12–1). The combined evidence from electron microscopy and studies of chemical composition, as well as physical studies of permeability and the motion of individual protein and lipid molecules within membranes, led to the development of the fluid mosaic model for the structure of biological membranes (Fig. 12–3). Phospholipids and sterols form a lipid bilayer in which the nonpolar regions of the lipid molecules face each other at the core of the bilayer and their polar head groups face outward. In this bilayer sheet, proteins are Oligosaccharide chains of glycoprotein Outside Glycolipid Lipid bilayer Nonpolar fatty acyl chains Phospholipid polar heads Sterol Inside Peripheral protein Integral protein (single transmembrane helix) Peripheral protein covalently linked to lipid Integral protein (multiple transmembrane helices) 392 Part II Structure and Catalysis embedded at irregular intervals, held by hydrophobic interactions between the membrane lipids and hydrophobic domains in the proteins. Some proteins protrude from only one side of the membrane; others have domains exposed on both sides. The orientation of proteins in the bilayer is asymmetric, giving the membrane “sidedness”; the protein domains exposed on one side of the bilayer are different from those exposed on the other side, reflecting functional asymmetry. The individual lipid and protein units in a membrane form a fluid mosaic whose pattern, unlike a mosaic of ceramic tile and mortar, is free to change constantly. The membrane mosaic is fluid because most of the interactions among its components are noncovalent, leaving individual lipid and protein molecules free to move laterally in the plane of the membrane. We now look at some of these features of the fluid mosaic model in more detail and consider the experimental evidence that confirms the model. A Lipid Bilayer Is the Basic Structural Element of Membranes figure 12–4 Amphipathic lipid aggregates that form in water. (a) In micelles, the hydrophobic chains of the fatty acids are sequestered at the core of the sphere. There is virtually no water in the hydrophobic interior. (b) In an open bilayer, all acyl side chains except those at the edges of the sheet are protected from interaction with water. (c) When a two-dimensional bilayer folds on itself, it forms a closed bilayer, a three-dimensional hollow vesicle (liposome) enclosing an aqueous cavity. Individual units are wedge-shaped (cross-section of head greater than that of side chain) Micelle (a) Glycerophospholipids, sphingolipids, and sterols are virtually insoluble in water. When mixed with water, they spontaneously form microscopic lipid aggregates in a phase separate from their aqueous surroundings, clustering together with their hydrophobic moieties in contact with each other and their hydrophilic groups interacting with the surrounding water. Recall that lipid clustering reduces the amount of hydrophobic surface exposed to water and thus minimizes the number of molecules in the shell of ordered water at the lipid-water interface (see Fig. 4–7), resulting in an increase in entropy. Hydrophobic interactions among lipid molecules provide the thermodynamic driving force for the formation and maintenance of these clusters. Depending on the precise conditions and the nature of the lipids, three types of lipid aggregates can form when amphipathic lipids are mixed with water (Fig. 12–4). Micelles are spherical structures containing a few dozen to a few thousand molecules arranged with their hydrophobic regions aggregated in the interior, excluding water, and their hydrophilic head groups at the surface, in contact with water. Micelle formation is favored when the cross-sectional area of the head group is greater than that of the acyl side chain(s), as in free fatty acids, lysophospholipids (phospholipids lacking one fatty acid), and detergents such as sodium dodecyl sulfate (SDS; p. 134). Individual units are cylindrical (cross-section of head equals that of side chain) Bilayer (b) Aqueous cavity Liposome (c) Chapter 12 Biological Membranes and Transport 393 A second type of lipid aggregate in water is the bilayer, in which two lipid monolayers form a two-dimensional sheet. Bilayer formation occurs most readily when the cross-sectional areas of the head group and acyl side chain(s) are similar, as in glycerophospholipids and sphingolipids. The hydrophobic portions in each monolayer, excluded from water, interact with each other. The hydrophilic head groups interact with water at each surface of the bilayer. Because the hydrophobic regions at its edges (Fig. 12–4b) are transiently in contact with water, the bilayer sheet is relatively unstable and spontaneously forms a third type of aggregate: it folds back on itself to form a hollow sphere called a vesicle or liposome. By forming vesicles, bilayers lose their hydrophobic edge regions, achieving maximal stability in their aqueous environment. These bilayer vesicles enclose water, creating a separate aqueous compartment. It is likely that the first living cells resembled liposomes, their aqueous contents segregated from the rest of the world by a hydrophobic shell. All evidence indicates that biological membranes are constructed of lipid bilayers. The thickness of membranes, measured by electron microscopy, is about that expected for a lipid bilayer 3 nm (30 Å) thick with proteins protruding on each side. X-ray diffraction studies of membranes show the distribution of electron density expected for a bilayer structure. And liposomes formed in the laboratory show the same relative impermeability to polar solutes as is seen in biological membranes (although the latter, as we shall see, are permeable to solutes for which they have specific transporters). Membrane lipids are asymmetrically distributed between the two monolayers of the bilayer, although the asymmetry, unlike that of membrane proteins, is not absolute. In the plasma membrane, for example, certain lipids are typically found primarily in the outer monolayer and others in the inner (cytosolic) monolayer, but rarely is a lipid found only on one side, not the other (Fig. 12–5). Membrane phospholipid Percent of total membrane phospholipid Distribution in membrane 100 Inner monolayer 0 Outer monolayer 100 Phosphatidylethanolamine 30 Phosphatidylcholine 27 figure 12–5 Sphingomyelin 23 Phosphatidylserine 15 Asymmetric distribution of phospholipids between the inner and outer monolayers of the erythrocyte plasma membrane. The distribution of a specific phospholipid is determined by treating the intact cell with phospholipase C, which cannot reach lipids in the inner monolayer but removes the head groups of lipids in the outer monolayer. (Other membrane-impermeant reagents could also be used; see Fig. 12 – 9.) The proportion of each head group released provides an estimate of the fraction of each lipid in the outer monolayer. Phosphatidylinositol Phosphatidylinositol 4-phosphate Phosphatidylinositol 4,5-bisphosphate Phosphatidic acid 5 394 Part II Structure and Catalysis Membrane Lipids Are in Constant Motion Paracrystalline state (solid) (a) Heat produces thermal motion of acyl side chains (solid → fluid transition) Fluid state (b) Lateral diffusion in plane of bilayer (c) Transbilayer diffusion (“flip flop”) t1/2 = hours to days (uncatalyzed) = seconds (flippase catalyzed) Although the lipid bilayer structure itself is stable, the individual phospholipid and sterol molecules have great freedom of motion within the plane of the membrane (Fig. 12–6). The interior of the bilayer is fluid; individual hydrocarbon chains of fatty acids are in constant motion produced by rotation about the carbon-carbon bonds of the long acyl side chains. The degree of fluidity depends on lipid composition and temperature. At low temperature, relatively little lipid motion occurs and the bilayer exists as a nearly crystalline (paracrystalline) array. Above a certain temperature, lipids can undergo rapid motion. The transition temperature, the temperature above which the paracrystalline solid changes to fluid, is characteristic for each membrane and depends on its lipid composition. Saturated fatty acids pack well into a paracrystalline array, but the kinks in unsaturated fatty acids (see Fig. 11–1) interfere with this packing, hindering the formation of a paracrystalline state. The higher the proportion of saturated fatty acids, the higher is the transition temperature of the membrane. The sterol content of a membrane is another important determinant of transition temperature. The rigid planar structure of the steroid nucleus, inserted between fatty acyl side chains, has two effects on fluidity. Below the membrane’s transition temperature, sterol insertion prevents the highly ordered packing of fatty acyl chains and thus increases membrane fluidity. Above the thermal transition point, the rigid ring system of the sterol reduces the freedom of neighboring fatty acyl chains to move by rotation about their carbon-carbon bonds and thus reduces the fluidity in the core of the bilayer. Sterols therefore tend to moderate the extremes of solidity and fluidity of membranes. Cells regulate their lipid composition to achieve a constant membrane fluidity under various growth conditions. For example, bacteria synthesize more unsaturated fatty acids and fewer saturated ones when cultured at low temperatures than when cultured at higher temperatures (Table 12–2). As a result of this adjustment in lipid composition, membranes of bacteria cultured at high or low temperatures have about the same degree of fluidity. A second type of lipid motion involves not merely the flexing of fatty acyl chains but the movement of an entire lipid molecule relative to its neighbors (Fig. 12–6b). A molecule in one monolayer, or face, of the lipid bilayer—the outer face of the erythrocyte plasma membrane, for example— can diffuse laterally so fast that it circumnavigates the erythrocyte in seconds. This rapid lateral diffusion within the plane of the bilayer randomizes the positions of individual molecules in a few seconds. The combination of acyl chain flexing and lateral diffusion produces a membrane bilayer with the properties of a liquid crystal: a high degree of regularity in one dimension (perpendicular to the bilayer) and great mobility in the other (the plane of the bilayer). A third kind of lipid motion, much less probable than conformational motion or lateral diffusion, is transbilayer or “flip-flop” diffusion of a molecule from one face of the bilayer to the other (Fig. 12–6c). This motion figure 12–6 Motion of membrane lipids. Lipid motion within a bilayer includes (a) thermal motion of the fatty acyl groups in the bilayer interior and (b) lateral diffusion of individual molecules in one face of the bilayer. A single lipid molecule can diffuse from one end of an E. coli cell to the other in about one second at 37 C. Because of the thermal motion, the bilayer is fluid above a certain transition temperature; as temperature decreases, the bilayer becomes paracrystalline. (c) Movement of individual lipid molecules between the two faces of the bilayer (flip-flop diffusion) is very slow unless catalyzed by a specific lipid transporter, or flippase. Random distribution of the lipids in one monolayer between the two monolayers takes hours if uncatalyzed, but only seconds when a flippase is present. Chapter 12 Biological Membranes and Transport table 12–2 Human cell 395 Mouse cell Fatty Acid Composition of E. coli Cells Cultured at Different Temperatures Percentage of total fatty acids* Myristic acid (14:0) Palmitic acid (16:0) Palmitoleic acid (16:1) Oleic acid (18:1) Hydroxymyristic acid Ratio of unsaturated to saturated† 10 C 20 C 30 C 40 C 4 18 26 38 13 2.9 4 25 24 34 10 2.0 4 29 23 30 10 1.6 8 48 9 12 8 0.38 Artificially induced cell fusion Source: Data from Marr, A.G. & Ingraham, J.L. (1962) Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 84, 1260. *The exact fatty acid composition depends not only on growth temperature but on growth stage and growth medium composition. † Calculated as the total percentage of 161 plus 181 divided by the total percentage of 140 plus 160. Hydroxymyristic acid was omitted from this calculation. requires a polar (and possibly charged) head group to leave its aqueous environment and move into the hydrophobic interior of the bilayer, a process with a large, positive free-energy change. Yet this highly endergonic event is sometimes necessary, for example during synthesis of the bacterial plasma membrane, when phospholipids produced on the inside surface of the membrane must undergo flip-flop diffusion to enter the outer face of the bilayer. Similar transbilayer diffusion must also occur in eukaryotic cells as membrane lipids synthesized in one compartment move to other organelles. A family of proteins (flippases) facilitates flip-flop diffusion, providing a transmembrane path that is energetically more favorable than uncatalyzed diffusion. Membrane Proteins Diffuse Laterally in the Bilayer Many membrane proteins behave as though they were afloat in a sea of lipids. Like membrane lipids, these proteins are free to diffuse laterally in the plane of the bilayer and are in constant motion, as shown by the experiment diagrammed in Figure 12–7. Some membrane proteins associate to form large aggregates (“patches”) on the surface of a cell or organelle, in which individual protein molecules do not move relative to one another; for example, acetylcholine receptors (see Fig. 12–39) form dense patches on neuron plasma membranes at synapses. Other membrane proteins are anchored to internal structures that prevent their free diffusion. In the erythrocyte membrane, both glycophorin and the chloride-bicarbonate exchanger (p. 413) are tethered to a filamentous cytoskeletal protein, spectrin (Fig. 12–8). figure 12–8 Restricted motion of the erythrocyte chloride-bicarbonate exchanger. The protein spans the membrane and is tethered to the cytoskeletal protein spectrin by another protein, ankyrin, limiting the exchanger’s lateral mobility. Ankyrin is anchored in the membrane by a covalently bound palmitoyl side chain (see Fig. 12–13). Spectrin, a long, filamentous protein, is cross-linked at junctional complexes containing actin. A network of cross-linked spectrin molecules attached to the cytoplasmic face of the plasma membrane stabilizes the membrane against deformation. Lateral diffusion of membrane proteins figure 12–7 Demonstration of lateral diffusion of membrane proteins. Fusion of a mouse cell with a human cell results in the randomization of membrane proteins from the two cells. After fusion, the location of each type of membrane protein is determined by staining cells with species-specific antibodies. Anti-mouse and anti-human antibodies are specifically tagged with molecules that fluoresce with different colors. Observed with the fluorescence microscope, the colored antibodies are seen to mix on the surface of the hybrid cell within minutes after fusion, indicating rapid lateral diffusion of the membrane proteins throughout the lipid bilayer. Chloride-bicarbonate exchange proteins Plasma membrane Ankyrin Spectrin Junctional complex (actin) Inside Outside 396 Part II box 12–1 Structure and Catalysis Looking at Membranes Electron microscopy combined with techniques for preparing and staining tissues has been essential to studies of membrane structure. When thin sections of tissue are stained with osmium tetroxide, which accentuates differences in electron density, transmission electron microscopy shows the trilaminar structure (see Fig. 12–1). This level of resolution does not reveal individual membrane proteins, however. When a tissue sample is quick-frozen (to avoid the distortion that results when ice crystals form within cells) then cut with a fine knife, fracture lines frequently run along the surface of a membrane or through its center, splitting the bilayer into two monolayers (Fig. 1). The surfaces exposed by this freeze-fracture technique can be coated with a thin layer of carbon (evaporated from a carbon electrode under vacuum). Scanning electron microscopy of the resulting carbon “replica” shows details in bas relief of the membrane surface and the inside face of one monolayer exposed by splitting of the bilayer (Fig. 2). A variety of particles with the dimensions of single protein molecules or protein complexes protrude from a featureless background (the lipid bilayer). In many membranes, the particles are randomly positioned, but in specialized regions such as the postsynaptic membrane, they are clustered and ordered. In the myelin sheath, the particles are sparsely distributed. In membranes rich in enzyme complexes—the thylakoid membranes of chloroplasts, for instance— the surface is virtually filled with particles. 500 nm 75 nm figure 2 figure 1 Splitting of a membrane bilayer by the freeze-fracture technique. An erythrocyte membrane is frozen in water, etched (by sublimation of some water), and a carbon replica is viewed in the electron microscope. The replica shows integral membrane proteins as bumps of various sizes and shapes, which are more readily visible at higher magnification (inset). Some Membrane Proteins Span the Lipid Bilayer The individual protein molecules and multiprotein complexes of biological membranes can be visualized by electron microscopy of the freezefractured membranes (Box 12–1). Some proteins appear on only one face of the membrane; others, which span the full thickness of the bilayer, protrude from both inner and outer membrane surfaces. Among the latter are proteins that conduct solutes or signals across the membrane. Membrane protein localization has also been investigated with reagents that react with protein side chains but cannot cross membranes (Fig. 12–9). The human erythrocyte is convenient for such studies because it has no Chapter 12 Biological Membranes and Transport Erythrocyte membrane containing three proteins P1 EA (permeant) C P3 14 14 C-labeled IEA HOCH2CH3 NH CH3 P2 C-labeled EA OCH2CH3 NH H2N Protein CH3 C N H Protein EA labels intracellular and extracellular domains of proteins NH CH3 C OCH2CH2SO 3 HOCH2CH2SO 3 IEA (impermeant) (a) IEA labels only extracellular domains of proteins figure 12–9 Experiments to determine the transmembrane arrangement of membrane proteins. (a) Both ethylacetimidate (EA) and isoethionylacetimidate (IEA) react with free amino groups in proteins, but only the ethyl derivative (EA) diffuses freely through the membrane. (b) Comparison of the labeling patterns after treatment of erythrocytes with the two reagents reveals whether a given protein is exposed only on the outer surface or only on the inner surface. A protein (P2) labeled by both reagents, but more heavily by the permeant reagent (EA), is exposed on both sides and thus spans the membrane. Proteins (P1) not labeled by the impermeant reagent (IEA) are exposed on the inner surface only. membrane-bounded organelles; the plasma membrane is the only membrane present. If a membrane protein in an intact erythrocyte reacts with a membrane-impermeant reagent (IEA in Fig. 12–9), it must have at least one domain exposed on the outer (extracellular) face of the membrane. Proteases such as trypsin are unable to cross membranes and are commonly used to explore membrane protein topology, the disposition of protein domains relative to the lipid bilayer. Trypsin cleaves extracellular domains but does not affect domains buried within the bilayer or exposed on the inner surface only. Experiments like those described in Figure 12–9 show that the glycoprotein glycophorin spans the erythrocyte membrane. Its amino-terminal domain (bearing the carbohydrate) is on the outer surface and can be cleaved by trypsin. The carboxyl terminus protrudes on the inside of the IEA Excess IEA, EA removed; membranes isolated, dissolved in SDS; proteins separated by SDS polyacrylamide gel electrophoresis EA P2 Radioactive proteins detected by exposing x-ray film to gel (autoradiography) P1 P3 (b) 397 398 Part II Structure and Catalysis 1 figure 12–10 Transbilayer disposition of glycophorin in the erythrocyte. One hydrophilic domain, containing all the sugar residues, is on the outer surface, and another hydrophilic domain protrudes from the inner face of the membrane. The red hexagons represent a tetrasaccharide (containing two Neu5Ac (sialic acid), Gal, and GalNAc) O-linked to a Ser or Thr residue; the blue hexagon represents an oligosaccharide chain N-linked to an Asn residue. The relative size of the oligosaccharide units is larger than shown here. A segment of 19 hydrophobic residues (residues 75 to 93) forms an a helix that traverses the membrane bilayer (see Fig. 12–17a). The segment from residue 64 to 74 has some hydrophobic residues and probably penetrates into the outer face of the lipid bilayer. Amino terminus Tyr Ala Ala Thr Thr Thr Ser Glu Val Ala Met His Leu Ser Thr Thr Ser Ser Val Ser Thr Asp Arg Lys His Thr Asp Asn Thr Lys Gln Ser Ser Ile Tyr Ser Ser Val Ser Glu Ile Thr Pro Arg Ala His Glu Val 60 74 Outside Arg Thr Val Tyr Pro Arg Glu Glu Thr Glu Glu Pro Glu Val Thr Gln Leu Ile Leu Ile Ile Ala Glu Phe His Pro Gly Glu His Val Met Ser Phe Ala Gly Val Ile Gly Thr Ile Leu Leu Ile Ser Tyr Gly Ile Ser Pro Ser Lys Lys Arg Arg 95 Inside Ile Leu Asp Val Lys Pro Leu Pro Ser Pro Leu Ser Ser Val Thr Asp Val Pro Asp Glu Ile Glu Gln Asp Ser Thr Glu Pro Asn Carboxyl terminus 131 cell. Both the amino-terminal and carboxyl-terminal domains contain many polar or charged amino acid residues and are therefore quite hydrophilic. However, a long segment in the center of the protein contains mainly hydrophobic amino acid residues. These findings suggest that glycophorin has a transmembrane segment arranged as shown in Figure 12–10. One further fact may be deduced from the results of the experiments with glycophorin: its disposition in the membrane is asymmetric. Similar studies of other membrane proteins show that each has a specific orientation in the bilayer and that proteins reorient by flip-flop diffusion very slowly, if at all. Furthermore, glycoproteins of the plasma membrane are invariably situated with their sugar residues on the outer surface of the cell. As we shall see, the asymmetric arrangement of membrane proteins results in functional asymmetry. All the molecules of a given ion pump, for example, have the same orientation in the membrane and therefore pump in the same direction. Peripheral Membrane Proteins Are Easily Solubilized Membrane proteins may be divided into two operational groups (Fig. 12–11). Integral (intrinsic) proteins are very firmly associated with the membrane, removable only by agents that interfere with hydrophobic interactions, such as detergents, organic solvents, or denaturants. Peripheral (extrinsic) proteins associate with the membrane through electrostatic interactions and hydrogen bonding with the hydrophilic domains of integral proteins and with the polar head groups of membrane lipids. They can Chapter 12 Biological Membranes and Transport figure 12–11 Peripheral protein Peripheral and integral proteins. Membrane proteins can be operationally distinguished by the conditions required to release them from the membrane. Most peripheral proteins can be released by changes in pH or ionic strength, removal of Ca2 by a chelating agent, or addition of urea or carbonate. Peripheral proteins covalently attached to a membrane lipid, such as a glycosyl phosphatidylinositol (see Fig. 12–13), can be released by treatment with phospholipase C. Integral proteins can be extracted with detergents, which disrupt the hydrophobic interactions with the lipid bilayer and form micelle-like clusters around individual protein molecules. change in pH; chelating agent; urea; CO32 399 Ca2 detergent phospholipase C Lipid-linked protein Protein–glycan Integral protein (hydrophobic domain coated with detergent) be released by relatively mild treatments that interfere with electrostatic interactions or break hydrogen bonds. Peripheral proteins may serve as regulators of membrane-bound enzymes or may limit the mobility of integral proteins by tethering them to intracellular structures. Annexins, for example, are a family of peripheral membrane proteins that bind reversibly to acidic membrane phospholipids, such as those that enrich the cytosolic (inner) surface of plasma membranes and the cytosolic (outer) face of organelles. Ca2 ions, which interact simultaneously with negatively charged groups on annexin and the negatively charged head group of a phospholipid, are essential to the annexin-membrane interaction (Fig. 12–12). Roles for various annexins have been suggested in vesicle aggregation, membrane fusion, and interactions between membranes and cytoskeletal elements. A number of other proteins that act at a membrane– aqueous solution interface (blood-clotting proteins, for example) also undergo reversible Ca2-dependent membrane binding, perhaps by a similar mechanism. figure 12–12 Proposed structure of annexin V in association with the lipid head groups of a membrane. In this image, the protein backbone of annexin V (derived from crystallographic studies) is seen through a transparent mesh of the surface contour. The membrane modeled here is composed entirely of phosphocholine, with the choline moiety shown in blue, the phosphate groups red, and the fatty acid tails yellow. The bridging Ca2 ions are purple. Electrostatic interactions hold annexin to the membrane. 400 Part II Structure and Catalysis Covalently Attached Lipids Anchor Some Peripheral Membrane Proteins figure 12–13 Lipid-linked membrane proteins. Covalently attached lipids anchor membrane proteins to the lipid bilayer. A palmitoyl group is shown attached by thioester linkage to a Cys residue; an N-myristoyl group is generally attached to an amino-terminal Gly; the farnesyl and geranylgeranyl groups attached to carboxyl-terminal Cys residues are isoprenoids of 15 and 20 carbons, respectively. These three lipid-protein assemblies are found only on the inside surface of the plasma membrane. Glycosyl phosphatidylinositol (GPI) anchors are derivatives of phosphatidylinositol in which the inositol bears a short oligosaccharide covalently joined to the carboxyl-terminal residue of a protein through phosphoethanolamine. GPIlinked proteins are always on the extracellular surface of the plasma membrane. Some membrane proteins contain one or more covalently linked lipids of several types: long-chain fatty acids, isoprenoids, or glycosylated derivatives of phosphatidylinositol, GPI (Fig. 12–13). The attached lipid provides a hydrophobic anchor, which inserts into the lipid bilayer and holds the protein at the membrane surface. The strength of the hydrophobic interaction between a bilayer and a single hydrocarbon chain linked to a protein is barely enough to anchor the protein securely. Other interactions, such as ionic attractions between positively charged Lys residues in the protein and negatively charged lipid head groups, probably stabilize the attachment. The association of these lipid-linked proteins with the membrane is certainly weaker than that for integral membrane proteins and is, in at least some cases, reversible. Beyond merely anchoring a protein to the membrane, the attached lipid may have a more specific role. In the plasma membrane, proteins with GPI anchors are exclusively on the outer (extracellular) face, whereas other types of lipid-linked proteins (Fig. 12–13) are found exclusively on the inner (cytosolic) face. In epithelial cells, GPI-linked membrane proteins are far more common on the apical surface (the surface facing the external environment or interior cavity) than on the basal surface (the adjoining lowerlayer epithelium). The attachment of a specific lipid to a membrane protein may therefore have a targeting function, directing the newly synthesized protein to its correct membrane location. The association of lipid-linked proteins with the membrane may be mediated by specific membrane receptors. NH3 O C Cys CH2 S COO Palmitoyl group on internal Cys (or Ser) O O C C CH2 N H Integral Proteins Are Held in the Membrane by Hydrophobic Interactions with Lipids COO The firm attachment of integral proteins to membranes is the result of hydrophobic interactions between membrane lipids and hydrophobic domains of the protein. Some proteins have a single hydrophobic sequence in the middle (glycophorin, for example) or at the amino or carboxyl terminus. Others have multiple hydrophobic sequences, each of which, when in the a-helical conformation, is long enough to span the lipid bilayer (Fig. 12–14). The same techniques that have allowed us to determine the three-dimensional N-Myristoyl group on amino-terminal Gly O CH3O C CH CH2 S NH NH 3 O Farnesyl (or geranylgeranyl) group on carboxyl-terminal Cys O P O Inositol O GlcNAc O Man O CH2 Man C O CH2 C O CH2 Man Man NH 3 O O P O O GPI anchor on carboxyl terminus Inside Outside CH2 CH2 NH C O Chapter 12 Biological Membranes and Transport 401 figure 12–14 Integral membrane proteins. For known proteins of the plasma membrane, the spatial relationships of protein domains to the lipid bilayer fall into six categories. Types I and II have only one transmembrane helix; the aminoterminal domain is outside the cell in type I proteins and inside in type II. Type III proteins have multiple transmembrane helices in a single polypeptide. In type IV proteins, transmembrane domains of several different polypeptides assemble to form a channel through the membrane. Type V proteins are held to the bilayer primarily by covalently linked lipids (see Fig. 12–13), and type VI proteins have both transmembrane helices and lipid (GPI) anchors. In this figure, and in other figures throughout the book, we represent transmembrane protein segments in their most likely conformations: as a helices of six to seven turns. Sometimes these helices will be shown simply as cylinders. As relatively few membrane protein structures have been deduced by x-ray crystallography, our representation of the extra-membrane domains is arbitrary and not necessarily to scale, unless otherwise noted. structures of many soluble proteins can in principle be applied to membrane proteins. However, generally useful techniques for crystallizing membrane proteins have only recently been developed, and relatively few membrane protein structures have been solved crystallographically. One of the best-studied membrane-spanning proteins, bacteriorhodopsin, has seven very hydrophobic internal sequences and crosses the lipid bilayer seven times. Bacteriorhodopsin is a light-driven proton pump densely packed in regular arrays in the purple membrane of the bacterium Halobacterium halobium. When these arrays are viewed with the electron microscope from several angles, the resulting images allow a three-dimensional reconstruction of the bacteriorhodopsin molecule. X-ray crystallography yields a structure similar in all essential features (Fig. 12–15). Seven ahelical segments, each traversing the lipid bilayer, are connected by nonhelical loops at the inner and outer face of the membrane. In the amino acid sequence of bacteriorhodopsin, seven segments of about 20 hydrophobic residues can be identified, each segment just long enough to make an a helix that spans the bilayer. Hydrophobic interactions between the nonpolar amino acids and the fatty acyl groups of the membrane lipids firmly anchor the protein in the membrane. The seven helices are clustered together and oriented roughly perpendicular to the bilayer plane, providing a transmembrane pathway for proton movement. As we shall see in Chapter 13, this pattern of seven hydrophobic membrane-spanning helices is a common motif in membrane proteins involved in signal reception. + NH3 Type I –OOC COO– + Type II H3N Type III Type IV Type V Type VI Inside Outside Outside Amino terminus figure 12–15 Bacteriorhodopsin, a membrane-spanning protein. The single polypeptide chain folds into seven hydrophobic a helices, each of which traverses the lipid bilayer roughly perpendicular to the plane of the membrane. The seven transmembrane helices are clustered, and the space around and between them is filled with the acyl chains of membrane lipids. The light-absorbing pigment retinal (see Fig. 11–19) is buried deep in the membrane in contact with several of the helical segments (not shown). The helices are colored to correspond with the hydropathy plot in Figure 12–17b. Inside Carboxyl terminus 402 Part II Structure and Catalysis The photosynthetic reaction center from a purple bacterium was the first membrane protein structure solved by crystallography. Although a more complex membrane protein than bacteriorhodopsin, it is constructed on the same structural principles. The reaction center has four protein subunits, three of which contain a-helical segments that span the membrane (Fig. 12–16). These segments are rich in nonpolar amino acids, with their hydrophobic side chains oriented toward the outside of the molecule where they interact with membrane lipids. The architecture of the reaction center protein is therefore the inverse of that seen in most water-soluble proteins, in which hydrophobic residues are buried within the protein core and hydrophilic residues are on the surface (recall the structures of myoglobin and hemoglobin, for example). In Chapter 19 we will encounter several complex membrane proteins having multiple transmembrane helical segments in which hydrophobic chains are positioned to interact with the lipid bilayer. figure 12–16 Outside Inside Three-dimensional structure of the photosynthetic reaction center of Rhodopseudomonas viridis, a purple bacterium. This was the first integral membrane protein to have its atomic structure determined by x-ray diffraction methods. Eleven a-helical segments from three of the four subunits span the lipid bilayer, forming a cylinder 45 Å (4.5 nm) long; hydrophobic residues on the exterior of the cylinder interact with lipids of the bilayer. In this ribbon representation, residues that are part of the transmembrane helices are shown in yellow. The prosthetic groups (light-absorbing pigments and electron carriers; see Fig. 19– 45) are red. The Topology of an Integral Membrane Protein Can Sometimes Be Predicted from Its Sequence Determining the three-dimensional structure of a membrane protein or its topology is generally much more difficult than determining its amino acid sequence, which can be accomplished by sequencing the protein or its gene. More than a thousand sequences are known for membrane proteins, but relatively few three-dimensional structures. The presence of long hydrophobic sequences in a membrane protein is commonly taken as evidence that these sequences traverse the lipid bilayer, acting as hydrophobic anchors or forming transmembrane channels. Virtually all integral proteins have at least one such sequence. What can we predict about the secondary structure of the membranespanning portions of integral proteins? An a-helical sequence of 20 to 25 residues is just long enough to span the thickness (30 Å) of the lipid bilayer (recall that the length of an a helix is 1.5 Å (0.15 nm) per amino acid residue). A polypeptide chain surrounded by lipids, having no water molecules with which to form hydrogen bonds, will tend to form a helices or b sheets, in which intrachain hydrogen bonding is maximized. If the side chains of all amino acids in a helix are nonpolar, hydrophobic interactions with the surrounding lipids further stabilize the helix. Several simple methods of analyzing amino acid sequences yield reasonably accurate predictions of secondary structure for transmembrane proteins. The relative polarity of each amino acid has been determined experimentally by measuring the free-energy change accompanying the movement of that amino acid’s side chain from a hydrophobic solvent into water. This free energy of transfer ranges from very exergonic for charged or polar residues to very endergonic for amino acids with aromatic or Chapter 12 Biological Membranes and Transport 50 100 130 Hydrophobic Hydrophilic 0 3 0 50 100 Residue number 130 Glycophorin (a) Hydropathy index Hydropathy index 0 3 10 3 50 100 3 2 1 403 150 200 5 4 6 250 7 Hydrophobic Hydrophilic 0 3 10 50 100 150 200 250 Residue number Bacteriorhodopsin (b) aliphatic hydrocarbon side chains. The overall hydrophobicity of a sequence of amino acids is estimated by summing the free energies of transfer for the residues in the sequence, which yields a hydropathy index for that region (see Table 5–1). To scan a polypeptide sequence for potential membranespanning segments, one calculates the hydropathy index for successive segments (called windows) of a given size, which may be from seven to 20 residues. For a window of seven residues, for example, the indices for residues 1 to 7, 2 to 8, 3 to 9, and so on, are plotted as in Figure 12–17 (plotted for the middle residue in each window—residue 4 for the window 1 to 7, for example). A region with more than 20 residues of high hydropathy index is presumed to be a transmembrane segment. When the sequences of membrane proteins of known three-dimensional structure are scanned in this way, we find a reasonably good correspondence between predicted and known membrane-spanning segments. Hydropathy analysis predicts a single hydrophobic helix for glycophorin (Fig. 12–17a) and seven transmembrane segments for bacteriorhodopsin (Fig. 12–17b)—in agreement with experimental studies. Many of the transport proteins described in this chapter are believed, on the basis of their amino acid sequences and hydropathy plots, to have multiple membrane-spanning helical regions—that is, they are type III or type IV integral proteins (Fig. 12–14). When predictions are consistent with chemical studies of protein localization (such as those described above for glycophorin and bacteriorhodopsin), the assumption that hydrophobic regions correspond to membrane-spanning domains is better justified. Not all integral membrane proteins are composed of transmembrane a helices. Another structural motif common in membrane proteins is the b barrel (see Fig. 6–20d), in which 20 or more transmembrane segments form b sheets that line a cylinder (Fig. 12–18). The same factors that favor ahelix formation in the hydrophobic interior of a lipid bilayer also stabilize b barrels. When no water molecules are available to hydrogen bond with the carbonyl oxygen and nitrogen of the peptide bond, maximal intrachain hydrogen bonding gives the most stable conformation. Porins, proteins that allow certain polar solutes to cross the outer membrane of gram-negative bacteria such as E. coli, have many-stranded b barrels lining the polar transmembrane passage. figure 12–18 Porin FhuA, an integral membrane protein with b-barrel structure. This protein of the E. coli outer membrane includes a b barrel composed of 22 antiparallel b strands (gray), forming a transmembrane channel through which iron ion bound to the carrier ferrichrome enters from the surrounding medium. The residues on the outside surface of the barrel are hydrophobic and interact with the lipids and lipopolysaccharides in the outer membrane. figure 12–17 Hydropathy plots. Hydropathy index (see Table 5–1) is plotted against residue number for two integral membrane proteins. The hydropathy index for each amino acid residue in a sequence of defined length (called the window) is used to calculate the average hydropathy for the residues in that window. The horizontal axis shows the residue number for the middle of the window. (a) Glycophorin from human erythrocytes has a single hydrophobic sequence between residues 75 and 93 (yellow); compare this with Figure 12–10. (b) Bacteriorhodopsin, known from independent physical studies to have seven transmembrane helices (see Fig. 12–15), has seven hydrophobic regions. Note, however, that the hydropathy plot is ambiguous in the region of segments 6 and 7. Physical studies have confirmed that this region has two transmembrane segments. 404 Part II Structure and Catalysis A polypeptide is more extended in the b conformation than in an a helix; just seven to nine residues of b conformation are needed to span a membrane. Recall that in the b conformation, alternating side chains project above and below the sheet (see Fig. 6–7). In b strands of membrane proteins, every second residue in the membrane-spanning segment is hydrophobic and interacts with the lipid bilayer; aromatic side chains are commonly found at the lipid-protein interface. The other residues may or may not be hydrophilic. The hydropathy plot is not useful in predicting transmembrane segments for proteins with b barrel motifs, but as the database of known b barrel motifs increases, sequence-based predictions of transmembrane b sheets become more feasible. Integral Proteins Mediate Cell-Cell Interactions and Adhesion Several families of integral proteins in the plasma membrane provide specific points of attachment between cells, or between a cell and extracellular matrix proteins. Integrins are heterodimeric proteins (with two unlike subunits, a and b) anchored to the plasma membrane by a single hydrophobic transmembrane helix in each subunit (Fig. 12–19). The large extracellular domains of the a and b subunits combine to form a specific binding site for extracellular proteins such as collagen and fibronectin. As there are at least 14 different a subunits and at least eight different b subunits, a wide variety of specificities may be generated from various combinations of a and b. One common determinant of integrin binding present in several extracellular partners of integrins is the sequence Arg–Gly–Asp (RGD). Integrins are not merely adhesives; they serve as receptors and signal transducers, carrying information across the plasma membrane in both directions. Integrins regulate many processes, including platelet aggregation at the site of a wound, tissue repair, the activity of immune cells, and the invasion of tissue by a tumor (see Fig. 9–27). Mutation in an integrin gene encoding the b subunit known as CD18 is the cause of leukocyte adhesion deficiency in humans, a rare genetic disease in which leukocytes fail to pass out of blood vessels to reach sites of infection. Infants with a severe defect in CD18 commonly die of infections before the age of two. Immunoglobulin-like domains Ligand-binding region Adhesive domain S S S S Ca2 figure 12–19 Four examples of integral protein types that function in cell-cell interactions. Integrins consist of a and b transmembrane polypeptides; their extracellular domains combine to form binding sites for divalent metal ions and proteins of the extracellular matrix (such as collagen and fibronectin) or specific surface proteins of other cells. Cadherin has four extracellular Ca2-binding domains, the most distal of which contains the site that binds to cadherin on another cell surface. N-CAM (neuronal cell adhesion molecule) is one of a family of immunoglobulinlike proteins that mediate Ca2-independent interactions with surface proteins of nearby cells. Selectins bind tightly to carbohydrate moieties in neighboring cells; the binding is Ca2-dependent. b a Ca 2 Ca2 Ca Outside Lectin domain (binds carbohydrates) Ca2 S S S S 2 Ca2 S S Plasma membrane Inside Integrin Cadherin N-CAM Selectins Chapter 12 Biological Membranes and Transport At least three other families of plasma membrane proteins are also involved in surface adhesion (Fig. 12–19). Cadherins undergo homophilic (“with same kind”) interactions with identical cadherins in an adjacent cell. Immunoglobulin-like proteins can undergo either homophilic interactions with their identical counterparts on another cell or heterophilic interactions with an integrin on a neighboring cell. Selectins have extracellular domains that, in the presence of Ca2, bind specific polysaccharides on the surface of an adjacent cell. Selectins are present primarily in the various types of blood cells and in the endothelial cells that line blood vessels. They are an essential part of the blood-clotting process. Integral proteins play a role in many other cellular processes. They serve as transporters and ion channels (discussed later in this chapter) and as receptors for hormones, neurotransmitters, and growth factors (Chapter 13). They are central to oxidative phosphorylation and photosynthesis (Chapter 19) and to cell-cell and antigen-cell recognition in the immune system (Chapter 7). Integral proteins are also major players in the membrane fusion that accompanies exocytosis, endocytosis, and the entry of many types of viruses into host cells. Membrane Fusion Is Central to Many Biological Processes A remarkable feature of the biological membrane is its ability to undergo fusion with another membrane without losing its integrity. Although membranes are stable, they are by no means static. Within the endomembrane system (see Fig. 2–9), the membranous compartments constantly reorganize as small vesicles bud from the Golgi complex to carry newly synthesized lipids and proteins to other organelles and to the plasma membrane. Exocytosis, endocytosis, cell division, fusion of egg and sperm cells, and entry of a membrane-enveloped virus into its host cell all involve membrane reorganization in which the fundamental operation is fusion of two membrane segments without loss of continuity (Fig. 12–20). Specific fusion of two membranes requires that (1) they recognize each other; (2) their surfaces become closely apposed, which requires the removal of water molecules normally associated with the polar head groups of lipids; (3) their bilayer structures become locally disrupted; and (4) the two bilayers fuse to form a single continuous bilayer. Receptor-mediated endocytosis or regulated secretion also requires that (5) the fusion process is triggered at the appropriate time or in response to a specific signal. Integral proteins called fusion proteins mediate these events, bringing about specific recognition and a transient, local distortion of the bilayer structure that favors membrane fusion. (Note that these fusion proteins are unrelated to the products of two fused genes, also called fusion proteins, as discussed in Chapter 29). One of the best-studied cases of membrane fusion occurs during infection of a host cell by a membrane-enveloped virus, such as the influenza virus or HIV. Infection by influenza virus begins when an integral protein of Budding of vesicles from Golgi complex Exocytosis Endocytosis Fusion of endosome and lysosome Viral infection Fusion of sperm and egg Fusion of small vacuoles (plants) figure 12–20 Membrane fusion. The fusion of two membranes is central to a variety of cellular processes involving both organelles and the plasma membrane. Separation of two plasma membranes at cell division 405 406 Part II Structure and Catalysis the virus envelope, the hemagglutination protein, binds to sialic acid (Neu5Ac) residues of glycoproteins or glycolipids in the host cell’s plasma membrane (Fig. 12–21a). Endocytosis then conveys the virus into the cell within an endosome. The low pH in the endosome triggers a major alteration in the conformation of the hemagglutination protein, exposing a short hydrophobic peptide called the fusion peptide. Insertion of the fusion peptide into the lipid bilayer of the endosomal membrane produces a bridge between viral and endosomal membranes. This presumably brings about local distortion of both bilayers, leading to membrane fusion and release of the viral contents into the host cell. In HIV infection, a pair of glycoproteins in the HIV envelope (gp41 and gp120) bind to two “receptors”—CD4 and a coreceptor protein—on the surface of the host cell (Fig. 12–21b). CD4 alone is sufficient to allow binding of HIV to the cell, but for fusion (and thus infection) to occur the host cell also must have a coreceptor such as CCR5. (CCR5 normally functions as a receptor for growth factors called chemokines that direct leukocytes to sites of inflammation.) The requirement for both CD4 and a coreceptor in HIV infection explains the specificity of HIV for T lymphocytes and phagocytes of the immune system; they are the only cell types that have both proteins in their plasma membranes. Presumably, these “receptors” evolved to serve functions useful to the cell, but the virus has evolved to exploit them. Agents that interfere with the interaction of HIV with CD4 or the coreceptor may prove useful in the treatment of HIV infection or in the prevention of progression of HIV infection to AIDS. According to a current model for HIV entry by fusion with the plasma membrane, as shown in Figure 12–21b, the gp41-gp120 complex is normally anchored to the viral membrane by a hydrophobic sequence near the carboxyl terminus of gp41. Contact with CD4 and the coreceptor in the host cell’s surface triggers a large conformational change in gp41, which stretches into an extended structure and exposes the fusion peptide near its amino terminus. This fusion peptide is inserted into the plasma membrane of the host cell; gp41 thus forms a bridge between virus and cell. A second dramatic structural change now occurs: gp41 bends sharply at a hinge region near its center, forming a hairpin-like structure that brings its amino terminus (in the plasma membrane) close to its carboxyl terminus (in the viral membrane). According to this model, the insertion of the fusion peptide into the plasma membrane somehow disorganizes the lipid bilayer locally. Realignment of lipid molecules in this disorganized region results in coalescence of the two membranes, and fusion occurs. Membrane fusion is central to other cellular processes, too, such as the movement of newly synthesized membrane components through the endomembrane system from the endoplasmic reticulum through the Golgi complex to the plasma membrane via membrane vesicles, and the release of proteins, hormones, or neurotransmitters by exocytosis. The proteins required for these membrane fusions, called SNARES, resemble the viral fusion proteins in several respects. SNAREs (the family name derives from synaptosome-associated protein receptors) form hairpin-shaped rods that bring together protein domains bound to the plasma membrane and the vesicle membrane. SNAREs in the cytosolic face of the intracellular vesicles are called v-SNAREs; those in the target membranes with which the vesicles fuse are t-SNAREs. The extended forms of SNAREs, like those of the viral fusion proteins, have coiled coils, long regions in which several a helices are coiled about each other. These structural similarities very probably reflect a fundamental similarity in fusion mechanisms in viruses and intracellular vesicles. Chapter 12 Biological Membranes and Transport 407 figure 12–21 Membrane fusion during viral entry into a host cell. (a) Influenza virus adheres to carbohydrate moieties in the host cell’s surface (see Fig. 9–29b) and triggers endocytosis, which encloses the virus in an endosome. Proton pumps (p. 416) acidify the endosome contents, causing a pH-dependent change in the structure of the hemagglutination (HA) protein and thus exposing a hydrophobic fusion peptide. The fusion peptide penetrates the endosomal membrane and brings about fusion of the viral and endosomal membranes, releasing the viral genome into the host cell. (b) When HIV encounters a T lymphocyte with CD4 and a second coreceptor (cytokine receptor) protein in its plasma membrane, the virus adheres to the cell through two glycoproteins (gp41 and gp120) in its own membrane, releasing the viral contents into the host cell. HIV infects only those cells (lymphocytes) with CD4 in their plasma membrane. Sialic acid HIV Influenza virus HA protein CCR5 HIV attaches to host lymphocyte by interaction between gp120 in HIV envelope and CD4 in host cell plasma membrane. gp41 gp120 Viral membrane Host cell plasma membrane Interaction of HA protein with sialic acid in host cell membrane triggers endocytosis. Low pH in endosome causes extension of HA protein’s fusion peptide, which penetrates the endosome membrane. CD4 Host cell plasma membrane Cytokine receptor (CCR5) in host cell plasma membrane binds CD4 and gp120, allowing amino-terminal membrane fusion domain of gp41 to insert into host cell membrane. Amino terminus Conformational change in gp41 yields hairpin structure with coiled-coil domain, bringing the two membranes into contact. Viral and endosomal membranes fuse, releasing viral contents into host cell. Fusion of HIV and host cell membranes allows HIV contents (RNA genome and enzymes) to enter host cell. (a) (b) 408 Part II Structure and Catalysis Solute Transport across Membranes Every living cell must acquire from its surroundings the raw materials for biosynthesis and for energy production, and must release to its environment the byproducts of metabolism. The plasma membrane contains proteins that specifically recognize and carry into the cell such necessities as sugars, amino acids, and inorganic ions. In some cases, these components are imported against a gradient of concentration, electrical charge, or both—they are “pumped” in. Certain other materials are pumped out to keep their concentrations in the cytosol lower than in the surrounding medium. With few exceptions, the traffic of small molecules across the plasma membrane is mediated by proteins such as transmembrane channels, carriers, or pumps. Within the eukaryotic cell, different compartments have different concentrations of metabolic intermediates and products, and these, too, must move across intracellular membranes in tightly regulated, protein-mediated processes. Passive Transport Is Facilitated by Membrane Proteins figure 12–22 Movement of solutes across a permeable membrane. (a) Net movement of electrically neutral solutes is toward the side of lower solute concentration until equilibrium is achieved. The solute concentrations on the left and right sides of the membrane are designated C1 and C2. The rate of transmembrane movement is proportional to the concentration gradient, C1/C2, as indicated by the large arrows. (b) Net movement of electrically charged solutes is dictated by a combination of the electrical potential (Vm) and the chemical concentration difference across the membrane; net ion movement continues until this electrochemical potential reaches zero. C1 C1 C2 When two aqueous compartments containing unequal concentrations of a soluble compound or ion are separated by a permeable divider (membrane), the solute moves by simple diffusion from the region of higher concentration, through the membrane, to the region of lower concentration, until the two compartments have equal solute concentrations (Fig. 12–22a). When ions of opposite charge are separated by a permeable membrane, there is a transmembrane electrical gradient, the membrane potential, Vm (expressed in volts or millivolts). This membrane potential produces a force opposing ion movements that increase Vm and driving ion movements that reduce Vm (Fig. 12–22b). Thus, the direction in which a charged solute tends to move spontaneously across a membrane depends on both the chemical gradient (the difference in solute concentration) and the electrical gradient (Vm) across the membrane. Together, these two factors are referred to as the electrochemical gradient or the electrochemical potential. This behavior of solutes is in accord with the second law of thermodynamics: molecules tend spontaneously to assume the distribution of greatest randomness—that is, entropy will increase and the energy of the system will be minimized. In living organisms, simple diffusion of most solutes is impeded by selectively permeable barriers—membranes that separate intracellular C2 C1 >> C2 C1 = C2 Vm > 0 Vm = 0 Before equilibrium Net flux At equilibrium No net flux Before equilibrium At equilibrium (a) (b) Biological Membranes and Transport compartments and surround cells. To pass through a lipid bilayer, a polar or charged solute must first give up its interactions with the water molecules in its hydration shell, then diffuse about 3 nm (30 Å) through a solvent (lipid) in which it is poorly soluble (Fig. 12–23). The energy used to strip away the hydration shell and to move the polar compound from water into and through lipid is regained as the compound leaves the membrane on the other side and is rehydrated. However, the intermediate stage of transmembrane passage is a high-energy state comparable to the transition state in an enzyme-catalyzed chemical reaction. In both cases, an activation barrier must be overcome to reach the intermediate stage (Fig. 12–23; compare with Fig. 8–3). The energy of activation (DG‡) for translocation of a polar solute across the bilayer is so large that pure lipid bilayers are virtually impermeable to polar and charged species over periods of time relevant to cells. A few biologically important gases can cross membranes by simple diffusion: molecular oxygen, nitrogen, and methane, all of which are relatively nonpolar. Despite its polarity, water crosses some biological membranes slowly by simple diffusion, presumably as a result of its very high concentration (about 55 M). However, for tissues in which rapid transmembrane water movement is essential (in the kidney, for example), water diffuses through channels formed by specific integral proteins, the aquaporins, described below. Transmembrane passage of polar compounds and ions is made possible by membrane proteins that lower the activation energy for transport by providing an alternative path for specific solutes through the lipid bilayer. Proteins that bring about this facilitated diffusion or passive transport are not enzymes in the usual sense; their “substrates” are moved from one compartment to another, but are not chemically altered. Membrane proteins that speed the movement of a solute across a membrane by facilitating diffusion are called transporters or permeases. The kind of detailed structural information obtained for many soluble proteins by x-ray crystallography is not yet available for most membrane transporters; as a group, these integral membrane proteins are both difficult to purify and difficult to crystallize. However, from studies of the specificity and kinetics of transporters we have learned that their action is closely analogous to that of enzymes. Like enzymes, transporters bind their substrates with stereochemical specificity through many weak, noncovalent interactions. The negative free-energy change associated with these weak interactions, DGbinding, counterbalances the positive free-energy change that accompanies loss of the water of hydration from the substrate, DGdehydration, thereby lowering DG‡ for transmembrane passage (Fig. 12–23). Transporters span the lipid bilayer at least once, and usually several times, forming a transmembrane channel lined with hydrophilic amino acid side chains. The channel provides an alternative path for a specific substrate to move across the lipid bilayer without its having to dissolve in the bilayer, further lowering DG‡ for transmembrane diffusion. The result is an increase of orders of magnitude in the rate of transmembrane passage of the substrate. (a) Simple diffusion without transporter Diffusion with transporter ∆G‡transport figure 12–23 Energy changes accompanying passage of a hydrophilic solute through the lipid bilayer of a biological membrane. (a) In simple diffusion, removal of the hydration shell is highly endergonic, and the energy of activation (DG ‡) for diffusion through the bilayer is very high. (b) A transporter protein reduces the DG‡ for transmembrane diffusion of the solute. It does this by forming noncovalent interactions with the dehydrated solute to replace the hydrogen bonding with water, and by providing a hydrophilic transmembrane passageway. 409 Hydrated solute Free energy, G Chapter 12 (b) Transporter ‡ ∆Gsimple diffusion 410 Part II Structure and Catalysis table 12–3 Aquaporins Aquaporin Roles and location AQP-1 Fluid reabsorption in proximal renal tubule; secretion of aqueous humor in eye and cerebrospinal fluid in central nervous system; water homeostasis in lung Water permeability in renal collecting duct (mutations produce nephrogenic diabetes insipidus) Water retention in renal collecting duct Reabsorption of cerebrospinal fluid in central nervous system; regulation of brain edema Fluid secretion in salivary glands, lachrymal glands, and alveolar epithelium of lung Water uptake by plant vacuole, regulating turgor pressure AQP-2 AQP-3 AQP-4 AQP-5 -TIP Source: King, L.S. & Agre, P. (1996) Pathophysiology of the aquaporin water channels. Annu. Rev. Physiol. 58, 619–648. Aquaporins Form Hydrophilic Transmembrane Channels for the Passage of Water figure 12–24 Likely transmembrane topology of an aquaporin, AQP-1. This protein is also called CHIP-28. (a) Each monomer consists of six transmembrane helices. (b) In a proposed structure for the aquaporin channel (viewed perpendicular to, or through, the plane of the membrane), four AQP-1 monomers associate side by side, with their 24 transmembrane helices surrounding a central channel (shaded blue). The channel, through which water molecules diffuse one by one, is lined with hydrophilic side chains. A family of integral proteins, the aquaporins (AQPs), provide channels for rapid movement of water molecules across plasma membranes in a variety of specialized tissues. Erythrocytes, which swell or shrink rapidly in response to abrupt changes in extracellular osmolarity as blood travels through the renal medulla, have a high density of aquaporin in their plasma membranes (2 105 copies of AQP-1 per cell). Also rich in aquaporins are the plasma membranes of proximal renal tubule cells, which reabsorb water during urine formation, and the vacuolar membrane of plant cells, which require osmotic movement of water into the vacuole in order to maintain turgor pressure (see Fig. 2–10). Table 12–3 provides some examples of aquaporins. All aquaporins are type III integral proteins (Fig. 12–14) with six transmembrane helical segments (Fig. 12–24a). In AQP-1, four monomers of Mr 28,000 form a tetrameric transmembrane channel lined with hydrophilic side chains and having a sufficient diameter (3 Å) to allow passage of water molecules in single file (Fig. 12–24b). Water flows through an AQP-1 channel at the rate of about 5 108 molecules per second. For comparison, the highest known turnover number for an enzyme is that for catalase, 4 107 s1, and many enzymes have turnover numbers between 1 s1 and 104 s1 (see Table 8–7). The low activation energy for passage of water through aquaporin channels (DG‡ 15 kJ/mol) suggests that water moves through the channels in a continuous stream, flowing in the direction dictated 6 Outside + H3N (a) 1 Inside 1 2 3 4 5 6 COO– (b) 5 3 2 4 Chapter 12 Biological Membranes and Transport 411 by the osmotic gradient. (For a discussion of osmosis, see p. 93.) Like most aquaporins, AQP-1 does not allow the passage of ions or other small solutes. The Glucose Transporter of Erythrocytes Mediates Passive Transport Energy-yielding metabolism in erythrocytes depends on a constant supply of glucose from the blood plasma, where the glucose concentration is maintained at about 5 mM. Glucose enters the erythrocyte by facilitated diffusion via a specific glucose transporter at a rate about 50,000 times greater than the uncatalyzed diffusion rate. This well-studied transporter provides an excellent example of how passive transport is managed in cells. The glucose transporter of erythrocytes (called GluT1 to distinguish it from related glucose transporters in other tissues) is a type III integral protein (Mr 45,000) with 12 hydrophobic segments, each of which is believed to form a membrane-spanning helix. The detailed structure of GluT1 is not yet known, but one plausible model suggests that the side-by-side assembly of several helices produces a transmembrane channel lined with hydrophilic residues that can hydrogen bond with glucose as it moves through the channel (Fig. 12–25). Hydrophobic Polar Charged Outside Inside +NH 3 COO– (a) Ser Leu Val Thr Asn Phe Ile figure 12–25 Proposed structure of GluT1. (a) Transmembrane helices are represented as oblique (angled) rows of three or four amino acid residues, each row depicting one turn of the a helix. Nine of the 12 helices contain three or more polar or charged amino acid residues, often separated by several hydrophobic residues. (b) A helical wheel diagram shows the distribution of polar and nonpolar residues on the surface of a helical segment. The helix is diagrammed as though observed along its axis from the amino terminus. Adjacent residues in the linear sequence are connected with arrows, and each residue is placed around the wheel in the position it occupies in the helix; recall that 3.6 residues are required to make one complete turn of the a helix. In this example, the polar residues (blue) are on one side of the helix and the hydrophobic residues (yellow) on the other. This is, by definition, an amphipathic helix. (c) Side-by-side association of five or six amphipathic helices, each with its polar face oriented toward the central cavity, can produce a transmembrane channel lined with polar and charged residues. This channel provides many opportunities for hydrogen bonding with glucose moving through the transporter. The three-dimensional structure of GluT1 has not yet been determined by x-ray crystallography, but researchers expect that the hydrophilic transmembrane channels of this and many other transporters and ion channels will prove to resemble this model. 2 Leu 5 6 Asn 1 Phe Ser Glc Val 3 Ile Thr 7 4 (b) (c) Structure and Catalysis Vmax ) Kinetics of glucose transport into erythrocytes. (a) The initial rate of glucose entry into an erythrocyte, V0, depends upon the initial concentration of glucose on the outside, [S]out. (b) Double-reciprocal plot of the data in (a). The kinetics of facilitated diffusion is analogous to the kinetics of an enzyme-catalyzed reaction. Compare these plots with Figure 8–11, and Figure 1 in Box 8–1. Note that Kt is analogous to Km, the Michaelis constant. 1 2 Vmax ( figure 12–26 1 1 V0 M/min Part II Initial velocity of glucose entry, V0 (mM/min) 412 Kt Extracellular glucose concentration, [S]out (mM) (a) 1 Kt 1 [S]out 1 Vmax 1 mM ( ) (b) The process of glucose transport can be described by analogy with an enzymatic reaction in which the “substrate” is glucose outside the cell (Sout), the “product” is glucose inside (Sin), and the “enzyme” is the transporter, T. When the rate of glucose uptake is measured as a function of external glucose concentration (Fig. 12–26), the resulting plot is hyperbolic; at high external glucose concentrations the rate of uptake approaches Vmax. Formally, such a transport process can be described by the equations k1 k2 k3 k1 k2 k3 Sout T 3::4 Sout • T 3::4 Sin • T 3::4 Sin T D-Glucose T1 in which k1, k1, and so forth, are the forward and reverse rate constants for each step. The steps are summarized in Figure 12–27. The rate equations for this process can be derived exactly as for enzyme-catalyzed reactions (Chapter 8), yielding an expression analogous to the Michaelis-Menten equation (p. 260): V0 Step 1 T1 Step 2 T2 Vmax[S]out Kt [S]out in which V0 is the initial velocity of accumulation of glucose inside the cell when its concentration in the surrounding medium is [S]out, and Kt (Ktransport) is a constant analogous to the Michaelis constant, a combination of rate constants characteristic of each transport system. This equation describes the initial velocity—the rate observed when [S]in 0. As is the case for enzyme-catalyzed reactions, the slope-intercept form of the equation describes a linear plot of 1/V0 against 1/[S]out, from which we can obtain values of Kt and Vmax (Fig. 12–26b). Kt, like Km, is a measure of the affinity of transporter for substrate; the lower the Kt, the higher the affinity. Because no chemical bonds are made or broken in the conversion of Sout to Sin, neither “substrate” nor “product” is intrinsically more stable, and the process of entry is therefore fully reversible. As [S]in approaches [S]out, Step 3 figure 12–27 T2 T1 Inside Step 4 Outside Model of glucose transport into erythrocytes by GluT1. The transporter exists in two conformations: T1, with the glucose-binding site exposed on the outer surface of the plasma membrane, and T2, with the binding site on the inner surface. Glucose transport occurs in four steps. In step 1 , glucose in blood plasma binds to a stereospecific site on T1, thus lowering the activation energy for a conformational change (step 2 ) from Sout • T1 to Sin • T2, effecting the transmembrane passage of the glucose. In step 3 , glucose is released from T2 into the cytosol, and in step 4 , the transporter returns to the T1 conformation, ready to transport another glucose molecule. Chapter 12 Biological Membranes and Transport the rates of entry and exit become equal. Such a system is therefore incapable of accumulating the substrate (glucose) within cells at concentrations above that in the surrounding medium; it simply achieves equilibration of glucose on the two sides of the membrane at a much higher rate than would occur in the absence of a specific transporter. GluT1 is specific for Dglucose, having a measured Kt of 1.5 mM. For the close analogs D-mannose and D-galactose, which differ only in the position of one hydroxyl group, the values of Kt are 20 and 30 mM, respectively; and for L-glucose, Kt exceeds 3,000 mM! Thus, GluT1 shows the three hallmarks of passive transport: high rates of diffusion down a concentration gradient, saturability, and specificity. Because the concentration of blood glucose, 4.5 to 5 mM, is maintained at about three times Kt, GluT1 is nearly saturated with substrate and operates near Vmax. In liver, a different glucose transporter, GluT2, transports glucose out of hepatocytes when liver glycogen is broken down to replenish the blood glucose. GluT2 has a Kt of about 66 mM and can therefore respond to increased levels of intracellular glucose by increasing outward transport. Muscle and adipose tissue have yet another glucose transporter, GluT4, which is distinguished by its stimulation by insulin (Box 12–2). Chloride and Bicarbonate Are Cotransported across the Erythrocyte Membrane The erythrocyte contains another facilitated diffusion system, an anion exchanger, which is essential in CO2 transport to the lungs from tissues such as muscle and liver. Waste CO2 released from respiring tissues into the blood plasma enters the erythrocyte, where it is converted into bicarbonate (HCO3 ) by the enzyme carbonic anhydrase. The HCO3 reenters the blood plasma for transport to the lungs (Fig. 12–28). Because HCO3 is much more soluble in blood plasma than is CO2, this roundabout route increases the blood’s capacity to carry carbon dioxide from the tissues to the lungs. In the lungs, HCO3 reenters the erythrocyte and is converted to CO2, which is eventually released into the lung space and exhaled. This shuttle, to be effective, requires very rapid movement of HCO3 across the erythrocyte membrane. The chloride-bicarbonate exchanger, also called the anion exchange protein, increases the permeability of the erythrocyte membrane to HCO3 by a factor of more than a million. Like the glucose transporter, it is an integral protein that probably spans the membrane 12 times. This protein mediates the simultaneous movement of two anions; for each HCO3 ion that moves in one direction, one Cl ion moves in the opposite direction (Fig. 12–28). The coupling of Cl and HCO3 movement is obligatory; in the absence of chloride, bicarbonate transport stops. In this respect, the anion exchanger is typical of all systems, called cotransport systems, that simultaneously carry two solutes across a membrane. When, as in this case, the two substrates move in opposite directions, the process is antiport. In symport, two substrates are moved simultaneously in the same direction. Transporters that carry only one substrate, such as the erythrocyte glucose transporter, are uniport systems (Fig. 12–29). 413 Carbon dioxide produced by catabolism enters erythrocyte Bicarbonate dissolves in blood plasma Chloride-bicarbonate exchange protein CO2 HCO 3 Cl In respiring tissues carbonic anhydrase HCO3 H CO2 H2O HCO 3 H CO2 H2O Cl Cl carbonic anhydrase In lungs HCO3 CO2 Carbon dioxide leaves erythrocyte and is exhaled Cl Bicarbonate enters erythrocyte from blood plasma figure 12–28 Chloride-bicarbonate exchanger of the erythrocyte membrane. This cotransport system allows the entry and exit of HCO 3 without changes in the transmembrane electrical potential. Its role is to increase the CO2-carrying capacity of the blood. S S1 S2 S1 figure 12–29 Three general classes of transport systems. Transporters differ in the number of solutes (substrates) transported and the direction in which each is transported. Examples of all three types of transporters are discussed in the text. Note that this classification tells us nothing about whether these are energy-requiring (active transport) or energy-independent (passive transport) processes. S2 Uniport Symport Antiport Cotransport 414 Part II box 12–2 Structure and Catalysis Defective Glucose and Water Transport in Two Forms of Diabetes When ingestion of a carbohydrate-rich meal causes blood glucose to exceed the usual concentration between meals (5 mM), excess glucose is taken up by cardiac muscle and skeletal muscle (which store it as glycogen) and by adipocytes (which convert it to triacylglycerols). Glucose uptake into myocytes and adipocytes is mediated by the glucose transporter GluT4. Between meals, the plasma membrane of these cells contains some GluT4, but most is sequestered in the membranes of small intracellular vesicles (Fig. 1). Insulin released from the pancreas in response to high blood glucose triggers the movement of these intracellular vesicles to the plasma membrane, where they fuse, thus exposing GluT4 molecules on the outer surface of the cell. With more GluT4 molecules in action, the rate of glucose uptake increases 15-fold or more. When blood glucose levels return to normal, insulin release slows and most GluT4 molecules are removed from the plasma membranes and stored in vesicles. In type I (juvenile onset) diabetes mellitus, the inability to release insulin (and thus to mobilize glucose transporters) results in low rates of glu- cose uptake into muscle and adipose tissue. One consequence is a prolonged period of high blood glucose after a carbohydrate-rich meal. This condition is the basis for the glucose tolerance test used to diagnose diabetes (Chapter 23). The water permeability of epithelial cells lining the renal collecting duct in the kidney is due to the presence of an aquaporin (AQP-2) in their apical plasma membranes (facing the lumen of the duct). Antidiuretic hormone (ADH) regulates the retention of water by mobilizing AQP-2 molecules stored in vesicle membranes within the epithelial cells, much as insulin mobilizes GluT4 in muscle and adipose tissue. When the vesicles fuse with the epithelial cell plasma membrane, water permeability increases dramatically and more water is reabsorbed from the collecting duct and returned to the blood. When the ADH level drops, AQP-2 is resequestered within vesicles, reducing water retention. In the relatively rare human disease diabetes insipidus, a genetic defect in AQP-2 leads to impaired water reabsorption by the kidney. The result is excretion of copious volumes of very dilute urine. 2 Insulin When insulin interacts with its receptor, vesicles move to surface and fuse with the plasma membrane, increasing the number of glucose transporters in the plasma membrane. 3 When insulin level drops, glucose transporters are removed from the plasma membrane by endocytosis, forming small vesicles. Glucose transporter Insulin receptor Plasma membrane 1 Glucose transporters “stored” within cell in membrane vesicles. 5 figure 1 Regulation by insulin of glucose transport into a myocyte. Patches of the endosome enriched for glucose transporters bud off to become small vesicles, ready to return to the surface when insulin levels rise again. 4 The smaller vesicles fuse with larger endosome. Chapter 12 Biological Membranes and Transport 415 figure 12–30 S ADP Pi ADP Pi ATP Two types of active transport. (a) In primary active transport, the energy released by ATP hydrolysis drives solute movement against an electrochemical gradient. (b) In secondary active transport, a gradient of an ion X (often Na) has been established by primary active transport. Movement of X down its electrochemical gradient then provides the energy to drive cotransport of a second solute (S) against its electrochemical gradient. ATP X X S X X Primary active transport (a) Secondary active transport (b) Active Transport Results in Solute Movement against a Concentration or Electrochemical Gradient In passive transport, the transported species always moves down its electrochemical gradient and is not accumulated above the equilibrium point. Active transport, by contrast, results in the accumulation of a solute above the equilibrium point. Active transport is thermodynamically unfavorable (endergonic) and occurs only when coupled (directly or indirectly) to an exergonic process such as the absorption of sunlight, an oxidation reaction, the breakdown of ATP, or the concomitant flow of some other chemical species down its electrochemical gradient. In primary active transport, solute accumulation is coupled directly to an exergonic chemical reaction, such as conversion of ATP to ADP Pi (Fig. 12–30). Secondary active transport occurs when endergonic (uphill) transport of one solute is coupled to the exergonic (downhill) flow of a different solute that was originally pumped uphill by primary active transport. The amount of energy needed for the transport of a solute against a gradient can be calculated from the initial concentration gradient. The general equation for the free-energy change in the chemical process that converts S into P is G G° RT ln [P][S] (12–1) where R is the gas constant, 8.315 J/mol K, and T is the absolute temperature. When the “reaction” is simply transport of a solute from a region where its concentration is C1 to another region where its concentration is C2, no bonds are made or broken and the standard free-energy change, DG, is zero. The free-energy change for transport, DGt, is then DGt RT ln (C2/C1) (12–2) If there is a tenfold difference in concentration between two compartments, the cost of moving 1 mol of an uncharged solute at 25 C across a membrane separating the compartments is therefore DGt (8.315 J/mol • K)(298 K)(ln 10/1) 5,705 J/mol 5.7 kJ/mol Equation 12–2 holds for all uncharged solutes. 416 Part II Structure and Catalysis When the solute is an ion, its movement without an accompanying counterion results in the endergonic separation of positive and negative charges, producing an electrical potential; such a transport process is said to be electrogenic. The energetic cost of moving an ion depends on the electrochemical potential (p. 408), the sum of the chemical and electrical gradients: DGt RT ln (C2/C1) Z Dw (12–3) where Z is the charge on the ion, is the Faraday constant (96,480 J/V mol), and Dw is the transmembrane electrical potential (in volts). Eukaryotic cells typically have electrical potentials across their plasma membranes of the order of 0.05 to 0.2 V (with the interior negative relative to the outside), so the second term of Equation 12–3 can be a significant contribution to the total free-energy change for transporting an ion. Most cells maintain more than tenfold differences in ion concentrations across their plasma or intracellular membranes, and for many cells and tissues, active transport is therefore a major energy-consuming process. The mechanism of active transport is of fundamental importance in biology. As we shall see in Chapter 19, the formation of ATP in mitochondria and chloroplasts occurs by a mechanism that is essentially ATP-driven ion transport operating in reverse. The energy made available by the spontaneous flow of protons across a membrane is calculable from Equation 12–3; remember that for flow down an electrochemical gradient, the sign of DG is opposite to that for transport against the gradient. There Are at Least Four General Types of Transport ATPases O O P O O OH Phosphate O V O OH Vanadate In the course of evolution, several distinct types of ATP-dependent active transporters have arisen, differing in structure, mechanism, and localization in specific tissues and intracellular compartments (Table 12–4). P-type ATPases are ATP-driven cation transporters that are reversibly phosphorylated by ATP as part of the transport cycle. All P-type transport ATPases have similarities in amino acid sequence, especially near the Asp residue that undergoes phosphorylation, and all are sensitive to inhibition by the phosphate analog vanadate. Each is an integral protein with multiple membrane-spanning regions in a single polypeptide; some also have a second subunit (Fig. 12–31a). P-type transporters are very widely distributed. In animal tissues, the NaK ATPase, an antiporter for Na and K, and the Ca2 ATPase, a uniporter for Ca2, are ubiquitous, well-understood P-type ATPases that maintain disequilibrium in the ionic composition between the cytosol and the extracellular medium. Parietal cells in the lining of the mammalian stomach have a P-type ATPase that pumps H and K across the plasma membrane, thereby acidifying the stomach contents. In higher plants, a P-type ATPase pumps protons out of the cell, establishing a difference of as much as 2 pH units and 250 mV across the plasma membrane. A similar P-type ATPase in the bread mold Neurospora pumps protons out of cells to establish an insidenegative membrane potential, which is used to drive the uptake of substrates and ions from the surrounding medium by secondary active transport. Bacteria use P-type ATPases to pump out toxic heavy metal ions such as Cd2 and Cu2. A distinctly different class of proton-transporting ATPases is responsible for acidifying intracellular compartments in many organisms. The vacuoles of fungi and higher plants maintain a pH between 3 and 6, well below that of the surrounding cytosol (pH 7.5), by the action of V-type ATPases—proton pumps. V-type ATPases (V for vacuolar) are also responsible for the acidification of lysosomes, endosomes, the Golgi complex, A B B A H Chapter 12 A B V1 a d b b Biological Membranes and Transport F1 a figure 12–31 b G C E D a b Subunit structure of three types of ion-transporting ATPases. (a) The P-type ATPases generally have two types of integral protein subunits. The a subunit, which is essential, has the Asp residue that is phosphorylated during transport. (b) V-type ATPases have a peripheral domain, V1 (blue), composed of seven different types of subunits including three A and three B subunits, and an integral domain, Vo (yellow), with three types of subunits including multiple copies of c. (c) F-type ATPases have a peripheral domain, F1 (blue), homologous with V1 of the V-type ATPases. The integral portion of F-type ATPases, Fo (yellow), also has three types of subunits, with multiple copies of c. P-type pumps such as the NaK ATPase move two ions in opposite directions. V-type and F-type proton pumps move protons in one direction—from top to bottom in this diagram—into vacuoles for V-type, out of mitochondria for F-type. a d e b P-type (a) F c c c c c c c a g a b c Vo V-type (b) 417 Fo F-type (c) table 12–4 Four Classes of Transport ATPases Organism or tissue Type of membrane Role of ATPase NaK Animal tissues Plasma H K Plasma H Acid-secreting (parietal) cells of mammals Fungi (Neurospora) Maintains low [Na], high [K] inside cell; creates transmembrane electrical potential Acidifies contents of stomach H Ca2 Ca2 Higher plants Animal tissues Myocytes of animals Cd2, Hg2, Cu2 Bacteria P-type ATPases Plasma Plasma Plasma Sarcoplasmic reticulum (endoplasmic reticulum) Plasma Create H gradient to drive secondary transport of extracellular solutes into cell Maintains low [Ca2] in cytosol Sequesters intracellular Ca2, keeping cytosolic [Ca2] low Pumps heavy metal ions out of cell V-type ATPases H Animals H H Higher plants Fungi Lysosomal, endosomal, secretory vesicles Vacuolar Vacuolar Create low pH in compartment, activating proteases and other hydrolytic enzymes Eukaryotes Higher plants Prokaryotes Inner mitochondrial Thylakoid Plasma Catalyze formation of ATP from ADP Pi Animal tumor cells Plasma F-type ATPases H H H Multidrug transporter and secretory vesicles in animal cells. Structurally unrelated to P-type ATPases, the V-type ATPases do not undergo cyclic phosphorylation and dephosphorylation and are not inhibited by vanadate. All V-type ATPases have a similar complex structure, with an integral (transmembrane) domain Removes a wide variety of hydrophobic natural products and synthetic drugs from cytosol, including vinblastine, doxorubicin, actinomycin D, mitomycin, taxol, colchicine, and puromycin 418 Part II ATP H Structure and Catalysis ADP Pi H figure 12–32 Reversibility of F-type ATPases. An ATP-driven proton transporter also catalyzes ATP synthesis as protons flow down their electrochemical gradient. box 12–3 (Vo) that serves as a proton channel and a peripheral domain (V1) that contains the ATP-binding site and the ATPase activity (Fig. 12–31b). The mechanism by which V-type ATPases couple ATP hydrolysis to the uphill transport of protons is not understood in detail. The V-type proton pumps are related in structure, and probably in mechanism, to a third family of proton pumps, the F-type ATPases. F-type ATPases play a central role in energy-conserving reactions in bacteria, mitochondria, and chloroplasts; that role will be discussed in detail when we describe oxidative phosphorylation and photophosphorylation in Chapter 19. (The F in their name originated in their identification as energy-coupling factors.) They catalyze the uphill transmembrane passage of protons driven by ATP hydrolysis, as well as the reverse reaction, in which downhill proton flow drives ATP synthesis (Fig. 12–32). In the second case, the F-type ATPases are more appropriately named ATP synthases. The proton gradient in oxidative phosphorylation and photophosphorylation is established by other types of proton pumps powered by substrate oxidation or sunlight. The F-type ATPases/ATP synthases are multisubunit complexes that provide a transmembrane pore (the integral protein Fo) for protons and a molecular machine (the peripheral protein F1) A Defective Ion Channel Causes Cystic Fibrosis Cystic fibrosis (CF) is a serious and relatively common hereditary disease of humans. About 5% of white Americans are carriers, having one defective and one normal copy of the gene. Only individuals with two defective copies show the severe symptoms of the disease: obstruction of the gastrointestinal and respiratory tracts, commonly leading to bacterial infection of the airways and death due to respiratory insufficiency before the age of 30. In CF, the thin layer of mucus that normally coats the internal surfaces of the lungs is abnormally thick, obstructing air flow and providing a haven for pathogenic bacteria, particularly Staphylococcus aureus and Pseudomonas aeruginosa. The defective gene in CF patients was discovered in 1989. It encodes a membrane protein called cystic fibrosis transmembrane conductance regulator, or CFTR. Hydropathy analysis predicted that CFTR has 12 transmembrane helices and is structurally related to the multidrug transporters of drug-resistant tumors (Fig. 1). The normal CFTR protein proved to be an ion Oligosaccharide chains of glycoprotein Outside figure 1 Topology of the cystic fibrosis transmembrane conductance regulator, CFTR. There are 12 transmembrane helices, and three functionally significant domains extend from the cytosolic surface: NBF1 and NBF2 are nucleotide-binding folds to which ATP binds, and a regulatory domain (R-domain) is the site of phosphorylation by cAMP-dependent protein kinase. Oligosaccharide chains are attached to several residues on the outer surface on the segment between helices 7 and 8. The most commonly occurring mutation leading to CF is the deletion of Phe508, in the NBF1 domain. The structure of CFTR is very similar to that of the multidrug transporter of tumors, described in the text. Inside + NH3 NBF1 NBF2 Phe508 COO– R domain Chapter 12 Biological Membranes and Transport that uses the energy released by downhill proton flow through Fo to form the phosphoanhydride bonds of ATP. The ATP-synthesizing and ATPase activities reside in the F1 protein (Fig. 12–31c). In the mid-1980s it became apparent that some tumors are strikingly resistant to a number of generally effective antitumor compounds. Investigation revealed that the plasma membranes of these tumors contain an ATPdependent transporter that can export many different drugs, preventing their accumulation within the tumor cells and their growth-inhibitory effects. The transported drugs are chemically dissimilar but are generally hydrophobic. The multidrug transporter responsible for removing these drugs from the tumor cell cytosol is an integral protein (Mr 170,000) with 12 transmembrane segments and two ATP-binding sites. Export of the drugs is driven by ATP hydrolysis. The multidrug transporter is also an ion channel that specifically allows transmembrane diffusion of Cl down its concentration gradient; this activity is independent of ATP. In the genetic disease cystic fibrosis, the underlying defect is in the gene that encodes a Cl-transporting protein, the cystic fibrosis conductance transmembrane regulator (CFTR), which may be related to the multidrug transporter of tumor cells (Box 12–3). channel specific for Cl. The Cl channel activity increases greatly when phosphoryl groups are transferred from ATP to several side chains of the protein, catalyzed by cAMP-dependent protein kinase (Chapter 13). The mutation responsible for CF in 70% of cases results in deletion of a Phe residue at position 508, with the effect that the mutant protein is not correctly folded and inserted in the plasma membrane. Other mutations yield a protein that is inserted properly but cannot be activated by phosphorylation. In each case, the fundamental problem is a nonfunctional Cl channel in the epithelial cells that line the airways (Fig. 2), the digestive tract, and exocrine glands (pancreas, sweat glands, bile ducts, and vas deferens). figure 2 Mucus lining the surface of the lungs traps bacteria. In healthy lungs, these bacteria are killed and swept away by the action of cilia. In CF, the bactericidal activity is impaired, resulting in recurring infections and progressive damage to the lungs. Normally, epithelial cells that line the inner surface of the lungs secrete a substance that traps and kills bacteria, and the cilia on the epithelial cells constantly sweep away the resulting debris. According to one hypothesis, the bactericidal activity requires a relatively low NaCl concentration. In CF patients, the defect in the Cl channel of CFTR results in surface fluid containing a high concentration of NaCl, and this fluid is much less effective in killing bacteria. Frequent infections by bacteria such as S. aureus and P. aeruginosa progressively damage the lungs and reduce respiratory efficiency. 419 420 Part II Structure and Catalysis 3 Na figure 12–33 Na K ATPase. In animal cells, this active transport system is primarily responsible for setting and maintaining the intracellular concentrations of Na and K and for generating the transmembrane electrical potential. It does this by moving three Na out of the cell for every two K it moves in. The electrical potential is central to electrical signaling in neurons, and the gradient of Na is used to drive the uphill cotransport of solutes in a variety of cell types. Membrane potential 50–70 mV NaK ATPase ADP Pi ATP 2 K Cytosol [K] 140 mM [Na] 12 mM Extracellular fluid or blood plasma [K] 4 mM [Na] 145 mM A P-Type ATPase Catalyzes Active Cotransport of Na and K Jens Skou In virtually every animal cell, the concentration of Na is lower in the cell than in the surrounding medium, and the concentration of K is higher (Fig. 12–33). This imbalance is established and maintained by a primary active transport system in the plasma membrane. The enzyme NaK ATPase, discovered by Jens Skou in 1957, couples breakdown of ATP to the simultaneous movement of both Na and K against their electrochemical gradients. For each molecule of ATP converted to ADP and Pi, the transporter moves two K ions inward and three Na ions outward across the plasma membrane. The NaK ATPase is an integral protein with two subunits (Mr 50,000 and 110,000), both of which span the membrane. The detailed mechanism by which ATP hydrolysis is coupled to transport awaits determination of the protein’s three-dimensional structure, but a current model (Fig. 12–34) supposes that the ATPase cycles between two forms, a phosphorylated form (designated P–EnzII) with high affinity for K and low affinity for Na, and a dephosphorylated form (EnzI) with high affinity for Na and low affinity for K. The conversion of ATP to ADP and Pi occurs in two steps catalyzed by the enzyme. (1) Formation of phosphoenzyme: ATP EnzI 88n ADP P–EnzII (2) Hydrolysis of phosphoenzyme: P–EnzII H2O 88n EnzI Pi The net reaction for these two steps is ATP H2O 88n ADP Pi Because three Na ions move outward for every two K ions that move inward, the process is electrogenic—it creates a net separation of charge across the membrane. The result is a transmembrane potential of 50 to 70 mV (inside negative relative to outside), which is characteristic of most animal cells and essential to the conduction of action potentials in neurons. The central role of the NaK ATPase is reflected in the energy invested in this single reaction: about 25% of the total energy consumption of Chapter 12 Biological Membranes and Transport a human at rest! The steroid derivative ouabain (pronounced wah-bane; from waa bayyo, Somali for “arrow poison”) is a potent and specific inhibitor of the NaK ATPase. Ouabain and another steroid derivative, digitoxigenin, are the active ingredients of digitalis, an extract of the leaves of the foxglove plant. Digitalis has long been used in human medicine to treat congestive heart failure. Inhibition of Na efflux by digitalis leads to higher Na concentration in cells, activating a Na-Ca2 antiporter in cardiac muscle. The increased influx of Ca2 through this antiporter produces elevated cytosolic Ca2, which strengthens the contractions of heart muscle. O C ATP-Driven Ca Pumps Maintain a Low Concentration of Calcium in the Cytosol OH CH2 O O H H H OH OH OH H Ouabain Transporter binds 3 Na from the inside of the cell. 1 EnzI 3 Na 2 Phosphorylation favors P–EnzII. ATP ADP P–EnzII Transporter releases 3 Na to the outside and binds 2 K from the outside of the cell. P P–EnzII Postulated mechanism of Na and K transport by the 1 The process begins with the binding NaK ATPase. of three Na to high-affinity sites on the large subunit of the transport protein on the inner (cytosolic) surface of the membrane. This same part of the large subunit also has the ATP-binding site. Phosphorylation of the transporter changes its conformation 2 and decreases its 3 Na release on the outer affinity for Na, leading to (extracellular) surface. 4 K on the outside now binds to high-affinity sites on the extracellular portion of the large subunit; 5 the enzyme is dephosphorylated, 6 K is discharged on reducing its affinity for K; and the inside of the cell. The transport protein is now ready for another cycle of Na and K pumping. C H 3C H figure 12–34 CH3 HO HO CH3 The cytosolic concentration of free Ca2 is generally about 100 nM, far below that in the surrounding medium, whether pond water or blood plasma. The ubiquitous occurrence of inorganic phosphates (Pi and PPi) at millimolar concentrations in the cytosol necessitates a low cytosolic Ca2 concentration, because inorganic phosphate combines with calcium to form relatively insoluble calcium phosphates. Calcium ions are pumped out of the cytosol by a P-type ATPase, the plasma membrane Ca2 pump. Another P-type Ca2 pump in the endoplasmic reticulum moves Ca2 into the ER lumen, a compartment separate from the cytosol. In myocytes, Ca2 is normally sequestered in a specialized form of endoplasmic reticulum called the sarcoplasmic reticulum. The sarcoplasmic and endoplasmic reticulum calcium (SERCA) pumps are closely related in structure and mechanism, and both are inhibited by the tumor-promoting agent thapsigargin, which does not affect the plasma membrane Ca2 pump. The plasma membrane Ca2 pump and SERCA pumps are integral proteins that cycle between two conformations in a mechanism similar to that for NaK ATPase (Fig. 12–34). The Ca2 pump of the sarcoplasmic reticulum, which comprises 80% of the protein in that membrane, has been thoroughly characterized and is the prototype for Ca2 pumps of the P-type. It consists of a single polypeptide (Mr 100,000) that spans the membrane ten times. A large cytosolic domain includes a site for ATP binding and an Asp residue that undergoes reversible phosphorylation by ATP. Phosphorylation favors a conformation with a high-affinity Ca2-binding site exposed on the cytosolic side, and dephosphorylation favors a conformation with a low-affinity Ca2-binding site on the lumenal side. As a result of the cyclic changes in conformation, the transporter binds Ca2 on the side of the membrane where Ca2 concentration is low and releases it on the side where the concentration is high. In this way, the energy released by hydrolysis of ATP to ADP and Pi during one phosphorylation-dephosphorylation cycle drives Ca2 across the membrane against a large electrochemical gradient. O HC OH 2 421 3 Na 3 2 K 4 P Pi 5 6 2 K EnzI Dephosphorylation favors EnzI. Transporter releases 2 K to the inside. Inside Outside 422 Part II Structure and Catalysis table 12–5 Cotransport Systems Driven by Gradients of Na or H Organism or tissue E. coli Intestine, kidney of vertebrates Vertebrate cells (many types) Higher plants Fungi (Neurospora) Transported solute (moving against its gradient) Cotransported solute (moving down its gradient) Type of transport Lactose Proline Dicarboxylic acids Glucose Amino acids Ca2 K K H H H Na Na Na H H Symport Symport Symport Symport Symport Antiport Antiport Antiport Ion Gradients Provide the Energy for Secondary Active Transport Lactose uptake in E. coli. (a) The primary transport of H out of the cell, driven by the oxidation of a variety of fuels, establishes both a proton gradient and an electrical potential (inside negative) across the membrane. Secondary active transport of lactose into the cell involves symport of H and lactose by the galactoside transporter. The uptake of lactose against its concentration gradient is entirely dependent on this inflow of H, driven by the electrochemical gradient. (b) When the energy-yielding oxidation reactions of metabolism are blocked by cyanide (CN), the galactoside transporter allows equilibration of lactose inside and outside the cell via passive transport. The dashed line represents the concentration of lactose in the surrounding medium. Galactoside H H transporter H H H Lactose H H H (outside) Proton pump (inhibited by CN) Fuel H Lactose (inside) H H (a) CO2 2Naout glucoseout 88n 2Nain glucosein The energy required for this process comes from two sources: the greater concentration of Na outside than inside (the chemical potential) and the [Lactose]inside figure 12–35 The ion gradients formed by primary transport of Na or H can themselves provide the driving force for cotransport of other solutes. Many cells contain transport systems that couple the spontaneous, downhill flow of these ions to the simultaneous uphill pumping of another ion, sugar, or amino acid (Table 12–5). The galactoside transporter of E. coli, for example, allows the accumulation of lactose to levels 100 times higher than the concentration in the surrounding growth medium (Fig. 12–35). E. coli normally has a gradient of protons and charge across its plasma membrane produced by energyyielding metabolism; protons tend to flow back spontaneously into the cell down this electrochemical gradient. The lipid bilayer is impermeable to protons, but the galactoside transporter provides a route for proton reentry, and lactose is simultaneously carried into the cell by symport. The endergonic accumulation of lactose is thereby coupled to the exergonic flow of protons; the overall free-energy change for the coupled process is negative. In intestinal epithelial cells, glucose and certain amino acids are accumulated by symport with Na, using the Na gradient established by the NaK ATPase of the plasma membrane (Fig. 12–36). The apical surface of the intestinal epithelial cell is covered with microvilli, long, thin projections of the plasma membrane that greatly increase the surface area exposed to the intestinal contents. Na-glucose symporters in the apical plasma membrane take up glucose from the intestine in a process driven by the downhill flow of Na: Active transport CN inhibition of fuel oxidation Efflux [Lactose]medium Time (b) Chapter 12 Apical surface Biological Membranes and Transport figure 12–36 Basal surface Intestinal lumen 423 Glucose transport in intestinal epithelial cells. Glucose is cotransported with Na across the apical plasma membrane into the epithelial cell. It moves through the cell to the basal surface, where it passes into the blood via GluT2, a passive glucose transporter. The NaK ATPase continues to pump Na outward to maintain the gradient of Na that drives glucose uptake. Blood Microvilli 2 K Epithelial cell 3 Na 2 Na NaK ATPase Glucose Glucose Glucose uniporter GluT2 (facilitates downhill efflux) Na glucose symporter (driven by high extracellular [Na]) transmembrane potential (the electrical potential), which is inside-negative and therefore draws Na inward. The electrochemical potential of Na is G RT ln [Na]in n [Na]out DE where n 2, the number of Na ions cotransported with each glucose molecule. Given the typical membrane potential of 50 mV, an intracellular [Na] of 12 mM, and an extracellular [Na] of 145 mM, the energy, DG, made available as two Na ions reenter the cell is 25 kJ, enough to pump glucose against a large concentration gradient. G 25 kJ RT ln [glucose]in [glucose]out [glucose]in 30,000 [glucose]out That is, the cotransporter can pump glucose inward until its concentration within the epithelial cell is about 30,000 times that in the intestine. As glucose is pumped from the intestine into the epithelial cell at the apical surface, it is simultaneously moved from the cell into the blood by passive transport through a glucose transporter (GluT2) in the basal surface (Fig. 12–36). The crucial role of Na in symport and antiport systems such as these requires the continued outward pumping of Na to maintain the transmembrane Na gradient. Because of the essential role of ion gradients in active transport and energy conservation, compounds that collapse the ion gradients across cellular membranes are poisons, and those that are specific for infectious microorganisms can serve as antibiotics. Valinomycin is a small, cyclic peptide that surrounds K and neutralizes its positive charge (Fig. 12–37). The peptide then acts as a shuttle, carrying K across membranes down its concentration gradient and deflating that gradient. Compounds that shuttle ions across membranes in this way are called ionophores, literally “ion bearers.” Both valinomycin and monensin (a Na-carrying ionophore) are antibiotics; they kill microbial cells by disrupting secondary transport processes and energy-conserving reactions. figure 12–37 Valinomycin, a peptide ionophore that binds K. In this image, the surface contours are shown as a transparent mesh, through which a stick structure of the peptide and a K atom (green) are visible. The oxygen atoms (red) that bind K are part of a central hydrophilic cavity. Hydrophobic amino acid side chains (yellow) coat the outside of the molecule. Because the exterior of the K-valinomycin complex is hydrophobic, the complex readily diffuses through membranes, carrying K down its concentration gradient. The resulting dissipation of the transmembrane ion gradient kills microbial cells, making valinomycin a potent antibiotic. 424 Part II Structure and Catalysis Ion-Selective Channels Allow Rapid Movement of Ions across Membranes First recognized in neurons and now known to be present in the plasma membranes of all cells, as well as in the intracellular membranes of eukaryotes, the ion-selective channel is another mechanism for moving inorganic ions across membranes. Ion channels determine the plasma membrane’s permeability to specific ions and, together with ion pumps such as the NaK ATPase, regulate the cytosolic concentration of ions and the membrane potential. In neurons, very rapid changes in the activity of ion channels cause the changes in membrane potential—the action potentials—that carry signals from one end of a neuron to the other. In myocytes, rapid opening of Ca2 channels in the sarcoplasmic reticulum releases the Ca2 that triggers muscle contraction. We discuss the signaling functions of ion channels in Chapter 13. Here we describe the structural basis for ion channel function, using as examples a bacterial K channel, the acetylcholine receptor ion channel, and the voltage-dependent Na channel of neurons. Ion channels are distinguished from ion transporters in at least three ways. First, the rate of flux through channels can be orders of magnitude greater than the turnover number for a transporter—107 to 108 ions per channel per second, near the theoretical maximum rate for unrestricted diffusion. Second, ion channels are not saturable; their rates do not approach a maximum at high substrate concentration. Third, they are “gated”— opened or closed in response to some cellular event. In ligand-gated channels (which are generally oligomeric), binding of some extracellular or intracellular small molecule forces an allosteric transition in the protein, which opens or closes the channel. In voltage-gated ion channels, a charged protein domain moves relative to the membrane in response to a change in transmembrane electrical potential, causing the ion channel to open or close. Gating by either ligands or membrane potential can be very fast. A channel typically opens in a fraction of a millisecond, and may remain open for only milliseconds, making these molecular devices effective for very fast signal transmission in the nervous system. The Structure of a K Channel Shows the Basis for Its Ion Specificity The structure of a potassium channel from the bacterium Streptomyces lividans, determined by x-ray crystallography in 1998, provides much insight into the way ion channels work. This bacterial ion channel is related in sequence to all other known K channels and serves as the prototype for such channels, including the voltage-gated K channel of neurons described in the next chapter. Among the members of this protein family, the similarities in sequence are greatest in the “pore region,” which contains the ion selectivity filter that allows K (radius 1.33 Å) to pass 10,000 times more readily than Na (radius 0.95 Å), at a rate (about 108 ions per second) approaching the theoretical limit for unrestricted diffusion. The K channel consists of four identical subunits that span the membrane and form a cone within a cone surrounding the ion channel, with the cone’s wide end facing the extracellular space (Fig. 12–38). Each subunit has two transmembrane a helices as well as a third, shorter helix that contributes to the pore region. The outer cone is formed by one of the transmembrane helices of each subunit. The inner cone, formed by the other four transmembrane helices, surrounds the ion channel and cradles the ion selectivity filter. Both the ion specificity and the high flux through the channel are understandable from the channel’s structure. At the inner and outer plasma membrane surfaces, the entryways to the channel have several negatively charged amino acid residues, which presumably increase the local concen- Chapter 12 Biological Membranes and Transport tration of cations such as K and Na. The ion path through the membrane begins (on the inner surface) as a wide, water-filled channel in which the ion can retain its hydration sphere. Further stabilization is provided by the short a helices in the pore region of each subunit, with their carboxyl termini and the associated partial negative charges pointed at K in the channel. About two-thirds of the way through the membrane, this channel narrows in the region of the selectivity filter, forcing the ion to give up its hydrating water molecules. Carbonyl oxygen atoms in the backbone of the selectivity filter replace the water molecules in the hydration sphere of K, forming a series of perfect coordination shells through which the K moves. This favorable interaction with the filter is not possible with Na, which is too small to make contact with all the potential oxygen ligands. The preferential stabilization of K is the basis for the ion selectivity of the filter, and mutations that change residues in this part of the protein eliminate the channel’s ion selectivity. K ions pass through the filter in single file. In the crystallographic structure, two K ions are visible, one at each end of the selectivity filter, about 7.5 Å apart. Their mutual electrostatic repulsion most likely just balances the interaction of each with the selectivity filter and keeps them moving through it rapidly. Other K channels are similar in sequence, and presumably in structure and mechanism, to the S. lividans K channel. For example, the product of the Shaker gene, which is a K channel in Drosophila, and K channels from the nematode Caenorhabditis elegans, the ciliated protozoan Paramecium, and the plant Arabidopsis thaliana all have sequences very similar to those in the pore region of the S. lividans K channel. Furthermore, the amino acid sequences of Na and Ca2 channels suggest that they, too, share some structural and functional similarities with the bacterial K channel. Determination of this channel’s structure was therefore a landmark in channel biochemistry. 425 (a) figure 12–38 Structure of the K channel of Streptomyces lividans. (a) Viewed in the plane of the membrane, the channel consists of eight transmembrane helices (two from each of the four identical subunits), forming a cone with its wide end toward the extracellular space. The inner helices of the cone (lighter-colored) line the transmembrane channel, and the outer helices interact with the lipid bilayer. Short segments of each subunit converge in the open end of the cone to make a selectivity filter. (b) This view perpendicular to the plane of the membrane shows the four subunits arranged around a central channel just wide enough for a single K ion to pass. Carbonyl oxygens of the peptide backbone protrude into the channel, interacting with and stabilizing a K ion passing through. These ligands are perfectly positioned to interact with K, but not with the smaller Na ion. This preferential interaction with K is the basis for the channel’s ion selectivity. (c) Cross section showing the water-filled and relatively broad channel at the cytosolic side (stippled blue). In this region, a K ion (green) is stabilized by hydration and by the partial negative charge at the carboxyl-terminal end of the short helices projecting from each of the four subunits. The channel narrows at the selectivity filter, where the water of hydration is stripped from the K and replaced with carbonyl oxygens of the selectivity filter. (b) Selectivity filter Outside d d Inside (cytosol) (c) 426 Part II Structure and Catalysis The Acetylcholine Receptor Is a Ligand-Gated Ion Channel O CH3 CH3 C O CH2 CH2 N CH3 Acetylcholine CH3 Another very well-studied ion channel is the nicotinic acetylcholine receptor, which is essential in the passage of an electrical signal from a motor neuron to a muscle fiber at the neuromuscular junction (signaling the muscle to contract). (Nicotinic receptors were originally distinguished from muscarinic receptors by the sensitivity of the former to nicotine, the latter to the mushroom alkaloid muscarine.) Acetylcholine released by the motor neuron diffuses a few micrometers to the plasma membrane of a myocyte, where it binds to the acetylcholine receptor. This forces a conformational change in the receptor, causing the ion channel intrinsic to the receptor to open. The resulting inward movement of positive charges depolarizes the plasma membrane of the myocyte, triggering contraction. The acetylcholine receptor allows Na, Ca2, and K to pass through with equal ease, but other cations and all anions are unable to pass. Na movement through an acetylcholine receptor ion channel is unsaturable (its rate is linear with respect to extracellular Na concentration) and very fast—about 2 107 Na ions per second under physiological conditions. This receptor channel is typical of many other ion channels that produce or respond to electrical signals: it has a “gate” that opens in response to stimulation by acetylcholine, and an intrinsic timing mechanism that closes the gate after a split second. Thus the acetylcholine signal is transient—an essential feature of electrical signal conduction. The structural changes underlying gating in the acetylcholine receptor are known, but the mechanism of “desensitization”—of closing the gate even in the continued presence of acetylcholine—is not well understood. The nicotinic acetylcholine receptor (which is structurally and functionally distinct from the muscarinic acetylcholine receptors) is composed of five subunits: single copies of subunits b, g, and d, and two identical a subunits each with an acetylcholine-binding site. All five subunits are related in sequence and tertiary structure, each having four transmembrane helical segments (M1 to M4) (Fig. 12–39a). The five subunits surround a central pore, which is lined with their M2 helices. The pore is about 20 Å wide in the parts of the channel that protrude on the cytosolic and extracellular surfaces, but narrows as it passes through the lipid bilayer. Near the center of the bilayer is a ring of bulky hydrophobic Leu side chains from the M2 helices, positioned so close together that they prevent ions from passing through the channel. Allosteric conformational changes induced by acetylcholine binding to the two a subunits include a slight twisting of the M2 helices (Fig. 12–39b), which draws these hydrophobic side chains away from the center of the channel and opens it to the passage of ions. Based on similarities between the amino acid sequences of other ligand-gated ion channels and the acetylcholine receptor, the receptor channels that respond to the extracellular signals g-aminobutyric acid (GABA), glycine, and serotonin are classified in an acetylcholine receptor superfamily and probably share three-dimensional structure and gating mechanisms. The GABAA and glycine receptors are anion channels specific for Cl or HCO3 , whereas the serotonin receptor, like the acetylcholine receptor, is cation-specific. The subunits of each of these channels, like those of the acetylcholine receptor, have four transmembrane helical segments and form oligomeric channels. A second class of ligand-gated ion channels responds to intracellular ligands: 3,5-cyclic guanosine mononucleotide (cGMP) in the vertebrate eye, cGMP and cAMP in olfactory neurons, and ATP and inositol 1,4,5- Chapter 12 Biological Membranes and Transport 427 NH3 NH3 COO COO M4 M1 M3 Subunit folds into four transmembrane a helices M2 M1 M2 M3 Acetylcholine binding sites M4 Outside M1 M4 M2 M3 a Subunit (b,g,d are homologous) g a a d b Inside M2 amphipathic helices surround channel (a) Bulky hydrophobic Leu side chains of M2 helices close the channel. Binding of two acetylcholine molecules causes twisting of the M2 helices. With receptor sites occupied, the M2 helices have smaller, polar residues lining the channel. 2 Acetylcholine Closed Open (b) trisphosphate (IP3) in many cell types. These channels are composed of multiple subunits, each with six transmembrane helical domains. We discuss these channels in the context of their signaling functions in Chapter 13. figure 12–39 Structure of the acetylcholine receptor ion channel. (a) Each of the five subunits (a2bgd) has four transmembrane helices, M1 to M4. The M2 helices are amphipathic; the others have mainly hydrophobic residues. The five subunits are arranged around a central transmembrane channel, which is lined with the polar sides of the M2 helices. At the top and bottom of the channel are rings of negatively charged amino acid residues. Near the middle of the bilayer, five Leu side chains (one from each M2 helix) protrude into the channel, constricting it to a diameter too small to allow passage of ions such as Ca2, Na, and K. (b) This top view of a cross section through the center of the M2 helices shows the blocking of the channel by the bulky Leu side chains. When both acetylcholine receptor sites (one on each a subunit) are occupied, a conformational change occurs. As the M2 helices twist slightly, the five Leu residues rotate away from the channel and are replaced by smaller, polar residues. This gating mechanism opens the channel, allowing the passage of Ca2, Na, or K. 428 II I III IV Domain Selectivity filter (pore region) Outside 1 Inside 2345 6 Activation gate NH3 COO Inactivation gate III 4 1 2 3 5 Voltage sensor (a) Voltage sensor Selectivity filter (pore) 6 IV Activation gate Outside II Activation gate Inside Membrane polarized, channel closed Aqueous ion channel Na Outside Tether Inactivation gate (open) I (b) figure 12–40 Voltage-gated Na channels of neurons. Sodium channels of various tissues and organisms have a variety of subunits, but only the principal subunit (a) is essential. (a) The a subunit is a large protein with four homologous domains (I to IV), each containing six transmembrane helices. Helix 4 in each domain (blue) is the voltage sensor; helix 6 (orange) is believed to be the activation gate. The segments between helices 5 and 6, the pore region (red), form the selectivity filter, and the segment connecting domains III and IV (green) is the inactivation gate. (b) The four domains are wrapped about a central transmembrane channel lined with polar amino acid residues. The segments linking helices 5 and 6 (red) in each domain come together near the extracellular surface to form the selectivity filter, which is conserved in all Na channels. The filter gives the channel its ability to discriminate between Na and other ions of similar size. The inactivation gate (green) closes (dotted lines) soon after the activation gate opens. (c) The voltage-sensing mechanism involves movement of helix 4 (blue) perpendicular to the plane of the membrane in response to a change in transmembrane potential. The strong positive charge on helix 4 allows it to be pulled inward in response to the inside-negative membrane potential (Vm). Depolarization lessens this pull, and helix 4 relaxes by moving outward. This movement is communicated to the activation gate (orange), inducing conformational changes that open the channel in response to depolarization. Inside Voltage sensor Na Membrane depolarized, channel open (c) The Neuronal Na Channel Is a Voltage-Gated Ion Channel Na channels in the plasma membranes of neurons and of myocytes of heart and skeletal muscle sense electrical gradients across the membrane and respond by opening or closing. These voltage-gated ion channels are typically very selective for Na over other monovalent or divalent cations (by factors of 100 or more) and have a very high flux rate (107 ions per second). Normally in the closed conformation, Na channels are activated (opened) by reduction in the transmembrane electrical potential. They then undergo very rapid inactivation. Within milliseconds of the opening, the channel closes and remains inactive for many milliseconds. Activation followed by inactivation of Na channels is the basis for signaling by neurons (see Fig. 13–5). The essential component of Na channels is a single, large polypeptide (1,840 amino acid residues) organized into four domains (Fig. 12–40) clustered around a central channel, providing a path for Na through the Chapter 12 Biological Membranes and Transport 429 membrane. That path is made Na-specific by a “pore region” composed of the segments between transmembrane helices 5 and 6 of each domain, which fold into the channel. Helix 4 of each domain has a high density of positively charged residues. This segment is believed to move within the membrane in response to changes in the transmembrane voltage, from the “resting” potential of about 60 mV (inside negative) to about 30 mV. The movement of helix 4 triggers opening of the channel, and this is the basis for voltage gating. Inactivation of the channel is believed to occur by a ball-and-chain mechanism. A protein domain on the cytosolic surface of the Na channel, called the inactivation gate (the ball), is tethered to the channel by a short segment of the polypeptide (the chain) (Fig. 12–40b). This domain is free to move about when the channel is closed, but when it opens, a site on the inner face of the channel becomes available for the tethered ball to bind, blocking the channel. The length of the tether appears to determine how long an ion channel stays open; the longer the tether, the longer the open period. Inactivation of other ion channels may occur by a similar mechanism. Ion-Channel Function Is Measured Electrically figure 12–41 A single ion channel typically remains open for only a few milliseconds, beyond the limit of most biochemical measurements. Ion fluxes must therefore be measured electrically, either as changes in Vm (in the millivolt range) or as electrical currents I (in the microampere or picoampere range), using microelectrodes and appropriate amplifiers (Fig. 12–41). Patch-clamping, a technique in which very small currents are measured through a tiny region of the membrane surface containing only one or a few ion channel molecules, reveals that as many as 104 ions can move through a single ion channel in one millisecond. This represents a huge amplification of the initial signal; for the acetylcholine receptor, for example, the signal may have been only a few molecules of acetylcholine. Electrical measurements of ion channel function. The “activity” of an ion channel is estimated by measuring the flow of ions through it, using the patch-clamping technique. A finely drawn-out pipette (micropipette) is pressed against a cell’s surface, and negative pressure in the pipette is used to form a pressure seal between pipette and membrane. When the pipette is pulled away from the cell, it pulls off a tiny patch of membrane (which may contain one or a few ion channels). When the pipette and attached patch are placed in an aqueous solution, channel activity can be measured as the electrical current that flows between the contents of the pipette and the aqueous solution. In practice, a circuit is set up that “clamps” the transmembrane potential at a given value and measures the current that must flow to maintain this voltage. With highly sensitive current detectors, researchers can measure the current flowing through a single ion channel, typically a few picoamperes. The trace showing the current as a function of time (in milliseconds) reveals how fast the channel opens and closes, how frequently it opens, and how long it stays open. Clamping the Vm at different values permits a determination of the effect of membrane potential on these parameters of channel function. Channel Micropipette applied tightly to plasma membrane Patch of membrane pulled from cell Patch of membrane placed in aqueous solution Time 50ms Inward 10pA current Micropipette Electrodes Electronics to hold transmembrane potential (Vm) constant and measure current flowing across membrane 430 Part II Structure and Catalysis Defective Ion Channels Can Have Striking Physiological Consequences O HO O O H H H N N H H2N OH H H HO CH2OH H H OH Tetrodotoxin O H2N H H N O HN H2N NH2 N H N HO OH Saxitoxin H3C CH3 N CH2 H 2Cl OH H O H3CO OCH3 O OH D-Tubocurarine CH2 H chloride N CH3 The importance of ion channels to physiological processes is clear from the effects of mutations in specific ion channel proteins. Genetic defects in the voltage-gated Na channel of the myocyte plasma membrane result in diseases in which muscles are periodically either paralyzed (as in hyperkalemic periodic paralysis) or stiff (as in paramyotonia congenita). As noted earlier, cystic fibrosis is the result of a mutation that changes one amino acid in the protein CFTR, a chloride ion channel. The defective process here is not neurotransmission, but secretion by various exocrine gland cells whose activities are tied to Cl ion fluxes. Naturally occurring toxins often act on ion channels, and the potency of these toxins further illustrates the importance of normal ion channel function. Tetrodotoxin (produced by the puffer fish, Spheroides rubripes) and saxitoxin (produced by the marine dinoflagellate Gonyaulax, which causes the occasional phenomenon of “red tides”) are poisons that act by binding to the voltage-gated Na channels of neurons and preventing normal action potentials. Puffer fish is an ingredient of the Japanese delicacy fugu, which may only be prepared by chefs specially trained to separate succulent morsel from deadly poison. Eating shellfish that have fed on Gonyaulax can also be fatal; shellfish are not sensitive to saxitoxin, but they concentrate it in their muscles, which become highly poisonous to organisms farther up the food chain. The venom of the black mamba snake contains dendrotoxin, which interferes with voltage-gated K channels. Tubocurarine, the active component of curare (used as an arrow poison in the Amazon), and two other toxins from snake venoms, cobrotoxin and bungarotoxin, block the acetylcholine receptor or prevent the opening of its ion channel. By blocking signals from nerves to muscles, all of these toxins cause paralysis and possibly death. On the positive side, the extremely high affinity of bungarotoxin for the acetylcholine receptor (Kd 1015 M) proved useful when radiolabeled toxin was used to quantify the receptor during its purification. Porins Are Transmembrane Channels for Small Molecules In the outer membrane of gram-negative bacteria such as E. coli, protein channels called porins allow the passage of molecules much larger than ions, but by a mechanism more like a gated channel than a transporter. The porin FhuA (Fig. 12–18) serves in E. coli to bring iron (in the form of a chelate of ferrichrome) from the extracellular medium across the outer membrane and into the periplasmic space. (In gram-negative bacteria, the periplasm is the material between the inner (plasma) and outer membranes.) FhuA is composed of a large 22-stranded b-barrel domain of about 560 residues and an amino-terminal “cork” domain of 160 residues, which normally obstructs the barrel and keeps the channel closed (Fig. 12–42). Binding of the ferrichrome complex to a specific site on the outer surface of the cell triggers allosteric changes that move the ferrichrome into the barrel and allow interaction of the FhuA protein with proteins of the inner membrane and periplasm. This interaction moves the cork out of the barrel and allows passage of the ferrichrome through the channel. With the emptying of the ferrichrome binding site, the changes are reversed and the channel closes. The transport systems discussed in this chapter are summarized in Figure 12–43. Chapter 12 (a) Biological Membranes and Transport (b) figure 12–42 Structure of FhuA, an iron transporter from E. coli. This protein of the E. coli outer membrane transports iron ion into the cell in the form of a ferrichrome-iron chelate. Figure 12–18 shows a ribbon representation of the protein viewed parallel to the membrane. Here we see (a) ribbon and (b) space-filling models, viewed perpendicular to the membrane. The 22-stranded b barrel (gray) is seen as a hollow pipe, plugged at one end by the cork domain (red). The binding of a ferrichrome-iron molecule at a specific site on the outer surface of FhuA drives a conformational change that helps to move the plug out of the channel; the ferrichrome-iron then moves through the channel, and the transporter resumes its closed conformation. Sout Facilitated diffusion (down electrochemical gradient) Simple diffusion (nonpolar compounds only, down concentration gradient) Sout Primary active transport (against electrochemical gradient) Sin Sout ATP Sin Sin Ionophoremediated ion transport (down electrochemical gradient) ADP + Pi Ion Ion Sin Ion Ion Sout figure 12–43 Summary of transport types. Ion channel (down electrochemical gradient; may be gated by a ligand or ion) Ion Ion Secondary active transport (against electrochemical gradient, driven by ion moving down its gradient) 431 432 Part II Structure and Catalysis summary Biological membranes are central to life. They define cellular boundaries, divide cells into discrete compartments, organize complex reaction sequences, and act in signal reception and energy transformations. Membranes are composed of lipids and proteins in varying combinations particular to each species, cell type, and organelle. The fluid mosaic model describes features common to all biological membranes. The lipid bilayer is the basic structural unit. Fatty acyl chains of phospholipids and the steroid nucleus of sterols are oriented toward the interior of the bilayer; their hydrophobic interactions stabilize the bilayer but allow the structure to be flexible. Lipids and most proteins are free to diffuse laterally within the membrane, and the hydrophobic moieties of the lipids undergo rapid thermal motion, making the interior of the bilayer fluid. Fluidity is affected by temperature, fatty acid composition, and sterol content. Cells regulate their lipid compositions to maintain a constant fluidity when external circumstances change. Peripheral proteins are loosely associated with the membrane through electrostatic interactions and hydrogen bonds or by covalently attached lipid anchors. Integral proteins associate firmly with membranes by hydrophobic interactions between the lipid bilayer and their nonpolar amino acid side chains, which are oriented toward the outside of the protein molecule. Some membrane proteins span the lipid bilayer several times, with hydrophobic sequences of about 20 amino acid residues forming transmembrane a helices. Such hydrophobic sequences detected in the primary structures of proteins can be used to predict the secondary structure and transmembrane disposition of these molecules. Multistranded b barrels are also common in integral membrane proteins. The lipids and proteins of membranes are inserted into the bilayer with specific sidedness; thus, membranes are structurally and functionally asymmetric. Many membrane proteins contain covalently attached oligosaccharides of various degrees of complexity. Plasma membrane glycoproteins are always oriented with the carbohydrate-bearing domain on the extracellular surface. A variety of proteins mediate the fusion of two membranes, which accompanies processes such as endocytosis and exocytosis. Fusion requires close apposition of two mem- branes and local disruption of the lipid bilayers, both processes attributed to fusion proteins called SNAREs. The lipid bilayer is impermeable to polar substances. Water is an important exception; it can diffuse passively across the bilayer. Cells that require large fluxes of water have water-specific channels called aquaporins in their plasma membranes. Other polar species cross biological membranes only by way of specific membrane proteins—transporters and ion channels. Transporters (transport proteins), like enzymes, show saturation and substrate specificity. Transport via these systems may be passive (down the electrochemical gradient, hence independent of metabolic energy) or active (against the gradient and thus dependent on metabolic energy). Glucose transport into erythrocytes and water transport through aquaporins is passive. The energy input for active transport may come from light, oxidation reactions, ATP hydrolysis, or cotransport of some other solute. Some transporters carry out symport, the simultaneous passage of two substances in the same direction; others mediate antiport, in which two substances move simultaneously in opposite directions. The lactose transporter of E. coli and the glucose transporter of intestinal epithelial cells are symporters, both cotransporting Na along with the sugar. The chloride-bicarbonate exchanger of erythrocytes and the ubiquitous NaK ATPase are antiporters. In animal cells, the differences in cytosolic and extracellular concentrations of Na and K are established and maintained by active transport through the NaK ATPase. The resulting Na gradient across the plasma membrane is used as an energy source for a variety of secondary active transport processes, both symport and antiport. In primary active transport, ATP serves directly as the energy source. There are four general types of ATP-driven active transporters. P-type ATPases undergo reversible phosphorylation during their catalytic cycle and are inhibited by the phosphate analog vanadate; examples are the NaK ATPase of the plasma membrane and the Ca2 transporters of the sarcoplasmic and endoplasmic reticulum (the SERCA pumps). V-type ATPases produce gradients of protons across a variety of intracellular membranes, including plant vacuolar membranes. F-type proton pumps (ATP synthases) are central to Chapter 12 energy-conserving mechanisms in mitochondria and chloroplasts. A multidrug transporter in tumor cells uses ATP to drive the export of a variety of drugs. Ionophores are lipid-soluble molecules that bind specific ions and carry them passively across membranes, dissipating the energy of electrochemical ion gradients. Ion channels provide hydrophilic pores through which select ions can diffuse, moving down their electrical or chemical concentration gradients. They are characteristically unsaturable and have very high flux rates. Many are highly specific for one ion, and most are gated by either voltage or a ligand. The structure of the Biological Membranes and Transport 433 K channel is known and serves as the prototype for other channels such as the acetylcholine receptor ion channel and the voltage-gated Na channel. Several a-helical transmembrane domains cluster about a central aqueous channel. Ion passage is restricted by a selectivity filter and by a gate that obstructs the ion channel when it is not activated by voltage or a bound ligand. Porins are integral membrane proteins that consist of a b barrel with a central transmembrane opening. A globular protein domain hinders passage of ion, but the binding of a specific substrate transiently opens the central pore, allowing uptake of that substrate. further reading Membrane Architecture Membrane Fusion Devaux, P.F. (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30, 1163– 1173. Nichols, B.J. & Pelham, H.R. (1998) SNAREs and membrane fusion in the Golgi. Biochim. Biophys. Acta 1404, 9–31. Frye, L.D. & Ediden, M. (1970) The rapid intermixing of cell-surface antigens after formation of mouse-human heterokaryons. J. Cell Sci. 7, 319–335. The classic demonstration of membrane protein mobility. Haltia, T. & Freire, E. (1995) Forces and factors that contribute to the structural stability of membrane proteins. Biochim. Biophys. Acta 1241, 295–322. Good discussion of the secondary and tertiary structures of membrane proteins and the factors that stabilize them. Jacobson, K., Sheets, E.D., & Simson, R. (1995) Revisiting the fluid mosaic model of membranes. Science 268, 1441–1442. Discussion of the restricted mobility of membrane proteins, updating the model in paper by Singer and Nicolson (below). Singer, S.J. & Nicolson, G.L. (1972) The fluid mosaic model of the structure of cell membranes. Science 175, 720–731. The classic statement of the model. von Heijne, G. (1994) Membrane proteins: from sequence to structure. Annu. Rev. Biophys. Biomol. Struct. 23, 167–192. A review of the steps required to predict the structure of an integral protein from its sequence. White, S.H. & Wimley, W.C. (1999) Membrane protein folding and stability: Physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365. Transporters Lienhard, F.E., Slot, J.W., James, D.E., & Mueckler, M.M. (1992) How cells absorb glucose. Sci. Am. 266 (January), 86–91. Introduction to the glucose transporter and its regulation by insulin. Lingrel, J.B. & Kuntzweiler, T. (1994) Na, KATPase. J. Biol. Chem. 269, 19,659–19,662. Møller, J.V., Juul, B., & LeMaire, M. (1996) Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1286, 1–51. Mueckler, M. (1994) Facilitative glucose transporters. Eur. J. Biochem. 219, 713–725. Postle, K. (1999) Active transport by customized b-barrels. Nat. Struct. Biol. 6, 3–6. Introductory description of the structure of the E. coli porins and their coupling to metabolic energy. Sheppard, D.N. & Welsh, M.J. (1999) Structure and function of the CFTR chloride channel. Physiolog. Rev. 79, S23–S46. This issue of the journal has 11 reviews of the CFTR chloride channel, covering its structure, activity, regulation, biosynthesis, and pathophysiology. 434 Part II Structure and Catalysis Ion Channels Aidley, D.J. & Stanfield, P.R. (1996) Ion Channels: Molecules in Action, Cambridge University Press. Excellent chapters on molecular structure, gating, and drug interactions of ion channels, and human diseases that result from defective channels. Changeux, J.P. (1993) Chemical signaling in the brain. Sci. Am. 269 (November), 58–62. Structure and function of the acetylcholine receptor channel. Doyle, D.A., Cabral, K.M., Pfuetzner, R.A., Kuo, A., Gulbis, J.M., Cohen, S.L., Chait, B.T., & MacKinnon, R. (1998) The structure of the potassium channel: Molecular basis of K conduction and selectivity. Science 280, 69–77. The first crystal structure of an ion channel. Edelstein, S.J. & Changeux, J.P. (1998) Allosteric transitions of the acetylcholine receptor. Adv. Prot. Chem. 51, 121–184. Advanced discussion of the conformational changes induced by acetylcholine. Marban, E., Yamagishi, T., & Tomaselli, G.F. (1998) Structure and function of voltage-gated sodium channels. J. Physiol. 508, 647–657. Review of evolution, structure, gating, drug interactions, and synthesis of voltage-gated Na channels. Neher, E. & Sakmann, B. (1992) The patch clamp technique. Sci. Am. (March) 266, 44–51. Clear description of the electrophysiological techniques used to measure the activity of single ion channels, by their Nobel Prize-winning developers. Perozo, E., Cortes, D.M., & Cuello, L.G. (1999) Structural rearrangements underlying K channel activation gating. Science 285, 73–78. Physical studies of the mechanism by which the K channel opens and closes. Unwin, N. (1998) The nicotinic acetylcholine receptor of the Torpedo electric ray. J. Struct. Biol. 121, 181–190. Evidence for the structural change that accompanies channel closing. Hille, B. (1992) Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, Massachusetts. Emphasizes the function of ion channels; good complement to Aidley and Stanfield (above). problems Force applied here to compress monolayer (a) 40 Force (dyne/cm) 1. Determining the Cross-Sectional Area of a Lipid Molecule When phospholipids are layered gently onto the surface of water, they orient at the airwater interface with their head groups in the water and their hydrophobic tails in the air. An experimental apparatus (a) has been devised that reduces the surface area available to a layer of lipids. By measuring the force necessary to push the lipids together, it is possible to determine when the molecules are packed tightly in a continuous monolayer; when that area is approached, the force needed to further reduce the surface area increases sharply (b). How would you use this apparatus to determine the average area occupied by a single lipid molecule in the monolayer? 30 20 10 0.2 0.6 1.0 Area (nm2/molecule) 1.4 (b) 2. Evidence for Lipid Bilayer In 1925, E. Gorter and F. Grendel used an apparatus like that described in Problem 1 to determine the surface area of a lipid monolayer formed by lipids extracted from erythrocytes of several animal species. They used a microscope to measure the dimensions of individual cells, from which they calculated the average surface area of one erythrocyte. They obtained the data shown on the next page. Were these investigators justified in Chapter 12 Biological Membranes and Transport concluding that “chromocytes [erythrocytes] are covered by a layer of fatty substances that is two molecules thick” (i.e., a lipid bilayer)? Animal Volume of packed cells (mL) Number of cells (per mm3) Total surface area of lipid monolayer from cells (m2) Total surface area of one cell (mm2) Dog Sheep Human 40 10 1 8,000,000 9,900,000 4,740,000 62 6.0 0.92 98 29.8 99.4 Source: Data from Gorter, E. & Grendel, F. (1925) On bimolecular layers of lipoids on the chromocytes of the blood. J. Exp. Med. 41, 439–443. 3. Number of Detergent Molecules per Micelle When a small amount of sodium dodecyl sulfate (NaCH3(CH2)11OSO3 ) is dissolved in water, the detergent ions enter the solution as monomeric species. As more detergent is added, a concentration is reached (the critical micelle concentration) at which the monomers associate to form micelles. The critical micelle concentration of SDS is 8.2 mM. The micelles have an average particle weight (the sum of the molecular weights of the constituent monomers) of 18,000. Calculate the number of detergent molecules in the average micelle. free-energy change for ATP hydrolysis under cellular conditions is about 58 kJ/mol (as we will explain in Chapter 14). Ignore the effects of the transmembrane electric potential. 4. Properties of Lipids and Lipid Bilayers Lipid bilayers formed between two aqueous phases have this important property: they form two-dimensional sheets, the edges of which close upon each other and undergo self-sealing to form liposomes. 9. Action of Ouabain on Kidney Tissue Ouabain specifically inhibits the NaK ATPase activity of animal tissues but is not known to inhibit any other enzyme. When ouabain is added to thin slices of living kidney tissue, it inhibits oxygen consumption by 66%. Why? What does this observation tell us about the use of respiratory energy by kidney tissue? (a) What properties of lipids are responsible for this property of bilayers? Explain. (b) What are the consequences of this property with regard to the structure of biological membranes? 5. Length of a Fatty Acid Molecule The carbon– carbon bond distance for single-bonded carbons such as those in a saturated fatty acyl chain is about 1.5 Å. Estimate the length of a single molecule of palmitate in its fully extended form. If two molecules of palmitate were placed end to end, how would their total length compare with the thickness of the lipid bilayer in a biological membrane? 6. Temperature Dependence of Lateral Diffusion The experiment described in Figure 12–7 was performed at 37 C. If the experiment were carried out at 10 C, what effect would you expect on the rate of cell-cell fusion and the rate of membrane protein mixing? Why? 7. Synthesis of Gastric Juice: Energetics Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calculate the amount of free energy required to concentrate the H in 1 L of gastric juice at 37 C. Under cellular conditions, how many moles of ATP must be hydrolyzed to provide this amount of free energy? The 8. Energetics of the NaK ATPase The concentration of Na inside a vertebrate cell is about 12 mM, and that in blood plasma is about 145 mM. For a typical cell with a transmembrane potential of –0.07 V (inside negative relative to outside), what is the freeenergy change for transporting 1 mol of Na out of the cell and into the blood at 37 C? 10. Energetics of Symport Suppose that you determined experimentally that a cellular transport system for glucose, driven by symport of Na, could accumulate glucose to concentrations 25 times greater than in the external medium, while the external [Na] was only ten times greater than the intracellular [Na]. Would this violate the laws of thermodynamics? If not, how could you explain this observation? 11. Location of a Membrane Protein The following observations are made on an unknown membrane protein, X. It can be extracted from disrupted erythrocyte membranes into a concentrated salt solution, and the isolated X can be cleaved into fragments by proteolytic enzymes. Treatment of erythrocytes with proteolytic enzymes followed by disruption and extraction of membrane components yields intact X. However, treatment of erythrocyte “ghosts” (which consist of only membranes, produced by disrupting the cells and washing out the hemoglobin) with proteolytic enzymes followed by disruption and extraction yields extensively fragmented X. What do these observations indicate about the location of X in the plasma membrane? Do the properties of X resemble those of an integral or peripheral membrane protein? 435 436 Part II Structure and Catalysis 12. Membrane Self-Sealing Cellular membranes are self-sealing—if they are punctured or disrupted mechanically, they quickly and automatically reseal. What properties of membranes are responsible for this important feature? 13. Lipid Melting Temperatures Membrane lipids in tissue samples obtained from different parts of the leg of a reindeer have different fatty acid compositions. Membrane lipids from tissue near the hooves contain a larger proportion of unsaturated fatty acids than those from tissue in the upper leg. What is the significance of this observation? 14. Flip-Flop Diffusion The inner face (monolayer) of the human erythrocyte membrane consists predominantly of phosphatidylethanolamine and phosphatidylserine. The outer face consists predominantly of phosphatidylcholine and sphingomyelin. Although the phospholipid components of the membrane can diffuse in the fluid bilayer, this sidedness is preserved at all times. How? 15. Membrane Permeability At pH 7, tryptophan crosses a lipid bilayer about 1,000 times more slowly than does the closely related substance indole: N H Suggest an explanation for this observation. 16. Water Flow through an Aquaporin Each human erythrocyte has about 2 105 AQP-1 monomers. If water flows through the plasma membrane at a rate of 5 108 H2O molecules per AQP-1 tetramer per second, and the volume of an erythrocyte is 5 1011 mL, how rapidly could an erythrocyte halve its volume as it encounters the high osmolarity (1 M) in the interstitial fluid of the renal medulla? 17. Labeling the Galactoside Transporter The galactoside transporter of a bacterium, which is highly specific for its substrate lactose, contains a Cys residue that is essential to its transport activity. Covalent reaction of N-ethylmaleimide (NEM) with this Cys residue irreversibly inactivates the transporter. A high concentration of lactose in the medium prevents inactivation by NEM, presumably by sterically protecting the Cys residue, which is in or near the lactose binding site. You know nothing else about the transporter protein. Suggest an experiment that might allow you to determine the Mr of the Cys-containing transporter polypeptide. 18. Predicting Membrane Protein Topology from Sequence You have cloned the gene for a human erythrocyte protein, which you suspect is a membrane protein. From the nucleotide sequence of the gene, you know the amino acid sequence. From this sequence alone, how would you evaluate the possibility that the protein is an integral protein? Suppose the protein proves to be an integral protein, either type I or type II. Suggest biochemical or chemical experiments that might allow you to determine which type it is. 19. Intestinal Uptake of Leucine You are studying the uptake of L-leucine by epithelial cells of the mouse intestine. Measurements of the rate of uptake of Lleucine and several of its analogs, with and without Na in the assay buffer, yield the following results. What can you conclude about the properties and mechanism of the leucine transporter? Would you expect L-leucine uptake to be inhibited by ouabain? Substrate L-Leucine D-Leucine L-Valine Uptake in presence of Na Uptake in absence of Na Vmax K t (mM) Vmax K t (mM) 420 310 225 0.24 4.7 0.31 23 5 19 0.24 4.7 0.31 20. Effect of an Ionophore on Active Transport Consider the leucine transporter described in Problem 19. Would Vmax and/or Kt change if you added a Na ionophore to the assay solution containing Na? Explain. 21. Surface Density of a Membrane Protein E. coli can be induced to make about 10,000 copies of the galactoside transporter (Mr 31,000) per cell. Assume that E. coli is a cylinder 1 mm in diameter and 2 mm long. What fraction of the plasma membrane surface is occupied by the galactoside transporter molecules? Explain how you arrived at this conclusion. Biochemistry on the Internet 22. Membrane Protein Topology The receptor for the hormone epinephrine in animal cells is an integral membrane protein (Mr 64,000) that is believed to have seven membrane-spanning regions. (a) Show that a protein of this size is capable of spanning the membrane seven times. (b) Given the amino acid sequence of this protein, how would you predict which regions of the protein form the membrane-spanning helices? (c) Go to the Protein Data Bank Web site. Use the PDB identifier 1DEP to retrieve the data page for a portion of the b-adrenergic receptor (one type of epinephrine receptor) from turkey. Using Chime to explore the structure, predict where this portion of the receptor is located: within the membrane or at the membrane surface. Explain. (d) Retrieve the data for a portion of another receptor, the acetylcholine receptor of neurons and myocytes, using the PDB identifier 1A11. As in (c), predict where this portion of the receptor is located and explain your answer. If you have not used the PDB or Chemscape Chime, you can find instructions at http://www.worthpublishers.com/lehninger.
© Copyright 2026