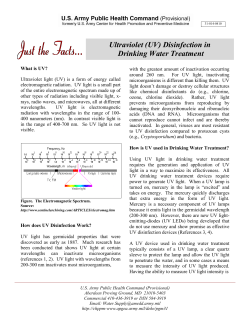
IMPROVING DISINFECTION EFFICIENCY IN SMALL DRINKING WATER TREATMENT PLANTS Water Research Commission
IMPROVING DISINFECTION EFFICIENCY IN SMALL DRINKING WATER TREATMENT PLANTS Report to the Water Research Commission by MNB Momba†, CL Obi♠ and P Thompson♣ † Department of Environmental, Water and Earth Sciences, Tshwane University of Technology, Arcadia Campus, P/Bag X680, Pretoria 0001 ♠ School of Agricultural and Life Sciences, University of South Africa, Sunnyside Campus, Pretoria, PO Box 392, UNISA 0003, South Africa ♣ Umgeni Water, PO Box 30800, Mayville 4058 WRC Report No 1531/1/08 ISBN 978-1-77005-683-1 Set No 978-1-77005-682-4 JULY 2008 This report forms part of a series of two reports. The other report is Guidelines for the Improved Disinfection of Small Water Treatment Plants (WRC Report TT 355/08). DISCLAIMER This report has been reviewed by the Water Research Commission (WRC) and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the WRC, nor does mention of trade names or commercial products constitute endorsement or recommendation for use ii EXECUTIVE SUMMARY 1. BACKGROUND OF THE STUDY AND PROBLEM STATEMENT In South Africa, water infrastructure is well developed in urban areas as opposed to rural areas where the infrastructure is either poorly developed or non-existent. Supply of water to rural communities is usually effected through small water treatment plants. Small water treatment plants (SWTPs) are defined as water treatment systems that are installed in areas which are not well serviced and which do not normally fall within the confines of urban areas. They include water supplies from boreholes and springs that are chlorinated, treatment plants of small municipalities and establishments such as rural hospitals, schools, clinics and forestry stations. However, the efficacy of drinking water treatment by small water treatment plants is fraught with several technical and management problems. This is corroborated by the extensive documentations on the supply of water of poor microbiological quality which is unsafe for human consumption in different provinces of South Africa. In order to unravel the intricacies around the operational and management parameters impinging on the disinfection efficiency of small water treatment plants and to ensure sustainability of potable water supply to rural communities, this study involved 181 small water treatment plants across seven provinces of South Africa. The goal was to determine the nature and full extent of the problems and provide practical and user-friendly guidelines for intervention. 2. PROJECT OBJECTIVES The objectives of the project were as follows: To identify and characterize the various types of disinfection equipment currently employed at small water treatment systems, as well as systems that could potentially be used. To identify the means of disinfection (i.e. the physical and/or chemical processes) employed at these systems, as well as the performance, chemical and electrical inputs, and ongoing maintenance requirements of each type. To identify or determine the current quality of treated water, procedures followed to monitor and control the disinfection processes and the adequacy and consistency of the levels of iii disinfectant added. To identify the frequency and adequacy of microbiological tests performed on the final treated water. To identify the main reasons for disinfection problems experienced at small water systems – both technical and non-technical. To estimate the cost of drinking water disinfection appropriate for the required standards in water sector using the required equipment, instrumentation and manpower. To identify major problems resulting in water quality changes in the distribution system by conducting a comprehensive study in the Fort Beaufort, Seymour and Alice distribution systems. To provide guidelines for the improved disinfection of final water at small water treatment plants which also include installation and operating costs for the different disinfection systems and chemicals. 3. METHODOLOGY In order to collect sufficient data on technical and management issues of small water treatment plants in South Africa, a detailed survey of 181 small water treatment plants across seven provinces was conducted. The seven provinces namely Limpopo, Mpumalanga, North-West, Free State, KwaZulu-Natal, Eastern Cape and Western Cape were selected on the basis of familiarity with the areas, economic status and rural areas experiencing technical and management problems. The geographic position system (GPS) co-ordinates of each site were logged during the survey to facilitate future incorporation into a geographical information system (GIS) that is being developed for the country. On-site visits of small water treatments plants in the designated provinces were conducted from June 2004 to December 2005. Wherever possible, some plants were visited at least twice during the study period. The methodology was two-pronged: use of a questionnaire and visual inspection, and determination of physicochemical and microbiological quality of drinking water. The questionnaire was used to gather information on the ownership and design capacity of each water treatment plant, the type of raw water sources and related characteristics, various methods of water treatment, the equipment currently employed, the performance of the plants, knowledge and skills of the operators as well as other technical and management issues. Physico-chemical and microbiological analyses of water samples collected from the raw water and final water at the points of treatment and consumption were performed using standard iv methods. Briefly, the chlorine residual concentrations and pH, the temperature, the turbidity and the conductivity of water samples were measured on-site using a multi-parameter ion specific meter (Hanna-BDH laboratory supplies), thermometer, microprocessor turbidity meter (Hanna instruments) and conductivity meter (Hanna instruments) respectively. Total and faecal coliforms were used to monitor bacteriological quality as stated in the South African National Standards (SANS 241:2005) and South African Water Quality Guidelines for Domestic Use (DWAF, 1996; Water Research Commission, 1998). To identify the major problems resulting in water quality changes in distribution systems, an on-site evaluation of the operating conditions at Fort Beaufort, Seymour and Alice water treatment plants in the Eastern Cape Province was conducted. This commenced with a reconnaissance visit to the plants and the network of smaller reservoirs in villages that were serviced by these plants. The relationship between the performance of the plant dosing systems and the water quality in the bulk distribution systems was established. To ensure effective free residual chlorine throughout the reticulation systems and the consumer’s taps, the chlorine dosages applied at the dosing points in each plant were assessed. The sampling regime for bacteriological quality of drinking water was designed to include points furthest to the satellite reservoirs servicing specific villages in order to provide a broad overview of the quality compliance of the water that got to the end users at these locations. The evaluation of these water treatment plants and their reticulation systems was performed from October to November 2005. 4. SUMMARY OF MAJOR FINDINGS AND CONCLUSIONS REACHED Small water treatment plant ownership – Four categories of the plant ownership were identified, viz Local/District Municipality, Department of Water Affairs and Forestry (DWAF), Department of Health (DOH) and Water Board (private company). Overall, 81% of the small water treatment plants surveyed in South Africa were owned by Local/District Municipality. Design capacity of small water treatment plants – The capacity of the plants surveyed during the investigation varied between 0.3 ML/d and 120 ML/d. Most of the plants were operating below the design capacity. Type of raw water sources – Overall 86% of the small water treatment plants surveyed abstracted their raw water from surface water, 10% used groundwater and 4% a combination of both water sources. v Water treatment practices – Conventional water treatment processes were generally used in the majority of the plants surveyed. In terms of coagulation, it was noted that polyelectrolyte (66%) was commonly used, followed by alum (18%) and ferric chloride (6%). Sixty percent of the small water treatment plants used rapid gravity filtration system while a further 24%, 9% and 2% of the plants made use of pressure filters, slow sand filtration and diatomaceous earth filters in that respective order. Chlorine gas was found to be the most popular disinfectant (69%), followed by sodium hypochlorite (15%) and calcium hypochlorite (HTH) (14%), among others. Physicochemical quality compliance – All water samples collected at various plants fell within SANS 241:2005 Class I in terms of pH (5 to 9.5) and conductivity (< 150 mS/m). Turbidity – At the point of treatment, 44% and 38% of the small water treatment plants surveyed in South Africa fell within SANS turbidity Class I (<1 NTU) and Class II (1-5 NTU), respectively. At the point of consumption, 46% and 41% of the plants fell within Class I and Class II, respectively. The highest turbidity compliance (Class I: 69-73%, Class II: 27 -31%) was noted in the Free State and the lowest turbidity compliance (Class I: 27-33%, Class II: 24-45%) was recorded in the Eastern Cape Province. Chlorine residual – Overall, the small water treatment plants surveyed had drinking water with free chlorine residual concentrations ranging between ≤ 0.1 and ≤ 0.5 mg/L. In most cases, the flow rate of the water and the initial chlorine dose were not known, resulting in under-chlorinated drinking water. During the on-site evaluation of the operating conditions at Fort Beaufort, Seymour and Alice water treatment plants, the following major problems impacted on the effectiveness of the disinfection process in the distributions: i) the distribution systems of the pipe network did not show acceptable levels of residual chlorine while the plant chlorination systems gave adequate dosage at the dosing points; ii) most of the chlorine dosed at the treatment plants was consumed by the floc sludge that accumulated in the reservoirs and the deposits that were present in the distribution networks . Microbiological compliance – For coliforms, 67% and 72% of the plants complied with the South African drinking water recommended limits for total coliforms and faecal coliforms at the point of treatment, respectively. The Eastern Cape Province produced the lowest drinking water quality in vi terms of both total (28% of the plants) and faecal (34% of the plants) coliforms while the Free State produced the best water quality (100% compliance). Control and monitoring – Generally, 50% of the operators and supervisors interviewed did not exude knowledge of the flow-rates at which their plants operated and more than 78% were unaware of the chemical doses used or how to correlate the required dose to the flow rate. In terms of instrumentation, only 46% of plants surveyed had the instruments to measure turbidity, pH and chlorine residual. Ninety–five percent of the plants reported that an external monitoring group visited the plants approximately once a month; however most plants complained about a lack of feedback. Non technical (management) aspects – Non technical issues affecting the efficiency of water supply by small water treatment plants included: inadequate training of manpower, poor maintenance practices, poor working conditions, insufficient financial capacity, poor recording, poor documentation and communication of data and information, as well as inadequate community involvement. Estimated cost of chlorination – The survey indicated that chlorine was commonly used in small water treatment plants. To improve the chlorination efficiency in water sector, this study suggests the following estimated costs for chlorine gas, sodium hypochlorite, and calcium hypochlorite, which are commonly used in rural small water treatment: Cost Comparison of Disinfection Alternatives Gas/liquid Sodium Calcium Chlorination Hypochlorite Hypochlorite Capital Cost R194 800 R317 455 R90 000 Direct Operating Cost c/kl 9.50 33.86 17.02 Maintenance c/kl 1.10 1.10 0.55 Total Operating Cost c/kl 13.77 34.79 36.01 For the improved disinfection of final water at small water treatment plants and distribution systems, a guide document was drawn up. It included practical steps and also installation and operating costs for the different disinfection systems and chemicals. This guide document is vii intended for use at operational and management levels by plant managers, supervisors, plant operators and plants owners, consultants and Municipal Water Local Authorities. 5. RECOMMENDATIONS Small water treatment plants should be equipped with a flow meter, jar stirrer, turbidity meter, pH meter and a chlorine meter for enhancement of disinfection efficiency. A programme for monitoring the physicochemical (at least pH, temperature, turbidity and free chlorine residual) and bacterial (coliform bacteria, especially faecal coliforms) quality of water at the point of treatment and various sites of the distribution systems should be established. Regular competency assessments and appropriate training programmes for water service providers and regulators should be conducted. The development and implementation of operational checklists and protocols are equally essential to ensure timely ordering of materials (particularly chemicals) and the maintenance of equipments. Increased funding of small water treatment plants and enhancement of working conditions of personnel are also recommended. 6. RECOMMENDATION FOR FUTURE STUDY A future investigation has to be conducted on the compliance of non-metropolitan South African potable water providers with the required management guidelines and norms including reasons for non-compliance. viii 7. LIST OF PRODUCTS 7.1 Book Momba MNB, Thompson P and Obi CL (2006). Practical and user-friendly guidelines for improving the efficiency of disinfection in small water treatment plants of South Africa 7.2 Article Momba MNB, Tyafa Z, Makala N, Brouckaert BM and Obi CL ( 2006 ) Safe drinking water still a dream in rural areas of South Africa. Case Study: The Eastern Cape Province. Water SA 32 (5): 715-720 Obi CL, Momba MNB, Samie A, Igumbor JO, Green E and Musie E (2007) Microbiological, physico-chemical and management parameters impinging on the efficiency of small water treatment plants in the Limpopo and Mpumalanga Provinces of South Africa. Water SA 33(2): 1-9 7.3 Conference Presentation Obi CL, Samie A, Green E, Musie E, Masebe T, Mashota M, Ndou S, Momba MNB, Thompson P, Charles K (2004) The efficiency of Disinfection of the Drinking Water in Small Water Treatment Plants in Limpopo and Mpumalanga Provinces of South Africa: Preliminary report. The 2nd International Conference on Safe Water, November 4-7, Johannesburg, South Africa. Z Tyafa, MNB Momba, N Makala, BM Brouckaert and CL Obi (2006) Safe drinking water still a dream in rural areas of South Africa - Case study: The Eastern Cape Province. The Water Institute of South Africa, Biennial conference and exhibition, Durban, May, South Africa. Obi CL, Momba MNB, Samie A, Green E, Musie E, Masebe T, Mashota M, Igumbor JO and Ndou S (2006) Water Quality Indicator Indices and Management Issues of Small Water Treatment Plants in Limpopo and Mpumalanga Provinces of South Africa. The International Water Association World Congress, China, September. ix ACKNOWLEDGEMENTS The following persons and organisations are thanked for their contribution to this report: Financial Support Water Research Commission Members of the Steering Committee Dr G Offringa Water Research Commission (Chairman) Prof D Key University of Western Cape Mr C Swartz Chris Swartz Engineer Mr K Charles CSIR Ms M du Preez CSIR Mr M Ramba Emanti Mr PL Chimloswa Amatola Water Project Team and Technical Support Prof M Momba Tshwane University of Technology (Project Leader) Mr P Thompson Umgeni Water (Project Team) Prof CL Obi University of South Africa (Project Team) Ms ZN Makala University of Fort Hare (Project Team) Ms Tyafa University of Fort Hare (Project Team) Mr K Charles CSIR (Project Team) Dr A Okoh University of Fort Hare (Technical Support) Dr BM Brouckaert University of KwaZulu-Natal (Technical Support) Mr C Mfenyana University of Fort Hare (Technical Support) Miss A Okeyo University of Fort Hare (Technical Support) Mr N Sibewu University of Fort Hare (Technical Support) Mr A Bosrotsi University of Fort Hare (Technique Support) Mr A Samie University of Venda (Technical Support) Mr E Green University of Venda (Technical Support) Mr E Musie University of Venda (Technical Support) Xolani Ngcemu Umgeni Water (Technical Support) x TABLE OF CONTENTS EXECUTIVE SUMMARY..............................................................................................................................iii ACKNOWLEDGEMENTS..............................................................................................................................x LIST OF TABLES..........................................................................................................................................xiv LIST OF FIGURES.........................................................................................................................................xv CHAPTER I: GENERAL INTRODUCTION……………..…………………………..…………………….1 CHAPTER II: LITERATURE REVIEW………………….………..……………………………………….4 2.1: BACKGROUND ON RURAL WATER SUPPLY AND SMALL WATER SYSTEMS IN SOUTH AFRICA ………………………………………………………………………………….4 2.2: GENERIC UNIT PROCESSES AND BARRIERS IN WATER TREATMENT ….……………5 2.2.1: Selection of water sources………………………………………………………………………5 2.2.2: Treatment processes …………………………………………………………………………..6 2.2.2.1: Treatment of surface water ……………………………………………………………….9 2.2.2.2: Treatment of groundwater ………………………………………………………………..9 2.2.2.3: Treatment of spring water ………………………………………………………………...10 2.3: DISINFECTION PRACTICES IN THE PRODUCTION OF POTABLE WATER ………………11 2.3.1: Chemical agents …………………………………………………………………………….11 2.3.1.1: Chlorine and chlorine based compounds 2.3.1.2: Ozone …………………………………………....12 …………………………………………………………………………………..17 2.3.1.3: Use of alternative chemical disinfectants…………………………………………………18 2.3.2: Physical agents (Ultraviolet irradiation)……………………………………………………….20 2.3.3: Physical processes……………………………………………………………………...………21 2.4 WATER DISTRIBUTION AND STORAGE……………………………………………………….23 2.5: MICROBIOLOGICAL QUALITY OF THE FINAL WATER & PUBLIC HEALTH SIGNIFICANCE ……………………………………………………………………………….….24 2.5.1: Monitoring the safety of water supplies ……………………………………………….…25 2.5.2: Quality at water works and in distribution systems…………………………………………….26 2.5.3: Impact of microbiological quality of treated water on public health…………………………...26 2.6: IMPROVING DISINFECTION EFFICIENCY IN SMALL WATER TREATMENT PLANTS.....28 2.6.1: In-service training of water personnel and management…………………………………..…...28 2.6.2: Hygiene education and community based management……………………………………..…29 CHAPTER III: SURVEY OF DISINFECTION EFFICIENCY OF SMALL DRINKING WATER TREATMENT PLANTS…………………………………………………………………………...31 3.1: SURVEY AREA…………………………………………………………………………………….31 3.2: SURVEY METHODOLOGY……………………………………………………………………….31 3.3: RESULTS OF THE SURVEY AND DISCUSSION………………………………………………..32 xi 3.3.1: Small water treatment plant ownership…………………………………………………………32 3.3.2: Design capacity of small water treatment plants………………………………………………..32 3.3.3: Type of raw water sources………………………………………………………………………34 3.3.4: Water treatment practices……………………………………………………………………….35 3.3.5: Quality of drinking water produced by small water treatment plants……………………….….38 3.3.5.1: Physicochemical compliance……...……………………………………………………….38 3.3.5.2: Microbiological compliance……………………………………………………………….42 3.3.6: Control and monitoring…………………………………………………………………………46 3.4: CONCLUSIONS AND RECOMMENDATIONS FROM THE SURVEY…………………………50 CHAPTER IV: WATER QUALITY CHANGES IN THE DISTRIBUTION SYSTEM…………………...52 4.1: METHODOLOGY………………………………………………………………………………… 52 4.1.1: Measurement of the flow of raw water and coagulant dose………………………………….....52 4.1.2: Physicochemical and Microbiological Analysis ………………………………………….…..53 4.2: RESULTS AND DISCUSSION…………………… …………………………………………..…53 4.2.1: Distribution networks……………………………………………………………………….…..53 4.2.2: Water Treatment Plant operation conditions……………………………………………….…...55 4.2.2.1: Fort Beaufort water treatment plant…………………………………………………….….55 4.2.2.2: Seymour Water Treatment Plant …………………………………………………….56 4.2.2.3: Alice Water Treatment Plant……………………………………………………………....57 4.2.3: Drinking water quality in the distribution systems……………………………………………..57 4.2.3.1: Turbidity compliance ……………………………………………………….…………...57 4.2.3.2: Free chlorine residual concentration and microbiological characteristics in the distribution systems………………………………………………………………………………..........59 4.3: CONCLUSIONS………………………………...……………………………………………..……63 4.4: RECOMMENDATIONS ……………………………………………………………….….…..63 CHAPTER V: MANAGEMENT ISSUES AFFECTING THE EFFICIENCY OF DISINFECTION IN SOUTH AFRICAN SMALL WATER TREATMENT PLANTS……………………………….…65 5.1: INTRODUCTION………………………………………………………………………….………..65 5.2: METHODOLOGY……………………………………………………………………….………….65 5.3: RESULTS AND DISCUSSION…………………………………………………………….…….....66 5.3.1: Poor Maintenance Practices…………………………………………………………….……….66 5.3.2: Training and Capacity Building…………………………………………………….…………...67 5.3.3: Poor Working Conditions……………………………………………………………………….68 5.3.4: Insufficient financial capacity…………………………………………………………………...68 5.3.5: Inadequate community involvement…….....................................................................................69 5.3.6: Streamlining Duties and Job Description……………………………………………………..…69 xii 5.3.7: Poor Recording, Documentation and Communication ……………………………………….....71 5.3.8: Emergency plans….......................................................................................................................71 5.4 :RECOMMENDATIONS………………………………………………………………………………..72 CHAPTER VI: GENERAL CONCLUSIONS AND RECOMMENDATIONS…………………………….82 REFERENCES……........................................................................................................................................84 APPENDIXES……………………………………………………………………………………………….89 xiii LIST OF TABLES Table 2.1 Water sources and potential health hazards ……………………………………….6 Table 2.2 Typical treatment steps in potable water production ……………………………….8 Table 2.3 Chloramine formation ………………………………………………………..…….16 Table 2.4 Membrane process classification Table 2.5 Impact of poor water quality on human health (2001-2005) ………………..…….27 Table 3.1 Example of a process control shift log sheet ………………………………..…….49 Table 3.2 Example of filter washing and clarifier solids management ………………..…….49 Table 3.3 Example of daily operator log………………………………………………..…….50 Table 5.1 Some non-technical issues impacting on quality of water services delivery in small ………………………………………..…….23 water treatment plants in south africa ………………………………………..…….67 Table 5.2 Calcium hypochlorite capital costs………………………………………… …..….73 Table 5.3 Calcium hypochlorite operating costs…………………………………………..….74 Table 5.4 Total operating costs for calcium hypochlorite dosing system…………….............74 Table 5.5 Design of typical sodium hypochlorite dosing system Table 5.6 Operating costs of typical sodium hypochlorite dosing system Table 5.7 Fixed capital and annual operating costs of sodium hypochlorite dosing system….77 Table 5.8 Gas chlorination operating costs Table 5.9 Gas chlorination system – capital cost ...........................................................79 Table 5.10 Total operating costs for gas chlorinator ...........................................................80 Table 5.11 Cost comparison of disinfection alternatives ...........................................................80 Table 5.12 Allocation of duties and responsibilities for personnel in water sector ...................................75 .......................76 .......................................................................78 xiv ...........81 LIST OF FIGURES Fig 2.1 Schematic of a Conventional Water Treatment Plant ……………………………………….7 Fig. 3.1 Category of Ownership of Small Treatment Plants surveyed in South Africa ……….34 Fig. 3.2 Types of Water Sources in Small Treatment Plants surveyed in South Africa ……….35 Fig. 3.3 Types of Coagulants used in Small Treatment Plants Surveyed in South Africa ……….37 Fig. 3.4 Types of Filters used in Small Treatment Plants surveyed in South Africa ………………..37 Fig. 3.5 Types of Disinfectants used in Small Treatment Plants in South Africa Fig. 3.6 Turbidity Compliance of Small Treatment Plants surveyed in South Africa at ………………..38 the Point of Treatment …………………………………………………………………….39 Fig. 3.7 Turbidity Compliance of Small Treatment Plants Surveyed in South Africa in Distribution system …………………………………………………………………….40 Fig. 3.8 Free Chlorine per Province at Point of Use and Point of Treatment ………………..41 Fig. 3.9 Free Chlorine Histogram for all Provinces at Point of Treatment ………………………...41 Fig. 3.10 Free Chlorine Histogram at Point of use across all Provinces Fig. 3.11 Bacteriological Compliance at the Point of Treatment …………………………………44 Fig. 3.12 Bacteriological Compliance in Distribution System …………………………………45 Fig. 4.1 Schematic Diagrams of Fort Beaufort Distribution Network………………………………54 Fig. 4.2 Schematic Diagrams of Alice Distribution Network Fig. 4.3 Fort Beaufort Turbidity Histogram Fig. 4.4 Histogram of Turbidity in Seymour Distribution System …………………………………58 Fig. 4.5 Histogram of Turbidity in the Alice Drinking Water Distribution System………………...58 Figs. 4.6-4.9 Histogram of the Free Chlorine Residual and Indicator Bacteria in the Fort Beaufort ………………………...42 ……………………………………55 …………………………………………………..58 Drinking Water Distribution System.....................................................................................60 Figs 4.10-4.13 Histogram of the Free Chlorine Residual and Indicator Bacteria in the Seymour Drinking Water Distribution System......................................................................................61 Figs 4.14-4.17 Histogram of the Free Chlorine Residual and Indicator Bacteria in the Alice Drinking Water Distribution System.....................................................................................62 Fig 5.1 Organogram of for Water Service management....................................................................70 xv xvi CHAPTER I GENERAL INTRODUCTION The main objective of water supply systems is to provide consumers with drinking water that is sufficiently free of microbial pathogens to prevent waterborne diseases. In addition to this requirement, water purification for domestic use must produce an aesthetically acceptable (in terms appearance, taste and odour) and chemically stable water (i.e. it must not case corrosion or form deposit in pipes or fixtures such as geysers). The key to produce water of such desired quality is to implement multiple barriers, which control microbiological pathogens, and chemical contaminants that may enter the water supply system. This includes adopting sound management practices and continuously reviewing both the state of the water treatment and the distribution infrastructure, and the quality of the water produced. Microbial pathogens which include bacteria, viruses and protozoan parasites can be physically removed as particles in many individual water treatment processes such as coagulation, flocculation, sedimentation and filtration (also called unit processes and unit operations) or inactivated in disinfection processes. Application of a disinfection barrier is a critical component of primary treatment of drinking water (LeChevallier, 1998). Disinfection is important because the turbidity removal by sedimentation and filtration does not remove all microbial pathogens from water. The disinfectant residual in the drinking water distribution system is also one of the key factors controlling the microbial quality of water, preventing bacterial proliferation in the water phase (regrowth) and limiting viability of bacteria released from pipe wall biofilms (Momba and Makala, 2004). The practice of disinfection of water supplies has been, in general, used since the beginning of the century and has given rise to substantial reduction in the occurrence of water-related diseases. The most commonly used technology to achieve disinfection has been chlorination. This method of disinfection has been proved to be reliable, appropriate and effective worldwide (Solsona and Pearson, 1995). In South Africa, the larger cities that are supplied with water from waterworks that are managed by Water Boards and Metropolitan Councils generally have high quality potable water (Nevondo and Cloete, 1999). Although, chlorination is commonly used in the majority of South African rural water treatment plants, recent studies have shown that these plants do not produce the quality or quantity of drinking water that they were designed to produce (MacKintosh and Colvin, 2002; Momba et al., 2004a; 2004b). Small water systems have difficulty in complying with the ever-expanding number of regulations or applying the best available technology due to poor 1 financial management, inadequate capital funding and limited technical capacity (Swartz, 2000; Momba et al., 2005). There are three major inter-related challenges facing small water systems that need to be addressed: i) financing, ii) lower-cost technology and iii) sustainability. For example, a water treatment technology is appropriate if it has a relatively lower capital or operation and maintenance cost, it is simpler to operate, it is convenient to monitor and produces fewer disinfection byproducts. The concept of sustainability is important because it focuses on the underlying causes of most problems experienced by small water systems. A major issue that has not been resolved for small systems is the inability to develop and evaluate low-cost treatment technologies. Complex technologies that require a higher level of operation, education and supervision cannot be easily applied to small systems. In addition, it has been noticed that the small water systems problems that are encountered in developing countries are also dominant even in developed countries (USEPA, 1998). In 2000, The Center for Disease Control (CDC) in the United States reported that there were 39 waterborne disease outbreaks; 2.068 illnesses; 122 hospitalizations and 2 deaths. Eighteen (46%) of the 39 outbreaks were linked to small water systems. Providing safe drinking water can immediately and dramatically improve the health of many communities and can also lead to the elimination of diseases. While municipal water treatment has eliminated threats from many waterborne illnesses of the past, such as cholera and typhoid fever, outbreaks of waterborne diseases still occur. New pathogens, some of which are resistant to the conventional treatment processes, continue to emerge (USEPA, 1998). Even with optimum treatment, contamination can also occur in the water distribution system, leaving the consumer vulnerable. Attempts at providing safe and adequate quantities of water to the developing regions of the world must thus be properly integrated with other aspects of development such as sanitation and education. PROJECT OBJECTIVES The project aimed at providing guidelines for the improved disinfection of final water at small water treatment plants which also include installation and operating costs for the different disinfection systems and chemicals. To achieve this goal, the following objectives were pursued: i) To identify and characterize the various types of disinfection equipment currently employed at small water treatment systems, as well as systems that could potentially be used. ii) To identify the means of disinfection (i.e. the physical and/or chemical processes) 2 employed at these systems, as well as the performance, chemical and electrical inputs, and ongoing maintenance requirements of each type. iii) To identify or determine the current quality of treated water, procedures followed to monitor and control the disinfection processes and the adequacy and consistency of the levels of disinfectants added. To identify the frequency and adequacy of microbiological tests performed on the final treated water. iv) To identify the main reasons for disinfection problems experienced at small water systems – both technical and non-technical. v) To estimate the cost of drinking water disinfection appropriate for the required standards in water sector using the required equipment, instrumentation and manpower. vi) To identify major problems resulting in water quality changes in the distribution system by conducting a comprehensive study in Fort Beaufort, Seymour and Alice distribution systems. 3 CHAPTER II LITERATURE REVIEW In developing countries, several disease outbreaks are associated with the use of untreated surface water, contaminated well water, treatment plant deficiencies and contaminated distribution systems. For the purpose of this study, the first section of this chapter provides information on rural water supply and water treatment systems, the second section briefly discusses various unit processes and multiple barriers used in the treatment of each type of water source. The third section, which is the most important in terms of this investigation, discusses in detail various disinfection practices used worldwide with emphasis on developing countries and small rural systems. This is followed by an overview of the process of drinking water distribution and storage (fourth section). The fifth section deals with the general quality of treated water at the plant and in the distribution system, which impacts on the health of consumers. Practical strategies, which can lead to improving the disinfection efficiency in small water treatment plants, are discussed in the sixth section. 2.1 BACKGROUND ON RURAL WATER SUPPLY AND SMALL WATER SYSTEMS IN SOUTH AFRICA Generally, water infrastructure in South Africa is well developed in urban areas and the majority of the urban population utilizes potable water. In rural communities, water infrastructure is either poorly developed or non existent and the majority of the populace depend on water sources such as rivers and ponds for their drinking water. These water sources are usually not treated, faecally contaminated and unsafe for human consumption (Muyima and Ngcakani, 1998; Obi et al., 2002; 2003 a, b; Momba and Kaleni, 2002; Momba and Notshe, 2003; Momba et al., 2005a; Momba et al., 2006). Contaminated water sources are vehicles for the transmission of waterborne diseases such as cholera, shigellosis and Campylobacteriosis (Ashbolt, 2004; Momba et al., 2006). The World Health Organization (WHO) estimated that about 1.1 billion people globally drink unsafe water and the vast majority of diarrhoeal diseases in the world (88%) are attributable to unsafe water, sanitation and hygiene. Approximately 3.1% of annual deaths (1.7 million) and 3.7% of the annual health burden (disability adjusted life years [DALYs]) world-wide (54.2 million) are attributable to unsafe water, sanitation and hygiene (WHO 2003). In order to prevent waterborne diseases, water 4 is treated to eliminate pathogens. In rural and peri-urban areas, water sources are usually treated in units called Small Water Treatment Plants (SWTPs). Small water treatment plants are defined as water treatment systems that are installed in areas, which are not well serviced, and which do not normally fall within the confines of urban areas. They are therefore mostly plants in rural and peri-urban areas and include water supplies from boreholes and springs that are chlorinated, small treatment systems for rural communities, treatment plants of small municipalities and treatment plants for establishments such as rural hospitals, schools, clinics and forestry stations. Most of these applications fall within the category of small plants of less than 2.5 Ml/d, although larger capacity plants operating in poorly serviced areas also fall into this category for purposes of this study. The following section briefly discusses the unit processes and multiple barrier strategy. 2.2 GENERIC UNIT PROCESSES AND BARRIERS IN WATER TREATMENT The primary purpose of water treatment is to render the water fit for human consumption. This requires the improvement of microbiological quality and the control of dangerous chemical substances and metals. Secondary purposes include the protection of distribution and plumbing systems and the maintenance of aesthetic quality (e.g. taste, odour, colour and hardness) (WHO, 1982). Meeting the goal of clean, safe drinking water requires a multi-barrier approach that includes: protection of source water from contamination, appropriately treating raw water and ensuring safe distribution of treated water to consumer taps. The treatment requirement for potable water supply in rural areas will therefore depend on water quality required and the quantity, and variation in quality of the source. 2.2.1 Selection of water sources Rational selection requires a review of the alternative sources available and their respective characteristics. Factors to consider when selecting a water supply source include: i) safe yield, ii) water quality, iii) collection requirements, iv) treatment requirements, v) transmission and distribution requirements. The ability of water sources to provide both quality and quantity requirements must be considered. Although the quality of water is variable from source to source, surface waters have qualities in common. Likewise, groundwater supplies have many similar characteristics. Table 2.1 shows the range of water sources together with the ingredients that may be present in each. 5 TABLE 2.1 WATER SOURCES AND POTENTIAL HEALTH HAZARDS Source Potential health hazards Deep groundwater (boreholes) Fe, Mn, Colour, H2S, NO3, NH4, CO2 (pH) Shallow groundwater Fe, microorganisms, NO3, NH4 Infiltration water Fe, colour, organic matter, taste Spring waters Fe, CO2 (pH) Rainwater (cisterns) Microorganisms, constituents of atmospheric pollution, pH Surface water (streams, rivers, lakes and Suspended solids, microorganisms, colour, algae, taste, reservoirs) odours, organic matter, NO3, NH3 Source (WHO, 1982) In a water scarce country such as South Africa, the selection of water sources may pose a problem. Nevertheless, groundwater sources, which remain the main water supply for many rural small communities, must be considered as a first priority due to the fact that many small villages are geographically scattered. Although the use of groundwater source supply will reduce the cost of water treatment, the removal of nitrate and the disinfection process must be taken into account. When small streams, open ponds, lakes, or open reservoirs must be used as sources of surface water supply, the danger of contamination and of consequent spread of enteric diseases such as typhoid fever and dysentery are increased. As a rule, surface water should be used only when groundwater sources are not available or are inadequate. The physical and bacteriological contamination of surface water makes it necessary to regard such sources of water supply as unsafe for domestic use unless reliable treatment, including filtration and disinfection, is provided (Anon, 1995). The treatment of surface water to ensure a constant, safe supply requires diligent attention to operation and maintenance by the owner of the system. 2.2.2 Treatment processes The need for reduction of microbial and chemical ingredients will depend on their initial concentrations and the target quality. It must be recognized, however, that a treatment process that may be appropriate for use under certain conditions may not be necessary appropriate elsewhere. It will depend on the quality and availability of operator skills and on the availability of other resources such as materials and electricity (WHO, 1982). Treatment practices vary from system to system but there are five generally accepted basic techniques: coagulation, flocculation, sedimentation, filtration and disinfection. After treatment, the drinking water flows into the 6 distribution system. These typical treatment steps are summarized in Fig. 2.1 and Table 2.2 describes the purpose of each step. The incidence for waterborne disease associated with protozoan parasites (Giardia or Cryptosporidium) and the resistance of some pathogens to conventional disinfection presents a challenge to the water industry. Because of its resistance to chlorination and its small size, making it difficult to be removed by filtration, Cryptosporidium represents a unique challenge to drinking water industry. Use of a single barrier such as disinfection alone, or operation of a conventional treatment plant that had not been optimized has contributed to several disease outbreaks (USEPA, 1998). A multiple barrier approach (which involves a number of physical and chemical removal processes) is recommended for the optimal removal of Giardia or Cryptosporidium. Chemical Addition and Flash Mixing Abstraction Raw Water Storage Tank coagulant pH adjustment Flocculation Flow Splitting F1 pre-oxidation recycle stream F4 Settling Tanks sludge Sludge Settling Pond backwash water Filters On-site Finished Water Reservoir F3 F2 post-disinfectant Fig. 2.1 Schematic of a conventional water treatment plant (Momba and Brouckaert, 2005) Disinfection methods that involve chemical and physical processes such as gravity separation (sedimentation and flotation) and filtration treatment barriers for the removal of pathogens and especially protozoa will be discussed in section 2.3 of this chapter. 7 TABLE 2.2 TYPICAL TREATMENT STEPS IN POTABLE WATER PRODUCTION Step Pre- Description Purpose Addition of chlorine to the raw water. chlorination Remove of colour, iron and/or manganese. Prevent biofilm growth in channels, settling tanks and filters. pH Addition of chemicals such as lime, soda ash Adjust the pH to fall in a required range for adjustment/ or carbon dioxide which change the pH. good floc formation and/or to prevent stabilisation corrosion or excessive scaling in the distribution system. Coagulation Flocculation Settling Addition and flash mixing of coagulants (also Add chemicals which produce floc. Floc called flocculants) such as alum and/or contains many of the contaminants present polymer solutions to raw water. in the original raw water. Formation of floc in channels or pipes between Form flocs which are easily removed in the coagulant addition and the settling tanks. settling tanks. Floc sinks to the bottom of the settling tank Removal of floc formed in coagulation and while settled water flows over the top into the flocculation steps. settled water channels. Filtration Water is filtered through a granular media Removal of floc or particles not removed in (sand and/or anthracite). the settling tanks. Disinfection/ Addition of chlorine to the filtered water or Kill off any microbes in the filter water and post- Final water storage reservoir. provide chlorine residual to prevent later re- chlorination infection. Finished After disinfection, the treated water flows to a Allow sufficient time for the chlorine to act water storage reservoir on or near the plant. and ensure an adequate supply of water storage during periods of high demand or disruptions to the operation of the plant. Sludge Dirty backwash and or sludge from the settling Reduces water losses on the plant and settling tanks is held in settling ponds where the sludge avoids and settles to the bottom of the ponds and the backwash water to either natural water washwater supernatant is recycled to the top of the plant. bodies (which is illegal) or to the sewer recovery discharging sludge (which requires a permit). Source: Momba and Brouckaert, 2005 8 and spent 2.2.2.1 Treatment of surface water Surface water can be divided into rivers, streams, lakes, small dams and ponds. These water sources are subjected to frequent dramatic changes in microbial quality as a result of a variety of activities on a watershed. Consequently any surface water source must be either well protected or treated before it can be used for drinking. For small communities, it is generally preferable to protect groundwater source that requires little or no treatment than to treat surface water that has been exposed to faecal contamination. In many circumstances, however, surface water is the only practicable source of supply and requires affordable treatment and disinfection (WHO, 1997). Surface water may contain organic and mineral particulate matter that could harbour protozoan parasites such as Cryptosporidium parvum and Gardia lambia (Chlorine Chemistry Council, 2003). The aim of surface water treatment is to achieve the required standard of final water quality regardless of the quality of the intake source water. However, the extent of water treatment for domestic use depends on the source water quality. Some sources such as a river require more extensive treatment. Consequently the treatment will involve two types of processes: physical removal of solids (mainly minerals and organic particulate matter) and chemical disinfection (killing or inactivating microorganisms). 2.2.2.2 Treatment of groundwater Groundwater is the water that is found in cracks and spaces in soil, sand and rocks. The area where the water fills these spaces is called a saturated zone. It can be found almost everywhere. Groundwater consists of wells and boreholes. Wells are suitable when they are located near the surface aquifers and weathered materials and where the population is widely dispersed. Most wells need an inner lining, which provides protection against caving and helps to prevent polluted water from seeping in from the surface. Boreholes are suited for low volume water supplies (Momba, 1997; Ndaliso, 2000). Groundwater is one of the primary sources of potable water in developing areas. In South Africa, more than 280 towns and villages derive their water supply from groundwater. Groundwater therefore accounts for 15% of water supply (Momba, 1997). Groundwater is usually bacteriologically safe unless a contaminant source exists nearby. Contaminants such as organic and inorganic particles collected during its flowpath are removed or degraded by filtration, oxidation, adsorption, cation exchange and dilution. Frequently encountered problems include excessive fluoride, iron, manganese, hardness and salts. The most common treatment is oxidation by aeration followed by sedimentation and chlorination, or if iron content is high, by aeration followed by sedimentation and rapid sand filtration. Slow sand filtration 9 is also effective at treating groundwater with iron and sulphate. Manganese removal may be improved by chlorination prior to filtration. The removal of Nitrate and nitrite should be considered together because conversion from one form to the other occurs in the environment. It is of primary health concern as nitrite causes methanoglobinaemia, which is particularly dangerous to infants under the age of 3 years. The maximum recommended value for South Africa is a total nitrite and nitrate of 6 mg/l, expressed as nitrogen (DWAF, 1996). However, some dissolved minerals may not be removed, and chemical reactions related to the geologic nature of the aquifers may also be a problem. The character of groundwater changes only slowly with time so that terminating the cause of contamination will not restore its quality for an indefinite period. Gravity springs and deep groundwater sources may be quite safe without disinfection if the source can be protected against contamination. This needs to be affirmed during sanitary survey and by chemical analysis of the water. In rural areas of South Africa, recent studies have linked some water outbreaks with the microbiological quality of groundwater, necessitating the need for disinfection (Momba and Kaleni, 2002; Momba et al., 2006). Groundwater with disinfection as the only treatment to eliminate bacteriological contamination is one of the most common types of water supply for rural communities. Calcium hypochlorite or bleaching powder is the most appropriate agent, sodium hypochlorite or liquid chlorine is also used. A free chlorine residual of 0.3 mg/l after 30 minutes standing time is adequate where turbidity and p+ are low. 2.2.2.3 Treatment of spring water Springs are commonly found in rolling topography which is incised with water courses, and they generally occur at the heads of the drainage network. Springs may appear as: seepage springs where water percolates over a wide area in porous ground, fracture springs issuing from joints or fractures, or tubular springs where the outflow is more or less like a pipe (Sami and Murray, 1998). The water from a well-protected spring should all originate from groundwater. Often this will be safe and will not need any disinfection other than what may be desirable to protect it in a distribution system. Once the spring water has been channeled into a pipe, a number of chlorination systems may be appropriate. These will be similar to those used for treating surface water for piped systems. The discharge of springs may be fairly constant although there may be some gradual seasonal variations that may require periodic changes in the dosing rate. If the discharge changes rapidly after rain it may indicate a risk that the spring is being polluted by surface water. 10 2.3 DISINFECTION PRACTICES IN THE PRODUCTION OF POTABLE WATER Disinfection is the process by which disease-causing pathogens are destroyed. Disinfection also provides additional protection for any contamination that may occur in the distribution system but this is dependent on the concentration of residual disinfectant that remains in the system once the water leaves the treatment plant. The importance of disinfecting potable water cannot be underestimated. The true value of disinfection first became evident as early as 1893 when Mills and Reincke, both public health researchers, after studying a large number of communities, discovered that when a contaminated water supply was replaced with a purified water source, the general health of the community improved significantly, far beyond what would be expected by accounting for the reduced incidence of typhoid and other typical waterborne diseases. In 1903, Allen Hazen, found that when a community water supply of bad quality was replaced with adequately treated water there was a reduction in morbidity and mortality due to water borne diseases (White, 1999). In general microorganisms can be removed, inhibited or killed using either a chemical disinfection process (e.g. chlorine), a physical disinfection process (e.g. ultra violet radiation) or a physical removal process (e.g. slow sand filtration). Although the first two methods are commonly known as disinfection processes, this section also focuses on physical removal processes. 2.3.1 Chemical agents The most commonly used chemical agents in the water industry are: i) chlorine and chlorine based compounds which include chlorine gas (Cl2), calcium hypochlorite [Ca(OCl)2], sodium hypochlorite (NaOCl), chlorine dioxide (ClO2), and mono-chloramines (NH2Cl); ii) ozone (O3), iii) hydrogen peroxide (H2O2), iv) potassium permanganate (KMnO4), v) iodine (I2) and vi) bromine (Br2). The commonly used chemical disinfectants in large water works are chlorine gas, chlorine dioxide, monochloramine and ozone. In small water works chlorine gas, hypochlorites, iodine, bromine and mixed oxidant gases are typically used as disinfection agents (Van Duuren, 1997). Chlorine disinfection has been practiced for over a century and has been credited with saving a significant number of lives worldwide on a daily basis. Although chlorine and chlorine compounds have been historically the most popular chemical disinfection agents, the special properties of ozone have caused a rapid increase in its use worldwide (White, 1992). The discussion in this section will be limited to chemical disinfectants most commonly used in South African small water treatment plants: chlorine gas, sodium hypochlorite (supplied as a liquid or generated on site by electrolysis of a salt solution), calcium hypochlorite (usually supplied as granular and commonly 11 known as HTH), chlorine dioxide, chloramines and ozone. A brief review of alternative chemical disinfectants is also discussed in this section. 2.3.1.1 Chlorine and chlorine based compounds Chlorine is one of the most effective disinfectants, it is relatively easy to handle, and the capital cost of chlorine installation is low. It is cost effective, simple to dose, measure and to control, and it has a relatively good residual effect. Moreover, it reduces many objectionable taste and odour compounds (chlorine oxidizes many naturally occurring substances such as foul-smelling algae secretions, sulfides and odours from decaying vegetation), removes chemical compounds that have unpleasant taste (ammonia and other nitrogenous compounds) and odour (hydrogen sulfide which has a rotten egg odour) and hinder disinfection (ammonia and other nitrogenous compounds). Although, there are certainly other disinfectants (such as monochloramine, ozone and ultraviolet) that are equal to or even better than chlorine (this will be discussed later), chlorine is the most widely used disinfectant for drinking water in rural developing areas. Chlorine disinfection is generally carried out using one of the three forms of chlorine (elemental chlorine (chlorine gas), sodium hypochlorite solution (bleach), and dry calcium hypochlorite - HTH) or it can be generated on site. The form of chlorine added to water will depend on the locally available chemicals and equipment. Chlorinating water requires that the operator be trained in making up the correct solution strengths. He must also ensure that sufficient chemicals are always available. On a cost per mass of active chlorine basis, chlorine in the form of a liquefied gas is the most cost effective option. Using liquefied gas carries the risk of accidental leakage of the gas, which is why some plants opt for more expensive sodium hypochlorite solution. On-site generated hypochlorite is well-suited to remote areas close to a cheap source of brine; however an electrical supply will be required. When chlorine gas is added to water, two species known together as free chlorine are formed. These species, hypochlorous acid (HOCl, electrically neutral) and hypochlorite ion (OCl-, electrically negative), behave very differently. Cl 2(g) H 2 O HOCl H Cl Hypochlorous acid dissociates (splits up) to form hydrogen and hydrochlorite ions (OCl-) HOCl ¾ H OCl All of the disinfectant capability of the chlorine gas resides with either the undissociated HOCl or the OCl- ion – the chloride ion (Cl-) has no ability to kill microbes at the concentrations which occur 12 in drinking water. If either sodium or calcium hypochlorite is used as the source of chlorine, each will yield OCl- upon dissociation in water. Hypochlorous acid is not only more reactive than the hypochlorite ion, but it is also a stronger disinfectant and oxidant. The ratio of hypochlorous acid to hypochlorite ion in water is determined by the pH. At low pH (higher acidity), hypochlorous acid dominates while at high pH hypochlorite ion dominates. Thus, the speed and efficacy of chlorine disinfection against pathogens may be affected by the pH of the water being treated (Chlorine Chemistry Council, 2003). Fortunately, bacteria and viruses are relatively easy targets for chlorination over a wide pH range. Furthermore, operators of surface water systems treating raw water contaminated by parasitic protozoa (e.g. Giardia) may take advantage of the pH-hypochlorous acid relationship and lower the pH to be more effective against Giardia, which is much more resistant to chlorination than either viruses or bacteria (Chlorine Chemistry Council, 2003). Within the permissible pH range of 6.5-9.5, the efficacy of chlorine decreases as the pH increases. Hypochlorous acid is generally considered to be a destructive, non-selective oxidant, which reacts with all biological molecules. One of the major disadvantages of chlorine is the relatively short half life of hypochlorous acid in water which effectively reduces the residual contact time to 18-24 hours. This has led the industry to rely on the use of chloramines to increase the lifespan of the combined chlorine residual from 24 hours to 3-7 days. Although chlorine is the most common disinfectant used for the treatment of drinking water in South African rural areas, existing chlorine disinfection practices are unreliable and often not monitored in many of the water treatment plants and small water supply schemes (Pearson and Idema, 1998; Momba et al., 2004b). Studies (Swartz, 2000; Mackintosh and Colvin, 2002; Momba and Kaleni, 2002a; Momba et al., 2004a) have shown that the majority of small water works in South Africa have difficulty providing adequate treatment and disinfection with the result that consumers are at risk of waterborne diseases even from treated water supplies. Both technical and human factors have been reported to be the major causes of failure of small rural water treatment plants to provide potable water to their consumers (Momba et al., 2004b). According to these authors, chlorine dosing and the delivery of chlorine to the plant remain a major on-going problem in most of rural water works. A lack of a proper chlorine dosing procedure and monitoring programme results in insufficient chlorine residuals at the point of treatment. However to have an effective disinfection, the chlorine dose has to be ratioed to the plant flow rate. In other words, before applying chlorine to the water, it is important to know the chlorine demand of the water. A number of countries including South Africa have issued guidance to water suppliers on disinfectant residual that should be aimed for in distribution. These countries indicate that the 13 residuals should be kept to a minimum consistent with ensuring that microbiological standards are met and some countries also include to minimize by-products formation, to minimize biofilm formation and to avoid problems with chlorine tastes and odours and to provide some protection against re-contamination within the distribution system (Hydes, 1999). Chlorine demand measurement in small water treatment plants in rural areas is seldom undertaken by operators. Operators generally use a fixed chlorine dose irrespective of changes in chlorine demand often leading to overdosing or under-dosing of the chlorine. It has been observed that the chlorine decay is influenced by the chlorine demand of the water and the reactions with deposits such as organic and inorganic sediments. It is therefore important for operators to understand and compensate for the way disinfectant decreases in the distribution system. Although chlorine is used to reduce bacterial numbers, the mere use of chlorine does not guarantee the removal microbiological pathogens; it is essential to apply the correct dose at the correct frequency (Momba and Brouckaert, 2005). Moreover, any point of chlorine application must be such as to give adequate time of contact for the chlorine with the water before it leaves the water works. Although the minimum contact time required is determined by the dosage of chlorine applied, it is desirable to make provision for a contact time of not less than an hour (Momba and Brouckaert, 2005). A number of different ways of dosing exist. The following important points must be taken into consideration in designing and controlling chlorine dosing systems: uninterrupted dosing, uniform distribution to all parts of the water mass, adjustment of the dosage to the chlorine demand of the water being treated and control of the dosage to produce safe water without spoiling the taste (Swartz, 2000). Chlorine gas – Chlorine gas is commonly used on large and medium scale plants. It is supplied as a liquid in pressurized gas cylinders and is evaporated prior to injection into the water being treated. Due to the hazardous nature of the gas, appropriate safety measures are required (Voortman and Reddy, 1997). Therefore gas chlorinators should not be operated and controlled by unskilled persons who are not fully conversant with the apparatus or dangers of the gas. Sodium hypochlorite – Sodium hypochlorite is used in many small water treatment plants and is fed into the system by means of constant head drip feeders or dosing pumps. The constant head drip feeders have the advantage that they do not require electrical power but one disadvantage is that the solution can not be introduced into the system under pressure. Storage conditions are important when using hypochlorite solutions as it decomposes on exposure to heat, light and impurities. The stock solution should not be stored for more than 1 month, even when sealed and stored under dark 14 and cool conditions. The diluted dosing solution should be prepared in quantities sufficient for 1 to 3 days of operation (Sami and Murray, 1998). Calcium hypochlorite – Calcium hypochlorite in the form of a dry powder or proprietary tablet- type dispenser can be used in small water treatment plants. This is more expensive than gaseous chlorine or hypochlorite solution, but can offer advantages in terms of convenience and low installation cost. Dosing of the dry granules or powder is not recommended since calcium hypochlorite is hygroscopic and decomposes in air to form chlorine gas. It can be dosed in the same way as sodium hypochlorite by dissolving the granules to create solution of appropriate strength. Granular calcium hypochlorite contains up to 70% chlorine and is more stable, losing only 3-5% of its chlorine per year, so its dosage is easily regulated (Sami and Murray, 1998). A number of proprietary in-line and free floating dispensers are available for industrial and swimming pool chlorination. These devices generally make use of tablets containing mixture of calcium hypochlorite and calcium carbonate and they function as saturators from which the solution is gradually released into the line (in-line-dispensers) or surrounding water (free floating dispensers) (Voortman and Reddy, 1997). Chloramine – The use of chloramines as a disinfectant is not a new process and has been used for decades in many cities of developed countries. It has been reported that 20 to 25% of the local governments within the United States currently use chloramines and the number is expected to grow due to new regulations (USEPA, 1999). Although chloramines have been used as a disinfectant in drinking water for many years, their use has not been widespread or highly publicized until recently. The process of chloramination has usually been referred to as combined residual chlorination or the ammonia-chorine process (combination of free chlorine and ammonia). The process is based upon the reaction of hypochlorous acid with ammonia. There are three types of chloramine that can be formed by the reaction of chlorine with ammonia depending on the ratio of free chlorine and ammonia: monochloramine, dichloramine and trichloramine as shown in Table 2.3. Dichloramine and trichloramine are less stable than monochloramine and are present only at low p+ values with high chlorine -to- oxygen ratios. At p+ levels above 8, monochloramine is the only chloramine of any consequence. 15 TABLE 2.3 CHLORAMINE FORMATION Chlorine/Ammonia Reaction Chloramine species ratio 5:1 NH3 + HOCl =H2O + NH2Cl Monochloramine 7:1 NH2 + HOCl =H2O + NHCl2 Dichloramine 9:1 NHCl2 + HOCl =H20 + NCl3 Trichloramine Chloramines are more commonly used in systems with long retention times as a secondary disinfectant, especially in high chlorine demand waters where they can provide bactericidal protection, control of algae, and bacterial after-growth in lieu of free chlorine which would be exhausted in the extremities of the distribution systems. Advantages of chloramination include the following: i) Chloramines do not react as readily with organic matter in the treated water supplies thereby dramatically reducing the potential formation of disinfection by-products (DBPs), such as trihalomethanes (THMs) and halo-acetic acids (HAAs) (USEPA, 1999); ii) They are less corrosive (on materials) and have less noticeable taste and odour than free chlorine (USEPA, 1999). iii) Although chloramine treatment may not be effective in all systems, the primary aim of many utilities in using chloramine as a primary or secondary disinfectant is to reduce the formation of THMs (Solsona and Pearson, 1995). However, chloramines are less effective bactericides and virucides than either hypochlorous acid or hypochlorite ion. Neither enteric viruses nor protozoan cysts are effectively inactivated by chloramines over a short period of time (10 min) at low dosages. Chloramines when improperly applied can lead to nitrification problems in distribution systems (American Water Works Association, 1982). The cost of using chloramines for disinfection is about the same as the cost for chlorine. The use of chloramines is 20% less expensive than alternative methods of treatment. Monochloramine for water disinfection is a safe and proven method when used as a secondary disinfectant. It provides adequate disinfectant residual that will enable compliance with bacteriological water quality standards (Momba and Binda, 2002b; Momba et al., 2003). 16 Chlorine dioxide – Chlorine dioxide is a disinfectant now being used fairly extensively throughout the world, but particularly in Europe and United States (Solsona and Pearson, 1995). It has been used in South Africa for the disinfection of mine service water where ammonia levels are high and for drinking water treatment where algal problems occur. It has also been used experimentally where iron and manganese problems occur. Its major advantages over the use of chlorine include the following: i) generally more powerful bactericide, sporicide and virucide; ii) does no react with ammonia or aromatic organics is capable of destroying certain precursors of THMS; iii) in general produces less taste and odours, and more effectively oxidises organic tastes and odours; iv) the residual in the distribution system is better maintained than with free chlorine; v) chlorine dioxide is not as affected by variations in p+ as is chlorine and vi) it is more effective for the removal of iron and manganese. Chlorine dioxide is an unstable gas and must be generated on site. It can be produced from sodium chlorite in combination with chlorine and / or a strong acid (HCl or H2SO4). The production process must be carefully monitored and controlled to produce high levels of chlorine dioxide. Hence, although used extensively in other countries, chlorine dioxide does present a number of limitations for its use in small water systems and these include the following: i) high cost of precursor (NaCl2); ii) sensitive to light and hence should not be used where water is contained in open tanks; iii) the by-products of chlorine dioxide disinfection include chlorite and chlorate which may have health implications for consumers. 2.3.1.2 Ozone Ozone is a naturally occurring form of activated oxygen produced during lightning storm discharges and is continuously occurring in the stratosphere by ultraviolet action. Ozone can also be artificially produced by the action of high voltage discharge in the presence of air or pure oxygen (O2). Due to the fact that the gas breaks down rapidly, users must generate the gas on site. Ozone is a powerful oxidant and disinfectant, with thermodynamic oxidation potential that is the highest of all common oxidants. High oxidation potential of ozone increases its reactivity with other elements and compounds and the better high kill rates of fungus, bacteria, parasites (i.e. Giardia and Cryptosporidium) and viruses are achieved. Control of more resistant cysts can be achieved at 10 mg/l (Laughton et al., 2001). It is well known that the addition of a strong oxidant such as ozone to humic waters reduces the colour significantly. Ozone treatment is reported to have no harmful residuals that would need to be removed after treatment. The most important disadvantage of the ozone disinfection is the lack of a residual 17 disinfectant that will need to protect the treated water against the bacterial regrowth or afterregrowth (Momba et al., 1998). Consequently, a secondary disinfectant will always be required to provide a residual. The perception that ozone is a more expensive treatment is one apparent reason for it not being more commonly used in the developing countries especially in small water treatment plants. Also, widespread availability and distribution of information or publicity on ozone treatment has been very lacking in the developing countries (Eagleton, 1999). 2.3.1.3 Use of alternative chemical disinfectants Every water treatment process has limitations and requires specific operation, maintenance and replacement of critical components, in accordance with manufacturer’s recommendations. To meet growing public demand for safe and clean potable water, many water utilities are exploring the use of alternative disinfection methods, which will correspond to the requirement of each and every water treatment plant. To meet this requirement, a disinfection technology which has the following characteristics should be employed: i) it should be affordable, ii) it should be simple yet give accurate doses, iii) it should be simple to operate and maintain, iv) any materials or chemicals which are required for operation should be readily available at close proximity to the place of usage. This section deals with the use of alternative chemical disinfectants that are currently employed in industrialized countries in some of the small and large water treatment plants: bromine disinfection, hydrogen peroxide disinfection and potassium permanganate disinfection. Bromine – Like chlorine, bromine is a halogen and acts in much the same way as chlorine. It can be supplied either as liquid bromine (but is highly corrosive), as bromine chloride (less corrosive) in a slow releasing organic complex (easy to handle but costly), or as NaBr salt which must then be oxidized to bromine on site (e.g. by addition to a chlorine solution) (Solsona and Pearson, 1995). Bromine has a number of advantages which make it a suitable disinfectant under specific conditions. These include the following: i) more reactive than chlorine for inactivating enteric viruses; ii) bromamines \which form when ammonia is present are significantly more effective than chloramines; iii) the disinfection effectiveness of bromine is not so dependant on p+ as for chlorine; iv) bromine and bromamines are less stable than their chlorine equivalents; v) being a liquid at ambient temperatures, bromine is less volatile than chlorine, and hence can be stored and handled more easily than chlorine gas. However, the disadvantages are its low oxidation ability, its higher price than chlorine and the caustic effect of the elementary bromine. 18 Hydrogen peroxide – Hydrogen peroxide is a weak acid, clear colourless liquid, miscible with water in all proportions. It is a physiological compound, which forms two stable and toxicological inert decomposition products (water and oxygen) (Solsona and Pearson, 1995). It is commercially available in aqueous solutions over a wide concentration range. Although it is a strong oxidant, it is proven a poor disinfectant. Hydrogen peroxide can be used to disinfect drinking water at a maximum dosage of 17 mg/l (100% hydrogen peroxide) and the residual in the consumable water must be lower than 5 mg/l (100% hydrogen peroxide) (Momba, 1997). To achieve adequate disinfection, higher dosages are required as compared to chlorine (15 000 to 50 000 mg/l for H2O2 vs 0.5 to 2 mg/l for chlorine), with extended contact times (Momba, 1997). In comparison to chlorine, the cost of the hydrogen peroxide product is higher (up to 8 times more costly than chlorine for the same dose), and the availability at more remote areas is very poor. The measurement of a residual for monitoring purposes is also very difficult. Advantages of hydrogen peroxide for use in small water systems include: i) ability to store large quantities under minimal storage regulations; ii) not hazardous to the environment and iii) effective over a wide p+ range. In general hydrogen peroxide is unsuitable as a drinking water disinfectant even in the developed world and could be considered for drinking water when used in conjunction with another oxidant or catalyst. Like monochloramine, hydrogen peroxide is better at attaining and maintaining a disinfectant residual than chlorine and ozone (Momba, 1997). Hydrogen peroxide has not been applied in South Africa to any extent to date for disinfection of drinking water. Potassium permanganate – Potassium permanganate is primarily used as an oxidant in water treatment processes, and not as a disinfectant. As an oxidizing agent, potassium permanganate is effective in controlling tastes and odours, as well as at removing hydrogen sulfide, iron and manganese. It has been used in South Africa as an oxidant for the removal of iron and manganese at certain water treatment plants (Solsona and Pearson, 1995). It is also not readily available in the remote areas. Although it does demonstrate some disinfection properties, the die-off rates are lower than for chlorine. Potassium permanganate decomposes to manganese dioxide, which is a precipitate which can cause colouring of washing, household utensils etc., and hence potassium permanganate is usually added before the coagulation/ flocculation step in water treatment (Solsona and Pearson, 1995). The primary disadvantages to its use as a disinfectant in small water systems are its high cost, and resulting residual which gives rise to discolourisation of washing, etc., unless removed in a coagulation/flocculation step. 19 2.3.2 Physical agents (Ultraviolet irradiation) Physical agents include heating (boiling) and irradiation. Boiling is practical only in small-scale applications; however the major disadvantage is the high energy cost of boiling water, especially where wood is the source of energy as well as the risk of scalding of children who handle the boiled water. Ultraviolet irradiation (UV) as a disinfection method is gaining popularity in the potable water industry (Momba et al., 1996). This section focuses only on UV disinfection process. Ultraviolet irradiation – Ultraviolet irradiation is a relatively old technology which was first used for disinfection purposes in 1910 and is becoming a common technique for disinfecting drinking water. The ability of UV to treat unfiltered water is dependent on water clarity and turbidity. Since most waters contain large amounts of inorganic and organic particles, which will decrease the effectiveness of the UV treatment, it is recommended that some type of filtration in addition to UV treatment be used to remove larger organisms and solid matter from the flowing water (Momba et al.,1998). To achieve the killing rate of a combined filtered/UV unit, an unfiltered/UV unit must expend more energy to be as effective. It has been recommended that a filter between 25-50 µm is ideal for removing sediment, and organisms larger than bacteria and viruses (Momba, 1997). The benefits of this technique in drinking water treatment include the following: i) UV does not alter taste, odour, colour or pH of the water; ii) no need to add chemicals in water and no formation of disinfection by-products; iii) UV systems are compact and easy to install; iv) they require very little maintenance and are cost effective (cost depends on the amount of water to be treated, pumping, pre-filtration and UV dose); v) the most important benefit in terms of disinfection is the ability of UV to inactivate Cryptosporidium oocysts which are generally resistant to most of the other chemical disinfectants. Ultraviolet treatment works to achieve disinfection efficiency by exposing target organisms to ultraviolet light (UV) energy waves. The exposure time and the intensity of the UV light application would determine the effectiveness of a lethal dose. In addition, the system performances would not only be affected by the dose and flow rate but also by the water quality of the water being treated. Ultraviolet absorbing constituents in water, such as organics, turbidity, and color, can influence the effectiveness of the system. As UV transmission decreases, additional UV energy would be required to maintain peak effectiveness. Typical maintenance procedures to maintain peak effectiveness in these systems include cleaning the quartz sleeves, replacing lamps, and checking proper function of the power module, inspecting the overall structural integrity of the system which includes a pretreatment unit. Training required for these procedures would be minimal. Although UV irradiation is a good disinfection process for killing parasites and other 20 organisms that are resistant to chlorination, like ozone, it has no residual protection that will be needed in treated water during distribution, and this means that the final water is at a high risk of being re-contaminated during storage. This technology is therefore good for point-source use, but not for developing communities. 2.3.3 Physical processes Physical processes include gravity separation and filtration. While gravity separation concerns mostly sedimentation and flotation, filtration processes include mechanical straining, absorption and adsorption, and, particularly in slow sand filters and biochemical processes. These processes play a very important role in removing viruses (White, 1992), schistosomes (bilharziasis) (Cairncross and Feachem, 1983) and protozoa cysts (Van Duuren, 1997). The removal of microorganisms, especially protozoan is very dependent upon the state and operation of sand filters. In other words, depending on the size, type and depth of the filter media and the flow rate and physical characteristics of the raw water, filters can remove suspended solids, pathogens and certain chemicals, tastes and odours. Straining and settlement are treatment methods that usually precede filtration to reduce the amount of suspended solids that enter the filtration stage (CDC, 2002). The size of microbes and other colloidal particles is of utmost importance when using filtration as a means of water purification. Viruses are the smallest of waterborne microbes, with a size of 20 to about 100 nanometers (nm). These viruses are the most difficult to remove by filtration. Bacteria are larger than viruses, being about 500 to 3000 nm in size. Bacteria are still too small to be removed from water by sedimentation or settling (WHO, 2002b). Although these microbes are too small to settle out, they often clump together with other particles, thereby increasing the size. This increases the efficiency of the filtration methods. A 95% to 100% reduction of faecal coliforms can be expected in water that has been filtered through slow sand filtration while rapid sand filters are capable of reducing turbidities and enteric bacteria by as much as 90%. Enteric viruses are not effectively removed by this method. To increase the reduction of viruses and bacteria using this method, coal can be added to the sand. Then positively-charged salts, such as alum, iron or manganese are added. This media then can reduce bacteria and viruses by up to 99% (WHO, 2002c). Membrane processes – Membrane processes offer an attractive alternative for primary disinfection of water. Disinfection is achieved by removal of the viruses and bacteria from the water system. One of the key aspects related to water treatment equipment performance verification is the range of 21 feedwater quality that can be treated successfully, resulting in treated water quality that meets drinking water quality standards. The influence of feedwater quality on the quality of treated water produced by the equipment should always be considered. The ultra-filtration membrane was designed to withstand feedwater turbidity of less than 20 NTU, but it should preferably be used or operated below 10 NTU (Pillay and Jacobs, 2004). Membrane systems have small land requirements, use fewer chemicals, and are not as complicated as conventional treatment systems. However, a secondary disinfectant will always be required to provide a residual which is indispensable to protect final water against re-contamination. A number of different types of membranes are available and are generally classified by the pore size as indicated in Table 2.4. Typical membranes processes are: microfiltration, ultrafiltration nanofiltration and reverse osmosis. Hence appropriate selection of a membrane will ensure the removal of the target organism thus ensuring efficient disinfection. The removal of much smaller viruses seems to be possible with these membranes as well since most of the viruses are attached to larger bacteria or other particles. The advantages of membrane processes are that there is no need for chemicals, and water clarification can take place simultaneously. However, membranes do become clogged with time despite the ongoing cross-flow cleaning process. Hence pre-treatment of the water by conventional means is advocated to lengthen the life of the costly membranes. Capital costs are high and operation is complex, requiring a high level of skills. Future developments may enable this to be considered as appropriate in the future. In South Africa microfiltration has not been used specifically for disinfection, but has been applied with some success to the concentration of water treatment sludge. Because of the cost of the membrane units when compared to other techniques such as chlorination that is commonly used in the water treatment field, Government has been adamant about subsidizing the unit. It is important for the Government to positively consider installing such membrane facilities that can produce water of high quality in order to counteract the enormous negative consequences that could arise from the consumption of poorly treated drinking water (Pillay and Jacobs, 2004). 22 TABLE 2.4 MEMBRANE PROCESS CLASSIFICATION Membrane Process Membrane Pore Size (nm) Microfiltration 77.5 to 550 Ultrafiltration 3.25 to 325 Nanofiltration 1 to 32.5 Reverse Osmosis 0.1 to 5.5 Note: Virus size 20-100 nm and bacteria 500 to 3000 nm 2.4 WATER DISTRIBUTION AND STORAGE The water distribution system comprises the network of pipes that transports water to the consumers. The size and distance of a water distribution system depends on the area it serves, the size of the population and the distance from its source. The main objective of the distribution is to supply the community with potable water as well as to ensure sanitary conditions. Assuring that drinking water remains safe after leaving a water treatment facility to the point of delivery to consumers is still a major challenge for drinking water providers. For most water systems, there is a larger investment in the infrastructure and operations of water delivery than water treatment, yet the vulnerability of water distribution is often overlooked. There is increasing evidence that finished water may undergo substantial changes in quality while being transported through the distribution system to the consumer. The distribution system is thus the final barrier in preventing waterborne disease outbreaks on one hand and may contribute to water quality deterioration on the other (Momba et al., 1998; Momba and Binda, 2002). It is believed that many of the waterborne disease outbreak problems associated with cholera were related to improperly operated and poorly maintained distribution systems (Clark et al., 1993). Among 619 waterborne diseases (chemical and microbial) outbreaks reported in the U.S. for public water systems, from 1971 to 1998, 113 (18%), were caused by distribution system deficiencies. Cross connections or back siphonage, corrosion or leaching of metals, broken or leaking water mains, contamination during storage, contamination of mains or repair, contamination of household plumbing or inadequate separation of water mains and sewers have been reported to be distribution system failures (Craun and Calderon, 2001). Among the outbreaks caused by distribution system failures, 66% have been reported to involve microbial pathogens (Craun and Calderon, 2001). 23 Fortunately, the importance of maintaining water quality during distribution has received growing attention in research and practice over the past decade. One specific matter of distribution water quality that deserves mention, concerns the maintenance of a disinfectant residual throughout the distribution system (Hrudey and Hrudey, 2004). The disinfectant residual is designed as a measure of protection against harmful microbes encountered after water leaves the treatment facility. This has been a requirement in a number of jurisdictions where chlorination has been most common. In the intrusion of pathogens resulting for example from a broken water main, the level of the average chlorine residual will be insufficient to disinfect contaminated water. In such cases, it is the monitoring of the sudden drop in the chlorine residual that provides the critical indication to water system operators that there is a source of contamination in the system (Chlorine Chemistry Council, 2003). Drinking water in storage tanks can be easily contaminated if storage tanks are not properly closed. Often if the tanks are underground, possibly in close proximity to sewage, cross contamination due to sewage overflow may occur. It is therefore recommended that reservoirs for storage of potable water be situated and built in such a way as to lower the risk of sewage or any other contaminants from being introduced into the water distribution system (Momba, 1997). It is important to note that the proper maintenance and operation of water supply, treatment and distribution systems are essential parts of any effort to ensure the on-going production and delivery of the highest quality drinking water possible (Momba et al., 2004b). 2.5. MICROBIOLOGICAL QUALITY OF THE FINAL WATER & PUBLIC HEALTH SIGNIFICANCE Lack of access to adequate drinking water supply is largely responsible for more than 1 billion estimated of diarrhoeal diseases and 2.2 million associated deaths worldwide (WHO, 2001; Gleick, 2002). The vast majority of people lacking access to safe water reside in developing nations (UN World Water Assessment Program, 2003). This implies that many people in the developing world still depend on contaminated water sources for their daily water needs. This is therefore a challenge to the water industry to provide clean and safe drinking water to their consumers. Drinking water quality can be assessed in terms of the senses (smell, taste, colour), physico-chemical and microbiological properties, both quantitatively and qualitatively. This section discusses the monitoring strategy used in South Africa to assess the safety or the quality of drinking water at the plant and in the distribution system, and the public health significance associated with the 24 microbiological quality of water supplied by small water treatment plants. 2.5.1 Monitoring the safety of water supplies In South Africa, the South Africa National Standard (SANS 241, 2005) is used as the official specification for assessing the quality of drinking water. Moreover, the Water Quality Guidelines for Domestic Use published by DWAF in 1996 provide a comprehensive discussion of all quality aspects relevant to water for domestic use with recommended quality ranges for different situations. The Assessment Guide published by DWAF, the WRC and the Department of Health in 1998 also gives a user-friendly presentation of the assessment procedure for drinking water. Consequently, the safety of treated water used for domestic purpose is assessed according to the lists of quality criteria, standards and guidelines stipulated in the above official guide documents. In order to ensure that drinking water is microbiologically safe to drink, there must be no pathogens in the water. The detection of waterborne and water-related pathogens requires expensive, complex, and time consuming techniques, while others are not detectable by conventional methods at all. Water quality monitoring programs are, therefore, usually based on the test of indicator microorganisms. These organisms, such as certain coliform groups, occur in the intestine of humans and are easy to detect. Their presence in treated water is therefore taken as an indication that drinking water was not adequately treated or disinfected. An ideal indicator microorganism should fulfill the following criteria: i) it should always be present when the pathogen is present and should be absent in clean, uncontaminated water; ii) it should be present in numbers greater than the pathogen it indicates; iii) its survival to the environment and resistance to the treatment processes should be comparable to that of pathogens; iv) it should not be harmful to human health; v) it should be easy to identify and to isolate and vi) it should be suitable in all types of water (Grabow, 1996). This section only discusses groups of indicator organisms recommended by SANS (241, 2005) to assess the effectiveness of disinfection. Total coliforms – Total coliforms are a group of bacteria that are commonly used to monitor the microbiological quality of drinking water as they satisfy many of the ideal characteristics of an ideal indicator organism. They are used to assess the effectiveness of the disinfection process and the integrity of the distribution system. It is generally assumed that water that is free of coliforms should have no pathogens present, however it should be remembered that the coliform bacteria are only used as an indicator of the possible presence of pathogens, and detailed analysis for the presence of pathogens is required when coliforms are detected. 25 Faecal coliforms and E. coli – Faecal coliform and E. coli are subgroups of the total coliforms that are typically present in contaminated water. SANS 241 (2005) recommend that the water industry uses E. coli as the preferred indicator organism for possible presence of faecal pollution and to ensure that the objectives of disinfection have been achieved. 2.5.2 Quality at water works and in distribution systems Three basic mechanisms govern the occurrence of pathogenic microorganisms in treated drinking water: i) the microbes break through the treatment process from the source water supply, ii) the microbes regrow from very low levels, typically in biofilms, and iii) the organisms result from a recontamination of the treated water within the distribution pipeline system. These mechanisms are incorporated in the multiple-barrier concept for water treatment as described earlier in this chapter. The discussion here will focus on the effect of the inadequate treatment on the quality of the treated water. Multiplication of bacteria in drinking water during distribution is usually referred to as regrowth. This term implicates the microbial growth process in the phenomenon of increased microbial cell numbers. Regrowth of coliform bacteria in distribution systems has been reported as a major problem for a number of water utilities (LeChevallier et al., 1991; Momba et al., 1998). High levels of bacteria in small water distribution systems could also be related to the inefficiency of the initial treatment barriers. The failure of rural schemes in South Africa to provide potable water to consumers has been a matter of concerns. Conventional water treatment processes could be capable of reducing the number of indicator microorganisms if they are well applied. However, it has been noted that low or high doses of coagulants could produce high turbidity in water. High turbidity increases the potential for the transmission of infectious disease due to shielding of the bacteria within the particulate matter (Momba et al., 2004a). Moreover, most finished waters in rural developing areas are characterized by the absence of chlorine residual throughout the distribution system, which leads to total and faecal coliform counts equal to that of raw water before treatment (Swartz, 2000; Mackintosh and Colvin, 2002; Momba et al., 2004, a; b). It is important that small water treatment plants maintain adequate concentrations of chlorine to prevent regrowth and repair of bacteria. 2.5.3 Impact of microbiological quality of treated water on public health Diarrhoeal diseases attributed to poor water supply, sanitation and hygiene account for 1.73 million deaths each year and contribute over 54 million disability adjusted life years, a total equivalent to 26 37% of the global burden of disease (WHO, 2002). Diarrhoeal diseases are therefore considered as the 6th highest burden of disease on a global scale, a health burden that is largely preventable (WHO, 2002). A significant portion of residents in rural communities of South Africa are exposed to waterborne diseases and their complications (Obi et al., 2002). The impact of poor water quality on human health is shown in Table 2.5. Cholera, typhoid fever, salmonellosis, shigellosis, gastroenteritis and hepatitis outbreaks have been linked to contaminated drinking water (Grabow, 1996). Between 1980 and 1989, approximately 25 251 cases of cholera were reported in KwaZuluNatal, and 845 cases in Eastern Cape of South Africa (Department of Water Affairs and Forestry, 2002). Most severely affected community were people in rural areas living in conditions of poverty, poor sanitation and poor domestic supply. Typhoid fever, salmonellosis and shigellosis are enteric disease commonly transmitted by drinking water. Areas affected by typhoid fever are Mpumalanga, Eastern Cape and KwaZuluNatal. These provinces are regarded as very poor with domestic water supply and poor sanitation facilities (Department of National Health and Population Development, 2001). Heterotrophic bacteria have been isolated from the in-plant reservoirs and distribution systems, these include Flavobacterium species (responsible for neonatal meningitis, meningitis in adults and respiratory tract infections), Enterobacter cloacae (opportunistic pathogen isolated from urine, sputum, pus, and spinal fluids is responsible for urinary tract, lower respiratory tract and bone joint infections) and Pseudomonas aeroginosa (causes eye and ear infections and also infections of wounds and burns) (Momba and Kaleni, 2002; Momba et al.,2004; 2005). TABLE 2.5 IMPACT OF POOR WATER QUALITY ON HUMAN HEALTH (2001-2005) Waterborne diseases No of reported cases Average number of deaths Cholera 667 300 92 000 Typhoid Fever 151 000 6 000 Paratyphoid Fever 35 000 2 000 Hepatitis A virus 112 000 1 000 Source: Department of Health, 2006 In addition, the coccodian genus Cryptosporidium is increasingly recognized as an important agent of gastrointestinal disease. Infection with this protozoan parasite can lead to chronic, life27 threatening conditions in immunocompromised individuals and to acute gastroenteritis in healthy people. Taking into account the impact of drinking water of low microbiological quality on the health of rural communities, it is important for local water authorities to develop strategies leading to safe drinking water. 2.6 IMPROVING DISINFECTION EFFICIENCY IN SMALL WATER TREATMENT PLANTS To develop a health protection strategy, it is vital that all the factors that indicate the quality of water supply service should be reported including monitoring the efficiency of treatment plant for indicators of pollution. The principal objective of drinking water quality surveillance is to reduce the risk of water related diseases by developing and implementing cost-effective surveillance that will identify and facilitate the elimination of the sources of contamination of water supply. Water quality surveillance has been defined as the continuous and vigilant public health assessment and overseeing of the safety and acceptability of drinking water supplies. The surveillance solutions to disinfection problems include source water selection and basic monitoring, bacteriological and chemical analysis. Remedial action strategies should be formulated and also routine-monitoring programs should be established. The selection of water sources, the use of alternative disinfectants as an upgrading strategy, process optimization as a remedial strategy and the education of local water authorities are discussed in this chapter. In addition, hygiene and education of rural community are highlighted. 2.6.1 In-service training of water personnel and management The greatest protection that consumers can achieve from dangers posed by contaminated water is to be assured that the operators of their drinking water system know and fully understand the system, its capabilities and its limitations. No amount of regulation or stringency in drinking water quality criteria will serve consumers better than having drinking water providers well-trained with the ability to learn effectively from their mistakes, external challenges and previous water quality so that future problems can be avoided or minimized. Ultimately, drinking water providers must accept that providing a technologically challenging undertaking continues to grow in its sophistication. These are characteristics of knowledge based industry. Consumers would not tolerate having their telephone system or home computer serviced by inadequately trained personnel. This concept could 28 also be applied to water industry. Water safety thus is highly dependent on the actions of the operators who have their hands on the control panels. Smith (1995) observed in a critique of the parties involved in the Milwaukee outbreak that some states in the U.S. have more rigorous training requirements for hairdressers than they do for water treatment operators. The article emphasizes the point that water treatment operators should be seen as holding a responsibility for public health at least as vital as any other health professional. Likewise, the provision of safe drinking water demands technologically and scientifically sophisticated management and leadership. Water authorities driven strictly by their economic bottom line, without regard to their scientific competence, sooner or later invite serious water failures. Therefore, to assure that effective performance is achieved, responsibility and accountability must be established at all levels within the organization of a drinking water provider (Hrudey and Hrudey, 2004). 2.6.2 Hygiene education and community based management Water and sanitation programs will not lead to improvements in health unless they are accompanied by effectively designed programs of health education which promote: i) community participation on decisions involved in the installation of water supply schemes; ii) encouragement of the appropriate use, transport and storage of water, iii) maintenance of water supply programs; iv) encouragement of the construction, use and maintenance of latrines, v) hygiene education programs directed at washing hands, preparation of weaning foods and disposal of children’s faeces. Hygiene is the maintenance of health standards. Hygiene education comprises a broad range of activities, aimed at changing attitudes and behaviours, to break the chain of disease transmission associated with inadequate water and sanitation. In rural areas, communities are situated far from the water supply distribution points. They thus store water in containers for long periods of time and this increases the probability of contamination (Momba and Kaleni, 2002; Momba and Notshe, 2002). Hygiene education informs the rural community about the correct use, storage and disposal of water. In most parts of rural South Africa the following problems are common: i) information is not readily available, ii) literacy levels are low, iii) there is a lack of scientific understanding of the implications of poor water quality, iv) there is a lack of public and political support for education, v) severe economic constraints are prevalent, vi) policies and instrument are out-dated, vii) institutional arrangements are poor (Mtetwa and Schutte, 2002). The above factors thus present a major challenge to the efficient management of rural water supply. It calls for new approaches that 29 are relevant, effective and that will increase sustainability and effective decision making in datapoor environments. In South Africa it is essential to understand the attitudes and behaviors of developing communities towards water and sanitation. Most rural communities rely on government to make sure that their projects are sustainable, but it is necessary for them to contribute themselves towards sustainability of their projects, as well as the development of appropriate hygiene education awareness programs. Community involvement is important in the management of water supply to ensure that: i) local leadership is representative and accountable, ii) the technology of choice is supported by the community, iii) all community members understand their tariffs and operation and maintenance (O&M), iv) the best people are selected for operation, management & training. Community based organizations are encouraged to be involved in the management of their water supply systems which should include the management of the water quality aspects. Community based organization management systems have been implemented in many countries such as Uganda and the United States of America (Bwengye, 2002) Similar systems have been encouraged in South Africa with community based organizations such as Mvula Trust situated in KwaZulu-Natal province (Vermeulen, 2002). It is therefore important for rural communities and local municipality to work together with local water authorities on infrastructure design and management system to ensure that improved water and sanitation services meet people’s needs and that they are sustained. The following chapter focuses on the survey of disinfection efficiency of rural small drinking water treatment plants in South Africa. 30 CHAPTER III SURVEY OF DISINFECTION EFFICIENCY OF SMALL DRINKING WATER TREATMENT PLANTS In an endeavour to collect sufficient data to formulate guidelines on improving the efficiency of disinfection of small water treatment plants in South Africa, a detailed survey of 181 small water treatment plants across seven provinces was conducted. The seven provinces were selected on the basis of familiarity with the areas, economic status, and rural areas experiencing technical and management problems. Information gathered included various methods of disinfection, equipment currently employed, performance of the treatment plants, knowledge and skills of the operators as well as other technical and management issues. 3.1 SURVEY AREA To ensure a comprehensive coverage of the country, small water treatment plants mainly located in rural communities of the following provinces were surveyed: Limpopo, Mpumalanga, North-West, Free State, KwaZulu-Natal, Eastern Cape and Western Cape. The geographic position system (GPS) co-ordinates of each site were logged during the survey to facilitate future incorporation into a geographical information system (GIS) that is being developed for the country. 3.2 SURVEY METHODOLOGY A questionnaire (Appendix 3.1) was designed to obtain the required information such as: the ownership and design capacity of each water treatment plant, the type of raw water sources and related characteristics, the pre-disinfection and disinfection processes, the state of the equipment and other technical and management issues. To achieve these, on-site visits of small water treatments plants in the designated provinces were conducted from June 2004 to December 2005. Wherever possible, some plants were visited at least twice during the study period. Microbiological and physico-chemical analyses of water samples collected from the raw water and final water at the point of treatment and at the point of consumption were performed using standard methods (APHA, 1998). Briefly, the chlorine residual concentrations, pH, temperature, turbidity and the conductivity of water samples were measured on-site using a multi31 parameter ion specific meter (Hanna-BDH laboratory Supplies) thermometer, microprocessor turbidity meter (Hanna instruments) and conductivity meter (Hanna instruments) respectively. Total and faecal coliforms were used to monitor bacteriological quality as stated in the South African Water Quality Guidelines for Domestic Use (DWAF, 1996) and SANS 241 (2005). 3.3 RESULTS OF THE SURVEY AND DISCUSSION 3.3.1 Small water treatment plant ownership Figure 3.1 illustrates the percentage and categories of plant ownerships in various provinces during the study period. Four categories of ownership were identified viz Local Municipality, Department of Water Affairs and Forestry (DWAF), Department of Health (DOH) and Water Board (private company). In Mpumalanga, the Local Municipalities were the major owners with the exception of the water treatment plant of Nelspruit that is now owned by Biwaters, which is a private company. In Limpopo, plant ownership was divided between the Local Municipalities and DWAF. In NorthWest, the ownership of the plants was distributed into two categories viz Local Municipalities and Water Boards (Botshelo Water and Magalies Water). Sixty seven percent of the plants were owned by Local Municipalities while Botshelo and Magalies Water Boards owned 22% and 11% of the plants respectively. Five (Taung, Wolmaranstad, Kudumane, Stella, Vryburg) of the towns in the Province were supplied by Sedibeng’s water treatment plant, which was situated in the Free State Province. Eighty six percent of small water treatment plants were owned by District Municipalities in the KwaZulu-Natal province. In the Eastern Cape Province, 88% of plants were owned by the District Municipalities with the remainder distributed amongst the Department of Water Affairs and Forestry, the Department of Health and a private company (Water and Sanitation Services of South Africa - WSSA) which managed the plants on behalf of the District Municipality. All the plants surveyed in the Free State Province and in the Western Cape Province were owned by the District Municipalities. Overall 81% of the small water treatments surveyed in South Africa were owned by the District Municipalities (Fig. 3.1). 3.3.2 Design capacity of small water treatment plants Small water treatment plants in rural areas are generally classified as plants having a maximum capacity of 2.5 Ml/d although plants of up to 25 Ml/d may sometimes also fall into this category. The survey did not limit itself to this classification as some of the plants supplying water to rural areas were found to exceed this capacity. The capacity of the plants surveyed during the investigation varied between 0.3 Ml/d and 120 Ml/d. Most plants were operating below the design 32 capacity. Some of the plants visited were in the process of being upgraded or had recently completed upgrades. In Mpumalanga, small water treatment plants situated in peri-urban regions usually provide water to the towns, townships as well as to some villages. Package plants were observed to be rare and few cases were recorded. The largest plant visited in Mpumalanga was Witbank with a design capacity of about 120 Ml/day. Most of the plants had a capacity of more than 1 Ml/day. Some plants were in the process of being upgraded or had recently been upgraded. However in Limpopo, most of the plants were package plants with a capacity of less than 1 Ml/day. The largest plant was found in Mhinga with a design capacity of 37 Ml/day. In the North-West Province, the design capacities of the plants ranged between 1.3 and 60 Ml/d. The Mafikeng water treatment plant was the largest plant and has a design capacity of 60 Ml/day. The majority of the plants were operating below the design capacity. The capacity of the plants investigated in the Free State Province ranged between 0.5 Ml/d and 11.5 Ml/d. The largest plant was at Parys with a capacity of 11.5 Mld. The capacity of the plants was determined by the capacity of the clarifiers (up-flow velocity of 1 m/h) and the rapid gravity sand filters (flow rate of 5 m/h) as the flow meters at 7 of the 13 plants investigated were inoperative. The plant with the lowest capacity (Oranjeville) was in the process of being upgraded. In KwaZulu-Natal, the design capacities of the plants visited ranged from small waterworks with capacities of 100 m3/d (Manjokeni waterworks situated near Bergville) to largest capacity plant of 32 Ml/d (Ezakheni Waterworks in Estcourt. Due to the lack of plant records and flowmeters in most plants especially in rural and peri-urban areas, the design capacities were assessed based on the dimensions of the unit operations. In the Eastern Cape, the Umtata plant was the largest waterworks visited during the survey period, with a design capacity of 60 Ml/d; however, most of other plants were designed for more than 1 Ml/d. Most of these plants were operating below design capacity. Nearly half of the plants were undergoing some sort of upgrading or had recently completed the upgrading. In the Western Cape, the design capacities of the plants visited ranged between 1 and 60 Ml/d. The largest plant visited was George water treatment plant with a design capacity of 60 Ml/d. While 62% of the plants were designed for less than 10 Ml/d, 38% of the plants had the design capacity ranging between 11 and 30 Ml/d. The majority of the plants were operating below the design capacity. The Sandhoogte plant was the only plant that was undergoing upgrade. 33 Municipality DWAF No of Plants Surveyed Private 200 181 100 180 160 80 140 120 % 60 100 80 40 60 54 36 20 N S o u r o v f e y P e l d a n t s 40 19 28 18 20 13 13 R SA W estern Cape Eastern Cape KwaZulu Natal Free State North W est M pum alanga 0 Lim popo 0 Fig. 3.1 Category of Ownership of Small Treatment Plants surveyed in South Africa 3.3.3 Type of raw water sources Figure 3.8 illustrates the types of raw water sources used by small water treatment plants in the designated provinces. Appendix 3.2 gives full details on the type of water sources used as intake raw water by each plant visited in various provinces. This information can also be found in the Database. Overall 86% of the small water treatment plants surveyed in South Africa abstracted their raw water from surface water, although 10% of the plants used groundwater or a combination of both water sources (4%). In Mpumalanga, only 5% of the waterworks surveyed abstracted intake water from groundwater. In Limpopo, 6% of the plants abstracted intake water from groundwater and 3% used a combination of both surface and ground water sources. The North-West province had the highest usage of groundwater (24%). A few of the plants draw water from unprotected springs. The surface water in the province was generally characterized by high turbidity and some of the dams were highly polluted with algae. In the Western Cape, the majority of the plants were designed for the removal of colour, iron and manganese removal. 34 Ground Water Surface Water Combined No of Plants Surveyed 200 100 181 90 180 80 160 N o 70 140 o f 60 120 50 100 40 80 % 60 30 54 20 19 10 40 28 36 18 P l a n t s S u r v e y e d 20 13 13 0 0 Limpopo Mpumalanga North West Free State KwaZulu Natal* Eastern Cape Western Cape RSA Fig. 3.2 Types of Water Sources in Small Treatment Plants surveyed in South Africa 3.3.4 Water treatment practices Conventional water treatment processes were generally used in the majority of the plants surveyed. In some of the plants that abstracted groundwater, the only form of treatment practiced was disinfection. Interestingly, one of the small water treatments surveyed was a desalination plant which was close to Port Alfred in the Eastern Cape and fell under the control of the Albany Water Board. The plant used membrane filtration followed by disinfection with chlorine. The unit processes and methods of disinfection used in the various treatment plants are summarized in Fig. 3.3 -3.11 with the details captured in appendix 3.2. Coagulation – In terms of coagulation, the survey has confirmed a general trend in South Africa where there has been a strong move towards the use of polyelectrolyte as a substitute for alum and ferric chloride with 69% of the plant surveyed now using these chemicals. The Free State had the highest usage of polyelectrolyte where 92% of the plants are currently using these coagulants. The Western Cape was the highest user of Alum (61%) and the North-West province showing the 35 highest user of ferric chloride (22%) (Fig. 3.3). Filtration – Sixty percent of the small treatment plants surveyed used rapid gravity filtration systems with a further 24% using pressure filters. Only nine percent of the plants were using slow sand filtration systems. It was interesting to find that 14 plants were using diatomaceous earth filters which are predominantly used in large municipal swimming pool systems. The North-West province had the highest (31%) application of slow sand filtration while the Western Cape had the lowest (8%). No sand filtration systems were used in Limpopo, Mpumalanga and Free State which had the highest application of rapid gravity filtration (Fig. 3.4). Disinfection – Chlorine gas was found to be the most popular disinfectant with 69% of the small treatment plants using this chemical, followed by sodium hypochlorite (15%) and calcium hypochlorite (HTH) (14%). The highest application of sodium hypochlorite was found in KwaZulu-Natal (40%) followed by Free State (31%). The highest application of HTH was found in the Eastern Cape (33%). No application of sodium hypochlorite was found in Limpopo, Mpumalanga and Western Cape. Only one instance of the application of chlorine dioxide, sodium bromide and ozone was found in the country at the following plants: Wild Coast Casino, Libode and Jeffreys Bay, respectively (Fig. 3.5). 36 Polyelectrolyte Alum Ferric Chloride Ferric + Polyelectrolyte Alum + Polyelectrolyte No of Plants Surveyed 100 200 181 90 % 180 80 160 70 140 60 120 50 100 40 80 30 60 54 20 40 36 28 10 19 18 20 13 13 0 0 Limpopo Mpumalanga North West Free State Kwazulu Natal Eastern Cape Western Cape RSA Fig. 3.3 Types of Coagulants used in Small Treatment Plants Surveyed in South Africa Fig. 3.4 Types of Filters used in Small Treatment Plants in South Africa 37 N o o f P l a n t s S u r v e y e d Chlorine Gas HTH Sodium hypochlorite Chlorine Dioxide Ozone Bromine No of Plants Surveyed 90 200 181 80 180 160 70 N o 140 o f 60 P 120 l a n 50 100 t % s 40 80 30 60 54 20 40 36 S u r v e y e d 28 10 19 18 20 13 13 0 0 Limpopo Mpumalanga North West Free State KwaZulu Natal Eastern Cape Western Cape RSA Fig. 3.5 Types of Disinfectants used in Small Treatment Plants in South Africa 3.3.5 Quality of drinking water produced by small water treatment plants 3.3.5.1 Physicochemical compliance Appendix 3.3 shows in detail the physicochemical quality of drinking water produced by small waterworks visited during the study period. These include pH, conductivity, turbidity and chlorine residual. The results of the analyses for all water samples collected at various plants fell within SANS Class I in terms of pH (5 to 9.5) and conductivity (<150 mS/m) (SANS 241, 2005). For efficient disinfection, the DWAF, HOD and WRC’ s Assessment Guide for the Quality of Domestic Water Supply (1998) recommends that the turbidity of drinking water should be less than 1 NTU and preferably less than 0.5 NTU. The maximum turbidity limit allowable by SANS 241 ranges between 1 and 5 NTU (2005). Water samples collected at the point of treatments showed that 44% and 38% of the small water treatments surveyed in South Africa fell within SANS Class I (< 1 NTU) and Class II (1-5 NTU), respectively. The remained plants had turbidity values higher than 5 NTU (Fig.3.12). At the point of consumption, 46% and 41% of the plants fell within Class I and Class II, respectively (Fig. 3.7). The highest turbidity compliance was noted in Free State at both point of treatment (Class I: 73% of the plants and Class II: 27%) and consumer’s taps (Class I: 69% of the plants, Class II: 31% of the plants). In this province, no small water treatment plant exceeded the maximum turbidity limits allowed by SANS 241. 38 Class 1 Class 2 < 1 NTU Class 2 1 to 5 NTU > 5 NTU No of Plants Surveyed 80 200 181 70 180 160 60 140 50 120 % 40 100 80 30 54 60 20 40 36 N o o f P l a n t s S u r v e y e d 28 10 19 18 20 13 13 0 0 Limpopo Mpumalanga North West Free State Kwazulu Natal Eastern Cape Western Cape RSA Name of Province Fig. 3.6 Turbidity Compliance of Small Treatment Plants surveyed in South Africa at the Point of Treatment The high turbidity compliance in the Free State Province might be attributed to the partnership that has been established between this province and a technical support organization (CSIR). This technical support visits each plant at least once per month to advise the operators on process control issues. The lowest turbidity compliance was noted in the Eastern Cape Province with 40% and 31% of the plants showing the highest turbidity values at the point of treatment and at the point of use, respectively. The turbidity values recorded from most water samples collected in these plants exceeded the allowable maximum limits set by SANS 241:2005. 39 Class 1 < 1 NTU Class 2 1 to 5 NTU Class 2 > 5 NTU No of Plants Surveyed 80 200 181 70 180 160 60 140 50 120 40 100 % 80 30 54 20 60 36 40 28 19 10 18 13 13 N o o f P l a n t s S u r v e y e d 20 0 0 Limpopo Mpumalanga North West Free State Kwazulu Natal Eastern Cape Western Cape RSA Name of Province Fig. 3.7 Turbidity Compliance of Small Treatment Plants Surveyed in South Africa in Distribution system North-West had 28% of the plants not complying with the maximum turbidity limits at the point of treatment as well as at the point of use. Mpumalanga and Western Cape had 39% and 42% of the plants falling within Class II at both points of treatment and points of use, respectively (Figs. 3.12 13). As indicated in Appendix 3.2, high turbidity values interfered with the concentrations of free chlorine residual at the point of treatment as well as at the point of use. This made it more difficult to maintain an adequate residual which could protect the drinking water against pathogenic microorganisms. Free chlorine residual is the primary indicator of microbial safety used in process controls. Adequate disinfection then requires a free chlorine concentration of at least 0.5 mg/l in the final water leaving the plant after a contact time of at least 30 min at pH less than 8 (Water Research Commission, 1998; WHO, 2004) . The free chlorine residual concentration at the point of delivery should be at least 0.2 mg/l under normal circumstances and 0.5 mg/l during periods of high risk of microbial contamination (WHO, 2004). To combat any possible contamination in the network and to protect public health, the South African’s Assessment Guide for the Quality of Domestic Water Supply recommends the ideal target range of 0.3 -0.6 mg/L free chlorine residual at the consumer’s tap water (Water Research Commission, 1998). This was not the case in 44% of the plants visited during the survey (Figs.3.14- 3.16). 40 Chlorine at Point of Treatement <= 0.5 Chlorine at Point of Treatement <= 0.1 Chlorine at Point of Use <= 0.2 Chlorine at Point of Use <= 0.1 90 80 Percentage Below Stipualted Limit 70 60 50 40 30 20 10 0 Limpopo Mpumalanga North West Free State Kwazulu Natal Eastern Cape Western Cape RSA Fig. 3.8 Free Chlorine per Province at Point of Use and Point of Treatment Water samples collected at the point of treatment indicated that 16% of the plants had a minimum free chlorine concentration ≤ 0.1 mg/l and 56% had a free chlorine concentration ≤ 0.5 mg/l. At the point of use 32% of the plants had free chlorine concentrations below 0.1 mg/l and 48% had below 2 mg/l in each province as shown in Fig. 3.8. Figures 3.15 and 3.16 show the histograms of the free chlorine concentrations as distributed across the entire country at the point of treatment and at the point of use. 60 120.00% 50 100.00% Frequency Cumulative % Frequency 40 80.00% 30 60.00% 20 40.00% 10 20.00% Free Chlorine mg/l Fig. 3.9 Free Chlorine Histogram for all Provinces at Point of Treatment 41 4 M or e 3. 5 3 2. 5 2 1. 5 1 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0.00% 0. 2 0. 1 0 It was noted that many operators were not aware of the chlorine dose added in the raw water after filtration and in most of the plants the flow rate of the intake water was not known. Many of the plants either overdosed the chlorine or under chlorinated the drinking water and this led to chlorine values outside the recommended limits (Appendix 3.3). Frequency Frequency Cumulative % 60 120.00% 50 100.00% 40 80.00% 30 60.00% 20 40.00% 10 20.00% 0 0.00% 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 1.5 Free Chlorine mg/l 2 2.5 3 3.5 4 More Fig. 3.10 Free Chlorine Histogram at Point of use across all Provinces 3.3.5.2 Microbiological compliance It is important to note that the quality of water reaching consumers depends not only on operating conditions at the treatment plant but also on changes that can occur in the distribution system. During the survey period, it was noted that final drinking water of the highest quality might be leaving some plants but its condition deteriorated to some extent before it reached the consumers (Appendix 3.4). Given sufficient time, the chlorine residual concentration decreased to a very low level that did not comply with the South African standard. High turbidity in the finished water, old pipes, breaks in distribution pipelines, biofilm growth, sludge accumulation in the storage reservoirs and the availability of nutrients for microbiological growth could be among the factors that accelerate the chlorine residual decay of final drinking water at the point of use (Momba et al., 2000). To ensure the absence of bacterial pathogens, the drinking water should be free of faecal organisms. The primary bacterial indicator recommended for this purpose is the coliform group of organisms (DWAF, 1996; WHO, 2004; SANS 241, 2005). Total and faecal coliforms are used much more than any other indicator group for monitoring drinking water quality because they address both health and treatment efficiency objectives. Although as a group, they are not 42 exclusively of faecal origin, they are universally present in large numbers in the faeces of human and other warm blooded animals. The presence of faecal coliforms in treated water indicates poor or inadequate treatment of drinking water. Higher concentrations of faecal coliforms in treated water indicate a high risk of contracting waterborne disease, even if small amounts of the water are consumed (DWAF, 1996). Figures 3.17-3.18 and Appendix 3.4 illustrate the microbiological quality of water supplied by plants surveyed. It must however be stressed that these results were once off surveys which were repeated in some provinces. Due to logistics and the costs involved, repeated analyses could not be done over the entire country. Despite these shortcomings it must be emphasized that once off surveys are a good indicator of the microbiological quality of the water produced by the plants surveyed. In many instances this was also the first time water samples from these all treatment plants were being analyzed and this is an indicative of major shortcomings in the legislation in South Africa. Recent developments within DWAF do however show that there is now increased emphasis on monitoring of drinking water quality. The Delmas incident in South Africa in 2005 resulted in 600 cases of typhoid, five documented deaths, and 3300 cases of diarrhoea. Escherichia coli were found in one of the town’s reservoirs and the lack of treatments, especially chlorination, was found to be a major contributing factor to the tragedy (Water Wheel, November/December 2005). In Mpumalanga, 95% of the plants at the point of treatment and 84% at point of use complied with the South African water quality standard in terms of total colifoms. Seventy four percent of the plants were within the limits recommended by South African standards in terms of faecal coliforms at both the point of treatments and the point of use. Total coliform counts ranged between 0 and 380 cfu/100 ml at the point of treatment and between 0 and 180 cfu/100 ml at the point of use, while faecal coliform counts ranged between 0 and 3 cfu/100 ml at the point of treatment and between 0 and 12 cfu/100 ml at the point of use. In Limpopo, 64% of the plants at the pint of treatment and 94% at point of use of the plants complied with the South African recommended standard in terms of total coliforms. The total coliform counts ranged between 0 and 3.6 × 103 cfu/100 ml at the point of treatment and between 0 and 250 cfu/100 ml at the point of use. In terms of faecal coliforms, 73% and 88% of the plants complied with South African drinking water recommended limits at the point of treatment and at the point of use, respectively. Faecal coliform counts ranged between 0 and 60 cfu/100 ml and between 0 and 7 cfu/100 ml at the point of treatment and at the point of use, respectively. 43 Total Plants Surveyed Faecal Coliform Compliance (%)(<1 cfu/100 ml) Total Coliform Compliance (%) (<10 cfu/100ml) 120 200 180 173 100 160 140 80 o f P l 120 a % n t 100 s 60 80 40 60 53 20 N o 40 33 S u r v e y e d 28 19 11 20 17 12 0 0 Limpopo Mpumalanga Free State North West KZN Eastern Cape Western Cape RSA Name of Province Fig. 3.11 Bacteriological Compliance at the Point of Treatment In the North-West province, 76% of the samples from the plants at point of treatment and 53% at the point of use complied with the South African recommended limits in terms of total coliforms. Total coliforms ranged between 0 and 83 cfu/100 ml at the point of treatment and between 0 and 288 cfu/100 ml at the point of use. A total of 94% and 83% of the plants were found within the recommended limits for faecal coliforms at the point of treatment and at the point of use respectively. Faecal coliform counts ranged between 0 to 5 cfu/100 ml and between 0 to 13 cfu/ 100 ml at the point of treatment and at the point of use, respectively. 44 Total Coliform Compliance (%) (<10 cfu/100ml) Faecal Coliform Compliance (%)(<1 cfu/100 ml) Total Plants Surveyed 120 200 180 174 100 160 N o 140 o f 80 120 P l a n 100 t s % 60 80 40 60 53 40 20 33 28 19 11 S u r v e y e d 20 17 13 0 0 Limpopo Mpumalanga Free State North West KZN Eastern Cape Western Cape RSA Name of Province Fig. 3.12 Bacteriological Compliance in Distribution System The microbiological data of the plants visited in the Free State province resulted in 100% compliance with the SANS Standard in terms of total coliforms and faecal coliforms at the point of treatment. At the point of use 92% compliance was obtained in terms of total coliforms and 85% compliance in terms of faecal coliforms. The above non-compliance represented two points in the distribution system and it must be noted that overall the plants in the Free State were producing excellent water quality. In KwaZulu-Natal, 57% of the plants at point of treatment complied with the SANS Standard in terms of total coliforms and 61% complied in terms of faecal coliforms. At the point of use, 64% plant compliance was obtained in terms of total and faecal coliforms. Total coliform counts ranging up to 866 cfu/100 ml were detected at one site at the point of treatment with corresponding faecal coliform counts of 53 cfu/100 ml. At the point of use an alarmingly high total coliform count of 2419 cfu/100 ml was detected at one of the sites with a corresponding faecal coliform count of 36cfu/100 ml. The primary cause of this failure was due to either lack of chlorination facilities or under-dosing of chlorine. In the Eastern Cape Province, at the point of treatment 28% of the plants complied with the SANS standard in terms of total coliforms and 34% complied in terms of faecal coliforms. At the point of use 20% of the plants complied in terms total coliforms and 29% complied in terms of faecal coliforms. Total coliform counts ranging up to 240 cfu/100 ml were noted at the point of treatment of one of the plants and 223 cfu/100 ml at one of the points in the distribution system. A maximum faecal coliform count of 25 cfu/100 ml was detected at one of the points of treatment while 98 cfu/100 ml was noted at one of the points in the distribution system. 45 In the Western Cape, at the point of treatment 50% of the plants complied in terms of total coliforms and 25% complied in terms of faecal coliforms. In the distribution system, there was 25% compliance in terms of both total and faecal coliforms. Total coliform counts ranged between 0 and 59 cfu/100 ml at the point of treatment and between 1 and 67 cfu/100 ml at the point of use, while the faecal coliform counts ranged between 0 and 25 cfu/100 ml and between 0 and 50 cfu/100 ml at the point of treatment and at the point of use, respectively. Compared to other provinces, the Eastern Cape Province produced the lowest potable drinking water quality in terms of both total and faecal coliforms while the Free State produced the best drinking water quality. These results confirm the poor microbiological quality of the drinking water in the Eastern Cape that was also noted by Momba and co-workers (2004) in a previous study conducted in the Alice Water Treatment plant. 3.3.6 Control and monitoring Water treatment plant operators are all aware that the characteristics of the raw water they are treating changes from time to time. While the quality of borehole water tends to remain fairly constant, the quality of raw water extracted directly from rivers can change drastically, especially in terms of suspended solids. Even the change in temperature from winter to summer can also influence the treatment of drinking water (e.g. it is harder to form floc in cold water than in summer). Furthermore, there are variations in water demand which may require changes in raw water flow rate. Consequently, operators need to make adjustments to the operation of the plant from time to time in order to meet changing treatment requirements. They also need to check that the adjustments made are consistent with the desired effect (Momba and Brouckaert, 2005). In Limpopo and Mpumalanga, most of the operators and supervisors interviewed did not have a good knowledge of the flow rate at which their plants were being operated. Generally the chemical dosing rates were determined by experience. Very few knew what their chemical dose rates were or how to calculate them. Coagulant doses were adjusted manually, usually based on the appearance of the floc and sometimes also based on the taste of the water when alum was used. Chlorine doses were set manually and some plants were overdosing chlorine. Nearly all of the plants reported that an external monitoring group (the District Municipality, BNK, MMP or some private company) visited the plants approximately once a month although most plants complained about a lack of feedback from the external monitors. Most of the plants were partially automated which facilitated the operator's work and limited some overdosing errors and ensured that remote reservoirs were kept full. In Mpumalanga, most plants had instruments to measure turbidity, pH and 46 chlorine residual although these were not always used. The maintenance of equipment in some plants was not taken into account. In the North-West Province, 60% of the plants were equipped with raw water flow meters; however readings were only recorded in plants owned by Water Boards and in three of the municipal owned plants. It was noted that 66% of the plants had jar stirrers on-site; however these were only used in four of the plants. Less than 20% of the supervisors and operators knew the required chemical dosing rates or how to calculate them. Chemical dosages were adjusted based on the appearance of the floc and the colour of the finished water. All the plants were measuring chlorine residuals, however less than half of them were measuring turbidity and pH. Only one plant was testing the general chemical and microbiological quality of water. Four municipal plants were monitored by local health inspectors; however the plant staffs were not getting any feedback on the water quality results. Nearly all of the plants reported that an external monitoring group visited the plants approximately once a month, but they did not know what kind of tests they were doing and they were not getting any feedback. The Botshelo water owned plants were equipped with a telemetry system and Supervisory Control And Data Analysis (SCADA) system for the monitoring of the whole plant. In the Free State, some of the operators and supervisors interviewed knew the flow rates at which their plants operated but very few knew what their chemical dose rates were or how to calculate them. Seventy percent of the plants had the instruments to measure turbidity, pH and chlorine residual. Fourteen percent of the plants measured chlorine only, and 14% do not have any on-site monitoring programme. All of those plant operators interviewed were aware of the importance of measuring these parameters but were typically unable to persuade the municipality to buy the instruments (however, basic instruments usually came with major upgrades). A number of supervisors were using swimming pool test kits to measure pH and chlorine. Coagulant doses were adjusted manually usually based on the appearance of the floc. However in one case, the dosage of the flocculants was automatically controlled by an ion charge analyzer. Chlorine doses were also set manually. In one case the Supervisor at a plant was reluctant to increase the chlorine dosage even though the chlorine levels in the town were low. All of the plants visited during the survey period were monitored by an external monitoring group (CSIR) on a monthly basis. In KwaZulu-Natal, 15% of the plants had functioning raw water meters, 10% had installed meters but were non-functional, and operators indicated that the meters had been non-functional for many years. Lack of maintenance of equipment was reported in 80% of the plants. Seventy percent of the plants were not able to calculate the chemical doses and the operators were running the plants by visual observation of the clarifier overflow. Only 20% of the plants had on-line instruments for 47 measuring turbidity and pH and 70% had bench scale equipment for measuring pH, turbidity and chlorine. On-site jar test equipment was recorded in 5% of the plants, however only 2% were capable of conducting a jar test. None of the plants were undertaking chlorine demand tests on site. External Process Audits were undertaken at 30% of the plants and involved a detailed assessment of the plant optimization. These plants were mainly plants managed by Water Boards such as Umgeni Water and Umhlatuze Water. Seventy percent of the plants were measuring process parameters and capturing onto daily log-sheets, however limited quality control of this data was practiced. At plants managed by Water Boards, Process Technicians visited the plants at least once a month to assist with optimization of the plant. The Process Technicians were responsible for a number of plants and rotated their service between the plants. In the larger Water Boards, Process Engineers and Process Scientists were available at short notice for trouble shooting process problems as well as to follow up on water quality failures. Supervisors and Works Managers were only available at 20% of the plants that were visited. In the Eastern Cape Province, 50% of the operators and supervisors interviewed knew the flow-rates at which their plants operated, 78% did not know the chemical doses used or how to correlate the required dose to the flow rate, 46% had the instruments to measure turbidity, pH and chlorine residual, 3% of the supervisors were using swimming pool test kits to measure pH and chlorine, 95% of the plants reported that an external monitoring group visited the plants approximately once a month; however most plants complained about a lack of feedback and 20% of the plants were partially automated. In the Western Cape, all the plants were equipped with raw water flow meters. Most of the operators reported that raw water flow was adjusted if there was high demand of water or during rain seasons. The majority of the plants owned jar stirrers. Almost all the plants were measuring chlorine residuals and pH, however less than half of the plants were measuring the turbidity of the water. Some of the plants were testing the general chemical quality of water and only one plant was testing E. coli onsite. Nearly all of the plants reported that an external monitoring group visited the plants approximately once a month and they were getting feedback. More than a half of the plants visited during the study period were equipped with a SCADA and telemetry system to monitor the whole plant. To provide a guide for a simple log sheet system for effective control and monitoring, the Tables presented below are suggested. The purpose of this type of log is to record all process changes that take place on the plant. This helps to ensure that sufficient information is recorded to pass onto the next operator that comes on shift. Typical details that would be recorded include, Time of arrival on plant, condition of the plant on arrival, reservoir levels, record of person visiting 48 the plant, reasons for making process control changes, etc. TABLE 3.1 Date Time EXAMPLE OF A PROCESS CONTROL SHIFT LOG SHEET Plant Name: Operator Name: Raw water Turbidity Raw water pH Coagulant Dose Clarifier turbidity Chlorine dose Final water chlorine Lime dose 06H00 08H00 10H00 12H00 14H00 16H00 18H00 20H00 22H00 00H00 02H00 04H00 TABLE 3.2 EXAMPLE OF FILTER WASHING AND CLARIFIER SOLIDS MANAGEMENT Date Plant Name: Operator Name: Time Filter No Backwash Volume Used (m3) Clarifier No Time Desludged 06H00 6 53.5 3 5 minutes 08H00 10H00 12H00 14H00 16H00 18H00 20H00 22H00 00H00 02H00 04H00 49 Final water pH TABLE 3.3 EXAMPLE OF DAILY OPERATOR LOG Date Plant Name Operator Name Time Description of Situation in the plant 06H00 Arrived on plant – everything in order, final water turbidity 0.3 NTU, final chlorine 1.2 mg/l, final pH 8.4 06H35 Call received from City Engineer – Water shortage in city reservoir – started pumps to city 07H10 Raw water turbidity increased from 45 NTU to 90NTU – undertook jar test – optimum dose of 11 mg/l – set plant on new dose 08H30 3.4 New dose working well – good clarifier overflow turbidity RECOMMENDATIONS FROM THE SURVEY It is concluded that although ownership of the plants belonged to 4 categories owners such as the District/Local Municipalities, DWAF, DOH and private companies (Water Board), over 80% of the plants were noted to be owned by the District Municipalities. In addition, about 84% of the small water treatment plants in the designated provinces abstracted their raw water from surface water. Conventional water treatment processes were generally employed; over 80% of the small water treatments employed the rapid gravity and pressure filtration systems. Chlorine gas was observed to be the most common disinfectant used (69%), followed by sodium hypochlorite (15%) and calcium hypochlorite (14%). Furthermore, a substantial number of the small water treatment plants engaged operators with limited technical understanding of the treatment processes, leading to either an overdose or an under-dose of coagulant and chlorine. Generally in terms of microbiological parameters employed, Free State and Eastern Cape Provinces produced the safest and poorest water quality respectively. Finally some of the small water treatment plants were devoid of basic monitoring equipment such flow meter, pH meter, jar test apparatus, turbidity meter, chlorine meter and colorimeter. These have led to lack of flow rate, turbidity, pH and chlorine residual measurements. It is strongly recommended that the following operational practices be implemented: 1. All small water treatment plants must be provided with basic functioning raw and final water flow meters installed. 2. Accurate records of flow into and out of the plant must be recorded on a daily basis or whenever a change in flow rate is made. 50 3. All the treatment plants must acquire jar stirrer apparatus to determine the optimum dose of the coagulants. Jar tests must be conducted at least once per week or when the raw water quality changes. 4. Plant operators must monitor pH at various points in the plant for coagulation control. The turbidity of the final water must be monitored and falls within Class 1. This can only be achieved if the operators target a filtrate turbidity of < 1NTU. 5. The chlorine dose has to be applied proportionally to the plant flow rate. To ensure effective disinfection, measurement of the chlorine demand of water is highly recommended. 6. For a monitoring programme to be effective, each small water treatment plant must be equipped with a jar stirrer, turbidity meter, pH meter and a chlorine meter. A programme for monitoring the physico-chemical (at least pH, temperature, turbidity and free chlorine residual) and bacterial (coliform bacteria, especially faecal coliform) quality of water at the point of treatment and various sites of the distribution systems must be established. 51 CHAPTER IV WATER QUALITY CHANGES IN THE DISTRIBUTION SYSTEM The distribution system is often vital in determining the final quality of potable water. Deterioration of drinking water quality during storage or in distribution systems is one of the major difficulties experienced by potable water supplies worldwide (Momba et al., 2000). The proper understanding, characterization and prediction of water quality behavior in drinking water distribution systems are critical in meeting regulatory requirements and ensuring customers oriented expectation. Realizing that the water quality after treatment deteriorates with time, the distributor cannot always guarantee the quality at the end of the distribution network. Moreover the reservoirs which are an important part of the distribution network represent the final point at which the water quality can be modified before it reaches the consumer (Momba et al., 2000). This part of the study provides a reference compendium on parameters affecting the quality of final treated drinking water in the distribution systems of three small water supplies (Seymour, Fort Beaufort and Alice). The Fort Beaufort, Seymour and Alice water treatment plants are all located in the Nkonkobe Municipality, in the Eastern Cape Province of South Africa. The Fort Beaufort plant has the highest number of distribution reservoirs (26) serving 15 villages in its distribution system. The water distribution system of Seymour consists of two storage reservoirs located in the water treatment plant and an associated primary and secondary distribution network. The Alice water treatment plant was selected to evaluate the effectiveness of previous operator training that had been conducted at the plant in 2004. 4.1 METHODOLOGY To identify the major problems resulting in water quality changes in distribution systems, an on-site evaluation of the operating conditions at Fort Beaufort, Seymour and Alice water treatment plants in the Eastern Cape Province was conducted by visual inspection, interviews and use of questionnaires from October 2005 to November 2005. 4.1.1 Measurement of the flow of raw water and coagulant dose The flow rates were recorded and in the absence of the flow meter (Seymour did not have a flow meter while the one for Alice was malfunctioning at the time of the visit), the flow of the raw water was calculated using the existing 90° V notch weir according to Kawamura (1991). For a 90o Vnotch weir, the total flow rate ( Q) is related to the height of the crest over the weir (H) as follows: 52 Q (m3/s) = 1.40 H2.5 The jar test was used to determine the optimum dose of the coagulant. 4.1.2 Physicochemical and Microbiological Analysis Physicochemical parameters determined on-site included: chlorine concentrations, pH, temperature and turbidity. The initial chlorine dose and free chlorine residual concentrations were determined at the point of the treatment and in the distribution networks using a multi parameter ion specific meter (Hanna-BDH laboratory supplies). The pH, temperature and the turbidity were measured at each step in the treatment processes and in the distribution networks using a pH meter, thermometer and a microprocessor turbidity meter (HACH company, model 2100P) respectively. For microbiological analysis, water samples were collected according to standard procedures using sterile bottles, which contained sodium thiosulphate (ca 17.5 mg/l), placed in cooler boxes and transported to the laboratory for analysis within 2 to 4 h after collection. Standard methods were used to quantify total and faecal coliforms and heterotrophic plate count bacteria. 4.2 RESULTS AND DISCUSSION 4.2.1 Distribution networks Schematic diagrams of the Fort Beaufort and Alice distribution systems are shown in Fig. 4.1 and 4.2, respectively. The Fort Beaufort plant had three main reservoirs (No1-3) with a combined capacity of 6.3 Ml. These reservoirs were filled by high lift pumping of the clear water (Fig. 4.1). From the main reservoirs, water was fed by either gravity or pumping to a number of service reservoirs. The topography of the distribution allowed gravity feed from service reservoirs. The Seymour plant had two storage reservoirs with a total capacity of about 400 m3. Reservoirs, ventilation, scour valves and overflows were adequately designed. However, there were no bulk sale meters or reservoir level indicators. The water was supplied by gravity to the community in which the service levels were mainly yard and house connections. During interviews, operators reported that the locations of the scour valves in the network were not known. Hence the network could be flushed and it was suspected that the reservoirs had high levels of silt. 53 R8 Hillside R1 PS2 R3 R5 Bhofolo R2 WW PS2 PS3 R9 R9 New town Fort Beaufort Town Fig. 4.1 Schematic diagrams of the Fort Beaufort Distribution Network (WW – Water Works, R1 – Rectangular balancing reservoir [2.3 ML], R2 - Circular Reservoir near plant [2.0 Ml], R3 – Circular reservoir near plant [2.0 Ml], R4 – Hillside reservoir, R5 – Reservoir feeding Ntobela, R7 – Bhofolo Reservoirs [2.5 Ml supply], R8 – Ntobela Reservoir supplying 14 Satellite reservoirs, R9 – Newtown Reservoir, PS1 – Pump station No 1, PS2 - Pump station No 2, PS3 - Pump station No 3, R1-3 – Has 1.6 Ml/d supply). The Alice Plant had a capacity of 7 Ml/d. Five of the town’s seven reservoirs, including the main reservoir were gravity fed. Float valves regulated the flows into the reservoirs and they operated essentially at 100% capacity under normal conditions. The flow to Ntselamanzi and Mavuso reservoirs was augmented by pumping which was initiated automatically based on the signals from level sensors in their reservoirs. 54 S a m p lin g p o in ts AW TP Pum p S ta tio n M e te r 1 ML B a la n c in g Tank M e te r M a v u so 2 .5 M L M a v u so V illa g e G o lf C o u rse D e v e lo p m e n t M a in 6 ML A lice N ew H appy R e st 0 .7 M L D a v id so n P rim a ry M a in L in e M e te r H a p p y R e st H illc re st F o rte F la ts N tse la m anzi 1 .5 M L M e te r M e te r M e te r Pum p S ta tio n O ld H appy R e st 0 .2 M L C lin ic V ic to ria E a st C lin ic 0 .6 M L V ic to ria E a st N tse la m a n z i V illa g e U FH Fig 4.2 Schematic diagrams of the Alice distribution network 4.2.2 Water Treatment Plant operation conditions 4.2.2.1 Fort Beaufort water treatment plant The Fort Beaufort water treatment plant abstracted its intake water from Kat River. The raw water turbidity varied between 60 and 70 NTU, but reached 100 NTU during the rainy season. The flow rate of the raw water measured by an ultrasonic flow meter varied between 66 and 80 l/s during the investigation. The accuracy of the flow meter checked by measuring the height over the V-notch weir was found to be acceptable (0.069 m3/s = 69 L/s). After coagulation with a blended polyamine, water passed through two circular clarifiers. Settled water was then filtered in seven rapid gravity filters followed by final chlorination. The supplier was the only person who made adjustments to the chemical dose. The standby dosing pump had been removed for repairs a year ago. A jar test done during the study period confirmed a dosage of 45 ml/30 s which was adequate for the flow of 66 l/s. Dosing took place downstream of a weir proving good mixing energy. Of concern was the fact that the operators were not in a position to measure the applied dose while a jar stirrer was available on-site. Calculations undertaken showed that the clarifier surface loading rates ranged between 1.8 and 2.21 m/h which was well above the design rate of 1 m/h. Sludge removal valves were malfunctioning and the clarifier overflow turbidity ranged between 8 and 10 NTU, well above the guideline of 5 NTU. 55 Filtration rates were found to be between 1.2 and 1.4 m/h well below the design of 5 m/h. This impacted on the turbidity of filtered water which was above 1 NTU. Gas chlorination was used for disinfection. One chlorinator was available with no standby. There were no emergency stocks of HTH. At the time of the visit the chlorinator was not working and a makeshift HTH dosing system was in use, however there was no means of measuring the dose. A the point of treatment, the Fort Beaufort chlorine dosing unit available read a dosing rate of 1.2 kg/h (333.3 mg/s). The dosage in mg/l from the plant inflow when chlorine gas was being dosed corresponded to 5.05 mg/l. Typical dosages usually do not exceed 1.5 - 2.0 mg/l. The high dosage calculated could be due to the following reasons: i) the dosing equipment being malfunctioning and ii) high chlorine demand of the water. The average free chlorine residual concentration was therefore ≤ 1.5 mg/l. 4.2.2.2 Seymour Water Treatment Plant The Seymour water treatment plant abstracted its intake water from Kat River. The raw water had the turbidity values ranging between 50 and 70 NTU. The raw water flow measurement took place at the head of waterworks and was found to be functional. At the time of visit, an average inflow rate of 5.38 l/s was recorded. A blended polymeric coagulant was dosed at a weir followed by the baffled flocculation basin and the horizontal flow rectangular sedimentation basin. The Surface Loading Rate (SLR) of the sedimentation tanks was found to be 0.3 m/h well within the guideline of 1 m/h. One dosing pumps was available with no standby and coagulant control was done by the suppliers. No jar stirrer was available at the plant, but the jar tests undertaken during the study period indicated that the dose was adequate (8.5 ml/60 s) for the flow rate of 5.38 l/s. Following slow sand filtration, the water was chlorinated before distribution. The two slow sand filters were operating at 0.3 m/h which was just on the upper limit for these types of filters. Filtered water had turbidity values below 1 NTU. Filter cleaning was done monthly and the procedure was in-line with standard practice. On-site electrolytic chlorination using salt as a feed was used for disinfection. Operators could not calculate the applied dose. During the visit, the chlorine dosage could not be determined as the operation and the specification manual for the equipment were not available. However, the free residual chlorine determined from the in-plant reservoir was 0.5 mg/l. According to the operators, occasional shortages of salt were common. No emergency or backup HTH stock was available. Bench pH, chlorine and turbidity meters that cost about R20 000 each were available but were surprisingly not in use due to the lack of batteries. 56 4.2.2.3 Alice Water Treatment Plant Raw water was abstracted from a dam, and after coagulation with a polymer the water passed through a flocculator and horizontal sedimentation tanks. This was followed by filtration through two valveless filters and gas chlorination. Raw water turbidity at the time of the assessment was 20 NTU. An ultrasonic flow meter was used to measure the flow rate of the raw water over a Vnotch at the head of works. The flow rate of raw water was 75 l/s and jar tests confirmed that the applied dose was correct (15 ml/30 s). Flocculation was poor and the poor flow distribution was overloading some of the sedimentation tanks. The chlorination system for Alice water treatment plant was similar to that of Fort Beaufort. Residual chlorine levels at the point of treatment ranged between 0.24 and 0.89 mg/lL after the contact tank. 4.2.3 Drinking water quality in the distribution systems 4.2.3.1 Turbidity compliance Figure 4.3 summarizes the turbidity results obtained in the Fort Beaufort distribution system. None of the water samples had the turbidity values below 0.2 NTU, with 27% of the samples having a turbidity ≤ 0.5 NTU. Most of the samples (86%) had turbidity values below 1 NTU with only 6% being above 2.5 NTU. An uncharacteristically high turbidity was observed once from the Henrietta control point (49.3 NTU), which was suspected to be a consequence of a damaged pipe along the route. In Seymour distribution networks, the turbidity of the drinking water at the various sampling points varied very markedly in the range of 0.55 to 27 NTU. Figure 4.4 shows that none of the samples had turbidity values below 0.2 NTU, 13% of the samples were below 0.5 NTU, 67% of the samples had turbidity greater than 1 NTU and 14% had turbidity higher than 5 NTU. Figure 4.5 depicts a histogram of turbidity in the Alice distribution system. The data show that none of the samples had turbidity below 0.2 NTU; 58% of the samples had turbidity values ≤ 1.0 NTU and 30% of the samples had turbidity values higher than 2.5 NTU. This indicates that the consumers were receiving unsafe water and the primary reasons for this were poor plant management as there was always a shortage of the primary coagulant and chlorine at this water works. 57 35 120.00% 30 100.00% 25 Frequency Cumulative % Frequency 80.00% 20 60.00% 15 40.00% 10 20.00% 5 0 0.00% 0.1 0.2 0.5 0.75 1 1.5 NTU 2 2.5 5 10 More Fig. 4.3 Histogram of Turbidity in the Fort Beaufort Drinking Water Distribution System 8 120.00% 7 100.00% 6 Frequency Cumulative % 80.00% Frequency 5 4 60.00% 3 40.00% 2 20.00% 1 0 0.00% 0.1 0.2 0.5 0.75 1 1.5 2 2.5 5 10 More NTU Fig. 4.4 Histogram of Turbidity in the Seymour Drinking Water Distribution System 8 120.00% 7 100.00% 6 Frequency Cumulative % 80.00% Frequency 5 4 60.00% 3 40.00% 2 20.00% 1 0 0.00% 0.1 0.2 0.5 0.75 1 1.5 2 2.5 5 10 More NTU Fig. 4.5 Histogram of Turbidity in the Alice Drinking Water Distribution System 58 4.2.3.2 Free chlorine residual concentration and microbiological characteristic in the distribution systems Fort Beaufort - Figure 4.6 depicts a histogram of the free chlorine concentrations and it was noted that 28% of the results were below 0.1 mg/l, indicating that there was a large reduction in the concentration of free chlorine residual from the treatment plant which supplied water with a free chlorine residual concentration up to 1.5 mg/l. Ninety two percent of the samples had free chlorine concentrations below 0.5 mg/l and were thus within the recommended levels of between 0.3 and 0.6 mg/l (Water Research Commission, 1998). No coliform bacteria were observed in the in-plant reservoirs of the Fort Beaufort water treatment plant during the study period. However in the distribution system, 60% of the samples had less than 10 cfu/100 ml total coliforms. Of concern was the 7% of the samples that had more than a 100 cfu/100 ml total coliforms (Fig. 4.7). Figure 4.8 shows a histogram of the faecal coliforms in the Fort Beaufort system, where 4% of the samples had five or more faecal coliforms, due to the depletion of chlorine residuals in the distribution networks. In general 88% of the water samples had more than the DWAF (1996) limits of 100 cfu/mL HPC (Fig.4.9). These results were an indicative of low chlorine residuals, possible regrowth of bacteria and poor maintenance of the distribution system. 59 Figure 4.6: Fort Beaufort Free Chlorine Histogram Figure 4.7 Fort Beaufort Total Coliform Histogram Cumulative % Frequency 25 F re q u e n c y 20 100.00% 20 120.00% 100.00% 80.00% Cumulative % Frequency 15 120.00% 25 80.00% 15 60.00% 60.00% 10 10 40.00% 5 40.00% 5 20.00% 0 0.00% 0.1 0.2 0.5 0.75 1 1.5 2 2.5 20.00% 0 0.00% 0 More 1 10 100 Free Chlorine 500 1000 Mor e Figure 4.9 Fort Beaufort Histogram of Heterotrophic Plate Counts Figure 4.8 Fort Beaufort Faecal Coliforms Histogram 60 120.00% 30 50 100.00% 25 Frequency 100.00% 20 Cumulative % 80.00% 40 80.00% Frequency Frequency Frequency 200 Tot al Colif or m Cumulative % 30 60.00% 20 40.00% 10 20.00% 0 1 5 10 15 20 15 60.00% 10 40.00% 5 20.00% 0 0.00% 0 120.00% 0.00% 100 More 1000 10000 100000 1000000 More Heterotrophic plate count Faecal Coliform Bins Figs 4.6-4.9 Histograms of Free Chlorine Residual and Indicator Bacteria in the Fort Beaufort Drinking Water Distribution System Seymour - In terms of free chlorine, the Seymour histogram (Fig. 4.10) shows that 20% of the samples had a free chlorine concentration below 0.1 mg/l, a further 20% had concentrations between 0.1 and 0.2 mg/l. Overall all the water samples had a concentration below 0.5 mg/L, while two samples had concentrations of 0.75 and 1.0 mg/l respectively. Eight of the 14 water samples analysed (60%) had no total coliforms, however 22% had 10 or more total coliforms (Fig.4.11). Figure 4.12 depicts the Seymour histogram for faecal coliforms which shows that 12 of the 14 samples (85%) had no faecal coliforms whereas only two samples had five or more. In terms of faecal coliforms, this system showed the most promising results and was an indicative of the smaller distribution network associated with shorter retention times. In terms of HPC, Fig. 4.13 shows that 12% of the samples had HPC densities greater than 105 cfu/ml, 50% of the samples had more than 104 cfu/ml HPC and 58% had more than 103 cfu/ml. Overall 65% of the 60 water samples did not meet the guideline value of 100 cfu/ml also showing that the distribution network was in need of a good clean out. Figure 4.11 Histogram of Total Colif orms in the Seymour Distribution System Figure 4.10 His togram of Free Chlorine in the Seym our Dis tribution Sys tem 6 Frequency Cumulative % 100.00% 100.00% 7 80.00% 4 40.00% 2 1 20.00% 0 0.00% 0.1 0.2 0.5 0.75 1 1.5 2 80.00% 6 60.00% 3 5 60.00% 4 40.00% 3 2 20.00% 1 0 2.5 More 0.00% 0 1 10 Free Chlorine 14 5 100.00% 95.00% 4 80.00% 3 60.00% 2 40.00% 80.00% 1 20.00% 75.00% 0 90.00% 6 85.00% 4 2 0 15 20 Cumulative % 100.00% 8 10 1000 More 120.00% Frequency Frequency Frequency 10 5 500 6 Cumulative % 12 1 200 Figure 4.13 Histogram of HPC in Seymour Distribution System 105.00% 0 100 Total Colif orm Figure 4.12 Histogram of Faecal Colif orms in Seymour Distribution System Frequency Cumulative % Frequency 8 Frequency Frequency 5 120.00% 9 120.00% 7 More 0.00% 100 Faecal Colif orm 1000 10000 1000001000000 More Heterotrophic plate count Fig 4.10 - 4.13 Histograms of Free Chlorine Residual and Indicator Bacteria in the Seymour Drinking Water Distribution System Alice - Figure 4.14 shows that 27% of the samples had residual free chlorine concentration below 0.1 mg/l, 62% below 0.2 mg/l and none of the water samples having more than 1.0 mg/l. In terms of total coliforms (Fig. 4.15), 27% of the water samples had no coliforms with 56% having more than the recommended limit of 10 cfu/100 ml. Of major concern was the fact that 6% of the aforementioned samples had more than 200 cfu/100 ml, representing a major health risk to the community. 61 Figure 4.14 Histogram of Residual Free Chlorine in Distribution system 8 120.00% 7 Frequency Cumulative % 6 120.00% 100.00% 6 Frequency 80.00% 4 60.00% 3 40.00% 2 80.00% 5 60.00% 4 3 40.00% 2 1 0 0.1 0.2 0.5 0.75 1 1.5 2 2.5 20.00% 1 0.00% 0 20.00% 0.00% 0 More 1 10 Figure 4.16 His togram of Faecal Coliform s in the Alice Dis tribution Sys tem 12 200 500 1000 More Figure 4.17 His togram of HPC in Alice Dis tribution Sys tem 120.00% 10 7 Frequency 60.00% 4 40.00% 2 100.00% 8 80.00% 6 Cumulative % Frequency 9 100.00% 8 120.00% 10 Cumulative % Frequency 100 Total Colif orm Free Chlorine Frequency Cumulative % Frequency 7 100.00% 5 Frequency Figure 4.15 Histogram of Total Colif orm in Alice Distrbution System 80.00% 6 60.00% 5 4 40.00% 3 2 20.00% 20.00% 1 0 0.00% 0 1 5 10 15 20 0 More 0.00% 100 Faecal Colif orm 1000 10000 1000001000000 More HPC Fig 4.14 - 4.17 Histograms of Free Chlorine Residual and Indicator Bacteria in the Alice Drinking Water Distribution System The faecal coliform histogram of the Alice distribution system in Figure 4.16 shows that 55% of the samples had no faecal coliforms present, however 34% of the samples had more than one faecal coliform. Of the aforementioned samples, 6% had more than 5 faecal coliforms confirming the major consumer risk mentioned above. In terms of HPC in Alice (Figure 4.17), 6% of the samples had more than 104 cfu/ml, 17% had more 103 cfu/ml and 50% of the samples had more than the recommended limit of 100 cfu/ml. Based on the chlorine rates at the dosing points and the concentrations of free residual chlorine in networks systems, relationships were then established between both points. When in operation, the plant chlorination systems always provided sufficient residual chlorine for areas near the main reservoirs. For the areas that were far away from the main reservoirs, the plant chlorination systems usually provided sufficient residual free chlorine on the days of supply/pumping to the service reservoirs. The dosages at the plants were not sufficient enough to combat the depletion of 62 free chlorine residual in most reservoirs when the final water was detained for more than 24 h. This was attributed to the chlorine consumption by floc/sediment accumulations in most reservoirs. Moreover, it was found that the reservoir designs were generally adequate. They were equipped with overflows, air vents and drainage facilities. The operation of reservoirs, however, seemed to be inadequate. Although the plant chlorination systems gave adequate dosage at the point of dosing, these dosages were not sustained in the distribution systems. 4.3 CONCLUSIONS In general, the assessment of the quality of drinking water in distribution systems of Fort Beaufort, Seymour and Alice revealed that most of the sampling points in distribution systems did not comply with the required standards in terms of indicator bacteria such as heterotrophic plate counts, total coliforms and faecal coliforms. This was a reflection of the inconsistency in the plant operations, which made impossible for the plants to ensure sustainable production of good quality of drinking water. Several factors could be responsible for this phenomenon: i) The design and operational of the flocculation processes were not optimized at all plants. This led to inadequate floc formation for the sedimentation process. ii) The monitoring and adjustment of chemical dosages (coagulants and chlorine) were not conducted on-site; operators should be capacitated to adjust dosing rates with respect to inflow and quality changes iii) Filtration process monitoring and control was not optimized. The filtration rate of the SSF in Seymour was 100% higher than the accepted upper limit. In all the plants, there was no standby dosing pumps for the coagulants and disinfection chemicals. The stocking of disinfection chemicals was not sustainable and lack of on-site water quality data which should assist in making critical decisions and also estimations in times of emergency. The above factors in water treatment plants impacted on the effectiveness of the disinfection process in the distribution systems and the following major problems were recorded: i) the distribution system of the pipe network did not show acceptable levels of residual free chlorine and ii) most of the chlorine dosed at the treatment plant was consumed by the floc sludge that accumulated in the reservoirs and the deposits that were present in the distribution pipe network. 4.4 RECOMMENDATIONS To improve disinfection efficiency in Fort Beaufort, Seymour and Alice drinking water treatment plants, this study suggests the following recommendations: 63 Floc sludge removal need to be done twice for the Seymour WTP and adequate flocculation basins need to be introduced at the Fort Beaufort WTP. The quality of inflow to each filter should be monitored to prevent overloading the filtration process and the quality of the effluent be monitored from each filter. The concept of filtering-to-waste must be investigated after the backwashing of the slow sand filters at Fort Beaufort and Seymour. The effectiveness of these filters with respect to turbidity removal within 1 to 2 h after backwashing was usually low and the filtered water should then be sent to waste until turbidity levels become acceptable (< 1 NTU). The reservoirs need to be maintained and cleaned regularly to ensure that there is no sludge accumulation. The sludge consumes the disinfectant thus reducing the residual chlorine and compromising the reliability of the disinfection in the network. Some reservoirs showed leakages. Replacement of screens on vents needs to be done annually. The pipe network system needs to be drained or flashed as required to remove deposits that also consume disinfectants. Scour valves for various sections of the network must be located and put to use. A comprehensive water quality monitoring programme needs to be developed and supported by the Municipality. Water distribution networks need to be documented in order to understand and optimize their operation. At the time of visit, network drawings could not be obtained and there were no records of pressure and bulk water meter readings. Record keeping and interpretations need to be enhanced at all plants as this would assist in taking critical operation, maintenance and management decisions. Operators should be trained to perform key tasks such residual monitoring, adjustment of chemical dosages, flow measurements and visual analysis of the water quality from each unit process, independently. Each shift should have two operators; this is important to reduce the consequences of accidents and also for morale and motivation purposes. The Alice case is clearly one of a loss of team towards compliance of the operational procedures, having been beneficiary of a training programme in the past year that significantly improved the plant’s performance and the quality of the final water produced (Momba et al., 2004; 2005). This underscores the need for a regular auditing of the plants performance to ensure continuous improvement and safe drinking water. 64 CHAPTER V MANAGEMENT ISSUES AFFECTING THE EFFICIENCY OF DISINFECTION IN SOUTH AFRICAN SMALL WATER TREATMENT PLANTS 5.1 INTRODUCTION The capacity of any water treatment plant to provide acceptable drinking water quality mainly depends on the performance of each functional unit in the plant including coagulation-flocculation, sedimentation, filtration and disinfection. Management and administration of water treatment plants also play an important role in determining the quality of the final water. In urban areas, drinking water quality complies with the South African National Standards (SANS) 241 Drinking Water Specification (2005). This water, therefore, does not pose a significant risk to public health over a lifetime of consumption. The difficulties experienced in training and retaining adequately skilled people to run water treatment plants in impoverished rural municipalities, as well as lack of revenues needed to hire experienced managers and to maintain and upgrade water supplies have been among the major hurdles to providing safe and clean drinking water in these areas. Inadequate manpower training and periodic updating of knowledge and skills affect institutional capacity to implement sustainable water resource management. Poor maintenance practices, inadequate improvement in working conditions and poor water programme monitoring are hallmarks of inadequate manpower training or institutional capacity. This chapter therefore seeks to explore management issues facing safe and reliable water supply in rural areas and some peri-urban areas of South Africa. 5.2 METHODOLOGY A survey of management issues of 181 small water treatment plants across seven provinces of South Africa (including Limpopo, Mpumalanga, North-West, Free State, KwaZulu-Natal, Eastern Cape and Western Cape) was conducted from June 2004 to December 2005. The survey started with a short introductory meeting following by interview with the plant operators, superintendents and supervisors of the plants. Information concerning management issues such as the training of the operators, their salaries, benefits, decision making, maintenance practices and financial capacity (for the purchase of chemicals/upgrading of infrastructure), data recording, documentation and communication were sought through the use of questionnaires. The above-mentioned plant staffs present at the time of survey were interviewed individually. 65 5.3 RESULTS AND DISCUSSION 5.3.1 Poor Maintenance Practices Lack of maintenance of equipment was noted to be a major management problem. About 60% of the SWTP operators interviewed in all the provinces studied (Eastern Cape, Free State, Western Cape, Mpumalanga and Limpopo Provinces) mentioned that equipments were not regularly maintained. This led to periodic equipment failures and the consequences of poor water quality. Indeed some operators asserted that the culture in most SWTPs was a culture of repairs or replacement of equipments and not maintenance of equipment. Several factors have been implicated to fan the poor maintenance culture. Such factors included lack of technical skills and appropriate training, inadequate or lack of relevant experience, inadequate funds and personnel. For example in North-West, Western Cape, Free State, KwaZulu-Natal, Eastern Cape, Mpumalanga and Limpopo Provinces, between 5.88-46.30% of the operators reportedly had educational qualifications of standard 8, 42-62% with Matric (with the exception of KwaZulu-Natal – 80%) whereas 0-53% were enrolled in post Matric qualifications. The implications of these trends are enormous because they typify the shortcomings and potential dangers in the water delivery system due to lack of appropriate qualification and training. The in-service training component is exemplified by the fact that in all the SWTPs studied, in the respective provinces, about 7-63% of the operators had not undergone relevant and appropriate training to enable them acquire technical skills for the job (Table 5.1). The main role of operators is to control the equipment and processes that remove or destroy harmful chemical compounds and micro-organisms from the water. This role is therefore mired in controversy because most of the operators lack technical knowledge of the equipment and technical processes. Some operators were not aware of how to determine flow rates, chlorine dosage or even the concept of chlorination as well as maintenance of technical equipments, measurement and documentation of processes. Above all, they lacked computational skills in an era of a rapidly changing information technology system. The ripple effects of these shortcomings can not therefore be ignored and should indeed be placed in the context of the reported management problems in some Local Government Authorities (LGAs) in South Africa. 66 TABLE 5.1 SOME NON-TECHNICAL ISSUES IMPACTING ON QUALITY OF WATER SERVICES DELIVERY IN SMALL WATER TREATMENT PLANTS IN SOUTH AFRICA Province Operator’s qualification (%) Std 8 Matric Post Experience (years) (%) <5 5-10 11+ Matric Salary (pm) (%) (Rand) 1000- 3000- 5000 2000 4000 + Training % Yes No LP 28 .0 56.0 22.0 32.0 34.2 38.0 32.3 41.0 16.0 53.0 47.0 MP 23 .0 51.0 20.0 29 .3 33.0 31.0 30.0 44.3 14.2 56.0 44.0 NW 18.37 61.22 20.41 37.50 34.38 28.12 36.59 48.78 14.63 50.0 50.0 FS 39.13 60.87 0 27.28 36.36 36.36 35.70 57.15 7.15 75.0 25.0 KZN 5.88 80.39 13.73 32.26 25.81 41.94 30.43 47.83 21.74 36.84 63.16 EC 46.30 42.60 11.10 30.51 23.73 45.76 26.0 66.0 8.0 36.73 63.27 WC 10.53 36.84 52.63 28.57 38.10 33.33 12.50 50 37.50 92.31 7.61 5.3.2 Training and Capacity Building Lack of technical skill has been highlighted as one of the major challenges to sustained quality water provision. Potential areas for capacity development include technical, managerial, marketing and public relations. This challenge underscores the need for upgrading and training of personnel but this has not been actively pursued by SWTPs in all the provinces studied (Table 1). For better coverage of the training programme, a training of training model could be used. This training should be task specific and guided by the contents of related policy documents and guidelines such as the Water Services Act – 1997, Strategic Framework for Water Services and Guidelines for Compulsory National Norms and Standards for Water tariff. The need for training is underscored by the inability of plant operators to calculate chlorine doses and calibrate or maintain equipments (Momba et al., 2004a; b; Momba et al., 2005b; c). This issue is compounded by shortage of human resource capacity in over 70% of SWTPs visited in the designated provinces. Coordinated efforts should equally be put in place to maximize the human resource capacity available in water provision support systems in the provinces. Such efforts could be achieved through strategic partnerships with relevant support agencies. Such partners could include academic institutions, research bodies, community social networks, CBOs, NGOs and relevant government departments. Partnership with research and academic institutions also offers opportunities for technical assistance and manpower through internship programmes where suitably prepared students and research fellows can take part in water supply activities (Momba and Brouckaert, 67 2005; Momba et al., 2005c). Capacity building should therefore cover a range of issues such as technical, social, finance, managerial and institutional (WHiRL, 2003). Specific technical needs of the treatment plants could include development of computational skills for plant operators, dosing calculations, calibration, operation and maintenance of technical equipments, measurement and documentation of processes and the interpretation of records or reports, measuring flow rate and use of instruments. 5.3.3 Poor Working Conditions Poor working conditions were also cited to hamper water services delivery. Poor working conditions were reflected as lack of comprehensive Medical Aid Scheme for operators, inadequate in-service training and capacitation, lack of motivation of operators by senior management, bureaucratic processes and poor salaries. In terms of salaries, about 12% - 37% of the operators earned between R1 000 and R2 000 per month while 40% to 66% earned between R3 000 and R4 000 per month and less than 40% earned above R5 000 in the SWTPs studied in the designated provinces (Table 5.1). In the wake of inflation, a salary range of between R1 000 and R2 000 for a staff member with over 10 years of working experience on the same job may not be adequate. Further salary increases are contingent on additional education attainments but this is apparently unattainable because job entry qualifications were rudimentary and current schemes for upward educational mobility or in-service capacitation are either non-existent or not implemented in some of the SWTPs visited across the designated provinces. This creates a situation of frustration and burnout due to lack of relevant education, training, skills development and performance of routine duties over years and poor incentives. 5.3.4 Insufficient financial capacity South Africa promotes free access to a safe and reliable source of water. The implementation of this policy operates by placing deferential tariffs on the rich to cater for the indigents. This policy challenges the capital requirement for water service operation and maintenance given the chances of inadequate production cost recovery. The viability of this policy and possible service expansion are predicated on the number of the rich who can afford to pay for water supply and sanitation as well as continued government commitment. Inadequate budgeting and financing was also noted to hamper service delivery in SWTP studied in the province. In virtually all the SWTPs in the designated provinces studied, inadequate funding for operational and implementation activities was mentioned as huge drawbacks for effective and efficient water services delivery. For example, 68 inadequate funding will affect maintenance culture, adequate stocking of water treatment chemicals, repairs and replacement of faulty equipments, recruitment of personnel to tackle routine and emergency issues. Although personnel interviewed were not aware of the level of funding or budgeting for SWTPs, they were unanimous in asserting that funding was grossly inadequate or mismanaged because, in most cases, operational activities were delayed or obviated due to lack of funds. Results of the survey have shown that chlorine is commonly used in small water treatment plants. To improve for example the chlorination efficiency in water sector, estimated costs was calculated taking into account the size and the design of the plant as well as the type of the disinfectants. These estimated costs are illustrated in Tables 5.2-5.11. 5.3.5 Inadequate community involvement Poor involvement of local communities was noted to be rampant at sub-national water service provision levels. Community participation could inform technology choices, quality of service, project citing and management structures. Experiences from community projects support that community participation and involvement is critical to quality improvement and project sustainability. Personnel from SWTPs mentioned that they did not interact regularly with their respective communities to ascertain currency in problems encountered, concerns and to proffer solutions. At best, they asserted that community involvement were informal and not coordinated. Inadequate community involvement causes a lapse in relaying information on water quality, management issues that may affect water distribution and inability to avoid or manage community concerns and displeasures before they spill into protest actions or crises situations. Sensitizing management practices to the above challenges is critical to their resolution and the enhancement of safe and reliable water supply. On this note, the above factors solicit peculiar management strategies and priorities. 5.3.6 Streamlining Duties and Job Description Although the job profiles of the role players in the water services delivery are clearly defined (Table 4.12), respondents at the various SWTPs maintained that the organogram was not often adhered to and this results in perceived overlap of activities. The significance of the organogram is that it facilitates accountability, efficient tracing of possible causes of system failures and precision in response. For instance, in the event of poor maintenance resulting to system failures, factors such as lack of funding or skills or negligence at a particular level of water distribution could be easily 69 implicated. This framework would ultimately suggest clear cut ways of dealing with the identified problems. The need for streamlining duties and responsibilities is reiterated by the potential dangers of dereliction of duty due to reported overlap of responsibilities of water plant operators and supervisors. It is important to highlight that the lack of manpower challenges the streamlining of duties and functions, as already discussed elsewhere in this chapter. Recruitment and retention of competent staff is hence vital. Figure 5.1 below illustrates a typical organogram for water service production. To facilitate the performance of the roles and responsibilities above, competent individuals should be recruited to serve in the various positions; position specific roles and responsibilities as well as organisational chain of command should be clearly stated to respective employees. Employees should also be conversant with disaster and emergency plans as well routine operational activities. District Municipality (WSA) Local Municipality (WSP) Municipal Manager Manager Engineering Services Supervisor Maintenance Department Operator/Shift Worker Operator/Shift Worker Plant 1 Plant 2 Fig. 5.1 Organogram for Water Service management 70 5.3.7 Poor Recording, Documentation and Communication Beyond the mechanical components in safe water supply, maintaining the quality of water supply is equally affected by the availability of adequate stocks of water treatment chemicals. For instance, an interruption in the supply of coagulants or disinfectants was the case in some SWTPs studied either due to system failures or unavailability would constitute a major emergency. To avoid this danger, proper recording of the rate of use of the various chemicals and actual stock taking by water treatment plant operators and timely replenishment is essential. This should also be closely monitored by higher authorities. However, respondents of the various SWTPs studied noted poor recording and documentation by different levels of management as the principal causes for stock depletion or interruption of supply of chemicals, reagents and equipments. In most cases, operators lacked knowledge of the exact inventory of chemicals, reagents and equipments. This lack of knowledge will obviously lead to various stock depletions, with ripple effects on quality of service delivery. In the event of the distribution of unsafe water, appropriate emergency plan should be instituted to avert or minimize the effect of the poor water quality. Such plans would initially consist of emergency prevention measures which are mostly related to plant maintenance, strikes and sabotage, natural disasters, equipment failures, ensuring adequate supply of chemicals, and various measures to protect the water treatment and distribution systems. Unfortunately over 50% of the operators were not aware of the existence of emergency prevention methods. This was generally attributed to poor communication between operators and management or operators and consumers. In fact poor water quality is not communicated to consumers. Suspected poor water quality or other problems should be communicated to the community and management in order to avoid disease outbreaks as was recently reported in Delmas, Mpumalanga. Effective communication ensures buy-in of relevant stakeholders, community sensitization and awareness and building of strategies to address pertinent issues such as the need to boil water or to treat water, by ant other method, in households for suspected poor water quality. 5.3.8 Emergency plans The plan should equally have a detailed description of the role and responsibilities of the different participants in water service provision (from the district WSA to plant operators/shift workers and community members). Efficient communication protocols should also be built into the emergency and routine plans. Such communication protocols should include internal communication procedures and public communication procedures. Outside emergency situations, communication between water service providers and the 71 public is critical in ensuring quality service. Through this, community participation and empowerment are facilitated. The sustainability of water supply projects is promoted by proper communication. Different community communication methods include education and awareness programmes, efficient consumer complain channels and emergency warning systems. It has been reported that internal and external communications were not in their best in most SWTPs (WHiRL, 2003). 5.4 RECOMMENDATIONS Given the above water resource management improvement priorities, there is a need to conduct competency assessments and appropriate training programmes for water service providers and regulators. The training programmes should also cover roles and responsibility clarification, information and communication systems. The development and implementation of operational checklists and protocols are equally essential to ensure timely ordering of materials (particularly chemicals) and the maintenance of equipment. Increased funding to SWTPs as well as enhancement of working conditions of personnel is also recommended. An urgent adoption of suitable water quality management protocols by the WSAs is critical for quality improvement and assurance. 72 TABLE 5.2 CALCIUM HYPOCHLORITE CAPITAL COSTS Typical Calcium Hypochlorite (HTH) Dosing System No. Basis for design Size Process flow rate 2.50 Ml/d Chlorine dose required 7 mg/l Cl2 No of days per year 365 Daily consumption 17.50 kg/d Cl2 Solution strength 5 % (m/v) No. batches prepared per day 1 Volume solution required per day 0.35 m3 Tank diameter (assume diameter 1 m) 1 m Area of tank 0.79 m2 Height of tank 0.45 m Allow free board of 0.3 m 0.30 m Total height of tank 0.75 m Required Cost (R) Estimated cost on 1 m3 tank No. Number of tanks required Unit Cost Required Cost Preparation tank size 1 Cost of preparation tank R5 000 2 R10 000 Baffles for make up tank R2 000 2 R4 000 Constant head tank size 0.02 Cost of constant head tank R5 000 2 R10 000 Axial flow mixers R15 000 2 R30 000 20 mm PVC pipework 1 000 1 R1 000 Electrical 10 000 1 R10 000 Fitting and installation 20 000 1 R20 000 m3 m3 Total capital costs R85 000 73 TABLE 5.3 CALCIUM HYPOCHLORITE OPERATING COSTS Design Flow Rate Process Ml/d Units 50 kg drums (incl VAT) 16.00 R/kg Purity % Cl2 68% %(m/m) Unit cost (R/kg) as chlorine (Cl2) 23.53 R/kg Cl2 Dose 7 mg/l Cl2 No. days per year 365 Daily consumption 17.50 kg/day Annual consumption as chlorine (Cl2) 6387.50 kg/year Annual consumption as chlorine (Cl2) 6.39 tons per year Annual cost Rands per year 150 294.12 R/year Cost per kl 16.47 Cents/kl Calcium hypochlorite unit of supply Unit cost (R/kg) calcium hypochlorite as delivered TABLE 5.4 TOTAL OPERATING COSTS FOR CALCIUM HYPOCHLORITE DOSING SYSTEM Fixed Capital Costs Item description Total Purchased equipment (installed) R85 000 Civil & structural R5 000 Total fixed capital investment R90 000 Operating costs Item Description R/y cents/kl Direct chemical cost R150 294 16.47 Maintenance R5 000 0.55 Direct operating costs R155 294 17.02 Depreciation 20% Fixed charges: depreciation (20%) R18 000 1.97 Total annual operating costs R328 588 36.01 74 TABLE 5.5 DESIGN OF TYPICAL SODIUM HYPOCHLORITE DOSING SYSTEM Average daily inflow Ml/d 2.5 Average inflow Ml/y 913 Chlorine dose mg/l 7 Chlorine dose kg/Ml 7 Chlorine consumption tons p/a 6.39 Mass balance Min Average Max Inflow Ml/d 1.50 2.50 3.00 Chlorine dosage mg/l 7.00 7.00 7.00 Chlorine dosage rate required kg/d Cl2 10.50 17.50 21.00 NaOCl specific gravity kg/l 1.05 1.05 1.05 Sodium hypochlorite - available % (m/v) 10.00 10.00 10.00 chlorine sold as 13-16%, but loses strength very quickly - use 10 % as average Sodium hypochlorite dosage l/d 105.00 175.00 210.00 hypochlorite dosage l/h 4.38 7.29 8.75 Min Average Max required Sodium required Storage capacity NaOCl consumption l/d 105.00 175.00 210.00 NaOCl consumption m3/d 0.11 0.18 0.21 Maximum supplier off-line time D 15.00 15.00 15.00 1.58 2.63 3.15 Min storage required cap. m3 Required Minimum bulk tank size Tanker load (as 12% delivered but m3 NaOCl 2 calculations based on 10% as it loses strength quite quickly) Safety factor 0.25 Tank size for accepting delivery m3 2.50 Equivalent chlorine delivered kg Cl2 200.0 75 TABLE 5.6 OPERATING COSTS OF TYPICAL SODIUM HYPOCHLORITE DOSING SYSTEM l 25 Cost as delivered R/l NaOCl R2.40 Sodium hypochlorite R/kg NaOCl R2.29 Sodium hypochlorite empty container price R/l container R57.00 Contribution of cost of container to overall price R/l container 2.28 Sodium hypochlorite total price as delivered R/l NaOCl R4.68 Chlorine total price as delivered R/kg Cl2 R46.80 Chlorine cost (delivered) R/ton Cl2 R46 800 Chlorine cost per annum R/y R298 935 Total inflow (based on average inflow) Ml/y 912.50 Chemical costs R/annum R298 935 Direct annual operating costs R308 935 Total annual operating costs R317 455 76 TABLE 5.7 FIXED CAPITAL AND ANNUAL OPERATING COSTS OF SODIUM HYPOCHLORITE DOSING SYSTEM Item Description No. of Unit Cost Total Purchased equipment (installed) R15 800 Bulk storage tanks, 2 m3 GRP 2 R3 ,000 R6 000 Day tank, 0.1 m3, GRP 1 R800 R800 Dosing pumps 2 R4 000 R8 000 Piping & fittings (PVC, C16) R1 000 Electrical & instrumentation R6 800 Level transmitter 2 R3 000 R6 000 Installation 1 R800 R800 Civil & structural R20 000 Bund & plinth 1 R4 000 R4 000 Total fixed capital investment R42 600 Annual Operating Costs R/y cents/kl Chemical cost of available chlorine R298 935 32.76 Maintenance R10 000 1.10 Direct operating costs R308 935 33.86 Fixed charges : depreciation (20% pa) R8 520 0.93 Total operating costs R317 455 34.79 77 TABLE 5.8 GAS CHLORINATION OPERATING COSTS Gas Chlorination Design Parameters Quantity Units Unit cost (R/kg) 12.00 R/kg Treated water flow rate 2.50 Ml/d Purity (% Cl2) 100.00 %(m/m) Dose (mg/l Cl2) 7 mg/l Cl2 Dose (kg/Ml) 7 kg/Ml Daily chlorine gas consumption 17.50 kg/d No of days per year 365 d/y Annual consumption 6387.50 kg/y Cl2 Annual cost R76 650 R/y Cost per kl 8.40 cents/kl Size of chlorinator 0.7 kg/h 78 TABLE 5.9 Description GAS CHLORINATION SYSTEM – CAPITAL COST Quantity Unit Rate Amount Cylinder manifold 2 R5 000 R10 000 Chlorinators, one duty and one standby. 2 R15 000 R30 000 Chlorine leak detector and alarm system dual sensor 1 R20 000 R20 000 chlorinator including connections 2 R4 000 R8 000 Heating & lagging 1 R5 000 R5 000 and chlorine injection sets, complete per spec. 1 R10 000 R10 000 1 Set breathing apparatus (gas respirators) 1 R20 000 R20 000 2 No. chlorine cylinders, 65 kg each filled 2 R8 400 R16 800 Scales 2 R10 000 R20 000 Exhaust fan, wall mounted capacity including controls 1 R15 000 R15 000 pipe, isolation valve and floor drainage system 1 R4 000 R4 000 1 No. first aid kit 1 R1 000 R1 000 1 Set warning and instruction signboards 1 R1 000 R1 000 4 No. filter cartridges for breathing apparatus. 4 R600 R2 400 2 No. chlorine gas injector. 2 R2 600 R5 200 4 No. set of 'O' rings for vacuum and dosing unit. 4 R4 100 R16 400 Duty/standby operating water booster bumps for Supply, delivery, installation and testing of the chlorination pipework, valves, fittings, water take-over Emergency shower including eyewash, water supply Spare Parts and Repair Fittings Total R184 800 79 TABLE 5.10 TOTAL OPERATING COSTS FOR GAS CHLORINATOR Fixed Capital Costs Item Description Total Purchased equipment (installed) R184 800 Civil & structural R10 000 Total fixed capital investment R194 800 Operating costs R/yr cents/kl Chemical cost R76 650 8.40 Maintenance R10 000 1.10 Direct operating costs R86 650 9.50 Fixed charges: depreciation (20%) R38 960 4.27 Total operating costs R125 610 13.77 TABLE 5.11 COST COMPARISON OF DISINFECTION ALTERNATIVES Gas/liquid Sodium Calcium Chlorination Hypochlorite Hypochlorite Capital cost R194 800 R317 455 R90 000 Direct operating Cost (c/kl) 9.50 33.86 17.02 Maintenance (c/kl) 1.10 1.10 0.55 Total operating cost (c/kl) 13.77 34.79 36.01 80 TABLE 5.12 ALLOCATION OF DUTIES AND RESPONSIBILITIES FOR PERSONNEL IN WATER SECTOR Position Duties and Responsibilities Plant operator Conduct daily water treatment activities in treatment plants Supervisors Oversee plant activities and operations; procurement of materials; requisition for repairs and maintenance and process control. Plant operators are answerable to them Water or engineering service manager Staff training; ensure water sufficiency, monitor the viability of treatment plants, co-ordination of plant maintenance, and handling of consumer complains, promotion norms and standards, sharing of information from external sources. Supervisors are answerable to them. Municipality manager Responsible for water safety, communication with the public on water issues. 81 CHAPTER VI: GENERAL CONCLUSIONS AND RECOMMENDATIONS In general, the majority (over 80%) of the small water treatment plants surveyed in the designated provinces were owned by the district municipalities. Virtually, the designed capacity of these plants varied between 1 and 60 Ml/day; the smallest had 100 m3/day and the largest had 120 Ml/day. The small water treatment plants abstracted their raw water from either surface or ground water or a combination of both water sources with greater preponderance for surface water sources (over 84%). Water treatment practices were noted to be the conventional type, mainly coagulation, flocculation, sedimentation, filtration and disinfection. Two types of coagulants, namely polyelectrolyte and alum, were commonly used by the variance water treatment plants across the provinces studied. Rapid gravity filtration, pressure filter and slow sand filtration systems accounted for 60%, 23% and 9% of the filtration systems across the provinces. Although disinfection practices were commonly used by the majority of the plants, the most predominant types employed were chlorine gas (69%) followed by calcium hypochlorite (14%). Chlorine dioxide, sodium bromide and ozone were least used. Over 50% of the various small water treatment plants investigated did not comply with the SANS (2005) Class I (< 1 NTU) and Class II (1-5 NTU) recommended turbidity values. The recommended target range of 0.3-0.6 mg/l free chlorine residual concentrations at the point of use were not met, indicating the possibility of microbial contamination and its ripple effect on disease transmission. It is concluded that about 70% of the small water treatment plants surveyed complied with the SANS (2005) criteria of microbiological safety of drinking water, vis-à-vis total and fecal coliforms. Operational problems affecting the efficiency of small water treatment plants included: i) inability to appropriately determine the flow rate, chemical dosage and turbidity, ii) lack of chlorine residual at the point of use and lack of water quality monitoring. Management issues affecting the efficiency of small water treatment plants included: i) lack of skilled operators and inadequate training operators, ii) poor maintenance practices, iii) inadequate improvement in working conditions and iv) poor water programme monitoring. To produce safe drinking water and enhance the profile and quality of service delivery of small water treatment, this study recommends the following: Operational practices must be implemented in all small water treatment plants. Operators 82 need to make adjustments to the operation of the plant from time to time in order to meet changing treatment requirements. Flow meters should be installed at all small water treatment plants for periodic monitoring of flow rates and for accurate determination of chemical dosages such as coagulants and chlorine. All small water treatment plants should be endowed with basic microbiological and physicochemical equipment for monitoring of water quality parameters. Evaluation and monitoring programmes should be done routinely as quality control measures. Adherence to a maintenance culture and increased budgetary allocation and in-service training of the operators should be prioritized. 83 REFERENCES AMERICAN WATER WORKS ASSOCIATION (1982) Disinfection Committee Report. J.AWWA 74 (7) 376-380. ANON (1995) Surface water for rural use. Watertek Info 61-71. APHA (1998) Standards Methods for the examination of Water and Wastewater (20th) edition American Public Health Association J.AWWA. 2005-2605. ASHBOLT NJ (2004) Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 198 (1-3) 229-238. BWENGYE E (2002) Development and sustainability of water supply scheme in Uganda. Workshop on sustainability of small water systems in Southern Africa 23. CAIRNCROSS S and FEACHEM R (1993) Environmental Health Engineering in the Tropics, 2nd edn, Wiley, Chichester. CDC (2002) Centre for Disease Control - Safe Water System Manual. CHLORINE CHEMISTRY COUNCIL (2003) Drinking water chlorination, a review of disinfection practices and issues. http://c3.org/chlorine_issues/disinfection/c3white2003.html. CLARK RM, GOODRICH JA and WYMER LJ (1993) Effect of the distribution system on drinking-water quality. J. Water SRT. 42: 03-38. CONSTITUTION OF THE REPUBLIC OF SOUTH AFRICA (1996) Act 108 of 1996, Republic of South Africa CRAUN GF and CALDERON RL (2001) “Waterborne disease outbreaks caused by distribution system deficiencies.” J. AWWA 93(9):64-75 DEPARTMENT OF NATIONAL HEALTH AND POPULATION DEVELOPMENT (2001) Cholera epidemic, Eastern Cape. DEPARTMENT OF WATER AFFAIRS AND FORESTRY (DWAF) (1996) South African Water Quality Guidelines for Domestic Use (2nd edition), Pretoria. DEPARTMENT OF WATER AFFAIRS AND FORESTRY (DWAF) (2002) Water is life; Sanitation is Dignity - White Paper on Water Services. Department of Water and Forestry, Pretoria, South Africa. 84 EAGLETON J (1999) Ozone in drinking water treatment a brief overview 106 years and still growing. Draft-JGE-2/1/99 http:www.hydroserve.com/ozone%20 documents/ozone_in_drinking_water_treatment.pdf. GLEICK, PH (2005) Dirty Water: Estimated death from water-related diseases 2000-2020. Pacific Institute Research Report. August 15, 2002. Pacific Institute for studies in development, environment and security. www.pacinst.org GRABOW WOK (1996) Waterborne diseases: Update on water quality assessment and control. Water SA. 22: 193-202. HRUDEY SE and HRUDEY EJ (2004) Safe drinking water: Lessons from recent outbreaks in affluent nations. IWA Publishing. London SW1H 0QS, UK. www.iwapublishing.com. HYDES O (1999) “European regulations on residual disinfectant”. J. Am. Water Works Assoc. 91 (1): 70-74. KAWAMURA S (1991) Integral design of water treatment facilities. John Wiley & Sons, Inc. New York. LECHEVALLIER MW (1998) Committee Report: Emerging pathogens: Names to know and bugs to watch out for. Microbial Contaminant Research Committee, American Water Works Association. LECHEVALLIER, SCHUZAND W and LEE RG (1991) Bacterial nutrients in drinking water. Appl. & Environ. Microbiol. 57: 857-862 MACKINTOSH GS and COLVIN C (2002) Failure of rural schemes in South Africa to provide potable water. Environ. Geology; 1-9. MOMBA MNB (1997) The Impact of Disinfection processes on Biofilm formation in Potable Water Distribution Systems. PhD thesis, University of Pretoria, South Africa. MOMBA MNB, CLOETE TE, VENTER SN and KFIR R (1998) Evaluation of the impact of disinfection processes on the formation of biofilms in potable surface water distribution systems. Water Sci. Technol, 38 (8-9), 283-289 MOMBA MNB and KALENI P (2002) Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 36 3023-3028. MOMBA MNB and P KALENI (2002a) Regrowth and survival of indicator micro-organisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 36, 3023-3028. 85 MOMBA MNB and BINDA (2002b) Combining chlorination and monochloramination processes for the inhibition of biofilm formation in drinking surface water system models. J.Appl. Microbiol. 92, 641-648. MOMBA MNB, NDALISO S and BINDA MA (2003a) Effect of a combined chlorinemonochloramine process on the inhibition of biofilm regrowth in potable water systems. Journal of Water Supply 3 (1-2), 215-221. MOMBA MNB and MAKALA (2004) Comparing the effect various pipe materials on biofilm formation in chlorinated and combined chlorine-chloraminated water systems. Water SA. 30 (2), 175-182. MOMBA MNB and NOTSHE TL (2003) The effect of long storage and household containers on the microbiological quality of drinking water in rural communities of South Africa J. Water Suppl. Res.& Technol.-AQUA, 52 (1): 67-76. MOMBA MNB, Z TYAFA and N MAKALA (2004)a Rural water treatment plants fall to provide potable water to their consumers: the Alice water treatment plant in the Eastern Cape Province of South Africa. S.A J. Sc. 100: 307-310. MOMBA MNB, MAKALA N, B BROUKAERT, TYAFA Z, THOMPSON P and CA BUCKLEY (2004)b Improving the efficiency and sustainability of disinfection at a small rural water treatment plant. Water SA. 30 (5): 617-622. MOMBA MNB and BROUCKAERT MB (2005) Guidelines for ensuring sustainable effective disinfection in small water supply systems. TT 249/05 WRC. ISBN No 1-77005-321-2. RSA. MOMBA MNB, MALAKATE VK and J THERON (2006) Abundance of pathogenic Escherichia coli, Salmonella typhimurium and Vibrio cholerae in Nkonkobe drinking water sources. J. Water & Health 04: 289-296. MTETWA S and SCHUTTE CF (2002) An interactive and participative approach to water quality management in agro-rural watersheds. Water SA. 28 (3) 334-337. MUYIMA NYO and NGCAKANI F (1998) Indicator bacteria and regrowth potential of the drinking water in Alice, Eastern Cape. Water SA. 24 (1) 29-34. NDALISO S (2000) The disinfection efficacy of combined chlorine-monochloramine process against bacterial regrowth I a groundwater laboratory-scale system. Honours Thesis, Department of Microbiology, University of Fort Hare, South Africa. NEVONDO TS and CLOETE TE (1999) Bacterial and chemical quality of water supply in the Dertig village settlement. Water SA 25 (2):215-220. 86 OBI CL, POTGIETER N, BESSONG PO and MATSAUNG G. (2002) Assessment of microbial quality of River water sources in rural Venda communities in South Africa. Water SA 28 (3) 287-292. OBI CL, POTGIETER N, BESSONG PO, IGUMBOR EO and GREEN E (2003a) Prevalence of pathogenic bacteria and retroviruses in the stools of patients presenting with diarrhoea from rural communities in Venda, South Africa. SA. J. Sc. 99 589-591 OBI CL, POTGIETER N, BESSONG PO and MATSAUNG G (2003b) Scope of potential bacterial agents of diarrhea and microbial assessment of quality of river water sources in rural Venda communities in South Africa. Water Sci. Technol 47 (3) 59-64. PEARSON and IDEMA (1998) An assessment of common problems associated with drinking water disinfection in developing areas. Division of Water, Environment and Forestry Technology. Pretoria. Water Research Commission Report No 649/1/98. PILLAY V and JACOBS EP (2004) The development of small-scale Ultra-filtration systems for potable water production. WRC Report. 20-29. SAMI K and MURRAY EC (1998) Guidelines for the evaluation of water resources for rural development with an emphasis on groundwater. Water Research Report No. 677/1/98. SANS 241 (2005) South African National Standard-Drinking Water, 6 edn. Standards South Africa. ISBN 0-626-17752-9. SMITH V (1995) “Complacency caused Milwaukee’s crypto outbreak”. J. AWWA 87 (1):8-9. SOLSONA F and PEARSON I (1995) Non-conventional disinfection technologies for small water systems. Water Research Commission Report No. 449/1/95. SWARTZ CD (2000) Guidelines for the upgrading of existing small water treatment plants. WRC Report No. 730/1/00. UN WORLD WATER ASSESSMENT PROGRAM (2003) World Water Development Report: Water for people, Water for life. United Nations Educational and Scientific Organisation 9, Paris USEPA (1998) Optimizing Water Treatment Plant Performance Using the Composite Correction Program. Cincinnati, Ohio, U.S. Environmental Protection Agency: 245pp. USEPA (1999) Alternative Disinfectants and Oxidants Guidance Manual. Washington, D.C., U.S. Environmental Protection Agency: 346pp 87 VAN DUUREN FA (1997) Water purification works design. Water Research Commission (WRC project No 504). ISBN 1 86845 345 6. Republic of South Africa. VERMEULEN A (2000) Institutional framework for water services provision in rural areas. Workshop on sustainability of small water systems in Southern Africa, 4. VOORTMAN WJ and REDDY CD (1997) Package water treatment plant selection. Water Research Commission report No. 450/1/97. WATER, HOUSEHOLD AND RURAL LIVELIHOODS (WHIRL) (2003) Institutional challenges for water resources management: India and South Africa. WHiRL Project Working Paper 7 (Draft). Available at http://www.nri.org/whirl accessed on 30/09/2005 WATER RESEARCH COMMISSION (1998) Quality of domestic water supplies: Volume 1; Assessment guide. WRC Report no TT 101/98, Water Research Commission, Pretoria, RSA WHITE GC (1992) The handbook of chlorination and Alternative Disinfectants (3rd edn). Van Nostrand Reinhold, New York WHITE GC (1999) Handbook of chlorination and Alternative Disinfectants (4th edn.) Wiley Interscience, John Wiley and Sons Inc., NY. WHO (1982) Rural water supplies. Stevenage, World Health Organisation. WHO (1997) Guidelines for drinking water quality-surveillance and control of community supplies. Geneva, World Health Organisation. WHO (2002a) Water, sanitation and hygiene links to health. Online at: http://www.who.int/docstore/water-sanitation -health/General/facts&fig.pdf WHO (2002b) Managing Water in the Home: Accelerated Health Gains from Improved Water Supply. World Health Organisation. WHO (2002c) Global water supply and sanitation- Assessment 2000 Report. World Health Organisation WHO (2003) Right to Water. World Health Organisation. www.who.org. 88 Appendix 1 Section A: Plant Number A01 Plant No. A02 Database # OFFICE USE ONLY Section B: General Information B01 Plant name B02 Previous plant name (if changed during the past 10 years) B03 Owner name B04 Owner type Municipal DWAF Other gov. Urban Peri-urban Rural Private Postal address B05 B06 Postal code B07 Town / nearest town B08 Locality B09 Province B10 Coordinates B11 Contact person B12 Telephone B13 Fax B14 E-mail NS EW Section C: Plant Manager / Supervisor C01 Name C02 Qualifications C03 Experience yrs Section D: Valid Date D01 Survey Date 89 Farm Park Other (specify) Section E: Plant History E1 Year built E2 Designer / supplier E3 Age of plant E4 Year upgraded E5 Designer / supplier E6 Age of upgrading E7 Description of upgrading yrs Section F: Capacity F1 Design capacity Ml/day F2 Inflow Ml/day F3 Current flow Ml/day F4 Losses F5 Population served F6 DWAF Classification of plant Don't know Outflow Ml/day Don't know % Don't know People Section G: Raw water characteristics Turbidity G1 G2 Raw water type (can be one or more than one) Raw water source G3 Name of source G4 Abstraction method G5 G6 Colour Salinity Raw water turbidity G8 Raw water pH G9 Raw water colour Algae Alkalinity x Iron Manganese Nitrate Fluoride Other (specify) Dam River Spring Borehole Sea x Presence of treated wastewater Presence of industrial effluent G7 Pollution NTU mg/l as Pt/Co / Hazen / colour units 90 G10 Raw water alkalinity mg/l as CaCO3 G11 Raw water conductivity G12 Chemical properties (mg/l) G13 chlorophyll a ug/l G14 Raw water DOC mg/l G15 Taste and odour TON G16 Temperature G17 DO G18 Other (specify) mS/m TDS Iron Fluoride Manganese Nitrate Hardness °C Section H: Treatment methods and chemicals, coagulation and disinfection H1 Main treatment process (can be more than one) Turbidity removal Colour removal Desalination Stabilisation /softening Algae removal Nitrate removal Fluoride removal Manganese & Iron removal Taste & odour removal Other (specify) Chlorination Ozonation Aeration Solids removal Other (specify) H2 Pretreatment processes H3 Coagulation H4 Coagulation Condition H5 Flocculation H6 Visible Floc Yes No pH adjustment chemical Lime Soda ash CO2 Other (specify) Alum Ferric chloride Ferric sulphate Poly-electrolite (specify) PAC Aluminium Sodium Alum Other (specify) Hydraulic (open channel) Good Poor Hydraulic (open channel) Hydraulic (static in-line) Good Poor Mechanical Good Hydraulic (pipe flocculation) H9 Coagulant type 91 Poor Mechanical H7 H8 Disinfection H10 Cost of Coagulation System H11 Installation Cost H12 Maintenance Cost H13 H14 H15 Dosing Pump Make Dosing Pump Model Dosing Pump Model H16 Dosing Point H17 Dose mg/l H18 H19 Condition of Equipment Power Consumption Watts H20 H21 Flocculant type H23 Disinfection/ Oxidation type H24 Cost of Disinfection System H25 Installation Cost H26 Maintenance Cost H27 H28 H29 Dosing Point H31 Dose mg/l H33 H34 H35 H36 Poor Poor Good Liquid Solid Aeration Chlorination Dosing Pump Make Dosing Pump Model Dosing Pump Model H30 H32 Good Condition of Equipment Power Consumption Watts Backup Disinfection Disinfection Contact Chamber Poor Good Baffling 92 Specify type Ozonation Potassium Permanganate Hydrogen peroxide Other (specify) H37 Disinfection Demand H37 Settling type H38 H39 H40 Flotation type Horizontal Upflow Dissolved air Induced air Roughing Rapid sand CATRIGE BAG Act Carbon Act Alumina Filter type Adsorption type H41 Disinfection chemical H42 Stabilisation chemical H43 Advanced treatment type Chlorine (gas) Chlorine (liq) Lime Soda ash Sludge blanket N/A SLOW SAND GRAITY SAND N/A Chlorine (solid) Other (specify) Pulsator High rate UPFLOW PRECOAT OTHER (SPECIFY) Ultraviolet CO2 Limestone RO NF Other (specify) Ozone Other (specify) X IX ED UF MF Section J: Residuals management J1 Methods of treatment J2 Method of disposal J3 Recycling Coagulant recovery Recycling of residuals streams Discharge to sewer Drying bed Sludge dam Sewer Settling Thickening Other (specify) Land application Section K: Operation Fully equipped Partially equipped Basic instr. Only K1 Laboratory details K2 Analyse acc to SABS YES X No Don't Know K3 Frequency of on-site monitoring Turbidity Residual chlorine pH 93 None Colour Metals Hardness M: monthly, 2: every 2 months, 3: every 3 months, A: annually W: once weekly, D: once daily Microbiological Other (specify) None Turbidity Frequency of external monitoring K4 M: monthly, 2: every 2 months, 3: every 3 months, A: annually W: once weekly, D: once daily Residual chlorine pH Microbiological Colour M NAME OF COMPANY/ AUTHORITY K5 Number of operators required Fulltime Daytime Part-time K6 Number of actual operators Fulltime Daytime Part-time K7 Operator Qualification Number of actual Operators Number of Support & Maintenance Staff K9 Plant Operation Manual Available on Plant K10 Hardness Other (specify) K5 K8 Metals NQF2 - Std 8 NQF2 NQF4 NQF5 NQF4 - Std 10 (Matric) Std 5 Mech Tech Elec Tech NQF5 - Matric + 2 Proc Tech Workshop Tools Available on Plant Available in Municipal Stores Section S: Staff Retention Operator 1 S01 Salary S02 Experience on this plant - Years S03 Experience as an Operator incl other plants S04 Do the operators udergo training ? S05 Training Provider S06 Frequency of Operator 2 Operator 3 Yes Internal External 6 mths 12 mths 94 24 mths Operator 4 Operator 5 Training S07 Type of Training S08 Automation S09 External monitoring (Name of Company / Authority) Technical Admin Management Fully Partial Manual Section T: Filtration T01 No of Filters T02 Type of media T03 Media Size T04 Filter type T05 T06 Flow Pattern onto Filter Frequency of Backwash T07 Recycle T08 Automated Section R: Costs R01 Original Capital Cost of Plant R02 Chemical treatment Costs R03 R04 Human Resource Costs Maintenance Costs Per Annum Per m3 Per Annum Per m3 Per Annum Per m3 Section V: Water Quality of Treated Water V01 Treated water turbidity V02 Treated water pH V03 V04 Treated water Free Chlorine Treated water alkalinity NTU mg/l as Pt/Co / Hazen / colour units mg/l as CaCO3 95 Financial Telemetric V05 Treated water conductivity V06 Chemical properties (mg/l) V07 chlorophyll a ug/l V08 Treated water DOC mg/l V09 Taste and odour TON V10 Temperature V10 DO V10 Other (specify) Total Coliforms (CFU/100 ml) Fecal Coliforms (CFU/100ml) Standard Plate Count (CFU/100 ml) V11 V12 V13 mS/m TDS Iron Fluoride Manganese Nitrate Hardness °C Section W: Water Quality of Distribution System W01 W02 W03 W04 W05 W06 W07 Sample Taken From Distribution water turbidity Distribution water pH Distribution water Free Chlorine Distribution water alkalinity Distribution water conductivity Shop Garage Clinic Other (specify) NTU mg/l as Pt/Co / Hazen / colour units mg/l as CaCO3 mS/m TDS Iron W08 Chemical properties (mg/l) W09 chlorophyll a ug/l W10 Distribution water DOC mg/l W11 Taste and odour TON W12 Temperature W13 DO W14 Other (specify) Fluoride °C 96 Manganese Nitrate Hardness if available Appendix 3.2 Water Treatment Processes and Equipment The Disinfection processes and equipment used by the small water treatment plants in Waterberg, Sekhukuni, Capricorn, Mopani, Vhembe (Limpopo Province) and Ehlanzeni, Nkangala (Mpumalanga Province) Districts and local municipalities. Mpumalanga District Municipality (Local Municipality) Name of Plants (Owner type) Water source Treatment processes (Disinfection practices) Equipment Lydenburg (Municipality) Surface water Sabie (Municipality) Ground water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Chlorination Sud floc Open channel flocculators, Lime Circular settlers, Rapid gravity sand filters, Chlorine gas, Alldos pumps Alldos pump Nelspruit (Private: Biowater's) Surface water Coagulation, Stabilisation, Flocculation, Sedimentation, Filtration, Chlorination White river (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Sudfloc Lime Open channel flocculators, Circular settlers, Rapid gravity sand filters, Aldos pumps, Chlorine gas Telemetric control panel, Basic laboratory instruments Sudfloc, Open channel flocculators, Lime Circular settling tanks, Rapid gravity sand filters, Pressure filters, Chlorine gas, Basic laboratory instruments Ferric chloride, Static mixing Lime Circular settling tank Pressure filters HTH Ferric chloride, Open channel flocculators, Soda ash, Circular settling tanks, Rapid gravity sand filters, Aldos pumps, Chlorine gas Basic laboratory instruments Ehlanzeni (Thaba Chweu) (Mbombela) Chlorination White river C E (Municipality) Surface water Hazyview (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination 97 Malelane (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Matsulu (Private: Biowater's) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Nka Nyamazane re (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Nka Nyamazane old (Municipality) Surface water Coagulation, (Emalahleni) Witbank (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc, Open channel flocculators, LIME, Mechanic feeders, Circular settling tanks, Rapid gravity sand filters, Aldoz pumps, Chlorine gas, Telemetric control panel, Basic laboratory equipment (Highlands local) Belfast (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc, Seiko pump Open channel flocculators, LIME, Mechanic feeder, Horizontal settling tanks, Rapid gravity sand filters, Soni X100 pumps, Chlorine gas, Telemetric control panel, Dullstroom (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Polyelectrolyte, Open channel flocculators, LIME, Horizontal settling tanks, Rapid gravity sand filters, HTH Manual control (Nkomazi) Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc 3450 Open channel flocculators, Lime, Horizontal settling tanks, Rapid gravity sand filters, TEKNA pumps, Chlorine gas, Sud floc 3450, Open channel flocculators, Lime, Horizontal settling tanks, Rapid gravity sand filters, TEKNA pumps, Chlorine gas, Telemetric control panel Sud floc 3450, Open channel flocculators, LIME Horizontal settling tanks, Rapid gravity sand filters, Aldoz pumps, Chlorine gas, Telemetric control panel, Fully equipped laboratory Sud floc 3450, PFINZTAL DN632N. Open channel flocculators, LIME Horizontal settling tanks, Pressure filters, Aldoz pumps, Chlorine gas, Telemetric control panel, Nkangala 98 Machadodorp (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc 3456, Open channel flocculators, LIME, Horizontal settling tanks, Rapid gravity sand filters, pumps, Chlorine gas, Manual operation Waterval Boven (Municipality) Surface water Coagulation, Polyelectrolyte U 3800, TEKNA 400 pump, Open channel flocculators, LIME, Horizontal settling tanks, Rapid gravity sand filters, TEKNA pump, Chlorine gas, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination (Steve Tshwete local) Middelburg (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc, Open channel flocculators, LIME, Circular settling tanks, Rapid gravity sand filters, Prominent pumps, Chlorine gas, Kruger Dam (Municipality) Surface water Coagulation, Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc, Open channel flocculators, LIME, Mechanic feeders, Circular settling tanks, Rapid gravity sand filters, Aldoz pumps, Chlorine gas, Telemetric control panel, Presidentsrus (Municipality) Surface water Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Sud floc, HELDEL pump Open channel flocculators, LIME, Circular settling tank, Pressure filters, HTH Hendrina (Municipality) Surface water Coagulation Aluminium chloride, Milton Roy pump Open channel flocculators, LIME, Horizontal settling tank, Rapid gravity sand filters, Chlorine gas, Wallace and Tierman pump Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination 99 Limpopo Province Vhembe district Municipality (Messina local) Messina (DWAF) Coagulation Surface water Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination (Makhado) Makhado (Municipality) Tshakhuma Regional (DWAF) Tshakhuma (DWAF, BioWaters) Tshedza (DWAF) Mutshedzi (DWAF) Tshifhire (DWAF) (Thulamela) Vondo (DWAF) Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Surface water Coagulation Surface water Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Surface water Surface water Chlorination Coagulation Surface water Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Coagulation Surface water Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Coagulation Surface water 100 Aluminium sulphate , ALDOS pump Open channel flocculator Lime Rapid gravity sand filters Chlorine gas, HTH, Polyelectrolyte, Milton Roy pump Lime Open channel flocculator, Rapid gravity sand filters Chlorine gas Ferric Floc HC 500, ALDOS pump, Open channel flocculators Lime Rapid gravity sand filters Chlorine gas, HTH Static mixer Open channel flocculator Lime Rapid Gravity Sand Filters, Pressure filters, HTH, HYDROCARE pump Polyelectrolyte, HYDROCARE pump Open channel flocculator Soda ash Horizontal settlers Pressure filters HTH Aluminium sulphate, ALDOS pump, Pen channel flocculator Lime Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Rapid Gravity Sand Filters Chlorine gas and HTH Polyelectrolyte, Hydrocare, ALDOS pump Static mixers Soda ash Horizontal settlers Pressure filters HTH Coagulation Flocculation, Sedimentation, Filtration, Polyelectrolyte Open channel flocculator Horizontal settlers Rapid gravity sand filters Dzingahe (DWAF) Phiphidi (DWAF) Surface water Chlorination Coagulation Surface water Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Damani (DWAF) Malamulele (DWAF) Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Coagulation Surface water Surface water Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Mudaswali (DWAF) Surface water Dzindi (DWAF) Surface water Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation Filtration, Chlorination Mhinga (DWAF) Coagulation Surface water Flocculation, Stabilisation Filtration, Chlorination Shikundu (DWAF) Coagulation Surface water Flocculation, Stabilisation Filtration, Chlorination 101 Chlorine gas, HTH Aluminium sulphate, Syberg74 Rahier Static mixers Lime Circular settling tank Pressure filters HTH Polyelectrolyte, Hydro-care AC 500, Milton Roy pump Open channel flocculators Lime Horizontal settling tank Rapid Gravity Sand Filters Chlorine gas, HTH, Wallace and Tierno pumps Alu Floc, Open channel flocculators Lime Rapid Gravity Sand filters Chlorine gas, HTH Ferric Floc HC 500, ALDOS D76327 Open channel flocculators Lime Rapid Gravity Sand Filters Chlorine gas, HTH, CONTROL MATIC M20 pump Telemetric control panel Aluminium sulphate Pipe flocculation Lime Circular settling tank Pressure filters HTH, SEIKO pump Aluminium chloride, DESAPRO MILTON ROY pump Static Mixers Soda ash Pressure filters Chlorine gas, C103 GECO ALLDOS Polyelectrolyte (Hydrocare), Milton Roy G020 -611M pump Static mixer Soda ash Pressure filters Chlorine gas, HTH, ALDOS C103 GECO Polyelectrolyte, HYDROCARE, ALDOS S/NO2/16562 Open channel flocculators Lime Rapid Gravity Sand Filters Chlorine gas, Chlorinator Inc (Mutale local) Mutale (DWAF, Municipality) Surface water Tzaneen (Municipality) Surface water Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Polyelectrolyte, Hydrocare CEP 153/39/NM Open channel Lime Horizontal settlers Slow sand filters Chlorine gas, HTH Mopani district (Greater Tzaneen local) George’s Valley (Municipality) Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Surface water Nkowankowa (DWAF) Surface water Semerela (DWAF) Surface water Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Thapane (DWAF) Surface water Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Nkambako (DWAF) Surface water Letsetele (Municipality) Surface water Coagulation Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Coagulation Flocculation, Stabilisation Sedimentation, 102 Ferri floc Open channel floculators Lime Rapid sand filters Chlorine gas, HTH Ferri floc, Seiko pump Open channel flocculators Lime Rapid Gravity Sand filters Chlorine gas Polyfloc, Encore 100 Pump Open channel flocculator Lime Horizontal settling tanks Rapid gravity sand filters Chlorine gas, HTH, Wallace and Tienan pump Poly Floc, ENCORE 100 pump, Static mixer Open channel flocculators Soda ash Horizontal settlers Pressure filters Chlorine gas, HTH, Wallace and Tienan pump Telemetric control panel Poly Floc, ENCORE 100 PUMP, Open channel floculators Soda ash Horizontal settlers Rapid gravity sand filters Chlorine gas, HTH, Wallace and Tienan pump Poly Floc, ALDOS pump Open channel flocculators Lime Horizontal settlers Rapid gravity sand filters Chlorine gas, HTH Aluminium sulphate, ALDOS pump Open channel flocculators, Lime Horizontal settlers Capricorn (Polokwane Filtration, Chlorination Rapid gravity sand filters Chlorine gas, HTH Pietersburg (Polokwane Municipality) Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Seshego (Municipality) Surface water Maratapilu (DWAF) Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Coagulation Flocculation, Sedimentation, Filtration, Chlorination Poly, ELATRON pump, Open channel flocculation, Horizontal settling tanks Rapid gravity sand filter, Chlorine gas, WALLACE and TIENAN pump Poly, Prominent pump Open channel flocculators Horizontal settling tanks Rapid gravity sand filters Chlorine gas, HTH Polyelectrolyte Open channel flocculators, Horizontal settling tanks Slow sand filters HTH (Modimole local) Nylstroom: Modimole (Municipality) Surface water Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Ferric floc, Prominent pump Open channel flocculators, LIME, Horizontal settling tank, Rapid gravity sand filters, HTH, (Bela Bela local) Bela Bela (Municipality) Surface water Coagulation Flocculation, Stabilisation, Sedimentation, Ferric floc, Prominent pump Open channel flocculators, LIME, Horizontal settling tank, Circular settling tank Rapid gravity sand filters, HTH, Cartridge filling, ECO JET 130 pump local) Waterberg Filtration, Chlorination (Mogalakwena local) Potgietersrus (Mokopane) Surface water (Thabazimbi local) Thabazimbi (Municipality) Surface water and ground water Chlorination Chlorine gas, Prominent pump (Mookgopong local) Naboomspruit (Municipality) Surface water Coagulation Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Polyelectrolyte, ASA Water pump Open channel flocculator, Lime, mechanic feeder, Horizontal settling tanks Slow sand filters Chlorine gas, Wallace and Tienan pump Burgersfort (Municipality) Surface water Coagulation Flocculation, Ferri Floc, SECO pump Pipe flocculator Sekhukuni District (Greater tubatse local) 103 Sedimentation, Filtration, Chlorination Coagulation Tubatse (Praktiseer) (Municipality) Surface water Ohrigstad (Municipality) Steelpoort (Municipality) Ground water Chlorination Chlorination Ground water No treatment Flocculation, Sedimentation, Filtration, 104 Horizontal settling tanks Rapid gravity sand filters Chlorine gas, Wallace and Tienan pump Aluminium Chloride, Milton Roy pump Open channel flocculators Horizontal settling tanks, Rapid gravity sand filters, Slow sand filters Chorine gas, Choroquip HTH, SERA C21 pump Plant Water source North West Province. Disinfection Equipment practices Bloemhof Surface water-river Coagulation Surface water-river Flocculation Sedimentation Filtration Chlorination Pre-chlorination Christiana Aquafloc 1090&3070 ALLDOS pump and Kemix mixer systems Circular scraper clarifier Coagulation Pudumong Surface water-river Dissolved Air Flotation Sedimentation Filtration Chlorination Coagulation Slow sand filters Chlorine gas, ALLDOS oxygraph 304 pump Chlorine gas ALLDOS pump Powdered Activated Carbon &ALLDOS Premus serie pump DAF system Horizontal settling tanks Slow sand filters Chlorine gas &ALLDOS pump Aluminium chloride & ALLDOS Primus serie pump Open channel flocculator Flocculation SchweizerReneke Lichtenburg Groot Mariko Surface water-dam Ground waterboreholes Surface water-river Sedimentation Filtration Chlorination Coagulation Flocculation Sedimentation Dissolved Air Flotation Filtration Chlorination Chlorination Solid removal Coagulation Circular scraper clarifier Slow sand filters Chlorine gas - AllDOS Sud-floc3TL & ALLDOS pump Open channel flocculators Horizontal settling tanks DAF system Slow sand filters Chlorine gas& ALLDOS pump Chlorine gas & ALLDOS primus serie pump Leave strainer Sudfloc3484 & ALLDOS pumps Soda ash and dry chemical feeder pH stablisation Flocculation Sedimentation Filtration Chlorination Pela Surface water-dam Coagulation pH stabilization Flocculation Sedimentation Chlorination Filtration 105 Pipe flocculation system Upflow settlers Pressure filters Chlorine gas & S10K Wallace and Terran pump Aluminium sulphate and ultrafloc 5082, ALLDOS primus serie pumps Soda ash Pipe flocculation system Cyclone clarifiers Granular HTH & ALLDOS pump Rapid sand filters Madikwe Surface water -dam Coagulation pH stabilisation Flocculation Sedimentation Chlorination Mmabatho Surface water-dam Filtration Solid removal Coagulation pH stabilisation Mafikeng Itsoseng Koster Swartruggens Ground waterboreholes and springs Ground waterboreholes Surface water –dam and ground waterboreholes Surface water-dam Surface water-river DAF system Rapid sand filters Chlorine gas & ALLDOS pumps Coagulation Ultrafloc & ALLDOS pump Hydraulic (Open channel) flocculators Horizontal settling tanks Rapid sand filters Chlorine gas & ALLDOS pump Flocculation Sedimentation Filtration Chlorination Coagulation Flocculation Sedimentation Filtration Chlorination Coagulation Flocculation Dissolved Air Flotation Sedimentation Filtration Chlorination Hartbeespoort Surface water - dam Ventersdorp Ground water- spring Potchefstroom Surface water - dam Leave strainer Ferric chloride and ALLDOS pump Soda ash-dry chemical feeders Circular scraper clarifier Flocculation and sedimentation Dissolved Air Flotation Filtration Chlorination Screening Filtration Chlorination Chlorination pH stabilization Madibeng Calcium chloride and ultra-floc – ALLDOS pumps Soda ash Pipe flocculation system Upflow settlers Granular HTH – ALLDOS pump Pressure filters Coagulation Flocculation Dissolved Air flotation Filtration Chlorination Filtration Chlorination Coagulation 106 Pressure filters Chlorine gas and ALLDOS pumps Chlorine gas and ALLDOS pumps Ferric chloride and ALLDOS pump Lime and dry chemical feeders Pulsators Horizontal settling tanks Pressure filters Sodium hypochlorite& ALLDOS pumps Aluminium chloride U3400, Plaestol 611BC and Powdered Activated carbon- ALLDOS pumps Open channel flocculators DAF system Pulsator Clarifiers Rapid sand filters Chlorine gas –ALLDOS pump Aluminium chloride-U3500 and Powdered Activated carbon- ALLDOS dosing pump Open Channel Flocculators DAF system Rapid sand filters Chlorine gas –Regal dosing pumps Slow sand filters Chlorine gas- Hydrogas chlorinator Ferric chloride- ALLDOS pump Flocculation Chlorination Sedimentation Filtration Chlorination 107 Open channel flocculators Chlorine gas- ALLDOS pump Circular scraper clarifiers Rapid gravity sand filters Chlorine gas-ALLDOS pump Free State Province Local Municipality Plants Water source Disinfection practices Equipment HarrismithWilge Plant Wilge River Botanical plant Dam and Wilge River Phumelela Warden Dam Phumelela Memel Dam Coagulation, Flocculation, Sedimentation, Filtration, Chlorination pH correction Coagulation, Flocculation, Sedimentation, Filtration, Chlorination pH correction Coagulation, Flocculation, Sedimentation, Filtration, Chlorination pH correction Coagulation, Flocculation, Sedimentation, Filtration, Chlorination Villiers River Polyelectrolyte Open channel flocculation, Horizontal flow clarifiers, Rapid gravity sand filters, Chlorine gas, dosing pump Dry lime feeder Polyelectrolyte Flocculation in clarifier, Radial flow clarifier, Rapid gravity sand filters, Chlorine gas, dosing pump Dry lime feeder Polyelectrolyte Open channel flocculation, Horizontal flow clarifier, Rapid gravity sand filters, Chlorine gas, dosing pump Dry lime feeder Polyelectrolyte Open channel flocculation, Vertical flow clarifier, Dual media pressure filters, Chlorine gas, Alldos chlorinator Polyelectrolyte (Prominent pumps) Open channel flocculation, Horizontal and vertical flow clarifiers, Rapid gravity sand and pressure filters, Ecometric gas chloronator, Dry lime feeder Polyelectrolyte (ACL pump) Open channel flocculation, Radial flow clarifier, Rapid gravity sand filters, Wallace and Tienan gas chlorinator Dry lime feeder Polyelectrolyte (Alldos pumps) Open channel flocculation, Horizontal flow clarifiers, Rapid gravity sand filters, Ecometric gas chloronator, Dry lime feeder Polyelectrolyte (Alldos pump) Pipe flocculation, Vertical flow clarifier, Dual media pressure filters, Maluti-A-Phofung Coagulation, Flocculation, Sedimentation, Filtration, Mafube Metsimaholo Chlorination pH correction Coagulation, Flocculation, Sedimentation, Filtration, Chlorination pH correction Tweeling River Frankfort River Coagulation, Flocculation, Sedimentation, Filtration, Chlorination pH correction Oranjeville Dam Coagulation, Flocculation, Sedimentation, Filtration, Chlorination 108 pH correction Ngwathhe Parys River Koppies - River Vredefort - River Pre-chlorination Coagulation Flocculation Sedimentation Filtration Chlorination pH correction Coagulation Flocculation Sedimentation Filtration Chlorination Edenville Coagulation, Flocculation, Sedimentation, Filtration, Chlorination Borehole Disinfection 109 Calcium, hypoChlorite gas; Alldos pump Soda ash; Alldos pump Sodium hypochlorite pump Ployeletrolyte (Alldos pump) Flocculation in clarifiers, Radial flow clarifier, Rapid gravity sand filters, Sodium hypochlorite pump Dry lime feeder Polyelectrolyte (Alldos pump) Open channel flocculation, Horizontal and vertical flow clarifiers, Rapid gravity sand filters, Sodium hypochlorite pump, Alldos pump Polyelectrolyte (pump) Open channel flocculation, Horizontal flow clarifiers, Rapid gravity sand filters, Sodium hypochlorite Alldos pump Sodium hypochlorite chemical pump Plant KwaZulu- Natal Water source Disinfection practices Equipment Assegaai Belgrade Bethesda (umkuze) Ceza Enyokeni Frischgewaagd Greytown Hlanganani Hlokozi Itshelejuba Ixopo Jozini Kwazibusele Manguzi Pongola River Belgrade Dam Umkuze River Vungu River Usuthu Spring Pongola River Merthley Lake & Spring No info No info Borehole Sodbux Dam Jozini Dam Kwazibusele Borehole Shengeza River/Gezisa Spring/Borehole Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (gaseous Cl2) No info No info Chlorination (Sodium hypo) Chlorination (gaseous Cl2) Chlorination (gaseous Cl2) No info Chlorination (Sodium hypo) 1 Dosing pump 2 Dosing pumps 1 Dosing pump 1 Dosing pump 1 Dosing pump 1 Dosing pump 1 Chlorinator & Injector No info No info 1 Dosing pump 1 Chlorinator & Injector 1 Chlorinator & Injector No info 1 Dosing pump Mbazwana Middeldrift Mpungamhlophe Mseleni Mtwalume Nongoma Pongola Richmond Thulasizwe Hospital Tongaat Hullet Ulundi Sibaya Lake Tugela River White Umfolozi River Sibaya Lake Mtwalume River Vuna Dam Pongola River River/Spring Sikhulule River Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (Sodium hypo) Chlorination (gaseous Cl2) Chlorination (gaseous Cl2) Chlorination (Sodium hypo) Chlorination (gaseous Cl2) Chlorination (Sodium hypo) 1 Dosing pump 2 Dosing pump 1 Dosing pump 1 Dosing pump 1 Chlorinator & Injector 1 Chlorinator & Injector 1 Dosing pump 1 Chlorinator & Injector 1 Dosing pump Tongaat & Emone Rivers Ulundi weir (on White Umfolozi River) No info No info No info No info Chlorination (gaseous Cl2) No info 1 Chlorinator & Injector Chlorination (gaseous Cl2) Chlorination (Sodium hypo) Chlorine dioxide No info Dosing pump Dosing pump Umbumbulu Vulamehlo Wild Coast 110 Eastern Cape Province District Municipality Cacadu Name of Plants (Owner type) Water source Treatment processes (Disinfection practices) Equipment Graaff -Reinet Ground water and Surface water Coagulation, Flocculation, Sedimentation, Filtration, Chlorination Aberdeen Groundwater Willowmore Ground water ChlorinationSodium hypochlorite Coagulation, Sud floc Open channel flocculators, Horizontal settlers, Rapid gravity sand filters, Chlorine gas, Chlorochol 14383 dosing pumps Hypochlorite dosing pumps - HPVM Sud floc 3450 & Lime UMP model 1620 Open channel flocculators, Horizontal settling tank, Slow sand filters ALLDOS pumps Flocculation, Sedimentation, Filtration, Chlorination Pearston Ground water Chlorination Somerset East Surface water Coagulation, Flocculation, Sedimentation, Chlorination Cookhouse Coagulation, Surface water Sedimentation, Filtration, Chlorination Joubertina Coagulation, Surface water pH Adjustment Flocculation, Stabilisation, Sedimentation, Filtration, Chlorination Louterwater Surface water Kareedouw Surface water Hankey Surface water and ground water 111 Filtration, Chlorination Pre-Filtration, Secondary filtration Chlorination Coagulation, Flocculation, Sodium Hypochlorite, Electrical pumps for abstraction Ultrafloc 3500, Milton Roy dosing pump- CEG020515H Open channel flocculators, Upflow Settlers, Chlorine gas, ALLDOS pump Ultrafloc 3500, POMPA dosing POMPA HPVM 1004 FP 230 VAC Open rapid sand filters, ALLDOS pump Telemetry system employed Powdered activated carbon dosed by ALLDOS pump and Lime dosed dry chemical feeders Open channel flocculators, Circular scrapper settlers Pressure filters Chlorine gas, Ecometrics stries 480 pump Pressure filters HTH- hypochlorite filters Lime stone filters, Pressure filters Sud Ultrafloc 3500 Open channel flocculators, Sedimentation, Filtration, Chlorination pH stabilization Patensie Surface water and ground water Coagulation, Chlorination Flocculation, Sedimentation, pH stabilization Filtration Humansdorp Jeffreys bay Coagulation and pH stabilization Flocculation, Sedimentation, Filtration Chlorination Pre-treatment- Solid removal Chlorination Ozonation and pH Stabilization Filtration Ozonation Coagulation, Flocculation, Sedimentation, Chlorination Filtration Ground water Bathurst Surface water Port Alfred Surface water Coagulation, Flocculation, Sedimentation, Filtration, Chlorination Sea Field Surface water Coagulation, Albany water board Amathole Surface water and ground water Idutywa Elliotdale Sedimentation, Chlorination Filtration Coagulation and pH Stabilisation, Flocculation, Sedimentation, Filtration, Chlorination Surface water Coagulation and pH Stabilisation, Flocculation, Sedimentation, Filtration, Chlorination Surface water Coagulation, Chlorination Surface water 112 Horizontal settling tanks, Rapid g sand filtersvalveless, ALLDOS pumps Lime, Polyelectrolyte and ALLDOS coagulant dosing pump ALLDOS pumps Open Channel Horizontal settlers Lime Conventional Sand filters Alum and Lime Open Channel flocculators Horizontal settlers Slow sand filters Conventional Sand filters Filtration with net ALDOS pump, Ozone generator and caustic Soda Pressure Filters Ozone generator Ferifloc 820- ALLDOS pump Pipe flocculation Horizontal settlers ALLDOS pump Pressure Filters Ferifloc 820- ALLDOS pump Open channel flocculators Horizontal settlers Slow sand filters HTH Ferrifloc-Microprecessor dosing pump ALLDOS pump Pressure filters Alum and Soda ash Open channel Horizontal settlers Rapid sand filters-valveless HTH and HTH dosing pumps Alum and Soda ash Open channel Horizontal settlers Rapid sand filters-valveless HTH and HTH dosing pumps Alum HTH and HTH dosing Butterworth Surface water Flocculation, Sedimentation, Filtration, pumps Open channel flocculator Horizontal settlers Slow sand filter Coagulation, Alum and Primco 735 dosed by dry screw feeder and Prominent dosing pump Upflow settlers Rapid sand filters Advanced seventrew series capital contracts Model 480, chlorine gas Profloc 150, ALLDOS P150 pump Pipe flocculation Horizontal settler (swimming pool) Pressure filters HTH, CMSIC pulse meter Diomite filters HTH and Milton Roy CEP053 HTH and Siemens dosing pump Sudfloc 3890 & 475 and Milton Roy A 952-86 Circular settling tanks Pressure filters Sodium hypochlorite generated onsite Sud floc 3899, and ALLDOS 205 Sedimentation, Filtration, Chlorination Cintsa Coagulation, Surface water Flocculation, Sedimentation, Kei Mouth Surface water Morgans Bay Surface water Filtration, Chlorination Filtration, Chlorination Prechlorination Coagulation, Sedimentation, Filtration, Chlorination Hagahaga Pre-Sedimentation, Coagulation Flocculation, Sedimentation, Filtration, Chlorination Surface water Komga Surface water Cathcart Surface water Pre-Sedimentation, Coagulation and pH stabilization Flocculation, Sedimentation, Disinfection Coagulation Flocculation, Sedimentation, Filtration, Disinfection Adelaide Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination 113 Pipe flocculators, Radial flow settlers, Pressure filters, HTH and CMSIC pulse meter Radial flow settlers, Alum and soda ash – dry chemical feeders Open channel flocculator Radial flow settlers, HTH Sud floc 3880, and Concept plus pump CNPA0704 Open channel flocculator Horizontal settling tanks Rapid gravity sand filters Chlorine gas and Hydrogas pump Sud floc 3456, and ALLDOS pump Open channel flocculator Horizontal settlers Rapid gravity sand filters Chlorine gas and ALLDOS pump Bedford Surface water and ground water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Chris Hani Cradock Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination Queenstown Coagulation Surface water Flocculation, Sedimentation, Chlorination Sada Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination Cofimvaba Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination Dordrecht Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Macubeni Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination pH stabilization Ngcobo Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination All saints hospital Coagulation and pH Surface water 114 Zetafloc A100 Etatran DS dosing pump Open channel Horizontal flow settlers Rapid gravity sand filters Sodium hypochlorite and CMSIC pulse meter Sud floc 3456, and ALLDOS pump Pipe flocculation Vertical flow sedimentation tanks Rapid gravity sand filters Chlorine gas and advance chlorinator 480 Primco 735 and ALLDOS dosing pump Open channel flocculation Horizontal settlers Chlorine gas sucked by the propeller Polyfloc 5075 and Promonent dosing pump Open channel flocculation Horizontal settling tanks Rapid sand filters Chlorine gas -ALLDOS pump Ultrafloc 3100 and ALLDOS dosing pump Pipe flocculation, Radial flow settlers, Rapid valveless sand filters HTH- ALLDOS dosing pump P101 and ALLDOS dosing pump Open channel flocculation Upflow settlers Rapid Gravity Sand Filters Chlorine gas and advances chlorinator Ultrafloc500 Prominent dosing pump Open channel flocculation Horizontal settlers Pressure filters Chlorine gas and ALLDOS pump Lime Polyelectrolyte (unknown)ALLDOS pump Pipe flocculation Horizontal and Upflow settlers Rapid Sand Filtersvalveless HTH- ALLDOS dosing pump Alum and lime. Alum dosed adjustment Molteno Flocculation, Sedimentation, Filtration, Chlorination Coagulation Flocculation, Sedimentation, Filtration, by constant level feeder and lime by dry feeder Open channel flocculators Horizontal Pressure filters HTH level feeder Ultrafloc. Prominent dosing system Mechanical stirrer Vertical flow sedimentional tanks Rapid Sand Filtersvalveless Chlorine gas and ALLDOS dosing pump M7005 NS ALLDOS pump Open channel flocculation Horizontal and Upflow settlers Pressure Filters Chlorine gas and ALLDOS dosing pump M7005 NS ALLDOS pump Pipe flocculation Upflow settlers Pressure Filters Chlorine gas and ALLDOS dosing pump Horizontal settlers Chlorine gas and ALLDOS dosing pump Pressure Filters Flocculation, Sedimentation, Filtration, Chlorination Coagulation Surface water Flocculation, Sedimentation, Filtration, Chlorination Ukwakhahlamba Maclear Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Sonwabile Surface water Coagulation Ugie Surface water Lady Grey Surface water Coagulation Flocculation, Sedimentation Filtration, Sudfloc 3860, ALLDOS Pipe flocculation Upflow settlers Pressure Filters Aliwal North Surface water Coagulation Ultrafloc 5100 and ALLDOS dosing pump Open channel flocculation, Horizontal settlers and Radial flow, Slow sand filters and pressure filters Chlorine gas and ALLDOS dosing pump Profloc 3860, ALLDOS Open channel Horizontal settlers Rapid gravity sand filters Chlorine gas and ALLDOS dosing pump Profloc 3860, ALLDOS Open channel flocculation Horizontal Pressure Filters HTH dosed manually Ultrafloc, ALLDOS pump Flocculation, Sedimentation, Filtration, Chlorination Burgersdorp Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Barkley East Surface water Elliot Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Coagulation and pH Stabilisation 115 OR Tambo Umtata Flocculation, Sedimentation, Filtration, Chlorination Coagulation and pH Stabilisation Surface water Flocculation, Sedimentation, Mqanduli Surface water Tsolo Surface water Ngqeleni Surface water Filtration, Chlorination Coagulation and pH Stabilisation Flocculation, Sedimentation, Filtration, Chlorination Coagulation and pH Stabilisation Flocculation, Sedimentation, Filtration, Chlorination Coagulation and pH Stabilisation Flocculation, Sedimentation, Chlorination Lutsheko Surface water Coagulation and pH Stabilisation Flocculation, Sedimentation, Chlorination Libode Surface water Coagulation Flocculation, Sedimentation, Filtration, Chlorination Umzimvibu Coagulation Surface water Flocculation, Stabilisation Sedimentation, Filtration, Chlorination Bulolo Coagulation and pH Stabilisation Flocculation, Sedimentation, Chlorination Surface water 116 Open channel floculators Horizontal settlers Rapid valveless sand filters Chlorine gas and ALLDOS Zetafloc LP226 and Lime dosed by ALLDOS pump and dry chemical feeders Open channel floculators Upflow settlers Diatomceous filter and Rapid Gravity Sand filters Chlorine gas and ALLDOS Alum and Soda ash dosed by manually Open channel flocculation Horizontal setters Slow Sand filters HTH dosed manually Sudfloc and Soda ash dosed by CMSIC pulse meter Open channel flocculation Horizontal settlers Pressure filters HTH dosed by CMSIC pulse meter Alum and Lime dosed manually Open channel flocculation Horizontal settlers HTH dosed manually Zetafloc LP226 ALLDOS pump Open channel floculators Upflow settlers Diatomceous filter and Rapid Chlorine gas and ALLDOS Polyelectriolyte (Not known) dosed by prominent pump Open channel flocculators Horizontal settlers Slow sand filters Bromine tablets (potabrom) Polyelectriolyte (Not known) dosed by prominent pump Open channel flocculators Upflow settlers Pressure filters HTH dosed by ALLDOS pump Profloc 3890, and lime dosed Open channel flocculation Upflow settlers Rapid gravity sand filters Chlorine gas Mt Ayliff Surface water Mt Frere Surface water Chlorination, Coagulation and pH Stabilisation Sedimentation, Filtration Screening Coagulation and pH Stabilisation Flocculation, Sedimentation, Filtration, Chlorination 117 HTH, Alum and lime dosed manually Radial settlers Rapid valveless sand filters Polyfloc 5015 dosed by prominent dosing pump and lime Open channel flocculator, Horizontal settlers Rapid conventional filters Chlorine gas. Western Cape Province Local Municipality (Owner) Plettenberg Bay Knysna Name of Plants Water source Plettenberg Bay Surface water (river and Dam) Rheenendal Surface water Knysna Surface water (river and Dam) Ruigter Surface water Treatment Process Disinfection practices Coagulation, Flocculation, Sedimentation, Flotation, Filtration, Disinfection Stabilization Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Coagulation, Flocculation, Stabilization Sedimentation, Filtration, Disinfection George George Surface water (Dam) Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Mossel Bay Wilderness Surface water (River) Klein Brak River Surface water (Dam) Great Brak River Surface water (Dam) 118 Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Coagulation, Flocculation, Sedimentation, Equipment Alum (ALLDOS) and PAC Open channel flocculation, Horizontal settlers, DAF Rapid gravity sand filters, Chlorine gas, ALLDOS lime (Dry chemical feeders) Alum (ALLDOS) Open channel flocculation, Horizontal Gravity sand Chlorine gas ALLDOS Alum, Gravity feed Open channel flocculation, Horizontal Gravity sand Chlorine gas ALLDOS Lime (Alum and Polyelectrolyte) Milton Roy Series GTM Open channel flocculation, Lime Horizontal settlers Pressure filters and rapid sand filters Chlorine gas and ALLDOS Ferric Chloride (ALLDOS) Polyelectrolyte Open channel flocculation, Horizontal Rapid sand filters, Horizontal Chlorine gas,(Wallace and Tienan, regulator) Lime Alum (ALLDOS) Pipe flocculation, Horizontal Rapid sand Chlorine gas ALLDOS Soda Ash Alum (ALLDOS) Open channel flocculation, Horizontal Rapid sand filters, Chlorine gas, Venture system Lime (Marlin and Watson) Alum (ALLDOS) Open channel flocculation, Horizontal Filtration, Disinfection Stabilization Sandhoogte Surface water (Dam) Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Fremersheim Surface water (Dam) Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Langeberg Riversdale Surface water (Dam) Albertinia Ground water (Borehole) Still Bay Ground water 119 Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Coagulation, Flocculation, Sedimentation, Filtration, Disinfection Stabilization Coagulation, Filtration, Disinfection Rapid sand filters, Chlorine gas (Venture system) Lime Alum (ALLDOS) Open channel flocculation, Horizontal Rapid sand filters, Chlorine gas, Venture system Lime (Marlin and Watson) Ultrafloc 3800 (Milton roy CEP043-531NM) Open channel Flocculation Upflow settlers Pressure filters Sodium hypochlorite (ALLDOS) Soda Ash Alum (ALLDOS) Open channel flocculation, Horizontal Slow sand Chlorine gas (ALLDOS) Lime and Soda Ash Alum Pipe flocculation, Upflow Upflow Chlorine gas (ALLDOS) Soda Ash Ultrafloc 3500 (Poly) Siemens Pressure Ozonation Malelane Matsulu KaNyamazane regional KaNyamazane old plant Sabie White river regional White river Country estate Nelspruit Hazyview Lydenburg Plant Final 0.82 0.7 1.77 10.9 0.18 0.82 0.32 1.56 7.15 1.81 Raw 28.5 26 87.4 86.7 0.19 10.09 3.17 53.7 18.6 2.88 0.79 0.62 1.33 0.37 0.17 0.21 4.13 POU 0.61 1.3 2.41 Turbidity (NTU) 16.4.5 10.3.2 50.0 60.2 20.4 5.6 21.4 Raw 31.2 25.0 27.8 18.0 11.5 52.1 92.1 18.4 9.1 28.6 Final 32.2 33.1 24.4 20.5 12.8 52.5 167.2 16.7 8.5 27.1 POU 35.3 34.5 24.6 Conductivity (PS/m) 120 7.89 8.05 7.98 6.9 8.00 8.13 7.96 Raw 8.06 8.05 7.94 8.1 7.62 7.30 9.1 7.81 9.37 7.94 Final 7.83 7.53 8.06 pH 8.03 7.77 7.81 7.84 7.76 7.63 8.07 POU 7.93 7.82 8.29 19.2 23.3 15.4 20.8 17.2 21.5 25.3 Raw 25 26.3 25.4 22.3 25.4 15.6 21.4 19.4 21.1 24.2 Final 27.1 27.7 26.2 24.2 24.4 16.0 23.4 20.1 25.2 22.3 POU 25.3 31.1 25.7 Temperature (oC) Ehlanzeni district Municipality Mpumalanga Province 0.38 0.68 2.13 0.16 0.25 1.4 1.6 0.46 4 3.36 Chlorine dosage (mg/l) 62 3.09 6 2 5.6 6 10 26 12 57 Current flow (Ml/D) 0.35 0.50 2.12 0.14 0.24 1.32 1.65 0.15 0.48 0.23 0.10 0.18 0.51 0.14 Residual Chlorine (mg/l) Final POU 0.27 0.21 2.48 0.51 1.75 0.59 Physico-chemical Quality of Raw Water and Treated Drinking Water in Various Surveyed Plants Appendix 3.3 Machadodorp Waterval Boven Belfast Dullstroom Hendrina Middelburg (Vaalbank) Middelburg (Kruger Dam) Presidentsrus Witbank 1.02 3.60 2.22 5.55 1.57 0.66 0.32 138.3 66.6 29.4 0.59 144.8 66.8 27.8 148.3 67.2 36.8 POU 6.6 13.1 35.9 10.8 16.8 71.0 25.1 Final 11.1 14.3 34.8 4.6 16.5 73.0 Raw 10.4.9 12.5 36.1 4.4 16.2 68.9 POU 16.3 11.5 4.23 3.70 0.62 0.71 Final 18.8 4.80 0.85 1.94 0.58 0.48 Raw 28.9 17.2 24.4 4.30 4.91 4.96 121 8.23 7.82 7.03 Raw 8.10 8.01 9.04 9.05 7.28 7.75 pH 8.15 7.51 7.06 Final 7.41 7.84 7.66 7.86 7.93 7.90 8.15 7.72 7.54 POU 8.12 7.77 7.76 7.01 7.97 7.70 24.5 23 21.8 Raw 18.1 21.2 20 23.6 18 21 24.6 26 21.5 Final 23.7 25.6 18 22 18 22 24.4 24.3 20 POU 23.7 26.5 17 21 18 24 Temperature (oC) Nkangala District Municipality Conductivity (mS/m) Turbidity (NTU) 0.25 3 1.55 Chlorine dosage (mg/l) 1.3 0.4 1.56 0 0.54 1.03 0.8 120 6.2 3.6 3.4 5.76 10 17.7 46.2 Current flow rate 0.2 0.22 0.29 Final 1.11 0.15 1.5 0.08 0.51 0.02 0.12 0.08 0.11 POU 0.1 0.37 0.07 0.04 0.52 0.11 Residual Chlorine Semerela Thapane Nkambako George’s valley Tzaneen Nkowankowa Vondo Phiphidi Dzingahe Damani Mudaswali Dzindi Mutshedzi Tshedza Tshifhire Tshakhumare Tshakhuma Makhado Musina Shikundu Mhinga Malamulele Mutale Plant Final 0.95 0.93 2.05 1.34 17.3 0.5 1.61 3.2 3.25 1.04 1.47 1 1.03 0.25 1.55 19.3 0.68 POU 0.71 0.40 1.4 0.81 10.2 0.69 1.78 6.6 2.2 0.67 1 1.2 1.03 0.35 2.53 1.02 0.46 Final 10.3 0.76 1.19 0.41 0.49 1.08 Raw 33.6 5.17 18.8 2.19 4.70 7.19 0.48 1.58 POU 7.52 0.98 0.94 0.48 Turbidity (NTU) Raw 2.18 2.25 5.29 8.93 25.2 4.88 3.2 21.8 6.62 2.41 3.51 18.8 3.2 15.2 8.93 19.3 3.32 Turbidity (NTU) POU 45 82.2 92.4 42.8 135.2 4.76 6.32 7.01 7.03 3.51 6.25 28.4 79.2 14.9 16.8 15.6 81.0 73.0 94.0 Raw 9.37 14.6 24.2 49.4 Final 10.3 14.6 22.1 73.2 110.1 115 Raw 7.55 7.02 7.83 6.46 7.36 6.9 7.5 7.16 7.15 7.32 7.05 7.25 7.5 7.23 7.71 7.37 7.82 Final 7.67 7.17 7.58 6.57 7.25 7.0 6.8 6.89 7.07 7.56 7.1 7.72 7.5 7.23 8.01 7.47 7.90 pH POU 7.61 7.14 7.72 6.83 7.24 7.2 7.0 6.93 7.07 7.66 7.18 7.68 7.13 7.35 8.14 7.15 8.00 Raw 27.4 25.7 27.9 31.0 29.6 24 21.2 20 19 18.2 19.2 19 28.9 21 22 20 31.4 Final 27.7 27.5 32 24.6 29.0 23.4 20 21 18.1 18 18 20 29 20 27 20 28.4 110.8 113.6 POU 11.0 13.9 22.1 110.8 122 6.93 7.96 Raw 7.29 7.29 8.18 7.64 8.93 9.45 Final 6.8 6.8 8.05 9.27 pH 9.21 9.23 POU 6.66 6.66 8.02 9.21 30.1 29.3 Raw 23 25 26.5 30.1 30.5 29.6 Final 22 25 26 30.5 24.7 29.0 POU 23.4 25.8 26.3 24.7 Temperature (oC) POU 28 29.6 31.7 29.6 26.7 26.2 22 20.5 19.6 18.7 19.1 22 29 21.6 26.2 21 30.4 Temperature (oC) Mopani district municipality Final 37.1 39.5 121.8 45.5 136.5 7.38 6.65 6.7 7.28 6.44 6.21 28 66.7 14.9 17.8 14.7 91.2 Conductivity (PS/m) Raw 34.1 86.9 120.5 40.5 152 5.48 6.63 5.9 6.51 5.92 5.84 25.9 66.3 14.7 14.2 14.8 76.2 Conductivity (PS) Vhembe District Municipal Limpopo Province 0.1 0.133 0.4 1.38 1.39 0.7 Chlorine dosage (mg/l) 0.35 0.24 0.37 0.52 0.75 0.9 0.34 1.54 0.27 0.28 1.2 0.8 0.4 1.2 0.06 0.6 0.35 Chlorine dosage (mg/l) 6 25 1 1 6.9 7.9 Current flow (Ml/D) 24 1.9 0.3 2.3 0.8 4 16 0.78 2.07 30 18 12 20 10 37 18 3.5 Current flow (Ml/D) 0.1 0.13 0.27 0.12 Residual Chlorine (mg/l) Final POU 0.4 0.12 1.3 0.8 0.56 0.50 0.3 0.24 Residual Chlorine (mg/l) Final POU 0.19 0.27 0.19 0.02 0.06 0.05 0.52 0.22 0.51 0.08 0.82 0.7 0.3 0.17 1.5 0.13 1.5 0.5 0.6 0.57 1.0 0.2 0.6 0.34 0.23 0.1 0.4 0.21 0.04 0.04 1.2 0.06 0.13 0.08 Maratapilu Pietersburg Seshego Burgersfort Ohrigstad Tubatse (Prakteseer) Steelpoort Bela Bela Thabazimbi Nylstroom (Modimole) Naboomspruit Potgietersrus (Mokopane) Letsetele 0.39 0.37 2.46 0.4 4.70 6.2 1.2 0.35 POU 1.74 0.63 1.05 Final 1.81 0.26 1.19 POU 6.92 0.26 1.08 Raw 24.4 1.68 96.6 Final 0.60 0.65 1.95 POU 0.50 0.74 0.50 Turbidity (NTU) Raw 61.9 0.3 23.1 Turbidity (NTU) Final 2.01 0.38 1.90 Raw 5.97 0.81 17.5 Turbidity (NTU) 5.73 982 41.0 45.4 POU 254 80.4 271 Raw 33.8 45.2 29.2 7.73 28.5 28.6 7.61 8.2 Raw 7.81 7.49 6.81 6.5 8.4 Final 9.26 7.99 7.01 pH 6.8 8.26 POU 8.84 7.73 7.5 POU 36.8 45.6 26.5 Raw 7.56 7.82 8.50 Final 8.48 7.73 8.11 pH POU 8.02 8.07 8.2 Final 36.4 57.7 41.2 POU 35.4 58.2 47.0 123 Raw 7.92 8.85 8.37 Final 8.62 8.93 7.86 pH POU 8.16 8.71 8.02 26 25.5 25.6 Raw 22.3 27 25.4 25 24.6 Final 23.5 29 25.6 Raw 23.1 20.1 21.8 Final 22.5 20.2 22.5 Raw 24.2 23.6 25.1 Final 24.5 23.7 23.8 POU 24.6 22.4 22.8 Temperature (oC) POU 23 20.5 23 Temperature (oC) 26.2 25.5 POU 27 27.5 25.2 Temperature (oC) Capricorn district Municipality Final 47.9 45.7 26.1 Conductivity (PS/m) Raw 20.7 45.9 25.4 9.64 Sekhukuni district Municipality 42.5 42.0 Final 97.2 78.8 81.5 Conductivity (PS/m) 94.2 37.2 Raw 63.8 94.6 53.9 7.83 Waterberg district municipality 153.5 Conductivity (PS/m) 116.5 0.16 0.21 0.34 Chlorine dosage (mg/l) 0.29 0.23 0.35 Chlorine dosage (mg/l) 0.92 0.28 0.1l 0.42 0.32 Chlorine dosage (mg/l) 0.87 0.6 17.2 3.6 Current flow (Ml/D) 3.9 0.5 13.7 Current flow (Ml/D) 2.6 - 5.86 5.8 5.76 Current flow (Ml/D) 1 0.37 1.01 0.11 Residual Chlorine (mg/l) Final POU 0.12 0.08 0.15 0.08 0.28 0.16 Residual Chlorine (mg/l) Final POU 0.27 0.22 0.18 0.22 0.25 0.21 2.3 0.21 Residual Chlorine (mg/l) Final POU 0.11 0.2 0.39 0.24 0.27 0.16 0.8 1.68 2.03 1.81 2.11 18 Pela 24.4 Final 14.5 0.64 5.28 13.8 POU 11.8 6.59 1.34 Raw 16.8 16.0 33.3 Final 0.81 1.03 1.54 POU 0.78 2.63 4.32 Turbidity (NTU) Raw 50.3 167 33.7 Madibeng Hartbeespoort Temba 0.86 0.35 POU 3.78 0.37 1.51 4.76 Turbidity (NTU) Final 4.27 2.01 0.52 4.11 Raw 50.2 15.9 9.17 4.11 Turbidity (NTU) Madikwe Koster Swartruggens Bloemhof Christiana Potchefstroom SchweizerReneke Ventersdorp Itsoseng Plant 28.5 31.3 POU 72.0 89.9 16.1 53.7 Raw 31.5 24.4 40.7 7.32 7.58 Final 7.39 7.32 7.66 8.36 7.77 7.35 POU 7.42 7.43 7.58 8.29 25.0 19.7 Raw 23.0 28.4 24.4 28.5 19.4 20.3 Final 25.7 27.1 25.0 27.7 8.54 POU 12.3 4.3 52.9 7.46 Raw 7.8 8.1 8.05 6.39 Final 8.07 7.65 7.05 pH 7.1 POU 8.1 7.63 7.42 23.4 Raw 26.0 24.0 29.2 Final 35.7 18.3 40.7 POU 37.3 23.6 39.4 124 Raw 7.9 8.5 8.17 Final 7.61 8.98 7.61 pH POU 7.64 8.62 7.57 24.2 POU 26.2 25.0 20.0 Raw 26.0 25.9 26.8 Final 26.0 26.3 25.8 POU 26.1 27.3 24.8 Temperature (oC) 24.5 Final 25.9 24.4 26.0 Temperature (oC) 25.0 22.2 POU 25.5 30.4 24.7 27.1 Temperature (oC) Eastern District Municipal 8.54 Final 12.3 8.1 33.9 Conductivity (PS) 8.38 Raw 11.8 16.5 42.9 7.6 7.26 Raw 8.25 8.36 8.04 8.15 pH Rustenburg District Municipality 25.3 30.5 Final 76.5 59.6 16.6 50.6 Conductivity (mS/m) 24.1 29.4 Raw 46.7 50.3 17.1 23.0 Conductivity (PS/m) Southern District Municipality North West Province 3 4.8 3 Chlorine dosage (mg/l) Chlorine dosage (mg/l) 3.5 3.5 Not Working 8.3 1.37 9.6 6.2 Not Working 2.1 3.85 Chlorine dosage (mg/l) 48 10.35 40 Current flow (Ml/D) 1.1 24.0 2.0 3.4 Current flow rate 9 0.9 5.7 3.5 20 4.01 Current flow (Ml/D) 0.19 0.02 0.89 POU 0.62 0.23 0.00 Residual Chlorine (mg/l) Final POU 1.45 0.03 0.52 0.07 1.92 0.59 3.52 Final 2.6 1.74 0.00 Residual Chlorine 1.98 0.11 Residual Chlorine (mg/l) Final POU 0.12 0.00 3.19 0.23 0.52 0.07 0.19 0.08 Pudumong 1.4 11 Mafikeng Mmabatho 1.18 2.14 Final 12.9 1.98 1.78 POU 11 Raw 9.13 Final 1.05 POU 2.31 Turbidity (NTU) Raw 14.2 Groot-Marico Turbidity (NTU) 29.2 44.1 POU 13.2 Raw 63.9 7.5 8.94 Raw 7.88 8.74 7.98 Final 7.85 8.03 7.6 POU 7.85 27.3 25.6 Raw 25.3 24.4 26.1 Final 25.5 Final 61.9 POU 62 125 Raw 8.24 Final 7.86 pH POU 7.75 Raw 26.3 Final 27.6 POU 27.0 Temperature (oC) 25.0 23.0 POU 24.5 Temperature (oC) Buphirima District municipality 27.5 43.2 Final 12.5 Conductivity (PS/m) 32.7 46 Raw 12.7 pH Central District Municipality Conductivity (PS/m) 2 Chlorine dosage (mg/l) Not Working 3.5 2.8 Chlorine dosage (mg/l) 10 Current flow (Ml/D) 42 20 0.9 Current flow (Ml/D) 0.69 0.12 Residual Chlorine (mg/l) Final POU 0.91 0.08 2.88 1.66 Residual Chlorine (mg/l) Final POU 0.00 0.00 Assegaai Belgrade Bethesda Ceza Enyokeni Frischgewaagd Greytown Hlanganani Hlokozi Itshelejuba Ixopo Jozini Kwazibusele Manguzi Mbazwana Middeldrift Mpungamhlophe Mseleni Mtwalume Nongoma Pongola Richmond Thulasizwe Tongaat Hullet Ulundi Umbumbulu Vulamehlo Weenen Wild Coast Plant 23.1 25.8 23.3 315 0.11 17.5 23.4 4.84 28 0.20 5.00 4.36 0.17 9.15 0.89 1.52 1487 2.4 N.D 1610 70.6 8.5 4.5 49.7 829 No info 28 ND 2.75 1.22 0.87 27.3 11.3 0.28 10.4 0.21 2.46 7.19 2.37 0.94 0.82 0.1 7.95 0.88 0.99 1.15 1.08 0.22 7.20 3.22 2.47 2.41 0.34 0.77 0.23 1.40 1.13 0.48 Final 1.62 0.2 1.41 6.51 0.42 11.1 0.14 2.52 6.06 0.77 1.21 0.71 0.13 7.67 1.15 1.19 1.36 1.48 0.25 7.38 3.21 1.34 2.60 0.5 0.61 0.34 1.09 ND 0.49 POU Turbidity (NTU Raw 11.8 26.9 154 5.79 63.6 5.58 6.79 7.46 7.41 13.1 ND 31.4 18.5 24.81 60.6 21.4 19.9 59.4 ND 27.5 8.44 5.74 4.19 14.6 21.7 No info 10.1 ND 20.9 Raw 12.5 27.8 119 7.19 62.4 5.71 7.45 9.42 7.51 13.7 23.4 31.5 19 27.5 62.3 22.1 20.2 60.6 22.8 28.6 8.87 9.44 5.24 18.1 20.8 15.2 11.5 ND 23.2 Final 126 12.1 28.7 64.3 7.06 61.8 5.89 7.78 10.2 7.56 13.8 22.6 31.8 18.9 27.7 62.5 22.3 21 61.4 23.1 28.4 9.01 9.56 5.18 19.6 20.3 15.8 11.6 ND 23.7 POU Conductivity (mS/m) 6.88 7.5 7.77 7.35 8.03 7.28 7.05 7.69 10.1 6.99 7.00 8.15 6.34 6.59 7.66 7.6 7.94 7.78 ND 7.55 7.59 7.32 6.55 7.62 7.64 No info 7.41 ND 7.5 Raw 6.83 7.68 7.8 7.34 8.09 7.35 7.02 7.67 7.48 6.96 7.12 7.92 6.4 6.60 7.99 7.87 7.99 8.06 ND 7.28 7.36 7.01 6.92 7.73 7.52 7.43 7.85 ND 7.49 Final pH KwaZulu-Natal Province 6.86 7.68 8.36 7.42 8.15 7.36 6.6 7.8 7.48 6.96 7.23 7.94 7.5 7.67 8.26 7.97 7.81 8.14 7.5 7.38 7.26 7.54 7.04 7.72 7.65 No info 7.81 ND 7.65 POU 0.68 0.00 0.00 1.7 0.00 0.51 <0.10 0.01 <0.01 1.09 <0.10 0.84 <0.40 0.51 0.72 <0.10 0.00C 0.63 <0.10 2.20+ 1.67 0.70 1.23 <0.10 1.24 0.50 <0.10 0 ND Final 0.00 0.00 1.85 0.39 0.00 0.14 <0.10 0.04 <0.1 1.71 <0.10 0.00 <0.20 0.17 0.97 <0.10 0.00 C 0.09 <0.10 2.20+ 0.60 0.20 0.20 <0.10 0.65 0.10 <0.10 ND ND POU Residual (mg/l) Plettenberg bay Knysna Rheenendal Ruigter George Wilderness Sandhoogte Friemersheim Kleinbrak River Great River Riversdale Still Bay Albertinia Plant 2.02 1.09 0.95 6.66 3.93 0.80 29.2 11.9 1.03 78.0 0.73 0.39 1.06 0.12 0.6 1.02 2.32 0.95 1.09 0.97 7.1 1.03 0.87 0.66 1.39 0.52 3.23 1.71 11.7 18.2 1.62 1.47 1.27 12.1 POU 0.80 Final 0.71 Raw 2.11 Turbidity (NTU) 6.75 6.48 6.35 6.5 7.13 6.97 6.49 7.01 7.18 4.53 4.61 5.26 Raw 4.9 7.5 7.40 11.10 8.69 7.68 6.83 7.12 6.52 10.23 8.95 8.11 Final 10.24 pH 7.06 8.81 9.99 9.5 8.99 7.13 8.88 7.9 9.63 9.48 8.57 POU 9.78 17 14.4 14.8 19.8 23 23 12.3 14 12.6 13.8 12.7 14.2 Raw 25 127 20.4 13 14.7 16.4 23 14.6 14.5 13.4 14.7 13.8 13.8 Final 14.8 17.3 15.4 16.9 14.7 14.6 16.1 16.2 16.7 15.2 14.5 15.1 POU 15.5 Temperature (oC) m) 5 3.2 4 1.5 11 0.67 4 60 2 5 1 30 27 Design Capacity (Ml/day) Ehlanzeni district Municipality Western Cape Province 2.5 3.2 1 7 0.4 1.9 6.6 1.5 3.6 2.88 23 25 Current flow (mg/l) 1.4 4.86 1.26 1.32 2.9 2.14 2.5 1.54 1.28 1.32 2.1 2.3 Chlorine dose (mg/l) 0.21 0.67 0.09 1.07 0.31 1.26 0.56 0.27 1.04 0.06 0.32 0.13 0.15 0.00 0.12 0.20 0.14 0.89 0.22 0.42 0.18 0.11 Residual Chlorine (mg/l) Final POU 0.1 0.14 Malelane Matsulu KaNyamazane regional KaNyamazane old plant Sabie White River regional White River Country estate Nelspruit Hazyview Lydenburg Plant 0 0 0 132 38 24 128 0 0 0 15 83 220 0 7 0 18 80 95 0 0 0 0 0 0 0 0 0 0 0 0 0 7 0 180 72 120 204 0 64 0 7.7 × 104 180 Total Coliforms (cfu/100 ml) Raw Final POU 220 0 22 90 0 0 64 0 0 Heterotrophic Plate Counts (cfu/ml) Raw Final POU 432 × 104 3.8 × 102 22 1.0 × 103 0 0 2.0 × 103 0 0 Ehlanzeni District Municipality Mpumalanga Province 132 38 24 18 80 95 64 0 0 0 0 0 0 0 0 0 0 0 2 0 0 Faecal coliform (cfu/100 ml) Raw Final POU 220 0 12 90 0 0 64 0 0 Microbiological water quality data for the district municipalities of Mpumalanga and Limpopo Provinces of South Africa Appendix 3.4 Machadodorp Waterval Boven Belfast Dullstroom Hendrina Middelburg (Vaalbank) Middelburg (Kruger Dam) Presidentsrus Witbank 72 92 40 100 129 84 7.2 ×104 0 0 0 1 0 2.2 × 106 7.5 × 107 0 400 2.9 × 104 1.8 × 106 6.5 × 103 Total Coliforms (cfu/100 ml) Raw Final POU 51 0 14 480 8 0 780 0 0 89 7 0 200 0 0 70 3 2 Nkangala District Municipality Heterotrophic Plate Counts (cfu/ml) Raw Final POU 1.94 × 104 0 170 3.6 × 105 79 4 3.84 × 106 2 0 7.2 × 104 85 20 2.56 × 105 1 0 1.2 × 103 180 82 10 360 132 0 2 3 0 8 5 Faecal coliform (cfu/100 ml) Raw Final POU 44 0 5 156 2 0 72 0 0 5 2 0 13 0 0 27 2 0 Semarela Thapane Mudaswali Nkambako George’s Valley Tzaneen Nkowankowa Letsetele 4.3 × 103 800 3.6 × 103 0 250 0 130 Total Coliforms (cfu/100 ml) Raw Final POU 1.8 × 104 0 0 3.4 × 103 0 0 1.4 × 103 0 0 370 70 10 460 92 5 700 80 0 1.7 × 103 10 0 1.8 × 104 48 0 Mopani District Municipality 3 × 103 140 Heterotrophic Plate Counts (cfu/ml) Raw Final POU 5.2 × 105 0 0 6 × 102 0 0 3.8 × 104 120 0 7.2 × 103 260 30 4.6 × 105 7.8 × 103 79 4.2 × 105 3.1 × 104 3 3.6 × 104 170 20 3.6 × 106 8.0 × 103 10 6.5 × 103 2.0 × 103 6.2 × 105 1.6 × 104 Total Coliforms (cfu/100 ml) Raw Final POU 570 28 10 250 21 5 2.5 × 103 172 0 200 0 0 2.1 × 103 0 0 5 × 102 0 0 1.3 × 104 0 0 0 0 0 0 0 0 0 0 0 0 0 0 5 × 102 0 0 1.4 × 103 0 0 Vhembe District Municipal Heterotrophic Plate Counts (cfu/ml) Raw Final POU 5.7 × 104 2.5 × 103 280 4 × 103 2.7 × 103 1.7 × 103 5 1 × 10 180 14 1.1 × 104 0 0 7 × 103 0 0 0 0 0 5.6 × 106 0 0 0 0 0 0 0 0 0 0 0 2 × 102 0 0 3.2 × 105 0 0 4 × 102 0 0 Mhinga Malamulele Mutale Vondo Phiphidi Dzingahe Damani Dzindi Mutshedzi Tshedza Tshifhire Tshakhumare Tshakhuma Makhado Shikundu Plant Limpopo Province 0 0 0 0 Faecal coliform (cfu/100 ml) Raw Final POU 0 0 0 0 0 0 680 0 0 220 1 0 150 0 0 60 0 0 330 0 0 1.1 × 103 23 0 2.1 × 103 610 Faecal coliform (cfu/100 ml) Raw Final POU 500 12 5 150 0 1 138 15 0 100 0 0 102 0 0 0 0 0 4.5 × 103 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.8 × 105 0 0 7.8 × 103 0 0 Maratapilu Pietersburg Seshego Burgersfort Ohrigstad Tubatse (Prakteseer) Steelpoort Bela Bela Thabazimbi Nylstroom (Modimole) Naboomspruit Potgietersrus (Mokopane) 0 0 Total Coliforms (cfu/100 ml) Raw Final POU 7.5 × 104 30 75 160 20 0 1.2 × 104 0 0 0 Heterotrophic Plate Counts (cfu/ml) Raw Final POU 1.6 × 104 39 1.8 × 105 0 0 3.4 × 104 279 130 131 Total Coliforms (cfu/100 ml) Raw Final POU 270 2 2.4 × 103 0 0 1.9 × 103 0 40 Capricorn District Municipality Heterotrophic Plate Counts (cfu/ml) Raw Final POU 5.4 × 105 180 112 204 34 24 2.1 × 104 26 0 0 Sekhukuni District Municipality 0 3 2.5 × 103 1.9 × 103 0 0 9.0 × 105 4.5 × 103 240 72 Total Coliforms (cfu/100 ml) Raw Final POU 600 20 0 2.2 × 104 6 0 820 16 2 Waterberg District Municipality Heterotrophic Plate Counts (cfu/ml) Raw Final POU 2.8 × 104 240 20 1.5 × 105 7 2 5.7 × 104 96 16 0 0 0 0 Faecal coliform (cfu/100 ml) Raw Final POU 100 0 80 0 0 210 0 7 Faecal coliform (cfu/100 ml) Raw Final POU 448 3 4 7 1 2 200 1 0 0 250 60 Faecal coliform (cfu/100 ml) Raw Final POU 100 2 0 660 3 0 610 60 0 Wilge Botanical Warden Vrede Memel Villiers Tweeling Frankfort Oranjeville Koppies Parys Vredefort Edenfort Plant - 0 0 0 0 0 0 0 0 0 0 0 0 0 132 0 0 0 5 12 0 0 0 0 0 0 0 0 0 Total coliforms (cfu/100 ml) Raw Final POU Free State Province Raw - 0 0 0 0 0 0 0 0 0 0 0 0 - 0 0 0 3 5 0 0 0 0 0 0 0 0 0 Faecal Coliforms (cfu/100 ml) Final POU Putumong Groot Marico Mafikeng Mmabatho Madibeng Hartbeespoort Temba Madikwe Koster Swartruggens Pela Bloemhof Christiana Potchefstroom Schweizer-Reneke Ventersdorp Itsoseng Plant 83 0 2 9 0 4 123 0 5 70 0 32 16 0 3 16 37 0 4 31 3 8 4 3 288 2 17 0 1 31 0 5 3.8×102 133 0 15 Buphirima District Municipality Total coliforms (cfu/100 ml) Raw Final POU 278 14 402 Central District Municipality Total coliforms (cfu/100 ml) Raw Final POU 5.9×102 6.0×102 3.3×102 Eastern District Municipality Total coliforms (cfu/100 ml) Raw Final POU 8.1×102 8.8×102 133 262 Rustenburg District Total coliforms (cfu/100 ml) Raw Final POU 5.6×102 5.0× 02 3.0×102 4.9×102 9.0×102 2.9×102 Total coliforms (cfu/100 ml) Raw Final POU Southern District Municipality North West Province Raw Raw 0 0 0 5 0 0 13 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 23 0 1 Faecal Coliforms (cfu/100 ml) Final POU 35 3 30 Faecal Coliforms (cfu/100 ml) Final POU 0 0 0 Faecal Coliforms (cfu/100 ml) Final POU 65 30 24 38 Faecal Coliforms (cfu/100 ml) Final POU 60 30 64 36 0 0 0 4×102 24 Raw Raw Raw Faecal Coliforms (cfu/100 ml) Final POU Assegaai Belgrade Bethesda Ceza Enyokeni Frischgewaagd Greytown Hlanganani Hlokozi Itshelejuba Ixopo Jozini Kwazibusele Manguzi Mbazwana Middeldrift Mpungamhlophe Mseleni Mtwalume Nongoma Pongola Richmond Thulasizwe Tongaat Hullet Tugela Estate Ulundi Umbumbulu Vulamehlo Wild Coast Sun Plant Raw ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND 134 0 0 0 44 82 44 0 32 866 0 ND 0 No info 0 0 16 435 0 1 0 2 ND 0 No info ND 0 0 11 1 1 0 6 0 125 0 ND ND ND 0 ND 0 ND 0 0 2419 2419 0 ND 0 0 ND 0 No info ND 0 ND ND ND Total coliforms Final POU Raw KwaZulu-Natal Province ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND ND 0 0 0 0 30 0 0 5 53 0 ND 0 No info 0 0 0 9 0 ND 0 0 ND 0 No info ND 0 0 1 0 Faecal Coliforms Final POU 0 0 0 0 36 0 ND ND ND 0 ND 0 ND 0 0 4 3 0 ND 0 0 ND 0 No info ND 0 ND ND ND Idutywa Elliotdale Butterworth Cintsa Kei Mouth Morgans Bay Hagahaga Cathcart Adelaide Bedford Graaff-Reinet Aberdeen Willowmore Pearston Somerset East Cookhouse Joubertina Louterwater Kareedouw Hankey Patensie Humansdorp Jeffreys Bay Port Alfred Sea Field Boesmanrivier Plant 12 ND 1 ND 64 11 9 132 14 17 5 3 1 6 81 6 15 5 3 16 82 19 17 227 22 23 13 4 4 18 122 11 476 216 273 207 ND 175 ND 41 346 167 135 89 11 ND 9 27 32 68 19 22 153 22 29 183 23 19 15 13 146 52 Amathole District Municipality Total coliforms (cfu/100 ml) Raw Final POU 69 ND 8 ND ND 73 63 427 30 304 22 34 46 63 123 16 Total coliforms (cfu/100 ml) Raw Final POU Cacadu District Municipality Eastern Cape Province Raw 5 ND 0 ND 15 0 6 12 1 6 3 0 0 1 11 0 7 1 1 4 21 0 12 91 3 7 5 2 0 2 19 0 98 19 25 0 ND 4 ND 17 25 25 2 0 6 0 9 4 ND 3 4 9 6 0 9 98 0 6 0 7 10 11 Faecal Coliforms (cfu/100 ml) Final POU 13 ND 1 ND ND 5 43 101 6 27 19 5 0 17 25 0 Faecal Coliforms (cfu/100 ml) Raw Final POU Umtata Mqanduli Tsolo Mngqeleni Lutsheko Libode Umzimvubu Bulolo Mt Ayliff Mt Frere Maclear Sonwabile Ugie Lady Grey Aliwal North Burgersdorp Barkley East Elliot Cradock Molteno Queenstown Sada Cofimvaba Dordrecht Machubeni Ungcobo All Saints Hospital 2 84 7 68 14 100 0 11 13 3 ND 18 73 26 140 8 55 40 Raw 2 0 40 13 13 32 32 27 23 0 62 8 18 7 41 32 519 411 09 220 69 171 212 632 157 156 136 150 56 89 51 17 36 72 240 27 0 223 ND ND 101 12 71 142 420 103 2 OR Tambo District Municipality Total coliforms (cfu/100 ml) Raw Final POU 320 75 252 87 234 194 236 169 Raw Ukwakhahlamba District Municipality Total coliforms (cfu/100 ml) Raw Final POU Raw 237 ND 66 268 321 378 298 354 215 Chris Hani District Municipality Total coliforms (cfu/100 ml) Raw Final POU 0 16 0 6 0 0 0 7 2 0 16 0 6 6 0 0 8 7 4 0 8 0 0 0 3 7 9 0 ND 0 0 0 3 5 80 92 160 20 4 6 31 20 174 32 25 18 17 2 1 0 3 2 4 0 38 27 15 5 2 0 15 4 7 0 Faecal Coliforms (cfu/100 ml) Final POU 27 30 15 11 29 0 13 19 Faecal Coliforms (cfu/100 ml) Final POU 32 ND 5 22 17 16 13 20 19 Faecal Coliforms (cfu/100 ml) Final POU Plettenberg bay Knysna Rheenendal Ruigte George Wilderness Sandhoogte Friemersheim Kleinbrak Rivier Great River Riversdale Still Bay Albertinia Plant 6.6×102 9.4×102 1.1×102 1.3×102 5.9×102 7.5×102 5.2×102 2.4×102 1.02×102 2.05×102 3.0×102 ND 2.6×102 137 59 39 34 12 11 1 2 0 8 9 12 ND 8 67 34 20 30 13 8 3 1 13 14 17 ND 26 Total coliforms (cfu/100 ml) Raw Final POU Western Cape Province 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 3.3×102 7 0 25 0 0 0 0 0 0 10 0 ND 12 9 50 35 22 47 2 0 0 8 25 0 ND 28 Faecal Coliforms (cfu/100 ml) Raw Final POU
© Copyright 2026