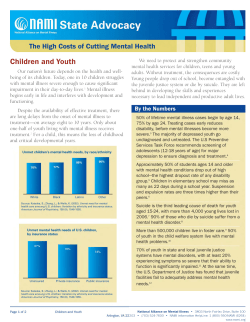
COMPREHENSIVE INTEGRATED STRATEGY FOR CHOLERA PREVENTION AND CONTROL
COMPREHENSIVE INTEGRATED STRATEGY FOR CHOLERA PREVENTION AND CONTROL Coalition for Cholera Prevention and Control August 2013 TABLE OF CONTENTS FOREWORD 1 ACKNOWLEDGMENTS 3 EXECUTIVE SUMMARY 5 DEVELOPMENT OF NATIONAL PLANS FOR CHOLERA PREVENTION AND CONTROL 11 SURVEILLANCE AND DISEASE DETECTION 17 DIAGNOSIS AND TREATMENT OF CHOLERA 25 DRINKING WATER INTERVENTIONS 35 SANITATION INTERVENTIONS 41 HYGIENE INTERVENTIONS 47 USE OF ORAL CHOLERA VACCINE 53 INFORMATION, EDUCATION, AND COMMUNICATION AND COMMUNITY HEALTH WORKER TRAINING 59 PROCUREMENT AND LOGISTICS 63 OPERATIONAL RESEARCH 75 MONITORING AND EVALUATION OF ALL INTERVENTIONS 81 ABBREVIATIONS AND ACRONYMS USED IN THIS DOCUMENT 91 BIBLIOGRAPHY 93 FOREWORD Alan R. Hinman and Paul E. Farmer Cholera, an ancient scourge, remains an important cause of morbidity and mortality around the world, with an estimated 100,000—or more—deaths each year. Many countries in Africa and southern Asia experience periodic epidemics, which dramatically impact these countries. Cholera was reintroduced to the Americas in 1991 and caused major epidemics throughout Latin America. Over a decade, major efforts to improve water and sanitation led to the expulsion of cholera from the Americas. Unfortunately, in the wake of the major earthquake in 2010, cholera was introduced to Haiti, which had been free of the disease for many decades. An explosive outbreak ensued, causing more than 650,000 cases and 8,000 deaths as of 28 May 2013 (1,2). Effective interventions to prevent and control cholera during past decades include effective disease detection and diagnosis; effective treatment with rehydration (oral or intravenous) and, when appropriate, antibiotics; provision of safe drinking water; proper sewage disposal; and improved hygiene (WaSH) (3). Unfortunately, these interventions may take many years to implement fully, as was the case in Latin America during the 1990s. An additional intervention has been developed in the last 10–15 years—oral cholera vaccine (OCV)—but it has not been widely implemented. Reasons for its limited use include lack of awareness of its possible impact; low vaccine production capacity; and concern that adding another intervention might divert efforts and resources from “traditional” approaches, which historically have been under-resourced. Moreover, there is no single statement of a comprehensive integrated strategy to prevent and control cholera incorporating appropriate use of OCV. Individual guidelines exist for a number of interventions, but no single document ties them all together. Thus, tragically, although we now have better tools than ever, cholera remains a serious burden that, in some areas, is getting worse. To address this issue, in October 2011, the Bill & Melinda Gates Foundation awarded a grant to The Task Force for Global Health and Harvard Medical School/Partners in Health to form the Coalition for Cholera Prevention and Control (CCPC) to “accelerate development of a comprehensive global strategy to prevent and control cholera, building on recent developments in thinking about the appropriate use of oral cholera vaccines in preventing and controlling epidemic and endemic cholera.” CCPC held its first meeting in Atlanta, Georgia, USA, on March 15–16, 2012, with 45 participants, including many of the most eminent investigators of cholera and those involved in its prevention and control. At the end of the meeting, participants issued a consensus statement that concluded: “The Coalition urges implementation of a comprehensive package of cholera prevention and control measures, including early detection, treatment and prevention with WaSH and oral cholera vaccines, appropriately tailored to the situation in specific countries/regions.” The statement subsequently was published as a letter to the editor of Vaccine (4). After the March 2012 meeting, CCPC members set about developing the Strategic Framework that is the body of this publication. This Framework summarizes existing recommendations and guidelines for preventing and controlling cholera, identifies outdated recommendations and gaps, and recommends new guidelines as suggested by existing research (or identifying areas for further research). 1 The process for developing the Framework was as follows: 11 individual sections were identified, and one or two CCPC members or consultants drafted each section. Each draft section was reviewed and commented on by two to five other CCPC members, after which the authors made appropriate revisions. The revised documents were then sent to an experienced writer-editor for review and revision to try to ensure relative uniformity. The entire document was sent out to all members of the Coalition in advance of the second meeting of the Coalition on June 3–4, 2013. At the second meeting the Framework was discussed thoroughly and endorsed in principle. Further minor revisions based on comments received at or after the meeting are incorporated into this final version. The Framework represents the sense of the Coalition and should not be taken to imply institutional endorsement by any of the agencies represented on the Coalition. Authors of individual sections are not identified. The Acknowledgments section lists the authors and the reviewers. Karen Foster served as the writer-editor and Alan Hinman as the overall editor of the work. The Strategic Framework is intended to be suitable for different audiences: policy makers, policy implementers, donors, clinicians, researchers, and others. We hope it can be useful for identifying the key issues around cholera for policy makers, as well as in providing links to the detailed documents that policy implementers would use. REFERENCES 1. Barzilay EJ, Schaad N, Magloire R, et al. Cholera surveillance during the Haiti epidemic— the first 2 years. N Engl J Med 2013:368:599–609. www.ncbi.nlm.nih.gov/pubmed/?term=Cholera+surveillance+during+the+Haiti+epidemic%E 2%80%94the+first+2+years (accessed May 18, 2013). 2. United Nations Office for the Coordination of Humanitarian Affairs (OCHA). Haiti cholera snapshot [published June 5, 2013]. http://gallery.mailchimp.com/ae620ada5956c2460fcad49f8/files/hti_cholera_Snapshot_June _2013.pdf (accessed June 20, 2013). 3. Waldman RJ, Mintz ED, Papowitz HE. The cure for cholera—improving access to safe water and sanitation. N Engl J Med 2013:368:592–4. www.nejm.org/doi/full/10.1056/NEJMp1214179 (accessed May 18, 2013). 4. Hinman AR, Farmer PE. The Coalition for Cholera Prevention and Control meeting [letter]. Vaccine 2013;31:2323. 2 ACKNOWLEDGMENTS We acknowledge the hard work of members of the Coalition for Cholera Prevention and Control and consultants who drafted the individual sections of this Framework, as well as those who reviewed drafts and suggested revisions. We also thank Katie Baer and Tanner Hendrick for their help. Section Authors Section Reviewers Jalaluddin Ahmed Pradip Kumar Bardhan William Carter Libertad Gonzalez Robert Hall Jan Heeger Louise Ivers Azharul Islam Khan Pinar Keskinocak Helen Matzger Martin Mengel Dima Nazzal Carmen Paradiso Firdausi Qadri David Sack Monica Villareal Sharmin Akhter Zahan John Clemens Vance Dietz Robert Hall Thomas Handzel Derek Hardy Myriam Henkens Myron Levine Martin Mengel Eric Mintz David Olson Jared Omolo Jean William Pape Firdausi Qadri Edward Ryan David Sack Lorenz von Seidlein Jesus Trelles Ronald Waldman Peter Wright Zabulon Yoti 3 4 EXECUTIVE SUMMARY COMPREHENSIVE INTEGRATED STRATEGY FOR CHOLERA PREVENTION AND CONTROL Cholera is an acute diarrheal illness caused by intestinal infection with the bacterium Vibrio cholerae. Although sometimes mild, cholera can be severe, resulting in profuse watery diarrhea and other symptoms. Rapid loss of body fluids can result in dehydration, shock, and even death. Worldwide, more than 100,000 people die of cholera each year. Cholera is most likely to occur in places with poor sanitation and inadequate water treatment and hygiene (1). Despite efforts to control cholera, global incidence is on the rise, and current response to cholera outbreaks tends to be primarily reactive. This document pulls together recommendations from a variety of sources to outline a comprehensive integrated strategy for cholera prevention and control. NATIONAL PLANS FOR CHOLERA PREVENTION AND CONTROL Development of a national plan for cholera prevention and control has the potential to provide direction to a country or region in combating cholera. Some countries have developed national plans, although few incorporate the complete range of prevention and control strategies, including water, sanitation, and hygiene (WaSH) interventions and oral cholera vaccines (OCVs). Ideally, a national plan for cholera prevention and control should bring together key stakeholders: community leaders, the media, relevant government bodies, public health agencies and organizations, environmental agencies, and others. Active involvement of all key stakeholders can mobilize support for the goals and help ensure successful implementation. Progress in cholera prevention and control will be faster if national guidelines can recommend best practices based on existing research. SURVEILLANCE AND DISEASE DETECTION Reliable, timely, accurate, and relevant information about cholera is critical to learn where, when, and in whom the disease occurs. Cases of cholera are reported to the World Health Organization (WHO) through the Integrated Disease Surveillance and Response systems and published in WHO’s Weekly Epidemiological Record (WER). However, countries with a high incidence of cholera often do not report complete data because they fear stigmatization and economic losses if the world learns about their cholera problem. In general, countries vary in the extent to which they provide complete reporting. Official notifications to WHO represent only a fraction of actual cases. Some countries have experimented with tools to reduce the delay in notification of outbreaks to national authorities; for example, Kenya uses short text messages sent by mobile phone. To declare a cholera outbreak, WHO requires laboratory confirmation of cholera for the first cases of acute watery diarrhea. Confirming cholera cases in remote areas without laboratory 5 facilities is challenging; several rapid diagnostic tests have been developed to address this problem. DIAGNOSIS AND TREATMENT OF CHOLERA Cholera can be fatal; every third person who has severe cholera is at risk of dying unless he or she receives prompt clinical diagnosis and management. Oral rehydration solution (ORS) therapy and intravenous fluid replacement are important approaches for replenishing a patient’s loss of body fluids caused by diarrhea. Antibiotics can further limit the duration of disease. Ongoing clinical research suggests that zinc therapy may help reduce illness and death. Immunoprophylaxis with OCV is another promising approach. Clinical evaluation focuses on the type of diarrhea and on other clinical signs and symptoms. Rapid diagnostic tests are being studied to determine their usefulness in detecting cholera epidemics. They are quick to conduct and do not require laboratory facilities. As noted above, ORS is a key part of treatment for mild to severe cholera. ORS replaces body fluids and electrolytes. Patients with more severe cholera might require emergency intravenous administration of adequate volumes of polyelectrolyte rehydration solution. Antibiotics can curb the excretion of V. cholera and decrease the total purging volume. Adjunct therapy, such as zinc and vitamin A administration, has been shown to decrease the duration and severity of diarrhea. It typically is given to children. Major complications of cholera include hypoglycemia, hypovolemic shock, septic shock, hypothermia, and other severe conditions, including acute renal failure. ENVIRONMENTAL APPROACHES TO CHOLERA PREVENTION AND CONTROL (WaSH) Drinking Water Interventions Cholera is transmitted by the fecal–oral route, i.e., a person contracts cholera by ingesting something (usually water or food) that has been contaminated with fecal matter infected with V. cholerae. Cholera can be reliably prevented and controlled only by stopping this contamination cycle. Key elements of interrupting the cycle include providing safe drinking water, improving sanitation conditions, and ensuring proper hygiene (personal hygiene and food preparation). In particular, water of good quality needs to be provided in sufficient quantity so the population can practice good hygiene. Ensuring a safe and sufficient water supply should be a key element in planning a cholera prevention and control strategy. Sanitation Interventions Proper and safe disposal of human waste (urine and feces) is essential to preventing and controlling cholera. Improved sanitation that hygienically separates human excreta from human contact can substantially improve the health of individuals and communities. Provision of latrines in communities—especially in public spaces, such as markets and schools—generally will benefit cholera control. A ratio of one latrine for every 20 people in 6 crowded settings, such as refugee camps, is ideal. Constructing latrines or setting up solidwaste systems might not be practical in acute outbreak situations. However, a later improvement in the sanitary conditions is likely to decrease future risk for cholera outbreaks. Hygiene Interventions Promoting hygiene necessarily involves community participation in prevention and control efforts. Community ownership is essential for the successful implementation of environmental changes that will benefit cholera prevention and control. Individual and community action typically includes promotion of hand-washing, use of latrines, consumption of safe water, proper disposal of dead bodies, and proper food hygiene (preparation, storage, and consumption). Experience has shown that a participatory approach to promoting hygiene is more effective than a message-based approach that simply raises awareness about cholera prevention and control. Mobilizing the community to adhere to safe hygiene practices is the key to behavior change. Techniques to improve community mobilization include using social media, peer connections, and participatory training. Understanding the public’s perceptions about the risk for cholera and the potential for prevention and control also is important. USE OF OCV OCV, a relatively new addition to the cholera prevention and control toolkit, can work synergistically with other interventions. The vaccine used most often in developing countries is Shanchol. It works by reducing the internal colonization of V. cholerae, thus making people less likely to spread infection. It is safe and effective, with virtually no side effects, and relatively inexpensive. Generally, two vaccine doses are given 2 weeks apart. Despite its promise, OCV is not widely promoted among public health agencies. Some agencies worry that focusing on OCV will divert already limited resources from more traditional (WaSH) interventions. However, the two approaches can be complementary and enhance the impact of each intervention. Two real constraints in using OCV more widely are 1) the need for a cold chain and 2) a limited supply of vaccine. Studies are exploring whether a full cold chain during field distribution is necessary. Once OCV is accepted more widely, production is likely to be increased so there will be fewer shortages. Creation of an emergency stockpile of OCV also might help increase production. In 2010, WHO recommended that OCV be used in conjunction with other prevention and control strategies in areas to which cholera is endemic. The public health community is still grappling with the best way to implement this recommendation. INFORMATION, EDUCATION, AND COMMUNICATION AND COMMUNITY HEALTH WORKER TRAINING Informing and training communities and community health workers is a necessary part of a cholera prevention and control plan. Synchronizing efforts and ensuring uniformity of message 7 content is critical. Evidence-based information, education, and communication activities emphasize an approach that includes individual behavior change or reinforcement, as well as changes in social and community norms. When carefully carried out, health communication strategies can help foster positive health practices. Key actions include developing, implementing, and monitoring a communication plan. The United Nations Children’s Fund toolkit is an excellent comprehensive guide to public communication activities for cholera outbreaks (2). The toolkit does not specifically address OCV or communication issues related to OCV as part of an outbreak response or as part of a national immunization program. PROCUREMENT AND LOGISTICS Procurement and logistics encompass a broad range of operations that aim to efficiently and effectively match the demand for a product (or set of products) to its supply. Emergency procurement and logistics operations span the timeline from preparedness through response and recovery to exit strategy. These operations are similar to those faced by traditional businesses, but key differences make them more challenging. Demand is highly unpredictable in terms of timing, location, and quantity; stakes are high; and resources are constrained. Advance planning and capacity building, effective management of response activities, and collaboration and coordination across agencies increase efficiencies of logistics and procurement activities—and ultimately increase the number of lives that can be saved. Cholera is not a complicated disease to treat, but it is transmitted quickly. Supplies needed to prevent and respond to a cholera outbreak, such as chlorine tablets; washing soap; and medical supplies like ORS, antibiotics, and vaccine (OCV), should be readily available. Planning should address various phases of response: needs assessment; planning, forecasting, and preparation; resource mobilization; procurement; transportation and storage; distribution; and measurement and evaluation. Each phase includes a checklist of items and actions to ensure a smooth implementation. Prevention efforts can—and should—incorporate vaccination as a part of an overall strategy. One logistical issue this raises is the need for a cold chain to maintain proper vaccine temperatures during storage and handling to preserve potency. The last mile often is the most challenging when communities lack a power source. Research is ongoing to determine the best way to preserve optimal shelf life of, for example, the Shanchol vaccine. OPERATIONAL RESEARCH Operational research seeks to use advanced analytic methods to improve decision making. It is intended to evaluate the impact of interventions in diverse routine care settings rather than in defined groups of patients. Operational research addresses problems related to specific programs by using systematic data collection. Operational research can help address questions on oral cholera vaccine, such as the following: 8 How do different settings (e.g., refugee camps, urban slums, rural mountain areas) affect feasibility, effectiveness, and cost-effectiveness? Within countries, where should OCV be targeted to save the most lives? What is optimal timing for vaccination in various cholera situations? Research focusing on specific vaccines is important. For example, Shanchol, the most recently prequalified vaccine, is less expensive and easier to administer than Dukoral. Critical knowledge gaps remain about vaccination strategies in endemic, epidemic, and outbreak scenarios, as well as about integrated approaches to cholera treatment and control that include vaccination, WaSH interventions, and treatment. MONITORING AND EVALUATION OF ALL INTERVENTIONS The effectiveness of any response marks the difference between a humanitarian disaster and a scenario with minimal deaths. All organizations and agencies involved in cholera response should adopt rigorous monitoring and evaluation procedures and report results to Ministries of Health and WHO. Sharing information about their cholera response will help others facing a similar dilemma. Feedback is critical to learning about the optimal response to cholera outbreaks. Key actions relate to the different phases of a cholera epidemic, including the early stages of an outbreak (managing cases, minimizing transmission), the epidemic itself (treating cases, minimizing transmission), and monitoring and evaluation of interventions, such as WaSH and OCV. Similarly, managing endemic cholera involves a different set of interventions, which also need to be evaluated. CONCLUSIONS This document represents combined efforts of members of the Coalition for Cholera Prevention and Control and consultants. It does not generate new recommendations but instead pulls together existing guidelines from a variety of sources to describe a comprehensive integrated strategy for cholera prevention and control. REFERENCES 1. Centers for Disease Control and Prevention. Cholera—Vibrio cholerae infection. General information. www.cdc.gov/cholera/general/ (accessed May 19, 2013). 2. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 19, 2013). 9 10 DEVELOPMENT OF NATIONAL PLANS FOR CHOLERA PREVENTION AND CONTROL DEFINITION OF ISSUE Although considerable efforts have been made to control cholera, global incidence has been increasing steadily, and current responses to cholera outbreaks tend to be primarily reactive. Lack of a definitive national action plan; lack of appropriate community resilience, lack of knowledge about cholera prevention, and lack of a coordinated multisectoral approach are major factors impeding optimal control of cholera in countries to which it is endemic (1). The recent resurgence of cholera, uselessness of quarantine of people and restrictions on trade as a means of controlling spread of cholera in countries, and fact that notification of cholera is not mandatory through the International Health Regulation (2005) (2) all require a strong surveillance system and national preparedness of countries to quickly identify and control the spread of cholera. Oral cholera vaccine (OCV) has proven to be safe and effective as a part of a comprehensive and multidisciplinary approach to prevent and control cholera (3). This section documents elements in developing a national plan for cholera prevention and control in light of currently available knowledge, facts and figures, and recommendations from various agencies on the issue. RECOMMENDED ACTIONS A national plan for cholera prevention and control has the potential to provide necessary directions for a country or region based on strong epidemiologic findings and surveillance data available over time. It should contain suggested activities based on successful models and best practices elsewhere. Several countries and organizations have developed national guidelines that, although not uniform, have captured the best practices and information applicable to the country or more generally. With few exceptions, these guidelines do not describe appropriate integration of OCV into cholera control strategies that use water, sanitation, and hygiene (WaSH) interventions. Examples of Cholera Control Plans (with and without OCV information) Kenya The Ministry of Public Health and Sanitation developed Multisectoral Cholera Prevention and Control Plan 2011–2016 in collaboration with the Centers for Disease Control and Prevention (CDC), United Nations Children’s Fund (UNICEF), Red Cross, Médecins Sans Frontières (MSF), WHO, African Field Epidemiology Network (AFENET), US Agency for International Development (USAID), and other key partners and in consultation with other government agencies and private sectors. WHO Guidelines Others (4) This detailed implementation plan prioritizes cholera activities under 11 different thematic areas, such as advocacy, WaSH, disease prevention and health promotion, and disease outbreak preparedness and response. The plan details short-term and long-term actions and a monitoring and evaluation framework. It also includes an estimated budget. Starting with country background and cholera situation, this plan advocates fighting cholera through a well-coordinated multisectoral approach that emphasizes continuous prevention rather than the traditional focus on outbreak response only. However, the plan does not describe integration of OCV into overall activities. Democratic Republic of the Congo (DRC) WHO Guidelines The DRC Ministry of Health (MoH) released its National Plan for the Elimination of Cholera 2013–2017 in March 2013. This plan was based on studies of different levels of risk in the country that indicate that targeting certain lake-bordering areas in eastern DRC could have lasting impact on preventing recurrent cholera epidemics in the entire country. Use of OCV was not specifically addressed. Zimbabwe Others (5) WHO Guidelines Others The Ministry of Health and Child Welfare and WHO developed Zimbabwe Cholera Control Guidelines, 2009, during an ongoing cholera outbreak, mainly to guide outbreak responders. The plan emphasized decreasing attack rates and case-fatality rates. The structure for multisector and multiagency coordination was through establishment of the Cholera Command and Control Centre (C4) in which organizations, such as UNICEF, Red Cross, MSF, and United Nations Office for the Coordination of Humanitarian Affairs, participated under the overall leadership of the Ministry of Health and Child Welfare. The document provides background information about cholera and technical guidance on cholera prevention, preparation for an outbreak, management of early response to the threat of an outbreak, management of a patient with cholera, prevention of the spread of an outbreak, role of the laboratory, and reporting and surveillance. (6) The document contains useful practical annexes but mentions OCV use only as information, not as a definitive recommendation. The OCV information needs to be updated to include adoption of OCV in the cholera control guideline. Southern Sudan Prepared by the MoH, Cholera Epidemic Preparedness and Response Guidelines for Southern Sudan, March, 2011, provides details of cholera epidemic preparedness and response plans and activities, including multisectoral strategic approach. The guideline contains information about 12 WHO Guidelines Others (7) OCV and mentions that WHO-recommended immunization with currently available cholera vaccines be used in conjunction with the usually recommended control measures in areas to which cholera is endemic and in areas at risk for outbreaks. Zanzibar WHO Guidelines Others The MoH; WHO; and the University of California, Los Angeles, Fielding School of Public Health, developed Zanzibar Programme to Eliminate Cholera, a time-limited (10-year) plan for sustainable elimination of cholera in Zanzibar. It recommends possible use of OCV combined with improved WaSH. Because of the negative impact of cholera on the health, economy, and development of the population, the Zanzibar government is keen to improve cholera control by using approaches based on evidence from its pilot project called Preemptive Use of Oral Cholera Vaccination in HighRisk Populations in Zanzibar (CHOZAN). The proposed 10-year program aims to eliminate indigenous transmission of cholera in Zanzibar by combing OCV and improved WaSH. WHO The 2004 WHO cholera outbreak guideline covers outbreak detection; outbreak confirmation; organization of the response; management of information; case management; reduction in mortality; hygiene measures in health care facilities; community involvement to limit disease spread; control of the environment; funeral practices; surveillance; and involvement of international partners. It includes a number of useful annexes. OCV is mentioned as a new strategy for cholera preparedness, and further assessment is recommended. OXFAM GB In June 2012, OXFAM GB published Cholera Outbreak Guidelines— Preparedness, Prevention and Control. These guidelines were originally developed as an internal resource drawing on experience from OXMFM’s cholera response programs in Ethiopia, Sudan, Somalia, Haiti, Zimbabwe, and DRC. These practical field-level guidelines aim to provide quick, stepby-step guidance to inform cholera outbreak interventions and ensure public health programs that are rapid, community-based, well-tailored, and sex- and diversity-aware. However, the guidelines lack comprehensiveness and must be used together with existing OXFAM and WASH Cluster public health guidelines. Integration of OCV also is not a focus of OXFAM GB published cholera intervention guideline. (8) WHO Guidelines Others (9) WHO Guidelines Others (10) 13 UNICEF The UNICEF toolkit aims to provide UNICEF offices, counterparts, and partners with one source of information for prevention (or risk reduction) and cholera outbreak preparedness, response, and recovery—including integration with regular/development programs. The guidelines pull together recommendations regarding a range of interventions to avoid the continuation of “silo” approaches for cholera prevention, preparedness, and response. Nevertheless, preemptive use of OCV in endemic, at-risk, and humanitarian settings and reactive use during outbreaks has been recommended on the basis of a sound risk assessment for clear decision making. However, the UNICEF toolkit does not provide great detail about the use of OCV. WHO Guidelines Others (11) The combination of programmatic and multidisciplinary approaches to cholera as part of prevention and control activities for diarrheal diseases has proven effective in decreasing the case-fatality rate during outbreaks and reducing the occurrence of epidemics. In general, a national plan for prevention and control of cholera should entail collaboration between various actors and sectors, such as ministries of health, environment, and planning to develop the guideline and should be adapted to local and regional needs. Careful attention should be paid to allocating and using public and private resources. The plan should describe briefly the processes of developing a multisectoral action plan and analyze country-specific strengths and weaknesses, opportunities, and threats as it guides the nation to reduce the risk for cholera. Effective collaboration and links among different government departments, multilateral and bilateral development partners, and nongovernment organizations (NGOs) should be demonstrated clearly to emphasize a coordinated and harmonious prevention effort at local and national levels. The community should be put at the center of this effort to create ownership of the problem and contribute to reducing risk for cholera through community-based prevention and control programs. Provisions are needed for updating the plan at timely intervals. Thematic areas for developing a comprehensive national plan for cholera prevention and control could be Context: Current cholera situation; relevance for the national plan (endemic cholera); costeffectiveness, timeliness, responsiveness, etc. Objective of the national plan: What do the authors of the national plan hope to achieve, e.g., cholera control or elimination in certain areas or at-risk populations? Stakeholders and actors relevant to cholera prevention and control. Advocacy and communication. Available resource/needs assessment Resource mobilization: Who does what (i.e., logistics)? Cholera prevention and control: from WaSH to vaccination in endemic, epidemic, and highrisk settings. Clinical guidelines for cholera management. Cholera surveillance and outbreak control. Monitoring and assessment/ evaluation. Knowledge gaps: identifying research areas. 14 PROCESS FOR DEVELOPING A COMPREHENSIVE MULTISECTOR NATIONAL PLAN Multidisciplinary engagement is required for developing a national plan for cholera prevention and control to help with standardized development and implementation of the interventions. Consultation should be sought with relevant stakeholders from all possible areas: The community (including community leaders and persons of both sexes) to get input of individuals in protecting their own health. The media: getting insight for disseminating information widely to the general public. Government bodies from different ministries and directorates; water, sanitation and public health; education; finance; local government; social welfare; community development; information and communication; disaster management; and other ministries according to the local need. United Nations bodies (UNICEF, WHO): these act both as donors and as technical support for other stakeholders. Civil society organizations, including NGOs, community-based organizations, and faithbased organizations. Private sector: representatives from hospitals, clinics, and pharmaceutical and vaccine industries. COMMENTS Key stakeholders, such as MoHs, United Nations’ bodies (e.g., UNICEF, WHO), donor agencies, medical and nonmedical NGOs, political leaders, religious leaders, schoolteachers and community members of both sexes, need to work together on an agreed plan for cholera prevention and control. This collaboration can be coordinated with government and NGOs through existing forums in health and WaSH programs. Involvement of public in the implementation process will ensure greater awareness of cholera prevention and prioritization of their participation in activities. Active involvement of all key stakeholders can mobilize support for the intended goals and help ensure that any intervention the guideline suggests is acceptable. Implementation of a national plan for cholera prevention and control will have a tremendous effect in mitigating the risk for cholera in vulnerable populations, provided it is accompanied by the resources necessary for its implementation. Many of the best practices involving the multidisciplinary approach will reflect the principles of good practice for any multiagency public health effort. Progress in cholera prevention and control will be faster if a national guideline can recommend for the preventing and controlling cholera, identifying gaps and outdated recommendations, and recommending new guidelines as suggested by existing research or identifying areas for further research. Because cholera does not recognize national boundaries, it is important to take a regional, not just national, view in developing plans and ensure that neighboring countries have compatible approaches. If external agencies are involved in developing plans, it is important to ensure that national capacity exists to update and implement the plans after these agencies depart. Development of national plans with sustainable and focused approaches for cholera prevention and control within existing country health systems is important for epidemic, high endemicity, and low endemicity settings. Such plans provide a platform for collaboration, resource mobilization, and deployment of interventions to prevent and control this fatal disease. 15 REFERENCES 1. World Health Assembly. Cholera: mechanism for control and prevention. 24 May 2011. http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_R15-en.pdf (accessed February 7, 2013). 2. WHO. International Health Regulations (2005). 2nd ed. www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=4015 (accessed July 29, 2013). 3. WHO. Cholera 2011. Wkly Epidemiol Rec 2012;87:289–304. www.who.int/wer/2012/wer8731_32.pdf (accessed February 7, 2013). 4. Republic of Kenya. Multi-sectoral cholera prevention and control plan, 2011 to 2016. Draft. September, 2011. www.unicef.org/cholera/Annexes/Supporting_Resources/Annex_6D/Kenya_M_Public_Healt h_and_Sanitation-preparedness_plan.docx (accessed August 26, 2013). 5. MOH DRC. National plan for the elimination of cholera 2013–2017 [in French]. www.africhol.org/sites/africhol.org/files/files/RDC_PlanEliminationCholera20132017_20130228.pdf (accessed April 24, 2013). 6. Ministry of Health and Child Welfare/WHO. Zimbabwe cholera control guidelines. 3rd ed. http://medmissio.de/surf/proxy/alfrescosystem/api/node/content/workspace/SpacesStore/dd738528-1187-4575-847d7de7eb6a4c24/Zimbabwe%20cholera%20control?a=true (accessed April 30, 2013). 7. MoH Southern Sudan. Cholera epidemic preparedness and response guidelines for southern Sudan, March, 2011. www.google.com.bd/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&sqi=2&ved=0CCsQFjA A&url=http%3A%2F%2Fwww.mwrigoss.org%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid%3 D19%26Itemid%3D8&ei=u6B_UfHLoTRrQfaooGwAQ&usg=AFQjCNGuaraEUXvX_x7GijaqUXDrCqNWA&bvm=bv.45921128,d.bmk (accessed April 30, 2013). 8. Dr. M Jiddawi. [email protected]. Zanzibar Program to Eliminate Cholera (ZPEC): a 10-year, time-limited plan for sustainable elimination of cholera. 23 January, 2013. 9. WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFk_2004.4_eng.pdf (accessed February 7, 2013). 10. Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012 www.cmamforum.org/Pool/Resources/Cholera-outbreakguidelines-OXFAM-2012.pdf (accessed February 7, 2013). 11. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 16 SURVEILLANCE AND DISEASE DETECTION DEFINITION OF ISSUE Surveillance is often described as gathering information for action. This description emphasizes the need for a reliable supply of timely, accurate, and relevant information to efficiently prevent and control diseases. Applied to cholera, this means that we have to know quickly, where, when, and in whom the disease occurs. The classical model of surveillance includes three major processes: capture and collation of data, analysis and interpretation of data, and dissemination of information. Many consider response also to be a component of surveillance. RECOMMENDED ACTIONS World Health Organization: Role and Materials for Surveillance Within the World Health Organization (WHO), the Global Task Force on Cholera Control coordinates cholera-related activities. The Task Force’s website contains a variety of technical guidelines, country information, maps, and current developments related to epidemics (1) and policies and recommendations for the prevention and control of cholera outbreaks (2). Cholera cases and deaths are officially reported to WHO through the national Integrated Disease Surveillance and Response systems (IDSR) and published in the Weekly Epidemiological Record (3). In addition, the Weekly Epidemiological Record provides annual summary tables of cholera cases and deaths and short notes on cholera outbreaks. Reports of major outbreaks also appear on the WHO website under Disease Outbreak News. However, countries with high incidence of cholera do not report or report only limited data because of fear of trade and travel embargos that might follow such news. International Health Regulations Countries need to report cases to WHO when “they are unusual or unexpected or when they pose a significant risk of international spread” (4). The 2005 revised International Health Regulations explicitly state that no travel or economic restrictions are to be established if a country notifies cholera. Yet, reluctance to notify remains because of concerns about stigmatization and subsequent economic losses. Many countries notify only sporadic cholera cases. Countries to which cholera is highly endemic, such as India, Bangladesh, and Ethiopia, did not notify any cholera cases to WHO in 2011 (3) although periodic epidemics did occur in these areas. Integrated Disease Surveillance and Response Cholera was included as one of three notifiable diseases in the International Health Regulations 1969. The IDSR guidelines classify cholera as a disease with “highly epidemic potential.” These guidelines define the modalities of reporting from the local to the national level and state in detail which reporting competences should be available at each level (5). 17 The IDSR guidelines for community health workers ask them to report cases “with plenty of watery diarrhea” to the district health officers that feed these notifications into the IDSR systems. In Cameroun, a change in the case definition (which, before 2010, was “cases with acute watery diarrhea AND renal failure”) to the WHO standard case definition led to a considerable increase in cases. In summary, modalities, completeness, and case definition of reporting to WHO varies from country to country. These variations decrease the completeness and specificity of the resulting data. National Cholera Plans National cholera plans are unfortunately the exception, even among countries to which cholera is considered endemic. Kenya recently issued a draft Multi-Sectoral Cholera Prevention and Control Plan for 2011–2016 (not publicly available) and an Integrated Drought and Cholera Preparedness and Response Operational Plan (6). Exemplary work is also done in the Democratic Republic of the Congo (DRC), where the national cholera team has been in place for approximately 10 years but only recently published its first public yearly report (7). During the recent epidemic in Guinea-Conakry, authorities circulated a weekly newsletter to partners involved in cholera prevention and control called “Infochol” (not publicly available) (8). Diffusion at district and local levels could not be ascertained. Regular comprehensive reporting and feedback to the regional level, the general public, and the health worker in individual cholera treatment centers remains sporadic and usually relies on outside funding that is mostly not sustainable long term. Such was the case in DRC during the epidemic of 2007–2008. The Department for Disease Control there published a regular bulletin called “Belichol“ funded by UNICEF (9). After funding ceased, the publication was suspended. Cross-Border Collaboration WHO recommends that countries bordering a country with epidemic cholera prepare before they detect cases in their own countries (10). Cross-border collaboration, at least in the many countries affected by cholera in Africa, is not yet well developed. A recent encouraging example is the epidemic in Guinea-Conakry and Sierra Leone that has been ongoing since February 2012. The health authorities responsible for cholera control in both countries established a cross-border collaboration committee comprising senior technical staff from disease control departments and national reference laboratories. During October 31–November 2, 2012, they met to facilitate a platform for preparing for and responding to outbreaks of cholera and other priority communicable diseases along their common borders (11). Similar initiatives need to be encouraged in other countries where cholera is endemic or where new epidemics are anticipated (i.e., in Asia, Africa, and now in the Americas). Cholera epidemics do not stop at borders; thus, surveillance should not stop there either. Limitations of WHO Data Case Definition WHO uses the following case definition for reporting suspected cases of cholera (2): 18 “in an area where the disease is not known to be present, a patient aged 5 years or more develops severe dehydration or dies from acute watery diarrhea; “in an area where there is a cholera epidemic, a patient aged 5 years or more develops acute watery diarrhea, with or without vomiting.“ Underestimation of Disease Burden in Children <5 Years of Age The WHO case definition excludes cases in children aged <5 years despite several studies that have shown considerable disease and death in that age group. According to several studies, children aged <5 years account for a considerable proportion of cholera cases (12). Prospective, laboratory-confirmed cholera surveillance conducted in the early 2000s by the Diseases of the Most Impoverished (DOMI) Program in four sites (rural Matlab, Bangladesh; Beira, Mozambique; slum areas of Kolkata, India; and slums in North Jakarta, Indonesia) showed that the annual incidence rates for children <5 years old were two to four times higher (2.0–8.8 cases/1,000 children) than rates in the overall population (13). Consequently, the official notifications to WHO represent only a fraction of actual cases. Programs such as DOMI have tried to compensate for these shortcomings by drawing on data from sources other than WHO and by using modeling for more precise estimations. Studies that examined disease incidence through active case finding report many-fold higher case numbers, amounting to 2,548,227 (14) to 2,800,000 (15) cases and 91,000 (13) to 209,216 (12) deaths (16,17). Limited Spatial Resolution of Aggregate Data Another important limitation of the aggregated WHO data is that they give only an impression of the epidemics at national level. Analysis of case notification at district or subdistrict level clearly indicates that the spatial distribution of cases can differ significantly from national-level trends (6,18). Reporting Delay The data are reported to WHO and published with considerable delay from the time a case is detected until it is notified at district, regional, and national levels and from there to WHO (19). Hence, these data cannot be called data for action because usually they arrive too late to inform action. For adequate reaction to cholera outbreaks, timely notification is crucial. For example, in 1994 during a major epidemic in a refugee camp in Goma, DRC, an estimated 58,000–80,000 cases and 23,800 deaths occurred within only 1 month (20). In an analysis of 78 cholera outbreaks in Mozambique during 2009–2011 for the African Cholera Surveillance Network project (Africhol [www.africhol.org, funded by the Bill & Melinda Gates Foundation and led by the Agence de Médecine Préventive), 68% of all cases and 89% of deaths occurred within the first 6 weeks of an outbreak. New tools for notification have been employed to reduce the delay in notification of outbreaks to the national authorities. For example, in Kenya coded short-text messages sent by mobile phones are used (21). Such tools also have been developed for the transmission of laboratory results (22). New technologies also have been used during natural disasters, such as in Bandah Aceh after the tsunami in 2004 (23). Their use and further development and refinement will be important. 19 Laboratory Confirmation of Cholera Cases Laboratory confirmation of cholera for the first cases of acute watery diarrhea is a requirement for the declaration of a cholera outbreak in accordance with WHO guidelines. The standard is confirmation by growth of colonies of Vibrio cholerae isolated from patient stool samples on standard culture media and on the selective thiosulfate citrate bile salts sucrose (TCBS) agar (24). WHO recommends laboratory confirmation only at the beginning of an outbreak to verify the outbreak. Thereafter, only sporadic sampling and confirmation is recommended to monitor strains’ antibiotic resistance profiles and toward the end of an outbreak to ensure that no further cases of cholera are occurring. Confirming cholera cases in remote areas without laboratory facilities is challenging and can delay confirmation of a cholera outbreak and subsequently control efforts. To overcome this challenge, several rapid diagnostic tests have been developed and successfully tested and have been found to have a sensitivity of 67%–100% and a specificity of 71%–97% (25–34). New diagnostic tools are under development, such as biosensors that are easier and less expensive (35) Another possibility is use of mobile laboratories in which strains can be isolated and culture confirmation can be performed at any place at any time (36). Surveillance in Humanitarian Emergencies and Natural Disasters Surveillance is even more complicated when it has to be implemented in already overburdened health systems that additionally face a humanitarian emergency caused by a natural or humanmade disaster. WHO has developed several guides (37). A good overview of practical considerations for surveillance in complex emergencies can be found in a dedicated chapter of the book Infectious Disease Surveillance (38). KNOWLEDGE GAPS AND RESEARCH PRIORITIES Surveillance Reliable surveillance data and laboratory confirmation of suspected cases generally are scarce. However, both are crucial for designing reactive and preventive interventions as efficiently as possible. To better understand cholera epidemiology, affected countries need to notify cases by Place, on subdistrict level and Sex and age group, e.g., <5 years (preschool-age), 5–15 (school-age), 16–45 (working age), >45 (retired). Africhol, an ongoing research project, is addressing the problem of underreporting and absence of data on cholera in Africa. Given the limited vaccine supply, these surveillance data are crucial in guiding the place and target populations for vaccine interventions. The surveillance sites of Africhol can further be used in impact assessment for oral cholera vaccine (OCV) campaigns and other interventions and for other enteric disease-related interventions. 20 Novel Methods for Diagnosis of V. cholerae (16,17) Diagnosing V. cholerae infection remains difficult and costly in patients with diarrhea and in field settings, which results in delays in reporting to health authorities and implementing control measures. The international community should encourage implementation of existing rapid diagnostic tests and development of new, less expensive, and easier-to-use diagnostic methods (particularly those that might be performed at the point of care) and strengthening of district laboratory capacities, whether stationary or mobile. (V. cholerae diagnosis is one of the easiest and least expensive of the enteric pathogens). Novel Methods for Timely Notification (18–20) The novel technologies described above have not been evaluated specifically in connection with cholera outbreaks or their prevention and control. Because these technologies already exist, they should be implemented to render cholera prevention and control efforts more efficient. Beyond notification, new technologies can be used to integrate surveillance and case management, for example, by using electronic handheld records and other specially designed applications for mobile phones and other mobile devices (39). Health Systems Strengthening and Capacity Building The management of epidemics could be improved considerably at low cost if the health authorities in countries with epidemic cholera acquired the habit of regular and thorough reporting and analysis of data on cholera and other enteric diseases. The introduction of additional variables beyond reports of just suspected cases and deaths also would greatly improve existing data and should require little additional cost, given that national surveillance systems are already in place. Efforts to publish national surveillance data from the past decade from nine African countries to which cholera is endemic showed that only one had staff processing cholera data with the intent of publishing them. Only three of those countries had a coherent database on cholera. Surveillance data from countries in Asia also is needed urgently. Simple training interventions and implementation of standard operating procedures for surveillance in cholera-prone countries would already be a major improvement. Use of WHO definitions would also simplify efforts. REFERENCES 1. WHO. Cholera. www.who.int/cholera/en/ (accessed April 12, 2013). 2. WHO Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. www.who.int/cholera/technical/prevention/control/en/index.html (accessed April 12, 2013). 3. WHO. Weekly Epidemiological Record. www.who.int/wer/ (accessed April 12, 2013) 4. WHO. International health regulations (2005). 2nd ed. www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=4015 (accessed April 12, 2013) 5. WHO. Global Alert and Response (GAR). Integrated disease surveillance. www.who.int/csr/labepidemiology/projects/diseasesurv/en/index.html (accessed April 12, 2013). 21 6. WESCOORD Drought Response Kenya. Preparedness and response. Integrated drought and cholera operational plan. 21st September 2011. http://wescoord.or.ke/documents/Keydocs/WESCOORD_CholeraPlan_20110921.pdf (accessed April 12, 2013). 7. Africhol. Report on cholera epidemiology in the Democratic Republic of Congo in 2011 [in French]. www.africhol.org/news/drc/report-cholera-epidemiology-democratic-republic-congo2011 (accessed April 12, 2013). 8. Africhol. Africhol in Guinea [processed content partly available]. www.africhol.org/country/guinea (accessed April 25, 2013). 9. Ministry of Public Health, Authority for Disease Control; BELICHOL: bulletin du projet d’elimination du choléra en RDC. www.minisanterdc.cd/Articles/d4/BELICHOL%20DEC3.pdf (accessed April 13, 2013). 10. WHO Global Task Force on Cholera Control. First steps for managing an outbreak of acute diarrhoea. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CSR_NCS_2003.7_Rev.2_eng.pdf (accessed April 12, 2013). 11. WHO. Sierra Leone & Guinea concludes cross border meeting on cholera prevention & control. www.afro.who.int/en/sierra-leone/press-materials/item/5091-sierra-leone--guineaconcludes-cross-border-meeting-on-cholera-prevention--control.html (accessed April 12, 2013). 12. Lanata CF, Mendoza W, Black RE. Improving diarrhoea estimates. Geneva: World Health Organization; 2002. 13. Deen J, von Seidlein L, Clemens J. Multidisciplinary studies of disease burden in the Diseases of the Most Impoverished Programme. J Health Popul Nutr 2004;22:232–9. www.ncbi.nlm.nih.gov/pubmed/?term=Multidisciplinary+studies+of+disease+burden+in+the+ Diseases+of+the+Most+Impoverished+Programme (accessed May 19, 2013). 14. Sack DA, Cholera burden of disease estimates. www.jhsph.edu/departments/internationalhealth/globalhealthresearch/cholera/index.html (accessed November 15, 2012). 15. Ali M, Lopez AL, You YA, et al. The global burden of cholera. Bull World Health Organ 2012;90:209–18A. www.who.int/bulletin/volumes/90/3/11-093427/en/ (accessed May 19, 2013). 16. Shikanga OT, Mutonga D, Abade M, et al. High mortality in a cholera outbreak in western Kenya after post-election violence in 2008. Am J Trop Med Hyg 2009;81:1085–90. www.ncbi.nlm.nih.gov/pubmed/?term=High+mortality+in+a+cholera+outbreak+in+western+ Kenya+after+post-election+violence+in+2008 (accessed May 19, 2013). 17. Zuckerman J, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect Dis 2007;7:521–30. www.ncbi.nlm.nih.gov/pubmed/?term=The+true+burden+and+risk+of+cholera%3A+implicati ons+for+prevention+and+control (accessed May 19, 2013). 18. Gujral L, Sema C, Gessner B, Jani I. Cholera epidemiology in Mozambique using national surveillance data. J Infect Dis 2013. In press. 19. WHO. WHO report on global surveillance of epidemic-prone infectious diseases. Geneva: WHO; 2000:40. www.who.int/csr/resources/publications/surveillance/en/cholera.pdf (accessed December 7, 2012). 20. Goma Epidemiology Group. Public health impact of the Rwandan refugee crisis: what happened in Goma, Zaire in July 1994? Lancet 1995;345:359–61. www.ncbi.nlm.nih.gov/pubmed/?term=Public+health+impact+of+the+Rwandan+refugee+cris is%3A+what+happened+in+Goma%2C+Zaire+in+July+1994%3F (accessed May 19, 2013). 21. Zurovac D, Talisuna AO, Snow RW. Mobile phone text messaging: tool for malaria control in Africa. PLoS Med 2012;9:e1001176. www.ncbi.nlm.nih.gov/pmc/articles/PMC3283546/ (accessed May 19, 2013). 22 22. Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS ONE 2009;4:e6320. www.ncbi.nlm.nih.gov/pubmed/?term=Mobile+phone+based+clinical+microscopy+for+global +health+applications (accessed May 13, 2013). 23. Ministry of Health, Indonesia; WHO; Global Outbreak Alert and Response Network (GOARN) partners; Centers for Disease Control and Prevention—USA; Epicentre—France; European Programme for Intervention Epidemiology Training (EPIET)—Sweden, et al. Epidemic-prone disease surveillance and response after the tsunami in Aceh, Indonesia. Euro Surveill. 2005;10:pii=2700. www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2700 (accessed May 19, 2013). 24. WHO. Laboratory methods for the diagnosis of epidemic dysentery and cholera, 1999. www.who.int/topics/cholera/publications/WHO_CDS_CSR_EDC_99_8_EN/en/index.html (accessed April 12, 2013). 25. Qadri F, Hasan JA, Hossain J, et al. Evaluation of the monoclonal antibody–based kit Bengal SMART for rapid detection of Vibrio cholerae O139 synonym Bengal in stool samples. J Clin Microbiol 1995;33:732–4. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+the+monoclonal+antibody%E2%80%9 3based+kit+Bengal+SMART+for+rapid+detection+of+Vibrio+cholerae+O139+synonym+Ben gal+in+stool+samples (accessed May 19, 2013). 26. Nato F, Boutonnier A, Rajerison Grosjean MP, et al. One-step immunochromatographic dipstick tests for rapid detection of Vibrio cholerae O1 and O139 in stool samples. Clin Diagn Lab Immunol 2003;10:476–8. www.ncbi.nlm.nih.gov/pubmed/?term=Onestep+immunochromatographic+dipstick+tests+for+rapid+detection+of+Vibrio+cholerae+O1+ and+O139+in+stool+samples (accessed May 19, 2013). 27. Wang X-Y, Ansaruzzaman M, Vaz R, et al. Field evaluation of a rapid immunochromatographic dipstick test for the diagnosis of cholera in a high-risk population. BMC Infect Dis 2006;6:17. www.ncbi.nlm.nih.gov/pubmed/?term=Field+evaluation+of+a+rapid+immunochromatographi c+dipstick+test+for+the+diagnosis+of+cholera+in+a+high-risk+population (accessed May 19, 2013). 28. Kalluri P, Naheed A, Rahman S, et al. Evaluation of three rapid diagnostic tests for cholera: does the skill level of the technician matter? Trop Med Int Health 2006;11:49–55. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+three+rapid+diagnostic+tests+for+chol era%3A+does+the+skill+level+of+the+technician+matter%3F (accessed May 19, 2013). 29. Mukherjee P, Ghosh S, Ramamurthy T, et al. Evaluation of a rapid immunochromatographic dipstick kit for diagnosis of cholera emphasizes its outbreak utility. Jpn J Infect Dis 2010;63:234–8. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+a+rapid+immunochromatographic+dip stick+kit+for+diagnosis+of+cholera+emphasizes+its+outbreak+utility (accessed May 19, 2013). 30. Harris JR, Cavallaro EC, de Nobrega AA, et al. Field evaluation of crystal VC Rapid Dipstick test for cholera during a cholera outbreak in Guinea-Bissau. Trop Med Int Health 2009;14:1117–21. www.ncbi.nlm.nih.gov/pubmed/?term=Field+evaluation+of+crystal+VC+Rapid+Dipstick+test +for+cholera+during+a+cholera+outbreak+in+Guinea-Bissau (accessed May 19, 2013). 31. Centers for Disease Control and Prevention. Global disease detection (GDD) manual. Rapid diagnostic tests for epidemic enteric diseases. Watery diarrhea. Differential diagnosis: outbreaks of acute watery diarrhea. www.cdc.gov/cholera/pdf/gdd_manual_cholera_chapters_2012_1_11-508c.pdf (accessed April 25, 2013). 23 32. Bhuiyan NA, Qadri F, Faruque AS, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol 2003;41:3939– 41. www.ncbi.nlm.nih.gov/pubmed/?term=Use+of+dipsticks+for+rapid+diagnosis+of+cholera+ca used+by+Vibrio+cholerae+O1+and+O139+from+rectal+swabs (accessed May 19, 2013). 33. Centers for Disease Control and Prevention. Laboratory methods for the diagnosis of epidemic dysentery and cholera. www.cdc.gov/cholera/pdf/Laboratory-Methods-for-theDiagnosis-of-Epidemic-Dysentery-and-Cholera.pdf (accessed April 12, 2013). 34. Sinha A, Sengupta S, Ghosh S, et al. Evaluation of a rapid dipstick test for identifying cholera cases during the outbreak. Indian J Med Res 2012;135:523–8, www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+a+rapid+dipstick+test+for+identifying+ cholera+cases+during+the+outbreak (accessed May 19, 2013). 35. Columbia University, Professor Virginia Cornish working to engineer yeast to detect cholera. http://news.columbia.edu/research/2806 (accessed April 12, 2013). 36. Ouedraogo RT, Njanpop-Lafourcade B-M, Jaillard P, et al. Mobile laboratory to improve response to meningitis epidemics, Burkina Faso epidemic season 2004, Field Actions Science Reports 2008;1. http://factsreports.revues.org/144 (accessed April 12, 2013). 37. WHO. Acute diarrheal diseases in complex emergencies: critical steps, www.who.int/cholera/publications/criticalsteps/en/index.html (accessed April 12, 2013). 38. Valenciano M, Moren A. Communicable disease surveillance in complex emergencies. In: M'ikanatha M, Lynfield R, Van Beneden CA, de Valk H, editors. Infectious disease surveillance. Malden, Massachusetts: Blackwell Publishing; 2007:265–80. 39. The University of Vermont. Medical student Wilkie builds portable EHR system for Doctors Without Borders. www.uvm.edu/~uvmpr/?Page=news&storyID=12670&category=ucommall (accessed April 12, 2013). 24 DIAGNOSIS AND TREATMENT OF CHOLERA DEFINITION OF ISSUE Cholera was—and still can be—a fatal disease, and every third person affected with the cholera gravis form of the disease can die if he or she does not receive prompt proper clinical diagnosis and management. Oral rehydration therapy and intravenous (IV) fluid replacement can save lives. Antibiotics can further limit the duration of disease and the total purging volume of diarrhea. Research to improve clinical outcomes is ongoing; noteworthy among this research has been work on zinc therapy to further reduce morbidity and mortality related to cholera and other diarrheal diseases. Another approach for the control of cholera has been the concept of immunoprophylaxis with oral cholera vaccines (OCVs). RECOMMENDED ACTIONS Diagnosis WHO guidelines Others (1) (2–4) Stool can be of rice-water consistency, but yellow watery or loose stools also occur. Exclusion criteria for cholera evaluation include signs of invasive diarrhea, such as blood and mucus in stool. (1) (3) Diarrhea accompanied by fever, marked abdominal cramps, tenesmus, and gross blood are features of invasive diarrhea. (5) (6) (7) (3,4) Clinical evaluation and presentation Evaluation of patients is based on type of diarrhea, clinical signs and symptoms, and different categories of dehydration (i.e., none; mild-tomoderate; and severe, with overt signs of dehydration). Vomiting and high rates of rice-water stools are useful as clinical features for suspecting cholera. Assessment of Dehydration Assessment is based on clinical evaluation and examination of level of consciousness, eyes, tongue, thirst, skin-pinch and strength, and rate of radial pulse. (See Annex table.) Stool Characteristics Painless purging of voluminous stools resembling rice water is characteristic of cholera stool. Fishy odor is another feature. (3,4) Rapid diagnostic tests 25 Rapid diagnostic tests (RDTs) have been evaluated in hospital- and field-based studies in Bangladesh and Madagascar. Five RDTs that have been tested are the coagglutination test, Institut Pasteur (IP) cholera dipstick, Sensitive Membrane Antigen Rapid Test, and IP dipstick and Medicos The rapid tests are quick to conduct and do not require laboratory facilities. Evaluation of these tests is under way for use during seasonal epidemics and outbreaks. Tests can be conducted by using stool or rectal swab specimens. The specificity of the tests is 60%–100%, and sensitivity is 58%–100%. The RDTs are useful for detecting cholera in diarrheal epidemics and not for individual patient diagnosis. (4) (8) (9) (10) (11) (12–14) (15) (2,16) Dark-field microscopy Because of its high motility, Vibrio cholerae O1 can be detected by using dark-field microscopy. Serogroup and serotype specificity can be determined by inhibiting motility with specific antibodies, either polyclonal or monoclonal. The method has a sensitivity of around 60%. Microbiological culture Stool and/or rectal swabs are cultured on selective media, such as thiosulfate citrate bile salt sucrose (TCBS agar) or taurocholate-tellurite gelatin agar (TTGA). The specimens also are used in alkaline peptone water, which serves as an enrichment broth; after 8–12 hours, the enrichment broth is subcultured on TCBS or TTGA. Isolated colonies are tested by slide agglutination with specific antiserum or monoclonal antibodies to detect V. cholerae O1 and O139 serogroups. V. cholerae O1 colonies are further typed to determine whether they are Inaba or Ogawa serotypes. The biotype of V. cholerae O1, classical or El Tor or altered variants, are detected by biochemical, immunochemical, or molecular methods. Antimicrobial resistance Because of widespread resistance to antibiotics, surveillance is conducted to detect changing sensitivity patterns for determining the drug of choice for treating cholera. The pattern of resistance fluctuates as it does for other pathogens in accordance with withdrawal of use in the community. For the surveillance of resistance, drugs tested include chloramphenicol, ampicillin, ciprofloxacin, ceftriaxone, trimethoprim– sulphamethoxazole, and azithromycin and performed by disc diffusion and by determining minimum inhibitory concentrations of antimicrobial agents. Molecular analyses also are conducted to determine the mechanism of resistance and its spread globally. 26 Treatment Management of dehydration WHO guidelines Others (1,17) (3,4) (1,11) (3,4) (1,17) (3,4) Oral Rehydration Solution (ORS) For mild-to-moderate cases, ORS is used both for rehydration (to replace fluid and electrolytes) and for maintenance of hydration once the deficit has been replaced. In addition to glucose ORS, use of rice ORS is useful in reducing stool output. IV Fluid All cholera patients with severe dehydration, and many with moderate dehydration, require emergency administration of adequate volumes of polyelectrolyte rehydration solution rapidly administered intravenously to expand their intravascular volume, raise blood pressure, and enhance renal blood flow. Once IV rehydration has replaced the fluid deficit, ORS can be initiated to keep up with ongoing fluid losses from continuing purging of watery diarrhea. If the purge rate exceeds 500 mL per hour in an adult, keeping up with ongoing losses is not possible by oral rehydration alone. Purging rates <500 mL per hour often can be replaced with ORS of glucose/electrolytes. The basic principles of rehydration therapy are rapid replacement of fluid and electrolyte deficits followed by replacement to keep up with ongoing losses. Reexpansion of intravascular volume and enhanced renal perfusion lead to correction of acidosis. Ringer's lactate is a commonly used rehydration fluid. Antimicrobial Therapy Patients with clinically significant cholera should receive a single dose of antibiotic to shorten the duration of illness and reduce community transmission. Antibiotics not only curtail excretion of V. cholerae but also decrease the total purging volume and volume of rehydration fluids needed and shorten hospital stay. In Bangladesh, azithromycin is the antibiotic of choice (1000 mg for adults and 20 mg/kg of body weight for children, administered as a single dose). Single-dose antibiotics are recommended. The drugs of choice are doxycycline, azithromycin, and ciprofloxacin, although the latter is avoided because of increasing drug resistance. Doxycycline is preferred as a single-dose therapy when an outbreak is caused by tetracycline-sensitive V. cholerae, whereas azithromicin is the antibiotic of choice when tetracycline resistance is documented, as in Bangladesh (1000 mg for adults and 20 mg/kg of body weight for children, both as single doses). 27 Adjunct Therapy—Zinc and Vitamin A Zinc has been shown to decrease the duration and severity of diarrhea. It is given to children (6 months–to 5 years of age) with diarrhea, including those with cholera, as an adjunct therapy to rehydration and antibiotics (where indicated, as for treatment of cholera) for 10 days. For severely malnourished children with diarrhea, zinc treatment is recommended for 14 days. (18) (19) WHO guidelines Others (1,11,17) (3,20,21) (18) (3,16) Vitamin A is given to children who have not received it under the national biyearly program in Bangladesh (6 months–1 year—100,000 units; >1 year—200,000 units). Normal diet can be resumed as soon as the patient is stable and can swallow, particularly in children. Critical management issues Diagnosis and Management of Complications It is Important to determine severity of disease in managing complications of cholera cases. The major complications of cholera are hypoglycemia, hypovolemic shock, septic shock (rare), hypothermia, hypernatremia, acidosis, hypokalemia, and abdominal distension. Other serious complications are acute renal failure and circulatory failure. Spontaneous abortion and stillbirth have been reported in pregnant women. Special attention should be paid to children <5 years of age and to pregnant women, both of whom suffer from complications and high fatality (including fetal loss [stillbirth and spontaneous abortion]). Managing Cholera Outbreaks and Epidemics Cholera outbreaks need to be assessed. Proper management of cases includes evaluation of dehydration status, rehydration therapy (IV and ORS), appropriate antibiotic use, prevention measures (including behavior changes to diminish risk), and provision of safe water and health education. Health care staff should be skilled, or trained, in administrating IV fluids to severely dehydrated persons. Correct knowledge is essential about the type of infusion needed for rehydration (if normal saline is used instead of cholera saline or Ringer’s lactate solution). Training is needed for diagnosis of dehydration status (severe/some/none) so that patients can be properly managed. Cholera treatment units are necessary that use available resources, including trained personnel and standardized operating manuals and 28 (22,23) guidelines. Immunoprophylactic Measures Using Vaccines Use of OCVs during outbreaks and epidemics, especially when cholera occurs in new settings with limited expertise of clinical management, is one strategy for controlling and preventing the spread of cholera. (17) (24,25) WHO guidelines Others KNOWLEDGE GAPS Research on care of pregnant women with cholera is needed to avoid complications resulting in fetal loss. Because available cholera vaccines are not registered for use in pregnant women, safety data are not available. Studies are needed on use of antibiotics to prevent spread of cholera, especially among household contacts. Interventions are needed after discharge. Awareness needs to be created on the mode of spread of cholera to patients and attendants; (21,22) OCV can be used in outbreaks and epidemics. Zinc should be given to children with cholera. OCV should be given to household contacts and those in the cluster of homes around the epicenter of cholera cases (and water supply) in the community. Emphasis should be placed on children, rather than on adults, of household members with a cholera index patient (because children are more susceptible). First-degree relatives are more at risk than more distant relatives. REFERENCES 1. WHO. Treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: WHO; 1990. 2. Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet 2012;379:2466–76. 3. Bardhan PK. Acute infectious diarrhoea. In: Rakel RE, Bope ET, editors. Conn’s current therapy 2005. Philadelphia: Saunders; 2005:18–24. 4. ICDDR.B. Management of diarrhea at ICDDR.B Hospital. www.icddrb.org/what-wedo/publications/cat_view/52-publications/10042-icddrb-periodicals/10078-glimpse/10292vol-31-no-2-2009/11072-management-of-diarrhoea-at-icddrb-hospital (accessed January 3, 2010). 29 5. WHO. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. http://whqlibdoc.who.int/publications/2005/9241592330_eng.pdf (accessed April 8, 2013). 6. Speelman P, McGlaughlin R, Kabir I, Butler T. Differential clinical features and stool findings in shigellosis and amoebic dysentery. Trans R Soc Trop Med Hyg 1987;81:549–51. www.ncbi.nlm.nih.gov/pubmed/?term=Differential+clinical+features+and+stool+findings+in+ shigellosis+and+amoebic+dysentery (accessed May 19, 2013). 7. WHO. Cholera 2009. Wkly Epidemiol Rec 2010;85:293–308. www.who.int/wer/2010/wer8531.pdf (accessed May 19, 2013). 8. Bhuiyan NA, Qadri F, Faruque AS, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol 2003;41;3939– 41. http://www.ncbi.nlm.nih.gov/pubmed/?term=Use+of+dipsticks+for+rapid+diagnosis+of+chole ra+caused+by+Vibrio+cholerae+O1+and+O139+from+rectal+swabs (accessed May 19, 2013). 9. Benenson AS, Islam MR, Greenough WB 3rd. Rapid identification of Vibrio cholerae by darkfield microscopy. Bull World Health Organ 1964;30:827–31. http://apps.who.int/iris/bitstream/10665/73717/1/bulletin_1964_30%286%29_827-831.pdf (accessed May 19, 2013). 10. Nelson EJ, Chowdhury A, Harris JB, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in transmission of infectious diarrhea. Proc Natl Acad Sci U S A 2007;104:19091–6. www.ncbi.nlm.nih.gov/pubmed/?term=Complexity+of+ricewater+stool+from+patients+with+Vibrio+cholerae+plays+a+role+in+transmission+of+infectio us+diarrhea (accessed May 19, 2013). 11. WHO. Guidelines for cholera control. https://apps.who.int/chd/publications/cholera/cholguid.htm (accessed April 4, 2013). 12. Rahman M, Sack DA, Mahmood S, Hossain A. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J Clin Microbiol 1987;25:2204–6. www.ncbi.nlm.nih.gov/pubmed/3693549 (accessed May 19, 2013). 13. Sjoling A, Wiklund G, Savarino SJ, Cohen DI, Svennerholm AM. Comparative analyses of phenotypic and genotypic methods for detection of enterotoxigenic Escherichia coli toxins and colonization factors. J Clin Microbiol 2007;45:3295–301. www.ncbi.nlm.nih.gov/pubmed/?term=Comparative+analyses+of+phenotypic+and+genotypi c+methods+for+detection+of+enterotoxigenic+Escherichia+coli+toxins+and+colonization+fa ctors (accessed May 19, 2013). 14. Schwartz BS, Harris JB, Khan AI, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg 2006;74:1067–73. www.ncbi.nlm.nih.gov/pubmed/?term=Diarrheal+epidemics+in+Dhaka%2C+Bangladesh%2 Cduring+three+consecutive+floods%3A+1988%2C+1998%2C+and+2004 (accessed May 19, 2013). 15. Sack DA, Lyke C, McLaughlin C, Suwanvanichkij V. Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. www.who.int/drugresistance/Antimicrobial_resistance_in_shigellosis_cholera_and_cam.pdf (accessed April 2, 2013). 16. Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet 2004;363:223–33. www.ncbi.nlm.nih.gov/pubmed/14738797 (accessed May 19, 2013). 17. WHO Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. www.who.int/cholera/technical/prevention/control/en/index.html (accessed November 26, 2012). 30 18. WHO, UNICEF. WHO/UNICEF joint statement on clinical management of acute diarrhea. www.who.int/maternal_child_adolescent/documents/who_fch_cah_04_7/en/index.html (accessed April 2, 2013). 19. Fischer Walker CL, Fontaine O, Young MW, Black RE. Zinc and low osmolarity oral rehydration salts for diarrhea: a renewed call to action. Bull World Health Organ 2009;87:780–6. www.who.int/bulletin/volumes/87/10/08-058990/en/ (accessed May 19, 2013). 20. Khan MR, Rahman ME. Community pediatrics. In: Essence of pediatrics. 3rd ed. Dhaka: Mrs. Anwara Khan; 2004:143–98. 21. Hirschhorn N, Chowdhury AK, Lindenbaum J. Cholera in pregnant women. Lancet 1969;293:1230–2. 22. Médecins Sans Frontières. Cholera guidelines. 2nd ed. New York: Médecins Sans Frontières; 2004. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed April 23, 2013). 23. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 24. Shin S, Desai SN, Sah BK, Clemens JD. Oral vaccines against cholera. Clin Infect Dis 2011:52;1343–9. www.ncbi.nlm.nih.gov/pubmed/21498389 (accessed May 19, 2013). 25. Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection–derived immunity in an endemic setting. J Infect Dis 2011:204:912–8. www.ncbi.nlm.nih.gov/pubmed/?term=Natural+cholera+infection%E2%80%93derived+immu nity+in+an+endemic+setting (accessed May 19, 2013). 31 Annex: Diagnosis and Management of Diarrhea Assessment of Dehydration of Diarrhea Assessment of dehydration Condition Normal Assess Eyes Tongue Thirst Skin-pinch Radial pulse Diagnose Treat Normal Normal Normal Normal Normal No sign of dehydration Prevent dehydration Reassess periodically Irritable/Less active than Lethargic/Comatose* usual* Sunken Dry Thirsty (drinks eagerly) Unable to drink* Goes back slowly* Low volume* Uncountable or absent* If at least 2 signs, including 1 of the asterisk (*)-marked signs, are present, diagnose some dehydration. Rehydrate with ORS. Frequent reassessment. If some dehydration + 1 of the asterisk (*)-marked signs are present, diagnose severe dehydration. Rehydrate with IV fluids and ORS. Frequent reassessment . Management Rehydration No sign of dehydration Send patient home with packets of ORS after observation for 2–4 hours and counseling of mothers about the use of ORS and continued feeding. Advise mothers on the volume of ORS to give in accordance with the following schedule: o Children <2 years: 50–100 mL after each liquid stool. o Children 2–9 years: 100–200 mL after each liquid stool. o Persons >10 years: as much as wanted. Some dehydration Treat with ORS, 75 mL/kg over ~4 hours. The patient should be kept under observation. The following age-specific plan can be used for giving ORS: o Infants <4 months: 200–400 mL in 4 hours. o Infants 4–11 months: 400–600 mL in 4 hours. o Children 11–23 months: 600–800 mL in 4 hours. o Children 2–4 years: 800–1200 mL in 4 hours. o Children 5–14 years: 1200–2200 mL in 4 hours. o Persons >15 years: 2200–4000 mL in 4 hours. AND o Reassess dehydration status periodically. o Manage most cases by using ORS only. 32 o o o In case of frequent vomiting (>3 times in 1 hour) with persistent dehydration, consider treatment with IV fluid. If signs of severe dehydration appear, treat with IV fluids. Continue normal feeding, including breastfeeding. Severe dehydration Start IV fluid immediately (100 mL/kg). o Young children <1 year: 30 mL/kg in first 1 hour. 70 mL/kg in next 5 hours. o Children (>1 year) and adults: 30 mL/kg in first 30 minutes. 70 mL/kg in next 2½ hours. Encourage the patient to take ORS as soon as he/she is able to drink. The IV fluid of choice for management of severe dehydration is cholera saline. For both adults and children, initial bolus therapy should be repeated if danger signs (i.e., shock) are present after the initial bolus. For children <5 years of age, use 20 mL/kg over 20 minutes, to be repeated up to 3 times, until signs of shock resolve (goal-directed therapy), after which the 70 mL/kg can be delivered in continuous infusion. Note that volume loss that continues during rehydration therapy has to be accounted for. Although there are practical issues with being entirely accurate, each stool that occurs during rehydration should be replaced with a volume similar to that given in “Maintenance therapy” below. Ideally, rehydration would be with ORS, but it can be done with IV fluid in addition to that prescribed initially. Maintenance therapy Should be done with ORS. Use the following volume of ORS after each liquid or watery stool: Children <2 years: 50–100 mL. Older children: 100–200 mL. Adults: Allow them to drink as much ORS as they want. In case of frequent vomiting (>3 times in 1 hour) with persisting dehydration, treatment with IV fluid can be considered. Antibiotic therapy For presumed cases of cholera (profuse watery stools, typically looking like rice water, that result in dehydration; many persons affected in the same locality), give antibiotics as follows: Children: azithromycin, 20 mg/kg body weight, single dose orally. Adults: azithromycin, 1 g single dose orally. Zinc treatment for episodes of diarrhea Children 6 months–5 years: zinc 20 mg once daily for 10 days. 33 34 DRINKING WATER INTERVENTIONS DEFINITION OF ISSUE Cholera is transmitted mainly through the fecal–oral route (1), and the ingestion of water contaminated with feces plays a primary role in spreading the disease, especially during epidemics. Cholera can be reliably prevented and controlled only by stopping the fecal–oral contamination cycle, where ensuring use of appropriate sanitation and proper hygiene (personal and food) and access to safe drinking water for the whole population is of utmost importance (2–8). In an epidemic, cholera can be contracted in only one way: by swallowing something (usually water or food) that has been contaminated with fecal matter containing Vibrio cholerae. Consequently, if fecal material is not ingested orally, the spread of cholera can be completely stopped and infection can be entirely prevented (9). The World Health Organization/United Nations Children’s Fund (WHO/UNICEF) Joint Monitoring Program for Water Supply and Sanitation monitors access to safe drinking water through the proxy indicator of improved drinking water sources, which are defined as “Those that are by nature of their construction protected from outside contamination, in particular from contamination with fecal matter” (10). During a cholera outbreak, water treatment and safe water storage are commonly recommended additional measures. Although the provision of safe water for drinking and food preparation is crucial to cholera prevention and control, some references suggest that during an outbreak, water used for all domestic purposes (including washing and bathing) should be safe because it could be ingested and thus be a potential vehicle for cholera transmission. Water of good quality needs to be available in sufficient quantity to enable the population to exercise healthy hygiene practices. RECOMMENDED ACTIONS Prevention WHO guidelines Others Implement protection of at-risk water sources identified through sanitary surveys or water safety plans as situations and locations with increased potential for outbreak. (5,7,11) (12) Develop water sources to reliably provide a minimum quantity of safe water for all domestic purposes, including personal hygiene. (5–7,11) (9,12) Promote acceptable household water treatment; recommended treatment is chlorination or boiling. (5–7,11) (9,12) Water supply actions Ensure Safe and Sufficient Water Supply 35 Ensure safe water handling and storage (e.g., through provision of adequate narrow-mouth water containers or covered containers with taps combined with promotion of their use) (6,7,11) (9,12) Monitor water quality at all main water points or systems, and take appropriate action where needed. (5–7,11) (9) WHO guidelines Others Ensure effective coordination, collaboration, and information sharing with authorities and responders from relevant sectors (cholera task force). (1,4,7,11,13) (8,12,14) Conduct a risk assessment to identify cholera season, priority areas of intervention, key actions, and gaps in capacity. (1,4) (9,12,14) (1,7,13) (9) (7,11) (9,12,14) Control Water supply actions Preparedness Contribute to the development of a comprehensive and effective response strategy in collaboration with relevant stakeholders. Strategically preposition sufficient stocks of identified WASH materials, supplies, and equipment for community- and facility-based outbreak response. Identify and train people on effective community- and facility-based outbreak response, particularly in regard to water treatment and infection control. Contribute to the identification and preparation of potential sites for isolation to ensure water and sanitation standards can be met. (e.g., does the selected site have access to a safe water supply?). (9) (1,7,11) (14) (7,11) (9) Identify contaminated water sources, and ensure follow-up action (e.g., treatment, rehabilitation, or closing if alternative source of water can be provided). (5–7,11) (9,12,14) Provide sufficient safe water (20 liters per person per day can be used as indicator). (6,11,13) (9,12,14) Response—community-based Identify with relevant authorities and stakeholders the main transmission routes, and agree on responding for priority interventions with authorities and stakeholders. 36 Ensure that all domestic water (for drinking, cooking, and bathing) is treated using a culturally acceptable proven method, and monitor water quality. If household water treatment products are provided, avoid gaps. Preferred method of treatment is chlorination. Recommended levels of residual chlorine are as follows: (5,6) (9) Provide people with the knowledge and the means to safely handle and store water (e.g., by providing narrow-mouth storage vessels or covered containers with taps combined with promotion of their use). (6,7,11) (9,12) Monitor water quality at all main water points or systems, and take appropriate action where needed. (5–7,11) (9) (15) (9,14) (15,16) (9,14) Ensure sufficient functioning hand-washing facilities are in place (e.g., for examination rooms, kitchens, toilets) and are appropriately managed. (15,16) (9,14) Ensure sufficient shower facilities in each area of cholera treatment center. (Guidance: one shower per 50 patients; ensure minimum one for male, one for female; and minimum one for male and one for female staff). (15) (9,14) 0,5mg/L at sample point of piped water system. 1,0 mg/L at stand posts in system with stand posts. 2,0 mg/L in tanker trucks at filling. Response—health facility–based (cholera treatment center, cholera treatment unit, oral rehydration points) Ensure provision of sufficient safe treated water with substantial storage capacity to avoid shortages. (60 litres of water is needed per patient per day, including for cleaning, laundry, bathing, food preparation, etc.). Ensure frequent production of various chlorine solutions: 0.05% chlorine for hand washing, dish rinsing, and bathing of soiled patients. 0.2% chlorine for disinfecting floors, beds, clothes, and footbaths. 2% for disinfecting of vomit, faeces, and corpses. At ORP, ensure sufficient safe/treated water for maintaining hygiene and preparation of ORS (estimated at 10 liters per patient per day) (14,15) OUTDATED GUIDELINES, SUGGESTED GUIDELINES In some literature, person-to-person transmission was considered of minor importance (8). However, new evidence showing the protective factor of washing hands with soap (18–20) 37 suggests that person-to-person transmission can be a possible transmission route. The provision of appropriate and well-managed hand-washing stations at critical places is, therefore, vital. A guideline on water needs during a vaccination campaign is needed. KNOWLEDGE GAPS AND RESEARCH PRIORITIES Potential areas for research the context of cholera prevention and control are as follows: Different guidelines recommend different strengths of chlorine solutions for disinfection. A study to determine the correct doses for various purposes would avoid confusion.(21). More research might be required to better understand seasonality and environmental factors that lead to increased risk for cholera outbreaks to allow targeted prevention actions. Questions can be raised about the risk factors (21) that lead to outbreaks and rapid spread of cholera in certain slums. Some slums have been seriously affected in the last years, e.g., Harare, Zimbabwe (2008–2009); Lusaka, Zambia (2005, 2008–2009); and Kampala, Uganda (1997), whereas other slums with possibly similar or worse environmental conditions have not yet experienced any mayor outbreak (Nairobi, Kenya; Addis, Ethiopia). Most guidelines recommend chlorination and boiling at point of use when system chlorination is not feasible. Few evaluations of interventions exist about distributing chlorine tablets or promoting boiling or about the effectiveness of other alternative household water treatment products, such as the biosand filter or the ceramic pot filter that, compared with boiling, might have less risk of post-treatment contamination. REFERENCES 1. WHO. Outbreak surveillance and response in humanitarian emergencies—WHO guidelines for EWARN implementation. Geneva: WHO; 2012. 2. WHO. Cholera 2011. Wkly Epidemiol Rec 2012;87;289–304. www.who.int/wer/2012/wer8731_32.pdf (accessed April 21, 2013). 3. WHO. Cholera fact sheet no. 107. July 2012. www.who.int/mediacentre/factsheets/fs107/en/index.html (accessed April 21, 2013). 4. Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what is next? Report: WHO meeting, 14–16 December 2005, Cairo, Egypt. www.who.int/cholera/publications/cholera_vaccines_emergencies_2005.pdf (accessed April 20, 2013). 5. WHO. Guidance on formulation of national policy on the control of cholera. www.who.int/csr/resources/publications/cholera/whocddser9216rev1.pdf (accessed April 20, 2013). 6. WHO. Guidelines for cholera control. http://www.mona.uwi.edu/cardin/virtual_library/docs/1273/1273.pdf (accessed April 20, 2013). 7. WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFk_2004.4_eng.pdf (accessed April 23, 2013). 8. WHO. Global defense against the infectious disease threat, chapter on cholera. Geneva: WHO; 2003; 74–9, 38 9. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 10. WHO, UNICEF. Rapid assessment of drinking water quality. A handbook for implementation. October 2012. www.wssinfo.org/fileadmin/user_upload/resources/RADWQHandbookv1final.pdf (accessed August 20, 2013). 11. Global Task Force on Cholera Control. Acute diarrhoeal diseases in complex emergencies: critical steps. Decision making for preparedness and response. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CPE_ZFK_2004.6_Rev.1_eng.pdf (accessed: April 20, 2013. 12. Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://reliefweb.int/sites/reliefweb.int/files/resources/mlcholera-guidelines-preparedness-prevention-and-control-030512-en.pdfOXFAM (accessed May 15, 2013). 13. WHO Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. www.who.int/cholera/technical/prevention/control/en/index.html (accessed April 20, 2013). 14. Médecins Sans Frontières. Cholera guidelines. 2nd ed. New York: Médecins Sans Frontières; 2004. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed May 13, 2013). 15. WHO. Water, sanitation and hygiene in cholera treatment centres in emergencies. Technical notes on drinking water, sanitation and hygiene in emergencies. No. 18. www.washclustermali.org/sites/default/files/wash_in_cholera_treatment_centers_in_emerge ncies_tech_brief_who.pdf (accessed April 24, 2013). 16. WHO. Water, sanitation and hygiene (WASH) in healthcare facilities in emergencies. Adams J, Chartier Y, Harvey B, Maison D, editors. Geneva: WHO; 2012. www.washclustermali.org/sites/default/files/wash_in_health_facilities_in_emergencies_who. pdf (accessed May 19, 2013). 17. Centers for Disease Control and prevention. Oral rehydration points (ORPs). Planning and guidance. www.cdc.gov/haiticholera/pdf/ORP_Guidance&Planning_finalcleared.pdf (accessed May 15, 2013). 18. Dubois AE, Sinkala M, Kalluri P, Makasa-Chikoya M, Quick RE. Epidemic cholera in urban Zambia: hand soap and dried fish as protective factors. Epidemiol Infect 2006;134:1226–30. www.ncbi.nlm.nih.gov/pmc/articles/PMC2870514/ (accessed May 15, 2013). 19. Quick RE, Thompson BL, Zuniga A, et al. Epidemic cholera in rural El Salvador: risk factors in a region covered by a cholera prevention campaign, Epidemiol Infect 1995;114:249–55. http://europepmc.org/articles/PMC2271272/pdf/epidinfect00050-0027.pdf (accessed May 15, 2013). 20. Sasaki S, Suzuki H, Igarashi K, Tambatamba B, Mulenga P. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urbanarea of Lusaka, Zambia. Am J Trop Med Hyg 2008;79:414–21. http://public.beuthhochschule.de/~kred/Literatur/GIS_Med_Geo/PDF/Sasaki_et_al_20081744621058/Sasaki_et_al_2008.pdf (accessed May 15, 2013). 21. Emergency Environmental Health Forum 2013 5th Conference Report, Public Health Promotion in Water and Sanitation Programmes www.shareresearch.org/LocalResources/EEHF_17th_and_18th_Meeting_Report_150113.p df (accessed July 19, 2013). 39 40 SANITATION INTERVENTIONS DEFINITION OF ISSUE Sanitation generally refers to the provision of facilities and services for the safe disposal of human urine and feces, wastewater, and other wastes that can negatively impact human health and well-being. Inadequate sanitation is a major cause of infectious diseases, and improved sanitation that hygienically separates human excreta from human contact substantially improves the health of individuals and communities. Cholera is transmitted mainly through the fecal–oral route, and the ingestion of fecally contaminated water plays a major role in the spread of the disease. Cholera can be reliably prevented and controlled only by stopping the fecal–oral contamination cycle, where ensuring use of appropriate sanitation and proper hygiene (personal and food) and access to safe drinking water for the whole population is of the utmost importance (1–6). In an acute outbreak situation, constructing latrines or setting up solid-waste management systems may not be realistic or practical as a priority measure because of time requirements (4,7,8); however, a later improvement in the sanitary conditions is likely to significantly decrease future outbreak risks. Immediate isolation of cholera patients in specialized health facilities (cholera treatment centers [CTCs], cholera treatment units [CTUs]) is advisable to improve patient care and reduce the risk for further spread of the disease. These facilities should be established and operating within 24 hours after an outbreak is confirmed (9). Timely identification and preparation of adequate sanitation in these facilities is therefore required, including strategic prepositioning of materials, supplies, and equipment, as well as staff training. RECOMMENDED ACTIONS Prevention Sanitation actions WHO guidelines Others (1–6,10,11) (8,9) Ensure Adequate Sanitation and a Fecal Matter–Free Environment Improve access to adequate and culturally acceptable sanitary facilities and services. Integrate cholera into planning and implementation of development programs. Provide more than one latrine per 20 people in camps and crowded situations. (8) (11) 41 Strengthen quality control of sewage treatment plants. (4) Control use of wastewater for agricultural purposes. (4) Emphasize the need for adequate disposal of children’s feces. (5,6) (8) Control WHO guidelines Others (3,6,10–12) (7–9) (3,12) (7–9) (6,10,12) (7,9) (6,12) (7–9) Contribute to the identification and preparation of potential sites for isolation to ensure water and sanitation standards can be met (waste and wastewater management, drainage). (6,11,12) (9) Identify and train people in safe handling of dead bodies and in burial practices. (6,11) Sanitation actions Preparedness Ensure effective coordination, collaboration, and information sharing with authorities and responders from relevant sectors (Cholera Task Force). Conduct a risk assessment to identify cholera season, priority areas of intervention, and capacity gaps. Contribute to the development of a comprehensive and effective response strategy in collaboration with relevant stakeholders. Strategically preposition sufficient stocks of identified water, sanitation, and hygiene (WaSH) materials, supplies, and equipment for community- and facility-based outbreak response. Establish inventory of existing sanitation facilities to evaluate risks. (6) Response, Community-Based Promote use of latrines, and ensure that they are cleaned and maintained. Construct latrines at public places where needed (markets, schools), and ensure that they are cleaned and maintained. Dispose of cholera wastes in latrines. 42 (5,6,13) (8) (7–9) (9) Coat latrine pits with unslaked/chlorinated lime. (5) (7) Ensure drainage and sewers are functional. (7–9) Provide materials for latrine disinfection. (7,8) Collect so-called “flying toilets” separately from other wastes, and dispose of them safely. Remind people of the importance of hand-washing with soap at critical times (e.g., after using latrines, after handling patients, before preparing food, before eating). Ensure the provision of sufficient hand-washing facilities with soap or ash at public toilets or latrines and at markets, restaurants, and eateries. (8) (4,5,10,11,13) (8,9) (6) (8,9) Conduct simple fly control measures. Clean and disinfect market places. Ensure appropriate handling and management of dead bodies. (9) (12) (9) (6,11,13) (7,9) Identify appropriate burial grounds. Control funeral procedures. Provide hand-washing facilities with soap or ash at funerals. (7) (6,11,12) (9) (5) (7,8) Disinfect soiled surfaces and materials with water and chlorine. (8) Response, Health Facility–Based (CTC, CTUs) Provide sufficient separate sanitary facilities (e.g., sex-segregated toilets and showers, laundry, waste disposal) for patients at isolation units. (6,14,15) Do not connect toilets to a main sewer system. (7,9) (8,9) Provide separate toilets for staff. (14) (7–9) Make sure that toilets and latrines are regularly cleaned and disinfected. (14) (7–9) (5,6,15) (8,9) Ensure provision of sufficient hand-washing facilities with soap or chlorinated water (0.05%) at critical areas: entrances; areas of patient 43 care; latrines/toilets; kitchen; morgue; exits for staff, patients, and caretakers. Remind users of correct hand-washing procedures though visual aids (e.g., posters). (15) Dispose of wastewater from hand-washing facilities in latrines or soakpits. (14,15) (8,9) Ensure appropriate management of liquid waste from cholera patients, including treatment and disinfection and safe disposal (pit latrine or burial). (5) (7–9) Segregate wastes, and ensure their appropriate treatment, storage, and disposal. (14) (8,9) Equip and train cleaners and waste handlers. (14) Safely manage and incinerate semisolid wastes. (5,6) Install a waste area inside the CTU/CTC, consisting of drum burner, organic pit, ash pit, sharps pit. Pits should be lined. (8,9) Frequently disinfect materials, supplies, and equipment used for patient care before reuse. (6,13–15) Appropriately prepare and dispose of dead bodies as soon as possible. (6,15) Seek specialist advice on issues such as installation of pit latrines and sludge management from CTCs. (14) Regularly clean and disinfect contaminated surfaces. (14) Consider the use of plastic sheeting on floors to facilitate cleaning and disinfection. (14) (9) Dispose of wastewater from hand-washing, laundry, cleaning, and morgue in soak-away pits. (14) (8,9) (14,15) (9) (14) (9) Provide adequate drainage to avoid flooding and contact of rainwater or runoff with infectious materials. Control disease vectors; seek specialist advice if necessary. Disinfect means of transporting patients. Ensure an enabling environment for managing menstrual hygiene. 44 (7–9) (7–9) (8) Contribute to the appropriate decommissioning of sanitary facilities at CTCs/CTUs before closure. (9) OUTDATED GUIDELINES, SUGGESTED GUIDELINES Some reference documents (5,7) recommend the use of lime in latrines pits; others do not suggest this practice. Some response organizations continue to carry out or promote the spraying of disinfectants at cholera patients’ homes. Such interventions are now discouraged (7,8). References (6) and (8) recommend the use of footbaths for disinfection of soles on entry, within, and on exit of a CTC/CTU. Reference (15) highlights that footbaths can quickly become dirty and suggests the use of backpack sprayers for the same purpose. There is little evidence to support either practice. Because more and more vaccination campaigns are being conducted, guidance should be developed regarding sanitation needs during the campaigns, including presence of latrines with hand-washing stations, disinfection protocols for areas of mass vaccination, handling of solid waste. KNOWLEDGE GAPS AND RESEARCH PRIORITIES The role of flies in transmitting cholera is generally acknowledged as being small (5,9). However flies can carry pathogens after contact with cholera liquid and contaminate food. The importance of flies in cholera transmission might be the subject of further study. More research might be needed for a better understanding of seasonality and specific environmental factors that increase the risk for cholera outbreaks to enable implementation of targeted preventive actions at critical times. Research into alternatives to the commonly recommended management of wastewater from CTCs/CTUs (disinfection and/or burial) is required for areas where such practices are not possible (e.g., high groundwater table, insufficient space). Reference (16) describes the piloting of two potential methods which warrant further study. (Treated) wastewater for irrigation is mentioned as a possible source of transmission (6), but no concrete actions are recommended other than from “careful control” (4). A better understanding of cholera transmission risks from the reuse of human wastes, as practiced particularly in parts of Asia, might be useful. REFERENCES 1. WHO. Cholera 2011. Wkly Epidemiol Rec 2012;87:289–304. www.who.int/wer/2012/wer8731_32.pdf (accessed April 25, 2013). 2. WHO. Cholera fact sheet no. 107. July 2012. www.who.int/mediacentre/factsheets/fs107/en/index.html (accessed April 25, 2013). 45 3. Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what is next? Report: WHO meeting, 14–16 December 2005, Cairo, Egypt. www.who.int/cholera/publications/cholera_vaccines_emergencies_2005.pdf (accessed April 25, 2013). 4. WHO. Guidance on formulation of national policy on the control of cholera. www.who.int/csr/resources/publications/cholera/whocddser9216rev1.pdf (accessed April 23, 2013). 5. WHO. Guidelines for cholera control. www.mona.uwi.edu/cardin/virtual_library/docs/1273/1273.pdf (accessed: April 23, 2013). 6. WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/who_cds_cpe_zfk_2004.4_eng.pdf (accessed April 23, 2013). 7. Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://reliefweb.int/sites/reliefweb.int/files/resources/mlcholera-guidelines-preparedness-prevention-and-control-030512-en.pdf (accessed May 14, 2013). 8. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 9. Médecins Sans Frontières. Cholera guidelines. 2nd ed. New York: Médecins Sans Frontières; 2004. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed May 13, 2013). 10. WHO Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. www.who.int/cholera/technical/prevention/control/en/index.html (accessed April 24, 2013). 11. Global Task Force on Cholera Control. Acute diarrhoeal diseases in complex emergencies: critical steps. Decision making for preparedness and response. http://whqlibdoc.who.int/hq/2010/who_cds_cpe_zfk_2004.6_rev.1_eng.pdf (accessed April 24, 2013). 12. WHO. Outbreak surveillance and response in humanitarian emergencies—WHO guidelines for EWARN implementation. Geneva: WHO; 2012. 13. WHO Global Task Force on Cholera Control. First steps for managing an outbreak of acute diarrhoea. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CSR_NCS_2003.7_Rev.2_eng.pdf (accessed April 24, 2013). 14. WHO. Water, sanitation and hygiene in cholera treatment centres in emergencies. Technical notes on drinking water, sanitation and hygiene in emergencies. No18. www.washclustermali.org/sites/default/files/wash_in_cholera_treatment_centers_in_emerge ncies_tech_brief_who.pdf (accessed April 24, 2013). 15. WHO. Water, sanitation and hygiene (WASH) in healthcare facilities in emergencies. Adams J, Chartier Y, Harvey B, Maison D, editors. Geneva: WHO; 2012. www.washclustermali.org/sites/default/files/wash_in_health_facilities_in_emergencies_who. pdf (accessed May 19, 2013). 16.Taylor H, Fesselet J-F, Sozzi E, Curtis T, Mahama A. Cutting the cholera risk—alternative approaches for medical centre wastewater treatment. Water21 2011;13.4;46. www.iwaponline.com/w21/01304/w21013040047.htm (accessed May 14, 2013). 46 HYGIENE INTERVENTIONS DEFINITION OF ISSUE Hygiene promotion in the emergency context is defined by the WASH [Water Sanitation Hygiene] Cluster (1) as “the planned, systematic attempt to enable people to take action to prevent or mitigate water, sanitation, and hygiene related diseases and provides a practical way to facilitate community participation and accountability in emergencies.” Hygiene promotion also involves ensuring optimal use of the water and sanitation facilities provided during cholera prevention and control interventions (1). Previous experience shows that behavior cannot be fully changed if hardware provision is not combined with an enabling environment that ensures proper operation and maintenance of the facilities and provides a strong sense of community ownership. RECOMMENDED ACTIONS Hygiene Promotion Actions Operation and maintenance of emergency water and sanitation facilities, including hand-washing stations WHO guidelines Provide feedback to engineers about design and acceptability of emergency water and sanitation facilities. Others (1)* Establish a system of cleaning and maintenance of emergency water and sanitation facilities, preferably voluntary. (2) (1,3) Encourage a sense of ownership and responsibility. (2) (1) (2,4,5) (1,3,6–8) Community and Individual Action Ensure hygiene promotion is planned around five hygiene domains: Hand-washing at key times (i.e., after visiting the toilet, before preparing food, after cleaning baby’s bottom, after caring for patients or handling their belongings). Use of (household or communal) latrines or any form of excreta containment. Consumption of safe water (from an improved water source and/or use of household water treatment) and transport, storage, and handling of water safely. * The WASH Cluster produced a set of technical documents on hygiene promotion in emergency in 2007. Those materials were designed to help clarify the purpose and outcomes of a hygiene promotion program during emergency. Most of the recommended actions in this table were validated under that initiative. 47 Funeral rituals and dead body disposal. Food hygiene (food preparation, storage, and consumption) at domestic level. Apply principles of behavior change communication in emergencies. Produce a sound communication strategy. (2,9) (1,6) Use available mass media (e.g., radio, TV, newspapers), new media (e.g., Short Message Service, call centers) and traditional media (e.g., story-telling, songs, drama) to provide quick information about how to prevent and control cholera. (2,5) (1,3,6) Recruit and train outreach system of hygiene promoters and/or community-based volunteers to facilitate participatory discussion with the community through more interpersonal channels, such as home visits, focus group discussions, community meetings, participatory learning activities (e.g., voting chart, three-pile sorting, community maps). (5) (1,3,6) (2,5) (3,6–8) Ensure the optimal use of items distributed at household level, such as soap, household water treatment products, and jerry cans for water transport and storage. (5) (3,6,10) As much as possible, consult with the affected population about the design of emergency water and sanitation facilities and community outreach systems. (4) (1) Develop and/or repackage a set of pretested cholera-focused Information, Education, Communication materials (e.g., posters, leaflets) for the intended audience to support promotional activities. Identify and respond to vulnerability, e.g., young children, pregnant women, persons with HIV/AIDS, chronically ill and elderly persons. (1) Food Hygiene in the Public Domain Control food hygiene (preparation, storage, and consumption) in food stalls in public places, markets, restaurants, and street vendors. (2,5) (3,6–8) (2,5) (3,6,7) Health Facilities (cholera treatment centers, cholera treatment units, and oral rehydration points) Ensure health facilities have hygiene and disinfection protocols in place. Train and supervise all staff, and make equipment (e.g., buckets, soap, chlorine) available. Ensure proper maintenance of emergency water and sanitation facilities within health institutions, including hand-washing stations (e.g., cleaning, refilling, linking to engineer for potential repairs) through 48 (3,6) paid attendants. Provide information and talk with patients and families on arrival, during their stay, and at discharge about how to prevent cholera transmission. Discuss their home context (identify key risky practices) on arrival, and provide follow-up through community outreach system. (6,7) Coordination with WaSH Stakeholders Train women’s groups/cooperatives and national nongovernment organizations. (5) (1,3,6) Collaborate with and/or orientate government workers. (2,4,5) (1.3,6) Link with national cholera task forces and WaSH and health clusters. (2,4,5) (1,3,6) WHO guidelines Others Monitoring Conduct a mini-cholera survey at household level for baseline data on hygiene knowledge, attitudes, and practices. Establish a monitoring system, ensuring the collection and analysis of data on optimal use of relief items and emergency water and sanitation facilities and on the uptake of safe hygiene practices. (2,4) (2) (1,3,6,7) OUTDATED GUIDELINES, SUGGESTED GUIDELINES Message-Based Approach vs. Participatory Approach In the past, hygiene promotion in the context of cholera prevention and control has been understood as simply delivering hygiene messages or any activity that informs people in the affected community about how to prevent acquiring cholera and spreading it to others. A message-based approach to disease prevention and control might be effective in some circumstances and has been used widely in cholera interventions because it reaches larger audiences in a short period of time at low cost. Most current technical guidance on cholera control and prevention, except for the United Nations Children’s Fund (UNICEF) cholera toolkit (6) and COMBI toolkit from WHO (9), refers to “messaging” or “provision of information” as the main approach to hygiene promotion. Consensus exists within the WaSH sector that “whatever the focus of hygiene promotion, the emphasis must be on enabling and mobilizing women, men, and children to take action to prevent or mitigate cholera (by adhering to safe hygiene practices) rather than simply raising awareness about the possible transmission paths of the disease” (1). A message-based approach always must be accompanied by other promotional activities that lead to effective behavior change, such as social marketing, peer influence, and participatory 49 learning and action. In the UNICEF cholera toolkit is a crucial statement: “communication is not just about providing information to affected communities; it should also facilitate participatory discussion in order to trigger community action and contribute to building rapport between communities and service providers.” Behavior Change Communication in Emergencies Understanding the public’s risk perceptions, views, and concerns is critical to effective communication in emergencies. Without knowing how people understand and perceive the risk for cholera transmission and their existing beliefs and practices, correct decisions and required behavior changes necessary to protect health may not occur, and societal or economic disruption may be more severe. Therefore, understanding what motivates people to make healthy choices in the context of cholera prevention and control is important because often health gain is not the primary motivating factor for change. Instead, other factors analyzed during cholera interventions in Haiti in 2010 (11), such as convenience for practicing a specific behavior, fear of imminent death or fear of a disease often associated with poverty, nurturing and protective feelings, and affiliation or social norms, might be the driving forces behind change. KNOWLEDGE GAPS AND RESEARCH PRIORITIES Potential areas for research in hygiene promotion in the context of cholera prevention and control are as follows: Impact of different communication channels and approaches for hygiene promotion in cholera prevention and control. Formative research on motivation factors associated with hygiene behavior in cholera outbreaks. REFERENCES 1. WASH Cluster. Hygiene promotion in emergency—a briefing paper. Introduction to hygiene promotion in emergency: Tools and approaches. www.washcluster.info/?q=content/introduction-hygiene-promotion-0 (accessed April 4, 2013). 2. Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://policy-practice.oxfam.org.uk/publications/choleraoutbreak-guidelines-preparedness-prevention-and-control-237172 (accessed April 4, 2013). 3. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 4. Médecins Sans Frontières. Cholera guidelines. 2nd ed. New York: Médecins Sans Frontières; 2004. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed April 4, 2013). 5. Rajasingham A, Bowen A, O’Reilly C, et al. Cholera prevention training materials for community health workers, Haiti, 2010–2011. Emerg Infect Dis 2011;17:2162–5. http://dx.doi.org/10.3201/eid1711.110806 50 6. WHO Global Task Force on Cholera Control. First steps for managing an outbreak of acute diarrhoea. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CSR_NCS_2003.7_Rev.2_eng.pdf (accessed April 4, 2013). 7. WHO. Acute diarrhoeal diseases in complex emergency. Critical steps. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CPE_ZFK_2004.6_Rev.1_eng.pdf (accessed April 4, 2013). 8. WHO. Outbreak communication. Best practices for communicating with the public during an outbreak. www.who.int/csr/resources/publications/WHO_CDS_2005_32web.pdf (accessed April 4, 2013). 9. WHO. Communication for behavioural impact (COMBI). A toolkit for behavioural and social communication in outbreak response. http://apps.who.int/iris/bitstream/10665/75170/1/WHO_HSE_GCR_2012.13_eng.pdf (accessed April 4, 2013). 10. Piarroux R, Barrais R, Faucher B, et al. Understanding the cholera epidemic, Haiti. Emerg Infect Dis 2011;17:1161–8. http://dx.doi.org/10.3201/eid1707.110059 11. WASH Cluster. WASH related non food item – A briefing paper. Introduction to hygiene promotion in emergency: Tools and Approaches. www.washcluster.info/?q=content/introduction-hygiene-promotion-0 (accessed April 4, 2013). OTHER MATERIALS REVIEWED WHO. Epidemic diarrhoeal disease preparedness and response. Training and practice. Facilitator’s guide. www.who.int/topics/cholera/publications/WHO_EMC_DIS_97_4Rev_1/en/ (accessed April 4 2013). UNICEF. Cholera / AWD emergency preparedness and response training. www.unicef.org/cholera/Chapter_6_preparedness/10_ESARO_OXFAMTraining_package_introduction.doc (accessed May 16, 2013). 51 52 USE OF ORAL CHOLERA VACCINE DEFINITION OF ISSUE The availability of safe and effective oral cholera vaccine (OCV) provides optimism for accelerating control of this devastating infection. The vaccines have great potential for reducing rates of cholera and cholera-related deaths; however, the public health community is only beginning to understand how and when to most effectively use OCV. OCV is not a “silver bullet” but it is a major new tool for cholera control that can work synergistically with other interventions. This discussion focuses on the use of Shanchol because it is the vaccine most suitable and cost effective for use in developing countries and in emergencies. Dukoral is also World Health Organization (WHO) prequalified but is less appropriate for field use because of higher costs and the need for buffer. Other vaccines (including other formulations of killed whole-cell vaccines and single-dose attenuated vaccines) are under development but are not yet licensed and prequalified. Description of the Vaccines The modern development of OCV began in the late 1970s, resulting in a large phase-three trial in Bangladesh initiated in 1985 (1,2). Subsequently, many other studies were been carried out with both Dukoral and Shanchol vaccines, and other studies are still under way (3–9). The currently licensed OCV consists of a mixture of killed Vibrio cholerae strains, which stimulates an intestinal immune response to the cell wall of V. cholerae and limits the ability of the bacteria to colonize the intestine (10). By reducing intestinal colonization, OCV decreases the likelihood that the disease will develop in persons exposed to V. cholerae. OCV also lowers the number of bacteria in patients’ stool and renders people less likely to spread infection. Furthermore, when a large proportion of a population has been vaccinated, OCV confers herd protection. In several controlled trials, the efficacy of the vaccine has been about 70%, but computer models suggest that public health effectiveness could be even higher if the vaccine was used widely. Two vaccine doses are given generally 2 weeks apart, although the interval can be longer if necessary. Benefits and Constraints of OCV Because the vaccines are given orally and are safe and effective with essentially no side effects, WHO recommended their use (11). Furthermore, one OCV (Shanchol) is relatively inexpensive (currently US$1.85 per dose). Therefore, these vaccines would be expected to be used widely. However, factors exist that limit their use. Some of these constraints are perceived; others are actual logistical constraints that are expected to be 53 addressed soon. Perceived Constraints Because WHO fairly recently prequalified and recommended OCV (November 2011), the leaders of many health agencies do not yet know about it, and few agencies have experience using it. This lack of experience has resulted in reluctance to add it to other interventions, especially when resources are limited (12). Concern exists that implementing a new vaccine program interferes with established interventions; the extent to which this interference might occur needs to be documented, but recent experience suggests that the two can be carried out simultaneously, and joint intervention might actually enhance each intervention (13). Another perceived constraint is the interval between vaccination and protection. During prospective controlled trials, the study designs have generally specified that only cases occurring 2 weeks after the second dose should be included in the data analysis. Thus, some health care workers have assumed that protection does not start until a month after the first dose (assuming 2 weeks between the two doses). If protection indeed starts this late, the vaccine might have limited impact on an outbreak because many cases already would have occurred before onset of protection. Establishing exactly when protection starts requires additional research; however, analysis of current immunogenicity data finds a vigorous serologic response after a single dose, which might suggest that protection begins soon after the first dose, even if full or more long-lasting protection requires a second dose. Actual Constraints Two actual constraints are expected to be addressed in the near future. These are 1) the requirement for maintaining a cold chain into the field and 2) a limited supply of vaccine. Studies are under way to determine whether the cold chain is necessary for OCV. It seems likely that vaccine programs will not need to maintain a full cold chain during field distribution, although long-term cold storage most likely will continue to be implemented. Eliminating the need to keep the vaccine cold would greatly simplify logistical requirements for vaccine programs. The limited supply of vaccine is related to two factors: the limited existing production capacity of the manufactures and the low production because of low demand. With increasing demand from agencies and ministries of health (MoHs) and with development of the WHO stockpile, OCV production should increase. Still, vaccine shortages are likely for several years until other manufacturers producing similar or improved products appear. Because of low supplies, the available vaccine will need to be targeted to specific groups and be used strategically to be most effective. RECOMMENDED ACTIONS WHO Recommendations In 2010, WHO published new recommendations on the use of OCV (11). The recommendations stated, “Given the availability of 2 oral cholera vaccines and data on their efficacy, field effectiveness, feasibility and acceptance in cholera-affected populations, immunization with 54 these vaccines should be used in conjunction with other prevention and control strategies in areas where the disease is endemic and should be considered in areas at risk for outbreaks.” Uncertainties of the WHO Recommendation Although this recommendation appears to be clear—OCV should be used in certain circumstances—the public health community is still learning how to implement these recommendations. For example, MoHs and agencies may not know whether cholera in a given “area” is “endemic.” Because many cholera-prone regions do not have high-quality surveillance or may not officially report cholera, the MoH may not know how to determine whether cholera is endemic to a particular area. Given the current low availability of vaccine, vaccinating an entire country is not possible, even in countries where cholera outbreaks occur regularly. Similarly, “areas at risk” for outbreaks imply the availability of tools able to identify such risky areas, but these tools are still under development. Even when developed, they are unlikely to precisely predict when and where new outbreaks will occur. However, OCV is now being used in several situations, and information from these implementations will build a knowledge base about how to use OCV most effectively. Because of OCV’s inherent safety and the higher risk for complications from cholera during pregnancy, WHO recommends that OCV be given during pregnancy. However, OCV manufacturers have not included this recommendation in their package inserts because of lack of specific safety data about OCV use during pregnancy. In fact, the Shanchol insert seems to discourage OCV administration during pregnancy. Integration of OCV in a Comprehensive Program OCV is part of an overall comprehensive approach to cholera control or, as stated in the WHO recommendation, “used in conjunction with other prevention and control strategies.” This statement implies that a cholera vaccine project needs to be integrated with an overall program that at least includes 1) detecting and diagnosing disease; 2) ensuring high-quality case management for patients; 3) communicating messages about where treatment is available, use of oral rehydration solution (ORS) in the community, and reduction of risk; and 4) improving water and sanitation. Although these are separate components of the overall approach, they need to be integrated into a unified program so that they reinforce, rather than compete with, each other. For example, vaccine teams can reinforce messages about safe water and sanitation, ORS, and health seeking in case of illness. Similarly, teams improving safe water can reinforce messages about the value of OCV and case management. Programs implementing such a comprehensive approach should document the positive (and potentially negative) interactions between the different components of the overall program. Integration of OCV also implies integration into other health programs, e.g., primary health care and the Expanded Program on Immunization, as well as integration into other aspects of the community. For example, OCV needs to be implemented in cooperation with other community structures, including education, religion, and community development. Not only do these other elements need to feel included in decision making, they may also provide innovative strategies for identifying persons who will most benefit from OCV and can facilitate delivery to these groups. Similarly, they need to be included in the range of interventions for cholera control including case management and water and sanitation. 55 Use of OCV in Situations in which Cholera is Epidemic and Endemic OCV can be part of both a crisis management plan and a long-term strategy for cholera elimination. Crisis management is needed for outbreak response and should be part of a general response to an emergency situation, particularly when improvements in water and sanitation are difficult and can be achieved only in the long term. On the other hand, some countries do have long-term development plans for improving water and sanitation in an area that has been cholera prone. If the infrastructure plan will require 10 years to complete, a vaccine program can be used as an interim measure to control cholera while the infrastructure project is under way—at which point the vaccinations might be stopped. Recent OCV trials have shown long-term protection (up to 5 years). With this long duration of protection, areas threatened with cholera should realize benefits over the longer term. Thus, the success of a vaccine campaign is assessed not only by its ability to control an individual outbreak but rather by its benefits over the longer term, even if implemented in response to a specific outbreak. The World Health Organization is now establishing a stockpile, initially with 2 million doses, which will make OCV available for emergency use. It is being organized in a manner similar to the already established stockpiles for yellow fever and meningitis vaccines. In addition to making OCV available for emergency use, the stockpile will raise the profile of OCV and will facilitate the availability of OCV for endemic use. KNOWLEDGE GAPS AND RESEARCH PRIORITIES Practical Research Needs Some important research topics remain about OCV use: 56 Development of a rapid risk assessment tool to evaluate when and where OCV may be most effectively used. Enunciation of optimal use of OCV in settings in which cholera is endemic. Ways to communicate the potential benefits of OCV to key stakeholders and policy makers. Targeting OCV to achieve desired outcomes: o Reduce the number of cholera cases in the most cost-effective manner. o Reduce the number of cholera-related deaths in the most cost-effective manner. o Use OCV to maximize herd protection. Definition of simple methods to track interactions between OCV programs within an integrated cholera control strategy. Determination of when protection starts after the first OCV dose, which will guide use of vaccine during emergencies. Development of OCV that does not require cold chain. Development of OCV formulations that are easier to use and taste better. Data on protection from regimens and dosing strategies other than two doses. For example: o Efficacy and duration of protection from a single dose. o Possibility of increased efficacy from a third dose, especially when given to populations that have never experienced cholera and are immunologically naïve. o If a limited number of OCV doses are available, ways to best use those doses. For example, will more cases and more deaths be prevented if a single dose is given to more people rather than two doses to fewer people? Whether a second dose is a “booster” or whether it is a means of protecting people who did not respond to a single dose (as with measles vaccine). o Assuming a single dose is protective, timing of a second or a booster dose to extend protection. o Appropriate, convenient, and effective schedule for second or booster doses of OCV for routine programs in areas to which cholera is endemic and ways this schedule varies by age group. Studies before prequalification and vaccine use if other companies begin producing a wholecell vaccine similar to Shanchol or if other formulations are developed. Additional studies on herd protection to understand whether certain vaccination strategies can actually stop the spread of an epidemic. Fast track studies with attenuate OCVs that might provide protection with a single dose. o Looking Ahead OCV will be used increasingly in coming years. A key development to facilitate its increasing use is creation of the WHO stockpile in cooperation with other international agencies. The stockpile will ensure the availability of OCV for emergency use. Simultaneously, OCV is likely to be used in areas with more predictable seasonal outbreaks. In fact, it seems likely that these relatively predictable episodes of seasonal cholera are responsible for more cholera cases than are the cholera outbreak emergencies, but because they are somewhat expected, they do not receive the publicity. Each of these OCV projects will increase understanding of optimal methods for OCV use during emergencies and during seasonal cholera events. Documenting experiences will increase confidence in ways to deliver OCV to persons who are most vulnerable and who will most benefit from them. REFERENCES 1. Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet 1986;2:124–7. www.ncbi.nlm.nih.gov/pubmed/2873397 (accessed May 19, 2013). 2. Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990;335:270–3. www.ncbi.nlm.nih.gov/pubmed/1967730 (accessed May 19, 2013). 3. Li Z, Nickkholgh A, Yi X, et al. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res 2009;46:365–72. www.ncbi.nlm.nih.gov/pubmed/?term=Melatonin+protects+kidney+grafts+from+ischemia%2 Freperfusion+injury+through+inhibition+of+NFkB+and+apoptosis+after+experimental+kidney+transplantation (accessed May 19, 2013). 4. Moser B, Barella L, Mattei S, et al. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J 1993;294(Pt. 1):285–92. www.ncbi.nlm.nih.gov/pubmed/?term=Expression+of+transcripts+for+two+interleukin+8+rec eptors+in+human+phagocytes%2C+lymphocytes+and+melanoma+cells (accessed May 19, 2013). 5. Trach DD, Clemens JD, Ke NT, et al. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 1997;349:231–5. www.ncbi.nlm.nih.gov/pubmed/?term=Field+trial+of+a+locally+produced%2C+killed%2C+or al+cholera+vaccine+in+Vietnam (accessed May 19, 2013). 6. Thiem VD, Deen JL, von Seidlein L, et al. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine 2006;24:4297–303. 57 www.ncbi.nlm.nih.gov/pubmed/?term=Longterm+effectiveness+against+cholera+of+oral+killed+wholecell+vaccine+produced+in+Vietnam (accessed May 19, 2013). 7. Lucas ME, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352:757–67. www.ncbi.nlm.nih.gov/pubmed?term=Effectiveness%20of%20mass%20oral%20cholera%20 vaccination%20in%20Beira,%20Mozambiqu[all]&cmd=correctspelling (accessed May 19, 2013). 8. Ribeiro-Neto FA, Mattera R, Hildebrandt JD, et al. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol 1985;109:566–72. 9. Khatib AM, Ali M, von Seidlein L, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet infect Dis 2012;12:837–44. www.ncbi.nlm.nih.gov/pubmed/?term=Effectiveness+of+an+oral+cholera+vaccine+in+Zanzi bar%3A+findings+from+a+mass+vaccination+campaign+and+observational+cohort+study (accessed May 19, 2013). 10. DiCesare D, DuPont HL, Mathewson JJ, et al. A double blind, randomized, placebocontrolled study of SP-303 (Provir) in the symptomatic treatment of acute diarrhea among travelers to Jamaica and Mexico. Am J Gastroenterol 2002;97:2585–8. www.ncbi.nlm.nih.gov/pubmed/?term=A+double+blind%2C+randomized%2C+placebocontrolled+study+of+SP303+%28Provir%29+in+the+symptomatic+treatment+of+acute+diarrhea+among+travelers+t o+Jamaica+and+Mexico (accessed May 19, 2013). 11. WHO. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010;85:117–28. 12. Date KA, Vicari A, Hyde TB, et al. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis 2011;17:2105–12. wwwnc.cdc.gov/eid/article/17/11/11-0822_article.htm (accessed May 19, 2013). 13. Andrews JR, Basu S. Transmission dynamics and control of cholera in Haiti: an epidemic model. Lancet 2011;377:1248–55. www.ncbi.nlm.nih.gov/pubmed/21414658 (accessed May 19, 2013). 58 INFORMATION, EDUCATION, AND COMMUNICATION AND COMMUNITY HEALTH WORKER TRAINING Informing and training communities and community health workers—key components of cholera control and prevention—are necessary to ensure that cholera-infected persons are treated promptly and effectively. To synchronize efforts and ensure uniformity and standardization of communication content during cholera outbreaks, the different actors involved in cholera response (and information, education, and control [IEC] in particular) need to trust each other. Sometimes the diversity of players results in unnecessary and distracting competition, as manifested by the dissemination of, or emphasis on, different messages during the same outbreak. Without a singular approach, IEC guidelines may be difficult to implement. The following materials provide general guidance, but experts in various settings must tailor their communications plans and approaches to their varied audiences at varied times and in varied circumstances. DEFINITION OF ISSUE Excellent, evidence-based IEC activities that are essential for success generally can be defined and explained as follows: [IEC] initiatives are concerned with individual behaviour change or reinforcement, and/or changes in social or community norms, public health education and communication seek to empower people vis-à-vis their health actions, and to garner social and political support for those actions. IEC can be defined as an approach which attempts to change or reinforce a set of behaviours in a “target audience” regarding a specific problem in a predefined period of time. It is multidisciplinary and client-centred in its approach, drawing from the fields of diffusion theory, social marketing, behaviour analysis, anthropology, and instructive design. IEC strategies involve planning, implementation, monitoring and evaluation. When carefully carried out, health communication strategies help to foster positive health practices individually and institutionally, and can contribute to sustainable change toward healthy behaviour. (1)* In cholera emergencies, the need for response in a very short time underscores the importance of advance planning. * This document, produced by WHO Department of Reproductive Health and Research, examines lessons learned in IEC from 2 decades of experience in reproductive health, but the principles established and lessons learned apply throughout the health sector. It is a good basic reference for IEC principles. 59 KEY ACTIONS IEC Developing, Implementing, and Monitoring a Communications Plan The United Nations Children’s Fund (UNICEF) toolkit (2) is an excellent, comprehensive guide to public communication activities for cholera outbreaks and cholera preparedness and provides examples of strategies for communication, a communications plan template, a checklist for communication, and key messages, all drawn from various country experiences. According to UNICEF, effective communication strategies respond to five main criteria (in no particular order): they are based on evidence; they are measurable; they integrate a variety of different channels; they mobilize a width of different actors; and they involve communities. Pursuant to the last point, communication should not be unidirectional—involvement of affected communities is critical. Merely providing information to a community is not sufficient for success. UNICEF recommends “participatory discussion….to trigger community action.” A key recommendation is to have an authoritative, informed designated official spokesperson on cholera who gives consistent messages that are transparent and use minimal or no technical terms or jargon. Working with local media and local radio, mobilizing communities, identifying and addressing false rumors, and engaging a variety of formal and informal community leaders are strongly recommended. The toolkit provides examples of how these have worked. Tools and Materials The UNICEF toolkit links to examples of tools for IEC and provides eight comprehensive annexes as examples and templates. These annexes are as follows: type of communication strategies; communication plan template and example; communication preparedness checklist for cholera outbreak; information about different communication activities and channels; key messages and actions for cholera prevention; preparedness and response; community beliefs and perceptions in relation to cholera; working with communities and troubleshooting; and IEC work plan template. Training for Community Health Workers Materials for training community health workers on case identification, early treatment, referral and hygiene and waste management are available from a number of sources. In addition, the UNICEF toolkit (2) addresses training of health workers (Human Resources section) and provides comprehensive reference to training materials (3–10). KNOWLEDGE GAPS AND RESEARCH PRIORITIES IEC: Gaps in the UNICEF Toolkit The toolkit does not address the specific issues surrounding communication on the use of oral cholera vaccine (OCV) either as part of outbreak response or as part of routine national immunization programs. Because the routine use of OCV is relatively new, publically available materials related to IEC for this activity are limited; this gap should be filled on the basis of recent experience with OCV use in outbreak response in Haiti and Guinea and with large 60 prevention campaigns in Zanzibar and Bangladesh. (See OCV section.) Training for Community Health Workers: Gaps in Available Materials Most materials for training on OCV use in communities is in English. Materials suitable for translation into other languages are needed. Partners In Health had drafted a Haitian Creole training guide for health workers on the administration of OCV. REFERENCES 1. WHO. Information, education and communication. Lessons from the past; perspectives for the future. www.who.int/reproductivehealth/publications/health_systems/WHO_RHR_01_22/en/ (accessed April 27, 2013). 2. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 17, 2013). 3. Ministry of Health and Population in Haiti/Centers for Disease Control and Prevention. Haiti cholera training manual: a full course for healthcare providers [also in French]. www.cdc.gov/haiticholera/pdf/haiticholera_trainingmanual_en.pdf (accessed April 27, 2013). 4. Centers for Disease Control and Prevention. Community health worker training materials for cholera prevention and control [also in French]. www.cdc.gov/haiticholera/pdf/Haiti_Eng_MASTER_Haiti.pdf (accessed April 27, 2013). 5. COTS Program. Cholera outbreak training and shigellosis (COTS). www.cotsprogram.com/wordpress/ (accessed April 17, 2013). 6. International Federation of Red Cross and Red Crescent Societies. Epidemic control for volunteers; a training manual. www.ifrc.org/Global/Publications/Health/epidemic-controlen.pdf (accessed April 27, 2013). 7. Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://policy-practice.oxfam.org.uk/publications/choleraoutbreak-guidelines-preparedness-prevention-and-control-237172 (accessed April 27, 2013). 8. UNICEF. Guidelines on external staff in emergencies. Standby arrangements. www.washcluster.info/?q=download/file/fid/472 (accessed April 27, 2013). 9. WHO. Five keys to safer food: train the trainers course. www.who.int/foodsafety/consumer/TrainingCourse.pdf (accessed April 27, 2013). 10. Partners In Health. Community health worker cholera training. www.pih.org/publications/entry/community-health-worker-cholera-training-manuals (accessed April 27, 2013). 61 62 PROCUREMENT AND LOGISTICS DEFINITION OF ISSUE Procurement and logistics encompass a broad range of operations that aim to efficiently and effectively match the demand for a product (or set of products) to its supply. Emergency procurement and logistics operations span the timeline from preparedness through response and recovery to exit strategy. These operations are similar to those faced by traditional businesses, but key differences make them more challenging. Demand is highly unpredictable in terms of timing, location, and quantity; stakes are high; and resources are constrained (1). Advance planning and capacity building, effective management of response activities, and collaboration and coordination across agencies increase efficiencies of logistics and procurement activities—and ultimately increase the number of lives that can be saved. Cholera is not a complicated disease to treat, but it acts quickly. Several supplies are needed to prevent and respond to a cholera outbreak. Hygiene supplies, such as chlorine tablets and washing soap; medical supplies, such as oral rehydration solution (ORS), antibiotics, vaccines, and intravenous (IV) fluids; and all the logistic materials needed to equip the cholera treatment facilities should be readily available. For instance, early rehydration using ORS is critical for patient survival (2), and products, such as chorine, are crucial for safe drinking water and prevention of further outbreaks. Vaccines also are becoming part of a comprehensive approach to cholera prevention and control, adding complexities of dosing schedule and cold-chain requirements (3). Effective care depends on the availability of supplies and equipment, but also should be cost-effective (4). To make these supplies available to affected and at-risk populations in an efficient way, responders need to answer such questions as What products will be needed? How much and when? How should they be procured? Where should they be stored? How they should be distributed to the population? RECOMMENDED ACTIONS The recommendations are organized along the emergency timeline starting from needs assessment through measurement and evaluation. WHO Guidelines Others Define necessary supplies to address needs according to the emergency context. Standard requirement lists are available: cholera treatment centers (CTCs) supply requirements, water and sanitation, and hygiene (WaSH) promotion. (2) (5–8) Consider preconfigured kits. Kits are designed to facilitate epidemic response, especially at the beginning, because they save time. Examples include interagency diarrheal disease kits, blanket cholera prevention kits, and WaSH kits. (9) (5,7,10–12) Needs Assessment Phase Whenever possible when selecting supplies (7) 63 Observe cultural norms and sensitivities, particularly in relation to sanitation and personal hygiene items. Involve community representatives in the selection. Consider the environmental impact of different material options and the long-term impact of temporary emergency structures. Determine the minimum requirements of supplies. Follow recommended guidelines. For instance, the Epidemic Diarrhoeal Disease Preparedness and Response manual reports the estimated minimum supplies needed to treat 100 persons during a cholera outbreak. Consider the special needs of people with disabilities and with HIV infection and AIDS, the elderly, women, girls, and children in this specification. (2,13) Assess the accessibility of the area (e.g., roads, harbors, airports, security issues) and customs duties and restrictions to bring and distribute supplies. (2) Planning, Forecasting, and Preparing Phase Estimate the number of cases, their timing, and patient demographics. Consider the permanent population, displaced and refugee populations, and estimated number and expected dates of new arrivals. Use standard figures for attack rate, peak time, proportion of severe cases, and average length of stay in CTCs to organize supplies. (5) WHO Guidelines Others (2) (11) Consider different demand scenarios for population, risk level, attack rate, and severity of cases. (5) Identify potential sites for treatment. Several criteria are available for selecting an existing building and/or a site for erecting a temporary shelter or a tent, such as drainage, distance to population, trucks, and patients’ ease of access. Check and, if necessary, improve these sites’ sanitation and water supply facilities. Location is important so people can be transported on time; when local transportation infrastructure is not adequate for ill people, consider alternatives such as pedal-powered ambulances or tricycle stretchers. Health authorities and communities should be involved in selecting and preparing sites. Provide emergency stocks of such items as ORS, IV fluids, transport medium, rehydration equipment, antibiotics, laboratory reagents, and chlorine (for instance, a diarrheal disease kit that can treat 100 people with severe cholera), and cholera cots (which should be made of materials that can be disinfected) in at-risk areas. Keep a small amount of stock at local-level health centers and a larger amount at the district level. During an outbreak, 64 (2) (5,11) (2,13,14) (7,10) district-level supplies can be quickly moved to where they are most needed. Consider stockpiling strategies, particularly when 1) the evolving emergency situation is unpredictable, 2) a prolonged response is expected, 3) fluctuations in supply and distribution are likely, 4) high turnover of supplies is anticipated, 5) transport is limited, or 6) transport infrastructure is poor or badly damaged. Prepare education, communication, and mass media campaigns in advance. (14) Have stock of cholera prevention supplies (e.g., cholera prevention and hygiene kits) ready to distribute to households. (14) Ensure that sufficient chlorine-based disinfectants and residual chlorine test kits are available within the countries to treat water points (urban drinking water networks, wells, boreholes, reservoirs), as well as soap and basic WaSH supplies. (14) Investigate potential regional and local suppliers in affected regions and locations; consider prices, quality and reputation, and lead time. Ensure agreements are in place with partners and contractors for the delivery and distribution of supplies. (15) (14) Pre-position supplies according to numbers of populations at risk and to what is stockpiled at the regional level. Map locations for pre-positioning as well as for the logistics involved from these locations to different regions (include lead times). (5) These emergency measures serve as a bridge between preemergency supplies in the country or region and post-emergency transition to more sustainable and more widely accessible supplies. Identify vaccination posts in case of a mass vaccination strategy. Develop a detailed cold-chain inventory, including number of facilities available, locations and back-up options, and distance between vaccine stocks and vaccination posts. Resource Mobilization Phase Secure funds to procure hygiene and health supplies. Consider costs of the investigation and the response. Establish sources of funding. Mobilize medical and WaSH supplies and supplies for establishing CTCs targeting cholera-affected areas. (16) WHO Guidelines Others (17) (10) (14) (5) Ensure that a proper procedure exists to follow up on stock use and 65 to avoid shortages. Plan staff responsibilities and reassign staff according to needs. Establish responsibilities of staff at each level. (17) Procurement Procure locally where possible by scaling up existing programs rather than by importing foreign materials that could be more costly and less sustainable, that could undermine local markets, and with which the population could be unfamiliar. This is particularly important in the case of logistics and some WaSH supplies. In addition, livelihood opportunities might be created through local production and sourcing of some supplies. Restricting the import of supplies that are unavailable locally to emergency materials and equipment only may be advisable. (7) Emphasize the importance of coordinating suppliers and/or donors when providing materials for water treatment or other fundamental tasks. Different materials in different doses from different manufacturers can create confusion and make appropriate use much more challenging. Transportation and storage Make adequate allowance for transportation times and delays. Transport and distribution options may be affected by damaged or poor infrastructure or security or by weather conditions or lack of drivers or vehicles. Where transportation is likely to be a major constraint, try to minimize the weight, volume, and overall quantities of materials requiring distribution. (7) Manage requirements of warehousing and distribution of emergency materials. Even when this is primarily the responsibility of the local government, in many situations local governments may lack the capacity. (7) Distribution Locate CTCs inside existing hospital compounds when possible. A CTC is meant to offer the best care to patients and to protect other people from contamination. If the hospital compound is not adequate, another site (e.g., football ground, school) must be found. A CTC should have all the resources needed for treating cholera cases (ranging from no dehydration to severe dehydration, with special patient sections according to clinical presentation). (17) (5,11) Provide early access to ORS through oral rehydration points (17) (5,11) 66 (ORPs). ORS could be given by family members at home or by volunteers who could make house calls in the community or be located at designated ORPs. Having one CTC and several ORPs is preferable to having multiple CTCs. Identify patients’ level of dehydration, and refer them to the appropriate site for further care. ORPs should accompany the CTC (~50 beds) and cholera treatment units (CTUs, ~5 beds) for patient treatment. Provide household supplies to the main family caregiver (usually a woman). Information about the use of unfamiliar items also should be provided. (10) Establish CTUs as a valid intermediate step to CTCs, where persons with severe cholera can receive IV rehydration, particularly in isolated and rural regions. The CTU should be located inside the local health center, if possible, or in tents. (17) (11) Define the number of facilities of each type required, according to the population needs, taking into account capacity overload and distance from population. However, also consider that when ORPs and/or CTUs are multiplied, human resources organization, supervision, and supplies become difficult. (2) (11) In case of mass vaccination, send vaccines to vaccination posts at the very last moment and only in the required amounts. Vaccines should be kept as long as possible in secured cold-chain conditions (cold room or refrigerator with back-up generator), and a strict cold chain should be ensured at all steps by using cold boxes with sufficient ice packs. (16) Follow up on and map necessary supplies to address current needs. Distribute timely available supplies to affected areas according to needs and risks. Measurement and Evaluation Phase (5) WHO Guidelines Regularly track and monitor inventory and replenishment of supplies according to prevailing situation. Map available resources, and estimate existing gaps. Collaboration and Coordination (Throughout the Timeline) Establish a separate subgroup or working group to coordinate material logistics (storage, transport, and distribution) and procurement needs. Others (5,10) WHO Guidelines Others (7) 67 OUTDATED GUIDELINES, SUGGESTED GUIDELINES Actions Focused Across Different Infection Phases: At-Risk, Outbreak, Endemic Current recommended actions to control cholera (as listed in section 3) focus on the outbreak response or epidemic phases of the disease. During the initial outbreak, the main objective of the recommended actions is to offer a quick response to minimize deaths and to control the spread, and given the fast pace of cholera, these actions are enabled greatly by actions taken during the preventive phase, such as pre-positioning emergency stock. However, for endemic cholera, recommended actions should focus on ensuring longer-term sustainable solutions for cholera elimination, cost-control, and efficiency. Also, in endemic situations, more time is available to tailor the intervention from the start and customize standardized interventions for the local needs. Conducting risk and needs assessments is especially important for better prevention, preparation, and response to cholera. However, this assessment generally occurs after the fact. Challenges associated with such actions include the following: Funds are harder to get for prevention and preparedness than for outbreaks. Most individual donors and funding agencies provide project-based funds with a limited timeline (e.g., response for a specific outbreak in a specific region). As a result, trained staff managing the installed resources may seek alternate positions with other agencies or leave as funds become more limited or exhausted. Guidelines and metrics for the exit strategy are scarce and may provide guidance to relief organizations on how to exit an effected region after the right logistics system has been put in place and human capacity has been built to an acceptable level so that local governments can successfully continue in more autonomously. Exit strategy also must address shorterterm issues, such as taking down tents, temporary latrines, and other structures and reversing the supply chain of reusable, unused, or discarded materials. Cholera is unpredictable; diagnosing few cases in a cholera-endemic region does not necessarily indicate a future outbreak. Furthermore, although supplies are commonly prepositioned at the local level, one challenge is having an adequate surveillance system for early detection that would effectively trigger the planning and ordering of additional supplies before a big outbreak. Vaccination for Cholera Prevention and Control Strategy The importance of cholera prevention and elimination and the associated logistical issues needs to be emphasized. Prevention actions include improving water sources and sanitation and changing behavior to decrease infection risk. However, implementation of these preventive actions has been affected by population dynamics, inadequate infrastructures, and shift to other public health priorities. As a result of new developments in oral cholera vaccines (OCVs), prevention efforts are now incorporating vaccination as a complement of these efforts (3,18); hence, OCVs need to be considered in a complete package of cholera prevention and response. WHO recommends that OCVs be considered particularly in cholera-endemic areas and be used preemptively to protect vulnerable groups. Also, under certain conditions, OCVs may be used reactively to reduce extension of an ongoing outbreak (19). OCVs need to be not only safe and effective but also cost-effective and easy to deliver (20). Procurement and logistics operations profoundly affect vaccination costs. For instance, expediting OCVs by air to Aceh after roads were destroyed by the tsunami increased vaccination costs by more than 68 100% (21). OCVs have important characteristics that differentiate them from other medical supplies used in cholera prevention and response: OCVs need to be distributed by using cold chains. Labels with VMMs [vaccine vial monitors] that change color with heat exposure help to ensure that vaccines have been maintained in the cold chain throughout their distribution (22). The last mile is particularly challenging in a cold chain, especially when the local level has no power source. To overcome this challenge, innovative cold-chain equipment has been proposed to enable handling of larger volumes and reach areas without electricity (23). Moreover, OCVs are more stable than other vaccines, possibly enabling them to be taken out of the cold chain into a controlled temperature chain, at least during the last mile. For instance, for Shanchol vaccine, the shelf life stated on the label is 2 years at 2ºC–8ºC, but current studies indicate that the shelf life could be extended to 3 years. Also, stability tests indicate that the vaccine can remain outside of the cold chain (up to 37ºC) for 21 days, although this information is not yet on label. Moreover, Shanchol does not need buffer, which facilitates its distribution. The shelf life of the Dukoral vaccine is 3 years at 2ºC–8ºC, but testing has shown that it can remain stable at up to 25ºC for 2 weeks. Stability testing up to 40ºC is under way (21). Some recommendations point at incorporating OCVs into the cold chain of other vaccines (or even other cold-chain drugs), which already are part of the national immunization programs, including the current installed regional distribution hubs (23). OCVs require two doses. Shanchol requires two doses 2 weeks apart, and Dukarol requires two doses 1–6 weeks apart. This regimen implies additional resources to reach at-risk populations twice, not only for organizations but also for the population (for instance, if persons have to walk long distances to the vaccination post). Moreover, there is a delay for the vaccine to take effect (in case of Shanchol, earliest protection occurs 7–10 days after the second dose), so it is crucial that in a humanitarian crisis, vaccination is administered as soon as possible (16,21). For these reasons, single-dose and fast-acting OCVs are under development or moving toward licensure (21). A person receiving OCV needs a booster to maintain level of protection (21), which extends logistical efforts. Certainly the real long-term solution is access to safe water and sanitation infrastructure, but OCVs can buy time until these permanent solutions are achieved. For control of cholera outbreaks, global and/or regional stockpile strategies should be explored, considering trade-offs of consolidating inventories in fewer stockpile locations and possibly reducing lead times by speeding access through more stockpile locations. An initial cholera vaccine stockpile could be used in response to emergency situations, and the remaining stocks could be used for prevention campaigns, in particular while countries develop their national immunization programs (21). However, to control endemic cholera, vaccination should eventually become part of the structure of national immunization programs (24). Nevertheless, an OCV stockpile in itself will not constitute sufficient preparedness for a large and/or sustained cholera epidemic and should not take attention away from the key responses to cholera outbreaks (detection, diagnosis, and treatment of cases; establishment of a safe water supply; implementation of adequate sanitation; and communication and social mobilization) (25). Previous experiences in cholera mass vaccination campaigns (20,26) can offer valuable insight, such as the importance of designing vaccination posts that optimize the number of people vaccinated per hour, as well as vaccination strategies that achieve the best possible coverage at the least cost (for instance, a fixed-point strategy), and the need for a strong communication with the field operations and sufficient trained field operators who can administer the vaccine properly, particularly given cold temperature requirements and expiration dates. 69 Cooperation and Coordination: Synergy Opportunities A variety of organizations support governments’ responses to cholera outbreaks. Coordination among organizations and with Ministry of Health supply and logistics systems is critical to avoid duplications of supplies and logistics: use of limited warehouse space should be allocated; aggregate inventory monitoring is needed for a clear understanding of needs for or gaps in supplies; logistic coordination is required to avoid duplications, such as two organizations reaching the same community. Specific mechanisms should be put in place so activities are not unnecessarily duplicated and resources wasted. The WHO publication, Cholera Outbreak: Assessing the Outbreak Response and Improving Preparedness (17) asked several questions about coordination across organizations such as: Is there a list of needs that might be supported by each organization? Are there formal mechanisms to avoid funding and supplies procurement competition? What are the established tasks and responsibilities per organization? A committee on cholera best includes national and local government agencies, such as health, water and sanitation, military, communication, and education, as well as nongovernment organizations, religious leaders, and civil societies. Synergies across organizations and across different campaigns and projects could be achieved, and best practices can be borrowed from the private sector (for instance, in the U.S. courier delivery services partnerships occur between providers, such as the U.S. Postal Service, UPS [United Parcel Service] and Federal Express [27]) bringing savings in procurement, transportation, storage, and distribution. The following questions should be answered: Who makes decisions and shares responsibilities at each level? What tools are available to create and follow-up on inventory of support and responsibilities that enable this cooperation and coordination among different organizations? How should the information flow? What are potential synergies and how can they be implemented? These questions should be addressed before, during, and after an outbreak. Prevention, preparedness, and response to cholera need to be more strategic rather than addressed after a large uncontrolled outbreak. The Inter-Agency Standing Committee cluster mechanism can be used to provide consistency to the participants in emergency response, both agency and personnel, increasing the level of experienced responders participating in emergencies. The clusters can bring advantages, such as access to funds, but require strong leadership to achieve operational synergies and success. Structures like the Cholera Command and Control Centre (C4) in Africa can be established to 1) give technical guidance and coordination support to the response; 2) bring together the support of a WASH Cluster, Health Cluster, and Logistics Cluster; 3) mobilize technical and financial resources; and 4) provide information to guide the decision-making of the national task force (28,29). KNOWLEDGE GAPS AND RESEARCH PRIORITIES Possible areas for development in procurement and logistics in the context of cholera prevention and control are as follows: 70 Develop adequate information systems that facilitate cooperation, coordination and accountability among organizations and agencies. For instance, in a single electronic platform, WHO’s Communicable Disease Global Atlas brings together standardized data and statistics for infectious diseases at the country, regional, and global levels (30). Inventory control for cholera treatment supplies is a life-and-death matter, and “death by stock-out” is a preventable tragedy. Electronic tools and information technology systems are already widely and successfully used in the private sector for inventory management. A standard information system platform between agencies during the response would be helpful to track and control the inventory of available supplies and capacities. Real-time inventory management information not only brings opportunities for timely restocking but also facilitates transparency during planning and response operations. Develop predictive models (31) to determine the best strategies for deploying OCVs and other cholera supplies, including target population and timing, inventory, and stockpile policies. Deployment of OCVs must be guided by epidemiologic, technical, and operational evidence, some of which is incomplete but should be consolidated with experience (32). Effective documentation and fast publication of this evidence are fundamental. Develop and/or incorporate models and tools to improve the design and operation of the cholera prevention and control supply chain (23). Consider, for instance, HERMES (Highly Extensible Resource for Modeling Supply Chains), a software that can rapidly generate a simulation model of any supply chain (33). This software can help the decision maker answer questions such as: How would different transport and storage methods change performance? How do operational decisions (e.g., numbers of storage units, shipping frequency, adding or removing storage levels) affect performance and cost? Develop and standardize adequate metrics for different phases along the cholera timeline (prevention and response) and for the different settings (endemic and epidemic), and tools to help compute and distribute such metrics. For instance, Project Optimize developed a vaccine supply chain costing tool by using Microsoft Excel that translates operational metrics into economic metrics of interest (e.g., cost per dose administered, cost by location, and cost by activity) (33). Develop funding frameworks and guidelines that consider long-term sustainable (i.e., selfsufficient) solutions for eliminating, preventing, and controlling cholera. For instance, funding guidelines for building infrastructure type projects (e.g., cold-chain warehouses and water and sanitation infrastructures), and capacity building (e.g., continuous training programs supported by technology) in addition to response projects. Donors supporting outbreak response often focus on specific metrics, such as the number of supplies distributed to the affected population, but this may not be an appropriate indication of effectiveness or efficiency during some projects or emergencies. These guidelines also should include suitable metrics for longer-term funded project objectives. Showing results to donors is key to ensure continuation of these harder-to-get funds. REFERENCES 1. Logistics Cluster. Logistics operational guide. http://log.logcluster.org/ (accessed April 29, 2013). 2. WHO. Acute diarrhoeal diseases in complex emergencies. Critical steps: decision-making for preparedness and response. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CPE_ZFK_2004.6_Rev.1_eng.pdf (accessed April 30, 2013). 3. WHO. Cholera 2011. Wkly Epidemiol Rec 2012;87:289–304. www.who.int/wer/2012/wer8731_32.pdf (accessed April 30, 2013). 4. UNICEF. Cholera prevention: 10 key factors. www.unicef.org/wcaro/07UNICEF_Cholera_Prevention.ppt (accessed April 30, 2013). 5. Somali Health Cluster. AWD/Cholera Preparedness and Response Plan [to be updated monthly]. http://healthsomalia.org/alerts_weekly_documents/health_cluster_awdcholera_preparednes s_and_response_plan_feb2012.pdf (accessed April 30, 2013). 71 6. UNICEF. Supply catalog https://supply.unicef.org/unicef_b2c/app/displayApp/(layout=7.012_1_66_67_115&carea=%24ROOT)/.do?rf=y (accessed April 30, 2013). 7. WASH Cluster. Hygiene promotion. www.unicefinemergencies.com/downloads/eresource/docs/Clusters/WASH_Cluster_Coordi nator_Handbk_FINAL_VERSION_Jan09.pdf (accessed April 30, 2013). 8. List of essential hygiene promotion equipment for communication. WASH, 2007. http://pakresponse.info/LinkClick.aspx?fileticket=XvwOOVT_F9k%3D&tabid=92&mid=639 9. WHO. Interagency diarrhoeal disease kit. www.who.int/topics/cholera/publications/interagency_diarrhoeal_disease_kit_2009_en.pdf (accessed April 30, 2013). 10. Lamond E, Kinyanjui J. Cholera outbreak guidelines. Preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://reliefweb.int/sites/reliefweb.int/files/resources/mlcholera-guidelines-preparedness-prevention-and-control-030512-en.pdf (accessed April 13, 2013). 11. Médecins Sans Frontières. Cholera guidelines. 2nd ed. New York: Médecins Sans Frontières; 2004. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed April 30, 2013). 12. Paramount Industries. Family hygiene kits (UNICEF type). http://paramounttent.com/familyhygiene-kit-unicef-type/ (accessed April 30, 2013). 13. WHO. Epidemic diarrhoeal disease preparedness and response. Training and practice. participant’s manual. www.who.int/csr/resources/publications/cholera/whoemcdis973rev1e.pdf (accessed April 30, 2013). 14. UNICEF, WHO. Cholera epidemics preparedness. www.unicef.org/wcaro/07Cholera_Prepareness_Joint_UNICEF_WHO_Recommendations.doc (accessed April 30, 2013). 15. Pan American Health Organization. Controlling cholera: a checklist for planners. http://bvs.per.paho.org/texcom/colera/controlling.pdf (accessed April 30, 2013). 16. WHO. Oral cholera vaccines in mass immunization campaigns. Guidance for planning and use. http://whqlibdoc.who.int/publications/2010/9789241500432_eng.pdf (accessed April 30, 2013). 17. WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFk_2004.4_eng.pdf (accessed April 30, 2013). 18. WHO. Cholera fact sheet no. 107. July 2012. www.who.int/mediacentre/factsheets/fs107/en/index.html (accessed April 30, 2013). 19. WHO. Cholera 2010. Wkly Epidemiol Rec 2011;86:325–39. www.who.int/wer/2011/wer8631.pdf (accessed April 30, 2013). 20. Global Task Force on Cholera Control. Cholera vaccines: a new public health tool? http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFK_2004.5.pdf (accessed April 30, 2013). 21. WHO. WHO consultation on oral cholera vaccine (OCV) stockpile strategic framework: potential objectives and possible policy options, http://whqlibdoc.who.int/hq/2012/WHO_IVB_12.05_eng.pdf (accessed April 30, 2013). 22. WHO. What is VVM and how does it work? www.who.int/immunization_standards/vaccine_quality/What%20is%20VVM%20and%20how %20does%20it%20work.pdf (accessed April 30, 2013). 23. PATH [Program for Appropriate Technology in Health], WHO. Project Optimize. Achieving the 2020 global vision for immunization supply and logistics. September 2012. www.who.int/immunization_delivery/optimize/Achieving_global_vision_for_future_immunizati on_supply_and_logistics_systems.pdf (accessed April 30, 2013). 72 24. UNICEF. The Global Immunization Vision and Strategy. www.unicef.org/immunization/index_27089.html, [also available at www.who.int/immunization/givs/en/index.html] (accessed April 30, 2013). 25. WHO. WHO Technical Working Group on creation of an oral cholera vaccine stockpile. www.who.int/cholera/publications/oral_cholera_vaccine/en/index.html (accessed April 30, 2013). 26. Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what is next? Report: WHO meeting, 14–16 December 2005, Cairo, Egypt. www.who.int/cholera/publications/cholera_vaccines_emergencies_2005.pdf (accessed April 30, 2013). 27. Rohrlich J. Why FedEx and UPS want the Postal Service to survive. www.minyanville.com/business-news/editors-pick/articles/postal-service-usps-post-officepost/8/3/2012/id/42951#ixzz2LfF1Aewdhttp://www.minyanville.com/business-news/editorspick/articles/postal-service-usps-post-office-post/8/3/2012/id/42951 (accessed April 30, 2013). 28. WHO. Global Alert and Response (GAR). Cholera in Sierra Leona—update. www.who.int/csr/don/2012_10_08/en/ (accessed April 30, 2013). 29. Zimbabwe Ministry of Health and Child Welfare, WHO. Intersectoral actions in response to cholera in Zimbabwe: from emergency response to institution building. www.who.int/sdhconference/resources/draft_background_paper23_zimbabwe.pdf (accessed April 30, 2013). 30. WHO. Communicable disease global atlas. http://apps.who.int/globalatlas/ (accessed April 30, 2013). 31. Longini IM Jr, Nizam A, Ali M, et al. Controlling endemic cholera with oral vaccines. PLoS Med 2007;4:e336. www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0040336 (accessed April 30, 2013). 32. Martin S, Costa A, Perea W. Stockpiling oral cholera vaccine. Bull World Health Organ 2012;90:714. www.who.int/bulletin/volumes/90/10/12-112433/en/index.html (accessed April 30, 2013). 33. PATH [Program for Appropriate Technology in Health]. Project Optimize. Building next‐generation vaccine supply systems. www.path.org/publications/files/TS_opt_evm_hermes_fs.pdf Interviews with Experts Kate Alberti, Epidemiologist, Epicentre, Médecins Sans Frontières (February 27, 2013). Eric Mintz MD, Leader of the Global Water, Sanitation, and Hygiene Epidemiology team, Centers for Disease Control and Prevention (February 20, 2013). Heather Papowitz, MD, MPH, Senior Advisor Health—Emergencies, UNICEF Health Section (February 26, 2013). Derek Hardy, Outbreak Response Regional Logistics Advisor for US Agency for International Development—Deliver Project on the Emerging Pandemic Threats (February 19, 2013). David Olson, MD, Deputy Medical Director Doctors Médecins Sans Frontières (March 1, 2013). Jonathan Colton PhD, Professor of Mechanical Engineering, Georgia Institute of Technology, with expertise in cold chains (February 20, 2013). 73 74 OPERATIONAL RESEARCH DEFINITION OF ISSUE AND INTERVENTIONS Operational research is broadly defined as the use of advanced analytical methods to improve decision-making (1). In the context of public health, operational research has been referred to as “the search for knowledge on interventions, strategies, or tools that can enhance the quality, effectiveness, or coverage of programmes in which the research is being done” (2). Médecins Sans Frontières (MSF) indicates that operational research helps its organization evaluate the feasibility of new strategies, improve existing prevention and care programs, and develop evidence-based advocacy messaging (3,4). Operational research can be distinguished from randomized controlled trials because operational research is intended to evaluate the impact of interventions in diverse routine care settings rather than in defined groups of patients (2). Thus, operational research includes such methods as cross-sectional studies, case–control studies, and prospective or retrospective cohort studies. The World Health Organization (WHO) specifies that operational research differs from other research in that it addresses problems related to specific programs that are within the control of managers, employs systematic data collection, depends on collaboration between researchers and managers for research design, and requires application of research results to programs (5). Operational research is often conducted by global organizations, such as the U.S. Centers for Disease Control and Prevention (CDC), WHO, and the United Nations Children’s Fund (UNICEF), and by humanitarian nongovernment organizations (NGOs), such as MSF and Partners in Health (PIH). Outstanding Questions on Cholera Vaccine Use Operational research can help answer open questions about how to use cholera vaccines and whether implementing new vaccination programs in different settings is logistically feasible (6). Open questions include the following: How do feasibility, effectiveness, and cost-effectiveness results change in endemic, epidemic, and outbreak scenarios in different settings (e.g., refugee camp, urban slum, rural mountainous region in/not in crisis)? Can data be collected in a standardized manner? Within countries, where should cholera vaccine be targeted to save the most lives: rural areas, urban slum areas? Within urban and rural areas, where should the vaccine be targeted to have the most impact (i.e., avert cases, save lives)? Will experience in one country be generalizable to other countries and regions? Are there specific epidemiologic measures that can be collected as the epidemics evolve that can be useful in implementing interventions? In supply-constrained situations, does targeting specific age groups make sense? If so, which age groups? What is optimal timing for vaccination in various cholera manifestations (e.g., epidemic, endemic)? How are trade-offs between treatment and vaccination, or both, determined? When is it too late to vaccinate in an epidemic or outbreak setting? What is the optimal initial and subsequent vaccination strategy with regard to revaccination? Can such factors as cell phone technology, social media, satellite technology, and environmental determinants be used to help predict cholera outbreaks, timing, and severity and/or appropriately alert communities? 75 Are persons who received cholera vaccination more likely or less likely to follow public health advice about water, sanitation, and hygiene (WaSH) processes? How do implementers quickly and accurately assess whether use of the vaccine in cholera response activities will not disrupt the provision of other services? Operational Research Completed Across All Cholera Control Measures Current operational research across the broad spectrum of cholera control measures are summarized in specific sections of this document. Vaccine-specific: Shanchol and Dukoral Shanchol (Shantha/Sanofi), the most recently prequalified vaccine, is less expensive and easier to administer in resource-constrained settings than is Dukoral (Crucell). Most information about the operational feasibility of Shanchol is from clinical research settings; however, operational lessons can be learned from recent vaccination programs and programs in process (see section on OCV). Demonstration projects including Dukoral garnered valuable early lessons on use of the vaccine. Legros et al. reviewed the feasibility of a mass vaccination campaign with Dukoral in a Sudanese refugee camp in Uganda, specifically to determine whether the campaign might disrupt other priority interventions. The study concluded that vaccination was feasible and recommended considering a preemptive vaccination strategy in stable refugee settings and in urban slums in high-risk areas if the supply and cost are accessible (7). Khatib et al. assessed the effectiveness of OCV in high-risk populations of Zanzibar by offering Dukoral vaccine to persons >2 years of age at six sites. At least 50% of the test cohort received the recommended two doses, and a subsequent cholera outbreak in the population suggested that OCV offered direct protection in those who received two doses and indirect protection in the rest of the population (8). Schaetti et al. assessed factors associated with uptake of Dukoral in Zanzibar; competing obligations and priorities were the most prominent barriers to uptake, which suggested that innovative vaccine delivery strategies (e.g., later hours, alternatives to mass campaigns) may need to be considered (9). Chaignat et al. studied the government’s decision to conduct a mass vaccination campaign with Dukoral in Aceh province, Indonesia, after the 2004 tsunami. The two-dose product had not been widely used in complex emergencies, and the evidence demonstrated feasibility of delivery and relevance of vaccine (10). Vaccine Modeling Although direct evidence of the effectiveness of the vaccine in epidemic settings is limited, mathematical modeling has suggested that the vaccine would be effective under certain conditions: 76 Chao et al. used a mathematical cholera transmission model to assess different vaccination strategies to show that randomly prevaccinating a fraction of the population well before an epidemic begins could reduce the number of cases roughly in proportion to the number of persons vaccinated and delay the epidemic peak. Vaccinating during the epidemic also would reduce cases significantly (For example, vaccinating 30% of the high-risk population in Haiti could have reduced the number of cases by 55% and saved 3,320 lives) (11). Reyburn et al. used existing data from cholera outbreaks in Zimbabwe to simulate the number of cholera cases preventable by reactive mass vaccination and concluded that as many as 40% of cases and 40% of deaths could have been prevented (12). Azman et al, found that targeting vaccination efforts to an urban hotspot in Guinea-Bissau (where transmission was most efficient) would have averted most of the cases in the hotspot area and throughout the city (13). Modeling can help guide future strategies for operational research around use of the vaccine. However, modeling does not account for the political, economic, and humanitarian realities under which most governments and organizations work. Ongoing Operational Research Several recent vaccination campaigns with the recently prequalified vaccine, Shanchol, have been ongoing in different settings that can increase understanding about the effectiveness and operational feasibility of vaccine use: The International Vaccine Institute is working with local health officials in a rural area of Orissa, India, to conduct and evaluate a mass oral cholera vaccination campaign, along with concurrent sociobehavioral studies, acceptability studies, and social mobilization activities. The International Centre for Diarrhoeal Disease Research, Bangladesh, is conducting an impact evaluation of cholera vaccine and WaSH-related behavior change interventions with approximately 160,000 persons living in urban Dhaka, Bangladesh. The Ministry of Health (MoH) Haiti partnered with PIH and GHESKIO to use the vaccine with approximately 100,000 persons in a rural area and an urban slum in Haiti in 2012. The MoH Guinea partnered with MSF to use the vaccine with approximately 200,000 persons in two rural coastal areas of Guinea in 2012. Use of OCV is being planned in a refugee setting along the Thai–Myanmar border in a collaboration among Première Urgence–Aide Médicale Internationale (PU-AMI), CDC, and the Thailand MoH. The MoH Solomon Islands, in partnership with CDC, conducted a preemptive vaccination campaign in an area neighboring Papua New Guinea. Preliminary results from several of the programs show high rates of second-dose completion and acceptance of the vaccine. However, because these programs are ongoing, results from these initial uses are not yet published. KNOWLEDGE GAPS AND RECOMMENDED ACTIONS It is important that findings from operational research are tailored into practical guidelines that can be understood by the wider community, especially those working in cholera prevention and response at the country level. Critical knowledge gaps remain about vaccination strategies in endemic, epidemic, and outbreak scenarios, as well as integrated approaches to cholera treatment and control that include vaccination, WaSH interventions, and treatment. The following areas need wider study: 77 Identification and targeting of interventions (vaccine, WaSH) to the highest risk populations in endemic, epidemic, and outbreak settings in ways that are most equitable and costeffective. An evidence-based understanding of best practices for case management with regard to appropriate use of antibiotics in different settings (e.g., jails) and various formulations of ORS and zinc. An evidence-based understanding of links between epidemic response and long-term development and sociobehavioral and intervention acceptability studies. Funding for operational research to inform global and country level policy and public health interventions is needed for continued use if best practices in cholera control activities. REFERENCES 1. Institute for Operations Research and Management Sciences. Operations research: the science of better. What operations research is. www.scienceofbetter.org/what/index.htm (accessed July 11, 2012). 2. Zachariah R, Harries AD, Ishikawa K, et al. Operational research in low-income countries: what, why, and how? Lancet Infect Dis 2009;9:711–7. www.ncbi.nlm.nih.gov/pubmed/19850229 (accessed August 2, 2013). 3. MSF. Operational research in Médecins Sans Frontières. www.msf.lu/fileadmin/WEBLibrary/2_Au_Luxembourg/OR_Brochure_EN.pdf (accessed July 11, 2012). 4. Zachariah R, Ford N, Draguez B, Yun O, Reid T. Conducting operational research within a non-governmental organization: the example of Médecins Sans Frontières. International Health 2010;2:1–8. 5. WHO. Expanding capacity for operations research in reproductive health: summary report of a consultative meeting. December 10–12, 2001. www.who.int/iris/handle/10665/67936 (accessed April 2, 2013). 6. Desai NS, Clemens JD. An overview of cholera vaccines and their public health implications. Curr Opin Pediatr 2012;24:85–91. www.ncbi.nlm.nih.gov/pubmed/?term=An+overview+of+cholera+vaccines+and+their+public +health+implications (accessed May 19, 2013). 7. Legros D, Paquet C, Marty I, et al. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp. Bull World Health Organ 1999;77:837–42. www.who.int/bulletin/archives/77%2810%29837.pdf (accessed May 19, 2013). 8. Khatib AM, Ali M, von Seidlein L, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort. Lancet Infect Dis 2012;12:873–44. www.ncbi.nlm.nih.gov/pubmed/?term=Effectiveness+of+an+oral+cholera+vaccine+in+Zanzi bar%3A+findings+from+a+mass+vaccination+campaign+and+observational+cohort+study (accessed May 19, 2013). 9. Schaetti C, Ali SM, Chaignat C-L, et al. Improving community coverage of oral cholera mass vaccination campaigns: lessons learned in Zanzibar. PLoS ONE 2012;7:e41527. www.ncbi.nlm.nih.gov/pubmed/?term=Improving+community+coverage+of+oral+cholera+m ass+vaccination+campaigns%3A+lessons+learned+in+Zanzibar (accessed May 19, 2013). 10. Chaignat CL, Monti V, Soepardi J, et al. Cholera in disasters: do vaccines prompt new hopes? Expert Rev Vaccines 2008;7:431–35. www.ncbi.nlm.nih.gov/pubmed/?term=Cholera+in+disasters%3A+do+vaccines+prompt+new +hopes%3F (accessed May 19, 2013). 78 11. Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. PNAS Early Edition: www.pnas.org/cgi/doi/10.1073/pnas.1102149108 (accessed April 2, 2013). 12. Reyburn R, Deen JL, Grais RF, et al. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis 2011;5:e952. www.ncbi.nlm.nih.gov/pubmed/21283614 (accessed August 2, 2013). 13. Azman AS, Luquero FJ, Rodrigues A, et al. Urban cholera transmission hotspots and their implications for reactive vaccination: evidence from Bissau City, Guinea Bissau. PLoS Negl Trop Dis 2012;6:e1901. www.ncbi.nlm.nih.gov/pubmed/?term=Urban+cholera+transmission+hotspots+and+their+im plications+for+reactive+vaccination%3A+evidence+from+Bissau+City%2C+Guinea+Bissau (accessed May 19, 2013). 79 80 MONITORING AND EVALUATION OF ALL INTERVENTIONS DEFINITION OF ISSUE The difference between a cholera epidemic that results in a humanitarian disaster and a cholera epidemic that results in minimal deaths is the speed and effectiveness of intervention. Best practices among interventions are established by operational research, with results assured by monitoring a minimum set of simple metrics and evaluating success at reaching targets for those metrics. Relief agencies vary widely in the data they provide on their activities, from specific metrics and targets to qualitative statements without measurable outcomes. The integration of vaccine adds urgency to defining simple metrics and targets for all interventions. Monitoring and evaluating the ability to reach targets in prevention, treatment, and control are key to ensuring the most rapid, effective, and sustainable response to cholera. RECOMMENDED ACTIONS Relief organizations should Communicate details of their activities, the underpinning operational research, and the metrics and targets through which activities are monitored and evaluated. Enhance integration: agencies in the field should use surveys to measure coordination of activities, metrics, targets, monitoring, and evaluation. Develop approaches to share and integrate setting-specific and qualitative information into improved program delivery. Strengthen cholera risk profiling to facilitate the transition from reactive response to sustainable prevention. Summary of Monitoring and Evaluation by Stage of Epidemic Cholera is difficult to enumerate and characterize within vulnerable populations that are themselves difficult to define. Whereas relief agencies can set, monitor, and evaluate internal organizational targets, assessing the overall impact of interventions in the field during a cholera epidemic is hostage to the uncertain and dynamic denominator of the size of the population at risk. The case-fatality rate is a crucial but imperfect metric, with a consensus target set at <1%, for example, by the third week of an epidemic. Additional metrics tabulated below can be tracked through the epidemic curve. The United Nations Children’s Fund (UNICEF) Cholera Toolkit (1), developed in collaboration with other major relief agencies, is the authoritative compendium of best practices. A few simple, essential metrics with targets should be identified from recent operational research and included both in the UNICEF Cholera Toolkit and the best practices of all agencies involved in cholera relief. The most thorough monitoring and evaluation of metrics and targets is evident in the cholera treatment centers (CTCs). Relief agencies should collaborate closely with each other and CTCs to measure the effectiveness of combined interventions in terms of cases prevented. The ultimate evaluation metric for all interventions is sustained, accurate, and thorough case reporting through national Ministries of Health (MoHs) to the World Health Organization (WHO) after relief organizations depart. 81 Pre-epidemic cholera Features The risk for cholera is real when Access to safe water and sanitation is poor. Local health care systems are inadequate to identify declining environmental and health indicators (2). Evidence exists of high infant mortality, high rates of diarrhea, or persistent enteric infection (2). Social structure, safe water, and sanitation are severely disrupted. Behavior, lack of knowledge, and environmental contamination expose the public to human feces in the environment. Supplies, expertise, and infrastructure are inadequate for prevention, treatment, and management of cholera (3,4). In displaced or migrant persons from cholera-affected regions, cholera is not adequately diagnosed or treated, and these persons are not adequately accommodated. Key Actions Strengthened surveillance and increased risks for cholera are reported to MoHs, WHO, and relief agencies to establish coordination and preparedness (3,5). Experts establish baseline epidemiology, identify the most vulnerable communities, and measure public awareness. Resources—expertise, staff, training, facilities, supplies, and transport—are assessed and appropriately reinforced (3). Preventive water, sanitation, and hygiene (WaSH) programs are activated. Process is initiated to acquire the data necessary to provide access to the oral cholera vaccine (OCV) stockpile. Data include epidemiology; modeling; target groups; required facilities, staff, training, and resources; and increased public awareness (6). MoH acquires data and alerts the WHO International Coordinating Group (ICG) for OCV (7). Monitoring and Evaluation of Interventions WHO Guidelines Others Establishment of cholera risk Establish appropriate cholera case definitions. (3,8) (1,2,9,10) Calculate incidence by using comprehensive surveillance, estimates of underreporting or overreporting, and census data. (3,8) (1,2,9,10) 82 Evaluate health systems, and quantify and characterize the at-risk population. Establish the level of public awareness and compliance with WaSH and OCV through training assessed with questionnaires. Measure the adequacy of supplies for CTCs and OCV against the at-risk population and the effectiveness of the supply chain. (9,11,12) (3,4) (11,13) (3) (1,9,12) Assess WaSH programs by metrics of user understanding, levels of coverage, and compliance. MoH will collect population, epidemiologic, and resource data to access OCV stockpile from WHO/ICG/Thematic Working Group (TWG). (10,13) (7) Collect baseline data to predict OCV impact by using metrics established in Bangladesh, Zanzibar, and Haiti. (2,11,12,14,15) Collect epidemiologic and environmental metrics to predict and assess impact of OCV in terms of cases averted and lives saved. (16) Evaluation Begin evaluation with a sensitivity analysis to determine the quality and bias of case report data from the field. Evaluate staff skills and training by testing. (2) (3) Evaluate public awareness and outreach by questionnaires. (1,13) (13) WHO/ICG/TWG will monitor and evaluate the quality of field data in support of OCV stockpile release. (7) Access and review the WHO Tools for Evaluation. (4) Early stage of outbreak Features The immediate needs in cholera outbreaks are to Manage cases. Minimize transmission. Perform prevention actions in at-risk areas. 83 Key Actions Managing outbreaks begins with coordinating and sharing epidemiologic information, establishing CTCs, optimizing patient access and emergency care, and educating convalescents at discharge (3,4). Cholera transmission can be interrupted by using WaSH packages and OCV. Minimizing transmission by WaSH programs includes drilling wells; installing hand-pumps; improving water collection; and using point-of-use water storage, chlorination, and sanitation improvements (e.g., digging pit latrines). Minimizing transmission through WaSH remains a key intervention until the end of the epidemic. WaSH is the core from which sustained improvement in sanitation drinking water are built into permanent infrastructure (3). WaSH programs have been introduced to many countries over many years. Improved systematic data on effectiveness and sustainability are needed. Communication among relief agencies, MoHs, and WHO/ICG/TWG is needed to ensure that data supporting application for OCV stockpile is up to date and that planning and implementation are expedited (7). Monitoring and Evaluation of Interventions Identify outbreaks by monitoring hospital admissions for patients whose illnesses meet the cholera case definition and for changing caseload and clinical presentation (e.g., age structure and severity of dehydration). WHO Guidelines Others (3) (1,9) Coordinate activities of relief agencies and staff, including training, situational awareness, education, logistics, outreach, case monitoring through and data sharing of geographic information system (GIS) indexing of cases and intervention points (e.g., WaSH facilities and vaccinees). (5,13) Coordinate establishment, design, and location of CTCs. Assess accessibility of CTC by recording mode of patient transport, distance traveled, and extent of dehydration on admission. (1,9,17) Measure effectiveness of emergency care by case-fatality rates, bedoccupancy rates, and supply consumption rates and inventory levels. (1,9) Limiting transmission by WaSH programs. Measure community coverage, awareness, supply replenishment rates, follow-up compliance, free chlorine, microbiological quality of drinking water, delinquency, and sustainability. (1,13) Limiting transmission by OCV programs: Measure coverage rates among highest transmitting groups, levels of acceptability, vaccine effectiveness, indirect protection, and cases averted. (14,15) 84 Management of epidemic cholera Features The key feature of epidemic cholera is an explosion of cases with the potential for disintegrating public order and severe disruption of the supply chain. Security problems may exist that prevent humanitarian intervention (3). Key Actions The first requirement in establishing the feasibility of intervention by WHO is a satisfactory security assessment (3,6). Treating cases The primary goal during cholera epidemics is to provide excellent patient care in the face of rapidly increasing caseload. Management and control procedures depend on settings and resources: rural, urban, and closed (e.g., camps for internally displaced persons). WHO, UNICEF, Médecins Sans Frontières (MSF), and others focus on collecting, analyzing, interpreting, and applying contemporaneous data on the supply chain and bed occupancy rates, etc., during epidemics to rapidly respond to and improve clinical care, with the ultimate metric being favorable patient outcomes. Minimizing transmission Limiting transmission by WaSH programs: WaSH interventions are expanded to keep pace or keep ahead of the geographic spread of disease. Limiting transmission by OCV programs: Interventions in Guinea, Haiti, Mozambique, Zanzibar, and Indonesia showed high levels of acceptance of and enthusiasm for OCV (8,9 12,13). Modeling shows that the sooner the vaccination occurs, the more deaths are averted. Understanding how best to employ OCV requires monitoring and evaluation. Monitoring and Evaluation of Interventions WHO Guidelines Others Treatment The case-fatality rate is the definitive metric for the effectiveness of cholera treatment. Additional metrics include caseload (admissions, bed occupancy, and length of stay), time of death, and rate of use and inventory of supplies (Ringer’s lactate, oral rehydration solution [ORS], antimicrobial drugs). (1,9) Prevention 85 Implementation of WaSH and OCV requires monitoring and evaluation. The overarching principle is to document each WaSH or OCV intervention (ideally including GIS), then monitor and evaluate at least a proportion of them for effectiveness through site visits and clinical surveillance. (1,9,13,16) WaSH Comparisons should be made in cholera rates; awareness; behavior change; and if possible, non-cholera enteric infections among households and communities receiving and not receiving WaSH interventions. Microbiological assays and residual chlorine should be included. Extending monitoring and evaluation beyond the end of the epidemic measures sustainability and assists future cholera risk assessment. (4) (13) OCV Mass vaccination demonstration projects have shown feasibility and acceptability. Monitoring and evaluation involves comparing cholera rates among individuals, households, and communities at different levels of vaccine coverage. Additional valuable metrics include impact on Expanded Program on Immunization vaccination rates, awareness, and behavior. Immunologic assays (e.g., saliva immunoglobulin) from a subset of persons is valuable for assessing immunologic status and population risk. (11,12,15,16,) Epidemiology The metric for the end of an epidemic where cholera was previously absent is a transition to endemicity with continuing sporadic cases. The metric for the end of an epidemic where cholera is endemic is a return to the epidemiologic pattern in nonepidemic years (e.g., inversion of the dominant age groups in hospital admissions from adults during epidemics to children). (9) Management of endemic cholera Features Global incidence and mortality from cholera are poorly understood because of scant epidemiology and national reporting to WHO, resulting in a gross underestimation of disease. Many cholera epidemics grow from poorly reported outbreaks and undocumented endemic disease. Managing endemic cholera involves raising awareness of the disease, its grave danger 86 (particularly to young children), and the availability of effective treatment. Many examples exist of successful awareness-raising efforts (e.g., ORS in Bangladesh and substantial investment in WaSH programs in many countries). OCV as a WHO prequalified vaccine provides a safe and effective tool for preventing and mitigating cholera. Licensure and WHO prequalification of OCV was supported by numerous large effectiveness studies. Large-scale OCV demonstration projects and field trials have shown that vaccination can be closely monitored and precisely evaluated in the face of an expanding epidemic. Combining WaSH and OCV has been proposed as a potentially decisive and mutually supportive combination for eliminating cholera (12). Evidence is needed to advocate for large, extended, and sustained programs supported by monitoring and evaluation. WHO Guidelines Key Actions Others Monitoring and evaluation of interventions Monitoring Epidemiology: Monitoring and evaluating interventions for endemic cholera first requires accurate national reporting. Risk factors: Risk factors for cholera should be monitored, including by measuring behavior, safe water, and sanitation. Awareness: Popular understanding of cholera is crucial to ensuring prevention, diagnosis, and treatment. Awareness campaigns can be enhanced, measured, and evaluated by using questionnaires. Cholera avoidance can be taught to patients on discharge from hospital, and the effectiveness of this education can be measured. More data are needed to improve public understanding of prevention. (18) (3) (2,16) (11–14,17) Evaluation Evaluation is crucial to show efficacy, field effectiveness, cost effectiveness, and sustainability of all interventions. (19) Retrospective analyses of WaSH and OCV programs should be established to demonstrate the long-term impact on awareness, behavior, incidence, diagnosis, treatment, morbidity, and mortality. Whether WaSH programs measurably impact other enteric infections needs to be determined. (10,20,21) Methods to determine the field effectiveness of OCV have been established and published, including 5-year protection data. (19) 87 Exit strategies Features ost agencies require a predetermined exit strategy before mobilizing against a cholera epidemic. Cholera epidemics usually follow a downward epidemic curve and resolve into endemicity with relatively low levels of sporadic cases. The exit of MSF is based on the capacity of the local health system to manage these sporadic cases (9). The exit strategy of WHO and Oxfam GB involves linking the downward emergency response to the affected country’s cholera program to build preparedness for future cholera outbreaks, improve local capacity to manage public health activities, and encourage MoHs to sustainably improve water and sanitation (13,14). Endemic cholera typically develops a seasonal pattern corresponding with precipitation and inundations. Key Actions The ultimate exit strategy is the universal provision of safe water and sanitary management of human feces (20). Monitoring and Evaluation of Exit Strategies WHO Guidelines Others MSF uses the following indicators to signal the impending end of cholera epidemics: declining numbers of hospital admissions, declining demand for clinical resources, building of surpluses in the supply chain, and declining bed-occupancy rates. (9) Monitoring and evaluating the termination of epidemic and endemic cholera requires effective cholera reporting. (18) WHO defines the end of an epidemic as 6 weeks’ absence of laboratory-confirmed cases in a specified district. (13) The elimination of endemic cholera is defined by WHO as the lack of cases other than those introduced into the community. (8) MoHs that participate in cholera control activities need to commit to accurate case reporting to WHO. Data are lacking on the sustainability of cholera interventions. The field effectiveness of WaSH and OCV programs needs to be monitored and evaluated by follow-up studies on safe drinking water sources and treatment, suitable sanitation, and immune responses (OCV). 88 Microbiological surveillance and risk assessment must be a part of sustainability. (3) REFERENCES 1. UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed May 19, 2013). 2. Guerra J, Mayana B, Djibo A, Manzo ML, Llosa AE, Grais RF. Evaluation and use of surveillance system data toward the identification of high-risk areas for potential cholera vaccination: a case study from Niger. BMC Res Notes 2012;5:231. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+and+use+of+surveillance+system+data+t oward+the+identification+of+highrisk+areas+for+potential+cholera+vaccination%3A+a+case+study+from+Niger (accessed May 13, 2013). 3. WHO. Acute diarrhoeal diseases in complex emergencies: critical steps. Decision-making for preparedness and response. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CPE_ZFK_2004.6_Rev.1_eng.pdf 4. WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFk_2004.4_eng.pdf (accessed April 20, 2013). 5. United Nations High Commissioner for Refugees. Epidemic preparedness and response in refugee camp settings: guidance for public health officers. October 2011. www.unhcr.org/4f707f509.pdf (accessed April 17, 2012). 6. Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what next? Report of WHO meeting, 14–16 December 2005. Cairo, Egypt. www.who.int/cholera/en/ (accessed April 20, 2013). 7. Martin S, Costa A, Perea W. Stockpiling oral cholera vaccine. Bull World Health Organ 2012;90:714. www.who.int/bulletin/volumes/90/10/12-112433/en/index.html# (accessed April 17, 2013). 8. WHO Regional Office for Africa; Centers for Disease Control and Prevention. Technical guidelines for integrated disease surveillance and response in the African Region. www.cdc.gov/idsr/focus/surv_sys_strengthening/tech_guidelines-integrateddiseaseENG.pdf (accessed April 17, 2013). 9. Blauernfeind A, Crosier A, Fesselet J-F, et al. Cholera guidelines, 2004. 2nd ed. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf (accessed April 17, 2013). 10. Centers for Disease Control and Prevention Guidelines Working Group. Updated guidelines for evaluating public health surveillance systems. MMWR Recomm Rep 2001;50(RR13). www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm (accessed April 17, 2013). 11. Ivers LC, Farmer PE, Pape WJ. Oral cholera vaccine and integrated cholera control in Haiti. Lancet. 2012;379:2026–8. 12. International Centre for Diarrhoeal Disease Research, Bangladesh. Introduction of cholera vaccine project in Bangladesh (ICVB) [press release]. www.icddrb.org/work-with-us/tender- 89 notices/doc_download/85-introduction-of-cholera-vaccine-project-in-bangladesh-icvb (accessed April 17, 2013). 13. Lamond E, Kinyanjui J, Cholera outbreak guidelines—preparedness, prevention, and control. Oxford, UK: Oxfam GB; 2012. http://policypractice.oxfam.org.uk/publications/cholera-outbreak-guidelines-preparedness-preventionand-control-237172 (accessed April 17, 2013). 14. Schaetti C, Ali SM, Chaignat CL, Khatib AM, Hutubessy R, Weiss MG. Improving community coverage of oral cholera mass vaccination campaigns: lessons learned in Zanzibar. PLoS ONE 2012a;7:e41527. 15. Schaetti C, Ali SM, Hutubessy R, Khatib AM, Chaignat CL, Weiss MG. Social and cultural determinants of oral cholera vaccine uptake in Zanzibar. Hum Vaccin Immunother 2012b;8:1223–9. 16. Grad YH, Miller JC, Lipsitch M. Cholera modeling: challenges to quantitative analysis and predicting the impact of interventions. Epidemiology 2012;23:523–30. 17. COTS Program. Cholera outbreak training and shigellosis (COTS). http://www.cotsprogram.com/wordpress/ (accessed April 17, 2013). 18. Ali M, Lopez AL, You YA, et al. The global burden of cholera. Bull World Health Organ. 2012;90:209–18A. 19. International Vaccine Institute. An investment case for the accelerated introduction of oral cholera vaccines. www.ivi.int (accessed April 17, 2913). 20. Bliss K, Bowe K. Paths forward for the global water sanitation and hygiene (WaSH) sector. A report of the CSIS Global Water Futures Project. Washington, DC: Center for Strategic and International Studies; 2010. www.csis.org/files/publication/100630_Bliss_PathsForward_Web.pdf (accessed April 17, 2013). 21. Fry SK. Cholera prevention and control: guidelines for assessing the options in water supply, sanitation and hygiene education. 1992. USAID WASH field report no. 380. http://pdf.usaid.gov/pdf_docs/PNACA703.pdf (accessed April 20, 2013). 90 ABBREVIATIONS AND ACRONYMS USED IN THIS DOCUMENT AFENET Africhol C4 CDC CHOZAN CTC CTU DOMI DRC EPI GHESKIO GIS HERMES ICDDR,B ICG ICVB IDSR IHR IP IV IVI MIC MoH MSF NGO OCV ORP ORS PIH PU-AMI RDT SMART TCBS TTGA TWG UNICEF USAID WaSH WHO African Field Epidemiology Network African Cholera Surveillance Network Cholera Command and Control Centre [in Africa] US Centers for Disease Control and Prevention Preemptive Use of Oral Cholera Vaccination in High-Risk Populations in Zanzibar cholera treatment center cholera treatment unit Diseases of the Most Impoverished Democratic Republic of the Congo Expanded Program on Immunization Haitian Group for the Study of Kaposi's Sarcoma and Opportunistic Infections geographic information system Highly Extensible Resource for Modeling Supply Chains International Centre for Diarrhoeal Disease Research, Bangladesh WHO International Coordinating Group Introduction of Cholera Vaccine in Bangladesh Integrated Diseases and Response Systems International Health Regulations Institut Pasteur intravenous International Vaccine Institute minimal inhibitory concentration Ministry of Health Médecins Sans Frontières nongovernment organization oral cholera vaccine oral rehydration point oral rehydration solution Partners in Health Première Urgence–Aide Médicale Internationale rapid diagnostic test Sensitive Membrane Antigen Rapid Test thiosulfate citrate bile salt sucrose [agar] taurocholate-tellurite gelatin agar WHO Thematic Working Group United Nations Children’s Fund U.S. Agency for International Development water, sanitation, and hygiene World Health Organization 91 92 BIBLIOGRAPHY (Alphabetical List of References Cited in this Document) Africhol. Africhol in Guinea [processed content partly available]. www.africhol.org/country/guinea Africhol. Report on cholera epidemiology in the Democratic Republic of Congo in 2011 [in French]. www.africhol.org/news/drc/report-cholera-epidemiology-democratic-republiccongo-2011 Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection–derived immunity in an endemic setting. J Infect Dis 2011:204:912–8. www.ncbi.nlm.nih.gov/pubmed/?term=Natural+cholera+infection%E2%80%93derived+i mmunity+in+an+endemic+setting Ali M, Lopez AL, You YA, et al. The global burden of cholera. Bull World Health Organ 2012;90:209–18A. www.who.int/bulletin/volumes/90/3/11-093427/en/ Andrews JR, Basu S. Transmission dynamics and control of cholera in Haiti: an epidemic model. Lancet 2011;377:1248–55. www.ncbi.nlm.nih.gov/pubmed/21414658 Azman AS, Luquero FJ, Rodrigues A, et al. Urban cholera transmission hotspots and their implications for reactive vaccination: evidence from Bissau City, Guinea Bissau. PLoS Negl Trop Dis 2012;6:e1901. www.ncbi.nlm.nih.gov/pubmed/?term=Urban+cholera+transmission+hotspots+and+their +implications+for+reactive+vaccination%3A+evidence+from+Bissau+City%2C+Guinea+ Bissau Bardhan PK. Acute infectious diarrhoea. In: Rakel RE, Bope ET, editors. Conn’s current therapy 2005. Philadelphia: Saunders; 2005:18–24. Barzilay EJ, Schaad N, Magloire R, et al. Cholera surveillance during the Haiti epidemic—the first 2 years. N Engl J Med 2013:368:599–609. www.ncbi.nlm.nih.gov/pubmed/?term=Cholera+surveillance+during+the+Haiti+epidemic %E2%80%94the+first+2+years. Benenson AS, Islam MR, Greenough WB 3rd. Rapid identification of Vibrio cholerae by darkfield microscopy. Bull World Health Organ 1964;30:827–31. http://apps.who.int/iris/bitstream/10665/73717/1/bulletin_1964_30%286%29_827831.pdf Bhuiyan NA, Qadri F, Faruque AS, et al. Use of dipsticks for rapid diagnosis of cholera caused by Vibrio cholerae O1 and O139 from rectal swabs. J Clin Microbiol 2003;41;3939–41. www.ncbi.nlm.nih.gov/pubmed/?term=Use+of+dipsticks+for+rapid+diagnosis+of+cholera +caused+by+Vibrio+cholerae+O1+and+O139+from+rectal+swabs Blauernfeind A, Crosier A, Fesselet J-F, et al. Cholera guidelines, 2004. 2nd ed. www.bvsde.paho.org/texcom/cd045364/choleraguide.pdf Bliss K, Bowe K. Paths forward for the global water sanitation and hygiene (WaSH) sector. A report of the CSIS Global Water Futures Project. Washington, DC: Center for Strategic and International Studies; 2010. www.csis.org/files/publication/100630_Bliss_PathsForward_Web.pdf Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS ONE 2009;4:e6320. www.ncbi.nlm.nih.gov/pubmed/?term=Mobile+phone+based+clinical+microscopy+for+gl obal+health+applications CDC. Cholera—Vibrio cholerae infection. General information. www.cdc.gov/cholera/general/ 93 CDC. Community health worker training materials for cholera prevention and control [also in French]. www.cdc.gov/haiticholera/pdf/Haiti_Eng_MASTER_Haiti.pdf CDC. Global disease detection (GDD) manual. Rapid diagnostic tests for epidemic enteric diseases. Watery diarrhea. Differential diagnosis: outbreaks of acute watery diarrhea. www.cdc.gov/cholera/pdf/gdd_manual_cholera_chapters_2012_1_11-508c.pdf CDC. Laboratory methods for the diagnosis of epidemic dysentery and cholera. www.cdc.gov/cholera/pdf/Laboratory-Methods-for-the-Diagnosis-of-Epidemic-Dysenteryand-Cholera.pdf CDC Guidelines Working Group. Updated guidelines for evaluating public health surveillance systems. MMWR Recomm Rep 2001;50(RR13). www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm Chaignat CL, Monti V, Soepardi J, et al. Cholera in disasters: do vaccines prompt new hopes? Expert Rev Vaccines 2008;7:431–35. www.ncbi.nlm.nih.gov/pubmed/?term=Cholera+in+disasters%3A+do+vaccines+prompt+ new+hopes%3F Chao DL, Halloran ME, Longini IM. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. PNAS Early Edition: www.pnas.org/cgi/doi/10.1073/pnas.1102149108 Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet 1986;2:124–7. www.ncbi.nlm.nih.gov/pubmed/2873397 Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990;335:270–3. www.ncbi.nlm.nih.gov/pubmed/1967730 Columbia University, Professor Virginia Cornish working to engineer yeast to detect cholera. http://news.columbia.edu/research/2806 COTS Program. Cholera outbreak training and shigellosis (COTS). www.cotsprogram.com/wordpress/ Date KA, Vicari A, Hyde TB, et al. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis 2011;17:2105–12. wwwnc.cdc.gov/eid/article/17/11/11-0822_article.htm Deen J, von Seidlein L, Clemens J. Multidisciplinary studies of disease burden in the Diseases of the Most Impoverished Programme. J Health Popul Nutr 2004;22:232–9. www.ncbi.nlm.nih.gov/pubmed/?term=Multidisciplinary+studies+of+disease+burden+in+t he+Diseases+of+the+Most+Impoverished+Programme Desai NS, Clemens JD. An overview of cholera vaccines and their public health implications. Curr Opin Pediatr 2012;24:85–91. www.ncbi.nlm.nih.gov/pubmed/?term=An+overview+of+cholera+vaccines+and+their+pu blic+health+implications DiCesare D, DuPont HL, Mathewson JJ, et al. A double blind, randomized, placebo-controlled study of SP-303 (Provir) in the symptomatic treatment of acute diarrhea among travelers to Jamaica and Mexico. Am J Gastroenterol 2002;97:2585–8. www.ncbi.nlm.nih.gov/pubmed/?term=A+double+blind%2C+randomized%2C+placebocontrolled+study+of+SP303+%28Provir%29+in+the+symptomatic+treatment+of+acute+diarrhea+among+travele rs+to+Jamaica+and+Mexico Dubois AE, Sinkala M, Kalluri P, Makasa-Chikoya M, Quick RE. Epidemic cholera in urban Zambia: hand soap and dried fish as protective factors. Epidemiol Infect 2006;134:1226– 30. www.ncbi.nlm.nih.gov/pmc/articles/PMC2870514/ Fischer Walker CL, Fontaine O, Young MW, Black RE. Zinc and low osmolarity oral rehydration salts for diarrhea: a renewed call to action. Bull World Health Organ 2009;87:780–6. www.who.int/bulletin/volumes/87/10/08-058990/en/ 94 Fry SK. Cholera prevention and control: guidelines for assessing the options in water supply, sanitation and hygiene education. 1992. USAID WASH field report no. 380. http://pdf.usaid.gov/pdf_docs/PNACA703.pdf Goma Epidemiology Group. Public health impact of the Rwandan refugee crisis: what happened in Goma, Zaire in July 1994? Lancet 1995;345:359–61. www.ncbi.nlm.nih.gov/pubmed/?term=Public+health+impact+of+the+Rwandan+refugee +crisis%3A+what+happened+in+Goma%2C+Zaire+in+July+1994%3F Grad YH, Miller JC, Lipsitch M. Cholera modeling: challenges to quantitative analysis and predicting the impact of interventions. Epidemiology 2012;23:523–30. www.ncbi.nlm.nih.gov/pubmed/?term=Cholera+modeling%3A+challenges+to+quantitativ e+analysis+and+predicting+the+impact+of+interventions Guerra J, Mayana B, Djibo A, Manzo ML, Llosa AE, Grais RF. Evaluation and use of surveillance system data toward the identification of high-risk areas for potential cholera vaccination: a case study from Niger. BMC Res Notes 2012;5:231. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+and+use+of+surveillance+system+dat a+toward+the+identification+of+highrisk+areas+for+potential+cholera+vaccination%3A+a+case+study+from+Niger Gujral L, Sema C, Gessner B, Jani I. Cholera epidemiology in Mozambique using national surveillance data. J Infect Dis 2013. In press. Harris JR, Cavallaro EC, de Nobrega AA, et al. Field evaluation of crystal VC Rapid Dipstick test for cholera during a cholera outbreak in Guinea-Bissau. Trop Med Int Health 2009;14:1117–21. www.ncbi.nlm.nih.gov/pubmed/?term=Field+evaluation+of+crystal+VC+Rapid+Dipstick+ test+for+cholera+during+a+cholera+outbreak+in+Guinea-Bissau Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet 2012;379:2466– 76. Hinman AR, Farmer PE. The Coalition for Cholera Prevention and Control meeting [letter]. Vaccine 2013;31:2323. Hirschhorn N, Chowdhury AK, Lindenbaum J. Cholera in pregnant women. Lancet 1969;293:1230–2. ICDDR.B. Management of diarrhea at ICDDR.B Hospital. www.icddrb.org/what-wedo/publications/cat_view/52-publications/10042-icddrb-periodicals/10078glimpse/10292-vol-31-no-2-2009/11072-management-of-diarrhoea-at-icddrb-hospital Institute for Operations Research and Management Sciences. Operations research: the science of better. What operations research is. www.scienceofbetter.org/what/index.htm International Centre for Diarrhoeal Disease Research, Bangladesh. Introduction of cholera vaccine project in Bangladesh (ICVB) [press release]. www.icddrb.org/work-withus/tender-notices/doc_download/85-introduction-of-cholera-vaccine-project-inbangladesh-icvb International Federation of Red Cross and Red Crescent Societies. Epidemic control for volunteers; a training manual. www.ifrc.org/Global/Publications/Health/epidemic-controlen.pdf International Vaccine Institute. An investment case for the accelerated introduction of oral cholera vaccines. www.ivi.int Ivers LC, Farmer PE, Pape WJ. Oral cholera vaccine and integrated cholera control in Haiti. Lancet. 2012;379:2026–8. Kalluri P, Naheed A, Rahman S, et al. Evaluation of three rapid diagnostic tests for cholera: does the skill level of the technician matter? Trop Med Int Health 2006;11:49–55. /www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+three+rapid+diagnostic+tests+for+ cholera%3A+does+the+skill+level+of+the+technician+matter%3F 95 Khan MR, Rahman ME. Community pediatrics. In: Essence of pediatrics. 3rd ed. Dhaka: Mrs. Anwara Khan; 2004:143–98. Khatib AM, Ali M, von Seidlein L, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis 2012;12:837–44. www.ncbi.nlm.nih.gov/pubmed/?term=Effectiveness+of+an+oral+cholera+vaccine+in+Za nzibar%3A+findings+from+a+mass+vaccination+campaign+and+observational+cohort+ study Lamond E, Kinyanjui J. Cholera outbreak guidelines, preparedness, prevention and control. Oxford, UK: OXFAM GB; 2012. http://reliefweb.int/sites/reliefweb.int/files/resources/mlcholera-guidelines-preparedness-prevention-and-control-030512-en.pdfOXFAM Lanata CF, Mendoza W, Black RE. Improving diarrhoea estimates. Geneva: World Health Organization; 2002. Legros D, Paquet C, Marty I, et al. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp. Bull World Health Organ 1999;77:837–42. www.who.int/bulletin/archives/77%2810%29837.pdf Li Z, Nickkholgh A, Yi X, et al. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res 2009;46:365–72. www.ncbi.nlm.nih.gov/pubmed/?term=Melatonin+protects+kidney+grafts+from+ischemia %2Freperfusion+injury+through+inhibition+of+NFkB+and+apoptosis+after+experimental+kidney+transplantation Logistics Cluster. Logistics operational guide. http://log.logcluster.org/ Longini IM Jr, Nizam A, Ali M, et al. Controlling endemic cholera with oral vaccines. PLoS Med 2007;4:e336. www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0040336 Lucas ME, Deen JL, von Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352:757–67. www.ncbi.nlm.nih.gov/pubmed?term=Effectiveness%20of%20mass%20oral%20cholera %20vaccination%20in%20Beira,%20Mozambiqu[all]&cmd=correctspelling Martin S, Costa A, Perea W. Stockpiling oral cholera vaccine. Bull World Health Organ 2012;90:714. www.who.int/bulletin/volumes/90/10/12-112433/en/index.html Médecins Sans Frontières. Operational research in Médecins Sans Frontières. www.msf.lu/fileadmin/WEBLibrary/2_Au_Luxembourg/OR_Brochure_EN.pdf Ministry of Health, Democratic Republic of the Congo. National plan for the elimination of cholera 2013–2017 [in French]. www.africhol.org/sites/africhol.org/files/files/RDC_PlanEliminationCholera20132017_20130228.pdf Ministry of Health, Indonesia; WHO; Global Outbreak Alert and Response Network (GOARN) partners; Centers for Disease Control and Prevention—USA; Epicentre—France; European Programme for Intervention Epidemiology Training (EPIET)—Sweden, et al. Epidemic-prone disease surveillance and response after the tsunami in Aceh, Indonesia. Euro Surveill. 2005;10:pii=2700. www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2700 96 Ministry of Health, Southern Sudan. Cholera epidemic preparedness and response guidelines for southern Sudan, March, 2011. www.google.com.bd/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&sqi=2&ved=0CCsQ FjAA&url=http%3A%2F%2Fwww.mwrigoss.org%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid %3D19%26Itemid%3D8&ei=u6B_UfHLoTRrQfaooGwAQ&usg=AFQjCNGuaraEUXvX_x7GijaqUXDrCqNWA&bvm=bv.45921128,d.bmk Ministry of Health and Child Welfare/WHO. Zimbabwe cholera control guidelines. 3rd ed. http://medmissio.de/surf/proxy/alfrescosystem/api/node/content/workspace/SpacesStore/dd738528-1187-4575-847d7de7eb6a4c24/Zimbabwe%20cholera%20control?a=true Ministry of Health and Population in Haiti/Centers for Disease Control and Prevention. Haiti cholera training manual: a full course for healthcare providers [also in French]. www.cdc.gov/haiticholera/pdf/haiticholera_trainingmanual_en.pdf Ministry of Public Health, Authority for Disease Control; BELICHOL: bulletin du projet d’elimination du choléra en RDC. www.minisanterdc.cd/Articles/d4/BELICHOL%20DEC3.pdf Moser B, Barella L, Mattei S, et al. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J 1993;294(Pt. 1):285– 92. www.ncbi.nlm.nih.gov/pubmed/?term=Expression+of+transcripts+for+two+interleukin+8 +receptors+in+human+phagocytes%2C+lymphocytes+and+melanoma+cells Mukherjee P, Ghosh S, Ramamurthy T, et al. Evaluation of a rapid immunochromatographic dipstick kit for diagnosis of cholera emphasizes its outbreak utility. Jpn J Infect Dis 2010;63:234–8. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+a+rapid+immunochromatographic+ dipstick+kit+for+diagnosis+of+cholera+emphasizes+its+outbreak+utility Nato F, Boutonnier A, Rajerison Grosjean MP, et al. One-step immunochromatographic dipstick tests for rapid detection of Vibrio cholerae O1 and O139 in stool samples. Clin Diagn Lab Immunol 2003;10:476–8. www.ncbi.nlm.nih.gov/pubmed/?term=Onestep+immunochromatographic+dipstick+tests+for+rapid+detection+of+Vibrio+cholerae+ O1+and+O139+in+stool+samples Nelson EJ, Chowdhury A, Harris JB, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in transmission of infectious diarrhea. Proc Natl Acad Sci U S A 2007;104:19091–6. www.ncbi.nlm.nih.gov/pubmed/?term=Complexity+of+ricewater+stool+from+patients+with+Vibrio+cholerae+plays+a+role+in+transmission+of+infe ctious+diarrhea Ouedraogo RT, Njanpop-Lafourcade B-M, Jaillard P, et al. Mobile laboratory to improve response to meningitis epidemics, Burkina Faso epidemic season 2004, Field Actions Science Reports 2008;1. http://factsreports.revues.org/144 Pan American Health Organization. Controlling cholera: a checklist for planners. http://bvs.per.paho.org/texcom/colera/controlling.pdf Paramount Industries. Family hygiene kits (UNICEF type). http://paramounttent.com/familyhygiene-kit-unicef-type/ Partners In Health. Community health worker cholera training. www.pih.org/publications/entry/community-health-worker-cholera-training-manuals PATH [Program for Appropriate Technology in Health], WHO. Project Optimize. Achieving the 2020 global vision for immunization supply and logistics. September 2012. www.who.int/immunization_delivery/optimize/Achieving_global_vision_for_future_immun ization_supply_and_logistics_systems.pdf 97 PATH [Program for Appropriate Technology in Health]. Project Optimize. Building next‐generation vaccine supply systems. www.path.org/publications/files/TS_opt_evm_hermes_fs.pdf Piarroux R, Barrais R, Faucher B, et al. Understanding the cholera epidemic, Haiti. Emerg Infect Dis 2011;17:1161–8. http://dx.doi.org/10.3201/eid1707.110059 Qadri F, Hasan JA, Hossain J, et al. Evaluation of the monoclonal antibody–based kit Bengal SMART for rapid detection of Vibrio cholerae O139 synonym Bengal in stool samples. J Clin Microbiol 1995;33:732–4. www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+the+monoclonal+antibody%E2%80 %93based+kit+Bengal+SMART+for+rapid+detection+of+Vibrio+cholerae+O139+synony m+Bengal+in+stool+samples Quick RE, Thompson BL, Zuniga A, et al. Epidemic cholera in rural El Salvador: risk factors in a region covered by a cholera prevention campaign, Epidemiol Infect 1995;114:249–55. http://europepmc.org/articles/PMC2271272/pdf/epidinfect00050-0027.pdf Rahman M, Sack DA, Mahmood S, Hossain A. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J Clin Microbiol 1987;25:2204–6. www.ncbi.nlm.nih.gov/pubmed/3693549 Rajasingham A, Bowen A, O’Reilly C, et al. Cholera prevention training materials for community health workers, Haiti, 2010–2011. Emerg Infect Dis 2011;17:2162–5. http://dx.doi.org/10.3201/eid1711.110806 Republic of Kenya. Multi-sectoral cholera prevention and control plan, 2011 to 2016. Draft. September, 2011. www.unicef.org/cholera/Annexes/Supporting_Resources/Annex_6D/Kenya_M_Public_H ealth_and_Sanitation-preparedness_plan.docx Reyburn R, Deen JL, Grais RF, et al. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis 2011;5:e952. www.ncbi.nlm.nih.gov/pubmed/21283614 Ribeiro-Neto FA, Mattera R, Hildebrandt JD, et al. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol 1985;109:566–72. Rohrlich J. Why FedEx and UPS want the Postal Service to survive. www.minyanville.com/business-news/editors-pick/articles/postal-service-usps-postoffice-post/8/3/2012/id/42951#ixzz2LfF1Aewdhttp://www.minyanville.com/businessnews/editors-pick/articles/postal-service-usps-post-office-post/8/3/2012/id/42951 Sack DA, Cholera burden of disease estimates. www.jhsph.edu/departments/internationalhealth/globalhealthresearch/cholera/index.html Sack DA, Lyke C, McLaughlin C, Suwanvanichkij V. Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. www.who.int/drugresistance/Antimicrobial_resistance_in_shigellosis_cholera_and_cam. pdf Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet 2004;363:223–33. www.ncbi.nlm.nih.gov/pubmed/14738797 Sasaki S, Suzuki H, Igarashi K, Tambatamba B, Mulenga P. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urban area of Lusaka, Zambia. Am J Trop Med Hyg 2008;79:414–21. http://public.beuthhochschule.de/~kred/Literatur/GIS_Med_Geo/PDF/Sasaki_et_al_20081744621058/Sasaki_et_al_2008.pdf Schaetti C, Ali SM, Chaignat C-L, et al. Improving community coverage of oral cholera mass vaccination campaigns: lessons learned in Zanzibar. PLoS ONE 2012;7:e41527. www.ncbi.nlm.nih.gov/pubmed/?term=Improving+community+coverage+of+oral+cholera +mass+vaccination+campaigns%3A+lessons+learned+in+Zanzibar 98 Schaetti C, Ali SM, Hutubessy R, Khatib AM, Chaignat CL, Weiss MG. Social and cultural determinants of oral cholera vaccine uptake in Zanzibar. Hum Vaccin Immunother 2012b;8:1223–9. www.ncbi.nlm.nih.gov/pubmed/?term=Social+and+cultural+determinants+of+oral+choler a+vaccine+uptake+in+Zanzibar Schwartz BS, Harris JB, Khan AI, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg 2006;74:1067–73. www.ncbi.nlm.nih.gov/pubmed/?term=Diarrheal+epidemics+in+Dhaka%2C+Bangladesh %2C+during+three+consecutive+floods%3A+1988%2C+1998%2C+and+2004 Shikanga OT, Mutonga D, Abade M, et al. High mortality in a cholera outbreak in western Kenya after post-election violence in 2008. Am J Trop Med Hyg 2009;81:1085–90. www.ncbi.nlm.nih.gov/pubmed/?term=High+mortality+in+a+cholera+outbreak+in+wester n+Kenya+after+post-election+violence+in+2008 Shin S, Desai SN, Sah BK, Clemens JD. Oral vaccines against cholera. Clin Infect Dis 2011:52;1343–9. www.ncbi.nlm.nih.gov/pubmed/21498389 Sinha A, Sengupta S, Ghosh S, et al. Evaluation of a rapid dipstick test for identifying cholera cases during the outbreak. Indian J Med Res 2012;135:523–8, www.ncbi.nlm.nih.gov/pubmed/?term=Evaluation+of+a+rapid+dipstick+test+for+identifyi ng+cholera+cases+during+the+outbreak Sjoling A, Wiklund G, Savarino SJ, Cohen DI, Svennerholm AM. Comparative analyses of phenotypic and genotypic methods for detection of enterotoxigenic Escherichia coli toxins and colonization factors. J Clin Microbiol 2007;45:3295–301. www.ncbi.nlm.nih.gov/pubmed/?term=Comparative+analyses+of+phenotypic+and+geno typic+methods+for+detection+of+enterotoxigenic+Escherichia+coli+toxins+and+coloniza tion+factors Somali Health Cluster. AWD/Cholera Preparedness and Response Plan [to be updated monthly]. http://healthsomalia.org/alerts_weekly_documents/health_cluster_awdcholera_prepared ness_and_response_plan_feb2012.pdf Speelman P, McGlaughlin R, Kabir I, Butler T. Differential clinical features and stool findings in shigellosis and amoebic dysentery. Trans R Soc Trop Med Hyg 1987;81:549–51. www.ncbi.nlm.nih.gov/pubmed/?term=Differential+clinical+features+and+stool+findings+ in+shigellosis+and+amoebic+dysentery Taylor H, Fesselet J-F, Sozzi E, Curtis T, Mahama A. Cutting the cholera risk—alternative approaches for medical centre wastewater treatment. Water21 2011;13.4;46. www.iwaponline.com/w21/01304/w21013040047.htm Thiem VD, Deen JL, von Seidlein L, et al. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine 2006;24:4297–303. www.ncbi.nlm.nih.gov/pubmed/?term=Longterm+effectiveness+against+cholera+of+oral+killed+wholecell+vaccine+produced+in+Vietnam Trach DD, Clemens JD, Ke NT, et al. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 1997;349:231–5. www.ncbi.nlm.nih.gov/pubmed/?term=Field+trial+of+a+locally+produced%2C+killed%2C +oral+cholera+vaccine+in+Vietnam UNICEF. Cholera prevention: 10 key factors. www.unicef.org/wcaro/07UNICEF_Cholera_Prevention.ppt UNICEF. Cholera toolkit 2013. www.unicef.org/cholera/Cholera-Toolkit-2013.pdf UNICEF. Guidelines on external staff in emergencies. Standby arrangements. www.washcluster.info/?q=download/file/fid/472 99 UNICEF. Supply catalog https://supply.unicef.org/unicef_b2c/app/displayApp/(layout=7.012_1_66_67_115&carea=%24ROOT)/.do?rf=y UNICEF. The Global Immunization Vision and Strategy. www.unicef.org/immunization/index_27089.html [also available at www.who.int/immunization/givs/en/index.html] UNICEF, WHO. Cholera epidemics preparedness. www.unicef.org/wcaro/07Cholera_Prepareness_Joint_UNICEF_WHO_Recommendations.doc United Nations High Commissioner for Refugees. Epidemic preparedness and response in refugee camp settings: guidance for public health officers. October 2011. www.unhcr.org/4f707f509.pdf United Nations Office for the Coordination of Humanitarian Affairs (OCHA). Haiti cholera snapshot [published June 5, 2013]. http://gallery.mailchimp.com/ae620ada5956c2460fcad49f8/files/hti_cholera_Snapshot_J une_2013.pdf University of Vermont. Medical student Wilkie builds portable EHR system for Doctors Without Borders. www.uvm.edu/~uvmpr/?Page=news&storyID=12670&category=ucommall Valenciano M, Moren A. Communicable disease surveillance in complex emergencies. In: M'ikanatha M, Lynfield R, Van Beneden CA, de Valk H, editors. Infectious disease surveillance. Malden, Massachusetts: Blackwell Publishing; 2007:265–80. Waldman RJ, Mintz ED, Papowitz HE. The cure for cholera—improving access to safe water and sanitation. N Engl J Med 2013:368:592–4. www.nejm.org/doi/full/10.1056/NEJMp1214179. Wang X-Y, Ansaruzzaman M, Vaz R, et al. Field evaluation of a rapid immunochromatographic dipstick test for the diagnosis of cholera in a high-risk population. BMC Infect Dis 2006;6:17. www.ncbi.nlm.nih.gov/pubmed/?term=Field+evaluation+of+a+rapid+immunochromatogr aphic+dipstick+test+for+the+diagnosis+of+cholera+in+a+high-risk+population WASH. List of essential hygiene promotion equipment for communication. WASH, 2007. http://pakresponse.info/LinkClick.aspx?fileticket=XvwOOVT_F9k%3D&tabid=92&mid=63 9 WASH Cluster. Hygiene promotion. www.unicefinemergencies.com/downloads/eresource/docs/Clusters/WASH_Cluster_Co ordinator_Handbk_FINAL_VERSION_Jan09.pdf WASH Cluster. Hygiene promotion in emergency—a briefing paper. Introduction to hygiene promotion in emergency: Tools and approaches. www.washcluster.info/?q=content/introduction-hygiene-promotion-0 WASH Cluster. WASH related non food item—a briefing paper. Introduction to hygiene promotion in emergency: tools and approaches. www.washcluster.info/?q=content/introduction-hygiene-promotion-0 WESCOORD Drought Response Kenya. Preparedness and response. Integrated drought and cholera operational plan. 21st September 2011. http://wescoord.or.ke/documents/Keydocs/WESCOORD_CholeraPlan_20110921.pdf WHO. Cholera. www.who.int/cholera/en/ WHO. Cholera 2009. Wkly Epidemiol Rec 2010;85:293–308. www.who.int/wer/2010/wer8531.pdf WHO. Cholera 2010. Wkly Epidemiol Rec 2011;86:325–39. www.who.int/wer/2011/wer8631.pdf WHO. Cholera 2011. Wkly Epidemiol Rec 2012;87:289–304. www.who.int/wer/2012/wer8731_32.pdf WHO. Cholera fact sheet no. 107. July 2012. www.who.int/mediacentre/factsheets/fs107/en/index.html 100 WHO. Cholera outbreak: assessing the outbreak response and improving preparedness. http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFk_2004.4_eng.pdf WHO. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010;85:117–28. WHO. Communicable disease global atlas. http://apps.who.int/globalatlas/ WHO. Communication for behavioural impact (COMBI). A toolkit for behavioural and social communication in outbreak response. http://apps.who.int/iris/bitstream/10665/75170/1/WHO_HSE_GCR_2012.13_eng.pdf WHO. Epidemic diarrhoeal disease preparedness and response. Training and practice. Facilitator’s guide. www.who.int/topics/cholera/publications/WHO_EMC_DIS_97_4Rev_1/en/ WHO. Expanding capacity for operations research in reproductive health: summary report of a consultative meeting. December 10–12, 2001. www.who.int/iris/handle/10665/67936 WHO. Five keys to safer food: train the trainers course. www.who.int/foodsafety/consumer/TrainingCourse.pdf WHO. Global Alert and Response (GAR). Cholera in Sierra Leona—update. www.who.int/csr/don/2012_10_08/en/ WHO. Global Alert and Response (GAR). Integrated disease surveillance. www.who.int/csr/labepidemiology/projects/diseasesurv/en/index.html WHO. Guidance on formulation of national policy on the control of cholera. www.who.int/csr/resources/publications/cholera/whocddser9216rev1.pdf WHO. Guidelines for cholera control. https://apps.who.int/chd/publications/cholera/cholguid.htm WHO. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. www.who.int/topics/shigella/en WHO. Outbreak surveillance and response in humanitarian emergencies—WHO guidelines for EWARN implementation. Geneva: WHO; 2012. WHO. Information, education and communication. Lessons from the past; perspectives for the future. www.who.int/reproductivehealth/publications/health_systems/WHO_RHR_01_22/en/ WHO. Interagency diarrhoeal disease kit. www.who.int/topics/cholera/publications/interagency_diarrhoeal_disease_kit_2009_en.p df WHO. International health regulations (2005). 2nd ed. www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=4015 WHO. Laboratory methods for the diagnosis of epidemic dysentery and cholera, 1999. www.who.int/topics/cholera/publications/WHO_CDS_CSR_EDC_99_8_EN/en/index.htm l WHO. Oral cholera vaccines in mass immunization campaigns. Guidance for planning and use. http://whqlibdoc.who.int/publications/2010/9789241500432_eng.pdf WHO. Outbreak communication. Best practices for communicating with the public during an outbreak. www.who.int/csr/resources/publications/WHO_CDS_2005_32web.pdf WHO. Sierra Leone & Guinea concludes cross border meeting on cholera prevention & control. www.afro.who.int/en/sierra-leone/press-materials/item/5091-sierra-leone--guineaconcludes-cross-border-meeting-on-cholera-prevention--control.html WHO. Treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: WHO; 1990. WHO. Water, sanitation and hygiene in cholera treatment centres in emergencies. Technical notes on drinking water, sanitation and hygiene in emergencies. No. 18. www.washclustermali.org/sites/default/files/wash_in_cholera_treatment_centers_in_eme rgencies_tech_brief_who.pdf WHO. Water, sanitation and hygiene (WASH) in healthcare facilities in emergencies. Adams J, Chartier Y, Harvey B, Maison D, editors. Geneva: WHO; 2012. 101 www.washclustermali.org/sites/default/files/wash_in_health_facilities_in_emergencies_w ho.pdf WHO. What is VVM and how does it work? www.who.int/immunization_standards/vaccine_quality/What%20is%20VVM%20and%20 how%20does%20it%20work.pdf WHO. WHO consultation on oral cholera vaccine (OCV) stockpile strategic framework: potential objectives and possible policy options, http://whqlibdoc.who.int/hq/2012/WHO_IVB_12.05_eng.pdf WHO. WHO report on global surveillance of epidemic-prone infectious diseases. Geneva: WHO; 2000:40. www.who.int/csr/resources/publications/surveillance/en/cholera.pdf WHO. WHO Technical Working Group on creation of an oral cholera vaccine stockpile. www.who.int/cholera/publications/oral_cholera_vaccine/en/index.html WHO Regional Office for Africa; Centers for Disease Control and Prevention. Technical guidelines for integrated disease surveillance and response in the African Region. www.cdc.gov/idsr/focus/surv_sys_strengthening/tech_guidelines-integrateddiseaseENG.pdf WHO Global Task Force on Cholera Control. Acute diarrheal diseases in complex emergencies: critical steps, Decision making for preparedness and response. http://whqlibdoc.who.int/hq/2010/who_cds_cpe_zfk_2004.6_rev.1_eng.pdf WHO Global Task Force on Cholera Control. Cholera vaccines: a new public health tool? http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_ZFK_2004.5.pdf WHO Global Task Force on Cholera Control. First steps for managing an outbreak of acute diarrhoea. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CSR_NCS_2003.7_Rev.2_eng.pdf WHO Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what is next? Report: WHO meeting, 14–16 December 2005, Cairo, Egypt. www.who.int/cholera/publications/cholera_vaccines_emergencies_2005.pdf WHO Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. www.who.int/cholera/technical/prevention/control/en/index.html WHO, UNICEF. Rapid assessment of drinking water quality. A handbook for implementation. October 2012. www.wssinfo.org/fileadmin/user_upload/resources/RADWQHandbookv1final.pdf WHO, UNICEF. WHO/UNICEF joint statement on clinical management of acute diarrhea. www.who.int/maternal_child_adolescent/documents/who_fch_cah_04_7/en/index.html World Health Assembly. Cholera: mechanism for control and prevention. 24 May 2011. http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_R15-en.pdf Zachariah R, Ford N, Draguez B, Yun O, Reid T. Conducting operational research within a nongovernmental organization: the example of Médecins Sans Frontières. International Health 2010;2:1–8. Zachariah R, Harries AD, Ishikawa K, et al. Operational research in low-income countries: what, why, and how? Lancet Infect Dis 2009;9:711–7. www.ncbi.nlm.nih.gov/pubmed/19850229 Zimbabwe Ministry of Health and Child Welfare, WHO. Intersectoral actions in response to cholera in Zimbabwe: from emergency response to institution building. www.who.int/sdhconference/resources/draft_background_paper23_zimbabwe.pdf Zuckerman J, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect Dis 2007;7:521–30. www.ncbi.nlm.nih.gov/pubmed/?term=The+true+burden+and+risk+of+cholera%3A+impli cations+for+prevention+and+control 102 Zurovac D, Talisuna AO, Snow RW. Mobile phone text messaging: tool for malaria control in Africa. PLoS Med 2012;9:e1001176. www.ncbi.nlm.nih.gov/pmc/articles/PMC3283546/ 103 104
© Copyright 2026