Calculating Molality - I
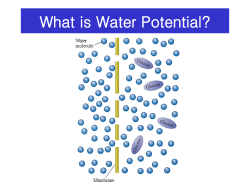
Calculating Molality - I To calculate Molality (mol/kg), we need the number of moles of solute, and the mass of solvent used to dissolve the solute! Normally, we are given the mass of solute, and mass of solvent, therefore we calculate the moles of the solute from the mass, then use the mass of the solvent to calculate the molality. Remember: Molality is different from Molarity Mass (g) of solute M (g/mole) Molality is based on mass, and is independent of temperature or pressure (unlike molarity) Because 1 L of H2O weighs 1 kg, molality and molarity of dilute aquous solutions are nearly identical Moles of Solute divide by kg water Molality (m) of solution 1 Calculating Molality - II Problem: Determine the molality and molarity of a solution prepared by dissolving 75.0g Ba(NO3)2 (s) in to 374.00g of water at 250C. Plan: We convert the quantity of Ba(NO3)2 to moles using the molar mass and then divide by the volume of H2O in liters (using water density = 0.99707 g/ml3). Solution: molar mass of Ba(NO3)2 = 261.32 g/mol 75.0 g moles Ba(NO3)2 = = 0.28700 mole 261.32 g/mol molality = 0.28700 mole = 0.76739 m = 0.767 m 0.37400 kg molarity - we need the volume of solution, and can assume that addition of the salt did not change the total volume. 374.00 g H2O = 375.099 ml = 0.375099 l 0.99707 g/ml M = 0.28700 mole = 0.765 M 0.375099 l Expressing Concentrations in Parts by Mass Problem: Calculate the PPB by mass of Iron in a 1.85 g Iron supplement pill that contains 0.0543 µg of Iron. Plan: Convert µg Fe to grams and then use Fe/ mass pill and multiply by 109 to obtain PPB. Solution: 0.0543µg Fe = 5.43 x 10 - 8 g Fe 5.43 x 10 - 8 g Fe x 109 = 2.94 PPB 1.85 g 2 Expressing Concentrations in Parts by Volume Problem: The label on a can of beer (340 ml) indicates “4.5% alcohol by volume”. What is the volume in liters of alcohol it contains? Plan: we know the vol% and the total volume so we use the definition to find the volume of alcohol. Solution: Vol Alcohol = 4.5 ml alcohol x 340 ml beer 100 ml beer = 15.3 ml alcohol Expressing Concentrations in Mole Fraction Problem: A sample of alcohol contains 118g of ethanol (C2H5OH), and 375.0g of water. What are the mole fractions of each? Plan: we know the mass and formula of each compound so we convert both to moles and apply the definition of mole fraction. Solution: Moles Ethanol = 118g Ethanol = 2.744 mol Ethanol 43g Ethanol/mol Moles Water = 395g H2O = 21.94 mol H O 2 18g H2O/mol XEthanol = XWater = 2.744 = 0.11117 21.94 +2.744 21.94 21.94 +2.744 = 0.88883 3 Converting Concentration Units Problem: Commercial concentrated Hydrochloric acid is 11.8 M HCl and has a density of 1.190g/ml. Calculate the (a) mass% HCl, (b) molality and (c) mole fraction of HCl. Plan: We know Molarity and density. (a) For mass% HCl we need the mass of HCl and water (the solvent). Assume 1L of solution, from the density we know the mass of the solution, and from the molecular mass of HCl we calculate its mass. (b) We know moles of HCl and mass of water (c) we use moles HCl from(a) and use the mass of water to get moles of water then calculate mole fractions and add them to check! Solution: (a) assume 1L of HCl solution 11.8 moles HCl x 36.46g HCl mole HCl 11.8 moles HCl = 430.228g HCl Converting Concentration Units - II (a) cont. 1L solution x 1000 mL x 1.190 g soln = 1190g solution 1 L solution mL soln mass % HCl = 430.228 g HCl x 100% = 36.1536 % HCl 1190.g solution (b) mass of H2O = mass of solution - mass of HCl = 1190g solution - 430.228g HCl = 759.772g H2O 1190g solution 11.8 moles HCl = 15.53 m HCl 0.759772 kg H2O (c) 759.772g H2O = 42.172 mole H2O 18.016g H2O/mol H2O XHCl = 11.8 = 0.219 54.0 XH2O =42.172 = 0.781 54.0 Total moles = 42.172 + 11.8 = 53.972=54.0 4 Definitions Saturated - A solution containing the maximum amount of solute that will dissolve under a given set of conditions. Unsaturated - A solution that contains less than the saturation quantity of solute. More solute may be added and it will dissolve. Supersaturated - A solution prepared at an elevated temperature and then slowly cooled so that more than the usual maximum amount of solute remains dissolved. Equilibrium in a Saturated Solution 5 For all gasses: ∆Hsolute ≈ 0, so ∆Hsoln < 0 Gas solubility always decreases with increasing T! The Effect of Pressure on Gas Solubility: Henry’s Law: Sgas = kH x Pgas 6 Henry’s Law of Gas Solubility Problem: The lowest level of oxygen gas dissolved in water that will support life is ~ 1.3 x10-4 mol/L. At the normal atmospheric pressure of oxygen is there adequate oxygen to support life in water? Plan: We will use Henry’s law and the Henry’s law constant for oxygen in water with the partial pressure of O2 in the air to calculate the amount of O2 dissolved in water under atmospheric conditions. Solution: The Henry’s law constant for oxygen in water is 1.3 x 10 -3 mol liter atm and the partial pressure of oxygen gas in the atmosphere is ~21%, or 0.21 atm. Soxygen = kH x PO2 = 1.3 x 10 -3 mol x (0.21 atm) liter atm . . SOxygen = 2.7 x 10 - 4 mol O2 / liter This is adequate to sustain life in water! Effect of Temperature: Heat of Solution Solubility increases with temperature if the solution process is endothermic: Solute + Solvent + Heat Solution Solubility decreases with temperature if the solution process is exothermic: Solute + Solvent Solution + Heat 7 Predicting the Effect of Temperature on Solubility - I Problem: From the following information, predict whether the solubility of each compound increases or decreases with an increase in temperature. (a) CsOH Hsoln = -72 kJ/mol (b) When CsI dissolves in water the water becomes cold (c) KF(s) H2O K+(aq) + F -(aq) + 17.7 kJ Plan: Write a chemical reaction that includes heat being absorbed (left) or released (right). If heat is on the left, a temperature increase shifts to the equilibrium to the right, so more solute dissolves, and visa versa. Solution: (a) The negative H indicates that the reaction is exothermic, so when one mole of Cesium Hydroxide dissolves, 72 kJ of heat is released. 8 Predicting the Effect of Temperature on Solubility - II (a) continued CsOH(s) H2O Cs+(aq) + OH -(aq) + Heat A higher temperature (more heat) decreases the solubility of CsOH. (b) When CsI dissolves, the solution becomes cold, so heat is absorbed. H2O CsI(s) + Heat Cs+(aq) + I -(aq) A higher temperature increases the solubility of CsI. (c) When KF dissolves, heat is on the product side, and is given off so the reaction is exothermic. H2O K+(aq) + F -(aq) + Heat KF(s) A higher temperature decreases the solubility of KF Colligative Properties of Solutions 17.4) Vapor Pressure Lowering - Raoult’s Law 17.5) Boiling Point Elevation and Freezing Point Depression 17.6) Osmotic Pressure We’ll start by focusing on nonvolatile nonelectrolitic solutes (e.g. sucrose in H2O) Later: volatile solutes, electrolytic solutes 9 Raoult’s Law: Psolvent = Xsolvent . P°solvent X is the mole fraction See Figure 17.9 Figure 17.8, Zumdahl 10 Example: Vapor Pressure Lowering Problem: Calculate the vapor pressure lowering (∆P) when 175g of sucrose is dissolved into 350.00 ml of water at 750C. The vapor pressure of pure water at 750C is 289.1 mm Hg, and it’s density is 0.97489 g/ml. Plan: Calculate the change in pressure from Raoult’s law using the vapor pressure of pure water at 750C. We calculate the mole fraction of sugar in solution using the molecular formula of sucrose and density of water at 750C. Solution: molar mass of sucrose ( C H O ) = 342.30 g/mol 12 22 11 175g sucrose = 0.51125 mol sucrose 342.30g sucrose/mol 350.00 ml H2O x 0.97489g H2O = 341.21g H2O ml H2O 341.21 g H2O = 18.935 mol H2O 18.02g H2O/mol Vapor Pressure Lowering (cont) Vapor Pressure lowering: ∆P = Xsolute . P°solvent Xsucrose = = mole sucrose moles of water + moles of sucrose 0.51125 mole sucrose = 0.2629 18.935 mol H2O + 0.51125 mol sucrose ∆P = Xsucrose * PoH2O = 0.2629 x 289.1 mm Hg = 7.600 mm Hg 11 Boiling Point Elevation Recall: The normal boiling point (Tb) of a liquid is the temperature at which its vapor pressure equals atmospheric pressure. Nonvolatile solutes lower the vapor pressure of a liquid Æ greater temperature required to reach boiling point Boiling Point Elevation: ∆Tb = Kbmsolute Kb = Molal boiling point constant for given liquid msolute = molal solute concentration Kb = Molal boiling point constant for given liquid See Table 17.5 12 Freezing Point Depression: ∆Tf = Kfmsolute See Figure 17.3 Kf = Molal freezing point constant for given liquid See Table 17.5 13 See Figure 17.12 Example: Boiling Point Elevation and Freezing Point Depression in an aqueous solution Problem: We add 475g of sucrose (sugar) to 600g of water. What will be the Freezing and Boiling points of the solution? Plan: Find the molality of the sucrose solution, and apply the equations for FP depression and BP elevation using the constants from table 17.5. Solution: Sucrose (C12H22O11) has molar mass = 342.30 g/mol 475g sucrose = 1.388 mole sucrose 342.30gsucrose/mol molality = 1.388 mole sucrose = 2.313 m 0.600 kg H2O oC ∆Tb = Kb . m = 0.512 m (2.313m)= 1.18oC BP = 100.00oC + 1.18oC = 101.18oC o ∆Tf = Kf . m = 1.86 C(2.313 m) = 4.30oC m FP = 0.00oC -4.30oC= -4.30oC 14 Example: Boiling Point Elevation and Freezing Point Depression in a Non-Aqueous Solution Problem: Calculate the Boiling Point and Freezing Point of a solution having 257g of napthalene (C10H8) dissolved into 500.00g of chloroform (CHCl3). Plan: Just like the first example. Solution: napthalene = 128.16g/mol chloroform = 119.37g/mol molesnap = 257g nap =2.0053 mol nap 128.16g/mol molality = moles nap = 2.0053 mol= 4.01 m kg(CHCl3) 0.500 kg o ∆Tb = Kb . m = 3.63 C(4.01m) = 14.56oC normal BP = 61.7oC m new BP = 76.3oC o ∆Tf = Kf . m =4.70 C (4.01m) =18.85oC normal FP = - 63.5oC m new FP = - 82.4oC Osmosis: The flow of solvent through a semipermeable membrane into a solution The semipermeable membrane allows solvent molecules to pass, but not solute molecules See Figures 17.15, 17.16 15 Osmotic Pressure, Π = MRT (similar to ideal gas law!) R = gas constant M = molar concentration of solute Determining Molar Mass from Osmotic Pressure Problem: A physician studying hemoglobin dissolves 21.5mg of the protein in water at 5.0oC to make 1.5 ml of solution in order to measure its osmotic pressure. At equilibrium, the solution has an osmotic pressure of 3.61 torr. What is the molar mass (M) of the hemoglobin? Plan: We know Π, R, and T. We convert Π from torr to atm, and T from oC to K, and then use the osmotic pressure equation to solve for molarity (M). Then we calculate the number of moles of hemoglobin from the known volume and use the known mass to find M. Solution: P = 3.61 torr . 1 atm = 0.00475 atm 760 torr Temp = 5.00C + 273.15 = 278.15 K 16 Molar Mass from Osmotic Pressure Concentration from osmotic pressure: M = Π = 0.00475 atm RT 0.082 L atm (278.2 K) mol K = 2.08 x10 - 4 M Finding # moles of solute: -4 n = M . V = 2.08 x10 mol L soln . 0.00150 L soln = 3.12 x10 - 7 mol Calculating molar mass of Hemoglobin (after changing mg to g): M = 0.0215 g = 6.89 x104 g/mol 3.12 x10-7 mol Colligative Properties of Solutions 17.4) Vapor Pressure Lowering - Raoult’s Law 17.5) Boiling Point Elevation and Freezing Point Depression 17.6) Osmotic Pressure We started with nonvolatile nonelectrolytes (e.g. sucrose/H2O) Now let’s look at volatile solutes and electrolytes 17 Colligative Properties of Volatile Nonelectrolyte Solutions From Raoult’s law: Psolvent = Xsolvent . P0solvent and Psolute = Xsolute . P0solute Consider a solution having equal molar quantities of acetone and chloroform, Xacetone = XCHCl3 = 0.500. At 350C, the vapor pressure of pure acetone = 345 torr and pure chloroform = 293 torr. ¾ Determine the vapor pressure of the solution and the partial pressure of each component. What are the mole fractions, X, of each component in the vapor phase? Pacetone = Xacetone . P0acetone = 0.500 . 345 torr = 172.5 torr PCHCl3 = XCHCl3 . P0CHCl3 = 0.500 . 293 torr = 146.5 torr P From Dalton’s law of partial pressures we know that XA = PA Total P Xacetone = Pacetone= 172.5 torr = 0.541 Total 172.5 + 146.5 torr Total Pressure = 319.0 torr P 146.5 torr XCHCl3 = CHCl3 = = 0.459 PTotal 172.5 + 146.5 torr Vapor is enriched in acetone! 18 Non-ideal solutions Ideal behavior is approached when solute and solvent are involved in similar intermolecular interactions (∆Hsoln = 0) When weaker solvent-solute interactions occur, heat is removed upon dissolving (∆Hsoln > 0) Æ the observed vapor pressure is higher than ideal When stronger solute-solvent interactions occur, heat is released upon dissolving (∆Hsoln < 0) Æ the observed vapor pressure is lower than ideal Figure 17.11 Colligative Properties of Ionic Solutions For ionic solutions we must take into account the number of ions present! i = van’t Hoff factor = “ionic strength”, or the number of ions present For Electrolyte Solutions: vapor pressure lowering: P = i XsoluteP 0solvent boiling point elevation: Tb = i Kb m freezing point depression: osmotic pressure: Tf = i Kf m π = i MRT 19 Non-ideal Behavior of Electrolyte Solutions (0.05m aqueous) See Table 17.6 Non-ideal Behavior of Electrolyte Solutions 20 Gallery Section (p. 508) Plane de-icing and car antifreezingethylene glycol (C2H6O2) “Biological antifreeze” in fish- glycerol (C2H8O3) Salts for de-icing streets- CaCl2 Water volume regulation in cellsextracellular Na+ and osmosis (also, salt cured foods!) Zone refinement- purification of crystals Key theme in each: the inability of solutes to cross a phase barrier changes the properties of the solvent. 21 The structure and function of a soap “Micelles”- stabilize nonpolar colloids in polar solvents 22
© Copyright 2026