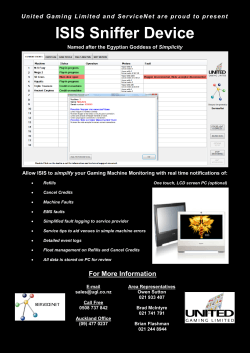
1a 1b 2 3 4 5 6 Yes No 1mg
Hepatitis C Enrollment Form Corpus Christi Location 3301 S. Alameda, Ste.100B Corpus Christi, TX.78411 877-793-6240 Phone: Fax to: (855) 646-0035 PATIENT INFO • Date: • Ship To: Name: □ Patient □ □ DOB: Address: □ Male Office Female City, State, Zip: Telephone: Other Phone: INSURANCE INFO: Diagnosis (ICD-9) Code: □ SS#: PLEASE FAX COPY OF INSURANCE CARD (FRONT & BACK) & PATIENT DEMOGRAPHIC SHEET 070.54 Hepatitis C (chronic) • Date of Diag: • HCV RNA (Baseline) CLINICAL INFORMATION • Date Needed: • Weight: IU/mL • HCV RNA (after ___ wks treatment) □ 1a □ 1b □ 2 □ 3 □ 4 □ □ Yes □ □ Yes □ • Is patient ineligible for treatment with Interferon? □6 kg/lb IU/mL □ NKDA • Allergies: • Date of lab: • HCV Genotype: 5 • Has patient been previously treated for Hepatitis C? No • Other medications: ____________________________________________ No • Length of treatment: • Pre-treatment ALT: • If yes, reason for ineligibility: • Liver Biopsy Results: ___________________/Date: _______________ _______________________________ • Fibroscan Results: ___________________/Date: ____________ • Does patient have any of the following findings (based on HCV Guidelines - www.hcvguidelines.org): HIGHEST PRIORITY: Advanced Fibrosis (Met F3) Compensated cirrhosis (Met F4) Post-liver transplant □ □ □ □ Type 2/3 essential mixed cyroglobulinemia w/ end-organ manif. □ Proteinura □ Nephrotic syndrome □ Glomerulonephritis HIGH PRIORITY: □ Fibrosis (Met F2) □ HIV co-infection □ Hepatitis B co-infection □ Other co-existent liver disease (eg, NASH) □ Debilitating fatigue □ Type 2 Diabetes mellitus □ Porphyria cutanea tarda OTHER: □ Q80K polymorphism □ Metavir F0-F1 (eg, vasculitis) • IF TAKING RIBAVIRIN, is the patient (or patient's partner) pregnant or unwilling to use adequate contraception, or is there a history of hemoglobinopathies or renal insufficiency (crcl < 50mL/min)? □ Yes □ No COMBINATION THERAPIES - HARVONI COMBINATION THERAPIES - SOVALDI/OLYSIO □ 1. SOVALDI™ 400mg (sofosbuvir) | □ HARVONI® 90mg/400 (ledipasvir/sofosbuvir) • Directions: □ 1 tab po QD □ Qty: 28-day supply mg PRESCRIPTION INFORMATION • Choose Patient type below: □ GTP 1: Tx. Naïve with or without cirrhosis □ GTP 1: Tx. Experienced, no cirrhosis □ GTP 1: Tx. Experienced, with cirrhosis Tx. Naïve, no cirr, HCV RNA < 6 mi u,GTP 1* □ □ GTPs 4 & 6: Tx. Naïve or Tx. Experienced *Prescriber decision - 8 weeks of treatment can be considered in tx naïve pts with HCV RNA < 6mill IU/ml Tx. Duration Refills 12 weeks 2 12 weeks 2 24 weeks 5 8 weeks 1 12 weeks 2 COMBINATION THERAPIES - VIEKIRA PAK □ VIEKIRA PAK™ • Qty: _______ □ 28-day supply (2 paritaprevir/ritonavir/ ombitasvir tabs po qam & 1 dasabuvir tab po BID) • Choose Patient type below: Tx. Duration Refills □ GTP 1a, no cirrhosis (add ribavirin) □ GTP 1a, with cirrhosis (add ribavirin) □ GTP 1b, no cirrhosis □ GTP 1b, with cirrhosis (add ribavirin) □ Post-liver transplant, GTP 1a or 1b (add riba) □ GTP 4: Tx. Naïve or Tx. Experienced □ RIBAVIRIN: 12 weeks 5 12 weeks 2 12 weeks 2 24 weeks 5 12 weeks 2 If approp, please choose product under 'RIBAVIRIN' HEPATITIS B ORAL THERAPIES Diagnosis (ICD-9) Code: □ □ BARACLUDE® ® □ □ 0.5mg • Quantity: Qty: _______ □ 28-day supply □ □ GTP 1: Cirrhosis/Tx. Naïve or Tx. Experienced □ GTP 4: Tx. Naïve or Tx. Experienced GTP 1: No Cirrhosis/Tx. Naïve or Tx. Exper. PRESCRIBER INFORMATION Refills 12 weeks 2 24 weeks 5 12 weeks 2 COMBINATION THERAPIES - SOVALDI/RIBAVIRIN □ 1. SOVALDI™ 400mg (sofosbuvir) | Directions: □ 1 tab po QD □ 2. RIBAVIRIN: Please choose product under 'RIBAVIRIN' • Choose Patient type below: □ GTP 2: Tx. Naïve, no cirrhosis □ GTP 2: Tx. Naïve, cirrhosis; Tx. Experienced □ GTP 3: Tx. Naïve or Tx. Experienced □ GTP 4: Tx. Naïve or Tx. Experienced Tx. Duration Refills 12 weeks 2 16 weeks 3 24 weeks 5 24 weeks 5 RIBAVIRIN □ RIBAPAK™ □ □ <75kg/172lbs 1000mg/day □ ≥75kg/173lbs 1200mg/day • Qty: MODERIBA™ tablet dose pack Take 600mg po qAM and 400mg po qPM Take 600mg po qAM and 600mg po qPM □ 28-day supply; □ Other: _____ □ 1mg □ tablets • Refills: ______ □ capsules □ □ OTHER MEDICATIONS □ Medication: ______________________ Strength: ______ • Directions: _____________________; Qty: _____; Refills: ____ Prescriber's Name: Contact person: Telephone: Fax: Office Address: Prescriber's Signature Tx. Duration Directions: Take _____ tabs/caps po qam and _____ tabs/caps po qpm • Qty: 28-day supply; Other: _____ • Refills: ______ □ Take 1 tablet po QD □ Other: _________ □ 30-day supply; □ Other: _____ • Refills: ____ NPI#: Directions: □ 1 cap po QD • Choose Patient type below: RIBAVIRIN 200mg 070.32 Hepatitis B VIREAD 300mg • Directions: 2 24 weeks **For patients with HIV co-infection, please follow mono-infection guidelines □ 2. OLYSIO™ 150mg (simeprevir) | Qty: _______ □ 28-day supply (paritaprevir 75/ritonavir 50-ombitasvir 12.5mg & dasabuvir 250mg) • Directions: □ Take as directed by mouth on PAK Directions: □ 1 tab po QD Qty: _______ □ 28-day supply City: License #: State: Zip: Medicaid Provider #: (DATE) *I f brand drugs are preferred, handw rite "Brand M edically N ecessary" above I authorize ReCept Pharmacy and its representatives to act as an agent to initiate and execute the insurance prior authorization process. IMPORTANT NOTICE: This facsimile transmission is intended to be delivered only to the named addressee and may contain material that is confidential, privileged, proprietary or exempt from disclosure under applicable law. If it is received by anyone other than the named addressee, the recipient should immediately notify the sender at the address and telephone number set forth herein and obtain instructions as to disposal of the transmitted material. In no event should such material be read or retained by anyone other than the named addressee, except by express authority of the sender to the named addressee. REF.006.60.12.14
© Copyright 2026