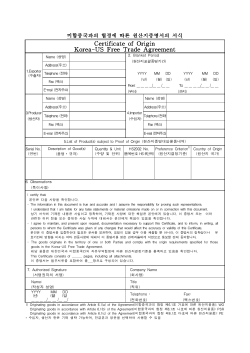
How to use the protocol template
How to use the protocol template The template is provided as is i.e. although the template was written to the best of our knowledge CTU Bern assumes no liability when using this template and does not guarantee for accuracy of information. Any suggestions or corrections are welcome and should be sent to [email protected]. The template was cross-checked with the SPIRIT guideline (http://www.spiritstatement.org/) and contains all SPIRIT items. What follows is an exact match of the original MS Word template but with additional content, explanations, and examples. Only headings are provided in the MS Word template. Most content provided in this guiding document is mandatory for all trial protocols. Such content is provided with a comment or highlighted yellow (to be copied as is; sometimes place-holders need to be replaced). Other text is provided for illustrative purposes. Explanations are highlighted in grey. It should be stressed that formatting should not be changed in the MS Word template unless you know MS Word well e.g. you know how to handle list styles or you know the difference between character and paragraph styles. You should not delete any of the headings. If you do not need a section with specific heading put in "not applicable" as text. Use the styles starting with "ProtocolTemplate_ …" provided within the template to format headings, text, lists, and tables. Clinical study protocol Version #.## (dd.mm.yyyy) Study title Short title (NCT###) Kommentar [TS1]: The title should identify: Study design Aim Population Intervention(s) Example: "A multi-center, investigatorblinded, randomized, 12-month, parallel-group, non-inferiority study to compare the efficacy of 1.6 to 2.4 g Asacol® (once daily) versus divided dose (twice daily) in the maintenance of remission of ulcerative colitis" Kommentar [TS2]: Trial acronym or other short name Sponsor Prof. Dr. Martina Trialist Department of Clinical Trials Inselspital, Bern University Hospital CH-3010 Bern Switzerland Kommentar [TS3]: Provide clinicaltrials.gov number or alternative registry number. Ensure that the registry is a WHO approved registry. This is often only be available after approval and should be added then. Study title Content 1 Protocol summary ...................................................................................................... 6 2 Zusammenfassung ...................................................................................................... 8 3 Protocol signature page ............................................................................................. 9 4 Abbreviations ........................................................................................................... 10 5 Background ............................................................................................................... 11 5.1 Standard treatment ............................................................................................ 11 5.2 Rationale of the study ....................................................................................... 14 5.3 Other relevant on-going studies ........................................................................ 15 6 Objectives of the study ............................................................................................. 16 7 Investigational plan .................................................................................................. 17 8 9 7.1 General study design......................................................................................... 17 7.2 Categorization of the study according to HFV-1/2........................................... 17 7.2.1 Rationale for categorization .................................................................... 17 7.3 Organization of the study.................................................................................. 17 7.3.1 Study Steering Committee ...................................................................... 17 7.3.2 Study coordination .................................................................................. 18 7.3.3 Data Monitoring Board ........................................................................... 18 7.3.4 Procedures for early stopping of the study .............................................. 19 7.4 Time schedule ................................................................................................... 19 Study population and patient registration ............................................................. 21 8.1 Recruitment....................................................................................................... 21 8.2 Pre-study assessments (screening/baseline) ..................................................... 21 8.3 Inclusion criteria ............................................................................................... 21 8.4 Exclusion criteria .............................................................................................. 22 8.5 Patient registration ............................................................................................ 23 8.6 Sample size and feasibility ............................................................................... 23 Study treatment ........................................................................................................ 27 9.1 Administration of study treatment .................................................................... 27 9.2 Dose modifications ........................................................................................... 28 9.3 Early withdrawal from study treatment ............................................................ 28 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 9.3.1 Procedures ............................................................................................... 28 9.4 10 Concomitant interventions ................................................................................ 28 Information on study interventions ........................................................................ 30 10.1 Compliance ....................................................................................................... 30 10.2 Intervention I (Investigational Medicinal Product) .......................................... 30 10.2.1 Summary of findings from non-clinical studies ................................. 30 10.2.2 Summary of findings from clinical studies ........................................ 30 10.2.3 Summary of known and potential risks .............................................. 30 10.2.4 Storage and handling .......................................................................... 30 10.3 Intervention II .................................................................................................. 30 10.4 Summary of findings from non-clinical studies ............................................... 30 10.4.1 Summary of findings from clinical studies ........................................ 30 10.4.2 Summary of known and potential risks .............................................. 31 10.4.3 Storage and handling .......................................................................... 31 10.4.4 Product supply and retrieval of unused products ............................... 31 10.5 Blinding procedures .......................................................................................... 31 10.6 Labelling ........................................................................................................... 31 10.7 Unblinding ........................................................................................................ 32 11 Study assessments .................................................................................................... 34 11.1 Baseline assessments ........................................................................................ 34 11.1.1 Case history and clinical examination ................................................ 34 11.1.2 Laboratory diagnostics ....................................................................... 34 11.1.3 Imaging diagnostics ............................................................................ 34 11.2 Assessments during treatment .......................................................................... 34 11.2.1 Clinical examination ........................................................................... 34 11.2.2 Laboratory diagnostics ....................................................................... 34 11.2.3 Imaging diagnostics ............................................................................ 34 11.3 Assessments at the end of treatment or early withdrawal from treatment ........ 34 11.3.1 Clinical examination ........................................................................... 34 11.3.2 Laboratory diagnostics ....................................................................... 35 11.3.3 Imaging diagnostics ............................................................................ 35 11.4 Assessments during follow-up (after end of treatment) ................................... 35 11.4.1 Clinical examination ........................................................................... 35 11.4.2 Laboratory diagnostics ....................................................................... 35 11.4.3 Imaging diagnostics ............................................................................ 35 11.5 Assessments at the end of study (final visit) .................................................... 35 11.5.1 Clinical examination ........................................................................... 35 11.5.2 Laboratory diagnostics ....................................................................... 35 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 11.5.3 12 Imaging diagnostics ............................................................................ 36 Study outcomes ......................................................................................................... 37 12.1 Definition of primary outcomes........................................................................ 37 12.2 Definition of secondary outcomes .................................................................... 37 12.3 Definition of other outcomes of interest ........................................................... 37 13 Adverse events .......................................................................................................... 38 13.1 Surveillance of adverse events.......................................................................... 38 13.2 Exceptions for rapid notification of serious adverse events ............................. 38 13.3 Adverse events that require rapid notification (besides SAEs/SUSARs) ......... 38 14 Statistics .................................................................................................................... 41 14.1 Randomization .................................................................................................. 41 14.2 Datasets to be analyzed ..................................................................................... 41 14.2.1 Full analysis dataset ............................................................................ 41 14.2.2 Per-Protocol analysis dataset .............................................................. 41 14.3 Statistical analysis ............................................................................................. 42 14.3.1 Primary analysis ................................................................................. 42 14.3.2 Key secondary analyses ...................................................................... 42 14.3.3 Interim analyses and stopping rule of the study ................................. 42 15 Ethical and regulatory aspects ................................................................................ 43 15.1 Ethics committee approval ............................................................................... 43 15.2 Regulatory authority approval .......................................................................... 43 15.3 Study registration .............................................................................................. 43 15.4 Informed consent .............................................................................................. 43 15.4.1 Amendments ....................................................................................... 44 15.5 Participant privacy and confidentiality ............................................................. 44 16 Administrative aspects ............................................................................................. 45 16.1 Protocol amendments........................................................................................ 45 16.2 Insurance ........................................................................................................... 45 16.3 Funding and other support for the trial ............................................................. 45 17 Data handling and record keeping ......................................................................... 47 17.1 Investigator site file .......................................................................................... 47 17.2 Case Report Forms ........................................................................................... 47 17.3 Record keeping ................................................................................................. 48 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 17.4 Data management ............................................................................................. 48 17.4.1 Hardware and software ....................................................................... 48 17.4.2 Data security, access and back-up ...................................................... 48 17.4.3 Analysis and archiving ....................................................................... 49 18 Quality assurance and control ................................................................................ 50 18.1 Electronic and central data validation............................................................... 50 18.2 On-site monitoring ............................................................................................ 50 18.3 Inspections ........................................................................................................ 50 18.4 Closure of study centers.................................................................................... 50 19 Dissemination policy ................................................................................................ 51 19.1 Public access to the full protocol, dataset, and statistical code ........................ 51 19.2 Publication policy and final report ................................................................... 51 20 Post-trial care ........................................................................................................... 52 20.1 Individual information of participants after end of trial ................................... 52 20.2 Access to effective trial interventions............................................................... 52 21 Accompanying scientific projects ........................................................................... 53 22 References ................................................................................................................. 54 23 Appendices ................................................................................................................ 55 23.1 Study flow diagram........................................................................................... 56 23.2 Study assessments overview ............................................................................. 57 23.3 Insurance certificate .......................................................................................... 58 23.4 Study contacts ................................................................................................... 59 23.4.1 Sponsor ............................................................................................... 59 23.4.2 Principal Investigator.......................................................................... 59 23.4.3 Trial coordination/management.......................................................... 59 23.4.4 Monitoring .......................................................................................... 59 23.4.5 Drug supply ........................................................................................ 59 23.4.6 Data management ............................................................................... 59 23.4.7 Statistics .............................................................................................. 60 23.5 Declaration of interests ..................................................................................... 61 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 1 Protocol summary Sponsor Prof. Dr. Martina Trialist Department of Clinical Trials Inselspital, Bern University Hospital CH-3010 Bern Switzerland Principal Investigator Prof. Dr. Peter Trialist Department of Clinical Trials Inselspital, Bern University Hospital CH-3010 Bern Switzerland Study title Study title Investigators Investigators will be documented in the trial master file Study sites Sites will be documented in the trial master file Time schedule First patient in: dd.mm.yyyy Last patient in: dd.mm.yyyy Last patient out: dd.mm.yyyy Interim analyses: dd.mm.yyyy Database closure: dd.mm.yyyy Statistical analysis: dd.mm.yyyy Study report: dd.mm.yyyy Objectives Primary objectives To assess whether intervention X improves survival in patients with disease D as compared to intervention Y Secondary objectives Design Number of patients To assess the adverse effects of intervention X as compared to intervention Y in patients with disease D This trial is an international, multicenter, open-label, twoarm, parallel-group, randomized-controlled trial. The proposed trial is a superiority trial. We assume a mean baseline score on the NCCN distress thermometer of around 3.5 with a standard deviation of 2.5 and a difference between groups of 1 point as clinically relevant. Based on a repeated measures ANCOVA (correcting for baseline values), a Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Kommentar [TS4]: Ensure that the summary exactly matches to the main protocol (including front page) and the German translation. It is recommended that the summary is written after the main protocol is finalized. Study title power of 90% and a two-sided alpha of 0.05 we calculated a required sample size of 55 patients per group. To account for drop-outs and losses to follow-up we set the final sample size to 75 per group or 150 patients overall. Main eligibility criteria Do not list all but only the most important ones Interventions Intervention I (name, dose, route of administration, frequency, duration) Intervention II (name, dose, route of administration, frequency, duration) Intervention III (name, dose, route of administration, frequency, duration) Follow-up/assessments Outcomes will be assessed every other month over the first six months using postal questionnaires. In case of nonresponse outcomes will be assessed via telephone interviews. Outcomes Primary outcomes Overall survival defined as time from randomization to death or last follow-up available Secondary outcomes Statistical analysis Incidence of any adverse events Survival time will be compared between groups using Kaplan-Meier curves and a Cox proportional hazards model. Adverse events will be described using descriptive statistics. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 2 Kommentar [TS5]: Ensure that the Zusammenfassung exactly matches to the main protocol (including front page) and the English summary. It is suggested that the German translation is done as the final step (after the English summary was written). Zusammenfassung Sponsor: Principal Investigator Studientitel PrüferInnen Eine Liste aller PrüferInnen wird im Trial Master File ausgewiesen. Studienzentren Eine Liste aller Studienzentren wird im Trial Master File ausgewiesen Zeitplan Einschluss erster Patient: dd.mm.yyyy Einschluss letzter Patient: dd.mm.yyyy Studienende letzter Patient: dd.mm.yyyy Interimanalysen: dd.mm.yyyy Abschluss Datenbank: dd.mm.yyyy Statistische Analyse: dd.mm.yyyy Studienreport: dd.mm.yyyy Studienziele Primäre Studienziele Sekundäre Studienziele Design Anzahl Studienteilnehmer Haupteinschlusskriterien Interventionen Nachbeobachtung/ Untersuchungen Endpunkte Primäre Endpunkte Sekundäre Endpunkte Statistische Analyse Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 3 Protocol signature page Study title (same as on title page!) Reviewed and approved by the following: Sponsor Name (same as on title page!) ................................................... Inselspital Bern Date, Signature Study site: Name ................................................... Site name Date, Signature Kommentar [TS6]: Maybe deleted if there are separate contracts for each site where adherence to the protocol is mentioned Please note that study responsibilities are documented in the Trial Master and Investigator Site File. Contribution (chapter) Background, Objectives, Investigational plan, Study population, Study treatment, Study assessments, Study outcomes Background, Study population, Study treatment, Study assessments, Study outcomes Objectives, Study outcomes, Statistics Name Martina Trialist Information on study interventions Matthias Pharmacist Quality assurance and control Brigitte Quality Peter Trialist Claudia Maths Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Signature Kommentar [TS7]: Provide contributorship for the most relevant parts of the protocol Add appropriate rows as needed Study title 4 Abbreviations AE ................................ Adverse event CRF.............................. Case report form EDC ............................. Electronic Data Capture GCP ............................. Good Clinical Practice ICH .............................. International Conference on Harmonization IEC ............................... Independent ethics committee ITT ............................... Intention to treat SAE .............................. Serious adverse event SOP .............................. Standard operating procedure SSL .............................. Secure Sockets Layer SUSAR ........................ Suspected unexpected serious adverse reaction Complete as applicable Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 5 Background Describe disease of interest EXAMPLE: INIS trial Background Neonatal sepsis is a major cause of mortality and morbidity and has been implicated in the causation of perinatal brain damage and cerebral palsy, both in term and preterm infants1 2. Although antibiotics are the mainstay of therapy, increasing numbers of bacteria are resistant to them3 4. Effective adjunctive strategies are therefore needed. Incidence, potential impact on mortality and problems in diagnosis In a prospective study in seven Australian neonatal intensive care units (NICUs), Isaacs and colleagues reported an annual incidence of sepsis of 6.6 per 1000 live births, of which 75% were late onset (more than 48 hours after birth). Overall hospital mortality for sepsis was 10%5. In a cohort of 54 UK neonatal units in 1998 (www.child-health.dundee.ac.uk/research/ukneonatalstaffing/)6, 204 (5%) of 3,963 consecutive admissions to neonatal units had a positive blood culture. Of these, 16 (8%) died. Of 3,759 (95%) babies with negative blood cultures, 95 babies died (2.5%). For very low birthweight (VLBW) infants with positive blood cultures, mortality was 14% (see table 1, p 10). In a recent North American cohort, mortality in VLBW infants with septicaemia was 21%7. However, these figures may underestimate the true incidence of neonatal sepsis. Blood cultures may often be negative if less than 1 ml of blood is sampled8. Furthermore, while sepsis was the primary cause of death in most infants under 1000g at autopsy, it was clinically undiagnosed in 61% of cases 9. Sepsis-specific mortality rates should therefore be interpreted with caution, as the diagnosis may often be inaccurate. More reliable evidence would be provided by randomised comparisons of the effects of specific interventions on mortality from all causes. Potential impact of sepsis on the perinatal brain Recent evidence suggests that sepsis is also important in the pathogenesis of neurodevelopmental impairment of perinatal origin. In a case-control study of 424 births, Grether and Nelson found that infants exposed in utero to maternal infection at term were 9 times more likely to have cerebral palsy than controls1. In another case-control study of 96 term infants, levels of cytokines in neonatal blood spots were consistently higher in children diagnosed with cerebral palsy at 3 years of age than in controls, suggesting that an inflammatory response may be important in the aetiology of cerebral impairment10. In preterm infants, sepsis is also associated with subsequent adverse neuro-developmental outcome2. Dammann and Leviton have suggested that infection remote from. 5.1 Standard treatment Describe how you searched for systematic reviews or primary studies Describe results of any systematic reviews or the systematic literature search Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: MAGPIE trial Systematic review of anticonvulsants for women with pre-eclampsia A recent systematic review of randomised trials demonstrates that, currently, there is insufficient evidence to either support or refute the use of prophylactic anticonvulsants13. Twelve trials have evaluated anticonvulsants for women with pre-eclampsia. Two small studies were excluded from the review because either no clinical outcomes were reported, or they were not reported separately for pre-eclampsia and eclampsia14,15. Two trials have compared magnesium sulphate with no anticonvulsant16,17 (228 women, South Africa; 64 women, Taiwan) and two have compared magnesium sulphate with placebo18,19 (822 women, South Africa; 135 women, USA). One quasi-randomised study (59 women, Tanzania)20 compared oral diazepam with no anticonvulsant. The remaining trials compared magnesium sulphate with diazepam21,22 (38 women, Mexico; 28 women, Malaysia) or with phenytoin12,23,24 (2138 women, USA; 115 women, USA; 54 women, USA). Results Overall, the methodological quality of these trials was average to poor. In most, concealment of the allocation at trial entry was inadequate, and two studies excluded from their analyses >10% of women randomised18,23. In the comparison of magnesium sulphate with no anticonvulsant/placebo, three women allocated magnesium sulphate had a fit, compared to 13 amongst those allocated no anticonvulsant/placebo (Relative Risk 0.33; 95% CI 0.11-1.02). There was also a non-significant trend towards a small increase in the risk of Caesarean section for women allocated magnesium sulphate (RR 1.04; CI 0.92-1.17). There is little information about possible effects on other important outcomes. In the comparison of magnesium sulphate with phenytoin, women allocated magnesium were less likely to develop eclampsia (RR 0.09; CI 0.01-0.72), but more likely to have a Caesarean section (RR 1.21; CI 1.05-1.41). There were no statistically significant differences in stillbirths (RR 0.62; CI 0.27-1.41) or neonatal deaths (RR 0.84; CI 0.41-1.74) amongst the babies allocated magnesium sulphate rather than phenytoin in utero. There is little information about other relevant outcomes. No woman in the comparison of magnesium sulphate with diazepam developed eclampsia. No trials have reported follow-up of the children beyond the perinatal period, an economic evaluation, or an assessment of the costs to the health services. Discussion To date, 1200 women with pre-eclampsia have been entered into trials comparing an anticonvulsant with none. The results of these trials, when taken together, are promising in terms of a reduction in the risk of eclampsia associated with the use of anticonvulsants. These data should be interpreted with caution, however, as the number of events was small and the largest study18 had a large proportion of post randomisation exclusions. Also, apart from the suggestion of a small increase in the risk of Caesarean section associated with the use of magnesium sulphate, there is little information about possible effects on other important outcomes, including toxicity and side effects. Nearly 2400 women have been randomised into trials comparing different anticonvulsants, most of whom were in one study comparing magnesium sulphate with phenytoin12. Women allocated magnesium sulphate were less likely to fit than those allocated phenytoin but this study provides no insight into whether giving magnesium sulphate is preferable to withholding it. Also, the number of events was small (0 versus 10) and, apart from an increase in the risk of Caesarean section, there are few data on other measures of maternal morbidity. Finally, 1% of the women allocated phenytoin developed eclampsia. This is an unusually high incidence for the reported severity of disease (only 18% had ³2+ proteinuria and 4% had been given an antihypertensive) and may, at least in part, be due to chance. In conclusion this review, combined with evidence that magnesium sulphate is the drug of choice for women with eclampsia25, supports magnesium sulphate as the best choice of anticonvulsant to evaluate for women with pre-eclampsia. There is a suggestion that magnesium sulphate may be associated with an increase in the risk of Caesarean section, which may be a tocolytic effect26. If true, this tocolytic action might have other consequences such as an increase in length of labour, postpartum haemorrhage and retained placenta. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: MAGPIE trial Current use of prophylactic anticonvulsants Internationally there is considerable variation in the use of anticonvulsants for women with preeclampsia. Amongst those who do use them, there is no consensus on either choice of agent or which women are most likely to benefit. In the USA, for example, 99% of obstetricians use magnesium sulphate which is given to an estimated 5% of pregnant women before delivery12. In contrast, 23% of UK obstetricians never use any prophylactic anticonvulsants27. In the past, only 2% used magnesium sulphate, others preferring diazepam, phenytoin or chlormethiazole6,28,29. There has recently been a substantial shift towards using magnesium sulphate in the UK, with 40% of obstetricians now reporting that they use it27. Mode of action for magnesium sulphate Exactly how magnesium sulphate might control eclamptic convulsions is unclear. Magnesium may have a localised cerebral effect. For example, it may cause vasodilatation with subsequent reduction of cerebral ischaemia15, and/or block some of the neuronal damage associated with ischaemia30,31. A possible mechanism for vasodilatation is relaxation of smooth muscle, and it has been suggested that magnesium may have a generalised effect on all smooth muscle, including the peripheral vasculature and uterus. Alternatively, any effects of magnesium sulphate in control of eclamptic convulsions may be, wholly or partially, through its role as a blocker of N-methyl-Daspartate (NMDA) receptors in the brain. These NMDA receptors are activated in response to asphyxia, leading to calcium influx into the neurones which causes cell injury. It is suggested that magnesium may block these receptors, so reducing calcium influx and protecting the neurones from damage. Possible benefits of magnesium sulphate Magnesium sulphate may reduce the risk of eclampsia. This effect may be reflected in a lower risk of other complications of eclampsia, such as renal failure, cerebrovascular accident and liver failure, as well as improved blood pressure control. If real, these potential benefits may lead to less time spent in hospital and less use of intensive care facilities. Suggestions about the possible mode of action for magnesium sulphate have also led to hypotheses about potential benefits for children who are exposed while in utero. For example, if magnesium sulphate administration does delay progression of pre-eclampsia this may be reflected in a reduction in preterm delivery. The mechanism for such a reduction could be either later onset of spontaneous labour or less obstetric intervention. In addition, it has been suggested that magnesium sulphate administration may improve the outcome for asphyxiated babies 32. For preterm babies, exposure to magnesium sulphate may also be associated with a lower risk of cerebral haemorrhage33, which in turn could be reflected in a reduction in the risk of cerebral palsy for these low birthweight infants34,35,36. All these hypotheses are based on observational data and, although they are being tested in randomised trials, these data await confirmation37,38. Possible hazards of magnesium sulphate Potential hazards of magnesium sulphate include, for the woman, respiratory depression, respiratory arrest and hypotension39. Cardiac arrest is a theoretical risk, but in practice is likely to be rare as a complication of magnesium sulphate. If magnesium does relax smooth muscle it may also have a tocolytic effect26 leading to an increase in a length of labour, postpartum haemorrhage, retained placenta and blood transfusion. In addition, potential side effects include nausea, vomiting, thirst, flushing of the skin, hypotension, arrythmias, drowsiness, confusion, and muscle weakness. We therefore need reassurance that magnesium sulphate, when used for women with pre-eclampsia, is well tolerated. Also magnesium sulphate may have an effect on mood post partum and so it is important to check whether it has any influence on the incidence of postpartum depression. For the baby, possible hazards related to hypermagnesaemia are similar to the mother and include respiratory depression, hypotonia and hypotension32,40. Recently it has been suggested that in utero exposure to magnesium sulphate for prevention of preterm delivery may increase the risk of mortality for the baby41, when compared to other tocolytic agents. However there are several other possible explanations for these data, as the number of events was very small and there were imbalances between the treatment groups37,38. Also, the dose of magnesium sulphate was very high (median 49.5g). Whatever the effects of magnesium sulphate on the outcome for low birthweight children, reassurance will still be required about possible effects for bigger babies, as the pathophysiology of cerebral palsy in these two groups is very different36. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 5.2 Rationale of the study Summarize the need for the study. The rationale should include the perceived benefits of any intervention which is to be evaluated. It should outline how the intervention under investigation might work, especially if there is little or no previous experience. Also provide justification for placebo-control if applicable. EXAMPLE: MAGPIE trial Why is a trial needed now? In 1995, magnesium sulphate was shown to be the anticonvulsant of choice for women with eclampsia (see table). These results had a major impact on both practice and policy throughout the world. Magnesium sulphate for the treatment of eclampsia is now included in the essential drugs list of the World Health Organisation and is recommended in the practice guideline produced by the Royal College of Obstetricians and Gynaecologists, London. Table: Data from the Collaborative Eclampsia Trial on maternal deaths and recurrence of convulsions *RR = relative risk; CI = confidence interval; * outcome not known for 1 woman Having switched to magnesium sulphate for women with eclampsia, many clinicians are also reviewing their policies for anticonvulsant prophylaxis. As discussed above further evidence is required to help them decide whether its use would be beneficial and, if so, for whom. Nevertheless many clinicians have begun using magnesium sulphate for women with preeclampsia, and others are considering starting to use it. There is currently a window of opportunity for properly evaluating this use of magnesium sulphate. Results of the Magpie Trial will provide a reliable basis for decision-making about the care of women with preeclampsia. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: PVAG-14 pilot trial III. RATIONALE OF THE STUDY Although huge progress has been achieved in the treatment of patients with Hodgkin's lymphoma in the last decades, the prognosis of patients with intermediate-stage disease is still unsatisfactory. Unlike the progress in patients with early- and advanced-stage disease, the standard regimen ABVD for these patients has remained unchanged for many years. Therefore, clinical trials are warranted to further improve the treatment of these patients incorporating new and possibly more effective agents. A new regimen derived from the ABVD-regimen was developed for this study (PVAG-14). No prospective trial has been published about the proposed chemotherapy regimen so far but preliminary data from an ongoing phase I/II trial showed promising results with favorable safety profile in a three-weekly schedule (I.2.2, p. 21). Given the experience of earlier trials with gemcitabine-combinations (I.2.2, p. 21) the proposed approach seems promising and feasible. Radiation will be administered after chemotherapy at a dose of 30 Gy of IF-RT since no definitive results are available from the HD11 yet justifying a lower radiation dose. No phase I dose-finding trial has been conducted to determine the optimal dose of gemcitabine administered concomitantly with doxorubicin, vinblastine, and prednisone in a bi-weekly regimen. According to pharmacokinetic studies a weekly dose of at least 350 mg/m² is required for optimal tumor control. Given the extensive data of clinical trials with gemcitabine (either administered as single-agent or in combinations) a dose of 1000 mg/m² (corresponding to a dose intensity of 500 mg/m²/week) seems to be most promising. However, the dose of doxorubicin in the ABVDregimen has not been challenged since its introduction in the 1970's. Recent trials in nonHodgkin lymphoma indicate that higher dose-intensities may be more effective than standard dosing regimens for the treatment of lymphoma. Doxorubicin doses of 50 mg/m² seem to be too high to be given in combination with other myelosuppressive agents like gemcitabine and vinblastine. Given the dose-intensities administered in trials of gemcitabine and doxorubicin combinations the most promising doses seem to be 25 mg/m² and 35 mg/m². Since complete response rate with ABVD is more than 90% in this population, a phase I dose-escalation study was deemed unfeasible by the trial steering committee. Therefore, a randomized phase II trial is the appropriate design to evaluate the activity and optimal dose level of this new regimen. FDG-PET performed early during treatment (e.g., after two cycles of chemotherapy) may be a promising approach for response-adapted treatment strategies. However, no data are available from prospective studies of patients with HD treated with a uniform regimen regarding its predictive value – a prerequisite to develop such strategies. Therefore, assessment of the predictive power of early FDG-PET will be another objective of the trial. Since assessment and quantification of predictive value is limited if the results of early FDG-PET evaluations guide subsequent treatment of patients no consequences regarding treatment will be drawn in this trial. 5.3 Other relevant on-going studies Briefly describe other relevant studies currently enrolling patients identified at clinicaltrials.gov or other study registers and the difference to the proposed trial. EXAMPLE Other relevant studies The International Clinical Trials Registry Platform (ICTRP) was last searched on May 4th, 2009 using the following search terms: 'osteoarthritis' (in Condition) AND 'glucosamine' (in Intervention) AND 'recruiting' (in Recruitment status). The search identified no ongoing placebo controlled trials. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 6 Objectives of the study Describe the aims of the trial. This does not necessarily require exact descriptions of the outcomes (i.e. definitions) but the concept of the outcome measures (e.g. activity, effectiveness, safety). When stating the aims of a trial, consider using the PICO (Population, Intervention, Control, Outcome) approach. Note that exact definition of outcomes should be described in 12. Think about classifying the trial according to ICH Harmonized Tripartite Guideline E8 as human pharmacology, therapeutic exploratory, therapeutic confirmatory, or therapeutic use trial. EXAMPLE: SYTRUST trial Overall Goal To determine the feasibility, accuracy, cost-effectiveness and impact on pregnancy outcome of on-site syphilis testing and related health promotion strategies in the community antenatal clinics of a rural South African health district. Specific Objectives 1. Through a pre-intervention phase, to document the current strategies for screening and treating maternal syphilis and other STDs in the antenatal clinics, and to determine their effectiveness. 2. To gain an understanding of women’s health seeking behaviours during pregnancy through an assessment of 1) awareness and understanding of syphilis and other STDs and their impact on pregnancy, and 2) reasons why women often book late for antenatal care, or do not book at all. 3. To implement in six intervention clinics, on-site syphilis testing and health promotion strategies aimed at 1) increasing awareness of the impact of STDs, particularly syphilis, on pregnancy, 2) reducing the gestational age that women book for antenatal care, 3) increasing the number of women that book for antenatal care, 4) increasing the proportion of infected women who complete a course of treatment for syphilis. 4. To develop effective partner notification strategies within the context of antenatal care. 5. To evaluate the feasibility, accuracy, and cost-effectiveness of this strategy. 6. To implement the strategy in control clinics if the findings are positive. 7. To disseminate this data and thereby inform reproductive health policy and practice, and hence improve reproductive health status. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 7 Investigational plan 7.1 General study design Use "labels" (no details) to provide an overall picture of the study EXAMPLE: ASCEND trial Design ASCEND is a mail-based, multi-centre, randomised controlled trial which aims to demonstrate whether aspirin and/or omega-3 fatty acids reduce the risk of cardiovascular events in individuals with diabetes who do not already have diagnosed occlusive arterial disease, and whether such benefits outweigh any potential risks. In order to do this reliably, at least 10,000 patients with diabetes and no clinical evidence of occlusive arterial disease will be randomly allocated to receive 100mg aspirin daily or matching placebo tablets and 1g omega-3 fatty acid or matching placebo capsules for about 5 years. A study of this size should have excellent power to detect a 20% proportional reduction in the cardiovascular event rate among such patients. In order to be cost-effective, this study is being undertaken predominantly by mail, with back-up from a 24-hour Freefone Service. A flowchart of the study is provided in the appendix (p. 55). 7.2 Categorization of the study according to HFV-1/2 This study is considered to fall under category A/B/C according to HFV-1/2. 7.2.1 Rationale for categorization Provide evidence and justification for categorization. Provide sound evidence in case of downgrading 7.3 Organization of the study Describe the general organization of the study i.e. number of centers, types of centers/settings, coordination, data management, etc. EXAMPLE Organization of the study This multicenter trial is centrally coordinated by CTU Bern and conducted in 56 tertiary care centers in Switzerland, France, Germany, and Italy. 7.3.1 Study Steering Committee A steering committee/board is usually responsible for the scientific oversight of a study e.g. was responsible for the initiation of the study, wrote (main parts of) the protocol, decides about amendments. Describe general organization, members, responsibilities, etc. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: RECORD trial The Steering Committee The trial is supervised by an MRC Steering Committee. This is made up of three independent members selected by the MRC, together with those originally granted funds to mount the trial. Observers from the MRC and host university (University of Aberdeen) may also attend. Other members of the Project Management Group may attend as observers at the invitation of the Chair of the Steering Committee. 7.3.2 Study coordination Describe the study coordination including all tasks centrally coordinated EXAMPLE: RECORD trial The Trial Office The Trial Office is in the Health Services Research Unit at Aberdeen and gives day to day support to the clinical centres. It is responsible for collection of data (in collaboration with the local study nurses), data processing and analysis. It is also responsible for randomisation, despatch of trial materials to participants, and unblinding, as clinically necessary. EXAMPLE: CRASH trial Co-ordinating Centre responsibilities • Provide study materials and a 24-hour randomisation (and unblinding) service (Figure 5) • Give collaborators regular information about the progress of the study • Help ensure complete data collection at discharge and at six months • Respond to any questions (e.g. from collaborators) about the trial 7.3.3 Data Monitoring Board A Data (and Safety) Monitoring Board/Committee is a group of independent experts who monitor aspects of a study while it is running. Most often safety data is monitored but other aspects such as efficacy data, recruitment, overall quality of the study performance, centerspecific performance might also be monitored by the DSMB. Note that the DSMB acts independently from the sponsor or any other study organization (such as the steering committee) and provides recommendations but no decisions. The final responsibility for the study remains always with the sponsor. Describe organization, participants, responsibilities and procedures. Data and safety monitoring is closely related to interim analyses and requires a detailed statistical analysis approach. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: RECORD trial The Data Monitoring Committee A data monitoring committee will be established. This will be independent of the trial organisers. During the period of recruitment to the trial, interim analyses will be supplied, in strict confidence, to the data monitoring committee, together with any other analyses that the committee may request. This may include analyses of data from other comparable trials. In the light of these interim analyses, the data monitoring committee will advise the Steering Committee if, in its view, one or more of the randomised comparisons in the trial has provided both (a) proof beyond reasonable doubt1 that for all or some types of patients one particular type of intervention is clearly indicated in terms of a net reduction in fracture risk without any increased risk of death (or clearly contraindicated because of a net increase in fracture risk or mortality), and (b) evidence that might reasonably be expected to influence materially the care of people who sustain a fracture by clinicians who know the results of this and comparable trials. The Steering Committee can then decide whether or not to modify intake to the trial. Unless this happens, however, the steering committee, project management group, clinical collaborators, and trial office staff (except those who supply the confidential analyses) will remain ignorant of the interim results. The frequency of interim analyses will depend on the judgement of the chairman of the committee, in consultation with the Steering Committee. Initially, the principal concern will be possible adverse effects. Data on new fractures will accumulate slowly so the committee is unlikely to be able to consider these reliably until some way into the trial. Appropriate criteria for proof beyond reasonable doubt cannot be specified precisely. A difference of at least three standard deviations in the interim analysis of a major endpoint may be needed to justify halting, or modifying, such a study prematurely. If this criteria were to be adopted, it would have the practical advantage that the exact number of interim analyses would be of little importance, and so no fixed schedule is proposed (Peto R et al. Br J Cancer 1976; 34: 584-612). 1 7.3.4 Procedures for early stopping of the study Describe procedures when formal stopping rules are met if not described above. (see also chapter 14.3.3 Interim analyses and stopping rule of the study) 7.4 Time schedule First patient in: ............. dd.mm.yyyy Last patient in: ............. dd.mm.yyyy Last patient out: ........... dd.mm.yyyy Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title Interim analyses: .......... dd.mm.yyyy Database closure: ......... dd.mm.yyyy Statistical analysis: ...... dd.mm.yyyy Study report: ................ dd.mm.yyyy EXAMPLE: PVAG-14 pilot trial Time schedule First patient in: 01.04.2006 Inclusion last patient (Randomization): 30.11.2007 Interim analysis: 31.05.2007 Analysis of primary endpoints: 31.08.2008 Last patient out (end of 5-year follow up): 31.05.2013 Final statistical analysis: 30.11.2013 (final analysis) Study report: 31.12.2013 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 8 Study population and patient registration Patients with main diagnoses will be included in this study. Patients must meet all of the inclusion criteria and none of the exclusion criteria to be enrolled in the study. No study-specific procedures (i.e., assessments/treatments not required in clinical practice) may be performed until an eligible patient has signed an approved informed consent to participate in the study. 8.1 Recruitment Describe the process of recruitment (for example advertisements with place(s) of publication, consecutive patients with no selective recuitment) and enrolment. 8.2 Pre-study assessments (screening/baseline) See section 11.1, p. 34 for a description of required pre-study assessments. Note that assessments done before study inclusion are only allowed if part of routine clinical practice. These assessments are done to establish eligibility of patients. Study-specific procedures must only be done after study inclusion i.e. after the informed consent form was signed. Pre-study assessments should be performed within 28 days prior to registration. 8.3 Inclusion criteria 1. List criteria that describe the overall nature of the population of interest 2. Signed informed consent with understanding of the study procedures and the investigational nature of the study Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: RECORD trial 2.1 Who will be considered for trial entry? The trial will involve people aged 70 or over who have had a previous fracture: I. Those who have sustained a fracture (except cervical spine, skull or face) in a fall from no more than a standing height within the previous two years (while eligible patients may have sustained more than one fracture, those who have high energy transfer injury will not be eligible) II. Those with a clinical vertebral fracture (defined as a definite clinical event with radiological evidence* of an incident vertebral fracture) Potential participants will be identified by study nurses based in each clinical centre from amongst patients attending hospital as outpatients or inpatients. A log will be kept of patients meeting these criteria, describing the reasons if they are not subsequently recruited to the trial. (Depending on the numbers of people attending hospital who are recruited, additional patients may also be identified from medical records as having sustained an eligible fracture in the preceding two years.) EXAMPLE: PVAG-14 pilot trial Inclusion criteria - Age: 18-60 years - Histologically confirmed diagnosis of Hodgkin's lymphoma - Clinical Stage IA, IB, IIA with at least one of the riskfactors a-d given below - Clinical Stage IIB with one or both riskfactors c-d given below Riskfactors: a) Large mediastinal mass (≥ 1/3 of the greatest thorax diameter as measured by chest x-ray) b) Extranodal involvement c) High erythrocyte sedimentation rate (≥ 50 mm/h in patients without B-symptoms, ≥ 30 mm inpatients with B-symptoms) d) Three or more involved lymph node areas - No prior therapy for Hodgkin's lymphoma (exception: pre-phase treatment with corticosteroids and vinca-alkaloids for a maximum of seven days may not preclude trial participation if clinically indicated and all staging examinations have been performed; all forms of prior radiotherapy preclude trial participation) - Life expectancy > 3 months according to investigator judgement. - Signed informed consent with understanding of the study procedures and the investigational nature of the study. - Patient agrees that personal data and tissue samples are provided to the GHSG 8.4 Exclusion criteria 1. List criteria that exclude patients from the study although inclusion criteria are met e.g. specific laboratory values 2. Pregnant or nursing women 3. Known intolerance to any protocol intervention 4. Participation in another clinical trial Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 5. 8.5 Patient's lack of accountability, inability to appreciate the nature, meaning and consequences of the study and to formulate his/her own wishes correspondingly Patient registration All patients who meet the eligibility criteria for study entry can be enrolled into the study. Eligible patients will be registered and randomized in the study centrally via the Internet: URL: enter the URL of the study database For randomization, the following is required: All inclusion criteria satisfied No exclusion criterion satisfied Patient briefing and signed informed consent form EXAMPLE: CRASH trial Randomisation Patients eligible for inclusion should be randomised, and the study treatment started, as soon as possible. Randomisation is done by telephoning a 24-hour toll-free service ### and takes only about two minutes. The questions that will be asked by the telephone operator prior to allocation of the treatment packs are shown in Appendix 2. The study computer will then randomly assign a treatment pack number that will identify one of the CRASH treatment packs stored in the emergency department. Once a patient has been randomised, we will definitely wish to learn the outcome in hospital, even if the trial treatment gets interrupted or is not actually given. 8.6 Sample size and feasibility Provide the number of participants to be enrolled (including a projection per study site). Describe sample size calculation and feasibility of recruitment. A justification of sample size requires details on: 1) Outcome(s) on which sample size calculation is based; 2) Null Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title hypothesis; 3) level of type I error; 4) level of type II error; 5) minimal clinically relevant difference; 6) allocation ratio; 7) underlying statistical test/methods; 8) software used for sample size calculation; 9) corrections for drop-outs/withdrawals; 10) Assumptions of any parameter used EXAMPLE: Magpie trial Sample size estimates Sample size estimates For women with severe pre-eclampsia the risk of convulsions is around 1%. To demonstrate a halving in this risk would require 14,000 women (alpha 0.05, beta 0.1). The aim is therefore to recruit 14,000 women. The trial may include women with a lower seizure risk and, if this was 0.75%, the power to demonstrate a halving would then be 80%. Most women (90%) are likely to be randomised before delivery and, if total mortality for the babies was 12%, this would give a power of 90% to detect a 15% proportional reduction to 10.2% (alpha 0.05). If total mortality for the babies was reduced from 10% to 8.5% (15% reduction), the power would be 80% (alpha 0.05). Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: INIS trial Sample size Table 4 shows positive blood culture rates (including probable contaminants) in 3,963 consecutive infants of all birthweights admitted to a randomly selected, nationally representative cohort of 54 UK neonatal units between 1st March 1998 and 4th September 1998 in a study of organisation and outcomes of neonatal care funded by the NHS Executive6. Table 4 Number with positive cultures Mortality (all causes) Number with negative cultures Mortality (all causes) 204 / 3,963 (5%) 16 / 204 (8%) 3,759 / 3,963 (95%) 95 / 3,759 (2.5%) Among VLBW infants with positive blood cultures, mortality was 14%. In a recent North American cohort, mortality in VLBW infants with septicaemia was 21%7. Assuming combined rates of mortality and major morbidity of 10-20% for all infants and 20-30% for VLBW infants, Table 5 outlines estimated sample sizes. Table 5 Mortality or major morbidity in control group Mortality or major morbidity in IVIG group Relative risk reduction 30% 26% 30% Total sample size required to detect difference with 95% confidence 80% power 90% power 13% 4,052 5,392 25% 17% 2,580 3,428 30% 20% 33% 626 824 25% 21% 16% 3,572 4,748 25% 20% 20% 2,266 3,006 25% 15% 40% 540 708 20% 16% 20% 2,994 3,972 20% 15% 25% 1,890 2,502 20% 12.5% 37.5% 810 1,066 15% 12% 20% 4,204 5,582 15% 10% 33% 1,450 1,914 12% 9% 25% 3,408 4,516 10% 7.5% 25% 4,166 5,524 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: INIS trial Feasibility About 5,000 infants will be needed to demonstrate moderate reductions in mortality or survival with major developmental delay with adequate power. Over a three year recruitment period, assuming that 7-10% of all admissions are diagnosed with clinical sepsis and considered eligible for recruitment, 150 NICUs with an average of 300 admissions per year will be required to achieve the recruitment target, assuming a 40-50% rate of recruitment of eligible infants. Neonatal units will initially be recruited in the UK, Australia and New Zealand. A census of all 186 UK neonatal intensive care units (NICUs) and 60 special care baby units in 199662 (which is a 100% response rate) found that the median number of admissions per year per NICU was 317. If a broadly representative sample of about half all UK NICUs and SCBUs participate, then over 50% of the projected recruitment rate for the trial will be possible within the UK, leaving the additional 50% to be recruited from the rest of the world. This study reflects the philosophy that the only practicable way to achieve comparisons which are sufficiently large to minimise the risk of being seriously misled by the play of chance is to design trials that are extremely simple and flexible63. Experience in the OSIRIS64 and MRC ORACLE study48 49 suggest that a large, simple trial of this scale of a potentially important intervention supplied free of charge to participating centres is feasible. Furthermore, systematic reviews of RCTs of IVIG therapy in neonatal sepsis suggest a substantial reduction in mortality. This contrasts with the systematic reviews of RCTs of antibiotics in threatened preterm birth which led to the ORACLE study, as these showed no evidence of a difference in neonatal mortality. This preliminary evidence that IVIG may reduce mortality may further enhance the appeal of the study. The estimate of the incidence of the outcome (the event rate) for the trial is imprecise, particularly as the threshold at which clinicians will enter patients cannot be estimated. If clinicians enter babies where the likelihood of serious sepsis is lower then the event rate will also be lower. If clinicians restrict entry to only those babies who are very sick, then the event rate will be high. Either of these two scenarios is reasonable because it will define a population to which the trial result can be generalised. However, it does mean that until the trial has recruited sufficient numbers of babies it will not be possible to determine the optimum trial sample size with any certainty. As a consequence the trial sample size currently represents the minimum size desirable. Assuming the trial recruits for three years, the maximum number of babies which can be recruited during this time will be recruited and it is possible that this number may exceed 5,000. During recruitment to the trial the accumulating data will be seen by an independent Data Monitoring and Ethics Committee at least once per year (see above) and they will advise the Trial Steering Committee whether the trial has answered the clinical question being addressed. If not, the trial will continue to recruit until 5,000 babies have been recruited, or until funding is exhausted. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 9 Study treatment Patients are expected to begin treatment at the day of randomization. Study treatment must be administered in a hospital setting. Outpatient treatment is allowed but depends on the investigator's judgment. 9.1 Administration of study treatment Describe administration of study treatment. Include details about: type of intervention, dosage, route of administration, frequency, duration. If intervention is a drug (including placebo) or device provide details about manufacturer, labelling etc. in chapter 10. EXAMPLE: CRASH trial Study treatment (Table 2) Each CRASH treatment pack contains: • • • • • 11 x 2g vials of methylprednisolone (MP) or placebo 1 x 20mL sterile water for injection (for use with the loading dose) 1 x 100mL bag of 0.9% NaCl (for use with the loading dose) CRASH stickers (for attaching to infusion bags and patient notes) Patient information leaflet and Early Outcome Forms Loading 2g MP (or matching placebo) over 1 hour in 100mL infusion: 1. Add 20mL water for injection to one 2g vial and mix well 2. Add contents of vial to the 100mL bag of 0.9% NaCl provided 3. Infuse over one hour Daily Maintenance 1. 2. 3. 4. 5. 6. 0.4 g/hour for about 24 hours in 20 mL/hour infusion (MP or matching placebo): Remove 100 mL from a 500 mL bag of 0.9% NaCl (to make room for the steroid) Add 20 mL water for injection to each of five 2 g vials and mix well Add all five (about 100 mL) to the 500 mL bag of 0.9% NaCl Infuse at 20 mL/hour for about 24 hours Repeat for maintenance day 2 N.B. As children under 16 are excluded, a simple fixed-dose treatment can be used. The dosing regimen is that used in the NASCIS-2 and NASCIS-3 trials of MP in acute spinal cord injury. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: Bortezomib in HD trial Study drug administration Patients receive 1.3 mg of bortezomib per square meter of body-surface area (SA) twice weekly for two weeks (day 1, 4, 8, 11) followed by one week without treatment (one cycle). The maximum number of cycles per patient is eight (24 weeks). Bortezomib is given as a short intravenous bolus injection (3-5 seconds). 9.2 Dose modifications Describe dose modification: how, under which circumstances etc. 9.3 Early withdrawal from study treatment Describe circumstances under which treatment should/must be stopped prematurely and subsequent procedures. EXAMPLE: Bortezomib in HD trial Early withdrawal from study treatment Protocol therapy will be discontinued at any time if any of the following situations occurs: • The development of toxicity which, in the investigator’s judgment, precludes further therapy, • Progressive disease, • Intercurrent illness precluding further therapy, in the investigator’s opinion, • Pregnancy, • Patient refusal to continue, • Patient lost to follow-up or non-compliance. 9.3.1 Procedures Investigators will make every reasonable effort to keep each patient on the study until all planned treatments and assessments have been performed. When a patient ends the treatment phase of the study prematurely, the date and reason for early treatment discontinuation will be noted in the source document and the appropriate portion of the CRF add additional notification if required e.g. direct information to sponsor with timeframe. Investigators will make any reasonable effort to collect and report end-oftreatment evaluations (11.3) and all follow-up evaluations (11.4). The end-of-treatment evaluation should be performed before any other therapeutic intervention. For procedures for patients withdrawing consent at a later stage see also 11.5. 9.4 Concomitant interventions Describe concomitant interventions received by all patients in the study. If there is no regulation/restriction on concomitant interventions state this explicitly. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: Bortezomib in HD trial Concomitant medication In addition to bortezomib, patients will take dexamethasone 20 mg p.o. on every treatment day (day 1, 4, 8, 11). Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 10 Information on study interventions 10.1 Compliance Describe how compliance will be ensured AND assessed e.g. pill counting. EXAMPLE: HWI trial Compliance Patients will have to record their compliance in the diary. In addition patients are asked to send back all study medications (including Monuril® 3g), which were not used, as well as empty packages, together with the diary to the CTU Bern after the interview on day 10. 10.2 Intervention I (Investigational Medicinal Product) 10.2.1 Summary of findings from non-clinical studies One may refer to the Investigator’s Brochure (IB), Investigational Medicinal Product Dossier (IMPD), Summary of Product Characteristics (SPC) or a similar document (if applicable), by mentioning the relevant pages in that document. Be sure that the information is up to date and references to peer reviewed papers in (biomedical/scientific) journals should be given where appropriate. 10.2.2 Summary of findings from clinical studies See 10.2.1 10.2.3 Summary of known and potential risks See 10.2.1 10.2.4 Storage and handling Describe any storage and handling guidelines if appropriate, including keeping a temperature log. 10.3 Intervention II 10.4 Summary of findings from non-clinical studies One may refer to the Investigator’s Brochure (IB), Investigational Medicinal Product Dossier (IMPD), Summary of Product Characteristics (SPC) or a similar document (if applicable), by mentioning the relevant pages in that document. Be sure that the information is up to date and references to peer reviewed papers in (biomedical/scientific) journals should be given where appropriate. 10.4.1 Summary of findings from clinical studies See 10.4 Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Kommentar [TS8]: Repeat chapter for multi-arm trials as appropriate Study title 10.4.2 Summary of known and potential risks See 10.4 10.4.3 Storage and handling Describe any storage and handling guidelines if appropriate 10.4.4 Product supply and retrieval of unused products Describe procedures for study product supply and retrieval of unused products (mention drug accountability log) 10.5 Blinding procedures Describe how blinding is ensured i.e production and packaging. 10.6 Labelling Provide a graph of the label for all trial interventions. According to Annex 13 of the EU Guidance "Manufacture of investigational medicinal products" the label should include: a) Name of the sponsor; b) Pharmaceutical dosage form, route of administration, quantity of dosage units (and name/identifier of the product and strength/potency in case of open trial); c) The batch and/or code number to identify the contents and packaging operation; d) The trial subject identification number, where applicable; e) Directions for use; f) Imprint "For clinical trial use only"; g) The name of the investigator (if not included as a code in the trial reference code); h) A trial reference code allowing identification of the trial site and investigator; i) The storage conditions; j) The period of use (use-by date, expiry date or re-test date as applicable), in month/year); k) "Keep out of reach of children" except when the product is for use only in hospital; Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title The outer packaging may include symbols or pictograms to clarify certain information, mentioned above and the request “return empty packaging and unused products”. Additional information for example any warnings and handling instructions, where applicable may be displayed according to the order. A copy of each type of label should be kept in the batch record. On the immediate packaging when the outer packaging carries the particulars mentioned above (a-k), the particulars a-f shall be given. The label needs to be in the home language of the canton of each study site unless the intervention is applied by the investigator directly (no information to study participants required) in which case an English label is sufficient. If regular market products are used an additional label with the following information is sufficient: a) Name of the sponsor; d) The trial subject identification number, where applicable; f) Imprint "For clinical trial use only"; h) A trial reference code allowing identification of the trial site and investigator; EXAMPLE: Lichen trial 10.7 Unblinding Describe unblinding procedures if applicable. Refer to a SOP if appropriate. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title EXAMPLE: HWI trial 9.6 Unblinding In general, breaking the statistical blind should be considered only when knowledge of the treatment assignment is deemed essential for the study patient's care by the participant's physician or a regulatory body. Unblinding should be restricted to emergency situations and is expected to be extremely rare in this trial. It may be required in the following circumstances: - Occurrence of a serious adverse event where the event appears possibly related to one of the interventions and the event is not listed in one of the package inserts i.e. the serious adverse event is considered to be a potential suspected unexpected serious adverse drug reaction (SUSAR) and would require expedited reporting. - Occurrence of a severe allergic reaction and knowledge of the treatment assignment is deemed essential for the participant's care. - Use of the investigational medicinal product by someone not participating in the clinical trial e.g. if a child in the household of a participant accidentially takes a study medication. - Knowledge of the treatment assignment is deemed essential for the participant's care for any reason. Code breaks/emergency envelopes will be held in the pharmacy. The on-call pharmacist at the Institute for Hospital Pharmacy of the Inselspital Bern will be available to perform the code break at any time (contact by the central number of the Inselspital Bern, 031 632 21 11). Only healthcare professionals involved in the care of the relevant study patient can require unblinding. The healthcare professional should immediately contact the Institute for Hospital Pharmacy and request code breaking by giving specific reasons. The responsible pharmacist will provide the information as required. On receipt of the relevant information, the treating healthcare professional should attend to the participant's medical emergency. Where possible, it is important that all other members of the study team e.g. other investigators, statisticians etc. remain blinded. The responsible pharmacist of the Institute for Hospital Pharmacy will inform the principal investigator of any request for unblinding and the reasons for the request. The investigator responsible for the care of the study patient will record the unblinding and reasons in the medical chart and in the eCRF. The responsible pharmacist will also record any unblinding and the reasons in the code list. Any unblinding will be documented in the study report. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 11 Study assessments 11.1 Baseline assessments Baseline assessments should be performed within 14 days of randomization but before any treatment. 11.1.1 11.1.2 11.1.3 Case history and clinical examination List what elements of the history and clinical examination are required Describe specific clinical examinations in detail Laboratory diagnostics List all laboratory diagnostics Imaging diagnostics List all imaging diagnostics Chest X-raya 11.2 Assessments during treatment The following assessments have to be performed every month during treatment. 11.2.1 11.2.2 11.2.3 Clinical examination List what elements of the clinical examination are required Describe specific clinical examinations in detail Laboratory diagnostics List all laboratory diagnostics Imaging diagnostics List all imaging diagnostics 11.3 Assessments at the end of treatment or early withdrawal from treatment The following assessments have to be performed at the end of treatment or if a patient is withdrawn prematurely from treatment. 11.3.1 a Clinical examination List what elements of the clinical examination are required Describe specific clinical examinations in detail This assessment is trial-specific Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Kommentar [TS9]: All study-specific procedures i.e. not done routinelyin clinical practice should be marked clearly e.g. with a star and explained in a footnote Study title 11.3.2 11.3.3 Laboratory diagnostics List all laboratory diagnostics Imaging diagnostics List all imaging diagnostics 11.4 Assessments during follow-up (after end of treatment) If assessments vary depending on the type of follow-up visit structure this chapter accordingly. The following assessments have to be performed schedule e.g. every 6 months after the end of treatment. 11.4.1 11.4.2 11.4.3 Clinical examination List what elements of the clinical examination are required Describe specific clinical examinations in detail Laboratory diagnostics List all laboratory diagnostics Imaging diagnostics List all imaging diagnostics 11.5 Assessments at the end of study (final visit) The following assessments have to be performed at the final visit ($$$ years/months after end of treatment). If a patient withdraws consent after the end of treatment investigators will make every reasonable effort to keep each patient on the study until all assessments have been performed. When a patient still wants to end the study prematurely, the date and reason for early discontinuation will be noted in the source document and the appropriate portion of the CRF. Investigators will make any reasonable effort to collect and report all end-ofstudy evaluations in these patients. Patients should be informed that data collected until the withdrawal will be kept in the study database and used for analysis. 11.5.1 11.5.2 Clinical examination List what elements of the clinical examination are required Describe specific clinical examinations in detail Laboratory diagnostics List all laboratory diagnostics Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 11.5.3 Imaging diagnostics List all imaging diagnostics Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 12 Study outcomes Describe exact definitions for each outcome: what (method) is assessed by whom (independent adjudication?), at which timepoint under which conditions (blinded?). The following components need to be described explicitly (for each outcome if different): 1) domain (e.g. pain, quality of life etc.); 2) specific measure/instrument (e.g. "Hamilton Anxiety Rating Scale", "blood pressure measured in sitting position on both upper arms after resting for 5 minutes using a sphygmomanometer", etc.); 3) metric i.e. the analytic approach (e.g. risk ratio, difference in absolute values, repeated-measures using all followup values, etc.); 4) cut-off for continuous/quantitative measures if used; 5) time frame i.e. the follow-up visit from which the outcome will derived (e.g. 2 years after randomization); 6) outcome assessor (e.g. patient, blinded and independent adjudication committee). 12.1 Definition of primary outcomes Description as detailed above for all primary outcomes (usually one but not more than three as a rough guidance). The primary outcome(s) should be the most relevant one(s) regarding the objective of the trial i.e. for phase III trials the most clinically relevant efficacy outcomes, for phase II trials activity outcomes or safety outcomes to determine dosing, for phase I trials pharmacokinetic/-dynamic or tolerability outcomes, and for phase 0/proof-of-concept trials pharmacodynamics or activity outcomes. EXAMPLE 11.1.1. Hematological toxicity Toxicity will be assessed according to NCI Common Terminology Criteria version 3.0 (CTCAE) published June 10, 2003 (see Study Manual). The primary toxicity outcome is defined as the percentage of patients with at least one episode of a hematological toxicity grade III/IV between treatment start until 28 days after the last study drug administration as assessed by each investigator based on regular laboratory studies as defined in chapter 10. For the purpose of this study an episode of a hematological toxicity grade III/IV is defined as one or more laboratory studies with: • Neutrophils < 1000/μL or • Leukocytes < 2000/μL or • Platelets < 50,000/μL or • Hemoglobin < 8.0 g/dl An episode ends if all hematological parameter(s) return to at least toxicity grade II. 12.2 Definition of secondary outcomes Ditto (approximately four other (clinically) relevant outcomes addressing the objective) 12.3 Definition of other outcomes of interest Ditto. Any other outcomes of interest e.g. to disentangle how interventions might work (different survival outcomes, components of composite event outcomes, subscales. Exploratory outcomes, or outcomes with unclear validity. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title 13 Adverse events Pre-existing diseases present prior to administration of study intervention, will be documented as concomitant diseases as part of participant history in the CRF. Any disease newly occurring or increasing in severity during the course of the trial will be documented as an AE. The most up to date version of the CTU Bern SOP "Safety reporting in clinical studies with drugs (CS_CI_SOP-01)" (current version: 01, 1.1.2013) will be used to manage adverse event related issues in this trial. The SOP contains Definitions of terms Instructions on assessing adverse events Instructions for handling, documenting, and reporting adverse events Guidance on responsibilities Guidance on annual safety reports Kommentar [TS10]: If the SOP is not used for the trial provide all required details. The SOP might be a starting point. 13.1 Surveillance of adverse events Provide details about study specific surveillance of adverse events: passive ("Watch and wait" approach with spontaneous reports by participants), unspecific active, specific active. Take into consideration already known and clinically relevant harms. Patients will regularly be assessed for development of adverse events during study visits. The safety office/sponsor will be timely informed about all serious adverse events as defined in the SOP mentioned above. The following potential adverse events will be actively monitored during this study: List all laboratory assessments List all other tests/questionnaires that apply 13.2 Exceptions for rapid notification of serious adverse events The instructions for rapid notification of SAEs as outlined in the SOP mentioned above do not apply for the following events: Adverse reactions listed in the package insert or investigator's brochure List any other adverse events 13.3 Adverse events that require rapid notification (besides SAEs/SUSARs) For the purpose of this study the following adverse events will be handled like a SUSAR (regarding reporting requirements) as described in the SOP mentioned above: List all adverse events that require rapid notification (which are not necessarily a SAE/SUSAR) Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Kommentar [TS11]: Ensure that this section agrees with study assessments in section 10 Study title The flow chart on the next page shows graphically the process of expedited safety reporting. Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Study title Expedited Safety Reporting No EC reporting LOCAL Investigator Serious Adverse Event No No Safety Signal Continued Vigilance No Causality Assessment Fatal? Life threatening? No Yes Related? Safety Signal? Yes Possibly Probably Definitely Yes Report to LOCAL EC within 7 days if fatal or life threatening 1 Report within Report to LOCAL EC ® within max 7 days 24 h Report within 24 h to - Sponsor - LOCAL EC New Risks and / or new Aspects of known adverse reactions Serious Adverse Event 15 days all other Events to Sponsor Unlikely Not related No SUSAR Related? Report within 24 h to - Swissmedic - All Investigators No Possibly Probably Definitely No Investigators report to their LOCAL EC (24 h) Sponsor Sponsor / Investigator No Yes Unexpected? Yes SUSAR 1 Independent Ethics Committee Serious? If different from Investigator see App 1 Multicentre Studies Report to LEAD EC ® within max 7 days Short study title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx Report to: 1.Swissmedic 2. LEAD EC 3. All participating study sites ® within 7 days if fatal or life threatening days for all other Events ® within 15 40 | 62 Study title 14 Statistics 14.1 Randomization Describe the exact randomization method. The following elements need to be included: The unit of randomization; usually patients but sometimes body parts or clusters (hospital wards, physicians) are randomized What is randomized i.e. the interventions Allocation ratio Mechanism of how random numbers are generated Blocking: whether simple randomization or randomization in blocks is planned. For block randomization, block size(s) and the mechanism of variation need to be specified Stratification: whether or not randomization is stratified. For stratified randomization, all stratification variables need to be specified Who is responsible for generating and implementing the randomization list A statement that randomization list(s) is concealed from any study personnel involved in patient recruitment EXAMPLE: Randomization SOP CTU Bern Randomization After patient registration in the web-based data entry system and confirmation of all inclusion and exclusion criteria patients are randomized using the same system thus ensuring concealment of allocation (central web-based randomization). Patients are randomized in a 1:1 ratio to one of the two intervention groups: Intervention 1, intervention 2. The allocation sequence is generated by an independent statistician at CTU Bern not involved in the final analysis of the trial. It is based on computer generated random numbers in randomly varying blocks of 4, 6, and 8 using the statistical software package Stata (StataCorp LP, College Station, TX, USA). Randomization is stratified by the following factors: study center, age (<60years vs. >/= 60 years). Correct implementation of the randomization list in the web-based system is quality controlled by the responsible statistician. The randomization list is kept electronically at CTU Bern in a password protected file. Study personnel involved in patient recruitment will have no access to the list. The statistician responsible for the final analysis will also have no access to the list until the primary analysis of the trial is finished. 14.2 Datasets to be analyzed 14.2.1 Full analysis dataset The main analysis will be based on all randomized patients regardless of whether they actually received the allocated intervention or not or any other protocol deviations. Patients will be analyzed in the group they were randomized regardless of any cross-overs (intention-to-treat principle). 14.2.2 Per-Protocol analysis dataset Secondary analyses will allow for a per-protocol analysis where only patients will be included who actually received the allocated product for at least (give minimum amount of Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 41 | 62 Kommentar [TS12]: Provide a definition of the intention-to-treat analysis set for this trial. Study title intervention received e.g. two weeks) and other details that need to be fulfilled (e.g. the number of follow-up visits attended). 14.3 Statistical analysis All analyses will be done using provide details on statistical package(s) that will be used. All confidence intervals will relate to the 95% level and the two-sided significance level will be set at 0.05. No adjustment for multiple testing will be done. A detailed statistical analysis plan will be developed before the final analysis describing the exact procedures including assessments of model fit and consequences. 14.3.1 Primary analysis Describe how the primary outcomes will be analysed e.g. statistical model. Describe how missing data will handled. 14.3.2 Key secondary analyses Describe how the secondary analyses will be done e.g. statistical model. 14.3.3 Interim analyses and stopping rule of the study No interim analysis is foreseen in this study. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 42 | 62 Study title 15 Ethical and regulatory aspects This trial is conducted in accordance with the Declaration of Helsinki {World Medical Association, 2008 #2}, the International Conference on Harmonization Good Clinical Practice Guidelines (ICH-GCP) {International Conference on Harmonisation, 1996 #1} and all applicable national and cantonal laws. The participating investigators/institutions permit study-related monitoring, audits, independent ethics committee (IEC) review, and regulatory inspections and provide direct access to source documents/data. 15.1 Ethics committee approval The responsible investigator at each site ensures that an appropriately constituted independent ethics committee (IEC) is responsible for the initial and continuing review and approval of the clinical study. Before initiation of the study, the investigator forwards copies of the protocol, consent form and any other required document to the IEC for its review and approval. The investigator ensures that all changes in the research activity and all unanticipated problems involving risks to human subjects are reported promptly to the IEC, and that no changes are made to the protocol without prior sponsor and IEC approval, except where necessary to eliminate apparent immediate hazards to study participants. 15.2 Regulatory authority approval The sponsor ensures that the clinical study is approved by all relevant regulatory authorities according to local laws. 15.3 Study registration The sponsor ensures that the study is registered in a WHO approved clinical study register, preferably at clinicaltrials.gov, before participant recruitment in the study starts. 15.4 Informed consent The investigator assumes the responsibility of obtaining written informed consent for each participant before any study-specific procedures are performed and before any study intervention is administered. Persons meeting the criteria set forth in the protocol are offered the opportunity to participate in the study. To avoid introduction of bias, the investigator must exercise no selectivity with regard to offering eligible persons the opportunity to participate in the study. Eligible persons or parents/legal guardians of candidate persons receive a comprehensive explanation of the proposed treatment, including the nature of the therapy, alternative therapies available, any known previously experienced adverse reactions, the investigational status of the study interventions, and other factors that are part of obtaining a proper informed consent. Persons are given the opportunity to ask questions concerning the study, and adequate time to consider their decision whether to or not to participate. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 43 | 62 Study title Informed consent is documented by the use of a written consent form that includes all the elements required by regulations and as approved by the relevant ethics committee. The form is to be signed and dated by the participant or participant's legally authorized representative and by the person who administers the consent process. A copy of the signed form is given to the person who signed it, the original signed consent form is filed in the investigator site file An additional copy may be filed in the participant's medical chart. The following information must be recorded in the source documents: Date and time of the informed consent interview including any agreements if applicable e.g. participant will come back next day with a decision Confirmation that participant received the written participant information Any witnesses if applicable Any other information that seems important Persons can make a free decision on participation in the study at any time. If study participation is refused, the choice of treatment is at the discretion of the study participant. 15.4.1 Amendments If an amendment to the protocol changes the study schedule in scope or activity, or changes the potential risk to the participant, the informed consent document must be revised or amended with clear reference to the original document, submitted to the IEC for review and approved by the IEC before use. The revised or amended informed consent document must be used to obtain re-consent from any participants currently enrolled in the study if the participant is affected by the amendment, and must be used to document consent from any new participant enrolled after the approval date of the amendment. 15.5 Participant privacy and confidentiality The investigator affirms and upholds the principle of the participant's right to privacy. Investigators shall comply with applicable privacy laws. The written informed consent form will explain that the study data will be stored in a computer database, maintaining confidentiality in accordance with local data legislation. Subjects in this database will be identified by participant identifier or pseudonym. The participant information will also explain that for data verification purposes, authorized representatives of the sponsor, a regulatory authority, or an ethics committee may require direct access to parts of the medical records relevant to the study, including participants’ medical history. Especially, anonymity of the participants will be guaranteed when presenting the data at scientific meetings or publishing them in scientific journals. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 44 | 62 Study title 16 Administrative aspects 16.1 Protocol amendments In order to maintain comparable conditions in all study sites and to obtain an unobjectionable data analysis changes of the protocol are not intended. If nonetheless changes become necessary they are reported as amendment. Any change or addition to this protocol requires a written protocol amendment that must be approved by the study sponsor before implementation. Amendments affecting the safety of participants, the scope of the investigation or the scientific quality of the study, require additional full approval by the IEC of all sites, and by regulatory authorities. Examples of amendments requiring such approval are: Increase in intervention dosage or duration of exposure of participants Changes in the study design Increase in the number of invasive procedures to which participants are exposed Addition or deletion of a test procedure for safety monitoring The requirements for approval should in no way prevent any immediate action from being taken by the investigator or the study sponsor in the interests of preserving the safety of all participants included in the study. If an immediate change to the protocol is felt to be necessary by the investigator and is implemented by him/her for safety reasons the study sponsor should be notified immediately and the IEC at the centre should be informed within ten working days. Amendments affecting only administrative aspects of the study do not require formal protocol amendments or IEC approval but the IEC of each site must be kept informed of such administrative changes. Examples of administrative changes not requiring formal protocol amendments and IEC approval that can be treated as administrative amendments include but are not limited to: Changes in the staff used to monitor the study Changes in addresses 16.2 Insurance Insurance will be provided by the Sponsor. A copy of the certificate is filed in each investigator site file and the trial master file. 16.3 Funding and other support for the trial Provide sources and types of financial, material, and other support for the trial Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 45 | 62 Study title Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 46 | 62 Study title 17 Data handling and record keeping 17.1 Investigator site file At each site an Investigator Site File will be maintained including at least the following documents/information: Signed protocol and amendments Sample case report forms (CRF) Current informed consent form and all revisions Current patient information and all revisions Any other written information Financial aspects of the study Insurance statement All signed agreements/contracts Dated, documented approval of ethics committee and regulatory authorities Signed CVs of all investigators and any study personnel (update regularly) Instructions for handling of investigational product and study related materials Monitoring reports Study initiation report Follow-up letters of on-site visits Study close out report Relevant communication Letters Meeting notes Notes of telephone calls Signed informed consent forms Signed, dated and completed case report forms if applicable SAE reporting Notification by sponsor of safety information Interim and annual reports to ethics committee and regulatory authorities Subject screening log Subject identification code list Subject enrolment log Investigational product accountability Authorisation/signature sheet Record of retained body fluids/tissue samples Documentation of investigational product destruction Clinical study report 17.2 Case Report Forms All protocol required procedures along with information necessary to report the observations and tests described in this protocol are recorded in the case report forms (CRFs). CRFs must be completed and signed in a timely and accurate manner by study site personnel (usually within ten working days after completion of a visit). Dedicated study personnel at CTU Bern monitors the data entry progress per center to ensure timely data collection. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 47 | 62 Study title All data entered on the CRFs must be documented in a source document. The case report form is not a source document. If data is directly entered in the CRF without any source document this needs to be explicitly mentioned in the CRF. All the information on which the entries in the CRF are based must be available in the patient files e. g. results of laboratory investigations. The investigator must review all CRFs for accuracy and consistency with the protocol, and sign the CRFs upon completion. The investigator or a designee is responsible for the presence of the data in the patient file and the correct entry of the data into the CRF. After prior agreement, a check of the consistency of data between the patient files, raw data and CRF as well as with other documents related to the study may be conducted by the responsible authorities and/or by monitors (inspection/audit/monitoring). 17.3 Record keeping A copy of the final completed CRFs is retained by the investigator, who must ensure that it is stored with other study documents, such as the signed informed consent forms, protocol, the investigator’s brochure and any protocol amendments, in a secure place for 10 (15 for trials with implants) years. 17.4 Data management 17.4.1 Hardware and software The CRFs in this trial are implemented electronically using a dedicated electronic data capturing (EDC) system (WebSpirit®/RedCAP). The EDC system is activated for the trial only after successfully passing a formal test procedure. All data entered in the CRFs are stored on a Linux/Windows server in a dedicated PostgreSQL database. Responsibility for implementing and hosting the EDC system and the database lies with CTU Bern. 17.4.2 Data security, access and back-up The server hosting the EDC system and the database is kept in a locked server-room. Only the system administrators have direct access to the server and back-up tapes. A role concept with personal passwords (site investigator, statistician, monitor, administrator etc.) regulates permission for each user to use the system and database as he/she requires. All data entered into the CRFs are transferred to the database using Secure Sockets Layer (SSL) encryption. Each data point has attributes attached to it identifying the user who entered it with the exact time and date. Retrospective alterations of data in the database are recorded in an audit table. Time, table, data field, original value and altered value, and the person are recorded (audit trail). A multi-level back-up system is implemented. Back-ups of the whole system including the database are run internally several times per day and on external tapes once a day. The back-up tapes are stored in a secure place in a different building. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 48 | 62 Study title 17.4.3 Analysis and archiving At interim (if applicable) and final analyses, data files will be extracted from the database into statistical packages to be analyzed. The status of the database at this time is recorded in special archive tables. The study database with all archive tables will be securely stored by CTU Bern for at least 15 years. The sponsor also keeps the Trial Master File and interim and final reports both in electronic and in hard copy form for at least 10 (15 in case of implants) years. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 49 | 62 Study title 18 Quality assurance and control A multilevel data validation/monitoring plan is conceived in order to guarantee the correctness and consistency of the data in the CRFs and database. 18.1 Electronic and central data validation Data can be entered into the database only after a check of completeness and plausibility. Furthermore, selected data points are cross-checked for plausibility with previously entered data for that participant. In addition, data will be extracted from the database regularly to perform central data validation checks using statistical software packages. Any detected inconsistencies and relevant missing data will be queried at the respective center. 18.2 On-site monitoring In order to guarantee a high quality of the study and data retrieval, participating centers may be visited on site by independent monitors. Data protection rights are respected. All findings and comments are documented in a monitoring report and communicated to the investigator and to the sponsor. The investigator in the participating centers supports the monitor in his/her activities. Before study start (first participant in) a monitoring plan detailing all on-site monitoring related procedures is developed. On-site monitoring will be conducted by CTU Bern. 18.3 Inspections A regulatory authority or independent ethics committee may wish to conduct an inspection (during the study or even after its completion). If an inspection is requested, the investigator must inform the sponsor immediately that this request has been made. The investigators in the participating sites will support the inspectors in their activities. 18.4 Closure of study centers The study sponsor may exclude participating sites/investigators from further participation in the study because of fraud or non-compliance with the study protocol, ICH-GCP guidelines, or applicable laws. Study sites or investigators may stop recruiting patients to this study when the investigator deems inclusion of patients into this study to be no longer ethical for medical or organizational reasons. In this case, the investigator should give detailed reasons to the study sponsor. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 50 | 62 Study title 19 Dissemination policy 19.1 Public access to the full protocol, dataset, and statistical code Describe whether and how access to the protocol, dataset, and statistical code is enabled. 19.2 Publication policy and final report The final report will present the results of the study, including appropriate tables and figures in the spirit of an unbiased objectivity. The sponsor will provide the IEC with a summary of the study’s findings, and if applicable the regulatory authorities with any reports required. The report, or parts of it, may be submitted in the form of a summary, a synopsis, published article or in some other similar way. The sponsor assures that all results of this study are published in a peer-reviewed journal in line with the Declaration of Helsinki {World Medical Association, 2008 #2}. All results relate to all outcomes as defined in this protocol regardless of the direction or statistical significance. It will be assured that the manuscript follows the principle of the CONSORT guidelines or any other applicable reporting guideline (www.equator.org). Co-authorship on any of the publications will be based on contribution to the study and manuscript according to the criteria of the International Committee of Medical Journal Editors {International Committee of Medical Journal Editors, 2009 #3}. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 51 | 62 Study title 20 Post-trial care 20.1 Individual information of participants after end of trial Describe whether any procedures are in place to inform participants about the results of the trial. Describe whether and how participants are allowed to be informed about the intervention received during the trial in case of a blinded trial. 20.2 Access to effective trial interventions Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 52 | 62 Study title 21 Accompanying scientific projects Provide details on accompanying scientific project such as nested studies, biomarker/genetic studies etc. This might require additional sub-headings identifying the population, describing the outcomes, assessments, analyses, etc. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 53 | 62 Study title 22 References Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 54 | 62 Study title 23 Appendices Study flow diagram ............................................................................................................. 56 Study assessments overview ................................................................................................ 57 Insurance certificate............................................................................................................. 58 Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 55 | 62 Study title 23.1 Study flow diagram Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 56 | 62 Study title 23.2 Study assessments overview Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 57 | 62 Study title 23.3 Insurance certificate Provide a scan of the insurance certificate Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 58 | 62 Study title 23.4 Study contacts 23.4.1 Sponsor Name: ................................... Address: ................................ Tel: ........................................ E-mail: .................................. 23.4.2 Principal Investigator Name: ................................... Address: ................................ Tel: ........................................ E-mail: .................................. 23.4.3 Trial coordination/management Name: ................................... Address: ................................ Tel: ........................................ E-mail: .................................. 23.4.4 Monitoring Responsible for study site:.... Name: ................................... Address: ................................ Tel: ........................................ Kommentar [TS13]: Add if more than one monitor is responsible E-mail: .................................. 23.4.5 Drug supply Name: ................................... Address: ................................ Tel: ........................................ E-mail: .................................. 23.4.6 Data management Name: ................................... Address: ................................ Tel: ........................................ Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 59 | 62 Study title E-mail: .................................. 23.4.7 Statistics Name: ................................... Address: ................................ Tel: ........................................ E-mail: .................................. Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 60 | 62 Study title Kommentar [TS14]: Provide financial and other competing interests of sponsor and (principal) investigators for the overall trial and each study site 23.5 Declaration of interests Short title, Version #.## (dd.mm.yyyy) Based on CS_GEN_TEM-01_RCTProtocolTemplate_CTUBern_v01.docx 61 | 62
© Copyright 2026