Wilfrid Laurier University, Waterloo, Ontario Session: Winter 2009 (SAMPLE TEST) Location:
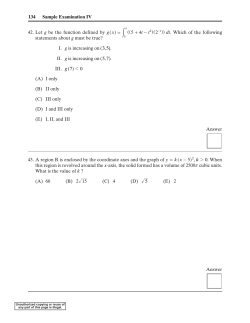
Wilfrid Laurier University, Waterloo, Ontario Session: Winter 2009 (SAMPLE TEST) Course No: CH234 Course Title: Aquatic Chemistry Professor: Dr. Scott Smith (not including coverpage) Length of Test: 50 minutes Examination Aids Allowed: calculators are allowed Location: Name: ID #: Number of pages 4 (14 questions) Answer all questions in the booklet provided. All questions are of equal weight and part marks will be assigned. Remember to put your name and id no. on your booklet(s). Official regulations for Laurier exams are given below: The doors of the examination room will be opened approximately 10 minutes before the start of the examination. Candidates will be permitted to enter the examination room quietly up to one half hour after the scheduled start of the exam. Candidates arriving late will not be allowed any extra time. Candidates must not begin the examination or attempt to read the examination questions until instructed to do so. Candidates once having entered, may not leave the exam room before completing and submitting the exam unless accompanied by a Proctor. Candidates are not permitted to submit their examination and leave the examination room until 1 hour after the examination has begun, and in no case before their attendance has been taken. In no case may a candidate leave the room temporarily, for any reason, until 30 minutes after the start of the examination. In order that remaining candidates are not disrupted, candidates must remain seated and may not leave the examination room during the last 15 minutes of the examination session. At the close of the examination period, candidates must stop writing immediately. The Presiding Officer may seize the papers of candidates who fail to observe this requirement, and a penalty may be imposed at the discretion of the instructor. Candidates must submit all their work, according to the instructions of the Presiding Officer, including all materials and a copy of the examination paper with their name and student ID number written on it. Unused examination booklets may not be taken from the examination room. A candidate who leaves before the examination is over must hand in all completed and attempted work, notes made during the exam, and a copy of the examination paper with their name and student ID number on it. Talk or any form of communication between candidates is absolutely forbidden. No information of any kind is to be written on the question paper or on scrap paper for the purpose of assisting other candidates. Responses to questions must not be done in an exaggerated way or in a manner that will involve transmission of information to others. Candidates must remain seated during the examination period. A candidate needing to speak to the proctor (e.g. to ask for additional supplies or to request permission to leave the examination room for any reason) should so indicate by raising his or her hand. Questions concerning possible errors, ambiguities or omissions in the examination paper must be directed to the proctor who will investigate them through the proper channels. The proctor is not permitted to answer questions other than those concerning the examination paper. Candidates must not use or attempt to use any improper source of information. No candidates for an examination may bring into the examination room any books, notes or other material containing information pertaining to the examination unless the examiner has given instructions that such material will be allowed and this instruction is specified on the examination paper. Any item brought into the examination room is subject to inspection. No briefcases, backpacks or other bags and carriers may be brought to the desk site where the candidate is writing the examination. These bags should be left outside the examination room. If books, notes etc. cannot be left outside the examination room, they must be put at the front of the examination room in a place designated by the proctor before a candidate takes a seat. Candidates are advised not to bring valuables to the examination room. No electronic or communication devices will be allowed in the examination room, including cell phones, blackberries, pagers, etc. Calculators are not allowed unless specified by the instructor and indicated on the examination paper. Only non-programmable models authorized by the instructor will be allowed. It is the candidate’s responsibility to ascertain whether the use of calculators is permitted, and, if it is, whether any restrictions are imposed on the types of calculators that may be brought to the examination. Translation dictionaries (e.g. English-French) or other dictionaries, (thesaurus, definitions, technical) are not allowed unless specified by the instructor and indicated on the examination paper. Electronic dictionaries are never allowed. Except for bottled water, no food or drink is allowed in the examination room. Candidates with health problems that warrant relaxation of this regulation should provide medical documentation to the presiding officer prior to the beginning of the examination. Such students should restrict themselves to those items and packaging that will least distract other examinees. Candidates are expected to write their examinations in an honest and straightforward manner. Where there are reasonable grounds for believing a violation of exam protocol has occurred, the candidate will be subject to the disciplinary procedures and sanctions according to the University Calendar. Only currently registered students will be permitted to write the final exam. Examinations conducted at Wilfrid University will be bound by WLU regulations, regardless of where the candidate is registered. Approved by Senate (Oct. 27/2003) CH234 Sample Test winter 2009 1. (5 points) What is the ANC (generalized alkalinity) for the following ? (a) A sample of pure water in equilibrium with carbon dioxide gas. At equilibrium the total dissolved carbon dioxide is 1.05 ×10−4 M and the pH is acidic. Assume there are no species other then carbonate species. (b) 0.1 M Na2 SO4 (c) 0.001 M HCl mixed with 0.005 M NaOH (d) 0.001 M HCl mixed with 0.005 M Na2 CO3 (e) 0.01 M NH4 Cl and 0.01 M NaCl 2. (5 points) What is the pH of a 0.034 M H2 SO4 solutions? 3. (5 points) Two waters are mixed in a volume ratio of 1:1. The alkalinity of the first water is 1.23 mM and the alkalinity of the second water is 2.34 mM. The alkalinity of the mixed water is 1.785 mM. Explain how the alkalinity value of the mixed waters is calculated and why this is a valid calculation. 4. (5 points) What is the alkalinity of a 3 mM sodium citrate solution? Citric acid is a triprotic acid H3 L. 5. (5 points) Silicic acid (H4 SiO4 ) results from the weathering of minerals. Silicic acid can be used by various microorganisms, such as diatoms, to grow their cell walls. The ability of diatoms to utilize aqueous silicic acid is dependent on pH though. Diatoms can utilize H3 SiO− 4 but not H4 SiO4 or 2− H2 SiO4 . For silicic acid the pKa values are pKa1 = 9.83 and pKa2 = 13.17. At what pH would you expect diatoms to grow best? 6. (5 points) List all of the processes given below that increase alkalinity? photosynthesis 6CO2(g) + 6H2 O → C6 H12 O6 + 6O2(g) denitrification + 5CH2 O + 4NO− 3 + 4H → 5CO2(g) + 2N2 + 7H2 O dissolution of carbonate CaCO3(s) + 2H+ → Ca2+ + H2 O + CO2(g) precipation of carbonate Ca2+ + CO2− 3 → CaCO3(s) Al-silicate weathering 2KAlSi3 O8(f eldspar) + 2CO2(g) + 11H2 O → Al2 Si2 O5 (OH)4(kaolinite) + 2K+ + 2HCO− 3 + 4H4 SiO4(aq) CH234 Sample Test winter 2009 7. (5 points) To analyze total sulphide in aqueous samples a “purge and trap” method is used. This method involves acidifying the sample while blowing argon gas through the sample. The outlet for the gas passes through a very basic sodium hydroxide solution. The sodium hydroxide solution is analyzed for sulphur using a colorimetric technique. Henry’s law constant for the reaction H2 S(g) * ) H2 S(aq) is KH = 10−0.991 . H2 S has two acid dissociations, pKa1=7 and pKa2=13.9. Explain the chemical basis for this technique. 1 mark bonus What is the analytical advantage if the basic “trap” solution is 10 mL and the “purge” sample is 500 mL? Be quantitiative. 8. (5 points) Paleolimnology (such as diatom analysis) can be used to determine water chemistry in the geologic past. If paleolimnology determines that sediments were deposited from water with a pH of 5.8, assuming that that water was in equilibrium with atmospheric carbon dioxide, what was the partial pressure of CO2(g) at the time of deposition? 9. (5 points) Cadmium can be complexed by chloride. A maximum of four chlorine atoms can bind to a central cadmium atom. Five sets of possible step-wise dissassociation constants are given below. Which one set of logK values is the only one that can be true. (a) log K1 = −0.05, log K2 = −0.09, log K3 = −0.9, log K4 = −1.32 (b) log K1 = −1.32, log K2 = −0.09, log K3 = −0.9, log K4 = −0.05 (c) log K1 = −1.32, log K2 = −0.90, log K3 = −0.09 (d) log K1 = −0.05, log K2 = −0.09, log K3 = −0.9, log K4 = −1.32, log K5 = −2 (e) log K1 = −1.32, log K2 = −0.90, log K3 = −0.09, log K4 = −0.05 10. (5 points) Benzoic acid, pKa=4.2, can be used to inhibit biological growth. The following data show the dependence of the minimum toxic concentration on the pH of the solution: pH 3.5 4.0 4.5 5.0 5.5 6.0 Minimum benzoic acid required (mM) 1.2 1.6 3.0 7.3 21.0 64.0 Interpret the concentration required for growth inhibition in terms of the acid-base chemistry of benzoic acid. Page 2 of 4 CH234 Sample Test winter 2009 2− 3− 11. (5 points) For the diagram below the chemical species (H3 AsO4 , H2 AsO− 4 , HAsO4 , AsO4 , HAsO2 , AsO− 2 ) corresponding to the labels are ... 2− 3− − (i) VI=H3 AsO4 , V=H2 AsO− 4 , VI=HAsO4 , III=AsO4 , II=HAsO2 , I=AsO2 2− 3− − (ii) I=H3 AsO4 , II=H2 AsO− 4 , III=HAsO4 , IV=AsO4 , V=HAsO2 , VI=AsO2 2− 3− − (iii) II=H3 AsO4 , III=H2 AsO− 4 , IV=HAsO4 , V=AsO4 , VI=HAsO2 , I=AsO2 2− 3− − (iv) IV=H3 AsO4 , III=H2 AsO− 4 , II=HAsO4 , I=AsO4 , VI=HAsO2 , V=AsO2 (v) none of the above Eh pH diagram for arsenic 12. (5 points) The toxic amount for a given metal at fixed pH and solution chemistry is 3 µM. If 1 mM Ca(NO3 )2 and 1 mM Mg SO4 are added the toxic amount is found to be 12 µM. Qualitatively explain this observation. 13. (5 points) The over formation constants for zirconium (Zr4+ ) with chloride are 0.92, 1.32, 1.51 and 1.2 for the formation of ZrCl, ZrCl2 , ZrCl3 and ZrCl4 (charges omitted) respectively. If seawater is 0.56 M total chloride, what are the most significant zirconium species? List the chloride species from most to least concentrated? Page 3 of 4 CH234 Sample Test winter 2009 14. (5 points) From the following diagram, determine the K values for the following reactions. Note: Fe(OH)3 and Fe(OH)2 are pure solid species. * Fe3+ + e− (i) Fe2+ ) (ii) Fe3+ + 3H2 O * ) Fe(OH)3(s) + 3H+ * Fe(OH)2(s) + 2H+ (iii) Fe3+ + 2H2 O + e− ) (iv) Fe(OH)2 + OH− * ) Fe(OH)3 + e− Fe pe-pH diagram Page 4 of 4
© Copyright 2026