Document 284588
Sample Problem
Using Boyle's Law to Calculate Volume
Problem
A weather balloon with a volume of 2.00 x 103 L at a pressure of 96.3 kPa rises to an
altitude of 1.00 x 103 m, where the atmospheric pressure is measured to be 60.8 kPa.
Assuming there is no change in temperature or amount of gas, what is the final
volume of the weather balloon?
What Is Required?
You need to find the volume, V2, after the pressure on the balloon has decreased.
What Is Given?
You know the pressure and volume for the first set of conditions and the pressure
for the final set of conditions.
P, = 96.3 kPa
V, = 2.00 x 103
P2 = 60.8 kPa
You know the temperature does not change.
Plan Your Strategy
Act on Your Strategy
Pressure and volume are changing, at constant temperature
and amount of gas. Therefore, use the equation for
Boyle's law.
Isolate the variable V*2 by dividing each side of the equation
byP 2 .
PiVi
J>2
Substitute numbers and units for the known variables in
the formula and solve. Make certain that the same units for
pressure are used in the equation.
PjVl
Pi
(96.3>Pa)(2.00 x 10 3 L)
= 3.17 X 10 3 L
According to Boyle's law, when the amount and temperature of a gas are constant, there is
an inverse relationship between the pressure and volume of a gas: V a —
Alternative Solution
Plan Your Strategy
Act on Your Strategy
According to Boyle's law, a decrease in pressure will cause
an increase in volume. Determine the ratio of the initial
pressure and the final pressure that is greater than 1 .
P, = 96.3 kPa
P2 = 60.8 kPa
pressure ratio ^ 1 is ^^
To find the final volume, multiply the initial volume of the
balloon by the ratio of the two pressures that is greater
than ] .
V2 = V, x pressure ratio
Q£T
P 00 x 10 3 L)
I1 Xx
"I LD-i
96 3 kftr
-60.8J<PS-
= 3.17 x 10 3 L
Check Your Solution
The units cancel out to leave the correct unit of volume, L. You would expect the volume
to increase when the pressure decreases, which is represented by the value determined.
Chapter 11 Properties of Gases • MHR
513
Practice Problems
Note: Assume that the temperature and amount of gas
are constant in all of the following problems.
1. 1.00 L of a gas at 1.00 atm pressure is compressed to
0.437 L. What is the new pressure of the gas?
2. A container with a volume of 60.0 mL holds a sample
of gas. The gas is at a pressure of 99.5 kPa. If the
container is compressed to one-quarter of its volume,
what is the pressure of the gas in the container?
2.29 atm
398 kPa
24 atm
0.27 L
1.3 x 102kPa
440 L
7. 14.3 mL
2
8. 1.1 X 10 L
3
9. 1.73 x 10 L
3. Atmospheric pressure on the peak of Mount Everest
can be as low as 0.20 atm. If the volume of an oxygen
tank is 10.0 L, at what pressure must the tank be
filled so that the gas inside would occupy a volume
of 1.2 x 103 L at this pressure?
2
10. a. 3.6 x 10 L
b. 5.6 x 102 min
6. The volume of carbon dioxide in a fire extinguisher
is 25.5 L. The pressure of the gas in this can is
260 psi. What is the volume of carbon dioxide
released when sprayed if the room pressure is 15 psi?
7. A 50.0 mL sample of hydrogen gas is collected at
standard atmospheric pressure. What is the volume of
the gas if it is compressed to a pressure of 3.50 atm?
8. A portable air compressor has an air capacity of
15.2 L and an interior pressure of 110 psi. If all the
air in the tank is released, what volume will that air
occupy at an atmospheric pressure of 102 kPa?
4. If a person has 2.0 x 102 mL of trapped intestinal gas
at an atmospheric pressure of 0.98 atm, what would
the volume of gas be (in litres) at a higher altitude
that has an atmospheric pressure of 0.72 atm?
9. A scuba tank with a volume of 10.0 L holds air at a
pressure of 1.75 X 104 kPa. What volume of air at
an atmospheric pressure of 101 kPa was compressed
into the tank if the temperature of the air in the tank
is the same as the temperature of the air before it was
compressed?
5. Decaying vegetation at the bottom of a pond contains
trapped methane gas. 5.5 x 102 mL of gas are released.
When the gas rises to the surface, it now occupies
7.0 x 102 mL. If the surface pressure is 101 kPa, what
was the pressure at the bottom of the pond?
10. An oxygen tank has a volume of 45 L and is
pressurized to 1200 psi.
a. What volume of gas would be released at 765 torr?
b. If the flow of gas from the tank is 6.5 L per
minute, how long would the tank last?
Kinetic Molecular Theory and Boyle's Law
Co to scienceontario
to find out more
Figure 11.12 The kinetic
molecular theory can
explain the relationship
between pressure
and volume. (d} and
d2 represent average
distances of molecules
from the container wall.)
514
M H R - U n i t 5 Gases
Pressure on the walls of a gas-filled container is caused by collisions of gas molecules
with the walls. Each collision of a gas molecule exerts a force on the wall. The average
force exerted by all the gas molecules divided by the surface area of the container is
equivalent to the pressure on the walls of the container. Examine Figure 11.12 to see
what happens when you change the external pressure on the gas. The containers have
pistons that will move until the external pressure and the internal pressure are equal.
If you increase the external pressure, the piston will move down, reducing the volume
available to the gas molecules. The gas molecules are now closer together and collide
with one another and the walls of the container more often. As the number of collisions
over time increases, the average force exerted by all the molecules increases; thus,
the gas pressure increases. If the temperature remains constant and no gas escapes or
enters, the decrease in the volume of the container will be inversely proportional to the
increase in the gas pressure.
Pe<t increases,
fand n fixed
Higher Pia causes
lower V, which causes
more collisions,
increasing the pressure
""til Pga$ = Pm
The Kelvin Temperature Scale and Absolute Zero
In 1848, twenty-five years after the death of Jacques Charles, Scottish physicist Lord
Kelvin (William Thomson, 1824-1907) interpreted the significance of the extrapolated
temperature at zero volume of a gas. Kelvin suggested that -273.15°C was the lowest
possible temperature, or absolute zero. He then established a new temperature scale
based on absolute zero as the starting point on the scale. The temperature scale was
named the Kelvin scale in his honour.
Figure 11.14 compares the Celsius and Kelvin temperature scales. The name of a
unit in the Kelvin scale is the kelvin (K). The size of the kelvin is the same as the size of
a Celsius degree, but the term "degree" is not used when reporting temperatures on the
Kelvin scale. As well, the starting points for these two temperature scales are different.
Notice that there are no negative values on the Kelvin scale. What would happen if
you tried to calculate a temperature that is twice as warm as -5°C? Mathematically,
the answer would be -10°C, but this is a colder temperature. When mathematical
manipulations are involved in studying gas behaviour, you need to convert
temperatures from the Celsius scale to the Kelvin scale.
absolute zero the
lowest theoretical
temperature, equivalent
to-273.15°C; the
temperature at which
the volume of a gas
approaches zero
For converting Celsius to kelvin: K = °C + 273.15
For converting kelvin to Celsius: °C = K — 273.15
The rounded-off value of 273 is often used as the conversion factor relating K and °C.
Celsius Scale
Kelvin Scale
100T
100°
80°
373 K
•'''imsmmHamaaammmm^
Boiling Water
60°
333
40°
20°
313
273 K
0"C
00
293
273
Freezing Water
-20°
IT
373
353
253
-40°
233
-60°
213
-80°
193
-100°
173
-120°
153
-140°
133
-160°
113
-180°
93
IT -200°
73
-220°
53
-240°
— -260°
33
-273 T
OK
13
Absolute Zero
.-.
Figure 11.14 There are 273 temperature units between absolute zero and the freezing
temperature of water on the Celsius and Kelvin scales. There are also TOO temperature units
between the freezing and boiling temperatures of water on both scales.
Apply If you double a Celsius temperature, by how much does the Kelvin temperature increase?
Chapter 11 Properties of Gases • MHR
517
Learning Check
13. What is the relationship between the temperature
and volume of a gas at constant pressure and amount?
14. What is absolute zero, and what is its significance?
15. Examine the graph in Figure 11.14. What do all the
graph lines have in common?
16. Make the following temperature conversions,
a. 27.3°C t o K
c. 373.2 K to °C
b.-25°CtoK
d. 23.5Kto°C
Activity
17. Why is it necessary to keep the pressure of a gas
constant when studying the relationship between
temperature and volume of a gas?
18. A teacher pours liquid nitrogen at a temperature of
77 K over a balloon. Predict the changes that would
occur to the balloon.
Analyzing the Temperature-Volume Relationship of a Gas
In this activity, you will use data from the table below and
the graph that you construct from them to analyze the
relationship between the temperature of a gas and its volume
and to infer the importance of the Kelvin temperature scale.
Procedure
1. Copy and complete the data table. For the second
column, you must calculate the Kelvin equivalent. For
the last two columns, you must calculate the quotient
of volume divided by temperature.
Volume versus Temperature Data
Tempe- TempeVolume
Volume (cm3)
Volume (cm3)
rature rature
3
(cm ) Temperature ("C) Temperature (K)
(°C)
(K>
8
29.5 |
20
30.8
30
32.1
40
34
50
37
60
42
70
49
Materials
• graph paper
• ruler
1
2. Draw one graph using the data from columns 1 and 4.
Draw a second graph using the data from columns 2 and 5.
Questions
1. Use a Venn diagram to describe how the two graphs that
you drew are similar and how they are different.
2. What is the x-intercept on each graph? What does each
represent?
3. Analyze your calculated values of -= (°C) and -= (K) in the
data table. What do you notice about the values?
4. How do the values of j (°C) compare to the values of
j (K)? Explain the significance of these sets of data.
5. Based on the data in this activity, what relationship seems to
exist between the volume and temperature of a gas, when
pressure and amount of gas remain constant? How is that
relationship affected by the temperature scale that is used?
Charles's Law and the Kelvin Temperature Scale
Charles's law a gas law
stating that the volume
of a fixed amount of gas
at a constant pressure is
directly proportional to
the Kelvin temperature
ofthegas: Voc T
518
MHR-Unit5 Gases
You learned at the start of this section that the volume of a gas is proportional to its
temperature, when pressure and amount of gas are constant. This relationship between
temperature and volume has become known as Charles's law. This law is often stated
in terms of a directly proportional relationship between temperature and volume. This
statement only holds true, however, if the temperature is expressed in Kelvin units. To
understand why, examine the graphs in Figure 11.5.
Both graphs show that the plot of temperature versus volume is a straight line, but
notice that Graph A—in which temperature is in degrees Celsius—does not show a
direct proportion. The graph of the line does not pass through the origin, and doubling
the temperature does not double the volume. Graph B does show a direct proportion;
temperature is in kelvins, and the graph of the line passes through the origin. A
temperature of 0 K corresponds to 0 ml. Doubling the temperature doubles the volume.
Sample Problem
Using Charles's Law to Calculate Volume of a Gas
Problem
A balloon inflated with air in a room in which the temperature of the air is 295 K has a
volume of 650 mL. The balloon is put into a refrigerator at 277 K and left long enough for
the air in the balloon to reach the same temperature as the air in the refrigerator. Predict
the volume of the balloon, assuming that the amount of air has not changed and the air
pressure in the room and in the refrigerator are the same.
What Is Required?
You need to find the volume, V2, of the balloon after it has been cooled to 277 K.
What Is Given?
You know the volume and temperature of the air sample for the first set of conditions
and the temperature for the second set of conditions:
V, = 650 ml
T, = 295 K
T2 = 277 K
Plan Your Strategy
Act on Your Strategy
Temperature and volume are changing at constant
pressure and amount of gas. Therefore, use the
equation for Charles's law.
V~i
Isolate the variable V2 by multiplying each side of the
equation by T2 and rearranging the equation.
^-(r2) = ^CK)
V2
r,
T2
n
<i
v
Substitute numbers and units for the known variables
in the formula and solve. Since the lowest number of
significant digits in values in the question is two, the
final volume is reported to two significant digits.
±^
V
v
>
v
^
T,
(650 mL)(277X)
(295XJ
= 610mL
According to Charles's law, when the amount and pressure of a gas are constant, there is a
directly proportional relationship between the volume of the gas and its Kelvin temperature:
Vex T
Alternative Solution
Plan Your Strategy
Act on Your Strategy
According to Charles's law, a decrease in temperature
will cause a decrease in volume. Determine the ratio
of the initial temperature and the final temperature
that is less than 1.
T2 = 277 K
TI = 295 K
To find the final volume, multiply the initial volume of
the balloon by the ratio of the two Kelvin temperatures
that is less than 1.
V2 = Vi x temperature ratio
277 K
£73 IX
= 610 ml
Check Your Solution
Volume units remain when the other units cancel out. Because the temperature decreases,
the volume is expected to decrease. The answer represents a lower value for the volume.
520
MHR-Unit5 Gases
Sample Problem
Using Charles's Law to Calculate Temperature of a Gas
Problem
A birthday balloon is filled to a volume of 1.50 L of helium gas in an air-conditioned room
at 294 K. The balloon is then taken outdoors on a warm sunny day and left to float as a
decoration. The volume of the balloon expands to 1.55 L. Assuming that the pressure and
amount of gas remain constant, what is the air temperature outdoors in kelvins?
What Is Required?
You need to find the outdoor air temperature, 72, in K.
What Is Given?
You know the volume and temperature of the air sample for the initial set of conditions and
the volume for the final set of conditions:
V, = 1.50 L
Ti = 294 K
V 2 = 1.55 L
Act on Your Strategy
Plan Your Strategy
Temperature and volume are changing at constant pressure
and amount of gas. Therefore, use the equation for
Charles's law.
V2
T2
Isolate the variable T2 by multiplying each side of the
equation first by T2 and then by -rp-.
V2r,
Substitute numbers and units for the known variables in the
formula and solve.
Since the number of significant digits in values in the
question is three, the final volume is reported to three
significant digits.
r,=
V,
_ (1.55/K294K)
(1.50,0
= 304K
Alternative Solution
Act on Your Strategy
Plan Your Strategy
According to Charles's law, a decrease in temperature will
cause a decrease in volume. Determine the ratio of the initial
volume and the final volume that is greater than 1 .
V, = 1.50 L
V 2 = 1.55 L
To find the final temperature, multiply the initial temperature T2 = T] x volume ratio
of the balloon by the ratio of the two volumes that is greater
pod
m v L-J§~±
UJ1K}X
1.50J/
than 1.
= 304K
Check Your Solution
The unit for the answer is kelvins. When the other units cancel out, kelvins remain. Because
the volume of the balloon had increased, you would expect that the temperature had increased.
The answer represents an increase in temperature.
Chapter 11 Properties of Gases-MHR
521
Practice Problems
Note: Assume that the pressure and amount of gas are
constant in all of the problems except question 20.
11. A gas has a volume of 6.0 L at a temperature of
250 K. What volume will the gas have at 450 K?
12. A syringe is filled with 30.0 mL of air at 298.15 K. If
the temperature is raised to 353.25 K, what volume
will the syringe indicate?
13. The temperature of a 2.25 L sample of gas decreases
from 35.0°C to 20.0°C. What is the new volume?
14. A balloon is inflated with air in a room in which the
air temperature is 27°C. When the balloon is placed
in a freezer at -20.0°C, the volume is 80.0 L. What
was the original volume of the balloon?
15. At a summer outdoor air temperature of 30.0°C, a
particular size of bicycle tire has an interior volume
of 685 cm3. The bicycle has been left outside in the
winter and the outdoor air temperature drops to
-25.0°C. Assuming the tire had been filled with air
in the summer, to what volume would the tire be
reduced at the winter air temperature?
16. At 275 K, a gas has a volume of 25.5 mL. What is its
temperature if its volume increases to 50.0 mL?
11. 11L
12. 35.5 mL
13. 1.29 L
14. 95 L
15.561cm 3
16. 539 K
17. 308 K
18. 27°C
19. 1.25 times room temperature
20. -214°C
Figure 11.16 When the
temperature of a gas
increases, the speed of the
gas molecules increases.
The gas molecules collide
with the walls of the
container more frequently,
thus increasing the
pressure. If the external
pressure remains the
same, the gas pushes the
piston up and increases the
volume of the container.
522
MHR-Unit5 Gases
17. A sealed syringe contains 37.0 mL of trapped air.
The temperature of the air in the syringe is
295 K. The sun shines on the syringe, causing
the temperature of the air inside it to increase. If
the volume increases to 38.6 mL, what is the new
temperature of the air in the syringe?
18. A beach ball is inflated to a volume of 25 L of air
in the cool of the morning at 15°C. During the
afternoon, the volume changes to 26 L. What was
the Celsius air temperature in the afternoon?
19. The volume of a 1.50 L balloon at room temperature
increases by 25.0 percent when placed in a hot-water
bath. How does the temperature of the water bath
compare with room temperature?
20. Compressed gases can be condensed when they
are cooled. A 5.00 x 102 mL sample of carbon
dioxide gas at room temperature (assume 25.0°C) is
compressed by a factor of four, and then is cooled
so that its volume is reduced to 25.0 mL. What
must the final temperature be (in °Q? (Hint: Use
both Boyle's law and Charles's law to answer the
question.)
Kinetic Molecular Theory and Charles's Law
Applying the kinetic molecular theory to Charles's law is shown in Figure 11.16.
The Kelvin temperature of a gas is directly proportional to the average kinetic energy
of the gas molecules. An object's kinetic energy is related to its speed (£k = — mv1).
As the temperature of a gas increases, the molecules move at higher speeds. As a result,
they collide with the walls of the container and with one another more frequently and
with greater force. Therefore, they exert a greater pressure on the walls of the container.
If, however, the external pressure on the gas stays the same, the gas pressure causes the
container to increase in size. As the volume of the container gets larger, the gas molecules
must travel farther to collide with the walls of the container and with one another. As
the collisions become less frequent, the pressure drops. The process continues until the
pressure inside the container is once again equal to the external pressure.
V increases
• ..Jk.
Higher T increases speed
and thus collision frequency:
V
> Pa,
V increases until
m
Go to scienceontario
to find out more
Developing a Mathematical Expression for Gay-Lussac's Law
Gay-Lussac's law states that the pressure of a fixed amount of gas, at constant volume,
is directly proportional to its Kelvin temperature. The relationship can be expressed
as P a T, where T is given in kelvins.
Using the general expression for a straight line (y = rax + b) and applying the
same mathematical treatment used for Charles's law, a mathematical expression for
Gay-Lussac's law is
"i _ "2
Ti ~ T2
For this equation, PL and 7\ represent the initial pressure and temperature conditions
and P2 and T2 represent the final pressure and temperature conditions. The relationship
applies as long as the volume and amount of a gas are constant and the temperature is
expressed in kelvins.
The following Sample Problem and Practice Problems will reinforce your
understanding of Gay-Lussac's law.
Sample Problem
Using Gay-Lussac's Law To Calculate Pressure of a Gas
Problem
The pressure of the oxygen gas inside a canister with a fixed volume is 5.0 atm at 298 K.
What is the pressure of the oxygen gas inside the canister if the temperature changes to 263 K?
Assume the amount of gas remains constant.
What is Required?
You need to find the new pressure, P2, of the oxygen gas inside the canister resulting from a
decrease in temperature:
What is Given?
You know the initial pressure of the oxygen gas in the canister, as well as the initial and final
air temperatures:
PI = 5.0 atm
T! = 298 K
T2 = 263 K
Act on Your Strategy
Plan Your Strategy
Temperature and pressure are changing at constant
volume and amount of gas. Therefore, use the
equation for Gay-Lussac's law.
Isolate the variable P2 by multiplying each side of
the equation by T,
PL_P
T,
2
T,
fa)
= fyfft
Pft - p
TT- P2
Substitute numbers and units for the known variables
in the formula and solve.
Since the lowest number of significant digits in values
in the question is two, the final pressure is reported to
two significant digits.
524
MHR-Unit5 Gases
Pz
PiT2
T,
(5.0atm)(263K)
298 K
= 4.4 atm
According to Gay-Lussac's law, when the amount and volume of a gas are constant,
there is a directly proportional relationship between the pressure of the gas and its
Kelvin temperature:
PCX T
Alternative Solution
Act on Your Strategy
Plan Your Strategy
According to Gay-Lussac's law, a decrease in
temperature will cause a decrease in pressure.
Determine the ratio of the initial temperature and the
final temperature that is less than 1.
T, = 298 K
T2 = 263 K
To find the final pressure, multiply the initial pressure
of the gas by the ratio of the two temperatures that is
less than 1.
PI = P} x temperature ratio
Jf.'l
\f
= 4.4 atm
Check Your Solution
The result shows the expected decrease in pressure. With kelvin units cancelling out,
the remaining unit, atm, is a pressure unit.
Practice Problems
Note: Assume that the volume and amount of gas are
constant in all of the following problems.
21. A gas is at 105 kPa and 300.0 K. What is the pressure
of the gas at 120.0K?
22. The pressure of a gas in a sealed canister is 350.0 kPa
at a room temperature of 298 K. The canister is
placed in a refrigerator and the temperature of the
gas is reduced to 278 K. What is the new pressure
of the gas in the canister?
23. A propane barbeque tank is filled in the winter at
-15.0°C to a pressure of 2500 kPa. What will the
pressure of the propane become in the summer
when the air temperature rises to 20.0°C?
24. A rubber automobile tire contains air at a pressure
of 370 kPa at 15.0°C. As the tire heats up, the
temperature of the air inside the tire rises to 60.0°C.
What would the new pressure in the tire be?
25. A partially filled aerosol can has an internal pressure
of 14.8 psi when the temperature is 20.0°C.
a. What would the pressure in the can be, in kPa,
if it were placed into an incinerator for disposal,
which would have the effect of raising the
temperature inside the can to 1800°C?
b. Approximately how many times higher is that
new pressure compared to standard atmospheric
pressure?
26. A sealed can of gas is left near a heater, which causes
the pressure of the gas to increase to 1.4 atm. What
was the original pressure of the gas if its temperature
change was from 20.0°C to 90.0°C?
27. Helium gas in a 2.00 L cylinder has a pressure of
1.12 atm. When the temperature is changed to
310.0 K, that same gas sample has a pressure of
2.56 atm. What was the initial temperature of the
gas in the cylinder?
28. A sample of neon gas is contained in a bulb at 150°C
and 350 kPa. If the pressure drops to 103 kPa, find
the new temperature, in °C.
29. A storage tank is designed to hold a fixed volume of
butane gas at 2.00 x 102 kPa and 39.0°C. To prevent
dangerous pressure buildup, the tank has a relief
valve that opens at 3.50 x 102 kPa. At what Celsius
temperature does the valve open?
30. If a gas sample has a pressure of 30.7 kPa at 0.00°C,
by how many degrees Celsius does the temperature
have to increase to cause the pressure to double?
24. 430 kPa
21. 42.0 kPa
22. 327 kPa
23. 2800 kPa
25. a. 720 kPa
26.
27.
28.
29.
30.
b. about 7 times higher
1.1 atm
136 K
-150°C
273°C
273°C
Chapter 11 Properties of Gases • MHR
525
Chapter 11
SELF-ASSESSMENT
Select the letter of the best answer below.
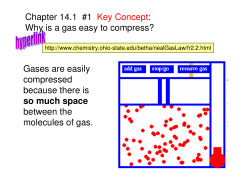
1. •:wfr Which of the following statements best explains
why gases can be easily compressed?
a. Molecules of a gas exhibit random translational
motion.
b. Molecules of a gas have negligible intermolecular
forces.
c. Molecules of a gas have small amounts of space
between them.
d. Molecules of a gas are in constant motion.
e. Molecules of a gas have little volume.
J
E
fi
0
bJ
X
a
II
E
M
2
3
OH
V
._
^
|
!-!
5
li
~ -<
\
* ~
r*j <^
! IN 00 .i «- •- M
g JD
1
IN . .
(N (N
,
CM
2. •:fl'fr Which of the following best describes a gas?
a. It assumes the volume and shape of the container,
and it has weak intermolecular attractions.
b. It assumes the volume and shape of the container,
and it has strong intermolecular attractions.
c. It has a distinct shape and volume, and it has strong
intermolecular attractions.
d. It has a distinct volume and assumes the shape of
the container, and it has moderate intermolecular
attractions.
O
e. It has a distinct volume and assumes the shape of
the container, but it lacks intermolecular attractions.
5 ol r*
o'
*>
in
111
a
g£ -^rs'
3. •:«»» Which of the following assumptions are made
by the kinetic molecular theory of gases?
I. Gas molecules move randomly in all directions.
II. Gas molecules exhibit negligible intermolecular
forces.
III. Gas molecules have negligible volume.
rt
oo*
M
IA
01
M
^
>0
tO
a. I and II only
b. I and III only
--
01
O
0s
w
a
19 ""
1
I
g « *
-s E
o
°- S
"'
S
2
C
r^ m
J
u
5
IA
u
<T3
E1
g
°3
^
t^
IT) \O
d. II only
e. I, II, and III
C. I only
4. BLfllJfr Which of the following represents the greatest
pressure?
10 ^ 0 TJ
a. 2.5 atm
b. 200 kPa
C. 960 mmHg
d. 21 psi
e. 790 torr
5. iilfi'a Which description best describes the situation
shown in the diagram below?
•%
_1kg
1kg
80°C
536
MHR-Unit5 Gases
a. a gas expanding as temperature increases and
pressure remains constant
b. a gas expanding as temperature and pressure rema
constant
c. a gas contracting as temperature increases and
pressure remains constant
d. a gas expanding as pressure remains constant
e. a gas expanding as temperature and pressure cham
6. •»• A sample of gas is in a sealed flexible container
at a fixed temperature. If the pressure on the containe
is reduced by half, the volume will
a. increase by a factor of 2
b. increase by a factor of 4
c. increase by a factor of 1
d. decrease by a factor of 2
e. decrease by a factor of 4
7. •!«'• A sample of nitrogen gas is placed in a sealed
2 L flexible container. Which of the following will
occur if the temperature of the gas is increased?
I. The pressure of the gas will increase.
II. The volume of the gas will decrease.
III. The speed of the gas molecules will increase.
a. I and II
b. I and III
c. I only
d. II only
e. I, II, and III
8. •«• What temperature on the Kelvin scale
corresponds to — 35°C?
a. 238
d. 333
b. 293
e. 35
c. 308
9- •:««» Identify the choice that best describes this
statement: "The volume of a fixed amount of gas is
directly proportional to its temperature at a constant
pressure."
a. Boyle's law
b. Charles's law
C. Gay-Lussac's law
d. kinetic molecular theory of gases
e. Avogadro's law
10. •«• A sample of argon gas is stored in a container
with a fixed volume at 1.00 atm of pressure. The
temperature, in K, of the gas is doubled. What is the
new pressure of the gas assuming the amount of gas is
constant?
a. 0.5 atm
d. 3.00 atm
b. 1.00 atm
e. 4.00 atm
c. 2.00 atm
Use sentences and diagrams, as appropriate, to answer the
questions below.
Pressure and Volume Measurements of a Gas
12. flCSP Each of the following observations relate to
properties of a gas. Name the property observed, and
explain the observation.
a. Gaseous oxygen and carbon dioxide are placed in
a sealed flask. After several minutes, the gases are
evenly distributed through the flask.
b. Air bubbled through water in a fish tank rises to the
surface and is released above the water.
15. •:«»» Torricelli and Pascal performed many important
studies of atmospheric pressure.
a. What is meant by the terms atmospheric pressure
and standard atmospheric pressure?
p( Explain what the term "millimetres of mercury"
refers to and how it relates to the discovery of
atmospheric pressure.
16. VUUP Determine the following conversions.
a. 551 kPatopsi
b. 6.0 psi to mrnHg
C. 0.52 atm to kPa
d. 902 mbar to mmHg and kPa
17. QdS An investigation to verify Boyle's law was
conducted. The data for the investigation are shown in
the table at the top of the next column.
100
2.5 X 102
75
3.3 X 102
50
25
15
5.0 X 102
l . O X 103
X
a. Calculate the missing value for volume, indicated
as x in the table.
b. Use the data to construct a graph that shows the *S
relationship between pressure and volume of a gas. I
Explain how it demonstrates Boyle's law.
/
C
18.
13. •:«»• In your own words, describe the kinetic
molecular theory of gases and its assumptions.
flESfe When food is being preserved by canning, a jar
is filled with very hot food, leaving a space at the top of
the jar. A rubber seal is placed on top of the jar, and the
lid is screwed shut. After several minutes, a "pop" is
heard, and the metal lid is observed to be dented
inward. Explain these observations using the kinetic
molecular theory and the properties of gases.
Volume (ml)
Pressure (kPa)
11. flQB Construct a graphic organizer to compare the
properties of gases with those of liquids. Include major
similarities and differences.
A sample of neon has a volume of 239 mL at
202.7 kPa of pressure. What is the pressure when the
volume is 500 mL? Assume the amount and
temperature of the gas are constant.
19. •«• What is absolute zeroj Dewribe a series of
experiments that could be performed that would
permit you to be able to determine its value.
2Q. flOB Use a graphic organizer to compare and contrast
the Kelvin and Celsius scales.
21. mum A sample of gas is heated from 273 K to 290 K.
If its original volume was 2.0 x 102 mL, what is the
volume after being heated? Assume amount and
pressure of the gas are constant.
22. mum A ball filled with air has a volume of 3.4 L at
25°C. What is its volume at 3.0°C, assuming constant
amount and pressure of air?
23. C3B Xenon gas is placed in a light bulb at 300 atm
and 20°C. When the bulb is in use, the bulb
temperature rises to 85°C. What does the pressure in
kPa become when the bulb is in use, assuming the
amount and volume of gas are constant?
24. mam Air in a ball has a pressure of 11.0 psi and a
temperature of 25.0°C. The temperature of the air rises
to 45.0°C. Calculate the new pressure of the air,
assuming a constant amount and volume of air.
25. flOB Describe one common occurrence or
technology that illustrates each of the gas laws that you
learned about in this chapter. (JUULotfULA jH^JL&B
Self-Check
If you
missed
question ...
1
2
Review
11.1 11.1
section (s)...
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19 | 20
21 I 22
23 24
!
1
\
n.il.ii.2 11.2 11.2 11.2, 11.3 11.3 11.3 11.1 11.1 11.1
11.3
25
|
|
11.1 11.2 11.2 11.2 11.2 11.2U1.3 11.3lll.3 11.3 11.3J11.2,
1
11.2
\
11.3
|
Chapter 11 Properties of Gases • MHR
537
© Copyright 2026