Supplemental Information
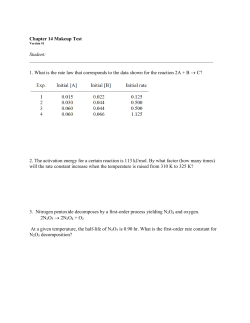
Molecular Cell, Volume 55 Supplemental Information Cytosolic pH Regulates Cell Growth through Distinct GTPases, Arf1 and Gtr1, to Promote Ras/PKA and TORC1 Activity Reinhard Dechant, Shady Saad, Alfredo J. Ibáñez, and Matthias Peter Supplementary material: Cytosolic pH regulates cell growth through distinct GTPases, Arf1 and Gtr1, to promote Ras/PKA and TORC1 activity Reinhard Dechant, Shady Saad, Alfredo J. Ibáñez and Matthias Peter cell size (fl) A 55 50 45 40 35 10-4 10-3 10-2 10-1 1 10 glucose (%) growth rate (1/h) B 0.5 uc r 0.4 Ra Gl Gl ff Va l 0.3 uc n Ga Pr l o 0.2 0.1 Fr Se Le u Gl yc 0 30 40 50 60 70 80 90 100 cell size (fl) C 8 Le cytosolic pH u Pr o Se r Gln 7.5 Gl uc 7 Fr uc Ra ff Ga l 6.5 40 50 60 70 80 90 cell size (fl) Dechant et al., Figure S1 A B extracellular pH 7.4 7.35 Gluc Gal Gluc Gal + + PMA1-TAP control Gluc Gal 7.3 7.25 PMA1-TAP 7.2 PGK1 7.15 0 1 2 3 4 5 6 time (min) PMA1-GFP - CHX D tetO7-PMA1 + dox + Whi5-GFP C m Dechant et al., Figure S2 0.16 0.14 OD600 0.12 0.10 pRS415 ctrl pRS415 dox pMET-Ras2 ctrl pMET-Ras2 dox 0.08 0.06 0.04 0.02 0 0 1 2 3 4 5 6 7 8 9 10 11 time (h) Dechant et al., Figure S3 B rel. Arf1-GFP membrane loc. (A.U.) 1.1 1.0 0.9 0.8 0.7 0.6 WT WT ctrl C-starv D Arf2-GFP C-starv E 1 0.85 0.75 0.65 WT 2-DOG gluocse 0 0 5 10 15 20 25 1.05 2 1.00 0.95 1 0.90 0.85 0.80 WT gluc 0 15 30 45 glucose (%) ctrl 0.95 time (min) rel. Arf2-GFP membrane loc. (A.U.) C 2 1.05 glucose (%) 2-DOG (%) Arf1-GFP rel. membrane localization (A.U.) A 0 time (min) F WT YPD 100mM Ca2+ GArf1 TVWDVGGQDRIRSLWRHYY Arf2 TVWDVGGQDRIRSLWRHYY Arl1 NVWDLGGQTSIRPYWRCYY Arl3 KFWDVGGQESLRSMWSEYY Dechant et al., Figure S4 A ctrl C-starv +CHX +Rap C Sch9-HA C-term B ctrl C-starv +CHX +Rap P-Sch9 (%) 125% WT 100% 75% 50% 25% P-Sch9 0% ctrl 5' 15' +gluc E 125% 125% P-Sch9 (%) 100% 75% 50% 25% 0% low gluc F 100% 75% ctrl dox 50% 25% 0% low gluc + 2-DOG Gtr1 WT Gtr1 GTP 150% P-Sch9 (%) P-Sch9 (%) 15' C-starv Pgk1 D 5' 100% WT 50% 0% pH 4.6 pH 7.0 Dechant et al., Figure S5 A GTR1 ctrl -C GTR1-GTP -N ctrl -C -N Sch9-HA C-term C B 3 P < 0.002 GTP/GDP ratio GTP/GDP ratio 3 2 1 0 SD-full D 1 Gluc P < 0.02 12 8 C GDP 13 ion count 6 4 2 0 Gal E THC-PMA1 GTP/GDP ratio 2 0 C-starv 10 n.s. ctrl 13 C GTP C GDP m/Z dox F Glucose pH GTP GDP Galactose ? pH GTP GDP C-starv ? Gtr1/2 Gtr1/2 TORC1 GTP GDP pH ? Gtr1/2 TORC1 TORC1 Dechant et al., Figure S6 A YPD WT 100mM Ca2+ WT B Rtg1-GFP tetO7-PMA1 + dox m Dechant et al., Figure S7 Supplementary Figure Legends Figure S1: Cytosolic pH tightly correlates with cell size on different Csources, related to Figure 1 (A) Correlation of cell size with glucose concentration. Cells expressing pHluorin were grown as in Figure 1A and cell size was determined. Cell size was plotted as a function of glucose concentration as in Figure 1A. (B) Correlation of growth rate and cell size with different nutrient conditions. Cells were grown as in Figure 1C and cell size was determined. Growth rates from Figure 1C were plotted as a function of cell size. (C) Correlation of growth rate and cytosolic pH on media containing alternative carbon sources or amino acids. Cells were grown as in Figure 1C and cell size was determined. Cytosolic pH was plotted as a function of cell size and data are represented as in Figure 1C. In all panels, error bars represent SEM. Figure S2: Regulation of Pma1 by C-source, related to Figure 2 (A) Measurement of net-proton export from cells. Representative example of raw data for the assay presented in Figure 2A. Acidification of the buffer is shown as a function of time upon addition of C-source. (B) Expression of PMA1 on different C-sources. Cells expressing Pma1-TAP were grown in SCmedia containing 2% glucose or 2% galactose and expression of Pma1 was determined by western-blotting. Pgk1 serves as a loading control. (C) Newly synthesized Pma1 is secreted into developing buds. Cells expressing Pma1GFP were grown and scored for Pma1 localization with or without treatment of cycloheximide (1h) to block new protein synthesis. Note that newly synthesized Pma1-GFP has low fluorescence due to the slow maturation of GFP. Thus, Pma1-GFP is barely visible in the absence of cycloheximide (CHX) in developing buds, but is readily detectable upon CHX treatment, which arrests growth and thus allows for maturation of the GFP fluorescence. (D) Localization of Whi5-GFP upon suppression of PMA1 expression. tetO7PMA1 cells expressing Whi5-GFP were grown in the presence of 10µg/mg doxycycline and scored for Whi5-GFP localization. A single mother cell (m) generating 3 daughter cells (arrow heads) is shown. Figure S3: Expression of wild-type Ras2 partially suppresses growth defect associated with suppression of Pma1 expression, related to Figure 3 tetO7-PMA1 cells expressing wild-type Ras2 or a control plasmid were grown in the presence or absence of doxycycline. Growth was determined by measuring OD600 as a function of time. Doxycycline was used at 10 µg/ml. Error bars represent SEM. Figure S4: Arf1 and Arf2 are regulated by glucose and V-ATPase activity, related to Figure 4 (A) Quantification of Arf1 membrane localization. Cells of the indicated genotype expressing Arf1-GFP were grown in SD media and membrane localization of Arf1 was quantified. Cells starved for glucose for 15 min are included as control. (B) Regulation of Arf1 by glucose metabolism. Cells expressing Arf1-GFP were grown in SC media containing 0.2% glucose, loaded into a microfluidic chip and Arf1 membrane localization was followed over time during addition of 2-DOG (solid red line). (C and D) Regulation of Arf2 membrane localization by glucose. Cells expressing Arf2-GFP were grown in SD media, loaded into a microfluidic chip and analyzed for Arf2 localization upon glucose starvation and readdition. (C) Representative images before and 15 min after glucose starvation and (D) quantification of time-lapse analysis are shown. (E) Synthetic lethality of arf1∆ and arf2∆ mutations. Diploid cells heterozygous for both arf1∆ and arf2∆ were sporulated and tetrads were dissected on YPD plates. Genotypes of the resulting spores are indicated. (F) Arf1 is not required for V-ATPase function. Cells of the indicated genotype were grown in YPD, tenfold serial dilutions of cells were spotted onto control plates (YPD) or plates containing 100 mM Ca2+ and scored for cell growth after 3 days. (G) Sequence alignment of ARF1 and related GTPases from S. cerevisiae. The conserved glutamine residue required for interaction with Arf-GAPs that was mutated to generate the hyperactive allele is indicated in red. In all panels, error bars represent SEM. Figure S5: Regulation of Sch9 phosphorylation by glucose is mediated by cytosolic pH and requires V-ATPase and Gtr1, related to Figure 5 and 6 (A) Cells expressing Sch9-HA were grown in SC medium containing 2% glucose, and Sch9-phosphorylation was analyzed by gel-shift assay following NTCB cleavage after glucose starvation, addition of 20 µg/ml Cycloheximide (CHX) and addition of 200nM Rapamycin. Note that CHX treatment increases TORC1 activity and serves as a control. (B) Wild-type cells were grown and treated as in (A) and analyzed for phosphorylation of Sch9 at T737 by western-blotting using a phosphospecific antibody. (C and D) Activation of Sch9 is regulated by glucose metabolism and Gtr1. Quantification of relative phosphorylation of Sch9 from the experiment shown in Figure 5A (C) and Figure 5B (D) is displayed as the mean +/- SEM of three independent experiments. (E) Activation of Sch9 is regulated by cytosolic pH through Gtr1. Quantification of relative phosphorylation of Sch9 from the experiment shown in Figure 5C is displayed as in (C). (F) V-ATPase is required for TORC1 activation. Quantification of relative phosphorylation of Sch9 from the experiment shown in Figure 6A is displayed as in (C). In all panels, error bars represent SEM. Figure S6: Two glucose dependent signals might regulate TORC1 dependent Sch9 phosphorylation, related to Figure 5 (A) Cells expressing wild-type Gtr1 or dominant active Gtr1-Q65L (GTR1GTP) were grown in SD media and starved for glucose and nitrogen for 15 min. Sch9 phosphorylation was analyzed by western blotting following NCBT cleavage. (B-D) Measurement of GTP/GDP ratio in cells after perturbations of cytosolic pH. (B) Wild-type cells were grown in SD medium, washed and resuspended in SD medium (SD-full) or in medium w/o glucose (C-starv) and the ratio of GTP/GDP was determined as described in the supplemental Materials and Methods. (C) Wild-type cells were grown in media containing glucose or galactose and GTP/GDP ratio was determined. (D) Cells harboring the tetO7-PMA1 allele were grown in the presence or absence of doxycycline and GTP/GDP ratio was determined. Doxycycline was used at 10 µg/ml. The P-values obtained from a Student’s t-test are indicated. n.s.: not significantly different (P>0.1). (E) Schematic representation of the analysis of intrinsic GTP fragmentation into GDP using 13 C and 12 C labeled standards. See supplemental Materials and Methods for details. (F) Model depicting the proposed integration of different glucose dependent signals by TORC1. Glucose promotes activation cell growth by two independent signals. In addition to cytosolic pH, another signal exists that regulates Sch9 phosphorylation at the level of, or downstream of Gtr1. This signal may be the ratio of GTP/GDP, or an associated metabolic signal. While growth on alternative C-sources mostly affects the pH signal, complete glucose starvation inactivates both signals, thus leading to inactivation of TORC1 even in cells expressing dominant-active Gtr1. In all panels, error bars represent SEM. Figure S7: Regulation of V-ATPase activity by Gtr1 and pH dependent regulation of Rtg1, related to Figure 6 and 7 (A) Cells of the indicated genotype were grown in YPD, tenfold serial dilutions of cells were spotted onto control plates (YPD) or plates containing 100 mM Ca2+ and scored for cell growth after 3 days. (B) tetO7-PMA1 cells expressing Rtg1-GFP were grown in SD medium in the presence of doxycycline and Rtg1 localization was analyzed by fluorescence microscopy. A mother cell (m) generating multiple daughter cells (arrowheads) is shown. Doxycycline was used at 10 µg/ml. Supplementary Tables Table S1: plasmids used in this study Plasmid No. Genotype Source pRD22 pRS306-pGPD-pHluorin (Dechant et al., 2010) pRD23 pRS415-pADH-pHluorin (Dechant et al., 2010) pBL98 YEP213-Ras2-V19 C. DeVirgilio pB1510 MET25-GFP-Ras2 (Wang and Deschenes, 2006) pRD30 pYX212-EGFP-RBD-3 (Leadsham et al., 2009) pRD50 pRS415-pADH1-ARF1-Q71L This study pRD32 pGAL-GST-Arf1 Thermo Scientific pRD33 pGAL-GST-Gtr1 Thermo Scientific pJU660 pRS415 GTR1 (Binda et al., 2009) pMB1483 pRS415 GTR1-Q65L (Binda et al., 2009) pJU656 pRS415 GTR1-S20L (Binda et al., 2009) pJU676 pRS416 SCH9-5HA (Urban et al., 2007) pRD40 pRS413 SCH9-5HA This study pMKi102 pRS413 GFP-ATG8 M. Kijanska Table S2: yeast strains used in this study Strain No. Relevant genotype Source BY4741 MATa ura3∆0; leu2∆0; his3∆1; met15∆0 Openbiosystems BY4742 MATα ura3∆0; leu2∆0; his3∆1; met15∆0 Openbiosystems FY4 MATa, isogenic prototrophic parent of BY4741 (Winston et al., 1995) FY3 FY4 ura3-52 (Winston et al., 1995) yRD204 BY4741, tetO7-PMA1::KanR; pCMV-TA*::URA3 R Openbiosystems yRD202 BY4741, tetO7-PMA1::Kan ; pCMV-TA*::URA3, WHI5-GFP::HIS3 This study yRD215 BY4741, PMA1-TAP::HIS3 Openbiosystems yRD216 BY4741, PMA1-GFP::HIS3 Invitrogen yRD135 BY4741, vma2∆::KanR (Dechant et al., 2010) yRD222 BY4741, ARF1-GFP::HIS3 Invitrogen yRD341 BY4741, ARF2-GFP::HIS3 yRD220 BY4741, ARF1-GFP::HIS3; vma2∆::Kan Invitrogen R This study yRD224 BY4741, ARF1-GFP::HIS3; gea1∆::KanR This study yRD65 BY4741, VMA5-GFP::HIS3 (Dechant et al., 2010) yRD267 BY4741, arf1∆::KAN R Openbiosystems yRD294 BY4741, arf2∆::KANR Openbiosystems yRD295 BY4742, arf2∆::KAN R Openbiosystems yRD268 BY4741, VMA5-GFP::HIS3; arf1∆::KanR This study yRD195 BY4741, TMD-RFP::HIS3 This study yRD201 BY4742, TMD-RFP::HIS3 This study yRD279 R BY4741, arf1∆::KAN , TMD-RFP::HIS3 R yRD210 BY4741, vma2∆::Kan ; TMD-RFP::HIS3 yRD269 R BY4741, arf1∆::Kan ; vma2∆::Kan R R R Openbiosystems Openbiosystems This study yRD280 BY4741 arf1∆::Kan ; vma2∆::Kan ; TMDRFP::HIS3 Openbiosystems yRD288 BY4741, stv1∆::KanR; TMD-RFP::HIS3 Openbiosystems R R yRD289 BY4741, arf1∆::Kan ; stv1∆::Kan ; TMDRFP::HIS3 This study yRD231 BY4741, STV1-TAP::HIS3 Openbiosystems yRD232 BY4741, VPH1-TAP::HIS3 Openbiosystems yRD258 BY4741, RTG1-GFP::HIS3 Invitrogen yRD259 BY4741, GAT1-GFP::HIS3 Invitrogen yRD243 BY4741, gtr1∆::KanR yRD256 R BY4741, gtr1∆::Kan ; vma2∆::Kan yRD305 BY4741, gtr1∆::KanR;VMA5-GFP::HIS3 R yRD307 BY4741, npr2∆::Kan yRD233 BY4741, CUP5-TAP::HIS3 yRD209 Openbiosystems R This study This study Openbiosystems Openbiosystems R This study R BY4741, tetO7-PMA1::Nat ; pCMV-TA*::URA3 yRD321 BY4741, tetO7-PMA1::Nat ; pCMV-TA*::URA3; gtr1∆::KanR This study yRD265 BY4741, tetO7-PMA1::KanR; pCMV-TA*::URA3, RTG1-GFP::HIS3 This study yRD311 BY4741, PGK1-GFP::HIS3 Invitrogen Supplementary Materials and Methods: Determination of GTP/GDP ratios using MALDI-MS Cells were harvested by directly mixing 0.5 ml of cell suspension with 1 ml 75% MeOH, which was precooled to -400C. Cells were collected by centrifugation (1 min at 4000g, -100C) and cell pellets were flash frozen in liquid N2 and stored at -800C. For MALDI MS, cells were reconstituted in 500 µl of a MeOH/ddH2O mixture (60:40) + 0.85% (w/V) ammonium bicarbonate buffer, pre-cooled at -400C, and carefully shaken to avoid cell aggregation. The samples were centrifuged (1 min at 1000g, -100C) and the cell pellet was reconstituted in 500 µl of 60% MeOH in ddH2O (-400C) and slightly shaken. 150 µl of the cell suspension (~1,000,000 cells per mL) were spiked with 13Clabeled GTP and mixed with 50 µl of the MALDI matrix, i.e. 9-aminoacridine (10 mg/mL dissolved in 60% MeOH in ddH2O). To avoid any changes to the metabolism during the subsequent spotting of the samples onto the MALDI steel target, the MALDI target was cooled (-400C) on a dry ice – ethanol bath in a nitrogen-rich atmosphere (with a constant flow of N2 gas) to avoid condensation. The MALDI MS measurements were carried out on a commercial MALDITOF/TOF mass spectrometer (AB Sciex TOF/TOF 5800, AB Sciex, Concord/ON, Canada). This instrument is equipped with a solid-state Nd:YAG (neodymium-doped yttrium aluminum garnet) laser. The laser emits pulses of ~4 ns duration at a wavelength of a 349-nm and at a repetition of 400 Hz. For ion generation, a fixed laser intensity of 4800 au (TOF/TOF series explorer) was applied. Measurements were conducted in the negative-ion reflection mode, analyzing a mass range between 70 and 900 m/z, while MS/MS analyses were conducted using the collision-induced dissociation (CID) cell, using air as the collision gas. During our measurements, we avoided sweet spot effects by using a center biased rastering pattern with 900 shots (30 subspectra x 30 shots). The intrinsic, partial fragmentation of GTP into GDP during laser desorption was determined as described previously (Steinhoff et al., 2013). In brief, a 1:1 mixture of 13 C-labelled GTP and unlabeled GDP (12C-GDP) was measured using MALDI-MS and relative intensities of 12C-GDP, 13C-GDP and 13C-GTP were determined. Based on this, we calculated a rough correction factor and confirmed that the intrinsic fragmentation of GDP is negligible under our experimental conditions. The correction factor was then fine-tuned for each individual experiment. Importantly, the observed correction factor was unaffected by growth conditions of the cells prior to quenching. Data treatment and data analysis software Spectral data (i.e., accurate mass, signal intensity, etc.) were calculated from the raw spectra using a MATLAB (MathWorks, Natick, MA, USA) peak recognition software that was kindly made available by Uwe Sauer and Nicola Zamboni (Institute of Molecular Systems Biology, ETH Zürich) (Fuhrer et al., 2011). Prior to using the MATLAB program, the raw data were transformed into an mzXML format using the freeware program Peak List Conversion Tool, available from http://www.proteomecommons.org. Chemical assignments (identities) were made on the basis of accurate mass measurements (accuracy of ~50 ppm or better). Supplementary References: Binda, M., Péli-Gulli, M.-P., Bonfils, G., Panchaud, N., Urban, J., Sturgill, T.W., Loewith, R., and De Virgilio, C. (2009). The Vam6 GEF controls TORC1 by activating the EGO complex. Molecular cell 35, 563-573. Dechant, R., Binda, M., Lee, S.S., Pelet, S., Winderickx, J., and Peter, M. (2010). Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. Embo J 29, 2515-2526. Fuhrer, T., Heer, D., Begemann, B., and Zamboni, N. (2011). High-throughput, accurate mass metabolome profiling of cellular extracts by flow injectiontime-of-flight mass spectrometry. Anal Chem 83, 7074-7080. Leadsham, J.E., Miller, K., Ayscough, K.R., Colombo, S., Martegani, E., Sudbery, P., and Gourlay, C.W. (2009). Whi2p links nutritional sensing to actin-dependent Ras-cAMP-PKA regulation and apoptosis in yeast. J Cell Sci 122, 706-715. Steinhoff, R.F., Krismer, J., Eyer, K., Fagerer, S.R., Ibanez, A., Pabst, M., and Zenobi, R. (2013). Rapid estimation of the energy charge from cell lysates using MALDI-MS: role of in-source fragmentation. Analytical biochemistry. Wang, G., and Deschenes, R.J. (2006). Plasma membrane localization of Ras requires class C Vps proteins and functional mitochondria in Saccharomyces cerevisiae. Mol Cell Biol 26, 3243-3255. Winston, F., Dollard, C., and Ricupero-Hovasse, S.L. (1995). Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11, 53-55.
© Copyright 2026