Document 3320
Compendium of Lectures
Winter School
on
Chemical Analysis of Value Added Dairy
Products and Their Quality Assurance
January 11-31, 2011
Editing and Compilation
Dr. Rajan Sharma
Dr. (Mrs.) Bimlesh Mann
DAIRY CHEMISTRY DIVISION
NATIONAL DAIRY RESEARCH INSTITUTE
(Deemed University)
Karnal – 132 001 (Haryana) INDIA
Dr. Rajan Sharma
Senior Scientist & Director, Winter School
Dr. (Mrs.) Bimlesh Mann
Principal Scientist & Co-Director, Winter School
Course Advisors
Dr. (Mrs.) B.K. Wadhwa
Dr. Darshan Lal
Dr. Raman Seth
ALL RIGHTS RESERVED
No part of the lecture compendium may be reproduced or transmitted in any form or by
any means, electronic or mechanical, including photocopy, recording, or any information,
storage and retrieval system without the written permission of Director, NDRI, Karnal.
FOREWORD
The increased concern of consumers for improving overall health and reducing risk for specific
diseases through food, gives an opportunities for expanding the dairy products to provide benefits
beyond their traditional nutritional value. Milk is considered as an ideal vehicle for developing valueadded products, as it already contains a number of beneficial major and minor micro-nutrients and
bioactive peptides. In society, as incomes rise and economic conditions improve, the demand for more
varied foodstuffs increases. Consequently, a large number of new products are being brought to the
market every year. The organized dairy industry is constantly looking for technologies for product
diversification that can enhance its competitive edge and increase profitability on sustainable basis.
The commodities like milk powder and ghee which remain the main stay of the dairy sector at the
moment, does not appear to be sustainable in future and hence a major shift in products for organized
dairy industry seems inevitable. Empirical evidences also suggest that the composition of an average
Indian's food basket is gradually shifting towards value added products. Purchasing power of the
consumers is on the upswing and the Indian middle class looks for value and quality and is willing to
pay extra bit for this purpose.
Dairy products enriched with the health attributes of functional ingredients, which is considered
as potential novel foods for health promotion should be safe. However, the level of health claim with
optimum sensory and textural properties of such foods has yet to be investigated. As the demand for the
value added dairy foods is increasing, so is the requirement for developing analytical methodologies
for assuring the consumer about the health claims.
In the present Winter School, the curriculum has been designed comprehensively to cover various
aspects for assuring the quality of value added dairy products. The emphasis will be given on analytical
techniques for the validation of health claims made in the value added dairy products and also to
provide hands-on-practical training to the participants on latest techniques being used in the area of
Dairy Chemistry.
I am sure that the deliberations in the 21 days Winter School on “Chemical Analysis of Value
Added Dairy Products and Their Quality Assurance” will be highly useful for the participants in the
area of Quality Assurance of Value Added Dairy Products. Further the information compiled by the
organizers in the form of compendium will also benefit the Faculty, Scientists and Students of Dairy
Chemistry and Allied Disciplines and serve as a guide to solve their problem at their respective place
of work.
I wish winter school a great success.
(A.K. Srivastava)
DAIRY CHEMISTRY DIVISION
NATIONAL DAIRY RESEARCH INSTITUTE
(Deemed University)
Karnal – 132 001 (Haryana) INDIA
Dr. (Mrs.) B.K. Wadhwa
Principal Scientist & Head
PREFACE
Dairy Chemistry Division, the host division of this Winter School is one of the oldest divisions of
the institute. The division has made significant achievements viz. kit for detection of 12 adulterants,
tests for detection of synthetic milk, technologies for calcium enriched milk and low cholesterol ghee.
The knowhow of these tests and technologies has been commercialized/transferred. Three analytical
methods viz. 10.75 ml milk pipette for the accurate estimation of fat in buffalo milk/mixed milk; lactometer
for SNF and dual purpose Gerber butyrometer for quantitative and purity determination of fat in milk
have been adopted by BIS. The other significant achievements are rapid methods for detection
of soya milk; detection of vegetable oils in ghee; technologies for low calorie artificially sweetened
dairy products; antioxidant rich fruit whey beverages; value addition through fortification of herbs and
cereals in milk and milk products; synbiotic ice cream; protein rich powder from buffalo colostrum,
bioactive peptides from whey protein hydrolysates and many more. Number of protocols have been
developed for the analysis of contaminants flavor compounds and bioactive components of milk and
milk products. The division has also contributed significantly in basic studies namely ghee flavor
chemistry, bioactive peptides like lactoferrin, osteopontin, caseinophosphopeptides etc. Currently, the
division is progressing towards quality control aspects viz. detection of adulterants at microlevel using
nanotechnology; application of nanotechnology in dairy foods, validation of methods for the detection
of foreign fats in ghee and development and evaluation of multiple micronutrients fortified milks. The
division has completed four externally funded projects and now engaged in four more such projects.
The faculty has successfully conducted about 20 trainings/national seminars/summer schools/Winter
Schools on fat rich dairy products, on quality control aspects, analytical techniques and bioactive
components etc. The faculty has published more than 1000 publications constituting research papers,
popular articles, review articles, books, compendium etc. The faculty has also autoured 5 books, 15
book chapters and10 teaching manuals.
Milk and milk products serve as an ideal delivery system for micronutrients. The demand for the
value added dairy products is continuously increasing because of consumer awareness about health
and nutrition. It is also important to ensure the consumer about the quality and health and nutrition
claims of such products. This can be achieved by analytical methods and techniques. I am sure the
knowledge gained through this Winter School on “Chemical Analysis of Value Added Dairy Products
and Their Quality Assurance” will be of immense use and of great interest for all the participants.
I wish you all a great success and a very happy and prosperous new year.
(B.K. Wadhwa)
ACKNOWLEDGEMENT
We feel honored that ICAR has entrusted us with the responsibility of organizing a Winter School on
“Chemical Analysis of Value Added Dairy Products and Their Quality Assurance” to Dairy Chemistry
Division at NDRI, Karnal. We are highly thankful to Dr. Kusumakar Sharma, Assistant Director General
(HRD), ICAR, New Delhi for giving us this opportunity to organize the Winter school at NDRI, Karnal and
for timely release of funds.
We want to place on record our deep sense of gratitude for Dr. A.K. Srivastava, Director NDRI Karnal
for his keen interest, valuable guidance and encouragement. He personally monitored the arrangements for
smooth conduct of the programme without which it would have not been possible to host the school in a befitting
manner. We are also thankful to the Dr. S. L. Goswami and Dr. G.R. Patil Joint Directors of NDRI for their
constructive suggestions and valuable support.
The kind cooperation and overwhelming support of Dr. (Mrs.) B.K. Wadhwa, Head, Dairy Chemistry
Division for the conduct of Winter School is gratefully acknowledged. We also express our thanks to other Course
Advisors Dr. Darshan Lal and Dr. Raman Seth and other Scientists of Dairy Chemistry Division, who served
on various committees and helped in planning and organizing this activity.
We take this opportunity in thanking honoured guest speakers who traveled all the way to Karnal to
share their knowledge and expertise with us. The faculty for this winter school transcended the boundaries of
conventional Divisions at NDRI and was spread to different Divisions i.e. Dairy Microbiology, Dairy Chemistry,
Dairy Technology, Animal Biochemistry, Animal Biotechnology, Dairy Cattle Nutrition and Dairy Cattle
Breeding. We express our gratitude to the faculty from all these disciplines for delivering lectures and conducting
practical classes during the winter school.
All our research scholars assiduously and enthusiastically worked for the success of this program, and they
deserve high acclaim and appreciation for the same. We also place on record our appreciation for secretarial
services of Mr. Ajit Singh and Mrs. Shakuntla Rani and helpful hand extended by Mr. Rajiv Sharma, and Mr.
Deepak, Mr. Mahinder Yadav and Mr.Chanderpal in day to day work.
We are highly thankful to all the participants for making it to Karnal. We highly appreciate the cooperative
spirit displayed by the participants. We are also grateful to the Heads of various Institutions and Departments
for sponsoring the participants.
(Bimlesh Mann)
Principal Scientist & Co-Director Winter School
(Rajan Sharma)
Senior Scientist & Director Winter School
Committees for Organisation of Winter School
Organizing Committee
Dr. (Mrs.) B.K. Wadhwa, Head & Principal Scientist
Dr. Darshan Lal, Principal Scientist
Dr. Raman Seth, Principal Scientist
Dr. (Mrs) Bimlesh Mann, Principal Scientist
Dr. Sumit Arora, Senior Scientist
Dr. Vivek Sharma, Senior Scientist
Dr. Rajesh Kumar, Senior Scientist
Dr. Rajan Sharma, Senior Scientist (Convener)
Registration Committee
Dr. Raman Seth (Chairman)
Dr. Rajesh Kumar (Convener)
Dr. Sumit Arora
Sh. P.C. Singh
Sh. Ajit Singh
Technical Comiittee
Dr. Darshan Lal (Chairman)
Dr. (Mrs) Bimlesh Mann (Convener)
Dr. Raman Seth
Dr. Rajesh Kumar
Dr. Rajan Sharma
Hospitality Committee
Dr. (Mrs.) B.K. Wadhwa (Chairman)
Dr. Vivek Sharma (Convener)
Dr. (Mrs.) Bimlesh Mann
Sh. Rajeev Sharma
Purchase Committee
Dr. (Mrs) Bimlesh Mann (Chairman)
Dr. Rajan Sharma (Convener)
Dr. Rajesh Kumar
Dr. Vivek Sharma
Contents
THEORY
1.
Novel and Emerging Food Technologies for Defence Food Supplies
1
A. S. Bawa
2.
An Overview of Designer Functional and Health Foods
5
A. K. Srivastava
3.
Prospects of Value Addition Through Functional Ingredients
10
G. R. Patil
4.
Technological and Nutritional Aspects of Milk Phospholipids
17
B. K. Wadhwa and Rajesh Kumar
5.
Methods of Cholesterol Removal to Develop Low –
Cholesterol Dairy Products
22
Darshan Lal and Vivek Sharma
6.
Fortification of Milk and Milk Products for Value Addition
29
Sumit Arora
7.
Packaging of Value Added Foods and Their Storage Stability
36
P. P. Gothwal
8.
Novel Technologies for Processing and Packaging of
Health Foods and Beverages
40
H. N. Mishra
9.
Glycomacropeptide – Biological Properties and its Application
49
Rajan Sharma and Neelima Sharma
10. New Approaches to Detect the Adulteration of Ghee
with Animal Body Fats and Vegetable Oils/ Fats
54
Vivek Sharma, Darshan Lal, Arun Kumar and Amit Kumar
11. Colostrum Powder and its Health
Benefits
59
Raman Seth and Anamika Das
12. Cow Ghee Protects from Mammary Carcinogenesis: Mechanism
68
Vinod K. Kansal, Rita Rani and Ekta Bhatia
13. Lateral Flow Assay- Principle and its Application in
Analytical Food Science
72
Rajan Sharma and Priyanka Singh Rao
14. Separation Strategies for Bioactive Milk Proteins
77
Rajesh Kumar
15. SDS-PAGE – Principle and Applications
81
Y. S. Rajput and Rajan Sharma
16. Western Blot: Theoretical Aspects
Y. S. Rajput and Rajan Sharma
85
17. Enzyme Linked Immunosorbent Assay - Theory
88
Rajeev Kapila and Suman Kapila
18. Experimental Determination of Thermal Stability of
Proteins: A Theoretical Background
93
Jai K. Kaushik
19. Species-Specific Identification of Milk and Milk
Products: A Molecular Approach
97
Archana Verma
20. Proteomic Techniques for Application in Food Science
100
Ashok K. Mohanty
21. Evaluation of Probiotic Attributes of Dairy Starter
Cultures Using Various Test Methods
106
Rameshwar Singh
22. Identification of Lactobacillus spp by PCR based Molecular
Methodology
110
Sachinandan De and Rupinder Kaur
23. Antimicrobial Substances produced by Lactic
Acid Bacteria (LAB)
114
Shilpa Vij, Subrota Hati and Minakshi Dahiya
24. Microbiological Risk Assessment: A New
Concept to Ensure Food Safety
117
Naresh Kumar and Raghu H. V.
25. Biopreservation of Dairy Products: Role of Bacteriocins
of Lactic Acid Bacteria
126
R. K. Malik and Gurpreet Kaur
26. Regulatory Aspects of Functional Foods
135
Bimlesh mann , Rajesh Kumar and Prerna Saini
27. Nanomaterials - Their Applications and Safety
Aspects in Foods
142
Bimlesh Mann , Rajesh Kumar and Prabhakar Padgham
28. Strategies for Animals Studies to Assess the
Safety Aspects and Bioavailability of Netraceuticals
145
Ayyasamy Manimaran and Bimlesh Mann
29. Recent Advances in Synbiotic Dairy Foods and
Their Safety Evaluation
151
Chand Ram, Manju and Santosh Anand
30. Physical Characterization of Dairy Foods with
Reference to Viscosity, Colour and Water Activity
160
R. R. B. Singh and Prateek Sharma
31. Malt Based Milk Foods as “Value Added
Functional Dairy Products”
Laxmana Naik, Rajan Sharma, Manju G. and Amit K. Barui
165
PRACTICAL
32. Preparation and Characterization of Gold Nanoparticles,
Their Conjugation with Antibodies and Construction
of Lateral Flow Devices
170
Priyanka Singh Rao, Swapnil Sonar, Y.S. Rajput and Rajan Sharma
33. Use of Lateral Flow Technique for Detecting Melamine in Milk
173
Raman Seth and Anamika Dass
34. Rancimat (Accelerated and Automated) Method for
Evaluation of Oxidative Stability of Fats and Oils
177
Sumit Arora
35. Estimation of Cholesterol Content in Ghee Using a
Cholesterol Estimation Kit
182
Vivek Sharma and Darshan Lal
36. Rapid Methods for Detection of Adulterants in Milk
184
Rajan Sharma, Raman Seth and Amit K. Bauri
37. Detection of Foreign Fats/Oils in Milk and Ghee
Using Newer Approaches
189
Darshan Lal, Vivek Sharma, Arun Kumar and Amit Kumar
38. Determination of Total Polyphenolic Content in Fruit
Enriched Dairy Product
195
Rajesh Kumar and Richa Singh
39. Separation and Identification of Low Molecular
Weight Proteins Using Tricine SDS-PAGE
197
Neelima Sharma, Rajan Sharma and Y. S. Rajput
40. Identification of Proteins Through Western Blotting – Practical
200
Neelima Sharma, Amit K.Barui and Y.S. Rajput
41. Typing of Milk for A1 and A2 beta Casein
204
Sachinandan De, C. M. Hari Kishore, Ayan Mukherjee and Rupinder Kaur
42. Enzyme-Linked Immunosorbent Assay-Practical
206
Suman Kapila and Rajeev Kapila
43. Evaluation of Biological Activity of Milk Protein Ingredients
208
Bimlesh Mann, Prerna Saini, Prabhakar Padghan, Anuradha Kumari
44. Purification of Bioactive Proteins from Milk
212
Neha Mishra, Rajesh Kumar and Jai K Kaushik
45. Immunological Method to Detect Buffalo Milk in Cow Milk
214
Archana Verma
46. Conjugated Linoleic Acid and Its Estimation
217
A. K. Tyagi, A. Hossain, A. Tyagi
47. Importance and Estimation of Vitamins A & E
in Value Added Dairy Products
Harjit Kaur
221
48. Use of Atomic Absorption Spectrophotometer for the
Estimation of Minerals in Milk and Milk Products
225
Veena Mani
49. Pesticides: Their Analysis in Milk Using High
Performance Liquid Chromatography
230
Chander Datt and Monica Puniya
50. Estimation of Microbial GOS by High Performance
Liquid Chromatography
233
Vikas Sangwan and Sudhir Kumar Tomar
51. Estimation of Trehalose Production by Propionibacteria
236
Poonam and Sudhir Kumar Tomar
52. Spore Based Biosensor as A Quality Control Tool in
Dairy Industry
239
Naresh Kumar, Raghu H. V. and Avinash
53. Detection and Evaluation of Antimicrobial Activities of
Bacteriocins and Bioactive Peptides Produced by LAB
Shilpa Vij, Subrota Hati and Meenakshi Dahiy
248
Programme Schedule for Winter School
Programme Schedule for Winter School
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
January 11-31, 2011
11th January 2011(Tuesday)
9.00 AM – 9.30 AM
Registration of Participants
9.30 AM -12.30 PM
Inauguration of Winter School
Novel and Emerging Food Technologies for Defence Food Supplies – Inagural Lecture
by Dr. A.S.Bawa, Director, Defence Food Research Laboratory, Mysore
12.30 PM -1.00 PM
Visit to ATIC/Institute Film
Lunch
2.15 PM – 3.15 PM
Achievements of Dairy Chemistry Division
Dr. (Mrs.) B.K. Wadhwa
3.15 PM – 4.30 PM
Prospects of Value Addition Through functional Ingredients
Dr. G.R. Patil
12th January 2011 (Wednesday)
9.45 AM -10.45 AM
Method of Cholesterol Removal to Develop Low Cholesterol
Dairy Products – Theory
Dr. Darshan Lal
11.00 AM – 12.00 PM
Fortification of Milk and Milk Products for Value Addition –
Theory
Dr. Sumit Arora
12.00 PM – 1.00 PM
Cow Ghee Protects from Mammary Carcinogenesis:
Mechanism – Theory
Dr.V.K. Kansal
Estimation of Cholesterol Content in Ghee Using a
Cholesterol Estimation Kit– Practical
Dr. Vivek Sharma
Lunch
2.15 PM -5.00 PM
13th January 2011 (Thursday)
9.45 AM -10.45 AM
Evaluation of Probiotic Attributes of Dairy Starter Cultures
using Various Test Methods – Theory
Dr. Rameshwar Singh
11.00 AM – 12.00 PM
Separation Strategies for Bioactive Milk Proteins – Theory
Dr. Rajesh Kumar
12.00 PM – 1.00 PM
New Approaches to Detect the Adulteration of Milk Ghee
with Animal Body Fats and Vegetable Oils/ Fats – Theory
Dr. Vivek Sharma
Lunch
2.15 PM – 3.15 PM
3.15 PM – 5.00 PM
Quality and Food Safety in Yoghurt Industry – Guest Lecture
Detection of Foreign Fats/Oils in Milk and Ghee Using Newer
Approaches - Practical
Mr. Anuj Mehta
(Danone India Ltd.)
Dr. Darshan Lal
14th January 2011 (Friday)
9.45 AM -10.45 AM
Technological and Nutritional Aspects of Milk Phospholipids Theory
Dr.(Mrs.) B.K. Wadhwa
11.00 AM – 12.00 PM
Colostrum Powder and its Health benefit - Theory
Dr. Raman Seth
12.00 PM – 1.00 PM
Novel Technologies for Processing and Packaging of Health
Foods and Beverages – Guest Lecture
Dr. H.N. Misra
IIT, Kharagpur
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Lunch
2.15 PM – 5.00 PM
Purification of Bioactive Proteins from Milk – Practical
Dr. Jai K. Kaushik
15th January 2011 (Saturday)
9.45 AM -10.45 AM
Nanomaterials - Their Applications and Safety Aspects in
Food – Theory
Dr. (Mrs.) Bimlesh Mann
11.00 AM – 12.00 PM
Recent Advances in Synbiotic Dairy Foods and their Safety
Evaluation – Theory
Dr. Chand Ram
12.00 PM – 1.00 PM
Determination of Total Polyphenolic Content in Fruit Enriched
Dairy Product– Theory & Practical
Dr. Rajesh Kumar
2.15 PM – 3.15 PM
contd…. Determination of Total Polyphenolic Content in Fruit
Enriched Dairy Product – Practical
Dr. Rajesh Kumar
3.15 PM – 5.15 PM
Rancimat (Accelerated and Automated) Method for
Evaluation of Oxidative Stability of Fats and Oils – Theory &
Practical
Dr. Sumit Arora
Lunch
16th January 2011(Sunday)
17th January 2011 (Monday)
9.45 AM -10.45 AM
SDS-PAGE – Principle and Applications -Theory
Dr. Y.S. Rajput
11.00 AM – 1.00 PM
Separation and Identification of Low Molecular Weight
Proteins using SDS-PAGE – Practical
Dr. Y.S. Rajput
2.15 PM – 3.15 PM
Western Blot: Theoretical Aspects – Theory
Dr. Y.S. Rajput
3.15 PM- 5.00 PM
Identification of Proteins through Western Blotting – Practical
Dr. Y.S. Rajput
Lunch
18th January 2011 (Tuesday)
9.45 AM -10.45 AM
Lateral Flow Assay- Principle and its Application in Analytical
Food Science – Theory
Dr. Rajan Sharma
11.00 AM – 1.00 PM
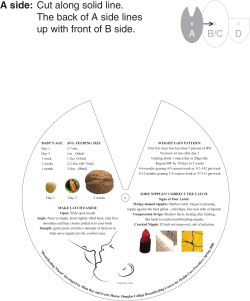
Preparation and Characterization of Gold Nanoparticles, their
Conjugation with Antibodies and Construction of Lateral Flow
Devices – Practical
Dr. Rajan Sharma
2.15 PM – 3.15 PM
contd…. Preparation and Characterization of Gold
Nanoparticles, their Conjugation with Antibodies and
Construction of Lateral Flow Devices - Practical
Dr. Rajan Sharma
3.15 PM - 4.00 PM
Use of Lateral Flow Technique for Detecting Melamine in
Milk – Practical
Dr. Raman Seth
4.00 PM – 5.00 PM
Regulatory Aspects of Functional Foods
Dr. Bimlesh Mann
Lunch
19th January 2011 (Wednesday)
9.45 AM -10.45 AM
Importance and Estimation of Vitamin A & E in Value Added
Dairy Products – Theory
Dr. (Mrs.) Harjeet Kaur
11.00 AM – 1.00 PM
contd…. Importance and estimation of vitamin A & E in Value
Added Dairy Products – Practical
Dr. (Mrs.) Harjeet Kaur
Lunch
Programme Schedule for Winter School
2.15 PM – 3.30 PM
3.30 PM – 5.00 PM
Estimation of Microbial GOS by HPLC - Theory and Practical
Estimation of Trehalose Production by Propionibacteria –
Theory and Practical
Dr. S.K. Tomar
Dr. S.K. Tomar
20th January 2011 (Thursday)
9.45 AM -10.45 AM
Microbiological Risk Assessment: A New Concept to Ensure
Food Safety – Theory
Dr. Naresh Kumar
11.00 AM – 1.00 PM
Spore Based Biosensor as A Quality Control Tool in Dairy
Industry – Practical
Dr. Naresh Kumar
2.15 PM – 3.15 PM
Enzyme Linked Immunossorbent Assay –Theory
Dr. Rajeev Kapila
3.15 PM – 5.00 PM
Enzyme Linked Immunossorbent Assay – Practical
Dr. Suman Kapila
Lunch
21st January 2011 (Friday)
9.45 AM -10.45 AM
Experimental Determination of Thermal Stability of Proteins: A
Dr. Jai K Kaushik
Theoretical Background
11.00 AM- 12.00 PM
Biopreservation of Dairy Products: Role of Bacteriocins of
Lactic Acid Bacteria – Theory
Dr. R.K. Malik
11.00 AM- 1.00 PM
Glycomacropeptide – Biological Properties and its Application
Dr. Rajan Sharma
2.15 PM – 3.15 PM
Pesticides: Their Analysis in Milk Using High Performance
Liquid Chromatography– Theory
Dr. Chander Datt
3.15 PM – 5.00 PM
Contd…. Pesticides: Their Analysis in Milk Using High
Performance Liquid Chromatography – Practical
Dr. Chander Datt
Lunch
22nd January 2011(Saturday)
Exposure of Participants of Winter School to “Brain Storming Session on Promotion of Indigenous Dairy Products in
International Market” being organized by Alumni Association, NDRI, Karnal
23rd January 2011 (Sunday)
24th January 2011(Monday)
2.15 PM – 3.30 PM
Identification of Lactobacillus spp by PCR based Molecular
Methodology – Theory & Practical
Dr. Sachinandan De
3.30 PM – 5.00 PM
Typing of Milk for A1 and A2 beta casein - Theory & Practical
Dr. Sachinandan De
2.15 PM – 3.15 PM
Use of Atomic Absorption Spectrophotometer for the
Estimation of Minerals in Milk and Milk Products – Theory
Dr. (Mrs.) Veena Mani
3.15 PM- 5.00 PM
Contd…. Use of Atomic Absorption Spectrophotometer for the
Dr. (Mrs.) Veena Mani
Estimation of Minerals in Milk and Milk Products – Practical
Lunch
25th January 2011(Tuesday)
9.45 AM -12.00 PM
12.00 AM – 1.00 PM
Lunch
Physical Characterization of Dairy Foods with Reference to
Viscosity, Colour and Water Activity – Theory & Practical
Allergen Mangement in Foods - Emerging Trends
Dr. R.R. B. Singh
Rajesh Kumar Sharma
(Cadbury India Ltd.)
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
2.15 PM – 3.15 PM
Common Statistical Techniques for Analytical Dairy and Food
Science – Theory
Dr. A.P. Ruhil
3.15 PM- 5.00 PM
contd…. Common Statistical Techniques for Analytical Dairy
and Food Science – Practical
Dr. A.P. Ruhil
26th January 2011(Wednesday) – Republic Day
27th January 2011 (Thursday)
9.45 AM -10.45 AM
Strategies for Animals Studies to Assess the Safety Aspects
and Bioavailability of Netraceuticals – Theory
Dr. Ayyasamy
Manimaran
11.00 AM – 12.00 PM
Rapid Methods for Detection of Adulterants in Milk – Practical
Dr. Rajan Sharma
12.00 PM – 1.00 PM
Visit to Model Dairy
Mr. G. Mutreja
2.15 PM – 3.15 PM
Immunological Method to Detect Buffalo Milk in Cow Milk –
Practical
Dr. (Mrs.) Archana
Verma
3.15 PM- 5.00 PM
Species-Specific Identification of Milk and Milk Products: A
Molecular Approach - Theory
Dr. (Mrs.) Archna Verma
Lunch
28th January 2011 (Friday)
Packaging of Value Added Foods and Their Storage Stability
– Guest Lecture
P.P. Gothwal (CFTRI,
Regional Center,
Lucknow)
Food Additives and Quality Issues – Guest Lecture
Ravinder Kumar
(Danisco India Ltd.)
Proteomic Techniques for Application in Food Science
Dr. Ashok K. Mohanty
2.15 PM – 3.15 PM
Evaluation of Biological Activity of Milk Protein Ingredients –
Theory
Dr. (Mrs.) Bimlesh Mann
3.15 PM – 5.00 PM
contd…. Evaluation of Biological Activity of Milk Protein
Ingredients – Practical
Dr. (Mrs.) Bimlesh Mann
9.45 AM -10.45 AM
11.00 AM – 12.00 PM
12.00 PM – 1.00 PM
Lunch
29th January 2011 (Saturday)
9.45 AM -10.45 AM
Antimicrobial Substances Produced by Lactic Acid Bacteria
(LAB) - Theory
Dr. (Mrs.) Shilpa Vij
11.00 AM – 1.00 PM
Detection and Evaluation of Antimicrobial Activities of
Bacteriocins and Bioactive Peptides Produced by LAB –
Theory & Practical
Dr. (Mrs.) Shilpa Vij
2.15 PM – 3.15 PM
Conjugated Linoleic Acid and Its Estimation – Theory
Dr. Amrish Tyagi
3.15 PM- 5.00 PM
Contd…. Conjugated Linoleic Acid and Its Estimation –
Practical
Dr. Amrish Tyagi
Lunch
30th January 2011 (Sunday)
31st January 2011(Monday)
9.45 AM -10.45 AM
10.45 AM – 1.00 PM
Lunch
Course Evaluation
Dr. (Mrs.) Bimlesh Mann
and Dr. Rajan Sharma
Interaction with Faculty
Chaired by Head, DC
Division
Novel and Emerging Food Technologies for Defence Food Supplies
Novel and Emerging Food Technologies
for Defence Food Supplies
Dr. A. S. Bawa
Director
Defence Food Research Laboratory, Mysore
The Defence Food Research Laboratory (DFRL) was established in December, 1961 under the
aegis of Defence Research & Development Organisation (DRDO), Ministry of Defence to cater to the
strategic operational requirements of our Services and to provide logistical support to the Armed
forces in the area of food supplies. Our troops often operate in far flung in hospitable treacherous
terrains under inclement and hostile weather conditions. In such operational situations, not only are
they deprived of the fresh produce needed to sustain life processes even normal regime of cooking
becomes extremely cumbersome and difficult. The R & D efforts at DFRL are aimed at designing and
engineering light weight convenient, pack rations for Army,Navy,Air force and other paramilitary
forces which do not require any elaborate cooking or preparation at the consumer’s end and remain
shelf-stable under varying climate condition for periods ranging from 6 months to 1 year. Through
enormous and substantive contribution, DFRL has developed a wide verity of food products of Indian
dietary matching the mainframe palate tastes of the country. Many of the DFRL foods, born out of
innovative state of the art technology, lend themselves eminently suitable to industrial scale commercial
exploitation by enterprising entrepreneurs of different genre. DFRL also has products which are export
worthy and amenable to working women. Owing to its singular dedicated contributions in processed
foods, DFRL can be reckoned as the leader in convenience food and packed ration developments in
this country. Indigenous ingenuity is the hallmark of most of the technologies developed at DFRL.
Over the decades, the technological advancements have resulted in several innovative technologies
for various applications. Among the dehydration techniques freeze-drying maintains the quality of
products which is quite close to that of fresh one. During freeze drying the thermal evaporation of
moisture is through sublimation at low temperatures and under high vacuum. Hurdle technology helps
to preserve foods for a period of 2-4 months and is applicable to fruits, vegetables and their products
as well as meat and fish products and is sparingly used for cereal products preservation. Hurdle
technology is an intelligent combination of hurdles such as pH, temperature, water activity, redox
potential, preservative etc. to ensure the microbial safety as well as sensory and nutritional acceptance.
Membrane technology is used in the manufacture of clarified juices, for initial concentration through
ultra filtration, nano-filtration and reverse osmosis processes.
Thermal treatment is the most widely used technology for preservation of foods. Thus retort
processing of foods has been the most promising technique for preservation of both vegetarian and
non-vegetarian foods in the ready-to-eat form. The temperatures in the range of 110 – 125ºC are used
for low acid foods with the main objective of inactivating the undesirable micro-organisms to achieve
commercial sterilization. High pressure technology is a novel non-thermal processing method of food
preservation where the food is subjected to high hydrostatic pressures in the range of 100-600 Mpa at
room temperature. The Armed Forces are the biggest consumer of processed foods and approximately
13 thousand tonnes of processed food is used annually. They have to subsist mainly on pack rations
during operational situations. With the advancements in technological methods, Defence Food
Research Laboratory (DFRL), Mysore, has contributed significantly to develop suitable technologies
for preserving traditional Indian foods in light weight flexible packages so that pack rations could
be designed based on such items to meet the nutritional requirements of the Defence personnel for
operational situations and this has also paved the way for providing variety of foods suiting to their
taste. These efforts led to the development of convenience foods based on cereals, pulses, fruits and
vegetables with a long shelf-life in flexible packs.
1
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Fruit and vegetable technologies
The Indian Army operates on hazardous terrain inclusive of Siachen Glacier and the sandy deserts
of Rajasthan. Similarly, the Indian Navy is a blue water navy and the operations go deeper in the
oceans to protect the maritime zones used for international shipping. The concept of fruit and vegetable
storage as such has undergone a change and the troops favors precut fruits and vegetables in packaged
form on operational rations due to the logistic utility and convenience. Therefore, minimal processing
of precut fruits and vegetables needs to be emphasized and the unit packages can be formulated as per
the ration scales and logistic requirements.
The futuristic technologies encompass non-thermal processing i.e. high pressure processing and
pulsed electric field applications. Eco-friendly and energy saving technologies are envisaged to occupy
their rightful place in the area of fruit and vegetable products. Use of biodegradable packaging for
fresh and processed fruits and vegetables is a certainty and an absolute necessity. It is a common site
to notice heavy accumulation of wastage and spent packaging material even in partially inhabitated
areas including the high altitude locations. Use of biodegradable plastics and other materials of organic
or inorganic origin need to be stressed upon to minimize the pollution hazards in the army locations
as well as the high seas of naval operations.
Minimal processing of fruits and vegetables
Supply of fruits and vegetables in precut and packaged form is a challenging task as the precutting
operations impose severe physiological stress on the commodity. Minimal processing of fruits and
vegetables had been contemplated as a ‘bridge technology’, touching technologies concerned with
post harvest handling of fresh produce on one side and conventional process technologies on the
other side. It is well accepted notion that minimally processed products can be defined as ‘lightly
processed’ products. This does not describe either the living or non living nature of the plant tissue. In
other words, it enlarges the horizons of minimally processed products giving scope for use of minute
thermal treatments and also application of anti-metabolic substances. As such the emphasis is on
‘fresh like’ sensory attributes of the products and any minimal process strategy shall keep the same as
the main objective.
Microbiological aspects
Minimally processed fruits and vegetables encounter incidence of enhanced microbial attacks due
to the elimination of natural barriers of the plant tissue and enhanced accessibility to moisture and
nutrition on the surface of the plant tissue. A number of contaminating microorganism including
spoilage organisms and pathogens were isolated from precut fruits and vegetables. The minimally
processed products were successfully subjected to field trials in different Naval commands and the
field trials on zero energy cooling devices were successfully completed in the forward locations of
desert areas in Rajasthan. Freezing of fruits and vegetables in whole or precut form is a major problem
during peak winters in high altitude locations such as Ladakh sector. Antifreeze containers with the
rated capacities of 30 and 80 kg were field evaluated in Ladakh sector and the feed back was highly
encouraging for the induction of the same in Armed Forces. As such, the time is ripe for consideration
of supply of precut fruits and vegetables to Armed Forces in packaged form and the strategies of the
transport and storage are encompassed to make the supply chain flexible enough to be accommodated
in the existing infrastructure prevailing in the areas of army deployment.
Ultra high pressure processing
The search for newer methods of food processing aims at processing of food without resorting
to thermal processing. The concept of high pressure processing had emerged from the depth of the
oceans as the sea beds are devoid of the usual microorganisms that one can find at sea level. Only a
few microorganisms can survive under high pressure conditions and the lethality grows manifold
from 500 MPa onwards. Ultra high pressure processing is an innovative technological concept
under the category of non thermal processing with minimal or no heat treatment. It is a process
2
Novel and Emerging Food Technologies for Defence Food Supplies
aimed at controlling growth of microbial populations and also inactivation of quality deteriorating
enzymes. High pressure processing involves instantaneous and uniform transmission of the pressure
throughout the product independent on the product volume. Upon reaching the desired pressure
level, the pressure can be maintained without further inputs of energy. Liquid foods such as fruit
juices can be subjected to high pressure processing holding the required pressure for specific duration
and decompressing for further aseptic filling as per the standard procedures of aseptic packaging.
Apart from these aspects, high pressure processing can also be used for pressure shift freezing, high
pressure thawing, texture modifications and enhancement of nutritive value of foods. High pressures
result in the physical confirmation of biological entities such as proteins, resulting in positive changes
in the bio-accessibility of nutrients.
Infrared processing of cereals and pulses
The infrared processing is also known as ‘micronising processes and is widely used for cooking
cereals, oil seeds, pulses and also for the processing of cocoa. Micronisation is used for the development
of different types of consumer foods, animal feeds inclusive of pet foods and several brewed products.
It is one of the most flexible and efficient means of processing for the development of value added
products.
Infrared radiation has wavelengths between 0.7 and 500 µm. Radiation with wavelengths
just below 0.7 µm consists visible light, whereas radiation with wavelengths just above 500 µm is
microwave radiation. Infrared radiation with shorter wavelengths transmits more thermal energy to
foods in shallow-bed radiators designed for in-depth processing. Such radiators are equipped with
glass-encapsulated heaters operating at about 3,000 kW. Microniser consists of a long flat moving belt
of approximately 5 meters in length onto which cereals (wheat, ragi, barley, soy, etc.) are fed at one
end. Above the belt and along its length are suspended gas burners which emit infrared energy on
the grains which is carried through the machine by the belt. Infrared energy is absorbed by the moist
grains, causing expansion of starch gelatinization. Extent of gelatinization depends upon magnitude
of infrared heat and the time the material takes to travel from one end to the other. The expanded
grain upon processing is subjected to flaking, cooling and subsequent packaging. Infrared processing
improves starch accessibility for easy digestion and the same could be attributed to opening up of
crystalline starch structurally. Conventional cooking methods also improve the accessibility of starch
for digestion but the process may result in nutrient losses besides being a long duration process.
Micronization is highly reliable and consistent “Short Time High Temperature Process” using
humidity, temperature and mechanical pressure to achieve high levels of starch gelatinization and
elimination of anti nutritional factors, without any significant loss in nutrient value. Infrared energy
makes the starch soft and turgid, causing it to swell, fracture and gelatinize. Immediate rolling /
flaking or secondary processing enhance the digestibility and nutritional value. The nutritive value or
protein quality of a food / feed protein depends not only on its content of amino-acids but also their
bio-availability. The products as such could be made ready-to-eat or instantized to suit the logistic
requirement of defence forces.
Retort processing technology
Retort processing of foods in rigid, semi rigid and flexible packaging systems is the most acceptable
form of food preservation. It represents a unique combination of product, process and package
technologies with potential, functional, quality and economical benefits. The increasing consumer
awareness and inhibition/dislike to accept other methods of food preservation such as use of chemical
preservatives, irradiation etc. has offered a vast scope for retort processed foods.
Although retort pouch processing of foods is similar to conventional canning, it has certain major
advantages like (i) Consumes less energy for processing (ii) enhances the quality attributes and (iii)
reduces the cost of transportation and storage.
Retort processing is generally carried out for low acid foods with a pH more than 4.5 at a temperature
of 121.1ºC using moist heat. During heat treatment, undesirable spoilage as well as pathogenic
3
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
microorganisms is inactivated / killed and thereby the food products become commercially sterile.
Thermal destruction of microorganisms is measured and monitored by time-temperature history,
lethality and Fo-value.
Despite distinct advantages, retort pouch processing of foods till recent years did not become
popular in India as compared to countries like Japan mainly because of the high cost of processing
equipment and non-availability of indigenous multi layer flexible packaging materials.
DFRL, Mysore has been a pioneer in developing the retort processing technology indigenously
in the country. Over the past two decades, research and development work has been carried out
in developing multilayer flexible packaging materials as well as designing a simple low cost retort
(semi-automatic and automatic) amenable to Indian food industry. Due to continuous efforts, DFRL
has so far successfully transferred the retort pouch processing technology to 40 firms for commercial
exploitation.
Functional foods
Functional food is a three way concept wherein the (i) agricultural or animal origin serves as
raw material, (ii) specific ingredients components of the products exerting functionality and (iii)
physiological effects with respect to human system. Hence the balanced view of the three factors,
with specific ingredient action, imparts the needful strategic effect. Thus functional food is a recent
strategic application in the food field and a driving force for the product development in this century.
The functional foods viz. antioxidant rich herbal tea, squash, baked foods, anti-ulcerative fruit spread,
low calorie squash for diabetics, fibre rich ash gourd juice, etc. are some of the recent developments
made in the field.
Appetisers are another class of functional foods which improve the appetite. The physiological
mechanism in brief is stimulation of trignomial nerves to increase the secretion of digestive juices.
On the other hand, the hormone leptin formed at hypothalmous in the brain for the appetite control
increases at high altitude stay; thereby satiety setting is signaled and results in lack of appetite. DFRL
has developed several appetisers for high altitudes which have proved its efficacy for the cause.
In conclusion, the food technologies from the ancient to the advanced technologies adopted in the
present, along with the emerging, promising technologies as well as the present day requirement of
functional foods have been reviewed in brief. Based on the raw materials i.e., fruits, vegetables, cereals,
nuts, medicinal but natural herbs as well as the food requirements of the Defence Forces along with
the logistic convenience of longer shelf life, ease of transportation, DFRL has developed more than
100 processed foods with varied technologies adopted. Packed rations with ready-to-eat products,
emergency rations with calorie dense products, logistic based foods with functionality, energy dense
food bars, functional food bars for low intensity conflicts, convenient processing machine such as
automatic chapathi making machine, automatic coconut processing system, on-line continuous
blancher for vegetables, soy paneer making plant, etc. are the important contributions of DFRL for
Defence Forces, besides the need based techniques and quick test kits for meat and processed foods
which are adopted by them.
4
An Overview of Designer Functional and Health Foods
An Overview of Designer Functional and Health Foods
Prof. A. K. Srivastava
Director & Vice-Chancellor
National Dairy Research Institute, Karnal
Introduction
Designer foods can be defined as “foods that are tailor-made to meet any specific requirement
in terms of functionality, nutrition, convenience and therapeutic aspects”. They are prepared by
manipulating the formulations or engineered genetically or by other conventional means to provide
the desired function. In last decades a lot of emphasis is given to designer foods mainly developed
to deliver the nutritional and Functional foods and nutraceuticals provide a means to reduce the
increasing cost on the health care system by a continuous preventive mechanism. The interest in
functional foods has started in early 1990s, becoming one of the fast growing sectors of global food
industry. Epidemiological studies and randomized clinical trials carried out in different parts of the
world have been demonstrated or at least suggested numerous health effects related to functional
food consumption, such as reduction of cancer risk, improvement of heart health, enhancement of
immune functions, lowering of menopause symptoms, improvement of gastrointestinal health, antiinflammatory effects, reduction of blood pressure, antibacterial & antiviral activities, reduction of
osteoporosis etc.
Foods for improved gastrointestinal health
Gastrointestinal (GI) organ system in human body is an important link between the food and
resultant health benefit. GI tract is known to harbor more than 70% of our immune system. The delicate
balance between the intestinal microflora and the host organism is very critical and any disturbance
may lead to acute gastro enterititis and more chronic disorders like inflammatory bowel syndrome
(IBD), peptic ulcer, colon cancer etc. Many factors influence the gut microflora including medication,
age, stress, life-style and above all diet. Hence, dietary management strategies that helps in maintaining
or even improving the normal GI microflora need to be prioritized. Probiotics are the well-known
means to target the GI microbes with proven disease preventing/curing attributes. Viable probiotic
bacteria such as Lactobacilli and Bifidobacteria can survive in sufficient numbers to assist the GI tract to
become metabolically active. Their therapeutic effects have been confirmed in clinical trials and they
have been utilized effectively in formulation of certain functional and nutritional foods. Probiotics
primarily targets immune system through exerting anti-microbial activity, enhancing the proliferation
of immune-defense cells, regulating certain metabolic enzymes and inhibiting the degenerative
processes. The exact mechanisms related to beneficial effects of probiotics vary with target group
and microorganisms. The food products which assist in improving the GI health are also termed as
“colonic foods” and include probiotics, prebiotics and synbiotics. Requirement of colonic mucosa for
multitude nutrients including Short chain fatty acids (SCFA), vitamins, amino-acids, poly amines,
growth factors and antioxidants is met from the beneficial microflora. Colonic foods meet the typical
nutritional demand of mucosal cells. The probiotic bacteria partly synthesize them using wide variety
of raw material while utilizing them as food such as prebiotics. Complex carbohydrates including
dietary fibers, resistant starch and oligosaccharides not only contribute as prebiotic, but also perform
certain physiological functions that are beneficial like relieve form constipation, inhibit cholesterol
absorption and increase the micronutrient bioavailability. Oligosaccharides, a common constituent
of plant and animal cellular constituents have been recognized with number of health attributes
and termed as “New age fiber”. Moreover, dietary fibers, resistant starch and oligosaccharides, also
exhibit novel functionalities like water binding; gelation and emulsification that can be utilized for the
development of low fat variants of probiotic products.
5
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Functional foods for infant and weaning purpose
India is among the nations with higher incidence of child malnutrition and deficiency diseases.
According to an estimate more than 50% of children are born with low birth weight resulting in
stunted growth. Lack of key nutrients and bio-protective components in infancy led to prevalence of
anaemia and infectious disease among children. Mother’s milk is considered as perfect food of nature
but in many incidences maternal nursing is not possible and new born has to feed with infant formula.
Infant formula is the best example of designer foods. Normal infant formulas are manufactured
from cow’s milk, but this requires substantial alteration to parallel the composition of breast milk.
These modifications include reduction in protein and minerals, an increase in carbohydrates and the
addition of vitamins and trace elements. In recent years, studies have indicated that infants may have
an impaired ability of synthesizing taurine and carnitine, and a dietary source is therefore required.
Carnitine is necessary for the transportation of long chain fatty acids into cell for the β-oxidation and
energy production. Fatty acid profile of different fat sources do not meet the complexity of mature
breast milk, therefore mixture of different fat sources is preferred. Most manufacturers use a mixture
of vegetable oils (Simmer, 2000). The fat source must also provide the essential fatty acids linoleic
(C18:2, ω-6) and α-linolenic acid (C18:3, ω-3). A ratio of 5:1 of ω-6:ω-3, as occurs in breast milk, is
being suggested. Short chain as well as medium chain fatty acids should also be present in sufficient
quantities as they are easy to absorb and assimilate. However there is a need for more short-and
long-term studies before the optimum ratio and its effects on growth are evaluated. Linoleic acid and
α-linolenic acid are the precursors of the very-long-chain (C20 - C22), polyunsaturated fatty acids
(LCPUFA): Arachidonic and docosahexaenoic acid (DHA). LCPUFA are involved in the neural and
vascular development of the fetus and neonates and are present in human milk.
Nucleotides, a component of non-protein nitrogen in human milk, may be important for normal
immune function. Supplementation of infant formula with nucleotides seems to be beneficial in
clinical trials, although further research is needed before routine nucleotide supplementation of infant
formula can be considered. The success of commercially prepared infant formulas has stimulated the
development of numerous formulations and several hundred varieties of proprietary infant formulas
are now available throughout the world. In addition, special formulas for use in clinical situations or
for premature infants or for infants with special inborn errors of metabolism are available as special
dietary foods.
The GI tract of infant is dominated by Bifidobacteria which provides health promoting and
protective properties such as activation of immune system, inhibition of pathogens by the secretion of
substances which are directly inhibitory towards several bacteria, lowering of pH by the production of
acids such as acetate and lactic acid, leading to an antibacterial environment, production of digestive
enzymes such as casein phosphatase and lysozyme and production of vitamins. For these reasons it
seems desirable to also increase the numbers of Bifidobacteria in the intestinal flora of formula-fed
infants. Administration of prebiotic oligosaccharides and probiotic supplements appear to be the most
effective way to increase the number of the Bifidobacteria in the intestine. Human milk oligosaccharides
are mainly responsible for Bifidogenic effects of breast milk. Several commercial formulations have
been developed with the view of providing a predominance of Bifidobacteria in the intestinal flora
formula-fed infants. However the inclusion of such unconventional ingredients in formulation of
infant formula needs long-term investigations before being approved.
Inadequate nutrition during first 2-3 years often leads to problems associated with malnutrition in
several developing nations in the world. Complementary nutrition is must for the normal and healthy
growth of a child after the age of 6 months, owing to increased requirement of nutrition in addition
to those provided by breast milk. Moreover the food preparations consumed as weaning foods do
not contain adequate nutrients desired for children. Traditional infant-feeding practiced, in countries
like India, is usually cereal based. For the preparation of such foods grains are often germinated,
6
An Overview of Designer Functional and Health Foods
fermented, processed and cooked in various ways to improve digestibility, and mixed with oilseeds or
animal products to enhance their nutritional profile, however most of these complementary foods are
reported to be less energy dense and less safer for children because of the higher proportion of antinutrients. Cereals in combination with milk solids are generally used for the preparation of weaning
foods. Milk-Cereal-millet based complementary foods appear to be unique in the sense that they can
deliver multitude of nutrients to children and complement each other as well. The correct form of
incorporation, effective concentration and required technological inputs determine the effectiveness
of the resulted complementary food. Such products could be an attractive option for mass children
feeding programmes.
Specialized foods with plant bioactive
Nutritional significance of plant molecules is well documented and increasing cases of cancers,
coronary heart diseases, diabetes and many other chronic diseases, have been attributed to under
consumption of fruits and vegetables in our diet. But beyond these known nutrients i.e. vitamins,
fibers, plants have clearly more to offer and scientists are scurrying to discover exactly which plant
components might fend off specific diseases. An ever-expanding array of previously unknown plant
molecules with hard to pronounce names are being uncovered. But there exact metabolic role and how
these can be utilized in designer food, need to be clarified.
The number of identified physiologically has increased dramatically in the last decades and
overwhelming evidence from epidemiological, in vivo, in vitro and clinical trial indicate that plant rich
diet can reduce the risk of certain chronic diseases (Hasler, 2000) Health professionals are gradually
recognizing the role of phytochemicals in health improvement. The major mechanism associated with
therapeutic aspects of plant bioactive is their ability to act as antioxidants.
There are certain other compounds present in plant foods, with significant health promoting effect
include plant fatty acids, tocotrienols, phenolic derivatives and dietary fibers etc. Docosahexaenoic
acid (DHA), which is one of the most important structural component of brain and retina, and de-novo
synthesis of this compound, is very rare. The decline in DHA intake could have serious implications for
public health, since low plasma, DHA concentrations have been correlated with increased incidence of
number of important chronic diseases such as depression, attention deficit disorders and Alzheimer’s
dementia. Crypthecodinium cohmii strain of marine algae is used for the commercial production of DHA
rich oil. Spirulina, termed as wonder alga is one of riches source of omeg-3-fatty acids, quality protein
and many other therapeutic molecules.
Plant polyphenols are secondary metabolites widely distributed in higher plants. Polyphenols
historically have been considered as anti-nutrients by nutritionists, because some, eg. tannins have
such adverse effects as decreasing the activities of digestive enzymes, energy, protein and amino acid
availabilities, mineral uptake and having other toxic effects. Recognition of the antioxidant activities
of many polyphenols has realigned thinking toward the health benefits provided by many of these
compounds. Phytoestrogens are a broad group of plant-derived compounds that are structural
mimics of endogenous 17 beta-estradiol. Two major phytoestrogens, which are of great importance
from nutritional and health perspectives, include lignans (Flaxseed) and isoflavones (soy bean).
These compounds either compete with or antagonize estrdiol action. Exact biochemical mechanism
involving CYP3A monoxygenase activity in presence of phase I enzyme inducers such as dixamethane.
Phytosterols are another important terpene subclass. Two sterol molecules that are synthesized by
plants are β - sitosterol and its glycoside. In animals, these two molecules exhibit anti-inflammatory,
anti-neoplastic, anti-pyretic and immune-modulating activity. In the body, phytosterols can compete
with cholesterol in the intestine for uptake, and aid in the elimination of cholesterol from the body.
Saturated phytosterols appear to be more effective than unsaturated ones in decreasing cholesterol
concentrations in the body. Certain designer foods like phytosterol containing yoghurt, β-glucan rich
dairy drink, DHA containing infant foods etc. have already reach to the stage of commercialization.
7
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Milk proteins and peptides based nutraceuticals
Dietary proteins possess nutritional, functional and biological properties, and the technological
processes used in food manufacture and processing often affect these properties. The role of proteins
as physiologically active components in the diet has been increasingly acknowledged in recent years.
Such proteins or their precursors may occur naturally in raw food materials, exerting their physiological
action directly or upon enzymatic hydrolysis in vitro or in vivo. Several dietary proteins, can act as a
source of biologically active peptides. These peptides inactive within remain the parent protein, and
released during gastrointestinal digestion or food processing. Once liberated, the bioactive peptides
may provide different functions in vitro or in vivo.
Bioactive peptides have to be released from the parent protein by enzymatic hydrolysis. This
can be achieved by the use of isolated enzymes, as well by microbial fermentation. Biologically
active peptides are of particular interest for pharma industry because they have been shown to play
different physiological roles, including opioid like activity, antimicrobial, immunomodulatory and
antihypertensive. Such peptides can be released during hydrolysis by digestive or microbial enzymes.
Microbial enzymes from lactic acid bacteria have demonstrated to be able to liberate theses peptides
from milk proteins, in various fermented milk products.
Upon oral administration bioactive peptides may affect the major body systems- namely the
cardiovascular, digestive, immune and nervous systems. For this reason, the potential of certain
peptides sequences to reduce the risk of chronic diseases or boost natural immune protection has
aroused a lot of scientific interest over the past few years. These beneficial health effects may be
attributed to known peptide sequences exhibiting, e.g., antimicrobial, antioxidative, antithrombotic,
antihypertensive and immunomodulatory activities. Milk proteins are considered the most important
source of bioactive peptides and an increasing number of bioactive peptides have been identified in
milk protein hydrolysates and fermented dairy products.
Over the last few years a number of investigations have been carried out across the world to
elucidate the bioactivity of milk proteins and derivatives. These components may be either serve as
functional ingredients in development of functional foods or can be utilized by pharma industry as
nutraceuticals. Most of the claimed physiological properties of milk proteins and derivatives have
been carried out in in-vitro or animal models, these hypothesized properties remains to be proven in
humans. Whey proteins are becoming an important constituent in the recipe of wide range of functional
and health foods because of the unique amino acid composition and bioactivity. Whey proteins based
commercially available food products include sports supplements, low fat dairy desserts, medical
foods, infant formulations and geriatric foods. Antihypertensive bioactive peptides may be utilized in
development of mood drinks and also foods for cardiac patients.
Other prospective designer foods
Beverages are another range of products that offer tremendous market potential for Indian food
industry because of being nutritionally-rich. Similarly, minor cereals and millets based milk beverages
seem to be lucrative products for school feeding programmes. Liquid milk fortification with vitamins A
and/D is mandatory in several countries. However, the milk fortification usually impaired its sensory
and processing quality characteristics. Moreover, bio-availability of fortified nutrients is another major
concern. Investigations carried out at NDRI suggest possibilities of fortification of liquid milk with
calcium and iron. Beverages and soups based on whey continue to receive a considerable amount
of attention nowadays. These indicate the growing awareness among consumers and manufacturers
alike for the enormous potential these offered for diversifying product profile. Other designer foods
include low calories/low fat variants, low sodium foods and fun foods etc.
Conclusion
Consumer interest in the relationship between diet and health has increased the demand for
information on functional foods. Rapid advances in science and technology, increasing healthcare
8
An Overview of Designer Functional and Health Foods
costs, changes in food laws affecting label and product claims, an aging population, and rising interest
in attaining wellness through diet are among the factors fueling interest in functional foods. Credible
scientific research indicates many potential health benefits from milk components.
References
Finley, J.W. 2005. Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids,
glucosinolates, polyphenols and selenocompounds. Annals of Botany, 95:1075-1096 pp.
Hasler, C.M. 1998. Functional Foods: Their role in disease prevention and health promotion. Food Technology 52(11),
63-70 pp
Hasler, C.M. 2000. The changing face of functional foods. Journal of American College of Nutrition 19 (5), 499S-506S pp.
Hirayama, M. 2002. Novel physiological functions of oligosaccharides. Pure Appl. Chem. 74 (7) 1271-1279 pp
Shah, N. P. 2000. Probiotic Bacteria: Slective Enumeration and survival in dairy foods. J. Dairy Science, 88:894-907
Simmer, K. 2000 a. Long-chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst.
Rev., -HD-(2): CD 000375 2000.
Wollowski, I. 2001. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutrition: 73 (Suppl):451S-5S
9
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Prospects of Value Addition Through
Functional Ingredients
G. R. Patil
Dairy Technology Division, NDRI, Karnal
Introduction:
In recent years, there has been a vast and rapidly growing body of scientific data showing that
diet plays an important part in diseases. Diet is thought to contribute to six of the 10 leading causes of
death. Nutrients and nonnutritive food components have been associated with the prevention and/
or treatment of chronic diseases such as cancer, coronary heart disease, diabetes, hypertension, and
osteoporosis. Up to 70% of certain cancers may be attributed to diet. As the data supporting the role
of diet in health promotion and disease prevention continue to mount, it is likely that the quantity of
enhanced foods will expand substantially. There is an increasing demand by consumers for quality
of life, which is fueling the functional foods revolution. Functional foods are viewed as one option
available for seeking cost-effective health care and improved health status. Moreover, the large babyboomer segment of the population is aging and considerable health care budget in most country is
focused on treatment rather than prevention. Thus, the use of nutraceuticals in daily diets can be seen
as means to reduce escalating health care costs that will contribute not only to a longer lifespan, but
also more importantly, to a longer health span. Development of functional food products will continue
to grow throughout the 21st century as consumer demand for healthful products grows.
The exploding area of functional foods and probiotics shows considerable promise to expand
the industry into new arenas. Both convenience and better for you attitudes are selling. Consumers
clearly believe in the concept of functional nutrition, or specific association between foods/nutrients
and health functions. They are interested in foods that boost the immure system, reduce the risk of
disease and enhance health, which consumers self-prescribe for themselves and their families. Hence,
there are clear opportunities to offer consumers dietary alternatives to medical solutions. These
opportunities, however, will be highly consumer driven and success will ultimately be dependent
upon defining your segment and knowing your target group.
The markets of traditional dairy products are increasingly getting overcrowded and our future
success will depend on our ability to provide innovative products, which consumers want and need.
Whatever the innovation - products, processing method or packaging - it should meet the real consumer
need. We know today’s families want “grab-and-go” convenience. They are also concerned about
nutrition and health. Different ages and demographics want different things. Therefore, investment at
this level is essential if we are to respond rapidly to customers who are increasingly demanding new and
different taste experiences from products that are also competitively priced. Thanks to advancements
in technology, researchers have shown that specific components of milk, as well as ingredients can
be readily added to dairy products, which contribute to health and wellness, and assist consumers
with feeling balanced and satisfied. There is a golden opportunity for dairy marketers to formulate
innovative products to meet consumers’ needs and to effectively market the product’s value. New
variants of sweets can be developed. Dairy products containing health-promoting ingredients may be
developed and promoted. Host of ingredients with health benefits are available for value addition of
dairy products. Some of these issues are discussed hereunder.
Functional ingredients for value addition
Functional nutrition is a broad topic, and covers many ingredient categories. The functional
components used in formulation of these formulated foods are given in Table 1.
10
Prospects of Value Addition Through Functional Ingredients
Table 1: Examples of Functional Ingredients*
Class/ Ingredients
Source*
Potential Benefit
Carotenoids
Beta-carotene
carrots, various fruits
neutralizes free radicals which may
damage cells; bolsters cellular
antioxidant defenses
Lutein, Zeaxanthin
kale, collards, spinach, corn, eggs,
citrus
may contribute to maintenance of
healthy vision
Lycopene
tomatoes and processed tomato
products
may contribute to maintenance of
prostate health
Insoluble fiber
wheat bran
may contribute to maintenance of a
healthy digestive tract
Beta glucan
oat bran, rolled oats, oat flour
may reduce risk of coronary heart
disease (CHD)
Soluble fiber
psyllium seed husk
may reduce risk of CHD
Whole grains
cereal grains
may reduce risk of CHD and cancer;
may contribute to maintenance of
healthy blood glucose levels
Monounsaturated fatty acids
(MUFAs)
tree nuts
may reduce risk of CHD
Polyunsaturated fatty acids (PUFAs)
- Omega-3 fatty acids—ALA
walnuts, flax
may contribute to maintenance of
mental and visual function
PUFAs - Omega-3 fatty acids—DHA/
EPA
salmon, tuna, marine and other fish
oils
may reduce risk of CHD; may
contribute to maintenance of mental
and visual function
PUFAs - Conjugated linoleic acid
(CLA)
beef and lamb; some cheese
may contribute to maintenance of
desirable body composition and
healthy immune function
Anthocyanidins
berries, cherries, red grapes
bolster cellular antioxidant defenses;
may contribute to maintenance of
brain function
Flavanols—Catechins, Epicatechins,
Procyanidins
tea, cocoa, chocolate, apples,
grapes
may contribute to maintenance of
heart health
Flavanones
citrus foods
neutralize free radicals which may
damage cells; bolster cellular
antioxidant defenses
Flavonols
onions, apples, tea, broccoli
neutralize free radicals which may
damage cells; bolster cellular
antioxidant defenses
Proanthocyanidins
cranberries, cocoa, apples,
strawberries, grapes, wine, peanuts,
cinnamon
may contribute to maintenance of
urinary tract health and heart health
Dietary (functional and total) Fiber
Fatty Acids
Flavonoids
Isothiocyanates
Sulforaphane
cauliflower, broccoli, broccoli sprouts, may enhance detoxification of
cabbage, kale, horseradish
undesirable compounds and bolster
cellular antioxidant defenses
Phenols
Caffeic acid, Ferulic acid
apples, pears, citrus fruits, some
vegetables
may bolster cellular antioxidant
defenses; may contribute to
maintenance of healthy vision and
heart health
11
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Class/ Ingredients
Source*
Potential Benefit
Plant Stanols/Sterols
Free Stanols/Sterols
corn, soy, wheat, wood oils, fortified
foods and beverages
may reduce risk of CHD
Stanol/Sterol esters
fortified table spreads, stanol ester
dietary supplements
may reduce risk of CHD
some chewing gums and other food
applications
may reduce risk of dental caries
Polyols
Sugar alcohols—xylitol, sorbitol,
mannitol, lactitol
Prebiotic/Probiotics
Inulin, Fructo-oligosaccharides
(FOS), Polydextrose
whole grains, onions, some fruits,
garlic, honey, leeks, fortified foods
and beverages
may improve gastrointestinal health;
may improve calcium absorption
Lactobacilli, Bifidobacteria
yogurt, other dairy and non-dairy
applications
may improve gastrointestinal health
and systemic immunity
Isoflavones—Daidzein, Genistein
soybeans and soy-based foods
may contribute to maintenance
of bone health, healthy brain and
immune function; for women,
maintenance of menopausal health
Lignans
flax, rye, some vegetables
may contribute to maintenance of
heart health and healthy immune
function
soybeans and soy-based foods
may reduce risk of CHD
Diallyl sulfide, Allyl methyl trisulfide
garlic, onions, leeks, scallions
may enhance detoxification of
undesirable compounds; may
contribute to maintenance of heart
health and healthy immune function
Dithiolthiones
cruciferous vegetables
contribute to maintenance of healthy
immune function
Phytoestrogens
Soy Protein
Soy Protein
Sulfides/Thiols
Source: IIFC (2004)
Examples are not an all-inclusive list.
Several functional dairy products can be developed using either single or combination of
ingredients given in the table targeting specific health benefits. Besides these functional ingredients,
which are mostly obtained from plant source, there are other ingredients such as fat replacers, artificial
sweeteners, micronutrients like vitamins and minerals, which can be used for value addition.
3.0 What are the possibilities?
Innovative milk beverages:
Recently, a whole new generation of beverages containing milk and dairy ingredient are emerging.
Thanks to new technologies, including processes and ingredients, such dairy based beverages not only
offer a wider range of flavour, texture and other sensory properties than are current present but also
provides new marketing opportunities for these products in the healthy/ neutraceutical/ bioactive
foods category foods today’s consumer’s want. Some of the ingredients highlighted above, along with
other ingredients that are currently used or can be used for development of such beverages. Dairy
manufacturers can develop a signature formula to appeal to specific market segments.
Select European countries use whey as a base for nutritional, fruity dairy-based beverages. A refreshing
beverage made from fermented milk and whey and containing fruit juice, or a probiotic beverage from
whey and fruit juice that is fortified with vitamins and calcium are being marketed in these countries.
NDRI has also recently developed formulations from whey such as whey-jaljeera beverage, whey-bael
beverage, and whey –mango beverage, which are available for commercial exploitation.
12
Prospects of Value Addition Through Functional Ingredients
Probiotic dairy products:
“Probiotic, food products in generals and “probiotic “ organism in particular are in the center of
current R & D activities all over the world. “Functional foods” segment that is registering a steady
and consistent growth at present, among processed food products, gathered the momentum primarily
from the scientific investigations based on “probiotic” food products. A probiotic is a mono-or mixed
culture of live microorganisms which benefits man or animals by improving the properties of the
indigenous microflora. Viable counts delivered to the gastrointestinal tract are key to the functionality
of probiotics. The consumption of probiotic culture positively affect the composition of this microflora or extends a range of host benefits including.
1.
Pathogen interference, exclusion and antagonism.
2.
Immunostimulation and immunomodulation.
3.
Anticarcinogenic or antimutagenic activities.
4.
Alleviation of symptoms of lactose intolerance.
5.
Reductiion in serum cholesterols.
6.
Reduction in blood pressures.
7.
Decreased incidence & duration of diarrhoea.
8.
Prevention of vaginitis.
9.
Maintenance of mucosal integrity.
Industrial interest in developing probiotics and probiotic functional foods is thriving, driven
largely by the market potential for foods that target general health or well being. NDRI has made
some progress in this area by developing probiotic dahi, lassi and probiotic cheese. There is possibility
of developing other milk based fermented traditional dairy products such as probiotic shrikand and
Rabadi – a milk-cereal based fermented product.
Fat-replacement in dairy products:
High fat consumption has been linked to several chronic diseases including cardiovascular diseases,
obesity and certain forms of cancer. Nutrition experts recommend a total fat intake of less than 30 per
cent of total daily calories. These dietary recommendations are one reason for the increasing demand
for lower fat food products of the world market has been flooded with the food products carrying the
labels “low fat”, ‘no fat’ or ‘reduced fat’. Fat mimics or fat substitutes are normally used to produce
low-fat foods, fat mimics are substances that help replace the mouthfeel of fat but can not substitute
for fat on a gram for gram basis and can not be used for applications involving frying. Substances
whose physical or thermal properties resemble fat are termed as fat substitutes and can replace fat on
a gram-for gram basis and can also be used for frying applications.
Categories of fat replacers
Fat mimics
Protein based
Fat replacers
Carbohydrate based
- whey protein conc.
- Microparticulated protein
- Starches
- Maltodextrins
- Polydextrose
-Emulsifiers
-Medium chain triacylglycerols.
-Structural lipids.
-Acaloric synthetic compounds.
* fatty alcohol esters of alkyl
malonic or malonic acid.
* esterified propoxylated glycerols
* trialkoxy tricarballylate
* poly carboxylic acid.
* Sucrose polyesters
Low-fat cheese, processed cheese, cultured products, frozen desserts, butters and spreads have
been successfully developed using commercially available fat mimics/replacers. Using similar
technique several low fat varieties of traditional dairy products can be developed. An attempt has
been made to develop low fat burfi at this institute.
13
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Dairy products for providing satiety:
There are a few dairy products currently in the marketplace, which claim to provide satiety. This
is an opportunity dairy product manufacturers need to tap into, too. Satiety is the state of being full
or gratified to the point of satisfaction. Scientific studies indicate that satiety is dependant on not only
how much food you eat, but what type of food you eat as well. Satiety is being addressed on food labels
with synonymous terms such as “hearty” and “controls or reduces hunger.” Unabsorbed nutrients in
the ileum, which is the final section of the small intestine, inhibit gastric emptying, providing a sense
of satiety. Fat, in particular, penetrates the ileum when a person has eaten too much for the body to
process. When this happens the ileum triggers a “full” message to the brain. That full message is the
result of the secretion of cholecystokinin (CCK), a peptide hormone of the gastrointestinal system
responsible for stimulating the digestion of fat and protein. It is secreted by the duodenum, the first
segment of the small intestine, and causes the release of digestive enzymes and bile from the pancreas
and gall bladder, respectively.
A satiety ingredient concept is available to dairy foods manufacturers. A patented combination of
oat and palm oils has been formulated into a novel emulsion with the oat oil extract containing a large
quantity of polar lipids that coat the palm oil droplets. This coating prevents digestion of the palm oil
in the stomach until it reaches the ileum.
Fiber ingredient suppliers, too, are touting some of their products for satiety value. For example,
research shows consumers on diets supplemented with inulin and oligofructose report higher levels
of satiety, longer feelings of fullness and lower calorie intake, which can all assist with weight loss.
Research also shows that foods high in fiber and protein slow digestion and extend the release of CCK.
With knowledge of this relationship between fiber, protein and satiety, several convenient, nutritious
and delicious products can be created for obese people, which can help them feel full and thus prevent
unhealthful snacking between meals.
A heart-healthy opportunity
With the functional food market abuzz about the heart-health benefits of plant sterols, dairy foods
formulators have excellent opportunity to develop variety of dairy products with heart healthy benefit.
Plant sterols can help lower serum low-density lipoprotein (LDL)—or bad—cholesterol levels, which
are well recognized as impacting heart disease risk. Eating foods low in saturated fat and cholesterol
and high in sterols can reduce LDL cholesterol by 20%. Plant sterols provide an effective, dietary
method for countering elevated cholesterol, a crisis facing millions of Indians.
Plant sterols are relatively easy to formulate into existing dairy applications, and sterols are
available in different forms to aid in the ease of processing.
Likewise, plant sterols can be used in virtually any dairy application. If included in the amounts
specified for health claim, plant sterols also enhance a finished dairy product’s nutritional profile
without altering its flavor or texture. The qualified claim states that foods containing at least 0.4g per
serving of plant sterols, eaten twice a day with meals for a daily total intake of at least 0.8g, as part of
a diet low in saturated fat and cholesterol, may reduce the risk of heart disease.
Arjuna Ghee: Arjuna ghee, with functionalities like resistance against heart diseases and blood
pressure regulating properties was developed at this institute. The developed ghee was found
sensorily similar to the market ghee. It had overall acceptability score of 85.1 compared to the control
(90.84). The Arjuna ghee was found to be 4 times more stable to oxidative deterioration as compared to
control ghee. This is due to the fact that Arjuna extract contains several antioxidants like polyphenols,
terpenoids in addition to phytosterol, which are beneficial in case of Cardio-vascular Diseases (CVD),
high blood pressure and to boost up our immune system.
Dietetic dairy products
The dairy industry has responded to the growing needs of health conscious consumers for lowcalorie foods. Consequently, a large number of dairy products made with low-calorie and nonnutritive
14
Prospects of Value Addition Through Functional Ingredients
sweeteners have been witnessed in the market. Low calorie sweeteners have become sugar alternatives
to replace sucrose in a wide variety of dairy products. Kumar (2000) developed a low calorie lassi by
using aspartame and reported that aspartame at a level of 0.08% was required to replace 15% of cane
sugar in lassi.
The technology for the production of rasogolla, the most popular channa based Indian sweetmeat,
was developed by Jayaprakash (2003) using sorbitol (40%) and aspartame (0.08%). Chetana, et al.
(2004) developed gulabjamun, a popular khoa based sweet, using sorbitol. Burfi, another khoa based
sweet delicacy was developed by completely replacing sugar using acesulfame-K (Yarrakula, 2006),
aspartame (Muralidhar, 2006), saccharin (Narendra, 2006), sucralose (Singh, 2006), and sucralose
and bulking agents (Prabha, 2006). Kalakand and flavored milk were developed using acesulfame-K
(Yarrakula, 2006), aspartame (Muralidhar, 2006), saccharin (Narendra, 2006), and sucralose (Singh,
2006). The Indian counterpart for ice cream, kulfi was developed by Pandit (2004) using sorbitol
(5.5%), maltodextrin (4.26%) and aspartame (742 ppm).
Dairy products fortified with dietary fiber
Milk and most dairy products are devoid of dietary fiber. With the growing interest in dietary fiber
and its health benefits, dairy industry has geared up for fortifying the dairy products with fiber.
Yogurt is one of the dairy products whose sales continues to increase due to diversification in
the range of yogurt-like products including reduced fat content yogurts, yogurt shakes, drinkable
yogurts, yogurt mousse, yogurt ice cream, etc. (Fiszman and Salvador, 1999).
In India, there are few traditional dairy products that contain significant quantities of fiber e.g.,
Gajar-pak (carrot halwa), Giya-ka-halwa (bottle gourd halwa), Doda-burfi, and Kaju-burfi. Traditionally
made cereals-based milk desserts like kheer and dalia-dessert are other dairy food sources of dietary
fiber in Indian diets (Patel and Arora, 2005). Recently, dahi (Chandrakant, 2002), lassi and other dairy
products have been fortified with fruits and commercial dietary fibers to give the benefits of dietary
fiber. Kantha (2005) developed a low fat paneer using soy fiber and inulin and reported that milk with
2.5% fat and 0.56% soy fiber or 1.8% fat and 4.5% inulin yielded a paneer similar to that prepared from
full cream milk (6% fat) in respect to sensory quality. Amul has launched a new variety of isabgolenriched ice cream. Isabgol is the seed derived from Plantago ovata. Being a ‘true dietary fibre’, the
isabgol husk is considered to be a natural laxative that aids easy bowel movement. Besides it is also
known to possess serum cholesterol reducing properties (Mann and Singh, 2005)
Targeting a successful product launch
In order to launch the product successfully in the market it is necessary to look in to following
points:
•
Identifying where the gaps are in a specific market--what the new/unmet consumer needs are.
•
Developing product concepts and consumer value propositions to fill the gaps.
•
Prper ingredient selection, formulating prototypes and evaluating product concepts at an inhouse pilot plant.
Fundamentally the product needs to pass the taste test. If it does not taste good, it is not possible
to get that repeat buy from consumer. Any product that has been developed by hitting a bull’s eye in
each one of these areas (health and wellness, simplicity and taste) will certainly have a stronger chance
for a successful product launch.
What are the prospects for functional foods in india?
Population growth, rising incomes, increasing awareness on health, urbanization, lifestyle changes
(“on-the-go” eating) and growing organized retailing are contributing to the potential for functional
foods. Just as for processed foods in general, India will be the largest potential markets for functional
foods with their GDP growth, demographics and burgeoning consumption.
15
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Thanks to the growing acceptance of functional foods, India could hope to leverage the country’s
key resources in this area to gain a foothold in the global market. Functional foods are among the
New Age drugs that are being developed to provide better health. Functional foods are gaining public
acceptance in many developed countries in recent times. Looking at the changing trends, the market
of functional foods has huge potential. These days, industries are showing interest in the functional
foods area. Within few years this potential can turn into a healthy growing market.
The ingredients used in health/functional foods are mainly plant-based products and most of them
being predominantly herbal. Hence clues to these functional ingredients could be got from our ancient
and traditional systems of medicine like Ayurveda, Siddha and Unani. The ‘Rasayan’ and ‘Vajikarna’
therapeutics of Ayurveda are essentially nutraceuticals and therefore there is ample scope for India to
develop a range of health food products. And to succeed, these products have to be standardized and
with scientific validation to ensure safety and efficacy so as to instill confidence in the customers to
use them not as an alternative medicine but as a well defined system of medicine. For this to happen,
there has to be research carried out on these products. Thus India’s own traditional knowledge base
gathered from Unani, Ayurveda and Siddha can help out in research work on nutraceuticals. And we
can take a lead on this from the western world.
What are the key challenges for functional foods in india?
In India, we have traditional products touted as functional but have little scientific validation.
Regulations will thus have to evolve to weigh R&D, ensure validation and prevent exploitation of
consumers. Companies will also have to be sincere and honest in their claims while marketing and
communicating with consumers till appropriate regulations for scientific validation are evolved.
Processors will need to provide an optimal merger between taste, convenience and health attributes.
References:
Chandrakant, P.N. (2002) Development of Technology for Fruit Dahi. M. Tech Thesis submitted to National Dairy
Research Institute, Deemed University, Karnal.
Chetana, R., Manohar, B. and Reddy S.R. (2004) Process optimization of Gulab Jamun, an Indian traditional sweet,
using sugar substitutes. Eur. Food Res. Technol. 209:386 – 392
Fiszman, S.M. and Salvador, A. (1999) Effect of gelatine on the texture of yoghurt and of acid-heat-induced milk gels.
Z. Lebensm. Unters. Forsch. 208: 100 – 05.
IIFC (2004) Background on functional foods. http://www.ific.org/nutrition/ functional/ upload/FuncFdsBackgrounder.
pdf
Jayaprakash, K.T. (2003) Technological Studies on the Manufacture of Rasogulla Using Artificial Sweeteners. M. Tech
Thesis submitted to National Dairy Research Institute, Deemed University, Karnal.
Kantha, K.L. (2005) Enhancement of Sensory and Functional Properties of Low-fat Paneer Using Dietary Fibre. M. Tech.
Thesis submitted to National Dairy Research Institute, Deemed University, Karnal.
Kumar, M. (2000) Physico-chemical Characteristics of Low-Calorie Lassi and Flavoured Dairy Drink Using Fat Replacer
and Artificial Sweetener. M. Tech Thesis submitted to National Dairy Research Institute, Deemed University,
Karnal.
Mann, R.S. and Singh, P.K. (2005) Specialty frozen products. In: lecture compendium of “Recent Developments in
Health Foods and Nutraceuticals” 18th Short Course organized by Centre of Advanced stuies in Dairy Technology,
NDRI, Karnal, pp 127 – 132.
Muralidhar, G.H. (2006) Determination of Aspartame and its Stability in Indigenous Dairy Products. M.Sc. Thesis
submitted to National Dairy Research Institute, Deemed University, Karnal.
Narendra, K. (2006) Estimation and Stability of Saccharin in Indigenous Dairy Products. M.Sc. Thesis submitted to
National Dairy Research Institute, Deemed University, Karnal.
Pandit, P. (2004) Technological Studies on manufacture of Kulfi using Artificial Sweeteners. M. Tech. Thesis submitted
to National Dairy Research Institute, Deemed University, Karnal.
Patel, A. A. and Arora, S.K. (2005) Fibre fortification of dairy products.Proceedings of the Seminar on Value Added
Dairy Products held at NDRI, Karnal from Dec. 21 – 22, 2005.
Prabha, S. (2006) Development of Technology for the Manufacture of Dietetic Burfi. Ph. D. Thesis submitted to National
Dairy Research Institute, Deemed University, Karnal.
Singh, V.P. (2006) Analysis of Sucralose and its Stability in Indigenous Dairy Products. M.Sc. Thesis submitted to
National Dairy Research Institute, Deemed University, Karnal.
Yarrakula, S. (2006) Analysis of Acesulfame-K and its Stability in Indigenous Dairy Products. M.Sc. Thesis submitted to
National Dairy Research Institute, Deemed University, Karnal.
16
Technological and Nutritional Aspects of Milk Phospholipids
Technological and Nutritional
Aspects of Milk Phospholipids
B. K. Wadhwa and Rajesh Kumar
Dairy Chemistry Division, NDRI, Karnal
Introduction
Milk fat in the lactating cow is secreted as myriads of lipid droplets of size 0.1 to 15 µm. These
micro lipid droplets are encircled by a special membrane composed of lipid bilayer and proteins.
This membrane has been designated the milk fat/ lipid globule membrane (MFGM). Milk fat globule
membrane is composed of proteins and lipids in a 1:1 weight ratio. Bovine MFGM is a potential
nutraceutical. The health beneficial factors are contributed by both protein and non protein components
of bovine MFGM. Among the health-beneficial components of the MFGM are cholesterolemia-lowering
factor, inhibitors of cancer cell growth, vitamin binders, inhibitor of Helicobacter pylori, inhibitor of betaglucuronidase of the intestinal Escherichia coli, xanthine oxidase as a bactericidal agent, butyrophilin
as a possible suppressor of multiple sclerosis, and phospholipids as agents against colon cancer,
gastrointestinal pathogens, Alzheimer’s disease, depression, and stress (Spitsberg, 2005).
Sources of phospholipids
Until recently commercially available phospholipids were predominantly made from vegetable
lecithin, the by-products of vegetable oil refining. Phospholipids of animal origin were extracted from
egg yolk or from fish roe, but played less important role compared to the plant derived products,
which mainly found applications as food additives.Bovine milk is a very new source for a commercial
production of phospholipids as milk fat globule membrane
Table 1: Phospholipid profile (%)
phospholipids are very unique in terms of phospholipid
of different raw materials
composition and application profile (Schneider, 2007).
Soya Egg Milk Marine Vegetable lecithin is a complex mixture of phospho- and
glycolipids, some carbohydrates and optionally triglycerides.
PC
23
73
27
82
Most animal lecithin does not contain glycolipids, nor
PE
22
18
22
04
carbohydrates. Vegetable oilseeds are solvent extracted to
PI
14
2
08
03
obtain the oil. Traces of phospholipids are co-extracted and
PA
7
need to be removed during the refining process in order to
PS
Traces
12
01
improve the oil for clarity and stability reasons. This is done
SPM
3
27
02
by the addition of small amounts of water; the phospholipids
Glycolipids
12
07
start swelling which makes them insoluble in oil. Mechanical
separation and drying of the so-called wet
Table 2: Fatty acid profile (%) of phospholipids
gums finally gives vegetable lecithin. To
from different raw materials
produce egg and/or milk phospholipids
Soya
Egg
Milk
Marine
the process is much more sophisticated.
Especially in the case of milk, it is a multi
Saturated
22
41
50
17
step approach to separate them from the
Mono-unsaturated
12
35
35
21
milk fat globule membrane - predominantly
Poly-unsaturated
66
24
15
62
milk processing technology, followed by
some solvent based steps. Concentrated or
isolated phospholipids are made by solvent-based extraction or fractionation processes from lecithin,
often followed by chromatographic purification steps. The qualitative and quantitative profile
of phospholipids varies with the type of raw materials used (Table 1). Milk phospholipids contain
sphingomyelin whereas soya phospholipids do not contain sphingomyelin (Schneider, 2007). Also, the
fatty acid profile of phospholipids from different raw materials is variable (Table 2). Milk phospholipids
are richer in saturated and MUFA but poorer in PUFA in comparison to soya phospholipids.
17
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Properties of phospholipids
The molecular structure of phospholipids is bipolar and amphiphilic, means it combines a lipophilic
part (the fatty acid tails linked to the glycerol backbone) and a hydrophilic part (the polar head group, a
phosphoric acid ester mostly of an amino alcohol like choline, ethanolamine, etc.).
Technological applications
Their bipolar structure makes phospholipids excellent natural emulsifiers, widely used in foods,
cosmetics and also pharmaceutical applications. The application properties of the food additive lecithin
relates to a great extent to their phospholipid content and profile. The most prominent applications
are for margarine, chocolate, baked products, instant powders, etc. The cosmetic industry appreciates
their emulsifying, skin friendly and moisturising properties; pharmaceuticals use them as emulsifiers
for intravenous fat emulsions, but also for complex drug delivery systems (Iiposome).
Liposomes are versatile delivery systems, originally for pharmaceutical uses, but also widely used
in cosmetics. Liposomes are tiny, artificial cell-like structures, surrounded by either one (unilamellar)
or many phospholipid double layers. Inside the vesicular structure, or between the different
phos¬pholipid membranes, water compartments are entrapped, carrying and protecting water-soluble
actives. But also lipophilic payloads can be entrapped and protected from the environment inside the
lipophilic domains of the phospholipid double layers.
Nutritiona1 profile of phospholipids
Besides their widely used technological properties, phospholipids have a very Interesting
nutritiona1 profile. Lots of clinical studies have shown
•
Cholesterol reducing properties ( soya phosphatidylcholine - PC)
•
Improvement of cognitive performance - stress symptom (soya phosphatidylserlne - PS)
•
Liver tissue detoxification and regeneration ( soya PC).
•
Egg phospholipids are used to supplement infant formulae because of their content of long
chain polyunsaturated fatty acids (docosahexaenoic and arachidonic acid DHA and ARA).
Milk polar lipids
Another biologically interesting lipid group in milk fat is the polar lipids, which are mainly
located in the milk fat globule membrane (MFGM). It is a highly complex biological structure that
surrounds the fat globulestabilizing it in the continuous aqueous phase of milk and preventing it from
enzymatic degradation by lipases (Spitsberg, 2005 ). The membrane consists of about 60% proteins and
40% lipids that are mainly composed of triglycerides, cholesterol, phospholipids, and sphingolipids.
The polar lipid content of raw milk is reported to range between 9.4 and 35.5 mg per 100 g of milk.
The major phospholipid fractions are phosphatidylethanolamine and phosphatidylcholine followed
by smaller amounts of phosphatidylserine and phosphatidylinositol. The major sphingolipid
fraction is sphingomyelin with smaller portions of ceramides and gangliosides. In processing milk
into different dairy products, the polar lipids are preferentially enriched in the aqueous phases like
skimmed milk, buttermilk and butter serum.
The polar lipids in milk are gaining increasing interest due to their nutritional and technological
properties. These compounds are secondary messengers involved in transmembrane signal
transduction and regulation, growth, proliferation, differentiation, and apoptosis of cells. They also
play a role in neuronal signaling and are linked to age - related diseases, blood coagulation, immunity,
and inflammatory responses. In particular, sphingolipids and their derivatives are considered highly
bioactive components possessing anticancer, cholesterol - lowering, and antibacterial activities.These
promising results from cell culture and animal - model studies warrant further confirmation and
human clinical studies but suggest that sphingolipid - rich foods or supplements could be beneficial in
the prevention of breast and colon cancers and bowel - related diseases (Korhonen, 2010).
18
Technological and Nutritional Aspects of Milk Phospholipids
Properties of milk phospholipids
Milk phospholipids are different from all other commercial lecithin and phospholipid products,
both in phospholipid pattern and fatty acid profile (Tables 1 and 2). Both differences make them very
attractive for a variety of new and innovative applications.
Technological applications of milk phospholipids
Because of the relatively high degree of saturated (50%) or mono-unsaturated fatty acids
(approximately 35%) milk phospholipids are quite stable against oxidation and are
•
Very Important for food applications
•
Very stable against hydrolytic break-down in aqueous environments.
•
Hence, the taste and flavour profile is not negatively affected by liberated free fatty acids (as
with soya phospholipids which are richer in PUFA).
•
Milk phospholipids are versatile ingredients for functional cosmetics. They are excellent
emulsifiers, creating a very good and soft skin feel, avoid trans-epidermal water loss and allow
preparing efficient Iiposomal systems with good entrapment stability.Milk phospholipids now
have a clear advantage over all other phospholipids used so far for liposome production.
•
They are relatively stable against oxidation
•
They have a phase transition temperature of approximately 28°C, ideal for a lot of cosmetic and
food applications.
•
(The phase transition temperature is the temperature at which the membrane undergoes
transition from an organized fatty acid region (a kind of crystalline structure) to an unorganized
one (a kind of liquid structure).
•
At ambient temperature milk phospholipids liposome membranes are crystalline with excellent
entrapment characteristics . At higher temperature they tend to release their payload – a simple
approach to protect sensitive ingredients and to release them at targeted conditions (Schneider,
2007).
Preparation of liposomes from milk fat globule membrane phospholipids using a microfluidizer
The isolation of MFGM material from buttermilk on a commercial scale has provided a new
ingredient rich in phospholipids and sphingolipids. An MFGM-derived phospholipid fraction was
used to produce liposomes via a high-pressure homogenizer (Microfluidizer). This technique does not
require the use of solvents or detergents, and is suitable for use in the food industry. The liposome
dispersion had an average hydrodynamic diameter of 95 nm, with a broad particle-size distribution.
Increasing the number of passes through the Microfluidizer, increasing the pressure, or reducing
the phospholipid concentration all resulted in a smaller average liposome diameter. Changing these
variables did not have a significant effect on the polydispersity of the dispersion. Electron microscopy
showed that the dispersions formed had a range of structures, including unilamellar, multilamellar,
and multivesicular liposomes. The composition of the MFGM phospholipid material is different from
that of the phospholipids usually used for liposome production in the pharmaceutical and cosmetic
industries. The MFGM-derived fraction comprises approximately 25% sphingomyelin, and the fatty
acids are primarily saturated and monounsaturated These differences are likely to affect the properties
of the liposomes produced from the phospholipid material, and it may be possible to exploit the unique
composition of the MFGM phospholipid fraction in the delivery of bioactive ingredients in functional
foods ( Thompson and Singh, 2006).
19
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Nutritional applications
Phospholipids
The consumption of the MFGM alone as a nutraceutical or as a dairy food, or the consumption of
food products enriched by the MFGM has health benefits due to the presence of phospholipids in the
MFGM. Phospholipids of bovine MFGM constitute almost 30% of the total MFGM lipids. The three
main MFGM phospholipids are sphingomyelin, phosphatidyl choline, and phosphatidyl ethanolamine
comprising (weight %) 19.2 to 23.0, 25.7 to 41.1, and 27.0 to 35.0% of total MFGM phospholipids,
respectively. Currently, it is considered that phospholipids, including milk-derived, affect numerous
cell functions including growth and development, molecular transport systems, absorption processes,
memory, stress responses, development of Alzheimer’s disease, and myelination in the central nervous
system . Phospholipids also affect the development of colon cancer as discussed above (Spitsberg,
2005).
Phospholipids and glycosphingolipids
Phospholipids and glycosphingolipids accounts to about 1% of total milk lipids. They have
functional roles in a number of reactions, such as binding enzymes on the globule surface, cellcell interactions, differentiation, proliferation, immune recognition. Gangliosides are one of these
components found in milk. The small amount of gangliosides (very complex neuraminic acid derivatives
of a glycosylatcd ceramide) in milk polar lipid fractions has triggered interest to incorporate milk
phospholipid compounds into infant formula products. Gangliosides have been confirmed as having
immune stimulating effects and can modulate the binding of microbial toxins in the intestinal tract
( Haug, 2007).
Sphingomyelin
Sphingomyelin (N-acylsphingosine-l phosphocho line or ceramide phosphocholine) is a phospho
lipid preferentially located in the outer leaflet of the plasma membrane of most mammalian cells. In
bovine milk, phospholipids account for 0.2-1.0 g/l00 g of total lipids, where they are as sociated with
the milk fat globule membrane. Sphingomyelin represents about one third of total milk phospholipids,
variation in content is influenced by season and the stage of lactation, Digestion products of
sphingomyelin and other sphingolipids, the ceramides(fatty acid amides of sphingosine), sphingosines
and sphingosine-phosphates are highly bioactive compounds that are associated with cell regulation.
They arrest cell growth and induce differentiation and apoptosis mechanisms that are deregulated
in carcinogenesis. Ceramide and sphingosine are referred to as tumor sup¬pressor lipids. The major
metabo-lites, ceramide and sphingosine, pass from the lumen to intestinal cells where they are utilized
to resynthesize sphingomyelin and other sphingolipids, which than largely pass to the circulation.
Because ceramide and sphingosine participate in major anti proliferative pathways of cell regulation
that suppress oncogenesis, they have been termed tumor suppressor lipids. Both sphingolipids and
their active metabolites, ceramides and sphingosines, were determined as effective bactericidal agents
on pathogens like Listeria monocytogenes. In addition, studies with experimental animals have
shown that feeding sphingolipids inhibits colon carcinogenesis, reduces serum LDL cholesterol and
regulates immune system. A series of study showed that dietary milkderived sphingomylein (0.025
to 0.1%) of diet inhibited chemically induced colon tumors development in mice, reduced aberrant
crypt foci (ACF) (ACF -early precursors of colon cancer) formation and suppressed the conversion of
benign adenomas to malignant adenocarcinomas. Feeding the milk derived sphingolipids, ceramide
monohexoside (glucosyl) ceramide dihexoside (lactosyl) and the ganglioside to mice at 0.025 to 1.0
g/100g diet has shown that there complex sphingolipids were hydrolysed to ceramide by colonic
enzymes. Supplementation reduced proliferation particularly in the upper-half of the colonic crypt
cells and reduced the number of ACF by 50-60 per cent. The reduction in the ACF formation is similar
to that previously obtained with sphingomyelin. Another aspect confirmed in human clinical trials
is sphingomyelin cholesterol lowering activity by inhibiting intestinal absorption of food based
cholesterol (Sibel et al, 2006; Schneider, 2007; Chaudhary et al, 2008).
20
Technological and Nutritional Aspects of Milk Phospholipids
Conclusion
Milk phospholipids are a new class of natural phospholipids now commercially available. They
offer a broad spectrum of both technological and nutritional properties which are unique to this kind of
polar lipid extract and which are different from all other phospholipid products on the market. These
are potent emulsifier, stable liposome forming compound, cholesterol lowering, improving cognitive
performance, stress dampening effects, colon cancer preventive effects, additive for infant formulae.
References
Chaudhry, I; Kathirvelan, C; Tyagi, A.K. (2008).Anticancer property of milk. Indian Dairyman, 60(5)37-53.
Haug, A; Hestmark, A.T; Harstad, O.M. (2007).Bovine milk in human nutrition-a review.Lipids in health and disease.
p1-26.
Korhonen, H.J. (2010).Bioactive components in bovine milk. Chapter2 p.15-42.cited from book- Bioactive components
in milk and dairy products Edt. By Young W.Park. Willey blackwell A John Wiley & Sons, Ltd., Publication.
Schneider, M.(2007). Milk phospholipids - technological and nutritional aspects.Bulletin of the IDF 413/2007.p.35-39.
Sibel Akal, Gönç and Gülfem Ünal (2006). Functional Properties of Bioactive Components of Milk Fat in Metabolism.
Pakistan Journal of Nutrition 5 (3): 194-197.
Spitsberg,V.L.(2005).Bovine MFGM as a potential nutraceutical. J. Dairy Sci. 88:2289-2294.
Thompson, A.K and Singh, H.(2006). Preparation of Liposomes from Milk Fat Globule Membrane Phospholipids Using
a Microfluidizer. J.Dairy Sci. 89:410-419.
21
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Methods of Cholesterol Removal to Develop
Low – Cholesterol Dairy Products
Darshan Lal and Vivek Sharma
Dairy Chemistry Division, NDRI, Karnal
Introduction:
The importance of milk and milk products, in India, has been recognized since Vedic times.
Milk is considered to be a complete food as it contains almost all essential nutrients required for
human health and growth. Lipids, the most important constituent of milk, play significant role in the
nutrition, flavour and physico-chemical properties of milk and milk products. They are also rich source
of fat-soluble vitamins (A, D, E & K) and essential fatty acids, apart from having pleasant sensory
attributes. Milk fat is easily digestible than other oils and fats. It contains number of components
which show anticarcinogenic activity, e.g. sphingomyeline, conjugated linoleic acid, β-carotene etc.
So one (especially vegetarians) cannot avoid it in one’s diet. But recent trend, in the society, is against
fat-rich dairy products due to the presence of saturated fat & cholesterol as these are known to increase
the incidence of coronary heart disease (CHD).
CHD is one of the common causes of heart attack. Through a period of time, many researchers have
shown that dietary cholesterol, serum cholesterol and occurrence of coronary heart disease (CHD)
have positive correlation. Milk fat contains about 0.25 to 0.40% cholesterol. Consumption of ghee
and other fat-rich dairy products makes appreciable contribution to cholesterol intake. Furthermore,
some cholesterol oxidation products (COPs) have been reported to be more harmful than cholesterol
itself as they are cytotoxic, atherogenic, mutagenic and carcinogenic. Recent wave against cholesterolcontaining foods has damaged the image and market growth of fat-rich dairy products. The educated
and urban society, in particular, is more conscious about the presence of cholesterol in their diet.
This segment of the society is the major consumer of dairy and other food items manufactured by the
organized sector. In recent years, demand of cholesterol-free foods has increased tremendously. This
has led to increase in market of margarine, vegetable fat filled dairy products, milk fat replaced dairy
products, etc.
Owing to the adverse affects of cholesterol on human health, various physical, chemical and
biological methods have been developed for reducing cholesterol in foods. These include blending of
milk fat with vegetable oils, extraction with organic solvent, adsorption with activated charcoal and
saponin, vacuum distillation, molecular distillation, degradation of cholesterol by enzyme (cholesterol
oxidase) and removal of cholesterol by supercritical carbon dioxide. Recently, β- cyclodextrin (a starch
hydrolysed product) has been effectively used for cholesterol removal from milk, cream, cheese, lard
and egg-yolk. Beta cyclodextrin is reported to be non-toxic, non-hygroscopic, chemically stable and
edible.
Cholesterol:
Cholesterol is a waxy material found in all cells of the body
and is a necessary part of cell membranes, some hormones
and other body components. In particular, it participates in
the formation of myelin sheaths in the brain and peripheral
nerves, and modulates the absorption of dietary fats in the
intestine. It also acts as a precursor in the biosynthesis of
bile acids, steroid hormones and vitamin D. The body makes
all the cholesterol it needs; it is not necessary to get any
cholesterol from the diet. A high level of cholesterol in the
blood is a major risk factor for CHD and heart attack.
22
Methods of Cholesterol Removal to Develop Low – Cholesterol Dairy Products
Structure and properties of cholesterol:
The term cholesterol was derived from the Greek words chole and stear, which mean “bile”
and “hard fat,” respectively. The origin of the term is a reflection of the fact that the substance was
first identified as a hard & white solid in gallstones. Though discovered by Poulletier de la Salle in
1769, cholesterol was not named until 1818, when Michel Chevreul rediscovered it and dubbed it as
cholesterine, believing that the material was like a fat (Sabine, 1977). Cholesterol is a hydrophobic sterol
consisting of a four-ring structure (Figure A) with molecular weight 386.66 and molecular formula:
C27H46O.
Cholesterol is insoluble in water, sparingly soluble in cold alcohol or petroleum ether, and soluble
in hot alcohol and most other organic solvents. Cholesterol melts at 148.5ºC. It can be sublimed and
distilled under high vacuum. The polar hydroxyl group, which gives cholesterol a slightly hydrophilic
nature, can be esterified to a fatty acid, producing cholesterol ester. Both cholesterol and cholesterol
ester are important structural components of cell membranes. Cholesterol is also a major determinant
of membrane fluidity due to its hydrophobic and hydrophilic regions (Webb et al, 1987).
Sources of cholesterol in body:
In the body, cholesterol appears through endogenous synthesis and from the diet. Cholesterol
synthesis in the body is most active in the liver and intestine and averages 11 mg per kg body weight
per day. This equals 770 mg for a 70 kg man on a low (less than 300 mg per day) cholesterol diet
(McNamara, 1987). Normally, liver makes 80% of the total blood cholesterol and only 20% comes from
the diet (Renner and Gurr, 1991; Allred, 1993). Cholesterol is not considered as an essential dietary
nutrient because of its endogenous synthesis. On the other hand, Thomas and Holub (1994) reported
that if less dietary cholesterol is consumed, the body compensates by making more cholesterol.
Digestion, absorption and transportation of cholesterol in the blood:
Digestion and absorption of cholesterol occurs in the small intestine (Grundy, 1983). Cholesterol
ester is broken down by a pancreatic cholesterol esterase into free cholesterol, which, absorbed into the
cells lining of the intestine. The absorption of endogenous cholesterol (as bile acids) is more efficient
than dietary cholesterol absorption.
Fat, including cholesterol, absorbed from the diet, is insoluble in the aqueous medium of the
blood. To enable transport through blood system, the various fat components are incorporated into
particles called lipoproteins (Grundy, 1983; Mahley and Innerarity, 1983). Lipoproteins consist of a
lipid core of triglyceride and cholesterol ester with a surface of mainly phospholipids, protein and
some free cholesterol. The four major lipoprotein fractions found in the blood are chylomicrons, verylow density lipoprotein (VLDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL).
Chylomicrons are very rich in triglycerides (about 85%) but also contain absorbed cholesterol in
the free or esterified form. VLDL is also rich in triglyceride (about 50%) and contains a substantial
portion of cholesterol mainly as cholesterol ester. VLDL transport about 15% of the total cholesterol
found in the blood.
LDL is enriched in cholesterol and accounts for about 60% of the total blood cholesterol level. It is
deposited in artery walls, increasing the buildup of plaque and hence also known as bad cholesterol.
HDL carries as much as 20% of the total blood cholesterol level. HDL is thought to be antiatherogenic
since it picks up cholesterol from peripheral tissues for delivery to the liver and excretion. Consequently,
HDL is called good cholesterol. A better indicator of risk for CHD is the LDL/HDL cholesterol ratio
(Thomas and Holub, 1994; Gurr, 1995).
Synergistic effect of cholesterol with saturated fatty acids on plasma cholesterol level:
Some saturated fatty acids are reported to affect total plasma cholesterol concentration. While,
stearic acid has little effect on plasma cholesterol concentration, myristic and palmitic acids have
been reported to have the greatest cholesterol raising potential (Hegsted et al., 1965). Some evidence
suggests that the effect of myristic and palmitic acids depends on the concomitant intake of dietary
23
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
cholesterol (National Academy of Sciences, 1989). Such an interaction is clear in several experimental
mammals (Spady et al., 1993) and has also been found in some human studies (Fielding et al., 1995).
The above reports suggesting an interaction between cholesterol and saturated fat intake; provide a
further reason to limit dietary cholesterol.
Coronary heart disease and atherosclerosis:
Coronary heart disease (CHD) is a condition in which the main coronary arteries which supply
blood to the heart are no longer able to supply sufficient blood and oxygen to the heart muscle. CHD,
the common cause of heart attack, is one of the most frequent causes of death in the developed and
developing countries (AHA, 1989). The rates of mortality due to CHD throughout the world vary.
For example, in one study among men aged 40-59 years, the annual incidence rate varied from 15 per
100,000 in Japan to 198 per 100,000 in Finland (Lovegrove and Jackson, 2003). According to Chopra
(1997), 2.5 million Indians become victims of heart disease every year, and Indian women are the
fastest rising group of coronary patients in the world. He further observed that 33 per 1000 Indians
have a greater chance of requiring treatment and intervention for heart disease than either European
or Americans.
Atherosclerosis is a silent, painless process and the main cause of CHD characterized by build up
of cholesterol-rich fatty deposits on the inner lining of the coronary arteries, which decrease blood
flow to the heart muscle by narrowing the arteries substantially (Tabas, 2002). The atherosclerosis
plaques usually develop at a point of minor injury in the arterial wall.
Cholesterol in milk and milk products:
Animal food products like milk and milk products, meat and meat products and eggs are the
major sources of cholesterol in our diet. Among these, chicken egg contains highest amount (about 215
mg/egg) of cholesterol. Normally, most of the dieticians believe milk fat as a main source of dietary
cholesterol and the main culprit for CHD disease. Cholesterol accounts for 0.25-0.45% of the total
lipids in milk. Cholesterol concentrates in the milk fat globule membrane (MFGM). In milk, 80% of the
cholesterol is associated with the milk fat globules and the remaining 20% is partitioned into the skim
milk phase where it is associated with fragments of cell membrane (Patton & Jensen, 1975). However,
any event disrupting the membrane structure, e.g. churning of cream will result in the partial passing
of cholesterol alongwith ruptured membrane material to the aqueous phase. Arul et. al., (1987) studied
the distribution of cholesterol in various milk fat fractions viz., solid fraction (m. pt. 39ºC), semisolid
fraction (m. pt. 21°C) and liquid fraction (m. pt. 12ºC) and reported that 80% of the total cholesterol
content was present in the liquid fraction of the milk fat. 80-90% of the cholesterol is present in milk in
the free form, while 10-20% is esterified (Bindal and Jain, 1973; Wood and Bitman, 1986; Jensen, 1987;
Schlimme & Kiel, 1989).
Pantulu and Murthy (1982) observed 8-10 times higher content of cholesterol in whey than in
whole milk. Srinivasan (1984) reported the average cholesterol content of cow and buffalo milk as
2.8 and 1.9 mg/g fat, respectively. However, Prasad and Pandita (1990) showed that buffalo milk (20
mg%) contained more cholesterol than cow milk (15.5 mg%). Similarly, they found that dahi from
buffalo milk contained more cholesterol as compared to dahi from milk of different breed of cows. In
general, dahi had lower cholesterol values than the fresh milk (Ismail and Ahmad, 1978; Prasad and
Pandita, 1990). Cholesterol in channa samples exhibited a highly significant variation, being minimum
in buffalo, while such species variations were not observed in case of khoa calculated on dry weight
basis (Prasad and Pandita, 1990).
Cheese was found to contain 52.3-76.6 (av. 69.3) mg of cholesterol/100 g of cheese and 198-298
(av. 273) mg/100g fat in cheese (Fuke and Matsuoka, 1974). Tylkin et al., (1975) reported 9 times
higher cholesterol/g fat in butter milk than butter. Aristova and Bekhova (1976) observed cholesterol
content in unsalted butter as 244 mg/100 g. Vyshemirskii et al., (1977) reported that 80-90% cholesterol
initially present in cream passed into butter and 10-20% to butter milk. Masson and Martinez (1984)
reported cholesterol content as 177–208 mg/100 g fat in butter. Bindal and Jain (1972) estimated free
24
Methods of Cholesterol Removal to Develop Low – Cholesterol Dairy Products
and esterified cholesterol in Desi ghee, using TLC method and reported their contents as 0.288 and
0.038% and 0.214 and 0.056% in cow and buffalo ghee, respectively. Prasad and Pandita (1987) observed
cholesterol content of ghee prepared from milk of Haryana, Sahiwal and Sahiwal X Friesian cows and
from Murrah buffaloes, to be 303, 310, 328 and 240 mg/100 g fat, respectively.
Factors affecting level of cholesterol in milk and milk products
Effect of Species/Breeds:
Bindal and Jain (1973) reported that cow ghee (0.31%) contained higher cholesterol than buffalo ghee
(0.267%). Bernolak (1979) observed that cow milk, with 2.8% fat, contained 237 mg total sterols/100 g fat
(92.8% cholesterol of total sterols). Prasad and Pandita (1987, 1990) also reported higher cholesterol content
in cow ghee compared to that in buffalo ghee. Singh and Gupta (1982) observed that goat ghee contain
higher cholesterol (0.236 g/100 g fat) than cow (0.230 g/100 g fat) and buffalo (0.196 g/100 g fat) ghee.
Effect of Season/Stage of lactation:
Season has also been reported to affect the cholesterol content of milk fat. Treiger (1979) reported
that total cholesterol content of cow milk fat ranged from 0.24-0.29 g/100 g fat in spring and 0.18-0.25
g/100 g fat in summer season. Prasad and Pandita (1987, 1990) observed that cholesterol content of
ghee was higher in winter than in summer (301 vs 291 mg/100g fat). Krzyzewski et al. (2003) also
observed a significantly lower (by about 16%) concentration of cholesterol in milk during winter
season. Ghee prepared from milk of old animals (Lal, 1982) and late lactation milk (Nigam, 1989) was
found to contain highest level of cholesterol.
Effect of Heat:
Bector and Narayanan (1975) observed that when cow and buffalo ghee were heated at 225°C for 2
h, respectively 26.1 and 27.3% of cholesterol was lost. Similarly, Rai and Narayanan (1984) also reported
28.2 and 49% loss of cholesterol after 12 h of intermittent frying in aluminium and iron container.
Methods of cholesterol removal from milk fat:
Since dairy products contain significant amounts of cholesterol, a number of processes for removal
of cholesterol have been developed to produce low-cholesterol dairy products. These include steam
stripping, molecular distillation, solvent or super-critical extraction, reaction with cyclic anhydride,
enzymatic method and treatments with adsorbents like saponin, activated charcoal and cyclodextrin.
These are briefly discussed below.
1. Steam stripping
This process is similar to that used in the deodorization of vegetable oils and removal of
unsaponifiable matter. To remove cholesterol by steam stripping, the fat is first deairated under
vacuum after which it is heated with steam upto 232ºC and then subjected to steam at low pressure
in cylindrical tall chamber. The anhydrous milk fat (AMF) passing over a series of plates is spread
in many thin layers, which increases the stripping efficiency. The steam rises and carries with it the
evaporated cholesterol to be condensed and collected with other volatiles. This process can remove
upto 93% of cholesterol though with 5% fat losses. The major disadvantage to the process is that it
removes flavouring compounds also (Schlimme & Kiel, 1989).
2. Molecular distillation
In this process, AMF is molecularly distilled at temperature 190 and 210ºC at a vacuum of 10-4
Torr. Fractions distilled at 190 and 210ºC represented 3.43 and 3.99 % of the initial mass and contained
more than 93% of the total cholesterol (Lanzani et al, 1994 and Sharma et al., 1999). Arul et al. (1988)
fractionated AMF into four fractions at temperatures of 245 and 265ºC and pressure of 220 and 100 mm
Hg. Two low melting point fractions were blended together to yield a total of three fractions (liquid,
intermediate and solid). About 78% of the total cholesterol was found in the liquid fraction while the
remaining was found in the intermediate (18%) and solid (4%) fractions in the esterified form. But,
because of the high heat used in the process, the quality of the end product is adversely affected.
25
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
3. Solvent extraction:
In this process butter oil is mixed with propane and ethanol in the mixing vessel. The low viscous
mixture of butter fat, ethanol and propane is fed into the extraction column. A mixture of ethanol and
water, containing a small amount of propane is used as extractant. The extract, a solution of cholesterol
and butter fat in a mixture of ethanol, water and some propane is withdrawn at the bottom of the
extraction column, which is splitted into two phases. The upper phase consists of fat and cholesterol,
which are subsequently separated, in a further processing step. Around 90 to 95% of the cholesterol is
extracted in this counter-current procedure operated at 30ºC and 10 bar (Czech et al., 1993).
4. Supercritical carbon dioxide extraction:
Some studies have shown that supercritical carbon dioxide (SC-CO2) can be used to fractionate
AMF with evidence that cholesterol can be concentrated into selected fractions. Kaufmann et al., (1982)
obtained two fractions of milk fat by SC-CO2 extraction at a pressure of 200 bars and temperature of
80ºC. In this process, the liquid fractions were enriched in total cholesterol. However, Huber et al.
(1996) observed that direct supercritical extraction of cholesterol from AMF is not feasible because
of the low selectivity of cholesterol and poor solubility of AMF. Moreover, under these conditions,
important milk flavours also get separated with the cholesterol. Therefore, they proposed another
process for cholesterol removal from AMF, dissolved in SC-CO2 under high solubility conditions for
AMF (40 MPa at 70ºC) to achieve rapid extraction. In this process, the dissolved AMF in SC-CO2 is
passed isobarically and isothermally through a high-pressure column, filled with a suitable adsorbent
(e.g. silica gel) to eliminate cholesterol. Finally, the supercritical mixture is fractionated by either
descending or ascending temperature profile in separators connected in series. Karkare and Alkio
(1993) found that over 99% of cholesterol from milk fat could be removed using an SC-CO2 extraction
system equipped with a silica gel column.
5. Reaction with cyclic anhydride:
Gu et al. (1994) developed a method for cholesterol removal from milk fat based on the reaction
between the hydroxyl group of cholesterol and a cyclic anhydride such as succinic anhydride. The
conversion of cholesterol into an acid derivative makes it possible to remove these from fats by
extraction with aqueous alkali. Addition of acetic acid increases the rate of reaction and prevents the
distillation of cyclic anhydride from reaction mixture. They removed 50% cholesterol from animal fats
but alongwith it α- tocopherol (50%), γ- and δ- lactones also get removed.
6. Enzymatic method:
McDonald et al. (1983) have described an enzymatic process using cholesterol reductase for
conversion of cholesterol to biologically inactive, e.g., non-toxic, non-absorbable products like
coprosterol, which is either not or is only poorly adsorbed by the body. This approach, which is
theoretically suitable for reducing the cholesterol content of milk fat, has been verified biologically
at least in part, by the finding that a portion of the intestinal cholesterol is reduced to coprosterol by
intestinal bacteria and subsequently eliminated.
7. Adsorption methods
Cholesterol can be removed by its adsorption on certain material. Adsorbents, which are used to
remove cholesterol, are activated charcoal, saponins and β cyclodextrin.
(A) Activated charcoal
Bindal et al., (1994) could remove half of the cholesterol present in milk fat through treatment
of liquid fat with activated charcoal. Another activated charcoal method claimed 95% of cholesterol
removal from AMF but many other compounds including yellow pigments were also removed
simultaneously (Sharma et al., 1999).
(B) Saponins
Saponins are naturally occurring plant compounds that can be used to selectively bind to
cholesterol and precipitate it out. 80% and 90% cholesterol reduction in cream and anhydrous milk
26
Methods of Cholesterol Removal to Develop Low – Cholesterol Dairy Products
fat was obtained by using this method (Riccomini et al., 1990). Oh et al. (1998) found 70.5% of the
cholesterol removal when milk was treated with 1.5% saponin at 45ºC for 30 min. Further, addition
of 0.25% celite increased cholesterol removal to 72%. However, the methods using activated charcoal
or saponins are relatively non-selective and remove flavour and nutritional components also when
cholesterol is removed (Lee et al., 1999; Sharma et al., 1999).
(C) β-cyclodextrin
Beta cyclodextrin, one of the well known members of cyclodextrin family, is a cyclic oligosaccharide
of seven glucose units joined ‘head to tail’ by α-1, 4 linkage and is produced by the action of enzyme
cyclodextrin glycosyl transferase (CGT) on hydrolyzed starch syrup. Beta cyclodextrin has torus like
structure. The central cavity is hydrophobic, giving the molecule its affinity for non-polar molecules
such as cholesterol (Szejtli, 2004). The radius of the cavity can accommodate a cholesterol molecule
almost exactly, explaining the highly specific nature of β-cyclodextrin’s ability to form an inclusion
complex with cholesterol (Hettinga, 1996).
References:
AHA (1989) Heart Facts. American Heart Association. Dallas, A. Heart. A.
Ahn, J. and Kwak, H. S. (1999) Optimizing cholesterol removal in cream using beta-cyclodextrin and response surface
methodology. J. Food Sci. 64(4): 629-632.
Allred, J. B. (1993) Lowering serum cholesterol. Who benefits? J. Nutr. 123: 1453.
Aristova, V. P. and Bekhova, E. K. (1976) Cholesterol in milk and milk products. Trudy, Vsesoyuznyi Nanchnoissledovatel’skii Institut Malochoi Promyshlennosti No. 42: 45 (cf. DSA 1977(39), 2748).
Arul, J. A., Boudreau, A., Makhlouf, J., Tardif, R. and Grenier, B. (1988). Distribution of cholesterol in milk fat fractions.
J. Dairy Res. 55: 361-371.
Bector, B.S. and Narayanan, K.M. (1975) Comparative stability of unsaponifiable constituents of ghee during thermal
oxidation. Indian J. Nutr. Dietetics. 12(6): 178-180.
Bindal, M. P. and Jain, M. K. (1973) Studies on cholesterol content of cow and buffalo ghee. Indian J. Anim. Sci. 43(10):
918-924.
Bindal, M. P., Wadhwa, B. K., Lal, D., Rai, T. and Aggarwal, P. K. (1994) Removal of cholesterol from milk and milk
products: Application of biotechnical processes. NDRI Annual Report. pp. 98-99.
Czech, B., Peter, S. and Weidner E. (1993) Effective removal of cholesterol from butter fat. Scandinavian Dairy Information,
7(4): 56-58.
Fielding, C. J., Havel, R. J., Todd, K. M., Yeo, K. E., Schloetter, M.C., Weinberg, V. and Frost, P. H. (1995) Effects of
dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young
men. J. Clin. Invest. 95: 611-618.
Fuke, Y. and Matsuoka, H. (1974) Cholesterol content and identification of foreign fats in processed cheese. J. Jap Soc.
Food Nutr. 27: 269.
Grundy, S. M. (1983) Absorption and metabolism of dietary cholesterol. Annu. Rev. Nutr. 3: 71-96.
Gu, Y. F., Chen, Y. and Hammond, E. G. (1994) Use of cyclic anhydrides to remove cholesterol and other hydroxy
compounds from animal fats and oils. J. Am. Oil Chem. Soc. 71: 1205-1207.
Gurr, M. I. (1995) Dietary lipids in health and disease. In Advanced Dairy Chemistry-2: Lipids, 2nd edn, (eds P. F. Fox),
Chapman & Hall, New York, pp. 349-402.
Hegsted, D.M., McGandy, R.B., Myers, M.L. and Stare, F.J. (1965) Quantitative effects of dietary fat on serum cholesterol
in man. Am. J. Clin. Nutr. 17: 281-95.
Hettinga, D. (1996) Butter. in Bailey’s industrial oil and fat products, Vol. 3, 5th edn, (eds Y. H. Hui), John Wiley & Sons,
INC. New York, pp. 1-23.
Huber, W., Molero, A., Pereyra, C. and Martinez de la Ossa E. (1996) Dynamic supercritical carbon dioxide extraction
for removal of cholesterol from anhydrous milk fat. Int. J. Food Sci. Tech. 31: 143-151.
Ismail, A.A. and Ahmad, N.S. (1978) Cholesterol content in buffalo milk and its distribution in some dairy products.
Egypt. J. Dairy Sci. 6: 92-95.
Jensen, R. J. (1987) Cholesterol in human milk. in Human Lactation. 3. The effect of human milk on the recipient infant
(eds A. S. Goldman, S. A. Atkinson and S. S. Hanson), Plenum Press, New York, pp. 151.
Karkare, V. and Alkio, M. (1993) Removal of cholesterol during milk fat fractionation by supercritical carbon dioxide.
Agric. Sci. Finland. 2(5): 387-393. (Cited in DSA, 56: 1522).
Kaufmann, W. , Biernoth, G., Frede, E., Merk, W., Precht, D. and Timmens, W. (1982) Fractionation of butter fat by
extraction with supercritical carbon dioxide. Milchwissenschaft. 37: 92-96.
Krzyzewski, J., Strzakowska, N., Jozwik, A., Bagnicka, E. and Ryniewicz, Z. (2003) Effect of nutrition and season on
cholesterol level in milk of Holstein-Friesian cows. Annals Anim. Sci. 3: 45-49.
Lal, D. (1982) Effect of lactation number on the physicochemical status of milk lipids. Ph.D. Thesis, Kurukshetra Univ.,
Kurukshetra.
Lanzani, A., Bondioli, P. Mariai, C., Folegatti, L., Venturii, S., Fedeli, E. and Barreteau, P. (1994) A new short-path
distillation system applied to the reduction of cholesterol in butter and lard. J. Am. Oil Chem. Soc. 71(6): 609-614.
27
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Lee, D. K., Ahn, J. and Kwak, H. S. (1999) Cholesterol removal from homogenized milk with beta-cyclodextrin. J. Dairy
Sci. 82: 2327-2330.
Lovegrove, J. and Jackson, K. (2003) Coronary heart disease. In Functional Dairy Products (eds Tina Mattila- Sandholm
and Maria Saarela), CRC Press, Boca Raton, pp: 54-93.
Masson, L and Martinez, M. (1984) Determination of cholesterol content in butter. International Dairy Federation Bulletin
no 7: 165.
McDonald, I. A., Bokkenheuser, V. D., McErnon, A. M., Mosbach, E. H. and Winter, J. (1983) Degradation of steroids in
human gut. J. Lipid Res. 24: 675-700.
McNamara, D. J. (1987) Diet and heart disease: The role of cholesterol and fat. J. Am. Oil Chem. Soc. 64: 1565-74.
Mahley, R. W. and Innerarity, T. L. (1983) Lipoprotein receptors and cholesterol homeostasis. Biochim. Biophys. Acta,
737: 197-222.
Nigam, S. (1989) Studies on the physico-chemical status of milk lipids from cross breed cattle. Ph.D. Thesis, Kurukshetra
Univ., Kurukshetra.
Oh, H. I., Chang, E. J. and Kwak, H. S. (1998) Conditions of the removal of cholesterol from milk by treatment with
saponin. Korean J. Dairy Sci. 20: 253-260.
Pantulu, P. C. and Murthy, M. K. R. (1982) Lipid composition of skimmed milk and whey. Asian. J. Dairy Res. 1(1): 1720.
Patton, S. and Jensen, R.G. (1975). Lipid metabolism and membrane functions of the mammary gland. In Progress in the
chemistry of fats and other lipids (eds R.T. Holman), Pergamon Press Oxford. pp. 163-277.
Prasad, R. and Pandita, N. N. (1990) Cholesterol content of milk and its fractionation during processing. Indian J. Dairy
Sci. 43(2):190-193.
Prasad, C.R., Subramanian, R. and Ramaprasad, C. (1992) Qualitative and comparative studies of cholesterol oxides in
commercial and home-made Indian ghee. Food Chemistry. 45(1): 71-73.
Prasad, R. and Pandita, N. N. (1987) Variations in the cholesterol content of dairy fat. Indian J. Dairy Sci. 40(1): 55-57.
Rai, T. and Narayanan, K.M. (1986) Unsaponifiable constituents of ghee as affected by intermittent frying. Indian j.
Anim. Sci. 56 (5): 610-611.
Renner, E. and Gurr, M.I. (1991) Do we need cholesterol reduced dairy products? Dairy Industry International. 56: 34.
Riccomini, M. A., Wick, C., Peterson, A, Jimenez-Flores, R. and Richardson, T. (1990) Cholesterol removal from cream
and anhydrous milk fat using saponins. J. Dairy Sci. 73(1): 107.
Sabine, J. R. (1977) Methods in cholesterol research. in Cholesterol, (eds J.R. Sabine) Marcel Dekker, Inc. New York and
Basel. pp. 29-55.
Schlimme, E. and Kiel, D. (1989) Removal of cholesterol from milk fat. European Dairy Magazine, 12-21.
Seth, R. and Singh, A. (1994-95) Removal of cholesterol from milk using β-cyclodextrin and preparation of milk products
from such treated milk. NDRI Annual Report. pp. 95.
Sharma, R., Nath, B. S. and Lal D. (1999) Approaches for cholesterol removal from milk fat: An overview. Indian J. Dairy
and Biosciences. 10:138-146.
Singh, I. and Gupta, M. P. (1982) Physico-chemical characteristics of ghee prepared from Goat milk. Asian J. Dairy Res.
1: 201-205.
Spady, D. K. Woollett, L. A. and Dietschy, J. M. (1993) Regulation of plasma LDL-cholesterol levels by dietary cholesterol
and fatty acids. Annual. Rev. Nutr. 13: 355-381..
28
Fortification of Milk and Milk Products for Value Addition
Fortification of Milk and Milk
Products for Value Addition
Sumit Arora
Dairy Chemistry Division, NDRI, Karnal
Introduction
Food fortification is thought to be a highly effective solution and among the most cost effective
public health interventions currently available. It may be defined as the addition of one or more
essential nutrients to food whether or not it is normally contained in the food, for the purpose of
preventing /correcting a demonstrated deficiency of one / more nutrients in the population or specific
population groups (Codex Alimentarius Commission, 1994). It is practiced in those areas where the
problems of malnutrition are prevalent.
According to FAO/WHO guidelines (1995) essential nutrients may be added (i) to replace losses
that occur during manufacture, storage and handling of food (restoration). For example the removal
of cream from milk takes almost all the natural vitamins A and D and therefore skimmed milk may be
fortified with the same vitamins at levels as fluid whole milk. (ii) To ensure nutritional equivalence in
imitation or substitute foods. (iii) To compensate for naturally occurring variations in nutrient levels.
For instance, milk and butter are subjected to seasonal variations in vitamins A & D contents. Some
dairy products are fortified with the vitamins A & D in order to maintain constant vitamin levels. (iv)
To provide levels higher that those normally found in a food. For example, margarine is fortified with
vitamins A & D (in western countries) to render it nutritionally equivalent to butter, and (v) to provide
a balanced intake of micronutrient in special case (dietetic foods) for example infant formulas, special
food for athletics, medical food etc.
General criteria for fortification
•
The intake of nutrients is below the desirable level in the diet of significant number of people.
•
The vehicle used for fortification should be consumed in significant quantities by target
population.
•
Addition of nutrient should not create an imbalance of essential nutrients.
•
The added nutrients should be stable under proper conditions of storage and use.
•
Biological availability of added nutrients should be high.
•
There should be reasonable insurance against excessive intake to a level of toxicity.(Food and
Nutrition Board, 1973)
Milk and milk products as a suitable vehicle for fortification
Milk in its natural form is almost unique as a balanced source of man’s dietary need (Table 1). The
various steps in processing and storage have a measurable impact on some specific nutrients. Milk
also provides a convenient and useful vehicle for addition of certain nutrients to man’s diet and has
following benefits:
-
Since milk is centrally processed so that the quality control can be effectively implemented.
-
Milk and milk products are widely consumed regularly in predictable amounts by people of all
age groups.
-
Cost is affordable by target population.
-
The stability and bioavailability of the added micronutrients to the milk remains high.
-
Since milk is nearly a complete food and all nutrients exist in almost fully available form, the
bioavailability of added nutrients remains high.
Addition of fortificants usually caused minimum change in colour, taste and appearance.
-
29
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Nutrients generally added to milk
Liquid milk fortification with vitamins A and/D is mandated in several countries. β-carotene is
added as a colour-enhancing agent to some milk products such as butter. Dried milk is often fortified
with vitamins A and D, calcium, and iron. Milk based infant formula and weaning foods are fortified
with a range of vitamins, minerals, and other nutrients such as polyunsaturated fatty acids. Powdered
milk used for complementary feeding in Chile is fortified with vitamin C, iron, copper and zinc.
Fortification of milk & milk products with vitamins
Under ambient conditions the water soluble vitamin C and vitamins of the B-complex group
such as thiamin, riboflavin, vitamin B6, niacin, pantothenic acid, folic acid, biotin and vitamin B12 are
powdered and thus relatively easy to work with when producing most dairy products. The fat soluble
vitamins which include vitamin A, D, E and K, however, exist either as an oil or as crystals, which may
cause processing difficulties during the production of certain types of dairy products (Mortensen and
Gotfredson, 1996).
One of the problem encountered with the vitamins, is their limited stability in presence of heat,
humidity and oxygen. Among the water soluble vitamins, vitamin C, folic acid, vitamin B6 and vitamin
B12 are the less stable. While in the case of fat soluble vitamins vitamin A, D and E are least stable.
In order to improve the stability of these vitamins, a number of different coating technologies
have been developed. One of the most important methods to protect the fat soluble vitamins is
microencapsulation, which results in a highly sophisticated powder, where the vitamin is kept protected
from degradation by the coating material used for the encapsulation. During microencapsulation, the
fat soluble vitamins are brought from the form of oil or a crystal – which in some processes would be
difficult to handle – to the form of a free flowing powder much easier to handle and mix with other
dry ingredients (Mortensen and Gotfredson, 1996).
When two or more vitamins are added to a food product at the same manufacturing stage, this
is commonly done in the form of premix or as blend. Premix is a homogenous mixture of desired
vitamins in a dry powder from, whereas a blend is the same for the fat soluble vitamins, but in an oily
form. A premix can consist of both water soluble and fat soluble vitamins and carotenoids, in which
case the fat soluble vitamins have to be microencapsulated.
Fortification of milk and milk products with iron, calcium and other minerals
Selection of an appropriate mineral fortificant (iron, calcium etc) is based on its organoleptic
considerations, bioavailability, cost and safety. The colour of iron compounds is often a critical factor
when fortifying milk and milk products. The use of more soluble iron compounds often leads to
the development of off-colours and off-flavours due to reactions with other components of the food
material. Infant cereals have been found to turn grey or green on addition of ferrous sulphate. Offflavours can be the result of lipid oxidation catalysed by iron. The iron compounds themselves may
contribute to a metallic flavour. Some of these undesirable interactions with the food matrix can be
avoided by coating the fortificant with hydrogenated oils or ethyl cellulose (Jackson and Lee, 1991).
Bioavailability of iron compounds is normally stated relative to a ferrous sulphate standard. The
highly water soluble iron compounds have superior bioavailability (Richardson, 1990). Bioavailability
of the insoluble or very poorly soluble iron compounds can be improved by reducing particle size.
Unfortunately this is accompanied by increased reactivity in deteriorative processes. The problem
of low bioavailability of some of the less reactive forms of iron is often circumvented by the use of
absorption enhancers like, ascorbic acid, sodium acid sulphate and orthophosphoric acid, added along
with the fortificant.
The other important mineral for the fortification of milk and milk products, which has been studied,
is calcium. Several commercial calcium salts are available for calcium fortification, which include
carbonate, phosphate, citrate, lactate and gluconate. In general, organic acid salts of calcium are more
bioavailable than inorganic salts (Labin-Godscher and Edelstein, 1996). The pH of the milk should be
30
Fortification of Milk and Milk Products for Value Addition
taken care of during Ca fortification. Manufacturers of calcium fortified milk products should consider
adding, magnesium, riboflavin and perhaps vitamin D as well, in amounts that would normally be
obtained in a serving of vitamin D fortified milk (Weaver, 1998).
Milk and milk products can also be fortified with a range of other mineral salts such as Mg, P,
Zn, Cu and Mn. Prudent selection of mineral compounds is based largely on consideration of mineral
reactivity and solubility of the salt. To overcome problems of flavour, texture and colour deterioration
due to addition of minerals, some companies have engineered new fortificant preparations, which
generally involve the use of stabilisers and emulsifiers to maintain the mineral in solution (FAO,
1995).
Technology for fortification:
1. Liquid milk
The technology of milk fortification is relatively simple and no additional equipments are needed
or can be practiced with minor modifications in the existing plant. Mineral/vitamin fortification can be
practiced at several stages in the production. But liquid milk is usually fortified prior to pasteurization
or ultra-heat treatment. Homogenization is essential for oily preparations of vitamins. Usually two
methods of additions are practiced i.e. batch process for small operations and metered additions for
continuous process. A metered injection of the vitamin preparation upstream to the homogenizer has
been the standard set up in continuous operation plants (Cornell University, 1994).
Oily preparations are diluted with 10 parts of warm oil (45 – 50°C), usually butter oil and
homogenized with a suitable quantity of skim milk or it can be mixed with appropriate quantity of milk
and cream and finally homogenized. In the case of water soluble or water dispersible micronutrients,
a premix can be made by diluting the nutrients to 20 times their weight with milk at 45°C, followed by
stirring and thorough mixing (USAID, 2001).
A simple procedure for fortification of skim milk with vitamins A without using homogenizer was
developed by Bector and Rani (1998). This process is basically a batch process and is suitable for small
plants of low capital cost.
Many iron compounds have been assessed in the fortification of pasteurised whole milk. The
best fortification procedure was judged to be the addition of ferric ammonium citrate followed by
pasteurisation at 81 °C. In this way fortified milk containing 30 ppm iron was found to be acceptable
after 7 days storage. Levels of vitamin E, vitamin A and carotene were not affected by the presence
of iron. At pasteurisation temperatures below 79 °C off-flavours developed due to lipolytic rancidity
(Edmondson et al, 1971). De-aeration of the milk prior to the addition of iron compounds was also found
to reduce flavour problems. In the production of iron fortified evaporated milk, ferric orthophosphate
was shown to be useful (FAO, 1995).
Calcium fortificant preparations including stabilizers and emulsifiers have been used for
fortification of milk and milk-based beverages. It maintains calcium in suspension so as to improve
mouth feel and appearance of products (FAO, 1995). In Germany a milk-based fruit beverage has been
marketed which is fortified with calcium, phosphorous as well as vitamins A, E, B and C.
Dried milk
Here particle size of the fortificant as well as density of the fortificant has to be taken care as large
and heavier size particles will lead to separation. In order to achieve stability of vitamins, the safest way
to fortify dried milk is to blend dry forms of premix with the dried milk powder, thereby protecting
the effect of microencapsulation. However, this requires an effective mixing system. If blends are used,
they are added directly to milk, provided homogenization is done before spray drying. If vitamins are
added before spray drying, overage addition (Table 2) will be necessary in order to compensate the
losses (Mortensen and Gotfredsen, 1996).
31
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Iron fortification of powdered non-fat dry milk, ferrous sulphate at a level of 10 ppm was found to
be stable for a period of 12 months. Ferric ammonium citrate and ferric chloride at a level of 20 ppm
iron in the reconstituted product gave acceptable results (FAO, 1995).
Infant formulas
The mineral content of cow milk, from which many formulas are produced, is highly variable.
Production methods have been adapted to control this source of variability. Operations have been
included which remove most of the minerals, but at the same time some vitamins and other components
of the milk are lost: technologies used include ion exchange, ultra filtration, electrodialysis, reverse
osmosis and gel filtration. Mineral compounds are then added at the required levels. There must be
careful selection of mineral compounds added to the formulas, as cereal products are highly susceptible
to lipid oxidation during storage. The use of ferrous fumarate and ferrous succinate is recommended
for fortification of infant cereals as they gave rise to no objectionable flavours/odours or colours on
storage. Ferrous sulphate coated with hydrogenated fats, mono- or di-glycerides and ethyl cellulose
caused discolouration on reconstitution with hot milk and hot water.
Although some allowance is made for the natural vitamin content of the ingredients used, most of
the vitamins are added to the formula. The Codex Alimentarius Commission (FAO/WHO, 1994) has
published an advisory list of mineral salts and vitamin compounds which can be added to formulas.
Predetermined excesses of vitamins have to be added to allow for processing and storage losses. UHT
processing followed by aseptic packaging has been preferred to in-can sterilisation since less nutrient
losses occur in the former case. Losses have been noted particularly for vitamin C, thiamin, folic acid
and vitamin B6.
Iron absorption from formulas has been reported to be 5-10% compared to 50% for human milk.
It has been suggested that bovine milk proteins or elevated calcium and phosphorus levels account
for this difference. Zinc levels in formulas are also higher than in human milk to make up for reduced
bioavailability.
Ice-cream:
The unit operations used in the manufacture of ice-cream is not highly destructive to vitamins.
Vitamins are added in the dry form to the mix. Since whipping and consequent operation of the mix
is carried out around freezing temperature, oxidative losses of vitamins are minimized. The greatest
processing losses, which occur during manufacture of fortified ice-cream, are during pasteurization of
ice cream mix. Calcium enriched ice-cream is also available in USA and is marketed under the name
of TruCal.
Fermented milk products:
In the production of yoghurt, the low pH renders it unsuitable as a carrier of vitamins such
as vitamin A. Water soluble vitamins are best used in a encapsulated form, protected for odour
and flavour considerations. Some vitamin losses can occur through metabolism by microorganisms
during fermentation (O'Brien and Roberton, 1993). The sensory quality of iron fortified yoghurt was
acceptable to when tested by a consumer panel. No significant difference in the appearance, mouthfeel,
flavour, or overall quality was observed between iron fortified and unfortified yoghurts (Hekman and
McMahon, 1997). In Germany, enrichment of cheese with iodine through the use of iodised salt has
been approved.
Considerations while fortification of milk & milk products
1. Bioavailability of commercial preparations: Bioavailability of different compounds facilitates
the selection of the optimal compound. Bioavailability refers to the rate of absorption and
utilization of a nutrient from a given matrix.
2. Nutrient–nutrient reaction: Interaction among the nutrients and other food components is
a key factor in nutrient addition. For example, Vitamin C will improve the absorption of iron
32
Fortification of Milk and Milk Products for Value Addition
(Kiran et al, 1977). On the other hand, the iron will accelerate vitamin degradation. Fortification of
calcium in milk may interfere with absorption of iron or zinc (Weaver, 1998).
3. Nutrient-matrix reaction: The added nutrient must not react with any component of the milk.
For example, iron is a pro-oxidant and can accelerate the development of fat rancidity, destroy
some of the vitamins and form coloured products.
4. Shelf-life & packaging: Many of the fortified milk and milk products may have limited shelf
life and thus may need different types of packaging which can be either oxygen impermeable
or opaque to light. This is particularly true for the fortification of liquid milk with vitamin A as
vitamin A fortified milk develops off flavour within 6 h when exposed to light, compared to 12 h
for control (Fellman et al, 1991). All the fortified products require proper labelling on the pack.
5. Process considerations: The stability of all the vitamins is well known during various processing
conditions and the same knowledge can be applied while processing the vitamin fortified milk.
6. Cost factor: Cost may not be a crucial factor in the manufacture and marketing of fortified milk
and milk products.
7. Safety factor: There should be sufficient insurance against excessive intake of the fortificant.
Unlike water soluble vitamins, fat soluble vitamins exhibited toxicity at higher concentrations.
Conclusion
Fortification should not alter the organoleptic properties (taste, smell, colour, consistency) and shelf
life (conditions related to storage, transport) of the product. Often there is a delicate balance between
bioavailability and other properties of fortified food. Milk and milk products provide a convenient and
useful vehicle for fortification with micronutrients. The risks associated with fortification are minimal
except if good manufacturing practices are not followed and only isolated incidents of this type have
ever been reported. Improved understanding of interactions between food ingredients and health and
ingenuity of food technologists in food formulation and fabrication will contribute to the advances in
food fortification.
Table 1: Micronutrient content of cow milk vs recommended dietary allowances (RDA)
Micronutrient
Quantity /Litre
RDA
Men
Women
Vitamin A (IU)
1300
1000
800
Vitamin D (IU)
42
5-10
5-10
Vitamin E (IU)
1.5
10
8
Vitamin K (μg)
41
45-80
45-65
Vitamin B1(mg)
0.4
1.5
1.1
Vitamin B2 (mg)
1.7
1.7
1.3
Vitamin B6 (mg)
0.4
2
1.6
Folic acid (μg)
62
200
180
Niacin (mg)
1
19
15
Vitamin B12 (mg)
3
2
2
Vitamin C (mg)
15
60
60
Iron (mg)
0.52
28
30
Calcium (mg)
1300
400
400
Copper (mg)
0.1
2.2
2.2
4
15.5
15.5
Zinc (mg)
(OMNI, 2001)
33
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Table 2: Micronutrient content of cow milk vs recommended overages
Micronutrient
Quantity /Litre
Recommended Overages (%)
Pasteurised
UHT
Dry Milk
Milk Desserts
Vitamin A (IU)
1300
20
30
40
20
Vitamin D (IU)
42
20
30
40
20
Vitamin E (IU)
1.5
10
30
20
10
Vitamin K (μg)
41
-
-
-
-
Vitamin B1(mg)
0.4
25
50
20
25
Vitamin B2 (mg)
1.7
15
40
20
15
Vitamin B6 (mg)
0.4
30
30
20-30
30
Folic acid (μg)
62
20
40
40
20
Niacin (mg)
1
15
20
20
15
Vitamin B12 (mg)
3
15
30
40
20
Vitamin C (mg)
15
30
100
50
30
Iron (mg)
0.52
5
5
5
5
Calcium (mg)
1300
5
5
5
5
Copper (mg)
0.1
-
-
-
-
4
-
-
-
-
Phosphorus (mg)
960
-
-
-
-
Iodine (ųg)
237
-
-
-
-
Zinc (mg)
(OMNI, 2001)
References
Anderson, H. C. and Thomas, E. L. (1974) Fortification of fluid milk products with ascorbic acid. J Dairy Sci. 57:5.
Bector, B.S. and Rani, S. (1998) A methodology to fortify low fat milk with vitamin A without using a homogenizer.
Indian J. Dairy Sci. 51: 69-72.
Bender, A. E. (1993) Food fortification. In. Encyclopedia on Food Science, Food Technology and Nutrition. Vol 3. p.19901993.
Bullock, D. H., Singh, S. and Pearson, A. M. (1968) Stability of vitamin C in enriched commercial evaporated milk. J.
Dairy Sci. 51: 921-923.
Conochie, J. (1958) The stability of sodium ascorbate in evaporated milk. Australian J. Dairy Technol. 80-82.
Cornell University: Dairy Science Facts (1999) Vitamin fortification of fluid milk. Department of Food Science, Stocking
Hall, New York.
Edmondson, L. F., Douglas, F. W. and Avants, J. K. (1971) Enrichment of pasteurized whole milk with iron. J. Dairy Sci.
54: 1422-1426.
Elliott, J. G. (1999) Application of antioxidant vitamins in foods and beverages. Food Technol. 53: 46-48.
El-Sayed, M. M., Abd-Rabou, N. S., Sayed, A. F. and El-Samragy, Y. A. (1995) Iron fortification of processed Ras cheese.
Egyptian J. Dairy Sci. 35: 289-298.
FAO (1995) Micronutrient fortification of food: Technology and quality control. FAO Technical Consultation of Food
Fortification: Technology and Quality Control. Rome, Italy, 20-23 November.
Fellman, R.L., Dimick, P,S and Hollender, R. (1991) Photooxidative stability of vitamin A fortified 2% low fat milk and
skim milk. J. Food Protection 54:113-116.
Food and Nutrition Board (1973) General policies in regard to improvement of nutritive quality of foods: policy
statement. National academy of Sciences Washington DC. p. 6.
Food Fortification Roundtable. (1996) Food Technol. 86-94.
Ford, J. E., Porter, J. W. G. and Thompson, S. Y. (1974) Influence of added ascorbic acid on the stability of folic acid and
vitamin B12 in milk during UHT processing and subsequent storage. Proceedings 19 th Intr. Dairy Congress, 567569.
Goldscher, R. L. and Edelstein, S. (1996) Calcium citrate: A revised look at calcium fortification. Food Technol. 96-98.
Graham, D. M. (1974) Alteration of nutritive value resulting from processing and fortification of milk and milk products.
J. Dairy Sci. 57: 738-745.
Head, M. K. and Hansen, A. P. (1979) Stability of L- ascorbic acid added to whole, chocolate, and low fat milks. J. dairy
Sci. 62: 352-354.
Hekmat, S and McMahon, D.J. (1997) Manufacture and quality of iron fortified yoghurt. J. Dairy Sci. 80: 3114-3122.
34
Fortification of Milk and Milk Products for Value Addition
Hekmat, S. and McMahon, D. J. (1997) Manufacture and quality of iron fortified yoghurt. J. Dairy Sci. 80: 3114-3122.
Hicks, T. Hansen, A. P. and Rushing, J. E. (1996) Procedures used by north Carolina dairies for Vitamins A and D
fortification of milk. J. Dairy Sci. 79: 329-333.
Ilic, D. B. and Ashoor, S. H. (1988) Stability of vitamins A and C in fortified yoghurt. J. Dairy Sci. 71:1492-1498.
Indyk, H., Littlejohn, V. and Woodland, D. C. (1996) Stability of Vit D3 during spray drying of milk. Food Chem. 57:
283-286.
Jackson, L. and Lee, K. (1991) Microencapsulated iron for food fortification. J. Food Sci. 56: 1047-1050.
Johnson, L. E. (1994) Vitamin and mineral fortification in foods. Food Technol. 124.
Kiran, R., Amma. M. K. P. and Sareen, K. N. (1977) Milk fortification with a system containing both iron and ascorbic
acid. The Indian J. Nutr. Dietet. 14:260-266.
Kiran, R., Kaur, I. P. and Vaneja, K. (19860 Studies on the acceptability and availability of fortificants present in milk
and whey. J. Food Sci. 23: 110-111.
Labin-Goldscher, R and Edelstein, S (1996) Calcium citrate: a-revised look at calcium fortification. Food Technol. 96-97.
Lopez, G. B., Terre, J. M. Q., Lopez, B. G. and Terre, J. M. Q. (1996) Calcium enrichment of skim milk subjected to UHT
treatment. Almentaria 34: 79-82.
McNamara, S. H. (1995) Food fortification in United States: a legal and regulatory perspective. Nutr. Reviews 53: 140144.
Mertz, W. (1997) Food fortification in United States. Nutr. Reviews 55: 44-49.
Mortensen, B. L. and Gotfredsen, P. (1996) Fortification- nutritional improvement of dairy products. Danish Dairy Food
Industry..worldwide 64-65.
Newsome, R.L. (1997) Use of vitamins as additives in processed food. Food Technol. 163-168.
Nordmark, B. (1999) A brief history of fortification. Food Technol.15-16.
O’Brien, A. and Robertson. D. (1993) In. The technology of vitamins in Food. P. Berry Ottaway (Ed.) p-30.
Olivares, m., Pizarro, F., Pineda, O., Name, J. J. Hertrampf, E. and Walter, T. (1997) Milk inhibits and ascorbic acid
favors ferrous bis-glycine chelate bioavilability in humans. American Society Nutr. Sci. 1407-1411.
OMNI/Roche/USAID (2001) Fortification basics: Milk. http://www. roche.com
Rao, B. V. R. and Mathur, B. N. (1988) Stability of vitamins A, D and E in spray dried infant formula during storage.
Indian J. Dairy Sci. 41:86-88.
Richardson, D. P (1990) Food fortification. Proceedings of the Nutrition Society. 49: 39-50.
Rosenthal, I., Rosen, B. and Bernstein, S. (1993) effects of milk fortification with ascorbic acid and iron. Milchwissenschaft
48: 676-679.
Saini, S. P. S., Jain, S. C. and Bains, G. S. (1987) Effect on iron fortification on flavour of buffalo milk. Indian J. Dairy Sci.
40: 88-93.
Sloan, A. E. and Stiedemann, M. K. Food Technol. 100-108.
Subbulakshmi, G. and Nai(1996) Food fortification: from public health solution to contemporary demand. l, M. (1999)
Food fortification in developing countries- current status and strategies. J. Food Sci. Technol. 36: 371-395.
Sweeney, M. A. and Ashoor, S. H. (1989) Fortification of cottage cheese with vitamins A and C. J. Dairy Sci. 72: 587590.
Tateo, F., Bononi, M., Testolin, G., Ybarra, L. and Fumagalli, M. (1997) Experiments in the use of calcium and magnesium
lactates in milk enrichment. Industrie-Almentari 36: 614-617.
Vahcic, N., Palic, A. and Ritz, M. (1992) Mathematical evaluation of relationship between copper, iron, ascorbic acid
and redox potential of milk. Milchwissenschaft 47:228-230.
Weaver, C.M. (1998) Calcium in food fortification strategies. Int. Dairy J. 8: 443-449.
Ziegler, E. E. and Fomon, S. J. (1996) Strategies for the prevention of iron deficiency: iron in infant formulas and baby
foods. Nutr. Reviews. 54: 348-354.
35
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Packaging of Value Added Foods
and Their Storage Stability
P. P. Gothwal
Central Food Technological Research Institute, Reseach Centre, Lucknow
Introduction
India produces nearly 300 million MT of food products which comprise cereals, pulses, fruits,
vegetables, mushrooms, algae, spices and plantation, meat, fish, poultry, milk and dairy based
products. It is estimated that nearly 30% of produce is lost due to poor handling, processing and
packaging. In the present scenario packaging has been identified as an integral part of processing in
the food industry. Packaging sector is an important global industry representing about 2% of GNP of
developed countries. The value of packaging industry is expected about 345 Million Euros world wide
with 50% for packaging of food materials. Scientific method of packaging and safe transportation of
food materials plays a significant role in reducing the post harvest processing losses. Food, both in its
fresh and processed form needs appropriate packaging to facilitate storage, preservation, transportation
and distribution. Packaged foods offer enormous export opportunities and foreign exchange earnings
to the country. Research and development in the area of food packaging has resulted in building up a
database on deteriorative characteristics and packaging needs of a large number and varieties of food
products stored under different environmental conditions. Accelerated testing conditions drastically
cut down the time required to identify suitable packaging materials to increase shelf life of processed
food products under various controlled conditions.
Packaging is a coordinated system of preparing food for transportation, distribution, storage,
retailing and end use. It is mean to ensure safe delivery of product to the ultimate consumer in sound
condition at minimum cost. Projected growth rate of demand and consumption for packaging in India
is 10%. Value added processed food products comprising of fruits & vegetables based, cereal and
pulses based, meat/fish/poultry based products, milk and dairy based products need specialized
types of packages depending upon the type of preservation method used and extent of storage desired.
All successful food processing industries continually develop and launch new value added products
with new attracting packaging. To ensure that these new value added products perform well in the
market, the food industries have to follow product development with food packaging procedures,
which maximize their chances of success and reduce their risk of failure. Some food is made possible
by the introduction of new technology and new packaging technologies. New or latest technologies
under active development or in the early stages of adoption as such can be expected to impact on the
type of value added products developed in the future.
Different packaging materials:
Packaging of fresh produce in consumer unit packs at the producing centers or terminal markets
protects the produce against damage and excess moisture loss. The packaging materials used i) should
have sufficient permeability to oxygen, carbon dioxide and water vapor ii) should have desired
protective physical properties, iii) should be transparent. The permeability requirement depends
upon rate of respiration of the produce, the package bulk density and temperature of storage. Food
packaging can be categorized in to primary, secondary and tertiary types.
•
Primary packaging is the material that first envelopes the product and holds it. This usually
is the smallest unit of distribution or use and is the package which is in direct contact with the
contents. Primary packaging is the main package that holds the food that is being processed.
•
The secondary packaging is outside the primary packaging, perhaps used to group primary
packages together. Secondary packaging combines the primary packages into one box being made.
Corrugated fiber board is most commonly used to make secondary shipping cartons.
36
Packaging of Value Added Foods and Their Storage Stability
•
Tertiary packaging is used for bulk handling, warehouse storage and transport shipping. The
most common form is a palletized unit load that packs tightly into containers. Tertiary packaging
combines all of the secondary packages into one pallet. Examples•
Form-Fill-Seal packaging
•
Bag-in-box
•
Dip-a- sauca packaging
•
Combi system of packaging
•
Boil in bag packs
•
Metals cans
•
Retort able pouches and trays
Packaging material consumption pattern:
With changing consumer preferences, the composition of substrate used in packaging industry
has also changed. The consumption pattern of various packaging material is shown below:
Selection of packaging system for processed foods:
•
•
Drastic changes are seen in the system of food
supply. The conventional means of harvesting
processing and handling are slowly replaced by
improved systems, bringing in modernization. It
is obvious that the produce in its natural or value
added processed form should also be properly
stored, so that it is available during non-seasonal
period and in emergency.
Material
India%
Global%
Paper and paperboard
40
29
Glass
16
8
Metal
5
19
Plastic
15
39
Others
24
05
The selection of package for a food product is to Source: Indian Institute of Packaging, New Delhi
identify the properties of the food, its sensitivity (2010 report)
to environment, the length of life desired, the
market condition, consumer needs and existing regulation. The technological need is to evaluate
the ‘product-package compatibility’. A product can be sensitive and susceptible to different
factors like bio-chemical changes and microbial changes; physical and chemical (including toxic
and traces elements), flavor loss, odor pick up, texture, moisture and gases.
Packaging used for fresh produce:
Corrugated Fiber Board boxes (CFB):
These are most commonly used shipping container. Their major attributes are;
•
Low cost to strength and weight ratio
•
Smooth non abrasive surface that is minimum bruising damage
•
Good cushioning characteristics
•
Excellent printability
•
Easy to set up and collapsible for storage
•
Reusability and recycle
•
Easy handling and stack ability
•
Can be turned out quickly in to highly precise and accurate sizes; can be appropriately punched
for ventilation and the most acceptable form in international markets.
•
Most of the perishables exported from India are packed in CFB cartons.
•
Plastic corrugated boxes: In recent years plastic corrugated boxes made of polypropylene (PP)
and high density polyethylene (HDPE) are partly replacing CFB. Its advantage over CFB is low
weight to strength ration high degree of wet resistance and its reusability.
37
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Modified atmosphere packaging:
MAP is the method for extending the shelf life of perishables by altering the relative proportion of
atmospheric gases that surround the food. This is becoming increasingly popular technique to meet
both distribution and retailing needs of fruits and vegetables. Modified atmospheric conditions are
created inside the packages by the commodities themselves by controlling respiration and selecting
suitable permeable films or by using carbon dioxide and ethylene absorbers (scavengers) within
the package to prevent the build up of a particular gas. Compounds like hydrated lime, activated
charcoal, and magnesium oxide are used to absorb carbon dioxide, iron powder for absorbing oxygen
and potassium permanganate, squalene and phenyl methyl silicone for absorbing ethylene within the
package. The modified atmosphere desirable for vegetables comprises reduced oxygen (2-3%) and
increased carbon dioxide levels (10%).
Future scope of tinplate container:
•
As long as the tin metal exists in the earth’s crust, tinplate continues to be an ideal packaging
material for processed foods and beverages. There is a good scope in reducing the thickness
of tin coating if suitable lacquer is coated, which may help in reducing the cost of container.
Recycling of tinplate container is another important aspect to be considered.
Packages for specific processed food products:
Bakery based processed food such as breads, buns, cakes, biscuits, pastries, etc. ensured by very
short life. The goal of these products packaging is partial moisture control especially in breads, but the
main purpose is to allow the product to be distributed safely and hygienically. Still cakes and pastries
are often packs in cardboard boxes. The packaging material recommended for bakery industry is
of good quality waked papers, cellophane etc. There is a good scope in the development of good
packaging material for bakery and confectionary products.
Statutory marking, international packaging regulations:
Standards and Regulatory issues are dealt with by multiple agencies: Most of the packaging related
regulatory initiatives are concerned with the product quality, public health and hygiene, safety, Export
promotion, transportation and consumer protection.
BIS
: All Agricultural products
DMI
: Spices, walnuts, casings, fruits and vegetables
PFA
: Pesticides residues, contaminants
EIC
: Only export inspection
APEDA: Only export standards
PPA
: Issue of phytosanitary certificates
Codex : International standards on processed foods
Areas of research and development in food packaging:
•
Design and development of suitable packages based on processed food products characteristics
and performance properties of packaging materials, and finished package forms
•
Development of economical, flexible packages for processed food/agro based products
•
Development of indigenous aluminum containers for processed food/agro based products
foods and beverages
•
Pre-packaging and bulk-packaging of fresh as well as processed produce
•
Safety evaluation of packaging components and plastic packaging materials for food contact
applications
Development of environment-friendly packaging materials today need
•
•
38
Utilization of agricultural waste materials and eco-friendly natural and synthetic materials
Development of bio-film from secretion of insects
Packaging of Value Added Foods and Their Storage Stability
•
Design and fabrication of packaging machinery such as bio-plate making machine
•
Development of vacuum packaging equipment; volumetric machine for filling free-flowing
solid materials; continuous heat sealers for flexible films; and vibration testers with variable
amplitude
•
Functional and economical (and suitable for marketing and distribution) package design for
a variety of processed food products including traditional foods, infant foods, bakery and
confectionery products
•
Design of transport packages for fresh produce and processed foods, and development of costreduction techniques in transport package design
•
Development of computer-aided package design techniques
•
Modeling and computer simulation of package performance
•
Standardization of process schedules for thermal processing of foods in cans, glass, tin-free
steel and aluminum containers, and retort able pouches based on heat penetration studies and
sterilization value
•
Development of quality standards and government regulation
Work carried out at CFTRI:
•
Technology packages for convenient and ready-to-eat foods
•
Economical and functional packages to contain edible oils and fats
•
Shelf-life prediction methods and generation of data on flexible packaging
•
Materials
•
Process modification and in-retort exhaust-cum-sterilization system for
•
Heat processing of food products in plastic containers
•
Transport package from traditional indigenous materials
•
Improvements in metal containers for processed food products and beverages
•
Migration aspects of plastic constituents into food simulants under use conditions
•
Development of rigid aluminum containers for packaging of processed foods and beverages
•
Development of bulk packages for storage and transportation of commercially important fruit
and vegetables
•
Studies on the suitability of alternatives to tin plate containers for packing processed food
products
Technical services offered for:
•
Unit packages, transport packages, fresh-produce packages; packaging materials; computeraided and graphic package designs; fabrication of canning and other packaging machinery
•
Sorption isotherm studies and shelf-life studies in controlled environments for inland and export
markets
•
Thermal processing of foods: Establishment of processes and evaluation of containers
•
Testing of packaging materials for their physico-chemical properties, safety and transport
worthiness
•
Migration testing for food-grade quality of plastic packaging materials
•
Evaluation of finished packages for performance under simulated storage and distribution
conditions
•
Routine quality control services
39
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Novel Technologies for Processing and
Packaging of Health Foods and Beverages
H. N. Mishra
Indian Institute of Technology, Kharagpur
Introduction
Food is basically energy – a form of solar energy - stored in plant and animal foods in chemical
forms. On consumption, this stored form gets converted to physiological energy. Processing and storage
of food become imperative because availability of food is mostly seasonal, whereas its consumption
goes on throughout the year unfettered by any types of seasonal bounds. Over the last five decades,
India has made great strides in the production of food grains, milk, fruits and vegetables etc., and
there is semblance of self-sufficiency, albeit fragile in view of the burgeoning population. Even so,
the net amount of the produce for consumption is further reduced due to insufficient storage and
processing.
Food processing industry is one of the largest manufacturing industries worldwide and possesses
global strategic importance. With the advancement of science and technology, new food processing
technologies are capturing the attention of many scientists in academia and industry. Consumers
prefer high-quality foods with longer shelf life and, clearly, some of the new technologies can meet
these demands. Newer strategies have been devised to modify the existing food processing techniques
and the adoption of novel processing technologies.
In industrialized countries the market for processed foods is changing. Consumers no longer
require a shelf life of several months at ambient temperature for the majority of their foods. Changes in
family lifestyle, and increased ownership of freezers and microwave ovens, are reflected in demands
for foods that are convenient to prepare, are suitable for frozen storage or have a moderate shelf life at
ambient temperature. There is also an increased demand by some consumers for foods that have fewer
changes during processing and thus either closely resemble the original material or have a healthy
image. New preservation technologies, such as high pressure processing and pulsed electric fields
offer advantages in meeting consumer demands of freshness, convenience and safety.
Minimally processed foods
In recent years the consumers have become more health conscious in their food choices but have
less time to prepare healthful meals. As a result the market demand for “minimally processed” or
“lightly processed” foods has rapidly increased. Consumers increasingly demand foods which retain
their natural flavor, colour and texture and contain fewer additives such as preservatives. In response
to these needs, one of the most important recent developments in the food industry has been the
development of minimal processing technologies designed to limit the impact of processing on
nutritional and sensory quality and to preserve food without the use of synthetic additives.
Minimal processed foods have been defined as products that include all the operations which
add some value to conventional food preservation processes like washing, selecting, peeling, slicing,
chopping, coring and packaging that cause fewer possible changes in food quality and maintain their
quality attributes similar to those of fresh produce, but at the same time provide the food enough
useful life to transport it from production site to the consumer. Minimal processed foods may be
meant for direct consumption or can be later transformed in to the final products by any conventional
techniques.
The demand for minimally processed, easily prepared and ready-to-eat ‘fresh’ food products,
globalization of food trade, and distribution from centralized processing pose major challenges for
food safety and quality. Recent food-borne microbial outbreaks are driving a search for innovative
40
Novel Technologies for Processing and Packaging of Health Foods and Beverages
ways to inhibit microbial growth in the foods while maintaining quality, freshness, and safety. One
option is to use packaging to provide an increased margin of safety and quality. The next generation
of food packaging may include materials with antimicrobial properties. These packaging technologies
could play a role in extending shelf-life of foods and reduce the risk from pathogens. Antimicrobial
polymers may find use in other food contact applications as well
Traditional thermal processing techniques can be both beneficial to foods in such areas as
preservation and flavor formation but detrimental in damaging other sensory and nutritional
properties. Minimizing undesirable changes can be achieved in a number of ways, whether through
more effective process control, the use of High Temperature Short Time (HTST) techniques such as
aseptic processing, or newer technologies such as volume heating methods. The various approaches and
the range of technologies such as infrared heating, dielectric methods such as the use of microwaves,
and ohmic heating is complemented by the following alternatives to thermal processing, ranging from
irradiation to high pressure processing and the use of pulsed electric fields.
Ultrasound method (USM)
Ultrasound is probably the most simple and most versatile method for the disruption of cells
and for the production of extracts. It is efficient safe and reliable. Ultrasound techniques have the
relatively low cost and robust process. Ultrasound cavitation creates shear forces that break cell walls
mechanically and improves material transfer. This effect is being used in the extraction of liquid
compounds from solid cells. The compound to be dissolved into a solvent is enclosed in an insoluble
structure. In order to extract it, the cell membrane must be destructed. For the purpose, ultrasound is
faster and more completed than maceration or stirring. The particle size reduction by the ultrasonic
cavitation increases the surface area in contact between the
solid and liquid phase, significantly. The mechanical activity
of this technique enhances the diffusion of the solvent into
the tissue; Ultrasound breaks the cell wall mechanically by
the cavitation shear forces at it facilitate the transfer from the
cell into the solvent. This technique has potential advantages
over other techniques including freedom from radiation
hazards, which may appear in some of the existing nondestructive methods. The presence of the small gas bubbles
in a sample can greatly attenuate ultrasound making signal
detection impossible. This can be solved by using reflection
Figure 1 Ultrasound set up for crystallization
measurements rather than transmission measurement.
Figure 1 shows an ultrasound set up for crystallization.
In United States, ultrasound techniques are being used for processing of fresh juices like oranges,
mango, grape fruit, plum, purees, sauces and dairy products. Oil extraction from oil seed, cell membrane
permeabilization of fruits like grapes, plums and mango, extraction of lipids and proteins from plant seeds
such as soybean, extraction of phenolic compounds from vascular structures by disrupting plant tissues
etc are also achieved by this method. The most effective use is for microbial and enzyme inactivation. This
technique is used even in the emulsification, dispersing and homogenizing as well as to improve chemical
reactions and surface chemistry or to influence crystallization process.
Oscillating Magnetic Fields (OMF)
Inactivating microbes has the potential to pasteurize food with an improvement in the quality and
shelf-life compared to conventional pasteurization processes. Strong static or oscillating magnetic fields
(5-50 TesLa) have the potential to inactivate the vegetative microorganisms. The impulse duration is in
between 10us and several milliseconds and the frequencies are maximally 500 MHz because above this
items begin to warm up noticeably. The preservation of foods with oscillating magnetic field involves
sealing of foods in a plastic bags and subjecting it into 1-100 pulses in can OMF at temperature of 0°C to
50°C for a total exposure time ranging from 25 to 100 minutes and for this no special preparation of food
41
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
is required. Magnetic field treatments are carried out at atmospheric pressure and at a temperature
that stabilizes the food material. It does not have any influence on the organoleptic properties as the
temperature raises only 2-5°C.
High pressure processing (HPP)
In this food processing method the food is subjected to very high pressure (up to 8.4 kg/cm2) to
kill bacteria present in the raw food. This technique can improve food safety by destroying the bacteria
that can cause food borne illness and spoilage and parasites
that causes diseases. High pressure works like heat to kill
bacteria, but the food remains fresh and rich. In a typical
process, pre-packaged raw product is placed in a pressure
chamber and subjected to very high pressures for specific
time (< 10 minutes). This process causes high changes in the
characteristics of food. The foods can be kept for a longer
period under better condition. Small molecules which are
the characteristics of flavouring and nutritional components
typically remain unchanged by pressure. These pressure
processed foods have better texture, nutrient retention
and colour compared to heat processed foods. Any food
with sufficient moisture can be subjected to high pressure
processing. This technique can be used to process both the Figure 2 : A typical high-pressure processing
liquid and solid foods expect for food materials containing system for treating pre-packaged foods
large quality of air pockets.
This technique was first time used by Royer (1895) to kill bacteria and subsequently in 1899 by
Hite to see its effect on milk, meat, fruits and vegetables. In Japan, in 1990 first commercial products
like fruit juices, jams, fruit topping and tenderized meats were introduced. HPP treatment consumes
less energy e.g. energy required pressurization at 400 MPa is equivalent to heating the same material
at 300 °C. The main benefits of HPP in food processing include inactivation of microorganisms,
structural modification of biopolymers and depression of freezing point of water. These could be used
advantageously in several segments of food industry including sea food meat and meat industry.
Since 1990 onwards, in Japan, HPP treated
jam prepared from strawberries, kiwi
fruit and apples are available without
any application of heat treatment. HPP
treated orange juices, pickles, soybean
paste, rice, seaweeds are available in
Japanese markets. The key components of
a high-pressure system are the pressure
vessel, pressurizing system, and ancillary
components (Figure 2).
The processing by HPP is carried out
usually in a low compressibility liquid
such as water. The second principle
is that of Lechatelier which states that
phenomenon of phase transition chemical
changes etc are accompanied by decrease Figure 3: Schematic representation of microwave drying process
in volume are favoured by pressure and
vice versa. Pressure influences most biochemical reactions occurring in foods since they often involve
a change in volume. Pressure may also inhabit the availability of energy by affecting energy producing
enzymatic reactions.
42
Novel Technologies for Processing and Packaging of Health Foods and Beverages
Microwave processing
The increasing consumer demands for foods which offer more convenience in usage and time
savings in preparation made microwave over as an alternative for conventional thermal ovens. The
microwave processing has been made use of for drying of fruit juices, pulps, apple segments and
finished drying of potato chips. Microwaves are endowed with some special characteristics such as, high
penetrating quality which results in the uniform heating of materials, selective absorption of radiation
by liquid water and capacity for easy control. These impart some unique effects to the dehydrated
material such as improved quality and good texture. In the wider field of preservation, microwaves
have been used in drying, blanching and vacuum drying. Typical product areas where microwaves
have been used commercially include blanching of vegetables, where it is claimed that there is less
need for mechanical handling with consequent better product. Also, microwaves in combination with
hot air have been shown to be a positive route to drying of food stuffs, in selective product areas,
where, other methods cannot be employed. Finally, microwave vacuum drying has found some outlets
in producing fruit juices and meat extracts. By aim of using microwave processing in preservation
in general and pasteurization or sterilization in particular is to deliver a more homogeneous heat
treatment at a faster rate than conventional method of heating. Figure 3 is a schematic representation
of a microwave drying system.
Ohmic heating
This technology has been around since early 1900s. The
food processing researcher, however, began investigating the
potential of ohmic heating on food quality and cost and energy
savings in 1980s. In this method an AC current is pass through
a food sample which leads to generate internal energy in foods.
As a result an inside out heating patterns is generated. Ohmic
heating is some what similar to microwave heating but with
Figure 4: Ohmic heating equipment
very different frequencies. The advantage of this technique is
that it uniformly heats food with different densities such as chicken soup. The quality product with
minimal structural nutritional and organoleptic changes can be produced. Potential application of
this technique includes blanching, evaporation, dehydration, fermentation and extraction. It saves
significant time energy in hot air and freeze drying of foods and enhances extraction yields some
processing operations. The parameters used during ohmic heating such as frequency of alternating
current applied voltage and the temperature to which the sample was heated have a significant effect
on it’s success. The electrical conductivity is also a significant factor. The ohmic heating is useful for
value added processing, and it has great potential for use in wide variety of food processing operations
involving a heat and mass transfer. Ohmic heating equipment is shown in figure 4.
Ohmic heating is currently used in Europe, Asia and North America to produce a variety of high
quality low and high acid products containing particulates. Electrical resistance heating allows particles
and liquids to heat at the same rate and permits the rapid heating of mixtures of high solid fractions.
The technique has been applied to a number of food processes, and has recently been developed into
a commercial process for the sterilization of food mixtures. Ohmic heating occurs, when an electric
current is passed through an electrically conducive product. Low frequency current from domestic
supply could be effectively used for ohmic heating. The ohmic heating has many advantages over
conventional heating. Continuous processing is possible without any heat transfer surface. Liquid-solid
mixture can be rapidly and evenly heated with minimal heat damage and residence time difference.
Nutrient retention will also be more. This process can obtain fresher-tasting, high quality products with
high microbiological safety. Maintenance cost is minimum due to absence of any moving parts. The
process is easy to control. Ambient temperature storage and distribution is possible when combined
with an aseptic filling system.
43
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Membrane technology
With the inception of new composite membranes and tubular system, reverse osmosis (RO) and
ultra filtration (UF) are being used extensively in food and dairy industries. RO is a single phase
concentration process which uses a pressure gradient across a semi permeable membrane to squeeze
water through membrane. RO process is extremely energy efficient compared to both evaporation
and freeze concentration. Ultra filtration uses much lower pressure 1 to 10 bars and much more open
membranes, which pass salts, sugars and organics in the molecular range typically from 5,000 to
1,00,000 depending on the membrane type. It is limited by osmotic pressures, since the sugars are not
concentrated.
Both RO and UF have promising uses in fruit and vegetable juice industry as a unit operation
for concentration or aroma
recovery and clarification
of juices respectively. The
primary goal of UF in
fruit industry is to replace
the
holding
filtration
and decantation steps of
traditional process. Enzyme
treatment is required to
reduce viscosity of juice by
partially hydrolyzing. It is
Figure 5: A simplified general design of a pulsed electric field apparatus.
a clarification process to
remove pectin, enzyme and
other fibrous components,
constituting the clear juice. For separation
of molecules, semi permeable membrane
is used at a temperature of 50 -55 °C (high)
on 10-15 °C (low), depending on the type of
juice and sensitivity. Tubular modules can
be used for viscous partially depectinized
juice whereas where as pre filtration of juice
is necessary when this channel on boiled fibre
UF system is used. Many case studies have
done on apple juice. UF system generates cost
savings and manpower reduction, uses only
electrical energy to raise the pressure of juice
feed, operating costs are typically 5-10 times Figure 6: Treatment chamber with different electrode geometries
lower than normal operations, process control and enhanced electric fields in the insulator channel
is simple, no cooling water equipment is
needed and products have better flavour.
In nutshell, UF has become economical
viable alternative for clarification of juice
in comparison to conventional method of
clarification. RO is a well established process
for concentration/pre concentration of raw
and clear depectinized juice from fruits and
vegetable. It consumes 10 times less energy
for renouncing water when compared with
conventional evaporators.
Figure 7: Continuous PEF chamber with baffles
44
Novel Technologies for Processing and Packaging of Health Foods and Beverages
Pulsed electric field (PEF)
It was in US where in 1920s first attempt to treat milk with electro impulse was made. Further,
experimentations followed in the 1960s primarily with in molecular biological research for incorporation
of foreign gene materials into microorganisms. This technique involves application of pulse of high
voltage (typically 20-80 KV/cm) to foods placed between two electrodes. Only pumpable food products
can be treated. This is the more novel process. PEF imposes a strong electric field on a flowering fluid
for a very short time. Above critical field strength of about 15,000 V/cm, vegetable cells are killed.
Generally higher field strength up to about 35,000 V/cm for disinfection like destruction of bacteria,
fungi and other microbes. When exposed to high electric field pulses, cell membranes develop pores
either by enlargement of existing pores or by creation of new ones. The pores increase membrane
permeability allowing loss of cell contents or inclusion of surrounding media either of which can cause
cell death. It has limited effect on pores and only appears to affect a few enzymes. Figure 5 shows a
simplified design of pulsed electric field and figures 6 and 7 describe different electrode geometries in
the treatment chamber and Continuous PEF chamber with baffles respectively.
PEF offers a five log reduction of most pathogens and is considered as a pasteurization process
so products must be refrigerated. PEF also applies to fruit and vegetables cell well, concentration of
sewage sludge. It kills live cells and reduces their ability to retain water, greatly improves filtration.
Extraction of sugars from beats and starches from potatoes can also be improved by PEF. The important
process variables of PEF include the electric field, temperature, pressure and time of exposure. PEF
units differ primarily in their fluid handling capacity: OSU-4, has 0.5-1.0 cm diameter tubing; OSU-5,
has 1 cm diameter tubing; and OSU-6, has 1-1.2 cm diameter tubing.
Food irradiation
Food Irradiation is the new addition to the methods of food preservation. A great deal of work
is being carried out at the utilization of ionizing radiation. The irradiation of foods does destroy the
microorganisms and enzymes. It may be desirable to inactivate some enzymes by other means, in
complementation to irradiation action. Irradiation does not leave any residue in foods like chemical
and hence is safe. The sterilization of food with ionizing radiation involves a major consideration,
the food products and suitable radiation source, since the temperature remains 4-50C. It is also called
“cold sterilization” technique. These techniques in controlling the ripening process of fruits and also
for checking sprouting of roots, tubers and bulbs apart from general food preservation techniques.
Modified atmosphere packaging (MAP) and controlled atmosphere storage
(CAS)
The main objective of modified atmosphere packaging (MAP) is to interrupt or slow down
the derivative processes and also to prevent the attack of pathogens until the food is consumed.
Controlled atmosphere (CA) is the alteration of the natural gaseous environment and maintenance of
this atmosphere at pre specified conditions throughout the storage time. Modified atmosphere (MA)
is the initial alteration of the gaseous environment in the immediate vicinity of stored and packaged
product. These are used for retail distribution and for consumer product packages. The CA and MAP
extend the shelf life of the product. Lot of work has been carried out and further research is on.
RTE health foods
In our country processed food product are available in both organized and unorganized sectors.
Developments in production technology, emergence of new products like ready-to-eat (RTE) mixes,
enhancement of product shelf life and packaging are driving the shift from the non organized to
organized commercial business. Due to growing urbanization and changing food habits, the
demand has been rising at a good pace and there is enough latent market potential waiting to be
exploited through developmental efforts. It is high time for the organized sector to take initiative
in technology improvement, process modeling and automation, overall improvement in quality,
investment in R&D to develop new products and enhance shelf life of existing products and further
45
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
improvement in packaging. The size of the industry might increase substantially if the product
portfolio were to include at least one each of the daily staples; ready chapatti from the chapatti-subzi
staple and precooked or concentrated dal from the dal–chawal combination. India is a vast country
with different eating habits and yet North Indian cuisine is an acceptable restaurant fare and the
ubiquitous idli and dosa are available in every part of country. From dishes consumed throughout
the country at different eating occasions of breakfast, lunch, tea time and dinner, it should be possible
to systematically examine similarities and come up with a short list of products that would find a
place in the menu of a major part of the country, which could then be developed as processed food.
The Western concept of hamburger or a sandwich and its local equivalent chapatti-subzi takeaways
cooked in perceived hygienic surrounding are a boon for the working women and can be nurtured into
big business. With the increasing dominance of large and technically more sophisticated companies
in food processing, attention also should be paid to small operations, which do not enjoy the same
economies of scale. To this end, an increase in the number of regionally centralized facilities offering
the latest processing technique and advice should be encouraged. It would be necessary to develop the
appropriate technology to produce an authentic product, with low cost or reusable packaging and an
efficient distribution system to market them at an acceptable price. The prime aim will be to provide
all consumers with increasing levels of convenience.
Health / nutritional benefits of functional foods
During recent years importance of B-complex vitamins, beta-carotene and vitamin C has been
realized in terms of their antioxidative and anticarcinogenic properties. Fruits and vegetables are
the rich sources of these vitamins. Fermented foods and beverages possess various nutritional and
therapeutic properties. Lactic acid bacteria play a major role in determining the positive health effects
of fermented milks and related products. The L. acidophilus and Bifidobacteria spp are known for
their use in probiotic dairy foods. Cultured products sold with any claims of health benefits should
meet the criteria of suggested minimum number of more than 106 cfu / g at the expiry date. Other
health benefits of fermented milk products include prevention of gastrointestinal infections, reduction
of serum cholesterol levels and anti -mutagenic activity. They are recommended for lactose intolerant
individuals and patients suffering from atherosclerosis.
Health claims
Health claims describe a relationship between a food, food component, or dietary supplement
ingredient, and reducing risk of a disease or health-related condition. There are three ways by which
FDA exercises its oversight in determining which health claims may be used on a label or in labeling for
a food or dietary supplement: (i) the 1990 Nutrition Labeling and Education Act (NLEA), (ii) the 1997
Food and Drug Administration Modernization Act (FDAMA) and (iii) the 2003 FDA Consumer Health
Information for Better Nutrition Initiative. Such health claims must be qualified to assure accuracy and
non-misleading presentation to consumers. FDA authorizes these types of health claims based on an
extensive review of the scientific literature, generally as a result of the submission of a health claim
petition, using the significant scientific agreement standard to determine that the nutrient/ disease
relationship is well established.
Nutrient content claims
Nutrient content claims describe the level of a nutrient or dietary substance in the product, using
terms such as free, high, and low, or they compare the level of a nutrient in a food to that of another
food, using terms such as more, reduced etc. An accurate quantitative statement (e.g., 200 mg of
sodium) that does not “characterize” the nutrient level may be used to describe any amount of a
nutrient present.
Structure/ function claims
Structure/ function claims describe the role of a nutrient or dietary ingredient intended to affect
normal structure or function in humans, for example, “calcium builds strong bones”. In addition, they
46
Novel Technologies for Processing and Packaging of Health Foods and Beverages
may characterize the means by which a nutrient or dietary ingredient acts to maintain such structure or
function, for example, “fiber maintains bowel regularity,” or “antioxidants maintain cell integrity,” or
they may describe general well-being from consumption of a nutrient or dietary ingredient. Structure/
function claims may also describe a benefit related to a nutrient deficiency disease (like vitamin C and
scurvy), as long as the statement also tells how widespread such a disease is in the United States.
Technology of formulation of health foods
There are several methods of manufacturing functional foods, based either on the method used to
produce them or on their purpose. Functional foods may be processed by modification or they may be
fortified with different substances and the functionality of a product can be targeted to a special disease
or just to improve overall well being. A particular food may be made more functional by increasing or
adding a potential health promoting entity. Alternatively concentration of adverse components may be
reduced or there may be a partial interchange between toxic and beneficial ingredients. Health drinks
are formulated taking into account the nutritional requirements or recommended dietary allowances
for the target group. It is not only essential to balance the energy protein and vitamin requirements,
but also to make it palatable, sparkling and thirst quenching.
Challenges in formulation of health foods
There are many obstacles within food system that hinder the development processes of these specific
foods. There are considerable processing losses of vitamins, and information on vitamin contents
of processed foods is essential for assessing the adequacy of vitamin intakes. Problems associated
with the iron fortification of fruit juices and drinks have been outlined as: accelerated loss of vitamin
C, flavour and taste deterioration in the presence of thiamine, folic acid, vitamin A and vitamin C,
levels of fortification beyond 2.7 mg per serving result in metallic off-flavors, decolourisation of some
pigments.
Fortification of beverages with calcium has become a popular practice. Insoluble Ca and Mg salts
cause lightening of food colour, whereas soluble salts may interact with other food components such as
tannins to cause darkening. The prooxidant effect of many minerals has caused rancidity development
in lipid containing beverages. In beverages with high protein content, the addition of Ca or Mg salts
have caused destabilization of the protein component. The use of soy lecithin to coat calcium ions for
use in the calcium fortification of soymilk prevented the Ca induced precipitation of soy proteins. In
the production of yoghurt, the low pH conditions render it unsuitable as a carrier for vitamins such
as vitamin A.
Health foods for control of cardiovascular disease (CVD) & diabetes
Functional foods that are marketed with claims to reduce heart disease focus primarily on the risk
factors of blood cholesterol, homocysteine and hypertension. This can be done by a reduced content of
food components that are known to increase risk, saturated fat or sodium. More recently products have
been designed that are enriched in components thought to reduce risk. The most common protective
ingredients include fibres, ώ-3 fatty acids, phytostanols, phytosterols and (antioxidant) vitamins.
Replacement of saturated or trans fat in the diet by carbohydrates or other types of fat reduces the risk
of coronary heart disease (CHD). High intakes of tea rich in catechins and other flavonoid polyphenols
have also been associated with a reduced risk of CHD. Many well-controlled trials have documented
the efficacy of sterols and stanols for lowering low density lipoprotein (LDL) cholesterol. A high
consumption of soy protein has been associated with a low risk of cardio vascular disease (CVD) in
ecological studies. Besides soy protein, isoflavonones (phytoestrogens) such as genistein might be
responsible for the effects on CVD risk. Thus, any dietary pattern combining a high intake of natural
antioxidants, a low intake of saturated fatty acids, a high intake of oleic acid, a low intake of ώ-6 fatty
acids and a high intake of ώ-3 fatty acids would logically produce a highly cardio protective effect.
Diabetes mellitus is a heterogeneous metabolic syndrome with several different causes characterized
by chronic hyperglycaemia with partial or total lack of insulin secretion and a reduced sensitivity to
47
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
the hormone in peripheral tissues. If monitored inadequately and associated with other lipid and
protein disorders, long term complications may develop in several organs and systems, resulting in
both high morbidity and mortality rates. Type 1 diabetes is the result of complete β-cell destruction.
Type 2 diabetes is the primary result of either insulin resistance or deficiency in insulin secretion,
and having a completely different clinical perspective and presentation is usually characterized by
a mixture of the two. It has been suggested that for type 1 diabetes an early exposure to cows’ milk
proteins may play a role in triggering the immune response that destroys pancreatic β-cells. In general
a good nutritional diet that is low in fat and salt is important. For someone with type 1 diabetes,
regular meal times and snacks and the right proportion of nutrients should be emphasized. Someone
with type 2 diabetes, where in most cases weight reduction is necessary, not only should consider
calorie intake but also the component and type of food that is eaten.
Conclusion
Improving food technology not only improves health, but reduces poverty. When food products
are safe, nutritious, well marketed, and competitively priced thanks to efficient manufacturing, they
attract consumers. Rising consumer demand, in turn, expands a nation’s entrepreneurial base in food
products, creating jobs and raising family incomes. Larger family food budgets then contribute to a
further drop in malnutrition. New preservation technologies, such as high pressure processing and
pulsed electric fields offer advantages in meeting consumer demands of freshness, convenience and
safety. There is no single process that will allow the high-quality production of every food product
while ensuring safety; all of these processes, as well as thermal processing, have their own set of
limitations and advantages.
References
Cánovas, B. G. V, Gongora-Nieto, M.M., Pothakamury, U. R. and Swanson, B. G. (1999). Preservation of foods with pulsed
electric fields. 1-9, 76-107, 108-155. Academic Press Ltd. London.
Chandrasekhar, U. (2004) Soy proteins - an ideal functional food for growth promotion.
Proc Nutr Soc Aust , Vol. 28. Asia Pac J Clin Nutr 2004; 13 (Suppl):S118.
Gonçalo,E. B. (2003) Certificação de sistemas de qualidade na indústria de laticínios, Revista do Instituto de Laticinios
Cândido Tostes 58 , (333): 9–14
http://www.fao.org/ ag/ags/agsi/Nonthermal/nonthermal_1.htm
Ilbery, B. and Kneafsey, M. (2000). Producer constructions of quality in regional speciality food production: a case
study from South West England. Journal of Rural Studies 16 2 (2000), pp. 217–230.
Ruecroft, G. (2007) Power Ultrasound, Crystals and Particle Engineering, Paper presented in “Chemsource Symposium
2007” during 27th & 28th June at RAI, Amsterdam.
Zhang, M and Xu, Y. Y. (2003) Research developments of combination drying technology for fruits and vegetables at
home and abroad, Journal of Wuxi University of Light Industry 22, (6): 103–106.
48
Glycomacropeptide – Biological Properties and its Application
Glycomacropeptide – Biological
Properties and its Application
Rajan Sharma and Neelima Sharma
Dairy Chemistry Division, NDRI, Karnal
Introduction
Whey proteins have been singled out as a super star ingredient for health promoting products
including ones formulated for weight loss, infant nutrition and immune support. The major whey
proteins are α-lactalbumin (α-la), β-lactoglobulin (β-lg), bovine serum albumin, immunoglobulins
and lactoferrin. In addition, sweet whey/rennet whey also contains glycomacropeptide (GMP) which
is a C-terminal hydrophilic glycopeptide released from κ-casein (κ-CN) by the action of chymosin
during cheese making. GMP lacks aromatic amino acids (phenylalanine, tyrosine, and tryptophan),
and contains varying amounts of sugars which are made up of N-acetylneuraminic acid (sialic acid or
NANA), galactose, and N-acetylgalactosamine.
GMP found in sweet whey is an acidic glycopeptide. The bond sensitive to chymosin (rennin)
hydrolysis occurs between the phenylalanine (Phe) residue at position 105 and the methionine (Met)
residue at position 106. The hydrolytic products are para κ-CN (residue 1-105) and macropeptide
(residue 106-169). Next to β-lg and α-la, GMP is the most abundant protein/peptide in whey
protein isolate (WPI) and whey protein concentrate (WPC) produced from cheese whey with typical
concentrations between 20-25% of the proteins (Thoma-Worringer et al., 2006). It is a heterogeneous
peptide of 64 amino acids formed by κ-CN (Delfour et al., 1964). It contains 47% (w/w) indispensable
amino acids, but contains no histidine (His), tryptophan (Trp), tyrosine (Tyr), arginine (Arg), cysteine
(Cys) or Phe (Laclair et al. 2009). There are four hydrophobic domains in GMP and most of them are
masked by the strong charge density of the glutamic acid (Glu) and asparatic acid (Asp) residues
over a wide range of neutral and basic pH, therefore the hydrophobic domains cannot interact. Only
the N-terminal hydrophobic domain (amino acid 1-5) is not covered by the negative charge and is
available for interaction (Kreub et al., 2009).
GMP has received much attention in recent years because of its several biological properties.
The various biological activities of GMP include its ability to bind cholera toxin and Escherichia coli
enterotoxins, inhibition of bacterial and viral adhesion, modulation of immune system responses,
promotion of bifido-bacterial growth, suppression of gastric secretions and regulation of blood
circulation etc. Further, GMP can find application in various food systems because of its functional
characteristics, mainly its high solubility and emulsifying properties.
Chemical properties of GMP
GMP is one of the various names given to the peptide formed by κ-CN rupture. This peptide is also
known as caseinomacropeptide (CMP) or casein-derived peptide (CDP). Usually GMP refers to the
glycosylated form, due to its high carbohydrate content, and CMP to the peptide´s non-glycosylated
form. Its composition varies and depends particularly on the whey source and on the technology used
for its isolation (Martín-Diana et al., 2006). The major chemical properties of GMP include:
•
Glycosylation;
•
Isoelectric point and UV absorption;
•
Molecular weight;
•
UV Characteristic
1. Glycosylation: The glycosylated form represents 50 to 60% of total GMP and the carbohydrate
part is composed of galactose, (Gal) N-acetylgalactosamine (GalNAc) and N-acetylneuraminic acid
(NeuAc) (Thoma et al., 2006). The most predominant is NeuAc, known as sialic acid. GMP purified to
49
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
90% is highly glycosylated with 7 to 8% sialic acid (Martín-Diana et al., 2003). Sialic acid present in the
GMP gives the peptide anionic character and may react with other compounds like thiobarbituric acid
(TBA) and ninhydrin to give chromophores which can be spectrophotometrically determined. This
property can be utilized for the estimation of GMP as the sialic acid content and can be correlated with
the GMP concentration. In one approach Nakano and Ozimek (1999) determined the sialic acid content
for the estimation of GMP in cheese whey by using TBA. The other approach for the determination
of sialic acid content in GMP has been given by the use of acidic ninhydrin by spectrophotometric
method (Fukuda et al., 2004).
2. Isoelectric point and UV absorption: The exact isoelectric point of GMP is still unclear. Using
anion exchange chromatography Nakano and Ozimek (2000) suggested that all sialylated GMP had
an apparent pI < 3.8. GMP is rich in branched-chain amino acids (valine and isoleucine) and it lacks
aromatic amino acids, including phenylalanine, tryptophan and tyrosine (Oliva et al., 2002).
3. Molecular weight: Several works have informed that the theoretical molecular weight of GMP
is between 7 and 8 kDa. Some authors suggest that GMP has the ability of associating and dissociating
under selected pH conditions. Kawasaki et al. (1993a) proposed that the monomer of k-casein GMP of
molecular weight 9 kDa is obtained at pH ≤ 4 and the polymer of k-casein GMP of molecular weight
between 45- 50 kDa is obtained at pH higher than 4. Later, Nakano and Ozimek (1998) also studied
the influence of pH on GMP behaviour. Using chromatography in Sephacryl S-200 gel, the authors
collected fractions that were monitored for sialic acid. The results suggested that GMP is an aggregate
of three monomers and the molecular weight was not affected by changes in pH. Farias et al. (2009)
studied self assembly of 3-5% (w/w) GMP at pH 3-6.5 using DLS (Dynamic Light Scattering). The
hydrodynamic diameter increased when decreasing the pH from 6.5 to 3. GMP solution at a pH below
4.5 shows time dependent self assembly at room temperature, which over time leads to gelation.
Currently there is an overall consensus that the experimental molecular weight of GMP is higher than
the theoretical weight.
4. UV Characteristic: Because of the absence of aromatic amino acids, GMP has no absorption
at 280 nm. It is known that GMP is only detected in the range of 205-217 nm and differences in the
absorption at 210/280 nm are frequently used to characterize GMP (Oliva et al., 2002).
Biological activities and nutritional properties
Biological activity of bovine GMP has received much attention in recent years. The various
physiological functions attributed to GMP include:
•
Binding of cholera toxin (CT);
•
Inhibition of bacterial and viral adhesion;
•
Suppression of gastric secretions;
•
Promotion of Bifidobacterial growth;
•
Modulation of immune system response;
•
Regulation of blood circulation through antihypertensive and antithrombotic activity;
1. Binding of cholera toxin (CT): Kawasaki et al. (1992) showed that GMP is capable of binding
CT. Normal Chinese hamster ovary (CHO)-K1 cells are spherical but in the presence of CT, CHO-K1
cells take on a spindle shape. As little as 20 ppm GMP is enough to cause considerable rounding of
CHO-K1 cells and 100 ppm GMP results in almost completely rounded CHO-K1 cells which indicate
that GMP has bound to CT. When the GMP was treated with sialidase, which hydrolyses the sialic
acid, complete loss of CT inhibiting activity occurred. The peptide chain must also participate in the
binding as partial loss of CT inhibiting activity occurred after treatment with proteases. CT binding
activity of purified GMP from bovine κ-CN was also detected by Oh et al. (2000) using ELISA. The CT
binding activity is rapidly lost by carbohydrase treatment.
50
Glycomacropeptide – Biological Properties and its Application
2. Inhibition of bacterial and viral adhesion: Many bacteria and viruses bind themselves to
their hosts as a part of the colonization process. Binding to the intestine or other mucosal surfaces is
achieved by adhesins, capsular material on the bacterial cell surface or hair-like fimbriae or pili which
are specific for the various ceramide and ganglioside glycoconjugates which make up epithelial cell
membranes (Simon, 1996). Nakajima et al. (2005) studied the prevention of intestinal infection by GMP.
The binding ability of GMP to intestinal pathogenic bacteria was evaluated by a binding assay with
biotinylated bacteria. GMP showed the ability to bind to Salmonella enteritidis and enterohemorrhagic
E.coli O157:H7. This binding ability was decreased by a sialidase treatment and completely eliminated
by periodate oxidation indicating that sialic acid in GMP are involved in binding to the bacteria.
Neeser et al. (1988a) investigated the mechanism by which milk components prevent dental caries.
They evaluated the role of GMP in inhibiting adhesion of cariogenic bacteria (Streptococcus mutans,
S. sanguis, S. sobrinus and Actinomyces viscosus) to oral surfaces. Haemagglutination by S. mutans, S.
sanguis and A. viscosus is prevented by GMP with a disaccharide (Gal β1 → 3GalNAc - O – R).
3. Suppression of gastric secretions: Guilloteau et al. (1994) while investing the effect of GMP
on gastric secretion in preruminant calves found that intravenous injection of GMP had no inhibition
of gastric secretion. Beucher et al. (1994b) found that feeding GMP fraction, stimulated the intestinal
hormone cholecystokinin which, in turn, regulates gastrointestinal functions. Yvon et al. (1994)
demonstrated that GMP acts by triggering receptors on the intestinal mucosa.
4. Promotion of bifidobacterial growth: Supplementation of milk with 2% GMP, either of
bovine, ovine or caprine origin increased the counts of Bifidobacterium lactis by 1.5 log cycles after 24
h incubation at 37°C when compared with unsupplemented milk (Janer et al., 2004).
5. Modulation of immune system response: Splenocyte (spleen lymphocyte) proliferation is a
step in the inflammatory response. Inhibition of splenocyte proliferation can be used to demonstrate
suppression of an immune response such as an allergic reaction. Research by Otani et al. (1992)
demonstrated that casein inhibits mouse splenocyte proliferation induced by the mitogen Salmonella
typhimurium lipopolysaccharide (LPS). Inhibitory activity was due to κ-casein, which upon rennet
hydrolysis, results in inhibitory activity being found in the GMP fraction. Para- κ-casein had no
inhibitory activity. Upon sialidase digestion, GMP lost its inhibitory activity, indicating that sialic acid
is critical to the phenomenon (Otani and Monnai, 1993). Inhibitory activity was reduced after GMP
digestion with chymotrypsin but inhibitory activity increased after GMP digestion with trypsin or
pronase so the peptide chain must also participate.
6. Regulation of blood circulation through antihypertensive and antithrombotic activity:
Bovine, ovine, and caprine GMP can inhibit platelet aggregation and, therefore, the formation of
thrombi, because the region 106–116 of κ-casein (casoplatelin) is analogous to the fragment 400–411
of fibrinogen γ-chain (Jolles et al., 1986). Peptides with in vitro angiotensin I converting enzyme
(ACE)-inhibitory activity were liberated from bovine, ovine and caprine GMP either by proteolysis
with trypsin or simulation of the GMP digestion under gastrointestinal conditions (Manso and
Lopez-Fandino, 2003). This suggests that intact GMP and its tryptic peptides may play a role in the
physiological regulation of blood pressure, although tryptic hydrolysates exhibited higher levels of
ACE inhibitory activity than did intact GMP during subsequent digestion, which justifies their use as
food components.
Along with the above mentioned physiological effects GMP has been found to be beneficial for
overall growth and development. GMP is rich in branched-chain amino acids and low in Met, which
makes it a useful ingredient in diets for patients suffering from hepatic diseases (Abd El-Salam et al.,
1996). Additionally, the fact that GMP has no Phe in its amino acid composition makes it suitable for
nutrition in cases of phenylketonuria. Nevertheless, because of its high content of Thr, GMP can cause
hyperthreoninemia (Fanaro and Vigi, 2002; Rigo et al., 2001). GMP supplementation has also been
found to increas zinc absorption (Kelleher et al., 2003). The sialic acid content of GMP is also interesting
in terms of bioactivity. Large amounts of this carbohydrate are found in the brain and in the central
nervous system in the form of gangliosides and glycoproteins, which contribute to the functioning of
51
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
cell membranes and membrane receptors and to normal brain development. An in vivo experiment
with laboratory animals has shown that the exogenous administration of sialic acid increased the
production of ganglioside sialic acid in the brain, improving learning ability (Wang et al., 2001). This
effect could also be achieved with dietary GMP (Wang et al., 2004).
Functional and technological properties/applications of GMP
GMP can also be taken up as a functional ingredient in various speciality products like infant
formulas, nutrition bars, medical foods (PKU), diet foods, oral care products and dietary supplements
because the development of innovative foods with an additional health benefit is a goal for the food
industry.
The characteristics of GMP not only permit medicinal and dietary applications, but also give
the molecule a great potential as a structural agent for food, since its glycosidic structure suggests
emulsifying and foaming properties (Kulozik and Guilmineau, 2003). GMP showed to be stable in the
pH range of 1 to 10, with minimal solubility (88%) between pH 1-5 and maximum (98%) between pH
5-10. The emulsifying activity was stronger at acid pH rather than alkaline. After letting the emulsion
stand for 24 hours and heating a decrease in the emulsifying activity (22-60%) at pH below 4 (Chobert
et al., 1989) was observed. Wong et al. (2006) determined the functionality, foaming capacity and
emulsifying activity of GMP after conjugation to fatty acids. The authors observed that the foaming
capacity was lost, whereas the emulsifying activity enhanced. The addition of GMP to fermented
goat milk favored gel formation in a more orderly and structured manner compared to the addition
of whey protein concentrate (Martín-Diana et al., 2003). However, Veith and Reynolds (2004) verified
in their work that the presence of GMP had a negative impact on gel strength and water retention
capacity. On the other hand, Martín-Diana et al. (2005) studied GMP of cow, ewe and goat cheese
whey, concluding that GMP had an emulsifying activity, more stable to pH variation, compared to
whey protein concentrate. This suggests the possibility to use GMP as an emulsifier in foods that
undergo great pH variation during processing, such as fermented dairy products. GMP obtained
from goat milk was modified with lactose through Maillard reaction under relative humidity 44% and
temperature of 40°C for periods of 0 to 11 days, thus obtaining different forms of lactosylated GMP.
At these conditions, the most abundant form of lactosylated GMP was the Monolactosylated (55-60%),
followed by the di-, tri- and tetralactosylated species. Solubility, heat stability and emulsifying capacity
of native and modified GMP were investigated. Lactosylation enhanced emulsifying capacity but did
not improve the outstanding solubility and heat stability of native GMP (Moreno et al., 2002).
References
Abd El-Salam, M.H.; El-Shibini, S. and Buchheim, W. (1996). Characteristics and potential uses of the casein macropeptide.
Int. Dairy J. 6:327–341.
Beucher, S.; Levenez, F.; Yvon, M. and Corring, T. (1994b) Effect of caseinomacropeptide (CMP) on cholecystokinin
(CCK) release in rat. Reproduction Nutrition Development. 34: 613-614.
Chobert, J.; Touati, A.; Bertrandharb, C.; Dalgalarrondo, M. and Nicolas, M. (1989) Solubility and emulsifying properties
of kappa-casein and its caseinomacropeptide. J. Food Biochem. 13:457-473.
Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrel, H.M.Jr.; Harwalkar, V.R.; Jennes, R. and Whitney, R.M. (1984)
Nomenclature of proteins of cow’s milk: Fifth Revision. J. Dairy Sci. 67:1599-1631.
Fanaro, S. and Vigi, V. (2002). Protein quality and quantity in infant formulas: A critical look. Minerva Pediatrica. 54:203–
209.
Farias, M.E.; Martinez, M.J. and Pilosof, A.M.R. (2010) Casein Glycomacropeptide pH-dependent self assembly and
cold gelation. Int. Dairy J. 20:79-88.
Fukuda, S.P.; Roig, S.M. and Prata, L.F. (2004) Correlation between acidic ninhydrin and HPLC methods to evaluate
fraudulent addition of whey in milk. Le Lait. 84:502-512.
Guilloteau, P.; Le Huerou-Luron, I.; Chayviaille, J.A.; Toullec, R.; Legeas, M.; Bernard, C.; Roger, L. and Mendy, F. (1994)
Effect of caseinomacropeptide (CMP) on gastric secretion and plasma gut regulatory peptides in preruminant
calves. Reproduction Nutrition Development. 34:612-613.
Janer, C.; Pelaez, C. and Requena, T. (2004) Caseinomacropeptide and whey protein concentrate enhance Bifidobacterium
lactis growth in milk. Food Chem. 86: 263-267.
Jolles, P.; Levy-Toledano, S.; Fiat, A. M.; Soria, C.; Gillensen, D.; Thomaidis, A.; Dunn, F.W.; Caen, J.P. (1986). Analogy
between fibrinogen and casein: Effect of an undecapeptide isolated from k-casein on platelet function. European
J. Biochem. 158:379–382.
52
Glycomacropeptide – Biological Properties and its Application
Kawasaki, Y., Isoda, H., Tanimoto, M.; Dosako, S. Idota, T. and Ahiko, K. (1992) Inhibition by lactoferrin and kappacasein
glycomacropeptide of binding of cholera toxin to its receptor. Biosci. Biotech. Biochem. 56: 195-198.
Kawasaki, Y.; Kawakami, H.; Tanimoto, M.; Dosako, S.; Tomizawa, A.; Kotake, M. and Nakajima, I. (1993a) pH
independent molecular weight changes of k-casein glycomacropeptide and its preparation by ultrafiltration.
Milchwissenschaft. 48:191-195.
Kelleher, S.L.; Chatterton, D.; Nielsen, K. and Lonnerdal, B. (2003) Glycomacropeptide and α-lactalbumin
supplementation of infant formula affects growth and nutritional status in infantis rhesus monkeys. Am. J. Clin.
Nutr. 77:1261-1268.
Kulozik, U. and Guilmineau, F. (2003) Food process engineering and dairy technology at the Technical University of
Munich. Int. J. Dairy Tech. 56:191-198.
Manso, M. A. and Lopez-Fandino, R. (2003). Angiotensin I converting enzyme-inhibitory activity of bovine, ovine and
caprine k-casein macropeptides and their tryptic hydrolyzates. J. Food Protection. 66:1686–1692.
Martin-Diana, A. B.; Frias J. and Fontecha J. (2005) Emulsifying properties of whey protein concentrate and
aseinomacropeptide of cow, ewe and goat. Milchwissenschaft. 60:363-366.
Martín-Diana, A.B.; Gomez-Guillén, M.C.; Montero, P. and Fontecha, J. (2006) Viscoelastic properties of
caseinmacropeptide isolated from cow, ewe and goat cheese whey. J. Sci. Food Agric. 86:1340-1349.
Martín-Diana, A.B.; Pelaez, C. and Requena, T. (2003) Rheological and structural properties of fermented goat’s milk
supplemented with caseinomacropeptide and whey protein concentrate. J. Dairy Sci. 86:1535-1540.
Moreno, F.J.; López-Fandiño, R. and Olano, A. (2002) Characterization and functional properties of lactosyl
caseinomacropeptide conjugates. J. Agric. Food Chem. 50:5179-5184.
Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y. and Hattori,
M. (2005) Prevention of intestinal infection by Glycomacropeptide. Biosci. Biotechnol. Biochem. 69:2294-2301.
Nakano, T. and Ozimek, L. (1998) Gel chromatography of glycomacropeptide (GMP) from sweet whey on Sephacryl
S-200 at different pH’s and on Sephadex G-75 in 6M guanidine hydrochloride. Milchwissenschaft. 53:629-632.
Nakano, T. and Ozimek, L. (1999) Purification of glycomacropeptide from non-dialyzable fraction of sweet whey by
anion exchange chromatography. J. Biotech. Tech. 13:739-742.
Nakano, T. and Ozimek, L. (2000) Purification of glycomacropeptide from caseinate hydrolysate by gel chromatography
and treatment with acidic solution. J. Food Sci. 65:588-590.
Neeser, J.R.; Chambaz, A.; Vedovo, S.D.; Prigent, M.J. and Guggenheim, B. (1988a) Specific and nonspecific inhibition of
adhesion of oral actinomyces and streptococci to erythrocytes and polystrene by caseinoglycopeptide derivatives.
Infection and Immunity. 56:3201-3208.
Oliva, Y., Escobar, A. and Ponce, P. (2002) Caseinomacropéptido bovino: una alternative para la salud. Rev. Salud Anim.
24:73-81.
Oh, S.; Worobo, R.D.; Kim, B.C.; Rheem, S. and Kim, S. (2000) Detection of cholera toxin binding activity of κ-casein
macropeptide and optimization of its production by the response surface methodology. Biosci. Biotechnol. Biochem.
64:516-522.
Otani, H. and Monnai, M. (1993) Inhibition of proliferative responses of mouse spleen lymphocytes by bovine milk
k-casein digests. Food and Agricultural Immunology. 5:219-229.
Otani, H.; Monnai, M. and Hosono, A. (1992) Bovine κ-casein as inhibitor of the proliferation of mouse splenocytes
induced by lipopolysaccharide stimulation. Milchwissenschaft. 47:512-515.
Rigo, J.; Boehm, G.; Georgi, G.; Jelinek, J.; Nyambugabo, K.; Sawatzki, G. and Studzinski, F. (2001). An infant formula
free of glycomacropeptide prevents hyperthreoninemia in formula-fed preterm infants. J. Pediatr. Gastroenterol
Nutr. 32:127–130.
Saito, T.; Yamaji, A. and Itoh, T. (1991) A new isolation method of caseinoglycopeptide from sweet cheese whey. J. Dairy
Sci. 74: 2831-2837.
Simon, P.M. (1996) Pharmaceutical oligosaccharides. Drug Discovery Today. 1:522-528.
Thomä-Worringer, C.; Sorensen, J. and López-Fandinõ, R. (2006) Health effects and technological features of
caseinomacropeptide. Int. Dairy J. 16: 1324-1333.
Tolkach, A. and Kulozik, U. (2005) Fractionation of whey proteins and caseinomacropeptide by means of enzymatic
crosslinking and membrane separation techniques. J. Food Eng. 67: 13-20.
Tullio, L. T.; Karkle, E. N. L. and Candido, L.M.B. (2007) Review: Isolation and Purification of Milk Whey
Glycomacropeptide. B. CEPPA. 25:121-132.
Wang, B.; Brand-Miller, J.; McVeagh, P. and Petocz, P. (2001). Concentration and distribution of sialic acid in human
milk and infant formulas. Am. J. Clin. Nutr. 74:510-515.
Wang, B.; Staples, A.; Sun, Y.; Karim, M. and Brand-Miller, J. (2004). Effect of dietary sialic acid supplementation on
saliva content in piglets. Asia and Pacific J. Clin. Nutr. 13:75.
Wong, P.Y.Y.; Nakamura, S. and Kitts, D.D. (2006) Functional and biological activities of casein glycomacropeptide as
influenced by lipophilization with medium and long chain fatty acid. Food Chem. 97:310-317.
Xu, Y.; Sleigh, R.; Hourigan, J. and Johnson, R. (2000) Separation of bovine immunoglobulin G and glycomacropeptide
from dairy whey. Process Biochem. 36:393-399.
Yvon, M.; Beucher, S.; Guilloteau, P.; Huerou-Luron, I.L. and Corring, T. (1994) Effects of caseinomacropeptide (CMP)
on digestion regulation. Reprod. Nutr. Dev. 34:527-537.
53
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
New Approaches to Detect the Adulteration of Ghee
with Animal Body Fats and Vegetable Oils/ Fats
Vivek Sharma, Darshan Lal, Arun Kumar and Amit Kumar
Dairy Chemistry Division, NDRI, Karnal
Introduction
Ghee is the most widely used milk product in the Indian sub- continent. It is a valuable source of
fat-soluble vitamins and essential fatty acids, apart from having rich and pleasant sensory attributes.
Due to its increased demand, cost and variable chemical composition, the unscrupulous people get
tempted to adulterate it with cheaper foreign fats like vegetable oils ⁄ fats and animal body fats, etc.,
which is an unethical practice. Earlier, ghee used to be adulterated with foreign oils and fats, and
accordingly several methods were developed for detection of adulteration in ghee. These methods
were based on differences in the nature and contents of major/minor components of ghee and
adulterant fats/oils. Methods presently used for detecting foreign fats in milk fat are mainly based on
the physico-chemical constants, fatty acid profile, sterol analysis, and partial solidification behavior.
However, all these methods fail when milk fat is adulterated with a mixture of body fats and vegetable
oils. In addition to this, now a days tailored vegetable oils with R.M/ P.V and B.R close to that of milk
fat are available to the unscrupulous people in the unspecified market for adulteration purposes. To
counter this approach some new methods have been developed, though these methods are also not
fool proof, but can be handy in the testing laboratories.
Methodology
There are two approaches for the detection of adulteration of milk fat. First approach is based on
the classical methods like B.R reading, R.M- value, P.V – value, Phytosterol acetate test, Gas – liquid
chromatographic analysis. Second approach is based on some innovative and rapid methods like
furfural test for vanaspati, Opacity test, crystallization test, Number of carbons by GLC of triglycerides
, color based test for vegetable oil detection, apparent solidification time test and complete liquification
time test. In all the cases, tests are applied on the extracted fat, accept the modified Gerber test, where
aspecially designed dual purpose Gerber butyrometer is used and B.R reading of the fat is measured.
Hence, the first step is to isolate the fat and then apply the test (Kumar et al, 2002). In this article the
following methods have been discussed.
1. Detection of animal body fats and vegetable oils/fats by the Opacity Test
Melt the sample of fat (5 gm) isolated by heat clarification method at 50 +1ºC in a test tube and
maintain for 3 min to equilibrate. Then transfer the test tube at 23 + 0.2ºC water bath and record the
opacity time (Time taken by fat sample to acquire either O.D. at 570 nm between 0.14-0.16 or Klett
reading using red filter between 58-62 after adjusting the instrument to 100% transmittance). The
opacity time of pure buffalo ghee is 14-15 min, cow ghee is 18-19 min and that of ghee from cotton tract
area is 11-12 min. The opacity time of buffalo ghee adulterated at 10% level with vanaspati is 10-11
min, with pig body fat is 8-9 min, with buffalo body fat is 2-3 min, with cow body fat is 3-4 min and
with refined oils is 20-25 min (Singhal, 1980).
2. Detection of vanaspati in ghee
Isolate the fat from milk by heat clarification method. Take about 5 g of the melted fat in a test
tube. Add 5 ml of concentrated HCl. Add 0.4 ml furfural solution (2% in alcohol) and shake the tube
thoroughly for 2 min. Allow the mixture to separate. The development of pink or red colour in the acid
layer indicates presence of vanaspati. Confirm by adding 5 ml distilled water and shaking again. If the
colour in acid layer persists, vanaspati is present. If the colour disappears, it is absent [SP:18 (1987)].
3. Detection of vegetable oils by B.R. Reading
Clean the prisms of the Butyro-refractometer with petroleum ether. Allow the ether to evaporate
54
New Approaches to Detect the Adulteration of Ghee with Animal Body Fats and Vegetable Oils/ Fats
to dryness. Maintain temperature of the prisms at 40ºC by circulating water. Calibrate the B.R.
apparatus by applying a drop of fluid of known B.R. and adjusting B.R. by moving the adjustment
screw. Clean the prisms. Apply a drop of sample of clear fat obtained by any of the three methods
between the prisms. Wait for 2 min before taking the reading so that sample should attain the constant
temperature of about 40ºC.
B.R. reading decreases and increases with the rise and fall of temperature, respectively. Normally,
the temperature of observation should not deviate by more than 2ºC. A correction of 0.55 is added to
the observed B.R. reading for each degree above 40ºC or subtracted for each degree below 40ºC to get
corrected B.R. reading of the sample.
If fat is isolated by the Gerber method, B.R. is depressed due to hydrolytic effect of H2SO4 on the
fat. Therefore, observed B.R. reading is corrected as follows:
Corrected B.R. = 1.08 x observed B.R.
B.R. reading of milk fat isolated by one of the above mentioned methods should be consistent
with the values given for ghee as per PFA requirement. Any deviation from the standard value
indicates adulteration of milk with vegetable oils. However, this method has limitation of detection
of adulteration with two oils i.e. coconut oil and palm oil whose values are close to that of milk fat
(Arora et al, 1996).
4. Detection of animal body fats and vegetable oils by crystallization test
Take 0.8 ml of melted fat in a stoppered test tube (10 x 1.0 cm internal diameter). Add 2.5 ml of
solvent mixture consisting of acetone and benzene (3.5:1.0). Mix the contents slowly. Place the test
tube in a water bath maintained at 20ºC for 3 min to equilibrate the temperature. Then transfer the
tube in another water bath maintained at 17 + 0.2ºC till the onset of crystallization. Note the time for
occurrence of crystallization. The crystallization time of pure buffalo ghee is 18-20 min and that of
cotton tract ghee is 10.5-12.5 min, whereas that of buffalo ghee adulterated at 10% level with pig body
fat is 11.5-12.5 min, with cow body fat 4.5-5.5 min and buffalo body fat 3.0-4.0 min, and with vegetable
oils is 26 to 36 min (Panda, 1996).
5. Detection of adulteration of vegetable oils in ghee by iodine value
Iodine value, which is a measure of extent of unsaturation of fat, can be determined by the Wij’s
method as described in SP:18 (Part XI)1981. This property is particularly useful for detection of
adulteration in ghee with vegetable oils, as these oils have higher iodine values than milk fat and body
fats. It can be measured, as follows:
Accurately 0.4 g of sample is weighed in a clean and dry iodine flask and is dissolved in 15 ml of
carbon tetrachloride. Then 25 ml of the Wij’s reagent are added and the flask is stoppered. The contents
are then mixed and kept in dark for one hour. After one hour, 20 ml of 10 per cent potassium iodide
solution and about 150 ml of distilled water are added to the iodine flask and mixed. The contents are
titrated against 0.1 N sodium thiosulphate solution using starch solution as an indicator. A blank test
is also carried out using the same quantities of the reagents. From this, the iodine value is calculated
as follows:
Iodine Value = 12.69 (B – S) N / W
Where;
B = Vol. (in ml) of standard sodium thiosulphate solution required for the blank
S = Volume (in ml) of standard sodium thiosulphate solution required for the sample
N = Normality of the standard sodium thiosulphate solution, and
W = Weight (in g) of the sample taken for the test
The iodine values for cow and buffalo pure ghee ranges between 30.12 to 40.26. Any deviation
from these values indicates adulteration (Kumar, 2008).
55
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
6. Detection of adulteration by apparent solidification time (AST) test.
The apparent solidification time (AST) of the fat sample is defined as the time taken by the melted
fat sample to get solidified apparently at a particular temperature. The test can be carried out as:
Take 3.0 gm of completely melted fat sample in a test tube (10 × 1.0 cm ID) and maintain at 60°C for
5 min. Transfer the test tube in a refrigerated water bath maintained at 18 ± 0.2°C and simultaneously
start the stop watch. Observe the test tube constantly till the apparent solidification of the fat sample
takes place which is confirmed by non- movement of fat sample on tilting the test tube. At this stage
stop the stopwatch and record the time taken for the apparent solidification of the fat. Pure ghee
sample of both cow and buffalo shows AST in the range of 2 min 31 sec to 3 min 25 sec. Any deviation
from these values gives an indication of adulteration of milk fat (Kumar, 2009)
7. Detection of adulteration using dry fractionation technique coupled with AST
By employing dry fractionation technique, the different fractions enriched with body fats or
vegetable oils are obtained and subsequently used to estimate AST. The aim is to enrich the solid
fraction with animal body fats and liquid fraction with vegetable oils. Vanaspati, if added, will also be
fractionated along with animal body fats.
Take 100 gm of clarified melted fat and keep it in a BOD incubator maintained at 20 ± 0.1°C. After
about 1.50 to 1.75 h of incubation, approximately one third of the whole fat gets solidified. Separate the
solid fraction (S20) from the remaining liquid portion by filtration inside a BOD incubator maintained at
20 ± 0.1°C. Further fractionate the liquid portion thus obtained in another BOD incubator maintained
at 18 ± 0.1°C. for 2 hr so as to obtain another solid (S18) and liquid (L18) fraction by filtering inside a
BOD incubator maintained at 18 ± 0.1°C. Analyze S20, S18 and L18 fractions of ghee for AST as described
above. S20, S18 and L18 fractions of pure ghee of both cow and buffalo show AST values of 1 min 40
sec to 2 min 50 sec; 2 min 30 sec to 3 min 40 sec and 2 min 50 sec to 3 min 50 sec, respectively. Any
deviation from these values gives an indication of adulteration (Kumar, 2003).
8. Detection of adulteration by complete liquification time (CLT) test
The complete liquification time (CLT) test of the fat sample is defined as the time taken by the
solidified fat sample to get melted completely at a particular temperature. The test can be performed,
as follows:
Take 3.0 gm of completely melted fat sample in a test tube (10 × 1.2 cm) and maintain at 60°C for 5
min. Keep the test tube containing fat sample in a refrigerator (6- 8ºC) for 45 min for solidification of the
melted fat sample. Transfer the test tube in a water bath maintained at 44 ± 0.1ºC and simultaneously
start the stop watch. Observe the test tube constantly till the fat sample is completely liquefied. At
this stage stop the stopwatch and record the time taken for complete liquification of the fat. Pure ghee
sample of both cow and buffalo shows CLT in the range of 2 min 12 sec to 3 min 15 sec. Any deviation
from these values gives an indication of adulteration of milk fat (Kumar, 2008).
9. Detection of adulteration using solvent fractionation technique coupled with CLT and Iodine value
Using solvent fractionation technique, the different fractions enriched with body fats or vegetable
oils can be obtained and used subsequently to estimate CLT. Here also, the aim is to concentrate
animal body fats in to solid fraction and vegetable oils into liquid fraction. Vanaspati, if added, will
also be concentrated in solid fraction along with animal body fats.
Take 30 gm of melted ghee sample in a 100 ml graduated glass tube, and then add 60 ml acetone
and mix well to dissolve the fat. After mixing, keep the sample at 40°C for equilibration for 5 min. Then
subject the sample in a refrigerated water bath to three temperatures/time combinations, viz., 16 ±
0.1°C/25 min, 8 ± 0.1°C/25 min and 4 ± 0.1°C/60 min, successively, after filtration at each stage of time/
temperature combination. After about 25 min at 16 ± 0.1°C, approximately one-fourth of the whole
fat gets solidified. This first solid fraction (S16) obtained at 16 ± 0.1°C is separated from the remaining
liquid portion (L16) of the whole fat by filtration through ordinary filter paper. The remaining liquid
portion (L16) thus obtained after filtration is further fractionated at 8 ± 0.1°C. in refrigerated water bath.
56
New Approaches to Detect the Adulteration of Ghee with Animal Body Fats and Vegetable Oils/ Fats
After about 25 min, it gets partitioned into two fractions, one solid (S8) and one liquid (L8), which can
be separated by filtration through ordinary filter paper. At last, L8 fraction is further fractionated at
4 ± 0.1°C for 60 min and filtered to get two fractions, one solid (S4) and one liquid (L4). Finally at the
end of fractionation, three solid fractions (S16, S8 and S4) and one liquid fraction (L4) are obtained from
ghee sample containing a mixture of adulterants. Solvent from liquid fraction is removed by using
rotary evaporator at about 40ºC, followed by nitrogen flushing to evaporate solvent completely from
the liquid fraction. To get rid of entrapped acetone, respective solid fractions are heated to 110ºC for
about 2 hr in an oven.
(a) Analysis of first fraction (S16) for CLT at 46ºC
Analyse S16 fraction for CLT at 46 ± 0.1ºC (instead of 44± 0.1ºC used for CLT of whole fat) as
described above. CLT values of S16 fraction at 46ºC range between 4 min 5 sec to 9 min for both cow
and buffalo pure ghee. Any deviation from these values gives an indication of adulteration of milk fat
(Kumar, 2008).
(b) Analysis of last fraction (L4) for Iodine value
Analyse L4 fraction for iodine value as described above. The iodine values for L4 fraction of pure
cow and buffalo ghee are found to vary between 37.85- 46. 48. Any deviation from these values gives
an indication of adulteration of milk fat (Kumar, 2008).
10. Detection of liquid paraffin in milk fat
Isolate the fat from milk by heat clarification method as described above. Saponify 1 g of fat taken
in a test tube with 5 ml of 0.5 N ethanolic KOH solutions by heating on direct flame, using wire gauge
for 5 min. Add about 5 ml of distilled water to the hot saponified solution. Appearance of turbidity
indicates the presence of mineral oil (Kumar, 2005)
11. Rapid color based test for detection of vegetable oils
One ml of clear molten fat was dissolved with 1.5 ml of hexane in a tightly capped test tube. To this
was added 1.0 ml of color developing reagent (distilled water, Sulphuric acid - Sp.gr.1.835 and Nitric acid
- Sp. gr. 1.42 in the ratio of 20:6:14), shaken vigorously and kept undisturbed till it is separated into two
layers. The appearance of a distinct orange tinge in the upper layer indicates the presence of vegetable
oils / fats including vanaspati (Sharma et al., 2007).
12. Detection of adulteration of rice bran oil in ghee
Rice bran oil contains gamma oryzanol, which can be used as a marker for the detection of its addition
to ghee. It can be done by thin layer chromatographic method as well as colorimetric method.
a)
Thin layer chromatographic method
A simple thin layer chromatographic method can be employed to detect the adulteration of ghee
with rice bran oil, as follows:
Gamma oryzanol is extracted from 10.0 gm of molten fat using 20.0 ml of a solvent system
consisting of methanol: water (9:1). The contents are vortexed for 2 min and centrifuged at 2000 rpm. /
10 min. The alcohol layer is drawn. Extraction protocol is repeated thrice and all the alcoholic extracts
are combined and evaporated at 60 – 70°C in a rotary evaporator. The residue is finally dried. The
dried residue is redissolved in 0.5 ml of developing solvent (toluene: ethyl acetate: methanol 90:8:2;
v/v) and 5-10 µl were applied on silica gel TLC plate and plates are developed in the developing
solvent. Properly developed plates are removed from the chamber and air dried followed by spraying
with color developing reagent (50% sulfuric acid) and heating at 120°C/ 10 - 15 min. Presence of the
gamma oryzanol band confirms the adulteration of rice bran oil in milk fat. Addition of rice bran oil in
ghee at 5% level is easily detected by this method. (Kumar, et al., 2008).
b)
Colorimetric method
Take 1ml of melted ghee sample in a dry test tube. Add 1.5 ml of hexane to dissolve the fat. Then,
in sequence, add 0.5 ml of dilute (25%) hydrochloric acid and 0.5 ml of 5% sodium nitrite solution
57
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
and mix, followed by the addition of 1 ml of 10% sodium hydroxide solution. Rice bran oil produces
orange-red color while other vegetable oils produce no color. Hence, this method is specific for the
detection of rice bran oil in ghee. As low as 2% rice bran oil added in ghee, can be detected by this
method.
13. Detection of beef body fat and margarine in butterfat by differential scanning calorimetry:
In this case melting and crystallization curves of different fats are studied by cooling fats from
70°C to - 40°C ( Aktas and Kaya, 2001). Based on new endothermic peak, more than 10 percent goat
body fat in ghee could be detected qualitatively with the help of melting diagram and determined
quantitatively from crystallization diagram. The method, however, failed to detect coconut oil, cotton
tract ghee and other animal body fats.
14. Temperature controlled attenuated total reflectance- mid- infrared (ATR-MIR) spectroscopy:
This is a spectroscopic technique used for the rapid estimation of butter adulteration. The
methodology is typically based on the infra red spectroscopic technique ( Koca et al., 2010). These
workers collected the Fourier transform infrared spectra of the samples between 4000 and 650 cm-1 on
a FTIR spectrophotometer. Here the temperature was controlled, which allowed the stabilization of
analysis temperature at 65± 2°C. The data was analyzed by using statistical tool namely Multivariate
data analysis and calibration models were developed covering all possible adulteration ratios. In this
case adulteration of butter with margarine @ 2.5% could be predicted.
15. Tryacyl glycerol analysis by evaporative light scattering detection (ELSD):
This method is an HPLC method where major and minor triglyceride species could be sparated in 33
min using reverse phase C18 column, ELSD and mbile pahse ( Dichloromethane: Acetonitrile) in a gradient
mode.
16. Analysis of triglycerides by GLC:
This method is based on the principle of specific distribution of fatty acid moieties on the glycerol
backbone. Tryglycerides of 28 - 54 carbons are identified and quantified. The data generated is analysed
by using multi variant analysis. The detection limit varied according to the source of fat added and
found to be < 10% ( Gutierrez et al., 2009).
References:
Aktas.N and Kaya.M ( 2001) Detection of beef body fat and margarine in butter fat by differential scanning calorimetry.
Journal of Thermal Analysis and calorimetry. 66. 795- 801.
Arora, K.L.; Lal. D, Seth. R and Ram, J. (1996). Platform Test for detection of refined mustard oil adulteration in milk.
Indian Journal of Dairy Sci., 49(10): 721-723.
Gutierrez.R; Vega.S; Daiz. G; Sanchez.J; Coronado.M; Ramirez.A; Perez.J; Gonzalez.M and Schettino.B ( 2009) Detection
of non milk fat by gas chromatography and linear discriminate analysis. J. Dairy Sci. 92: 1846- 1855.
ISI (1981). Handbook of Food Analysis. IS: SP:18, Part XI. Dairy Products. Bureau of Indian Standards, New Delhi.
Koca.N; Kocaogulu-Vurma.N.A; Harper.W.J; Rodriguez-Saona. L.E ( 2010) Application of temperature controlled
attenuated total reflectance – mid- infrared (ATR-MIR) spectroscopy for rapid estimation of dutter adulteration.
Food Chemistry. 121: 778- 782.
Kumar.A; Lal.D; Seth.R and Sharma.R (2002) Recent trends in detection of adulteration in milk fat – A Review. Indian
J Dairy Sci., 55 (6): 319 - 330.
Kumar. A, Lal, D, Seth, R and Sharma. V (2005) Turbidity test for detection of liquid paraffin in ghee. Indian J Dairy
Sci., 58 (4): 298.
Kumar. A; Sharma. V and Lal.D (2008) Development of a thin layer chromatography based method for the detection of
rice bran oil as an adulterant in ghee. Ind. Journal . Dairy Sci. 61,2: 113 – 115.
Kumar. Amit; (2008) Detection of adulterants in ghee. Ph. D thesis submitted to NDRI, Karnal (Deemed University).
Kumar. A; Ghai, D. L; Seth, R and Sharma, V (2009) Apparent solidification time test for detection of foreign oils and
fats adulterated in clarified milk fat, as affected by season and storage. International J . Dairy Tech. 62: 33 –38.
Lal, D.; Seth, R.; Arora, K.L. and Ram, J. (1998) Detection of vegetable oils in milk. Indian Dairyman., 50(7): 17-18.
Panda, D.K. (1996). Detection of adulteration of foreign fats in milk fat. M.Sc. thesis, submitted to N.D.R.I. Deemed
University, Karnal.
Sharma. V; Lal, D and Sharma. R. (2007) Color based platform test for the detection of vegetable oils/fats in ghee. Ind.
Journal . Dairy Sci. 60,1: 16 – 18.
Singhal, O.P. (1980). Adulteration & Methods for detection. Indian Dairyman, 32: 771-774.
SP:18 (1987). Handbook of Food Analysis Part XI, Dairy Products. Bureau of Indian Standards, Manak Bhawan, New
Delhi.
58
Colostrum Powder and its Health Benefits
Colostrum Powder and its Health Benefits
Raman Seth and Anamika Das
Dairy Chemistry Division, NDRI, Karnal
Introduction
Since the time immemorial, man has sought some alternative methods to enhance and improve the
immune system of human body in order to fight against diseases. Historically, Ayurvedic physicians
have used bovine colostrum for therapeutical application in Asia, particularly in India for thousands of
years. Increased awareness of the diet - health relationship in many countries has stimulated a trend in
nutrition science whereby more attention is given to the health effects of individual foods. Colostrum
is the first lacteal secretion from the mammary glands after parturition during the first 24-72 hours.
Colostrum is a complex fluid rich in nutrients and is also characterized by its high level of bioactive
components e.g. immunoglobulins (Igs), particularly IgG1, growth factors, i.e. insulin like growth
factors-1, transforming growth factor β2 and growth hormone in addition to lactoferrin, lysozyme and
lactoperoxidase. Because of its poor heat stability, colostrum is an under utilized product in the dairy
industry. Heat processing may affect the functionality of bioactive components present in colostrum.
Knowledge concerning the influence of processing and isolation procedures on bioactive compounds
in colostrum based products is, however, limited. Due to less heat stability of colostrum, its addition
in raw milk affects further processing. Colostrum addition to milk causes elevated protein and mineral
content which might render milk unsuitable for certain dairy processing operations such as UHT
or milk powder production. But, during the past three decades, there has been increased interest in
human consumption of bovine colostrum or its supplemented products based on the prophylactic and
immuno-therapeutic benefits of absorbed immunoglobulin especially IgG Thus, colostrum has been
processed into products designed for pharmaceutical and nutraceutical purposes which provide the
consumer with an identified health benefit over basic nutritional value. Internationally, colostrumderived products have become valuable niche products and are currently being sold into highly
competitive markets with current focus on protein components because of their physiological effects
and hence their commercial value.
Processes involved in drying of colostrum powder
Low-heat pasteurization
The high-heat pasteurization and drying processes used by many producers of colostrum powders
can denature the sensitive PRPs and IgG proteins in colostrum. Only low-heat flash pasteurization
and low-heat indirect drying can be used to preserve the efficacy and bioactivity of colostrum. Using
flash pasteurization (161ºF or 72ºC for 15 seconds) all potentially harmful pathogens are removed,
while immunoglobulins and other biologically important proteins retain their bioactivity.
Low-pressure processing
Similar to high-heat processing, high-pressure processing of colostrum will denature proteins and
reduce the bioactivity of the finished product. So low pressure processing is applied to manufacture
colostrum powder.
Indirect steam drying
Colostrum is spray dried using indirect steam and with low pressure and temperatures (less than
145°F or 63ºC) to produce a high quality powder while protecting the colostral proteins. Toxic nitrogen
oxides components produced in direct fired dryers used by other manufacturers are not produced in
indirect steam dryer.
Freeze drying (lyophilization)
Freeze-drying (lyophilization) has been one of the most useful methods for producing high quality
colostrum powder from colostrum. However, lyophilization has high capital and process costs. Freeze59
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
drying works by freezing colostrum and then reducing the surrounding pressure and adding enough
heat to allow the frozen water in the material to sublime directly from the solid phase to the gas
phase.
Composition of colostrum powder
Often colostrum powder is priced on the basis of IgG content. Standardized colostrum powders
are available having 10 and 40 percent IgG levels and from 45 to 80 percent minimum protein levels.
Typical composition of colostrum powder is as depicted below
Immune factors
Immunoglobulins
Immunoglobulins (Igs) are a family of globular proteins with antimicrobial and other protective
bioactivities. They exist at different concentrations
in milk and colostrum. Qualitative and quantitative Chemical Composition
4-5%
differences are dependent on species, and are Moisture
0.5-1.5%
found in various isotypes, with immunological Fat
activities that are dependent on the Ig class. The Protein
55-80%
Igs are the principal agents that protect the gut Lactose
15-23%
mucosa against pathogenic microorganisms. IgG Ash
5-7%
antibodies express multifunctional activities, IgG
14-55%
including complement activation, bacterial
Physical properties
opsonisation and agglutination, and act by
Colour
Creamy, yellow
binding to specific sites on the surfaces of most
Free flowing spray dried
infectious agents or products, either inactivating Appearance
powder
them or reducing infection. In bovine colostrum
Bulk Density
0.4 – 0.5 g/ml (when packed)
and milk, immunoglobulin G (IgG; subclasses
(25 g)A Disc
IgG1 and IgG2) is the major immune component, Sediment
although low levels of IgA and IgM are also Microbiological properties
<50
present IgG1 constitutes approximately 80% Standard Plate Count
(1
g)
of the total Ig content of bovine milk. IgG is a
Not detected
monomeric glycoprotein consisting of two heavy Coliforms (1 g)
Not detected
(long) polypeptide chain of 53 KDa and two Coagulase +ve S.
aureus (1 g)
light (short) polypeptide chains of 23 KDa that
Absent
are linked by disulfide bonds. The polypeptide Salmonella (25 g)
chains contain both constant (Fc) and variable Yeast and moulds (1 g) <50
(Fab) regions of amino acid sequence, with E. coli (1 g)
Not detected
the antigen-binding sites located in the Fab Listeria Species (25 g) Absent
N-terminal region.
The concentration of immunoglobulins in colostrum and normal milk
Lactoferrin
Lactoferrin is an 80 kDa iron-binding
Immunoglobulin Colostrum (g/L) Normal milk(g/L)
glycoprotein present in colostrum and shows
IgG1
52. 0 – 87.0
0. 31 – 0 .40
antiviral,
antibacterial,
anti-inflammatory
IgG2
1.6 – 2 .1
0. 03 – 0 .08
properties. The concentration of lactoferrin in
3.7 – 6 .1
0.03 – 0 .06
bovine colostrum and mature milk is about 1.5-5 IgM
3. 2 – 6 . 2
0.04 – 0 .06
mg/mL and 0.1 mg/mL respectively. Lactoferrin IgA
has been implicated in the treatment of diseases
like cancer, HIV, herpes, chronic fatigue, Candida albicans and other infections. Lactoferrin’s affinity
for iron is very high (about 260 times that of blood serum transferrin). The cDNA for bovine lactoferrin
has been isolated. and the deduced amino acid sequence (708 amino acids) is homologous with human
60
Colostrum Powder and its Health Benefits
lactoferrin (68%) and human transferrin (60%) another iron-binding protein predominantly present in
serum. Lactoferrin has been shown to inhibit the growth of several microbes, including E.coli, Salmonella
typhimurium, Shigella dysenteria, Listeria monocytogenes ,Streptococcus mutans, Bacillus stearothermophilus
and Bacillus subtilis. In a recent study it was shown that human and bovine lactoferrin and their
N-terminal peptides were germicidal against Giardia lamblia in vitro. It has been proposed that the
antimicrobial effect of lactoferrin is based on its capacity to bind iron, which is essential for the growth
of bacteria.. Lactoferrin exerts its antimicrobial activity by modifying bacterial cell membranes. In
addition to its antibacterial activity, lactoferrin has antiviral effects against herpes simples virus type-l
(HSV-1) human immunodeficiency virus-l (HIV-l) and human cytomegalovirus in vitro. Lactoferrin
plays a role in iron uptake in the intestine. and the activation of phacocytes and immune responses.
Receptors for lactoferrin are found on intestinal tissues, monocytes, macrophages, neutrophils,
lymphocytes, platelets and on some bacteria .Studies have shown that lactoferrin can bind DNA and
activate transcription, which might explain the molecular basis of growth regulation.
Lysozyme
Lysozyme is a well-known antibacterial and lytic enzyme present in many mammalian body
fluids, including colostrum. The concentration of lysozyme in colostrum and in normal milk is about
0.14-0.7 and 0.07-0.6 mg/L, respectively. The natural substrate of the enzyme is the peptidoglycan
layer of the bacterial cell wall and its degradation results in lysis of the bacteria. Some recent results
suggest that the antibacterial activity of lysozyme is not only due to its enzymatic activity, but also to
its cationic and hydrophobic properties The presence of lactoferrin enhances the antibacterial activity
of lysozyme against E.coli, which also supports the hypothesis that lactoferrin damages the outer
membrane of Gram-negative bacteria.
Lactoperoxidase
Lactoperoxidase is a major antibacterial enzyme in colostrum. Bovine colostrum and milk contain
about 1l-45 mg/L and 13-30 mg/ L lactoperoxidase, respectively.It is a basic glycoprotein containing
a heme-group with Fe3+ and catalyzes the oxidation of thiocyanate (SCN-) in the presence of hydrogen
peroxide (H2O2), producing a toxic intermediary oxidation product. This product inhibits bacterial
metabolism via the oxidation of essential sulphydryl groups in proteins. The lactoperoxidase system is
also toxic to other Gram-positive and Gram negative bacteria such as Pseudomonas aeruginosa,Salmonella
typhimurium,Listeria monocytogenes, Streptococcus mutans, Staphylococcus aureus and psychrotrophic
bacteria in milk. Lactoperoxidase system inactivates polio virus and human immunodeficiency virus
type 1 in vitro. The single peptide chain (612 amino acids) includes 15 half-cystines and 4- 5 potential
N-glycosylation sites and the heme group is suggested to bind to the peptide chain via a disulphide
linkage. Bovine lactoperoxidase also contains a site with high affinity for calcium. The lactoperoxidase
is partly activated by forming a complex with lysozyme and this interaction appears to be quite specific.
The lactoperoxidase system and lactoferrin have been shown to have an additive but not a synergistic,
antibacterial effect against Streptococcus mutans.
Proline-Rich Polypeptides (PRP)
A hormone that regulates the thymus gland, stimulating an underactive immune system or
down-regulating an overactive immune system as seen in autoimmune disease(Multiple sclerosis,
rheumatoid arthritis, lupus, scleroderma, chronic fatigue syndrome, allergies, etc.). PRP stimulates
immature thymocytes to turn into functionally active T-cells. Studies revealed that the addition of PRP
isolated from colostrum led to the inhibition of vesicular stomatitis virus (VSV) replication in resident
peritoneal cells. Furthermore, PRP acts as an immuno regulator by changing surface markers and
functions of cells .It is a mixture of peptides(polypeptide-clostrinin) derived from colostrum which
could help to slow the progression of Alzheimer’s disease by reducing the build-up of beta amyloid, a
toxic protein that accumulates in the brains of Alzheimer’s sufferers.
61
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Growth factors
Growth factors are so called because historically they have been identified by their ability to
stimulate the growth of various cell lines in vitro but, in reality, the functions of these peptide based
molecules are considerably more diverse.
Concentration ranges in growth factors reported for bovine colostrum and milk
Epidermal growth factor
EGF is a 53–amino acid peptide produced by
Concentration
the salivary glands and the Brunners glands of the
(ng/ml)
Growth factors
duodenum in adults. In vitro experiments using
Colostrum
milk
gastric juice from preterm infants indicate that milk4 – 325
1- 150
borne EGF is not deactivated under typical gastric Epidermal growth factor
Betacellulin
<
5
<5
proteolytic conditions. In contrast, we showed
100 - 2000 5-100
that adult gastric juice digests EGF to an EGF form Insulin like growth –I
that has only 25% of the biological activity of the Insulin like growth factor-II
150 – 600
5-100
intact EGF molecule. Once EGF enters the small Transforming growth factor-β1 10 – 50
<5
intestine, it is susceptible to proteolytic digestion Transforming growth factor- β2 150 -1150
10-70
under fasting conditions but is preserved in Fibroblast growth factor
NAb
<1
the presence of ingested food proteins . There
Platelet-derived growth factor
NA
NA
is controversy over the physiologic function of
EGF in the gastrointestinal lumen under normal
(nondamaged) conditions. EGF acts as a “luminal surveillance peptide” in the adult gut, readily available
to stimulate the repair process at sites of injury. The EGF in colostrum and milk may therefore play a role
in preventing bacterial translocation and stimulating gut growth in suckling neonates.
Transforming growth factor α
In contrast with EGF, TGF- α is produced within the mucosa throughout the gastrointestinal tract.
Systemic administration of TGF- α stimulates gastrointestinal growth and repair, inhibits acid secretion,
stimulates mucosal restitution after injury, and Increases gastric mucin concentrations. Within the
small intestine and colon, TGF- α expression occurs mainly in the upper (nonproliferative) zones,
which suggests that its physiologic role may be to influence differentiation and cell migration rather
than cell proliferation. TGF-a may therefore play a complementary role to that of TGF-β in controlling
the balance between Proliferation and differentiation in the intestinal epithelium. Up-regulation of
TGF-α expression has been shown to occur in the gastrointestinal mucosa at sites of injury as well as
in the liver after partial hepatectomy, supporting a role for TGF-α in mucosal growth and repair. Other
findings support the role of TGF- α in maintaining epithelial continuity but suggest that the relative
importance of peptides such as this might vary from one region of the gut to another. Taken together,
most studies suggest that the major physiologic role of TGF- α is to act as a mucosal-integrity peptide,
maintaining normal epithelial function in the undamaged mucosa.
Transforming growth factor β
This family of molecules is structurally distinct from TGF- α and, in most systems, actually inhibits
proliferation. There are 5 different isoforms of TGF- β and their major site of expression in the normal
gastrointestinal tract is in the superficial zones, where they may inhibit proliferation once the cells have
left the crypt region. TGF- β has many diverse functions; it is a potent chemoattractant for neutrophils
and stimulates epithelial cell migration at wound sites. It is therefore likely to be a key player in
stimulating restitution, the early phase of the repair process during which surviving cells from the
edge of a wound migrate over the denuded area to reestablish epithelial continuity. TGF- β and TGF- β
-like molecules are present in high concentrations in both bovine milk (1–2 mg/L) and colostrum (20–
40 mg/L). These concentrations are sufficient to prevent indomethacin-induced gastric injury in rats,
suggesting that the TGF- β in colostrum may be a key component in mediating its ability to maintain
gastrointestinal integrity in suckling neonates.
62
Colostrum Powder and its Health Benefits
Platelet-derived growth factor
Platelet-derived growth factor (PDGF) is an acid-stable molecule that was originally identified
from platelets but is also synthesized and secreted by macrophages. It consists of 2 disulfide linked
polypeptides: chain A (14 kDa) and chain B (17 kDa). The dimer, therefore, exists in 3 isoforms (AA,
AB, and BB) that bind to tyrosine kinase–type receptors. PDGF is a potent mitogen for fibroblasts
and arterial smooth muscle cells and administration of exogenous PDGF has been shown to facilitate
ulcer healing when administered orally to animals. Although PDGF is present in bovine milk and
colostrum, most of the PDGF-like mitogenic activity in bovine milk is actually derived from bovine
colostral growth factor, which shares sequence homology with PDGF.
Insulin-like growth factors (somatomedins) and their binding proteins
IGF-I and IGF-II promote cell proliferation and differentiation and are similar in structure to
proinsulin and it is possible that they also exert insulin-like effects at high concentrations. Bovine
colostrum contains much higher concentrations of IGF-I whereas lowered concentrations is found
in mature bovine milk (10 mg/L). These growth factors are relatively stable to both heat and acidic
conditions. They therefore survive the harsh conditions of both commercial milk processing and
gastric acid to maintain their biological activity. IGF-I is known to promote protein accretion, ie, it is
an anabolic agent (50) and is at least partly responsible for mediating the growth-promoting activity of
growth hormone (GH). IGF-II is present in bovine milk and colostrum at much lower concentrations
than is IGF-I, but like IGF-I, it has anabolic activity and has been shown to reduce the catabolic state
in starved animals. IGFs in bovine colostrum and milk are present in both free and bound forms. The
amount of free IGF varies during the perinatal period, with most of the IGF-I in bovine colostrums
being present in the free form (ie, not associated with its binding protein), whereas the reverse is
true in the antepartum period and in mature milk. It was initially thought that the main function
of IGFBPs was to act as carrier proteins, reducing the proteolytic digestion of IGF and limiting its
biological activity because only the free forms of IGF are thought to have any major proliferative
activity. Additional roles for IGFBPs have been suggested because it has been shown that different
IGFBPs have distinct patterns of distribution in different tissues and their concentrations are altered in
response to hormonal or nutrient status.
The detailed functions of IGFBPs are unclear, although it is probable that one of the roles of
secreted or soluble IGFBP is to inhibit IGF-mediated proliferation or amino acid uptake by limiting
the availability of free IGF to bind to its receptors. Conversely, cell surface and cell matrix–associated
IGFBPs may potentiate the actions of IGF by increasing local concentrations of IGF-I and IGF-II next
to their receptors.
Clinical applications of colostrum
Gut related infections
Short-bowel syndrome
Some patients have an insufficient length of bowel to digest and absorb food adequately, usually
as a result of massive intestinal resection for vascular insufficiency or after repeated operations for
inflammatory bowel disease. Current therapeutic options are unpleasant and associated with a
high risk of morbidity or mortality, eg, long-term parenteral (intravenous) feeding and small-bowel
transplantation. Strategies to optimize the function of residual bowel and ultimately wean patients
off total parenteral nutrition would therefore be of great benefit. There is evidence that growth factors
could be instrumental in achieving this goal; e.g systemic administration of individual growth factors
such as EGF have been shown to stimulate bowel growth in rats receiving total parenteral nutrition. In
addition, oral administration of EGF helped restore glucose transport and phlorizin binding in rabbit
intestines after jejunal resection, and colostrum supplementation of piglet feeding regimens resulted
in a significant increase in intestinal proliferation. Colostrum supplementation may be of particular
value in young children who have undergone intestinal resection because gut adaptation is more
likely during early childhood than it is in adulthood.
63
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Nonsteroidal antiinflammatory drug–induced gut injury
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely prescribed and are effective in the
treatment of musculoskeletal injury and chronic arthritic conditions. Nevertheless, <2% of subjects
taking NSAIDs for 1y suffer from gastrointestinal adverse effects, including bleeding, perforation,
and stricture formation of the stomach and intestine. Acid suppressants and prostaglandin analogues
have been shown to be effective in reducing gastric injury induced by NSAIDs but are less effective in
preventing small intestinal injury. EGF and TGF-α and TGF-β have all been shown to reduce NSAID
induced gastric injury. It was shown recently that a defatted colostrum preparation, which is rich in
the growth factors discussed earlier, reduced NSAID-induced gastric and intestinal injury in rats and
mice. This material was also shown to effectively reduce gastric erosions in human volunteers taking
NSAIDs. Further support for this approach comes from our recent finding that this defatted colostrum
preparation reduced small intestinal permeability, which was used as a marker of intestinal damage in
human volunteers taking clinically relevant doses of the drug indomethacin. Clinical trials involving
patients taking NSAIDs long term are under way.
Chemotherapy-induced mucositis
Current regimens for the treatment of cancers require patients to take much higher doses of
chemotherapeutic agents than were used previously. As a result of these higher doses, toxic adverse
effects on the bone marrow and gastrointestinal tract can be the factor limiting the dose or duration
of treatment. Strategies to protect these tissues and encourage their recovery may facilitate the use
of higher doses of chemotherapy, with greater potential for cure. For example, EGF enhances the
repair of rat intestinal mucosa damaged by methotrexate , TGF-b ameliorates chemotherapy-induced
mucositis , and administration of a cheese whey–derived preparation reduces methotrexate-induced
gut injury in mice . Not all studies have shown favorable results, however, because EGF had only a
minor beneficial effect in reducing mouth ulceration in a phase I clinical study of patients undergoing
chemotherapy . If peptides with growth stimulatory or inhibitory effects are to be used, the timing of
administration is likely to be critical; growth-arresting factors might protect bone marrow or gut from
the damaging effects of chemotherapy, which tend to affect areas with the highest cell turnover, if given
before chemotherapy. In contrast, growth-stimulating factors might “rescue” recovery of injured areas
if administered after chemotherapy. This latter approach is already being used clinically, eg, colonystimulating growth factor is being used to stimulate bone marrow recovery after chemotherapy.
Inflammatory bowel disease
The etiology of ulcerative colitis and Crohn disease is unknown and, therefore, current treatment
of these severe, incapacitating conditions has to be on an empiric basis. Studies examining the effect of
administration of EGF, PDGF, TGF-b or IGF-I in animal models of colitis have had encouraging results
and a cheese whey growth factor extract containing several of these growth factors had positive results
in a similar model . Other peptides, not present in milk or colostrum in significant concentrations,
under study as potential therapeutic agents for these conditions include keratinocyte growth factor
and trefoil peptides. These studies are in the very early (animal model) stages and the agents are
unlikely to be in standard clinical use for many years. Milk-derived products are already in clinical use
for the treatment of inflammatory bowel disease; casein-based enteral feeds are used for the treatment
of Crohn disease and their efficacy might be due, in part, to the presence of MDGFs in the preparation,
which are preserved during the processing of the milk protein (see above). In addition, clinical trials
of the use of colostrum enemas for the treatment of ulcerative colitis and resistant proctitis are under
way and the results are awaited with interest.
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a severe life-threatening illness of young children that causes
severe ulceration of the small and large bowel. Its etiology is unclear, although there are many possible
risk factors, including prematurity, enteric infections, intestinal ischemia, and abnormal immune
responses. Although many proinflammatory molecules are likely to be involved in the etiology of
NEC, there is currently interest in the role of the phospholipid-mediator platelet activating factor
64
Colostrum Powder and its Health Benefits
(PAF), which is produced by intestinal flora and inflammatory cells during the development of NEC.
The finding that human colostrum contains the enzyme PAF acetylhydrolase, which degrades PAF,
might therefore be relevant in explaining why human milk feeds protect against the development
of NEC. These areas are discussed further by others (91–93). Although the molecular mechanisms
underlying the development of NEC are unclear, there is no doubt that once it is established, it is
associated with a very high mortality rate. Current treatment consists of general supportive measures
consisting of fluid-replacement and antibiotic therapy, although intestinal resection is often required.
There is therefore a need for novel therapeutic approaches, e.g. the use of peptides to stimulate the
repair process. Support for this idea comes from a recent case study in which a continuous infusion of
EGF resulted in a remarkable restorative effect on gut histology in a child with NEC.
Infective diarrhea
Hyperimmune milk or colostrum preparations have been shown to be of benefit in the prevention
and treatment of infection and to increase weight gain in both clinical and veterinary practice, eg,
vaccination of cows with specific viruses or bacteria to produce hyperimmune milk has been shown
to be beneficial in the prevention and treatment of enteropathic infections due to Escherichia coli and
rotavirus. The use of whole hyperimmune colostrum rather than specific antibodies purified from milk
or other sources has the added value of potentially stimulating the repair process (due to the presence
of growth factors) as well as facilitating the eradication of the infection by mechanisms involving
nonspecific antibacterial factors in colostrum and milk.
The ultimate antioxidant
Colostrum is rich in Glutathione, a powerful antioxidant which is often described as ‘the ultimate
antioxidant’. Antioxidants play an important part in overall good health and the prevention of disease,
by scavenging for free radicals which cause disease, muscle damage, and inflammation. It has been
shown that glutathione enhances athletic performance by increasing muscle strength, and increasing
the capacity to exercise before fatigue sets in. Oxidative stress in the form of training and exercise
contributes to muscle fatigue. Glutathione and its precursors present in colostrum, have been shown
to increase the capacity of exercise prior to the onset of fatigue.
Anti-inflammatory
Inflammation is associated with strenuous exercise and anti-inflammatories are the most commonly
prescribed class of drug to athletes. Inflammation is typically centered in the joints and in the digestive
tract. Inflammation is a protective response to an injury, invading foreign substance, or an internally
produced substance (e.g. in auto-immune disorders like rheumatoid arthritis). Colostrum reduces the
need for damaging medication, and because it is a natural food, unlike NSAIDS, it has absolutely no
negative side-effects and has a multitude of benefits.
Increased Brain Function
Phospholipids, components of alpha lipid, help in increasing brain function and have been
associated with improved memory. They have also shown to elevate moods and reduce the symptoms
of depression.
Viral illnesses
About 75% of the antibodies in the body are produced by the GI component of the immune system.
The ability of AIDS/HIV patients to fight infectious disease is severely compromised due to damage to
the gut from chronic inflammation and diarrhea. Recent studies report colostrum’s role in the reversal
of this chronic problem stemming from opportunistic infections like Candida albicans, Cryptosporidia,
rotavirus, Herpes simplex, Pathogenic Strains of E. Coli and intestinal flu infections. All gut pathogens
are handled well by colostrum without side effects.
Allergies and autoimmune diseases
PRP from colostrum can work as a regulatory substance of the thymus gland. It has been
demonstrated to improve or eliminate symptomatology of both allergies and autoimmune diseases
65
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
(Multiple Sclerosis, rheumatoid arthritis, lupus, and myasthenia gravis). PRP inhibits the overproduction
of lymphocytes and T-cells and reduces the major symptoms of allergies and autoimmune disease:
pain, swelling and inflammation.
Heart Disease
Colostrum PRP have a role in reversing heart disease very much like it does with allergies
and autoimmune diseases. Additionally, IgF-1 and GH in colostrum can lower LDL-cholesterol
while increasing HDL-cholesterol concentrations. Colostrum growth factors promote the repair
and regeneration of heart muscle and the regeneration of new blood vessels for collateral coronary
circulation.
Cancer
The cytokines like Interleukins 1, 6, 10, Interferon G and Lymphokines found in colostrum are
involved in the treatment of cancer. Colostrum lactalbumin has been found to be able to cause the
selective death (apoptosis) of cancer cells, leaving the surrounding non-cancerous tissues unaffected.
Lactoferrin has similarly been reported to possess anti-cancer activity. The mix of immune and growth
factors in colostrum can inhibit the spread of cancer cells.
Diabetes
Juvenile diabetes (TypeI, insulin dependent) is thought to be brought about through an autoimmune
mechanism, possibly initiated by an allergic reaction to the protein GAD found in cow’s milk.
Colostrum contains several factors, which can offset this and other allergies. Human trials reported
that IgF-1 stimulates glucose utilization, effectively treating acute hyperglycemia and lessening a Type
II diabetic dependence on insulin.
Helps in weight loss
IgF-1 is required by the body to metabolize fat for energy through the Krebs cycle. With aging, less
IgF-1 is produced in the body. Inadequate levels are associated with an increased incidence of Type
II diabetes and difficulty in losing weight despite a proper nutritional intake and adequate exercise.
Colostrum provides a good source of IgF-1 as a complementary therapy for successful weight loss.
Athletic stress
Exhaustive workouts and athletic competition can temporarily depress the immune system, decreasing
the number of T-lymphocytes and NK cells. Athletes are therefore, more prone to develop infections,
including Chronic Fatigue Syndrome. Many of colostrum’s immune factors can help significantly reduce
the number and severity of infections caused by both physical and emotional stress.
Leaky Gut Syndrome
One of the major benefits of colostrum supplementation is enhanced gut efficiency due to the
many immune enhancers that control clinical and subclinical GI infections. Colostral growth factors
also play a role by keeping the intestinal mucosa sealed and impermeable to toxins. Healing leaky gut
syndrome reduces toxic load and helps in the reversal of many allergic and autoimmune conditions.
For the healthy individual or athlete in training, colostrum supplementation enhances the efficiency
of amino acid and carbohydrate fuel uptake by the intestine. One of the reasons for the energy boost
seen in most healthy individuals who use colostrum as a food supplement is this ability of colostrum
to improve nutrient availability and the correction of subclinical leaky gut syndrome.
Wound healing
Several colostrum components stimulate wound-healing. Nucleotides, EGF, TGF and IGF-1
stimulate skin growth, cellular growth and repair by direct action on DNA and RNA. These growth
factors facilitate the healing of tissues damaged by ulcers, trauma, burns, surgery or inflammatory
disease. The tissues affected beneficially by colostrums wound healing properties are skin, muscle,
cartilage, bone and nerve cells. Powdered colostrum can be applied topically to gingivitis, sensitive
teeth, aphthous ulcers, cuts, abrasions and burns after they have been cleaned and disinfected.
66
Colostrum Powder and its Health Benefits
Conclusion
The world-wide trend towards the development of health-promoting foods offers interesting
opportunities for applications which contain specific antibody ingredients derived from immunised
cows. It is anticipated that colostrums based preparations may have remarkable potential to contribute
to human health care as part of health promoting diet and as an alternative or a supplement to the
medical treatment of specified human diseases. Bovine colostrum virtually contain all compounds of
human cellular and humoral immune defence. They are ideal sources of these defence molecules for
industrial production because of their ready availability and safety as compared with blood derived
analogues. The ongoing success of colostrums based products speaks for itself. However, the challenge
for manufacturers still remains as how to process colostrums in a cost effective way.
References
Blum, J. W., and Hammon, H. 2000. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and
metabolic parameters in neonatal calves. Livest. Prod. Sci., 66: 151.
Chen, C.C., Tu, Y.Y., and Chang, H.M. 2000. Thermal stability of bovine milk immunoglobulin G(IgG) and the effect of
added thermal protectants on the stability. J. Food Sci., 65: 188.
Donovan, S. M. and Odle, J. 1994. Growth factors in milk as mediators of infant development. Ann. Rev of Nutr., 14:
147.
Elfstrand, L., Lindmark-Mansson, H., Paulsson, M., Nyberg, L. and Akesson, B. 2002. Immunoglobulins, growth factors
and growth hormone in bovine colostrum and the effects of processing. Int. Dairy J., 12: 879.
Gapper, L., Copestake D., Otter, D., Indyk, H., 2007. Analysis of bovine immunoglobulin G in milk, colostrum and
dietary supplements: a review Anal Bioanal Chem.,389:93.
Godden, S. M., Smith, S., Feirtag, J. M., Green, L. R., Wells, S. J., and Fetrow, J. P. 2003. Effect of on-farm commercial
batch pasteurization of colostrum on colostrum and serum immunoglobulin concentrations in dairy calves. J. Dairy
Sci., 86: 1503.
Korhonen, H., Marnila, P. and Gill, H. S. 2000. Bovine milk antibodies for health. Br J Nutr., 84: S135.
Kurokowa, M., Lynch, K. and Podolsky, D. K. 1987. Effects of growth factors on an intestinal epithelial cell line:
transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochemical and Biophysical
Research Communications 142:775-182.
67
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Cow Ghee Protects from Mammary
Carcinogenesis: Mechanism
Vinod K. Kansal, Rita Rani and Ekta Bhatia
Animal Biochemistry Division, NDRI, Karnal
During the past several years, epidemiological studies have indicated the influence of environment
and life-styles on the development of certain forms of cancer. About 35 % of all cancer mortality in US may
be attributable to dietary factors. The association between dietary fat and cancer has been consistently
supported by experimental evidence. Limiting the intake of fats and oils reduces the risk of cancer. This
has now been expanded to limit saturated fat intake through reduction of animal fat intake.
Epidemiological associations between dietary fat intake and cancer in humans are highly
controversial. Some of this controversy stems from the limited ability to accurately assess total energy
and fat consumption, and the difficulty in assessing the effects of dietary fat independent of total
energy or micronutrient intake and other environmental factors such as physical activity.
A chorus of establishment voices, including the American Cancer Society, the National Cancer
Institute and the Senate Committee on Nutrition and Human Needs, claim that animal fat is linked not
only with heart disease, but also with cancers of various types. However, when researchers from the
University of Maryland analyzed the data, they found that vegetable fat consumption was correlated
with cancer, not the animal fat (Enig et al., 1978).
The mechanisms supporting a relationship between dietary fat and cancer can be classified as
either direct or indirect. Potential direct mechanisms include: 1) peroxidation of double bond in
PUFAs, leading to persistant oxidative stress and generation of reactive lipid peroxidation products
(malondialdehyde, 4-hydroxyalkenals), which induce DNA damage; 2) conversion of essential fatty
acids to eicosanoids (short lived hormone synthesized from n-6 unsaturated fatty acids); and 3)
interaction between fatty acids with signal transduction pathways leading to altered gene expression.
Potential indirect mechanisms include: 1) effect on membrane bound enzymes such as cytochrome
P450 (CYP) that regulate xenobiotic and estrogen metabolism; 2) structural and functional changes
in cell membranes that can alter the hormone activity and growth factor receptors; and 3) effects on
immune function.
Dietary fat and breast cancer
Breast cancer is the most commonly diagnosed cancer in women and is the leading cause of cancer
mortality in females in the world. There is strong positive correlation between fat intake and mortality
from breast cancer. It is likely that sex hormones especially estrogen, play a promotional role in breast
carcinogenesis, stimulating mitotic division of initiated cells and proliferation.
An increased amount of both vegetable and animal fat accelerates mammary tumor growth.
Different types of fat also have different effects on mammary tumorigenesis. Meta-analysis of 97
reports that studied the effects of dietary fatty acids on mammary tumor incidence and found: 1) n-6
polyunsaturated fatty acids have a strong tumor-enhancing effect; 2) saturated fats have weaker tumor
enhancing effects; 3) n-3 polyunsaturated fatty acids have a small non-significant protective effect; 4)
the effects of n-6 polyunsaturated fats are stronger than that of saturated fats even at low levels; and
5) there is no effect of monounsaturated fats on mammary carcinogenesis (Fay et al., 1997). A high fat
diet rich in n-6 polyunsaturated fatty acid in animal models could enhance metastasis of human breast
cancer cells (Rose et al., 1991).
Dairy products and breast cancer
The major hypotheses suggesting an increased risk of breast cancer risk associated with the
consumption of dairy product include: 1) a high consumption of dairy products results high dietary
68
Cow Ghee Protects from Mammary Carcinogenesis: Mechanism
fat intake particularly saturated fat which in turn has been associated with breast cancer risk; 2) milk
product may contain contaminants, such as pesticide that are potentially carcinogenic; and 3) milk
may contain growth factors, such insulin like growth factor 1 (IGF-1), which have been shown to
promote breast cancer cell growth.
The hypotheses suggesting inverse relation between dairy product consumption and breast cancer
risk have focused on the anticarcinogenic effects of vitamin D and calcium, conjugated linoleic acid
and butyric acid. Dairy products have high calcium content and are also a major dietary source of
vitamin D in countries where milk and other dairy products are fortified, such as the United States.
In breast cancer cell lines, vitamin D exerts antiproliferative effects by causing arrest in phase G0 /
G1 of the cell cycle (Colston and Hansen, 2002). The cellular functions of vitamin D are closely linked
to calcium. Calcium is a pivotal regulator of a wide variety of cellular functions, including cellular
proliferation and differentiation. Several investigations have shown that animals fed diet deficient in
calcium and vitamin D develops mammary hyperplasia and hyperproliferation (Lipkin and Newmark,
1999). Furthermore, animal studies have shown that supplementation with calcium and vitamin D
reduces the risk of mammary tumors in animals fed a high fat diet and prevents the development of
mammary tumors in animals induced with the carcinogen 7,12-dimethylbenz (a)anthracene (DMBA)
(Mehta et al., 2000).
A third potential mechanism to suggest that dairy products may reduce breast cancer risk involves CLA.
Animal studies suggest that CLA confers protection against the development of mammary tumors (Ip et al.,
1996). It is interesting to note that tumor formation was inhibited in animals fed CLA, regardless of the type
or amount of fat in their diets. Another compound found in dairy products, known to have protective effect
against mammary carcinogenesis is butyric acid.
Epidemiological studies
Most of the epidemiological studies showed no consistent pattern of increased or decreased breast
cancer risk with a high consumption of dairy products (Moorman and Terry, 2004). Two of the cohort
studies and 10 of the case-control studies investigated the association between breast cancer and butter
consumption and no consistent pattern was observed with reported butter intake. In a cohort study
conducted in Finland (Knekt et al., 1996), an inverse association that was not statistically significant
was reported; whereas a slight positive association was reported in a cohort study in the Netherlands
(Voorrips et al., 2002). In the case-control studies, odds ratios both > and < 1.0 were reported, but
generally differences between cases and controls were not statistically significant. Persons with a
high consumption of butter, cheese and other high-fat dairy products may also be more likely to
consume large amounts of meat or other high fat-foods that could also contribute to an increased risk
of breast cancer. Further, milk fat is rarely used in isolation from other dietary items, and other milk
components (milk protein, calcium, lactic acid bacteria) also have anticarcinogenic properties; hence it
is not possible to separate the effect of milk fat as such.
Animal studies
There are a few studies in which milk fat or butter was compared with vegetable oils or margarines
in animal models of carcinogenesis. The vegetable oils (soybean oil, sunflowers oil, corn oil, cotton oil)
were reported to enhance DMBA induced mammary adenocarcinomas more than butter and some
saturated fats (coconut oil, tallow, lard) in rodents (Carroll and Khor, 1971; Yanagi et al., 1989; Cope
and Reeve 1994). The milk fat was more effective when introduced in the diet at weaning (Klurfeld et
al., 1983).
The work done in this laboratory (Bhatia and Kansal, in press) showed that ghee (clarified butter fat)
opposed to soybean oil attenuated the gastrointestinal and mammary carcinogenesis. Gastrointestinal
carcinogenesis was induced by DMH in weanling male rats fed diet containing at 10% level of soybean
oil or buffalo ghee or cow ghee. The incidence was considerably higher in animal on soybean oil
(73.30%) than on cow ghee (55%) or buffalo ghee (40%). Tumor multiplicity and tumor volume were
less on ghee diets than on soybean oil, and cow ghee was more efficacious than buffalo ghee in reducing
69
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
tumor volume. Increased accumulation of CLA and decreased lipid peroxidation (measured by the
level of TARS) in liver and colorectal tissue on ghee opposed to soybean oil correlated with decreased
tumor incidence, tumor multiplicity and tumor volume on ghee diets. Similar dietary treatment was
given to weanling female rats and mammary carcinogenesis was induced by DMBA. Considerable
number of animal died in all dietary groups due to acute DMBA toxicity and mortality incidence was
greater on soybean oil than on ghee groups. Cow ghee opposed to soybean oil decreased the tumor
multiplicity, tumor volume and non-neoplastic disorders.
In recent studies (Rani and Kansal, 2010) we evaluated the mechanism of protective effect of
cow ghee versus soybean oil in DMBA induced mammary carcinogenesis rat model. Four groups of
female rats were fed for 44 weeks diet containing cow ghee or soybean oil. Mammary carcinogenesis
was induced with 7,12-dimethylbenz(a)anthracene (DMBA) given through oral intubation. The two
groups, which were not given DMBA but fed similarly, served as controls. The animals challenged
with DMBA developed tumors in both cow ghee fed and soybean oil fed rats. The tumor latency
period was greater on cow ghee (27 weeks) than on soybean oil diet (23 weeks). The tumor incidence
was considerably higher in animals on soybean oil (65.4%) than on cow ghee diet (26.6%). The tumor
volume and tumor weight were significantly less on cow ghee (1925 mm3, 1.67 g) than on soybean
oil diet (6285 mm3, 6.18 g). The progression of carcinogenesis was more rapid on soybean oil than on
cow ghee diet. While no adenocarcinoma was observed in cow ghee group, 8% of tumors in soybean
oil group were adenocarcinoma.
Cyclooxygenase-2 (COX-2) is a rate-limiting step in synthesis of prostaglandin E2, and the
excessive production of the latter promotes mammary carcinogenesis. The expression of COX-2 was
observed in DMBA treated rats, but not in untreated rats. The expression of COX-2 was significantly
greater on soybean oil than on cow ghee diet in tumor tissue as well as in uninvolved tissue of tumor
bearing animals (Rani and Kansal, 2010).
The cyclin A and D up regulate cell proliferation and promote tumerogenesis in carcinogen treated
animals. The expression of cyclin A in carcinogen treated rats was significantly greater in soybean oilfed rats than in cow ghee-fed rats, both in tumor bearing and no tumor bearing rats, and in tumor
tissue and in uninvolved tissue of tumor bearing rats. The expression of cyclin D was also significantly
greater on soybean oil diet than on cow ghee diet, both in control and carcinogen treated rats (Rani et
al., 2010)
The peroxisome proliferators activated receptor-γ (PPAR-γ) down regulates cell proliferation and
up regulates apoptosis, and thus prevents tumorogenesis. The expression of PPAR-γ was significantly
greater on cow ghee diet than on soybean oil diet, both in control and carcinogen treated rats. Further,
its expression was greater on cow ghee than on soybean oil both in tumor bearing and no tumor bearing
rats of treated animals (Rani and Kansal, 2010).
The Bax up regulates apoptosis and thus prevents progression of tumorogenesis. Its expression
was decreased in tumor tissue and uninvolved tissue of tumor bearing rats. However, the dietary
treatment with cow ghee or soybean oil has no effect on expression of Bax (Rani et al., 2010)
The expression of both bcl2 and PKC genes was not effected by dietary treatments with cow ghee
or soybean oil in control as well as in no tumor bearing treated animals. However, their expression
was significantly less on cow ghee than on soybean oil in tumor tissue as well as in uninvolved tissue
of tumor bearing rats (Rani et al., 2010)
Apoptotic signal decreased in tumor bearing animals. In uninvolved tissue of tumor bearing
animals the decrease was significantly more on soybean oil than on cow ghee, while in tumor tissue
the decline in apoptotic signal was similar on cow ghee and soybean oil. In control animals and no
tumor bearing animals, the apoptotic signal was not affected by dietary treatment with cow ghee or
soybean oil. Hence, cow ghee feeding decreases expression of genes involved in cell proliferation, and
increases apoptotic signal (Rani et al., 2010)
Most of carcinogens in nature occur in inactive form, and these are activated by cytochrome
70
Cow Ghee Protects from Mammary Carcinogenesis: Mechanism
P450 activities present in liver. Several enzymes present in liver and the target tissue detoxify the
active carcinogen. The balance of these two activities determines the active carcinogen present in the
body at a given moment. Feeding cow ghee opposed to soybean oil decreased carcinogen activating
cytochrome P4501A1, CYP1A2, CYP1B1 and CYP2B1 activities in liver. Further, feeding cow ghee
opposed to soybean oil also increased carcinogen-detoxifying activities, γ-glutamyltranspeptidase,
uridinediphospho-glucuronosyl transferase and quinone reductase in liver and mammary gland
tissue of control as well as DMBA treated rats (Rani and Kansal, 2011) .
The present study shows that compared to vegetable oil, cow ghee confers protection against
mammary gland carcinogenesis. The mechanism involves modulation of xenobiotic metabolism and
expression genes involved in cell proliferation and apoptosis. Nutraceutical importance of cow ghee
over vegetable oils in conferring protection against mammary cancer has been validated. This counters
the propaganda against dairy ghee and indiscriminate promotion of vegetable oil as health food.
References
Bhatia, E. 2005. Effects of dairy Ghee versus soybean oil on 1,2-dimethylhydrazinedihydrochloride induced
gastrointestinal tract carcinogenesis and lipid peroxidation in rats. Ind. J. Med. Res (in press)
Bhatia, E. and Kansal, V. K. (2010) Dairy Ghee opposed to soybean oil attenuates diet-induced hypercholesterolemia in
rats. Milchwissenschaft (Germany), in press
Carroll, K.K. and Khor, H.T. 1971. Effects of level and type of dietary fat on incidence of mammary tumors induced in
female Sprague-Dawley rats by 7,12-dimethylbenz[a] anthracene. Lipids, 6: 415-420.
Colston, K.W. and Hansen, C.M. 2002. Mechanisms implicated in the growth regulatory effects of vitamin D in breast
cancer. Endocr. Relat. Cancer, 9: 45-49.
Cope, R.B. and Reeve, V.E. 1994. Modification of 7,12-dimethylbenzanthra-cene (DMBA) / ultraviolet radiation (UVR)
co-carcinogenesis, UVR carcinogenesis and immune suppression due to UVR and cis urocanic acid by dietary fats.
Photochem. Photobiol., 59: 24S.
Enig, M.G., Munn, R.J. and Keeney, M. 1978. Dietary fat and cancer trends - a critique. Fed. Proc., 37(9): 2215-2220
Fay, M.P., Freedman, L.S., Clifford, C.K. and Midthune, D.N. 1997. Effect of different types and amounts of fat on the
development of mammary tumors in rodents : A review. Cancer Res., 57: 3979-3988.
Ip, C., Briggs, S.P., Haegele, A.D., Thompson, H.J., Storkson, J. and Scimeca, J.A. 1996. The efficacy of conjugated
linoleic acid in mammary cancer prevention is independent of the level or type of fat in the diet. Carcinogenesis.,
17: 1045-1050.
Klurfeld, D.M., Weber, M.M. and Kritchevsky, D. 1983. Comparison of semi-purified and skim milk protein containing
diets on DMBA-induced breast cancer in rats. Kiel. Milchwirtschaft. Forschun., 35: 421-422.
Knekt, P., Jarvinen, R., Seppanen, R., Pukkala, E. and Aromaa, A. 1996. Intake of dairy products and the risk of breast
cancer. Br. J. Cancer, 73: 687-691.
Lipkin, M. and Newmark, H.L. 1999. Vitamin D, calcium and prevention of breast cancer : A review. J. Am. Coll. Nutr.,
18: 392S-397S.
Mehta, R., Hawthorne, M., Uselding, L., Albinescu, D., Moriarty, R., Christov, K. and Mehta, R. 2000. Prevention of
N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats by γ-hydroxy vitamin D5. J. Natl. Cancer Inst.,
92(22): 1836-1840.
Moorman, P.G. and Terry, P.D. 2004. Consumption of dairy products and the risk of breast cancer: A review of the
literature. Am. J. Clin. Nutr., 80: 5-14.
Rani, R and Kansal, V. K. (2010) Dietary intervention of cow Ghee versus soybean oil on 7,12dimethylbenz(a)anthracene induced mammary carcinogenesis and expression of cyclooxygenase-2
and peroxisome proliferators activated receptor- γ in rats. Indan. Journal of Medical Research
(in press)
Rani, R., Kansal, V. K., Kaushal, D and De, D. (2010) Dietary intervention of cow Ghee and soybean oil on expression
of cell cycle and apoptosis related genes in normal and carcinogen treated rat mammary gland. Molecular Biology
Reports (Netharlands) DOI: 10.1007/S11033-010-0435-1
Rani, R and Kansal, V. K. (2010) Dietary intervention of cow Ghee versus soybean oil on 7,12-dimethylbenz(a)anthracene
induced mammary carcinogenesis and expression of cyclooxygenase-2 and peroxisome proliferators activated
receptor- γ in rats. Indan. Journal of Medical Research (in press)
Rose, D.P., Connolly, J.M. and Meschter, C.L. 1991. Effect of dietary fat on human breast cancer growth and lung
metastasis in nude mice. J. Natl. Cancer Inst., 83: 1491-1495.
Voorrips, L.E., Brants, H.A.M., Kardinaal, A.F.M., Hiddink, G.J., van den Brandt, P.A. and Goldbohm, R.A. 2002. Intake
of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer : The Netherlands
Cohort Study on Diet and Cancer. Am. J. Clin. Nutr., 76: 873-882.
Yanagi, S., Yamashita, M., Sakamoto, M., Kumazawa, K. and Imai, S. 1989. Comparative effects of butter, margarine,
safflower oil and dextrin on mammary tumorigenesis in mice and rats. In: The Pharmacological Effects of Lipids.
III. The role of Lipids in Cancer Research (J.J. Kabara, ed.). Lauricidin Inc., Galena, IL, pp.159-169.
71
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Lateral Flow Assay- Principle and its
Application in Analytical Food Science
Rajan Sharma and Priyanka Singh Rao
Dairy Chemistry Division, NDRI, Karnal
Introduction
The lateral flow assay (LFA), also called
the immunochromatographic assay or the strip
assay is a simple device intended to detect
the presence (or absence) of a target analyte
in sample (matrix). This technique is based
on an immunochromatographic procedure
that utilizes antigen–antibody properties and
enables rapid detection of the analyte. It includes
several benefits, such as a user-friendly format,
rapid results, long-term stability over a wide
range of weather conditions, and relatively
low manufacturing costs. These characteristics
render it ideally suited for on site testing by
untrained personnel. The main application of
Figure 1. Typical configuration of a lateral flow
this technology had been the human pregnancy
immunoassay test strip
test which came in picture in the 1970s. However,
to fully develop the lateral flow test platform,
a variety of other enabling technologies were also required. These include technologies as diverse
as nitrocellulose membrane manufacturing, antibody generation, fluid dispensing and processing
equipment, as well as the evolution of a bank of knowledge in development and manufacturing
methodologies. Many of these facilitative technologies had evolved throughout the early 1990s, the
first lateral flow products were introduced to the market in the late 1980s. Since then, as of 2010, over
200 companies worldwide are producing a range of testing formats. The world market for LF-based
tests (Rosen, 2009) is estimated at $2,270 million in 2005 and, with a compounded annual growth rate
(CAGR) of 10%, it will reach $3,652 million in 2012. This estimate includes LF-based tests used in
human and veterinary medicine, food and beverage manufacturing, pharmaceutical, medical biologics
and personal care product production, environmental remediation, and water utilities.
Architecture and working of a lateral flow immunoassay
Figure 1 shows the typical configuration of a LFA which is composed of a variety of materials,
each serving one or more purposes. The parts overlap onto one another and are mounted on a backing
card using a pressure-sensitive adhesive. The assay consists of several zones, typically constituted
by segments made of different materials. When a test is run, sample is added to the proximal end
of the strip, the sample pad. Here, the sample is treated to make it compatible with the rest of the
test. The treated sample migrates through this region to the conjugate pad, where a particulate
conjugate has been immobilized. The particle can typically be colloidal gold, or a colored, fluorescent,
or paramagnetic monodisperse latex particle. This particle has been conjugated to one of the specific
biological components of the assay, either antigen or antibody depending on the assay format. The
sample re-mobilizes the dried conjugate, and the analyte in the sample interacts with the conjugate
as both migrate into the next section of the strip, which is the reaction matrix. This reaction matrix
is a porous membrane, onto which the other specific biological component of the assay has been
immobilized. These are typically proteins, either antibody or antigen, which have been laid down in
bands in specific areas of the membrane where they serve to capture the analyte and the conjugate as
72
Lateral Flow Assay- Principle and its Application in Analytical Food Science
they migrate by the capture lines. Excess reagents move past the capture lines and are entrapped in
the wick or absorbent pad. Results are interpreted on the reaction matrix as the presence or absence of
lines of captured conjugate, read either by eye or using a reader.
Lateral flow assay formats
This test can be performed on two platforms, either direct (sandwich) or competitive (inhibition)
and also can be used to accommodate qualitative, semi quantitative and in limited cases, fully
quantitative determination.
Direct assay format: Direct assays (Figure 2) are typically used when testing for larger analyte with
multiple antigenic sites i.e. analyte presenting several epitopes. This system (equivalent to sandwich
ELISA) employs two different antibodies (polyclonal and monoclonal) that bound distinct epitopes of
the analyte: a labelled polyclonal antibody is placed in a dehydrated state onto a glass-fiber membrane
(conjugate pad) to serve as detector reagent and a monoclonal antibody specific to the analyte is sprayed
at the test line of the nitrocellulose membrane to serve as capture reagent. An additional antibody specific
to the detection antibody (species specific) could be used to produce a control signal at control line.
Figure 2. Direct Lateral Flow Assay
Figure 3. Competitive Lateral Flow Assay
When a sample extract is applied to sample
pad, the liquid migrates up by capillary force and the detector reagent is then released. Some of the
analyte bind to the detection antibody and some remain free in the solution. Subsequently, the mixture
passes through the capture zone (test line) and both unbound analyte and bound analyte bind to the
capture antibody. The response in the capture zone (test line) is directly proportional to the amount of
analyte in the sample.
Competitive assay: Competitive assay formats (Figure 2) are typically used when testing for small
molecules with single antigenic determinants, which cannot bind to two antibodies simultaneously. In
this format, an analyte-protein conjugate coated on the test zone of a nitrocellulose membrane captures
a labelled anti-analyte monoclonal antibody complex, allowing colour particle (e.g. colloidal gold) to
concentrate and form a visible line on the test zone. Another specific antibody coated on the control
line allows the capture of the excess antibody complex. One band colour will therefore be visible in
the control zone regardless of the presence of target analytes, confirming correct test development.
Conversely, a negative sample will result to the formation of two band colours visible (test line and
control line)
Materials and processes in lateral flow immunoassay development and
construction
A typical test strip consists of the following components:
Membrane/Analytical Region: The purpose of the analytical region in a lateral flow immunoassay
is to bind proteins at the test and control areas and to maintain their stability and activity over the
73
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
shelf-life of the product. The membrane material is typically a hydrophobic nitrocellulose or cellulose
acetate membrane onto which anti-target analyte antibodies are immobilised in a line across the
membrane as a capture zone or test line. A control zone may also be present, containing antibodies
specific for the conjugated antibodies. Nitrocellulose, while extremely functional, the only material
that has been successfully and widely applied for LIFA because of it’s relatively low cost, true capillary
flow characteristics, high protein-binding capacity, relative ease of handling.
Conjugate Pad or reagent pad: This contains antibodies specific to the target analyte conjugated to
coloured particles (usually colloidal gold particles, or latex microspheres). The role of the conjugate pad
in a lateral flow immunoassay is to accept the conjugate, hold it stable over its entire shelf life, and release
it efficiently and reproducibly when the assay is run. The materials of choice are glass fibers, polyesters,
or rayons.
Sample Pad: Sample pad is an absorbent pad onto which the test sample is applied. One of the
major advantages of the lateral flow concept is that these assays can be run in a single step with many
different sample types in a variety of application areas. The role of the sample pad is to accept the
sample, treat it such that it is compatible with the assay, and release the analyte with high efficiency.
The materials used for the sample pad depend on the requirements of the application. Examples of
such materials are cellulose, glass fiber, rayon, and other filtration media.
Wick or waste reservoir: The wick is the engine of the strip. It is designed to pull all of the fluid
added to the strip into this region and to hold it for the duration of the assay. It should not release
this fluid back into the assay or false positives can occur. The material is typically a high-density
cellulose.
Backing Materials: All components of the lateral flow assay are laminated to the backing material
to provide rigidity and easy handling of the strip. The backing material is coated with a pressuresensitive adhesive to hold the various components in place. The backing materials are typically
polystyrene or other plastic materials coated with a medium to high tack adhesive.
Labels for Detection: The most commonly used particulate detector reagents in lateral flow systems
are colloidal gold and monodisperse latex. Latex particles coupled with a variety of detector reagents,
such as colored dyes, fluorescent dyes, and magnetic or paramagnetic components, are available
commercially.
Applications of lateral flow assays in food quality assurance
In the past 3–5 years, food safety issues and concerns for public health have led to more stringent
legislation in food safety requirements. Legislation has produced increased demand for pathogen
and toxin tests in just about every segment of the food production industry – processed food, meats,
poultry, beverages, and dairy; and by all major food producers worldwide. For monitoring residue
contaminants such as veterinary, pesticide and antibiotic residues, an analytical strategy has been
recommended using two different methods. This strategy comprises: (i) screening with a first method
optimized to prevent false negative results, with a high sample throughput (e.g. ELISA), an acceptable
percentage of false positive results and low cost, and (ii) confirmation with an independent second
method optimized to prevent false positive results. Confirmatory methods are generally separative
techniques coupled with various detectors such as HPLC and GC–MS. Chromatography methods
are sensitive and specific, but suffer from being time consuming, laborious and multi-complex. In
addition, these technologies are unaffordable to the farmers and some laboratories in the developing
countries. Therefore there have been emergent needs for developing highly accurate, rapid and cheap
analytical tools. Lateral flow tests provide advantages in simplicity and rapidity when compared to
the conventional detection methods. LFT has also been confirmed to be a rapid and sensitive method
in the detection of food borne pathogens such as Salmonella, Listeria, Campylobacter, Clostridium
and Escheriachia coli. Apart form these pathogens, LFA also has been employed for the detection of
bacterial toxins and zoonotic viruses such as Avian Influenza (AIV). LFA have also been used for the
detection of potentially allergenic peanut and hazelnut in raw cookie dough and chocolate. The
74
Lateral Flow Assay- Principle and its Application in Analytical Food Science
Table :- applications of lateral flow assays in food analysis
Analyte
Assay format
Labels
Sample
Sensitivities
Reference
Detection of pathogen bacteria and related toxins
Staphylococcus
aureus
Sandwich
Colloidal gold
Pork, Beef, Fried
Chicken
<25 CFU/g
(93.0–100%)
[4]
Escherichia coli
Sandwich
Colloidal gold
Milk Powder, Flour,
Starch, Etc
105CFU/ml
[18]
Listeria
monocytogenes
Sandwich
Carbon black
Dairy Products
10 CFU/25
mL
[1]
Salmonella enteritidis
Sandwich
Colloidal gold
Raw Eggs
107CFU/ mL
[15]
Staphylococcus
aureus enterotoxin B
Sandwich
Fluorescent
immunoliposomes
Water, Apple Juice,
Ham , Milk, Cheese
0.02–0.6 pg/
ml
[5]
Detection of veterinary drug residues mycotoxins and pesticides
Veterinary Drug Residue
Progesterone
Competitive
Colloidal gold
Bovine Milk
0.6–1.2 μg/L
[3]
Deoxynivanelol and
Zearalenone
Competitive
Colloidal gold
Wheat
100–1500
μg/kg
[6]
Deoxynivanelol
Competitive
Colloidal gold
Wheat and Maize
50 ng./mL
[22]
Aflatoxin B1
Competitive
Colloidal gold
Rice, Corn ,Wheat
0.05–2.5 ng/
ml
[23]
Aflatoxin B2
Competitive
Magnetic nanogold
microsphere
Peanut, Hazelnut,
Pistacia, Almond
0.9 ng/ ml
[17]
Total B fumonisins
(B1, B2 and B3)
Competitive
Colloidal gold
Maize
4,000 μg/ kg
[8]
Ochratoxin
Competitive
Colloidal gold
Coffee
5 ng/ml
[21]
Ochratoxin
Competitive
Colloidal gold
Barley, Wheat, Oat,
Corn, Rice etc
1 ng/ mL
[19]
Methamidophos
Competitive
Colloidal gold
Green Vegetables
1.0 μg/ mL
[2]
Thiabendazole and
Methiocarb
Competitive
Carbon black
Fruit Juices
0.005–0.5
mg/kg
[16]
Carbaryl
Competitive
Colloidal gold
Rice And Barley
50–10 μg/L
[20]
Hazelnut Protein
Competitive
Unknown
Chocolates
3.5 mg/kg
[13]
Doughs
2.6 mg/kg
Allergenic Peanut
Protein Ara H1
Competitive
Unknown
Chocolates
0.8 mg/kg
Doughs
0.6 mg/kg
Milk and milk powder
15 ng/ml
[7]
0.50 (%,
w/w)
[12]
Mycotoxins
Pesticides
Detection of Allergens
Detection of Adulteration
Rennet whey in milk& Sandwich
milk powder
Latex beads
Raw
Beef in
chicken
Cooked
Sterilized
Thermal-stable
ruminant-specific
muscle protein,
troponin I
Competitive
Coloured particles
Lamb-inpork
Beef-inturkey
Raw
Cooked
0.05 (%,
w/w)
Sterilized
Raw
0.10 (%, w/w)
Cooked
Sterilized
75
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Table summarizes the published reports on LFT applications in this field. A driver in the demand
for rapid and LF tests in food production is the adoption of Hazard Analysis and Critical Control
Points (HAACP) regulations that prescribe test procedures throughout the manufacturing process.
A number of manufacturers have come with LF type tests. No one company dominates the market
for LF food tests. The leaders are Strategic Diagnostics, Inc. (Newark, DE), Neogen, Idexx Labs and
Biocontrol Systems (Brownsville, CA). Other companies include Celsis International PLC (Chicago,
IL), Medical Wire & Equipment Co. (Wiltshire, United Kingdom), Merck KgaA (Dermstadt, Germany),
and M-Tech Diagnostics Ltd. (Cheshire, United Kingdom).
Conclusions
A variety of analytical methods available for detecting pathogen organisms or hazardous
chemicals related to food safety, human health and environment suffers from being time-consuming,
too expensive and too complicated to use. Major advantages found on LFT are low-cost, speed,
portable, do not require complicated equipment and technical expertise, which are critical
components during testing in the field. Since its initial development in the 1980s, the technology of
Lateral Flow Immunoassay has gained wide acceptance. The main reason for its popularity is the
simplicity of the test design. The lateral flow immunoassay devices are compact and easily portable.
Most of them do not require external reagent for results. Results are quick and easy to interpret,
usually without the help of an instrument. The technology is also powerful. Multiple analytes can be
tested simultaneously with a single device. It can also be easily combined with other technology to
provide a comprehensive analysis like simultaneous drug and alcohol determinations by the police
force in a roadside testing situation. Manufacturing of the test is relatively easy and inexpensive.
Advancement in the detection moieties, improvement in material components, availability of better
processing equipment, and increased attention to quality manufacturing all these factors contribute
to increase in the reliability, accuracy, and applications of the technology. However, the continuing
demand for quantitative result and sensitivity has presented great challenge for assay developer.
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
76
Blaskoza M, Koet M, Rauch P, Amerogen AV (2009) Eur Food Res Technol 229:867–874
Chenggang S, Suqing Z, Kun Z, Guobao H, Zhenyu Z (2008) J Environ Sci 20:1392–1397
Geertruida A, Posthuma T, Jakob K, Amerogen AV (2008) Anal Bioanal Chem 392:1215-1223
Huang S-H, Wei H-C, Lee Y-C (2007) Food Control 18:893–897
Khreich N, Lamourette P, Boutal H, Deveilliers K, Creminon C, Vollad H (2008) Anal Biochem 377:182–188
Kolosova AY, De Saeger S, Sibanda L, Verheijen R, Peteghem CV (2007) Anal Bioanal Chem 389:2103–2107
Martı´n-Herna´ndez, C.; Mun˜oz, M.; Daury, C.; Weymuth, H.; Kemmers-Voncken, A.E.M,; Corbato´n, T.; Toribio,
T. and Bremer, M.G.E.G. (2009). Int Dairy J. 19:205–208.
Molinelli A, Grossalber K, Krska R (2009) Anal Bioanal Chem 395:1309–1316
Ngom, B., Guo, Y. Wang, X and Bi, D. (2010) Anal. Bioanal. Chem. 397: 1113-1135.
O’Farrell, B. (2009) Evolution in lateral flow-based systems. In: Lateral flow immunoassay. (Ed. R.C. Wong and
H.Y. Tse). Springer, NY, USA.
Ponti, J.S. (2009)Material platform for the assembly of lateral flow immunoassay test strips. In: Lateral flow
immunoassay. (Ed. R.C. Wong and H.Y. Tse). Springer, NY, USA.
Q. Rao, Y.-H. Peggy Hsieh. (2007) Meat Science. 76: 489–494
Röder M , Vieths S, Holzhauser T (2009) Anal Bioanal Chem 395:103–109
Rosen, S. (2009) Market Trends in Lateral Flow Immunoassays. In: Lateral flow immunoassay. (Ed. R.C. Wong and
H.Y. Tse). Springer, NY, USA.
Seo K-H, Holt PS, Gast RK, Stone HD (2003) Int J Food Microbiol 87:139–144
Smidova Z, Blazkova M, Fukal L, Rauch P (2009) Czech J Food Sci 27:S414–S416
Tang D, Sauceda JC, Lin Z, Basova SOE, Goryacheva I, Biselli S, Lin J, Niessner R, Knopp D (2009) Biosens Bioelectron
25:514
Wang J, Chen WN, Hu KX, Li W (2006) Chinese J 35:439–441
Wang XH, Liu T, Xu N, Zhang Y, Wang S (2007) Anal Bioanal Chem 389:903–911
Wang S, Zhang C, Wang J, Zhang Y (2005) Anal Chim Acta 546:161–166
Wang J, Yu F-Y (2008) Anal Chem 80:7029–7035
Xu Y, Huang Z-B, He QH, Deng SZ, Li LS, Li YP (2010) J Food Chem 119:834–839
Xiulan S, Xiaolian Z, Jian T, Xiaohong G, Jun Z, Chu FS (2006) Food Control 17:256–262
Separation Strategies for Bioactive Milk Proteins
Separation Strategies for Bioactive Milk Proteins
Rajesh Kumar
Dairy Chemistry Division, NDRI, Karnal
Introduction:
Over the past three decades, the dairy industry globally has moved from being based solely on
commodity food production to earning a significant income from specialty proteins. The introduction
of large scale membrane processing in the early 1970’s made it possible not only to reduce waste but to
produce new products such as lactose and whey protein concentrate. A logical extension of the latter
product is whey protein isolate (WPI), produced by single-stage batch capture of proteins on anion
exchange resins. WPI is a crude mixture of acidic whey proteins, containing mainly α-lactalbumin,
β-lactoglobulin, bovine serum albumin and immunoglobulins. Two whey proteins not captured
during WPI production by anion exchange chromatography because of their high isoelectric points
are lactoferrin (LF) and lactoperoxidase (LP). These basic proteins are instead captured from whey or
skim milk by cation exchange chromatography and sold as specialty ingredients.
Although production of high-value whey proteins is a commercial reality, two aspects of dairy
processing may not be optimal for their production. First, the proteins are subjected to a series of
processing steps prior to being extracted. It is a generally accepted principle of bioseparation process
design that proteins should be separated from a source material as fast and in as few steps as possible to
avoid loss of activity and yield. Currently, high-value dairy proteins are viewed as a by-product, with
the major income of the industry coming from commodity dairy foods such as milk powder, cheese
and butter. Economies of scale for production of commodity dairy products mean that centralized
processing is the industry norm.
Separation technologies provide the basis for adding value to milk through the production of bioactive
components that provide the food industry with nutraceuticals to develop functional foods. The global
functional food and nutraceutical market is currently worth about US$50 billion and is growing at some
8 – 10 % annually. This huge and rapidly growing market, driven by consumer demands for healthpromoting foods, is creating an almost insatiable desire on the part of food manufacturers for new and
novel ingredients with which to formulate these foods. Dairy constituents, notably the proteins and
peptides, provide the food technologist with a rich selection of potential ingredients for functional foods.
Dairy proteins and peptides are truly multi-functional components, providing desirable features such
as physical functional traits, nutritional qualities and an increasing array of substantiated bioactivities.
Their promise is clear. The challenge for science and technology is to isolate these ingredients in a costeffective manner while maintaining their inherent bio functional traits.
Protein bioseparation:
Protein bioseparation refers to the recovery and purification of protein products from various
biological feed streams is an important unit operation in the food pharmaceutical and biotechnological
industry. Protein bioseparation is at present more important than at any time before due to
phenomenonal developments in recent years in the frontiers of separation technology.
Novel separation techniques:
Separation technologies used to produce protein ingredients derived from milk include
screening based on size differences: centrifugation based on density differences; membrane
processes based on size differences, such as ultrafiltration, diafiltration, nanofiltration, and reverse
osmosis; ion exchange based primarily on charge differences; and affinity chromatography based on
specific binding to a matrix. Owing to unique functional and biological properties of many of the whey
proteins, a number of pilot and industrial scale technological methods have been developed for their
isolation in a purified form. Improved separation technologies and emerging markets have resulted
77
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
in fractionation of milk proteins into ingredients that are enriched in specific proteins, or peptides, or
both to fill those new opportunities. This is especially true for milk protein fractions that are in very
low concentrations in the native state that require further concentration. These ingredients may be
especially useful in the developing market of physiologically functional foods, or nutraceuticals.
Separation technologies are available to prepare fractions that are enriched in the following
milk components: alpha-CN, beta-CN, beta-LG, alpha-LA, casein phosphopeptides, lactoferrin,
lactoperoxidase and immunoglobulins and other minor proteins with special functional properties.
Many of these products are commercially available in limited quantities. Bioactive peptides derived
from food proteins are often intermediates and are isolated from a very complex peptidic hydrolysates
in which their concentration are very low. The preparation of such peptides generally requires time
consuming steps. Accordingly commercial production of bioactive peptide from milk proteins has
been limited by lack of suitable large scale techniques. Until now membrane based separation tech. has
provided best technique for enrichment of peptides.
Chromatographic techniques:
Chromatographic techniques have been widely used for the isolation of milk proteins, and high
performance methodology now forms the basis for several accurate methods of analysis. Different
types of separation chemistries are used for chromatographic separation of milk proteins. In its simplest
form, a chromatographic separation system consists of a column filled with separation adsorbent
beads. Chromatography has been known since the turn of the century, but its primary use has been
in the analytical sector, where the excellent separation capability has been a valuable investigative
tool. The industrial use of this technology has however, been fairly limited and mainly used for high
value added products in the pharmaceutical industry. Low processing rates and difficulties in scaling
up chromatographic separation from laboratory to production scales has hampered the broader use
and acceptance of the technology. However, now with the advancement in chromatography based
separation technology, it has greatly improved the profitability of dairy industry through the best
possible utilization of raw material especially whey in a cost effective manner.
Chromatographic systems:
The chromatographic systems are in many ways similar to ion-exchange systems. The variety
in adsorbent types and the range of applications are however far beyond what is known for ion
exchange, and this has created a need for more sophisticated systems such as membrane adsorber
based chromatography, stirred tank batch process and expanded bed chromatography.
Ion-exchange Chromatography
Ion-exchange chromatography is the most popular method for protein purification.
The theory of it is to use the difference of charges on proteins at a given pH. The solid adsorbents
are charged, positive or negative. Then the charged protein will be adsorbed by the charged adsorbents.
According to the difference of the interaction forces between the protein and adsorbent, different
protein is bounded differently by the adsorbent. Then, when we use some other buffer to replace
the protein, they (the proteins) will be washed out of the adsorbents in different velocity: the less the
interaction between the adsorbent and the proteins, the faster they will be washed out. Then, proteins
can be separated according to the sequence of their elution. There are two kinds of ion exchangers:
anion exchangers, which have positively charged matrix, and will adsorb the proteins with negative
charge; cation exchanger, which have negative charged matrix, and will adsorb the proteins with
positive charge. The most common anion exchangers are DEAE- ,TEAE- and QAE-, and the cation
exchangers often being used are CM- , S-.
Membrane Chromatography:
In order to overcome the limitations of traditional beads column, synthetic microporous or
macroporous membranes have been used as chromatography media. This method is called membrane
chromatography. Membrane chromatography can overcome the limitations associated with packed
78
Separation Strategies for Bioactive Milk Proteins
beds based chromatography. In membrane chromatographic processes, the transport of solutes to their
binding sites take place predominantly by convection and the pore diffusion is very small comparing
with the beads column, thereby the mass transfer resistance is tremendously reduced. The result of
this advantage is to reduce process time including adsorption, washing, elution and regeneration
time, which save time and improve efficiency. Most importantly, fast process can avoid the inactivity
of biomolecules. As we all know, all the biomolecules have activities. The faster is the process, the less
possibility for the biomolecules lose activity. The id ea of membrane chromatography is especially
suited for large scaleprocess since the column volume of membrane can be made from less than 0.1
ml and larger than thousands of liters. Due to the macroporous structure of the membrane support,
membrane chromatography has a lower pressure drop, higher flow rate and higher productivity.
There are many advantages of using membrane chromatography over column chromatography. The
rate of association between target proteins and functional groups in ion exchange membranes is very
rapid, unlike the slow rate of diffusion through packed columns. The fast convective flow combined
with negligible pressure drop lim itations exhibited by the thin membranes mean that processing
times are dramatically reduced compared with packed columns. A distinct advantage of pressure
driven ion exchange processes is that there are no heat-treatments, extremes of pH, or chemical
pretreatment that could compromise protein structure and functionality. Hence, development of ion
exchange membranes would be most desirable in terms of purifying individual whey proteins for use
in functional foods and pharmaceutical products (Goodall et al., 2008).
Application of membrane chromatography for protein purification
Usually, the membranes used for membrane chromatography have functional ligands attached to
their inner pore surface as adsorbents. There are many types of adsorptive membranes including ionexchange membranes, affinity membranes, reverse-phase membranes and hydrophobic interaction
membranes. All these membranes have been developed for the purification of proteins, enzymes, and
antibodies from various sources.
The stirred tank system:
In the stirred tank system for protein fractionation, the liquid to be treated is slurried with the
chromatographic adsorbent material, in this case an ion-exchange resin, - until adsorption has occurred.
The deproteinised solution is then draining from the stirred tank. The adsorbent is then washed with
water, prior to desorption of the proteins using an acid, alkali and/ or mineral salt solution. Compared
to the column system, the disadvantage of the stirred tank system is the fact that all the target proteins
are not removed, due to the equilibrium conditions. This problem can be partly solved by refeeding the
deproteinised solu tion to the adsorbent material, before the next batch of whey is treated. The stirred
tank system allows the use of larger adsorbent particles, since the contact time between adsorbent and
liquid can be increased. This facilitates
the removal of the process solu tion, and makes the process less sensitive to fat and suspended
solids. Also less overall changes in pH are required during the complete adsorption / desorption
cycle, which reduces the risk of protein denaturation, improving the final p roduct quality.
Expanded bed chromatography:
In expanded bed chromatography, viscous and particu late-containing feeds that would foul a
traditional packed column are accommodated by introducing the feed upward through a column packed
with media designed to be suspended and dispersed in the upward flow. The bed expansion creates
an increased void space between adsorbent particles allowing passage of particulate contaminants in
the feed and preventing unacceptable pressure buildup within the column. Once the feed is loaded
and the target product is bound to the adsorbent, a wash step is performed, also in expanded bed
upward flow mode, to remove particulates and unbound contaminants. Elution of the target product
is then performed via downward flow in traditional packed bed mode. Some studies relate elution
performed with an expanded bed upward flow mode. This tends to increase the elution volume. A
clean-in-place procedure is normally required after elution to prepare the column for another loading
79
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
step. An expanded bed is essentially a cross between a packed bed, with stationary p articles and low
flu id dispersion, and a fluidized bed, with randomly mixing particles and high flu id dispersion.
Process design variables for optimizing expanded bed chromatographic separations include chemical
and physical properties of the packing media and of the feed solutions. Chromatographic media with
the appropriate density, particle size distribution, and mechanical stability required for expanded bed
operation are commercially available with several different chemistries including anion and cation
exchange. The chemical properties of the feed such as pH, ionic strength, and buffer type that affect
selectivity and capacity of the process have essentially the same effect in expanded bed mode as they
do in traditional packed bed chromatography.
High Gradient Magnetic Fishing (HGMS):
Heeboll-Nielsen et al., (2004) described the process for separation of lactoferrin and lactoperoxidase
from whey using superparamagnetic ion exchangers. HGMS is employed to simply remove product
or contaminants from process or waste streams in mineral processing. The term HGMF covers the
integrated process from product adsorption to magnetic separation and finally recovery of the product
in a clarified and partly purified form, in contrast to merely the collection of particles in HGMS. The
objectives of the individual steps in HGMF are similar to those of any chromatographic purification
process, and the process generally consists of: (i) mixing of the feedstock with the superparamagnetic
adsorbents to bind the target protein; (ii) separation of the protein laden supports from the spent
feedstock supernatant using high gradient magnetic separation technology; (iii) washing of the
adsorbents and elu tion of bound
protein from the adsorbents using multip le cycles of support capture and release and finally (iv)
a procedure for cleaning and regeneration of the supports. In contrast to chromatography all steps are
operated in a batchwise manner, and each operation contains a step to capture the supports in high
gradient magnetic field s. For such operation the HGMF system may be +designed with stirred reactor
tanks and closed recycled loops.
Conclusion:
Dairy manufacturing technology has expanded tremendously in recent years and the emphasis
on identifying, recovering, and/ or supplementing bioactive proteins and peptides as functional
ingredients will remain at forefront of future. Ultimately these approaches will improve the quality of
food products containing such constituents.
References:
Bajaj, R.K. and Sangwan, R.B. (2002) Health enhancing potential of whey proteins – a review. Indian J. Dairy Sci., 55(5):
253-260.
Bargemana,G., Koopsb, G.-H. , Houwinga , J. , Breebaartb, I. , van der Horsta, H.C. and Wessling, M. (2002). The
development of electro-membrane filtration for the isolation of bioactive peptid es: the effect of membrane selection
and operating parameters on the transport rate. Desalination. 149: 369–374.
Clare, D.A. , Catignani, G.L. and Swaisgood, H.E. (2003) Biodefense properties of milk: The role of antimicrobial p
roteins and p eptid es. Current Pharmaceutical Design, 9: 1239- 1255.
Expanded bed adsorption; princip les and methods, Amersham Pharmacia Biotech, (1997).
Goodall, S., Grandison, A. S., Jauregi, P. J. and Price J. (2008). Selective separation of the major whey proteins u sing ion
exchange membranes. J. Dairy Sci. 91: 1-10
Hauffman, L.M. and Harper, W.J (1999). Maximizing the value of milk through separation technologies. J. Dairy Sci.,
82(10): 2238-2244
Heebøll-Nielsen,A., Justesen,S.F.L, Hobley,T.J. and Thomas, O.R.T. (2004). High Gradient Magnetic Fishing recovery of
basic whey proteins using superparamagnetic cation-exchangers. J. Biotech. 113: 247-262.
Korhonen, H. (1998) Colostrum immunoglobulins and the complement system- potential ingredients of functional
foods. IDF bulletin, 336: 36-40.
Nielsen, W.K., Morten, A.O. and Lihme, A. Expanding the fronteiers in separation technology in Scandinavian dairy
Information 2 / 02.
Olander, M.A., Jakobsen, U.L., Hansen M.B. and Lihme, A. Fractionation of high value whey Proteins. in Scandinavian
dairy Information 2 / 01.
Roper, D.K. and Lightfoot, E.N. (1995) Separation of biomolecules using adsorptive membranes. J. of Chromatography
A 702: 3
80
SDS-PAGE – Principle and Applications
SDS-PAGE – Principle and Applications
Y. S. Rajput1 and Rajan Sharma2
1
Animal Biochemistry Division, 2Dairy Chemistry Division, NDRI, Karnal
Introduction and Principle
The purpose of sodium dodecyl sulphate – polyacrylamide gel electrophoresis (SDS-PAGE) is to
separate proteins according to their size. SDS-PAGE is the most widely used method for analyzing
protein mixture qualitatively. It is particularly useful for monitoring protein purification and, because
the method is based on the separation of proteins according to size, it can be used to determine the
relative molecular mass of proteins. SDS (CH3-(CH2)10-CH2OSO3-Na+) is an anionic detergent and when
proteins are treated with SDS in presence of a reducing agent like β-mercaptoethanol or dithiothreitol,
SDS binds to hydrophobic regions of protein molecule and provides net negative charge on protein
molecule. The binding of SDS to per-unit-length of protein molecules is almost constant for large
number of different proteins and this brings charge-to–mass ratio almost constant for most proteins.
The electrophoretic movement of protein in acrylamide gel is determined by molecular weight of
proteins. Lower molecular weight proteins move faster than high molecular weight proteins. The
method described by Laemmli (1970) is widely used. In this method, discontinuous buffer system
is employed. A continuous buffer system has only single separating gel and uses same buffer in the
tanks and gel. In discontinuous buffer system, large pore gel (stacking gel) is layered over small pore
gel (separating or running gel). For preparation of stacking gel and separating gel, different buffers are
used and also tank buffers are different from gel buffers. When electrophoresis is started, ions from
stacking gel (leading ion), ions from buffer tank (trailing ion) and proteins start moving into stacking
gel. In stacking gel, protein moves between leading ion and trailing ion and this leads to concentration
of protein in a thin zone referred as stack. The protein molecules continue to move in the stack until
they reach the separating gel.
Formation of Polyacrylamide Gels: Crosslinked polyacrylamide gels are formed from the
polymerization of acrylamide monomer in the presence of smaller amounts of N,N’-methylene-bisacrylamide (normally referred to as “bis-acrylamide”) (Fig. 1). Note that bis-acrylamide is essentially
two acrylamide molecules linked by a methylene group and is used as a crosslinking agent.
Acrylamide monomer is polymerized in a head-to-tail fashion into long chains, and occasionally a bisacrylamide molecule is built into the growing chain, thus introducing a second site for chain extension.
Proceeding in this way, a crosslinked matrix of fairly well-defined structure is formed (Figure 1). The
polymerization of acrylamide is an example of free-radical catalysis, and is initiated by the addition of
ammonium persulfate and the base N,N,N’,N’tetramethylenediamine (TEMED). TEMED catalyzes the
decomposition of the persulfate ion to give a free radical (i.e., a molecule with an unpaired electron):
S2O82- + e-
------ → S2O82-
+ SO4-. (1)
If this free radical is represented as R. (where the dot represents an unpaired electron) and M as an
acrylamide monomer molecule, then the polymerization can be represented as follows:
R. + M → RM.
RM. + M → RMM.
RMM. + M → RMMM. and so forth (2)
In this way, long chains of acrylamide are built up, being crosslinked by the introduction of the
occasional bis-acrylamide molecule into the growing chain. Oxygen “mops up” free radicals, and
therefore the gel mixture is normally degassed (the solutions are briefly placed under vacuum to
remove loosely dissolved oxygen) prior to addition of the catalyst.
Use of Stacking Gels: For both SDS-PAGE and native-PAGE, samples may be applied directly to
the top of the gel in which protein separation is to occur (the separating gel). However, in these cases,
81
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
the sharpness of the protein bands produced in the gel is
limited by the size (volume) of the sample applied to the gel.
Basically the separated bands will be as broad (or broader,
owing to diffusion) as the sample band applied to the gel.
For some work, this may be acceptable, but most workers
require better resolution than this. This can be achieved by
polymerizing a short stacking gel on top of the separating gel.
The purpose of this stacking gel is to concentrate the protein
sample into a sharp band before it enters the main separating
gel, thus giving sharper protein bands in the separating gel.
This modification allows relatively large sample volumes
to be applied to the gel without any loss of resolution. The
stacking gel has a very large pore size (4% acrylamide) which
Figure 1. Polymerization of acrylamide
allows the proteins to move freely and concentrate, or stack
under the effect of the electric field. Sample concentration is produced by isotachophoresis of the
sample in the stacking gel. The band-sharpening effect (isotachophoresis) relies on the fact that the
negatively charged glycinate ions (in the reservoir buffer) have a lower electrophoretic mobility than
the protein-SDS complexes, which in turn, have lower mobility than the C1- ions if they are in a region
of higher field strength. Field strength is inversely proportional to conductivity, which is proportional
to concentration. The result is that the three species of interest adjust their concentrations so that [C1-]
> [protein-SDS] > [glycinate]. There are only a small quantity of protein--SDS complexes, so they
concentrate in a very tight band between the glycinate and C1- ion boundaries. Once the glycinate
reaches the separating gel, it becomes more fully ionized in the higher pH environment and its
mobility increases. (The pH of the stacking gel is 6.8 and that of the separating gel is 8.8.) Thus, the
interface between glycinate and the C1- ions leaves behind the protein-SDS complexes, which are left
to electrophorese at their own rates.
Procedure: The below mentioned procedure is for the separation of proteins using glycine-SDSPAGE. For the separation of low molecular weight proteins, Tricine-SDS-PAGE is used and procedure
is mentioned in the compendium.
Equipments and Chemicals
Mini-vertical gel electrophoresis dual model with glass plates, spacer, comb and power-supply;
orbital shaker; 1 ml glass syringe with 2”22G needle; acrylamide, N,N1 methylene bisacrylamide;
ammonium persulfate; β-mercaptoethanol; sodium dodecyl sulfate; molecular weight markers;
coomassie brilliant blue R-250; TEMED; tris; glycine; dithiothreitol.
Stock Solutions
Acrylamide / Bisacrylamide (30%): 29.2 g acrylamide and 0.8 g bisacrylamide are dissolved in
distilled water and total volume was made to 100 ml. The solution is filtered and filtered solution can
be stored at 4ºC in dark bottle up to 3 months.
4x Running Gel Buffer (1.5 M Tris-HCl, pH 8.8): 18.15 g Tris is dissolved in about 80 ml distilled
water. pH is adjusted to 8.8 with 1 N HCl and total volume is made to 100 ml with distilled water.
Prepared buffer can be stored up to 3 months at 4ºC in dark bottle.
4x Stacking Gel Buffer (0.5M Tris-HCl, pH 6.8): 3.0 g Tris is dissolved in about 40 ml distilled
water. pH is adjusted to 6.8 with 1 N HCl and total volume is made to 50 ml with distilled water.
Prepared buffer can be stored up to 3 months at 4ºC in dark bottle.
10% SDS: 10 g sodium dodecyl (lauryl) sulfate is dissolved in distilled water and total volume is
made to 100 ml with distilled water. Prepared solution can be stored at room temperature.
5 x Electrode Buffer (125 mM Tris, 960 mM Glycine, 0.5 % SDS, pH 8.3: 15 g Tris, 72 g glycine and 5
g SDS are dissolved in distilled water and total volume is made to 1 litre with distilled water. The pH
of buffer should be 8.3 ± 0.2. Stock electrode buffer is diluted five times with distilled water before use.
82
SDS-PAGE – Principle and Applications
The stock buffer can be stored at room temperature up to 1 month. The diluted stock buffer is 25 mM
tris, 192 mM glycine, and 0.1% SDS.
10% Ammonium Persulfate: 100 mg ammonium persulfate is dissolved in 1.0 ml distilled water.
The solution is always prepared fresh.
2 x Sample Buffer (0.125 M Tris, 4% SDS, 20% glycenol 0.2 M DTT, 0.02% bromophenol blue, pH
6.8: 2 x sample buffer is prepared by mixing following solutions/chemical.
4 x stacking gel buffer
-
2.5 ml
glycerol
-
2.0 ml
10% SDS
-
4.0 ml
Bromophenol blue
-
2.0 mg
Dithiothreitol (DTT)
-
0.31 g
Distilled water
-
1.5 ml
2 x sample buffer can be stored in small aliquots at - 200C up to 6 months. Instead of DTT, 1.0 ml
of β-mercaptoethanol can be used but the volume of water is reduced to 0.5 ml.
Overlay Buffer (0.375 M Tris, 0.1%, SDS, pH 8.8): Overlay buffer is prepared by mixing 25 ml
running gel buffer, 1 ml 10% SDS and 74 ml distilled water. This buffer can be stored up to 3 months
at 4ºC in the dark bottle.
Procedure
Glass Sandwich: One notched glass plate is placed on a flat surface. One spacer (1.0 mm) each is
then placed along the each of two edges so that spacer aligns with the notch. Subsequently, rectangular
glass-plate is placed over it. The sandwich is held firmly between thumb and fingers. Side-ways of
both spacers were sealed with appropriate tape to overcome any possible gel-leak during gel plate
preparation. There is always the possibility of leakage at the bottom of the plate. This is taken care
by placing molted agar (1% in water) up to 5 mm height in trough of gel-casting unit. The plate in
standing position is then quickly placed in casting unit and screws are finger tightened.
Preparation of Running Gel: Running gel of desired concentration is prepared by mixing
appropriate volumes of solutions as shown below
Acrylamide / bisacrylamide, running gel
buffer, SDS and distilled water are added to
conical flask and degassed. Then ammonium
persulfate and TEMED are added and
contents mixed gently. With the help of glass
pipette, the running gel solution is delivered
to sandwich to a level about 3 cm below the
top of rectangular plate. Air should not be
trapped while filling sandwich with running
gel solution. A small volume of water or
overlay-buffer (~ 200 µl) is layered over gel
solution with the help of glass syringe with 22
G needle. This prevents exposure to oxygen.
Solutions
Final Gel Concentrations
7.5%
10%
12.5%
15%
Acrylamide /
bisacrylamide (30%)
5.0 ml
6.7 ml
8.3 ml
10.0 ml
4 x Running gel
buffer
5.0 ml
5.0 ml
5.0 ml
5.0 ml
10% SDS
0.2 ml
0.2 ml
0.2 ml
0.2 ml
Distilled water
9.7 ml
8.0 ml
6.4 ml
4.7 ml
10% Ammonium
persulfate
0.1 ml
0.1 ml
0.1 ml
0.1 ml
TEMED
6.7 µl
6.7 µl
6.7 µl
6.7 µl
Preparation of Stacking Gel: Stacking gel of 4% concentration is prepared by mixing appropriate
volumes of solutions as shown below
The preparation of stacking-gel solution is similar to preparation of running-gel solution. After
removal of water or overlay buffer, stacking-gel solution is layered over running gel. Appropriate
comb is inserted into the stacking gel to make wells for sample application. Comb is removed after
polymerization of gel.
83
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Sample Preparation: Protein samples (1 mg/ml) are Solutions
Volume
centrifuged (10,000 g, 5 min.) to remove any insoluble material Acrylamide / bisacrylamide (30%)
1.3 ml
and are mixed with equal volume of 2 X sample buffer. The 4 x Stacking gel buffer
2.5 ml
resultant solution is boiled for 3 to 5 min. Molecular weight 10% SDS
0.1 ml
markers are also prepared in a similar way.
Distilled water
6.1 ml
Electrophoresis: Gel plates are then tightly attached
10% Ammonium persulfate
50 µl
to electrophoresis unit. Stock electrode buffer is five times
TEMED
10 µl
diluted with cold water. Anode and cathode chambers are
filled with buffer. 5 to 20 µl of sample is applied to each
well. Electrophoresis is carried out at constant voltage of 50V till sample crosses stacking gel. When
sample enters running gel, voltage is increased to 100V. Complete electrophoretic run takes around
2.5 to 3.0 h. During electrophoresis, temperature is kept low by circulating water in electrophoretic
assembly. After electrophoretic run, stacking gel is removed. Small cut on top left side in running
gel is made to remember the orientation of the gel.
Staining of proteins in gel: The gel is placed in glass tray containing coomassie brilliant blue solution
(0.25%) prepared in methanol : acetic acid : water (40:7:53) mixture. Glass tray is then placed on orbital
shaker for 4 h at room temperature. After staining for 4 h, the gel is transferred to the destaining solution
I (methanol, acetic acid and water mixture in ratio of (40:7:53) for 30 min. Subsequently gel is placed
in destaining solution II (methanol, acetic acid and water mixture in ratio of 7:5:88) till bands become
visible against light background. During staining and destaining, gel should float free in glass tray.
Helpful-hints
•
A particular concentration of acrylamide gel is used
for separating proteins of particular range of molecular
weights. Whereas low acrylamide gel concentration is
used for separating high molecular weight proteins,
low molecular weight proteins are resolved in high
gel concentration. Use following table in deciding gel
concentration in separating gel.
Per Cent
gel
Molecular weight of proteins
to be separated (KD)
7.5
24 – 205
10.0
14 – 205
12.5
14 – 66
15.0
14 – 45
•
Spacers can absorb heat and thus lowers the temperature of gel at edges. If the gel is hotter in
the middle than at the edges, the mobility of dye front at edges will be lower as compared to
mobility in the middle. This can be avoided by (i) using cooled electrode buffer and (ii) not
allowing buffer to warm up during run. Thus, during electrophoretic run either use cooling
device or use low current.
•
If gel is not polymerized properly at edges, current can leak down the edges resulting in more
mobility at edges. Air- bubbles at the bottom of glass plates can block current flow resulting
abnormal dye front.
•
While placing comb in stacking gel, care should be taken not to allow air-trap. Air inhibits
polymerization and sample wells will be distorted.
•
All stock solutions required for gel preparation are stored at refrigerated temperature and these
should be brought to room temperature. At low temperature, polymerization is inhibited. Oxygen
also inhibits polymerization of acrylamide and these solutions should be degassed before use.
•
Sometimes boiling of sample in sample buffer may lead to irreversible precipitation and such
samples remain at the top of separating gel. For such samples one can try incubating sample in
sample buffer at 70ºC instead of 100ºC.
Reference:
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227L
680-685.
Walker, J.M. (2006) Electrophoretic techniques. In Principles and Techniques of Biochemistry and Molecular biology (K.
Wilson & J. Walker Eds). Cambridge University Press, New York.
84
Western Blot: Theoretical Aspects
Western Blot: Theoretical Aspects
Y. S. Rajput1 and Rajan Sharma2
1
Animal Biochemistry Division, 2Dairy Chemistry Division, NDRI, Karnal
Western blotting is the transfer of proteins from the SDS-PAGE gel to a solid supporting membrane.
For analysis based on antibody reactivity or nucleic acid hybridization, the separated molecules are
made free of electrohoretic matrix. This can be simply achieved by slicing the gel followed by elution in
buffer. But, this process is slow and resolution is also poor. An alternative efficient method is ‘blotting’
technique in which molecules are separated on a slab gel and separated molecules are eluted through
the broad face of the gel onto a membrane that binds the molecules as they emerge. Proteins and
nucleic acids stay on the surface and can be detected.
The membrane materials frequently employed in blotting are nitrocellulose, nylon and
polyvinylidene difluoride (PVDF). The choice of membrane depends on the type of analysis and
characteristics of detection system. Nitrocellulose is the most widely used since it works well with
both protein and nucleic acids. Some nylons do not bind protein reliably. PVDF, is often used when
bound proteins are analysed for sequencing.
The transfer of the proteins or nucleic acids from the gel to the membrane can be achieved by
capillary flow of buffer or by transverse’ electrophoresis. The use of capillary flow to transfer DNA
from agarose gels to nitrocellulose membrane was first described by Southern (1975) and thus referred
as Southern blotting. Using the same method for transfer of RNA is referred as Northern blotting.
Western blot refers to transfer of protein from gel to membrane and this technique was described in
1979-80 by many workers but the method described by Towbin et al. (1979) is most cited.
Western blotting essentially comprises of three techniques which are applied in sequence. The
first one is referred as SDS-PAGE through which proteins are separated based on the molecular size
of molecules in acrylamide gel. Sodium dodecyl sulphate (SDS) is an anionic detergent that denatures
proteins by wrapping around the polypeptide backbone. This results in net negative charge to
polypeptide in proportion to its length. Laemmli system (Laemmli, 1970) employing discontinuous
buffer is most widely used electrophoretic system. The resolution in Laemmli’s method is excellent
because treated peptides are concentrated in stacking gel before entering the separating gel. The
technique, which follows SDS-PAGE, is transfer of protein/from gel to membrane. There are two
types of equipments for electrophoretic transfer of proteins: the semi-dry blotting apparatus and
‘tank’ buffer apparatus. The third technique used in sequence is for identification of protein (antigen)
by performing” antigen-antibody (first antibody) reactions on the membrane itself. Second antibody
enzyme conjugates were then allowed to interact with immobilized first antibody and, then using
appropriate substrate, protein bands are detected. Although, antigen-antibody interactions are widely
employed in Western blot, other kind of interactions such as glycoprotein-lectin and biotin-avidin
have allowed research workers to employ this technique for other applications including carbohydrate
staining of glycoprotein, protein sequencing etc.
Semi-dry electrophoretic transfer
In semi-dry electrophoretic transfer, a stack of wetted filter papers surrounding the gel and the
blotting membrane is used as a buffer reservoir, instead of tank as in conventional electrophoretic
transfer. The electrodes, consist of conductive plates made of graphite or stainless steel or a conducting
polymer. The size of the plates is at least the same size as that of gel to provide homogeneous electric
field. The main advantages with semi¬dry transfer are the ease of handling, the short time (30 min, to
1 h) required for the transfer and low buffer consumption. Another important feature is that different
buffers can be used at the anodic and cathodic sides to improve the transfer. The short electrode
distance gives a high voltage gradient despite low power. Cooling is not normally required since heat
production is negligible. Transfer can be performed from several gels at a time, either by placing them
85
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
beside each other if the electrodes are large enough or by placing several transfer units on top of each
other. Because of the short electrode distance, voltage applied is most often 10-20V. On the other hand,
because of large cross-sectional area, the current passing through the transfer sandwich is fairly high,
in the range 0.1-1A.
Tank-buffer electrophoretic transfer
In tank-buffer electrophoretic transfer, transfer cassette is submerged in a ‘tank’ of buffer. Gel,
membrane, filter paper, porous foam sheet are arranged in cassette as per instructions of manufacturer.
The details have been provided in write-up for practicals.
Blot membranes
Numerous types of papers and membranes have been utilized for protein blotting. Nitrocellulose
paper (film of nitric acid esterified cellulose) has been the most frequently used membrane.The binding
of proteins to nitrocellulose is probably hydrophobic. For electrophoretic transfer of small proteins,
membranes with 0.1or 0.2 µm pore size are selected. If membranes stick to low concentration gels after
transfer, membranes with pore size of 0.45 µm are selected. A drawback with nitrocellulose membrane
is, however, that they are very brittle when dry.
The other membrane, which is in use, is polyvinylidene fluoride (PVDF). PVDF membrane is a
teflon-type polymer composed of the basic repeating unit (- +δCH2--δCF2-)n and has good mechanical
strength. Proteins interact with the polymer non-covalently through dipolar and hydrophobic
interactions. PVDF is chemically compatible with the aqueous buffer systems. Since PVDF is resistant to
most organic solvents, it can withstand harsh chemical conditions in which nitrocellulose membranes
dissolve or decompose. These membranes are expensive. Some PVDF membranes have additional
components. Western consists of PVDF cast on a polyester web. The web does not interfere with
electroblotting or alter the characteristics of the PVDF. Immobilon-CD is PVDF membrane in which
surface is chemically modified to have a cationic charge. Although hydrophobic and dipolar interactions
with the Immobilon-CD may contribute to protein binding, the primary binding interaction is ionic.
The membranes with high internal surface area (>2000 cm2 per cm2 of frontal area) bind substantially
more protein (400 µg BSA/cm2) as compared to membrane with low internal surface area (~400 cm2
per cm2 of frontal area) that binds to around 130 µg BSA/cm2. Low internal surface area membranes
usually function better in immunodetection. They are comparatively easy to block and antibodies are
better able to penetrate the more open pore structure. Membranes with high internal surface structure
are more difficult to block effectively and less open pore structure often limits antibody accessibility.
Besides immunodetection, PVDF membranes are used for amino acid sequencing, amino acid analysis
and peptide mapping. For these applications, blocking is not required and there is no steric hindrance
encountered by antibodies. Higher internal surface membranes and Immobilon-CD are suitable for
amino acid sequencing and amino acid analysis. Peptide mapping is more effective on low internal
surface area membranes. PVDF membrane is compatible with protein staining and immune-chemical
protocols.
Positively charged nylon membranes arc mechanically strong and have a high binding capacity.
A disadvantage is their high non-specific binding which results in a high background after
immunodetection. Most general protein stains are anionic dyes and can not be used with nylon
membranes since they bind to these membranes.
Transfer buffer
A major concern in transferring proteins onto nitrocellulose membrane is the composition of the
transfer buffer. The original protocol of Towbin et al. (1979) uses a transfer buffer containing methanol,
which was added to, counteract swelling of the gel. Methanol also decreases gel pore size, removes SDS
from proteins. Methanol may precipitate the proteins within the gel, however, it increases the capacity
and the affinity of nitrocellulose membrane for proteins. PVDF membrane is activated by placing
it in 100% methanol for 1-2 sec. This allows the hydrophobic surface of PVDF to wet with aqueous
86
Western Blot: Theoretical Aspects
solvent. Addition of 20% methanol to transfer buffer is recommended for low molecular weight
proteins. Methanol is not required for transfer to charged nylon membranes. Methanol facilitates the
dissociation of SDS-Protein complexes and increases the hydrophobic interaction between protein and
membrane. On the other hand, for high molecular weight proteins, methanol can decrease the elution
efficiency by denaturing the proteins or retarding the elution from the gel. In contrast to low molecular
weight proteins, high molecular weight proteins do not require methanol for adequate binding to the
membrane.
The presence of SDS in transfer buffer increases the mobility of protein from gel to membrane. This
is especially useful for transfer of protein after isoelectric focussing, when proteins have no net charge.
However, SDS decreases the binding of the protein to both nitrocellulose and PVDF membrane. It is
sometimes necessary to add SDS (0.01-0.02%) to aid transfer of high molecular weight proteins. Transfer
buffer generally used is 25 mM Tris, 192 mM glycine, pH 8.3 and 20% methanol. If membrane is to
be used for protein sequencing or amino acid, analysis, CAPS buffer (10 mM 3-(cyclohexylamino)-1¬
propanesulfonic acid, 10% methanol, pH 11.0 is recommended. Application of protein blotting for
characterization of antigens will require antigen specific antiserum. By simultaneously running
molecular weight markers and proteins (extracted from biological materials) in SDS-PAGE and
subsequent detection after electrophoretic transfer provides information about molecular weight of
antigen. Antibodies should be specific otherwise cross-reaction is observed and interpretation is more
difficult. Affinity purified antibodies or monoclonal
antibodies provide good result. Through these
reactions, one can detect presence or absence of
such antigens in related and unrelated biological
materials. Now tools are available for ascertaining
carbohydrate moiety in proteins on membrane.
These proteins can be oxidized by periodate
resulting in generation of free aldehyde groups
(Figure 1). The generated groups are reacted with
biocytin hydrazide leading to biotinylation of
glycoproteins. Using appropriate probe such as
avidin-peroxidase and substrate, glycoproteins
are detected. Alternately lactins specific for
carbohydrate residues can be employed. In this Figure 1. Detection of Biotin Labeled Glycoproteins on
Western blots
approach antibody (against lectins) enzyme
conjugates or lactin - enzyme conjugates can
be used for staining glycoproteins. Asn-linked oligosaccharides can be cleared from protein onto
membrane by hydrazinolysis. The released oligosaccharides can be characterized by using biochemical
techniques. Proteins onto membrane can be hydrolyzed for determining amino acid composition.
Peptide mapping and protein sequencing are other useful applications where proteins on membrane
are the starting material for subsequent steps.
References:
Kurien, B.T and ScoWeld, R. H. (2006) Western blotting. Methods: 38 (2006) 283–293.
Towbin, H.; Stachelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: procedure and some applications. Proc. Nat. Acad. Sci. USA. 76: 4350-4354.
87
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Enzyme Linked Immunosorbent Assay - Theory
Rajeev Kapila and Suman Kapila
Animal Biochemistry Division, NDRI, Karnal
Enzyme immunoassay (EIA) and enzyme-linked immunosorbent assay (ELISA) have become
household names for medical laboratories, manufacturers of in vitro diagnostic products, regulatory
bodies, and external quality assessment and proficiency-testing organizations. Analytes such as
peptides, proteins, antibodies and hormones can be detected selectively and quantified in low
concentrations by ELISA. ELISAs are rapid, sensitive, cost effective and can be performed in a highthroughput manner. In contrast, techniques like immunofluorescence and RIA are tedious, time
consuming, having short shelf-life of the reagents, requiring sophisticated expensive equipments and
the strict regulatory controls on the use of isotope. Though, this technique is relatively less sensitive as
compared to radio-immuno-assay (RIA) but efforts are continuing to increase its sensitivity.
An ELISA is used in a vast variety of different types of assays (e.g. direct ELISA, indirect ELISA,
sandwich ELISA, competitive ELISA). Nevertheless, all ELISA variants are based on the same principle,
the binding of one assay component – antigen or specific antibody – to a solid surface and the selective
interaction between both assay components. Molecules not specifically interacting with the assay
component are washed away during the assay. For the detection of the interaction, the antibody or
antigen is labeled or linked to an enzyme (direct ELISA). Alternatively, a secondary antibody conjugate
can be used (indirect ELISA). The assay is developed by adding an enzymatic substrate to produce a
measurable signal (colorimetric, fluorescent or luminescent). Such a substrate is called a chromogenic
or luxogenic substrate. The strength of the signal indicates the quantity of analytes in the sample.
A number of enzymes have been employed for ELISA, including alkaline phosphatase, horseradish
peroxidase and β-galactosidase. These assays approach the sensitivity of Radioimmunoassay (RIA)
and have the advantage of being safer and less costly.
Direct ELISA
The direct ELISA uses the method of directly labeling the antibody itself. Microwell plates are
coated with a sample containing the target antigen, and the binding of labeled antibody is quantitated
by a colorimetric, chemiluminescent, or fluorescent end-point. This technique has advantages of
direct detection, quick methodology since only one antibody is used. Cross-reactivity of secondary
antibody is eliminated. Major disadvantages of direct detection is reduced Immunoreactivity of the
primary antibody as the result of labeling. Labeling of every primary antibody is time-consuming and
expensive. No flexibility in choice of primary antibody label from one experiment to another. Little
signal amplification
Indirect ELISA
The indirect ELISA utilizes an unlabeled primary antibody in conjunction with a labeled secondary
antibody. Since the labeled secondary antibody is directed against all antibodies of a given species
(e.g. anti-mouse), it can be used with a wide variety of primary antibodies (e.g. all mouse monoclonal
antibodies). Indirect detection method of ELISA is versatile, since many primary antibodies can be
made in one species and the same labeled secondary antibody can be used for detection. Moreover,
wide variety of labeled secondary antibodies are available commercially. Immunoreactivity of the
primary antibody is not affected by labeling. Sensitivity is increased because each primary antibody
contains several epitopes that can be bound by the labeled secondary antibody, allowing for signal
amplification. Cross-reactivity may occur with the secondary antibody, resulting in nonspecific
signal.
88
Enzyme Linked Immunosorbent Assay - Theory
Sandwich ELISA
The sandwich ELISA measures the amount of antigen between two layers of antibodies. The
antigens to be measured must contain at least two antigenic sites, capable of binding to antibody,
since at least two antibodies act in the sandwich. So sandwich assays are restricted to the quantitation
of multivalent antigens such as proteins or polysaccharides. Sandwich ELISAs for quantitation of
antigens are especially valuable when the concentration of antigens is low and/or they are contained
in high concentrations of contaminating protein.
Competitive ELISA
In this unlabeled antibody is incubated in the presence of its antigen. These bound antibody/
antigen complexes are then added to an antigen coated well. The plate is washed and unbound
antibody is removed. The secondary antibody, specific to the primary antibody is added. This second
antibody is coupled to the enzyme. A substrate is added, and remaining enzymes elicit a chromogenic
or fluorescent signal. For competitive ELISA, the higher the original antigen concentration, the weaker
the eventual signal.
Selection of enzymes for labelling
Antigen-antibody interaction is basis on which Elisa works and extent of interaction is measured
by measuring the activities of enzyme linked to antigen or antibody. In ELISA, a soluble substrate that
is converted to soluble coloured product is used. Absorbance of colour is read in ELISA plate reader,
which can read samples in 96-well microtitre plates. Enzymes are selected on the basis of availability of
purified enzyme at cheaper rate, turn over number and availability of cheaper chromogenic substrates.
The most common enzymes used are horseradish peroxidase and alkaline phosphate.
Applications in dairy
Since the advent of pasteurization, the dairy industry has been a leader in food safety and
aggressively proactive in its commitment to ensure the safety of dairy products. Few areas of attention
are pathogens, toxins, adulterants and, more recently, allergens. Thanks to new technological advances
in convenient-to-use, rapid screening tests, these safety issues can now be addressed as part of a total
dairy quality control program.
Detection of pathogens
Bacterial pathogen contamination of dairy products is usually monitored via agar plate counting
techniques, which generally take from one to five days—too long to be an effective pathogen screening
tool. One of the most popular immunoassay techniques for screening milk for pathogens and toxins is
the enzyme-linked immunosorbent assay (ELISA) method. Highly automated and sensitive bench-top
instruments based on immunoassay methods are now available and have significantly reduced the
time and labor required to obtain results. According to Vasavada (2001), the rapidity and sensitivity
of immunoassay-based test kits and systems have come a long way in the past few years due to
development in immunoprecipitation devices, lateral flow devices and immunomagnetic separation
(IMS) techniques. Immunoassay tests offer three important advantages: speed of analysis, sensitivity
and high specificity for detecting the target pathogen.
Detection of allergens
Allergens are another area of food safety concern. Approximately 2 to 3% of adults and 5 to 8%
of children are allergic to foods. Food allergies are caused by proteins that can trigger an immune
response in sensitized individuals. As the number of different ingredients used in formulated
foods continues to grow, it is becoming more common for dairy processing plants to handle a
wider spectrum of ingredients than they did a few years ago. This has increased the likelihood of
cross-contamination of products with inappropriate ingredients—i.e., ingredients that can cause
allergic reactions and are not indicated on product labels. Whether it’s peanuts, tree nuts, milk,
eggs, wheat or soybeans, nearly every processed food has an identified allergen in it. Currently,
89
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
the most popular type of food allergen testing is based on sandwich enzyme-linked immunoassays
(S-ELISAs). A target allergen protein is extracted from samples with a buffered salt solution. The
extracted protein is sampled and added to antibody-coated microwells, where it binds to the
antibody during an incubation period. After a wash step, enzyme-labeled antibody (conjugate) is
added to the antibody wells and is allowed to attach to the bound allergen, forming an “antibody
sandwich” around the allergen. After another wash step, substrate is added which reacts with the
conjugate to produce blue color. The intensity of color is proportional to the amount of allergen.
It is important to realize some of the limitations inherent in this type of testing. Because high
temperature may denature protein, test antibodies may not capture a sample’s allergenic component.
This is especially true with egg products, which denature at a relatively low time and temperature
combination. Since denatured protein may remain allergenic, it is recommended that products be
tested prior to baking or cooking. Another problem can occur when testing samples that have high oil
content. Although low levels of protein may be present in edible oils, they may be difficult to extract
with standard extraction solutions and may not be detected by the test. Also, it is important to note
that using a test kit designed for testing the presence of peanut protein is not appropriate to use for
screening samples for almonds, pecans or other tree nuts. Care must be exercised when testing for
egg allergens. Some test kits only test for egg white proteins; egg yolk proteins can also cause allergic
reaction in people sensitized to egg yolk proteins.
Detection of of Aflatoxin M1 (AFM1) in milk and milk products
Kim et al (2000) examined the occurrence of aflatoin M1 in pasteurized milk and dairy products
like milk infant formula, milk powders and yoghurt. Recoveries of AFM1 from sample spiked at levels
between 5 and 500pg/ml were 88-106% for pasteurized milk and 84-94% for yoghurt by ELISA. Limits
of detection were 2pg/ml. The occurrence of Aflatoxin M1 (AFM1) contamination in Indian infant
milk products and liquid milk samples was investigated by competitive ELISA technique. The range of
contamination of AFM1 was comparatively higher in infant milk products (65–1012 ng/l) than liquid
milk (28–164 ng/l). Almost 99% of the contaminated samples exceeded the European Communities/
Codex Alimentarius recommended limits (50 ng/l), while 9% samples exceeded the prescribed limit
of US regulations (500 ng/l). The extrapolation of AFM1 data to estimate the Aflatoxin B1 (AFB1)
contamination in dairy cattle feedstuffs indicate that the contamination may range from 1.4 to 63.3 μg/
kg with a mean of 18 μg/kg which is substantially higher than the directive of European Communities
regulation (5 μg/kg).
Detection of adulteration of goat, sheep and buffalo milk and cheese
An indirect ELISA successfully developed for the detection of defined amounts of cows’ milk
(1-50%) in sheeps’ milk and cheese. The assay used polyclonal antibodies raised in rabbits against bovine
caseins (BC). The antibodies were biotinylated and rendered cows’ milk specific by mixing them with
lyophilized ovine and caprine caseins. Extravidin- peroixidase used to detect the biotinylated anti-BC
antibodies bound to BC immobilized on 96-well plates. Subsequent enzymic conversion of substrate
gave clear absorbance differences when assaying mixtures of sheeps’milk and cheese containing
variable amounts of cows’ milk. The indirect competitive ELISA had a lower sensitivity when applied
to cheese, compared with milk. A sandwich ELISA was developed utilising the monoclonal antibody
in combination with a polyclonal goat anti-bovine IgG antibody. Once optimised, the ELISA was
found to be highly specific. Detection limits in milk were 0.001% cows’ milk adulteration of sheep
or buffalo milk, and 0.01% cows’ milk adulteration of goat milk. Detection limits in soft cheese were
0.001% in goat cheese and 0.01% in sheep or buffalo cheese. The ELISA performance makes it suitable
for development as a kit for use in routine surveillance of milk and soft cheese.
Detection of insecticide and pesticide in milk
Polyclonal antibodies against an aldrin/dieldrin immunogen have been raised in rabbits and used
as the basis of an enzyme-linked immunosorbent assay (ELISA). This assay can detect dieldrin in milk
90
Enzyme Linked Immunosorbent Assay - Theory
in the range 5 μg/ml to 1ng/ml reliably. This range differs in skimmed and semi-skimmed milk, and
in cream, reflecting the differences in fat content between these samples (Ibrahim et al, 1993).
Detection of melamine
Recent food safety scares, such as the discovery of melamine in milk, have sharpened the global
awareness of the links between profit, the food chain and the global food supply. A cheap industrial
chemical, melamine has been used to artificially increase the amount of protein content in diluted
milk, thereby increasing the price for
the milk. The combination of melamine
and a degradation product, cyanuric
acid, results in crystals that can create
blockages in the kidneys. In March
2007, public awareness of melamine
contamination was heightened when
Figure: General principle of the competitive ELISA assay. Enzyme- the contaminant was found in pet food
conjugated melamine competes with the melamine from the ingredients imported from China,
sample for binding to melamine antibody. This enzyme activity and
absorbance values decrease according to increasing amount of the causing the death of many animals.
Following this scare, it was revealed that
unlabeled melamine from the unknown sample.
melamine-tainted fodder may have been
used to feed animals, including chickens, swine and catfish intended for human consumption. As
melamine is still being found in eggs, fish and a variety of processed foods imported from China, the
scrutiny of products for melamine is intense.
ELISA, is a high-throughput technique for screening food for melamine. The general principle of
these competitive ELISA assays is shown in Figure .
Detection of histamine in cheese
Aygun et al. (1999) used a competitive direct ELISA for determining histamine in cheese. Cheese
was homogenized with phosphate buffered saline, centrifuged and filtered and the supernatant was
diluted with phosphate buffered saline. Detection limit and mean recoveries were 2 mg/kg and 93%.
Quantification of immunoglobulins and cytokine in milk and colostrum
A double sandwich enzyme-linked immunosorbent assay (ELISA) procedure for the quantification
of IgG in bovine milk was developed for detecting the concentration of IgG in various homogenized
HTST, UHT, evaporated and raw milk samples as well as skim milk powder. Using this procedure,
homogenized, HTST pasteurized milk was found to contain from 65 to 79% of the IgG found in raw
milk. Skim milk powder also retained a major portion of IgG, while evaporated and UHT pasteurized
milk were virtually devoid of IgG (Kummer et al., 1992)
Colostrum contains factors that are protective for the neonate and may be a source of
immunomodulary molecules that positively influence the immune status of the neonate. To confirm
that colostrum contains a variety of cytokines with immunomodulatory properties, a bovine cytokine
specific ELISA and five cytokines (IL-1β, IL-6, TNF-α, INF-γ or IL-1 receptor antagonist, IL-1ra) in
the whey samples from cows at different stages of lactation were monitored. The concentrations
of cytokines in colostrum were significantly higher concentrations than those in the mature milk.
Colostrum contains high levels of cytokines that could be produced and secreted in the mammary
gland and that may have an immunomodulatory activity and influence neonatal immunity (Hagiwara
et al., 2000)
Detection of antibiotics in milk
β-Lactam antibiotics, particularly penicillins are widely used in medicine and veterinary medicine,
this being the reason why residual amounts of penicillins may be found in foodstuffs of animal origin.
Antibiotics contained in milk may adversely affect the health of human consumers (e.g., by inducing
91
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
allergic reactions). Moreover, the presence in milk of antibiotics and other compounds suppressing the
development of microorganisms disrupts technological processes of production of cheese (including
soft cheese) and sour milk beverages by retarding or blocking lactic acid fermentation. Therefore,
milk should be carefully controlled for the presence of residual amounts of penicillins, and such
control requires reliable and readily available analytical methods. ELISAs are widely used abroad
for determining penicillin in milk (Usleber et al., 1994; Rohner, et al., 1995) including as commercially
available kits (Sternesjo and Johnsson, 1998). Determination of penicillin G, ampicillin, and isoxazolyl
penicillins (cloxacillin and dicloxacillin) in milk has been described, with detection limits in the
range 10–30 ng/ml A modification of an ELISA in which a fluorescent probe replaces conventional
chromogens and capillaries are used for simultaneous determination of six penicillins in milk has been
described (Huth, 2002).
Conclusion
The increase in food borne illnesses and specific types of pathogens is due to various consumer,
manufacturing and regulatory trends that may set the stage for contamination or minimal screening
methods. For example, there are changes in consumer consumption habits and preferences for minimally
processed convenience foods, as well as an increase in certain risk groups who are most vulnerable to
diseases. Also adding to the problem are changes in food production, distribution and globalization of
supply that expands the potential for imports tainted with pathogens or pesticide residues. Compounding
the problem are new types of pathogens, as well as new strains of recognized pathogens, and both are
appearing in food products where they have never before been identified. It is likely that technologies
based on immunoassays and PCR methods will emerge as the two favorite types of rapid pathogen
screening tests for dairy products because of their enhanced specificity, sensitivity and efficiency
compared to other methods. PCR tests that measure pathogen DNA or RNA are more sensitive but
also are more expensive than immunoassay methods. To continue to deliver safe dairy products to the
public, dairy processors will be increasingly dependent on new rapid, accurate, sensitive and specific
screening tests for pathogens, toxins, adulterants and allergens.
References:
Aygun,o.,Schneider,E.,Suhener,R. and Usleber, E. and Martlbauer, E. J. agril. & food Chem.,1999, 47(5):1961-1964.
Hagiwara, K.i Kataoka, S. ,Yamanaka, H.. Kirisawa, R. and Iwai, H. Veterinary Immunology & Immunopathology,
2000, 76(3-4):183-190
Huth, S.P., Warholic, P.S., Devou, J.M., Chaney, L.K.,and Clark, G.H., J. AOAC Int., 2002, 85 (2): 355–364.
Ibrahim,A.M.A., Hewedi, M.M. and Smith, C.J. Food and Agril Immunol, 1993 5 (3): 145 - 154
Kim, E.K., Shon, D.A. Dyer, D., Park, J.W., Hwang, H.J. and Kim, Y.B.. Food additives and Contaminants, 2000, 17(1):5964.
Kummer, A., Kitts, D.D.,Li-Chan, E., Losso, J.N., Skura, B.J.and Nakai, S. Food and Agricultural Immunology, 1992, 4
(2): 93 - 102
Rastogi, S., Dwivedi, P. D., Khanna, S. K., Das, M. Food control, 2004 15(4)287-290
Rohner, P., Schallibaum, M., and Nicolet, J., J. Food Prot., 1995, 48(1):59–62.
Sternesjo, A. and Johnsson, G., J. Food Prot., 1998, 61 (7) :808–811.
Usleber, E., Lorber, M., Straka, M., Terplan, G., and Martlbauer, E., Analyst, 1994, 119 (12): 2765–2768
Vasavada, P.C. Food Safety, 2001; 7(3):29-38
92
Experimental Determination of Thermal Stability of Proteins: A Theoretical Background
Experimental Determination of Thermal Stability
of Proteins: A Theoretical Background
Jai K. Kaushik
Animal Biotechnology Centre, NDRI, Karnal
One of the most crucial aspects in protein science is the solution properties affecting the structure,
stability and activity of proteins in solutions. Most of the enzymes and many structural proteins are
globular while some structural proteins are fibrous in structure. The stability of proteins originates from
their detailed three dimensional structures. The covalently linked amino acids in a linear fashion results in
the primary structure that folds into a unique 3-D structure, which is responsible for the specific function
and activity of a protein molecule. The 3-D structure of proteins is defined by weak intermolecular
interactions and therefore the native state of proteins is only marginally stable by ca 5-15 kcal/mol.
The small free energy is the difference of large changes, which can be several hundreds of kcal/mol, in
enthalpy and entropy over folding. Therefore, determining these large changes in enthalpy and entropy
accurately is the central problem in protein physical chemistry to evaluate the precise free energy (Gibbs
energy) of stabilization of proteins.
With the great advancement in generation and production of recombinant proteins, there has
been a new interest in understanding the molecular basis of protein stability so that more rugged and
stable proteins functional over a wide range of environmental conditions can be designed. Therefore
it is critically important to evaluate the stability of proteins precisely and accurately to compare and
relate the experimentally determined stability data to theoretically determined stability for a set of
mutant proteins to understand the role of molecular interactions. There are two standard methods to
determine the protein stability, viz. kinetic methods, which include chemical denaturant jump, pH jump
and temperature jump to determine the rate of folding and unfolding, and equilibrium methods which
include the solvent denaturation and thermal denaturation procedures. The easiest and the most widely
used method is the thermal denaturation of proteins and determining the equilibrium thermodynamic
parameters linked with the process. The obtained thermodynamic parameters like enthalpy, heat
capacity and entropy evaluated at the midpoint of denaturation can be used to determine the stability
profile (Free Energy versus temperature) for a given protein.
There are several techniques to monitor the denaturation of proteins under varying external agents
like increasing concentration of chemical denaturants or increasing temperature or changing the pH. To
monitor the changes in thermodynamic parameters related to thermal denaturation, the most direct method
is the calorimetry, which measures the enthalpy of denaturation as a function of temperature. Calorimetry
can provide the precise enthalpy, entropy and heat capacity of denaturation. Figure 1 shows a typical
thermal denaturation and renaturation scans as a function of temperature measured by (A) calorimetry
and (B) spectrophotometry. In a
calorimetrically measured phase
transition (Native ↔ Denatured)
profile of a protein the peak of the
endotherm indicates the midpoint
of transition. The total area under
the curve provides a direct measure
of enthalpy of transition, whereas
the differences in the baselines for
pure native and pure denatured
Figure 1: Typical thermal denaturation profiles of a protein determined by (A)
species measured at the midpoint of
differential scanning calorimetry (DSC) measuring the excess heat capacity
transition
provide the heat capacity of
as a function of temperature, and (B) spectrophotometry measuring change
in absorbance.
denaturation.
93
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
For a two state transition N ↔ D, where N is the native state and D the denatured state, the ∆H(T)
- the enthalpy of transition at a temperature (T) can be directly evaluated using equation 1:
∆H(T) = ∆Hº + ∆Cp (T – Tm)
(1)
where, ∆Hº is the enthalpy of transition at Tm; ∆Cp - the heat capacity of transition; and Tm, the
midpoint of transition. This is the most direct method of evaluating the thermodynamic parameters
like enthalpy, while the entropy can be evaluated using the relation:
∆S(T) = ∆Sº + ∆Cp ln (T/Tm)
(2)
where ∆Sº is the entropy at the midpoint of transition and can be derived using the relation:
∆Sº = ∆Hº/Tm
(3)
The Gibbs energy of protein folding (free energy of stabilization) therefore can be defined by the
equation:
∆G(T) = ∆H(T) – T ∆S(T)
(4)
Substituting the values of ∆H and ∆S from equations (1-3) in to (4) and rearranging we obtain:
∆G(T) = ∆Hº (1 – T/Tm) – ∆Cp (Tm – T + T ln (T/Tm))
(5)
which is a modified form of Gibbs Helmholtz equation and can be used to determine the protein
stability at any given temperature.
Similarly, using the indirect methods which can measure the change in conformation of the native
state of protein, we can determine the thermodynamic parameters linked with a phase transition
(N↔D). The change in conformation can be monitored by various spectroscopic methods; e.g. circular
dichroism, spectrophotometry, fluorescence, IR, light scattering, hydrogen exchange or anything
which are sensitive enough to record the phase transitions in proteins; even viscometric or volumetric
methods which can measure the solution properties of proteins due to phase transitions can also
be used. Figure 1B shows the typical change in tertiary interactions of RNase A over denaturation
measured by change in absorbance in the aromatic region. For a two state reversible phase transition
undergoing equilibrium conditions, the equilibrium constant can be defined as follows:
K = [Unfolded] / [Native] = α/ 1– α
(6)
or
α = K / 1+K
(7)
where α is the fractional denatured state concentration and K is the equilibrium constant.
Also we know,
K = e–∆Gº/RT
(8)
Substituting the values of ∆Gº, the standard free energy, and K from equation (5) and (8),
respectively, in to equation (7), we obtain:
α(T) =
e{–1/R[∆Hº (1/T – 1/Tm) – DCp (Tm/T – 1 + ln (T/Tm))]}
1 + e{–1/R[∆Hº (1/T – 1/Tm) – ∆Cp (Tm/T – 1 + ln (T/Tm))]}
(9)
Equation (9) can be used to fit the experimental data to directly evaluate the thermodynamic
parameters like ∆Hº, ∆CP, and Tm to evaluate the Gibbs energy of protein denaturation. The enthalpy
of denaturation (∆Hº) is known as van’t Hoff enthalpy (∆HvH) distinct from the calorimetric enthalpy
(∆Hcal) determined by DSC. For a two state reversible denaturation process for a single domain and/or
monomer protein the ratio of ∆HvH and ∆Hcal should be equal to unity. The solid lines shown in Figure
1B are the nonlinear least square fittings to the experimentally determined data points represented
by solid and open symbols. It is important to use good quality of data to evaluate the free energy of
stabilization. Even small differences in the free energy of stabilization due to single amino acid change
in proteins can be reliably evaluated by using any of the methods mentioned above. These methods
also require that phase transition must be reversible and undergoing equilibrium conditions.
94
Experimental Determination of Thermal Stability of Proteins: A Theoretical Background
Apart from the above mentioned methods which depend upon the heat-denaturation, other isothermal
methods employing the chemical denaturant to induce the phase transition of protein conformation can
also be used to evaluate the Gibbs energy of protein folding. It has been known for years that proteins
can be unfolded in aqueous solutions by high concentrations of urea or guanidine hydrochloride.
Denaturation with these chemicals is one of the primary ways of measuring the conformational stability
of proteins and comparing the stabilities of mutant proteins. Figure 2 shows the typical denaturation
reaction mediated by guanidine hydrochloride. It has been observed that the free energy of denaturation,
∆Gº = – RT ln K, depends upon the denaturant concentration as follows:
d(∆Gº)/d(GdnCl) = RTn/(GdnCl)½
(10)
where (GdnCl)½ is the midpoint of transition and n the slope of the curve. Later linear extrapolation
method (LEM) became popular as ∆Gº was found to vary linearly with denaturant concentration as
follows:
∆Gº = ∆G(H2O) – m[denaturant]
(11)
where ∆G(H2O) is an estimate of the conformational stability of a protein that assumes that the
linear dependence continues to 0 M denaturant concentration, and m the slope of the line measures
the dependence of ∆Gº on denaturant concentration. The values of K can be calculated from the
curve plotted in Figure 2A to calculate the ∆Gº as a function of denaturant concentration followed by
estimation of ∆G(H2O) using the LEM. However, an equation to directly fit the raw data can also be
written by substituting the value of K and ∆Gº from equations 8 and 11, respectively, in to equation
7 to give:
α(T) =
e–(∆G(H2O) – m[denaturant] )/RT
1 + e–(∆G(H2O) – m[denaturant] )/RT
(12)
Nonlinear least square fitting of the above equation to the raw data provide us the value of m and
∆G(H2O ).
Figure
2A
shows
the typical equilibrium
denaturation
and
renaturation curves of a
protein as monitored by
change in fluorescence, it
is clear that the process is
in equilibrium and highly
reversible, while the Figure
2B shows the increase in the
stability of wild type (WT)
due to engineering (Mutants
NuG1 and NuG2).
Figure 2. Typical equilibrium transition curves for protein unfolding and refolding
induced by guanidine hydrochloride. (A) The open triangles and solid circles are the
unfolding curves while the open squares represent the refolding equilibrium curves.
Panel (B) shows the increase in the midpoint of transition [GdnHCl]½ due to protein
engineering (mutants NuG1 and NuG2) of the wild type protein (WT).
All the above methods are based on equilibrium conditions; however, it is also possible to evaluate
the free energy of protein stability using the kinetic methods which can measure the rates of folding and
unfolding, since equilibrium constant K is the ratio of rate of unfolding (ku) and rate of folding (kf):
K = ku / kf
(13)
K can be used to calculate ∆Gº and various other thermodynamic parameters using the same
procedure mentioned above. The value of rate constants can be known by unfolding and refolding
reactions induced by jump studies using temperature, pH or denaturant concentrations (Figure 3). For
a two state process N↔D, all these methods should provide the same value of Gibbs energy of protein
folding within the experimental errors and therefore any of the technique available at ones disposal
can be used reliably for analysis of protein stability.
95
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Figure 3: Temperature dependence of kinetic and thermodynamic parameters of Pyrrolidone carboxyl peptidase (a)
Temperature dependence of ku and kf and relaxation (kr = ku + kf) rate constants. Dashed line represents kr values, (b)
Solid circles represent the unfolding Gibbs energies obtained from the equilibrium constant (K = ku/kf). The solid line is the
stability curve fitted to the data points using equation 5, whereas the dashed line is that generated from the thermodynamic
parameters obtained by DSC, mk(c) log of rates of folding (circles) and unfolding (squares) as a function of urea conc. kf =
3600sec-1 and ku = 27 sec-1 were obtained by extrapolation to 0 M urea. In the inset, the dotted line shows the denaturation
curve simulated by using the kinetic parameters and crosses indicate the experimentally obtained equilibrium data.
Reference
Hughues-Despointes, B. M. Scholtz, J. M. and Pace, C. N. (1999). Protein conformational stabilities can be determined
from hydrogen exchange rates. Nat. Struct. Biol. 6: 910-912.
Kaushik, J. K. and Bhat, R. (1998). Thermal stability of proteins in aqueous polyol solutions. J. Phys. Chem. Sect. B. 102:
7058-7066.
Kaushik, J. K. and Bhat, R. (1999). A mechanistic analysis of the increase in the thermal stability of proteins in aqueous
carboxylic acid salt solutions. Protein Science. 8: 222-233.
Kaushik, J. K., Ogasahara, K. and Yutani, K. (2002). The unusually slow relaxation kinetics of the folding-unfolding of
pyrrol.idone carboxyl peptidase from a hyperthermophile, Pyrococcus furiosus. J. Mol. Biol. 316: 989-1001.
Kaushik, J. K. and Bhat, R. (2003). Why is trehalose an exceptional protein stabilizer?: An analysis of the thermal stability of
proteins in the presence of compatible osmolyte trehalose. J Biol Chem, 278: 26458-26465.
Kaushik et al. (2006) Completely-buried, Non-ion-paired glutamic acid contributes favorably to the conformational
stability of pyrrolidone carboxylic peptidases from hyperthermophiles, Biochemistry. 45: 7100-7112.
Nauli, S., Kuhlman, B. and Baker, D. (2001). Computer-based redesign of a protein folding pathway. Nat. Struct. Biol.
8: 602-605.
Pace, C. N. (1986). Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methed
Enzymol. 131: 266-280.
Pace, C. N. and Shaw, K. L. (2000). Linear extrapolation method of analyzing solvent denaturation curves. Proteins:
Struct. Funct. Genet. (Suppl). 4: 1-7.
Santoro, M. M. and Bolen, D. W. (1988). Unfolding free energy changes determined by the linear extrapolation method.
1. Unfolding of phenylmethanesulphonyl α-chymotrypsin using different denaturants. Biochemistry. 27: 80638068.
Tanford, C. (1970). Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv. Protein Chem.
24: 1-95.
96
Species-Specific Identification of Milk and Milk Products: A Molecular Approach
Species-Specific Identification of Milk and
Milk Products: A Molecular Approach
Archana Verma
Dairy Cattle Breeding Division, NDRI, Karnal
Introduction
Intermixing of milk from different species of origin is a common practice depending on the demand
of the consumer for liquid milk or the manufacturing of milk products. Different methods based
on protein analysis are currently used for milk species identification, including chromatographic,
electrophoretic and immunological techniques. Among these, capillary electrophoresis, twodimensional electrophoresis, isoelectric focusing of milk caseins, HPLC, mass spectrometry and ELISA
are widely reported. All techniques are based on strategies suited to evaluate the protein patterns
originating from the major whey proteins or casein fraction. All these analytical methods are able to
detect bovine milk proteins to the minimum level of 0.5–1 %. Still, the success of analytical tools that
rely on protein detection for species identification may be in some cases is affected by proteolysis or
denaturation of milk proteins as a result of heat treatment during processing.
In the last years, full attention has been turning towards application of DNA-based approaches for
the authentication of food. Particularly, the polymerase chain reaction (PCR) is increasingly used for the
specific detection of the animal origin in milk and milk products. DNA from somatic milk cells, principally
represented by leucocytes persists in milk products and may be analysed for species identification.
Several PCR-based techniques (DNA hybridization assay; restriction enzyme analysis, RFLP; singlestranded conformation polymorphism analysis, SSCP; duplex polymerase chain reaction, duplex-PCR)
have been reported to be performed to amplify nuclear genome obtained from milk and milk products.
These methods currently represent valid complements to protein electrophoretic and immunochemical
analyses. Their reliability and very low thresholds of detection make them promising as routine tools.
The methods developed so far rely mostly on PCR-amplification of various regions of the mitochondrial
genome. Only 2 of them assure protection from false negative results, as the mix contains primers for all
the identified species in a single tube. Other published methods use primers for single species or apply
restriction analysis of the obtained PCR-product. On the basis of various studies demonstrating that DNA
is not degraded after thermal and enzymatic processes, this has come up as a new strategy for the detection
of low amounts of interspecies milk. Present paper will focus on DNA based techniques.
Basic methodology
DNA Extraction from whole blood /milk is isolated using standard protocol of lysis, proteinase
K digestion, phenol - chloroform extraction, ethanol-precipitation.
Primers Designing of specific primer pairs for detection of cow and buffalo genomic/mitochondrial
DNA is done taking care to avoid significant Tm differences between the primers, thereby preventing
the generation of unspecific products. Some of the primer pairs from literature have been tabulated:
Table: Some of the Primer Pairs with respective Annealing Temperatures and PCR Products *
S.No
Forward Primer
5’------------3’
Reverse Primer
5’--------------3’
Ta°C
PCR Product
(bp)
1
GGTAAATCTCGTGCCAGCCA
TCCAGTATGCTTACCTTGTTACGAC
56
300
2
GAACTCTGCTCGGAGACGAC
AGCACCAATTATTAGGGGAAC
56
134
3
CAATAACTCAACACAGAATTTGC
CGTGATCTAATGGTAAGGAATA
52
300
4
CCAACATGCGTATCCCGT
AGCGGATGCATGATGAAATG
52
444
5
CTAGAGGAGCCTGTTCTATAATCGATAA
TGGTTTCATAATAACTTTCGCGCT
63
223
Lo´ pez-Calleja, et al. (2004); .2. FELIGINI et al.(2005); 3-4: Kotowicz et al. (2007)l 5. D´ıaz et al., (2007)
97
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Figure 1: Comparison of Qualitative and Quantitative PCR based species Identification (Ballin et al., 2009)
[Some workers used universal primer, cytochrome b (cytb; cytochrome b1 and cytochrome
b2). to differentiate milk from animal species (cattle, buffalo, goat and sheep) each according to its
mitochondrial DNA (mtDNA). The PCR product was digested by restriction endonuclease and
yielded a species-specific restriction profile. The assay was more rapid than conventional methods
and showed considerable sensitivity.]
PCR amplification performed in a 50 L reaction mixture containing template DNA, 200 µM dNTPs,
10 mM Tris-HCl, pH=8.3, 50 mM KCl, 1.5 mM MgCl2, 100 nM primers, 1.5 U DNA polymerase with
PCR conditions of initial denaturation step at 95 °C for 10 min followed by 35 cycles of 95 °C for 30 s,
annealing temperatures indicated against each primer and 72 °C for 30 s; final extension step at 72 °C
for 10 min. Agarose Gel Electrophoresis is carried out to check for the amplicons.
Analysis is carried out based on nucleotide-nucleotide BLAST similarity search (http://www.
ncbi.nlm.nih.gov/ BLAST) was conducted with the bovine and bubaline specific primer sequences.
98
Species-Specific Identification of Milk and Milk Products: A Molecular Approach
Conclusion
Modern molecular techniques based on DNA analysis have found good applicability in detecting
adulteration and they represent valid complements to the methods relying on protein analysis for the
identification of animal species. DNA-based techniques have become effective and reliable also for
commercial dairy products. Possible applications in DNA analysis includes traditional and real time
PCR. PCR amplified sequence can originate from either mitochondrial or genomic DNA, where both
single copy and repetitive sequences can be used. The choice of analytical technique and especially the
DNA sequence has a large influence on the limit of detection. However, quantitative methods based
on genome/genome equivalents that rely on a fixed copy number and use of repetitive sequences is
also an option.
References
Ballin N.Z., et al. (2009). Species determination – Can we detect and quantify meat adulteration? Meat Science. 83:165–
174.
El-Rady, A. et al., (2006). Identification of milk source by polymerase chain reaction-restriction fragment length
polymorphism analysis. Journal of Rapid Methods and Automation in Microbiology 14 (2) 146–155.
Feligini M., et al. (2005). Detection of Adulteration in Mozzarella Cheese, Food Technol. Biotechnol. 43 (1) 91–95.
Kotowicz M, et al. (2007). Application of a duplex-PCR for detection of cows’ milk in goats’ milk. Ann Agric Environ
Med 2007, 14, 215-218.
Lo´ pez-Calleja I., et al. (2004). Rapid Detection of Cows’ Milk in Sheeps’ and Goats’ Milk by a Species-Specific
Polymerase Chain Reaction Technique. J. Dairy Sci. 87:2839–2845.
L´opez-Calleja I., et al., (2007). Application of a polymerase chain reaction to detect adulteration of ovine cheeses with
caprine milk. Eur Food Res Technol. 225: 345–349.
99
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Proteomic Techniques for Application in Food Science
Ashok K. Mohanty
Animal Biotchnology Centre, NDRI, Karnal
Introduction
In recent years, a number of ‘‘omics’’ technologies (genomics, proteomics, metabolomics, and
others) have become available that hold promise for increasing the understanding of the complexities
of pathogen behavior at the molecular level and for the development of improved pathogen detection
and typing systems. Genomics is the study of genes and their function, transcriptomics refers to global
analyses of gene expression, and proteomics is the study of the complete set of proteins produced by
a species and their modifications, expression, involvement in metabolic pathways, and interactions.
Omics-based tools enable researchers to explore complex biological processes in a quantitative and
integrative manner via a systems biology approach. These methods of analysis are facilitating the
identification of genes that are responsible for survival and persistence in specific environments, and
revealing genes that are potential targets for interventions and that play a role in pathogenesis, stress
responses, and biofilm formation.
Proteomics is the study of the proteome, the protein complement of the genome. Proteomics or, more
appropriately functional proteomics refers to the branch of discovery of science focusing on proteins.
The term ‘proteome’ is used to describe the complete set of proteins that is expressed, and modified
following expression, by the entire genome in the lifetime of a cell. The term “proteomics” and “proteome”
were coined by Marc Wilkins and colleagues in the early 1990s and mirror the terms “genomics” and
“genome”, which describe the entire collection of genes in an organism. Today, proteomics is a scientific
discipline that promises to bridge the gap between our understanding of genome sequence and cellular
behaviour. It can be viewed as more of a biological assay or tool for determining gene function.
Initially the term was used to describe the study of the expressed proteins of a genome using twodimensional (2D) gel electrophoresis, and massspectrometry (MS) to separate and identify proteins
and sophisticated informatics approaches for deconvoluting and interrogating data. This approach is
now referred to as “expression” or “global profiling” proteomics. The scope of proteomics has now
broadened to embrace the study of “protein-protein” interactions (protein complexes), referred to as
cell-mapping proteomics (Blackstock and Weir, 1999).
The many faces of proteomics
Proteomic analysis (or analytical protein chemistry). The large-scale identification and
characterization of proteins, including their posttranslational modifications, such as phosphorylation
and glycosylation. Analysis is done with the aid of mass spectrometry or Edman degradation.
Expression proteomics (or differential display proteomics). Two-dimensional gels are used for
global profiling of expressed proteins in cell lysates and tissues. This conventional approach is being
challenged by non-2D gel methods, such as liquid-based isoelectric focusing (IEF) or ion-exchange
chromatography / reversed-phage high-performance liquid chromatography (RP-HPLC). Proteins are
typically identified by massspectrometry (MS). In many situations, these methods are complemented
by DNA-based array methods.
Cell-mapping proteomics (or cataloging of protein-protein interactions). Protein-protein interactions
and intracellular signaling are determined by identification of protein complexes (obtained by affinity
purification and protein identification by MS) or by direct DNA readout (e.g. yeast two-two hybrid,
phage display, ribosome display, and RNA-peptide fusions).
Proteomics vis-a vis Genomics
Large-scale genome sequencing:
One of the most biological achievements to emerge during the last 40 years has been the completion
100
Proteomic Techniques for Application in Food Science
of draft DNA sequences of the human genome, published by
the International Human Genome Sequencing Consortium
(a publicly) funded project and by Celera Genomics (a
commercial effort). This has provided a blue print of the
information needed to create a human being and revealed
for the first time the organization of a vertebrate’s DNA.
The public project estimates that there are 31, 000 proteinencoding genes, where as Celera finds ~26,000, with many
more still to be found (a current estimate suggests that the
number of protein-encoding genes may be on the order of
60,000).
Interestingly, the number of coding genes in the human
sequence is not dramatically different from the numbers Biological context of Genomics and Proteomics
reported for phylogenetically remote organisms: 6, 000 for
a yeast cell, 13,000 for a fly, 18,000 for a worm, and 26,000 for a plant (Genomes Online Databases
at http://wit.integratedgenomics.com/GOLD). The number of genes reported for multicellular
organisms is not highly accurate because of limitations of existing abinitio gene prediction methods used
to identify genes. The existence of an open reading frame (ORF) in genomic data does not necessarily
imply the existence of functional gene. In human DNA, gene prediction by abinitio methods is difficult
because of extensive alternative splicing, lower density exons, and high proportions of interspersed
repetitive sequences. Given the unreliability of abinitio gene prediction software, all genes will need
to be experimentally identified and annotated. Hence, verification of a gene product by proteomic
analysis is an important first step in annotating the genome.
Disparity between mRNA Profiling and protein profiling:
There is no simple correlation between changes in mRNA expression levels (transcriptomics) and
those in protein levels (proteomics). The link between transcript levels and protein levels in a given cell
or tissue is difficult. It is also understood that array-based gene expression monitoring or other gene
expression methods for measuring mRNA abundances, alone are insufficient for analyzing the cell’s
protein complement. There is a marked disparity between the relative expression levels of mRNAs
and those of their corresponding proteins. Differing stability of mRNAs and different efficiencies
in translation can affect the generation of new proteins. Once formed, proteins differ significantly
in stability and turnover rates. Many proteins involved in signal transduction, transcription factor
regulation, and cell-cycle control are rapidly turned over as a means of regulating their activities.
Also mRNA levels tell us nothing about the regulatory status of the corresponding proteins, whose
activities and functions are subject to many endogenous posttranslational modifications and other
modifications by environmental agents. Therefore, the complication arises when considering the
complementarity of genomics and proteomics. Despite the notion that one gene gives rise to one
protein, the situation in eukaryotic cells is more likely six to eight proteins per gene. Thus, there
may be several hundred thousand human proteins after splice variants and essential posttranslational
modifications are included. For example, 22 different forms of human α-1-antitrypsin have been
observed in human plasma. Such biological complexities can be unraveled using proteomic studies to
understand how cells modulate and integrate signals.
Identification and analysis of proteins
Four key platform technologies are crucial to any proteomics strategy aimed at elucidating the
function of an unknown gene.
•
Sample preparation & handling
•
Determination of partial amino acid sequence information
•
Protein identification and quantification
•
Cell mapping
101
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
SEC: size exclusion chromatography; IEX: ion-exchange chromatography; RP-HPLC: reversed
–phase high-performance liquid chromatography; HIC: hydrophobic interaction chromatography;
2DE: two-dimensional gel electrophoresis; 1DE: one dimensional gel electrophoresis; FFE: free-flow
electrophoresis; CZE: capillary zone electrophoresis; FRET: fluorescence resonanace energy transfer
Protein separation strategies:
One of the rate-limiting steps
in any proteomic analysis study
is obtaining, and then handling,
sufficient quantities of target protein
(s) from its original biological source.
The classical method of quantitative
and
qualitative
expression
Technology platforms for Proteomics
proteomics combines protein separation by high-resolution 2D gel electrophoresis with MS or MS/MS
identification of selected protein spots. Because, even the best 2D gels can routinely separate no more
than 1500 proteins, this technique is limited to the most abundant proteins if a crude protein mixture
(whole-cell lysate) is used. 2D electrophoresis is limited by the amount of material that can be applied to
the first-dimension immobilized pH gradient gel (~150 ug to low milligram quantities). Hence 2D gels
have limited ‘scale up’ capability. For this reason, it is often desirable to “trace enrich” for a particular
subclass of proteins. By
analyzing proteins in a cellular
compartment or organelle,
it is possible to reduce the
complexity and differences
in abundance of a subset of
proteins within a cell.
Two-Dimesional
SDSPAGE: This separation method
has
become
synonymous
with proteomics and remains
the single best method for
resolving highly complex
protein
mixtures.
Twodimensional SDS-PAGE is
Proteomics strategies for the identification and analysis of proteins
actually a combination of two
different types of separations.
In the first, the proteins are
resolved on the basis of isoelectric point by IEF. In the second, focused proteins are then are further
resolved by electrophoresis on a polyacrylamide gel. Thus 2D-SDS-PAGE resolves proteins in the first
dimension by isoelectric point and in the second dimension by molecular weight. Dedicated 2D-SDSPAGE systems are available that use immobilized pH gradient (IPG) strips and relatively foolproof
hardware to facilitate the transfer of proteins from the IPG strip into the SDS-PAGE slab gel. The IPG
strip is based on the use of immobilized pH gradients, in which polycarboxylic acid ampholytes are
immobilized on supports to reproducibly create stable pH gradient. The use of narrow pH ranges
facilitates the separation of proteins with highly similar isoelectric points. Proteins separated by 2D
gels are visualized by conventional staining techniques, including silver, Coomassie, amido black
stains and fluorescent staining. Silver-staining and newer fluorescent dyes are the most sensitive.
Cell mapping and identification of proteins in complexes
One way to observe interacting proteins involved in a given biological process is to specifically
enrich for these proteins. Typically, this requires knowledge of the activity of atleast one protein in
102
Proteomic Techniques for Application in Food Science
the multiprotein complex. Under nondenaturing conditions, interacting proteins can be enriched from
complex protein mixtures (e.g., cell lysates) using methods such as:
•
Coimmunoprecipitation or “pull-down” techniques using antibodies directed against one of the
component proteins.
•
Coprecipitation using affinity-tagged recombinant proteins and antibodies directed against the
“tag” epitope
•
Protein-affinity-interaction chromatography (e.g using recombinant glutathione S-transferase (GST)fusion proteins and glutathione-affinity chromatography).
•
Isolation of intact multiprotein complexes (e.g., nuclear pore complex, ribosome complexes,
spliceosomes).
Determination of Partial Amino Acid Sequence
Usually, the final step of most proteomic studies, independent of the purification method
employed, utilizes either SDS-PAGE or 2D acrylamide gels to separate the proteins for identification
and characterization. Following electroblot and transfer to an inert membrane, such as polyvinylidine
difluoride (PVDF), intact proteins can be identified directly by amino- or carboxy-terminal amino
acid sequence analysis or indirectly from peptides generated by in-gel or on-membrane digestion
of the protein with a protease usually trypsin. MS-based methods usually identify a protein, not by
analyzing it directly, but by analyzing the peptides derived from proteolytic digestion. Usually, a
small number of peptides yield sufficient information to permit protein identification (by peptide mass
finger printing (PMF) and/or MS/MS of individual peptides. In contrast to peptides, the molecular
mass of intact proteins is usually insufficient to allow database identification.
MALDI-MS is used to
determine the accurate mass
of a group of peptides derived
from a protein by digestion
with a sequence-specific
protease, usually trypsin,
thus generating a peptide
mass map or peptide mass
fingerprint. Because trypsin
cleaves proteins at the amino
acids arginine and lysine, the
masses of tryptic peptides can
be predicted theoretically for
Cell mapping: affinity capture
any entry in a protein sequence
database. Electrospray ionization and tandem mass spectrometry is used to sequence the isolated
peptides from a peptide mixture.
Differential display proteomics
A fundamental aspect of proteomic research is the determination of protein expression levels
between two different states of a biological system (e.g. relative quantification of protein levels), such
as that encountered between a normal and diseased cells or tissues. This is referred to as differential
display or comparative proteomics. This can be done in two ways such as running and comparing
samples in 2D-SDS-PAGE or with LC-MS and Isotope tags.
For differences in the protein-expression profiling, we compare 2D-gels from two different
samples for differences in the occurrence or intensity of protein spots. This approach provides a useful
means of comparing proteomes. However, identification of protein is cumbersome and difficult by
this procedure. Application of peptide mass fingerprinting and LC-MS-MS analysis now makes it
possible to identify essentially any protein one can detect by staining the gel. Therefore, the critical
103
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
task in comparative proteomics with 2D gels is identifying the features that differ between the gels.
The LC-MS approach to proteome comparisions is conceptually the opposite of the 2D gel approach.
Whereas the 2D gel approach separates proteins and begins with an image comparision, the LC-MS
approach separates peptides and ends with data mining to assess differences between samples. Two
protein samples are treated with reagents to “tag” them. The tags are chemically identical, except
that one contains heavy isotopes (e.g. 2H, 15N, 13C, 18O etc.) and the other contains light isotopes. The
samples are digested and the peptides are analyzed by LC-MS-MS. Analysis of the MS-MS data allows
identification of the protein present. Examination of the full-scan spectra corresponding to each MSMS scan then allows measurement of the ratio of the light- and heavy- isotope tagged peptides. This
ratio corresponds to the ratio of that protein in the two samples. This approach provides a relative
quantification of the level of a particular protein in two samples.
Mapping protein modifications:
Vast majority of all eukaryotic proteins are posttranslationally modified and more than 200
posttranslational modifications (PTMs) of amino acids have been reported so far. Practically all PTMs
are associated with either an increase or a decrease in molecular mass. The two major PTMs of proteins
are phosphorylation and glycosylation. Phosphorylation of proteins is a ubiquitous regulatory
mechanism in both eukaryotes and prokaryotes. Intracellular phosphorylation is regulated by protein
kinases (dephosphorylation is regulated by protein phosphatases), which are activated in response
to extracellular signals and trigger cells to switch on or off many diverse processes such as metabolic
pathways, kinase cascade activation, membrane transport, gene transcription, and motor mechanisms.
Protein phosphorylation can be examined in several ways such as ‘phosphopeptide mapping of 32Plabelled proteins and peptides’, amino-terminal sequencing using Edman degradation procedure and
Mass spectrometry. While all the three methods are equally good, MS is an ideal tool in proteomics
studies of PTM identification and characterization because of its high sensitivity.
Application in food science
Microarray-based comparative genomics research, which takes advantage of information
available from whole genome sequences, is leading to an increased understanding of the evolution
and pathogenesis of food-borne pathogens and is providing critical information for the development
of improved detection and genotyping methods.DNAmicroarray technology provides accurate
measurements of gene expression for every gene in a genome and allows this expression to be
analyzed in response to specific environmental variables. Further, this technology can be utilized
to identify genes that are controlled by specific regulators by comparing gene expression in mutant
and wild-type bacteria. However, the potential for the analysis of gene expression of pathogens in
food environments has not yet been completely realized due to the substantial technical challenges
associated with accurately measuring bacterial gene expression in complex matrices. Genomotyping
involves the comparison of whole genomes of bacteria using DNA microarrays and has been utilized
to identify potential genes associated with virulence, disease severity, and adaptation to different
hosts and ecological niches. Used as diagnostic tools, DNA microarrays offer the capability to detect
and characterize a broad spectrum of pathogens simultaneously in a relatively short period of time.
Various food grade bacteria with specific reference to lactic acid bacteria are used for production
of fermented foods. Different lactic acid bacteria produce different proteins in the system during
fermentation. Global analysis of proteome of useful lactic acid bacteria using proteomic approaches
will help to identify various proteins expressed in the system. Systematic analysis of the proteins
expressed will give an idea about the importance of various proteins during fermentation. Differential
expression proteomics in between various useful microorganisms will help us to identify useful
biomolecules specific for a particular microorganism. This will help us to identify organism specific
cellular markers for future application in food product development.
Milk constitutes an important ingredient of food system. This is constituted of number of growth
104
Proteomic Techniques for Application in Food Science
promoting
proteins,
enzymes and signaling
molecules. Global and
differential expression
analysis of proteins
in milk of various
animal species will
help us to identify
various new proteins
of health importance.
Until now, most of
the abundant proteins
have been studied in
milk. However, many
low abundant proteins
which
are
secreted
in milk are generally
ignored and have not
been studied in detail.
Proteomic approaches will help to identify useful low abundant proteins, which can be studied further
for understanding their beneficial properties. Thus proteomic techniques are extremely useful for
discovery of novel biomolecules for future applications in food science. Global genetic-based analyses
provide information regarding which genes an organism contains or which genes are expressed under
specific conditions; however, examining the posttranslational protein output of an organism allows
one to query the ultimate outcome of the organism’s genetic and regulatory activities. Techniques
that fall within the category of either proteomics or protein arrays are used for global analysis of
cellular protein output under different conditions and potentially those relevant to food and foodprocessing environments. The integration of both genetic- and protein based approaches provides a
global analysis of treatment- or environment-related changes at the molecular level, thus presenting
a more comprehensive view of cellular activities. The development of high-throughput analysis
techniques will make possible multi-omics approaches to understanding complete biological systems,
a field known as systems (integrated) biology. Although omics technologies are becoming standard
research tools that offer tremendous opportunities, there are also significant challenges. There is a
need to properly manage the large quantity of complex raw data generated by these technologies in
a manner such that it can be adequately analyzed, scrutinized, and compared for the benefit of the
scientific community. There are various omics standardization activities underway, which are critical
for the integration and interpretation of data from different data sources. Lastly, there is a need to
bridge the gap between knowledge of the genome, proteome, and metabolome, and results obtained
in relevant systems by studying the behavior of pathogens in foods and in the animal host, not only
in model systems under laboratory conditions. The knowledge garnered from omics-based research
in the coming years will play an important role in understanding how pathogens survive food safety
barriers and interact with host species. Each new advance in our understanding will potentially give
rise to improved and novel strategies for detection, identification, and control of food-borne pathogens,
as well as for diagnosis and control of infections.
105
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Evaluation of Probiotic Attributes of Dairy
Starter Cultures Using Various Test Methods
Rameshwar Singh
Dairy Microbiology Division, NDRI, Karnal
Probiotics – friendly bacteria with a host of benefits
“Let food be the medicine and medicine be the food,” the age-old quote by Hippocrates, is certainly
the dogma of today. With the growing interest in self-care, recognition of the link between diet and
health is becoming stronger day by day. As a result, the market for functional foods, or foods that
promote health beyond providing basic nutrition, is flourishing. Within the functional foods is the
rapidly expanding arena of probiotics.
Probiotics (according to the currently adopted definition by FAO/WHO) are: “Live microorganisms
which when administered in adequate amounts confer a health benefit on the host”. The term probiotics
is derived from the Greek word ‘pro’ means ‘for’ and ‘bio’ means ‘life’. Lilly and Stillwell were the
first one to introduce the term probiotics in the year 1965 to describe the growth promoting factors
produced by the microorganisms. However over the years, the term probiotics has been linked to
several definitions. As per the version of Parker, probiotics can be defined as those organisms and
substances, which contribute to the intestinal microbial balance. Later, Fuller redefined probiotics as
foods containing live microorganisms, which actively enhance the health of consumers by improving
the balance of microflora in the gut, when ingested live in sufficient numbers.
Types of probiotics
Many types of bacteria have been used as probiotics since time immemorial. Today food products
containing probiotics are almost exclusively dairy products – fluid milk, dahi, soy yogurt, yogurt due to
the historical association of lactic acid bacteria with fermented milk. The most frequently used bacteria
in these products include the Lactobacillus and Bifidobacterium species. Some Enterococcus species, yeasts
like Saccharomyces species too find a place in the long list of probiotics. In particular lactobacilli are
generally used as probiotics. This may have historical reasons since Metchnikoff proposed that the
lactobacilli present in yoghurt would have a health promoting effect.
Potential health benefits of probiotic
The list of potential health promoting traits attributed in particular to LAB is quite impressive.
Health benefit: proposed mechanism(s)
1.
Alleviation of lactose intolerance:
Bacterial β-galactosidase acts on lactose
2.
Positive influence on intestinal flora:
Lactobacilli influence activity of overgrowth flora, decreasing toxic metabolite production
Antibacterial characteristics
a.
b.
3.
a.
b.
c.
d.
e.
f.
g.
h.
106
Prevention of intestinal tract infections:
Adjuvant effect increasing antibody production
Stimulation of the systemic or secretory immune response
Competitive exclusion
Alteration of intestinal conditions to be less favorable for pathogenicity (pH, short chain fatty
acids, bacteriocins)
Alteration of toxin binding sites
Gut flora alteration
Adherence to intestinal mucosa, preventing pathogen adherence
Competition for nutrients
Evaluation of Probiotic Attributes of Dairy Starter Cultures Using Various Test Methods
4.
a.
b.
c.
d.
e.
f.
g.
Improvement of the immune system:
Strengthening of non-specific defense against infection
Increased phagocytic activity of white blood cells
Increased serum IgA after attenuated Salmonella typhimurium challenge
Increase in IgA production
Proliferation of intra-epithelial lymphocytes
Adjuvant effect in antigen-specific immune responses
Regulation of the Th1/Th2 balance, induction of cytokines
a.
b.
c.
Reduction of inflammatory or allergic reactions:
Restoration of the homeostasis of the immune system
Regulation of cytokine synthesis
Prevention of antigen translocation into blood
a.
b.
c.
d.
e.
Anti-colon cancer effect
Mutagen binding
Carcinogen deactivation
Alteration of activity of colonic microbes
Immune response
Influence on secondary bile salt concentration
a.
b.
c.
Blood lipids, heart disease :
Assimilation of cholesterol
Alteration of activity of bile salt hydrolase enzyme
Antioxidative effect
5.
6.
7.
8.
b.
Antihypertensive effect:
Peptidase action on milk results in antihypertensive tripeptides (angiotensin converting
enzyme inhibitors)
Cell wall components act as angiotensin converting enzyme inhibitors
a.
b.
c.
Urogenital infections:
Adhesion to urinary and vaginal tract cells
Competitive exclusion
Inhibitor production (H2O2, bio-surfactants)
a.
9.
10.
a.
b.
c.
Infection caused by Helicobacter pylori:
Competitive exclusion
Lactic acid production
Decreased urease activity of H. pylori in humans after administration of a supernatant of a
Lactobacillus culture
11.
Regulation of gut motility (constipation)
Characteristics expected of potential probiotic strains
•
Non toxic and non-pathogenic
•
Accurate taxonomic identification
•
Normal inhabitant of the targeted species
•
Capable of survival, proliferation and metabolic activity in the target site, which
• resistance to gastric acid and bile
• ability to persist, albeit for short periods, in the gastrointestinal tract
• adherence potential preferred
• ability to compete with the resident flora
•
Production of antimicrobial substances
•
Antagonism towards pathogenic bacteria
•
Ability to modulate immune responses
•
Ability to exert at least one clinically documented health benefit
implies:
107
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
•
•
•
•
Genetically stable
Amenability of the strain and stability of the desired characteristics during processing, storage
and delivery
Viability at high populations
Desirable organoleptic and technological properties when included in fermentation
processes
Evaluation of the probiotic attributes of lactic acid bacteria
(1) Acid tolerance: The ability of lactic acid bacteria (LAB) to resist acidic conditions (Clark et al., 1997) is
tested. The LAB are grown in their respective broth overnight at their respective growth temperatures.
The actively grown cells (8 log10 cfu ml-1) are harvested by centrifugation and resuspended in equal
volume of broth with pH adjusted to pH 4.0, pH 3.0, pH 2.0 with 1 M HCl and simultaneously in broth
with pH 7.0 as control. Survival is evaluated by determining the viable counts of the samples serially
diluted in peptone water after 0, 30, 60 and 120 min in acidic conditions, which is subsequently plated
on their respective agar and incubated at their respective temperature.
(2) Bile tolerance : Tolerance for bile acids is tested according to the method of Gilliland et al. (1984).
The LAB are grown in their respective broth overnight at their respective growth temperature. The
actively grown cells (8 log10 cfu/ ml) are harvested by centrifugation and resuspended in equal volume
of their broth supplemented with 0.5%, 1%, 2% w/v ox bile and without supplement as a control .
Survival is evaluated by plate count on their respective agar, after 0, 1, 3 and 12h of incubation in broth
containing bile salts reflecting the time spent by food in the small intestine and subsequently the plates
were incubated at their respective temperature.
(3) Cell surface hydrophobicity : Ability of the organisms to adhere to hydrocarbons is a measure
of their adherence to the epithelial cells in the gut i.e. cell surface hydrophobicity. Cell surface
hydrophobicity of LAB is determined according to the method described by Rosenberg et al., (1980)
with slight modification using n-Hexadecane, n-Octane and Xylene. Cultures of the strains are grown
in their respective broth overnight at their respective growth temperatures. The cells (8 log10 cfu ml-1)
are harvested in their early log phase by centrifugation at 12,000 x g for 5 min at 5ºC, washed twice
and resuspended in 5 ml phosphate urea magnesium (PUM) buffer (pH 6.5) and the cell suspension
is adjusted to an absorbance value (A610) of approx. 0.8 - 1.0. Three ml of the bacterial suspension are
put in contact with 1 ml of each of the hydrocarbons. The cells are pre-incubated at their respective
temperature for 10 min and then vortexed for 120 s. The suspension is then kept undisturbed at their
respective temperatures for 1h to allow phase separation and the hydrocarbon layer is allowed to rise
completely. After 1h, aqueous phase is removed carefully with a Pasteur pipette and the absorbance
(A610) is measured using Spectrophotometer . The decrease in the absorbance is taken as a measure of
the cell surface hydrophobicity (%H) calculated with the given equation.
Where, ODinitial and ODfinal are the absorbance (at 610nm) before and after extraction with the
three hydrocarbons.
(4) Antibiotic susceptibility : Pattern of resistance/susceptibility to antibiotic of LAB is studied by
disc diffusion method. Various antibiotic discs of ampicillin, amoxycillin, bacitracin, chloramphenicol,
ciprofloxacin, cotrimoxazole, erythromycin, gentamicin, kanamycin, nalidixic acid, penicillinG,
rifampicin, streptomycin, tetracycline, and vancomycin are used. Mueller Hinton agar 2 (Himedia)
plates are poured in petri plates and allowed to solidify. These are subsequently over laid with 4 ml of
Mueller Hinton agar 2 soft agar tempered at 45ºC and seeded with 200 µL of active cultures. Petriplates
are allowed to stand at room temperature for 15 min and then the antibiotic discs are dispensed onto
agar using forceps under aseptic conditions. The agar plates are incubated at 37ºC aerobically for 24 h.
Diameter (mm) of zone of inhibition is measured using antibiotic zone scale and results are expressed
108
Evaluation of Probiotic Attributes of Dairy Starter Cultures Using Various Test Methods
in terms of resistance, moderate susceptibility or susceptibility by comparing with the interpretative
zone diameters given by Performance Standards for Antimicrobial Disk Susceptibility tests (CLSI,
2007) for disc diffusion antibiotic susceptibility test.
(5) Antimicrobial activity : LAB are screened for their antibacterial activity and inhibitory spectra
against a broad range of Gram-positive and Gram-negative strains by spot-on-lawn assay (Uhlman
et al., 1992). Active pure cultures of LAB are grown in broth for 16-18 h at 37ºC. Cell free culture
supernatants (CFCS) are prepared by centrifuging the broth at 10,000 rpm for 10 min at 4ºC. The
culture supernatants thus obtained are heat treated (90ºC, 5-7 min) to kill any live cell. Fresh culture of
indicator bacteria grown for 16-18 h are further inoculated for its active growth at optimum temperature
for 3-4 h (absorbance at 660 nm = 0.01). Fifty microlitres of this culture is mixed with 7 ml of melted and
tempered (45ºC) TGE soft agar and poured onto the previously surface dried TGE agar plates. The soft
agar is allowed to solidify and 5 μl of CFCS is directly spotted on the lawns of indicator organism. The
plates are kept undisturbed for 2 h and subsequently incubated at 37oC. After 24 h of incubation, a 5
mm or more diameters (mm) of the growth inhibition zones are considered positive inhibition.
(6) Cholesterol reduction test : For the ability of probiotic culture to reduce cholesterol present in the
media, 20 mL of respective broth (containing 3% oxgall and 1% lipid cholesterol rich) is inoculated
with culture and incubate at respective temperature for 16 h. Uninoculated broth (control) is processed
in the same way. Cells are removed by centrifugation at 8000 g for 5 min. 0.5 mL supernatant is placed
into a clean glass tube and 3 mL of 95% ethanol is added to each tube, followed by 2 mL of 50%
potassium hydroxide and mixed. Tubes are placed in water bath at 60°C for 10 min. and cooled at
room temperature (20°C). After this, 5 mL of hexane is carefully added and mixed vigorously with a
vortex for 20s. A 3 mL H2O is added and mixing is done with the vortex. Then the tubes are allowed to
settle at room temperature for 15 min or until complete phase separation (aqueous and organic phase).
Next, 2.5 mL of the hexane layer (upper phase) is transferred into a clean tube and dried at 60°C under
nitrogen gas flow. The residues formed are resuspended in 4 mL of o-phthalaldehyde reagent. Tubes
are kept at room temperature for 10 min and then 2 mL of concentrated sulfuric acid is pipette slowly
down the inside of each tube. These tubes are mixed thoroughly. After standing at room temperature
for an additional 10 min, absorbance is recorded at 550 nm (A550) against the reagent blank. The
results are expressed as micrograms (μg) of cholesterol per milliliter.
References
Clark, P.A., Cotton, L.N. and Martin, J.H. 1997. Selection of bifidobacteria for use as delivery adjuncts in cultured dairy
foods. II. Tolerance to stimulated pH of human stomachs. Cult. Dairy Prod. J., 28(4): 11-14.
Gilliland, S.E., Staley, T.E. and Bush, L.J. 1984. Importance of bile tolerance of Lactobacillus acidophilus used as dietary
adjunct. J. Dairy Sci., 67: 3045-3051.
Rosenberg, M., Gutnick, D. and Rosenberg, E. 1980. Adherence of bacteria to hydrocarbons: A simple method for
measuring cell-surface hydrophobicity. FEMS Microbiol. Lett., 9: 29-33.
Performance Standards for Antimicrobial Disk Susceptibility tests, Clinical and Laboratory Standards Institute (CLSI),
27(1), 2007.
Uhlman, U., Schillinger, U., Rupnow, J.R. and Holzapfel, W.H. 1992. Identification and characterization of two
bacteriocin-producing strains of Lactococcus lactis isolated from vegetables, Int. J. Food Microbiol., 16: 141-151.
Spencer, J. and Spencer, A. 2001. Methods in Biotechnology: Food Microbiology protocols, 14: 174-181.
109
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Identification of Lactobacillus spp by
PCR based Molecular Methodology
Sachinandan De and Rupinder Kaur
Animal Biotechnology Centre, NDRI, Karnal
Lactic Acid Bacteria (LAB) constitute an important class of organism widely distributed in nature
and occurring naturally as indigenous microflora in raw milk. They play an important role in food and
feed fermentation. Lactobacillus forms the most numerous genus in the -heterogeneous group of LAB.
Members of the genus Lactobacillus are also found in plants and in plant-derived materials, such as
silage, grains and foods, but also in the gastrointestinal tract (GIT) of humans and animals (Stewart,
1997). Lactobacillus species are used industrially for the production of yogurt, cheese, sauerkraut,
pickles, beer, wine, cider, kimchi, chocolate and other fermented foods, as well as animal feeds, such as
silage. The genus Lactobacillus currently consists of over 149 species and 29 subspecies and encompasses
a wide variety of organisms (http://www.bacterio.cict.fr/l/lactobacillus.html). Members of the genus
Lactobacillus are Gram positive, non motile, non spore forming rods or cocci bacteria that produce
mainly lactic acid after carbohydrate fermentation (Kandler and Weiss 1986).
Quality assurance programs associated with research, development, production and validation
of the health or technological benefits of these bacteria require their relevant isolation, counting and
identification. The precise identification of these bacteria to the genus and species level is quite laborious.
At present the identification of Lactobacilli largely depend on selective growth in microaerophilic
MRS (deMan Rogosa - Sharpe) media and an array of biochemical tests like Gram staining, catalase
test, carbohydrate fermentation. These microbiological / culture based methods are time consuming
and often give rise to ambiguous results. Polyphasic approaches combining biochemical, molecular,
and morphological data are important for the accurate classification of lactic acid bacteria (Klein et
al., 1998). Lactobacillus species may be difficult to identify by conventional biochemical methods,
although simplified approaches are useful for presumptively assigning organisms to this genus.
Many DNA based methods have been applied to the identification of Lactobacilli. The ribosomal
RNA gene sequences (16 S rRNA and 23S rRNA) have been used by many workers for the identification
of LAB. This is possible because of the conserved nature of the 16S rRNA gene sequence and a vast
repertoire of online rRNA gene sequences reported by the scientific community against which the rRNA
could be compared. The 16S rRNA gene based classification has revealed considerable diversity in this
genus (Vela et al. 2008). New Lactobacillus species are continually being described (www.bacterio.
cict.fr), with 10 new species in 2007 and four in 2008. Some Lactobacillus species have been renamed
over the years. These changes cause confusion and some previous identification of Lactobacilli may
yet be subject to change . Dubernet et al (2002) have developed a PCR assay using a genus specific
primer, targeted to the genes encoding the 16S rRNA. As we enter into the genomics era the rRNA
based microbial phylogeny is under critical scrutiny. Other useful genotypic studies using protein
encoding genes tuf gene (encoding elongation factor Tu, involved in protein biosynthesis, Chavagnat
et al 2002), rpoB (RNA polymerase beta subunit) , gyr B (B subunit of DNA gyrase), hsp 65, ( heat
shock protein 65) dnaJ (asoociated with DnaK chaperone machinery) , recA (encoding recombinase A),
groEL (groEL, encoding a 60-kDa heat shock protein) pheS (phenylalanyl-tRNA synthase) have been
published recently. Being housekeeping genes from biosynthetic pathways, they retained the amino
acid structure more or less conserved without modifying the product of translation substantially by
tolerating silent point mutations, which lead to a greater degree of variability at the nucleotide level.
Characterisation and identification of lactobacilli from genus level to
strain level
For decades, differentiation between genera has been based on phenotypic characters. Under a
light microscope, lactobacilliare generally regularly shaped, non-motile, non-spore-forming, Gram110
Identification of Lactobacillus spp by PCR based Molecular Methodology
positive rods. However, cell morphology varies widely, from long, straight or slighty crescent
shaped rods to coryneform coccobacilli. Numerous genera display such morphological features.
However, we can separate by simple tests such as tests for the oxygen tolerance, presence of catalase
and growth on acidified MRS. Classical phenotypic tests for identification of lactobacilli are based
on physiological characteristics such as respiratory type, motility, growth temperature and growth
in NaCl, and on biochemical characteristics such as homo/hetero-fermentative, production of lactic
acid isomers, metabolism of carbohydrate substrates, coagulation of milk and presence of particular
enzymes (e.g. arginine dihydrolase, antibiotic susceptibilities, and so on). Lactobacilli are typically
chemoorganotrophic and ferment carbohydrates, producing lactic acid as a major end product.
Analysis at genus level
The genus Lactobacillus is heterogeneous, with the G+C content of the DNA of its species varying
from 33 to 55% (Hammes and Vogel 1995). However, it is generally thought that G+C content should
vary by no more than a 10% range within a well-defined genus (Vandamm et al., 1996). The nucleotide
sequences of Lactobacillus 16S ribosomal DNA (rDNA) provide an accurate basis for identification. The
sequence obtained from an isolate can be compared with those of Lactobacillus species held in databases.
Recently, Dubernet et al. (Dubernet et al., 2002) defined a genus-specific primer by analysing similarities
between the nucleotide sequences of the spacer region between the 16S and 23S ribosomal RNA genes
of Lactobacillus. The specificity of this genus-specific primer combined with a universal primer was
tested against 23 strains of lactobacilli of varied origin (corresponding to 21 species) Escherichia coli, two
leuconoctocs species, Carnobacterium piscicola, Pediococcus pentosaceus, Bifidobacterium bifidum, Weissella
confusa, Enterococcus faecalis, Staphylococcus aureus and Listeria monocytogenes. Positive amplification
was only obtained with the lactobacilli strains.
Analysis at species level Phenotypical micro methods
Several combinations of tests and ready-to-inoculate identification kits such as API 50 CH, LRA
Zym and API Zym enzymatic tests can be used for the rapid and theoretically reproducible phenotypic
identification of pure cultures. They have been used for the characterisation and identification of
lactobacilli in milks [Medina et al., 2001], yoghurts and other fermented milks (Andrighetto et al., 1998)
and in cheeses (Andrighetto et al., 1998, Tilsala and Alatossava 1997). However, the reliability of these
tests has been questioned, especially for API 50 CH, initially developed for the identification of medical
Lactobacillus strains. In addition, the manufacturer’s database is not updated and some Lactobacillus
species are missing. Andrighetto et al.(Andrighetto et al., 1998) used API 50 CH to analyse 25 strains of
thermophilic lactobacilli isolated from yoghurt and from semi-hard and hard cheeses (Lb. delbrueckii
ssp. lactis and ssp. bulgaricus, Lb. helveticus and Lb. acidophilus). For most of the strains, clear assignment
to a particular species or subspecies was not possible because ambiguous results were obtained for the
sugar fermentation profile. Nigatu (Nigatu 2000) also reported a lack of agreement between the API
50 CH grouping pattern of isolates and RAPD clusters. Tynkkynen et al. (Tynkkynen et al., 1999) used
API 50 CH for identifying strains of the Lb. casei group (Lb. rhamnosus, Lb. zeae and Lb. casei). The exact
identifications of these closely related species were not reliable; some were doubtful or unacceptable
and some strains were misidentified with a good identification level. Furthermore, variability may
be observed within a single strain. For example, the Lb. rhamnosus GG strain has traditionally been
detected, counted and identified on the basis of cultures in selective anaerobic conditions on MRS or
Rogosa agar (37°C for 78 h), colony morphology (large, white, creamy and opaque), Gram staining
and cell morphology (Gram-positive and uniform rods in chains) and the carbohydrate fermentation
profile in the API 50 CHL test. However, it has been pointed out that the colony morphology and the
carbohydrate fermentation pattern of strain GG are not always typical, due to variation (Charteris et
al 1997). This variation may result from the loss or gain of plasmids, leading to inconsistency in the
metabolic traits of a strain, as most of the proteins involved in carbohydrate fermentation are plasmidencoded (Arhné et al., 1989).
111
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Sequencing
Comparison of rRNA gene sequences is currently considered to be the most powerful and accurate
method for determining the degree to which microorganisms are phylogenetically related (Woese
1987). Advances in molecular biological techniques have made it possible to sequence long stretches of
rRNA genes. Initially, reverse transcriptase was used to generate DNA from rRNA, and this DNA was
then sequenced. It is now possible to sequence 16S or 23S rDNA molecules by direct PCR sequencing,
and this method has generated large sequence databases. Although the speciesspecific sequences are
located in the first half of the 16S rRNA gene (V1-V3 region), identification is more accurate if the whole
gene is sequenced (Stackebrandt and Goebel 1994). This requires the sequencing of about 1.5 kb of
DNA. Tannock et al. (Tannock et 1999) showed that comparison of the16S-23S spacer region sequences
of lactobacilli can be used in practical situations for strain identification. The spacer region sequences
is sequencing rapidly and accurately identifies Lactobacillus isolates obtained from gastrointestinal,
yoghurt and silage samples. The 16S-23S spacer sequences of lactobacilli are small, only about 200 bp
in length. These short sequences are easy to sequence on both strands and provide reliable information
forcomparative work. The spacer region method has the advantage of distinguishing between Lb.
rhamnosus and Lb. casei strains . (Tannock et 1999) It can be used to distinguish Lb. plantarum, from
Lb. paraplantarum, these two closely related species belonging to the Lb. plantarum group [12]. Chen
et al. (Chen et al., 2000) analysed the 5S-23S rRNA intergenic spacer regions (ISRs) of the Lactobacillus
group. This method was found to be an effective way of discriminating Lb. rhamnosus from Lb. casei/
Lb. paracasei because spacer length polymorphism results in a 76/80 bp insertion with respect to the
16S V2-V3 sequences.
Conclusion
It is widely recognised that the identification of lactobacilli to species or strain level on the basis
of physiological and biochemical criteria is very ambiguous and complicated. Numerous taxonomic
changes have been observed in the Lactobacillus genus as qualification of old species in new genera
or description of new species. This leads to a problematic genus characterization by phenotypic tests
and to an increasing use of classical culture-based molecular methods. New molecular techniques for
microbial community analysis that do not require isolation of the microorganisms are very promising.
They provide a complementary picture of the population obtained using culture-based techniques
when applied to the analysis of milks and dairy products. However, these molecular approaches have
several limitations, including the design of adequate primers, and the possibility that DNA isolation,
amplification and cloning might be biased by certain strains and sequences. There is also dependence
on the detection threshold and on the number of lactobacilli, unfortunately low in high quality raw
milks.
Numerous techniques, culture- dependent or culture-independent, are based on the use of probes
and primers. For these techniques the discrimination level depends on the existence or not of the probes
and primers at the taxonomic level desired. To date we are very far from having specific primers and
probes for the 88 lactobacilli species, and regarding those which have been designed, their specificity
and validity should be checked one by one with closed genera, species or strains. Another problem
results from the given list of 88 lactobacilli species since it is not an official list (it does not exist) and
thus to bypass possible misidentification all probes and primers should be validated against the same
reference strain at the beginning to ensure their common specificity. Moreover, all the techniques
mentioned in this review have not been applied to the lactobacilli using the same objectives.
The genus primer designed by Dubernet et al. (2002), has been used for PCR and PCR-TGGE,
but not for hybridisation, but it is clear that it could be used. The difficulty of choosing a technique
that has good discrimination power depends not only on the techniques but also on the species
or strains. Results also depend on the quality and exhaustivity of a database. Finally, only a few
limited techniques can be applied with a high degree of confidence although they are dependent on
112
Identification of Lactobacillus spp by PCR based Molecular Methodology
database robustness: sequencing to identify at genus and species level, and sequencing or pulsed
field gel electrophoresis to discriminate strains. In conclusion, analysis of lactobacilli in cheeses and
other dairy products is very complicated and the use of different techniques, especially molecularbased phenotypic or genomic techniques, is recommended.
Reference
Hammes W.P., Vogel R.F., The genus Lactobacillus, in: Wood B.J.B., Holzapfel W.H. (Eds.), The lactic acid bacteria. The
genera of lactic acid bacteria, Blackie Academic, London, UK, 1995, pp. 19–54.
Vandamme P., Pot B., Gillis M., de Vos P., Kersters K., Swings J., Polyphasic taxonomy, a consensus approach to
bacterial systematics, Microbiol. Rev. 60 (1996) 407–438.
Dubernet S., Desmasures N., Guéguen M., A PCR-based method for identification of lactobacilli at genus level, FEMS
Microbiol. Lett. 214 (2002) 271
Medina R., Katz M., Gonzalez S., Oliver G., Characterization of the lactic acid bacteria in ewe’s milk and cheese from
northwest Argentina, J. Food Prot. 64 (2001) 559–563
Andrighetto C., De Dea P., Lombardi A., Neviani E., Rossetti L., Giraffa G., Molecular identification and cluster analysis
of homofermentative thermophilic lactobacilli isolated from dairy products, Res. Microbiol. 149 (1998) 631–643
Tilsala-Timisjarvi A., Alatossava T., Development of oligonucleotide primers from the 16S-23S rRNA intergenic
sequences for identifying different dairy and probiotic lactic acid bacteria by PCR, Int. J. Food Microbiol. 35 (1997)
49–56
Nigatu A., Evaluation of numerical analyses of RAPD and API 50 CH patterns to differentiate Lactobacillus plantarum,
Lact. fermentum, Lact. rhamnosus, Lact. sake, Lact. parabuchneri, Lact. gallinarum, Lact. casei, Weissella minor and related
taxa isolated from kocho and tef, J. Appl. Microbiol. 89 (2000) 969–978.
Tynkkynen S., Satokari R., Saarela M., Mattila-Sandholm T., Saxelin M., Comparison of ribotyping, randomly amplified
polymorphic DNA analysis, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L. casei
strains, Appl. Environ. Microbiol. 65 (1999) 3908–3914
Charteris W.P., Kelly P.M., Morelli L., Collins J.K., Selective detection, enumeration and identification of potentially
probiotic Lactobacillus and Bifidobacterium species in mixed bacterial populations, Int. J. Food Microbiol. 35 (1997)
1–27
Arhné S., Molin G., Stahl S., Plasmids in Lactobacillus strains isolated from meat and meat products, Syst. Appl. Microbiol.
11 (1989) 320–325.
Woese C.R., Bacterial evolution, Microbiol. Rev. 51 (1987) 221–271
Stackebrandt E., Goebel B.M., Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis
in the present species definition in bacteriology, Int. J. Syst. Bacteriol. 44 (1994) 846–849
Tannock G.W., Tilsala-Timisjarvi A., Rodtong S., Ng J., Munro K., Alatossava T., Identification of Lactobacillus isolates
from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence
comparisons, Appl. Environ. Microbiol. 65 (1999) 4264–4267
113
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Antimicrobial Substances produced
by Lactic Acid Bacteria (LAB)
Shilpa Vij, Subrota Hati and Minakshi Dahiya
Dairy Microbiology Division, NDRI, Karnal
Introduction
Lactic acid bacteria (LAB) are found in many nutrient rich environments and occur naturally in various
food products such as dairy, meat products and vegetables. They have traditionally been used as natural
biopreservatives of food and feed. Biopreservation refers to extended shelf life and enhanced safety of
foods obtained by using the natural or added microflora and their antimicrobial products. Lactic acid
bacteria have traditionally been used as natural biopreservatives in food and animal feed, sauerkraut and
silage. Their preserving effect relates mainly to the formation of organic acids and hydrogen peroxide,
competition for nutrients and production of antimicrobial substances. Lactic acid bacteria are able to
produce antimicrobial compounds such as organic acids, hydrogen peroxides, bacteriocins etc. Antifungal
compounds such as proteinaceous compounds, phenyllactic acid, cyclic dipeptides and hydroxylated
fatty acids and Bacteriocin-like substances (BLIS) and other low and medium molecular weight mass
compounds are also produced by LAB.
Organic acids
Organic acids occurring in foods are additives or end-products of carbohydrate metabolism of LAB.
Lactic and acetic acids are the main products of the fermentation of carbohydrates by LAB. Acetic acid
is the strongest inhibitor and has wide range of inhibiting activity against bacteria, yeast and molds.
These acids generally recognised as safe agents for the preservation of foods (El-Ziney, 1998), diffuse
through the membrane of the target organisms because they are lipid soluble. After entering the cell,
the acid gets dissociated. The release of protons in the cytoplasm leads to acidification and inhibition
of the cell growth.
Hydrogen peroxide (H2O2)
Most LAB have flavoprotein oxidases, enabling them to produce hydrogen peroxide (H2O2)
in the presence of oxygen. Hydrogen peroxide accumulates in the environment since LAB do not
produce catalase. The antimicrobial effect of hydrogen peroxide attributes to a strong oxidizing
effect on the bacterial cell, and to the destruction of basic molecular structures of cellular proteins.
The antimicrobial effect of hydrogen peroxide at non-inhibitory concentrations is potentiated by
lactoperoxidase and thiocyanate present in milk and saliva (Condon, 1987). The lactoperoxidase–
thiocyanate–peroxide system involves the reaction of hydrogen peroxide and thiocyanate through
catalysed by lactoperoxidase. Hypothiocyanate (OSCN–) and other intermediary products then inhibit
other microorganisms. The structural damage and changes in bacterial membrane due to exposure to
OSCN –. Occurs. It inhibits glucose transport and some enzyme activity due to oxidation of sulfahydral
in the metabolic enzymes.
SCN–+ H2O2 → OSCN–+H2O
The Gram –negative bacteria are rapidly killed whereas, the Gram-positive bacteria are inhibited.
Lactoperoxidase and thiocyanate are present in milk, and when some LAB are grown in milk or milk
products, the third needed component, hydrogen peroxide, is added.
Diacetyl
Diacetyl (2, 3-butanedione), the characteristic aroma compound of butter, has antimicrobial effects
at low pH (Jay, 1982) and is produced by strains of some genera of LAB during citrate fermentation.
However, the amounts of diacetyl needed to exert antimicrobial activity (close to 200 mM) dramatically
alters both the taste and aroma of the product. It has antimicrobial activity against Bacillus sp.
114
Antimicrobial Substances produced by Lactic Acid Bacteria (LAB)
Bacteriocins
Bacteriocins are bacterial ribosomally synthesized peptides or proteins with antimicrobial activity
and kill very closely related bacteria upon binding to the inner membrane or other cytosolic targets.
Nowadays, the term bacteriocin is mostly used to describe the small, heat-stable cationic peptides
synthesized by Gram positive bacteria, namely lactic acid bacteria (LAB), which display a wider
spectrum of inhibition. Based on their cationic and their hydrophobic nature, most of these peptides
act as membrane permeabilizers. Pore formation leads to the total or partial dissipation of the proton
motive force, ultimately causing cell death. Bacteriocin pore formation seems to be target mediated.
Nisin and other lantibiotics use the cell wall precursor lipid II as a docking molecule. Thereby, two
modes of action, i.e. inhibition of cell wall biosynthesis and pore formation, are combined within one
molecule for potent antimicrobial activity.
Class
General Features
Produced by LAB
I-lantibiotics: la-Linear, lb-Globular
and lc-Multi component
Modified, heat stable, <15kDa, Pore
forming, cationic Enzyme inhibitors
Nisin, lacticin 481, Plantaricin C,
Lct3147
II-Unmodified peptides:lla-Pediocin
like, llb-Miscellaneous, llcMulticomponent
Heat stable, <15 kDa, antilisteria,
two peptides, non-pediocin like
Pediocin PA1/AcH, Enterocin
A, Sakacin A, Lactococcin G,
Plantaricin S, Lactacin F
III-Large Proteins: llla-Bacteriolytic,
lllb-Non-lytic
Heat Stable, >30 KDa, Cell wall
degradation, cytosolic targets
Enterolysin A, Colicins E2-E9
IV-Circular peptides
Heat stable, tail-head peptide bond
AS-48, Gassericin A, Acidocin B
(Heng and Tagg, 2006)
Reuterin
Reuterin is produced from glycerol by starving cells under anaerobic conditions, and the active
reuterin is an equilibrium mixture of monomeric, hydrated monomeric and cyclic dimeric forms of
3-HPA (3-hydroxypropionaldehyde). Reuterin is active against Gram-positive and Gram-negative
bacteria, yeast and fungi. Antifungal activity has been shown against species of Candida, Torulopsis,
Saccharomyces, Aspergillus and Fusarium. The production of reuterin (3-HPA) has also been reported
from L. brevis and L. buchneri, L. collinoides and L. coryniformis (Magnusson, 2003). A sourdough isolate
of L. reuteri has also been shown to produce the antibiotic reutericyclin, a tetramic acid active against
many Gram-positive bacteria, including common sourdough LAB, but lacking activity against yeast.
Glycerol addition to all L. coryniformis strains dramatically increase their antifungal activity. During
isolation of glycerol metabolites from L. coryniformis detects equal amounts of 3-hydroxypropionic
acid and 1,3-propanediol and only trace amounts of 3-HPA. They proposed a mechanism for glycerol
breakdown by LAB through dehydration of glycerol to 3-HPA, that might be oxidized further to
3-hydroxypropionic acid or reduced to 1, 3-propanediol. First step is catalysed by a glycerol dehydratase
and the second step by a NAD-linked reductase, whereas the oxidation to 3-hydroxypropionic acid
appears to be spontaneous.
Bioactive Peptides
Bioactive peptides are described as ‘food-derived components (genuine or generated) that,
in addition to their nutritional value, exert a physiological effect in the body’. Biological activities
associated with such peptides include immunomodulatory, antibacterial, anti-hypertensive and
opioid-like properties. Milk proteins are recognized as a primary source of bioactive peptides, which
can be encrypted within the amino acid sequence of dairy proteins, requiring proteolysis for release
and activation. Fermentation of milk proteins using the proteolytic systems of lactic acid bacteria
(LAB) is an attractive approach for generation of functional foods enriched in bioactive peptides given
the low cost and positive nutritional image associated with fermented milk drinks and yoghurt. Thus,
fermentation of milk and milk proteins by proteolytic lactic acid bacteria can lead to development
of functional foods conferring specific health benefits to the consumer beyond basic nutrition. The
starter culture applied in the manufacture of `Festivo’ cheese, a novel bioactive cheese is a mixture
of commercial starter cultures containing 12 different strains of the following genera or species:
115
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Lactococcus sp. and Leuconostoc sp. (BD type cultures), Propionibacterium sp. Lactobacillus sp. as well as
Lactobacillus acidophilus and Bifidobacterium.
Antifungal compounds of LAB
Proteinaceous compounds: Ribosomally synthesized antimicrobial peptides generally have a
hydrophobic and a hydrophilic end, a size of 20–50 amino acids, and cationic properties. Many LAB
produce bacteriocins, antibacterial, ribosomally synthesized peptides or proteins. Antifungal activity
of the compounds produced by LAB are well known. Lactococcus lactis subsp. lactis and Lactobacillus
casei produced proteinaceous compounds with antagonistic activity against several filamentous fungi.
The anti-aflatoxigenic properties of LAB depend on adherence of fungal toxins to cells of LAB. A
proteinaceous compound from Lactobacillus coryniformis subsp. coryniformis strain Si3 as antifungal
effect against several moulds and yeasts. The peptide is small (approximately 3 kDa), heat stable,
active in the pH range 3–6 and totally inactivated by proteinase K. Similar characteristics are found
among the heat stable, unmodified bacteriocins of subclass II.
Phenyllactic acid: Phenyllactic acid and 4-hydroxy-phenyllactic acid from L. plantarum 21B have
antifungal activity against several species of filamentous fungi (Lavermicocca et al. (2000). Phenyllactic
acid has also been identified from culture supernatants of L. plantarum MiLAB 393, L. coryniformis strain
Si3, and strains of Pediococcus pentosaceus and L. sakei. Phenyllactic acid is only active against yeasts
and moulds. However, this metabolite certainly contributes to the overall antifungal effect in synergy
with other compounds produced by LAB.
Cyclic dipeptides and other low-molecular-mass inhibitory compounds: New types of antimicrobial
compounds from the culture filtrate of L. plantarum VTT E-78076 was found (Niku-Paavola et al. (1999)
. The active fraction include benzoic acid, 5-methyl-2,4-imidazolidinedione (methylhydantoine),
tetrahydro-4-hydroxy-4-methyl-2H-pyran-2- one (mevalonolactone), and cyclo(glycyl-L-leucyl). Two
cyclic dipeptides are cyclo (Phe-Pro) and cyclo (Phe-OH-Pro) in the supernatant of L. plantarum MiLAB
393. The antimicrobial effect of several different cyclic dipeptides has been found that cyclo(Phe-Pro)
and cyclo(Phe-OH-Pro) are also produced by strains of P. pentosaceus, L. sakei and L. coryniformis and
thus might be common LAB metabolites.
Phenolic compounds: This phenolic compound produced by P. acidilactici LAB 5 and showed
varying degrees of antifungal activity against a number of foods and plant pathogenic fungi.
Hydroxy fatty acids: Some LAB can produce antimicrobial fatty acids that improve the sensory
quality of fermented products. Caproic acid isolated from Lb. sanfrancisco CB1 is a potent antifungal
substance produced by this strain. This compound can act in synergy with other acids such as propionic,
butyric and valeric acids. Among these fatty acids, the most active is shown to possess a 12-carbon
atom chain length. Hydroxylated fatty acid compounds present a very broad inhibition spectrum and
are efficient against moulds and yeasts. The minimum inhibitory concentration (MIC) of hydroxylated
fatty acids ranges between 10 and 100 µg/ml (Sjögren et al., 2003).
Reference:
Heng, N. C. K. and Tagg, J. R. (2006). What is in a name? Class distinction for bacteriocins. Nature Reviews Microbiology,
4. doi:10.1038/nrmicro1273-c1. Correspondence (February 2006).
El-Ziney, M. (1998). Antimicrobial activity of lactic acid bacteria metabolites: The role of lactic acid enterocin 5701 and reuterin.
Ph.D. Thesis, University of Gent (pp. 3– 23).
Sjögren, J., Magnusson, J., Broberg, A., Schnürer, J. and kenne, L. (2003). Antifungal 3 - hydroxyl fatty acids from
Lactobacillus plantarum MiLAB14. Applied and Environmental Microbiology, 69, 7554–7557.
Meisel, H. and Bocklmann W. (1999). Bioactive pepitdes encrypted in milk proteins; proteolytic activation and throphofuntional properties. Antonie van Leeuwenhoek, 76: 207-215.
Magnusson, J., Ström, K., Roos, S., Sjögren, J. and Schnürer, J. (2003). Broad and complex antifungal activity among
environmental isolates of lactic acid bacteria. FEMS Microbiology Letters, 219, 129–135.
Lavermicocca, P., Valerio, F. and Visconti, A. (2003). Antifungal activity of phenyllactic acid against molds isolated
from bakery products. Applied and Environmental Microbiology, 69, 634–640.
Niku-Paavola, M. L., Laitila, A., Mattila-Sandholm, T. and Haikara, A. (1999). New types of antimicrobial compound
produced by Lactobacillus plantarum. Journal of Applied Microbiology, 86, 29–35.
Condon, S. (1987). Responses of lactic acid bacteria to oxygen. FEMS Microbiology Reviews, 46, 269–280.
Jay, J.M. 1982. Antimicrobial properties of diacetyl. Applied and Environmental Microbiology, 44, 525– 532.
116
Microbiological Risk Assessment: A New Concept to Ensure Food Safety
Microbiological Risk Assessment: A New
Concept to Ensure Food Safety
Naresh Kumar and Raghu H. V.
Dairy Microbiology Division, NDRI, Karnal
The significance of milk in human nutrition in now well established as it is considered as the
best, ideal and complete food for all age groups. Milk can also serve not only as a potential vehicle
for transmission of some pathogens but also allows these organisms to grow, multiply and produce
toxins. A variety of pathogenic organisms may gain access in milk and milk products from different
sources and cause different types of food born illnesses which includes food infection, intoxication
and toxio-infection (Aneja et al., 2002). Recent development regarding Quality and safety management
systems such as ISO and Hazard Analysis Critical Control Point (HACCP) has reduced such incidences.
The safety of milk and milk products has been extensively reviewed by regulatory agencies in India
and internationally. A large number of risk assessments and risk profiles have been undertaken,
examining the risks across the entire dairy supply chain and conducting in-depth evaluations of
specific pathogen-product combinations. This risk assessment will summarize the major body of
relevant work undertaken to date.
Evolution of food safety systems: When it was accepted that people can contract disease from contaminated food, hygiene control laws were introduced and examples can be seen in old legal records.
Table 1 gives an overview of the more important milestones in developing food safety systems. In
the absence of knowledge about the causes of serious foodborne diseases and their etiology, use was
made of the ‘prohibition’ principle. This means that it was prohibited to produce and/or to consume
certain type of food after it was realized that the foods could be a cause of high mortality. The principle
was used particularly to protect special groups of individuals within society, such as soldiers. After
the recognition at the end of the nineteenth century that microbial agents were often responsible for
foodborne illness, systems for controlling the safety of the food supply began to be introduced. First,
use was made of microbiological testing of foods and this became widely accepted as a means of
assessing food safety during the early part of the twentieth century. Eventually, statutory microbiological requirements relating to food safety were established in many parts of the world. Further
progress occurred when Esty and Meyer (1922) developed the concept of setting process performance
criteria for heat treatment of low-acid canned food products to reduce the risk of botulism. Later,
many other foods processed in this way were controlled in the same manner. An outstanding example
is the work of Enright et al. (1956, 1957) who established performance criteria for the pasteurization of
milk that provided an appropriate level of protection against Coxiella burnetii, the causative agent of
Q fever. Studies for tuberculosis have been carried out earlier. The work is an early example of the use
of risk assessment principles in deriving process criteria.
The ability of different bacteria to multiply in foods is influenced by several key factors, including
pH, water activity and storage temperature. The effects of these factors, both singly and in combination,
have been studied extensively in laboratory media and model food systems, and this has led to the
development of mathematical models for predicting bacterial growth in commercial food products.
Although not a food safety system on its own, predictive modelling is a valuable tool, which has helped
to make possible the introduction of QRA. The latter has been used for many years in other disciplines
and its use in food microbiology has been stimulated by the decision of the World Trade Organisation
(WTO) to promote free trade in safe food (Anon, 1995). It has been emphasized, however, that control
of food safety in this context must be based on the application of sound scientific principles, and risk
analysis is seen as the basis for ensuring that the requirement is met.
Setting public health goals – The concept of Appropriate Level of Protection (ALOP): During
the past decade, there has been increased interest and effort in developing tools to more effectively link
117
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
the requirements of food safety programs with their expected public health impact. This document
introduces two such tools, the "Food Safety Objective" (FSO) and the “Performance Objective” (PO).
These can be used to communicate food safety
Severity
Description
requirements to industry, trade partners, consumers
and other countries. Good practices and HACCP Moderate
Not usually life threatening; no
sequelae; normally short duration;
remain essential food safety management systems to
symptoms are self-limiting; can be
achieve FSOs or POs. Setting goals for public health
severe discomfort
are the right and responsibility of governments.
Incapacitating but not life
These goals may specify the maximum number Serious
threatening; sequelae infrequent;
of harmful bacteria that may be present in a food.
moderate duration
Where possible, the determination of this number
Severe
Life threatening, or substantial
should be based on scientific and societal factors.
sequelae, or long duration
The level of risk can be expressed in a qualitative
way (e.g., high, medium or low risk), or when
possible, as the number of cases of foodborne disease per number of people per year. The ICMSF
ranking scheme categorizes hazards by the severity of the threat they pose to human health, taking
into consideration the: likely duration of illness; likelihood of death; and potential for ongoing adverse
health effects. The severity of adverse health effects caused by a hazard is ranked as moderate, serious
or severe according to the following definitions:
Under the ICMSF ranking, severe hazards are further divided into those applying to the general
popu-lation and those applying to specific sub-populations, that is, susceptible individuals (for
example, the very young and old, the immunocompromised, and pregnant women and their unborn
children). This takes into account those situations where a hazard considered to be of moderate or
serious to the general population may cause a severe illness in certain susceptible sub-populations.
The estimates of the risk level have to be based on clinical information available (e.g., how many stool
samples have been found to contain salmonellae) in combination with results from microbiological
surveys of foods, evaluations of the types of foods that are produced, how they are produced and how
they are stored, prepared and used. A few countries may use scientific techniques such as Quantitative
Microbiological Risk Assessment (QMRA) to estimate the risk of illnesses using detailed knowledge
of the relationship between the number of microorganisms in foods and the occurrence of foodborne
diseases. Whatever method is used to estimate the risk of foodborne illness, the next step is to decide
whether this risk can be tolerated or needs to be reduced. The level of risk a society is willing to accept
is referred to as the "Appropriate Level Of Protection" (ALOP). Importing countries with more strict
requirements for a particular hazard (e.g., harmful bacteria) may be asked to determine a value for the
ALOP according to the SPS agreement. When a country is willing to accept the current risk of illnesses,
that level is the ALOP. However, most countries will wish to lower the incidence of foodborne disease
and may set targets for future ALOPs. For instance, the current level of listeriosis could be 6 per
million people per year and a country may wish to reduce this to 3 per million people per year.
A Food Safety Objective (FSO):
When a government expresses public health goals relative to the incidence of disease, this does not
provide food processors, producers, handlers, retailers or trade partners with information about what
they need to do to reach this lower level of illness. To be meaningful, the targets for food safety set
by governments need to be translated into parameters that can be assessed by government’s agencies
and used by food producers to process foods. The concepts of food safety objectives (FSOs) and performence objectives (POs) have been proposed to serve this purpose. The position of these concepts
appearing in the food chain can be seen in Figure 1. An FSO is “The maximum frequency and/or
concentration of a hazard in a food at the time of consumption that provides or contributes to the
ap-propriate level of protection (ALOP)” It transforms a public health goal to a concentration and/
or fre-quency (level) of a hazard in a food. The FSO sets a target for the food chain to reach, but does
not specify how the target is to be achieved. Hence, the FSO gives flexibility to the food chain to use
118
Microbiological Risk Assessment: A New Concept to Ensure Food Safety
dif-ferent operations and processing techniques that best suit their situation, as long as the maximum
hazard level specified at consumption is not exceeded.
FSO and Product/pathogen/Pathway Analysis:
The ICMSF has introduced a simple equation that summarises the fate of a hazard along the food
chain as follows:
Ho - SR + SI = FSO
Where:
FSO = Food Safety Objective
Ho
= Initial level of the hazard
SR = the cumulative (total) decrease in level
SI
= the cumulative (total) increase in level
≤
= preferably less than, but at least equal to
FSO, Ho, R, and I are expressed in log10 units
I (increase) is determined by growth (G) as well as by recontamination (RC). Since the FSO is the
level of a hazard at the
moment of consumption,
another term is needed
to describe the level
at another point in the
food chain. The term
Performance Criterion
has been proposed by
the ICMSF, but this
term is also used to describe the outcome of a processing step (for example a 6 decimal reduction of a
pathogen). For this reason the term Performance Standard is used in this document to reflect the level
of a hazard and Performance Criterion to describe the impact of a process on the level of a hazard.
As a consequence of this, the following equation is proposed:
Where:
FSO = Food Safety Objective
PS = Performance Standard
Ho
= Initial level of the hazard
SR = the cumulative (total) decrease of the hazard
SIRC = the cumulative (total) recontamination with the hazard
SI G = the cumulative (total) growth of the hazard
≤
= preferably less than, but at least equal to
Note that the PS of one point of the food chain may be the Ho of the following one.
This equation is helpful to determine the effect of control measures necessary to meet a FSO. It
is im-portant to recognise that data used in PPP analyses that can be used to determine the various
values of Ho, R, IRC, IG and PS, may differ according to their source and use.
A Performance Objective (PO): For some food hazards, the FSO is likely to be very low, sometimes
referred to as "absent in a serving of food at the time of consumption". For a processor that makes
ingredients or foods that require cooking prior to consumption, this level may be very difficult to use
as a guideline in the factory. Therefore, it is often required to set a level that must be met at earlier
steps in the food chain. This level is called a performance objective (PO). A PO may be obtained from
an FSO, as will be explained below, but this is not necessarily always the case. Foods that need to be
119
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
cooked before consumption may contain harmful bacteria that can contaminate other foods in a kitchen.
Reducing the likelihood of cross-contamination from these products could be important in achieving
a public health goal. The level of contamination that should not be exceeded in such a situation is a
PO. For example, raw chicken may be contaminated with Salmonella. Although thorough cooking will
make the chicken safe (absence of Salmonella in a serving), the raw chicken may contaminate other
foods during preparation of a meal. A PO of “no more than a specified percentage of raw chicken
carcasses may contain Salmonella” may reduce the likelihood that Salmonella will contaminate other
foods. In products, such as ready-to-eat foods, the POs can be calculated from the FSO by subtracting
expected bacterial contamination and/or growth between the two points.
Process performance criteria for heat pasteurisation of milk: The work of Enright et al. (1957) led
to the development of process standards for controlling Cox. burnetii in milk. The heat treatments used
initially for milk were designed to inactivate any tubercle bacilli present and these were considered to
be the most heat-resistant of the nonsporing pathogenic bacteria likely to occur in the product. The
treatments were based on information from many studies on the heat-resistance of both human and
bovine strains (Mycobacterium tuberculosis and Myc. bovis respectively). In the USA, the heating regime
adopted in 1924 for the conventional process was 142°F (61.1°C) - 145°F (62.8°C) for 30 min. In 1933 a
heating regime was introduced for the High-Temperature, Short-Time (HTST) process: 161°F (71.7°C)
for 15 s. In practice, Cox. burnetii appears to be slightly more heat-resistant than the tubercle bacilli and,
following recognition that the organism, which causes Q fever in man, could be transmitted by raw
milk, it was necessary to check on the adequacy of existing pasteurisation processes for inactivating
the organism. The work undertaken by Enright and colleagues (1956, 1957) fulfilled this requirement
and, although no formal MRA was employed,
elements of the MRA approach were implicit in
their studies. These aspects are discussed below.
Meeting the FSO: Since the FSO is the
maximum level of a hazard at the point of
consumption, this level will frequently be very
low. Because of this, measuring this level is
impossible in most cases. Compliance with POs
set at earlier steps in the food chain can sometimes
be checked by microbi-ological testing. However,
in most cases, validation of control measures,
verification of the results of monitoring critical
control points, as well as auditing good practices
Figure 1. Model food chain indicating the position of a
and HACCP systems, will provide the reliable food safety objective and derived performance objectives
evidence that POs and thus the FSO will be met.
Microbiological criteria can be derived from
FSOs and POs, if such levels are available. If such
levels are not stated, microbiological criteria can
be developing, if appropriate. The ICMSF (2002)
has provided guidance on the establishment of
microbiological criteria.
Risk assessment & HACCP: The relation
between Risk assessment and Hazard analysis and
critical control point (HACCP) system has been
the source of much confusion. HACCP is the food
safety management tool applied in a production,
processing, used to continuously control hazards
and thus, to reduce risks. Control measures are
put into place at critical control points in the
120
Figure 2. FSOs and POs are means of communicating
public health goals to be met by food processors by good
practices and HACCP. Also, industry can set POs to ensure
that FSOs are met.
Microbiological Risk Assessment: A New Concept to Ensure Food Safety
production process to prevent or eliminate a food safety hazard or reduce it to an acceptable level.
Risk assessment, on the other hand, is scientific processes of compiling and analysing information
objectively, systematically and transparently estimate risk. A HACCP study is done for a particular
product on a particular process line, sold and used under a specific set of conditions.
It is some times thought that Risk Assessment is a part of a HACCP study, may be because the
first activity in HACCP is Called “Hazards Analysis”. In HACCP this includes identifying potential
hazards and determining which are significant, i.e. those that need to be controlled. In Risk Assessment
the first activity is called”Hazard identification”. This is one of the reasons for confusion.
Coxiella burnetii is a small, Gram-negative bacterium, originally classified as a rickettsia that cannot
be grown in axenic culture but can now be cultivated in vitro in various cell lines (Maurin and Raoult,
1999). Q fever is characterised by fever, chills and muscle pain, with occasional long-term complications. It was first described by Derrick (1937). And is known to occur worldwide. The organism infects many wild and domestic animals, which often remain asymptomatic. Domestic animals, such as
cattle, sheep and goats, are considered the main sources of infection for humans (Maurin and Raoult,
1999) and, when shed in milk, Coxiella burnetii is often present in relatively high numbers.
Contact with infected animals was known to result in transmission of Coxiella burnetii to man,
with subsequent development of illness, and the likelihood of the organism contaminating raw milk
was recognised. Early on, there was a lack of epidemiological evidence for transmission via milk, but
this was suspected in several outbreaks and there was strong supporting evidence from a UK outbreak
in 1967 (Brown et al. 1968). Thus, the hazard was the presence of Coxiella burnetii in milk intended for
human consumption.
To determine the significance of potential hazards, the HACCP study team assesses the probability
of contamination, survival and growth of the pathogen in the food during and after processing, as well
as in the production environment. This part of the HACCP study is similar to the product pathogen
pathway analysis that is used in risk assessment; however, the aim and output are different. In HACCP,
it is done to introduce control measures at critical control point to prevent, eliminate or reduce hazards.
In risk assessment, it is done to assess exposure. In HACCP, the input is product and population line
specific. After implementation of HACCP plan, a “residual” level of a hazard can remain and this is
the input for exposure assessment in risk assessment.
A full risk assessment as defined by the Codex can be useful when acceptable level (or Food Safety
Objectives) have not been established, and when dealing with a production line that does not reduce
pathogens (i.e. when HACCP is not fully effective). The risk assessor may estimate the effectiveness
of changes in control measures or the introduction of new control measures in terms of reduction of
estimated illnesses. The result of such a risk assessment might help the HACCP-team to determine
CCPs or the critical limits at CCPs. This limited form of Risk Assessment could better be called Safety
Assessment, and can be used as a tool for product and process development.
This approach is not normally taken in industrial settings. CCPs and critical limits are determined
on the basis of previous experience. Such experience includes both incidents that initiated corrective
actions and the safety record for the particular product and processing line. For new products, the
experiences with similar products, or challenge and storage tests, are used.
Hazard analysis and critical control point (HACCP) technique is the foremost system for the control
of microbiological hazards in food. The first phase of both MRA and HACCP is the identification of
Hazard. Consequently, there is potential confusion between the two concepts. However, HACCP is
really a risk management system, thus the role of MRA is to provide the information that HACCP
system developers need to make more informed decisions on. In addition to enhancing the hazard
identification phase of HACCP, Risk Assessment can be used to help identify critical control points
(CCPs), establish the critical limits, and determine the extent of hazard associated with a product during
periods of CCP deviation (ICMSF, 1998).
Microbiological Risk Profile: Risk analysis (RA) and its component parts (risk assessment, risk
121
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
management and risk communication) should be used as a tool in evaluating and controlling microbiological hazards. A risk-management based approach is required to develop recommendations to
ensure consumer protection and facilitate fair practices in the food trade. This structured approach
may employ microbiological risk assessment and may utilize a spectrum of risk communication
products including guidance documents, codes of hygiene practice, food safety objectives (FSO) and
microbiological criteria (CCFH, 2004).
Principle of Risk assessment described by Codex Alimentarius Commission: Protocol for Risk
assessment originally developed to manage chemical hazards. Principle of microbiological Risk Assessment as described by Codex Alimentarius and the FAO/WHO report on Risk management. According to the principle the risk manager who selects the hazards (Figure 3), while the risk assessor describes the behavior and other important characteristics of the selected hazards. Subsequently, the risk
assessor determines the level of exposure to the hazard either by analyzing products or by describing
the complete route from the raw materials, transport, processing, and storage to consumption. This
is called a Dynamic Flow Tree model, process risk Model, Pathogen –Product Pathway or Farm-to
Fork-Model. It allows to estimate the various levels of hazards in various situations/circumstances
and the probability that the population is exposed to them. Finally risk assessor combine the exposure
data with data on the dose response relationship and severity of the effects, in a final risk estimate the
probability and the severity of the illness due to a particular pathogen in a particular food, in a specific
group of consumers.
Some general guidelines used to manage pathogens in foods have been described by ICMSF (2002),
indicating the respective roles of industry and government. A series of steps is described, including:
a) analysis of epidemiological data which may give rise to concern for public health or a need for
im-proved controls; b) risk evaluation by an expert panel or through quantitative risk assessment; c)
es-tablishment of a FSO when necessary; d) assessing whether the FSO is technologically achievable
through preliminary process and/or product formulation criteria; and e) if the FSO is achievable, establishment of process/product requirements.
An explicit description of an ALOP may be in terms of the probability of an adverse public
health consequence or the incidence of disease (e.g. the number of cases per 100,000 populations
per year). Translation of an ALOP into a Food Safety Objective (FSO), expressed in terms of the
required level of hazard control in food, provides a measurable target for producers, manufacturers
and control au-thorities. An FSO is defined as “the maximum frequency and/or concentration
of a microbiological hazard in a food at the time of
consumption that provides an appropriate level of
protection” (ICMSF, 2002). An alternative definition
of an FSO might be a limit to the prevalence and
the average concen-tration of a microbial hazard
in food, at an appropriate step in the food chain
at or near the point of consumption that provides
the appropriate level of protection (Havelaar et al.,
2004). The assessment of risks to public health and
safety from microbiological hazards in milk and
milk products has been undertaken in the form of
a Microbiological Risk Profile. It provides a broad
overview of risks asso-ciated with consumption of
dairy products. The risk profile identifies key food
safety hazards and as-sesses where in the primary Figure. 3 Principle of Risk assessment described by
production and processing supply chain these Codex Alimentarius Commission
hazards might be introduced, increased, reduced
or eliminated.
The WTO/SPS agreement (WHO, 1997) describes the rules for the international trade in safe food
122
Microbiological Risk Assessment: A New Concept to Ensure Food Safety
and has introduced the term "appropriate level of protection" (ALOP) to express what is mentioned
in the first bullet point above. This ALOP has also been called "acceptable level of risk". This term is
similar to the expression "tolerable level of risk" (TLR) preferred by the ICMSF, because it recognises
that risks related to the consumption of food are seldom accepted, but at best tolerated. Also implied
is that for a number of food safety hazards, “zero risk” does not exists and/or too costly (financial,
societal) to achieve.
Risk Characterization in Dairy Products: The risk involved in consuming raw milk could not be
estimated because of the absence of dose response data. The data for the prevalence of contaminated
milk, the maximum level of contamination and the fact that milk would have been consumed
regularly by the majority of the population were probably implicit factors in an assumption that the
risks associated with inadequate heat treatment were high.
The studies of Enright et al. (1956, 1957) led to the conclusion that heating at “143°F for 30 min
was wholly inadequate to eliminate viable Coxiella burnetii from whole, raw milk, while heating at
145°F ensures elimination of these organisms with a high level of confidence” (Enright et al., 1957).
This led to the adoption of the higher temperature for vat pasteurisation in the USA. The work on
the HTST process indicated that the recommended standard of 161°F for 15 s was sufficient for total
elimination.
In preparing the Dairy Risk Profile, previous risk assessments conducted by other scientific agencies
were reviewed and evaluated in this document. There have been few assessments undertaken for
dairy products, and typically they address specific pathogen: commodity pairs. This profile considers
the entire dairy supply chain, including the wide range of milk and milk products. Dairy products
likely to support the growth of pathogens and prone to contamination after pasteurization may be
categorised as higher risk than other dairy products. Alternatively, dairy products that do not support
the growth of pathogens, if correctly formulated, can be classified as low risk.
The actual ranking of the dairy products is quite variable. Once a shelf-stable UHT product is
opened, it may become contaminated and when subjected to temperature abuse it could become a
high-risk food. In contrast, the low pH and low water activity of extra hard cheese means its will be
very robust and unlikely to support the growth of any pathogen that adventitiously contaminates the
surface. Dried milk powders and infant formulae are inherently stable products due to their low water
activity, however these products may be prone to contamination, and upon reconstitution become
higher risk, especially if improperly reconstituted and stored. Following criteria in food matrix may
be considered while characterizing the risk:
•
Intrinsic properties of the product (i. e. the impact of aw, pH, salt concentration, and their effect
on the growth of contaminating microorganism)
•
Extent to which food is exposed to factory environment or handling after heat treatment
•
Hygiene and control during distribution and retail sale
•
Degree of reheating or cooking before consumption (many dairy products are RTE, so this is
rarely a factor).
Attribution of Food-borne Illness to Dairy Products: While there is enhanced quantitative
data on the incidence of illness due to specific pathogens, there is often not the ability or capacity to
identify or distinguish specific food vehicles. The causative agent of an illness is usually determined
through epidemiological studies, but confirming the identity of a key ingredient or the original source
of product contamination, or critical factors contributing to their occurrence is problematic. This
inability to attribute cases of food-borne illness to causal vehicles is a major issue internationally, and
is especially difficult where illness is linked to foods with multiple ingredients. Critical in this process
is the capacity to link epidemiological data to animal and food monitoring data. The development
of public health interventions requires accurate data defining the source from which humans are
acquiring pathogens and how specific foods contribute to the total burden of food-borne illness.
However, outbreak data represents only a small component of actual cases of food-borne illness, as
123
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
many outbreaks go unrecognized. People do not always seek medical attention for mild forms of
gastroenteritis, and not all food-borne illnesses require notification to health authorities.
Dose response: There was no information on the dose response in humans, since challenge trials
had not been carried out and epidemiological data were lacking in this respect.
Exposure assessment: Information relevant to this step in MRA was obtained by injecting guinea
pigs to determine the presence and titre of Coxiella burnetii in milk. The organism was found in
33% of 376 samples of raw milk from California, USA. “The maximum number of Coxiella burnetii
demonstrated in the milk of an infected dairy cow was the number of organisms contained in 10,000
infective guinea pig doses of Coxiella burnetii per millilitre” (Enright et al., 1957). Similar titres were
found in milk that had been frozen and thawed. However, the study did not involve testing of all
breeds of dairy cattle, and it is possible that even higher levels of shedding may have occurred in some
breeds that were not examined. Nevertheless, it was concluded that the maximum level of consumer
exposure would be represented by the highest infective dose demonstrated in this study and that
the pasteurisation process should bring about thermal inactivation of such a number (Enright et al.,
1957).
Risk Management Issues and Control Strategies for Dairy foods: The critical factors having the
most significant impact on the safety of processed dairy products are as follows:
•
The quality of raw materials
•
Correct formulation
•
Effective processing
•
The prevention of recontamination of product
•
Maintenance of temperature control through the dairy supply chain.
Risk ranking of pathogen:
Product
L. Monocytogenes
EHEC
Campylobacter
S. Aureus
TBEV
Dairy
Normal
Susceptible
Pasteurized Milk Cheese
Medium
High
low
low
low
low
Raw milk cheese
Medium
High
high
medium
medium
low
While pathogenic microorganisms may contaminate raw milk supplies, pasteurization is a very effective Critical Control Point (CCP) in eliminating pathogens; good manufacturing practices must also
be employed to ensure that post-pasteurization contamination does not occur. The effectiveness of
pasteurization is dependent
upon the microbiological
status of the incoming
raw
milk.
Control
measures at the primary
production level involve
minimizing the likelihood
of
microbiological
hazards
contaminating
the raw milk. This is
achieved through the
implementation
of
a
food
safety
program
incorporating
good
The relative risk from dairy products may also be expressed graphically as a
agricultural
practices
continuum:
(GAP). These measures are
124
Microbiological Risk Assessment: A New Concept to Ensure Food Safety
effective in reducing the microbial load of milk being sent for processing.
However, should microbial contamination of raw milk occur, it is critical that milk is stored at
a temperature that minimizes the opportunity for the bacteria to multiply. Temperature abuse of the
milk may allow growth of pathogenic bacteria to the extent where the pasteurization process may not
eliminate all pathogenic bacteria and/or toxins. The Aflatoxins can be formed and ingested by dairy
cattle during feeding, eventually contaminating the milk. Aflatoxin contamination of milk is more
common where intensive supplementary feeding of dairy herds is conducted.
Concluding Remarks: FSOs and POs are new concepts that have been introduced to further assist
government and industry in communicating and complying with public health goals. These tools are
additional to the existing programmes of GAPs, GHPs and HACCP which are the means by which the
levels of POs and FSOs will be met. Hence FSOs and POs build on, rather than replace, existing food
safety practices and concepts.
References:
Aneja, R. P., B. N. Mathur, R. C. Chandan, A. K. Banerjee, 2002. Technology of Indian Milk Products. A Dairy India
Publication, Delhi.
Brown G L, Colwell D C and Hooper, W L, ‘An outbreak of Q fever in Staffordshire. Journal of Hygiene, Cambridge 1968
66 649-655.
Codex Committee on Food Hygiene (CCFH, CX/FH 04/5/6), 2004. Proposed draft process by which the committee on
food hygiene could undertake its work in microbiological risk assess-ment/risk management, Alinorm 04/27/13.
Derrick E H, ‘”Q” fever, A new fever entity: Clinical features, diagnosis, and laboratory investi-gation. Med J Australia,
1937 2 281-299
Enright, J. B., Sadler, W. W. and Thomas, R. C. ‘Thermal inactivation of Coxiella burnetii and its relation to pasteurisation
of milk’, Public Health Service Publication No. 517. United States Gov-ernment Printing Office, Washington, D C,
1957
Enright, J. B., Sadler, W. W. and Thomas, R. C. ‘Observations on the thermal inactivation of the organism of Q fever in
milk’, J Milk Food Technol, 1956 10 313-318.
Havelaar, A. H., Nauta, M. J., Jansen, J. T., 2004. Fine-tuning food safety objectives and risk as-sessment. International
Journal of Food Microbiology 93, 11–29
ICMSF, 1998, Principles for the establishment of microbiological food safety objectives and related control measures.
Food Control 9, 379-384.
ICMSF, 2002. Microorganisms in Foods 7. Microbiological testing in food safety management. Kluwer Academic /
Plenum Publishers, New York, USA.
Jansson, E., Moir, C., Richardson, K., 1999. Final Report Review of Food Safety Systems devel-oped by the NSW Dairy
Corporation. Food Science Australia Report.
Maurin M and Raoult D, ‘Q Fever’. Clinical Microbiology Reviews, 1999 12 518-553.
WHO, 1997. Food Safety and Globalization of Trade in Food, a challenge to the public health sector. WHO/FSF/
FOS/97.8 Rev. 1, WHO, Geneva.
Zottola, E.A., Smith, L.B., 1991. Pathogens in cheese. Food Microbiology 8, 171-182.
125
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Biopreservation of Dairy Products: Role of
Bacteriocins of Lactic Acid Bacteria
R. K. Malik and Gurpreet Kaur
Dairy Microbiology Division, NDRI, Karnal
In spite of modern advances in technology, the preservation of foods is still a debated issue, not
only for developing countries (where implementation of food preservation technologies are clearly
needed) but also for the industrialized world. Amelioration of economic losses due to food spoilage,
lowering the food processing costs and avoiding transmission of microbial pathogens through the
food chain while satisfying the growing consumers demands for foods that are ready to eat, freshtasting, nutrient and vitamin rich, and minimally-processed and preserved, are major challenges for
the food industry. The extent of microbiological problems in food safety was clearly reflected in the
WHO food strategic planning meeting (WHO, 2002):
•
The emergence of new pathogens and pathogens not previously associated with food
consumption is a major concern;
•
Microorganisms have the ability to adapt and change, and changing modes of food production,
preservation and packaging have, therefore, resulted in altered food safety hazards.
The consumers in the developed world often question the safety of the thousands of non-food
preservatives and other additives that are incorporated in food. It has also encouraged them to voice
their feelings against the use of these chemicals in foods and also to look for foods that are “natural”,
“healthy” and not treated with harsh contaminants. At the same time they are also concerned about
the loss of nutritional value of the “harshly processed foods” and the possible health risks of food
preserved with chemicals.
The empirical use of microorganisms and/or their natural products for the preservation of foods
(biopreservation) has been a common practice in the history of mankind (Ross et al., 2002). Food
fermentations have a great economic value and it has been accepted that these products contribute
in improving human health. Lactic Acid Bacteria (LAB) have contributed in the increased volume of
fermented foods world wide especially in foods containing probiotics or health promoting bacteria.
Potential risk from psychrotrophic pathogenic and spoilage organisms
Until relatively recently it was assumed that refrigeration at or below 4oC was sufficient to prevent
the growth of infectious and toxigenic food borne organisms. However, this assumption has changed
with the reports that several food borne pathogens are psychotrophs e.g. Listeria monocytogenes,
Yesinia enterocolitica, Aeromonas hydrophila, some enterotoxigenic E. coli and Clostridium botulinum
B & E. Moreover, it has been often observed that during distribution and before consumption of
refrigerated foods, some temperature abuse may occur which may permit conditions for the growth
of several other pathogens that can grow at 5 and 12oC. (Del Giudice, 1991; Snyder et al., 1991). Long
storage of refrigerated foods, even at low temperatures will allow the psychrotrophic and spoilage
microorganisms to multiply and reach a high level even from a very low initial population.
Safety concerns of the chemical preservatives
The consumers’ preference for the refrigerated foods that do not contain any preservative(s) is
based on their perception of these foods to be nutritious, healthy and closed to natural as opposed to
harshly processed and chemically preserved foods. This is because the safety of some preservatives
as well as some other additives has been questionable such as NO2, sulphides, sodium diacetate, beta
propiolactones and therapeutic antibiotics. Reports on possible health hazard from the consumption
of some preservatives and other additives currently being used such as nitrite and saccharine, and
additives previously used but currently not been permitted for use such as cyclomate and some dyes
have shaken the faith of consumers, thus prompting them to question the safety and wholesomeness
126
Biopreservation of Dairy Products: Role of Bacteriocins of Lactic Acid Bacteria
of food they eat.
Antimicrobial chemical preservatives inhibit or retard the growth of spoilage and pathogenic
microorganisms and are used to enhance the safety and shelf life of foods. They produce their
antimicrobial effect by interfering with the structural and functional components of microorganisms.
Their effectiveness is dependant on the type of chemical, the concentration of chemicals used, the
microorganism present, the type of microorganisms and their physiological state (vegetative cell or
spore), the composition and pH of food, and the temperature and duration of storage. Moreover, in
recent years the chemicals used in food items have increased exponentially to several thousands. The
effect of actual consumption of these chemicals in multiple products over substantial length of time
should be an important consideration in judging their safety.
Therefore, the basis of selection of antimicrobial biopreservatives to be used in foods should not
only be their effectiveness against both Gram positive and Gram negative pathogens and spoilage
organisms and other desired characteristics of preservatives but also their proven safety records
and their acceptance by the health conscious consumers and health regulatory agencies. Among the
compounds that have generated considerable interest in the recent years are several antimicrobial
metabolites of lactic acid bacteria used to produce, or associated with, fermented foods.
Lactic acid bacteria
Lactic acid bacteria belong to a group of Gram-positive anaerobic bacteria that excrete lactic acid
as their main fermentation product into the culture medium. LAB were among the first organisms to
be used in food manufacturing. Today LAB play crucial role in the manufacturing of fermented milk
products, vegetables and meat, as well as in the processing of other products such as wine. Lactic acid
bacteria which include the genera Lactococcus, Streptococcus, Lactobacillus, Pediococcus, Leuconostoc,
and Carnobacterium (Nettles and Barefoot, 1993), play an essential role in food fermentations. The most
important contribution of these microorganisms to the product is to preserve the nutritive qualities of
Fig.1 Production of various metabolites by a lactic culture including acid, H2O2, diacetyl and bacteriocin
the raw material through an extended shelf life and the inhibition of spoilage and pathogenic bacteria.
This is due to competition for nutrients and the presence of inhibitors produced by the starter. The
Lactic Acid Bacteria produce an array of antimicrobial substances (such as organic acids, diacetyl,
acetoin, hydrogen peroxide, reuterin, reutericyclin, antifungal peptides, and bacteriocins (Holzapfel et
al., 1995; El-Ziney et al., 2000; Holtzel et al., 2000; Magnusson and Schnürer, 2001).
Bacteriocins of lactic acid bacteria
There are several examples of the inhibition of spoilage and pathogenic bacteria by LAB. Extensive
investigations, over the last few decades, into the antagonistic behaviour of such strains have led to
the identification and characterization of numerous bacteriocins produced by LAB (Jack et al, 1995;
127
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Nettles and Barefoot, 1993; Klaenhammer, 1993). Such investigations have led to the discovery of a
range of different bacteriocin-producing strains, many of which have potential in food applications.
Given the ease with which bacteriocin-producing strains can be isolated from food sources, it is clear
that many of these bacteriocins have been safely consumed for decades and thus it could be argued
that reintroduction of such cultures should have negligible associated safety or toxicological problems
when consumed (Kelly et al., 1996).
Bacteriocin could be highly advantageous as even in small amount; these peptides are sufficient to
kill or inhibit bacteria competing for the same ecological niche or the same nutrient food. Bacteriocins
produced by bacteria can be defined as biologically active protein and protein complex (protein
aggregates), lipo-carbohydrate proteins, glycoproteins, etc displaying a bactericidal mode of action
exclusively towards Gram positive bacteria and particularly against closely related species. They form a
heterogenous group with respect to producing bacterial species, molecular size, physical and chemical
properties, stability, anti microbial spectrum and mode of action, etc. The bacteriocins produced by
LAB are of particular interest to the food industry (Nettles and barefoot, 1993), since these bacteria
have generally been regarded as safe (GRAS status). Moreover, majority of bacteriocin producing LAB
are natural food isolates, they are ideally suited for food preservation. The production of bacteriocins
by LAB is not only advantageous to the bacteria themselves but could also be exploited by the food
industry as a tool to control undesirable bacteria in a food grade and natural manner, which is likely
to be more acceptable to consumers.
Bacteriocins are antimicrobial peptides or small proteins which inhibit, by a bactericidal or
bacteriostatic mode of action, micro-organisms that are usually closely related to the producer strain
(De Vuyst and Vandamme 1994; Schillinger and Holzapfel 1996). A bacteriocin producer protects
itself against its own antimicrobial compound by means of a system referred to as immunity, which
is expressed concomitantly with the antimicrobial peptide (Nes et al., 1996). The bacteriocin family
includes a diverse group of proteins in terms of size, microbial targets, modes of action, and immunity
mechanisms. The bacteriocins produced by LAB offer several desirable properties that make them
suitable for food preservation:
Inhibitory spectrum of bacteriocins of lactic acid bacteria
In the original definition of Jacob et al (1953), bacteriocins were characterized by predominate intra
species killing activity. While this is true for most of the bacteriocins of LAB especially those produced
by a large number of lactococci and lactobacilli, others have been found to exhibit a broad range of
inhibitory activity extending across numerous Gram positive bacteria. Thus Klaenhammer (1998)
defined two types of bacteriocins of lactic acid bacteria; one type exhibiting a classical bacteriocin
antibacterial spectrum affecting only closely related bacteria and the second type effective against a
wide range of Gram positive bacteria. Inhibition of Gram negative bacteria in their native state has not
been reported for any of the purified and thoroughly characterized bacteriocins. Similarly, inhibition
of yeast and molds has not been observed.
Classification of bacteriocins of lactic acid bacteria
Most LAB bacteriocins are small (< 6 kDa), cationic, heat-stable, amphiphilic, membranepermeabilizing peptides that may be divided into three main groups: the modified bacteriocins, known
as lantibiotics (Class I), the heat-stable unmodified bacteriocins (Class II), and the larger heat-labile
bacteriocins (Class III) as proposed by Klaenhammer (1993). A fourth group (Class IV) with complex
bacteriocins carrying lipid or carbohydrate moieties is often included in bacteriocins classifications.
Recently a fifth class of circular bacteriocins has been included in the classification scheme with lesser
amount of modified amino acids (Table 2).
128
Biopreservation of Dairy Products: Role of Bacteriocins of Lactic Acid Bacteria
Table 2. General classification of LAB Bacteriocins. a Category excluded from Nes’
classification (Nes et al., 1996) but included in Garneau’s (Garneau et al., 2002)
CLASS
CHARACTERISTICS AND SUBCATEGORIES
Class I. Lantibiotics
Ribosomally synthesized peptides that undergo posttranslational modifications.
Molecular weight 2– 5 kDa. Contain lanthionine and β-methyl lanthionine.
Class II. Nonlantibiotics—
Unmodified Bacteriocins.
Heat stable peptides formed exclusively by unmodified amino acids.
Ribosomally synthesized as inactive pre peptides to get activated by
posttranslational cleavage of the N- terminal leader peptide. Molecular weight
< 10 kDa.
Class III. Nonlantibiotics—Large,
Heat-labile Bacteriocins.
Heat-labile proteins. Molecular weight >30 kDa.
Class IVa
Complex bacteriocins carrying lipid or carbohydrate moieties
Class V
Circular Bacteriocins
Bacteriocins of LAB as potential food biopreservatives
The term “biopreservative” includes the
antimicrobial compounds that are of plant,
animal and microbial origin and have been
used in human food for long time, without
any adverse effect on human health. They
are used to enhance safety and extend
shelf life of food and can thus be regarded
as “biopreservatives”. Fermented foods
are good examples of biopreserved foods
in which the starter cultures are allowed
Fig 2. Influence of different factors on the efficacy of in situ bacteriocin
to grow so that they can produce anti production for biopreservation. (Galvez et al., 2007)
microbial metabolites. In fermentation the
raw materials are converted by desirable
microorganisms such as bacteria, yeast and molds to products that have acceptable qualities of food.
In controlled fermentation, the starter cultures are added to the raw material in large number and
then incubated under conditions to stimulate the growth and production of desirable products. An
example of food produced by controlled fermentation is yoghurt in which Lactobacillus bulgaricus
and Streptococcus thermophilus are added to achieve the fermentations. The lactic acid and other
metabolites produced by these desirable bacteria in ‘Sauerkraut’ and yoghurt prevent the growth
of undesirable microorganisms present in the non sterile raw materials and make the products shelf
stable. (Ray, 1992)
Biopreservation by bacteriocins of LAB
The small heat-stable bacteriocins of lactic acid bacteria have been recognized as perhaps the
most promising entities for use in applications of food preservation for a number of reasons. They
are widely distributed and established as food-grade bacteria and often have a suitable spectrum
of bacterial targets, for example, strains of Listeria, Clostridium and other Gram-positive bacteria
including LAB. Furthermore, these Bacteriocins can endure harsh treatments, such as boiling, without
loosing much of their activity.
Bacteriocins produced by lactic acid bacteria have received particular attention in recent years
due to their potential application in the food industry as natural preservatives. This trend reflects the
increasing consumer awareness of the risks derived not only from food borne pathogens, but also
from the artificial chemical preservatives used to control them (Abee et al., 1994). Some bacteriocins
have well established their action as potential antimicrobial and also their possible applications in food
129
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
preservation systems. Nisin A already showed effective inhibitory activity on the Listeria monocytogenes
growth in cheese up to eight weeks. Enterocin, when inoculated in ham, pig meat, meat chicken, and
sausage, showed inhibitory capacity on the L. monocytogenes growth. Lactocin also had inhibitory
capacity on the same microorganism, when applied in ground meat (Vignolio et al., 1996; Davies, 1999;
Aymerich et al., 2000). There are numerous applications of nisin as food preservative, including shelflife extension of dairy products, canned foods, vacuum-packed meat and cold smoke salmon (Hurst,
1981; Davies, 1999; Nilsson et al., 2000).
Nisin presents almost all the desirable characters of a potential biopreservative like:
1. It is non toxic
2. It is natural and safe (produced by Lactococcus lactis having GRAS status)
3. Heat and storage stable
4. It can be degraded by digestive enzymes so pose no harmful side-effect to human
5. It does not confer any undesirable taste and flavor to foods and
It show prominent antimicrobial spectrum against Gram-positive microorganisms (Kominsky, 1999;
Fiorentini et al., 2001).
The accumulation of studies carried out in recent years clearly indicate that the application of
bacteriocins in food preservation can offer several benefits, still the use of food grade bacteriocins
as biopreservative is in its infancy. Civilization has reaped the benefits of Bacteriocins unknowingly
for 1000s of years, yet nisin is the only bacteriocin bio-preservative that has received acceptance in
countries worldwide. Among the major bacteriocins apart from nisin, pediocin, acidocin, bavaracin,
curavaticin, and sakacin, can be other alternatives though there is need to well characterize them
with respect to their use in food preservation along with safety issues associated with them. Nisin
is the single bacteriocin commercially used as natural agent of food conservation (biopreservation)
and considered safe by World Health Organization (WHO) and has received the denomination of
Generally Recognized as Safe (GRAS) and also by Food and Drug Administration (FDA). Nisin is
produced by Lactococcus lactis subsp. lactis and is used in various countries (Abee et al., 1994).
Dairy Products
Nisin is used in pasteurized, processed cheese products to prevent outgrowth of spores such
as those of Clostridium tyrobutyricum that may survive heat treatments as high as 85–105°C. Use of
nisin allows these products to be formulated with high moisture levels and low NaCl and phosphate
contents, and also allows them to be stored outside chill cabinets without risk of spoilage. The level of
nisin used depends on food composition, likely spore load, required shelf life and temperatures likely
to be encountered during storage. (Hirsch et al., 1951).
Nisin is also used to extend the shelf life of dairy desserts which cannot be fully sterilized without
damaging appearance, taste or texture. Nisin can significantly increase the limited shelf life of such
pasteurized products.
Nisin is added to milk in the Middle East where shelf-life problems occur owing to the warm climate,
the necessity to transport milk over long distances and poor refrigeration facilities. It can double the
shelf life at chilled, ambient and elevated temperatures and prevent outgrowth of thermophilic heatresistant spores that can survive pasteurization. It can also be used in canned evaporated milk.
Canned foods
Nisin may also be added to canned foods at levels of 100–200 IU g–1 to control thermophilic sporeformers such as Bacillus stearothermophilus and Clostridium thermosaccharolyticum which may survive and
grow in canned foods stored at high temperatures. It also allows a reduction in heat processing required
without compromising food safety. It is used in canned potatoes, peas, mushrooms, soups, and cereal
puddings. It increased activity at acid pH levels makes it ideally suitable in low pH foods such as canned
tomatoes, to inhibit acid-tolerant spoilage flora such as B. macerans and C. pasteurianum. (Eckner, 1992).
130
Biopreservation of Dairy Products: Role of Bacteriocins of Lactic Acid Bacteria
Meat
Concern about the toxicological safety of nitrite used in cured meat has led to investigation into the
use of nisin to allow a reduction in nitrite levels. However, uneconomically high levels are required
to achieve good control of Clostridium botulinum, perhaps as a consequence of nisin binding to meat
particles, uneven distribution, poor solubility in meat systems, or possibly interference in activity by
meat phospholipids. (Nielson et al., 1990; Eckner, 1992).
Cereals
Little research has been conducted on the use of bacteriocins in cereal or cereal related products.
Delves- Broughton (1996) discussed the use of bacteriocins in cereal puddings. Another possible
use could be foreseen in pasta. Pasta dough is susceptible to contamination and growth of S. aureus.
Bacteriocins, with their activity against Gram positive microbes, may be able to inhibitgrowth of this
pathogen in the dough. (Eckner, 1992).
Wine
The insensitivity of yeasts to nisin allows its use to control spoilage lactic acid bacteria in beer or
wine. It can maintain its activity during fermentation without any effect on growth and fermentative
performance of brewing yeast strains and with no deleterious effect on taste. It can therefore be used
to reduce pasteurization regimens and to increase shelf life of beers. It has similar applications in wine
except for those that require a desirable malolactic fermentation. However, nisin-resistant bacterial
starter cultures such as resistant strains of Leuconostoc oenos, in conjunction with nisin, can be used to
actually control the malolactic fermentation. Nisin may also be used to reduce the amount of sulphur
dioxide used in winemaking to control bacterial spoilage. (Todorov et al., 2003).
Pediocin-like bacteriocins
Pediocin-like bacteriocins are members of the Class II bacteriocins, a group of bacteriocins in
which there is considerable commercial interest. They are small, heat-resistant peptides that are not
post-translationally modified to the same extent as the Class I bacteriocins, apart from the cleavage
of a leader sequence from a double glycine site upon export of the bacteriocin from the cell, and the
presence of disulphide bridges in some molecules. All of the pediocins share certain features,including
a seven amino acid conserved region in the N-terminal of the active peptide (-Tyr-Gly-Asn-Gly-ValXaa-Cys-). Perhaps the best-known is pediocin PA-1, which is produced by Pediococcus acidilactici. A
commercial formulation has been introduced under the trade name ALTA. Pediococci are important
in the fermentation of vegetables and meat for both acid production and flavour development. The
pediocin-like bacteriocins (which are also produced by genera other than the pediococci) are active
against other lactic acid bacteria but are particularly effective against Listeria monocytogenes, a foodborne pathogen of increasing concern to the food industry. Listeria may be found in raw milk,
dairy products, vegetables and meat products and can grow under conditions such as refrigeration
temperatures (growth has been reported at temperatures as low as –1°C), high salt concentrations (up
to 10%), low pH (pH 5.0), and high temperatures (44°C). Pediocin PA-1 has been observed to inhibit
Listeria in dairy products such as cottage cheese, ice cream, and reconstituted dry milk. It has also
been demonstrated as a biocontrol agent on meat systems. In situ production in dry fermented sausage
inhibits L. monocytogenes throughout fermentation and drying, possibly owing to a combination of the
reduction in pH and bacteriocin production. Pediococcus acidilactici is also used as a low-level inoculum
in reduced-nitrite bacon to prevent the outgrowth of Clostridium botulinum spores and subsequent
toxin production.
Potential uses for other bacteriocins
As more and more bacteriocin producers are being isolated and characterized, usually
from food environments, the potential for their use increases. Lactobacillus plantarum produces
plantaricins S and T in the Spanish-style green olive fermentation. Many traditional African
foods are fermented by lactic acid bacteria before consumption. Naturally occurring bacteriocin131
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
producing strains in these products may have the potential to improve the quality and shelf life
of other African fermented foods which are often plagued by problems such as inconsistent
quality, hygienic risks and premature spoilage.
Other bacteriocins which have been isolated from food environments include plantaricin F, from
chilled processed channel catfish; acidocin B, produced by Lactobacillus acidophilus with a narrow
spectrum of activity which includes Clostridum sporogenes and a narrow range of other lactobacilli;
and salivaricin B, produced by Lactobacillus salivarus with a very broad host range including Listeria
monocytogenes, Bacillus cereus, Brochothrix thermosphacta, Enterococcus faecalis and many lactobacilli,
which may have a more widespread application. Another recently identified bacteriocin with a
broad host range similar to that of nisin is lacticin 3147, produced by a strain of Lactococcus lactis.
Since lacticin 3147 is also an effective inhibitor of many Gram-positive food pathogens and spoilage
microorganisms, these starters may provide a very useful means of controlling the proliferation of
undesirable microorganisms during Cheddar cheese manufacture.
Microgard (Wesman Foods Inc., USA) is commercially produced from grade A skim milk
fermented by a strain of Propionibacterium shermanii, and has a wide antimicrobial spectrum including
some Gram-negative bacteria, yeasts and fungi. This product is added to 30% of the cottage cheese
produced in the USA as an inhibitor against psychrotrophic spoilage bacteria. It is added to a variety
of dairy products such as cottage cheese and yoghurt and a nondairy version is also available for use in
meat and bakery goods. The inhibitory activity almost certainly depends primarily on the presence of
propionic acid, but there has also been a role proposed for a bacteriocin-like protein produced during
the fermentation. This use of milk fermented by a bacteriocin producer as an ingredient in milk-based
foods may be a useful approach for introducing bacteriocins into foods at little cost.
Bacteriocins and hurdle technology
Hurdle technology refers to the manipulation of multiple factors (intrinsic and extrinsic) designed to
prevent bacterial contamination or control growth and survival in food. A combination of preservation
methods may work synergistically or at least provide greater protection than a single method alone,
thus improving the safety and quality of a food. While in certain foods intrinsic properties such as high
salt may provide adequate protection, the conscious addition of an extra hurdle(s) can ensure safety.
(Leistner, 2000)
The concept of hurdle technology began to apply in the food industry in a rational way after the
observation that survival of microorganisms greatly decreased when they were confronted with multiple
antimicrobial factors (Leistner, 1978; Leistner and Gorris, 1995; Leistner, 2000). Over 60 potential
hurdles have been described to improve food stability and/or quality (Leistner, 1999). The application of
bacteriocins as part of hurdle technology has received great attention in recent years (Chen and Hoover,
2003; Ross et al., 2003; Deegan
et al., 2006), since bacteriocins
can be used purposely in
combination with selected
hurdles in order to increase
microbial inactivation (Fig
3). The combination of
hurdles to be applied will
depend greatly on the type
of food and its microbial
composition. This must be
carefully considered, since
different hurdles usually
have different effects on
the members of a microbial
Fig 3. Application of bacteriocins as part of hurdle technology.
community.
132
Biopreservation of Dairy Products: Role of Bacteriocins of Lactic Acid Bacteria
Antimicrobial packaging
Bio-active packaging is a further potential application in which bacteriocins can be incorporated
into packaging destined to be in contact with food. This system combines the preservation function of
bacteriocins with conventional packaging materials, which protects the food from external contaminants.
Spoilage of refrigerated foods usually begins with microbial growth on the surface, which reinforces
the attractive use of bacteriocins being used in conjunction with packaging to improve food safety and
improve shelf-life (Collins-Thompson & Hwang, 2000). Bio-active packaging can be prepared by directly
immobilizing bacteriocin to the food packaging, or by addition of a sachet containing the bacteriocin
into the packaged food, which will be released during storage of the food product. Studies investigating
the effectiveness of bio-active cellulose- based packaging inserts and a vacuum packaging pouch made
with polyethylene/polyamide to improve shelflife and safety aspects have proved promising. When
considering bio-active packaging, the stability and the ability to retain activity while immobilised to the
packaging film is of vital importance.
While many LAB bacteriocins possess significant antimicrobial qualities that could greatly enhance
the safety of a food, it may yet emerge that industrially they will be most frequently applied as a ‘finalhurdle’ in a food system where another hurdle(s) already exists to eliminate pathogens and spoilers
that survive only in adventitious circumstances.
Bacteriocin resistance among pathogens and food spoilage bacteria
Although the use of bacteriocins for preservation is a novel approach to eliminating or controlling
pathogens in food, the development of highly tolerant or resistant strains remains the main concern
and decreases the efficiency of bacteriocins as biopreservatives. Resistant food-borne pathogens are
posing a global problem which is further facilitated by international trade of raw and processed foods.
In foods with a long shelf life, even a small number of these resistant cells can multiply to very high
number and thus may lead to food-borne outbreaks and food spoilage.
Future prospects
A large number of bacteriocins from LAB have been characterized to date, and many different
studies have indicated the potential usefulness of bacteriocins in food preservation. Bacteriocins are a
diverse group of antimicrobial proteins/peptides, and, therefore, are expected to behave differently on
different target bacteria and under different environmental conditions. Since the efficacy of bacteriocins
in foods is dictated by environmental factors, there is a need to determine more precisely the most
effective conditions for application of each particular bacteriocin. Bacteriocinogenic cells may also act
as living factories in foods. The antimicrobial effects of bacteriocins and bacteriocinogenic cultures
in food ecosystems must be understood in terms of microbial interactions. Among the food borne
pathogens, knowledge of the characteristics of bacteriocin resistant variants and the conditions that
prevent their emergence will help in determining the optimal conditions for application of bacteriocins
in foods and minimize the incidence of resistance.
References
Abee T., Rombouts FM., Hugenholtz J., Guihard G., and Letellier L. 1994. Mode of action of nisin Z against Listeria
monocytogenes Scott A grown at high and low temperatures. Applied Environmental Microbiology, 60, 1962-1968.
Aymerich T., Garriga M., Ylla J., Vallier J., Monfort JM., and Hugas M. 2000. Effect of sausage ingredients and additives
on the production of enterocin A and B by Enterococcus faecium CT492. Optimization of in vitro production and
anti-listerial effect in dry fermented sausages. Journal of Applied Microbiology, 88, 686-694.
Chen H., and Hoover DG. 2003. Bacteriocins and their food applications. Comprehensive Reviews in Food Science and
Food Safety, 2, 82–100.
Collins-Thompson D., and Hwang CA. 2000. Packaging with antimicrobial properties. In Robinson RK., Batt CA.,and
Patel PD. (eds.), Encyclopedia of food microbiology,. Academic press, London, UK, Pp. 416–420.
Davies EA. 1999. Effective use of nisin to control lactic acid bacterial spoilage in vacuum-packed bologna-type sausage.
Journal of Food Protection, 9, 1004-1010.
Deegan LH., Cotter PD., Hill C., and Ross P., 2006. Bacteriocins: biological tools for bio-preservation and shelf-life
extension. International Dairy Journal, 16, 1058–1071.
Del Giudice, VJ. 1991. We must need the 21st century standards, Natl. Provisioner, 204 (6), 12
133
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Delves-Broughton, J., Blackburn, P., Evans, RJ. and Hugenholtz, J. 1996. Applications of the bacteriocin, nisin. Antonie
Leeuwenhock, 69: 193-202.
DeVuyst L. and Vandamme E. 1994. Bacteriocins of Lactic acid bacteria: microbiology, genetics and application.
Chapman & Hill Ltd., London, UK.
Eckner KF. Bacteriocins and food applications. 1992. Dairy Food and Environment Sanitation,12 (4), 204-209
El-Ziney MG., Debevere J., and Jakobsen M. 2000. Reuterin. 2000. In: Naidu AS. (ed.), Natural Food Antimicrobial Systems,
CRC Press, London, Pp. 567–587.
Fiorentini, AM., Ernani SS., Porto ACS., Jaciara ZM., and Franco BDGM. 2001. Influence of bacteriocins produced by
Lactococcus plantarum BN in the shelf-lie of refrigerated bovine meat. Brazilian Journal of Microbiology, 32, 42-46
Gálvez A., Abriouel H., López ., and Ben Omar N. 2007. Bacteriocin-based strategies for food biopreservation, Int. J.
Food Microbiol. 120, 51–70.
Hirsch A., Grimstead E., Chapman HR., and Mattic ATR. 1951. A note on the inhibition of an anaerobic sporeformer in
Swiss cheese by a nisin producing streptococcus, Journal of Dairy Science, 18, 205-206
Holtzel A., Ganzle MG., Nicholso, G.J., Hammes W.P., and Jung G. 2000. The first low-molecular-weight antibiotic from
lactic acid bacteria: reutericyclin, a new tetramic acid. Angewandte Chemie, International Edition, 39, 2766–2768.
Holzapfel WH., Geisen R., and Schillinger U. 1995. Biological preservation of foods with reference to protective cultures,
bacteriocins and food-grade enzymes. International Journal of Food Microbiology, 24, 343–362.
Hurst A. 1981. Nisin. Applied Microbiology, 27, 85-122.
Jack RW., Tagg JR. and Ray B. 1995 Bacteriocins of Gram-Positive Bacteria. Microbiology Reviews, 59, 171-200.
Jacob F., Lwaff A., Siminonvich A., and Wallman E. 1953. Definition de qualques terms relatifs à la lysogenie. Annales
de Institut Pasteur, 84, 222-224.
Kelly WJ., Asmundson RV., and Huang CM. 1996. Isolation and characterization of bacteriocin-producing lactic acid
bacteria from ready-to-eat food products. International Journal of Food Microbiology, 33, 209–218.
Klaenhammer TR. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiology Reviews, 12,
39–86.
Klaenhammer TR.1998. Bacteriocins of lactic acid bacteria, Biochimie 70, 337–349.
Kominsky GMR. 1999, Avaliação da sensibilidade de microrganismos selecionados a diferentes concentrações de nisina
em salsichas comuns e de frango. Higiene Alimentar, 13, 56-62.
Leistner L. 1978. Hurdle effect and energy saving. 1978 In: Downey, WK. (ed.), Food Quality and Nutrition. Applied
Science Publishers, London, UK, Pp. 553–557.
Leistner L. 1999. Combined methods for food preservation. 1999 In: Shafiur Rahman M. (ed.), Handbook of Food
Preservation. Marcel Dekker, New York, Pp. 457–485.
Leistner L. 2000. Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology, 55,
181–186.
Leistner L., and Gorris LGM. 1995. Food preservation by hurdle technology. Trends in Food Science & Technology, 6,
41–46.
Magnusson J., and Schnürer J. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broadspectrum proteinaceous antifungal compound. Applied and Environmental Microbiology, 67, 1–5.
Nes IF., Bao Diep D., Havarstein LS., Brurberg MB., Eijsink V., Holo H. 1996. Biosynthesis of bacteriocins of lactic acid
bacteria. Antonie van Leeuwenhoek 70, 113-128.
Nettles CG., and Barefoot SF. 1993 Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid
bacteria. Journal of Food Protection, 56, 338-346.
Nielsen JW., Dickson JS., and Crouse JD. 1990. use of bacteriocin product by Pediococcus acidilactici to inhibit Listeria
monocytogenes associated with fresh meat, Applied and Environmental Microbiology, 56, 2142-2145
Nilsson L., Chen Y., Chikindas ML., Huss HH., Gran L., and Monteville TJ. 2000.Carbon dioxide and nisin act
synergistically on Listeria monocytogenes. Applied and Environmental Microbiology, 66, 769-774.
Ray B., and Daeschel MA. 1992. Food Biopreservatives of Microbial Origin. CRC Press, Boca Raton, FL.
Ross AIV., Griffiths MW., Mittal G.S., and Deeth HC. 2003. Combining on thermal technologies to control foodborne
microorganisms. International Journal of Food Microbiology, 89, 125–138.
Schillinger, U. and Holzapfel, W.H. 1996. Guidelines for manuscripts on bacteriocins of lactic acid bacteria. Int. J. Food
Microbiol., 33: 3–5.
Snyder, op., and Poland, DM. 1991. America’s safe food-Part II, Dairy Food Environment Sanit., 11, 14
Todorov S., Vaz-Velho M., and Dicks LMT. 2003. Isolation and partial characterization of bacteriocins produced by four
lactic acid bacteria isolated from traditional South African Beer. Electronic Journal of Environmental and Agricultural
Food Chemistry, 2(4), 1-6.
Vignolio G., Fadda S., Kairuz MN., Ruizholgado AA., and Oliver G. 1996. Control of Listeria monocytogenes in ground
beef by lactocin 705, a bacteriocin produced by lactobacillus casei CRL 705. International Journal of Food Microbiology,
29, 3997-4002.
WHO. 2002. Food safety strategic planning meeting: report of a WHO strategic planning meeting, WHO headquarters,
Geneva, Switzerland.
134
Regulatory Aspects of Functional Foods
Regulatory Aspects of Functional Foods
Bimlesh mann , Rajesh Kumar and Prerna Saini
Dairy Chemistry Division, NDRI, Karnal
Introduction:
The term “Functional Foods” was first introduced in Japan in the mid-1980s and refers to processed
foods containing ingredients that aid specific body functions, in addition to being nutritious. Currently,
there is no universally accepted term for functional foods. A variety of terms have appeared worldwide such as nutraceuticals, medifoods, vita foods and the more traditional dietary supplements and
fortified foods. However, the term Functional foods have become the predominant one even though
several organizations have attempted to differentiate this emerging food category. Consumer interest in
the relationship between diet and health has increased substantially. There is much greater recognition
today that people can help themselves and their families to reduce the risk of illness and disease and
to maintain their state of health and well being through a healthy lifestyle, including the diet. Ongoing
support for the important role of foods such as fruits and vegetables and wholegrain cereals in disease
prevention and the latest research on dietary antioxidants and combinations of protective substances
in plants has helped to provide the impetus for further developments in the functional food market.
Current research suggests that functional foods can make a positive contribution to addressing those
challenges. Behind functional food research and development, the key drivers are the food industry,
consumers and governments. The growth of the functional foods sector not only represents significant
benefits to the health sector but also offers opportunities for processing and manufacturing companies.
Manufacturers and their search for added-value, higher margin products provided key impetus
for the growth of functional products. However, the potential for financial gain resulted in many
unsupported claims for functional ingredients by commercial enterprises whose interests lie more in
profit rather than sound science. As a result, the functional foods field has been tarnished and suffers a
credibility gap. Many academic, scientific and regulatory organizations are actively working on ways
to establish the scientific basis to support claims for functional components or the foods containing
them. Any regulatory framework will need to protect consumers from false and misleading claims
and to satisfy the needs of industry for innovation in product development, marketing and promotion.
For functional foods to deliver their potential public health benefits, consumers must have a clear
understanding of, and a strong confidence level in, the scientific criteria that are used to document
health effects and claims. As interest in this category of foods has grown, new products have appeared
and interest has turned to the development of standards and guidelines for the development and
promotion of such foods.
Existing national and international regulatory systems governing the
production and distribution of functional foods:
WHO (1991) published a seminal report “Guidelines for the Assessment of Herbal Medicines”
which set out “to define basic criteria for the evaluation of quality, safety and efficacy” of all herbal
(including mushrooms) medicines. “As a general rule in this assessment, traditional experience means
that long-term use as well as the medical, historical and ethnological background of those products
shall be taken into account.” Depending on each country’s situation, “the definition of long-term use
may vary, but would be at least several decades … Prolonged and apparently uneventful use of a
substance usually offers testimony of its safety”. The Guidelines call for various assessments of quality,
efficacy and the intended use, and reference should be made to pharmacopoeia monographs where
they exist. If none exist, then the manufacturer should be required to produce a similar statement.
Procedures should all correspond to Good Manufacturing Practices and include stability testing of the
final product as packaged. With regard to safety “A guiding principle should be that if the product has
been traditionally used without demonstrated harm, no specific restrictive regulatory action should
be undertaken unless new evidence demands a revised risk-benefit assessment” (Alkerele, 1992).
135
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
It is recommended that consumer product information should include a quantitative list of active
ingredients, dosage, dosage form, indications, mode of administration, duration of use, any major
adverse effects, contraindications, warnings, etc. (Wasser et al., 2000a). In this article the preliminary
recommendations by FAO about regulation of functional foods followed by an overview of the
functional foods regulatory systems of Europe, USA, Canada, Australia, Japan , other Asian countries
and guidelines of Codex Alimentarius as well as food safety act 2006 (India) are presented.
Some preliminary recommendations:
Some recommendations have previously been made by FAO (FAO, 2004).
1. Functional foods should be clearly defined:
An international definition for functional foods should be adopted: Functional foods should be “a
food similar in appearance to a conventional food (beverage, food matrix), consumed as part of the
usual diet which contains biologically active components with demonstrated physiological benefits
and offers the potential of reducing the risk of chronic disease beyond basic nutritional functions”;
•
An international database from dietary active compounds should be encouraged:
•
The basic principles for the addition of dietary active compounds in foods could be based on the
principles for the addition of essentials nutrients to foods as stated by the Codex Alimentarius
Commission:
2. Health claims vs structure/functions claims vs nutrition claims should be clearly defined
•
Nutrition Claims could be referred to what the product contains;
•
Health Claims: could be related to what the food or food components does or do. The Codex
Alimentarius guidelines for use of nutrition and health claims in foodlabelling should be
encouraged.
3. Health claims should require scientific validation and substantiation Substantiation of a claim
should be based on human data using rigorous scientific protocols:
•
There is a need to define guidelines for safety and efficacy assessment of functional foods.
EUROPE :
In December 2006, the regulation on the use of nutrition and health claims for foods was adopted
by the Council and Parliament of Europe.
For the purposes of this regulation, the following definitions have been proposed:
•
“Claim”: any message or representation, which is not mandatory under Community or national
Legislation, including pictorial, graphic or symbolic representation, in any form, which states,
suggests or implies that a food has particular characteristics;
•
“Nutrition claim”: means any claim which states, suggests or implies that a food has particular
beneficial nutritional properties due to: a) the energy (calorific value) it (i) provides; (ii) provides
at a reduced or increased rate; or (iii) does not provide; and/or b) the nutrients or other substances
it (i) contains; (ii) contains in reduced or increased proportions; or (iii) does not contain;
•
“Health claim”: means any claim that states, suggests or implies that a relationship exists
between a food category, a food or one of its constituents and health;
•
“Reduction of disease risk claim”: means any health claim that states, suggests or implies that
the consumption of a food category, a food or one of its constituents significantly reduces a risk
factor in the development of a human disease.
•
European Agencies: European Commission, European Food Safety Authority (EFSA), European
Food Information Council (EUFIC) and International Life Sciences Institute (ILSI)
•
USA: Currently, Food and Drug Administration (FDA) has neither a definition nor a specific
Regulatory rubric for foods being marked as “functional foods”, they are regulated under the
136
Regulatory Aspects of Functional Foods
same regulatory framework as other conventional foods under the authority of the Federal Food
Drug and Cosmetic Act. There are three categories of claims that can be used on food:
•
Health Claims - Health claims describe a relationship between a food substance and a disease or
health-related conditions. There are three sets of legislation by which FDA exercises its oversight
in determining which health claims may be used on a label or in labeling for a food or dietary
supplement:
•
NLEA Authorized Health Claims – Under the provisions of the Nutrition Labeling and Education
Act (NLEA) of 1990, the Dietary Supplement Act of 1992, and the Dietary Supplement Health
and Education Act of 1994 (DSHEA), FDA may authorize a health claim for a food or dietary
supplement based on an extensive review of the scientific literature, generally as a result of the
submission of a health claim petition, using the significant agreement standard to determine the
nutrient/disease relationship is well established
•
Health Claims Based on Authoritative Statements – Under the 1997 Food and Drug
Administration Modernization Act (FDAMA), a health claim may be authorised for a food
based on an authoritative statement of a scientific body of the U.S. government or the National
Academy of Sciences. FDA has prepared a guide on how a firm can make use of authoritative
statement-based health claims on food
•
Qualified Health Claims – FDA’s 2003 Consumer Health Information for Better Nutrition
Initiative provides for the use of qualified health claims when there is emerging evidence for
a relationship between a food, food component, or dietary supplement and reduced risk of a
disease or health-related condition.
•
Nutrient Content Claims - The Nutrition Labelling and Education Act of 1990 (NLEA) permits
the use of label claims that characterize the level of a nutrient in a food (i.e., nutrient content
claims) made in accordance with FDA’s authorizing regulations.
•
Structure/Function Claims - Structure/function claims have historically appeared on the labels of
conventional foods and dietary supplements as well as drugs. However, the Dietary Supplement
Health and Education Act of 1994 (DSHEA) established somespecial regulatory procedures for
such claims for dietary supplement labels. Manufacturers of dietary supplements that make
structure/function claims on labels or in labelling must submit a notification to FDA no later
than 30 days after marketing the dietary supplement that includes the text of the structure/
function claim.
•
Agencies: The Food and Drug Administration (FDA), American Heart Association (AHA), The
Institute of Medicine (IOM).
CANADA:
•
In Canada, Health Canada regulates the functional foods and nutraceutical industry and the
Canadian Food Inspection Agency enforces these regulations. Within Health Canada’s Health
Products and Food Branch, the Food Directorates regulates functional foods, while the Natural
Health Products Directorate regulates other natural health products including vitamins, minerals;
herbal remedies; homeopathic medicines; traditional medicines such as traditional Chinese
medicines; probiotics, and other products like amino acids and essential fatty acids. Briefly, the
term “health claim”is not defined in Canada but currently, there are 3 types of nutrition claims
allowed:
•
Nutrient Content Claims- Nutrient content claims are the simplest label statement as they
identify/quantify the amount of a nutrient contained in a food. In addition, comparative
nutrient content claims (e.g. reduced, less, light) are allowed based on thestandardized reference
amount.
•
Biological Role/ Structure Function Claims – The second category of nutrition claims are referred
to as biological role or structure/function claims. Biological role claims are for nutrients, not a
137
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
food containing the nutrient. These statements identify the generally recognized function of a
nutrient as an aid in maintaining the functions of the body necessary for the maintenance of
good health, or for normal growth and development.
•
Risk Reduction Health Claims – Health Canada began to consider the possibility of risk reduction
claims for foods in 1999, by reviewing the ten U.S. approved health claims. In 2003, the Food
and Drug Regulations were amended to introduce the first series of authorized health claims
in Canada. In the meantime Health Canada developed a proposed regulatory framework for
product-specific authorization of health claims In Canada, the most of nutraceuticals fall under
the Natural Health Products Regulations of the Food and Drugs Act which came into effect on
January 1, 2004. In addition, a compliance policy is in place to ensure the safety of Canadians
until all natural health products have undergone Health Canada’s approval process.
•
Agencies: Health Canada, the Canadian Food Inspection Agency
JAPAN:
The Japanese Ministry of Health, Labour, and Welfare (MHLW) set up ‘Foods for Specified Health
Use’ (FOSHU) in 1991 as a regulatory system to approve the statements made on food labels concerning
the effect of the food on the human body. FOSHU refers to foods containing ingredient with functions
for health and officially approved to claim its physiological effects on the human body. The regulatory
range of FOSHU was broadened in 2001 to accept the forms of capsules and tablets in addition to
those of conventional foods. FOSHU increased the total to about 330 items in January 2003. In April
2001, the MHLW enacted a new regulatory system, ‘Foods with Health Claims’, which consists of the
existing FOSHU system and the newly established ‘Foods with Nutrient Function Claims’ (FNFC).
FNFC refers to all food that is labeled with the nutrient function claims specified by the MHLW.
The labelling of functional foods should always be based on scientific evidence and be in harmony
with international standards. The nutrient–function claim was adopted in the guidelines for nutrition
claims by the Codex Alimentarius in 1997. The claims of the Japanese FNFC are equivalent to the
nutrient function claims standardized by The Codex Alimentarius.
•
Agencies: Ministry of Health, Labour and Welfare
Other asian countries:
Recently, the Asian-Pacific Network for Food and Nutrition (ANFN) of the FAO regional office for
Asia and the Pacific held its regional expert consultation on functional foods and their implications in
the daily diet and published a report on the development and status of Functional foods in different
asian countries including China, India, Bangladdesh, Indonesia, Nepal, Malaysia, Philippines,
Thailand, Sri Lanka, and Vietnam (FAO, 2004). In Korea, the term “health/functional food” (HFF)
refers to food supplements containing nutrients or other substances (in a concentrated form) that have
a nutritional or physiological effect whose purpose is to supplement the normal diet. The Korean
Health/Functional Food Act that came into effect in 2004 requires these products to be marketed in
measured doses, such as in pills, tablets, capsules, and liquids.
Codex Alimentarius: The Codex Alimentarius Commission (CAC) was created in 1961/62 by
Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization
(WHO), to develop food standards, guidelines and related texts such as codes of practice under the
Joint FAO/WHO Food Standards Programme. The main purpose of this Programme is to protect
the health of consumers, ensure fair practices in the food trade, and promote coordination of all food
standards work undertaken by international governmental and non-governmental organizations.
“Codex India” the National Codex Contact Point (NCCP) for India, is located at the Directorate
General Of Health Services, Ministry of Health and Family Welfare (MOH&FW), Government of
India. It coordinates and promotes Codex activities in India in association with the National Codex
Committee and facilitates India’s input to the work of Codex through an established consultation
process.The Codex Alimentarius has defined two types of nutrition claims- Nutrition content claim
138
Regulatory Aspects of Functional Foods
and Nutrient comparative claim- and three types of health claims- nutrient function claims; enhanced
function claims and reduction of disease risk (Codex Alimentarius Commission, 2004).
•
Nutrition Claims -Guidelines for the use of nutrition claims by the Codex Committee on Food
Labeling proposed that ‘nutrient claim means any representation which states, suggests or
implies that a food has particular nutritional properties including but not limited to the energy
value and to the content of protein, fat and carbohydrate, as well as the content of vitamins
and minerals’. Nutrition Claims include two types: (i) Nutrient content claim that is a nutrition
claim that describes the level of a nutrient contained in a food and (ii) Nutrient comparative
claim is a claim that compares the nutrient levels and/or energy value of two or more foods.
•
Health Claims - Guidelines for the use of nutrition claims by the Codex Committee in Food
Labelling proposed that “health claim means any representation which states, suggests
or implies that a relationship exits between a food or a constituent of that food and health”.
Health claims include three types: (i) Nutrient Function Claim that is the claim that describes
the physiological role of the nutrient in growth, development, and the normal function of the
body’; (ii) Enhanced Function Claim concerns specific beneficial effects of the consumption of
foods and their constituents in the context of the total diet and relate to a positive contribution
to health or to improvement of a function or to modifying or preserving health and (iii) Disease
Risk Reduction Claim relates to the consumption of a food or food constituent, in the content
of the total diet, to the reduced risk of developing a disease or a health-related condition. Risk
reduction means significantly altering a major risk factor(s) for a disease or a health related
condition. Diseases have multiple factors and altering one of these risk factors may or may not
have a beneficial effect. The presentation of Risk Reduction Claims must ensure, for example, by
use of appropriate language and reference to other risk factors, that consumers do not interpret
them as prevention claims.’
Food Safety and Standards Act, 2006 (India): Several Acts and orders prevailed in India to
safeguard food safety and the health of the consumer. They were introduced to complement and
supplement each other in achieving total food safety and quality. However due to variation in the
specifications/standards in different Acts/Orders, and administration by different Departments and
Ministries, there were implementation problems and the lack of importance given to safety standards
over a period of time. The food industries were facing problems as different products were governed
by different orders and ministries and the rules and regulations in the Country needed consolidation.
The Food Safety and Standards Act 2006 was introduced to overcome these shortcomings and to give
more importance to safety standards. This Act consolidates the laws relating to food and establishes
the Food Safety and Standards Authority of India (FSSAII) for laying down science-based standards
for articles of food and to regulate their manufacture, storage, distribution, sale and import, to
ensure availability of safe and wholesome food for human consumption. This Act provides for the
establishment of the FSSAII which is an autonomous body under the Ministry of Health and Family
Welfare, Government of India. FSSAII’s work programs ensure the provision of appropriate scientific,
technical and administrative support for scientific committees and scientific panels, ensuring that the
FSSAII carries out its tasks in accordance with the requirements of its users, prepares statements of
revenue and expenditure and executes the budget, while developing and maintaining contact with
the Central Government and ensuring a regular dialogue with its relevant committees. The FSSAI
also constitutes scientific panels and scientific committees to address various technical issues such
as food additives, pesticides and antibiotics, genetically modified foods, functional foods, biological
hazards, contaminants, labeling and methods of sampling. The Scientific Committee is responsible
for providing scientific opinions to FSSAI. Finally the FSSAI is also responsible for regulating and
monitoring the manufacture, processing, distribution, sale and import of food so as to ensure safe
and wholesome food to consumers. The general principles to be followed by the Central Government,
State Governments and FSSAI while implementing the provisions of this Act shall be guided by the
following seven principles:
139
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
1. To endeavour to achieve appropriate levels of protection of human life and health and protection
of consumers’ interests including fair practices in all kinds of food;
2. Carry out risk management based on risk assessment;
3. Adopt risk management measures necessary to ensure appropriate levels of health protection;
4. Measures adopted shall be proportionate and no more trade restrictions shall be imposed than
required;
5. Measures adopted shall be revised within a reasonable period;
6. In case of suspected risks of the public consuming contaminated food, the FSSAI shall take
appropriate steps to inform the general public of the risk to health; and
7. If any lot of food fails to comply with food safety requirements it shall be presumed that the
whole consignment fails to comply with these requirements.
Genetically modified foods, organic foods, functional foods, nutraceuticals and proprietory foods
are regulated by this Act. Packaged foods, labelling requirements and advertising requirements are
adequately covered along with import regulations for food articles. There is a provision for the FSSAI
to establish various Scientific Panels such as:
•
Food additives, flavours, processing aids, materials in contact with food;
•
Pesticides and antibiotic residues;
•
Genetically modified organisms and foods;
•
Functional foods, nutraceuticles and foods for special dietary purposes;
•
Biological hazards, other contaminants; and
•
Food labelling and methods of sampling and analysis.
The Act has laid down certain broad principles for implementing the food safety, viz;
1. To lay down food safety standards and to ensure fair trade practices while achieving an
Appropriate Level of Protection (ALOP) of human life and health; for contaminants and
hazards,
2. To carry out risk analysis so as to ensure an appropriate level of protection to the consumers as
well to see that such measures are least trade restrictive and are in accordance with SPS and TBT
measures of WTO;
3. Wherever appropriate, food standards are to be specified on the basis of risk analysis;
4. Risk assessment is to be based on the available toxicological evaluation (e.g. JECFA) and extensive
open and transparent discussion with all stakeholders, and the underlying principle is to ensure
protection of consumers by preventing fraudulent, deceptive or unfair trade practices.
The Act also prescribes general provisions for articles of food:
•
Food additives / processing aids are to be added only in accordance with provisions / regulations
under the Act;
•
Foods are not to contain any contaminants such as toxic metals, toxins, pesticide residues,
antibiotics and veterinary drugs, in excess of limits prescribed under the regulation;
•
Regulations will be made for the manufacture, distribution or trade of any novel foods, GM foods,
irradiated foods, organic foods, foods for special dietary uses, functional foods, nutraceuticals,
health supplements, proprietary foods etc.
The onus of safety of food production, processing, import, distribution and sale lies with the food
business operator. The Commissioner of Food Safety of the state will implement rules under this Act at
state level. The FSSAI and the State Food Authorities will maintain a system of control, involving risk
communication, food safety surveillance and other monitoring activities covering all stages of food
business. The FSSAI is empowered to recognise any agency to conduct food safety audits which are
140
Regulatory Aspects of Functional Foods
a systematic and functionally independent examination of food safety measures based on food safety
management systems consisting of Good Manufacturing Practices, Good Hygienic Practices, Hazard
Analysis and Critical Control Points or any other such measures specified by regulation. Food testing
laboratories are required to be accredited by any accreditation agency so that the analytical results are
reliable and consistent. The FSSAI and State Food Safety Authorities are responsible for enforcement
of this Act. Both shall monitor and verify that the relevant requirements of law are fulfilled by food
business operators at all stages of food business. There is provision for food recall by the business
operator if the food does not comply with the Act. The Food Safety Authority in the State (Health
Ministry) appoints a Commissioner of Food Safety, designated officers (district level) and food safety
officers to implement the programmes under the provisions of this Act. The FSSIA will notify food
laboratories and research institutions accredited by the National Accreditation Board for testing and
calibration laboratories. It may also recognise more referral food laboratories by this Act. This Act gives
more importance for ensuring a very safe food product to consumers by providing quicker disposal of
cases within the state. Punishments offered are very severe which would make the retailer/wholesaler
be more cautious in their dealings. The standards for quality and safety laid down in this Act are
harmonised standards and applicable throughout the country, and all other standards/specifications
become null and void. This system provides for quicker corrective actions by the regulators as the
problems are localised and traceable.
Conclusion/ Recommendatrions:
Several approaches to the use of health claims on foods have been made around the world, and
the common theme is that any health claim will require scientific validation and substantiation. There
is also broad consensus that any regulatory framework should protect the consumer, promote fair
trade and encourage innovation in the food industry. However, there is a clear need to have uniform
understanding, terminology and description of types of nutrition and health claims.
References:
Food Safety and Standards Act , 2006 of India Ministry of Health and Family Welfare, New Delhi, Government of India
http://www.mohfw.nic.in/pfa.htm
Food Safety in India Ministry of Health and family Welfare, New Delhi, Government of India http://www.
foodsafetyindia.nic.in/
P. Roupas_ P. G. Williams (2007) Regulatory aspects of bioactive dairy ingredients . Food Science Australia University
of Wollongong, http://ro.uow.edu.au/
Report on Functional Foods Food Quality and Standards Service (AGNS) Food and Agriculture Organization of the
United Nations (FAO) November, 2007
141
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Nanomaterials - Their Applications
and Safety Aspects in Foods
Bimlesh Mann , Rajesh Kumar and Prabhakar Padgham
Dairy Chemistry Division, NDRI, Karnal
Nanomaterials which include nanoparticles, nano-emulsions and nano-capsules are now
being used in processed foods, food packaging and food contact materials. Because of their unique
properties, nanomaterials offer many new opportunities for the food industries, as potent colourings,
flavourings, nutritional additives and antibacterial ingredients. Due to their very large surface area,the
nanoparticles have better chemical reactivity, biological activity and catalytic behaviour as compared
to larger particles of the same chemical composition (Garnett and Kallinteri, 2006).
The Chemical Selection Working Group of the U.S. Food and Drug Administration (FDA) defined
nanomaterials as “particles with dimensions less than micrometer scale [i.e. less then 1,000 nm]
that exhibit unique properties not recognized in micron or larger sized particles” (U.S. FDA 2006).
Australia’s Commonwealth Scientific and Industrial Research Organisation (CSIRO) food scientists
have also defined nanomaterials as measuring up to 1,000 nm (Sanguansri and Augustin, 2006). In
another report on nanomaterials FDA chose not to offer a size-based definition at all (U.S. FDA, 2007).
Nanomaterials also have far greater bioavailability than larger particles, resulting in greater uptake
into individual cells, tissues and organs. Materials which measure less than 300 nm can be taken up by
individual cells (Garnett and Kallinteri, 2006), while nanomaterials which measure less than 70 nm can
even be taken up by cells’ nuclei, where they can cause major damage (Chen and Mikecz, 2005).
Nanotechnology has potential applications in all aspects of food processing, food packaging and
food monitoring.These includes:
•
Developments of the methods for the production of foods such as soft drinks, ice cream,
chocolate or chips to be marketed as ‘health’ foods by reducing fat, carbohydrate or calorie
content or by increasing protein, fibre or vitamin content.
•
Production of stronger flavourings, colourings, and nutritional additives.
•
Development of foods capable of changing their colour, flavour or nutritional properties
according to a person’s dietary needs, allergies or taste preferences.
•
Development of packaging materials to increase food shelf life and which can detect spoilage,
bacteria, or the loss of food nutrient.
One of the earliest commercial applications of nanotechnology within the food sector is in packaging
(Roach, 2006). Between 400 and 500 nanopackaging products are estimated to be in commercial use
now. A key purpose of nano packaging is to deliver longer shelf life by improving the barrier functions
of food packaging to reduce gas and moisture exchange and UV light exposure (Sorrentino et al., 2007).
Nano packaging can also be designed to release antimicrobials, antioxidants, enzymes, flavours and
nutraceuticals to extend shelf-life (Cha and Chinnan, 2004; LaCoste et al., 2005). Packaging equipped
with nano sensors is designed to track either the internal or the external conditions of food products,
pellets and containers throughout the supply chain. The use of nanomaterials to strengthen bioplastics
(plant-based plastics) may enable bioplastics to be used instead of fossil-fuel based plastics for food
packaging and carry bags (Sorrentino et al., 2007; Technical University of Denmark, 2007).
Unfortunately, the greater chemical reactivity and bioavailability of nanomaterials may also result
in greater toxicity of nanoparticles compared to the same unit of mass of larger particles of the same
chemical composition (Hoet et al., 2004; Oberdörster et al., 2005b).Other properties of nanomaterials
that influence toxicity include: chemical composition, shape, surface structure, surface charge, catalytic
behaviour, extent of particle aggregation (clumping) or disaggregation, and the presence or absence of
other groups of chemicals attached to the nanomaterial (Brunner et al., 2006; Magrez et al., 2006; Sayes
et al., 2006).The potential health risks associated with nanomaterials in foods has mainly focused on
142
Nanomaterials - Their Applications and Safety Aspects in Foods
manufactured nanomaterial food or food packaging additives but ignored the nanoparticles created
during processing. Thus nanoparticles are also present in many foods because of the technology
used to process the foods, rather than because they are food additives or ingredients. Although food
processing technologies that produce nanoparticles are not new, the rapidly expanding consumption of
highly processed foods is most certainly increasing our exposure to nanoparticles in foods. Processing
techniques which produce nanoparticles, particles up to a few hundred nanometres in size, and nanoscale emulsions are used in the manufacture of salad dressings,chocolate syrups, sweeteners, flavoured
oils, and many other processed foods (Sanguansri and Augustin, 2006). The formation of nanoparticles
and nanoscale emulsions can result from food processing techniques such as high pressure valve
homogenisation, dry ball milling, dry jet milling and ultrasound emulsification. Although many food
manufacturers may remain entirely unaware that their foods contain nanoparticles, it is likely that
these processing techniques are used precisely because the textural changes and flow properties they
produce are attractive to manufacturers.
Recent research has found that many food products contain insoluble, inorganic nanoparticles
and microparticles that have no nutritional value, and which appear to have contaminated foods
unintentionally, for example as a result of the wear of food processing machines or through
environmental pollution. The health implications of food processing techniques that produce
nanoparticles and nanoscale emulsions also warrant the attention of food regulators. The potential for
such foods to pose new health risks must be investigated in order to determine whether or not related
new food safety standards are required. Just as a better understanding of the health risks of incidental
nanoparticles in air pollution have resulted in efforts to reduce air pollution, improved understanding
of the health risks associated with incidental nanoparticle contaminants in foods may also warrant
efforts to reduce incidental nanoparticles’ contamination of processed foods.
The commercial manufacturing of food products, food packaging and food contact materials should
be after the introduction of nanotechnology specific regulation which protects the public workers and
the environment from their risks. Because of their potentially serious health risks, environmental risks
and social implications, the following points have to be ascertained, before the commercial applications
of these nanomaterials.
•
These manufactured food nanomaterials must be subject to new safety assessments as new
substances, even where the properties of their larger scale counterparts are well-known.
•
The manufactured nanomaterials must be subject to rigorous nano-specific health and
environmental impact assessment and demonstrated to be safe prior to approval for commercial
use in foods, food-packaging and food contact materials.
•
All particles up to 300nm in size must be considered to be ‘nanomaterials’ for the purposes of
health and environment assessment, given the early evidence that they pose similar health risks
as particles less than 100nm in size which have to date been defined as ‘nano’.
•
The data related to safety assessments, and the methodologies used to obtain them, must be
placed in the public domain.
•
The nano ingredients must be clearly indicated on product labels to allow the public to make an
informed choice about product use.
•
The public, including all stakeholder groups affected, must be involved in all aspects of decision
making regarding nanotechnology in food. This includes in the development of regulatory
regimes, labeling systems, and prioritization of public funding for food and agricultural
research.
References:
Cha D, Chinnan M. 2004. Biopolymer-based antimicrobial packaging: A review. Critic RevFood Sci Nutrit 44:223-237.
Chen M, von Mikecz A. 2005. Formation of nucleoplasmic protein aggregatesimpairs nuclear function in response to
SiO2 nanoparticles. Experiment Cell Res 305:51-62.
Friends of the Earth International. 2009. OUT OF THE LABORATORY AND ON TO OUR PLATES Nanotechnology in
Food & Agriculture Amsterdam. Available at: http://www.foei.org
143
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Garnett M, Kallinteri P. 2006. Nanomedicines and nanotoxicology: some physiological principles. Occup Med 56:307311. Gatti A. Undated. “Nanopathology : a new vision of the interaction environment-human life”. Available at:
http://ec.europa.eu/research/qualityoflife/ ka4/pdf/report_nanopathology_en.pdf (accessed 11 September
2007).
Hoet P, Bruske-Holfeld I, Salata O. 2004. Nanoparticles – known and unknown health risks. J Nanobiotechnol 2:12.
Hund-Rinke K, Simon M. 2006. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids.
Environ Sci Poll Res 13(4):225-232.
Invest Australia. 2007. Nanotechnology: Australian Capability Report, Third Edition.
Commonwealth of Australia, Canberra. Available at: http://www. investaustralia.gov.au/media/NANOREPORT07.
pdf(accessed 17 January 2008).
LaCoste A, Schaich K, Zumbrunnen D, Yam K. 2005. Advanced controlled release packaging through smart blending.
Packag Technol Sci 18:77-87.
Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D,
Olin S, Monteiro-Riviere N, Warheit D, Yang H. 2005b. Principles for characterising the potential human health
effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol 2:8
Roach S. 2006. Most companies will have to wait years for nanotech’s benefits. Foodproductiondaily.com 21 August
2006. Available at: http://www.foodproductiondaily.com/news/ng.asp?id=69974 (accessed 17 January 2008).
Sanguansri P, Augustin M. 2006. Nanoscale materials development – a food industry perspective. Trends Food Sci
Technol 17:547-556.
Sayes C, Wahi R, Kurian P, Liu Y, West J, Ausman K, Warheit D, Colvin V. 2006. Correlating nanoscale titania structure
with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung
epithelial cells. Toxicol Sci 92(1):174–185.
Sorrentino A, Gorrasi G, Vittoria V. 2007. Potential perspectives of bio-nanocomposites for food packaging applications.
Trends Food Sci Technol 18:84-95.
Technical University of Denmark. 2007. Bioplastic developed into food packaging through nanotechnology. News 23
March 2007. Available at: http://risoe-staged.risoe.dk/News_archives/News/2007/0322_bioplast.aspx (accessed
17 January 2008).
U.S. FDA. 2006. Nanoscale Materials [no specified CAS] Nomination and Review of Toxicological Literature. December
8, 2006. Prepared by the Chemical Selection Working Group, U.S. Food & Drug Administration. Available at:
http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Nanoscale_materials.pdf (accessed 15
January 2008).
U.S. FDA. 2007. Nanotechnology: A Report of the U.S. Food and Drug Administration Nanotechnology Task Force. July
25, 2007. Available at: http://www.fda.gov/ nanotechnology/taskforce/report2007.html (accessed 15 January
2008).
144
Strategies for Animals Studies to Assess the Safety Aspects and Bioavailability of Netraceuticals
Strategies for Animals Studies to Assess the Safety
Aspects and Bioavailability of Netraceuticals
Ayyasamy Manimaran1 and Bimlesh Mann2
1
Livestock Production Management and 2Dairy Chemistry Division, NDRI, Karnal
Introduction
The word “nutraceutical” was first coined by DeFelice (2007) who defined it as “a substance that is
a food or a part of food and provides medical and health benefits, including prevention and treatment
of disease.” Research and awareness about nutraceuticals has been increased in last few decades
and expected to continue to increase. Basic research using laboratory animals is critical to furthering
understanding of the impact of nutraceuticals on health promotion and disease management, apart
from regulatory prerequisite for conducting further human clinical trials. A variety of laboratoryand small-animals are used for evaluation of functional food and nutraceutical efficacy/metabolic
evaluation. These pre-clinical trials can be conducted utilizing either immune competent or
immunocompromised animals like nude mice which are not having cell mediated immune response.
Although significant evidence exists that functional foods and nutraceuticals can play key roles in
disease prevention and health promotion, as in decreasing the risk of certain chronic diseases, safety
considerations must not be ignored. Safety pharmacology studies for developing nutraceuticals are
necessary and compulsory to support human clinical trials of a given scope and duration as well
as marketing authorization for pharmaceuticals. The objective is to identify possible, undesirable
pharmacodynamic effects of the nutraceuticals which are unrelated to the main pharmacological
activity, after therapeutic administration or overdose. Safety pharmacology and pharmacodynamic
studies includes the assessment of effects on cardiovascular, central nervous and respiratory
systems and should generally be conducted before human exposure. Safety pharmacology studies
can be performed as independent studies or can be incorporated as a part of toxicological studies
thus reducing the number of animals used in accordance with the 3R’s principles. Incorporation into
toxicological studies may offer the additional advantage that the effect of the nutraceuticals can be
evaluated not only after a single administration but also after repeated administration for a given
period of time. However, one objection is that the dose levels involved can be much higher than the
therapeutic dose. In safety pharmacology studies, the low dose should be equal to or slightly higher
than the therapeutic dose. The purpose of toxicity testing of animals is to know the biological effects
of substances, so that precautions can be taken to protect humans, animals and the environment.
Mice are the most widely used species accounting for more than 50% of animals use. Mice biology is
well known than any other laboratory animal and this is widely used by immunologist, oncologist
and geneticist. Rats are second most widely used a laboratory animal species and they are generally
preferred over mice by toxicologist and pharmacologists due to convenient size and they do not have
many virally-induced tumors as mice. Rabbits have been widely used for antisera production, pyrogen
testing and reproductive studies particularly for teratogenicity.
Analysis of nutraceuticals
Milk and dairy products are important source for proteins, peptides and amino acids. They
have angiotension converting enzymes (ACE) inhibitory activities, antibacterial, antioxidant,
immunomodulating, antithrombotic, absorption of minerals and anti-inflammatory activities. These
health promoting effects make these compounds as nutraceuticals for prevention and treatment of
hypertension, diabetes and hepatitis etc. Their identification requires advanced analytical techniques
due to complexity of these compounds. In general, analysis of milk compounds are carried out by liquid
chromatography coupled to mass spectrometry (LC-MS) or capillary electrophoresis (CE) (Campanella
et al., 2009; Contreras et al., 2008; Meltretter et al., 2008; Simone et al., 2009) and immunosensors for
particular determination of lactoferrin and immunoglobulin G in milk (Campanella et al., 2009).
145
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Further, an omics-rooted study of milk proteins has been carried out using advanced analytical
techniques (HPLC-MS/MS, 2D-PAGE, MALDI-TOFMS) showing the great potential of this modern
approach (Casado et al., 2009). Polyacrylamide gel electrophoresis (PAGE), sodium dodecyl sulfate
PAGE (SDS-PAGE) and 2D-PAGE have been employed to analyze proteins in milk, (Casado et al.,
2009). However, these more classical techniques do not provide an identification of these biomolecules
as accurate as CE or HPLC coupled to mass spectrometry. Thus, mass spectrometry alone or coupled
to HPLC has been used to characterize, identify and analyze proteins, peptides and amino acids in
several sources including milk. Gas chromatography (GC) and HPLC are preferred analytical tools
for analyzing bioactive compounds, probably due to their versatility, generalized availability, lowcost and simplicity. Other techniques such as CE, MS, Nuclear Magnetic Resonance (NMR) or Fourier
Transformed Infrared Spectroscopy (FTIR) have also given good results, although their use is not as
widespread as GC or HPLC.
Table.1 Nutraceutical and analytical techniques employed for their analysis
Nutraceutical
Source
Possible health effect
Analytical
techniques
Reference
Phytosterols and
phytostanols
Milk and
yoghurt
Decrease cholesterol levels
GC-MS
Santos et al., 2007
Milk lipids
(triglycerides,
diacylglycerides,
saturated fatty acids
and PUFAs).
Milk
Immuno-suppressive, antiinflammatory, andantimicrobial
properties.
HPLC-MS/MS,
GC/LC.
Casado et al., 2009
Gangliosides
Dairy
products
(milk)
Protect against enteric
pathogens, and prebiotic
functions.
MALDI-TOFMS,
HPTLC, HPLCMS
Lacomba, et al., 2010;
Mocchetti, 2005
Milk proteins, peptides,
Lactoferrin and
immunoglobulin G.
Milk and
derived
products
Antihypertensive, antimicrobial,
antiinflammatory and
inmunostimulating activities.
Important source of amino
acids
HPLC-MS/
MS, 2D-PAGE,
MALDI-TOFMS,
Inmunosensors,
CE (UV, MS),
Lacomba, et al., 2010;
Campanella et al., 2009;
Contreras et al., 2008;
Meltretter et al., 2008;
Simone et al., 2009
Pharmacological characterization
The pharmacological characterization of a nutraceutical is simply the determination of its efficacy
and safety. Since many nutraceuticals are considers as food items (Dietary Supplement Health and
Education Act, 1994), currently, many nutraceuticals (e.g., botanicals) do not require efficacy and
safety testing before marketing. However, Morrow et al. (2005) reported that there is a concern that
many nutraceuticals have pharmacological activity that can endanger the public health and that certain
nutraceuticals (e.g., botanicals) should be regulated similarly to prescription nutraceuticals. Therefore,
future marketing of nutraceuticals may require more rigorous testing of safety and efficacy before
marketing. In fact, the FDA (2007) developed a current good manufacturing practice requirement for
dietary supplements that obligates manufacturers to evaluate the composition, identity, quality, and
strength of their marketed products. With future increased regulation of nutraceuticals on the horizon,
pharmacological characterization of nutraceuticals will be useful.
Drug development, testing and review process
The drug review process is roughly divided into preclinical and clinical testing. The preclinical test
is primarily in vitro and animal studies, whereas clinical are human studies.
Preclinical testing in animal model (one rodent, one non-rodent) is useful to evaluate acute and
short term toxicity. Doses will be at normal levels for short and long term or increasingly high levels
to induce toxicity. It is useful to determine lethal dose. Pre-clinical studies will be useful to assess
how drug/chemicals is absorbed, distributed, metabolized, and excreted in animals. Further, clinical
studies will be conducted in human being in order to verify the mechanism and efficacy. It includes
146
Strategies for Animals Studies to Assess the Safety Aspects and Bioavailability of Netraceuticals
the following phases (FDA, 2002; Berkowitz, 2007).
•
Phase I: 20–80 human subjects, safety, pharmacokinetics
•
Phase II: 36–300 human subjects, efficacy
•
Phase III: 300–3,000 human subjects, efficacy, double-blind studies
•
Phase IV: post-marketing surveillance
Preclinical testing
Preclinical safety testing assesses the potential toxicity of a drug in in vitro and animal studies
(FDA, 1985; Berkowitz, 2007). Preclinical testing involves pharmacological profile tests and it can be
further divided into the following (Berkowitz, 2007)
1. Molecular: receptor binding, enzyme inhibition
2. Cellular: cell cultures, isolated tissues
3. Disease models: pain, seizures
Safety studies required by the FDA
1. Pharmacology studies: determine ED50
2. Acute toxicity studies: determine LD50
3. Multi-dose toxicity studies
a. Subchronic toxicity: duration of one to three months
b. Chronic toxicity: duration of six months
c. Carcinogenicity: duration of two years
4. Special toxicity studies: route of administration
5. Reproduction studies: birth defects
6. Mutagenicity studies: Ames test
7. Pharmacokinetics studies: absorption, distribution, metabolism and excretion (ADME)
Acute toxicity
Acute toxicity tests are generally provide data on the relative toxicity likely to arise from a single
or fractionated doses up to 24 hrs for oral and dermal studies, while 4-hr exposure for inhalation
studies. Rats are preferred for oral and inhalation tests where as rabbits preferred for dermal tests.
Young adults of 5 of each sex per dose level with minimum three dose levels were recommended.
Animals should be monitored for 14 days for any clinical symptoms.
Subacute study (repeated dose exposure)
It is performed to obtain dose for subchronic studies typical protocol is to give 3-4 dosages, and
10 animals for each sex per dose are often used. For non-rodents species, usually dogs (3-4 of each sex
per dose).
Subchronic toxicity
Subchronic toxicity tests are employed one month to three months (90 days are common).
Detailed clinical observations and pathology examinations should be conducted. Two species are
recommended (rodents and non-rodents). Young adult rodents’ (10-20 animals for each sex per dose)
and non-rodents species, usually dogs (4 of each sex per dose) should be used for experimentation. At
least 3 dose levels, in which high dose produce toxicity but not more than 10 per cent mortality, low
dose not produce toxicity and intermediate dose. The principal goal of this test is development of No
Observable Adverse Effects Level (NOAEL) and sometimes these protocols can be used for further
like chronic and developmental toxicity studies.
147
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Chronic toxicity
Long-term or chronic toxicity tests determine toxicity from exposure for a substantial portion of a
subject’s life. They are similar to the subchronic tests except that they extend over a longer period of
time, which is depend upon intended period (short or long) of exposure to human and involve larger
groups of animals. In rodents, chronic exposures are usually for 6 months to 2 yrs and in non rodents
1 yr or more. It is useful to assess the cumulative toxicity of chemicals particularly carcinogencity.
Dose selection for the chronic study is generally based on the results of a series of subchronic (90 day)
toxicity studies. Result of weight gain, survival information, pharmacokinetic, metabolism data, and
histopathology from these experiments that are used for the dose selection. Highest dose employed
should be the maximum tolerated dose (MTD) which is defined as “the highest dose of the test agent
during the chronic study that can be predicted not to alter the animals’ normal longevity from effects
other than carcinogenicity”. Carcinogenicity tests are similar to chronic toxicity tests. Testing in two
rodent species (mice and rats), 50 of each sex per dose level are preferred due to short life span. The
exposure period is at least 18 months for mice and 24-30 month for rats. They should be observed for
18-24 months for mice and 24-30 month for rats.
Developmental and reproductive toxicity
Developmental toxicity testing detects the potential for substances to produce embryotoxicity
and birth defects. Developmental toxicity is the study of adverse effects on the developing organism
occurring at any time during life span form before conception, during prenatal development or
postnatally until puperty. Teratology study involves from conception to birth. Reproductive toxicity
testing is intended to determine the effects of substances on gonadal function, conception, birth, and
the growth and development of the offspring. The oral route is preferred.
Pharmacological studies
Apart from safety and efficacy of nutraceuticals, the bioavailability studies are important.
Bioavailability is the measurement of the rate and extent of the active ingredient that reaches the
systemic circulation. This can be determined by measuring the active ingredient of nutraceuticals or
its metabolites from the blood. Active ingredient can be accurately quantitated pharmacokinetically
in the plasma (tmax, Cmax and AUC) or urine (rate of drug excretion) gives the most objective
data on bioavailability. Pharmacokinetic studies are preferred over pharmacodynamic (deals about
mechanism of action) studies. When both pharmacokinetic and pharmacodynamical studies are not
possible, then a clinical study can be used in human or suitable animals model with assumption
of therapeutic success occurred because there was enough bioavailability when the nutraceuticals
was administered. However, various factors such as diet, disease, or genetics, which can make it
difficult to understand the success or failure (Shargel, 1993; FDA, 2003). Whenever potentially active
metabolites found during human cell culture studies, these metabolites can be studied in laboratory
animals to determine their safety and efficacy, which can help determine future in vitro or in vivo
human studies. Moreover, animal studies can be used to examine nutraceuticals-drug interactions
with regard to parent drug and its metabolites. Since several nutraceuticals (example, Grapefruit
and St. John’s Wort) are inhibit or induce a cytochrome P450 which could affect subsequently
administrated drug concentrations.
Assays for ACE-inhibitory and antihypertensive activity
Determination of the ACE inhibitory activity is the most common strategy followed in the selection of
antihypertensive peptides derived from milk proteins. In order to facilitate the characterisation of ACE
inhibitory peptides, the establishment of a simple, sensitive and reliable in vitro ACE inhibition assay
like, spectrophotometric, fluorimetric, radiochemical, HPLC and capillary electrophoresis methods
can be used to measure ACE activity. This is usually expressed as the IC50, i.e. concentration needed
to inhibit 50% of the enzyme activity. The spectrophotometric method of Cushman and Cheung (1971)
is most commonly utilized. The in vivo effects are tested in spontaneously hypertensive rats (SHR),
148
Strategies for Animals Studies to Assess the Safety Aspects and Bioavailability of Netraceuticals
which constitute an accepted model for human essential hypertension. In addition, in many in vivo
studies it is also checked that antihypertensive peptides from milk proteins do not modify the arterial
blood pressure of Wistar-Kyoto (WKY) rats that are the normotensive control of the SHR. The results
of hypotensive effects caused by the short-term administration to SHR of milk protein hydrolysates,
fermented products and isolated milk-derived peptides have been shown to lack of correlation between
the in vitro ACE inhibitory activity and the in vivo action. This poses doubts on the use of the in vitro
ACE inhibitory activity as the exclusive selection criteria for potential antihypertensive substances,
as it does not take into consideration of the bioavailability of the peptides or other mechanism like
antioxidant effects. On other hand, long-term intake of milk products on blood pressure of SHR was
shown that dose dependent attenuation of the development of hypertension in SHR during 14 weeks
of treatment with milk containing the potent ACE inhibitory peptides (Nakamura et al., 1995; Sipola
et al., 2002).
Hypertension animal models
Rats are the most popular species in hypertension. The rat models of hypertension thus
provide ample opportunity not only to investigate the mechanisms involved in the pathogenesis
of hypertension, but also to learn about the critical balance between stress and coping. Among
rats spontaneously hypertensive rat (SHR) is most widely used rat model, although it reflects only
a rare subtype of primary human hypertension, which is due to genetic inheritance. SHR stroke
prone (SHR-SP) is a further developed sub-strain, with even higher levels of blood pressure,
and a strong tendency to die from stroke. Other rat models of hypertension are Dahl (due to
genetic inheritance like SHR), deoxycorticosterone acetate (DOCA)-salt, cause hypertension due
to hormonal alterations (Contreras et al., 2009).
Type 2 diabetic animal models
Chemical induced diabetes model can be produced by administrating drugs like alloxan in rat
(40-200 mg/kg, iv or ip), mice (50-200 mg/kg, iv or ip), rabbit (100-150 mg/kg, iv), dog (50-75 mg/
kg, iv) can cause diabetes. Administration of streptozotocin to rat (35-65 mg/kg, iv or ip), mice (100200 mg/kg, iv or ip), hamster (50 mg/kg, ip), dog (20-30 mg/kg, iv) can induce diabetus in these
animals. Selective loss of pancreatic beta cells, residual insulin secretion and ketosis makes less
mortality. Comparatively cheaper, easier to make and maintenance of animals. Disadvantages are,
direct cytotoxic action on the beta cells and insulin deficiency rather than consequence of insulin
resistance, less stable and reversible. Further, toxic actions on other body organs are constraints in
long-term experiments. Though spontaneous type 2 diabetic models are resemble to human being
and minimum variability of results with minimum sample size, they are limited availability, costly
and required sophisticated maintenance. In dietary or nutrition induced type 2 diabetic models as a
result of overnutrition, toxicity of other vital organ can be avoid. However, long period is required
to create diabetes and no frank hyperglycaemia develops upon simple dietary treatment. Surgical,
transgenic and knock out models of diabetes animals are need cumbersome technical procedure and
costly procedure (Srinivasan and Ramarao, 2007).
Conclusion
Experiments using laboratory animals should be well designed, efficiently executed, correctly
analyzed, clearly presented, and correctly interpreted if they are to be ethically acceptable. Laboratory
animals are nearly always used as models or surrogates of humans or other species. Animals should be
used only if the scientific objectives are valid (i.e. high probability of meeting the stated objectives and
reasonable contributing to human or animal welfare, possibly in the long term), no other alternative,
and the cost to the animals is not excessive. The reason for choosing their particular animal model
and the species, strain, source, and type of animal used should be clear. The “3Rs” rules (replacement,
refinement and reduction) should be followed to humane use of animals. However, it is important to
recognizing biological effects with sufficient numbers animals in experiments. The number of animals
to be used in an experiment depends on a variety of factors, including experiment objectives, degree
149
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
of precision required, the expected difference between the effects of treatments and structure and
methods of analysis. Development and application of the biomarkers to clarify functionality and
risk are important to understand the fundamental molecular mechanism concerning health care and
disease prevention of nutraceuticals. Through use of advanced technologies to study the relationship
between nutrition intake and health associated with genes can be useful for better understanding of
nutraceuticals.
References
Berkowitz, B. A. 2007. Development and Regulation of Drugs. In Basic and Clinical Pharmacology. 10th edition. Edited by
Katzung, B. G. New York, NY: McGraw-Hill, pp. 64–73.
Campanella, L., Martini, E., Pintore, M. and Tomassetti, M. (2009). Determination of lactoferrin and immunoglobulin G
in animal milks by new immunosensors. Sensors, 9: 2202-2221.
Casado, B., Affolter, M. and Kussmann, M. (2009). OMICS-rooted studies of milk proteins, oligosaccharides and lipids,
J. Proteomics, 73: 196-208.
Contreras, M. del. M., Carro´n, R., Montero, M. J., Ramos, M. and Recio, I. (2009). Novel casein-derived peptides with
antihypertensive activity. International Dairy Journal, 19: 566–573.
Contreras, M. M., López-Expósito, I., Hernández-Ledesma, B., Ramos, M. and Recio, I. (2008). Application of mass
spectrometry to the characterization and quantification of food bioactive peptides. J. AOAC Int., 91 (4): 981-994.
Cushman, D.W. and Cheung, H. S. (1971). Spectrophotometric assay and properties of the angiotensin-converting
enzyme of rabbit lung. Biochem.Pharmacol., 20: 1637–1648.
DeFelice, S. 2007. The Foundation for Innovation in Medicine. http://www.fi mdefelice.org.
Food and Drug Administration. (1985). Guidance for industry: Guideline for the format and content of the nonclinical
pharmacology/toxicology section of an application. http:// www.fda.gov/cder/guidance/old032fn.pdf.
Food and Drug Administration. (2002). The FDA’s drug review process: Ensuring drugs are safe and effective. http://
www.fda.gov/fdac/features/2002/402_drug.html.
Food and Drug Administration. (2003). Guidance for industry. Bioavailability and bioequivalence for orally administered
drug products: General considerations. http://www.fda. gov/cder/guidance/5356fnl.pdf.
Food and Drug Administration. (2007). Final rule promotes safe use of dietary supplements. http://www.fda.gov/
consumer/updates/dietarysupps062207.html.
Meltretter, J., Schmidt, A., Humeny, A., Becker, C.M. and Pischetsrieder, M. (2008). Analysis of the peptide profile of
milk and its changes during thermal treatment and storage, J. Agric. Food Chem., 56: 2899-2906.
Morrow, J., T. Edeki, M. El Mouelhi, R. Galinsky, R. Kovelesky, and C. Preuss. (2005). American Society for Clinical
Pharmacology and Therapeutics position statement on dietary supplement safety and regulation. Clin. Pharmacol.
Ther. 77:113–122.
Nakamura, Y., Yamamoto, N., Sakai, K. and Takano, T. (1995). Antihypertensive effect of sour milk and peptides
isolated from it that are inhibitors to angiotensin I-converting enzyme. J. Dairy Sci., 78: 1253–1257.
Shargel, L. and A. Yu. (1993). Bioavailability and Bioequivalence. In Applied Biopharmaceutics and Pharmacokinetics.
3rd edition. Norwalk, CT: Appleton and Lange, pp. 193–223.
Simone, C. D., Picariello, G., Mamone, G., Stiuso, P., Dicitore, A., Vanacore, D., Chianese, L., Addeo, F. and Ferranti, P.
(2009). Characterisation and cytomodulatory properties of peptides from Mozzarella di Bufala Campana cheese
whey. J. Pept. Sci., 15: 251-258.
Sipola, M., Finckenberg, P., Korpela, R., Vapaatalo, H., and Nurminen, M.-L. (2002). Effect of long-term intake of milk
products on blood pressure in hypertensive rats. J. Dairy Res., 69: 103–111.
Srinivasan K. and Ramarao P. (2007). Animal models in type 2 diabetes research: An overview Indian J Med Res 125:
451-472.
150
Recent Advances in Synbiotic Dairy Foods and Their Safety Evaluation
Recent Advances in Synbiotic Dairy
Foods and Their Safety Evaluation
Chand Ram, Manju and Santosh Anand
Dairy Microbiology Division, NDRI, Karnal
Introduction
The human gut habitats >500 species of bacteria and their proper balance is pre requisite for well
being of humans and animals. It is established that beneficial microflora must be viable and should
remain adhered to the inner surface of the epithelial cells to confer desired health benefits to the host.
Various factors such as nutritional requirements, transit time, infections, availability of metabolizable
substrates affect microbial ecology of large intestine. Dietary habits influence gut microflora e.g.
bifidobacteria dominate breast fed infants which gradually decrease with advancement in age. In
addition, food substrate form also plays vital role in determining composition of gut microflora for
instance, presence of N- acetyglucosamine, galactose, certain glycoproteins and fucose oligomers
in human milk act as specific growth factors for bifidobacteria. Further, low protein and high
lactoferrin content in human milk elevate growth of bifidobacteria and inhibit growth of undesirable
microorganisms, respectively. Elie Metchnikoff (1907) hypothesized longevity of Bulgarian peasants
associated with continuous consumption of fermented milks, the concept of probiotics as known
today. Imbalance of microbial ecology of gut can be restored by administration of probiotics. World
Health Organization (WHO) has advocated application of probiotics in the form of functional foods
for treatment and/or prevention of various ailments. This decade has witnessed advancement in the
field of probiotics in the form of synbiotic dairy foods. Hence, it is worth to discuss safety issues
related to probiotic vis a vis synbiotic dairy foods.
Functional foods and related concepts:
Dairy foods have always been a choice of innovation to remain competitive in the market as
well as changes in the consumer preference. The primary role of these foods is to provide nutrition
and satisfaction feeling to the consumer. However, in recent times apart from nutrition, the trend is
towards consumption of functional foods with beneficial microbes to have a state of good health and
reduce risk of disease. Dairy products have a distinct role in delivering the probiotics to the host, as
these products provide suitable environment for survival and growth.
Functional food- Food that satisfactorily demonstrate beneficial effect on one or more target
functions in the host, beyond the adequate nutritional effects in a way that is relevant to either an
improved state of health and well-being and/or reduction of risk of disease (European Functional
Food Science programme, Diplock, 1999).
Probiotics- Live microorganisms, which when administrated in adequate amount confer health
benefits to the host (FAO/WHO,2002).
Probiotic food- That contains viable probiotic microorganisms in adequate numbers incorporated
in a suitable matrix so that upon ingestion claimed health effect is obtained in the host beyond regular
nutrition. Probiotics adhere to epithelial cell and colonise thereby, improve metabolism, immunity
and gut physiology.
Prebiotics- Non digestible food ingredients that beneficially affect the host by selectively stimulating
the growth and/or activity of one or a limited number of bacteria in the colon. Synbiotics- A mixtures
of pro and prebiotics that beneficially affect the host by improving the survival and implantation of
selected live microbial strains in the gastrointestinal tract.
Probiotics:
Lactic acid bacteria (LAB) including bifidobacteria, natural inhabitant of human gut have been in
use for preparation of various functional foods. The product can be called probiotic functional only if
151
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
the effective dose of live organisms present and its health benefit has been shown upon consumption.
As per WHO recommendations food must contain 106 cfu/g or 108 cfu/day of viable microorganisms
in-take for better probiotic efficacy. Health benefits can be ascribed to probiotics/synbiotics i.e.
alleviation of lactose intolerance, improvement in Ca, Fe and Mg absorption, cardiovascular health,
anticancer effect, cholesterol assimilation, modulation of immune function, constipation alleviation,
prevention/ treatments of diarrhoea and infections, increase in nutrient bioavailability, regularisation
of intestinal flow, production of vitamins etc. Some of commercially available probiotics foods in
global market are enlisted in Table 1.
Prerequisite of probiotic cultures:
Probiotic cultures should be selected on the following criteria, irrespective of the intended host or
site of application:
•
Survival in GIT and exhibit health effects in the host.
•
Proliferation and colonization under the host environmental condition.
•
Survival in association with the host immune system and non inflammatory.
•
Immuno-stimulatory for the mucosal immune system.
•
Production of antimicrobial substances against food spoilage and pathogenic bacteria.
•
Non-pathogenic, non-toxic, non-allergic, non-mutagenic or anti-carcinogenic, even in immunecompromised hosts.
•
Genetically stable, non-plasmid transfer and technologically suitable for process applications.
•
Potential for delivery of recombinant proteins and peptides.
•
Desirable metabolic activity and antibiotic resistance / sensitivity.
New generation probiotics:
Due to advancement in health and nutrition science, new cultures and novel probiotic products
are being introduced in market. These will require well established safety assessment procedures, e.g.
in the European Novel Foods Directive and in the US Premarketing Approval Clearances are must.
• Novel probiotic species: Majority of probiotics belong to the genera Lactobacillus, Bifidobacterium,
Lactococcus, Leuconostoc, and Propionibacterium with GRAS status. However, other organisms such
as Oxalobacter formigenes, Enterococcus fecalis and Escherichia coli do not enjoy the same status and
possibly more strict safety assessments are necessary to give clearance to novel probiotics for
incorporation into synbiotic products.
•
Genetically modified probiotics: Dairy food containing genetically modified (GM) probiotics
have low consumer acceptance in many countries e.g. Europe. However, GM probiotics posses
potential in clinical applications e.g. delivery of antigens for vaccines and thus are more
readily accepted. This would provide a safer method of vaccination than the use of attenuated
pathogens e.g. GM, Lactococcus lactis, produce IL-10 in the mouse intestine. This may provide
new treatment strategies for inflammatory bowel disease, and similar applications may be
useful for other diseases. The safety of such organisms that produce very powerful bioactive
substances is of major concern as excess production of these substances in a healthy individual
may be detrimental.
•
Non-viable probiotics: Generally probiotics are live microbes, however, non-viable probiotics
may also have beneficial health effects. These are likely to be in the market due to practical and
economic advantages; longer shelf-life, transportation and storage, safety etc. These could be
considered safe when used in extremely high-risk immune-suppressed patients.
•
Novel applications: The main application for probiotics is their use in foods, aiming at affecting
the composition or activity of the intestinal microflora or directly affecting the function of
the intestine. However, the probiotic principle should be expected to work in any part of the
body that has a normal microflora. With the exception of the urogenital tract, extra-intestinal
152
Recent Advances in Synbiotic Dairy Foods and Their Safety Evaluation
applications of probiotics have received little attention. Such probiotic preparations would
clearly need different safety requirements.
•
Animal probiotics: Probiotics application in animals requires more strict safety assessment and
should be safe to both. Because of probiotics application in farm animals, these may enter the
food chain. Intimate relationship between pet and its owner result spread of probiotic from
animal to human is possible. Enterococci are commonly used in animal preparations and this
may be reason for some concern. The safety requirements for animals are different from those
for humans. Few studies suggest that Lactococcus garvieae is associated with mastitis in cows and
septicaemia in fish as well as disease in humans. However, its true pathogenesis for humans
remains to be determined.
Challengages for use of probiotics in food systems:
•
Acid sensitivity is principal factors for poor viability of probiotic cultures particularly
bifidobacteria in fermented dairy foods. However, microencapsulation technique can be used to
improve viability of acid sensitive cultures in food systems.
•
Oxygen sensitivity is of particular relevance to bifidobacteria as they are strict anaerobes. Toxic
effects of oxygen can be overcome; milk may be deaerated prior to fermentation. Alternatively,
use of impermeable packaging may eliminate the toxic effects of oxygen during product storage.
Addition of reducing agents such as cysteine or oxygen scavengers such as ascorbic acid and
selection of oxygen tolerating strains may also improve the tolerance of probiotic cultures to
oxygen sensitivity.
•
Processing parameters such as thermo tolerance is an important parameter when considering
microbial survival in food processes such as spray-drying. Within the genera most often employed
as probiotics, certain strains and species are more heat tolerant than others e.g. “thermophilic”
lactobacilli.
Safety issues of probiotics for humans:
•
Probiotic cultures used for preparation of functional dairy foods should be safe even in immunecompromised individuals. Long history of LAB usage in preparation of fermented food provides
them generally regarded as safe (GRAS) status. WHO and LABIP outlined following parameters
for their safety (Table 2.).
Table 1. Commercial probiotic foods in the global market
SN
Commercial preparation
Probiotic cultures
1
Acidophilus milk
Lactobacillus acidophilus
2
Sweet acidophilus milk
Lb. acidophilus
3
Acidophilin
Lb. acidophilus, Lc. lactis subsp. lactis, kefir yeasts.
4
Nu-Trish A/B
Lb. acidophilus, Bifidobacterium spp
5
Diphilus milk
Lb. acidophilus, B. bifidum
6
Biomild
Lb. acidophilus, B. bifidum
7
Cultura® or A/Bmilk
Lb. acidophilus, B. bifidum
B. longum (CKL 1969) or B. longum (DSM2054)
8
Bifighurt
9
Acidophilus buttermilk
Lb. acidophilus, Lactococcus lactis subsp. lactis, subsp.
cremoris, subsp. lactis biovar. diacetylactis
10
Acidophilus-yeastmilk
Lb. acidophilus, Saccharomyces lactis
11
Bifidus milk
B. bifidum or longum
12
Yakult
Lb. casei Shirota
13
Yakult Miru-Miru
Lb. casei, B. bifidum or B. bereve, Lb. acidophilus
14
A-38 fermented milk
Lb. acidophilus, mesophilic lactic cultures
15
Onaka He GG,
Str. thermophilus,
Lb. delbrueckii subsp. bulgaricus,
16
Gefilus (Valio Ltd)
Lactobacillus rhamnosus GG
®
153
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
17
CHAMYTO
Lb. johnsonii, Lb. helveticus
18
Vitagen
Lb. acidophilus
19
Procult drink
B. longum BB536, Str. thermophilus,
Lb. delbrueckii subsp. bulgaricus
20
Actimel
Lactobacillus casei ImmunitasTM
21
AKTfit, Biola, BioAktiv, YOMO, LGG+,
Actif Yoplait 360º , Kaiku
Lb. rhamnosus GG
22
Gaio
Lb. casei F19
23
Verum
Lb. rhamnosus LB21
24
ProViva
Lactobacillus plantarum 299v
Adopted from Ozer and Kirmaci, 2009
Guidelines for evaluation of probiotics (FAO/WHO, 2002):
•
Strain identification by phenotypic and genotypic methods (Section 3.1)
•Genus, species, strain
•Deposit strain in international culture collection
•
Functional characterization (Section 3.2)
• In vitro tests
• Animal studies
•
Safety assessment (Section 3.3)
• In vitro and/or animal
• Phase 1 human study
•
Double blind, randomized, placebo-controlled (DBPC) phase 2 human trial or other appropriate
design with sample size and primary outcome appropriate to determine if strain/product is
efficacious (Section 3.4)
•
Preferably second independent DBPC study to confirm results
•
Phase 3, effectiveness trial is appropriate to compare probiotics with standard treatment of a
specific condition
•
Labeling (Section 3.5)
•Contents – genus, species, strain designation
•Minimum numbers of viable bacteria at end of shelf-life
•Proper storage conditions
•Corporate contact details for consumer information.
Table 2. Safety assessment scheme for probiotic cultures
SN
Attribute
Safety issues for assessment
1
Intrinsic strain properties
Adhesion factors, antibiotic résistance, plasmid transfer, enzyme profile
2
Metabolic products
Concentrations, safety, and other effects
3
Toxicity
Acute and sub acute effect of ingestion of large amounts of cultures
4
Infective properties
In vitro with cell lines ; in vivo with animal models
5
Dose- response effects
Oral administration in volunteers
6
Clinical assessments
Potential for side effects and disease-specific effects; nutritional studies
7
Epidemiological studies
Surveillance of large populations following introduction of new strains and products
Prebiotics:
The prebiotic application is directed to support growth of LAB due to their proposed health promoting
properties. The latest definition results in an equalization of ‘prebiotic’ and ‘bifidogenic’ which includes
154
Recent Advances in Synbiotic Dairy Foods and Their Safety Evaluation
in the definition the prebiotic index (i.e. absolute increase in fecal bifidobacteria concentration/g of daily
consumed prebiotics. Some of the properties of food ingredient to be classified as prebiotic are listed in
the Table 3.
Table 3. Desirable attributes of functional prebiotics
Desirable attribute in prebiotic
Properties of oligosaccharides
Active at low dosage and lack of side effects
Selectively and efficiently metabolised by ‘beneficial’ bacteria
but not by gas producers, putrefactive organisms, etc.
Persistence through the colon
Controlled molecular weight distribution
Protection against colon cancer
Stimulate butyrate production in the colon
Enhance the barrier effect against pathogens
Structural basis unknown
Inhibit adhesion of pathogens
Possess receptor sequence
Targeting at specific probiotics
Selectively metabolised by restricted species of Lactobacillus
and/or Bifidobacterium
Table 4: List of some selected prebiotics
SN
Recognized prebiotics
Emergent prebiotics
1
Fructo-oligosaccharides (FOS)
Genti-oligosaccharides
2
Galactooligosaccharides (GOS)
Gluco-oligosaccharides
3
Galacto-oligosaccharides (GOS)/ transgalactosylatedoligosaccharides (GOS/TOS)
Isomalto-oligosaccharides (IMO)
4
Inulin
Lactosucrose
5
Isomalto-oligosaccharides
Levans
6
Lactulose
Pectic-oligosaccharides
7
Pyrodextrins
Resistant starch
8
Soy-oligosaccharides (SOS)
Sugar alcohols
9
Xylo-oligosaccharides (XOS)
Synbiotics:
Another promising approach to manage correct balance of gut microflora is the use of synbiotics.
These also improve survival of bacteria during storage and passage of upper part of GIT, thereby
enhancing their health effects in the large intestine. The combined effects of synbiotics can be additive
or even synergistic.
Synbiotics foods with defined health benefits:
All the commercial probiotics are highly selected to have useful properties such as resistance
to acid and bile and technological stability to freeze-drying and product preparation. However, to
transfer health benefits to the host synbiotic approach holds promise such as immune stimulation,
cancer prevention, anti-pathogen activity etc. It would be expected that synbiotic versions of probiotic
strains made with targeted prebiotics would display better survival and colonisation in the gut. It
would be highly desirable to develop targeted prebiotics at particular species of microorganisms.
Infant formulae and weaning foods: Bifidogenic factors in milk stimulate the growth of bifidobacteria
that result health benefits to the infant, including a decreased susceptibility to microbial infections. Breast
fed infants’ gut is dominated by bifidobacteria while of formula-fed with mixed microflora resemble that
of an adult. The supplementation of infant milk formula with non-digestible compounds would support
growth of bifidobacteria. Hence, it would be of great interest to produce prebiotics with high selectivity
towards growth of bifidobacteria that are present in the gut of breast-fed infants as the basis of novel
infant food formulations.
155
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Functional foods for healthy ageing: Bifidobacterial population decreased markedly in the colon
of elderly person (55–60 years of age) as compared with those of young adults. Species of Bifidobacterium
are reasonable target for prebiotic viz., B. infantis and B. breve are predominant in infants, whereas
B. adolescentis and B. longum in adults. Decrease in bifidobacterial numbers results in reduction in
resistance to gastrointestinal infections and thus elderly people suffer more with such ailments.
Development of targeted prebiotic that promotes the probiotic strains able to inhibit gastrointestinal
pathogens viz., E. coli, Salmonella sp. and Campylobacter jejuni.
Development of targeted prebiotics:
Targeted prebiotics for probiotic can be developed firstly by screening a wide range of
oligosaccharide for their prebiotic attributes which will provide information about their selectivity
towards particular species. Structural diversity and cost effective manufacture technology for complex
oligosaccharides is most important. The second approach is enzymes expressed probiotics which can
act as synthetic catalysts. These enzymes will produce a mixture of oligosaccharide, which inturn
may be more readily metabolised by the producing organism, resulting in higher selectivity. Novel
β-galacto-oligosaccharide mixtures have been synthesised from lactose using β-galactosidases from a
range of prebiotics.
Technologies for manufacturing prebiotics
First generation prebiotics are either extracted from plants or manufactured from cheap, readily
available sources, generally by means of enzymatic hydrolysis or synthesis reactions.
Second approach is enzyme hydrolysis of polysaccharide. Fungal inulinase is used to hydrolyse
chicory inulin to oligosaccharides with low monosaccharide contents. Fructo-oligosaccharides and
xylo-oligosaccharides are both manufactured by hydrolysis of their parent polysaccharides. Fructooligosaccharides can also manufactured by synthesis from sucrose. Consequently, FOS produced
from inulin have reducing activity. The probiotic like Galactooligosaccharides, lactosucrose, isomaltooligosaccharides (IMO) and some fructooligosaccharides are manufactured by enzymic glycosyl
transfer reactions from cheap sugars such as sucrose and lactose or from starch. All of the sucrosederived FOS terminate in a non-reducing glucose residue. Ion-exchange chromatography can be used
to remove glucose and sucrose.
Second generation prebiotics: If the full potential of enhanced prebiotics is to be realised, new
technological innovations will be required. The challenge, as ever, for biotechnologists is to achieve the
manufacturing technologies at economically viable costs. Two areas of development are being explored
in laboratories in Europe at the current time. Controlled polysaccharide hydrolysis: Polysaccharide
hydrolysis is a commercial manufacturing approach for prebiotics. In this a more controlled partial
hydrolysis carried out in order to achieve control over the molecular weight distribution of the products.
Different IMO with average molecular weights up to 12,000 Da can be prepared by controlled partial
hydrolysis of dextran and pectins by endo-dextranase in an enzyme membrane reactor by controlling
residence time and ratio of enzyme to substrate. The fractions displayed good prebiotic fermentation
in vitro.
Safety of pre & probiotics:
Probiotics mainly belongs to genera of Lactobacillus or Bifidobacterium, have been in use to confer
health effects as they enjoy status of Generally Regarded as Safe (GRAS) due to their long history
of safe use. Various in vitro tests are available to evaluate efficacy and safety of pro and prebiotics.
However, most probiotics do not have a documented history of safe use hence, safety evaluation is
quite necessary. Some of the issues of probiotics concerned to safety are as below:
•
156
Antibiotic resistance: Presence of antibiotic resistance encoding genes must be determined
in order to prevent transmission of drug resistance to undesirable organism. The antibiotic
resistance gene specially vancomycin resistance should not be unstable plasmid encoded in
probiotic organisms as this is one of the last antibiotics used as an effective tool against multidrug-
Recent Advances in Synbiotic Dairy Foods and Their Safety Evaluation
resistant staphylococci. It is recommended not to use any vancomycin-resistant Enterococci as
either human or animal probiotics.
•
Strain Identification: This is not possible that all strains genus would confer probiotic health
benefits to the host. Proper identification of the organism is desirable by using internationally
accepted molecular tools such as DNA-DNA hybridization, 16S rRNA, pulsed field gel
electrophoresis (PFGE) or randomly amplified polymorphic DNA (RAPD), newer system such
as terminal restriction fragment-length polymorphism (T-RFLP) etc to give proper designation
so that it can be easily accessible by researcher. After identification the strain must be deposited
in a collection centre so that it can be easily available for workers.
•
Metabolic activities: Certain probiotics are capable to convert food components or biological
secretions into secondary metabolites which could be potentially harmful to the host. Hence,
these should be assessed for the following parameters:
•
Biogenic amines: These are produced during degradation of food proteins by certain LAB due
to deaminase activity, whish is considered as detrimental effects of probiotics. The candidate
probiotic can be evaluated for this activity decarboxylase broth using Bover-Cid and Holzapfel’s
method.
•
Bile salt deconjugation: Bile salts are water soluble end products of cholesterol metabolism
in liver and assist in the lipid digestion. They are absorbed actively in the terminal ileum and
are subsequently re-secreted, thereby form an enterohepatic cycle. During the microbial bile
acid metabolism first step is deconjugation as these are less effective in solubilisation of dietary
lipids. Further, too early and too much deconjugation, particularly in the upper small intestine
may disturb the lipid digestion and subsequent uptake of fat-soluble vitamins. Primary bile
acids can subsequently be dehydroxylated to yield secondary bile acids. The latter are most
hydrophobic and toxic to hepatocytes and the gastric and intestinal mucosa, and have been
suggested to be cancer promoters and to be involved in the formation of gal stones. Considering
the detrimental properties of secondary bile acids, no increase in 7α- dehydroxylase activity can
be accepted anywhere in the intestine. Potential probiotics and starters should not exhibit this
property.
•
D (-) lactic acid Production: Mammalian tissues lack D-lactate dehydrogenase (DLDH) enzyme
to metabolize D(-)-lactic acid. Production of D(-)-lactic acid by probiotic bacteria, is also a
concern to use them in children, due to D(-)-lactic acidosis. Acidosis is a pathologic condition
characterized by neurological alterations.
•
Others-binding: The binding of probiotics to mucosal layer is one of the prime selection
criteria as it is more important for immune modulation by competitive exclusion of pathogens.
However, binding is also a first step for the pathogenesis. Probiotics adhere to the extracellular
matrix (ECM) proteins typically exposed in wound tissue. Pathogens often have affinity for
these proteins, which also serve as receptors for invading microbes. Many lactic acid bacteria
have been observed to be able to reduce bioavailability of certain toxins by absorption viz.,
absorb environmental toxins; mycotoxins, heterocyclic amines etc. Although, absorbing
these compounds is desirable trait, it is important that such organisms do not be able bind to
therapeutic compound or essential nutrients.
•
If the strain under evaluation belongs to a species with known hemolytic potential,
determination of hemolytic activity is required
•
Assessment of lack of infectivity by a probiotic strain in immunocompromized consumers
(add a measure of confidence in the safety of a probiotic)
•
Animal and human studies: Assessment of side-effects, epidemiological surveillance (postmarket) and degradation of mucines must be carried out.
157
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Evaluation of prebiotic:
•
Prebiotic characterisation: The component which is claimed to have prebiotic attribute(s) must
be characterised for source, origin, purity, chemical structure, composition, concentration and
amount required to be delivered to the host.
•
Functionality evaluation: Correlation of physiological effect and modulation of intestinal
microflora should be substantiated based on studies tested in the target host with the final
product type alongwith time framework. A prebiotic can be a fiber but a fiber need not be a
prebiotic.
FAO has recommended the following guidelines for safety evaluation and substantiation of
prebiotic (Figure 1):
•
If the product has long history of safe usage then it should be considered as GRAS status and
thus no need for further human and animal trials. If it is a new candidate, safe levels must be
determined.
•
Levels of consumption for safe and minimum side effects must be established.
•
The product must be free from contaminants and impurities, characterisation of contaminant
should be done with toxicological studies.
•
The prebiotic must not alter the gut microbiota in a way detrimental to the host.
Global regulatory status:
The dietary supplement market for probiotics/synbiotic is gaining momentum at a very fast pace
over the globe, hence, regulation of these products is must consumers safety.
UNITED STATES: FDA controls the safety of foods of dietary supplements and probiotics that
are sold as components of conventional
foods or as dietary supplements. The safety
of traditional LAB has been granted GRAS
status. However, new and less traditional
strains of microbes have to be more carefully
assessed prior to distribution to consumers
with potentially compromised health. In the
US, Title 21 of the Code of Federal Regulations
(21 CFR).
EUROPEAN
UNION:
Probiotics/
synbiotic dairy foods fall under EC Novel
Foods Regulation (258/97) to ensure the free
movement of novel foods, while protecting
the interests of consumers, especially with
respect to safety, health and information.
GERMANY: Occupational foundation of
chemical industry has established an expert
group to assess the safety of microbiology
and biotechnology. This expert group has
also assessed the safety of microbes and Figure1 Guidelines to evaluate and substantiate a product as
published a list containing the classification of prebiotic (FAO Technical Meeting, 2007)
bacteria used by different industries including
food and feed industries (Berufsgenossenschaft der chemischen Industrie, 1998).
JAPAN: Probiotics/ functional foods fall under the Food for Specified Health Uses (FOSHU)
regulation. FOSHU regulations do not specifically define the safety aspects for probiotic microbes but
for functional foods in general and to get FOSHU status company has to get it from Ministry of Health
and Welfare.
158
Recent Advances in Synbiotic Dairy Foods and Their Safety Evaluation
International Dairy Federation(IDF): An expert action team has been constituted in collaboration
with European Food and Feed Culture Association to prepare a position document on properties of
dairy starters and probiotics to be used by the dairy industry..
Conclusion and future perspectives:
The current safety record of food starter cultures and probiotics appears to be excellent in developed
countries. Although, safety regulation related to functional/ probiotic / symbiotic foods yet to be
formulated in India. The future development of probiotics/ synbiotics and also industrial dairy starters
requires stringent guidelines for safety assessment of such organisms. Hence, constant surveillance of
probiotics/synbiotics is essential specially in clinical applications. Development of safety assessment
tools is extremely important for both premarket safety assessment and post-marketing surveillance of
human populations to guarantee safety of future products in humans and animals including immunecompromised. This will enable the future use of microbes and microbial fermentations for a widening
area in food technology and in functional and clinical food areas.
References:
Diplock A. T. 1999. Scientific concepts of functional foods in Europe: Consensus Document. Br J Nutr, 81(Suppl 1),
S1–S27.
Granato D., Branco, G.F., Cruz, A. G., Faria, J.A.F. and Shah, N.P. 2010. Probiotic dairy products as functional foods.
Comp. Rev. Food Sci. Safety.9:455-470
Ozer, B.H. and Kirmaci, H.A. 2009. Functional milks and dairy beverages. Int. J. Dairy Tech. 63(1):1-15.
Ross, R.P., Desmond, C., Fitzgerald, G.F. and Stanton, C. 2005. Overcoming the technological hurdles in the development
of probiotic foods. J. Appl. Micro. 98:1410-1417.
Suvarna, V.C. and Boby, V.U. 2005. Probiotics in human health: A current assessment. Curr. Sci. 88(11):1744-1748.
159
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Physical Characterization of Dairy Foods with
Reference to Viscosity, Colour and Water Activity
R. R. B. Singh and Prateek Sharma
Dairy Technology Division, NDRI, Karnal
Physical properties of foods are manifestations of its inherent compositional make up and structural
organization of its molecules. While the intrinsic properties of the foods are largely determined by
factors controlled by the material itself, the extrinsic factors are influenced by external conditions. The
composition of foods could be determined by either genetically or technologically induced factors.
The physical properties of milk components affect the functional properties of the processed foods
and are therefore of significant importance with regard to new product development or selection
of new processing technologies for designing a new product formulation or packaging condition.
The principal physical properties of processed dairy foods could include rheology, color, and water
activity.
Viscosity:
Texture and rheology are two important physical properties of foods. While texture refers to sensory
perception of the force-deformation relationship, rheology generally refers to response of foods as
exemplified by flow properties to application of definite stress or strain. Therefore instrumental data
can be usefully related to sensory data for interpreting the consumer assessment of the sensory quality
of a product. Instrumental data can be alternatively also used for designing equipments, packaging
requirements, processing conditions and evaluation of the finished product quality.
Instrumental methods measuring rheology can be classified as (i) empirical or (ii) fundamental
methods based on the test conditions in relation to the applied force and the resulting deformation
(or flow) coupled with the sample geometry. The empirical methods represent large-deformation
data (destructive tests) generated under specified test conditions and are highly product-specific. The
data generated are useful when a comparison is to be made between different products tested under
identical test conditions. Therefore these data are relevant when effect of processing variables or storage
conditions on the rheological properties is to be evaluated and are also appropriate for quality tests
carried out routinely. On the other hand the fundamental methods are based on small deformations
(non-destructive tests) and the data are generated in well defined (physical or engineering) units
of mechanical properties viz., viscosity for fluids and various modulii for solids. The results are
independent of the test conditions and appropriate for engineering design considerations. However,
rheological parameters measured using fundamental methods are relevant to pure engineering
materials rather than complex materials such as foods. Furthermore, while the fundamental rheological
measurements are made in a compression, tension, torsion or shear mode, empirical methods make
measurements in terms of penetration, extrusion, pressing etc.
Liquid foods are often characterized in terms of viscosity. The rheological behavior of fluids may
be Newtonian, pseudoplastic, or Bingham, depending upon the manner in which shear stress varies
with shear rate and time (Fig. 1). While the Newtonian fluids exhibit a viscosity which does not vary
with the shear rate, the viscosity of non-Newtonian foods is shear-dependent. Non-Newtonian foods
are hence classified as either shear-thinning type (decreasing viscosity with increasing shear rate) or
shear-thickening (dilatant) type (viscosity increases with increasing shear rate), the former being most
common to fluid foods. For example, milk with 30% or more total solids is shear-thinning. Alternately,
a series of measurements may be made on a non-Newtonian product using a range of shear rates and
the stress-shear rate relationship from the set of data generated expressed in terms of parameters of an
appropriate mathematical expression, such as ‘consistency coefficient’ and ‘flow behaviour index’ in
the most frequently used ‘power law’ model.
160
Physical Characterization of Dairy Foods with Reference to Viscosity, Colour and Water Activity
η=K.γn-1
Where η=viscosity, γ= shear rate, K=consistency co-efficient and n=flow behaviour index
Pseudoplastic fluids which exhibit shear
thinning are the most common among the nonNewtonian fluids and include emulsions and many
types of dispersions (Fig. 2). Dilatant fluids contain
higher levels of deflocculated solids such as corn
starch in water. Plastic fluids behave as a solid when
static and flow only upon application of certain
amount of force referred as yield value. Tomato
ketch up is a good example of such fluids. Plastic
fluids may display Newtonian, pseudoplastic or
dilatant properties. Many high viscosity fluids
exhibit thixotrophic behaviour which implies
that the viscosity drops even at constant shear
rate as a result of structural breakdown of food
components until a point when it attains a constant
Fig. 1. Typical time independent fluids curves
value. Subsequently upon quiescent storage of
such fluids, structure rebuilds and the viscosity
is restored to a limited extent generally below the
original value. Rheopexy fluids which are rarely
encountered behave opposite to thixotrophy
a n d
viscosity increases with time when it is sheared
at constant rate. In many cases both rheopexy
a n d
thixotrophy may occur in combination with any
o
f
the flow behaviours described above. However
time
is a critical factor when shear rate is constant
and therefore while some fluids may take only
f e w
seconds for the viscosity to become constant,
other
may take much longer time. Most pseudoplastic
liquid
foods follow the power law but the value of
t h e
exponent, which reflects the extent of digression from Newtonian flow, has not been determined for many
dairy and food components. These flow properties Fig 2. Rheogram of pseudoplastic fluids
of these fluid foods affect the mouthfeel and
therefore are very important in determining the acceptability of food.
Colour:
Colour is another important physical parameter that is important from the point of view of
characterizing the dairy products. This is also an indicator of chemical reactions that occur leading to
formation of chemical compounds during processing or subsequent storage of the processed foods.
These compounds are generally absent or are present in negligible quantities in the raw products and
are formed when these are thermally processed. Therefore often the formation of these compounds
is linked to measurement of severity of heat treatment. Storage induced enzymatic or non-enzymatic
changes are also associated with formation of compounds that lead to change in the colour profile of
the products. Color measurement techniques are used for recording desirable color changes in canning
salmon with higher oil content, defining translucency of the tissues and green pigment degradation
after blanching treatment of green peas, studying browning kinetics, or determining the influence of
particle sizes in the final color of powders. Characterization of the color of ingredients can also help to
predict the color of the final product—for example, control of raw strawberries for processing into jam.
In red wine, the percentage of brown component and the relative loss of anthocyanin can be followed
by reflectance measurement during storage.
161
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
The color of foods as perceived by the human eye is related to the factors such as the spectral
composition of the source of light, the food properties, and the spectral sensitivity properties of the eye.
Therefore instrumental measurement of colour of any food requires that at least two of these factors are
standardized. As such the human eye can give fairly good estimate of the colour properties of the food
however replacing it with instrumental sensor or photocell is known to give a more consistent and
reproducible measure of this attribute. Early instrumental methods for color measurement were based
on transmission, or reflection, spectrophotometry. However modern system of color measurement is
based on CIE, Munsell, Hunter, and Lovibond systems. The important factors in these systems are
source of light, geometry of viewing, and background colour. Colour may be specified in terms of
three characteristics of light: Hue, saturation and brightness. Hue represents dominant wave-length in
a mixture of light waves and therefore relates to the dominant color as perceived by the eye. Saturation
measures relative purity or the amount of white light whereas brightness refers to chromaticity of the
intensity. Hue and saturation are together called chromaticity.
In CIE system, spectral curves as illustrated in Fig. 3 indicate the response of observers eyes to
various spectral light types in the visible portion of the spectrum. It demonstrates that any colour
can be matched by mixing different proportion of red, green, and blue. These primary combinations
are called tristimulus values of color. The definite colour of an object can be thus defined in terms of
chromacity coordinates x and y, and by the luminous transmittance or lightness. A chromacity diagram
defines different color points that define the standard color
of a food. The Munsell system (Fig. 4) describes all colors
by three attributes: hue (or type of color), lightness (relative
to the proportion of light emitted), and saturation or purity
(associated with clear to dark perception). The hue scale has
ten hues distributed on a circumference (scaled 1 to 10); the
lightness ranges from black to white (0 to 10) and is distributed
on a perpendicular line; the purity is of irregular length
beginning with 0 for the central gray to the limit of purity
obtainable by available pigments in the Munsell book of color.
The Hunter system is also a three-dimensional system using
parameters L*, a*, and b* in each dimension: L* is the lightness
(nonlinear), a* is redness or greenness, and b* is yellowness
or blueness. Combination of L*, a*, and b* can be converted
to a single color. The Lovibond system is generally used for
determining the color of vegetable oils. It makes comparison Fig. 3 CIE color system
of visual light transmitted through a glass couvette using
color filters. The oil samples oils are generally expressed in
terms of red to yellow. The Lovibond index are also used to
measure color in wines and juices. Modern instruments use
software to convert light transition spectra into CIE, Munsell,
Hunter, and Lovibond color indices.Color can be measured
instrumentally with Colorimeters are also frequently used to
measure colour and can be broadly classified as tristimulus
colorimeters and spectrophotometers. The difference between
spectrophotometers and colorimeters is that the former
measures intensity of light through the completely visible
spectrum, and colorimeters are designed to measure only
some parameters related to sensory colors. Colorimeters are
particularly suitable for quality control of foods, and give
Fig. 4. Munshell colour system
results correlated with visual measurements.
162
Physical Characterization of Dairy Foods with Reference to Viscosity, Colour and Water Activity
Water activity:
The water activity of food can be conveniently expressed as the equilibrium water vapour pressure
(PW) over the food system divided by the vapour pressure of pure water (Pow) at the same temperature
and atmospheric pressure:
The water activity is correlated to the moisture content of foods. This relationship is known as water
sorpition isotherm. The water sorption isotherm for most of the food products is sigmoid in shape. It
can be divided into segments representing the aw ranges in which the three principal types of water
binding predominates. The region lying between aw value of 0 and 0.25 is believed to be dominated
by water bound by ionic groups such as NH3 associated with
proteins and -COO- groups associated with proteins, pectins Table 1. Approximate aw levels
of some dairy products
and other polyuronic acids. The moisture in this region is
tightly bound (known as monolayer moisture), which is PRODUCT
aW at 25°C
generally not available for either chemical or microbiological Dried Milk Products
0.1-0.3
activities. The region lying between aw values of 0.25 and 0.75 Butter, Unsalted
>0.99
appears to be related primarily to covalently bound water, such Salted
0.91-0.93
as amide groups in proteins and -OH groups in proteins and Sweetened Condensed Milk 0.77-0.85
carbohydrate polymers such as pectins, starch, hemicellulose Cheese, Hard
0.86-0.97
and cellulose. In this region, water is bound sufficiently Soft
0.96-0.98
tightly that it is unavailable to most of the microorganisms but Fresh
0.98-0.99
available for chemical activity. The region lying between aw Cream
>0.99
values of 0.75 and 1.0 is believed to represent water multilayers Frozen Desserts
0.98-0.99
on protein and carbohydrate polymers, in addition to water Fermented Milk Products
0.97-0.99
in which the vapour pressure is reduced by dissolved solutes, Milk And Whey
1
such as free amino-acids, sugars, and/or capillary attraction in Khoa
0.96
the microstructure.The water in this region is loosely bound
Paneer
>0.99
and is available for chemical and microbial activities. The water
Fried Paneer
0.97
activity of various dairy products is presented in Table 1.
Information on water binding is helpful in determining the energy requirements and conditions
for drying of specific materials; controlling the growth of microorganisms to minimize quality
deterioration; ensure safety, and selecting the appropriate microflora in certain foods, e.g. ripening
cheese; for evaluation of water uptake, porosity, sorption/desorption enthalpies; estimation of specific
surface area, crystalline state of components (lactose), and facilitating control of several chemical,
physical and quality attributes of stored foods in addition to ensuring microbial stability. Besides
determinant role played by aw in influencing the stability of quality parameters, aw has many other
practical applications such as prediction of packaged product moisture gain/loss and prediction of
shelf-life of packed food.
Measurement of water activity: Analytical instruments or methods for aw value determination
are many. The important ones are: Hair hygrometers, Isopiestic methods, Electronic hygrometers
etc.
Hair Hygrometry: Measurements is based on the magnitude of longitudinal change in length of
water-sorbing fibre in the same container at equilibrium. This measurement is based on the principle
that the keratinaceous proteins in hair strands stretch under tension, when they absorb moisture. If
the hair strand is fixed at one end and attached to an indicating lever arm at the other end, the relative
humidity within an enclosure can be read directly. The hair hygrometer is a dial-type polyamide
thread hygrometer. This type of hygrometer is relatively inexpensive. Its accuracy is comparable to
others (i.e. + 0.02 upto 0.01).
163
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Isopiestic Methods: This method provides the measurement of equilibrium relative humidity of
any aqueous systems in a closed container at a specified temperature. This is based on a principle
that most food substances are consistently adjusting their moisture content through absorption or
desorption processes depending upon the moisture condition and temperature of the environment
until the food substances approach equilibrium. In other words, the equilibrium vapour pressure of
the reference salt slush will be identical to the vapour pressure of the sample at equilibrium condition.
In this method, multiple samples must be measured at different equilibrium conditions using different
salt slushes in desiccators. Upon equilibrium the sorption isotherm is drawn and the aw is measured
against moisture content of the food.
Electronic Hygrometers: This type of instrument features the use of calibrated aluminium oxide
or lithium chloride humidity sensors. Recalibration or standardization of sensor response is by a set of
reference salt slushes. Water activity measurement is carried out by connecting the appropriate sensor
to an airtight food sample container and equlibrated at a specified temperature. Since the sensor
is sealed in a small container, it usually takes less than 2 hr for a sample to approach equilibrium
conditions. These instruments provide a better and convenient means of aw measurement with
adequate accuracy and precision. However, they are susceptible to contaminants such as SO2, H2S,
Chlorine and oil vapours. Temporary contaminants include ammonia, acetic acid, alcohols, glycol,
glycerols, etc. depending upon the sensor material used. Foods usually contain these contaminants,
thereby reducing the useful life of the sensors.
The instrument using principle of chilled mirror dew point measurement to calculate aw of a given
food sample is one of the frequently used systems. When a sample is placed in the instrument, a stainless
steel mirror within the chamber is repeatedly cooled and heated. The mirror temperature is controlled by
a thermoelectric (Peltier) cooler. A fan placed in the instrument continually circulates air in the sensing
chamber to hasten the equilibration process. The precise determination of the temperature at which
the condensation first appears is done with a photoelectric cell. The photodetector senses the change
in reflectance when condensation occurs on the mirror. The temperature at which the condensation
occurs is recorded with the help of a thermocouple attached to the mirror. Simultaneously, the sample
temperature is also measured. Both the temperature of sample and the mirror temperature are used
for calculating the aw. The aw is calculated and compared with the previous measurement and the
process terminates with a beep only when two consecutive readings does not differ by more than
0.001. The instrument thus displays the temperature of sample and aw.
References:
Sogi D. S (2008). Fundamentals of rheology. In compendium of the short course on “Sensory and related techniques for
evaluation of dairy products” under Centre of Advanced studies held at Karnal from June 17-July 07, 2008214-220,
pp.66-71.
Rao M. A (2005) Rheological properties of liquid foods. In: Engineering properties of foods (Ed M. A. Rao, A. K. Datta
and S. S. H. Rizvi) CRC Press, USA.
Patil G. R. (2003) Water activity of foods in relation to packaging. In compendium of the short course on “Advances
in Packaging of Dairy and Food Products” under Centre of Advanced studies held at Karnal from February 13 –
March 05, 2003, pp.66-71.
Francis F J (2005) Color properties of foods. In: Engineering properties of foods (Ed M. A. Rao, A. K. Datta and S. S. H.
Rizvi) CRC Press, USA.
164
Malt Based Milk Foods as “Value Added Functional Dairy Products”
Malt Based Milk Foods as “Value Added
Functional Dairy Products”
Laxmana Naik, Rajan Sharma, Manju G. and Amit K. Barui
Dariy Chemestry Division, NDRI, Karnal
Introduction:
Food is a basic nutritional requirement, but as a result of substandard diet, approximately 925
million people are suffering from undernutrition in different regions of the world. Consequently a
larger population in the underdeveloped world fall prey to the protein deficiency On the other hand
busy lifestyles fragmented eating habits, change in consumer perception towards physical appearance,
dietary choices and more importantly demanding an ideal wholesome food that address many diet and
health related issues. Thus there is a need for developing a value added nutritional food supplement.
Much of scientific evidence has shown that there is a strong positive relationship between consumed
foods and human health, and that there is a beneficial correlation between the function of various
food components to the treatment and prevention of specific illnesses. Therefore, consumer interest
has focused on a diet with the capability to promote good health and to extend a healthy life span, this
strongly promoted in the functional foods development.
What makes a Functional food and what is the best source?
Functional foods may be defined as any food, in a natural or processed form, that contains, in
addition to its nutritional components, substances which favor the good health, physical capacity and
mental wellbeing of an individual. (Vasconcelles, 2001). Most of the individual foods are deficient in one
or other type of constituents which are very essential to human health. To prepare a diet nutritionally
complete it is essential to make a complement of two or more foods which synergistically makeup the
deficiency of each other. Milk is an ideal food but milk proteins are deficient in sulfur containing amino
acids like methionine and cystein. Cereals on the other hand are generally deficient in lysine, threonine
and tryptophan. Thus in order to develop a balanced food cereal protein should be supplemented with
milk protein. Nutritional merits of milk are well acclaimed. The malted barley is rich source of readily
digestible carbohydrates, proteins and provides a carbohydrate splitting α-amylase enzyme which
hydrolyses the insoluble starch ingredients into readily soluble sugars like maltose, dextrose, glucose
etc. This is also a good source of soluble fiber like β-Glucans and other health-promoting components.
Recently U.S. Food and Drug Administration finalized a rule that allows foods containing barley to
carry a claim that they may reduce the risk of coronary heart disease. Hence special supplement and
enrichment is made by use of milk and malted barely. These major ingredients synergistically fulfill
most of the nutritional and technological requirements in making an ideal wholesome food.
Malt based milk foods fall in the category of nutritional functional health foods, these are prepared
by a mixture consisting of standardized milk or milk solids with the fluid separated from the mash of
ground barley malt. Depending on the intended use these foods are prepared either in liquid form for
direct consumptions or in dried powdered form to be use as an ingredient for reconstitution and some
times a base material for other food recipe.
Historical background of Malted Milk:
In 1878 William Horlick and James Horlick, brothers, established a Horlick Food plant in the
outskirts of Racine, Wisconsin USA. They began to manufacture a product known as Horlick’s Food
by enriching milk with malted barley, they under took a research on this product at the request of
some physician who wanted to have an infant food combining milk and cereal, First successful malted
milk food was developed in 1883 and the product commercially marketed in 1887. This product
gained favorable attention from the medicinal professionals and public due to its nutritional value,
convenience, digestibility and palatability.
165
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Myth about Malted Barley:
Barley is one of the seven internationally grown cereal grains, currently ranking fourth in world
production (FAO 2006). Belongs to the genus Hordeum and the major cultivated barley species is
Hordeum vulgare. Malted barley means the product obtained from soaking or steeping the whole
barley kernel followed by germination and drying in a controlled environment.
What makes barley so special; Barley is a rich source of both soluble and insoluble fiber and it is
one of the dieter’s delight” component. However, researchers have identified β-glucan as the primary
component in barley that is responsible for lowering cholesterol. Based on scientific evidence, the Food
and Drug Administration (FDA) finalized a rule in 2006 allowing barley foods to carry a health claim
specific to soluble fiber and relating to both for reducing cardiovascular disease risk and modifying
glycemic responses for treatment and prevention of diabetes (Lazaridou and Biliaderis 2007).
Qualifying products may use the following claim: “Soluble fiber from foods such as [name of
food], as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease. A
serving of [name of food] supplies [x] grams of the soluble fiber necessary per day to have this effect.”
Specifically, a food made from eligible barley sources must contain at least 0.75 g of β-glucan (soluble
fiber) per serving (FDA 2006).
Malt based milk food: Defination
Defined as “Malt based milk food means the product obtained by mixing whole milk, partly
skimmed milk or milk powder with the wort separately from a mash of ground barley malt, any
other malted cereal grain and wheat flour or any other cereal flour or malt extract with or without
addition of flavouring agents and spices, emulsifying agents, eggs, protein isolates, edible common
salt, sodium or potassium bicarbonate, minerals and vitamins and without added sugar in such a
manner as to secure complete hydrolysis of starchy material and prepared in a powder or granule or
flake form by roller drying, spray drying, vacuum drying or by any other process. It may contain cocoa
powder. It shall be free from dirt and other extraneous matter. It shall not contain any added starch
(except starch natural to cocoa powder) and added non-milk fat. It shall not contain any preservative
or added colour. Malted milk food containing cocoa powder may contain added sugar” (PFA 1955).
The requirements according to PFA 1955 and Bureau of Indian Standards (IS-1806-1975) it
shall confirm the fallowing standards.
Characteristics
Malted Milk Food without
Cocoa powder
Malted Milk Food with Cocoa
powder
Moisture, % by mass
Not More Than 5.0
Not More Than 5.0
Total Protein (Nx6.25), % by weight
Not Less Than12.5
Not Less Than 11.25
Fat, % by weight
Not Less Than7.5
Not Less Than 6.0
Total Ash, % , dry basis
Not More Than 5.0
Not More Than 5.0
Acid Insoluble ash, in Dilute HCl, %
Not More Than 0.1
Not More Than 0.1
Alcoholic Acidity, % H2SO4 in 90% alcohol
Not More Than 0.3
Not More Than 0.3
Solubility, % by weight
Not Less Than 85.0
Not Less Than 85.0
Cocoa Powder, % dry basis
Test for Starch
Bacterial Count, Per gram
N/A*
Not Less Than 5.0
Negative
N/A*
Not More Than 50000
Not More Than 50000
Coliform Count, Per gram
Not More Than 10
Not More Than 10
Yeast and Mold Count, per gram
Not More Than 100
Not More Than 100
Salmonella and Shigella
Absent in 25 gm
Absent in 25 gm
E. Coli
Absent in 10.0 gm
Absent in 10.0 gm
Vibrio cholera and V. paraheamolyticus
Absent in 0.1 gm
Absent in 0.1 gm
Faecal streptococci and Staphylococcus
aureas
* N/A: Not applicable.
Absent in 0.1 gm
Absent in 0.1 gm
166
Malt Based Milk Foods as “Value Added Functional Dairy Products”
Basic Ingredients of malted dairy foods:
1. Malted Barley: Provide appropriate levels of α-amylase enzyme required to convert all the starch
into simple sugars and also imparts typical malty flavor to the finished product.
2. Malt Extract: This is a concentrated extract from roasted malted barley with a solid of
approximately 80 per cent, imparts desired level of colour and typical caramelized flavor.
3. Milk and Milk solids: Enhance nutritional value of the product by providing high quality protein,
vitamins, minerals and milk fat provides unique flavor.
4. Wheat flour, wheat gluten, isolated soy protein, malto dextrin, vitamin premixes, salts and other
micro ingredients are supplemented to improve the nutritional requirement, provide optimum
pH for better digestibility, enhancing flavor and for value addition.
Method of Preparation:
In the manufacturing of malt based milk food (Figure 1) mashing is the first step fallowed by
mix preparation, pasteurization of mix and concentration of mix in first stage up to 50 per cent solids
then this base material can be directly spray dried or further concentrated up to 80 per cent solids in
multiple effect evaporator then vacuum oven dried or either band dried (Dhillon, 2005).
Figure 1: Flow chart for production of malt based milk food.
167
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Quality related Issues:
Quality related issue either of physical, chemical or microbiological problems hampers
manufacturing and business performance and ultimately to the safety of consumer health. Hence it
is very much essential to address at the root level. Critical to quality issue arises mainly due to 5 M
factors; these are Man, Machine, Methods of preparation, Materials quality and Mother nature of
products. It is often overlooked fact that just about every food item we eat is biological in origin,
consumer expect our food should to be fresh, wholesome, and not to contain any unnecessary added
additives. In ordered to preserve food from microbes; processing at high temperature is essential but
leads to loss of nutritional and volatile compounds, hence it is optimally processed so that nutritional
identity is retained but many of these components are very heat sensitive, creates problem during
processing, major bottleneck are their bioactivity will diminishes and loss of volatile aroma compounds
but fortification in final stage is possible. Encapsulation of sensitive ingredients helps in protecting
from thermal shock.
Functional ingredients and Health benefits of malted milk foods:
Malted barley has received attention from health professionals hence barley is used as the main
cereal grain for the development of functional foods, as it contains two classes of compounds of strong
nutritional interest: β-glucans (dietary fiber) and tocols (antioxidant - vitamin E).
Dietary fibers, such as β-glucans, are defined as the edible parts of plants and analogous
carbohydrates that are resistant to digestion and absorption in the human small intestine with complete
or partial fermentation in the large intestine. Dietary fiber includes polysaccharides, oligosaccharides,
lignin, and associated plant substances (AACC 2001). A diet rich in fiber has health benefits including
lowered energy density, prolonged satiety, and effects related to an increase in fecal bulk. Foods
containing soluble dietary fiber have been shown to lower serum cholesterol levels, postprandial
blood glucose, and insulin response (Jenkins et al., 2000).
There are three mechanisms for barley’s hypocholesterolemic effects are: (1) reduced absorption
of dietary lipids including cholesterol; (2) reduced absorption of bile acids; and (3) production of
volatile fatty acids in the large intestine that are reabsorbed, and act as inhibitors of β-hydroxy-βmethyl glutaril coenzyme A (HMG-CoA) reductase in the liver (McIntosh and Oakenfull 1990).
It is important for diabetics to know the glycemic potential of food carbohydrates. The glycemic
index (GI) is a powerful method for nutritional characterization of carbohydrates and has been
proposed to diabetic subjects as a tool for managing their diet. Epidemiological data indicates that a
diet characterized by a low GI reduces insulin resistance and improves certain metabolic consequences
of insulin resistance. This suggests a potential role against both the development of non-insulindependent diabetes mellitus (NIDDM) and cardiovascular diseases (Björck et al., 2000).
Tocols (tocopherols and tocotrienols) are well recognized for their biological effects, including
antioxidant activity (Kamal-Eldin and Appelvist 1996) and reduction of serum LDL-cholesterol.
While tocopherols, mainly α-tocopherol, are considered to have the greater biological activity,
tocotrienols have been the focus of growing research interest as unique nutritional compounds for
their hypocholesterolemic action. Among the four tocotrienol isomers, γ-tocotrienol and δ-tocotrienol
seem to be more effective than α-tocotrienol. Tocotrienols are reported to be capable of reducing
serum LDL-cholesterol in chickens, swine, and human subjects. They may act as inhibitors of the
HMG-CoA reductase, a rate-limiting enzyme of cholesterol biosynthesis (Qureshi et al., 1991). Some
studies indicate that the antioxidant potential of tocotrienols is even greater than that of α-tocopherol
in certain types of fatty cell membranes and in some brain cells. Moreover, recent studies suggest
that tocotrienols may affect the growth and/or proliferation of several types of human cancer cells
(Nesaretnam et al., 1998).
168
Malt Based Milk Foods as “Value Added Functional Dairy Products”
Conclusion:
There is no room for second thought to it that food is going to be a medicine, consumers
demanding that they need a food which can overcome all the health related risk. Opportunities before
the technologist is that formulation and design of a product containing bioactive substances, but many
of these components are very heat sensitive, creates problem during processing, major bottleneck
are their bioactivity will diminishes and loss of volatile aroma compounds. Some efforts are made
like; Probiotic malted milk made by encapsulation of probiotics and subsequent drying. At present
Indian malted milk industry growing at a rate of 8 to10 per cent, because of marketing strategy and
advertising, brand image, the health claims, variants, ease of convenience.
References:
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
AACC (American Association of Cereal Chemists). 2001. The definition of dietary fiber. AACC Report., 46:3,112126.
Baldwin, A.J., Baucke, A.G. and Sanderson, W. B. 1980. New Zealand J. Dairy Sci.Tec., 15: 286.
Björck, I., Liljeberg, H. and Ostman, E. 2000. Low glycemic-index foods. British Journal. of Nutrition., 83:149-155.
Dhillon, L.S. 2005. Manufacturing of malt based food products. Ind. Dairyman., 57:4, 59-66.
FAO. 2006. World crop production. Published online at http://www.faostat.fao.org.
FDA (U.S. Food and Drug Administratin). 2006. Food labeling: health claims; soluble dietary fiber from certain
foods and coronary heart disease. Fed. Reg. 71:29248–29250.
Food Safety and Standards Regulations, 2010. Final Regulations Hand Book, 349-351.
Jenkins, D.J.A., Axelsen, M., Kendall, C.W.C., Augustin, L.S.A, Vuksan, V. and Smith, U.2000. Dietary fibre, lente
carbohydrates and the insulin-resistant diseases. British Journal of Nutrition., 83:S1,157-163.
Kamal-Eldin, K. and Appelqvist, L.A. 1996. The chemistry and antioxidant properties of tocopherols and
tocotrienols. Lipids., 31:7, 671-701.
Lazaridou and Biliaderis. 2007. Barley products to carry heart health claim. Food Navigator-USA. Published online
at http://www.foodnavigator-usa.com.
McIntosh, G.H. and Oakenfull, D. 1990. Possible health benefits from barley grain. Chemistry in Australia., 57: 294296.
Nesaretnam, K., Stephen, R. Dils, R and Dabre, P. 1998. Tocotrienols inhibit the growth of breast cancer cells,
irrespective of estrogen status. Lipids., 33: 461-469.
PFA. 1955. The Prevention of Food Adulteration Act & Rules Hand Book, as on 1.10.2004. 329-330.
Qureshi, A.A., Qureshi, N. Wright, J.J. Shen, Z. Kramer, G. Gapor, A. Chong, Y.H. DeWitt, G. Ong, A. and
Peterson, D.M. 1991. Lowering serum cholesterol in hypocholesterolemic humans by tocotrienols. The American
Journal of Clinical Nutrition., 53: 1021s-1026s.
Rosemary, K. N and Walter, C.N. 2008. Text Book of: Barley for Food and Health: Science, Technology, and Products.
John Wiley & Sons Inc. pub.
Salooja, M. K and Balachandran, R. 1988. Physical properties of spray dried malted milk powder, Ind. J. of Dairy
Sci., 41:4, 456-461.
The Hindu, 2010. U. N. Warns of food crisis. 15-09-2010, ISSN 0971-751X 133:220:9.
Vasconcellos, J.A. 2001. Functional foods. Concepts and benefits. The World of Food Science.. www.worldfoodscience.
org:80/vol.1_3/feature1-3b.html.
169
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Preparation and Characterization of Gold
Nanoparticles, Their Conjugation with Antibodies
and Construction of Lateral Flow Devices
Priyanka Singh Rao1, Swapnil Sonar2, Y.S. Rajput2 and Rajan Sharma1
1
Dairy Chemistry Division, 2Animal Biochemistry Division, NDRI, Karnal
Lateral Flow Assays also known as Immunochromatographic assays are a simple device intended
to detect the presence (or absence) of a target analyte in sample (matrix). Traditionally designed assays
are composed of a variety of materials, each serving one or more purposes. Colloidal gold is the most
widely used label today in commercial lateral flow immunoassays for many reasons. It is fairly easy and
inexpensive to prepare in the laboratory. The color is intense, and no development process is needed
for visualization. A large body of protocols exist in the literature for its conjugation and application.
Gold colloids are formed by the reduction of gold tetrachloric acid through a “nucleation” process.
The size and shape of the colloids depend on the type and amount of reducer used. The label is very
stable in liquid or dried form and is non-bleaching after staining on membranes. An accurate and
reproducible lateral-flow assay requires the use of high-quality gold conjugates. Gold particles can
be produced that range in size from 5 to 100 nm in diameter. The most common size of colloidal gold
particle used is 40 nm. In addition, colloidal gold in unconjugated forms (which are ready for labeling)
and conjugated forms (conjugated with biologicals) are now readily available from many commercial
sources. In addition to the dry parts of a lateral-flow assay, there are also the biological components that
allow the visualization of the results. By virtue of their high levels of specificity and binding affinities,
antibodies are the ideal choice of agent for detection. In Lateral Flow Assay an antibody molecule is
conjugated to a colloidal gold particle. Antibodies can be polyclonal or monoclonal. Once the antibody
has been conjugated, the quality of the gold conjugate must be assessed before incorporation into the
rapid-test assay. Usually, electron microscopy is employed as a quality-control measure. Conjugation
of colloidal gold particles and antibodies depends on the availability and accessibility of three amino
acid residues—lysine, tryptophan, and cysteine. Once a high-quality antibody–gold conjugate is
formed, it can be applied to the conjugate pad either by soaking or by spraying. The drying process that
follows is essential. The lateral flow immunoassay devices are compact and easily portable. A test strip
typically consists of a plastic backing holding together a sample pad for deposition of sample fluids,
a conjugate pad pre treated with sample detection particles, a microporous membrane containing
sample capturing reagents, and an absorbent pad at the distal end serving to collect excess fluids.
A.
Preparation of Gold Nano Particle
Material: All the chemicals required for the preparation for gold nanoparticles can be procured
from Sigma-Aldrich Ltd.
Reagents:
1. Stock gold chloride (tetrachloroauric acid trihydrate, Mol.Wt. 393.83; 200 mM) solution- 787.6
mg of HAuCl4.3H2O is dissolved in Millipore water and volume is made up to 10 ml. The stock
solution is stored at room temperature.
2. Working gold chloride solution (50 mM) - Stock gold chloride solution is diluted four times with
Millipore water.
3. Trisodium citrate dihydrate (38.8 mM; M.W. 294) - 114 mg of trisodium citrate dihydrate is
dissolved in 10 ml Millipore water.
Procedure:
1. 1. Prepare aqua regia by mixing 3:1 concentrated HCl:HNO3 in a large beaker in a fume hood.
Be extremely careful when preparing and working with aqua regia. Wear goggles and gloves,
170
Preparation and Characterization of Gold Nanoparticles, Their Conjugation with Antibodies and Construction of Lateral Flow Devices
and perform the experiment in a fume hood. Aqua regia should be freshly prepared and
should never be stored in a closed vessel. The capped aqua regia bottle may explode. Render
it safe by dilution and neutralization.
2. Soak the 200 ml two-neck flask, magnetic stir bar, stopper and condenser in aqua regia for at
least 15 min. Rinse the glassware with copious amount of deionized water and then Milliporefiltered water. Obtaining high-quality nanoparticles is the first important step towards
the success of the experiment. Care should be taken to make sure that no contamination is
introduced during nanoparticle synthesis.
3. Load 98 ml of Millipore water into the two-neck flask. Add 2 ml of 50 mM HAuCl4 solution so
that the final HAuCl4 concentration is 1 mM.
4. Connect the condenser to one neck of the flask, and place the stopper in the other neck. Put the
flask on the hot plate to reflux while stirring.
5. When the solution begins to reflux, remove the stopper. Quickly add 10 ml of 38.8 mM sodium
citrate, and replace the stopper. The color should change from pale yellow to deep red in 1
min. Allow the system to reflux for another 20 min.
6. Turn off heating and allow the system to cool to room temperature (23–25°C) under stirring.
B. Characterization of gold nanoparticles
The diameter of such prepared nanoparticles is ~13 nm. The extinction value of the 520 nm plasmon
peak is 3.8, and the nanoparticle concentration is ~13 nM. The colour should be burgundy red, and
the nanoparticle shape should be spherical under transmission electron microscopy (TEM). All gold
sols display a single absorption peak in the visible range between 510 and 550 nm, and the absorption
maximum shifts to a longer wavelength with increasing particle size. The relative uniformity of the
particles or the range of particles can be gauged by the width of the absorption spectra: the sharper the
band, the more uniform the particles. The relative concentration of each batch of colloidal gold can be
determined by absorbance at 520 nm. Various batches can be brought to the same relative concentration
by the addition of de-ionized water.
C.
Labelling of gold nanoparticles with antibody
Reagents
i.
NaOH (0.2 N)
ii.
Carbonate Buffer (5 mM)
iii.
Tris-HCl buffer (pH 8.2) containing 1% BSA and 0.1 sodium azide
Procedure: Adjust the pH of Gold nanoparticle using 0.2 N NaOH to 8.5. 6 µl (20 µg) of affinity
purified antibody (against glycomacropeptide) is diluted to 160 µl with carbonate buffer and added to
nanoparticles. The mixture is incubated overnight at 4ºC. Centrifuge the contents at 7,000 rpm at 15ºC
for 15 minutes. Suspend the pellet in carbonate buffer and again centrifuge at 7,000 rpm at 15ºC for
15 minutes. Decant the supernatant and
dissolve the pellet in Tris-HCl buffer.
Store the antibody labelled nanoparticles
at 4°C till further use.
D. Construction and working
of lateral flow strip
Materials: All the material required
for the construction of lateral flow strip,
can be purchased from Milipore India
Pvt. Ltd. Bangalore.
Procedure: Lateral flow assays are
Side view of test-strip construction
171
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
composed of a variety of materials, each serving one or more purposes. The parts overlap onto one
another and are mounted on a backing card using a pressure-sensitive adhesive. Each component of the
test, including membrane, backing substrate, and each of the pad materials, has a defined dimension. On
conjugation pad Gold nanoparticles are coated with antigen specific antibodies.Test line is also coated
with the antigen specific antibody and Control line with species specific anti-antibody for the antibody
in the particulate conjugate.
The sample is treated to make it compatible with the rest of the test. The treated sample migrates
through this region to the conjugate pad, where a particulate conjugate has been immobilized. The
sample interacts with the conjugate as both migrate into the next section of the strip, which is the
membrane. Excess reagents move past the capture lines and are entrapped in the wick or absorbent
pad. The control line indicates that the test developed properly and test line indicates that the test is
positive.
172
Use of Lateral Flow Technique for Detecting Melamine in Milk
Use of Lateral Flow Technique for
Detecting Melamine in Milk
Raman Seth and Anamika Dass
Dairy Chemistry Division, NDRI, Karnal
Introduction
Monitoring of milk and milk product for quality and safety during entire food chain is of major
concern to the food producers and to the consumers. In the present scenario, consumers always prefer
high quality and safe foods. Dairy industry always looks forward for innovative tests to access dairy
product for their quality. Therefore a holistic approach for checking the quality of any food product
is an essential requirement. The Indian dairy industry is passing through a phase of adulteration
in milk and milk product. Poor quality raw milk is either neutralized or preserved with different
preservatives. Recently the menace of melamine addition in milk powder in order to enhance its protein
content has been reported in China which caused death of many infants. Nitrogenous compounds like
urea, ammonium salts can be detected by simple and rapid test but presence of melamine cannot be
detected by simple test. In recent years incidence of melamine addition in infant milk formula has
been reported in media especially in China.
Melamine is a chemical used primarily for the production of melamine resins. Because of its high
nitrogen level (66% by mass), melamine is illegally added to milk product especially to milk powder
in order to compensate for protein content when estimated on the basis of total nitrogen. Ingestion of
melamine at levels above the safe limit (2.5 ppm in the USA and 1 ppm in European Union for infant
milk powder) can induce renal failure and even death in infants. Melamine, a non protein nitrogen
substance when added to any food product increases the total nitrogen content. Adulteration of protein
rich foods with melamine increases the crude protein content .This reminds us the recent scandal
in China where attempts were made to increase the nitrogen content in infant food with melamine
which accumulates in the body on feeding melamine contaminated milk and caused toxicity problem,
there by forming solid stone deposit within kidneys or bladder which ultimately damage kidneys.
Infants fed regularly with milk containing melamine were more susceptible to urinary infections.
Thousands of infants were affected and several died in China due to melamine contamination in milk.
However, the existing analytical methods for estimation of melamine in milk such as low temperature
plasma probe combined with tandem mass spectroscopy (LTP/MS), liquid chromatography–mass
spectrometry (LC/MS), Electrospray ionization coupled with mass spectrometry (EESI-MS), Enzyme
linked immunosorbent assay (ELISA) and High performance liquid chromatography (HPLC) methods
involves cumbersome steps along with expensive instrumentation thus making difficult to take decision
whether to accept or reject the milk for further processing into milk products. Therefore there is an
urgent need to develop a simple test for detection of melamine in milk to stop unscrupulous person to
adulterate milk with such harmful chemicals. Development of simple and rapid test for detection of
melamine in milk and food has become a challenge for researchers. Color indicator for the presence of
melamine in milk using gold nanoparticles may find important application in detecting melamine. In
2008 in China, reports appeared in media where attempts were made to increase the protein content
in infant milk food using melamine .Thousands of infants were affected and several died in China.
Milk protein contributes 30% of total milk solids. At present in India, the payment of milk in
dairy industry is made on the basis of fat, and SNF. Milk supplier’s uses various adulterants such
as urea, ammonium salts, starch, sugar, skim milk powder, maltodextrin, etc to increase the total
solids in milk. Chemical tests are available to detect such common adulterants. Melamine is one of the
recent adulterant which is reported in China and is used as a source to enhance the protein content in
infant milk powder. Melamine contains high nitrogen level (66% by weight),easily available and is not
much costlier. For such reasons melamine adulteration in milk is rampant. Melamine is commercially
173
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
available as white powder, odourless and does not impart any undesirable sensory attributes when
added to milk. So it becomes very difficult to detect its presence when organoleptic and platform tests
are performed at the reception dock of milk plant.
Melamine
Melamine is an organic base and a trimer Properties of melamine
of cyanamide, with a 1,3,5-triazine skeleton. 1 Other name
Cyanurotriamide , cyanurotriamine,
Like cyanamide, it contains 66% nitrogen
cyanuramide
by mass and when mixed with resins, has 2 IUPAC Name
1,3,5 triazine 2,4,6 triamine
fire retardant properties due to release of
3
Molecular formula C3H6N6
nitrogen gas when burned or charred.It has
4
Molar mass
126.12
several other industrial uses also.
1574 kg/ m3
The chemical term melamine was 5 Density
3500C
coined by combining the names of two 6 Melting point
german words Melam (a distillation 7 Solubility in water 3.240 g / L at 200 C
derivative of ammonium thiocyanate)
and amine .Melamine when combined with formaldehyde produces melamine resin, a very durable
thermosetting plastic used in formica, and melamine foam, a polymeric cleaning product. Melamine
resin is used in the production of dry erase boards, fabrics, glues, kitchen housewares and flame
retardants. Melamine is one of the major component in pigment yellow 150, a colorant in ink and
plastic Melamine is also used in the fabrication of melamine poly-sulfonate as a superplasticizer for
making high-resistance concrete (www. Wikipedia. com).Sulfonated melamine formaldehyde (SMF)
is a polymer used as cement admixture to reduce the water content in concrete while increasing the
fluidity and the workability of the mix during its handling and pouring. It results in concrete with
a lower porosity and a higher mechanical strength exhibiting an improved resistance to aggressive
environments and therefore has a longer durability. The use of melamine as a fertilizer for crops had
been envisaged during 1960 because of its high nitrogen content. To be effective as a fertilizer, it is
essential that the plant nutrients should be made available in a manner that matches the needs of
the growing crop. The nitrogen mineralization process for melamine is extremely slow, making this
product both economically and scientifically impractical for use as a fertilizer. Melamine derivatives
of arsenical drugs are potentially important in the treatment of African trypanosomiasis. In 1958,
melamine was used as a source of Non-Protein Nitrogen (NPN) for cattle.
Melamine toxicity
Melamine is described as being harmful if swallowed, inhaled or absorbed through the skin.
Chronic exposure may cause cancer or reproductive damage. Melamine can also affect eye, skin and
respiratory system. However, the toxic dose is at par with common table salt with an LD50 of more
than 3 grams per kilogram of bodyweight. Melamine and cyanuric acid can also be absorbed into
the bloodstream, concentrate and interact in the urine-filled renal microtubules, then crystallize and
form large numbers of round, yellow crystals, which in turn block and damage the renal cells that line
the tubes, causing kidneys failure. The European Union has set a standard for acceptable human
consumption of melamine at 0.5 milligrams per kg of body weight, Canada has declared a limit of 0.35
mg and the USFDA’s limit was put at 0.63 mg, but was later reduced to 0.063 mg/kg body weight
daily. The amount of melamine a person could withstand per day known as the "tolerable daily intake"
(TDI), is 0.2 mg per kg of body mass without incurring a major health risk.
Melamine is reported to have an oral LD50 of 3248 mg/kg body weight in rats, while in rabbits it
was reported to be more than 1000 mg/ kg body weight for rabbits . A melamine cyanurate commonly
used as an fire retardant could be more toxic than either melamine or cyanuric acid alone. For rats and
mice, the reported LD50 for melamine cyanurate was 4.1 g/kg body weight and 3.5 g/kg body weight
when compared to 6.0 g and 4.3 g/kg body weight for melamine and 7.7 g and 3.4 g/kg body weight
for cyanuric acid, respectively. .
174
Use of Lateral Flow Technique for Detecting Melamine in Milk
Ingestion of melamine may lead to reproductive damage or bladder or kidney stones, which can
lead to bladder cancer . Dogs when fed with 3% melamine in diet regularly for one year showed
changes in their urine i.e. reduced specific gravity, increased urine output, melamine crystalluria,
protein occult blood (WHO Report 1999). Crystals were formed in the kidneys when melamine
combined with cyanuric acid was fed to dogs.
In April 2007,the newspaper The New York Times reported that the addition of "melamine scrap"
into fish and livestock feed gave the false appearance of a higher level of protein which became an
"open secret" in many parts of China.
In China, several companies were implicated in a scandal involving milk and infant formula
which had been adulterated with melamine, leading to kidney stones and other renal failure especially
among young children. Melamine may have been added to milk to fool government with regard to
protein content test after water was added to fraudulently to dilute the milk. Because of melamine's
high nitrogen content (66% by mass), it can cause the protein content of food to appear higher than
the true value. About 20 percent of the dairy companies tested in China sell products tainted with
melamine (Guan et. al 2009).
2.1.4 Methods for testing melamine in milk
Until 2007, melamine had not been routinely monitored in food, except in the context of plastic
safety or insecticide residue. This could be due to the previously assumed low toxicity of melamine
and the relatively expensive method of detection. Different methods for the analysis of melamine in
food and milk developed by (USFDA 2008 ,http://www.wikipedia.org/wiki/melamine) have been
mentioned in Table1
Table1: different methods for the analysis of melamine in food and milk developed by
(usfda 2008 ,http://www.Fsis.Usda.Gov )
Method
Application
Limit of detection
Analogues detected
GC / MS
Various foods
2ppm
Melamine,Ammeline Ammelide
Cyanuric Acid
ELISA
Used for wheat gluten, moist
pet food, dried pet food, milk
and milk powder
10 ppm for wheat gluten, 2 ppm
for moist pet food, 4 ppm for
dried pet food, 2 ppm for milk
and 10 ppm for milk powder
Melamine
HPLC/UV
USFDA for meat
25 ppb
Melamine / Cyanuric Acid
HPLC/UV
Used for wheat Gluten and
rice protein
100ppm
Melamine, Ammeline, and Cyanuric
acid
HPLC/UV
Used for beverage
50 ppb
Melamine
HPLC/UV
Used for cereal flour
5 ppm for Melamine, Ammeline,
Ammelide, 90ppm for Cyanuric
acid
Melamine
HPLC/UV
Used for meat and pet food
10 ppb
Melamine,Cyanuric acid
Detection of melamine in milk using melamine strip
Melamine strips procured from cusabiotech were used for detection of melamine in milk. Presence
of melamine in milk was detected with the appearance of one pink or purple band in the test region
while two pink or purple bands were observed in case of control milk. This test is very simple, rapid
and could detect up to 100ppm melamine in milk .Fig 1 and 2 shows the pattern of pink bands
appearing on melamine strip when prepared milk filtrate were applied. Another advantage of this
test is that only 3-4 drops of sample is required and no instrument is required.
175
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Preparation of sample for melamine strip
Skim Milk Powder extraction protocol:
1. Add 1ml of methanol: water mixture ( 60:40) to 1g skim milk powder sample and Vortex the
tube vigorously.
2. Sonicate for 1min and then shake for 1 min. Allow it to stand for 5 min.
3. Transfer the supernatant to another tube, centrifuge at 10000 rpm for 5min.
4. Filter the supernatant through Whatman filter paper No 1, the filtrate is ready for the assay.
Liquid milk extraction protocol (1:2.5)
1. Add 150μl of methanol : water mixture ( 60:40) to 100μl liquid milk sample.Vortex the tube
vigorously.
2. Sonicate for 1min and then shake for 1 min. Allow it to stand for 5 min
3. Transfer the supernatant to another tube, centrifuge at 10000 rpm for 5min.
4. Filter the supernatant through Whatman filter paper No 1, the filtrate is ready for the assay.
Detection procedure for melamine in milk filtrate using strip.
1. Bring the melamine test strip and samples to room temperature (25-30ºC).
2. Add four drop the prepared sample to the sample area of the test strip.
3. Observed the formation of pink band in assay area within 5 min.
Fig 1. Photograph showing pattern of pink or purple bands on melamine strip in
melamine adulterated milk samples.
Fig 2. Photograph showing different level of detection of melamine in milk using strip.
176
control milk and
Rancimat (Accelerated and Automated) Method for Evaluation of Oxidative Stability of Fats and Oils
Rancimat (Accelerated and Automated) Method for
Evaluation of Oxidative Stability of Fats and Oils
Sumit Arora
Dairy Chemistry Division, NDRI, Karnal
Introduction
Rancimat is a modern, computer controlled analytical instrument for the comfortable determination
of oxidative stability index to predict oxidation stability of oils and fats, and hence, their shelf life. It was
developed by Hador and Zurcher in 1974 to replace the time consuming active oxygen method (AOM)
and other such methods. Oxidative stability is an important criterion for evaluating the quality of oils, fats
and fatty acid methyl esters (biodiesel). Lipid oxidation in foodstuffs is one of the most important critical
factors affecting major quality parameters such as colour, flavour, aroma and nutritive value, which
reduces their shelf life and influence its suitability for consumption. Therefore, it has great importance
in food industry to predict the shelf life of foods especially fatty foods. Determining oxidative stability is
a tedious and time-consuming process when performed at room temperature, thus it is necessary to use
accelerated methods to obtain the oxidative stability in a shorter time. For this reason, several accelerated
methods have been developed such as Schaal oven test, Active Oxygen Method (AOM) and Rancimat
Method. AOM and Schaal oven test are non-reproducible and time-consuming methods, however,
Rancimat method is comparatively more popular because of its ease of handling and reproducibility of
results. The unique temperature extrapolation allows an approximate estimation of the storage stability
of a product, thus saving both time and money.
Advantages:
•
Automated computer-controlled instrument, therefore, is easy to operate
•
Conversion of induction time to other temperatures i.e. extrapolation to predict the shelf life of
samples
•
Excellent data security and reproducibility
•
Time and money saving
•
Evaluation can be done at two different temperatures simultaneously
•
Independent heating blocks having individual start of each position
Principle:
Oil or fat sample is heated at higher temperature in a sealed reaction vessel. Stream of air is passed
through the oil or fat sample which results in oxidation of lipid molecules. The volatile products
formed upon oxidation are transported through the stream of air to a second vessel containing distilled
water, whose conductivity increases with increase in content of oxidation products. A graph is plotted
between conductivity and time which can be used to estimate the induction time or oxidative stability
index of oil or fat, thus predicting the shelf life of sample.
Standards
The Rancimat method is included in various national and internationals standards, such as:
•
AOCS Cd 12b-92 (Sampling and analysis of commercial fats and oils: Oil Stability Index)
•
ISO 6886 (Animal and vegetable fats and oils– Determination of oxidation stability by accelerated
oxidation test)
•
2.4.28.2-93 (Fat stability test on Autoxidation. CDM, Japan)
•
Swiss Food Manual (Schweizerisches Lebensmittelbuch), section 7.5.4
177
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Determination of Oxidation Stability:
Prepare fat/oil sample
Switch on 743 Rancimat as suggested by the manufacturer
Select method
Start heating
Insert and connect reaction vessels when temperature is reached
Start determination
Determination finished when stop criteria* is reached
Figure 1: Flow diagram showing working of 743 Rancimat
Result display
Clean vessels and accessories
*Stop criteria may be induction time, conductivity or end point (point at which conductivity starts
increasing abruptly)
RANCIMAT:
A.
Instrumentation:
A.
Heating blocks:
The 743 Rancimat has two independent heating blocks that allow up to eight samples to be
analyzed at one or two temperatures. Up to four Rancimats can be connected to one computer, so
that the maximum number of samples that can be analyzed in parallel can be increased to 32. Each
measuring position can be started individually. As soon as the measurement has been completed the
measuring position is immediately ready for a new sample, which means that the instrument can be
used to its full capacity.
B.
Reaction vessel:
Weighing out the sample and assembling the reaction vessel are extremely simple and safe. Reaction
vessel does not need to be expensively cleaned at the end of the measurement, thus reducing the analysis
costs.
C.
Measuring vessel:
Figure 2: Reaction Vessel
178
Figure 3: Measuring Vessel
Figure 4: Conductivity cell
Rancimat (Accelerated and Automated) Method for Evaluation of Oxidative Stability of Fats and Oils
Easy-to-clean polycarbonate beakers are used for the automatic conductivity measurement. Glass
beakers are available as an alternative.
D.
Cover with built-in conductivity cell:
The conductivity cell is incorporated in the measuring vessel cover. When the cover is placed in
position the cell is immersed in the water. At the same time electrical contact is made to the electronics
in the instrument. The use of fragile glass conductivity electrodes with lengthy connecting cables went
out of fashion a long time ago. The new conductivity cell is also very easy to clean.
e.
Connections:
In order to make operation as simple as possible, there are no controls at all on the instrument.
All its functions are controlled from the computer. Apart from the power switch, the only features
you will find on the instrument are the RS-232 socket for connection to a computer and a socket for
connecting the Pt-100 temperature sensor.
Figure 5: Connections
f.
Figure 6: Air inlet filter and molecular sieve
Figure 7: GLP Set
Air inlet filter and molecular sieve:
The air used for the measurement is aspirated through a filter that prevents particles from entering
the instrument. The molecular sieve removes water vapour from the aspirated air; as water contributes
to the hydrolytic decomposition of the fat molecules, it could interfere with the measurement.
g.
Air supply line:
The amount of air that passes through the sample is automatically controlled via the rotation
rate of the built-in pump according to the method settings. A separate supply of compressed air is
not necessary.
B. Validation with the glp set:
The optionally available GLP Set facilitates the validation of 743 Rancimat. It contains a certified
Pt-100 temperature sensor with accessories that can be used for testing the temperature regulation of
the heating block. A test plug for checking the conductivity measurement inputs is also supplied.
C. Software functions:
All functions of the 743 Rancimat are controlled by the Rancimat software, which excels by its userfriendliness. All the functions are clearly arranged in just a few windows, the operation is intuitive.
D. Rancimat control:
This is where the measuring parameters can be called up and edited. The instrument functions are
controlled directly from here; the measurements are also started and shown in the live display field.
The arrangement of this window corresponds to a view of the instrument from above. This means that
the assignment of sample information and measuring position is perfectly clear. The timer function
can be used to automatically switch on the heating blocks before the start of work, so that it is no
longer necessary to wait while they warm up.
179
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
The functions in a nutshell:
•
Individual start/stop for each position
•
Live display
•
Temperature display
•
Method definition
•
Instrument controls
•
Calculation formulas, automatic result transformation to other temperatures
•
Timer function
E. Results:
At the end of each determination, the measured data is stored in a database and can be viewed by
the user in the results window. Sample information and results are shown in tabular form and can be
exported in various formats. The measured curves can be shown individually or in groups. It is also
possible to edit the automatic evaluation and recalculate the results. The temperature extrapolation
function for estimating the storage stability is available in this section of the software. All the displayed
data can be sorted or filtered and display can be adapted to meet our requirements. Results can be
obtained in the following forms:
• Overview table
• Curve display: individual or multiple plots
• Re-evaluation: induction time, stability time and manual tangent method
• Report printout
• Temperature extrapolation (estimation of storage stability)
• Database functions: filtering, sorting
• Data export
F. Applications:
Determination of oxidation stability of foods:
Just like the pure substances, the oils and fats contained in foods are also subject to oxidation,
which contributes to their spoilage. In such cases the Rancimat can be used to determine the oxidation
stability of foods containing oils and fats. Meltable foods with a high fat content, such as ghee, butter,
margarine, lard or tallow, can be analyzed directly without any further sample preparation. For liquid
or semi-liquid foods, such as salad dressings or mayonnaise, it is better to split the emulsion and
analyze the separated fat phase. For solid, non-meltable foods it is also necessary to separate off the fat
phase. In this case the fat is normally cold-extracted with petroleum ether and the isolated fat is then
analyzed.
Following food samples can be analysed: butter, margarine, ghee, vegetable oils, baby foods, icecream, cereals, chocolate, nuts and biscuits.
G. Technical specifications:
1. Heating blocks:
Two aluminium heating blocks; electrically heated; can be set to different temperatures
2. Number of samples:
Eight samples (4 measuring positions per heating block)
3. Temperature control and measurement
Temperature range
Temperature correction
180
: 50 to 220°C, adjustable in 1ºC steps
: -9.9 to +9.9°C, adjustable in 0.1ºC
Rancimat (Accelerated and Automated) Method for Evaluation of Oxidative Stability of Fats and Oils
Reproducibility of set temperature
Temperature variation
Temperature difference between
different measuring positions
Instrument heating-up time from 20°C
To 120ºC
Instrument heating-up time from 20°C
to 220°C
Outer temperature of instrument
Response temperature of thermal
protection device
steps
: Typically better than ±0.2 °C*
: Typically <0.1 °C*
: Typically <0.3 °C*
: ~ 45min (to ±0.1°C temp. stability)
: ~ 60min (to ±0.1°C temp. stability)
: <50°C (at an operating temp. 220°C)
: 260ºC
*When operating temperature has been reached, with inserted reaction vessels with an identical
filling and 20 L/h air throughput.
4. Air throughput:
Pump
: Diaphragm pump
Output range
: 7 to 25 L/h
5. Conductivity measurement:
Measurement range
Electrodes
6. Temperature:
Nominal working range
Storage
Transport
7. Line power
Voltage
Frequency
Power consumption
8. Dimensions
Width
Height
Depth
9. Weight
27.6 kg (with accessories)
10. PC requirements
Processor
Operating system
Memory
RAM
Graphics resolution
Interface
Printer
: 0 to 400 μS/cm
: 6.0913.130 conductivity cell with double steel pin electrode
built into vessel cover
: +5ºC to +40ºC (at 20 to 80% relative humidity)
: -20ºC to +70ºC
: -40ºC to +70ºC
: 2.743.0014/2.743.0114: 230 V (220...240 V ±10%)
2.743.0015/2.743.0115: 115 V (100...120 V ±10%)
: 50 to 60 Hz
: <450 VA (depending on heating power)
: 405 mm
: 268 mm (without accessories)
353 mm (with accessories)
: 466 mm
: Pentium III with 700 MHz or higher
: Windows TM NT, Windows TM 2000 or Windows TM XP
: 20 MB for program files, 200 MB recommended for measuring
data storage
: Working memory 128 MB, recommended 256 MB or higher
(particularly for Windows TM XP)
: min. 800 x 600, recommended 1024 x 768 or higher
: 1 free RS-232C interface (COM)
: All printers supported by WindowsTMt advantage
181
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Estimation of Cholesterol Content in Ghee
Using a Cholesterol Estimation Kit
Vivek Sharma and Darshan Lal
Dairy Chemistry Division, NDRI, Karnal
Principle
Cholesterol is extracted in unsaponifiable matter as free cholesterol. The aliquot of unsaponifiable
matter is made to react with the reagents of the cholesterol estimation kit and the color developed
is measured at 505nm. The absorbance values in the sample and control are used to calculate the
cholesterol content in a given sample of ghee.
Materials
Ghee, Enzymatic Diagnostic kit, Methanol, Potassium hydroxide, Hexane, Teflon line screw
capped tubes.
Equipment
Water bath, Spectrophotometer, Cuvettes.
Protocol for cholesterol estimation in milk fat after saponification using enzymatic diagnostic kit:
Milk fat (0.1-0.15 g) in test tube with teflon lined screw cap
Add 5 ml 5% methanolic KOH and mix
Incubate capped tubes in water bath for 90 °C/ 20 min with shaking every 5 min.
Cool contents by tap water
Add 1 ml distilled water
Add 5 ml hexane
Vortex the contents for 1 min
182
Estimation of Cholesterol Content in Ghee Using a Cholesterol Estimation Kit
Centrifuge at 2000 rpm/ 2 min
Pipette out upper hexane layer
Take 0.2 ml of aliquot in dry test tube
Evaporate solvent under nitrogen at 60-70°C
Add 10 µl of absolute ethanol to dissolve dried residue
Add 1.0 ml cholesterol reagent provided in kit and incubate at 37°C/10min
Cool to room temp (28 – 30°C).
Measure colour (pink) intensity at 505 nm
Calculation:
Where,
0.02 is the concentration (mg) of cholesterol in 10 µl of standard solution provided in the kit.
183
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Rapid Methods for Detection of Adulterants in Milk
Rajan Sharma, Raman Seth and Amit K. Bauri
Dairy Chemistry Division, NDRI, Karnal
Introduction
Addition of neutralizers and adulterants in milk has become a common feature for fulfilling the milk
demands of over populated country. Now for dairy industry it seems to be difficult to run the plant
without neutralization of milk. For milk vendors and shop-keepers, adulteration of milk with water to
increase the quantity in order to supply milk in large number of house-holds also has become a common
practice. The lack of timely action against the adulterators by the Public Health Departments and lack of
easier and rapid methods for detection of adulteration further encouraged this menace. Common man
i.e. consumers are not aware of the methods and chemicals used in the methods. Now in NDRI Karnal,
the procedures for the detection of various adulterants and neutralizers have been simplified to be easily
adopted by the house-holds. The prepared reagents as well as a KIT for the detection of adulterants and
neutralizers are available in the Dairy Chemistry Division of NDRI, Karnal.
Preservatives
A.
Test for formaldehyde
Formalin (40% water solution of formaldehyde) is generally used by Public Health Departments to
preserve the milk samples for chemical analysis purpose. Formaldehyde is very poisonous chemical.
Though, it can preserve the milk for very long time, it should never be added to milk meant for
processing due to its poisonous property. Moreover, it affects the quality of the milk products. If milk
kept at room temperature (25 to 35ºC) for longer time, did not sour, then that milk must be tested for
formaldehyde by the following simple method:
Method 1: Leach test
1. Take about 5 ml of milk in a test tube.
2. Add to it equal volume of Conc. HCl containing 1 ml of 10% ferric chloride solution to each 500
ml of the acid.
3. Keep the tube in boiling water bath for about 3-4 min.
4. Observe the colour of the solution in the tube. The tube containing pure sample will turns
yellowish. The positive sample (i.e. containing HCHO) will turn violet to brown black.
Method II: Chromotropic acid test
Reagent: Saturated solution of 1,8-dihydroxynaphthalene-3,6-disulphonic acid in about 72%
sulphuric acid (about 500 mg/100 ml). Light straw-coloured solution should result.
1. Take one ml of milk sample in a test tube. Add 1 ml of the Chromotropic acid reagent and mix well.
2. Appearance of yellow colour confirms the presence of formalin in the sample, whereas; control
sample will remain colourless.
B.
Test for hydrogen peroxide
Hydrogen Peroxide is a preservative, but as per PFA rule it is not permitted to be added in milk.
Hence if it is found, then milk is said to be adulterated.
Method I
Reagent: Para-phenylenediamine solution (2%, Aq, w/v).
Procedure:
1. Add to about 5 ml of milk in a test tube, an equal volume of raw milk, followed by five drops of
a 2 % of para-phenylenediamine.
184
Rapid Methods for Detection of Adulterants in Milk
2. A blue colour is developed in the presence of hydrogen peroxide.
Note: It is unlikely that the addition of less than 0.1% of H2O2 to milk could be detected after 24 h,
owing to the action of peroxidase and catalase which stimulate its conversion into water. If moe than
0.2% H2O2 is added, some will persist for considerable long time. Owing to the fact that larger amount
of H2O2 are known to destroy peroxidase, it is always advisable to add to the sample an equal volume
of raw unpreserved milk and to follow with a few drops of a 0.2% solution of para-phenylenediamine.
Under these circumstances a blue colour will develop immediately if H2O2 is added.
Method II
A method using potassium iodide and starch was standardized for the detection of hydrogen
peroxide in milk.
Procedure: Take one ml milk sample in a test tube. Add one ml of potassium iodide-starch
reagent (mix equal volumes of 20% potassium iodide solution and 1% starch solution) to the test tube.
Appearance of blue colour indicates the presence of hydrogen peroxide in the milk sample whereas
control samples remain colourless.
C. Detection of Neutralizers
Alkali in various forms like sodium carbonate, sodium bicarbonate, sodium hydroxide and lime
are used to neutralize developed acidity in milk. Detection of such neutralizers can be made by the
following two tests.
Method I. Rosalic Acid Test:
Reagents: Ethanol (95%), Rosalic acid solution (1% in alcohol).
Procedure:
1. Take in test tube about 5 ml milk and mix with 5-ml ethanol followed by 2-3 drops of rosalic
acid solution.
2. Formation of rose red colouration indicates the presence of alkali as neutralizer. Pure milk
produces brownish or brownish yellow colour only.
Rosalic acid is an organic dye, which is used as an indicator-changing colour at pH 7.0 to 8.0.
Hence, milk made even faintly alkaline by addition of neutralizers can be detected due to formation of
rose red colour with rosalic acid solution.
Method II. Ash alkalinity test
Neutralization of milk whether with lime, soda, or caustic soda, invariably increases the ash
content and the total alkalinity of the ash from a fixed quantity.
Reagent: HCl (standard, 0.1 N), Phenolphthalein indicator.
Procedure:
1. Pipette 20 ml of milk into a porcelain basin and evaporate to dryness on boiling water bath.
2. Remove the basin, cool to room temperature and ignite the residue by heating over Bunsen
flame until gray-white ash is obtained.
3. Cool the basin to room temperature. Add to the residue 10-ml of water and disperse the ash in
water by stirring with a glass rod.
4. Titrate the ash dispersate by standard HCl using phenolphthalein indicator. If the volume of 0.1
N HCl required to neutralize the ash dispersate exceeds 1.20 ml; the milk is suspected to contain
neutralizers.
D. Detection of starch or cereal flours
Reagent: Iodine solution (1%), Dissolve 2.5 g potassium iodide in 100 ml water, add to it 1 g pure
iodine crystal, shake well to give a clear solution.
185
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Procedure:
1. Take about 3 ml of well-mixed milk sample in a test tube.
2. Heat the milk to just boiling by holding the tube over flame, and thereafter cool to room time.
3. Add 1-2 drops of 1% iodine solution.
4. Observe the development of colour. Formation of blue-violet colour indicates presence of starch
cereal flours.
E.
Detection of cane sugar
Sugar or cane-sugar, is generally added to milk in order to raise the lactometer reading of the
milk which was diluted with water, so that by lactometer reading, the detection of added water is
prevented. In suspected samples, sugar can be easily detected by following method:
Reagent: Resorcinol, conc. HCl. (or prepare sucrose detecting reagent by dissolving 0.5 g of
resorcinol in about 40 ml of distilled water. Then add 35 ml of 12 N conc. HCl. Make up the volume
to 100 ml using distilled water.)
Procedure:
1. To about 5 ml of milk in a test tube, add 1 ml of conc. HCl and 0.1 g of resorcinol and mix.
2. Place the tube in boiling water bath for 5 min.
3. In the presence of cane sugar, red colour is produced.
Note: The test can be simplified by taking 1 ml of suspected sample of milk is a test tube followed by
the addition 1 ml of sucrose detecting reagent. In the presence of cane sugar, red colour is produced.
F. Detection of glucose
Glucose being a reducing sugar poses many problems in its detection. Moreover, it is easily
available in commercial form as concentrated syrup. These days adulteration of milk with glucose is
increasing. Now it has become possible to detect Glucose in milk by the following method:
Reagents:
1. Barfoed’s reagent: Dissolve 24 g cupric acetate in 450 ml boiling water and immediately add 25
ml of 8.5% lactic acid to the hot solution. Shake to dissolve almost all precipitate, cool and dilute
with water to 500 ml. If necessary decant of filter to get a clear solution.
2. Phosphomolybdic acid reagent: Take 35 g ammonium molybdate and 5 g sodium tungstate in a
large beaker; add 200 ml of 10% NaOH solution and 200 ml water. Boil vigorously (20-60 min)
so as to remove nearly whole of ammonia. Cool, dilute with water to about 350 ml. Add 125 ml
conc. H3PO4 (85%) and dilute further to 500 ml.
Procedure:
1. Take 1 ml of milk sample in a test tube. Add 1 ml of modified Barefoed’s reagent.
2. Heat the mixture for exact 3 min in a boiling water bath and then rapidly cool under tap water.
3. Add one ml of phosphomolybdic acid reagent to the turbid solution and observe the colour.
4. Immediate formation of deep blue colour indicates the presence of added glucose. In case of
pure milk only faint bluish colour is formed due to the dilution of Barefoed’s reagent.
G.
Detection of nitrates (pond water)
Pond water is heavier than the tap water; some unscrupulous persons for adulteration in milk
usually prefer it. However, it can be easily detected by the following method. This method actually
detects nitrates present in the pond water. In the pond water nitrates may come from fertilizers used
in the fields.
Reagent: Diphenylamine: Prepare 2% solution of diphenylamine in conc. sulfuric acid.
186
Rapid Methods for Detection of Adulterants in Milk
Procedure:
Take 2 ml of milk in a test tube. Rinse the tube with the milk and drain the milk from the test tube.
Add two-three drops of the reagent along the side of the test tube. Deep blue colour will be formed in
presence of nitrate.
H. Detection of Urea in milk
Urea is a natural constituent of milk and it forms a major part of the non-protein nitrogen of
milk. Urea concentration in milk is variable within herd. Urea is one of the ingredients of synthetic
milk along with caustic soda, detergent, sugar and foreign fats. Adulteration of natural milk with
synthetic milk increases the level of urea to such an extent that on consumption of this adulterated
milk causes toxicological hazards. Estimation of urea concentration in milk may serve as a tool for
checking the menace of adulteration of natural milk with synthetic milk. The average urea content
in milk of Karan Swiss, Karan Fries and Sahiwal cows was reported to be 28.57, 28.79 and 25.39
mg/100 ml (range 20 to 35 mg/100 ml). In buffalo milk, the average urea content was found to be
35.10 mg (range 25 to 40 mg/100 ml). The addition of urea to milk can be detected by using DMAB
method. This method is based on the principle that urea forms a yellow complex with p-dimethyl
aminobenzaldehyde (DMAB) in a low acidic solution at room temperature. The intensity of yellow
colour is measured at 425 nm. Here only qualitative method is described
Urea + DMAB
Reagent:
1.6% DMAB reagent: Dissolve 1.6 g DMAB in 100-ml ethyl alcohol and add 10-ml conc. HCl.
Procedure:
1. Take equal quantity of milk and equal quantity of 24% TCA in a glass stoppered test tube. Mix
and filter it.
2. Take 3 ml of filtrate in a test tube and add 3 ml of 1.6% DMAB reagent in ethyl alcohol and HCl.
Note the colour obtained.
3. The occurrence of distinct yellow colour indicates the presence of added urea in milk.
Note: The control (milk sample containing no added urea) showed a slight yellow colour due to
the presence of natural urea in milk.
I. Maltodextrin
To 5 ml milk sample in a test tube, 2 ml of dilute iodine solution (0.05 N) is added. Appearance of
chocolate red brown colour developed indicates the presence of maltodextrin.
J. Sodium chloride
Take 5 ml of milk and 1 ml of silver nitrate solution (0.1 N). Mix well and add two drops of a
solution of 10% potassium chromate. Yellow colour indicates the presence of added salt. Otherwise,
red colour will appear.
K. Ammonium salts
The added ammonium salts e.g ammonium chloride, ammonium sulfate, ammonium nitrate and
ammonium dihydrogen orthophosphate can be detected in milk by two methods i.e Nessler’s reagent
method and turmeric paper method.
Method I: Nessler’s reagent method
Reagent : Nessler’s reagent: Dissolve the following chemicals separately.
a.
8.0 g of mercuric chloride in 150 ml distilled water.
187
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
b.
60.0 g of sodium hydroxide in 150 ml distilled water.
c.
16.0 g of potassium iodide in 150 ml distilled water.
Add reagent a to reagent b and mix well. To this mixture, add reagent c, mix and dilute the contents
to 500 ml. Leave this solution undisturbed and decant the clear upper layer of the solution and store
in a stoppered glass bottle.
Procedure: Pipette 5 ml of suspected milk sample into a test tube and add 1 ml of Nessler’s reagent.
Mix the contents of the tube thoroughly. Appearance of yellowish or grey colour confirms the presence
of added ammonium salts in milk
Method II. Turmeric paper method
This method is based on the principle that ammonium salts on addition of strong alkali liberate
ammonia and the liberated ammonia turns turmeric paper to pinkish red.
Reagents:
•
Turmeric paper: Dissolve 10 g of pure turmeric powder in 100 ml distilled water and dip
Whatman filter paper Grade 1 strips into it for 2 min. Dry the paper at room temperature. The
dried filter paper is wetted with distilled water before use.
•
Sodium hydroxide solution: 10% (aq.)
•
Procedure: Pipette 5 ml of suspected milk sample in a test tube and add 1 ml of 10% sodium
hydroxide solution in such a manner that should not touch the rim of the test tube while adding.
Mix the contents of the tube. Place a piece of wet turmeric paper on the rim of the test tube
and keep the test tube undisturbed. Observe the change in the colour of the turmeric paper.
Appearance of pinkish red colour confirms the presence of ammonium salt in milk.
L. Sulfate salts
Presence of sulfates in milk can be detected by using barium chloride.
Reagents:
a.
Barium chloride (BaCl2.2H2O) solution: 5% (w/v, aq.)
b.
Trichloroacetic acid (TCA): 24% (w/v, aq.).
Procedure: Take 10 ml of milk in a 50 ml stoppered test tube and add 10 ml of TCA solution. Filter
the coagulated milk through Whatman filter paper Grade 42. Take 5 ml of clear filtrate and add few
drops of barium chloride solution. Formation of milky-white precipitates indicates the presence of
added sulfates like ammonium sulfate, sodium sulfate, zinc sulfate and magnesium sulfate etc. to
milk
M. Detection of refined oil in milk
This method is based on the principle that BR reading of milk fat is comparatively lower than
that of most of the foreign fats/oils. Its adulteration with vegetable and/or animal body fats/oils
significantly increases the BR reading/
For taking BR reading of the milk fat the milk fat is isolated from the specially designed butyrometer
which has both ends open. Milk fat after centrifugation is taken with the help of a capillary and BR
reading is noted at 40°C. A correction factor is added to the observed BR reading. This is done to
eliminate the inherent hydrolytic effect of H2SO4.
Actual BR at 40°C = Observed BR at 40°C + (0.08X observed BR at 40°C)
References:
Manual in Dairy Chemistry, NDRI, Karnal.
IS:1479 (Part II) – 1961 Methods of test for Dairy Industry-Part II Chemical analysis of milk.
188
Detection of Foreign Fats/Oils in Milk and Ghee Using Newer Approaches
Detection of Foreign Fats/Oils in Milk
and Ghee Using Newer Approaches
Darshan Lal, Vivek Sharma, Arun Kumar and Amit Kumar
Dairy Chemistry Division, NDRI, Karnal
Introduction
The menace of adulteration in food products has reached an alarming stage in recent years. Even
the milk (most sacred food) has not been spared. Milk fat, the costliest edible fat, increasingly catches
the attention of the unscrupulous elements for an easy adulteration with far cheaper oils and fats of
vegetable and animal origin. Under the circumstances, the dairy industry is in dire need for some
rapid and simple methods to check the menace of adulteration in milk and milk products. Earlier, ghee
used to be adulterated with foreign oils and fats, and accordingly several methods were developed for
detection of adulteration in ghee. These methods were based on differences in the nature and contents
of major/minor components of ghee and adulterant fats/oils. Now days, a new trend of addition of
foreign fats/oils directly into milk has been gaining momentum. Unfortunately, the tests, which are
applicable for detecting adulteration in ghee, cannot be directly applied to milk because milk is not a
single-phase emulsion. Rather, it is an oil-in-water type emulsion. Therefore, the fat phase of milk has
to be separated from its aqueous phase before applying any test for checking the adulteration of milk
fat. Moreover, since no single test can detect all types of adulterants (oils and fats), therefore, often
more than one tests have to be employed to confirm the purity of milk fat.
Methodology
There are two approaches for the detection of adulteration of milk fat. First approach is based
on the classical methods like B.R reading, R.M value, P. value, Phytosterol acetate test, Gas – liquid
chromatographic analysis. Second approach is based on some innovative and rapid methods like furfural
test for vanaspati, Opacity test, crystallization test, color based test for vegetable oil detection, apparent
solidification time test and complete liquification time test. In all the cases, tests are applied on the extracted
fat, except the modified Gerber test, where especially designed dual purpose Gerber butyrometer is used
and B.R reading of the isolated fat is measured. Hence, the first step is to isolate the fat and then apply the
test (Kumar et al, 2002).
A) Detection of foreign oils and fats in milk:
Keeping in view the need for a rapid test which can be applied to milk for detecting the adulteration
right at the platform where the milk is to be either accepted or rejected, the approach suggested by
Lal et al (1998) involves the isolation of fat from milk followed by determination of B.R. reading of the
isolated fat. This test is specifically useful for detection of vegetable oils in milk.
Isolation of fat from milk
Isolation of fat from milk can be done by any of the three methods:
•
Solvent extraction method
•
Heat clarification method
•
Modified Gerber method.
Solvent extraction method
Take 100 ml of milk sample in a 500 ml flask. Add 15 ml of NH4OH, and shake thoroughly. Add
50 ml ethyl alcohol, 100 ml solvent ether and 100 ml petroleum ether and shake thoroughly after each
addition. Allow to stand for half an hour. Decant the ethereal layer in another conical flask of 250 ml
capacity.
Add about 50 g of anhydrous Na2SO4 to remove the traces of moisture from the ethereal layer.
Collect the ether extract and add 1 or 2 glass beads. Evaporate ether extract to dryness on boiling water
bath taking care to prevent bumping and then transfer in oven maintained at 102ºC.
189
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Heat clarification method
Obtain about 50g of cream by separation using cream separator or by centrifuging the milk at 4000
rpm for 10 min. Convert the cream into ghee by heat clarification.
Modified gerber method
Isolate the fat from milk by Gerber method using specially designed dual purpose milk butyrometer,
which is open at both ends. Close the stem side opening with a good quality acid resistant silicon cork.
From the neck side, add 10 ml of 90% H2SO4, 10.75 ml milk and 1 ml amyl alcohol. Close the neck side
with lock stopper; mix the contents and centrifuge for 5 min to get clear fat in the column. Remove
the silicon cork and take out the fat from the stem of butyrometer with the help of a capillary tube or
a syringe (Lal et al, 1998).
B) Detection of foreign oils and fats in ghee:
Available tests:
1. Detection of animal body fats and vegetable oils/fats by the opacity test
Melt the sample of fat (5 gm) isolated by heat clarification method at 50 +1oC in a test tube and
maintain for 3 min to equilibrate. Then transfer the test tube at 23 + 0.2oC water bath and record the
opacity time (Time taken by fat sample to acquire either O.D. at 570 nm between 0.14-0.16 or Klett
reading using red filter between 58-62 after adjusting the instrument to 100% transmittance). The
opacity time of pure buffalo ghee is 14-15 min, cow ghee is 18-19 min and that of ghee from cotton tract
area is 11-12 min. The opacity time of buffalo ghee adulterated at 10% level with vanaspati is 10-11
min, with pig body fat is 8-9 min, with buffalo body fat is 2-3 min, with cow body fat is 3-4 min and
with refined oils is 20-25 min (Singhal, 1980).
2. Detection of vanaspati in ghee
Isolate the fat from milk by heat clarification method as described above. Take about 5 g of the
melted fat in a test tube. Add 5 ml of concentrated HCl. Add 0.4 ml furfural solution (2% in alcohol)
and shake the tube thoroughly for 2 min. Allow the mixture to separate. The development of pink or
red colour in the acid layer indicates presence of vanaspati. Confirm by adding 5 ml distilled water
and shaking again. If the colour in acid layer persists, vanaspati is present. If the colour disappears, it
is absent [SP:18 (1987)].
3. Detection of vegetable oils byButyro-Refractometer (B.R.) Reading
Clean the prisms of the Butyro-refractometer with petroleum ether. Allow the ether to evaporate to
dryness. Maintain temperature of the prisms at 40ºC by circulating water. Calibrate the B.R. apparatus
by applying a drop of fluid of known B.R. and adjusting B.R. by moving the adjustment screw. Clean
the prisms. Apply a drop of sample of clear fat obtained by any of the three methods between the
prisms. Wait for 2 min before taking the reading so that sample should attain the constant temperature
of about 40ºC.
B.R. reading decreases and increases with the rise and fall of temperature, respectively. Normally,
the temperature of observation should not deviate by more than 2ºC. A correction of 0.55 is added to
the observed B.R. reading for each degree above 40ºC or subtracted for each degree below 40ºC to get
corrected B.R. reading of the sample.
If fat is isolated by the Gerber method, B.R. is depressed due to hydrolytic effect of H2SO4 on the
fat. Therefore, observed B.R. reading is corrected as follows:
Corrected B.R. = 1.08 x observed B.R.
B.R. reading of milk fat isolated by any one of the above mentioned methods should be consistent
with the values given for ghee as per PFA requirement. Any deviation from the standard value
indicates adulteration of milk with vegetable oils. However, this method has limitation of detection
of adulteration with two oils i.e. coconut oil and palm oil whose values are close to that of milk fat
(Arora et al, 1996).
190
Detection of Foreign Fats/Oils in Milk and Ghee Using Newer Approaches
4. Detection of animal body fats and vegetable oils by crystallization test
Isolate the fat from milk by heat clarification method as described above. Take 0.8 ml of melted
fat in a stoppered test tube (10 x 1.0 cm internal diameter). Add 2.5 ml of solvent mixture consisting of
acetone and benzene (3.5:1.0). Mix the contents slowly. Place the test tube in a water bath maintained at
20ºC for 3 min to equilibrate the temperature. Then transfer the tube in another water bath maintained
at 17 ± 0.2ºC till the onset of crystallization. Note the time for occurrence of crystallization. The
crystallization time of pure buffalo ghee is 18-20 min and that of cotton tract ghee is 10.5-12.5 min,
whereas that of buffalo ghee adulterated at 10% level with pig body fat is 11.5-12.5 min, with cow body
fat 4.5-5.5 min and buffalo body fat 3.0-4.0 min, and with vegetable oils is 26 to 36 min (Panda, 1996).
5. Detection of adulteration of vegetable oils in ghee by Iodine value
Iodine value, which is a measure of extent of unsaturation of fat, can be determined by the
Wij’s method as described in SP:18 (Part XI)1981. This property is particularly useful for detection of
adulteration in ghee with vegetable oils, as these oils have higher iodine values than milk fat and body
fats. It can be measured, as follows:
Accurately 0.4 g of sample is weighed in a clean and dry iodine flask and is dissolved in 15 ml of
carbon tetrachloride. Then 25 ml of the Wij’s reagent are added and the flask is stoppered. The contents
are then mixed and kept in dark for one hour. After one hour, 20 ml of 10 per cent potassium iodide
solution and about 150 ml of distilled water are added to the iodine flask and mixed. The contents are
titrated against 0.1 N sodium thiosulphate solution using starch solution as an indicator. A blank test
is also carried out using the same quantities of the reagents. From this, the iodine value is calculated
as follows:
Iodine Value = 12.69 (B – S) N / W
Where;
B = Vol. (in ml) of standard sodium thiosulphate solution required for the blank
S = Volume (in ml) of standard sodium thiosulphate solution required for the sample
N = Normality of the standard sodium thiosulphate solution, and
W = Weight (in g) of the sample taken for the test
The iodine value for cow and buffalo pure ghee ranges between 30.12 to 40.26. Any deviation from
these values indicates adulteration (Kumar, 2008).
6. Detection of adulteration by apparent solidification time (AST) test
The apparent solidification time (AST) of the fat sample is defined as the time taken by the melted
fat sample to get solidified apparently at a particular temperature. The test can be carried out as:
Take 3.0 gm of completely melted fat sample in a test tube (10 × 1.0 cm ID) and maintain at 60ºC for
5 min. Transfer the test tube in a refrigerated water bath maintained at 18 ± 0.2ºC and simultaneously
start the stop watch. Observe the test tube constantly till the apparent solidification of the fat sample
takes place which is confirmed by non- movement of fat sample on tilting the test tube. At this stage,
stop the stopwatch and record the time taken for the apparent solidification of the fat. Pure ghee
sample of both cow and buffalo shows AST in the range of 2 min 31 sec to 3 min 25 sec. Any deviation
from these values gives an indication of adulteration of milk fat (Kumar, 2009)
7. Detection of adulteration using dry fractionation technique coupled with AST
By employing dry fractionation technique, the different fractions enriched with body fats or
vegetable oils are obtained and subsequently used to estimate AST. The aim is to enrich the solid
fraction with animal body fats and liquid fraction with vegetable oils. Vanaspati, if added, will also be
fractionated along with animal body fats.
Take 100 gm of clarified melted fat and keep it in a BOD incubator maintained at 20 ± 0.1ºC. After
about 1.50 to 1.75 h of incubation, approximately one third of the whole fat gets solidified. Separate the
solid fraction (S20) from the remaining liquid portion by filtration inside a BOD incubator maintained at
191
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
20 ± 0.1°C. Further fractionate the liquid portion thus obtained in another BOD incubator maintained
at 18 ± 0.1°C. for 2 hr so as to obtain another solid (S18) and liquid (L18) fraction by filtering inside a
BOD incubator maintained at 18 ± 0.1°C. Analyze S20, S18 and L18 fractions of ghee for AST as described
above. S20, S18 and L18 fractions of pure ghee of both cow and buffalo show AST values of 1 min 40
sec to 2 min 50 sec; 2 min 30 sec to 3 min 40 sec and 2 min 50 sec to 3 min 50 sec, respectively. Any
deviation from these values gives an indication of adulteration (Kumar, 2003).
8. Detection of adulteration by complete liquification time (CLT) test
The complete liquification time (CLT) test of the fat sample is defined as the time taken by the
solidified fat sample to get melted completely at a particular temperature. The test can be performed,
as follows:
Take 3.0 gm of completely melted fat sample in a test tube (10 × 1.2 cm) and maintain at 60°C for 5
min. Keep the test tube containing fat sample in a refrigerator (6- 8ºC) for 45 min for solidification of the
melted fat sample. Transfer the test tube in a water bath maintained at 44 ± 0.1ºC and simultaneously
start the stop watch. Observe the test tube constantly till the fat sample is completely liquefied. At
this stage stop the stopwatch and record the time taken for complete liquification of the fat. Pure ghee
sample of both cow and buffalo shows CLT in the range of 2 min 12 sec to 3 min 15 sec. Any deviation
from these values gives an indication of adulteration of milk fat (Kumar, 2008).
9. Detection of adulteration using solvent fractionation technique coupled with CLT and Iodine
value
Using solvent fractionation technique, the different fractions enriched with body fats or vegetable
oils can be obtained and used subsequently to estimate CLT. Here also, the aim is to concentrate
animal body fats in to solid fraction and vegetable oils into liquid fraction. Vanaspati, if added, will
also be concentrated in solid fraction along with animal body fats.
Take 30 gm of melted ghee sample in a 100 ml graduated glass tube, and then add 60 ml acetone
and mix well to dissolve the fat. After mixing, keep the sample at 40°C for equilibration for 5 min. Then
subject the sample in a refrigerated water bath to three temperatures/time combinations, viz., 16 ±
0.1°C/25 min, 8 ± 0.1°C/25 min and 4 ± 0.1°C/60 min, successively, after filtration at each stage of time/
temperature combination. After about 25 min at 16 ± 0.1°C, approximately one-fourth of the whole
fat gets solidified. This first solid fraction (S16) obtained at 16 ± 0.1°C is separated from the remaining
liquid portion (L16) of the whole fat by filtration through ordinary filter paper. The remaining liquid
portion (L16) thus obtained after filtration is further fractionated at 8 ± 0.1°C. in refrigerated water bath.
After about 25 min, it gets partitioned into two fractions, one solid (S8) and one liquid (L8), which can
be separated by filtration through ordinary filter paper. At last, L8 fraction is further fractionated at
4 ± 0.1°C for 60 min and filtered to get two fractions, one solid (S4) and one liquid (L4). Finally at the
end of fractionation, three solid fractions (S16, S8 and S4) and one liquid fraction (L4) are obtained from
ghee sample containing a mixture of adulterants. Solvent from liquid fraction is removed by using
rotary evaporator at about 40ºC, followed by nitrogen flushing to evaporate solvent completely from
the liquid fraction. To get rid of entrapped acetone, respective solid fractions are heated to 110ºC for
about 2 hr in an oven.
(a)
Analysis of first fraction (S16) for CLT at 46ºC
Analyse S16 fraction for CLT at 46 ± 0.1oC (instead of 44± 0.1oC used for CLT of whole fat) as
described above. CLT values of S16 fraction at 46oC range between 4 min 5 sec to 9 min for both cow
and buffalo pure ghee. Any deviation from these values gives an indication of adulteration of milk fat
(Kumar, 2008).
192
Detection of Foreign Fats/Oils in Milk and Ghee Using Newer Approaches
(b)
Analysis of last fraction (L4) for Iodine value
Analyse L4 fraction for iodine value as described above. The iodine values for L4 fraction of pure
cow and buffalo ghee are found to vary between 37.85- 46. 48. Any deviation from these values gives
an indication of adulteration of milk fat (Kumar, 2008).
10. Detection of mineral oil in ghee
Isolate the fat from milk by heat clarification method as described above. Take 1 g of fat in a
standard joint test tube and add 5 ml of 0.5 N ethanolic KOH solution and reflux by heating in
boiling water bath, using condenser for 10 min. or more till saponification process is complete.
Add about 5 ml of distilled water to the hot saponified solution. Appearance of turbidity indicates
the presence of mineral oil.
11. Rapid color based test for detection of vegetable oils
One ml of clear molten fat was dissolved with 1.5 ml of hexane in a tightly capped test tube. To this
was added 1.0 ml of color developing reagent (distilled water, Sulphuric acid - Sp.gr.1.835 and Nitric acid
- Sp. gr. 1.42 in the ratio of 20:6:14), shaken vigorously and kept undisturbed till it is separated into two
layers. The appearance of a distinct orange tinge in the upper layer indicates the presence of vegetable
oils / fats including vanaspati (Sharma et al, 2007).
12. Detection of adulteration of rice bran oil in ghee
Rice bran oil contains gamma oryzanol, which can be used as a marker for the detection of
its addition to ghee. It can be done by thin layer chromatographic method as well as colorimetric
method.
a)
Thin layer chromatographic method
A simple thin layer chromatographic method can be employed to detect the adulteration of ghee
with rice bran oil, as follows:
Gamma oryzanol is extracted from 10.0 gm of molten fat using 20.0 ml of a solvent system
consisting of methanol: water (9:1). The contents are vortexed for 2 min and centrifuged at 2000rpm. /
10 min. The alcohol layer is drawn. Extraction protocol is repeated thrice and all the alcoholic extracts
are combined and evaporated at 60 – 70 °C in a rotary evaporator. The residue is finally dried. The
dried residue is redissolved in 0.5 ml of developing solvent (toluene: ethyl acetate: methanol 90:8:2;
v/v) and 5-10 µl were applied on silica gel TLC plate and plates are developed in the developing
solvent. Properly developed plates are removed from the chamber and air dried followed by spraying
with color developing reagent (50% sulfuric acid) and heating at 120°C/ 10 -15min. Presence of the
gamma oryzanol band confirms the adulteration of rice bran oil in milk fat. Addition of rice bran oil in
ghee at 5% level is easily detected by this method. (Kumar, et al, 2008).
b)
Colorimetric method
Take 1ml of melted ghee sample in a dry test tube. Add 1.5 ml of hexane to dissolve the fat. Then,
in sequence, add 0.5 ml of dilute (25%) hydrochloric acid and 0.5 ml of 5% sodium nitrite solution
and mix, followed by the addition of 1 ml of 10% sodium hydroxide solution. Rice bran oil produces
orange-red color while other vegetable oils produce no color. Hence, this method is specific for the
detection of rice bran oil in ghee. As low as 2% rice bran oil added in ghee, can be detected by this
method.
193
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Standards of ghee under PFA rules
Sr.
No.
Name of the State & U.T.
1.
Bihar, Chandigarh, Delhi, Punjab, Haryana (Areas other than cotton
tract areas), West Bengal (Areas other than Bishnupur sub-division),
Sikkim, Jharkhand.
2.
Manipur, Meghalaya, Mizoram, Arunachal Pradesh, Orissa,
Uttaranchal, Nagaland, Tripura, Assam, Goa, Kerala, Himachal
Pradesh, U.P., J & K, Rajasthan (Areas other than Jodhpur Divn),
Haryana (Cotton tract areas), Lakshadweep, Maharashtra(Areas
other than cotton tract areas).
BR
Reading
at 40ºC
RM
value
(Min)
% of
FFA (as
Oleic
acid)
(Max)
% of
Moisture
(Max)
40-43
28
3
0.5
40-43
26
3
0.5
3.
Karnataka (Belgaum district), Madhya Pradesh (Areas other than
cotton tract areas), Pondicherry, Chhatisgarh.
40-44
26
3
0.5
4.
Andhra Pradesh, Daman & Diu, Dadar & Nagar Haveli, Karnataka
(Areas other than Belgaum distt.)
40-43
24
3
0.5
5.
Andaman & Nicobar Island, Tamil Nadu.
41-44
24
3
0.5
6.
Gujarat (areas other than cotton tract).
40-43.5
24
3
0.5
7.
Gujarat (cotton tract areas), Madhya Pradesh (Cotton tract areas),
Maharashtra (cotton tract areas), Rajasthan (Jodhpur sub division),
West Bengal (Bishnupur sub division).
41.5-45
21
3.0
0.5
Baudouin test shall be negative
By cotton tract is meant the areas in the state where cotton seed is extensively fed to the cattle and so notified by the
State Govt. concerned.
Usually such cotton tract areas ghee has low RM value and high BR reading compared to other areas
Ghee may contain BHA not more than 0.02% as antioxidant.
References:
Singhal, O.P. (1980). Adulteration & Methods for detection. Indian Dairyman, 32: 771-774.
Arora, K.L.; Lal. D, Seth. R and Ram, J. (1996). Platform Test for detection of refined mustard oil adulteration in milk.
Indian J. Dairy Sci., 49(10): 721-723.
Panda, D.K. (1996). Detection of adulteration of foreign fats in milk fat. M.Sc. thesis, submitted to N.D.R.I. Deemed
University, Karnal.
Lal, D.; Seth, R.; Arora, K.L. and Ram, J. (1998) Detection of vegetable oils in milk. Indian Dairyman., 50(7): 17-18.
Kumar.A; Lal.D; Seth.R and Sharma.R (2002) Recent trends in detection of adulteration in milk fat – A Review. Indian
J. Dairy Sci., 55 (6): 319 - 330.
Sharma. V; Lal, D and Sharma. R. (2007) Color based platform test for the detection of vegetable oils/fats in ghee. Indian
J. Dairy Sci. 60,1: 16 – 18.
Kumar. A; Sharma. V and Lal.D (2008) Development of a thin layer chromatography based method for the detection of
rice bran oil as an adulterant in ghee. Indian J. Dairy Sci. 61,2: 113 – 115.
Kumar. A; Ghai, D. L; Seth, R and Sharma, V (2009) Apparent solidification time test for detection of foreign oils and
fats adulterated in clarified milk fat, as affected by season and storage. International J . Dairy Tech. 62: 33 –38.
Kumar. A; Lal, D.; Seth, R and Sharma, V (2010) Detection of milk fat adulteration with admixture of foreign oils and
fats using a fractionation technique and the apparent solidification time test. International J . Dairy Tech. 63 (3): 457
–462.
Kumar. Amit; (2008) Detection of adulterants in ghee. Ph. D thesis submitted to NDRI, Karnal (Deemed University).
ISI (1981). Handbook of Food Analysis. IS: SP:18, Part XI. Dairy Products. Bureau of Indian Standards, New Delhi.
Lal, D.; Seth, R and Sharma, R; Kumar. A. (2005) Approaches for detection of adulteration in milk fat-An overview.
Indian Dairyman 57(10): 31-43.
194
Determination of Total Polyphenolic Content in Fruit Enriched Dairy Product
Determination of Total Polyphenolic Content
in Fruit Enriched Dairy Product
Rajesh Kumar and Richa Singh
Dairy Chemistry Division, NDRI, Karnal
Introduction:
Polyphenols are plant secondary metabolites commonly found in herbs and fruits, such as berries,
apples, citrus fruit, cocoa, grapes, vegetables like onions, olives, tomatoes, broccoli, lettuce, soybeans,
grains and cereals, green and black teas, coffee beans, propolis, and red and white wines. Many of
these polyphenols are responsible for the attractive colour of leaves, fruits and flowers. Further,
polyphenolics are classified as:- Simple phenols: Phenol acids are phenols that possess one carboxylic
acid functionality such as the hydroxycinnamic and hydroxybenzoic acid; Flavonoids: Polyphenol
possessing at least two phenol subunits and Tannins: Polyphenol possessing three or more phenol
subunits. The quantification of polyphenol content in foods and beverages is critical for understanding
the potential health benefits of polyphenols.
Principle: The polyphosphotungstates are colorless in the fully oxidized 6+ valance state
of the metal, and the analogous molybdenum compounds are yellow. They form mixed
heteropolyphosphotungstates- molybdatesthey exist in acid solution as hydrated octahedral complexes
of the metal oxides coordinated around a central phosphate sequence of reversible one or two electron
reductions lead to blue species such as ( PMoW11O40)4- . In principle, addition of an electron to a
formally nonbonding orbital reduces nominal MoO4+ units to “isostructural” MoO3+. Tungstate forms
are considered to be less easily reduced but more susceptible to one electron transfer. Molybdates are
considered to be reduced more easily to blue forms. Mixed complexes as in Folin-Ciocalteu reagent
are intermediate. Blue products of phosphomolybdate reduction can have Mo6+ to Mo5+ ratios of 9.6 to
0.6. The 4 e- reduced species is the most stable blue form and develops readily from mixture of Mo6+
and Mo5+.
Folin: Mo(VI) (yellow) + e- (from AH) → Mo(V) (blue)
Reagents:
a) Folins Ciocalteu’s reagent: (0.2N): The Folin-Ciocalteu reagent (FCR) is a mixture of
phosphomolybdate and phosphotungstate used for the colorimetric assay of polyphenols
and polypolyphenols antioxidants. 2N Folin-Ciocalteu’s phenol reagent (SRL) is diluted with
distilled water in the ratio 1:10.
b) Sodium carbonate solution: (7.5% (w/v)
7.5 g of sodium carbonate is dissolved in distilled water and make-up the Volume to 100ml
using volumetric flask.
c)
Sample: Two gm of fruit fortified dahi sample was placed in 10ml volumetric flask and
diluted with distilled water and subjected to centrifugation at 4000g for 10 min at 4°C and the
supernatant was collected.
d)
Gallic acid stock solution (1mg/ml)
1g of Gallic acid (Sigma) was dissolved in 10ml ethanol and made up the volume to 1000 ml
with distilled water using volumetric flask.
Procedure:
a.
Take 400μl of appropriately diluted sample/gallic acid standard in a test tube.
b.
To it add 2000μl of diluted Folin-Ciocalteu’s reagent and mix with vortex mixer.
195
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
c.
After 3 minutes add 1600 μl of sodium carbonate solution and incubate under dark at room
temperature for 30 min.
d.
For blank preparation take 400μl of distilled water instead of sample.
e.
Measure the absorbance of the samples against blank at 765nm using SPECORD-200 double
beam spectrophotometer (Analytical zena).
C. Standard curve preparation:
Standard curve is prepared by using 10-100 μg/ml concentration of gallic acid solution.
D. Results:
Express the results in terms of μmol gallic acid equivalent (GAE) /g of fruit pulp.
196
Separation and Identification of Low Molecular Weight Proteins Using Tricine SDS-PAGE
Separation and Identification of Low Molecular
Weight Proteins Using Tricine SDS-PAGE
Neelima Sharma1, Rajan Sharma1 and Y. S. Rajput2
1
Dairy Chemistry Division, 2Animal Biochemistry Division, NDRI, Karnal
The purpose of SDS-PAGE is to separate proteins according to their size. SDS-PAGE is the most
widely used method for analyzing protein mixture quantitatively. It is particularly useful for monitoring
protein purification and, because the method is based on the separation of proteins according to size,
it can be used to determine the relative molecular mass of proteins. SDS (CH3-(CH2)10-CH2OSO3-Na+)
is an anionic detergent and when proteins are treated with SDS in presence of a reducing agent like
β-mercaptoethanol or dithiothreitol, SDS binds to hydrophobic regions of protein molecule and
provides net negative charge on protein molecule. The binding of SDS to per unit length of protein
molecules is almost constant for large number of different proteins and this brings charge-to-mass
ratio almost constant for most proteins. The electrophoretic movement of protein in acrylamide gel is
determined by molecular weight of proteins. Lower molecular weight proteins move faster than high
molecular weight proteins.
Glycine-SDS-PAGE (also known as Laemmli-SDS-PAGE) and Tricine-SDS-PAGE, based on
glycine-Tris and Tricine-Tris buffer systems, respectively are the commonly used SDS electrophoretic
techniques for separating proteins.
Tricine-SDS-PAGE is commonly used to separate proteins in the mass range 1-100 kDa. It is the
preferred electrophoretic system for the resolution of proteins smaller than 30 kDa. Tricine, used as the
trailing ion, allows a resolution of small proteins at lower acrylamide concentrations than in glycineSDS-PAGE systems. A superior resolution of proteins, especially in the range between 5 and 20 kDa,
is achieved without the necessity to use urea. The omission of glycine and urea prevents disturbances
which might occur in the course of subsequent amino acid sequencing.
Requirements
(A)
Reagents
1. Acrylamide solution (49.5% T, 3%C)
•
Acrylamide
: 48 g
•
N, N’- Methylene-bis-acrylamide
: 1.5 g
•
Dissolved in distilled water to a final volume of 100 ml. Filter the solution and refrigerate
(7-10°C). Gentle warming may be required for complete dissolution after refrigeration.
•
%T = Total acrylamide percentage of both monomers (acrylamide and the crosslinker
bisacrylamide)
•
%C = Percentage concentration of the crosslinker relative to total concentration
2. Gel buffer (3X)
•
Tris
: 36.34 g
•
SDS
: 0.3 g
•
HCl
•
Dissolve in 60 ml water. Adjust the pH to 8.45 with concentrated HCl. Make up the final
volume upto 100 ml with water. Store at 20 – 25°C.
3. Cathode buffer (1X)
•
Tris
: 12.11 g
•
Tricine
: 17.92 g
197
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
•
SDS
: 1g
•
Dissolve in distilled water and make volume upto 1 L. The pH of the solution should be approx
8.2. Do not correct the pH.
4. Anode buffer
•
Tris
6.05 g
•
Dissolve in 50 ml. Adjust pH upto 8.9 with conc. HCl/ 1N HCl and then makeup volume up
to 500 ml with dist water.
5. Sample buffer (4x)
•
SDS (12%, w/v)
:
12 ml of 20% SDS
•
Glycerol (30%, w/v)
:
6g
•
Mercaptoethanol (6%.v/v)
:
1.2 ml
•
Coommassie blue G 250 (0.05%)
:
0.01g
•
Tris/HCl (pH 7) (150 mM)
:
3 ml of 1 M Tris-HCl
•
Make up the volume upto 20 ml.
6. Marker (2.5µl/well)
•
Take 3.5µl marker and add 10µl sample buffer (1.33x). For 1.33x sample buffer, dilute 1ml of 4x
sample buffer with 2 ml distilled water.
7. Ammonium persulfate
•
Dissolve 100 mg of ammonium persulfate in 1 ml of distilled water immediately before use.
8. Separating gel solution (16%)
•
Acrylamide solution
:
5 ml
•
Gel buffer
:
5 ml
•
Glycerol
:
1.5 ml
•
Water
:
3.5 ml
•
APS
:
30 μl
•
TEMED
:
10 μl
•
TEMED - N,N,N’,N’-Tetramethylethylenediamine.
• APS – This is the last to be added in the solution.
9. Stacking gel solution (4%)
•
Acrylamide solution
:
1 ml
•
Gel buffer
:
3 ml
•
Water
:
8 ml
•
APS
:
60 μl
•
TEMED
:
10 μl
10. Fixing solution
•
10% TCA (Trichloroacetic acid)
11. Staining solution (0.025% Coommassie brilliant blue g250 in 10% acetic acid)
•
Dissolve 25 mg of the dye in 100 ml of 10% acetic acid.
12. Destaining solution
•
10% Acetic acid. Destain the gel twice. Each incubation should last for 15-60 min. Then transfer
the gel to distilled water.
•
198
(B) Mini vertical gel electrophoresis unit.
Separation and Identification of Low Molecular Weight Proteins Using Tricine SDS-PAGE
Method
1. Gel preparation
•
Prepare gel plates of the size 8 x 10 cm in casting stand for gel electrophoresis.
•
Prepare the separating gel (16.5%) was, pipette it down into each of the gel cassettes to a height
of 4 cm.
•
Overlay the gel mix with water or butanol to cuy oxygen action and to give a flat gel surface for
flat sample bands.
•
After polymerization carefully remove water with the help of blotting paper.
•
Then overlay stacking gel solution over it.
•
Insert preparative comb into the stacking gel solution to make troughs and wells and keep the
whole system undisturbed (1 -2 hours) for the setting of gel.
2. Sample preparation
•
Dissolve 3 mg sample in 500 µl of Tris buffer (150 mM, pH 7.0) - Solution A.
•
Take 3 x 15 µl of Solution A and 3 x 5 µl of 4x buffer and load 10 µl in each well. Each well
would contain 45 µg of protein.
•
Then take 100 µl of the Solution A and mix with equal volume of Tris buffer (150 mM, pH
7.0). Now again take 3 x 15 µl of this sol in 3 x 5µl of 4x buffer and load 10 µl in each well.
Each well would contain 22.5 µg of protein.
•
Incubate samples and marker at 37°C for 15 min
3. Electrophoresis
•
Place the gel sandwich after removing the comb in the mini vertical gel electrophoresis unit.
•
Clamp the sandwich in place.
•
Load 15 µl of protein samples and molecular weight markers to these wells.
•
Fill the lower buffer chamber with anode buffer. Check that the lower electrode is completely
submerged.
•
Fill the upper buffer chamber with cathode buffer and also layer it over the applied samples
carefully. Place the safety lid on the unit.
•
Run the experiment at 10°C by keeping the whole assembly in the refrigerator.
•
Carried out electrophoresis at constant current of 20 mA till the sample crosses the stacking gel.
Then the increase the current to 25 mA and maintain throughout the remainder of the run until
the marker dye was within 1 cm of the anodic end of the gel.
•
Remove the gel carefully and then transfer it to the fixative solution. Keep it over an orbital
shaker for 60 min.
•
Stain the gel with staining solution for twice the length of time used for fixing again using orbital
shaker.
•
Transfer the gel to the destaining solution till bands become visible against light background
renewing the solution every 30 min.
References
Schagger (2006 )Nature Protocol, 1 (1): 16-22.
Shagger, H. and Jagow, G.V. (1987) Tricine-Sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the
separation of proteins in the range from 1 to 100 kDa. 166:368-379.
199
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Identification of Proteins Through
Western Blotting – Practical
Neelima Sharma1, Amit K.Barui1 and Y.S. Rajput2
1
Dairy Chemistry Division, 2Animal Biochemistry Division, NDRI, Karnal
Introduction
The technique of Western blotting refers to identification of specific proteins, which are
first separated on acrylamide gel using electrophoresis and then subsequently transferred to
nitrocellulose membrane and identified. The identification of protein (antigen) is carried out by
performing antigen-antibody (first antibody) reactions on the membrane itself. Second antibodyenzyme conjugates were then allowed to interact with immobilized first antibody and then using
appropriate substrate, protein bands are detected. Although, antigen-antibody interactions are
widely employed in Western blot, other kind of interactions such as glycoprotein-lectin and biotinavidin have allowed research workers to employ this technique for other applications including
carbohydrate staining of glycoprotein, protein sequencing etc.
During 1979-80, this technique was described simultaneously by many workers but the method
described by Towbin et al. (1979) is most cited. The technique of Western blotting involves two distinct
techniques viz. (i) SDS-PAGE and (ii) electrophoretic transfer of protein from gel to membrane and
immuno-detection of proteins.
For Western blot, SDS-PAGE is carried out in mini-gel units. The separation of protein in minigel unit is similar to large-gel unit. In mini-gel units, volumes and separation times are considerably
reduced. The resolution in mini-gel is adequate for most routine applications.
Electrophoretic transfer of proteins from gel to membrane
Mini-trans blot assembly (Bio-rad), power pack, orbital shaker, tris, glycine, methanol, nitrocellulose
membrane, Whatman No. 3 paper.
Transfer buffer (25 mM tris, 192 mM glycine, 20% methanol, pH 8.3)3.03 g tris and 14.4 g glycine are dissolved in distilled water. 200 ml methanol is then added.
Volume is made up to 1 litre with distilled water. The pH of buffer will range from 8.1 to 8.4 depending
on quality of tris, glycine and methanol. Methanol should be analytical grade as metallic contaminants
in low grade methanol will plate on the electrode. The pH of buffer is not adjusted with acid or base.
Procedure•
Membrane and gel are handled only after wearing gloves.
•
After completion of SDS-PAGE, spacer gel is removed. A small cut on top left side in running
gel is made to remember the orientation of gel.
•
The running gel is equilibrated with transfer buffer for 30 min. to remove salts and SDS. Transfer
buffer is changed at least once during equilibration. Membrane of appropriate size is cut from
sheet. A small cut on top left side of membrane (glossy side facing worker) is made to remember
orientation of the membrane.
•
While the gel is equilibrating, nitrocellulose membrane is activated by placing it in transfer buffer
at an angle of 450. Also, fiber pads and pre-cut filter papers (Whatman No. 3) are immersed in
transfer buffer. Air bubbles trapped in fiber pads and filter papers are removed.
•
Gel holder cassete is opened and placed in glass vessel so that the gray panel is flat on the
bottom of the vessel and clear panel rests at an angle against wall of the vessel.
•
Gel holder cassette is assembled in following sequence : gray panel (cathode), fiber pad,
filter paper, gel, nitrocellulose membrane (glossy side facing the gel), filter paper, fiber pad,
200
Identification of Proteins Through Western Blotting – Practical
clear panel (anode). For easy remembrance of orientation, cut portions of gel and membrane
is aligned. This arrangement allows transfer of proteins on membrane where well position
remains the same as that in acrylamide gel. While assembling, care is taken not to allow
trapping of air-bubbles. This is achieved by assembling cassette under buffer and when each
layer is added, all air pockets are removed by rolling clean test tube over the layer. Nearly
adhesive contact is essential between the membrane and gel otherwise swirled or missing
transfer patterns and overall high background will be observed.
•
Buffer tank is filled with transfer buffer (4ºC). Bio-freeze cooling unit containing ice is placed in
buffer tank.
•
Gel holder cassette is closed and placed in the buffer tank such that gray panel of the cassette
faces the gray cathode electrode panel. The whole of blotting assembly is then placed over the
magnetic stirrer.
•
Electrophoretic transfer is carried out at constant voltage of 30 V overnight at 4ºC. The starting
current should be around 40 mA. At the end of transfer, the current should be 90 mA. In case
final value of current is less than 90 mA, a constant voltage of 100 V is additionally applied for
1 h.
•
After run, nitrocellulose membrane is stained with different reagents for visualization of proteins
or antigens. For ascertaining transfer of proteins from gel, the gel is also stained with coomassie
brilliant blue as described in earlier section.
Detection of transferred protein on nitrocellulose membrane
In Western blot, molecular weight markers and protein (antigen) samples are loaded in separate
lanes in SDS-PAGE. Whereas, methods used for staining of molecular weight markers are based on
non-specific reaction of dye with protein, antigenic proteins are detected employing antigen-antibody
interaction. Therefore, after electrophoretic transfer, the membrane-portion containing molecular
weight marker is cut from rest of membrane containing protein antigens. The molecular weight
markers can be stained by Ponceau S or congo-red dye. The proteins (antigens) are stained using
primary antibody and secondary antibody-enzyme conjugates.
Visualization of molecular weight markersPonceau S StainingStock Ponceau S dye solution is prepared by dissolving 200 mg Ponceau S in 10 ml of 3%
trichloroacetic acid. The stock dye solution can be stored at room temperature. The stock solution is
diluted ten fold with distilled water before use. The membrane is added slowly to vessel containing
diluted dye solution so that membrane absorbs dye uniformly. The membrane is then sub-merged for
5 to 10 min with mild shaking. After staining, the membrane is then rinsed with water or PBS until a
clear contrast between the bands (pink) and background (white) is observed. Staining of proteins with
Ponceau S is reversible.
Congo-red StainingStock Congo-red solution is prepared by dissolving 1 g Congo-red in 100 ml distilled water. This
solution is stable at room temperature. The working congo-red solution is prepared just before use by
diluting 1 ml of stock dye solution with 9 ml of 0.2 M acetate buffer, pH 3.5. The membrane is submerged in working congo-red solution for 5 min. at room temperature. The destaining is carried out
by immersing the membrane in distilled water until brown bands become visible against light pink
background. During staining and destaining, mild shaking is employed.
Visualization of Protein (Antigen)-
ReagentsPrimary antibodies directed against antigen and raised in rabbit, secondary antibody enzyme
conjugates such as goat anti rabbit immunoglobulin-peroxidase goat, anti rabbit immunoglobulin201
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
alkaline phosphatase, diamino benzedine, hydrogen peroxide, bovine serum albumin, nitro blue
tetrazolium (NBT) bromochloroindolyl phosphate (BCIP), dimethyl formamide (DMF).
Visualization of antigen using secondary antibody peroxidase conjugateAll steps are carried out at room temperature.
•
Membrane is washed with PBS (3x10 min.)
•
The membrane is treated with blocking solution (3% BSA prepared in PBS) for 1 h.
•
The membrane is treated with diluted rabbit antiserum for 1 h. The antiserum in rabbit is raised
against the antigen. The dilution is decided by antibody litre in immune serum and is carried
out in 1% BSA – PBS- (0.05%) Tween – 20. The membrane is washed with PBS -0.05% Tween 20
(3x10 min.).
•
The membrane is treated with goat anti-rabbit immunoglobulin-peroxidase conjugate (1:1000
diluted with 1% BSA – PBS – Tween 20) for 1 h. The dilution of conjugate is done as per instruction
from manufacturer.
•
The membrane is washed with PBS (4 x 10 min.).
•
The membrane is immersed in enzyme substrate DAB - H2O2 (6 mg diaminobenzidine in 10 ml
of 0.05 M Tris-HCl buffer, pH 7.6 containing 100 µl of 3% H2O2 ) till brown bands become visible.
The membrane at that stage is washed with distilled water and air-dried.
•
Membrane strips containing molecular weight markers and proteins (antigens) are aligned and
photographed.
Visualization of antigen using secondary antibody-alkaline phosphatase
conjugate
The method is similar to the method described using secondary antibody-peroxidase conjugate
except the followings.
•
Appropriately diluted secondary antibody-alkaline phosphatase conjugate is used instead of
antibody-peroxidase conjugate.
•
Instead of DAB-H2O2, the enzyme substrate used is BCIP-NBT. Stock solutions of nitroblue
tetrazolium (NBT) and bromochloro indolyl phosphate (BCIP) are prepared and stored at
–200C. Stock NBT is prepared by dissolving 30 mg NBT in 1 ml of 70 per cent DMF. Stock
BCIP is prepared by dissolving 15 mg BCIP in 1 ml of DMF. The working substrate solution
is prepared by addition of 200 µl of stock NBT and 200 µl of stock BCIP to 20 ml of 100 mM
Tris-HCl, pH 9.5 containing 100 mM NaCl and 5 mM MgCl2. When membrane is treated with
enzyme substrate, light violet colour blots become visible against light background.
Helpful-hints
•
The one major problem in Western blot is incomplete transfer of protein from gel to nitrocellulose
membrane. Transfer efficiency is improved by decreasing gel concentration which leads to
more porous gel. In more porous gel, the resolution of proteins is decreased. Gel containing
low molecular weight proteins should not be excessively washed after SDS-PAGE and before
transfer to avoid removal of these proteins in washing.
•
Methanol in transfer improves binding of SDS-proteins to nitrocellulose membrane but it causes
acrylamide gel pores to contract resulting in fixation of large molecular weight proteins within
the gel matrix. In case of poor transfer of large molecular weight proteins, one can try transfer in
transfer buffer containing reduced concentration of methanol.
•
Gel and membrane must make good contact. Thus excess moisture in the gel-membrane interface
should be removed by rolling test tube over membrane while gel holder cassette is assembled.
•
Poor transfer can occur if the protein is basic (ie pI > 9) as protein will have net positive charge
at the pH of transfer buffer (pH 8.5).
202
Identification of Proteins Through Western Blotting – Practical
•
Lower concentration of methanol (< 15%) does not facilitate removal of SDS from the gel and
proteins.
•
Nitrocellulose membrane is compatible with enzyme immuno assay. Blocking of free protein
binding sites is easy and thus background problems are not observed. No activation of the
membrane is required. However, some proteins (<20 KD) may be lost during post transfer
washes.
•
Zeta-Probe positively charged nylon membrane allow binding of SDS protein complexes in
absence of methanol. These membranes are of choice when elution of high molecular weight
protein or protein having high negative charge is required. Small proteins bind tightly.
The capacity of Zeta-Probe nylon membrane (480 µg/cm2) is much higher as compared to
nitrocellulose membrane (80-100 µg/cm2). Blocking of membrane (Zeta-Probe) is difficult and
results in high background.
References
Kurien, B.T and ScoWeld, R. H. (2006) Western blotting. Methods: 38 (2006) 283–293.
Towbin, H.; Stachelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: procedure and some applications. Proc. Nat. Acad. Sci. USA. 76: 4350-4354.
203
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Typing of Milk for A1 and A2 beta Casein
Sachinandan De, C. M. Hari Kishore, Ayan Mukherjee and Rupinder Kaur
Animal Biotechnology Centre, NDRI, Karnal
Milk contains numerous components of nutritional and health benefit. Calcium is one example.
Milk is also a significant source of dietary fat. An additional risk factor present in some bovine milk
related to the beta casein has been discovered. Initially, three variants of beta casein were discovered
and denoted as A, B and C. It was later found that the A variant could be resolved into A1, A2 and
A3 by gel electrophoresis. The beta casein variants now known are A1, A2, A3, B, C, D, E and F, with
A1and A2 being present in the milk in the highest proportions. The debate about A1 and A2 milk types
has been in the public arena for more than ten years. There have been lots of claims and counter claims
about whether ‘ordinary milk’, which is a mixture of A1 and A2 milk, is linked to a range of disease
conditions, and whether selecting for cows that produce only A2 milk can avoid these problems. We
have successfully developed a method for typing DNA from cells available from the milk. We are
also in the process of detecting A1 A2 beta casein variants from the milk sample. This is an innovative
process for the isolation of DNA from milk and milk products. The DNA samples obtained from
the milk and milk products were used for differentiation of A1 and A2 beta-casein by simple PCR
technique.
Cows’ milk b-casein contains 209 amino acids. The A1 and A2 variants differ only at position
67, which is histidine in A1 or proline in A2 milk. (Another variant B b-casein also has histidine at
positive 67. It is less frequent than A1 or A2 in the milk of cows of European origin.) A bioactive sevenamino-acid peptide, b-casomorphin-7 (BCM-7) can be released by digestion in the small intestine of
A1 b-casein with pepsin, leucine aminopeptidase and elastase but the alternative proline at position
67 prevents a split at this site.
Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-His67
b-casomorphin-7 (BCM-7)
BCM-7 has opioid and cytomodulatory properties. Synthetic BCM-7 can inhibit responses of
lymphocytes to stimulants in vitro (Elliott, 1992; Elliott et al, 1997). Elliott et al (1997) reported that
NOD mice fed A1 b-casein did not develop diabetes if they were also given naloxone (the morphine
antagonist). The antibody response to ovalbumin was prevented in NOD mice if they were also
given injections of (synthetic) BCM-7; this prevention did not happen in Swiss mice. They suggested
that appearance of diabetes in genetically susceptible NOD mice fed A1 bcasein— not those fed A2
b-casein—might be due to release from A1 b-casein of the bioactive peptide, BCM-7 which had a
strong inhibitory effect on immune function.
Some 75% of the world’s 300 million strong dairy herd produces milk that contains the protein
beta casein A1. There is a somewhat controversial claim, backed by 16 years of research, that this
milk, which is drunk by most people in the western world, could be a cause of diabetes, heart disease,
autism and schizophrenia in people with immune deficiencies. It is also claimed that the protein beta
casein A2 is benign in this respect. Cows in the well-known dairy breeds can produce either or both
of the beta casein proteins. They can be A1/A1, A1/A2, or A2/A2. Genotyping has shown that about
80% of Indian (Bos indicus) cows produce only beta Casein A2. In Australia, A2 milk was launched
(A2 Corporation) quietly into the world marketplace. A2’s backers believe it will help prevent disease
and make them fortunes. A1 proponents argue that the evidence against ordinary milk has not been
proved and that they are the victims of a scare campaign. The New Zealand Medical Journal published
a paper in 2003 entitled ‘The influence of consumption of A1 ß-casein on heart disease and Type
204
Typing of Milk for A1 and A2 beta Casein
1 diabetes’, (http://www.nzma.org.nz/journal/116-1168/295/) by Murray Laugesen, and Robert B
Elliott. We all know the well documented and proven benefits of drinking milk which is a mixture
of A1/A2. The general view is that there may be quite some way to go before the hypothesis can be
proved by evidence of cause and effect.
A PCR based method was developed to detect the A1 and A2 beta casein variant forms in cattle
and buffalo milk. Buffalo milk is of A2 type so far the numbers of samples are tested in our laboratory.
Different proportions of A1 and A2 alleles are found in Indian cattle milk. This A1 allele is represented
in heterozygous A1A2 type as well as in A1A1 type. Some animals are homozygous for example
bovines that are A1A1 for Beta casein and those A2A2 for beta casein. In bovine, a mutation in the
DNA sequence coding for the beta casein protein at nucleotide position 200 has resulted in the
replacement of a cytidine base with an adenine base. Thus, the triplet codon affected by this change
codes for histidine (CAT) rather than for proline (CCT) at amino acid position 67 of the protein. Thus,
the histidine at position 67 results in the cow producing beta casein A1 type while the proline results
in the cow producing beta casein A2 type. A high proportion of the common domestic cattle breeds,
such as Holstein, express the beta casein A1type. It was estimated that in the late 1980s more than 70%
of the Californian Dairy herd carried the A1 allele. If the hypothesis of undesirable role of A1 betacasein is confirmed, consumers may wish to reduce or remove this allele from their diet. In this way,
we systematically try to monitor the frequency of beta-casein alleles in bulls and indirectly in cows.
205
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Enzyme-Linked Immunosorbent Assay-Practical
Suman Kapila and Rajeev Kapila
Animal Biochemistery Division, NDRI, Karnal
Enzyme-linked immunosorbent assay, commonly known as ELISA is a heterogeneous EIA based
on the same principle as the radioimmunoassay but depends on an enzyme rather than a radioactive
label. An enzyme conjugated with an antibody reacts with a colorless substrate to generate a colored
reaction product. Such a substrate is called a chromogenic substrate. A number of enzymes are being
used for ELISA like alkaline phosphatase, horseradishperoxidase and β-galactosidase. The specificity,
sensitivity and ease to perform these techniques have made these methods popular. A number of
variations of ELISA have been developed.
Indirect ELISA
Coat well with
Addition of specific
Ag
Ab
Addition of secondary
Ab (Enzyme -conjugated)
Addition of substrate
measure color
Sandwich ELISA
Coat well with
Addition of Ag
Ab
Addition of secondary
Ab (Enzyme -conjugated)
Addition of substrate
measure color
Competitive ELISA
Incubation of antibody
with the Ag
206
Addition of Ag-Ab
mixture
Addition of secondary
Ab(Enzyme -conjugated)
Addition of substrate
measure color
Enzyme-Linked Immunosorbent Assay-Practical
Assay for immunoglobulins in colostrums/milk/serum by sandwich ELISA
Reagents:
Coating buffer: 50mM Carbonate-Bicabonate Buffer, pH 9.6
Washing buffer : 0.05 percent Tween in PBS (PBS/T).
Blocking solution : 1 percent BSA (fraction V) in PBS/T.
Coating antibody : Sheep anti-Bovine IgG
Standard : Bovine reference serum
Detection antibody : Sheep anti-Bovine IgG HRP conjugate
Substrate : TMB/ H2O2 (0.02%) substrate
Stop solution : H2SO4 (2M)
Procedure
Coating
1. Dilute capture antibody at a ratio of 1:100 with coating buffer and add 100ul of diluted capture
antibody to coat each well.
2. Incubate for at least 1h at room temperature
3. After incubation, aspirate the solution of each well and wash the wells three times with washing
buffer.
Blocking
1. Add 200ul of blocking solution to each well
2. Incubate for at least 1h at room temperature.
3. After incubation, aspirate the solution of each well and wash the wells three times with washing
buffer.
Reacting Standards and Samples
For preparation of sample, take 20ml of milk/colostrums, warm it and add 0.5ml of rennet solution
(0.5%). After 10 minutes, filter the coagulated sample using Whatman 42. Take filtrate for quantification
of antibodies.
1. Dilute the standards and samples in blocking solution at 1:2 serial dilutions
2. Transfer 100ul of standard or sample to assigned wells.
3. Incubate for at least 1h at room temperature.
4. After incubation, aspirate the solution of each well and wash the wells five times with washing
buffer..
Detection Antibody
1. Dilute the detection antibody in blocking solution.
2. Add 100ul per well and incubate for 1h at room temperature.
3. After incubation, aspirate the solution of each well and wash the wells five times with washing
buffer.
Colour reaction
1. Add 150ul of substrate solution containing 50ul TMB and 100ul H2O2 to each well. Mix well by
shaking slightly.
2. After incubation for 10-15 minutes at room temperature add 50ul stop solution
3. Using a microtiter plate reader, read the plate at 450nm.
207
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Evaluation of Biological Activity of
Milk Protein Ingredients
Bimlesh Mann, Prerna Saini, Prabhakar Padghan, Anuradha Kumari
Dairy Chemistry Division, NDRI, Karnal
With the growing popularity of high protein dairy products among health conscious consumers,
many dairy manufacturers are looking for ways to boost the protein level of foods such as yoghurt,
dairy beverages and frozen desserts. Milk protein ingredients which include sodium caseinate, whey
protein concentrate (WPC), whey protein isolates (WPI) and milk protein hydrolysates, not only
improve the nutritional profile of dairy foods, but also provide the functionality. From a nutritional
perspective, all the milk ingredients are complete, high quality protein with all the essential amino
acids required for the human nutrition. From functional perspective, WPC and WPI are highly soluble
over a wide pH range and contribute emulsifying, water binding, thickening, foaming, gelling, and
film forming properties to food and beverages system. While the milk protein hydrolysate are fully
soluble and less likely to gel at high concentration in the high protein beverages compared to intact
milk proteins. From the biofunctional point of view, milk proteins are the potential sources of bioactive
peptides with antimicrobial, ACE- inhibitory, cholesterol lowering, antioxidant, immunomodulatory
and opioid properties. These peptides are inactive within the protein sequence and require enzymatic
proteolysis for their release. Bioactive peptides usually contain 3-20 amino acid residues per molecule.
These milk derived bioactive peptides are considered as prominent ingredients for various health
promoting functional foods targeted at heart, bone and digestive system health as well as improving
immune defense, mood and stress control.
1) Antioxidant activity:Free radicals are generated through normal reactions within the body during respiration in aerobic
organisms, particularly vertebrates and humans. In addition to the physiological production of
oxidants and their secondary reactions, there are other sources for production of oxidants. Oxidation
of fats and oils during processing and storage of food products worsen the quality of their lipid content
and nutritive values. Consumption of these potentially toxic products can give rise to several diseases.
Under normal conditions, antioxidant defense systems can remove reactive species through enzymatic
antioxidants like superoxide dismutase and glutathione peroxidase and non-enzymatic antioxidants
such as proteins and peptides, antioxidant vitamins, trace elements, coenzymes and cofactors. The
milk derived bioactive peptides show antioxidant activity by sequestering free radicals, chelating
metal and regulating the level of antioxidant enzymes in body.
Principle:
Based on the principle of interaction of antioxidant with chemically generated ABTS˙ (blue
coloured) oxidant by persulfate oxidation of 2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)
(ABTS2-) indicated by decrease in absorbance at 734nm with concomitant decrease in blue colour of
the oxidant.
Sample preparation:
5% solution of whey protein concentrate preheated at 65°C /30 min is hydrolyzed by using alcalase
at 65°C for 5 hrs by maintaining pH at 8.5. The hydrolysate is centrifuge at 10000 rpm/30 min and
supernatant is collected.
208
Evaluation of Biological Activity of Milk Protein Ingredients
Reagents:
a) Potassium persulphate solution (140 mM)
b) ABTS [2, 2’-azinobis (3 ethyl benzothiazoline)-6-sulfonic acid] stock solution
Dissolve 19.2 mg of ABTS in 5 ml of double distilled water; add 88 µl of 140 mM potassium
persulphate and keep the solution in an amber colour bottle in dark for 12-16 hours.
c) Phosphate buffer saline (pH 7.4)
PBS was prepared by dissolving 8.0 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and 0.24 g of
KH2PO4 in 800 ml distilled water, adjusted pH to 7.4 with 1 N HCl and made the volume to 1 liter with
distilled water.
d) ABTS working solution
Dilute 1 ml of ABTS stock solution with phosphate buffer saline (approx 1:90) till it gives an
absorbance of 0.70±0.02 at 734 nm. The stock solution of ABTS is stable upto 2 days for analytical
purpose.
e) Trolox solution (5 mM)
Dissolve 12.5 mg of Trolox [6-hydroxy. 2, 5, 7, 8 – tetramethyl chroman-2-carboxylic acid] in 10 ml
of ethanol. Dilute with distilled water to varying concentrations (25 - 250µM).
Procedure:
•
Pipette out 3 ml of ABTS working solution in 3 ml cuvette
•
Pipette out 3 ml of phosphate buffer saline in another cuvette
•
Insert the curette into the respective slots in the double beam spectrophotometer
•
Add 10 µl of appropriate diluted sample / Trolox to both reference and ABTS solution
•
Mix the contents for 10 seconds
•
Measure the decrease in the absorbance at 734nm over a period of 10 minutes at 10 sec interval.
•
Plot the standard curve with concentration (µM) of Trolox (X-axis) vs % inhibition (Y-axis)
•
Express the results as trolox equivalent antioxidant capacity (TEAC) values i.e. μM trolox
equivalent / mg of protein using standard curve.
2) Antihypertensive activity:Angiotensin I converting enzyme (ACE; kinases II peptidyldipeptide hydrolase, EC 3.4.15.1) is
important for blood pressure regulation. In the event where decreased blood volume or decreased blood
flow to the kidneys is sensed, renin acts on angiotensinogen to form angiotensin I. ACE then catalyses
the hydrolysis of the inactive prohormone angiotensin I (decapeptide) to angiotensin II (octapeptide).
The result is an increase in blood pressure through vasoconstriction, via increased systemic resistance
and stimulated secretion of aldosterone resulting in increased sodium and water resorption in the
kidneys. ACE also inactivates the vasodilating peptide bradykinin (nonapeptide) and endogenous
opioid peptide Met-enkephalin. Biologically active peptides derived from milk proteins are having an
affinity to modulate blood pressure by inhibit ACE activity.
Principle:
The method is based on the liberation of hippuric acid from hippuryl-L-histidyl-L-leucine (HHL)
catalyzed by ACE.
Sample preparation:
5% solution of whey protein concentrate preheated at 65°C /30 min is hydrolyzed by using alcalase
at 65°C for 5 hrs by maintaining pH at 8.5. The hydrolysate is centrifuge at 10000 rpm/30 min and
supernatant is collected.
209
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Reagents:
Hippuryl- histidyl-leucine (HHL) (5 mM)
10.74 mg of HHL (Sigma, U.S.A) was dissolved in 5 mL of 0.1 M sodium borate buffer (pH 8.3) with
0.3 M NaCl, pH 8.3
Sodium Borate buffer (0.1 M, pH 8.3) containing 0.3 M NaCl
Sodium tetra borate 3.81 g and NaCl 1.75 g were dissolved in 80 mL of distilled water, pH was
adjusted to 8.3 and finally volume was made to 100 mL with distilled water.
Angiotensin converting enzyme (ACE)
ACE from rabbit lung (Sigma, U.S.A) 1 unit was dissolved in 5 mL of distilled water and stored at
-20ºC.
Procedure:
1. Add 20 μl of sample to 110 μl of substrate (in 5 mM HHL in 0.1 M borate buffer)
2. Add 20 μl ACE (4 mU), mix and incubate the mixture at 37ºC for 30 min.
3. Add 250 μl of 1M HCl to terminate the reaction
4. Extract the hippuric acid formed with 1.5 ml ethyl acetate centrifuging at 3000 g for 10 min
5. Dry one ml of upper organic layer by heating at 95ºC for 20min and redissolve in 1 ml of distilled
water
6. Measure the absorbance at 228nm
7. Prepare positive control with distilled water in place of sample
8. Prepared blank with substrate and water (ACE volume is replaced by equal amount of water)
9. Calculate
% ACE =
Express the results as peptide concentration required to inhibit 50 percent of the original ACE
activity (IC50).
3) Caseinophosphopeptides as mineral binding peptides:Caseinophosphopeptides(CPPs) are casein derived peptides contains phosphorus bound
via monoester linkages to seryl residues. They contain a common motif i.e. a sequence of three
phosphoseryl groups followed by two glutamic acid residues Ser (p)- Ser (p)- Ser (p)- Glu- Glu.
These peptides are highly negatively charged structures and soluble at pH 4.6. The highly anionic
phosphorylated regions and the a.a. sequence around this hydrophilic region part play a significant
role in mineral binding and absorption in body. These peptides are able to bind macroelements such
as Ca, Mg and Fe along with trace elements such as Zn, Ba, Cr, Ni, Co and Se.
Principle:
CPPs are soluble at pH 4.6 and they are aggregated with divalent cation such as calcium at neutral
pH and precipitated by using ethanol.
Procedure:
•
Prepare 5 % casein suspension by mixing casein on a magnetic stirrer
•
Adjust the pH to 7 using 0.5 N NaOH.
•
Add enzyme tripsin at Enzyme: substrate ratio of 1:25
•
Hydrolysis is carried out by mixing the suspension using electric stirrer in water bath at 37ºC
for 4 hours
•
The pH of solution is kept constant at 7.0 by addition of 0.1N NaOH solution
210
Evaluation of Biological Activity of Milk Protein Ingredients
•
After complete hydrolysis remove the mixture from water bath
•
Adjust the pH of casein hydrolysate to 4.6 using 2N HCl
•
Centrifuge at 3000 rpm for 10 min to remove the unhydrolyze protein.
•
Collect the supernatant and adjust pH to 7.0 using 2.0 N NaOH.
•
Add calcium chloride at 1% level to the supernatant and allow it for 1 hour at room
temperature.
•
Add Ethanol 50%(V/V)
•
The precipitate is collected by centrifugation at 6000 rpm for 10 min.
•
The CPPs thus obtained is lyophilized.
Flow Diagram for Isolation of CPPs:
Whole casein
Hydrolysis with enzymes at 37ºC
Adjustment of pH to 4.6 with 2N HCl
Removal of unhydrolyze protein by centrifugation
at 3000 rpm /10min
Adjustment of pH to 7.0 using 2N NaOH
Calcium chloride aggregation and ethanol extraction by
Centrifuging at 6000 rpm/10min
Enriched CPPs
211
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Purification of Bioactive Proteins from Milk
Neha Mishra1, Rajesh Kumar2 and Jai K Kaushik1
1
Animal Biotechnology Centre, 2Dairy Chemistry Division, NDRI, Karnal
Colostrum secretion in the mammary gland during the first few days after parturition provides the
calves with nourishment and passive immunity. Differences between relative protein concentration
in colostrum and milk reflect differences in immunoglobin transfer. It is a source of nutrients and
contains many kinds of bioactive molecules which are essential for specific functions. The major whey
proteins are β-lactoglobulin, α-lactalbumin, lactoperoxidase, lactoferrin and immunoglobins etc.
Lactoferrin and immunoglobin G are two of the most important bioactive components in colostrum
and both are contained in over 10 fold higher concentrations in colostrum as compared to normal
milk. Several proteins with antimicrobial activity, such as immunoglobulins, κ-casein, lysozyme,
lactoferrin, haptocorrin, β-lactalbumin, and lactoperoxidase, are relatively resistant against proteolysis
in the gastrointestinal tract. Lactoferrin is nearly 80-kDa glycoprotein belonging to transferin family
with characteristic red color due to iron binding. Apart from being present in high concentration
in colostrum, lactoferrin is also an important component of many external secretions such as saliva,
tears, semen, mucosal secretions and neutrophilic granules of leucocytes. Membrane separation and
chromatography are commonly used techniques for isolation of high purity lactoferrin. Compared
with other chromatographic methods, ion-exchanger is an advantageous technique due to its low cost,
reduced number of steps and easy to scale up. Lactoferrin is subjected to cation exchanger by taking
advantage of its basic nature, as LF has isoelectric point of ~ 9, while major whey proteins alphalactalbumin and ß-lactoglobulin have pI values of 4.2 and 5.4, respectively. Therefore, by employing
weak cation exchanger at neutral pH, lactoferrin is allowed to bind to the resin followed by elution
using a linear gradient of NaCl.
Materials and method
Fresh buffalo or cattle colostrum, 7 gms CM-sephadex per liter colostrum, Tris-HCl - 50mM, pH8.0 equilibration buffer, Tris-HCl (50mM, pH 8.0) + 0.2M NaCl washing buffer, Tris-HCl (50mM, pH
8.0) + 0.5M NaCl elution buffer, Tris-HCl (50mM, pH-8.0) +1M NaCl. Glass column of ca 1.5-2.5 cm
diameter and 50 cm in height can be employed at the first step of purification of lactoferrin in a batch
mode. High resolution purification requires prepacked cation-exchanger like CM-sepharose or MonoS
(GE Biosciences) connected with a medium pressure protein purification system, e.g. AktaPrimer or
Akta Explorer (GE Biosciences). The cation exchanger column and purification system from other
suppliers may also be used without any effect on purification; however in our lab, the protocol for
high resolution purification of lactoferrin has been optimized on HiLoad 16/10 SP-Sepharose high
performance cation exchange column from GE Biosciences.
Procedure
CM-Sephadex (7g/litre) ion exchanger resin is equilibrated with 2 volume of Tris-HCl 50mM,
pH 8.0. 2 liters of fresh colostrum is defatted by centrifuging at 3-5000 rpm for 15 mins. Fat layer can
be removed from centrifuge bottles by spatula followed by filteration of the defatted milk through
a double layered cheese cloth. The skimmed milk is then resuspended in 2-3X volume of 50 mM
Tris-HCl pH 8.0. Pre-equilibrated CM-sephadex matrix (50 mM Tris-HCl pH 8.0) is then added and
lactoferrin and other cationic proteins are allowed to bind to the matrix by continuous manual stirring
for 2-3 hours. Mixture was left overnight on the magnetic stirrer at 4 ºC for effective binding which
was observed by change in color of the gel. Stirring is stopped and the matrix gel is allowed to settle
in the bottom of the vessel. Whey can be decanted carefully without disturbing the gel. The settled gel
is then washed with 3-4 volumes of Tris-HCl 50mM, pH 8.0 until whey has been completely removed
212
Purification of Bioactive Proteins from Milk
from the matrix, which can be packed in the column. Lactoperoxidase and immunoglobins are eluted
by washing the matrix with Tris-HCl 50mM, pH 8.0 + 0.2M NaCl. Lactoperoxidase is eluted as a
blue-greenish layer. This is followed by elution of lactoferrin with 0.5M NaCl with Tris-HCl 50 mM at
pH 8.0. Isolated lactoferrin is then dialyzed in 50mM Tris-HCl pH 8.0 to remove the NaCl, followed
by concentration by using 30kDa ultrafiltration devices (4000rpm for 30 mins) like Centricon from
Millipore or equivalent from other manufacturers. Highly purified lactoferrin can be obtained by
loading it into SP-Sephrose column equilibrated with 0.4M NaCl + 50mM Tris-HCl, pH 8.0 and eluted
through a linear gradient of 0.4M NaCl -0.7M NaCl with 50 mM Tris-HCl pH 8.0. Level of purity of the
sample can be analyzed by SDS-PAGE.
Flow diagram of purification of lactoferrin from colostrum
Fresh colostrum
Centrifuged at 4000 rpm (250-500 ml bottle fixed rotor)
Remove fat by filtering through cheese cloth
Dilute whey 2-3 times with 50 mM Tris-HCl, pH 8.0
Add CM-Sephadex C-50, pre-equilibrated with 0.05M Tris- HCl, pH 8
Stir the gel in the colostrums manually for 2-3 hours, leave it O/N with stirring
Decant the whey, and wash the settled gel with 0.05M Tris HCl pH 8.0
Gel washing followed by decantation is repeated at-least thrice
Proteins like LP and Ig are washed from gel with 0.05 M Tris-HCl / 0.2 M NaCl (pH 8.0)
The lactoferrin is eluted as a red solution with 0.05 M Tris-HCl/ 0.5 M NaCl (pH 8.0).
Lactoferrin eluted at 0.5 M NaCl is pooled, ultrafiltered and passed through Hiload SP-Sephrose
column equilibrated with 0.4 M NaCl/ 50mM Tris-HCl (pH 8.0)
Run SDS-PAGE for evaluating the quality of purified lactoferrin
The purified sample can be desalted for biochemical, biophysical or structural analysis work or
storage at -20ºC
213
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Immunological Method to Detect
Buffalo Milk in Cow Milk
Archana Verma
Dairy Cattle Breeding Division, NDRI, Karnal
Introduction
Since buffalo milk constitutes a major share of total milk production in our country, sale of watered
down buffalo milk in the name of cow milk is commonly practiced. Due to pricing policy in most states,
liking for cow milk by a specific group of users and also for manufacturing some quality products
from cow milk it has to be made sure that there is no admixing of buffalo milk in the milk /products. If
buffalo milk is manipulated in terms of fat percentage and addition of pale yellow coloration, it is very
difficult to distinguish the milk with regards to its species of origin by any physico–chemical method.
The answer to the problem of such manipulations or admixing of milk from different species lies with
a test known as the ‘Hansa Test’ named after the mythological bird ‘hans’, which detects the admixing
of buffalo milk in cow milk. This is based on the principle of antigen-antibody reaction. The antiserum
produced after immunizing rabbit with buffalo casein, gives visible reaction only with buffalo milk.
This is the “Hansa Test Serum” specific to buffalo milk. The test may also be applied to some of the
milk products after reconstitution.
Material required
•
Rabbits (Adult, healthy and preferably male).
•
Centrifuge- High speed upto 12000 rpm.
•
Laboratory (upto 5000 rpm).
•
Autoclave.
•
Homogenizer.
•
General laboratory items and chemicals (centrifuge tubes, beakers, conical flasks, Scalpel blade,
xylene, saline, phenol, slides, pipettes, cotton, tooth-picks, adrenaline 1:50000 I.U etc.).
•
Syringes (2 ml and 5 ml)-needles (21G and 23G).
•
Source of pure cow and buffalo milk.
•
Pure cow casein.
Procedure
The test is carried out in following three steps:
Preparation of antigen
•
Take 50 ml of pure buffalo milk in polypropylene tubes and centrifuge at 3000 rpm for 30
minutes.
•
Pierce the top layer of fat to pour skimmed milk in a beaker.
•
Centrifuge the skim milk at 12000 rpm for 30 minutes.
•
Remove the clear whey and scrap out the packed casein in a clean glass beaker containing
distilled water.
•
Homogenize in a mechanical homogenizer and make the final volume equal to the quantity of
skim milk used.
•
Filter through several layers of muslin cloth and store in a refrigerator under clean sterilized
condition and may be used for one week.
•
Casein, thus prepared, acts as an antigen to produce antibodies against it.
214
Immunological Method to Detect Buffalo Milk in Cow Milk
Immunization of rabbits
•
Select adult and healthy rabbits for immunization. with buffalo casein according to a definite
schedule for a specific period of time (Table).
•
Intra-peritoneal injections are administered taking care that needle(21G) does not pierce the
viscera.
•
Intra-venous injections and blood collection are done through marginal vein of the ear using
23G needle.
•
The injections should be very slow and always use one year for injections and other one for
blood collection.
•
A period of 3 to 6 weeks of immunization is required to get the antisera of desired titre.
Blood collection and testing the titre
•
After immunizing the rabbits for two consecutive weeks, the blood is collected from each rabbit
on the first day of 3rd week, before injection and the serum is separated.
•
Dilute cow and buffalo milk 1:10 with water.
•
Place one drop of diluted milk on a clean slide. Add one drop of serum to be tested and mix
thoroughly with toothpick.
•
Observe the agglutination giving swirling movement to the slide (Figure). Those rabbits, whose
sera give good titre, further immunization is stopped and blood is collected to the maximum
extent to get the antiserum. For other rabbits, injections are continued. In case, any rabbit does
not show titre, suspend immunization after 6 weeks.
•
Sometimes, due to cross- reacting antibodies in cow and buffalo caseins, the titre might be seen
in both the species. In such case, cow casein component of the antisera is absorbed using dried
cow casein, leaving the test valid only for buffalo milk.
Preservation of serum
The anti-serum is preserved without getting its efficacy affected by adding 5% solution of phenol
@ 3% of the volume of the serum.
Precautions
•
For casein (antigen) preparation, utmost care should be taken to use only pure buffalo milk to
get anti-serum specific to buffalo milk adulteration.
•
Intravenous injections should be administered very slowly to avoid shock to the animal. If
the animal shows any sign of shock, 1 ml of adrenaline (1:50000 I.U.) should be given intramuscularly.
•
Store serum at 4ºC.
•
Repeated freezing and thawing of the serum should be avoided.
•
While testing the titre, use separate pipettes for cow milk, buffalo milk and the serum.
•
Homogenize milk products thoroughly before testing.
Benefits of the technology
•
Adulteration of buffalo milk in cow milk (or any other milk) can be detected with accuracy.
•
The test is very fast i.e. less than 30 seconds for one lot of milk.
•
Only one drop of antiserum is required to test whole lot of milk.
•
Benefits of pricing policy may be obtained by cow breeders.
•
Test may be performed with equal efficacy with the formalin preserved milk also.
•
Good and acceptable quality products may be manufactured using pure cow milk whenever
required.
215
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
•
Test is equally effective for milk products as well.
•
The technology is applicable to detect admixing of milk of any species provided the antigen i.e.
casein is injected from that species.
Table: Immunization Schedule
Week of the
Immunization
*Dose of antigen in a week (ml)
**1st Day
2nd Day
3rd Day
I
0.5
0.5
1.0
II
1.0
1.0
1.5
III
1.5
1.5
2.0
IV
2.0
2.0
2.5
V
2.5
2.5
3.0
VI
3.0
3.0
3.5
*The three days should be 3 consecutive days of the
week and the same schedule should be followed for all
the weeks of immunization.
**All the injections are intravenous except the 1st day
of week II to week VI, which are intra-peritoneal.
216
Figure: observations using hansa test
a) Agglutination reaction means Positive Test i.e. the
sample is either buffalo milk or admixed with buffalo
milk
b) Clear solution means Negative Test i.e. no admixing
of buffalo milk.
Conjugated Linoleic Acid and Its Estimation
Conjugated Linoleic Acid and Its Estimation
A. K. Tyagi, A. Hossain, A. Tyagi
Dairy cattle Nutrition, NDRI, Karnal
Introduction
Conjugated linoleic acid:
CLA refers to mixture of positional and geometric isomers of LA (cis-9, cis-12 octadecadienoic acid)
with a conjugated double bond system, instead of the usual methylene-separation. Each double bond
can be of cis or trans configuration giving rise to possible CLA isomers (Kelly et al., 1998). Conjugation
of double bond occurs as part of free radical mediated oxidation of LA. CLA is a true isomer of LA,
in that it does not possess additional oxygen (Vandenberg et al., 1995). The presence of fatty acid with
conjugated double bond was first demonstrated in food products derived from ruminants by Booth
et al. (1935) who observed that fatty acids obtained from summer butter differed from those obtained
from winter butter by exhibiting a much stronger spectrophotometric absorption at 230 mm. It was
subsequently concluded that the absorption at this wavelength was due to a conjugated double bond
pair (Moore, 1939). Parodi (1977) was the first to identify cis-9, trans-11 octadecadienoic acid as a fatty
acid in milk fat that contained the conjugated double bond pair. The discovery of “role of CLA as a
functional food” occurs decade ago when ground beef contained anti-carcinogen factor that consisted
of a series of conjugated dienoic isomers of LA (Pariza et al., 1979 and Ha et al., 1989).
Isomers of CLA:
Numerous isomers of CLA have been identified and these differ by position or geometric orientation
of the double bond pair (Fig. 2.1). CLA includes more than 28 positional and geometrical isomers of
which only cis-9, trans-11 and trans10, cis-12 have thus far been proven to have biological activities
(Banny and Martin 1994; Park et al., 2003). Of the two physically important isomers, c-9, t-11 is the
most prevalent, comprising 80-90% of the total CLA in food products from ruminants where as t-10,
c-12 is present in small amounts at 3-5% of total CLA (Parodi, 2003). The trivial name “Rumenic Acid”
(RA) has been proposed for cis-9, trans-11 CLA due to its unique relationship to ruminants (Kramer et
al., 1998). Other isomer of CLA are present at low concentration, generally representing less than 0.5
percent of the total CLA in ruminant fat.
Sources of CLA:
CLA is present in a great variety of feeds, although usually in residual quantities (Chin et al.,
1992). Food products from ruminants (Table 1) are the major dietary sources of CLA for humans. The
highest CLA concentration was found in adipose tissue (38 mg/g fatty acid) of kangaroo (Engelke et
al., 2004).
Potential health benefits of CLA:
•
Anticarcinogenic
•
Antiatherogenic
•
Altered nutrient partitioning and lipid metabolism
•
Antidiabetic (type II diabetes)
•
Immunity enhancement
•
Improved bone mineralization
217
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Table 1. Total CLA (mg/g fat) content of different types of selected foods
Category of food
Total CLA
(mg/g fat)
Percent of cis-9,
trans-11 isomer
Category of food
Dairy products
Total CLA
(mg/g fat)
Percent of cis-9,
trans- 11 Isomer
Meat (fresh)
Homogenized Milk
5.5
92
Fresh Ground Beef
4.3
85
Butter
4.7
88
Beef Round
2.9
79
Sour Cream
4.6
90
Veal
2.7
84
Plain Yogurt
4.8
84
Lamb
5.6
92
Non-fat Yogurt
1.7
83
Pork
0.6
82
Ice-cream
3.6
86
Poultry (fresh)
Cheddar Cheese
3.6
93
Chicken
0.9
84
Cottage Cheese
4.5
83
Vegetable oils
Mozzarella
4.9
95
Soybean
0.6
25
Sunflower
0.4
38
Seafood (fresh)
Salmon
0.3
—
Mustard
0.3
42
Shrimp
0.6
—
Corn
0.2
39
Source: Chin et al. (1992)
Biosynthesis of CLA:
Kepler et al. (1966) had identified CLA as the first intermediate of linoleic acid biohydrogenation
in the rumen by Butyrivibrio fibrisolvens. Griinari and Bauman (1999) concluded that CLA synthesis
occurred in the rumen only. CLA found in milk and meat of ruminants originates from two sources.
One source is CLA formed during ruminal biohydrogenation of linoleic acid. The second source is
CLA synthesized by animal tissues (mammary gland epithelial tissue) from trans-11 C18:1 or TVA,
another intermediate in the biohydrogenation of unsaturated fatty acid. Hence, the uniqueness of CLA
in ruminant edible products relates to incomplete biohydrogenation of dietary unsaturated fatty acids
in the rumen.
Estimation of conjugated linoleic acid in milk
a) Extraction of fat
Fat is extracted from milk by the method of Ha et al. (1989). Fresh milk (3ml) is vortexed with 3 ml
of methanol and 1.5 ml of chloroform. The mixture is vortexed with 1.5 ml chloroform for an additional
2 min. The homogenate is centrifuged at 2200 rpm for 10 min. The upper (methanol-water) layer is
removed through aspiration and the bottom layer (chloroform layer) is passed through anhydrous
sodium sulphate on Whatman filter paper No.1.The filter paper is rinsed with 3 ml of chloroform and
the extract is evaporated to dryness under vacuum and then under the stream of nitrogen.
b) Hydrolysis of fat
Extracted fat is hydrolyzed with 1 ml of 1N methanolic sodium hydroxide in a boiling water bath
for 15 minutes and then cooled to room temperature for 5 minutes. 1 ml Hydrochloric acid (2N) and
2 ml chloroform are added to the tube containing methanolic sodium hydroxide and vortexed for 4
minutes, followed by centrifugation for 10 minutes at 2200 rpm. The organic layer (lower layer) is
collected and evaporated to dryness under vacuum and then under the steam of nitrogen.
c) Preparation of standard
A stock solution of CLA (1mg/ml) in acetonitrile is prepared. A working standard solution is
prepared by adding (500ml) stock solution to 2000 ml of acetonitrile and it gives 4mg of CLA in 20ml
of standard to be injected.
218
Conjugated Linoleic Acid and Its Estimation
d) High performance liquid chromatography (HPLC)
HPLC conditions:
Column
:
C18 micro Bondapack
Flow rate
:
1.5 ml per minute
Wave length
:
234 nm
20 µl.
Inject volume :
Eluent used for the separation of CLA consists of acetonitrile containing 0.12 per cent glacial acetic
acid (v/v) and double distilled water in the ratio of 70:30.
The peak of CLA is eluted at 13 to 18 min. The retention time of samples are compared with that
of standard CLA (Sigma Chemical Co., St. Louis, MO, USA).
CLA estimation by Gas chromatography (GC)
Estimation of CLA and other fatty acids in feedstuffs, plasma, ruminal liquor, milk and muscle
are analyzed as per direct transestrification method of O’Fallon et al. (2007) with slight modification
using GC fitted with flame ionization detector. For the methyl ester formation 1 g feedstuff, 1.5 ml
plasma, rumen liquor, milk and 1.5 g muscle samples are taken.
Preparation of standard
A stock solution of CLA (1mg/ml) in acetonitrile is prepared. A working standard solution is
prepared by adding (500µl) stock solution to 2000 µl of acetonitrile and it gives 4µg of CLA in 20µl of
standard to be injected.
Conditions
Oven temperature
:
Initial 15°C, Final 24ºC
FID
:
26ºC
Injector
:
24ºC
Flow rate
:
30 ml/min.
Attenuation
:
1
Split ratio
:
1:10
Inject volume
:
0.5 µl
Helium can be used as a carrier gas at constant inlet pressure (205 kPa). Conjugated linoleic acid
is identified by comparing its retention time with that of standard CLA and concentration of CLA is
calculated considering the peak area.
Afterword
The evident beneficial potential of CLA along with other PUFAs augmented interest in its
enhancement in milk and meat products as a consequence. This has caused a great deal of effort
to be expended toward increasing the concentration of CLA in the milk and tissues of ruminant
foods because these are the predominant source of CLA in human diets. Among more than a dozen
isomers of CLA detected in foods of ruminant origin, c-9, t-11, t-10 and c-12 are the ones with known
physiological importance. While c-9, t-11 comprises 80 to 90% of total CLA and the major source of
its occurrence is endogenous synthesis via desaturation of VA by Δ9-desaturase. , t-10, c-12 comprises
3 to 5% of the total. As has been shown in many studies reported in the limited review above, there
are several ways to increase CLA levels in milk and meat products from ruminants, hence, products
with enhanced CLA content which can effectively discharge their beneficial role in humans can be
219
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
designed, however, To date, statements about health promoting effects of CLA are mainly based on
animal trials and remain to be proven largely in humans. In human trials synthetic CLA supplements
are usually used and these do not reflect natural isomer composition in foodstuffs. Whether natural
CLA sources (meat and milk from ruminants) have a similar impact on human health warrants further
research.
Refrences
Kelly ML, Kolver E S, Bauman DE, Van Amburgh ME, Muller LD (1998) Effect of intake of pasture on concentrations of
conjugated linoleic acid in milk of lactating cows. J Dairy Sci 81:1630–1636
Booth RG, Kon SK, Dann WJ & Moore T (1935) A study of seasonal variation in butter fat. A seasonal spectroscopic
variation in the fatty acid fraction. Biochem J 29, 133-37.
P. Parodi, Conjugated Linoleic Acid: an anticarcinogenic fatty acid present in milk. Australian Journal of Dairy Technology
49 (1977), pp. 49-93.
Pariza PW, Ashoor SH, Chu FS & Lund DB (1979) Effects of temperature and time on mutagen formation in panfried
hamburger. Cancer Lett 7, 63-69.
Ha YL, Grimm NK, Pariza MW (1989) Newly recognized anticarcinogenic fatty acids: identification and quantification
in natural and processed cheese. J Agric Food Chem 37:75-81
Parodi, P., 2003. Conjugated linoleic acid in food. In J. Sebedio, W.W. Christie and R. Adolf (ed) Advances in Conjugated
Linoleic Acid Research, Vol. 2, pp: 101-121. AOCS Press, Champaign, IL.
Banny, S and Martin, J.C. 1994. Conjugated Linoleic Acid and metabolites in trans fatty acids in human nutrition, The
oily Press, Dundee, Scotland : 261-302.
Park, S.J., Park, C.W., Kim, S.J., Kim, J.K., Kim., Y.R. Kim, Y.S and Ha, Y.L. 2003. Divergent cytotoxic effects of Conjugated
Linoleic Acid isomers on NCI-N87 cells, ACS Symp. Series, 85:1113-118.
Chin, S.F., Liu, W., Storkson, J.M., Ha, Y.I. and Pariza, M.W. 1992. Dietary sources of conjugated dienoic isomers of
linoleic acid, a newly recognized class of anticarcinogens. J. Food Composition Analysis, 15: 185-197.
Kepler, C.R., Hirons, K.P., McNeil, J.J. and Tove, S.B. 1966. Intermediates and products of the biohydrogenation of
linoleic acid by Butyrivibrio fibrisolvens . J. Biol. Chem. 241: 1350–1354.
Griinari, G.M. and Bauman, D.E. 1999. Biosynthesis of conjugated linoleic acid and its composition, incorporation in to
meat and milk in ruminants. Advance in CLA research. AOCS Press, Champaign,II. Pp: 180-200.
O’Fallon, J.V., Busboom, J.R., Nelson, M.L. and Gaskins, C.T. 2007. A direct method for fatty acid methyl ester synthesis:
Application to wet meat tissues, oils and feedstuffs. J. Anim. Sci. 85: 1511-1521.
Vandenberg, J.J., Cook N.E. and Tribble, D.L. 1995. Reinvestigation of the antioxidant properties of Conjugated Linoleic
Acid. Lipids, 30: 599-605.
Moore, T., 1939. Spectroscopic changes in fatty acids. VI. General. Biochem. J. 33: 1635-1638.
Kramer, J.K.G., Parodi, P.W., Jensen, R.G., Mossoba, M.M., Yurawecz, M.P., and Adlof, R.O. 1998. Rumenic acid: A
proposed common name for the major conjugated linoleic acid isomer found in natural products. Lipids. 33: 835.
Engelke, C.F., Siebert, B.D., Gregg, K., Wright, A.D.G. and Vercoe, P.E. 2004. Kangaroo adipose tissue has higher
concentration of cis-9, trans-11 conjugated linoleic acid than lamb adipose tissue. J. Anim. Feed Sci., 13: 689-692.
220
Importance and Estimation of Vitamins A & E In Value Added Dairy Products
Importance and Estimation of Vitamins A & E
in Value Added Dairy Products
Harjit Kaur
Dairy Cattle Nutrition Division, NDRI, Karnal
Vitamin A, or retinol, is a colorless, alcohol compound. It is essential to the immune system,
providing antioxidants that benefit growth, healing, reproduction and skin. If this vitamin is deficient,
the epithelium becomes keratinized and cracks occur, giving easy access to bacteria and viruses,
resulting in infectious diseases. Vitamin A plays an important role in the chemical processes which
occur in the eye and are essential for vision. This vitamin combines with proteins in the retina of the eye
and forms the pigment called visual purple. These cells, together with the lens pigment, are responsible
for vision in dim light. During this process, some of the vitamin A is excreted and has to be replenished
from the blood, if normal vision is to be maintained. Vitamin A affects bone development through
its effect on bone metabolism. Essentially, Vitamin A deficiency results in unchecked bone growth
that in turn manifests as malformed bones and joints. Vitamin A directly affects immunity through
both production of antibodies and through maintaining an adequate barrier to infection with healthy
epithelial cells. Its deficiency also affects reproduction by interfering with the production of sperm
in males, and by causing resorption of the foetus in females. The symptoms of vitamin A deficiency
are scouring, low resistance to bacterial infection, stiffness of joints and uncoordinated movements,
lesions around the eyes and dull watery eyes followed by night blindness at more advanced stages.
Excess vitamin A has been demonstrated to have toxic effects in most species.
VITAMIN E: Vitamin E has very strong antioxidant properties and is involved in the mammalian
antioxidant defense system where it stimulates the immune response. For certain purposes, the
antioxidant functions of vitamin E can be performed by Se, which is present in glutathione peroxidase
and decomposes peroxides. Most species hydrolyze dietary tocopheryl esters effectively at the mucosal
surface of the small intestine. Vitamin E is absorbed as the free alcohol, tocopherol. The vitamin is
insoluble in the aqueous environment of the intestinal lumen. Its enteric absorption, like that of other
fat-soluble nutrients, therefore is dependent upon its micellar solubilization. Consequently, impairment
of pancreatic function or bile production results in impaired absorption of vitamin E. The efficiency
of absorption of tocopherols is relatively low at 20 to 40 percent. Absorption is increased by mediumchain triglycerides and is decreased by high levels of linoleic acid. In mammals, absorbed tocopherol is
transported by chylomicrons via the lymphatic circulation to the liver and subsequently to the general
circulation in very low density lipoproteins (VLDL). Most species show normal plasma α-tocopherol
concentrations in the range of 1-5 μg/ml. The dietary requirements for vitamin E are estimated in the
range of 5 to 50 IU/kg of diet for most animal species. Vitamin E is generally considered to be one of
the least toxic of the vitamins. Dietary intakes of at least 20 times the nutritionally adequate levels
should be well tolerated by most species.
Estimation of vitamins A and E by HPLC
Evaluation of vitamin A in dairy products is highly required because of its important roles in
vision, maintenance of epithelial lining and immunity in man and animals. Vitamin E (α-tocopherol),
another non enzymatic antioxidant, is involved in maintenance of immunity status Due to the critical
role of these vitamins, their quantitative analysis is very important to know their content in the diet as
well as to know the changes in their concentration under different storage and processing conditions.
High performance liquid chromatography is extensively used for measuring vitamins in milk
and milk products. Earlier methods of vitamin estimation such as, colorimetric lack the ability to
221
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
differentiate between the vitamin congeners with varying activity. In milk, vitamin A occurs mainly
as a mixture of fatty acid retinyl esters. β-carotene, the provitamin A, is the predominant form of
vitamin A in the milk. Evaluation of vitamin A and β-carotene together enables the evaluation of total
vitamin A activity of the milk. The major form of vitamin E in the milk is α-tocopherol. Vitamins
A, β-carotene and vitamin E are fat soluble vitamins and are found in the fat fraction of the milk. It
is therefore necessary to extract the vitamins from the sample. Saponification (alkaline hydrolysis)
provides an effective means of removing the preponderance of neutral lipids (mainly triglycerides)
from the sample. Saponification involves refluxing the sample with ethanolic KOH solution and added
antioxidant for 30 min. The hydrolysis attacks ester linkages and releases the fatty acids from the
glycerol moiety of glycerides and phospholipids and from esterified sterols and carotenoids. Hence
ester forms of the vitamins are hydrolysed to their respective alcohols which allow the estimation of
total vitamin content in the sample.
1. HPLC method for simultaneous estimation of retinol (Vitamin A), β-carotene and α-tocopherol
(Vitamin E) (Chawla and Kaur, 2001)
Principle
The sample is saponified with ethanolic potassium hydroxide solution and vitamins A, β-carotene
and vitamin E are extracted into petroleum ether. The petroleum ether is removed by evaporation and
the residue is dissolved in mobile phase. The vitamins A, β-carotene and vitamin E concentrations are
determined simultaneously by reverse-phase liquid chromatography.
1. Reagents : Potassium hydroxide solution, 60%, Ethanol, 95 % , Petroleum ether, boiling
range 40º C to 60º C, 0.5 N potassium hydroxide, All-trans-retinol, vitamin A alcohol, α-tocopherol,
β-carotene, Water, HPLC grade,Methanol, HPLC grade,Acetonitrile, HPLC grade, Tetrahydrofuran,
HPLC grade, Ascorbic acid, Whatman phase separator filter paper, Inert gas, nitrogen.
Preparation of Standards: Prepare stock solutions of α-tocopherol (300 µg/ml) and retinol (30 µg/
ml) in 100 % ethanol. Prepare stock solution of β-carotene (30 µg/ml) in chloroform. Take requisite
aliquots of individual stock solutions in amber coloured tubes and dry under nitrogen at room
temperature. Reconstitute the dried standards in mobile phase (mobile phase is prepared by mixing
acetonitrile, tetrahydrofuran and HPLC water in the ratio of 47: 42:11). Prepare a working standard
solution containing 100µg/ml α-tocopherol, 10µg/ml retinol and 10 µg/ml β-carotene, at the time of
use. Store all the vitamin stock standards at –20ºC.
Extraction of samples: Take 2-3 ml milk in a 50 ml stoppered test tube. Add 5 ml of absolute
ethanol containing 0.1% (w/v) ascorbic acid or 1% pyrogallol (w/v) and 2ml of 50% KOH. The tubes
are agitated carefully and placed in a water bath at 80ºC for 20 min. After saponification, cool the
tubes with running water and place in an ice water bath. Add 10 ml petroleum ether (40-60ºC) and
shake for 15 minutes. Transfer the upper ether layer in another tube. Repeat the extraction thrice and
collect the ether portion. Transfer the combined ether extract to a separating funnel, wash with 10 ml
of 0.5 N KOH and subsequently with distilled water (2-3 times) to remove excess alkali. Pass the ether
extract through phase separator filter paper to remove water, if any. Evaporate the ether extract under
nitrogen in a water bath maintained at 37°C. Perform all the extractions under subdued incandescent
light using amber coloured glassware.
HPLC system and procedure: The HPLC system consists of a model 510 pump, UV visible
absorbance detector 486, rheodyne injector with 20 µl loop, using multiwavelength detector. A reverse
phase Discovery C-18 (15 cm x 4.6 mm) column is used. The flow rate is 1.5ml/minute. The programme
for the separation of retinol, α-tocopherol and β-carotene using millennium software method is given
in Table 1.
222
Importance and Estimation of Vitamins A & E In Value Added Dairy Products
Fig. 1 Chrotomatogram of standard Retinol, α-Tocopherol and β-Carotene
Table 1. Program for HPLC analysis of Retinol, α-Tocopherol and β-Carotene
Vitamin
Wavelength (nm)
Change Time (min)
Retention time (min)
Retinol
325
0.00
1. 73
α-tocopherol
290
2.5
3.37
β-carotene
450
4.5
5.67
Reconstitute the residue in the mobile phase prior to injection on HPLC. Filter the reconstituted
extract through a 0.45-µm filter and inject 20 µl into the HPLC column. The run time is 6 minutes
per sample. Measure the area of the retinol, α-tocopherol and β-carotene peaks. Determine their
concentrations in the extracted sample with reference to the peak area of respective standards.
2. Simultaneous estimation of vitamins A and E in milk by HPLC
Follow the same procedure as mentioned above (for simultaneous estimation of α-tocopherol,
retinol and β-carotene) upto extraction of samples. Prepare mobile phase by mixing methanol and
Fig. 2 Chromatogram of standard retinol and α-tocopherol
water in the ratio of 95:5 and filter through a membrane filter.
Separation of Vitamins A and E
Reconstitute the dried residue in Table 2: Program for HPLC analysis of retinol and α-tocopherol
the mobile phase and filter through
0.45µ membrane filter for injection Vitamin
Wavelength Change Time
Retention time
(nm)
(min)
(min)
on HPLC column. Inject 20 µl of
sample extract onto the column of the Retinol
325
0.00
1.98
liquid chromatograph. A programme
4.00
7.35
α-tocopherol 290
for the separation of retinol and
α-tocopherol at different wavelengths
simultaneously is presented in Table 2.
The chromatographic separation of vitamins A and E is shown in Fig. 2.
223
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Calculate the mean peak area from replicate injections of the sample extract and determine the
retinol and α-tocopherol concentrations of the extract by reference to the mean peak area found from
replicate injections of standards. Table 3 shows the average values of vitamin A and E in the milk of
different species.
Table 3. Vitamin A and E content of milk /serving (244 g)
Vitamin
Cow Milk,
3.25% fat
Cow,
Skim milk
Goat
Sheep
Buffalo
A, ug
68
149
139
108
129
E, mg
0.15
0.02
0.17
ND
ND
Reference
Chawla, Rajiv and Harjit Kaur (2001). Isocratic HPLC method for simultaneous determination of carotene, retinol and
tocopherol in feeds and blood plasma. Indian J. Dairy Sci., 54: 84 - 90.
224
Use of Atomic Absorption Spectrophotometer for the Estimation of Minerals in Milk and Milk Products
Use of Atomic Absorption Spectrophotometer for the
Estimation of Minerals in Milk and Milk Products
Veena Mani
Dairy Cattle Nutrition, NDRI, Karnal
By atomic absorption spectrophotometer, the metals in water /organic sample can be analyzed. It
is the highly sensitive technique highly useful for the determination of the presence and concentrations
(even at very low levels) of metals in liquid samples. Thus, prior to the estimation the solid samples
should be accurately weighed and then dissolved often using strong acids. Metals include Ca, mg
,Fe, Cu, Al, Pb, Zn, Cd and many more. Typical concentrations range in the low mg/L (ppm) range.
As the elements in the sample to be analyzed are not in the free state but are combined with other
elements invariably to make a so-called molecule. For the analysis the combination must be cut off by
some means to free the atoms. This is called atomization. The most popular method of atomization
is dissociation by heat. Samples are heated to a high temperature so that molecules are converted
into free atoms. This method is classified into the flame method, in which a chemical flame is used as
the heat source; and an electro thermal atomization method, in which a very small electric furnace is
used
The technique is designed to determine the amount (concentration) of an object element in a
sample, utilizing the phenomenon that the atoms in the ground state absorb the light of characteristic
wavelength passing through an atomic vapour layer of the element of interest and attain excited
states. During excitation element being analyzed is dissociated from its chemical bond and is placed
in an unionized state. This is normally achieved by aspirating the sample into the flame or graphite
furnace. Each metal has a characteristic wavelength that will be absorbed. . The AAS instrument
looks for a particular metal by focusing a beam of UV light at a specific wavelength through a flame
and into a detector. The instrument measures the change in intensity As a result of absorption,
the intensity of light decreases, which is proportional to the number of the examined atoms being
present. The more concentrated the solution, the more light energy is absorbed!
In atomic absorption, the method is based on the attenuation (weakening) of a beam of nearly
monochromatic light as a consequence of its interaction with and partial absorption by the ground state
atoms of the element being analyzed. The amount of light absorbed at the characteristic wavelength
increases with the number of atoms of the selected element in the light path. By comparison with
suitable standards, the concentration of the element in the sample can be inferred from the amount of
light absorbed. A computer data system converts the change in intensity into an absorbance.
Quantitative analysis by Atomic absorption depends on: 1)accurate measurement of the intensity
of the light ,2) the assumption that the radiation absorbed is proportional to atomic concentration
Instrumentation
There are six basic components of an atomic absorption spectrometer
1. A light source(hollow cathode lamp), which emits a beam of radiation of a wavelength
characteristic of the element to be determined.
2. An "absorption cell" or atomizer section for atomizinig the sample, in which the sample solution
is reduced to a cloud of ground state atoms, for example a flame or graphite furnace.
3. A monochromator to select the wavelength that is being absorbed by the element to be
measured.
4.
A photomultiplier tube ,which is a detector for converting the light into an electrical signal.
Thus, it detects and measures the intensity of the resultant beam of radiation. The PMT
determines the intensity of photons of the analytical line exiting the monochromator
225
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
5. An amplifier and associated electronics to amplify and process the signal from the photomultiplier
tube.
6. A display and/or a recorder which shows the
measured signal after it has been processed.
Hollow cathode lamp To produce the proper
monochromatic light necessary for the AAS, so called“hollow
cathode lamps” are used which is situated in the centre of the
lamp and is normally composed of either the pure form of
the element or an alloy of the element It means that different Schematic Diagram for atomic absorption
lamps are used for the determination of each element
spectrophotometer
(source
:http://www.
thebritishmuseum.ac.uk/science/techniques/
sr-techaas.html)
It is named after the cylindrical shape of the cathode
that gives direction to emerging beam, and helps re-deposit
sputtered atoms back on cathode. The hollow cathode lamp for the light source consists of a hollow
cathode and an anode(made of tungsten) enclosed in a glass (quartz) tube and neon or argon gas is
filled at around 10 Torr. in pressure in it. Being made up of the element to be measured or its alloy,
it emits the light its wavelength is equal to that absorbed by the atoms of the sample. These lamps
are available for about 70 elements. They are generally reliable and most have operating lives well in
excess of 5000 mA hours. The life of lamps with cathodes constructed of alloys of volatile elements
such as arsenic or selenium is shorter. Multi-element lamps are also available and can be useful both
from the point of view of economy in lamp numbers and also in reduced warm-up time.
Absorption Cell/Nebulizer
It sucks up liquid sample at a controlled rate. Create a fine aerosol spray for introduction into the
flame. It mixes the aerosol and fuel and oxidant thoroughly for introduction into the flame
The following atomization methods are known:
1) Flame atomization 2) Graphite furnace atomization
Flame: The source of atoms is usually flame (“flame atomisation”). Metals could be measured at
ppm concentration (part per million, that is mg kg-1 or mg dm-3 in case of dilute solutions). The most
commonly employed technique in atomic absorption spectrometry is that in which the sample, either
as a liquid or in solution, is sprayed into a flame as a mist of droplets. The liquid or solution is reduced
to mist by being drawn through a pneumatic nebulizer which normally employs an impact bead.
Once in the flame, the liquid droplets are dried to give solid particles which are then decomposed to
give molecules in the gaseous phase. The molecules then dissociate to give free atoms. Only about
10% of the solution actually reaches the flame. This is one reason for the limited sensitivity of flame
techniques. The other important reason for the limited sensitivity is the rather short residence time of
free atoms in the flame (and especially in the light path).The sensitivity, however, could be increased
when the light travels for longer in the flame. Therefore most of the burners are about 5-10 cm long.
The air/acetylene flame is the most used type of flame which provides a temperature high enough
for the determination of many elements. There is no considerable ionization in the flame (with the
exception of the alkali elements) and almost no absorption at wavelengths above 230 nm. Furthermore
light emission by this flame is rather low. The nitrous oxide/acetylene flame with its high temperature
is recommended for the determination of elements which need a high energy for dissociation (that
is, formation of free atoms). For those elements, this flame provides suitable chemical, thermal and
optical conditions. Background emission, however, is rather high at certain wavelengths and the high
temperature leads to a considerable risk of ionization for certain elements. The air/propane or air/
hydrogen flames are mainly recommended for the analysis of alkali elements since the temperature
is low enough to prevent larger ionization effects. The hydrogen/argon-diffusion flame is especially
suitable for the determination of arsenic and selenium, since its absorption at wavelengths below
200 nm is much smaller than absorption of other flame types. Due to its low temperature, chemical
226
Use of Atomic Absorption Spectrophotometer for the Estimation of Minerals in Milk and Milk Products
interferences must be expected.
Fuel gas Oxidant Flame temp. (ºC)
Graphite furnace atomisation The graphite Fuel Gas
Oxidant
Flame Temp. (ºC)
furnace AAS (GFAAS), a more recent technique is Hydrogen
Argon/ air(diffusion)
400 (350-1000)
even more sensitive than the traditional, cheaper
Propane
Air
1930
AAS using flame. Measurements could be done at
Hydrogen
Air
2000-2050
ppb level (part per billion, ppb = 10-3 ppm, that
Acetylene
Air
2100-2400
is µg kg-1 or µg dm-3 in case of dilute solutions!).
Nitrous oxide
2650-2800
Since the presence of toxic heavy metal i.e as Acetylene
contaminants even in very low concentration may
be of concern from human health point of view, therefore the technique has the importance with this
reference.
In graphite furnace AAS, a heatable graphite tube as atomization device is located in the ray
path. A droplet of the sample is pipetted into the graphite tube, where it dries through electrical
heating and the residues are ashed. The temperature of the tube can be increased in a stepwise
fashion such that in the first stage, the tube will be heated to a relatively low temperature in order
to vaporize the solvent. In the second stage, the temperature will be increased by increasing the
current such that the solid residue is dry ashed without the loss of analyte. Finally, the tube is rapidly
heated to a temperature of up to 3000°C in order to atomize the analyte. The heated graphite furnace
provides the thermal energy to break chemical bonds within the sample held in a graphite tube, and
produce free ground state atoms. Ground-state atoms then are capable of absorbing energy, in the
form of light, and are elevated to an excited state. The amount of light energy absorbed increases as
the concentration of the selected element increases.The atomic absorption signal is then measured at
this stage. A purge gas of argon or nitrogen is passed through the tube during the drying and ashing
stages but normally the flow of inert gas will be stopped during the atomization stage so that the
free atoms will remain in the absorption cell for a longer period. It is important to control the drying
and ashing stages such that drying comes about without spitting or spreading of the sample and
that ashing occurs in such a way as not to interfere with the final atomization stage.
The use of automatic background correction is essential when using a graphite furnace, since the
level of non-specific background absorption is much more significant than is the case with the flame
atomization. Matrix effects can be compensated for by the method of standard additions and/or
by the addition of matrix or analyte modifiers. The addition of analyte modifiers usually decreases
the volatility of the analyte so that it is not lost during the ashing stage or increases the volatility
of the matrix making it more readily removable Matrix modifiers, on the other hand, decrease the
volatility of interfering compounds and contribute to time resolved analyte/background signals.
Monochromator: the main purpose of monochromator is to isolate the absorption lines from
the background light
due to the interferences. Comparison of Flame and Electrothermal atomization method
Thus,
monochrometer
Flame Atomization
Electrothermal Atomization
is used for selecting the
ppm level in the solution
ppb level in the solution
analysis wavelength of Sensitivity
about 1mL for one analysis
5 - 50 µL for one analysis
the target element, and Sample Volume
a detector for converting Atomizing efficiency about 10%
More than 90%
the light into an electrical
Matrix effect
Small
large
signal. Thus,it Isolate the
10 - 30 sec. For one sample 2 - 5 min. for one sample
analytical line photons Time for analysis
passing through the flame
and remove scattered light of other wavelengths from the flame In doing this, only a narrow spectral
line impinges on the PMT.
227
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Photomultiplier tube (PMT)
This is the detector. The PMT determines the intensity of photons of the analytical line exiting the
monochromator. The PMT is the most commonly used detector for atomic absorption spectroscopy.
They consists of a photocathode and a series of dynodes in an evacuated glass enclosures. However,
solid state detectors are now replacing conventional vacuum-type photomultipliers. High tech
electronics amplify, filter, and process the electrical signal, using a series of chips and microprocessors,
transmitting the result to an internal or external computer which handle all data-handling and
display
Analysis of minerals in milk and milk products using Atomic absorption spectrophotometer
They can be divided into macro minerals (major elements) and micro minerals (trace elements).
The macro minerals such as sodium, potassium, magnesium, calcium and phosphorus are required
by the body in amounts greater than 100mg per day whereas the micro minerals such as iron, copper,
zinc and manganese are required in amounts less than 100mg per day.
Sampling and processing of samples
The processing of milk/ products samples for mineral analysis can be done dry ashing or wet
digestion, since milk is not totally homogenous therefore, it is utmost important that bulk sample
should be sufficiently homogenized to ensure that the aliquot/sub-sample which is taken for analysis
is representative of the whole. The size of the sample should be proportional to the bulk. Thorough
mixing of sub-samples from a large bulk is preferred in representative sampling
(a) Dry Ashing Generally, 5 to 25 ml sample (depending upon the concentration of the desired
mineral) is taken in a silica crucible and weighed. For powdered milk/product, 1-2 gm sample is
taken. Then, the sample is placed in the muffle furnace and the temperature is brought to 550ºC and
held for 4 hr. After cooling, the ash obtained is dissolved in dilute HCl (6M) and then made upto
suitable volume. The resulting solution is used in mineral element determination.
Advantages and Disadvantages: Dry ashing is a convenient and versatile method using relatively
large sample size for the sample preparation. It also minimizes the contamination due to reagents.
Because ,the ashing is usually done between 400-600ºC, therefore, some elements such as Se, Pb, As
and Hg, are lost through volatilization or by adsorption on the walls of the crucible. Other metals such
as, tin may form un-soluble refractory compounds during ashing
(b) Wet digestion: Wet digestion procedure requires the use of strong oxidizing acid mixture
of nitric, sulphuric and perchloric acid. A mixture of HNO3 and HClO3 is useful for many routine
applications. The use of mixtures containing H2SO4 is particularly useful when sample containing
fats are to be oxidized. The addition of H2SO4 increases speed of wet oxidation process as it raises
the temperature of digestion. Extra care should be taken to avoid charring of samples when elements
like As, Se or Hg are to be determined. It is essential that HNO3 should be in excess at all time during
digestion. Even H2SO4 and HNO3 mixture can be used, but complete digestion of fat may take hours.
Generally, milk (10-20ml) or 1-2 g milk product is taken in 60 ml test tubes/ kjeldahl flask and
weighed. To this, 10 ml triacid mixture (HNO3: HClO4: H2SO4:: 3 : 2 : 1) is added. Tubes are heated till
the contents become clear and perchloric acid fumes cease to come out. The volume is made to 25 ml
with double distilled water.
Advantages/ Disadvantages: When compared with dry ashing, this method gives better recovery
for most of the elements as there is less danger of loss of volatile elements (Pb, Cd, As). But the chances
of contamination are high as relatively large volumes of reagents are required to be added. The other
drawback with this method is that it is suitable only for small size samples.
Analysis
HCl extract or wet digested samples after suitable dilution are used for the estimation of major
and trace elements by atomic absorption spectrophotometer.
228
Use of Atomic Absorption Spectrophotometer for the Estimation of Minerals in Milk and Milk Products
•
Take 1 ml of digested sample / HCl extract in a clean test tube.
•
For Ca estimation, add 1 ml 2% SnCl2 to 1ml of digested sample to eliminate interfering elements
(such as Al, Be, P, Si, Ti) and make the volume to 10 ml with doubled distilled water. The
concentration of 0.2% Sn is required in the test sample as well as in standards. This sample
is ready for estimation on AAS at 422.7 nm wavelength using acetylene as fuel and air as an
oxidant.
•
For Mg estimation, add 1 ml 2% SnCl2 to 1ml of digested sample to eliminate interfering
elements (such as Al, Be, P, Si, Table 1 Conditions for various minerals
Ti) and make the volume to 10
ml with doubled distilled water. Mineral
Wavelength
Lower range
Upper range
(nm)
(ppm)
(ppm)
The concentration of 0.1% Sn is
required in the test sample as Calcium
422.7
1.8
18
well as in standards. This sample
Magnesium
285.2
0.06
0.6
is ready for estimation on AAS
589
0.26
2.6
at 285.2 nm wavelength using Sodium
acetylene as fuel and air as an Potassium
766.5
0.24
2.4
oxidant.
Iron
248.3
0.5
8
•
•
For other mineral the estimations
Copper
324.7
are carried out in the and suitably
213.9
diluted digested sample. The Zinc
conditions for the estimation Manganese
279.6
and range for preparation of
standards of different minerals are given in Table 1.
0.08
8
0.5
5
0.58
5.8
Plot the standard curve for a particular mineral and find out the concentration in the unknown
sample from the standard curve considering dilution factor. Express the concentration as percent
in case of Ca and Mg and ppm for other minerals.
The samples and standards are often prepared with duplicate acid concentrations to replicate the analyte's
chemical matrix as closely as possible. Acid contents of 1% to 10% are common. High acid concentrations
help keep all dissolved ions in solution.
Preparation of standards for some trace minerals of nutritional significance
Zinc standard stock solution, 1 mg/ml - Take 0.25 g pure zinc metal in 250 ml volumetric flask add
about 50 ml distilled water and then add 1 ml sulphuric acid. Heat to dissolve Zn. Dilute to volume and
store in pyrex bottle. Or dissolve 4.3984 g ZnSO4.7H20 in 0.1N HCl and dilute to 1 liter. This solution
will give 1000 ppm concentration. Make working stock solution of 100 ppm and then prepare working
standards.
Cu Standard stock solution,1 mg/ml - Dissolve 1.9645 g CuSO4.5H2O in distilled H2O and dilute
to 500 ml. One ml of the solution contains 1 mg Cu. Make further dilutions with double glass distilled
water.
Fe Standard stock solution, 100 µg/ml - Dissolve 0.7022 g (FeSO4 (NH3)2 SO4.6H2O in 100 ml water.
Add 5 ml concentrated H2SO4, warm it slightly and add potassium permanganate solution (0.1 N)
until solution shows slight pink colour. Make volume to 1 liter. It will give 100 µg/ml solution of Fe.
Make serial dilutions to get standards in the range
Mn Standard solution ,100 µg/ml - Dissolve 0.5756 g dry KMnO4 in 50 ml water. Add 40 ml conc.
H2SO4 and reduce the permanganate by careful addition of sodium metabisulphite solution until Mn
solution is colorless. Oxidise excess of H2SO4 in hot solution by addition of little HNO3. Cool, and
transfer the contents to 2 liter volumetric flask. Make volume upto the mark. This solution contains 0.1
mg of Mn/ ml. Solution must be protected from light. Make further dilutions.
Standard calcium solution – Dissolve 100.1 mg of dry calcium carbonate in 30 ml of 0.1 N HCl and
dilute to 200 ml with water. This solution contains 20 mg of calcium per 100 ml. Further dilutions are
made to get required working standards
229
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Pesticides: Their Analysis in Milk Using High
Performance Liquid Chromatography
Chander Datt and Monica Puniya
Dairy Cattle Nutrition Division, NDRI, Karnal
Pesticides are defined as the substances intended to repel, kill or control any species designated
as pest including weeds, insects, rodents, fungi, bacteria or other organisms. The family of pesticides
includes herbicides, insecticides, rodenticide, bacteriocide, nematocide etc. Pesticides that may
contaminate feeds originate from most of the major groups including organochlorine, organophosphate
and carbamates. Although the residues of pesticides are potentially toxic to farm livestock, the
primary focus of concern is centered on residues in animal products destined for human consumption.
Organochlorine pesticides residues (OCP) are lipophilic in nature and relatively stable. Most of the
OCPR’s and their metabolites are readily excreted in milk. Unlike organochlorines, organophophate
pesticide (OPP) compounds are readily decomposed by physico-chemical and enzymatic processes
in plant and animal systems, therefore, these are less persistent but could lead to acute toxicity if
consumed beyond toxic levels. Normally, the milk and milk products get contaminated with OPPR if
the animals are given a feed which is treated with pesticides during storage or the feed is manufactured
from the plant material treated during its cultivation. The other sources of contamination of feeds,
milk and milk products can be- direct treatment of animals against parasites, control of flies/insects
in the milk processing area and use of contaminated water for drinking of animals and for processing
of milk. The carry over rate of these pesticides from feed to milk varies from 20 to 80% depending
upon the nature and stability of the pesticide, method of its application, duration of exposure and
metabolism within the animal
The OCPR’s damage the peripheral nerves, cause blindness and sometimes show tumerogenic effects.
In addition, an induction of liver enzymes, hydrolases and mixed function oxidases also occurs. A negative
influence on the reproductive functions is also observed. The toxicity of these compounds leads to cardiac
and respiratory impairment due to disorders of autonomous nervous system. Some of the OPPR,s induce
myopathy in exposed human beings and animals, which is characterized by muscle cell degeneration and
respiratory muscles are affected. Human erythrocyte cholinesterase activity is inhibited and pathological
alterations are observed in several tissues. Acute poisoning leads to respiratory distress, nervousness,
convulsions, paralysis and death.
During the past few years, there has been a drastic reduction in the level of residues of organochloro
pesticides in feeds as well as in milk. For the protection of consumers, Codex Committee on Pesticide
Residues of Codex Alimentarius Commission of FAO/WHO takes care to establish the MRL for
different pesticides for animal feeds and foods of animal origin. In India, there is an urgent need to
monitor the level of pesticide residues in milk with special reference to organophosphates. Though
methods for multiresidue analysis of pesticide residues have been developed but these have not
been tried for simultaneous analysis of most commonly used OPP and OCP compounds in India.
Therefore, a technique (Singh and Chhabra, 2004) has been evolved in DCN Division for simultaneous
determination of commonly used OCP and OPP compounds.
Methodology
Requirements
1. Pesticide standards (Sigma-Aldrich): Eighteen OPP including acephate (ACP), chlorpyrifos
(CPP), chlorpyrifos-methyl (CPP-me), diazinon (DZN), dichlorvos (DCV), dicrotophos (DCP),
dimethoate (DMT), fenitrothion (FTN), malaoxon (MOX), malathion (MTN), monocrotophos
(MCP), paraoxon-ethyl (POX), parathion-methyl (PTN), phorate (PRT), phosphamidon (PMD),
230
Pesticides: Their Analysis in Milk Using High Performance Liquid Chromatography
profenophos (PFP), quinalphos (QNP), tetrachlorvinphos (TCV) and 10 OCP namely aldrin
(ADR), dieldrin (DER), endosulfan (ESF), endrin (EDR), heptachlor (HCR), lindane (LDN), 2,4
DDE, 2,4 DDT, 4,4 DDD and 4,4 DDT.
2. HPLC system: Waters HPLC with binary gradient solvent delivery module, injector, C18
µBondaPak column (300 mm x 10 mm i.d.; particle size: 10 µ) with heater to provide column
temperature of 39ºC, UV-VIS absorbance detector, Millennium 32 chromatography software.
3. Acetonitrile, toluene, methanol, water (all HPLC grade) and sodium sulphate (anhyd.)
4. Solvent filtration apparatus with membrane filters (47 mm dia.) viz., aqueous HVLP 04700 and
organic FHLP 04700 (Millipore India Pvt. Ltd., Bangaluru), Whatman filter paper No. 41, Solid
phase extraction (SPE) cartridge
5. Other equipments: Analytical balance, mixture/blender, vortex mixture, solvent evaporatory
apparatus, vacuum manifold and vacuum pump
HPLC conditions for analysis
Gradient programme
A gradient programme has been developed for separation of various pesticides at 200 nm detection
wavelength with column temperature of 39ºC. All the pesticides are separated within a run time of 60
min., however, for proper cleaning the total duration is increased to 86 min, The subsequent sample
can be injected at or after 90 min. of run. Acephate is the first pesticide to be eluted while aldrin is the
last one.
Limit of detection (LOD)
LOD is the minimum concentration of each pesticide that produces a response which is equal to
twice the short term noise at 200 nm. For each pesticide, it was worked out from the average response/
area of 4 injections of standard pesticide mixture. The LOD was calculated by keeping a min. area of
1000 units for each pesticide.
Linearity
Linearity for each pesticide is determined by injecting upto 5 times different concentrations of
mixed standard pesticide solution. From the area units and concentration for each pesticide, the
correlation coefficients are calculated. The correlation coefficient for most of the residues was about
0.99.
Repeatability
For validation, area units for individual pesticides, 5 samples of standard mixture run during the
entire analysis period are randomly selected for the determination of coefficient of variation (CV). The
CV values for different OPP and OCP ranges from 0.86 to 8.13 and 1.24 to 19.2%, respectively.
Extraction, drying and clean up procedures for sample preparation for injection in HPLC
Extraction: Milk sample (25 ml) is mixed with 100 ml of acetonitrile at high speed for 5 min. It is
then mixed with 10g and 20g anhydrous sodium sulphate each time for 2 min. in 2 steps. The sample
is kept undisturbed for 2 min. Supernatant is filtered through Whatman No. 41 and kept overnight in
a dark place. For determination of per cent recoveries, 1 to 5 ppm of individual pesticides are added
to 25 ml milk sample and after mixing, it is kept overnight in dark before carrying out the extraction
with solvent as explained above.
Drying: A 50 ml portion of the extract is taken in 500 ml solvent evaporatory apparatus and dried
under a stream of N2 gas and vacuum in 500 ml capacity beaker containing water at 45-50ºC. Then 5
ml of acetonitrile was added to flask and contents were mixed using a vortex mixture. Acetonitrile is
evaporated to dryness.
231
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Clean up using SPE cartridge: Fresh SPE cartridges were conditioned over vacuum manifold
assembly using 3 ml methanol thrice followed by acetonitrile (3 ml thrice). When acetonitrile phase
was just near to the level of sorbent, the individual valves of the vacuum manifold assembly were
closed. The dried sample extract was dissolved in 1.0 ml acetonitrile and transferred to preconditioned
cartridge. Subsequent rinsing twice with 0.5 ml acetonitrile was also transferred to SPE cartridge. Then
a flask was kept below SPE cartridge in vacuum manifold assembly. The contents were collected in
the flask after addition of methanol twice. The sample is then evaporated to dryness and the residue
is dissolved in 1.0 ml acetonitrile. After filtering it through membrane filter, it is injected in HPLC
system or stored in a refrigerator until used. Likewise, pesticide standards are run in order to know
the concentration of each pesticide in unknown samples.
232
Estimation of Microbial GOS by High Performance Liquid Chromatography
Estimation of Microbial GOS by High
Performance Liquid Chromatography
Vikas Sangwan and Sudhir Kumar Tomar
Dairy Microbiology Division, NDRI, Karnal
Introduction
Galacto-oligosaccharides belong to the group of non-digestible oligosaccharides (NDO), which
can be regarded as soluble dietary fibres because they are completely soluble and are fermented by
specific bacteria present in the colon, resulting in the production of short-chain fatty acids (propionate,
acetate and butyrate). Their chemical formula is (Galactose)n - Glucose, with n ranging from 1 to 4. The
galactose-galactose linkage is a β-(1-3), β-(1-4), β-(1-6) linkage, with the β-(1-4) being predominant:
the galactose-glucose linkage is mainly β-(1-4). Some disaccharides are also present in GOS (e.g.
allolactose and galactobiose).
GOS have a high solubility and a relative sweetness about 35 % that of sucrose. They are more
viscous than high-fructose corn syrups, decrease the water activity and freezing point, and show good
moisture retention capacities. They also have remarkable stability at high temperatures and variable pH
levels. In particular, the stability of GOS in acidic and high-temperature conditions enables them to be
applied without decomposition in a wider variety of foods. GOS remain unchanged after treatment at
160ºC for 10 min at pH-2, where about a half or more of the sucrose is degraded. Even in acidic conditions
at room temperature, GOS tend to be stable during long-term storage. Galacto-oligosaccharides (GOS)
are carbohydrate-based food ingredients that can enhance health- related physiological activities,
which can provide protection from infection; decrease the number of potentially pathogenic bacteria;
facilitate the normal functions of the gut; stimulate the absorption of some minerals and decrease
blood lipids content (Dias and others 2009).
Production of GOS
GOS molecules (for example, Gal (β1→4) Gal (β1→4) Glc) are typically synthesised by the
enzymatic activity of β-galactosidase on lactose in a reaction known as transgalactosylation (Gosling
and others 2010). β-D-Galactosidases (β-D-galactoside galactohydrolase, EC 3.2.1.23), which are also
referred to as lactases, hydrolyze the β(1→4) linkage of lactose (galactosyl β(1→4) glucose) to glucose
and galactose and transfer the galactose formed from lactose cleavage onto the galactose moiety of
another lactose to yield galacto-oligosaccharides (Park and others, 2009).
The use of lactic acid bacteria (LAB) as producers of β galactosidase enzymes offers substantial
potential for the production of GOS. First, LAB are known to be good producers of extracellular
β-galactosidases that enable GOS production from lactose. Second, LAB have a safe tradition in food
Fig. 1. Production of GOS
Fig 2. Schematic diagram of HPLC
fermentations and exhibit rapid anaerobic growth on agricultural substrates including waste products
such as whey. Therefore, GOS may be produced from crude cellular extracts without costly downstream
processing. Moreover, GOS may be produced in situ during food fermentations or by using whey to
produce food-grade GOS preparations.
233
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Detection of GOS by High Performance Liquid Chromatography (HPLC)
High performance liquid chromatography is basically a highly improved form of column
chromatography. Instead of a solvent being allowed to drip through a column under gravity, it is
forced through under high pressure that makes it much faster.
Flow Scheme for HPLC
Liquid chromatography involves a sample being
dissolved in a mobile phase (liquid, acetonitrile: w1ter, 80:20,
in case of GOS). The mobile phase is then forced through
an immobile, immiscible stationary phase. The stationary
phases are chosen such that components of the sample have
different solubility in each phase. A component, which is
quite soluble in the stationary phase, will take longer to travel
through it than a component, which is not very soluble in the
stationary phase but very soluble in the mobile phase. As a
result of these differences in mobility, sample components
will become separated from each other as they travel through
the stationary phase. Figure 3 represents a chromatogram Fig. 3. HPLC chromatogram of GOS sample:
Glc: glucose, Gal: galactose, Lac: lactose, a(1–6)
for GOS obtained in HPLC.
galactobiose, DP: degree of polymerisation.
Detection limits of substances using HPLC in general
are largely dependent on the compound being analyzed as well as the sensitivity of detector used.
Whereas the RI detector is not the most sensitive, for example being 100 to 1000 less sensitive than
UV detectors, its suitability with detection range of micrograms per milliliter (μg/mL) is convenient
enough for GOS detection and analysis (Otieno, 2010)
Materials of chromatographic separation
Different types of materials can be used as a solid phase for chromatographic separation. The
stationary phase is the key element in a chromatography system. Separation is enhanced by using
stationary phases that present the shortest possible diffusion pathways to the solutes, have low
resistance to mass transfer, reasonably narrow particle size distribution, and are uniformly packed in
the column. The most frequent materials used in sugar separation are:
a) Active carbon
In sugar industry, the most used adsorbent is active carbon, because is the cheapest and does not
require a difficult pretreatment. Use of active carbon for the separation of GOS has shown that the carbon
has higher affinity for oligosaccharides and low affinity for monosaccharides (glucose and galactose).
b) Ion exchange materials
Cation exchange resins have been used HPLC program for GOS detection
extensively in the sugar industry for different
Acetonitrile: Water (80:20)
types of separation. The use of cation-exchange Mobile phase
Amino (NH2) column
resins together with water as the mobile phase Column
resulted in a better separation of saccharides Detector
Refractive Index Detector
than when anion exchangers were used. Use Run time
20 min
of a method for the separation of sugars on the Flow rate
0.4 ml/min
cation-exchange resin Dowex 50W-X4 (K+),
Column temperature
800C
using water as the eluent separate various
sugars including oligosaccharides, hexoses, pentoses, acetals, methyl-α-D-glycosides and other sugar
derivatives, with recoveries of greater than 95%.
Fractionation
The separation of carbohydrates plays an important role in food production and in cosmetic and
pharmaceutical industries. Also, 90% of the cost in food production is related with separation processes.
234
Estimation of Microbial GOS by High Performance Liquid Chromatography
Liquid chromatography offers high selectivity, efficiency and loading capacity of the stationary phase
and speed of process. Purification of GOS is important because by removing monosaccharide and
lactose from GOS, there is a decrease in sweetness and calorie value. Different techniques are being
used for the fractionation of oligosaccharides including
•
Diafiltration
•
Yeast treatment,
•
Activated charcoal treatment
•
Size exclusion chromatography (SEC) (Hernandez and others 2009).
Other methods of detection
Quemener and others (1997) developed a method based on high performance anion-exchange
chromatography with pulsed amperometric detection (HPAE-PAD) to measure GOS in food and
feed products. A few years later, de Slegte (2002) organized collaborative study of this method in
which galactose and other sugars were separated on a CarboPacTM PA1 column and detected by
pulsed amperometric detection (PAD) using a triple potential waveform. Thin layer chromatography
(TLC) and spectrophotometric (UV spectra) methods are also used for GOS detection. TLC is only
qualitative and less sensitive as compared to HPLC. The UV method together with chemometric
models of calibration is an acceptable analytical method for a fast, simple and inexpensive monitoring
of total GOS production in a predefined fermentation process, allowing to promptly verifying if the
fermentation is running as expected, or if some correction action is needed, which is crucial when
an industrial GOS production is envisaged, being a possible alternative to the standard analytical
methods usually used (Dias and others 2009).
Conclusion
Liquid chromatography has been largely used depending on the matrix from which GOS is to be
extracted and analyzed. However, HPAE-PAD has been found to be more superior in the detection
of GOSs than high-performance liquid chromatography with RI detection. Nevertjeless, in the event
that HPAE-PAD is not available for use, HPLC-RI can be reliably used instead. Concerning the
chemometric methods analyzed, in general, the ANN multiple is robust and present the best global
prediction performance. However, further studies are needed in order to obtain better results with
these chemometric models. Although the RI detector has several limitations, namely the dependence
of sensitivity on changes in solvent composition, temperature, and pressure, it however remains the
most useful tool so far in the determination of sugar concentrations in foods.
References
de Slegte J. 2002. Determination of trans-galactooligosaccharides in selected food products by ion-exchange
chromatography. J AOAC Int 85:417–23.
Dias LG, Veloso ACA, Correia DM, Rocha O, Torres D, Rocha I, Rodrigues LR, Peres AM. 2009. UV spectrophotometry
method for the monitoring of galacto-oligosaccharides production. Food Chemistry 113:246–252.
Gosling A, Stevens GW, Barber AR, Kentish SE, Gras SL. 2010. Recent advances refining galactooligosaccharide
production from lactose. Food Chemistry 121:307–318.
Hernandez O, Matute AIR, Olano A, Moreno FJ, Sanz ML. 2009. Comparison of fractionation techniques to obtain
prebiotic galactooligosaccharides. International Dairy Journal 19:531–536.
Morales V, Sanz ML, Olano A, Corzo N. 2006. Rapid separation on activated charcoal of high oligosaccharides in honey.
Chromatographia 64:233–238.
Otieno DO. 2010. Synthesis of β-Galactooligosaccharides from Lactose Using Microbial β-Galactosidases. Comprehensive
Reviews in Food Science and Food Safety 9:471-482.
Park AR, Oh DK. 2009. Galacto-oligosaccharide production using microbial β-galactosidase: current state and
perspectives. Appl Microbiol Biotechnol.
235
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Estimation of Trehalose Production by Propionibacteria
Poonam and Sudhir Kumar Tomar
Dairy Microbiology Division, NDRI, Karnal
Introduction
Trehalose also known as mycose, is a non reducing disaccharide in which two glucose molecules
are linked together in a 1,1-glycosidic linkage. Although there are three possible anomers of trehalose,
that is, α,β-1,1, β,β-1,1, and α,α-1,1, only the α,α -trehalose (Figure 1) has been isolated from and
biosynthesized in living organisms.
This naturally occurring disaccharide is widespread throughout the biological world with the
first tentative report being in ergot of rye. Trehalose is
found naturally in insects, plants, fungi, and bacteria,
the major natural dietary source is mushrooms. It is
implicated in anhydrobiosis—the ability of plants and
animals to withstand prolonged periods of desiccation.
It has high water retention capabilities and is used
in food and cosmetics. The sugar forms a gel phase
Fig. 1. Structure of naturally occurring isomer of
as cells dehydrate, which prevents disruption of
trehalose, α,α-1,1 trehalose
internal cell organelles by effectively splinting them
in position. Rehydration then allows normal cellular
activity to be resumed without the major, lethal damage that would normally follow a dehydration/
rehydration cycle. Trehalose has the added advantage of being an antioxidant. Trehalose is a naturally
occurring reducer of cell stress, protecting these organisms from extremes in heat shock and osmotic
stress (Crowe, 2002). It acts by altering or replacing the water shell that surrounds lipid and protein
macromolecules. It is thought that its flexible glycosidic bond allows trehalose to conform to the
irregular polar groups of macromolecules. In doing so, it is able to maintain the 3-dimensional structure
of these biologic molecules under stress, preserving biologic functions. Furthermore, trehalose is very
promising as a sugar substitute in food: it is repeated as being anti-cariogenic and can be considered
as a dietetic sugar since it is only partially digested in the human intestine (Neta et al., 2000). These
properties place trehalose in the category of compounds known as nutraceuticals, defined as foods
and food components with benefits for human and animal health (Hugenholtz et al., 2002).
Trehalose production by propionibacteria
Dairy Propionibacteria are important starter organisms involved in typical flavor and eyes
formation in Swiss-type of cheeses and in the production of other dairy products. Besides their
technological role, these bacteria can also be used as cell factory for the production of a variety of
biomolecules like vitamin B12, folate, riboflavin and propionic acid. They also have been recognized
as potential probiotics in recent years. In cheese manufacturing and during other applications,
Propionibacteria are subjected to different kind of stress conditions and a part of this defense system
involves the intracellular accumulation of trehalose.
Pathways for trehalose biosynthesis
At least four pathways for the synthesis of trehalose in biological system have been reported
thus far: (i) the OtsA–OtsB pathway, the most common route, involves the transfer of glucose from
UDPglucose to glucose 6-phosphate to yield trehalose 6- phosphate, which is subsequently converted
to trehalose (ii) the TreS pathway, Trehalose synthase (TS) catalyses an intramolecular arrangement
of maltose, in order to convert the glycosidic bond α-(1-4) of this disaccharide to the α -(1-1) trehalose
bond (iii) the TreY–TreZ pathway, a two-step reaction involving maltooligosyl-trehalose synthase
(TreY) catalyzing the conversion of maltodextrines to maltooligosyl-trehalose and subsequently the
maltooligosyl-trehalose trehalohydrolase (TreZ) breaks this intermediate to generate trehalose and
236
Estimation of Trehalose Production by Propionibacteria
(iv) a single-step pathway involving trehalose glycosyltransferring
synthase, that catalyses the reversible conversion of glucose and
NDP-glucose into trehalose.
Pathways leading to trehalose accumulation in Propionibacteria
freudenreichii have been studied (Cardoso et al., 2007). P.
freudenreichii uses the OtsA–OtsB pathway for trehalose synthesis,
whereas trehalose catabolism proceeds via TreS. Maltose derived
from TreS activity can be processed by amylomaltase, releasing
glucose which is further catabolized via glycolysis (Figure 2).
Given the beneficial properties of trehalose, there is a need
to estimate the level of trehalose accumulated by different
Propionibacteria strains so that high trehalose accumulating strains
can be selected which will act as robust cheese starter and can be Fig.2. Proposed scheme of trehalose
metabolism in P. freudenreichii.
used for enhanced trehalose production at industrial level.
1, Glucokinase; 2, trehalose-6-
Estimation of trehalose production
phosphate synthase; 3, trehalose-6phosphate phosphatase; 4, trehalose
synthase; 5, amylomaltose; PolyP,
polyphosphate; G, glucose.
Different types of methods are available for estimation
of trehalose production by Propionibacterium strains. All these
methods include growth of culture in YELA medium, extraction of
intracellular trehalose from the cells and then estimating the level of trehalose.
Growth conditions
For investigating trehalose biosynthesis, culturing of the cells is required in Erlenmeyer flasks
(500mL). At the beginning of the experiment, a 3% inoculums (v/v) is introduced in 200 mL of YELA
(Yeast extact lactae agar) medium and strieedd at 50 rpm in a water bath at 30 ºC. To establish anaerobic
conditions, the medium is aseptically gassed with argon during the 15-min preceding inoculation. A
culture grown until exponential phase is used as inoculum to obtain an initial optical density of about
0.06.
Trehalose extraction from the cell
Trehalose extraction using ethanol extraction: Cells are centrifuged (13800 g, 10 min) and washed in
an isotonic solution. The cell pellets are transferred into a glass tube with 80 % ethanol and stirred
by vortex for 30 min at ambient temperature. They are evaporated to dryness by means of a rotary
evaporator. The residues obtained are then dissolved in the 1.5 ml of acetonitrile : water (70:30) and
directly taken up for HPLC analysis. (Ferreira et al., 1996).
Trehalose extraction using TCA: The cell pellet obtained is mixed with 3 volumes of TCA 0.5 M for
1hr at room temperature. Then the suspension is centrifuged at 20000 g for 10 min. The supernatant is
neutralized before trehalose conc. Measurement (Cola et al., 2010).
Trehalose extraction using hot water: The cell pellet obtained is washed with 1 ml of water. Cells are
resuspended in 0.2 ml of water and transferred to a boiling water bath, followed by incubation for 10
min to extract intracellular compounds. After centrifugation of the boiled sample, the supernatant is
obtained and used for measuring the trehalose content (Mahmud et al., 2010).
Trehalose extraction using Bead mill: The test tubes are directly taken up from the freezer and
immersed in boiling water for 10 min. followed by cooling on ice. Glass beads are added to tubes
and cells are disintegrated in the bead mill for 30 min. Cell debris are removed by centrifugation and
supernatant is taken for further analysis (Schulze et al., 1995).
Estimation of trehalose from the cell extract
HPLC method: Trehalose concentration in the supernatant obtained is measured using a HPLC
system equipped with refractometer detector and a silica-amino column (250x4 mm i.d.) coupled to a
guard-column (10x4 mm i.d.). Mobile phase used is acetomitrile : water (70:30) at 1mL. min (Deborde
237
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
et al., 1996)
Enzymatic (Trehalase) method: Trehalose can also be determined by incubating the cell free extracts
with a stable trehalase preparation. Extracts (100 µl) plus 25 µl trehalase are incubated overnight (14
h) at 40ºC. The resulting glucose is assayed with glucose oxidase by adding to the spectrophotometer
cell (2.0 ml final volume), the following reagents: 1% odianisidine, prepared by diluting in 0.1 M
phosphate buffer pH 6.0; digested trehalose sample; peroxidase, 200 lg/ml in distilled water (1.5 U/
ml of the assay mixture); and glucose oxidase (1 mg/ml in distilled water, to obtain 21 U/ml in 2 ml
of the assay mixture). Absorbance changes are measured in the spectrophotometer at 460 nm and 25ºC
and compared to a glucose standard curve (Gonzalez-Hernandez et al., 2005).
Anthrone method: Trehalose extracts are submitted to the reaction with anthrone and the colour
formed measured at 620 nm, according to the procedure described by Brin (1966). Quantification is
achieved using trehalose as external standard.
NMR method: After overnight ethanol extraction, the samples are dried in a desiccator for at least
48 h. Dried samples are resuspended in D2O (Aldrich Chemical) and analyzed by NMR spectroscopy
using a Bruker AMX300 spectrometer with a 5-mm inverse detection probe head, 32 K data points, a
90j flip angle and a repetition delay of 42.7 s. The water resonance is suppressed with a pre-saturation
pulse. The trehalose peak is identified against that of the pure substance. (Cardoso et al., 2004).
Megazyme kit method: The trehalose content in the supernatant can also be measured using a
enzymatic assay kit from Megazyme International Ireland Ltd. (Wicklow, Ireland). The basic principle
includes hydrolysis of trehalose to D-glucose by trehalase and then D-Glucose released is measured
by phosphorylating with enzyme hexokinase (HK).
Cell protein determination: For expressing the trehalose content in the cell, the total protein in the
cell is determined by the method of Lowry et al. (1951) after cell lysis with 1 M NaOH (85ºC, 5 min),
and using bovine serum albumin as a standard. Trehalose content of the cell is expressed in terms of
protein content of the cell (Cardoso et al., 2004).
References
Brin, M. (1966). Transketolase: clinical aspects ed. In. S. P. Colowick & N. 0. Kaplan, Methods in Enzymology, 9,
Academic Press, pp. 506514.
Cardoso, F. S., Castro, R. F., Borges, N., Santos, H. (2007). Biochemical and genetic characterization of the pathways
for trehalose metabolism in Propionibacterium freudenreichii and their role in stress response. Microbiology, 153, 270–
280.
Cardoso, F. S., Gaspar, P., Hugenholtz, J., Ramos, A., Santos, H. (2004). Enhancement of trehalose production in dairy
Propionibacteria through manipulation of environmental conditions. International Journal of Food Microbiology, 91,
195– 204.
Colla, E., Pereira, A. B., Hernalsteens, S., Maugeri F., Rodrigues, M. I. (2010). Optimization of trehalose production
by Rhodotorula dairenensis following a sequential strategy of experimental Design. Food Bioprocess Technol, 3,
265–275.
Crowe, L. M. (2002). Lessons from nature: the role of sugars in anhydrobiosis. Comp Biochem Physiol AMol Integr Physiol,
131, 505–13.
Deborde, C., Coree, C., Rolin, D. B., Nadal, L., de certaines, J. D., Boyaval, P. (1996) Trehalose biosynthesis in dairy
Propionibacterium. Journal of magnetic resonance analysis, 2, 297-304.
Ferreira, J. C., Paschoalin, V. M. F., Panek, A. D., Trugo, L. C. ( 1997). Comparison of three different methods for
trehalose determination in yeast extracts. Food Chemistry, 60(2), 251-254.
Gonzalez-Hernandez, J. C., Jimenez-Estrada, M. (2005). Comparative analysis of trehalose production by Debaryomyces
hansenii and Saccharomyces cerevisiae under saline stress. Extremophiles, 9, 7–16.
Hugenholtz, J., Hunik, J., Santos, H., Smid, E. (2002). Nutraceutical production by propionibacteria. Lait, 82, 103– 112.
Mahmud, S. A., Hirasawa, T., Shimizu, H. (2010). Differential importance of trehalose accumulation in Saccharomyces
cerevisiae in response to various environmental stresses. Journal of Bioscience and Bioengineering, 109 (3), 262–266.
Neta, T., Takada, K., Hirasawa, M. (2000). Low-cariogenicity of trehalose as a substrate. Journal of Dentistry, 28, 571–
576.
Schulze, U., Larsen, M. E., Villadsen, J. (1995). Determination of intracellular trehalose and glycogen in Saccharomyces
cerevisiae. Analytical Biochemistry, 228, 143-149.
238
Spore Based Biosensor as A Quality Control Tool in Dairy Industry
Spore Based Biosensor as A Quality
Control Tool in Dairy Industry
Naresh Kumar, Raghu H. V. and Avinash
Dairy Microbiology Division, NDRI, Karnal
The development of sensors for detecting foodborne pathogens has been motivated by the need
to produce safe foods and to provide better healthcare (Irudayaraj, 2009). Improving food and water
safety and security depends on the ability to detect, identify, and trace food and water pathogens.
As milk is a compulsory part of daily diet and being nutritious food for human beings, also serves
as a good medium for the growth of many microorganisms which cause spoilage of milk and milk
products. Earlier for detection of pathogens conventional methods which rely on specific media to
enumerate and isolate viable bacterial cells in food were used, and are considered as gold-standard
for their detection. These methods are very sensitive, inexpensive and can give both qualitative and
quantitative information and involve the basic steps: pre-enrichment, selective enrichment, selective
plating, and biochemical screening and serological confirmation. Hence, a complete series of tests are
often required before any identification can be confirmed (Mandal et al., 2011) Although methods are
powerful, error-proof, and dependable but are lengthy, cumbersome and are often ineffective because
they are not compatible with the speed at which the products are manufactured and the short shelf
life of products. To overcome these challenging criteria of time and sensitivity rapid methods which
include nucleic acid, fluorescent antibody or immuno-based techniques have been developed which
gives instant or real time results but requiring additional expensive devices and equipments (Ivnitski
et al., 1999). Biosensor based tools offer the most promising solutions and address some of the modernday needs for fast and sensitive detection of pathogens in real time.
Biosensors are defined as analytical devices integrating biological elements and signal transducers.
The biological elements interact specifically with an analyte, producing a signal that the transducer
recognizes and converts into measurable parameters as shown in fig.1 (Rasooly and Herold, 2006).
Currently biosensor is defined as a sensor that integrates a biological element with a physiochemical
transducer to produce an electronic signal proportional to a single analyte which is then conveyed to
a detector.
Biosensor achievements have revolutionized the detection method and provide us with simple to
use device, cost-effective, rapid and appropriate detection method that give immediate and accurate
results comparable to or better than the conventional analytical systems in terms of performance
i.e.
reliability,
sensitivity,
selectivity,
specificity and robustness and can identify
the contaminants much faster, more efficient
and can give effective real time monitoring
of pathogens and most importantly ensuring
customer safety (Scott, 1998).
History of biosensor: In 1956, Leland
C Clark Jr., who is known as the father of
Biosensors and he published his definitive
paper on the oxygen electrode. In 1962, he Fig. 1. Diagrammatic representation of Biosensor and its
described "how to make electrochemical working principle
sensors more intelligent" by adding Source:www.realtimebiosensor.com (Mattias Rudh, 2007)
"enzyme transducers as membrane enclosed
sandwiches”. The year wise development in the field of biosensor is as follows:
1922: First glass pH electrode;1956: Invention of the oxygen electrode;1962: First description of a
biosensor- an amperometric enzyme electrode for glucose; 1969: First potentiometer biosensor- Urease
239
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
immobilized on an ammonia electrode to detect urea; 1972/5: First commercial biosensor-Yellow
Springs Instruments glucose biosensor;1975: First microbe-based biosensor, First immunosensorovalbumin on a platinum wire, Invention of the pO2/pCO2 optode; 1982: First fibre optic-based
biosensor for glucose; 1983: First surface plasmon resonance (SPR) immunosensor; 1984: First
mediated amperometric biosensor: ferrocene used with glucose Oxidase for the detection of glucose;
1996: Glucocard launched; 1998: Launch of LifeScan Fast Take blood glucose biosensor; 2001: LifeScan
purchases Inverness Medicals glucose testing business for $1.3 billion.
Types of biosensors:
1. Optical biosensors: Technique used in this biosensor is based on surface plasma resonance.
2. Electrochemical sensing biosensors: Technique used in this biosensor is based on amperometric
sensing, Conductometric sensing. In amperometric sensing, increasing potential is applied to
the cell until oxidation of the substance to be analysed occurs. This in turn increases the cell
current and gives a peak current. The height of this peak current will be directly proportional
to the concentration of electroactive substances or molecules. In conductive sensors substrate
concentration is measured using relationship between conductance and concentration of ionic
species.
3. Enzymatic biosensors: This type of sensors is widely used as they are easy to use. For example
glucose biosensors. In glucose biosensor enzyme acts as a biorecognition element and recognizes
only the glucose molecule. These enzymes are present in the electrode surface. When enzyme
recognizes the glucose molecule it act as biocatalyst and produces gluconic acid and hydrogen
peroxide using glucose and oxygen from the air. This reaction leads to the flow of electrons
from hydrogen peroxide/oxygen coupling. This flow of electron is directly proportional to the
number of glucose, molecules present in the biological fluid such as blood.
Classification of biosensor:
It may be classified according to the biological specificity conferring mechanism or to the mode of
signal transduction, or alternatively a combination of both (Belluzo et al., 2008).
Classification based on bioreceptors: A Bioreceptor is a biological molecular species or a
living biological system that utilizes a biochemical mechanism for recognition (Tantilipikara, 2005).
Depending upon the mechanism of biochemical interaction between the receptor and the analyte the
biosensor can categorised into two types:
1. Biocatalytic sensors: They are based on the recognition and binding of an analyte followed by
a catalyzed chemical conversion of the analyte from a non-detectible form to detectible form
which are detected and recorded by a transducer. This includes:
i) Monoenzyme, multienzyme ii) Microorganisms (such as bacteria, fungi, yeast), or sub cellular
organelles and particles (mitochondria, cell walls); iii) Animal / plant tissue slice
2. Bioaffinity sensors: They are based on the interaction of the analyte with biological components,
such as antibodies, nucleic acid, lectins, cell membrane receptor or harmone receptor (Rogers,
2000).
Classification based on transduction system: The transducing element of a biosensor is used to
convert the biological recognition step into the measurable signal that can be detected and displayed.
It is further classified into following types:
a) Electrochemical : Electrochemical detectors measure changes in electron transfer caused by
an oxidation/reduction reaction involving the analyte at the surface of a suitable electrode
(Thevenot et al., 2001). It further includesi) Amperometric : It detects the changes in current as a function of concentration of electro
active species e.g. - Solid electrolyte gas sensors, electronic noses.
ii) Potentiometric : It depends on changes in potential of a system at constant current (I=0) or
it detects the change in distribution of charge. e.g. - Ion-selective electrodes (such as pH
240
Spore Based Biosensor as A Quality Control Tool in Dairy Industry
meter), Ion-selective field effect transistors, LAPS.
iii) Conductometric : It measures the change in conductance of the biological complex
situated between electrodes. or it involves the measurement of changes in conductance
due to the migration of ions. e. g.-Optical fibers, surface plasmon resonance, absorbance,
luminescence.
b) Optical : In optical biosensors, the optical fibers allow detection of analyte on the basis of
absorption, fluorescence or light scattering (Chauhan et al., 2004) e.g.-Surface plasmon
resonance
c) Piezoelectric: The change in frequency is proportional to the mass absorbed material or
Sensitive to changes in mass, density, viscosity and acoustic coupling phenomena e. g.
Surface acoustic wave sensors
d) Calorimetric : Many enzyme catalyzed reactions are exothermic, generating heat which may
be used as a basis for measuring the rate of reaction and, hence, the analyte concentration.
Whole-cell bacterial biosensors
Bacteria can be used as biosensors to demonstrate the toxicity of a variety of environmental
media including soil, sediment, and water by coupling bacteria to transducers that convert a cellular
response into detectable signals (Biran et al., 2003). These bacterial biosensors are engineered by
pairing a reporter gene that generates a signal with a contaminant-sensing component that responds
to chemical or physical change, such as exposure to a specific analyte. When the biosensor is exposed
to such a change, the sensing component stimulates the reporter gene through a biochemical pathway
in the cell. The reporter gene then produces a measurable response, such as emitting visible light,
which is indicative of the degree of chemical or physical change (Biran et al., 2003; Tauriainen et al.,
2000, Turpeinen et al., 2003; Daunert et al., 2000). Several biosensors have been developed that indicate
toxicity of any chemical or physical change; new biosensors are being developed to respond to
particular analytes. Such biosensors have been developed for heavy metals and metalloids including
arsenic, cadmium, mercury, and lead (NRC, 2003).
Biosensors measure the bioavailability concentration for the contaminant they are designed to
detect (Tauriainen et al., 2000). To test the measurements made by biosensors, a chelating agent known
to decrease bioavailability of lead was added to a lead solution. Measurements of the lead solution
containing chelating agents were taken and compared to measurements of the lead-only solution. A
decrease in the biosensors luminescence matched a decrease in bioavailability concentration of lead
in the solution. This demonstrates that biosensors are sensitive to the bioavailability fraction of the
contaminant and their luminescence reflects the bioavailability concentration (Tauriainen et al., 2000).
Spores based biosensor: Bacterial spores appears to have great potential for their application as
bio-sensor as they have the ability to sense environmental changes and to respond using explosive
molecular mechanisms that transform dormant spores into rapid growing cells. There are a great
number of bacterial species which produce spores for example; genus Bacillus (widely dispersed in soil,
plant matter, and air) may be readily grown in the laboratory to form spores: B. cereus, B. licheniformis,
B. megaterium, B. sphaericus, B. stearothermophilus, B. subtilis, and B. thuringiensis. They can also survive
in a very harsh condition. For the development of bacterial spore as a biosensor, it is a prerequisite to
have a complete or descriptive knowledge regarding their germinants (carbohydrates, nucleotides,
amino acids etc.) which by their action on the dormant spores convert them into vegetative cells. The
germination process of a whole population of spore may be completed in a very short duration of
time (15-30min) followed by a sequence of metabolic reactions and synthesis of enzymes resulting in
outgrowth of vegetative cells. After germination de novo acetyl esterase is released from the core of
the spore which act upon DAF and its hydrolysis results in flouroscence and the signal can be captured
using optical device to quantify the presence of target analyte (Rotman, 2001).
Characteristic features of spores: Bacillus species have inherent characteristics to produce
241
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
endospore. These are the dormant form of life having no metabolic activity. They are resistant to
environmental stress like heat, desiccation, irradiation and chemical compounds and can be stored
in a medium for long time even in the absence of nutrients. Spores are resistant due to the Calciumdipicolinate present in the spores that stabilize and protects the DNA from denaturation. DNA-binding
proteins helps in protecting the DNA from heat, drying, chemicals, and radiations. While dehydration
process that is the loss of water provides them resistance toward heat and radiation. And finally during
the germination process damaged DNA they get repaired by DNA repair enzymes (Setlow, 2003).
Sporulation: Spore formation, sporogenesis or sporulation, normally commences when growth
ceases due to lack of nutrients. It is a complex process and may be divided into seven stages .An axial
filament of nuclear material forms (Stage-I), followed by an inward folding of the cell membrane to
enclose part of the DNA and produce the forespore septum (Stage-II). The membrane continues to
grow and engulfs the immature spore in a second membrane (Stage-III). Next, cortex is laid down in
the space between the two membranes, and both calcium and dipicolinic acid is accumulated (StageIV). Protein coats then are formed around the cortex (Stage-V), and maturation of the spore occurs
(Stage-VI). Finally, lytic enzymes destroy the sporangium releasing the spore (Stage-VII). Diagram
representing the different stages of sporulation (Prescott et al., 2002).
Germination: In the presence of favorable growth conditions spores get germinated. The
germination process is essentially a biophysical and degradative one – the spore’s inner membrane
increases in fluidity and ion fluxes resume; monovalent cations, potassium and sodium, move across
the spore membrane, and calcium ions and dipicolinate are excreted. The peptidoglycan of the
spore cortex is degraded, and the coat layers are partially degraded. ATP synthesis and oxidative
metabolism resume, DNA damage is repaired and the DNA-complexing small acid-soluble proteins
(SASPs) are degraded by a specific protease, providing a source of amino acids for outgrowth. As
germination events precede any de novo synthesis of macromolecules, the apparatus required for
spore germination must be already present in the mature spore (Moir et al., 2002).
Application of spores as biosensor: Bacterial spores are suitable for use as biosensor because they
have the ability to sense environmental changes in response to specific “germinant” and transform
into rapid growing cells. The spores are heat resistant and can remain in non metabolic state for many
years. This characteristic can effectively be used as a biosensor for tracking these residues in milk and
milk products and the details of biosensor developed are as follows:
242
Spore Based Biosensor as A Quality Control Tool in Dairy Industry
1. Development of analytical process for detection of antibiotic residues in milk using bacterial
spores as biosensor.(Patent no Reg# IPR /4.9.1/05074/2006)(Kumar et al., 2006)
2. A kit for detection of β-lactam antibiotic group in milk using bacterial spore as biosensor (Patent
Reg # IPR/ 4.14.1/08073/del/2009) (Kumar et al., 2009)
3. Development of Spore Inhibition Based -Enzyme Substrate Assay (SIB-ESA) for monitoring
Aflatoxin M1 in milk (Patent Regd.# IPR /4/14.4/10045)
4. Development of Enzyme Substrate Assay (ESA) for Monitoring Enterococci in Milk
(Setlow, P, 2003.)
Development of analytical process for detection of antibiotic residues in
milk using bacterial spores as biosensor
The bacterial spores have unique ability to sense environmental changes in response to specific
“germinant” and transform rapidly into growing vegetative cells. The spores are heat resistant and can
remain in non metabolic state for many years. This characteristic can effectively be used as a biosensor
for tracking these residues in milk and milk products. In the present invention, an analytical process
of transformation of dormant spore of Bacillus stearothermophilus into active vegetative cell through
activation, germination and outgrowth has been developed .This analytical process can track major
groups of antibiotic residues in milk within 2.30-3.0 hours at MRL / or above levels recommended by
codex.
Brief of Invention: An analytical process which involves sporulation & activation of dormant
spores of B.stearothermophilus in newly developed medium & their germination/ outgrowth in
presence of selective germinant mixture has been developed (Patent Reg # IPR/ 4.9.1.4/ 05074/ 1479
/DEL /2006). The validated process is in line with AOAC approved charm 6602 system & can be
used effectively for semi-quantitative detection of antibiotic residues in different types of milk system
within 2.30-3.0 hrs at MRL/ or above levels as recommended by the codex /EU. This cost effective
process can also find applications in targeting spoilage and pathogenic organisms in dairy and non
dairy foods.
Market Potential: For monitoring of drug residues in milk well defined test / rapid assay technique
are not available in India. MDR test Kit was offered to various stake holders like m/s Duke Thomson
Pvt. Ltd., Indore; Hi-media Pvt. Ltd, Mumbai, NDDB; M/s Neugen diagnostic secunderabad etc. The
product was appreciated by all these potential customers and finally one non-exclusive license with
fee of Rs. 2.50 lakhs, royalty 2.0% & validity of license for period of 7 years was given to M/s Neugen
diagnostic Secunderabad who currently is selling our product to different dairy units like Mother
Dairy, Delhi; Paras Dairy (3 units); Bholebaba Dairy; Hatsun Dairy, TN; Aavin Dairy, TN; Kolar Dairy,
Karnataka; Shipra Lab, Bengaluru; Delhi Milk Scheme etc. Microbial Drug Residues Test Kit (15 set
test) developed at dairy microbiology division were sold to different dairy units through M/s Neugen
Diagnostic Pvt. Ltd. @ 1200/- + CST @ 10.30% in last six months period. Further steps are required for
243
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
AOAC approval of this product which will be costing around 10 lakhs and may expand its application
at domestic level by incorporating in our legal standard (PFA standard) as well as in export.
Novel features of process:
1.
Cost effective
2.
Better sensitivity
3.
Semi-quantitative detection
4.
No false positive /negative results
5.
Insensitivity towards detergents / sanitizers
6.
Consistency in color development with in 3.0 hrs
7.
Validated with AOAC approved charm 6602 system
8.
Wide spectrum of application for different types of milk
244
Spore Based Biosensor as A Quality Control Tool in Dairy Industry
A kit for detection of β-lactam antibiotic group in milk using bacterial spore as biosensor:
This invention relates to application of dormant bacterial spores as biosensor. The study is
based on the resistance mechanism of some β-lactamase generating Bacillus spp. Some spore forming
bacteria such as B. cereus and B. licheniformis produce β-lactamase enzyme due to induction by β-lactam
antibiotics and the enzyme production is proportional to the concentration of inducer present in milk.
A real time microbial assay based on β-lactamase enzyme using starch iodine as color indicator has
been developed. The microbial assay is working on principle of non competitive enzyme action on
inducer (β-Lactam) resulting in indirect reduction of starch iodine mixture through penicilloic acid. A
comparison of the intensity of the test reaction with that of a control was taken as criteria to determines
whether the sample is positive or negative (Kumar et al., 2009) .The assay can detect specifically βlactam groups in spiked milk with in 15-20 min at regulatory codex limits with negligible sensitivity
towards non β- lactam groups. The presence of Inhibitors other than antibiotic residues in milk did not
interfere with the working principle of microbial assays. A significant correlation between microbial
assay & receptor based assay (charm 6202) was established in survey work with raw, pasteurized
milk and dried products with no false positive/ negative results. Spore suspension was found stable
up to 5 months when stored under refrigeration conditions. The microbial assay (Rs 20.54/- test) is
cost effective can find immense application in dairy industry as “ON FARM” milk screening test for
β- lactam group (Kumar et al., 2009).
The impact of innovation on life of Rural India: The invention was carried out to test drug residues
at farm level. These drug residues have immense public health and processing implications. The field
level testing will be of public heath and processing value to dairy farmers and entrepreneurs who are
involved in dairy small business.
Development of spore inhibition based–enzyme substrate assay (sib-esa)
for monitoring aflatoxin M1
Brief about Innovation: Aflatoxins are toxic, carcinogenic, mutagenic immuno-suppressive
agents produced as secondary metabolites by the fungi Aspergillus flavus & A. parasiticus. Four major
Aflatoxins B1, B2, G1, and G2 have been isolated from feeds. Aflatoxin M1 is hydroxylated derivative
Patent on development of spore inhibition based–enzyme substrate assay (SIB-ESA) for monitoring aflatoxin
M1 in milk has been filed at NDRI and is under processing(Patent Regd.# IPR /4/14.4/10045)
245
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
of Aflatoxin B1.The bacterial spores as nano-detector have unique ability to sense environmental
changes in response to specific “germinant” and transform rapidly into growing vegetative cells.
This characteristic can be effectively used as biosensor for tracking microbial and non–microbial
contaminants (Kumar et al.,2005; Rotman 2001 & 2003).The present hypothesis is based on the specific
spore germination inhibition principle in presence of specific analyte i.e. aflatoxin M1. In case where
analyte is absent in milk system, specific indicator enzyme (s) are produced by active bio-sensing
molecules which will act specifically on chromogenic/or fluorogenic substrate resulting in colored
reaction/or fluorescence as end product which is measured semi-quantitatively by either visually/or
using optical system at specific excitation/emission spectra (Kumar et al., 2010).
The end product response is significantly different in case of buffer/ or milk system containing
specific analyte i. e. aflatoxin M1. The developed test assay was validated by analyzing 25 samples of
each raw and pasteurized milk procured from different organized/or private own dairies and other
reputed brands using AOAC approved RIA and ELISA based system and a significant correlation
with ELISA at Codex MRL Limit (0.5 ppb) of Aflatoxin M1 was established.
Development of Enzyme Substrate Assay (ESA) For Monitoring Enterococci in Milk: An
Enzyme Substrate Assay (ESA) based on β-D-glucosidase activity was attempted for specific
detection of Enterococci to meet the emerging demand of dairy industry. Four enrichment broths
commercially available in the market were screened for selective recovery of Enterococci based on
β-D-glucosidase activity. One of these broths namely Chromocult Enterococcus Broth (CEB) showed
better performance in terms of selectivity and enzyme activity with partial inhibition of contaminants
other than Enterococci. The selected medium was further improved for desired features by increasing
the concentration of sodium azide from 0.06 to 0.15 g/100 ml resulting in significant inhibitory
effect on growth pattern of L. lactis, L. casei, Leuconostoc mesenteroides and L. monocytogenes. Other
media components and supplements were also optimized for enhanced sensitivity and selectivity
of Enterococcus sp. The optimized selective enrichment medium i. e. Esculin Based Sodium Azide
Medium (EBSAM) demonstrated superior features in terms of sensitivity, selectivity, fastness, accuracy
etc. and may be a suitable substitute for existing media used for routine monitoring of Enterococci in
R&D institutions. Developed assay was screened for Enterococci count with 32 samples of raw milk
and it could detect 2.67, 3.50, 4.25 and 4.8 log counts within incubation period of 12, 7½, 6½ and 5 hr
respectively. ESA could also detect Enterococci log counts of 2.84 in pasteurized milk within 12 hrs
of incubation; however, assay was insensitive at very low level of 1.13 and 0.915 log counts. As such
ESA developed in current investigation may find industrial application as Hygiene Indicator test for
detection of Enterococci in raw milk & pasteurized milk with in 5-12 hrs as against 36-48hrs required
246
Spore Based Biosensor as A Quality Control Tool in Dairy Industry
in conventional method (Thakur et al., 2010).
Concluding remarks: Biosensors are making a great impact on the development of rapid, sensitive
assays for the detection of microbial and non – microbial contaminants in food system. Kits are now
available for several organisms such as E. coli O157:H7 and Salmonella typhimurium and it is hoped that
more will become available shortly. The most viable openings in the food industry will arise where
a biosensor can rapidly detect total microbial contamination. The largest area of application for the
environment lies in the development of biosensors for monitoring bacteria in drinking and waste
water, rivers, reservoirs and supplies. Spores have a great potential to be used as a biosensor and
the bioassay are cost effective, rapid, easy to perform and require almost negligible infra-structural
facilities.
References:
Belluzo, M. S., Ribone, M. E., Lagier, C. M., 2008. Assembling Amperometric Biosensors for Clinical Diagnostics. Sensors
8, 1366-1399.
Biran, I., Rissin, D., Ron, E. and D. Walt. 2003. Optical imaging fiber-based live bacterial cell array biosensor. Analytical
Biochemistry, 315:1, pp. 106-113.
Chauhan, S., Rai, V., Singh, H. B., 2004. Biosensors. Resonance. 33-44.
Daunert S., Barrett G., Feliciano J., Shetty R., Shrestha S., and W. Smith-Spencer. 2000. Genetically Engineered WholeCell Sensing Systems: Coupling Biological Recognition with Reporter Genes. Chem. Rev., 100, pp. 2705-2738.
Irudayaraj, J., 2009. Pathogen Sensors. Sensors 9, 8610-8612.
Ivnitski, D., Abdel-Hamid, I., Atanasov, P., Wilkins, E., 1999. Biosensors for detection of pathogenic bacteria. Biosensors
& Bioelectronics 14, 599–624.
Kumar, N., Das, S., Manju, G., 2009. A kit for detection of β-lactam antibiotic group in milk using bacterial spore as
biosensor (Patent Reg # IPR/115/del/2009).
Kumar, N., Sawant, S., Malik, R.K., Patil, G.R., 2005. Development of analytical process for detection of antibiotic
residues in milk using bacterial spores as biosensor (Patent Reg # IPR/4.9.1.4/05074/1479/del/2006).
Kumar, N., Singh, N., Singh, V.K., Bhand, S., Malik, R.K., 2010. Development of spore inhibition based–enzyme substrate
assay (SIB-ESA) for monitoring aflatoxin M1 in milk (Patent Regd.# IPR /4/14.4/10045).
Mandal, P. K., Biswas, A. K., Choi, K., Pal, U. K., 2011. Methods for Rapid Detection of Foodborne Pathogens: An
Overview. American Journal of Food Technology 6(2), 87-102
Moir, A., Corfe, B. M., Behravan, J., 2002. Spore germination. Cell Mol Life Sci 59, 403–409.
National Research Council (NRC), 2003. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and
Applications. The National Academies Press.
Prescott, L. M., Harley, Klein., 2002. Microbiology. 5th Edition. The McGraw-Hill companies. (Chapter 3)
Rasooly and Herold, 2006. Biosensors for the Analysis of Food- and Waterborne Pathogens and their Toxins. Journal of
AOAC international 89(3), 873-883.
Rogers, K. R., 2000. Principles of affinity-based biosensors. Molecular Biotechnology 14(2), 109-129.
Rotman, B., 2001. Using living spores for real-time biosensing. Gen. Eng. News 21, 65.
Rotman, B., Cote, M. A., 2003. Application of a real-time biosensor to detect bacteria in platelet concentrates. Biochem.
Biophys. Res. Comm 300, 197-200.
Scott, A. O., 1998. Biosensor for food analysis. Published by Royal Society of chemistry, Cambridge, UK. (Chapter 1)
Setlow, P., 2003. Spore germination. Current Opinion in Microbiology 6, 550–556.
Tantilipikara, P., 2005. Optical biosensor for microalbumin determination. A thesis submitted in partial fulfilment of the
requirement for the degree of Master of Science. Mahidol University.
Tauriainen, S., Virta, M. and M. Karp. 2000. Detecting Bioavailable Toxic Metals and Metalloids from Natural Water
Samples Using Luminescent Sensor Bacteria. Water Research, 34:10, pp. 2661-2666.
Thakur, G., Kumar, N., Raghu, H. V., Malik, R. K., (2010). Development of Off-Line Enzyme Substrate Based Assay for
Monitoring Enterococci in Milk. NDRI Newsletter Apr – June 2010. Pp 2-3.
Thevenot, D. R., Toth, K., Durst, R. A., Wilson, G. S., 2001. Electrochemical biosensors: recommended definitions and
classification. Biosensors & Bioelectronics 16(1), 121-131.
Turpeinen R., Virta M., and M. Haggblom. 2003. Analysis of Arsenic Bioavailability in Contaminated Soils. Environmental
Toxicology and Chemistry, 22:1, pp. 1-6.
247
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
Detection and Evaluation of Antimicrobial Activities of
Bacteriocins and Bioactive Peptides Produced by LAB
Shilpa Vij, Subrota Hati and Meenakshi Dahiya
Dairy Microbiology Division, NDRI, Karnal
Lactic Acid Bacteria (LAB) have been used for centuries in the fermentation of a variety of dairy
products. The preservative ability of LAB in foods is attributed to the production of anti-microbial
metabolites including organic acids and bacteriocins. Bacteriocins generally exert their anti-microbial
action by interfering with the cell wall or the membrane of target organisms, either by inhibiting
cell wall biosynthesis or causing pore formation, subsequently resulting in death. Bioactive Peptides
are released from milk proteins on enzymatic hydrolysis by the proteolytic enzymes such as trypsin,
pepsin , chymotrypsin and LAB fermentation of milk proteins i.e. whey and casein by proteolytic LAB
such as L.helveticus, L.fermentum, L.acidophilus, L casei and Lactococci. A large variety of techniques have
evolved to assess the ability of microorganisms to produce antagonistic substances.
Antimicrobial activities of bacteriocins/ Bioactive Peptides produced by LAB
Materials Required:
Sterile Petri-plates, sterile pipettes, Micropipettes, Sterile Micro tips, incubator, MRS agar, TGE
agar, TGE soft Agar (0.8 % agar) tubes, indicator strains, the glass tube with a suction bulb, sterile filter
paper discs, forceps, ethyl alcohol (70%).
Indicator strain
Culture Condition
Pediococcus acidilactici LB42
30/37ºC MRS / TGE Broth
Lactobacillus plantarum NCDO 955
37ºC MRS / TGE Broth
Lactobacillus helveticus
37ºC Whey/ Sodium Caseinate
Important:
•
Only freshly grown (3-4 hours incubation) active indicator strains should be used for determining
antibacterial activity.
•
Do not use stored (refrigeration temperature) indicator strain for the assay.
•
Do not use the over night incubated culture for the assay.
Procedure:
•
Grow LAB/ Bacteriocin producing LAB cultures in MRS / M17 broth for 18-24 H as its optimal
growth temperature (30/37ºC).
•
Grow proteolytic strains of LAB in whey or sodium caseinate (supplemented with 0.5% glucose)
for 24 - 48 h as its optimal growth temperature (30/37ºC) for bioactive peptide production.
•
Remove the cells by centrifugation at 12000 rpm for 20 min at 5ºC.
•
Sterilize the supernatant from broth by passing through a 0.22 µm membrane or heat treat the
supernatant at 90ºC for 3 min in a dry bath/ water bath.
•
Alternatively cells are killed by boiling for 3-5 min and heat killed cultures can be employed.
•
Ultrafilterate whey qnd sodium caseinate fermentate from 10K Da membrane for separating
bioactive peptides of less than 10 KDa molecular weight.
•
Prepare agar plates by pouring melted agar (MRS/M17) in sterile Petri plates
•
After solidification of the agar transfer the plates to the incubator at 37ºC overnight for drying
of the agar surface.
248
Detection and Evaluation of Antimicrobial Activities of Bacteriocins and Bioactive Peptides Produced by LAB
•
Overlay 5-7 ml of soft agar (0.8% agar) which had been seeded with 50 µl of the freshly grown
(3-4 h) indicator strain. This will generate a potential mat of the indicator bacteria.
•
Refrigerate the plates at 5ºC for 1-2 h before the wells are punched out of the agar.
•
Punch out the wells with the broad end of a sterile Pasteur pipette and remove the agar
buttons.
•
Fill the wells 100 µl of the prepared culture supernatant/ heat killed cultures and less than 10 K
Da bioactive peptides from whey and sodium caseinate
•
Put the plates in the refrigerator (5-7ºC) for 3-4 h to facilitate the diffusion of the antimicrobial
compound (Do not invert the plates).
•
Incubate the plates at optimum temperature of the indicator strain for 18-24 h (Do not invert the
plates).
•
Observe the plates for zone of clearance (if any) around the edge of the wells.
•
A clear zone of 1 mm or greater extending laterally from the edge of the wells is considered
positive inhibition. Use sterile distilled water as a control.
Detection of bacteriocin (Nisin) produced by LAB
This is a faster MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] colorimetric
assay (MCA) for quantitative measurement of polypeptide bacteriocins in solutions with nisin as an
example. After an initial incubation of nisin and indicator bacterium Micrococcus luteus NCIB 8166 in
tubes, MTT was added for another incubation period. After that, nisin was quantified by estimating
the number of viable bacteria based on measuring the amount of purple formazan produced by
cleavage of yellow tetrazolium salt MTT. Then MCA was compared to a standard agar diffusion
assay (ADA).
1. Indicator bacterial strain and cultivation: Inoculate a loop of Micrococcus luteus on the S1 agar
(0.8% tryptone , 0.5% yeast extract 0.5% D-glucose 0.5% NaCl and 0.2% Na2HPO4. In agar
medium add 1.5% (w/v) Tween 20.) plate and incubate at 37°C for 18~24 h. Then, transfer a
single colony of bacteria from the S1 agar to S1 broth and incubate at 37°C for 12 h.
2. Dissolve MTT in phosphate buffered saline (pH 7.2) to a concentration of 5 mg/ml, and then
filtere through a 0.2-μm syringe filter.
3. Add 100µl of MTT solution into each of 2 ml fresh S1 broth with indicator bacteria (from 106 to
101 CFU/ml) and then incubate at 37°C for 1, 2, 3, 4, 5 and 6 h, respectively.
4. Keep the broth culture in boiling water for 5 min to stop reaction.
5. After cooling, centrifuge the cultures at 1 500×g for 20 min to precipitate formazan crystals
6. Remove the supernatant. To dissolve the formazan crystals, add 2 ml of dimethylsulfoxide
(DMSO) and then incubate the mixture for 10 min at room temperature.
7. Measured the optical density (OD) of the formazan solution at the wavelength of 510 nm.
249
Chemical Analysis of Value Added Dairy Products and Their Quality Assurance
List of Selected Participants for Winter School
1.
Sh. Shiv Shanker Chasta
7.
Assistant Professor,
DFT Department, College of Dairy & Food
Science Technology, MPUAT, Udaipur-313001
(Rajasthan)
[email protected], 09828566021
2.
Dr. M. Ilamaran
Assistant Professor (FSN)
Department of Food Science and Nutrition,
Home Science College and Research Institute,
Tamil Nadu Agricultural University,
Madurai - 625 104
[email protected], 09865175206
3.
Dr. R. Saravanakumar
Assistant Professor (FSN) Department of Food
Science and Nutrition, Home Science College
and Research Institute,
Tamil Nadu Agricultural University,
Madurai - 625 104
[email protected], 09942893107
4.
Mr. Durga Shankar Bunkar
Assistant Professor,
Center of Food Science & Technology,
Institute of Agricultural Sciences,
Banaras Hindu University,
Varanasi- 221 005
[email protected], 09389835175
5.
Dr. Raj Kumar Duary
Assistant Professor,
Department of Food Processing Technology,
School of Engineering, Tejpur University,
Napaam, Sonitpur, Assam -784 028
[email protected], 09957669564
6.
Dr. Ashim Kumar Biswas
Dr. S.G. Narwade
Assist. Prof. (Dairy Science)
Department of Animal Husbandry & Dairy
Science, College of Agriculture M.A.U.
Parbhani.431 402
[email protected], 09028584537
8.
Dr. R.A. Patil
Assistant Professor (AHDS)
Dept. Animal Husbandry & Dairy Science,
College of Agriculture, Latur-412 513 (M.S.)
[email protected], 09422189001
9.
Dr. D.D. Patange
Assistant Professor, College of Agriculture,
Kohlapur-416004 (MS)
[email protected], 09421800941
10. Dr. K.D. Chavan
Assistant Professor,
Animal Science and Dairy Science Section,
College of Agriculture, Pune, Behind Mariai
Gate , Khadki, Pune 411 003 (MS)
[email protected], 09422058693
11. Mr. Ramachandra. B
Assistant Professor, Dairy Science College,
KVAFSU, Bidar-585 401
[email protected], 09481191728
12. Mr. Harsh Prakash Sharma
Assistant Professor
Department of Food Engineering
College of Food Processing Technology & Bio
Energy AAU,Anand-388110 Gujarat,
[email protected], 09408398737
13. Dr. Pawas Goswami
Department of Livestock Products Technology,
COVS, Gadvasu,
Ludhiana-141 004 (Punjab)
Assistant Professor
Department of Microbiology
Maharshi Dayanand Saraswati University
Ajmer – 305009
[email protected], 09463320622
[email protected], 09829273453
List of Selected Participants for Winter School
14. Dr. Rakesh Kumar
20. Mr. Devraja Naika H.
SMS (Dairy Technology) 303-C, Aradhana
Enclave, Khajpura, Bailey Road, Patna-800 014
Veterinary college, Koravangala Gate,
Arsikere road, Hassan-573201, Karnataka
[email protected], 09934263033
[email protected], 9900704695
15. Sh. Yogesh Khetra
Scientist, Dairy Technology Division,
NDRI, Karnal
[email protected], 09813902989
16. Dr. P. Narender Raju
Scientist, Dairy Technology Division,
NDRI, Karnal
[email protected], 09896038983
17. Mr. Awanish Kr. Srivastava
Unipex Dairy Product Co. Ltd. UAE
PO Box 5646, Sharjah, United Arab Emirate
[email protected], 0097150 3637840
18. Er. Tariq Ahmad
Assistant Professor
Department of Food Technology
Islamic University of Science and Technology
Avantipora (J & K)
[email protected], 09906480112
19. Dr. Arun Goel,
Assistant Professor
DFT Department,
College of Dairy & Food Science Technology,
MPUAT, Udaipur-313001 (Rajasthan)
[email protected], 09887182750
21. Dr. S. Shive Kumar
Assistant Scientist (DT)
College of Dairy Science & Technology
GADVASU, Ludhiana-141 004 (Punjab)
[email protected], 09646434238
22. Mr. Vilas Mahadeorao Thakre
Programme Coordinator
KVK, Sindewahi, Distt. Vhandrpur
(Maharashatra)
[email protected], 09881149896
23. Mr. Saraff Sripad
Asstt. Professor & Head,
Department of Dairy Chemistry,
Dairy Technology Programme,
SVVU, Kamareddy, Distt. Nizamabad-AP 503
111 [email protected], 09848721561
24. Ms. Nikam Pranali Balkishan
College of Dairy Technology, Warud,
Pusad -445204
[email protected], 09225235250
25. Dr. Vishakha Singh
Assistant Professor
Department of Foods & Nutrition,
College of Home science, Maharana Pratap
University of Agriculture & Technology,
Udaipur 313001(Rajasthan)
[email protected], 09414029748
© Copyright 2026