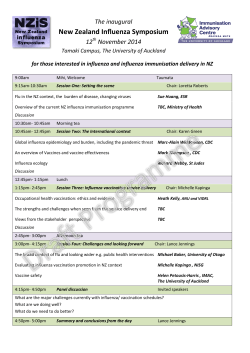
2012-14 CENTRE FOR RESPIRATORY DISEASES AND MENINGITIS
CENTRE FOR RESPIRATORY DISEASES AND MENINGITIS 2012-14 CENTRE FOR RESPIRATORY DISEASES AND MENINGITIS TABLE OF CONTENTS 1. 2. 3. 4. 5. 6. 7. 8. Message from the director What is pneumonia? What can be done about pneumonia? Goals of the CRDM CRDM timeline Vaccine reducing disease in children and adults Using a vaccine to reduce antibiotic resistance Who is most at risk of pneumococcal disease in the pneumococcal vaccine era? 9. HIV-infected individuals and infants <1 year of age are at increased risk of severe influenza 10. Influenza vaccination is the best way to prevent influenza 11. Emerging respiratory viruses: Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and avian influenza A(H7N9) 02 04 05 06 08 10 11 12 12 14 15 12. Ten years of tracking meningococcal meningitis and sepsis 13. Preventing meningococcal disease 14. Burden of respiratory syncytial virus (RSV) in South Africa and risk groups 15. Discovering pertussis in South Africa 16. Staying ahead with advanced diagnostics 17. Sharing skills and experience 18. CRDM surveillance sites 19. Calendar of events for 2012-2014 20. CRDM staff 21. CRDM Publications 2012-2014 22. Contacts and resources 16 17 19 19 20 22 24 26 30 38 46 12th November 2014 marks the sixth anniversary of World Pneumonia Day, an initiative which was established to advocate for greater attention to be focused on what is termed by the UNICEF as the “forgotten epidemic”. Despite the significant progress in reducing under-five childhood mortality globally over the past decade, approximately 942,000 children died of pneumonia in 2013. This placed pneumonia as the leading cause of under-5 childhood mortality, with >95% of these deaths occurring in low-income countries. Although a number of bacteria, viruses and fungi have been established to cause pneumonia, the majority of deaths are thought to occur due to bacterial infections. In addition, Pneumocystis jirovecii and cytomegalovirus also contribute to pneumonia deaths among HIV-infected and other immunocompromised children. Among the bacterial causes of pneumonia, a large proportion is attributable to Streptococcus pneumoniae and previously (prior to widespread immunization) to Haemophilus influenzae 02 CRDM | 2012-2014 type B (Hib). The fact that these bacteria are among the leading causes of pneumonia mortality is more an indictment on the poor health infrastructure which exists in low-middle income countries, than due to any major challenges related to antibiotic resistance associated with these bacteria. It has been estimated that less than one third of children with pneumonia have access to first-line antibiotic therapy in low-income countries, which if improved could reduce pneumonia mortality by up to 40-50% alone. This is a serious indictment of failure to provide basic care to the most vulnerable in the population. This inequity in access to health care is further accentuated among those children living in the poorest economic quintile of the population, and who suffer disproportionately from pneumonia due to other factors such as increased exposure to air pollutants and malnutrition. MESSAGE FROM THE DIRECTOR The ongoing, accelerated introduction of pneumococcal conjugate vaccine (PCV) into public immunisation programmes of low-income countries, coupled with the recently established widespread introduction of Hib conjugate vaccine, has the potential for accelerating a decline in pneumonia hospitalisation and mortality in low-middle income countries. The South African government has been exemplary in the timeous introduction of HibCV and PCV into its public immunisation programmes, being the first in Africa to self-fund these vaccines for its public immunisation programme since 1999 and 2009 respectively. Although the impact of these vaccines on childhood mortality is difficult to quantify in countries such as South Africa, where a number of other changes related to improved HIV management occurred concurrently with the introduction of these vaccines, the introduction of these vaccines have likely contributed to the ongoing decline in under-five childhood mortality in South Africa. In 2013, the under-five childhood mortality was 37 per 1,000 live births, the lowest rate in the history of the country and almost half of what it was a decade earlier. However, much still remains to be done in South Africa to prevent children from developing and dying from pneumonia. Included among these are the strengthening of primary health care to ensure early detection and timeous treatment with the correct antibiotics, as well as a focus on reducing risk factors such as indoor pollution and malnutrition, especially in the rural areas of South Africa. The Centre for Respiratory Diseases and Meningitis (CRDM) at the National Institute for Communicable Diseases (NICD) has been stalwart in providing epidemiological data on the burden of disease due to bacterial and viral causes of pneumonia in South Africa. Through its decade-old laboratory-based and more recently active, hospital-based surveillance, the centre provides burden of disease data on the causes of childhood and adult pneumonia which are unsurpassed on the African continent. This has gained it international recognition and also allows for it to contribute to multiple World Health Organization programmes, and is used as a reference laboratory for training of other African scientists and a source of isolates to help inform WHO vaccine recommendations on influenza virus. The activities of the CRDM also contribute to measuring the impact which the introduction of vaccines has on childhood and adult pneumonia morbidity and mortality. The CRDM, like many other centres at the NICD, is a national asset whose mission remains to improve the lives of all South Africans by providing updated epidemiological data to refine treatment strategies and prioritise the introduction of future vaccines into the public immunization programme of South Africa. Also, its programme ensures ongoing surveillance to timeously identify any novel viruses, including influenza viruses with pandemic potential, to inform public health policy and assist the Department of Health with its programme. The activities of the centre contribute to the mission of World Pneumonia Day in highlighting the burden and success of preventative strategies such as vaccination in South Africa. This also provides a tool for advocacy on pneumonia prevention elsewhere on our continent. The CRDM staff members are commended for their dedication and the success of their programme on this World Pneumonia Day. Prof. Shabir Madhi Executive Director: National Institute for Communicable Diseases CRDM | 2012-2014 03 WHAT IS PNEUMONIA? Pneumonia is an infection of the lungs that is usually caused by bacteria or viruses, and can also be caused by fungi Pneumonia is the leading killer of children under five years old • • • • • 04 In 2012 alone, 1.1 million children died from this preventable and treatable illness, accounting for 17-18% of child mortality. In 2008, there were an estimated 541,000 deaths due to Streptococcus pneumoniae (pneumococcus) and 203,000 deaths due to Haemophilus influenzae type b (Hib) in children under five globally. 99% of child pneumonia deaths occur in developing countries. “Influenza and pneumonia” was the second most common underlying cause of death in South Africa in 2011, after tuberculosis. Respiratory syncytial virus is the most common viral cause of childhood pneumonia. Pneumonia creates an economic burden for families, communities, and governments • • • Preventing pneumonia averts treatment costs, other losses due to illness and allows children to become healthy, productive adults. Scaling up coverage of vaccines against Hib and pneumococcus in the world’s 73 poorest countries (2011-2020) would avert $51 billion in treatment costs and productivity losses. This increase in vaccine coverage would also save 2.9 million lives and prevent 52 million cases of illness. WHAT CAN BE DONE ABOUT PNEUMONIA? Global Pneumonia Interventions Controlling childhood pneumonia requires an integrated package of interventions to protect, prevent and treat the disease. Fortunately, many of the interventions targeted at pneumonia also help control other childhood diseases, such as diarrhoea, and should be part of a comprehensive approach to child survival. The Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD) formulated by the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) in 2013 aims to stop preventable childhood deaths from pneumonia and diarrhoea by 2025. Implementation of this plan by national governments will allow them to achieve the Millennium Development Goal (MDG) to save children under the age of 5 (MDG4). Protect Against Pneumonia Treat Pneumonia • • • • Exclusive breastfeeding during the first six months of life and adequate nutrition through age five protect babies from pneumonia, diarrhoea, malnutrition and other diseases. Regular hand washing, access to clean water and sanitation protect children against pathogens that cause pneumonia, diarrhoea and other diseases. Eliminating household air pollution, especially smoke from unsafe cooking stoves reduces the risk of severe pneumonia in children. • • Normal Pneumonia Antibiotics such as amoxicillin can prevent the majority of pneumonia deaths and cost only about $US 0.21-0.42 per treatment course. Effective integrated case management strategies ensure that children receive proper and timely treatment for pneumonia. Improving access to services and increasing awareness and demand for services within communities is crucial to controlling pneumonia as only around 30% of children with bacterial pneumonia receive the antibiotics they need. Prevent Pneumonia • • • • • • Vaccines against pneumococcus, Hib, pertussis, influenza and measles can prevent a significant portion of pneumonia cases. The Hib vaccine was introduced into the South African Expanded Programme on Immunization (EPI) in 1999 and the pneumococcal conjugate vaccine in 2009. The measles vaccine is also given as part of the EPI. Annual influenza vaccination is recommended for everyone ≥6 months of age in South Africa. Other preventative strategies include: adequate nutrition, zinc supplementation for children with diarrhoea, prevention of HIV infection in children and antibiotic prophylaxis for HIV-infected or -exposed children. Efavirenz-based programmes for the prevention of mother-to-child transmission of HIV were rolled out in South Africa from April 2012 and cotrimoxazole prophylaxis for HIV-infected children is recommended. Addressing socioeconomic and environmental factors associated with increased risk to contract pneumonia emphasises personal hygiene practices like regular hand washing with soap and implementing measures to reduce household air pollution. CRDM | 2012-2014 05 GOALS OF THE CRDM In 2012, the NICD was restructured and the Centre for Respiratory Diseases and Meningitis (CRDM) was formed. Our goals are: 1. 2. 3. 4. 5. 6. 7. 06 To conduct surveillance for communicable respiratory diseases and meningitis within South Africa in order to provide data on the burden, severity and seasonality. To characterise pathogens contributing to respiratory diseases and meningitis in order to understand the contribution of genetic factors to the cause and transmission of disease, and guide vaccine development, treatment and prevention policy. To monitor trends in antiviral and antibacterial drug resistance in respiratory and meningeal pathogens in order to inform empiric treatment guidelines. To monitor the impact and effectiveness of interventions to reduce respiratory diseases and meningitis. To provide reference laboratory functions for specialised organism identification and characterisation related to respiratory diseases and pathogens causing meningitis nationally and regionally. To identify and characterise novel respiratory and meningeal pathogens with potential to cause outbreaks and to assist with response to respiratory disease outbreaks. To be a source of local and regional expertise on respiratory diseases and meningitis. CRDM | 2012-2014 8. 9. To engage in directed and relevant research to answer questions related to regional respiratory disease and meningitis disease problems and their surveillance and management. To build local and regional capacity in epidemiology and laboratory diagnostics for respiratory diseases and meningitis. CRDM | 2012-2014 07 CRDM TIMELINE 2009 2009 • • • • • • • • Pneumococcal conjugate vaccine (PCV) introduced into the Expanded Programme on Immunisation (EPI) Haemophilus influenzae serotype b (Hib) vaccine booster at nine months introduced into the EPI Pertussis vaccine changed from whole cell to acellular pertussis vaccine in the EPI Syndromic surveillance programme for Severe Acute Respiratory Illness (SARI) started in three provinces to test for pneumococcus, influenza, respiratory syncytial virus and eight other common respiratory viruses New molecular diagnostic assays implemented to detect and serogroup/type Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and ten respiratory viruses First laboratory-confirmed case of pandemic influenza A(H1N1)pdm09 identified in South Africa First edition of the annually updated “Healthcare Workers Handbook on Influenza” published Enhanced viral watch programme for surveillance of hospitalised patients in all nine provinces introduced 2010 • • • SARI surveillance site in Klerksdorp-Tshepong started enrolling patients Case definition for SARI surveillance expanded at Edendale and Klerksdorp-Tshepong sites to include chronic cases Case-control study initiated to evaluate effectiveness of PCV-7 in routine use through the EPI 2010 2011 • • • 13-valent PCV replaced 7-valent vaccine in the EPI Avian influenza A(H7N1) outbreak in ostriches in the Western Cape Guidelines published for the Management, Prevention and Control of Meningococcal Disease in South Africa 2011 08 CRDM | 2012-2014 2012 2012 • • • • 2013 2013 • • • Healthcare utilisation survey conducted in Pietermaritzburg Multi-year study of household transmission of influenza in HIVinfected and -uninfected individuals started CRDM biosafety level 3 laboratory established Nature; Vol. 312, Aug, 2014 NEWS IN FOCUS SOURCE: ASTRONOMICAL OBSERVATORY OF LA PLATA • Centre for Respiratory Diseases and Meningitis established Expanded testing to include atypical bacterial causes of pneumonia, pertussis, tuberculosis and Pneumocystis jirovecci at Edendale and Klerksdorp-Tshepong SARI sites Molecular diagnostic assays implemented for detection of Bordetella pertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella species Healthcare utilisation surveys conducted in Soweto and Klerksdorp Surveillance for influenza-like illness implemented at Edendale and Klerksdorp-Tshepong sites Avian influenza A(H7) outbreak in ostriches in the Western and Eastern Cape areas CRDM appointed as WHO/AFRO (World Health Organization Regional Office for Africa) Regional Reference Laboratory for vaccine-preventable invasive bacterial diseases (VP-IBD) for the southern and eastern African region STELLAR SHOW Scientists have struggled to explain the erratic behaviour of the binary star η Carinae, which brightened unexpectedly in the 1840s and again more recently. –2 –1 Visual apparent magnitude* • • Great eruption 0 1 2 3 4 5 Lesser eruption Recent brightening 6 7 8 9 1820 1860 1900 1940 1980 2020 *Lower numbers represent greater brightness. • • 2014 CRDM featured in the journal “Nature” for the work being done on monitoring the impact of the pneumococcal vaccine in South Africa. Nature; Vol. 312, Aug., 2014; News in Focus SARI surveillance implemented at Rahima Moosa Mother and Child and Helen Joseph Hospitals A nurse prepares to immunize a young child with pneumococcal and rotavirus vaccines in Ghana. VACCINES Hidden bonus from vaccination Immunization against pneumococcus in Africa also reduces levels of antibiotic resistance. B Y E W E N C A L L A WAY T his summer, Eritrea, Côte d’Ivoire and Niger will join a growing list of countries where infants receive a vaccine to prevent pneumonia, meningitis and other deadly diseases caused by the pneumococcus bacterium (Streptococcus pneumoniae). Pneumonia is a leading killer of young children in low-income countries; vaccinations from 2010 to the end of this year are estimated to have averted 500,000 deaths, according to the GAVI Alliance in Geneva, an international organization that facilitates vaccination. Data from South Africa also point to another benefit of vaccination: stemming a rising tide of antibiotic resistance in the developing world. The country’s introduction of a pneumococcal conjugate vaccine (PCV) in 2009 has not only reduced the overall incidence of invasive pneumococcal disease by about two-thirds in infants (the age group vaccinated) and in adults, but has also reduced penicillin-resistant infections in both groups. This is the first time such benefits have been observed outside the developed world. ALFREDO CALIZ/PANOS 2014 X-ray production peaked in mid-July and has since plummeted to near zero — probably as the colliding winds, where the X-rays are born, have become entirely unstable and collapsed. The Hubble Space Telescope and other instruments are also tracking dramatic changes in the chemical-element signatures found in η Carinae’s light spectrum. The interaction between the two approaching stars can strip electrons from elements such as iron and helium, ionizing them more strongly than in normal celestial environments. “You have these bare helium nuclei — that’s awfully hard to make in normal circumstances,” says Gull. Watching this process over time helps to reveal how the stellar winds are interacting. At the Pico dos Dias Observatory in southern Brazil, astronomer Augusto Damineli has been spending every night since 25 July trying to catch a glimpse of η Carinae through the winter clouds. On 29 July, his team finally caught a brief opening and managed to gather data showing that a helium spectral line is dropping in just the pattern that Damineli expected. “TOUCHDOWN!” he wrote in an e-mail. In 2009, when η Carinae had its most recent close encounter, the system’s X-ray production plunged and then shot back up in half the time it did in 2003. That could be because the primary star’s winds are slowing down, so it takes less time for the whole system to recover. If the wind speeds have continued to drop, X-ray emissions might shoot up even faster than last time. Seeing such big differences from one close encounter to the next is “what everyone is waiting for”, says Andrea Mehner, an astronomer at the European Southern Observatory in Santiago, Chile, who is monitoring η Carinae with Hubble. “We cannot make the star do something exciting if it doesn’t want to.” ■ 1 4 | NAT U R E | VO L 5 1 2 | 7 AUG U S T 2 0 1 4 © 2014 Macmillan Publishers Limited. All rights reserved CRDM | 2012-2014 09 VACCINE REDUCING DISEASE IN CHILDREN AND ADULTS 2012 overall decline in IPD was almost 70 % In 2009, all infants in South Africa started receiving the pneumococcal conjugate vaccine as part of the government’s immunisation programme. Because we had documented levels of severe pneumococcal disease as part of our national surveillance programme before the vaccine was rolled out to all children (von Gottberg et al., Vaccine, 2013), we were able to monitor the changes in disease as a result of bringing this vaccine into all South African vaccination clinics. In 2010, we already noticed some declines in disease at a very young age (<2 years of age), but by 2012, disease due to the serotypes contained in the vaccine had decreased by almost 90% and even invasive pneumococcal disease overall (including all the other serotypes that are not included in the vaccine) showed a decline of almost 70%. 10 CRDM | 2012-2014 Although only infants are receiving this vaccine, due to the fact that this age group spreads disease to other age groups, disease has declined across all age groups. This was not an unexpected finding, as this protection for adults has been seen in countries such as the United States and England. It is the first time however, that such additional protection in age groups not vaccinated has now also been documented in a low- or middle-income country. Our centre started an additional research study to measure the effectiveness of this vaccine more carefully and we were able to confirm more accurately that this vaccine prevented disease due to the serotypes that it was designed to prevent by approximately 75% in HIV-uninfected children (Cohen et al., Clin Infect Dis, 2014). We were not able to show clear protection in HIV-infected children, but these children are also benefiting from the overall reduced transmission of this organism. USING A VACCINE TO REDUCE ANTIBIOTIC RESISTANCE In South Africa, we are fortunate in that we can monitor antibiotic resistance among pneumococcal isolates - the most important bacterium to cause pneumonia in children, as part of the national surveillance programme. Throughout the country, laboratories that receive clinical specimens from sick patients who may have pneumonia or meningitis, and that grow this bacterium during the processing of these specimens, are then requested to send these isolates to our laboratory so that we can characterise the isolates and monitor antibiotic resistance. As a result, more than 40,000 cases have been recorded on our database since 2003 and many of these cases have isolates saved for further testing. Approximately 40% of pneumococcal isolates causing severe disease in all ages were penicillin resistant when the CRDM reviewed data from 2003 through 2008 (Crowther-Gibson et al., Antimicrob Agents Chemother, 2012). This type of antibiotic resistance was more common among isolates causing disease in children (~60% resistance). Approximately 20% of isolates were so-called multidrug resistant, in that they were classified as resistant to a few antibiotic groups. This further limits choices of antibiotics for treatment. Due to resistance being more common among children, it is also more common among the serotypes that cause disease in children, which are also the serotypes specifically targeted by the pneumococcal conjugate vaccines currently being used in South Africa. By reducing these childhood serotypes, we have effectively also reduced the more resistant pneumococcal strains, and antibiotic resistance has declined substantially among the isolates that we are subsequently receiving in our laboratory. So far we have been able to measure more than 80% reduction in penicillin-resistant and multi-drug-resistant disease among very young children (<2 years) following introduction of pneumococcal conjugate vaccines. CRDM | 2012-2014 11 WHO IS MOST AT RISK? Who is most at risk of pneumococcal disease in the pneumococcal vaccine era? As part of our research study to monitor vaccine effectiveness in routine practice (Cohen et al., Clin Infect Dis, 2014), we examined groups of children who were at highest risk of contracting pneumococcal disease with and without vaccination (von Mollendorf et al., Antimicrob Agents Chemother, 2014). We found that children who were HIV-uninfected and had disease caused by any serotypes, were at higher risk if they had young siblings, underlying medical conditions, poorer socio-economic backgrounds, were not up-to-date for vaccination and had been exposed to HIV perinatally. In contrast HIV-infected children who were ill, i.e. malnourished, diagnosed with tuberculosis and not on antiretroviral therapy were more likely to get pneumococcal disease. Due to improvement in programmes to reduce the perinatal transmission of HIV from mothers to their children, there are a growing number of children who are HIV-exposed but uninfected. We showed that these children are at increased risk of being hospitalised with pneumococcal disease as well as dying from pneumococcal disease if they are very young (<6 months of age) when they fall ill. HIV-INFECTED INDIVIDUALS HIV-infected individuals and infants <1 year of age are at increased risk of severe influenza It is important to know who is at highest risk of severe influenza in order to decide who to target with vaccination campaigns. The CRDM analysed national mortality and surveillance statistics and combined this with modelling approaches to address this important question. We estimated that influenza causes 2,500 - 5,700 respiratory deaths and 17,000 - 22,000 respiratory hospitalisations in South Africa each year (Tempia et al., Clin Infect Dis, 2014). Children aged <1 year have the highest risk of being admitted to hospital or dying from influenza (Tempia et al., Clin Infect Dis, 2014 and Cohen et al., Clin Infect Dis, 2014). Analysis of data from more 12 CRDM | 2012-2014 than 10,000 people enrolled into the pneumonia surveillance programme demonstrated that HIV-infected individuals are three to six times more likely to be admitted to hospital with severe influenza than HIV-uninfected individuals (Cohen et al., Clin Infect Dis, 2014). Once admitted to hospital, HIV-infected individuals also stay in hospital for longer and are more likely to die. CRDM | 2012-2014 13 INFLUENZA VACCINATION IS THE BEST WAY TO PREVENT INFLUENZA Vaccination with the trivalent influenza vaccine (TIV) is the best way to prevent influenza infection. The NICD publishes annual recommendations for the risk groups which should be targeted for influenza vaccination (Walaza, S Afr Med J, 2014). The main risk groups include pregnant women (including the first two weeks of the post-partum period), infants and young children (particularly <2 years of age), persons with chronic diseases (including HIV) and persons aged ≥65 years. The National Department of Health started providing influenza vaccines to the target groups in 2010 and before this time, South African citizens could access the influenza vaccine from private practitioners and pharmacies at a cost. Each year, before the start of the influenza season, the CRDM assists the Department of Health with the training of health care workers in preparation for the delivery of the influenza vaccine. To help raise awareness among health practitioners about the importance of influenza vaccination, the CRDM hosts the annual influenza symposium before the start of the influenza season. The health care worker’s handbook on influenza, published annually, also provides guidance on clinical management and treatment considerations for patients with respiratory illness during the influenza season. 14 CRDM | 2012-2014 Influenza vaccine effectiveness depends on the characteristics of the person being vaccinated (age and health) and on whether there is a good match between the circulating viruses and the viruses contained in the vaccine. In general, influenza vaccines work best among children >2 years and healthy adults. Recent studies using data from the Viral Watch influenza surveillance programme which is coordinated by the CRDM, have generated annual estimates of the effectiveness of the influenza vaccine as administered in South Africa (Ntshoe et al., PLoS One, 2014). In 2013, TIV was estimated to be 87% effective against laboratory-confirmed influenza. This study showed that the vaccine was significantly protective in 2010, 2011 and 2013, but not in 2012 when the circulating A(H3N2) strain showed genetic drift. A recent study conducted in South Africa showed that influenza vaccination of pregnant HIV-infected and-uninfected women was safe and effective. In addition it provided protection to HIV-unexposed infants up to 24 weeks of age (Madhi et al., N Engl J Med, 2014). The effectiveness in infants is important because the influenza vaccine is not licensed for children aged <6 months and this group is at high risk of influenza-associated hospitalisation and death. EMERGING RESPIRATORY VIRUSES Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and avian influenza A(H7N9) Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was identified in 2012 as the cause of severe acute respiratory syndrome cases in Saudi Arabia. All cases of MERS have been linked to countries in and near the Arabian Peninsula and travel history to those countries. The source of human infection with MERS-CoV is still unknown, although the virus has been found in some camels. Persons at highest risk to develop severe MERS include people with diabetes, kidney failure, chronic lung disease, and people whose immune systems are compromised such as transplant and HIVinfected individuals. Human infections with a new avian influenza A(H7N9) virus were first reported in China in March 2013. Most human infections are believed to result from exposure to infected poultry or contaminated environments such as live bird markets in China. This virus is defined as a low pathogenic avian strain as it does not cause disease symptoms in poultry and may continue unnoticed. The possibility that H7N9 could gain the ability to spread sustainably among people should not be ignored. Human-to-human transmission of MERS-CoV and avian influenza A(H7N9) is through close contact with confirmed cases, but no evidence of sustained spread between humans has been reported. For emerging infectious agents of concern, all countries should have the ability to quickly identify potential cases as part of their pandemic preparedness planning. South Africa conducts active surveillance for imported cases of MERS-CoV and influenza A(H7N9). Case definitions are updated regularly and are published on the NICD webpage. The CRDM offers diagnostic testing for MERS-CoV and influenza A(H7N9) as well as a number of additional influenza types and subtypes which have been identified in animals. Support for diagnostic testing is also offered to neighbouring countries that do not have in-country ability to test for emerging viruses. No cases of MERS-CoV or influenza A(H7N9) have been identified in South Africa to date. CRDM | 2012-2014 15 TEN YEARS OF TRACKING MENINGOCOCCAL MENINGITIS AND SEPSIS Even though meningococcal infection occurs infrequently in the population (incidence on average of 1 per 100,000 persons), it is a devastating illness with a sudden onset of severe symptoms that can be life-threatening, even to previously healthy individuals. Meningococcal disease is endemic to South Africa and has the potential to cause outbreaks in crèches, schools, university residents, and on the mines. Since 1999, the CRDM has been conducting surveillance for all laboratory-confirmed cases of meningococcal disease in South Africa. Ten years of meningococcal surveillance data from South Africa were presented at the 16th International Congress of Infectious Diseases, held in Cape Town in 2014. These data clearly show the cyclical nature of the disease as it waxes and wanes over a ten-year period. Currently disease rates are at a nadir, therefore South Africa should be on the alert for a possible upsurge of meningococcal disease over the next few years. 16 CRDM | 2012-2014 Much work has been done to characterise the molecular make-up of the meningococcal isolates causing disease in South Africa by multilocus sequence typing (MLST) and comparing them to isolates from other countries with isolates registered on the global database (http://pubmlst.org/ neisseria/). Meningococci causing disease in South Africa have been characterised and these data have been published in various peer-reviewed journals (du Plessis et al., J Clin Microbiol, 2012; Moodley et al., J Clin Microbiol, 2012; du Plessis et al., J Infect, 2014 and Ndlangisa et al., PLoS One, 2014). PREVENTING MENINGOCOCCAL DISEASE Meningococcal vaccine uptake in South Africa is minimal, however with the recent registration of conjugate meningococcal vaccines CRDM staff have participated in various expert advisory groups in preparation for the use of these new vaccines. The unit is also undertaking a cost-effectiveness analysis to determine potential approaches for routine quadrivalent conjugate meningococcal vaccination introduction in South Africa. Seventy-five percent of the meningococcal cases occurring in South Africa would be prevented by these vaccines which are targeted at preventing disease due to serogroups A, C, Y and W. Potential options for routine vaccination would be to target infants, where disease burden is highest, or teenagers, a group where nasopharyngeal carriage is highest and who contribute most to ongoing transmission. Vaccines based on outer-membrane proteins (OMP) of meningococci have been developed, particularly targeting serogroup B strains which account for a large proportion of disease in developed countries and almost 25% of meningococcal disease in South Africa. Altough this vaccine is targeted at preventing serogroup B disease, it may protect against other serogroups. We have described the distribution of vaccine antigens in isolates from other meningococcal serogroups (Mothibeli et al., Vaccine, 2011). This knowledge should help guide decision makers whether these new OMP vaccines would be of benefit to our population. The CRDM will continue to use the surveillance data to describe the epidemiology of laboratoryconfirmed, invasive disease and to monitor for emerging antimicrobial resistance of the isolates. It will also advocate, where necessary, for the prevention of disease through vaccination. Molecular characterisation will continue in a effort to better understand the lineages that commonly cause disease as we commit to fighting meningococcal disease on a global scale. CRDM | 2012-2014 17 18 CRDM | 2012-2014 BURDEN OF RESPIRATORY SYNCYTIAL VIRUS (RSV) IN SOUTH AFRICA AND RISK GROUPS Respiratory syncytial virus (RSV) is the most common cause of viral pneumonia in children and most children will have been infected by the age of two years. Many children will have a mild common cold-like infection, and re-infection with RSV is common. Surveillance for RSV in hospitalised individuals in South Africa has demonstrated that during the annual RSV season 50-60% of all respiratory admissions in children are due to RSV. The RSV season in South Africa begins in early February until mid May/early June each year. This season precedes the influenza season in South Africa. times more likely to die from RSV infection than HIV-uninfected children. The only prophylaxis and treatment available for RSV is a very expensive monoclonal antibody called palivizumab. Although palivizumab is not available in the public health care sector, when used it is usually given to children at risk of severe illness and given just prior to the RSV season. There are several candidate vaccines in development. In South Africa children under the age of one year admitted to hospital are three times more likely to have RSV-associated pneumonia than non-RSV pneumonia. HIV-infected children are three to five times more likely to be admitted to hospital with RSV pneumonia compared to HIV-uninfected children (Moyes et al., J Infect Dis, 2013). In addition, hospitalised HIV-infected children are 30 DISCOVERING PERTUSSIS IN SOUTH AFRICA Pertussis, commonly known as whooping cough, is caused by the bacterium Bordetella pertussis. It is a highly infectious disease caused by person-to-person spread of infectious respiratory droplets. Bordetella pertussis can cause severe disease in neonates and infants, as well as in older children and adults. It is a vaccine-preventable disease and use of the vaccine has significantly reduced the burden of disease globally. However, in countries with high vaccine coverage, the disease still occurs in neonates and infants that have not received the full primary vaccination schedule. In addition, countries such as the United States, that have replaced the whole-cell pertussis vaccine with the acellular pertussis vaccine are observing an increase in disease in adolescents, potentially due to waning immunity. In South Africa, the whole-cell pertussis vaccine was introduced in 1950 and was replaced by the acellular vaccine in 2009. However, there are limited data on the prevalence of pertussis in South Africa. We have established laboratory methods for the identification of Bordetella species from clinical specimens. We provide specialised diagnostics for suspected cases of pertussis in South Africa and report all cases to the Department of Health. In 2012, we initiated ongoing surveillance for pertussis and are collecting data on the epidemiology of pertussis disease to increase our understanding of pertussis in South Africa, detect outbreaks and advise on policy. We are currently performing surveillance at three sites in Gauteng, KwaZulu-Natal and North West. In 2015, surveillance will be expanded to Mpumalanga and the Western Cape. CRDM | 2012-2014 19 STAYING AHEAD WITH ADVANCED DIAGNOSTICS Molecular diagnostics We have developed highly sensitive and specific molecular diagnostic assays for the detection and characterisation of respiratory and meningeal pathogens including Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis (Wang et al., J Clin Microbiol, 2012), Bordetella pertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella species, parainfluenza virus 1-3, respiratory syncytial virus, influenza virus A and B, enterovirus, human metapneumovirus, adenovirus and rhinovirus (Pretorius et al., J Infect Dis, 2012). These real-time PCR assays enable us to perform rapid diagnostics from clinical specimens, and particularly culture-negative specimens. 20 CRDM | 2012-2014 To ensure reliable and accurate results, we participate in internal and external quality assessment programmes including the United Kingdom National External Quality Assessment Service (UK NEQAS), Quality Control for Molecular Diagnostics (QCMD), exchange programmes with the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). The use of molecular diagnostics and multi-pathogen detection platforms for both bacteria and viruses enables the identification of co-infections and is increasing our understanding of multi-pathogen infections and pathogen interactions. Emerging viral pathogens TaqMan Array Card (TAC) The National Influenza Centre (NIC) is a WHO reference laboratory for influenza surveillance and research that is housed within our centre and continues to provide data on circulating influenza strains to WHO collaborating centres for annual updating of the southern hemisphere vaccine, as well as data on influenza strains that cannot be typed or emerging respiratory viruses. We are conducting studies to evaluate the TaqMan Array Card (TAC) technology. TAC functions as a multiple-pathogen detection system using an array of vessels for the amplification of nucleic acids using real-time PCR. Individual wells of the card contain lyophilised primers and probes to detect pathogen-specific targets. TAC is currently being used in the Sepsis Aetiology in Neonates in South Africa (SANISA) study to detect and identify pathogens causing early-onset neonatal sepsis and community-acquired sepsis in infants at Chris Hani Baragwanath Hospital, as well as in the TAC-KID study, a multi-centre study to determine the cause of pneumonia in children less than 5 years of age in South Africa, India, Peru and Malawi. The following techniques have been established: • Influenza virus isolation using MDCK-SIAT1 cell lines; • Re-establishing the use of embryonated eggs for virus isolation, to increase our ability to generate viable viruses of which representative isolates are sent to the WHO collaborating centres; • Real-time reverse-transcription PCR assays for the diagnosis of infections caused by H5, H7 and H9 avian influenza viruses in order to monitor virus transmission at the animal-human interface; • Serum-based haemagglutination inhibition, to investigate evidence of virus trans mission to humans after avian influenza virus outbreaks in 2011 and 2012; • Full genome sequencing of influenza viruses from clinical samples; and • Real-time RT-PCR testing of the novel Middle East Respiratory Syndrome Coronavirus (MERS-CoV). CRDM | 2012-2014 21 SHARING SKILLS AND EXPERIENCE The CRDM was appointed as a Southern African Regional Reference Laboratory for the Global WHO Vaccine-Preventable Invasive Bacterial Diseases network CRDM is a South African National Accreditation System (SANAS) accredited laboratory since 2008, and complies with the quality management requirements of ISO 15189 (Facility accreditation number M0029G and M0029F). The centre undertakes teaching and training activities for laboratory and clinical staff. The centre also assists with training of among others medical technologists, medical scientists, epidemiologists, public health and microbiology registrars and SA-FELTP residents. Regular surveillance site visits provide training opportunities to engage with laboratory and hospital staff participating in national surveillance. 22 CRDM | 2012-2014 In 2012, the CRDM was appointed as a Southern African Regional Reference Laboratory for the Global WHO Vaccine-Preventable Invasive Bacterial Diseases network and contributes by providing training and technical support to African laboratories in order to improve laboratory capacity and expertise in these countries. Workshops and training interventions hosted by CRDM at the NICD, as well as throughout South Africa and Africa, have provided participants with skills to enhance surveillance and monitoring of respiratory and meningitis pathogens. CRDM | 2012-2014 23 CRDM SURVEILLANCE SITES Enhanced GERMS sites SARI sites Enhanced GERMS and SARI sites 24 CRDM | 2012-2014 Enhanced GERMS sites • • • • • • • • • • • • • Addington, King Edward and RK Khan Hospitals, Durban, KwaZulu-Natal Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, Gauteng Dr George Mukhari Hospital, Pretoria, Gauteng Greys Hospital and Northdale Hospital, Pietermaritzburg, KwaZulu-Natal Kimberley Hospital, Kimberley, Northern Cape Natalspruit Hospital, Johannesburg, Gauteng (only cryptococcus surveillance and invasive pneumococcal disease case-control study) Nelson Mandela Academic Hospital and Mthatha Provincial Hospital, Mthatha, Eastern Cape Polokwane and Mankweng Hospitals, Polokwane, Limpopo Red Cross Children’s Hospital and Tygerberg Hospital, Cape Town, Western Cape Rob Ferreira and Themba Hospitals, Nelspruit, Mpumalanga Rustenberg Hospital, Rustenberg, North West (closed in 2013) Steve Biko (Pretoria Academic Hospital) and Tshwane Hospital, Pretoria, Gauteng Universitas and Pelonomi Hospitals, Bloemfontein, Free State SARI sites • Mapulaneng and Matikwana Hospitals (Agincourt Site), Nelspruit, Mpumalanga Enhanced GERMS and SARI sites • • • • Chris Hani Baragwanath Academic Hospital, Soweto, Gauteng (for SARI 2009-2013) Edendale Hospital, Pietermaritzburg, KwaZulu-Natal Helen Joseph Hospital and Rahima Moosa Mother and Child Hospital, Johannesburg, Gauteng Klerksdorp Tshepong Hospital Complex, Klerksdorp, North West CRDM | 2012-2014 25 CALENDAR OF EVENTS 2012-2014 2012 Conferences hosted National and international conferences 1. Annual Influenza Symposium, NICD, Johannesburg in March each year. 2. 3rd Annual African Network for Influenza Surveillance and Epidemiology (ANISE) Meeting, Nairobi, Kenya, 1 - 3 March 2012. 8th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD), Iguaçu Falls, Brazil, 11 - 15 March 2012. East African Paediatric Conference: Pre-conference meeting on rotavirus, Mombasa, Kenya, 14 April 2012. Southern African Society for Veterinary Epidemiology and Preventative Medicine conference, Farm Inn Hotel, Pretoria, 1 - 2 August 2012. 13th Conference of the International Society for Veterinary Epidemiology and Economics, Maastricht, Netherlands, 20 - 24 August 2012. 11th Biennial Conference of the Society for Tropical Veterinary Medicine (STVM) and the final meeting of the EU-funded project Arbo-Zoonet, Orvieto, Italy, 19 - 22 September 2012. Joint Conference on emerging and re-emerging epidemics affecting Global Health, Ovieto, Italy, 19 - 22 September 2012. 15th Annual Meeting of the European Society for Clinical Virology and Joint Meeting with the European Society for Veterinary Virology, Madrid, Spain, 4 - 7 September 2012. 18th International Pathogenic Neisseria Conference (IPNC), Würzburg, Germany, 9 - 14 September 2012. 8th International Annual Respiratory Syncytial Virus Symposium, Santa Fe, New Mexico, USA, 26 - 30 September 2012. 1st International African Vaccinology Conference (IAVC), Lagoon Beach Hotel, Cape Town, South Africa, 9 - 11 October 2012. 1st International Conference of the African Society for Laboratory Medicine (ASLM), Cape Town, South Africa, 1 - 7 December 2012. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 26 CRDM | 2012-2014 2013 2014 1. The African Influenza and Emerging Respiratory Virus Preparedness Meeting, Westin Hotel, Cape Town, 4 September 2013. 1. 4th African Network for Influenza Surveillance and Epidemiology (ANISE) Meeting, Cape Town, South Africa, 5 - 6 December 2014 . 2. International Conference of the Association of Institutes for Tropical Veterinary Medicine, Johannesburg, South Africa, 25 - 29 August 2013. Options for the Control of Influenza VIII conference (Options), International Convention Centre, Cape Town, 5 - 9 September 2013. 5th Federation of Infectious Diseases Societies of Southern Africa (FIDSSA) Congress, Champagne Sports Resort Drakensberg, KwaZulu-Natal, South Africa, 10 - 12 October 2013. RSVVW 2013 - RSV Vaccines for the World Conference, Porto, Portugal, 14 - 16 October 2013. 8th World Congress of the World Society for Paediatric Infectious Disease (WSPID) Conference, Cape Town, South Africa, 19 - 22 November 2013. 2. 9th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD), Hyderabad, India, 9 - 13 March 2014. 16th International Congress on Infectious Diseases conference Cape Town, South Africa, 2 - 5 April 2014. 5th African Pneumococcal Paediatric Summit, Zimbali Lodge, KwaZulu-Natal, South Africa, 2 August 2014. UNIPATH Conference, Pretoria, South Africa, 19 - 21 September 2014. North West Provincial Research conference, South Africa, 1 - 2 October 2014. 19th International Pathogenic Neisseria Conference (IPNC), Asheville, North Carolina, USA, 12 - 17 October 2014. 9th International RSV Symposium, Stellenbosch, South Africa, 9 - 13 November 2014. 2nd International Conference of the African Society for Laboratory Medicine (ASLM), Cape Town, South Africa, 30 November - 3 December 2014. 3. 4. 5. 6. 3. 4. 5. 6. 7. 8. 9. CRDM | 2012-2014 27 CALENDAR OF EVENTS 2012-2014 2012 Workshops and meetings 1. 2. 3. 4. 5. 6. 7. 8. 28 CRDM | 2012-2014 SARIMA (Southern African Research and Innovation Management Administration) workshop on the role of research managers, Farm Inn, Pretoria, South Africa, 4 - 6 June 2012. Workshop on Health and Economic Impact of Influenza, Intercontinental Hotel, Bali, Indonesia, 5 - 7 June 2012. The Second Southern African Postgraduate Student Symposium, Pretoria, South Africa, 16 - 18 September 2012. The Global Meeting on Immunization Monitoring and Surveillance, Washington DC, United States of America, 9 - 11 October 2012. The Invasive Bacterial Vaccine-Preventable Disease (IB-VPD) Laboratory Technical Working Group, Washington DC, United States of America, 12 October 2012. 1st NICD Pneumococcal Surveillance Workshop, NICD, Johannesburg, South Africa, 17 - 19 October 2012. The Pfizer SA Pneumococcal Disease at the Media Master class meeting, Sandton, Johannesburg, South Africa, 29 October 2012. The Vaccine Effectiveness Methods meeting, Hilton Paddington, London, United Kingdom, 28 - 29 November 2012. 2013 2013 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. The Consortium for the Standardization of Influenza Seroepidemiology (CONCISE) regional influenza seroepidemiology expert meeting, Hong Kong University, Hong Kong SAR, China, 22 - 23 January 2013. International Pneumococcal Disease Expert Panel (Pfizer International Corporation), Johannesburg, South Africa, 11 February 2013. The Pfizer Pneumococcal Expert Summit, Cape Town, South Africa, 16 - 17 March 2013. The Meningitis Expert Forum: A focus on MenACWY-CRM, convened by Novartis, Milan, Italy 28 May 2013. The Global meeting on Implementing New and Under-utilized Vaccines, meeting convened by WHO, Dominican Republic, 4 - 6 June 2013. Sanofi Pasteur Advisory Board Meeting, Radisson Gautrain Hotel, Sandton, Johannesburg, South Africa, 31 August 2013. Consortium for Standardization of Influenza Seroepidemiology (CONSISE) international meeting, Westin Hotel, Cape Town, South Africa, 3 - 5 September 2013. New and Underutilised Vaccines Implementation (NUVI) strategic Review meeting, Geneva, Switzerland, 16 - 20 September 2013. The Respiratory Syncytial Virus Global Estimates Network, Edinburgh, Scotland, 4 - 6 November 2013. Laboratory Technical Working Group (TWG) for WHO IB-VPD Surveillance Network (Invasive Bacterial Vaccine Preventable Disease), Bangkok, Thailand, 13 – 14 November 2013. A Pfizer Advisory Board Meeting on Pneumococcal Vaccination in Adults, Hilton Hotel, Cape Town, South Africa, 16 November 2013. The 2nd GMI (Global Meningococcal Initiative) summit meeting, Cape Town, South Africa, 17 - 18 November 2013. The Influenza Expert meeting, WHO, Geneva, Switzerland, 10 - 13 December 2013. 2014 1. 2. 3. 4. 5. 6. 7. 8. 9. The Academy of Family Practice/ Sandton Clinic CPD Meeting, Bryanston Country Club, Johannesburg, South Africa, 15 February 2014. The 9th Annual Sequencing, Finishing, and Analysis in the Future Meeting, Santa Fe, New Mexico, 28 - 30 May 2014. The 12th regional meeting of The Fogarty International Centerinitiated Multinational Influenza Seasonal Mortality Study (MISMS), an Influenza Research Workshop, Washington DC, United States of America, 30 June - 3 July 2014. Sanofi Pasteur Advisory Board Meeting, Sandton Convention Centre, Sandton, Johannesburg, South Africa, 12 July 2014. National Influenza Surveillance Vaccine Effectiveness meeting at Red Cross Children’s Hospital, Cape Town, South Africa, 22 - 24 July 2014. Good Emergency Management Practice workshop: “Strengthening capacity to respond to animal diseases emergencies” The workshop was held at St George’s Hotel, South Africa, 25 - 29 August 2014. Meningitis Expert Panel Meeting, Rhodes Memorial Restaurant, Cape Town, South Africa, 1 September 2014. The First South African Pertussis Interest Group Meeting, Protea Hotel North Warf, Cape Town, South Africa, 2 September 2014. Global IB VPD Sentinel Surveillance meeting (iTAG and TWG), Geneva, Switzerland, 27 - 30 October 2014. CRDM | 2012-2014 29 CDRM STAFF 30 CRDM | 2012-2014 DISEASES AND MENINGITIS CENTRE FOR RESPIRATORY Bacteriology Dr. Anne von Gottberg Centre head Ms. Linda de Gouveia Laboratory manager Dr. Mignon du Plessis Senior medical scientist Dr. Nicole Wolter Senior medical scientist Medical scientists (x7) Ms. Olga Hattingh Medical technologist Ms. Happy Skosana Medical technologist Medical technologists (x2) Data clerks (x2) Medical technicians (x2) Administrator Virology Dr. Florette Treurnicht Centre head (Acting) Ms. Orienka Hellferscee Medical scientist Medical scientists (x4) Dr. Ziyaad Valley-Omar Senior medical scientist Intern medical scientist Ms. Amelia Buys Medical technologist Medical technologists (x4) Dr. Sibongile Walaza Medical epidemiologist Epidemiology Dr. Cheryl Cohen Centre head Dr. Claire von Mollendorf Medical epidemiologist Dr. Jocelyn Moyes Medical epidemiologist Medical technician Laboratory assistants (x2) Nurse epidemiologist Field project co-ordinator Surveillance officers (x9) Research assistants (x3) Administrator Medical officer Data manager Data analyst Data clerks (x10) Surveillance officer Research assistant 31 STAFF LIST 32 Allam, Mushal Baloyi, Keitumetsi Buthelezi, Nelisiwe Buys, Amelia Carrim, Maimuna Bacteriology unit (Bioinformatician) Epidemiology unit (Surveillance officer) Epidemiology unit (Surveillance officer) Virology unit (Medical technologist) Bacteriology unit (Medical scientist) Cohen, Cheryl de Gouveia, Linda Dlamini, Wesley Duma, Noluthando du Plessis, Mignon Epidemiology unit (Centre head) Bacteriology unit (Laboratory manager) Virology unit (Medical technician) Bacteriology unit (Medical technologist) Bacteriology unit (Medical scientist) Fourie, Cardia Ganesh, Karistha Harvey, Lynn Hattingh, Olga Hellferscee, Orienka Virology unit (Medical technologist) Bacteriology unit (Medical Scientist) Virology unit (Laboratory assistant) Bacteriology unit (Medical technologist) Virology unit (Medical scientist) CRDM | 2012-2014 Hyde, Phyllis Khasane, Pontsho Lengana, Sarona Letlape, Boitumelo Makamba, Wanga Bacteriology unit (Administrator) Epidemiology unit (Administrator) Epidemiology unit (Medical officer) Epidemiology unit (Database administrator) Epidemiology unit (Data clerk) Photograph not available Makhele, Lifuo Malapane, Julia Malinga, Nhlakanipho Mamorobela, Ernest Mampa, Nthabiseng Bacteriology unit (Medical technician) Epidemiology unit (Research assistant) Epidemiology unit (Research assistant) Virology unit (Medical technologist) Epidemiology unit (Data clerk) Manaka, Mokupi Maraka, Dimakatso Mashaba, Theresa Matshogo, Seipati McAnerney, Jo Epidemiology unit (Field project coordinator) Epidemiology unit (Data clerk) Virology unit (Laboratory assistant) Epidemiology unit (Research assistant) Epidemiology unit (Nurse epidemiologist) CRDM | 2012-2014 33 STAFF LIST Photograph not available Mdluli, Cleopatra Mgidlana, Kholiwe Mhlarhi, Shirley Miller, Beulah Moerdyk, Alexandra Epidemiology unit (Data clerk) Epidemiology unit (Research assistant) Epidemiology unit (Data clerk) Virology unit (Medical technologist) Virology unit (Medical technologist) Photograph not available 34 Mogale, Dineo Judith Mohale, Thabo Moleleki, Malefu Moodley, Anna Alleta Moosa, Fahima Bacteriology unit (Medical technologist) Bacteriology unit (Medical scientist) Bacteriology unit (Medical scientist) Epidemiology unit (Surveillance officer) Bacteriology unit (Medical scientist) Moremi, Myrah Motsepe, Kelebogile Moyes, Jocelyn Mthembu, Thembi Mthembu, Thulisile Epidemiology unit (Surveillance officer) Epidemiology unit (Data clerk) Epidemiology unit (Medical epidemiologist) Bacteriology unit (Data clerk) Epidemiology unit (Surveillance officer) CRDM | 2012-2014 Mtshali, Senzo Musetha, Robert Naicker, Dshanta Naidoo, Prabha Ncwana, Bekiwe Virology unit (Medical scientist) Epidemiology unit (Data clerk) Virology unit (Medical scientist) Bacteriology unit (Medical technician) Epidemiology unit (Surveillance officer) Ndlangisa, Kedibone Ngubane, Wendy Nguweneza, Athermon Ntoyi, Mpho Papo, Makatisane Bacteriology unit (Medical scientist) Epidemiology unit (Surveillance officer) Epidemiology unit (Data analyst) Epidemiology unit (Data clerk) Epidemiology unit (Data manager) Photograph not available Phalatse, Louisa Rangunwala, Azeeza Salie-Bassier, Yusrah Sambo, Andrina Sekhula, Pertunia Epidemiology unit (Surveillance officer) Virology unit (Medical scientist) Virology unit (Intern medical scientist) Epidemiology unit (Surveillance officer) Epidemiology unit (Data clerk) CRDM | 2012-2014 35 STAFF LIST Photograph not available Seleka, Mpho Shangase, Khadija Skosana, Happy Treurnicht, Florette Tshabalala, Judith Virology unit (Medical scientist) Epidemiology unit (Surveillance officer) Bacteriology unit (Medical technologist) Virology unit (Acting centre head) Bacteriology unit (Data clerk) von Mollendorf, Claire Walaza, Sibongile Valley-Omar, Ziyaad Venter, Marietjie von Gottberg, Anne Virology unit (Medical scientist) Virology unit (Centre head 2009- March 2014) Bacteriology unit (Centre head) Wolter, Nicole Bacteriology unit (Medical scientist) 36 CRDM | 2012-2014 Epidemiology unit (Medical epidemiologist) Epidemiology unit (Medical epidemiologist) CRDM PUBLICATIONS 2012-2014 1. Abrahams, M. R., F. K. Treurnicht, N. K. Ngandu, S. A. Goodier, J. C. Marais, H. Bredell, R. Thebus, R. D. de Assis, K. Mlisana, C. Seoighe, S. A. Karim, C. M. Gray, and C. Williamson. 2013. Rapid, complex adaptation of transmitted HIV-1 full-length genomes in subtype C-infected individuals with differing disease progression. AIDS. 27:507-518. 2. Adler, D., F. Laher, M. Wallace, K. Grzesik, H. Jaspan, L.-G. Bekker, G. Gray, Z. Valley-Omar, B. Allan, and A.-L. Williamson. 2013. High rate of multiple concurrent human papillomavirus infections among HIV-uninfected South African adolescents. J Immunol Tech Infect Dis 2:1. 3. Ampofo, W. K., S. Al Busaidy, N. J. Cox, M. Giovanni, A. Hay, S. Huang, S. Inglis, J. Katz, T. Mokhtari-Azad, M. Peiris, V. Savy, P. Sawanpanyalert, M. Venter, A. L. Waddell, G. Wickramasinghe, W. Zhang, and T. Ziegler. 2013. Strengthening the influenza vaccine virus selection and development process: outcome of the 2nd WHO Informal Consultation for Improving Influenza Vaccine Virus Selection held at the Centre International de Conferences (CICG) Geneva, Switzerland, 7 to 9 December 2011. Vaccine 31:3209-3221. 4. Archer, B. N., G. A. Timothy, C. Cohen, S. Tempia, M. Huma, L. Blumberg, D. Naidoo, A. Cengimbo, and B. D. Schoub. 2012. Introduction of 2009 pandemic influenza A virus subtype H1N1 into South Africa: clinical presentation, epidemiology, and transmissibility of the first 100 cases. J Infect Dis 206 Suppl 1:S148-53. 5. Archer, B. N., S. Tempia, L. F. White, M. Pagano, and C. Cohen. 2012. Reproductive number and serial interval of the first wave of influenza A(H1N1)pdm09 virus in South Africa. PLoS One 7:e49482. 6. Cockeran, R., H. C. Steel, N. Wolter, L. de Gouveia, A. von Gottberg, K. P. Klugman, A. T. Leanord, D. J. Inverarity, T. J. Mitchell, C. Feldman, and R. Anderson. 2012. Effects of clarithromycin at sub-minimum inhibitory concentrations on early ermB gene expression, metabolic activity and growth of an ermB-expressing macrolide-resistant strain of Streptococcus pneumoniae. Open J Respir Dis 2 :1-8. 7. Cohen, A. L., O. Hellferscee, M. Pretorius, F. Treurnicht, S. Walaza, S. Madhi, M. Groome, H. Dawood, E. Variava, K. Kahn, N. Wolter, A. von Gottberg, S. Tempia, M. Venter, and C. Cohen. 2014. Epidemiology of influenza virus types and subtypes in South Africa, 2009-2012. Emerg Infect Dis 20:1162-1169. 8. Cohen, C., C. von Mollendorf, L. de Gouveia, N. Naidoo, S. Meiring, V. Quan, V. Nokeri, M. Fortuin-deSmit, B. Malope-Kgokong, D. Moore, G. Reubenson, M. Moshe, S. A. Madhi, B. Eley, U. Hallbauer, R. Kularatne, L. Conklin, K. L. O’Brien, E. R. Zell, K. Klugman, C. G. Whitney, and A. von Gottberg. 2014. Effectiveness of 7-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in HIVinfected and -uninfected children in South Africa: a matched case-control study. Clin Infect Dis 59:808-818. 9. Cohen, C., L. Simonsen, J. Sample, J. W. Kang, M. Miller, S. A. Madhi, M. Campsmith, and C. Viboud. 2012. Influenza-related mortality among adults aged 25-54 years with AIDS in South Africa and the United States of America. Clin Infect Dis 55:996-1003. 10. Cohen, C., J. Moyes, S. Tempia, M. Groome, S. Walaza, M. Pretorius, H. Dawood, M. Chhagan, S. Haffejee, E. Variava, K. Kahn, A. Tshangela, A. von Gottberg, N. Wolter, A. L. Cohen, B. Kgokong, M. Venter, and S. A. Madhi. 2013. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009-2011. Emerg Infect Dis 19:1766-1774. 11. Cohen, C., S. Walaza, J. Moyes, M. Groome, S. Tempia, M. Pretorius, O. Hellferscee, H. Dawood, M. Chhagan, F. Naby, S. Haffejee, E. Variava, K. Kahn, S. Nzenze, A. Tshangela, A. von Gottberg, N. Wolter, A. L. Cohen, B. Kgokong, M. Venter, and S. A. Madhi. 2014. Epidemiology of Viral-Associated Acute Lower Respiratory Tract Infection among Children <5 Years of Age in a High HIV Prevalence Setting, South Africa, 2009-2012. Pediatr Infect Dis J. In Press. 12. Crowther-Gibson, P., C. Cohen, K. P. Klugman, L. de Gouveia, and A. von Gottberg. 2012. Risk factors for multidrug-resistant invasive pneumococcal disease in South Africa, a setting with high HIV prevalence, in the prevaccine era from 2003 to 2008. Antimicrob Agents Chemother 56:5088-5095. CRDM | 2012-2014 37 CRDM PUBLICATIONS 2012-2014 13. Dangor, Z., A. Izu, D. P. Moore, M. C. Nunes, F. Solomon, N. Beylis, A. von Gottberg, J. M. McAnerney, and S. A. Madhi. 2014. Temporal association in hospitalizations for tuberculosis, invasive pneumococcal disease and influenza virus illness in South African children. PLoS One 9:e91464. 14. du Plessis, M., C. Moodley, K. M. Mothibeli, A. Fali, K. P. Klugman, and A. von Gottberg. 2012. Population snapshot of invasive serogroup B meningococci in South Africa from 2005 to 2008. J Clin Microbiol. 50:2577-2584. 15. du Plessis, M., N. Wolter, P. Crowther-Gibson, H. J. Hamstra, K. Schipper, C. Moodley, C. Cohen, D. van de Beek, P. van der Ley, A. von Gottberg, and A. van der Ende. 2014. Meningococcal serogroup Y lpxL1 variants from South Africa are associated with clonal complex 23 among young adults. J Infect 68:455-461. 16. Feikin, D. R., E. W. Kagucia, J. D. Loo, R. Link-Gelles, M. A. Puhan, T. Cherian, O. S. Levine, C. G. Whitney, K. L. O’Brien, and M. R. Moore. 2013. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 10:e1001517. 17. Frean, J., O. Perovic, V. Fensham, K. McCarthy, A. von Gottberg, L. de Gouveia, B. Poonsamy, L. Dini, J. Rossouw, K. Keddy, W. Alemu, A. Yahaya, A. Pierson, V. Dolmazon, S. Cognat, and J. B. Ndihokubwayo. 2012. External quality assessment of national public health laboratories in Africa, 2002-2009. Bull World Health Organ 90:191-199A. 18. Gumede, N., O. Lentsoane, C. C. Burns, M. Pallansch, E. de Gourville, R. Yogolelo, J. J. Muyembe-Tamfum, A. Puren, B. D. Schoub, and M. Venter. 2013. Emergence of vaccine-derived polioviruses, Democratic Republic of Congo, 2004-2011. Emerg Infect Dis 19:1583-1589. 19. Haynes, A. K., A. P. Manangan, M. K. Iwane, K. Sturm-Ramirez, N. Homaira, W. A. Brooks, S. Luby, M. Rahman, J. D. Klena, Y. Zhang, H. Yu, F. Zhan, E. Dueger, A. M. Mansour, N. Azazzy, J. P. McCracken, J. P. Bryan, M. R. Lopez, D. C. Burton, G. Bigogo, R. F. Breiman, D. R. Feikin, K. Njenga, J. Montgomery, A. L. Cohen, J. Moyes, M. Pretorius, C. Cohen, M. Venter, M. Chittaganpitch, S. Thamthitiwat, P. Sawatwong, H. C. Baggett, G. Luber, and S. I. Gerber. 2013. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis 208 Suppl 3:S246-54. 20. Hunt, G. M., J. Ledwaba, A. E. Basson, J. Moyes, C. Cohen, B. Singh, S. Bertagnolio, M. R. Jordan, A. Puren, and L. Morris. 2012. Surveillance of transmitted HIV-1 drug resistance in Gauteng and KwaZulu-Natal Provinces, South Africa, 2005-2009. Clin Infect Dis 54 Suppl 4:S334-8. 21. Keddy, K. H., A. Sooka, P. Crowther-Gibson, V. Quan, S. Meiring, C. Cohen, T. Nana, C. Sriruttan, S. Seetharam, A. Hoosen, P. Naicker, E. Elliott, S. Haffejee, A. Whitelaw, and K. P. Klugman. 2012. Systemic shigellosis in South Africa. Clin Infect Dis 54:1448-1454. 22. Liu, M. K., N. Hawkins, A. J. Ritchie, V. V. Ganusov, V. Whale, S. Brackenridge, H. Li, J. W. Pavlicek, F. Cai, M. Rose-Abrahams, F. Treurnicht, P. Hraber, C. Riou, C. Gray, G. Ferrari, R. Tanner, L. H. Ping, J. A. Anderson, R. Swanstrom, M. Cohen, S. S. Karim, B. Haynes, P. Borrow, A. S. Perelson, G. M. Shaw, B. H. Hahn, C. Williamson, B. T. Korber, F. Gao, S. Self, A. McMichael, and N. Goonetilleke. 2013. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. J Clin Invest 123:380-393. 23. Lwande, O. W., Z. Irura, C. Tigoi, E. Chepkorir, B. Orindi, L. Musila, M. Venter, A. Fischer, and R. Sang. 2012. Seroprevalence of Crimean Congo hemorrhagic fever virus in Ijara District, Kenya. Vector Borne Zoonotic Dis 12:727-732. 38 CRDM | 2012-2014 24. Lwande, O. W., J. Lutomiah, V. Obanda, F. Gakuya, J. Mutisya, F. Mulwa, G. Michuki, E. Chepkorir, A. Fischer, M. Venter, and R. Sang. 2013. Isolation of tick and mosquito-borne arboviruses from ticks sampled from livestock and wild animal hosts in Ijara District, Kenya. Vector Borne Zoonotic Dis 13:637-642. 25. Madhi, S. A., C. Cohen, and A. von Gottberg. 2012. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine 30 Suppl 3:C21-7. 26. Madhi, S. A., S. Dittmer, L. Kuwanda, M. Venter, H. Cassim, E. Lazarus, T. Thomas, A. Liberty, F. Treurnicht, C. L. Cutland, A. Weinberg, and A. Violari. 2013. Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS 27:369-379. 27. Madhi, S. A., C. L. Cutland, L. Kuwanda, A. Weinberg, A. Hugo, S. Jones, P. V. Adrian, N. van Niekerk, F. Treurnicht, J. R. Ortiz, M. Venter, A. Violari, K. M. Neuzil, E. A. Simoes, K. P. Klugman, and M. C. Nunes. 2014. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 371:918-931. 28. Madhi, S. A., L. Kuwanda, M. Venter, and A. Violari. 2014. Prospective cohort study comparing seasonal and H1N1(2009) pandemic influenza virus illnesses in HIV-infected children during 2009. Pediatr Infect Dis J 33:174-176. 29. Magomani, V., N. Wolter, S. Tempia, M. du Plessis, L. de Gouveia, and A. von Gottberg. 2014. Challenges of using molecular serotyping for surveillance of pneumococcal disease. J Clin Microbiol 52:3271-3276. 30. McAnerney, J. M., C. Cohen, J. Moyes, T. G. Besselaar, A. Buys, B. D. Schoub, and L. Blumberg. 2012. Twenty-five years of outpatient influenza surveillance in South Africa, 1984-2008. J Infect Dis 206 Suppl 1:S153-8. 31. Meiring, S. T., V. C. Quan, C. Cohen, H. Dawood, A. S. Karstaedt, K. M. McCarthy, A. C. Whitelaw, and N. P. Govender. 2012. A comparison of cases of paediatric-onset and adult-onset cryptococcosis detected through population-based surveillance, 2005-2007. AIDS 26:2307-2314. 32. Metcalf, C. J., C. Cohen, J. Lessler, J. M. McAnerney, G. M. Ntshoe, A. Puren, P. Klepac, A. Tatem, B. T. Grenfell, and O. N. Bjornstad. 2013. Implications of spatially heterogeneous vaccination coverage for the risk of congenital rubella syndrome in South Africa. J R Soc Interface 10:20120756. 33. Moodley, C., M. du Plessis, K. Ndlangisa, L. de Gouveia, K. P. Klugman, and A. von Gottberg. 2012. Clonal analysis of Neisseria meningitidis serogroup B strains in South Africa, 2002 to 2006: emergence of new clone ST-4240/6688. J Clin Microbiol 50:3678-3686. 34. Moyes, J., C. Cohen, M. Pretorius, M. Groome, A. von Gottberg, N. Wolter, S. Walaza, S. Haffejee, M. Chhagan, F. Naby, A. L. Cohen, S. Tempia, K. Kahn, H. Dawood, M. Venter, and S. A. Madhi. 2013. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010-2011. J Infect Dis 208 Suppl 3:S217-26. 35. Msimang, V. M., N. Page, M. J. Groome, J. Moyes, M. M. Cortese, M. Seheri, K. Kahn, M. Chagan, S. A. Madhi, and C. Cohen. 2013. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr Infect Dis J 32:1359-1364. 36. Nair, H., E. A. Simoes, I. Rudan, B. D. Gessner, E. Azziz-Baumgartner, J. S. Zhang, D. R. Feikin, G. A. Mackenzie, J. C. Moisi, A. Roca, H. C. Baggett, S. M. Zaman, R. J. Singleton, M. G. Lucero, A. Chandran, A. Gentile, C. Cohen, A. Krishnan, Z. A. Bhutta, A. Arguedas, A. W. Clara, A. L. Andrade, M. Ope, R. O. Ruvinsky, M. Hortal, J. P. McCracken, S. A. Madhi, N. Bruce, S. A. Qazi, S. S. Morris, S. El Arifeen, M. W. Weber, J. A. Scott, W. A. Brooks, R. F. Breiman, and H. Campbell. 2013. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 381:1380-1390. CRDM | 2012-2014 39 CRDM PUBLICATIONS 2012-2014 37. Ndlangisa, K. M., M. du Plessis, N. Wolter, L. de Gouveia, K. P. Klugman, A. von Gottberg, and GERMS-SA. 2014. Population snapshot of Streptococcus pneumoniae causing invasive disease in South Africa prior to introduction of pneumococcal conjugate vaccines. PLoS One 9:e107666. 38. Ntshoe, G. M., J. M. McAnerney, B. N. Archer, S. B. Smit, B. N. Harris, S. Tempia, M. Mashele, B. Singh, J. Thomas, A. Cengimbo, L. H. Blumberg, A. Puren, J. Moyes, J. van den Heever, B. D. Schoub, and C. Cohen. 2013. Measles outbreak in South Africa: epidemiology of laboratory-confirmed measles cases and assessment of intervention, 2009-2011. PLoS One 8:e55682. 39. Ntshoe, G. M., J. M. McAnerney, S. Tempia, L. Blumberg, J. Moyes, A. Buys, D. Naidoo, M. Venter, T. Besselaar, B. D. Schoub, B. N. Harris, and C. Cohen. 2014. Influenza epidemiology and vaccine effectiveness among patients with influenza-like illness, Viral Watch sentinel sites, South Africa, 2005-2009. PLoS One 9:e94681. 40. Nunes, M. C., T. Shiri, N. van Niekerk, C. L. Cutland, M. J. Groome, A. Koen, A. von Gottberg, L. de Gouveia, K. P. lugman, P. V. Adrian, and S. A. Madhi. 2013. Acquisition of Streptococcus pneumoniae in pneumococcal conjugate vaccine-naive South African children and their mothers. Pediatr Infect Dis J 32:e192-e205. 41. Nzenze, S. A., T. Shiri, M. C. Nunes, K. P. Klugman, K. Kahn, R. Twine, L. de Gouveia, A. von Gottberg, and S. A. Madhi. 2013. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J 32:1270-1278. 42. Nzenze, S. A., T. Shiri, M. C. Nunes, K. P. Klugman, K. Kahn, R. Twine, L. de Gouveia, A. von Gottberg, and S. A. Madhi. 2014. Temporal association of infant immunisation with pneumococcal conjugate vaccine on the ecology of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonisation in a rural South African community. Vaccine 32:5520-5530. 43. Pimenta, F. C., A. Roundtree, A. Soysal, M. Bakir, M. du Plessis, N. Wolter, A. von Gottberg, L. McGee, M. G. Carvalho, and B. Beall. 2013. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 51:647-652. 44. Ping, L. H., S. B. Joseph, J. A. Anderson, M. R. Abrahams, J. F. Salazar-Gonzalez, L. P. Kincer, F. K. Treurnicht, L. Arney, S. Ojeda, M. Zhang, J. Keys, E. L. Potter, H. Chu, P. Moore, M. G. Salazar, S. Iyer, C. Jabara, J. Kirchherr, C. Mapanje, N. Ngandu, C. Seoighe, I. Hoffman, F. Gao, Y. Tang, C. Labranche, B. Lee, A. Saville, M. Vermeulen, S. Fiscus, L. Morris, S. A. Karim, B. F. Haynes, G. M. Shaw, B. T. Korber, B. H. Hahn, M. S. Cohen, D. Montefiori, C. Williamson, and R. Swanstrom. 2013. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol 87:7218-7233. 45. Pretorius, M. A., S. A. Madhi, C. Cohen, D. Naidoo, M. Groome, J. Moyes, A. Buys, S. Walaza, H. Dawood, M. Chhagan, S. Haffjee, K. Kahn, A. Puren, and M. Venter. 2012. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness-South Africa, 2009-2010. J Infect Dis 206 Suppl 1:S159-65. 46. Pretorius, M. A., S. van Niekerk, S. Tempia, J. Moyes, C. Cohen, S. A. Madhi, and M. Venter. 2013. Replacement and positive evolution of subtype A and B respiratory syncytial virus G-protein genotypes from 1997-2012 in South Africa. J Infect Dis 208 Suppl 3:S227-37. 47. Pretorius, M. A., S. Tempia, F. K. Treurnicht, S. Walaza, A. L. Cohen, J. Moyes, O. Hellferscee, E. Variava, H. Dawood, M. Chhagan, S. Haffjee, S. A. Madhi, C. Cohen, and M. Venter. 2014. Genetic diversity and molecular epidemiology of human rhinoviruses in South Africa. Influenza Other Respir Viruses 8:567-573. 40 CRDM | 2012-2014 48. Radin, J. M., M. A. Katz, S. Tempia, N. N. Talla, R. Davis, J. Duque, A. Adedeji, M. J. Adjabeng, W. K. Ampofo, W. Ayele, B. Bakamutumaho, A. Barakat, A. L. Cohen, C. Cohen, I. T. Dalhatu, C. Daouda, E. Dueger, M. Francisco, J. M. Heraud, D. Jima, A. Kabanda, H. Kadjo, A. Kandeel, S. K. Bi Shamamba, F. Kasolo, K. C. Kronmann, M. L. Mazaba Liwewe, J. J. Lutwama, M. Matonya, V. Mmbaga, J. A. Mott, M. A. Muhimpundu, P. Muthoka, H. Njuguna, L. Randrianasolo, S. Refaey, C. Sanders, M. Talaat, A. Theo, F. Valente, M. Venter, C. Woodfill, J. Bresee, A. Moen, and M. A. Widdowson. 2012. Influenza surveillance in 15 countries in Africa, 2006-2010. J Infect Dis 206 Suppl 1:S14-21. 49. Riou, C., F. Treurnicht, M. R. Abrahams, K. Mlisana, M. K. Liu, N. Goonetilleke, R. Koup, M. Roederer, K. S. Abdool, G. de Bruyn, C. Williamson, C. M. Gray, and W. A. Burgers. 2012. Increased memory differentiation is associated with decreased polyfunctionality for HIV but not for cytomegalovirus-specific CD8+ T cells. J Immunol 189:3838-3847. 50. Sartorius, B., C. Cohen, T. Chirwa, G. Ntshoe, A. Puren, and K. Hofman. 2013. Identifying high-risk areas for sporadic measles outbreaks: lessons from South Africa. Bull World Health Organ 91:174-183. 51. Sartorius, B., C. Cohen, T. Chirwa, G. Ntshoe, A. Puren, and K. Hofman. 2013. The impact of prior vaccination coverage and failure, susceptible population accumulation and association with a sporadic national measles outbreak, South Africa, 2009-2011: a framework to predict future targetable high risk areas. Bull World Health Organ 91:174-183. 52. Sayer, J., T. Sunderland, J. Ghazoul, J. L. Pfund, D. Sheil, E. Meijaard, M. Venter, A. K. Boedhihartono, M. Day, C. Garcia, C. van Oosten, and L. E. Buck. 2013. Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses. Proc Natl Acad Sci USA. 110:8349-8356. 53. Shiri, T., K. Auranen, M. C. Nunes, P. V. Adrian, N. van Niekerk, L. de Gouveia, A. von Gottberg, K. P. Klugman, and S. A. Madhi. 2013. Dynamics of pneumococcal transmission in vaccine-naive children and their HIV-infected or HIV-uninfected mothers during the first 2 years of life. Am J Epidemiol 178:1629-1637. 54. Soeters, H. M., A. von Gottberg, C. Cohen, V. Quan, and K. P. Klugman. 2012. Trimethoprim-sulfamethoxazole prophylaxis and antibiotic nonsusceptibility in invasive pneumococcal disease. Antimicrob Agents Chemother 56:1602-1605. 55. Tempia, S., S. Walaza, C. Viboud, A. L. Cohen, S. A. Madhi, M. Venter, J. M. McAnerney, and C. Cohen. 2014. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting-South Africa, 1998-2009. Clin Infect Dis 58:1241-1249. 56. Valley-Omar, Z., S. Sibeko, J. Anderson, S. Goodier, L. Werner, L. Arney, V. Naranbhai, F. Treurnicht, M. R. Abrahams, G. Bandawe, R. Swanstrom, Q. A. Karim, S. S. Karim, and C. Williamson. 2012. CAPRISA 004 tenofovir microbicide trial: no impact of tenofovir gel on the HIV transmission bottleneck. J Infect Dis 206:35-40. 57. Valley-Omar, Z., R. Muloiwa, N. C. Hu, B. Eley, and N. Y. Hsiao. 2013. Novel respiratory syncytial virus subtype ON1 among children, Cape Town, South Africa, 2012. Emerg Infect Dis 19:668-670. 58. van Eeden, C., J. H. Williams, T. G. Gerdes, E. van Wilpe, A. Viljoen, R. Swanepoel, and M. Venter. 2012. Shuni virus as cause of neurologic disease in horses. Emerg Infect Dis 18:318-321. 59. van Tonder, A. J., S. Mistry, J. E. Bray, D. M. Hill, A. J. Cody, C. L. Farmer, K. P. Klugman, A. von Gottberg, S. D. Bentley, J. Parkhill, K. A. Jolley, M. C. Maiden, and A. B. Brueggemann. 2014. Defining the estimated core genome of bacterial populations using a Bayesian decision model. PLoS Comput Biol 10:e1003788. 60. Venter, M., D. Naidoo, M. Pretorius, A. Buys, J. McAnerney, L. Blumberg, S. A. Madhi, C. Cohen, and B. Schoub. 2012. Evolutionary dynamics of 2009 pandemic influenza A virus subtype H1N1 in South Africa during 2009-2010. J Infect Dis 206 Suppl 1:S166-72. 61. Venter, M., P. J. van Vuren, J. Mentoor, J. Paweska, and J. Williams. 2013. Inactivated West Nile Virus (WNV) vaccine, Duvaxyn WNV, protects against a highly neuroinvasive lineage 2 WNV strain in mice. Vaccine 31:3856-3862. 62. von Gottberg, A., C. Cohen, A. Whitelaw, M. Chhagan, B. Flannery, A. L. Cohen, L. de Gouveia, M. du Plessis, S. A. Madhi, and K. P. Klugman. 2012. Invasive disease due to Haemophilus influenzae serotype b ten years after routine vaccination, South Africa, 2003-2009. Vaccine 30:565-571. CRDM | 2012-2014 41 CRDM PUBLICATIONS 2012-2014 63. von Gottberg, A., C. Cohen, L. de Gouveia, S. Meiring, V. Quan, A. Whitelaw, P. Crowther-Gibson, S. A. Madhi, C. G. Whitney, and K. P. Klugman. 2013. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003-2008. Vaccine 31:4200-4208. 64. von Mollendorf, C., C. Cohen, L. de Gouveia, N. Naidoo, S. Meiring, V. Quan, S. Lindani, D. P. Moore, G. Reubenson, M. Moshe, B. Eley, U. M. Hallbauer, H. Finlayson, S. A. Madhi, L. Conklin, E. R. Zell, K. P. Klugman, C. G. Whitney, and A. von Gottberg. 2014. Risk factors for invasive pneumococcal disease among children less than 5 years of age in a high HIV-prevalence setting, South Africa, 2010 to 2012. Pediatr Infect Dis J. In Press. 65. von Mollendorf, C., C. Cohen, L. de Gouveia, V. Quan, S. Meiring, C. Feldman, K. P. Klugman, and A. von Gottberg. 2014. Factors associated with ceftriaxone nonsusceptibility of Streptococcus pneumoniae: analysis of South African national surveillance data, 2003 to 2010. Antimicrob Agents Chemother 58:3293-3305. 66. Walaza, S. 2014. Recommendations pertaining to the use of viral vaccines: influenza 2014. S Afr Med J 104:166-167. 67. Wang, X., M. J. Theodore, R. Mair, E. Trujillo-Lopez, M. du Plessis, N. Wolter, A. L. Baughman, C. Hatcher, J. Vuong, L. Lott, A. von Gottberg, C. Sacchi, J. M. McDonald, N. E. Messonnier, and L. W. Mayer. 2012. Clinical validation of multiplex real-time PCR assays for detection of bacterial meningitis pathogens. J Clin Microbiol 50:702-708. 68. Wolter, N., S. Tempia, C. Cohen, S. A. Madhi, M. Venter, J. Moyes, S. Walaza, B. Malope-Kgokong, M. Groome, M. du Plessis, V. Magomani, M. Pretorius, O. Hellferscee, H. Dawood, K. Kahn, E. Variava, K. P. Klugman, and A. von Gottberg. 2014. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. In Press. 69. Wolter, N., C. Cohen, and A. von Gottberg. 2014. Causes of fever in outpatient Tanzanian children. N Engl J Med 370:2243. 70. Wolter, N., C. Cohen, S. Tempia, S. A. Madhi, M. Venter, J. Moyes, S. Walaza, B. Malope-Kgokong, M. Groome, M. du Plessis, M. Pretorius, H. Dawood, K. Kahn, E. Variava, K. P. Klugman, and A. von Gottberg. 2014. HIV and influenza virus infections are associated with increased blood pneumococcal load: a prospective, hospital-based observational study in South Africa, 2009-2011. J Infect Dis 209:56-65. 71. Wyres, K. L., L. M. Lambertsen, N. J. Croucher, L. McGee, A. von Gottberg, J. Linares, M. R. Jacobs, K. G. Kristinsson, B. W. Beall, K. P. Klugman, J. Parkhill, R. Hakenbeck, S. D. Bentley, and A. B. Brueggemann. 2012. The multidrug-resistant PMEN1 pneumococcus is a paradigm for genetic success. Genome Biol 13:R103-R113. 72. Wyres, K. L., L. M. Lambertsen, N. J. Croucher, L. McGee, A. von Gottberg, J. Linares, M. R. Jacobs, K. G. Kristinsson, B. W. Beall, K. P. Klugman, J. Parkhill, R. Hakenbeck, S. D. Bentley, and A. B. Brueggemann. 2013. Pneumococcal capsular switching: a historical perspective. J Infect Dis 207:439-449. 73. Zaayman, D. and M. Venter. 2012. West Nile virus neurologic disease in humans, South Africa, September 2008 - May 2009. Emerg Infect Dis 18:2051-2054. 42 CRDM | 2012-2014 CRDM | 2012-2014 43 CONTACTS AND RESOURCES Contact information Centre for Respiratory Diseases and Meningitis National Institute for Communicable Diseases, division of the National Health Laboratory Service 1 Modderfontein Road, Sandringham, Johannesburg Tel: 27-11 386 6400 Fax: 27-11 882 0596 E-mail: [email protected] Website: www.nicd.ac.za Centre Heads Anne von Gottberg (Bacteriology) Tel: 27-11 555 0316 E-mail: [email protected] Cheryl Cohen (Epidemiology) Tel: 27-11 386 6593 E-mail: [email protected] Florette Treurnicht (Virology) Tel: 27-11 386 6392 E-mail: [email protected] 44 CRDM | 2012-2014 Resource information World pneumonia day website: http://worldpneumoniaday.org/ WHO Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD): http://www.who.int/woman_child_accountability/news/gappd_2013/en/ UNICEF Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD): http://www.unicef.org/media/files/Final_GAPPD_main_Report-_EN-8_April_2013.pdf UNICEF Pneumonia and diarrhoea: Tackling the deadliest diseases for the world’s poorest children, 2012: http://www.unicef.org/media/files/UNICEF_P_D_complete_0604.pdf UNICEF Committing to Child Survival: A Promise Renewed Progress Report 2013: http://www.unicef.org/publications/files/APR_Progress_Report_2013_9_Sept_2013.pdf United Nations Foundation. Every Woman, Every Child. Commission on Life-Saving Commodities 2012: http://www.everywomaneverychild.org/resources/un-commission-onlife-saving-commodities/life-saving-commodities PATH Tackling the deadliest diseases for the world’s poorest children 2012: http://worldpneumoniaday.org/wp-content/uploads/2012/11/pneumonia-diarrhea-disease-Infographic_2012.pdf Pneumonia: Open Access Journal: https://pneumonia.org.au/ IVAC Pneumonia and Diarrhea Progress Report 2013: http://www.jhsph.edu/research/centers-and-institutes/ivac/resources/IVAC-2013-Pneumonia-Diarrhea-Progress-Report.pdf Centers for Disease Control and Prevention website: http://www.cdc.gov/pneumonia/ National Institute for Communicable Diseases annual reports and publications: http://www.nicd.ac.za/assets/files/2012_GERMS-SA_Annual_Report.pdf http://www.nicd.ac.za/?page=centre_for_respiratory_and_meningitis&id=171 http://www.nicd.ac.za/assets/files/NICD_AR2012-13(1).pdf CRDM | 2012-2014 45 NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES
© Copyright 2026