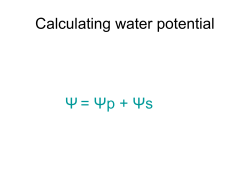Osmosis and Water Potential Lab
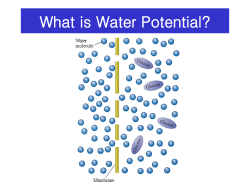
Osmosis and Water Potential Lab Background Information Water moves through membranes from areas of high potential (high free water concentrations) to areas of low potential (low free water concentrations) in a process called osmosis. The direction in which water will move in any given environment can be predicted by determining a system's water potential. Water potential, then, measures the tendency of water to leave one place in favor of another place. Water potential not only measures the tendency of water to move from one place to another, but is also a measure of water’s potential to do work. Water has the ability to dissolve polar molecules, such as salts. A high water potential means there are more free water molecules to do this work. When water moves from an area of high potential to low, it does work in the form of breaking down solutes. In botany, the term water potential is used when predicting the movement of water into or out of plant tissue. Water potential is represented by the Greek letter psi (ψ) and has two components; pressure potential ψ , and solute potential ψ . ψ=ψ +ψ Water potential = Pressure potential + Solute potential p s p s Water potential is affected by two physical factors: 1. Solute Potential –The addition of solutes lowers the water potential of the system (fewer free water molecules.). Solute Potential can be calculated using the following formula: ψs = -iCRT i = Ionization constant (for sucrose this is 1.0 because sucrose does not ionize in water) C = Molar concentration R = Pressure constant (R = 0.0831 liter bars/mole K) T = Temperature in K (273 + C of the solution) o Movement of water into and out of a cell is influenced by the solute potential (relative concentration of solute) on either side of the cell membrane. If a cell is immersed in a hypotonic solution, where the solute potential is low (high water potential), water will move into the cell by osmosis. In contrast, if a cell is immersed in a hypertonic solution where the solute potential is high (low water potential), water will move out of the cell. It is important to realize that water potential and solute concentration are inversely related. In summary, solute potential is the effect that solutes have on a solution’s overall water potential. 2. Pressure Potential- Water movement is directly proportional to the pressure on a system. An increase in pressure will increase the water potential. . Movement of water into and out of a cell is also influenced by the pressure potential (physical pressure) on either side of the cell membrane. In plant cells this physical pressure is exerted when the cell presses against its cell wall and cause the plant to stiffen, this is known as turgor pressure. Pressure potential is usually positive in living cells: in dead xylem tissue it is often negative. (Note: A bar is a metric measure of pressure, measured with a barometer and is about the same as 1 atmosphere. Another measure of pressure is the megapascal (MPa.) 1 MPa = 10 bars.) Water potential values can be positive, zero, or negative. An increase in pressure potential results in a more positive water potential value and a decrease in pressure potential (tension or pulling) results in a more negative water potential value. Solute potential on the other hand is always negative; since pure water has a water potential of zero, any addition of solutes will result in a lower (negative) water potential of the solution. Generally, increasing the solute potential makes the water potential value more negative and increasing the pressure potential makes the water potential more positive. When a solution, such as that inside a carrot cell, is immersed in a second solution and is separated by a selectively permeable cell membrane, water will move (by osmosis) from the solution where water potential is higher, to the side of the membrane where water potential is lower (more negative) due to the solute potential (ψs). In this lab you will use this concept to measure the net movement water into/out of carrot tissue, through osmosis, in sucrose solutions of varying molarities to determine the water potential of carrot cells. Learning Objectives 2.1.d I can represent graphically or model quantitatively the exchange of molecules between and organism and its environment, and the subsequent use of these molecules to build new molecules that facilitate dynamic homeostasis, growth and reproduction. [SP 1.1, 1.4] 2.4.a I can use representations and models to pose scientific questions about the properties of cell membranes and selective permeability based on molecular structure. [SP 1.4, 3.1] 2.4.b I can construct models that connect the movement of molecules across membranes with membrane structure and function [SP 1.1, 7.1, 7.2] 2.5.a I can use representations and models to analyze situations or solve problems qualitatively and quantitatively to investigate whether dynamic homeostasis is maintained by the active movement of molecules across membranes. [SP 1.4] Materials • Ruler • 200 ml beaker/metric measuring cup • Scissors or Knife • Food Scale • • • • • thermometer 6- 3 oz. Cups 24 Petite Baby Carrots Sucrose (Table Sugar) Distilled Water Procedure Part 1-Determining Molar Concentration of Carrots 1. Prepare 100 ml of distilled water, 0.2 M, 0.4M, 0.6M, 0.8M, and 1.0M sucrose solution using steps 2-3. 2. Use the following formula to determine the moles of sucrose needed for each molar solution. Then convert moles to grams. 1) Determine moles of sucrose needed using the Molarity Formula: i. Molarity= moles of solute/Liters of Solution or M = mol/L • Solve for moles. (Don’t forget to convert 100 ml to L first) 2) Convert moles of sucrose to grams of sucrose: i. Multiply the molar mass of sucrose (342.30g) by the moles of sucrose calculated above. This total is the mass (g) of sucrose that will be needed to make 100 ml of solution. ( ____mol x 342.30 g/mol = ___grams of sucrose) 3. Add appropriate mass of sucrose to a beaker or measuring cup, then fill with distilled water up to the 100 ml mark and stir until all sucrose is dissolved. Pour solution into one 3 oz. cup until cup is ¾ full. Label the molarity and discard the excess solution. 4. Repeat steps 2-3 for each solution. 5. Carefully cut the ends off 4 baby carrots for each cup (24 total). You should cut enough off each end so that the resulting length is 2 cm. Keep carrot pieces in a separate covered cup until you are ready to mass them. 6. Using a food scale, determine the mass of the four carrot pieces. Record this mass on your data table. Place these pieces into their respective cup of solution. Write the initial mass of the carrots on the cup. Repeat this step for each cup of solution. 7. Cover each cup and let them stand for 24 hours. 8. Pour off the solution in each cup, blot the carrot pieces with a paper towel and take their final mass. 9. Record the final mass in the data table below. Calculate the percentage change using the formula below. (Note: If final mass is less than initial, you will have a negative % change in mass) % Change in Mass of Carrot Tissue Solution in Cup Initial Mass of Carrots Final Mass of Carrots Mass Difference % change in mass 0.0 M Distilled water 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose 10. Graph your individual data to show Molarity of Solution vs. Percentage Change in Mass. 11. Determine the molar concentration of the carrots. This would be the sucrose molarity in which the mass of the carrot pieces does not change. To find this, draw the straight line on the graph that best fits your data. The point at which this line crosses the x-axis represents the molar concentration of sucrose with a water potential that is equal to the carrot tissue water potential. At this concentration there is no net gain or loss of water from the tissue. Indicate this concentration of sucrose in the space provided below. Molar concentration of sucrose = ____________ M Part 2-‐ CALCULATION OF WATER POTENTIAL FROM EXPERIMENTAL DATA 1. Calculate the solute potential of this sucrose solution using the following formula: ψs = -iCRT i = Ionization constant (for sucrose this is 1.0 because sucrose does not ionize in water) C = Molar concentration of solution resulting in 0% change in mass of carrots R = Pressure constant (R = 0.0831 liter bars/mole K) T = Use thermometer to find Temperature in K (273 + C of the solution) o The units of measure will cancel as in the following example: 2. The pressure potential of this sucrose solution is 0, since it is an open container. 3. Calculating the solute potential of the solution (ψs) and knowing that the pressure potential of the solution is zero (ψp = 0) allows you to calculate the water potential of the solution. ψ = 0 + ψs ψ = ψs 4. The water potential of the solution at equilibrium (point at which there is a 0% change in carrot mass) will be equal to the water potential of the carrots. What is the water potential of the carrot cells from Part 1? Show your work. Lab Discussion Questions 1. Describe what happened when the carrot samples were immersed in hypotonic solutions? • Initially, where was water potential the highest, in the carrot cells or in the hypotonic solution? • Which solutions were hypotonic? 2. Describe what happened to the carrot samples when immersed in hypertonic solutions? • Initially, where was water potential the highest, in the carrot cells or in the hypotonic solution? • Which solutions were hypotonic? 3. Explain the relationship between the molarity of the solutions and the % change in mass of the carrots. 4. Explain how you used the percent change in mass of the carrot samples to determine the water potential of the carrot cells. 5. What would happen to the water potential of the carrot cells if the carrots were allowed to dehydrate. Why? Practical Situation Questions Using what you’ve learned about water potential, hypertonic, hypotonic, and isotonic solutions, discuss the following are common practices in everyday life: 1. Why do grocery stores spray their produce with water on a regular basis even though they are separated from the rest of the plant? 2. How does salt or sugar curing meat prevent the growth of bacteria and fungus? 3. Why is it important to determine the water potential of human cells before developing standard IV solution concentrations. What might happen if human cells are immersed in extremely hypotonic and hypertonic solutions? Adapted from Lab One: Diffusion and Osmosis, College Board, 2001, Biology Lab Manual .
© Copyright 2026