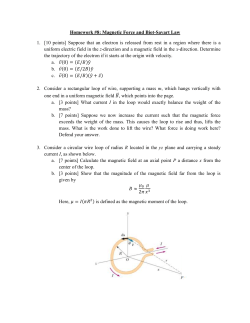
Structure-Magnetic Relationships in the Fe-Mn-P-Si System for Energy Applications
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1197 Structure-Magnetic Relationships in the Fe-Mn-P-Si System for Energy Applications VIKTOR HÖGLIN ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2014 ISSN 1651-6214 ISBN 978-91-554-9091-1 urn:nbn:se:uu:diva-234516 Dissertation presented at Uppsala University to be publicly examined in Polhemsalen, Ångströmlaboratoriet, Lägerhyddsvägen 1, Uppsala, Friday, 12 December 2014 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Dr Mogens Christensen (Aarhus University, Denmark). Abstract Höglin, V. 2014. Structure-Magnetic Relationships in the Fe-Mn-P-Si System for Energy Applications. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1197. 74 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9091-1. Demands for new, energy-efficient appliances have greatly increased in response to our growing need for a more environmentally friendly society. Magnetic refrigeration is a technique that utilizes the magnetocaloric effect, with possible energy savings of up to 30% compared to commercial gas compression refrigerators. A material appropriate for commercial magnetocaloric devices should be both cheap and non-toxic; it should also exhibit a firstorder magnetic transitions close to room temperature. The magnetic properties of Fe2P-related materials can be relevant in this context, since their magnetic properties can be finely tuned through the substitution of Fe by Mn and P by Si, As, Ge or B to meet the general requirements for a magnetocaloric device. An in-depth study has therefore here been made of the structural and magnetic properties of the (Fe,Mn)2(P,Si)-system. The phase diagram of the FeMnP1-xSix-system has been carefully reexamined. It is found to contain two single-phase regions: an orthorhombic Co2P-type structure (x < 0.15) and a hexagonal Fe2P-type structure (0.24 ≤ x < 0.50). Selected compounds within the Fe2P-type region of the phase diagram have been shown to exhibit potential for use in magnetic refrigeration applications. Neutron powder diffraction has here been used to determine the magnetic structures of selected crystalline compositions within the FeMnP1-xSix-system to gain a better understanding of its magnetic properties. The Fe2P-type region is mainly ferromagnetic, but an incommensurate antiferromagnetic structure has also been identified close to the Co2P/Fe2P-type phase border for x ≈ 0.25. The so-called ''virgin effect'' in the Fe2P-type region of the FeMn(P,Si) phase diagram is found to be accompanied by an irreversible structural phase transition induced by magnetostriction. This new phase is found to be preserved during successive cooling-heating cycles. Furthermore, the magnetic properties of the substituted Fe2P-type structure changes significantly for metal:non-metal ratios away from 2:1. Such deviations could well explain the apparently conflicting structure-property relationships described in earlier literature for the FeMnP1-xSixsystem. Keywords: Magnetocaloric, X-ray powder diffraction, Neutron powder diffraction, Magnetization measurements, Phase diagram, Crystal structure, Magnetic structure, Incommensurate structure, Ferromagnetic, Antiferromagnetic, Fe2P, Fe-Mn-P-Si. Viktor Höglin, Department of Chemistry - Ångström, Inorganic Chemistry, Box 538, Uppsala University, SE-751 21 Uppsala, Sweden. © Viktor Höglin 2014 ISSN 1651-6214 ISBN 978-91-554-9091-1 urn:nbn:se:uu:diva-234516 (http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-234516) ”Tittar man noga efter så ska man finna små, små skillnader som faktiskt gör varje gump till ett unikum.” Tage Danielsson Till Johanna och August List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I The crystal and magnetic structure of the magnetocaloric compound FeMnP0.5 Si0.5 V. Höglin, M. Hudl, M. Sahlberg, P. Nordblad, P. Beran, Y. Andersson. Journal of Solid State Chemistry, 184, 2434-2438 (2011). II Strongly enhanced magnetic moments in ferromagnetic FeMnP0.5 Si0.5 M. Hudl, L. Häggström, E. K. Delczeg-Czirjak, V. Höglin, M. Sahlberg, L. Vitos, O. Eriksson, P. Nordblad, Y. Andersson. Applied Physics Letters, 99, 152502 (2011). III Detailed study of the magnetic ordering in FeMnP0.75 Si0.25 V. Höglin, M. Hudl, L. Caron, P. Beran, M. H. Sørby, P. Nordblad, Y. Andersson, M. Sahlberg. Journal of Solid State Chemistry, 221, 240-246 (2015). IV Irreversible structure change of the as prepared FeMnP1−x Six structure on the initial cooling through the Curie Temperature V. Höglin, J. Cedervall, M. S. Andersson, T. Sarkar, P. Nordblad, M. Sahlberg. Journal of Magnetism and Magnetic Materials, 374, 455-458 (2015). V Phase diagram, structures and magnetism of the FeMnP1−x Six system V. Höglin, J. Cedervall, M. S. Andersson, T. Sarkar, M. Hudl, P. Nordblad, Y. Andersson, M. Sahlberg. Submitted. Reprints were made with permission from the publishers. My contributions Paper I. I have planned the study, synthesized the samples and performed all structural characterizations. I wrote the main part of the manuscript and was involved in all discussions. Paper II. I have synthesized the samples and taken part in the planning, discussion and writing of the manuscript. Paper III. I have planned the study, synthesized the samples and performed all structural characterizations except the representational analysis. I wrote the main part of the manuscript and was involved in all discussions. Paper IV. I have planned the study, synthesized the samples and taken part in the structural characterizations, discussion and writing of the manuscript. Paper V. I have planned the study, synthesized a majority of the samples and performed all structural characterizations. I wrote the main part of the manuscript and was involved in all discussions. Other publications to which the author has contributed. Magnetocrystalline anisotropy and the magnetocaloric effect in Fe2 P L. Caron, M. Hudl, V. Höglin, N. H. Dung, C. P. Gomez, M. Sahlberg, E. Brück, Y. Andersson, P. Nordblad. Physical Review B, 88, 094440, (2013). Thermodynamics around the first-order ferromagnetic phase transition of Fe2 P single crystals M. Hudl, D. Campanini, L. Caron, V. Höglin, M. Sahlberg, P. Nordblad, A. Rydh. Physical Review B, 90, 144432, (2014). Contents 1 Introduction 1.1 The magnetocaloric effect . . . . . . . . . . . . . . . . . . . 1.2 Material systems . . . . . . . . . . . . . . . . . . . . . . . 1.2.1 The mother compound Fe2 P . . . . . . . . . . . . . 1.2.2 Substituted Fe2 P-type compounds . . . . . . . . . . 1.2.3 Fe2 P-type compounds in magnetocaloric applications . . . . . 13 14 16 16 17 18 2 Methods 2.1 Material synthesis . . . . . . . . . . . . . . . . . . . . . . 2.2 X-ray diffraction techniques . . . . . . . . . . . . . . . . 2.2.1 X-ray powder diffraction . . . . . . . . . . . . . . 2.2.2 Synchrotron radiation X-ray powder diffraction . . 2.3 Neutron powder diffraction . . . . . . . . . . . . . . . . . 2.4 Determination of crystal and magnetic structures . . . . . 2.4.1 The Rietveld method . . . . . . . . . . . . . . . . 2.4.2 Representational analysis . . . . . . . . . . . . . . 2.5 Magnetic characterization . . . . . . . . . . . . . . . . . . 2.6 Other experimental methods . . . . . . . . . . . . . . . . 2.6.1 Differential thermal analysis and thermogravimetry 2.6.2 Mössbauer spectroscopy . . . . . . . . . . . . . . 2.6.3 Theoretical methods . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19 19 19 20 21 22 24 25 25 25 26 26 26 27 3 Results and discussion 3.1 The phase diagram of FeMnP1−x Six 3.2 Compounds of Fe2 P-type . . . . . . 3.2.1 FeMnP0.50 Si0.50 . . . . . . . 3.2.2 FeMnP0.75 Si0.25 . . . . . . . 3.3 The virgin effect . . . . . . . . . . . 3.4 Phosphorus deficient samples . . . . 3.5 In field XRD experiments . . . . . . . . . . . . . . . . . . . . 29 29 35 35 39 49 53 56 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 Summary and concluding remarks 59 5 Sammanfattning på svenska 61 6 Acknowledgments 65 References 67 Abbreviations CPA DFT DTA EMTO EPMA EDS f.u. FOMT µB M Msat MCE MPMS NPD PPMS RA RT SQUID SR-XRD XRD Coherent potential approximation Density functional theory Differential thermal analysis Exact muffin-tin orbital method Electron probe micro analysis Energy-dispersive X-ray spectroscopy Formula unit First order magneto-structural phase transition Bohr magneton Magnetization Saturated magnetization Magnetocaloric effect Magnetic property measurements system Neutron powder diffraction Physical property measurements system Representational analysis Room temperature Superconducting quantum interference device Synchrotron radiation X-Ray powder diffraction X-ray powder diffraction 11 1. Introduction Alloying of metals is a craft with historical heritage back to around 2000 B.C. when humans learned to melt copper and tin to prepare bronze [1]. The aspiration to find new and to improve existing materials is still present today when researchers of the world continuously strive to develope new materials with extreme properties. The phase diagram of silver and copper reported by W. Chandler Roberts in 1875 [2] is believed to be the first ”temperature-composition”-diagram [3]. The first compilation of binary phase diagrams was published by Max Hansen in German in 1936 and was translated, expanded and published in English in 1958 [4]. The amount of phase related data grew rapidly during the second half of the 1900s and in 1970 the CALPHAD (CALculation of PHAse Diagrams) method to predict phase equilibrium in not yet experimentally investigated system was described by Kaufman and Bernstein [5]. Calculations are fast and economical compared to experimental phase determination and consequently computational simulation has grown to become the dominant method in present time [3]. The work to study alloy systems and to construct phase diagrams is very time consuming and is today rarely performed experimentally, since the majority of the binary compounds already have been studied and the ternary and quaternary phase diagrams take unreasonably large efforts to produce experimentally. Nevertheless, it is possible to construct parts of ternary or quaternary phase diagrams to put focus on certain areas to be studied. Because if one possesses knowledge of the structural characteristics of a certain alloy system this can be used to understand and predict other qualities such as the magnetic properties. The primary objective of this thesis has been to explore materials which have potential to be used in magnetocaloric applications, e.g. magnetic refrigeration. The work has been made from a crystallographic point of view and detailed studies of the structures of the investigated materials have been performed using a broad spectrum of characterization techniques with focus on diffraction methods. All materials in this thesis are of (Fe,Mn)2 (P,Si)-type and efforts have been made to derive phase diagrams of the structural and magnetic orderings of FeMnP1−x Six . This work has been part of an interdisciplinary project on magnetocalorics where the focus of this thesis has been on sample preparation and complete determination of the structural and magnetic properties of the FeMnP1−x Six -system. 13 1.1 The magnetocaloric effect Total entropy The magnetocaloric effect (MCE) is a phenomena experimentally discovered by Weiss and Piccard in 1918 [6]. Warburg and his observations from 1881 [7] have by tradition been cited as the discovery of the MCE but in a review of literature by Smith [8], Weiss and Piccard are proposed as the real discoverers of the MCE when they found a reversible heating close to the Curie temperature (TC ) of nickel when a magnetic field was applied (a temperature rise of 0.7 K in a field of 1.5 T). Thus, in contrast to Warburg, they identified the two main features of the magnetocaloric effect: it is reversible and it is largest at temperatures close to the TC . The MCE is quantified by two parameters, the entropy change per mass unit ∆S (for an isothermal field change) and the temperature change ∆Tad (for an adiabatic field change). ∆Tad is defined as the temperature difference when a sample is initially exposed to a magnetic field Hi which is changed to the final field H f (Hi < H f ) under adiabatic conditions. Similarly, ∆S is defined as the entropy change when a sample is exposed to an magnetic field change Hi to H f under isothermal conditions, see figure 1.1. Hi Hf ∆Tad(T;Hi,Hf) ∆S(T;Hi ,Hf) Temperature TC Figure 1.1: The isotropic entropy and adiabatic temperature in the initial (Hi ) and final (H f ) magnetic fields at the Curie temperature, TC . The isothermal entropy change can be calculated as a function of field and temperature from magnetization (M) measurements using the Maxwell relation ∂S ∂H T = µ0 ∂M ∂T (1.1) H as starting point (where µ0 is the vacuum permeability = 4π ·10−7 V·s·A−1 m−1 ). The entropy change can be calculated according to 14 ∆S = µ0 ZH f Hi ∂M ∂T (1.2) dH H and the adiabatic temperature change can be estimated from magnetization and heat capacity (C) experiments using ∆Tad = −µ0 ZH f Hi T CH ∂M ∂T dH. (1.3) H The derivation and numerical methods to calculate ∆S and ∆Tad are described by Thishin and Spichkin [9]. Experimental methods for direct measurements of ∆S and ∆Tad are continuously being developed [10–17]. ∆S and ∆Tad are quantitative quality measures of the efficiency of a magnetocaloric material. However, the two quantities are directly coupled and has to be optimized simultaneously [18]. The actual performance of a material is better appreciated if also the refrigerant capacity (RC) is derived: Thigh RC = Z ∆S dT (1.4) Tcold as it gives a measure of how much energy a given amount of material can transfer betweeen the desired low temperature (Tcold ) and ambient temperature (Thigh ) [19; 20] Figure 1.2 schematically illustrates the refrigeration cycle of a magnetocaloric system. The magnetic moments of the magnetocaloric material are initially randomly oriented (1), then a magnetic field is applied and the magnetic moments align along the field reducing the magnetic entropy, which results in heating of the working material (2). The heat is removed from the material using heat transfer (with a transfer medium such as water, air or helium) (3) and a subsequent removal of the magnetic field will cool the material below the initial temperature (4). This final step is used to extract heat from the system to be cooled by using heat transfer. The efficiency of the magnetocaloric refrigeration cycle promises possible energy savings of up to 30% if compared with gas compression refrigerators [21]. The discovery of the giant MCE in Gd5 (Ge,Si)4 compounds by Pecharsky and Gschneidner [22] boosted the interest in magnetic refrigeration. A large number of material systems such as La(Fe,Si)13 , MnAs, Heusleralloys and related compounds have been studied in order to find an optimal 15 Figure 1.2: Scheme of the magnetic refrigeration cycle. material for use in magnetocaloric applications [23–25]. An optimal material must show large ∆S and ∆Tad and the first materials showing giant MCE were rare earth compounds. But in order to go commercial, an ideal material must show advantageous magnetocaloric characteristic, be economically favorable and be synthesized from non toxic elements. A commercialization of magnetic refrigeration has not yet been achieved even if there are ongoing efforts to start large scale material synthesis of magnetocaloric materials. 1.2 Material systems 1.2.1 The mother compound Fe2 P In 1929 Hägg [26] performed the first examinations of Fe2 P using X-ray powder diffraction (XRD). He determined the lattice parameters and proposed a hexagonal structure. A complete structure was not determined until 1959 when Rundqvist and Jellinek [27] stated that Fe2 P has the symmetry of the P62m space group. The structure of Fe2 P (from now called the Fe2 P-type structure in the text) has two iron sites, one on the crystallographic 3f site surrounded by four phosphorus atoms in a tetrahedral ordering. The second iron site is situated on the 3g position surrounded by five phosphorus atoms in a pyramidal arrangement. The two phosphorus sites (2c and 1b) are surrounded by trigonal prisms of iron atoms. Layer arrangements are formed by the 3f iron and 2c phosphorus and the 3g iron and 1b phosphorus respectively, see table 1.1 and figure 1.3. 16 Table 1.1: Atomic parameters of the Fe2 P-type structure, space group P62m. Numerical data are from ref. [28] Atom Site x y z Fe I Fe II PI P II 3g 3f 2c 1b 0.2568(1) 0.5946(1) 0 0 1 2 1 3 2 3 0 0 0 0 1 2 Fe I (3f) Fe II (3g) P I (2c) P II (1b) c a b a b Figure 1.3: Unit cell of the hexagonal Fe2 P-type structure, space group P62m A first order magneto-structural phase transition (FOMT) is observed for Fe2 P at TC = 216 K. Sample series of Fe2−x P have shown that the lattice parameters and, consequently, the magnetic properties are very sensitive to deviations in sample composition [28–30]. This makes Fe2 P a very delicate material system to study since small changes in the sample composition may alter the characteristics heavily. 1.2.2 Substituted Fe2 P-type compounds Elemental substitutions in Fe2 P manipulate the magnetic properties. For example, TC is very sensitive to substitution of phosphorus and is increased to ∼350 K [31], ∼450 K [32] and ∼350 K [33] for 10% substitution of silicon, boron and arsenic respectively retaining Fe2 P-type structure. On the metal sites substitution of iron with manganese ((Fe1−x Mnx )2 P) will retain pure ferromagnetic ordering for x ≤ 0.015 while for 0.0015 < x ≤ 0.03 there is fer17 romagnetism for low temperatures (<100K) and an incommensurate screwantiferromagnetism at high temperatures (>150K). Pure antiferromagnetic ordering is found for x > 0.03 [34]. The hexagonal Fe2 P-type structure is preserved until x > 0.26 where a phase transition to the orthorhombic Co2 P-type structure takes place [35; 36]. The magnetic moments on the metal sites are increased by manganese substitution and the manganese atom at the pyramidal 3g site of FeMnP possesses a magnetic moment of 2.6 µB [37] compared to 1.78 µB for the iron atom on the 3g site in Fe2 P [38]. The increased magnetic moment on the metal atoms can be combined with a tunable TC by creating a quaternary system of (Fe,Mn)2 (P,A)-type (A = Si, As and/or Ge). During the 1990s, comprehensive studies of the FeMnP1−x Asx system were performed regarding structural and magnetic properties as well as magnetic structures [39–43]. This material system paved the way for numerous new compounds to be studied or re-investigated for magnetocaloric applications during the 2000s. 1.2.3 Fe2 P-type compounds in magnetocaloric applications The (Fe,Mn)2 P1−x Asx -system was found to be very promising for use in magnetic refrigeration [44–46] due to its giant MCE and transition temperature tunable close to room temperature. Further studies to improve (Fe,Mn)2 P1−x Asx -type materials were made by adding a fifth alloy element (germanium) in order to enhance the MCE, increase TC and reduce the thermal hysteresis for selected compounds [47–49]. However, the use of arsenic-rich compounds in products meant for commercial use is always received with aversion. It was therefore crucial to substitute arsenic with a more environmentally friendly element while keeping the advantageous magnetocaloric properties found in this material system. Arsenic was found to be satisfactory replaced by silicon or a Si/Ge combination [50–53]. The latest approach of magnetocaloric materials based on the Fe2 P-type structure are samples with metal deficiencies of (Fe,Mn)1.95 (P,Si)-type [54– 57]. These samples have exhibit less thermal hysteresis which is a known problem for many compounds of Fe2 P-type. 18 2. Methods 2.1 Material synthesis The materials studied in this thesis are from the (Fe,Mn)2 P1−x Six -system and samples were prepared for 0.05-steps of x except at selected phase borders were smaller steps of x were used. Since manganese and phosphorus are highly volatile elements the drop synthesis technique [28] was used to ensure low losses of the raw materials due to evaporation during sample synthesis. The equipment used for the drop synthesis technique is shown in figure 2.1. Proper amounts of iron and silicon were put in crucibles of alumina and melted using induction heating. The temperature of the melt was measured using a pyrometer. After a stable melt of iron and silicon was formed, pieces of manganese and phosphorus were dropped into the melt causing an immediate reaction with the iron-silicon melt. Successful experiments produce samples of 10-30 g with losses < 0.5 wt%. This technique for phosphide synthesis is well described in the literature and only minor changes have been made compared to the techniques described by Haughton [58] and Hägg [26]. Selected compositions (FeMnP, FeMnP0.25 Si0.75 , FeMnP0.50 Si0.50 , FeMnP0.25 Si0.75 , FeMnSi, FeMnP0.40 Si0.50 and (Fe0.375 Mn0.625 )2 P0.50 Si0.50 .) were prepared using the drop synthesis method while the intermediate compositions were prepared from mixtures of the larger batches (most characterization techniques used in this thesis only need orders of mg to work satisfyingly, except for neutron powder diffraction where the amount of sample preferably should be > 1 cm3 ). All samples were grounded and heat treated in evacuated silica ampules and finally either slowly cooled to room temperature or quenched in water. 2.2 X-ray diffraction techniques X-ray diffraction is undoubtedly one of the most important methods for characterizing crystal structures. Diffraction occurs when X-rays are scattered coherently by atomic planes in a crystal. The scattering strength of the X-rays depends on the atomic number and falls of with increased diffraction angle. 19 a) (1) b) (2) (3) (4) (5) (6) (7) (8) (9) (10) Figure 2.1: The experimental set-up for the drop synthesis method. Photo of the furnace in (a) and schematic view in (b). (1) viewing window, (2) volatile elements (such as phosphorus and manganese), (3) soft magnetic pusher, (4) Pyrex glass tube, (5) silica funnel, (6) silica tube, (7) alumina crucibles, (8) RF work coil, (9) starting materials (such as iron and silicon), (10) vacuum connection. 2.2.1 X-ray powder diffraction An ideal powder is defined as a polycrystalline sample with randomized crystals arranged in every possible orientation. By varying the incident angle θ of the X-ray beam, a sufficiently large number of randomly oriented crystals in the studied polycrystalline material will be oriented in such way that some of the hkl planes in the crystals will be oriented in the Bragg angle. A sweep over a selected θ -range will produce a so called powder pattern. Every crystalline phase has an unique powder pattern which can be used for identification. XRD intensities were collected on a Bruker D8 diffractometer equipped with a Våntec position sensitive detector (PSD) with 4◦ opening using CuKα1 radiation (λ = 1.540598 Å). Measurements were performed in temperatures from 16 K to 403 K and in a 2θ -range of 20-90◦ . Measurements above RT were performed using an Anton Paar XRK 900 reaction chamber and measurements below RT were performed using an Oxford Phenix cryostat. 20 2.2.2 Synchrotron radiation X-ray powder diffraction Synchrotron radiation X-Ray powder diffraction (SR-XRD) is a technique using accelerated electrons to produce high intensity X-rays. The high intensity makes it possible to perform ”one shot” experiments and collect X-ray intensities over the whole 2θ -range within seconds. The ability of this method to collect complete data sets makes SR-XRD an excellent technique for structure investigations vs. temperature. SR-XRD experiments were performed on the I711 beamline at the MAX IV Laboratory in Lund, Sweden [59]. Wavelength and sample to detector distances were determined using a LaB6 standard and collected data were handled in the FIT2D software [60]. Pulverized samples were carefully loaded in a thin quartz capillary and placed in a single crystal sapphire tube which was attached to the sample cell. The sample cell [61] was heated using resistive heating (tungsten or kanthal wire) and the temperature was measured using a thermocouple placed inside the sapphire tube. A custom built add-on to the sample cell featuring a strong permanent magnet of NdFeB-type, see figure 2.2, made it possible to perform XRD experiments in a magnetic field of 0.35 T. (2) (1) Figure 2.2: Sample cell for SR-XRD experiments in field. A permanent magnet of NdFeB-type is placed inside the aluminum box (1). Samples placed in the gap (2) are exposed for a magnetic field of 0.35 T. 21 2.3 Neutron powder diffraction Neutron powder diffraction (NPD) is an elastic scattering technique with similarities to XRD but complementary information can be determined since the interaction with matter is different. The neutrons interact with the nucleus of the atom in contrast to XRD where the X-rays are scattered by the electrons surrounding the nucleus. Therefore, while the scattering length of the X-rays has a linear dependence on the atomic number the scattering amplitude of the neutrons depends on two factors. Firstly, the contribution called ”potential scattering” which depends on the size of the nucleus. Secondly there may be ”resonance scattering” which depend on the detailed structure of the nucleus which can vary greatly from element to element and even from isotope to isotope [62]. The summarized neutron scattering amplitude has an irregular dependence on the atomic number, see figure 2.3. This makes it possible to separate iron from manganese which has been of importance for the work in this thesis. X-rays 5 X-rays = 0° (sin )/ -1 = 0.5 Å Scattering amplitude (10 -12 cm) 6 4 3 2 58 Sc Cl 1 0 7 1 -1 H Li 20 40 56 Ni Fe Mn Neutrons 60 Ti 62 Ni 80 100 Atomic weight Figure 2.3: The irregular variation of the neutron scattering amplitude as a function of atom weight. The regular increase for X-rays is shown for comparison. The neutron is an uncharged particle, but it carries a magetic moment and therefore interacts with the unpaired electrons which arises in the electron cloud around the nucleus in a magnetic material. This makes it possible to determine the microscopic magnetic structure of a material. In a simple case with a ferromagnetic structure where all magnetic moments, within a domain, align in the same direction, the magnetic diffraction peaks will occur at the same angular positions as the nuclear peaks. Thus, the magnetic scattering in a ferromagnetic material (see figure 2.4a) will merely increase the intensity of the nuclear peaks (as seen in figure 2.5a). In a case with a simple antiferromagnetic structure (see figure 2.4b) the magnetic moments point alternately 22 up and down in the structure making the magnetic unit cell twice as large as the chemical cell along the b-axis. This type of antiferromagnetic material will give diffraction peaks of purely magnetic origin, see figure 2.5b. An important feature of the magnetic scattering is that it decreases with increased scattering angle in contrast to the nuclear scattering. Consequently, high intensity peaks from magnetic scattering occurs at low 2θ angles. a) b) c b b a Figure 2.4: Unit cells of a ferromagnetic (a) and an antiferromagnetic (b) structure. The dashed lines in the ferromagnetic case indicate a second unit cell. The directions of the arrows indicate the directions of the magnetic moments. Intensity a) 2θ b) A B C 2θ Figure 2.5: Neutron diffraction intensities from a ferromagnetic (a) and antiferromagnetic (b) ordered sample. The additional magnetic scattering (shaded) contributes to the nuclear peaks for a ferromagnetic material and appears as new peaks A, B and C for an antiferromagnetic material. 23 The iteration of the a magnetic structure can (simplified) be described be the propagation vector k = (qx qy qz ). The simple ferromagnetic structure above can be described by the vector k = 0 since the chemical and magnetic unit cells are the same. The propagation vector in the antiferromagnetic case is k = (0 1/2 0) since the magnetic cell propagates in the b-direction and there is two crystallographic unit cells needed to describe the magnetic unit cell. In more complicated cases when the terms of the propagation vector can not be described by rational numbers the structure is incommensurate. These types of magnetic structures are infinite and can be e.g. helical or sinusoidal, see figure 2.6. a) b) Figure 2.6: Models of a sinusoidal (a) and a helical (b) magnetic structure. The NPD experiments presented in paper I and III were performed using thermal neutrons at the Nuclear Physics Institute in Rez, Czech Republic. Intensities were collected using the MEREDIT diffractometer and the neutron beam was monochromatized by a copper mosaic monochromator (reflection 220) providing a wavelength of λ = 1.46 Å. A 2θ -range of 4-144 (and 148)◦ was used at 16 K, 298 K and 450 K. Intensities in paper III were also collected using thermal neutrons on the PUS diffractometer at the Institute for Energy Technology in Kjeller, Norway. The neutron beam was monochromated by a germanium mosaic monochromator (reflection 511) giving a wavelength of λ = 1.556 Å. A 2θ -range of 10-105◦ was used at 8 K and 298 K. 2.4 Determination of crystal and magnetic structures There is a long list of parameters that can be derived from powder diffraction data. Most essential are the lattice parameters which were determined from Bragg peak positions of XRD intensities using a non-linear least square method implemented in the software UNITCELL [63]. Methods for in depth studies of the powder diffraction intensities are described below. 24 2.4.1 The Rietveld method The method to perform structural analysis using the the whole pattern from powder diffraction experiments was developed by Hugo M. Rietveld [64] during the 1960s. The structural model and the instrument parameters are refined in a least-square procedure where the function M = ∑ Wi i 1 yi (obs) − yi (calc) c 2 (2.1) is minimized over the whole profile. Wi is a weighting factor, yi (obs) and yi (calc) are the observed and calculated intensities respectively and c is a scale factor. This procedure is carried out by varying selected parameters until a satisfactory fit is obtained. The fit is numerically calculated and the parameters R p , Rwp , RBragg , Rexp , RF and χ 2 are used as guides for the progress of the refinement [65; 66]. Nevertheless, the user must still evaluate all parameters manually and determine if they are reasonable. All refinements to resolve crystallographic and magnetic structures were performed according to the Rietveld method using the software FULLPROF [67; 68] on intensities generated from XRD and NPD measurements. 2.4.2 Representational analysis The determination of a magnetic structure can be very time consuming and frustrating if using a trial and error method. The number of possible structures can be reduced by only investigating structures that are allowed by symmetry. A method to perform this selection is representational analysis (RA) and this approach to study magnetic structures rests on a mathematical idea first used by Dzyaloshinskii [69; 70]. RA is based on the Landau thermodynamic theory of second-order phase transitions [71] and the method implements a systematic decomposition of the magnetic representation Γ of the little group Gk into irreducible representations (IR). The number of symmetry allowed magnetic structures for the system will be equal to the number of non-zero IR in the final decomposition of Γ. In this thesis, the symmetry analysis of the incommensurate antiferromagnetic structure in paper III was performed using simulated annealing and representational analysis (SARAh) [72]. 2.5 Magnetic characterization The magnetic properties of a material can be characterized by several techniques. In this thesis, the magnetization has been studied as a function of temperature and applied magnetic field. A superconducting quantum interference 25 device (SQUID) magnetometer was used to measure the magnetic moment of the samples. A SQUID uses the quantization of magnetic flux in a closed loop of a superconducting material containing one or two Josephson junctions in order to measure the magnetic fields. Magnetization experiments in paper II, III, IV and V were performed using a Quantum Design MPMS SQUID magnetometer and a Quantum Design PPMS with the VSM option. The MCE was determined from magnetization measurements by calculation of the magnetic entropy change, ∆S, using equation 1.2. 2.6 Other experimental methods 2.6.1 Differential thermal analysis and thermogravimetry Differential thermal analysis (DTA) and thermogravimetry (TG) are techniques for studying thermal effects in a material such as polymorphic transitions and mass changes. The studied sample and a reference are arranged with identical thermocouples which are connected back-to-back. When heated, the sample and reference will be at the same temperature and result in a ∆T = 0 output. When a thermal effect occurs in the sample, ∆T will deviate from 0 and the output shows if the thermal event was exo- or endothermic. DTA and TG measurements were performed using a Netzsch STA 409 PC Luxx TG-DTA/DSC instrument. All samples were measured under a flowing Ar atmosphere and Ar was also used as purge gas. Measurements were performed with an increased temperature from 303 to 1373 K using a heating rate of 10 K/min. The temperature was cycled three times for all samples. 2.6.2 Mössbauer spectroscopy Mössbauer spectroscopy is a method to study chemical bonding, magnetic properties and oxidation states for isotopes with sufficiently large mass and excitation energies lower than ∼100 keV. A unique Mössbauer nucleus is the isotope 57 Fe. The technique is based on the resonant emission and absorption of γ -rays in solid matter. To perform such experiments, the γ -source must match the studied material, e.g., the 57 Fe isotope is studied using a 57 Co source and Mössbauer spectroscopy is an element specific technique. The interactions between the nucleus and the surrounding electrons are indicated by hyperfine interactions which is described by the hyperfine field. Quantification of the magnetic moments can be achieved within an order of magnitude accuracy. 26 Mössbauer absorption spectra in paper II were recorded in the constant acceleration mode at temperatures between 5 K and 440 K using a 57 CoRh source. 2.6.3 Theoretical methods The foundation of Density Functional Theory (DFT) is that the total energy of a system can be described solely by the electron density [73]. Over time, this theory has been developed to reproduce physical parameters of realistic materials [74]. A majority of the DFT based theoretical methods are based on solving the Kohn-Sham equation [75] and there are several methods to find a solution depending on the set up. In the exact muffin-tin orbital method (EMTO) [76; 77] the potentials are built using overlapping spherical potentials and the Kohn-Sham equation is solved exactly for these potentials. The EMTO method can be combined with the coherent potential approximation (CPA) [78; 79] to allow for chemical disorder. The real atomic potential is replaced by the coherent potential built from the atomic potentials from the components of the studied material. The electronic structure and total energy calculations in this thesis were performed using the EMTO method combined with CPA. Ab initio calculations were performed for three different phases of FeMnP0.50 Si0.50 . Two phases were set as ordered: one with manganese atoms occupying the pyramidal 3g site and one with the manganese atoms occupying the tetrahedral 3f site. A third disordered phase was also considered where the iron and manganese atoms were randomly distributed on the two metal sites in the Fe2 Ptype structure. Internal positions and lattice parameter were taken from NPD data in Paper I and all numerical details of the calculations are similar to the ones reported in Ref. [80] 27 3. Results and discussion The results have been divided into five parts. In part one overall characteristics of the FeMnP1−x Six phase diagram will be presented. In part two the focus is put on the compounds FeMnP0.50 Si0.50 and FeMnP0.75 Si0.25 which are found on the borders of the single phase Fe2 P-type region in the FeMnP1−x Six phase diagram. The virgin effect observed in (Fe,Mn)2 (P,Si)-type compounds is discussed in part three. The properties of phosphorus deficient samples of (Fe,Mn)2 (P,Si)-type are presented in part four. Finally, the results from XRD measurements in a magnetic field are discussed in part five. 3.1 The phase diagram of FeMnP1−x Six Careful phase analysis has been performed in order to study the phase diagram of the FeMnP1−x Six -system. The FeMnP1−x Six phase diagram exhibits five regions out of which two are single phase, see figure 3.1. The single phases are one orthorhombic of Co2 P-type (Pnma) for x < 0.15 and one hexagonal of Fe2 P-type (P62m) for 0.24 ≤ x < 0.50. A two phase region of the Co2 Pand Fe2 P-type structures was found for 0.15 ≤ x < 0.24. For 0.50 ≤ x < 1.00, a three phase region, which consists of the Fe2 P-type, hexagonal Mn5 Si3 -type (P63 /mcm) and cubic Fe3 Si-type (Fm3m) structures, was found. Finally, x = 1.00 was shown to result in the Mn5 Si3 - and Fe3 Si-type structures. The phase fraction of x ≥ 0.50 is shown in figure 3.2 and the amount of the Fe2 P-type phase linearly decreases with increased silicon concentration (x). As seen in figure 3.1, the phase border at x = 0.50 is temperature dependent and thus the composition obtained at room temperature is related to the heat treatment and cooling rate. Single phase samples of Fe2 P-type close to this phase border were prepared by quenching in cold water after the heat treatments. Quenched and slowly cooled samples of FeMnP0.52 Si0.48 are compared in figure 3.3 and reflexions confirmed to belong to the Mn5 Si3 - and Fe3 Si-type structures were observed in the slowly cooled sample. This indicates that the three phase region seen in figure 3.1 for 0.50 ≤ x < 1.00 is present for low temperatures at x = 0.48 and that quenched samples are needed to reach a single phase of Fe2 P-type. DTA-data collected from x = 0.50 are seen in figure 3.4 where a kink is observed at about 950 K on heating at the first temperature cycle. The subsequent cycles do not show any thermal activities indicating an oxidation or decom29 Fe P 2 hex. Co P - orth. Temperature (K) 1473 K Fe P 2 2 0,0 Fe Si + Mn Si 3 5 3 hex. 0,2 0,4 0,6 0,8 1,0 x Si conc. ( ) Figure 3.1: Phase diagram of the FeMnP1−x Six -system. White regions represent single phases while regions with multiple phases are grey. Detailed phase analysis of the quenched and slowly cooled samples with compositions close to x = 0.50 indicates a temperature dependence of the phase boundary of the three-phase region for 0.50 ≤ x < 1.00 to extend slightly below x = 0.50 at lower temperatures. Figure 3.2: Phase fractions of the Fe2 P-, Mn5 Si3 - and Fe3 Si-type structures in the composition range 0.50 ≤ x ≤ 1.00 for FeMnP1−x Six position of the samples. XRD experiments before and after the DTA-analysis confirm these observations with several oxide phases and a decomposed Fe2 P structure likely due to losses of phosphorus. Similar observations were seen for the samples of composition x = 0.40, 0.48 and 0.65. It is shown that selected samples of Fe2 P-type are stable at temperatures up to ∼950 K. The unit cell parameters (measured at 298 K) and magnetic properties for the two single phase regions of Co2 P- and Fe2 P-type are summarized in table 3.1 and are visualized in figures 3.5 and 3.6. The unit cell distortion observed 30 Quenched Slowly cooled Intensity (arb. units) Mn Si -type 5 3 Fe Si-type 3 39 40 41 42 43 44 45 46 47 2-theta (deg.) T)/dT (arb. units) Figure 3.3: XRD intensities of quenched and slowly cooled samples of FeMnP0.52 Si0.48 . Peaks of the Mn5 Si3 - and Fe3 Si-type structures are observed in the slowly cooled sample. Data were collected at 298 K. d( 1st cycle 2nd cycle 3rd cycle 300 600 900 1200 1500 Temperature (K) Figure 3.4: The derivated DTA-signal of FeMnP0.50 Si0.50 vs. temperature. A kink (indicated by a vertical arrow) is observed on heating at about 950 K on the first heating/cooling cycle indicating a thermal activity. at x = 0.35 originates from magnetostriction effects which occur when the magnetic state changes from para- to ferromagnetic at room temperature (TC reaches values above RT), table 3.1: TC for x = 0.30 is 275 K and 308 K for x = 0.35. The magnetic ordering in the Co2 P-type region is mainly antiferromagnetic with TN in a range from 260 K to 285 K. The magnetic transitions of FeMnP agree with previous work [34; 36; 81]. An orthorhombic (Co2 P-type) to hexagonal (Fe2 P-type) phase transition has been reported to occur at 1473 31 3,6 cell parameter length (Å) b T 6,7 C at 275 K 3,5 c 6,6 3,4 a 6,2 T C at 308 K 6,1 3,3 c 6,0 a 5,9 cell parameter length (Å) 298 K 6,8 3,2 0,0 0,1 0,2 0,3 0,4 0,5 x Si conc. ( ) 400 160 320 120 0,0 0,2 3-phase region 2 Fe P (hex.) 80 2-phase region 2 Co P (orth.) 160 2 240 M (Am /kg) Transition temperature (K) Figure 3.5: Unit cell parameters of the orthorhombic (0.00 ≤ x < 0.15) and hexagonal (0.24 ≤ x < 0.50) region of FeMnP1−x Six . A unit cell distortion is observed for x = 0.35 due to magnetostriction. Shadowed regions contain two phases. Data were collected at 298 K. 0,4 80 T N T* T 40 C M T = 20 K; µ0H = 1 T 0,6 0,8 1,0 x Si conc. ( ) Figure 3.6: Magnetic phase diagram for the FeMnP1−x Six -system. Transition temperatures of all samples and saturation magnetization of the ferromagnetic samples at T = 20 K and µ0 H = 1 T are indicated K in FeMnP [82]. This occurrence is indicated in the phase diagram in figure 3.1. The magnetic transition temperatures are shown vs. silicon concentration in figure 3.6 where T∗ indicates the temperature of a minimum in the M vs. T curves (measured in a field of 1 T). A linear dependency is seen for TC vs sili32 Table 3.1: Structural and magnetic properties of FeMnP1−x Six crystallizing in the Co2 P-type structure (0.00 ≤ x < 0.15) and the Fe2 P-type structure (0.24 ≤ x < 0.50). Unit cell parameters are determined at 298 K and TC is measured at heating. x a (Å) b (Å) c (Å) V (Å3 ) TC /TN (K) ∆Thys (K) 0.00 0.05 0.10 0.15 5.9603(3) 5.9611(2) 5.9574(2) 5.9582(2) 3.5722(2) 3.5786(2) 3.5826(2) 3.5982(2) 6.7415(2) 6.7519(2) 6.7548(2) 6.7653(2) 143.53(1) 144.03(2) 144.17(1) 144.77(1) 261∗ 255∗ 284∗ 281∗ 7 8 9 9 0.20 0.20 5.9584(2) 5.9816(2) 3.5927(2) 6.7672(2) 3.4936(2) 144.86(1) 108.25(1) 0.24 0.25† 0.25 0.30 0.35‡ ” 0.40 0.45 0.48 0.50 5.9823(2) 5.9879(2) 5.9907(2) 6.0025(1) 6.0118(3) 6.1731(5) 6.1801(2) 6.1921(2) 6.2016(2) 6.2075(2) 3.4914(2) 3.4937(2) 3.4996(3) 3.4922(1) 3.4833(4) 3.3102(4) 3.3088(2) 3.2995(2) 3.2931(1) 3.2906(2) 108.21(1) 108.48(1) 108.77(1) 108.96(1) 109.03(2) 109.24(2) 109.44(2) 109.56(1) 109.68(2) 109.81(2) 215 150∗ 250 275 308 ” 350 370 392 395 96 ∗ Néel 90 74 57 ” 42 27 24 18 temperature † Antiferromagnetic phase of FeMnP0.75 Si0.25 different unit cells are observed at x = 0.35 because of a unit cell distortion due to magnetostriction effects. ‡ Two con concentration in the Fe2 P-type region. In the same region a plateau is observed for the saturated magnetization (Msat ) which decreases abruptly close to the phase border at lower concentration of silicon (x ∼ 0.25). In the three phase region for x ≥ 0.50, Msat decreases linearly with increased amount of silicon and TC abruptly decreases close to x = 1.00 where the Fe2 P-type phase disappears. Hence, the presence of the Fe2 P-type structure strongly influences the magnetic properties in this composition range and Msat correlates with the amounts of the Fe2 P-type phase in the material (cf. figure 3.2). The sample of nominal composition FeMnSi contains two phases in equilibrium: one with the cubic Fe3 Si-type structure (Fm3m, a = 5.6687(2) Å) and the other with hexagonal Mn5 Si3 -type structure (P63 /mcm, a = 6.8499(1) Å, c = 4.7564(1) Å). The unit cell parameters are similar to the compositions 33 ∼(Fe0.4 Mn0.6 )3 Si [83] and ∼(Fe0.5 Mn0.5 )5 Si3 [84; 85]. This indicates that the Fe/Mn ratio in the FeMnSi samples is close to one. Two magnetic transition temperatures are observed for FeMnSi with TN ∼ 60 K (for (Fe,Mn)3 Si) and TC = 169 K (for (Fe,Mn)5 Si3 ), these transition temperatures agree with the values reported in earlier studies of (Fe,Mn)3 Si [86] and (Fe,Mn)5 Si3 [85]. 34 3.2 Compounds of Fe2 P-type The main work in this thesis has been to study compounds of Fe2 P-type in the FeMnP1−x Six -system. Therefore this section will go more deeply into the magnetic and crystallographic properties of this part of the system. 3.2.1 FeMnP0.50 Si0.50 The compound FeMnP0.50 Si0.50 was found to be at the upper border of the phase region of Fe2 P-type in the FeMnP1−x Six phase diagram. Traces of the Mn5 Si3 - and Fe3 Si-type structures can be observed even for quenched samples which indicate that the limit concentration of silicon in the single phase region is reached. The unit cell parameters are determined to a = 6.2090(3) Å and c = 3.2880(2) Å at 296 K in paper I. High temperature XRD experiments show an iso-structural phase transition at approx. 385 K as shown in figure 3.7. This transition originates from magnetostrictive effects when the magnetic state is changed. Magnetization measurements of FeMnP0.50 Si0.50 are seen in figure 3.8a and indicate a first order para- to ferromagnetic transition with TC = 395 K (measured on heating). In figure 3.8b, the magnetization at 5 T and 295 K is 156 Am2 /kg which corresponds to 3.9 µB /f.u. The saturated moment at 5 K is determined to be 4.4 µB /f.u. which is higher than previous experimental studies (3.8 µB /f.u.) [52] and slightly higher than previous calculations (4.2 µB /f.u.) [87] have reported. The ∆S of FeMnP0.50 Si0.50 was estimated from magnetization data and a magnetic entropy change of 8 J/kgK was obtained for a magnetic field change of 1.8 T. The magnetic structure of FeMnP0.50 Si0.50 was determined using NPD and it was found that the iron atoms occupy the tetrahedral 3f site while the manganese atoms preferably occupy the pyramidal 3g site. The magnetic moments are aligned along the hexagonal a/b-axis and the direction of the moments has changed from the c-axis to the basal plane if compared to the mother compound Fe2 P [29; 30], see figure 3.9. The size of the magnetic moments of FeMnP0.50 Si0.50 was found to be 1.9(1) µB on the iron site and 2.5(1) µB on the manganese site giving a total magnetic moment of 4.4(2) µB /f.u. which is significantly higher than Fe2 P having a total magnetic moment of 2.9(1) µB /f.u. [38; 88–91]. The size of the magnetic moments determined from NPD intensities agree with magnetization experiments and the theoretical calculations shown in figure 3.10. The total calculated energies are presented as a function of the lattice parameter a for a fixed c/a = 0.5296 for three different phases (described in section 2.6.3). As seen in figure 3.10, the case with manganese occupying the pyramidal 3g site has the lowest energy for all volumes (lattice parameters) and this concords with the NPD experiments. However, the Mn-pyramidal case has a energy minimum for a lattice constant a around 6.16 Å while the experimental value is 6.2090(3) Å in paper I and 6.2075(2) Å in paper V. 35 403 K 111 201 300 210 211/002 Intensity (arb. units) 393 K 388 K 383 K 373 K 303 K 111 201 210 40 300 211 002 50 60 2-theta (deg.) Figure 3.7: XRD intensities of FeMnP0.50 Si0.50 from 303 to 401 K. A structural transition due to magnetostriction is seen to occur at approx. 385 K. λ = 1.540598 Å. 150 200 150 120 b) 295 K 100 2 Magnetization (Am /kg) a) 50 90 0 60 -50 -100 30 -150 µ H = 1 T 0 0 320 360 400 Temperature (K) 440 -200 -4 -2 0 2 4 µ H (T) 0 Figure 3.8: Field cooled cooling and heating magnetization experiments of FeMnP0.50 Si0.50 . (a): magnetization vs. temperature measured at 1 T. (b): magnetization vs. magnetic field measured at 295 K. The occupancies of the iron and manganese atoms were also confirmed by Mössbauer spectroscopy experiments. The Mössbauer spectra for FeMnP0.50 Si0.50 are shown in figure 3.11. The upper panel shows a broad single line centered at 0.17 mm/s. This together with an average electric quadrupole splitting of 0.32 mm/s show that the iron atoms in the samples only occupy the tetrahedral 3f site. A marked shoulder should have been 36 a) Fe (3f) Mn (3g) P/Si I (2c) P/Si II (1b) c a b b) Fe I (3f) Fe II (3g) P I (2c) P II (1b) c a b Figure 3.9: The magnetic structure of (a) FeMnP0.50 Si0.50 and (b) Fe2 P. The length of the arrows correspond to the magnitude of the magnetic moments. 5 Mn pyramidal Disordered Fe pyramidal E (mRy/site) 0 -5 -10 magnetic moment (µB/site) -15 -20 Mn pyramidal Mn tetrahedral Fe tetrahedral Fe pyramidal 3.5 3.0 2.5 2.0 1.5 1.0 6.0 6.1 6.2 6.3 6.4 a-axis (Å) Figure 3.10: Theoretical results for FeMnP0.50 Si0.50 . Total energy per site (upper panel) and site-projected magnetic moments (lower panel) of the Mn- and Fepyramidal and Mn- and Fe-tetrahedral ordered phases as a function of the unit cell parameter a and for fixed c/a = 0.5296. The dashed line indicates the experimental unit cell parameter. present on the resonance line if there had been any iron on the pyramidal 3g site [42; 92]. The spectrum collected in the ferromagnetic regime at 298 K (see lower panel in figure 3.11) shows a six-line pattern along with a broad central line. Two sextets were needed to fit the spectrum due to differences in the line intensities in the outer lines, an electric quadrupole split doublet was used to fit the broad central line and the amount of the doublet was ∼5% at 37 295 K and it had disappeared at 77 K and below. The observed co-existence of para- and ferromagnetic ordering in FeMnP0.50 Si0.50 may indicate local non-stoichiometry [38]. (a) PM T = 440 K (b) FM T = 295 K Figure 3.11: Mössbauer spectra for FeMnP0.50 Si0.50 measured at (a) 440 K and (b) 295 K. Inset: magnetic hyperfine field distribution measured at 295 K. The compound FeMnP0.50 Si0.50 has been investigated by several techniques in order to study its structural and magnetic properties. Evaluations of the experiments show that FeMnP0.50 Si0.50 exhibits a rather large magnetocaloric effect in combination with the smallest thermal hysteresis of the samples in the Fe2 P-type region (see table 3.1). This makes FeMnP0.50 Si0.50 one of the most promising compounds in the FeMnP1−x Six -system for use in magnetocaloric applications. 38 3.2.2 FeMnP0.75 Si0.25 The compound FeMnP0.75 Si0.25 is close to the lower silicon limit in the Fe2 Pregion of the FeMnP1−x Six phase diagram. Samples of FeMnP0.75 Si0.25 were first synthesized and properly analyzed. Then followed another heat treatment of the analyzed samples and a second round of characterizations. The flowchart of the work with FeMnP0.75 Si0.25 is shown in figure 3.12. It should be emphasized that the sample labeled ”sample II” is a re-heat-treated batch of the sample labeled ”sample I”. Characteriza"on Fabrica"on Drop synthesis technique Heat treatment 1 Heat treatment 2 (Sample I) (Sample II) Sintering at 1373 K (2h) Annealing at 1273 K (10d) Slow cooling (36h) Annealing at 1273 K (25d) Slow cooling (24h) X-ray diffrac"on X-ray diffrac"on Magne"za"on Magne"za"on Neutron diffrac"on Neutron diffrac"on Figure 3.12: Flowchart showing the order of sample preparation and experimental work for the samples I and II of FeMnP0.75 Si0.25 . Table 3.2: Unit cell parameters of FeMnP0.75 Si0.25 at (a) 298 K and (b) 16 K for sample I (phase A, the majority phase in sample I) and sample II (phase B, the majority phase in sample II), as determined from X-ray powder diffraction data. λ = 1.540598 Å. Sample T (K) a (Å) c (Å) V ( Å3 ) I I 298 16 5.9907(2) 6.1630(2) 3.4996(3) 3.3035(2) 108.77(1) 108.66(1) II II 298 16 5.9879(1) 5.9756(3) 3.4937(2) 3.4878(2) 108.48(1) 107.86(1) Collected XRD intensities at 298 K and 16 K of sample I and II are shown in figure 3.13 and 3.14. Careful analysis of the intensity profiles detects overlapping reflexions over the whole 2θ range. Two phases of Fe2 P-type (labeled 39 Intensity (arb. units) a) 298 K A B 50 51 52 53 54 55 56 A B 20 30 40 50 60 70 80 90 2-theta (deg.) Intensity (arb. units) b) 16 K A B 50 51 52 53 54 55 56 A B 20 30 40 50 60 70 80 90 2-theta (deg.) Figure 3.13: X-ray powder diffraction intensities of sample I at (a) 298 K and (b) 16 K. Red dots and black lines correspond to observed and calculated data respectively. Blue lines show the difference between the observed and calculated data. Tick marks indicate the position of Bragg reflections of the two phases of Fe2 P-type (phase A and B). The low angle intensities of the 16 K data have been excluded because of high background from the cryostat. Insets: Zoom showing phase A and B in detail. λ = 1.540598 Å. 40 Intensity (arb. units) a) 298 K A B 50 51 52 53 54 55 56 A B 20 30 40 50 60 70 80 90 2-theta (deg.) Intensity (arb. units) b) 16 K A B 50 51 52 53 54 55 56 A B 20 30 40 50 60 70 80 90 2-theta (deg.) Figure 3.14: X-ray powder diffraction intensities of sample II at (a) 298 K and (b) 16 K. Red dots and black lines correspond to observed and calculated data respectively. Blue lines show the difference between the observed and calculated data. Tick marks indicate the position of Bragg reflections of the two phases of Fe2 P-type (phase A and B) and the phase of (Fe,Mn)3 Si-type. The low angle intensities of the 16 K data have been excluded because of high background from the cryostat. Insets: Zoom showing phase A and B in detail. λ = 1.540598 Å. 41 A and B) with only narrowly different lattice parameters are found with the ratio A/B ∼70/30 in sample I and ∼10/90 in sample II. The unit cell parameters of the majority phases in sample I and II are shown in table 3.2 and the large changes of the unit cell dimensions of sample I at 16 K indicates a low temperature ferromagnetic ordering as observed in FeMnP0.50 Si0.50 and pure Fe2 P [30]. 140 a) Virgin cycle Second cycle 120 0 2 Magnetization (Am /kg) µ H = 2 T 100 80 60 40 20 0 0 50 100 150 200 250 300 Temperature (K) 7 b) ZFC FC 2 Magnetization (Am /kg) 6 µ H = 1 T 0 5 4 1,7 3 1,6 1,5 2 1,4 1 125 0 150 50 175 100 200 150 200 250 300 Temperature (K) Figure 3.15: Magnetization vs. temperature data. (a): Magnetization data of sample I measured in a magnetic field of 2 T. Black squares correspond to the first coolingheating event and the red circles to the second. (b): Magnetization data of sample II measured in a magnetic field of 1 T. The antiferromagnetic ordering temperature TN ∼ 150 K is evident from the weak bump in both the ZFC and FC magnetization curves. Inset: blow-up of the temperature range near TN of the FC curve. Magnetization vs. temperature curves of sample I are shown in figure 3.15a and a para- to ferromagnetic transition is indicated at 250 K (measured on heating). When measured on cooling and re-heating, the magnetic transition temperature shows a strong thermal hysteresis of the order of 100 K and the cooling cycles exhibit a considerable virgin effect on the magnetic transition 42 140 120 sample I 2 M (Am /kg) 100 sample II 80 T = 5 K 60 40 20 0 0 1 2 µ H (T) 0 Figure 3.16: Magnetization vs. magnetic field data of sample I and II measured at 5K. between the first|second (and subsequent) cooling cycles. The saturation magnetization of sample I at 5 K is about 125 Am2 /kg (measured at 1 T), see figure 3.16. Similar magnetization measurements on sample II show a strongly altered behavior. As seen in figure 3.15b, the ferromagnetic amplitude is substantially lower and an indication of an antiferromagnetic transition is observed at 150 K noticed as a weak bump on both cooling and heating. The large thermal hysteresis of the ferromagnetic transition temperature, which was observed in sample I, is preserved. The decreased ferromagnetic component is unexpected (the only difference between sample I and II is an additional heat treatment) but clearly illustrated in the low temperature magnetization vs. temperature curves in figure 3.16 where the magnetization of sample II only reaches 5 Am2 /kg at 1 T. This is about 25 times smaller compared to the corresponding magnetization of sample I. The NPD data collected on sample I agrees with results from XRD and magnetization measurements. Observed and calculated NPD profiles of sample I are shown in figure 3.17 and the two phases A and B of the Fe2 P-type (determined from XRD intensities) are observed in the paramagnetic region measured at 298 K. In accordance with FeMnP0.50 Si0.50 and other Fe2 P-type compounds [56; 57], the iron atoms in phase A preferably occupy the tetrahedral 3f site while the manganese atoms mainly are occupying the pyramidal 3g site. In the 8 K profiles, magnetic intensities from a ferromagnetic structure were observed for phase A. There were also small amounts of phase B together with a few extra peaks that could not be indexed by any of the crystal structures known to occur in the FeMnP1−x Six -system. The magnetic moments of the ferromagnetic phase were determined to be aligned in the hexagonal basal 43 211 a) 298 K Y obs Y calc Y -Y obs calc Intensity (arb. units) Bragg position 001 110 111 101 A B 20 40 60 80 100 2-theta (deg.) b) 8 K Y 211 obs Y calc Y Intensity (arb. units) obs -Y calc Bragg position 110 001 111 101 A B 20 40 60 80 100 2-theta (deg.) Figure 3.17: Neutron powder diffraction intensities of sample I at 298 K and 8 K. Red dots and black lines correspond to observed and calculated data respectively. Blue lines show the difference between the observed and calculated data. Tick marks indicate the position of Bragg reflections of the two structural phases of Fe2 P-type (phase A and B) at 298 K in (a) and the majority phase of Fe2 P-type (phase A), the ferromagnetic part of the majority phase, the minority phase of Fe2 P-type (phase B) and the antiferromagnetic low T phase derived from the minority phase in (b). λ = 1.556 Å. 44 211 a) 298 K Y obs Y calc Y -Y obs calc Intensity (arb. units) Bragg position 001 110 A B 20 40 60 80 100 2-theta (deg.) 211 b) 16 K Y obs Y calc Y -Y calc 010 - k 010 + k 110 + k 110 - k 000 ± k 001 110 100 - k Intensity (arb. units) obs Bragg position A B 20 40 60 80 100 2-theta (deg.) Figure 3.18: Neutron powder diffraction intensities of sample II at 298 K and 16 K. Red dots and black lines correspond to observed and calculated data respectively. Blue lines show the difference between the observed and calculated data. Tick marks indicate the position of Bragg reflections of the two structural phases of Fe2 P-type (phase A and B) and the structural phase of (Fe,Mn)3 Si-type at 298 K in (a) and the minority phase of Fe2 P-type (phase A), the ferromagnetic part of the minority phase, the majority phase of Fe2 P-type (phase B), the antiferromagnetic part of the majority phase and the structural phase of (Fe,Mn)3 Si-type in (b). λ = 1.46 Å. 45 plane (as for FeMnP0.50 Si0.50 ) with a moment of 2.1(1) µB on the iron site and 2.8(2) µB on the manganese site giving a total moment of 4.9(2) µB /f.u. In sample II, the observed and calculated NPD profiles (shown in figure 3.18) showed similar sample characteristics at 298 K as for sample I, except the altered phase ratio. At 16 K, phase B was observed together with small amounts of the ferromagnetically ordered phase A and several peaks that could be indexed as an incommensurate antiferromagnetic ordering of phase B. The propagation vector of the incommensurate antiferromagnetic structure was determined to qx = 0.363(1) and the amplitude of the magnetic moments were found to propagate sinusoidally along the a-axis. The magnetic moments on the iron sites are aligned along the b-axis and the magnetic moments on the manganese site align along the a-axis, see figure 3.19. The maximum amplitude of the magnetic moments were 2.2(2) µB and 2.0(2) µB on the iron and manganese sites respectively. Sinusoidal arrangements of the magnetic moments can be very hard (if not impossible) to separate from a helical ordering when the magnetic structure is determined from NPD intensities [93]. But several magnetic structures were evaluated (including helical arrangements) using representational analysis (RA) and the present antiferromagnetic structure was found to be the most reasonable ordering. b a Fe (3f) Mn (3g) P/Si (2c/1b) Figure 3.19: The antiferromagnetic and incommensurate (qx = 0.363(1)) low temperature structure of sample II. The magnetic moments of the iron and manganese atoms are aligned in the basal plane along the the a- and b-axis respectively and the amplitude of the moments propagate sinusoidally along the the a-axis. This is the first time this type of antiferromagnetic structure has been reported in the (Fe,Mn)2 (P,Si)-system. However, a similar structure has been reported in the analogous FeMnP1−x Asx -system for x = 0.15-0.22 [40], but the corresponding arsenic compound (x = 0.25) is strictly antiferromagnetic and there are no reports on any order-disorder behavior as seen for FeMnP0.75 Si0.25 . Co-existing ferromagnetic and incommensurate ordering is unusual but is still observed occasionally, a related example is for pure Fe2 P where a simultaneous order-disorder behavior has been observed in a narrow temperature range below TC [89]. A similar dramatic change in the magnetic ordering has been observed for Fe2 P caused by narrow differences of the composition where just a few percent substitution of manganese in Fe2 P switch the magnetic ordering from ferromagnetic to antiferromagnetic [34]. The strength and direction of the magnetic interactions in Fe2 P-type structures have been reported from experiments [31; 36] and calculations [94] and are found to critically depend on changes of the Me-Me distances. As seen in 46 table 3.3, the Fe-Fe and Mn-Mn distances in sample I and II are significantly different in the paramagnetic state. Since the magnetic low temperature ordering of sample I and II are different, the Me-Me distances of the two samples are considerably different due to the magnetoelastic effect in the ferromagnetic sample I. The observations of the two samples with compositions close to FeMnP0.75 Si0.25 present a new magnetic ordering not observed before in the (Fe,Mn)2 (P,Si)-system. Even if the antiferromagnetic ordering makes this compound a weak candidate for magnetocaloric applications; the findings are not less important for the fundamental understanding of the (Fe,Mn)2 (P,Si)-system which has been found more complicated than expected. 47 48 II I P AF 16 F 8 298 P 298 2.264(1) x 2 2.313(1) x 2 < 2.288(1) > 2.271(1) x 2 2.317(1) x 2 < 2.294(1) > 2.291(1) x 2 2.319(1) x 2 < 2.305(1) > 2.264(1) x 2 2.328(1) x 2 < 2.296(1) > 2.456(3) x 1 2.510(1) x 4 < 2.499(1) > 2.474(5) x 1 2.512(1) x 4 < 2.504(2) > 2.491(4) x 1 2.495(1) x 4 < 2.494(2) > 2.451(5) x 1 2.520(1) x 4 < 2.506(2) > 2.634(2) x 2 2.638(2) x 2 2.753(2) x 2 2.662(2) x 2 3.126(2) x 4 3.128(2) x 4 3.241(1) x 4 3.143(1) x 4 2.652(5) x 2 2.766(1) x 4 < 2.728(4) > 2.650(4) x 2 2.779(1) x 4 < 2.736(3) > 2.652(3) x 2 2.737(1) x 4 < 2.709(2) > 2.662(2) x 2 2.768(1) x 4 < 2.733(2) > Table 3.3: Interatomic distances of the majority phases in sample I (at 298 K (paramagnetic state, P) and 8 K (ferromagnetic state, F)) and sample II (at 298 K (paramagnetic state, P) and 16 K (antiferromagnetic state, AF)) in Å. Mean distances inside <>. Derived from neutron powder diffraction data. Sample T (K) State Fe-P/Si Mn-P/Si Fe-Fe Mn-Mn Fe-Mn 3.3 The virgin effect Further studies of the first order magnetic transitions of polycrystalline samples of Fe2 P-type from the FeMnP1−x Six -system were performed in Paper IV to gain understanding of the magnetic phenomenon called the virgin effect. In figure 3.20, magnetization vs. temperature cycles in field cooled cooling (FCC) and field cooled heating (FCH) protocols of FeMnP0.75 Si0.25 in a magnetic field of 1 T are shown. On the first cooling, TC is found to occur at a lower temperature than on subsequent cycles, while the transition temperature on heating remains unaffected through subsequent cycles. 160 2 Magnetization (Am /kg) FCC (I) FCH (I) 120 FCC (II) FCH (II) FCC (III) FCH (III) 80 FCC (IV) FCH (IV) 40 0 0 µ H = 1 T 0 100 200 300 400 Temperature (K) Figure 3.20: Magnetization vs. temperature curves for FeMnP0.75 Si0.25 under a magnetic field of 1 T. The labels FCC (I) and FCH (I) refer to the initial cooling and heating events; FCC (II, III and IV) and FCH (II, III and IV) to the second, third and fourth cooling and heating events, respectively. The ferromagnetic transition is followed by structural changes due to magnetostrictive effects. The room temperature XRD intensities of FeMnP0.75 Si0.25 are seen in figure 3.21 and the inset shows intensities collected on cooling the samples through TC . A magnetostrictive transition is observed as abrupt changes of the peak intensities occur with the appearance of extra peaks. The first cooling through TC introduces an irreversible change of the as prepared structure. When the sample is re-heated to the paramagnetic regime this minute structural change persists which is decisive on the magnetic properties. This permanent structure change is demonstrated in figure 3.22 where the XRD intensities collected before and after the first cooling/heating cycle are shown. The inset shows observed peak splits at high diffraction angles which occur after the virgin cooling. This split is an indication that the sample decomposes into two very similar phases with slightly different lattice parame49 298 K 45 60 Intensity (arb. units) 75 * 120 135 150 175 40 45 50 298 2 theta (deg.) * * * 20 105 * 40 60 * * 80 2-theta ( ) Figure 3.21: X-ray powder diffraction intensities of FeMnP0.75 Si0.25 at 298 K. The inset shows a sequence of diffractograms, at temperatures according to the color code, on cooling the samples through TC . Silicon (*) was used as an internal standard. ters. It is possible that the origin of this phase decomposition is the magnetic domain formation at low temperature which alters the local strain distribution to a more stable formation than in the high temperature situation of the as prepared sample. The irreversible change is further illustrated in figure 3.23 where diffractograms recorded at 150 K on the virgin and second cooling cycles are shown. For the second cycle, the peaks of the magnetically ordered phase to have higher intensities than in the virgin cycle since a larger fraction of the sample has ordered at 150 K in the second cycle. In a further view of the virgin effect in the FeMnP1−x Six -system, it is shown that the observed virgin effect is strongly dependent of the magnitude of TC . As seen in figure 3.25, samples with TC close to or above room temperature show minor or zero virgin effect while samples with TC below room temperature show an increased virgin effect along with a decreased TC . The temperature dependence of the virgin effect is further illustrated in figure 3.24 where a sample of composition FeMnP0.52 Si0.48 exhibits a partial virgin effect. On the first cooling, a high temperature reversible part is observed above RT and an irreversible part showing the virgin effect is seen below RT. This shows that the virgin effect is driven by temperature and take place when the as prepared sample is first cooled through the first order magnetic transition. 50 298K Intensity (arb. units) * 84 86 88 90 2-theta (deg.) * Virgin After * * 40 50 60 70 * * 80 90 2-theta (deg.) Figure 3.22: Room temperature X-ray powder diffraction intensities of FeMnP0.75 Si0.25 recorded before and after the virgin cooling cycle. The inset shows the minute changes of the high angle peaks. Silicon (∗) was used as internal calibration standard. After 150 K Virgin Intensity (arb. units) Si 40 45 50 2-theta (deg.) Figure 3.23: X-ray powder diffraction intensities during the transition at the virgin cooling cycle and the 2nd cycle measured at 150 K. The contribution from the magnetically ordered phase (arrows) is higher for the 2nd cycle than for the virgin cycle, confirming that a larger fraction of the sample has ordered in the second cycle. The data was normalized against ”unaltered” diffraction peaks so that the contribution of the magnetically ordered phase could be extracted. Silicon was used as an internal calibration standard of the peak positions. 51 2 Magnetization (Am /kg) 160 120 80 FCC (I) 40 FCH (I) FCC (II) FCH (II) 0 0 H = 1 T 100 200 300 400 Temperature (K) Figure 3.24: Magnetization vs. temperature curves for FeMnP0.52 Si0.48 under a magnetic field of 1 T. The vertical line is a reference line showing T = 300 K. Nomenclature as in figure 3.20. 400 50 virgin effect (K) 20 200 150 10 100 0 in first cooling cycle 250 C 300 30 T 350 40 50 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1,0 Si conc. (x) Figure 3.25: Virgin effect and TC in the first cooling cycle vs. temperature for ferromagnetic samples of the FeMnP1−x Six -system. 52 3.4 Phosphorus deficient samples There are two major studies of the FeMnP1−x Six -system, one by Cam Thanh et al. [52] and another by Katagiri et al. [53]. The reported results in refs. [52] and [53] are coherent but do not agree with the observations found in paper V. These three studies are here compared in detail and a central difference is the techniques used for sample preparation. In Paper V, the drop synthesis method has been utilized while ball milling was used in refs. [52] and [53]. Comparing the phase analysis of the three investigations show that relatively large amounts of a secondary phase of the Fe3 Si-type structure was present in the samples of refs. [52] and [53]. In the thesis by Cam Thanh [95], selected samples of Fe2 P-type in the FeMnP1−x Six -system were reported to contain as much as 18% of the phase of Fe3 Si-type. 298 K Y Y obs calc Intensity (arb. units) Difference Bragg position 20 30 40 50 60 70 80 90 2-theta (deg.) Figure 3.26: X-ray powder diffraction intensities of FeMnP0.40 Si0.50 at 298 K. Red dots and black lines correspond to observed and calculated intensities respectively. Blue lines show the difference between the observed and calculated data. Tick marks indicate the position of Bragg reflections of two phases of Fe2 P-type (RBragg = 8.67/11.41%) and a phase of Fe3 Si-type (RBragg = 4.87%). λ = 1.540598 Å. R p = 1.02%, Rwp = 1.34%, χ 2 = 1.53 It has been suggested that losses of phosphorus could explain the divergent results in-between the different investigations. The drop synthesis technique prevents major losses of the volatile elements phosphorus and manganese. Therefore a sample with composition FeMnP0.40 Si0.50 was prepared in order to study how deficiency of phosphorus influences the structural and magnetic properties. Observed and calculated XRD intensities of FeMnP0.40 Si0.50 are shown in figure 3.26. The sample was found to contain three phases, two similar phases of Fe2 P-type and one phase of Fe3 Si-type. The phase ratio of the 53 two Fe2 P-type structures and the Fe3 Si-type structure were ∼ 70/30. This indicates that the solid solubility of silicon has been exceeded and a two phase region of Fe2 P-type and a minority phase of Fe3 Si-type is formed. The magnetization vs. temperature curves of FeMnP0.40 Si0.50 and FeMnP0.50 Si0.50 in figure 3.27 shows that the first order transition temperature of FeMnP0.40 Si0.50 is reduced to 271 K. The saturation magnetization at T = 20 K was reduced as well. 150 a) 2 Magnetization (Am /kg) 100 50 FeMnP 0.50 0 T C Si 0.50 = 395 K b) 100 50 FeMnP 0.40 0 T 0 C Si 0.50 = 271 K 100 200 300 400 500 Temperature (K) Figure 3.27: Magnetization vs. temperature curves recorded under a magnetic field of 1 T for (a) FeMnP0.50 Si0.50 and (b) FeMnP0.40 Si0.50 . The red and black arrows indicate that the data was recorded on heating and cooling respectively. Unit cell parameters from Refs. [52] and [53] are compared with the FeMnP0.40 Si0.50 compound in figure 3.28. It was found that the unit cell parameters of FeMnP0.40 Si0.50 are similar to those with composition FeMnP0.50 Si0.50 by Katagiri et al. [53] and FeMnP0.52 Si0.48 by Cam Thanh et al. [52]. The magnetic parameters of FeMnP0.40 Si0.50 and FeMnP0.52 Si0.48 [52] are also found to closely agree. The TC of FeMnP0.40 Si0.50 is 271 K and is reported to be 268 K for FeMnP0.52 Si0.48 [52]. The thermal hysteresis for FeMnP0.40 Si0.50 was 23 K and reported as 21 K for FeMnP0.52 Si0.48 in ref. [52]. This comparison has given further insight on how the structural and magnetic properties are correlated in the FeMnP1−x Six -system and a possible explanation to why nominally similar samples exhibit vastly different structural and magnetic properties in different studies. 54 This work cell parameter length (Å) 6,3 Cam Thanh et al. (2008) Katagiri et al. (2013) FeMnP 6,2 6,1 0.40 Si 0.50 a-axis 6,0 3,5 3,4 c-axis 3,3 0,3 0,4 0,5 0,6 Si conc. (x) Figure 3.28: Unit cell parameters of the hexagonal Fe2 P-type structure in the FeMnP1−x Six -system determined in this thesis (FeMnP0.50 Si0.50 and FeMnP0.40 Si0.50 ) compared with previous reported values of Cam Thanh et al. [52] and Katagiri et al. [53]. 55 3.5 In field XRD experiments 2-theta (deg.) The concept to perform XRD measurements in an applied field of 0.35 T using synchrotron radiation was tested on a compound with composition (Fe0.45 Mn0.55 )2 P0.50 Si0.50 . The integrated 2D diffractograms vs. temperature are seen in figure 3.29 where the intensities were collected in a field of 0.35 T. A structural transition due to magnetostriction is observed at approx 352 K on heating. The experiment is further illustrated in figure 3.30 where the relative changes of the unit cell parameters are plotted vs. temperature on heating and subsequently cooling. A thermal hysteresis was observed in the in-field measurements and was also observed in magnetization vs. temperature measurements in the same applied field, 0.35 T, see figure 3.31. The transition temperature from the in-field XRD experiments correlates with the transition temperature observed in the magnetization experiments. 25 µ H = 0.35 T o 30 35 40 310 320 330 340 350 360 370 Temperature (K) Figure 3.29: Diffraction intensities vs. temperature for (Fe0.45 Mn0.55 )2 P0.50 Si0.50 measured on heating. Intensities were collected in a field of 0.35 T. 0,03 µ H = 0.35 T 0 0,02 0,01 a a 0,00 -0,01 c c -0,02 -0,03 300 320 340 360 380 Temperature (K) Figure 3.30: The relative changes of the unit (Fe0.45 Mn0.55 )2 P0.50 Si0.50 measured in a field of 0.35 T. 56 cell parameters of FCC FCH Long moment (emu) 0,03 µ0H = 0.35 T 0,02 0,01 0,00 300 320 340 360 Temperature (K) Figure 3.31: Magnetization vs. temperature data of (Fe0.45 Mn0.55 )2 P0.50 Si0.50 measured in a magnetic field of 0.35 T. 57 4. Summary and concluding remarks The work presented in this thesis shows how the crystallographic and magnetic properties of the compound Fe2 P can be tuned by substitution of manganese and silicon to form compounds of (Fe,Mn)2 (P,Si)-type. The characteristics of compounds with equal amount iron and manganese and varied concentration of phosphorus and silicon (FeMnP1−x Six ) have been studied using techniques such as X-ray powder diffraction, neutron powder diffraction, synchrotron radiation X-ray powder diffraction, SQUID-magnetometry, differential thermal analysis, Mössbauer spectroscopy and electronic and total energy calculations. Samples have been synthesized using the drop synthesis method in order to prevent losses of the volatile elements manganese and phosphorus. The phase diagram of the FeMnP1−x Six -system consists of five regions out of which two are single phase: one with the orthorhombic Co2 P-type structure (x < 0.15) and one with the hexagonal Fe2 P-type structure (0.24 ≤ x < 0.50). The FeMn(P,Si)-phase of Fe2 P-type is ferromagnetic with the magnetic moments on the two metal sites aligned in the basal plane along the hexagonal a/b-axis. Increased silicon content of the Fe2 P-type increases the Curie temperature from 215 to 395 K and decreases the thermal hysteresis from 96 K to 18 K. The growing hysteresis co-exists with an increased so called virgin effect which, in the Fe2 P-type structure within this material system, is accompanied by an irreversible structure change, induced by magnetostriction effects, that persists on succeeding cooling-heating cycles. For x = 0.25, a low temperature incommensurate antiferromagnetic ordered structure is shown to co-exist along with the ferromagnetic structure. The magnetic structures arise from two phases of Fe2 P-type with narrow differences regarding structural properties in the paramagnetic regime. Certain compounds with high silicon content in the Fe2 P-type phase region have been found promising for use in magnetocaloric applications. The TC is tunable and close to room temperature for x = 0.35 and the magnetic entropy change 8 J/kgK is obtained for a magnetic field change of 1.8 T for x = 0.50. A study of a phosphorus deficient sample (FeMnP0.40 Si0.50 ) has brought more knowledge to the conflicting properties of the FeMnP1−x Six -system reported in literature. It is indicated that the disagreements are due to slight differences in composition of the investigated compounds of Fe2 P-type. A two-phase region with the Fe2 P- and Fe3 Si-type structures is reached even for small losses of phosphorus during preparation. This knowledge is im- 59 portant for future investigations in order to find the optimum compounds of (Fe,Mn)2 (P,Si)-type in magnetic cooling applications. A concept to perform experiments in an applied field of 0.35 T has been tested for use in synchrotron radiation X-ray powder diffraction. The quality of the collected intensities was satisfactory and crystal structural properties can be obtained in a magnetic field. 60 5. Sammanfattning på svenska Dagens energisamhälle ställer hela tiden högre krav på att teknik och elektronisk utrustning ska vara mer energisnål. Något av det mest energiförbrukande som finns i våra hem idag är kylanläggningar som till exempel luftkonditionering och kylskåp. En ny teknik kallad magnetisk kylning är under utveckling för att på lång sikt kunna ersätta dagens kommersiella kylskåp. Tekniken utnyttjar den magnetokaloriska effekten och kan vara upp till 30% mer effektiv än den teknik som används idag. Den magnetokaloriska effekten bygger på att de atomära magnetiska momenten i ett ferromagnetiskt material linjeras upp av ett momentant pålagt magnetiskt fält, vilket innebär att materialets magnetiska entropi (oordning) minskar. Samtidigt kräver termodynamikens lagar att materialets totala entropi inte ändras, vilket betyder att atomernas vibrationsrörelse (temperatur) ökar, det vill säga materialets temperatur stiger. På motsvarande sätt: när ett pålagt magnetiskt fält tas bort från materialet minskar upplinjeringen av de magnetiska momenten och oordningen i det magnetiska systemet ökar. Detta betyder att atomernas vibrationsrörelse måste minska, det vill säga materialet kyls. Den magnetokaloriska effekten är störst nära materialets ferromagnetiska fasövergångstemperatur (Curie temperaturen). Detta kan utnyttjas i den magnetokaloriska cykeln (se figur 1) där ett oordnat magnetiskt material (1) placeras i ett magnetiskt fält vilket leder till att materialet blir varmare (2). Det varmare materialet kyls sedan ner genom att värmen leds bort (3) och har nu ungefär samma temperatur som vid det inledande steget. När materialet sedan förs ut ur det magnetiska fältet kommer temperaturen i materialet att vara lägre än ursprungstemperaturen (4). Det ”kalla” materialet kan nu användas för att leda bort värme från t.ex. ett kylskåp vilket gör att materialet får samma temperatur som vid det inledande steget och cykeln sluts och kan börja om igen. Ett lämpligt material för magnetisk kylning bör vara billigt, fritt från giftiga ämnen samt ha egenskapen att gå från magnetiskt oordnat till magnetiskt ordnat nära rumstemperatur. Genom att byta ut järn och fosfor mot andra grundämnen i järnfosfiden Fe2 P kan dess egenskaper skräddarsys till att vara lämpligt för magnetiskt kylning. Material av typen (Fe,Mn)2 (P,Si) (där järn delvis har ersatts av mangan och fosfor delvis har ersatts av kisel) har visat sig vara särskilt intressant för magnetokaloriska tillämpningar. Den här avhandlingen handlar om hur den atomära strukturen hos material av typen (Fe,Mn)2 (P,Si) påverkar de magnetiska egenskaperna. 61 Materialet förs in i ett magnetiskt fält Värme leds bort Värme leds bort Kylskåp Materialet förs ut ur det magnetiska fältet Figur 5.1: Illustration av den magnetokaloriska cykeln. Eftersom fosfor och mangan förångas vid relativt låga temperaturer har en särskild metod använts för att tillverka prover där dessa grundämnen ingår tillsammans med järn (och i detta fall kisel). Korrekta mängder av järn och kisel hettas upp tills provet smälter. Detta sker vid temperaturer över 1400◦ C. Sedan har små bitar av fosfor och mangan ”knuffats ner” i smältan. Reaktionen mellan de nedknuffade bitarna och smältan sker så fort att fosfor och mangan inte hinner förångas. När allt fosfor och mangan reagerat i smältan sänks temperaturen och det önskade materialet har bildats. Proverna har studerats med en mängd olika analystekniker men med störst fokus på röntgen- och neutronpulverdiffraktion och magnetiseringsexperiment. Ett fasdiagram av FeMnP1−x Six (för 0.00 ≤ x ≤ 1.00) har sammanställts och det kunde visas att det består av två enfasiga områden; ett område med ortorombisk struktur av Co2 P-typ (för x < 0.15) och ett område med hexagonal struktur Fe2 P-typ (för 0.24 ≤ x < 0.50). Det kunde också visas att utvalda prover i området av Fe2 P-typ är lämpliga för användning inom magnetisk kylning. För sammansättningen x = 0.35 är exempelvis den magnetiska övergångstemperaturen nära rumstemperatur. I material som är magnetiska får varje magnetisk atom en riktning. Dessa atomer kan peka åt olika håll och sammantaget bilda en magnetisk struktur. Den magnetiska ordningen eller magnetiska strukturen bestäms genom att bestråla materialet med neutroner med tekniken neutronpulverdiffraktion. Utvalda prover av Fe2 P-typ har studerats med denna teknik och sammansättningen för x = 0.50 har visats vara ferromagnetisk, det vill säga att alla magnetiska atomer är riktade åt samma håll. Detta gäller för nästan alla sammansättningar av Fe2 P-typ i FeMn(P,Si)-fasdiagrammet. Dock, provet med sammansättning x = 0.25 skiljer sig ur mängden. Detta prov bestod av två faser av Fe2 P-typ där 62 den ena var ferromagnetisk och den andra var antiferromagnetisk, vilket betyder att riktningarna på de magnetiska atomerna är motriktade och sammantaget tar ut varandra. Det visade sig också att ordning i den antiferromagnetisk strukturen uppvisar en komplex magnetisk struktur som varierar likt en våg genom kristallen, se figur 5.2. b a järn mangan fosfor kisel Figur 5.2: Den antiferromagnetiska strukturen för FeMnP0.75 Si0.25 . Studier av prover av Fe2 P-typ visade också att temperaturen där materialet ordnade sig magnetiskt förändrades sig mellan den första och andra kylningscykeln, för att sedan vara densamma för alla påföljande cykler. På svenska kan denna temperaturberoende effekt översättas från engelskans ”virgin effect” med jungfrueffekt. Röntgendiffraktionexperiment visade att även strukturen i materialet påverkas av temperaturcyklingen. Efter den första cykeln syns en förändring i strukturen som inte går tillbaka efter att temperaturcykeln är genomförd. Denna förändring av strukturen kan vara en bidragande orsak till att den magnetiska övergångstemperaturen förändras efter den första temperaturcykeln. En studie av Fe2 P-typ med otillräcklig mängd fosfor (FeMnP0.40 Si0.50 ) genomfördes för att studera hur brist på fosfor påverkar strukturen och de magnetiska egenskaperna. Det visades att brist på fosfor skapar stora förändringar hos såväl strukturen som hos de magnetiska egenskaperna och detta kan indikera varför det har förekommit avvikelser mellan olika studier av material av (Fe,Mn)2 (P,Si)-typ. Sammantaget är (Fe,Mn)2 (P,Si) ett materialsystem där utvalda sammansättningar kan vara konkurrenskraftiga inom magnetisk kylning. Den här avhandlingen har också visat att materialsystemet är komplicerat med olika typer av magnetisk ordning och att små variationer i sammansättning av de olika grundämnena kraftigt påverkar de önskade magnetiska egenskaperna. 63 6. Acknowledgments Till att börja med vill jag tacka mina handledare för allt jobb ni har lagt ner för att hjälpa mig fram till mitt slutmål. Tack Yvonne för alla diskussioner och för att du har lärt mig allt från hur man tacklar bångstyriga parametrar i fullprof till den engelska stavningen av fosfor (phosphou... phosphorou... phosphorus?). Tack Per för din expertis och all assistans med ”politiska” dokument, det har verkligen varit till stor hjälp. Ett särskilt tack till Martin för att du tidigt hoppade på tåget och för allt stöd du har gett mig både i arbetet och på det personliga planet. Ni har alla tre varit ett stort stöd och har gjort att jag kunnat nå högre mål än vad jag någonsin trodde var möjligt. Tack! Thank you Matthias, Mikael A, Tapati and Luana for all your help and support with the magnetic measurements (you have been great!); Johan for helping me with various work in the basement and in the X-ray lab; Premek and Magnus for all your hard work and assistance with the neutron diffraction experiments; Erna, Olle and Levente for all your work with the computational calculations; Girma for your efforts to help me with the single crystal measurements; Lennart, Tore, Rolf, Sergey, Torbjörn and Josh for support, suggestions and helpful discussions. Tack Mattis för att du alltid har varit en så trevlig kontorskamrat. Tack Jonas ”back seat refiner” Å för all hjälp vid 711:an (R.I.P.) och för att vi har blivit så pass goda kamrater under åren som gott. Tack Rickard för alla trevliga (och fåniga) stunder. Även de tyngsta av dagar har blivit lättsamma efter en vistelse i lunchrummet. Tack Fredde, David, Adam, Tomas, Linus, Daniel m.fl. för alla diskussioner som faktiskt har haft hyfsad höjd vid några få enstaka tillfällen. Tack Eva och Tatti för all hjälp med allt som rör administration. Förlåt Eva och Tatti för att jag alltid kommit till er med allt som rör all administration. Tack Pedro och Janne för all teknisk hjälp, stort som smått. Ett särskilt tack till Anders och Mikael O för all den support ni har gett mig och för att ni har haft tålamod med alla mina ständiga små problem. I also want to thank all my colleagues at the department. I am grateful that I had the opportunity to make my PhD at a place where I always have felt good spirit and joy. It is all of you that have made it possible! Tack också till min familj och mina svärföräldrar, tack för att ni finns. Men, framför allt vill jag tacka min underbara fru Johanna för allt stöd och all glädje du ger mig. Du och August är det viktigaste jag har och jag älskar er verkligen över allt annat. 65 References [1] V. Fabritius Buchwald. Iron and Steel in Ancient Times. Copenhagen : Det Kongelige Danske Videnskabernes Selskab (2005). [2] W. C. Roberts. On the Liquation, Fusibility, and Density of certain Alloys of Silver and Copper. Proc. R. Soc. London, 23 (1875). [3] J. C. Zhao (editor). Methods for Phase Diagram Determination. First edition. Elsevier Science Ltd (2007). [4] M. Hansen and K. Anderko. Constitution of Binary Alloys. McGrawHill (1958). [5] L. Kaufman and H. Bernstein. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals. Academic Press, New York (1970). [6] P. Weiss and A. Piccard. Sur un nouveau phénomène magnétocalorique. Comptes Rendus Hebdo- madaires des Séances l’Académie des Sci., 166, 352–354 (1918). [7] E. Warburg. Magnetische Untersuchungen. Ueber einige Wirkungen der Coercitivkraft. Ann. Phys., 13 (1881). [8] A. Smith. Who discovered the magnetocaloric effect? Eur. Phys. J. H, 38, 507–517 (2013). [9] A. M. Tishin and Y. I. Spichkin. The Magnetocaloric Effect and its applications. IOP Publishing (2003). [10] T. Plackowski, Y. Wang and A. Junod. Specific heat and magnetocaloric effect measurements using commercial heat-flow sensors. Rev. Sci. Instrum., 73, 2755 (2002). [11] F. Casanova, A. Labarta, X. Batlle, F. J. Pérez-Reche, E. Vives, L. Mañosa and A. Planes. Direct observation of the magnetic-fieldinduced entropy change in Gd5 (Six Ge1−x )4 giant magnetocaloric alloys. Appl. Phys. Lett., 86, 262504 (2005). [12] A. Schilling and M. Reibelt. Low-temperature differential-thermal analysis to measure variations in entropy. Rev. Sci. Instrum., 78, 033904 (2007). 67 [13] M. Kuepferling, C. P. Sasso, V. Basso and L. Giudici. An Isothermal Peltier Cell Calorimeter For Measuring the Magnetocaloric Effect. IEEE Trans. Magn., 43, 2764–2766 (2007). [14] J. C. B. Monteiro, R. D. dos Reis, A. M. Mansanares and F. G. Gandra. Determination of the magnetocaloric entropy change by field sweep using a heat flux setup. Appl. Phys. Lett., 105, 074104 (2014). [15] J. Sun, F. Hu and B. Shen. Comment on ”Direct Measurement of the ’Giant’ Adiabatic Temperature Change in Gd5 Si2 Ge2 ”. Phys. Rev. Lett., 85, 4191–4191 (2000). [16] D. V. Christensen, R. Bjørk, K. K. Nielsen, C. R. H. Bahl, A. Smith and S. Clausen. Spatially resolved measurements of the magnetocaloric effect and the local magnetic field using thermography. J. Appl. Phys., 108, 063913 (2010). [17] H. Yibole, F. Guillou, L. Zhang, N. H. van Dijk and E. Brück. Direct measurement of the magnetocaloric effect in MnFe(P,X) (X = As, Ge, Si) materials. J. Phys. D. Appl. Phys., 47, 075002 (2014). [18] A. Smith, C. R. H. Bahl, R. Bjørk, K. Engelbrecht, K. K. Nielsen and N. Pryds. Materials Challenges for High Performance Magnetocaloric Refrigeration Devices. Adv. Energy Mater., 2, 1288–1318 (2012). [19] M. E. Wood and W. H. Potter. General analysis of magnetic refrigeration and its optimization using a new concept: maximization of refrigerant capacity. Cryogenics, 25, 667–683 (1985). [20] V. K. Pecharsky and K. A. Gschneidner. Some common misconceptions concerning magnetic refrigerant materials. J. Appl. Phys., 90, 4614 (2001). [21] K. A. Gschneidner Jr, V. K. Pecharsky and A. O. Tsokol. Recent developments in magnetocaloric materials. Reports Prog. Phys., 68, 1479– 1539 (2005). [22] V. K. Pecharsky and K. A. Gschneidner, Jr. Giant Magnetocaloric Effect in Gd5 (Si2 Ge2 ). Phys. Rev. Lett., 78, 4494–4497 (1997). [23] X. Moya, S. Kar-Narayan and N. D. Mathur. Caloric materials near ferroic phase transitions. Nat. Mater., 13, 439–50 (2014). [24] V. Franco, J. S. Blázquez, B. Ingale and A. Conde. The Magnetocaloric Effect and Magnetic Refrigeration Near Room Temperature: Materials and Models. Annu. Rev. Mater. Res., 42, 305–342 (2012). 68 [25] O. Gutfleisch, M. A. Willard, E. Brück, C. H. Chen, S. G. Sankar and J. P. Liu. Magnetic materials and devices for the 21st century: stronger, lighter, and more energy efficient. Adv. Mater., 23, 821–42 (2011). [26] G. Hägg. X-ray studies of the binary systems of iron with phosphorus, arsenic, antimony and bismuth. Nov. Acta Regiae Soc. Sci. Ups., 7 (1929). [27] S. Rundqvist and F. Jellinek. The structures of Ni6 Si2 B, Fe2 P and some related phases. Acta Chem. Scand., 13, 425–432 (1959). [28] B. Carlsson, M. Gölin and S. Rundqvist. Determination of the homogeneity range and refinement of the crystal structure of Fe2 P. J. Solid State Chem., 8, 57–67 (1973). [29] L. Lundgren, G. Tarmohamed, O. Beckman, B. Carlsson and S. Rundqvist. First Order Magnetic Phase Transition in Fe2 P. Phys. Scr., 17, 39 (1978). [30] H. Fujii, T. Hokabe, T. Kamigaichi and T. Okamoto. Magnetic Properties of Fe2 P Single Crystal. J. Phys. Soc. Jpn., 43, 41 (1977). [31] P. Jernberg, A. A. Yousif, L. Häggström and Y. Andersson. A Mössbauer study of Fe2 P1−x Six (x ≤ 0.35). J. Solid State Chem., 53, 313–322 (1984). [32] R. Chandra, S. Bjarman, T. Ericsson, L. Häggström, C. Wilkinson, R. Wäppling, Y. Andersson and S. Rundqvist. A Mössbauer and Xray study of Fe2 P1−x Bx compounds (x < 0.15). J. Solid State Chem., 34, 389–396 (1980). [33] A. Catalano, R. J. Arnott and A. Wold. Magnetic and crystallographic properties of the system Fe2 P1−x Asx . J. Solid State Chem., 7, 262–268 (1973). [34] H. Fujii, T. Hokabe, K. Eguchi, H. Fujiwara and T. Okamato. Magnetic Properties of (Fe1−x Mnx )2 P Compounds. J. Phys. Soc. Jpn., 51, 414–419 (1982). [35] R. Fruchart, A. Roger and J. P. Senateur. Crystallographic and Magnetic Properties of Solid Solutions of the Phosphides M2 P, M = Cr, Mn, Fe, Co, and Ni. J. Appl. Phys., 40, 1250 (1969). [36] B. K. Srivastava, T. Ericsson, L. Häggström, H. R. Verma, Y. Andersson and S. Rundqvist. A Mössbauer study of the (Fe1−x Mnx )2 P system. J. Phys. C Solid State Phys., 20, 463–472 (1987). [37] T. Suzuki, Y. Yamaguchi, H. Yamamoto and H. Watanabe. Magnetic Structure of FeMnP. J. Phys. Soc. Jpn., 34, 911–916 (1973). 69 [38] R. Wäppling, L. Häggström, T. Ericsson, S. Devanarayanan, E. Karlsson, B. Carlsson and S. Rundqvist. First order magnetic transition, magnetic structure, and vacancy distribution in Fe2 P. J. Solid State Chem., 13, 258–271 (1975). [39] R. Zach, M. Guillot and R. Fruchart. The influence of high magnetic fields on the first order magneto-elastic transition in MnFe(P1−y Asy ) systems. J. Magn. Magn. Mater., 89, 221–228 (1990). [40] M. Bacmann, J.-L. Soubeyroux, R. Barrett, D. Fruchart, R. Zach, S. Niziol and R. Fruchart. Magnetoelastic transition and antiferroferromagnetic ordering in the system MnFeP1−y Asy . J. Magn. Magn. Mater., 134, 59–67 (1994). [41] R. Zach, B. Malaman, M. Bacmann, R. Fruchart, S. Niziol, G. Le Caër, J.-L. Soubeyroux, J. Zukrowski and D. Fruchart. Magnetic study of the hexagonal FeMnP1−x Asx system. J. Magn. Magn. Mater., 147, 201–204 (1995). [42] B. Malaman, G. Le Caër, P. Delcroix, D. Fruchart, M. Bacmann and R. Fruchart. Magneto-elastic transition and magnetic couplings: a 57 Fe Mössbauer spectroscopy study of the MnFeP1−x Asx system. J. Phys. Condens. Matter, 8, 8653–8667 (1996). [43] R. Zach, M. Bacmann, D. Fruchart, P. Wolfers, R. Fruchart, M. Guillot, S. Kaprzyk, S. Niziol and J. Tobola. Magneto-elastic properties of MnFeP1−x Asx (0.15 ≤ x ≤ 0.66) and MnRhP1−x Asx isostructural series of compounds. J. Alloys Compd., 262-263, 508–511 (1997). [44] O. Tegus, E. Brück, K. H. J. Buschow and F. R. de Boer. Transitionmetal-based magnetic refrigerants for room-temperature applications. Nature, 415, 150–2 (2002). [45] O. Tegus, E. Brück, L. Zhang, W. Dagula, K. H. J. Buschow and F. R. de Boer. Magnetic-phase transitions and magnetocaloric effects. Physica B, 319, 174–192 (2002). [46] E. Brück, O. Tegus, X. W. Li, F. R. de Boer and K. H. J. Buschow. Magnetic refrigeration–towards room-temperature applications. Physica B, 327, 431–437 (2003). [47] X. W. Li, O. Tegus, L. Zhang, W. Dagula, E. Brück, K. H. J. Buschow and F. R. de Boer. Magnetic properties of MnFeP0.5 As0.5−x Gex . IEEE Trans. Magn., 39, 3148–3150 (2003). [48] E. Brück, O. Tegus, L. Zhang, X. Li, F. R. de Boer and K. H. J. Buschow. Magnetic refrigeration near room temperature with Fe2 P-based compounds. J. Alloys Compd., 383, 32–36 (2004). 70 [49] O. Tegus, B. Fuquan, W. Dagula, L. Zhang, E. Brück, P. Z. Si, F. R. de Boer and K. H. J. Buschow. Magnetic-entropy change in Mn1.1 Fe0.9 P0.7 As0.3−x Gex . J. Alloys Compd., 396, 6–9 (2005). [50] D. T. Cam Thanh, E. Brück, O. Tegus, J. C. P. Klaasse, T. J. Gortenmulder and K. H. J. Buschow. Magnetocaloric effect in MnFe(P,Si,Ge) compounds. J. Appl. Phys., 99, 08Q107 (2006). [51] D. T. Cam Thanh, E. Brück, O. Tegus, J. C. P. Klaasse and K. H. J. Buschow. Influence of Si and Ge on the magnetic phase transition and magnetocaloric properties of MnFe(P,Si,Ge). J. Magn. Magn. Mater., 310, e1012–e1014 (2007). [52] D. T. Cam Thanh, E. Brück, N. T. Trung, J. C. P. Klaasse, K. H. J. Buschow, Z. Q. Ou, O. Tegus and L. Caron. Structure, magnetism, and magnetocaloric properties of MnFeP1−x Six compounds. J. Appl. Phys., 103, 07B318 (2008). [53] K. Katagiri, K. Nakamura and H. Wada. Magnetocaloric properties and magnetic refrigerant capacity of MnFeP1−x Six . J. Alloys Compd., 553, 286–290 (2013). [54] N. H. Dung, Z. Q. Ou, L. Caron, L. Zhang, D. T. Cam Thanh, G. A. de Wijs, R. A. de Groot, K. H. J. Buschow and E. Brück. Mixed Magnetism for Refrigeration and Energy Conversion. Adv. Energy Mater., 1, 1215–1219 (2011). [55] N. H. Dung, L. Zhang, Z. Q. Ou and E. Brück. From first-order magnetoelastic to magneto-structural transition in (Mn,Fe)1.95 P0.50 Si0.50 compounds. Appl. Phys. Lett., 99, 092511 (2011). [56] N. H. Dung, L. Zhang, Z. Q. Ou, L. Zhao, L. van Eijck, A. M. Mulders, M. Avdeev, E. Suard, N. H. van Dijk and E. Brück. High/low-moment phase transition in hexagonal Mn-Fe-P-Si compounds. Phys. Rev. B, 86, 045134 (2012). [57] Z. Q. Ou, L. Zhang, N. H. Dung, L. van Eijck, A. M. Mulders, M. Avdeev, N. H. van Dijk and E. Brück. Neutron diffraction study on the magnetic structure of Fe2 P-based Mn0.66 Fe1.29 P1−x Six melt-spun ribbons. J. Magn. Magn. Mater., 340, 80–85 (2013). [58] J. L. Haughton. Alloys of iron research. VIII. Constitution of alloys of iron and phosphorus. J. Iron Steel Inst., 115, 417–433 (1927). [59] Y. Cerenius, K. Ståhl, L. A. Svensson, T. Ursby, A. Oskarsson, J. Albertsson and A. Liljas. The crystallography beamline I711 at MAX II. J. Synchrotron Radiat., 7, 203–8 (2000). 71 [60] A. P. Hammersley. FIT2D: An Introduction and Overview. ESRF Internal Report, ESRF97HA02T (1997). [61] T. R. Jensen, T. K. Nielsen, Y. Filinchuk, J. Jørgensen, Y. Cerenius, E. M. Gray and C. J. Webb. Versatile in situ powder X-ray diffraction cells for solid-gas investigations. J. Appl. Crystallogr., 43, 1456–1463 (2010). [62] G. E. Bacon. Neutron Diffraction. Third edition. Oxford University Press (1975). [63] T. J. B. Holland and S. A. T. Redfern. Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral. Mag., 61, 65–77 (1997). [64] H. M. Rietveld. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr., 2, 65–71 (1969). [65] L. B. McCusker, R. B. Von Dreele, D. E. Cox, D. Louër and P. Scardi. Rietveld refinement guidelines. J. Appl. Crystallogr., 32, 36–50 (1999). [66] R. A. Young (editor). The Rietveld Method. Oxford Science Publications (1996). [67] J. Rodríguez-Carvajal. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B, 192, 55–69 (1993). [68] J. Rodríguez-Carvajal. Fullprof.2k computer program. ver 5.30 (2012). [69] I. E. Dzyaloshinskii. Thermodynamic theory of weak ferromagnetism in antiferromagnetic substances. Sov. Phys. JETP, 5, 1259 (1957). [70] I. E. Dzyaloshinskii. Theory of Helicoidal Structures in Antiferromagnets. I. Nonmetals. Sov. Phys. JETP, 19, 960 (1964). [71] Y. A. Izyumov and V. E. Naish. Symmetry analysis in neutron diffraction studies of magnetic structures. J. Magn. Magn. Mater., 12, 239–248 (1979). [72] A. S. Wills. A new protocol for the determination of magnetic structures using simulated annealing and representational analysis (SARAh). Physica B, 276-278, 680–681 (2000). [73] L. H. Thomas. The calculation of atomic field. Proc. Camb. Philol. Soc., 23 (1927). [74] P. Hohenberg and W. Kohn. Inhomogeneous Electron Gas. Phys. Rev., 136, B864–B871 (1964). 72 [75] W. Kohn and L. J. Sham. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev., 140, A1133–A1138 (1965). [76] V. Kumar, O. K. Andersen and A. Mookerjee (editors). Lectures on Methods of Electronic Structure Calculations. World Scientific, Singapore (1994). [77] L. Vitos. Total-energy method based on the exact muffin-tin orbitals theory. Phys. Rev. B, 64, 014107 (2001). [78] P. Soven. Coherent-Potential Model of Substitutional Disordered Alloys. Phys. Rev., 156, 809–813 (1967). [79] B. Gyorffy. Coherent-Potential Approximation for a NonoverlappingMuffin-Tin-Potential Model of Random Substitutional Alloys. Phys. Rev. B, 5, 2382–2384 (1972). [80] M. Hudl, P. Nordblad, T. Björkman, O. Eriksson, L. Häggström, M. Sahlberg, Y. Andersson, E. K. Delczeg-Czirjak and L. Vitos. Orderdisorder induced magnetic structures of FeMnP0.75 Si0.25 . Phys. Rev. B, 83, 134420 (2011). [81] M. Bacmann, D. Fruchart, B. Chenevier, R. Fruchart, J. A. Puertolas and C. Rillo. Magnetic phase diagram of the (Fe1−x Mnx )2 P system. J. Magn. Magn. Mater., 83, 313–314 (1990). [82] B. Chenevier, J.-L. Soubeyroux, M. Bacmann, D. Fruchart and R. Fruchart. The high temperature orthorhombic ⇋ hexagonal phase transformation of FeMnP. Solid State Commun., 64, 57–61 (1987). [83] S. Yoon and J. G. Booth. Magnetic properties and structures of some ordered (FeMn)3 Si alloys. J. Phys. F Met. Phys., 7, 1079–1095 (1977). [84] B. Aronsson. An Investigation of the Me5 Si3 -MeSi Region of the MnFe-Si and some Related Systems. Acta Chem. Scand., 12, 308–313 (1958). [85] V. Johnson, J. F. Weiher, C. G. Frederick and D. B. Rogers. Magnetic and Mössbauer effect studies of Mn5 Si3 :Fe5 Si3 solid solutions. J. Solid State Chem., 4, 311–323 (1972). [86] J. V. Leitão, Y. Xinmin, L. Caron and E. Brück. Magnetostructural study of the (Mn,Fe)3 (P,Si) system. J. Alloys Compd., 520, 52–58 (2012). [87] M. Diviš and I. Turek. Electronic structure and magnetism of MnFeP1−x Six alloys from first-principles calculations. Physica B, 403, 3276–3278 (2008). 73 [88] D. Scheerlinck and E. Legrand. Neutron diffraction study of the magnetic structure of Fe2 P. Solid State Comm., 25, 181–184 (1978). [89] S. Fujii, S. Ishida and S. Asano. Electronic structures and magnetic properties of Fe2 P, Co2 P and CoMnP. J. Phys. F Met. Phys., 18, 971– 980 (1988). [90] A. Koumina, M. Bacmann, D. Fruchart, J.-L. Soubeyroux, P. Wolfers, J. Tobola, S. Kaprzyk, S. Niziol, M. Mesnaoui and R. Zach. Crystallographic and magnetic properties of Fe2 P. Ann. Chim. Sci. des Mater., 23, 177–180 (1998). [91] J. Tobola, M. Bacmann, D. Fruchart, S. Kaprzyk, A. Koumina, S. Niziol, J.-L. Soubeyroux, P. Wolfers and R. Zach. Magnetism of Fe2 P investigated by neutron experiments and band structure calculations. J. Magn. Magn. Mater., 157/158, 708–710 (1996). [92] R. Hermann, O. Tegus, E. Brück, K. Buschow, F. de Boer, G. Long and F. Grandjean. Mössbauer spectral study of the magnetocaloric FeMnP1−x Asx compounds. Phys. Rev. B, 70, 214425 (2004). [93] M. Markkula, A. M. Arevalo-Lopez, A. Kusmartseva, J. A. Rodgers, C. Ritter, H. Wu and J. P. Attfield. Incommensurate spin order in the metallic perovskite MnVO3 . Phys. Rev. B, 84, 094450 (2011). [94] E. K. Delczeg-Czirjak, Z. Gercsi, L. Bergqvist, O. Eriksson, L. Szunyogh, P. Nordblad, B. Johansson and L. Vitos. Magnetic exchange interactions in B-, Si-, and As-doped Fe2 P from first-principles theory. Phys. Rev. B, 85, 1–7 (2012). [95] D. T. Cam Thanh. Magnetocalorics and Magnetism in MnFe(P,Si,Ge) materials. Ph.D. thesis, University of Amsterdam (2009). 74 Acta Universitatis Upsaliensis Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1197 Editor: The Dean of the Faculty of Science and Technology A doctoral dissertation from the Faculty of Science and Technology, Uppsala University, is usually a summary of a number of papers. A few copies of the complete dissertation are kept at major Swedish research libraries, while the summary alone is distributed internationally through the series Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology. (Prior to January, 2005, the series was published under the title “Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology”.) Distribution: publications.uu.se urn:nbn:se:uu:diva-234516 ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2014
© Copyright 2026