IDD System Implant Billing Worksheet
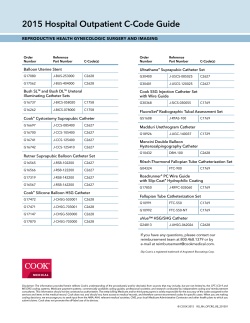
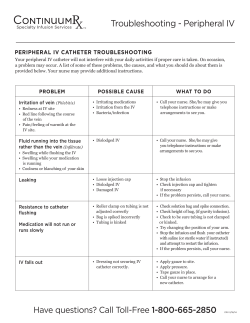
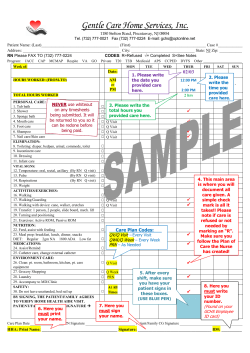
Implant Billing Worksheet Patient Name:______________________________________________________ Physician Name:_______________________________________________________ Intrathecal Drug Delivery System Date:________________________________ Time:_______________________ □ Trial □ Implant □ Replacement Payer:_________________________________ Physician Signature:___________________________________________________ (Check applicable boxes within each category) Inpatient Facility Outpatient Facility Trial Trial ICD-9 Procedure ττ #03.90 Catheter insertion ττ #03.92 Injection of other agent into spinal canal CPT Code ττ 62311 Injection, single (not via indwelling catheter) ττ 62319 Injection, including catheter placement, continuous infusion or Intermittent bolus Implant or Replacement of Intraspinal Catheter Implant, Revision, or Replacement Intraspinal Catheter ICD-9 Procedure ττ #03.90 Catheter insertion ττ #03.92 Injection of other agent into spinal canal CPT Code ττ 62350 Catheter implantation without laminectomy ττ 62351 Catheter implantation with laminectomy ττ 77003 Fluoroscopy, guidance of catheter tip Implant or Replacement of Infusion Pump Implantation or Replacement of Pump ICD-9 Procedure ττ #86.06 Insertion of totally implantable infusion pump CPT Code ττ 62362 Programmable pump implantation Removal of Catheter Removal of Catheter or Pump ICD-9 Procedure ττ #03.99 Catheter removal CPT Code ττ 62355 Removal of Intrathecal Catheter ττ 62365 Removal of Pump Removal of Pump C-Code (Medicare-outpatient only) ICD-9 Procedure ττ #86.05 Pump removal ττ C1772 Programmable Infusion pump ττ C1755 Intraspinal Catheter E-Code (As required by payer) Medicare’s consolidated device edits require that when specific CPT procedure codes for device implantation are billed, the associated HCPCS (C-code) must also be billed. ττ E0783 Entire Infusion pump system ττ E0786 Programmable pump only replacement ττ E0785 Intraspinal Catheter only-replacement myPTM ττ A9900 myPTM-misc DME supply-used during system replacement only-otherwise included in E0783 Current Procedural Terminology (CPT) is copyright 2010 American Medical Association, all rights reserved. No fee schedules, basic units, relative values, or related listings are included in CPT. The AMA assumes no liability for the data contained herein. Medtronic has compiled this coding information for your convenience. It is always the provider’s responsibility to determine coverage and submit appropriate codes, modifiers, and charges for the services that were rendered. Please contact your local Medicare Administrative Contractor/payer for interpretation of appropriate coverage and coding policies. Accessories and Supplies Product # NDC HCPCS II Codes and Description Concentration Total Volume in Kit Total Drug Qty Billing Units Total Billable per Kit Units Used (1 unit = 10 mg) 8561 58281-0560-01 J0475 Injection, baclofen, 10 mg 500 mcg/mL 20 mL 10 mg 1 8565 58281-0560-02 J0475 Injection, baclofen, 10 mg 500 mcg/mL 40 mL 20 mg 2 8564 58281-0563-01 J0475 Injection, baclofen, 10 mg 2000 mcg/mL 20 mL 40 mg 4 8566 58281-0563-02 J0475 Injection, baclofen, 10 mg 2000 mcg/mL 40 mL 80 mg 8 8562 58281-0561-02 J0475 Injection, baclofen, 10 mg 2000 mcg/mL 10 mL 20 mg 2 8563S 58281-0562-01 J0476 Injection, baclofen, 50 mcg for intrathecal trial 50 mcg/mL 1 mL .05 mg 1 Coverage of discarded drugs applies only to single-use vials. Multi-use vials are not subject to payment for discarded amounts of drug. An itemized bill should be submitted with the claim to verify how drug was supplied. The claim should include amount of drug administered and amount wasted. check with payer if JW modifier required. Reference CMS Manual 100-4, Chapter 17, subsections 40 or other payer requirements. Trial Catheter ττ 8516 Percutaneous intraspinal trial catheter kit ττ 8590-8 Catheter accessory kit ττ 220289 ambIT cassette ττ 8590-9 Catheter accessory repair kit ττ 220246 ambIT lock box ττ 8709SC InDura 1P intrathecal catheter (sutureless pump connector) ττ 220275 ambIT pole clamp ττ 8731SC Intrathecal catheter (sutureless pump connector) ττ 220287 External Epidural ambIT pump ττ 8711 InDura free-flow intrathecal catheter ττ 220249 ambIT pump carrying pouch ττ 81192 AlgoLine catheter with closed tip ττ 8590-1 SynchroMed mesh pouch ττ 8575 40 degree pump connector ττ 8577 90 degree pump connector ττ 8578 sutureless pump connector ττ 863720 SynchroMed II infusion pump ττ 863740 SynchroMed II infusion pump ττ 81102 AlgoLine catheter kit with closed-tip long strain-relief ττ 8583 Catheter passer (38 cm hollow stainless steel tunneling device with polypropylene obturator for use with the Model 8731SC intrathecal catheter) ττ 8586 Catheter passer (60 cm hollow stainless steel tunneling device with polypropylene obturator for use with the Model 8731SC intrathecal catheter) ττ 8591-38 Catheter passer (38 cm hollow stainless steel tunneling device with removable handle and polypropylene obturator for use with the Models 8709 and 8709SC intrathecal catheters) ττ 8591-60 Catheter passer (60 cm hollow stainless steel tunneling device with removable handle and polypropylene obturator for use with the Models 8709 and 8709SC intrathecal catheters) ττ 8596SC Pump segment revision kit (sutureless pump connector) Catheter Access Port Kits ττ 8598A Spinal segment revision kit ττ 8540 SynchroMed catheter access port (CAP) kit Analgesics Notes/Other: NDC HCPCS II Codes and Description Concentration Total Billable Units Used J2275 Injection, morphine sulfate (preservative-free sterile solution), per 10 mg J2278 Ziconotide, injection, 1 mcg Current Procedural Terminology (CPT) is copyright 2010 American Medical Association, all rights reserved. No fee schedules, basic units, relative values, or related listings are included in CPT. The AMA assumes no liability for the data contained herein. Medtronic has compiled this coding information for your convenience. It is always the provider’s responsibility to determine coverage and submit appropriate codes, modifiers, and charges for the services that were rendered. Please contact your local Medicare Administrative Contractor/payer for interpretation of appropriate coverage and coding policies. UC201004233b EN NP9342b © 2011 Medtronic, Inc. Printed in USA. SynchroMed Pump
© Copyright 2026