Chapter 15 Lesson 1 Worksheet
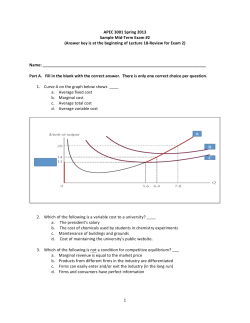
Chapter 15 Lesson 3 Worksheet Multiple Choice – Pick the best answer to the question 1. At 400 K, the equilibrium constant for the reaction: Br2 (g) + Cl2 (g) 2BrCl (g) is Kp = 7.0. A closed vessel at 400 K is charged with 1.00 atm of Br2 (g), 1.00 atm of Cl2 (g), and 2.00 atm of BrCl (g). Use Q to determine which of the statements below is true. A) The equilibrium partial pressures of Br2, Cl2, and BrCl will be the same as the initial values. B) The equilibrium partial pressure of Br2 will be greater than 1.00 atm. C) At equilibrium, the total pressure in the vessel will be less than the initial total pressure. D) The equilibrium partial pressure of BrCl (g) will be greater than 2.00 atm. E) The reaction will go to completion since there are equal amounts of Br2 and Cl2. 2. Which of the following statements is true? A) Q does not change with temperature. B) Keq does not change with temperature, whereas Q is temperature dependent. C) K does not depend on the concentrations or partial pressures of reaction components. D) Q does not depend on the concentrations or partial pressures of reaction components. E) Q is the same as Keq when a reaction is at equilibrium. 3. How is the reaction quotient used to determine whether a system is at equilibrium? A) The reaction quotient must be satisfied for equilibrium to be achieved. B) At equilibrium, the reaction quotient is undefined. C) The reaction is at equilibrium when Q < Keq. D) The reaction is at equilibrium when Q > Keq. E) The reaction is at equilibrium when Q = Keq. 4. Consider the following chemical reaction: CO (g) + 2H2 (g) CH3OH(g) At equilibrium in a particular experiment, the concentrations of CO and H2 were 0.15 M and0.36 M, respectively. What is the equilibrium concentration of CH3OH? The value of Keq for this reaction is 14.5 at the temperature of the experiment. A) 14.5 D) 3.72 × 10-3 B) 7.61 × 10-3 E) 1.34 × 10-3 C) 2.82 × 10-1 5. A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen and iodine: 2HI (g) H2(g) + I2(g) When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2 = 0.0960 atm. The value of Kp at this temperature is __________. E) Kp cannot be calculated for this gas A) 6.80 × 10-2 reaction when the volume of the reaction B) 1.30 × 10-2 vessel is not given. C) 54.3 D) 1.84 × 10-2 6. Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC2H3O2 (aq) C2H3O2- (aq) + H+ (aq) At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC2H3O2] = 0.0990 M, [C2H3O2-] = 1.33 × 10-3 M, and [H+] = 1.33 × 10-3 M. The equilibrium constant, Keq, for the ionization of acetic acid at 25°C is __________. A) 5.71 × 104 D) 1.79 × 10-5 B) 0.100 E) 5.71 × 106 C) 1.75 × 10-7 7. Dinitrogentetraoxide partially decomposes according to the equilibrium: N2O4 (g) 2NO2 (g) A 1.00-L flask is charged with 0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________. D) 0.022 A) 2.2 × 10-4 E) 0.87 B) 13 C) 0.22 8. Given the following reaction: CO (g) + 2H2(g) CH3OH (g) In an experiment, 0.42 mol of CO and 0.42 mol of H2 were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.29 mol of CO remaining. Keq at the temperature of the experiment is __________. A) 2.80 D) 17.5 B) 0.357 E) none of the above C) 14.5 9. Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: PCl3 (g) + Cl2 (g) PCl5 (g) An equilibrium mixture at 450 K contains: PPCl3 = 0.124 atm, PCl2 = 0.157 atm, and PPCl5 = 1.30 atm. What is the value of Kp at this temperature? A) 66.7 D) 1.02 -2 E) 4.63 B) 1.50 × 10 C) 2.53 × 10-2 10. Consider the following chemical reaction: H2 (g) + I2 (g) 2HI (g) At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were 0.25 M, 0.035 M, and 0.55 M, respectively. The value of Keq for this reaction is __________. A) 23 D) 5.1 B) 63 E) 34 C) 0.0090 Short Answer 11. The number obtained by substituting starting reactant and product concentrations into an equilibriumconstant expression is known as the __________. 12. If the reaction quotient Q for a reaction is less than the value of the equilibrium constant K for that reaction at a given temperature, __________ must be converted to __________ for the system to reach equilibrium. 13. If the reaction quotient Q for a reaction is greater than the value of the equilibrium constant K for that reaction at a given temperature, __________ must be converted to __________ for the system to reach equilibrium. 14. At 100C the equilibrium constant for the reaction COCl2 (g) CO (g) + Cl2 (g) has the value Kc = 2.19 x 10-10. Is a mixture of [COCl2] = 2.00 x 10-3 M, [CO] = 3.30 x 10-6 M and [Cl2] = 6.62 x 10-6 M at equilibrium? If it isn’t at equilibrium, which way will the reaction shift? 15. At 448C the equilibrium constant Kc for the reaction: H2 (g) + I2 (g) 2HI (g) is 50.5. Predict in which direction the reaction will proceed to reach equilibrium at 448C if we start with 2.0 x 10-2 mol of HI, 1.0 x 10-2 mol H2 and 3.0 x 10-2 mol I2 in a 2.00 L container. 16. At 800C the equilibrium constant for I2 (g) 2I (g) is Kc = 3.1 x 10-5. If an equilibrium mixture in a 10.0 L vessel contains 2.67 x 10-2 g of I (g), how many grams of I2 (g) are in the mixture? 17. The reaction A2 + B2 2AB has an equilibrium constant Kc = 1.5. a. Which reaction mixture is at equilibrium? b. For those mixtures not at equilibrium, how will the reaction proceed to reach equilibrium? 18. The reaction A2 + B AB + A has an equilibrium constant of Kp = 2. How many B atoms should be added to the diagram to illustrate an equilibrium mixture?
© Copyright 2026