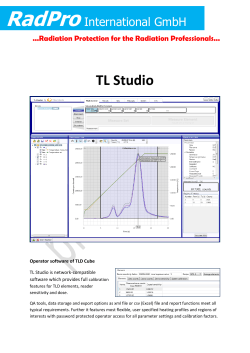
QA of a Hematology Analyzer
May 30th 2014 M R Tiwari B.Sc. (Microbiology), PGDMLT, M.Sc.(TQM) Scientific Assistant ‘D’ Hematology, Composite Laboratory, ACTREC, TMC Laboratory test results (Good laboratory practice, GLP) Clinical diagnosis Patient management Quality can be assured at ◦ Pre-analytical stage ◦ Analytical stage ◦ Post-analytical stage Requisition form Sample collection & transport Sample receiving & rejection Sample preparation = Definition Reproducibility of a result Achieved with ◦ Controls samples (Commercially procured or whole blood) When required ◦ Calibration ◦ Daily quality control ◦ After troubleshooting ◦ After change of reagent Definition Closeness of a result to the true value Achieved with ◦ Calibrator When required ◦ Calibration Mean – The target Standard deviation – The acceptable limits (±2SD) Coefficient of variation (CV %) variation at different levels can be compared. X-axis - the days of the month (time interval) Y-axis. - control observations Control Chart's Inventor In 1931, Dr. Walter Shewhart, a In 1950, scientist at the Bell Telephone S. Levey & Laboratories, proposed applying statistical based E.R. Jennings control charts to interpret suggested the use in industrial manufacturing the clinical laboratory. processes. L-J chart interpretation Westgard rules Selection Purchase Installation Use Replace Selection Comparative studies Cost per test Through put Reagent consumption Sample volume Good logistics etc…. Purchase Reagent rental / direct purchase Loan availability etc…. Installation Installation Qualification (IQ) Parts, accessories, manuals, computer, software, filter, labour, consumables shipped & received, vendor’s verification against all materials received against the initial order, hardware installation, environmental requirements, space, electricity, temperature. Operational Qualification (OQ) System power-up & shutdown, fluidic start-up & shutdown, no detectable leaks, absence of bubble in the sheath fluid, sheath and waste level sensors are operational, pneumatic pressures are in range, sample & sheath fluid rates meet their specifications, computer & its operating system starts up with no errors, flowcytometer’s control software, photodiode & photo multiplier tubes (PMT) voltage controls & detector responses should be tested, laser power of each laser line or wavelength to be tested, path between the laser and flow cell is checked, manual and automated sample delivery, testing of barcode reader, etc. Performance Qualification (PQ) Linearity, Carryover, Precision, Calibration, Method validation,. ◦ ◦ ◦ ◦ ◦ WBC (0.02 to 400) RBC (0.0 to 7.0) HGB (0.0 to 22.5) PLT (0.2 to 3500) RETIC% (0.2 to 24.5) ◦ %Carryover = [(L1-L3)/H]x100 ◦ L1– the count of first aspiration of cell-free plasma ◦ L3- the count of third aspiration of cell-free plasma ◦ H – the count of high pool Carryover is defined as a number of cells remaining behind following the cycling of a blood sample. This test is performed to determine if one sample interferes with the accurate analysis of the next sample. Ideally, carryover shall be very low. Calibrator Certified Reference Material (CRM) used to calibrate a measurement on an analyzer. Cal-Factors If any deviation from calibration references is observed necessary calibration correction factors are applied to set the accuracy of the instrument. Pre-calibration check ◦ Total maintenance of the instrument ◦ Reagents (replenish or replace) ◦ Calibrator (Check for expiry) ◦ Controls (Check for expiry) Calibration procedure ◦ Carryover check ◦ Precision check ◦ Calibration in both (open and closed modes) Post-calibration validation ◦ Run calibrator as samples ◦ Run 3 level controls Use Internal Quality control (IQC) External Quality Assurance (EQA) Sample analysis Hematology analyzers • Start up status and background count check First step (Monitor for acceptable background…..if required take corrective action) • QC monitoring using multilevel controls (Low level, normal level and high level) Done once (Monitor L-J chart, apply Westergard’s interpretation, monitor %CV…..if required take corrective action) • QC monitoring using retained sample Periodic in b/w check till shut down (Monitor %CV…..if required take corrective action) • Start up status and background count check First step (Monitor for acceptable background…..if required take corrective action) Done once • QC monitoring using multilevel controls (Low level, normal level and high level) (Monitor L-J chart, apply Westergard’s interpretation, monitor %CV…..if required take corrective action) Warning rule = use other rules to inspect the control points Rejection rule = “out of control” ◦ Do not run patient samples ◦ Identify and correct problem ◦ Do not report patient results until problem is solved and controls indicate proper performance • QC monitoring using retained sample Periodic in b/w check till shut down (Monitor %CV…..if required take corrective action) Comparative study b/w the analyzers (monitor % variation) Rerun on same sample in different mode Lot validation (after change of reagent) with retained sample – precision check Correlation with peripheral smears All haematology analyzers ◦ Moving average analysis (Bull’s algorithm) High end analyzers ◦ ◦ ◦ ◦ ◦ WBCP – WBCB HGB (spectrophotometricaly)-HGB(cellular-laser) MCHC – CHCM PMN – (Neutrophils, Eosinophils) MN – (Lymphocytes, Monocytes) Follow the manufacturer’s manual Perform maintenance regularly ◦ ◦ ◦ ◦ ◦ ◦ Daily Weekly Biweekly Monthly Annually As if required Maintain record of maintenance Try to correct at operator level if possible or Call the service engineer for help Maintain a record of any breakdown observed ◦ ◦ ◦ ◦ ◦ ◦ Nature Time Error detected Corrective action QC checked Service report Normal Distribution Curve or Gaussian curve Describes events or data that occur symmetrically about the mean. Out of 100 events 68.7 will fall within ±1 SD 95.4 will fall within ± 2 SD 99.7 will fall within ±3 SD Z-Score A-1 A-2 A-3 A-4 A-5 A-6 A-7 A-8 A-9 A-10 A-11 A-12 WBC 5.9 6.3 6.2 5.2 6.3 6.2 5.9 6.3 HGB 13.1 12.9 12.7 13.1 12.9 12.7 13.1 PLT 262 246 255 262 246 255 Z-SCORE A-1 A-2 A-3 A-4 A-5 A-6 MEAN SD 6.2 5.9 6.3 6.2 6.1 0.3 12.9 12.7 13.1 12.9 12.7 12.9 0.2 262 246 300 262 246 255 258.1 14.9 A-7 A-8 A-9 A-10 A-11 A-12 WBC -0.55 0.70 0.39 -2.74 0.70 0.39 -0.55 0.70 0.39 -0.55 0.70 0.39 HGB 1.17 0.00 -1.17 1.17 0.00 -1.17 1.17 0.00 -1.17 1.17 0.00 -1.17 PLT 0.26 -0.81 -0.21 0.26 -0.81 -0.21 0.26 -0.81 2.82 0.26 -0.81 Z SCORE SCALING :< ±0.5 - Excellent performance ±0.5 to ±1.0 - Satisfactory ±1 to ±2 - Acceptable > ±2 - Defect requiring attention -0.21 Check for the IQC results during the period when the EQAS sample was analyzed. Follow the instructions from the nodal organization. A formal way of testing for aberrant results is known as `delta check`. The blood count parameters should not differ from recent tests in the previous 2-3 weeks by more than a certain amount. For Hb and RBC For WBC For Platelet count 10 % 20-25 % 50 % Assuming that the patient’s clinical condition has not altered significantly The laboratory The requester i.e. takes responsibility for reporting the results within the specified turn Coag4 hrs CBC2hrs the clinician is notified in case of delay in BMA3wd around time. examination only in such cases where the delay can compromise TAT patients care. Quality Indicator – “TAT shall be monitored on periodic basis for continual improvement” Laboratory test results ◦ Pre-analytical stage Clinical diagnosis ◦ Analytical stage ◦ Post-analytical stage Quality Patient management Good Laboratory Practice
© Copyright 2026