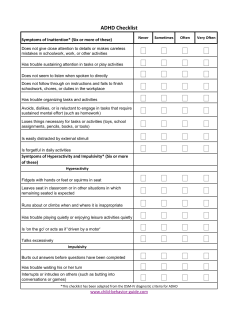
N B P C
NEUROBIOLOGICAL AND BEHAVIORAL PHENOTYPES AND COCAINE SELF‐ADMINISTRATION IN ADULT RHESUS MONKEYS EXPOSED TO COCAINE THROUGHOUT GESTATION B Y LINDSEY R. HAMILTON A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVERSITY GRADUATE SCHOOL OF ARTS AND SCIENCES In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY Program in Neuroscience August 2010 Winston‐Salem, North Carolina Approved By: Michael A. Nader, Ph.D., Advisor ____________________________________ Examining Committee: Joseph R. Tobin, M.D., Chair ____________________________________ Paul W. Czoty, Ph.D. ____________________________________ Anthony Liguori, Ph.D. ____________________________________ Linda J. Porrino, Ph.D. ____________________________________ ACKNOWLEDGEMENTS Although this dissertation will have only my name on the cover, a great number of people have contributed to its production. I owe many thanks to all those people who have made this dissertation possible. First and foremost, I would like to express my deepest gratitude to my advisor, Dr. Mike Nader, who has given me the freedom to explore on my own and, at the same time, the guidance to better refine my ideas. He has impressed upon me all the important traits necessary to become a behavioral pharmacologist: perseverance (“Stay the course!”), proper terminology (“Don’t call it a DRL schedule.”), critical analysis (“The data are the data.”), and high caffeine intake (“Is decaf coffee a reinforcer?”). I also greatly appreciate the people that made coming to work everyday a treat, not a chore: my labmates with whom I shared an office, frustrations, successes, and many great conversations, Dr. Matt Banks, Dr. Jenn Martelle, Dr. Natallia Riddick, Rob Gould, and Brandi Blaylock; and my colleagues who trained me and were always available to assist me in anyway, Sue Nader, Tonya Calhoun, Michelle Icenhower, Susan Martelle, Michael Coller, and Whitney Wilson. I also would be remiss if I did not thank my presidential monkeys who taught me patience and always gave me a good laugh. ii I am grateful to my dissertation committee for their helpful suggestions, comments, critiques, and alternate viewpoints: Dr. Paul Czoty, Dr. Tony Liguori, Dr. Linda Porrino, and Dr. Joe Tobin. Finally, I wholeheartedly thank my friends and family for their support, patience, and love during my graduate school years. My parents have always been my biggest cheerleaders and I deeply appreciate their belief in me. The friends who make up my extended family have given me strength throughout difficult times, celebrated with me in happy times, and helped me stay focused on completing this dissertation. I truly value their friendship. This research was supported by Grant R01 DA 025120, R37 DA 10584, and an Individual Predoctoral National Research Service Award F31 DA 024485 from the National Institute on Drug Abuse. iii TABLE OF CONTENTS PAGE ACKNOWLEDGEMENTS ii LIST OF ABBREVIATIONS v LIST OF TABLES AND FIGURES viii ABSTRACT x CHAPTERS: I. Introduction II. Characterization of the dopamine receptor system in adult 86 rhesus monkeys exposed to cocaine throughout gestation. Published in Psychopharmacology, accepted March 2010 III. Increased impulsivity in male, but not female, adult rhesus 115 monkeys exposed to cocaine throughout gestation. Submitted to Psychopharmacology, April 2010 IV. Increased vulnerability to selfadminister cocaine in adult rhesus monkeys exposed to cocaine throughout gestation. To be submitted to Science, May 2010 156 V. Discussion 182 1 SCHOLASTIC VITAE 218 iv LIST OF ABBREVIATIONS 5‐HIAA 5‐hydroxyindole acetic acid 5‐HT Serotonin ACC Anterior Cingulate Cortex ANOVA Analysis of Variance BSID Bayley Scale of Infant Development COMT Catechol‐O‐methyl transferase CSF Cerebrospinal Fluid DA Dopamine DAT Dopamine Transporter DVR Dopamine E Embryonic Day EDTA Ethylenediaminetetraacetic acid e.g. Exempli gratia or For example FCP Fluoroclebopride FR Fixed Ratio HV Highly Vulnerable HVA Homovanillic acid i.e. id est or That is i.m. Intramuscular IQ Intelligence Quotient i.v. Intravenous v LV Low Vulnerable MAO Monoamine oxidase MDI Mental Development Index MRI Magnetic Resonance Imaging nM Nanomole NAc Nucleus Accumbens NBAS Neonatal Behavioral Assessment Scale NE Norepinephrine NHP Nonhuman Primates NIDA National Institute on Drug Abuse NMDA N‐methyl‐D‐aspartate NSDUH National Survey on Drug Use and Health OTB Operant Test Battery PCP Phencyclidine PR Progressive Ratio ROI Region of Interest SAMHSA Substance Abuse and Mental Health Services Administration SEM Standard Error of the Mean SN Substantia Nigra Pars Compacta TH Tyrosine hydroxylase TO Timeout vi VR Variable Ratio VTA Ventral Tegmental Area vii LIST OF TABLES AND FIGURES CHAPTER I PAGE Table 1. Subject Demographics CHAPTER II 40 Figure 1. Distribution Volume Ratios of [18F]FCP in the 104 Caudate, Putamen, and Amygdala Figure 2. A) Yawning Elicited by Quinpirole in Female Prenatally Cocaine Exposed and Control Monkeys B) Yawning Elicited by Quinpirole in Male Prenatally Cocaine Exposed and Control Monkeys C) Group Comparison for Yawning Elicited by Quinpirole 105 106 Figure 3. Correlation of Peak Yawns Elicited by Quinpirole and Maximal Daily Dose of In Utero Cocaine 107 Figure 4. A) Effects of SKF 81297 on Eye‐Blinking Rates in Control Monkeys B) Effects of SKF 81297 on Eye‐Blinking Rates in Prenatally Cocaine Exposed Monkeys C) Group Comparison of the Effects of SKF 81297 on Eye‐Blinking Rates 108 109 CHAPTER III Table 1. Comparison of CSF Concentrations of 5‐HIAA and HVA 139 Table 2. Response Rates During the Delay Discounting Task 140 Figure 1. A) Latency to Touch a Novel Object Placed in the Home Cage B) Grid Crossings in a Novel Environment 141 Figure 2. Number of Sessions to Extinguish Food‐Reinforced Behavior 142 Figure 3. Representative Delay Value – Percentage Choice for the Delayed Reinforcer Curves for a Prenatally Cocaine Exposed Monkey and a Control Monkey 143 Figure 4. Indifference Points on the Delay Discounting Task 144 viii Figure 5. Overall Impulsivity Score Measured Across Four Impulsivity Measures CHAPTER IV 145 Figure 1. Percentage of Monkeys Reaching Criteria to Acquire Cocaine Self‐Administration at Various Doses of Cocaine 174 Figure 2. Amount of Cocaine Intake Prior to Reaching Criteria to Acquire Cocaine Self‐Administration in Prenatally Cocaine Exposed and Control Monkeys 175 Figure 3. Amount of Cocaine Intake Prior to Reaching Criteria to Acquire Cocaine Self‐Administration in Highly and Less Vulnerable Monkeys 176 Figure 4. A) Comparison of Impulsivity Scores Between Highly and Less Vulnerable Monkeys B) Peak Yawns Elicited by Quinpirole in Highly and Less Vulnerable Monkeys C) Relationship Between Peak Yawns and Amount of Cocaine Intake Prior to Reaching Criteria to Acquire Cocaine Self‐Administration 177 178 CHAPTER V Figure 1. Representative Response Rates for Food and Drug Under an FR 30 Schedule of Reinforcement 185 Figure 2. Acquisition and Maintenance Cocaine Dose‐ Response Curves for Prenatally Cocaine‐Exposed and Control Monkeys 187 Figure 3. Alterations in D2 and D3 Receptors Could Result in Similar FCP DVRs in Prenatally Cocaine Exposed and Control Monkeys 200 Figure 4. Percentage of Male and Female Monkeys Reaching Criteria to Acquire Cocaine Self‐Administration at Various Doses of Cocaine 206 ix ABSTRACT Hamilton, Lindsey R. NEUROBIOLOGICAL AND BEHAVIORAL PHENOTYPES ASSOCIATED WITH VULNERABILITY TO COCAINE SELF‐ADMINISTRATION IN ADULT RHESUS MONKEYS EXPOSED TO COCAINE THROUGHOUT GESTATION Dissertation under the direction of Michael A. Nader, Ph.D., Professor of Physiology and Pharmacology Maternal cocaine addiction is a significant public health problem yet the effects of cocaine use during pregnancy on long‐term postnatal outcomes have not been well established. This dissertation utilized an animal model of prenatal cocaine exposure in rhesus monkeys to evaluate the long‐term neuropharmacological consequences, behavioral impulsivity, and vulnerability to stimulant self‐administration. These adult monkeys had been exposed to cocaine throughout gestation and were compared to a population of control monkeys. In Chapter II, the dopamine (DA) receptor system was characterized using several in vivo models. There were no differences between groups in D2‐like receptor availability, as determined with positron emission tomography imaging, or in D1‐like receptor function, as assessed by unconditioned behaviors elicited by an agonist. Prenatally cocaine‐exposed monkeys had higher D3 receptor function compared to controls, as assessed by quinpirole‐elicited yawning. Additionally, a relationship was found x between gestational cocaine dose and D3 receptor function, demonstrating that prenatal cocaine exposure had long‐lasting neurobiological effects. The studies in Chapter III examined several measures hypothesized to assess impulsivity. In general, unconditioned behaviors did not show differences in impulsivity, while behaviors involving schedule‐controlled responding were more sensitive. Overall, male prenatally cocaine‐exposed monkeys were more impulsive than controls while there were no differences in overall impulsivity observed in females. Chapter IV was aimed at determining whether the neurobiological and behavioral outcomes observed earlier would result in altered sensitivity to the reinforcing effects of cocaine. Prenatally cocaine‐exposed monkeys acquired cocaine self‐administration at lower doses than controls. Vulnerability to self‐administer cocaine was found to be related to an individual phenotype involving increased D3 receptor function and increased impulsivity. In conclusion, the research presented in this dissertation suggests that cocaine use during pregnancy can have long‐lasting neurobiological and behavioral effects. These results indicate that a vulnerable phenotype for cocaine reinforcement exists and that prenatal cocaine exposure may predispose individuals towards this phenotype in adulthood. Greater understanding of this phenotype could lead to identification of pharmacological and behavioral targets for prevention and treatment of cocaine abuse. xi CHAPTER I INTRODUCTION COCAINE ABUSE Cocaine is a powerful central nervous system stimulant that inhibits the synaptic uptake of dopamine (DA), norepinephrine (NE), and serotonin (5‐HT) by binding to the transporters for these neurotransmitters (Benowitz, 1993). Cocaine also activates the peripheral sympathetic nervous system and has local anesthetic effects (Krug, 1989). Physical and psychological symptoms can arise from both acute and chronic cocaine use. Physical consequences include myocardial infarctions, ischemic complications, arrhythmias, seizures, loss of consciousness, and migraine headaches (Gradman, 1988; Klonoff et al., 1989; Romero et al., 2002). The acute behavioral effects of cocaine use include irritability, impaired judgment, aggressiveness, sexual disinhibition, increased impulsivity, and manic excitement (Taylor and Ho, 1977; Estroff and Gold, 1985; Hurlbut, 1991; Das, 1993). Chronic use of cocaine has been associated with somatic complaints, depression, anxiety, paranoia, memory loss, and problems with executive function (Jovanovski et al., 2005; Minnes et al., 2008). The peak of the cocaine epidemic in the United States occurred in the mid‐1980s, when about 8 million Americans were using cocaine regularly (Cregler and Mark, 1986). The 1988 National Household Survey on Drug Abuse (NHSDA) found that the number of heavy cocaine users increased signficantly from 1985 to 1988 (SAMHSA, 1988). During this 3 year period, there was a 33% increase among those using cocaine once a week or more (SAMHSA, 1988). The 1 number of Americans who used cocaine within the preceding month was at an all time high of 7.1 million in 1985 (SAMHSA, 1986) and 1 in 3 young adults reported using cocaine at least once (O’Malley et al., 1991). Today, cocaine use is still widespread in the United States with 2.4 million Americans confirming current cocaine use (SAMHSA, 2006). Additionally, epidemiologic data suggest that cocaine dependence may be growing in Western Europe and Africa, evidenced by the largest recent increase in cocaine seizures (United Nations, 2007), making this a global public health problem as well. Cocaine use among females of childbearing age is significant public health concern. The most recent National Survey on Drug Use and Health (NSDUH) found that in 2006‐2007, 5.6% of pregnant women ages 15 to 44 reported using illicit substances in the past month and almost 1.0% reported current use of cocaine (SAMHSA, 2008). Of all the illicit substances, cocaine, second only to marijuana, remains one of the most widely used illicit substances among women during pregnancy and is commonly used concurrently with tobacco and alcohol (NIDA, 1996; SAMHSA, 2006). In fact, in the 1990s, typically up to 10‐20% of live births in some urban, primarily low socioeconomic status areas, tested positive for cocaine exposure (Church et al., 1991; Kandel et al., 1998; Wetherington and Roman, 1998) although it was reported as high 40% in one study (Ostrea et al., 1992). Reports from the most recent National Pregnancy and Health Survey (NPHS) estimate that 45,000 infants prenatally exposed to cocaine are born annually (NIDA, 1996). However, since the NPHS is based on self‐report, it is likely that it is an 2 underestimate of the scope of the problem (Markovic et al., 2000; Savitz et al., 2002, Bessa et al., 2010). Even with conservative estimates, widespread use of cocaine in the United States has resulted in upwards of 1.5 million children prenatally exposed to cocaine, many of whom are now entering adolescence or young adulthood, a time when many experiment with drugs of abuse. Young adolescents with parents who abuse drugs are 2‐3 times more likely to try substances (Newcomb et al., 1983; Jackson et al., 1997; Kaplow et al., 2002). Furthermore, these children are 2‐4 times more likely to have developed a substance use disorder during adolescence (Biederman et al., 2000). Since 1 in 5 Americans ages 21‐25 years old have tried cocaine at least once in their lifetime (SAMHSA, 2008), this is a large public health problem if prenatal exposure to cocaine increases vulnerability to drug dependence. The focus of the research in this dissertation is on the longterm effects of prenatal cocaine exposure, specifically vulnerability to selfadminister drugs of abuse. DOPAMINE The behavioral and reinforcing effects of cocaine are thought to be primarily mediated by the DA system (Roberts et al., 1977, 1980; Wise, 1984; Ritz et al., 1987; Bergman et al., 1989; Madras et al., 1989; Kuhar et al., 1991). DA neurons are widely distributed in the adult central nervous system and DA 3 serves a variety of functions in the mature brain, including control of movement and the regulation of endocrine, cardiovascular, and limbic systems. DA is synthesized from conversion of L‐tyrosine into L‐dopa by tyrosine hydroxylase, the rate‐limiting enzyme. L‐dopa is converted to DA by activity of DOPA decarboxylase. DA receptors are characterized by an intracelluar C‐ terminus region, an extracellular N‐terminus region, and seven membrane spanning regions. The receptors are coupled intracellularly to guanine nucleotide binding proteins that induce intracellular signaling cascades, which can influence regulation of calcium and potassium channels on the postsynaptic membrane. The DA receptors can be divided into two superfamilies based on their pharmacological profiles and sequence homology: D1‐like receptors and D2‐like receptors. D1‐like receptors, including the D1 and D5 receptor subtypes, catalyze the synthesis of cyclic adenosine monophosphate (cAMP) from the action of adenylate cyclase on adenosine triphosphate. Stimulation of D2‐like receptors, including the D2, D3, and D4 receptor subtypes, has opposite effects, inhibiting cAMP synthesis (Kebabian and Calne, 1979; Missale et al., 1998). DA transmitter action is terminated by re‐uptake into the presynaptic terminal by a high affinity plasma membrane dopamine transporter (DAT) and enzymatically degraded by monoamine oxidase (MAO) or catechol‐O‐methyl transferase (COMT). There are several major dopaminergic pathways. The nigrostriatal tract consists of dopaminergic neurons in the substantia nigra (SN) pars compacta that terminate in the striatum, a major DA‐containing area of the brain. The 4 striatum is a component of the extrapyramidal motor system and plays an essential role in the coordination of locomotor activity. The mesolimbic and mesocortical DA pathways are two midbrain dopaminergic pathways implicated in behaviors associated with motivation, reward (both endogenous systems and drug abuse), and reinforcement. Both pathways begin in the midbrain ventral tegmental area (VTA) and provide input to the nucleus accumbens (NAc) and frontal cortex (both medial prefrontal (mPFC) and anterior cingulate (ACC)), respectively (Olson et al., 1973). In monkeys, it has also been observed that a subset of VTA neurons provide innervation to the caudate nucleus of the striatum (Lynd‐Balta and Haber, 1994a, 1994b; Haber et al., 1995). It is thought that stimulation of DA receptors in mesolimbic pathway is in part responsible for the reinforcing effects of cocaine (Wise and Rompre, 1989; Volkow et al., 1999). The subjective effects of cocaine are also thought to be mediated through this same pathway (Di Chiara and Imperato, 1988; Wise and Rompre, 1989; Wise, 1996). D1‐ like receptors are positively coupled to adenylyl cyclase through stimulatory Gα proteins, resulting in an increase in cAMP concentrations. DA D1 receptors are primarily localized in the nucleus accumbens, olfactory tubercles, and the amygdala, while DA D5 receptors are primarily localized in the hippocampus, hypothalamus, and parafascicular nucleus. It is thought that D1‐ like receptors may play a role in the reinforcing effects of cocaine. Several groups have demonstrated that antagonism of D1‐like receptors results in increases in cocaine self‐administration under a fixed‐ratio (FR) schedule of 5 reinforcement in rats (Koob et al., 1987; Corrigall and Coen, 1991; Hubner and Moreton, 1991; Ranaldi and Wise, 2001). D2‐like receptors are negatively linked to adenylate cylase through inhibitor Gi/o proteins and stimulation results in inhibition of cAMP concentrations. These receptors are found throughout the brain including the caudate nucleus, putamen, nucleus accumbens, olfactory tubercles, and cerebral cortex. A prominent role for D2‐like receptors in the reinforcing effects of psychostimulants has been established. D2‐like receptor antagonists attenuate the reinforcing effects of self‐administered cocaine (Bergman et al., 1990; Britton et al., 1991; Corrigall and Coen, 1991; Hubner and Moreton, 1991; Caine and Koob, 1993; Campbell et al., 1999; Nader et al., 1999). Additionally, Volkow et al. (1999) determined that people with low D2‐ like receptor availability as assessed with positron emission tomography (PET) found methylphenidate to be more pleasant than people with high D2‐like receptor availability. Additionally, cocaine abusers have lower D2‐like receptor availability compared to age‐matched, non‐drug abusing individuals (Volkow et al., 1993; Martinez et al., 2004). Monkey with lower D2‐like receptor availability self‐administer cocaine at higher rates than monkeys with higher D2‐like receptor availability (Morgan et al., 2002; Nader et al., 2006). It appears that there may be opposing effects of D1‐like and D2‐like receptors on the reinforcing effects of cocaine. 6 DOPAMINERGIC RECEPTOR SYSTEM DURING GESTATION Since the focus of this dissertation is on the long‐term effects of prenatal cocaine exposure, it is important to understand the development of the DA system during gestation. Tyrosine hydroxylase (TH), the rate‐limiting enzyme in DA synthesis, is a useful marker for identifying DA neurons. TH is first apparent at Embryonic day (E) 12‐13 of an approximate 21 day gestational period in the rat midbrain, and is present by E14 of an approximate 30 day gestational period in the rabbit. DA is also likely to have early biological activity in the primate brain. In the monkey, DA neurons of the SN/VTA are produced between E36 and E43 of a 165 day gestational period (Levitt and Rakic, 1982). In humans, midbrain DA neurons appear during the first trimester in the second month of gestation (Olson and Seiger, 1972). This input is thus already present in the cortex even while more superficial cortical layers (II‐IV) are beginning to form, consistent with a morphogenic role of DA. Axons of dopaminergic cells reach the cortex a few days after their initial detection in the midbrain in monkeys (Lambe et al., 2000). Limbic cortical regions, such as the ACC and mPFC receive the densest dopaminergic innervation. The density of tyrosine hydroxylase‐positive axons in the cortex increases gradually over development then declines postnatally to reach adult levels during puberty (Lambe et al., 2000). Transcripts for the D1, D2 and D3 receptors can be detected in the striatum and cortex by E14 in the rat and by E12 in the mouse (Jung and Bennett, 1996; Araki et al., 2007). D1‐like and D2‐like receptors are measurable 7 at these early prenatal time‐points and increase in great quantity throughout prenatal and early postnatal development to reach adult levels of expression between postnatal day 14 and 21 in rodents (Sales et al., 1989; Rao et al., 1991; Schambra et al., 1994; Caille et al., 1995). In the monkey, DA receptors appear in target regions of DA input by week 12 of gestation (Lidow et al., 1991; Lidow, 1995a) and in humans DA receptor binding sites have been detected by week twelve of gestation (Aubert et al., 1997). In all species examined, DA receptors are present very early in prenatal development, consistent with a role for DA in regulating neuronal differentiation and circuit formation. Therefore, the DA system is a potential modulatory target for cocaine to act on during prenatal development when the drug is used during pregnancy (Malanga and Kosofsky, 2003; Stanwood and Levitt, 2004). “CRACK BABIES” In the 1980s, the 'war on drugs' associated with the crack cocaine epidemic focused national attention on the relationship between drug use and social and economic problems in society. An early report in The New England Journal of Medicine suggested that prenatal cocaine exposure could cause behavioral and learning problems (Chasnoff et al., 1985) and a media frenzy over "crack babies" ensued. Despite Chasnoff and colleagues’ warnings that more research was needed to determine the long‐term effects of prenatal cocaine exposure, media reports described children exposed to cocaine in utero as a "bio‐underclass" and forecasted that "theirs will be a life of certain suffering, 8 of probably deviance, of permanent inferiority” (Krauthammer, 1989). The rising trends of cocaine use among pregnant women, coupled with these types of dire reports in the popular press, resulted in a rush to judgement with regard to the fate of prenatally cocaine exposed children (Leshner, 1998). The persistence of the mythology that prenatally cocaine‐exposed children suffer from a myriad of devastating effects may negatively shape clinical investigations. Observers of children who are cocaine‐exposed may be influenced by media reports that frequently over‐generalize from anecdotal descriptions. Media anecdotal descriptions have been used in a manner that implies that these children are representative of all children who are cocaine‐ exposed (Neuspiel, 1993; Day and Richarson, 1993; Elyer and Behnke, 1995; O’Neill and Carter, 1999; Will, 1999; Rotzoll, 2000). The label cocaine‐exposed may then impact the expectations of clinicians and researchers working with children. Woods et al. (1998) designed a study that examined the association between the label cocaine‐exposed and observers’ perceptions. College students from education, nursing, general education, and psychology programs were asked to rate behavioral parameters from videotapes of healthy infants who were, in fact, not cocaine‐exposed. One group of observers was told that the child they were about to watch was “born without any known problems.” A second group was told that the same child was “born without any known problems other than that her mother used cocaine during pregnancy.” The results of the study showed that the same infants in the same tapes when labeled as cocaine‐ 9 exposed during pregnancy were rated more negatively than when they had not been labeled as cocaine‐exposed. This prejudice is damaging in clinical settings and highlights the crucial need for research on the subject to be done thoroughly and without bias. Prenatal cocaine exposure in animal models allows for such objective assessments across a wide range of behaviors. PRENATAL COCAINE EXPOSURE: HUMAN STUDIES Maternal cocaine use during pregnancy continues to be of great concern for health care professionals. It has been suggested that gestational exposure to drugs of abuse is the single largest preventable cause of in utero developmental compromise of infants in the United States today (Lester and Twomey, 2008). Findings in the literature demonstrate inconsistencies in regard to the physiologic and developmental outcomes of infants/young children prenatally exposed to cocaine. Further research is warranted, as it is evident from studies that not all investigators are controlling for confounding variables such as poly‐drug use, which is necessary in order to isolate cocaine's effects. However, the majority of research on the topic points to a myriad of clinical manifestations that present in the infant/young child. Cocaine use during pregnancy poses a risk to both mother and fetus and has been associated with significant obstetric complications. Pregnant women who use cocaine have been found to have a higher incidence of poor weight gain and cardiac complications, such as hypertension, arryhythmia, 10 cardiac ischemia, and hemorrhagic stroke (Plessinger and Woods, 1998; Kuczkowski, 2003; Vidaeff and Mastrobattista, 2003). Cocaine is known to have a negative additive effect to the already stressed cardiovascular system of the pregnant woman (Wagner et al., 1998). During pregnancy, the toxicity of cocaine is increased, thus increasing the risk for cardiovascular events such as stroke and seizures (Plessinger and Woods, 1998). Other adverse effects, including uterine rupture, hepatic rupture, placental abruption and maternal death, are known to occur more frequently in those using cocaine (Plessinger and Woods, 1998; Kuczkowski, 2003). Prenatal cocaine exposure is hypothesized to directly affect the development of the fetal central nervous system through teratologic effects on brain growth and development. The fetus is at significant risk from cocaine exposure secondary to maternal use. Cocaine has a low molecular weight and is both hydrophilic and lipophilic. These properties allow significant levels of cocaine to cross the placenta and pass through the blood brain barrier. Fetal cocaine concentrations are equivalent to maternal cocaine concentrations within 1‐2 minutes, raising fetal heart rate, and decreasing internal and placental blood flow (Woods et al., 1987). The metabolism of cocaine in the fetus is known to be considerably slower than that of the adult. Thus, fetal exposure to cocaine is prolonged (Schenker et al., 1993; Wagner et al., 1998). A study by Mahone and colleagues (1994) found cocaine and its derivatives to not only be transferred to the fetus via diffusion into the umbilical cord and within the placental vessels, but also to be in the amniotic fluid and then swallowed by the fetus. In fact, 11 amniotic fluid can act as a repository for cocaine exposure to the fetus. Sanberg and Olsen (1992) demonstrated that cocaine concentrations in amniotic fluid were 3‐4 times greater than in fetal blood. Cocaine can then enter the fetal blood at a rate of 3% from the amniotic fluid (Mahone et al., 1994). If cocaine enters the amniotic fluid early in pregnancy, when water transport across fetal skin is unrestricted, fetal exposure may be markedly enhanced at a critical time period during which neurotransmitter development is being initiated (Woods, 1998). The dose, the duration of drug ingestion, and the point in gestation at which the fetus is exposed to cocaine determines the effect the drug may have on the fetus. One of the major difficulties in studying prenatal cocaine exposure in humans is the problem of controlling for confounding and moderating variables. For example, pregnant women who use cocaine also tend to use other illicit substances like marijuana as well as licit substances that can have toxic effects like nicotine and alcohol (Frank et al., 1988; Zuckerman and Frank, 1989; Hurt et al., 1995). There are also many biological and social confounds that must be taken into account, including factors that precede conception like maternal age, educational status, nutritional status, and disease status (Frank et al., 1988). Determining criteria for whether prenatal cocaine exposure has occurred differs from study to study. Ascertainment of cocaine use during pregnancy is commonly done by self‐report, although that is not the most reliable method (Zuckerman et al., 1989). More accurate ascertainment techniques include urine toxicology (Ambre et al., 1982; Weiss and Gawin, 1988), hair radioimmunoassay 12 (Graham et al., 1989; Kline et al., 1992), and meconium analysis (Ostrea et al., 1989; Lewis et al., 1995). The dose of cocaine the fetus was exposed to is also challenging to determine in human studies. An interest in dose has emerged, though, as it is thought that greater cocaine exposure can disrupt brain development more so and may have greater behavioral and physiological consequences. One issue in the human literature is that there is no consensus for what defines heavy use of cocaine (Frank et al., 1998). It is not clear whether heavy users in one population are comparable to heavy users in another population because of variability in potency, route of administration, and modes of ascertainment of dose of prenatal cocaine exposure. In fact, some of the interviews designed to identify prenatal cocaine exposure use a criteria of heavy use (using more than two days a week or more than one line of cocaine a day) that does not distinguish between frequency and quantity (Richardson et al., 1993; Singer et al., 1994; Jacobson et al., 1996). Also, the dose used during pregnancy may vary throughout gestation, as there have been reports that women’s use decreases as the pregnancy progresses (Richardson et al., 1993). Beyond the issue of determining dose of cocaine exposure, it is not yet known whether the primary determinant of adverse outcomes is cumulative dose or maximum dose used on a single occasion (Frank et al., 1998). Despite these challenges, it does appear that there is a cocaine dose effect on neonatal size at birth, on neonatal behavior, and on infant informational processing (Jacobson et al., 1996; Hurt et al., 1997; Chiriboga et al., 1999; Bateman and Chiriboga, 2000). However, these studies 13 need to be more thoroughly investigated while controlling for confounding biological and social variables. PHYSIOLOGIC EFFECTS OF PRENATAL COCAINE EXPOSURE IN NEONATES Cocaine's physiological effects, such as vasoconstriction, hypertension, and tachycardia during pregnancy, may have profound effects on the fetus. During pregnancy, cocaine causes vasoconstriction of the maternal uterine blood vessels causing an increase in uterine vascular resistance and decreased uterine blood flow (Woods et al., 1987). Oxygen and nutrients normally transferred to the fetus via these vessels are unable to reach the placenta and fetus resulting in uteroplacental insufficiency and fetal hypoxemia (Woods et al., 1987). This uterine vasoconstriction may be the primary cause for such complications as spontaneous abortion, premature labor and delivery, abruptio placentae, and fetal intracranial hemorrhage (Acker et al., 1983; Bingol et al., 1987; Cohen et al., 1991; Wootton and Miller, 1994; Plessinger and Woods, 1998; Fajemirokun‐ Odudeyi and Lindow, 2004). Although, one of the metabolites of cocaine, benzoylecgonine, does not cross the placenta very readily, the placenta is still a direct target for cocaine. Therefore, the placental transfer systems are adversely affected for essential nutrients as well. Placental perfusion can be compromised by as much as 50% in mothers who ingest cocaine (Woods et al., 1987). Decreased 14 fetal oxygen levels have also been noted with maternal cocaine exposure (Chao, 1996). In animal studies, a decreased level of oxygenation was not seen with direct administration of cocaine to the fetus (Chao, 1996). Therefore, this problem suggests decreased oxygenation results from poor placental perfusion. It has recently been shown that cocaine is pharmacologically active in the nonhuman primate fetal brain (Benveniste et al., 2010). This indicates that prenatal cocaine exposure may not just have harmful effects on a fetus due to placental circulation but also due to cocaine directly acting on the developing brain. Therefore, deleterious effects of cocaine on brain development can occur either directly, by influencing both structural and functional aspects of fetal brain development, or indirectly, through hypoxic states which can alter neural development. Direct teratologic mechanisms can affect the neurogenesis of neural systems, including alterations on monoaminergic system development and neural growth factors, destruction of ion channel and monoamine systems, and possible alteration of gene expression (Mayes, 2002). Recent animal and human imaging data have begun to reveal even more specific areas of insult attributable to prenatal cocaine exposure. Lee et al. (2008) have isolated a mechanism (down‐regulation of the cell cycle regulatory protein cyclin A) for the inhibition of neural progenitor cell proliferation observed in fetal rodents exposed to cocaine in utero. Physiologic changes to 15 human brains after fetal cocaine exposure have been identified as reduced cerebral blood flow (Rao et al., 2007), reduced corpus callosum (Singer et al., 2006), and smaller caudate (Avants et al., 2007) in cocaine‐exposed individuals compared to controls. Monamines have been the focus of several studies as they have a trophic influence on developing brain cells. Blockade of monoamines may also be a mechanism by which prenatal cocaine perturbs the brain architecture (Cabrera‐ Vera et al., 2000). Several studies have examined areas of the brain that are monoamine‐rich in prenatally cocaine exposed animals and have found evidence of alterations in brain cell development, including the maturation and progression of brain development beyond the fetal period (Ren et al., 2004; Morrow et al., 2005; Lee et al., 2008). Therefore, monoamine circuitry that influences risk‐taking behavior and regulates substance use, emotional and behavioral reactivity to stress, attention, and executive functioning is of particular relevance to the study of prenatally cocaine‐exposed adolescence and young adults. In Chapter II, the long‐term effects of prenatal cocaine exposure on the dopamine receptor system were examined. PHYSIOLOGICAL EFFECTS OF PRENATAL COCAINE EXPOSURE IN INFANTS Human research involving very large sample sizes, multivariate statistical analyses, and control for important confounds has found consistent patterns of deficit among newborns and infants exposed to cocaine. Intrauterine growth 16 retardation, prematurity, low birth weight, and deficits in birth length and head circumference for gestational age have been consistently related to fetal cocaine exposure in infants despite differing methodologies (Singer et al., 1994, 2002; Chiriboga et al., 1999; Richardson et al., 1999; Bateman and Chiriboga, 2000; Kuhn et al., 2000; Bandstra et al., 2001; Bada et al., 2002, 2005; Behnke et al., 2006). Singer and colleagues (2002) compared 218 infants who were prenatally exposed to cocaine to 197 who were not and reported that cocaine use during pregnancy was associated with an increase in premature labor and delivery, lower birth weight, smaller head circumference, a decrease length at birth, and shorter gestational age. Similarly, Bandstra et al. (2001) noted cocaine‐ associated deficits in birth weight and length but not head circumference. Prematurity was not found by Bandstra et al. (2001), as the infants studied were full‐term. Both Singer et al. (2002) and Bandstra et al. (2001) included a target population of primarily African‐American pregnant women of low‐ socioeconomic status with cocaine use, identified confounding variables including poly‐drug use (i.e., alcohol, marijuana, tobacco, etc.), and controlled for these variables in comparison groups. In a study examining 295 infants prenatally cocaine‐exposed whose mother received no prenatal care as compared to 98 infants whose mother did receive prenatal care by the fifth month of pregnancy, Richardson et al. (1998) noted that during early pregnancy infants from both groups exhibited lower gestational age, birth weight, length, and head circumference. The authors concluded prenatal cocaine exposure was associated with restricted 17 intrauterine growth regardless of prenatal care. Confounding factors (alcohol, tobacco, marijuana, other illicit drugs, maternal and infant variables) were controlled and at the end of each trimester both groups were questioned about their use of cocaine/crack, alcohol, marijuana, tobacco and other drug use. Bateman and Chiriboga (2000) investigated the relationship between birth weight and head circumference with the quantity of cocaine exposure in 240 non‐randomized full‐term infants from a single inner‐city hospital. Levels of cocaine exposure were measured using radioimmuno analyses of hair in the third trimester of pregnancy. Measurements indicated no exposure (136 infants), low exposure (52 infants), and high exposure (52 infants). Adjustments were made for the confounding variables of use of alcohol, tobacco, marijuana, and opiates during pregnancy as well as infant and maternal characteristics. A dose‐response effect of cocaine on the neonates' head circumference was only found in the high‐exposure group. Chiriboga et al. (1999) and Kuhn et al. (2000) reported similar findings. Chirboga et al. (1999) also found a decrease in birth lengths among infants prenatally exposed to cocaine at high levels of exposure. In summary, research indicates prenatal exposure to cocaine may affect the infant's gestational age at birth, length, weight, and head circumference. As described in more detail later in this chapter, the prenatally cocaine exposed monkeys used in this dissertation showed decreased infant body weight, body length, and crown circumference at birth compared to controls (Morris et al., 1997). 18 However, there were no differences in postnatal growth of the offspring over the first 18 months between the two groups (Morris et al. 1996). The mean weight of the two groups has not been different since 12 months of age. NEUROBEHAVIORAL AND NEUROLOGICAL OUTCOMES In infants and preschoolers, a pattern of neurocognitive processes (Azuma et al., 1993; Mayes et al., 1993; Jacobson et al., 1996; Lester et al., 1998; Singer et al., 2000) has been shown to be negatively affected by fetal cocaine exposure in the domains of attention (Singer et al., 2000; Gaultney et al, 2005), arousal modulation (Morrow et al., 2001; Mayes, 2002), cognitive and language processing (Alessandri et al., 1993; 1998; Singer et al., 2001; Morrow et al., 2003; Lewis et al., 2004), and tone and motor function (Arendt et al., 1999). However, some of these neurobehavioral reports are conflicting. In an early study, Mayes et al. (1993) administered the Brazelton Neonatal Behavioral Assessment Scale (NBAS) to 56 newborns (24‐48 hours old) prenatally exposed to cocaine and 20 matched newborns who were not exposed. The authors found the infants prenatally cocaine‐exposed only demonstrated a lower score on the habituation cluster of the NBAS, which indicates that the infants required more stimulus exposures before they demonstrated a decreased response to that stimulation. A 1995 study examined the association between 61 3‐month‐old infants prenatally exposed to cocaine and 47 non‐exposed infants (Mayes et al., 1995). Controlling for poly‐drug use, the authors documented no 19 difference in informational processing by habituation and response to novelty related to prenatal cocaine exposure assessed by a visual habituation and novelty responsiveness procedure. A retrospective study found 464 infants heavily exposed to cocaine prenatally to have poorer scores on the novelty preference test at 6.5 and 12 months and faster reactivity on the visual expectancy paradigm at 6.5 months (Jacobson et al., 1996). While examining neonates prenatally cocaine‐exposed, Morrow et al. (2001) and Delaney‐Black et al. (1996) found, after controlling for covariates, significant differences in all NBAS cluster scores. However, Morrow et al. (2001) found no difference in abnormal reflexes and Delaney‐Black et al. (1996) reported no difference in habituation in the neonates prenatally cocaine‐exposed. Delaney‐Black et al. (1996) also found infants with higher meconium concentrations of cocaine metabolites had poorer motor skills and regulation of state. Tronick et al. (1996) examined 251 full‐term infants prenatally cocaine‐exposed and found poorer regulation arousal (state regulation and excitability) among only the infants "heavily" cocaine‐exposed at 3 weeks, but not at 3 days of age on the NBAS while controlling for covariates. Infants heavily cocaine‐exposed were also found by Singer et al. (2000) to be four times as likely to be "jittery" and have more movement and tone abnormalities and sensory asymmetries than the lightly or non‐exposed neonates. The authors statistically controlled for other drug exposures. 20 King et al. (1995) found neurobehavioral abnormalities (increased jitteriness, hyperactive responses, and excessive sucking) on day 1 and 2 of life in neonates exposed to cocaine in utero, even while controlling for confounding variables. While King et al. (1995) identified these results as consistent with withdrawal, a report from the American Academy of Pediatrics (1998) suggested that these signs of neurobehavioral and neurological alterations were a result of the toxic effects of cocaine itself rather than evidence of withdrawal. Overall, it does appear that prenatally cocaine‐exposed infants have deficits in arousal and novelty responsiveness. In this dissertation, several models of impulse control, including reaction to novelty, were examined in adult monkeys prenatally cocaine exposed to cocaine and controls (Chapter III). COGNITION The results of studies to determine an association between a cognitive delay and prenatal cocaine exposure are conflicting. Several studies documented no statistically significant differences on cognitive development assessed by the Bayley Scale of Infant Development's (BSID) Mental Development Index (MDI) among infants/young children exposed to cocaine prenatally when tested at 6‐24 months (Jacobson et al., 1996; Kilbride et al., 2000; Frank et al., 2002; Messinger et al., 2004). 21 Similarly, Koren et al. (1998) investigated the intelligence quotient (IQ) of 2 1/2‐year‐old children prenatally cocaine‐exposed (n = 23) and adopted at birth into middle to upper class families to a control group (n = 23). The authors claim that their design provided exclusion of postnatal environment influences on the cognitive functioning of infants prenatally exposed to cocaine. After administering the Bayley and McCarthy IQ tests, they found no differences in global IQ between the two groups; however, they reported a trend towards lower IQ of the children in the cocaine‐ exposed group. In contrast, Singer and colleagues (2002) reported infants prenatally exposed to cocaine were twice as likely to have significant cognitive delays throughout their first 2 years of life than non‐exposed infants. Alessandri et al. (1998) and Lewis et al. (2004) reported lower MDI scores among infants/young children heavily prenatally cocaine‐exposed. Alessandri and colleagues (1998) examined the cognitive functioning of infants prenatally cocaine‐exposed (heavily exposed [n = 30) and lightly exposed [n = 30] to a matched control group [n = 169] at 8 and 18 months of age) using the BSID’s MDI. They documented a decrease in MDI scores only at 18 months of age among all infants prenatally cocaine exposed, but the infants heavily exposed had the poorest scores. Lewis and associates (2004) examined the effects of prenatal cocaine exposure and cognitive development at 12, 18, 24, and 36 months of 147 young children who were exposed and 89 who were not. After controlling for confounding factors such as socioeconomic status and maternal educational 22 level and using the BSID’s MDI, the authors found the young children with higher meconium concentrations of cocaine metabolites had lower test scores with a decline at 18 months on mental development. With regard to older children, prenatal cocaine exposure appeared to result in more specific rather than general cognitive effects. For example, seven year old prenatally cocaine exposed children were determined to be 2.8 times more likely to experience learning disabilities than control subjects (Morrow et al., 2006). Singer and colleagues found that prenatally cocaine exposed children at ages 4 and 9 years old performed more poorly on tasks requiring visual perceptual reasoning than non‐cocaine exposed children despite no Full Scale IQ differences between groups (Singer et al., 2004; Singer et al., 2008). Additionally, decreased visual motor skills (Arendt et al., 2004), visual spatial working memory (Schroder et al., 2004; Mayes et al., 2007), and deficits in visual spatial integration (Singer et al., 2004) have been observed in prenatally cocaine‐ exposed children. While not examined in this dissertation, both groups of monkeys will be studied in cognition tasks in the coming years. ATTENTION/EXECUTIVE FUNCTION Attention and executive function domains have been extensively investigated in prenatally cocaine exposed children and a pattern of difficulties with sustained and selective attention (Bandstra et al., 2001; Savage et al., 2005; Linares et al., 2006) have been revealed. In studies of selective attention, children exposed to cocaine prenatally made more commission errors (Noland 23 et al., 2005) and omission errors (Accornero et al., 2007) compared to controls. The Stroop Task has been used to investigate executive function in these children as well. Prenatally cocaine exposed children generated longer responses to words in this task than non‐exposed children, suggesting that early cocaine exposure may inhibit the specialization and efficiency of frontal functioning (Mayes et al., 2005). At the age of four years old, increased rates of commission errors, which indicate greater impulsivity, were found using an adapted version of the Connors’ Continuous Performance Test (Minnes et al., 2010). In the same cohort at 6 years old, the prenatally cocaine exposed children were reported to demonstrate more symptoms of inattention than a control group (Linares et al., 2006). ANTISOCIAL/AGGRESSIVE BEHAVIOR Behavioral problems have been reported in prenatally cocaine‐exposed children in several large well‐controlled studies. Studies using a variety of measures including self report (Linares et al., 2006), caregiver report (Sood et al., 2005; Accornero et al., 2006; Warner et al., 2006; Bada et al., 2007; Minnes et al., 2008), teacher report (Delaney‐Black et al., 2000; Nordstrom Bailey et al., 2005), or a combination (Bendersky et al., 2006), have revealed a pattern of behavior disturbances involving inhibitory control, externalizing, aggressive and delinquent behavior problems with specific gender effects revealed in some studies (Delaney‐Black et al., 2000; 2004; Nordstrom Bailey et al., 2005; Sood et al., 2005; Bendersky et al., 2006; Minnes et al., 2008). For example, with regard 24 to impulsivity, Bendersky and Lewis (1998) found that 2 year‐old prenatally cocaine‐exposed children had less impulse control compared to those exposed to other substances but not cocaine in utero. Children were seated at a table upon which an experimenter had placed a cookie and were told not to take the cookie until the experimenter returned. The experimenter gave the child a novel toy, left the room, and retuned after two minutes. It was found the prenatally cocaine exposed children had shorter latencies to reach for, to take, and to eat the cookie compared to controls (Bendersky and Lewis, 1998), indicating that gestation cocaine exposure alters impulse control. However, there have been a limited number of studies that found either no differences (Accornero et al., 2006) or attributed the negative behavioral findings to poor environments, maternal psychopathology, gestational weaknesses, and/or environmental lead exposure rather than the direct effects of cocaine (Accornero et al., 2002; Warner et al., 2006). Early research focusing on caregiver reports indicated higher rates of behavioral problems in children prenatally exposed to cocaine compared to non‐ cocaine‐exposed children (Richardson, 1998; Chasnoff et al., 1998). Several studies have recently found an increase in hyperactivity and externalizing behaviors in prenatally cocaine‐exposed children (Linares et al., 2006; Bendersky et al., 2006). These behavioral issues appear to be exaggerated in male children exposed to cocaine in utero, with prenatally cocaine‐exposed boys often demonstrating more aggression, hyperactivity, and disruptive behavior than non‐exposed boys, whereas girls do not show these differences (Bendersky 25 et al., 2006; Bennett et al., 2007; Delaney‐Black et al., 2004). However, a recent study indicates the increased odds of delinquent behavior associated with prenatal cocaine exposure to be found only in girls (Minnes et al. 2010). This discordance with earlier studies has been attributed to a trend towards greater ease of reporting antisocial and aggressive behavior among females, especially among those known to be at some biologic risk such as prenatal cocaine exposure. In summary, important patterns of differences have emerged between prenatally cocaine‐exposed children and controls in human cohort studies. Developmental differences have been well documented and include deficits from prenatal cocaine exposure on visual recognition and memory (Singer et al., 1999; 2005), attention (Singer et al., 2000; Noland et al., 2005; Linares et al., 2006), birth outcomes (Singer et al., 2002), early cognitive and language development (Singer et al., 2001; 2002), perceptual reasoning (Singer et al., 2004; 2008), and aggression/impulse control (Linares et al., 2006). The differences that have been observed between prenatally cocaine exposed children and non‐exposed children have meaningful implications. While the developmental outcomes in which differences have been found in the human cohort studies have small to moderate effect sizes (ranging from 0.2‐0.5), these differences continue to be observed as a consistent pattern of deficits attributed specifically to prenatal cocaine exposure. Additionally, it has been reported that even small differences and subtle effects have been shown to result in a substantial number of cocaine‐ 26 exposed children requiring special education or therapeutic intervention (Lester et al., 1998). To date, there are no studies that have documented the persistence of early cognitive impairment and behavioral problems due to prenatal cocaine exposure through the adolescent and young adult period. Yet significant disruption in prenatal neuronal differentiation and in the development of neuronal circuitry and anatomy (Dow‐Edwards et al., 2006; Singer et al., 2006) can have permanent effects on long‐term outcomes. Additionally, some long‐ term outcomes, like substance abuse and mental illness, may not emerge until later in life. It is hypothesized that behaviors such as impulsivity, sensation seeking, and cognitive impairments in decision‐making may manifest in adolescence and young adulthood in such activities as drug abuse, risky sexual behaviors, criminality, teen pregnancy, and school drop‐out. For example, some cognitive effects of prenatal cocaine exposure may not appear until these children’s cognitive abilities along certain dimensions are challenged as they advance in their schooling. If pervasive compensatory adjustments occur for the impact of prenatal cocaine exposure, there may be substantial recovery in any compromised brain systems allowing for retention or return of function. However, a long‐term cost of such compensatory neural reorganization may be a decrease in adaptability (Hughes and Sparber, 1978; Spear, 1996). Some behavioral and physiological functions may appear normal under basal testing conditions and deficits may only become unmasked when these rectified neural systems are taxed by stressors or other challenges. For example, self‐regulating 27 problems observed in infants and children prenatally cocaine exposed may be early markers for the development of mental health concerns, not apparent until onset of drug use or stressful transitions associated with adolescent development. Central to the problem of risk‐taking behavior among prenatally cocaine‐ exposed adolescents and young adults is whether it is related to predisposition toward drug seeking and addiction in this group. Research efforts that identify early risk and protective factors for substance dependence among prenatally cocaine‐exposed children can ultimately prevent human suffering and reduce public health expenditures. It can also be argued that the patterns of differences found, rather than the number of differences found, have important implications for disruptions in adult behavior. For example, attention and externalizing behavior problems have consistently been found among prenatally cocaine‐ exposed children. This pattern of behavior has been associated with the development of substance use disorders and conduct problems. Research efforts focusing on examining group differences in substance use risk and protective factors among prenatally cocaine exposed adolescence and young adults help provide a foundation for prevention and intervention of drug abuse. Currently, the human cohort studies have only followed the prenatally cocaine‐exposed children and non‐exposed controls through age 13 years. It is not yet known whether prenatal cocaine exposure is associated with increased risk of specific types of substance dependence, mental health problems, or other high‐risk behaviors. Additionally, researchers with humans, unlike those using animal 28 models, struggle to refine their methods to ascertain accurately whether cocaine exposure occurred as well as to determine gestational timing of exposure, the acute and cumulative doses to which the fetus was exposed to, and to control other potentially confounding variables. Therefore, the studies in this dissertation investigating the longterm effects of prenatal cocaine exposure on risk factors for drug abuse in adult monkeys offer an important complement to the human studies. PRENATAL COCAINE EXPOSURE: ANIMAL STUDIES Paralleling the human longitudinal studies, the animal literature indicates that prenatal cocaine exposure has persistent, specific, negative effects on brain anatomy, organization, and neurotransmitter function. Learning, memory, attention, emotional reactivity to stress, and vulnerability to substance abuse have been investigated in prenatally cocaine‐exposed animals. Different animal models, designed to mimic human drug use during gestation, confirm that prenatal cocaine exposure results in specific and long‐lasting behavioral, cellular, and molecular changes (Mayes, 2002; Lidow, 2003; Harvey, 2004; Stanwood and Levitt, 2004). However, the extent and nature of the cellular alterations vary across model systems. Deficits range from alterations in basic processes of neocortical development that result in altered cell production, migration, and genetic regulation (Lidow, 1995a, 1995b; Lidow and Song, 2001a, 2001b; Crandall et al., 2004; Ren et al., 2004; Guerriero et al., 2005; Lee et al., 2008; Novikova et al., 2008), to more subtle changes in cellular morphology, and molecular signaling cascades within DA‐rich regions of the cerebral cortex 29 (Jones et al., 1996; 2000; Stanwood et al., 2001; Stanwood and Levitt, 2003; 2007). Molecular analyses have determined that the DA D1 receptor exhibits permanent reduced coupling to its G‐protein following prenatal cocaine exposure (Wang et al., 1995; Friedman et al., 1996; Jones et al., 2000). This reduction in coupling is a result of DA D1 receptor remaining internalized and not trafficking properly to the cell membrane (Stanwood and Levitt, 2007). Adult rabbits exposed to cocaine prenatally also exhibit greatly reduced psychostimulant‐induced stereotypies, consistent with diminished D1 receptor signaling (Simansky and Kachelries, 1996; Stanwood and Levitt, 2003). It is important to emphasize that other receptor signaling does not appear to be altered, nor is D2 coupling altered in the DA‐rich brain regions (Wang et al., 1995; Friedman et al., 1996). This selective reduced coupling of the D1 receptor has been implicated in the cellular, morphological, and behavioral changes observed following prenatal cocaine exposure in this teratologic model. Additional evidence to support a role for altered D1 receptor signaling at the cellular level comes from studies of the D1 receptor knockout mouse, which exhibits similar cellular and morphological changes to the prenatal cocaine exposed rabbits (Stanwood et al. 2005). In contrast to the relatively few cellular effects detected, consistent behavioral changes including deficits in attention tasks, emotional reactivity, and the reinforcing effects of drugs of abuse that correspond with the human clinical literature are observed in a variety of animal models of prenatal cocaine 30 exposure (Morrow et al., 2002; Rocha et al., 2002; Gabriel et al., 2003; Stanwood and Levitt, 2003; Thompson et al., 2005; Malanga et al., 2008). Prenatally cocaine exposed rodents were found to have long‐term alterations in working memory, spatial memory (Inman‐Wood et al., 2000), non‐spatial short‐term memory (Morrow et al., 2002), and learning impairments in a water‐maze test (Bashkatova et al., 2005). Additional evidence for enduring effects of prenatal cocaine exposure in rats on visual attention (Gendle et al., 2004) and sustained attention (Gendle et al., 2003) can be found in the animal literature. In response to environmental or social stress, prenatally cocaine‐exposed rats have shown suppressed levels of play (Wood et al., 1994), less behavioral adaptation (Campbell et al., 2000), and more aggressive behavior (Wood and Spear, 1998). Increased aggression (Johns et al., 1994; McMurray et al., 2008) and anxiety with decreased socialization (Overstreet et al., 2000) as well as alterations in regulatory and coping behavior with elevated responsivity to acute and chronic stress (Wood et al., 1994; 1995) has been observed in prenatally cocaine‐ exposed rodents. Prenatally cocaine‐exposed rodents also show an altered propensity to become involved in self‐administration of drugs or respond differently to cocaine than rodents not exposed to cocaine (Heyser et al., 1992a, 1992b; Keller et al., 1994, 1996; Hecht et al., 1998; Rocha et al., 2002; Crozatier et al., 2003; Guerriero et al., 2005; Malanga et al., 2009), raising questions regarding increased sensitivity to drugs and increased substance abuse risk for prenatally cocaine‐exposed humans. Kosofsky and colleagues have documented that 31 prenatally cocaine‐exposed mice do not habituate to novelty and in response to repeated cocaine injections, show blunted locomotor sensitization, increased stereotypic behavior, and increased DA release in the nucleus accumbens (Crozatier et al., 2003; Guerriero et al., 2005; Malanga et al., 2009). This suggests that prenatal cocaine exposure alters the adaptation of brain reward systems to chronic psychostimulant exposure in adulthood. Other groups have examined in rodents the reinforcing effects of cocaine in adulthood following prenatal cocaine exposure. Heyser et al. (1992a, 1992b) found that prenatally cocaine‐exposed rats did not acquire cocaine conditioned place preference suggesting a reduction in cocaine reward. In contrast, Keller et al. (1994, 1996) determined that prenatally cocaine‐exposed rats had higher basal DA levels and had significantly higher rates of responding compared to controls for a low dose of cocaine made available under a FR 1 schedule of reinforcement. The dose of cocaine was actually so low that it did not appear to function as a reinforcer in control animals (Keller et al., 1996). In an important control to this study, no differences were observed in acquisition to acquire water‐reinforced responding under the FR 1 schedule of reinforcement. This finding suggests that prenatally cocaine‐exposed rats are more sensitive to reinforcing effects of cocaine but not to other reinforcers. Hecht et al. (1998) extended the work of the previous groups to measure the reinforcing strength of cocaine using a progressive‐ratio (PR) schedule of reinforcement. Prenatally cocaine‐exposed rats had significantly lower break points than controls suggesting that these animals were less sensitive to the 32 reinforcing strength of cocaine. However, Rocha et al. (2002) reported conflicting data in mice. Different doses of cocaine (0.25‐2.0 mg/kg/injection) were made available under an FR 1 schedule of reinforcement to examine vulnerability to self‐administer cocaine. While there were no differences between prenatally cocaine‐exposed and control mice in acquisition of food‐ reinforced responding or to cocaine self‐adminstration, a greater number of the prenatally cocaine‐exposed mice reached the criteria for acquisition at all the doses tested (Rocha et al., 2002). This suggests that prenatally cocaine exposed subjects may be more vulnerable to cocaine reinforcement. NONHUMAN PRIMATE MODELS This dissertation work utilizes laboratory animals and behavioral pharmacology methodology to study the long‐term effects of prenatal cocaine exposure and vulnerability to self‐administration of cocaine. Human studies involving prenatal cocaine exposure have resulted in inconsistent findings that can be attributed to difficulties in controlling for potentially confounding variables, such as level of pre‐ and post‐natal care, inadequate nutrition during pregnancy, multiple drug use during pregnancy, and drug dosage (Karmel and Gardner, 1996; Richardson et al., 1996; Gingras and O'Donnell, 1998; Dow‐ Edwards et al., 1999; Mayes et al., 2003; Singer et al., 2004); these variables can be controlled in animal studies. Women who use cocaine while pregnant use greater amounts of other drugs (Singer et al., 2000; 2001), indicating that simultaneous control for other 33 prenatal drug exposures is important when evaluating the direct effects of cocaine on developmental outcomes later in life. For example, attentional measures, which have been examined in prenatal cocaine studies, have been shown to be vulnerable to the effects of prenatal alcohol (Fried et al., 1992), marijuana (Leech et al., 1999), and tobacco (Fried et al., 1992). In addition, prenatal tobacco exposure has been associated with conduct disorder in boys (Wakschlag and Hans, 2002) and externalizing behaviors, anxiety, and depression (Cornelius et al., 2001). Research on fetal alcohol exposure has also shown a range of cognitive and behavioral effects with exposed children having more internalizing and externalizing behavior problems (Bailey et al., 2004; Sood et al., 2001) and psychiatric disorders (O’Connor et al., 2002). Further obfuscating the direct effects of prenatal cocaine exposure, caregivers who use drugs may expose their children to chaotic rearing environments and/or passive drug exposure through secondhand tobacco, marijuana, or cocaine smoke. The behavioral outcome evaluations of children exposed to cocaine in utero have generated equivocal results regarding the domains affected, which highlights the need for longitudinal studies that control for confounding environmental and biologic factors such as prenatal exposure to other drugs and alcohol. Therefore, investigating the neurobiological and behavioral consequences of prenatal cocaine exposure in highly controlled animal models is an important complement to the human studies. Nonhuman primates (NHP), especially rhesus macaques (Macaca mulatta), offer numerous advantages compared to other animals for the study of prenatal cocaine exposure. For instance, NHP are very 34 similar to humans in physiology and brain organization (Goldman‐Rakic and Brown, 1982; Schneider and Suomi, 1992; Silk et al., 1993). NHP have approximately 95% gene homology to humans (Hacia et al., 1998) and greater homology in DA, 5‐HT, and NE systems than rodents (Weerts et al., 2007). Additionally, NHP have similar in utero development as humans over a long gestation period (24‐26 weeks) (Silk et al., 1993), making them especially valuable for prenatal cocaine exposure studies. Another line of evidence that NHP may be preferential compared to rodents for prenatal cocaine exposure studies is that the behavioral and neurochemical response to psychostimulants may be different between the species. There is evidence suggesting species differences between NHP and rodents in the metabolic effects of cocaine (Lyons et al., 1996), the behavioral effects of psychostimulants (Roberts et al., 1999; Lile et al., 2003), and the DA receptor distribution (Richfield et al., 1987; Camps et al., 1990). Finally, NHP can be utilized for longitudinal studies due to their relatively long lifespan compared to rodents so the long‐term effects of prenatal cocaine exposure can be fully examined. Therefore, the major strengths of the studies in this dissertation are that NHP models are more analogous to the human condition than any other animal model, that NHP allow for the study of long‐term effects in adults (beginning 13 years after gestational cocaine exposure), that NHP have similar neurochemical, hormonal, and neuroanatomical functions, and that NHP allow for the study of multiple behaviors, making these extremely translational studies. 35 PRENATAL COCAINE EXPOSURE: NONHUMAN PRIMATES There have been four research groups that have examined the effects of prenatal cocaine exposure in NHP. The majority of the work with prenatally cocaine‐exposed NHP has concentrated exclusively on the physiological consequences. Ronnekleiv and colleagues mainly focused on fetal brain development during in utero cocaine exposure. Briefly, 3.0 mg/kg cocaine was administered i.m. to the mothers four times daily while control subjects received saline injections four times daily. The treatment regimen began on day 18 of pregnancy and continued until researchers removed the fetus at day 60. Ronnekleiv and Naylor (1995) reported that TH messenger ribonucleic acid (mRNA) content, as measured by quantitative in situ hybridization, was reduced in the substantia nigra and ventral tegmental areas after 60 days of prenatal cocaine exposure, which suggests reduced DA synthesis. Also, increases in DA D1, D2, and D5 receptor subtype mRNA levels in the frontal cortex and striatal areas were observed (Choi and Ronnekleiv, 1996). In vitro receptor autoradiography later revealed signficant increases in D1‐ and D2‐like receptor densities in the striatum and substantia nigra (Fang et al., 1997) and significant increases in DA transporter mRNA and densities (Fang and Ronnekliev, 1999). While Ronnekleiv and colleagues have not reported any functional consequences of this prenatal cocaine exposure regimen, they have noted that these alterations in DA neurocircuitry could affect motivation and reward (Fang et al., 1997). A second research group has examined prenatal cocaine exposure in NHP using a different treatment regimen. Lidow and colleagues treated pregnant 36 monkeys with 10 mg/kg cocaine p.o. twice a day from E40‐E102 and allowed them to deliver the offspring at full‐term. No differences in weight of offspring, signs of atrophy, or hemorrhages were seen between the prenatally cocaine exposed infants and saline‐treated controls (Lidow, 1995b). However, the volume and weight of the brains of the cocaine‐treated infants were about 20% lower compared to the brains of the saline‐treated infants (Lidow, 1995b). Other studies from this group have described higher incidence of cell death in the developing cerebrum of cocaine‐exposed infants (He et al., 1999) and abnormal neurocortical cytoarchitecture (Lidow and Song, 2001a, 2001b). Both the Lidow and Ronnekleiv research groups have shown that prenatal cocaine exposure has detrimental effects on the developing brain in NHP. A third group that has examined the effects of cocaine exposure during gestation used osmotic minipumps to infuse cocaine (0.3 mg/kg/hr) or saline continuously in pregnant rhesus monkeys (Howell et al., 2001). Compared to a pair‐fed control group, signficantly lower survival rates were found for the cocaine‐exposed fetuses (Howell et al., 2001), which is consistent with the documentation of increased stillbirths, spontaneous abortions, and in utero death from human studies (Chasnoff et al., 1985; Bingol et al., 1987; Lutiger et al., 1991). Of the monkeys that survived to full‐term, there were no physiological differences observed between prenatally cocaine exposed and control subjects, including body weight, body length, and heart rate (Howell et al., 2001). None of these studies in NHP have investigated the long‐term consequences of the gestational cocaine exposure. The monkeys used in the 37 experiments included in this dissertation are the first prenatally cocaine exposed NHP to be examined for biological or behavioral effects beyond infancy. The twenty monkeys used in these dissertation studies have been part of research by Paule and colleagues since inception (for descriptions see Morris et al., 1996, 1997). Female rhesus monkeys were administered 1.0 mg/kg cocaine i.m. three times a day, five days a week. This dosing began prior to mating. The dose of cocaine was increased weekly so that by the end of gestation, the pregnant monkeys were receiving between 4.5‐8.5 mg/kg/injection cocaine three times per day. The mean cumulative intake of monkeys in this group was 1131.5 mg/kg (Table 1). The dose and frequency of cocaine over the pregnancy was carefully controlled and rigorous controls for factors such as nutritional status of the mothers, stress from drug injections, and postnatal rearing were in place increasing the likelihood that prenatal cocaine was the primary independent variable influencing long‐term behavioral assessments. Compared to controls, no differences were observed in the maternal characteristics of body weight, food intake, and length of pregnancy. However, decreased infant body weight, body length, and crown circumference were noted in the prenatally cocaine exposed monkeys at birth compared to controls (Morris et al., 1997). No differences were observed between groups though with respect to postnatal growth of the offspring over the first 18 months (Morris et al., 1996) and the mean weight of the two groups has not been significantly different since 12 months of age. 38 Morris and colleagues investigated the behavioral consequences of prenatal cocaine exposure using an Operant Test Battery (OTB), which has been described in detail (Morris et al., 1996). The OTB consists of five components: 1) motivation as assessed by PR responding maintained by banana‐flavored food pellets; 2) color and position discrimination; 3) short‐term memory using a delayed matching‐to‐sample task; 4) timing behavior which was assessed with a temporal response differentiation task; and 5) learning using a repeated acquisition task (Paule et al, 2000). No differences in the acquisition of any of the five behavioral components were observed between the prenatally cocaine exposed monkeys and controls (Morris et al., 1996). In fact, the only behavioral difference Paule and colleagues ever documented between the two groups was that the prenatally cocaine exposed monkeys perseverated on the simple visual discrimination task longer than control monkeys when the stimuli on this task were reversed after six years of performing the task (Chelonis et al., 2003). This deficit in task performance was still apparent 2.5 years after the rule reversal indicating that prenatal cocaine exposure may permanently impair the ability of the subjects to respond to environmental changes (Chelonis et al., 2003). The monkeys from Paule and colleagues’ laboratory came to Wake Forest University in 2006 around the age of 12 years old and began testing in the experiments described in this dissertation at this point. From the time they began testing, there were no differences in weight between the two groups (Table 1). 39 TABLE 1. SUBJECT DEMOGRAPHICS CONTROLS ID Sex 1553 F 1556 F 1558 F 1559 F 1561 F 1564 M 1566 M 1661 M 1662 M 1663 M Mean (± SEM) PRENATALLY COCAINE EXPOSED Weight (kg) In utero ID Sex Cocaine (mg/kg) 5.9 0.0 1554 F 6.3 0.0 1555 F 7.3 0.0 1557 F 6.0 0.0 1560 F 5.6 0.0 1563 M 7.6 0.0 1565 M 8.4 0.0 1567 M 9.0 0.0 1568 M 7.2 0.0 1569 M 8.2 0.0 1570 M 7.15 0.0 Mean (± 0.37) (± 0.00) (± SEM) Weight (kg) In utero Cocaine (mg/kg) 6.1 982.5 5.0 912.0 6.4 1248.0 6.0 1132.5 8.2 1084.5 8.7 1138.5 7.5 1404.0 8.1 885.0 7.3 1147.5 8.5 1380.0 7.18 1131.45 (± 0.39) (± 56.10) Approximately half of the subjects in each group (prenatally cocaine exposed and controls) are female monkeys. It is known that hormonal changes across the menstrual cycle may have a large effect on stimulant drugs, particularly cocaine. A consistent and greater mood‐altering effect of stimulant use during the follicular phase of the menstrual cycle has been observed (for review, see Terner and de Wit, 2006) and it is thought that progesterone level fluctuations may account, in part, for this menstrual phase difference (Evans and Foltin, 2006; Evans, 2007). Evans et al. (2002) found that cocaine effects on heart rate and ratings of “good drug effect” were increased more in the follicular phase than in the luteal phase, demonstrating that cocaine’s effects can vary as a function of menstrual cycle phase. Additionally, Czoty et al. (2009) found that D2 40 –like receptor availability varied across the menstrual cycle in macaques with D2‐like receptor availability in the striatum being lower in the follicular phase than in the luteal phase. Therefore, specific considerations were taken into account and the experiments in this dissertation were designed to examine the dopaminergic system (Chapter II) and acquisition of cocaine self‐administration (Chapter IV) in female subjects in the follicular phase of the menstrual cycle. IMPULSIVITY Animal studies and human magnetic resonance imaging (MRI) have documented specific regions (caudate nucleus, corpus callosum, prefrontal and posterior cortices) (Avants et al., 2007; Rao et al., 2007; Singer et al., 2006) and mechanisms (Buxhoeveden et al., 2006; Lee et al., 2008; Morrow et al., 2005; Ren et al., 2004) of brain damage related to fetal cocaine exposure. These data, combined with early patterns of cognitive deficits and behavioral problems in humans, indicate that prenatal cocaine exposure exerts specific teratologic effects on human development during early childhood years through its effect on areas of the brain related to higher order thinking, impulse control, and sensation or pleasure seeking. It has been hypothesized that some negative effects of cocaine, particularly those governed by monoamine rich areas of the brain such as emotional regulation and impulse control (Bandstra et al., 2007), will ultimately lead to higher rates of substance dependence. It is possible that the early deficits in 41 attention and executive function seen in the human cohort studies at ages 4, 6, and 9 years old may be associated with disturbances in inhibitory control in adolescence and adulthood. Reduced impulse control could indicate that prenatal exposure to cocaine may predispose adolescents to greater drug use experimentation and a trajectory toward substance dependence. High impulsivity has been strongly associated with drug addiction (Jentsch and Taylor, 1999; Bickel and Marsch, 2001; de Wit and Richards, 2004). However, the cause and effect relationships between impulsivity and substance abuse have been challenging to determine. Prospective studies in both humans and animals indicate that preexisting impulsive traits may predispose individuals to drug use (Tarter et al., 2003; Perry et al., 2005, 2008; Dalley et al., 2007; Diergaarde et al., 2008; Marusich and Bardo, 2009). However, other studies in animals have found that chronic stimulant use may cause deficits in impulse control (Simon et al., 2007; Stanis et al., 2008; Dandy and Gatch, 2009). Some of the difficulty in evaluating impulsivity is due to it being conceptualized as a broad spectrum of behaviors rather than a single trait. Due to the multidimensional aspect of impulsivity, it has been difficult to classify in the scientific literature although a common definition is ‘a predisposition toward rapid, unplanned reactions to internal or external stimuli with diminished regard to the negative consequences of these reactions to the impulsive individual or to others’ (Moeller et al., 2001; Chamberlain and Sahakian, 2007). Although it is well accepted that 42 impulsivity is not a unitary construct but rather a spectrum of behaviors, there is not much agreement on how to measure impulsivity in animal models. In human studies, impulsivity is typically assessed by questionnaires. Several distinct measures have been developed to assess impulsivity in animals, including 1) choosing an immediate, low magnitude reward over a delayed, large magnitude reward, 2) lack of behavioral inhibition, and 3) exaggerated response to novelty. These measures are the focus of studies in Chapter III. Impulsive choice is most commonly assessed in human studies using a delay discounting task in which subjects are surveyed and asked to choose between a small, immediate reinforcer and a larger, delayed reinforcer. The subjective value of the larger reinforcer is decreased (e.g. discounted) as the length of time the subject must wait to receive it increases. By using a series of choices between varying delay values, the indifference point can be calculated as the delay value at which the smaller, immediate reinforcer is chosen as often as the larger, delayed reinforcer. Delay discounting has been adapted for animal studies by presenting delay discounting choices as differing reinforcement schedules. Animals are trained to make an operant response on one manipulandum to obtain a small magnitude reinforcer and to respond on another manipulandum to obtain a larger magnitude reinforcer after a set delay. It has been shown that drug users discount the value of both real and hypothetical delayed reinforcers moreso than nonusers (Madden et al., 1997; Bickel et al., 1999; Kirby et al., 43 1999; Coffey et al., 2003), which indicates a possible relationship between impulsive choice and drug abuse. Demonstrating the utility of delay discounting procedures in assessing the relationship between impulsive choice and drug abuse, Perry et al. (2005) used delay discounting measures in rats to show that impulsiveness was directly related to vulnerability to acquire cocaine self‐administration. Rats were divided into high and low impulsive choice groups based on their percentage of responses on the lever associated with the larger, delayed reinforcer. When cocaine acquisition was examined, the high impulsiveness rats acquired self‐administration more rapidly and at higher levels than the low impulsiveness rats. Delay discounting studies have been extended to nonhuman primates (Newman et al., 2008; Woolverton et al., 2007; Woolverton and Anderson, 2006; Anderson and Woolverton, 2003). Woolverton et al. (2007) demonstrated that rhesus monkeys self‐administering cocaine intravenously show similar discounting rates comparable to humans, suggesting they may be an ideal model to study the long‐term effects of drug abuse on impulsivity. The studies in Chapter III are the first to use NHP in food‐reinforced delay discounting studies. Response perseveration is the tendency to continue responding for a reinforcer despite the responses currently being either unrewarded or punished (McCleary, 1966). Response perseveration tasks first establish a dominant response set for an initial high rate of reward that subjects then 44 have to alter as the response set becomes unrewarded or punished more than it is rewarded (Matthys et al., 2004). It is thought that increased response perseveration is a measure of behavioral inhibition because in these tasks subjects must stop their ongoing behavior (Matthys et al., 1998). Increased behavioral inhibition, as assessed by response perseveration tasks, has been shown in studies with patients with externalizing disorders where impulsivity is often a symptom. In nonhuman primates, perseverative responding can be assessed in a variety of ways. One of the simplest measures is to examine continued responding during extinguishing of food‐ reinforced behavior on a simple FR schedule. A subject that takes many sessions to extinguish responding (unrewarded responding) would be considered more impulsive than a subject that takes only a few sessions to extinguish responding, as described in Chapter III. Differential behavioral response to novel stimuli has been attributed to impulsivity (Goldber, 1990; Zuckerman, 1996). In rodents, a strong correlation between novelty preference and impulsive reactivity with both self‐administration rates and rewarding efficacy of psychomotor stimulants has been observed (Hooks et al., 1991; Abreu‐Villaça et al., 2006; Davis et al., 2008). Measuring impulsivity by exposing subjects to a novel object has also been used successfully in nonhuman primate studies (Bolig et al., 1992; reviewed in Clark and Boinski, 1995; Coleman et al., 2005). Responsiveness in an open field is another method commonly used to assess impulsivity. Piazza and colleagues demonstrated that responsiveness in an open field was 45 associated with vulnerability to stimulant self‐administration (Piazza et al., 1989; 1990; reviewed in Piazza and Le Moal, 1998). It has been proposed that locomotor response to a novel field is closely related to behavioral disinhibition (Stoffel and Cunningham, 2008). Humans have also been shown to display differences in reactivity levels when exposed to a novel environment (Alessi et al., 1999). In Chapter III, the effect of prenatal cocaine exposure on multiple measures of impulsivity, including both unconditioned and conditioned behaviors, will be discussed. ACQUISITION OF COCAINE SELFADMINISTRATION Although a number of prospective, longitudinal studies are currently investigating the role of cocaine in contributing to adverse outcomes in prenatally exposed children, the impact of prenatal cocaine exposure regarding vulnerability for addiction has not been systematically examined. Children who were born during the crack cocaine epidemic of the mid‐1980s and early 1990s are now entering young adulthood, a time when experimentation with drugs of abuse typically occurs. Given the apparent links between prenatal cocaine exposure and long‐term behavioral and neural outcomes that are ostensibly involved in determining sensitivity to cocaine, it is reasonable to examine the possible relationship between cocaine exposure during gestation and the acquisition of cocaine self‐administration. It is impossible to ethically study acquisition of drug taking behavior in cocaine‐naïve humans. Therefore, animal 46 models are extremely valuable in allowing researchers to examine variables, like prenatal cocaine exposure, that may enhance or impede the initiation of drug‐ taking and predict vulnerability for drug abuse. Acquisition studies have primarily been conducted in rodents, due to the prohibitive expense of between‐subject designs using NHP. However, there have been a few studies examining drug history variables in acquisition of self‐administration of a second drug in monkeys (Pickens et al., 1973; Young and Woods, 1981; Carroll et al., 1984; Beardsley et al., 1990; Nader and Mach, 1996; Wojnicki and Glowa, 1996; Lile et al., 2000). For example, acquisition of the selective dopamine reuptake inhibitor GBR 12909 self‐ administration was studied in different groups of rhesus monkeys that were either experimentally naïve or had a history of cocaine self‐administration. It was determined that self‐administration was maintained under a multiple FR30 schedule with alternating components of either food or drug presentation only in the monkeys with previous cocaine self‐administration history (Wojnicki and Glowa, 1996). However, experimentally naïve monkeys failed to acquire GBR 12909 self‐adminstration at low doses (Wojnicki and Glowa, 1996). Similarly, Beardsley et al. (1990) demonstrated that MK‐801, the N‐methyl‐D‐aspartate (NMDA) receptor antagonist, was self‐administered by rhesus monkeys previously trained to self‐administer phencyclidine (PCP), a different NMDA receptor antagonist, but was not self‐ administered by rhesus monkeys previously trained to self‐administer cocaine. This finding supports the general conclusion that prior exposure to a 47 drug with similar pharmacological actions facilitates acquisition of self‐ adminstration in NHP. These studies also imply that in utero cocaine exposure, acting as a prior exposure, may increase vulnerability to self‐ administer cocaine later in life. In rodent studies, many types of variables have been identified as affecting rate of acquisition of drug self‐adminstration. Like in NHP, drug history is an important variable. Pretreatment with amphetamine (Piazza et al., 1989; 1990), cocaine (Horger et al., 1990; Childs et al., 2006), naltrexone (Carroll et al., 1986), and caffeine (Horger et al., 1991) can enhance acquisition of psychomotor stimulant self‐administration. Environmental conditions such as restricted access to food (DeVry et al., 1989) and a history of restricted feeding (Specker et al., 1994), physical stress (Shaham et al., 1992; Goeders and Guerin, 1994), or social stress (Haney et al., 1995) have all been shown to enhance acquisition of drug taking in rats. Additionally, characteristics of the animals have been noted as predictive of faster acquisition of stimulant self‐administration. For example, high rate of novelty‐induced locomotor activity (Piazza et al., 1989), preference of sweets (Gosnell et al., 1995), and increased impulsivity as assessed by a delay‐of‐ reward paradigm (Poulos et al., 1995, Perry et al., 2005) have all been determined to predict acquisition of self‐administration of stimulants. While acquisition studies typically hold the dose of the drug constant at low or moderate levels and manipulate other variables, another method of examining acquisition is to test multiple doses of the drug and use rate of 48 acquisition or probability of acquisition at various doses as the dependent measure (van Ree et al., 1978; Gerrits and van Ree, 1995; Carroll and Lac, 1997; Zhao and Becker, 2009). The focus of Chapter IV was to determine if prenatal cocaine exposure would facilitate rate of acquisition of self‐administration of cocaine in adulthood. Since it has been argued that the rate of acquisition of drug self‐administration may serve as a predictor of later drug‐taking behavior, possibly influencing the transition from drug use to addition (Rocha et al., 2002; 2005), the studies in Chapter IV were designed to test acquisition by examine multiple doses of cocaine and focusing on rate of acquisition. Importantly, two factors that are thought to predict vulnerability to self‐administration drugs of abuse, basal dopaminergic function (Chapter II) and impulsivity (Chapter III), were also examined. 49 REFERENCES Abreu‐Villaça Y, Quieroz‐Gomes FE, Dal Monte AP, Filgueiras CC, Manhães AC (2006) Individual differences in novelty‐seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res 167:175‐ 182. Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES (2007) Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr 28:195‐205. Accornero VH, Anthony JC, Morrow CE, Xue L, Bandstra ES (2006) Prenatal cocaine exposure: an examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiol Psychiatr Soc 15:20‐29. Accornero VH, Morrow CE, Johnson AL, Anthony JC (2002) Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol 27:259‐269. Acker D, Sachs BP, Tracey KJ, Wise WE (1983) Abruptio placentae associated with cocaine use. Am J Obstet Gynecol 146:220‐221. Alessandri SM, Sullivan MV, Imaizumi S, Lewis M (1993) Learning and emotional responsivity in cocaine‐exposed infants. Dev Psychol 29:989‐997. Alessandri SM, Bendersky M, Lewis M (1998) Cognitive functioning in 8‐ to 18‐month‐old drug‐exposed infants. Dev Psychol 24:565‐573. Alessi SM, Greenwald M, Johanson CE (2003) The prediction of individual differences in response to D‐amphetamine in healthy adults. Behav Pharmacol 14:19‐32. Ambre JJ, Ruo TI, Smith GL, Backes D, Smith CM (1982) Ecgonine methyl ester, a major metabolite of cocaine. J Anal Toxicol 6:26‐29. 50 American Academy of Pediatrics (AAP), Committee on Drugs (1998) Neonatal drug withdrawal. Pediatrics 101:1079‐1088. Anderson KG, Woolverton WL (2003) Effects of dose and infusion delay on cocaine self‐administration choice in rhesus monkeys. Psychopharmacology 167:424‐430. Araki KY, Sims JR, Bhide PG (2007) Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre‐ and postnatal development. Brain Res 1156:31‐45. Arendt RE, Angelopoulos J, Salvator A, Singer L (1999) Motor development of cocaine‐exposed children at age two years. Pediatrics 103:86‐92. Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, Carlson L, Min MO, Klein N, Flannery D (2004) Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr 25:83‐90. Asanbe CB, Lockert E (2006) Cognitive abilities of African American children with prenatal cocaine/polydrug exposure. J Health Care Poor Underserved 17:400‐412. Aubert I, Brana C, Pellevoisin C, Giros B, Caille I, Carles D, Vital C, Bloch B (1997) Molecular anatomy of the development of the human substantia nigra. J Comp Neurol 379:72‐87. Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC (2007) Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol 37:275‐279. Azuma SD, Chasnoff IJ (1993) Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three‐year data. Pediatrics 92:396‐402. Bada HS, Bauer CR, Shankaran S, Lester B, Wright LL, Das A, Poole K, Smeriglio VL, Finnegan LP, Maza PL (2002) Central and autonomic system signs with in utero drug exposure. Arch Dis Child Fetal Neonatal Ed 87:F106‐112. Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, Wright LL, LaGasse L, Higgins R (2005) Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol 25:631‐637. 51 Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, LaGasse L, Hammond J, Wright LL, Higgins R (2007) Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 119:348‐359. Bailey BN, Delaney‐Black V, Covington CY, Ager J, Janisse J, Hannigan JH, Sokol RJ (2004) Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. Am J Obstet Gynecol 191:1037‐1043. Bandstra ES, Accornero VH, Morrow CE, Xue L, Simpson GR, Glavach MK, Anthony JC (2007) Longitudinal study of visual attention in prenatally cocaine‐exposed children and adolescents. Pediatric Academic Societies’ Annual Meeting, Toronto, Canada. 6715.3 Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA (2001) Longitudinal investigation of task persistance and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol 23:545‐559. Bandstra ES, Morrow CE, Anthony CE, Churchill SS, Chitwood DC, Steele BW, Ofir AY, Xue L (2001) Intrauterine growth of full‐term infants: impact of prenatal cocaine exposure. Pediatrics 108:1309‐1319. Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, Xue L, Anthony JC (2002) Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol 24:297‐ 308. Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC (2004) Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent rowth curve analysis. Subst Use Misuse 29:25‐59. Bashkatova V, Meunier J, Maurice T, Vanin A (2005) Memory impairments and oxidative stress in the hippocampus of in‐utero cocaine‐exposed rats. Neuroreport 16:1217‐1221. Bateman DA, Chiriboga CA (2000) Dose‐response effect of cocaine on newborn head circumference. Pediatrics 106:E33. Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ (2000) Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J Neurosci 20:8902‐8908. Beardsley PM, Hayes BA, Balster RL (1990) The self‐administration of MK‐ 801 can depend on drug‐reinforcement history, and its discriminative 52 stimulus properties are phencyclidine‐like in rhesus monkeys. J Pharmacol Exp Ther 252:953‐959. Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K (2006) Outcome from a prospective longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol 31:41‐ 49. Bendersky M, Bennet DS, Lewis M (2006) Aggression at age five as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol 31:1‐14. Bendersky M, Lewis M (1998) Arousal modulation in cocaine‐exposed infants. Dev Psychol 34:555‐564. Bendersky M, Lewis M (1998) Prenatal cocaine exposure and impulse control at two years. NY Academy of Sci 846: 365‐367. Bennett D, Bendersky M, Lewis M (2007) Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr 28:467‐472. Benowitz NL (1993) Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol 72:3‐12. Benveniste H, Folwer JS, Rooney WD, Scharf BA et al (2010) Cocaine is pharmacologically active in the nonhuman primate fetal brain. Proc Natl Acad Sci 107:1582‐1587. Bergman J, Kamien JB, Spealman RD (1990) Antagonism of cocaine self‐ administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol 1:355‐363. Bergman J, Madras BK, Johnson SE, Spealman RD (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self‐administration by squirrel monkeys. J Pharmacol Exp Ther 251:150‐155. Bessa MA, Mitshuhiro SS, Chalem E, Barros MM, Guinsburg R, Laranjeira R (2009) Underreporting of use of cocaine and marijuana during the third trimester of gestation among pregnant adolescents. Addictive Behaviors 35:266‐269. Bettinardi V, Pagani E, Gilardi MC et al (1999) An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med 26:447‐458. 53 Bhide PG (2009) Dopamine, cocaine, and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol 20:395‐402. Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73‐86. Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: delay discounting in current, never, and ex‐smokers. Psychopharmacology 146:447‐454. Biederman J, Faraone S, Monuteaux M, Feighner J (2000) Patterns of alcohol and drug use in adolescents can be predicted by parental substance use disorder. Pediatrics 106:792‐797. Bingol N, Fuchs M, Diaz V, Stone RK, Gromisch DS (1987) Teratogenicity of cocaine in humans. J Pediatr 110:93‐96. Bolig R, Price CS, O’Neill PL, Suomi SJ (1992) Subjective assessment of reactivity level and personality traits of rhesus monkeys. Int J of Primatology 13:287‐306. Booze RM, Wallace DR, Silvers JM, Strupp BJ, Snow DM, Mactutus CF (2006) Prenatal cocaine exposure alters alpha2 receptor expression in adolescent rats. BMC Neurosci 7:33. Brown GL, Linnoila MI (1990) CSF serotonin metabolite (5‐HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry 51: Suppl 31‐ 43. Buxhoeveden DP, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF (2006) Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Dev Neurosci 24:335‐341. Cabrera‐Vera TM, Garcia F, Pinto W, Battaglia G (2000) Neurochemical changes in brain serotonin neurons in immature and adult offspring prenatally exposed to cocaine. Brain Res 870:1‐9. Caille I, Dumartin B, Le Moine C, Begueret J, Bloch B (1995) Ontogeny of the D1 dopamine receptor in the rat striatonigral system: an immunohistochemical study. Eur J Neurosci 7:714‐722. Caine SB, Koob GF (1993) Modulation of cocaine self‐administration in the rat through D3 dopamine receptors 260:1814‐1816. 54 Campbell JO, Bliven TD, Silveri MM, Snyder KJ, Spear LP (2000) Effects of prenatal cocaine on behavioral adaptation to chronic stress in adult rats. Neurotoxicol Teratol 22:845‐850. Campbell UC, Rodefer JS, Carroll ME (1999) Effects of dopamine receptor antagonists (D1 and D2) on the demand for smoked cocaine base in rhesus monkeys. Psychopharmacology 144:381‐388. Camps M, Kelly PH, Palacios JM (1990) Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J Neural Transm Gen Sect 80:105‐127. Carroll ME, Lac ST (1997) Acquisition of IV amphetamine and cocaine self‐ administration in rats as a function of dose. Psychopharmacology 129:206‐214. Carroll ME, Stotz DC, Kliner DJ, Meisch RA (1984) Self‐administration of orally‐ delivered methohexital in rhesus monkeys. Pharmacol Biochem Behav 20:137‐143. Carroll ME, Lac ST, Walker M, Kragh R, Newman T (1986) The effects of naltrexone on intravenous cocaine self‐administration in rats during food satiation and deprivation. J Pharmacol Exp ther 238:1‐7. Chamberlain SR, Sahakian BJ (2007) The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20:255‐261. Chao C (1996) Cardiovascular effects of cocaine during pregnancy. Semin Perinatol 20:107‐114. Chasnoff IJ, Burns WJ, Schnoll SH, Burns KA (1985) Cocaine use in pregnancy. New Eng J of Med 313:66‐669. Chelonis JJ, Gillam MP, Paule MG (2003) The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol 25:437‐446. Childs E, Shoaib M, Stolerman IP (2006) Cocaine self‐administration in rats with histories of cocaine exposure and discrimination. Psychopharmacology 186:168‐176. Chiriboga CA, Brust JC, Bateman D, Hauser WA (1999) Dose‐response effect of fetal cocaine exposure on newborn neurological function. Pediatrics 103:79‐85. 55 Choi WS, Ronnekleiv OK (1996) Effects of in utero cocaine exposure on the expression of mRNAs encoding the dopamine transporter and the D1, D2, and D5 dopamine receptor subtypes in fetal rhesus monkey. Brain Res Dev Brain Res 96:249‐260. Christian BT, Vandehey NT, Fox AS et al (2009) The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage 44:1334‐1344. Church MW et al (1991) Effects of prenatal cocaine exposure. In: Biochemistry and Physiology of Substance Abuse, Vol. III. Chapter 8, (Watson RR, ed), Boca Raton, FL: CRC Press, pp179‐204. Clarke AS, Boinski S (1995) Temperament in nonhuman primates. Am J Primatol 37:103‐125. Coccaro EF, Lee R (2010) Cerebrospinal fluid 5‐hydroxyindolacetic acid and homovanillic acid: reciprocal relationships with impulsive aggression in human subjects. J Neural Trasm 117:241‐248. Code RA, Tang AH (1991) Yawning produced by dopamine agonists in rhesus monkeys. Eur J Pharmacol 201:235‐238. Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003) Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine‐dependent invididuals. Exp Clin Psychopharmacol 11:18‐25. Cohen HR, Green JR, Crombleholme WR (1991) Peripartum cocaine use: estimating risk of adverse pregnancy outcome. Int J of Gynecol and Obstet 25:51‐54. Coleman K, Tully LA, McMillan JL (2005) Temperament correlates with training success in adult rhesus macaques. Am J Primatol 65:63‐71. Collins GT, Newman AH, Grundt P et al (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology 193:159‐170. Collins GT, Witkin JM, Newman AH et al (2005) Dopamine agonist‐induced yawning in rats: a dopamine D3 receptor‐mediated behavior. J Pharmacol Exp Ther 314:310‐319. Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA (2001) Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr 22:217‐225. 56 Corrigall WA, Coen KM (1991) Cocaine self‐administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav 39:799‐802. Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG (2004) Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex 14:665‐675. Cregler LL, Mark H (1986) Medical complications of cocaine abuse. N Engl J Med 315:1495‐1500. Cremniter D, Jamain S, Kollenbach K, Alvarez JC, Lecrubier Y, Gilton A, Jullien P, Lesieur P, Bonnet F, Spreux‐Varoquaux O (1999) CSF 5‐HIAA levels are lower in impulsive as compared to nonimpulsive violent suicide attempters and control subjects. Biol Psychiatry 45:1572‐ 1579. Crozatier C, Guerriero RM, Mathieu F, Giros B, Nosten‐Bertrand M, Kosofsky BE (2003) Altered cocaine‐induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain Res Dev Brain Res 147:97‐ 105. Czoty PW, Gage HD, Nader MA (2010) Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology 208: 585‐592. Czoty PW, McCabe C, Nader MA (2005) Assessment of the reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther 312: 96‐102. Czoty PW, Morgan D, Shannon EA, Gage HD, Nader MA (2004) Characterization of dopamine D1 receptor function in socially housed cynomolgus monkeys. Psychopharmacology 174: 381‐388. Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA (2009) Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Psychopharmacology 34:548‐554. Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 215:1267‐1270. 57 Dandy KL, Gatch MB (2009) The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol 20:400‐405. Das G (1993) Cocaine abuse in North America: a milestone in history. J Clin Pharmacol 33:296‐310. Davis BA, Clinton SM, Akil H, Becker JB (2008) The effects of novelty‐seeking phenotypes and sex differences on acquisition of cocaine self‐ administration in selectively bred High‐Responder and Low‐ Responder rats. Pharmacol Biochem Behav 90:331‐338. Day N, Richardson G (1993) Cocaine use and crack babies: science, the media, and miscommunication. Neurotoxicol Teratol 15:293‐294. DeVry J, Donselaar I, van Ree JM (1989) Food deprivation and acquisition of intravenous cocaine self‐administration in rats: effects of naltrexone and haloperidol. J Pharmacol Exp Ther 251:735‐740. de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22‐31. de Wit H, Richards JB (2003) Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv 50:19‐55. Delaney‐Black V, Covington C, Nordstrom B et al (2004) Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr 25:254‐263. Delaney‐Black V, Covington C, Ostrea E Jr et al (1996) Prenatal cocaine and neonatal outcome: evaluation of dose‐response relationship. Pediatrics 98:735‐740. Delaney‐Black V, Covington C, Templin et al (2000) Teacher‐assessed behavior of children prenatally exposed to cocaine. Pediatrics 106:782‐791. Dellu F, Piazza PV, Mayo W, LeMoal M, Simon H (1996) Novelty‐seeking in rats –biobehavioral characteristics and possible relationship with the sensation‐seeking trait in man. Neuropsychobiology 34:136‐145. Dennis‐Tiwary T, Bendersky M, Ramsay D et al (2006) Reactivity and regulation in children prenatally cocaine exposed. Dev Psycho 42:688‐ 697. 58 Di Chiara G, Imperato A (1988) Drugs abused by human preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274‐5278. Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, de Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301‐308. Dow‐Edwards D (2010) Sex differences in the effects of cocaine abuse across the lifespan. Physiology and Behavior 100:208‐215. Dow‐Edwards D, Mayes L, Spear L, Hurd Y (1999) Cocaine and development: clinical, behavioral, and neurobiological perspectives – a symposium report. Neurotoxicol Teratol 21:481‐490. Dow‐Edwards DL, Benveniste H, Behnke M, Bandstra ES, Singer LT, Hurd YL, Stanford LR (2006) Neuroimaging of prenatal drug exposure. Neurotoxicol Teratol 28:386‐402. Estroff TW, Gold MS (1985) Medical and psychiatric complications of cocaine abuse with possible points of pharmacological treatment. Adv Alcohol Subst Abuse 5:61‐76. Evans SM (2007) The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol 15:418‐426. Evans SM, Foltin RW (2006) Exogenous progesterone attenuates the subjective effects of smoked cocaine in women but not in men. Neuropsychopharmacology 31:659‐674. Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology 159:397‐406. Evenden J (1999) Impulsivity: a discussion of clinical and experimental findings. Journal of Psychopharmacology 13:180‐192. Eyler FD, Behnke M (1995) Prenatal cocaine exposure: consequences for child and family. J Fla Med Assoc 82:603‐606. Fairbanks LA, Fontenot MB, Phillips‐Conroy JE, Jolly CJ, Kaplan JR, Mann JJ (1999) CSF monoamines, age, and impulsivity in wild grivet monkeys (Cercopithecus aethiops). Brain Behav Evol 53:305‐312. 59 Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ (2004) Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol 64:1‐17. Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT (2001) Neuropsychopharmacology 24:370‐378. Fajemirokun‐Odudeyi O, Lindow SW (2004) Obstretric implications of cocaine use in pregnancy: a literature review. Eur J of Obstet, Gynecol, and Reprod Bio 112:2‐8. Fang Y, Janowsky A, Ronnekleiv OK (1997) Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1‐ and D2‐like receptor binding densities. Brain Res Dev Brain Res 104:163‐174. Fang Y, Ronnekleiv OK (1999) Cocaine upregulates the dopamine transporter in fetal rhesus monkey brain. J Neurosci 19:8966‐8978. Farde L, Hall H, Pauli S, Halldin C (1995) Variability in D2‐dopamine receptor density and affinity: A PET study with [11C]raclopride in man. Synapse 20:200‐208. Fang Y, Janowsky A, Ronnekleiv OK (1997) Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1‐ and D2‐like receptor binding densities. Brain Res Dev Brain Res 104:163‐174. Fang Y, Ronnekleiv OK (1999) Cocaine upregulates the dopamine transporter in fetal rhesus monkey brain. J Neurosci 19:8966‐8978. Frank DA, Jacobs RR, Beeghly M, Augustyn M, Bellinger D, cabral H, Heeren T (2002) Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics 110:1143‐1152. Frank DA, Zuckerman BS, Amaro H, Aboagye K et al (1988) Cocaine use during pregnancy: prevalence and correlates. Pediatrics 82:888‐895. Fried PA, Watkinson B, Gray R (1992) A follow‐up study of attentional behavior in 6‐year‐old children exposed prenatally to marijuana, cigarettes, and alcohol. Neurotoxicol Teratol 14:299‐311. Friedman E, Yadin E, Wang HY (1996) Effects of prenatal cocaine on dopamine receptor‐G protein coupling in mesocortical regions of the rabbit brain. Neuroscience 70:739‐747. 60 Gabriel M, Taylor C, Burhans L (2003) In utero cocaine, discriminative avoidance learning with low‐salient stimuli and learning‐related neuronal activity in rabbits (Oryctolagus cuniculus). Behav Neurosci 117:912‐926. Gaultney JF, Gingras JL, Marin M, DeBrule D (2005) Prenatal cocaine exposure and infants’ preference for novelty and distractibility. J Dev Behav Pediatr 166:385‐406. Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ (2003) Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res 147:85‐96. Gendle MH, White TL, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ (2004) Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav Neurosci 118:290‐297. Gerrits MAFM, van Ree JM (1995) Assessment of motivational aspects involved in initiation of ocaine and heroin self‐administration in rats. Pharmacol Biochem Behav 52:35‐42. Goeders NE, Guerin GF (1994) Non‐contingent electrical foot‐shock facilitates the acquisition of intravenous cocaine self‐administration in rats. Psychopharmacology 114:63‐70. Goldman‐Rakic PS, Brown RM (1982) Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res 256:339‐349. Gosnell BA, Lane KE, Bell SM, Krahn DD (1995) Intravenous morphine self‐ administration by rats with low versus high saccharin preference. Psychopharmacology 117:248‐252. Gradman AH (1988) Cardiac effects of cocaine: a review. Yale J Biol Med 61:137‐147. Graham K, Koren G, Klein J, Schneiderman J, Greenwald M (1989) JAMA 262:3328‐3330. Gringas JL, O’Donnell KJ (1998) State control in the substance‐exposed fetus. I. The fetal neurobehavioral profile: an assessment of fetal state, arousal, and regulation competency. Ann NY Acad Sci 846:262‐276. 61 Guerriero RM, Rajadhyaksha A, Crozatier C, Giros B, Nosten‐Bertrand M, Kosofsky BE (2005) Augmented constitutive CREB expression in the nucleus accumbens and striatum may contribute to the altered behavioral response to cocaine of adult mice exposed to cocaine in utero. Dev Neurosci 27:235‐248. Haber SN, Ryoo H, Cox C, Lu W (1995) Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol 362:400‐410. Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SP, Brody LC, Collins FS (1998) Evolutionary sequence comparisons using high‐ density oligonucleotide arrays. Nat Genet 18:155‐158. Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self‐administration in male and female rats. Brain Res 698:46‐52. Harvey JA (2004) Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev 27:751‐764. He N, Song ZM, Lidow MS (1999) Cocaine induces cell death within the primate fetal cerebral wall. Neuropathol Appl Neurobiol 25:504‐512. Hecht GS, Spear NE, Spear LP (1998) Alterations in the reinforcing efficacy of cocaine in adult rats following prenatal exposure to cocaine. Behav Neurosci 112:410‐418. Heidbreder C (2008) Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets 7:410‐421. Heyser CJ, Goodwin GA, Moody CA, Spear LP (1992a) Prenatal cocaine exposure attenuates cocaine‐induced odor preference in infant rats. Pharmacol Biochem Behav 42:169‐173. Heyser CJ, Miller JS, Spear NE, Spear LP (1992b) Prenatal exposure to cocaine disrupts cocaine‐induced conditioned place preference in rats. Neurotoxicol Teratol 14:57‐64. Higley JD, Bennett AJ (1999) Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non‐human primates. Alcohol 34:402‐418. 62 Higley JD, Linnoila M (1997) Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann NY Acad Sci 836:39‐56. Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M (1996) CSF testosterone and 5‐HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40:1067‐1082. Hooks MS, Jones GH, Smith AD, Neill DB, Justice DB Jr. (2001) Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse 9:121‐128. Horger BA, Shelton K, Schenk S (1990) Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav 37:707‐711. Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S (1991) Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport 2:53‐60. Howell LL, Schama KF, Ellis JE, Grimley PJ, Kitchens AJ, Byrd LD (2001) Fetal development in rhesus monkeys exposed prenatally to cocaine. Neurotoxicol Teratol 23:133‐140. Hubner CB, Moreton JE (1991) Effects of selective D1 and D2 dopamine antagonists on cocaine self‐administration in the rat. Psychopharmacology 105:151‐156. Hughes JA, Sparber SB (1978) d‐Amphetamine unmasks postnatal consequences of exposure to methylmercury in utero: methods for studying behavioral teratogenesis. Pharmacol Biochem Behav 9:365‐ 375. Hurlbut KM (1991) Drug‐induced psychoses. Emerg Med Clin North Am 9:31‐ 52. Hurt H, Brodsky NL, Braitman LE, Giannetta J (1995) Natal status of infants of cocaine users and control subjects: a prospective comparison. J Perinatol 14:297‐304. Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta J (1997) A prospective evaluation of early language development in children with in utero cocaine exposure and in control subjects. J Pediatr 130:310‐312. 63 Inman‐Wood SL, Williams MT, Morford LL, Vorhees CV (2000) Effects of prenatal cocaine on Morris and Barnes maze tests of spatial learning and memory in the offspring of C57BL/6J mice. Neurotoxicol teratol 22:547‐557. Jackson C, Henricksen L, Dickinson D, Levine D (1997) The early use of alcohol and tobacco: its relation to children’s competence and parents’ behavior. Am J Public Health 87:359‐364. Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM (1996) New evidence for neurobehavioral effects of in utero cocain exposure. J Pediatr 129:581‐590. Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward‐related stimuli. Psychopharmacology 146:373‐390. Johns JM, Lubin DA, Lieberman JA, Lauder JM (2002) Developmental effects of prenatal cocaine exposure on 5‐HT1A receptors in male and female rat offspring. Dev Neurosci 24:522‐530. Johns JM, Means MJ, Bass EW, Means LW, Zimmerman LI, McMillan BA (1994) Prenatal exposure to cocaine: effects on aggression in Sprague‐Dawley rats. Dev Psychobiol 27:227‐239. Jones L, Fischer I, Levitt P (1996) Nonuniform alternation of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb Cortex 6:431‐445. Jones LB, Stanwood GD, Reinoso BS et al (2000) In utero cocaine‐induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci 20:4606‐4614. Jovanovski D, Erb S, Zakzanis KK (2005) Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol 27:189‐204. Jung AB, Bennett JP Jr (1996) Development of striatal dopaminergic function. I. Pre‐ and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Brain Res Dev Brain Res 94: 109‐120. Jutkiewicz EM, Bergman J (2004) Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther 1999:649‐661. 64 Kandel DB, Warner MPP, Kessler RC (1998) The epidemiology of substance abuse and dependence among women. In: Drug addiction research and the health of women. Rockville, MD: United States Department of Health and Human Resources. Kaplow JB, Curran PJ, Dodge KA (2002) Child, parent, and peer predictors of early‐onset substance use: a multisite longitudinal study. J Abnorm Child Psychol 30:199‐216. Karmel BZ, Gardner JM (1996) Prenatal cocaine exposure effects on arousal‐ modulated attention during the neonatal neurobehavioral status. Neurotoxicol Teratol 18:519‐528. Kebabian JW, Caine DB (1979) Multiple receptors for dopamine. Nature 277:93‐96. Keller RW Jr, LeFevre R, Raucci J, Carlson JN, Glick SD (1996) Enhanced cocaine self‐administration in adult rats prenatally exposed to cocaine. Neurosci Lett 205:153‐156. Keller RW Jr, Maisonneuve IM, Nuccio DM, Carlson JN, Glick SD (1994) Effects of prenatal cocaine exposure on the nigrostriatal dopamine system: an in vivo microdialysis study in the rat. Brain Res 634:266‐274. Kilbride H, Castor C, Hoffman E, Fuger KL (2000) Thirty‐six‐month outcome of prenatal cocaine exposure for term or near‐term infants: impact of early case management. J Dev Behav Pediatr 21:19‐26. King TA, Perlman JM, Laptook AR, Rollins N, Jackson G, Little B (1995) Neurologica manifestation of in utero cocaine exposure in near‐term and term infants. Pediatrics 96:259‐264. Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128:78‐87. Kline J, Ng SK, Schittini M, Levin B, Susser M (1997) Cocaine use during pregnancy: sensitive detection by hair assay. Am J Public Health 87:3520‐358. Klonoff DC, Andrews BT, Obana WG (1989) Stroke associated with cocaine use. Arch Neurol 46:989‐993. 65 Koe BK (1976) Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther 199:649‐661. Koob GF, Le HT, Creese I (1987) The D1 dopamine receptor antagonist SCH 23390 increases cocaine self‐administration in the rat. Neurosci Lett 79:315‐320. Koren G, Nulman I, Rovet J, Greenbaum R, Loebstein M, Einarson T (1998) Long‐term neurodevelopmental risks in children exposed in utero to cocaine. The Toronto Adoption Study. Ann NY Acad Sci 846:306‐313. Kraemer S (2000) The fragile male. Br Med J 321:1609‐1612. Krauthammer C (1989) Children of Cocaine. Washington Post. 7/30/1989. Krug SE (1989) Cocaine abuse: historical, epidemiologic, and clinical perspectives for pediatricians. Adv Pediatr 36:369‐406. Kuczkowski KM (2003) Anesthetic implications of drug abuse in pregnancy. J Clin Anesth 15:382‐394. Kuhar MJ, Ritz MC, Boja JW (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299‐302. Kuhn L, Kline J, Ng S, Levin B, Susser M (2000) Cocaine use during pregnancy and intrauterine growth retardation: new insights based on maternal hair tests. Am J Epidemiol 152:112‐119. Laakso A, Vilkman H, Bergman J et al (2002) Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Soc Biol Psychiatry 52:759‐763. Lambe EK, Krimer LS, Goldman‐Rakic PS (2000) Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci 20:8780‐ 8787. Le Foll B, Goldberg SR, Sokoloff P (2005) The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology 49:525‐541. Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine D2/D3 receptor availability 66 is reduced in methamphetamine dependence and is linked to impulsivity. J of Neurosci 29:14734‐14740. Lee C‐T, Chen J, Hayashi T, Tsai S‐Y, Sanchez JF, Errico SL, Amable R, Su TP, Lowe RH, Huestis MA, Shen J, Becker KG, Geller HM, Freed WJ (2008) A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Medicine 5:987‐1004. Leech SL, Richardson GA, Goldschmidt L, Day NL (1999) Prenatal substance exposure: effects on attention and impulsivity of 6‐year‐olds. Neurotoxicol Teratol 21:109‐118. Leshner AI (1998) Foreword in Cocaine: effects on the developing brain. Annals of NY Acad of Sci 846:xv‐xvii. Lester BM, Twomey JE (2008) Treatment of substance abuse during pregnancy. Women’s Health 4:67‐77. Levitt P, Rakic P (1982) The time of genesis, embryonic origin and differentiation of the brain stem monoamine neurons in the rhesus monkey. Brain Res 256:35‐57. Lewis DE, Moore CM, Leikin JB, Koller A (1995) Meconium analysis for cocaine: a validation study and comparison with paired urine analysis. J Anal Toxicol 19:148‐150. Lewis MW, Misra S, Johnson HL, Rosen TS (2004) Neurological and developmental outcomes of prenatally cocaine‐exposed offspring from 12 to 36 months. Am J Drug Alcohol Abuse 30: 299‐320. Lidow MS (1995a) D1‐ and D2 dopaminergic receptors in the developing cerebral cortex of macaque monke: a film autoradiographic study. Neuroscience 65:439‐452. Lidow MS (1995b) Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse 21:332‐341. Lidow MS (1998) Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann NY Acad Sci 846:182‐ 193. Lidow MS (2003) Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res 147:23‐36. Lidow MS, Goldman‐Rakic PS, Gallager DW, Rakic P (1991) Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative 67 autoradiographic analysis using [3H]spiperone and [3H]SCH23390. Neuroscience 40:657‐671. Lidow MS, Song ZM (2001a) Effect of cocaine on cell proliferation in the cerebral wall of monkey fetuses. Cereb Cortex 11:545‐551. Lidow MS, Song ZM (2001b) Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol 435:263‐275. Lile JA, Morgan D, Freedland CS, Sinnott RS, Davies HM, Nader MA (2000) Self‐administration of two long‐acting monoamine transport blockers in rhesus monkeys. Psychopharmacology 152:414‐421. Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA (2003) The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther 307:356‐366. Lin D, Kellogg CK (1996) Neonatal exposure to cocaine enhances the reward‐ potentiating properties of the drug in young adult animals. Behav Neurosci 110:791‐801. Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S (2006) Mental health outcomes of cocaine‐exposed children at 6 years of age. J Pediatr Psychol 31:85‐97. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK (1983) Low cerebrospinal fluid 5‐hydroxyindoleacetic acid concentration differentiates impulsive from non‐impulsive violent behavior. Life Sci 33:2609‐2614. Logan J, Fowler JS, Volkow ND et al (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834‐840. Lutiger B, Graham K, Einarson TR, Koren G (1991) Relationship between gestational cocaine use and pregnancy outcome: a meta‐analysis. Teratology 44:405‐414. Lynd‐Balta E, Haber SN (1994a) The organization of midbrain projections to the striatum in the primate: sensorimotor‐related striatum versus ventral striatum. Neuroscience 59:625‐640. Lynd‐Balta E, Haber SN (1994b) The organization of midbrain projections to the ventral striatum in the primate. Neuroscience 59:609‐623. 68 Lyons D, Friedman DP, Nader MA, Porrino LJ (1996) Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci 16:1230‐1238. Mach RH, Nader MA, Ehrenkaufer RL et al (1996) Comparison of two fluorine‐18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse 24:322‐333. Mach RH, Luedtke RR, Unsworth CD et al (1993) 18F‐labeled benzamides for studying the dopamine D2 receptor with positron emission tomography. J Med Chem 36:3707‐3720. Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self‐ control choices in opioid‐dependent patients and non‐drug‐using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256‐262. Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD (1989) Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate‐putamen. J Pharmacol Exp Ther 251:131‐141. Mahone PR, Scott K, Sleggs G, D’Antoni T, Woods JR (1994) Cocaine and metabolites in amniotic fluid may prolong fetal drug exposure. Am J Obstet Gynecol 171:465‐469. Malanga CJ, Kosofsky BE (2003) Does drug abuse begat brug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Brain Res Dev Brain Res 147:47‐57. Malanga CJ, Ren JQ, Guerriero RM, Kosofsky BE (2009) Augmentation of cocaine‐sensitized dopamine release in the nucleus accumbens of adult mice following prenatal cocaine exposure. Dev Neurosci 31:76‐ 89. Malanga CJ, Riday TT, Carlezon WA, Kosofsky BE (2008) Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry 63:214‐221. Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME (2003) Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomologus monkeys (Macaca fascicularis). Am J Primatol 61:187‐194. 69 Markovic N, Ness RB, Cefilli D, Grisso JA, Stahmer S, Shaw LM (2000) Substance use measures among women in early pregnancy. Am J Obstet Gynecol 183:627‐632. Martelle JL, Claytor R, Ross JT et al (2007) Effects of two novel D3‐selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 321:573‐582. Martinez D, Broft A, Foltin RW et al (2004) Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine‐seeking behavior. Neuropsychopharmacology 29:1190‐1202. Marusich JA, Bardo MT (2009) Differences in impulsivity on a delay‐ discounting task predict self‐administration of a low unit dose of methylphenidate in rats. Behav Pharmacol 20:447‐454. Matthys W, van Goozen SH, de Vries H, Cohen‐Kettenis PT, van Engeland H (1998) The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J Child Psychol Psychiatry 39:642‐651. Matthys W, van Goozen SH, Snoek H, van Engeland H (2004) Response perseveration and sensitivity to reward and punishment in boys with oppositional‐defiant disorder. Eur Child Adolesc Psychiatry 13:361‐ 364. Mayes L, Snyder PJ, Langlois E, Hunter N (2007) Visuospatial working memory in school‐aged children exposed in utero to cocaine. Child Neuropsychol 13:205‐218. Mayes LC (2002) A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol 24:385‐395. Mayes LC, Granger RH, Frank MA, Schottenfeld R, Bornstein MH (1993) Neurobehavioral profiles of neonates exposed to cocaine prenatally. Pediatrics 91:778‐783. Mayes LC, Grillon C, Granger R, Schottenfeld R (1998) Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann NY Acad Sci 846: 126‐143. 70 Mayes LC, Molfese DL, Key AP, Hunter NC (2005) Event‐related potentials in cocaine‐exposed children during a Stroop task. Neurotoxicol Teratol 27:797‐813. McCleary R (1966) Response‐modulating function of the limbic system: Initiation and suppression. In Stellar E and Sprague J (Eds.), Progress in physiological psychology, Vol 1. Academic Press, New York, pp 209‐ 271. McMurray MS, Joyner PW, Middleton CW et al (2008) Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress 11:398‐410. Messinger DS, Bauer CR, Das A, Seifer R, Lester BM et al (2004) The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine‐ exposed and opiate‐exposed infants through three years of age. Pediatrics 113:1677‐1685. Minnes S, Singer LT, Kirchner HL, Satayanthum S, Short EJ et al (2008) The association of prenatal cocaine use and childhood trauma with psychological symptoms over 6 years. Arch Womens Ment Health 11:181‐192. Minnes S, Singer LT, Kirchner HL, Short EJ, Lewis B, Satayathum S, Queh D (2010) The effects of prenatal cocaine exposure on problem‐behavior in children 4‐10 years. Neurotoxicol Teratol doi:10.1016/j.ntt.2010.03.005. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189‐225. Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001) Psychiatric aspects of impulsvity. Am J Psychiatry 158:1783‐1793. Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC (2002) Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend 68:105‐111. Moody CA, Frambes NA, Spear LP (1992) Psychopharmacological responsiveness to the dopamine agonist quinpirole in normal weanlings and in weanling offspring exposed gestationally to cocaine. Psychopharmacology 108:256‐262. 71 Moore RJ, Vinsant SL, Nader MA et al (1998) Effect of cocaine self‐ administration on dopamine D2 receptors in rhesus monkeys. Synapse 30:88‐96. Morgan D, Grant KA, Gage HD et al (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self‐administration. Nat Neurosci 5:169‐174. Morris P, Binienda Z, Gillam MP et al (1996) The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 18:147‐154. Morris P, Gillam MP, Allen RR, Paule MG (1996) The effect of chronic cocaine exposure during pregnancy on the acquisition of operant behaviors by rhesus monkey offspring. Neurotoxicol Teratol 18:155‐166. Morrow BA, Elsworth JD, Roth RH (2002) Prenatal cocaine exposure disrupts non‐spatial, short‐term memory in adolescent and adult male rats. Behav Brain Res 129:217‐223. Morrow BA, Elsworth JD, Roth RH (2005) Prenatal exposure to cocaine selectively disrupts the development of parvalbumin containing local circuit neurons in the medial prefrontal cortex of the rat. Synapse 56:1‐11. Morrow BA, Hajszan T, Leranth C, Elsworth JD, Roth RH (2007) Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse 61:862‐865. Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes MB (2001) Influence of prenatal cocaine exposure on full‐term infant neurobehavioral functioning. Neurotoxicol Teratol 23:533‐544. Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes MB (2003) Influence of prenatal cocaine exposure on early language development: longitudinal findings from four months to three years of age. J Dev Behav Pediatr 24:39‐50. Morrow CE, Culbertson JL, Accornero VH, Zue L, Anthony JC, Bandstra ES (2006) Learning disabilities and intellectual functioning in school‐ aged children with prenatal cocaine exposure. Dev Neuropsychol 30:905‐931. 72 Munro CA, McCaul ME, Wong DF et al (2006) Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966‐974. Nader MA, Czoty PW (2005) PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry 162:1473‐1482. Nader MA, Daunais JB, Moore T et al (2002) Effects of cocaine self‐ administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 21:589‐596. Nader MA, Grant KA, Gage HD et al (1999) PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane‐ and ketamine‐induced anesthesia. Neuropsychopharmacology 21:35‐46. Nader MA, Green KL, Luedtke RR, Mach RH (1999) The effects of benzamide analogues on cocaine self‐administration in rhesus monkeys. Psychopharmacology 147:143‐152. Nader MA, Mach RH (1996) Self‐administration of the dopamine D3 agonist 7‐OH‐DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology 125:13‐22. Nader MA, Morgan D, Gage HD et al (2006) PET imaging of dopamine D2 receptors during chronic cocaine self‐administration in monkeys. Nat Neurosci 9:1050‐1056. Nair BS, Watson RR (1991) Cocaine and the pregnant woman. J Reprod Med 36:862‐867. National Institute on Drug Abuse (1996) National Pregnancy and health survey. http://www.drugabuse.gov/NIDA_Notes/NNVol12N1/Survey.html Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP (2004) Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res 153:213‐223. Neuspiel D (1993) Cocaine and the fetus: mythology of severe risk. Neurotoxicol Teratol 15:305‐306. Newell‐Morris L, Fahrenbruch CE (1985) Practical and evolutionary considerations for use of nonhuman primate model in prenatal 73 research. In: Nonhuman primate models for human growth and development (Watts ES, ed), New York: Liss, pp9‐40. Newman JL, Perry JL, Carrol ME (2008) Effects of altering reinforce magnitude and reinforcement schedule on phencyclindine (PCP) self‐ administration in monkeys using an adjusting delay task. Pharmacol Biochem Behav 90:778‐786. Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C (2005) Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol 27:429‐438. Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, Covington C, Delaney‐Black V (2005) Gender and alcohol moderate prenatal cocaine effects on teacher‐report of child behavior. Neurotoxicol Teratol 27:181‐189. Novikova SI, He F, Nai J, Cutrufello NJ, Lidow MS, Undieh AS (2008) Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One 3:e1919. O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J (2002) Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. Am J Drug Alcohol Abuse 28:743‐754. O’Malley PM, Johnston LD, Bachman JG (1991) Quantitative and qualitative changes in cocaine use among American high school seniors, college students, and young adults. NIDA Res Monogr 110:19‐43. O’Neill AM, Carter K (1999) Desperate measures. People Magazine 145‐149. 9/27/1999. Olson L, Nystrom B, Seiger A (1973) Monoamine fluorescence histochemistry of human post mortem brain. Brain Res 63: 231‐247. Olson L, Seiger A (1972) Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch 137:301‐316. Ostrea EM, Brady M et al (1992) Drug screening of newborns by meconium analysis: A large‐scale, prospective, epidemiological study. Pediatrics 89:107‐113. 74 Ostrea EM, Brady MJ, Parks PM, Asensio DC, Naluz A (1989) Drug screening of meconium in infants of drug‐dependent mothers: an alternative to urine testing. J Pediatr 115:474‐477. Overstreet DH, Moy SS, Lubin DA, Gause LR, Liberman JA, Johns JM (2000) Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav 70:149‐156. Paule MG, Gillam MP, Allen RR, Chelonis JJ (2000) Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann NY Acad Sci 914:412‐417. Paule MG, Gillam MP, Binienda Z, Morris P (1996) Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci 801:301‐309. Peng XQ, Ashby CF Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX (2009) The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine‐ triggered relapse to drug‐seeking behaviors in rats. Neuropharmacology 56:752‐760. Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self‐ administration in female rats. Psychopharmacology 178:193‐201. Perry JL, Nelson SE, Carroll ME (2008) Impulsive choice as a predictor of acquisition of IV cocaine self‐administration and reinstatement of cocaine‐seeking behavior in male and female rats. Exp Clin Psychopharmacol 16:165‐177. Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self‐administration. Science 245:1511‐1513. Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H (1990) Individual reactivity to novelty predicts probability of amphetamine self‐administration. Behav Pharmacol 1:339‐345. Piazza PV, Le Moal M (1998) The role of stress in drug self‐administration. Trends Pharmacol Sci 19:67‐74. Pickens R, Thompson T, Muchow DC (1973) Cannabis and phencyclidine self‐ administration by animals. In: Goldberg L, Hoffmeister F (eds) Psychic dependence. Springer, New York, pp 78‐86. 75 Plessinger MA, Woods JR (1998) Cocaine in pregnancy. Recent data on maternal and fetal risks. Obstet Gynecol Clin North Am 25:99‐118. Pohjalainen T, Rinne JO, Nagren K et al (1998) Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155:768‐773. Poulos CX, Le AD, Parker JL (1995) Impulsivity predicts individual susceptibility to high levels of alcohol self‐administration. Behav Pharmacol 6: 810‐814. Pulsifer MB, Butz AM, O’Reilly FM, Belcher HM (2008) Prenatal drug exposure: effects on cognitive functioning at 5 years of age. Clin Pediatr 47:58‐65. Ramsey NF, Niesink RJM, Van Ree JM (1993) Prenatal exposure to morphine enhances cocaine and heroin self‐administration in drug‐naïve rats. Drug Alcohol Depend 33:41‐51. Ranaldi R, Wise RA (2001) Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 21:5841‐5846. Rao PA, Molinoff PB, Joyce JN (1991) Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Brain Res Dev Brain Res 60:161‐177. Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H (2007) Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics 120:1245‐1254. Ren JQ, Malanga CJ, Tabit E, Kosofsky BE (2004) Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci 22:309‐320. Retz W, Rosler M, Supprian T, Retz‐Junginger P, Thome J (2003) Dopamine D3 receptor gene polymorphism and violent behavior: relation to impulsiveness and ADHD‐related psychopathology. J Neural Transm 110:561‐572. Richardson GA (1998) Prenatal cocaine exposure. A longitudinal study of development. Ann NY Acad Sci 846:144‐152. 76 Richardson GA, Day NL, McGauhey PJ (1993) The impact of prenatal marijuana and cocaine use on the infant and child. Clin Obstet Gynecol 36:302‐318. Richardson GA, Hamel SC, Goldschmidt L, Day NL (1996) The effects of prenatal cocaine use on neonatal neurobehavioral status. Neurotoxicol Teratol 18:519‐528. Richardson GA, Hamel SC, Goldschmidt L, Day NL (1999) Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics 103:E18. Richfield EK, Young AB, Penney JB (1987) Comparative distribution of dopamine D‐1 and D‐2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol 262:446‐463. Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA (2009) Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience 158:1257‐1265. Ritz MC, Lamb RK, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self‐administration of cocaine. Science 237:1219‐1223. Roberts DC, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self‐administration of cocaine. Pharmacol Biochem Behav 6:615‐620. Roberts DC, Koob GF, Klonoff P, Fibiger HC (1980) Extinction and recovery of cocaine self‐administration following 6‐hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12:781‐787. Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H (1999) Self‐administration of cocaine analogs by rats. Psychopharmacology 144:389‐397. Rocha A, Valles R, Cardon AL, Brafton GR, Nation JR (2005) Enhanced acquisition of cocaine self‐administration in rats developmentally exposed to lead. Neuropsychopharmacology 30:2058‐2064. Rocha BA, Mead AN, Kosofsky BE (2002) Increased vulnerability to self‐ administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology 163:221‐229. 77 Rogers JG, Harrop R, Kinahan PE (1987) The theory of three‐dimensional image reconstruction for PET. IEEE Trans Med Imaging 6:239‐243. Romero VE, Vazquez J, Bermudez M, Negrin D, Antequera R (2002) Cocaine long‐term administration induces myocardial depressant effects and adrenoceptors desensitization. Acta Cient Venez 53: 225‐231. Ronnekleiv OK, Naylor BR (1995) Chronic cocaine exposure in the fetal rhesus monkey: consequences for early development of dopamine neurons. J Neurosci 15:7330‐7340. Rotzoll BW (2000) Costs increase as crack babies mature. Chicago Sun Times. 4/23/2000. Sales N, Martres MP, Bouthenet ML, Schwartz JC (1989) Ontogeny of dopaminergic D2 receptors in the rat nervous system: characterization and detailed autoradiographic mapping with [125I]iodosulpride. Neuroscience 28:673‐700. Sanberg JA, Olsen GD (1992) cocaine and metabolite concentrations in the fetal guinea pig after chronic maternal cocaine administration. J Pharmacol Exp Ther 260:587‐591. Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H (2005) Attentional functioning and impulse control in cocaine‐exposed and control children at age ten years. J Dev Behav Pediatr 26:42‐47. Savitz DA, Henderson L, Dole N, Herring A, Wilkins DG, Rollins D, Thorp JM (2002) Indicators of cocaine exposure and preterm birth. Obstet Gynecol 99:458‐465. Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT Jr (1994) Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience 62: 65‐85. Schenker S, Yang Y, Johnson RF et al (1993) The transfer of cocaine and its metabolites across the term human placenta. Clin Pharmacol Ther 53:329‐339. Schneider ML, Suomi SJ (1992) Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behav Dev 15:155‐ 177. 78 Schroder MD, Snyder PJ, Sielski I, Mayes L (2004) Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cogn 55:409‐412. Shaham Y, Klein LC, Alvares K, Grunberg NE (1992) Effect of stress on oral morphine and fentanyl self‐administration in rats. Pharmacol Biochem Behav 48: 1025‐1029. Sher L, Oquendo MA, Li S, Huang YY, Grunebaum MF, Burke AK, Malone KM, Mann JJ (2003) Lower CSF homovanillic acid levels in depressed patients with a history of alcoholism. Neuropsychopharmacology 28:1712‐1719. Silk J, Short J, Roberts J, Kusnitz J (1993) Gestation length in rhesus macaques (Macaca mulatta). Intern J Primatol 14:95‐104. Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM (2006) Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol 28:173‐180. Simansky KJ, Kachelries WJ (1996) Prenatal exposure to cocaine selectively disrupts motor responding to D‐amphetamine in young and mature rabbits. Neuropharmacology 35:71‐78. Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long‐term increases in impulsive choice. Behav Neurosci 121:543‐549. Singer LT, Arendt R, Minnes S, Farkas K, Salvator A (2000) Neurobehavioral outcomes of cocaine‐exposed infants. Neurotoxicol Teratol 22:653‐ 666. Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kircher HL, Kliegman R (2002) Cognitive and motor outcomes of cocaine‐exposed infants. JAMA 287:1952‐1960. Singer LT, Arendt R, Song LY, Warshawsky E, Kliegman R (1994) Direct and indirect interactions of cocaine with childbirth outcomes. Arch Pediatr Adolesc Med 148:959‐964. Singer LT, Eisengart LJ, Minnes S, Noland J, Jey A, Lane C, Min MO (2005) Prenatal cocaine exposure and infant cognition. Infant Behav Dev 28:431‐444. 79 Singer LT, Hawkins S, Huang J, Davillier M, Baley J (2001) Developmental outcomes and environmental correlates of very low birthweight, cocaine‐exposed infants. Early Hum Dev 64:91‐103. Singer LT, Lewin J, Minnes S, Weishampel P, Drake K, Satayathum S, Boll DT, Zijdenbox A, Paus T, Evans A. (2006) Neuroimaging of 7 to 8 year‐old children exposed prenatally to cocaine: Neurobehavioral teratology society, special edition 2004 meeting. Neurotoxicol Teratol 28:386‐ 402. Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kircher HL (2004) Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA 291:2448‐2456. Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S (2008) Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr 153:105‐111. Smith S (2002) Fast robust automated brain extraction. Hum Brain Map 17:143‐155. Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5:25‐43. Sood B, Delaney‐Black V, Covington C, Nordstrom‐Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ (2001) Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose‐response effect. Pediatrics 108:E34. Sood BG, Nordstrom Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, Hannigan JH, Delaney‐Black V (2005) Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol 27:191‐201. Spear LP (1996) Assessment of the effects of developmental toxicants: pharmacological and stress vulnerability of offspring. NIDA Res Monogr 164:125‐145. Specker S, Carroll ME, Lac ST (1994) Food deprivation history and cocaine self‐administration: an animal model of binge eating. Pharmacol Biochem Behav 48:1025‐1029. Staley JK, Mash DC (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100‐ 6106. 80 Stanis JJ, Marquez Avila H, White MD, Gulley JM (2008) Dissociation between long‐lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay‐discounting task. Psychopharmacology199:539‐548. Stanwood GD, Levitt P (2001) Prenatal cocaine exposure as a risk factor for later developmental outcomes. JAMA 286:45‐47. Stanwood GD, Levitt P (2003) Repeated i.v. cocaine exposure produces long‐ lasting behavioral sensitization in pregnant adults, but behavioral tolerance in their offspring. Neuroscience 122:579‐583. Stanwood GD, Levitt P (2004) Drug exposure early in life: functional repercussions of changing neuropharmacology during sensitive periods of brain development. Curr Opin Pharmacol 4:65‐71. Stanwood GD, Levitt P (2007) Prenatal exposure to cocaine produces unique developmental and long‐term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci 27:152‐157. Stanwood GD, Parlaman JP, Levitt P (2005) Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J Comp Neurol 487:270‐282. Stoffel EC, Cunningham KA (2008) The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend 92:69‐78. Stuerenburg HJ, Petersen K, Baumer T, Rosenkranz M, Buhmann C, Thomasius R (2002) Plasma concentrations of 5‐HT, 5‐HIAA, norepinephrine, epinephrine, and dopamine in ecstasy users. Neuro Endocrinol Lett 23:259‐261. Suto N, Austin JD, Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self‐administer nicotine. Psychopharmacology 158:175‐180. Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D (2003) Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry 160:1078‐1085. Taylor D, Ho BT (1977) Neurochemical effects of cocaine following acute and repeated injection. J Neurosci Res 3:95‐101. 81 Terner JM, de Wit H (2006) Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend 84:1‐13. Thompson BL, Levitt P, Stanwood GD (2005) Prenatal cocaine exposure specifically alters spontaneous alternation behavior. Behav Brain Res 164:107‐116. Tronick EZ, Frank DA, Cabral H, Mirochnick M, Zuckerman B (1996) Late dose‐response effects of prenatal cocaine exposure on newborn neurobehavioral performances. Pediatrics 98:76‐83. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1986) Latest National Survey on Drug Use and Health. Ref Type: Report. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1988) Latest National Survey on Drug Use and Health. Ref Type: Report. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1996) Results from the 1992 National Pregnancy and Health Survey. Ref Type: Report. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (2006) Latest National Survey on Drug Use and Health. Ref Type: Report. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (2008) Results from National Survey on Drug Use and Health. Ref Type: Report. Unterwald EM, Ivkovic, Cuntapay M et al (2003) Prenatal exposure to cocaine decreases adenylyl cyclase activity in embryonic mouse striatum. Brain Res Dev Brain Res 147:67‐75. van Ree JM, Slangen JL, de Wied D (1978) Intravenous self‐administration of drugs in rats. J Pharmacol Exp Ther 204:547‐557. 82 Vidaeff AC, Mastrobattista JM (2003) In utero cocaine exposure: a thorny mix of science and mythology. Am J Perinatol 20:165‐172. Volkow ND, Fowler JS Wang GJ et al (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169‐177. Volkow D, Wang GJ, Fowler JS et al (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291:409‐415. Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine‐seeking and cocaine‐enhanced brain reward in rats. J Neurosci 22:9595‐9603. Wagner CL, Katikaneni LD, Cox TH, Ryan RM (1998) The impact of prenatal drug exposure on the neonate. Obstet Gynecol Clin North Am 25:169‐ 194. Wakschlag LS, Hans SL (2002) Maternal smoking during pregnancy and conduct problems in high‐risk youth: a developmental framework. Dev Psychopathol 14:351‐369. Wang HY, Runyan S, Yadin E, Friedman E (1995) Prenatal exposure to cocaine selectively reduces D1 dopamine receptor‐mediated activation of striatal Gs proteins. J Pharmacol Exp Ther 273:492‐498. Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ (2006) Diffusion tensor imaging of frontal white matter and executive functioning in cocaine‐exposed children. Pediatrics 118:2014‐2024. Weerts EM, Fantegrossi WE, Goodwin AK (2007) The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15:309‐ 327. Weiss RD, Gawin FH (1988) Protracted elimination of cocaine metabolites in long‐term high‐dose cocaine abusers. Am J Med 85:879‐880. Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD (2003) Physiological correlates of aggression and impulsivity in free‐ranging female primates. Neuropsychopharmacology 28:1045‐1055. 83 Westergaard GC, Suomi SJ, Higley JD, Mehlman PT (1999) CSF 5‐HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology 146:440‐446. Wetherington CL, Roman AB (1998) Drug addiction research and the health of women. DHHS publication no. 98‐4290. Rockville, MD: US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse. Will GF (1999) Paying addicts not to have kids is a good thing. Baltimore Sun. 11/01/1999. Wise RA (1984) Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr 50:15‐33. Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243‐251. Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191‐225. Wojnicki FHE, Glowa JR (1996) Effects of drug history on the acquisition of responding maintained by GBR 12909 in rhesus monkeys. Psychopharmacology 123:34‐41. Wood RD, Bannoura MD, Johanson IB (1994) Prenatal cocaine exposure: effects on play behavior in the juvenile rat. Neurotoxicol Teratol 16:139‐144. Wood RD, Spear LP (1998) Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci 112:419‐431. Woods JR (1998) Maternal and transplacental effects of cocaine. NY Acad Sci 846: 1‐11. Woods JR, Plessinger MA, Clark KE (1987) Effect of cocaine on uterine blood and fetal oxygenation. JAMA 257:957‐961. Woods N, Eyler FD, Conlon M, et al (1998) Pygmalion in the cradle: observer bias against cocaine‐exposed infants. J Dev Behav Pediatr 19:283‐285. Woods RP, Mazziotta JC, Cherry SR (1993) MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17:536‐546. Woolverton WL, Anderson KG (2006) Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology 186:99‐106. 84 Woolverton WL, Myerson J, Green L (2007) Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol 15:238‐244. Wooten J, Miller SI (1994) Cocaine: a review. Pediatrics in Review 15:89‐92. Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR Jr, Gitain L, Gardner EL (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine‐induced reinstatement of drug‐seeking behavior in rats. Neuropsychopharmacology 31:1393‐1405. Young AM, Woods JH (1981) Maintainance of behavior by ketamine and related compounds in rhesus monkeys with different self‐ administration histories. J Pharmacol Exp Ther 218: 720‐727. Zhao W, Becker JB (2009) Sensitization enhances acquisition of cocaine self‐ administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm and Behav doi:10.1016/j.yhbeh.2009.09.005. Zuckerman B, Frank DA, Hingson R, Amaro H et al (1989) Effects of maternal marijuana and cocaine use on fetal growth. N Eng J Med 320:762‐768. Zuckerman M (1996) The psychobiological model for impulsive unsocialized sensation‐seeking: a comparative approach. Neuropsychobiology 34:125‐129. 85 CHAPTER II CHARACTERIZATION OF THE DOPAMINE RECEPTOR SYSTEM IN ADULT RHESUS MONKEYS EXPOSED TO COCAINE THROUGHOUT GESTATION Lindsey R. Hamilton, Paul W. Czoty, H. Donald Gage, Michael A. Nader The following manuscript was accepted for publication by Psychopharmacology March 2010. Stylistic variations are due to the requirements of the journal. Lindsey R. Hamilton performed the experiments, analyzed the data, and prepared the manuscript. H. Donald Gage aided in the analysis of the positron emission tomography data. Paul W. Czoty and Michael A. Nader acted in an advisory and editorial capacity. 86 ABSTRACT RATIONALE: Cocaine use during pregnancy is associated with alterations in the dopamine (DA) system in the fetal brain. However, little is known about the effects of prenatal cocaine exposure on the postnatal dopaminergic system. OBJECTIVES: The objective of the study was to examine DA receptor function in adult monkeys that were prenatally exposed to cocaine. MATERIALS AND METHODS: Male and female rhesus monkeys (approximately 13 years old) that had been prenatally exposed to cocaine (n = 10) and controls (n = 10) were used in all studies. First, DA D2‐like receptor availability was assessed using positron emission tomography and the D2‐like receptor radiotracer [18F]fluoroclebopride (FCP). Next, D3 receptor function was assessed by measuring quinpirole‐induced yawning (0.03‐0.3 mg/kg). Finally, D1‐like receptor function was examined by measuring eye blinking elicited by the high‐efficacy D1‐like receptor agonist SKF 81297 (0.3‐3.0 mg/kg). RESULTS: There were no differences between groups or sexes in D2‐like receptor availability in the caudate nucleus, putamen or amygdala. However, quinpirole elicited significantly more yawns in prenatally cocaine‐exposed monkeys compared with control monkeys. A significant correlation between gestational dose of cocaine and peak effects of quinpirole was observed. In all monkeys, administration of SKF 81297 elicited dose‐dependent increases in eye blinks that did not differ between groups. CONCLUSIONS: These findings suggest that prenatal cocaine exposure can have long‐term effects on DA D3 receptor function in adults. KEYWORDS: Prenatal cocaine D2 receptors PET imaging Quinpirole – SKF 81297 Rhesus monkey 87 It has been estimated that over 45,000 infants born each year have been prenatally exposed to cocaine (National Pregnancy and Health Survey 1996). Cocaine use during pregnancy is associated with several physical deficits including reduced body weight, body length, and head circumference at birth (Nair and Watson 1991). However, the effects of cocaine use during pregnancy on postnatal development and long‐term neurobiological and behavioral outcomes have been less thoroughly investigated. The present study compared a population of rhesus monkeys that were prenatally exposed to cocaine throughout gestation to control monkeys with nearly identical pharmacological and experimental histories (Morris et al. 1996, 1997). At the start of the present study, these monkeys were adults (13 years old), with minimal drug exposure since birth (see Paule et al. 1996, 2000; Morris et al. 1996). Despite the escalating cocaine intakes of the mothers and the lower infant weights at birth (Morris et al. 1997), over the first 18 months, no differences were observed between cocaine and control groups with respect to postnatal growth (Morris et al. 1996). A particular advantage of using nonhuman primates in prenatal cocaine exposure studies is the relatively long gestational period. In rhesus macaques, the average gestational period is approximately 24 weeks (Silk et al. 1993). Despite this advantage, there are no studies involving prenatal cocaine exposure in rhesus monkeys that have examined the consequences of gestational drug exposure in adults. 88 For the present studies, the dopamine (DA) neurotransmitter system was examined using several in vivo measures. Within the DA system, there are two superfamilies of DA receptors, the D1‐like receptors with two receptor subtypes D1 and D5 and D2‐like receptors with D2, D3 and D4 receptor subtypes. Both D1‐ and D2‐like receptors have been shown to be affected by chronic cocaine exposure in adult humans and nonhuman primates (e.g., Moore et al. 1998a, 1998b; Martinez et al. 2004; Nader et al. 2002; Volkow et al. 1999). As it relates to effects on the fetus, elevation of extracellular monoamine concentrations during development may lead to alterations in receptor signaling mechanisms at birth and perhaps throughout life. Since DA is among the first neurochemical pathways to develop in the fetal brain (reviewed in Bhide 2009), the long‐lasting effects of cocaine exposure on the dopaminergic system during this crucial development stage are of particular interest. In the present study, DA D2‐like receptor availability was assessed using positron emission tomography (PET) and the tracer [18F]fluoroclebopride (FCP), which does not differentiate between D2‐like receptor subtypes (Mach et al. 1996). In adult rhesus monkeys, D2‐like receptor availability has been shown to decrease as a consequence of chronic cocaine exposure (Nader et al. 2006). We hypothesized that D2‐like receptor availability would be lower in adult monkeys who had been exposed to cocaine throughout gestation. While data suggest that D2‐like receptors are reduced due to cocaine exposure, post‐mortem studies found D3 receptors to 89 be higher in cocaine overdose victims compared to age‐matched controls (Staley and Mash 1996). Thus, we used the D3/D2 agonist quinpirole and the unconditioned behavior yawning to assess D3 receptor function in vivo. Earlier work in rodents has shown that the ascending limb of the quinpirole‐ elicited yawning dose‐response curve, including the peak of the curve, is mediated by D3 receptors (Collins et al. 2005). As it relates to D1 receptors, Jones et al. (2000) demonstrated that prenatal cocaine exposure induced early desensitization of DA D1‐like receptors in fetal rabbit anterior cingulate cortex and caudate nucleus that occurred without alterations of the receptor protein itself, suggesting that the D1‐like receptors become uncoupled from their G‐protein (Lidow 1998; Jones et al. 2000). Importantly, D1‐like receptor alterations in rabbits and rodents prenatally exposed to cocaine have been shown to persist into adolescence and adulthood (Bayer et al. 2000; Stanwood and Levitt 2007). Therefore, in the present study, D1‐like receptor function was investigated by assessing the ability of the high‐efficacy agonist SKF 81297 to elicit eye blinking (Jutkiewicz and Bergman 2004). For these studies, there was a near equal distribution of male and female monkeys, so the effects of prenatal cocaine exposure and sex were factors in all analyses. MATERIALS AND METHODS SUBJECTS. Twenty adult rhesus monkeys (Macaca mulatta), born between 1993 and 1995 and raised at the FDA facility in Little Rock, AR until their 90 arrival at Wake Forest University in 2007, served as subjects. Ten monkeys (6 male, 4 female) were prenatally exposed to cocaine and 10 monkeys (5 male, 5 female) were controls, as described previously (Morris et al. 1996, 1997). Briefly, mothers of cocaine‐exposed monkeys received intramuscular injections of escalating doses of cocaine three times per day for the entire course of gestation; the mean cumulative cocaine intake was 1131.5 (± 56.1 SEM) mg/kg (Morris et al. 1996). At 6 months of age, all monkeys were housed individually in the same colony room and began behavioral training involving an operant test battery (Morris et al. 1997). Other than their prenatal drug histories, all monkeys had identical experimental histories, including acute exposure to cocaine, amphetamine, haloperidol, quinpirole, SCH‐23390, spiperone, and MK‐801 (see Paule et al. 1996; Morris et al. 1997; personal communication from M. Paule). At the time of the present studies, there were no significant differences between the prenatally cocaine exposed and control monkeys in age (12.4 ± 0.3 years vs. 12.9 ± 0.3 years, respectively) or weight (7.8 ± 0.7 kg vs. 6.4 ± 0.4 kg, respectively). Monkeys were individually housed in stainless‐steel cages with water available ad libitum and had visual and auditory contact with each other. During a 2‐month quarantine, a free‐feeding weight was determined and monkeys’ body weights were maintained at approximately 95% of that value throughout these studies (LabDiet Monkey Chow and fresh fruit). Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate 91 Products) using a specially designed stainless‐steel pole that attached to the collar. All experimental and environmental enrichment protocols were approved by the Wake Forest University Institutional Animal Care and Use Committee. The experiments are listed in the order that each animal was tested. MENSTRUAL PHASE DETERMINATION. Since we have previously shown that D2 receptor availability differs across the menstrual cycle (Czoty et al. 2009), all experiments were conducted in the follicular phase. Menstrual cycle was assessed by daily vaginal swabs. Days of bleeding were recorded as indicative of menses. PET scans were scheduled to occur during the follicular phase (days 2‐12). To confirm cycle phase, on the day of a PET study, 3 ml of blood was drawn from the femoral vein and analyzed for progesterone at the Biomarkers Core Laboratory of the Yerkes National Primate Research Center of Emory University in Atlanta, GA (see Czoty et al. 2009 for details). EXPERIMENT 1: EFFECTS OF PRENATAL COCAINE EXPOSURE ON D2 RECEPTOR AVAILABILITY. Magnetic resonance imaging (MRI) scans were acquired for each monkey. Twenty minutes before the MRI, subjects were anesthetized with ketamine (15‐20 mg/kg, i.m.) and transported to the MRI facility. Anesthesia was maintained during the scanning procedure with ketamine supplements when necessary. T1‐weighted images of the entire brain were acquired with a 1.5‐Tesla GE Signa NR scanner (GE Medical 92 Systems). Images were used to anatomically define regions of interest (ROIs), including the caudate nucleus, putamen, amygdala, and cerebellum, for later co‐registration with PET images. PET data were acquired using a GE Advance NXi PET scanner (~4.8 mm3 resolution) and the radiotracer [18F]fluoroclebopride (FCP), which binds with high affinity to D2‐like receptors (Mach et al. 1993) with a test/retest variability of ~2% (Nader et al. 1999). Methodological details regarding the data acquisition protocol, blood sampling and metabolite analysis for FCP have been described previously (Mach et al. 1996; Nader et al. 1999). Approximately 30 min before the scan, monkeys were anesthetized with ketamine (10 mg/kg, i.m.), intubated, and maintained throughout the scan by inhaled isoflurane (1.5%). This induction protocol does not alter D2 receptor availability as measured with FCP (Nader et al. 1999). Catheters were placed in an external artery and vein by percutaneous sticks and saline was delivered to the monkey throughout the scan. Body temperature was maintained at 38°C and vital signs (heart rate, blood pressure, respiration rate, and temperature) were monitored throughout the scanning procedure. A paralytic (0.07 mg/kg vecuronium bromide) was administered i.v. and respiration was maintained by a ventilator. Supplemental doses of vecuronium bromide (0.1 mg/h) were administered throughout the study. A 5‐min transmission scan was acquired in 2D mode. Next, the monkey received a bolus dose of [18F]FCP (2‐5 mCi) followed by a 3 ml flush with heparinized saline and a 180 min dynamic acquisition scan was 93 acquired. Twenty‐six frames were acquired over 3 hr (5 x 1 min, 5 x 2 min, 5 x 5 min, 8 x 10 min, 3 x 20 min) in 3D mode (i.e., septa retracted). Image reconstruction of 3D data was done using the 3D‐reprojection method (Rogers et al. 1987) with full quantitative corrections. Once the scanning was compete, the transmission scan data were smoothed transaxially using a 4‐ mm Gaussian filter and segmented (Bettinardi 1999). Emission data were corrected for attenuation and reconstructed into 129 x 128 matrices using a Hanning filter with a 4‐mm cutoff transaxially and a ramp filter with an 8.5‐ mm cutoff axially. The first five frames of each study's PET image data were then added together. This summed image represents tracer uptake in the early part of the study and approximates a blood flow image. The image was then registered to the animal’s MRI using the AIR algorithm (Woods et al. 1993) after extracting the brain image from the MRI, using the method of Smith (2002). Time‐activity curves were generated for radiotracer concentrations in ROIs defined on each subject’s co‐registered MRI. Distribution volume ratios (DVR) were calculated for each ROI using the cerebellum as the reference region and the graphical method of Logan et al. (1996). The DVR thus served as an index of specific FCP binding in each ROI. For all regions, the right and left sides’ DVRs did not differ and were averaged. EXPERIMENT 2: EFFECTS OF PRENATAL COCAINE EXPOSURE ON QUINPIROLEINDUCED YAWNING. A quinpirole dose‐response curve was 94 determined for each monkey. Before each experimental session, the monkey was placed in a primate chair and given an injection of saline (1.0 ml) or quinpirole (0.03, 0.1, or 0.3 mg/kg, i.m.); doses were tested in random order with at least 2 days between testing. These doses of quinpirole do not induce hypothermia in our monkeys (unpublished observations), an effect described as D2 receptor‐mediated effect observed in rodents (Boulay et al. 1999a, b; Chaperon et al. 2003; Collins et al. 2007). Immediately after the injection, occurrences of yawning were counted for 30 minutes. Full extension of the jaws, withdrawal of the lips, and exposure of the teeth characterized yawning (Code and Tang 1991). Sessions were videotaped and two people who were blind to the monkeys’ prenatal history scored these sessions with an inter‐ observer variability of <5%. EXPERIMENT 3: EFFECTS OF PRENATAL COCAINE EXPOSURE ON SKF 81297INDUCED EYE BLINKING. Monkeys were seated in a primate chair in a testing room. Following a 15 minute acclimation period, saline (1.0 ml) was administered into the saphenous vein and blinking was counted during the last 2.5 minutes of the following 15 minute period. Subsequently, cumulative doses of SKF 81297 (0.3, 1.0, and 3.0 mg/kg, i.v.) were administered and blinking was counted in the last 2.5 minutes of the 15 minute period following each dose. Total session length was 75 minutes. Sessions were videotaped and two people, one of whom was blind to the monkeys’ prenatal drug history, scored these sessions with an inter‐observer variability of <8%. 95 Drugs. Quinpirole (Sigma‐Aldrich, St. Louis, MO) was dissolved in sterile saline to a concentration of 1.0 mg/ml. SKF 81297 (Sigma‐Aldrich, St. Louis, MO) was dissolved in sterile saline to a concentration of 5.0 mg/ml. All doses are expressed as the salt. STATISTICAL ANALYSIS. In Experiment 1, for each ROI, data were analyzed using a two‐way repeated measures ANOVA with group (prenatal cocaine and control) and sex as factors. In Experiments 2 and 3, three‐way repeated measures ANOVAs with group, sex and dose (quinpirole or SKF 81297) as factors were conducted. In all cases, significance was accepted at the 95% level of confidence (p < 0.05). RESULTS EXPERIMENT 1: EFFECTS OF PRENATAL COCAINE EXPOSURE ON D2 RECEPTOR AVAILABILITY. For all monkeys, there was a high level of uptake of [18F]FCP in all three regions of interest and a linear rate of washout, as shown previously (e.g. Morgan et al. 2002). In the cerebellum, [18F]FCP uptake was low with a rapid rate of washout (not shown). For both groups of monkeys, [18F]FCP DVRs in the caudate nucleus and putamen were higher than DVRs observed in the amygdala in all monkeys (Fig. 1). There were no 96 significant main effects of prenatal cocaine exposure or sex and no group x sex interactions for any ROI. EXPERIMENT 2: EFFECTS OF PRENATAL COCAINE EXPOSURE ON QUINPIROLEINDUCED YAWNING. Using a 3‐way repeated measures ANOVA, there was a significant effect of quinpirole dose [F(3, 48)=7.13, p<0.001], and group [F(1, 16)=8.51, p<0.01] and a significant sex x quinpirole dose interaction [F(3, 48)=3.42, p<0.05] (Fig. 2). All quinpirole doses elicited more yawns in the prenatal cocaine‐exposed monkeys compared to controls (Fig. 2, right panel). Male monkeys, irrespective of their prenatal condition, yawned more following 0.03 mg/kg quinpirole compared to female monkeys whose quinpirole curve peaked at 0.1 mg/kg (Fig. 2, left and middle panels). For control and prenatally cocaine‐exposed monkeys (male and female), all quinpirole doses elicited more yawns than saline, but the dose‐response curves were relatively flat (Fig. 2, right panel). Finally, combining the data for all prenatally cocaine‐exposed monkeys (Fig. 3) revealed a significant positive correlation between the maximal effect of quinpirole and maximal daily in utero cocaine exposure (r2 = 0.84, p < 0.0005). However, when cumulative gestational cocaine dose was used in the analysis, the correlation was not signficant. EXPERIMENT 3: EFFECTS OF PRENATAL COCAINE EXPOSURE ON SKF 81297INDUCED EYE BLINKING. Following saline administration, rates of 97 blinking ranged from 4.8‐17.2 blinks per minute but did not differ between prenatally cocaine exposed and control monkeys (Fig 4). A repeated‐ measures three‐way ANOVA revealed a significant main effect of SKF 81297 dose [F(3,39)=45.80, p<0.001] and SKF 81297 dose x sex interaction [F(3,39)=3.11, p<0.05]. For both groups and across all SKF81297 doses, males blinked more than females. DISCUSSION The purpose of the present studies was to determine if there were long‐term alterations in dopamine function in adult monkeys that were exposed to cocaine in utero. Ten monkeys (male and female) prenatally exposed to cocaine were compared to 10 age‐matched control monkeys who had nearly identical postnatal experimental histories. There were no differences between groups in D1‐like receptor function, as assessed by SKF 81297‐elicited eye blinks, or in D2‐like receptor availability as determined with PET imaging. In contrast, the D3/D2 receptor agonist quinpirole elicited significantly more yawns in monkeys prenatally exposed to cocaine compared to control monkeys. Furthermore, a significant correlation was observed between maximal daily gestational dose of cocaine and peak effects of quinpirole. These findings suggest long‐lasting effects of prenatal cocaine exposure on DA D3 receptor function. Accumulating evidence suggests that chronic cocaine exposure can produce significant reductions in DA D2‐like receptor availability in adult 98 humans and animals (e.g., Volkow et al. 1999; Martinez et al. 2004; Nader et al. 2002, 2006). However, earlier work suggested that the effects of chronic cocaine on fetal DA receptor densities may be different from those observed in adults. For example, Fang et al. (1997) observed significantly higher levels of D2‐like receptor densities in the fetal monkey striatum following gestational cocaine exposure. Data from the present PET imaging study suggest that any changes in D2‐like receptor availability that may have occurred in utero or in the developing brain have recovered in adulthood. Compared to the Fang et al. (1997) rhesus monkey study, the present study involved longer in utero treatments (approximately 6 months), full‐term pregnancy and 13 years of abstinence. Future longitudinal PET imaging experiments conducted at multiple points during a monkey’s lifespan following in utero cocaine exposure would directly address the time course of recovery. No significant sex differences were observed in D2‐like receptor availability in any of the regions of interest. This is consistent with the lack of sex differences seen in striatal D2/D3 receptor binding using [18F]‐fallypride in adolescent rhesus monkeys (Christian et al. 2009) and with previous reports of women and men showing equivalent D2‐like receptor availability (Farde et al. 1995; Pohjalainen et al. 1998; Munro et al. 2006). However, it has been suggested that female sex hormones may enhance presynaptic dopamine turnover (Laakso et al. 2002) and the radiotracer used in this experiment (FCP) is sensitive to fluctuations in menstrual cycle phase (Czoty 99 et al. 2009). In addition, sex differences have been reported in a study using [11C]raclopride and PET in healthy men and women of ages ranging from 19‐ 82 yrs old (Pohjalainen et al. 1998). Therefore, it remains possible that differences in D2‐like receptor availability in males and females may have been observed at earlier time points or may yet be seen as these monkeys age. The PET radiotracer used in the present study does not differentiate between D2, D3 and D4 subtypes of the D2‐like receptor superfamily. Thus, it is conceivable that prenatal cocaine exposure could have long‐term effects on subtypes of this superfamily which would be obscured by opposite adaptations in another subtype. For example, in vitro receptor autoradiography studies have shown lower D2‐like receptor densities (e.g., Moore et al. 1998; Nader et al. 2002) and higher D3 receptor densities (e.g., Staley and Mash 1996) in cocaine‐exposed individuals compared to age‐ matched controls. To determine if there were differences in D3 receptor function, the D3 receptor agonist quinpirole was used to examine the sensitivity of behavior related to this subtype in both groups of monkeys and as a function of sex. Collins et al. (2005, 2007) have shown that the ascending limb of the quinpirole dose‐response curve is mediated by D3 receptors while the descending limb is mediated by D2 receptors. Based on previous experiments in rhesus monkeys (Martelle et al. 2007) the dose range of quinpirole administered in the present study is situated on the ascending limb of the dose‐response curve and therefore is thought to assess primarily 100 D3 receptor function. The greater ability of quinpirole to elicit yawning in the prenatally cocaine‐exposed monkeys is similar to results from Moody et al. (1992), who demonstrated that rat pups exposed to cocaine throughout gestation exhibited a supersensitivity to the stimulating effects of quinpirole with respect to behaviors such as forward locomotion, rearing and directed oral movements compared to control pups. Additionally, when all monkeys prenatally exposed to cocaine were used in the analysis, we found that D3 receptor sensitivity correlated with the maximum dose of cocaine each individual monkey received in utero. Taken together, the present results provide evidence for long‐term neuropharmacological consequences of prenatal cocaine exposure on D3 receptor function under conditions in which no difference in D2‐like receptors was observed using PET imaging. The combination of effects lead to interesting hypotheses regarding differential sensitivity to the reinforcing effects of cocaine. For example, because PET imaging studies in monkeys have shown a relationship between D2 receptor availability and cocaine reinforcement (see Nader et al. 2008), the PET imaging data would suggest no differences between prenatal cocaine exposed and control monkeys in vulnerability to cocaine reinforcement. However, D3 receptor sensitivity has been associated with impulsivity (e.g. Dodd et al. 2005; Sokoloff et al. 2006), which would suggest differential sensitivity of cocaine‐exposed monkeys compared to controls in acquisition of cocaine self‐administration. Additional behavioral studies in these monkeys, including assessing the reinforcing effects of cocaine, will provide 101 important information as to the long‐term consequences of prenatal cocaine exposure and the role of D2‐like receptor subtypes in these behavioral outcomes. In an effort to more fully characterize DA receptor activity in vivo, functional studies of the D1‐like receptor were also undertaken in these same monkeys. D1‐like receptor densities have previously been shown to be affected by chronic cocaine exposure in adult monkeys (Moore et al. 1998) and not necessarily in a manner similar to the effects of cocaine on D2‐like receptors (Nader et al. 2002). Fang et al. (1997) reported that cocaine treatment from gestational day 22 to 70 resulted in significant increases in D1‐like receptor densities in day‐70 fetal monkey striatum. In rodent and rabbit models, several studies suggest that prenatal cocaine exposure uncoupled the D1 receptor from its G‐protein resulting in an attenuation of D1 receptor signaling (Friedman et al. 1996; Wang et al. 1995; Lidow 1998; Jones et al. 2000; Unterwald et al. 2003). However, there are no data assessing D1‐like receptor function in adults who had been prenatally exposed to cocaine. In the present study, no differences in potency or effects of SKF 81297‐elicited eye blinks were observed in adult monkeys prenatally exposed to cocaine versus controls. Because it has been argued that this unconditioned behavior is a sensitive measure of D1‐like signaling (Jutkiewicz and Bergman 2004), these data suggest that any functional differences in D1 receptor sensitivity observed in prenatally cocaine‐exposed animals shortly after birth are no longer apparent in these animals as adults. 102 It should be noted that under other conditions in socially housed monkeys, SKF 81297‐elicited eye blinking did not differentiate monkeys based on social rank (Czoty et al. 2004), even though differences in sensitivity to cocaine reinforcement were observed (Czoty et al. 2005). It remains possible that other functional measures of D1‐like receptor activity (e.g., drug discrimination or drug self‐administration) may yield differential sensitivity due to prenatal cocaine exposure. The present findings are also the first to note sex differences in sensitivity to the D1‐like agonist effects elicited by SKF 81297. It is important to note that D3 receptor function (quinpirole‐ elicited yawning) was also differentially affected by sex. The present findings add to a growing body of evidence for sex differences in the behavioral effects of drugs. Taken together, these findings indicate that prenatal cocaine exposure can have long‐lasting effects on DA receptor function and that males and females are equally sensitive to these perturbations. ACKNOWLEDGMENTS This research was supported by National Institute on Drug Abuse grants R01 DA25120, R37 DA10584, and K31 DA024485. The authors report no conflict of interest and would like to acknowledge the technical assistance of Tonya Calhoun, Kimberly Black, Holly Smith and Whitney Wilson. The authors also thank Dr. Merle Paule for providing information related to the histories of these monkeys and Dr. Anthony Liguori for statistical consultation. 103 FIGURE 1. Distribution volume ratios (DVRs) of [18F]FCP in control (open symbols) and prenatally cocaine‐exposed (shaded symbols) monkeys in the caudate nucleus, putamen, and amygdala. Each bar represents mean ± SEM values from 10 monkeys. 104 105 FIGURE 2. Yawning induced by quinpirole (0.03‐0.3 mg/kg) in female (A) and male (B) control (open symbols) and prenatally cocaine‐exposed (closed symbols) adult rhesus monkeys. Panel C represents mean data (male and female) for each group. Data are represented as the mean ± SEM number of yawns in a 30‐min observation period. 106 FIGURE 3. Relationship between peak number of yawns elicited by quinpirole and maximal daily dose of cocaine received in utero (from Morris et al., 1996). Different symbols represent males (triangles) and females (circles). 107 108 FIGURE 4C. Effects of SKF 81297 on rate of eye blinking in control (A) and prenatally cocaine‐exposed (B) male (filled symbols) and female (open symbols) monkeys. Panel C represents mean data (male and female) for each group. Each point represents mean ± SEM values. 109 REFERENCES Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ (2000) Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. J Neurosci 20:8902‐8908. Bettinardi V, Pagani E, Gilardi MC et al (1999) An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med 26:447‐458. Bhide PG (2009) Dopamine, cocaine, and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol 20:395‐402. Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ (1999a) Dopamine D2 receptor knock‐out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology 38:1389–1396. Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ (1999b) Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock‐out and wild‐type mice. Neuropharmacology 38:555–565. Chaperon F, Tricklebank MD, Unger L, Neijt HC (2003) Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 44:1047–1053. Christian BT, Vandehey NT, Fox AS et al (2009) The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage 44:1334‐1344. Code RA, Tang AH (1991) Yawning produced by dopamine agonists in rhesus monkeys. Eur J Pharmacol 201:235‐238. Collins GT, Newman AH, Grundt P et al (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology 193:159‐170. Collins GT, Witkin JM, Newman AH et al (2005) Dopamine agonist‐induced yawning in rats: a dopamine D3 receptor‐mediated behavior. J Pharmacol Exp Ther 314:310‐319. 110 Czoty PW, McCabe C, Nader MA (2005) Assessment of the reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther 312: 96‐102. Czoty PW, Morgan D, Shannon EA, Gage HD, Nader MA (2004) Characterization of dopamine D1 receptor function in socially housed cynomolgus monkeys. Psychopharmacology 174: 381‐388. Czoty PW, Riddick NV, Gage HD (2009) Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34:548‐554. Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE (2005) Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol 62: 1377‐1381. Farde L, Hall H, Pauli S, Halldin C (1995) Variability in D2‐dopamine receptor density and affinity: A PET study with [11C]raclopride in man. Synapse 20:200‐208. Fang Y, Janowsky A, Ronnekleiv OK (1997) Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1‐ and D2‐like receptor binding densities. Brain Res Dev Brain Res 104:163‐174. Friedman E, Yadin E, Wang HY (1996) Effects of prenatal cocaine on dopamine receptor‐G protein coupling in mesocortical regions of the rabbit brain. Neuroscience 70:739‐747. Jones LB, Stanwood GD, Reinoso BS et al (2000) In utero cocaine‐induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci 20:4606‐4614. Jutkiewicz EM, Bergman J (2004) Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther 1999:649‐661. Koe BK (Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther 199:649‐661. Laakso A, Vilkman H, Bergman J et al (2002) Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Soc Biol Psychiatry 52:759‐763. 111 Lidow MS (1998) Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann NY Acad Sci 846:182‐ 193. Logan J, Fowler JS, Volkow ND et al (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834‐840. Mach RH, Nader MA, Ehrenkaufer RL et al (1996) Comparison of two fluorine‐18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse 24:322‐333. Mach RH, Luedtke RR, Unsworth CD et al (1993) 18F‐labeled benzamides for studying the dopamine D2 receptor with positron emission tomography. J Med Chem 36:3707‐3720. Martelle JL, Claytor R, Ross JT et al (2007) Effects of two novel D3‐selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 321:573‐582. Martinez D, Broft A, Foltin RW et al (2004) Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine‐seeking behavior. Neuropsychopharmacology 29:1190‐1202. Moody CA, Frambes NA, Spear LP (1992) Psychopharmacological responsiveness to the dopamine agonist quinpirole in normal weanlings and in weanling offspring exposed gestationally to cocaine. Psychopharmacology 108:256‐262. Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (1998a) Effect of cocaine self‐administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse 28: 1‐9. Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (1998b) Effect of cocaine self‐administration on dopamine D2 receptors in rhesus monkeys. Synapse 30: 88‐96. Morgan D, Grant KA, Gage HD et al (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self‐administration. Nat Neurosci 5:169‐174. 112 Morris P, Binienda Z, Gillam MP et al (1996) The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 18:147‐154. Morris P, Binienda Z, Gillam MP et al (1997) The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 19: 47‐57. Munro CA, McCaul ME, Wong DF et al (2006) Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966‐974. Nader MA, Czoty PW, Gould RW, Riddick NV (2008) PET imaging studies of dopamine receptors in primate models of addiction. Phil Trans Royal Soc B 363: 3223‐3232. Nader MA, Daunais JB, Moore T et al (2002) Effects of cocaine self‐ administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 21:589‐596. Nader MA, Grant KA, Gage HD et al (1999) PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane‐ and ketamine‐induced anesthesia. Neuropsychopharmacology 21:35‐46. Nader MA, Morgan D, Gage HD et al (2006) PET imaging of dopamine D2 receptors during chronic cocaine self‐administration in monkeys. Nat Neurosci 9:1050‐1056. Nair BS, Watson RR (1991) Cocaine and the pregnant woman. J Reprod Med 36:862‐867. Paule MG, Gillam MP, Allen RR, Chelonis JJ (2000) Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann NY Acad Sci 914:412‐417. Paule MG, Gillam MP, Binienda Z, Morris P (1996) Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci 801:301‐309. Pohjalainen T, Rinne JO, Nagren K et al (1998) Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155:768‐773. Rogers JG, Harrop R, Kinahan PE (1987) The theory of three‐dimensional image reconstruction for PET. IEEE Trans Med Imaging 6:239‐243. 113 Silk J, Short J, Roberts J, Kusnitz J (1993) Gestation length in rhesus macaques (Macaca mulatta). Intern J Primatol 14:95‐104. Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5: 25‐43. Staley JK, Mash DC (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100‐ 6106. Stanwood GD, Levitt P (2007) Prenatal exposure to cocaine produces unique developmental and long‐term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci 27: 152‐157. Smith S (2002) Fast robust automated brain extraction. Hum Brain Map 17:143‐155. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1996) Results from the 1992 National Pregnancy and Health Survey. Ref Type: Report. Unterwald EM, Ivkovic, Cuntapay M et al (2003) Prenatal exposure to cocaine decreases adenylyl cyclase activity in embryonic mouse striatum. Brain Res Dev Brain Res 147:67‐75. Volkow D, Wang GJ, Fowler JS et al (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291:409‐415. Wang HY, Runyan S, Yadin E, Friedman E (1995) Prenatal exposure to cocaine selectively reduces D1 dopamine receptor‐mediated activation of striatal Gs proteins. J Pharmacol Exp Ther 273:492‐498. Woods RP, Mazziotta JC, Cherry SR (1993) MRI‐PET registration with automated algorithm. J comput Assist Tomogr 17:536‐546. 114 CHAPTER III INCREASED IMPULSIVITY IN MALE, BUT NOT FEMALE, ADULT RHESUS MONKEYS EXPOSED TO COCAINE THROUGHOUT GESTATION Lindsey R. Hamilton, Paul W. Czoty, Michael A. Nader The following manuscript was submitted to Psychopharmacology in April 2010. Stylistic variations are due to the requirements of the journal. Lindsey R. Hamilton performed the experiments, analyzed the data, and prepared the manuscript. Paul W. Czoty and Michael A. Nader acted in an advisory and editorial capacity. 115 ABSTRACT RATIONALE: In utero cocaine exposure has been associated with alterations in the dopamine (DA) system in monkeys. However, the behavioral outcomes of prenatal cocaine exposure in adulthood are poorly understood. OBJECTIVES: To assess behavioral measures of impulsivity in 14 year‐old rhesus monkeys exposed to cocaine in utero and controls (n=10 per group). MATERIALS AND METHODS: To assess impulsivity, two unconditioned behavioral tasks, novel object reactivity and locomotor activity, and two conditioned behavioral tasks, response extinction and delay discounting, were examined. In addition, cerebrospinal fluid (CSF) samples were analyzed for concentrations of the monoamine metabolites homovanillic acid (HVA) and 5‐hydroxyindoleacetic acid (5‐HIAA). RESULTS: No differences in CSF concentrations of 5‐HIAA and HVA, latencies to touch a novel object or locomotor activity were observed between groups or sexes. However, prenatally cocaine‐exposed monkeys required a significantly greater number of sessions to reach criteria for extinction of food‐reinforced behavior than control monkeys. On the delay‐discounting task, male prenatally cocaine‐ exposed monkeys had a significantly larger mean indifference point than male control monkeys; no differences were observed in females. When an overall impulsivity score was determined taking into account the two unconditioned and two conditioned behavioral measures, male prenatally cocaine‐exposed monkeys were more impulsive than male control monkeys. No differences in overall impulsivity were observed in females. A significant 116 negative correlation between CSF concentration of HVA and overall impulsivity score was observed. CONCLUSIONS: These findings suggest that prenatal cocaine exposure has long‐term neurobehavioral deficits with neurobiological correlates that are influenced by sex of the individual. KEYWORDS: Prenatal cocaine – Impulsivity – Delay discounting – CSF– HVA – Rhesus monkey 117 Maternal cocaine addiction is a significant public health problem with almost 50,000 infants born each year having been exposed to cocaine in utero (National Pregnancy and Health Survey 1996). Several investigators have examined the physiological consequences of cocaine use throughout gestation in nonhuman primate models (e.g. Howell et al. 2001; Lidow 2003). The present study utilized prenatal cocaine exposure in rhesus monkeys in order to evaluate the behavioral and neurochemical consequences of in utero drug exposure in adults. These animals had been exposed to cocaine throughout the 25 weeks of gestation or were controls (Morris et al. 1996, 1997) and were studied as adults (14‐15 years old). We recently examined dopamine (DA) receptor function in these adult monkeys (Hamilton et al. 2010) using agonist‐elicited behaviors and PET imaging. There were no differences in DA D1‐ and D2‐like receptor function, but differences were noted in the behavioral effects of the D3/D2 agonist quinpirole in monkeys prenatally exposed to cocaine. In the present study, we extended the examination of neurobiological characteristics to include CSF concentrations of the DA metabolite homovanillic acid (HVA) and the serotonin metabolite 5‐hydroxyindole acetic acid (5‐HIAA), and extended behavioral assessments to measures thought to reflect aspects of impulsivity. Cocaine use during pregnancy has been associated with physical deficits in the offspring including reduced body weight, body length, and head circumference of the infants at birth (Nair and Watson 1991) and developmental deficits including memory (Singer et al. 2005, 2008), 118 attention (Singer et al. 2000; Noland et al. 2005; Linares et al. 2006), cognition (Singer et al. 2001; Morrow et al. 2006), and impulse control (Savage et al. 2005; Linares et al. 2006; Accornero et al. 2007; Pulsifer et al. 2008). Investigating the differences in impulse control in a highly controlled animal model is an important complement to the human studies. However, some of the difficulty in evaluating impulsivity is due to it being conceptualized as a broad spectrum of behaviors rather than a single trait (Moeller et al. 2001; Chamberlain and Sahakian 2007). While research with humans has typically assessed impulsivity using standardized questionnaires (Evenden 1999), there is no agreement on how to measure impulsivity in animal models. Several distinct measures have been developed to assess impulsivity in animals, including response to novelty, locomotor activity, behavioral inhibition, and choice between an immediate, low‐magnitude reward over a delayed large‐magnitude reward. In the present study, each of these dependent variables was assessed to directly compare these various measures of impulsivity. The first set of behavioral measures to assess impulsivity used in the present study involved the unconditioned behaviors of response to a novel object (Dellu et al. 1996; Zuckerman 1996) and locomotor activity in an open field. Reactivity to a novel object has been used as a measure of impulsivity in rodents (Hooks et a. 1991; Suto et al. 2001; Davis et al. 2008) and nonhuman primates (Bolig et al. 1992; reviewed in Clarke and Boinski 1995; Coleman et al. 2005; Czoty et al. 2010). Regarding locomotor activity, Piazza and 119 colleagues demonstrated that responsiveness in an open field was associated with vulnerability to stimulant self‐administration (Piazza et al. 1989; 1990; reviewed in Piazza and Le Moal 1998). Interestingly Dalley et al. (2007) did not find a relationship between locomotor activity and other measures of impulsivity. In the present study, we directly compared locomotor activity of adult rhesus monkeys with several other measures hypothesized to assess impulsivity. A third measure of impulsivity is response perseveration, which is the tendency to continue emitting a formerly reinforced response despite the response currently being either unrewarded or punished (McCleary 1966). It is thought that response perseveration is a measure of deficient behavioral inhibition because in these tasks subjects must stop their ongoing behavior (Matthys et al. 1998). In the present study, perseverative responding was assessed by examining responding during extinction of previously food‐ reinforced fixed‐ratio (FR) responding in monkeys and subjects requiring a greater number of sessions to extinguish responding (unrewarded responding) was classified as more impulsive than subjects that took fewer sessions to extinguish responding. As a final measure of impulsivity, we assessed choice behavior involving delays. Impulsive choice is most commonly assessed in human studies using a delay‐discounting task in which subjects are asked to choose between a small, immediate reinforcer and a larger, delayed reinforcer. The subjective value of the larger reinforcer is decreased (i.e., discounted) as the 120 length of time the subject must wait to receive it increases. By using a series of choices between varying delay values, an indifference point can be calculated as the delay value at which the smaller, immediate reinforcer is chosen as often as the larger, delayed reinforcer. Delay discounting has been adapted for animal studies (e.g. Perry et al. 2005; Woolverton et al. 2007). Although several studies have examined delay discounting in monkeys (Anderson and Woolverton 2003; Woolverton and Anderson 2006; Woolverton et al. 2007; Newman et al. 2008), these studies have involved choice between drug reinforcers. The present study extended this work to delay discounting involving different magnitudes of non‐drug reinforcers. Such information allows for the general assessment of impulsivity and allows for delay discounting values to be compared to other non‐drug related behavioral measures of impulsivity. Finally, in addition to these behavioral measures, we examined the relationship between measures of DA receptor function and monoamine metabolite levels and the various measures of impulsivity in both male and female monkeys. MATERIAL AND METHODS SUBJECTS. Twenty adult rhesus monkeys (Macaca mulatta), born between 1993 and 1995 and raised at the FDA facility in Little Rock, AR until their arrival at Wake Forest University in 2007, served as subjects. Ten monkeys (6 male, 4 female) were prenatally exposed to cocaine and 10 monkeys (5 male, 5 female) were prenatally exposed to saline, as described previously 121 (Morris et al. 1996, 1997). Briefly, the mothers of the monkeys used in this study received intramuscular injections of saline or escalating doses of cocaine three times per day for the entire course of gestation, with mean cumulative cocaine intake of 1131.3 (± 56.1 SEM) mg/kg (Morris et al. 1996). When the monkeys were 6 months of age, they were housed individually in the same colony room and began behavioral training involving an operant test battery (Morris et al. 1997). Other than their prenatal drug histories, all monkeys had nearly identical experimental histories (see Paule et al. 1996; Morris et al. 1996). At the start of this experiment, monkeys were individually housed in stainless‐steel cages with water available ad libitum and had visual and auditory contact with each other. Since we have previously shown that monoamine function is influenced by menstrual cycle (Czoty et al. 2009), we monitored menstrual cycle phase throughout the experiment by daily vaginal swabs. Days of bleeding were recorded as indicative of menses. During quarantine, a free‐feeding weight was determined and monkeys’ body weights were maintained at approximately 95% of that value throughout these studies (LabDiet Monkey Chow and fresh fruit). Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products) using a specially designed stainless‐steel pole that attached to the collar. All manipulations were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the 122 Wake Forest University Institutional Animal Care and Use Committee. The order of experiments accurately depicts the order of testing for each monkey. APPARATUS. The apparatus consisted of a ventilated, sound‐attenuating chamber (1.5 x 0.74 x 0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two response keys (5 cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each response key and a food receptacle was located between the response keys. The receptacle was connected with tygon tubing to a pellet dispenser (Gerbarands Corp., Arlington, MA) located on the top of the chamber for delivery of 1‐g banana‐flavored food pellets (P.K. Noyes Co., Lancaster, NH). An infusion pump (Cole‐Palmer, Inc., Chicago, IL) was located on the top of the chamber. SURGERY. Each monkey was prepared with a chronic indwelling venous catheter and subcutaneous vascular port (Access Technologies, Skokie, IL) under sterile surgical conditions. Anesthesia was induced and maintained with ketamine (15 mg/kg) and butorphanol (0.025 mg/kg). Vital signs were monitored for the duration of the surgery. Briefly, a catheter was inserted into the femoral vein to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was then attached to the vascular access port and placed in a pocket formed by blunt dissection. 123 Each port and catheter was filled with heparinized saline solution (100 Units/ml) after every experimental session to prolong the patency. Prior to each saline self‐administration session, the back of the animal was cleaned with betadine and 95% EtOH and the port was connected to the infusion pump located outside the chamber via a 20‐gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 sec to fill the port and catheter line with saline prior to starting the session. CEREBROSPINAL FLUID (CSF) MEASURES OF 5HIAA AND HVA. Monkeys were anesthetized with 10 mg/kg ketamine, the aluminum collar was removed and the neck and the back of the skull were shaved and cleaned with betadine and 95% EtOH. A 25‐gauge, 1.5‐inch needle attached to a 3‐ml syringe was inserted through the cisterna magna and approximately 2 ml of CSF was removed within 10 min of induction of anesthesia. Females were studied only during the follicular phase. The samples were immediately transferred to vacutainer tubes on ice. Samples were centrifuged at 4º C for 30 min at 3000 rpm and then aliquoted into microcentrifuge tubes for storage at ‐30º C until they were analyzed using high‐pressure liquid chromatography with electrochemical detection. The mobile phase consisted of 9.6 g citric acid, 11.2 g sodium phosphate monobasic and 0.7 g 1‐ octanesulfonic acid in 860 ml of ultra‐pure water. 100 microliters of 0.5 M ethylenediaminetetraacetic acid (EDTA) was added and the pH of the solution was adjusted to 3. Next, 6 drops of triethylamine and 140 ml 124 acetonitrile were added and the mobile phase was filtered twice. Mobile phase was delivered to the system at a rate of 0.2 ml/min using an ESA 582 solvent delivery module (ESA Inc., Chelmsford, MA). Three 30‐microliter aliquots of each sample were loaded into a ESA 542 autoinjector and 20 microliters were injected. Separation was achieved with a C‐18 column (150 mm length, 3.2 mm i.d., 3 micrometer particle size; ESA, Inc.) and 5‐HIAA and HVA were detected in samples using an ESA Coulochem II detector. Concentrations of 5‐HIAA and HVA were determined by interpolation using a standard curve that was generated using standard solutions containing known amounts of the metabolites. Each sample was tested in triplicate and average values were used for data analysis. EXPERIMENT 1: EFFECTS OF PRENATAL COCAINE EXPOSURE ON IMPULSIVITY USING UNCONDITIONED BEHAVIORS. Approximately 2‐6 months after quarantine ended, monkeys were characterized on two measures of impulsivity that utilized unconditioned behaviors. For the novel object reactivity test, the monkey in the cage adjacent to the subject’s home cage was removed, the partition was removed from between the cages, and the subject was moved to the adjacent cage. Next, the partition was replaced and an opaque black Plexiglas box measuring 30.5 x 20.3 x 20.3 cm was placed in the monkey’s empty home cage. The partition was removed again and the latency to touch the object was recorded. If the monkey did not touch the object within 15 min, a score of 900 sec was assigned. All sessions were 125 videotaped and scored by an observer blind to the monkey’s prenatal condition. The 900‐sec maximum duration was based on data from our laboratory (Riddick et al. 2009). To assess locomotor activity, each monkey was placed in a 3.0 x 2.0 x 1.75 m enclosure with the field divided into 9 equal grid zones. Over the 30 minute test period, the monkeys’ activity was videotaped using a camera mounted overhead. The primary dependent measure was crossings between the zones, defined as >50% of the monkey’s body crossing into a new grid section, and was counted by an observer blind to the prenatal condition of the monkey. EXPERIMENT 2: EFFECTS OF PRENATAL COCAINE EXPOSURE ON RESPONSE EXTINCTION. Monkeys were initially trained to respond on the left and right keys by reinforcing each response with a 1‐g banana‐flavored pellet; a 30‐sec timeout followed each food presentation. The light above the response key signaled food availability; only one key was active during a session. Over the course of 2‐3 weeks, the number of responses required was increased until a FR 30 schedule of food presentation was achieved. Sessions ended after 30 reinforcers had been delivered or 60 min had elapsed. When responding was reliably maintained (i.e., mean response rate ± 20% for 3 consecutive sessions) on both keys and maximal food reinforcement was obtained consistently, intravenous catheters were implanted. 126 After implantation of the catheter, baseline food reinforcement rates were re‐ established over 5 sessions, but only on the response key associated with the highest rates. For control monkeys, 4 had higher rates on the left key and 6 on the right key and for prenatally cocaine‐exposed monkeys the distribution was 3 on the left key and 7 on the right key. Saline injections were substituted for food pellets for at least 5 consecutive sessions and until responding was deemed extinguished. The criteria for extinguishing food‐ reinforced responding were 3 consecutive sessions in which response rates were reduced by at least 80% of baseline food reinforced responding (i.e., mean response rate ± 20% for 3 consecutive sessions) with no trends in responding. The primary dependent measure was the number of sessions to meet these criteria. EXPERIMENT 3: EFFECTS OF PRENATAL COCAINE EXPOSURE ON DELAY DISCOUNTING. Monkeys were re‐exposed to the FR 30 schedule of food presentation, with each pellet delivery followed by a 30‐sec timeout (TO). At the start of the session, one light above one response key was illuminated to indicate it was active; the order of which key was active varied across sessions. Sessions ended after 30 reinforcers were received or 60 min elapsed. Subjects responded under these conditions until responding on the right and left response key was reliably maintained and was deemed stable on each lever (i.e., mean response rate ± 20% for 3 consecutive sessions). 127 The conditions were then changed to a concurrent schedule, which served as the schedule of reinforcement for the delay discounting procedure. The choice was initially between 1 and 3 food pellets delivered immediately after completion of the FR 30 requirement. The 1 pellet reinforcer was contingent on responding on the key associated with the highest rates, while responding on the other key delivered 3 pellets. Initially, the delay value associated with both reinforcers was 0 sec. The delay value associated with 1 pellet remained at 0 sec throughout the experiment, while the delay associated with 3 pellet reinforcer varied from 5‐300 sec. Sessions began with two forced‐choice (i.e., sampling) trials. During these sampling trials, only one response key was active with illumination of the light and completion of the FR 30 resulted in the reinforcer and delay. After a 30‐sec TO, the other response key was illuminated and completion of the FR resulted in the reinforcer and delay. For the duration of the delay, a red light above the response key flashed on and off each second. Once both sampling trials were completed and following a 30‐sec TO, the schedule changed to a concurrent schedule with both response keys being active. To limit the influence of response perseveration, a forced choice was implemented during the session if the monkey chose the same lever 5 times in a row and then returned to a concurrent schedule. Delay values were kept constant for at least 5 consecutive sessions, and until the percent choice of the larger, delayed reinforcer was deemed stable (mean percent choice ± 20% for 3 consecutive sessions). Sessions terminated after 30 free choices were 128 completed or 60 min had elapsed. Delay values were presented in a quasi‐ random order to determine a delay‐percent choice larger reinforcer curve. Based on each monkey’s individual curve, the indifference point (delay value that engendered 50% choice of the larger, delayed reinforcer and 50% choice of the smaller, immediate reinforcer) was calculated. The primary dependent measure for this experiment was the indifference point. DATA ANALYSIS. CSF concentrations, latency to approach a novel object, locomotor activity, responding during extinction, and the indifference point (from delay discounting) were each analyzed using a two‐way ANOVA using Group (Prenatal Cocaine and Saline) and Sex as Factors. Post‐hoc Bonferroni’s tests were conducted when significant main effects were indicated by the ANOVA. For some dependent variables, data were analyzed using Spearman’s correlation coefficient for ranked data. In all cases, significance was accepted at the 95% level of confidence (p < 0.05). At the end of all the experiments, the data were collated and an overall impulsivity score was calculated. To determine this index, all 20 monkeys were ranked from 20 (most impulsive) to 1 (least impulsive) on each dependent variable (latency to touch a novel object, locomotor activity, number of sessions to reach extinction criteria, and indifference point from delay discounting) and the average rankings across all tasks was calculated. These data were analyzed using a Mann‐Whitney test and correlated with CSF concentrations using Spearman’s correlation coefficient. 129 RESULTS EFFECTS OF PRENATAL COCAINE EXPOSURE ON CSF MEASURES OF 5HIAA AND HVA. A two‐way ANOVA indicated no significant effect of prenatal condition, sex or an interaction on CSF concentrations of 5‐HIAA and HVA (Table 1). EXPERIMENT 1: EFFECTS OF PRENATAL COCAINE EXPOSURE ON IMPULSIVITY USING UNCONDITIONED BEHAVIORS. A two‐way ANOVA revealed no significant main effect of prenatal cocaine exposure or sex and no significant interaction on latency to approach a novel object (Fig. 1A). Because there were no differences due to prenatal drug exposure, mean latencies for all the males were compared to mean latencies for the females and were not significantly different. Locomotor activity ranged from 5‐316 counts over the 30 min exposure and did not differ as a function of prenatal exposure or sex and there was no significant interaction (Fig. 1B). EXPERIMENT 2: EFFECTS OF PRENATAL COCAINE EXPOSURE ON EXTINGUISHING FOOD REINFORCED BEHAVIOR. Under baseline conditions, mean response rates under the FR 30 schedule of food presentation were not different in male monkeys with mean (± SEM) values of 3.89 (± 0.68) and 2.46 (± 0.54) resp/sec for control and prenatally cocaine‐exposed monkeys, respectively. Similarly, female monkeys did not differ in mean response rates 130 between groups (1.60 ± 0.30 and 2.64 ± 1.16 resp/sec, for control and prenatally cocaine‐exposed monkeys, respectively). Response extinction was studied by substituting saline for food presentation. A two‐way ANOVA revealed a significant main effect of prenatal cocaine exposure (F(1,17)=4.78, p=0.04) but no significant effect of sex and no significant interaction on number sessions to reach criteria for response extinction. Prenatally cocaine‐ exposed monkeys required a greater number of sessions to reach criteria for extinguishing food‐reinforced responding than control monkeys (Fig. 2). EXPERIMENT 3: EFFECTS OF PRENATAL COCAINE EXPOSURE ON DELAY DISCOUNTING. Under the concurrent schedule, when the delay was 0 sec, monkeys chose the larger magnitude food reinforcer on nearly 100% of the trials (see Fig. 3 for representative curves). On average, response rates were higher on the key associated with the larger magnitude of food reinforcement (1.56 ± 0.28 resps/sec and1.72 ± 0.23 resps/sec for the 1 pellet‐ and 3 pellet‐associated keys, respectively). Response rates did not differ between prenatally cocaine exposed and control monkeys nor between male and female monkeys and response rates did not change significantly from baseline at any delay value (Table 2). Increases in the delay value resulted in time‐dependent reductions in the percent of trials in which the larger reinforcer was chosen (Fig. 3). Indifference points were calculated as the delay value (sec) that engendered 50% choice of the larger, delayed reinforcer and the smaller, immediate reinforcer (Fig. 4). A two‐way ANOVA 131 revealed a signficant effect of prenatal cocaine exposure [F(1, 16)=10.56, p=0.005] and a significant interaction between prenatal condition and sex [F(1, 16)=4.8, p=0.04]. Post‐hoc Bonferroni tests indicated that male prenatally cocaine‐exposed monkeys had significantly shorter indifference points than male control monkeys (p=0.01), while there was no difference between female control and prenatally cocaine exposed monkeys (Fig. 4). EFFECTS OF PRENATAL COCAINE EXPOSURE ON OVERALL IMPULSIVITY. In order to characterize each monkey across the various dependent variables, on overall impulsivity score was calculated (see Methods). Based on these values, male prenatally cocaine‐exposed monkeys were more impulsive than male control monkeys (p=0.009), whereas there were no differences observed in female monkeys (Fig. 5). Spearman correlation analysis revealed that scores on none of the impulsivity tasks (i.e., novel object reactivity, locomotor activity, response extinction and delay discounting) correlated with scores on any other impulsivity task (data not shown). However, there was a significant negative correlation between CSF concentration of HVA and overall impulsivity score (rs=‐0.45, p=0.046); no significant correlation between CSF 5‐HIAA concentrations and impulsivity scores were observed. DISCUSSION The purpose of the present studies was to extend earlier work characterizing adult rhesus monkeys prenatally exposed to cocaine and 132 controls to include neurochemical correlates and behavioral endpoints related to measures of impulsivity. To accomplish this, CSF concentrations of the DA metabolite HVA and the 5‐HT metabolite 5‐HIAA were obtained from 10 cocaine‐exposed and 10 control monkeys. In addition, several unconditioned and conditioned behaviors believed to assess aspects of impulsivity were examined. Finally, the interaction between prenatal drug history and sex of the monkey on these various measures was assessed. There were no differences between groups or sexes in CSF concentrations of monoamine metabolites or in response to novelty or locomotor activity. In contrast, prenatally cocaine‐exposed monkeys were more impulsive than control monkeys on two conditioned behavioral measures of impulsivity, response extinction and delay discounting. When all the measures were combined and each animal was assigned an “impulsivity score”, the prenatally cocaine‐exposed male monkeys were significantly more impulsive. These findings suggest differential effects of prenatal cocaine exposure on measures of impulsivity that are influenced by sex. These monkeys represent a unique cohort of animals – adult male and female Old World macaques who had been exposed to cocaine throughout the 25 weeks of gestation (Morris et al. 1996) and grown up with minimal exposure to drugs of abuse. Using PET imaging, we previously reported that there were no group or sex differences in DA D2‐like receptor availability in these adults (Hamilton et al. 2010). Other pharmacological studies revealed no differences in D1‐like receptor function, but significant differences related 133 to prenatal drug exposure and sensitivity to DA D3 agonist effects. Comparing results from that study and the present data, there is a significant positive correlation between overall impulsivity rank and peak effects of yawning elicited by the D3 receptor agonist quinpirole in males (rs=0.61, p=0.04) but not females (data not shown). Additionally, a one‐tailed Spearman’s correlation found that in male subjects impulsivity ranking was negatively correlated (rs= ‐0.62, p=0.046) with D2‐like receptor availability in the caudate nucleus (from Hamilton et al. 2010). These findings suggest that long‐term neuropharmacological effects due to prenatal cocaine exposure can have behavioral consequences, especially in male subjects. The present findings are consistent with other studies showing an association between low striatal D2/D3 receptor availability and impulsivity in human methamphetamine‐dependent subjects (Lee et al. 2009) and in rodents (Dalley et al. 2007). The present findings also support the idea that the D3 receptor could be a promising pharmacological target for treating impulsivity‐related disorders, including substance abuse (for reviews see Le Foll et al. 2005; Sokoloff et al. 2006; Heidbreder 2008). We found a relationship between impulsivity and CSF concentrations of the DA metabolite HVA, but not the serotonin metabolite 5‐HIAA, in contrast to the extensive literature documenting an association between decreased 5‐HIAA levels and increased impulsivity in nonhuman primates (Higley et al. 1996; Westergaard et al. 1999, 2003; Fairbanks et al. 1999, 2001, 2004; Manuck et al. 2003). A likely explanation for the discrepancy 134 between findings is the use of different measures of impulsivity. As described below, the construct of impulsivity is multi‐faceted, such that differential contribution of 5‐HT and 5‐HIAA may be dependent on the behavioral measure. Nonetheless, these data suggest that 5‐HT is not necessarily a major contributor to behaviors deemed impulsive. The relationship we observed between HVA and impulsivity is a novel finding in nonhuman primates and concurs with a recent report that CSF HVA is inversely correlated with a form of impulsivity in human subjects with personality disorder (Coccaro and Lee 2010). This relationship between HVA and impulsivity provides further evidence that alterations in the dopaminergic system may regulate impulsivity. The present findings also extended our earlier work by showing higher levels of impulsivity in the male monkeys exposed to cocaine in utero but not in females, suggesting differential effects of prenatal cocaine exposure influenced by sex. Gender‐specific effects have also been found in animal studies with males more susceptible to the negative long‐term effects of prenatal cocaine exposure on 5‐HT receptors (Johns et al. 2002) and DA receptor binding and reactivity (Silvers et al. 2006; Dow‐Edwards 2010). Additionally, recent clinical studies reported males to be more adversely affected by prenatal cocaine exposure than females, specifically increasing their risk for problems of inhibitory control (Delaney‐Black et al. 2004; Bendersky et al. 2006; Dennis‐Tiwary et al. 2006; Bennet et al. 2007). It has been suggested that the male fetus is more vulnerable to in utero stressors 135 and neurotoxins than the female fetus (Kraemer 2000) which may account for the larger deficits observed in prenatally cocaine exposed males than females. It is also possible that other changes that occur during hormonal variations of adolescence may mask effects of prenatal cocaine exposure until maturation (Cabrera‐Vera et al. 2000). Our findings of increased impulsivity in male, but not female, monkeys exposed to cocaine throughout gestation support the idea that prenatal cocaine exposure outcomes are influenced by sex. This is the first report to investigate a wide range of impulsivity measures in the same cohort of nonhuman primates. Behavioral outcomes from the four tasks used in this study (novel object reactivity, locomotor activity, response extinction and delay discounting) did not correlate with one another, indicating that we were measuring different facets of the construct of impulsivity. Interestingly, differences in impulsivity between prenatally cocaine‐exposed and control monkeys were only observed in the conditioned behavioral measures. The two unconditioned behavioral measures are tasks that are typically used in the rodents to assess impulsivity and have been shown to correlate with drug use (Hooks et al. 1991; Klebar et al. 2001; Piazza et al. 1989, 1990; reviewed in Piazza and Le Moal 1998). It is possible that these unconditioned measures are not sufficient to see differences in impulsivity in nonhuman primates or that more complex, conditioned behavioral measures are necessary to unmask the subtle differences in impulsivity in prenatally cocaine‐exposed monkeys. 136 In future studies, we will be able to establish which impulsivity measures correlate with stimulant self‐administration in this same cohort of monkeys to determine which tasks have the most predictive ability. It has been hypothesized that some negative effects of cocaine, particularly those regulated by monoamine‐rich areas of the brain, such as deficits in impulse control (Bandstra et al. 2007), will ultimately lead to higher rates of substance dependence. Therefore, the relationship between addiction and impulsivity (Jentsch and Taylor 1999; Bickel and Marsch 2001; de Wit 2010) suggests that the increased impulsivity observed in the male monkeys exposed to cocaine in utero may predispose them to drug use. The female prenatally cocaine‐exposed monkeys did not differ from controls on overall impulsivity which indicates they may be protected from this potential vulnerability to substance abuse. Since we observed deficits in impulsivity 15 years after the cocaine exposure in utero, it is likely that the early deficits in attention and impulse control seen in the human cohort studies at ages 4, 6, and 9 years old (Savage et al. 2005; Linares et al. 2006; Pulsifer et al. 2008) may be associated with disturbances in inhibitory control in adolescence and adulthood that could contribute to an increased vulnerability to substance abuse. Taken together, the present results provide evidence for long‐term neurobehavioral consequences of prenatal cocaine exposure on impulse control and a neurobiological correlate for the increase impulsivity observed in male, but not female, subjects. 137 ACKNOWLEDGEMENTS This research was supported by National Institute on Drug Abuse grants R01 DA25120, R37 DA10584 and K31 DA024485. The authors report no conflict of interest and would like to acknowledge the excellent technical assistance of Tonya Calhoun and Whitney Wilson. The authors also thank Dr. William L. Woolverton for technical consultation and Dr. Merle Paule for providing information related to the histories of these monkeys. 138 TABLE 1. COMPARISON OF CSF 5‐HIAA AND HVA (MEAN ± SEM) BETWEEN MALE AND FEMALE PRENATALLY COCAINE‐EXPOSED MONKEYS AND CONTROLS 5‐HIAA (nM) HVA (nM) Male Prenatally Cocaine Exposed 148 ± 18.1 614.7 ± 68.5 Male Controls 153.5 ± 4.9 Female Prenatally Cocaine Exposed 177.1 ± 6.4 156.0 ± 24.4 734.2 ± 86.8 802.7 ± 113.1 785.1 ± 87.1 139 Female Controls TABLE 2. RESPONSE RATES (RESP/SEC) DURING THE DELAY DISCOUNTING TASK¶. Delay Value (sec) 0 10§ 30 60 120† Prenatally Cocaine Exposed Monkeys Immediate Delayed reinforcer key reinforcer key 1.85 ± 0.39 1.93 ± 0.29 1.30 ± 0.35 1.11 ± 0.28 1.53 ± 0.48 1.32 ± 0.35 1.84 ± 0.43 1.61 ± 0.40 1.36 ± 0.29 1.60 ± 0.35 Control Monkeys Immediate reinforcer key 1.27 ± 0.39 1.08 ± 0.43 0.96 ± 0.27 1.19 ± 0.29 1.38 ± 0.25 Delayed reinforcer key 1.45 ± 0.33 1.05 ± 0.33 1.03 ± 0.22 1.36 ± 0.23 1.55 ± 0.34 ¶ All points are means (± SEM) of 10 monkeys, except where noted § n=10 for prenatal cocaine group and n=7 for controls † n= 6 for prenatal cocaine group and n=9 for controls 140 FIGURE 1. A: Latency to touch a novel object placed in the monkey’s home cage (in sec). B: Number of gridline crossings in a novel environment over 30 min. Values shown are mean ± SEM for cocaine‐exposed (filled bars) and control (open bars) male and female monkeys. 141 FIGURE 2. Number of sessions to extinguish previously food‐reinforced responding in male and female monkeys prenatally exposed to cocaine (filled bars) and controls (open bars). Each bar represents mean ± SEM values. *p<0.05. 142 FIGURE 3. Percentage of trials in which the larger, delayed reinforcer was chosen over the smaller, immediate reinforcer as a function of delay value. Data are from representative prenatally cocaine exposed (left) and control (right) male monkeys. The delay value at which the curve intersects with the dashed line (50% choice of larger reinforcer) represents the indifference point. 143 FIGURE 4. Mean indifference points calculated from delay‐discounting procedures for monkeys prenatally exposed to cocaine (filled bars) and controls (open bars). Data are shown from male (left) and female (right) monkeys. Each bar represents mean ± SEM values. *p<0.05. 144 FIGURE 5. Overall impulsivity rank across 4 measures of impulsivity (novel object reactivity, locomotor activity, response extinction and delay discounting) in male (left) and female (right) prenatally cocaine exposed (filled bars) and control (open bars) monkeys. To calculate the score, monkeys were ranked from 1 (least impulsive) to 20 (most impulsive) and an overall mean was determined for each animal. Higher scores represent greater impulsivity. Bars represent mean ± SEM values. *p<0.05. 145 REFERENCES Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES (2007) Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr 28:195‐205. Alessi SM, Greenwald M, Johanson CE (2003) The prediction of individual differences in response to D‐amphetamine in healthy adults. Behav Pharmacol 14:19‐32. Anderson KG, Woolverton WL (2003) Effects of dose and infusion delay on cocaine self‐administration choice in rhesus monkeys. Psychopharmacology 167:424‐430. Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC (2007) Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol 37:275‐279. Bandstra ES, Accornero VH, Morrow CE, Xue L, Simpson GR, Glavach MK, Anthony JC (2007) Longitudinal study of visual attention in prenatally cocaine‐exposed children and adolescents. Pediatric Academic Societies’ Annual Meeting, Toronto, Canada. 6715.3 Bendersky M, Bennet DS, Lewis M (2006) Aggression at age five as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol 31:1‐14. Bennett D, Bendersky M, Lewis M (2007) Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr 28:467‐472. Bessa MA, Mitshuhiro SS, Chalem E, Barros MM, Guinsburg R, Laranjeira R (2009) Underreporting of use of cocaine and marijuana during the third trimester of gestation among pregnant adolescents. Addictive Behaviors 35:266‐269. Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73‐86. Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: delay discounting in current, never, and ex‐smokers. Psychopharmacology 146:447‐454. 146 Bolig R, Price CS, O’Neill PL, Suomi SJ (1992) Subjective assessment of reactivity level and personality traits of rhesus monkeys. Int J of Primatology 13:287‐306. Brown GL, Linnoila MI (1990) CSF serotonin metabolite (5‐HIAA) studies in depression, impulsivity, and violence. J Clin Psychiatry 51: Suppl 31‐ 43. Buxhoeveden DP, Hasselrot U, Buxhoeveden NE, Booze RM, Mactutus CF (2006) Microanatomy in 21 day rat brains exposed prenatally to cocaine. Int J Dev Neurosci 24:335‐341. Cabrera‐Vera TM, Garcia F, Pinto W, Battaglia G (2000) Neurochemical changes in brain serotonin neurons in immature and adult offspring prenatally exposed to cocaine. Brain Res 870:1‐9. Chamberlain SR, Sahakian BJ (2007) The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20:255‐261. Clarke AS, Boinski S (1995) Temperament in nonhuman primates. Am J Primatol 37:103‐125. Coccaro EF, Lee R (2010) Cerebrospinal fluid 5‐hydroxyindolacetic acid and homovanillic acid: reciprocal relationships with impulsive aggression in human subjects. J Neural Trasm 117:241‐248. Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003) Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine‐dependent invididuals. Exp Clin Psychopharmacol 11:18‐25. Coleman K, Tully LA, McMillan JL (2005) Temperament correlates with training success in adult rhesus macaques. Am J Primatol 65:63‐71. Cremniter D, Jamain S, Kollenbach K, Alvarez JC, Lecrubier Y, Gilton A, Jullien P, Lesieur P, Bonnet F, Spreux‐Varoquaux O (1999) CSF 5‐HIAA levels are lower in impulsive as compared to nonimpulsive violent suicide attempters and control subjects. Biol Psychiatry 45:1572‐1579. Czoty PW, Gage HD, Nader MA (2010) Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology 208:585‐592. Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA (2009) Effect of menstrual cycle phase on 147 dopamine D2 receptor availability in female cynomolgus monkeys. Psychopharmacology 34:548‐554. Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 215:1267‐1270. Dandy KL, Gatch MB (2009) The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol 20:400‐405. Davis BA, Clinton SM, Akil H, Becker JB (2008) The effects of novelty‐seeking phenotypes and sex differences on acquisition of cocaine self‐ administration in selectively bred High‐Responder and Low‐ Responder rats. Pharmacol Biochem Behav 90:331‐338. de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22‐31. Delaney‐Black V, Covington C, Nordstrom B et al (2004) Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr 25:254‐263. Dellu F, Piazza PV, Mayo W, LeMoal M, Simon H (1996) Novelty‐seeking in rats –biobehavioral characteristics and possible relationship with the sensation‐seeking trait in man. Neuropsychobiology 34:136‐145. Dennis‐Tiwary T, Bendersky M, Ramsay D et al (2006) Reactivity and regulation in children prenatally cocaine exposed. Dev Psycho 42:688‐ 697. Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, de Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301‐308. Dow‐Edwards D (2010) Sex differences in the effects of cocaine abuse across the lifespan. Physiology and Behavior Epub ahead of print. PMID: 20045010. Evenden J (1999) Impulsivity: a discussion of clinical and experimental findings. Journal of Psychopharmacology 13:180‐192. 148 Fairbanks LA, Fontenot MB, Phillips‐Conroy JE, Jolly CJ, Kaplan JR, Mann JJ (1999) CSF monoamines, age, and impulsivity in wild grivet monkeys (Cercopithecus aethiops). Brain Behav Evol 53:305‐312. Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ (2004) Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol 64:1‐17. Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT (2001) Neuropsychopharmacology 24:370‐378. Hamilton LR, Czoty PW, Gage HD, Nader MA (2010) Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology 210:481‐488. Heidbreder C (2008) Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets 7:410‐421. Higley JD, Bennett AJ (1999) Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non‐human primates. Alcohol 34:402‐418. Higley JD, Linnoila M (1997) Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann NY Acad Sci 836:39‐56. Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M (1996) CSF testosterone and 5‐HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40:1067‐1082. Hooks MS, Jones GH, Smith AD, Neill DB, Justice DB Jr. (2001) Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse 9:121‐128. Howell LL, Sachama KF, Ellis JE, Grrimley PJ, Kitchens AJ, Byrd LD (2001) Fetal development in rhesus monkeys exposed prenatally to cocaine. Neurotoxicol Teratol 23: 133‐140. Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward‐related stimuli. Psychopharmacology 146:373‐390. 149 Johns JM, Lubin DA, Lieberman JA, Lauder JM (2002) Developmental effects of prenatal cocaine exposure on 5‐HT1A receptors in male and female rat offspring. Dev Neurosci 24:522‐530. Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128:78‐87. Kraemer S (2000) The fragile male. Br Med J 321:1609‐1612. Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J of Neurosci 29:14734‐14740. Lee C‐T, Chen J, Hayashi T, Tsai S‐Y, Sanchez JF, Errico SL, Amable R, Su T‐P, Lowe RH, Huestis MA, Shen J, Becker KG, Geller HM, Freed WJ (2008) A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Medicine 5:987‐1004. Le Foll B, Goldberg SR, Sokoloff P (2005) The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology 49:525‐541. Lidow MS (2003) Consequences of prenatal cocaine exposure in nonhuman primates. Developmental Brain Res 147: 23‐36. Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S (2006) Mental health outcomes of cocaine‐exposed children at 6 years of age. J Pediatr Psychol 31:85‐97. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK (1983) Low cerebrospinal fluid 5‐hydroxyindoleacetic acid concentration differentiates impulsive from non‐impulsive violent behavior. Life Sci 33:2609‐2614. Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self‐ control choices in opioid‐dependent patients and non‐drug‐using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256‐262. Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME (2003) Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomologus monkeys (Macaca fascicularis). Am J Primatol 61:187‐194. 150 Marusich JA, Bardo MT (2009) Differences in impulsivity on a delay‐ discounting task predict self‐administration of a low unit dose of methylphenidate in rats. Behav Pharmacol 20:447‐454. Matthys W, van Goozen SH, de Vries H, Cohen‐Kettenis PT, van Engeland H (1998) The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J Child Psychol Psychiatry 39:642‐651. Matthys W, van Goozen SH, Snoek H, van Engeland H (2004) Response perseveration and sensitivity to reward and punishment in boys with oppositional‐defiant disorder. Eur Child Adolesc Psychiatry 13:361‐ 364. McCleary R (1966) Response‐modulating function of the limbic system: Initiation and suppression. In Stellar E and Sprague J (Eds.), Progress in physiological psychology, Vol 1. Academic Press, New York, pp 209‐ 271. Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001) Psychiatric aspects of impulsvity. Am J Psychiatry 158:1783‐1793. Morris P, Binienda Z, Gillam MP et al. (1996) The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 18:147‐154. Morris P, Binienda Z, Gillam MP et al. (1997) The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 19: 47‐57. Morrow BA, Elsworth JD, Roth HD (2005) Prenatal exposure to cocaine selectively disrupts the development of parvalbumin containing local circuit neurons in the medial prefrontal cortex of the rat. Synapse 56:1‐11. Morrow BA, Hajszan T, Leranth C, Elsworth JD, Roth RH (2007) Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse 61:862‐865. Morrow CE, Culbertson JL, Accornero VH, Zue L, Anthony JC, Bandstra ES (2006) Learning disabilities and intellectual functioning in school‐ aged children with prenatal cocaine exposure. Dev Neuropsychol 30:905‐931. 151 Nair BS, Watson RR (1991) Cocaine and the pregnant woman. J Reprod Med 36:862‐867. Newman JL, Perry JL, Carrol ME (2008) Effects of altering reinforce magnitude and reinforcement schedule on phencyclindine (PCP) self‐ administration in monkeys using an adjusting delay task. Pharmacol Biochem Behav 90:778‐786. Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C (2005) Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol 27:429‐438. Paule MG, Gillam MP, Allen RR, Chelonis JJ (2000) Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann NY Acad Sci 914:412‐417. Paule MG, Gillam MP, Binienda Z, Morris P (1996) Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci 801:301‐309. Peng XQ, Ashby CF Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX (2009) The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine‐ triggered relapse to drug‐seeking behaviors in rats. Neuropharmacology 56:752‐760. Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self‐ administration in female rats. Psychopharmacology 178:193‐201. Perry JL, Nelson SE, Carroll ME (2008) Impulsive choice as a predictor of acquisition of IV cocaine self‐administration and reinstatement of cocaine‐seeking behavior in male and female rats. Exp Clin Psychopharmacol 16:165‐177. Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self‐administration. Science 245:1511‐1513. Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H (1990) Individual reactivity to novelty predicts probability of amphetamine self‐administration. Behav Pharmacol 1:339‐345. Piazza PV, Le Moal M (1998) The role of stress in drug self‐administration. Trends Pharmacol Sci 19:67‐74. 152 Pulsifer MB, Butz AM, O’Reilly FM, Belcher HM (2008) Prenatal drug exposure: effects on cognitive functioning at 5 years of age. Clin Pediatr 47:58‐65. Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H (2007) Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics 120:1245‐1254. Ren JQ, Malanga CJ, Tabit E, Kosofsky BE (2004) Neuropathological consequences of prenatal cocaine exposure in the mouse. Int J Dev Neurosci 22:309‐320. Retz W, Rosler M, Supprian T, Retz‐Junginger P, Thome J (2003) Dopamine D3 receptor gene polymorphism and violent behavior: relation to impulsiveness and ADHD‐related psychophathology. J Neural Transm 110:561‐572. Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA (2009) Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience 158:1257‐1265. Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H (2005) Attentional functioning and impulse control in cocaine‐exposed and control children at age ten years. J Dev Behav Pediatr 26:42‐47. Sher L, Oquendo MA, Li S, Huang YY, Grunebaum MF, Burke AK, Malone KM, Mann JJ (2003) Lower CSF homovanillic acid levels in depressed patients with a history of alcoholism. Neuropsychopharmacology 28:1712‐1719. Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM (2006) Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol 28:173‐180. Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long‐term increases in impulsive choice. Behav Neurosci 121:543‐549. Singer LT, Arendt R, Minnes S, Farkas K, Salvator A (2000) Neurobehavioral outcomes of cocaine‐exposed infants. Neurotoxicol Teratol 22:653‐ 666. 153 Singer LT, Eisengart LJ, Minnes S, Noland J, Jey A, Lane C, Min MO (2005) Prenatal cocaine exposure and infant cognition. Infant Behav Dev 28:431‐444. Singer LT, Hawkins S, Huang J, Davillier M, Baley J (2001) Developmental outcomes and environmental correlates of very low birthweight, cocaine‐exposed infants. Early Hum Dev 64:91‐103. Singer LT, Lewin J, Minnes S, Weishampel P, Drake K, Satayathum S, Boll DT, Zijdenbox A, Paus T, Evans A. (2006) Neuroimaging of 7 to 8 year‐old children exposed prenatally to cocaine: Neurobehavioral teratology society, special edition 2004 meeting. Neurotoxicol Teratol 28:386‐ 402. Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S (2008) Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr 153:105‐111. Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5:25‐43. Stanis JJ, Marquez Avila H, White MD, Gulley JM (2008) Dissociation between long‐lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay‐discounting task. Psychopharmacology 199:539‐548. Stuerenburg HJ, Petersen K, Baumer T, Rosenkranz M, Buhmann C, Thomasius R (2002) Plasma concentrations of 5‐HT, 5‐HIAA, norepinephrine, epinephrine, and dopamine in ecstasy users. Neuro Endocrinol Lett 23:259‐261. Suto N, Austin JD, Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self‐administer nicotine. Psychopharmacology 158:175‐180. Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D (2003) Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry 160:1078‐1085. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1996) Results from the 1992 National Pregnancy and Health Survey. Ref Type: Report. 154 Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine‐seeking and cocaine‐enhanced brain reward in rats. J Neurosci 22:9595‐9603. Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD (2003) Physiological correlates of aggression and impulsivity in free‐ranging female primates. Neuropsychopharmacology 28:1045‐1055. Westergaard GC, Suomi SJ, Higley JD, Mehlman PT (1999) CSF 5‐HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology 146:440‐446. Woolverton WL, Anderson KG (2006) Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology 186:99‐106. Woolverton WL, Myerson J, Green L (2007) Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol 15:238‐244. Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR Jr, Gitain L, Gardner EL (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine‐induced reinstatement of drug‐seeking behavior in rats. Neuropsychopharmacology 31:1393‐1405. Zuckerman M (1996) The psychobiological model for impulsive unsocialized sensation‐seeking: a comparative approach. Neuropsychobiology 34:125‐129. 155 CHAPTER IV INCREASED VULNERABILITY TO SELFADMINISTER COCAINE IN ADULT RHESUS MONKEYS EXPOSED TO COCAINE THROUGHOUT GESTATION Lindsey R. Hamilton, Michael A. Nader The following manuscript is in preparation to be submitted to Science in May 2010. Stylistic variations are due to the requirements of the journal. Lindsey R. Hamilton performed the experiments, analyzed the data, and prepared the manuscript. Michael A. Nader acted in an advisory and editorial capacity. 156 ABSTRACT RATIONALE: Prenatal cocaine exposure has been associated with alterations in the dopamine (DA) system and increased impulsivity in adult monkeys. However, whether these neurobiological and behavioral outcomes of prenatal cocaine exposure results in altered sensitivity to the reinforcing effects of stimulants in nonhuman primates is not known. OBJECTIVES: To assess vulnerability to acquire cocaine self‐administration in 15 year‐old rhesus monkeys exposed to cocaine or saline in utero (n=10 per group). MATERIALS AND METHODS: Monkeys were trained to self‐administer food pellets (1 g) under a fixed‐ratio (FR) 30 schedule of reinforcement. Saline and ascending doses of intravenous cocaine (0.001‐0.3 mg/kg/injection) were substituted for food pellets. Acquisition of cocaine self‐administration was operationally defined as the lowest dose in which response rates were signficantly greater than saline‐contingent rates of responding. RESULTS: Prenatally cocaine‐exposed monkeys acquired cocaine self‐administration at lower doses than controls and required less cocaine history prior to finding a dose of drug reinforcing. Vulnerability to self‐administer cocaine was found to be related to an individual phenotype involving increased dopamine D3 receptor function and impulsivity. CONCLUSIONS: These findings suggest that prenatal cocaine exposure results in increased vulnerability to stimulant self‐adminstration. 157 Keywords: Prenatal cocaine – Cocaine – SelfAdministration – Predisposition – Acquisition – Rhesus monkey 158 Although rates of prenatal cocaine exposure peaked during the late 1980s and early 1990s, it continues presently with approximately 50,000 additional children born each year (National Pregnancy and Health Survey, 1996). Widespread use of cocaine in the United States has resulted in more than 1 million children prenatally exposed to cocaine, many of whom are now entering adolescence or young adulthood, a time when many experiment with drugs of abuse. For example, approximately 1 in 5 Americans between the ages of 21‐25 have tried cocaine at least once in their lifetime (SAMHSA, 2008). Children and young adults that were exposed to cocaine in utero may be sensitized to the reinforcing effects of cocaine and be more vulnerable to progressing to drug abuse. Currently, the human cohort studies have only followed the prenatally cocaine‐exposed children and non‐ exposed controls through 13 years old. It is not yet known whether prenatal cocaine exposure is associated with increased risk of substance dependence. There is accumulating evidence that prenatal exposure to substances predicts subsequent substance use later in life. An elevated risk of tobacco dependence and early adolescent smoking and tobacco smoking has been found in offspring of mothers who smoked during their pregnancy (Kandel et al., 1994; Buka et al., 2003; Cornelius et al., 2005). Similarly, prenatal marijuana exposure has been associated with initiation and use of marijuana among young adults (Porath and Fried, 2005). A prospective, longitudinal study observed a significant correlation between prenatal alcohol exposure and subsequent alcohol use and alcohol problems at 14 years (Baer et al., 159 1998) and at 21 years old (Baer et al., 2003), even after controlling for family history of alcohol abuse. While no human studies have examined the effect of prenatal cocaine exposure and vulnerability to substance abuse, studies in rodents suggest that the reinforcing efficacy of stimulants is altered by gestational cocaine exposure. Prenatal cocaine exposure enhances the cocaine‐induced potentiation of brain stimulation reward (Lin and Kellogg, 1996; Malanga et al., 2008). Prenatally cocaine exposed rats also showed significantly higher rates of responding compared to controls for a low dose of cocaine available under an FR 1 schedule of reinforcement (Keller et al., 1996). Additionally, Rocha et al. (2002) found that mice exposed to cocaine in utero acquired cocaine self‐administration more readily than controls despite observing no differences in rate of acquisition of food‐reinforced responding. However, there are conflicting reports about prenatally cocaine exposed animals’ propensity to be more sensitive to the reinforcing effects of cocaine. Heyser et al. (1992) found that rats exposed to cocaine throughout gestation did not acquire cocaine conditioned place preference which suggests a reduction in cocaine reward. Furthermore, Hecht et al. (1998) determined that prenatally cocaine‐exposed rats were less sensitive to the reinforcing strength of cocaine because these animals had signficantly lower break points for cocaine using a progressive‐ratio schedule of reinforcement. Therefore, it has not been well established whether prenatal cocaine exposure alters vulnerability to cocaine self‐administration. 160 In order to evaluate the consequences of in utero drug exposure in adulthood, the present study examined prenatal cocaine exposure in rhesus moneys. These animals had been exposed to cocaine or saline throughout the 25 weeks of gestation (Morris et al., 1996) and were studied as adults (14‐15 years old). We recently examined dopamine (DA) receptor function in these adult monkeys (Hamilton et al., 2010) using agonist‐elicited behaviors and PET imaging. There were no differences in DA D1 and D2 receptor function, but prenatally cocaine‐exposed animals were more sensitive to the behavioral effects of the D3 agonist quinpirole compared to controls. Additionally, we have previously found that male, but not female, prenatally cocaine‐exposed monkeys are more impulsive than controls (Hamilton et al., submitted). Long‐lasting alterations in the dopaminergic system and increased behavioral impulsivity are risk factors for increased vulnerability to self‐administer stimulants, suggesting that prenatally cocaine‐exposed monkeys may be more sensitive to the reinforcing effects of cocaine as adults. In the present study, we extended the behavioral assessment of this cohort to examine acquisition of cocaine self‐administration. METHODS SUBJECTS. Nineteen adult rhesus monkeys (Macaca mulatta), born between 1993 and 1995 and raised at the FDA facility in Little Rock, AR until their arrival at Wake Forest University in 2007, served as subjects. Ten monkeys (6 male, 4 female) were prenatally exposed to cocaine and 9 monkeys (5 161 male, 4 female) served as controls, as described previously (Morris et al., 1997). Briefly, the mothers of the monkeys used in this study received intramuscular injections escalating doses of cocaine three times per day for the entire course of gestation (Morris et al., 1996). The mean gestational cocaine exposure was 1131.5 mg/kg. Other than their prenatal drug histories, all monkeys had identical experimental histories (see Paule et al., 1996; Morris et al., 1996). Monkeys were individually housed in stainless‐ steel cages with water available ad libitum and had visual and auditory contact with each other. Since we have previously shown that monoamine function is influenced by menstrual cycle (Czoty et al. 2009), we monitored menstrual cycle phase throughout the experiment by daily vaginal swabs. Days of bleeding were recorded as indicative of menses. During quarantine, a free‐feeding weight was determined and monkeys’ body weights were maintained at approximately 95% of that value throughout these studies (LabDiet Monkey Chow and fresh fruit). Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products) using a specially designed stainless‐steel pole that attached to the collar. All manipulations were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee. 162 APPARATUS. The apparatus consisted of a ventilated, sound‐attenuating chamber (1.5 x 0.74 x 0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two response keys (5 cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each response key and a food receptacle was located between the response keys. The receptacle was connected with tygon tubing to a pellet dispenser (Gerbarands Corp., Arlington, MA) located on the top of the chamber for delivery of 1‐g banana‐flavored food pellets (P.K. Noyes Co., Lancaster, NH). An infusion pump (Cole‐Palmer, Inc., Chicago, IL) was located on the top of the chamber. SURGERY. Each monkey was prepared with a chronic indwelling venous catheter and subcutaneous vascular port (Access Technologies, Skokie, IL) under sterile surgical conditions. Anesthesia was induced and maintained with ketamine (15 mg/kg) and butorphanol (0.025 mg/kg). Vital signs were monitored for the duration of the surgery. Briefly, a catheter was inserted into the femoral vein to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was then attached to the vascular access port and placed in a pocket formed by blunt dissection. Each port and catheter was filled with heparinized saline solution (100 Units/ml) after every experimental session to prolong the patency. Prior to each drug self‐administration session, the back of the animal was cleaned 163 with betadine and 95% EtOH and the port was connected to the infusion pump located outside the chamber via a 20‐gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 sec to fill the port and catheter line with drug prior to starting the session. ACQUISITION OF COCAINE SELFADMINISTRATION. Monkeys were initially trained to respond on the left and right keys by reinforcing each response with a 1‐g banana‐flavored pellet; a 30‐sec timeout followed each food presentation. The light above the response key signaled food availability; only one key was active during a session. Over the course of 2‐3 weeks, the number of responses required was increased until a fixed‐ratio (FR) 30 schedule of food presentation was achieved. Sessions ended after 30 reinforcers had been delivered or 60 min had elapsed. When responding was reliably maintained (i.e., mean response rate ± 20% for 3 consecutive sessions) on both keys and maximal food reinforcement was obtained consistently, intravenous catheters were implanted. After implantation of the catheter, baseline food reinforcement response rates were re‐established over 5 sessions, but only on the response key associated with the highest rates. For control monkeys, 4 had higher response rates on the left key and 6 on the right key and for prenatal cocaine exposed monkeys the distribution was 3 on the left key and 7 on the right key. Saline injections were substituted for food pellets for at least 5 164 consecutive sessions and until responding was deemed extinguished. The criteria for extinguishing food‐reinforced responding were 3 consecutive sessions in which response rates were reduced by at least 80% of baseline food reinforced responding (i.e., mean response rate ± 20% for 3 consecutive sessions) with no trends in responding. The primary dependent measure was the number of sessions to meet these criteria. Next, baseline food‐maintained rates of responding were re‐ established. Injections of cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, dissolved in sterile 0.9% saline) were than substituted for the food pellets in ascending order from 0.001 mg/kg/injection increasing in half log units to 0.3 mg/kg/injection. Drug doses were available for at least 5 consecutive sessions until animals reached criteria for stability (response rate mean ±20% with no trends for 3 consecutive sessions). In between drug doses, monkeys returned to a food‐reinforced baseline for at least 3 consecutive sessions. The dose at which cocaine self‐administration was determined to be acquired was the first dose at which response rate was signficantly greater than the response rate when saline was available (see Data Analysis). For female subjects, drug was only made available during the follicular phase of the menstrual cycle (days 2‐12 where day 1 is the first day of menses). During the luteal phase, female subjects responded under an FR 30 schedule of food presentation. 165 DATA ANALYSIS. A t‐test was used to determine if response rates under each dose of cocaine was greater than saline‐contingent response rates. To determine if there were differences in the rate of acquisition between prenatally cocaine exposed and control animals, a logrank analysis of Kaplan‐ Meier survival curves was computed. Additionally, a t‐test was used to compare the cumulative exposure to cocaine prior to acquisition between the two groups. After the individual animals were classified into high or low vulnerability groups, t‐tests and Mann‐Whitney tests were used to compare the two groups for differences. In all cases, significance was accepted at the 95% level of confidence (p < 0.05). RESULTS Acquisition of food‐maintained responding on an FR 30 schedule did not differ between the two groups (sessions to acquire food‐maintained FR 30 was 34.5 ± 4.4 for the prenatally cocaine exposed animals and 35.5 ± 5.9 for the controls). Mean response rates under the FR 30 schedule of food presentation were not different in male monkeys with mean (± SEM) values of 3.89 (± 0.68) and 2.46 (± 0.54) resp/sec for control and prenatally cocaine‐ exposed monkeys, respectively. Similarly, female monkeys did not differ in mean response rates between groups (1.60 ± 0.30 and 2.64 ± 1.16 resp/sec, for control and prenatally cocaine‐exposed monkeys, respectively). Neither prenatally cocaine‐exposed or control monkeys differed in mean response 166 when saline was available (Males: 0.03 ± 0.01 and 0.04 ± 0.02, respectively; Females: 0.02 ± 0.01 and 0.02 ± 0.01, respectively). After testing all cocaine doses (0.001‐0.3 mg/kg/injection) once, 80% (8 of 10) of prenatally cocaine exposed monkeys acquired cocaine self‐ administration compared to only 55% (5 of 9) of controls (Fig. 1). The remaining 2 prenatally cocaine exposed monkeys and 3 of the 4 remaining control monkeys eventually acquired cocaine self‐administration once the doses were tested a second time. One female control monkey never found any dose of cocaine reinforcing. A logrank test of Kaplan‐Meier survival curves revealed no differences between prenatally cocaine‐exposed and control monkeys on the rate of acquisition of cocaine self‐administration (χ2 = 1.34, p = 0.25). The median acquisition dose (the dose by which 50% of the animals had acquired self‐administration) was 0.01 mg/kg for the prenatally cocaine exposed animals and 0.3 mg/kg for the control animals. A t‐test revealed greater cumulative cocaine exposure since beginning the experiment until acquisition of cocaine self‐adminstration in controls compared to prenatally cocaine‐exposed monkeys (p = 0.04) (Fig. 2). Based on each animal’s individual cumulative intake of cocaine prior to acquisition, animals were split into two groups: high vulnerability (HV, cumulative cocaine intake prior to acquisition < 3 mg/kg, n = 10) and low vulnerability (LV, cumulative cocaine intake prior to acquisition > 3 mg/kg, n = 9) (Fig. 3). A t‐test showed that the number of self‐administration sessions to reach acquisition criteria was signficantly greater in the LV group (40.8 ± 167 6.0 sessions) compared to the HV group (15.4 ± 2.5 sessions) (p = 0.0008) (data not shown). Using data generated from these monkeys in earlier studies (Hamilton et al., 2010, under review), a Mann‐Whitney test revealed that the HV group were more impulsive compared to the LV (p = 0.04) (Fig. 4, panel A) and a t‐test revealed the HV group had greater frequency of yawns elicited by quinpirole (p = 0.03) (Fig. 4, panel B) compared to the LV group. Additionally, there was significant correlation between quinpirole‐elicited yawning and cumulative cocaine intake prior to acquisition (r2 = 0.18, p = 0.05) (Fig. 4, panel C). There was no difference observed between the HV and LV groups for any other phenotypic variable examined including D2 receptor availability, D1 receptor‐elicited eye‐blinking, or 5‐HIAA or HVA basal cerebrospinal fluid concentration (data not shown). DISCUSSION The purpose of the present studies was to determine whether prenatally cocaine exposed adult rhesus monkeys were more vulnerable to stimulant self‐administration compared to controls. To study vulnerability, monkeys responded under an FR 30 schedule of food presentation and, when stable, saline and then increasing cocaine doses were examined. Between saline or various cocaine doses, responding was again maintained by food. In this way, acquisition of cocaine reinforcement could be operationally defined as the lowest dose in which cocaine‐maintained responding was significantly 168 higher than saline‐contingent responding (i.e., the lowest dose in which cocaine functioned as a reinforcer). Prenatally cocaine‐exposed monkeys acquired cocaine reinforcement at lower doses than control animals. This is not simply a difference in learning to acquire because acquisition of food‐ maintained responding on an FR 30 schedule did not differ between the two groups. Furthermore, the cumulative cocaine dose prior to acquisition (i.e., the amount of cocaine that had been self‐administered prior to a dose functioning as a reinforcer) was significantly lower for prenatal cocaine exposed monkeys compared to controls. This is of relevance because cocaine will function as a reinforcer in all monkeys; however, compared to controls, the amount of cocaine history necessary for the prenatally cocaine‐exposed monkeys is substantially less than the amount necessary for control monkeys, suggesting they are more sensitive to the reinforcing effects of cocaine than controls. After completing cocaine dose‐response curves for all animals, several phenotypic variables that have been thought to be related to cocaine self‐ administration were examined. To do this, individual monkeys were characterized as highly vulnerable (HV) or low vulnerable (LV) to cocaine reinforcement based on the amount of cocaine intake prior to acquisition (i.e., the 10 monkeys with the lowest intakes were considered HV, while the 9 with the highest intakes were considered LV). Of the 10 HV monkeys, 70% were prenatally cocaine exposed. We previously reported that there were no group or sex differences in DA D2‐like receptor function using PET imaging 169 in this cohort (Hamilton et al., 2010). Other pharmacological studies revealed no differences in D1‐like receptor function, but significant differences related to prenatal drug exposure and sensitivity to DA D3 agonist effects in male monkeys (Hamilton et al., 2010). Comparing results from that study and the present data, we determined that there were no significant differences in D2‐ like receptor availability or D1‐elicited eye‐blinking between the HV and LV monkeys. However, the HV monkeys previously displayed increased frequency of yawns elicited by quinpirole compared to LV monkeys and there was a significant correlation between peak yawns and the cumulative cocaine intake prior to acquisition, suggesting a role for D3 receptor function in cocaine reinforcement. We also compared the results from the present study to impulsivity measures previously examined. The prenatally cocaine exposed monkeys were more impulsive compared to controls (Hamilton et al., under review). As it relates to self‐administration, HV monkeys are more impulsive across multiple measures of impulsivity compared to LV monkeys, which suggests that impulsivity is a behavioral phenotype that predicts acquisition of stimulant self‐administration. By using a within subject design and several behavioral and neuropharmacological measures, we were able to identify a specific profile or phenotype that predicted vulnerability to stimulant self‐administration. Increased D3 receptor function appears to be a neurobiological risk factor that predisposes individuals to cocaine self‐administration. The present findings are consistent with other studies showing an association between 170 low striatal D2/D3 receptor availability and impulsivity in human methamphetamine‐dependent subjects (Lee et al., 2009) and in rodents (Dalley et al., 2007). Additionally, an upregulation of D3 receptors has been observed in individuals that died from a cocaine overdose (Staley and Mash, 1996), which indicates perhaps D3 function plays a critical role in the reinforcing effects of cocaine. The present findings also provide supporting evidence for the D3 receptor being a promising pharmacological target for treating impulsivity‐related disorders, including substance abuse (for reviews see Le Foll et al., 2005; Sokoloff et al., 2006; Heidbreder, 2008). Our findings that increased impulsivity appears to be a behavioral risk factor for vulnerability to self‐administer cocaine support the relationship between addiction and impulsivity that has been previously reported in the literature (Jentsch and Taylor, 1999; Bickel and Marsch, 2001; De Wit, 2010). In this study, only 68% (13 out of 19) of the subjects acquired cocaine self‐administration initially, which is very low in contrast to our laboratory’s typical acquisition rate of approximately 100%. However, this lower acquisition rate may be related to the experimental design and is a strength of the acquisition procedure. Typically, to have a monkey acquire cocaine self‐administration, subjects are trained to respond on an operant schedule with food reinforcement and an intermediate dose of cocaine (0.03 mg/kg/injection) is made available, which results in almost every subject acquiring self‐administration very quickly. In the present study, we started with an extremely low cocaine dose (0.001 mg/kg/injection), made many 171 doses available in an ascending order, and repeatedly went back to a food baseline between every dose available. This design resulted in repeated pairings of the cues such as the pump noise and lights changing associated with receiving an injection and very low doses of drug that were not reinforcing, which is the equivalent of repeatedly extinguishing responding in these subjects. Ultimately 95% (18 of 19) of the subjects in this cohort acquired self‐administration, although the median dose for control monkeys was one log‐unti higher than the typical 0.03 cocaine dose we use for training. The experimental design provided a more sensitive measure of cocaine acquisition and one that allowed us to determine individual differences in vulnerability for cocaine to function as a reinforcer. Individual differences in vulnerability to addiction is well accepted in the clinical literature but has not been thoroughly investigated in animal self‐ administration studies. When studying the etiology of drug addiction, an important question is why certain individuals report becoming addicted to a substance after their first dose whereas other individuals are able to use a drug for months or even years only sporadically before becoming addicted to the substance (O’Brien et al., 1986). The present findings suggest that a particular set of traits, increased impulsivity and increased D3 receptor function, can predict individual differences in the development of cocaine self‐administration in monkeys. The differences we observed in impulsivity measures, D3 receptor function, and predisposition to acquire stimulant self‐administration were 15 172 years after the cocaine exposure in utero. Our data suggest that prenatal cocaine exposure may increase the likelihood of an adult behavioral and neurobiological phenotype that predisposes individuals to substance abuse. This is supported by the human prospective longitudinal studies which have found early deficits in attention and impulse control in prenatally cocaine exposed children at ages 4, 6, and 9 years old (Savage et al., 2005; Linares et al., 2006; Pulsifer et al., 2008), indicating that these children may also be more vulnerable to substance abuse. Taken together, the present results provide supporting evidence for long‐term neurobehavioral consequences of prenatal cocaine exposure on vulnerability to self‐administer cocaine. ACKNOWLEDGEMENTS This research was supported by National Institute on Drug Abuse grants R01 DA25120, R37 DA10584 and K31 DA024485. The authors report no conflict of interest and would like to acknowledge the excellent technical assistance of Tonya Calhoun and Whitney Wilson. The authors also thank Dr. Merle Paule for providing information related to the histories of these monkeys and Dr. Beth Reboussin for her assistance with statistical analysis. 173 FIGURE 1. Percentage of prenatally cocaine exposed (closed symbols) and control (open symbols) monkeys that reached criteria to acquire cocaine self‐ administration at various doses of cocaine available under an FR 30 schedule of reinforcement. 174 FIGURE 2. Amount of cocaine intake prior to reaching criteria to acquire cocaine self‐administration in prenatally cocaine exposed and control monkeys. Bars represent mean ± SEM values. *p<0.05. 175 FIGURE 3. Amount of cocaine intake prior to reaching criteria to acquire cocaine self‐administration in high vulnerable and low vulnerable monkeys. Bars represent mean ± SEM values. *p<0.05. 176 177 FIGURE 4. a) Impulsivity score derived from 4 behavioral measures of impulsivity (1 = least impulsive, 20 = most impulsive) in high and low vulnerable monkeys as described in Hamilton et al., (under review). Squares represent prenatally cocaine exposed monkeys and triangles represent control monkeys. b) Peak yawns elicited by the D3 receptor agonist quinpirole in high and low vulnerable monkeys. Squares represent prenatally cocaine exposed monkeys and triangles represent control monkeys. Quinpirole‐elicited yawning data are from Hamilton et al. (2010). c) Relationship between peak yawns elicited by quinpirole and cumulative cocaine intake prior to reaching acquisition criteria. 178 REFERENCES Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissgurth AP (1998) Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol 59:533‐543. Baer JS, Sampson PD, Barr HM, Connor PD, Streissgurth AP (2003) A 21‐year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry 60:377‐385. Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73‐86. Cornelius MD, Leech SL, Goldschmidt L, Day NL (2005) Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow‐up study. Neurotoxicol Teratol 27:667‐676. Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA (2009) Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Psychopharmacology 34:548‐554. Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 215:1267‐1270. De Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22‐31. Hamilton LR, Czoty PW, Gage HD, Nader MA (2010) Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology doi:10.1007/s00213‐010‐1847‐2. Hecht GS, Spear NE, Spear LP (1998) Alterations in the reinforcing efficacy of cocaine in adult rats following prenatal exposure to cocaine. Behav Neurosci 112:410‐418. Heidbreder C (2008) Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets 7:410‐421. 179 Heyser CJ, miller JS, Spear NE, Spear LP (1992) Prenatal exposure to cocaine disrupts cocaine‐induced conditioned place preference in rats. Neurotoxicol Teratol 14:57‐64. Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward‐related stimuli. Psychopharmacology 146:373‐390. Kandel DB, Wu P, Davies M (1994) Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health 84:1407‐1413. Keller RW Jr, LeFevre R, Raucci J, Carlson JN, Glick SD (1996) Enhanced cocaine self‐administration in adult rats prenatally exposed to cocaine. Neurosci Lett 205:153‐156. Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J of Neurosci 29:14734‐14740. Le Foll B, Goldberg SR, Sokoloff P (2005) The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology 49:525‐541. Lin D, Kellogg CK (1996) Neonatal exposure to cocaine enhances the reward‐ potentiating properties of the drug in young adult animals. Behav Neurosci 110:791‐801. Malanga, CJ, Riday TT, Carlezon WA, Kosofsky BE (2008) Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry 63:214‐221. Morris P, Binienda Z, Gillam MP et al (1996) The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 18:147‐154. Morris P, Binienda Z, Gillam MP et al (1997) The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol 19:47‐57. O’Brien CP, Ehrman RN, Terres JW (1986) Classical conditioning in human opioid dependence. In: Behavioral Analysis of Drug Dependence. 180 (Goldberg SR, Stolerman IP, eds), London: Academic Press, pp329‐ 356. Paule MG, Gillam MP, Binienda Z, Morris P (1996) Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci 801:301‐309. Porath AJ, Fried PA (2005) Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol 27:267‐ 277. Pulsifer MB, Butz AM, O’Reilly FM, Belcher HM (2008) Prenatal drug exposure: effects on cognitive functioning at 5 years of age. Clin Pediatr 47:58‐65. Rocha BA, Mead AN, Kosofsky BE (2002) Increased vulnerability to self‐ administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology 163:221‐229. Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H (2005) Attentional functioning and impulse control in cocaine‐exposed and control children at age ten years. J Dev Behav Pediatr 26:42‐47. Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006) The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5:25‐43. Staley JK, Mash DC (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100‐ 6106. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (1996) Results from the 1992 National Pregnancy and Health Survey. Ref Type: Report. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies (2008) Ref Type: Report. 181 CHAPTER V DISCUSSION “Some addicts go for months or years using heroin or cocaine only on weekends before becoming a daily (addicted) user. Others report that they had such an intense positive response that they became addicted with the first dose…” O’Brien et al., 1986. Studying a complex, multi‐dimensional psychiatric disorder like substance abuse is made even more challenging by the fact that drug abusers are a highly heterogenous group. Determining individual vulnerability to go from misuse of drugs to addiction, as the O’Brien and colleagues quote indicates, is a critical line of research in the study of the etiology of drug addiction. It is believed that there are multiple factors that contribute to vulnerability, inherent or acquired (Le Moal, 2009). Quantitative traits that indicate a specific risk factor can be conceptualized as a behavioral or biological phenotype. The research in this dissertation was designed to examine several of the multiple determinants of an addiction phenotype and their interactions. These studies attempted to try to elucidate a vulnerable phenotype in order to identify characteristics (behavioral and neuropharmacological) that may predispose an individual to addiction by characterizing the long‐term effects of prenatal cocaine exposure in adult 182 rhesus monkeys. The dopamine receptor system, behavioral measures of impulsivity, and acquisition of cocaine self‐administration were examined in a cohort of adult rhesus monkeys that were exposed to cocaine throughout gestation. As will be described in this Chapter, there appears to be a relationship between DA D3 receptor function, impulsivity, and acquisition of cocaine self‐administration. The research described in this dissertation supports the idea that there are biological and behavioral phenotypes that seem to predispose individuals to drug‐taking behavior and that prenatal cocaine exposure can increase the likelihood of these phenotypes. ACQUISITION OF COCAINE SELFADMINISTRATION Measuring acquisition of drug self‐administration is a method of examining the etiology of drug‐taking behavior that allows for the investigation of variables that may attenuate or enhance initiation of drug use. Factors that impede acquisition may be targets for treatment. Likewise, variables that increase the rate of acquisition may be identified as risk factors that predispose to drug use. In typical acquisition studies, the dose of the drug is held contant and the criteria to reach acquisition are defined as a set number of injections received or set amount of drug taken (mg/kg). By defining acquisition as reaching set arbitrary criteria, it can be argued that these studies are assessing criterion performance not acquisition of the drug becoming a reinforcer. For example, Carroll and Lac (1997) investigated acquisition of amphetamine (0.06 mg/kg/injection) self‐administration and 183 defined acquisition as greater than 50 injections per session. One rat that did not reach this acquisition criterion had a mean of 44 injections per session over the 30 days of acquisition. It is not clear whether amphetamine was a reinforcer in this particular rat because there was no comparison to vehicle. It is possible that amphetamine was a reinforcer in this animal without reaching the defined arbitrary criteria. In Chapter IV, an acquisition procedure was used that operationally defined acquisition regardless of dose. We assessed multiple doses of cocaine in ascending order and defined acquisition as greater responding for cocaine than when saline was available (Figure 1). Each animal’s individual saline baseline rates of responding were used to determine whether or not the acquisition criterion had been met. This methodology allowed us to focus on individual differences in acquiring cocaine reinforcement. This method, which used very low doses of drug, also highlights more variability between subjects than training an animal to acquire cocaine self‐administration at a moderate or high dose. This increased variability allowed the groups of animals to differentiate from each other more and enhanced our ability to detect variables that affected acquisition rate. 184 FIGURE 1. Representative response rates for food (open symbols) and drug (closed symbols) under an FR 30 schedule of reinforcement during the acquisition of cocaine self‐administration for a prenatally cocaine exposed monkeys. The lower acquisition rate observed in these studies could be explained by latent inhibition, which is a reduction in conditioning to a stimulus that occurs as a result of preexposure to that stimulus without reinforcement (Weiner 1990). Since the acquisition procedure repeatedly paired doses of cocaine that were not reinforcing to the animal with the cues, the monkeys may have learned to ignore stimuli that are repeatedly presented because they were not previously followed by a meaningful consequence. It is possible that the latent inhibition processes were stronger 185 in control animals compared to prenatally cocaine‐exposed animals since they took longer to acquire cocaine self‐administration. The prenatally cocaine‐exposed monkeys may be less responsive to Pavlovian conditioning compared to the controls. Latent inhibition is a robust behavioral phenomenon and can be demonstrated in many species, including in humans, across a variety of classical and instrumental conditioning procedures, including avoidance, taste aversion, and discrimination learning (Lubow, 1989). It is thought that latent inhibition, since it is conserved across many species and is observed across a broad range of conditions, serves an important adaptive function, allowing for more efficient and rapid learning (Lubow, 1989). Additionally, latent inhibition is considered to reflect animals’ learning not to attend to or to ignore irrelevant stimuli and has a large attentional component. Therefore, in addition to displaying increased sensitivity to the reinforcing effects of low doses of cocaine, prenatally cocaine‐exposed monkeys may also be demonstrating altered attentional processes compared to control monkeys. Since it has been hypothesized that activation of the mesolimbic dopaminergic system disrupts the latent inhibition processes (Wiener, 1990) and prenatally cocaine‐exposed monkeys have increased D3 receptor function (Hamilton et al., 2010), it is possible that the prenatally cocaine‐ exposed monkeys acquired cocaine self‐administration more readily than controls due to alterations in their latent inhibition processes. 186 This explanation is supported by the preliminary data indicating that when the cocaine dose‐response curves are re‐established after acquisition has already occurred, there are no differences between prenatally cocaine‐ exposed monkeys and control monkeys (Figure 2). This would be consistent with the human studies that have found a pattern of difficulties with sustained and selective attention (Bandstra et al., 2001; Savage et al., 2005; Linares et al., 2006). FIGURE 2. Dose‐response curves for prenatally cocaine‐exposed (closed symbols) and control (open symbols) monkeys under an FR 30 schedule of reinforcement during the acquisition (left panel) and maintenance (right panel) of cocaine self‐administration. The prenatally cocaine‐exposed monkeys had higher response rates and the peak of their dose‐response curve was 0.003 mg/kg/injection compared to controls whose peak was a full log‐unit higher, 0.03 mg/kg/injection. This could indicate the prenatally cocaine‐exposed 187 monkeys are more sensitive to the reinforcing effects of cocaine. However, during maintenance sessions, the dose‐response curve shifted to the right for the prenatally cocaine‐exposed monkeys so it was now identical to the control monkeys’ dose‐response curve. Therefore, under an FR schedule of reinforcement, the initial increased sensitivity is no longer present. Other schedules of reinforcement, like choice studies or progressive‐ratio (PR) studies, should be examined to determine if the reinforcing strength of cocaine is the same between the two groups. PRENATAL COCAINE DOSERESPONSE EFFECTS In the prenatal drug exposure literature, there is debate about whether the cumulative dose over gestation or the maximum dose used on a single occasion is the primary determinant of adversive outcome (Frank et al., 1998). Based on neonatal size at birth, neonatal behavior, and infant informational processing, it appears that in the human literature there is a cocaine dose‐effect relationship (Jacobson et al., 1996; Hurt et al., 1997; Chiriboga et al., 1999; Bateman and Chiriboga, 2000). One of the advantages of the cohort examined in this dissertation is that the prenatally cocaine exposed monkeys varied in their cumulative dose of cocaine they were exposed to throughout gestation as well as varied in their maximal dose used on a single occasion. Therefore, we could examine whether there was a dose effect relationship that could be observed in adulthood. In Chapter II, we 188 determined that there was a significant positive correlation between the daily maximal dose of cocaine each monkey was exposed to in utero and D3 receptor function. However, when cumulative gestational cocaine exposure was used in the analysis, the correlation was no longer significant. All the other measures discussed in Chapters III and IV were also examined for an in utero cocaine dose effect relationship. Although there was a trend for a negative correlation between sessions to acquire cocaine self‐administration and cumulative gestational cocaine exposure dose, it was not significant (r2 = 0.16, p = 0.1; data not shown). No other measures were revealed to have a relationship to either cumulative gestational cocaine dose or maximal dose received on a single occasion. This suggests while there may a dose effect relationship present (as evident by the D3 function and acquisition of self‐ administration data), it is challenging to observe in adulthood, years after the prenatal cocaine exposure. MULTIPLE ASPECTS OF IMPULSIVITY It has been said that, “Impulsivity is key to understanding the phenomenology of externalizing disorders, such as addiction” (J.D. Jentsch, 2010). Impulsivity, as it relates to substance abuse, has been described as difficulty with interrupting and inhibiting automatic responses. Since drug dependence is characterized by risky drug‐taking behavior and repeated failure to reduce drug‐taking, dysfunction of the impulse control systems may play a key role in addiction. Willful and active suppression of drug‐ 189 taking behavior requires being able to voluntarily modulate impulsive thoughts and action to inhibit impulsive drug‐seeking and drug‐taking. Trait impulsivity is rapid, unplanned inflexible approach to novelty or to rewards and can be examined by novel object reactivity. High novelty‐ seeking individuals are considered impulsive (Cloninger, 1987) and this temperament has been linked in the general population to drug addiction and impulse control disorders (Kim and Grant, 2001). Discounting tasks are thought to measure a slightly different aspect of impulsivity, impulsive decision‐making or choice rather than impulsive behavior (Jentsch, 2008). Impulse control can be examined by reversal learning or perseveration tasks. Since impulsivity is a multi‐factorial construct, it stands to reason that various tasks to assess impulsivity rely upon only partially overlapping neural circuitry. Impulsive choice tasks like delay discounting have been linked to the anterior cingulate cortex, medial prefrontal cortex, and nucleus accumbens core (Cardinal et al., 2001; 2004) while impulse control of response adaptation (e.g., reversal learning) depends upon orbitofrontal cortex and its efferent targets in the medial striatum (Dias et al., 1996; Schoenbaum et al., 2002; Chudasama and Robbins, 2003; Fellows and Farah, 2003; Boulougouris et al., 2007; Clarke et al., 2008). If these tasks are neurally independent, then their individual relationships to addiction may vary as well. One cognitive component of the impulse control system is the ability to stop, withhold, or change responses. Reversal learning selectively 190 measures the ability to change or inhibit a conditioned response. Deficits in reversal learning has been shown to be predictive of the prognosis of drug‐ dependent individuals in treatment, with those with the most deficits on the task, who show the most perseveration of responding, being linked to relapse (Paulus et al., 2005). In animal studies, Jentsch and colleagues have determined that even short‐term (2 weeks) exposure to cocaine can produce long‐lasting impairments in reversal learning (Jentsch et al., 2002; Olausson et al., 2007). These impairments have also been observed in rats that self‐ administer cocaine (Calu et al., 2007) and human cocaine addicts (Fillmore and Rush, 2006; Ersche et al., 2008), indicating that this is a robust effect. Additionally, a recent study revealed that this deficit in inhibition of behavior may be specific to cocaine as individuals who abuse drugs other than cocaine did not show the same perseverative responding in a reversal learning task as cocaine abusers (Ersche et al., 2008). Exposure to drugs of abuse in adulthood can also impair impulsive choice. Rats chronically exposed to cocaine exhibit less ability to delay gratification than saline‐treated controls (Paine et al., 2003; Simon et al., 2007). Cocaine‐exposed rats also exhibit hypersensitivity to changes in reward delay and magnitude (Roesch et al., 2007). Collectively, the literature provides evidence that the direct pharmacological effects of drugs of abuse can alter impulsive responding and choice in laboratory animals. These changes may mirror similar deficits in performance in drug‐dependent individuals (Jentsch, 2008) which indicates 191 these laboratory measures may be useful analogous or homologous behavioral assessments. In Chapter III, we determined that prenatally cocaine‐exposed monkeys were more impulsive than controls across a variety of measures. Although we did not observe differences in novel object reactivity or locomotor activity in a novel environment, monkeys exposed to cocaine in utero displayed more response perseveration during extinction of food‐ reinforced behavior than controls. This is similar to earlier work with these same monkeys when they were much younger. Chelonis et al. (2003) showed the prenatally cocaine‐exposed monkeys displayed response perseveration for two and half years when the rules were reversed on a simple visual discrimination task compared to controls, which the authors believed indicated that these animals have greater difficulty adapting to important changes in their environment. In both Paule and colleagues’ study and in our experiments, all the monkeys acquired the original task equally well but the prenatally cocaine exposed monkeys (highly impulsive) perseverated much longer than control monkeys (low impulsive), indicating difficulty inhibiting what had become an automatic response. Additionally, male prenatally cocaine‐exposed monkeys exhibited greater discounting of a delayed food reward than controls, indicating impaired impulsive choice. These data demonstrate that the effects of cocaine exposure on impulsive responding and impulsive choice are incredibly long‐lasting (14‐15 years after prenatal exposure). 192 IMPULSIVITY AND COCAINE SELFADMINISTRATION It has been well‐established that increased impulsivity can be a direct consequence of chronic exposure to drugs of abuse, especially cocaine. However, it is also thought that impulsivity is a trait with naturally‐occurring variation that can be a risk‐factor for substance abuse. Impulsivity is at least in part under genetic control with more than 13% of natural variation in impulsive behavior attributed to variations in dopamine system‐related genes (Bailey et al., 2007; Groman et al., 2008) and this may be a crucial quantitative indicator of drug abuse liability. Dalley et al. (2007) showed that rats that exhibited greater impulsive action on a choice reaction‐time task subsequently took more cocaine. It is possible that there is a causal relationship between impulsivity and D2‐like receptor function. The rats that were most impulsive and most vulnerable to cocaine self‐administration behavior were also the ones with lower dopamine D2‐like receptor availability in the ventral striatum (Dalley et al., 2007). This same relationship between impulsivity and acquisition of nicotine self‐ administration has been observed in rodents (Diergaarde et al., 2008), suggesting this relationship may be true for all stimulants. Furthermore, blockade of D2‐like receptor function increases perseverative behavior on a reversal learning task in monkeys (Lee et al., 2007). Vulnerability to self‐administer drugs of abuse in rodents has been related to naturally occurring impulsivity. For example, rats that exhibit the 193 steepest delay discounting effects self‐administer more ethanol and cocaine than rats characterized as less impulsive (Poulos et al., 1995; Perry et al., 2005). In rats self‐administering nicotine, impulsive delay discounting performance predicts resistance to extinction and susceptibility to conditioned cue reinstatement (Diergaarde et al., 2008). Furthermore, naïve animals that are characterized as highly impulsive are more likely compared with low impulsive animals to transition sooner to inflexible cocaine‐taking that is resistant to punishment (Belin et al., 2008). In Chapter IV, we determined that prenatally cocaine exposed monkeys, who were previously characterized as more impulsive than controls, were more vulnerable to acquiring cocaine self‐administration. Additionally, the most impulsive monkeys, regardless of prenatal condition, acquired cocaine self‐administration more readily than the less impulsive monkeys. The studies we used to measure aspects of impulsivity were useful in defining a cocaine abuse “vulnerable” phenotype. Furthermore, we were able to establish that the biological basis of this predisposition to self‐ administer cocaine may be D3 receptor function, as animals that displayed the largest behavioral response to a D3 agonist were also more impulsive and more susceptible to cocaine functioning as a reinforcer than animals without as robust D3 receptor function. Although all four behavioral assays deemed to measure impulsivity were used in the calculation of the impulsivity score, certain tasks may have more predictive validity in NHP for which individuals may be most 194 vulnerable to self‐administration. The two conditioned behavioral measures, delay discounting and perseverative responding while extinguishing food‐ reinforced behavior, revealed significant differences between prenatally cocaine exposed and control monkeys by themselves while the two unconditioned behavioral measures, novel object reactivity and locomotor activity, only revealed trends towards a difference between the two groups. In NHP, more challenging, complex tasks like the conditioned behavioral measures may be more useful for parsing out differences in impulsivity than unconditioned behavioral measures, which have traditionally been used in rodent studies. Although no one impulsivity measure correlated with acquisition of self‐administration measures, in Chapter IV, we showed that monkeys classified as highly vulnerable were more impulsive overall than monkeys classified as less vulnerable. Although the conditioned behavioral measures might be better suited for detecting subtle differences in impulsivity, all measures used contribute towards predicting predisposition to self‐administration behavior. Therefore, there is still great value in using multiple assays to investigate and to create a clearer overall picture of impulsivity. Impulsive behavior has been shown to play a key role in our current concepts of drug abuse. Since animal models continue to demonstrate the nature of these relationships (i.e., chronic drug exposure causes increases in impulsivity but also that impulsivity is a risk factor for substance abuse), new pharmacological treatments should be selected for their ability to enhance 195 impulse control in these animal models in order to ideally reduce drug‐ seeking and drug‐taking behavior in addicts. D2LIKE RECEPTOR FUNCTION D2‐like agonists have a biphasic effect on yawning, with low‐doses producing a dose‐dependent increase in yawning and higher doses inhibiting yawning and inducing hypothermia (Collins et al., 2005; 2007). These effects have been attributed to the D3 and D2 receptors, respectively. Collins and colleagues (2005; 2007; 2008; 2009) have provided support for these subtype specific roles by antagonist interaction studies in which D3‐selective antagonists have been shown to produce dose‐dependent and selective rightward shifts of the ascending limb, whereas D2‐preferring antagonists have been shown to produce a selective rightward shift of the descending limb of the dose‐response curve for D2‐like agonist‐induced yawning at doses that also inhibit the induction of hypothermia. These differential roles of the D3 (induction) and D2 (inhibition) receptors in the mediation of yawning, allows for the determination of changes in these two receptor subtypes after exposure to cocaine since increases in D3 receptor density should result in leftward shifts of the ascending limb of the yawning dose‐ response curve while decreases in D2 receptor density should result in rightward shifts of the descending limb of the yawning dose‐response curve. Collins et al (in preparation) found that rats treated with 15 mg/kg cocaine i.p. once per day for seven days, a dose that results in locomotor 196 sensitization, displayed a progressive and persistant leftward and upward shift of the ascending limb of the pramipexole‐elicited yawning dose‐ response curve beginning as early as 72 hours after the first injection. This effect on the dose‐response curve was apparent even 6 weeks after the cocaine administration had ceased while the hypothermic response to pramipexole was unaffected by cocaine treatment. Furthermore, it was determined that there were increases in D3 receptor binding using in vitro [3H]7‐OH‐DPAT binding assays on membranes prepared from the ventral striatum tissue collected at 6 weeks post‐cocaine treatment suggesting the increased sensitivity in pramipexole‐elicited yawning was due to an upregulation of D3 receptors. This work supports previous research that found increases in D3 receptor binding in the nucleus accumbens core and ventral caudate‐putamen in rats that self‐administered cocaine (0.75 mg/kg/injection) on a variable‐ratio (VR) 5 schedule of reinforcement for 2 weeks after 32 days (but not 2 or 8 days) after their last self‐administration session (Neisewander et al., 2004). In Chapter II, we used D1‐like and D2‐like agonist‐induced behavioral effects in conjunction with PET measures of D2‐like receptor availability to determine if prenatal cocaine‐exposure affected the function of D1‐like or D2‐like receptors in adult rhesus monkeys. Interestingly, although the prenatally cocaine exposed monkeys did not differ from controls with respect to PET measures of D2‐like receptor availability or D1‐like agonist‐ induced eye blinking, a significant increase in quinpirole‐elicited yawning 197 was observed in monkeys that were exposed to cocaine in utero as compared to controls (Hamilton et al., 2010). Not only was this effect positively correlated with the maximal daily dose of cocaine received throughout gestation, but these increases in quinpirole‐elicited yawning were observed 13 years after in utero exposure. This suggests that cocaine exposure may result in long‐lasting, if not permanent, enhancement of the function and/or sensitivity of D3 receptors, which would support the finding of upregulation of D3 receptors in the nucleus accumbens of human cocaine overdose fatalities (Staley and Mash, 1996; Segal et al., 1997). The use of D2‐like PET ligands (e.g., [11C] raclopride, [18F] FCP, and [18F] fallypride) has improved the ability to conduct longitudinal, within subject studies of the relationship between striatal D2‐like receptor availability and behavior in humans, monkeys, and rats (Volkow et al., 1999; Morgan et al., 2002; Martinez et al., 2004; Nader et al., 2006; Dalley et al., 2007). However, it is difficult to parse the contributions of changes in D3 receptor availability and/or changes in the D2 or D3 receptor affinity as these radioligands do not discriminate between D2 and D3 receptors and are insensitive to changes in the functional state of D2‐like receptors. Therefore, another explanation for the lack of differences in D2‐like receptor availability as examined with PET is that if there were differences in D3 and D2 receptor density (upregulated D3, downregulated D2) in the prenatally cocaine exposed monkeys compared to controls it could have been masked by basal dopamine binding to receptors differentially. 198 Dopamine has a 70‐fold greater affinity for the D3 receptor compared to the D2 receptor (Sokoloff et al., 1992). Binding constants for the dopamine receptor for competition with dopamine in cloned human dopamine receptors in vitro [Ki (nM)] are D3 = 30, D5 = 230, D4 = 450, D2 = 2000, D1 = 2300 (Sokoloff et al., 1992). Therefore, at a resting dopamine concentration of 5 nM, these relative affinities would predict that D3 receptors would be 14% occupied while occupancy of D2 receptors would be about 0.2%. If the dopamine concentration was increased by the blockade of the DAT by a stimulant drug like cocaine to 250 nM, D3 receptor occupancy would be 90% while D2 receptors would be about 10% occupied. These marked differences in receptor occupancy could result in differences in FCP binding. If there was an upregulation of D3 receptor density, more dopamine would be bound to the D3 receptors, allowing FCP to bind more at D2 receptors in the prenatally cocaine exposed monkeys. If these monkeys also had a decrease in D2 receptor density, it could still appear the same using FCP as control animals where more D2 receptors are bound with dopamine (see Figure 3). 199 PRENATALLY COCAINE EXPOSED D3 receptor D2 receptor Dopamine FCP CONTROLS D3 receptor D2 receptor Dopamine FCP 200 FIGURE 3: Despite potential alterations in D3 and D2 receptors in prenatally cocaine exposed monkeys, D2‐like receptor availability as measured by [18F]FCP could still have been similar to controls due to differences in dopamine’s affinity for the receptor subtypes. To determine more definitively whether there was an upregulation of D3 receptors and/or a downregulation of D2 receptors, completive binding studies could be done. Administering a selective D2 receptor antagonist prior to injection of FCP would allow researchers to examine just the D3 receptor availability. Likewise, administration of a selective D3 receptor antagonist that would compete with dopamine to bind the D3 receptors prior to FCP injection would allow for examination of just D2 receptor availability. Another important point is that even though there were no changes detected in D2‐like receptor availability with PET (Chapter II), once the animals have self‐administered cocaine chronically, there could be differences in the reduction of D2‐like receptor availability observed between the two groups. For example, when the system is stressed by the chronic cocaine exposure in adulthood, the prenatally cocaine exposed monkeys may show more dramatic decreases in D2‐receptor availability compared to controls. D3 RECEPTOR FUNCTION AND SELFADMINISTRATION D3 receptors may play a crucial role in the reinforcing effects of stimulants. The earliest indicator was that D3 receptors have a unique 201 anatomical distribution with highest concentrations found in mesolimbic systems that have been implicated in drug abuse (Levesque et al., 1992). D3‐ preferring agonists can modulate cocaine self‐administration (Caine and Koob, 1993; 1995; Nader and Mach, 1996; Parsons et al., 1996). Moreover, it has been shown that the relative potencies of D2‐like receptor agonists to alter cocaine self‐administration is highly correlated with their relative potencies for increasing mitogenesis in vitro in cell lines expressing D3 but not D2 receptors (Caine et al., 1997). The D3‐preferring agonist 7‐OH‐DPAT functions as a reinforcer in monkeys with a cocaine self‐administration history but fails to support self‐administration in drug‐naïve monkeys (Nader and Mach, 1996). This suggests that some alteration in D3 receptor sensitivity and/or density occurs following cocaine exposure. Although D3‐selective antagonists have generally been found to be ineffective at decreasing self‐administration when cocaine is available under a low FR (i.e., FR 1 or FR 2) schedules of reinforcement (Gal and Gyertyan, 2003; Xi et al., 2005; Xi et al., 2006), there is growing evidence suggesting that the D3 receptor plays an important role in drug abuse‐related behaviors, like reactivity of laboratory animals to drug‐paired stimuli and drug‐seeking behaviors. D3‐preferring agonists like quinpirole have been shown to induce responding for stimuli that had been previously paired with cocaine reinforcement (Collins and Woods, 2009). Additionally, a variety of D3‐ selective antagonists and partial agonists have also been shown to inhibit the capacity of cues to reinstate responding after some period of abstinence from 202 stimulant self‐administration (Vorel et al., 2002; Gilbert et al., 2005; Gal and Gyertyan, 2006; Cervo et al., 2007; Khaled et al., 2009). Furthermore, in Chapter IV, we determined that monkeys that were most vulnerable to acquisition of cocaine self‐administration had greater sensitivity to D3 agonist‐elicited yawning than monkeys that were less vulnerable to cocaine self‐administration. Therefore, it appears that increased D3 receptor sensitivity and/or density may be a biological risk factor for progressing to stimulant addiction and that there is increase in D3 receptor density that occurs following cocaine exposure that may be linked to the reinforcing effects of cocaine and the development of cocaine dependence. IMPULSIVITY AND D3 RECEPTOR FUNCTION Recently, it has been observed that patients with Parkinson’s Disease being treated with DA agonists may develop impulse control disorders, such as pathological gambling, compulsive shopping, and hypersexuality (Pontone et al., 2006; Szarkman et al., 2006; Weintraub et al., 2006; Voon et al., 2007). A review of the Food and Drug Administration adverse events database revealed that treatment with DA agonists was a major correlate of pathological gambling and the most frequently identified medication was pramipexole, the D3 receptor preferring agonist (Szarfman et al., 2006). In fact, pramipexole was identified in 58% of the 67 pathological gambling reports in the database (which was not confined to Parkinson’s Disease patients) (Szarfman et al., 2006). A more recent review of all published cases 203 of Parkinson’s disease patients that developed an impulse control disorder, 174 out of 177 patients were being treated with a DA agonist (Gallagher et al., 2007). This review also concluded that incidence of pathological gambling was as high as 8% in Parkinson’s Disease patients treated with DA agonists compared to less than 1% in the general population (Gallagher et al., 2007). Although there have been questions regarding a causal relationship between DA agonists like pramipexole and pathological gambling, evidence for such a causative role is growing. Rigorous clinical evaluations have shown that patients treated with levodopa alone did not develop impulse control disorders while treatment with pramipexole was predictive of developing an impulse control disorder (Pontone et al., 2006; Weintraub et al., 2006). This indicates that the development of impulse control disorders may not be simply due to an increase in DA signal, but perhaps related to D3 receptor stimulation. In a follow‐up study with 12 Parkinson’s Disease patients that had developed an impulse control disorder, reducing the dose of the DA agonist and increasing levodopa dose to achieve the same benefit in relieving motor symptoms resulted in resolution of the impulse control disorder symptoms in all patients (Mamikonyan et al., 2008). Also, an association has been shown between a variant of the D3 receptor gene DRD3, but not the D2 receptor gene DRD2, and development of impulse control disorders in Parkinson’s patients (Lee et al., 2009). 204 It appears that this increased risk of impulse control disorders associated with DA agonists is not unique to Parkinson’s Disease patients. Reports are emerging of pathological gambling as a side effect in patients with restless legs syndrome being treated with pramipexole (Tippmann‐ Peikert et al., 2007; Driver‐Dunckley et al., 2007; Ondo and Lai, 2008, Cornelius et al., 2010). Ondo and Lai (2008) found that almost 20% of patients interviewed indicated increased impulsivity with the use of DA agonists, specifically pramipexole. Furthermore, a statistically significant correlation for pramipexole dose and impulse control disorders has been observed in restless legs syndrome patients (Ondo and Lai, 2008; Cornelius et al., 2010). Taken together, it appears that the administration of DA agonists, in particular the D3 preferring agonist pramipexole, can be a trigger for development of pathological gambling and other impulse control disorders, suggesting a causal role for increased D3 receptor stimulation and impulsivity. This conclusion is supported by the data in Chapters II and III that found prenatally cocaine exposed monkeys were more impulsive than controls and that increased D3 receptor function may be the neurobiological correlate for that behavioral change. SEX DIFFERENCES Other than DA agonist treatment, one of the main risk factors for the development of impulse control disorders in patients with parkinsonian‐ related diseases is male sex (Voon et al., 2007). This is interesting to note 205 considering the sex difference observed in Chapters III. The increase in overall impulsivity was observed in male, but not female, prenatally cocaine‐ exposed monkeys. Despite the fact that differences were observed in impulsivity, in Chapter IV we did not find any sex differences in acquisition of cocaine self‐administration (Figure 4), suggesting that increased D3 receptor function may be a larger risk factor for vulnerability to stimulant self‐administration than increased impulsivity, at least in females. FIGURE 4. Percentage of male (closed symbols) and female (open symbols) monkeys that reached criteria to acquire cocaine self‐ administration at various doses of cocaine available under an FR 30 schedule of reinforcement. 206 Furthermore, the relationship we found in individual animals between impulsivity and D3 receptor function and total cumulative cocaine intake prior to reaching acquisition criteria was found in both male and female subjects. However, we had fairly low power to detect differences in the survival analysis of acquisition of cocaine self‐administration due to such a low acquisition rate across all groups resulting in censored data. Therefore, it is possible that with a larger cohort, subtle sex differences in acquisition of cocaine self‐adminstration may be observable. Alternatively, other mechanisms may play a larger role in vulnerability to stimulant self‐administration behavior in female subjects than impulsivity and D3 receptor function. For example, it has been shown that estrogen can alter the response to cocaine in rats (Sell et al., 2000). Estrogen can also modulate DA neuron firing activity in VTA neurons induced by a cocaine injection (Zhang et al., 2008), and regulate mRNA expression for specific DA and 5‐HT receptors (Zhou et al., 2002). Studies in NHP have shown ovarian steroid can affect functional properties of the 5‐HT neural system (Bethea et al., 1998; Pecins‐Thompson et al., 1998; Pecins‐ Thompson and Bethea, 1999). Since there is evidence for a modulatory role of 5‐HT in the behavioral effects of cocaine (Cunningham and Callahan, 1994; Satel et al., 1995), ovarian hormones have the potential to alter both 5‐HT and DA neurotransmission resulting in modification of the response to stimulants. It is possible that the modulatory role of 5‐HT on response to cocaine may be more critical in females than in males for acquisition of self‐ 207 administration. It is also possible that if we had examined cocaine self‐ administration in the luteal phase when estrogen levels are lower than in the follicular phase or if we had examined self‐administration during adolescence, we may have observed sex differences in the acquisition of self‐ administration. Nevertheless, the findings in this dissertation that males exposed to cocaine throughout gestation are more impulsive compared to controls concurs with the human literature. The behavioral issues noted in children exposed to cocaine in utero appear to be exaggerated in males , with the prenatally cocaine‐exposed boys often demonstrating increased aggression, hyperactivity, and disruptive behavior than non‐exposed boys, whereas girls do not show these difference (Bendersky et al., 2006; Bennett et al., 2007; Delaney‐Black et al., 2004). Since these patterns of behavioral issues are associated with development of substance use disorders, it suggests that these male children may be more vulnerable to stimulant drug taking. CONCLUSION The research presented in this dissertation further extends our understanding of the neurobiological and neurobehavioral underpinnings of individual phenotypes related to vulnerability of drug‐taking behavior. The research in this dissertation also provides to the rationale for the development of D3 receptor compounds as pharmacological targets for 208 treating impulse control disruptions. Beyond pharmacological treatment, behavioral interventions could be implemented with this high risk population of children. For example, cognitive‐behavioral therapy has shown modest success in decreasing impulsive behavior in adults with impulse control disorders (Drysdale et al., 2009; Filomensky and Tavares, 2009; Okuda et al., 2009) and could be adapted for younger children. The data in this dissertation suggest that predisposition to acquire cocaine self‐ administration is associated with increased impulsivity and increased D3 receptor function. Since it appears that prenatal cocaine exposure increases the tendency of an individual having this vulnerable phenotype in adulthood, prenatally cocaine exposed children may be at risk for increased likelihood of stimulant use. 209 REFERENCES Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA (2007) The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatr Genet 17:23‐27. Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA (2001) Longitudinal investigation of task persistance and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol 23:545‐559. Bateman DA, Chiriboga CA (2000) Dose‐response effect of cocaine on newborn head circumference. Pediatrics 106:E33. Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine‐taking. Science 320:1352‐ 1355. Bethea CL, Pecins‐Thompson WE, Schutzer WE, Gundlah C, Lu ZN (1998) Ovarian steroids and serotonin neural function. Mol Neurobiol 18:87‐ 123. Boulougouris V, Dalley JW, Robbins TW (2007) Effects of orbitofrontal, infralimbic, and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res 179:219‐228. Caine SB, Koob GH (1993) Modulation of cocaine self‐administration in the rat through D3 dopamine receptors. Science 260:1814‐1816. Caine SB, Koob GF (1995) Pretreatment with the dopamine agonist 7‐OH‐ DPAT shifts the cocaine self‐administration dose‐effect function to the left under different schedules in the rat. Behav Pharmacol 6:333‐347. Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P (1997) D3 receptor test in vitro predicts decreased cocaine self‐administration in rats. Neuroreport 8: 2373‐2377. Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G (2007) Withdrawal from cocaine self‐administration produces long‐lasting deficits in orbitofrontal‐dependent reversal learning in rats. Learn Mem 14:325‐328. 210 Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499‐2501. Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ (2004) Limbic corticostriatal systems and delayed reinforcement. Ann NY Acad Sci 1021‐33‐50. Carroll ME, Lac ST (1997) Acquisition of IV amphetamine and cocaine self‐ administration in rats as a function of dose. Psychopharmacology 129:206‐214. Cervo L, Cocco A, Petrella C, Heidbreder CA (2007) Selective antagonism at dopamine D3 receptors attenuates cocaine‐seeking behaviour in the rat. Int J Neuropsychpharmacol 10:167‐181. Chelonis JJ, Gillam MP, Paule MG (2003) The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol 25:437‐446. Chiriboga CA, Brust JC, Bateman D, Hauser WA (1999) Dose‐response effect of fetal cocaine exposure on newborn neurological function. Pediatrics 103:79‐85. Chudasama Y, Robbins TW (2003) Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23:8771‐8780. Cloninger CR (1987) A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry 44:573‐588. Collins GT, Calinkski DM, Newman AH, Grundt P, Woods JH (2008) Food restriction alters pramipexole‐induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor‐mediated effects. J Pharmacol Exp Ther 325: 691‐697. Collins GT, Newman AH, Grundt P et al (2007) Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology 193:159‐170. Collins GT, Truccone A, Haji‐Abdi F et al (2009) Proerectile effects of dopamine D2‐like agonists are mediated by the D3 receptor in rats and mice. J Pharmacol Exp Ther 329:210‐217. 211 Collins GT, Witkin JM, Newman AH et al (2005) Dopamine agonist‐induced yawning in rats: a dopamine D3 receptor‐mediated behavior. J Pharmacol Exp Ther 314:310‐319. Collins GT, Woods JH (2009) Influence of conditioned reinforcement on the response‐maintaining effects of quinpirole in rats. Behav Pharmacol 20:492‐504. Cornelius JR, Tippmann‐Peikert M, Slocumb NL, Frerichs CF, Silber MH (2010) Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case‐control study. Sleep 33:81‐87. Cunningham KA, Callahan PM (1994) Neurobehavioral pharmacology of cocaine: role for serotonin in its locomotor and discriminative stimulus effects. NIDA Res Monogr 145:40‐66. Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 215:1267‐1270. Dias R, Robbins TW, Roberts AC (1996) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380:69‐72. Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, de Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301‐308. Driver‐Dunckley ED, Noble BN, Hentz JG, Evidente UG, Caviness JN, Parish J, Krahn L, Adler CH (2007) Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol 30:249‐255. Drysdale E, Jahoda A, Campbell E (2009) The manipulation of arousal on the intensity of urges to pull hair in a 16 year old female with trichotillomania: a single case study. Behav Cogn Psychother 37:115‐ 20. Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008) Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology 197:421‐431. 212 Fellows LK, Farah MJ (2003) Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain 126:1830‐1837. Filomensky TZ, Tavares H (2009) Cognitive restructuring for compulsive buying. Rev Bras Psiquiatr 31:77‐78. Frank DA, Jacobs RR, Beeghly M, Augustyn M, Bellinger D, cabral H, Heeren T (2002) Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics 110:1143‐1152. Gal K, Gyertyan I (2003) Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self‐administration in rats. Brain Res Bull 61:595‐601. Gal K, Gyertyan (2006) Dopamine D3 as well as D2 receptor ligands attenuate the cue‐induced cocaine‐seeking in a relapse model in rats. Drug Alcohol Depend 81:63‐70. Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A (2007) Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord 22:1757‐1763. Gilbert JG, Newman AH, Gardner EL, Ashby CR, Heidbreder CA, Pak AC, Peng XQ, Xi ZX (2005) Acute administation of SB‐277011A, NGB 2904, or BP 897 inhibits cocaine‐induced reinstatement of drug‐seeking behavior in rats: role of dopamine D3 receptors. Synapse 57:17‐28. Groman SM, James AS, Jentsch JD (2009) Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev 33:690‐698. Gundlah C, Pecins‐Thompson M, Schutzer WE, Bethea CL (1999) Ovarian steroid effects on serotonin 1A, 2A, and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res 63:325‐339. Hamilton LR, Czoty PW, Gage HD, Nader MA (2010) Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology 210: 481‐488. Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta J (1997) A prospective evaluation of early language development in children with in utero cocaine exposure and in control subjects. J Pediatr 130:310‐312. 213 Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM (1996) New evidence for neurobehavioral effects of in utero cocain exposure. J Pediatr 129:581‐590. Jentsch JD (2008) Impulsivity in animal models for drug abuse disorders. Drug Discov Today Dis Models 5:247‐250. Jentsch JD, Olausson P, De La Garza R, Taylor JR (2002) Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administration to monkeys. Neuropsychopharmacology 26:183‐190. Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B (2009) The selective dopamine D3 receptor antagonist SB 277011‐ A, but not the partial agonist BP 897, blocks cue‐induced reinstatement of nicotine‐seeking. Int J Neuropsychopharmacol 13:181‐190. Kim SW, Grant JE (2001) Personality dimensions in pathological gambling disorder and obsessive‐compulsive disorder. Psychiatry Res 104:205‐ 212. Le Moal M (2009) Drug abuse: vulnerability and transition to addiction. Pharmacopsychiatry 42: S42‐55. Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA (2009) Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J of Neurosci 29:14734‐14740. Lee J‐Y, Lee EK, Park SS, Lim J‐Y, Kim HJ, Kim JS, Jeon SB (2009) Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord 24:1803‐1810. Levesque D, Diaz J, Pilon C, Martes MP, Giros B, Souil E et al (1992) Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7‐[3H]hydroxy‐N,N‐di‐n‐propyl‐2‐ aminotetralin. Proc Natl Acad Sci USA 89:8155‐8159. Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S (2006) Mental health outcomes of cocaine‐exposed children at 6 years of age. J Pediatr Psychol 31:85‐97. 214 Lubow RE (1989) Latent inhibition and conditioned attentional theory. Cambridge University Press: New York. Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, Weintraub D (2008) Long‐term follow‐up of impulse control disorders in Parkinson’s disease. Mov Disord 23:75‐80. Martinez D, Broft A, Foltin RW et al (2004) Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine‐seeking behavior. Neuropsychopharmacology 29:1190‐1202. Morgan D, Grant KA, Gage HD et al (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self‐administration. Nat Neurosci 5:169‐174. Nader MA, Mach RH (1996) Self‐administration of the dopamine D3 agonist 7‐OH‐DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology 125:13‐22. Nader MA, Morgan D, Gage HD et al (2006) PET imaging of dopamine D2 receptors during chronic cocaine self‐administration in monkeys. Nat Neurosci 9:1050‐1056. Neisewander JL, Fuchs RA, Tran‐Nguyen LT, Weber SM, Coffey GP, Joyce JN (2004) Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self‐ administration: implications for cocaine‐seeking behavior. Neuropsychopharmacology 29:1479‐1487. O’Brien CP, Ehrman RN, Ternes JW (1986) Classical conditioning in human opiod dependence. In Behavioral Analysis of Drug Dependence. Goldberg SR and Stolerman IP, Eds. Academic Press: London, pp. 329‐ 354. Okuda M, Balan I, Petry NM, Oquendo M, Blanco C (2009) Cognitive‐ behavioral therapy for pathological gambling: cultural considerations. Am J Psychiatry 166:1325‐1330. Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR (2007) Orbitofrontal cortex and cognitive‐motivational impairments in psychostimulant addiction: evidence from experiments in the non‐ human primate. Ann NY Acad Sci 1121:610‐638. 215 Ondo WG, Lai D (2008) Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord 14:28‐ 32. Paine TA, Dringenberg HC, Olmstead MC (2003) Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147:135‐147. Paulus MP, Tapert SF, Schuckit MA (2005) Neural activation patterns of methamphetamine‐dependent subjects during decision making predict relapse. Arch Gen Psychiatry 62:761‐768. Pecins‐Thompson M, Bethea CL (1999) Ovarian steroid regulation of serotonin‐1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 89:267‐277. Pecins‐Thompson M, Brown NA, Bethea CL (1998) Regulation of serotonin reuptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res 53:120‐129. Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self‐ administration in female rats. Psychopharmacology 178:193‐201. Pontone G, Williams JR, Bassett SS, Marsh L (2006) Clinical features associated with impulse control disorders in Parkinson disease. Neurology 67:1258‐1261. Poulos CX, Le AD, Parker JL (1995) Impulsivity predicts individual susceptibility to high levels of alcohol self‐administration. Behav Pharmacol 6: 810‐814. Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G (2007) Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci 27:245‐250. Satel SL, Krystal JH, Delgado PL, Kosten TR, Chamey DS (1995) Trypotophan depletion and attenuation of cue‐induced craving for cocaine. Am J Psychiatry 152:778‐783. Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H (2005) Attentional functioning and impulse control in cocaine‐exposed and control children at age ten years. J Dev Behav Pediatr 26:42‐47. 216 Schoenbaum G, Nugent SL, Saddoris MP, Setlow B (2002) Orbitofrontal lesions in rats impair reversal but not acquisition of go, no‐go odor discriminations. Neuroreport 13:885‐890. Schoenbaum G, Saddoris MP, Stalnaker TA (2007) Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann NY Acad Sci 1121:320‐35 Segal DM, Moraes CT, Mash DC (1997) Up‐regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res 45:335‐339. Sell SL, Scalzitti JM, Thomas ML, Cunningham KA (2000) Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther 293:879‐886. Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long‐term increases in impulsive choice. Behav Neurosci 121:543‐549. Sokoloff P, Martes MP, Giros B, Bouthenet ML, Schwartz JC (1992) The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol 43:659‐666. Staley JK, Mash DC (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100‐ 6106. Szarfman A, Doraiswamy PM, Tonning JM, Levine JG (2006) Association between pathological gambling and Parkinsonian therapy as detected in the Food and Drug Administration Adverse Event Database. Arch Neurol 63:299‐300. Tippmann‐Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH (2007) Pathological gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology 68:301‐303. Volkow ND, Wang GJ, Fowler JS et al (1999) Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 156:1440‐1443. Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, Duff‐Canning S, Lang AE, Zurowski M (2007) Factors associated with dopaminergic drug‐related pathological gambling in Parkinson disease. Arch Neurol 64:212‐216. 217 Vorel SR, Ashby CR, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine‐seeking and cocaine‐enhanced brain reward in rats. 22:9595‐ 9603. Weiner I (1990) Neural substrates of latent inhibition: the switching model. Psych Bull 108:442‐461. Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB (2006) Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 63:969‐ 973. Xi ZX, Gilbert JG, Pak AC, Ashby CR, Heidbreder CA, Gardner EL (2005) Selective dopamine D3 receptor antagonism by SB‐277011A attenuates cocaine reinforcement as assessed by progressive‐ratio and variable‐cost‐variable‐payoff fixed‐ratio cocaine self‐ administration in rats. Eur J Neurosci 21:3427‐3428. Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Gitajn L, Gardner EL (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine‐induced reinstatement of drug‐seeking behavior in rats. Neuropsychopharmacology 31:1393‐1405. Zhang D, Yang S, Yang C, Jin G, Zhen X (2008) Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 199:625‐635. Zhou W, Cunningham KA, Thomas ML (2002) Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res 100:75‐ 83. 218 SCHOLASTIC VITAE LINDSEY REBECCA HAMILTON EDUCATION: 2001‐2004 2005‐2010 Bates College Lewiston, Maine B.S. Neuroscience; Magna Cum Laude Thesis: Medial septal lesions enhance cocaine‐induced suppression of saccharin intake in rats. Advisor: John E. Kelsey, Ph.D. Wake Forest University School of Medicine Winston‐Salem, North Carolina Ph.D. Neuroscience Dissertation: Neurobiological and neurobehavioral phenotypes associated with vulnerability to cocaine self‐administration in adult rhesus monkeys exposed to cocaine throughout gestation. Advisor: Michael A. Nader, Ph.D. EXPERIENCE: RESEARCH EXPERIENCE June 2006 – May 2010 Jan. 2006 – May 2006 Aug. 2005 – Dec. 2005 Jan. 2005 – July 2005 Graduate Research Michael A. Nader, Ph.D. Wake Forest University School of Medicine Graduate Research Rotation Anthony Liguori, Ph.D. Wake Forest University School of Medicine Graduate Research Rotation Paul W. Czoty, Ph.D. Wake Forest University School of Medicine Post‐Baccalaureate Research Assistant Roy A. Wise, Ph.D. National Institute on Drug Abuse 219 EXPERIENCE: (CONTINUED) May 2003 – Dec. 2004 SCHOLASTIC EXPERIENCE Undergraduate Research Assistant John E. Kelsey, Ph.D. Bates College 2008 – 2009 2007‐2008 2007 Winston‐Salem State University Life Science Department Lecturer and Laboratory Instructor BIO 1301: Principles of Biology, 5 hours/week Wake Forest University School of Medicine Graduate School Discussion Facilitator Ethics in Science, 1 hour/week Wake Forest University Department of Counseling Guest Lecturer Psychiatric Pharmacology, 3 hours 2005‐2010 2003‐2004 Winston‐Salem Public School System Brain Awareness Council Presentations Drug Addiction, 3 hours/month Bates College Department of Neuroscience Laboratory Instructor Physiological Psychology, 3 hours/week INSTITUTIONAL APPOINTMENTS AND SERVICE: Women’s Health Center of Excellence Student Representative, 2008‐2010 Program in Neuroscience Recruitment Committee Student Representative, 2007‐2009 Graduate School Professional Development Co‐Director, 2006‐2007 220 PROFESSIONAL MEMBERSHIPS: 2009 – Present 2006 – Present Society for the Stimulus Properties of Drugs American Society for Pharmacology and Experimental Therapeutics 2005 – Present Western North Carolina Society for Neuroscience Chapter 2005 – Present 2004 – Present Society for Neuroscience Sigma Xi HONORS AND AWARDS: 2010 2010 2010 2009 Graduate Student Travel Award American Society for Pharmacology and Experimental Therapeutics Graduate Student Best Abstract Award 2nd Place Behavioral Pharmacology Division American Society for Pharmacology and Experimental Therapeutics Best Integrative Science Poster Award Wake Forest University Graduate Student Research Day 2009 2009 Mary A. Bell Award for Best Poster and Presentation Behavioral Neuroscience Division Western North Carolina Society for Neuroscience Research Day Best Seminar Award Program in Neuroscience Tutorial Series Best Poster Award Fourth Annual Women’s Health Research Day 221 HONORS AND AWARDS: (CONTINUED) 2007 2006 2005 – 2006 2004 – 2005 2005 Best Graduate Student Poster Award Women’s Health Center of Excellence Western North Carolina Society for Neuroscience Research Day Best First‐Year Student Tutorial Presentation Program in Neuroscience Tutorial Series Graduate Student Fellowship Graduate School of Arts and Sciences Wake Forest University Post‐Baccalaureate Intramural Research Training Award National Institute on Drug Abuse Phi Beta Kappa GRANTS: ACTIVE National Institute on Drug Abuse F31 DA024485, 6/2008 – 6/2010 “Vulnerability to Addiction in Adult Rhesus Monkeys Exposed to Cocaine In Utero” PAST Hughes Student‐Faculty Research Grant, 5/2003‐8/2003 “The Effects of Lesions of the N. Accumbens Core or Shell on Context‐Specific Locomotor Sensitization to Nicotine” PUBLICATIONS: Hamilton LR, Nader MA. Increased vulnerability to self‐administer cocaine in adult rhesus monkeys exposed to cocaine throughout gestation. To be submitted May 2010 to Science. 222 PUBLICATIONS: (CONTINUED) Hamilton LR, Czoty PW, Nader MA. Increased impulsivity in male, but not female, adult rhesus monkeys exposed to cocaine throughout gestation. Submitted April 2010 to Psychopharmacology. Hamilton LR, Czoty PW, Gage HD, Nader MA (2010) Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology, 210(4):481‐488. Elmer GI, Pieper JO, Hamilton LR, Wise RA (2010) Qualitative differences between C57/BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self‐ adminstration. Psychopharmacology, 208(2):309‐21. ABSTRACTS: Hamilton LR, Wilson WD, Nader MA (2010) Increased vulnerability to cocaine self‐administration in adult rhesus monkeys exposed to cocaine throughout gestation. FASEB J 24:765.5. Hamilton LR, Czoty PW, Nader MA (2010) Impulsivity and vulnerability to cocaine self‐administration in adult rhesus monkeys exposed to cocaine throughout gestation. Wake Forest University Graduate School Student Research Day, Program No. 12. Hamilton LR, Czoty PW, Nader MA (2009) Impulsivity and vulnerability to cocaine self‐administration in adult rhesus monkeys exposed to cocaine in utero. Program No. 842.14, Neuroscience Meeting Planner. Hamilton LR, Czoty PW, Calhoun TL, Nader MA (2009) Impulsivity and vulnerability to cocaine self‐administration in adult rhesus monkeys exposed to cocaine in utero. FASEB J 23:588.9. Hamilton LR, Calhoun TL, Nader MA (2009) Increased impulsivity in male, but not female, adult rhesus monkeys exposed to cocaine in utero. Fourth Annual Women’s Health Research Day, Program No. 3. Hamilton LR, Calhoun TL, Nader MA (2008) Increased impulsivity in male, but not female, adult rhesus monkeys exposed to cocaine in utero. Western North Carolina Chapter of the Society for Neuroscience Poster Competition, Program No. 5. 223 ABSTRACTS: (CONTINUTED) Hamilton LR, Gage HD, Calhoun TL, Nader MA (2008) Characterization of the D1, D2, and D3 receptor systems in adult rhesus monkeys exposed to cocaine in utero. Johns Hopkins Bloomberg School of Public Health Second Annual Conference for the Dissemination of Research on Addiction, Infectious Disease, and Public Health, Program No. 6. Hamilton LR, Gage HD, Calhoun TL, Nader MA (2008) Characterization of the D1, D2, and D3 receptor systems in adult rhesus monkeys exposed to cocaine in utero. FASEB J 22:904.2. Hamilton LR, Gage HD, Calhoun TL, Nader MA (2008) Characterization of the D1, D2, and D3 receptor systems in adult rhesus monkeys exposed to cocaine in utero. Wake Forest University Graduate Student Research Day, Program No. 9. Hamilton LR, Gage HD, Calhoun TL, Nader MA (2008) Characterization of the D1, D2, and D3 receptor systems in adult rhesus monkeys exposed to cocaine in utero. Wake Forest University Women’s Health Research Day, Program No 5. Hamilton LR, Gage HD, Calhoun TL, Nader MA (2007) Characterization of the D1, D2, and D3 receptor systems in adult rhesus monkeys exposed to cocaine in utero. Western North Carolina Chapter of the Society for Neuroscience Poster Competition, Program No. 12. Hamilton LR, Gage HD, Nader MA (2007) Altered D2 receptor availability in adult rhesus monkeys exposed to cocaine in utero. FASEB J 21:885.13. Czoty PW, Nader SH, Reboussin BA, Calhoun TL, Hamilton LR, Nader MA (2006) Long‐term cocaine self‐administration under binge conditions or a second‐order schedule in monkeys: influence of self‐ administration. Program No. 123.2, Neuroscience Meeting Planner. Elmer GI, Pieper JO, Hamilton LR, Wise RA, Becker JB, Arnold AP (2005) Sex‐chromosome genes influence amphetamine potentiation of brain stimulation reward independently of gonadal secretions in mice. Program No. 541.5, Neuroscience Meeting Planner. 224 ABSTRACTS: (CONTINUTED) Hamilton LR, Elmer GI, Pieper JO, Wise RA, Becker JB, Arnold AP (2005) Sex‐chromosome genes influence amphetamine potentiation of brain stimulation reward independently of gonadal secretions in mice. Western North Carolina Chapter of the Society for Neuroscience Poster Competition, Program No. 16. SEMINARS AND PRESENTATIONS: Hamilton LR, (2010) Effects of prenatal cocaine exposure on long‐ term neurobiological and neurobehavioral phenotypes associated with addiction. United States Army Medical Research Institute of Chemical Defense, Invited Speaker. Hamilton LR (2010) Long‐term neurobiological and neurobehavioral phenotypes associated with addiction in rhesus monkeys exposed to cocaine in utero. Yale University, Department of Psychiatry, Invited Speaker. Hamilton LR (2010) Impulsivity and vulnerability to addiction in adult rhesus monkeys exposed to cocaine in utero. 31st Annual Meeting of the Society for the Stimulus Properties of Drugs, Keynote Speaker. Nader MA, Czoty PW, Nader SH, Hamilton LR, Martelle SE, Icenhower M, Riddick NV (2010) What is implied by potency differences in acquisition and maintenance of cocaine self‐administration? 53rd Annual Behavioral Pharmacology Society Meeting. Hamilton LR (2009) Long‐term effects of prenatal cocaine exposure in rhesus monkeys. Johns Hopkins University, Department of Psychiatry, Behavioral Pharmacology Research Unit, Invited Speaker. Hamilton LR (2009) Long‐term neurobiological and neurobehavioral phenotypes associated with addiction in rhesus monkeys exposed to cocaine in utero. Wake Forest University School of Medicine, Physiology and Pharmacology Departmental Seminar. Hamilton LR (2009) Increased impulsivity in adult rhesus monkeys exposed to cocaine in utero. Wake Forest University – Emory University Behavioral Pharmacology Symposium. 225 SEMINARS AND PRESENTATIONS: (CONTINUED) Hamilton LR (2009) Sex differences in the long‐term effects of prenatal cocaine exposure in rhesus monkeys. Wake Forest University Program in Neuroscience Tutorial Series. Hamilton LR (2008) Characterization of the dopaminergic receptor system in adult rhesus monkeys exposed to cocaine in utero. Wake Forest University School of Medicine, Physiology and Pharmacology Departmental Seminar. Hamilton LR (2008) Characterizing the dopaminergic receptor system in adult rhesus monkeys. Wake Forest University – Emory University Behavioral Pharmacology Symposium. Hamilton LR (2008) Characterization of the dopaminergic receptor system in adult rhesus monkeys. Wake Forest University Program in Neuroscience Tutorial Series. Hamilton LR (2007) Prenatal cocaine exposure and vulnerability to addiction in adult rhesus monkey. Wake Forest University School of Medicine, Physiology and Pharmacology Departmental Seminar. Hamilton LR (2007) Prenatal cocaine exposure and vulnerability to addiction in adult rhesus monkeys. Wake Forest University – Emory University Behavioral Pharmacology Symposium. Hamilton LR (2006) Effects of prenatal cocaine exposure in rhesus monkeys. Wake Forest University – Emory University Behavioral Pharmacology Symposium. Hamilton LR (2006) Comparison of marijuana and tobacco withdrawal during a two week abstinence period. Wake Forest University Program in Neuroscience Tutorial Series. 226
© Copyright 2026