Biological effect of magnetic field on the production of
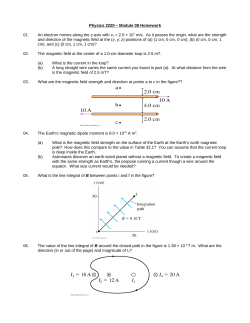
Sustain. Environ. Res., 25(2), 125-130 (2015) 125 Technical Note Biological effect of magnetic field on the production of polyhydroxyalkanoates Marzie Fatehi and Seyed Ahmad Ataei* Department of Chemical Engineering Shahid Bahonar University Kerman 7618891167, Iran Key Words: Municipal wastewater, excess activated sludge, polyhydroxyalkanoates, magnetic field ABSTRACT This study reports on the effect of magnetic field at 0 to 50 mT on polyhydroxyalkanoate (PHA) production in activated sludge. Tests were performed for excess carbon addition and PHA content and some other parameters determined. In summary, this research indicated that higher PHA contents of 0.75 g L-1 were produced at magnetic field intensities of 5 and 20 mT, whereas the lowest PHA content was recorded at 50 mT (0.55 g L-1). In addition, magnetic field influenced the type and amount of monomer produced of the copolymer PHA. Higher amounts of butyrate and valerate monomer were observed at magnetic field of 5 and 50 mT, respectively. Finally, in the present work, the magnetic field intensity of 5 mT was determined as the optimum magnetic field by considering the ratio of PHA produced to the amount of suspended solids in the activated sludge together with other economic factors (such as costs related to the production of PHA, reduction of sludge volume and production of magnetic field). INTRODUCTION Biodegradable plastics are mainly constituted of chemically or biologically produced polyesters. Polyhydroxyalkanoates (PHAs) are natural macro molecules of polyesters produced by a variety of microorganisms and are currently considered as an alternative to conventional plastics. PHAs are formed from 3-hydroxy fatty acid monomers and accumulated as a carbon/energy reserve source in microorganisms [1-3]. More than 300 types of microorganism are able to produce and store PHA under conditions of limited nutrients and excess carbon source [1]. Bacteria can synthesize a wide range of PHAs and approximately 150 different constituents of PHAs have been identified. Currently polyhydroxybutyrate (PHB) and copolymer P (HB/HV) are the only PHAs produced on a commercial scale [4-6]. Over the past decades, the intrinsic resistance of plastic materials to degradation has been increasingly regarded as a source of environmental and waste *Corresponding author Email: [email protected] management problems. Converting biodegradable components in municipal sludge under thermophilic conditions to volatile acids and further into PHAs has its merits for sustainable development and waste management such as less and safer sludge to be handled, less methane produced in landfill sites, lower cost for sludge disposal, if it can be partially utilized as raw materials to produce valuable products, and production of true biodegradable thermoplastics. Based on previous studies, excess sludge volume can be reduced to less than 30% by extracting of PHA from activated sludge [7]. The idea of PHA production using mixed culture arose from recognition of the PHA’s role as a metabolic intermediate in microbial processes for wastewater treatment (WWT). In most cases, a mixed culture generates PHA from organic acids in wastewater or organic acids that have been added from other sources of industrial waste [8-10]. Magnetic field application is a new technique in WWT for various objectives and its key advantages are those of protein recovery, treatment of cells, simulation 126 Fatehi and Ataei, Sustain. Environ. Res., 25(2), 125-130 (2015) of enzymes and biological WWT. There have been numerous studies on the effect of magnetic field on microbial performance, but the results were usually inconsistent. Some of studies showed a negative effect, while most of them showed an enhancement in growth, because the effect depended on the intensity of magnetic field and the type of microorganisms. Yavuz and Celebi indicated that a magnetic field tended to increase bacterial activity and this effect was far more noticeable in heterogeneous cultures (sewage) than in pure culture [11]. Several other studies have been conducted on the effect of magnetic field on chemical oxygen demand (COD) removal from domestic and industrial wastewater and they reported a strong relationship between type of wastewater and intensity of magnetic field [12-14]. To date, there has been no serious investigation on effect of magnetic field on PHA production, although magnetic field exposure has definite positive and negative effects on the storage capacity of PHA in microorganisms [10,13,15]. The effect of static magnetic field on production of PHAs from different short-chain volatile fatty acids (VFAs) by activated sludge process under the aerobic dynamic feeding technique was investigated in sequencing batch reactors with applied magnetic field intensities of 7-42 mT [16]. These tests reported that exposure to a static magnetic field had a definite influence on biosynthesis of PHAs, and the effect was dependent on field strength [16]. However, there have been very few reports on changes of enzymatic activity in cells and increased or decreased PHA production in microorganisms subject to a magnetic field exposure. The aim of the present research was to study the effect of magnetic field on PHA content, amount and type of monomer in the copolymer. An activated sludge from Kerman municipal wastewater plant in Kerman, Iran, was transferred into a PHA production reactor under magnetic field at the various strengths of 0 (control sample), 5, 10, 15, 20, 25 and 50 mT. MATERIALS AND METHODS 1.Batch Reactor Experiment Figure 1 shows a 1.5 L cylindrical plexi reactor with 1 L working volume. The reactor was fed with sodium acetate as the sole carbon source at 3000 mg L -1. Oxygen was supplied by an air compressor at the rate 1 L min-1. The reactor was operated at 15 ± 1 °C without pH control. Samples were taken from the sludge in the batch reactor at regular intervals, and analyzed for PHA, biochemical oxygen demand (BOD), pH and mixed liquor suspended solids (MLSS). The initial characterization of the activated sludge, Fig. 1. PHA production reactor. from Kerman municipal wastewater plant, Kerman, Iran is presented in Table 1. 2.Magnetic Field Exposure The reactor was operated under seven different intensities of magnetic field (0-50 mT). The magnetic field was generated by magnets and intensity of the magnetic field was produced at the center of reactor. The magnetic field intensity was measured by a Tesla meter placed in the middle of the reactor. 3.Analysis PHA measurement was measured according to the method described in Ataei et al. [17]. 5 mL of samples were centrifuged at 6000 rpm and 30 min. Then 2 mL of chloroform and 1 mL of acidified methanol containing benzoic acid as the internal standard were added to the sludge. Samples were heated for 2 h at 100 °C by COD reactor (model WTW) then cooled rapidly. One mL of distilled water was added to the solution and vortexed for 1 min. Organic and aqueous layers were allowed to separate. The bottom organic phase (methyl ester of alkanoates) was transferred into a fresh tube. Then 2 μL of this phase was injected into a gas chromatograph (model Varian CP 3800) at Table 1. Characterization of activated sludge Characterization C:N:P pH COD (ppm) BOD (ppm) Sludge volume index Volatile suspended solids/ Total suspended solids MLSS (g L-1) Range 24:0.14:1 7.65 5490 1290 90-95 0.75 3600 Fatehi and Ataei, Sustain. Environ. Res., 25(2), 125-130 (2015) 250 °C, which was equipped with a flame ionization detector and column (Capillary 8 CP, 30 m x 1 μm). The detector temperature was 280 °C. Helium was used as the carrier gas. Initial oven temperature was 80 °C, which was held constant for 1 min. Then temperature was increased to 150 °C at a rate of 25 °C min-1 and retained for 1 min. Calibrations of PHA were done with a standard poly (3-hydroxybutyric-co-3-hydroxyvaleric acid) (12 wt% PHV) (Sigma, USA). BOD was measured by the Standard Method [18]. 127 sodium acetate to the activated sludge as a carbon source. In the PHA production process, biodegradable organic material and excess carbon source are converted into VFAs. These VFAs are then consumed by microorganisms to produce PHA. Figure 2 shows the changes in BOD with time; reduction of 69% BOD at 30 h cultivation. 2.PHA Production with Magnetic Field The changes in BOD and PAH levels during the course of 48 h cultivation are shown in Fig. 2. The PAH content increased with time with the highest amount of PHA content (0.6 g L-1) at 30 h. Thereafter (30-48 h), the PHA production declined due to less substrate available with cells undergoing endogenous phase [19]. Therefore, a longer aeration time may select a microbial community with lower PHA production capacity than that selected under shorter aeration time [20]. At aeration time lower than 30 h, the system may be at the log-growth phase. There is an excess amount of food available during this period and the microorganism population is less than that during the stationary phase. As a result, an activated sludge processes operating in the stationary phase can produce more PHAs compared to productivity in endogenous or log-growth phases. Based on these results, optimum aeration time for PHA production for this system was determined to be 30 h. Evaluations for BOD of activated sludge were taken before and after additions of sodium acetate as excess carbon source, these were 1290 and 6110 ppm, respectively. The amount of carbon can be increased by adding In the second step of experiments, the influence of magnetic field on the production of PHAs was investigated at 30 h cultivation (same operating condition) and results of these tests were compared with those of the control sample (without magnetic field). Figure 3 plots show that magnetic field exposure influenced PHA production by the activated sludge. Results shown in Fig. 3 clearly demonstrate that magnetic field intensities of 5, 10, 15 and 20 mT had positive effects on PHA production, while intensities of 25 and 50 mT had negative effects. There are a number of possible causes for the effect of magnetic exposure on PHA production, and its influence on substrate consumption and enzyme activity [11,21]. Xu et al. indicated that magnetic exposure can change rate of biological reactions [15]. The first probable reason for decreased PHA production, especially at 10 and 15 mT compared to 5 and 20 mT, is that magnetic field enhanced the rate of consumption of the substrate resulting in cells entering into the death phase earlier than would be expected (30 h). The second reason is that magnetic field perhaps influences enzyme activity as reported previously [15]. Some studies have confirmed the effect of exposure to magnetic field on amplification pattern of microbes in the sludge [10]. At magnetic field intensities of 5 and 20 mT, enzymatic activity may be improved and PHA production increased. However, detailed study is Fig. 2. Effect of cultivation time on the PHA content (∆) and BOD changes (◊). Fig. 3. Effect of magnetic field on amount of PHA production. RESULTS AND DISCUSSION 1.PHA Production without Magnetic Field (Control Sample) 128 Fatehi and Ataei, Sustain. Environ. Res., 25(2), 125-130 (2015) required to further investigate this mechanism. Finally, a comparison was made between results of this part and those results described in others [16] as shown in Fig. 4. For magnetic field intensity lower than 21 mT, it had a positive influence on PHA produced by mixed culture. The result reported in Li et al. [22] demonstrate that magnetic field intensity of 25 mT had a positive effect on algal cultivation while this intensity had negative effect on mixed culture (activated sludge). PHB and PHV biosynthetic processes are quite similar but different enzymes activate each process. Previous researches have confirmed the effect of magnetic field on enzymatic activity [11,23,24]. Results of the present research show that variations of mass percentage of HB and HV are related to intensity of magnetic field (Fig. 5). The maximum HB occurred at 5 mT at 81% with the minimum of HB observed at 50 mT. The magnetic field intensity of 50 mT had a negative effect on PHA production (reduced to 8% PHA compared to the control sample). Considering that the purpose was to generate the maximum amount of HV in the copolymer, the negative effect of this magnetic field can be ignored. Due to the effect of HV or HB on mechanical properties of biopolymer and according to our aim of producing PHA (increase production or improve properties of the biopolymer) it is preferable to apply the magnetic field intensity with optimum effect. Figure 6 shows the PHA/MLSS ratio at different magnetic field intensities after 30 h cultivation in 1 L of sample. If PHA production and biomass volume reduction are the aims of process, it would be appropriate to determine a high ratio of PHA/MLSS. The highest ratio reached at 25 mT, but PHA content at in this magnetic field (0.58 g L-1) was less than that determined at the magnetic field intensity of 20 mT (0.75 g L-1). Consequently, according to PHA content and PHA/MLSS ratio, intensity of 20 mT was more efficient than that of 25 mT. In the other tests, PHA contents at 5 and 20 mT were approximately similar, while energy used to generate magnetic field intensity of 5 mT is much less than that for 20 mT. In general, the costs of PHA production and generation of a magnetic field have higher costs than that needed for volume reduction. Then magnetic field intensity of 5 mT was determined as the optimum intensity. Finally, it is suggested that according to economic factors such as cost of PHA production, generation of magnetic field and reduction of sludge volume, the most efficient intensity of magnetic field needs to be selected. CONCLUSIONS Fig. 4. Percentage of PHA production compared to control sample in this study and previous research [16]. The main results can be summarized as follows: • The best position to optimize PHA production is in the stationary phase. • The highest amount of PHA production was 0.6 g L-1 after 30 h aeration. • BOD removal during the PHA production process after 30 h aeration was 69%. • Magnetic field intensity less than 20 mT increased production of PHA (positive effect) and higher than Fig. 5. Mass percentage of HB and HV in the copolymer PHA. Fig. 6. Ratio of PHA/MLSS at different magnetic field intensity. Fatehi and Ataei, Sustain. Environ. Res., 25(2), 125-130 (2015) 20 mT it had a negative effect on PHA production. • These results demonstrate that exposure to magnetic field had a definite influence on PHA biosynthesis and the effect was dependent on field strength. The maximum and minimum PHA contents were observed at 20 and 50 mT to be 0.75 and 0.55 g L-1, respectively. • Magnetic field influenced on mass percentages of HB and HV in the copolymer PHA. Higher amounts of HV and HB in the copolymer occurred at 50 and 5 mT, respectively. • Magnetic field intensity of 5 mT was determined as the most efficient intensity when economic factors were also taken into consideration. ACKNOWLEDGEMENT Authors gratefully acknowledge Amir Sadeghi Pour Marvi from Shahid Bahonar University, Kerman, Iran for critical reading of the manuscript. REFERENCES 1. Chee, J.Y., S.S. Yoga, N.S. Lau, S.C. Ling, R.M.M. Abed and K. Sudesh, Bacterially produced polyhydroxyalkanoate (PHA): Converting renewable resources into bioplastics. In: A. Méndez-Vilas (Ed.). Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Formatex Research Center, Badajoz, Spain (2010). 2. N a i k , S . , S . K . V. G o p a l a n d P. S o m a l , Bioproduction of polyhydroxyalkanoates from bacteria: A metabolic approach. World J. Microb. Biot., 24(10), 2307-2314 (2008). 3. Khanna, S. and A.K. Srivastava, Recent advances in microbial polyhydroxyalkanoates. Process Biochem., 40(2), 607-619 (2005). 4. Suriyamongkol, P., R. Weselake, S. Narine, M. Moloney and S. Shah, Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants - A review. Biotechnol. Adv., 25(2), 148-175 (2007). 5. Brandl, H., R.A. Gross, R.W. Lenz and R.C. Fuller, Plastics from bacteria and for bacteria: Poly(βhydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Microb. Bioprod., 41, 77-93 (1990). 6. Finch, C.A., Chemistry and Technology of Biodegradable Polymers. Chapman & Hall, London, UK (1995). 7. Lee, S. and J. Yu, Production of biodegradable thermoplastics from municipal sludge by a twostage bioprocess. Resour. Conserv. Recy., 19(3), 129 151-164 (1997). 8. Tian, P.Y., L.A. Shang, H. Ren, Y. Mi, D.D. Fan and M. Jiang, Biosynthesis of polyhydroxyalkanoates: Current research and development. Afr. J. Biotechnol., 8(5), 709-714 (2009). 9. Dionisi, D., M. Majone, V. Papa and M. Beccari, Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng., 85(6), 569-579 (2004). 10. Chen, H., H.B. Li and Y.F. Xia, Acclimating PHA storage capacity of activated sludge with static magnetic fields. Enzyme Microb. Tech., 46(7), 594-597 (2010). 11. Yavuz, H. and S.S. Celebi, Effects of magnetic field on activity of activated sludge in wastewater treatment. Enzyme Microb. Tech., 26(1), 22-27 (2000). 12. Hattori, S., M. Watanabe, T. Endo, H. Togii and K. Sasaki, Effects of an external magnetic field on the sedimentation of activated sludge. World J. Microb. Biot., 17(3), 279-285 (2001). 13. Jagadeesh, A., Investigation of the Effect of Magnetic Field on the Chemical Oxygen Demand Removal of Wastewater. Master Thesis, Department of Chemical Engineering, Ryerson University, Toronto, Canada (2006). 14. K r z e m i e n i e w s k i , M . , M . D e b o w s k i , W. Janczukowicz and J. Pesta, Effect of sludge conditioning by chemical methods with magnetic field application. Pol. J. Environ. Stud., 12(5), 595605 (2003). 15. Xu, Z.L., H. Chen, H.Y. Wu and L.X.Y. Li, 7 mT static magnetic exposure enhanced synthesis of poly-3-hydroxybutyrate by activated sludge at low temperature and high acetate concentration. Process Saf. Environ., 88(4), 292-296 (2010). 16. Chen, H. and X.L. Li, Effect of static magnetic field on synthesis of polyhydroxyalkanoates from different short-chain fatty acids by activated sludge. Bioresource Technol., 99(13), 5538-5544 (2008). 17. Ataei, S.A., E. Vasheghani-Farahani, S.A. Shojaosadati and H.A. Tehrani, Isolation of PHA-producing bacteria from date syrup waste. Macromol. Symp., 269(1), 11-16 (2008). 18. Hach, C.C., R.L. Klein Jr. and C.R. Gibbs, Introduction to Biochemical Oxygen Demand. Hach Company, Loveland, CO (1997). 19. Metcalf and Eddy, Wastewater Engineering: Treatment and Reuse. McGraw-Hill, New York (2003). 20. Mokhtarani, N., Determination of Effective Factors on the Production of Poly-Hydroxyalkanoates by 130 Fatehi and Ataei, Sustain. Environ. Res., 25(2), 125-130 (2015) Activated Sludge. Ph.D. Dissertation, Department of Environmental Engineering, Tarbiat Modares University, Tehran, Iran (2005). 21. Berlot, M., T. Rehar, D. Fefer and M. Berovic, The influence of treatment of Saccharomyces cerevisiae inoculum with a magnetic field on subsequent grape must fermentation. Chem. Biochem. Eng. Q., 27(4), 423-429 (2013). 22. Li, Z.Y., S.Y. Guo, L. Li and M.Y. Cai, Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresource Technol., 98(3), 700-705 (2007). 23. Jung, J.T. and S. Sofer, Enhancement of phenol biodegradation by south magnetic field exposure. J. Chem. Technol. Biot., 70(3), 299-303 (1997). 24. Blanchard, J.P. and C.F. Blackman, Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics, 15(3), 217-238 (1994). Discussions of this paper may appear in the discussion section of a future issue. All discussions should be submitted to the Editor-in-Chief within six months of publication. Manuscript Received: February 5, 2014 Revision Received: July 16, 2014 and Accepted: August 18, 2014
© Copyright 2026