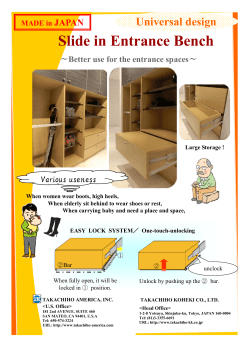
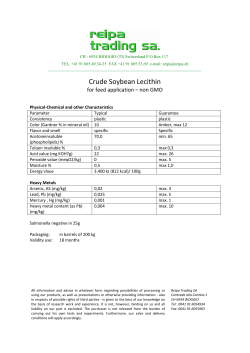
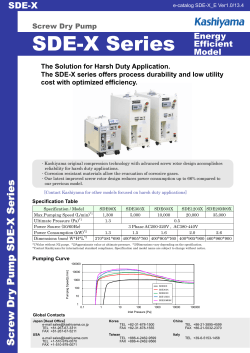
RoHS Substance Additions â Webinar Slides
RoHS Substance Addition Answering the Key Questions Krystal Cameron Regulatory Affairs [email protected] AGENDA ❖ ❖ ❖ ❖ Introduction Substance Addition - DBP, BBP, DEHP, DIBP: ➢ Where will you find these substances in your products? ➢ 5th prioritized substance that WASN’T added ➢ What is the scope? ➢ When do you have to comply to these new restrictions? ➢ What impact does this have on you and your products (CE MARKING)? ➢ How do you best comply? Exemption expiry and applications Scope Review Publication [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 3 Introduction - Assent ❖ ❖ ❖ Assent Compliance Manager Consulting Every Vertical - from Retail to Aerospace to Medical ❖ Testing Partners [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 4 Introduction ❖ 2011/65/EU - Published in the Official Journal of the European Union: July 2011 ❖ Major Differences ❖ Categories ❖ Timelines ❖ Scope [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 5 Official Journal has NOT been published yet! What does that mean? The proposed act is what we are working from. The notification has been made to the WTO, we know it WILL be published and we know a scope clarification that will be added in the published act. Where will you find these substances? Acronym EC Number CAS Number USE/Industry DEHP 204-211-0 117-81-7 EC proposed act for amendment states it is the most common plasticiser used in PVC. According to the European Council for Plasticisers and Intermediates (ECPI), Low orthophthalates are commonly used in medical devices (DEHP), general purpose PVC applications, adhesives and inks. Use of DEHP in medical applications is regulated by the Medical Devices Directive and not by REACH i.e. there is no need for REACH Authorisation for DEHP in medical applications – it can continue to be used subject to the Medical Devices Directive. DBP 201-557-4 84-74-2 Flooring, Automotive , Inks and waxes are main applications according to the European Council for Plasticisers and Intermediates (ECPI) . EC proposed act for amendment states similar to DEHP. BBP 201-622-7 85-68-7 Flooring is main application according to the European Council for Plasticisers and Intermediates (ECPI) . EC proposed act for amendment states similar to DEHP. DIBP 201-553-2 84-69-5 Automotive , Adhesives & Sealants, Inks and waxes are main applications according to the European Council for Plasticisers and Intermediates (ECPI) . EC proposed act for amendment states available data collected by the Commission’s consultants suggests that DIBP is currently not used in traditional EEE. It is however used as a plasticiser in glues and inks for paper and food packaging, and in toys, childcare articles and a wide range of consumer products, some of which indeed might be in the scope of RoHS 2. [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 7 Why wasn’t HBCDD Added? ❖ Known to be an issue and regulated in a multitude of ways and jurisdictions ❖ The EC considered a ban disproportionate and refrained from it - EEE is not used in the EU. ❖ A complete phase-out of HBCDD in electronics, whether imported or produced in Europe, will only be a matter of years. [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 8 Scope and Timelines 22 July 2019 - ALL Categories of EEE except 8 and 9 22 July 2021 - Categories 8 and 9 - medical devices including in vitro medical devices and monitoring and control instruments including industrial monitoring and control instruments. Cables or spare parts for products placed on the market before the relevant compliance deadline are excluded from the new substance restriction Crossover with REACH, Timelines impact? ❖ DEHP, BBP and DBP - Toys ❖ All 4 are on the Candidate AND Authorisation List - February 2015 Banned! [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 9 CE Marking RoHS falls under the CE Marking Directive ❖ Declaration of Conformity (DoC) ❖ Technical file ❖ CE Mark ➢ Finished products in scope of RoHS The CE Mark, when present on a product, indicates compliance to the regulations/directives for which the product is in scope at the time it is placed on the market. What does that mean? [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 10 Compliance Implications ❖ Measure Impact ➢ Substances ➢ Exemptions ❖ Supplier Education ❖ Yearly Audits ❖ Supplier Data Gathering, Testing and Risk Assessment ❖ FMDs? [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 11 Exemption Expirations Consultations Project “Pack 7” has started: 4 requests for renewal/amendment - 7b, 9b, 13a, 13b September 2015 NOTE: April 9th - Application to renew 7b was withdrawn Requests Requests to renew exemptions - several! http://ec.europa.eu/environment/waste/rohs_eee/pdf/renewal_exemptions_oct14-jan15.pdf IPC Submitted extension requests for 14 Exemptions Timelines Commission must decide no later than 6 months before the expiry date of the existing exemption. Consequences If you are using an exemption and it expires = [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 12 Exemption Additions 3 New Exemptions: ❖ Mercury in intravascular ultrasound imaging systems - 30 June 2019 ❖ Lead in polyvinyl chloride (PVB) sensors in in vitro diagnostic medical devices – 31 December 2018 ❖ Cadmium in downshifting cadmium-based semiconductor nanocrystal quantum dots for use in display lighting applications - 30 June 2018 1 Exemption Extension: ❖ Cadmium in colour-converting LEDs for illumination and display (suggested) until 30 June 2017 Sent to the European Parliament and the EU Council of Ministers - two months to oppose the measures - April 25th, 2015. [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 13 Scope Review Publication ❖ ❖ ❖ ❖ Published 12/03/2015 Covers: ➢ non-road mobile machinery without an on-board power source ➢ on windows and doors with electric functions ➢ on the refurbishment of medical devices Makes specific recommendations to the European Commission in regards to areas to be further clarified. Makes a recommendation for a change to the legal text to allow free circulation for refurbished medical devices Can be downloaded via: http://rohs.exemptions.oeko. info/fileadmin/user_upload/reports/20150312_RoHS_scope_review_final_a.pdf [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 14 SUMMARY The RoHS Directive is undergoing serious changes. To be reactive will be too late: ❏ Assess your areas of risk ❏ Assess the impact to your products ❏ Educate suppliers ❏ Test when needed ❏ Prepare for the update to the needed documentation. You must be tracking all of these changes and the impact to your products to stay in compliance, meet the needs of your customers and stay... [email protected] | www.assentcompliance.com | TEL: 1(866)964-6931 Slide 15 CANADA 5300 Canotek, Suite 30 Ottawa | ON | K1J 1A4 Krystal Cameron 1-866-964-6931 [email protected] 613-882-1429 USA 244 Fifth Avenue | Suite 1717 New York | NY | 10001 | USA UNITED KINGDOM Longcroft House, 2-8 Victoira Avenue, Bishopgate London | UK | EC2M 4NS
© Copyright 2026