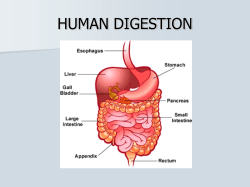
Chapter 40 Structure and Function of the Digestive System
Chapter 40 Structure and Function of the Digestive System Alexa K. Doig and Sue E. Huether http://evolve.elsevier.com/McCance/ • Review Questions and Answers • Animations CHAPTER OUTLINE The Gastrointestinal Tract, 1393 Mouth and Esophagus, 1395 Stomach, 1396 Small Intestine, 1400 Large Intestine, 1407 Intestinal Bacteria, 1408 Accessory Organs of Digestion, 1409 Liver, 1409 Gallbladder, 1413 Exocrine Pancreas, 1413 Tests of Digestive Function, 1415 Gastrointestinal Tract, 1415 Liver, 1416 Gallbladder, 1416 Exocrine Pancreas, 1416 AGING and the Gastrointestinal System, 1417 The digestive system breaks down ingested food, prepares it for uptake by the body’s cells, provides body water, and eliminates wastes. This system consists of the gastrointestinal tract and accessory organs of digestion: the liver, gallbladder, and exocrine pancreas. Food breakdown begins in the mouth with chewing and continues in the stomach, where food is churned and mixed with acid, mucus, enzymes, and other secretions. From the stomach, the fluid and partially digested food pass into the small intestine, where biochemicals and enzymes secreted by the liver, exocrine pancreas, and small intestinal epithelium break it down into absorbable components of proteins, carbohydrates, and fats. These nutrients pass through the small intestinal epithelium into underlying blood vessels and lymphatics that carry them to the liver via the hepatic portal circulation for further processing and storage. Ingested substances and secretions that are not absorbed in the small intestine pass into the large intestine, where fluid continues to be absorbed. Solid wastes pass into the rectum and are eliminated from the body through the anus. Except for chewing, swallowing, and defecation of solid wastes, the activities of the digestive system are controlled by hormones and the autonomic nervous system. As ingested substances move through the gastrointestinal tract, they trigger the release of hormones that stimulate or inhibit (1) the muscular contractions (gastrointestinal motility) that mix and propel food from the esophagus to the anus, and (2) the timely secretion of substances that aid in digestion. The autonomic innervation, sympathetic and parasympathetic, is controlled by centers in the brain and by local stimuli that are mediated by neural plexuses within the gastrointestinal walls. The Gastrointestinal Tract The gastrointestinal tract (alimentary canal) consists of the mouth, esophagus, stomach, small intestine, large intestine, 1393 1394 Unit XII The Digestive System Parotid gland Submandibular salivary gland Tongue Sublingual salivary gland Larynx Pharynx Esophagus Trachea Diaphragm Liver Transverse colon Stomach Spleen Hepatic flexure of colon Splenic flexure of colon Ascending colon Descending colon Sigmoid colon Ileum Cecum Vermiform appendix Anal canal Rectum FIGURE 40-1 Structures of the Digestive System. Mesentery Blood vessels Nerve rectum, and anus (Figure 40-1). It carries out the following digestive processes: 1.Ingestion of food 2.Propulsion of food and wastes from the mouth to the anus 3.Secretion of mucus, water, and enzymes 4.Mechanical digestion of food particles 5.Chemical digestion of food particles 6.Absorption of digested food 7.Elimination of waste products by defecation Histologically, the gastrointestinal tract consists of four layers. From the inside out, they are the mucosa, submucosa, muscularis, and serosa or adventitia (esophagus only). These concentric layers vary in thickness, and each layer has sublayers (Figure 40-2). Neurons forming the enteric nervous system are located solely within the gastrointestinal tract and are controlled by local and autonomic nervous system stimuli. The enteric nervous system comprises three nerve plexuses located in different layers of the gastrointestinal walls. The submucosal plexus (Meissner plexus) is located in the muscularis mucosae, the myenteric plexus (Auerbach plexus) between the inner circular and outer longitudinal muscle layers in the muscularis, and the subserosal plexus just beneath the serosa. The enteric (intramural) plexus neurons regulate motility reflexes, blood flow, absorption, secretions, and immune response.1 SEROSA Connective tissue layer Peritoneum Myenteric plexus Submucosal plexus Intramural plexus SUBMUCOSA Gland in submucosa Duct from gland MUCOSA Mucous epithelium Lamina propria Muscularis mucosae MUSCULARIS Lymph nodule Circular muscle layer Longitudinal muscle layer FIGURE 40-2 Wall of the Gastrointestinal (GI) Tract. The wall of the GI tract is made up of four layers with a network of nerves between the layers. Shown here is a generalized diagram of a segment of the GI tract. Note that the serosa is continuous with a fold of serous membrane called a mesentery. Note also that digestive glands may empty their products into the lumen of the GI tract by way of ducts. (From Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) Chapter 40 Structure and Function of the Digestive System Mouth and Esophagus The mouth is a reservoir for the chewing and mixing of food with saliva. As food particles become smaller and move around in the mouth, the taste buds and olfactory nerves are continuously stimulated, adding to the satisfaction of eating. The tongue’s surface and soft palate contains thousands of chemoreceptors, or taste buds, that can distinguish salty, sour, bitter, umami, and sweet tastes. Tastes and food odors help initiate salivation and the secretion of gastric juice in the stomach. There are 32 permanent teeth in the adult mouth, and they are important for speech and mastication. Salivation The three pairs of salivary glands (the submandibular, sublingual, and parotid glands) (Figure 40-3) secrete about 1 L of saliva per day. Saliva consists mostly of water that contains varying amounts of mucus, sodium, bicarbonate, chloride, potassium, and salivary α-amylase (ptyalin), an enzyme that initiates carbohydrate digestion in the mouth and stomach. The sympathetic and parasympathetic divisions of the autonomic nervous system control salivation. Because cholinergic parasympathetic fibers stimulate the salivary glands, atropine (an anticholinergic agent) inhibits salivation and makes the mouth dry. β-Adrenergic stimulation from sympathetic fibers also increases salivary secretion. The salivary glands are not regulated by hormones, although hormones are found in saliva.1a Parotid gland Parotid duct Submandibular gland Submandibular duct Sublingual gland FIGURE 40-3 Salivary Glands. (From Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) 1395 The composition of saliva depends on the rate of secretion. Aldosterone can decrease the rate of secretion by increasing an exchange of sodium for potassium. Sodium and water are conserved and potassium is excreted. The bicarbonate concentration of saliva sustains a pH of about 7.4, which neutralizes bacterial acids and prevents tooth decay. Saliva also contains immunoglobulin A (IgA), which helps prevent infection. Exogenous fluoride (e.g., fluoride in drinking water) is absorbed and then secreted in the saliva, providing additional protection against tooth decay. Swallowing The esophagus is a hollow muscular tube approximately 25 cm long that conducts substances from the oropharynx to the stomach (see Figure 40-1). The pharynx and upper third of the esophagus contain striated muscle that is directly innervated by skeletal motor neurons that control swallowing. The middle third contains a mix of striated and smooth muscle, and the lower third is smooth muscle that is innervated by preganglionic cholinergic fibers from the vagus nerve. Peristalsis is stimulated when afferent fibers distributed along the length of the esophagus sense changes in wall tension caused by stretching as food passes. The greater the tension, the greater the intensity of esophageal contraction. Occasionally, intense contractions cause pain similar to “heartburn” or angina. Each end of the esophagus is opened and closed by a sphincter. The upper esophageal sphincter (cricopharyngeal muscle) prevents entry of air into the esophagus during respiration.2 The lower esophageal sphincter (cardiac sphincter) prevents regurgitation from the stomach. The lower esophageal sphincter is located near the esophageal hiatus—the opening in the diaphragm where the esophagus ends at the stomach.3 Swallowing is a complex event mediated by the trigeminal nucleus, nucleus tractus solitarius, and reticular formation of the brainstem and also involves other brain regions, including the insula/claustrum and cerebellum.4 Swallowing occurs in two phases: the oropharyngeal (voluntary) phase and the esophageal (involuntary) phase. During the oral and pharyngeal phases of swallowing, food is segmented into a bolus by the tongue and forced posteriorly toward the pharynx as the tongue pushes upward against the hard palate. The superior constrictor muscle of the pharynx contracts, preventing movement of food into the nasopharynx. At the same time, respiration is inhibited and the epiglottis folds downward to prevent the bolus from entering the larynx and trachea. The movements of the tongue and pharyngeal constrictors propel the food into the esophagus in a series of coordinated events, taking less than 1 or 2 seconds. During the esophageal phase of swallowing, the bolus is transported to the stomach by the coordinated sequential contraction and relaxation of outer longitudinal and inner circular layers of smooth muscle. The wave of relaxation reduces resistance and allows food to pass, after which the wave of contraction pushes food farther along. The terminal 1 to 2 cm portion of the musculature forms the lower esophageal sphincter, which relaxes just before the arrival of a peristaltic wave. The sphincter muscles return to their resting tone after the bolus of food passes into the stomach. The esophageal phase of swallowing 1396 Unit XII The Digestive System takes 5 to 10 seconds, with the bolus moving 2 to 6 cm/second. Throughout swallowing, the sphincters and esophagus work in concert with the peristaltic wave that moves food from the mouth to the stomach. Peristalsis that immediately follows the oropharyngeal phase of swallowing is called primary peristalsis. If a bolus of food becomes stuck in the esophageal lumen, the distention of the esophageal wall stimulates secondary peristalsis, a wave of contraction and relaxation that is independent of voluntary swallowing. This is in response to stretch receptors that are stimulated by increased wall tension, causing an increase in impulses from the swallowing center of the brain. When it is closed, the lower esophageal sphincter serves as a barrier between the stomach and esophagus. Cholinergic vagal stimulation and the digestive hormone gastrin increase sphincter tone. Nonadrenergic, noncholinergic vagal impulses relax the lower esophageal sphincter, as do the hormones progesterone, secretin, and glucagon. Relaxation during swallowing is mediated by the vagus nerve.5 Stomach The stomach is a hollow muscular organ that stores food during eating, secretes digestive juices, mixes food with these juices, and propels partially digested food, called chyme, into the duodenum of the small intestine. The anatomy of the stomach is presented in Figure 40-4. Its major anatomic boundaries are the lower esophageal sphincter, where food passes through the cardiac orifice at the gastroduodenal junction into the stomach, and the pyloric sphincter, which relaxes as food is propelled into the duodenum. Functional areas of the stomach are the fundus (upper portion), body (middle portion), and antrum (lower portion). The stomach has three layers of smooth muscle: an outer, longitudinal layer; a middle, circular layer; and an inner, oblique layer (the most prominent) (see Figure 40-4). These layers become progressively thicker in the body and antrum, where food is mixed, churned, and pushed into the duodenum. The circular layer is most prominent and the oblique layer is the least complete; the longitudinal layer is absent on the anterior and posterior surfaces. The glandular epithelium is discussed in the section about secretory functions of the stomach (see p. 1398). Blood is supplied to the stomach by branches of the celiac artery. The blood supply is so abundant that nearly all arterial vessels must be occluded before ischemic changes occur in the stomach wall. A series of small veins (short gastric, left and right gastric, and left and right gastro-omental) drain blood from the stomach towards the hepatic portal vein. The stomach is innervated by sympathetic and parasympathetic divisions of the autonomic nervous system. Some of the autonomic nerves are extrinsic; that is, they originate in the central nervous system and are controlled by nerve centers in the brain. The vagus nerve provides parasympathetic innervation and branches of the celiac plexus innervate the stomach sympathetically. Other neurons are intrinsic; that is, they originate within the stomach and respond to local stimuli and are components of the enteric nervous system. Extrinsic sympathetic fibers reach the stomach through the celiac plexus (solar plexus), whereas extrinsic parasympathetic fibers enter through the gastric branch of the vagus nerve. Few substances are absorbed in the stomach. The stomach mucosa is impermeable to water, but the stomach can absorb alcohol and aspirin and other nonsteroidal anti-inflammatory agents. Esophagus Fundus Gastroesophageal opening Lower esophageal sphincter (LES) Body of stomach Cardia Serosa Longitudinal muscle layer rv atu re Pyloric sphincter Pylorus Lesser c Duodenal bulb Circular muscle layer u Oblique muscle layer Submucosa Mucosa Duodenum Antrum Rugae r at e Gre re tu rva u c FIGURE 40-4 Stomach. A portion of the anterior wall has been cut away to reveal the muscle layers of the stomach wall. Note that the mucosa lining the stomach forms folds called rugae. The dashed lines distinguish the fundus, body, and antrum of the stomach. (Modified from Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) Muscularis Chapter 40 Structure and Function of the Digestive System Gastric Motility In its resting state the stomach is small and contains about 50 ml of fluid. There is little wall tension, and the muscle layers in the fundus contract very little. Swallowing causes the fundus to relax (receptive relaxation) to receive a bolus of food from the esophagus. Relaxation is coordinated by efferent, nonadrenergic, noncholinergic vagal fibers and is facilitated by gastrin and cholecystokinin, two polypeptide hormones secreted by the gastrointestinal mucosa. (The actions of digestive hormones are summarized in Table 40-1.) Food is stored in vertical or oblique layers as it arrives in the fundus, whereas fluids flow relatively quickly down to the antrum. Gastric (stomach) motility increases with the initiation of peristaltic waves, which sweep over the body of the stomach toward the antrum. The rate of peristaltic contractions is approximately three per minute and is influenced by neural and hormonal activity. Gastrin and motilin (small intestine hormones) and the vagus nerve increase contraction by making the threshold potential of muscle fibers less negative. (The neural and biochemical mechanisms of muscle contraction are described in Chapter 43.) Sympathetic activity and secretin (another small intestine hormone) are inhibitory and make the threshold potential more negative. The rate of peristalsis is 1397 mediated by pacemaker cells that initiate a wave of depolarization (basic electrical rhythm), which moves from the upper part of the stomach to the pylorus. Gastric mixing and subsequent emptying of gastric contents (chyme) from the stomach take several hours (3 to 6 hours depending on the composition and volume of food intake). Mixing occurs as food is propelled toward the antrum. As food approaches the pylorus, the velocity of the peristaltic wave increases, forcing the contents back toward the body of the stomach. This retropulsion effectively mixes food with digestive juices, and the oscillating motion breaks down large food particles. With each peristaltic wave a small portion of the chyme passes through the pylorus and into the duodenum. The pyloric sphincter is about 1.5 cm long and is always open about 2 mm. It opens wider during antral contraction. Normally there is no regurgitation from the duodenum into the antrum. The rate of gastric emptying (movement of gastric contents into the duodenum) depends on the volume, osmotic pressure, and chemical composition of the gastric contents. Larger volumes of food increase gastric pressure, peristalsis, and rate of emptying. Solids, fats, and nonisotonic solutions delay gastric emptying.6 (Osmotic pressure and tonicity are described in Chapters 1 and 3.) Products of fat digestion, which are formed TABLE 40-1 SELECTED HORMONES AND NEUROTRANSMITTERS OF THE DIGESTIVE SYSTEM* SOURCE HORMONE† STIMULUS FOR SECRETION ACTION Mucosa of the stomach Gastrin Presence of partially digested proteins in the stomach Gastrin Acid in the stomach Vagus and local nerves in stomach Vagus and local nerves in stomach Stimulates gastric glands to secrete hydrochloric acid and pepsinogen; growth of gastric mucosa; promotes gastric motility Stimulates acid secretion Inhibits acid and pepsinogen secretion and release of gastrin Stimulates release of pepsinogen and acid secretion Stimulates gastrin and release of pepsinogen and acid secretion Presence of acid and fat in the duodenum Increases gastrointestinal motility Secretin Presence of chyme (acid, partially digested proteins, fats) in the duodenum Cholecystokinin Presence of chyme (acid, partially digested proteins, fats) in the duodenum Enteroglucagon Intraluminal fats and carbohydrates Entero-oxyntin Gastric inhibitory peptide (GIP) Peptide YY Presence of chyme in duodenum Fat and glucose in small intestine Pancreatic polypeptide Vasoactive intestinal peptide Protein, fat, and glucose in small intestine Intestinal mucosa and muscle Stimulates pancreas to secrete alkaline pancreatic juice and liver to secrete bile; decreases gastrointestinal motility; inhibits gastrin and gastric acid secretion Stimulates gallbladder to eject bile and pancreas to secrete alkaline fluid; decreases gastric motility; constricts pyloric sphincter; inhibits gastrin; delays gastric emptying Weakly inhibits gastric and pancreatic secretion and enhances insulin release, lipolysis, ketogenesis, and glycogenolysis; delays gastric emptying Delays gastric emptying Inhibits gastric secretion and gastric emptying, stimulates insulin release; inhibits intestinal motility Inhibits postprandial gastric acid and pancreatic secretion and delays gastric and small bowel emptying Decreases pancreatic and enzyme secretion Relaxes intestinal smooth muscle, increases blood flow Mucosa of the small intestine Histamine Somatostatin Acetylcholine Gastrin-releasing peptide (bombesin) Motilin Intraluminal fat and bile acids Modified from Johnson LR: Gastrointestinal physiology, ed 7, St Louis, 2007, Mosby. *Data from Schubert ML, Peura DA: Gastroenterology 134(7):1842–1860, 2008; Wren AM, Bloom SR: Gastroenterology 132(6):2116–2130, 2007. †Note: The digestive hormones are not secreted into the gastrointestinal lumen but rather into the bloodstream, in which they travel to target tissues. There are more than 30 peptide hormone genes expressed in the gastrointestinal tract and more than 100 hormonally active peptides. 1398 Unit XII The Digestive System in the duodenum by the action of bile from the liver and enzymes from the pancreas, stimulate the secretion of cholecystokinin.7 This hormone inhibits food intake, reduces gastric motility, and decreases gastric emptying so that fats are not emptied into the duodenum at a rate that exceeds the rate of bile and enzyme secretion. Osmoreceptors in the wall of the duodenum are sensitive to the osmotic pressure of duodenal contents. The arrival of hypertonic or hypotonic gastric contents activates the osmoreceptors, which delays gastric emptying to facilitate formation of an isosmotic duodenal environment. The rate at which acid enters the duodenum also influences gastric emptying. Secretions from the pancreas, liver, and duodenal mucosa neutralize gastric hydrochloric acid in the duodenum. The rate of emptying is adjusted to the duodenum’s ability to neutralize the incoming acidity. Peristaltic activity in the stomach is also affected by blood glucose levels. Low blood glucose levels stimulate the vagus nerve and gastric smooth muscles, increasing the rate of contraction.8 Gastric Secretion Stimulated by eating, the stomach produces large volumes of gastric secretions. Specialized cells located throughout the gastric mucosa produce mucus, acid, enzymes, hormones, intrinsic factor, and gastroferrin. Intrinsic factor is necessary for the intestinal absorption of vitamin B12 and gastroferrin facilitates small intestinal absorption of iron. The hormones are secreted into the blood and travel to target tissues in the bloodstream. The other gastric secretions are released directly into the stomach lumen under neural and hormonal regulation.9 Mucus covering the entire mucosa, intercellular tight junctions, bicarbonate secretion, and submucosal acid sensors form a protective barrier against acid and proteolytic enzymes, which otherwise would damage the gastric lining.10 In the fundus and body of the stomach the gastric glands of the mucosa are the primary secretory units (Figure 40-5). Several of these glands (three to seven) empty into a common duct known as the gastric pit. The parietal cells (oxyntic cells) within the glands secrete hydrochloric acid, intrinsic factor, and gastroferrin. The chief cells within the glands secrete pepsinogen, an enzyme precursor that is readily converted to pepsin (a proteolytic enzyme) in the gastric fluid. The pyloric gland mucosa in the antrum synthesizes and releases the hormone gastrin from G cells. Enterochromaffin-like cells secrete histamine, and D cells secrete somatostatin. The composition of gastric fluid depends on volume and flow rate (Figure 40-6). Potassium level remains relatively constant, but its concentration is greater in gastric secretions than in plasma. The rate of secretion varies with the time of day; lower in the morning and higher in the afternoon and evening. Loss of gastric secretions through vomiting, drainage, or suction may decrease body stores of sodium, potassium, hydrogen, and chloride. Acid. The major functions of gastric hydrochloric acid are to dissolve food fibers, act as a bactericide against swallowed organisms, and convert pepsinogen to pepsin. The production of acid by the parietal cells requires the transport of hydrogen and chloride from the parietal cells to the stomach lumen. Acid is formed in the parietal cells, primarily through the hydrolysis of water (Figure 40-7). At a high rate of gastric secretion, bicarbonate moves into the plasma, producing an “alkaline tide” in the venous blood, which also may result in a more alkaline urine.11 Surface mucous cells Gastric pit Lamina propria Gastric glands: Mucous neck cell Mucosa Chief cell Parietal cell Endocrine cell Muscularis mucosae Submucosa Blood vessels Oblique muscle layer Muscularis Circular muscle layer Serosa Longitudinal muscle layer Connective tissue Visceral peritoneum FIGURE 40-5 Gastric Pits and Gastric Glands. Gastric pits are depressions in the epithelial lining of the stomach. At the bottom of each pit is one or more tubular gastric glands. Chief cells produce the enzymes of gastric juice, and parietal cells produce stomach acid. (From Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) Concentration in gastric juice (mEq/L) 200 Cl H 100 K 0 Low Na Medium High Secretory rate FIGURE 40-6 Relationship Between Secretory Rate and Electrolyte Composition of the Gastric Secretions. Sodium (Na+) concentration is lower in the gastric secretions than in the plasma, whereas hydrogen (H+), potassium (K+), and chloride (Cl−) concentrations are higher. Gastric secretions are close to isotonic. Secretory rate increases during the cephalic and gastric phases of digestion. Chapter 40 Structure and Function of the Digestive System Acid secretion by parietal cells is stimulated by acetylcholine (ACh) (a neurotransmitter), gastrin (a hormone), and histamine (a biochemical mediator). The vagus nerve releases ACh and stimulates the secretion of histamine and gastrin-releasing peptide (GRP), which stimulates release of gastrin.12 Histamine secretion is also stimulated by gastrin. Histamine is stored in enterochromaffin cells (mast cells; see Chapter 7) in the gastric mucosa. Histamine receptors in the gastric mucosa are H2 receptors (unlike those in the bronchial mucosa, which are H1 receptors). Gastric lipase is produced by glands in the fundus of the stomach and is most effective in an acidic environment. Caffeine stimulates acid secretion, as does calcium. Prostaglandins, enterogastrones (such as gastric inhibitory peptide), somatostatin, and secretin inhibit acid secretion.13 Pepsin. Acetylcholine, through vagal stimulation during the cephalic and gastric phases, is the strongest stimulation Blood CO2 Parietal cell CO2 H2O H2CO3 HCO 3 Cl HCO 3 Stomach lumen H2O OH H K K H H HCl H2O Cl Cl FIGURE 40-7 Hydrochloric Acid Secretion by Parietal Cell. Phase Cephalic Gastric Intestinal All for pepsin secretion. The precursor pepsinogen is quickly converted to pepsin at a pH of 2. Acid also stimulates a local cholinergic reflex and stimulates chief cells to secrete pepsin. Gastrin and secretin are weaker pepsinogen secretagogues. Pepsin is a proteolytic enzyme that breaks down proteinforming polypeptides in the stomach. Once chyme has entered the duodenum, the alkaline environment of the duodenum inactivates pepsin. Mucus. The gastric mucosa is protected from the digestive actions of acid and pepsin by a coating of mucus called the mucosal barrier. Gastric mucosal blood flow is important to maintaining mucosal barrier function.14 The quality and quantity of mucus and the tight junctions between epithelial cells make gastric mucosa relatively impermeable to acid. Prostaglandins and nitric oxide protect the mucosal barrier by stimulating the secretion of mucus and bicarbonate and by inhibiting the secretion of acid. A break in the protective barrier may occur because of exposure to aspirin or other nonsteroidal anti-inflammatory drugs, Helicobacter pylori, ethanol, regurgitated bile, or ischemia. Breaks cause inflammation and ulceration. Intrinsic factor (IF), a mucoprotein produced by parietal cells, combines with vitamin B12 in the stomach. It is required for the absorption of vitamin B12 by the ileum. Atrophic gastritis and failure to absorb vitamin B12 result in pernicious anemia (see Chapter 28). Phases of Gastric Secretion. The secretion of gastric juice is influenced by numerous stimuli that together facilitate the process of digestion. The phases of gastric secretion are the cephalic phase, gastric phase, and intestinal phase (Figure 40-8). Stimulation at parietal cell Stimulus Chewing, swallowing, smell, taste Vagus ACh GRP G cell Local reflexes ACh Distention Digested protein G cell Digested protein Intestinal G cell Gastrin ACh 1399 ECL cell Gastrin Circulating amino acids Histamine FIGURE 40-8 Mechanisms for Stimulating Acid Secretion. ACh, Acetylcholine; ECL, enterochromaffin-like cell; GRP, gastrin-releasing peptide. (From Johnson LR: Gastrointestinal physiology, ed 7, St Louis, 2007, Mosby.) 1400 Unit XII The Digestive System Cephalic Phase. The anticipatory and sensory experiences of smelling, seeing, tasting, chewing, and swallowing food contribute to the cephalic phase of secretion.15 The cephalic phase of gastric secretion is mediated by the vagus nerve through the myenteric plexus. Acetylcholine stimulates the parietal and chief cells to secrete acid and pepsinogen, respectively. The G cells in the antrum release gastrin, which circulates through the bloodstream to the gastric glands and stimulates acid and pepsinogen secretion. Insulin secretion by the endocrine pancreas, stimulated by hyperglycemia, also is a strong stimulus for gastric secretion and is mediated by the vagus nerve through sensors located in the hypothalamus. Maintenance of steady serum glucose levels suppresses the gastric response to insulin. Gastric Phase. The gastric phase of secretion begins with the arrival of food in the stomach. Two major stimuli have a secretory effect: (1) distention of the stomach, and (2) the presence of digested protein. The vagus and enteric nerve plexuses are stimulated by distention and contribute to gastric secretion through a local reflex. Both neural reflexes are mediated by ACh and can be blocked by atropine. As digestion proceeds, products of protein break down, stimulating the release of more gastrin from G cells. Intestinal Phase. The movement of chyme from the stomach into the duodenum initiates the intestinal phase of secretion. This phase represents a deceleration of the gastric secretory response; however, the presence of digested protein and amino acids in the duodenum continues to stimulate some gastric secretion. Concurrently, in response to low duodenal pH and the presence of lipids, inhibitory vagal and enteric reflexes decrease gastric motility when chyme enters the duodenum. The release of secretin and cholecystokinin stimulate pancreatic secretions and inhibit gastric secretions. Small Intestine The small intestine is about 5 to 6 m long and is functionally divided into three segments: the duodenum, jejunum, and ileum (Figure 40-9). The duodenum begins at the pylorus and ends where it joins the jejunum at a suspensory ligament called the Treitz ligament. The end of the jejunum and beginning of the ileum are not distinguished by an anatomic marker. These structures are not grossly different, but the jejunum has a slightly larger lumen. The ileocecal valve (sphincter) controls the flow of digested material from the ileum into the large intestine and prevents reflux into the small intestine. The peritoneum is the serous membrane surrounding the organs of the abdomen and lining the abdominopelvic cavity. It is analogous to the pericardium and pleura that surround the heart and lungs, respectively. The visceral peritoneum lies Mucosa Villi mucosa Lamina propria Muscularis mucosae Duodenal glands extending into mucosa Circular muscle layer Longitudinal muscle layer Serosa Longitudinal section of duodenum Ligament of Treitz (Turned to left) Ileocecal valve Villus Cecum Appendix Lymph lacteal Artery Ileum Cell shedding Lymph lacteal Goblet cell Enterocytes (absorptive cells) Endocrine cell Differentiating cells Intestinal stem Intestinal crypt (of Lieberkühn) cells Jejunum Muscularis Vein mucosae FIGURE 40-9 Small Intestine. Paneth cell Lymph duct Chapter 40 Structure and Function of the Digestive System on the surface of the organs, and the parietal peritoneum lines the wall of the body cavity. The space between these two layers is called the peritoneal cavity. This cavity normally contains just enough fluid to lubricate the two layers and prevent friction during organ movement. Inflammation of the peritoneum, called peritonitis, may occur with perforation of the intestine or after abdominal surgery. As the inflammatory process resolves, scar tissue adhesions may form and cause intestinal obstruction. The duodenum lies behind the peritoneum, or retroperitoneally, and has an essential role in mixing food with digestive juices from the liver and pancreas. The ileum and jejunum are suspended from the posterior abdominal wall by a component of the peritoneum called the mesentery. The loose folds of the mesentery facilitate intestinal motility and support blood vessels, nerves, and lymphatics. The arterial supply to the duodenum arises primarily from the gastroduodenal artery, a small branch of the celiac artery. The jejunum and ileum are supplied by branches of the superior mesenteric artery. Blood flow increases significantly during digestion. The superior mesenteric vein drains blood from the entire small intestine and empties into the hepatic portal circulation. The regional lymphatics drain into the thoracic duct. Both divisions of the autonomic nervous system innervate the small intestine. Secretion, motility, and intestinal reflexes (e.g., relaxation of the lower esophageal sphincter) are mediated parasympathetically by the vagus nerve. Sympathetic activity inhibits motility and produces vasoconstriction. Intrinsic reflexive activity is mediated by the myenteric plexus (Auerbach plexus) and the submucosal plexus (Meissner plexus) of the enteric nervous system. The smooth muscles of the small intestine are arranged in two layers: a longitudinal, outer layer; and a thicker, inner circular layer (see Figure 40-9). Circular folds of the small intestine mucosa slow the passage of food, thereby providing more time for digestion and absorption. The folds are most numerous and prominent in the jejunum and upper ileum. Absorption occurs through villi, which cover the circular folds and are the functional units of the intestine (see Figure 40-9). A villus is composed of absorptive columnar epithelial cells (enterocytes) and mucus-secreting goblet cells of the mucosa. Each villus secretes some of the enzymes necessary for digestion and absorbs nutrients. Near the surface, columnar cells closely adhere to each other at sites called tight junctions. Water and electrolytes are absorbed through these intercellular spaces. The surface of each columnar epithelial cell on the villus contains tiny projections called microvilli (see Figure 40-9). Together the microvilli create a mucosal surface known as the brush border. The villi and microvilli greatly increase the surface area available for absorption. Coating the brush border is an “unstirred” layer of fluid that is important for the absorption of substances other than water and electrolytes. The lamina propria (a connective tissue layer of the mucosa) lies beneath the epithelial cells of the villi and contains lymphocytes; plasma cells, which produce immunoglobulins; and macrophages. Central arterioles ascend within each villus and branch into a capillary array that extends around the base of the columnar cells and cascades down to the venules that lead to the hepatic 1401 portal circulation. The opposing ascending and descending blood flow provides a countercurrent exchange system for absorbed substances and blood gases. A central lacteal, or lymphatic capillary, is also contained within each villus and is important for the absorption and transport of fat molecules. Contents of the lacteals flow to regional nodes and channels that eventually drain into the thoracic duct16 (see Figure 40-9). Between the bases of the villi are the crypts of Lieberkühn (intestinal glands), which extend to the submucosal layer. Undifferentiated (stem cells) and secretory cells and Paneth epithelial cells are located here. The stem cells are precursors of columnar epithelial and goblet cells. These premature cells produce alkaline fluids containing electrolytes, mucus, and water. These cells arise from the base of the crypt and move toward the tip of the villus, maturing in shape and function as they progress. After becoming columnar cells and completing their migration to the tip of the villus, they function for a few days and then are shed into the intestinal lumen and digested. The entire epithelial population is replaced about every 4 to 7 days. Many factors can influence this process of cellular proliferation. Starvation, vitamin B12 deficiency, and cytotoxic drugs or irradiation suppress cell division and shorten the villi. The decreased absorption that results can cause diarrhea and malnutrition. Nutrient intake and intestinal resection stimulate cell production. The Paneth cells located at the base of the crypts produce defensins and other antibiotic peptides and proteins.17 Other secretory cells produce digestive enzymes. Intestinal Digestion and Absorption The process of intestinal digestion is initiated in the stomach by the actions of gastric hydrochloric acid and pepsin, which break down food fibers and proteins. The chyme that passes into the duodenum is a liquid that contains small particles of undigested food. Digestion is continued in the proximal portion of the small intestine by the action of pancreatic enzymes, intestinal brushborder enzymes, and bile salts (Box 40-1). Here carbohydrates are broken down to monosaccharides and disaccharides; proteins are degraded further to amino acids and peptides; and fats are emulsified and reduced to fatty acids and monoglycerides (Figure 40-10). These nutrients, along with water, vitamins, and electrolytes, are absorbed across the intestinal mucosa and into the blood by active transport, diffusion, or facilitated diffusion. Products of carbohydrate and protein breakdown move into villus capillaries and then to the liver through the hepatic portal vein. Digested fats move into the lacteals and eventually reach the liver through the systemic circulation. Intestinal motility exposes nutrients to a large mucosal surface area by mixing chyme and moving it through the lumen. Different segments of the gastrointestinal tract absorb different nutrients. Digestion and absorption of all major nutrients occur in the small intestine. Sites of absorption are shown in Figure 40-11. Water and Electrolyte Transport by the Small Intestine. The epithelial cell membranes of the small intestine are formed of lipids and therefore are hydrophobic, or tend to repel water. (The properties of cell membranes are described in Chapter 1.) Therefore, water and electrolytes are transported in both directions (toward the capillary blood or toward the intestinal lumen) through the tight junctions and intercellular spaces 1402 Unit XII The Digestive System BOX 40-1 SOURCES OF DIGESTIVE ENZYMES Salivary Glands Amylase Lingual lipase Stomach Pepsin Gastric lipase Pancreas Amylase Trypsin Chymotrypsin Carboxypeptidase Elastase Lipase-colipase Phospholipase A2 Cholesterol esterase–nonspecific lipase Small Intestine Enterokinase Disaccharidases Maltase Sucrase Lactase α,α-Trehalase Isomaltase Peptidases Amino-oligopeptidase Dipeptidase From Johnson LR: Gastrointestinal physiology, ed 7, St Louis, 2007, Mosby. rather than across cell membranes. Water diffuses passively across hydrostatic pressure and osmotic gradients established by the active transport of sodium and other substances. Approximately 85% to 90% of the water that enters the gastrointestinal tract each day is absorbed in the small intestine. The remaining water and electrolytes are absorbed at a constant rate in the large intestine.18 Sodium passes through the tight junctions and is actively transported across cell membranes. Sodium and glucose share a common active transport carrier (sodium-glucose ligand transporter1 [SGLT1]) so that sodium absorption is enhanced by glucose transport18a (Figure 40-12). Potassium moves passively across the tight junctions with changes in the electrochemical gradient. Chloride is actively secreted throughout the large and small intestines. Carbohydrates. Carbohydrate (starch, table sugar [sucrose], milk sugar [lactose], cereal sugar [maltose]) accounts for at least 50% of the American diet. Because only monosaccharides (ribose, galactose, glucose, fructose) are absorbed by the intestinal mucosa, the complex carbohydrates (polysaccharides and oligosaccharides) must be hydrolyzed to their simplest form (see Figure 40-10). Salivary and pancreatic amylases break down starches to oligosaccharides by splitting α-1,4-glucosidic linkages of long-chain molecules. The major disaccharides are sucrose (glucose-fructose), maltose (glucose-glucose), and lactose (glucose-galactose). Approximately half of starch hydrolysis occurs in the stomach and about half in the duodenum. In the small intestine, disaccharides are hydrolyzed by brush-border enzymes (sucrase, maltase, and lactase) to their respective monosaccharides. The sugars are absorbed primarily in the duodenum and upper jejunum. The monosaccharides pass through the unstirred layer by diffusion. At the cell membrane, glucose and galactose are actively transported with a sodium carrier (SGLT1) and fructose absorption is facilitated by glucose transporter 5 (GLUT5) and GLUT7.19 Transport of all three monosaccharides from the cytosol to the bloodstream is facilitated by GLUT220 (see Figure 40-12). Insulin facilitates glucose transport into fat and muscle cells via glucose transporter 4 (GLUT4). Insulin is not required for the intestinal absorption of glucose. Cellulose is a glucose polysaccharide found in plants. Humans lack enzymes to digest cellulose, and the undigested fiber contributes to stool volume and stimulates large intestine motility. Proteins. Adults require 44 to 56 g of protein per day. Approximately 20 to 30 g of protein is derived endogenously from shed epithelial cells and small amounts of plasma proteins. Most ingested protein is absorbed; only 5% to 10% is eliminated in the stool. The site of digestion of protein depends on the source of the protein. For example, casein from bovine milk precipitates in the stomach and is digested by gastric pepsin and acid, whereas the soluble proteins whey and soy pass rapidly through the stomach and are digested by pancreatic enzymes.21 Major protein hydrolysis is accomplished in the small intestine by the pancreatic enzymes trypsin, chymotrypsin, and carboxypeptidase (see Figure 40-10). Trypsin and chymotrypsin (endopeptidase) hydrolyze the interior bonds of the large molecules, and carboxypeptidases cleave the end amino acids (exopeptidase). Hydrolysis of proteins is also carried out by the brush-border enzymes and enzymes in the epithelial cytosol (intracellular fluid). The brush-border enzymes hydrolyze the large oligopeptides (proteins composed of three to six amino acids) into smaller peptides, which can cross cell membranes. Enzymes in the cytosol then break them down to amino acids. Amino acids are actively transported from the cytosol into the bloodstream by a sodium-dependent carrier in the basolateral membrane. There also are free amino acids that can be absorbed directly from the intestinal lumen using a membrane transport protein. Like the sugars, proteins are absorbed primarily in the proximal area of the small intestine. Fats Approximately 90 to 100 g of fat is consumed daily by the average American. Fat is an important source of calories and is a primary structural component of cell membranes and organelles. Sources of dietary fat are reviewed in Box 40-2. Although triglycerides are the major dietary lipids, cholesterol, phospholipids, and fatsoluble vitamins also have nutritional importance. The digestion and absorption of fat occur in four phases: (1) emulsification and lipolysis, (2) micelle formation, (3) fat absorption, and (4) resynthesis of triglycerides and phospholipids. The mechanical action of the stomach and small intestine disperses the triglyceride droplets into small particles. Chapter 40 Structure and Function of the Digestive System Action Foodstuff Enzymes/source Site of action Starch Salivary amylase Mouth Dextrins, oligosaccharides Pancreatic amylase Carbohydrate digestion and absorption Lactose Maltose Sucrose Galactose Glucose Fructose Brush-border enzymes (lactase, maltase, sucrase) Small intestine Pepsin in presence of hydrochloric acid Stomach Absorbed by capillaries in the villi and transported to the liver by portal vein Proteins Proteases, peptones Protein digestion and absorption Pancreatic enzymes (trypsin, chymotrypsin, carboxypeptidase) Small intestine Brush-border enzymes (aminopeptidases and dipeptidases) Small intestine Emulsifying agents (bile acids, fatty acids, monoglycerides, lecithin, cholesterol, and protein) Small intestine Pancreatic lipases Small intestine Small polypeptides, dipeptides Amino acids Absorbed by capillaries in the villi and transported to the liver by hepatic portal vein Unemulsified fats Fat digestion Monoglycerides and fatty acids Glycerol and fatty acids Absorbed by lacteals in the villi and transported to the liver in the systemic circulation, which receives lymphatic flow from the thoracic duct or via the hepatic portal vein Glycerol and short-chain fatty acids absorbed by capillaries in the villi and transported to the liver by the portal vein FIGURE 40-10 Digestion and Absorption of Foodstuffs. 1403 1404 Unit XII The Digestive System Stomach Water Alcohol Duodenum Iron Calcium Fats Sugars Water Proteins Vitamins Magnesium Sodium Jejunum Sugars Proteins Ileum Bile salts Vitamin B12 Chloride Colon Water Electrolytes FIGURE 40-11 Sites of Absorption of Major Nutrients. Brush-border membrane Intestinal lumen Emulsification is the process by which emulsifying agents (fatty acids, monoglycerides, lecithin, cholesterol, protein, bile salts) in the small intestinal lumen cover the small fat particles and prevent them from re-forming into fat droplets. Emulsified fat is then ready for lipolysis (lipid hydrolysis) by pancreatic lipase, phospholipase, and hydrolase. Lipase breaks down triglycerides to diglycerides, monoglycerides, free fatty acids, and glycerol (see Figure 40-10). The action of lipase requires the presence of colipase, a pancreatic enzyme that allows lipase to penetrate the triglyceride molecule. Phospholipase cleaves fatty acids from phospholipids, and cholesterol esterase breaks cholesterol esters into fatty acids and glycerol. The products of lipid hydrolysis must be made water soluble if they are to be absorbed efficiently from the intestinal lumen. This is accomplished by the formation of water-soluble molecules known as micelles (Figure 40-13). Micelles are formed of bile salts (see p. 1411), the products of fat hydrolysis, fat-soluble vitamins, and cholesterol. The fats form the core of the micelle, and the polar bile salts form an outer shell, with the hydrophobic (“water-hating”) side facing the interior and the hydrophilic (“water-loving”) side facing the aqueous (water-like) content of the intestinal lumen. Because the unstirred layer of the brush border is aqueous, the micelles readily diffuse through it. The micelles maintain the fat molecules in the dissolved or solubilized form, which allows them to move more rapidly from the micelle toward the absorbing surface of the intestinal epithelium. The fat products of the micelle then readily diffuse through the epithelial cell membrane, while the bile salts remain in the lumen and proceed to the ileum, where they are absorbed into Intracellular region Capillary in villi Hydrolytic enzymes on surface of microvilli Fructose Fructose Sucrose Maltose Lactose Na (relatively high concentration) Glucose Galactose Na SGLT1 Passive transport Fructose Glucose (high concentration) Glucose Galactose Galactose Na (low concentration maintained by active extrusion) Na Basolateral membrane FIGURE 40-12 Monosaccharides and Sodium Transport. Schematic showing monosaccharides and sodium (Na+) transport through the small intestinal epithelium. Glucose, galactose, and sodium are transported into the epithelial cell by sodium-glucose ligand transporter 1 (SGLT1). See text for details. Chapter 40 Structure and Function of the Digestive System BOX 40-2 DIETARY FAT Saturated Fatty Acid (Palmitic Acid [C16H32O2]) Each carbon atom in the chain is linked by single bonds to adjacent carbon and hydrogen atoms; atoms are solid at room temperature and found in animal fat and tropical oils (coconut and palm oils); they increase low-density lipoprotein (LDL) cholesterol (“bad” cholesterol) blood levels and increase the risk of coronary artery disease. Unsaturated Fatty Acid Unsaturated fatty acids are soft or liquid at room temperature; omega-6 fatty acids are found in plants and vegetables (olive, canola, and peanut oils), and omega-3 fatty acids are found in fish and shellfish. 1.Monounsaturated fatty acids (e.g., oleic acid [C18H34O2]): Contain one double bond in the carbon chain and are found in plants and animals; may be beneficial in reducing blood cholesterol, glucose levels, and systolic blood pressure; do not lower high-density lipoprotein (HDL) cholesterol (“good” cholesterol) level; low HDL levels have been associated with coronary heart disease. 2.Polyunsaturated fatty acids (e.g., linoleic acid [C18H32O2]): Contain two or more double bonds in the carbon chain and are found in plants and fish oils; omega-6 fatty acids lower total and LDL cholesterol blood levels; high levels of polyunsaturated fatty acids may lower LDL level; omega-3 fatty acids lower blood triglyceride levels, reduce platelet aggregation and blood-clotting tendency, are necessary for growth and development, and may prevent coronary artery disease, hypertension, cancer, and inflammatory and immune disorders. Hydrophobic face Hydrophilic face OH groups Bile salts Peptide bonds Carboxyl or sulfonic acid A Cylindrical micelle B Cross section Bile acids Cholesterol Phospholipids Free fatty acids 2-Monoglycerides FIGURE 40-13 Structure of Bile Acid and Micelle. A, A bile salt molecule in solution. The molecule is amphipathic in that it has a hydrophilic face and a hydrophobic face. The amphipathic structure is key in the ability of the bile salts to emulsify lipids and form micelles. B, A model of the structure of a bile salt–lipid mixed micelle, an emulsified fat. (From Levy MN, Koeppen BM, Stanton BA: Principles of physiology, ed 4, St Louis, 2006, Mosby.) 1405 the circulation and returned to the liver via the enterohepatic circulation (Figure 40-14 and Figure 40-20, p. 1411). Almost all of the bile salts are recycled in this way. When the fat products reach the inside of the epithelial cell, they are resynthesized into triglycerides and phospholipids. The triglycerides are covered with phospholipids, lipoproteins, and cholesterol to become particles called chylomicrons. The chylomicrons travel to the basolateral membrane of the columnar epithelial cells, where they are extruded into the intercellular spaces of the villus. From here they enter the lacteals and lymphatic channels and, eventually, the systemic circulation. Minerals and Vitamins. The recommended intake of calcium ranges from 1000 to 1500 mg/day. Between 500 and 600 mg is secreted or shed into the lumen with desquamated epithelial cells. Not all of this calcium is absorbed. Daily absorption of calcium is approximately 600 mg. This amount increases with increased intake. When its concentration in the lumen is greater than 5 mmol/L, calcium is absorbed by passive diffusion. At concentrations less than 5 mmol/L, calcium is transported actively across cell membranes, bound to a carrier protein. The carrier formation requires the presence of the active form of vitamin D3 (1,25-dihydroxy-vitamin D). The calcium-protein complex moves into the epithelial cell, where the calcium binds to proteins or other substances. Then these complexes move through the basolateral membrane to the interstitial fluid by diffusion or active transport. Calcium is absorbed throughout the small intestine, but primarily in the ileum. Increased serum calcium concentration inhibits parathyroid hormone, which in turn decreases the formation of vitamin D3 by the kidney, thus regulating calcium absorption. Increased demand for calcium results in increased uptake, as evidenced by the fact that calcium is absorbed more rapidly in children and pregnant or lactating women. Bile salts enhance calcium absorption indirectly by facilitating the absorption of vitamin D, which is fat soluble. In addition, bile salts promote the absorption of free fatty acids that, at high concentrations, bind calcium and form soaps in the intestinal lumen. In older individuals calcium is absorbed less readily because of inadequate amounts of the active form of vitamin D.22 The recommended intake of magnesium for adults is 300 to 350 mg/day. Approximately 50% of it is absorbed by active transport or passive diffusion in the jejunum and ileum. Phosphate is also absorbed in the small intestine by passive diffusion and active transport. The levels of iron in the body are regulated primarily by intestinal absorption and secretion. The average intake ranges from 15 to 30 mg/day. Of this amount, menstruating women absorb 1 to 1.5 mg and men absorb 0.15 to 1 mg. Generally the amount of iron absorbed is equal to the amount required. Iron is absorbed more rapidly if a deficiency exists. A primary source of iron is heme from animal protein. This iron is rapidly absorbed by the epithelial cells primarily in the duodenum. Inorganic iron (e.g., iron in fruits, cereals, eggs, vegetables) is also readily absorbed. The presence of vitamin C reduces ferric iron to ferrous iron, which is the form more easily absorbed. Calcium phosphate and phosphoproteins (milk and antacids) in the intestinal lumen bind iron and reduce absorption. Tea also binds iron and inhibits absorption by forming iron tannate complexes. 1406 Unit XII The Digestive System Unstirred layer Brush-border membrane Microvillus Well-mixed luminal contents Diffusion of micelles through unstirred layer Fatty acids Cytosol Phospholipids Cholesterol Monoglycerides FIGURE 40-14 Lipid Absorption in the Small Intestine. Micelles of bile salts and products of lipid digestion diffuse through the unstirred layer and among the microvilli. As digestive products are absorbed from free solution by epithelial cells of the villi, more digestive products dissociate from the micelles. (From Levy MN, Koeppen BM, Stanton BA: Principles of physiology, ed 4, St Louis, 2006, Mosby.) TABLE 40-2 INTESTINAL ABSORPTION OF VITAMINS VITAMIN MECHANISMS OF ABSORPTION SITE OF ABSORPTION Fat-Soluble Vitamins A (retinal) D3 (1,25-dihydroxy-vitamin D) E (α-tocopherol) K Micelle formation with bile salts; lipid diffusion Ileum Active transport (sodium dependent) Unknown Passive diffusion Passive diffusion; active transport (sodium dependent) Active transport (sodium dependent) Active transport (intrinsic factor dependent) Passive diffusion Passive diffusion Unknown Duodenum and jejunum Duodenum and jejunum Jejunum Ileum Jejunum Terminal ileum Jejunum Duodenum and jejunum Unknown Water-Soluble Vitamins B1 (thiamine) B2 (riboflavin) Niacin (nicotinic acid) C (ascorbic acid) Folic acid B12 (cobalamin) B6 (pyridoxine, pyridoxamine, pyridoxal phosphate) Pantothenic acid Biotin Iron is bound to intestinal transferrin in the lumen and is absorbed and bound to the protein ferritin and to amino acid chelates in the cytosol of the enterocytes. Transport of iron across the basolateral membrane is determined by the amount of iron in the circulation. It is transported in the blood by plasma transferrin, a glycoprotein, and is carried to body tissues. When there is less need for iron, it remains in the enterocyte as ferritin and is carried into the lumen when the cell is sloughed from the end of the villus. Following hemorrhage, the intestinal cells require 3 days to increase their rate of iron absorption. This is because the need for iron is perceived by the precursor stem cells in the crypts of Lieberkühn, and they take 3 days to mature and migrate to the tips of the villi, where they absorb more iron. Hepcidin is a protein synthesized by the liver that inhibits apical uptake of iron by enterocytes and modulates iron trafficking.23 The absorption of vitamins is summarized in Table 40-2. Most of the water-soluble vitamins are absorbed passively or by sodium-dependent active transport. Most vitamin B12 (cobalamin) is bound to intrinsic factor (making it resistant to digestion) and absorbed in the terminal ileum, although a small amount of the vitamin is absorbed in its free (unbound) form. Intestinal Motility The movements of the small intestine facilitate digestion and absorption. Chyme coming from the stomach stimulates intestinal movements that mix in secretions from the liver, pancreas, and intestinal glands. A churning motion brings the luminal content into contact with the absorbing cells of the villi. Propulsive movements then advance the chyme toward the large intestine. Chapter 40 Structure and Function of the Digestive System Intestinal motility is regulated by the enteric nervous system, vagal stimulation, and hormones (see Table 40-1). Two movements promote motility: segmentation and peristalsis. Segmentation consists of localized rhythmic contractions of the circular smooth muscles and occurs more frequently than peristalsis.24 The contraction waves occur at different rates in different parts of the small intestine in segments of 1 to 4 cm. Frequency is greatest (12 per minute) in the upper small intestine and least (8 per minute) in the distal part of the ileum. Segmentation divides and mixes the chyme, bringing it into contact with the absorbent mucosal surface. It also helps to propel the chyme toward the large intestine. The frequency of the segmentation is regulated intrinsically by the frequency of the basic electrical rhythm (BER), which arises in the myenteric plexus of longitudinal smooth muscle and is controlled by the interstitial cells of Cajal, the pacemaker cells of the gastrointestinal (GI) tract. Although the basic rate of contraction is controlled intrinsically, the force of contraction can be enhanced extrinsically by vagal stimulation.25 Intestinal peristalsis involves short segments (about 10 cm) of longitudinal smooth muscle and propels chyme through the intestine. The wave of contraction moves slowly (1 to 2 cm/second) to allow time for digestion and absorption. Peptide hormones, including motilin, gastrin, secretin, and cholecystokinin, facilitate intestinal motility. Neural reflexes along the length of the small intestine facilitate motility, digestion, and absorption. Through reflex action, receptors in one part of the intestine transmit signals that influence the function of another part. The ileogastric reflex inhibits gastric motility when the ileum becomes distended. This prevents the continued movement of chyme into an already distended intestine. The intestinointestinal reflex inhibits intestinal motility when one part of the intestine is overdistended. Both of these reflexes require extrinsic innervation. The gastroileal reflex, which is activated by an increase in gastric motility and secretion, stimulates an increase in ileal motility and relaxation of the ileocecal sphincter. This empties the ileum and prepares it to receive more chyme. The gastroileal reflex is probably regulated by the hormones gastrin and cholecystokinin or through the autonomic nerves. During prolonged fasting or between meals, particularly overnight, slow waves sweep along the entire length of the intestinal tract from the stomach to the terminal ileum. This is known as the interdigestive myoelectric complex, and it appears to propel residual gastric and intestinal contents, including bacteria, into the colon. The intestinal villi move with contractions of the muscularis mucosae, a very thin layer of muscle that separates the mucosa and submucosa. Absorption is promoted by the swaying of villi in the luminal contents. Contractile activity also helps to empty the central lacteals, which contain products of fat digestion. The ileocecal valve (sphincter) marks the junction between the terminal ileum and the large intestine. This valve is intrinsically regulated and is normally closed. The arrival of peristaltic waves from the last few centimeters of the ileum causes the ileocecal valve to open, allowing a small amount of chyme to pass through. Distention of the upper large intestine causes the 1407 sphincter to constrict, preventing further distention or retrograde flow of intestinal contents. Large Intestine The large intestine is approximately 1.5 m long and consists of the cecum, appendix, colon, rectum, and anal canal (Figure 40-15). The cecum is a pouch that receives chyme from the ileum. Attached to the cecum is the vermiform appendix, an appendage having little or no physiologic function. From the cecum, chyme enters the colon, a four-part length of intestine that loops upward, traverses the abdominal cavity, and descends to the anal canal. The four parts of the colon are the ascending colon, transverse colon, descending colon, and sigmoid colon. Two sphincters control the flow of intestinal contents through the cecum and colon: the ileocecal valve, which admits chyme from the ileum to the cecum, and the rectosigmoid (O’Beirne) sphincter, which controls the movement of wastes from the sigmoid colon into the rectum. A thick (2.5 to 3 cm) portion of smooth muscle surrounds the anal canal, forming the internal anal sphincter. Overlapping it distally is the striated skeletal muscle of the external anal sphincter. In the cecum and colon the longitudinal muscle layer consists of three longitudinal bands called teniae coli (see Figure 40-15). The teniae coli are shorter than the colon, giving the colon its “gathered” appearance. The circular muscles of the colon separate the gathers into outpouchings called haustra. The haustra become more or less prominent with the contractions and relaxations of the circular muscles. The mucosal surface of the colon has rugae (folds), particularly between the haustra, and Lieberkühn crypts but no villi. Columnar epithelial cells and mucus-secreting goblet cells form the mucosa throughout the large intestine. The columnar epithelium absorbs fluid and electrolytes, and the mucus-secreting cells lubricate the mucosa. The enteric nervous system regulates motor and secretory activity independently of extrinsic nervous system control. Extrinsic parasympathetic innervation occurs through the vagus nerve and extends from the cecum up to the first part of the transverse colon. Vagal stimulation increases rhythmic contraction of the proximal colon. Extrinsic parasympathetic fibers reach the distal colon through the sacral parasympathetic splanchnic nerves. The internal anal sphincter is usually in a state of contraction, and its reflex response is to relax when the rectum is distended. The myenteric plexus provides the major innervation of the internal anal sphincter, but responds to sympathetic stimulation to maintain contraction and parasympathetic stimulation that facilitates relaxation when the rectum is full. Sympathetic innervation of this sphincter arises from the celiac and superior mesenteric ganglia and the sphincter nerve. The external anal sphincter is innervated by the pudendal nerve arising from sacral levels of the spinal cord. Voluntary control of the external anal sphincter is paralyzed after injury of the lower spinal cord, but the internal sphincter is still functional. Sympathetic activity in the entire large intestine modulates intestinal reflexes, conveys somatic sensations of fullness and pain, participates in the defecation reflex, and constricts blood vessels. The blood supply of the large intestine and rectum is derived primarily from branches of the superior and inferior mesenteric artery.26 1408 Unit XII The Digestive System Hepatic portal vein Aorta Transverse Inferior vena cava Splenic colon vein Superior mesenteric artery Hepatic (right colic) flexure Splenic (left colic) flexure Inferior mesenteric artery and vein Teniae coli Ascending colon Haustra Descending colon Mesentery Ileocecal valve Sigmoid artery and vein Ileum Cecum A Vermiform Rectum appendix External anal sphincter muscle Superior rectal artery and vein Anus Crypt of Lieberkühn Lamina propria Lymphoid nodule Muscularis mucosae Sigmoid colon Submucosa B Circular muscle of muscularis externa FIGURE 40-15 Large Intestine. A, Structure of the large intestine. B, Microscopic cross section illustrating cellular structures of the large intestine. The wall of the large intestine is lined with columnar epithelium in contrast to the villi characteristics of the small intestine. The longitudinal layer of muscularis is reduced to become the teniae coli. (A modified from Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby; B from Gartner LP, Hiatt JL: Color textbook of histology, Philadelphia, 2007, Saunders.) The primary type of colonic movement is segmental. The circular muscles contract and relax at different sites, shuttling the intestinal contents back and forth between the contracting and relaxing haustra. The movements massage the intestinal contents, then called the fecal mass, and facilitate the absorption of water. Propulsive movement occurs with the proximal-to-distal contraction of several haustral units. Peristaltic movements also occur and promote the emptying of the colon. The gastrocolic reflex initiates propulsion in the entire colon, usually during or immediately after eating, when chyme enters from the ileum. The gastrocolic reflex causes the fecal mass to pass rapidly into the sigmoid colon and rectum, stimulating defecation. Gastrin and cholecystokinin participate in stimulating this reflex. Epinephrine inhibits contractile activity. Approximately 500 to 700 ml of chyme flows from the ileum to the cecum per day. Most of the water is absorbed in the colon by diffusion and active transport. The electrochemical gradient established by sodium movement enhances the diffusion of serum potassium from the capillaries in the lumen. Aldosterone increases colon membrane permeability to sodium, thereby increasing both the diffusion of sodium into the cell and the active transport of sodium across the basolateral membrane to the interstitial fluid. (See Chapters 3, 21, and 37 for a discussion of aldosterone secretion.) This increases the cell-to-lumen diffusion gradient for potassium. Potassium moves outward, and chloride is absorbed with sodium as the complementary anion. Chloride also enters the cell in exchange for bicarbonate. By the time the fecal mass enters the sigmoid colon, the mass consists entirely of wastes and is called the feces. Feces, or excrement, consists of food residue, unabsorbed gastrointestinal secretions, shed epithelial cells, and bacteria. The movement of feces into the sigmoid colon and rectum stimulates the defecation reflex (rectosphincteric reflex). The rectal wall stretches and the tonically constricted internal anal sphincter relaxes, creating the urge to defecate. The defecation reflex can be overridden voluntarily by contraction of the external anal sphincter and muscles of the pelvic floor. The rectal wall gradually relaxes, reducing tension, and the urge to defecate passes. Retrograde contraction of the rectum may displace the feces out of the rectal vault until a more convenient time for evacuation. Pain or fear of pain associated with defecation (e.g., rectal fissures or hemorrhoids) can inhibit the defecation reflex. The defecation reflex is regulated by parasympathetic and cholinergic fibers. Voluntary inhibition or facilitation of defecation is mediated from cortical projections onto the medulla and down to sacral segments of the cord. Defecation is facilitated by squatting or sitting because these positions straighten the angle between the rectum and anal canal and increase the efficiency of straining (increasing intraabdominal pressure). Intra-abdominal pressure is increased by initiating the Valsalva maneuver. This maneuver consists of inhaling and forcing the diaphragm and chest muscles against the closed glottis. This increases both intrathoracic and intraabdominal pressure, which is transmitted to the rectum. Intestinal Bacteria The type and number of bacterial flora vary greatly throughout the normal gastrointestinal tract, with an increasing number Chapter 40 Structure and Function of the Digestive System Hepatic duct Cystic duct Liver Esophagus Pyloric sphincter Gallbladder Spleen 1409 digestion and absorption. Between meals bile is stored in the gallbladder. The exocrine pancreas produces enzymes needed for the complete digestion of carbohydrates, proteins, and fats. The exocrine pancreas also produces an alkaline fluid that neutralizes chyme, creating a duodenal pH that supports enzymatic action. The liver receives nutrients absorbed by the small intestine and metabolizes or synthesizes these nutrients into forms that can be absorbed by the body’s cells. It then releases the nutrients into the bloodstream or stores them for later use. Liver Stomach Common bile duct Accessory papilla Greater duodenal papilla (of Vater) Pancreas Accessory pancreatic duct Pancreatic duct Duodenum FIGURE 40-16 Locations of the Liver, Gallbladder, and Exocrine Pancreas, Which Are the Accessory Organs of Digestion. of bacteria from the stomach to the distal colon. The stomach is relatively sterile because of the secretion of acid that kills ingested pathogens or inhibits bacterial growth. Bile acid secretion, intestinal motility, and antibody production suppress bacterial growth in the duodenum, and in the duodenum and jejunum there is a low concentration of aerobes (10−1 to 10−4/ ml), primarily streptococci, lactobacilli, staphylococci, enterobacteria, and Bacteroides. Anaerobes are only found distal to the ileocecal valve. They constitute about 95% of the fecal flora in the colon and contribute one third of the solid bulk of feces. Bacteroides, clostridia, anaerobic lactobacilli, and coliforms are the most common microorganisms from the ileum to the cecum.27 The intestinal tract is sterile at birth but becomes colonized with Escherichia coli, Clostridium welchii, and Streptococcus within a few hours. Within 3 to 4 weeks after birth, the normal flora are established. The normal flora do not have the virulence factors associated with pathogenic microorganisms, thus permitting immune tolerances. The intestinal mucosal environment also produces a broad spectrum of protective antimicrobial agents.28 The intestinal bacteria play a role in the metabolism of bile salts (contributing to the intestinal reabsorption of bile and the elimination of toxic bile metabolites); the metabolism of estrogens, androgens, and lipids and conversion of unabsorbed carbohydrates to absorbable organic acids; the synthesis of vitamin K2 and B vitamins; and the metabolism of various nitrogenous substances and drugs.29 Accessory Organs of Digestion The liver, gallbladder, and exocrine pancreas all secrete substances necessary for the digestion of chyme. These secretions are delivered to the duodenum through ducts (Figure 40-16). The liver produces bile, which contains salts necessary for fat The liver, which weighs 1200 to 1600 g, is the largest solid organ in the body. It is located under the right diaphragm and is divided into right and left lobes. The larger, right lobe is divided further into the caudate and quadrate lobes (Figure 40-17). The falciform ligament separates the right and left lobes and attaches the liver to the anterior abdominal wall. A fibrous cord called the round ligament (ligamentum teres) extends along the free edge of the falciform ligament. The round ligament is the remnant of the umbilical vein and extends from the umbilicus to the inferior surface of the liver. The coronary ligament branches from the falciform ligament and extends over the superior surface of the right and left lobes, adhering the liver to the inferior surface of the diaphragm. The liver is covered by a fibroelastic capsule called the Glisson capsule. When the liver is diseased or swollen, distention of the capsule causes pain because it is innervated by afferent neurons. The metabolic functions of the liver require a large amount of blood. The liver receives blood from both arterial and venous sources. The hepatic artery branches from the celiac artery and provides oxygenated blood at the rate of 400 to 500 ml/ minute (about 25% of the cardiac output). The hepatic portal vein, which receives deoxygenated blood from the inferior and superior mesenteric veins and the splenic vein, delivers about 1000 to 1200 ml/minute of blood to the liver. The hepatic portal vein, which carries 70% of the blood supply to the liver, is rich in nutrients that have been absorbed from the digestive tract (Figure 40-18). Within the liver lobes are multiple, smaller anatomic units called liver lobules (Figure 40-19). The lobules are formed of cords or plates of hepatocytes, which are the functional cells of the liver. These cells are capable of regeneration; therefore, damaged or resected liver tissue can regrow. Hepatocytes secrete electrolytes, lipids, lecithin, bile acids, and cholesterol into the canaliculi. Plasma proteins are also synthesized and released into the bloodstream. Lipocytes are star-shaped cells that store lipids, including vitamin A. Small capillaries, or sinusoids, are located between the plates of hepatocytes. The sinusoids receive a mixture of venous and arterial blood from branches of the hepatic artery and hepatic portal vein. Blood from the sinusoids drains into a central vein in the middle of each liver lobule. Venous blood from all the lobules then flows into the hepatic vein, which empties into the inferior vena cava. The sinusoids of the liver lobules are lined with highly permeable endothelium. This permeability enhances the transport of nutrients from the sinusoids into the hepatocytes, where they are metabolized.30 The sinusoids are also lined with phagocytic 1410 Right lobe Unit XII The Digestive System Gallbladder Inferior vena cava Quadrate lobe Left lobe Falciform ligament Right lobe proper Left lobe Common hepatic duct Hepatic artery Falciform ligament Hepatic portal vein Round ligament Gallbladder Inferior vena cava A Caudate lobe B FIGURE 40-17 Gross Structure of the Liver. A, Anterior view. B, Inferior view. (From Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) Inferior vena cava Hepatic veins Liver Hepatic portal vein Duodenum Pancreas Superior mesenteric vein Ascending colon Appendix Stomach Sinusoid Plates of hepatic cells Central veins Gastric vein Spleen Pancreatic veins Splenic vein Gastroepiploic vein Bile duct Inferior mesenteric vein Descending colon Small intestine Branch of portal vein Hepatocytes Bile canaliculi Branch of hepatic artery FIGURE 40-18 Hepatic Portal Circulation. In this unusual circulatory route, a vein is located between two capillary beds. The hepatic portal vein collects blood from capillaries in visceral structures located in the abdomen and empties into the liver for distribution to the hepatic capillaries. Hepatic veins return blood to the inferior vena cava. (Organs are not drawn to scale.) (From Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) FIGURE 40-19 Diagrammatic Representation of a Liver Lobule. A central vein is located in the center of the lobule, with plates of hepatocytes disposed radially. Branches of the portal vein and hepatic artery are located on the periphery of the lobule, and blood from both vessels perfuses the sinusoids. Peripherally located bile ducts drain the bile canaliculi that run between the hepatocytes. (Modified from Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.) cells, known as Kupffer cells, which are part of the mononuclear phagocyte system (see Chapters 7 and 27) and are the largest population of tissue macrophages in the body. They are bactericidal and central to innate immunity.31 Stellate cells contain retinoids (vitamin A), are contractile in liver injury, regulate sinusoidal blood flow, may proliferate into myofibroblasts, and participate in liver fibrosis.32 They remove foreign substances from the blood and trap bacteria. Pit cells are natural killer cells found in the sinusoidal lumen; they produce interferon-γ and are important in tumor defense.33 Between the endothelial lining of the sinusoid and the hepatocyte is the Disse space, which drains interstitial fluid into the hepatic lymph system. Chapter 40 Structure and Function of the Digestive System Liver Hepatocytes synthesize cholesterol to form primary bile acids Bile acid pool Amino acids (glycine or taurine) conjugate bile acids to form bile salts in bile Gallbladder Some bile is stored for release during eating Hepatic portal vein 65% to 85% of bile salts and secondary bile acids enter the circulation with protein binding and are transported to liver Duodenum and jejunum Bile salts emulsify fats and form micelles to transport fats through the unstirred layer Micelles release fats at the brush border Free bile salts proceed through the intestinal lumen Ileum and colon Bile salts are actively transported across the intestinal lumen or are deconjugated by bacteria into secondary bile acids that diffuse passively across the lumen Rectum 15% to 35% of bile salts are excreted in feces FIGURE 40-20 The Enterohepatic Circulation of Bile Salts. Secretion of Bile The liver assists intestinal digestion by secreting 700 to 1200 ml of bile per day. Bile is an alkaline, bitter-tasting yellowish green fluid that contains bile salts (conjugated bile acids), cholesterol, bilirubin (a pigment), electrolytes, and water. It is formed by hepatocytes and secreted into the canaliculi, which are small channels adjacent to hepatocytes. Bile salts, which are conjugated bile acids, are required for the intestinal emulsification and absorption of fats. The bile canaliculi empty into hepatic bile ducts and eventually drain into the common bile duct (see Figure 40-19). The union of the common bile duct and pancreatic duct is at the papilla or ampulla of Vater, which empties into the duodenum through an opening called the major duodenal papilla and is surrounded by the sphincter of Oddi.34 Having facilitated fat emulsification and absorption in the small intestine, most bile salts are actively absorbed in the terminal ileum and returned to the liver through the portal circulation for resecretion. The pathway for recycling of bile salts is termed the enterohepatic circulation (Figure 40-20).35 1411 Bile has two fractional components: the acid-dependent fraction and the acid-independent fraction. Hepatocytes secrete the bile acid–dependent fraction of the bile. This fraction consists of bile acids, cholesterol, lecithin (a phospholipid), and bilirubin (a bile pigment). The bile acid–independent fraction of the bile, which is secreted by the hepatocytes and epithelial cells of the bile canaliculi, is a bicarbonate-rich aqueous fluid that gives bile its alkaline pH. Bile salts are conjugated in the liver from primary and secondary bile acids. The primary bile acids are cholic acid and chenodeoxycholic (chenic acid or chenodiol) acid. These acids are synthesized from cholesterol by the hepatocytes. The secondary bile acids are deoxycholic acid and lithocholic acid. These acids are formed in the small intestine by the action of intestinal bacteria, after which they are absorbed and flow to the liver (see Figure 40-20). Both forms of bile acids are conjugated with amino acids (glycine or taurine) in the liver to form bile salts. Conjugation makes the bile acids more water soluble, thus restricting their diffusion from the duodenum and ileum. The primary and secondary bile acids together form the bile acid pool. Bile secretion is called choleresis. A choleretic agent is a substance that stimulates the liver to secrete bile. One strong stimulus is a high concentration of bile salts. Other choleretics include secretin, which increases the rate of bile flow by promoting the secretion of bicarbonate from canaliculi and other intrahepatic bile ducts; cholecystokinin; and vagal stimulation. Metabolism of Bilirubin Bilirubin is a byproduct of destruction of aged red blood cells. It gives bile a greenish black color and produces the yellow tinge of jaundice. Aged red blood cells are taken up and destroyed by macrophages of the mononuclear phagocyte system, primarily in the spleen and liver. (In the liver these macrophages are Kupffer cells.) Within these cells, hemoglobin is separated into its component parts—heme and globin (Figure 40-21). The globin component is further degraded into its constituent amino acids, which are recycled to form new protein. The heme moiety is converted to biliverdin by the enzymatic (heme oxygenase) cleavage of iron. The iron attaches to transferrin in the plasma and can be stored in the liver or used by the bone marrow to make new red blood cells. The biliverdin is enzymatically converted to bilirubin in the macrophage of the mononuclear phagocytic system and then is released into the plasma. In the plasma, bilirubin binds to albumin and is known as unconjugated bilirubin, or free bilirubin, which is lipid soluble. Bilirubin also may have a role as an antioxidant and provide cytoprotection.36,37 In the liver, unconjugated bilirubin moves from plasma in the sinusoids into the hepatocyte. Within hepatocytes it joins with glucuronic acid to form conjugated bilirubin, which is water soluble. Conjugation transforms bilirubin from a lipid-soluble substance that can cross biologic membranes to a water-soluble substance that can be excreted in the bile. When conjugated bilirubin reaches the distal ileum and colon, it is deconjugated by bacteria and converted to 1412 Unit XII The Digestive System TABLE 40-3 PROTEINS IN THE BODY Red blood cell destruction Mononuclear phagocytes in spleen and liver Hemoglobin Globin Heme ROLE EXAMPLE Contraction Energy Fluid balance Protection Actin and myosin enable muscle contraction. Proteins can be metabolized for energy. Albumin is a major source of plasma oncotic pressure. Antibodies and complement protect against infection and foreign substances. Enzymes control chemical reactions; hormones regulate many physiologic processes. Collagen fibers provide structural support to many parts of the body; keratin strengthens skin, hair, and nails. Hemoglobin transports oxygen and carbon dioxide in the blood; plasma proteins serve as transport molecules; proteins in cell membranes control movement of materials into and out of cells. Hemostasis is regulated by proteins that balance coagulation and anticoagulation. Regulation Structure Iron Biliverdin Iron pool Unconjugated bilirubin (lipid soluble) Plasma Amino acid pool Coagulation Unconjugated bilirubin Albumin (bilirubin/albumin complex makes bilirubin soluble in blood) Hepatocytes in liver Unconjugated bilirubin Glucuronic acid Bile channels Conjugated bilirubin (conjugated bilirubin is water soluble) excreted with bile Intestine Conjugated bilirubin excreted with stools Bacterial action Liver Kidney Urobilinogen Feces Transport Urine FIGURE 40-21 Bilirubin Metabolism. See text for further explanation. urobilinogen. Urobilinogen is then reabsorbed in the intestines and transported to the kidney where it is excreted in the urine as urobilin, giving urine its yellow color. A small amount of urobilin is recirculated back into the liver and eliminated in feces as stercobilin, which contributes to the stool’s brown pigmentation. Vascular and Hematologic Functions Because of its extensive vascular network, the liver can store a large volume of blood. The amount stored at any one time depends on pressure relationships in the arteries and veins. The liver also can release blood to maintain systemic circulatory volume in the event of hemorrhage. Kupffer cells (macrophages) in the sinusoids of the liver remove bacteria and foreign particles from blood in the hepatic circulation. Because the liver receives all of the venous blood from the gut and pancreas, the Kupffer cells play an important role in destroying intestinal bacteria and preventing infections. The liver also has hemostatic functions. It synthesizes most of the clotting factors (see Chapters 7 and 27). Vitamin K1 (synthesized by plants and ingested in the diet) and vitamin K2 (synthesized by intestinal bacteria) are fat-soluble vitamins essential for the synthesis of clotting factors (prothrombin and factors VII, IX, and X). Because bile salts are needed for absorption of fats, vitamin K absorption depends on adequate bile production in the liver. Impairment of vitamin K absorption diminishes production of clotting factors and increases risk of bleeding. Metabolism of Nutrients Fats. Ingested fat absorbed by lacteals in the intestinal villi enters the liver through the lymphatics, primarily as triglycerides. In the liver the triglycerides can be hydrolyzed to glycerol and free fatty acids and used to produce metabolic energy (ATP), or they can be released into the bloodstream bound to proteins (lipoproteins). The lipoproteins are carried by the blood to adipose cells for storage. The liver also synthesizes phospholipids and cholesterol, which are needed for the hepatic production of bile salts, steroid hormones, components of plasma membranes, and other special molecules. Proteins. Protein synthesis requires the presence of all the essential amino acids (obtained only from food), as well as nonessential amino acids. Proteins perform many important roles in the body and are summarized in Table 40-3. Within hepatocytes, amino acids are converted to carbohydrates by the removal of ammonia (NH3), a process known as deamination. The ammonia is converted to urea by the liver and passes into the blood to be excreted by the kidneys. The plasma proteins, including albumins and globulins (with the exception of gamma globulin, which is formed in lymph nodes and lymphoid tissue), are synthesized by the liver. The liver also Chapter 40 Structure and Function of the Digestive System synthesizes several nonessential amino acids and serum enzymes that become elevated with liver injury (and other diseases): • Aspartate aminotransferase (AST) (previously called serum glutamate-oxaloacetate transaminase [SGOT]): also present in red blood cells and skeletal muscle; AST transfers an α-amino group between aspartate and glutamate. • Alanine aminotransferase (ALT) (previously called serum glutamate-pyruvate transaminase [SGPT]): also present in small amounts in the kidneys, heart, skeletal muscle, and pancreas; ALT transfers an amino group from alanine to α-ketoglutarate to form pyruvate and glutamate. • Lactate dehydrogenase (LDH): catalyzes the conversion of lactate to pyruvate; LDH is widely distributed throughout the body and different isoenzymes are found in different tissues. • Alkaline phosphatase: removes phosphate groups, particularly in an alkaline environment. • Gamma-glutamyltransferase: transfers the gammaglutamyl moiety of glutathione to an acceptor to form glutamate and is a pro-oxidant. Reference values for the above enzymes can be reviewed in Table 40-6 and are summarized on the inside back book cover. Carbohydrates. The liver contributes to the stability of blood glucose levels by releasing glucose during states of hypoglycemia (low blood glucose levels) and taking up glucose during states of hyperglycemia (high blood glucose levels) and storing it as glycogen (glyconeogenesis) or converting it to fat. When all glycogen stores have been used, the liver can convert amino acids and glycerol to glucose. Metabolic Detoxification The liver alters exogenous and endogenous chemicals (e.g., drugs), foreign molecules, and hormones to make them less toxic or less biologically active. This process, called metabolic detoxification (biotransformation), diminishes intestinal or renal tubular reabsorption of potentially toxic substances and facilitates their intestinal and renal excretion. In this way alcohol, barbiturates, amphetamines, steroids, and hormones (including estrogens, aldosterone, antidiuretic hormone, and testosterone) are metabolized or detoxified, preventing excessive accumulation and adverse effects. Although metabolic detoxification is usually protective, sometimes the end products of metabolic detoxification become toxins. Those of alcohol metabolism, for example, are acetaldehyde and hydrogen. Excessive intake of alcohol over a prolonged period causes these end products to damage hepatocytes. Acetaldehyde damages cellular mitochondria, and the excess hydrogen promotes fat accumulation. This is how alcohol impairs the liver’s ability to function (see Chapter 2). Storage of Minerals and Vitamins The liver stores certain vitamins and minerals, including iron and copper, in times of excessive intake and releases them in times of need. The liver can store vitamins B12 and D for several months and vitamin A for several years. The liver also stores vitamins 1413 E and K. Iron is stored in the liver as ferritin, an iron-protein complex, and is released as needed for red blood cell production. Gallbladder The gallbladder is a saclike organ that lies on the inferior surface of the liver (Figures 40-17 and 40-22). The wall of the gallbladder is composed of the mucous membrane, muscularis, and serosa. The primary function of the gallbladder is to store and concentrate bile between meals. During the interdigestive period, bile flows from the liver through the right or left hepatic duct into the common bile duct and meets resistance at the closed sphincter of Oddi, which controls flow into the duodenum and prevents reflux of duodenal contents into the pancreatobiliary system. Bile then flows into the gallbladder through the cystic duct where it is concentrated and stored. The mucosa of the gallbladder wall readily absorbs water and electrolytes, leaving a high concentration of bile salts, bile pigments, and cholesterol. The gallbladder holds about 90 ml of bile. Within 30 minutes after eating, the gallbladder begins to contract, forcing stored bile through the cystic duct and into the common bile duct. The sphincter of Oddi relaxes, and bile flows into the duodenum through the major duodenal papilla. During the cephalic and gastric phases of digestion, gallbladder contraction is mediated by cholinergic branches of the vagus nerve. Hormonal regulation of gallbladder contraction is derived primarily from the release of cholecystokinin secreted by the duodenal and jejunal mucosa in the presence of fat. Vasoactive intestinal peptide, pancreatic polypeptide, and sympathetic nerve stimulation relax the gallbladder. Exocrine Pancreas The pancreas is approximately 20 cm long, with its head tucked into the curve of the duodenum and its tail touching the spleen. The body of the pancreas lies deep in the abdomen, behind the stomach (see Figure 40-16). The pancreas is unique in that it has endocrine as well as exocrine functions. The endocrine pancreas secretes insulin, glucagon, somatostatin, and pancreatic polypeptide (see Chapter 21). The exocrine pancreas is composed of acinar cells that secrete enzymes and networks of ducts that secrete alkaline fluids with important digestive functions. The acinar cells are organized into spherical lobules (acini) around small secretory ducts (see Figure 40-22). Secretions drain into a system of ducts that leads to the pancreatic duct (Wirsung duct), which empties into the common bile duct at the ampulla of Vater and then through the duodenal papilla into the duodenum. In some individuals an accessory duct (the duct of Santorini) branches off the pancreatic duct and drains directly into the duodenum at an opening called the minor duodenal papilla (see Figure 40-22). Arterial blood is supplied to the pancreas by branches of the celiac and superior mesenteric arteries. Venous blood leaves the head of the pancreas through the tributaries to the hepatic portal vein, with the body and tail being drained through the splenic vein. All hormonal pancreatic secretions also pass through the hepatic portal vein into the liver. Pancreatic innervation arises from parasympathetic neurons of the vagus nerve, which stimulate enzymatic and hormonal 1414 Unit XII The Digestive System Neck of gallbladder Right and left hepatic ducts Body of gallbladder Cystic duct Fundus of gallbladder Common hepatic duct Common bile duct Duodenum Accessory Minor pancreatic duct duodenal Body of pancreas papilla Tail of pancreas Ampulla of Vater Major duodenal papilla Pancreatic duct Jejunum Head of pancreas Alpha cells (secrete glucagon) Beta cells (secrete insulin) Pancreatic islet Acini Duct cells secrete bicarbonate Vein Acinar cells secrete enzymes Pancreatic duct (to duodenum) Centroacinar cells secrete electrolytes and water FIGURE 40-22 Associated Structures of the Gallbladder, Pancreas, and Pancreatic Acinar Cells and Duct. (Modified from Patton KT, Thibodeau GA: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.) secretion. Sympathetic postganglionic fibers from the celiac and superior mesenteric plexuses innervate the blood vessels and cause vasoconstriction and inhibit pancreatic secretion.38 The aqueous secretions of the exocrine pancreas are isotonic and contain potassium, sodium, bicarbonate, magnesium, calcium, and chloride. Sodium and potassium concentrations are about equal to those in the plasma. The concentration of bicarbonate in pancreatic juice varies directly with the secretory flow rate. As bicarbonate secretion increases, chloride secretion decreases to maintain a constant anionic concentration. The highly alkaline pancreatic juice neutralizes the acidic chyme that enters the duodenum from the stomach and provides the alkaline medium needed for the actions of digestive enzymes and the absorption of fat in the intestine. In the pancreas, transport of water and electrolytes through the ductal epithelium involves active and passive mechanisms. The ductal cells actively transport hydrogen into the blood and bicarbonate into the duct lumen. Potassium and chloride are secreted by diffusion according to changes in electrochemical potential gradients. As the secretion flows down the duct, water is osmotically transported into the juice until it becomes isosmotic. At low flow rates, bicarbonate is exchanged passively for chloride, but at higher flow rates there is less time for this exchange and bicarbonate concentration increases. Because Chapter 40 Structure and Function of the Digestive System 1415 TABLE 40-4 SELECTED STUDIES OF GASTROINTESTINAL STRUCTURE TEST DESCRIPTION APPLICATION Plain roentgenograms Use of high-energy electromagnetic radiation to evaluate tissue structure by radiopacity or radiolucency Introduction of radiopaque substances into the upper or lower gastrointestinal tract Visualization of the position, size, and structure of abdominal contents Enhanced visualization of the contours, position, and size of the gastrointestinal tract to detect umbilical hernia, ulcers, diverticula, congenital anomalies, polyps, tumors, strictures, obstructions Visualization or biopsy of inflamed hernias, polyps, ulcers, strictures, varices, tumors, sites of bleeding, mucosal or neoplastic lesions and for culture of Helicobacter pylori from stomach Air or barium contrast roentgenograms Endoscopy Esophagoscopy (esophagus) Gastroscopy (stomach) Duodenoscopy (duodenum) Colonoscopy (large intestine) Sigmoidoscopy (sigmoid colon) Ultrasound Passage of rigid or flexible (fiberoptic) endoscope into the gastrointestinal tract for visualization or biopsy Computed tomography (CT) Use of a computer to integrate differences in absorption of a large number of x-rays to produce a cross-sectional image; may be done with contrast agents Projection of differences in magnetic properties of molecules within different cells and tissues, using the field of a large magnet Magnetic resonance imaging (MRI) Use of piezoelectric crystal to generate sound waves that are reflected from tissue interfaces to provide an image eating stimulates the flow of pancreatic juice, the juice is most alkaline when it needs to be—during digestion. Secretion of the aqueous and enzymatic components of pancreatic juice is controlled by hormonal and vagal stimuli. The pancreatic enzymes hydrolyze proteins (proteases), carbohydrates (amylases), and fats (lipases). The proteases include trypsin, chymotrypsin, carboxypeptidase, and elastase. These enzymes are secreted in their inactive forms—that is, as trypsinogen, chymotrypsinogen, and procarboxypeptidase—to protect the pancreas from the digestive effects of its own enzymes. For further protection the pancreas produces trypsin inhibitor, which prevents the activation of proteolytic enzymes while they are in the pancreas. Once in the duodenum, the inactive forms (proenzymes) are activated by enterokinase, an enzyme secreted by the duodenal mucosa. Trypsinogen is the first proenzyme to be activated. Its conversion to trypsin stimulates the conversion of chymotrypsinogen to chymotrypsin and procarboxypeptidase to carboxypeptidase. Each of these enzymes cleaves specific peptide bonds to reduce polypeptides to smaller peptides. Pancreatic α-amylase is secreted in active form and digests intestinal carbohydrate by cleaving interior α-1,4glucosidic bonds to yield glucose and maltose at an optimum pH of approximately 6.9. Pancreatic lipases hydrolyze triglycerides, cholesterol, and phospholipids to free fatty acids in the intestine. Secretin stimulates the acinar and duct cells to secrete the bicarbonate-rich fluid that neutralizes chyme and prepares it for enzymatic digestion. As chyme enters the duodenum, its acidity (pH of 4.5 or less) stimulates the S cells of the duodenum to release secretin, which is absorbed by the intestine and delivered to the pancreas in the bloodstream. In the pancreas, secretin causes ductal and acinar cells to release alkaline fluid. Secretin also inhibits the actions of gastrin, thereby decreasing gastric hydrochloric acid secretion and Imaging of abdominal organs (gallbladder, liver, pancreas, spleen), masses, stones, abscesses, structural abnormalities Imaging of gallbladder, liver, pancreas, spleen, cysts, hematomas, abscesses, stones, extrahepatic bile ducts, and portal vein Same applications as CT scan; also can detect blood flow and vessel patency motility. The overall effect is to neutralize the contents of the duodenum. Enzymatic secretion follows, stimulated by cholecystokinin, which activates ACh from the vagus nerve and release of ACh from pancreatic stellate cells.39 Cholecystokinin is released in the duodenum in response to the essential amino acids and fatty acids already present in chyme. Once in the small intestine, activated pancreatic enzymes inhibit the release of more cholecystokinin and ACh. This feedback mechanism inhibits the secretion of more pancreatic enzymes. Pancreatic polypeptide is released after eating and inhibits postprandial pancreatic exocrine secretion. (Table 40-1 summarizes hormonal stimulation of pancreatic secretions.) Tests of Digestive Function Gastrointestinal Tract Although important diagnostic information can be obtained from the patient’s medical history and presenting symptoms, numerous disease-specific tests must be performed to evaluate the structure and function of the gastrointestinal tract. A description of selected studies is presented in Tables 40-4 and 40-5. Radiography and imaging techniques—including radionuclide, positron emission tomography (PET), magnetic resonance (MR), computed tomography (CT) scanning, and ultrasound—are procedures for evaluating structure and function. Plain radiographs using contrast media such as barium- or iodine-containing compounds can be used to outline the gastrointestinal lumen, biliary tree and pancreatic ducts, fistulae, and arteriovenous systems. CT scanning is particularly useful for diagnosis of intestinal lesions and pancreatic or hepatic tumors or cysts. Ultrasonic scanning is a safe, simple, and relatively inexpensive technique used to detect gallstones and intraabdominal masses, particularly abscesses. 1416 Unit XII The Digestive System TABLE 40-5 SELECTED TESTS OF GASTROINTESTINAL FUNCTION TEST NORMAL FINDINGS CLINICAL SIGNIFICANCE OF ABNORMAL FINDINGS Stool studies Resident microorganisms: clostridia, enterococci, Pseudomonas, a few yeasts Detection of Salmonella typhi (typhoid fever), Shigella (dysentery), Vibrio cholerae (cholera), Yersinia (enterocolitis), Escherichia coli (gastroenteritis), Staphylococcus aureus (food poisoning), Clostridium botulinum (food poisoning), Clostridium perfringens (food poisoning), Aeromonas (gastroenteritis) Steatorrhea (increased values) can result from intestinal malabsorption or pancreatic insufficiency Large amounts of pus are associated with chronic ulcerative colitis, abscesses, and anorectal fistula Positive tests associated with bleeding Fat: 2-6 g/24 hr Pus: none Occult blood: none (orthotolidine or guaiac test) Ova and parasites: none d-Xylose absorption Gastric acid stimulation Manometry (use of water-filled catheters connected to pressure transducers passed into the esophagus, stomach, colon, or rectum to evaluate contractility) Culture and sensitivity of duodenal contents Breath tests Glucose or d-xylose breath test Urea breath test Lactose breath test 5-hr urinary excretion: 4.5 g/L Peak blood level: >30 mg/dl 11-20 mEq/hr after stimulation Values vary at different levels of the intestine Detection of Entamoeba histolytica (amebiasis), Giardia lamblia (giardiasis), and worms Differentiation of pancreatic steatorrhea (normal d-xylose absorption) from intestinal steatorrhea (impaired d-xylose absorption) Detection of duodenal ulcers, Zollinger-Ellison syndrome (increased values), gastric atrophy, gastric carcinoma (decreased values) Inadequate swallowing, motility, sphincter function No pathogens Detection of Salmonella typhi (typhoid fever) Negative for hydrogen or CO2 Negative for isotopically labeled CO2 Negative for exhaled hydrogen May indicate intestinal bacterial overgrowth Presence of Helicobacter pylori infection Lactose intolerance Fiberoptic endoscopy, using flexible endoscopes, allows direct visualization of the gastrointestinal tract. A biopsy channel allows tissue sampling, and suction can be applied to remove gastrointestinal secretions or blood. Analysis of stool, gastric secretions, tissue, and plasma provides important clues to infection, malabsorption syndromes, ulcerative lesions, and tumor growth. Liver A variety of diagnostic tests can be performed to evaluate liver function40,41 (Table 40-6). Imaging techniques similar to those described for the gastrointestinal tract also are useful for evaluating liver structure and function. Nuclear imaging is useful after liver transplant.42 Elevated plasma levels of liver enzymes are associated with many liver diseases because of the release of cytoplasmic enzymes into the circulation when there is damage to the hepatocyte. Of particular importance are elevations of aminotransferases and lactate dehydrogenase (LDH). Obstruction of bile canaliculi or ducts results in regurgitation of bile back into the hepatic sinusoids and into the circulation, manifesting with elevation of conjugated bilirubin levels. Prothrombin times (a measure of clotting tendency) are often prolonged with both hepatitis and chronic liver disease. In severe disease, other plasma proteins, such as albumin and globulins, may be diminished as a result of hepatocyte damage. Liver biopsies are often performed to evaluate the extent of liver involvement or degeneration with cirrhosis, hepatitis, or fatty liver disease. Gallbladder Evaluation of structural alterations in the gallbladder may be achieved by the use of various imaging techniques. Table 40-7 summarizes these techniques. Obstruction of the bile ducts from stones, tumors, or inflammation prevents the flow of bile from the liver and gallbladder from reaching the gastrointestinal tract. Both the conjugated and total serum bilirubin values are elevated, urine urobilinogen level is increased, stools are clay colored, and jaundice develops. Fat absorption can be impaired and the prothrombin time prolonged if vitamin K is not absorbed. With inflammation of the gallbladder, the white cell count is elevated. Exocrine Pancreas Tests of pancreatic function are summarized in Table 40-8. Evaluation of serum lipase and urinary amylase provides particularly significant measures of pancreatic injury. Inflammation or obstruction of the pancreas results in an early increase in serum amylase levels. Serum lipase level remains elevated after serum amylase has returned to normal levels and provides greater sensitivity with delayed presentation of pancreatitis. Elevation of urine amylase level also occurs later (after 48 hours) when serum amylase may have returned to normal levels. Urinary trypsinogen 2 (available as dipstick) is used to diagnose acute pancreatitis and is comparable to serum amylase and lipase.43 Increased stool fat can reflect pancreatic insufficiency caused by decreased lipase secretion when biliary function is normal. Chapter 40 Structure and Function of the Digestive System 1417 TABLE 40-6 COMMON LIVER FUNCTION TESTS TEST Serum Enzymes Alkaline phosphatase Gamma-glutamyltranspeptidase (GGT) Aspartate aminotransferase (AST; previously serum glutamate-oxaloacetate transaminase [SGOT]) Alanine aminotransferase (ALT; previously serum glutamate-pyruvate transaminase [SGPT]) Lactate dehydrogenase (LDH) 5′-Nucleotidase Bilirubin Metabolism Serum bilirubin Unconjugated (indirect) Conjugated (direct) total Urine bilirubin Urine urobilinogen Serum Proteins Albumin Globulin total Albumin/globulin (A/G) ratio Transferrin Alpha fetoprotein (AFP) Blood-Clotting Functions Prothrombin time (PT) Partial thromboplastin time (PTT) Bromsulphthalein (BSP) excretion NORMAL VALUE INTERPRETATION 13-39 units/L Male 12-38 units/L Female 9-31 units/L 5-40 units/L Increases with biliary obstruction and cholestatic hepatitis Increases with biliary obstruction and cholestatic hepatitis 5-35 units/L Increases with hepatocellular injury (and injury in other tissues, i.e., skeletal and cardiac muscle) Increases with hepatocellular injury and necrosis 90-220 units/L 2-11 units/L Isoenzyme LD5 is elevated with hypoxic and primary liver injury Increases with increase in alkaline phosphatase and cholestatic disorders <0.8 mg/dl 0.2-0.4 mg/dl <1.0 mg/dl 0 0-4 mg/24 hr Increases with hemolysis (lysis of red blood cells) Increases with hepatocellular injury or obstruction Increases with biliary obstruction Increases with biliary obstruction Increases with hemolysis or shunting of portal blood flow 3.5-5.5 g/dl 2.5-3.5 g/dl 6-7 g/dl 1.5:1 to 2.5:1 250-300 mcg/dl 6-20 ng/ml Reduced with hepatocellular injury Increases with hepatitis 11.5-14 sec or 90-100% of control 25-40 sec <6% retention in 45 min Increases with chronic liver disease (cirrhosis) or vitamin K deficiency Ratio reverses with chronic hepatitis or other chronic liver disease Liver damage with decreased values, iron deficiency with increased values Elevated values in primary hepatocellular carcinoma Increases with severe liver disease or heparin therapy Increased retention with hepatocellular injury TABLE 40-7 DIAGNOSTIC EVALUATION OF THE GALLBLADDER TEST APPLICATION Plain roentgenogram of the abdomen Oral cholecystogram (use of an oral contrast medium such as iopanoic acid, which is excreted with bile and concentrated in the gallbladder for visualization by radiography; may be administered as a double dose) Intravenous cholangiography (use of intravenous contrast agents for visualization of gallbladder and bile ducts) Cholecystography (ultrasound imaging of gallbladder and bile ducts) Visualization of calcified gallstones Visualization of gallstones; evaluation of filling and emptying of gallbladder Cholescintigraphy (radioisotope imaging of gallbladder) Endoscopic retrograde cholangiography (instillation of contrast medium through cannulation of ampulla of Vater with a duodenoscope) Computed tomography (CT) Aging and the Gastrointestinal System Age-related changes in gastrointestinal function can begin to occur before 50 years of age.44 Tooth enamel and dentin wear down, making the teeth vulnerable to cavities. Teeth are lost, often as a result of periodontal (gum) disease, recession of Diagnosis of acute gallbladder inflammation (cholecystitis) or disease of bile ducts Preferred method for detecting gallstones; differentiation of hepatic disease from biliary obstruction; diagnosis of chronic cholecystitis Diagnosis of cholecystitis in individuals allergic to iodine-containing contrast agents; diagnosis of cystic duct obstruction Differentiation of intrahepatic or extrahepatic obstructive jaundice Diagnosis of biliary obstruction or malignancy when ultrasound is not successful the gums, osteoporotic bone changes, and more brittle roots that fracture easily. Taste buds decline in number, and the sense of smell diminishes.45 Together these losses decrease the sense of taste. Salivary secretion decreases and contributes to dry mouth and loss of taste.46 In very old adults these oral and sensory changes make eating less pleasurable and reduce appetite. Food may not be chewed or lubricated sufficiently, making 1418 Unit XII The Digestive System TABLE 40-8 SELECTED TESTS OF PANCREATIC FUNCTION TEST NORMAL VALUE CLINICAL SIGNIFICANCE Serum amylase Serum lipase 60-180 Somogyi units/ml 1.5 Somogyi units/ml Urine amylase Secretin test 35-260 Somogyi units/hr Volume 1.8 ml/kg/hr Bicarbonate concentration: >80 mEq/L Bicarbonate output: >10 mEq/L/30 sec 2-5 g/24 hr Elevated levels with pancreatic inflammation Elevated levels with pancreatic inflammation (may be elevated with other conditions; differentiates with amylase isoenzyme study) Elevated levels with pancreatic inflammation Decreased volume with pancreatic disease as secretin stimulates pancreatic secretion Stool fat Measures fatty acids; decreased pancreatic lipase increases stool fat swallowing difficult. The esophagus develops decreased motility, and changes in the upper esophageal sphincter, history of stroke, and dementia may affect swallowing and contribute to gastroesophageal reflux.47 Age also diminishes gastric motility and volume, including secretion of bicarbonate and gastric mucus in some individuals. Acid content of gastric juice is related to gastric atrophy, which results in hypochlorhydria (insufficient hydrochloric acid), delayed gastric emptying, and compromise of the gastric mucosal barrier.48 Decreased production of intrinsic factor leads to inadequate small intestinal absorption of vitamin B12 and pernicious anemia.49 Aging may be associated with a change in the composition of the intestinal microflora and increased susceptibility to disease.50 The ileal villi of the small intestine may become broader and shorter, perhaps because of a decrease in cell turnover. Intestinal absorption, motility, and blood flow decrease, impairing nutrient absorption.51 Proteins, fats, minerals (including iron and calcium), and vitamins are absorbed more slowly and in lesser amounts, and absorption of carbohydrates is decreased. Intestinal transit time is delayed. Constipation is often described as a condition of old age, but it is probably caused by lifestyle factors (e.g., diet, lack of fluid intake) rather than physiologic decline although studies demonstrate there can be alterations in intestinal innervation.52 The rate of liver regeneration decreases with advancing age but the volume of the liver can be maintained.53 Alterations in liver function in older individuals are usually a sign of a pathologic condition. Liver blood flow and enzyme activity decrease with age and can influence the efficiency of drug and alcohol metabolism.54 However, liver function test results often remain within relatively normal ranges. Alterations in liver function in older individuals are usually a sign of a pathologic condition. The pancreas undergoes structural changes, such as fibrosis, fatty acid deposits, and atrophy. Pancreatic secretion decreases, but there is usually no observable dysfunction.55 Aging does not cause apparent changes in the structure and function of the gallbladder and bile ducts, but the incidence of gallstones increases.56 SUMMARY REVIEW The Gastrointestinal Tract 1.The major functions of the gastrointestinal tract are the mechanical and chemical breakdown of food and the absorption of digested nutrients. 2.The gastrointestinal tract is a hollow tube that extends from the mouth to the anus. 3.The walls of the gastrointestinal tract have several layers: mucosa, muscularis mucosa, submucosa, muscularis (circular muscle and longitudinal muscle), and serosa (adventitia in the esophagus). 4.Except for swallowing and defecation, which are controlled voluntarily, the functions of the gastrointestinal tract are controlled by extrinsic autonomic nerves (vagus, parasympathetic splanchnic, and sympathetic nerves) and intrinsic autonomic nerves (enteric nervous system) and intestinal hormones. 5.Digestion begins in the mouth, with chewing and salivation. The digestive component of saliva is α-amylase, which initiates carbohydrate digestion. 6.The esophagus is a muscular tube that transports food from the mouth to the stomach. The muscularis in the upper part of the esophagus is striated muscle, and that in the lower part is smooth muscle. 7.Swallowing is controlled by the swallowing center in the reticular formation of the brain. The two phases of swallowing are the oropharyngeal phase (voluntary swallowing) and the esophageal phase (involuntary swallowing). 8.Food is propelled through the gastrointestinal tract by peristalsis: waves of sequential relaxations and contractions of the muscularis. 9.The lower esophageal sphincter opens to admit swallowed food into the stomach and then closes to prevent regurgitation of food back into the esophagus. 10.The stomach is a baglike structure that secretes digestive juices, mixes and stores food, and propels partially digested food (chyme) into the duodenum. The smooth muscles of the stomach include the outer longitudinal, middle circular, and internal oblique. 11.The vagus nerve stimulates gastric (stomach) secretion and motility. 12.The hormones gastrin and motilin stimulate gastric emptying; the hormones secretin and cholecystokinin delay gastric emptying. 13.Gastric glands in the fundus and body of the stomach secrete intrinsic factor, which is needed for vitamin B12 absorption, Chapter 40 Structure and Function of the Digestive System 1419 S U M M A R Y R E V I E W—cont’d and hydrochloric acid, which dissolves food fibers, kills microorganisms, and activates the enzyme pepsin. 14.Chief cells in the stomach secrete pepsinogen, which is converted to pepsin in the acidic environment created by hydrochloric acid. 15.Acid secretion is stimulated by the vagus nerve, gastrin, and histamine and inhibited by sympathetic stimulation and cholecystokinin. Acetylcholine stimulates pepsin secretion. 16.Mucus is secreted throughout the stomach and protects the stomach wall from acid and digestive enzymes. 17.The three phases of acid secretion by the stomach are the cephalic phase (anticipation and swallowing), the gastric phase (food in the stomach), and the intestinal phase (chyme in the intestine). 18.The small intestine is 5 m long and has three segments: the duodenum, jejunum, and ileum. Digestion and absorption of all major nutrients and most ingested water occur in the small intestine. 19.The peritoneum is a double layer of membranous tissue. The visceral layer covers the abdominal organs, and the parietal layer extends along the abdominal wall. 20.Blood flow to the small intestine is primarily provided by the superior mesenteric artery. 21.The duodenum receives chyme from the stomach through the pyloric valve. The presence of chyme stimulates the liver and gallbladder to deliver bile and the pancreas to deliver digestive enzymes and alkaline secretions. Bile and enzymes flow through an opening guarded by the sphincter of Oddi. 22.Bile is produced by the liver and is necessary for fat digestion and absorption. Bile’s alkalinity helps neutralize chyme, thereby creating a pH that enables the pancreatic enzymes to digest proteins, carbohydrates, and sugars. 23.Enzymes secreted by the small intestine (maltase, sucrase, lactase), pancreatic enzymes (proteases, amylase, and lipase), and bile salts act in the small intestine to digest proteins, carbohydrates, and fats. 24.Digested substances are absorbed across the intestinal wall and then transported to the liver through the hepatic portal vein, where they are metabolized further. 25.The ileocecal valve connects the small and large intestines and prevents reflux into the small intestine. 26.Villi are small fingerlike projections that extend from the small intestinal mucosa and increase its absorptive surface area. 27.Carbohydrates, amino acids, and fats are absorbed primarily by the duodenum and jejunum; bile salts and vitamin B12 are absorbed by the ileum. Vitamin B12 absorption requires the presence of intrinsic factor. 28.Bile salts emulsify and hydrolyze fats and incorporate them into water-soluble micelles that transport them through the unstirred layer to the brush border of the intestinal mucosa. The fat content of the micelles readily diffuses through the epithelium into lacteals (lymphatic ducts) in the villi. From there fats flow into the lymphatics and into the systemic circulation, which delivers them to the liver. 29.Minerals and water-soluble vitamins are absorbed by active and passive transport throughout the small intestine. 30.Peristaltic movements created by longitudinal muscles propel the chyme along the intestinal tract, whereas contractions of the circular muscles (segmentation) mix the chyme and promote digestion. 31.The ileogastric reflex inhibits gastric motility when the ileum is distended. 32. The intestinointestinal reflex inhibits intestinal motility when one intestinal segment is overdistended. 33.The gastroileal reflex increases intestinal motility when gastric motility increases. 34.The large intestine consists of the cecum, appendix, colon (ascending, transverse, descending, and sigmoid), rectum, and anal canal. 35.The teniae coli are three bands of longitudinal muscle that extend the length of the colon. 36.Haustra are pouches of colon that are formed with alternating contraction and relaxation of the circular muscles. 37.The mucosa of the large intestine contains mucus-secreting cells and mucosal folds, but no villi. 38.The large intestine massages the fecal mass and absorbs water and electrolytes. 39.Distention of the ileum with chyme causes the gastrocolic reflex, or the mass propulsion of feces to the rectum. 40. Defecation is stimulated when the rectum is distended with feces. The conically contracted internal anal sphincter relaxes and, if the voluntarily regulated external sphincter relaxes, defecation occurs. 41.The largest numbers of intestinal bacteria are in the colon. They are anaerobes consisting of Bacteroides, clostridia, coliforms, and lactobacilli. 42.The intestinal tract is sterile at birth and becomes totally colonized within 3 to 4 weeks. 43.Endogenous infections of the gastrointestinal tract occur by excessive proliferation of bacteria, perforation of the intestine, or contamination from neighboring structures. Accessory Organs of Digestion 1.The liver is the largest organ in the body. It has digestive, metabolic, hematologic, vascular, and immunologic functions. 2.The liver is divided into the right and left lobes and is supported by the falciform, round, and coronary ligaments. 3.Liver lobules consist of plates of hepatocytes, which are the functional cells of the liver. 4.The hepatic artery supplies blood to the liver. The portal vein receives blood from the inferior and superior mesenteric veins. 5.Hepatocytes synthesize 700 to 1200 ml of bile per day and secrete it into the bile canaliculi, which are small channels between the hepatocytes. The bile canaliculi drain bile into the common bile duct and then into the duodenum through an opening called the major duodenal papilla (sphincter of Oddi). 1420 Unit XII The Digestive System S U M M A R Y R E V I E W—cont’d 6.Sinusoids are capillaries located between the plates of hepatocytes. Blood from the hepatic portal vein and hepatic artery flows through the sinusoids to a central vein in each lobule and then into the hepatic vein and inferior vena cava. 7.Kupffer cells, which are part of the mononuclear phagocyte system, line the sinusoids and destroy microorganisms in sinusoidal blood. 8.The primary bile acids are synthesized from cholesterol by the hepatocytes. The primary acids are then conjugated to form bile salts. The secondary bile acids are the product of bile salt deconjugation by bacteria in the intestinal lumen. 9.Most bile salts and acids are recycled. The absorption of bile salts and acids from the terminal ileum and their return to the liver are known as the enterohepatic circulation of bile. 10.Bilirubin is a pigment liberated by the lysis of aged red blood cells in the liver and spleen. Unconjugated bilirubin is fat soluble and can cross cell membranes. Unconjugated bilirubin is converted to water-soluble, conjugated bilirubin by hepatocytes and is secreted with bile. 11.Fats are synthesized by the liver from protein and carbohydrates and include glycerol, free fatty acids, phospholipids, and cholesterol. Fat absorbed by intestinal lacteals is primarily triglyceride, which is hydrolyzed to glycerol and free fatty acid. 12.Protein synthesis by the liver requires all essential amino acids. The liver synthesizes albumin, globulin, and several serum enzymes and can convert amino acids to carbohydrates by removal of ammonia. 13.Carbohydrates can be released as glucose, stored as glycogen, or converted to fat. 14.The liver performs many metabolic functions including detoxification of exogenous and endogenous chemicals and hormones. 15.The gallbladder is a saclike organ located in the inferior surface of the liver. The gallbladder stores bile between meals and ejects it when chyme enters the duodenum. 16.Stimulated by cholecystokinin, the gallbladder contracts and forces bile through the cystic duct and into the common bile duct. The sphincter of Oddi relaxes, enabling bile to flow through the major duodenal papilla into the duodenum. 17.The pancreas is a gland located behind the stomach. The endocrine pancreas produces hormones (glucagon and insulin) that facilitate the formation and cellular uptake of glucose. The exocrine pancreas secretes an alkaline solution and the enzymes (trypsin, chymotrypsin, carboxypeptidase, α-amylase, lipase) that digest proteins, carbohydrates, and fats. 18.Secretin stimulates pancreatic secretion of alkaline fluid, and cholecystokinin and ACh stimulate secretion of enzymes. Pancreatic secretions originate in acini and ducts of the pancreas and empty into the duodenum through the common bile duct or an accessory duct that opens directly into the duodenum. Tests of Digestive Function 1.Numerous diagnostic tests can evaluate structure and function (digestion, secretion, absorption) of the gastrointestinal tract. Radiographs and scans are most commonly used to evaluate structure, in addition to direct observation by endoscopy. Gastric and stool analysis and blood studies provide important information about digestion, absorption, and secretion. 2.Plasma chemistry levels and imaging procedures are commonly used to diagnose alterations in liver function. Of particular importance are the enzymes LDH, AST, and ALT. Plasma bilirubin levels reflect alterations in bilirubin and bile metabolism, and prothrombin times are prolonged in hepatitis and chronic liver disease. 3.Obstructive diseases of the gallbladder are evident by elevated serum bilirubin levels, elevated urine urobilinogen levels, and increased stool fat. The serum leukocyte levels become elevated with inflammation of the gallbladder. 4.The most significant indicators of pancreatic dysfunction are serum amylase and stool fat. Both values are increased with diseases of the pancreas. Aging and the Gastrointestinal System 1.Advancing age is often associated with the loss or deterioration of teeth, diminished senses of taste and smell, and diminished salivary secretions, all of which may make eating difficult and reduce appetite. 2.Aging reduces gastric motility and secretions, particularly of hydrochloric acid. These changes slow gastric digestion and emptying. 3.Intestinal motility and absorption of carbohydrates, proteins, fats, and minerals decrease with age. 4.Efficiency of drug and alcohol metabolism decreases with age and can be related to decreased liver perfusion and decreased liver enzymes. KEY TERMS Alanine aminotransferase (ALT), 1413 Alkaline phosphatase, 1413 Ampulla of Vater, 1411, 1413 Antrum of stomach, 1396 Ascending colon, 1407 Aspartate aminotransferase (AST), 1413 Bile, 1411 Bile acid–dependent fraction, 1411 Bile acid–independent fraction, 1411 Bile acid pool, 1411 Bile canaliculi, 1411 Bile salt, 1411 Bilirubin, 1411 Biliverdin, 1411 Body of stomach, 1396 Brush border, 1401 Calcium, 1405 Carboxypeptidase, 1402 Cardiac orifice, 1396 Cecum, 1407 Cephalic phase of secretion, 1400 Chief cell, 1398 Cholecystokinin, 1398, 1415 Choleresis, 1411 Choleretic agent, 1411 Cholesterol esterase, 1404 Chylomicron, 1405 Chyme, 1396 Chymotrypsin, 1402 Colipase, 1404 Colon, 1407 Common bile duct, 1411 Conjugated bilirubin, 1411 Chapter 40 Structure and Function of the Digestive System 1421 K E Y T E R M S —cont’d Crypts of Lieberkühn, 1401 Cystic duct, 1413 D cell, 1398 Deamination, 1412 Defecation reflex (rectosphincteric reflex), 1408 Descending colon, 1407 Disse space, 1410 Duodenum, 1400 Emulsification, 1404 Enteric nervous system, 1394 Enterochromaffin-like cell, 1398 Enterohepatic circulation, 1411 Enterokinase, 1415 Esophageal phase of swallowing, 1395 Esophagus, 1395 Exocrine pancreas, 1413 External anal sphincter, 1407 Fat, 1402 Fecal mass, 1408 Feces, 1408 Fundus of stomach, 1396 G cell, 1398 Gallbladder, 1413 Gamma-glutamyltransferase, 1413 Gastric emptying, 1397 Gastric gland, 1398 Gastric hydrochloric acid, 1398 Gastric phase of secretion, 1400 Gastric pit, 1398 Gastrin, 1397 Gastrocolic reflex, 1408 Gastroileal reflex, 1407 Gastrointestinal tract (alimentary canal), 1393 Haustrum (pl., haustra), 1407 Hepatic artery, 1409 Hepatic portal vein, 1409 Hepatic vein, 1409 Hepatocyte, 1409 Hepcidin, 1406 Histamine, 1398 Ileocecal valve (sphincter), 1400, 1407 Ileogastric reflex, 1407 Ileum, 1400 Internal anal sphincter, 1407 Intestinal peristalsis, 1407 Intestinal phase of secretion, 1400 Intestinointestinal reflex, 1407 Intrinsic factor (IF), 1399 Iron, 1405 Jejunum, 1400 Kupffer cell, 1410 Lactate dehydrogenase (LDH), 1413 Lacteal, 1401 Lamina propria, 1401 Large intestine, 1407 Lipase, 1404 Lipocyte, 1409 Lipolysis, 1404 Liver, 1409 Liver lobule, 1409 Lower esophageal sphincter (cardiac sphincter), 1395 Magnesium, 1405 Major duodenal papilla, 1411 Mesentery, 1401 Metabolic detoxification (biotransformation), 1413 Micelle, 1404 Microvillus (pl., microvilli), 1401 Motilin, 1397 Mouth, 1395 Mucosal barrier, 1399 Myenteric plexus (Auerbach plexus), 1394 Oral phase of swallowing, 1395 Pancreas, 1413 Pancreatic α-amylase, 1415 Pancreatic duct (Wirsung duct), 1413 Pancreatic lipase, 1415 Paneth cell, 1401 Parietal cell (oxyntic cell), 1398 Pepsin, 1398, 1399 Pepsinogen, 1398 Peristaltic movement, 1408 Peritoneal cavity, 1401 Peritoneum, 1400 References 1.Furness JB: The enteric nervous system and neurogastroenterology, Nat Rev Gastroenterol Hepatol 9(5):286–294, 2012. 1a.Gröschl M: The physiological role of hormones in saliva, Bioessays 31(8):843–852, 2009. 2.Lang IM, Shaker R: An overview of the upper esophageal sphincter, Curr Gastroenterol Rep 2(3):185–190, 2000. 3.Hershcovici T, Mashimo H, Fass R: The lower esophageal sphincter, Neurogastroenterol Motil 2011 23(9):819–830, 2011. 4.Lang IM: Brain stem control of the phases of swallowing, Dysphagia 24(3):333–348, 2009. 5. Goyal RK, Chaudhury A: Physiology of normal esophageal motility, J Clin Gastroentrol 42(5):610–619, 2008. 6.Hellström PM, Grybäck P, Jacobsson H: The physiology of gastric emptying, Best Pract Res Clin Anaesthesiol 20(3):397–407, 2006. 7.Dockray GJ: Cholecystokinin, Curr Opin Endocrinol Diabetes Obes 19(1):8–12, 2012. 8.Middleton SJ, Balan K: Idiopathic accelerated gastric emptying presenting in adults with post-prandial diarrhea and reactive hypoglycemia: a case series, J Med Case Rep 6(1):132, 2012. 9.Chu S, Schubert ML: Gastric secretion, Curr Opin Gastroenterol 28(6):587–593, 2012. 10.Palileo C, Kaunitz JD: Gastrointestinal defense mechanisms, Curr Opin Gastroenterol 27(6):543–548, 2011. 11.Longkumer T et al: Assessment of vagotomy status with postprandial urinary alkaline tide, Trop Gastroenterol 30(2):91–94, 2009. 12. Ramsay PT, Carr A: Gastric acid and digestive physiology, Surg Clin North Am 91(5):977–982, 2011. Pharyngeal phase of swallowing, 1395 Phases of gastric secretion, 1399 Phosphate, 1405 Phospholipase, 1404 Pit cell, 1410 Primary bile acid, 1411 Primary peristalsis, 1396 Pyloric sphincter, 1396 Rectosigmoid (O’Beirne) sphincter, 1407 Retropulsion, 1397 S cell, 1415 Saliva, 1395 Salivary α-amylase (ptyalin), 1395 Salivary gland, 1395 Secondary bile acid, 1411 Secondary peristalsis, 1396 Secretin, 1397 Segmentation, 1407 Sigmoid colon, 1407 Sinusoid, 1409 Small intestine, 1400 Somatostatin, 1398 Sphincter of Oddi, 1411, 1413 Stellate cell, 1410 Stem cell, 1401 Stomach, 1396 Submucosal plexus (Meissner plexus), 1394 Subserosal plexus, 1394 Swallowing, 1395 Teniae coli, 1407 Transverse colon, 1407 Trypsin, 1402 Trypsin inhibitor, 1415 Unconjugated bilirubin, 1411 Upper esophageal sphincter (cricopharyngeal muscle), 1395 Urobilinogen, 1412 Valsalva maneuver, 1408 Vermiform appendix, 1407 Villus (pl., villi), 1401 Vitamin, 1406 13.Schubert ML: Hormonal regulation of gastric acid secretion, Curr Gastroenterol Rep 10(6):523–527, 2008. 14.Tarnawski AS, Ahluwalia A, Jones MK: The mechanisms of gastric mucosal injury: focus on microvascular endothelium as a key target, Curr Med Chem 19(1):4–15, 2012. 15.Zafra MA, Molina F, Puerto A: The neural/cephalic phase reflexes in the physiology of nutrition, Neurosci Biobehav Rev 30(7):1032–1044, 2006. 16.Miller MJ, McDole JR, Newberry RD: Microanatomy of the intestinal lymphatic system, Ann N Y Acad Sci 1207(Suppl 1):E21–E28, 2010. 17. Santaolalla R, Abreu MT: Innate immunity in the small intestine, Curr Opin Gastroenterol 28(2):124–129, 2012. 18.Ashton KA et al: Basal and meal-stimulated colonic absorption, Dis Colon Rectum 39(8):865–870, 1996. 18a.Wright EM, Loo DD, Hirayama BA: Biology of human sodium glucose transporters, Physiol Rev 91(2):733–794, 2011. 19.Cheeseman C: GLUT7: a new intestinal facilitated hexose transporter, Am J Physiol Endocrinol Metab 295(2):E238–E241, 2008. 20.Leturque A, Brot-Laroche E, Le Gall M: GLUT2 mutations, translocation, and receptor function in diet sugar managing, Am J Physiol Endocrinol Metab 296(5):E985–E992, 2009. 21.Jahan-Mihan A et al: Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract, Nutrients 3(5):574–603, 2011. 22.Christakos S et al: Vitamin D and intestinal calcium absorption, Mol Cell Endocrinol 347(1-2):25–29, 2011. 23.Drakesmith H, Prentice AM: Hepcidin and the iron-infection axis, Science 338(6108):768–772, 2012. 24.Husebye E: The patterns of small bowel motility: physiology and implications in organic disease and functional disorders, Neurogastroenterol Motil 11(3):141–161, 1999. 1422 Unit XII The Digestive System 25.Lubbers T, Buurman W, Luyer M: Controlling postoperative ileus by vagal activation, World J Gastroenterol 16(14):1683–1687, 2010. 26. Byard RW: Acute mesenteric ischaemia and unexpected death, J Forensic Leg Med 19(4):185–190, 2012. 27.Manson JM, Rauch M, Gilmore MS: The commensal microbiology of the gastrointestinal tract, Adv Exp Med Biol 635:15–28, 2008. 28.Abraham C, Medzhitov R: Interactions between the host innate immune system and microbes in inflammatory bowel disease, Gastroenterology 140(6):1729–1737, 2011. 29. Resta SC: Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling, J Physiol 587(Pt 17): 4169–4174, 2009. 30.Yokomori H et al: Recent advances in liver sinusoidal endothelial ultrastructure and fine structure immunocytochemistry, Micron 43(2-3): 129–134, 2012. 31. Yang Q et al: The evolving story of macrophages in acute liver failure, Immunol Lett 147(1-2):1–9, 2012. 32.Tacke F, Weiskirchen R: Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques, Expert Rev Gastroenterol Hepatol 6(1):67–80, 2012. 33.Tian Z et al: Natural killer cells in liver disease, Hepatol 57(4):1654–1662, 2013. 34.Horiguchi S, Kamisawa T: Major duodenal papilla and its normal anatomy, Dig Surg 27(2):90–93, 2010. 35.Hofmann AF: The enterohepatic circulation of bile acids in mammals: form and functions, Front Biosci 14:2584–2598, 2009. 36.Jansen T et al: Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1— evidence for direct and indirect antioxidant actions of bilirubin, J Mol Cell Cardiol 49(2):186–195, 2010. 37.Jirásková A et al: Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer, Int J Cancer 131(7):1549–1555, 2012. 38. Rodriguez-Diaz R et al: Innervation patterns of autonomic axons in the human endocrine pancreas, Cell Metab 14(1):45–54, 2011. 39. Chandra R, Liddle RA: Recent advances in pancreatic endocrine and exocrine secretion, Curr Opin Gastroenterol 27(5):439–443, 2011. 40.Aranda-Michel J, Sherman KE: Tests of the liver: use and misuse, Gastroenterologist 6(1):34–43, 1998. 41.Johnston DE: Special considerations in interpreting liver function tests, Am Fam Physician 59(8):2223–2230, 1999. 42.Hoekstra LT et al: Physiological and biochemical basis of clinical liver function tests: a review, Ann Surg 257(1):27–36, 2013. 43.Mayumi T et al: Validity of the urinary trypsinogen-2 test in the diagnosis of acute pancreatitis, Pancreas 41(6):869–875, 2012. 44.Grassi M et al: Changes, functional disorders, and diseases in the gastrointestinal tract of elderly, Nutr Hosp 26(4):659–668, 2011. 45.Gaines AD: Anosmia and hyposmia, Allergy Asthma Proc 31(3):185–189, 2010. 46.Gupta A, Epstein JB, Sroussi H: Hyposalivation in elderly patients, J Can Dent Assoc 72(9):841–846, 2006. 47.Sura L et al: Dysphagia in the elderly: management and nutritional considerations, Clin Interv Aging 7:287–298, 2012. 48.Salles N: Is stomach spontaneously ageing? Pathophysiology of the ageing stomach, Best Pract Res Clin Gastroenterol 23(6):805–819, 2009. 49.Patel KV: Epidemiology of anemia in older adults, Semin Hematol 45(4):210–217, 2008. 50.Tiihonen K, Ouwehand AC, Rautonen N: Human intestinal microbiota and healthy ageing, Ageing Res Rev 9(2):107–116, 2010. 51.Drozdowski L, Thomson AB: Aging and the intestine, World J Gastroenterol 12(47):7578–7584, 2006. 52. Rayner CK, Horowitz M: Physiology of the ageing gut, Curr Opin Clin Nutr Metab Care 16(1):33–38, 2013. 53.Schmucker DL, Sanchez H: Liver regeneration and aging: a current perspective, Curr Gerontol Geriatr Res 2011:526379, 2011. 54.McLachlan AJ, Pont LG: Drug metabolism in older people—a key consideration in achieving optimal outcomes with medicines, J Gerontol A Biol Sci Med Sci 67(2):175–180, 2012. 55.Herzig KH et al: Fecal pancreatic elastase-1 levels in older individuals without known gastrointestinal diseases or diabetes mellitus, BMC Geriatr 11:4, 2011. 56.Stinton LM, Shaffer EA: Epidemiology of gallbladder disease: cholelithiasis and cancer, Gut Liver 6(2):172–187, 2012.
© Copyright 2026