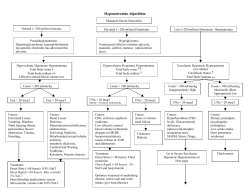
The use of an algorithm to aid diagnosis and treatment... patients with hyponatraemia secondary to SIADH
The use of an algorithm to aid diagnosis and treatment of patients with hyponatraemia secondary to SIADH Prof J G Verbalis, Georgetown University Medical Center, Washington DC, United States Dr E J Hoorn, Erasmus Medical Center, Rotterdam, Netherlands 1. Introduction Hyponatraemia (serum sodium [Na+] < 135 mmol/l) is the most common electrolyte disorder encountered in clinical practice, occurring in up to 30% of hospitalised patients.1 The condition is important clinically because it is often associated with a poor prognosis, increased length of hospital stay and higher rates of resource utilisation.1-6 Even mild so-called “asymptomatic” hyponatraemia can be accompanied by neurocognitive disturbances, gait instability and increased falls, possibly leading to increased bone fractures in elderly individuals.7-9 Among hospitalised patients, euvolaemic hyponatraemia is the most frequent presentation of abnormally low serum [Na+].10 Euvolaemic hyponatraemia is most commonly caused by the syndrome of inappropriate antidiuretic hormone secretion (SIADH),11 which is characterised by inappropriate secretion of, or response to, the pituitary hormone arginine vasopressin (AVP).12,13 SIADH can be caused by a myriad of different disease processes, including tumours, pneumonias and other lung infections, a variety of different central nervous system disorders, the postoperative state, HIV and many different drugs.13 Despite its frequency, hyponatraemia is often poorly managed 14 and optimal treatment strategies have not been well defined. The recent availability of vasopressin receptor antagonists, which facilitate a targeted approach to regulating body water and sodium balance, is stimulating a renewed interest in hyponatraemia and physicians are now reassessing the importance of this condition and the need to treat affected patients. A critical review of the clinical data related to correction of hyponatraemia using various therapeutic modalities has prompted the development of an algorithm for selecting the appropriate therapy for hyponatraemia secondary to SIADH based primarily on the presenting symptoms rather than on the level of serum sodium or the chronicity of the hyponatraemia, which is often difficult to ascertain. 2. History Tolvaptan is an oral selective AVP V2 receptor antagonist that effectively raises the serum [Na+] by increasing electrolyte-free water excretion (aquaresis).15 Tolvaptan was approved in August 2009 by the European Medicines Agency for the treatment of hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH).16 It is the first AVP receptor antagonist to be approved in Europe. 3. Patient selection The standard diagnostic pathway should be followed to confirm a diagnosis of SIADH as described in the table below. Criteria for the diagnosis of SIADH Essential • Decreased measured plasma osmolality (< 275 mOsm/kg H2O) • Urinary osmolality > 100 mOsm/kg H2O during hypo-osmolality • Clinical euvolaemia – No clinical signs of contraction of extracellular fluid (e.g., no orthostasis*, tachycardia, decreased skin turgor or dry mucous membranes) – No clinical signs of expansion of extracellular fluid (e.g., no oedema or ascites) • Urinary sodium > 30 mmol/l with normal dietary sodium intake † • Normal thyroid and adrenal function determined by both clinical and laboratory assessment • No use of diuretic agents within the week prior to evaluation Supporting • Plasma uric acid < 4 mg/dl (< 0.24 mmol/l) • Blood urea nitrogen < 10 mg/dl (< 3.57 mmol/l) • Fractional sodium excretion > 1%; Fractional urea excretion > 55% § • Failure to improve hyponatraemia after 0.9% saline infusion • Improvement of hyponatraemia with fluid restriction * Orthostatic changes in blood pressure and pulse rate are defined as a ≥ 20 mm decrease in systolic BP and/ or a ≥ 20 bpm increase in pulse rate upon going from a supine to a standing position. † § Although high urine sodium excretion generally occurs in patients with SIADH, its presence does not confirm the diagnosis, nor does its absence rule out the diagnosis; urine sodium can also be high in renal causes of solute depletion such as diuretic use or Addison’s disease, and conversely some patients with SIADH can have low urinary sodium if they become hypovolaemic or solute depleted, which are conditions sometimes produced by imposed sodium and water restriction. Fractional sodium excretion = (urinary sodium / plasma sodium) / (urinary creatinine / plasma creatinine) X 100; Fractional urea excretion = (urinary urea / plasma urea) / (urinary creatinine / plasma creatinine) X 100. References: 1. Upadhyay A, et al. Am J Med. 2006;119(7A):S30–S35. 2. Bissram M, et al. Intern Med J. 2007;37: 149–155. 3. Boscoe A, Paramore C, Verbalis JG. Cost Eff Resour Alloc. 2006, 4:10. 4. Gill G, et al. Clin Endo. 2006;64:246–249. 5. Sherlock M, et al. Postgrad Med J. 2009;85;171–175. 6. Wald R, et al. Arch Intern Med. 2010;170:294–302. 7. Renneboog B, et al. Am J Med. 2006;119:71.e1–8. 8. Kengne FG, et al. Q J Med. 2008;101:583–588. 9. Kinsella S, et al. Clin J Am Soc Nephrol. 2010;5:275–280. 10. Chung HM, et al. Arch Int Med 1986;146:33–336. 11. Ellison DH, Berl T. N Eng J Med. 2007;356:2064–2072. 12. Robertson GL, Aycinena P, Zerbe RL. Am J Med 1982;72:339–353. 13. Verbalis JG, et al. Am J Med. 2007;120(11A):S1–S21. 14. Hoorn EJ, Lindemans J, Zietse R. Nephrol Dial Transplant. 2006;21:70–76. 15. Schrier RW, et al. New Eng J Med. 2006;355:2099–2112. 16. Samsca (tolvaptan) Summary of prescribing characteristics. August 2009. 17. Sterns RH, et al. J Am Soc Nephrol 1994;4:1522–1530. Acknowledgements: The authors would like to thank ApotheCom, which is part of Huntsworth Health, for editorial support. Otsuka Pharmaceutical Europe Ltd. has had the opportunity to comment on the medical content and accuracy of this article, however final editorial content resides with the authors. The authors have not received any honorarium from Otsuka Pharmaceutical Europe Ltd. in relation to this poster. Conflicts of interest: Prof Verbalis and Dr Hoorn report having received consulting fees from Otsuka for their involvement in speaker and advisory board activities. 4. Hyponatraemia secondary to SIADH: Treatment algorithm Hyponatraemia secondary to SIADH Severe symptoms Moderate symptoms Mild symptoms or asymptomatic (e.g., seizures, coma, respiratory distress) (e.g., nausea, confusion, disorientation, unsteady gait) (e.g., mild neurocognitive symptoms, depression) Active therapy with hypertonic saline Active therapy should be strongly considered: t)ZQFSUPOJDTBMJOF t5PMWBQUBO t'MVJESFTUSJDUJPO t*GnVJESFTUSJDUJPOGBJMTPSJTOPU tolerated consider tolvaptan t.POJUPSresponse to therapy (symptom level, improvement in serum sodium concentration)† t"TTFTTOFFEGPSDPOUJOVFEUIFSBQZPSBDIBOHFJOUIFSBQZ § * Although moderate symptoms can be treated with either hypertonic saline or tolvaptan, if the hyponatraemia is known to be acute (i.e., < 48 hrs duration) hypertonic saline is preferred because of the possibility of rapid progression to more severe symptoms. Rule out or address correctable factors Assess need for chronic therapy Once diagnosed, treatment of hyponatraemia secondary to SIADH should be based on presenting symptoms • Severe symptoms usually imply a more acute onset or worsening of hyponatraemia and require immediate active treatment to reduce cerebral oedema and decrease the risk of potentially fatal brain herniation. • Moderate symptoms can be either acute or chronic, but allow time to elaborate a more deliberate approach to treatment. • Mild symptoms (or “asymptomatic”) indicate that the patient may have chronic or slowly evolving hyponatraemia. These symptoms necessitate a cautious approach, especially when patients have underlying comorbidities, to avoid osmotic demyelination syndrome (ODS) during therapy. Close monitoring of patients in a hospital setting during active therapy is warranted until symptoms improve or stabilise • All patients should have frequent monitoring (minimum every 8 hours, but more frequently in patients with risk factors for development of ODS) of serum [Na+] and extracellular fluid (ECF) volume status to ensure that the serum [Na+] does not exceed the recommended maximum levels during the active phase of correction.13 † Patients receiving active therapy should have serum [Na+] monitored at least every 6 hours during initial days of therapy. § Correction of hyponatraemia with active therapies should be limited to < 12 mmol/l in 24 hours and to < 18 mmol/l in 48 hours. present, a safe serum [Na+] (usually > 120 mmol/l) has been achieved, or the rate of correction has reached 12 mmol/l within 24 hours (reduced to 8 mmol/l in patients with risk factors for development of ODS, including hypokalaemia, malnutrition, alcoholism and very low serum [Na+]) or 18 mmol/l within 48 hours.13,17 • If overcorrection occurs, active treatment should be discontinued and consideration should be given to administering sufficient water, either orally or as intravenous D5W to bring the overall correction below 8–12 mmol/l, depending on the presence of risk factors for ODS. Dosing guidance for tolvaptan • Initiate tolvaptan therapy in hospital at a dose of 15 mg once daily (without regard to meals).16 • Titrate to 30 mg and 60 mg at 24 hour intervals if the serum [Na+] remains < 135 mmol/l or the increase in serum [Na+] has been ≤ 5 mmol/l in the previous 24 hrs.15,16 • Patients should be advised that they can and should continue ingestion of fluid in response to thirst; therefore, they should not be placed on a fluid restriction during the titration phase of tolvaptan therapy.16 • Active treatment should be stopped when the patient’s symptoms are no longer 5. Practical questions What constitutes being “resistant” to fluid restriction in patients with hyponatraemia? This is up to each physician’s clinical judgment. Our criterion is failure to increase the serum [Na+] by at least 2 mmol/l after 24 hours of a confirmed total fluid restriction of ≤ 1,000 ml per 24 hours. How long does a patient have to be in a hospital setting for initiation of tolvaptan therapy? Long enough to be sure that the rise in serum [Na+] will not exceed the accepted limits for maximal correction. As most of the increase in serum [Na+] usually occurs on day 1 after therapy, monitoring for ~ 24 hours will usually be sufficient, and 48 hours will almost always be sufficient. How long does a patient have to be off tolvaptan to require hospitalisation for “re-initiation” of therapy? Long enough for the patient to be at risk for ODS with overly rapid correction. Existing clinical experience suggests that reasonable criteria would be a sustained level of serum [Na+] < 125 mmol/l for > 48 hours. What are the guidelines for which patients are candidates for long-term tolvaptan therapy? There are none, so it is important to carefully evaluate the underlying aetiology of hyponatraemia in each patient prior to discharge (see section 6). If long-term therapy is felt to be required, the patient should be discharged on tolvaptan, but a trial of withdrawal within 2–4 weeks after discharge should be considered to see if hyponatraemia is still present. A reasonable period of tolvaptan cessation to evaluate the presence of continued SIADH is 7 days. Ideally, serum [Na+] should be monitored every 2–3 days following withdrawal so that the drug can be resumed quickly in those patients with recurrent hyponatraemia. What if a patient does not respond to therapy? Assess the need for a change in therapy or reconsider the underlying cause. If the criteria for SIADH are met, but the patient does not respond to tolvaptan, other causes for SIADH may be present. These include a reset osmostat or the nephrogenic syndrome of inappropriate antidiuresis, which is caused by an activating mutation of the V2 receptor. Consider specific tests for these disorders (water loading test, mutation analysis). 6. Assessing the need for long-term therapy Some patients who have an untreatable cause of SIADH will benefit from continued treatment of hyponatraemia following discharge from the hospital. However, many patients with in-patient hyponatraemia will have a transient form of SIADH without need for long-term therapy. The table below demonstrates the relative probability that patients with different causes of SIADH will have persistent hyponatraemia, which may benefit from long-term treatment with tolvaptan following discharge from the hospital. Probability of need for long-term treatment of SIADH depending on underlying aetiology Aetiology of SIADH Likely duration of SIADH* Tumours producing vasopressin ectopically (small-cell lung carcinoma, head and neck carcinoma) Indefinite Drug-induced, with continuation of offending agent (carbamazepine, SSRI) Subarachnoid haemorrhage Brain tumours Idiopathic (senile) Stroke Respiratory failure (chronic obstructive lung disease) Inflammatory brain lesions HIV infection Traumatic brain injury Nausea, pain, prolonged exercise Post-operative hyponatraemia Drug-induced, with cessation of offending agent Pneumonia Relative risk of chronic SIADH High Duration of drug therapy 1–4 weeks Indefinitely Indefinitely 1–2 weeks Dependent on response to therapy Dependent on response to therapy Dependent on response to therapy 0.5–2 years Variable depending on cause 2–3 days postoperatively Medium Duration of drug therapy 2–5 days Low *Time frames are based on clinical experience. 7. Conclusions The prompt identification and optimal management of abnormally low serum [Na+] is critical if we are to reduce the increased morbidity and mortality that accompany hyponatraemia in hospitalised patients. Application of this algorithm to clinical practice should aid physicians both with making a correct diagnosis of SIADH, and with selecting the most appropriate initial treatment for hyponatraemia secondary to SIADH based on the presence of symptoms known to be associated with hyponatraemia. However, careful monitoring of serum [Na+] responses is required in all cases to adjust therapy appropriately in response to changing clinical conditions. Future studies will enable assessment of the effects of using the algorithm to improve clinical outcomes across a variety of different disease states by virtue of reducing morbidity and mortality associated with hyponatraemia in patients with SIADH.
© Copyright 2026