FOR IMMEDIATE RELEASE ISORAY’S LIQUID CESIUM-131 (CESITREX®) RECIEVES FINAL REGULATORY
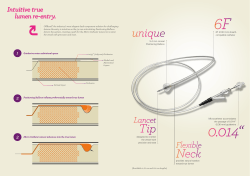
FOR IMMEDIATE RELEASE ISORAY’S LIQUID CESIUM-131 (CESITREX®) RECIEVES FINAL REGULATORY APPROVAL FOR USE IN GLIASITE® BRAIN CANCER TREATMENT SYSTEM Revolutionary Therapy Now Available For Brain Cancers Richland, WA (May 12, 2014) - - IsoRay Inc. (NYSE MKT: ISR), a medical technology company and innovator in seed brachytherapy and medical radioisotope applications, today announced Isoray has now received final approval from state of Washington Department of Health to manufacture its recently FDA cleared Liquid Cesium- 131 (Cesitrex®) for use with GliaSite® balloon catheter for the treatment of metastatic and glioblastoma brain cancers. The GliaSite® balloon catheter can now incorporate the use of Liquid Cesium-131 for the treatment of metastatic and glioblastoma brain cancers and management believes this combination will offer a number of advantages in treating these debilitating brain cancers. Liquid Cesium-131 eliminates the need for a thyroid block as required with Iodine-125, is safer for the patient and staff, and if a spill were to occur, it is an easier cleanup than with other isotopes. With a specified dose of liquid Cesium-131 within the balloon is placed in the tumor cavity most likely to contain cancer cells following surgery. This form of radiation is less likely to damage healthy brain tissue than other available alternatives. The ability for the tumor to recur is greatly diminished by treating the tumor bed immediately at the time of surgery with a single treatment, impacting patient longevity and quality of life. IsoRay CEO Dwight Babcock commented, "We are very excited to have our Liquid Cesium-131 isotope now available for use with our GliaSite® balloon catheter system. With reimbursement codes already available, we will immediately begin offering our newest innovation to major medical centers to establish patient studies, ultimately resulting in these thought leaders reporting their study findings for peer review and publication. As a company, we are witnessing a growing adoption of our products in hospitals and medical practices nationwide. Equally important, we are proud to have reached this new milestone for patients who are searching for treatment options and hope in their fight against brain cancer.” IsoRay’s additional products include Cesium-131 seeds (loose and stranded), sutured seeds and seeds in a stranded mesh. These products together with the GliaSite® balloon catheter system provide physicians with a new powerful weapon in the battle against cancer IsoRay is the exclusive manufacturer of Cesium-131. The pioneering brachytherapy therapy is one of the most significant advances in internal radiation therapy in 20 years. Cesium-131 allows for the precise treatment of many different cancers because of its unrivaled blend of high energy and its 9.7 day halflife (its unequaled speed in giving off therapeutic radiation). In addition to its CMS codes, Cesium-131 is FDA-cleared and holds a CE mark for international sales in seed form for the treatment of brain cancer, prostate cancer, lung cancer, ocular melanoma cancer, colorectal cancer, gynecologic cancer, head and neck cancer and other cancers throughout the body. The treatment can be deployed using several delivery methods including single seed applicators, implantable strands and seed sutured mesh. IsoRay also sells several new implantable devices, including the GliaSite® radiation therapy system. ### About IsoRay IsoRay, Inc., through its subsidiary, IsoRay Medical, Inc. is the sole producer of Cesium-131 brachytherapy seeds, which are expanding brachytherapy options throughout the body. Learn more about this innovative Richland, Washington company and explore the many benefits and uses of GliaSite® and Cesium-131 by visiting www.isoray.com. Join us on Facebook/Isoray. Follow us on Twitter @Isoray. Contact: IsoRay Medical [email protected] (509) 375-1202 Or Worldwide Financial [email protected] (954) 360-9998 Safe Harbor Statement Statements in this news release about IsoRay's future expectations, including: the advantages of our products and their delivery systems, whether IsoRay will be able to continue to expand its base beyond prostate cancer, whether sales of our products will continue at historic levels or increase, whether the use of our products will increase or continue, whether future studies of treatment of various cancers using our products will have favorable results, whether clinical results achieved with treatment using Cesium-131 in the GliaSite® radiation therapy system will be favorable, whether we will continue to partner with other companies, whether awareness of our productsll other statements in this release, other than historical facts, are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 ("PSLRA"). This statement is included for the express purpose of availing IsoRay, Inc. of the protections of the safe harbor provisions of the PSLRA. It is important to note that actual results and ultimate corporate actions could differ materially from those in such forward-looking statements based on such factors as physician acceptance, training and use of our products, our ability to successfully manufacture, market and sell our products, our ability to manufacture our products in sufficient quantities to meet demand within required delivery time periods while meeting our quality control standards, our ability to enforce our intellectual property rights, whether additional studies are released and support the conclusions of past studies, patient results achieved with our products, successful completion of future research and development activities, our ability and the ability of our distributors and customers to receive and maintain all required regulatory approvals in the U.S. and internationally, continued compliance with ISO standards as audited by BSI, the success of our sales and marketing efforts, changes in reimbursement rates, changes in laws and regulations applicable to our products, and other risks detailed from time to time in IsoRay's reports filed with the SEC.
© Copyright 2026