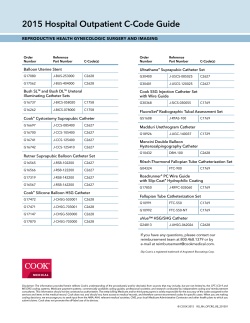
A Step Ahead in SFA Treatment L Why 035
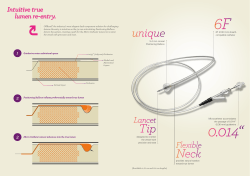
A Step Ahead in SFA Treatment Why Lutonix 035 ® The Lutonix® 035 difference is in our unique, proprietary formulation and coating, designed to maximize efficacy without compromising safety, as demonstrated through rigorously conducted clinical trials. Proven Efficacy Uniform drug delivery from in vivo administration of fluorescent-labeled Lutonix® 035 DCB Catheter, harvested one hour after delivery.1 Proven Formulation and Coating More than 250 formulations, 50,000 balloons, and 45 pre-clinical studies went into the development of the Lutonix® 035 DCB Catheter formulation and coating. The formulation is scientifically engineered to deliver an optimal therapeutic dose at the drug treatment site. · Delivers an optimal therapeutic dose, utilizing a paclitaxel concentration of 2 µg/mm2 · Scientifically designed to achieve high drug retention on the balloon during preparation and handling · Uniform, transfer-efficient coating optimizes drug delivery and uptake1 The Lutonix® 035 DCB Catheter has been studied in LEVANT 2, a global, prospective, randomized, multi-center, rigorous, level 1 clinical trial that was designed to minimize bias and isolate key variables. · Anti-restenotic effects demonstrated in level 1 clinical trial · In LEVANT 2, Lutonix® 035 demonstrated similar risks and ease of use as PTA, with superior primary patency outcomes3 Proven Safety The Lutonix® 035 DCB Catheter’s safety profile is comparable with PTA, as proven in LEVANT 1 and LEVANT 2 clinical trials. In LEVANT 2, at 12 months there was no increased risk compared to PTA observed after treatment with Lutonix® 035 in major events including amputations, thrombosis, embolization, reintervention, death and freedom from loss in patency.3 Its proprietary coating is designed to: · Minimize systemic paclitaxel exposure · Minimize downstream effects · Ensure durability during preparation and handling Bard and Lutonix are trademarks and/or registered trademarks of C. R. Bard, Inc. or an affiliate. Copyright 2014 C. R. Bard, Inc. All rights reserved. Advancing Lives and the Delivery of Health Care™ 75 cm Catheter Length .035" Guidewire Compatible Diameter (mm) 130 cm Catheter Length .035" Guidewire Compatible Length (mm) RBP † (ATM) Sheath Profile (F) 40 12 5F LX3575440 60 12 5F LX3575460 Diameter (mm) Product Codes 4 Length (mm) RBP † (ATM) Sheath Profile (F) 40 12 5F LX35130440 60 12 5F LX35130460 Product Codes 4 80 12 5F LX3575480 80 12 5F LX35130480 100 12 5F LX35754100 100 12 5F LX351304100 40 12 5F LX3575540 40 12 5F LX35130540 60 12 5F LX3575560 60 12 5F LX35130560 5 5 80 12 5F LX3575580 80 12 5F LX35130580 100 12 5F LX35755100 100 12 5F LX351305100 40 12 6F LX3575640 40 12 6F LX35130640 60 12 6F LX3575660 60 12 6F LX35130660 6 6 80 12 6F LX3575680 80 12 6F LX35130680 100 12 6F LX35756100 100 12 6F LX351306100 1 Animal data on file. Bench or pre-clinical data may not be indicative of clinical performance Nominal Pressure* 2 Rosenfield, et. al The LEVANT I (Lutonix® Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis) Trial for Femoropopliteal Revascularization JACC: Cardiovascular Interventions Vol 7, No. 1, 20143 Bench test data on file. Bench results may not be indicative of clinical performance. Different test methods may yield different results. 3 LEVANT 2 clinical trial data on file. N=476. At 12 months, treatment with Lutonix® 035 resulted in a primary patency rate of 73.5% versus 56.8% with PTA alone (p=0.001). Primary patency defined as absence of binary restenosis defined by DUS PSVR ≥ 2.5 and freedom from Target Lesion Revascularization (TLR). At 12 months, treatment with Lutonix® 035 resulted in a freedom from primary safety event rate of 86.7% versus 81.5% with PTA alone. Primary safety defined as composite of freedom from all-cause perioperative death and freedom at 1 year in the index limb from amputation (ATK or BTK), reintervention, and Index-limb related death. Percentages repoted are derived from Kaplan-Meier analyses, not pre-specified. 4, 5 mm 6 ATM 6 mm 7 ATM † RBP (Rated Burst Pressure): the pressure at which Bard has 95% confidence that 99.9% of the balloons will not burst at or below upon single inflation. * Nominal pressure: the pressure at which the balloon reaches its labeled diameter. REPRESENTATIVE’S NAME CONTACT PHONE NO. PHYSICIAN’S SIGNATURE Lutonix® 035 Drug Coated Balloon PTA Catheter Indications for Use: The Lutonix® 035 Drug Coated Balloon PTA catheter is indicated for percutaneous transluminal angioplasty, after pre-dilatation, of de novo or restenotic lesions up to 150 mm in length in native superficial femoral or popliteal arteries with reference vessel diameters of 4-6 mm. Contraindications: The Lutonix® Catheter is contraindicated for use in: 1) Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy. 2) Women who are breastfeeding, pregnant or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and there is a potential for adverse reaction in nursing infants from paclitaxel exposure. 3) Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system. Warnings: 1) Contents supplied STERILE using ethylene oxide (EO) process. Do not use if sterile barrier is damaged or opened prior to intended use. 2) Do not use if product damage is evident. 3) The Lutonix® Catheter is for use in one patient only; do not reuse in another patient, reprocess or resterilize. Risks of reuse in another patient, reprocessing, or resterilization include: – Compromising the structural integrity of the device and/or device failure which, in turn, may result in patient injury, illness or death. – Creating a risk of device contamination and/or patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to patient injury, illness or death. 4) Do not exceed the Rated Burst Pressure (RBP) recommended for this device. Balloon rupture may occur if the RBP rating is exceeded. To prevent over-pressurization, use of a pressure monitoring device is recommended. 5) Use the recommended balloon inflation medium of contrast and sterile saline (≤50% contrast). Never use air or any gaseous medium to inflate the balloon. 6) This product should not be used in patients with known hypersensitivity to paclitaxel or structurally related compounds. 7) The safety and effectiveness of the Lutonix® Catheter have not been established for treatment in cerebral, carotid, coronary, or renal vasculature. 8) The safety and effectiveness of using more than two Lutonix® drug coated balloons (i.e., a maximum drug coating quantity of approximately 7.6 mg paclitaxel) in a patient has not been clinically evaluated. Precautions: General Precautions: 1) The Lutonix® Catheter should only be used by physicians trained in percutaneous interventional procedures. 2) Consideration should be given to the risks and benefits of use in patients with a history of noncontrollable allergies to contrast agents. Potential Adverse Events: Potential adverse events which may be associated with a peripheral balloon dilatation procedure include: Additional intervention • Allergic reaction to drugs, excipients, or contrast medium • Amputation/loss of limb • Aneurysm or pseudoaneurysm • Arrythmias • Embolization • Hematoma • Hemorrhage, including bleeding at the puncture site • Hypotension/hypertension • Inflammation • Occlusion • Pain or tenderness • Pneumothorax or hemothorax • Sepsis/infection • Shock • Stroke • Thrombosis • Vessel dissection, perforation, rupture, or spasm Although systemic effects are not anticipated, refer to the Physicians’ Desk Reference for more information on the potential adverse events observed with paclitaxel. Potential adverse events, not described in the above source, which may be unique to the paclitaxel drug coating include: Allergic/immunologic reaction to the drug coating (paclitaxel) • Alopecia • Anemia • Blood product transfusion • Gastrointestinal symptoms • Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia) • Hepatic enzyme changes • Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis • Myalgia/Arthralgia • Myelosuppression • Peripheral neuropathy Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions. Bard, Advancing Lives and the Delivery of Health Care, and Lutonix are trademarks and/or registered trademarks of C. R. Bard, Inc., or an affiliate. All other trademarks are property of their respective owners. Copyright © 2014, C. R. Bard, Inc. All Rights Reserved. Illustration by Mike Austin. Copyright © 2014. All Rights Reserved. Bard Peripheral Vascular, Inc. 1625 W. 3rd Street Tempe, AZ 85281 USA www.bardpv.com Tel: 1 480 894 9515 / 1 800 321 4254 Fax: 1 480 966 7062 / 1 800 440 5376
© Copyright 2026