Biosimilars Newsletter Volume 6, April 2015
BIOSIMILARS
NEWSLETTER
01 HOT TOPIC
Signal Management in Drug
and Biosimilars Development
Vol ume 6 , Ap r il 2 0 1 5
05 BIOSIMILAR
APPLICATIONS
07 COMPANY NEWS
Pfizer to Acquire Hospira
First Biosimilar Approved
in the US
Welcome
Welcome to the sixth edition of Biosimilars Newsletter, a quarterly publication
dedicated to keeping you updated on current biosimilars news, including the global regulatory landscape, biosimilars articles and reports, and company news as reported by the company press releases.
Highlights at a Glance
•
•
•
•
Hot Topic - Signal Management in Drug and Biosimilars Development
Regulatory - First Biosimilar Approved in the US
Articles of Interest:
- Assessment of Pharmacists' Views on Biosimilar Naming Conventions
- Considerations in the Early Development of Biosimilar Products
- The Challenging Definition of Naïve Patient for Biological Drug Use
Company News - Pfizer to Acquire Hospira
HOT TOPIC
Signal Management in Drug and
Biosimilars Development
by Jacinta Aniagolu-Johnson, Phd, MSc.
Senior Director, Safety and Risk Management
INSID E T H I S I S S UE:
01. Biosimilars Newsletter
Welcome
Hot Topic
04. Regulatory Framework Update
Europe
United States
Rest of World
05. Approved & Under Review
Europe
United States
Rest of World
05. Regulatory Meetings
06. Articles & Reports of Interest
07. Company News
07. Next Edition
S
ignal management in drug development has been ongoing,
evolving, and improving for
many years. For the European Medicines Agency (EMA), Food and Drug
Administration (FDA) and other regulatory bodies immunogenicity is a
major area of safety concern for bio-
similars as well as for peptide, protein,
and drug therapeutics, all of which
have the potential to trigger some level
of antibody response1-7. Hence, there is
the regulatory requirement to conduct
immunogenicity testing and safety
assessment during pre-clinical and
clinical studies, and post-approval;
thereafter, usually requiring continued pharmacovigilance monitoring of
immunogenicity. During pre-clinical
and clinical studies, the critical focus
is to decipher the potential for immunotoxicity and allergenicity, supported by appropriate data collection in
order to evaluate impact of the pres-
01
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
ence of Anti-Drug Antibody (ADA) on
pharmacokinetics, pharmacodynamics, efficacy, and safety. While a target
focus is to ensure comparable immunogenicity, a biosimilar product with
lower immunogenicity than its innovator reference product would not be
excluded for biosimilarity approval1.
As most biosimilars are intended for
long-term use to treat chronic diseases, monitoring of safety concerns beyond clinical development will continue to be equally crucial. Therefore,
even though pre-clinical and clinical
trials are indispensable for determining identified and potential immunogenicity risks, not all such risks
(especially risks that are rare) can be
identified during pre-market phase,
and as such, customized risk management, pharmacovigilance, and post
market commitments such as Post
Authorization Safety Studies (PASS)
will continue to be essential8-10.
Iterative signal management process
is critical to demonstrate the safety of
biosimilars and comparability of their
safety profiles to innovator reference
products, and overall risk management to ensure that their benefits
continue to outweigh their risks. Likewise, post-approval, continued safety
monitoring is essential and required.
The EMA’s Good Pharmacovigilance
Practices guidelines define11-12 signal
management as follows, “includes
the following activities: signal detection, signal validation/confirmation,
signal analysis and prioritization,
signal assessment, and recommendation for action. It therefore is a set
of activities performed to determine
whether, based on an examination of
individual case safety reports (ICSRs),
aggregated data from active surveillance systems or studies, literature information or other data sources, there
are new risks causally associated
with an active substance or a medicinal product, or whether known risks
have changed”. The initial step in the
signal management process is detec-
tion of a signal, and the EMA defines
a signal as “information arising from
one or multiple sources, including
observations and experiments, which
suggests a new potentially causal association, or a new aspect of a known
association between an intervention
and an event or set of related events,
either adverse or beneficial, that is
judged to be of sufficient likelihood to
justify verificatory action12-13”.
An appropriate signal management
process needs to be designed to support both pre- and post-approval
phases of biosimilars development,
and should also support comparability assessment of safety and immunogenicity profile of the index
biosimilars to the innovator reference
product. Preceding this process would
be the development of Standard Operating Procedures (SOPs) and Work
Instructions (WIs), to ensure adequate
training (and documentation of such
training) of medical and safety pharmacovigilance professionals with relevant pre-requisite education and experience, preferably in the treatment
disease indication under investigation. Therefore, the set-up phase of the
signal management process should
encompass customization of all relevant documents including: strategy
document, signal management plan
(includes timelines, blinded/unblinded data handling, communication
and escalation process), templates,
trackers for documentation of medical review; subsequent steps should
include, signal detection, validation,
confirmation, analysis and prioritization, and recommendation for action.
Please note that during the conduct
of a blinded study, unblinding of data
for signal detection is not necessarily
required except in special situations
that warrant such action. However,
any existing unblinded data such as
expedited reports should be reviewed.
Situations warranting unblinding
should be defined in the protocol.
Quality control checks should be part
of the quality management system.
Overall, a signal management process
should define a quality tracking and
management system that supports
and ensures adequate evaluation of
operational quality from “A-to-Z”.
In designing a signal detection and
management strategy for a drug or biosimilar in development, a necessary
first step is thorough review and understanding of the current non-clinical and clinical safety data, including
pharmacokinetics/pharmacodynamics of the innovator reference product,
as well as legacy data of the index biosimilar in development. Then, carefully delineating the “assumptions” based
on documented scientific and medical
evidence on which signal detection
will be performed. This should include
clearly stating clinical trial study protocols and the safety population to be
included in the signal detection and
evaluation data. In addition, signal
detection strategies should factor in
index protocol designs, ensuring that
careful data screening is performed
on all available safety data, especially
during investigational product treatment switch from index biosimilar
to comparator as applicable (ie, reference biologic product) and vice versa.
Furthermore, well-defined and easy
to follow threshold algorithms, both
qualitative and quantitative, should
be specified, and also ensure that all
necessary data output specifications
delineated can support the application of the algorithms and the entire
signal detection process.
A dynamic and clearly outlined signal
management strategy, translated into
a plan, should be well-documented
and should consider all available data
sources and data output, as well as defined assumptions based on pre-clinical and clinical information. Such information includes the current safety
and immunogenicity profile (per index
innovator reference product) highlighting target medical events such
as: adverse events of special interest,
safety and medical review guidelines
including qualitative and quantitative
algorithms to be applied, relevant epidemiology and natural history data,
and other peer-reviewed literature to
be reviewed as required. A signal management plan should also delineate an
appropriate signal validation and confirmation process, in addition to a signal analysis and prioritization process,
and signal assessment method. Such a
plan should also include an approach
to ensure appropriate recommendation for action.
During the pre-approval phase, all signal management activities should be
conducted on a compound level, considering the known safety profile of
the index innovator reference product
per protocol design and indication, as
well as relevant approved products of
the same class. Overall, a signal detection and management process should
be designed to identify and characterize risks during pre- and post-approval phases of development, supporting
safety profiling, and description of
possible safety issues that may arise
with subject/patient exposure to a biosimilar relative to the reference product. Also, a comprehensive and well
designed signal management process
feeds into benefit-risk evaluation
(BRE) and risk management, as such,
should be well-documented, including documentation of qualification
and training of all involved medical
and safety pharmacovigilance professionals. In the European Union (EU),
a Risk Management Plan is mandated to support marketing authorization application, and should include
a safety specification encompassing
identified and potential risk characterization and safety concerns, as
well as pharmacovigilance planning,
and planning and implementation
of routine and additional (as needed)
risk minimization measures and assessment of the effectiveness of such
measures9,10.
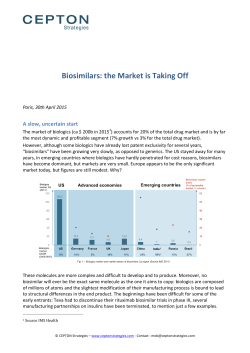
02
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
SIG NAL
M ANAG EM E N T
BE N E F I T RISK
AS S E S S M ENT
RISK
MA NAGEMENT
Iterative Signal Detection and Management Activities,
Signal-to-Risk Translation and
Benefit Risk Assessment
Evaluation for any need
for Risk Minimization(if any risk is identified)
SAFETY PROFILING:
Biosimilars vs. Reference
Innovator Product
Index Drug (relative to
drugs of the same class)
Benefit Risk Assessment
PH A S E I T O I I I C L I N IC A L T R I A L S (B LI NDE D/OPE N LA B E L)
DI S EA S E I N DIC AT ION S U N DE R I NV E S TIGATION
QUA L I T Y C ON T ROL / M A NAGE M E NT S YS TE M S
Integrated Process of Signal
Management, Benefit Risk
Evaluation, and Risk
Management
Overall, the signal management process should be governed by SOPs
which should include, signal detection, validation (and confirmation),
analysis and prioritization, assessment, and effective communication of
significant findings especially validated signals. In addition, there should
be appropriate documentation, and
tracking of activities and findings. Signal detection and management strategies translated into a plan should
be comprehensive in nature, in both
planned qualitative and quantitative
approaches, as well as tailored to specific biosimilar relative to its innovator
reference product and target indication, and taking into account regulatory guidelines and requirements. As
the focus on biosimilar development
continues to grow, so does the critical
need for a comprehensive, efficient
and well defined signal management
process, continued benefit risk evaluation, and an overall risk management
process.
References:
1. Hincal F. An introduction to safety issues in biosimilars/follow-on biopharmaceuticals. J Med CBR Def. 2009;7.
http://jmedcbr.org/issue_0701/Hincal/Hincal_09_09.pdf
2. EMA Guideline on Immunogenicity Assessment of Biotechnology-derived therapeutic proteins. [London, 13 December 2007
Doc. Ref. EMEA/CHMP/BMWP/14327/2006; Effective April 2008].
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003946.pdf
3. EMA Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. Committee for Medicinal Products for Human Use (CHMP), 24 May 2012 EMA/CHMP/BMWP/86289/2010. Effective December 1, 2012].
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128688.pdf
4. Tobias Blank, et al. 2013. Safety and toxicity of biosimilars—EU versus US regulation Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(3):144-50. [DOI: 10.5639/gabij.2013.0203.039].
http://gabi-journal.net/safety-and-toxicity-of-biosimilars-eu-versus-us-regulation.html
5. FDA Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products, August 2014.
http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf
6. FDA Guidance for Industry Immunotoxicology Evaluation of Investigational New Drugs, October 2002.
http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079239.pdf
7. Vera Brinks, 2013. Immunogenicity of biosimilar monoclonal antibodies Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(4):188-93.
http://gabi-journal.net/immunogenicity-of-biosimilar-monoclonal-antibodies.html.[DOI: 10.5639/gabij.2013.0204.052]
8. Guideline on good pharmacovigilance practices (GVP) Module VIII – Post-authorisation safety studies (Rev 1) (19 April 2013
EMA/813938/2011 Rev 1)
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129137.pdf
9. EMA Guideline on good pharmacovigilance practices (GVP) Module V – Risk management systems (Rev 1). 15 April 2014
EMA/838713/2011 (Rev 1).
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129134.pdf
10. EMA Guideline on good pharmacovigilance practices (GVP) Module XVI– Risk minimisation measures: selection of tools and
effectiveness indicators (Rev 1).
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162051.pdf
11. EMA Guideline on good pharmacovigilance practices (GVP) Annex I - Definitions (Rev 3) 15 April 2014 EMA/876333/2011 (Rev 3).
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143294.pdf
12. EMA Guideline on good pharmacovigilance practices (GVP) Module IX – Signal management 22 June 2012 EMA/827661/2011
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129138.pdf
13. Practical Aspects of Signal Detection in Pharmacovigilance: Report of CIOMS Working Group VIII (2010)
03
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
Regulatory Framework Updates
Europe
EMA Issues Finalized
Insulin Biosimilars
Guideline
T
he EMA has released its finalized guideline on the non-clinical and clinical development of insulin biosimilars.
The new guideline replaces the "Guidance on similar medicinal products
containing recombinant human
soluble insulin" (EMEA/CHMP/
BMWP/32775/2005), which came
into effect in June 2006. The new
guideline lays down the non-clinical
and clinical requirements for recombinant insulin-containing biosimilars,
including human insulin and insulin
analogues (both referred to as insulin).
Guideline on non-clinical and clinical development of similar biological medicinal products containing
recombinant human insulin and
insulin analogues EMEA/CHMP/
BMWP/32775/2005_Rev. 1.
United States
FDA Announces List of
Guidance Documents
for 2015
T
he US FDA's Center for Drug
Evaluation and Research (CDER)
is planning to release 4 new guidance
documents on biosimilars during calThe non-clinical section addresses endar year 2015.
the requirements of in vitro pharmacodynamic studies and cases when The guidance documents planned are
there is a need for additional in vivo as follows:
toxicological assessment. The clinical
•
Biosimilars: additional quessection addresses the requirements for
tions and answers regarding
pharmacokinetic, pharmacodynamic,
implementation of the Bioand safety studies, as well as the risk
logics Price Competition and
management plan.
Innovation Act of 2009.
•
Considerations in demonIn a change from the previously restrating interchangeability to
leased draft version, EMA has dropped
a reference product.
its requirement for "manufacturers
•
Labeling for biosimilar biowho are planning comparative clamp
logical products.
studies to consider reports that in•
Statistical approaches to evaldividuals of African, South Asian, or
uation of analytical similarity
Hispanic descent have reduced gludata to support a demonstracose clearance".
tion of biosimilarity.
Date: 26 February 2015
Effective date: 1 September 2015
Link to guideline:
www.ema.europa.eu
guidances is seen as a positive devel- Mexico Issues Rules
opment for industry groups who have On Biolimbos
been calling for FDA to promptly issue
appropriate guidance on the issue of
he Mexican regulatory body for
naming, as well as to issue, or finalize
approval of medicines, the Fedguidances on other outstanding issues
such as establishing interchangeability. eral Commission for the Protection
against Sanitary Risks (COFEPRIS),
Link:
has issued rules for older non-orighttp://www.fda.gov/downloads/Drugs/ inator biologicals registered prior
GuidanceComplianceRegulatoryInforma- to 19 October 2011, when the countion/Guidances/UCM417290.pdf
try’s guidelines for biocomparables
were first published, mandating that
companies conduct clinical trials to
prove biosimilarity. These products,
known as "biolimbos", have not undergone any marketing authorization review consistent with globally
Australia Reviewing Plans accepted standards for the approval
of biosimilars.
T
Rest of World
For Naming Biosimilars
The companies affected have until 31
ollowing recent international December 2015 to present their tests
developments in the area of bi- to the agency.
osimilar naming, the Therapeutic
Goods Administration (TGA) will not
be continuing with the previously
proposed naming convention for biosimilars while a review of the policy is
undertaken.
F
Link to TGA site, 20 Jan 2015:
The news of additional biosimilars www.tga.gov.au
04
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
Biosimilar Applications Approved & Under Review
Europe
alence trial in patients with moder- Hospira Submits
ate-to-severe RA. In Europe, Remi- Application to US FDA
cade is indicated for the treatment of
RA, adult Crohn’s disease, pediatric for Proposed Epoetin Alfa
Crohn’s disease, ulcerative colitis, Biosimilar
pediatric ulcerative colitis, psoriatic
ospira has submitted a Biologics
arthritis, ankylosing spondylitis, and
License Application (BLA) to
psoriasis.
If
authorized
by
the
EMA,
amsung Bioepis’s Marketing Authe US FDA for Retacrit™, a proposed
SB2
could
be
available
for
use
in
all
thorization Application (MAA)
biosimilar to Amgen's EPOGEN®
for its Enbrel (etanercept) biosimilar of the same indications as Remicade. (epoetin alfa) and Janssen's PROCRIT®
candidate, SB4 has been validated
Company press release 13 Mar 2015: (epoetin alfa).
and accepted for review by the EMA.
www.samsungbioepis.com
The acceptance of the MAA marks
The biosimilar application was subthe first Enbrel biosimilar to advance
mitted on 16 December 2014, under the
new 351(k) approval pathway created
into regulatory review in the Europe
by the Biologics Price Competition and
EU. The MAA is based on results from
Innovation Act of 2009 (BPCIA).
United
States
a phase III clinical trial in patients
with moderate-to-severe rheumatoid
Company Press release 12 Jan 2015:
FDA Approves First
arthritis (RA).
phx.corporate-ir.net
EMA Accepts
Samsung Bioepis’
Enbrel® Biosimilar
Candidate, SB4, for
Regulatory Review
H
S
Biosimilar Product
Apotex Announces FDA
Has Accepted For Filing its
Biosimilar Application for
Filgrastim (Grastofil™)
A
potex Inc., announced that on
the 13 February 2015, the US
FDA accepted for filing the company's
application for Filgrastim [Grastofil™], a biosimilar version of Amgen's
Neupogen®. This product has been
jointly developed with Intas Pharmaceuticals Ltd.
This is the second follow-on biologic
FDA submission for Apotex via the
351(k) abbreviated approval pathway
created by the Biosimilar Price Competition and Innovation Act (BPCIA).
Apotex also has a 351k biosimilar application for the long acting pegylated
formulation of filgrastim currently
under FDA review.
In addition to the European filings, Zarxio
Samsung Bioepis intends to move
US FDA Postpones
forward with additional applications
he US FDA have approved Zarxfor regulatory approvals in other terio (filgrastim-sndz), the first bio- Celltrion’s Remicade
similar
product approved in the US. Biosimilar Review
ritories worldwide.
Company press release 17 Feb 2015:
Zarxio has been approved as biosimi- Meeting
www.apotex.com
lar,
not
as
an
interchangeable
product.
Company press release 21 Jan 2015:
www.samsungbioepis.com
he FDA has postponed the meetSandoz, Inc.’s Zarxio is biosimilar to
ing of the Arthritis Advisory
Amgen Inc.’s Neupogen (filgrastim),
Committee scheduled for 17 March Rest of World
which was originally licensed in 1991
Samsung Bioepis
and is approved for the same indica- 2015. The postponement is due to in- None reported.
formation requests pending with the
Submits Marketing
tions as Neupogen.
sponsor of the application. A future
Authorization
For this approval, the FDA designated meeting date is still to be announced
ApplicationFor SB2, A
a placeholder non-proprietary name in the Federal Register.
Remicade (Infliximab)
for this product as “filgrastim-sndz.”
Biosimilar Candidate,
The FDA have stated that the provi- The US delay is unlikely to spell a
To The EMA
sion of a placeholder non-proprietary
major disruption to Celltrion’s global
name for this product should not be
plans, as Remicade, which brings in
viewed
as
reflective
of
the
agency’s
amsung Bioepis have announced
that the MAA for SB2, its Remi- decision on a comprehensive naming roughly $8.4 billion around the world
cade (Infliximab) biosimilar candi- policy for biosimilar and other bio- each year, isn't scheduled to lose US
date, has been submitted to the EMA. logical products. While the FDA has exclusivity until 2018. Celltrion is doThis is the second MAA biosimilar not yet issued draft guidance on how ing its best to move that date forward,
current and future biological products challenging some of Johnson’s and
that Samsung Bioepis has submitted
marketed in the US should be named,
Johnson’s (J&J), the originator compato the EMA.
the agency intends to do so in the
ny, patents in court but J&J is battling
near future.
The MAA is based on results from
back with legal claims of its own in an Europe/US/
an extensive head-to-head pre-clin- FDA news release 06 Mar 2015:
Rest of World
ongoing dispute.
ical data package comparing SB2 to http://www.fda.gov/NewsEvents/
None reported.
the originator, a head-to-head phase Newsroom/PressAnnouncements/ FDA press release 25 Feb 2015:
http:/
/www.fda.gov/advisorycomI study in healthy volunteers, and a ucm436648.htm
mittees/calendar/ucm433919.htm
robust head-to-head phase III equiv-
T
T
S
Regulatory
Meetings
05
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
Articles & Reports of Interest
UK Outlines Process For
Developing Biosimilars
Guidances
I
n response to the increasing availability and use of biosimilars by the
UK’s National Health Service (NHS),
the country’s healthcare watchdog,
the National Institute for Health and
Care Excellence (NICE), has updated
its methods for providing guidance
and advice on biosimilars.
NICE is an independent organization, set up by the UK Government
in 1999. The agency assesses the
clinical and cost-effectiveness of
drugs, and decides which drugs and
treatments are available on the NHS
in England and Wales.
substituting interchangeable biologics, the Academy of Managed Care
Pharmacy, the American Pharmacists Association, and the American
Society of Health-System Pharmacists fielded a survey to their membership, or a partial segment of their
membership. The survey consisted
of 2 sections: (1) current processes for
reporting biologics being dispensed,
and (2) familiarity and preferences
regarding biosimilars.
The Challenging Definition
of Naïve Patient For
Biological Drug Use
The results of this survey indicate that
the ultimate decision on the naming convention for biosimilars may
influence dispensing pharmacists,
with the majority of respondents being most comfortable with biosimilars having the same nonproprietary
name as the reference biologic.
B
Link to full free article:
Positive appraisals of biosimilars will
use the name of the active drug sub- www.amcp.org
stance, including the reference product and brand name, to inform clinical decisions.
Considerations In The
The decision by NICE to use the name
of the active drug substance aligns
with the majority of European Union
Member States, which have agreed
that biosimilars should have the same
International Nonproprietary Name
(INN) as their reference biological.
Link to article, 06 Jan 2015:
www.nice.org.uk
Assessment of Pharmacists'
Views On Biosimilar
Naming Conventions
Fernandez-Lopez. S. et al:
Journal of Managed Care
& Specialty Pharmacy,
March 2015, Vol. 21, No. 3
A
s the date for the introduction of
biosimilars in the US approaches, questions remain regarding the
naming, coding, and approval process
for these agents that will need to be
carefully considered.
Early Development of
Biosimilar Products
Abbas. R. et al: Drug
Discovery Today, In Press,
Uncorrected Proof, Available online 5 January 2015
T
he widespread use and patent
expiration of many biologics
have led to global interest in development of biosimilar products. Because the manufacture of biologics,
including biosimilars, is a complex
process involving living systems, the
development of a biosimilar is more
rigorous than the development of a
generic small molecule drug. Several
regulatory agencies have established,
or are proposing guidelines, that recommend a stepwise process to ensure
the efficacy and safety of a biosimilar are highly similar to the reference
product. This article also explores the
early clinical phase of biosimilar development, which is particularly important to resolving any uncertainties
that might remain following in vitro
and in vivo evaluations and to enable
a selective and targeted approach to
phase III clinical efficacy and safety
investigation.
To (a) ascertain pharmacists' awareness of and comfort level with biosimilars and (b) determine the impact of
identical or different nonproprietary Link to purchase site for article:
names on pharmacists' confidence in www.ncbi.nlm.nih.gov
Biggioggero. M. et al : Autoimmun Rev. 2015 Jan 31.
pii: S1568-9972(15)000312. doi: 10.1016/j.autrev.2015.01.016. [Epub
ahead of print]
A
district court judge from California has denied an injunction
by Amgen meant to stop the launch
of the first biosimilar approved by
the US FDA - Sandoz’s Zarxio.
"As the twelve-year exclusivity period
for Neupogen long ago expired, there
exists no substantive bar to market
entry for Sandoz’s biosimilar filgrastim and, consequently, no basis on
which Amgen is entitled to injunctive
relief or other remedies for disadiosimilar is defined by the EMA vantages it may suffer due to market
as a biological medicinal prod- competition from Sandoz."
uct, which is similar, but not identical
to the biological drug already autho- Amgen originally filed suit when
rized. The biosimilar and its refer- Sandoz failed to provide them with
ence product are expected to display certain information from its biosimthe same safety and efficacy profile, ilars application, including on the
and are generally used to treat the manufacturing processes. However, the judge ruled that in some insame conditions. The Italian Medistances this information does not
cines Agency considers biosimilars
have to be provided. The judge also
as a valid therapeutic option with
dismissed claims of harm by Amgen
an economic advantage, especial- that would come if Zarxio were to be
ly in primary naïve patients with no launched. Questions however, still
previous exposure to the originator, remain around the pricing of Zarxio,
or with a sufficiently long wash-out details of which were not included in
period ("secondary naïve"). The iden- the latest order.
tification of "secondary naïve" is not
well defined and can be subjected to It appears that Amgen may decide to
different variables, mainly the drug appeal. The time frame of Amgen's
biologic effect and its immunogenic- appeal seems unclear, and Novarity. The first one depends on the type tis has not specified whether it will
of biologics and on their mechanism launch Zarxio prior to completion of
of action. The second one is related the appeals process.
to the fact that biologicals may be
immunogenic and can trigger an an- Link to court order 19 Mar 2015:
www.fdalawblog.net
ti-drug antibody response (ADA).
In conclusion, the development and
use of biosimilars represent a tool for
increasing health system sustainability. However, it is of paramount importance to distinguish between the
pharmacodynamics of a given drug
and its immunogenicity being the 2
aspects unrelated. Thus a detailed
definition of "secondary naïve" patients is challenging, and may be related to both the 2 parameters.
Link to purchase site for article:
www.ncbi.nlm.nih.gov
US District Court Dismisses
Amgen's Petition To
Block Launch of Neupogen
Biosimilar Zarxio
06
B I OS I M I L A RS N E W S L E TTE R |
Vol u me 6 , Ap r i l 20 1 5
Company News
(The information provided is sourced directly from the company websites)
Pfizer To Acquire Hospira
P
fizer Inc. and Hospira, Inc. have
announced that they have entered into a definitive merger agreement under which Pfizer will acquire
Hospira for a total enterprise value of
approximately $17 billion. This strategically and complementary combination will add a growing revenue
stream, and a platform for growth for
Pfizer’s Global Established Pharmaceutical (GEP) business.
The transaction is subject to customary closing conditions, including regulatory approvals in several
jurisdictions and approval of Hospira’s shareholders, and is expected to
close in the second half of 2015.
Company press release 05 Feb 2015:
www.pfizer.com
Hospira Launches First
Biosimilar Monoclonal
Antibody (mAb) Inflectra
(infliximab) In Major European Markets
H
ospira announced the launch
of the first biosimilar mAb, Inflectra (infliximab), in major European markets. Inflectra is licensed
for the treatment of inflammatory
conditions including RA, psoriatic
arthritis, ankylosing spondylitis,
adult and pediatric Crohn's disease,
adult and pediatric ulcerative colitis, and plaque psoriasis.
Inflectra received its license from
the EC in September 2013, following
adoption of the EMA Committee for
Medicinal Products for Human Use
(CHMP) positive recommendation
for granting marketing authorization.
Review by the EMA included detailed analysis of biophysical proper-
ties and safety, efficacy, and tolerabili- data, as it is obtained by the company.
ty data from an extensive pre-clinical
and clinical trial program.
Amgen Announces PosiCompany press release 16 Feb 2015:
phx.corporate-ir.net
Oncobiologics ONS-3010
Meets Primary Endpoints
O
ncobiologics, Inc have announced that ONS-3010, its
adalimumab (Humira®) biosimilar
candidate met the primary endpoints in its first clinical study.
tive Results From Phase III
Study Of Biosimilar Candidate ABP 501
Proceeds from the financing will be
used to advance Innovent’s pipeline,
consisting of 8 antibody products,
mgen have announced a phase which include 1 approved IND and 3
III study evaluating the effica- additional filed applications, and the
cy and safety of biosimilar candidate company’s operations.
ABP 501 compared with Humira®
(adalimumab) in patients with mod- Company press release 22 Jan 2015:
erate-to-severe RA, met its primary www.innoventbio.com
and key secondary endpoints. The
primary endpoint compared the
ACR20 measurements (20 percent or
greater improvement in ACR assessment) at week 24. The ACR20 was
within the pre-specified margin for Look out for the next edition
ABP 501 compared to adalimumab, of the Biosimilars Newsletter
showing clinical equivalence. Safe- due out in July 2015.
ty and immunogenicity of ABP 501
were comparable to adalimumab.
Key secondary endpoints included
ACR50, ACR70, and DAS 28-CRP.
A
A 3-arm single-dose pharmacokinetic
(PK) study was performed in healthy
volunteers to compare ONS-3010 to
both the US- and EU-sourced Humira® reference products, and the 2 reference products to each other. All of
the PK endpoints met the bioequivalency and safety and immunogenicity
were similar across the 3 arms.
ABP 501 is being developed as a biosimilar candidate to adalimumab, an
Company press release 12 Feb 2015: anti-TNF-α monoclonal antibody,
oncobiologics.com
which is approved in many countries
for the treatment of a number of inflammatory diseases.
Mabion Submits
Registration Dossier in
Argentina
Amgen has 9 biosimilar molecules in
development and expects to launch
5 of these biosimilars between 2017
abion SA is one step closer and 2019.
to releasing its MabionCD20
drug in Argentina. Together with the Company Press release 03 Feb 2015:
intermediary company LKM SA, a www.ext.amgen.com
M
petition was submitted in Argentina
for approval to start the registration
procedure of MabionCD20, a drug
used in the treatment of blood cancers and RA.
complex, biologics to be marketed in
the rapidly growing Chinese market
and elsewhere worldwide, has raised
$100 million in Series C financing.
Next Edition
Previous
Editions
Please use the below link to
find previous editions of PRA
Health Sciences’ Biosimilars
Newsletters.
http://prahs.com/therapeutic-expertise/biosimilars/
Contact
Rodeina Challand,
Executive Director, Biosimilars
Innovent Biologics, Inc.
Development, Scientific Affairs
Completes Financing Funds [email protected]
to Advance Novel Biologic
Pipeline
Hazel Gorham,
The data currently available, is only
Director, Biosimilars
nnovent Biologics, Inc., a Chinese
sufficient to initiate the registration
biopharmaceutical company in the Development, Scientific Affairs
procedure. The documentation will
be regularly updated with additional development and manufacturing of [email protected]
I
07
© Copyright 2026