URGENT: PRODUCT CORRECTION
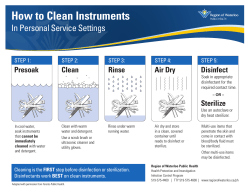
November 13, 2007 URGENT: PRODUCT CORRECTION Dear STERRAD® Sterilization System Customer: Advanced Sterilization Products (ASP) would like to inform you that all brand- and model-specific compatibility lists and associated instrument assessments provided to STERRAD® Sterilization System customers are now out-of-date. Information related to specific brands and models of medical devices, over time, can become outdated as manufacturers introduce new technologies and make materials, manufacturing and repair process changes. After such modifications, devices listed as compatible may nevertheless become functionally impaired after sterilization using the STERRAD System. This could lead to potential patient injury if the integrity of the device that was sterilized is compromised. As a result, refer to the Medical Device Manufacturers (MDM) for the most up-to-date information on whether their devices will remain functional after being sterilized in a STERRAD System. While we are evaluating improved means of updating the compatibility information we provide to you, we are also advising all STERRAD System customers as follows: 1. 2. 3. 4. If you have received any of the following STERRAD Sterilizers Compatible Medical Device Reference Lists that indicate specific brands and/or models of medical devices are compatible with STERRAD Systems, please destroy your copies: · STERRAD® Sterilizers (100S/50/200) Compatible Medical Device Reference List for US Customers (Document Number: 15-53229) · STERRAD® NX™ Sterilizers Compatible Medical Device Reference List for NX Customers (Document Number: 15-53231) · STERRAD® NX™ System Brochure (AD-53421) Please discontinue using and destroy any ASP instrument assessment documentation that describes the compatibility of specific devices. For general sterilization questions, refer to the User’s Guide for your STERRAD System model(s) for information about the characteristics of medical devices and materials that may be sterilized in your STERRAD System. To determine if a specific brand and model of device will remain functional following sterilization in your STERRAD System, please contact the MDM or refer to the Instructions For Use (IFU) for the device. Even if the MDM’s IFU references the STERRAD System, we recommend that you contact the MDM to ensure the IFU is upto-date. Please be assured that devices falling within the guidelines for lumen dimensions and compatible materials provided in the User’s Guide for your STERRAD System will be sterile if processed in accordance with the instructions in that User’s Guide. For additional information, you can also refer to the brochure titled: “What Can I Sterilize in the STERRAD (100S, 50, 200, NX) Sterilizer?” attached to this letter and located at www.sterrad.com. We understand this new information may pose challenges for you in your daily sterilization practices. We are committed to working with you to minimize any disruption these developments may have on your work and ensure we merit the trust you put in us and our products every day. Our ASP Customer Care Center is available to answer additional questions you may have at (888) 225-9961 or you can e-mail us at [email protected]. Thank you for your valued partnership as we work together to safely and effectively prevent infection. Sincerely, Mizanu Kebede Vice President, Worldwide Quality Assurance, Regulatory Affairs & Process Excellence Advanced Sterilization Products CO-1000590 CL-100363 Rev. A (11/07) WARNING: This is a controlled proprietary and confidential document. Verify revision is current prior to use.
© Copyright 2026