5.563"
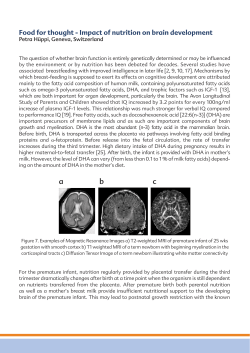
5.563" Vayacog® DESCRIPTION Vayacog® is an orally administered prescription medical food for the clinical dietary management of certain lipid imbalances associated with early memory impairment. Each capsule contains Lipicogen™ 310 mg, providing: Phosphatidylserine (PS) ............................................. 100 mg Docosahexaenoic acid (DHA) .................................... 19.5 mg Eicosapentaenoic acid (EPA) ...................................... 6.5 mg CHEMICAL STRUCTURE* O O O O O O P O O- CO2+NH3 CLINICAL EXPERIENCE Vayacog® was evaluated in a randomized, double-blind, placebo-controlled study of 15 weeks followed by an open label extension of an additional 15 weeks. In the double-blind phase, 157 men and women (aged 50-90 years) with memory complaints were randomly assigned to receive Vayacog® or placebo (three capsules a day)[13]. During the open-label extension, all participants consumed one capsule a day of Vayacog®. Cognitive performance was evaluated using Rey Auditory Verbal Learning Test (Rey-AVLT) at baseline and endpoint (week 15) of the double-blind phase and using a computerized cognitive assessment tool at baseline (week 15) and endpoint (week 30) of the open-label phase. Safety assessment was evaluated by clinical laboratory assessments including biochemical and hematological parameters at baseline and endpoint (week 15) of the double-blind phase and by adverse events recording, physical examination and measurement of vital signs and weight at baseline, week 7, and endpoint (week 15) of the double-blind phase and at the end of the open-label extension (week 30). * Schematic structure of one of the most abundant molecule present in Vayacog®. 2.0 Mean change from baseline (# words) INGREDIENTS Phosphatidylserine, Hypromellose, Maltodextrin, Silicon Dioxide, Rosemary Extract (preservative), Mixed Tocopherols (E306-E309), Ascorbyl Palmitate (E304), Titanium Dioxide (color). Rx Only Capsules Vayacog capsules contain soy and fish (e.g. herring, sprout, blue whiting, anchovy). ® Vayacog® Sample May contain shellfish. Vayacog® capsules do not contain sugar, lactose, yeast or gluten. Rx Only 8.938" • Mechanism of Action Administration of phosphatidylserine (PS) enriched with omega 3 fatty acids was found to significantly increase DHA levels in the brains of rats [1]. DHA brain and plasma levels are suggested to be positively associated with cognitive performance [2,3]. While the exact mechanism by which Vayacog® exerts its effects is not fully understood, PS in the mammalian nervous system, which is characterized by its substantial levels of omega-3 fatty acids, has been implicated in numerous membrane related functions, such as maintaining the integrity of cell membranes, cell excitability and cell-to-cell recognition and communication [4]. PS has been found to regulate key proteins in neuronal membranes, including sodium/calcium ATPase [5] and protein kinase C [6] which undertake crucial functions in diverse signal transduction pathways. Similarly, PS interacts with Raf-1 protein kinase to promote a cascade of reactions that are believed to be involved in cell survival [7]. Additionally, PS has been found to influence neurotransmitter activity, such as the release of acetylcholine, dopamine and noradrenaline [8, 9] and to increase brain glucose levels. • Absorption and Metabolism Following dietary ingestion of phospholipids, pancreatic digestive enzymes cleave specific fatty acids leading to the formation of lysophospholipids that are absorbed by the mucosal cells of the intestine and could be reacylated into phospholipids [10]. The fatty acids released can be further used for triglyceride synthesis. Because of the high activity of decarboxylases in the mucosal cells, the majority of the PS is converted into other phospholipids, primarily to phosphatidylethanolamine [11]. The reacylated PS, phosphatidylethanolamine and other phospholipids enter the lymph and circulation, and are redistributed. • Drug Interactions PS can potentially interact with some anticholinergic and cholinergic medications. It is recommended to consult with a physician about Vayacog® interactions that may apply to specific medical conditions. • Toxicity Vayacog®, similar to phosphatidylserine extracted from bovine cortex (BC-PS), contains saturated and monounsaturated fatty acids as well as DHA. The safety profile of BC-PS was determined in several non-clinical studies. Repeat-dose safety studies in rats and dogs show that oral administration of BC-PS at doses up to 1000 mg/kg/day for up to 6 months was without any significant adverse effects of toxicological concern [12]. The results of teratogenicity studies in rats at doses up to 200 mg/kg/day and in rabbits at doses up to 450 mg/kg/day showed that oral administration of PS did not affect embryonic and fetal development [12]. The mutagenic potential of BC-PS was investigated in several cell types and revealed no significant findings. In a micronucleus test, BC-PS was administered to mice at total dosages of 30, 150 and 300 mg/kg in two equal doses separated by 24-hours. The results of the study did not reveal any evidence of mutagenic potential or bone marrow toxicity [12]. Rev. 8 Front 1.6 1.4 1.2 1.0 0.8 0.6 0.4 Placebo Vayacog® Effect of Vayacog® (n=60) versus placebo (n=62) on Rey-AVLT results following 15 weeks of administration (per-protocol participants) during the double-blind phase. Values are presented as mean change from baseline ± standard error (SE). * p< 0.05 based on ANCOVA controlled for Mini-Mental State Examination (MMSE) and baseline mean score. Immediate Recall Delayed Recall Total Learning 8 3.0 Mean change from baseline (# words) Capsules PHARMACOLOGY Vayacog® is a prescription medical food used under medical supervision. 1.8 7 2.5 6 2.0 5 4 1.5 3 1.0 2 0.5 1 0 0.0 Placebo Vayacog® Effect of Vayacog® (n=40) versus placebo (n=38) on Rey-AVLT results following 15 weeks administration during the double-blind phase, within a subgroup of participants with relatively good cognitive status prior treatment. Subjects were included in this selected subset upon fulfillment of two out of the following criteria: I). A score in MMSE greater than 26. II) Baseline performance in the delayed recall trial above the per-protocol mean (>7). III) Number of education years greater than 12. Values are presented as mean change from baseline ± SE. * p<0.05 and ** p<0.01 based on two-tailed T-test comparison of the mean difference from baseline for independent samples. * * Mean score (pts.) Vayacog Sample ® Immediate Recall Effect of Vayacog® on parameters of the computerized cognitive assessment tool following 15 weeks administration during the open-label phase (n=55, participants who consumed placebo during the double-blind phase). Values are presented as mean ± SE. * P < 0.05 based on paired two tailed Student's t-test. 5.563" Safety assessment No significant differences were found in any of the tested safety parameters between the study groups, or within each group during the double-blind phase. At the end of the open label phase, there was a reduction in resting diastolic blood pressure and a slight weight gain among participants who consumed Vayacog® for 30 weeks [14] (additional safety information is detailed in the Adverse Events section). PHYSICIAN SUPERVISION Vayacog® is a medical food product dispensed by prescription and must be used under physician supervision. CONTRAINDICATIONS Vayacog® is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vayacog® or any of its components. 8.938" Lipicogen™ is a proprietary composition containing phosphatidylserine-omega 3, DHA enriched. Vayacog® and Lipicogen™ are trademarks of Enzymotec Ltd. US Patents No. 7,935,365, No. 7,968,112 and No. 5,965,413. Distributed by: VAYA Pharma™ Inc. REFERENCES 1. Vaisman, N. and D. Pelled, n-3 phosphatidylserine attenuated scopolamine-induced amnesia in middle-aged rats. Prog Neuropsychopharmacol Biol Psychiatry, 2009. 33(6): p. 952-9. 2. Soderberg, M., et al., Lipid composition in different regions of the brain in Alzheimer's disease/senile dementia of Alzheimer's type. J Neurochem, 1992. 59(5): p. 1646-53. 3. Conquer, J.A., et al., Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, PRECAUTIONS and cognitive impairment. Lipids, 2000. 35(12): p. 1305-12. • Safety and effectiveness of Vayacog® in pediatric patients or pregnant or lactating patients have not been established. 4. Mozzi, R., S. Buratta, and G. Goracci, Metabolism and Therefore, Vayacog® is not recommended for these functions of phosphatidylserine in mammalian brain. populations. Neurochem Res, 2003. 28(2): p. 195-214. • Vayacog® should be used with caution in patients with known hypersensitivity to soy and/or fish. 5. Wheeler, R.P.W., R. , ATPase activity of the sodium pump needs of phosphatidylserine. Nature, 1970. 225(5231): DRUG ABUSE p. 449-450. Vayacog® does not have any known drug abuse or withdrawal effects. 6. Bittova, L., R.V. Stahelin, and W. Cho, Roles of ionic residues of the C1 domain in protein kinase C-alpha ADVERSE EVENTS activation and the origin of phosphatidylserine specificity. The adverse events of Vayacog® were evaluated in a randomized, J Biol Chem, 2001. 276(6): p. 4218-26. double blind, placebo-controlled study of 15 weeks followed by an open label extension of additional 15 weeks [14] (additional 7. Vance, J.E., Phosphatidylserine and information is detailed in the Clinical Experience section). Phosphatidylethanolamine in Mammalian Cells: Two Metabolically-related Aminophospholipids. ASBMB, 2008: Adverse events reported during the course of the double-blind p. 1-48. phase: 10 participants from the Vayacog® group and 8 8. Pepeu, G., I.M. Pepeu, and L. Amaducci, A review of participants from the placebo group were classified by the phosphatidylserine pharmacological and clinical effects. Is study physicians as suffering from treatment related, or phosphatidylserine a drug for the ageing brain? Pharmacol probably related, adverse events (16 and 11 adverse events, Res, 1996. 33(2): p. 73-80. respectively). There were no significant differences between the study groups in the number of participants who reported an 9. Mazzari, S. and A. Battistella, Phosphatidylserine effects on adverse event (P=0.637) or in the number of reported adverse dopamine release from striatum synaptosomes. In: events (P=0.472). Multidisciplinary Approach to Brain Development Elsevier/North Holland, Amsterdam 1980: p. 569-570. Adverse events reported during the course of the open-label extension: 3 participants reported 3 adverse events that were classified by the study physicians as related or probably related 10. Tso, P., Intestinal lipid absorption. Physiology of the Gastrointestinal Trac, ed. L.R. Johnson. Vol. 56. 1994, New to the study treatment. York: Raven Press. Reported adverse events* 11. Wise, E.M., Jr. and D. Elwyn., Rates of reactions involved in Open-label phosphatide synthesis in liver and small intestine of intact Study design Double-blind phase extension rats. Journal of Biological Chemistry, 1965. 240: Treatment Group Vayacog® p. 1537-1548. Placebo Vayacog® [n=78] [n=79] [n=122] Adverse event 12. Heywood, R., D.D. Cozens, and M. Richold, Toxicology of a Gastrointestinal discomfort 13 2 1 PS preparation from bovine brain (BC-PS). Clinical Trials Rash 1 0 0 Journal, 1987. 24: p. 25-32. Increased appetite and weight Strange general feeling Headache Dizziness Mood swings Weight loss Tenesmus Redness in the mouth Pruritus Sum 0 0 1 0 0 1 0 0 0 16 events in 10 participants 1 1 2 2 1 0 0 1 1 11 events in 8 participants 0 0 1 0 0 0 1 0 0 3 events in 3 participants *Judged by the study physicians as related or probably related to the study treatment 13. Vakhapova, V., et al., Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 29(5): p. 467-74. 14. Vakhapova, V., et al., Safety of phosphatidylserine containing omega-3 fatty acids in non-demented elderly: a double-blind placebo-controlled trial followed by an open-label extension. BMC Neurol. 11: p. 79. Rev. 7/12 DOSAGE AND ADMINISTRATION Usual dose is one capsule daily or as directed by a physician. HOW SUPPLIED Available as hard shell capsules. Commercial product is supplied in bottles of 30 capsules. Commercial Product (30 capsules) Use under medical/ 75959-344-30* physician supervision. Sample Product Professional Samples (Packet of 2 capsules) 75959-344-02* -Not for sale. * VAYA Pharma™ does not represent these product codes to be actual National Drug Codes (NDCs). NDC format codes are product codes adjusted according to standard industry practice to meet the formatting requirements of pharmacy and health insurance computer systems. STORAGE Store at up to 77°F (25°C). Protect from light and moisture. WARNING Keep this product out of the reach of children. Rev. 8 Back
© Copyright 2026