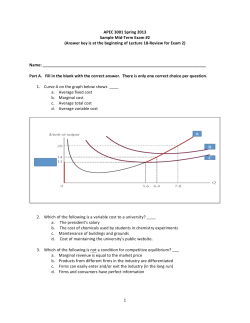
L A B O
CHE 1402 School of Science & Engineering LABORATORY MANUAL FOR GENERAL CHEMISTRY II Last Update: May 2014 Last update: June 2011 1 CHE 1402 Name: ________________________ Section: ________________________ LABORATORY MANUAL FOR GENERAL CHEMISTRY II Last Update: May 2014 Last update: June 2011 1 CHE 1402 TABLE OF CONTENTS Preface Laboratory Safety ii iii Experiment 1: Boyle’s law and molar mass of a vapor 1 Experiment 2: Determination of R: the gas law constant 6 Experiment 3: Colligative properties: freezing point depression and molar mass 10 Experiment 4: Rates of chemical reactions, a clock reaction 16 Experiment 5: An equilibrium constant 25 Experiment 6: Titration curves of polyprotic acids 34 Experiment 7: Determination of the solubility-product constant for a slightly soluble salt 38 Experiment 8: Molar solubility, common-ion effect 43 Experiment 9: Determination of orthophosphate in water 48 Experiment 10: pH-metric titration of an HCl-H3PO4 mixture 52 Experiment 11: Water analysis 56 Experiment 12: Galvanic cells, the Nernst equation 61 Appendix 70 Lab Manual i CHE 1402 PREFACE Chemistry is an experimental science that relies upon accurate measurements and observations from scientists. Thus, it is important that students of chemistry do experiments in the laboratory to more fully understand that the theories they study in lecture and in their textbook are developed from the critical evaluation of experimental data. The laboratory can also aid the student in the study of Science by clearly illustrating the principles and concepts involved. Finally, laboratory experimentation allows students the opportunity to develop techniques and other manipulative skills. The laboratory is designed to support and illustrate chemical concepts studied in the lecture portion of the course, as well as to introduce important laboratory techniques and encourage analytical thinking. The sequence of experiments in this laboratory manual is designed to follow the lecture notes. However, we cannot guarantee the synchronization of lectures and laboratory experiments. For this, certain experiments may come before or after the material that has been covered in the lecture. The lab manual contains background information and procedures for the experiments you will perform as part of your General Chemistry II course – CHE1402. Along with concepts and chemistry covered in the lecture, the laboratory portion of the course will present some additional chemistry, both theoretical and practical (e.g. water analysis). Questions are presented throughout each experiment. It is important that you try to answer each question as it appears in the manual, as it will help you understand the experiment as you do it. There is a lot of interesting chemistry to explore in the CHE 1402 Laboratory Manual. It is our hope that you enjoy learning it and that it will enhance your understanding of the material presented in lecture. Remember that what you get out of your laboratory experience will be directly proportional to what you put into it. Lab Manual ii CHE 1402 SAFETY IN THE LABORATORY Safety in the laboratory must be emphasized. The compounds you will work with do have some hazards associated with them. Therefore, it is important to follow the safety rules outlined in this lab manual. You should assume that all compounds encountered in the laboratory are toxic and handle them accordingly. Safety goggles for eye protection are recommended and lab coats are to be worn by all students at all times when entering the laboratory. Many chemicals, common in chemical laboratories, will make holes in clothing. Always wash your hands thoroughly when leaving the laboratory. The location and use of the safety equipment in laboratory were already discussed in CHE1401 and will be reminded by your instructor the first day the laboratory class meets. You should become familiar with the proper use of the safety shower, eye-wash fountain, fire blanket and fire extinguisher. Report any accidents which occur immediately to the laboratory supervisor (Dr. S. El Hajjaji). Safety rules to be strictly followed by all students. 1. Wear goggles when required. 2. Do not touch chemicals with your hands. Spatulas will be provided for handling solid materials. 3. Do not eat or drink in the laboratory. 4. Do not taste any chemical. 5. Do not smell any chemicals directly. Use your fingers to waft the odor to your nose. 6. Do not pipet solutions by mouth. Rubber pipet bulbs are provided at each lab station. 7. Do not put flammable liquids near an open flame. 8. When heating a test tube, make certain that the open end of the tube is directed away from the students. 9. When finished with your Bunsen Burner for a given portion of an experiment, turn it off. 10. Do not sit on the lab benches. 11. Do not engage in games in the laboratory. Failure to follow this rule will result in immediate dismissal from the lab and subsequent conduct action. 12. Do not pour any chemicals into a sink without authorization from the instructor. 13. Notify your instructor if a mercury spill should occur. 14. All broken glassware should be cleaned up immediately. The instructor should be notified of all breakage, especially if a thermometer is involved. 15. Do all reactions involving malodorous, noxious or dangerous chemicals in a fume hood. 16. If a chemical gets on your skin, immediately wash the affected area with large quantities of water. The instructor should be notified; no matter how insignificant the incident might seem. 17. When pouring one liquid into another, do so slowly and cautiously. To dilute an acid, pour the acid into the water; never pour water into an acid. 18. No student shall be permitted to work alone in the lab, you should be supervised by a laboratory instructor (or the lab technician during make up sessions). 19. Exercise good housekeeping practices in the laboratory. Be sure that the lab benches remain free of disorder during the experiment. In the event of a spill, clean the area immediately and be sure to use a wet sponge to wipe off the work station at the end of the lab session. 20. Know what you have to do before entering the lab. Read the experiment carefully before coming to the laboratory. For more information, a booklet titled “Student’s Chemistry Laboratory Safety Manual” will be provided to you in your first lab session. Please get acquainted with it. Be cautious and think about what you are doing ! Lab Manual iii CHE 1402 EXPERIMENT 1 Boyle’s Law & Molar Mass of a Vapor OBJECTIVES To observe how changes in pressure, for a fixed amount of a trapped gas at constant temperature, can affect the volume of the gas. To determine the molar mass of a gas based on a knowledge of its mass, temperature, pressure, and volume. Relates to chapter 10 of “Chemistry the Central Science, 12th Ed.”. APPARATUS AND CHEMICALS gas-law demonstration apparatus balance 125-mL Erlenmeyer flask 600-mL beaker boiling chips 250-mL graduated cylinder Bunsen burner ring stand and iron ring buret clamp wire gauze 3-mL sample of a volatile unknown liquid DISCUSSION The Effect of Pressure on the Volume of a Gas The effect of the pressure on the volume of a gas can be determined by using a gas buret connected to a U shaped manometer as shown in Figure1.1. When the stopcock is opened, air can enter in the buret, and the level of mercury will be equal in both arms of U shaped manometer. If the stopcock is then closed, a fixed volume of air is trapped in the buret at atmospheric pressure. Raising the leveling of mercury increases the pressure on the gas (enclosed air); the new pressure on the gas corresponds to the atmospheric pressure plus the difference in height of the mercury in both arms. At constant temperature, the volume of a given amount of gas is inversely proportional to the pressure. This is Boyle's law that may be expressed mathematically as [1] P1V1 P2V2 Therefore, one can calculate the volume of a gas at any pressure, provided that the initial volume of the gas at a given pressure is known. Behavior of Gases: Molar Mass of a Vapor The ideal gas law can be expressed as follows: Figure 1.1 PV nRT [2] R= 62400 mL-mmHg/mol-K or, in other units, R = 0.0821 L-atm/mol-K. The number of moles of a substance (n) equals its mass in grams, m, divided by the number of grams per mole (that is, its molar mass, M): n = m/M. Making this substitution into Equation [2] gives m PV RT M Lab Manual [3] 1 CHE 1402 The units of P, V, T, and m must be expressed in units consistent with the value of R. The first part of this experiment will be a demonstration of Boyle's law. You will vary the pressure of a fixed amount of trapped air at constant temperature, using a special gas buret. The second portion of this experiment concerns the determination of the molar mass of a volatile liquid using equation [3]. A small quantity of liquid sample is placed in a pre-weighed flask and vaporized so as to expel the air from the flask, leaving it filled with the vapor at a known temperature (temperature of water). The flask plus vapor is then cooled so that the vapor condenses. The flask plus condensed vapor plus air is then weighed. The mass of air, being nearly identical before and after cancels out and allows one to determine the mass of the vapor. The above data, in conjunction with the volume of the flask, permit the calculation of the molar mass. PROCEDURE : A. Verification of Boyle’s law Step 1: As shown in Figure 1.3a, with each leg of a U-tube manometer exposed to the atmosphere, the height of liquid in the columns is equal. At this moment, close the stopcock and record the height h1 that exists between the stopcock and the level of mercury. h1 corresponds to the height occupied by the gas (air) that is trapped in the left leg of the manometer, at pressure P1 (measure P1 by means of a barometer). Step 2: Next, shift the position of the right leg, either upwards or downwards; this will create a difference of pressure between the trapped gas in the left leg and the atmospheric pressure in the right leg. The new height and pressure of the trapped gas are named h2 and P2. Two situations can then occur (Figure 1.3b): - h2 > h1 which means that P2 < P1. In that case, the difference of heights between the right leg and the left leg, GP, called the gauge pressure, is negative. - h2 < h1 which means that P2 > P1. In that case, the difference of heights between the right leg and the left leg, GP, is positive. Figure 1.3a Figure 1.3b Gas is expanded Lab Manual Gas is compressed 2 CHE 1402 Once P1 and GP recorded, you can calculate P2: P2 = P1+ GP. Finally, verify Boyle’s law: P1V1 P2V2 P1 S h1 P2 S h2 P1h1 P2 h2 You will consider that the law is verified if the percent error is lower than 5%. Percent Error: % error P1h1 P2 h2 100 P1h1 P2 h2 B. Molar Mass of a Vapor Overview: A sample of a volatile liquid is added to a preweighed flask. The flask is submerged in a boiling water bath to vaporize the liquid. Because an excess amount of liquid is used, the volume of vapor produced is greater than the volume of the flask. Upon heating, the vapor that is created initially pushes the air out of the flask and then the vapor begins exiting the flask until the pressure inside the flask is equal to the atmospheric pressure. The mass of the vapor remaining in the flask is obtained by reweighing the flask. Additional measurements are made to determine the pressure, temperature and volume of the sample. Procedure: Place a rubber stopper (inside which a glass tubing is inserted) in the mouth of a clean, dry 125-mL Erlenmeyer flask; (see Figure 1.2). Remove the rubber stopper and add approximately 2 mL of unknown to the flask. Clamp the flask at the top of the neck and immerse it as deeply as possible in a 600-mL beaker nearly full of water. Place some boiling chips in the water and heat to boiling. Record the temperature of the boiling water (3) Figure 1.2 and the barometric pressure (4). As the water boils, watch the liquid in the flask. As soon as all of the liquid (including any that has condensed in the neck) has vaporized (about 3 min.), remove the flask by means of the clamp and set it aside to cool. After the flask has cooled to room temperature, wipe it dry and remove any water that may adhere to the rubber stopper. Weigh the flask, cap, rubber band, and condensed unknown liquid (5). Calculate the weight of the condensed liquid (6). Remove the cap and fill the flask completely with water. Measure the volume by pouring the water into a large graduated cylinder (7). Calculate the molar mass of the unknown using Equation [3] (8). Lab Manual 3 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1 How does the pressure of an ideal gas at constant volume change as the temperature increases? 2 How does the volume of an ideal gas at constant temperature change as the pressure increases? 3 How does the volume of an ideal gas at constant temperature and pressure change as the number of molecules changes? 4. Write the ideal-gas equation and give the units for each term when R = 0.0821 L-atm /mol-K. 5. Show by mathematical equations how one can determine the molar mass of a volatile liquid by measurement of the pressure, volume, temperature, and weight of the liquid. 6. If 0.75 g of a gas occupies 300 mL at 27°C and 700 mm Hg of pressure, what is the molar mass of the gas? 7. A sample of nitrogen occupies a volume of 300 mL at 30°C and 700 mm Hg of pressure. What will be its volume at STP? 8. Consider Figure 12.1. If the height of the mercury column in the leveling bulb is 30 mm greater than that in the gas buret and atmospheric pressure is 670 mm, what is the pressure on the gas trapped in the buret? 9. Consider Figure 1.3a. If the level of the mercury in the leveling bulb is lowered, what happens to the volume of the gas in the gas buret? 10. Show that Boyle's law, Charles's law, and Avogadro's law can be derived from the ideal-gas law. 11 Methane bums in oxygen to produce CO2 and H2O. CH4 (g) + 2 O2 (g) 2 H2O (l) + CO2 (g) If 3.7 L of gaseous CH4 is burned at STP, what volume of O2 is required for complete combustion? What volume of CO2 is produced? 12 Calculate the density of O2 at STP, (a) using the ideal-gas law and (b) using the molar volume and molar mass of O2. How do the densities compare? Lab Manual 4 CHE 1402 Experiment 1 Boyle’s Law & Molar Mass of a Vapor Name(s) Date Laboratory Instructor REPORT SHEET A. Boyle's Law : Effect of Pressure at Constant Temperature Trial 1 Trial 2 1. First pressure (atmospheric), barometer reading, mm Hg (P1) _______ _______ 2. First height, in mm (h1) _______ _______ 3. Second height in mm (h2) _______ _______ 4. Difference in mercury levels (+ or -), mm Hg (GP) _______ _______ 5. Second pressure, mm Hg (P2) _______ _______ 6. Percent error _______ _______ (Show calculations) B. Molar Mass of a Vapor 1. Unknown liquid number ____________ 2. Wt. of flask + rubber stopper + cap ____________ g 3. Temperature of water when liquid boiled ____________ °C 4. Pressure of the flask = PATM = ___________ mbar = ___________ mm Hg 5. Wt. of flask + rubber stopper + cap + condensed vapor ____________ g 6. Wt. of condensed vapor ____________ g 7. Volume of flask ____________ mL 8. Molar mass of vapor ____________ g/mol (Show calculations) Lab Manual 5 CHE 1402 EXPERIMENT 2 Determination of R: The Gas-Law Constant OBJECTIVE Determination of the ideal-gas-law constant R. Relates to chapter 10 of “Chemistry the Central Science, 12th Ed.”. APPARATUS & CHEMICALS 0.30 g CaCO3 5 mL of 4 M HCl test tube ring stand clamp pinch clamp 250-mL Erlenmeyer flask rubber bulb 250 mL beaker rubber stoppers rubber tubing balance 125 mL Erlenmeyer flask thermometer barometer 100 mL graduate cylinder DISCUSSION The ideal-gas equation, PV nRT is very useful in describing the behavior of ideal gases under normal condition (room temperature and atmospheric pressure). However, the behavior of a real gas can be described by the van der Waals equation: n2 P a 2 V nb nRT V where a and b are constants characteristic of a given gas. The term nb is a correction for the finite n2 volume of the molecules. The term a 2 is a correction to the pressure which takes into account the V intermolecular attractions. In this experiment you will determine the numerical value of the gas-law constant R, in its common units of L-atm/mol-K. This will be done using both the ideal-gas law and the van der Waals equation together with measured values of pressure, P, temperature, T, volume, V, and number of moles, n, of an enclosed sample of CO2. An error analysis will then be performed on the experimentally determined constant. The CO2 will be prepared by the decomposition of CaCO3 in presence of a concentrated solution of HCl: CaCO3 (s) + 2 HCl (aq) CO2 (g) + H2O (l) + CaCl2 (aq) The CO2 can be collected by displacing water from a bottle and the volume of gas can be determined from the volume of water displaced. Through use of Dalton's law of partial pressures, the vapor pressure of water, and atmospheric pressure, the pressure of the gas may be obtained. Dalton's law states that the pressure of a mixture of gases in a container is equal to the sum of the pressures that each gas would exert if it were present alone: Ptotal i Pi Lab Manual 6 CHE 1402 Since this experiment is conducted at atmospheric pressure, Ptotal Patmosphere PCO2 PH 2O vapor . PROCEDURE Place 0.30 g of CaCO3 in a test tube and carefully insert another smaller test tube in it containing 5mL of 4 M HCl (be sure NOT to mix CaCO3 and HCl before the experiment). Assemble the apparatus illustrated in Figure 2.1 but do not attach the test tube. Be sure that tube B does not extend below the water level in the bottle. Fill glass tube A and the rubber tubing with water by loosening the pinch clamp and attaching a rubber bulb to and applying pressure through tube B. Close the clamp when the tube is filled. Attach tube B as shown in Figure 2.1. When you attach the test tube, half-fill the beaker with water, insert glass tube A in it, open the pinch clamp, and lift the beaker until the levels of water in the bottle and the beaker are identical; then close the clamp, discard the water in the beaker, and dry the beaker. The purpose of equalizing the levels is to produce atmospheric pressure inside the bottle and test tube. Set the beaker with tube A in it on the desk and open the pinch clamp. A little water will flow into the beaker, but if the system is air tight and has no leaks, the flow will soon stop, and tube A will remain filled with water. If this is not the case, check the apparatus for leaks and start over again. Leave the water that has flowed into the beaker in the beaker; at the end of the experiment, the water levels will be adjusted, and this water will flow back into the bottle. By opening the pinch clamp, mix the solids (CaCO3) in the test tube with the liquid HCl, be sure that none of the mixture is lost from the tube. You will notice a slow but steady stream of gas produced, as evidenced by the flow of water into the beaker. When the rate of gas evolution slows considerably and no more CO2 is evolved stop the experiment and close the pinch clamp. Empty the water from the beaker into a 100 mL graduated cylinder and measure its volume which is equal to the volume of CO2 produced (assuming that the density of water is 1 g/mL). Record the barometric pressure. The vapor pressure of water at various temperatures is given in Table 2.1. Calculate the gas-law constant, R, from your data, using the ideal-gas equation PV nRT as well as the van der Waals equation P a n2 V nb nRT . V 2 a b CO2 CO2 3.59 L2 atm / mol 2 0.0427 L / mol PCO2 Patm PH 2O nCO2 nCaCO3 mCaCO3 M CaCO3 VCO2 Vwater displaced Using the ideal gas equation: PV nRT R PCO2 VCO2 nCO T 2 Using the van der Waals equation: n2 P a 2 V nb nRT V Lab Manual 2 nCO2 VCO2 nCO2 b R PCO2 a 2 n T V CO2 CO2 7 CHE 1402 TABLE 2.1 Vapor Pressure of Water at Various Temperatures Temperature H2O vapor pressure (°C) (mm Hg) 15 12.8 13.6 16 14.5 17 15.5 18 16.5 19 17.5 20 18.6 21 19.8 22 21.1 23 22.4 24 23.8 25 Figure 2.1 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. Under what conditions of temperature and pressure would you expect gases to obey the ideal-gas equation? 2. Calculate the value of R in L-atm/mol-K by assuming that an ideal gas occupies 22.4 L/mol at STP. 3. Why do you equalize the water levels in the bottle and the beaker? 4. Why does the vapor pressure of water contribute to the total pressure in the bottle? 5. What is the value of an error analysis? 6. Suggest reasons, on the molecular level, why real gases might deviate from the ideal gas law. 7. Newly devised automobile batteries are sealed. When lead storage batteries discharge, they produce hydrogen. Suppose the void volume in the battery is 100 mL at 1 atm of pressure and 25°C. What would be the pressure increase if 0.05 g H2 were produced by the discharge of the battery? Does this present a problem? Do you know why sealed lead storage batteries have not been used in the past? 8. Why is the corrective term to the volume subtracted and not added to the volume in the van der Waals equation? 9. A sample of pure gas at 20°C and 670 mm Hg occupied a volume of 562 cm3. How many moles of gas does this represent? (HINT: Use the value of R that you found in question 2) 10. A certain compound containing only carbon and hydrogen was found to have a vapor density of 2.550 g/L at 100°C and 760 mm Hg. If the empirical formula of this compound is CH, what is the molecular formula of this compound? 11. Which gas would you expect to behave more like an ideal gas Ne or HBr? Why? Lab Manual 8 CHE 1402 Experiment 2 Determination of R: The Gas-Law Constant Name(s) Date Laboratory Instructor REPORT SHEET 1. Mass of CaCO3 = ____________ g 2. Moles of CaCO3 = ____________ = Moles of CO2 3. Volume of 4M HCl = ____________ mL 4. Volume of water collected = ____________ mL = Volume of CO2(g) released 5. Barometric pressure = ____________ mbar = ____________ mm Hg Show calculation overleaf 6. Temperature of water = ____________ oC = ____________ K 7. Vapor pressure of water = ____________ mmHg 8. Pressure of the gas (show calculations) 9. Gas-law constant, R, from ideal-gas law (show calculations) 10. R from the van der Waals equation (show calculations) Accepted value of R Lab Manual _______ Justify your choice overleaf 9 CHE 1402 EXPERIMENT 3 Colligative Properties: Freezing-Point Depression & Molar Mass OBJECTIVE Determination of the molar mass of a substance by using the colligative properties of a solution. Relates to chapter 13 of “Chemistry the Central Science, 12th Ed.”. APPARATUS AND CHEMICALS ring and ring stand clamp wire gauze thermometer large test tube wire stirrer Bunsen burner and hose 600-mL beaker unknown solid (2 g) naphthalene (50 g) 2-hole rubber stopper with slit towel wide-mouth glass bottle weighing paper DISCUSSION A solution is a homogeneous mixture of a solute in a given solvent. Both solute and solvent are components of the solution. The solute is the smallest amount present in the solution while the solvent is the largest amount present in the solution. Since the solution is primarily composed of solvent, physical properties of a solution resemble those of the solvent. Some of these physical properties called colligative properties, are independent of the nature of the solute and depend only upon the solute concentration. The colligative properties include vapor-pressure lowering, boiling point elevation, freezing point lowering, and osmotic pressure. The vapor pressure is just the escaping tendency of the solvent molecules. When the vapor pressure of a solvent is equal to atmospheric pressure, the solvent boils. At this temperature the gaseous and liquid states of the solvent are in dynamic equilibrium, and the rate of molecules going from the liquid to the gaseous state is equal to the rate of molecules going from the gaseous state to the liquid state. It has been found experimentally that the dissolution of a non volatile solute (one with very low vapor pressure) in a solvent lowers the vapor pressure of the solvent, which in turn raises the boiling point and lowers the freezing point. This is shown graphically by the phase diagram given in Figure 3.1. Figure 3.1 Lab Manual 10 CHE 1402 You are probably familiar with some common uses of these effects: Antifreeze is used to lower the freezing point and raise the boiling point of coolant (water) in an automobile radiator; and salt is used to melt ice. These effects are expressed quantitatively by the colligative-property law, which states that the freezing point and boiling point of a solution differ from those of the pure solvent by amounts that are directly proportional to the molal concentration of the solute. This relationship is expressed by Equation [1] for the freezing-point lowering and boiling-point elevation: T Km [1] where T is the freezing-point lowering or boiling-point elevation, K is a constant that is specific for each solvent, and m is the molality of the solution (number of moles solute per/ 1000 g solvent). Some representative constants, boiling points, and freezing points are given in Table 3.1. For naphthalene, the solvent used in this experiment, the molal freezing-point depression constant (Kfp) has a value of 6.9218 °C/ m. Table 3.1: Molal freezing point and boiling point constants Solvent CH3COOH (acetic acid) C6H6 (benzene) CHCI3 (chloroform) C2H5OH (ethyl alcohol) H2O (water) C10H8 (naphthalene) C6H12 (cyclohexane) Freezing point (°C) 16.6 5.4 -63.5 -114.1 0.0 80.6 6.6 Kfp Boiling point (°C/m) (°C) 3.90 118.1 5.12 80.2 4.68 61.3 --78.4 1.86 100.0 6.9218 --20.4 80.7 Kbp (°C/m) 2.93 2.53 3.63 --0.51 --2.79 Since the molal freezing-point-depression constant is known, it is possible to obtain the molar mass of a solute by measuring the freezing point of a solution and the weight of both the solute and solvent. In this experiment you will determine the molar mass of an unknown. You will do this by determining the freezing-point depression of a naphthalene solution having a known concentration of your unknown. The freezing temperature is difficult to ascertain by direct visual observation because of a phenomenon called supercooling and also because solidification of solutions usually occurs over a broad temperature range. Temperature-time graphs, called cooling curves, reveal freezing temperatures rather clearly. Therefore, you will study the rate at which liquid naphthalene and its solutions cool and will construct cooling curves, for both the pure solvent and the solution, similar to the ones shown in Figure 3.2. Lab Manual 11 CHE 1402 Figure 3.2: Cooling curve. Figure3.2 shows how the freezing point of a solution must be determined by extrapolation of the cooling curve. Extrapolation is necessary because as the solution freezes, the solid that is formed is essentially pure solvent and the remaining solution becomes more and more concentrated. Thus its freezing point lowers continuously. Clearly, supercooling produces an ambiguity in the freezing point and should be minimized. Stirring the solution helps to minimize supercooling. PROCEDURE A. Cooling Curve for Pure naphthalene Weigh a large test tube to the nearest 0.01 g. Add about 15g of naphthalene and weigh again. The difference in weight is the weight of naphthalene. Assemble the apparatus as shown in Figure 3.3; be certain to use a split two-hole rubber stopper. Carefully insert the thermometer into the hole that has been slit. Bend the stirrer so that the loop encircles the thermometer. Fill your 600-mL beaker nearly full of water and heat it to about 85°C. Clamp the test tube in the water bath as shown in Figure 3. When most of the naphthalene has melted, insert the stopper containing the thermometer and stirrer into the test tube; make certain that the thermometer is not resting on the bottom of or touching the sides of the test tube. When all of the naphthalene has melted, stop heating, remove the beaker of water, and dry the outside of the test tube with a cloth towel. Place the test tube in a wide-mouth bottle that contains a piece of crumpled paper in the bottom to lessen the chance that impact of the test tube with the bottle will cause the bottle to break. The purpose of the wide-mouth bottle is to minimize drafts. Record temperature readings every 30 s while you are stirring. When the freezing point is reached, crystals will start to form, and the temperature will remain constant. Shortly after this, the naphthalene will solidify to the point where you can no longer stir it. Your lab instructor will direct you to perform either procedure B or procedure C. Lab Manual 12 CHE 1402 Figure 3.3 B. Determination of the Molar Mass of the unknown Using weighing paper, weigh to the nearest 0.01 g about 1.0 g of the unknown. Replace the test tube in the water bath and heat until all the naphthalene has melted. Gently remove the stopper, making sure that no naphthalene is lost, and add the unknown to the test tube. Replace the stopper and stir gently until the entire unknown has dissolved. Remove the water bath, dry the test tube with a towel, and insert the test tube in a wide-mouth glass bottle containing a crumpled piece of paper. Record the temperature every 30 s until all the naphthalene has solidified. C. Determination of the Molar Mass of the unknown Same as Part B, but use about 2.0 g of the unknown. Cleanup: To clean out the test tube at the end of the experiment, heat the test tube in a water bath until the naphthalene just melts. Care should be taken not to heat the thermometer beyond its temperature range. Be careful, because naphthalene is flammable. Remove the stopper and pour the molten naphthalene on a crumpled wad of paper. When the naphthalene has solidified, throw both the paper and solid naphthalene into a waste receptacle. DO NOT POUR LIQUID NAPHTHALENE INTO THE SINK! Lab Manual 13 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. Distinguish between solute and solvent. 2. List three colligative properties and suggest a rationale for the choice of the word colligative to describe these properties. 3. Distinguish between volatile and nonvolatile substances. 4. What effect does the presence of a nonvolatile solute have upon (a) the vapor pressure of a solution, (b) the freezing point, and (c) the boiling point? 5. What is the molality of a solution that contains 1.5 g urea (molar mass = 60 amu) in 200 g of benzene? 6. What is supercooling? How can it be minimized? 7. Calculate the freezing point of a solution containing 6.50 g of benzene in 160 g of chloroform. 8. A solution containing 1.00 g of an unknown substance in 12.5 g of naphthalene was found to freeze at 75.4°C. What is the molar mass of the unknown substance? 9. How many grams of NaNO3 would you add to 250 g of H2O in order to prepare a solution that is 0.200 molal in NaNO3? 10. Define molality and molarity. Lab Manual 14 CHE 1402 Experiment 3 Colligative Properties: Freezing-Point Depression & Molar Mass Name(s) Date Laboratory Instructor REPORT SHEET 1. Weight of naphthalene _____________ g 2. Weight of unknown _____________ g Cooling curve data: Pure naphthalene Time (s) 0 30 60 90 120 150 180 210 240 270 300 330 360 390 420 Temp. (oC) Naphthalene + unknown Time (s) 0 30 60 90 120 150 180 210 240 270 300 330 360 390 420 Temp. (oC) 3. Freezing point of pure naphthalene, from cooling curve 4. Freezing point of solution of the unknown in naphthalene T = o C 5. Molality of the unknown (show calculations) 6. Molar mass of unknown (show calculations) _______ Lab Manual 15 CHE 1402 EXPERIMENT 4 Rates of Chemical Reactions A Clock Reaction OBJECTIVES To determine the order of a reaction with respect to the reactant concentrations. To obtain the rate law for a chemical reaction. Relates to chapter 14 of “Chemistry the Central Science, 12th Ed.”. APPARATUS & CHEMICALS burets (2) 1-mL pipets (2) clock or watch with second hand 125-mL Florence flask 400-mL beaker test tube pipet bulb 250-mL Erlenmeyer flasks (4) buret clamp ring stand 25-mL pipet 50-mL pipet thermometer 0.2 M KI (200-mL) 0.4 M Na2S2O3 (100- mL) (freshly prepared) 1% percent starch solution, boiled 0.2 M KNO3 (300-mL) 0.1 M solution of Na2H2EDTA 0.2M (NH4)2S2O8, (200-mL) (prepared from fresh solid) DISCUSSION Kinetics is the study of how fast chemical reactions occur. Among the important factors that affect the rates of chemical reactions are: 1. Reactant concentration 2. Temperature 3. Catalyst Before a reaction can occur, the reactants must come into direct contact via collisions of the reacting particles. The reacting particles (ions or molecules) must have the right orientation and must collide with sufficient energy to result in a reaction. With these considerations in mind, we can qualitatively explain how the various factors influence the rates of reactions. Concentration Changing the concentration of a solution alters the number of particles per unit volume. The more particles present in a given volume, the greater the probability of their collision. Therefore, increasing the concentration of a solution increases the number of collisions per unit time and therefore the rate of reaction. Temperature Since temperature is a measure of the average kinetic energy, an increase in temperature increases the kinetic energy of the particles. An increase in kinetic energy increases the velocity of the particles and therefore the number of collisions between them in a given period of time. Thus, the rate of reaction increases. Also, an increase in kinetic energy results in a greater proportion of the collisions having the required energy for reaction. Catalyst Catalysts, in some cases, are believed to increase reaction rates by bringing particles into close juxtaposition in the correct geometrical arrangement for reaction to occur. In other instances, catalysts offer an alternative Lab Manual 16 CHE 1402 route to the reaction, one that requires less energetic collisions between reactant particles. If less energy is required for a successful collision, a larger percentage of the collisions will have the requisite energy, and the reaction will occur faster. Actually, the catalyst may take an active part in the reaction, but at the end of the reaction, the catalyst can be recovered chemically unchanged. Let's examine now precisely what is meant by the expression rate of reaction. Consider the hypothetical reaction A+B C + D [1] Speed of a reaction is measured by the change in concentration of reactants or products with time. Suppose A reacts with B to form C and D. For the reaction A and B there are two ways of measuring rate: 1. the speed at which the products appear (i.e. change in concentration of C or D per unit time), or 2. the speed at which the reactants disappear (i.e. the change in concentration of A or B per unit time). average rate change in concentrat ion A B C D time required for this change t t t t The units for average rate are mol/L-s or M/s. In general, the rate of the reaction will depend upon the concentration of the reactants. Thus, the rate of our hypothetical reaction may be expressed as x y [2] rate k A B where [A] and [B] are the molar concentrations of A and B, x and y are the powers to which the respective concentrations must be raised to describe the rate, and k is the specific rate constant. One of the objectives of chemical kinetics is to determine the rate law. Stated slightly differently, one goal of measuring the rate of the reaction is to determine the numerical values of × and y. Suppose that we found x = 2 and y = 1 for this reaction. Then 2 [3] rate k A B would be the rate law. It should be evident from equation [3] that doubling the concentration of B (keeping [A] the same) would cause the reaction rate to double. On the other hand, doubling the concentration of A (keeping [B] the same) would cause the rate to increase by a factor of 4, because the rate of the reaction is proportional to the square of the concentration of A. The powers to which the concentrations in the rate law are raised are termed the order of the reaction. In this case, the reaction is said to be second order in A and first order in B. The overall order of the reaction is the sum of the exponents, 2 + 1 = 3, or a third-order reaction. It is possible to determine the order of the reaction by noting the effects of changing reagent concentrations on the rate of the reaction. It should be emphasized that k, the specific rate constant, has a definite value that is independent of the concentration. k depends only on temperature. Once the rate is known, the value of k can be calculated. Reaction of Peroxydisulfate Ion with Iodide Ion SO 2- + 2 I- 2- I2 + 2 SO4 2 8 In this experiment you will measure the rate of the reaction [4] and you will determine the rate law by measuring the amount of peroxydisulfate, S2O82-, that reacts as a function of time. The rate law to be determined is of the form: Lab Manual 17 CHE 1402 rate of disappearance of S 2 O8 or S 2 O8 t 2 2 k S 2 O8 I 2 X Y [5] k S O I 2 X 2 Y 8 Your goal will be to determine the values of x and y as well as the specific rate constant, k. You will add to the solution a small amount of another reagent (sodium thiosulfate, Na2S2O3 which will cause a change in the color of the solution. The amount is such that the color change will occur when 2 × 10-4 mol of S2O82- has reacted. For reasons to be explained shortly, the solution will turn blue-black when 2 × 10-4 mol of S2O82- has reacted. You will quickly add another portion of Na2S2O3 after the appearance of the color, and the blue-black color will disappear. When the blue-black color reappears the second time, another 2 × 10-4 mol of S2O82- has reacted, making a total of 2× (2 × 10-4) mole of S2O82 that has reacted. You will repeat this procedure several times, keeping careful note of the time for the appearance of the blue-black colors. By graphing the amount of S2O82- consumed versus time, you will be able to determine the rate of the reaction. By changing the initial concentrations of S2O82- and I- and observing the effects upon the rate of the reaction, you will determine the order of the reaction with respect to S2O82- and I-. The blue-black color that will appear in the reaction is due to the presence of a starch-iodine complex that is formed from iodine, I2, and starch in the solution. The color therefore will not appear until a detectable amount of I2 is formed according to Equation [4]. The thiosulfate ion S2O32- that is added to the solution reacts extremely rapidly with the iodine, as follows: I2 + 2 S2O32- 2 I- + S4O62- [6] Consequently, until the same amount of S2O32- that is added is all consumed, there will not be a sufficient amount of I2 in the solution to yield the blue-black color (Figure 4.1). You will add 4 × 10-4 mol of S2O32each time (these equal portions are termed aliquots), and from the stoichiometry of equations [4] and [6] you can verify that when this quantity of S2O32- has reacted, 2 × 10-4 mol of S2O82- has reacted. Note also that although iodide, I-, is consumed according to equation [4], it is rapidly regenerated according to equation [6] and therefore its concentration does not change during a given experiment (Figure 4.1). S2O82- + 2 I- [4] BLUE-BLACK rch sta I2 2- (+ 2 SO4 ) 2S ve 2 O3 ry fa [6] st 2- COLORLESS S4O62- + 2 I- Figure 4.1 Starch is used as a colored indicator allowing us to know when reaction [6] is complete. Lab Manual 18 CHE 1402 Graphical Determination of Rate The more rapidly the 2 × 10-4 mol of S2O82- is consumed, the faster is the reaction. To determine the rate of the reaction, a plot of moles of S2O82- that have reacted versus the time required for the reaction is made. The best straight line passing through the origin is drawn, and the slope is determined. The slope, nS O 2 2 8 , t corresponds to the moles of S2O82- that have been consumed per second and is proportional to the rate. Since the rate corresponds to the change in the concentration of S2O82- per second, dividing the slope by the volume of the solution yields the rate of disappearance of S2O82-, that is, S 2 O8 t 2 . Helpful Comments 1. According to the procedure of this experiment, the solution will turn blue-black when exactly 2 ×10-4 mol of S2O82- has reacted. 2. The purpose of the KNO3 solution in this reaction is to keep the reaction medium the same in each run in terms of the concentration of ions; it does not enter into the reaction in any way. 3. The reaction studied in this experiment is catalyzed by metal ions. The purpose of the drop of EDTA solution is to minimize the effects of trace quantities of metal ion impurities that would cause spurious effects on the reaction. 4. You will perform a few preliminary experiments to become acquainted with the observations in this experiment so that you will know what to expect in the reactions. 5. The initial concentrations of the reactants have been provided for you on the report sheet. PROCEDURE Prepare four reaction solutions as follows (one at a time): Solutions 0.2 M KI (mL) 25.0 25.0 50.0 12.5 buret 0.2 M KNO3 (mL) 48.0 23.0 23.0 35.5 buret 0.4 M Na2S2O3 (mL) 1.0 1.0 1.0 1.0 5-mL pipet starch (mL) 1.0 1.0 1.0 1.0 5-mL pipet EDTA 1 drop 1 drop 1 drop 1 drop 25.0 mL 50.0 mL 25.0 mL 50.0 mL 100.0 100.0 100.0 100.0 0.2 M (NH4)2S2O8 (mL) Plastic dropper buret added at time zero Total Volume (mL) Lab Manual 19 CHE 1402 Each solution must be freshly prepared to begin the rate study - that is, prepare solutions 1, 2, 3, and 4 one at a time as you make your measurements. Equipment Setup Set up three burets held by a clamp on a ring stand. Use these burets to measure accurately the volumes of the KI, KNO3 and (NH4)2S2O8 solutions. Use two separate 5-mL pipets for measuring the volumes of the Na2S2O3 and starch solutions. Rate Measurements Procedure for solution 1: Prepare solution 1 in a 250-mL Erlenmeyer flask that has been scrupulously cleaned and dried. Pour 25.0 mL of (NH4)2S2O8 solution into a clean, dry 100-mL beaker. Be ready to begin timing the reaction when the solutions are mixed (READ AHEAD). The reaction starts the moment the solutions are mixed! BE PREPARED! ZERO TIME! Quickly pour the 25.0 mL of (NH4)2S2O8, solution into solution 1 and swirl vigorously; note the time you begin mixing to the nearest second. At the instant when the blue-black color appears, 2 × 10-4 mol of S2O82has reacted. IMMEDIATELY (be prepared!) add a 1.0 mL aliquot of Na2S2O3 solution from the pipet and swirl the solution; the color will disappear. If the students fill each of seven clean, dry test tubes with 1.0 mL of Na2S2O3 solution, they then can add these aliquots to their reactions at the appearance of the blue color without loss of time. Record the time for the reappearance of the blue-black color. Add another 1.0 mL aliquot of Na2S2O3 solution and note the time for the reappearance of the color. The time interval being measured is that between the appearance of the blue-black color. For good results, these aliquots of Na2S2O3 must be measured as quickly, accurately, and reproducibly as possible. Continue this procedure until you have added seven aliquots to solution 1.You are finished with solution 1 when you have recorded all your times on the report sheet. The time intervals are cumulative. Procedure for solution 3: Solution 3 should be treated in exactly the same manner as solution 1. CAUTION: Be on guard-solution 3 will react much more rapidly than solution l. Procedure for solutions 2 and 4: Solutions 2 and 4 should be treated in exactly the same manner except that 50.0 mL portions of (NH4)2S2O8 solutions should be added. In each of these reactions the final total solution volume is 100 mL. Calculations Tabulate on the data sheet for each aliquot of Na2S2O3 added to each of the four solutions: 1. The time interval from the start of the reaction (addition of S 2O82-) to the appearance of color for the first aliquot of S2O82- and the time interval from the preceding color appearance for each succeeding aliquot (column2) 2. The cumulative time from the start of the reaction to each appearance of color (column 3) 3. For each solution plot on the graph paper provided the moles of S2O82- consumed (as the ordinate, vertical axis) versus time in seconds (as the abscissa, horizontal axis), using the data in columns 3 and 4. Draw the tangent to the curve at time zero. Calculate the slope of each tangent, and from these calculations answer the questions on your report sheet. Lab Manual 20 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. What factors influence the rate of a chemical reaction? 2. What is the general form of a rate law? 3. What is the order of reaction with respect to A and B for a reaction that obeys the rate law rate k A B ? 2 3 4. Write the chemical equations involved in this experiment and show that the rate of disappearance of [S2O82-] is proportional to the rate of appearance of the blue-black color of the starch-iodine complex. C that doubling the concentration of either A or B 5. It is found for the reaction A + B quadruples the rate of the reaction. Write the rate law for this reaction. 6. If 2 × 10-4 mol of S2O82- in 50 mL of solution is consumed in 188 s, what is the rate of consumption of S2O82-? 7. Why are chemists concerned with the rates of chemical reactions? What possible practical value does this type of information have? 8. Suppose you were dissolving a metal such as zinc with hydrochloric acid. How would the particle size of the zinc affect the rate of its dissolution? 9. Assuming that a chemical reaction doubles in rate for each 100 temperature increase, by what factor would the rate increase if the temperature were increased by 40°C? Lab Manual 21 CHE 1402 Experiment 4 Rates of Chemical Reactions A Clock Reaction Name(s) Date Laboratory Instructor REPORT SHEET Solution 1. Initial [S2O82-] = 0.05 M; initial [I-] = 0.05 M. Time (s) between appearances of color Cumulative time (s) Total moles of S2O82consumed 1 __________________ _____________ 2.0 × 10-4 2 __________________ _____________ 4.0 × 10-4 3 __________________ _____________ 6.0 × 10-4 4 __________________ _____________ 8.0 × 10-4 5 __________________ _____________ 10 × 10-4 6 __________________ _____________ 12 × 10-4 7 __________________ _____________ 14 × 10-4 Aliquot no. Solution 2. Initial [S2O82-] = 0.10 M; initial [I-] = 0.05 M. Time (s) between appearances of color Cumulative time (s) Total moles of S2O82consumed 1 __________________ _____________ 2.0 × 10-4 2 __________________ _____________ 4.0 × 10-4 3 __________________ _____________ 6.0 × 10-4 4 __________________ _____________ 8.0 × 10-4 5 __________________ _____________ 10 × 10-4 6 __________________ _____________ 12 × 10-4 7 __________________ _____________ 14 × 10-4 Aliquot no. Lab Manual 22 CHE 1402 Solution 3. Initial [S2O82-] = 0.05 M; initial [I-] = 0.10 M. Time (s) between appearances of color __________________ Cumulative time (s) _____________ Total moles of S2O82consumed 2.0 × 10-4 2 __________________ _____________ 4.0 × 10-4 3 __________________ _____________ 6.0 × 10-4 4 __________________ _____________ 8.0 × 10-4 5 __________________ _____________ 10 × 10-4 6 __________________ _____________ 12 × 10-4 7 __________________ _____________ 14 × 10-4 Aliquot no. 1 Solution 4. Initial [S2O82-] = 0.10 M; initial [I-] = 0.025 M. Time (s) between appearances of color __________________ Cumulative time (s) _____________ Total moles of S2O82consumed 2.0 × 10-4 2 __________________ _____________ 4.0 × 10-4 3 __________________ _____________ 6.0 × 10-4 4 __________________ _____________ 8.0 × 10-4 5 __________________ _____________ 10 × 10-4 6 __________________ _____________ 12 × 10-4 7 __________________ _____________ 14 × 10-4 Aliquot no. 1 CALCULATIONS 1. Determine the slopes of the tangents and the corresponding rates of reactions. Complete the table below. Solution 1 [S2O82-]0 (M) 0.05 [I-]0 (M) 0.05 Tangent slope slope 1 = _________ mol/s Rate r1 = ____________ M/s 2 0.10 0.05 slope 2 = _________ mol/s r2 = ____________ M/s 3 0.05 0.10 slope 3 = _________ mol/s r3 = ____________ M/s 4 0.10 0.025 slope 4 = _________ mol/s r4 = ____________ M/s Lab Manual 23 CHE 1402 2. What effect does doubling the concentration of I- have on the rate of this reaction? (see table) 3. What effect does changing the [S2O82-] have on the reaction? (see table) 4. Determine the partial orders, x and y, and the overall order of the reaction. Show calculations 5. From your knowledge of the partial orders (as well as the rate in a given experiment), calculate the specific rate constant, k, from your data. Express the rate law of the reaction. ( Rate k S 2 O8 Show calculations Lab Manual I ). 2 X Y 24 CHE 1402 EXPERIMENT 5 An Equilibrium Constant OBJECTIVES To determine the equilibrium constant of a chemical reaction by spectrophotometric measurements. To use graphing techniques and data analysis to evaluate data. Relates to chapter 15 of “Chemistry the Central Science, 12th Ed.”. DISCUSSION A spectrophotometric method of analysis involves the interaction of electromagnetic radiation EM with matter. The most common regions of the EM spectrum used for analyses are the ultraviolet, visible, and the infrared regions. We are most familiar with the visible region of the spectrum, having a wavelength range from 400 to 800 nm. The colors of nature, the bluebonnet flowers, the red rocks, the green grass, and the changing colors of the leaves in fall are a consequence of visible light interacting with the compounds that are present in the material. Every chemical substance possesses its own unique set of electronic, vibrational, and rotational energy states. When EM radiation falls incident upon an atom or molecule, the radiation absorbed (the absorbed light) is an energy equal to the difference between two energy states in the atom or molecule, placing the atom or molecule in an "excited state". The remainder of the EM radiation passes through the sample (the transmitted light) and an EM radiation detector detects it. As the energy absorbed (and transmitted) equals the energy difference between the unique sets of energy states in an atom or molecule, absorption and emission spectrophotometry methods are used to detect its presence in a sample. The energy absorbed, E, by an atom or molecule is related to the wavelength, λ, of the EM radiation. Eh c h h is Planck's constant ,and is the frequency of light When a substance absorbs EM radiation from the visible region of the spectrum, it is usually an electron that is excited from a lower to a higher energy state. When white light (EM radiation containing all wavelengths of visible light) passes through the sample, our eyes detect the wavelengths of visible light not absorbed, i.e., the light transmitted. The light that is absorbed excites electrons of the sample. Therefore, the color we see is complementary to the one absorbed. If, for example, the atom or molecule absorbs energy from the violet region of the visible spectrum, the transmitted light (and the substance) appears yellow (violet's complementary color)-the higher the concentration of violet absorbing atoms or molecules, the more intense is the yellow. Thus the eye, one kind of EM radiation detector, detects only the transmitted light. Table 5.1 lists the colors corresponding to wavelength regions of light (and their complements) in the visible region of the EM spectrum. Lab Manual 25 CHE 1402 Table 5.1: Color and Wavelengths in the Visible Region of the Electromagnetic Spectrum Color Absorbed Wavelength (nm) Color Transmitted red 750-610 green-blue orange 610-595 blue-green yellow 595-580 violet green 580-500 red-violet blue 500-435 orange-yellow violet 435-380 yellow In this experiment, EM radiation is used to determine the concentration of an absorbing substance in an aqueous solution. The amount of transmitted light is measured using an instrument called a spectrophotometer, an instrument that measures light intensities with a photosensitive detector at specific (but variable) wavelengths. The wavelength at which maximum absorption of the EM radiation by the absorbing substance occurs is determined and set on the spectrophotometer. The ratio of the intensity of the transmitted light It to that of the incident light IO, is called the transmittance, T (Figure 5.2). This ratio, expressed as percent, is It 100% %T I0 [2] Figure 5.2 Incident light, Io, and transmitted light, It, for a sample of concentration c in a cuvet of thickness l. The spectrophotometer has a %T (percent transmittance of light) scale. Because it is linear, the %T scale is easy to read and interpolate. Chemists often perform calculations based on the amount of light absorbed by the sample, rather than the amount of light transmitted, because the extent of absorption is directly proportional to the concentration of the absorbing substance. The absorbance, A, of the substance is related to the intensity of the incident and transmitted light and the percent transmittance by the equation: A log I0 1 100 log log It T %T [3] Several factors control the amount of EM radiation (light energy) that a sample absorbs: - Concentration of the absorbing substance, c Lab Manual 26 CHE 1402 - Thickness of the sample containing the absorbing substance, l (determined by the width of the cuvet) - Probability of light absorption by the absorbing substance, ε (called the molar absorptivity coefficient or extinction coefficient) A l c This equation is commonly referred to as Beer's law. A is the absorbance, ε (the molar absorptivity coefficient) constant at any given wavelength for a thickness l, and c is the molar concentration of the absorbing substance. The absorbance value is directly proportional to the molar concentration of the absorbing substance, if the same (or a matched) cuvet and a set wavelength are used for all measurements. A plot of absorbance versus concentration data is linear; a calculated slope and absorbance data can be used to determine the molar concentration of the same absorbing species in an unknown solution. Measuring an Equilibrium constant The magnitude of an equilibrium constant, Kc, expresses the equilibrium position for a chemical system. For the reaction, aA + bB the mass action expression, X x Y y Aa Bb xX + yY equals the equilibrium constant, Kc, when a dynamic equilibrium has been established between reactants and products. The brackets in the mass action expression denote the molar concentration of the respective substances. The magnitude of the equilibrium constant indicates the principal species, products or reactants, that exist in the chemical system at equilibrium. For example, a large equilibrium constant indicates that the equilibrium lies to the right with a high concentration of products and correspondingly low concentration of reactants. The value of Kc is constant for a chemical system at a given temperature. This experiment determines Kc for a chemical system in which all species are soluble. The chemical system involves the equilibrium between iron(III) ion Fe3+, thiocyanate ion SCN-, and thiocyanatoiron(III) ion FeNCS2+ : Fe(H2O)63+ (aq) + SCN- (aq) Fe(H2O)5NCS2+ (aq) + H2O (l) [4] Because the concentration of water is essentially constant in dilute aqueous solutions, we omit the water of hydration and simplify the equation to read Fe3+ (aq) + SCN- (aq) FeNCS2+ (aq) [5] The mass action expression for the equilibrium system, equal to the equilibrium constant, is Kc FeNCS Fe SCN 2 2 In Part A you will prepare a set of standard solutions of the FeNCS2+ ion. As FeNCS2+ ion is a deep, bloodred complex with an absorption maximum at 447 nm, its concentration is determined spectrophotometrically. The absorbance for each solution is plotted versus the molar concentration of Lab Manual 27 CHE 1402 FeNCS2+; this establishes a calibration curve from which the concentrations of FeNCS2+ are determined for the chemical systems in Part B. In preparing the standard solutions, the Fe3+ concentration far exceeds the SCN-concentration. This huge excess of Fe3+ pushes the equilibrium (Equation 5) far to the right, nearly consuming all of the SCN- placed in the system. As a result the FeNCS2+ concentration at equilibrium approximates the original concentration. In other words, we assume that the position of the equilibrium is driven so far to the right by the excess Fe3+ that all of the SCN- is complexed, forming FeNCS2+ In Part B, the concentrations of the Fe3+ and SCN- ions in the various test solutions are nearly the same, thus creating equilibrium systems in which there is an appreciable amount of each of the species in the equilibrium. The chemical system is prepared by mixing known molar concentrations of Fe3+ and SCN-. By knowing the initial concentrations of Fe3+ and SCN-, and by measuring the equilibrium concentration of FeNCS2+ spectrophotometrically, the equilibrium concentrations of Fe3+ and SCN- are calculated. Using these equilibrium concentrations, the Kc for the system is calculated. At equilibrium, nFeNCS2+ = nFe3+ reacted = nSCN- reacted and [Fe3+]equilibrium = [Fe3+] initial _ [FeNCS2+]equilibrium [SCN-]equilibrium = [SCN-] initial _ [SCN-]equilibrium PROCEDURE One set of solutions having known molar concentrations of FeNCS2+ is prepared for a plot of absorbance versus concentration. A second set of standard solutions is prepared to determine unknown molar concentrations of FeNCS2+. By carefully measuring the initial amounts of reactants placed in the reaction systems, the mass action expression at equilibrium can be solved; this equals Kc. A. A Set of Standard Solutions to Establish a Calibration Curve These solutions are used to determine the absorbance of known molar concentrations of FeNCS2+. A plot of the data, known as a calibration curve, is used to determine the equilibrium molar concentrations of FeNCS2+, in Part B. Record on the Report Sheet the exact molar concentrations of the Fe(NO3)3 and NaSCN reagent solutions. 1. Prepare a Set of the Standard Solutions. Pipet 0, 2, 5, 10, and 15 mL of 0.002 M NaSCN into separate, labeled, and clean 100-mL volumetric flasks (Table 5.2). Pipet 25 mL of 0.2 M Fe(NO3)3 into each flask and dilute to the 100 mL "mark" with 0.25 M HNO3. Stir each solution thoroughly to ensure that equilibrium is established. Lab Manual 28 CHE 1402 Table 5.2: Compositions of Standard Solutions for Preparing the Calibration Curve. Solution 1(blank) 2 3 4 5 0.002 M NaSCN (in 0.25 M HNO3) 0 mL 2 mL 5 mL 10 mL 15 mL 0.200 M Fe(NO3)3 (in 0.25 M HNO3) 25 mL 25 mL 25 mL 25 mL 25 mL 0.25 M HNO3 [Fe3+]0 [SCN-]0 [FeSCN2+]equ dilute to 100 mL dilute to 100 mL dilute to 100 mL dilute to 100 mL dilute to 100 mL (mol/L) 5ₓ10-2 5ₓ10-2 5ₓ10-2 5ₓ10-2 5ₓ10-2 (mol/L) 0 4ₓ10-5 10ₓ10-5 20ₓ10-5 30ₓ10-5 (mol/L) 0 4ₓ10-5 10ₓ10-5 20ₓ10-5 30ₓ10-5 2. Initial set-up Turn on the spectrophotometer and let the light source (Tungsten halogen lamp) warm up for 10 minutes. Then, set the wavelength at 447 nm by using the UP and DOWN arrow keys (Figure 5.3). 2. Prepare the Blank Solution. Solution 1 is called the blank solution and will be used to calibrate the spectrophotometer. Rinse a cuvet with several portions of Solution 1. Dry the outside of the cuvet with a clean Kimwipe or Joseph paper, removing water and fingerprints. Handle the lip of the cuvet thereafter. 3. Calibrate the Spectrophotometer. Open the sample compartment and insert the cuvet containing your blank solution. Select the Absorbance mode by moving the cursor to the ABS mode using the LEFT or RIGHT arrow keys (Figure 5.3). The primary display will show the absorbance, with ABS units. Press the CAL key to initiate the calibration routine. First the routine performs a zero% transmission calibration (by automatically activating an internal shutter this part of the routine is therefore independent of the solution in the light path) and the instrument will display overrange absorbance “1. ABS” during this dark calibration. Then the instrument performs the 0.000 Absorbance calibration on your blank solution and will display “0.000 ABS”. 4. Record the Absorbance of the Standard Solutions. Empty the cuvet and rinse it thoroughly with several portions of Solution 2. Fill it approximately threefourths full. Carefully dry the outside of the cuvet with a clean Kim wipe. Remember, handle only the lip of the cuvet. Place the cuvet into the sample compartment; read the absorbance and record. Repeat with Solutions 3, 4, and 5. Disposal: Discard each test solution and each rinse into the appropriate disposal container. 5. Graph the Data. Plot, on linear graph paper, absorbance A (ordinate) versus [FeNCS2+] (abscissa) for the five solutions. Draw the best straight line through the five points and the origin. Ask your instructor to approve your graph. Record on the Report Sheet the exact molar concentrations of the Fe(NO3)3 and NaSCN reagent solutions. Lab Manual 29 CHE 1402 1. used to adjust values on the selected display 2. used to move the cursor horizontally between menu options 3. used to select the displayed menu option 4. initiates a calibration routine 5. Print key. Provides a printout of the current reading with an incremental sample number. When pressed for the first time after a calibration the print out will give calibration information. The incremental sample number will be reset after a calibration. 1. Primary display area - Transmission, Absorbance, Concentration 2. Primary display adjust annunciator 3. Secondary display area - Wavelength, Factor 4. Primary display units 5. Secondary display adjust annunciator 6. Operation with PC 7. Menu options - %T ABS CONC FACTOR UNITS 8. Menu pointers (for 7) Figure 5.3: Jenway 6320D Spectrophotometer employed in this experiment and its controls. B. Absorbance of the test solutions 1. Prepare the Test Solutions. In clean 150-mm test tubes (or 10-mL volumetric flasks), prepare the test solutions in Table 5.3. Use pipets for the volumetric measurements. Be careful not to “mix” pipets to avoid contamination of the reagents prior to the preparation. Also note that the molar concentration of Fe(NO3)3 for this set of solutions is 0.002 mol/L, not the 0.2 mol/L solution used in Part A. Lab Manual 30 CHE 1402 Table 5.3 Compositions of Test Solutions for determination of Kc. Solution 1’ 2’ 3’ 4’ 5’ 0.002 M NaSCN (in 0.25 M HNO3) 1 mL 2 mL 3 mL 4 mL 5 mL 0.002 M Fe(NO3)3 (in 0.25 M HNO3) 4 mL 5 mL 5 mL 5 mL 5 mL 0.25 M HNO3 [Fe3+]0 [SCN-]0 dilute to 10 mL dilute to 10 mL dilute to 10 mL dilute to 10 mL - (mol/L) 8ₓ10-4 10ₓ10-4 10ₓ10-4 10ₓ10-4 10ₓ10-4 (mol/L) 2ₓ10-4 4ₓ10-4 6ₓ10-4 8ₓ10-4 10ₓ10-4 2. Recalibrate the Spectrophotometer. Use the blank solution (Solution 1) from Part A to check the 0.000 Absorbance. 3. Determine the Percent Transmittance of the Test Solutions. Stir each test solution until equilibrium is reached (approximately 1 minute). Rinse the cuvet thoroughly with several portions of the test solution and fill it three-fourths full. Clean and dry the outside of the cuvet. Be cautious in handling the cuvets. Record the absorbance of each test solution. Disposal. Dispose of the waste thiocyanatoiron (III) ion solutions from the 100-mL volumetric flasks and the cuvets in the appropriate waste container. CLEANUP: Rinse the volumetric flasks, the pipets, and the cuvets twice with tap water and once with deionized water. Discard each rinse in the sink. 4. Use Data to Determine Equilibrium Concentrations. Using the calibration curve prepared in Part A.5 determine the equilibrium molar concentration of FeNCS 2+, for each test solution. 5. Do the Calculations. Complete the calculations as outlined on the Report Sheet. Complete an entire Kc calculation for Test Solution 1’ (Part B) before attempting the calculations for the remaining solutions. The equilibrium constant varies from solution to solution and from chemist to chemist in this experiment, depending on chemical technique and the accumulation and interpretation of the data. Consequently, it is beneficial to work with other colleagues through your own calculations, and then "pool" your final, experimental K, values to determine an accumulated "probable" value. Lab Manual 31 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. What effect does a dirty cuvet (caused by fingerprints, water spots, or lint) have on the percent transmittance reading for a FeNCS2+ solution? Does this error cause the Kc to be reported as being too high or too low? Explain. 2. In our calculations, the thickness of the solution (the cuvet) and the molar absorptivity of FeNCS 2+ are not considered. Explain. 3. If the percent transmittance reads less than 3.0%T on the spectrophotometer, how can the procedure be modified to obtain a higher %T reading? 4. Over a period of time the 0 %T (no sample in the sample compartment) and the l00%T(blank solution in the sample compartment) may drift from the initial calibration of the spectrophotometer. If the 100% T reading drifts downward (less than 100%T), how does his error affect, in Part B, the : a. absorbance readings? b. [FeNCS2+], equilibrium? c. [Fe3+], equilibrium? d. [SCN], equilibrium? Lab Manual 32 CHE 1402 Experiment 5 An Equilibrium Constant Name(s) Date Laboratory Instructor REPORT SHEET Vsolution = ________ L Solutions 1 2 3 4 5 Comment(s) [Fe3+]0 Ci ₓ Vi = [Fe3+]0 ₓVsolution [SCN-]0 Volume of Fe(NO3)3 (mL) Ci’ ₓ Vi‘= [SCN-]0 ₓ Vsolution / Moles of Fe3+ Volume of NaSCN (mL) Moles of SCN- (at t0) = (L) ₓ [SCN-]0 Absorbance : spectrophotometer [Fe(NCS2+], equilibrium : calibration curve Moles of Fe3+, reacted = ₓ Vsolution (L) Moles of Fe3+, equilibrium =- [ Fe3+], equilibrium = ÷ Vsolution (L) Moles of SCN- , reacted = = ₓ Vsolution (L) 11 Moles of SCN- , equilibrium 11 =- 12 [SCN-], equilibrium 12 = 11 ÷ Vsolution (L) 13 = ÷ ( ₓ 13 Kc 2 Lab Manual 5.0 5.0 5.0 5.0 = (L) ₓ [Fe3+]0 (at t0) FeNCS eq Fe eqSCN eq 3 4.0 1.0 2.0 3.0 4.0 5.0 / 12 ) 33 CHE 1402 EXPERIMENT 6 Titration Curves of Polyprotic Acids OBJECTIVE Determination of dissociation constants of a polyprotic acid. Relates to chapter 16 of “Chemistry the Central Science, 12th Ed.”. APPARATUS AND CHEMICALS pH meter with electrodes potassium acid phthalate (KHP) sodium hydroxide solution about 0.1 M 100 mL of approx. 0.1M phosphoric acid standard buffer solution phenolphthalein indicator solution weighing bottle buret clamp, and ring stand 25-mL pipet 150-mL beaker (3) 250-mL beaker DISCUSSION Consider the triprotic acid H3PO4 .It undergoes the following dissociations in aqueous solution: H3PO4 (aq) H2PO4- (aq) + H+ (aq) H2PO4- (aq) HPO42- (aq) + H+ (aq) H PO H Ka1 2 H 3 PO4 HPO H H PO PO H HPO 2 Ka 2 PO43- (aq) + H+ (aq) [2] 4 3 Ka3 [1] 4 2 HPO42- (aq) 4 4 2 [3] 4 The acid H3PO4 possesses three dissociable protons, and for this reason it is termed a triprotic acid. If you were to perform a titration of H3PO4 with NaOH, the following reactions would occur: H3PO4 + NaOH NaH2PO4 + H2O [4] NaH2PO4 + NaOH Na2HPO4 + H2O [5] Na2HPO4 + NaOH Na3PO4 + H2O [6] The resultant titration curve, when plotted as pH versus milliliters of NaOH added, would be similar to that shown in Figure 6.1. At the point at which one-half of the protons in the first dissociation step of H3PO4 have been titrated with NaOH, the H3PO4 concentration is equal to the H2PO4- concentration. Substituting [H3PO4] = [H2PO4-] into Equation [1] yields Ka1 = [H+], or pH = pKa1 at this point. Similarly, at one-half the second equivalence point, one-half of the H2PO4- has been neutralized and [H2PO4-] = [HPO42-]. Substituting this into Equation [2] yields Ka2 = [H+] or pKa2 = pH at this point. In the same manner, at one-half the third equivalence point, [HPO42-] = [PO43-]. Substituting this into Equation [3], we obtain the expression Ka3 = [H+], or pKa3 = pH. Lab Manual 34 CHE 1402 The same type of result is obtained for any polyprotic acid. If a titration of the acid is performed with a pH meter, the dissociation constants may be obtained from titration curves as long as the dissociation constants exceed the ion product of water Kw, which you should recall is 10-14 for the reaction. 2 H2O H3O+ + OH- Kw =10-14 In practice, if the acidity of the acid being studied approaches that of water, as in the case for the third proton of H3PO4 for which Ka3, is 4.2 × 10-13 it is difficult to determine the dissociation constant in this manner. Thus for H3PO4, both Ka1 and Ka2 are readily obtained in this way, but Ka3 is not. In this experiment you will determine the dissociation constants Ka1 and Ka2 of a phosphoric acid. PROCEDURE 1. Standardize the pH meter. 2. Fill your buret with 0.1 M NaOH solution. 3. Titrate three separate 50-mL aliquots of the phosphoric acid in three separate 250-mL beakers and plot the titration curves. 4. Determine the volumes necessary to reach the equivalence points. 5. Using the relations given above, and the relevant equations from this experiment, determine the K a and pKa and values for phosphoric acid. HINT: You can save time if you do your first titration rapidly so that you know the approximate volumes of the equivalence points; then you can do the next two titrations with large-volume increment away from the equivalence points and small-volume increments near the equivalence points). Figure 6.1: Titration curve of H3PO4. Lab Manual 35 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. What is a polyprotic acid? 2. If 20.2 mL of 0.122 M NaOH is required to reach the first equivalence point of a solution of citric acid (H3C6H5O7), how many mL of NaOH are required to completely neutralize this solution? 3. How many mmol of NaOH will react with 50 mL of 6.2 M H2C2O4? 4. How many moles of H3O+ are present in 50 mL of a 0.3 M solution of H2SO4? 5. Why is it necessary to standardize a pH meter? 6. If the pH at one-half the first and second equivalence points of a dibasic acid is 3.52 and 6.31, respectively, what are the values for pKa1 and pKa2? From pKa1 and pKa2 calculate the Ka1 and Ka2. 7. Derive the relationship between pH and pKa at one-half the equivalence point for the titration of a weak acid with a strong base. 8. Could Kb for a weak base be determined in the same way that Ka for the weak acid determined in this experiment? 9. If the Ka1 of a diprotic acid is 3.20, what is the pH of a 0.10 M solution of this acid? Lab Manual 36 CHE 1402 Experiment 6 Titration Curves of Polyprotic Acids Name(s) Date Laboratory Instructor REPORT SHEET Determination of pKa values of H3PO4 VNaOH (mL) pH VNaOH (mL) pH pKa1 __________ pKa2 __________ Ka1 __________ Ka2 Lab Manual VNaOH (mL) pH __________ 37 CHE 1402 EXPERIMENT 7 Determination of the Solubility-Product Constant for a Slightly Soluble Salt OBJECTIVE Determination of the value of the solubility-product constant for a slightly soluble salt. Relates to chapter 17 of “Chemistry the Central Science, 12th Ed.”. APPARATUS AND CHEMICALS Buret Ring stand and buret clamp Centrifuge 75-mm test tubes (3) Spectrophotometer and cuvets 5-mL pipets (2) 0.0024 M K2CrO4 0.004 M AgNO3 0.25 M NaNO3 100-mL volumetric flasks (4) no.1 corks (3) DISCUSSION Acids, bases, and salts are classified as inorganic substances. When an acid reacts with a base in aqueous solution, the products are a salt and water, as illustrated by the reaction of H2SO4 and Ba(OH)2: H2SO4 (aq) + Ba(OH)2 (aq) BaSO4 (s) + 2 H2O (l) [1] In general most common salts are strong electrolytes. The solubilities of salts span a broad spectrum, ranging from slightly soluble to very soluble. This experiment is concerned with heterogeneous equilibria of slightly soluble salts. In order for a true equilibrium to exist between a solid and solution, the solution must be saturated. For instance barium sulfate is a slightly soluble salt, and in a saturated solution this equilibrium may be rewritten as: BaSO4 (s) Ba2+ (aq) + SO42- (aq) [2] The equilibrium constant for Equation [2] is: Ksp = [Ba2+] [SO42-] [3] The value of Ksp is a constant at constant temperature. The solubility product for a slightly soluble salt can easily be calculated by determining the solubility of the substance in water. Suppose, for example, we determined that 2.42 × 10-4 g of BaSO4 dissolves in 100 mL of water. The molar solubility of this solution (that is, the molarity of the solution) is 1.04 × 10-5 M. Lab Manual 38 CHE 1402 s 2.42 10 g 2.42 10 g 233.39 g / mol mol 1.04 10 4 4 100 mL 0.100 L Ksp = s × s = s2 = (1.04 × 10-5)2 = 1.08 × 10-10 5 M at 25oC In a saturated solution the product of the molar concentrations of Ba2+ and SO42- cannot exceed 1.08 × 10-10 at 25oC. If the ion product [Ba2+][SO42-] exceeds 1.08 × 10-10 at 25oC, precipitation of BaSO4 would occur until this product is reduced to the value of Ksp or if, for example, a solution of Na2SO4 is added to a solution of Ba(NO3)2, BaSO4 would precipitate if the ion product [Ba2+]×[SO42-] is greater than Ksp. If we determined that the solubility of Ag2CO3 were 3.49mg/100mL, we could calculate the solubility-product constant for Ag2CO3 as follows: The solubility equilibrium involved is Ag2CO3 (s) 2 Ag+ (aq) + CO32- (aq) [4] and the corresponding solubility-product expression is Ksp = [Ag+]2 [CO32-]. The solubility of Ag2CO3 in moles per liter is: 3.49 10 3 g g s 276.6 1.27 10 4 M 3 mol 100 10 L [CO32-] = 1.27 × 10-4 M ; [Ag+]= 2 × 1.27×10-4 = 2.54 × 10-4 M Ksp= [Ag+]2 [CO32-] = [2.54 × 10-4]2 [1.27 × 10-4] = 8.19 × 10-12 M3 To determine the solubility-product constant for a slightly soluble substance, we need only to determine the concentration of one of the ions since the concentration of the other ion is related to the first ion's concentration by a simple stoichiometric relationship. In this experiment you will determine the solubility-product constant for Ag2CrO4. This substance contains the yellow chromate ion, CrO42- that should be determined experimentally by spectrophotometric measurement at 375 nm. To determine the solubility of Ag2CrO4, you will first prepare it by the reaction of AgNO3 with K2CrO4: 2 AgNO3 (aq) + K2CrO4 (aq) 15 min Ag2CrO4 (s) 15 min + 2 KNO3 (aq) 2 Ag+ (aq) + CrO42- (aq) Ksp = ? If a solution of AgNO3 is added to a solution of K2CrO4, precipitation will occur if the ion product [Ag+]2 [CrO42-] numerically exceeds the value of Ksp if not, no precipitation will occur. Then, the Ag2CrO4 formed is isolated by simple decantation and a new equilibrium is established between solid Ag2CrO4 and Ag+ and CrO42- ions. Ksp = [Ag+]equ2 [CrO4 2-] equ. Lab Manual 39 CHE 1402 PROCEDURE A. Preparation of a Calibration Curve Using a buret, add 1, 5, 10, and 15 mL of standardized 0.0024 M K2CrO4 to each of four clean, dry 100 mL volumetric flasks and dilute to the 100 mL mark with 0.25 M NaNO3. 1. Calculate the CrO42-concentration in each of these solutions. 2. Measure the absorbance of these solutions at 375 mn. 3. Plot the absorbance versus concentration to construct your calibration curve. B. Determination of the Solubility-Product Constant Accurately prepare three separate solutions in separate 150-mm test tubes by adding 5 mL of 0.004 M AgNO3 to 5 mL of 0.0024 M K2CrO4. Stopper each test tube and shake the solutions thoroughly at periodic intervals for about 15 min to establish equilibrium between the solid phase and the ions in solution. Transfer the contents of each test tube into 75-mm test tubes and centrifuge. Discard the supernatant liquid and retain the precipitate. To each of the test tubes add 2 mL of 0.25 M NaNO3. Shake each test tube thoroughly for another 15 minutes to establish equilibrium between the solid and the solution and centrifuge again. There must be some solid Ag2CrO4 remaining in these test tubes. If there is not, start over again. Transfer the clear, pale yellow supernatant liquid from each of the three test tubes to a clean, dry cuvette. Measure and record the absorbance of the three solutions. Using your calibration curve, calculate the molar concentration of CrO42- in each solution. Note on Calculations: You should note that at equilibrium [Ag+]eq = 2[CrO42-]eq. Therefore, having determined the concentration of chromate ions, you know the silver-ion concentration Lab Manual 40 CHE 1402 REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1 Write the solubility equilibrium and the solubility-product constant expression for the slightly soluble salt CaF2. 2 Calculate the number of moles of Ag+ in 5 mL of 0.004 M AgNO3 and the number of moles of 2CrO4 in 5 mL of 0,0024 M K2CrO4, If 10 mL of 0,004 M AgNO3 is added to 10 mL of 0,0024 M K2CrO4' is either Ag+ or CrO42- in stoichiometric excess? If so, which is in excess? 3 4. The Ksp for BaCrO4 is 1.2 × 10-4-10. Will BaCrO4 precipitate upon mixing 10 mL of 1 × 10-4 M Ba(NO3)2 with 10 mL of 1 × 10 M K2CrO4? 5. The Ksp for BaCO3 is 5.1 × 10-9. How many grams of BaCO3 will dissolve in 100 mL of water? 6. Distinguish between the equilibrium-constant expression and Ksp for the dissolution of a sparingly soluble salt. 7. List as many experimental techniques as you can that may be used to determine Ksp for a sparingly soluble salt. 8. Why must some solid remain in contact with a solution of a sparingly soluble salt in order to ensure equilibrium? 9. In general, when will a sparingly soluble salt precipitate from solution? 10. Sparingly soluble bases and salts, such as Fe(OH)2 and FeCO3 are more soluble in acidic than in neutral solutions. Why? Lab Manual 41 CHE 1402 Experiment 7 Determination of the Solubility-Product Constant for a Slightly Soluble Salt Name(s) Date Laboratory Instructor REPORT SHEET A. Calibration Curve Initial [CrO42-] = 0.0024 M Volume of 0.0024 M K2CrO4 Total volume of CrO42- Final [CrO42-] Absorbance Molar absorption coefficient (M-1 cm-1) (M) 1 1 mL 100 mL _________ _______________ ____________ 2 5 mL 100 mL _________ _______________ ____________ 3 10 mL 100 mL _________ _______________ ____________ 4 15 mL 100 mL _________ _______________ ____________ εaverage = ____________ B. Determination of Ksp Absorbance [CrO42-] [Ag+] Ksp of Ag2CrO4 1 ____________ ____________ ____________ ____________ 2 ____________ ____________ ____________ ____________ 3 ____________ ____________ ____________ ____________ Ksp average = ____________ Lab Manual 42 CHE 1402 EXPERIMENT 8 Molar Solubility, Common-ion Effect OBJECTIVES To determine the molar solubility and the solubility constant of calcium hydroxide. To study the effect of a common ion on the molar solubility of calcium hydroxide. Relates to chapter 17 of “Chemistry the Central Science, 12th Ed.”. DISCUSSION Salts that have a very limited solubility in water are called slightly soluble (or "insoluble") salts. A saturated solution of a slightly soluble salt is a result of a dynamic equilibrium between the salt and its ions in solution; however, because the salt is only slightly soluble, the concentrations of the ions are low. For example, in a saturated silver sulfate, Ag2SO4, solution, the dynamic equilibrium between solid Ag2SO4, the Ag+ and SO42- ions in solution lies far to the left because of the low solubility of silver sulfate. Ag2SO4 (s) 2 Ag+ (aq) + SO42- (aq) for which the solubility product Ksp is: [1] Ksp = [Ag+]2 [SO4 2-] What happens to the molar solubility of a salt when an ion, common to the salt, is added to the saturated solution? According to Le Chatelier's principle, the equilibrium for the salt shifts to compensate for the added ions; that is, it shifts left to favor the formation of more of the solid salt. This effect, the addition of an ion common to an existing equilibrium, is called the common-ion effect. As a result of the common ion addition and the corresponding shift in the equilibrium, fewer moles of the salt dissolve in solution, lowering the molar solubility of the salt. The molar solubility of a salt is the number of moles of that salt that dissolves per liter of (aqueous) solution. In this experiment, you will determine the molar solubility and the solubility constant for calcium hydroxide, Ca(OH)2. A saturated Ca(OH)2 solution is prepared; after an equilibrium is established between the solid Ca(OH)2 and the Ca 2+ and OH- ions in solution, the decanted solution is analyzed. The hydroxide ion, OH-, in the solution is titrated with a standardized HCl solution to determine its molar concentration. According to the following equation: Ca(OH)2 (s) Ca2+ (aq) + 2 OH- (aq) [2] For each mole of Ca(OH)2 that dissolves, 1 mole of Ca2+ and 2 moles of OH- are present in solution. Therefore, by determining the molar concentration of hydroxide ion, [Ca2+], Ksp, and the molar solubility s of Ca(OH)2(s) can be calculated. Ca 2 OH2 s ; Ksp Ca OH Lab Manual 2 2 43 CHE 1402 Likewise, we can use the same procedure to determine the molar solubility of Ca(OH) 2 in the presence of added calcium ion, an ion common to the slightly soluble salt equilibrium. PROCEDURE The decantate from a saturated calcium hydroxide solution is titrated with a standardized hydrochloric acid solution to the methyl orange endpoint. An analysis of the data results in the determination of the molar solubility and solubility constant of calcium hydroxide. The procedure is repeated on a decantate from a saturated calcium hydroxide solution containing added calcium ion. A. Molar Solubility and Solubility Constant of Calcium Hydroxide Three analyses are to be completed. To hasten the analyses in Parts A.4 and A.5, prepare three, clean labeled 125- or 250-mL Erlemneyer flasks and pipet 25 mL of the saturated Ca(OH)2 solution into each flask before you begin any titration. 1. Prepare the Stock Calcium Hydroxide Solution. Prepare a saturated Ca(OH)2 solution 1 week before the experiment by adding approximately 3 g of Ca(OH)2 to 120 mL of boiled, deionized water in a 125-mL Erlenmeyer flask. Stir the solution and stopper. The resulting saturated solution of calcium hydroxide is called limewater. This solution may have been prepared for you. Ask your instructor. 2. Set Up the Titration Apparatus. Prepare a clean, 50-mL buret for titration. Rinse the clean buret and tip with three 5-mL portions of the standard 0.05 M HCl solution and discard. Fill the buret with standardized 0.05 M HCl, remove the air bubbles in the buret tip, and, after 30 seconds, read and record the initial volume (± 0.02 mL). Record the actual concentration of the 0.05 M HCl on the Report Sheet. Place a sheet of white paper beneath the receiving flask. 3. Transfer the Saturated Calcium Hydroxide Solution. Allow the solid Ca(OH)2 to remain settled on the bottom of the flask (in Part A. 1). Carefully (try not to disturb the finely divided Ca(OH)2 solid) decant about 90 mL of the saturated Ca(OH)2 solution into a second 125-mL flask. 4. Prepare a Sample for Analysis. Rinse a 25-mL pipet twice with 1- to 2-mL portions of the saturated Ca(OH)2 solution and discard. Pipet 25 mL of the saturated Ca(OH)2 solution into a 125mL flask and add 2 drops of methyl orange indicator. 5. Titrate. Titrate the Ca(OH)2 solution to the methyl orange endpoint, where the color changes from yellow (=basic pH) to red (=acidic pH). Remember the addition of HCl should stop within onehalf drop of the endpoint. Read (± 0.02 mL) and record the final volume of standard HCl in the buret. 6. Repeat. Titrate two additional samples of the saturated Ca(OH)2 solution until a 0.50 % (maximum) reproducibility is achieved. reproducibility Lab Manual x 100 1 2 xi x n 100 x 44 CHE 1402 Example: After 3 trials, you get the following volumes of equivalence: V1= 10.15 mL, V2= 10.05 mL, V3= 10.15 mL. n3 x 10.12 mL reproducibility 1 2 2 2 10.15 10.12 10.05 10.12 10.15 10.12 3 100 0.47% 10.12 7. Do the Calculations. The reported values for the Ksp of Ca(OH)2 will vary from chemist to chemist. Complete your calculations as outlined on the Report Sheet. B. Molar Solubility of Calcium Hydroxide in the Presence of a Common Ion Again, three analyses are to be completed. Clean and label three 125- or 250-mL Erlenmeyer flasks. Prepare all three of the Ca(OH)2-CaCI2 samples at the same time. 1. Prepare the Stock Solution. Mix 3 g of Ca(OH)2 and 1 g of CaCl2.2H2O with 120 mL of boiled, deionized water in a 125-mL flask 1 weeks before the experiment. Stir and stopper the flask. 2. Prepare a buret for analysis, prepare the sample, and titrate. Repeat Parts A.2-A.6. Disposal: Discard all of the reaction mixtures in the sink, followed by a generous supply of water. CLEANUP: Discard the HCl solution in the buret into the sink. Rinse the buret twice with tap water and twice with deionized water. REVIEW QUESTIONS 1. How did the addition of CaCl2 affect the molar solubility of Ca(OH)2? 2. a. In Part A.3, suppose that some solid Ca(OH)2 was accidentally transferred to the titrating flask. What effect does this error have on the reported Ks value? b. As a result of the inadvertent transfer, will the calculated molar solubility for Ca(OH)2 be too high or too low? Explain. 3. If the endpoint in the titration is surpassed in Part A.5, will the reported Ks value be too high or too low? Explain. 4. Does adding boiled, deionized water to the titrating flask to wash the wall of the flask and the buret tip affect the Ks value of the Ca(OH)2? Explain. 5. How will tap water instead of boiled, deionized water affect the Ks value of Ca(OH)2 in Part A? Hint: How will the minerals in the water affect the solubility of Ca(OH)2 ? Lab Manual 45 CHE 1402 Experiment 8 Molar Solubility, Common-ion Effect Name(s) Date Laboratory Instructor REPORT SHEET A. Molar Solubility and Solubility Constant of Calcium Hydroxide Trial 1 1 Concentration of standard HCl solution (M) 2 Buret reading, initial (mL) 3 Trial 2 Trial 3 _______________ 0 0 0 Buret reading,final (mL) ________ ________ ________ 4 Volume of HCl used (mL) ________ ________ ________ 5 Amount of HCl added (mol) ________ ________ ________ 6 Amount of OH- in satd. solution (mol) ________ ________ ________ 7 Volume of satd. Ca(OH)2 solution (mL) 25.0 25.0 25.0 8 [OH-], equilibrium (M) ________ ________ ________ 9 [Ca2+] equilibrium (M) ________ ________ ________ 10 Molar solubility of Ca(OH)2 (M) , s ________ ________ ________ 11 Average molar solubility of Ca(OH)2 (M) 12 Ksp of Ca(OH)2 13 Average Ksp of Ca(OH)2 Lab Manual _______________ ________ ________ ________ _______________ 46 CHE 1402 Molar Solubility of Calcium Hydroxide in the Presence of a Common Ion B. Trial 1 1 Concentration of standard HCl solution (M) 2 Buret reading, initial (mL) 3 Trial 2 Trial 3 _______________ 0 0 0 Buret reading,final (mL) ________ ________ ________ 4 Volume of HCl used (mL) ________ ________ ________ 5 Amount of HCl added (mol) ________ ________ ________ 6 Amount of OH- in satd. solution (mol) ________ ________ ________ 7 Volume of satd. Ca(OH)2/CaCl2 solution (mL) 25.0 25.0 25.0 8 [OH-], equilibrium (M) ________ ________ ________ 9’ Molar solubility of Ca(OH)2 (M) , s’ ________ ________ ________ 10’ Average molar solubility of Ca(OH)2/CaCl2 (M) Lab Manual _______________ 47 CHE 1402 EXPERIMENT 9 Determination of Orthophosphate in Water OBJECTIVE To gain some familiarity with the techniques of spectrophotometric analysis by analysing a water solution for its phosphate content. APPARUTUS & CHEMICALS water sample KH2PO4 (oven-dried) conc. H2SO4 ammonium vanadomolybdate solution cuvets balance spectrophotometer 1-L volumetric flask l-, 5-, and 10-mL pipets 100-mL volumetric flasks (6) graduated 5-mL pipet CHCl3 DISCUSSION Tripolyphosphates have been found to be extremely effective in enhancing the cleansing ability of detergents; they also are very inexpensive. Their aid in cleaning is probably due, in part, to the stable complexes that they form with Ca2+ and Mg2+, thus softening the water. Their extensive use, however, has very serious side effects on nature. The accelerated eutrophication, or overfertilization, of our lakes has aroused a great deal of ecological concern. Nutrient enrichment enhances the growth of algae and other microscopic organisms. This produces the green scum of an algal bloom on the water surface, masses of waterweeds, and a depletion of dissolved oxygen; it also kills fish and other aquatic organisms and produces malodorous water systems. When the photosynthetically active algae population near a lake's surface rapidly expands, most of the oxygen produced escapes to the atmosphere. After the algae die, they sink to the lake bottom, where they are biochemically oxidized. This depletes the dissolved oxygen needed to support aquatic life. When oxygen is removed, anaerobic decomposition of the algae continues, producing foul odors. Although many factors affect algae growth, the only one that is readily subject to preventive control is the supply of nutrients. The many nutrients important to the growth of algae include phosphorus, carbon, nitrogen, sulfur, potassium, calcium, and magnesium. Many environmentalists have accepted the idea that phosphorus is generally the key nutrient that limits the plant growth that a body of water can support. There are at least four major sources of phosphorus associated with human activity: human and food wastes, fertilizers, industrial wastes, and detergents. Although detergent products contribute only about one-third of the phosphates entering our water systems, curtailing this particular source is a logical place to begin to combat eutrophication. The phosphate found in natural waters is present as orthophosphate, PO43-, as well as the polyphosphates P2O7-4 and P3O105-. The species present, PO43-, HPO42-, H2PO4-, or H3PO4, depend on the pH. Trace amounts are also present as organophosphorus compounds. Detergents usually contain triphosphate, P3O105- which slowly hydrolyses to produce orthophosphate, PO43-, according to the following reaction: P3O105- + 2 H2O 3 PO43- + 4 H+ In this experiment you will determine the amount of orthophosphate present in a sample of water of unknown concentration. Analytical Method In dilute phosphate solutions ammonium metavanadate, (NH4VO3), molybdate (MoO42-), and phosphate (PO43-) condense to form an intensely yellow colored compound called a heteropoly-acid, whose formula Lab Manual 48 CHE 1402 is thought to be (NH4)3PO4 NH4VO316MoO3. The intensity of the yellow color is directly proportional to the concentration of phosphate. The relative amount of color developed is measured with a spectrophotometer. The amount of light absorbed by the spectrophotometer is directly proportional to the concentration of the colored substance. This is stated by Beer’s law, also known as Beer-Lambert law: A l c A is the absorbance, ε (the molar absorptivity coefficient, in M-1 cm-1) constant at any given wavelength for a thickness l (in cm), and c is the molar concentration of the absorbing substance. The colored solutions that you study in this experiment have been found to obey the Beer-Lambert law in the region of wavelengths ranging from 350 nm to 410 nm. It is convenient to run this experiment at 400 nm, wavelength at which maximum absorption is detected for you solute. The amount of phosphate in the unknown sample of interest is determined by comparison with a calibration curve constructed by using a distilled water reference solution and solutions of known phosphate concentrations. The minimum detectable concentration of phosphate is about 0.01 mg/L (10 ppb). The usual experimental precision will lie within about ±l percent of the result obtained by an experienced analyst. Comparative Phosphate Levels in Water Systems Limiting nutrients and their critical concentrations are likely to differ in different bodies of water. Analysis of the waters of 17 Wisconsin lakes has led to the suggestion that an annual average concentration of 0.015 mg/L of inorganic phosphorus (0.05 mg phosphate/L) is the critical level above which algal blooms can be expected if other nutrients, such as nitrogen, are in sufficient supply. During the 1968-69 period, Lake Tahoe in Nevada had an average phosphate level of 0.006 mg/L, while its tributaries averaged 0.08mg/L. Lake Tahoe is one of the two purest lakes in the world. The other is Lake Baikal in Russia. This figure thus represents the lowest natural-water value of phosphate one is likely to find. In July of 1969 the phosphate level of Lahontan Reservoir (about 55 km east of Reno, Nevada) was 0.52 mg/L. By comparison, Lake Erie's phosphate level increased from 0.014mg/L in 1942 to 0.40 mg/L in 196768. The U.S. Public Health Service has set 0.1mg/L of phosphorus (0.3 mg phosphate/L) as the maximum value allowable for drinking water. Raw sewage contains an average of about 30 mg/L of orthophosphate, of which about 25 percent is removed by most secondary sewage-treatment plants. PROCEDURE A. Preparation of Calibration Curve Dissolve about 136 mg of oven-dried KH2PO4 (weigh accurately) in about 500 mL of water. Quantitatively transfer this solution to a 1-L volumetric flask, add 0.5 mL of 98% H2SO4, and dilute to the mark with distilled water. This yields a stock solution that is about 1×10-3 M in various phosphate species. From this stock solution prepare a series of six solutions with phosphate concentrations 2×10-5, 5×10-5, 1×10-4, 2×10-4, 5×10-4, and 7.5×10-4 M by appropriate dilution of the stock solution. You must know the precise concentrations of these solutions. Each point on the calibration curve is obtained by mixing 10 mL of the phosphate solution with 5 mL of the ammonium vanadomolybdate solution (see note below) and measuring the absorbance on the spectrophotometer at 400 nm. Your curve is constructed by plotting absorbance as the ordinate versus concentrations of phosphate as the abscissa. A straight line passing through the origin should be obtained. Your calibration curve should be handed in with your report sheet. B. Analysis of Water Sample The unknown samples (A, B, and C) to be analyzed are stored in three test tubes and tightly stoppered. Lab Manual 49 CHE 1402 Add 5 mL of the ammonium vanadomolybdate solution to 10 mL of the unknown and measure the absorbances at 400 nm. Note: Preparation of Ammonium vanadomolybdate solution: Dissolve 40 g of ammonium molybdate (molybdic acid, 85% MoO3) in about 400 mL of distilled water. Dissolve 1 g of ammonium metavanadate, NH4VO2 in about 300 mL of distilled water and add 200 mL of concentrated nitric acid. Mix the two solutions and dilute to 1 L. This solution is stable for about 90 days and will be provided for your analysis. REVIEW QUESTIONS Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. What volume of 1×10-3 M solution is required to make 50 mL of solution with the following concentrations: 2×10-5, 5×10-5, 1×10-4, 2×10-4, 5×10-4 and 7.5×10-4 M? 2. What species is thought to be the light-absorbing species in this experiment? 3. Write a balanced chemical equation for the formation of the light- absorbing species that results from reaction of phosphate and ammonium vanadomolybdate (AVM). 4. 5. 6. 7. State the Beer-Lambert law and define all terms in it. What are the five fundamental components of a spectrophotometer? Why is a calibration curve constructed? How? How do you know whether to measure the absorbance of a more dilute or more concentrated solution if the absorbance of your unknown solution is not within the limits of your calibration curve? 8. A 0.0750 M sample of CO(NO3)2 gave an absorbance of 0.38 at 505 nm in a l-cm cell. What is the cobalt concentration of a solution giving an absorbance of 0.52 in the same cell at the same wavelength? 9. A 17.28-ppm (1 ppm = 1 mg/L) a solution of FeSCN2+ has a transmittance of 0.59 when measured in a 1.00-cm cell at 580 nm. Calculate the extinction coefficient for FeSCN2+ at this wavelength. 10. Define eutrophication. 11. If raw sewage contains 30 mg/L of phosphate and a secondary sewage treatment plant removes 25% of the phosphate, would a secondary treatment plant provide potable water if 0.3 mg/L is the maximum phosphate concentration allowable in drinking water? 12. Write a balanced chemical equation for the hydrolysis of triphosphate, P3O105-, to orthophosphate, PO43-. Lab Manual 50 CHE 1402 Experiment 9 Determination of Orthophosphate in Water Name(s) Date Laboratory Instructor REPORT SHEET A. Preparation of Calibration Curve ÷ 1.5 × 1.5 [PO43-] (M) in the flask [PO43-] (M) in the cuvette Absorbance of PO43in the cuvette Absorbance of PO43in the flask Soln. 1 2×10-5 __________ __________ __________ Soln. 2 5×10-5 __________ __________ __________ Soln. 3 1×10-4 __________ __________ __________ Soln. 4 2×10-4 __________ __________ __________ Soln. 5 5×10-4 __________ __________ __________ Soln. 6 7.5×10-4 __________ __________ __________ B. Determination of the concentration of PO43- in the unknown × 1.5 unknown A unknown B unknown C Lab Manual Absorbance of PO43- in the cuvette [PO43-] (M) in the cuvette [PO43-] (M) in the sample test tube __________ __________ __________ __________ __________ __________ __________ __________ __________ 51 CHE 1402 EXPERIMENT 10 pH-metric titration of an HCl-H3PO4 mixture OBJECTIVE To determine the concentrations of acids in a mixture of phosphoric acid and hydrochloric acid. Relates to chapter 16 of “Chemistry the Central Science, 12th Ed.”. Background Frequently an acid or a base is quantitatively determined by titration using a pH meter to detect the equivalence point rather than using a visual indicator. This has the advantage that one actually monitors the change in pH at the equivalence point rather than just observing the change in color of a visual indicator. This eliminates any indicator blank error. Some laboratory workers complain that this method is more tedious than methods using visual indicators; they soon find, however, that after running one titration to find out the approximate location of the equivalence point, they only need to concern themselves with the dropwise addition of titrant close to the equivalence point on subsequent titrations. The titration of a mixture of phosphoric acid and hydrochloric acid is complicated by the fact that phosphoric acid is a triprotic acid with Ka1 = 7.5x10-3, Ka2 = 6.2x10-8, and Ka3 = 4.8x10-13. Ka1 is sufficiently large that the first proton from phosphoric acid cannot be differentiated from strong acids like hydrochloric acid. The second dissociation of phosphoric acid varies significantly from the first. The second proton can be neutralized and differentiated from the first phosphoric acid proton and the strong acid proton. The titration curve for a mixture of phosphoric and hydrochloric acids is illustrated above. The first break in the mixed acid curve indicates the amount of hydrochloric acid plus the amount of the phosphoric acid. The amount of phosphoric acid in the sample is indicated by the difference between first and second breaks in the titration curve. The first equivalence point volume (25.0 mL) permits calculation Lab Manual 52 CHE 1402 of the total concentration of HCl + H3PO4 /3 since the first proton of H3PO4 is neutralized. In this example, nacid 1st equ nHCl nH3PO4 1st equ n HCl nH 3 PO4 3 TOT nbase1st equ Cbase Vbase 0.100 M 25.0 mL 2.50 mmol Taking the difference between the first and second equivalence point volumes (10.0 mL) one obtains: nacid 2nd equ nH 3PO4 2 nd equ n H 3 PO4 TOT 3 Cbase Vbase 0.100 M 10.0 mL 1.00 mmol From these results one can calculate that the sample contains 3.00 mmol of H3PO4 and 1.50 mmol of HCl. This type of analysis is ideally suited for the determination of strong acid impurities in a weak acid and is unaffected by colored or suspended materials in the solution provided that these materials are not acids or bases. Interference in the analysis would be other weak or strong acids mixed into the sample. High concentrations of sodium ion or potassium ion in the sample can cause an error in the reading of the glass electrode, (i.e., the absolute pH values may be in error) but generally will not affect locating the equivalence points. PROCEDURE A. Standardization of the NaOH solution. Standardize the 0.100 M NaOH solution prepared prior to use. B. Titration of the mixture of acids. Titration of the H3PO4-HCl mixtures 1. In a clean 250.0 mL volumetric flask, dilute 10 mL of the unknown sample in the flask to the mark with distilled water. 2. Into 250 mL beakers pipet 50.00 mL portions of the acid sample. While stirring with magnetic stirrer, titrate with the standard NaOH solution using the pH meter to detect the equivalence point. Perform an approximate titration first, adding the titrant in 0.5 mL portions. Then on subsequent titrations add the NaOH in one portion up to within 2 mL of the equivalence point. Then add the titrant in 0.10 mL, 0.05 mL, or 1 drop portions. 3. Perform the titration accurately on three portions of the acid mixture. Lab Manual 53 CHE 1402 Treatment of the Data 1. On good graph paper, plot pH against mL titrant added. Look for the regions of rapidly changing pH. pH as a function of mL of titrant added; the equivalence point is the Vbase pH point on the curve where has its maximum value. Vbase 2. Also plot curves of 3. Duplicate titrations should agree to within 0.04 mL for excellent work. 4. Report total mmol H3PO4 and mmol HCl contained in the unknown. Recall that you titrate 50 mL portions of a 250 mL total sample. Calculations Two breaks will occur in the titration curves, the first corresponding to the titration of hydrogen ions from the HCl and the first hydrogen ion from the H3PO4. The second break corresponds to the titration of H2PO4- that resulted from the H3PO4. Therefore, the mmol of NaOH consumed up to the first endpoint is equal to mmol H3PO4 + mmol HCl. The mmol of NaOH consumed between the first endpoint and second endpoint equals mmol H3PO4. Subtract the mmol H3PO4 from mmol H3PO4 + mmol HCl to get mmol HCl. Multiply by the appropriate factor to get the total mmol HCl and total mmol H3PO4 in your 250 mL unknown. Report those values. REVIEW QUESTIONS 1. What volume (mL) of 0.100 N NaOH are required to titrate a sample containing 2.0 mmol HCl and 1.0 mmol H3PO4: (a)To the first equivalence point? and (b)To the second equivalence point? 2. Consider the titration of 20 mL of a sample that is 0.10 F in HCl and 0.05 F in H3PO4 with 0.100 N NaOH. Calculate the pH of the solution being titrated at each of the following points and plot the titration curve in your notebook. Titrant Volume pH 0.01 mL 15.00 mL 30.00 mL 35.00 mL 40.00 mL 45.00 mL Lab Manual 54 CHE 1402 EXPERIMENT 10 pH-metric titration of an HCl-H3PO4 mixture REPORT SHEET Name(s)(s)____________________________ Date V NaOH (mL) pH ________________________ pH Vbase 0 15 16 17 18 19 20 21 22 23 24 25.0 25.2 25.4 25.6 25.8 26.0 26.2 26.4 V NaOH pH (mL) pH Vbase 26.6 26.8 27.0 27.2 27.4 27.6 28.0 29 30 31 32 33 34 35 36 37 38 39 40 V1st equ = _________ Laboratory Instructor________________________ V NaOH pH (mL) 41 42 43 44 45.0 45.2 45.4 45.6 45.8 46.0 46.2 46.4 46.6 46.8 47.0 47.2 47.4 47.6 47.8 pH Vbase V NaOH pH (mL) pH Vbase 48.0 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 V2nd equ = ____________ (V2nd equ - V1st equ -) = _______________ nH3PO4 (in your initial 100 mL volumetric flask) = _______________ nHCl (in your initial 100 mL volumetric flask) = _______________ [H3PO4] (in your initial 100 mL volumetric flask) = _______________ [HCl] (in your 100 mL volumetric flask) = _______________ Show all calculations (overleaf): Lab Manual 55 CHE 1402 EXPERIMENT 11 Water analysis OBJECTIVES To learn how a water sample is collected and stored. Perform some chemical analyses on a water sample. Relates to chapter 18 of “Chemistry the Central Science, 12th Ed.”. APPARATUS AND CHEMICALS conductivitimeter 150-mL beakers 100 mL, graduate cylinders Buffer solutions vacuum filtration apparatus analytical balance hot plates pH meter with electrodes 100 mL, 25-mL pipets weiging papers filter paper INTRODUCTION Water is the universal solvent and many contaminants (impurities) are easily dissolved upon its contact. They may give water a bad taste, color, odor, or cloudy appearance (turbidity), and cause hardness, corrosiveness, or staining. They can also damage growing plants and transmit disease. At low levels, impurities generally are not harmful in water. Removing all contaminants would be extremely expensive and in nearly all cases would not provide greater protection of health. At high level (waste water) many of these impurities are treated and removed or rendered harmless. Chemists are concerned with the purity of water but regulatory agencies are concerned with setting standards to protect the environment and public health. One mean of establishing and assuring the purity and safety of water is to meet standards for various contaminants found in water. In this project you be able to get some practice on how water is collected and stored and to perform some routine chemical analysis. A. Sample Collection and Storage Site data should be recorded for all sampling locations. The information generally required includes time, date, grid references of site, weather, temperature, method of collection and information about any local activities that might influence the results. Some of these data may only be applicable for certain classes of water samples. A number of sampling devices are available for taking water samples from small ponds and from different depths in large stratified lakes. The simplest system uses a weighted bottle which is suspended at the required depth. The stopper is then removed by a sharp pull on a separate line. Water samples are especially subject to alteration in chemical composition due to microbiological activity and chemical reactions. Heavily polluted waters can undergo changes in composition within an hour of collection, and most natural waters are affected to some degree. Some tests (particularly pH and dissolved gases) should, if possible, be carried out in the field. Glass sample containers are frequently recommended for storage since polythene vessels can be porous to gaseous constituents and have been found to absorb phosphorus. In general, to minimize possible bias of results caused by any changes occurring during storage it is important to: 1 - Analyze the samples as soon as possible. Ensure that solution collectors at sites are emptied regularly. 2 - Fill containers to exclude air. 3 - Keep sample cool, but do not freeze. 56 CHE 1402 Physical preservation methods Fine filtration If the interest is only in the dissolved fraction then fine filtration, which removes many of the microorganisms, can be applied. It will also remove fine mineral matter and any traces of turbidity which could affect a later analytical stage. An alternative approach is centrifuging which can be used to separate various particle sizes. Temperature reduction Although some microbial activity appears to continue even at 0 °C the rapid cooling of samples after collection is generally to be recommended. Preliminary and general tests For reasons given in the previous section tests on waters should be made as soon as possible after sampling and in some cases in the field. This particularly applies to labile and gaseous constituents. Odor, turbidity, and color Odor can serve as a guide to gross pollution of water. For example, characteristic odors are associated with chlorination plants, untreated sewage and chemical industry effluents. Color in water may be a true color due to dissolved material or an apparent color when suspended material is present. The latter is quite common in natural waters, seen for example when algal blooms impart a greenish tinge. Turbidity may be used as estimate of undissolved substances in the sample. It is generally measured by visual comparison with standards or photometrically, using a neophelometer or spectrophotometer. Turbidity and color control light penetration in lake which in turn affect phytoplankton population. SOLID Total suspended solids (TSS) The finer suspended matter in natural waters is usually of an organic nature representing colloidal matter, which has been flocculated under the influence of bacteria and protozoa. Inorganic suspended matter is chiefly restricted to siliceous material resulting from the erosion of mineral soils. Total dissolved solids (TDS) It is often convenient to determine the dissolved solids in the filtrate remaining from the TSS determination. Total organic matter (TOM) TOM is all organic matter that can be found in a given water sample Alkalinity (and acidity) The alkalinity of water is its capacity to neutralize a strong acid, and the values obtained will depend on the pH of the titration end-point. In practice it is the bicaronate, carbonates and hydroxides in solution that largely determine the alkalinity although there are minor contributions from silicates and other anions. Total alkalinity is determined by titration to the equivalent point of carbonic acid which occurs between pH 4.2 and 5.4 depending on the carbon dioxide content of water. It has long been the practice in water analysis to determine solids as dissolved, suspended and organic. Conductivity Conductivity is a property of water governed by the total ionic content. Although it is non specific and varies with the proportion of species presents, it is often measured, because of its value in characterising waters. It expresses the resistance of 1 cm cube of water to the passage of a current, usually at 25°C (specific resistance). 57 CHE 1402 PROCEDURE 1. TSS determination Filtrate 100 mL of your sample and then determine the weight of the solid in the filter paper 2. TDS determination Evaporate the filtrate (liquid) to a small volume (from 100 to 50 mL) Transfer to a weighed 100 mL beaker for evaporation. Dry at 105°C to a constant weight. Cool and weigh. Express the result in mg/L . 3. TOM determination Transfer to a small pre-weighed evaporating beaker 50mL of your sample. Evaporate to dryness at constant temperature and weigh beaker plus contents. Ash in a muffle furnace, leaving at 500°C for 1 hour. The loss in weight of the residue gives the TOM in the sample. The method is only approximate and estimates of total organic carbon are preferable when organic contents are low. 4. Alkalinity (and acidity) Measure pH of water with a pH meter If pH> 7.5 add 3-4 drops of methyl-orange indicator and titrate against HCl. 5. Conductivity Add the unfiltered sample into two beakers and bring to the required temperature (preferably 25 °C) by immersion in a water bath. Immerse the electrodes in each beaker that contain water samples. Record the conductivity in the second tube (having used the first as a rinse). Check the sample temperature just after immersion of the electrode. Source: A.P. Rowland & H.M. Grimshaw, in Chemical Analysis of Ecological Materials (S.T. Allen, Editor) 2nd Edition, Oxford, UK: Blackwell, 1989, p. 62. 58 CHE 1402 Experiment 11 Water Analysis (I) – Field Trip Name(s) Date Laboratory Instructor REPORT SHEET 1/2 Observations (in the field) Water temperature (oC) pH Conductivity γ (µmhos/cm or µS/cm) Amount of plants, or living things you observe Think about it and ANSWER these questions in the field Areas with large amount of water insects and underwater plants are warmer because these living things produce heat. Compare your results with other groups that collected water from areas with and without plants/insects, is there a temperature difference? Plants need CO2 from the water as food. When CO2 dissolves in water, an acid is produced, if CO2 is removed from water by the plants. What would happen to the pH in areas where there is plenty of plants? Does this agree with your observations of pH? The conductivity is a measure of the amount of ions in water. Plants need CO32-, NO3- and PO43-ions and sunlight to produce food. Given the conductivity measurements that you have made on the water that you took and combining it with the pH measurements, do you think that there can be life in the area where you took the water? The solubility of ionic compounds in water depends on the temperature. Higher temperatures dissolve more of the compound to produce more ions. Does your measurement of conductivity and temperature respectively, when compared to that of other groups, agree with this observation? 59 CHE 1402 Experiment 11 Water Analysis (II) Name(s) Date Laboratory Instructor REPORT SHEET 2/2 (Water analysis will be performed at the AUI Chemistry laboratory) Part 1 - SOLID Mass of total suspended solids (TSS) in mg ________________ Mass of total dissolved solids (TDS) in mg ________________ Mass of total organic matter (TOM) in mg ________________ Alkalinity (and acidity) pH ________________ If pH is larger than 7.5 continue otherwise skip to conductivity measurements Part 2 – Determination of Alkalinity. Trial 1 Trial 2 1 [HCl] (mol/L) 2 Volume of HCL used (mL) _____________ _____________ 5 Amount of HCL added (mol) _____________ _____________ 6 Amount of OH- in satd solution (mol) _____________ _____________ 7 Volume of sample solution (mL) 8 [OH-], equivalence (mol/L) Part 3 - Conductivity γ _________________ 25.0 _____________ 25.0 _____________ γ = _____________ µmhos/cm (or µS/cm) 60 CHE 1402 EXPERIMENT 12 Galvanic Cells, the Nernst Equation OBJECTIVES To measure the relative reduction potentials for a number of redox couples. To develop an understanding of the movement of electrons, anions, and cations in a galvanic cell. To study factors affecting cell potentials. To estimate the concentration of ions in a solution by using the Nemst equation. Relates to chapter 20 of “Chemistry the Central Science, 12th Ed.”. INTRODUCTION When iron corrodes, a change in the oxidation number of the iron atoms occurs; when gasoline bums, a change in the oxidation number of the carbon atoms occurs. A change in the oxidation number of an atom is the result of an exchange of electrons between the reactants of the reaction. A decrease in an oxidation number requires a gain of electrons, whereas an increase in an oxidation number requires a loss of electrons. The substance that lowers its oxidation number gains electrons and is reduced; the substance that increases its oxidation number loses electrons and is oxidized. A chemical reaction that involves the transfer of electrons from one substance to another is an oxidation-reduction (redox) reaction. Experimentally, when copper metal is placed into a silver ion solution, copper atoms spontaneously lose electrons (copper atoms are oxidized) to the silver ions (which are reduced). Silver ions migrate to the copper atoms to pick up electrons and form silver atoms at the copper metal solution interface; the copper ions that form then move into the solution away from the interface. The overall reaction that occurs at the interface is Cu (s) + 2 Ag+ (aq) 2 Ag (s) + Cu2+ (aq) [1] This redox reaction can be divided into an oxidation and a reduction half-reaction. Each half-reaction, called a redox couple, consists of the reduced state and the oxidized state of the substance. Cu (s) + 2 Ag (aq) + 2 e- Cu2+ (aq) + 2 e- oxidation half-reaction [2] Ag (s) reduction half-reaction [3] A galvanic cell is designed to take advantage of this spontaneous transfer of electrons. Instead of electrons being transferred at the interface between the copper metal and the silver ions in solution, a galvanic cell separates the copper metal from the silver ions and forces the electrons to pass externally through a wire, an external circuit. Figure 12.1 is a schematic diagram of a galvanic cell setup for these two redox couples. 61 CHE 1402 Figure 12.1 The two redox couples are placed in separate compartments, called half-cells. Each half-cell consists of an electrode, usually the metal (reduced state) of the redox couple, and a solution containing the corresponding cation (oxidized state) of the redox couple. The electrodes of the half-cells are connected by a wire; this is where the electrons flow, providing current for the external circuit. A salt bridge, which connects the two half-cells, completes the construction of the galvanic cell (and the circuit). The salt bridge permits limited movement of ions from one half-cell to the other so that when the cell operates, electrical neutrality is maintained in each half-cell. For example, when copper metal oxidizes in the Cu2+/Cu half-cell, either anions must enter or copper ions must leave the half-cell to maintain neutrality. Similarly, when silver ions form silver metal in its half cell, either anions must leave or cations must enter its half-cell to maintain neutrality. The electrode at which reduction occurs is called the cathode; the electrode at which oxidation occurs is called the anode. As oxidation releases electrons to the electrode to provide a current in the external circuit, the anode is designated the negative electrode in a galvanic cell. The reduction process draws electrons from the circuit and supplies them to the ions in solution; the cathode is the positive electrode. This sign designation allows us to distinguish the anode from the cathode in a galvanic cell. Different metals, such as copper and silver, have different tendencies to oxidize; similarly, their ions have different tendencies to undergo reduction. The cell potential, Ecell , of a galvanic cell is due to the difference in tendencies of the two metals to oxidize (lose electrons) or of their ions to reduce (gain electrons). Commonly, a measured reduction potential, the tendency for the metal ion to gain electrons, is the value used to identify the relative ease with which a given metal ion undergoes reduction. A potentiometer, placed in the external circuit between the two electrodes, measures this relative tendency of the electrons to be transferred between the two redox couples. The potentiometer measures a cell potential, Ecell , a value that represents the difference between the tendencies of the metal ions in their respective halfcells to undergo reduction (i.e. the difference between the reduction potentials of the two redox couples). For the copper and silver redox couples, we can represent their reduction potentials as ECu2 / Cu and E Ag / Ag , respectively. 62 CHE 1402 The cell potential is therefore Ecell Ecathode Eanode E Ag / Ag ECu2 / Cu [4] Experimentally, silver ion has a greater tendency than does copper ion to be in the reduced (metallic) state; therefore, Ag+ has a greater reduction potential. As the cell potential, Ecell , is measured positive, E Ag / Ag is placed before ECu2 / Cu in Equation 4. o The measured cell potential Ecell corresponds to the standard cell potential Ecell when the concentrations of all ions are 1 mol/L and the temperature of the solutions is 25°C. o The standard reduction potential for the Ag+(l M)/Ag redox couple, E Ag , is +0.80 V, and the standard / Ag o reduction potential for the Cu2+(l M)/Cu redox couple ECu , is +0.34 V. Theoretically, a potentiometer 2 / Cu should show the difference between these two potentials, or, at standard conditions, o o o Ecell E Ag ECu 0.80 0.34 0.46V 460 mV 2 / Ag / Cu [5] Deviation from the theoretical value may be the result of surface activity at the electrodes or activity of the ions in solution. In Part A of this experiment, several cells are “built” from a selection of redox couples and data are collected. From an analysis of the data, the relative reduction potentials for the redox couples are determined and placed in an order of decreasing reduction potentials. In Part B, the effects of concentration changes and the formation of a complex on the reduction potential of a redox couple are determined. The presence of ammonia in a Cu2+ solution affects the potential of the Cu2+/Cu redox couple by the equation Cu2+ (aq) + 4 NH3 (aq) Cu(NH3)4 (aq) The Nernst equation is applicable to redox systems that are not at standard conditions, most often when the concentrations of the ions in solution are not 1 mol/L. At 25oC, the measured cell potential, Ecell , is related to o o and ionic concentrations by Ecell Ecell Ecell 0.0592 log Q n [6] where n represents the moles of electrons exchanged according to the cell equation. For the copper/silver cell, n 2 ; two electrons are lost per copper atom and two electrons are gained per two silver ions (see Equations 1-3). For dilute ionic concentrations, the reaction quotient Q , equals the mass action expression for the cell reaction, i.e. the product of the molar concentrations of the products divided by the product of the molar concentrations of the reactants, each concentration raised to the power of its coefficient in the balanced cell equation. For the copper/silver cell (see Equation .1) Q Cu Ag 2 2 In Part C of this experiment, we study, in depth, the effect of the change in concentration of an ion have on the potential of the cell. The cell potentials for a number of zinc/copper redox couples are measured; in each one the copper ion concentrations is varied but the zinc ion concentration is maintained constant. 63 CHE 1402 Zn (s) + Cu2+ (aq) Cu (s) + Zn2+ (aq) The Nernst equation for this reaction is o Ecell Ecell 0.0592 Zn 2 log 2 Cu 2 [7] Rearrangement of equation 7 yields an equation for a straight line y = ax + b: 0.0592 Zn 2 log 2 Cu 2 0.0592 o Ecell log Zn 2 log Cu 2 2 0.0592 0.0592 o 2 Ecell log Zn 2 log Cu Ecell 2 2 o Ecell Ecell y = a x × [8] b + A plot of Ecell versus log Cu 2 for solutions of known copper ion concentrations is a straight line that has a slope a of o b Ecell 0.0592 and an intercept b that leads to the determination of the concentration of the zinc ion. 2 0.0592 log Zn 2 2 Zn 10 2 2 0.0592 o b Ecell PROCEDURE The cell potentials for a number of galvanic cells are measured, and the redox couples are placed in order of decreasing reduction potentials, the effects of changes in ion concentrations on cell potentials are observed and analyzed. PART A: Reduction Potential of Several Redox Couples 1. Collect the Electrodes, Solutions, and Equipment. Obtain a 24-test tubes plate. Place about 2 mL (three-fourths full) of the solutions in test tubes A1, A2, B1, and B2. Polish 2-cm strips of copper, zinc, lead, and iron metal with steel wool, rinse briefly with dilute 0.1 M HNO3, and rinse with deionized water. These polished metals are used as electrodes. Check out a potentiometer (or a voltmeter) with two electrical wires (different colors) attached to "alligator" clips. 2. Set Up the Copper/Zinc Cell. Place a Cu strip (electrode) in test tube A1 and a Zn strip (electrode) in test tube B2. Roll and flatten a 2×2-cm piece of filter paper; wet the filter paper with a 0.1 M KNO3 solution. Fold and insert the ends of the filter paper into test tubes A1 and B2; this is the salt bridge. Connect one electrode to the negative terminal of the potentiometer and the other to the positive terminal. 3. Determine the Copper/Zinc Cell Potential. 64 CHE 1402 If the needle on the potentiometer swings the "wrong way", reverse the connections. Read and record the (positive) cell potential. Identify the cathode (positive terminal) and the anode. Write an equation for the reaction occurring at each electrode. 4. Repeat for the Remaining Cells. Determine the cell potentials for all possible galvanic cells that can be constructed from these four redox couples. Be sure to prepare a "new" salt bridge for each galvanic cell. You have now combined two half-cells to form a galvanic cell. 5. Determine the Relative Reduction Potentials. Assuming the reduction potential of the Zn2+(0. 1 M)/Zn redox couple is -0.79 V, determine the reduction potentials of all other redox couples. 6. Determine the Reduction Potential of the Unknown Redox Couple. Place a 0.1 M solution of unknown and electrodes obtained from your instructor in well A3. Determine the reduction potential, relative to the Zn2+(0.1 M/Zn) redox couple, for your unknown redox couple. Part B: Effect of Concentration and Complex Formation on Cell Potential 1. Effect of Concentration. Place about 2 mL of 1 M CuSO4 in test tube Dl and 0.001 M CuSO4 in test tube D2. Immerse a polished copper electrode in each solution. Prepare a new salt bridge to connect the two redox couples. Measure the cell potential. Determine the anode and the cathode. Write an equation for the reaction occurring at each electrode. 2. Effect of Complex Formation. Add 5-10 drops of 6 M NH3 to the 0.001 M CuSO4 solution.3 (Caution: do not inhale NH3) Observe any changes in the cell potential. Part C: The Nernst Fquation and an Unknown Concentration 1. Prepare the Diluted Solutions. Prepare Solutions 1 through 4 as outlined below using a 1-mL pipet and 100-mL volumetric flasks. Be sure to rinse the pipet with the more concentrated solution before making the transfer. Use deionized water for dilution "to the mark" in the volumetric flasks. Calculate the molar concentration of each solution. Solution 1: 10 mL of 0.1 M Cu(NO3)2 Solution 2: Take 10 mL of solution 1 and dilute to 100 mL Solution 3: Take 1 mL of solution 2 and dilute to 100 mL Solution 4: Take 1 mL of solution 3 and dilute to 100 mL 2. Measure and Calculate the Cell Potential for Solution 2. The Zn2+/Zn redox couple in test tube B2 is the reference half-cell for this part of the experiment. Place about 2 mL of Solution 2 in test tube C1 and a polished Cu strip. Connect the two half-cells with a "new" salt bridge. Connect the electrodes to the potentiometer and record the potential difference, Ecell . Calculate the theoretical cell potential. (Use a table of standard reduction potentials and the Nernst equation.) 3. Measure and Calculate the Cell Potentials for Solutions 3 and 4. Repeat Part C.2 with Solutions 3 and 4 in wells C2 and C3, respectively. Again, a freshly prepared salt bridge is required for each cell. 65 CHE 1402 4. Plot the Data. Plot Ecell , expt and Ecell , calc (ordinate) versus log Cu 2 abscissa) on the same piece of linear graph paper for the four concentrations of Cu(NO3)2 solutions in wells A1, Cl, C2, and C3. Have your instructor approve your graph. 5. Determine the Concentration of the Unknown. Obtain a Cu(NO3)2 solution with an "unknown" copper ion concentration from your instructor and place the unknown solution in test tube B3. Determine Ecell as in Part C.2. Using the graph, determine the unknown copper ion concentration in the solution. Disposal. Dispose of the waste zinc, copper, lead, and iron solutions in the "Waste Metal Solutions" container. Return the metals to appropriately marked containers. CLEANUP: Rinse the 24-test tubes plate twice with tap water and once with deionized water. Discard the first rinse in the "Waste Metal Solutions" container and the other rinses into the sink. REVIEW QUESTIONS 1. Identify the oxidizing and reducing agents in each cell in Part A. a. Cu-Zn b. Cu-Pb c. Zn-Pb d. Fe-Pb e. Zn-Fe 2. List two reasons why an experimentally measured reduction potential would not be equal to the theoretical reduction potential. See Part A.3 of the Report Sheet. 3. Why is it not possible to use a nonmetal as an electrode in a nonmetal-nonmetal ion redox couple,such as the Cl2/Cl- redox couple? Explain. 66 CHE 1402 Experiment 12 Galvanic Cells, the Nernst Equation Name(s) Date Laboratory Instructor REPORT SHEET A. Reduction Potentials of Several Redox Couples Fill in the following table with your observations and interpretations from the galvanic cells. Galvanic cell Measured Anode Cathode Anode reaction Cathode reaction Ecell (mV) 1) Cu-Zn _________ _______ _______ _____________ _____________ 2) Cu-Pb _________ _______ _______ _____________ _____________ 3) Zn-Pb _________ _______ _______ _____________ _____________ 4) Fe-Pb _________ _______ _______ _____________ _____________ 5) Zn-Fe _________ _______ _______ _____________ _____________ 1. Compare the sum of the Zn~Pb and Cu-Pb cell potentials with the Zn-Cu cell potential. 2. Compare the sum of the Zn~Fe and Fe-Pb cell potentials with the Zn-Pb cell potential. 67 CHE 1402 3. Arrange the four redox couples in order of decreasing (measured) reduction potentials. List the reduction potential for each redox couple relative to that of the Zn2+(0.1 M)/Zn couple, which is -0.79 V. Use a table of standard reduction potentials and the Nemst equation to calculate the reduction potentials for each of these redox couples. Reduction Potential (mV) Redox Couple (measured) (calculated) % Error Zn-Cu _________ _________ _________ Zn-Pb _________ _________ _________ Zn-Fe _________ _________ _________ 4. Reduction potential of the unknown redox couple: _____________ B. Effect of Concentration and Complex Formation on Cell Potential 1. Cell potential of "concentration cell": Anode reaction: ______________________ Cathode reaction: ______________________ 2. Cell potential from complex formation: Explain why the potential changes with the addition of NH3(aq). C. The Nernst Equation and an Unknown Concentration Complete the following table with the concentrations of the Cu(N03)2 solutions and the measured cell potentials. Use a table of standard reduction potentials and the Nernst equation to calculate the Ecell . 68 CHE 1402 Solution [Cu(NO3)2] (mol/L) Log [Cu 2+] Measured Ecell (mV) Calculated Ecell (mV) 1 0.1 -1 ____________ ____________ 2 __________ __________ ____________ ____________ 3 __________ __________ ____________ ____________ 4 __________ __________ ____________ ____________ Account for any significant difference between the measured and calculated Ecell values. 3. Ecell for the solution of unknown concentration: ______________________ Molar concentration of Cu2+ in the unknown: ______________________ 69 CHE 1402 APPENDIX 70
© Copyright 2026