Document 333298
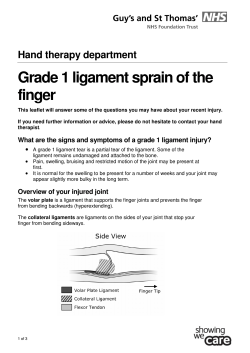
Nostrum October 2014 A newsletter from the Prescribing Support Team, NHS Dumfries and Galloway In this edition of Nostrum: • • • • • • • Endocrine matters Emergency Inhalers in Schools Formulary Review HBGM Aide Memoire Latest SMC Advice Specific Drug Issues Scriptswitch Update ScriptSwitch Update ScriptSwitch has now been running in 29 out of the 34 practices in NHS Dumfries and Galloway since November 2013. The graph below shows the variation in its use amongst practices. The dark dots within the bars represent actual savings made against the potential savings. There are obviously practices who are more inclined to accept the recommendations offered by ScriptSwitch than others. We recognise that some recommendations are never accepted and never likely to be, and are therefore working with ScriptSwitch to remove these. However, it is also evident where more savings could be made, including simple switches to formulary choices and avoiding branded prescribing where appropriate. Practice-based PST pharmacists and technicians can help facilitate these changes- just ask ☺! Emergency Inhalers in Schools From the 1st October, schools will be allowed to hold spare emergency inhalers. ‘Asthma UK’ report that 86% of children have been without their own inhaler because it was lost, forgotten, broken or had run out, so the change in legislation which has been made, will help to keep children with asthma safe. The Scottish Government is reviewing guidance for schools on managing medicines and this will contain advice for schools on emergency inhaler use for children with asthma. Schools will have the option to purchase their own inhaler and spacer devices for inclusion in their first aid kit, so that children with asthma are not at risk of being without emergency reliever treatment. Formulary Review Meetings Formulary review meetings will be taking place Please contact a member of the team if you have any comments or suggestions you would like to make regarding formulary drug choices and/or associated text. Quick Guide to Blood Glucose Monitoring Equipment Patient Type I Meter GlucoMen LX Plus (Menarini) Test Strip GlucoMen LX Sensor GlucoMen LX Ketone Type I Carb Counting Freestyle InsuLinx (Abbott) GlucoRx Nexus Freestyle Lite Type II GlucoRx Nexus Lancet Unilet ComforTouch OR Glucoject Lancet Plus Unilet ComforTouch GlucoRx Unilet ComforTouch GlucoRx Visually Impaired Nexus Voice GlucoRx Nexus NOTES o The needle of choice is Omnican (4mm, 6mm 8mm) o Type 2 patients not on insulin - only those on oral sulphonylureas should definitely be testing due to the recognised risk of hypoglycaemia o If patient is using a insulin pump please check with Diabetes Centre re BGM/strips o If patient is visually impaired or has dexterity problems a Unistix 3 lancet may be considered Healthcare Professionals should be using a CareSensN meter – strips have the same name and are available through PECOS 3457546, £6.91. Those not on Pecos should send their requisition to Procurement quoting the above product number. The needle is BD-Autoshield Duo and the lancet is Unistix 3. Hydrocortisone for Addison’s Disease A recent article in the BMJ in July of this year (BMJ 2014; 349:g48433 Amin et al) discussed the use of prednisolone as a potential glucocorticoid replacement therapy in the treatment of Addison’s disease. Coupled with the fact that the cost of hydrocortisone has escalated in the last year, would it be reasonable to use prednisolone as an alternative to hydrocortisone? Dr Fiona Green, Consultant Endocrinologist DGRI has advised that; hydrocortisone is preferable to prednisolone as it’s easily recognisable as replacement therapy and therefore less likely to be stopped/omitted (prednisolone potentially being used for several other indications). Also by using hydrocortisone, there is the potential to monitor replacement doses in blood and urine collections which is not possible with prednisolone; this is helpful in the context of monitoring under and over-replacement therapy. In addition, the use of hydrocortisone acts as a flag to double doses in times of stress/ill health.[Prednisolone may be used for those who struggle to take hydrocortisone two to three times daily.] Liothyronine As mentioned in last month’s edition, the price of liothyronine in comparison to levothyroxine has prompted the Health Board to look into the prescribing of this. There are no formal guidelines on the use of liothyronone vs levothyroxine; Dr Green states that there are a very small number of patients who request a trial of liothyronine (T3) based on the theory that in the context of another chronic illness, there is a lack of peripheral conversion of T4 (levothyroxine) to T3 and anecdotally patients feel better on it. However, the number of patients involved would be expected to be low and closely supervised by the Consultant specialist. Specific Drug Issues Domperidone for Enhancing Breast Milk Production This is an unlicensed use of domperidone although occasionally recommended by midwives. Prescribers are advised to exercise caution when this is requested in light of the recent MHRA safety advice relating to the cardiac side effects of domperidone and the fact that it can no longer be purchased over the counter. Full details at: http://www.mhra.gov.uk/N ewsCentre/Whatsnew/CON 452545 Antibiotic Awareness Day Latest SMC Decisions For full advice see www.scottishmedicnes.org.uk Accepted for Use in NHS Scotland Nostrum extra – wound Azelastine hydrochloride + fluticasone propionate (Dymista ). Indication under review: for the relief of symptoms of management. March 2006 or moderate to severe seasonal and perennial allergic rhinitis if monotherapy with either intranasal antihistamine ® glucocorticoid is not considered sufficient. For patients in whom the combination of azelastine hydrochloride and fluticasone propionate nasal spray is an appropriate choice of therapy, Dymista® provides the two ingredients in a single nasal spray. This SMC advice takes account of the benefits of a Patient Access Scheme (PAS) that improves the cost-effectiveness of Dymista®. This SMC advice is contingent upon the continuing availability of the Patient Access Scheme in NHS Scotland or a list price that is equivalent or lower. Accepted with PAS but NOT added to the D&G formulary Dabigatran etexilate (Pradaxa®). Indication under review: Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. Accepted but NOT added to the D&G formulary for this indication, pending a protocol Picato Gel (Ingenol mebutate 150mcg/g and 500mcg/g). Indication under review: Cutaneous treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis in adults. Accepted and added to the D&G formulary Accepted for Restricted use in NHS Scotland Alogliptin plus metformin (Vipdomet®). Indication under review:Treatment of adult patients aged 18 years and older with type 2 diabetes mellitus. Accepted Restricted but NOT added to the D&G formulary Antibiotic awareness day is now held on the 18th November every year; this is well-timed to discourage the inappropriate use of antibiotics. Some GPs have already undertaken the Scottish Reduction in Antimicrobial Prescribing (ScRAP) programme which focuses on appropriate prescribing in RTIs and recommends the use of delayed prescriptions. With the current focus on this coupled with the fact that NHS Dumfries and Galloway are performing poorly in this area compared to all other HBs in Scotland, next month there will be a ‘Nostrum Xtra’ dedicated entirely to antibiotic prescribing. Ocriplasmin (Jetrea®). Indication under review: In adults for the treatment of vitreomacular traction, including when associated with macular hole of diameter less than or equal to 400 microns. Accepted Restricted and approved for use in D&G pending a business case Prednisolone 25mg tablets Posaconazole (Noxafil®). Indication under review: for the treatment of fungal infections in adults. Accepted Restricted but NOT added to the D&G formulary pending a protocol Some GPs have noted the cost difference between prednisolone 25mg and 5mg strengths when issuing scripts for treatments that are initiated in hospital. DGRI are aware of this difference and try to limit the use of 25mg strength tablets where possible. Please ensure multiples of 5mg tablets are prescribed where appropriate. New Oral Anticoagulants (NOACs) NOAC therapy should only be initiated if an individual is intolerant/allergic to warfarin therapy or where there is poor INR control on warfarin (target INR <60% of the time). Warfarin is the first line anticoagulant in NHS D&G; the inconvenience of INR testing is not a valid reason to commence NOAC therapy. Capsaicin (Qutenza®). Indication under review: For the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. SMC restriction: For use in patients who have not achieved adequate pain relief from, or have not tolerated, conventional first and second line treatments Accepted Restricted but NOT added to the D&G formulary pending a protocol Dapagliflozin + metformin (Xigduo®). Indication under review: in adults aged 18 years and older with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control. Accepted Restricted but NOT added to the D&G formulary Empagliflozin (Jardiance®). Indication under review: Treatment of type 2 diabetes to improve glycaemic control in adults as add-on combination therapy: in combination with other glucose–lowering medicinal products including insulin, when these, together with diet and exercise, do not provide adequate glycaemic control. SMC restriction: to use in the following situations: dual therapy in combination with metformin, when a sulphonylurea is inappropriate; triple therapy in combination with metformin plus standard care; add-on to insulin therapy in combination with insulin plus standard care. Accepted Restricted but NOT added to the D&G formulary Lurasidone (Latuda®). Indication under review: For the treatment of schizophrenia in adults aged 18 years and over. SMC Restriction: as an alternative treatment option in patients in whom it is important to avoid weight gain and metabolic adverse effects. Accepted Restricted but NOT added to the D&G formulary pending a protocol Tocilizumab (RoActemra®). Indication under review: In combination with methotrexate for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have either responded inadequately to, or who were intolerant to previous therapy with one or more disease-modifying anti-rheumatic drugs or tumour necrosis factor (TNF) antagonists. Accepted Restricted with PAS and approved for use in D&G Not accepted for use in NHS Scotland Colestilan (BindRen®). Indication under review: treatment of hyperphosphataemia in adult patients with chronic kidney disease (CKD) stage 5 receiving haemodialysis or peritoneal dialysis. Not Recommended and NOT added to the D&G formulary Racecadotril (Hidrasec®). Indication under review: Complementary symptomatic treatment of acute diarrhoea in infants older than three months and in children, together with oral rehydration and the usual support measures, when these measures alone are insufficient to control the clinical condition and when causal treatment is not possible. If causal treatment is possible racecadotril can be administered as a complementary treatment. Not Recommended and NOT added to the D&G formulary Trastuzumab emtansine (Kadcyla). Indication under review: as a single agent, for the treatment of adult patients with human epidermal growth factor type 2 (HER2)-positive, unresectable locally advanced or metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either: Received prior therapy for locally advanced or metastatic disease, or Developed disease recurrence during or within six months of completing adjuvant therapy. Not Recommended and NOT added to the D&G formulary Contact the Prescribing Support Team @ Dr Paul Beardon [email protected]; Dr Jennifer Dillett [email protected] Dr Emily Kennedy [email protected]; Dorothy Kirkpatrick [email protected] Gordon Loughran [email protected]; Mandy Mackintosh [email protected] Susan Roberts [email protected]
© Copyright 2026