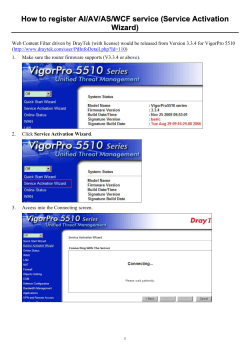
Monoclonal antibody F1 binds to the kringle domain of factor... induces enhanced susceptibility for cleavage by kallikrein
From www.bloodjournal.org by guest on October 15, 2014. For personal use only. 1995 86: 4134-4143 Monoclonal antibody F1 binds to the kringle domain of factor XII and induces enhanced susceptibility for cleavage by kallikrein DM Ravon, F Citarella, YT Lubbers, B Pascucci and CE Hack Updated information and services can be found at: http://www.bloodjournal.org/content/86/11/4134.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From www.bloodjournal.org by guest on October 15, 2014. For personal use only. Monoclonal Antibody F1 Binds To the Kringle Domain of Factor XI1 and Induces Enhanced Susceptibility for Cleavage by Kallikrein By Dorothea M. Ravon, Franca Citarella, Yvonne T.P. Lubbers, Barbara Pascucci, and C. Erik Hack In a previous study we have shown that monoclonal antibody F1 (MoAb Fl),directed against an epitope on theheavy chain of factor XI1 distinct from the bindingsite for anionic surfaces, is able t o activate factor XI1 in plasma (Nuijens JH, et al: JBio/Chem264;12941,1989).Here, we studiedin detail the mechanism underlying the activation of factor XI1 by MoAb F1 using purified proteins. Formation of factor Xlla was assessed by measuring its amidolytic activity towards in the chromogenic substrateH-D-Pro-Phe-Arg-pNA(S-2302) the presence of soybean trypsin inhibitor and by assessing cleavage on sodiumdodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Upon incubation with MoAb F1 alone, factor XI1 was auto-activated in a time-dependent fashion, activation being maximalafter 30 hours. Factor XI1 incubated in the absence of MoAb F1 was hardly activated by kallikrein, whereas in the presence of MoAb F1, but not in that of a control MoAb, the rate of factor XI1 activation by kallikrein was promoted at least 60-fold. Maximal activation offactor XI1 with kallikrein in thepresence of MoAb F1 was reached within 1 hour. This effect of kallikrein on the cleavage of factor XI1 bound t o MoAb F1 was specific because the fibrinolytic enzymes plasmin, urokinase, and tissue-type plasminogen activator couldnot substitute for kallikrein. Also, trypsin could easily activate factor XII, but in contrast t o kallikrein, this activation was independent of MoAb F1. SDS-PAGE analysis showed that theappearance of amidolytic activitycorrelated well with cleavage of factor XU. MoAb F1-induced activation offactor XI1 in this purified system wasnot dependent on the presence of high-molecular-weight kininogen (HK), in contrast to the activation of Experiments with the contact systemin plasma by MoAb Fl. deletion mutants revealed that theepitopic regionfor MoAb F1 on factor XI1 is located on the kringledomain. Thus, this study shows that binding of ligands t o t h ekringle domain, which does not contributeto theproposed binding site for negatively charged surfaces, may induce activation of factor XII. Therefore, these findings pointto theexistence of multiple mechanisms of activation of factor XII. 0 1995 by The American Societyof Hematology. T upon binding to an activator to be the key event for initiating activation of factor W and, hence, of the contact These conformational changes render factor XI1 much more susceptible to proteolytic activation by other plasma or cellular proteases, in particular plasma kallikrein. The site of factor XI1 involved in binding to an activator has been localized between amino acids 134 and 153, the fibronectin type I domain of factor XII," and between amino acids 2 and 12,1x.19 Preliminary experiments suggest an additional binding site in the epidermal growth factor-like domain and/or kringle domain.20Factor XI1 bound to an activator also displays auto-activation, an intermolecular process in which activator bound-single chain factor XI1 is activated by factor XIIa.2'~Z4 Previously, we described that the contact system in plasma can be activated by a monoclonal antibody (MoAb), MoAb F1, which is not directed against the binding site of factor XI1 for anionic surfaces.*' A detailed knowledge of the binding site for MoAb F1 on factor XI1 as well as that of the mechanism of activation by MoAb F1 may, therefore, provide more insight into the molecular changes during the activation process of factor XII. In this report we analyze in detail the activation of purified factor XI1 by MoAb F1 and localized its epitope on the heavy chain region. Our results indicate that MoAb F1 is directed against the kringle domain and that binding of MoAb F1 to this domain induces a conformational change in factor XI1 rendering it a better substrate for plasma kallikrein. HE CONTACT SYSTEM of human plasma consists of the zymogens factor XI1 (Hageman factor), prekallikrein (PK), factor XI, and the nonenzymatic cofactor highmolecular-weight kininogen (HK).' In vitro, factor XII, an 80-kD glycoprotein, readily binds to anionic surfaces such as silicates, dextran sulfate or sulfatides, and thereby activates the contact systemwhich inturn in may initiate activation of the intrinsic pathway of blood coagulation, the fibrinolytic system, the complement cascade and the production of kin ins.'^' Protein sequencing as well as cloning of full-length cDNA have shown that the heavy-chain region of factor XI1 contains a number of domains homologous to those recognized previously in other proteins, ie, (from the aminoterminal to the carboxyterminal region) a fibronectin type-I1 domain, an epidermal growth factor-like domain, a fibronectin type-l domain, a second epidermal growth factor-like domain, a kringle, and a proline-rich region, the latter being unique for factor XII. The light-chain region contains the catalytic domain characteristic for serine proteases.'-" Several investigators have proposed conformational changes in factor XI1 From the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service and Laboratory for Experimental and Clinical Immunology, University of Amsterdam, Amsterdam, The Netherlands; andthe Dipartimento di Biopatologia Umana, Sezione di Biologia Cellulare, Universita di Roma "La Sapienza, "Rome, Italy. Submitted November 15, 1994; accepted June 8. 1995. Supported by Grant No. 89.127 fromthe Dutch Heart Foundation. Address reprint request to C. Erik Hack, MD, c/o Publication Secretariat, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, PO Box 9406, 1006 AKAmsterdam, The Netherlands. The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 1995 by The American Society of Hematology. 0006-4971/95/8611-0115$3.00/0 4134 MATERIALS AND METHODS Reagents. Dextran sulfate (molecular weight 500.000; DXS 500), Protein G , CNBr-activated Sepharose 4B, and benzamidineSepharose were obtained from Pharmacia Fine Chemicals AB (Uppsala, Sweden); hexadimethrine bromide (Polybrene) from Janssen Chimica (Beerse, Belgium); Tween-20 from J.T. Baker Chemical CO (Phillipsburg, NJ); soybean trypsin inhibitor (SBTI) from BDH Biochemicals Ltd (Poole, UK); nitrocellulose membranes from Scheiler and Schull (Dassel, Germany); the chromogenic substrates H-D-Pro-Phe-Arg-p-nitroanilide (S-2302), H-D-Val-Leu-Lys-pNA Blood, Vol 86, No 1 1 (December I), 1995:pp 4134-4143 From www.bloodjournal.org by guest on October 15, 2014. For personal use only. MoAb INDUCED ACTIVATION OFFACTOR XI1 (S2251), and H-D-Ile-Pro-kg-pNA (S2288) were from Chromogenix AB (Mlilndal, Sweden); and restriction endonucleases, T4 DNA ligase and the Klenow fragment of DNA polymerase I were purchased from New England Biolabs GnbH (SchwalbachlTaunus, Germany). Proreins. The MoAbs F1 and F3, against human factor XII, and MoAb K15, against human (pre)kallikrein, have been described previously.2s.26MoAb B7C9, directed against an epitope on the first 28 amino-terminal amino acids of the heavy-chain of factor XII," was a kind gift from Dr R.A. Pixley (Temple University, Philadelphia, PA). Human urokinase (uPA) was obtained from Laboratoire Choay (Paris, France) and recombinant tissue-type plasminogen activator (r-tPA) from Boehringer Mannheim Biochemica (Mannheim, Germany). a-Factor XIIa was obtained from Kordia (Leiden, The Netherlands). The enzymatic activities were established usingthe chromogenic substrates S2288 for tPA and uPA, and S2302 for afactor XIIa. Plasminogen (PLG) prepared using MoAb APl, directed against human plasmin(ogen), according to Levi et al." Plasminogen was converted into plasmin using uPA (molar ratio of PLG to uPA was 1 0 0 to 1). Plasmin activity was established using the chromogenic substrate S225 l. Bovine trypsin was obtained from Sigma Chemical CO (St Louis, MO) and was further purified by cation-exchange chromatography on a Phast S column (Pharmacia). Factor XI1 was purified as described previously.**The preparation was found to contain approximately 0.7% of activated factor Xll as determined with S-2302 using the low-molecular-weight form of active factor X I , termed &factor XIIa as a reference (the term afactor XIIa is used herein to denote the 80-kD form of activated factor XII, p-factor XIfa refers to the 28-kD activated factor XIl fragment). 8-FactorXIla was obtained by incubating factor XIl with trypsin at a 500 to 1 molar ratio, followed by anion-exchange chromatography of the mixture on a Sephadex DEAE-A50 column (Pharmacia). The resulting p-factor XIIa preparation contained no detectable trypsin activity and migrated as a single protein band of approximately Mr 30,000 on nonreduced as well as reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Prekallikrein (PK) was purified according to a similar procedure as described for factor XI1 with some exceptions. Briefly, fresh citrated human plasma was filtered through a column of MoAb K15Sepharose. PK was eluted with 3 moVL KSCN and dialyzed against phosphate-buffered saline, pH 7.4 (PBS). Then, the PK preparation was sequentially applied onto columns of F3-Sepharose to remove traces of factor Xi1 species, protein G-Sepharose to remove Igs, and benzamidine-Sepharose to remove contaminating proteases. The final preparation was adjusted to pH 5.2 as described for factor ?Qfs and stored at -70°C. Purified PK (20 pg) migrated as a doublet of Mr 86,000 and Mr 88,000 on nonreduced as well as reduced SDSPAGE. PK was converted into kallikrein by incubation with f3-factor XIIa coupled to Sepharose 4B (molar of ratio p-factor XIIa to PK 1 to 100) for 1 hour at 37"C, after which the p-factorXIIa-Sepharose was removed by centrifugation. SDS-PAGE analysis showed complete conversion of PK into kallikrein. Protein concentrations were determined by radioimmunoassay (kallikrein and factor XUz6)or by spectrophotometer using = 16.1 for plasmin, 15.6 for trypsin and 14 for MoAb F1.All proteins were stored in aliquots at -70°C. Localization of the epitope for MoAb F1 on factor X l l . The epitopic region of MoAb F1 on factor XII was localized using the following factor XI1 variants: full-length recombinant (r) factor XI1 [rFXIIl; r-factor XI1 lacking the N-terminal fibronectin type II domain, the first epidermal growth factor-like domain, the fibronectin type I domain, and the second epidermal growth factor-like domain IrFXII-kringlel; and c-factor XI1 containing only the entire serineprotease region and part of the proline-rich hinge [rFXII-lpc]. Con- 4135 struction of cDNAs, vaccinia vectors, and expression of recombinant proteins by HepG2 cells have been described for rFXLI and for rFxU-1~c.Z~ The other variant was obtained in an analogous way. Briefly, to obtain a plasmid carrying the sequences coding for rFXIIkringle (PBFXILKringle), plasmids carrying the sequences coding for full-length FXII cDNA (pBFXII) were digested with HincIl and AvrII restriction endonucleases. The cDNA fragment comprising nucleotides 48 through 925 (numbering according toref 29) was removed and digested with Sty I restriction endonuclease to isolate the cDNA fragment comprising nucleotides 639 through 925. This latter fragment was ligated into pBFXII digested with HincII-AvrII, together with a synthetic deoxyribonucleotide obtained by annealing two chemically synthesized deoxyribonucleotides: 5'-AACACTTKGATTGACAC-3' and 3'"lTGTGAAAGCTAACTGTGGTTCS. The synthetic nucleotide was used to reconstitute the proper cleavage site after the leader peptide and the correct reading frame of the cDNA. As confirmed by sequence analysis according to Sanger et al?' the FXlI cDNA inserted in pBFXII.Kringle was 1,739 bp in length and codes for the complete leader peptide (amino acid residues - 19 to +l), the kringle domain, the proline-rich region, and the complete catalytic domain (amino acid residues +l93 to +596). The recombinant insert from pBFXII.Ktingle plasmid was subcloned into the HindIII-Sac I sites of vector p1 lkd6-131." Construction and selection of a recombinant vaccinia virus, v-rFXII.&ingle, containing the FXEKringle cDNA sequences, were performed as described for rFXII and r F X I l - l ~ c . ~ ~ The epitopic region of MoAb F1 on XI1 was localized as follows: human hepatoma cells (HepG2), grown to subconfluency in Dulbecco's modified Eagle's medium containing 1Wo(voYvol) fetal calf serum, were infected with wild-type vaccinia viruses or with recombinant vaccinia viruses carrying cDNA sequences coding for the different factor XI1 variants. Twenty-four hours after infection, cells were washed with minimum essential medium (MEM) without L-cysteine (GIBCO-BRL, Gaithersburg, MD) and incubated for 2 hours with MEM without L-cysteine. Then, themediumwas replaced with MEM without L-cysteine containing 50 pCimL of Promi~:L-[~'s]in vitro cell labeling mix (Amersham, Bucks, UK), which contains approximately 70% L-t3*S]methionine and 30% L["Slcysteine, and the cells were grown for an additional 6 hours. Then, approximately S X IO6 cells were washed with PBS and lysed with 1 mL of cold NP-40 lysis buffer (ie, 150 mmolfL NaCI, 1% (wt/vol) Nonidet P-40 (NP-40; ICN Biomedicals. Aurora, OH), and 50 mmoVL Tris, pH 8.0). The cell lysates were centrifuged for 30 minutes at 4°C at 10,OOOg and the supernatant collected. Labeled recombinant factor XI1 mutants were absorbed from the supernatant using MoAb Fl.OT2, and B7C9 coupled to CNBr-activated Sepharose 4B in the presence of bovine serum albumin (BSA) (0.3%,wt/ vol). After 4 hours of incubation, the samples were Centrifuged at 1,5OOg, the supernatant discarded and the Sepharose washed four times with PBS containing Tween-20 (0.1% wt/vol), and thereafter once with PBS containing 1 moVL NaCl and once with PBS containing SDS (0.196, wt/vd). The Sepharose beads were dried and sample buffer for gel electrophoresis was added. SDS-PAGE was performed in the presence of dithiotreitol (DTT) using a 5% (wt/ vol) polyacrylamide stacking gel and 12% (wt/vol) polyacrylamide separating gel. Thereafter, the gels were incubated for 30 minutes with Enlightening (Dupont, Boston, MA), dried, and protein bands were visualized by autoradiography using x-ray films. A detailed description of the construction and characterization of these recombinant factor XI1 variants is submitted (F. Citarella et al, manuscript submitted). Chromogenic assays. Assays were performed at37°Cin Tris buffer (ie, 100mmovL Tris containing 150 mmoW NaCl and 0.1 % [wt/vol] Tween-20, pH 8.0). H-D-Pro-Phe-kg-p-nitroanilide ( S 2302) was used to quantitate factor XIIa generated. The substrate was dissolved in distilled water and the concentrations of stock From www.bloodjournal.org by guest on October 15, 2014. For personal use only. 4136 solutions were determined by absorbance at 316 nm (molar extinctioncoefficient cman= 1.27 X IO4 [mol/L]".cm").Thesubstrate was usually dissolved at a concentration of approximately 4 mmol/ L and was stored at -20°C. Before use S-2302 was diluted to 0.5 mmol/L with Tris buffer containing 0.1% (wt/vol) Polybrene to prevent binding of factorXI1to surfaces, andSBTI (230 pg/mL) to inhibit kallikrein activity (substrate solution). The substances added to the Tris buffer had no influence on the hydrolysis of S-2302 by factor XIIa. Control experiments established that kallikrein trypand sin up to a concentration of 25 nmol/L (final concentration per well) was not able to convert S2302 in this substrate solution. Activation of MoAb Fl-bound factor XI1 in the presence of kallikrein orjbrinolytic proteases. Ten microliters of factor XI1 (final concentration 1,475 n m o m in Tris buffer) was preincubated for 30 minutesat37°Cwith S pL ofMoAbF1(finalconcentration 15 pmolL) in 1.5 mL polypropylene tubes (Eppendorf). Then, 5 pL of kallikrein,plasmin,uPA,r-tPA, or trypsin(finalconcentration 125 nmol/L), prewarmed at37T, were added and the mixtures were incubatedfor25minutesat37°C.Then,10 pL of samplewere transferred to a 96-well microtiter plate (Nunc, Roskilde. Denmark) containing 195 p L substrate solution per well, prewarmed at 37°C. Thereactionwasfollowedbyassessingthechangeinabsorbance at405nm,recordedevery 3 minutesusing a Titertektwinreader (Flow Laboratories, Irvine,UK), equipped with a thermostat (37°C). The amount of factor XlIa present in the mixtures was calculated from the rate of conversion of S-2302 as estimated from the changes in absorbance at 405 nm (AA405/min),by reference to a calibration curve of factor XIIa versus AA40Wmin. This calibration curve consisted of serial dilutions of factor XI1 maximally activated by kallikrein in the presence of MoAb F1. In a parallel experiment, MoAb F3,directedagainstthelightchainoffactorwasusedas a control antibody. Under the experimental conditions used, no hydrolysis of S-2302 byuPA, r-tPA, plasmin, kallikrein, or trypsin was observed in the absence of factor XII. Kinetic analysis of the activation of MoAb FI-bound factor XI1 by kallikrein. Six differentconcentrations of factor XI1 (125 to 1,250 nmol/L)in Tris buffer, were preincubated for 1 hour at 37°C with MoAb F1 (15 prnollL) in polypropylene tubes. Kallikrein (140 of 10 nmolL) was added to start the activation reaction. Samples pL, diluted 1/20 in substrate solution, were assayed for factor XI1 activityat10-secondintervalsduringthe first 60 seconds.Factor XIIa generation was linear with time during this period. The amount of factor XIIa generated was determined as described above. The initial ratesof activation were obtained from least-squares regression lines obtained by plotting generated factor XIIa versus incubation time. A double-reciprocal plot of these rates versus the initial factor XI1 concentration was used to determine Km, Vmax, and K,,of the activation reaction. Kinetics of auto-activation of factor XI1 in the presence of MoAb F l . Different amounts of factor XI1 (125 to 1,250 nmolk) in Tris buffer,wereincubatedfor30to 300 minutes with MoAb FI (15 MmolL) at 37°C in polypropylene tubes. At intervals (varying from 20 to 60 minutes)duringtheincubation,smallaliquots(7.5 &) were withdrawn from the mixtures, diluted 1/20 in substrate solution and assayed for generated active factorXI1 as described above. The change in absorbance at 405 nm were recorded every 10 minutes for up to 2 hours. RAVON ET AL of DTT using a 5% (wt/vol) polyacrylamide stacking gel and 10%' (wt/vol)polyacrylamideseparatinggel.Afterelectrophoresisproteins were transferred electrophoretically onto nitrocellulose membranes. Electroblotting was performed for 1 hour at 200 V in 0.192 m o l L glycine, 19.28 (vol/vol)ethanol and 0.025 mol/L Tris. pH 8.3. Subsequently, the nitrocellulose sheets were incubated overnight with 'Z'I-radiolabeled anti-factorXI1 antibodie? (5 X 10"cpm, ie, approximately150 ngof antibodies) in PETbuffer (ie, PBS-10 mmolL EDTA and 0.1% [wt/vol] Tween-20) containing S'% (wt/vol) nonfat drymilk to block remaining bindingsites on the nitrocellulose sheets. Blots were washed, air dried, and protein bands were visualized by autoradiography using preflashed Kodak X-Omat AR films. The extent of factor XI1 cleavage was judged from the intensity of the uncleaved factor XI1 band, as measured by densitometry. RESULTS Localization of the epitope for MoAb FI on factor XII. Previous experiments indicated that MoAb F1 reacts with an epitope on the heavy chain of factor XII.*' To further map the epitopic region of MoAb F1 on factor XII, we assessed binding of MoAb F1 to severaldeletionmutants lacking the various N-terminal heavy chain domains. As a control,bindingof MoAb OT2, whichisdirected against the catalytic domain of factor XII?' and B7C9, which is directed against an epitope on the aminoterminal 28 amino acids of factor XII," to the r-factor XI1 variants was also assessed. Figure 1 shows that MoAbF1 was able to immunoprecipitate rFXII of Mr 77,500 and 67,000 (lane 9; the band of Mr 67,000 representsincompletelyglycosylated factor XII, as was established in experiments with endoglycosidase F), and the mutant containing the kringle domain, the proline-richregion andthecatalyticdomain of factor XI1 (rFXII-kringle) (lane 10) with doublet of Mr of 46,500 and 46,000, which represent differential glycosylation, as confirmed by endoglycosidase F treatment. However, MoAb F1 did not bind to the r-factor XI1 variant containing only part of the proline-rich hinge in addition to the entire catalytic domain (rFXJI-lpc) (lane 11). As expected,MoAb OT2 boundall threefactor XI1 variants(lanes 5 through 7 ) whereas B7C9 bound only full-length factor XII (lane I), confirming that the other variants lacked the aminoterminal 28 amino acids. Thus, the epitope for MoAb F1 appeared to be located on the kringle domain of factor XII. Kinetics of activation of factor XII by kallikrein in the presence or absence of MoAb FI. To assess activation of factor XI1 by kallikrein in the presence or absenceof MoAb F1, we measured the conversion of the chromogenic substrate S2302 by factor XIIa. Toprevent conversion by kallikrein, SBTI was added to the substrate solution and in control experiments it was established that under the conditions usedkallikrein upto afinalconcentrationof 25 nmollL did not hydrolyze S2302. Purified factor XII, absorbed with benzamidine-Sepharose, had negligible amidolytic activity Assessment of cleavage of factor XI1 during auto-activation or against S-2302. Upon addition of kallikrein a slight increase activation by kallikrein inthe presence of MoAb FI. Factor XI1 of factor XI1 activity was observed (Fig 2) corresponding to was incubatedfor various times with MoAb F1 aloneor preincubated 2% activation of factor XII, and in agreement with observafor 30 minutes with MoAb F1 and then incubated for various times tions by others showing lessthan 5% activation of fluidwith kallikrein at 37°C in polypropylene tubes.At intervals, samples were withdrawn and assayed for factor XlIa activity using the S2302phase factor XI1 at a kallikrein-factor XI1 ratio of IO%.'' In contrast, in the presence of MoAb F1, kallikreinrapidly substrate solution. Simultaneously, samples were transferred to tubes cleaved factor XI1 yielding, for example, 7 1 % factor XIIa containing 1% (wt/vol) SBTI. Thereafter, sample buffer for gel elecunder the experimental conditions shown in Fig 2. The actitrophoresis was added. SDS-PAGE was performed in the presence From www.bloodjournal.org by guest on October 15, 2014. For personal use only. MoAb INDUCED ACTIVATION OF FACTOR XI1 41 37 40.5- Fig 1. Localization of the epitope for MoAb F1 on factor XII. Human hepatoma cells (HepG2) were infected with recombinant vaccinia viruses carring cDNA sequences coding for factorXI1 variants. After infection, cells were labeled with PRO-MIX:L-[35SIin vitro celllabeling mix and lysed with NP-40 lysis buffer. Cell lysates were then immunoprecipitated with MoAb B7C9 (lanes 1 through 41,OTZ (lanes 5 through 81, and F1 (lanes 9 through 121.SDS-PAGE was performed under reducing conditions, the gels were incubated for 30 min with Enlightening, dried, and protein bands were visualized by autoradiographyas described in Materials and Methods. Lanes 1,5, and 9 full-llength r-factor XII; lanes 2, 6, and 10: r-factor XII-kringle; lanes 3,7, and 11: r-factor XII-lpc; lanes 4, 8, and 12: wild-type vaccinia virus. The positions of molecular weight markers in kilodaltons are indicated at theleft. vation of factor XI1 by kallikrein in the presence of MoAb F1 was not inhibited by the presence of Polybrene (data not shown). Moreover. a similar activation of factor XI1 by kallikrein occurred in the presence of F(&')? and F(&') fragments of MoAb F1 (data not shown). To determine the initial rate of activation of factor XI1 by kallikrein in the presence or absence of MoAb FI, different amounts of factor XI1 were preincubated with MoAb FI or Tris buffer alone, andthereafterincubated with kallikrein for 10 to 60 seconds. During this time period up to 10% activation was observed for each concentration of factor XII. Figure 3 shows thatat increasing concentrations of factor 2.00 1.20 1.50 1.00 0.50 1 ./- h v) \ 3 c I ;/ 0 0.00 v -a 5 0.40 10 20 30 40 Time (min) Fig 2. Time-course of substrate hydrolysis by factor Xlla generated by kallikrein in the presence or absence of MoAb Fl.Factor XI1 11,475 nmollL in Tris buffer) was preincubated with MoAb F1 (15 pmollL1 or buffer alone for 30 minutes at 37°C. Then kallikrein (125 nmol/LI or buffer was added, and the mixtures were incubated for 25 minutes at 37°C. Finally, lO-@L samples were withdrawn and added to the S2302 in substrate solution and the change in absorbance at 405 nm wasmeasured as outlined in Materials and Methods. The mixtures contained factor XI1 and buffer ,(l. factor XII, MoAb F1 and kallikrein (01,factor XI1 and kallikrein ( l, and factor XI1 and MoAb F1 (A).The experiment shown was repeated three times with identicalresults. 0.00 Fig 3. Michaelis-Menten plot for the activation of factor XI1 in the presence or absence of MoAb F1. Six different concentrations of factor X11 (125 t o 1,250 nmollL in Tris buffer), preincubated with MoAb F1 for l hour at 37°C. were incubated for 10 t o 60 seconds with kallikrein (140 nmol/L). Generated factor Xlla, and therefrom the initial rates of activation, were determined as described in Materials and Methods.The symbols represent incubation mixtures containing factor XII, MoAb F1, and kallikrein (01;factor XI1 and kallikrein (01; and factor XI1 and MoAb F1 (Al. From www.bloodjournal.org by guest on October 15, 2014. For personal use only. 4138 XII, the rate at which factor XIIa was formed increased. Not surprisingly, the rate of activation was also dependent on the concentration of MoAb F1. However, in separate experiments we established that the rate of activation of factor XI1 reached a plateau at 4 p m o K of MoAb F1. Therefore, in further experiments we added MoAb F1 at a concentration of 15 pmol/L to rule out an influence of the concentration of MoAb F1 on the observed activation. It is to be noted that in separate experiments we established that the affinity (Kassoc,ation) of MoAb F1 for native factor XI1was 6 X IO6 (moK)". This low affinity is probably because of the fact that MoAb F1 has to induce a conformational change in factor XI1 upon binding, which explained the necessity for a molar excess of MoAb F1 in the experiments (we reported previously that MoAb F1 has an affinity of about 200 times higher for activated factor XI1 than native factor XII). Furthermore, there was no significant contribution of auto-activation to the total activation rate under the experimental conditions used: Samples containing the highest concentration of factor XI1 (1,250 nmoVL) and to which SBTI was added after a l-minute incubation with kallikrein, were assayed immediately and after 5 or 10 minutes for factor XIIa activity. The rate of substrate hydrolysis was the same in all cases (data not shown). From a Lineweaver-Burk plot, a double-reciprocal plot of the rates of activation versus the initial factor XI1 concentration, we calculated values of 10 pmol/L for the Km (Michaelis constant), 10 nmol/L for the Vmax (maximal rate of catalysis), and 0.07 S" for the L,, for the activation of factor XI1by kallikrein in the presence of MoAb F1. However, these values could not be determined very accurately using higher concentrations of factor XII, because the amount of factor XIIa present in the preparation was too high, resulting in high blank values in the absence of kallikrein. In addition, it was also impossible to determine the kinetic constants for the activation of factor XI1 incubated with MoAb F1 or kallikrein alone, because the rate at which factor XIIa was formed, was too low. Specijicity of the increased susceptibility of cleavage of factor XII bound to MoAb F1 for kallikrein. It has been suggested that factor XI1may be involved in fibrinolysis.8,27.3Z-3STherefore, we compared the rate of cleavage of MoAb Fl-bound factor XI1 by kallikrein with those by trypsin, a-factor XIIa and the fibrinolytic proteases plasmin, uPA and tPA. As shown in Fig 4, the fibrinolytic enzymes were hardly able to activate MoAb F1-bound factor XII. Activation of MoAb F1-bound factor XI1by these proteases resulted in less than 1% activation of factor XI1 during an incubation period of 25 minutes. Conversely, kallikrein and trypsin activated 70% and 100% of MoAb F1-bound factor XII, respectively. However, the activation by trypsinwas not dependent on MoAb F1 in contrast to that by kallikrein (Fig 4). We also examined the susceptibility of MoAb F1bound factor XI1 for a-factor XIIa. However, because a factor XIIa is able to hydrolyze the substrate S2302, we used a 10-fold lower concentration of a-factor XIIa, ie, 12.5 nmoV L (and also of kallikrein for comparison). a-Factor XIIa activated MoAb F1-bound factor XI1 at a much lower rate than kallikrein. About 0.4% activation was observed (in addition to that induced by MoAb F1) during an incubation RAVON ET AL n 80 S v PI (0 X Y 40 0 buffer pli uPA tPA kal tryp Fig 4. Specificity of activation MoAb F1-bound factor Xli. Factor XI1 (1,475 nmol/L in Tris buffer), preincubated for30 minutes at 37°C with MoAb F1 (15 pmol/L) orbufferalone, was incubated for 25 minutes with 125 nmol/L of plasmin (pli), uPA, r-tPA, kallikrein (kall. and trypsin (tryp). Tan-microliter samples were assayed for factor Xlla activity (fordetails, see Materials and Methods). Resultsare expressed as percentage of maximal activation. (a],Activation of factor XI1 in the absence of MoAb F1; (Dl, activation of factor XI1 in the presence of MoAb F1. periodof 20 minutes, whereas a similar concentration of kallikrein'yielded 12%activation under these conditions. DFactor XIIa at a similar concentration did not activate MoAb FI-bound factor XII. In all the experiments detailed above, MoAb F3, an antibody against the light chain of factor XI1 was studied as a control. In the presence of this MoAb, no significant changes in the rate of activation of factor XI1 by kallikrein or the other enzymes, except for trypsin, was observed (data not shown). Auto-activation offactor XII induced by MoAb F l , Purified factor XI1 in the presence of negatively charged surfaces may undergo autoactivation. Therefore, we studied the effect of various concentrations of factor XI1 on the rate of autoactivation induced by MoAb F1. It is to be noted that to study autoactivation, different conditions had to be used than those used to study susceptibility for kallikrein cleavage. For the latter an incubation of up to 1 hour was used, whereas for autoactivation at least 5 hours incubation was necessary. Figure 5A shows that in the presence of MoAb F1 concave upward progress curves were obtained with the highest factor XI1 concentrations, consistent with an continuously increasing rate of formation of activated factor XII. The purified factor XI1 was found to contain about 0.7% of activated enzyme, which could catalyze the autocatalytic process. Incubation of MoAb F1-bound factor XI1 with @factor XIIa did not support activation of factor XII. However, this autoactivation process was very slow compared with the activation of MoAb F1-bound factor XII by kallikrein. For example, with the highest concentration of factor XI1 (1,250 nmoV From www.bloodjournal.org by guest on October 15, 2014. For personal use only. 4139 MoAb INDUCED ACTIVATION OF FACTOR XI1 I 150 0 a0 160 240 320 160 200 Time (min) 100 B 75 h I -C v l3 50 II 25 0 0 60 100 Time (min) Fig 5. Progress curves for autoactivation of factor XI1 in the presence of MoAb F1. (A) Various concentrations of factor XI1 were incubated at 37°C with MoAb F1 for the times indicated. Aliquots (10 pL) of the incubation mixtures were assayed for factor XI1 activity. Experimental procedures are described in Materials and Methods. The initial concentrations of factor XI1 were 125 W, 250 IO), 500 (D), 750 (A),1,000 (0)and . 1250 (A)nmol/L. (Inset) Percentage of activated factor XI1 formed after 5 hours of incubation with buffer (B) Factor XI1 1 5 0 0 nmol/L) was incubated alone (a)or MoAb F1 (0). a t 37°Cwith buffer alone (01,DXS 500 (2 pgfmL final concentration; 0). MoAb F1 ( l 5 pmol/L final concentration; D), or together with MoAb F1 and DXS 500 (A) for the timesindicated. Factor XI1 activity was assayed as described above. L) used, only10%of the native factor XI1was activated after 5 hours of incubation (inset, Fig 5A), whereas 70% of factor XI1 was activated in the presence of kallikrein within 1 hour (Fig 6B). Autoactivation in the absence of MoAb F1 was even slower, yielding for example, 1.5% activation of the native factor XI1 after 5 hours at 37°C (inset, Fig 5.4). TO test the effects of negatively charged surfaces in the presence of MoAb F1, factor XI1 (500 nmol/L) was incubated with buffer alone, MoAb F1 (15 pmolk), DXS 500 (2 pg/mL), or together with MoAb F1 and DXS 500 at 37°C. As shown inFig 5B, under the conditions used no autoactivation was observed in the presence ofDXS 500 alone. In the presence of MoAb F1 autoactivation was observed, and this was potentiated in the presence of DXS 500: for example, after 3 hours of incubation, about 9% of factor XI1 was activated in the presence of MoAb F1 alone, whereas in the presence of both MoAb F1 and DXS, about 21% of factor XI1 was activated. The molar ratio of MoAb F1 to factor XI1 during the autoactivation experiments was atleast 10 to 1 (Fig 5). Therefore, most, if not all, of factor XII-MoAb F1 complexes consisted of one molecule factor XI1 and one molecule MoAb F1, andthis apparently was sufficient to allow autoactivation. Consistent herewith, a similar autoactivation was observed when F(ab')-fragments of MoAb F1 wereused (data not shown), definitely ruling out the possibility that the observed autoactivation was due to a spatial positioning of two factor XI1 molecules by one MoAb F1 molecule. Taken together, these findings indicated that autoactivation of factor XI1 is accelerated in the presence of MoAb F1. Cleavage of factor XII because of autoactivation or by kallikrein inthe presence of MoAb F1. The experiments shown above indicated that in the presence of MoAb F1 , factor XI1 obtained amidolytic activity, which process was enhanced by kallikrein. To assess whether this increase in amidolytic activity was due to generation of two-chain factor XIIa, we studied the coincidence of cleavage of factor XI1 and the generation of amidolytic activity during incubation of factor XI1 with MoAb F1 in the presence or absence of kallikrein. Cleavage of factor XI1 was assessed by SDSPAGE under reducing conditions. The electrophoresed factor XI1 species were visualized by immunoblotting with '"1polyclonal anti-factor XI1 antibodies followed by autoradiography. The extent of cleavage was determined by densitometry. Figure 6 shows the time course of the appearance of amidolytic activity of MoAb F1-bound factor XI1 in the absence (A) or presence of kallikrein (B) and the time course of cleavage of factor XI1 under these conditions (C). During the course of activation, factor XI1 was cleaved into a-factor XIIa as evidenced by the presence of the heavy chain with Mr 50,000 (Fig 6C). After 1 hour, about 70% of MoAb Flbound factor XI1 was cleaved in the presence of kallikrein (Fig 6C, lane 13), which correlated well with the generation of factor XIIa amidolytic activity (inset, Fig 6B). In contrast, only about 17% of the maximal amidolytic activity was observed when factor XI1 was incubated for 5 hours with MoAb F1 alone (Fig 6A), which was accompanied by a comparable extent of cleavage of factor XII, as was judged from the intensities of the uncleaved factor XI1 bands (Fig 6C; inset, Fig 6A). Upon prolonged incubation of MoAb F1-bound factor XI1 in the presence (not shown) and absence of kallikrein, cleavage products of Mr =50,000 and Mr =40,000 and minor products of Mr 30,000 and Mr 18,OOO were observed (Fig 6C, lane 5). In none of the experimental condi- From www.bloodjournal.org by guest on October 15, 2014. For personal use only. RAVON ET AL 41 40 B75 n 50 S v -m X Y 25 l 0 1 0 100 200 7 5 300 Time (min) 0 50 0 25 Time (min) n - 106 - 80 - 49.5 - 32.5 - 27.5 Fig 6. Comparison of cleavage of factor XI1 and appearance of amidolytic activity. (A)Factor XI1 (1,400 nmol/L) wasincubated with MoAbF1 (15 pmollL) alone for various times at37°C and generation of amidolytic activity wasassessed. The extent of cleavage was analyzed by SDSPAGE (under reducing conditions) followed by immunoblotting with '251-anti-factorX11 antibodies as described in Materials and Methods. Generation of factor XI1 amidolytic activity is expressed as percentage of maximal amidolytic activity. (B) In a parallel experiment, factor XI1 (1,400 nmollL) was incubated for 30 minutes at37°C with MoAbF1 (15 pmol/L) and activated by kallikrein(140 nmol/L) forvarious times. Factor XI1 amidolytic activity ( 0 )and the extent ofcleavage (inset A andB; CI) were assessed as described above. (Cl Autoradiographs of the cleavage of factor XI1 in the presence of MoAb F1 alone (lanes 1 through 5) and MoAb Fl-boundfactor XI1 in the presence of kallikrein (lanes 7 through 13) corresponding to the experiments shown in (A) and (B), respectively. Incubation time with MoAbF1 alone: 2 hours (lane l ) , 3 hours (lane 21, 4 hours (lane 31, 5 hours (lane 4). and 27 hours (lane5). Incubation time in thepresence of kallikrein: 1 minute (lane7). 3 minutes (lane 8 ) . 6 minutes (lane9). 10 minutes (lane10). 20minutes (lane 11). 30 minutes (lane121, and 60 minutes (lane 13). Lane 6, factor XI1 alone. The positions of the molecular weights markers (in kilodaltons) are indicated on the right. tions studied did we observe significant amidolytic activity accompanied by the presence of only the 80.000 daltons form of factor XII. DISCUSSION Previously wehave shown that MoAb F1 activates the contact system in plasma."As this MoAb is not directed against the surface-binding site,'< unraveling ofhow this MoAb induces activation may show an alternative mechanism of activation of factor XII. Therefore, in this study we performed a detailed analysis of the changes of factor XI1 that occurred after binding to MoAb FI. We found that in a purified system, factor XI1 was rapidly cleaved and activated by kallikrein in the presence of MoAb FI. in contrast to the slow activation that occurred in the absence of MoAb F t . The latter is consistent with the concept that factor XI1 in the fluid phase is a poor substrate for kallikrein."..'" Optimal activation of the contact system in plasma by MoAb F1 was dependent on the presence of both factor XII. PK.and HK,'5 whichreflect the importance of reciprocal activation of factor XI1 and PK in theMoAb FI-induced activation of plasma. We observed no significant effect of the presence of HK (up to concentrations of 80 nmol/L. data not shown). Thus, activation of MoAb FI-bound factor XI1 in this purified system by kallikrein was independent of HK. The explanation for these seemingly discrepant results is presumably that in this study we focused on the changes that occurred in factor XI1 after binding toMoAb Ft. and for From www.bloodjournal.org by guest on October 15, 2014. For personal use only. MoAb INDUCEDACTIVATION OF FACTOR XI1 this reason we added kallikrein rather than PK. Experiments are now underway to establish whether HK is required for contact activation by MoAb F1 when using factor XI1 and PK (rather than kallikrein) or in the presence of plasma inhibitors such as Cl esterase inhibitor. In vitro, factor XI1 can be activated by trypsin, plasmin, factor XIa, kallikrein,12s36-38 factor XIIa,21-24and microbial pro tease^.^^.^ In this study, the rate of activation of factor XI1 by a-factor XIIa and the fibrinolytic proteases plasmins, uPA and tPA, was at least 100-fold slower than that by kallikrein. As shown in Fig 4, MoAb F1-bound factor XI1 was specifically cleaved by kallikrein, consistent with the concept that kallikrein is the enzyme mainly responsible for the generation of active factor XII.'2,366,37,41 p-Factor XIIa had no effect on the activity of MoAb F1-bound factor XII, in agreement with the results of Silverberg et al?' who observed no cleavage of glass-bound factor XI1 upon incubation with p-factor XIIa, whereas cleavage occurred with a-factor XIIa. We observed that a-factor XIIa also is able to activate factor XI1 bound to MoAb F1, although less efficiently than kallikrein. Thus, these data together show that a-factor XIIa is the factor XI1 species that is able to propagate autoactivation induced by MoAb F1, whereas p-factor XI1 has no role in this process. One hypothesis to explain the initial generation of active factor XI1 during contact activation implies that factor XI1 gains intrinsic enzymatic activity without proteolytic cleavage, upon binding to a surface, because of a conformational change in the molecule.'",42We studied this concept by incubating benzamidine-Sepharose absorbed factor XI1 with MoAb F1, also absorbed with benzamidine-Sepharose. Under our experimental conditions, the generation of amidolytic activity correlated well with cleavage of factor XI1 as was assessed by SDS-PAGE, immunoblotting with 12'I-anti-factor XI1 antibodies and densitometry, upon incubation with MoAb F1 alone (Fig 6). Thus, we found no experimental evidence for the existence of a single-chain 80-kD enzymatically active species. In similar experiments with sulfatides>22 g l a ~ s , ~ ' .or ~ ' DXS," comparable findings have been reported. The surface-binding site of factor XI1 has been proposed to consist of residues 134 through 153 (belonging to the fibronectin type I region of factor XII), as proposed by Pixley et al," and residues 1-28.'8,19Using deletion mutants, we localized the epitope on factor XI1 for MoAb F1 on the kringle domain of factor XII, corresponding to the residues 193 through 276:" ie, a region not considered to contribute to the surface-binding site. Recent studies in our laboratory indicate that factor XI1 may have another or a complementary surface-binding site located in the second epidermal growth factor-like domain and/or kringle domain."However, MoAb F1 is not directed against any of these binding sites, because it easily recognizes factor XI1 bound to glass2' and is not able to inhibit binding of labeled factor XI1 to glass or other activators (F.C., unpublished observations, 1994). In agreement with the concept that the binding sites for MoAb F1 and negatively charged surfaces on factor XI1 are different, was the observation that MoAb F1 and DXS 500 together enhanced the observed increase in susceptibility for proteolytic cleavage (Fig 5B). 4141 Generally, kringle domains are thought to function as autonomously folding modules specialized in protein-protein interactions and to play regulatory roles in the functions of the proteins in which they have been identified. The kringle module has been implicated in the binding of fibrinolytic proteins to fibrin, which binding seems to be essential for effective activation of the p r ~ t e i n s ? ~Kringle -~l domains also mediate the interaction of plasminogen with a2-antiplasmin and tPA with plasminogen activator inhibitor type 1 (PAI1).52-5h Plasminogen binds specificallyand in a functional manner tohumanumbilicalvein endothelial cells (HUVECs), which binding is mediated by the lysine-binding sites present in its kringle Factor XI1 has also been shown to bindspecifically to H U V E C S , ~ ,although ~' the binding site on factor XI1 mediating this effect has not been identified. Schmeidler-Sapiro et a16' have reported mitogenic effects of factor XI1 on HepG 2 cells, although also the binding site necessary for this effect is not known. The most potent mitogen for mature hepatocytes is hepatocyte growth factor, a protein homologous to pla~minogen.'~Binding of hepatocyte growth factor to its receptor on hepatocytes is mediated by its first and second kringle domain.ma Therefore, it is tempting to speculate that some of the effects of factor XI1 on cells may occur via its kringle domain. Our studies with MoAb F1 predict that such an interaction of factor XI1 with cellular receptors may allow the stabilization of a conformation resulting in an enhanced susceptibility for proteolytic cleavage and an acceleration of autoactivation. Such an enhanced susceptibility of proteins for other proteases, after binding via their kringles, is known. For example, the enzymatic activity of single-chain uPAis potentiated upon binding to its receptor on monocytes.67Furthermore, plasminogen bound to its receptor, is more easily activated by tPAs7,h8 oru P A . ~ ~ . ~ ' The classical kringle structure contains specific lysinebinding sites. Recently, however, Stephens et a17' described a class of kringles with affinityfor polyanions. They reported that the uPA kringle domain contains a heparin binding site, which mediates the stimulation of activation of pro-uPA by plasmin and the activation of plasminogen by uPA in cellfree system^.^"^^ Because factor XI1 is also activated when bound to negatively charged surfaces, interactions of factor XI1 with proteoglycans in vivo via its kringle domain may also serve as the mechanism of factor XI1 activation. Whether these mechanisms may contribute to activation of factor XI1 under physiologic conditions remains to be established in further studies. In conclusion, we have shown that MoAb F1 binds to the kringle domain of factor XII, which domain does not contain the proposed binding sites for negatively charged surfaces, andthat this MoAb enhances susceptibility of factor XI1 for cleavage by kallikrein. These findings may point to the existence of alternative mechanisms of activation of factor XII. REFERENCES I . Tans G, Rosing J: Structural and functional characterisation of factor XII. Semin ThrombHemost 13:1, 1987 2. Tans G, Griffin JH: Properties of sulfatides in factor-XII-dependent contact activation. Blood 59:69, 1982 From www.bloodjournal.org by guest on October 15, 2014. For personal use only. 4142 3. van der Graaf F, Keus FJA, Vlooswijk RAA, Bouma BN: The contact activation mechanism in human plasma: Activation induced by dextran sulfate. Blood 59:1225, 1982 4. Espana F, Ratnoff OD: Activation of Hageman factor by sulfatides and other agents in the absence of plasma proteases. J Lab Clin Med102:31,1983 5. Hack CE, Dors DM: Activation and inhibition of factor XI1 (Hageman factor) in vivo. Cum Opin Invest Drugs 1:95, 1993 6. Fuhrer G. Gallimore MJ, Heller W, Hoffmeister HE: F X11. Blut 61:258, 1990 7. Kaplan AP, Silverberg M: The coagulation-kinin pathway of human plasma. Blood 70:1, 1987 8. Kaplan AP: The intrinsic coagulation, fibrinolytic and kininforming pathways of man, in KelleyWN, Harris ED, Ruddy S, Sledge CB (eds): Textbook of Rheumatology. Philadelphia, PA, Saunders, 1985, p 95 9. McMullen BA, Fujikawa K: Amino acid sequence of the heavy chain of human alpha-factor XlIa (activated Hageman factor). J Biol Chem 2605328, 1985 IO. Cool DE, Edgell CS, Louie CV, Zoller MJ, Brayer CD, Macgillivray RTA: Characterization of human blood coagulation Factor XI1 cDNA. Prediction of the primary structure of factor XI1 and the tertiary structure of beta-factor XIIa. J Biol Chem 260: 13666, 1985 I 1. Cool DE, Macgillivray RTA: Characterization of the human blood coagulation factor XI1 gene. J Biol Chem 262:13662, 1987 12. Griffin JH: Role of surface in surface-dependent activation of Hageman factor (blood coagulation factor XII). Proc Natl Acad Sci USA 75: 1998, 1978 13. Revak SD, Cochrane CG, Johnson AR, Hugli TH: Structural changes accompanying enzymatic activation ofhuman Hageman factor. J Clin Invest 54:619, 1974 14. Samuel M,Pixley RA, Villanueva MA, Colman RW, Villanueva GB: Human factor XI1 (Hageman factor) autoactivation by dextran sulfate circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J Biol Chem 267:19691, 1992 15. Heimark RL, KurachiK, Fujikawa K, Davie EW: Surface activation ofblood coagulation, fibrinolysis andkinin formation. Nature 286:456, 1980 16. Ratnoff OD, Saito H: Amidolytic properties of single chain activated Hageman factor. Proc Natl Acad Sci USA 76:1461, 1979 17. Pixley RA, Stumpo LC, Birkmeyer K, Silver L, Colman RW: A monoclonal antibody recognizing an icosapeptide sequence in the heavy chain of human factor XI1 inhibits surface-catalyzed activation. J Biol Chem 262:10140, 1987 18. Clarke BJ, CBtt HCF, CoolDE, Clark-Lewis I, Saito H, Pixley RA, Colman RW, Macgillivray RTA: Mapping of a putative surface-binding site of human coagulation factor XII. J Biol Chem 264:11497, 1989 19. Samuel E, Samuel M, Villanueva GB: Anticoagulant property and conformational flexibility of factor XU-derived peptides. Thromb Haemost 69: 1306, 1993 20. Citarella F, Felici A, Aiuti A, Pascucci B, Dors DM, Hack CE, Fantoni A: Recombinant human factor XII: Role of regulatory domains for the process of contact activation. Thromb Haemost 69:1235, 1993 21. Silverberg M, Dunn JT, Garen L, Kaplan AP: Autoactivation of human Hageman factor demonstration utilizing a synthetic substrate. J Biol Chem 255:7281, 1980 22. Tans G, Rosing J, Griffin JH: Sulfatide-dependent autoactivation of human blood coagulation factor XI1 (Hageman factor). J Biol Chem 258:8215, 1983 23. Tankersley DL, Finlayson JS: Kinetics of activation and autoactivation of human factor XII. Biochemistry 23:273, 1984 24. Mitropoulos KA, Esnouf MP: The autoactivation of factor XI1 in the presence of long-chain saturated fatty acids: A comparison RAVON ET AL with potency of sulphatides and dextran sulphate. Thromb Haemost 66:446, 1991 25. Nuijens JH, Huijbregts CCM, Eerenberg-Belmer AJM, Meijers JCM, Bouma BN, Hack CE: Activation ofthe contact system of coagulation by a monoclonal antibody directed against a neodeterminant in the heavy chain region of human coagulation factor XI1 (Hageman factor). J Biol Chem 264:12941, 1989 26. Nuijens JH, Huijbregts CCM, Eerenberg AIM, Abbink JJ, Strack van Schijndel RJM, Felt-Bersma RJF, Thijs LG, Hack CE: Quantitication of plasma factor XIIa-C1-Inhibitor and kallikrein-CI inhibitor complexes in sepsis. Blood 72:1841, 1988 27. LeviM,Hack CE, De Boer JP, Brandjes DPM. Biiller HR, ten Cate WJ:Reduction of contact activation related fibrinolytic activity in factor XI1 deficient patients. Further evidence for the role of the contact system in fibrinolysis in vivo. J Clin Invest 88: I 155, l99 1 28. Dors DM, Nuijens JH, Huijbregts CCM, Hack CE: A novel sensitive assay for functional factor XI1 based on the generation of kallikrein-Cl inhibitor complexes in factor XI1-deficient plasma by glass-bound factor X11. Thromb Haemost 67:644, 1992 29. Citarella F, AiutiA, La Porta C, Russo G, Pietropaolo C , Rinaldi M, Fantoni A: Control of human coagulation by recombinant serine proteases. Blood clotting is activated by recombinant factor XI1 deleted of five regulatory domains. Eur J Biochem 208:23, 1992 30. Sanger F, Nicken S, Coulson AR:DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463, 1977 3 1. Smith CL, Murphy BR, Moss B: Construction and characterization ofan infectious vaccinia virus recombinant that expresses the influenza hemoagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci USA 80:7155. 1983 32. Kluft C, Dooijewaard G, Emeis JJ: Role of the contact system in fibrinolysis. Thromb Haemost 13:50, 1987 33. Hauert J, Nicoloso G, Schleuning WD, Bachman F, Schapira M: Plasminogen activators in dextran sulfate-activated euglobulin fractions: A molecular analysis of factor XU-and prekallikreindependent fibrinolysis. Blood 73:994, 1989 34. Tsuda H, Miyata T, lwananga S, Yamamota T: Analysis of intrinsic fibrinolysis in human plasma induced by dextran sulfate. Thromb Haemost 67:44O, 1992 35. Miles LA, Rothschild Z, Griffin JH: Dextran sulfate-dependent fibrinolysis in whole plasma Dependence on factor XI1 and prekallikrein. J Lab Clin Med 101:214, 1983 36. Cochrane CC, Revak SD, Wuepper KD: Activation of Hageman factor in solid and fluid phases. J Exp Med 138:1564, 1973 37. Fujikawa K, Heimark RL, Kurachi K, Davie EW: Activation of bovine factor XI1 (Hageman factor) by plasma kallikrein. Biochemistry 19:1322, 1980 38. Kaplan AP, Austen KF: A prealbumin activator of prekallikrein. J Immunol 105:802, 1970 39. Yamamoto T. Shibuya Y, Nishino N, Okabe H, Kambara T: Activation of human Hageman factor by Pseudornonus ueruginosa elastase in the presence or absence of negatively charged substance in vitro. Biochim Biophys Acta Protein Struct Mol Enzymol 1038:231, 1990 40. Molla A, Yamamoto T, Akaike T, Miyoshi S, Maeda H: Activation of Hageman factor and prekallikrein and generation of kinin by various microbial proteases. J Biol Chem 264:10589, 1989 41. Meier HL, Pierce JV, Colman RW, KaplanAP: Activation and function of human Hageman factor: The role of high molecular weight kininogen and prekallikrein. J Clin Invest 60:18, 1977 42. MCMillen CR, Saito H, Ratnoff OD, Walto AG: The secondary structure of human Hageman factor (factor XII) and its alteration by activating agents. J Clin Invest 54:1312. 1974 43. Dunn JT. Silverberg M, Kaplan AP: The cleavage and forma- From www.bloodjournal.org by guest on October 15, 2014. For personal use only. MoAb INDUCEDACTIVATION OFFACTOR XI1 tion of activated human Hageman factor by autodigestion and by kallikrein. J Biol Chem 257:1779, 1982 44. Silverberg M, Kaplan A P Enzymatic activities of activated and zymogen forms of human Hageman factor (factor XU). Blood W64, 1982 45. De Vries C, Veerman H, Pannekoek H: Identification of the domains of tissue-type plasminogen activator involved in the augmented binding of fibrin after limited digestion with plasmin. J Biol Chem 264:12604, 1989 46. van Zonneveld AJ, Veerman H, Pannekoek H: On the interaction of the finger and the kringle-2 domain of tissue-type plasminogen activator with fibrin: inhibition of kringle-2 binding to fibrin by €-amino caproic acid. J Biol Chem 261:14214, 1986 47. Verheijen JH, Caspers MPM, Chang GTG, de Munk GAW, Pouwels PW, Enger-Valk BE: Involvement of finger domain and kringle-2 domain of tissue type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J 5:3525, 1986 48. Hoylaerts M, Rijken DC, Lijnen HR. Collen D: Kinetics of the activation of plasminogen by human tissue type plasminogen activator. J Biol Chem 257:2912, 1982 49. Tran-Thang C, Kuithof EKO, Atkinson J, Bachman F: High affinity binding sites for Glu-plasminogen unveiled by limited plasmic degradation of human fibrin. Eur J Biochem 160:599, 1986 50. Randby M Studies of the kinetics of plasminogen activation by tissue type plasminogen activator. Biochim Biophys Acta 704:461. 1982 5 1. Thorsen S : Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation influence of omega-aminocarboxylic acids. Biochim Biophys Acta 393:55, 1975 52. Wiman B, Lijnen HR, Collen D: On the specific interaction between the lysine-binding sites in plasmin and complementary sites in a2-antiplasmin and in fibrinogen. Biochim Biophys Acta 579 142, 1979 53. Moroi M, Aoki N: Inhibition of plasminogen binding to fibrin by a-plasmin inhibitor. Thromb Res 10:851, 1977 54. Kaneko M, Mimuro J, Matsuda M, Sakata Y: The plasminogen activator inhibitor-l binding site in the kringle-2 domain of tissue-type ptasminogen activator. Biochem Biophys Res Commun 178:1160. 1991 55. Wilhelm OG, Jaskunas RS, Vlahos CJ, Bang NU: Functional properties of the recombinant kringle-2 domain of tissue plasminogen activator produced by Escherichia coli. J Biol Chem 265: 14606, 1990 56. Kurokawa T, Toyoda Y, Iwasa S: Characterization of monoclonal antibodies against human tissue plasminogen activator (PA): Quantitation of free @A in human cell cultures by an ELISA. J Biochem 109:217, 1991 57. Hajjar K A , Harpel PC, Jaffe EA, Nachman RL: Binding of plasminogen to cultured human endothelial cells. J Biol Chem 262:11656, 1986 58. Plow EF, Freany DE, Plescia J, Miles L A The plasminogen system and cell surfaces: Evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol 103:2411, 1986 59. Shih GC, Hajjar KA: Plasminogen and plasminogen activator assembly on the human endothelial cell. Proc Soc Exp BiolMed 202258, 1993 41 43 60. Berrettini M, Mazzolai L, Bura A, Nenci GG: Receptors for blood coagulation factor XI1 on the surface of cultured human endothelial cells. Thrornb Haemost 69643, 1993 61. Reddigari SR,Shibayama Y, Brunde T, Kaplan AP: Human hageman factor (factor X I I ) and hig molecular weight kininogen compete for the same binding site on human umbilical vein endothelial cells. J Biol Chem 286:11982, 1993 62. Schmeidler-Sapiro KT, Ramoff OD, Gordon EM: Mitogenic effects of coagulation factor XJI and factor XIIa on HepG2 cells. Proc Natl Acad Sci USA 88:4382, 1991 63. Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S : Molecular cloning and expression of human hepatocyte growth factor. Nature 342:440, 1989 64. Martsumoto K, Takehara T, hone H, Hagiya M, Shimuzu S : Deletion of kringle domains or the N-terminal hairpin structure in hepatocyte growth factor results in marked decreases in related biological activities. Biochem Biophys Res Commun 181:691, 1991 65. Okigaki M, Komada M, Uehara Y, Miyazawa K, Kitamura N: Functional characterization of human hepatocyte growth factor mutants obtained by deletion of structural domains. Biochemistry 31:9555, 1992 66. Lokker NA, Mark MR, Luis EA, Bennett GL, Robbins KA, Baker JB, Godowski PJ: Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J 11:2503, 1992 67. Manchanda N. Schwartz BS: Single chain urokinase. Augmentation of enzymatic activity upon binding to monocytes. J Biol Chem 26614580, 1991 68. Bizik J, Lizonova A, Stephens RW, Grofova M, Vaheri A: Plasminogen activation by P A on the surface of human melanoma cells in the presence of a2-macroglobulin secretion. Cell Regulation 1:895, 1990 69. Meissauer A, Kramer MD, Schinmacher V, Brunner G: Generation of cell surface-bound plasmin by cell-associated urokinasetype or secreted tissue-type plasminogen activator: A key event in melanoma cell invasiveness in vitro. Exp Cell Res 199:179, 1992 70. Ellis V, Scully M F , Kakkar VV: Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. J Biol Chem 264:2185, 1989 71. Ronne E, Behrendt N, Ellis V, Ploug M, Dan0 K, HoyerHansen G. Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NHZterminal domain of the urokinase receptor. FEBS Len 288:233, 1991 72. Stephens RW, Bokman A M , Myohiinen HT, Reisberg T, Tapiovaara H, Pedersen N, Griindahl-Hansen J, Llinhs M, Vaheri A: Heparin binding to the Urokinase kringle domain. Biochemistry 31:7572, 1992 73. Andrade-Gordon P, Strickland S : Interaction of heparin with plasminogen activators and plasminogen: Effects on the activation of plasminogen. Biochemistry 25:4033, 1986 74. Lijnen HR, Collen D: Stimulation by heparin of the plasminmediated conversion of single-chain to two-chain urokinase-type plasminogen activator. Thromb Res 43:687, 1986 75. Paques E-P, Stohr H-A, Heimburger N: Study on the mechanism of activation of heparin and related substances in the fibrinolytic system: Relationship between plasminogen activators and heparin. Thromb Res 42:797, 1986
© Copyright 2026