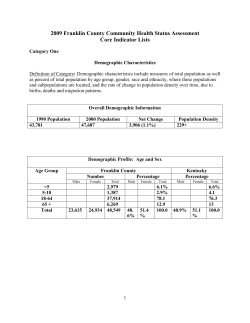
Sepsis Management Guidelines Supplement
Intensive Care Med (2001) 27: S 1±S 2 Charles L. Sprung Gordon R. Bernard R. Phillip Dellinger Introduction ) C. L. Sprung ( ) ´ G. R. Bernard ´ R. P. Dellinger E-mail: [email protected] Phone: +9 72-2-6 77 71 11 Fax: +9 72-2-5 63 54 43 This supplement to Intensive Care Medicine is the result of a sustained effort by the International Sepsis Forum (ISF) to bring together a body of practical recommendations for the management of patients with severe sepsis and septic shock. The ISF is a nonprofit, nongovernmental association whose members are healthcare professionals in critical care and infectious disease committed to improving the understanding and clinical management of patients with severe sepsis. While there is still a high morbidity and mortality associated with sepsis and its sequelae, new data on patient management are emerging that may ultimately significantly improve the current situation. Such findings need to be evaluated and incorporated, when appropriate, into existing treatment protocols. Headed by a Steering Committee of international experts and opinion leaders, the ISF focuses on improving the management of sepsis and, in particular, septic shock by developing an international consensus on the latest understanding of key scientific and clinical issues, and disseminating emerging practice guidelines to researchers, intensivists and other critical care professionals worldwide. The ISF educational program comprises three major elements: workshops on management strategies in sepsis, traveling fellowships and lecture series. Funding for the activities of the ISF is provided through international sponsors using unrestricted educational grants. The present supplement presents management guidelines for practicing clinicians and is primarily devoted to those patients with severe sepsis, typically hospitalized patients, many of whom are in intensive care units. Animal trials have been mentioned when relevant, but our major goal is clinical management and we have primarily focused on human studies. The current recommendations are based on an evidence-based methodology with categorization as previously described (Table 1). The recommendations represent the groups assessment of the evidence-based medicine literature together with clinical practice and personal experience. The evidence-based method is devised primarily for therapeutic trials, and some of the contributions to this Table 1 Grading of responses to questions and levels of evidence (adapted from [1]) Grading of responses to questions A. Supported by at least two level I investigations B. Supported by only one level I investigation C. Supported by level II investigations only D. Supported by at least one level III investigation E. Supported by level IV or V evidence Levels of evidence I. Large, randomized trials with clearcut results; low risk of false-positive (alpha) error or false-negative (beta) error II. Small, randomized trials with uncertain results; moderate to high risk of false-positive (alpha) and/or false-negative (beta) error III. Nonrandomized, contemporaneous controls IV. Nonrandomized, historical controls and expert opinion V. Case series, uncontrolled studies, and expert opinion S2 issue such as definitions, epidemiology of infection and experimental therapy are therefore not well suited to this approach. In addition, not all treatments used in septic patients have been the subject of randomized, controlled trials. Therefore the level of evidence may not be so strong, even though a particular clinical practice is well established and in routine use. Clinicians will need to be aware of this limitation and use their judgement in interpreting these recommendations. The methodology used included a Medline search for at least 10 years prior to the publication, supplemented by a manual search of other relevant journals. Keywords that were used are noted in each contribution to this issue. All nine ISF members or their collaborators was re- Reference 1. Sackett DL (1989) Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 95 [Suppl]:2S±4S quired to present a first draft of their respective contributions prior to a meeting in November 1999. At the meeting each author presented the data for the article, which were discussed. Based on suggestions, changes were made and contributions were revised. Each article was then reviewed by another ISF member and then edited by one of the three editors. We believe this supplement will be an extremely helpful source of practical information for the clinician. As the fields of infectious diseases, microbiology and intensive care are ever moving forward, the data and recommendations may change in the future. In the meantime, we believe this supplement will be an important addition to every clinician's library. Intensive Care Med (2001) 27: S 3±S 9 Definition of sepsis Idit Matot Charles L. Sprung ) I. Matot ´ C. L. Sprung ( ) Department of Anesthesiology and Critical Care Medicine, Hadassah University Medical Center, Hebrew University of Jerusalem, Jerusalem, Israel E-mail: [email protected] Phone: +9 72-2-6 77 71 11 Fax: +9 72-2-5 63 54 43 History of definitions Sepsis is the systemic inflammatory response to infection [1]. Sepsis and its sequelae represent progressive stages of the same illness in which a systemic response to an infection mediated by endogenous mediators may lead to a generalized inflammatory reaction in organs remote from the initial insult and eventually to end-organ dysfunction and/or failure [2]. Sepsis remains an important and life-threatening problem. Sepsis is the most common cause of death in the intensive care unit [3]. Because of increasingly aggressive treatment of patients in advanced stages of illness the incidence and mortality from sepsis in hospital patients remains high [2, 4, 5, 6]. New efforts to improve survival have highlighted the uncertainty of the specific diagnostic criteria used to define entry criteria for clinical trials. In the past the terms bacteremia, septicemia, sepsis, sepsis syndrome, and septic shock were used interchangeably, which led to an imprecise understanding of sepsis and its related disorders and to confusion in the interpretation of clinical trials [6]. A review of the literature published in the 1980s discloses remarkable disparity in the mortality of patients with sepsis, sepsis syndrome, and septic shock [5, 7, 8, 9, 10, 11]. The conflicting results were due in part to different definitions of the clinical entity under investigation. Bone, Sibbald, and Sprung [9, 10, 11] listed several reasons for the lack of firm definitions at that time, including the lack of epidemiological studies to evaluate the systemic response to infection, the lack of precise criteria for the different terms used, the early death of severely injured patients before the development of sepsis and septic shock, and the focus on patients with Gramnegative bacteremia rather than sepsis. Two decades ago the idea of a microbial cause of sepsis was established, and bacteremia was required for a patient to have ªsepsisº [12]. Later, investigators recognized that the host is not passive when invaded by an organism but in fact secretes a large spectrum of endogenous inflammatory mediators that may also result in injury. Moreover, it was also recognized that the same inflammatory response might result from noninfectious insults as well [13, 14]. Further studies [7, 15] found that the clinical response persists after eradication of the infection, and is itself associated with an increased mortality. Although the inflammatory response is beneficial in many patients, an exaggerated response, rather than the invasive infection, may be the more important determinant of outcome in critically ill patients [16]. Methods Consensus Conference definitions To evaluate studies of sepsis for definitions a computer-based review of the literature was undertaken using Medline from 1990 until September 1999 as the primary database. The specific subject heading keywords were definition, diagnosis, criteria, epidemiology, and classification and matching them with infection, sepsis, sepsis syndrome, septicemia, septic shock, and multiple organ failure. A new set of definitions was proposed by the Consensus Conference of the American College of Chest Physicians and the Society of Critical Care Medicine held in Chicago in August 1991 [6]. These definitions included patients in various stages of infection: bacteremia, sep- Introduction S4 Table 1 Clinical frequency of SIRS, sepsis, severe sepsis, and septic shock Reference Location Determination SIRS (%) Sepsis (%) Severe sepsis (%) Rangel-Frausto et al. [19] Pittet et al. [20] Medical, surgical ICUs and wards Surgical ICU Study period 68 26 18 Study period 93 49 16 7 Salvo et al. [21] General ICU Admission 52 5 2 3 Saez-Llorens et al. [22] Pediatric ICU Infection suspected ± 21 61 18 Proulx et al. [23] Jones and Lowes [24] Pediatric ICU Medical ward Study period Time of blood culture 82 55 23 16 4 5 2 3 Muckart and Bhagwanjee [4] Surgical ICU (trauma) 1st 24 h 88 14 14 20 Bossink et al. [25] Medical ward Fever onset 95 44 ± ± sis, severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS). Recommendations from the Consensus Conference provided both a conceptual and practical framework for the definition of the systemic inflammatory response to infection (sepsis). It was expected that the application of a more flexible and easily met definition would improve early bedside detection of sepsis, permit early intervention, standardize research protocols, and allow comparisons of results of clinical trials of new therapies. The conference proposed a new term, systemic inflammatory response syndrome (SIRS), to describe widespread inflammation that occurs following a wide variety of insults including infection, pancreatitis, trauma, burns, etc. The term SIRS validated the concept that endogenous mediators of inflammation play an important role in ªsepsisº together with microbial factors. The term sepsis was defined as a subset of patients with an inflammatory response, limited to those patients with documented infection. The systemic response included two or more abnormalities in temperature, heart rate, respiratory rate, and white blood count. Many patients have infection without a systemic response and are therefore not septic. SIRS, sepsis, severe sepsis, and septic shock represent a continuum of clinical and pathophysiological severity. The process begins with an infection, with or without a systemic inflammatory response, and may progress to a systemic response with severe sepsis (hypotension, hypoperfusion, or organ dysfunction) or septic shock (hypotension not responsive to adequate fluid resuscitation with hypoperfusion or organ dysfunction). It was believed that the phases of the disease process form a continuum of severity which characterize populations at increased risk of morbidity and mortality. The terms septicemia, sepsis syndrome, and refractory shock were eliminated by the Consensus Conference because they were believed to be confusing and nonspecific, applying to a variety of inflammatory states. Septic shock (%) 4 Epidemiology Prior to the Consensus Conference it was not possible to compare epidemiological or outcome studies of sepsis since the definitions were not standardized. Hypothermia, fever, tachycardia, and tachypnea or hypocapnea were included in many studies [7, 12, 17, 18] as well as hypotension [7, 17, 18]. Laboratory results indicative of infection were also included in many sepsis trials. They included abnormal white blood cell count, abnormal neutrophil count, and thrombocytopenia [7, 12, 18]. Manifestations of organ dysfunction in septic patients included altered mentation, hypoxemia, oliguria, coagulopathy, and increased lactate concentration [7, 17, 18]. Unfortunately, no studies had evaluated and compared the epidemiology, morbidity, and mortality of different definitions of sepsis. After the Consensus Conference several studies evaluated the epidemiology of sepsis [4, 19, 20, 21, 22, 23, 24, 25]. The frequency of the problem usually decreased as the severity increased from SIRS to sepsis to severe sepsis and to septic shock (Table 1). Unfortunately, the population of patients studied differed and the inflammatory entity was determined at different times (admission, during the first 24 or 48 h, at fever onset, or over the entire study period). As patients developed more SIRS criteria (from two to four), the incidence of sepsis increased [19, 24], as did mortality [24]. Correlation to mortality Sepsis in its evolution to severe sepsis and septic shock reflect increasing disease severity with increased patient morbidity and mortality. Knaus et al. [5] found that the presence or absence of sepsis (infection plus two of four SIRS criteria) failed to identify subgroups with increased risk of death and concluded that while categoric S5 Table 2 Mortality of SIRS, sepsis, severe sepsis, and septic shock Reference SIRS (%) Sepsis (%) Severe sepsis (%) Septic shock (%) Rangel-Frausto et al. [19] Pittet et al. [20] Salvo et al. [21] Saez-Llorens et al. [22] Jones and Lowes [24] Muckart and Bhagwanjee [4] Bossink et al. [25] 7 6 27 ± 23 8 6 16 0 36 16 ± 10 13 20 35 52 40 38 18 ± 46 58 82 62 56 53 ± definitions of sepsis may be useful in selecting patients for entry into clinical trials, they may not be useful in characterizing individual, or perhaps group, risks. Several studies, however, have demonstrated increasing organ failure (acute respiratory distress syndrome, disseminated intravascular coagulopathy, acute renal failure, and shock) with worsening severity of sepsis [19, 20]. In addition, most studies [4, 19, 20, 21, 22, 24, 25] have noted stepwise increases in mortality from SIRS to sepsis to severe sepsis and to septic shock (Table 2). Clinical characteristics defining the response to infection The Consensus definition [4] of sepsis has been criticized for its restrictive and incomplete nature [26, 27]. For example, it may be very difficult to detect a respiratory rate of more than 20 breaths/min in patients already on respiratory support. Moreover, it might be difficult to diagnose organ dysfunction, for example, CNS depression in a sedated patient [26]. The definition of ªseptic shockº is also somewhat subjective, as hypotension that persists after ªadequate fluid resuscitationº has not been defined and may be arbitrary. Moreover, a blood pressure based criterion may underestimate the number of patients with shock since shock physiologically represents the inability of oxygen delivery to support metabolic demands, which can occur in the presence of normal blood pressure [28]. Some recent epidemiological studies of sepsis have not used the consensus definitions [29, 30, 31, 32]. Several clinical trials have used consensus definitions [33, 34, 35] whereas others have not [36, 37, 38, 39, 40]. Despite certain problems with the Consensus definitions they define a continuum of progressive physiological decline toward multiple organ dysfunction/failure and mortality. Better understanding of the pathophysiology of sepsis will enable us in the future to begin therapy at an earlier stage in the disease process before the onset of shock and multiorgan dysfunction and failure. The Consensus Conference used specific clinical characteristics and thresholds to define sepsis, but there may be better combinations of clinical manifestations in septic patients. Unfortunately, none has proven more use- ful or has undergone such a rigorous evaluation of its epidemiology, morbidity, and mortality. Although the Consensus criteria have been useful in epidemiological studies, they should not be the sole basis for the clinical diagnosis of sepsis. It may be extremely difficult to diagnose sepsis in the patient who does not have the classical findings. The symptoms and signs that should lead the clinician to suspect sepsis are as follows: · Clinical signs · Fever/hypothermia · Unexplained tachycardia · Unexplained tachypnea · Signs of peripheral vasodilation · Unexplained shock · Changes in mental status · Invasive hemodynamic or laboratory parameters · Low systemic vascular resistance/Increased cardiac output · Increased oxygen consumption · Leukocytosis/neutropenia · Unexplained lactic acidosis · Unexplained alteration in renal or liver function tests · Thrombocytopenia/disseminated intravascular coagulation · Increased procalcitonin · Increased cytokines, C reactive protein Absolute thresholds are probably less important than combinations or severity levels. For example, sepsis should be suspected in all patients with unexplained shock. Consensus vs. controversy The Consensus Conference [5] agreed to a new set of definitions that could readily be applied to patients in various stages of sepsis. It was expected that application of these definitions would improve bedside detection of infections and permit earlier intervention before the onset of shock and multiorgan failure. Early recognition of S6 the inflammatory response to infections should lead to more effective therapeutic management. The term SIRS was introduced to provide a term other than sepsis to describe a patient who looked ªsepticº but was not infected. Some clinicians oppose the use of the term SIRS [4, 19, 20, 26, 27, 28, 41] as it is nonspecific, very common in hospitalized patients, and can lead physicians to become compliant with a ªdiagnosisº and not seek a potential infection. When a patient manifests a systemic response to infection, the terminology used is not as important as the actions. It is agreed that sepsis, severe sepsis, and septic shock represent a continuum in a disease process and are correlated with increasing organ dysfunction and failure and mortality. It is vitally important to search continuously for a source of infection and to make a diagnosis. Treatment of infection should eradicate the invading organism with antimicrobial therapy and surgical debridement or drainage. Early detection of infections in patients should lead to rapid and aggressive diagnostic and therapeutic interventions which should prevent circulatory compromise and organ dysfunction and improve survival. The exact criteria used to define these entities may change with time as more studies are performed. Biological markers of infection Early detection of sepsis is difficult because the first signs of this disease may be minimal and are similar to those of various noninfectious processes, and culture results are not immediately available. The availability of laboratory tests to accurately and more rapidly identify septic patients by the isolation of micro-organisms from body fluid specimens would be of considerable value. Several indicators measured in the bloodstream have been evaluated for the diagnosis of sepsis. A prominent and invariable component of the systemic inflammatory response is the induction and release of cytokines and acute-phase proteins, which rapidly increase in the serum. The principal cytokines [interleukin (IL) 1, tumor necrosis factor (TNF) a, IL-6, IL-8, IL-10] and their soluble receptors (soluble TNF receptor) have convincingly been demonstrated to increase during sepsis [42, 43, 44, 45, 46]. Both cytokines and their soluble receptors, however, are greatly elevated also in nonbacterial infections such as malaria [47] and in disease states such as burn injury [48], trauma [49], pancreatitis [50], heart failure [51], renal allograft rejection [52], and even in such minor injury states as elective surgery [53, 54]. Critics have therefore rightly questioned the diagnostic significance of elevated cytokine concentration because of its nonspecific character. In addition, the inflammatory response changes with time during the course of sepsis and a complex balance exists between cytokines and their inhibitors and antagonists, which require that the evaluation of inflammatory pathways take into account the pro- and anti-inflammatory factors that affect each pathway. Current efforts should therefore be directed at defining the cytokine balance that exists at the onset of sepsis, how this balance changes over time, and determining whether groups of cytokine variables (not a single cytokine) can be used to predict more accurately either the onset or the outcome of sepsis. Recently several studies have evaluated the use of Creactive protein (CRP) and procalcitonin (PCT) in differentiating conditions that may mimic sepsis. These studies highlight both CRP and PCT as specific markers of bacterial sepsis [55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69]. Both are acute-phase proteins, and their role is unknown. It has been reported that increased CRP concentrations differentiate between viral and bacterial infections [55], and that decreases in levels indicate resolution of bacterial sepsis [56]. Moreover, CRP has been shown to be superior to body temperature and white blood cell count in identifying bacterial sepsis [57]. Other studies have been less enthusiastic about CRP as a marker of infection. Increased levels were demonstrated in patients with stable and unstable angina pectoris and in patients with heart failure. Interestingly, increased preoperative levels of CRP were predictive of postoperative infection after cardiac surgery [58]. CRP was also markedly elevated in trauma patients, in children after head injury, and in patients undergoing various types of surgery. Some studies have also reported higher concentrations of PCT in patients with systemic manifestations of infection than in noninfected patients who fulfill the SIRS criteria [59] or patients with systemic viral and localized bacterial infection [60]. Other studies have shown that increased levels of PCT predict infected necrosis in patients with acute pancreatitis [61, 70] or bacterial or fungal infection in severe burn injuries [62] and can discriminate between infection and graft rejection in patients after organ transplantation [63, 64, 65, 71]. In patients having undergone cardiopulmonary bypass increases in PCT have been demonstrated only in infected patients, with low values in patients with SIRS alone [66]. Moreover, in injured patients [72] sustained increases in PCT plasma levels predict sepsis and MODS. In neonates [67] the sensitivity and specificity of PCT concentrations in the detection of early-onset sepsis was 92.6 % and 97.5 %, respectively, and 100 % for both for the diagnosis of late-onset sepsis. In a recent study of 111 infected and 79 noninfected patients Urgate et al. [68] found a slightly higher sensitivity and specificity for CRP than PCT in the diagnosis of infection: 71.8 % and 66.6 % for CRP and 67.6 % and 61.3 % for PCT, respectively. However, the combination of CRP and PCT was much more specific in ascertaining the diagnosis of infection. In contrast, PCT S7 was found to be a better marker of infection than CRP in patients with pancreatitis [61], and in patients after renal transplantation [63]. PCT has been found to be superior to the classical criteria of inflammation (CRP, TNFa, IL-6, leukocyte count, and body temperature) in identifying patients with sepsis [69, 73, 74]. How abnormal must PCT blood concentration be before it reliably predicts the presence of sepsis in the appropriate clinical setting? The answer is not known. Based on the data reviewed, a value higher than 5 ng/ ml would be a reasonable threshold. More importantly, PCT measurements might serve as a reasonable parameter to rule out sepsis. When blood levels are very low or undetectable, the patient is unlikely to have sepsis as a cause of organ dysfunction or shock. The definition of sepsis should not depend on any single arbitrary dichotomizing measurement. Rather, sepsis should be defined as the systemic inflammatory response, associated with an identified source of infection that develops under the appropriate clinical setting. If measurement of a marker such as procalcitonin is validated to be correlated with the presence of infection (and further studies are needed for this), a more precise set of criteria can be proposed for the diagnosis of sepsis: (a) systemic manifestations; (b) an appropriate clinical setting (the reason for the addition of this criterion is that the systemic manifestations are by themselves nonspecific markers of infection); (c) source of infection; and (d) laboratory marker. In the future a biological marker or markers of infection, alone or in combination, should be evaluated for their capability to predict/ diagnose sepsis. As discussed above, PCT, with an appropriate concentration threshold, may prove to be such a marker. Measurements of such a marker may have to be repeated daily as the sensitivity increases with time after the diagnosis of infection [68]. Other measures [75, 76] that have been proposed as markers of sepsis are not easily determined and therefore are difficult to apply to all patients. In the meantime these should serve as a research tool only. Sepsis is a diverse illness, and the heterogeneity of patient populations may account for some of the variability in the results of the published studies. Prospec- tive epidemiological studies and clinical trials of sepsis should include a larger number of patients, enough to permit stratification of the population by the source of infection (CNS, lungs, gastrointestinal, urinary, etc.) and the pattern of immunological response (hypo- vs. hyperactivation, and precise stage of inflammatory response). The former is a potentially important issue since the source of infection has been found in many studies to be statistically associated with increased mortality [77, 78]. In addition, to know whether the studied patient populations are similar, one must also develop two sets of appropriate tests: one that measures the severity of injury to the infected system and one that measures the overall severity of the patient's illness. Summary and recommendations Sepsis is the systemic inflammatory response to infection. In the past many different definitions of sepsis were used interchangeably, which led to confusion. Today, the definitions of sepsis and its clinical manifestations are still a source of controversy. No single physiological or laboratory parameter can universally identify sepsis. Not all patients with sepsis are equally ill. Sepsis, severe sepsis, and septic shock constitute different gradations in the continuum of a disease process manifested by a combination of changes in vital signs, laboratory parameters, hypoperfusion, and organ dysfunction. The continuum of sepsis, severe sepsis, and septic shock is correlated with increasing organ dysfunction and mortality. The source of infection and diagnosis of sepsis must be identified as early as possible to permit early intervention with antimicrobial therapy and surgical drainage to prevent disease progression, organ dysfunction, and mortality. Although the consensus criteria have been useful in epidemiological studies, they should not be the sole basis for the clinical diagnosis of sepsis. Future prospective clinical trials may lead to a more precise understanding of sepsis and its related disorders and result in clearer, more universally accepted and easily interpreted definitions. References 1. Rackow EC (1986) Clinical definition of sepsis and septic shock. In: Sibbald WJ, Sprung CL (eds) New horizons: perspectives of sepsis and septic shock. Society of Critical Care Medicine, Fullerton, pp 1±9 2. Bone RB, Grodzin CG, Balk RA (1998) Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 112: 235±243 3. Parrillo JE, Parker MM, Natanson C (1990) Septic shock; advances in understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 113: 227±242 4. Muckart DJJ, Bhagwanjee S (1997) American College of Chest Physicians/ Society of Critical Care Medicine Consensus Conference: definition of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med 25: 1789±1795 S8 5. Knaus WA, Xiaolu Sun, Nystrom PO, Wagner DP (1992) Evaluation of definitions for sepsis. Chest 101: 1656±1662 6. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RMH, Sibbald WJ (1992) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864±874 7. Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1989) The Methylprednisolone Severe Sepsis Study Group. Sepsis syndrome: a valid clinical entity. Crit Care Med 17: 389±393 8. Bihari DJ (1990) Septicaemia ± the clinical diagnosis. J Antimicrob Chemother 25 [Suppl C]:1±7 9. Sprung CL (1991) Definitions of sepsis ± have we reached a consensus? Crit Care Med 19: 849±850 10. Bone RC (1991) Let's agree on terminology: definitions of sepsis. Crit Care Med 19: 973±976 11. Bone RC, Sibbald WJ, Sprung CL (1992) The ACCP-SCCM Consensus Conference on sepsis and organ failure. Chest 101: 1481±1482 12. Kreger BE, Craven DE, McCabe (1980) Gram negative bacteremia. Reevaluation of clinical features and treatment in 612 patients. Am J Med 68: 344±355 13. Nystrom PO (1998) The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother 41 [Suppl A]:1±7 14. Canadian Multiple Organ Failure Study Group: Sibbald WJ, Marshall J, Christou N, Girotti M, McCormack D, Rostein O, Martin C, Meakins J (1991) ºSepsisº ± clarity of existing terminology...or more confusion? Crit Care Med 19: 996±998 15. Jonsson B, Berglund J, Skau T, Nystrom PO (1993) Outcome of intra-abdominal infection in pigs depends more on host responses than on microbiology. Eur J Surg 159: 571±578 16. Marshall JC, Sweeney D (1990) Microbial infection and the septic response in critical surgical illness. Sepsis, not infection, determines outcome. Arch Surg 125: 17±23 17. The Veterans Administration Systemic Sepsis Cooperative Study Group (1987) Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 317: 659±665 18. Ziegler EJ, Fisher CH, Sprung CL, et al (1991) Treatment of Gram-negative bacteremia and septic shock with HA-1 A human monoclonal antibody against endotoxin. N Engl J Med 324: 429±436 19. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP (1995) The natural history of the systemic inflammatory response syndrome (SIRS). JAMA 273: 117±123 20. Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, Jebson P, Wenzel RP (1995) Systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med 21: 302±309 21. Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, Montani C, Magni E: The Sepsis Study Group (1995) The Italian sepsis study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med 21:S244±S249 22. Saez-Llorens X, Vargas S, Guerra F, Coronado L (1995) Application of new sepsis definitions to evaluate outcome of pediatric patients with severe systemic infections. Pediatr Infect Dis J 14: 557±561 23. Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M (1996) Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 109: 1033±1037 24. Jones GR, Lowes JA (1996) The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. Q J Med 89: 515±522 25. Bossink AWJ, Groeneveld J, Hack CE, Thijs LG (1998) Prediction of mortality in febrile medical patients. How useful are systemic inflammatory response syndrome and sepsis criteria? Chest 113: 1533±1541 26. Vincent JL (1997) Dear SIRS, I'm sorry to say that I don't like you... Crit Care Med 25: 372±374 27. Benjamin E, Leibowitz AB, Oropello J, Iberti TJ (1992) Systemic hypoxic and inflammatory syndrome: an alternative designation for ªsepsis syndromeº. Crit Care Med 20: 680±682 28. Opal SM (1998) The uncertain value of the definition for SIRS. Chest 113: 1442±1443 29. Poole GV, Griswold JA, Muakkassa FF (1993) Sepsis and infection in the intensive care unit: are they related? Am Surg 59: 60±64 30. Kieft H, Hoepelman AIM, Zhou W, et al (1993) The sepsis syndrome in a Dutch university hospital. Arch Intern Med 153: 2241±2247 31. Conboy K, Welage LS, Walawander CY, et al (1995) Sepsis syndrome and associated sequelae in patients at high risk for gram-negative sepsis. Pharmacotherapy 15: 66±77 32. Sands KE, Bates DW, Lanken PN, et al (1997) Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 278: 234±240 33. Fisher CJ, Dhainaut J-FA, Opal SM, et al (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. JAMA 271: 1836±1843 34. Opal SM, Fisher CJ, Dhainaut J-FA, et al (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 25: 1115±1124 35. Abraham E, Glauser MP, Butler T, et al (1997) p55 tumor necrosis factor receptor fusion protein in the treatment of patient with severe sepsis and septic shock. JAMA 277: 1531±1538 36. Abraham E, Wunderink R, Silverman H, et al (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor a in patients with sepsis syndrome. JAMA 273: 934±941 37. Cohen J, Carlet J for the INTERSEPT study group (1996) INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor a in patients with sepsis. Crit Care Med 24: 1431±1440 38. Fisher CJ, Agosti JM, Opal SM, et al (1996) Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. N Engl J Med 334: 1697±1702 39. Bernard GR, Wheeler AP, Russell JA, et al (1997) The effects of ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med 336: 912±918 40. Eisele B, Lamy M, Thijs LG, et al (1998) Antithrombin III in patients with severe sepsis. Intensive Care Med 24: 663±672 41. Sibbald WJ, Doig G, Inman KJ (1995) Sepsis, SIRS and infection. Intensive Care Med 21: 299±301 42. Moscovitz H, Shofer F, Mignott H, et al (1994) Plasma cytokine determination in emergency department patients as a predictor of bacteremia and infectious disease severity. Crit Care Med 22: 1102±1107 S9 43. Damas P, Ledoux D, Nys M, et al (1992) Cytokine serum level during severe sepsis in human. IL-6 as a marker of severity. Ann Surg 215: 356±362 44. Damas P, Reuter A, Gysen P, et al (1989) Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med 17: 975±978 45. Hack CE, De Groot ER, Felt-Bersma RJF, et al (1989) Increased plasma levels of interleukin-6 in sepsis. Blood 74: 1704±1710 46. Van der Poll T, Jansen J, van Leenen D, et al (1993) Release of soluble receptors for tumor necrosis factor in clinical sepsis and experimental endotoxemia. J Infect Dis 168: 955±960 47. Brockhaus M (1997) Soluble TNF receptor: what is the significance? Intensive Care Med 23: 808±809 48. Nijsten MWN, de Groot ER, ten Duis HJ, et al (1987) Serum levels of interleukin-6 and acute phase responses. Lancet II:921±922 49. Ertel W, Keel M, Bonaccio M, Steckholzer U, et al (1995) Release of anti-inflammatory mediators after mechanical trauma correlates with severity of injury and clinical outcome. J Trauma 39: 879±885 50. Brivet FG, Emilie D, Galanaud P (1999) Pro- and anti inflammatory cytokines during acute severe pancreatitis: and early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med 27: 749±755 51. Testa M, Yeh M, Fanelli R, et al (1996) Circulating levels of cytokines and their endogenous modulators in patients with severe congestive heart failure due to coronary artery disease or heart failure. J Am Coll Cardiol 28: 964±971 52. Van Oers MHJ, van der Hyeden AAPAM, Aarden LA (1988) Interleukin 6 (IL-6) in serum and urine of renal transplant recipients. Clin Exp Immunol 71: 314±319 53. Shenkin A, Fraser WD, Series J (1989) The serum interleukin-6 response to elective surgery. Lymphokine Res 8: 123±127 54. Bistrian BR (1999) Acute phase proteins and the systemic inflammatory response. Crit Care Med 27: 452±453 55. Shaw AC (1991) Serum C-reactive protein and neopterin concentrations in patients with viral or bacterial infection. J Clin Pathol 44: 596±599 56. Yentis SM, Soni N, Sheldon J (1995) C-reactive protein as an indicator of resolution of sepsis in the intensive care unit. Intensive Care Med 21: 602±605 57. Povoa P, Almeida E, Moreira P, et al (1998) C-reactive protein as an indicator of sepsis. Intensive Care Med 24: 1052±1056 58. Fransen EJ, Maessen JG, Elenbaas TW, et al (1999) Enhanced preoperative C-reactive protein plasma levels as a risk factor for postoperative infections after cardiac surgery. Ann Thorac Surg 67: 134±138 59. Al-Nawas B, Krammer I, Shah PM (1996) Procalcitonin in diagnosis of severe infection. Eur J Med Res 1: 331±333 60. Assicot M, Gendrel D, Carsin H, et al (1993) High serum procalcitonin concentration in patients with sepsis and infection. Lancet 341: 515±518 61. Rau B, Steinbach G, Gansauge F, et al (1997) The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 41: 832±840 62. Von Heimburg D, Stieghorst W, Khorram S, et al (1998) Procalcitonin-a sepsis parameter in severe burn injuries. Burns 24: 745±750 63. Eberhard OK, Langefeld I, Kuse ER, et al (1998) Procalcitonin in the early phase after renal transplantation ± will it add to diagnostic accuracy? Clin Transplant 12: 206±211 64. Hammer C, Reichenspurner R, Meiser B (1998) Cytoimmunology in monitoring: the Munich experience. Transplant Proc 30: 873±874 65. Staehler M, Hammer C, Meiser B, et al (1997) Procalcitonin: a new marker for differential diagnosis of acute rejection and bacterial infection in heart transplantation. Transplant Proc 29: 584±585 66. Boeken U, Feindt P, Petzold T, et al (1998) Diagnostic value of procalcitonin: the influence of cardiopulmonary bypass, aprotinin, SIRS, and sepsis. Thorac Cardiovasc Surg 46: 348±351 67. Chiesa C, Panero A, Rossi N, et al (1998) Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin Infect Dis 26: 664±672 68. Urgate H, Eliezer Silva, Mercan Dany, et al (1999) Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 27: 498±504 69. Oberhoffer M, Vogelsang H, Russwurm S, et al (1999) Outcome prediction by traditional and new markers of inflammation in patients with sepsis. Clin Chem Lab Med 37: 363±368 70. Rau B, Steinbach G, Baumgart K, et al (2000) The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med 26:S159±S164 71. Kuse ER, Langefeld I, Jaeger K, et al (2000) Procalcitonin in fever of unknown origin after liver transplantation: a variable to differentiate acute rejection from infection. Crit Care Med 28: 555±559 72. Wanner GA, Keel M, Steckholzer U, et al (2000) Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med 28: 950±957 73. Cheval C, Timsit JF, Garrouste-Orgeas M, et al (2000) Procalcitonin (PCT) is useful in predicting the bacterial origin of an acute circulatory failure in critically ill patients. Intensive Care Med 26:S153±S158 74. Oberhoffer M, Ruûbwurm S, Bredle D, et al (2000) Discriminative power of inflammatory markers for prediction of tumor necrosis factor-a and interleukin-6 in ICU patients with systemic inflammatory response syndrome (SIRS) or sepsis at arbitrary time points. Intensive Care Med 26:S170±S174 75. Palombo JD, Bistrian BR (1999) Early diagnosis of septic shock: a simple test for a complex condition? Crit Care Med 27: 465±466 76. Martens A, Eppink GJM, Woittiez AJJ, et al (1999) Neutrophil function capacity to express CD10 is decreased in patients with septic shock. Crit Care Med 27: 549±553 77. Uzun O, Akalin HE, Hayran M, et al (1992) Factors influencing prognosis in bacteremia due to gram-negative organisms: evaluation of 448 episodes in a Turkish university hospital. Clin Infect Dis 15: 866±873 78. Barriere SL, Lowry SF (1995) An overview of mortality risk prediction in sepsis. Crit Care Med 23: 376±393 Intensive Care Med (2001) 27: S 10±S 32 Diagnosis of infection in sepsis Martin Llewelyn Jonathan Cohen ) M. Llewelyn ´ J. Cohen ( ) Department of Infectious Diseases, Imperial College School of Medicine, Hammersmith Hospital, London, UK E-mail: [email protected] Phone: +44-20-83 83 32 43 Fax: +44-20-83 83 33 94 Introduction Infection is a sine qua non of sepsis. Sepsis can complicate infection occurring at any site, most commonly the respiratory tract, abdomen and blood stream. More than 90 % of cases of sepsis are caused by bacteria, and Gram-negative and Gram-positive organisms occur with approximately equal frequency [1]. Fungi ± in particular Candida species ± are sometimes responsible, but a wide variety of other organisms have occasionally been implicated [2]. There are several reasons why it is important to try and make a microbiological diagnosis in septic patients. First, and most important, is to ensure that effective antimicrobial therapy is given. There is good evidence to support the intuitive belief that patients given appropriate therapy are more likely to survive than those given inadequate or inappropriate treatment [3]. Secondly, obtaining microbiological information will contribute to the local epidemiological database, without which logical prescribing is difficult, if not impossible. There are substantial differences between intensive care units in the microbial ecology, including the prevalence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis. Antimicrobial resistance patterns also vary widely, for example, penicillin resistance in Streptococcus pneumoniae, and gentamicin resistance in Enterobacteriaceae. Furthermore, these patterns are constantly changing, and an up-todate awareness of these patterns is obviously essential when considering empirical therapy. Finally, knowledge of the microbial cause of sepsis may be important in the choice of adjunctive therapies. This is not yet clinical reality but will clearly be important if, for instance, antiendotoxin agents ever enter the clinical arena. In this contribution we first consider the general approach to the diagnosis of infection in septic patients and then some aspects of infections at particular sites. We focus on the microbiological aspects of diagnosis, although where appropriate we also comment on other modalities such as imaging. The clinical management of these infections are discussed elsewhere. Methods We use where possible a systematic, evidence-based approach to provide answers to specific questions. A computer-based review of the literature was undertaken using Medline from 1991 until September 1999 as the primary database. The references obtained were searched manually for relevance. More recent clinical articles were identified by manual search of the relevant journals. Although the literature searches were not extended further back than 1991, key earlier papers are frequently cited. In reviewing this field we frequently observed that whereas there is extensive literature on microbiological aspects of infection in general, there is a paucity of data concerning sepsis in particular. Hence we have often been obliged to make recommendations based on the available literature and our own clinical experience, and that of others. The search strategies used were as follows. Bacteremia The primary search terms bacteremia/septicemia (diagnosis, microbiology) and blood (microbiology) were combined with secondary search terms sepsis/sepsis syndrome (diagnosis, microbiology), blood specimen collection (methods), bacteriological techniques and diagnostic tests, routine (methods). Studies addressing exclusively pediatric populations were excluded. S 11 Central venous catheter infection The primary search terms catheterization, central venous (adverse effects) and indwelling catheters (adverse effects) were combined with secondary search terms sepsis/sepsis syndrome (diagnosis, microbiology, etiology), bacteremia/septicemia (diagnosis, microbiology, etiology), blood specimen collection (methods), bacteriological techniques and diagnostic tests, routine (methods). Ventilator-associated pneumonia The primary search terms artificial respiration (adverse effects) ventilation, mechanical (adverse effects) and intensive care (methods) were combined with the secondary search terms pneumonia (diagnosis, microbiology, etiology), sepsis/sepsis syndrome (diagnosis, microbiology, etiology), bacteremia/septicemia (diagnosis, microbiology, etiology), bacteriological techniques and diagnostic tests, routine (methods). Surgical site infections and intra-abdominal sepsis The primary search terms wound infection (diagnosis, microbiology), abscess (diagnosis, microbiology) and abdomen were combined with secondary search terms sepsis/sepsis syndrome (diagnosis, microbiology, etiology), bacteremia/septicemia (diagnosis, microbiology, etiology), bacteriological techniques, diagnostic tests, routine (methods) and interventional radiology. General considerations in the diagnosis of sepsis There are three key difficulties associated with the diagnosis of infection in patients who have sepsis. Establishing infection as the primary cause The controversies surrounding the definition of sepsis are discussed elsewhere (see Matot and Sprung, ªIntroductionº). Establishing that the patient has an ongoing infection ± and therefore has sepsis rather than a noninfective cause of the systemic inflammatory response syndrome (SIRS) ± can be extremely difficult. An important first step is a systematic consideration of possible noninfective causes. Knowledge of other pathologies that may mimic sepsis and how they apply to the specific patient can make up for a relative paucity of clinical information in patients who may be sedated or critically ill. Noninfective causes of SIRS are the following: · Tissue injury · Surgery/trauma · Hematoma/venous thrombosis · Myocardial/pulmonary infarction · Transplant rejection · Pancreatitis · Erythroderma · Metabolic · Thyroid storm · Acute adrenal insufficiency · Therapy related · Blood products · Cytokines, especially granulocyte-macrophage colony stimulating factor · Anasthetic-related malignant hyperpyrexia, especially halothane · Neuroleptic malignant syndrome, for example, caused by haloperidol · Opiates/benzodiazepines · Malignancy · Hypernephroma/lymphoma · Tumor lysis syndrome · Neurological · Subarachnoid hemorrhage Tissue infarction or hematoma, for example, may need to be actively sought in a surgical or trauma patient who develops signs of SIRS and precipitation of thyroid storm or adrenal insufficiency should be considered in at-risk patients following trauma or surgery. Localizing the site of infection This may be straightforward, but frequently it is confounded by the fact there are multiple pathological processes occurring concurrently or by the frequent use of antibiotics which undermine microbiological diagnosis. Occasionally the site of infection is occult, for instance, when there is sinusitis or deep intra-abdominal infection. Interpreting the microbiological findings Conventional microbiology has several limitations in hospitalized patients who may be septic: · · · · · Distinguishing colonization from infection Prior antibiotic therapy Correctly identifying unfamiliar organisms Determining the significance of mixed culture results Interpreting the importance of organisms normally of low virulence Principal among these is the fact that many organisms isolated from nonsterile sites may represent either colonization or infection ± microbiology alone cannot answer this question. Conversely, the microbiology laboratory may report negative findings in samples from sites that are in fact infected, either because antibiotics have sterilized the specimen, or because special procedures need to be carried out (e.g., immunofluorescence to detect Pneumocystis carinii). S 12 A clinical approach Fever is a common sign in hospitalized patients and is often the first indication of sepsis. Practical guidelines for the evaluation of fever on the ICU have recently been published [4]. Focused clinical examination, guided by risk factors relevant to the individual patient, often reveal potential sources of sepsis and guide subsequent investigation. Surgical and traumatic wounds should be exposed and examined for signs of infection. Particular attention should be paid to vascular access sites for signs of phlebitis or cellulitis and to pressure areas or injection sites for evidence of soft tissue infection. Evidence of sinusitis should be sought, and fundoscopy is invaluable in detecting candidal endophthalmitis, a pathognomic feature of systemic fungal sepsis. Urine in the catheter may be frankly purulent, and the presence of diarrhea may indicate Clostridium difficile associated colitis. The importance of repeated, complete physical examination to detect the emergence of new signs cannot be overstated. Nonspecific markers of infection Traditional markers of infection such as neutrophilia lack sufficient sensitivity among hospitalized patients to be of value in distinguishing sepsis, although marked neutrophilia or failure to mount a neutrophil response may be of prognostic value. Levels of procalcitonin (PCT) and C-reactive protein (CRP) are straightforward to assay. The evidence that these acute-phase markers have specificity in differentiating infection from other causes of an inflammatory response has recently been reviewed [5]. Levels of CRP and PCT are correlated well with the degree of inflammatory response and are of particular value in monitoring response to treatment [6]. PCT may have some advantages over CRP in that it rises more quickly at the onset of inflammation and is cleared more quickly as inflammation resolves [7]. Levels of PCT are correlated more closely with severity of sepsis [8] and also are predictive of mortality [9]. A prospective study of ICU patients found that a CRP level of 50 mg/l or higher had a sensitivity of 98.5 % and specificity of 75 % in identifying probable or definite sepsis [10]. De Werra et al. [11], also in an ICU population, found PCT levels of 1.5 ng/ml or higher to have a sensitivity of 100 % and specificity of 72 % in identifying sepsis. Such markers therefore cannot alone differentiate sepsis from other causes of SIRS; rather they are a part of a systematic evaluation that includes clinical examination and directed diagnostic techniques. Daily, sequential measurement of inflammatory markers is likely to be of more value in diagnosis of infection than single measurements [10]. Detection of circulating endotoxin might be expected to be a specific test for sepsis. Assays differ in the sensitivity, cutoffs are not established, and the transient nature of endotoxemia makes timing of measurements essential. For these reasons measurement of endotoxin levels in sepsis patients remains experimental. Figure 1 outlines an algorithm for investigation of suspected sepsis into which may be fed data from clinical examination and nonspecific investigations along with the appropriate specific microbiology and imaging investigations discussed in detail below. Discussion: literature-based recommendations To answer each of the following important clinical questions, a review of the literature was performed as previously described. Bacteremia Are there specific indications for obtaining blood for culture? Answer: yes; grade D. Recommendation Fever, chills, hypothermia, leukocytosis, left-shift of neutrophils, neutropenia, and when infection is suspected, hypoalbuminemia, development of renal failure or signs of hemodynamic compromise are specific indications for obtaining blood for culture. Blood cultures should be taken as soon as possible after onset of fever or chills. Rationale Blood should be obtained for culture whenever there is reason to suspect blood stream infection, commonly when a patient develops a new fever. In practice, as a noninvasive, safe, and low-cost investigation, blood culture is often performed when there a few specific indications. However, a number of clinical and laboratory parameters are independently correlated with the presence of bacteremia in patients in whom infection is suspected, notably, chills, hypoalbuminemia, development of renal failure, and a diagnosis of urinary tract infection [12]. Other criteria are fever, hypothermia, leukocytosis, left-shift of neutrophils, neutropenia and signs of hemodynamic compromise [13]. Ideally patients should not be receiving parenteral antibiotics when blood cultures are performed. While we are aware of no published S 13 Fig. 1 Algorithm for systematic evaluation of nonneutropenic patients with suspected sepsis data which directly address this issue, blood cultures should be taken, where possible, immediately before a regular dose of antibiotic so that blood levels are minimized. In suspected fungemia, therapy with antibacterial agents clearly should not impact on yield. Otherwise the indications for performing blood culture are the same irrespective of whether the patient is receiving antibiotics or not. In this group of patients, media containing antibiotic adsorbing substances such as BacT/Alert FAN and BACTEC Plus/F should be used since they are associated with increased recovery of significant pathogens, particularly among patients on appropriate antibiotic regimes [14]. The literature contains no clinical data relating to timing of blood cultures with respect to timing of fever or chills. Nevertheless, bacteria are rapidly cleared from blood, and development of fever usually follows an episode of bacteremia by 30±90 min. Published expert opinion is that blood cultures should be taken as soon as possible following onset of fever [15]. Does the technique employed in obtaining blood cultures influence the sensitivity and specificity of this investigation? Answer: yes; grade D. Recommendation Blood should be obtained by fresh venipuncture. Sites associated with skin contamination (e.g., femoral site) or loss of skin integrity (e.g., burns or dermatological disease) should be avoided. Skin should be swabbed twice with either 70 % isopropyl alcohol or with an iodine containing solution prior to venipuncture. The blood culture stopper should also be sterilized prior to inoculation. An adequate volume (20±60 ml) of blood should be obtained per culture (10±30 ml per bottle). If insufficient blood is available, only the aerobic bottle should be inoculated. The needle used for venipuncture should be changed prior to inoculation of blood into culture bottles. Rationale When a decision has been made to take blood for culture, adherence to a protocol for obtaining the specimen results in lower contamination rates and improved yield [13, 16]. The cost of additional investigations, treatment and in-patient stay associated with each contaminated blood culture has been estimated as between U. S. $1,000 and $5,000 [17, 18]. Furthermore, with Grampositive organisms making up an increasing proportion of significant blood culture isolates, identifying such isolates as contaminants is more difficult than ever. Blood taken from a central venous catheter (CVC) is significantly more likely to be contaminated by skin flora [19], as is blood taken from previously placed periph- S 14 Table 1 Trials comparing skin sterilization techniques Protocol Conclusion Reference 10 % povidone iodine and 0.2 % chlorine peroxide No benefit 21 Alcohol then povidone iodine compared with alcohol alone No benefit 22 70 % iodophore compared with tincture of iodine Tincture of iodine associated with 50 % fewer contaminated cultures 23 70 % isopropyl alcohol/70 % povidone iodine compared with isopropyl alcohol, 10 % acetone and povidone iodine dispenser Isopropyl alcohol and 10 % acetone and povidone iodine dispenser associated with 50 % fewer contaminated cultures 24 eral cannulas, although contamination rates may not be higher for blood obtained through a peripheral cannula at the time of insertion, and this is acceptable as a means of minimizing number of venipunctures [20]. It may be possible to minimize contamination of blood obtained through venous catheters by adherence to meticulous sterile technique [21], but this approach should only be used when no site for venipuncture is available. Definitive evidence that skin disinfection reduces blood culture contamination rates is lacking, but this conclusion has been inferred from the findings of controlled trials which demonstrated superiority of one skin preparation agent over another. Two other trials have shown no benefit. One concluded that contamination occurs during laboratory specimen handling [22], a second found unexpectedly low contamination rates in both patient groups. The relevant trials since 1990 are summarized in Table 1. We are aware of no reason to revise our earlier recommendations that skin should be swabbed twice with either 70 % isopropyl or ethyl alcohol or with an iodine-containing solution [25]. The blood culture stopper should also be sterilized prior to inoculation of the blood sample. While we are aware of no data that directly address this issue, it is the recommendation of previously published expert opinion [15]. Inoculation of 3 cfu into blood culture is required to give 100 % culture positivity [26]. The concentration of bacteremia in adult patients is frequently less than one viable organism per milliliter [27] and may be less than 0.1 organisms per milliliter [28]. For these reasons it is not surprising that the volume of blood inoculated into culture is an important variable determining culture yield [13, 29]. This effect has also been demonstrated in clinical studies, although not specifically in sepsis patients [30, 31]. While the blood culture system employed determines the volume of blood that may be utilized, in adults a minimum sample size of 20 ml is required per venipuncture (10 ml per bottle) [15, 32] while increasing the sample volume above 30 ml is not associated with significantly improved culture rates [13]. In infants, in whom bacteremia is associated with higher number of colony-forming units per milliliter, a smaller volume of blood (0.5± ml or < 1 % of circulating blood volume) can be used for culture [33]. Anaerobic organisms now make up fewer than 5 % of blood culture isolates [34, 35]. Furthermore, aerobic culture bottles are more successful in culture of the overwhelming majority of organisms identified in blood [36]. Therefore, if insufficient blood is available to inoculate two culture bottles, only the aerobic bottle should be inoculated. Contrary to earlier reports [37, 38, 39], a recent metaanalysis has demonstrated lower contamination rates if a needle change is performed [18]. Guidelines regarding resheathing of needles must be strictly adhered to in order that the associated increased risk of needle-stick injury be avoided. Is there evidence to determine how many sets of blood cultures should be taken? Answer: yes; grade E. Recommendation A minimum of two and maximum of three sets of blood cultures should be obtained for each episode of suspected bacteremia. Rationale When bacteremia is associated with endocarditis, if one culture is positive, the probability of any subsequent culture being positive exceeds 95 %. In bacteremia associated with other sources of infection, sensitivity exceeding 99 % is reached with either two [40] or three [41] cultures. The taking of only one culture is rarely permissible since the rate of contamination of an individual set of blood cultures is finite, ranging from 1 % to 4.5 % [22, 23, 24]. Interpretation of a single isolate of a potentially contaminating organism may therefore be exceedingly difficult. S 15 Is there evidence that temporal separation of blood cultures is valuable? Answer: no; grade D. Recommendation In critically ill patients, in whom it may not be possible to delay treatment, no interval is required between taking sets of blood cultures. initions of CRB and CRS. In particular, most published studies that appear to show that it is possible to diagnose catheter infection without catheter removal for culture in reality only address cases of CRB. Can CVC infection be identified as a source of bacteremia without resorting to catheter removal for culture? Answer: yes; grade C. Rationale Recommendations In patients who are not critically unwell, published expert opinion has been that a 30- to 60-min interval should be allowed between obtaining sets of blood cultures [42]. However, the only published study directly to address the efficacy of serial versus simultaneous blood cultures, demonstrated that drawing blood cultures simultaneously or at intervals over a 24-h period did not effect yield [31]. When a CVC is suspected as a source of bacteremia, diagnosis of CVC infection may be made by blood culture based techniques if (a) the patient's clinical condition permits a potentially infected line to be left in place, (b) treatment of CVC infection is to be attempted, or (c) other potential sources of bacteremia are apparent. While the acridine orange leucocyte cytospin (AOLC) test offers the possibility of virtually immediate diagnosis, on the basis of currently available data its use should remain experimental. Central venous catheter infection Definition of terms Rationale It is clinically important to distinguish catheter-related bacteremia (CRB) from catheter-related sepsis (CRS). CRB is defined by the presence of three criteria [43]: When a patient who has a CVC in place develops bacteremia, the likelihood that the CVC is the source of the bacteremia depends on the organism cultured. One study of 311 patients who had CVCs found that 73 % of bacteremias were related to CVC infection, and that if the culture was of S. aureus, this figure rose to over 92 % [48]. Other organisms that are particularly associated with CVC infection as a source are coagulase-negative staphylococci, Corynebacterium (especially JK-1), Bacillus species and fungi, in particular, Candida species [49]. Two approaches to diagnosing CVC infection as a source of bacteremia, without catheter removal for culture, have been reported in the literature: (a) culture of CVC and peripheral blood and (b) AOLC test. · Positive catheter culture. · Positive peripheral blood culture taken before catheter removal. · The same micro-organism is identified in each of the above. A positive catheter culture has been defined [44] as the presence of 15 colonies or more on semiquantitative culture of the catheter tip [45] or of 10 colonies or more on quantitative culture [46]. The various laboratory techniques used to culture CVCs is beyond the scope of this article but have recently been reviewed [47]. CRS is defined as a positive catheter culture when this is considered to be the source of the patient's sepsis, but bacteremia does not occur [44]. The term catheterrelated blood stream infection is sometimes used, and refers to cases in which peripheral cultures are positive, but catheter tip cultures do not need to meet culture criteria as long as there is indirect evidence that the catheter is the source of infection, for example, defervescence following catheter removal. Unfortunately, many of the studies pertaining to diagnosis of CVC infection do not strictly adhered to def- Culture of central venous catheter blood This approach is based on the fact that the concentration of bacteria drawn through an infected catheter is between 4 [50] and 30 [51] times higher than the concentration in peripheral blood drawn simultaneously. The use of this approach in clinical diagnosis has been assessed in two ways. First, by quantitative or semiquantitive blood culture and, secondly, in continuous monitoring blood culture systems. Both methods rely on it being possible to aspirate at least 20 ml blood through the S 16 Table 2 Studies of continuous blood culture monitoring (CRB catheter-related bacteremia) Table 3 Studies of acridine orange leukocyte cytospin (AOLC) in adult patients (CRB catheter-related bacteremia, CVC central venous catheter, HDU high dependency unit) Population Finding Reference Retrospective review of 7 patients with suspected line related infection Time to positivity shorter for central catheter cultures than cultures from peripheral sites 59 Retrospective study of 11 patients in whom CRB was diagnosed Time to culture positivity 1±24 h earlier for central peripheral catheter cultures 61 Retrospective analysis of 64 cancer patients with suspected CRB 28 cases of CRB were identified; a cutoff of +120 min had 100 % specificity and 96.4 % sensitivity 62 Prospective study of 93 cancer patients on an ICU 28 cases of CRB were identified; a cutoff of +120 min had 91 % specificity and 94 % sensitivity 63 Setting Finding Reference 100 patients with suspected CRB, most on parenteral nutrition 35 cases of CRB, AOLC positive in 2/17; when used in conjunction with endoluminal brushing, AOLC identified 15/18 and was 100 % specific 4 cases of CRB, AOLC positive in 2; 10 colonized catheters, AOLC positive in 2 12 cases of catheter-related blood-stream infection identified, AOLC negative in all 64 50 cases of CRB, AOLC was 96 % sensitive and 92 % specific 67 55 CVC tips from ICU patients 49 patients with suspected catheterrelated blood-stream infection; mixed ICU/HDU 128 surgical patients with suspected CRB catheter in question, and it is estimated that blood cannot be aspirated from 12±50 % of potentially infected catheters [43, 52]. Furthermore, being culture based methods, both involve a delay of up to 48 h before cultures can be expected to become positive, time when a potentially infected catheter remains in situ. Quantitative blood cultures A series of studies in the 1980s demonstrated that quantitative cultures of central and peripheral blood can be used to diagnose CRB [53, 54, 55]. In the literature since 1991 two studies have attempted to determine the ratio of colony-forming units per milliliter that gives optimal sensitivity and specificity. Capdevila et al. [50] found that using a cutoff of 1:4 for the ratio of bacterial colonies in peripheral to central blood, sensitivity and specificity of 94 % and 100 %, respectively, could be achieved. Quilici et al. [56] used a ratio of 1:8 and found sensitivity of 92.8 % and specificity of 100 % in a prospective study of critical care patients. Published expert opinion is that quantitative culture of central and peripheral blood samples showing a greater than 5:1 ratio suggest CRB [57, 58]. A meta-analyis of various catheter culture techniques concluded that quantitative blood 65 66 cultures were more cost effective than any culture technique involving the catheter itself [47]. Continuous blood culture monitoring In this approach the time taken for a blood culture to become positive is related to the number of micro-organisms initially present. The higher the initial concentration, the faster the cutoff point is be reached to determine positivity [59]. Four clinical studies have been performed using paired central and peripheral blood samples and one of the commercially available continuous monitoring blood culture systems (e.g., BacT/Alert) which employs a colorimetric CO2 sensor [60]; findings are summarized in Table 2. Acridine orange leukocyte cytospin test This is currently the only good candidate for a rapid diagnostic test for CRB. The test requires only 50 ml blood to be withdrawn from a CVC and takes around 30 min and minimal specialist laboratory expertise. We are aware of four clinical trials in adults (Table 3). Two have yielded positive results [64, 67], both from the S 17 Table 4 Factors associated with risk of central venous catheter (CVC) infection (CRB catheter-related bacteremia) References Catheter site Subclavian lines are associated with significantly less catheter site colonization and infection than jugular or femoral lines although less CRB has not been demonstrated. Difficult catheter insertions with multiple attempts are associated with higher rates of infection. 71, 74, 75, 76 Catheter type It has been suggested that multilumen catheters may be associated with a higher risk of infection than single lumen catheters possibly because they are handled more frequently. This effect is not apparent in critical care patients. Tunnelled lines are associated with delayed and fewer incidences of CRB. 72, 74, 78, 79, 80 Antiseptic impregnated and antibiotic coated catheters There are conflicting studies assessing the extent to which incorporating antimicrobial agents into the manufacture of catheters reduces infection rates. When assessed in critical care patients, such catheters are associated with rates of colonization, local infection and CRB reduced by up to 70 %. When CVCs are used for parenteral nutrition this benefit may be lost. In an ICU setting the use of CVCs for parenteral nutrition has been shown to increase colonization rates. In a non-ICU setting higher infection rates have been found in CVCs used for parenteral feeding. The risk of a central venous catheter being infected increases as duration of catheterization increases beyond 1 week. Meta-analysis data suggest that ªguide-wireº replacement of CVC may be associated with a higher infection rate than replacement to a new site. The infection rate for a patient's first CVC is significantly lower than for any subsequent catheter whether replaced by ªguide-wireº or at a distant site. 81, 82, 83, 84 Trauma and burns patients at high risk, neurosurgical patients at lowest risk. 71 Catheter insertion Catheter use Duration of catheterization Previous catheters Underlying disease same group in Leeds, while two from elsewhere [65, 66] both produced disappointing results. None has specifically investigated sepsis patients, and only one specifically involved critical care patients [65]. Two small studies, again from the group in Leeds, have demonstrated that culture of a wire brush passed down the lumen of a potentially infected catheter is correlated well with subsequent culture of the catheter tip. The first looked at 115 CVCs used for parenteral nutrition in general surgical patients [68]. The second reported 95 % sensitivity and 84 % specificity among 22 surgical patients subsequently diagnosed with CRB [69]. Can CVC infection be identified as a source of sepsis in nonbacteremic patients without catheter removal for culture? Answer: no; grade E. Recommendation When a CVC is suspected as a source of sepsis in nonbacteremic patients, definitive diagnosis requires that the CVC should be removed and sent for culture. 77 85, 86 74, 87 74, 88, 89 90 91 Rationale Only a proportion (10±72 % depending on organisms involved) of infected CVCs are associated with bacteremia [70]. When critical care patients develop signs of sepsis, even in the absence of bacteremia, replacement of central venous catheters is frequently advised. However, using standard culture techniques, rates of proven catheter infection range from 8.9 % to 26 % [66, 71, 72], and therefore many more catheters are removed than are infected [66, 69, 73]. The decision whether to remove a CVC in this setting, where blood cultures are negative, is essentially a clinical one, and involves weighing up risk of catheter replacement against risk of leaving in place a potential source of infection. To help guide this decision a range of clinical parameters have been suggested as possible correlates of catheter infection. A number of individual associations have been identified, summarized in Table 4, and these have been used to make recommendations concerning CVC insertion and care [44]. Two studies have assessed the use of such parameters in guiding clinical judgement and have failed to show they are of value in increasing the proportion of removed CVCs that are infected [66, 92]. Clinical criteria have been incorporated with microbiological data and clinical response to catheter removal into a scoring system [93] which appears more sensitive and no less specific than the Hospital Infection Control Practices Advisory Committee diagnostic criteria [94] S 18 but nevertheless requires removal of the catheter for culture. Is infection at the catheter insertion site indicative of CVC infection? Answer: yes; grade C. Recommendation If infection is suspected at the catheter site, swabs should be taken from the insertion site for culture. The presence of purulence at the CVC site should prompt catheter replacement at a distant site irrespective of culture results. Rationale We are unaware of any studies of insertion site skin culture as a predictor of catheter-related infection in sepsis patients. In patients with long-term nutrition catheters, negative site cultures had a negative predictive value for line infection of 98 % [95] while positive culture, particularly of organisms other than coagulase-negative staphylococci is predictive of CVC infection [96]. In patients with nontunneled CVCs, quantitative skin cultures at the time of removal for suspected infection had a sensitivity of 75 %, a positive predictive value of 100 % and a negative predictive value of 92 % in detecting CVC infection [97]. Expert opinion in the literature is generally that inflammation and frank purulence around a catheter insertion site is a predictor of CVC infection [43, 57, 98]. Reed et al. [44] recommend that ªif the site appears to be infected, the catheter is removed.º While local evidence of infection is predictive of systemic infection, it is possible to find marked local signs of infection with no evidence of systemic infection [48]. Ventilator-associated pneumonia This section addresses specifically the diagnosis of ventilator-associated pneumonia (VAP) in sepsis patients. The diagnoses of community-acquired pneumonia [99, 100, 101] and pneumonia in the immunocompromised host [102] have recently been reviewed by others. The merits of various invasive diagnostic approaches to VAP have also been the subject of several recent editorials and reviews [103, 104, 105, 106, 107]. We therefore specifically exclude from this discussion a review of the extensive literature, which assesses the different modes of invasive airway sampling. Nevertheless, the question ªIs the lower respiratory tract the source of this patient's sepsis?º will, particularly in the ICU frequently apply to patients who develop sepsis while ventilated. We focus then on the evidence to support the recommendation of particular diagnostic tests in management of sepsis patients in whom VAP is suspected as the source. Can clinical parameters be used to diagnose pneumonia as a source of sepsis in a ventilated patient? Answer: uncertain; grade D. Rationale The clinical diagnosis of pneumonia in the non-ICU patient is usually based on the presence of fever, leukocytosis, purulent sputum and new radiographic infiltrates in such patients these criteria are sensitive and specific. In intubated patients these parameters are too nonspecific to be of diagnostic value. Purulent secretions, for example, are almost inevitably found in patients receiving prolonged mechanical ventilation and do not specifically indicate the presence of pneumonia [108]. A range of risk factors for the development of VAP have been identified. Cumulative incidence of VAP increases with time following intubation, but the daily increase in risk diminishes over time [109], such that rates are approximately 3 % per day in the first week, 2 % per day in the second and 1 % per day thereafter [109]. Other independent risk factors recently reviewed include witnessed aspiration, neurological disease and administration of a paralyzing agent impairing airway reflexes, presence of a nasogastric tube, enteral feeding and drugs used to raise gastric pH [110]. One study used by multivariate analysis of clinical parameters to generate a scoring system for risk of developing nosocomial pneumonia in ICU patients. In the patients studied the scoring system had a sensitivity of 85 % and a specificity of 66 % [111]. We are not aware of any prospective, comparative data to assess the usefulness of such a system in clinical practice; hence the answer to the question remains ªuncertain.º Should blood cultures be obtained in patients in whom VAP is suspected? Answer: yes; grade E. Recommendation Two sets of blood cultures should be sent in patients with suspected VAP. S 19 Rationale Recommendation Blood cultures are neither sensitive nor specific in the diagnosis of VAP. Between 3 % and 12 % of bacteremias which occur in ICU patients have a respiratory tract source [112, 113], but only one-quarter of cases of VAP are associated with bacteremia [114]. Bacteremia in patients with suspected VAP, in reality, usually arises from outside the chest [114, 115]. Meduri et al. [115] have demonstrated that two-thirds of patients with nosocomial pneumonia have at least one other focus of infection, usually urinary or CVC related. For this reason published expert opinion is that blood cultures are an essential part of the work up of a patient with suspected VAP [116]. Pleural effusions larger than 10 mm should be aspirated. Samples should be sent for immediate Gram and fungal stains, culture and biochemistry including protein, lactic dehydrogenase and glucose. Paired blood chemistry samples should also be sent for comparison. Are new chest radiographic infiltrates diagnostic of pneumonia in a ventilated patient? Answer: no; grade D. Recommendation A chest radiography should be performed. Rationale The development of a new chest radiographic infiltrate in a ventilated patient may have many causes other than infection [104, 117]. When assessed against diagnosis by bronchoscopy [118], final clinical diagnosis [104] or postmortem histology [119], chest radiographic changes alone are insufficiently specific for diagnosis of pneumonia in this group of patients. Computed tomography (CT) is more sensitive in detecting lung parenchymal changes than plain radiography and may better demonstrate fluid collections [120]. Even so, most causes of diffuse air-space shadowing cannot be reliably differentiated on CT, which therefore adds little diagnostic information in suspected VAP over and above plain radiography [121]. Not withstanding this lack of diagnostic specificity, chest radiography may provide valuable information, for example, to guide invasive diagnostic approaches and detect pleural effusion. Should thoracocentesis be performed in patients with pleural effusions in whom VAP is suspected? Answer: yes; grade E. Rationale Pleural effusions are uncommon in VAP, and empyema develops rarely. We are not aware of any data which determine rates at which diagnostic information is gained from analysis of pleural fluid in the context of VAP. Our recommendation is in line with published guidelines of the American Thoracic Society [116]. The presence of a parapneumonic effusion larger than 10 mm warrants diagnostic thoracocentesis. Parameters suggestive of an underlying pneumonia include: white blood cells higher than 5 109/l, more than 50 % polymorphonuclear cells, organisms seen on Gram stain, low glucose (< 40 g/dl), pH less than 7.3 and biochemical criteria of an exudate (protein > 3 g/l, raised lactic dehydrogenase) [122]. Do serological tests have a role in diagnosis of VAP? Answer: no; grade E. Recommendation Serology is not routinely indicated in the diagnosis of VAP. Rationale With the possible exception of Legionella infection, ªatypicalº organisms are not causes of VAP. Although nonepidemic Legionella infections made up 22 out of 286 episodes of hospital acquired pneumonia in one study, none developed in patients who were already ventilated [123]. Legionella infection developing in a ventilated patient would raise the possibility of acquisition from within the ICU. In rare cases in which Legionella infection is suspected, urinary antigen testing provides rapid and accurate diagnosis and is now beginning to replace serology. Should tracheal aspirates be obtained in patients in whom VAP is suspected? Answer: yes; grade C. S 20 Table 5 Studies assessing diagnostic accuracy of endotracheal aspirates (ETA) in diagnosis of ventilator-associated pneumonia (VAP) (BAL bronchoalveolar lavage, PSB protected specimen brush) Protocol Finding Reference 12 patients with suspected VAP; compares semiquantitative culture of ETA, PSB and BAL Similar range and quantities of organisms recovered by all techniques 127 52 patients with suspected VAP; compares ETA (cutoff 106 cfu/ml) and PSB Sensitivity 82 % vs. 64 %; specificity 83 % vs. 96 % 128 26 patients with ªdefinite VAP,º 48 ªpossible VAP,º 28 controls; compares ETA (cutoff 105 cfu/ml) and PSB/BAL Sensitivity 70 % vs. 60 %/57 %; specificity 72 % vs. 93 %/87 % 129 28 patients with suspected VAP; compares ETA (cutoff 105 cfu/ml) and PSB/BAL to postmortem histology Sensitivity 63 % vs. 57 %/47 %; specificity 75 % vs. 88 %/100 % 130 Recommendation A sample of secretions aspirated via the endotracheal tube should be sent for Gram stain and for bacterial and fungal culture. Rationale Studies which assess different approaches to the microbiological diagnosis of VAP have been hampered by the fact that there is no accepted diagnostic ªgold-standard.º Postmortem histology and quantitative tissue culture (104 cfu/g tissue) are generally regarded as the most precise techniques available but are often not practicable for use in clinical studies. Furthermore, characteristic histological changes in pneumonia may be found in lung which is sterile in culture, and bacterial counts up to 104 cfu/g lung tissue may be found in the absence of histological changes in pneumonia, due to rapid proliferation of organisms after death (reviewed in [124]). Published studies therefore frequently make comparisons between different techniques or use clinical response as confirmation of the diagnosis. The microbiology of endotracheal aspirates (ETA) exemplifies the problem of distinguishing colonization from infection in an ICU setting. Bacterial colonization of the lower respiratory tract is almost universal following intubation [125]. Consequently, negative ETA cultures have powerful negative predictive value in the diagnosis of VAP. When pneumonia is present, the causal agent is usually present in nonquantitative culture of ETA [126, 128, 129]. True pathogens may, however, be missed in culture if more numerous but possibly less pathogenic organisms over-grow the plates. Gram staining is an essential component of the evaluation of respiratory tract specimens. The presence of squamous cells (> 10 per high power field) and the absence of leukocytes (< 25 per high power field) suggests the specimen is contaminated with saliva and unsuitable for culture. The finding of certain organisms may influence initial choice of antibiotics. For example, large numbers of clustered gram positive cocci may emphasize the need to cover S. aureus pending culture results. Table 5 summarizes the studies of ETA in diagnosis of VAP since 1991. What is clear from these data is that ETA has the advantages of not only being the least invasive means of sampling the respiratory tract but also the most sensitive. This is a crucial point in favor of use of ETA in that the outcome of VAP is correlated with the adequacy of the initial antibiotic regimen used. Where the initial antibiotic regimen fails to cover pathogens subsequently identified by microbiology, changes aimed at broadening coverage of pathogens does not improve outcome [131]. ETAs may be of particular value combined with clinical and radiographic parameters in a formal scoring system [132]. Should lower respiratory tract specimens be obtained routinely for microbiology in suspected VAP? Answer: yes; grade B. Recommendations Samples from the lower respiratory tract should be obtained for microbiology. No significant advantage of one invasive diagnostic approach over another has been consistently demonstrated. Choice of technique depends in practice primarily on available expertise and equipment. Rationale Sampling of lower respiratory tract secretions for microbiology may impact on the management of VAP in several ways. First, the range of bacteria which cause VAP, and their susceptibility patterns, varies widely between different hospitals [133]. For this reason knowledge of S 21 Fig. 2 Algorithm for diagnosis of ventilator associated pneumonia pathogens present in an individual hospital has great importance in choice of empirical antimicrobial regimens. Secondly, the microbiological data may alter the outcome. A number of studies on the impact of invasive diagnostic techniques on mortality of VAP have demonstrated that invasive techniques often trigger a change in the antibiotic regimen but have failed to provide conclusive evidence of associated improvement in outcome [131, 134, 135, 136, 137]. However, the balance of evidence is beginning to accumulate in favor of invasive sampling. A preliminary report of a prospective randomized controlled trial comparing invasive to noninvasive strategies demonstrated that the invasive approach is significantly better [138]. A large randomized controlled trial recently been completed in France has demonstrated reduced 14- and 28-day mortality and reduced antibiotic use associated with invasive as oppose to noninvasive diagnosis of VAP [139]. Direct examination of a good-quality specimen helps to guide initial empirical therapy, and culture results allow subsequent modification of antibiotic regimen. The information obtained by invasive sampling of the lower respiratory tract secretions might be expected to allow use of broad spectrum antibiotics to be restricted. The studies cited above, however, show that in clinical practice this is rarely the case. Only in a small minority of cases do the changes in antibiotic regimen, which follow lower respiratory tract sampling, result in the use of narrower spectrum drugs. This need not be so. Where appropriate cutoff values for quantitative culture are set, sensitivity of bronchoalveolar lavage and protected specimen brush has exceeded 90 %. Two studies addressing outcome in patients with suspected VAP who have had antibiotics withdrawn on the basis of bronchoscopy findings showed that there was no increase in mortality associated with this strategy [140, 141]. A systematic approach to the diagnosis of VAP in sepsis patients is described in Fig. 2. When a ventilated S 22 patient develops sepsis, blood cultures should be drawn and chest radiography performed. A diagnostic bronchoscopy should be performed without delay unless either facilities or expertise are not available, or the procedure is contraindicated. In such cases diagnostic endotracheal aspiration is indicated. Respiratory samples should be sent for direct examination and the result used to guide choice of antibiotic therapy. When culture results become available, the antibiotic regimen may be modified. Should blood cultures be obtained in cases of suspected surgical site infection or deep abdominal collection? Answer: yes; grade E. Recommendation Two sets of blood cultures should be obtained. Rationale Surgical site infection and intra-abdominal sepsis National Nosocomial Infections Surveillance definitions for surgical site infection (SSI) have been in use in the United States for a decade [142]. These are as follows [143]: · Superficial SSI: Occurs within 30 days, involves skin or subcutaneous tissue of the incision, and any one of the following; purulence, organisms cultured from aspirate or biopsy specimen, clinical signs of local infection, diagnosis as SSI by attending clinician. Not stitch abscess. · Deep SSI: Occurs within 30 days, or up to 1 year if implant in place, infection appears to relate to surgery and involves fascia and deep muscle layers and any one of the following; purulence, dehiscence of deep incision, abscess found at reoperation, radiology or histology, diagnosis as deep SSI by attending clinician. · Organ/space SSI: Occurs within 30 days, or up to 1 year if implant in place, infection appears to relate to surgery, involves any part of the body other than the incision, and any of the following; purulence, organisms cultured from aspirate, abscess found at reoperation, radiology or histology, diagnosis as organ/ space SSI by attending clinician. Similar definitions have recently been adopted in Europe and the United Kingdom [144]. The literature on microbiological diagnosis of surgical site and wound infection has recently been reviewed [145], and we know of no more recent, relevant data. In brief, while culture of bacteria from an aseptically collected sample of deep fluid or tissue is diagnostic of infection, the contribution of qualitative culture of wound swabs is limited by inevitable contamination of any open wound. Certain organisms such as b-hemolytic streptococci can be considered as pathogenic when present at any concentration. Otherwise, on culture of tissue biopsy, growth of more than 105 bacteria per gram of tissue is considered diagnostic of wound infection. Superficial SSI rarely causes sepsis and uncommonly bacteremia. Although making up fewer than 5 % of all causes of bacteremia among hospitalized patients [113, 146], cases of deep SSI and localized intra-abdominal sepsis are frequently associated with bacteremia. Furthermore, empirical antibiotics may need to be given before samples from the suspected site of infection itself are available. However, such deep infections are frequently polymicrobial, comprising fecal organisms, and blood cultures may not identify the full range of organisms involved, particularly anaerobes. Are there specific indications for obtaining wound swabs or specimens of drain fluid? Answer: yes; grade E. Recommendations The presence of purulence or spreading cellulitis are indications for taking wound swabs. Infection should be suspected particularly at ªcontaminatedº or ªdirtyº surgical sites Rationale Certain clinical changes imply that a superficial surgical site has become infected. Discharge of purulent fluid is diagnostic of SSI, and spreading inflammation, in excess of that seen in normal healing, is present. The development of these features in the first 48 h after surgery or trauma (ªearly infectionº) suggests the presence of infection by virulent organisms such as b-hemolytic streptococci or Clostridium species. Most surgical site infections appear between the 4th and 6th postoperative days (ªlateº) and are polymicrobial. The National Research Council wound definitions set out in 1964 continue to be of value in risk assessment of wound infection. The category of wound is correlated well with the rate of wound infection (Table 6). S 23 Table 6 Wound categories and infection rates (modified from [147]) Wound category Definition Clean Elective surgery, primary closure, no breach in sterile technique, no contamination from potentially colonized body sites Nonelective surgery, controlled opening of colonized body site, minimal breach in sterile technique, reoperation through clean wound with-in 7 days Clean contaminated Infection rate 1.5 % 7.7 % Contaminated Nonpurulent inflammation at first surgery, major break in sterile technique or contamination from colonized body sites; penetrating trauma < 4 h old 15.2 % Dirty Purulent inflammation at first surgery, preoperative perforation of colonized body sites; penetrating trauma > 4 h old 40 % Can the contribution of anaerobic organisms to surgical site infection be determined in routine practice? Answer: no; grade D. Recommendation When contaminated or dirty abdominal wounds develop, features of wound infection, a diagnosis of anaerobic coinfection should be assumed irrespective of whether anaerobes are identified by routine microbiology. Is there evidence to support the preference of particular imaging modalities in the diagnosis of intra-abdominal infection? Answer: yes; grade E. Recommendation In most situations ultrasound is be the modality of first choice. When ultrasound is not diagnostic, CT should be considered. Rationale Rationale Detection of anaerobic organisms in clinical specimens is technically demanding. If anaerobic organisms are to be cultured, specific measures may need to be taken in obtaining samples, such as transporting pus in anaerobic conditions. In the laboratory, culture techniques are specialized and time consuming. For these reasons routine processing of samples in most microbiology laboratories does not include an extensive search for anaerobes. While infections which develop in clean wounds are frequently caused by skin flora such as S. aureus, when contaminated or dirty wounds become infected it is possible to identify, at least one anaerobic organism in 65±94 % of samples [148]. Similarly, over 50 % of abdominal abscesses are polymicrobial, and almost 80 % involve at least one anaerobic species [149, 150]. Consequently, while data from randomized controlled trials are lacking, it is accepted best practice to cover anaerobic organisms when treating sepsis arising contaminated or dirty surgical sites [151]. Gas liquid chromatography to detect bacterial shortchain fatty acids is a technique that allows rapid identification of anaerobes in a mixed culture. Although cost currently limits the availability of gas liquid chromatography, it may in future replace culture-based methods of anaerobe identification. Plain radiography of the abdomen may reveal free gas within the abdomen suggesting bowel perforation, or demonstrate the presence of gas within an abscess, but is only rarely yield definitive diagnostic information [152]. In most patients further imaging, usually by ultrasound or CT, is necessary to localize a source of infection within the abdomen [153]. Ultrasound is readily available, if necessary as a bed-side investigation. Its limitations are that gas-filled loops of bowel, commonly present in postoperative ileus may obscure underlying pathology. Wounds and drains may make access to the abdominal wall difficult. The sensitivity of ultrasound is particularly operator dependent. CT, by comparison, is more sensitive than ultrasound in detecting small foci of infection, but in certain areas of the abdomen, particularly the pancreas, distinguishing an abscess from inflammation may be difficult by CT [154]. Magnetic resonance imaging is in turn more sensitive than CT; a recent study reported 100 % sensitivity and 94 % specificity in detecting intraperitoneal abscess [155]. In many situations, for example in ventilated patients, the use of magnetic resonance imaging is limited in the diagnosis of sepsis by the need to keep all metallic instruments away from the scanner's magnetic field. S 24 Should abdominal fluid collections identified by imaging be aspirated as a matter of routine? Answer: yes; grade E. Recommendation Collections identified by radiology should, where technically possible, be aspirated and drained under radiological control, samples being sent for Gram-staining and culture. Rationale Differentiation of infected material from hematoma or inflammatory fluid is not possible on the basis of radiology alone [154]. Confirmation that infection is present and identification particularly of any drug-resistant organisms depends on obtaining samples for microscopy and culture [156, 157]. The pH of fluid obtained at ultrasound-guided aspiration (< 7.1) was found in one study to be a sensitive marker (92 %) of the presence of infection [158] and has been suggested as a bed-side means of identifying collections which require formal drainage. Acalculous cholecystitis Acute acalculous cholecystitis (ACC) is an infrequent but probably underdiagnosed complication in critically ill patients [159, 160]. It is caused by spontaneous gangrene of the gall bladder which without prompt diagnosis and treatment progresses to perforation. The cause appears to involve infection by Clostridium perfringens [161]. Although reported as a complication of a wide range of critical illnesses, the majority of cases of AAC follow trauma or biliary surgery [162, 163]. High doses of narcotic agents may be a contributory factor [164]. Is there a standard approach to the diagnosis of acalculous cholecystitis? Answer: no; grade E. Recommendations ACC should be suspected in any sepsis patient, particularly postoperatively, when there are either signs relating to the right upper quadrant of the abdomen or obstructive liver function tests. When ACC is suspected, ultrasound should be ordered urgently. If an initial ultrasound examination is not diagnostic, CT should be performed. If CT is unavailable, a repeat ultrasound should be performed after 24 h. Rationale Localizing right upper quadrant pain and tenderness is often absent in sedated or ventilated patients suffering from ACC. Diagnosis therefore requires a high index of suspicion. The only differentiating features in a sepsis patient may be elevation in alkaline phosphatase or gglutaryl transferase, in an at risk patient [165]. Although the ultrasound appearances of ACC (gallbladder distension, wall thickening and free fluid suggestive of perforation) are well established [166], such changes are not diagnostic and are frequently demonstrable in critically ill patients who do not go on to develop ACC [167]. The value of a single ultrasound study in diagnosis of ACC has been assessed in three retrospective studies. Two studies of ICU patients estimated sensitivity at 76 % [168] and 92 %, with a specificity of 96 % [169]. More recently among 27 cases of ACC, only half of which occurred in critically ill patients, a sensitivity of 29 % was found [165]. Two prospective studies looking specifically at the use of serial ultrasound examinations in patients with suspected ACC have demonstrated that when initial diagnosis is uncertain, failure of any abnormalities to progress on followup scans has excellent negative predictive value [170, 171]. The role of CT in diagnosis of ACC has not been thoroughly evaluated. Superior sensitivity of CT over ultrasound has been suggested by three retrospective studies of patients with a surgical diagnosis of ACC [165, 169, 172]. We are aware of two studies which examine the role of laparoscopy in diagnosis and treatment of ACC. Neither study compared diagnostic accuracy with radiology. In both the procedure was well tolerated. Laparoscopy has the advantage over CT that it may, in some units, be performed at the bedside and, if the diagnosis of ACC is confirmed, can proceed directly to laparoscopic cholecystectomy [162] or cholecystostomy [173]. Sinusitis Since being first reported in 1974 [174], ventilator-associated sinusitis has become an increasingly well recognized cause of sepsis. It occurs usually but not exclusively in patients who have nasotracheal, as opposed to orotracheal intubation [175, 176]. The true incidence of sinusitis among critical care patients is hard to establish since published estimates vary widely depending on the population studied and the diagnostic techniques used. In the only study to directly address the issue, one of 19 patients with occult sepsis on a surgical ICU had si- S 25 nusitis as the sole focus of infection [177]. This topic has recently been the subject of a general review [178]. Is there a standard approach to the diagnosis of ventilator-associated sinusitis? Answer: no; grade E. Recommendations Acute sinusitis should be suspected in any sepsis patient who has either a nasotracheal tube or a fine-bore nasogastric feeding tube, or who has suffered a head injury. When sinusitis is suspected, radiography of the maxillary sinuses should be performed to detect the presence of fluid. When radiography does not demonstrate fluid in the maxillary sinuses, CT should be performed. If either radiography or CT demonstrates the presence of fluid, antral puncture should be performed to allow definitive diagnosis and therapeutic drainage before antibiotic therapy is initiated. Rationale If sinusitis is the source of sepsis, specific physical signs are likely to be absent, although a mucopurulent nasal discharge may be noted. For this reason the diagnosis should be suspected in any patient who has a nasotracheal tube or an indwelling nasal device of any sort (even a fine-bore nasogastric feeding tube), or who has had a head injury. The definitive investigation of antral sinus disease is considered to be direct endoscopic examination [179]. In general the diagnosis is made on the basis of culture of bacteria from purulent material obtained from the sinus cavities [180]. Because clinical evidence to support a diagnosis of sinusitis is generally lacking in ICU patients, the first supportive evidence often comes from radiology, either plain radiographic sinus views, ultrasound or CT. For each of these modalities there is a discrepancy between radiological diagnosis of sinusitis (presence of fluid) and microbiological confirmation on any subsequent aspirate. While plain radiography is of value in diagnosis of maxillary sinusitis [181], five views are required to achieve 88 % sensitivity [182]. Similarly, ultrasound detected accumulation of fluid in the sinuses of 15 of 100 patients in a consecutive prospective series of intubated ICU patients but in only one of these patients could aspiration confirm sinusitis [183]. CT has two advantages over sinus radiography and ultrasound. CT is able to distinguish mucosal thickening from fluid within the sinuses [184] and can assess the other paranasal sinuses which may, albeit less frequently, be infect- ed in isolation [185]. The principal disadvantage is that CT usually requires the patient to be moved from the ICU. Although the ability of CT to detect mucosal abnormalities improves diagnostic accuracy, the discrepancy between CT diagnosis and diagnosis by antral puncture remains significant [184, 186]. Since the discrepancy between radiological diagnosis of sinusitis and confirmation on aspiration is unavoidable, abnormalities on radiology indicate further investigation by antral puncture to obtain fluid for culture. Interpretation of culture results, however, requires caution. Hospitalized patients have heavy colonization of the nose, and contamination of samples is virtually unavoidable [184]. In addition, while true infective sinusitis is frequently caused by mixed Gram-negative and anaerobic infections, fluid obtained from antra that do not have signs of infection quite frequently produces an apparently significant culture result [187]. Invasive Candida infection As a result of widespread use of broad-spectrum antibiotics and intravascular catheters, an increase in the incidence of nosocomial infection by Candida species has occurred in both the United States and Europe [188, 189, 190, 191]. Although C. albicans remains the most frequently isolated species, the incidence of other, potentially more drug-resistant species is also rising [192]. The importance of invasive fungal infection is further underlined by the considerable attributable mortality, 38 % in one study [193] and 21.7 % in another [194]. Invasive Candida infections begin by colonization of the gastrointestinal tract or skin [195]. Suppression of indigenous intestinal bacteria allows overgrowth of Candida in the gastrointestinal tract and mucosal adhesion. Once a critical level of colonization has been reached translocation, across intact small bowel mucosa may occur. In an ICU setting, where a range of physiological stresses may impair small bowel mucosal integrity, such translocation may occur at much lower concentrations. Similarly, skin colonization provides a source for invasive disease when integrity is breached either by intravascular catheters or burns. Finally, any disease or drug which inhibits cellular immunity, for example, diabetes mellitus, or corticosteroids, predisposes to invasive Candida infection [196]. Is there a standard approach to the diagnosis of invasive candidiasis? Answer: no; grade E. S 26 Table 7 Examples of suggested risk factors for invasive Candida infection and associated mortality (APACHE II Acute Physiology and Chronic Health Evaluation II) (data summarized from: [194, 201, 202, 203, 204]) Examples of risk factors for development of invasive Candida infection Examples of risk factors for mortality associated with invasive Candida infection Colonization with Candida species. Prior treatment with multiple antibiotics APACHE II score > 20 Delay between onset of candidemia and start of antifungal therapy > 48 h Total number of different antibiotics > 2 Total number of days on antibiotics > 14 Prior Hickman catheter Prior hemodialysis Recommendations There are no data to support a policy of routine screening of hospitalized patients for Candida colonization. However, in sepsis patients invasive fungal infection is more likely in patients who are heavily colonized. When sepsis develops in patients colonized by Candida species at two or more sites, blood cultures should be sent and lysis centrifugation performed if available. Isolates of Candida species from sterile sites should be sent for speciation and sensitivity testing. Rationale Clinical features of invasive Candida infection are in most cases nonspecific, ranging from unexplained fever to sepsis [197]. Specific clinical manifestations are rare. Candidal chorioretinitis occurs in fewer than 15 % of candidemic patients [198], but when found is an absolute indication for initiation of antifungal therapy. Skin lesions and septic arthritis occur less frequently still [199]. Retrospective studies have demonstrated a range of risk factors for invasive Candida infection (Table 7). One small prospective study has suggested that after 4 days of persisting fever despite antibacterial antibiotics, antifungal agents should be started empirically [200]. We are not aware of any studies that address a risk stratification approach to diagnosis of fungal infection. Development of invasive Candida infection is correlated with preceding colonization [201]. Sites at which colonization may be detected include urine, rectum, gastric aspirate, vascular access sites, sputum/throat swab, wounds and surgical drains. The number of sites has been found to be correlated with the risk of develop- ing invasive fungal infection [202, 203] A cutoff of two sites colonized, as an indication for beginning empirical antifungal therapy, has a high sensitivity but low specificity, 22 % in one study [203]. This may in part be because using the number of colonized sites alone as a measure of infection risk fails to take into account the intensity of colonization. Using semiquantitative culture techniques to produce a ªcorrected Candida colonization indexº; sensitivity and specificity of 100 % was achieved in a retrospective analysis of 29 critical care patients [203]. No evidence exists regarding how frequently samples should be taken to detect colonization in at-risk patients. Five days would seem a reasonable interval. At present there is no consensus on the value or use of routine screening for Candida in hospitalized patients, and the data are inadequate to support a general recommendation that it should be instituted. Conventional blood culture techniques are insensitive in detecting blood-borne Candida infections. For example, only 50 % of patients with disseminated candidiasis have positive blood cultures [196], but lysis centrifugation of blood cultures increases yield by 30±40 %. While growth of Candida species in blood is a clear indication for initiation of antifungal agents, failure to confirm candidemia in an at-risk patient in no way disproves the diagnosis. Candiduria in patients who have not had instrumentation of the renal tract is strongly suggestive of renal involvement in disseminated candidiasis [195]. The practical value of this is limited by the fact that the majority of ICU patients will have been catheterized at some stage. Furthermore, up to 50 % of patients who have disseminated candidiasis do not have candiduria [205]. In an ICU setting the finding of candiduria in a catheterized patient is no more significant an indicator of invasive disease than isolation from any other single site [206]. While a single colony of Candida species isolated from a sterile site such as blood or cerebrospinal fluid must be regarded as significant, the greatest obstacle to the diagnosis of invasive Candida infection by culture from nonsterile sites is distinguishing infection from colonization. Cut-off values for quantitative diagnosis of Candida infections are much less well established than for bacterial infections. Diagnosis of Candida infection by tissue biopsy is made on the basis of either quantitative culture of more than 105 organisms per gram of tissue or the presence of yeasts on microscopy pending culture results [196]. Although C. albicans continues to make up the majority of clinical isolates, the incidence of other species is increasing. When Candida is cultured from nonsterile sites or urine, differentiation of C. albicans from other species using the germ-tube technique is generally sufficient. However, when Candida is identified at sterile sites, speciation and sensitivity testing should be routine [157]. Relative sensitivity of these species to azole anti- S 27 fungals varies. In certain cases the use of azoles may be possible in place of amphotericin, with its associated toxicity (see Bochud et al., ªAntibiotics in sepsisº) [157, 207]. In addition, knowledge of the species and resistance patterns within a particular hospital is essential to choice of empirical antifungal agents. A number of PCR [208] and serological [209, 210, 211] assays have shown promise in preclinical trials, but two clinical trials in hospitalized patients have found that such techniques are unlikely to replace culture based diagnosis [212, 213]. References 1. Cohen J, Heumann D, Glauser MP (1995) Do monoclonal antibodies and anticytokines still have a future in infectious diseases? Am J Med 99: 45S±52S 2. Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328: 1471±1477 3. Kreger BE, Craven DE, McCabe WR (1980) Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med 68: 344±355 4. O'Grady NP, Barie PS, Bartlett JG, et al (1998) Practice guidelines for evaluating new fever in critically ill adult patients. Task Force of the Society of Critical Care Medicine and the Infectious Diseases Society of America. Clin Infect Dis 26: 1042±1059 5. Carlet J (1999) Rapid diagnostic methods in the detection of sepsis. Infect Dis Clin North Am 13: 483±494, xi 6. Yentis SM, Soni N, Sheldon J (1995) Creactive protein as an indicator of resolution of sepsis in the intensive care unit. Intensive Care Med 21: 602±605 7. Oberhoffer M, Karzai W, Meier-Hellmann A, et al (1999) Sensitivity and specificity of various markers of inflammation for the prediction of tumor necrosis factor-alpha and interleukin-6 in patients with sepsis. Crit Care Med 27: 1814±1818 8. Ugarte H, Silva E, Mercan D, et al (1999) Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 27: 498±504 9. Whang KT, Steinwald PM, White JC, et al (1998) Serum calcitonin precursors in sepsis and systemic inflammation. J Clin Endocrinol Metab 83: 3296±3301 10. Povoa P, Almeida E, Moreira P, et al (1998) C-reactive protein as an indicator of sepsis. Intensive Care Med 24: 1052±1056 11. de Werra I, Jaccard C, Corradin SB, et al (1997) Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med 25: 607±613 12. Leibovici L, Greenshtain S, Cohen O, et al (1991) Bacteremia in febrile patients. A clinical model for diagnosis. Arch Intern Med 151: 1801±1806 13. Smith-Elekes S, Weinstein MP (1993) Blood cultures. Infect Dis Clin North Am 7: 221±234 14. McDonald LC, Fune J, Gaido LB, et al (1996) Clinical importance of increased sensitivity of BacT/Alert FAN aerobic and anaerobic blood culture bottles. J Clin Microbiol 34: 2180±2184 15. Reimer LG, Wilson ML, Weinstein MP (1997) Update on detection of bacteremia and fungemia. Clin Microbiol Rev 10: 444±465 16. Boschman CR, Tucker LJ, Dressel DC, et al (1995) Optimizing detection of microbial sepsis: a comparison of culture systems using packaged sets with directions for blood collection. Diagn Microbiol Infect Dis 23: 1±9 17. Bates DW, Goldman L, Lee TH (1991) Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA 265: 365±369 18. Spitalnic SJ, Woolard RH, Mermel LA (1995) The significance of changing needles when inoculating blood cultures: a meta-analysis. Clin Infect Dis 21: 1103±1106 19. Bryant JK, Strand CL (1987) Reliability of blood cultures collected from intravascular catheter versus venipuncture. Am J Clin Pathol 88: 113±116 20. Weinstein MP (1996) Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin Infect Dis 23: 40±46 21. Souvenir D, Anderson DE Jr, Palpant S, et al (1998) Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol 36: 1923±1926 22. Shahar E, Wohl-Gottesman BS, Shenkman L (1990) Contamination of blood cultures during venepuncture: fact or myth? Postgrad Med J 66: 1053±1058 23. Strand CL, Wajsbort RR, Sturmann K (1993) Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA 269: 1004±1006 24. Schifman RB, Pindur A (1993) The effect of skin disinfection materials on reducing blood culture contamination. Am J Clin Pathol 99: 536±538 25. Lynn WA, Cohen J (eds) (1995) Microbiological requirements for studies of sepsis. In: Sibbald WJ, Vincent J-L (eds) Update in intensive care and emergency medicine, vol 19. Springer, Berlin Heidelberg New York, pp 71±85 26. Brown DR, Kutler D, Rai B, et al (1995) Bacterial concentration and blood volume required for a positive blood culture. J Perinatol 15: 157±159 27. Kreger BE, Craven DE, Carling PC, et al (1980) Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med 68: 332±343 28. Jonsson B, Nyberg A, Henning C (1993) Theoretical aspects of bacteremia as a function of the volume of blood cultured. APMIS 101: 595±601 29. Weinstein MP, Mirrett S, Wilson ML, et al (1994) Controlled evaluation of 5 versus 10 milliliters of blood cultured in aerobic BacT/Alert blood culture bottles. J Clin Microbiol 32: 2103±2106 30. Mermel LA, Maki DG (1993) Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 119: 270±272 31. Li J, Plorde JJ, Carlson LG (1994) Effects of volume and periodicity on blood cultures. J Clin Microbiol 32: 2829±2831 32. Wilson ML (1996) General principles of specimen collection and transport. Clin Infect Dis 22: 766±777 33. Kaditis AG, O'Marcaigh AS, Rhodes KH, et al (1996) Yield of positive blood cultures in pediatric oncology patients by a new method of blood culture collection. Pediatr Infect Dis J 15: 615±620 34. Lombardi DP, Engleberg NC (1992) Anaerobic bacteremia: incidence, patient characteristics, and clinical significance. Am J Med 92: 53±60 S 28 35. Pottumarthy S, Morris AJ (1997) Assessment of the yield of anaerobic blood cultures. Pathology 29: 415±417 36. Murray PR, Traynor P, Hopson D (1992) Critical assessment of blood culture techniques: analysis of recovery of obligate and facultative anaerobes, strict aerobic bacteria, and fungi in aerobic and anaerobic blood culture bottles. J Clin Microbiol 30: 1462±1468 37. Krumholtz H, Cummings S, York M (1990) Blood Culture phlebotomy: switching needles does not prevent contamination. Ann Intern Med 113: 290±292 38. Thamlikitkul V, Chokloikaew S, Tangtrakul T, et al (1992) Blood culture: comparison of outcomes between switch-needle and no-switch techniques. Am J Infect Control 20: 122±125 39. Smart D, Baggoley C, Head J, et al (1993) Effect of needle changing and intravenous cannula collection on blood culture contamination rates. Ann Emerg Med 22: 1164±1168 40. Weinstein MP, Reller LB, Murphy JR, et al (1983) The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis 5: 35±53 41. Washington JA 2nd (1975) Blood cultures: principles and techniques. Mayo Clin Proc 50: 91±98 42. Strand CL (1988) Blood cultures: consensus recommendations in 1988. American Society for Clinical Pathologists Check Sample Continuing Education Program 43. Dobbins BM, Kite P, Wilcox MH (1999) Diagnosis of central venous catheter related sepsis-a critical look inside. J Clin Pathol 52: 165±172 44. Reed CR, Sessler CN, Glauser FL, et al (1995) Central venous catheter infections: concepts and controversies. Intensive Care Med 21: 177±183 45. Maki DG, Jarrett F, Sarafin HW (1977) A semiquantitative culture method for identification of catheterrelated infection in the burn patient. J Surg Res 22: 513±520 46. Cleri DJ, Corrado ML, Seligman SJ (1980) Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis 141: 781±786 47. Siegman-Igra Y, Anglim AM, Shapiro DE, et al (1997) Diagnosis of vascular catheter-related bloodstream infection: a meta-analysis. J Clin Microbiol 35: 928±936 48. Gosbell IB, Duggan D, Breust M, et al (1995) Infection associated with central venous catheters: a prospective survey. Med J Aust 162: 210±213 49. Maki DG, Mermel LA (1998) Infections due to infusion therapy. In: Bennett JV, Brachman PS (eds) Hospital infections. Lippincott-Raven, Philadelphia, pp 689±722 50. Capdevila JA, Planes AM, Palomar M, et al (1992) Value of differential quantitative blood cultures in the diagnosis of catheter-related sepsis. Eur J Clin Microbiol Infect Dis 11: 403±407 51. Weightman NC, Simpson EM, Speller DC, et al (1988) Bacteremia related to indwelling central venous catheters: prevention, diagnosis and treatment. Eur J Clin Microbiol Infect Dis 7: 125±129 52. Sherertz RJ, Heard SO, Raad II (1997) Diagnosis of triple-lumen catheter infection: comparison of roll plate, sonication, and flushing methodologies. J Clin Microbiol 35: 641±646 53. Andremont A, Paulet R, Nitenberg G, et al (1988) Value of semiquantitative cultures of blood drawn through catheter hubs for estimating the risk of catheter tip colonization in cancer patients. J Clin Microbiol 26: 2297±2299 54. Benezra D, Kiehn TE, Gold JW, et al (1988) Prospective study of infections in indwelling central venous catheters using quantitative blood cultures. Am J Med 85: 495±498 55. Paya C, Guerra L, Marsh M, et al (1989) Limited usefulness of quantitative culture of blood drawn through the device for diagnosis of intravascular-device-related bacteremia. J Clin Microbiol 27: 1431±1433 56. Quilici N, Audibert G, Conroy MC, et al (1997) Differential quantitative blood cultures in the diagnosis of catheter-related sepsis in intensive care units. Clin Infect Dis 25: 1066±1070 57. Sherertz RJ (1996) Surveillance for infections associated with vascular catheters. Infect Control Hosp Epidemiol 17: 746±752 58. Raad I (1998) Intravascular-catheterrelated infections. Lancet 351: 893±898 59. Rogers MS, Oppenheim BA (1998) The use of continuous monitoring blood culture systems in the diagnosis of catheter related sepsis. J Clin Pathol 51: 635±637 60. Thorpe TC, Wilson ML, Turner JE, et al (1990) BacT/Alert: an automated colorimetric microbial detection system. J Clin Microbiol 28: 1608±1612 61. Bernard D, Verschraegen G, Claeys G, et al (1996) Diagnosis of intravascular device related sepsis by a continuous monitoring blood culture system. Intensive Care Med 22: 829±830 62. Blot F, Schmidt E, Nitenberg G, et al (1998) Earlier positivity of central-venous- versus peripheral-blood cultures is highly predictive of catheter-related sepsis. J Clin Microbiol 36: 105±109 63. Blot F (1999) Diagnosis of catheter-related-bacteremia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet 354: 1071±1077 64. Tighe MJ, Kite P, Thomas D, et al (1996) Rapid diagnosis of catheter-related sepsis using the acridine orange leukocyte cytospin test and an endoluminal brush. JPEN J Parenter Enteral Nutr 20: 215±218 65. von Baum H, Philippi P, Geiss HK (1998) Acridine-orange leucocyte cytospin (AOLC) test as an in-situ method for the diagnosis of central venous catheter (CVC)-related sepsis in adult risk patients. Zentralbl Bakteriol 287: 117±123 66. Gowardman JR, Montgomery C, Thirlwell S, et al (1998) Central venous catheter-related bloodstream infections: an analysis of incidence and risk factors in a cohort of 400 patients. Intensive Care Med 24: 1034±1039 67. Kite P, Dobbins BM, Wilcox MH, et al (1999) Rapid diagnosis of central-venous-catheter-related blood stream infection without catheter removal. Lancet 354: 1504±1507 68. Tighe MJ, Kite P, Fawley WN, et al (1996) An endoluminal brush to detect the infected central venous catheter in situ: a pilot study. BMJ 313: 1528±1529 69. Kite P, Dobbins BM, Wilcox MH, et al (1997) Evaluation of a novel endoluminal brush method for in situ diagnosis of catheter related sepsis. J Clin Pathol 50: 278±282 70. Aufwerber E, Ringertz S, Ransjo U (1991) Routine semiquantitative cultures and central venous catheter-related bacteremia. APMIS 99: 627±630 71. McKinley S, Mackenzie A, Finfer S, et al (1999) Incidence and predictors of central venous catheter related infection in intensive care patients. Anaesth Intensive Care 27: 164±169 72. Farkas JC, Liu N, Bleriot JP, et al (1992) Single- versus triple-lumen central catheter-related sepsis: a prospective randomized study in a critically ill population. Am J Med 93: 277±282 73. Raad II, Bodey GP (1992) Infectious complications of indwelling vascular catheters. Clin Infect Dis 15: 197±208 S 29 74. Gil RT, Kruse JA, Thill-Baharozian MC, et al (1989) Triple- vs single-lumen central venous catheters. A prospective study in a critically ill population. Arch Intern Med 149: 1139±1143 75. Brun-Buisson C, Abrouk F, Legrand P, et al (1987) Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med 147: 873±877 76. Pinilla JC, Ross DF, Martin T, et al (1983) Study of the incidence of intravascular catheter infection and associated septicemia in critically ill patients. Crit Care Med 11: 21±25 77. Sitzmann JV, Townsend TR, Siler MC, et al (1985) Septic and technical complications of central venous catheterization. A prospective study of 200 consecutive patients. Ann Surg 202: 766±770 78. Keung YK, Watkins K, Chen SC, et al (1994) Comparative study of infectious complications of different types of chronic central venous access devices. Cancer 73: 2832±2837 79. Timsit JF, Sebille V, Farkas JC, et al (1996) Effect of subcutaneous tunneling on internal jugular catheter-related sepsis in critically ill patients: a prospective randomized multicenter study. JAMA 276: 1416±1420 80. Randolph AG, Cook DJ, Gonzales CA, et al (1998) Tunneling short-term central venous catheters to prevent catheter-related infection: a metaanalysis of randomized, controlled trials. Crit Care Med 26: 1452±1457 81. Darouiche RO, Raad II, Heard SO, et al (1999) A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med 340: 1±8 82. Maki DG, Stolz SM, Wheeler S, et al (1997) Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med 127: 257±266 83. Kamal GD, Pfaller MA, Rempe LE, et al (1991) Reduced intravascular catheter infection by antibiotic bonding. A prospective, randomized, controlled trial. JAMA 265: 2364±2368 84. Kamal GD, Divishek D, Kumar GC, et al (1998) Reduced intravascular catheter-related infection by routine use of antibiotic-bonded catheters in a surgical intensive care unit. Diagn Microbiol Infect Dis 30: 145±152 85. Ciresi DL, Albrecht RM, Volkers PA, et al (1996) Failure of antiseptic bonding to prevent central venous catheter-related infection and sepsis. Am Surg 62: 641±646 86. Pemberton LB, Ross V, Cuddy P, et al (1996) No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Arch Surg 131: 986±989 87. Christensen ML, Hancock ML, Gattuso J, et al (1993) Parenteral nutrition associated with increased infection rate in children with cancer. Cancer 72: 2732±2738 88. Ena J, Cercenado E, Martinez D, et al (1992) Cross-sectional epidemiology of phlebitis and catheter-related infections. Infect Control Hosp Epidemiol 13: 15±20 89. Raad I, Umphrey J, Khan A, et al (1993) The duration of placement as a predictor of peripheral and pulmonary arterial catheter infections. J Hosp Infect 23: 17±26 90. Cook D, Randolph A, Kernerman P, et al (1997) Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med 25: 1417±1424 91. Badley AD, Steckelberg JM, Wollan PC, et al (1996) Infectious rates of central venous pressure catheters: comparison between newly placed catheters and those that have been changed. Mayo Clin Proc 71: 838±846 92. Bozzetti F, Bonfanti G, Regalia E, et al (1991) A new approach to the diagnosis of central venous catheter sepsis. JPEN J Parenter Enteral Nutr 15: 412±416 93. Lugauer S, Regenfus A, Boswald M, et al (1999) A new scoring system for the clinical diagnosis of catheter-related infections. Infection 27:S49±53 94. Pearson ML (1996) Guideline for prevention of intravascular device-related infections. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 17: 438±473 95. Snydman DR, Gorbea HF, Pober BR, et al (1982) Predictive value of surveillance skin cultures in total-parenteralnutrition-related infection. Lancet 2: 1385±1388 96. Armstrong CW, Mayhall CG, Miller KB, et al (1990) Clinical predictors of infection of central venous catheters used for total parenteral nutrition. Infect Control Hosp Epidemiol 11: 71±78 97. Raad II, Baba M, Bodey GP (1995) Diagnosis of catheter-related infections: the role of surveillance and targeted quantitative skin cultures. Clin Infect Dis 20: 593±597 98. Reimer LG (1994) Catheter-related infections and blood cultures. Clin Lab Med 14: 51±58 99. Brown PD, Lerner SA (1998) Community-acquired pneumonia. Lancet 352: 1295±1302 100. Finch R (1998) Community acquired pneumonia. J R Coll Physicians (Lond) 32: 328±332 101. Marrie TJ (1998) Community-acquired pneumonia: epidemiology, etiology, treatment. Infect Dis Clin North Am 12: 723±740 102. Conces DJ Jr (1998) Bacterial pneumonia in immunocompromised patients. J Thorac Imaging 13: 261±270 103. Bonten MJ, Gaillard CA, Wouters EF, et al (1994) Problems in diagnosing nosocomial pneumonia in mechanically ventilated patients: a review. Crit Care Med 22: 1683±1691 104. Meduri GU (1995) Diagnosis and differential diagnosis of ventilator-associated pneumonia. Clin Chest Med 16: 61±93 105. Wunderink RG (1998) Mortality and the diagnosis of ventilator-associated pneumonia: a new direction. Am J Respir Crit Care Med 157: 349±350 106. Bonten MJ (1999) Controversies on diagnosis and prevention of ventilator-associated pneumonia. Diagn Microbiol Infect Dis 34: 199±204 107. Gallego M, Rello J (1999) Diagnostic testing for ventilator-associated pneumonia. Clin Chest Med 20: 671±679 108. Griffin JJ, Meduri GU (1994) New approaches in the diagnosis of nosocommial pneumonia. Med Clin North Am 78: 1091±1105 109. Cook DJ, Walter SD, Cook RJ, et al (1998) Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 129: 433±440 110. Cook DJ, Kollef MH (1998) Risk factors for ICU-acquired pneumonia. JAMA 279: 1605±1606 111. Joshi N, Localio AR, Hamory BH (1992) A predictive risk index for nosocomial pneumonia in the intensive care unit. Am J Med 93: 135±142 112. Edgeworth JD, Treacher DF, Eykyn SJ (1999) A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit Care Med 27: 1421±1428 113. Weinstein MP, Towns ML, Quartey SM, et al (1997) The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 24: 584±602 S 30 114. Fagon JY, Chastre J, Hance AJ, et al (1988) Detection of nosocomial lung infection in ventilated patients. Use of a protected specimen brush and quantitative culture techniques in 147 patients. Am Rev Respir Dis 138: 110±116 115. Meduri GU, Mauldin GL, Wunderink RG, et al (1994) Causes of fever and pulmonary densities in patients with clinical manifestations of ventilatorassociated pneumonia. Chest 106: 221±235 116. Anonymous (1995) Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med 153: 1711±1725 117. Santos E, Talusan A, Brandstetter RD (1998) Roentgenographic mimics of pneumonia in the critical care unit. Crit Care Clinics 14: 91±104 118. Butler KL, Sinclair KE, Henderson VJ, et al (1999) The chest radiograph in critically ill surgical patients is inaccurate in predicting ventilator-associated pneumonia. Am Surg 65: 805±809 119. Wunderink RG, Woldenberg LS, Zeiss J, et al (1992) The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest 101: 458±463 120. Peruzzi W, Garner W, Bools J, et al (1988) Portable chest roentgenography and computed tomography in critically ill patients. Chest 93: 722±726 121. Lipchik RJ, Kuzo RS (1996) Nosocomial pneumonia. Radiol Clin North Am 34: 47±58 122. Light RW (1977) Pleural effusions. Med Clin North Am 61: 1339±1352 123. Carratala J, Gudiol F, Pallares R, et al (1994) Risk factors for nosocomial Legionella pneumophila pneumonia. Am J Respir Crit Care Med 149: 625±629 124. Brun-Buisson C (1995) Nosocomial pneumonia during mechanical ventilation: problems with diagnostic criteria. Thorax 50: 1128±1130 125. Cardenosa Cendrero JA, Sole-Violan J, Bordes Benitez A, et al (1999) Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 116: 462±470 126. Kirtland SH, Corley DE, Winterbauer RH, et al (1997) The diagnosis of ventilator-associated pneumonia: a comparison of histologic, microbiologic, and clinical criteria. Chest 112: 445±457 127. Middleton R, Broughton WA, Kirkpatrick MB (1992) Comparison of four methods for assessing airway bacteriology in intubated, mechanically ventilated patients Am J Med Sci 304: 239±245 128. Marquette CH, Georges H, Wallet F, et al (1993) Diagnostic efficiency of endotracheal aspirates with quantitative bacterial cultures in intubated patients with suspected pneumonia. Comparison with the protected specimen brush. Am Rev Respir Dis 148: 138±144 129. el-Ebiary M, Torres A, Gonzalez J, et al (1993) Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am Rev Respir Dis 148: 1552±1557 130. Marquette CH, Copin MC, Wallet F, et al (1995) Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med 151: 1878±1888 131. Sanchez-Nieto JM, Torres A, GarciaCordoba F, et al (1998) Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: a pilot study. Am J Respir Crit Care Med 157: 371±376 132. Pugin J, Auckenthaler R, Mili N (1991) Diagnosis of ventilator associated pneumonia by bacteriologic analysis and bronchoscopic and nonbronchoscopic ªblindº Bronchoalveolar lavage fluid. Am Rev Respir Dis 143: 1121±1129 133. Rello J, Sa-Borges M, Correa H, et al (1999) Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med 160: 608±613 134. Alvarez-Lerma F (1996) Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med 22: 387±394 135. Luna CM, Vujacich P, Niederman MS, et al (1997) Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111: 676±685 136. Rello J, Gallego M, Mariscal D, et al (1997) The value of routine microbial investigation in ventilator-associated pneumonia Am J Respir Crit Care Med 156: 196±200 137. Kollef MH, Ward S (1998) The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 113: 412±420 138. Perry C, Chandrasekhar A, Moorman DW, et al (1999) Ventilator associated pneumonia: therapy based on protected specimen brushing versus tracheal aspirate data. Chest 116: 278S 139. Fagon JY, Chastre J, Wolff M, et al (2000) Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 132: 621±630 140. Bonten MJ, Bergmans DC, Stobberingh EE, et al (1997) Implementation of bronchoscopic techniques in the diagnosis of ventilator-associated pneumonia to reduce antibiotic use. Am J Respir Crit Care Med 156: 1820±1824 141. Croce MA, Fabian TC, Schurr MJ, et al (1995) Using bronchoalveolar lavage to distinguish nosocomial pneumonia from systemic inflammatory response syndrome: a prospective analysis. J Trauma Inj Infect Crit Care 39: 1134±1139 142. Horan TC, Gaynes RP, Martone WJ, et al (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13: 606±608 143. Mangram AJ, Horan TC, Pearson ML, et al (1999) Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27: 97±132 144. Crowe MJ, Cooke EM (1998) Review of case definitions for nosocomial infection ± towards a consensus. Presentation by the Nosocomial Infection Surveillance Unit (NISU) to the Hospital Infection Liaison Group, subcommittee of the Federation of Infection Societies (FIS). J Hosp Infect 39: 3±11 145. Robson MC (1997) Wound infection: a failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 77: 637±650 146. Crowe M, Ispahani P, Humphreys H, et al (1998) Bacteremia in the adult intensive care unit of a teaching hospital in Nottingham, UK, 1985±1996. Eur J Clin Microbiol Infect Dis 17: 377±384 147. Sawyer RG, Pruett TL (1994) Wound infections. Surg Clin North Am 74: 519±536 S 31 148. Nichols RL, Smith JW (1993) Wound and intraabdominal infections: microbiological considerations and approaches to treatment. Clin Infect Dis 16:S266±272 149. Brook I, Frazier EH (1998) Aerobic and anaerobic microbiology of retroperitoneal abscesses. Clin Infect Dis 26: 938±941 150. Brook I, Frazier EH (1999) Microbiology of subphrenic abscesses: a 14-year experience. Am Surg 65: 1049±1053 151. de Lalla F (1999) Antimicrobial chemotherapy in the control of surgical infectious complications. J Chemother 11: 440±445 152. Fry DE (1994) Noninvasive imaging tests in the diagnosis and treatment of intra-abdominal abscesses in the postoperative patient. Surg Clin North Am 74: 693±709 153. Montgomery RS, Wilson SE (1996) Intraabdominal abscesses: image-guided diagnosis and therapy. Clin Infect Dis 23: 28±36 154. McDowell RK, Dawson SL (1996) Evaluation of the abdomen in sepsis of unknown origin. Radiol Clin North Am 34: 177±190 155. Noone TC, Semelka RC, Worawattanakul S, et al (1998) Intraperitoneal abscesses: diagnostic accuracy of and appearances at MR imaging. Radiology 208: 525±528 156. McClean KL, Sheehan GJ, Harding GK (1994) Intraabdominal infection: a review. Clin Infect Dis 19: 100±116 157. Anonymous (1994) Management of deep Candida infection in surgical and intensive care unit patients. British Society for Antimicrobial Chemotherapy Working Party. Intensive Care Med 20: 522±528 158. Wong JK, Mustard R, Sadler DJ, et al (1999) Predicting infection in localised intra-abdominal fluid infections: value of pH and PO2 measurements. J Vasc Interv Radiol 10: 421±427 159. Frazee RC, Nagorney DM, Mucha P Jr (1989) Acute acalculous cholecystitis. Mayo Clin Proc 64: 163±167 160. Imhof M, Raunest J, Rauen U, et al (1992) Acute acalculous cholecystitis in severely traumatized patients: a prospective sonographic study. Surgical Endoscopy 6: 68±71 161. Hill AG, Collins JP (1994) Acute acalculous cholecystitis. Aust N Z J Surg 64: 251±253 162. Almeida J, Sleeman D, Sosa JL, et al (1995) Acalculous cholecystitis: the use of diagnostic laparoscopy. J Laparoendosc Surg 5: 227±231 163. Hagino RT, Valentine RJ, Clagett GP (1997) Acalculous cholecystitis after aortic reconstruction. J Am Coll Surg 184: 245±248 164. Flancbaum L, Majerus TC, Cox EF (1985) Acute posttraumatic acalculous cholecystitis. Am J Surg 150: 252±256 165. Kalliafas S, Ziegler DW, Flancbaum L, et al (1998) Acute acalculous cholecystitis: incidence, risk factors, diagnosis, and outcome. Am Surg 64: 471±475 166. Beckman I, Dash N, Sefczek RJ, et al (1985) Ultrasonographic findings in acute acalculous cholecystitis. Gastrointest Radiol 10: 387±389 167. Molenat F, Boussuges A, Valantin V, et al (1996) Gallbladder abnormalities in medical ICU patients: an ultrasonographic study. Intensive Care Med 22: 356±358 168. Shapiro MJ, Luchtefeld WB, Kurzweil S, et al (1994) Acute acalculous cholecystitis in the critically ill. Am Surg 60: 335±339 169. Mirvis SE, Vainright JR, Nelson AW, et al (1986) The diagnosis of acute acalculous cholecystitis: a comparison of sonography, scintigraphy, and CT. AJR Am J Roentgenol 147: 1171±1175 170. Jeffrey RB Jr, Sommer FG (1993) Follow-up sonography in suspected acalculous cholecystitis: preliminary clinical experience. J Ultrasound Med 12: 183±187 171. Helbich TH, Mallek R, Madl C, et al (1997) Sonomorphology of the gallbladder in critically ill patients. Value of a scoring system and follow-up examinations. Acta Radiol 38: 129±134 172. Blankenberg F, Wirth R, Jeffrey RB Jr, et al (1991) Computed tomography as an adjunct to ultrasound in the diagnosis of acute acalculous cholecystitis. Gastrointest Radiol 16: 149±153 173. Yang HK, Hodgson WJ (1996) Laparoscopic cholecystostomy for acute acalculous cholecystitis. Surg Endosc 10: 673±675 174. Arens JF, LeJeune FE Jr, Webre DR (1974) Maxillary sinusitis, a complication of nasotracheal intubation. Anesthesiology 40: 415±416 175. Lum Cheong RS, Cornwell Eed (1992) Suppurative sinusitis in critically ill patients: a case report and review of the literature. J Natl Med Assoc 84: 1057±1059 176. Bach A, Boehrer H, Schmidt H, et al (1992) Nosocomial sinusitis in ventilated patients. Nasotracheal versus orotracheal intubation. Anaesthesia 47: 335±339 177. Borman KR, Brown PM, Mezera KK, et al (1992) Occult fever in surgical intensive care unit patients is seldom caused by sinusitis. Am J Surg 164: 412±415 178. Westergren V, Lundblad L, Hellquist HB, et al (1998) Ventilator-associated sinusitis: a review. Clin Infect Dis 27: 851±864 179. Laranne JE, Penttila MA, Paakkala TA, et al (1992) Diagnostic value of plain radiographs in chronic maxillary sinusitis: a comparison between radiological and endoscopic findings in 75 patients. Rhinology 30: 205±215 180. Garner JS, Jarvis WR, Emori TG, et al (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16: 128±140 181. Salord F, Gaussorgues P, Marti-Flich J, et al (1990) Nosocomial maxillary sinusitis during mechanical ventilation: a prospective comparison of orotracheal versus the nasotracheal route for intubation. Intensive Care Med 16: 390±393 182. Bert F, Lambert-Zechovsky N (1995) Microbiology of nosocomial sinusitis in intensive care unit patients. J Infect 31: 5±8 183. Kaups KL, Cohn SM, Nageris B, et al (1995) Maxillary sinusitis in the surgical intensive care unit: a study using bedside sinus ultrasound. Am J Otolaryngol 16: 24±28 184. Rouby JJ, Laurent P, Gosnach M, et al (1994) Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am J Respir Crit Care Med 150: 776±783 185. Bowers BL, Purdue GF, Hunt JL (1991) Paranasal sinusitis in burn patients following nasotracheal intubation. Arch Surg 126: 1411±1412 186. Holzapfel L, Chevret S, Madinier G, et al (1993) Influence of long-term oroor nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med 21: 1132±1138 187. Westergren V, Lundblad L, Forsum U (1998) Ventilator-associated sinusitis: antroscopic findings and bacteriology when excluding contaminants. Acta Otolaryngol (Stockh) 118: 574±580 188. Beck-Sague C, Jarvis WR (1993) Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980±1990. National Nosocomial Infections Surveillance System. J Infect Dis 167: 1247±1251 S 32 189. Pittet D, Wenzel RP (1995) Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med 155: 1177±1184 190. Vincent JL, Bihari DJ, Suter PM, et al (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 274: 639±644 191. Henderson VJ, Hirvela ER (1996) Emerging and reemerging microbial threats. Nosocomial fungal infections. Arch Surg 131: 330±337 192. Nguyen MH, Peacock JE Jr, Morris AJ, et al (1996) The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med 100: 617±623 193. Wey SB, Mori M, Pfaller MA, et al (1988) Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 148: 2642±2645 194. Nolla-Salas J, Sitges-Serra A, LeonGil C, et al (1997) Candidemia in nonneutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med 23: 23±30 195. Solomkin JS (1993) Pathogenesis and management of Candida infection syndromes in non-neutropenic patients. New Horiz 1: 202±213 196. Dean DA, Burchard KW (1996) Fungal infection in surgical patients. Am J Surg 171: 374±382 197. Pittet D, Garbino J (1995) Fungal infections in the critically ill. Curr Opin Crit Care 1: 369±380 198. Donahue SP, Greven CM, Zuravleff JJ, et al (1994) Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology 101: 1302±1309 199. Bodey GP, Luna M (1974) Skin lesions associated with disseminated candidiasis. JAMA 229: 1466±1468 200. Fass RJ, Goff DA, Sierawski SJ (1996) Candida infections in the surgical intensive care unit. J Antimicrob Chemother 38: 915±916 201. Vincent JL, Anaissie E, Bruining H, et al (1998) Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med 24: 206±216 202. Wey SB, Mori M, Pfaller MA, et al (1989) Risk factors for hospital-acquired candidemia. A matched casecontrol study. Arch Intern Med 149: 2349±2353 203. Pittet D, Monod M, Suter PM, et al (1994) Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 220: 751±758 204. Wenzel RP (1995) Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis 20: 1531±1534 205. Burchard KW, Minor LB, Slotman GJ, et al (1983) Fungal sepsis in surgical patients. Arch Surg 118: 217±221 206. Cornwell EE 3rd, Belzberg H, offne TV, et al (1995) The pattern of fungal infections in critically ill surgical patients. Am Surg 61: 847±850 207. Rex JH, Bennett JE, Sugar AM, et al (1994) A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med 331: 1325±1330 208. Shin JH, Nolte FS, Morrison CJ (1997) Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J Clin Microbiol 35: 1454±1459 209. Sendid B, Tabouret M, Poirot JL, et al (1999) New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J Clin Microbiol 37: 1510±1517 210. Walsh TJ, Merz WG, Lee JW, et al (1995) Diagnosis and therapeutic monitoring of invasive candidiasis by rapid enzymatic detection of serum D-arabinitol. Am J Med 99: 164±172 211. Pfaller MA (1992) Laboratory aids in the diagnosis of invasive candidiasis. Mycopathologia 120: 65±72 212. Petri MG, Konig J, Moecke HP, et al (1997) Epidemiology of invasive mycosis in ICU patients: a prospective multicenter study in 435 non-neutropenic patients. Paul-Ehrlich Society for Chemotherapy, Divisions of Mycology and Pneumonia Research. Intensive Care Med 23: 317±325 213. Pallavicini F, Izzi I, Pennisi MA, et al (1999) Evaluation of the utility of serological tests in the diagnosis of candidemia. Minerva Anesthesiol 65: 637±639 Intensive Care Med (2001) 27: S 49±S 62 Source control in the management of sepsis Maria F. Jimenez John C. Marshall ) M. F. Jimenez ´ J. C. Marshall ( ) Toronto General Hospital, University Health Network, Toronto, Ontario, Canada E-mail: [email protected] Phone: +1-4 16-3 40-52 05/52 04 Fax: +1-4 16-5 95 94 86 Introduction Antibiotics were first used as anti-infective therapy a mere 60 years ago. Even the administration of intravenous fluids to support the circulation is an innovation of the twentieth century. However, the surgical therapy of infection in the form of drainage of abscesses, removal of foreign bodies, or dØbridement of devitalized tissue dates to antiquity. The surgical drainage of ocular abscesses was sufficiently common in ancient Mesopotamia that the fees payable to its practitioners are laid out in the Code of Hammurabi, and the drainage of abscesses was a common practice at the temple of Asclepios in Greece in the fourth century BC. The surgical management of wounds dates back to prehistoric times; skulls showing evidence of trephination (craniotomy) with healing during life have been found in both Europe and the pre-Columbian western hemisphere. Surgical approaches to the treatment of infection have evolved through principle and tradition, and few have been evaluated by randomized controlled trials. Moreover the need to tailor therapy to circumstances that are often unique to a particular clinical situation makes it difficult to standardize surgical therapy sufficiently to conduct a randomized trial of surgical approaches in the management of severe sepsis and septic shock. For example, there has been considerable controversy regarding the relative merits of open versus closed management of the peritoneal cavity in the patient with diffuse peritonitis. Yet a randomized trial to answer this question has proven difficult if not impossible to organize [1]. Most patients with peritonitis have an infectious process that is at least relatively localized within the peritoneal cavity, and open management necessitates much greater use of nursing and operating room resources, raising important economical and ethical concerns in designing such a study. Conversely, for a number of patients with diffuse peritonitis it is not technically possible to close the abdomen because of increased intra-abdominal pressure or concomitant abdominal wall defects. Thus while the question is of theoretical interest, there is in reality only a very small number of patients for whom the two different approaches could reasonably be considered to be sufficiently appropriate to justify their random assignment to either therapeutic option. The paucity of data derived from level I or level II studies limits the development of solid evidence-based recommendations regarding optimal approaches to source control in patients with sepsis. The guidelines presented here are not definitive management algorithms, and their applicability is influenced by a number of factors best evaluated by a surgical consultant with experience in the complex process of surgical decision making in life-threatening sepsis. Methods A computer-based literature review was undertaken using Medline from 1985 to the present. While references were sought using specific subject heading keywords related to sepsis, i.e., sepsis, sepsis syndrome, septic shock, infection, multiple organ failure, critical care, and intensive care units, the paucity of relevant retrievals prompted us to repeat the search using headings specific for the questions asked, including soft tissue infections, pancreatitis, and diverticulitis. Additional references were identified from the references cited in these reports. Case reports and reviews were excluded, and clinical papers included only if they reported a least ten subjects and included some form of control population (often historical). S 50 Definitions and principles The term source control is used in preference to surgical therapy or surgery to emphasize that measures to control the source of an infection are not limited to surgery, but may include, for example, removal of an infected intravascular catheter or tube thoracostomy to treat an empyema of the pleural cavity. Advances in interventional radiology have permitted access to anatomic areas previously only amenable to surgical intervention, with the result that formal operative intervention is necessary less often than in the past. Source control measures encompass three broad types of intervention: Drainage of abscesses, dØbridement of devitalized infected tissue and removal of colonized foreign bodies, diversion, repair, or excision of an ongoing focus of contamination from a hollow viscus. Drainage of abscesses An abscess is a discrete collection of tissue debris, bacteria, and host leukocytes that has become walled off from adjacent healthy tissues. The local host inflammatory response activates the coagulation cascade, resulting in the generation of fibrin; this fibrin becomes organized to form a capsule of fibrous tissue that isolates the infectious focus from the surrounding tissues [2]. The contents of an abscess are usually liquid, because of the presence of tissue fluid, permitting the abscess to be drained to the exterior. This process externalizes the focus of infection: further microbial entry into host tissues is prevented by the abscess wall, and the clinical infectious process resolves. Drainage of an abscess converts a closed focus of infection to either a fistula if the abscess arises from perforation of an epithelially lined surface such as the gastrointestinal tract, or a sinus if it does not. Drainage of an abscess may occur spontaneously or may be a result of deliberate therapeutic intervention. The clinical consequences of such a process of spontaneous drainage depend on the site of drainage. In the preantibiotic era, conservative management of appendiceal abscesses was an accepted practice, since the abscess generally drained into the gastrointestinal tract, and was associated with no significant clinical sequelae. However, a diverticular abscess may drain spontaneously into the bladder, giving rise to a colovesical fistula, or the vagina, creating a colovaginal fistula. Therapeutic drainage of an abscess results in the creation of a controlled sinus or fistula. Intervention should be planned to minimize contamination of uninfected tissues adjacent to the abscess, to simplify nursing care needed during the resolution of the abscess cavity, and to minimize the long-term morbidity of the intervention. Thus, if percutaneous drainage of an abscess can be performed effectively, it is generally preferable to surgical intervention, for it reduces the contamination of surrounding tissues, obviates the need for postoperative wound management and avoids the later risks of incisional hernia. DØbridement of devitalized tissues DØbridement ± the removal of dead tissue ± can be accomplished by surgical excision, by the use of dressings that adhere to devitalized tissue, removing it as the dressing is changed, or by the use of enzymatic or biological agents, including, in the past, maggots. Dead tissue is an excellent culture medium for micro-organisms, and because it lacks a blood supply, permits microbial proliferation in an environment that is protected from host inflammatory cells. Thus dØbridement of infected necrotic tissue is a critical element in source control, eliminating a locus of unopposed microbial growth. The benefits of excising uninfected necrotic tissue are less clear, and must be weighed against the risks of attempted excision. In the early stages of tissue necrosis, viable tissue is not clearly demarcated from nonviable tissue, and an intermediate zone of hyperemic inflammatory tissue is present: bleeding during attempted excision can be considerable and is an important reason that delayed dØbridement of infected pancreatic necrosis is associated with a better clinical outcome [3]. With time the demarcation of viable from nonviable tissue becomes clearer, and intervention is facilitated; however, potential morbidity of intervention must be weighed against the risks of leaving dead, infected tissue. A foreign body such as a colonized intravascular catheter or a retained surgical sponge serves as a reservoir of micro-organisms that are relatively protected from endogenous host defenses. Experimental studies show that the presence of a foreign body significantly reduces the inoculum of micro-organisms required for the establishment of a focus of infection [4]. In addition, certain organisms, notably coagulase-negative Staphylococci, form biofilms on prosthetic materials that permit the establishment of microbial colonies, and protect the bacteria from host immune cells [5]. Removal of a colonized foreign body is therefore a key element of source control. Again, however, the clinician must balance risks and benefits. When the infected foreign body is a retained surgical sponge or a colonized Foley catheter, the decision to remove the device is obvious. However, removal of a prosthetic heart valve, a vascular graft, or even a peritoneal dialysis catheter in a patient with dialysis-dependent renal failure entails a greater degree of risk, and justifies a trial of noninterventional management. S 51 Definitive management of source of contamination When infection arises as a consequence of perforation of a hollow viscus, ongoing contamination occurs unless the anatomical problem is corrected. Ideally this is accomplished by surgical removal of the involved structure, for example, appendectomy for acute perforated appendicitis. Drainage alone can serve to create a controlled fistula, and is frequently a less morbid interim solution. Finally, contamination can be reduced by proximal diversion or defunctioning of the injured viscus. A colostomy created proximal to a colonic perforation, for example, reduces the volume of gastrointestinal effluent passing across the injured colon and thus minimizes the leakage of bacteria into tissues surrounding the perforation. Indications for source control in the management of sepsis The potential role of source control measures should be evaluated for all patients with severe sepsis. In many circumstances, for example, the patient with diffuse peritonitis from a perforated ulcer or clostridial myonecrosis following a traumatic injury, the need for source control is obvious. In others, it may be less so. The patient with pneumonia, for example, may have a concomitant empyema; even in the absence of this complication, tracheal suctioning, and physiotherapy to enhance clearance of contaminated secretions are source control measures that may be of benefit. Replacement of a colonized catheter can hasten the resolution of a urinary tract infection. The incremental benefits of instituting source control are largely unknown, even in the management of a number of common infectious processes. Analyses of cohorts of patients enrolled in trials of novel mediator-targeted therapies indicate that inadequate source control is associated with a worse clinical outcome [6]; however, the merits of surgical source control, as with those of antibiotic therapy, are largely based on expert opinion, informed by experience. Selection of the best method of providing definitive management of a focus of infection involves a principle common to the application of all forms of source control ± balancing the physiological costs and risks of intervention against the benefits of more definitive control. Timing of source control interventions Optimal timing of intervention requires that the benefits of intervention in providing definitive control of a focus of infection be balanced against the risks of doing so. General anesthesia or a reduction in the intensity of monitoring in the radiology suite result in increased risk for the patient who is not fully resuscitated, or who remains physiologically unstable despite resuscitation. Surgical intervention early in the course of necrotizing pancreatitis can eliminate a potential or actual focus of infection but entails a significantly greater risk of lifethreatening bleeding, because tissue planes are not demarcated. As a general principle, source control measures should only be undertaken once the patient has been appropriately stabilized, although resuscitation should proceed as rapidly as feasible. Rarely, source control is part of the process of resuscitation, for example, in emergency surgery for a ruptured mycotic aneurysm. Moreover in septic shock associated with intestinal infarction or infections such as clostridial myonecrosis, full resuscitation is not possible until the rapidly advancing tissue necrosis has been stopped. Nonetheless, even in these situations, aggressive and rapid resuscitation can reduce the risks of anesthesia. For most infections requiring source control, timing is an urgency, but not an emergency. The impact of the timeliness of source control on clinical outcome was evaluated for two distinct infectious processes. Necrotizing soft tissue infection Does the timing of surgery alter outcome in necrotizing fasciitis? Answer: yes, grade E. Recommendation Surgical intervention in the form of dØbridement of infected, devitalized, or nonbleeding tissue should be undertaken rapidly following hemodynamic stabilization in patients with necrotizing soft tissue infections. This is a grade E recommendation supported by level IV and level V evidence. Rationale The mortality of necrotizing fasciitis in reported series ranges from 9 % to 30 %. A number of case series and uncontrolled studies suggest that timely intervention in the patient with a necrotizing soft tissue infection is associated with a superior clinical outcome. Early reports suggested that delayed diagnosis and limited or inadequate resection results in increased mortality [7, 8, 9]. Both Majestic and Alexander [10] and Miller [11] have shown that mortality in patients with necrotizing fasciitis is less than that in historical controls when infection is diagnosed early, and surgical dØbridement is aggressive. S 52 More recent retrospective cohort studies [12, 13, 14, 15, 16, 17] have confirmed the benefits of early aggressive surgical dØbridement for the treatment of patients with necrotizing fasciitis. For example, Bilton and colleagues [12] demonstrated a mortality reduction from 38 % to 4.2 % when patients underwent radical surgical dØbridement at the time of initial presentation, and Freischlag and coworkers [14] reported that surgical intervention within 24 h of recognition of necrotizing soft tissue infection resulted in 36 % mortality, compared to 70 % when the surgical procedure was delayed more than 24 h. Various reports have suggested that improved outcome requires surgical intervention within 3 h of admission [18, 19] or 12 h of admission [20]. Two studies evaluating clinical and laboratory predictors of survival of patients with necrotizing soft tissue infections have suggested that surgical dØbridement does not influence outcome [21, 22], particularly for patients with coagulopathy at the time of admission [22]. Infected pancreatic necrosis and pancreatic abscesses Does early surgery improve outcome for patients with infected pancreatic necrosis? Answer: no, grade C. Recommendation The decision to intervene surgically in the patient with infected pancreatic necrosis must weigh the potential advantages of removing a source of ongoing bacterial proliferation against the inherent morbidity of early surgery. In general, surgery should be delayed in the stable patient to permit adequate demarcation of tissue planes. This is a grade C recommendation supported by a single randomized trial and expert opinion. Rationale Studies of the natural history of retroperitoneal infection in patients with severe acute pancreatitis demonstrate that culture positivity generally occurs during the second or third week after the onset of pancreatitis, and that the risk of infection is directly related to the initial clinical severity of the disease process [23]. Drainage of infected fluid collections or dØbridement of infected necrosis is intuitively appealing. However, the morbidity of early surgical intervention is increased because clear biological demarcation between viable and necrotic tissues is often not present early in the course of the disease, and significant retroperitoneal hemorrhage is a common complication. Earlier enthusiasm for early ac- tive surgical intervention is giving way to greater conservatism. Delayed surgical intervention in infected pancreatic necrosis was first suggested by Machado et al. [24], and later supported by a randomized trial showing a reduction in complication rates and mortality in patients in whom surgical intervention was delayed at least 2 weeks [3]. The benefits of further intentional delay in patients with known infected pancreatic necrosis are not established. The discordant recommendations for the management of infected necrosis of subcutaneous or peripancreatic tissues reflect several factors, the most important of which is the morbidity associated with intervention. Principles of drainage of infectious foci Surgical drainage of deep-seated foci of infection was an innovation of the twentieth century. The classical surgical principles for the drainage of intra-abdominal abscesses were established by Ochsner and Debakey in 1938 [25] in an era when the diagnosis of intra-abdominal infection was established primarily through the clinical findings, and antibiotics were not yet available as adjuvant anti-infective therapy. More recently improvements in diagnostic imaging and the development of techniques for radiologically guided abscess drainage have provided the clinician with a spectrum of therapeutic options for the management of deep space infections. Radiographic techniques for the diagnosis of deep site infections Are computed tomography and ultrasonography equally efficacious in detecting foci of intra-abdominal infection? Answer: yes, grade E. Recommendation The diagnosis of intra-abdominal infection amenable to source control measures can generally be made by either ultrasound or computed tomography (CT). Ultrasonography has the advantage of being portable and inexpensive, but is highly operator dependent; CT is especially useful in the evaluation of the retroperitoneum. Rationale The presence of infection within the thoracic or abdominal cavities is usually suspected on the basis of history S 53 and physical examination; however, radiographic documentation is almost always indicated. Not only does delineation of the process permit safer surgical approaches, in many cases deep site infections can be treated by radiographically guided percutaneous drainage. Three methods of radiological investigation are commonly used to define such infections ± ultrasonography, CT, and contrast studies. Rigorous comparative studies of their merits are generally not available. Abdominal ultrasound is a useful first line diagnostic modality for intra-abdominal collections. It is inexpensive and portable, and allows confirmatory needle aspiration of fluid collections or therapeutic percutaneous abscess drainage. The capacity to establish a diagnosis at the bedside in the ICU obviates the risks associated with transportation to the radiographic suite. However, ultrasound is highly operator dependent, and adequate visualization may be prevented by gas within the lumen of the intestine or external dressings. Abdominal computed tomography is the most sensitive and specific radiological study for the diagnosis of foci of intra-abdominal infection. It provides readily interpretable information about anatomical location, localizes gas collections as being intraluminal or extraluminal, and facilitates percutaneous aspiration of cysts and abscesses. It is especially useful in the evaluation of the retroperitoneum, and, when performed using intravenous contrast, can differentiate viable and nonviable tissue. However, CT is expensive and requires that the patient be transported to the radiology suite. The accuracy of CT without contrast is reduced because it is difficult to differentiate fluid-filled bowel from an abscess. When infection arises as a consequence of perforation of the gastrointestinal tract, studies using water-soluble contrast agents may show the site of perforation or demonstrate distortion of normal anatomy by an adjacent abscess. Sinograms performed by the injection of contrast solution into a therapeutic drain can document resolution of an abscess cavity and evaluate its connection with the gastrointestinal tract. Radionuclide scans using gallium or indium have largely given way to ultrasonography and CT. Two retrospective studies compared the accuracy of CT and ultrasonography for the diagnosis of intra-abdominal abscesses [26, 27]. The accuracy of ultrasound examination ranges from 75±96 %, while CT correctly diagnoses 71±100 % of intraperitoneal abscesses. No statistically significant difference was demonstrated between the two imaging modalities in identifying an abdominal abscess. However, with technological improvements to both imaging modalities, the CT has generally emerged as the preferred method for identifying intraabdominal infectious foci. Therapeutic options in intra-abdominal infection Is percutaneous drainage as efficacious as surgical intervention for the treatment of intra-abdominal abscesses? Answer: yes, grade E. Recommendation The initial approach to well-defined and accessible intra-abdominal abscesses should be percutaneous drainage. Catheter drainage can also be used as a temporizing measure to optimize the physiological and hemodynamic condition of an acutely ill patient prior to surgical exploration [28]. Laparotomy should be reserved for those circumstances in which there are no well-defined collections, dead tissue requires dØbridement, or residual collections cannot be treated percutaneously. Surgical intervention may also be indicated to control a source of ongoing peritoneal contamination. Rates of failure have increased as interventional radiologists have extended the indications for percutaneous drainage. If the clinical condition of the patient does not improve following the initial drainage, a follow-up CT should be performed to determine whether a residual or missed collection is present, and surgical intervention should be considered. Rationale The traditional approach to the treatment for intra-abdominal abscesses has been surgical drainage, often performed on clinical grounds alone, without definitive radiological confirmation. However, surgical drainage carries potential morbidity including bleeding, the development of fistulas, and wound infection. Improvements in radiological imaging techniques led to new techniques for percutaneous drainage of intra-abdominal abscesses that could be successfully employed with low mortality [29]. Percutaneous abscess drainage was initially restricted to unilocular well-defined collections, for which a safe drainage route could be established. Gerzof et al. [30] initially reported a 92 % success rate for percutaneous drainage when these criteria were satisfied. Indications and access routes for percutaneous drainage have been since expanded. Multiple, ill-defined, or complicated abscesses (for example, appendiceal, interloop, and pelvic abscesses), collections communicating to the gastrointestinal tract, and even abscesses whose drainage must traverse normal organs can be treated percutaneously, with a success rate of 73.6 % and 9 % mortality [31]. S 54 Much of the literature comparing of operative and percutaneous methods for the drainage of intra-abdominal abscesses uses historical surgical controls, without stratification of severity of illness prior to drainage [32, 33]. There are no randomized studies comparing percutaneous and operative drainage techniques. Uncontrolled case series show that percutaneous drainage is as effective as conventional surgery for the drainage of intra-abdominal collections [34, 35, 36]. In a retrospective case-control study Olak and colleagues [37] stratified patients undergoing percutaneous drainage of intra-abdominal abscesses using the acute physiology score, and matched for age, sex, diagnosis, abscess etiology, and location with a cohort of patients managed surgically. They found no differences in morbidity and mortality when patients with intra-abdominal infection were treated surgically or percutaneously. Are aggressive operative approaches (continuous postoperative peritoneal lavage, open abdomen) superior to conventional surgical treatment for intraabdominal infection? Answer: in continuous postoperative peritoneal lavage, uncertain, grade D; in open abdomen, no, grade D. Recommendation Current data support the concept that relaparotomy ªon demand,º as indicated by worsening of the clinical status, absence of improvement, or evolving organ dysfunction is as efficacious as a more aggressive approach. Planned relaparotomy is indicated for patients with ischemic bowel when intestinal viability is a concern (ªsecond lookº), for patients with necrotizing pancreatitis when demarcation of necrotic tissue demarcation is not distinct, or when bleeding precludes complete dØbridement. Rationale The substantial morbidity and mortality of unsuccessful surgical management of severe intra-abdominal infection has led to the development of more aggressive surgical procedures, in particular, continuous postoperative peritoneal lavage, and open abdomen management, with or without planned relaparotomy. The rationale for continuous postoperative peritoneal lavage is that the continuous removal of bacteria and fibrin may hasten the resolution of intra-abdominal infection and reduce the risk of persistence or recurrence [38]. The technique is labor intensive and time consuming, requires intensive care monitoring, and has been associated with the formation of enteric fistulas. It necessitates the perioperative placement of drains in the subphrenic spaces for infusion of dialysis solution, and of outflow catheters in the pelvis for evacuation of the effluent. The abdomen is closed and lavage is performed with large volumes of fluid, either continuously or intermittently, for a period of 1±5 days. The role of postoperative peritoneal lavage remains to be defined. A nonrandomized study by Washington et al. [39] showed that postoperative lavage with cefamandole, erythromycin, and heparin decreases postoperative abscess formation. On the other hand, a prospective randomized control study by Hallerback et al. [40] was unable to demonstrate benefit for postoperative lavage containing neither antibiotics nor heparin for patients who had received broad-spectrum systemic antibiotics. Similarly, a randomized trial of radical peritoneal dØbridement to remove fibrin from the peritoneal surface failed to show any benefit for this more aggressive form of therapy [41]. Laparostomy ± open management of the abdomen ± has been promoted in circumstances in which multiple reexplorations are required to control an intra-abdominal infection. The technique avoids the increased intraabdominal pressure associated with the closure of the abdomen and facilitates reintervention. Its complications include evisceration, massive fluid losses, fistula formation, and retraction of the abdominal wall resulting in postoperative hernias. The significant risk of these complications led to the development of a semiopen technique, in which temporary abdominal wall closure is accomplished by the use of polypropylene and polyglycolic acid meshes, or polytetrafluoroethylene patches with or without zippers or adhesive sheets. This semiopen technique facilitates reintervention without leaving the bowel exposed, prevents evisceration, avoids raised intra-abdominal pressures associated with abdominal wall closure, and minimizes damage to the abdominal wall [42]. Planned relaparotomy or staged abdominal repair (STAR), has been advocated for the management of patients with diffuse peritonitis to prevent the development of multiple organ failure and facilitate the resolution of intra-abdominal infection [43]. STAR implies a commitment at the first operation to perform multiple surgical procedures at fixed intervals (24±72 h), regardless of the patient's clinical condition. In theory, planned relaparotomy allows superior control of peritoneal contamination and earlier detection of anastomotic leaks. The disadvantages of this method are inadvertent visceral injury and fistula formation. Increased intra-abdominal pressure resulting from abdominal wall closure may compromise organ perfusion and pulmonary function [44]. Although favorable results with these aggressive surgical techniques have been reported worldwide [45, 46], S 55 objective evaluation is difficult because of the heterogeneity of patients in whom the method has been employed, and the inherent difficulties associated with performing a well-designed clinical trial. A semiopen technique with planned relaparotomy was found to reduce mortality to one third that predicted on the basis of Acute Physiology and Chronic Health Evaluation II scores for patients with diffuse peritonitis [47]. On the other hand, a multicenter case-control study undertaken by the Peritonitis Study Group of the Surgical Infection Society Europe compared planned relaparotomy to relaparotomy on demand for the treatment of intra-abdominal infections. The two groups of patients were comparable respect to the severity of illness, age, cause of infection, site of origin of peritonitis, and ability to eliminate the source of infection. No difference was found between the groups with respect to mortality or the need for unplanned relaparotomy. However, the incidence of anastomotic leaks, septicemia, and postoperative organ failure was increased in the patients who underwent planned relaparotomy [48]. In a collected review of 642 patients from 22 series Schein and colleagues [42] found an overall mortality rate of 33 % in patients treated with aggressive surgical management compared to a range of 30±76 % in patients with intra-abdominal infection treated with conventional methods. A prospective, nonrandomized trial comparing the closed-abdomen technique to open-abdomen technique in patients with severe peritonitis, suggested that the major determinant of mortality in patients with intra-abdominal infection is the host response to peritoneal contamination, rather than the approach used to control bacteria in the peritoneal cavity [1]. There currently is no clearcut evidence of the superiority of radical surgical procedures for the treatment of severe intra-abdominal infection. DØbridement and device removal DØbridement ± the surgical removal of injured, necrotic, and/or infected tissue ± is a fundamental principle of source control, and at least in the case of necrotizing soft tissue infections early surgical intervention is associated with a better prognosis. However, the identification of necrotic tissue may be difficult, particularly in deep-seated infections. Moreover, as discussed earlier for patients with pancreatic necrosis, dØbridement of sterile necrotic tissue may not be necessary to achieve a favorable clinical outcome. Can the presence of tissue necrosis be ruled out by means of nonoperative investigations? Answer: no, grade E. Recommendation Although tissue necrosis can often be detected by such characteristic radiographic findings as gas in the tissues, or nonenhancement of tissues following administration of intravenous contrast, there is no single test that can exclude the presence of tissue necrosis with certainty, and in circumstances in which necrosis may be life threatening (for example, intestinal ischemia) it is often necessary to establish the diagnosis operatively. Rationale The early diagnosis of necrotizing soft-tissue infections may be difficult because the initial appearance of the skin is normal, and local signs are mild, despite the presence of extensive deeper soft tissue necrosis. Local signs of deep infection may include edema, crepitus, and cyanosis or bronzing of the skin. Radiographic studies revealing the presence of air in the soft tissues support the diagnosis of underlying necrosis [10]. CT with intravenous contrast can also provide information about the viability of deeper tissues. The easy separation of the subcutaneous tissue at the level of the fascia when probing the wound through an incision in the skin has traditionally been considered pathognomonic of necrotizing fasciitis, and the diagnosis of necrotizing soft-tissue infection can be confirmed by operative exploration, with direct visualization of whether the incised tissue bleeds. Frozen-section biopsy early in the evolution of a suspected necrotizing soft tissue infection has been recommended in patients with rapidly advancing infections to provide a reliable diagnosis and define the extent of dØbridement [49]. Can a vascular catheter be safely changed over a guidewire? Answer: yes, grade B. Recommendation An infected central venous catheter can be safely changed over a guidewire, provided there is not significant local soft tissue infection at the exit site. This is a grade B recommendation supported by level II evidence. Rationale The diagnosis of catheter-related infection is based on the isolation of the same organism from the catheter and the blood, clinical and microbiological information S 56 ruling out another site of infection, and the presence of signs of systemic inflammatory response. A semiquantitative technique to differentiate contamination of a catheter from true infection was described by Maki et al. [50], based on the demonstration of at least 15 colonies of a given organism on an agar culture roll-plate. The presence of more than more than 103 cfu/25 cm2 or more than 102 colonies from cultures obtained by sonication is also indicative of catheter infection [51]. Cook and colleagues [52] performed a systematic review of 12 randomized trials that addressed the relative merits of catheter changes over a guidewire or catheter replacement at a new site for patients with central venous catheter infections. The guidewire exchange technique was associated with fewer mechanical complications (relative risk 1.72, 95 % confidence interval 0.89±3.33) compared to replacement at a new site, but also with a trend towards a higher rate of catheter exitsite infection (relative risk 1.52, 95 % confidence interval 0.34±6.73) and catheter-related bacteremia. Tunneled silastic catheters such as the Hickman or Broviac catheter have a lower rate of infection. Removal of the catheter is often required for tunnel infections (presence erythema, induration or purulence along the subcutaneous tract), whereas exit-site infection (signs of infection within 2 cm of the exit site) usually resolves with local wound care and antibiotics [53]. Tunneled hemodialysis catheters can also be safely and easily replaced over a guidewire through the preexisting subcutaneous tunnel, with infection rates comparable to de novo catheter replacement [54]. Removal of the catheter and excision of the affected vein leaving the wound open is the recommended approach for peripheral septic thrombophlebitis. Catheter-related septic central venous thrombosis requires systemic antibiotics, catheter removal, and surgical excision when medical measures fail [55]. Does a policy of scheduled replacement of indwelling central venous catheters reduce the risk of infectious complications? Answer: no, grade C. Recommendation There is no evidence that routine catheter replacement reduces the risk of catheter-related bacteremia. Venous catheters should be changed only as needed when evidence of infection is present (signs of inflammation, purulent discharge at the insertion site), or when the catheter is not working [52, 56]. This is a level C recommendation for central venous catheters, supported by level II evidence, and a level E recommendation for peripheral catheters, supported by level V evidence. Rationale A clinical trial in cardiac surgical patients evaluating the risk of infection associated with routine guidewire exchange from a pulmonary artery to a central venous catheter 48±72 h after the surgical procedure showed a higher incidence of catheter-related infection (35.3 % compared 12.5 %) in patients undergoing catheter exchange [57]. Similarly a randomized controlled trial of routine catheter exchanges every 3 days failed to show any reduction in rates of catheter-related infections [58]. Proximal diversion, defunctioning, and definitive therapy An anatomical defect in the gastrointestinal tract permits continuing contamination of sterile tissues by micro-organisms found within the gut lumen. Diverting the fecal stream or otherwise defunctioning of the gut minimizes such contamination. Regardless of the level of the leak proximal diversion of the gastrointestinal tract involves two elements: drainage of the infectious focus adjacent to the perforation and creation of a diverting stoma or ostomy proximal to the site of perforation. For mediastinitis secondary to an intrathoracic rupture of the esophagus, exercise of this principle entails mediastinal drainage and the creation of a cervical esophagostomy; in the case of a perforation of the colon secondary to cancer or diverticulitis, the principle would dictate drainage of the abscess and the creation of a colostomy or ileostomy proximal to the site of the leak. Application of this principle led to the classical approach to perforated diverticulitis ± a three-stage operation involving the sequential drainage of the abscess and creation of a transverse loop colostomy, followed by a second operation to resect the involved segment of sigmoid colon, and a final third operation to close the colostomy. While the principle of proximal diversion remains widely accepted as the safest and most conservative approach to complex perforations of the gastrointestinal tract, its utility is open to evaluation. In the first place, the approach is not applicable to many common sites of gastrointestinal perforation. Proximal diversion of the stomach, for example, would entail the creation of a cervical esophagostomy, and standard therapy for perforated ulcers now consists simply of an omental patch with or without an acid-reducing operation. Indeed a randomized clinical trial has suggested that nonoperative management is a reasonable alternative for most patients with perforated ulcers [59]. Secondly, drainage S 57 Table 1 Clinical Studies Comparing Resection with Colostomy and Drainage for Perforated Sigmoid Diverticulitis Author No. Patients Colostomy& Drainage Resection Deaths Deaths Complications Comment Complications Kronberg, 1993 [60] 62 6/31 (19 %) 8/31 (26 %) Smirniotis, 1992 [62] 38 4/14 (29 %) 1/24 (4 %) Finlay, 1987 [64] 78 24 % 20 % Fistulas 21 % 0 % Fistulas Auguste, 1985 [65] 116 10/51 20 % Stay- 52 days 8/65 12 % Stay- 36 days Nagorney, 1985 [66] 121 8/31 (26 %) and diversion, although technically simpler, leaves the source of contamination in situ. A number of case series have addressed the question of whether resection of the diseased segment of colon is superior to simple drainage and diversion, and whether anastomosis of the colon is safe in the face of perforation and active infection. Is resection of perforated colon preferable to simple drainage and proximal diversion for patients with perforated diverticulitis? Answer: yes, grade D. Recommendation Definitive resection is preferable to proximal diversion and drainage for perforated diverticulitis, and likely for other causes of intestinal perforation, when the more demanding procedure of resection can be performed safely. Extension of this principle to other sites of gastrointestinal peroration such as the esophagus requires balancing the risks of resection with the potential benefits. This is a grade D recommendation based on level III evidence. Rationale Resection of a perforated segment of colon eliminates a focus of ongoing contamination. However, the additional operative time and resulting physiological stress to a critically ill patient may result in higher perioperative morbidity. Moreover, definitive resection is more technically demanding; therefore the potential for significant perioperative complications is increased. Higher rate of colostomy closure with resection (84 %) than drainage (44 %) Shorter length of disability with resection (81 vs 148 days) 6/90 (7 %) Medline was searched from 1985 to the present, using as keywords, ªgastrointestinal perforation/surgery,º and restricting the search to papers published in English. The literature search was restricted to the past 15 years since changes in other aspects of supportive care may have rendered earlier study conclusions less relevant. Of the 639 references identified, 73 were selected for review. One randomized trial [60] and six retrospective case series [61, 62, 63, 64, 65, 66] compared outcomes for patients managed by drainage and diversion or definitive resection (Table 1). While the series were retrospective, and the subjects randomized in only one of the trials, consistent evidence of benefit was apparent for patients treated with definitive resection compared to proximal diversion and drainage. Pooled data showed a mortality of 22.8 % (38/167) for patients treated by proximal diversion and drainage compared with 12.5 % (31/248) for those managed with definitive resection and colostomy (odds ratio 2.06, 95 % confidence interval 1.22±3.48, p = 0.007). It should be emphasized that intrinsic differences in patient populations, or in adjuvant care may have created a spurious suggestion of superiority for resection. Resection also appears to be associated with lower morbidity, principally a shorter hospital stay and period of disability, a lower rate of postoperative fistulas, and a higher rate of colostomy reversal. Following colonic resection for perforated diverticulitis, is primary anastomosis a safe alternative to the construction of a colostomy? Answer: yes, grade D. S 58 Table 2 Clinical Studies Comparing Primary Anastomosis to Colostomy Following Resection for Perforated Diverticulitis Author Umbach, 1999 [67] Setti Carraro, 1999 [68] No. of Patients Primary Anastomosis Resection and Colostomy Deaths Complications Deaths Complications 33 0 1 anastomotic dehiscence N/A N/A No control group; mortality from literature 1±28 % for Hartmann procedure 105 ± ± ± ± No difference in outcome; mortality related to APACHE II Strada, 1993 [70] 73 4/73 (5.5 %) 11/73 (15 %) Saccomani, 1993 [71] 38 1/26 (3.8 %) 11/26 (42 %) Smirniotis, 1992 [62] 24 1/6 (17 %) Alanis, 1989 [73] 60 1/34 (3 %) 2/6 (33 %) ± Recommendation Primary anastomosis or colostomy are equally efficacious following colon resection for diverticulitis. The choice of procedure should be dictated by other factors such as severity of illness, presence of chronic disease, the degree or duration of peritoneal contamination, and the skill and experience of the surgical team. This is a grade D recommendation based on level III evidence. Rationale A primary anastomosis obviates the need for a second operative procedure to close the stoma, a procedure with a recognized morbidity. On the other hand, the risk of anastomotic leakage is generally held to be greater in the face of an acute inflammatory process, and the attendant morbidity resulting from an anastomotic leak may be formidable. Nine reports were identified that evaluated the safety of primary anastomosis in the clinical setting of colon perforation with established inflammation [61, 62, 67, 68, 69, 70, 71, 72, 73]; four of these reported rates for concomitant controls. Six studies were judged to be evaluable (Table 2). All studies were case series. In no study was primary anastomosis associated with an increased risk of mortality, and two reports suggested that the primary determinant of mortality was the degree of physiological derangement, rather than the specific method of therapy used. Strada and colleagues [70] reviewed the course of 73 patients injured on the battlefield during the Afghan War, all of whom were managed by primary anastomosis or direct suture repair, without creation of a stoma. Despite the fact Comment Battlefield management of colonic injuries during Afghan War 3/8 (38 %) 5/8 (63 %) 0/18 (0 %) 1/18 (6 %) 4/26 (15 %) ± 1 additional death with colostomy closure that nearly half the patients were hypotensive at presentation, and that management was undertaken more than 12 h after injury in 445, only 4 patients died (5.5 %). The independent morbidity and mortality associated with colostomy closure is an additional variable to be considered. Complications develop during one-third of colostomy closures [74, 75, 76], although the majority are relatively minor. In addition, colostomy closure necessitates an additional hospital stay and postoperative convalescence. Pooled data from the four studies that compared primary anastomosis with colostomy following colon resection for diverticulitis [62, 71, 72, 73] showed a modestly lower mortality for patients undergoing primary anastomosis (3/69 or 4.3 % vs. 8/55 or 14.5 %, odds ratio 0.27, 95 % confidence interval 0.07±1.06, p = 0.06). Publication bias and the absence of randomization preclude drawing firm conclusions from these data; however, they suggest that, contrary to classical teaching, primary anastomosis does not carry a higher mortality rate. Dealing with diagnostic uncertainty Does empirical or ªblindº laparotomy improve outcome? Answer: no, grade E. Recommendation Intra-abdominal infectious complications mandating source control are almost always evident using modern S 59 diagnostic imaging techniques. There is little if any role for empirical laparotomy to rule out undiagnosed infection in a critically ill patient in whom radiological examination has failed to demonstrate a surgically correctable problem. Rationale The first descriptions of the multiple organ dysfunction syndrome emphasized its association with uncontrolled and/or occult infection, particularly infection within the abdomen [77, 78, 79]. It remains a truism that the presence of remote organ dysfunction in critically ill patients should trigger an aggressive search for an unidentified focus of infection, especially when organ dysfunction evolves rapidly. The association between organ dysfunction and occult intra-abdominal infection stimulated led to the concept of ªblind laparotomy,º undertaken in the absence of radiological evidence of infection to search for an undrained abscess [80, 81]. However, improved radiological techniques have tempered the initial enthusiasm for this approach. Significant occult pathology is relatively uncommon, and when it is encountered, there is no convincing evidence that a more aggressive approach alters outcome [82, 83, 84]. With the spectrum of radiological diagnostic techniques currently available, interpreted within the specific clinical context of an individual patient, there is little justification for the temptation to ªhave a lookº for fear that a clinically important infection that might benefit from source control measures is being missed. Summary The process of surgical decision making is based on both general principles that are amenable to evaluation using rigorous techniques of clinical research and the intangible element of surgical judgment that seeks to apply those principles to the care of an individual patient. The role of surgical judgment is inescapable, even though it is intrinsically subjective and recalcitrant to objective evaluation, for a host of factors modify the application of principle in each patient, and render the circumstances of a given problem sufficiently distinctive, that evidence must be tempered with common sense. We have tried to provide, through an evidence-based approach to a series of questions, the rationale for the basic principles that should guide the clinician in initiating or modifying source control, recognizing that sound clinical judgement demands, at times, that these be set aside. In the individual patient, evidence of clinical improvement is the most important marker of the approach selected. Evaluation of the adequacy of source control in the critically ill patient can be difficult. As with other modes of anti-infective therapy, effective source control measures are expected to result in clinical improvement, reflected in: · Resolution of clinical signs of sepsis or systemic inflammation · Bacteriological resolution · Evidence of reversal of the metabolic sequelae of infection, with normal progression of wound healing, reflected in the formation of granulation tissue, and epithelialization · Radiographic evidence of control of an infectious focus · Prevention of further organ dysfunction, and resolution of existing organ dysfunction · Survival Evaluation of the adequacy of source control may necessitate planned reoperation. The adequacy of dØbridement of necrotizing soft-tissue infections can be assessed by repeat exploration under general anesthesia, continuing the process until there is evidence of healthy granulation tissue throughout the wound. Planned reexploration is also indicated for patients with diffuse intestinal ischemia to ensure bowel viability. The appropriate interventions to determine the adequacy of source control are dictated by the clinical circumstances. A residual or recurrent abscess can usually be demonstrated by CT or ultrasound examination, while resolution of an abscess cavity can be monitored using sinograms. The diagnosis of persistent or evolving tissue necrosis is guided by the clinical setting. Retroperitoneal necrosis can be detected by CT, while sigmoid ischemia following aortic aneurysmectomy can be evaluated by sigmoidoscopy. Occasionally diagnostic peritoneal lavage assists in establishing a diagnosis of gut ischemia; the lavage fluid appears bloody with established ischemia. The diagnosis of an infected foreign body requires an appropriate history and is supported by recurrent bacteremia or by positive cultures drawn retrograde through an indwelling vascular or peritoneal dialysis catheter. Finally, ongoing contamination from a breach of the gastrointestinal tract can be documented by appropriate contrast studies. The general principles that guide the use of source control techniques in the management of the patient with severe sepsis or septic shock are readily articulated. Their implementation in practice, however, is more complex, and does not, as a rule, lend itself to simple algorithms that are applicable in all cases. Moreover evidence-based support for these principles is weak. In the final analysis, the elusive process of experienced surgical judgement is invaluable for all but the most straightforward problems. S 60 References 1. Christou NV, Barie PS, Dellinger EP, Waymack JP, Stone HH (1993) Surgical Infection Society intra-abdominal infection study. Prospective evaluation of management techniques and outcome. Arch Surg 128: 193±198 2. Finlay-Jones JJ, Davies KV, Sturm LP, Kenny PA, Hart PH (1999) Inflammatory processes in a murine model of intra-abdominal abscess formation. J Leukoc Biol 66: 583±587 3. Mier J, Leon EL, Castillo A, Robledo F, Blanco R (1997) Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg 173: 71±75 4. Dougherty SH (1988) Pathobiology of infection in prosthetic devices. Rev Infect Dis 10: 1102±1117 5. Patrick CC, Plaunt MR, Hetherington SV, May SM (1992) Role of the Staphylococcus epidermidis slime layer in experimental tunnel tract infections. Infect Immun 60: 1363±1367 6. Sprung CL, Finch RG, Thijs LG, Glauser MP (1996) International sepsis trial (INTERSEPT): role and impact of a clinical evaluation committee. Crit Care Med 24: 1441±1447 7. Fisher JR, Conway MJ, Takeshita RT, Sandoval MR (1979) Necrotizing fasciitis. Importance of roentgenographic studies for soft tissue gas. JAMA 241: 803±806 8. Strasberg SM, Silver MS (1968) Hemolytic Streptococcus gangrene: an uncommon but frequently fatal infection in the antibiotic era. Am J Surg 115: 763±768 9. Rea WJ, Wyrick WJ (1970) Necrotizing fasciitis. Ann Surg 172: 957±964 10. Majeski JA, Alexander JW (1983) Early diagnosis, nutritional support, and immediate extensive debridement improve survival in necrotizing fasciitis. Am J Surg 145: 784±787 11. Miller JD (1983) The importance of early diagnosis and surgical treatment of necrotizing fasciitis. Surg Gynecol Obstet 157: 197±200 12. Bilton BD, Zibari GB, McMillan RW, Aultman DF, Dunn G, McDonald JC (1998) Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg 64: 397±400 13. Voros D, Pissiotis C, Georgantas D, Katsaragakis S, Antoniou S, Papadimitriou J (1993) Role of early and extensive surgery in the treatment of severe necrotizing soft tissue infections. Br J Surg 80: 1190±1191 14. Freischlag JA, Ajalat G, Busuttil RW (1985) Treatment of necrotizing soft tissue infections. A need for a new approach. Am J Surg 149: 751±755 15. Adant JP, Bluth F, Fissette J (1998) Necrotizing fasciitis: a life-threatening infection. Acta Chir Belg 98: 102±106 16. Sjo OH, Ivarsson LE, Sudhange JO, Mikkelsen EM (1999) Necrotizing fasciitis ± a surgical challenge. Tidsskr Nor Laegeforen 119: 2660±2663 17. Kujath P, Eckmann C, Benecke P (1996) Standardized treatment of necrotizing fasciitis. Zentralbl Chir 121: 35±43 18. Moss RL, Musemeche CA, Kosloske AM (1996) Necrotizing fasciitis in children: prompt recognition and aggressive therapy improve survival. J Pediatr Surg 31: 1142±1146 19. Kaiser RE, Cerra FB (1981) Progressive necrotizing surgical infections ± a unified approach. J Trauma 21: 349±355 20. Sudarsky LA, Laschinger JC, Copppa GF, Spencer FC (1987) Improved results from standardized approach in treating patients with necrotizing fasciitis. Ann Surg 206: 661±665 21. Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI (1995) Outcome prediction in patients with Fournier's gangrene. J Urol 154: 89±92 22. Hsiao GH, Chang CH, Hsiao CW, Fanchiang JH, Jee SH (1998) Necrotizing soft tissue infections. Surgical or conservative treatment? Dermatol Surg 24: 243±247 23. Beger HG, Bittner R, Block S, Büchler M (1986) Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 91: 433±438 24. Machado MC, Bacchella T, Monteiro da Cunha JE (1986) Surgical treatment of pancreatic necrosis. Dig Dis Sci 31: 25 25. Ochsner A, DeBakey M (1938) Subphrenic abscess: collective review and analysis of 3: 608 collected and personal cases. Surg Gynecol Obstet 66: 426 26. Korobkin M, Callen PW, Filly RA, Hoffer PB, Shimahak RR, Kressel HY (1978) Comparison of computed tomography, ultrasonography, and gallium-67 scanning in the evaluation of suspected abdominal abscess. Radiology 129: 89±93 27. Knochel JQ, Koehler PR, Lee TG, Welch DM (1980) Diagnosis of abdominal abscesses wih computed tomography, ultrasound, and 111In leukocyte scans. Radiology 137: 425±432 28. Karlson KB, Martin EC, Frankuchen EI (1981) Non-surgical drainage of intra-abdominal and mediastinal abscesses: a report of 12 cases. Cardiovasc Intervent Radiol 4: 170±176 29. Saini S, Kellum J, O'Leary M, O'Donnell T, Tally F, Carter B, Deterling RA, Curtis LE (1983) Improved localization and survival in patients with intra-abdominal abscesses. Am J Surg 145: 136±142 30. Gerzof SG, Robbins AH, Johnson WC, Birkett DH, Nabseth DC (1981) Percutaneous catheter drainage of abdominal abscesses. A five year experience. N Engl J Med 305: 653±657 31. Gerzof SG, Robbins AH, Johnson WC, Birkett DH, Nasbeth DC (1985) Expanded criteria for percutaneous abscess drainage. Arch Surg 120: 227±232 32. Johnson WC, Gerzof SG, Robbins AH, Nabseth DC (1981) Treatment of abdominal abcesses. Comparative evaluation of operative drainage versus percutaneous catheter drainage guided by computed tomography or ultrasound. Ann Surg 194: 510±520 33. Glass CA, Cohn Ijr (1984) Drainage of intraabdominal abscesses. A comparison of surgical and computerized tomography guided catheter drainage. Am J Surg 147: 315±317 34. Levison MA, Ziegler D (1991) Correlation of APACHE II score, drainage technique, and outcome in postoperative intraabdominal abscess. Surg Gynecol Obstet 172: 89±94 35. Hemming A, Davis NL, Robins RE (1991) Surgical versus percutaneous drainage of intra-abdominal abscesses. Am J Surg 161: 593±595 36. Bufalari A, Giustozzi G, Moggi L (1996) Postoperative intraabdominal abscesses: percutaneous versus surgical treatment. Acta Chir Belg 96: 197±200 37. Olak JO, Christou NV, Stein LA, Casola G, Meakins JL (1986) Operative versus pecutaneous drainage of intra-abdominal abscesses. Arch Surg 121: 141±146 38. Stephen M, Loewenthal J (1979) Continuing peritoneal lavage in high-risk peritonitis. Surgery 85: 603±606 39. Washington BC, Villalba MR, Lauter CB, Colville J, Starnes R (1983) Cefamandole erythromycin heparin peritoneal irrigation: an adjunct to the surgical treatment of diffuse bacterial peritonitis. Surgery 94: 576±581 S 61 40. Hallerback B, Andersson C, Englund N, Glise H, Nihlberg A, Solhaug J, Wahlstrom B (1986) A prospective randomized study of continuous peritoneal lavage postoperatively in the treatment of purulent peritonitis. Surg Gynecol Obstet 163: 433±436 41. Polk HC, Fry DE (1980) Radical peritoneal debridement for established peritonitis: the results of a prospective randomized clinical trial. Ann Surg 192: 350±355 42. Schein M, Hirshberg A, Hashmonai M (1992) Current surgical management of severe intraabdominal infection. Surgery 112: 489±496 43. Schein M (1991) Planned re-operations and open management in critical intraabdominal infections. Prospective personal experience in 52 cases. World J Surg 15: 537±545 44. Schein M, Wittmann DH, Aprahamian CC, Condon RE (1995) The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg 180: 745±753 45. Wittmann DH, Aprahamian C, Bergstein JM (1990) Etappenlavage: avanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and velcro analogue for temporary abdominal closure. World J Surg 14: 218±226 46. Mughal MM, Bancewicz J, Irving MH (1986) Laparostomy: a technique for the management of intractable intraabdominal sepsis. Br J Surg 73: 253±259 47. Garcia-Sabrido JL, Tallado JM, Christou NV, Polo JR, Valdecantos E (1988) Treatment of severe intra-abdominal sepsis and or necrotic foci by an ªopen abdomenº approach. Arch Surg 123: 152±156 48. Hau T, Ohmann C, Wolmershauser A, Wacha H, Yang Q (1995) Planned relaparotomy versus relaparotomy on demand in the treatment of intra-abdominal infections. Arch Surg 130: 1193±1196 49. Pruitt BA Jr (1984) Biopsy diagnosis of surgical infections. N Engl J Med 310: 1737±1738 50. Maki DG, Weise CE, Sarafin HW (1977) A semiquantitative culture method for identifying intravenous catheter-related infection. N Engl J Med 296: 1305±1309 51. Sherertz RJ, Heard SO, Raad II (1997) Diagnosis of triple-lumen catheter infection: comparison of roll-plate, sonication, and flushing methodologies. J Clin Microbiol 35: 641±646 52. Cook DJ, Randolph A, Kernerman P, et al (1997) Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med 25: 1417±1424 53. Clarke DE, Raffin TA (1990) Infectious complications of indwelling long-term central venous catheters. Chest 97: 966±972 54. Dusvak RJr, Haskal ZJ, Thomas-Hawkins C, Soulen MC, Baum RA, Shlansky-Goldberg RD, Cope C (1998) Replacement of failing tunneled hemodialysis catheters through pre-existing subcutaneous tunnels: a comparison of catheter function and infection rates for de novo replacements and over-thewire exchanges. J Vasc Interv Radiol 9: 321±327 55. Kaufman J, Demas C, Stark G (1986) Catheter-related septic central venous thrombosis: current therapeutic options. West J Med 145: 200±203 56. Bregenzer T, Conen D, Sakmann P, et al (1998) Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med 158: 151±156 57. Bach A, Bohrer H, Geis HK (1992) Safety of a guidewire technique for replacement of pulmonary artery catheters. J Cardiothorac Vasc Anesth 6: 711±714 58. Cobb DK, High KP, Sawyer RG, et al (1992) A controlled trial of scheduled replacement of central venous and pulmonary artery catheters. N Engl J Med 327: 1062±1068 59. Crofts TJ, Park KG, Steele RJ, Chung SS, Li AK (1989) A randomized trial of nonoperative treatment for perforated peptic ulcer. N Engl J Med 321: 970±973 60. Kronborg O (1993) Treatment of perforated sigmoid diverticulitis: a prospective randomized trial. Br J Surg 80: 505±507 61. Tucci G, Torquati A, Grande M, Stroppa I, Siaesi M, Farinon AM (1996) Major acute inflammatory complications of diverticular disease of the colon: planning of surgical treatment. Hepatogastroenterology 43: 839±845 62. Smirniotis V, Tsoutsos D, Fotopoulos A, Pissiotis AC (1992) Perforated diverticulitis: a surgical dilemma. Int Surg 77: 44±47 63. Corder AP, Williams JD (1990) Optimal operative treatment in acute septic complications of diverticular disease. Ann R Coll Surg Engl 72: 82±86 64. Finlay IG, Carter DC (1987) A comparison of emergency resection and staged management in perforated diverticular disease. Dis Colon Rectum 30: 929±933 65. Auguste L, Borrero E, Wise L (1985) Surgical management of perforated colonic diverticulitis. Arch Surg 120: 450±452 66. Nagorney DM, Adson MA, Pemberton JH (1985) Sigmopid diverticulitis with perforation and generalized peritonitis. Dis Colon Rectum 28: 71±75 67. Umbach TW, Dorazio RA (1999) Primary resection and anastomosis for perforated left colon lesions. Am Surg 65: 931±933 68. Setti Carraro PG, Magenta A, Segala M, Ravizzini C, Nespoli A, Tiberio G (1999) Predictive value of a pathophysiological score in the surgical treatment of perforated diverticular disease. Chir Ital 51: 31±36 69. Kriwanek S, Armbruster C, Beckerhinn P, Dittrich K (1994) Prognostic factors for survival in colonic perforation. Int J Colorectal Dis 9: 158±162 70. Strada G, Raad L, Belloni G, Setti Carraro P (1993) Large bowel perforations in war surgery: one-stage treatment in a field hospital. Int J Colorectal Dis 8: 213±216 71. Saccomani GE, Santi F, Gramegna A (1993) Primary resection with and without anastomosis for perforation of acute diverticulitis. Acta Chir Belg 93: 169±172 72. Medina VA, Papanicolaou GK, Tadros RR, Fielding LP (1991) Acute perforated diverticulitis: primary resection and anastomosis? Conn Med 55: 258±261 73. Alanis A, Papanicolaou GK, Tadros RR, Fielding LP (1989) Primary resection and anastomosis for treatment of acute diverticulitis. Dis Colon Rectum 21: 933±939 74. Del Pino A, Cintron JR, Orsay CP, Pearl RK, Tan A, Abcarian H (1997) Enterostomal complications: are emergently created enterostomas at greater risk? Am Surg 63: 653±656 75. Ghorra SG, Rzeczycki TP, Natarajan R, Pricolo P (1999) Colostomy closure: impact of preoperative risk factors on morbidity. Am Surg 65: 266±269 76. Belmonte C, Klas JV, Perez JJ, Wong WD, Rothenberger DA, Goldberg SM, Madoff RD (1996) The Hartmann procedure. First choice or last resort in diverticular disease? Arch Surg 131: 612±617 77. Polk HC, Shields CL (1977) Remote organ failure: a valid sign of occult intraabdominal infection. Surgery 81: 310±313 78. Fry DE, Pearlstein L, Fulton RL, Polk HC (1980) Multiple system organ failure. The role of uncontrolled infection. Arch Surg 115: 136±140 S 62 79. Bell RC, Coalson JJ, Smith JD, Johanson WG (1983) Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med 99: 293±298 80. Ferraris VA (1983) Exploratory laparotomy for potential abdominal sepsis in patients with multiple organ failure. Arch Surg 118: 1130±1133 81. Hinsdale JG, Jaffe BM (1984) Re-operation for intra-abdominal sepsis. Indications and results in modern critical care setting. Ann Surg 199: 31±36 82. Bunt TJ (1986) Non-directed relaparotomy for intraabdominal sepsis: a futile procedure. Am Surg 52: 294±298 83. Norton LW (1985) Does drainage of intraabdominal pus reverse multiple organ failure? Am J Surg 149: 347±350 84. McCrory C, Crowley K (1997) Is repeat laparotomy of value in patients with suspected intra-abdominal sepsis in the intensive care unit? Ir J Med Sci 166: 89±91 Intensive Care Med (2001) 27: S 63±S 79 Airway and lung in sepsis Greg S. Martin Gordon R. Bernard ) G. S. Martin ´ G. R. Bernard ( ) Center for Lung Research, Vanderbilt University Medical Center, Division of Allergy, Pulmonary and Critical Care Medicine, Nashville, Tenn., USA E-mail: [email protected] Phone: +1-6 15-3 43 00 77 Fax: +1-6 15-3 43 74 48 Introduction The development of respiratory dysfunction in patients with sepsis presents a myriad of complex interactions, which are yet to be completely understood. Similar to sepsis itself, the respiratory dysfunction that accompanies sepsis lies on a continuum from subclinical disease to overwhelming organ dysfunction. The most dreaded respiratory complication of sepsis is the acute respiratory distress syndrome (ARDS) ± a severe form of acute lung injury (ALI) at the end of the spectrum of respiratory dysfunction. Sepsis itself encompasses an entire range of host inflammatory responses, most frequently generated in humans by an infectious source. As the criteria defining the sepsis syndrome have become more established, the ability to determine specific epidemiological information associated with the syndrome has become more feasible. Recent compilations suggest a rising incidence of sepsis, likely resulting from advancing age of the population, potent immunosuppressive medications, and increasing numbers of invasive procedures [1]. In addition, data compiled by the Centers for Disease Control indicate that the incidence of sepsis increased more than 100 % from 1979 to 1987, although the lack of contemporaneous standardized definitions may make this statistic exaggerated. The sepsis syndrome remains one of the most commonly recognized predisposing conditions for ALI, accounting for approximately 40 % of cases [3, 4]. Pulmonary and intra-abdominal infections are the most commonly associated sites of infection identified in patients suffering ALI related to sepsis [5]. The development of ARDS in patients with sepsis is reported to occur in 25±42 % of patients, increasing with persistent arterial hypotension [6]. Since its description in 1967, the defining criteria of ARDS have varied. Most physicians include the presence of bilateral pulmonary infiltrates on frontal chest radiography, impaired gas exchange, and the absence of cardiac dysfunction. Many investigators believe reduced respiratory system compliance, increased extravascular lung water, or other biochemical markers of inflammation should be included [7]. The AmericanEuropean Consensus Conference on ARDS created a uniform definition for ALI and ARDS in 1994, outlined in Table 1. These criteria have allowed more precise epidemiological estimates to be made, although the incidence has been reported to vary from 5 to 71 per 100000 persons in the United States [8, 9]. Imprecision in these statistics makes quantification of the financial burden of this disorder difficult, although rational yearly estimates approach $5 billion in the United States alone. Broad, cooperative studies to obtain more precise estimates are underway. The morbidity and mortality associated with ALI and ARDS may be declining slowly, although it is widely considered to remain in excess of 40 %. Mortality is most often due to unresolved sepsis or multisystem organ failure (MOF) as opposed to progressive respiratory failure [5]. A recent review based on data from the period 1983±1993 in Seattle suggests that mortality rates have declined slowly over time, particularly in young patients with lung injury related to sepsis [10]. Several factors have been consistently found to affect mortality in patients with ALI, including age, severity of illness, cause of lung injury, presence of MOF, and preexisting comorbid conditions [11]. The degree of initial hypoxemia is not a reliable prognostic indicator, although changes in oxygenation over the first 48 h appear to dis- S 64 Table 1 Definitions of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (adapted from the American-European Consensus Conference) ALI ARDS Timing Degree of oxygenation defect Radiographic appearance Hydrostatic component Acute Acute PaO2/FIO2 £300 PaO2/FIO2 £200 Bilateral infiltrates Bilateral infiltrates PAOP < 18 mmHg PAOP < 18 mmHg Table 2 Calculation of the Lung Injury Score: the total number of points is divided by the number of components used Points 0 1 2 3 4 Chest radiography PaO2/FIO2 ratio PEEP (cmH2O) Cstat (ml/cmH2O) No infiltrates > 300 5 80 1 quadrant 225±299 6±8 60±79 2 quadrants 172±224 9±11 40±59 3 quadrants 100±174 12±14 20±39 4 quadrants < 100 15 19 criminate eventual outcomes. In 1988 the publication of the lung injury score (LIS, Table 2) provided a method of grading the severity of lung injury ± a system that has been validated prospectively for prognostic purposes. An initial LIS higher than 3.5 has been observed to be correlated with a survival rate of 18 %, while a score of 2.5±3.5 corresponds to a survival rate of 30 %, a score of 1.1±2.4 with a 59 % survival, and a score below 1.1 with a 66 % rate of survival [12]. This contribution addresses the respiratory system complications encountered in patients with sepsis, with a focus on clinically relevant diagnostic methods and management options for the practicing critical care physician. Methods A computer-based review of the literature was undertaken using Medline from 1993 to the present as the primary database. The specific subject heading keywords defined for each question were combined with the following general sepsis-related subject heading keywords: infection, sepsis, sepsis syndrome, septic shock, multiple organ failure, critical care, intensive care. An additional manual retrieval of pertinent cited articles from the retrieved literature was performed. Pathophysiology Biochemical and cellular mediators The hallmark of ALI/ARDS is alveolar epithelial inflammation, airspace flooding with plasma proteins and cellular debris, surfactant depletion and inactivation, and a loss of normal endothelial reactivity [13]. This article is not intended to serve as a reference for the biochemical and cellular mediators of sepsis-induced respiratory dysfunction, although their interplay is indisputably critical to the pathophysiology common to the syn- drome. The pathogenesis of ALI/ARDS is complex, with a consistently observed broad activation of the host inflammatory response. A well described pathophysiological model of ALI/ARDS is one of acute lung inflammation mediated by neutrophils, cytokines, and oxidant stress [14]. Bronchoalveolar lavage fluid from patients with ALI contains increased quantities of neutrophils and their enzymes, both of which are correlated with the severity of lung injury [15]. While it is clear that neutrophils exert a critical role in the evolution of the host inflammatory response, neutropenic patients can develop ALI, thus supporting a pivotal role for other effector cells such as alveolar macrophages [16]. Both of these cell types produce inflammatory mediators, catalyze the generation of reactive oxygen species, and encourage lipid peroxidation through arachidonic acid metabolism pathways. Persistent plasma elevations in the proinflammatory cytokines such as tumor necrosis factor-a and interleukins (ILs) 1, 6, and 8 are correlated with reduced survival, while increases in the bronchoalveolar lavage fluid anti-inflammatory cytokines such as IL-10 are correlated directly with survival [17, 18, 19, 20]. Evidence of lipid peroxidation and oxidant stress is uniformly present in patients with sepsis, with reported elevations in hypoxanthine and numerous arachidonic acid metabolites (e.g., isoprostanes) [21, 24]. Plasma thiol levels have been found to be correlated with survival in patients with ARDS, while lipid peroxidation products are correlated with severity of disease and survival [25]. Cytokine expression has been known to be regulated by oxidative stress mechanisms; antioxidants such as N-acetylcysteine and tocopherol derivatives have been shown experimentally to reduce the expression of proinflammatory cytokines [26, 27, 28]. Recent evidence exists that mechanisms independent of oxidant control also contribute to cytokine expression [29, 30]. In addition, endogenous (e.g., transferrin and ceruloplasmin) and exogenous substances (albumin) with the ability to S 65 chelate iron have been shown effectively to suppress proinflammatory cytokine production in vitro [31, 32]. Unfortunately, the endogenous antioxidants have been shown to be either insufficient or inactive, rendering the natural oxidant stress defense mechanisms ineffectual. As the lung functions to filter nearly the entire cardiac output, it may thus be injured as a passive participant in the systemic inflammatory cascade. Gas exchange The principal cause of hypoxemia associated with sepsis is extensive right-to-left intrapulmonary shunting of blood flow. Intrapulmonary shunting is normally limited to less than 5 % of the total cardiac output, whereas in ARDS it may consume more than 25 % of the total cardiac output. In ARDS, shunting is due to persistent perfusion of atelectatic and fluid-filled alveoli. Ordinarily, compensation occurs through hypoxic pulmonary vasoconstriction to limit the amount of shunt by reducing perfusion to poorly ventilated lung units. In states of lung injury, however, hypoxic pulmonary vasoconstriction may be ineffective or absent, thereby increasing the magnitude of the intrapulmonary shunt. After the initial insult to the lung, gradients appear along a gravitational axis, in which the dependent lung is extensively consolidated and the main source of venous admixture [33]. Another factor affecting the ability to compensate for intrapulmonary shunting may be differences among patients in the mixed venous oxygen concentration of blood perfusing the injured lung regions as a result of differences in cardiac output or tissue oxygen consumption. Shunting of blood through nonventilated lung units accounts for the relative refractory nature of hypoxemia in ARDS. As a means to improve oxygenation, manipulation of airway pressure is often required to restore ventilation to nonventilated lung units. Lung mechanics Decrements in lung compliance (the change in lung volume for a given change in transpulmonary pressure) related to small airway and alveolar collapse are nearly universal in patients with ALI/ARDS. When delivered by mechanical ventilation with no end-expiratory pressure, the static inflation pressure for typical tidal volumes of 8 ml/kg may exceed 25 cmH2O. This implies lung compliance approaching 20 ml/cmH2O, or less than one-fourth that of normal. To reflect the actual intrinsic elastic properties of lung tissue, compliance should be calculated with the quantity of lung participating in gas exchange. In early ARDS the volume of aeratable lung is reduced by alveolar edema and surfactant dysfunction. These changes account for the need for higher inflation pressures, exclusive of any change in the intrinsic elastic properties of lung. As such, the inflation pressure may function as an estimation of the amount of edema and atelectasis early in the course of ARDS. This is reflected in the concept of a ªsmallº lung early in ARDS versus a ªstiffº lung later in the course. Only if fibrosis develops in the later phases do increases in inflation pressures reflect true changes in lung compliance. In a person with normal lungs, a transpulmonary pressure of 30 cmH2O is sufficient to achieve total lung capacity ± thus the recommended pressure limit for mechanical ventilation adopted by the American College of Chest Physicians Consensus Conference [34]. Interestingly, this level of airway pressure has also been shown to induce lung injury in some animals [35]. Whereas the static inflation pressure is the best index of transalveolar pressure during mechanical ventilation, the mean airway pressure is the best predictor of an overall effect on oxygenation or hemodynamics. As the mean airway pressure increases, progressively greater amounts of potentially recruitable lung are recruited. Unfortunately, at the same time venous return can be impeded and cardiac output depressed. Because volume-related alveolar overdistension is now recognized to play a major role in airway pressure associated injury in ARDS, the term ªvolutraumaº (instead of barotrauma) has been coined. At times, peak airway pressures during mechanical ventilatory support of patients with ARDS are increased out of proportion to the increase in static inflation pressures. This finding suggests an increase in airway resistance. Airway secretions, edema, mediators that provoke bronchospasm, narrow endotracheal tubes, etc. can all increase airway resistance. Airway resistance, as with compliance, should be normalized for the amount of aeratable lung volume available and, although abnormal in part due to bronchoconstriction, the extent to which airway resistance is increased in ARDS is not completely known [36]. Work of breathing Because these changes in mechanical properties increase the airway pressure necessary to achieve a given tidal volume, the work of breathing (measured as the pressure-volume product during spontaneous breaths) is also increased in ARDS ± an effect that is multiplied by coincident tachypnea. One cause of increased deadspace ventilation is hyperventilation of still normal or relatively normal alveolar units, a process exaggerated by differences in the distribution of ventilation with mechanical ventilatory support and by overinflation of normal lung units when mean airway pressure is increased by positive end-expiratory pressure (PEEP) or other maneuvers. Normally, the dead space-to-tidal volume S 66 ratio (Vd/Vt) is 0.3, but in severe ARDS as much as 90 % (Vd/Vt = 0.9) of each tidal volume may fail to participate in effective gas exchange. As a consequence minute ventilation greater than five times normal may be necessary to maintain normal arterial CO2 concentrations. The increases in work of breathing are multifactorial, including hypoxemia, dead-space ventilation, and increased airflow resistance from bronchoconstriction. Although normal work of breathing accounts for only a small percentage of the body's overall oxygen consumption, the work of spontaneous breathing in patients with ARDS may require nearly 50 % of the body's total oxygen consumption. To supply the energy necessary to sustain this level of work, resources (i.e., relative blood flow) may have to be diverted from other vital organ systems. Patients with lung injury related to sepsis demonstrate heightened airway resistance, as least partially mediated by bronchoconstriction, although this phenomenon has not been shown to exist specifically in sepsis (see ªLung mechanics,º above). The combination of altered airflow and abnormal pulmonary vascular perfusion (with loss of hypoxic pulmonary vasoconstriction) contributes to dramatic alterations in ventilation ± perfusion matching, which may lead to clinically disproportionate hypoxemia relative to radiographic changes. Extravascular lung water The equation described by Starling in 1896 characterizes fluid flux across a semipermeable membrane and has been applied both experimentally and clinically to predict pulmonary edema formation in humans. The prime factors in this equation are the hydrostatic and oncotic gradients between the vasculature and interstitium coupled with the degree of capillary permeability. When fluid deposition exceeds the capacity of the lung to remove such fluid (i.e., lymphatic flow), accumulation of extravascular water occurs. Patients with sepsis demonstrate variable degrees of capillary permeability, increasing the effect of the hydrostatic pressure gradient relative to the oncotic pressure gradient as molecules responsible for maintaining oncotic pressure may be allowed freely to cross such leaky barriers. Accumulation of extravascular lung water and exudation of plasma proteins into the alveolar space creates the interstitial edema recognized as a complication of sepsis (i.e., ALI/ARDS). Pulmonary hemodynamics Increased pulmonary artery pressure is common in patients with ARDS, but pulmonary vascular resistance is usually only mildly to moderately elevated as a consequence of increased cardiac output. The prognosis of patients with significant elevations in pulmonary vascular resistance is worse, whether related to depressed cardiac function or worsening pulmonary hypertension. The cause of pulmonary hypertension in ARDS is multifactorial [37]. Vasoconstriction caused by alveolar hypoxia or other vasoactive mediators such as thromboxane and endothelin and intravascular obstruction from platelet thrombi or perivascular edema probably dominate initially. Later, sustained or worsening pulmonary hypertension probably reflects the degree to which fibrosis is responsible for obliteration of the vascular bed. Thus the poor prognosis associated with late pulmonary hypertension in ARDS may simply reflect the severity of fibrosis. Pathology and lung repair The pathological hallmark of ALI/ARDS, diffuse alveolar damage, changes dynamically as ARDS evolves [38, 39]. This occurs gradually over days to weeks, depending on the severity and resolution of the insult, and may not resolve for months or may result in chronic fibrotic changes along the alveolar interstitium. The changes that develop are conveniently divided into three phases: the early exudative phase (days 1±5), the fibroproliferative phase (days 6±10), and the fibrotic phase (after 10 days). These times are approximate, and the characteristic features in each phase often overlap. The initial pathological abnormalities are interstitial swelling, proteinaceous alveolar edema, hemorrhage, and fibrin deposition. Basement membrane disruption and denudation, especially of alveolar epithelial cells, can be seen with electron microscopy. After 1±2 days hyaline membranes (sloughed alveolar cellular debris mixed with fibrin) are commonly observed by light microscopy. Cellular infiltrates may be minimal or may be dominated by neutrophils. Fibrin thrombi can be seen in some of the alveolar capillaries and small pulmonary arteries. Although type I alveolar epithelial cells cover 95 % of the alveolar surface, they are terminally differentiated cells that cannot regenerate. Instead, several days after the onset of ARDS type II cells (the cell responsible for surfactant production) proliferate and then differentiate into new type I cells to reline the alveolar walls. After approximately 1 week most of the alveolar edema has resolved, hyaline membranes are much less prominent, mononuclear cells have replaced the neutrophilic infiltrate, and fibroblasts are proliferating within the interstitium and depositing new collagen. Pulmonary fibrosis in ARDS is often referred to as ªinterstitialº because structures between airspaces appear to be markedly widened by fibrotic material. Detailed inspection has revealed, however, that this fibrosis is often the S 67 result of either alveolar collapse or intra-alveolar fibrosis in which the proteinaceous edema and cellular debris of the exudative stage have been incorporated into the alveolar wall. Actual deposition of new collagen within the interstitial space appears to be relatively uncommon [40]. Eventually this healing of injured tissue may result in lung fibrosis, but the extent to which scarring develops is enormously variable. When parenchymal fibrosis does develop, intimal fibrosis and medial hypertrophy of pulmonary arterioles, along with complete obliteration of portions of the vascular bed, are also common. Clinical presentation Initial signs and symptoms When clinical manifestations of sepsis first appear, between 28 % and 33 % of patients meet the criteria for ARDS [41]. Few data exist regarding respiratory abnormalities prior to this time, although progression through a clinical spectrum of dysfunction is likely the case. Patients may experience severe dyspnea, tachypnea, and unremitting hypoxemia prior to meeting all the criteria for ALI/ARDS. Hypoxemia results from myriad causes, including cardiocirculatory dysfunction affecting global oxygen delivery and shifts in the oxyhemoglobin dissociation curve. Respiratory dysfunction contributes to hypoxemia as well, with increased work of breathing. The multifaceted increase in work of breathing is easier to recognize than to quantify. Changes occur through increased dead space ventilation, related to ventilationperfusion mismatching, respiratory muscle dysfunction, decreased thoracic compliance and increased airway resistance (bronchoconstriction). Both increased physiological dead-space ventilation and intrapulmonary shunting are responsible for the tachypnea and elevated minute ventilation required to achieve effective CO2 excretion in patients with ARDS. Patients may also experience altered mental status or extrapulmonary organ failure confounding their respiratory dysfunction. These physiological changes in pulmonary and cardiocirculatory function are most often radiographically inapparent. In practice, the radiographic findings associated with sepsis vary widely [42]. During the course of sepsis pulmonary edema may develop as a combination of increased pulmonary vascular permeability as described earlier, increased hydrostatic pressures related to resuscitation efforts, and/or lowered oncotic pressure gradients from any cause. During this time bilateral infiltrates may appear on the chest radiograph without overt evidence of fluid overload (i.e., increased vascular pedicle width or cardiothoracic ratio). When combined with appropriate thresholds of hypoxemia, the diagnosis of ALI or ARDS is secured. Unfortunately, standard chest radiographs are poor predictors of the severity of oxy- genation defect or clinical outcome. Although the classic pulmonary parenchymal changes associated with ALI are diffuse, bilateral, peripheral, and interstitial in nature, they may be asymmetric or even patchy and focal. Clinical course The natural history of ALI/ARDS tends to be dominated by the inciting event rather than the lung injury itself. As such, treatment of the underlying cause and support of the respiratory system remains the standard of care. Death from refractory respiratory failure is unusual, with the most common cause of death being from the development of multiple organ failure or (recurrent) sepsis [5]. In patients who resolve ARDS relatively rapidly (over a period of 10±14 days), minute ventilation and dead-space ventilation both decrease in tandem with improvements in oxygenation. Given the substantial delay to peak incidence of pneumothorax, the lung appears to withstand exposure to somewhat higher forces in the earliest phase of human ARDS without radiographically evident barotrauma [43, 44]. After this time further improvements in oxygenation depend on whether the fibroproliferative response can restore the normal lung architecture for gas exchange. In patients with more severe ARDS, i.e., those in whom significant lung fibrosis eventually develops, minute ventilatory requirements stay high even as oxygenation improves. As fibrosis develops, progressive amounts of the vascular bed are obliterated, which contributes to the increase in dead-space ventilation even as alveolar edema and the intrapulmonary shunt resolve. Prognostication As discussed above, respiratory dysfunction related to sepsis exists on a continuum from subclinical aberrations to overt respiratory failure. Quantifying the severity of respiratory system involvement has been of keen interest for more than a decade. To streamline the ability to conduct research in this area, a clinical definition of lung injury was proposed and adopted in 1994 (Table 1). The Consensus Conference definition of ARDS emphasizes the spectrum of abnormalities present from ALI to ARDS, using readily available clinical criteria to make the necessary distinction. Although the exact role of respiratory failure in multiple organ failure is not clear, it has been demonstrated that potentially injurious modes of mechanical ventilation can produce cytokine release in humans and end-organ damage in animal models [45]. A number of detailed models have been created in an attempt to accurately predict the clinical outcomes in respiratory failure and/or sepsis (Acute Physiology and S 68 Chronic Health Evaluation, Multiple-Organ Dysfunction Syndrome, Sequential Organ Failure Assessment, Injury Severity Score). Unfortunately, the prospective ability to recognize those patients who will survive is much closer to being an art than a science. Survival in sepsis appears to be slowly improving over the past 30 or more years, with a coincident decrease in the mortality associated with ALI/ARDS [11]. For most of the first two decades since ARDS was first reported, mortality remained relatively constant at 60±70 %. More recent reports, however, suggest that mortality has declined to roughly 40 % [11]. The explanation for this apparent improvement in patient outcomes is not clear but could be due to differences in patient populations, changes in ventilator support strategies, greater attention to fluid management, improved hemodynamic and nutritional support, improved antibiotics for nosocomial infection, corticosteroid use later in ARDS, or the potential benefits of protocol-driven patient management systems now implemented in many ICUs. General scoring systems provide an estimate of the probability of mortality on admission to the ICU [46]. A specific scoring system for ARDS has been developed; however, its predictive accuracy is debated [47]. The number of acquired organ system failures is often the most important prognostic indicator for patients requiring intensive care, including patients with ARDS. Not surprisingly, patients developing fibrosis have a poorer outcome than do patients in whom fibrosis does not develop. In addition, liver failure in association with ARDS carries a particularly poor prognosis. More specific predictors of outcome for patients with ARDS have been sought from measurements of various serum and lung lavage factors. As discussed above, concentrations of proinflammatory cytokines are correlated with outcome. The concentrations of von Willebrand factor antigen in serum and neutrophil-activating factor type 1, IL-8, and procollagen peptide in airspace lavage fluid are correlated with outcomes or progressive disease in some but not all studies. Increases in unsaturated serum acyl chain ratios appear to discriminate severity of illness and may serve as a marker of those most at risk of developing ALI/ARDS [48]. The integrity of the epithelial barrier in relation to resolution of alveolar edema also appears to be a determinant of outcome in patients with ARDS [49]. Patients who can concentrate the protein in the edema fluid during the first 12 h of illness (indicating an intact epithelial barrier with the ability to actively transport fluid out of the alveoli) are more likely to recover than those who cannot do so. Similarly, the change in the PaO22/FIO2 ratio following initial treatment of ARDS can discriminate between survivors and nonsurvivors [50]. At the present time none of these markers has been validated as an accurate method for predicting outcome in individual patients with ARDS. The long-term functional outlook for survivors of ARDS is generally good [51]. Long-term abnormalities in pulmonary function are more common if lung function is impaired for more than a few days after the onset of ARDS. Most of the improvement in pulmonary function and perceived health occurs in the first 3 months following an episode of ARDS. Recently more complete data concerning long-term outcomes in patients suffering severe respiratory complications suggest a reduction in the quality of life relative to their premorbid level of function, often attributed to objective or subjective declines in pulmonary function [52]. Management options No therapeutic intervention has been proven effective in reducing the incidence of respiratory failure in sepsis or its attributable mortality. Prevention of complications is of utmost importance while general supportive measures (e.g., antimicrobial therapy, nutrition) are undertaken. Control of the upper airway and consideration of the need for ventilatory assistance is an important first step in the management of patients with respiratory dysfunction related to sepsis. Although limited data exist, which are somewhat conflicting, noninvasive positive-pressure ventilation has not clearly been shown to be effective in this clinical circumstance [53, 56]. In addition, it is critically important to not impede the timing of other appropriate respiratory interventions, such as institution of mechanical ventilation, regardless of the availability and/or seeming adequacy of noninvasive positive-pressure ventilation (NIPPV). Given our knowledge of fluid flux in states of altered capillary permeability (i.e., complete equalization of oncotic forces with attendant magnification of effective hydrostatic forces predicted by Starling's equation), it seems prudent to advocate judicious fluid resuscitation and/or fluid restriction when possible in this condition [57]. Recently investigators have shown that improvements in physiology and outcome occur in patients who lose weight or whose microvascular pressures fall as a result of diuresis or fluid restriction [58, 59]. These improvements can be produced by strategies employing fluid restriction without any higher incidence of complications such as renal failure or hemodynamic compromise [60]. The intravenous solution of choice (i.e., crystalloid versus colloid) is still unclear despite years of detailed investigation. In hypo-oncotic patients with established lung injury, treatment with the combination of albumin and furosemide appears to improve physiology and may reduce the duration of mechanical ventilation, although evidence of improved outcomes requires further investigation [61]. If patients cannot adequately protect their airway, placement of an endotracheal tube is indicated. Based S 69 on increased rates of sinusitis, orotracheal intubation is the preferred route [62, 63]. Mounting evidence implicates nosocomial sinusitis in the development of ventilator-associated pneumonia (VAP) ± an entity with a significant independent contribution to mortality [64, 65, 66]. Once endotracheal tube placement has occurred, institution of mechanical ventilation is almost universally indicated due to coincident respiratory failure (i.e., severe hypoxemia and increased work of breathing). For this reason one of the chief benefits of mechanical ventilatory support in ARDS is to reduce the patient's work of breathing so that blood flow may be redirected to other vital organs. Commonly accepted indications for institution of mechanical ventilation from other causes of respiratory failure apply equally to this patient population, including refractory hypoxemia (PaO2 < 60 despite high flow oxygen), respiratory rate of more than 35 breaths/ min, and vital capacity below 15 ml/kg, among others. Mechanical ventilation is not a therapeutic option in patients with respiratory failure, and thus the goal is to support the individual respiratory requirements until the indication(s) requiring mechanical ventilation have reversed. No mode of ventilation has been proven superior to others in terms of outcomes in patients with sepsis-related respiratory failure, although complete ventilatory support is appropriate immediately after institution of mechanical ventilation. For this reason, volumecycled ventilation using the ªassist-controlº mode (controlled mandatory ventilation) is an appropriate mode to choose at the outset. Similar degrees of respiratory support can probably be achieved with intermittent mandatory ventilation or pressure-regulated volumecontrolled ventilation. Inhaled oxygen requirements are dictated by the degree of hypoxemia, with arterial oximetric saturations of approx. 90 % (an approximate pO2 of 60 mmHg) being desirable. To ameliorate the changes in closing volume and lung derecruitment, application of PEEP is appropriate and may provide dramatic improvements in PaO2. Although some data suggest that levels of PEEP should be chosen based on respiratory system pressurevolume curves, to select the level of PEEP above the lower inflection point of the curve and thus prevent cyclic alveolar collapse, this is impractical in current clinical practice [68]. Prone positioning has been shown to be safe and to result in improvements in oxygenation in approximately 65 % of patients with ALI/ARDS, although no data exist to predict which patients will respond in this manner [68, 69] Those who do respond (defined as an improvement in PaO2 > 10 % from baseline) often maintain higher oxygenation levels for up to 18 h after resuming supine positioning and are more likely to respond to subsequent attempts at prone positioning [70]. Tidal volume should be chosen based on ideal body weight [men = 50+2.3 (height, in.)±60; women = 45.5 +2.3 (height, in.)±60)], and should be targeted to prevent end-inspiratory plateau pressures from exceeding 30 cmH2O whenever possible. Permissive hypercapnia, the method of allowing pCO2 to rise while reducing tidal volume and minute ventilation to prevent alveolar overdistension or propagation of lung injury, has been shown to be safe and effective at reducing mortality without adverse consequences (noted increases in QS/QT and mean pulmonary artery pressure) in small nonrandomized series [71, 72, 73]. In this era of reduced tidal volumes based on the recent National Institutes of Health sponsored ARDS network trial, permissive hypercapnia has become accepted as a secondary phenomenon associated with the primary goal of avoiding dangerous airway pressures [73, 74, 75]. Gradual increases in pCO2 are generally well-tolerated, particularly if significant acidosis does not occur, although the reduction in mean airway pressure may adversely affect indices of oxygenation [77]. In cases of severe acidosis, intravenous bicarbonate or extracorporeal removal of CO2 may be employed. No trials have been conducted to specifically define the most effective method of liberating septic patients from mechanical ventilation, although it is reasonable to presume that the literature addressing discontinuation of mechanical ventilation in other patient populations would apply equally. In patients with significant hemodynamic instability or altered mental status, attempts at discontinuing mechanical ventilation are not recommended. Thus a two-step method of identifying patients ready to discontinue mechanical ventilation is required. A daily screening test (consisting of a brief evaluation of the resolution of the primary indication for mechanical ventilation and adequate oxygenation and ventilation) is the most efficient way to identify those patients potentially capable of breathing spontaneously [78]. This screening tool is intended to identify patients in whom the primary indication for mechanical ventilation has reverted, using data such as frequencyto-tidal volume ratio, oxygenation (PaO2/FIO2 ratio), maximal inspiratory pressure, maximal expiratory pressure, airway occlusion pressure, and vital capacity [79, 80]. Patients with adequate respiratory recovery according to the screening information should progress to a simple spontaneous breathing trial to assess the true ability of an individual patient to be liberated from mechanical ventilation. To that end, daily attempts at spontaneous breathing (through a T-piece connection or with minimal ventilatory support such as flow-by with PEEP of 5 cmH2O) should be offered to all hemodynamically stable patients with adequate mental status who pass the daily respiratory screening instrument. This simple assessment may be as short as 30 min, although roughly half of patients may fail such a trial after that time, suggesting 60±120 min as the appropriate duration [81]. In some circumstances pressure support S 70 may help ªbridgeº patients in the weaning process, by slow reductions in the applied level of support to determine the ability of the patient to effectively breath spontaneously before a trial with minimal support as described above. Experimental options It is a well-known fact that numerous agents have challenged the unyielding morbidity and mortality of ALI/ ARDS associated with sepsis in well-designed clinical trials, only to be added to the growing list of failed therapies. Use of systemic corticosteroids has been thoroughly tested for both prevention of lung injury as well as treatment of early phase ALI/ARDS and found to not be efficacious in either setting with possible increased mortality in patients with established ALI/ ARDS [82, 83, 84]. Uncontrolled or small randomized trials have suggested benefit to intravenous corticosteroid therapy in patients with prolonged (fibroproliferative phase) ALI/ARDS (Late Steroids Rescue Study. http://hedwig.mgh.harvard.edu/ardsnet/ards02.html.), with a large scale trial underway to definitively answer this important question [85]. Ketoconazole, having demonstrated potential benefit in the prevention of sepsis-induced lung injury [86] was intensely evaluated in a multicenter trial supported by the National Institutes of Health sponsored ARDS network in the United States and found to lack efficacy in treating established ARDS [87] Intravenous prostaglandin E1 [88, 89, 90, 91] and aerosolized prostagladin I2 (prostacyclin) [92, 93] have been shown to improve pulmonary physiology without improving outcome. Also completely tested has been intravenous lisofylline and strategies to achieve supranormal oxygen delivery DO2 [94, 95]. Incompletely tested strategies include antioxidants such as N-acetyl cysteine [98], blocking of tumor necrosis factor, IL-10 therapy [97], and platelet-activating factor antagonists. There are a few experimental options worthy of discussion based on promising clinical or preclinical data. Newer modes of ventilation such as pressure-controlled ventilation or airway pressure release ventilation [98] may play a role in select patients requiring high levels of ventilatory support, although no data support improved outcomes at this time. In addition, some newer modes of ventilation are potentially confusing to unfamiliar physicians, thus making it less than desirable to recommend. High frequency or oscillatory ventilation has been tested in adults with ARDS and not shown to be of any significant benefit, although trials are ongoing to determine whether certain patient subgroups may benefit from such modes [99, 100, 101, 102, 103]. In addition, liquid ventilation has been shown to result in physiological improvements in small series and animal mod- els, and phase III trials in humans are underway to evaluate the efficacy of this agent in humans with ALI/ ARDS. Nitric oxide (either alone, or in combination with the selective pulmonary vasoconstrictor almitrine) has been shown to improve oxygenation without any reduction in duration of mechanical ventilation or mortality [104, 105, 106, 107]. Extracorporeal membrane oxygenation [108] or extracorporeal removal of CO2 [109, 110, 111] have been shown to result in significant physiological improvements in severely ill patients with ARDS, without clear beneficial effects on the development of organ failure or survival. Liquid ventilation has been shown to improve pulmonary physiology and reduce inflammation, and is currently in phase III trials to evaluate its ability to improve outcomes [112, 113, 114]. Similarly, surfactant therapy has been shown to improve gas exchange without directly affecting days of mechanical ventilation or mortality [115]. Phase III investigations are currently in progress in North America and Europe to determine the efficacy of variations in protocolized surfactant therapy. A number of experimental therapies exist on the horizon for patients with sepsis-induced lung injury, including gene therapy [116, 117]. Modulation of cytokines continues to be a difficult but promising area of intervention, with a preliminary randomized trial administering the anti-inflammatory cytokine IL-10 in ALI/ ARDS patients demonstrating a trend towards reduced organ failure. Other relevant respiratory issues Despite the enormous potential of future therapies we should not ignore the simple and readily available potentially beneficial therapies. This includes the use of inhaled beta-agonists, which have been shown to reduce inspiratory pressure and increase lung compliance by reducing airway resistance without significant benefits in dead-space ventilation, oxygenation, or overall outcome [118]. Elevating the head of the bed at least 30 degrees at all times has been shown to reduce the incidence of gastric material migrating to the trachea [119]. Hospital beds capable of patient rotation and/or distribution of pressure points to prevent decubitus ulceration [120]. Finally, specific enteral nutritional formulae with antioxidants and amino acid compositions designed to reduce inflammatory lipid mediators have recently been demonstrated to improve gas exchange and reduce the duration of mechanical ventilation and intensive care stay in patients with ARDS [121]. S 71 Postextubation period Recommendation After removal of the endotracheal tube patients should be monitored closely for signs of respiratory compromise for a period of 6±24 h depending on the cause and severity of respiratory failure. Endotracheal intubation may cause upper airway injuries that result in immediate or delayed airway compromise. Insertion of an endotracheal tube with an internal stylet may tear the pyriform recesses beside the larynx and result in bleeding and hematoma formation [122]. Use of a tube that is too large can result in vocal cord injury, edema, and hematoma and overinflation or malpositioning of the endotracheal tube cuff can cause periglottic injury and stenosis. Prolonged intubation, coughing, or repeated endotracheal tube placements can cause the formation of obstructive arytenoid granulation tissue [123]. Stridor, related to upper airway injury or inflammation, occurs in 25±75 % of pediatric extubations but is rare in adults, occurring in a small fraction of endotracheally intubated patients [124]. Although in some circumstances this may be managed expectantly, a low level of tolerance should exist before replacement of an adequate airway to prevent respiratory compromise. Flexible fiber-optic examination of the larynx before extubation is often prudent in such patients. Typically, within a few hours, patients tolerate reintroduction of oral nutrition, although this should progress through stages demonstrating adequate swallowing and airway protective reflexes. For patients with significant respiratory secretions, assistance with ªpulmonary toiletº may be required either through airway suctioning (nasotracheal or orotracheal) or chest percussion with postural drainage. A subset of patients requiring prolonged mechanical ventilation demonstrate significant respiratory muscle weakness, in which case assisted coughing and/or hyperinflation therapy (e.g., intermittent positive pressure breathing) may be of benefit. Provide adequate supplemental oxygen to maintain an oximetric saturation of approximately 90 % through use of simple oxygen delivery systems (i.e., nasal cannula or face mask) if possible. For endotracheally intubated patients, use of PEEP to increase mean airway pressure may be employed to reduce concentrations of inspired oxygen below potentially toxic thresholds (FIO2 < 0.60). Discussion: literature-based recommendations To answer each of the following important clinical questions, a review of the literature was performed as previously described. Do manipulations of airway pressure improve (a) oxygenation or (b) outcome in patients with sepsis? Answer: (a) yes, grade C; (b) uncertain, grade B. Rationale The following specific subject heading keywords were used to answer this question: oxygen, oxygen inhalation therapy, anoxia, anoxemia, partial pressure, and pulmonary gas exchange. The broad spectrum of respiratory dysfunction encountered in sepsis only allows for answers in specific clinical circumstances. For instance, a significant proportion of the 60 % of septic patients who never develop ALI/ARDS have normal chest radiographs, and this patient population has not been intensely studied. Although the relationship between radiographic manifestations of pulmonary dysfunction and gas exchange is poor, it is reasonable to presume that hypoxemic patients with sepsis but without radiographic pulmonary infiltrates would respond similarly to patients with visible interstitial edema (i.e., patients with ALI/ARDS). It is clear that hypoxemia is modestly correlated with prognosis in ALI/ARDS related to sepsis, and that simple methods of oxygen supplementation raise PaO2. Raising mean airway pressure results in recruitment of additional lung units to participate in gas exchange while maintaining the patency of units once recruited and thus increases PaO2 [125, 126, 127]. It is equally clear that application of PEEP, either with an endotracheal tube in place or through a tight-fitting face mask, has been shown to improve oxygenation in hypoxemic patients through increases in airway pressure [128, 129, 130]. The underlying goal in providing such therapy is to ensure adequate oxygen delivery to critical tissue beds in states of altered microvascular flow. Unfortunately, there is no data on which to assess outcomes through manipulations of airway pressure. Studies of mechanically ventilated patients with ALI/ ARDS treated with different methods of ventilation designed to achieve different inspiratory pressures have shown differences in outcome, but attributing these improvements to the manipulation of airway pressure directly is impossible [67, 74]. Determinations made by the most routine measure of oxygenation, pulse oximetry, are correlated well with arterial oxygen saturation but may misrepresent arterial saturation by 7 % in patients with extremes of heart rate, cardiac index, or pulmonary arterial wedge pressure [131]. Despite this the S 72 use of this device is recommended to monitor arterial oxygenation in this patient population, with supplemental oxygen and PEEP administered to maintain saturation of approximately 88±90 % (approximating a PaO2 of 60 mmHg) with nontoxic concentrations of oxygen (ideally FIO2 2 < 0.60). Can noninvasive positive-pressure ventilation be safely and effectively used in ALI/ARDS related to sepsis? Answer: no, grade B. Recommendation Avoid the use of NIPPV in sepsis-related ALI/ARDS patients. Rationale The following specific subject heading keywords were used to answer this question: positive pressure respiration, artificial respiration, intermittent positive pressure ventilation, adult respiratory distress syndrome. There has been a surge of interest in applying noninvasive positive-pressure ventilation to all patients with respiratory failure, although it appears that patients with ALI/ ARDS are more likely to fail this therapy [53]. It is clear that NIPPV is most effective in selected patients (normal or near normal mental status without significant respiratory system secretions) with expected resolution of respiratory failure within 72 h ± a rare situation in ALI/ ARDS [55]. Although NIPPV may avoid the use of mechanical ventilation (and its attendant risks) in a small population of ALI/ARDS patients [54], the delay in institution of mechanical ventilation may be equally likely to result in untoward complications in the majority of patients. Does (a) placement of an endotracheal tube or (b) institution of mechanical ventilation improve outcome in respiratory failure related to sepsis? Answer: (a) no, grade E; (b) yes, grade E. Recommendation Early placement of an endotracheal tube and institution of mechanical ventilation in patients with sepsis is appropriate based upon standard clinical criteria heralding the onset of respiratory failure to avoid the recognized complications associated with respiratory failure and/or acute respiratory arrest. Indications for institution of mechanical ventilation include severe tachypnea (respiratory rate > 40 breaths/min), muscular respiratory failure (use of accessory muscles), altered mental status, and/or severe hypoxemia despite supplemental oxygen. Rationale The following specific subject heading keywords were used to answer this question: intubation, intratracheal intubation, respiratory insufficiency, artificial respiration, positive-pressure respiration, and adult respiratory distress syndrome. For ethical reasons there are no randomized trials evaluating the use of endotracheal intubation in critically ill patients. It is important to recognize that placement of an endotracheal tube is not a therapeutic maneuver. This step carries the attendant risks of anesthesia for the procedure and subsequent morbid events such as VAP and thus by itself does not improve outcome in this clinical circumstance. In addition, mechanical ventilation (independently of airway protection, etc.) has not been shown to improve outcome in patients with sepsis and respiratory failure, although this has not been studied in depth. In comparison with historical controls (i.e., the polio epidemic), mechanical ventilation does indeed provide significant tangible clinical benefits [132]. Alternatively, discontinuation of mechanical ventilation by removing an endotracheal tube in terminally ill patients results in more rapid expiration than simply withholding therapy, thus providing indirect evidence of clinical benefit from endotracheal intubation with mechanical ventilation [133]. The greatest morbidity associated with endotracheal tube placement relates to risk of VAP, which is increased in patients with burns, trauma, central nervous system disease, respiratory disease, cardiac disease, and witnessed aspiration [134]. Potentially VAP may be decreased by use of orotracheal intubation, subglottic secretion drainage, kinetic hospital beds, and increased by heated respiratory circuit humidifiers and histamine-2 receptor antagonists [135, 136]. It is well recognized that mechanical ventilation possesses the potential to initiate or propagate lung injury, and thus can be considered an independent source of patient morbidity. Is normalization of (a) pH or (b) pCO2 necessary in ALI/ARDS? Answer: (a) no, grade D; (b) no, grade D. S 73 Recommendation Implement permissive hypercapnia through reduced tidal volume ventilation in mechanically ventilated ALI/ARDS patients with high inspiratory pressures or otherwise at risk for barotrauma/volutrauma. Rationale The following specific subject keyword headings were used to answer this question: hypercapnia, artificial respiration, positive pressure respiration, adult respiratory distress syndrome. Permissive hypercapnia, the method of allowing pCO2 to rise while reducing tidal volume and minute ventilation to prevent alveolar overdistension or perpetuation of lung injury has been shown to be safe and effective at reducing mortality without adverse consequences in small nonrandomized series [75, 76, 77]. The upper limit for pCO2 has not been established, although arterial pH should be maintained at a level higher than 7.20. Based on these data, normalization of arterial blood gas values is not considered a valuable therapeutic maneuver. Does the use of (a) small tidal volume ventilation or (b) pressure limited ventilation strategies affect outcome in ALI related to sepsis? Answer: (a) yes, grade A; (b) uncertain, grade A. Recommendation Mechanical ventilation of patients with ALI should be conducted with small tidal volumes (approximately 6 ml/kg ideal body weight) with the goal to maintain end-inspiratory plateau pressures at levels less than 30 cmH2O. Rationale The following specific subject heading keywords were used to answer this question: tidal volume, lung compliance, positive pressure respiration, intrinsic positive pressure respiration, mechanical ventilator, adult respiratory distress syndrome. Well-designed large scale randomized trials designed to alter inspiratory pressure through variations in tidal volume have been conducted, with varying results [137, 138, 139, 140]. It is not completely understood why the results of these well-designed trials conflict, although the intergroup differential in airway pressure is a likely contributor. In a recent trial in the United States, absolute all-cause mortality was reduced by 10 % in ALI patients receiving mechanical ventilation with tidal volumes of 6 ml/kg ideal body weight [74]. This topic has also been recently reviewed by an international expert consensus conference [141]. Does prone positioning affect (a) gas exchange or (b) outcome in sepsis-related ALI? Answer: (a) yes, grade C; (b) uncertain, grade C. Recommendation Prone positioning may be considered in patients requiring high levels of inspired oxygen (FIO2 > 0.60) in whom positional changes are not contraindicated, and who are cared for at facilities experienced in the management of critically ill mechanically ventilated patients. Rationale The following specific subject heading keywords were used to answer this question: prone position and supine position. Although the evidence for prone positioning must be graded C because of the small size of randomized trials, strong data exists to confirm the physiological benefits of this intervention. Recent studies have made it clear that prone positioning of patients with ALI/ARDS results in improvements in oxygenation in approximately 65 % of patients (ªrespondersº) [68, 69, 70]. In addition, the improvements in gas exchange may persist up to 18 h, even after returning to the supine position, and such changes in position may be accomplished safely in intensive care units accustomed to managing critically ill mechanically ventilated patients. Because of the limited size of trials to date, no definitive comments can be made on the general applicability of these maneuvers to all patient care centers or their effect on overall mortality. Does inhaled nitric oxide affect (a) oxygenation or (b) outcome in ALI/ARDS? Answer: (a) yes, grade A; (b) no, grade A. Recommendation Restrict nitric oxide as an option for salvage therapy in patients with life-threatening hypoxemia not responding to traditional mechanical ventilation strategies or for evaluation in controlled clinical trials. S 74 Rationale The following specific subject heading keywords were used to answer this question: oxygen, nitric oxide, inhalation administration, and pulmonary gas exchange. Inhaled nitric oxide has been studied extensively in both preclinical models of lung injury and clinical trials of patients with ALI/ARDS. It has been shown to lower pulmonary artery pressures and improve right ventricular function in patients with pulmonary hypertension. Inhaled nitric oxide improves oxygenation and may reduce edema formation in patients with ALI/ARDS through effects on hydrostatic pressure. Unfortunately, it has not been found to significantly affect mortality [104, 105, 106]. These data support the observation that inhaled nitric oxide consistently improves pulmonary physiology in a large proportion of these patients, but fails to affect outcome. Is there a defined fluid management strategy in sepsisrelated ALI/ARDS? Answer: uncertain, grade C. Recommendation Judicious use of crystalloid fluid administration should be practiced in patients with ALI/ARDS, with colloid solutions considered in hypo-oncotic patients with established ALI/ARDS. It is not clear if volume restriction improves outcome. Rationale The following specific subject heading keywords were used to answer this question: fluid therapy, resuscitation, diuresis, intravenous infusions, hypertonic saline solution, sodium chloride, colloids, plasma substitutes, hetastarch, dextrans. Optimal fluid management has been considered a critical question in patients with sepsis and ALI/ARDS since these syndromes were first described. Conflicting data exist regarding the relative benefits of crystalloid and colloid administration in these patient populations despite years of research. Use of colloids in this patient population has been advocated and debated for decades, with evidence for potential benefit appearing only recently. Similarly, only recently has fluid balance been evaluated independently with respect to its contribution to overall morbidity and mortality [59]. Prospective, randomized trials have been conducted which support improved clinical outcomes based on direct manipulation of fluid balance variables in this critically ill patient population [60]. The details of the most appropriate intravenous solution and volume of administration requires large-scale investigation. Are corticosteroids indicated in the (a) prevention, (b) early treatment (exudative phase), or (c) late treatment (fibroproliferative phase) of ARDS? Answer: (a) no, grade A; (b) no, grade A; (c) uncertain, grade C. Recommendation Do not routinely administer corticosteroids to patients at risk for, or meeting current criteria for, ALI/ARDS. Consider intravenous methylprednisolone in patients with persistent or refractory ARDS after actively excluding infection, pending the results of ongoing trials. Rationale The following specific subject heading keywords were used to answer this question: steroids, adrenal cortex hormones, prednisone, methylprednisolone, hydrocortisone, dexamethasone. Corticosteroids have long been considered part of an appropriate treatment plan for patients with lung injury. There have been well-designed trials that fail to demonstrate any significant benefit for corticosteroids in the prevention or early treatment of ARDS [82, 83, 84]. A recent resurgence of interest has been generated by small trials suggesting benefit in the subpopulation of patients failing to progress in the late phase of ARDS (Late Steroids Rescue Study, http:// hedwig.mgh.harvard.edu/ardsnet/ards02.html). In an attempt to answer this pressing question, the National Institutes of Health have sponsored a large-scale trial in the United States randomizing patients with ARDS for more than 7 days to methylprednisolone therapy [85]. In conducting all of these trials, close attention was paid to excluding infection before or during corticosteroid therapy. Until definitive trials have been completed, a clear recommendation cannot be made regarding corticosteroid administration in patients with persistent ALI/ARDS. Do daily spontaneous breathing trials or weaning protocols reduce the duration of mechanical ventilation? Answer: yes, grade A. S 75 Recommendation It is recommended that all patients requiring acceptable levels of ventilatory support who are not overtly unstable should receive a spontaneous breathing trial on a daily basis to determine ability to breathe unassisted. Rationale The following specific subject heading keywords were used to answer this question: weaning, ventilator weaning, artificial respiration, and mechanical ventilator. The last 10 years has seen a surge in interest in determining the optimum method of discontinuing mechanical ventilation [142, 143, 144]. Recent large-scale trials have been conducted to demonstrate the benefits of daily trials of spontaneous breathing in reducing the duration of mechanical ventilation [78, 145, 146]. Identifying patients capable of breathing spontaneously requires a two-step process: a brief screen and a trial of spontaneous breathing. The screening procedure is designed to exclude patients requiring excessive levels of mechanical ventilatory support. Thus the following criteria may be employed to identify patients ready to accept a trial of spontaneous breathing: FIO2 < 0.50, PEEP 5 cmH2O, intact airway reflexes, hemodynamic stability and adequate mental status. The definition of spontaneous breathing trial is still a subject of debate, with both ªT-pieceº breathing and flow-triggered ventilation with continuous positive airway pressure of 5 cmH2O currently being acceptable methods of achieving ªspontaneous breathing.º Patients who tolerate such a breathing trial for 2 h have an approximately 85 % success rate with complete discontinuation of mechanical ventilation. Conclusion Despite significant advances in both the knowledge of sepsis-related respiratory failure and the care of critically ill patients, ALI/ARDS continues to be a complex problem with high mortality. The recommendations above represent the current state of knowledge for this condition, but equally serve to highlight the vast deficiencies of knowledge that remain. To provide our patients with the best possible outcome, a continued focus on physiological, therapeutic, and outcomes research is necessary. Acknowledgements This research was supported in part by the National Institutes of Health (NHLBI HL 07123). References 1. Hazinski MF, Iberti TJ, MacIntyre NR, et al (1993) Epidemiology, pathophysiology and clinical presentation of gram-negative sepsis. Am J Crit Care 2: 224±235 2. Anonymous (1990) Current trends increase in national hospital discharge survey rates for septicemia ± United States, 1979±1987. MMWR Morb Mort Wkly Rep 39: 31±34 3. Fowler AA, Hamman RF, Good JT, et al (1983) Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 98: 593±597 4. Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ (1982) Clinical predictors of the adult respiratory distress syndrome. Am J Surg 144: 124±130 5. Montgomery AB, Stager MA, Carrico CJ, Hudson LD (1985) Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 132: 485±489 6. Bernard GR, Artigas A, Brigham KL, et al (1994) The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818±824 7. Kollef MH, Schuster DP (1995) The acute respiratory distress syndrome. N Engl J Med 332: 27±37 8. National Heart and Lung Institute, National Institutes of Health (1972) Respiratory distress syndromes: Task Force on Problems, Research Approaches, Needs. US Government Printing Office, Washington. DHEW Publication No (NIH) 73±432, pp 165±180 9. Thomsen GE, Morris AH (1995) Incidence of the adult respiratory distress syndrome in the State of Utah. Am J Respir Crit Care Med 152: 965±971 10. Milberg JA, Davis DR, Steinberg KP, Hudson LD (1995) Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983±1993. JAMA 273: 306±309 11. Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA (1995) Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152: 1818±1824 12. Vasilyev S, Schaap RN, Mortensen JD (1995) Hospital survival rates of patients with acute respiratory failure in modern respiratory intensive care units: an international, multi-center, prospective survey. Chest 107: 1083±1088 13. Artigas A, Bernard GR, Carlet J, et al (1998) The American-European Consensus Conference on ARDS. II. Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Am J Respir Crit Care Med 157: 1332±1347 14. Demling RH (1995) The modern version of the adult respiratory distress syndrome. Annu Rev Med 46: 193±202 15. Steinberg KP, Milberg JA, Martin TR, et al (1988) Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am Rev Respir Dis 138: 720±723 S 76 16. Rinaldo JE, Christman JW (1990) Mechanisms and mediators of the adult respiratory distress syndrome. Clin Chest Med 11: 621±632 17. Marks JD, Marks CB, Luce JM, et al (1990) Plasma tumor necrosis factor in patients with septic shock: Mortality rate & incidence of adult respiratory distress syndrome. Am Rev Respir Dis 141: 94±97 18. Meduri GU, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A (1995) Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest 108 1315±1325 19. Calandra T, Baumgartner JD, Grau GE, et al (1990) Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon alpha, and interferon gamma in the serum of patients with septic shock. J Infect Dis 161: 982±987 20. Roumen RMH, Hendriks T, van der Ven-Jongekrijg J, et al (1993) Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma: relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg 218: 769±776 21. Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM (1997) Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med 155: 479±484 22. Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JM (1996) Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med 24: 241±246 23. Carpenter CT, Price PV, Christman BW (1998) Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 114: 1653±1659 24. Christman BW, Bernard GR (1993) Antilipid mediator and antioxidant therapy in adult respiratory distress syndrome. New Horiz 1: 623±630 25. Quinlan GJ, Margarson MP, Mumby S, Evans TW, Gutteridge JM (1998) Administration of albumin to patients with sepsis syndrome: a possible beneficial role in plasma thiol repletion. Clin Sci (Colch) 95: 4:459±465 26. Dong W, Simeonova PP, Gallucci R, et al (1998) Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol Appl Pharmacol 151: 359±366 27. Bulger EM, Helton WS, Clinton CM, Roque RP, Garcia I, Maier RV (1997) Enteral vitamin E supplementation inhibits the cytokine response to endotoxin. Arch Surg 132: 1337±1341 28. Tsan M, Danis E, Del Vechhio PJ, Rosano CL (1985) Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun 127: 270±276 29. Barcellos-Hoff MA, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077±1083 30. Neuschwander-Tetri BA, Bellezzo JM, Britton RS, Bacon BR, Fox ES (1996) Thiol regulation of endotoxin-induced release of tumor necrosis factor alpha from isolated rat Kupffer cells. Biochem J 320: 1005±1010 31. Lin M, Rippe RA, Niemela O, Brittenham G, Tsukamoto H (1997) Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol 272:G1355±G1364 32. Loban A, Kime R, Powers H (1997) Iron-binding antioxidant potential of plasma albumin. Clin Sci (Colch) 93: 445±451 33. Gattinoni L, Pesenti A, Bombino M, et al (1988) Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology 69: 824±832 34. Slutsky AS (1993) Mechanical ventilation: American College of Chest Physicians' Consensus Conference. Chest 104: 1833±1859 35. Tsuno K, Prato P, Kolobow T (1990) Acute lung injury from mechanical ventilation at moderately high airway pressures. J Appl Physiol 69: 956±961 36. Wright PE, Bernard GR (1989) The role of airflow resistance in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 139: 1169±1174 37. Jones R, Reid LM, Zapol WM, et al (1992) Pulmonary vascular pathology: human and experimental studies. In: Falke KJ (ed) Lung biology in health and disease. Philadelphia, Saunders 38. Meyrick B (1986) Pathology of the adult respiratory distress syndrome. Crit Care Clin 2: 405±428 39. Tomashefski JF Jr (1990) Pulmonary pathology of the adult respiratory distress syndrome. Clin Chest Med 11: 593±620 40. McDonald JA (1991) Idiopathic pulmonary fibrosis: A paradigm for lung injury and repair. Chest 99 [Suppl]:87±93 41. Bernard GR, Wheeler AP, Russell JA, et al (1997) The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med 336: 912±918 42. Wheeler AP, Carroll FE, Bernard GR (1993) Radiographic issues in the adult respiratory distress syndrome. New Horiz 1: 471±477 43. Gammon RB, Shin MS, Buchalter SE (1992) Pulmonary barotrauma in mechanical ventilation: patterns and risk factors. Chest 102: 568±572 44. Gammon RB, Shin MS, Groves RH, Hardin M, Hsu C, Buchalter SE (1995) Clinical risk factors for pulmonary barotrauma: a multivariate analysis. Am J Respir Crit Care Med 152: 1235±1240 45. Ranieri VM, Suter PM, Tortorella C, et al (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54±61 46. Schuster DP (1992) Predicting outcome after ICU admission. The art and science of assessing risk. Chest 102: 1861±1870 47. Murray JF, Matthay MA, Luce JM, et al (1988) An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 138: 720±723 48. Bursten SL, Federighi DA, Parsons P, et al (1996) An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Crit Care Med 24: 1129±1136 49. Matthay MA, Wiener-Kronish JP (1990) Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250±1257 50. Sloane PJ, Gee MH, Gottlieb JE, et al (1992) A multicenter registry of patients with acute respiratory distress syndrome: physiology and outcome. Am Rev Respir Dis 146: 419±426 51. McHugh LG, Milberg JA, Whitcomb ME, et al (1994) Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med 150: 90±94 S 77 52. Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP (1999) Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 281: 354±360 53. Alsous F, Amoateng-Adjepong Y, Manthous CA (1999) Noninvasive ventilation: experience at a community teaching hospital. Intensive Care Med 25: 458±463 54. Antonelli M, Conti G, Rocco M, et al (1998) A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 339: 429±435 55. Meduri GU, Turner RE, Abou-Shala N, Wunderink R, Tolley E (1996) Noninvasive positive pressure ventilation via face mask: first-line intervention in patients with acute hypercapnic and hypoxemic respiratory failure. Chest 109: 179±193 56. Patrick W, Webster K, Ludwig L, Roberts D, Wiebe P, Younes M (1996) Noninvasive positive-pressure ventilation in acute respiratory distress without prior chronic respiratory failure. Am J Respir Crit Care Med 153: 1005±1011 57. Schuster DP (1993) The case for and against fluid restriction and occlusion pressure reduction in adult respiratory distress syndrome. New Horiz 1: 478±488 58. Simmons RS, Berdine GG, Seidenfeld JJ, et al (1987) Fluid balance and the adult respiratory distress syndrome. Am Rev Respir Dis 135: 924±929 59. Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L (1990) Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest 97: 1176±1180 60. Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145: 990±998 61. Martin GS, Mangialardi RJ, Wheeler AP, Bernard GR (1999) Albumin and diuretics in ARDS. Am J Respir Crit Care Med 159:A376 62. Salord F, Gaussorgues P, Marti-Flich J, et al (1990) Nosocomial maxillary sinusitis during mechanical ventilation: a prospective comparison of orotracheal versus the nasotracheal route for intubation. Intensive Care Med 16: 390±393 63. Holzapfel L, Chevret S, Madinier G, et al (1993) Influence of long-term oroor nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med 21: 1132±1138 64. Holzapfel L, Chastang C, Demingeon G, Bohe J, Piralla B, Coupry A (1999) A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med 159: 695±701 65. Cook D, De Jonghe B, Brochard L, Brun-Buisson C (1998) Influence of airway management on ventilator-associated pneumonia: evidence from randomized trials. JAMA 279: 781±787 66. Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C (1999) The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med 159: 1249±1256 67. Amato MB, Barbas CS, Medeiros DM, et al (1995) Beneficial effects of the ªopen lung approachº with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med 152: 1835±1846 68. Stocker R, Neff T, Stein S, Ecknauer E, Trentz O, Russi E (1997) Prone positioning and low-volume pressurelimited ventilation improve survival in patients with severe ARDS. Chest 111: 1008±1017 69. Lamm WJ, Graham MM, Albert RK (1994) Mechanism by which prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med 150: 184±193 70. Jolliet P, Bulpa P, Chevrolet JC (1998) Effects of the prone position on gas exchange and hemodynamics in severe acute respiratory distress syndrome. Crit Care Med 26: 1977±1985 71. Hickling KG, Walsh J, Henderson S, Jackson R (1994) Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22: 1568±1578 72. Bidani A, Tzouanakis AE, Cardenas VJ, Zwischenberger JB (1994) Permissive hypercapnia in acute respiratory failure. JAMA 272: 957±962 73. Thorens JB, Jolliet P, Ritz M, Chevrolet JC (1996) Effects of rapid permissive hypercapnia on hemodynamics, gas exchange, and oxygen transport and consumption during mechanical ventilation for the acute respiratory distress syndrome. Intensive Care Med 22: 182±191 74. Anonymous (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301±1308 75. Hickling KG, Walsh J, Henderson S, Jackson R (1994) Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22: 1568±1578 76. Bidani A, Tzouanakis AE, Cardenas VJ, Zwischenberger JB (1994) Permissive hypercapnia in acute respiratory failure. JAMA 272: 957±962 77. Ely EW, Baker AM, Dunagan DP, et al (1996) Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 335: 1864±1869 78. Vallverdu I, Calaf N, Subirana M, Net A, Benito S, Mancebo J (1998) Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158: 1855±1862 79. Chatila W, Jacob B, Guaglionone D, Manthous CA (1996) The unassisted respiratory rate-tidal volume ratio accurately predicts weaning outcome. Am J Med 101: 61±67 80. Esteban A, Alia I, Tobin MJ, et al (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med 159: 512±518 81. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 317: 653±658 82. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA (1987) Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 92: 1032±1036 S 78 83. Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF (1988) Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 138: 62±68 84. Meduri GU, Headley AS, Golden E, et al (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 280: 159±165 85. Yu M, Tomasa G (1993) A doubleblind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med 21: 1635±1642 86. Anonymous (2000) Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA 283: 1995±2002 87. Abraham E, Park YC, Covington P, et al (1996) Liposomal prostaglandin E1 in acute respiratory distress syndrome: a placebo-controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med 24: 10±15 88. Bone RC, Slotman G, Maunder R, et al (1989) Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Prostaglandin E1 Study Group. Chest 96: 114±119 89. Holcroft JW, Vassar MJ, Weber CJ (1986) Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome: a prospective trial. Ann Surg 203: 371±378 90. Meyer J, Theilmeier G, Van Aken H, et al (1998) Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg 86: 4:753±758 91. Walmrath D, Schneider T, Pilch J, et al (1993) Aerosolized prostacyclin in adult respiratory distress syndrome. Lancet 342: 961±962 92. Zwissler B, Kemming G, Habler O, et al (1996) Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med 154: 1671±1677 93. Hayes MA, Timmins AC, Yau EHS, et al (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330: 1717±1722 94. Gattinoni L, Brazzi L, Pelosi P, et al (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med 333: 1025±1032 95. Bernard GR, Wheeler AP, Arons MM, et al (1997) A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 112: 164±172 96. Bernard GR, Wheeler AP, Naum CC, et al (1999) A placebo controlled randomized trial of IL-10 in acute lung injury. Chest 116: 260 S 97. Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J (1999) Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 159: 1241±1248 98. Carlon GC, Howland WS, Ray C, et al (1983) High-frequency jet ventilation: a prospective randomized evaluation. Chest 84: 551±559 99. Holzapfel L, Robert D, Perrin F, et al (1987) Comparison of high-frequency jet ventilation to conventional ventilation in adults with respiratory distress syndrome. Intensive Care Med 13: 100±105 100. Macintyre NR, Follett JV, Deitz JL, et al (1986) Jet ventilation at 100 breaths per minute in adult respiratory failure. Am Rev Respir Dis 134: 897±901 101. Borg UR, Stoklosa JC, Siegel JH, et al (1989) Prospective evaluation of combined high-frequency ventilation in post-traumatic patients with adult respiratory distress syndrome refractory to optimized conventional ventilatory management. Crit Care Med 17: 1129±1142 102. Hurst JM, Branson RD, Davis K, et al (1990) Comparison of conventional mechanical ventilation and high-frequency ventilation: a prospective randomized trial in patients with respiratory failure. Ann Surg 211: 486±491 103. Rossaint R, Gerlach H, SchmidtRuhnke H, et al (1995) Efficacy of inhaled nitric oxide in patients with severe ARDS. Chest 107: 1107±1115 104. Troncy E, Collet J-P, Shapiro S, et al (1997) Should we treat acute respiratory distress syndrome with inhaled nitric oxide? Lancet 350: 111±112 105. Dellinger RP, Zimmerman JL, Taylor RW, et al (1998) Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med 26: 15±23 106. Gallart L, Lu Q, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ (1998) Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med 1998 158: 1770±1777 107. Zapol WM, Snider MT, Hill JD, et al (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. JAMA 242: 2193±2196 108. Morris AH, Wallace J, Menlove RL, et al (1994) Randomized clinical trial of pressure controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149: 295±305 109. Brunet F, Belghith M, Mira JP, et al (1993) Extracorporeal carbon dioxide removal and low-frequency positivepressure ventilation. Improvement in arterial oxygenation with reduction of risk of pulmonary barotrauma in patients with adult respiratory distress syndrome. Chest 104: 889±898 110. Gattinoni L, Pesenti A, Mascheroni D, et al (1986) Low frequency positive pressure ventilation with extracorporeal CO2 removal In severe acute respiratory failure. JAMA 256: 881±886 111. Hirschl RB, Tooley R, Parent A, Johnson K, Bartlett RH (1996) Evaluation of gas exchange, pulmonary compliance, and lung injury during total and partial liquid ventilation in the acute respiratory distress syndrome. Crit Care Med 24: 1001±1008 112. Hirschl RB, Pranikoff T, Wise C, et al (1996) Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA 275: 383±389 113. Bartlett RH, Croce M, Hirschl RH, et al (1997) A phase II randomized, controlled trial of partial liquid ventilation (PLV) in adult patients with acute hypoxemic respiratory failure. Crit Care Med 25 [Suppl]:A35 114. Anzueto A, Baughman RP, Guntupalli KK, et al (1996) Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med 334: 1417±1421 115. Brigham KL, Stecenko AA (1995) Gene therapy in acute critical illness. New Horiz 3: 321±329 116. Bhattacharya J (2000) Gene therapy for pulmonary edema. Am J Respir Cell Mol Biol 22: 640±641 S 79 117. Wright PE, Carmichael LC, Bernard GR (1994) Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS) Chest 106: 1517±1523 118. Torres A, Serra-Batlles J, Ros E, et al (1992) Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med 116: 540±543 119. Ferrell BA, Osterweil D, Christenson P (1993) A randomized trial of lowair-loss beds for treatment of pressure ulcers. JAMA 269: 494±497 120. Gadek JE, DeMichele SJ, Karlstad MD, et al (1999) Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med 27: 1409±1420 121. Laporta D, Kleiman S, Begin L, et al (1993) Traumatic perforation of the cervical esophagus: A complication of endotracheal intubation. Intensive Care Med 19: 59±60 122. Keiser GJ, Bozentka NE, Gold BD (1991) Laryngeal granuloma: a complication of prolonged endotracheal intubation. Anesth Prog 38: 232±234 123. Demling RH, Read T, Lind TJ, et al (1988) Incidence and morbidity of extubation failure in surgical intensive care patients. Crit Care Med 16: 573±577 124. Marini JJ, Ravenscraft S (1992) Mean airway pressure: physiologic determinants and clinical importance. I. Physiologic determinants and measurements. Crit Care Med 20: 1461±1472 125. Gattinoni L, Marcolin R, Caspani ML, et al (1985) Constant mean airway pressure with different patterns of positive pressure breathing during the adult respiratory distress syndrome. Bull Eur Physiopathol Respir 21: 275±279 126. Pesenti A, Marcolin R, Prato P, et al (1985) Mean airway pressure vs positive end-expiratory pressure during mechanical ventilation. Crit Care Med 13: 34±37 127. Venus B, Jacobs HK, Lim L (1979) Treatment of the adult respiratory distress syndrome with continuous positive airway pressure. Chest 76: 257±261 128. Smith RA, Kirby RR, Gooding JM, Civetta JM (1980) Continuous positive airway pressure (CPAP) by face mask. Crit Care Med 8: 483±485 129. Rasanen J, Heikkila J, Downs J, Nikki P, Vaisanen I, Viitanen A (1985) Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol 55: 296±300 130. Smatlak P, Knebel AR (1998) Clinical evaluation of noninvasive monitoring of oxygen saturation in critically ill patients. Am J Crit Care 7: 370±373 131. Petty TL (1990) A historical perspective of mechanical ventilation. In: Tobin MJ (ed) Critical care clinics: mechanical ventilation. Saunders, Philadelphia, pp 489±504 132. Eidelman LA, Jakobson DJ, Pizov R, Geber D, Leibovitz L, Sprung CL (1998) Foregoing life-sustaining treatment in an Israeli ICU. Intensive Care Med 24: 162±166 133. Cook DJ, Walter SD, Cook RJ, et al (1998) Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 129: 433±440 134. Cook DJ, De Jonghe B, Brochard L, Brun-Buisson C (1998) Influence of airway management on ventilator-associated pneumonia: evidence from randomized trials. JAMA 279: 781±787 135. Kollef MH (1999) The prevention of ventilator-associated pneumonia. N Engl J Med 340: 627±634 136. Amato MBP, Barbas CS, Medeiros DM, et al (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347±354 137. Stewart TE, Meade MO, Cook DJ, et al (1998) Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 338: 355±361 138. Brochard L, Roudot-Thoraval F, Roupie E, et al (1998) Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med 158: 1831±1838 139. Brower RG, Shanholtz CB, Fessler HE, et al (1999) Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med 27 8: 1661±1663 140. Anonymous (1999) International Consensus Conferences in Intensive Care Medicine: ventilator-associated lung injury in ARDS. Am J Respir Crit Care Med 160: 2118±2124 141. Esteban A, Frutos F, Tobin MJ, et al (1995) A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med 332: 345±350 142. Brochard L, Rauss A, Benito S, et al (1994) Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 150: 896±903 143. Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324: 1445±1450 144. Kollef MH, Shapiro SD, Silver P, et al (1997) A randomized, controlled trial of protocol-directed versus physiciandirected weaning from mechanical ventilation. Crit Care Med 25: 567±574 145. Esteban A, Alia I, Gordo F, et al (1997) Extubation outcome after spontaneous breathing trials with Ttube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 156 (2 Pt 1):459±465 146. Esteban A, Alia I, Tobin MJ, et al (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 159: 512±518 Intensive Care Med (2001) 27: S 80±S 92 Jean-Louis Vincent Hemodynamic support in septic shock ) J.-L. Vincent ( ) Department of Intensive Care, Erasme University Hospital, Brussels, Belgium E-mail: [email protected] Phone: +32-2-5 55 33 80 Fax: +32-2-5 55 45 55 Introduction Shock, defined as an imbalance between oxygen demand and oxygen supply, results in alterations in tissue perfusion, with reduction in delivery of oxygen and other nutrients to tissues, causing cellular, and then organ, dysfunction. The ultimate goals of hemodynamic therapy in shock are to restore effective tissue perfusion and to normalize cellular metabolism. In hypovolemic, cardiogenic, and obstructive shock, hypotension occurs as the result of a decrease in cardiac output, with consequent anaerobic tissue metabolism. Septic shock, however, typically results from distributive alterations, so that alterations in tissue perfusion result from abnormal control of the microvasculature with abnormal distribution of a normal or increased cardiac output. Cellular alterations in sepsis also result from the important inflammatory response, with the involvement of many mediators, including nitric oxide. Hence the endpoints of therapy are much more difficult to define with certainty than in other forms of shock in which a reduction in blood flow is the dominant problem. The complex nature of the pathophysiology of sepsis has led to considerable confusion and controversy regarding optimal patient management. Nevertheless, it is possible to develop a basic approach to the hemodynamic support of sepsis, which will almost certainly change as our understanding of sepsis improves further. Methods This review of the literature enables recommendations to be established and graded according to the strength of the available evidence. Altered tissue perfusion in septic shock Septic shock is characterized by hypotension, which in adults generally refers to a mean arterial pressure below 65±70 mmHg. Hypotension is usually accompanied by signs of altered tissue perfusion, for example, oliguria, reduced capillary refill, and altered sensorium. Some caution is necessary in interpreting these signs in septic patients, however, since signs of peripheral vasoconstriction may be remarkably absent; even oliguria is not always present. The adequacy of regional perfusion is usually assessed clinically by evaluating indices of organ function, although none of these parameters alone has been validated as a reliable indicator of adequate resuscitation. These parameters include: coagulation abnormalities (disseminated intravascular coagulation); altered renal function with increased blood urea nitrogen and creatinine; central nervous system dysfunction indicated by a clouded sensorium; altered liver parenchymal function with increased serum levels of transaminases, lactic dehydrogenase, and bilirubin; and altered gut perfusion, manifest by ileus and malabsorption. Mixed venous oxygen saturation Mixed venous oxygen saturation (SvO2) can be measured in patients with a Swan-Ganz catheter in place. SvO2 is dependent on cardiac output, oxygen demand, hemoglobin, and arterial oxygen saturation. The normal SvO2 value is 70±75 % in critically ill patients but can be elevated in septic patients due to maldistribution of S 81 blood flow. Nevertheless, it is useful to measure SvO2 because if cardiac output becomes inadequate, SvO2 decreases. Ronco and colleagues [1] studied terminally ill patients in whom treatment was withdrawn; SvO2 decreased dramatically before oxygen consumption started to fall, indicating that oxygen extraction capabilities are not necessarily profoundly altered even in patients with terminal stages of the disease process. Hence SvO2, if normal or high does not necessarily indicate that everything is alright, while a low SvO2 should prompt rapid intervention to increase oxygen delivery to the tissues. Blood lactate levels Hyperlactatemia (> 2 mEq/l) is typically present and may be secondary to anaerobic metabolism due to hypoperfusion. However, the interpretation of blood lactate levels in septic patients is not always straightforward. Experimental studies have not always been able to show a reduction in high-energy phosphate levels in animal models of sepsis [2]. The differences between studies may be related to the severity of the septic model, with more severe sepsis being associated with depletion of ATP despite maintenance of systemic oxygen delivery and tissue oxygenation. Also, measurements of tissue PO2 in septic patients have not demonstrated tissue hypoxia in the presence of lactic acidosis [3]. However, if inhomogeneity in blood flow distribution is a real phenomenon, it is likely that cell hypoperfusion also exists with ischemia/reperfusion. A number of studies have suggested that elevated lactate levels may result from cellular metabolic failure rather than from global hypoperfusion in sepsis. Some organs may produce more lactate than others, in particular, the lungs in acute lung injury or acute respiratory distress syndrome [4, 5]. Elevated lactate levels can also result from decreased clearance by the liver. Nonetheless, the prognostic value of raised blood lactate levels has been well established in septic shock patients [6], particularly if the high levels persist [7, 8]. It is also of interest to note that blood lactate levels are of greater prognostic value than oxygenderived variables [9]. the serosa and muscularis [10], resulting in mucosal hypoxia. Any further reduction in splanchnic flow has a correspondingly greater effect on gut hypoxia. Second, the gut may have a higher critical oxygen delivery threshold than other organs [11]. Third, the tip of the villus is supplied by a central arteriole and drained by venules passing away from the tip. A countercurrent exchange mechanism operates in the villus, whereby a base to tip PO2 gradient exists, making the tip particularly sensitive to changes in regional flow and oxygenation. Fourth, constriction of the villus arteriole occurs during sepsis [12], rendering the villus even more sensitive to reductions in blood flow. Fifth, the capillary density at the villus tip is reduced during sepsis [13], impeding the transfer of oxygen. Finally, gut ischemia increases intestinal permeability, which may increase bacterial translocation, a suggested trigger or ªmotorº of the sepsis response and multiple organ failure. Gastric tonometry has been proposed as a method to assess regional perfusion in the gut by measuring DPCO2. Calculations of gastric intramucosal pH (pHi) have become obsolete as bicarbonate is global, nonspecific, and measured only intermittently. For these reasons gastric mucosal PCO2 may be more accurate than pHi because this measure is not confounded by arterial bicarbonate. Gastric mucosal PCO2 is influenced directly by systemic arterial PCO2, and some clinicians have proposed using the gastric-arterial PCO2 difference as the primary tonometric variable of interest [14]. Even this measure is not a simple measure of gastric mucosal hypoxia because either anaerobic metabolism decreased gastric blood flow in the absence of anaerobic metabolism, or a combination of the two can increase gastric mucosal PCO2 [14]. An early trial suggested that tonometry derived parameters may be useful in guiding therapy [15], but these findings were not confirmed recently [16], and many investigators have emphasized the limited sensitivity, and especially specificity, of these measurements. Various vasoactive agents have been shown to have divergent effects on PgCO2 and pHi that are neither consistent nor predictable [17]. In summary, each endpoint parameter should be considered in the appropriate context, and the combination of clinical parameters (mean arterial pressure, urine flow, skin perfusion, level of consciousness) with blood lactate levels is most useful. Gut tonometry The measurement of regional perfusion as a means of detecting inadequate tissue oxygenation has focused on the splanchnic circulation, as the hepatosplanchnic circulation is particularly sensitive to changes in blood flow and oxygenation for several reasons. First, under normal conditions the gut mucosa receives the majority of total intestinal blood flow. However, in sepsis there is a redistribution of flow away from the mucosa toward Fluid resuscitation in septic shock What is the endpoint of fluid resuscitation in septic shock? Answer: (a) adequate tissue perfusion, grade E. S 82 Recommendation The goal of fluid resuscitation in septic shock is restoration of tissue perfusion and normalization of cellular metabolism. Does fluid resuscitation increase cardiac output in septic shock patients? Can the use of pulmonary artery catheter guided therapy improve outcome from septic shock? Answer: uncertain, grade D. Recommendation Answer: yes, grade C. When central venous pressure increases, a pulmonary artery catheter is probably required, although its role has recently been questioned [24]. Recommendation Rationale Volume repletion in patients with septic shock produces significant increases in cardiac output and systemic oxygen delivery [18, 19], and fluids alone are sometimes sufficient to reverse hypotension and restore hemodynamic stability [20]. When pulmonary artery catheter measurements of cardiac output and SvO2 are available, filling pressures should be increased to a level associated with maximal cardiac output. In most patients with septic shock quite high pulmonary artery occluded pressures are required, despite the risk of pulmonary edema. Should fluid infusion be the initial step in the cardiovascular support of septic shock patients? Answer: yes, grade D. Recommendation Requirements for fluid infusion are not easily determined, and therefore the fluid challenge should be titrated to the clinical endpoints of blood pressure, heart rate, and urine output. Central venous pressure is initially required to evaluate the complex relationship between intravascular blood volume and cardiac function. It is difficult to give optimal values for cardiac filling pressures. Rationale Septic shock can be associated with either absolute or relative hypovolemia. Large fluid deficits can exist, as a consequence of external (e.g., diarrhea, sweating) or internal (e.g., peritonitis) losses. Relative hypovolemia is related to the maldistributive defect with vasodilation and peripheral blood pooling. The initial phases of experimental and clinical septic shock present as a low cardiac output syndrome with low filling pressures. Failure to appreciate the degree of underlying hypovolemia may result in a low cardiac output. The hyperdynamic state is apparent only after volume repletion [21, 22]. Increased blood and plasma volumes are associated with increased cardiac output and enhanced survival from septic shock [23]. Choice of fluid Patients with septic shock can be successfully resuscitated with crystalloid or colloid, although the choice of fluid continues to be a matter of debate, with colloids usually preferred in Europe, and crystalloids more widely used in North America. There are many different colloid solutions available including natural solutions (albumin, plasma protein fraction) and artificial solutions (gelatins, dextrans and hydroxyethyl starch). The most commonly used solutions are albumin and hetastarch. Is resuscitation with colloids or crystalloids associated with similar outcomes in septic shock? Answer: uncertain, grade C. Recommendation Increases in cardiac output and systemic oxygen delivery are proportional to the degree of intravascular volume expansion achieved. Rationale When crystalloids and colloids are titrated to the same level of filling pressure, they restore tissue perfusion to the same degree [25], but for the same effect, two to four times more volume of crystalloid is required than colloid, and slightly longer infusion periods may be nec- S 83 essary to achieve desired hemodynamic endpoints. Colloid solutions are, however, much more expensive than crystalloid solutions. Should albumin be avoided in resuscitation from septic shock? Answer: uncertain, grade C. Albumin is a naturally occurring plasma protein accounting for approximately 80 % of the plasma colloid osmotic pressure in normal subjects. In the presence of peripheral edema, mobilization of extravascular volume can be achieved by using hyperoncotic (20 or 25 %) albumin. Hydroxyethyl starch (HES, hetastarch) is a synthetic colloid available in a 6 % solution of normal saline. HES molecules may also affect endothelial cell function and reduce endothelial cell activation and injury [26], which could account in part for the preservation of microvascular structures with reduced leakage seen with HES in experimental sepsis models [27]. HES solutions can decrease factor VIII activity and prolong prothrombin time, so that the total amount of starch infused should be limited. The possibility that the long-term deposition of higher molecular weight hetastarch particles in the reticuloendothelial system could have immunosuppressive effects has caused some concern. However, in experimental studies, macrophage function and reticuloendothelial function were not altered by HES [28]. Gelatin solutions are cheaper but also less efficacious. They are not currently available in the United States, although they may soon become available there. Blood transfusion Can one recommend a minimum hemoglobin concentration in severe sepsis? Answer: yes, 7±8 g/dl, grade B. Can one recommend a minimum hemoglobin concentration in septic shock? Answer: uncertain, grade E. Recommendation The optimal hemoglobin and hematocrit for patients with septic shock is unclear. Most experts recommend hemoglobin levels of 9±10 gm/dl in patients with septic shock. This degree of anemia is usually well tolerated in most patients, even with cardiac impairment. Rationale Excessive tachycardia, very low SvO2, or electrocardiographic signs of myocardial ischemia may suggest the need for higher hemoglobin levels to be maintained. A large study by Hebert et al. [29] in a mixed group of ICU patients showed no benefit of transfusion to a hemoglobin level of 10 vs. 7 g/dl. Indeed, this trial found that use of the lower hemoglobin level trigger for transfusion, 7 g/dl, resulted in improved survival. When considering blood transfusion to improve oxygenation in critically ill patients, stored blood may be less effective than fresh blood [30]. Vasopressor therapy in septic shock Does adrenergic support improve outcome from septic shock? Answer: yes, grade E. Recommendation When fluid challenge fails to restore an adequate arterial pressure and organ perfusion, therapy with vasopressor agents should be started. Vasopressor therapy may also be required transiently to sustain life and maintain perfusion in the face of life-threatening hypotension, even when cardiac filling pressures are not elevated. Rationale In shock states the measurement of blood pressure using a cuff is often unreliable and inaccurate, and patients should have an arterial catheter in place for continuous blood pressure monitoring. This is even more the case in patients receiving vasopressor therapy for shock, as restoration of an adequate blood pressure is the required endpoint and measure of effectiveness. The precise level of mean arterial pressure to aim for is, however, not entirely certain and is likely to vary among patients. In animal studies a mean arterial pressure of less than 60 mmHg is associated with compromised autoregulation in the coronary, renal, and central nervous system vascular beds, and blood flow may be reduced. Some patients, however, especially the elderly, may require higher blood pressures to maintain adequate perfusion. Assessment of regional and global perfusion by a combination of the methods outlined previously is advisable. S 84 Effects of vasopressors on renal perfusion Although no prospective randomized studies have demonstrated a significant improvement in renal function with vasopressors, a number of open-label clinical series support an increase in renal perfusion pressure [31, 32, 33, 34, 35, 36, 37, 38, 39]. Excessive doses of vasopressors may shift the renal autoregulation curve to the right, necessitating a greater perfusion pressure for a specified renal blood flow. The precise target mean blood pressure level depends on the premorbid blood pressure but can be as high as 75 mmHg [31, 33, 34, 35, 36, 37, 38, 39]. However, individual levels should be kept at the minimum needed to reestablish urine flow, and in some patients this can be achieved with a mean arterial pressure of 60 or 65 mmHg. Certain patients may remain oliguric despite normalization of systemic hemodynamic variables [32, 33, 34, 37, 39]. This may be due to the absence of an increase in renal blood flow, a decrease in glomerular perfusion pressure, or irreversible ischemic renal lesions. Although in nonseptic conditions combination therapy with the use of low-dose dopamine (1±4 mg kg±1 min±1) in addition to norepinephrine in an anesthetized dog model and healthy volunteers resulted in significantly higher renal blood flow and lower renal vascular resistance [40, 41], such effects have not been conclusively demonstrated in septic shock, and there is no information available on the effects of such therapy on patient survival. blood flow, increases PgCO2 production, and decreases pHi, suggesting that the drug alters oxygen supply in the splanchnic circulation [42]. At low doses dopamine increases splanchnic oxygen delivery by 65 % but splanchnic oxygen consumption by only 16 %. Despite this, dopamine may decrease pHi, perhaps by a direct effect on the gastric mucosal cell. The effects of dopamine on cellular oxygen supply in the gut remain incompletely defined. The effects of norepinephrine on splanchnic circulation are hardly predictable. The combination of norepinephrine and dobutamine appears to be more predictable and more appropriate to the goals of septic shock therapy than the effects of epinephrine alone. Individual vasopressor agents Among adrenergic agents, are dopamine or norepinephrine the first line agents to correct hypotension in septic shock? Answer: yes, grade E. Should low-dose dopamine be routinely administered for renal protection? Answer: no, grade D. Dopamine Effects of vasopressors on the splanchnic circulation Is the combination of norepinephrine and dobutamine superior to dopamine in the treatment of septic shock? Answer: uncertain, grade C. Recommendation The effects of dopamine on cellular oxygen supply in the gut remain incompletely defined. The effects of norepinephrine on splanchnic circulation are hardly predictable. The combination of norepinephrine and dobutamine appears to be more predictable and more appropriate to the goals of septic shock therapy than the effects of epinephrine alone. Rationale Splanchnic perfusion and the integrity of the gut mucosa may play an important role in the pathogenesis of multiple organ failure. Epinephrine decreases splanchnic Recommendation The hemodynamic effects of dopamine in patients with septic shock are well established. Dopamine increases mean arterial pressure primarily by increasing cardiac index with minimal effects on systemic vascular resistance. The increase in cardiac index is due to an increase in stroke volume, and to a lesser extent, to increased heart rate [43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. Patients receiving dopamine at rates greater than 20 mg kg±1 min±1 show increases in right heart pressures as well as in heart rate, and therefore doses should not usually exceed 20 mg kg±1 min±1, at least not without adequate hemodynamic monitoring. Rationale Dopamine is the natural precursor of norepinephrine and epinephrine, and possesses several dose-dependent pharmacological effects. Generally, at doses less than 5 mg kg±1 min±1 dopamine stimulates dopaminergic DA1 receptors in the renal, mesenteric, and coronary beds, S 85 resulting in vasodilation. Infusion of low doses of dopamine causes an increase in glomerular filtration rate, renal blood flow, and sodium excretion. At doses of 5±10 mg kg±1 min±1, ª1-adrenergic effects become predominant, resulting in an increase in cardiac contractility and heart rate. Dopamine also causes the release of norepinephrine from nerve terminals, contributing to its cardiac effects. At higher doses (above 10 mg kg±1 min±1), ª1-adrenergic effects predominate, leading to arterial vasoconstriction and an increase in blood pressure. Dopamine's effect on gastric tonometric parameters has been evaluated with mixed results. Roukonen et al. [51] and Meier-Hellmann et al. [54] have documented that dopamine increases splanchnic blood flow. Neviere and colleagues [55] reported that dopamine is associated with a reduction in gastric mucosal blood flow; there were changes in gastric PCO2, gastric-arterial PCO2 difference, and calculated intramucosal pH. They [55] concluded that they could not determine whether the reduction in gastric mucosal blood flow was critical because there were no changes in the acid-base parameters of the patients. Recent studies have shown that dopamine may alter the inflammatory response in septic shock by decreasing the release of a number of hormones, including prolactin [56]. Norepinephrine Recommendation Summarizing the results of studies of norepinephrine, it can be concluded that norepinephrine markedly improves mean arterial pressure and glomerular filtration. This is particularly true in the high output-low resistance state of many septic shock patients. After restoration of systemic hemodynamics, urine flow reappears in most patients and renal function improves without the use of low-dose dopamine or furosemide. This fact supports the hypothesis that renal ischemia observed during hyperdynamic septic shock is not worsened by norepinephrine infusion and even suggests that this drug may effectively optimize renal blood flow and renal vascular resistance. Rationale Norepinephrine is a potent ª-adrenergic agonist with some ª-adrenergic agonist effects. The effects of norepinephrine have been studied in a number of studies on patients with septic shock. In open label trials norepinephrine has been shown to increase mean arterial pressure in patients with hypotension resistant to fluid resus- citation and dopamine. The potential that norepinephrine may have negative vasoconstrictive effects on regional vascular beds such as the liver and the kidney, with resultant regional ischemia, has meant that norepinephrine has commonly been reserved for use as a last resort, with predictably poor results. However, recent experience with the use of norepinephrine in patients with septic shock suggests that it can successfully increase blood pressure without causing the feared deterioration in organ function. Most studies have given septic patients fluid to correct hypovolemia before dopamine, with or without dobutamine, titrated to doses of 7±25 mg kg±1 min±1 to achieve the target blood pressure. Only if this regime failed was norepinephrine added [31, 32, 35, 39, 42, 51, 57, 58]. In older studies norepinephrine was added after the use of metaraminol, methoxamine, or isoproterenol [43, 59]. A few studies have used norepinephrine as the only adrenergic agent to correct sepsis-induced hemodynamic abnormalities [34, 37, 50, 51, 53]. In most studies the mean dose of norepinephrine was 0.2±1.3 mg kg±1 min±1, although the initial dose can be as low as 0.01 mg kg±1 min±1 [32], and the highest reported norepinephrine dose was 3.3 mg kg±1 min±1 [58]. Thus, large doses of the drug can be required in some patients with septic shock, which may be due to ª-receptor down-regulation in sepsis [60]. Norepinephrine therapy usually causes a statistically and clinically significant increase in mean arterial pressure due to its vasoconstrictive effects, with little change in heart rate or cardiac output, leading to increased systemic vascular resistance. Several studies have demonstrated increases in cardiac output ranging from 10 % to 20 % and increases in stroke volume index of 10±15 % [39, 43, 53]; other studies, however, have observed no significant changes in either cardiac output or stroke volume index after the use of norepinephrine in the presence of a significant increase in vascular resistance, suggesting that norepinephrine is exerting ª1receptor agonist effects [31, 32, 33, 35, 58, 61]. Obviously, since cardiac index is either increased or unchanged, and mean arterial pressure is consistently increased, left ventricular stroke work index is always statistically increased with norepinephrine. With regards to pulmonary capillary wedge pressure, no clinically significant changes are reported. Norepinephrine should be used only to restore normal values (or values in the lower part of the normal range) of mean arterial blood pressure and systemic vascular resistance. Higher values should be avoided during norepinephrine therapy, since elevated cardiac afterload could be deleterious in cases of severe underlying cardiac dysfunction. Due to methodological problems with the use of systemic vascular resistance as the sole measurement of peripheral resistance, the use of mean arterial pressure is more advisable, although, as men- S 86 tioned above, the optimal target mean arterial pressure is not known, and depends on many factors including age and premorbid condition. Norepinephrine is probably more effective than dopamine at reversing hypotension in septic shock patients. Martin et al. [50] carried out a study with the most striking findings. They prospectively randomized 32 volume-resuscitated patients with hyperdynamic sepsis syndrome to receive either dopamine (2.5±25 mg kg±1 min±1) or norepinephrine (0.5±5.0 mg kg±1 min±1) to achieve and maintain normal hemodynamic and oxygen transport parameters for at least 6 h. If the goals were not achieved with one agent, the other was added. The groups were similar at baseline. Dopamine administration (10±25 mg kg±1 min±1) was successful in only 31 % (5 of 16) of patients whereas norepinephrine (1.5 1.2 mg kg±1 min±1) resulted in success in 93 % (15 of 16) of patients (p < 0.001). Of the 11 patients who did not respond to dopamine 10 responded when norepinephrine was added. In contrast, the one patient who did not respond to norepinephrine failed to respond to dopamine. The survival rate differed between the two groups (59 % norepinephrine vs. 17 % dopamine) although the study was not statistically designed to examine this issue. In patients with hypotension and hypovolemia, for example, during hemorrhagic shock, norepinephrine and other vasoconstrictor agents have severe detrimental effects on renal hemodynamics. Despite the constant improvement in blood pressure, renal blood flow does not increase, and renal vascular resistance continues to rise [62]. Renal tissue oxygen tension can decrease markedly, worsening renal ischemia [63]. Indeed, norepinephrine has been demonstrated to cause ischemia-induced acute renal failure in rats [64]. In nonseptic shock patients norepinephrine has a marked vasoconstrictive effect in most vascular beds, reducing blood flow to the liver, skeletal muscle, and kidneys [65]. When used in normotensive and hypertensive patients, norepinephrine can decrease renal blood flow and increase renal vascular resistance [66]. However, in hyperdynamic septic shock, during which urine flow is believed to decrease mainly as a result of lowered renal glomerular perfusion pressure, the situation is different. Since norepinephrine has a greater effect on efferent arteriolar resistance and increases the filtration fraction, normalization of renal vascular resistance could effectively reestablish urine flow. The importance of this during norepinephrine infusion was shown by Schaer et al. [40], who demonstrated that while renal vascular resistance increased during norepinephrine infusion; renal blood flow remained stable or even slightly increased because the drug enhanced cardiac output and renal perfusion pressure. The effects of norepinephrine on renal function in sepsis have been evaluated in four studies. Desjars et al. [33] studied 22 septic shock patients treated with norepinephrine (0.5±1.5 mg kg±1 min±1) and dopamine (2±3 mg kg±1 min±1). Serum creatinine, blood urea nitrogen, free water clearance, and fractional excretion of sodium decreased significantly, while urine output, creatinine clearance, and osmolar clearance increased significantly. In this study [33] six of seven patients considered at risk for developing acute renal failure had improved renal function during norepinephrine treatment, and only one developed nonoliguric acute renal failure requiring dialysis. Martin et al. [37] studied 24 septic shock patients (treated with norepinephrine (1.1 mg kg±1 min±1 + dobutamine at 8±14 mg kg±1 min±1 + dopamine at 6±17 mg kg±1 min±1). No patient received low-dose dopamine or furosemide. Normalization of systemic hemodynamics was followed by reestablishment of urine flow, decrease in serum creatinine, and increase in creatinine clearance. Fukuoka et al. [34] studied 15 patients with septic shock treated with norepinephrine (0.05±0.24 mg kg±1 min±1), dopamine (9 mg kg±1 min±1) and dobutamine (5 mg kg±1 min±1). Only patients with a normal serum lactate concentration had an increase in systemic vascular resistance, and an increase in urine flow. Creatinine clearance was not affected (18.8+5.5 ml/min before and 20.1+6.6 ml/min after norepinephrine). Patients with elevated serum lactate concentrations had no change in vascular resistance, a decrease in creatinine clearance (32.6 6.4 to 11.9 4.9 ml/min), and required higher doses of furosemide. The authors concluded that the serum lactate concentration may predict which patients will experience potentially adverse renal effects with norepinephrine. However, this study included only a very limited number of patients and is at variance with the findings of other studies [39, 43, 50, 53, 57] in which vascular resistance and urine flow were increased in patients with elevated lactate concentrations (as high as 4.8 1.6 mmol/l [50]). Redl-Wenzel et al. [39] studied 56 patients with septic shock treated with norepinephrine (0.1±2.0 mg kg±1 min±1) and dopamine (2.5 mg kg±1 min±1). During norepinephrine infusion creatinine clearance increased significantly from 75 37 to 102 43 ml/ min after 48 h of treatment. The authors concluded that mean arterial pressure could be increased by norepinephrine with a positive effect on organ perfusion and oxygenation. The effects of norepinephrine on serum lactate concentrations have been assessed in five studies. Four studies assessed changes in serum lactate concentrations over a relatively short period of time, i.e., 1±3 h. Hesselvik et al. [35] reported unchanged lactate levels during norepinephrine therapy, but the actual values were not given. In the other three studies [51, 53, 57] mean values of serum lactate concentrations did not change over the 1- to 3-h study period. It should be noted that initial values were not very high (1.8±2.3 mmol/l). Since blood S 87 flow tended to significantly improve and lactic acid concentrations decreased (but not significantly) in one study, it is unclear whether sufficient time elapsed between measurements to see a significant norepinephrine-induced change in serum lactate concentrations. In the last study [50] initial lactate concentrations were elevated (4.8 1.6 mmol/l), and a statistically and clinically significant decrease in lactate levels was observed at the end of the 6-h study period. Norepinephrine thus does not worsen, and may even improve, tissue oxygenation, as assessed by serum lactate levels, in patients with septic shock. Ruokonen et al. [51] measured splanchnic blood flow and splanchnic oxygen consumption in septic shock patients receiving either norepinephrine (0.07±0.23 mg kg±1 min±1) or dopamine (7.6±33.8 mg kg±1 min±1) to correct hypotension. With norepinephrine no overall changes in splanchnic blood flow and splanchnic oxygen consumption or extraction were noted, and in individual patients its effects on splanchnic blood flow were unpredictable (increased in three patients, decreased in two). Dopamine caused a consistent and statistically significant increase in splanchnic blood flow. Meier-Hellman et al. [54] studied patients changed from dobutamine to norepinephrine. They observed a significant decrease in hepatic venous oxygen saturation. In another group of patients, they studied the effects of switching from dobutamine plus norepinephrine to the latter drug alone. They observed the previously reported changes in hepatic venous oxygen saturation together with a decrease in splanchnic blood flow (green dye dilution technique) and in cardiac output. Splanchnic oxygen consumption remained unchanged due to a regional increase in oxygen extraction. The decrease in splanchnic blood flow paralleled the decrease in cardiac output. The authors concluded that as long as cardiac output is maintained treatment with norepinephrine alone has no negative effects on splanchnic tissue oxygenation. This finding was confirmed by Marik and Mohedin [53] who observed a significant increase in pHi (from 7.16 0.07 to 7.23 0.07) over 3 h of norepinephrine treatment. During treatment with dopamine pHi decreased significantly (7.24 0.04 to 7.18 0.05). Reinelt et al. [67] tested the hypothesis that when dobutamine is added to norepinephrine to obtain a 20 % increase in cardiac index in septic shock patients, splanchnic blood flow and oxygen consumption increases and hepatic metabolic activity (hepatic glucose production) improves. Splanchnic blood flow and cardiac index increased in parallel, but there was no effect on splanchnic oxygen consumption and hepatic glucose production decreased. The conclusion of the authors was that splanchnic oxygen consumption was not dependent on delivery in septic shock patients well resuscitated with norepinephrine. Levy et al. [42] studied the effects of the combination of norepinephrine and dob- utamine on gastric tonometric variables in 30 septic shock patients. pHi and gastric PCO2 gap were normalized within 6 h, while in epinephrine-treated patients pHi decreased and gastric PCO2 gap increased. Changes in the epinephrine group were only transient and were corrected within 24 h but could potentially have caused splanchnic ischemia. The authors concluded that the combination of norepinephrine with dobutamine was more predictable than epinephrine. Clinical experience with norepinephrine in septic shock patients suggests that this drug can successfully increase blood pressure without causing deterioration in cardiac index or organ function. Norepinephrine (at doses of 0.01±3 mg kg±1 min±1), consistently improves hemodynamic variables in the large majority of patients with septic shock. The effects of norepinephrine on oxygen transport variables remain undefined from the available data, but most studies find other clinical parameters of peripheral perfusion to be significantly improved. Unfortunately only one published study was controlled [50] and a prospective, randomized clinical trial is still required to assess whether the use of norepinephrine in septic shock patients affects mortality compared to other vasopressors. The data are sufficiently strong to suggest that when contemplated in the treatment of septic shock patients, norepinephrine should be used early and not merely as a last resort. Epinephrine In patients who fail to respond to fluid administration or other vasopressors epinephrine can increase arterial pressure primarily by increasing cardiac index and stroke volume [38, 68, 69, 70]. Moran et al. [70] reported a linear relationship between epinephrine dose and heart rate, mean arterial pressure, cardiac index, left ventricular stroke work index, and oxygen delivery, and consumption. Epinephrine, however, has detrimental effects on splanchnic blood flow and causes transient decreases in pHi and increases in the PCO2 gap [42, 71]. Epinephrine administration has been associated with increases in systemic and regional lactate concentrations [42, 69, 72], although the cause of these increases is unclear. As the monitoring periods in all these studies were short, it is unclear whether these increases are a transient phenomenon. Other adverse effects of epinephrine include tachyarrhythmias. In summary, epinephrine clearly increases blood pressure in patients unresponsive to other agents. However, because of its negative effects on gastric blood flow and blood lactate concentrations its use should be limited. S 88 Other vasoconstricting agents Dobutamine Phenylephrine, a selective-1-adrenergic agonist, has been used in septic shock patients, although there are concerns about its potential to reduce cardiac output and lower heart rate in these patients. Doses of phenylephrine start at 0.5 mg kg±1 min±1 and reach a maximum dose of 5±8 mg kg±1 min±1. A few studies have evaluated the clinical use of phenylephrine in septic shock [73, 74, 75]. Reinelt et al. [75] reported reduced splanchnic blood flow and oxygen delivery in six septic shock patients treated with phenylephrine compared to norepinephrine. Dobutamine is an adrenergic agonist that stimulates b1-, b2-, and b1-adrenergic receptors. A number of studies have investigated the effect of dobutamine on cardiac function during sepsis or septic shock [82, 83, 84, 85, 86]. The doses utilized ranged from 2 to 28 mg kg±1 min±1. The majority of these studies found increases in cardiac index combined with increases in stroke volume and heart rate. Epinephrine See individual vasopressor agents, above. Inotropic therapy in septic shock Although the cardiac index is usually maintained in the volume resuscitated septic shock patient, cardiac function is impaired [76]. Characterized by ventricular dilatation, a decreased ejection fraction an impaired contractile response to volume loading, and a low peak systolic pressure/end-systolic volume [77, 78], the mechanism of the myocardial dysfunction is complex. Coronary blood flow is usually normal and there is no net lactate production across the coronary vascular bed, so myocardial ischemia is not implicated. Alterations in intracellular calcium homeostasis and in - -adrenergic signal transduction may be contributory factors. Several inflammatory mediators have been shown to cause myocardial depression in various animal models, including cytokines [79], platelet-activating factor, and nitric oxide [80]. Inotropic therapy in septic shock is thus not straightforward. Cardiac output is usually not decreased, and multiple factors may be involved in the depressed cardiac function. In patients with decreased cardiac output the goals of therapy are relatively clear and are aimed at restoring normal physiology. Because of the complexity of assessment of clinical parameters in septic patients, direct measurement of cardiac output by invasive hemodynamic monitoring is advisable, but other endpoints of global perfusion should be followed as well. When global hypoperfusion is manifest by a decreased SvO2, monitoring of SvO2 can be helpful to guide response to therapy. Similarly, although lactate production in sepsis is complex, a fall in blood lactate levels during inotropic therapy is a good prognostic sign [81]. Individual inotropic agents Is dobutamine the pharmacological agent of choice to increase cardiac output in the treatment of septic shock? Answer: yes, grade D. Dopexamine Dopexamine is a dopamine analog that stimulates b2adrenergic and dopamine 1 and 2 receptors. It is not approved for use in the United States. Several studies have evaluated short-term infusions of dopexamine in sepsis or septic shock and demonstrated significant improvements in cardiac index and left ventricular stroke work index [87, 88, 89]. In addition, mesenteric perfusion, as assessed by gastric tonometry, were improved compared to baseline values in initial studies [88], but this has not been confirmed in subsequent studies [90]. Phosphodiesterase inhibitors Phosphodiesterase inhibitors alone, such as amrinone and milrinone, have little place in the treatment of septic shock. They may be considered in combination with adrenergic agents. One study evaluating milrinone in pediatric patients with sepsis observed that cardiac index and right and left ventricular stroke work indices improved significantly, with little change in heart rate [91]. Other agents Calcium supplementation has been proposed in the management of myocardial dysfunction in septic shock. However, no consistent beneficial hemodynamic effect of calcium administration in septic patients has been reported [92], and increased mortality has been reported in animal models [93, 94]. Digoxin has been reported significantly to improve cardiac performance in hypodynamic septic patients [95]. S 89 The ¹supranormalª approach Are hyperkinetic patterns associated with better outcome in septic shock patients? Answer: yes, grade C. Systemic alterations occurring in sepsis including an increased oxygen demand, altered oxygen extraction, and myocardial depression, can explain how circulatory failure may persist despite a normal or high cardiac output (see alterations in distribution above). Hence, it may be valuable to further increase cardiac index (to 'supranormal' values) despite the fact that it is not typically decreased. This remains a controversial issue. Initial studies by Shoemaker et al. [96] seemed to support this approach, but in order to correlate with improved survival, the cardiac index needed to be greater than 4.5 l m±2 min±1, oxygen delivery greater than 600 ml m±2 min±1, and oxygen consumption greater than 170 ml m±2 min±1. Randomized studies [97, 98] to test this hypothesis in all critically ill patients have produced rather negative results, with increased mortality rates in the study by Hayes et al. [97] which sometimes involved the administration of very high doses of dobutamine. Moreover, it is possible that increases in cardiac index and oxygen delivery may simply reflect higher underlying physiological reserve of the patients, associated with an increased chance of survival. The main problem with studying this approach in a typical ICU population is the heterogeneity of the patients included. What may be beneficial in certain groups of patients could potentially be harmful in others, thus giving an overall negative result. Tuchschmidt et al. [99] included only patients with septic shock and obtained more positive results. However, the strategy of increasing oxygen delivery to predetermined elevated endpoints of cardiac index and oxygen delivery cannot be recommended routinely. Different interventions to increase oxygen delivery, such as fluid resuscitation, blood transfusion, or infusion of vasoactive agents, can have different effects on regional perfusion. This is an area of controversy and ongoing research; randomized controlled trials, with clear, reproducible treatment algorithms and use of defined measures of regional perfusion are necessary. In the meanwhile, one should define the goals and desired endpoints of inotropic therapy in septic patients and use these endpoints to monitor and titrate therapy. References 1. Ronco JJ, Fenwick JC, Tweeddale MG, Wiggs BR, Phang PT, Cooper DJ, Cunningham KF, Russell JA, Walley KR (1993) Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 270: 1724±1730 2. James JH, Luchette FA, McCarter FD, Fischer JE (1999) Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 354: 505±508 3. Boekstegers P, Weidenhofer S, Pilz G, Werdan K (1991) Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection 19: 317±323 4. Kellum JA, Kramer DJ, Lee K, Mankad S, Bellomo R, Pinsky MR (1997) Release of lactate by the lung in acute lung injury. Chest 111: 1301±1305 5. De Backer D, Creteur J, Zhang H, Norrenberg M, Vincent JL (1997) Lactate production by the lungs in acute lung injury. Am J Respir Crit Care Med 156: 1099±1104 6. Weil MH, Afifi AA (1970) Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 41: 989±1001 7. Vincent JL, Dufaye P, Berre J, Leeman M, Degaute JP, Kahn RJ (1983) Serial lactate determinations during circulatory shock. Crit Care Med 11: 449±451 8. Friedman G, Berlot G, Kahn RJ, Vincent JL (1995) Combined measurements of blood lactate concentrations and gastric intramucosal pH in patients with severe sepsis. Crit Care Med 23: 1184±1193 9. Bakker J, Coffernils M, Leon M, Gris P, Vincent JL (1991) Blood lactate levels are superior to oxygen derived variables in predicting outcome in human septic shock. Chest 99: 956±962 10. Vallet B, Lund N, Curtis SE, Kelly D, Cain SM (1994) Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. J Appl Physiol 76: 793±800 11. Nelson DP, King CE, Dodd SL, Schumacker PT, Cain SM (1987) Systemic and intestinal limits of O2 extraction in the dog. J Appl Physiol 63: 387±394 12. Schmidt H, Secchi A, Wellmann R, Bach A, Bohrer H, Gebhard MM, Martin E (1996) Effect of endotoxemia on intestinal villus microcirculation in rats. J Surg Res 61: 521±526 13. Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ (1996) Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res 61: 190±196 14. Russell JA (1997) Gastric tonometry: does it work? Intensive Care Med 23: 3±6 15. Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J, Klein F, San Roman E, Dorfman B, Shottlender J, Giniger R (1992) Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 339: 195±199 16. Gomersall CD, Joynt GM, Freebairn RC, Hung V, Buckley TA, Oh TE (2000) Resuscitation of critically ill patients based on the results of gastric tonometry: a prospective, randomized, controlled trial. Crit Care Med 28: 607±614 17. Silva E, De Backer D, Creteur J, Vincent JL (1998) Effects of vasoactive drugs on gastric intramucosal pH. Crit Care Med 26: 1749±1758 18. Haupt MT, Gilbert EM, Carlson RW (1985) Fluid loading increases oxygen consumption in septic patients with lactic acidosis. Am Rev Respir Dis 131: 912±916 S 90 19. Packman MI, Rackow LC (1983) Optimum left heart filling pressures during fluid resuscitation of patients with hypovolemic and septic shock. Crit Care Med 11: 165±169 20. Sugerman HJ, Diaco JF, Pollock TW, Miller LD (1971) Physiologic management of septicemic shock in man. Surg Forum 22: 3±5 21. Carroll G, Snyder J (1982) Hyperdynamic severe intravascular sepsis depends on fluid administration in cynomolgus monkey. Am J Physiol 243:R131±R141 22. Rackow EC, Kaufman BS, Falk JL, Astiz ME, Weil MH (1987) Hemodynamic response to fluid repletion in patients with septic shock: evidence for early depression of cardiac performance. Circ Shock 22: 11±22 23. Weil MH, Nishjima H (1978) Cardiac output in bacterial shock. Am J Med 64: 920±922 24. Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus W, SUPPORT Investigators (1996) The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA 276: 889±897 25. Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, Haupt MT, Kaufman BS, Putnam D (1983) Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Crit Care Med 11: 839±850 26. Boldt J, Müller M, Heesen M, Heyn O, Hempelmann G (1996) Influence of different volume therapies on platelet function in the critically ill. Intensive Care Med 22: 1075±1081 27. Morisaki H, Bloos F, Keys J, Martin C, Neal A, Sibbald WJ (1994) Compared with crystalloid, colloid therapy slows progression of extrapulmonary tissue injury in septic sheep. J Appl Physiol 77: 1507±1518 28. Schmand JF, Ayala A, Morrison MH, Choudry IH (1995) Effects of hydroxyethyl starch after trauma-hemorrhagic shock: Restoration of macrophage integrity and prevention of increased circulating IL-6 levels. Crit Care Med 23: 806±814 29. Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 340: 409±417 30. Marik PE, Sibbald WJ (1993) Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 269: 3024±3029 31. Desjars P, Pinaud M, Potel G, Tasseau F, Touze M-D (1987) A reappraisal of norepinephrine therapy in human septic shock. Crit Care Med 15: 134±137 32. Meadows D, Edwards JD, Wilkins RG, Nightingale P (1988) Reversal of intractable septic shock with norepinephrine therapy. Crit Care Med 16: 663±666 33. Desjars P, Pinaud M, Bugnon D, Tasseau F (1989) Norepinephrine therapy has no deleterious renal effects in human septic shock. Crit Care Med 17: 426±429 34. Fukuoka T, Nishimura M, Imanaka H, Taenaka N, Yoshiya I, Takezawa J (1989) Effects of norepinephrine on renal function in septic patients with normal and elevated serum lactate levels. Crit Care Med 17: 1104±1107 35. Hesselvik JF, Brodin B (1989) Low dose norepinephrine in patients with septic shock and oliguria: effects on afterload, urine flow and oxygen transport. Crit Care Med 17: 179±180 36. Bollaert PE, Bauer P, Audibert G, Lambert H, Larcan A (1990) Effects of epinephrine on hemodynamics and oxygen metabolism in dopamine resistant shock. Chest 98: 949±953 37. Martin C, Eon B, Saux P, Aknin P, Gouin F (1990) Renal effects of norepinephrine used to treat septic shock patients. Crit Care Med 18: 282±285 38. Lipman J, Roux A, Kraus P (1991) Vasoconstrictor effects of adrenaline in human septic shock. Anaesth Intensive Care 19: 61±65 39. Redl-Wenzl EM, Armbruster C, Edelmann G, Fischl E, Kolacny M, Wechsler-Fördös A, Sporn P (1993) The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 19: 151±154 40. Schaer GL, Fink MP, Parrillo JE (1985) Norepinephrine alone versus norepinephrine plus low-dose dopamine: enhanced renal blood flow with combination pressor therapy. Crit Care Med 13: 492±496 41. Richer M, Robert S, Lebel M (1996) Renal hemodynamics during norepinephrine and low-dose dopamine infusion in man. Crit Care Med 24: 1150±1156 42. Levy B, Bollaert PE, Charpentier C, et al (1997) Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock. Intensive Care Med 23: 282±287 43. Winslow EJ, Loeb HS, Rahimtoola SH, Kamath S, Gunnar RM (1973) Hemodynamic studies and results of therapy in 50 patients with bacteremic shock. Am J Med 54: 421±432 44. Wilson RF, Sibbald WJ, Jaanimagi JL (1976) Hemodynamic effects of dopamine in critically ill septic patients. J Surg Res 20: 163±172 45. Regnier B, Rapin M, Gory G, Lemaire F, Teisseire B, Harari A (1977) Haemodynamic effects of dopamine in septic shock. Intensive Care Med 3: 47±53 46. Drueck C, Welch GW, Pruitt BA (1978) Hemodynamic analysis of septic shock in thermal injury: treatment with dopamine. Am Surg 7: 424±427 47. Samii K, Le Gall JR, Regnier B, Gory G, Rapin M (1978) Hemodynamic effects of dopamine in septic shock with and without renal failure. Arch Surg 113: 1414±1416 48. Jardin F, Gurdjian F, Desfonds P, Margairaz A (1979) Effects of dopamine on intrapulmonary shunt fraction and oxygen transport in severe sepsis with circulatory and respiratory failure. Crit Care Med 7: 273±277 49. Regnier B, Safran D, Carlet J, Teisseire B (1979) Comparative haemodynamic effects of dopamine and dobutamine in septic shock. Intensive Care Med 5: 115±120 50. Martin C, Papazian L, Perrin G, Saux P, Gouin F (1993) Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 103: 1826±1831 51. Ruokonen E, Takala J, Kari A, Saxen H, Mertsola J, Hansen EJ (1993) Regional blood flow and oxygen transport in septic shock. Crit Care Med 21: 1296±1303 52. Hannemann L, Reinhart K, Grenzer O, Meier-Hellmann A, Bredle DL (1995) Comparison of dopamine to dobutamine and norepinephrine for oxygen delivery and uptake in septic shock. Crit Care Med 23: 1962±1970 53. Marik PE, Mohedin J (1994) The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA 272: 1354±1357 54. Meier-Hellmann A, Bredle DL, Specht M, Spies C, Hannemann L, Reinhart K (1997) The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock. Intensive Care Med 23: 31±37 55. Nevire R, Mathieu D, Chagnon JL, Lebleu N, Wattel F (1996) The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients. Am J Respir Crit Care Med 154: 1684±1688 S 91 56. Bailey AR, Burchett KR (1997) Effect of low-dose dopamine on serum concentrations of prolactin in critically ill patients. Br J Anaesth 78: 97±99 57. Schreuder WO, Schneider AJ, Groeneveld ABJ, Thijs LG (1989) Effect of dopamine vs norepinephrine on hemodynamics in septic shock. Chest 95: 1282±1288 58. Martin C, Saux P, Eon B, Aknin P, Gouin F (1990) Septic shock: a goal-directed therapy using volume loading, dobutamine and/or norepinephrine. Acta Anaesthesiol Scand 34: 413±417 59. Brown RS, Carey JS, Mohr PA, Monson DO, Shoemaker WC (1966) Comparative evaluation of sympathomimetic amines in clinical shock. Circulation 34: 260±271 60. McMillan M, Chernow B, Roth BL (1986) Hepatic alpha 1-adrenergic receptor alteration in a rat model of chronic sepsis. Circ Shock 19: 185±193 61. Martin C, Perrin G, Saux P, Papazian L, Gouin F (1994) Effects of norepinephrine on right ventricular function in septic shock patients. Intensive Care Med 20: 444±447 62. Mills LC, Moyer JH (1960) The effects of various catecholamines on specific vascular hemodynamics in hypotensive and normotensive subjects. Am J Cardiol 5: 652±659 63. Murakawa K, Kobayashi A (1988) Effects of vasopressors on renal tissue gas tensions during hemorrhagic shock in dogs. Crit Care Med 16: 789±792 64. Conger JD, Robinette JB, Guggenheim SJ (1981) Effect of acetylcholine on the early phase of reversible norepinephrine-induced acute renal failure. Kidney Int 19: 399±403 65. Eckstein JW, Abboud FM (1962) Circulatory effects of sympathomimetic amines. Am Heart J 63: 119±135 66. Gombos EA, Hulet WH, Bopp P, Goldring W, Baldwin DS, Chasis H (1962) Reactivity of renal and systemic circulations to vasoconstrictor agents in normotensive and hypertensive subjects. J Clin Invest 41: 203±217 67. Reinelt H, Radermacher P, Fischer G, Geisser W, Wachter U, Wiedeck H, Georgieff M, Vogt J (1997) Effects of a dobutamine-induced increase in splanchnic blood flow on hepatic metabolic activity in patients with septic shock. Anesthesiology 86: 818±824 68. Mackenzie SJ, Kapadia F, Nimmo GR, Armstrong IR, Grant IS (1991) Adrenaline in treatment of septic shock: effects on hemodynamics and oxygen transport. Intensive Care Med 17: 36±39 69. Wilson W, Lipman J, Scribante J, Kobilski S, Lee C, Krause P, Cooper J, Barr J (1992) Septic shock: does adrenaline have a role as a first-line inotropic agent? Anaesth Intensive Care 20: 470±474 70. Moran JL, O'Fathartaigh MS, Peisach AR, Chapman MJ, Leppart P (1993) Epinephrine as an inotropic agent in septic shock: a dose-profile analysis. Crit Care Med 21: 70±77 71. Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L (1997) Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med 25: 399±404 72. Day NPJ, Phu NH, Bethell DP, Mai NTH, Chau TTH, Hien TT, White NJ (1996) The effects of dopamine and adrenaline infusion on acid-base balance and systemic hemodynamics in severe infection. Lancet 348: 219±223 73. Gregory JS, Bonfiglio MF, Dasta JF, Reilley TE, Townsend MC, Flancbaum L (1991) Experience with phenylephrine as a component of the pharmacologic support of septic shock. Crit Care Med 19: 1395±1400 74. Flancbaum L, Dick M, Dasta J, Sinha R, Choban P (1997) A dose-response study of phenylephrine in critically ill, septic surgical patients. Eur J Clin Pharmacol 51: 461±465 75. Reinelt H, Radermacher P, Kiefer P, Fischer G, Wachter U, Vogt J, Georgieff M (1999) Impact of exogenousadrenorecptor stimulation on hepatosplanchnic oxygen kinetics and metabolic activity in septic shock. Crit Care Med 27: 325±331 76. Vincent JL, Gris P, Coffernils M, Leon M, Pinsky MR, Reuse C, Kahn RJ (1992) Myocardial depression characterizes the fatal course of septic shock. Surgery 111: 660±667 77. Vincent JL, Reuse C, Frank RN, Contempre B, Kahn RJ (1989) Right ventricular dysfunction in septic shock: assessment by measurements of right ventricular ejection fraction using the thermodilution technique. Acta Anaesthesiol Scand 33: 34±38 78. Parker MM, McCarthy KE, Ognibene FP, Parrillo JE (1990) Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest 97: 126±131 79. Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE (1996) Tumor necrosis factor and interleukin Ib are responsible for in vitro myocardial depression induced by human septic shock serum. J Exp Med 183: 949±958 80. Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, Schulz R, Parrillo JE (1999) Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol 276:R265±R276 81. Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL (1996) Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 171: 221±226 82. Jardin F, Sportiche M, Bazin M, Bourokba A, Margairaz A (1981) Dobutamine: a hemodynamic evaluation in human septic shock. Crit Care Med 9: 329±332 83. Fisher CJJ, Horowitz Z, Albertson TE (1985) Cardiorespiratory failure in toxic shock syndrome: effect of dobutamine. Crit Care Med 13: 160±165 84. Tell B, Majerus TC, Flancbaum L (1987) Dobutamine in elderly septic shock patients refractory to dopamine. Intensive Care Med 13: 14±18 85. Shoemaker WC, Appel PL, Kram HB, et al (1989) Comparison of hemodynamic and oxygen transport effects of dopamine and dobutamine in critically ill surgical patients. Chest 96: 120±126 86. Vincent JL, Roman A, Kahn RJ (1990) Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med 18: 689±693 87. Colardyn FC, Vandenbogaerde JF, Vogelaers DP, Verbeke JH (1989) Use of dopexamine hydrochloride in patients with septic shock. Crit Care Med 17: 999±1003 88. Smithies M, Yee TH, Jacson L, Beale R, Bihari D (1994) Protecting the gut and the liver in the critically ill: effects of dopexamine. Crit Care Med 22: 789±795 89. Hannemann L, Reinhart K, MeierHellmann A, et al (1996) Dopexamine hydrochloride in septic shock. Chest 109: 756±760 90. Meier-Hellmann A, Bredle DL, Specht M, Hannemann L, Reinhart K (1999) Dopexamine increases splanchnic blood flow but decreases gastric mucosal pH in severe septic patients treated with dobutamine. Crit Care Med 27: 2166±2171 91. Barton P, Garcia J, Kouatli A, Kitchen L, Zorka A, Lindsay C, Lawless S (1996) Hemodynamic effects of IV milrinone lactate in pediatric patients with septic shock. Chest 109: 1302±1312 92. Woo P, Carpenter MA, Trunkey D (1979) Ionized calcium: the effect of septic shock in the human. J Surg Res 26: 605±610 S 92 93. Malcolm DS, Zaloga GP, Holaday JW (1989) Calcium administration increases the mortality of endotoxic shock in rats. Crit Care Med 17: 900±903 94. Zaloga GP, Sager A, Black KW, Prielipp R (1992) Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock 37: 226±229 95. Nasraway SA, Rackow EC, Astiz ME, Karras G, Weil MH (1989) Inotropic response to digoxin and dopamine in patients with severe sepsis, cardiac failure, and systemic hypoperfusion. Chest 95: 612±615 96. Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94: 1176±1186 97. Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330: 1717±1722 98. Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med 333: 1025±1032 99. Tuchschmidt J, Fried J, Astiz M, Rackow E (1992) Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 102: 216±220 Intensive Care Med (2001) 27: S 93±S 103 J. Carlet Immunological therapy in sepsis: currently available ) J. Carlet ( ) Intensive Care Unit, Fondation-Hôpital Saint-Joseph, Paris, France E-mail: [email protected] Phone: +33-1 44-12 37 83 Fax: +33-1 44-12 34 15 Should corticosteroids be used during septic shock at low doses and for a prolonged period of time? Answer: yes, grade C. Recommendations Introduction Many therapies used in our daily practice are known to have significant effects on inflammation. These drugs influence the activation of the inflammatory network that occurs during severe sepsis and related syndromes as disseminated intravascular coagulation and acute respiratory distress syndrome (ARDS). Many of these compounds (Table 1) have already been used during experimental models of sepsis and/or human studies. Corticosteroids should not be used in severe sepsis or septic shock at high doses (30 mg/kg) and for a short course (1±2 days). On the other hand, corticosteroids may be used during ªrefractoryº septic shock but not during severe sepsis without shock or mild shock. It should then be used at low doses (100 mg hydrocortisone three times a day) for 5 days or more and then with subsequent tapering of the dose according to the hemodynamic status and the need for vasopressors. Rationale Methods This contribution reviews those drugs that are available in the daily management of severe sepsis and septic shock. A computer-based review of the literature was undertaken using Medline from 1990 to September 1999 as the primary database. The subject heading keywords defined for each of the compounds listed in Table 1 were combined with the following general sepsis-related subject heading keywords: sepsis, severe sepsis, septic shock, and ARDS. Anti-inflammatory agents Should corticosteroids be used in the treatment of severe sepsis or septic shock at high doses (30 mg/kg) for a short course (one or 2 days)? Answer: no, grade A. An extensive literature is available for corticosteroids. Steroids have been used for many years, and their efficacy is controversial. Numerous animal studies performed during experimental septic (endotoxic) shock or acute lung injuries showed a very significant reduction in both intensity of shock, acute respiratory failure and mortality [1, 2]. They have been used at very high doses (30 mg/kg per dose for a maximum of 24±48 h). The ability of these high doses of corticosteroids to reduce complement activation and to inhibit leukocyte aggregability and adherence was at that time a very logical rationale for their efficacy [3]. Very promising initial findings have been published regarding humans [12]. However, two well designed, prospective, multicenter, randomized, double-blind studies demonstrated very clearly their inability to decrease mortality [5, 8]. Some studies mention positive trends when looking at subgroups of infections due to Gram-negative rods [5, 8, 13]. S 94 Table 1 List of therapies currently available for eventually treating severe sepsis Therapy References Anti-inflammatory agents Corticosteroids (high or low doses) Ibuprofen Prostaglandin E1 Pentoxifylline Oxygen scavengers N-Acetylcysteine Selenium Drugs modifying coagulation Antithrombin III Drugs enhancing host defenses Immunoglobulins Interferon-g Granulocytes stimulating factors Immunonutrition 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 25a 27, 28, 29, 30, 31 32, 33, 34, 35 36, 37, 38 39, 40, 41, 42, 43, 44, 45, 46, 47 39, 40, 41, 42, 43, 44, 45 46, 47 48, 49, 50, 51, 52 48, 49, 50, 51, 52 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 54, 55, 56, 57, 58 59, 60, 61, 62, 63, 64 65, 66, 67, 68, 69, 70 ±a Other drugs Growth hormone Antibiotics Including ketoconazole Including polymyxin B Taurolidine Fresh frozen plasma Anesthetic sedative and analgesic agents Catecholamines 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 71 72, 73, 74, 75, 76, 77 73, 74 72 78 79 80 81, 82, 83, 84, 85 Hemofiltration, plasma filtration, plasma exchange 86, 87, 88, 89, 90, 91 a See PØrez and Dellinger, ªOther supportive therapies in sepsisº Two recent meta-analyses [13, 14] reviewing the studies confirm that corticosteroids at the dose of 30 mg/kg (one or two doses) are ineffective [13] or even harmful [14]. The design and the results of the nine randomized studies are summarized in Tables 2 and 3. Similar negative results have been obtained during ARDS [15]. Pooling the results only from those patients with Gram-negative infections, as in the meta-analysis by Lefering et al. [13], yields a rate difference of ±5.6 % [confidence interval (CI): ±21.4 to 10.1) in favor of steroids, based on 413 patients. Those patients with Gram-positive infections (n = 306) had an overall effect of +1.8 % (CI: ±15.8 to 18.6). Most persons stopped using steroids when these large trials were published. Several studies performed over the years, however, have maintained interest in the use of corticosteroids. Mortality was reduced using steroids during severe typhoid fever [16], and neurological sequelae were reduced during meningitis [17]. Two large double-blind case control studies demonstrated that prolonged treatment (10±15 days) of relatively low doses of steroids (120±240 mg hydrocortisone) dramatically reduced mortality during severe Pneumocystis carinii pneumonia in AIDS patients [18, 19]. In addition, Meduri et al. [20] showed that the course of late, fibrotic ARDS was improved by steroid use which was confirmed in a re- cent randomized double-blind study showing a significant reduction in mortality [21]. Two small, randomized, double-blind studies of steroids in patients with severe and refractory septic shock recently demonstrated positive results [22, 23]. Corticosteroids were used at small doses (100 mg hydrocortisone three times per day in one [22] and 100 mg followed by a continuous infusion of 0.18 mg/kg per hour in the other [23], for longer periods of time than in past studies: 5 days in one [22] and 5±10 days in the other, with tapering of the doses according to hemodynamic status and need for vasopressors. Both studies showed a significant reversal of shock and organ failures and a trend in reduction in mortality. Additional studies are necessary, and a French multicenter randomized, controlled, double-blind study reported that low dose steroids decrease mortality in patients with septic shock [25 a]. Several factors may explain the recent positive effects of corticosteroids during sepsis [24]. These include the treatment of ªrelativeº adrenal insufficiency [25] and the potentiation of adrenergic receptivity [26] in addition to the anti-inflammatory effect. The lower immunosuppressive doses and a more prolonged duration of therapy than in the initial studies could also explain discrepancies. S 95 Table 2 Design of the nine randomized studies used in the meta-analysis (from Cronin et al. [14]) (DB double blind, M methylprednisolone, B betamethasone, D dexamethasone, H hydrocortisone) Reference n Type of study Product Dose Duration Endpoints Cooperative Study Group [6] Klastersky et al. [10] Schumer et al. [12] 194 Open H 6d 85 Open B 300 mg then 50 mg/d 1 mg/kg 172 DB M 30 mg/kg 1 dose or 2 Thompson et al. [11] Lucas and Ledgerwood [9] Sprung et al. [4] 60 DB M 30 mg/kg 48 Open D 2 mg/kg Max. 6 doses in 24 h 2d 59 Open M 30 mg/kg 1 dose (or 2) Mortality, complications Mortality (20 d), complications Mortality (28 d), complications Mortality, complications Mortality (14 d), complications Hospital mortality, complications 3d Bone et al. [5] 381 DB M 30 mg/kg 1d Mortality (14 d), complications Veteran Administration [8] 223 DB M 30 mg/kg 9d Mortality (14 d), complications 75 DB M 30 mg/kg4 1d Hospital mortality, ARDS complications Luce et al. [7] Table 3 Results of the nine randomized studies used in the metaanalysis (from Cronin et al. [14]) Cooperative study group [6] Klastersky et al. [10] Schumer et al. [12] Thompson et al. [11] Lucas et al. [9] Sprung et al. [4] Bone et al. [5] Veteran Administration [8] Luce et al. [7] n Risk ratio 95 % CI 194 85 172 60 48 59 381 223 75 1.72 0.97 0.30 1.01 1.09 1.11 1.35 0.95 1.07 1.23±2.41 0.65±1.45 0.13±0.72 0.77±1.31 0.36±3.27 0.74±1.67 0.98±1.84 0.57±1.58 0.72±1.60 Should ibuprofen be used in the treatment of severe sepsis and septic shock? Answer: no, grade B. Recommendations Ibuprofen should not be used during severe sepsis or septic shock. Additional studies are needed to determine whether some patients, for example, those with hypothermia, could benefit from the drug. Rationale Ibuprofen is a powerful anti-inflammatory agent, acting on the prostaglandin metabolism as a cyclo-oxygenase inhibitor. It has been used with controversial effects in animals during both experimental sepsis and ARDS [27, 28]. Two small randomized, double-blind studies in patients showed some hemodynamic effect and a normalization of pH without any significant effect upon mortality [29, 30]. Mortality was decreased significantly in a post hoc analysis of hypothermic patients [30]. A large multicenter randomized, controlled, double blind study, however, failed to demonstrate any effect upon mortality, reversal of shock or acute respiratory failure [31]. Ibuprofen was able to reduce the levels of prostacyclin and thromboxane and to decrease fever, tachycardia and oxygen consumption [31]. The drug was not associated with adverse affects. Should prostaglandins be used in the treatment of ARDS due to severe infections and sepsis? Answer: no, grade B. Recommendations Prostaglandins, in particular prostaglandin E1 or liposomal prostaglandin E1 should not be used during ARDS due to sepsis. There are no specific data allowing recommendations in severe sepsis. S 96 Rationale Oxygen scavengers Several prostaglandins which have both an anti-inflammatory and a vasoactive effect have been studied including prostaglandin I2 and particularly prostaglandin E1 [32, 33, 34, 35] during ARDS. The vast majority of these patients had ARDS due to severe infections or sepsis. An early, small, randomized study showed promising results [32]. However, a large multicenter, randomized, controlled, double blind study failed to show any difference in survival [33]. An increase in oxygen delivery and oxygen consumption was noted in treated patients who survived [34]. A recent, multicenter randomized, controlled, double-blind study with liposomal prostaglandin E1 (TLC C-53) showed that indices of oxygenation of treated ARDS patients were improved compared with controls, but without any effect upon duration of mechanical ventilation or 28 days mortality [35]. Again, most ARDS was due to sepsis in these two large studies. No data are really available concerning an overall group of patients with severe sepsis. Several oxygen scavengers are currently available, including N-acetylcysteine (NAC), vitamin E, vitamin C, and selenium. Vitamins E and C have been only poorly studied in humans, and we focus on N-acetylcysteine and selenium. Should pentoxifylline be used in the treatment of severe sepsis in (a) adults, (b) infants? Answer: (a) no, grade B; (b) no, grade C. Recommendations Pentoxifylline should not be used in adults with severe sepsis unless new studies show a significant effect. The positive effect of a small study in infants should be confirmed before clinical use. Rationale Pentoxifylline, which has a powerful anti-inflammatory effect including a strong inhibition of tumor necrosis factor secretion, has been used successfully in many animal studies with the prevention of the transition from a hyperdynamic to hypodynamic state, although no effect upon mortality has been shown [36]. Human studies are more scarce. A multicenter, randomized, controlled, double-blind study during sepsis showed an increase in PaO2/FIO2 ratio but no effect upon cytokines levels or mortality [37]. A recent double-blind study performed in premature infants with sepsis showed a decrease in cytokines levels and a significant decrease in mortality (1/40 vs. 6/38 p = 0.046) [38]. However, the size of this study was rather small, and additional large studies are mandatory. Should N-acetylcysteine be used in the treatment of severe sepsis? Answer: no, grade C. Recommendations NAC should not be used in severe sepsis until new data are available, focusing in particular on very early therapy. Rationale During acute lung injury an improvement in oxygenation and reduction in the required length of mechanical ventilation was found in patients treated with NAC compared to controls [39]. However, several randomized studies have shown no difference in mortality, gas exchange, and development of respiratory failure in patients treated with NAC [39, 40]. Several studies have also been performed during severe sepsis, with heterogeneous results [41, 42, 43, 44]. Depressed cardiac performance has been described in septic patients treated with NAC [42]. A very recent multicenter, randomized, controlled, double-blind study showed that a prolonged infusion of NAC is unable to prevent multiple organ failure in consecutively admitted critically ill patients [43]. In this study treatment used more than 24 h after the initial insult worsened the prognosis compared to controls. Better results were obtained when the drug was use before the insult, as during cardiac surgery [44]. These results suggest that this compound could be helpful when started before (or perhaps shortly after) the insult, but possibly harmful when started too late. Combinations of several antioxidants have also been published, but data are too limited to allow recommendations [45]. Should selenium be used in the treatment of severe sepsis? Answer: no, grade C. S 97 Fig. 1 Effect of proinflammatory cytokines. Upon coagulation cascade during sepsis leading to an activation of tissue factor, a depletion in protein C (via a decrease in thrombomodulin levels) antithrombin III and C1 inhibitor, and a decrease in fibrinolysis (via the effect of plasminogen activator inhibitor 1) Recommendations Selenium should not be used for severe sepsis. Additional studies are warranted to confirm initial positive data. Rationale A profound depletion in selenium levels has been demonstrated in many severe septic patients [46]. Mortality and morbidity are far higher in patients with a very low selenium level [46]. A recent prospective, randomized, but nonblinded study performed in septic patients showed that selenium replacement is able to reduce severity indexes at day 3 and reduce the need for hemodialysis but has no significant effect upon mortality (52 % in controls and 33, 5 % in treated patients, p = 0.13) [47]. Additional large studies are needed to confirm initial promising results. Drugs modifying coagulation There are complex interactions between the inflammation and coagulation systems (Fig. 1). Proinflammatory cytokines activate coagulation cascades, in particular via an effect upon tissue factor which is a key player in the coagulation cascade. They can also reduce fibrinolysis and profoundly reduce the levels of protein C and of antithrombin III which are important anticoagulant agents. Antithrombin III inhibits several coagulation factors of the extrinsic pathway such as factors IXa, XIa, XIIa in addition to factors Xa, IIa, and plasmin. Activated protein C inhibits factors Va, Vlla, and plasminogen activator inhibitor 1. The overall effect during sepsis is a marked procoagulant balance. Conversely, coagulation products can activate the inflammation network which creates numerous amplification loops. For example, thrombin can induce an up-regulation of Pand E-selectin, and contact factor activation can induce the production of bradykinin, worsening hypotension and tissue hypoperfusion. In humans studies, both anti- S 98 thrombin III and protein C levels are sharply decreased [48], and mortality of septic patients is inversely correlated with the levels of those two products. This makes the rationale for studying those types of compounds, such as antithrombin III, protein C, and tissue factor protein inhibitor very strong. Only antithrombin III is currently available. Should antithrombin III be used in the treatment of severe sepsis? Answer: no, grade B. Recommendations Antithrombin III should not be used during severe sepsis. Countries which allow the free use of this drug in this setting should reconsider their position. Rationale Antithrombin III is a drug which is widely used for septic patients in several countries. Three randomized, small, double-blind studies were published [49, 50, 51]. Duration of disseminated intravascular coagulation was reduced [49] as well as the number of organ failures [51], but mortality was not different although a positive trend was clearly noted. A meta-analysis was also performed [51] showing a 22.9 % reduction in mortality but which did not reach statistical significance. Unfortunately a large multicenter, prospective, double-blind study has recently been completed which showed no significant improvement in survival [52]. The complete data have not yet been published. Other drugs such as activated protein C and tissue factor inhibitors are not currently available and are discussed elsewhere (see Arndt and Abraham, ªImmunological therapy of sepsis: experimental therapiesº). Drugs enhancing host defenses After the initial activation of the proinflammatory network, a profound immunodepression can occur in septic patients [53]. This could influence outcome increasing the risk of nosocomial infections. Several strategies have been used to increase host defenses, including polyvalent immunoglobulins, interferon-g, stimulating factors for granulocytes [including granulocyte colony stimulating factor (G-CSF)], and immunonutrition. The latter is discussed elsewhere (see PØrez and Dellinger, ªOther supportive therapy in sepsisº). Should intravenous immunoglobulins be used in the treatment of severe sepsis in (a) adults or (b) neonates? Answer: (a) no, grade C; (b) no, grade C. Recommendations Immunoglobulins should not be used either in adult patients or in neonates with sepsis, unless additional large studies confirm some positive data in small-sized metaanalyses. Countries which allow a wide use of these compounds should reconsider their position and encourage these studies. Rationale Intravenous immunoglobulins (IVIG) are widely used in both infants and adults in the treatment of severe sepsis, at least in certain countries. Reports which support their empirical use, however, are still rather weak. The rationale is to restore immunoglobulins levels, which may be depressed in sepsis, and to provide patients with specific antibodies against micro-organisms. No individual well designed clinical study has been performed in adults with severe sepsis. A recent study was performed in patients with streptococcal toxic shock syndrome [54]. This was a comparative nonblinded study performed in 21 patients which demonstrated a significantly reduced mortality (67 % vs. 34 %, p = 0.02). Both Acute Physiology and Chronic Health Evaluation II scores and IVIG were prognostic factors in the multivariate analysis. The odds ratio associated with IVIG was 8.1 (95 % CI: 1.6±45). A recent meta-analysis by the Cochrane group [55] looking at 23 studies (some of them unpublished) on immunoglobulins, antiendotoxins, and anticytokines, extracted from the small size studies already published, evaluated a population of 413 patients receiving polyclonal immunoglobulins. Mortality was significantly reduced (relative risk: 0.6; 95 % CI: 0.47±0.76). Results were even more positive when only sepsis related deaths were considered. A large, well designed, multicenter, randomized, doubleblind study is, however, warranted before making firm conclusions. Two prophylactic studies have been published recently [56, 57]. A study performed in cardiac surgery patients showed no difference in the occurrence of sepsis between polyvalent IVIG and IgM-enriched immunoglobulin [56]. A prospective comparative study showed that IVIG and not placebo is able to prevent nosocomial infections after major surgery [57]. Such prophylactic studies are needed in this field in nonsurgical critically ill patients. In neonatal sepsis, a recent meta-analysis of 110 newborns in three studies showed that IVIG is able to re- S 99 duce mortality significantly (odds ratio: 0.173; 95 % CI: 0.031±0.735; p = 0.007) [58]. However, the size of the overall population was very small, and large studies are urgently warranted. In the same meta-analysis the effect of IVIG in the prevention of sepsis in 4933 evaluable newborns was significant (p = 0.0193, two-tailed), although heterogeneity of the studies precluded estimation of an overall odds ratio. Other Drugs Interferon-g Growth hormone should not be used in patients with sepsis because it increases mortality. Interferon-g has been used successfully in animals models of Gram-negative sepsis [59, 60]. Few data are available in human sepsis. The drug has been used with positive results to prevent infection during chronic granulomatous disease [61] and trauma [62, 63]. The drug, however, was unable to prevent infections in burn patients [64]. Data are insufficient for therapy of severe sepsis to allow recommendations. Should growth hormone be used in the treatment of severe sepsis? Answer: no, grade A. Recommendations Rationale Answer: no, grade C. The administration of growth hormone could in theory attenuate the catabolic response to injury, surgery or sepsis. Two prospective double-blind studies with more than 200 patients each were recently reported in critically ill patients with cardiac or abdominal surgery, multiple trauma or acute respiratory failure [71]. Mortality was increased significantly in treated patients. The relative risk in these two pooled studies was 1.9 (95 % CI: 1.3±2.9). Length of stay and duration of mechanical ventilation were longer in treated survivors than in controls. Recommendations Antimicrobial compounds G-CSF should not be used in nonneutropenic patients with severe sepsis. Polymixin B. Polymixin B is able to neutralize endotoxin via strong antilipid A activity [72]. Since it is very toxic, it is difficult to use intravenously in humans, although some derivates are less toxic. Extracorporeal techniques, in which polymyxin is coated on membranes, are under investigation. Should granulocyte colony stimulating factor be used in the treatment of severe infections? Rationale G-CSF is very efficient and reduces mortality in animal models of abdominal sepsis [65, 66]. During pneumonia models in rats the drug has been shown to exert different effects according to the micro-organisms involved [67]. Preliminary studies have been performed in community or hospital acquired pneumonia with controversial results [68, 69]. In patients with head trauma and receiving mechanical ventilation G-CSF prophylaxis did not improve outcome nor lower the risk of nosocomial pneumonia [70]. Ketoconazole. Ketaconazole, one of the new imidazoles, has a strong effect upon thromboxane synthase inhibition and has been shown to prevent ARDS in septic patients in a small double-blind randomized study [73]. A recent study performed in 234 patients, however, failed to demonstrate any effect upon mortality and duration of mechanical ventilation in ARDS patients [74]. No data are available in patients with sepsis. Other antibiotics Immunonutrition See PØrez and Dellinger, ªOther supportive therapies in sepsis.º Some antibiotics have anti-inflammatory effects, in particular in decreasing cytokine release. Effects have been shown for vancomycin [75] trovafloxacin [76] and ciprofloxacin [77]. S 100 Taurolidine Hemofiltration and plasma filtration Taurolidine is an anti-infective agent (nonantibiotic), used either locally, or intravenously, which has some antibacterial effect associated with an antiendotoxin effect. A randomized placebo-controlled study failed to demonstrate any effect on outcome in sepsis [78]. Should hemofiltration be used in the treatment of patients with severe sepsis, without renal indications? Answer: no, grade C. Recommendations Other drugs currently used Many other drugs that we use daily could have important effects upon inflammation, including heparin, fresh frozen plasma [79], and anesthetic, sedative, and analgesic agents [80]. A recent review [80] describes the potential effects of these agents upon immunomodulation. Catecholamines and inflammation. It is well known that inotropic agents such as catecholamines have a significant impact upon inflammation [81]. Epinephrine inhibits tumor necrosis factor and potentiates interleukin10 leading to a significant anti-inflammatory effect [82], via an effect upon macrophages [83]. Dopamine increases interleukin-6 release but decreases tumor necrosis factor [84]. Recent data support the concept that the anti-inflammatory effect of catecholamines explains the possible beneficial effects of supranormal oxygen delivery in critically ill surgical patients [85]. These data do not enable clinicians to take into account the effect of catecholamines upon inflammation in deciding which is the best to use. Hemofiltration should not be used in patients with sepsis without renal indications unless ongoing studies provide positive results. Rationale Hemofiltration has been shown to decrease cytokines levels significantly, although temporarily during severe sepsis in humans. The technique is widely used in Europe and many authors have strong opinions [86] regarding its use, although the data are weak. A randomized, still unpublished study found no effect upon mortality [87]. Another randomized controlled study [88] reported a 15 % (nonsignificant) increase in survival for filtrated patients. Favorable results have been described for cardiac surgery patients [89]. Large multicenter studies are currently under way. Plasma filtration induced a significant attenuation of acute-phase response in a randomized, prospective study recently performed in 22 adults with sepsis [90]. However, no difference in mortality and only a trend toward fewer organ failures were noted. Plasma exchange has also been used in severe meningococcemia in children [91] with varying results. References 1. Hinshaw LB, Archer LT, Beller Todd BK, et al (1981) Survival in primates in lethal septic shock following delayed treatment with steroid. Circ Shock 8: 291±300 2. Hinshaw LB, Beller Todd BK, Archer LT, et al (1979) Recovery from lethal Escherichia coli shock in dogs. Surg Gynecol Obstet 149: 545±553 3. Hammerschmidt DE, White JG, Craddock PR, et al (1979) Corticosteroids inhibit complement-induced granulocyte aggregation: a possible mechanism for their efficacy in shock states. J Clin Invest 63: 798±803 4. Sprung CL, Caralis PV, Marcial EH, et al (1984) The effect of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med 311: 1137±1143 5. Bone RC, Fisher CJ Jr, Clemmer TP, et al (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 317: 653±658 6. Cooperative Study Group, Bennet IL, Finland M, et al (1963) The effectiveness of hydrocortisone in the management of severe infections. JAMA 183: 462±465 7. Luce JM, Montgomery AB, Marks JD, et al (1988) Ineffectiveness of highdose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am J Respir Crit Care Med 138: 62±68 8. The Veterans Administration Systemic Sepsis Cooperative Study Group (1987) Effect of high-dose glucocorticosteroid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 317: 659±665 9. Lucas C, Ledgerwood A (1984) The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg 119: 537±541 10. Klastersky J, Cappel R, Debusscher L (1971) Effectiveness of betamethasone in management of severe infections. N Engl J Med 284: 1248±1250 11. Thompson WL, Gurley H, Lutz B, et al (1976) Inefficacy of corticosteroids in shock. Clin Res 24: 258A 12. Schumer W (1976) Steroids in the treatment of clinical septic shock. Ann Surg 184: 333±341 S 101 13. Lefering R, Neugebauer EAM (1995) Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med 23: 1294±1303 14. Cronin L, Cook DJ, Carlet J, et al (1995) Corticosteroid treatment for sepsis: a critical appraisal and metaanalysis of the literature. Crit Care Med 23: 1430±1439 15. Bernard GR, Luce JM, Sprung CL, et al (1987) High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 317: 1565±1570 16. Hoffman SL, Punjabi NH, Kumala S, et al (1984) Reduction in mortality in chloramphenicol treated severe typhoid fever by high-dose dexamethasone. N Engl J Med 310: 82±88 17. Lebel MH, Freij BJ, Syrogainnopolous GA, et al (1988) Dexamethasone therapy for bacterial meningitis: results of two double-blind placebo-controlled trials. N Engl J Med 319: 964±971 18. Montaner JS, Lawson LM, Levitt N, et al (1990) Corticosteroids prevent early deterioration in patients with moderate severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome. Ann Intern Med 113: 14±20 19. Gagnon S, Boota AM, Fischl MA, et al (1990) Corticosteroids as adjunctive therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 323: 1440±1450 20. Meduri U, Chinn AJ, Leeper KV, et al (1994) Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS: patterns of response and predictors of outcome. Chest 105: 1516±1527 21. Meduri GU, Headley ASH, Golden E, et al (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA 280: 159±165 22. Bollaert PE, Charpentier C, Levy S, et al (1998) Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26: 645±650 23. Briegel J, Forst H, Haller M, et al (1999) Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single center study. Crit Care Med 27: 723±732 24. Carlet J (1999) From mega to more reasonable doses of corticosteroids: a decade to recreate hope. Crit Care Med 27: 672±674 25. Hatherill M, Tibby SM, Hilliard T, et al (1999) Adrenal insufficiency in septic shock. Arch Dis Child 80: 51±55 25 a. Annane D (2001) Effects of combination hydrocortisone (HC) ± fludro-cortisone (FC) on mortality in septic shock. Crit Care Med (in press) 26. Davies AO, Lefkowitz RJ (1980) Corticosteroids induced differential regulation of beta-adrenergic receptors in circulating human polymorphonuclear leukocytes and mononuclear leukocytes. J Clin Endocrinol Metab 51: 599±605 27. Snapper JR, Hutchison AA, Obletree ML, Brigham KL (1983) Effects of cyclooxygenase inhibitors on the alterations of lung mechanics caused by endotoxemia in unanesthetized sheep. J Clin Invest 72: 63±76 28. Elinger JH, Seyde WC, Longnecker DE (1984) Methylprednisolone plus ibuprofen increase mortality in septic rats. Circ Shock 14: 203±208 29. Haupt MT, Jastremski MS, Clemmer TP, et al (1991) Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. Crit Care Med 19: 1339±1347 30. Arons MM, Wheeler AP, Bernard GR, et al (1999) Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med 27: 699±707 31. Bernard GR, Wheeler AP, Russell JA, et al (1997) The effects of Ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med 336: 912±918 32. Holcroft JW, Vassar MJ, Weber CJ (1986) Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome. A prospective trial. Ann Surg 203: 371±378 33. Bone RC, Slotman G, Maunder R, et al (1989) Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Chest 96: 114±119 34. Silverman JH, Slotman G, Bone RC, et al (1990) Effects of prostaglandin E1 on oxygen delivery and consumption in patients with the adult respiratory distress syndrome. Results from the prostaglandin E1 multicenter trial. The Prostaglandin E1 Study group. Chest 98: 405±410 35. Abraham E, Baughman R, Fletcher E, et al (1999) Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med 27: 1478±1485 36. Yang S, Zhou M, Koo DJ, et al (1999) Pentoxifylline prevents the transition from the hyperdynamic to hypodynamic response during sepsis. Am J Physiol 277: 1036±1044 37. Staubach KH, Schröder J, Stüber F, et al (1998) Effect of pentoxifylline in severe sepsis: results of a randomized, double-blind, placebo-controlled study. Arch Surg 133: 94±100 38. Lauterbach R, Pawlik D, Kowalczyk D, et al (1999) Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, doubleblind trial. Crit Care Med 27: 807±814 39. Suter PM, Dominghetti G, Schaller MD, et al (1994) N-Acetylcysteine enhances recovery from acute lung injury in man. Chest 105: 190±194 40. Jepsen S, Herlevsen P, Knudsen P, et al (1992) antioxidant treatment with Nacetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med 20: 918±923 41. Spies CD, Reinhart K, Witt I, et al (1994) Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double-blind study. Crit Care Med 22: 1746±1748 42. Peake SL, Moran JL, Leppard PI (1996) N-Acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit Care Med 24: 1302±1310 43. Molnar Z, Shearer E, Lowe D, et al (1999) N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med 27: 1100±1104 44. De Backer WA, Amsel B, Jorens PG, et al (1996) N-Acetylcysteine pretreatment of cardiac surgery patients influences plasma neutrophil elastase and neutrophil influx in bronchoalveolar lavage fluid. Intensive Care Med 22: 900±908 45. Ortolani O, Conti A, De Gaudio AR, et al (2000) The effect of glutathione and N-acethylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med 161: 1907±1911 46. Forceville X, Vitoux D, Gauzit RØmy, et al (1998) Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med 26: 1536±1544 47. Angstwurm MWA, Schottdorf J, Schopohl J, et al (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27: 1807±1813 S 102 48. Vervloet MG, Thijs LG, Hack CE (1998) Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost 24: 33±44 49. Fourrier F, Chopin C, Huart JJ, et al (1993) Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 104: 882±888 50. Baudo F, Caimi TM, de Cataldo F, et al (1998) Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Med 24: 336±342 51. Eisele B, Lamy M, Thijs LG, et al (1998) Anti-thrombin III in patients with severe sepsis: a randomized, placebo-controlled, double-blind, multicenter trial plus a meta-analysis on all randomized placebo-controlled, doubleblind trials with anti-thrombin III in severe sepsis. Intensive Care Med 24: 663±672 52. Riess H (2000) Antithrombin in severe sepsis. ªNewº indication of an ªoldº drug. Intensive Care Med 26: 657±665 53. Volk HD, Reimke P, Krausch D, et al (1996) Monocyte deactivation ± rationale for a new therapeutic strategy in sepsis. Intensive Care Med 22:S471±S481 54. Kaul R, McGeer A, Norrby-Teglund A, et al (1999) Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome ± a comparative observational study. Clin Infect Dis 28: 800±807 55. Alejandria MM, Lansang MA, Dans LF, Mantaring JB (2000) Intravenous immunoglobulins for treating sepsis and septic shock. Cochrane database Syst Rev (2) CD001090 56. Pilz G, Appel R, Kreuzer E, Werdan K (1997) Comparison of early IgM-enriched immunoglobulin vs. polyvalent IgG administration in score-identified postcardiac surgical patients at high risk for sepsis. Chest 111: 419±426 57. The Intravenous Immunoglobulin Collaborative Study Group (1992) Prophylactic intravenous administration of standard immune globulin as compared with core-lipopolysaccharide immune globulin in patients at high-risk of postsurgical infection. N Engl J Med 327: 234±240 58. Jenson HG, Pollock BH (1997) Metaanalyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 99:E2 59. Livingston DH, Loder PA, Kramer SM, et al (1994) Interferon gamma administration increases monocyte HLA-DR antigen expression but not endogenous interferon production. Arch Surg 129: 172±178 60. Kohler J, Heumann D, Garotta G, et al (1993) IFN-gamma involvement in the severity of gram-negative infections in mice. J Immunol 151: 916±921 61. The International Chronic Granulomatous Disease Cooperative Study Group (1999) A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med 1324: 509±516 62. Dries DJ, Jurkovich J, Maier RV, et al (1994) Effect of interferon gamma on infection-related death in patients with severe injuries. Arch Surg 129: 1031±1042 63. Polk HC Jr, Cheadle WG, Linvingston DH, et al (1992) A randomized prospective clinical trial to determine the efficacy of interferon-g in severely injured patients. Am J Surg 63: 191±196 64. Wasserman D, Ioannovich JD, Hinzmann RD, et al (1998) Interferon-g in the prevention of severe burn-related infections: a European phase III multicenter trial. Crit Care Med 26: 434±439 65. Zhang P, Bagby GH, Stoltz DA, et al (1998) Enhancement of peritoneal leukocyte function by granulocyte colonystimulating factor in rats with abdominal sepsis. Crit Care Med 26: 315±321 66. Lundblad R, Nesland JM, Giercksky KE (1996) Granulocyte colony-stimulating factor improves survival rate and reduces concentrations of bacteria, endotoxin, tumor necrosis factor, and endothelin-1 in fulminant intra-abdominal sepsis in rats. Crit Care Med 24: 820±826 67. Karzai W, Ulrich von Specht B, Parent C, et al (1999) G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am J Respir Crit Care Med 159: 1377±1382 68. Nelson S, Farkas S, Fotheringham N, et al (1996) Filgrastim in the treatment of hospitalized patients with community acquired pneumonia (CAP) (abstract). Am J Respir Crit Care Med 151:A535 69. Wunderink RG, Leeper KV, Schein RMH, et al (1996) Clinical response to figrastim in pneumonia with severe sepsis. Am J Respir Crit Care Med 153:A123 70. Heard S, Fink MI, Gamelli RE, et al (1998) The effect of prophylactic administration of recombinant human GCSF on the incidence of nosocomial infections in patients with acute traumatic brain injury or cerebral hemorrhage. Crit Care Med 26: 748±754 71. Takala J, Ruokonen E, Webster NR, et al (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785±792 72. Doig GS, Martin CM, Sibbald WJ (1997) Polymixin-dextran anti entodotin pre-treatment in an ovin model of normotensive sepsis. Crit Care Med 25: 1956±1961 73. Yu M, Tomasa G (1993) A doubleblind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med 21: 1635±1642 74. The ARDS network (2000) Ketaconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomised controlled trial. JAMA 283: 1995±2002 75. Siedlar M, Szczepanik A, Wieckiewicz J, et al (1997) Vancomycin down-regulates lipopolysaccharide-induced tumor necrosis factor alpha (TNF-alpha) production and TNF alpha mRNA a accumulation in human blood monotyes. Immunopharmacology 35: 265±271 76. Khan AA, Slifer TR, Remington JS (1998) Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob Agents Chemother 42: 1713±1717 77. Riesbeck K, Gullberg M, Forsgren A (1994) Evidence that the antibiotic ciprofloxacin counteracts cyclosporin-dependent suppression of cytokine production. Transplantation 57: 267±272 78. Willatts SM, Radford S, Leitermann M (1995) Effect of the antiendotoxic agent, taurolidine, in the treatment of sepsis syndrome: a placebo-controlled, double-blind trial. Crit Care Med 23: 1033±1039 79. Eisenfeld L, Krause PJ, Herson VC, et al (1992) Enhancement of neonatal neutrophil motility (chemotaxis) with adult fresh frozen plasma. Am J Perinatol 9: 5±8 80. Galley HF, DIMatteo MA, Webster NR (2000) Immunomodulation by anaesthetic, sedative and analgesic agents: does it matter? Intensive Care Med 26: 267±274 S 103 81. Severn A, Rapson NT, Hunter CA, et al (1992) Regulation of tumor necrosis factor production by adrenaline and badrenergic agonists. J Immunol 148: 3441±3445 82. Van Der Poll T, Coyle SM, Barbosa K, et al (1996) Epinephrine inhibits tumor necrosis factor-a and potentiates interleukin 10 production during human endotoxemia. J Clin Invest 97: 713±719 83. Spengler RN, Chensue SW, Giacherio DA, et al (1994) Endogenous norephinephrine regulates tumor necrosis factor-a production from macrophages in vitro. J Immunol 152: 3024±3031 84. Ritchie PK, Ashby M, Knight HH, et al (1996) Dopamine increase interleukin 6 release and inhibits tumor necrosis factor release from rat adrenal zona glomerulosa in vitro. Eur J Endocrinol 134: 610±616 85. Unsaro A, Russell JA (2000) Could anti-inflammatory actions of catecholamines explain the possible effect of supranormal oxygen delivery in critically ill surgical patient. Intensive Care Med 26: 299±304 86. Bellomo R, Ronco C (1999) Continuous renal replacement therapy in the intensive care unit. Intensive Care Med 25: 781±789 87. Mehta R, McDonald B, Gabbai F, et al (1996) Continuous versus intermittent dialysis for acute renal failure in the ICU: results from a randomized multicenter trial. J Am Soc Nephrol 5: 1457 88. Silvester W (1998) Outcome studies of continuous renal replacement therapy in the intensive care. Kidney Int 53:S138±S141 89. Journois D (1999) Hemofiltration during cardiopulmonary bypass. Minerva Anestesiol 65: 427±432 90. Reeves JH, Warwick WB, Shann F, et al (1999) Continuous plasmafiltration in sepsis syndrome. Crit Care Med 27: 2096±2104 91. Churchwell KB, McManus ML, Kent P, et al (1995) Intensive blood and plasma exchange for treatment of coagulopathy in meningococcemia. J Clin Apheresis 10: 171±177 Intensive Care Med (2001) 27: S 93±S 103 J. Carlet Immunological therapy in sepsis: currently available ) J. Carlet ( ) Intensive Care Unit, Fondation-Hôpital Saint-Joseph, Paris, France E-mail: [email protected] Phone: +33-1 44-12 37 83 Fax: +33-1 44-12 34 15 Should corticosteroids be used during septic shock at low doses and for a prolonged period of time? Answer: yes, grade C. Recommendations Introduction Many therapies used in our daily practice are known to have significant effects on inflammation. These drugs influence the activation of the inflammatory network that occurs during severe sepsis and related syndromes as disseminated intravascular coagulation and acute respiratory distress syndrome (ARDS). Many of these compounds (Table 1) have already been used during experimental models of sepsis and/or human studies. Corticosteroids should not be used in severe sepsis or septic shock at high doses (30 mg/kg) and for a short course (1±2 days). On the other hand, corticosteroids may be used during ªrefractoryº septic shock but not during severe sepsis without shock or mild shock. It should then be used at low doses (100 mg hydrocortisone three times a day) for 5 days or more and then with subsequent tapering of the dose according to the hemodynamic status and the need for vasopressors. Rationale Methods This contribution reviews those drugs that are available in the daily management of severe sepsis and septic shock. A computer-based review of the literature was undertaken using Medline from 1990 to September 1999 as the primary database. The subject heading keywords defined for each of the compounds listed in Table 1 were combined with the following general sepsis-related subject heading keywords: sepsis, severe sepsis, septic shock, and ARDS. Anti-inflammatory agents Should corticosteroids be used in the treatment of severe sepsis or septic shock at high doses (30 mg/kg) for a short course (one or 2 days)? Answer: no, grade A. An extensive literature is available for corticosteroids. Steroids have been used for many years, and their efficacy is controversial. Numerous animal studies performed during experimental septic (endotoxic) shock or acute lung injuries showed a very significant reduction in both intensity of shock, acute respiratory failure and mortality [1, 2]. They have been used at very high doses (30 mg/kg per dose for a maximum of 24±48 h). The ability of these high doses of corticosteroids to reduce complement activation and to inhibit leukocyte aggregability and adherence was at that time a very logical rationale for their efficacy [3]. Very promising initial findings have been published regarding humans [12]. However, two well designed, prospective, multicenter, randomized, double-blind studies demonstrated very clearly their inability to decrease mortality [5, 8]. Some studies mention positive trends when looking at subgroups of infections due to Gram-negative rods [5, 8, 13]. S 94 Table 1 List of therapies currently available for eventually treating severe sepsis Therapy References Anti-inflammatory agents Corticosteroids (high or low doses) Ibuprofen Prostaglandin E1 Pentoxifylline Oxygen scavengers N-Acetylcysteine Selenium Drugs modifying coagulation Antithrombin III Drugs enhancing host defenses Immunoglobulins Interferon-g Granulocytes stimulating factors Immunonutrition 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 25a 27, 28, 29, 30, 31 32, 33, 34, 35 36, 37, 38 39, 40, 41, 42, 43, 44, 45, 46, 47 39, 40, 41, 42, 43, 44, 45 46, 47 48, 49, 50, 51, 52 48, 49, 50, 51, 52 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 54, 55, 56, 57, 58 59, 60, 61, 62, 63, 64 65, 66, 67, 68, 69, 70 ±a Other drugs Growth hormone Antibiotics Including ketoconazole Including polymyxin B Taurolidine Fresh frozen plasma Anesthetic sedative and analgesic agents Catecholamines 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 71 72, 73, 74, 75, 76, 77 73, 74 72 78 79 80 81, 82, 83, 84, 85 Hemofiltration, plasma filtration, plasma exchange 86, 87, 88, 89, 90, 91 a See PØrez and Dellinger, ªOther supportive therapies in sepsisº Two recent meta-analyses [13, 14] reviewing the studies confirm that corticosteroids at the dose of 30 mg/kg (one or two doses) are ineffective [13] or even harmful [14]. The design and the results of the nine randomized studies are summarized in Tables 2 and 3. Similar negative results have been obtained during ARDS [15]. Pooling the results only from those patients with Gram-negative infections, as in the meta-analysis by Lefering et al. [13], yields a rate difference of ±5.6 % [confidence interval (CI): ±21.4 to 10.1) in favor of steroids, based on 413 patients. Those patients with Gram-positive infections (n = 306) had an overall effect of +1.8 % (CI: ±15.8 to 18.6). Most persons stopped using steroids when these large trials were published. Several studies performed over the years, however, have maintained interest in the use of corticosteroids. Mortality was reduced using steroids during severe typhoid fever [16], and neurological sequelae were reduced during meningitis [17]. Two large double-blind case control studies demonstrated that prolonged treatment (10±15 days) of relatively low doses of steroids (120±240 mg hydrocortisone) dramatically reduced mortality during severe Pneumocystis carinii pneumonia in AIDS patients [18, 19]. In addition, Meduri et al. [20] showed that the course of late, fibrotic ARDS was improved by steroid use which was confirmed in a re- cent randomized double-blind study showing a significant reduction in mortality [21]. Two small, randomized, double-blind studies of steroids in patients with severe and refractory septic shock recently demonstrated positive results [22, 23]. Corticosteroids were used at small doses (100 mg hydrocortisone three times per day in one [22] and 100 mg followed by a continuous infusion of 0.18 mg/kg per hour in the other [23], for longer periods of time than in past studies: 5 days in one [22] and 5±10 days in the other, with tapering of the doses according to hemodynamic status and need for vasopressors. Both studies showed a significant reversal of shock and organ failures and a trend in reduction in mortality. Additional studies are necessary, and a French multicenter randomized, controlled, double-blind study reported that low dose steroids decrease mortality in patients with septic shock [25 a]. Several factors may explain the recent positive effects of corticosteroids during sepsis [24]. These include the treatment of ªrelativeº adrenal insufficiency [25] and the potentiation of adrenergic receptivity [26] in addition to the anti-inflammatory effect. The lower immunosuppressive doses and a more prolonged duration of therapy than in the initial studies could also explain discrepancies. S 95 Table 2 Design of the nine randomized studies used in the meta-analysis (from Cronin et al. [14]) (DB double blind, M methylprednisolone, B betamethasone, D dexamethasone, H hydrocortisone) Reference n Type of study Product Dose Duration Endpoints Cooperative Study Group [6] Klastersky et al. [10] Schumer et al. [12] 194 Open H 6d 85 Open B 300 mg then 50 mg/d 1 mg/kg 172 DB M 30 mg/kg 1 dose or 2 Thompson et al. [11] Lucas and Ledgerwood [9] Sprung et al. [4] 60 DB M 30 mg/kg 48 Open D 2 mg/kg Max. 6 doses in 24 h 2d 59 Open M 30 mg/kg 1 dose (or 2) Mortality, complications Mortality (20 d), complications Mortality (28 d), complications Mortality, complications Mortality (14 d), complications Hospital mortality, complications 3d Bone et al. [5] 381 DB M 30 mg/kg 1d Mortality (14 d), complications Veteran Administration [8] 223 DB M 30 mg/kg 9d Mortality (14 d), complications 75 DB M 30 mg/kg4 1d Hospital mortality, ARDS complications Luce et al. [7] Table 3 Results of the nine randomized studies used in the metaanalysis (from Cronin et al. [14]) Cooperative study group [6] Klastersky et al. [10] Schumer et al. [12] Thompson et al. [11] Lucas et al. [9] Sprung et al. [4] Bone et al. [5] Veteran Administration [8] Luce et al. [7] n Risk ratio 95 % CI 194 85 172 60 48 59 381 223 75 1.72 0.97 0.30 1.01 1.09 1.11 1.35 0.95 1.07 1.23±2.41 0.65±1.45 0.13±0.72 0.77±1.31 0.36±3.27 0.74±1.67 0.98±1.84 0.57±1.58 0.72±1.60 Should ibuprofen be used in the treatment of severe sepsis and septic shock? Answer: no, grade B. Recommendations Ibuprofen should not be used during severe sepsis or septic shock. Additional studies are needed to determine whether some patients, for example, those with hypothermia, could benefit from the drug. Rationale Ibuprofen is a powerful anti-inflammatory agent, acting on the prostaglandin metabolism as a cyclo-oxygenase inhibitor. It has been used with controversial effects in animals during both experimental sepsis and ARDS [27, 28]. Two small randomized, double-blind studies in patients showed some hemodynamic effect and a normalization of pH without any significant effect upon mortality [29, 30]. Mortality was decreased significantly in a post hoc analysis of hypothermic patients [30]. A large multicenter randomized, controlled, double blind study, however, failed to demonstrate any effect upon mortality, reversal of shock or acute respiratory failure [31]. Ibuprofen was able to reduce the levels of prostacyclin and thromboxane and to decrease fever, tachycardia and oxygen consumption [31]. The drug was not associated with adverse affects. Should prostaglandins be used in the treatment of ARDS due to severe infections and sepsis? Answer: no, grade B. Recommendations Prostaglandins, in particular prostaglandin E1 or liposomal prostaglandin E1 should not be used during ARDS due to sepsis. There are no specific data allowing recommendations in severe sepsis. S 96 Rationale Oxygen scavengers Several prostaglandins which have both an anti-inflammatory and a vasoactive effect have been studied including prostaglandin I2 and particularly prostaglandin E1 [32, 33, 34, 35] during ARDS. The vast majority of these patients had ARDS due to severe infections or sepsis. An early, small, randomized study showed promising results [32]. However, a large multicenter, randomized, controlled, double blind study failed to show any difference in survival [33]. An increase in oxygen delivery and oxygen consumption was noted in treated patients who survived [34]. A recent, multicenter randomized, controlled, double-blind study with liposomal prostaglandin E1 (TLC C-53) showed that indices of oxygenation of treated ARDS patients were improved compared with controls, but without any effect upon duration of mechanical ventilation or 28 days mortality [35]. Again, most ARDS was due to sepsis in these two large studies. No data are really available concerning an overall group of patients with severe sepsis. Several oxygen scavengers are currently available, including N-acetylcysteine (NAC), vitamin E, vitamin C, and selenium. Vitamins E and C have been only poorly studied in humans, and we focus on N-acetylcysteine and selenium. Should pentoxifylline be used in the treatment of severe sepsis in (a) adults, (b) infants? Answer: (a) no, grade B; (b) no, grade C. Recommendations Pentoxifylline should not be used in adults with severe sepsis unless new studies show a significant effect. The positive effect of a small study in infants should be confirmed before clinical use. Rationale Pentoxifylline, which has a powerful anti-inflammatory effect including a strong inhibition of tumor necrosis factor secretion, has been used successfully in many animal studies with the prevention of the transition from a hyperdynamic to hypodynamic state, although no effect upon mortality has been shown [36]. Human studies are more scarce. A multicenter, randomized, controlled, double-blind study during sepsis showed an increase in PaO2/FIO2 ratio but no effect upon cytokines levels or mortality [37]. A recent double-blind study performed in premature infants with sepsis showed a decrease in cytokines levels and a significant decrease in mortality (1/40 vs. 6/38 p = 0.046) [38]. However, the size of this study was rather small, and additional large studies are mandatory. Should N-acetylcysteine be used in the treatment of severe sepsis? Answer: no, grade C. Recommendations NAC should not be used in severe sepsis until new data are available, focusing in particular on very early therapy. Rationale During acute lung injury an improvement in oxygenation and reduction in the required length of mechanical ventilation was found in patients treated with NAC compared to controls [39]. However, several randomized studies have shown no difference in mortality, gas exchange, and development of respiratory failure in patients treated with NAC [39, 40]. Several studies have also been performed during severe sepsis, with heterogeneous results [41, 42, 43, 44]. Depressed cardiac performance has been described in septic patients treated with NAC [42]. A very recent multicenter, randomized, controlled, double-blind study showed that a prolonged infusion of NAC is unable to prevent multiple organ failure in consecutively admitted critically ill patients [43]. In this study treatment used more than 24 h after the initial insult worsened the prognosis compared to controls. Better results were obtained when the drug was use before the insult, as during cardiac surgery [44]. These results suggest that this compound could be helpful when started before (or perhaps shortly after) the insult, but possibly harmful when started too late. Combinations of several antioxidants have also been published, but data are too limited to allow recommendations [45]. Should selenium be used in the treatment of severe sepsis? Answer: no, grade C. S 97 Fig. 1 Effect of proinflammatory cytokines. Upon coagulation cascade during sepsis leading to an activation of tissue factor, a depletion in protein C (via a decrease in thrombomodulin levels) antithrombin III and C1 inhibitor, and a decrease in fibrinolysis (via the effect of plasminogen activator inhibitor 1) Recommendations Selenium should not be used for severe sepsis. Additional studies are warranted to confirm initial positive data. Rationale A profound depletion in selenium levels has been demonstrated in many severe septic patients [46]. Mortality and morbidity are far higher in patients with a very low selenium level [46]. A recent prospective, randomized, but nonblinded study performed in septic patients showed that selenium replacement is able to reduce severity indexes at day 3 and reduce the need for hemodialysis but has no significant effect upon mortality (52 % in controls and 33, 5 % in treated patients, p = 0.13) [47]. Additional large studies are needed to confirm initial promising results. Drugs modifying coagulation There are complex interactions between the inflammation and coagulation systems (Fig. 1). Proinflammatory cytokines activate coagulation cascades, in particular via an effect upon tissue factor which is a key player in the coagulation cascade. They can also reduce fibrinolysis and profoundly reduce the levels of protein C and of antithrombin III which are important anticoagulant agents. Antithrombin III inhibits several coagulation factors of the extrinsic pathway such as factors IXa, XIa, XIIa in addition to factors Xa, IIa, and plasmin. Activated protein C inhibits factors Va, Vlla, and plasminogen activator inhibitor 1. The overall effect during sepsis is a marked procoagulant balance. Conversely, coagulation products can activate the inflammation network which creates numerous amplification loops. For example, thrombin can induce an up-regulation of Pand E-selectin, and contact factor activation can induce the production of bradykinin, worsening hypotension and tissue hypoperfusion. In humans studies, both anti- S 98 thrombin III and protein C levels are sharply decreased [48], and mortality of septic patients is inversely correlated with the levels of those two products. This makes the rationale for studying those types of compounds, such as antithrombin III, protein C, and tissue factor protein inhibitor very strong. Only antithrombin III is currently available. Should antithrombin III be used in the treatment of severe sepsis? Answer: no, grade B. Recommendations Antithrombin III should not be used during severe sepsis. Countries which allow the free use of this drug in this setting should reconsider their position. Rationale Antithrombin III is a drug which is widely used for septic patients in several countries. Three randomized, small, double-blind studies were published [49, 50, 51]. Duration of disseminated intravascular coagulation was reduced [49] as well as the number of organ failures [51], but mortality was not different although a positive trend was clearly noted. A meta-analysis was also performed [51] showing a 22.9 % reduction in mortality but which did not reach statistical significance. Unfortunately a large multicenter, prospective, double-blind study has recently been completed which showed no significant improvement in survival [52]. The complete data have not yet been published. Other drugs such as activated protein C and tissue factor inhibitors are not currently available and are discussed elsewhere (see Arndt and Abraham, ªImmunological therapy of sepsis: experimental therapiesº). Drugs enhancing host defenses After the initial activation of the proinflammatory network, a profound immunodepression can occur in septic patients [53]. This could influence outcome increasing the risk of nosocomial infections. Several strategies have been used to increase host defenses, including polyvalent immunoglobulins, interferon-g, stimulating factors for granulocytes [including granulocyte colony stimulating factor (G-CSF)], and immunonutrition. The latter is discussed elsewhere (see PØrez and Dellinger, ªOther supportive therapy in sepsisº). Should intravenous immunoglobulins be used in the treatment of severe sepsis in (a) adults or (b) neonates? Answer: (a) no, grade C; (b) no, grade C. Recommendations Immunoglobulins should not be used either in adult patients or in neonates with sepsis, unless additional large studies confirm some positive data in small-sized metaanalyses. Countries which allow a wide use of these compounds should reconsider their position and encourage these studies. Rationale Intravenous immunoglobulins (IVIG) are widely used in both infants and adults in the treatment of severe sepsis, at least in certain countries. Reports which support their empirical use, however, are still rather weak. The rationale is to restore immunoglobulins levels, which may be depressed in sepsis, and to provide patients with specific antibodies against micro-organisms. No individual well designed clinical study has been performed in adults with severe sepsis. A recent study was performed in patients with streptococcal toxic shock syndrome [54]. This was a comparative nonblinded study performed in 21 patients which demonstrated a significantly reduced mortality (67 % vs. 34 %, p = 0.02). Both Acute Physiology and Chronic Health Evaluation II scores and IVIG were prognostic factors in the multivariate analysis. The odds ratio associated with IVIG was 8.1 (95 % CI: 1.6±45). A recent meta-analysis by the Cochrane group [55] looking at 23 studies (some of them unpublished) on immunoglobulins, antiendotoxins, and anticytokines, extracted from the small size studies already published, evaluated a population of 413 patients receiving polyclonal immunoglobulins. Mortality was significantly reduced (relative risk: 0.6; 95 % CI: 0.47±0.76). Results were even more positive when only sepsis related deaths were considered. A large, well designed, multicenter, randomized, doubleblind study is, however, warranted before making firm conclusions. Two prophylactic studies have been published recently [56, 57]. A study performed in cardiac surgery patients showed no difference in the occurrence of sepsis between polyvalent IVIG and IgM-enriched immunoglobulin [56]. A prospective comparative study showed that IVIG and not placebo is able to prevent nosocomial infections after major surgery [57]. Such prophylactic studies are needed in this field in nonsurgical critically ill patients. In neonatal sepsis, a recent meta-analysis of 110 newborns in three studies showed that IVIG is able to re- S 99 duce mortality significantly (odds ratio: 0.173; 95 % CI: 0.031±0.735; p = 0.007) [58]. However, the size of the overall population was very small, and large studies are urgently warranted. In the same meta-analysis the effect of IVIG in the prevention of sepsis in 4933 evaluable newborns was significant (p = 0.0193, two-tailed), although heterogeneity of the studies precluded estimation of an overall odds ratio. Other Drugs Interferon-g Growth hormone should not be used in patients with sepsis because it increases mortality. Interferon-g has been used successfully in animals models of Gram-negative sepsis [59, 60]. Few data are available in human sepsis. The drug has been used with positive results to prevent infection during chronic granulomatous disease [61] and trauma [62, 63]. The drug, however, was unable to prevent infections in burn patients [64]. Data are insufficient for therapy of severe sepsis to allow recommendations. Should growth hormone be used in the treatment of severe sepsis? Answer: no, grade A. Recommendations Rationale Answer: no, grade C. The administration of growth hormone could in theory attenuate the catabolic response to injury, surgery or sepsis. Two prospective double-blind studies with more than 200 patients each were recently reported in critically ill patients with cardiac or abdominal surgery, multiple trauma or acute respiratory failure [71]. Mortality was increased significantly in treated patients. The relative risk in these two pooled studies was 1.9 (95 % CI: 1.3±2.9). Length of stay and duration of mechanical ventilation were longer in treated survivors than in controls. Recommendations Antimicrobial compounds G-CSF should not be used in nonneutropenic patients with severe sepsis. Polymixin B. Polymixin B is able to neutralize endotoxin via strong antilipid A activity [72]. Since it is very toxic, it is difficult to use intravenously in humans, although some derivates are less toxic. Extracorporeal techniques, in which polymyxin is coated on membranes, are under investigation. Should granulocyte colony stimulating factor be used in the treatment of severe infections? Rationale G-CSF is very efficient and reduces mortality in animal models of abdominal sepsis [65, 66]. During pneumonia models in rats the drug has been shown to exert different effects according to the micro-organisms involved [67]. Preliminary studies have been performed in community or hospital acquired pneumonia with controversial results [68, 69]. In patients with head trauma and receiving mechanical ventilation G-CSF prophylaxis did not improve outcome nor lower the risk of nosocomial pneumonia [70]. Ketoconazole. Ketaconazole, one of the new imidazoles, has a strong effect upon thromboxane synthase inhibition and has been shown to prevent ARDS in septic patients in a small double-blind randomized study [73]. A recent study performed in 234 patients, however, failed to demonstrate any effect upon mortality and duration of mechanical ventilation in ARDS patients [74]. No data are available in patients with sepsis. Other antibiotics Immunonutrition See PØrez and Dellinger, ªOther supportive therapies in sepsis.º Some antibiotics have anti-inflammatory effects, in particular in decreasing cytokine release. Effects have been shown for vancomycin [75] trovafloxacin [76] and ciprofloxacin [77]. S 100 Taurolidine Hemofiltration and plasma filtration Taurolidine is an anti-infective agent (nonantibiotic), used either locally, or intravenously, which has some antibacterial effect associated with an antiendotoxin effect. A randomized placebo-controlled study failed to demonstrate any effect on outcome in sepsis [78]. Should hemofiltration be used in the treatment of patients with severe sepsis, without renal indications? Answer: no, grade C. Recommendations Other drugs currently used Many other drugs that we use daily could have important effects upon inflammation, including heparin, fresh frozen plasma [79], and anesthetic, sedative, and analgesic agents [80]. A recent review [80] describes the potential effects of these agents upon immunomodulation. Catecholamines and inflammation. It is well known that inotropic agents such as catecholamines have a significant impact upon inflammation [81]. Epinephrine inhibits tumor necrosis factor and potentiates interleukin10 leading to a significant anti-inflammatory effect [82], via an effect upon macrophages [83]. Dopamine increases interleukin-6 release but decreases tumor necrosis factor [84]. Recent data support the concept that the anti-inflammatory effect of catecholamines explains the possible beneficial effects of supranormal oxygen delivery in critically ill surgical patients [85]. These data do not enable clinicians to take into account the effect of catecholamines upon inflammation in deciding which is the best to use. Hemofiltration should not be used in patients with sepsis without renal indications unless ongoing studies provide positive results. Rationale Hemofiltration has been shown to decrease cytokines levels significantly, although temporarily during severe sepsis in humans. The technique is widely used in Europe and many authors have strong opinions [86] regarding its use, although the data are weak. A randomized, still unpublished study found no effect upon mortality [87]. Another randomized controlled study [88] reported a 15 % (nonsignificant) increase in survival for filtrated patients. Favorable results have been described for cardiac surgery patients [89]. Large multicenter studies are currently under way. Plasma filtration induced a significant attenuation of acute-phase response in a randomized, prospective study recently performed in 22 adults with sepsis [90]. However, no difference in mortality and only a trend toward fewer organ failures were noted. Plasma exchange has also been used in severe meningococcemia in children [91] with varying results. References 1. Hinshaw LB, Archer LT, Beller Todd BK, et al (1981) Survival in primates in lethal septic shock following delayed treatment with steroid. Circ Shock 8: 291±300 2. Hinshaw LB, Beller Todd BK, Archer LT, et al (1979) Recovery from lethal Escherichia coli shock in dogs. Surg Gynecol Obstet 149: 545±553 3. Hammerschmidt DE, White JG, Craddock PR, et al (1979) Corticosteroids inhibit complement-induced granulocyte aggregation: a possible mechanism for their efficacy in shock states. J Clin Invest 63: 798±803 4. Sprung CL, Caralis PV, Marcial EH, et al (1984) The effect of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med 311: 1137±1143 5. Bone RC, Fisher CJ Jr, Clemmer TP, et al (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 317: 653±658 6. Cooperative Study Group, Bennet IL, Finland M, et al (1963) The effectiveness of hydrocortisone in the management of severe infections. JAMA 183: 462±465 7. Luce JM, Montgomery AB, Marks JD, et al (1988) Ineffectiveness of highdose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am J Respir Crit Care Med 138: 62±68 8. The Veterans Administration Systemic Sepsis Cooperative Study Group (1987) Effect of high-dose glucocorticosteroid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 317: 659±665 9. Lucas C, Ledgerwood A (1984) The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg 119: 537±541 10. Klastersky J, Cappel R, Debusscher L (1971) Effectiveness of betamethasone in management of severe infections. N Engl J Med 284: 1248±1250 11. Thompson WL, Gurley H, Lutz B, et al (1976) Inefficacy of corticosteroids in shock. Clin Res 24: 258A 12. Schumer W (1976) Steroids in the treatment of clinical septic shock. Ann Surg 184: 333±341 S 101 13. Lefering R, Neugebauer EAM (1995) Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med 23: 1294±1303 14. Cronin L, Cook DJ, Carlet J, et al (1995) Corticosteroid treatment for sepsis: a critical appraisal and metaanalysis of the literature. Crit Care Med 23: 1430±1439 15. Bernard GR, Luce JM, Sprung CL, et al (1987) High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 317: 1565±1570 16. Hoffman SL, Punjabi NH, Kumala S, et al (1984) Reduction in mortality in chloramphenicol treated severe typhoid fever by high-dose dexamethasone. N Engl J Med 310: 82±88 17. Lebel MH, Freij BJ, Syrogainnopolous GA, et al (1988) Dexamethasone therapy for bacterial meningitis: results of two double-blind placebo-controlled trials. N Engl J Med 319: 964±971 18. Montaner JS, Lawson LM, Levitt N, et al (1990) Corticosteroids prevent early deterioration in patients with moderate severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome. Ann Intern Med 113: 14±20 19. Gagnon S, Boota AM, Fischl MA, et al (1990) Corticosteroids as adjunctive therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 323: 1440±1450 20. Meduri U, Chinn AJ, Leeper KV, et al (1994) Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS: patterns of response and predictors of outcome. Chest 105: 1516±1527 21. Meduri GU, Headley ASH, Golden E, et al (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA 280: 159±165 22. Bollaert PE, Charpentier C, Levy S, et al (1998) Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26: 645±650 23. Briegel J, Forst H, Haller M, et al (1999) Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single center study. Crit Care Med 27: 723±732 24. Carlet J (1999) From mega to more reasonable doses of corticosteroids: a decade to recreate hope. Crit Care Med 27: 672±674 25. Hatherill M, Tibby SM, Hilliard T, et al (1999) Adrenal insufficiency in septic shock. Arch Dis Child 80: 51±55 25 a. Annane D (2001) Effects of combination hydrocortisone (HC) ± fludro-cortisone (FC) on mortality in septic shock. Crit Care Med (in press) 26. Davies AO, Lefkowitz RJ (1980) Corticosteroids induced differential regulation of beta-adrenergic receptors in circulating human polymorphonuclear leukocytes and mononuclear leukocytes. J Clin Endocrinol Metab 51: 599±605 27. Snapper JR, Hutchison AA, Obletree ML, Brigham KL (1983) Effects of cyclooxygenase inhibitors on the alterations of lung mechanics caused by endotoxemia in unanesthetized sheep. J Clin Invest 72: 63±76 28. Elinger JH, Seyde WC, Longnecker DE (1984) Methylprednisolone plus ibuprofen increase mortality in septic rats. Circ Shock 14: 203±208 29. Haupt MT, Jastremski MS, Clemmer TP, et al (1991) Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. Crit Care Med 19: 1339±1347 30. Arons MM, Wheeler AP, Bernard GR, et al (1999) Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med 27: 699±707 31. Bernard GR, Wheeler AP, Russell JA, et al (1997) The effects of Ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med 336: 912±918 32. Holcroft JW, Vassar MJ, Weber CJ (1986) Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome. A prospective trial. Ann Surg 203: 371±378 33. Bone RC, Slotman G, Maunder R, et al (1989) Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Chest 96: 114±119 34. Silverman JH, Slotman G, Bone RC, et al (1990) Effects of prostaglandin E1 on oxygen delivery and consumption in patients with the adult respiratory distress syndrome. Results from the prostaglandin E1 multicenter trial. The Prostaglandin E1 Study group. Chest 98: 405±410 35. Abraham E, Baughman R, Fletcher E, et al (1999) Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. Crit Care Med 27: 1478±1485 36. Yang S, Zhou M, Koo DJ, et al (1999) Pentoxifylline prevents the transition from the hyperdynamic to hypodynamic response during sepsis. Am J Physiol 277: 1036±1044 37. Staubach KH, Schröder J, Stüber F, et al (1998) Effect of pentoxifylline in severe sepsis: results of a randomized, double-blind, placebo-controlled study. Arch Surg 133: 94±100 38. Lauterbach R, Pawlik D, Kowalczyk D, et al (1999) Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, doubleblind trial. Crit Care Med 27: 807±814 39. Suter PM, Dominghetti G, Schaller MD, et al (1994) N-Acetylcysteine enhances recovery from acute lung injury in man. Chest 105: 190±194 40. Jepsen S, Herlevsen P, Knudsen P, et al (1992) antioxidant treatment with Nacetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med 20: 918±923 41. Spies CD, Reinhart K, Witt I, et al (1994) Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double-blind study. Crit Care Med 22: 1746±1748 42. Peake SL, Moran JL, Leppard PI (1996) N-Acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit Care Med 24: 1302±1310 43. Molnar Z, Shearer E, Lowe D, et al (1999) N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med 27: 1100±1104 44. De Backer WA, Amsel B, Jorens PG, et al (1996) N-Acetylcysteine pretreatment of cardiac surgery patients influences plasma neutrophil elastase and neutrophil influx in bronchoalveolar lavage fluid. Intensive Care Med 22: 900±908 45. Ortolani O, Conti A, De Gaudio AR, et al (2000) The effect of glutathione and N-acethylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med 161: 1907±1911 46. Forceville X, Vitoux D, Gauzit RØmy, et al (1998) Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med 26: 1536±1544 47. Angstwurm MWA, Schottdorf J, Schopohl J, et al (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27: 1807±1813 S 102 48. Vervloet MG, Thijs LG, Hack CE (1998) Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost 24: 33±44 49. Fourrier F, Chopin C, Huart JJ, et al (1993) Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 104: 882±888 50. Baudo F, Caimi TM, de Cataldo F, et al (1998) Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Med 24: 336±342 51. Eisele B, Lamy M, Thijs LG, et al (1998) Anti-thrombin III in patients with severe sepsis: a randomized, placebo-controlled, double-blind, multicenter trial plus a meta-analysis on all randomized placebo-controlled, doubleblind trials with anti-thrombin III in severe sepsis. Intensive Care Med 24: 663±672 52. Riess H (2000) Antithrombin in severe sepsis. ªNewº indication of an ªoldº drug. Intensive Care Med 26: 657±665 53. Volk HD, Reimke P, Krausch D, et al (1996) Monocyte deactivation ± rationale for a new therapeutic strategy in sepsis. Intensive Care Med 22:S471±S481 54. Kaul R, McGeer A, Norrby-Teglund A, et al (1999) Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome ± a comparative observational study. Clin Infect Dis 28: 800±807 55. Alejandria MM, Lansang MA, Dans LF, Mantaring JB (2000) Intravenous immunoglobulins for treating sepsis and septic shock. Cochrane database Syst Rev (2) CD001090 56. Pilz G, Appel R, Kreuzer E, Werdan K (1997) Comparison of early IgM-enriched immunoglobulin vs. polyvalent IgG administration in score-identified postcardiac surgical patients at high risk for sepsis. Chest 111: 419±426 57. The Intravenous Immunoglobulin Collaborative Study Group (1992) Prophylactic intravenous administration of standard immune globulin as compared with core-lipopolysaccharide immune globulin in patients at high-risk of postsurgical infection. N Engl J Med 327: 234±240 58. Jenson HG, Pollock BH (1997) Metaanalyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 99:E2 59. Livingston DH, Loder PA, Kramer SM, et al (1994) Interferon gamma administration increases monocyte HLA-DR antigen expression but not endogenous interferon production. Arch Surg 129: 172±178 60. Kohler J, Heumann D, Garotta G, et al (1993) IFN-gamma involvement in the severity of gram-negative infections in mice. J Immunol 151: 916±921 61. The International Chronic Granulomatous Disease Cooperative Study Group (1999) A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med 1324: 509±516 62. Dries DJ, Jurkovich J, Maier RV, et al (1994) Effect of interferon gamma on infection-related death in patients with severe injuries. Arch Surg 129: 1031±1042 63. Polk HC Jr, Cheadle WG, Linvingston DH, et al (1992) A randomized prospective clinical trial to determine the efficacy of interferon-g in severely injured patients. Am J Surg 63: 191±196 64. Wasserman D, Ioannovich JD, Hinzmann RD, et al (1998) Interferon-g in the prevention of severe burn-related infections: a European phase III multicenter trial. Crit Care Med 26: 434±439 65. Zhang P, Bagby GH, Stoltz DA, et al (1998) Enhancement of peritoneal leukocyte function by granulocyte colonystimulating factor in rats with abdominal sepsis. Crit Care Med 26: 315±321 66. Lundblad R, Nesland JM, Giercksky KE (1996) Granulocyte colony-stimulating factor improves survival rate and reduces concentrations of bacteria, endotoxin, tumor necrosis factor, and endothelin-1 in fulminant intra-abdominal sepsis in rats. Crit Care Med 24: 820±826 67. Karzai W, Ulrich von Specht B, Parent C, et al (1999) G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am J Respir Crit Care Med 159: 1377±1382 68. Nelson S, Farkas S, Fotheringham N, et al (1996) Filgrastim in the treatment of hospitalized patients with community acquired pneumonia (CAP) (abstract). Am J Respir Crit Care Med 151:A535 69. Wunderink RG, Leeper KV, Schein RMH, et al (1996) Clinical response to figrastim in pneumonia with severe sepsis. Am J Respir Crit Care Med 153:A123 70. Heard S, Fink MI, Gamelli RE, et al (1998) The effect of prophylactic administration of recombinant human GCSF on the incidence of nosocomial infections in patients with acute traumatic brain injury or cerebral hemorrhage. Crit Care Med 26: 748±754 71. Takala J, Ruokonen E, Webster NR, et al (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785±792 72. Doig GS, Martin CM, Sibbald WJ (1997) Polymixin-dextran anti entodotin pre-treatment in an ovin model of normotensive sepsis. Crit Care Med 25: 1956±1961 73. Yu M, Tomasa G (1993) A doubleblind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med 21: 1635±1642 74. The ARDS network (2000) Ketaconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomised controlled trial. JAMA 283: 1995±2002 75. Siedlar M, Szczepanik A, Wieckiewicz J, et al (1997) Vancomycin down-regulates lipopolysaccharide-induced tumor necrosis factor alpha (TNF-alpha) production and TNF alpha mRNA a accumulation in human blood monotyes. Immunopharmacology 35: 265±271 76. Khan AA, Slifer TR, Remington JS (1998) Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob Agents Chemother 42: 1713±1717 77. Riesbeck K, Gullberg M, Forsgren A (1994) Evidence that the antibiotic ciprofloxacin counteracts cyclosporin-dependent suppression of cytokine production. Transplantation 57: 267±272 78. Willatts SM, Radford S, Leitermann M (1995) Effect of the antiendotoxic agent, taurolidine, in the treatment of sepsis syndrome: a placebo-controlled, double-blind trial. Crit Care Med 23: 1033±1039 79. Eisenfeld L, Krause PJ, Herson VC, et al (1992) Enhancement of neonatal neutrophil motility (chemotaxis) with adult fresh frozen plasma. Am J Perinatol 9: 5±8 80. Galley HF, DIMatteo MA, Webster NR (2000) Immunomodulation by anaesthetic, sedative and analgesic agents: does it matter? Intensive Care Med 26: 267±274 S 103 81. Severn A, Rapson NT, Hunter CA, et al (1992) Regulation of tumor necrosis factor production by adrenaline and badrenergic agonists. J Immunol 148: 3441±3445 82. Van Der Poll T, Coyle SM, Barbosa K, et al (1996) Epinephrine inhibits tumor necrosis factor-a and potentiates interleukin 10 production during human endotoxemia. J Clin Invest 97: 713±719 83. Spengler RN, Chensue SW, Giacherio DA, et al (1994) Endogenous norephinephrine regulates tumor necrosis factor-a production from macrophages in vitro. J Immunol 152: 3024±3031 84. Ritchie PK, Ashby M, Knight HH, et al (1996) Dopamine increase interleukin 6 release and inhibits tumor necrosis factor release from rat adrenal zona glomerulosa in vitro. Eur J Endocrinol 134: 610±616 85. Unsaro A, Russell JA (2000) Could anti-inflammatory actions of catecholamines explain the possible effect of supranormal oxygen delivery in critically ill surgical patient. Intensive Care Med 26: 299±304 86. Bellomo R, Ronco C (1999) Continuous renal replacement therapy in the intensive care unit. Intensive Care Med 25: 781±789 87. Mehta R, McDonald B, Gabbai F, et al (1996) Continuous versus intermittent dialysis for acute renal failure in the ICU: results from a randomized multicenter trial. J Am Soc Nephrol 5: 1457 88. Silvester W (1998) Outcome studies of continuous renal replacement therapy in the intensive care. Kidney Int 53:S138±S141 89. Journois D (1999) Hemofiltration during cardiopulmonary bypass. Minerva Anestesiol 65: 427±432 90. Reeves JH, Warwick WB, Shann F, et al (1999) Continuous plasmafiltration in sepsis syndrome. Crit Care Med 27: 2096±2104 91. Churchwell KB, McManus ML, Kent P, et al (1995) Intensive blood and plasma exchange for treatment of coagulopathy in meningococcemia. J Clin Apheresis 10: 171±177 Intensive Care Med (2001) 27: S 104±S 115 Immunological therapy of sepsis: experimental therapies Patrick Arndt Edward Abraham ) P. Arndt ´ E. Abraham ( ) Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado Health Sciences Center, 4200 E. Ninth Avenue, Denver, CO 80262, USA E-mail: [email protected] Phone: +1-3 03-3 15 70 47 Fax: +1-3 03-3 15 56 32 Introduction An excessive inflammatory response accompanies the initial stages of severe infection and appears to contribute to associated organ system failure and death [1, 2, 3]. A number of immunomodulatory therapies aimed at decreasing the dysregulated inflammatory response have been examined in patients with sepsis (Table 1). The published literature from 1985 to 2000 was reviewed in preparing this summary of the major approaches to modifying immunological pathways that have been examined in clinical trials, using the key words of sepsis, septic shock, endotoxemia, cytokines, immunology, coagulation, tumor necrosis factor (TNF) a, interleukin (IL) 1, and human. Pathophysiology Although microbial products, such as endotoxins, have been targets for therapy in sepsis, a fundamental concept is that the constellation of abnormalities in these patients is not due to the direct effect of the infectious agent or its products but rather results from the patient's own inflammatory response to infection. Initially, activation of complement was considered causal, particularly the fifth component of complement, which is a potent neutrophil activator and produces a capillary hyperpermeability syndrome [4, 5]. The release of platelet-activating factor (PAF) was also thought to be responsible for hypotension and organ system dysfunction in sepsis, particularly since PAF is a potent hypotensive agent [6, 7, 8, 9, 10]. Using specific inhibitors of PAF, animals given lethal bacterial toxins survive [8, 9]. Similar results were obtained when cyclo-oxygenase inhibitors were administered to animals lethally challenged with endotoxin or bacteria, implicating cyclo-oxygenase products as a contributing cause to septic shock [11, 12, 13]. In the absence of infection, high doses of TNF administered to animals induced circulatory collapse and organ necrosis which were similar to those observed in humans with septic shock [13]. Similar results were observed with high doses of IL-1 [13]. Injecting a combination of low doses of IL-1 plus TNF revealed synergistic effects in inducing shock [13]. Neutralizing TNF activity with antibodies [14, 15, 16] or soluble receptor fusion constructs [17], or blocking IL-1 receptors [18, 19] was effective in preventing death in animal models of lethal bacteremia or endotoxemia. The findings in animal models were confirmed when humans were injected with either IL-1 or TNF as cancer chemotherapy [20, 21, 22], the most impressive physiological consequence of which was the fall in blood pressure. The hypotension was dose-dependent and, despite a short plasma halflife of less than 10 min, the biological consequences could be observed for days. The logical clinical conclusion from these data was that reducing the biological effects of systemic TNF or IL-1 would reduce the risk of dying from septic shock since these cytokines appeared to be essential for the manifestation of the disease. The biological basis for the development of a shocklike state after systemic IL-1 or TNF has been established at the molecular level. Both cytokines activate the transcription of genes that increase the production of small, potent, proinflammatory mediator molecules. For example, IL-1 and TNF increase gene expression and synthesis for phospholipase A2 (PLA2) leading to increased PAF synthesis [23]. Similarly, an increase in cyclo-oxygenase type II (COX-2) by IL-1 or TNF results S 105 Table 1 Immunomodulatory therapies examined in sepsis Agent Number of patients enrolled in each trial of the agent Antiendotoxin antibodies HA-1A E5 543 [58], 2199 [61] 488 [60], 847 [59], 1102 [118] Interleukin-1 receptor antagonist 99 [71], 893 [72], 696 [113] Bradykinin antagonist Anti-TNF-a monoclonal antibodies Murine anti-TNF Murine anti-TNF-a Fab2'fragments 251 [3], 504 [112] 971 [6], 564 [7], 1879 [10] 122 [76], 39 [3], 446 [3] TNF receptor fusion proteins p75 TNF receptor fusion protein p55 TNF receptor fusion protein 141 [78] 498 [8], 1340 [79] Platelet-activating factor antagonists Ibuprofen (cyclo-oxygenase inhibitor) 262 [114], 668 [115] 29 [116], 30 [113], 455 [117] in elevated levels of prostaglandin E2 [24, 25, 26] Nitric oxide (NO) is a potent vasodilator and thought to be primarily responsible for the hypotension and myocardial suppression in septic shock [27]. IL-1, TNF, and interferon-g, particularly the combination of the three, activate gene expression and synthesis of inducible NO synthase (iNOS) [13, 28, 29, 30, 31]. Compared to IL-1 and TNF, no other cytokine has been shown to reproduce the dramatic hypotension and pathophysiological parameters of septic shock in animals or in humans. This does not mean that other cytokines do not participate in the pathogenesis of the event. For example, animals treated with neutralizing antibodies to interferon-g are protected against lethal endotoxemia [29]. Neutralizing antibodies to IL-8 in models of inflammation reduce neutrophil infiltration in the lung, kidneys, and myocardium [32]. Other cytokines, both proinflammatory, such as IL-12 [33] and macrophage-inducing factor [34], and anti-inflammatory, such as IL-10 [35, 36, 37], as well as other mediators of inflammation such as adhesion molecules [38, 39, 40, 41], also appear to have a role in modulating cellular function in models of endotoxemia or bacteremia. Antiendotoxin therapies Antiendotoxin therapies, both nonspecific, such as intravenous immunoglobulins, and specific, such as human antiserum to heat killed Escherichia coli J5 and murine (E5) and humanized (HA1A) antibodies directed against the lipid A component of endotoxin, have been investigated in large populations of adult patients with presumed sepsis [42, 43, 44, 45, 46, 47, 48, 49, 50, 51]. Although initial results were encouraging, particularly in patients with Gram-negative sepsis, larger clinical trials showed no benefit. In part, the lack of effect of the monoclonal E5 and HA1A antibodies may have been due to the inability of these antibodies to effectively block the effects of endotoxin. In particular, when cultured macrophages are first incubated with either E5 or HA1A, and endotoxin added, there is no decrease in the release of either IL-1 or TNF by these cells [52]. These findings indicate that neither of these antibodies is able to prevent endotoxin from activating cells to produce proinflammatory cytokines. Given these in vitro results, it is in retrospect not surprising that clinical trials with these antibodies were also negative. Bactericidal/permeability increasing (BPI) protein is a member of a group of naturally occurring proteins that bind to the lipid A portion of endotoxin [53]. BPI is produced in neutrophils and stored in their primary granules. In in vitro studies BPI effectively binds to lipopolysaccharide, prevents the LPS-induced inflammatory response from occurring (i.e., release of TNF-a and IL-6 from cells), and significantly reduces Gramnegative bacteria viability [54, 55]. In animal models of septic shock the administration of BPI significantly improved hemodynamics, lowered endotoxin levels, decreased markers of inflammation (serum levels of TNF-a and IL-6), and improved survival when given up to 30 min after an infusion of LPS or E. coli [56, 57]. In a small, phase I/II, open-labeled clinical trial in patients with severe meningococcal sepsis, BPI given by continuous infusion improved survival compared to that predicted by the Glasgow Meningococcal Prognostics Septicemia Score (96 % compared to < 70 %) [58]. Unfortunately, these results were unable to be confirmed in a larger, double-blind, randomized phase III study of BPI in the therapy of pediatric patients with severe meningococcal disease. There are several important issues that may limit the utility of any antiendotoxin therapy, no matter how potent. First, high levels of endotoxin are associated primarily with Gram-negative bacterial infections, and ideally patients with a high probability of such infections would therefore be targeted for antiendotoxin thera- S 106 pies. However, it is very difficult to identify such patients since the clinical presentation of Gram-negative infection is often not significantly different from that of Gram-positive, or culture-negative sepsis. Second, endotoxin often initiates inflammatory cascades leading to organ system dysfunction in septic patients. By the time these patients are first seen and antiendotoxin therapy considered, cellular dysfunction may be established and not amenable to correction by such therapy, unless endotoxin continues to be present and to drive such pathophysiological sequelae. Animal models investigating BPI and other antiendotoxin therapies have generally used pretreatment strategies. It is unclear whether benefit can be shown in preclinical models or in the clinical setting when such therapies are administered several hours to days after the onset of endotoxemia or Gram-negative sepsis. Interleukin-1 receptor antagonist The interleukin-1 receptor antagonist (IL-1ra) is a naturally occurring inhibitor of IL-1 which competitively binds to the IL-1 receptor [18, 19]. In preclinical studies, involving primarily rabbits and mice, infusion of IL-1ra starting before or shortly after the onset of endotoxemia or bacteremia improve survival [18, 19]. Interestingly, in baboon studies [59, 60] IL-1ra therapy appears to have a minimal effect in blunting endotoxemia-induced increases in IL-6, TNF-a, or circulating levels of TNF receptors, suggesting that in primates IL-1 does not have a central role in the sepsis-induced inflammatory response. Although a small (n = 99) nonblinded phase II trial [61] suggested that IL-1ra can improve survival in septic patients, two large subsequent phase III studies [62, 63] were unable to demonstrate similar efficacy. In part, the inability of IL-1ra to provide benefit to septic patients may be because IL-1 does not occupy a central, pivotal role in perpetuating the inflammatory response and producing organ system dysfunction in human sepsis, as was suggested by the baboon endotoxemia studies [59, 60] reviewed above. Additionally, the failure of IL1ra to reduce mortality may reflect a timing issue, generic in sepsis studies. In particular, even if IL-1 is important in initiating a proinflammatory response, its role may be minimal by the time the patients are recognized and entered into a clinical trial. Indeed, very few septic patients have elevated plasma levels of IL-1, and therefore the number of patients who truly had increased IL-1 tissue expression at the time of enrollment into the IL-1ra studies is unknown. Such information is of obvious importance because therapies inhibiting IL-1 would be expected to have a beneficial effect only in patients with increased expression of this cytokine. Anti-tumor necrosis factor therapies The two major approaches taken to neutralizing TNF have involved either monoclonal anti-TNF antibodies or fusion protein constructs in which the extramembrane portion of the p55 (type I) or p75 (type II) TNF receptor is joined to the Fc fragment of a human IgG1 antibody. Pretreatment of endotoxemic or bacteremic animals with anti-TNF-a antibodies or TNF receptor fusion protein constructs results in clear improvements in survival and amelioration of organ system dysfunction [14, 15, 16, 17]. In some models of Gram-negative or Gram-positive bacteremia, administration of anti-TNFa antibodies at the time of initiation of the bacteremic insult or even shortly thereafter (i.e., within the first hour) is still associated with a significant survival benefit [64]. However, the use of such antibodies at later time points in endotoxemic or bacteremic models does not appear to be associated with any clear benefits. Although several small studies [65, 66] have suggested that anti-TNF-a antibody therapy improved certain physiological parameters, such as cardiac output, in septic patients, they were too small to detect any survival benefit. The initial study powered to examine day 28 survival with such therapy was the North American Sepsis Trial (NORASEPT I), which examined a murine IgG1 monoclonal antibody in the treatment of severe sepsis and septic shock [67]. A total of 994 patients were enrolled, of whom approximately one-half were in shock at the time of randomization. Overall, there was no statistically significant benefit associated with anti-TNF therapy. However, in the prospectively defined subgroup of patients with septic shock a statistically significant reduction in mortality was present during the first 2 weeks after administration of monoclonal anti-TNF-a antibody compared to placebo. At day 28 after anti-TNF-a therapy the reduction in mortality among septic shock patients was 17 % compared to those receiving placebo. By contrast, no benefit was found with anti-TNF-a therapy in patients not in shock at study entry. In the NORASEPT I shock patients the beneficial effect of anti-TNF-a antibodies on survival appeared within the first 24 h after enrollment; the greatest separation between the survival curves for placebo and anti-TNF antibody-treated patients occurred during this time [67]. Approximately 60 % of the placebo deaths occurred within the first 3 days of the study. Treatment with 7.5 mg/kg monoclonal anti-TNF-a antibodies was associated with a 49 % reduction in mortality versus placebo at day 3 after study enrollment. A second study, the International Sepsis Trial (INTERSEPT), using the same murine monoclonal antiTNF-a antibody as NORASEPT I, was undertaken in 14 primarily European countries [68]. Although the INTERSEPT study initially enrolled septic patients with S 107 and without shock, after the results of NORASEPT I were available, only shock patients were entered into INTERSEPT. A total of 564 patients, of whom 420 were in septic shock, were enrolled. Day 28 mortality was reduced by 14.5 % in patients who received 3 mg/ kg monoclonal anti-TNF-a antibody, with no reduction in mortality found in those receiving 15 mg/kg. There was no evidence of early survival benefit (i.e., within the first 3 days after anti-TNF antibody infusion), a similar finding to that seen in NORASEPT I. Additionally, whereas 60 % of the placebo deaths among patients in shock occurred within the first 3 study days in NORASEPT I, fewer than 45 % of placebo deaths occurred within this period in the INTERSEPT study. A recently completed study (NORASEPT II) enrolled 1900 patients with septic shock and examined the potential utility of 7.5 mg/kg the murine monoclonal anti-TNF-a antibody [69]. No improvement in survival was found in the actively treated group, all-cause mortality at day 28 being 40.3 % in monoclonal anti-TNF-a antibody treated patients compared to 42.8 % in those receiving placebo. Although the Acute Physiology and Chronic Health Evaluation II scores, day 28 mortality rates, sex ratio, and percentage of patients with one or more organ failures present at baseline were similar in NORASEPT I and NORASEPT II, there did appear to be substantial differences in patient survival patterns. Whereas more than 60 % of the deaths in the placebo arm of NORASEPT I occurred in the first 3 days after study entry, the mean time to death in the placebo group of NORASEPT II was delayed, averaging 6.8 days. These differences in survival may reflect improvements between the two studies in the supportive care provided to patients with septic shock, resulting in better survival from the initial hypotensive episode and associated immediate complications, a period in which proinflammatory cytokine release, including that for TNF-a, may be greatest. If advances in management have permitted critically ill septic patients to better survive the initial state of accelerated cytokine expression, this would diminish the efficacy of therapies aimed at modulating the early proinflammatory response. An additional concern in interpreting the NORASEPT II data revolves about the efficacy of the antiTNF antibody used. Even though only a minority of patients had detectable levels of circulating TNF-a at baseline and posttreatment time points, review of posttreatment plasma TNF-a levels showed continued presence of circulating TNF-a in the antibody treated group. Therefore a question remains as to the ability of the anti-TNF antibody employed in the doses used in NORASEPT I, INTERSEPT, and NORASEPT II, to actually block cytokine activity. The utility of administering F(ab')2 fragments of a murine IgG3 monoclonal antibody to TNF-a has been examined in patients with severe sepsis or septic shock [70, 71]. There were 122 patients entered in the initial clinical trial, and no increase in survival from sepsis for the patients receiving anti-TNF treatment was detected overall [70]. However, a retrospective stratification of patients according to their plasma interleukin-6 (IL-6) concentrations suggested beneficial effects for the drug in patients (n = 37) with baseline levels greater than 1000 pg/ml. In patients with IL-6 levels greater than 1000 pg/ml, mortality decreased from 80 % in the placebo group to 35 % in patients who received the highest dose (1 mg/kg) of the anti-TNF-a therapy. Two larger unpublished studies in Europe and North America have shown an aggregate reduction in mortality of approximately 3.5 % for patients receiving anti-TNF-a antibody fragments. These results were consistent with those found in NORASEPT II [69], INTERSEPT [68], and the p55 TNF receptor fusion protein [72] studies which found no relationship between IL-6 levels and response to anti-TNF therapy. Three clinical studies have reported results using soluble TNF receptor constructs as anti-TNF agents. In the first of these clinical trials [73] the molecule used consisted of the extramembrane components of the human type II (p75) receptor joined to the Fc portion of a human IgG1 antibody molecule [17]. Patients (n = 141) with septic shock, with or without associated organ system dysfunction, were entered into the study. A significant dose-dependent increase in mortality was found in patients treated with this p75 soluble TNF receptor construct, with mortality rising from 30 % in the placebo group to 53 % in the patients treated with the highest dose (1.5 mg/kg) of the anti-TNF compound. The enhanced mortality associated with treatment with the p75 TNF receptor molecule may be related to the extremely high doses used in the study. Although potency estimates are difficult to quantitate, soluble TNF receptor fusion proteins appear to inactivate TNF-a more than 50 times as effectively as the monoclonal antibodies [72], and therefore therapy with a dose of 1.5 mg/kg of the p75 TNF receptor fusion protein would be expected to completely neutralize TNF-a for a prolonged period, especially given the long halflife of the compound (> 60 h). TNF-a is an essential component of normal inflammatory responses, and prolonged neutralization of its activity may have potent immunosuppressive effects leading to increased mortality. Two clinical trials have examined the role of a p55 TNF receptor fusion protein construct in septic patients. In the initial 498 patient study, separate randomization lists were used for patients with severe sepsis with or without early shock, shock and for those with refractory septic shock [72]. The doses of the p55 TNF receptor complex used in this study (0.008, 0.042, and 0.08 mg/ kg) were substantially lower than those administered in the p75 TNF receptor complex clinical trial [73]. Therapy with 0.08 mg/kg of the p55 TNF receptor fusion pro- S 108 tein complex, but not other doses, was associated with a 36 % reduction (p = 0.07) in day 28 mortality in the prospectively defined patient group with severe sepsis with or without early septic shock. By contrast, no beneficial effects were apparent with any dose of the p55 receptor complex in patients with refractory septic shock. Because of the apparent benefit of the p55 TNF receptor fusion protein in severe sepsis with or without early septic shock, a 1340 patient, phase III study was undertaken in this patient population [74]. No improvement in day 28 all cause mortality or in surrogate endpoints, such as organ failure scores, was found in patients treated with the p55 TNF receptor construct compared to placebo. Notably, the p55 TNF receptor fusion protein used in this phase III study was from a different batch than that in the phase II study, with differences in glycosylation, and had slightly lower TNF neutralizing ability, and therefore higher doses (approximately 0.125 mg/kg) were used. It is unknown what role if any these alterations in molecular structure or binding potency played in the different outcomes between the phase II and phase III clinical trials. Phospholipase A2 inhibition Nonpancreatic PLA2 (sPLA2) is released into the systemic circulation after endotoxin exposure and reproduces the hemodynamic profile of severe sepsis when administered intravenously to animals [75]. sPLA2 catalyzes the hydrolysis of membrane phospholipids resulting in the production of PAF as well as other lysophospholipids [75]. PLA2 levels are increased in the serum of humans with severe sepsis, without significant increases in patients hospitalized with other diagnoses or trauma patients. Levels are correlated with hypotensive episodes and survival in patients with severe sepsis [75, 77, 78, 79, 80]. These results suggest that inhibition of sPLA2 would be of benefit in patients with severe sepsis. Inhibitors of sPLA2 activity decrease PAF and leukotriene levels and improve survival in murine models of septic shock [76]. A human phase II trial of the use of a sPLA2 inhibitor in severe sepsis has recently been completed and demonstrated no benefit in all enrolled patients. Results of subgroup analyses should be available in the near future. Nitric oxide inhibition NO, previously called endothelium-derived relaxing factor, is synthesized by endothelial cells by way of NOS, can be released into the systemic circulation, and then can function to regulate blood flow to tissues [81]. Exposure to endotoxin increases endothelial secretion of NO [82, 83], predominately by upregulating the in- ducible form of NOS (iNOS) [84, 85]. Specific inhibitors of iNOS, in particular NG-methyl-L-arginine (LNAME), have reduced the hypotensive response in animals infused with endotoxin, but have not improved their survival [85, 86]. The adverse effects of iNOS inhibitors, including a decrease in cardiac output and an increase in pulmonary artery pressure, have resulted in questions regarding their potential benefit in humans with severe sepsis [81, 87]. Additionally, continuous infusion of L-NAME resulted in an increase in mortality in an animal model of septic shock [87]. Two small, open-label studies of L-NAME infusion have reported reproducible and sustained increases in mean arterial pressure in humans with severe sepsis [81, 88]. However, both studies were associated with increases in pulmonary artery pressures and a fall in cardiac output requiring an increase in dobutamine infusion in one study [81, 88]. The fall in cardiac output was greatest in the high-dose group (20 mg/kg per hour) and was associated with electrocardiographic evidence of cardiac ischemia in 27 % of the patients in this group [88]. A recent phase III clinical trial utilizing continuous L-NAME infusion in patients with severe sepsis was discontinued due to an increase in adverse effects, including statistically significantly increased mortality in the L-NAME group [89]. Anticoagulation The coagulation system is activated in animal models and in humans with severe sepsis as evidenced by the presence of intravascular thrombi in vessels on tissue specimens and the frequent occurrence of disseminated intravascular coagulation (DIC) [90, 91]. The activation of the coagulation system is associated with decreased levels of fibrinogen, increased levels of activated factor X, and increased levels of tissue factor and its inhibitor, tissue factor pathway inhibitor (TFPI) [90]. Additionally, activation of coagulation in the setting of severe infection appears to potentiate proinflammatory responses, primarily through the activation of endothelial cells, which then produce inflammatory mediators, including cytokines such as TNF-a. Several studies have investigated modulation of the coagulation system including the administration of TFPI, anti-Xa, and activated protein C (APC). TFPI is a naturally occurring, circulating protein which can inhibit the procoagulant effects of tissue factor by binding to factor Xa and then to the tissue factor±VIIa complex [90, 92]. Circulating levels of TFPI increase after exposure to endotoxin [90, 92]. A phase II study investigating the infusion of TFPI in patients with severe sepsis was recently completed with a trend toward clinical benefit [93]. A large international phase III study of TFPI in severe sepsis is presently underway. S 109 Blockade of factor Xa in the extrinsic coagulation pathway has been investigated in animal models of severe sepsis. In animals administered endotoxin, dansyl glutamyl-glycyl-arginyl chloromethyl ketone-treatedXa, a factor Xa inhibitor, prevented endotoxemia induced DIC but did not affect survival [94]. Early human trials are underway investigating the potential benefit of factor Xa inhibition in severe sepsis. APC is an anticoagulant formed when protein C is cleaved by thrombin [95, 96]. In children and adults with severe meningococcemia and/or purpura fulminans, serum APC levels are depressed and are correlated with clinical outcome [97, 98]. Several small nonrandomized trials utilizing infusions of protein C (50±100 IU/kg every 6 h) have been performed in adults and children with severe meningococcal disease and purpura fulminans [96, 99, 100, 101]. Although these studies were not powered to detect a difference in mortality, protein C infusion normalized sera protein C levels, increased fibrinogen levels and was accompanied by resolution of DIC [96, 99, 100, 101]. A phase II trial of APC in sepsis showed a nonsignificant trend towards improved survival in APC-treated patients [95]. A large phase III study of APC in sepsis was recently stopped after approximately 1500 patients were enrolled because of efficacy associated with APC therapy [101 a]. Such positive results with APC suggest that infusions of APC will become part of the standard therapy in severely ill septic patients. Definitions and entry criteria Entry criteria for sepsis trials have been designed primarily to include patients with clinical evidence of infection associated with the recent development of organ system dysfunction believed to be due to this infectious process [102, 103]. Because of the perceived need to enroll patients early in their clinical course, positive microbiological cultures have not been required. Indeed, in a number of recent studies only a minority of patients had positive blood cultures, with bacteremia present in about 30 % [63, 67, 68, 69]. The definitions of sepsis and septic shock used in most clinical trials did not include consideration of the length of time that the infective process had been present, nor of its anatomic site. Such a classification may be particularly important since animal studies have shown differing patterns of response to anticytokine therapies, such as anti-TNF-a monoclonal antibodies, for intra-abdominal infections compared to bacteremias, and for rapidly initiated infectious processes, such as acute bacteremia, compared to more slowly developing infections, such as peritonitis [104]. No microbiological classifications were used prospectively in clinical trials of immunomodulatory agents even though re- sponses to anti-inflammatory therapies may differ between Gram-positive and Gram-negative infections, and also may be more effective in patients with documented infections [73]. Even though recent clinical trials have targeted specific mediators, including endotoxin, IL-1, or TNF-a, which were postulated to have a pivotal role in the inflammatory cascade leading to organ system failure and death in sepsis, the actual presence of excessive levels of these mediators was not required for entry into the study. Rather, clinical criteria, such as the presence of one or more organ system failures, with or without shock, were used. Although elevation in the mediator of interest was postulated to accompany organ system dysfunction in septic patients, data gathered from the IL-1ra and anti-TNF clinical trials suggested that such a correlation was present in only a minority of cases. For example, in the North American Sepsis Trial (NORASEPT II) of a murine monoclonal antibody for septic shock only 40 % of patients had detectable circulating TNF-a levels at the time of enrollment [69]. In contrast, decreased levels of protein C were present in approximately 90 % of patients with signs of severe infection with associated organ failures, indicating that the Consensus Conference criteria were highly useful in identifying patients with deficiency of protein C, who would be an appropriate target population for APC therapy. The lack of a requirement to recruit demonstrably infected patients with elevated plasma levels of endotoxin or of the cytokine of interest may therefore have adversely affected outcome in trials examining immunomodulatory agents in sepsis. For example, improved efficacy of therapies, such as monoclonal anti-TNF-a antibodies, was found when blinded data safety monitoring committees eliminated patients without clear evidence of infection [105]. In the NORASEPT II trial of murine monoclonal anti-TNF-a antibodies there was an 18 % relative reduction in day 28 mortality compared to placebo in patients with detectable circulating TNF-a levels at the time of enrollment. By contrast, no effect of such anti-TNF therapy was seen in patients without elevated circulating TNF-a concentrations. Future directions Although a meta-analysis combining most of the clinical trials using anti-inflammatory agents has suggested that benefit in survival could be achieved with such therapies, the magnitude of such an effect was small [3]. By contrast, therapies directed against specific proinflammatory cytokines such as TNF-a and IL-1 have produced remarkable clinical response in diseases such as rheumatoid arthritis and Crohn's disease [106, 107, 108]. S 110 The failure of immunomodulatory therapies to improve outcome in sepsis raises several questions about drug development strategies and the design of clinical trials in this area. First, there are reasons to believe that some of the agents tested were insufficiently potent to block the mediator of interest, or had properties directed at mediators which were not of central importance in determining clinical outcome. It is therefore not surprising that clinical studies with these agents were negative. Second, most of the immunomodulatory therapies used in the treatment of patients with sepsis showed impressive efficacy in animal models. Their subsequent failure in clinical trials raises concerns about the relevance of preclinical experimental models. Specifically, differences between the biochemical and immunological responses of patients with a clinical diagnosis of sepsis and animals with known bacterial infections or endotoxemia may explain the divergence of results between experimental and clinical studies. Third, the fact that agents such as anti-TNF-a antibodies are clinically effective in the setting of rheumatoid arthritis and Crohn's disease raises questions about the heterogeneous nature of patients with sepsis. Overly broad definitions for sepsis may have diluted out any effect that such therapies could have in more clinically limited patient populations. Thus, whereas rheumatoid arthritis or Crohn's disease are well described entities with specific immunological, radiological, and pathophysiological diagnostic criteria, the defining features of sepsis are based mainly on organ system dysfunction developing in the appropriate clinical setting. Almost all clinical trials of immunomodulatory therapies have enrolled patients on the basis of clinical evidence of infection with associated organ system dysfunction, rather than requiring evidence that the immunological abnormality of interest is present. Such inclusion criteria may permit the entry of excessively heterogeneous patient populations and thereby prevent detection of beneficial effects that might be apparent in more homogeneous subgroups. Positive results associated with anti-TNF therapy in preventing Jarisch-Herxheimer reactions [109] suggest that there are specific groups of infected patients who do respond to immunomodulatory agents. Classification of infected patients based on demonstrable abnormalities in immunological or biochemical pathways may permit inclusion into therapeutic studies at earlier points in their clinical course, before organ dysfunction develops and becomes irreversible. In this case, patients with clinical evidence of infection and alteration in plasma levels of the mediator of interest, such as TNF-a, would be eligible for study enrollment. Such a classification scheme, in addition to allowing appropriate targeting of patient populations who may benefit from a specific therapeutic agent, would also mean that the development of clinically relevant organ system dysfunction can be used as an endpoint, rather than as entry criteria for clinical trials. There are substantial differences in the intracellular signaling cascades initiated by Gram-negative and Gram-positive infections [110], as well as by the site of infection. It would therefore appear reasonable to limit the nature and site of the infection in patients enrolled in future trials of immunomodulatory agents. For example, an immunomodulatory therapy would initially be investigated only in patients with evidence of meningococcemia or pneumococcal pneumonia. The use of mechanistic definitions to define patient populations who are at risk for infection-initiated organ system dysfunction lacks the reductionist simplicity of the Consensus Conference definitions of sepsis [101, 102], which generate large numbers of patients presenting with similar constellations of clinical findings. However, mechanistic definitions should provide more homogeneous groups of patients with activation of similar immunological or biochemical pathways, at earlier stages in their clinical course and who should respond to interruption of immunological or other cascades. Although the approach taken in almost all clinical trials has been to block completely the effects of the mediator of interest, it may be more appropriate to modulate, rather than to ablate biochemical responses [111]. Improved techniques to monitor immunological markers of inflammatory and host defense responses will be important in assessing the effects of future therapies on central mechanisms contributing to organ dysfunction in sepsis. The extent to which such a mechanistic approach is beneficial will depend upon how important the mediators generated are in contributing to the subsequent clinical course, particularly the development of organ system dysfunction and mortality. Using more stringent definitions in clinical trials would of course restrict the numbers of patients eligible for a specific therapy until subsequent studies examine larger at risk populations. However, unless benefit can first be shown in a rigorously defined patient population in which there is specific evidence of mediator activation, there is little reason to anticipate that efficacy will be achieved in larger, more heterogeneous patient groups. S 111 References 1. Abraham E. Therapies for sepsis (1997) Emerging therapies for sepsis and septic shock. West J Med 166: 195±200 2. Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP (1990) Septic shock in humans. Ann Intern Med 113: 227±242 3. Zeni F, Freeman B, Natanson C (1997) Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 25: 1095±1100 4. Mollnes TE, Redl H, Hogasen K, Bengtsson A, Garred P, Speilberg L, Lea T, Oppermann M, Gotze O, Schlag G (1993) Complement activation in septic baboons detected by neoepitope-specific assays for C3b/iC3b/C3c, C5a and the terminal C5b-9 complement complex (TCC). Clin Exp Immunol 91: 295±300 5. Granowitz EV, Porat R, Gelfand JA, Wolff SM, Dinarello CA (1994) Administration of low-dose endotoxin to healthy humans increases C5a binding to circulating neutrophils. J Infect Dis 169: 480±482 6. Coughlan AF, Hau H, Dunlop LC, Berndt MC, Hancock WW (1994) Pselectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med 179: 329±334 7. Dhainaut JF, Tenaillon A, Le Tulzo Y, Schlemmer B, Solet JP, Wolff M, Holzapfel L, Zeni F, Dreyfuss D, Mira JP, et al (1994) Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. BN 52021 Sepsis Study Group. Crit Care Med 22: 1720±1728 8. Koltai M, Hosford D, Braquet PG (1993) Platelet-activating factor in septic shock. New Horiz 1: 87±95 9. Kruse-Elliott KT, Albert DH, Summers JB, Carter GW, Zimmerman JJ, Grossman JE (1996) Attenuation of endotoxin-induced pathophysiology by a new potent PAF receptor antagonist. Shock 5: 265±273 10. Ishii S, Nagase T, Tashiro F, Ikuta K, Sato S, Waga I, Kume K, Miyazaki J, Shimizu T (1997) Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J 16: 133±142 11. Coran AG, Drongowski RA, Paik JJ, Remick DG (1992) Ibuprofen intervention in canine septic shock: reduction of pathophysiology without decreased cytokines. J Surg Res 53: 272±279 12. Mansilla-Rosello A, Ferron-Orihuela JA, Ruiz-Cabello F, Garrote-Lara D, Fernandez-Mondejar E, DelgadoCarrasco ML (1997) Differential effects of IL-1b and ibuprofen after endotoxic challenge in mice. J Surg Res 67: 199±204 13. Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA (1988) Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest 81: 1162±1172 14. Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature 330: 662±664 15. Fong Y, Tracey KJ, Moldawer LLL, et, et al (1989) Antibodies to cachectin/TNF reduce interleukin-1 and interleukin-6 appearance during lethal bacteremia. J Exp Med 170: 1627±1633 16. Hinshaw LB, Tekamp-Olson P, Chang AC, et al (1990) Survival of primates in LD 100 septic shock following therapy with antibody to tumor necrosis factor (TNF). Circ Shock 30: 279±292 17. Van Zee KJ, Moldawer LL, Oldenburg HAS, Thompson WZ, Stackpole SA, Montegut WJ, Rogy MA, Meschter C, Gallati H, Schiller CD, et al (1996) Protection against lethal Escherichia coli bacteremia in baboons (Papio anubis) by pretreatment with a 55-kDa TNF receptor (CD120a)-Ig fusion protein, Ro 45-2081. J Immunol 156: 2221±2230 18. Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC (1990) Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348: 550±552 19. Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA (1991) A specific receptor antagonist for interleukin-1 prevents Escherichia coli-induced shock. FASEB J 5: 338±343 20. Chapman PB, Lester TJ, Casper ES, et al (1987) Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol 5: 1942±1951 21. Smith JW, Urba WJ, Curti BD, et al (1992) The toxic and hematologic effects of interleukin-1 alpha administered in a phase I trial to patients with advanced malignancies. J Clin Oncol 10: 1141±1152 22. Van der Poll T, Buller HR, ten Cate HT (1990) Activation of coagulation after administration of TNF to normal subjects. N Engl J Med 322: 1622±1627 23. Endo S, Inada K, Nakae H, et al (1995) Plasma levels of type II phospholipase A2 and cytokines in patients with sepsis. Res Commun Mol Pathol Pharmacol 90: 413±421 24. Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA (1998) Differential regulation of cyclooxygenases 1 and 2 by interleukin-1b, tumor necrosis factor-a, and transforming growth factor-b1 in human lung fibroblasts. Exp Cell Res 241: 222±229 25. Newton R, Stevens DA, Hart LA, Lindsay M, Adcock IM, Barnes PJ (1997) Superinduction of COX-2 mRNA by cycloheximide and interleukin-1b involves increased transcription and correlates with increased NFk B and JNK activation. FEBS Lett 418: 135±138 26. Guan Z, Baier LD, Morrison AR (1997) p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1b. J Biol Chem 272: 8083±8089 27. Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109±142 28. Dinarello CA (1996) Biological basis for interleukin-1 in disease. Blood 87: 2095±2147 29. Heremans H, van Damme J, Dillen C, Dikman R, Billiau A (1990) Interferon-g, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock in mice. J Exp Med 171: 1853±1861 30. Huang S, Hendriks W, Althage A, et al (1993) Immune response in mice that lack the interferon-gamma receptor. Science 259: 1742±1745 S 112 31. Car BD, Eng VM, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B (1994) Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med 179: 1437±1444 32. Herada A, Mukaida N, Matsushima K (1996) Interleukin-8 as a novel target for intervention therapy in acute inflammatory diseases. Mol Med Today 2: 482±489 33. Zisman DA, Kunkel SL, Strieter RM, Gauldie J, Tsai WC, Bramson J, Wilkowski JM, Bucknell KA, Standiford TJ (1997) Anti-interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8: 349±356 34. Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377: 68±71 35. Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL (1995) Neutralization of IL-10 increases lethality in endotoxemia. J Immunol 155: 2222±2229 36. Howard M, Muchamuel T, Andrade S, Menon S (1993) Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 177: 1205±1208 37. Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T (1993) Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med 177: 547±550 38. Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC (1994) Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med 180: 95±109 39. Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, Sugerman HJ, Fowler AA (1995) Circulating ICAM1 is increased in septic shock. Am J Respir Crit Care Med 151: 1420±1427 40. Watanabe S, Mukaida N, Ikeda N, Akiyama M, Harada A, Nakanishi I, Nariuchi H, Watanabe Y, Matsushima K (1995) Prevention of endotoxin shock by an antibody against leukocyte integrin beta 2 through inhibiting production and action of TNF. Int Immunol 7: 1037±1046 41. Kayal S, Jais JP, Aguini N, Chaudiere J, Labrousse J (1998) Elevated circulating E-selectin, intracellular adhesion molecule 1, and von Willebrand factor in patients with severe infection. Am J Respir Crit Care Med 157: 776±784 42. Nydegger UE (1997) Sepsis and polyspecific intravenous immunoglobulins. J Clin Apheresis 12: 93±99 43. Werdan K, Pilz G (1996) Supplemental immune globulins in sepsis: a critical appraisal. Clin Exp Immunol 104 [Suppl 1]:83±90 44. Ziegler EJ, McCutchan JA, Fierer J, Glauser MP, Sadoff JC, Douglas H, Braude AI (1982) Treatment of gramnegative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med 307: 1225±1230 45. Zanetti G, Glauser MP, Baumgartner J (1991) Use of immunoglobulins in prevention and treatment of infection in critically ill patients: review and critique. Rev Infect Dis 13: 971±972 46. Calandra T, Glauser MP, Schellekens J, Verhoef J (1988) Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind, randomized trial. J Infect Dis 158: 312±319 47. J5 Study group (1992) Treatment of severe infectious purpura in children with human plasma from donors immunized with Escherichia coli J5: a prospective double-blind study. J Infect Dis 165: 695±701 48. The HA-1A Sepsis Study Group (1991) Treatment of gram-negative bacteremia and septic shock with HA1A human monoclonal antibody against endotoxin: a randomized, double-blind placebo-controlled trial. N Engl J Med 324: 429±436 49. Bone RC, Balk RA, Fein AM, Perl TM, Wenzel RP, Reines HD, Quenzer RW, Iberti TJ, MacIntyre N, Schein RM (1995) A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. Crit Care Med 23: 994±1006 50. Greenman RL, Schein RM, Martin MA, et al (1991) A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. JAMA 266: 1097±1102 51. McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR (1994) Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med 121: 1±5 52. Warren HS, Amato SF, Fitting C, Black KM, Loiselle PM, Pasternack MS, Cavaillon JM (1993) Assessment of ability of murine and human antilipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med 177: 89±97 53. Tobias PS, Mathison JC, and Ulevitch R (1988) A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J Biol Chem 263: 13479±13481 54. Marra M, Wilde C, Griffith J, Snable J, Scott R (1990) Bactericidal/permeability-increasing protein has endotoxinneutralizing activity. J Immunol 144: 662±666 55. Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, Theofan G (1992) Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Invest 90: 1122±1130 56. Jin H, Yang R, Marsters S, Ashkenazi A, Bunting S, Marra MN, Scott RW, Baker JB (1995) Protection against endotoxic shock by bactericidal/permeability-increasing protein in rats. J Clin Invest 95: 1947±1952 57. Rogy M, Oldenburg HS, Calvano SE, Montegut WJ, Stackpole SA, Van Zee KJ, Marra M, Scott R, Seilhammer JJ, Moldawer LL, Lowry SF (1994) The role of bactericidal/permeability-increasing protein in the treatment of primate bacteremia and septic shock. J Clin Immunol 14: 120±133 58. Giroir BP, Quint PA, Barton P, Kirsch EA, Kitchen L, Goldstein B, Nelson BJ, Wedel NJ, Carroll SF, Scannon PJ (1997) Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet 350: 1439±1443 S 113 59. Hawes AS, Fischer E, Marano MA, Van Zee KJ, Rock CS, Lowry SF, Calvano SE, Moldawer LL (1993) Comparison of peripheral blood leukocyte kinetics after live Escherichia coli, endotoxin, or interleukin-1 alpha administration. Studies using a novel interleukin-1 receptor antagonist. Ann Surg 218: 79±90 60. Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, DeForge L, Kenney JS, Remick DG, Bloedow DC, et al (1992) Interleukin1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest 89: 1551±1557 61. Fisher CJ Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC Emmanuel G, Ng D, Bloedow DC, Catalano MA (1994) Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. The IL-1RA Sepsis Syndrome Study Group. Crit Care Med 22: 12±21 62. Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebocontrolled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271: 1836±1843 63. Opal SM, Fisher CJ, Pribble JP, Dhainaut J-F, VincentJ-L, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, LaBrecque JF, Lookabaugh J, Donovan H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M, and the Interleukin-1 Receptor Antagonist Sepsis Investigator Group (1997) The confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 25: 1115±1124 64. Hinshaw LB, Emerson TE Jr, Taylor FB Jr, Chang ACK, Duerr M, Peer GT, Flournoy DJ, White GL, Kosanake SD, Murray CK, Xu R, Passey RB, Fournel MA (1992) Lethal S. aureus shock in primates: prevention of death with anti-TNF antibody. J Trauma 33: 568±573 65. Vincent JL, Bakker J, Marecaux G, Schandene L, Kahn RJ, Dupont E (1992) Administration of anti-TNF antibody improves left ventricular function in septic shock patients. Results of a pilot study. Chest 101: 810±815 66. Dhainaut J-F A, Vincent J-L, Richard C, Lejeune P, Martin C, Fierobe L, Stephens S, Ney UM, Sopwith M (1995) CDP571, a humanized antibody to human tumor necrosis factor-a: safety, pharmacokinetics, immune response, and influence of the antibody on cytokine concentrations in patients with septic shock. Crit Care Med 23: 1461±1469 67. Abraham E, Wunderink R, Silverman H, et al (1995) Monoclonal antibody to human tumor necrosis factor alpha (TNF MAb): efficacy and safety in patients with the sepsis syndrome. JAMA 273: 934±941 68. Cohen J, Carlet J (1996) INTERSEPT: an international, multicenter, placebocontrolled trial of monoclonal antibody to human tumor necrosis factora in patients with sepsis. Crit Care Med 24: 1431±1440 69. Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S, for the NORASEPT II Study Group (1998) Monoclonal antibody to human tumor necrosis factor alpha (TNF Mab) in the treatment of patients with septic shock: a multi-center, placebo controlled, randomized, double-blind clinical trial. Lancet 351: 929±933 70. Reinhart K, Wiegand-Lohnert C, Grimminger F, et al (1996) Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med 24: 733±742 71. Panacek E (2000) Results of the Monarcs Study. American Thoracic Society International Conference, ªThe Heart in Sepsisº, May 2000 72. Abraham E, Glauser MP, Butler T, et al (1997) p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. JAMA 277: 1531±1534 73. Fisher CJ Jr, Agosti JM, Opal SM, et al (1996) Treatment of septic shock with the tumor necrosis factor receptor Fc fusion protein. N Engl J Med 334: 1697±1702 74. Abraham E, Reynaert M, Lew D, Pingleton S, Butler I, Dugernier I, Margolis B, Kudsk K, Zimmerli W, Anderson P, Laterre PF, Lesslauer W, Cooper P, Burdeska A, Passe S, Modi M, Leighton A, Salgo M, Van der Auwera P, for the Tenefuse Study Group (2001) Lenercept (p55-Tumor Necrosis Factor Receptor Fusion Protein, Ro 452081, Tenefuse) patients with severe sepsis or early septic shock. A randomized double-blind placebo-controlled multicenter phase III trial with 1342 patients. Crit Care Med (in press) 75. Vadas P (1984) Elevated plasma phospholipase A2 levels: correlation with the hemodynamic and pulmonary changes in gram-negative septic shock. J Lab Clin Med 104: 873±881 76. Marshall LA, Hall RH, Winkler JD, Badger A, Bolognese B, Roshak A, Flamberg PL, Sung CM, ShabotFletcher M, Adams JL, Mayer RJ (1995) SB 203347, an inhibitor of 14 kDa phospholipase A2, alters human neutrophil arachidonic acid release and metabolism and prolongs survival in murine endotoxin shock. J Pharmacol Exp Ther 274: 1254±1262 77. Vadas P, Pruzanski W, Stefanski E, Sternby B, Mustaqrd R, Bohnen J, Fraser I, Farewell V, Bombardier C (1988) Pathogenesis of hypotension in septic shock: correlation of circulating phospholipase A2 levels with circulatory collapse. Crit Care Med 16: 1±7 78. Endo S, Inada K, Nakae H, Takakuwa T, Yamada Y, Suzuki T, Taniguchi S, Yoshida M, Ogawa M, Teraoka H (1995) Plasma levels of type II phospholipase A2 and cytokine in patients with sepsis. Res Commun Mol Pathol Pharmacol 90: 413±421 79. Sorenson J, Kald B, Tagesson C, Lindahl M (1994) Platelet-activating factor and phospholipase A2 in patients with septic shock and trauma. Intensive Care Med 20: 555±561 80. Nyman KM, Uhl W, Forsstrom J, Buchler M, Beger HG, Nevalainen TJ (1996) Serum phospholipase A2 in patients with multiple organ failure. J Surg Res 60: 7±14 81. Avontuur JA, Tutein-Nolthenius RP, Bodegom JW, Bruining H (1998) Prolonged inhibition of nitric oxide synthesis in severe septic shock: a clinical study. Crit Care Med 26: 660±667 82. Salvemini D, Korbut R, Anggard E, Vane J (1990) Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Eschericha coli lipopolysaccharide. Proc Natl Acad Sci USA 87: 2593±2597 S 114 83. Evans T, Carpenter A, Kinderman H (1993) Evidence of increased nitric oxide production in patients with the sepsis syndrome. Circ Shock 41: 77±81 84. Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109±142 85. Kilbourn RG, Jubran A, Gross SS, Griffith OW, Levi R, Adams J, Lodato RF (1990) Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun 172: 1132±1138 86. Kilbourn RG, Gross SS, Jubran A, Adams J, Griffith OW, Levi R, Lodato RF (1990) NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci USA 87: 3629±3632 87. Cobb JP, Natanson C, Hoffman WD, Lodato RF, Banks S, Koev CA, Solomon MA, Elin RJ, Hosseini JM, Danner RL (1992) NW-amino-L-arginine, an inhibitor of nitric oxide synthase, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. J Exp Med 176: 1175±1182 88. Grover R, Zaccardelli D, Colice G, Guntupalli K, Watson D, Vincent JL (1999) An open-label dose escalation study of the nitric oxide synthase inhibitor, NG-methyl-L-arginine hydrochloride (546C88), in patients with septic shock. Crit Care Med 27: 913±922 89. Nasraway SA (1999) Sepsis research: we must change course. Crit Care Med 27: 427±430 90. Camerota A, Creasey A, Patla V, Larkin V, Fink M (1998) Delayed treatment with recombinant human tissue factor pathway inhibitor improves survival in rabbits with gram-negative peritonitis. J Infect Dis 177: 668±676 91. Park T, Creasey A, Wright S (1997) Tissue factor pathway inhibitor blocks cellular effects of endotoxin by binding to endotoxin and interfering with transfer to CD14. Blood 89: 4268±4274 92. Creasey AA, Change AK, Feigen L, Wun TC, Taylor FB, Hinshaw LB (1993) Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 91: 2850±2860 93. Abraham E (2000) New therapies in sepsis. American Thoracic Society International Conference, ªNew insights into Acute Lung Injuryº, May 2000 94. Taylor FB, Change AC, Peer GT, Mather T, Blick K, Catlett R, Lockhart MS, Esmon CT (1991) DEGR-factor Xa blocks disseminated intravascular coagulation initiated by Escherichia coli without preventing shock or organ damage. Blood 78: 364±368 95. Abraham E (2000) Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol 22: 401±404 96. Smith OP, White B, Vaughan D, Rafferty M, Claffey L, Lyons B, Casey W (1997) Use of protein-C concentrate, heparin, and haemodiafiltration in meningococcus-induced purpura fulminans. Lancet 350: 1590±1593 97. Fourrier F, Lestavel P, Chopin C, Marey A, Goudemand J, Rime A, Mangalaboy J (1990) Meningococcemia and purpura fulminans in adults: acute deficiencies of proteins C and S and early treatment with antithrombin III concentrates. Intensive Care Med 16: 121±124 98. Leclerc F, Hazelzet J, Jude B, Hofhuis W, Hue V, Martinot A, Van der Voort E (1992) Protein C and S deficiency in severe infectious purpura of children: a collaborative study of 40 cases. Intensive Care Med 18: 202±205 99. Rivard G, David M, Farrell C, Schwarz H (1995) Treatment of purpura fulminans in meningococcemia with protein C concentrate. J Pediatr 126: 646±652 100. Kreuz W, Veldman A, Escuriola-Ettingshausen C, Schneider W, Beeg T (1998) Protein-C concentrate for meningococcal purpura fulminans. Lancet 351: 986±987 101. Rintala E, Seppala OP, Kotilainen P, Pettila V, Rasi V (1998) Protein C in the treatment of coagulopathy in meningococcal disease. Crit Care Med 26: 965±968 101 a. Bernard GR, la Rosa SP et al (2001) The efficacy and safety of recombinant human activated protein C for the treatment of patients with severe sepsis. Crit Care med (in press) 102. Bone RC, Balk RA, Cerra FB, et al (1992) ACCP/SCCM consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644±1655 103. Bone RC, Fisher Jr CJ, Clemmer TP, et al (1989) Sepsis syndrome: a valid clinical entity. Crit Care Med 17: 389±393 104. Zanetti G, Heumann D, Gerain J, et al (1992) Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. J Immunol 148: 1890±1897 105. Sprung CL, Finch RG, Thijs LG, Glauser MP (1996) International sepsis trial (INTERSEPT): role and impact of a clinical evaluation committee. Crit Care Med 24: 1441±1447 106. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. N Engl J Med 337: 1029±1035 107. Lorenz HM, Antoni C, Valerius T, Repp R, Grunke M, Schwedtner N, Nusslein H, Woody J, Kalden JR, Manger B (1996) In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol 156: 1646±1653 108. Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, Woody JN (1994) Repeated therapy with monoclonal antibody to tumor necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 344: 1125±1127 109. Fekade D, Knox K, Hussein K, Melka A, Lalloo DG, Coxon RE, Warrell DA (1996) Prevention of JarischHerxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med 335: 311±315 110. Bone RC (1994) Gram-positive organisms and sepsis. Arch Intern Med 154: 26±34 111. Kopf M, Baumann H, Freer G, et al (1994) Impaired immune and acute phase responses in interleukin-6-deficient mice. Nature 368: 339±342 112. Fein AM, Bernard GR, Criner GJ, et al (1997) Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, Deltibant (CP-0127) JAMA 277: 482±487 113. Natanson C, Esposito CJ, Banks SM (1998) The sirens' songs of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med 26: 1927±1931 S 115 114. Dhainaut JF, Tenaillon A, Le Tulzo Y, et al (1994) Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med 22: 1720±1728 115. Dhainaut JF, Tenaillon A, Hemmer M, et al (1998) Confirmatory platelet-activating factor receptor antagonist trial in patients with severe Gram-negative bacterial sepsis: a phase III, randomized double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med 26: 1963±1971 116. Bernard GR, Reines HD, Halushka PV (1991) Prostacyclin and thromboxane A2 formation is increased in human sepsis syndrome: effects of cyclooxygenase inhibition. Am Rev Respir Dis 144: 1095±1101 117. Bernard GR, Wheeler AP, Russell JA, et al (1997) The effects of ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med 336: 912±918 118. Angus DC, Birmingham MC, Balk RA, et al (2000) E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. JAMA 283: 1723±1730 Intensive Care Med (2001) 27: S 116±S 127 Other supportive therapies in sepsis Javier PØrez R. Phillip Dellinger ) J. PØrez ´ R. P. Dellinger ( ) Division of Cardiovascular and Critical Care Medicine, Rush-Presbyterian-St. Luke's Medical Center, Chicago, Ill., USA E-mail: [email protected] Phone: +1-3 12-9 42 33 30 Fax: +1-3 12-9 42 63 59 Introduction Because sepsis is associated with multisystem organ failure, there are many other supportive therapies used to treat these patients that do not directly relate to the sepsis process. Although most of these have been studied in randomized controlled clinical trials in hospitalized patients, few have been tested specifically in sepsis. Thus research and the literature support some, while others are extrapolations from other ill populations. Deep vein thrombosis (DVT) prophylaxis, nutritional support, and stress ulcer prophylaxis (SUP) are important adjunctive considerations in the management of sepsis. Consumption coagulopathy makes the septic patient at risk for development of venous clots. Nutritional support, especially enteral, is recognized as important in supporting the critically ill septic patient who is unable to eat. In addition, sepsis and the associated organ dysfunction put the patient at increased risk for development of stress ulcers. Methods We performed a comprehensive Medline literature search from January 1966 to February 2000. The following terms were independently searched: DVT, deep vein thrombosis, thrombophlebitis, venous thrombosis, thromboembolic disease, pulmonary embolism, anticoagulation, warfarin, heparin, low-molecular weigh heparin, DVT prophylaxis, mechanical compression devices, external pneumatic compression, nutritional support, parenteral nutrition, total parenteral nutrition, enteral nutrition, immunoenhancing diets, immunomodulating diets, stress ulcers, gastrointestinal bleeding, SUP, gastrointestinal bleeding prophylaxis, gastrointestinal bleeding prevention, antacids, histamine antagonists, sucralfate, antiulcer agents, omeprazole, and proton-pump inhibitors. Each one of those terms was searched and crossed with the following: critical care, intensive care, infection, systemic inflammatory response syndrome, sepsis, severe sepsis, sepsis syndrome, septic shock, and multiple organ dysfunction syndrome. Deep vein thrombosis prophylaxis in sepsis The use of DVT prophylaxis in higher risk postoperative patients has been universally accepted since the early 1970s when it was found to reduce the risk of thromboembolic phenomena in this group [1]. Many subsequent trials have continued to emphasize the value of DVT prophylaxis in most postoperative patients [2, 3, 4, 5]. In addition to postoperative patients, subcutaneous heparin has also proven efficacious in reducing the risk of thromboembolism among myocardial infarction [6, 7, 8, 9] and ischemic stroke patients [10, 11, 12]. Only a few studies of venous thromboembolism prophylaxis have been carried out on general medical wards, and medical intensive care units. In those studies, patients treated with subcutaneous heparin [13, 14, 15, 16, 17] or with low molecular weight heparin (LMWH) [18, 19] reduced the risk of thromboembolic events. Does DVT prophylaxis improve clinical outcome in patients with sepsis? Answer: yes, grade A. Recommendations Considering the frequent occurrence of independent risk factors for DVT in septic patients and the high per- S 117 Table 1 DVT prophylaxis studies performed in general populations of the acutely ill: percentage of sepsis/infected patients Reference Design, methods Setting n Infection, sepsis n % Pingleton et al. [13] Prospective/historical controls, V/Q angio, autopsy Respiratory care unit 188 53 28 Reduction in incidence of pulmonary embolism Cade [14] Prospective, double-blind placebo-control, I-fibrinogen scan ICU/medical ward 119/131 ND ± Reduction in incidence of DVT (29 % vs. 13 %) Halkin et al. [15] Randomized prospective control, no data Medical ward 1358 138 10 Reduction in mortality (10.9 % vs. 7.8 %) Belch et al. [16] Prospective randomized, control, I-fibrinogen scan ICU 100 52 52 Reduction in incidence of DVT (26 % vs. 4 %) Gardlund et al. [17] Prospective randomized, no data Medical ward 11693 1610 14 Minor thromboembolic events reduced Samana et al. [18] Placebo-control, doubleblind, randomized, venography, ultrasound, V/Q angio, CAT, autopsy Medical ward/ ICU 1102 584 53 Reduction in incidence of DVT (14.9 % vs. 5.5 %) Dahan et al. [19] Placebo-control randomized, I-fibrinogen Medical ward 270 11 4 Reduction in incidence of DVT (9 % to 3 %) centage of sepsis/infected patients included in studies that have demonstrated efficacy of DVT prophylaxis in general, septic patients should be treated with DVT prophylaxis. Even though there is not a randomized study that establishes the impact of DVT prophylaxis on morbidity and mortality specifically in septic patients, the significant number of septic patients included in the populations of patients enrolled in other prospective randomized trials supports that the use of DVT prophylaxis reduces morbidity and mortality in septic patients. Moreover, septic patients, especially those with severe sepsis and multiple organ failure, have less cardiopulmonary reserve, and the impact of a minor thromboembolic event in this group of patients could be very compromising. Rationale Patients in the intensive care unit are at high risk of development of thromboembolic phenomena [14, 20, 21]. Septic patients as described above are expected to be in the intensive care unit (ICU) and to be part of the population at risk. No definitive study restricted to the incidence of DVT in septic patients has been carried out. The significance of DVT prophylaxis on morbidity and mortality in septic patients needs to be implied based on the analysis of proportion of the septic patients included in the studies of the acutely ill patient in general (Table 1). Pingleton et al. [13], observed a reduction in the incidence of pulmonary embolism in patients admitted to the respiratory intensive care unit. Cade [14] Results found a reduction in the risk of thromboembolic events from 29 % to 13 % among patients admitted to ICU and treated with subcutaneous heparin. In the latter study, using a control group consisting of patients admitted to the medical ward and coronary care unit, a significantly higher incidence of thromboembolic events was found among patients admitted to the ICU. Halkin and coworkers [15], in a randomized prospective study of patients admitted to medical wards, compared treatment with low-dose unfractionated heparin to patients who did not receive any treatment. They found a significant reduction in mortality in heparin-treated patients (7.8 % vs. 10.9 %). Belch et al. [16], in a study carried out in medical patients admitted to the intensive care unit, found a significantly reduced incidence of thromboembolic events (4 % vs. 26 %) in the group treated with unfractionated heparin. Mortality was not addressed in the study. Gardlund et al. [17], found a significant reduction in minor embolic events in patients admitted to the hospital with infectious disease diagnoses who were treated with subcutaneous heparin versus those not treated, although there was no difference in mortality or major thromboembolic events. In a recently published trial [18] 1102 patients received either LMWH (in two different doses) or placebo. Although the patients included in this trial were not admitted to the ICU, many of them suffered from complicated conditions. Patients receiving 40 mg enoxaparin had a significant reduction in the incidence of thromboembolic phenomena (5.5 % vs. 14.9 %). No significant difference was found in mortality among any of the groups, but a trend toward decreased mortality in S 118 patients receiving 40 mg enoxaparin was reported. Dahan et al. [19] compared medical patients using treatment with LMWH versus placebo in a double-blind, placebo-controlled randomized trial. LMWH reduced the incidence of thromboembolic phenomena (9.1 % vs. 3 %). Hirsch and colleagues [20] studied 100 patients admitted to ICU and found the incidence of DVT to be 33 %. There was an association with increased mortality in patients suffering DVT (although it is not possible to determine whether death was caused by DVT or was a consequence of the deteriorated state of those patients). Although it is difficult to demonstrate mortality benefit from DVT prophylaxis unless either a very large study or patients at very high risk are studied, many argue that demonstrating a decrease in DVT without increase in bleeding complications implies that mortality benefit could be demonstrated if higher powered studies were performed. Septic patients, especially those admitted to the ICU, frequently have one or more risk factors for thromboembolic phenomena. These have been widely described in postoperative, medical and critically ill patients [14, 22, 23, 24.]. These factors are: age (> 40 years), history of venous thromboembolism, malignancy, bed rest (> 5 days), major surgery, congestive heart failure, fracture (pelvic, hip or leg), estrogen replacement, stroke, myocardial infarction, multiple trauma, and hypercoagulable states. The concurrence of two or more factors increases the risk of thromboembolic events [23, 24]. Other risk factors frequently present in septic patients include use of central venous catheters [20, 25, 26, 27], use of neuromuscular blockade, use of deep sedation [28], and presence of coagulopathy [29]. Is there any pharmacological method for DVT prophylaxis preferred in septic patients? Answer: no, grade A. Recommendations Septic patients who do not have a contraindication to heparin use should receive prophylaxis with either lowdose unfractionated heparin (5,000 U either two or three times daily) or LMWH (at recommended doses; grade A). For those septic patients who have an absolute contraindication for heparin use (i.e., thrombocytopenia, severe coagulopathy, active bleeding, recent intracerebral hemorrhage), the use of a mechanical prophylactic device is advised since this method has proven to be effective in postsurgical patients and therefore would likely work in septic patients (grade E). Rationale Unfractionated subcutaneous heparin (UH) is widely used for the prevention of DVT among postoperative patients and in medical high-risk patients. UH is inexpensive and has been demonstrated in critically ill medical and surgical patients to be safe and to be associated with minor bleeding complications (bruising, hematoma at the site of injections) and rarely with heparin-induced thrombocytopenia [13, 14, 24, 30, 31]. Although smaller studies have suggested that LMWH may be either as effective as UH with less bleeding complications or more effective with the same bleeding complications [31, 32, 33] in the treatment of thromboembolic disease, larger studies have not demonstrated statistically significant differences between the drugs. However, LMWH has been demonstrated to be more effective than UH in several high-risk populations for prophylaxis of DVT [34]. Enoxaparin has been demonstrated to be safe and efficacious in treatment of thromboembolic disease in medical patients with minimal adverse events [18]. Two other randomized studies [35, 36, 37] in acutely ill medical patients compared LMWH and UH and showed equal effectiveness in the prevention of DVT. Each hospital should assess which form of heparin is most cost effective at that institution. Both are effective in presenting DVT in at-risk patients. Special considerations: patients with sepsis-induced coagulopathy Sepsis is frequently associated with hemostatic defects [29, 40]. The sepsis milieu may include consumptive coagulopathy and liver dysfunction leading to predisposition for both clotting and bleeding. Thrombocytopenia is frequently present in septic patients. In the setting of active hemorrhage or in septic patients with significant abnormality in clotting function we recommend the use of mechanical leg compression devices as a preferred alternative to heparin. Intermittent pneumatic compression devices applied to legs have been demonstrated to be efficacious in postoperative patients [38, 39], and the use of these devices is recommended in septic patients with contraindication to the use of heparin (grade E). Nutrition in sepsis Septic patients are characterized by having increased energy expenditure and enhanced catabolism [41, 42]. The need to provide adequate nutritional support to septic patients is thus generally accepted as part of standard care in the ICU. However, many issues regarding nutrition to septic patients remain controversial. This controversy is enhanced by the fact that most nutritional S 119 data available come from studies performed in trauma or postsurgical patients, as opposed to a population of septic patients alone. Does institution of nutritional support improve clinical outcome of patients with sepsis? body mass index and mortality in critically ill patients [55] whereas another placebo-controlled study demonstrated no difference in clinical outcome between patients receiving enteral nutrition and those receiving intravenous crystalloid [56]. Answer: yes, grade E Are there any nutritional routes or formulations preferred for patients with sepsis? Recommendations Answer: yes, grades C, E, B (based on different populations) Based on the assumption that sepsis produces a hypercatabolic state and leads to protein-energy malnutrition, and given that protein loss is associated with poor outcome, nutritional support in septic patients is recommended. The correlation of nutritional support with outcome in septic patients comes from data extrapolated from studies performed in perioperative patients and from expert opinion that allow us to establish this recommendation. Many important questions remain regarding what kind of nutrition and when in the course of sepsis should nutrition begin. Rationale Nutritional status has been closely related with outcome of critically ill patients. Malnutrition has been associated with increased morbidity and longer hospital stays [43]. Mullen et al. [44] in 1980 demonstrated a reduction in perioperative complications in surgical patients with the use of adequate nutritional support. Scientific evidence supports the important role of nutritional status in the outcome of septic and other critically ill patients [45, 46, 47]. Decreased gastrointestinal mucosal permeability [48], improved healing function [49], and lower infection rates [47] have been attributed to the use of enteral feeding in critically ill patients. The activation of the inflammatory cascade in sepsis alters the body's metabolism. Patients with sepsis have elevated energy requirements, net catabolism, and rapid loss of lean mass [50, 51]. For this reason the use of nutritional support has been axiomatically accepted. The ability of nutrition to alter the clinical outcome of critically ill patients, however, is controversial [52, 53]. Studies have identified enteral nutrition as a major factor in maintaining normal gut mucosal function [48, 54], both in humans and in animals. Thus the use of enteral formulas would be expected to maintain mucosal integrity in the critically ill septic patient. Most of the studies investigating metabolic changes and effects of nutrition have been carried out in postoperative patients and have provided conflicting conclusions. For example, one study demonstrated a direct relationship between Recommendations Enteral nutrition is the preferred method of nutritional support in the catabolic critically ill patient in general, inclusive of the septic patient (grade C). For those patients who cannot tolerate enteral nutrition for a prolonged time or when contraindications do not allow its use (mesenteric ischemia, mechanical bowel obstruction), parenteral nutritional support should be used (grade E). Immune-enhancing formulas may be better than other enteral formulations in critically ill patients, but effects on ultimate outcome (i.e., survival) remain to be demonstrated in large randomized trials (grade B). Rationale Although controversy exists, most authorities advocate the use of enteral nutrition in critically ill patients [57, 58]. Several studies have compared enteral and parenteral nutrition in critically ill patients, most of them perioperative. Cerra and coworkers [53] compared standard nutrition with total parenteral nutrition in septic patients. No difference was found in clinical outcome. However, enteral nutrition has proven superior to parenteral nutrition in reduction in stress ulcers [59], gut protection [48], and costs [52, 60]. In addition, catheter placement and indwelling catheters have been associated with increased complications [61, 62]. A recent meta-analysis [47] found an increased rate of complications and mortality in ICU medical patients receiving parenteral nutrition when compared with those receiving enteral feeding. The advantage of enteral nutrition versus total parenteral nutrition in some high-risk groups has been demonstrated [63, 64]. Recent studies have also examined the potential advantage of enriched mixtures of enteral feeding formulas compared with standard formulas [50, 65, 66, 67, 68]. Bower et al. [50], published a prospective randomized clinical trial in septic patients comparing standard enteral feeding versus an immunomodulatory formula that contained arginine, nucleotide, and fish oil. S 120 Although mortality was not modified, a significant reduction in length of stay and infections was noted in the immunomodulatory formula group. Galbµn and coworkers [67] concluded a benefit of immune-modulatory diets in septic patients from their study which revealed a decrease in mortality from 32 % to 19 %, and in infection from 20 % to 7 %. Atkinson and colleagues [68] published a controlled double-blind clinical trial involving medical and surgical ICU patients. They compared different formulations of enteral nutrition. The use of immunomodulatory formula reduced mechanical ventilation time, ICU stay, hospital length of stay and duration of systemic inflammatory response syndrome. A recent study by Gadek et al. [69] in patients with acute respiratory distress syndrome, including a proportion of septic patients, resulted in significant differences in outcome in those patients who received an immunomodulatory diet. Are there any preferred range of calories and/or proportion of elements in nutritional support in sepsis? Answer: yes, grade E Recommendations The following are specific recommendations for septic patients, according to the guidelines established by the American College of Chest Physicians [58] and American Society of Parenteral and Enteral Nutrition [70] consensus conferences: · Daily caloric intake: 25±30 kcal/kg usual body weight · Protein: 1.3±2.0 g/kg per day · Glucose: 30±70 % of total nonprotein calories, to maintain serum glucose level below 225 mg/dl · Lipids: 15±30 % of total nonprotein calories. w6Polyunsaturated fatty acid should be reduced in septic patients, maintaining that level which avoids deficiency of essential fatty acids (7 % of total calories ± generally 1 g/kg per day). No specific recommendations are offered for use of medium-chain triglycerides, branched-chain amino acids, or specific microelements added to the nutritional formulas. The use of any of these strategies, although supported in concept, does not have enough investigational evidence to determine any clinical benefit in outcome of septic patients. Rationale No randomized clinical trial has addressed optimal total caloric requirements or the amount of fat and protein needed in the diet of septic patients. Much of our knowledge regarding these issues derives from studies carried out in patients with trauma, burns, and surgery, who, as in the case of septic patients, are frequently hypercatabolic. Despite a lack of clinical outcome evidence from randomized trials, expert panels have offered recommendations for general critically ill patients and for septic patients as well. In 1993 the American Society of Parenteral and Enteral Nutrition used an evidence-based approach to publish practice guidelines for nutritional support in the ICU [70]. Although the guidelines do not address specific recommendations for septic patients, they provide a grade B recommendation for total caloric requirements in critically ill patients. In presenting the results of a more recent conference the authors emphasize the use of branched-chain amino acids in the composition of enteral formulas although the existing data did not allow establishing specific recommendations [71] The American College of Chest Physicians (ACCP) in 1997 published a consensus statement of nutrition guidelines in ICU patients [58]. Specific recommendations on caloric requirements in septic patients as well as proportion of nutrients in formulations were offered. Since then these recommendations have found agreement by most experts, but large gaps remain in our scientific basis for recommending enteral feeding in the short-term critically ill patient [72, 73, 74]. Stress ulcer prophylaxis in sepsis The use of SUP to prevent upper gastrointestinal bleeding in critically ill patients has become a routine in the ICU. However, there are controversial points in this practice: (a) SUP has not demonstrated a benefit in mortality [23]; (b) there are many definitions of upper gastrointestinal bleeding in critically ill patients that could be responsible for the heterogeneity in results in several controlled studies [75, 76]; (c) the use of SUP has been implicated in the development of ventilator-associated pneumonia although the impact of this complication on mortality and morbidity has not been established [77, 78]; (d) only specific subgroups of patients in the ICU are likely to benefit from SUP [79]. Comparing the various studies is made difficult by the varied criteria used for diagnosing stress ulcer bleeding. The use of microscopic bleeding (either orthotoluidine or guaiac in nasogastric aspirate or feces) as a marker of stress ulcer bleeding entails several problems that have already been identified: (a) guaiac is S 121 Table 2 Proportion of septic patients in different studies of SUP (R randomized trial, P placebo, C control, SU stress ulcer, MV mechanical ventilation) Study Trial n Septic patients (%) Sepsis definition Summary of results Cook et al. [79] Cohort 2252 1.6 Fever-hypothermia, leukocytosis/leukopenia, + blood culture Risk factors for SU bleeding: prolonged MV and coagulopathy Schuster et al. [82] Cohort 179 7.8 Not listed Risk factors for SU bleeding: coagulopathy, hypotension and MV Zandstra and Stoutenbeek [83] Cohort 167 40 Severe bacterial infection Pinilla et al. [84] R-C 259 3.8 2 criteria of: fever; WBC > 15,000, shift to the left, + culture Minimal SU bleeding episodes; prolonged MV identified as a risk factor No difference between patient treated with antacids and control Peura and Johnson [85] R/P-C 39 15 Not listed Cimetidine superior to placebo in preventing SU; fewer transfusions required in treated group Groll et al. [86] R/P-C 221 30±15a Not listed No significant differences between placebo and cimetidine Basso et al. [87] R/C 168 22 Foci of infection or septicemia and fever, leukocytosis, elevated sed rate and culture + Cimetidine and antacid decreased the risk of SU bleeding compared to placebo Ben-Menachem et al. [88] R/C 300 21 Not listed No differences between cimetidine and sucralfate vs. control Borrero et al. [89] R 155 30 Not listed No differences between sucralfate and antacids Bressalier et al. [90] R 74 23 Systemic infection with + cultures or hypotension Sucralfate advantages vs. antacids (both in safety and effectiveness) Cook et al. [91] R 1200 6.5 Not listed Ranitidine offers better protection than sucralfate; no differences in ventilator-associated pneumonia Poleski and Spanier [92] R 37 45 Blood culture with evidence of infection (fever, leukocytosis) Cimetidine and antacids equally effective Stothert et al. [93] R 123 28 Culture and clinical evidence; sepsis confirmed at autopsy or surgery Antacids and cimetidine equally effective a Referred to 30 % of septic patients in the placebo group and 5 % in the cimetidine group nonspecific [80]; (b) cimetidine may produce false-positive results in gastric aspirates [81]; (c) the clinical relevance of microscopic bleeding is usually minimal, and a minority of cases progress toward overt or clinically significant bleeding. The use of overt bleeding (hematemesis, gross blood, or coffee ground material in nasogastric aspirates, hematochezia, or melena) or clinically important bleeding (associated with a decrease in systolic blood pressure > 20 mmHg, orthostatic changes, decrease in hemoglobin > 2 g/dl, transfusion of at least 2 U blood in 24 h caused by the bleeding episode, or the need of surgical intervention) seems more reason- able when evaluating the impact of stress ulcers in morbidity and mortality and the efficacy of the prophylactic measures. Although there are no specific studies of SUP in septic patients, many randomized trials have been carried out in critically ill patients that include some number of septic patients. Unfortunately, only few studies do allow identification of the precise number of septic patients enrolled (Table 2 lists the proportion of septic patients in prospective studies). Furthermore, it is possible to compare the frequency of occurrence of stress ulcer bleeding in septic patients with that of patients at higher S 122 risks, because many of the risk factors for development of stress ulcer bleeding are common in septic patients. Does SUP improve clinical outcome in patients with sepsis? Answer: yes, grade C Recommendations No randomized trial has evaluated the effect of SUP on clinical outcome in septic patients. Examination of successful clinical trials of SUP does not allow precise identification of patients with diagnosis of sepsis. Therefore no definitive data exist in septic patients on the effectiveness of SUP in diminishing episodes of overt or clinically significant bleeding. The clinical utility of SUP as it affects clinical outcome in septic patients is therefore not clear. Septic patients have been assumed to have an increased risk for SUP since they have multiple risk factors known to increase the risk of stress ulcer bleeding. Since data do support SUP as being efficacious in preventing upper gastrointestinal bleeding in populations of critically ill patients, which would be expected to contain large proportions of septic patients, the use of SUP is recommended in this group (see below). Rationale The use of SUP has become accepted practice in the great majority of ICUs. Early studies associated sepsis with stress ulcer bleeding and with an increased risk of mortality in critically ill patients [94]. The initial study by Skillman et al. [94] retrospectively reported a mortality of 87 % in patients admitted to the ICU (medical and surgical) who developed stress ulcer related gastrointestinal bleeding. The use of SUP has become accepted practice in the great majority of ICUs. However, recent studies report significantly less mortality related to stress ulcer bleeding [79, 83, 91]. Schuster et al. [82] reported a 14 % incidence of bleeding in patients admitted to a respiratory intensive care unit. Although the mortality was significantly higher among patients who bled (64 % vs. 9 %), death was related to bleeding only in 3 of the 25 patients who bled. Other studies [85, 95] comparing histamine receptor antagonists or antacids versus placebo report similar results. Moreover, several authors believe that the modernization of anesthesia and ventilation techniques and, in general, the improvement in the management of critically ill patients have decreased the incidence of stress ulcers and therefore prophylaxis is not warranted [79, 80, 81, 82, 83]. Lacroix and colleagues [96] in a meta-analysis observed a range of overt bleeding from 1.6 % to 52.8 % of in control groups and from 0 to 23.1 % in antacids groups. The conclusion of the study was that cimetidine and antacids are effective in preventing stress ulcer bleeding (33 % and 43 % better than control, respectively). Collectively these studies support the assertion that patients who develop bleeding from stress ulcers require more transfusions. However, no difference in clinical outcome has been noted. Patients with stress ulcer bleeding who do not receive SUP often show two factors: coagulopathy and liver failure. A recent meta-analysis [97] reporting risk reduction for bleeding in critically ill patients with antacids, sucralfate, or histamine-2 receptor antagonists could not establish any impact on clinical outcome compared with control groups. BenMenachem et al. [88], in a randomized single-blind, control trial, reported that the incidence of bleeding did not differ among three groups of 100 patients (control, sucralfate, and cimetidine). The mortality and hospital length of stay did not vary with prophylaxis. Is there any specific subgroup of septic patients who should receive SUP? Answer: yes, grades A, C Recommendations Although no large randomized trial has addressed septic patients alone, abundant data exist regarding subgroups of septic patients with prolonged mechanical ventilation, hypotension, and coagulopathy. For these patients the use of SUP is recommended (grade A). For other septic patients in whom these factors are not present SUP is recommended based on several small randomized trials in which SUP has proven efficacious in preventing bleeding and therefore reducing morbidity in critically ill patients (grade C). Rationale Cook et al. [79] in a prospective study found an increased risk of stress ulcer bleeding in patients with prolonged mechanical ventilation (> 48 h) and those with coagulopathy. The low number of septic patients in this study does not allow the determination of the true impact of sepsis as an independent risk factor for the development of stress ulcer bleeding. Schuster et al. [82] found increased risk of bleeding associated with coagulopathy, prolonged mechanical ventilation, and sepsis. The authors of this study did not perform a multivariate analysis that would help to determine the true impact of sepsis as a single variable risk factor for stress ulcer S 123 bleeding. Coagulopathy, frequently found in severe sepsis, has been classically associated with increased incidence of bleeding [98]. Risk factors for stress ulcer bleeding have been demonstrated to be additive [95, 96, 97, 98, 99]. A score has been offered to predict the risk of SU bleeding [100]. Are some methods to be preferred over others in the prevention of stress ulcers in patients with sepsis? Answer: uncertain, grade B. Recommendations Several trials have confirmed the efficacy of antacids, sucralfate, or histamine-2 receptor antagonists in preventing stress ulcer bleeding. Since the data are conflicting, no single one can be determined as preferable. General recommendations should be based on the individual experience in the use of one or another, the availability, or cost-analysis in individual centers. In septic patients with risk factors the use of enteral nutrition following the preventive strategies currently available may be beneficial for preventing stress ulcer bleeding. less in patients treated with ranitidine, without increased associated pneumonia. These findings have been corroborated in other studies [105, 106]. A metaanalysis published in 1996 found sucralfate to be associated with a trend toward a lower incidence of pneumonia compared with both antacid and histamine-2 receptor antagonists. In this meta-analysis sucralfate was associated with less mortality. A recently published cost effectiveness analysis [107] pointed out the high costs involved in SUP. In 1999 a national survey in the United States [108] found a wide variation in the forms of SUP among intensivists. The costs of prophylaxis in low-risk patients were considered by these authors as prohibitive. In this survey the authors called for the creation of hospital-based algorithms, based on individualization of cost and care issues at the institution as it applies to patients with higher risks of bleeding. There are also data supporting the use of enteral nutrition as SUP [59]. The beneficial effect of enteral feeding has been demonstrated with distal enteral nutrition rather than gastric. Patient's position, type of tube (orogastric versus nasogastric, small-bore versus largebore), and continuous versus intermittent delivery, are factors implicated by the findings of various studies that could modify stress ulcer bleeding in patients receiving enteral nutrition [109]. Rationale Summary There are many studies comparing the efficacy of histamine-2 receptor antagonists, antacids, and sucralfate in the prevention of stress ulcer bleeding [85, 86, 87, 88, 90, 100, 101, 102]. Cook et al. [103] in a meta-analysis of SUP studies found histamine-2 receptor antagonists more effective than antacids in controlling overt bleeding. No data about nosocomial pneumonia were presented. There was no difference in mortality between the three methods, and no difference was found when compared with no prophylaxis. Similar results have been reported by two other meta-analysis [96, 97]. A controversy related to SUP stems from the ability of both antacids and histamine-2 receptor antagonists to raise the gastric pH, which may be associated with increase in gastric bacterial colonization. Increased bacterial presence in the gastrointestinal tract can lead to an increase in pneumonia if it is a route that leads to pharyngeal colonization. This area is controversial, and although some studies have demonstrated an increase in ventilator-associated pneumonia with the use of histamine-2 blockers and antacids, these data have not been validated in all clinical trials [77, 91, 103]. Furthermore, prospective studies suggest that gastric colonization is not a frequent route to pharyngeal colonization [104]. In a recently published Canadian trial [91] the risk of bleeding (in 1200 patients studied) was significantly Patients who survive the circulatory and organ deficits in sepsis may still fall victim to complications such as pulmonary embolism and stress ulcer bleeding. Although there is no clearcut evidence to quantitate the impact of such complications on mortality, the anticipated impact is grave when considering the compromised physiological reserve of these patients. For this reason it is important to institute effective prophylaxis to minimize the impact. In addition, catabolism associated with sepsis likely influences the recovery of patients with sepsis and moreover can compromise the response of the immune system against an infectious insult. Early and adequate nutritional support therefore appears important. There is much controversy and lack of prospective research regarding effect of supportive therapies on outcome in patients with severe sepsis. This research is needed. S 124 References 1. Kakkar VV, Corrigan TP, Fossard DP and the International Multicentre Trial (1975) Prevention of fatal postoperative pulmonary embolism by low doses of heparin: an international multicentre trial. Lancet II:45±51 2. Upchurch GR, Demling RH, Davies J, Gates JD, Knox JB (1995) Efficacy of subcutaneous heparin in prevention of venous thromboembolic events in trauma patients. Am Surg 61: 749±755 3. Fauno P, Suomalainen O, Rehnberg V, Hansen TB, Kroner K, Soimakallio S, Nielsen E (1994) Prophylaxis for the prevention of venous thromboembolism after total knee arthroplasthy. J Bone Joint Surg Am 76: 1814±1818 4. Amstutz HC, Friscia DA, Dorey F, Carney BT (1989) Warfarin prophylaxis to prevent mortality from pulmonary embolism after total hip replacement. J Bone Surg Am 71: 321±326 5. Fisher CG, Blachut PA, Salvian AJ, O'Brien PJ (1995) Effectiveness of pneumatic leg compression devices for the prevention of thromboembolic disease in orthopaedic trauma patients: a prospective, randomized study of compression alone versus no prophylaxis. J Orthop Trauma 9: 1±7 6. Medical Research Council (1969) Assessment of short-term anticoagulant administration after cardiac infarction. BMJ 1: 335±e42 7. Drapkin A, Merskey C (1972) Anticoagulant therapy after acute myocardial infarction. JAMA 222: 541±548 8. Warlow C, Beattie AG, Terry G, Ogston D, Kenmure ACF, Douglas AS (1973) A double-blind trial of low doses of subcutaneous heparin in the prevention of deep vein thrombosis after myocardial infarction. Lancet II: 934±936 9. Kierkegaard A, Norgren L (1993) Graduated compression stockings in the prevention of deep vein thrombosis in patients with acute myocardial infarction. Eur Heart J 14: 1365±1368 10. Prins MH, Gelsena R, Sing AK, van Heerde LR, den Ottolander GJ (1989) Deep vein thrombosis prophylaxis with a low molecular weight heparin in stroke patients. Haemostasis 19: 245±250 11. McCarthy ST, Turner JJ, Roberston D, Hawkey CJ, Macey DJ (1977) Low dose heparin as prophylaxis against deep vein thrombosis after acute stroke. Lancet II:800±801 12. Turpie AG, Levine MN, Hirsh J, Carter CJ, Jay RM, Powers PJ, Andrew M, Magnani HN, Hull RD, Gent M (1987) A double blind randomized trial of ORG 10172 low molecular weight heparinoid in the prevention of deep vein thrombosis in thrombotic stroke. Lancet I:523±526 13. Pingleton SK, Bone RC, Pingleton WW, Ruth WE (1981) Prevention of pulmonary emboli in a respiratory intensive care unit. Chest 79: 647±650 14. Cade JF (1982) High risk of the critically ill for venous thromboembolism. Crit Care Med 10: 448±450 15. Halkin H, Goldberg J, Modan M, Modan B (1982) Reduction in mortality in general medical in-patients by lowdose heparin prophylaxis. Ann Intern Med 96: 561±565 16. Belch JJ, Lowe DO, Ward AG, Forbes CD, Prentice CRM (1981) Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott Med J 26: 115±117 17. Gardlund B and the Heparin Prophylaxis Study Group (1996) Randomized, controlled trial of low-dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. Lancet 347: 1357±1361 18. Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Nguyen H, Olsson CG, Turpie AG, Weisslinger N (1999) A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med 341: 793±800 19. Dahan R, Houlbert D, Caulin C, Cuzin C, Viltart C, Woler M, Segrestaa JM (1986) Prevention of deep vein thrombosis in elderly medical in-patients by a low molecular weight heparin: a randomized double-blind trial. Haemostasis 16: 159±164 20. Hirsch DR, Ingenito EP, Goldhaber SZ (1995) Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA 274: 335±337 21. Gallus AS, Hirsh J, Tuttle RJ, Trebilcock R, O'Brien S, Carroll JJ, Minden JH, Hudecki S (1973) Small subcutaneous doses of heparin in prevention of venous thrombosis. N Engl J Med 288: 545±551 22. Thromboembolic Risk Factors Consensus Group (1992) Risk of and prophylaxis for venous thromboembolism in hospital patients BMJ 305: 567±574 23. Saint S, Matthay M (1998) Risk reduction in the intensive care unit. Am J Med 105: 515±523 24. Anderson FA, Wheeler HB (1995) Venous thromboembolism. Risk factors and prophylaxis. Clin Chest Med 16: 235±251 25. Wheeler HB, Anderson FA Jr, Cardullo PA, Patwardhan NA, Jian-Ming L, Cutler BS (1982) Suspected deep vein thrombosis: management by impedance plethysmography. Arch Surg 117: 1206±1209 26. Randolph AG, Cook DJ, Gonzales CA, Andrew M (1998) Benefit of heparin in central venous and pulmonary artery catheters. A meta-analysis of randomized controlled trials. Chest 113: 165±171 27. Borow M, Crowley JG (1985) Evaluation of central venous catheter thrombogenicity. Acta Anaesthesiol Scand 81 [Suppl]:59S±64S 28. Durbec O, Viviand X, Potie F (1997) Lower extremity deep vein thrombosis: a prospective, randomized, controlled trial in comatose or sedated patients undergoing femoral vein catheterization. Crit Care Med 25: 1982±1985 29. Carvalho AC, Freeman NJ (1994) How coagulation defects alter outcome in sepsis: survival may depend on reversing procoagulant conditions. J Crit Illness 9: 51±75 30. Clagett GP, Reisch JS (1988) Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg 208: 227±240 31. Levine MN, Raskob G, Landfield S, Hirsh J (1995) Hemorrhagic complications of anticoagulant treatment. Chest 108 [Suppl]:276S±290S 32. Warkentin TE, Levine MN, Hirsh J (1995) Heparin induced thrombocytopenia in patients treated with low molecular weight heparin or unfractionated heparin. N Engl J Med 332: 1330±1335 33. Hirsh J, Raschke R, Warkentin, Dalen JE, Poller L (1995) Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 108 [Suppl]:258S±275S 34. Green D, Hirsh J, Heit J, Prins M, Davidson B, Lensing AWA (1994) Low molecular weight heparin: a critical analysis of clinical trials. Pharmacol Rev 46: 89±121 S 125 35. Bergman JF, Neuhart E (1988) A multicenter randomized, double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. Thromb Haemost 60: 407±410 36. Haremberg J, Kallenbach B, Martin CE, Dempfle E, Zimmermann R, Kübler W, Heene DL (1990) Randomized controlled study of heparin and low molecular weight heparin for prevention of deep-vein thrombosis in medical patients. Thromb Res 59: 639±650 37. Simmonneau G, Sors H, Charbonnier B, Page Y, Laaban JP, Azarian R, Laurent M, Hirsch JL, Ferrari E, Bosson JL, Mottier D, Beau B (1997) A comparison of Low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med 337: 663±669 38. Anglen JO, Goss K, Edwards J (1998) Foot pump prophylaxis for deep vein thrombosis: the rate of effective usage in trauma patients. Am J Orthop 27: 580±582 39. Black PM, Crowell RM, Abbott WM (1986) External pneumatic calf compression reduces deep thrombosis in patients with ruptured intracranial aneurysms. Neurosurgery 18: 25±28 40. Mammen EF (1994) Coagulation defects in liver disease. Med Clin North Am 78: 545±553 41. Cerra FB, Siegel JH, Coleman B (1980) Septic autocanibalism: a failure of exogenous nutritional support. Ann Surg 192: 570±580 42. Bartlett RH, Dechert RR, Mault JR, Ferguson SK, Kaiser AM, Erlandson EE (1982) Measurement of metabolism in multiple organ failure. Surgery 92: 771±779 43. Dempsey DT, Mullen JL, Buzby GP (1988) The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr 47: 351±356 44. Mullen JL, Busby GP, Matthews DC, Smale BF, Rosato EF (1980) Reduction of operative morbidity and mortality by combined pre-operative and post-operative nutritional support. Ann Surg 192: 604±613 45. Young B, Ott L, Twyman D, Norton J, Rapp R, Tibbs P, Haack D, Brivins B, Dempsey R (1987) The effect of nutritional support on outcome from severe head injury. J Neurosurg 67: 668±676 46. Border JR, Hasset J, LaDuca J, Seibel R, Steinberg S, Mills B, Losi P, Border D (1987) The gut origin septic states in blunt multiple trauma in the ICU. Ann Surg 206: 427±448 47. Moore FA, Feliciano DV, Andrassy RJ (1992) Early enteral feeding compared with parenteral, reduces septic complications: the results of a meta-analysis. Ann Surg 216: 172±183 48. Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW (1995) Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med 152: 1545±1548 49. Schroeder D, Gillanders L, Mahr K, Hill GL (1991) Effects of immediate postoperative enteral nutrition on body composition, muscle function, and wound healing. JPEN J Parenter Enteral Nutr 15: 376±383 50. Bower RH, Cerra FB, Bershadsky B, Licari JJ, Hoyt DB, Jensen GL, Van Buren CT, Rothkopf MM, Daly JM, Adelsberg BR (1995) Early enteral administration of a formula (impact registered trademark) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med 23: 436±449 51. Müller TF, Müller A, Bachem MG, Lange H (1995) Immediate metabolic effects of different nutritional regimens in critically ill medical patients. Intensive Care Med 21: 561±566 52. Wheeler A, Bernard GR (1999) Current concepts: treating patients with severe sepsis. N Engl J Med 340: 207±214 53. Cerra FB, McPherson JP, Konstantinides FN, Konstantinides NN, Teasley KM (1988) Enteral nutrition does not prevent multiple organ failure syndrome (MOFS) after sepsis. Surgery 104: 727±733 54. Steiner M, Burges HR, Freeman LS, Gray SJ (1968) Effect of starvation on the tissue composition of intestine in the rat. Am J Physiol 215: 75±77 55. Galanos AN, Pieper CF, Kussin PS, Winchell MT, Fulkerson WJ, Harrell FE Jr, Teno JM, Layde P, Connors AF Jr, Phillips RS, Wenger NS (1997) Relationship of body mass index to subsequent mortality among seriously ill hospitalized patients. Crit Care Med 25: 1962±1968 56. Heslin M, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, Shike M, Brennan MF (1997) A prospective randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg 226: 567±580 57. Zaloga GP (1999) Early enteral nutritional support improves outcome: hypothesis or fact? Crit Care Med 27: 259±261 58. American College of Chest Physicians Consensus Statement. Applied nutrition in ICU patients (1997) Chest 111: 769±778 59. Pingleton SK, Hadzima SK (1983) Enteral alimentation and gastrointestinal bleeding in mechanically ventilated patients. Crit Care Med 11: 13±16 60. Frost P, Bihari D (1997) The route of nutritional support in the critically ill: physiological and economical considerations. Nutrition 13 [Suppl]:58S±63S 61. Orr ME, Ryder MA (1993) Vascular access devices: perspective on designs, complications and management. Nutr Clin Pract 8: 145±152 62. Richet H, Hubert B, Nitemberg G, Andremont A, Buu-Hoi A, Ourbak P, Galicier C, Veron M, Boisivon A, Bouvier AM (1990) Prospective multicenter study of vascular-catheter-related complications and risk factors for positive central-catheter cultures in intensive care unit patients. J Clin Microbiol 28: 2520±2525 63. Heyland DK, Cook DJ, Guyatt GH (1993) Enteral nutrition in the critically ill patient: a critical review of the evidence. Intensive Care Med 19: 435±442 64. Braga M, Gianotti L, Vignalli A, Cestari A, Bisagni P, Di Carlo V (1998) Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med 26: 24±30 65. Moore FA, Moore EE, Kudsk KA, Brown RO, Bower RH, Koruda MJ, Baker CC, Barbul A (1994) Clinical benefit of an immune-enhancing diet for early postinjury feeding. J Trauma 37: 607±615 66. Cerra FB, Lehmann S, Konstantinides N, Dzik J, Fish J, Konstantinides F, LiCari JJ, Holman RT (1991) Improve in immune function in ICU patients by enteral nutrition supplemented with arginine, RNA and menhaden oil is independent of nitrogen balance. Nutrition 7: 193±199 S 126 67. Galbµn C, Montejo JC, Mesejo A, Marco P, Celaya S, Sanchez-Segura J, Farre M, Bryg DJ (2000) An immuneenhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 28: 643±648 68. Atkinson S, Sieffert E, Bihari D (1998) A prospective, randomized, doubleblind, controlled clinical trial of enteral immunonutrition in the critically ill. Crit Care Med 26: 1164±1172 69. Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wenneberg AK, Nelson JL, Noursalehi M (1999) Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med 27: 1409±1420 70. American Society of Parenteral and Enteral Nutrition Board of Directors (1993) Guidelines for the use of parenteral and enteral nutrition in adult and paediatric patients. JPEN J Parenter Enteral Nutr 17: 1SA±26SA 71. Klein S, Kinney J, Jeejeebhoy K, Alpers D, Hellerstein M, Murray M, Twomey P (1997) Nutrition support in clinical practice: review of published data and recommendations for future research directions. JPEN J Parenter Enteral Nutr 21: 133±156 72. Wojnar MM, Hawkins WG, Lang CH (1995) Nutritional support of the septic patient. Crit Care Clin 11: 717±733 73. Preiser JC, BerrØ J, Carpentier Y, Jolliet P, Pichard C, Van Gossum A, Vincent JL (1999) Management of nutrition in European intensive care units: results of a questionnaire. Intensive Care Med 25: 95±101 74. Mizock BA, Troglia S (1997) Nutritional support of the hospitalized patient. Dis a month 43: 351±426 75. Fisher RL, Pipkin GA, Wood JR (1995) Stress-related mucosal disease. Crit Care Clin 11: 323±345 76. Tryba M (1999) Research on stress ulcer prophylaxis: wrong questions, wrong answers? Crit Care Med 27: 16±17 77. Tryba M (1987) Risk of acute stress bleeding and nosocomial pneumonia in ventilated intensive care unit patients: sucralfate verus antacids. Am J Med 83: 117±124 78. Garvey BM, McCambley JA, Tuxen DV (1989) Effects of gastric alkalinization on bacterial colonization in critically ill patients. Crit Care Med 17: 211±216 79. Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P (1994) Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med 330 377±381 80. Layne EA, Mellow MH, Lipman TO (1981) Insensitivity of guaiac slide test for detection of blood in gastric juice. Ann Intern Med 94: 774±776 81. Schentag JJ (1980) False positive ªhemoccultº reaction with cimetidine. N Engl J Med 303: 110 82. Schuster DP, Rowley H, Feinstein S, McGue MK, Zuckerman GR (1984) Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit. Am J Med 76: 623±629 83. Zandstra DF, Stoutenbeek CP (1994) The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis: a prospective cohort study. Intensive Care Med 20: 335±340 84. Pinilla JC, Oleniuk FH, Reed D, Malik B, Laverty WH (1985) Does antacid prophylaxis prevent upper gastrointestinal bleeding in critically ill patients? Crit Care Med 13: 646±650 85. Peura DA, Johnson LF (1985) Cimetidine for prevention and treatment of gastroduodenal mucosal lesions in patients in an Intensive care unit. Ann Intern Med 103: 173±177 86. Groll A, Simon JB, Wigle RD, Taguchi K, Todd RJ, Depew WT (1988) Cimetidine prophylaxis for gastrointestinal bleeding in an intensive care unit. Gut 27: 135±140 87. Basso N, Bagarani M, Materia A, Fiorani S, Lunardi P, Speranza V (1981) Cimetidine and antacid prophylaxis of acute upper gastrointestinal bleeding in high risk patients. Am J Surg 141: 339±342 88. Ben-Menachem T, Fogel R, Patel RV, Touchette M, Zarowitz BJ, Hadzijahic N, Divine G, Verter J, Bresalier RS (1994) Prophylaxis for stress-related gastric hemorrhage in the medical intensive care unit: a randomized, controlled, single-blind study. Ann Intern Med 121: 568±575 89. Borrero E, Bank S, Margolis I, Schulman ND, Chardavoyne R (1985) Comparison of antacid and sucralfate in the prevention of gastrointestinal bleeding in patients who are critically ill. Am J Med 79: 62±64 90. Bresalier RS, Grendell JH, Cello JP, Meyer AA (1987) Sucralfate versus titrated antacid for the prevention of acute stress-related gastrointestinal hemorrhage in critically ill patients. Am J Med 83: 110±116 91. Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, Wood G, Kirby A (1998) A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med 338: 791±797 92. Poleski MH, Spanier AH (1986) Cimetidine versus antacids in the prevention of stress erosions in critically ill patients. Am J Gastroenterol 81: 107±111 93. Stothert JC, Simonowitz DA, Dellinger EP, Farley M, Edwards WA, Blair AD, Cutler R, Carrico CJ (1980) Randomized prospective evaluation of cimetidine and antacid control of gastric pH in the critically ill. Ann Surg 192: 169±174 94. Skillman JJ, Bushnell LS, Goldman H, Silen W (1969) Respiratory failure, hypotension, sepsis and jaundice: a clinical syndrome associated with lethal hemorrhage from acute stress ulceration of the stomach. Am J Surg 117: 523±530 95. Hastings PR, Skillman JJ, Bushnell L, Sillen W (1978) Antacid titration in the prevention of acute gastrointestinal bleeding. N Engl J Med 298: 1041±1045 96. Lacroix J, Infante-Rivard C, Jenicek M, Gauthier M (1989) Prophylaxis of upper gastrointestinal bleeding in intensive care units: a meta-analysis. Crit Care Med 17: 862±886 97. Cook DJ, Reeve BK, Guyatt GH, Heyland DK, Griffith LE, Buckingham L, Tryba M (1996) Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analysis. JAMA 275: 308±314 98. MacDougall BRD, Bailey RJ, Williams R (1977) H-2 receptor antagonists and antacids in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure. Lancet I:617±619 99. Metz CA, Livingston DH, Smith JS, Larson GM, Wilson TH (1993) Impact of multiple risk factors and ranitidine prophylaxis on the development of stress-related upper gastrointestinal bleeding: a prospective, multicenter, double-blind randomized trial. Crit Care Med 21: 1844±1849 S 127 100. Tryba M, Zevounou F, Torok M, Zenz M (1985) Prevention of acute stress bleeding with sucralfate, antacids or cimetidine. Am J Med 79 [Suppl 2C]:55±60 101. Luk GD, Summer WR, Messersmith JF (1982) Cimetidine and antacid in prophylaxis of acute gastrointestinal bleeding: a randomized, double blind, controlled study (abstract). Gastroenterology 82: 1121 102. Priebe HJ, Skillman JJ, Bushnell LS, Long PC, Silen W (1980) Antacid versus cimetidine in preventing acute gastrointestinal bleeding. N Engl J Med 302: 426±430 103. Cook DJ, Witt LG, Cook RJ, Guyatt GH (1991) Stress ulcer prophylaxis in the critically ill: a meta-analysis. Am J Med 91: 519±527 104. Bonten MJM, Gaillard CA, Van der Geest S, van Tiel FH, Beysens AJ, Smeets HG, Stobberingh EE (1995) The role of intragastric acidity and stress ulcus prophylaxis on colonization and infection in mechanically ventilated ICU patients. Am J Respir Crit Care Med 152: 1825±1834 105. Prod'hom G, Leuenberger P, Koerfer J, Blum A, Chiolero R, Schaller MD, Perret C, Spinnler O, Blondel J, Siegrist H (1994) Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine or sucralfate for stress ulcer: a randomized controlled trial. Ann Intern Med 120: 653±662 106. Hanisch EW, Encke A, Naujoks F, et al (1998) A randomized, double-blind trial for stress ulcer prophylaxis shows no evidence of increased pneumonia. Am J Surg 176: 453±457 107. Ben-Menachem T, McCarthy BD, Fogel R, Schiffman RM, Patel RV, Zarowitz BJ, Nerenz DR, Bresalier RS (1996) Prophylaxis for stress-related gastrointestinal hemorrhage: a cost effectiveness analysis. Crit Care Med 24: 338±345 108. Lam NP, Le PDT, Crawford SY (1999) National survey of stress ulcer prophylaxis. Crit Care Med 27: 98±103 109. American Thoracic Society (1995) Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy and preventive strategies. Am J Respir Crit Care Med 153: 1711±1725 Intensive Care Med (2001) 27: S 128±S 134 Summary of recommendations Definition of sepsis (I. Matot, C. L. Sprung) · In the past many different definitions of sepsis were used interchangeably, which led to confusion. · Sepsis is the systemic inflammatory response to infection. · No single physiological or laboratory parameter can universally identify sepsis. · Not all patients with sepsis are equally ill. Sepsis, severe sepsis, and septic shock constitute different gradations in the continuum of a disease process manifested by a combination of changes in vital signs, laboratory parameters, hypoperfusion, and organ dysfunction. · The continuum of sepsis, severe sepsis, and septic shock is correlated with increasing organ dysfunction and mortality. · The source of infection and diagnosis of sepsis must be identified as early as possible to permit early intervention with antimicrobial therapy and surgical drainage to prevent disease progression, organ dysfunction, and mortality. Diagnosis of infection in sepsis (M. Llewelyn, J. Cohen) Bacteremia · Fever, chills, hypothermia, leukocytosis, left-shift of neutrophils, neutropenia, and when infection is suspected, hypoalbuminemia, development of renal failure or signs of hemodynamic compromise are specific indications for obtaining blood for culture. · Blood cultures should be taken as soon as possible after onset of fever or chills. · Blood should be obtained by fresh venipuncture. Sites associated with skin contamination (e.g., femoral site) or loss of skin integrity (e.g., burns or dermatological disease) should be avoided. · Skin should be swabbed twice with either 70 % isopropyl alcohol or with an iodine containing solution prior to venipuncture. The blood culture stopper should also be sterilized prior to inoculation. · An adequate volume (20±60 ml) of blood should be obtained per culture (10±30 ml per bottle) · If insufficient blood is available, only the aerobic bottle should be inoculated. · The needle used for venipuncture should be changed prior to inoculation of blood into culture bottles. · A minimum of two and a maximum of three sets of blood cultures should be obtained for each episode of suspected bacteremia. · In critically ill patients in whom it may not be possible to delay treatment, no interval is required between taking sets of blood cultures. Central venous catheter infections · When a central venous catheter (CVC) is suspected as a source of bacteremia, diagnosis of CVC infection may be made by blood culture based techniques if (a) the patients clinical condition permits a potentially infected line to be left in place, (b) treatment of CVC infection is to be attempted, or (c) other potential sources of bacteremia are apparent. · While the acridine orange leukocyte cytospin test offers the possibility of virtually immediate diagnosis, on the basis of currently available data, its use should remain experimental. · When a CVC is suspected as a source of sepsis in nonbacteremic patients, definitive diagnosis requires that the CVC should be removed and sent for culture. · If infection is suspected at the catheter site, swabs should be taken from the insertion site for culture. · The presence of purulence at the CVC site should prompt catheter replacement at a distant site irrespective of culture results. S 129 Ventilator-associated pneumonia Acute cholecystitis · Ventilator-associated pneumonia (VAP) should be considered as a source of sepsis in any ventilated patient particularly in the first week following intubation, following aspiration, when a nasogastric/entral feeding tube is in place, or when drugs have been given to raise gastric pH. · Investigation of suspected VAP should include the taking of two sets of blood cultures and a chest radiograph. · Pleural effusions larger than 10 mm should be aspirated. Samples should be sent for immediate Gram and fungal stains, culture and biochemistry including protein, lactic dehydrogenase and glucose. Paired blood chemistry samples should also be sent for comparison. · Serology is not routinely indicated in the diagnosis of VAP. · A sample of secretions aspirated via the endotracheal tube should be sent for Gram stain and for bacterial and fungal culture. · Bronchoscopy should be performed unless contraindicated or unavailable. · No significant advantage of one invasive diagnostic approach over another has been consistently demonstrated. Choice of technique depends in practice primarily on available expertise and equipment. · Acute acalculous cholecystitis should be suspected in any sepsis patient, particularly postoperatively, when there are either signs relating to the right upper quadrant of the abdomen or obstructive liver function tests. · When acute acalculous cholecystitis is suspected, ultrasound should be ordered urgently. · If an initial ultrasound examination is not diagnostic, computed tomography should be ordered. · If computed tomography is unavailable, a repeat ultrasound should be performed after 24 h. Surgical site infection and intra-abdominal sepsis · Blood cultures should be sent when investigating suspected surgical site infection or deep abdominal infection. · The presence of purulence or spreading cellulitis are indications for taking wound swabs. · Infection should be suspected particularly at ºcontaminatedº or ªdirtyº surgical sites. · When contaminated or dirty abdominal wounds develop features of wound infection, a diagnosis of anaerobic coinfection should be assumed irrespective of whether anaerobes are identified by routine microbiology. · In most situations ultrasound is be the modality of first choice. When ultrasound is not diagnostic, computed tomography should be considered. · Collections identified by radiology should, where technically possible, be aspirated and drained under radiological control, samples being sent for Gramstaining and culture. Sinusitis · Acute sinusitis should be suspected in any sepsis patient who has either a nasotracheal tube or a finebore nasogastric feeding tube, or who has suffered a head injury. · When sinusitis is suspected, radiography of the maxillary sinuses should be performed to detect the presence of fluid. · When radiography does not demonstrate fluid in the maxillary sinuses, computed tomography should be performed. · If either radiography or computed tomography demonstrates the presence of fluid, antral puncture should be performed to allow definitive diagnosis and therapeutic drainage before antibiotic therapy is initiated. Invasive Candida infection · There are no data to support a policy of routine screening of hospitalized patients for Candida colonization. However, in sepsis patients, invasive fungal infection is more likely in patients who are heavily colonized. · When sepsis develops in patients colonized by Candida species at two or more sites, blood cultures should be sent and lysis centrifugation performed if available. · Isolates of Candida species from sterile sites should be sent for specificity and sensitivity testing. Antibiotics in sepsis (P.-Y. Bochud, M. P. Glauser, T. Calandra) · Retrospective studies have shown that early administration of appropriate antibiotics reduces the mortality in patients with bloodstream infections caused by Gram-negative bacteria. S 130 · By analogy with the observations made in patients with Gram-negative sepsis and despite the lack of substantial clinical data in the literature, it is likely that appropriate antibiotic therapy reduces the morbidity and the mortality of Gram-positive sepsis. · Antifungal therapy is recommended for patients with candidemia. Whether early treatment is associated with better outcome is unknown, and additional studies are needed to evaluate this question. · Monotherapy with carbapenem antibiotics is as effective as combination therapy with a b-lactam and an aminoglycoside for the empirical treatment of nonneutropenic patients with severe sepsis. · Monotherapy with third- or fourth-generation cephalosporins is as effective as combination therapy with a beta-lactam and an aminoglycoside for the empirical treatment of nonneutropenic patients with severe sepsis. · Extended-spectrum carboxypenicillins or ureidopenicillins combined with b-lactamase inhibitors have been shown to be effective for the treatment of suspected infections in febrile, neutropenic cancer patients, and in patients with peritonitis or nosocomial pneumonia. However, similar studies have not yet been carried out in patients with severe sepsis or shock. · Monotherapy with aztreonam appears to be as effective as combination of a b-lactam and an aminoglycoside for the treatment of patients with documented Gram-negative sepsis. The fact that aztreonam lacks any appreciable activity against Gram-positive or anaerobic bacteria precludes its use as empirical single-agent therapy in patients with severe sepsis. · Fluoroquinolones have been shown to be highly effective for the treatment of documented Gram-negative bloodstream infections. However, data are lacking to support their use as single-agent treatment of severe sepsis, especially as first-generation fluoroquinolones display suboptimal activities against Grampositive bacteria. · Third- and fourth-generation cephalosporins and carbapenem antibiotics are equally effective as empirical therapy in patients with severe sepsis. · The indiscriminate use of glycopeptide antibiotics (i.e., vancomycin or teicoplanin) for presumed Gram-positive infections in patients with severe sepsis and septic shock should be avoided. However, glycopeptides are appropriate in severely ill patients with catheter-related infections or in centers in which methicillin-resistant staphylococci predominate. The possible clinical benefit associated with the empirical use of glycopeptides should be weighed against the risks of selecting resistant organisms and of increased toxicity. Most cases require additional Gram-negative coverage, at least until microbiological results are available. · Antifungal agents, such as fluconazole, should not be used on a routine basis as empirical therapy in patients with severe sepsis and septic shock. · Fluconazole is as effective as and less toxic than amphotericin B for the treatment of candidemia in nonneutropenic patients. However, if the patient is unstable or has been treated previously with fluconazole, it might be prudent to begin therapy with amphotericin B while waiting for the identification of the Candida species and for the results of susceptibility testing. Whether 5-fluorocytosine should be combined with amphotericin B in unstable patients is debatable. Hemodynamic support in septic shock (J.-L. Vincent) · The goal of fluid resuscitation in septic shock is restoration of tissue perfusion and normalization of cellular metabolism. · Volume repletion in patients with septic shock produces significant increases in cardiac output and systemic oxygen delivery, and fluids alone are sometimes sufficient to reverse hypotension and restore hemodynamic stability. · Requirements for fluid infusion are not easily determined, and therefore that the fluid challenge should be titrated to the clinical endpoints of blood pressure, heart rate, and urine output. Central venous pressure is initially required to evaluate the complex relationship between intravascular blood volume and cardiac function. It is difficult to give optimal values for cardiac filling pressures. · When central venous pressure increases, a pulmonary artery catheter is probably required, although its role has recently been questioned. · Increases in cardiac output and systemic oxygen delivery are proportional to the degree of intravascular volume expansion achieved. · The optimal hemoglobin and hematocrit for patients with septic shock is unclear. Most experts recommend hemoglobin levels of 9±10 gm/dl in patients with septic shock. This degree of anemia is usually well tolerated in most patients, even with cardiac impairment. · When fluid challenge fails to restore an adequate arterial pressure and organ perfusion, therapy with vasopressor agents should be started. Vasopressor therapy may also be required transiently to sustain life and maintain perfusion in the face of life-threatening hypotension, even when cardiac filling pressures are not elevated. · The effects of dopamine on cellular oxygen supply in the gut remain incompletely defined. The effects of norepinephrine on splanchnic circulation are hardly predictable. The combination of norepinephrine and S 131 dobutamine appears to be more predictable and more appropriate to the goals of septic shock therapy than the effects of epinephrine alone. · The hemodynamic effects of dopamine in patients with septic shock are well established. Dopamine increases mean arterial pressure primarily by increasing cardiac index with minimal effects on systemic vascular resistance. The increase in cardiac index is due to an increase in stroke volume, and to a lesser extent, to increased heart rate. Patients receiving dopamine at rates greater than 20 mg kg±1 min±1 show increases in right heart pressures as well as in heart rate, and therefore doses should not usually exceed 20 mg kg±1 min±1, at least not without adequate hemodynamic monitoring. · Norepinephrine markedly improves mean arterial pressure and glomerular filtration. This is particularly true in the high output-low resistance state of many septic shock patients. After restoration of systemic hemodynamics, urine flow reappears in most patients and renal function improves without the use of low-dose dopamine or furosemide. This fact supports the hypothesis that renal ischemia observed during hyperdynamic septic shock is not worsened by norepinephrine infusion and even suggests that this drug may effectively optimize renal blood flow and renal vascular resistance. · Dobutamine is an adrenergic agonist that stimulates b1, b2, and b1 adrenergic receptors. A number of studies have investigated the effect of dobutamine on cardiac function during sepsis or septic shock. The doses utilized ranged from 2 to 28 mg kg±1 min±1. The majority of these studies found increases in cardiac index combined with increases in stroke volume and heart rate. Source control in the management of sepsis (M. F. Jimenez, J. C. Marshall) · Surgical intervention in the form of dØbridement of infected, devitalized, or nonbleeding tissue should be undertaken rapidly following hemodynamic stabilization in patients with necrotizing soft tissue infections. This is a grade E recommendation supported by level IV and level V evidence. · The decision to intervene surgically in the patient with infected pancreatic necrosis must weigh the potential advantages of removing a source of ongoing bacterial proliferation against the inherent morbidity of early surgery. In general, surgery should be delayed in the stable patient to permit adequate demarcation of tissue planes. This is a grade C recommendation supported by a single randomized trial and expert opinion. · The diagnosis of intra-abdominal infection amenable to source control measures can generally be made by either ultrasound or computed tomography. Ultrasonography has the advantage of being portable and inexpensive, but is highly operator dependent; computed tomography is especially useful in the evaluation of the retroperitoneum. · The initial approach to well-defined and accessible intra-abdominal abscesses should be percutaneous drainage. Catheter drainage can also be used as a temporizing measure to optimize the physiological and hemodynamic condition of an acutely ill patient prior to surgical exploration. Laparotomy should be reserved for those circumstances in which there are no well-defined collections, dead tissue requires dØbridement, or residual collections cannot be treated percutaneously. Surgical intervention may also be indicated to control a source of ongoing peritoneal contamination. Rates of failure have increased as interventional radiologists have extended the indications for percutaneous drainage. If the clinical condition of the patient does not improve following the initial drainage, follow-up computed tomography should be performed to determine whether a residual or missed collection is present, and surgical intervention should be considered. · Current data support the concept that relaparotomy ªon demand,º as indicated by worsening of the clinical status, absence of improvement, or evolving organ dysfunction is as efficacious as a more aggressive approach. Planned relaparotomy is indicated for patients with ischemic bowel when intestinal viability is a concern (ªsecond lookº), for patients with necrotizing pancreatitis when demarcation of necrotic tissue demarcation is not distinct, or when bleeding precludes complete dØbridement. · Although tissue necrosis can often be detected by such characteristic radiographic findings as gas in the tissues, or nonenhancement of tissues following administration of intravenous contrast, there is no single test that can exclude the presence of tissue necrosis with certainty, and in circumstances in which necrosis may be life threatening (for example, intestinal ischemia), it is often necessary to establish the diagnosis operatively. · An infected central venous catheter can be safely changed over a guidewire, provided there is not significant local soft tissue infection at the exit site. This is a grade B recommendation supported by level II evidence. · There is no evidence that routine catheter replacement reduces the risk of catheter-related bacteremia. Venous catheters should be changed only as needed when evidence of infection is present (signs of inflammation, purulent discharge at the insertion site), or when the catheter is not working. This is a level C S 132 recommendation for central venous catheters, supported by level II evidence, and a level E recommendation for peripheral catheters, supported by level V evidence. · Definitive resection is preferable to proximal diversion and drainage for perforated diverticulitis, and likely for other causes of intestinal perforation, when the more demanding procedure of resection can be performed safely. Extension of this principle to other sites of gastrointestinal peroration such as the esophagus requires balancing the risks of resection with the potential benefits. This is a grade D recommendation based on level III evidence. · Primary anastomosis or colostomy are equally efficacious following colon resection for diverticulitis. The choice of procedure should be dictated by other factors such as severity of illness, presence of chronic disease, the degree or duration of peritoneal contamination, and the skill and experience of the surgical team. This is a grade D recommendation based on level III evidence. · Intra-abdominal infectious complications mandating source control are almost always evident using modern diagnostic imaging techniques. There is little if any role for empirical laparotomy to rule out undiagnosed infection in a critically ill patient in whom radiological examination has failed to demonstrate a surgically correctable problem. · Mechanical ventilation of patients with ALI should be conducted with small tidal volumes (approximately 6 ml/kg ideal body weight) with the goal to maintain end-inspiratory plateau pressures at levels less than 30 cmH2O. · Prone positioning may be considered in patients requiring high levels of inspired oxygen (FIO2 > 0.60) in whom positional changes are not contraindicated, and who are cared for at facilities experienced in the management of critically ill mechanically ventilated patients. · Restrict nitric oxide as an option for salvage therapy in patients with life-threatening hypoxemia not responding to traditional mechanical ventilation strategies. · Judicious use of crystalloid fluid administration should be practiced in patients with ALI/ARDS, with colloid solutions considered in hypo-oncotic patients with established ALI/ARDS. It is not clear whether or not volume restriction improves outcome. · Do not routinely administer corticosteroids to patients at risk for, or meeting criteria for, ALI/ARDS. Consider intravenous methylprednisolone in patients with persistent or refractory ARDS after actively excluding infection. · All patients requiring acceptable levels of ventilatory support who are not overtly unstable should receive a spontaneous breathing trial on a daily basis to ascertain their ability to breathe unassisted. Airway and lung in sepsis (G. S. Martin, G. R. Bernard) · Provide adequate supplemental oxygen to maintain an oximetric saturation of 90 % through use of simple oxygen delivery systems (i.e., nasal cannula or face mask), if possible. In endotracheally intubated patients, use of positive end-expiratory pressure to increase mean airway pressure may be employed to reduce concentrations of inspired oxygen below potentially toxic thresholds (FIO2 < 0.60). · Avoid the use of noninvasive positive-pressure ventilation in patients with sepsis-related acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). · Use early placement of an endotracheal tube and institution of mechanical ventilation in patients with sepsis. Indications for institution of mechanical ventilation include severe tachypnea (respiratory rate > 40 bpm), muscular respiratory failure (use of accessory muscles), altered mental status, and severe hypoxemia despite supplemental oxygen. · In mechanically ventilated ALI/ARDS patients with high inspiratory pressures or otherwise at risk for barotrauma or volutrauma, implement permissive hypercapnia through reduced tidal volume ventilation. Immunological therapy in sepsis: currently available (J. Carlet) · Corticosteroids should not be used in severe sepsis or septic shock at high doses (30 mg/kg), and for a short course (1±2 days). On the other hand, corticosteroids may be used during ªrefractoryº septic shock but not during severe sepsis without shock or mild shock. They should then be used at low doses (100 mg hydrocortisone three times a day) for 5 days or more (up to 10 days) and then with subsequent tapering of the dose according to the hemodynamic status. The results of a large trial will be available shortly and must be considered before definite recommendations can be made. · Ibuprofen should not be used during severe sepsis and septic shock. Additional studies are needed to determine whether some patients, for example, those with hypothermia, could benefit from the drug. · Prostaglandins, in particular prostaglandin E1 or liposomal prostaglandin E1 should not be used during acute respiratory distress syndrome due to sepsis. There are no specific data allowing recommendations in severe sepsis. S 133 · Pentoxifyilline should not be used in adults with severe sepsis unless new studies show a significant effect. The positive effect of a small study in infants should be confirmed before clinical use. · N-Acetylcysteine should not be used in severe sepsis until new data are available, focusing in particular on very early therapy. · Selenium should not be used for severe sepsis. Additional studies are warranted to confirm initial positive data. · Antithrombin III should not be used during severe sepsis. Countries which allow the free use of this drug in this setting should reconsider their position. · Immunoglobulins should not be used either in adult patients or in neonates with sepsis, unless additional large studies confirm some positive data in smallsized meta-analyses. Countries which allow a wide use of these compounds should reconsider their position and encourage these studies. · Granulocyte colony stimulating factor should not be used in nonneutropenic patients with severe sepsis. · Growth hormone should not be used in patients with sepsis because it increases mortality. · Hemofiltration should not be used in patients with sepsis, without renal indications unless ongoing studies provide positive data. Other supportive therapies in sepsis (J. PØrez, R. P. Dellinger) · Considering the frequent occurrence of independent risk factors for Deep vein thrombosis (DVT) in septic patients and the high percentage of sepsis/infected patients included in studies that have demonstrated efficacy of DVT prophylaxis in general, septic patients should be treated with DVT prophylaxis. Even though there is not a randomized study that establishes the impact of DVT prophylaxis on morbidity and mortality specifically in septic patients, the significant number of septic patients included in the populations of patients enrolled in other prospective randomized trials supports that the use of DVT prophylaxis reduces morbidity and mortality in septic patients. Moreover, septic patients, especially those with severe sepsis and multiple organ failure, have less cardiopulmonary reserve, and the impact of a minor thromboembolic event in this group of patients could be very compromising. · Septic patients who do not have a contraindication to heparin use should receive prophylaxis with either low-dose unfractionated heparin (5,000 U either two or three times daily) or low molecular weight heparin (at recommended doses; grade A). · For those septic patients who have an absolute contraindication for heparin use (i.e., thrombocytopenia, severe coagulopathy, active bleeding, recent intracerebral hemorrhage), the use of a mechanical prophylactic device is advised since this method has proven to be effective in postsurgical patients and therefore would likely work in septic patients (grade E). · Based on the assumption that sepsis produces a hypercatabolic state and leads to protein-energy malnutrition, and given that protein loss is associated with poor outcome, nutritional support in septic patients is recommended. The correlation of nutritional support with outcome in septic patients comes from data extrapolated from studies performed in perioperative patients and from expert opinion that allow us to establish this recommendation. Many important questions remain regarding what kind of nutrition and when in the course of sepsis should nutrition begin. · Enteral nutrition is the preferred method of nutritional support in the catabolic critically ill patient in general, inclusive of the septic patient (grade C). For those patients who cannot tolerate enteral nutrition for a prolonged time or when contraindications do not allow its use (mesenteric ischemia, mechanical bowel obstruction), parenteral nutritional support should be used (grade E). Immune-enhancing formulas may be better than other enteral formulations in critically ill patients, but effects on ultimate outcome (i.e., survival) remain to be demonstrated in large randomized trials (grade B). · The following are specific recommendations for septic patients, according to the guidelines established by the American College of Chest Physicians and American Society of Parenteral and Enteral Nutrition consensus conferences: · Daily caloric intake: 25±30 kcal/kg usual body weight · Protein: 1.3±2.0 g/kg per day · Glucose: 30±70 % of total nonprotein calories, to maintain serum glucose level below 225 mg/dl · Lipids: 15±30 % of total nonprotein calories. w 6 Polyunsaturated fatty acid should be reduced in septic patients, maintaining that level which avoids deficiency of essential fatty acids (7 % of total calories ± generally 1 g/kg per day). · No specific recommendations are offered for use of medium-chain triglycerides, branched-chain amino acids, or specific microelements added to the nutritional formulas. The use of any of these strategies, although supported in concept, does not have enough investigational evidence to determine any clinical benefit in outcome of septic patients. · No randomized trial has evaluated the effect of stress ulcer prophylaxis (SUP) on clinical outcome in septic patients. Examination of successful clinical trials of S 134 SUP does not allow precise identification of patients with diagnosis of sepsis. Therefore no definitive data exist in septic patients on the effectiveness of SUP in diminishing episodes of overt or clinically significant bleeding. The clinical utility of SUP as it affects clinical outcome in septic patients is therefore not clear. Septic patients have been assumed to have an increased risk for SUP since they have multiple risk factors known to increase the risk of stress ulcer bleeding. Since data do support SUP as being efficacious in preventing upper gastrointestinal bleeding in populations of critically ill patients, which would be expected to contain large proportions of septic patients, the use of SUP is recommended in this group (see below). · Although no large randomized trial has addressed septic patients alone, abundant data exist regarding subgroups of septic patients with prolonged mechan- ical ventilation, hypotension, and coagulopathy. For these patients the use of SUP is recommended (grade A). For other septic patients in whom these factors are not present, SUP is recommended based on several small randomized trials in which SUP has proven efficacious in preventing bleeding and therefore reducing morbidity in critically ill patients (grade C). · Several trials have confirmed the efficacy of antacids, sucralfate or histamine-2 receptor antagonists in the prevention of stress ulcer bleeding. Since the data are conflicting, no single one can be determined as preferable. General recommendations should be based on the individual experience in the use of one or another, the availability, or cost-analysis in individual centers. In septic patients with risk factors, the use of enteral nutrition following the preventive strategies currently available may be beneficial for preventing stress ulcer bleeding.
© Copyright 2026