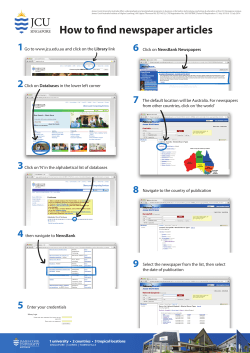
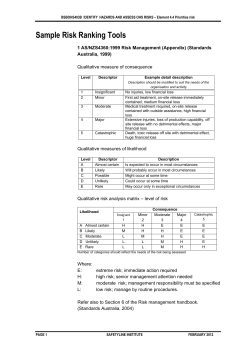
AS/NZS 3003:2011 Electrical installations Patient areas IHEA seminar– July 2011
IHEA seminar– July 2011 AS/NZS 3003:2011 Electrical installations Patient areas Lawrie Knuckey Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 1 New edition • Still has requirements for: • body-protected electrical areas • cardiac-protected electrical areas • Some locations must now be • Some other locations must now be • Some socket-outlets and medical electrical equipment must still be protected by LPDs: • high speed, high sensitivity residual current devices (RCDs), or • isolation transformers with line isolation and overload monitors. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 2 Major changes in the new edition • Specific socket-outlets now require LPD protection: • A circuit can no longer supply more than one room and its ensuite. • A circuit can no longer supply socket-outlets for cleaning purposes and other socket-outlets. • Socket-outlets within 5 m of a patient area must now be protected. • UPS status indicators are now required. • RCDs must now be readily accessible. • Socket-outlets for cleaning purposes no longer required within Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 3 Major changes in the new edition • EP terminals no longer required in • New section on special locations: • Specific requirements for locations with patients susceptible to selfharm. • Requirements for home care use of medical electrical equipment. • Practical new requirements for home dialysis installations. • New section on alterations, additions and repairs: • “Alterations” and “additions” range from installing a new socketoutlet to replacing a CT scanner. • Routine testing must be up-to-date before alterations or additions can begin. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 4 Major changes in the new edition • New section on alterations, additions and repairs (cont): • All socket-outlets must be upgraded to correct colour if total number increased >10%. • EP earthing system must be upgraded/replaced for new equipment >2 Kw. • New definition of “essential supply”: • No longer includes generator-backed circuits subject to load shedding. • Some existing red socket-outlets no longer on essential supplies. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 5 Major changes in the new edition • New section on magnetic field testing: • Specifies limits for for ECG monitoring and recording. • Must be applied in specified locations. • New detailed requirements for routine electrical safety testing. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 6 Scope of AS/NZS 3003 Scope is still predicated by the definition of “patient area”: • Patient areas must be wired as body-protected or cardiac-protected electrical areas. • Requirements are still limited to patient areas and locations associated with patient areas. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 7 Scope of AS/NZS 3003 Historical confusion: Which institutions: ✔✔ Hospital ✔? X-ray practice ✔? Dental surgery ✔? Nursing home ?? Aged care facility Which locations: ? Hospital corridors ? Staff base in ICU/CCU/A&E ? Control rooms for Cath lab/CT room Further attempt to define scope: New title Amended definition of patient area Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 8 New title 1976 edition Electrical installations in electromedical treatment areas 1985 edition Electrical installations — Patient treatment areas of hospitals and medical and dental practices 1999 edition Electrical installations — Patient treatment areas of hospitals and medical and dental practices 2003 edition Electrical installations — Patient areas of hospitals, medical and dental practices and dialyzing locations 2011 edition Electrical installations — Patient areas Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 9 New definition of patient area 2003 Locations in hospitals and medical and dental practices in which it is intended that mains powered medical electrical equipment will be used. Locations in patients’ homes and other facilities intended for dialysis are also included. 2011 Locations where it is intended that mains powered medical electrical equipment will be used on a patient. This does not include areas such as corridors and lifts where medical equipment is only used in an emergency or for transporting patients. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 10 Extent of the patient area 2003 The boundary of the cardiac-protected electrical area extends to the walls enclosing the patient location(s) or, if such walls are incomplete (e.g. entryway without a door) to the boundary formed if such walls or projections were extended. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 11 Extent of the patient area Draft: Where the walls of a body-protected electrical area are incomplete (e.g. entryway without a door) and the opening is greater than 2 m, the adjacent area is part of the body-protected electrical area. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 12 Extent of the patient area 0 1 2 Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 13 Extent of the patient area Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 14 Extent of the patient area Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 15 Extent of the patient area 2011 The boundary of the cardiac-protected electrical area extends to the walls enclosing the patient location(s) or, if such walls are incomplete (e.g. an entryway without a door) to the boundary formed if such walls or projections were extended. In this context, the following are not considered walls or boundaries: partitions, dividers, screens, benches, moveable walls or curtains. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 16 Socket-outlets near patient areas 2003 Socket-outlets within 2 m of the entrance to a patient area, if located in an area that freely communicates* with the patient area, must be: • protected by an LPD, and • connected to the EP earthing system if *no door Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 17 Socket-outlets near patient areas 2011 Socket-outlets located within 5 m of the entrance* to a patient area, in any room, corridor, etc, opening directly off the patient area, must be: • protected by an LPD, and • connected to the EP earthing system if *Whether or not the entryway has a door Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 18 Extent of the patient area 2011 The boundary of the cardiac-protected electrical area extends to the walls enclosing the patient location(s) or, if such walls are incomplete (e.g. an entryway without a door) to the boundary formed if such walls or projections were extended. In this context, the following are not considered walls or boundaries: partitions, dividers, screens, benches, moveable walls or curtains. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 19 Extent of the patient area 2011 The boundary of the cardiac-protected electrical area extends to the walls enclosing the patient location(s) or, if such walls are incomplete (e.g. an entryway without a door) to the boundary formed if such walls or projections were extended. In this context, the following are not considered walls or boundaries: partitions, dividers, screens, benches, moveable walls or curtains, and entryways wider than 10% of the perimeter of the patient area. (Proposed ESV ruling.) Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 20 Socket-outlets near patient areas 2011 The boundary of the cardiac-protected electrical area extends to the walls enclosing the patient location(s) or, if such walls are incomplete (e.g. an entryway without a door) to the boundary formed if such walls or projections were extended. In this context, the following are not considered walls or boundaries: partitions, dividers, screens, benches, moveable walls or curtains, and entryways wider than 10% of the perimeter of the patient area. (Proposed ESV ruling.) Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 21 Socket-outlets near patient areas Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 22 New magnetic field requirements • Health care facility must ensure magnetic fields do not exceed “safe” limits in areas used for sensitive monitoring or recording equipment. • Maximum field strength and test methods are specified for ECG monitoring and recording. • More demanding requirements are required for some other tests but neither maximum field strength nor test methods are specified. • “Requirements” only apply in: Biomec Australia A&E departments Cardiac cath labs CCU ICU OPD rooms for diagnostic electrocardiography Resuscitation units Stress testing units © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 23 New magnetic field requirements • “Requirements” do not apply (!) in locations such as: - Operating theatres - Procedure rooms intended for general anaesthesia - Diagnostic ECG rooms (except in OPD) - Cardiac ultrasound rooms - Imaging rooms with ECG monitoring or ECG-synchronised injectors/angiography/scanning - ECT rooms • “Testing” seems to be required in any location intended for ECG monitoring or recording. • But it is unclear what “testing” is required in locations where the “requirements” do not apply. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 24 New magnetic field requirements • Where the requirements do apply: New installations: - Wiring must be designed to achieve maximum specified field strength. - Installation must be tested upon completion. Alterations or additions: - Location must be tested before commencing work. - Installation must be tested again upon completion. Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 25 Socket-outlets for cleaning purposes Still required in each cardiac-protected electrical area Can be outside the room – but every part of a bodyprotected electrical area must be within 15 m of a socketoutlet marked for cleaning purposes MRI rooms Exempt Ensuites Still required Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 26 Routine in-service testing • Must be up-to-date before starting any alteration or addition. • Detailed normative requirements included in new standard. • Associated report form is full of errors. • We recommend: - Review contracts for routine testing of patient areas - Nominate compliance with new requirements (Section 9) - Disallow use of associated report form (Appendix G) - Disallow test stickers for alterations/additions Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 27 Routine in-service testing Disallow test stickers required by new standard: Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 28 Outstanding issues plug-in medical electrical equipment Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 29 Outstanding issues such as rooms – not roof spaces! Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 30 Outstanding issues Body-protected electrical areas of nursing homes Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 31 Outstanding issues socket-outlets in cardiac-protected electrical areas Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 32 Outstanding issues readily accessible Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 33 Outstanding issues Which requirements of AS/NZS 3009? Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 34 Outstanding issues Available: • in the hospital? • in the particular building of the hospital? • at the switch-board supplying the UPS? • in the room housing the UPS? Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 35 Thank you Biomec Australia © 2011 Biomedical Engineering Company of Australia Pty Ltd Consulting engineers – medical device standards and regulatory affairs 36
© Copyright 2026