Phosphatidylinositol-3-kinase activity is required for the anti-ig-
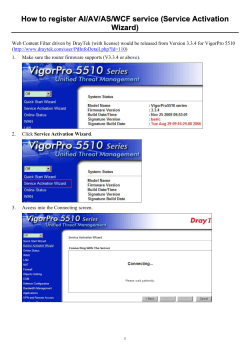
From www.bloodjournal.org by guest on November 7, 2014. For personal use only. 1996 87: 202-210 Phosphatidylinositol-3-kinase activity is required for the anti-igmediated growth inhibition of a human B-lymphoma cell line M Beckwith, RG Fenton, IM Katona and DL Longo Updated information and services can be found at: http://www.bloodjournal.org/content/87/1/202.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From www.bloodjournal.org by guest on November 7, 2014. For personal use only. Phosphatidylinositol-3-Kinase Activity Is Required for the Anti-IgMediated Growth Inhibition of a Human B-Lymphoma Cell Line By Margaret Beckwith, Robert G. Fenton, lldy M. Katona, and Dan L. Longo Stimulation of B lymphocytes through the Ig receptor initimannin, can completely abrogateanti-lg-medmted growth ates acascade of biochemicalchanges, which can ultimately inhibitionwithout affecting tyrosine kinase induction or prolead to either activation and growth, or cell-cycle arrest and tein kinase C (PKC) activation. Treatment of intact cells with celldeath.Oneof the critical events that occurs in both Wortmannin results in an irreversible decrease in anti-lgcases isthe activation of tyrosinekinases, and the resulting induced P13K activity, suggesting that the effect of Wortphosphorylationof a variety of proteins on tyrosine residues. mannin on anti-lg-mediated growth inhibition is caused by In this report we identify one ofthe substrates of phosphory- its inactivation ofP13K activity. Taken together, these data lation as the 85-kD subunit ofthe enzyme phosphatidylinosi- show that activation of P13K is a critical component of the tol-3 kinase (P13K), andshow that both anti-lgM and anti-lg-initiated signaling cascadethat leads to growth inhianti-lgD stimulation results in anincrease in the antibition of human B lymphoma cells. phosphotyrosine-precipitable P13K activity.Furthermore, This is a US government work. There areno restrictions on we show that the potent and specific inhibitor of P13K. Wortits use. T HE Ig RECEPTOR ON B lymphocytes has been shown to consist of a complex of proteins similar to those identified as members of the T-cell receptor The IgM and IgD heavy and light chains are associated with a heterodimer consisting of Iga and Igp or Igy,"' products of the mb-l and B29 genes, re~pectively.~.~ These small phosphoproteins serve to link the Ig receptor with several src-family tyrosine kinases that are activated after receptor ligati~n.~"' This results in the phosphorylation of several proteins on tyrosine,'*.l3including the ~ 2 1 GTPase-activat"~ ing protein ( ~ 2 1 "GAP),I4 ~ phospholipase C y l (PLCyl)lS and y2 (PLC ~ 2 ) , ' VAV," ~ , ' ~ phosphatidylinositol-3 kinase (P13K),19 in addition to the mb-l and B29 gene products.2a22 The phosphorylation of proteins on tyrosine is a key regulatory process inmany different pathways shown tobe involved in activation and proliferation of B lymphocytes,2'-25 andthe functional role of these phosphorylated signaling proteins is under intense investigation. One of these proteins that has recently been the focus of many studies in a variety of cell types is P13K.26This enzyme is comprised of a 110-kD (p1 10) catalytic subunit and an 85- From the Biological Carcinogenesis and Development Program, Science Applications International CorporatiodFrederick; and Biological Response Mod$ers Program, the Division of Cancer Treatment, National Cancer Institute, Frederick CancerResearch and Development Center, Frederick;and Departments of Pediatrics and Medicine, Uniformed ServicesUniversity ofthe Health Sciences, Bethesda. MD. Submitted May 22, 1995; accepted August 22, 1995. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, or the Department of Defense, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Goverment. Address reprint requests to Margaret Beckwith, PhD, NCIFCRDC, Bldg 567, Room 204, Frederick, MD 21702. The publication costsof this article were defrayedin part by page chargepayment. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C.section 1734 solelv to indicate this fact. This is a US government work. There are no restrictions on its use. 0006-4971/96/8701-0033$0.00/0 202 kD (p85) regulatory and it phosphorylates inositol lipids on the D-3 hydroxyl position." Activation of PI3K has been shown to occur after receptor ligation inmany different systems, including growth factor receptors containing intrinsic tyrosine kinase activit~,'~-~' as well as receptors associated with src-like kinase^.'^^'"^^ For example, the 85-kD subunit of PJ3K has been shown to associate with the SH3 domaid6 of the lyn tyrosine kinase in B cells,19 to be phosphorylated on tyrosine after stimulation through surface Ig," and to bind to purified Ig-aGST fusion proteins, via an associated src-like k i n a ~ eLigation .~ of CD19 on B cells has also been shown to recruit active PDK to the CD19 comp l e ~ . 'In ~ addition, in T lymphocytes, stimulation through CD28 results in association of CD28 with p85, phosphorylation of p85 on tyrosine, and activation of P13K.3s,'9 Several approaches have been used to determine the functional importance of PI3K in receptor-mediated biologic responses. By mutating or deleting the residues in the plateletderived growth factor (PDGF) receptor critical for PI3K p85 binding,"" or by overexpressing a mutant p85 to which the 110 subunit cannot bind:' investigators have demonstrated a requirement for PI3K activity in PDGF-mediated receptor internali~ation,~'membrane ruffling:' and mit~genesis.~' Recently, the functional significance of PI3K has also been demonstrated using inhibitors of PBK activity. One compound that has been shown to be a potent and specificinhibitor of PI3K in vivo and in vitro is the fungal metabolite Wortmannin.4'." Yano et a14' showed that Wortmannin blocks the IgE-mediated histamine release from RBL-2H3 cells through a direct interaction of this compound with the 1 10 subunit of PI3K. Taken together, these results strongly suggest that PI3K is a critical, and shared, signaling component of many different receptor-activated pathways. We have been investigating anti-Ig-mediated growth inhibition of a human B-lymphoma cell line that expresses endogenous IgM and IgD, and is negative for Epstein-Barr virus (EBV). We have previously shown that tyrosine phosphorylation appears to be critical for growth inhibition induced by anti-IgM antibodies bound to acrylamide beads.45 In this report we demonstrate that both anti-IgM and antiIgD induce tyrosine phosphorylation of the regulatory, p85, subunit of PI3K, and stimulation of enzyme activity. Further- Blood, Vol 87, No 1 (January l), 1996: pp 202-210 From www.bloodjournal.org by guest on November 7, 2014. For personal use only. 203 P13K ACTIVITY IN HUMAN B LYMPHOMA CELLS more, w e show that Wortmannin can specifically block antiIg-mediated stimulation of PDK activity, and anti-Ig-induced growth inhibition, with no effect on the activation of tyrosine kinases or protein kinase C (PKC). These results show that PI3K activity is a critical element in the Ig-activated signaling pathway leading togrowth inhibition in these cells. MATERIALS AND METHODS Cell lines and culture conditions. The RL cell line was grown from the ascites of a patient with diffuse large cell lymphoma. It is an IgM’, IgD+, B-cell line that has been shown by Southern blot analysis to be EBV genome negative, and has been described previously.46The cells are passed twice weekly in RPM1 1640 (CellGro; Mediatech, Washington, DC), containing 2 mmoVL L-glutamine, 1,OOO U/mL penicillin, and 100 pg/mL streptomycin, and 10% fetal calf serum (FCS), all obtained from GIBCO (Grand Island, NY), with no other growth factors added. Reagents. Acrylamide-conjugatedrabbit-antihumanIgM (RcrIgM) (p-chain specific) and control rabbit y-globulin (Ryy)-acrylamide beads were purchased from Bio-Rad (Richmond, CA). Murine antihuman IgM monoclonal antibody (MoAb) (DA4-4) was obtained from the American Type Culture Collection (ATCC) (Rockville, MD). Murine antihuman IgD MoAb (6IA6.2) and control murine MoAb of the IgG2a subclass (UPC-10) were a kind gift of Dr Fred Finkelman (Uniformed Services University of the Health Sciences [USUHS], Bethesda, MD). Anti-IgM (MaIgM), anti-IgD (MaIgD), and UPC-l0 conjugated to high-molecular-weight dextran as previously described4’ were a kind gift of Dr Andrew Lees (USUHS, Bethesda, MD). Rabbit antisera specific for PI3K was obtained from Upstate Biotechnology, Inc ([UBI], Lake Placid, NY). Wortmannin was obtained originally from Dr Allen Oliff (Merck & CO,Inc, West Point, PA), and then purchased from Sigma Chemical CO (St Louis, MO). Wortmannin was dissolved in dimethyl sulfoxide (DMSO) to 2 or 10 m o m stocks, stored at -20°C. and diluted in media immediately before use. Proliferation assays. Lymphoma cells were split 18 to 24 hours before assays were performed. RL cells were resuspended in culture medium to a concentration of 1 X l@/mL, and 100 pL was plated in 96-well, flat-bottomed, microtiter plates already containing 100 pL of appropriately diluted reagents. In some experiments RL cells were pretreated with Wortmannin before adding to the assays. One microcurie of ’H-thymidine/well (6.7 Cdmmol; New England Nuclear Research Products, Boston, MA) was added for the final 8 to 18 hours of a 72-hour culture. Cultures were harvested onto glass fiber filters with a PhD Cell Harvesting system (Cambridge Technology, Inc, Cambridge, MA), and isotope uptake was assessed by liquid scintillation on a beta counter (LKB/Wallac, Inc, Gaithersburg, MD). Immunoprecipitation and Western blotting. RL cells were incubated with Ryy or RaIgM beads, or MaIgM or MaIgDdextran for various lengths of time, pelleted, and lysed in buffer containing 20 m o m Tris, 2 m o m EDTA, 1% NP40, 137 m o w NaCI, and 10% glycerol, with aprotinin (2 pg/mL), phenylmethylsulfonyl fluoride (PMSF) (100 pmom), leupeptin (5 pg/mL), and Na,V04 (1 mol/ L) added immediately before lysing. Lysates were incubated on ice for 15to30 minutes, and cleared by spinning 12,OOOg in a microcentrifuge for 30 minutes at 4°C. Protein concentrations were determined by BCA protein assay (Pierce Chemical CO, Rockford, IL), and 50 pg/lane was used for straight immunoblotting. For immunoprecipitations, 200 pg to 500 pg (approximately 2 to 5 X lo6 cell equivalents, respectively) of lysate was added to Eppendorf tubes containing Protein A Sepharose (Pharmacia LKB Biotech, Piscataway, NJ) and 10 pL of appropriate antisera. Tubes were rotated 2 hours to overnight, and pellets were washed three times in lysis buffer, and one time in 10 m o m Tris containing . l % NP40. Electrophoresis sample buffer was added to the washed pellets, samples were boiled for 10 minutes, and run by 8% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) on 8% mini-gels (Novex, Encinitas, CA). Gels were transfered to Hybond-ECL nitrocellulose (Amersham Corp, Arlington Heights, IL), and blocked for 1 hour in Tris-buffered saline containing 0.1% Tween-20 (TBST), 5% powdered milk, and 5% normal goat serum (TBSThIBiNGS). Primary antibodies were added, and blots were incubated for 2 hours at room temperature. After washing (three times, 15 minutes) in TBST, horseradish peroxidase-linked secondary antibodies were added to the blots in TBSThIBiNGS. Blots were then washed (three times, 15 minutes) in TBST, and developed using enhanced chemiluminescent (ECL) kits from Amersham. PI3 kinase assays. Assays for PI3K activity were performed by U cells were cultured in the absence the method of Kaplan et al.@I or presence of Wortmannin, and then immunoprecipitates were prepared from control or anti-Ig-stimulated RL cells exactly as described above. They were washed three times in lysis buffer, and three times in 10 m o m Tris, pH 7.4. Wortmannin was added to the pellet in various concentrations, and samples were incubated at room temperature for 30minutes. Ten microliters of sonicated PI substrate (Avanti Polar Lipids, Inc, Alabaster, AL) was added to each sample and samples were incubated for 10 minutes on ice. Forty microliters of kinase buffer containing 30 m o m HEPES pH 7.4, 30 m o m MgCI2,50 pmoVL adenosine triphosphate (ATP), and 200 p m o m adenosine was added, followed by 10 pCi y-”P ATP (New England Nuclear). Samples were incubated for 20 minutes at room temperature, and the reaction was ended with 100 pL 1 m o m HCI. Phospholipids were extracted with 200 pL chlorofondmethanol (1: 1). Equal volume aliquots from the bottom chloroform layer were spotted onto thin-layer chromatography plates (MCB reagents; Merck & CO,Inc, Gibbstown, NJ), and developed in chlorofondmethanol/dH20/ammonium hydroxide (45:35:7.5:2.5). Plates were dried briefly,and placed with x-ray film (Eastman Kodak, Rochester, NY) for 1 to 7 days at -70°C. RESULTS Phosphorylation of P13K p85 on tyrosine occurs after anti-Ig stimulation of RL cells. The RL cell line can be induced to undergo activation-induced growth inhibition in the presence of phorbol anti-IgD (data not shown), or anti-IgM45antibodies. The latter response was shown to depend on induction of tyrosine phosphorylation after receptor ligati~n.~’ To better understand signaling pathways that might be involved in anti-Ig-induced growth inhibition, we were interested in identifying specific substrates of tyrosine phosphorylation. We initially examined the ability of antiIg toinducephosphorylationof PDK p85. Lysates from RL cells ( 2 to 5 X lo6 cell-equivalents) treated with Ryy-, RaIgM-beads, UPC-dextran, or MaIgM-dextran were immunoprecipitated with antibodies specific for PDK (p85), electrophoresed, transferred to nitrocellulose, and analyzed for phosphotyrosine-containing proteins. As shown in Fig 1A, anti-IgM-beads induced phosphorylation of the regulatory subunit (p85) of PDK. In addition, we observed phosphorylation of a protein a t 110 M), which was not reactive with an antibody specific for the1 IO-kD catalytic PI3K subunit (data not shown), and an unidentified protein at greater than 200 kD. Probing the same blot with an anti-p85 anti- From www.bloodjournal.org by guest on November 7, 2014. For personal use only. BECKWITH ET AL 204 IP: IP: Anti-PI3K I 0 10' 20' --n M M ' I M M CB pB CB pB A - 47 - 33 - 0 10' 20' n n - ' IP: Anti-PI3K I CB pB CD pD CB pB CB pB -.- C B 11084 Anti-PI3K p85 +p85 +p85 Fig 1. Phosphorylationof P13K by anti-lgM beads or by anti-IgM conjugated to high-molecular-weightdextran. RL cells were treated with R y y beads (CB),anti-lgM-beads (CB), UPC-dextran (CD), or anti-lgM-dextran (pD) for 10 or 20 minutes (A and B), or l 0 minutes (C). The 0 timepoint is a media only control (M).Three hundred micrograms (a2to 5 x 10' cells) oflysate per sample was immunoprecipitatedovernight at 4°C as described, and pellets were washed, run on 8% gels, and transferred to nitrocellulose. Blots were then probed with anti-PTyr (A and C). The blot shown in (A) was stripped and reprobed with anti-P13K(p85) (B). These data are representativeof at least three experiments, with the exception of the anti-lgM-dextran, which was performed twice. body (Fig 1B) demonstrated that equal amounts of PI3K pendent kinases," at concentrations ranging from 5.5 pmol/ (p85) were immunoprecipitated in every lane. In addition, L (c-src) to 270 pmoVL (PLC). When 1 pmol/L Wortmannin when we analyzed lysates from RL cells treated for 10 minwas included in the PI3K assay (Fig 2A, lanes 5 through Utes with high concentrations of anti-IgM-dextran (5 p&! 8), the anti-PTyr-precipitable PI3K activity from anti-lgtreated RL cells was completely abolished. Furthermore, a mL) (Fig IC), we were also able to detect phosphorylation dose response of Wortmannin in the in vitro assay showed of PI3K ( ~ 8 5 )and . associated proteins. Similar results were a significant reduction of PI3K activity at 1 nmol/L (Fig 2B, obtained with anti-IgD-dextran (data not shown). In similar lane 3), with a complete inhibition observed at 10 nmoVL experiments using anti-IgM-beads, we were able to measure (lane 4). These inhibitory concentrations of Wortmannin are tyrosine phosphorylation of VAV, GAP, and PLC-y (data consistent with published reports demonstrating the specinot shown), all of which have been implicated in signaling ficity of Wortmannin for PI3K at nanomolar concentrathrough the Ig re~eptor.'~"" tions?'." Furthermore, the specificity of the in vitro kinase Anti-IgM and anti-IgD induce increases in anti-PTyrprecipitable PI3K activiol in RL cells, and this activity is assay for PI3K activity was determined by including adenosine in the assay buffer as an inhibitor of PI4K;* and by inhibited in vitro by thePI3K inhibitor, Wortmannin. We showing that NP40 also inhibits the precipitated PI3K activnext performed in vitro PI3K assays to measure directly the ity (data not shown). These data show that both anti-IgM ability of anti-Ig to stimulate PI3K activity in RL cells. RL and anti-IgD stimulate PI3K activity in RL cells, and that cells were stimulated for 10 minutes with Ryy-beads, this activity can be specifically inhibited in vitro by the PI3K RdgM-beads, UPC-dextran, or anti-IgD-dextran. Lysates inhibitor Wortmannin. were immunoprecipitated overnight with anti-PTyr, and precipitates were analyzed for PI3K activity. As previously In vivo treatment of RL cells with nanomolar concentrashown by Gold et aljSfor normal B lymphocytes, we demontions of Wortmannin reverses the anti-Ig-induced growth arrest, and inhibits PI3K activio. As previously menstrate here (Fig 2A) that anti-Ig stimulation of RL cells with tioned, PI3K activation has been shown to occur after ligaanti-IgM-beads (lane 2), or anti-IgD-dextran (lane 4) retion of different growth factor the and sulted in an increase in anti-PTyr-precipitable PI3K activity many p85 subunit has been shown to associate with cell-surface over control lysates (lanes 1 and 3). Wenextwanted to proteins involved in costimulation of T cells'R.'') and B cells.37 measure the effect of the PI3K inhibitor Wortmannin in this To determine if PI3K activity is a critical functional composystem. Wortmannin was isolated as an antifungal compound from the bacterial culture broth of Penicillium ~ortmannii.~' nent of anti-Ig-induced growth inhibition, we used the PI3K inhibitor, Wortmannin. RL cells were treated with various It has been shown to specifically inhibit PI3K at nanomolar concentrations of Wortmannin for 30minutes at 37°C before concentration^^^." by binding to the catalytic, 1 I O - k D subthe addition of control or anti-IgM-beads. In two representaunit?' It has been shown to have no effect on many other intracellular signaling enzymes, including PI4K," PKC,4'." tive experiments shown in Fig 3, it is clear that Wortmannin was able to significantly reduce the anti-Ig-mediated inhibic-src-like tyrosine kinases?" phosphoinositide-specific tion of RL cells at a concentration of 5 nmol/L, and comphospholipase C," c-AMP-, c-GMP-, and calmodulin-de- From www.bloodjournal.org by guest on November 7, 2014. For personal use only. P13KACTIVITY 205 IN HUMAN B LYMPHOMA CELLS 2 1 3 5 4 7 8 8 - A PI-3P Kinase Assay: Pretreatment: Fig 2. Anti-lgMandanti-lgD Activator: induce anti-PTyr-precipitable P13K activity in RL cells, and this activity is inhibitedin vitro with the P13K inhibitor, Wortmannin. (A) RL cells were stimulatedwith Ryy-beads (CBI, RalgM-beads (FBI, UPC-dextran (CD),or anti&dextran (&D) (Activator). Lysates wereprecipitated overnight with anti-PTyr, and assayed for P13K activity as described in Materials and Methods, in the absence or presence of 1p m o l l L Wortmannin (Kinase Assay). Position of phosphatidylinositol 3-phosphate indiis cated. (B) Dose response of Wortrnannin inhibitionof in vitro P13K assay. RL cells were stimulated with Ryy-beads (CB) or RalgM-beads (FBI. Lysates were immunoprecipitated overnight with anti-PTyr, and assayed for P13K activity in the absence or presence of various concentraWM in Assay tions of Wortmannin, as indicated under the figure. Position WM Pretreat: ofphosphatidylinositol 3-phosActivator: phate is indicated. 1 2 3 4 5 6 B - PI-3P (PM): 0 CB pletely abrogate the response at concentrations of 10 to 50 nmol/L. These results show that Wortmannin interferes with signal transduction through surface Ig, presumably via its effect on PI3K, and thus abrogates growth arrest. Wenext assayed the in vivo effect of Wortmannin on PI3K precipitated from anti-lg-treated RL cells. RL cells were treated with various concentrations of Wortmannin for 30 minutes at 37"C, followed by stimulation with anti-Ig beads for I O minutes.As shown in Fig 4A, whenPI3K activity was measured in anti-PTyr immunoprecipitates from RL cells treated in vivo with concentrations of Wortmannin ranging from 0.5 to 5,000 nmolL, inhibition of enzyme activity was observed. In Fig 4B, anti-PTyr immunoprecipitates from the same lysates were run on a 10% SDS-PAGE mini-gel, andblottedwithanti-P13Kp85MoAbtoshow that equivalent amounts of phosphorylated PI3K p85 were brought down in every lane where samples had been treated with anti-Ig. A phosphotyrosine immunoblot (Fig 4C) of the lysates fromwhich the immunoprecipitates weremade shows that there was no effect of Wortmannin at anyconcentration on anti-lg-induced tyrosine phosphorylation, and immunoblots of these lystates with anti-PI3K p85 (Fig 4D) From www.bloodjournal.org by guest on November 7, 2014. For personal use only. 206 BECKWITH ET AL 40 50 35 30 45 n I 0 25 x 5 20 40 /+' U 15 35 10 5 30 I 0 3.0 10.0 100.0 1000.0 I/+ I b .O 0.5 50.0 5.0 [Wortmannin] nM shows that there were equal amounts of PBK in each sample. These data, taken together, strongly suggest that Wortmannin interferes with anti-Ig-induced signaling and growth arrest of RL cells via its inhibitory effect on PI3K, thus implicating PI3K as a critical functional component of this response. Wortmannin does not interfere with anti-lg-induced tyrosine kinase activation or stimulation of PKC. We next wished to monitor the specificity of Wortmannin in our system, especially because our initial experiments were performed with a relatively high concentration (5 pmol/L). We further assessed the effect of Wortmannin on tyrosine kinase activation after anti-Ig stimulation of RL cells (see also Fig 4C). RL cells were cultured overnight in the absence or presence of 5 prnoVL Wortmannin. Cells were then treated for 10 minutes with media, Ryy-beads, or RaIgM-beads in the continued absence or presence of 5 pmol/L Wortmannin. Lysates were either tested directly in immunoblots for induction of phosphotyrosyl-containing proteins (Fig SA), or were first precipitated with an anti-PI3K p85 antibody before probing with an anti-PTyr antibody (Fig 5B). In neither case did we see any reduction in phosphorylation in lysates made from Wortmannin-treated cells, demonstrating that Wortmannin is not interfering with anti-Ig-induced tyrosine kinase activation or tyosine phosphorylation of P13K p85. We previously showed that RL cells were growth inhibited in the presence of phorbol esters, and this inhibition could be partially reversed by PKC inhibitors,% suggesting that activation of PKC was occumng. To test the effect of Wortmannin on PKC activation, RL cells were incubated overnight in the absence or presence of 5 pmoVL Wortmannin, followed by the addition of various concentrations of DMSO or PMA, and fresh Wortmannin. 3H-thymidine uptake was measured 72 hours later. As shown in Fig 6, although Wortmannin was able to completely reverse inhibition induced by anti-Ig-beads, 5 pmoW Wortmannin had no effect on the PMA-induced growth inhibition of RL cells at any concentration of PMA tested. These data support previous invest i g a t i o n ~demonstrating ~~ that Wortmannin is not interfering with PKC activation. Taken together, these data show that Fig 3. In vivo treatment ofRL celhwith Wortmannin completely abrogates enti-lgM-inducedgrowth arrest. RL cells were pretreated 30 minutes in the pmence of various concentrations of Wortmannin in microtiter wells, followedthe byaddition of media (A), control beads(0). or RaIgM-beads ( 0 ) .Cultures harvested at were 72 hours, with 'H-thymidine added for the last 18 hours. The results of two -Derate experiments are shown, and these are representative of four experiments. in our system, 5 pmol/L Wortmannin has no effect on PKC or protein tyrosine kinase activation, and therefore support other reports43." of the relative specificity of Wortmannin for PI3K. DISCUSSION Our previous res~lts,4~ as well as other s t ~ d i e s ? demon~-~~ strated a critical role for tyrosine phosphorylation in Igmediated signal transduction resulting both in proliferation and growth arrest of B lymphocytes. In this report, we show that both anti-IgM and anti-IgD result in tyrosine phosphorylation and activation of P13 kinase after receptor stimulation, and that PI3Kactivity is critical for anti-Ig-mediated growth inhibition. It has become clear in the past few years that the membrane kinase PDK is an important signaling molecule shared by many different receptor complexes. In the case of receptors containing intrinsic tyrosine kinase activity, association with p85 occurs via p85 SH2 domain binding to p85-specific Y-X-X-M motifs within the kinase insert d ~ m a i n . * ~For *~'.~ receptors that lack intrinsic kinase activity, the association involves binding of p85 to SH3 domains of receptor-associated In B lymphocytes, Gold et aiJ5 showed an increase in PDK activity after stimulation with anti-IgM or anti-IgD. Yamanishi et a l l 9 also showed an anti-Ig-mediated increase in PI3K associated with the lyn tyrosine kinase. Enzyme activation was shown by Pleiman et a136to occur via binding of p85 to the SH3 domains of lyn or fyn, which can themselves bind via SH2 domains to the Ig-associated signaling molecule Ig-a.'.' Similarly, we show here that antiIgM or anti-IgD stimulation of a human B-lymphoma cell line results in phosphorylation of PI3K p85 on tyrosine, and enzyme activation (Figs 2 and3). In preliminary experiments, we have also shown an association of p85 with the lyn kinase in anti-Ig-activated cells (not shown). The biologic significance of PI3K activation is unclear in many systems. Most of the data demonstrating a functional role for PDK in cellular responses to external stimuli have been obtained from investigations of PDGF-induced re- From www.bloodjournal.org by guest on November 7, 2014. For personal use only. P13KACTIVITY IN HUMAN B LYMPHOMA CELLS 207 A 1 3 2 4 5 6 8 7 7 - - P1 -3P WM Pretreat (nM): Activator: 0 M 0 CB B 1 2 3 4 5 6 7 8 2009768 - Fig 4. In vivo treatment ofRL cells with Wortmannin inhibits anti-PTyr-precipitable P13K activity.(A) RL cells were pretreated for 30 minutes with various concentrations of Wortmannin, followed by stimulation with control beads (CB)or antiIgM-beads (pB) for 10 minutes at 37'C. Lysates were immunoprecipitated overnight at 4°C with anti-PTyr MoAb and assayed for P13K activity as described in Materials and Methods. As a positive control for P13K activity, lysates from anti-lgM-treated RL cells wereimmunoprecipitated with anti-PI3K p85 (data not shown). (B) Lysates fromthe above experiment were immunoprecipitated with anti-PTyr as described, run on 10% SDSPAGE mini-gels, transferred t o nitrocellulose, andblotswere probed with anti-P13K MoAb. (C and D) Lysates from the above 1096 experimentwererunon SDS-PAGE mini-gels and immunoblots were probed with antiPTyr IC) or anti-PI3K p85 (D) antibodies. 43 - m- " " " - p85 , ' ./ C 200 97 68- - 43 D sponses. Fantl et alJoshowed that PI3K p85binds specifically to two phosphorylated tyrosine residues withinthe kinase insert domain of the PDGF receptor, and that mutation of these sites abrogates both the binding of p85, and the mitogenic activity of PDGF. Using similar approaches, Wennstrom et a14' and Joly et al" demonstrated a critical role for p85 binding in PDGF-initiated membrane ruffling and receptor internalization and trafficking in the endocytic pathway, respectively. In a T-cell system, investigators have also recently demonstrated that mutation of the cytoplasmic Y-XX-M domain of the costimulatory molecule, CD28, abolishes anti-CD28-stimulated binding and activation ofPI3K. as well as interleukin-2 production."' Recent studies using specific inhibitors of PI3K activity 1 2 3 4 5 6 7 8 200 97 68- - - . " C " " - p85 43 - have confirmed and extended these r e s ~ l t s ~ ' ~One ~ " of ~~'~~~' these inhibitors, Wortmannin, has been shown to selectively inhibit PI3K activity, without affecting P14K,PKC, W K . and several other intracellular signaling In addition to the mutational analysis discussed above, Wennstrom et a14' showed that Wortmannin could abolish PDGF-stimulated membrane ruffling. In addition, using Wortmannin and another PI3K inhibitor, LY294002, other investigators have shown thatPI3K activation. is required for insulin or PDGF activation of pp70ShK,.5"andinsulinstimulated DNA synthesis and glucose transporter function." Finally, in a very detailed analysis of Wortmannin effects, Yano et alJ demonstrated a role for PI3K in IgE-mediated histamine and leukotriene release fromtheratbasophilic From www.bloodjournal.org by guest on November 7, 2014. For personal use only. BECKWITH 208 IP: Anti-P13K Pretreatment: - - + - + Pretreatment: Activator: M CB CB Activator: CB pB pB - - + + pB CB pB " A 200 97 ... . - - 97 p85 68 - 29 - - 68 - 43 - 43 leukemia cell line, RBL-2H3. In these studies, Wortmannin treatment of intact cells resulted in irreversible inhibition of PI3K activation at nanomolar concentrations, probably via binding of the drug to the pi IO catalytic subunit:' In this report we have shown that activation of PI3K also appears to play a critical role in signaling through the antigen receptors on a human B-lymphoma cell line. Stimulation with anti-IgM or anti-IgD results in the tyrosine phosphorylation of PI3K p85, activation of PI3K, and growth inhibition (not shown for anti-IgD). Treatment of the cells with Wortmannin completely abrogated both growth arrest (Fig 3 ) and PI3K activity (Fig 4), without affecting p85 phosphorylation (Fig S). Complete abolition of the anti-Ig-induced growth arrest and induction of PI3K activity occurred at nanomolar concentrations of Wortmannin (Figs 3 and 4). consistent with the observed in vitro inhibition of PI3K activity by 1 to 10 nmol/L Wortmannin (Fig 2). Furthermore, consistent with other results on the specificity of Wortmannin for PI3K. in our system Wortmanninhadno effect on overall anti-lginduced tyrosine kinase activation (Figs 4 and S), or PMA- Fig 6. Wortmannin does not interfere with PMAinduced growth inhibition of RL cells. RL cells were pretreated overnight the in absence (open symbols) or presence (closed symbols) of 5 pmol/L Wortmannin. Cells werespun down, andplated in microtiter wells containing various concentrations of DMSO (0,W or PMA (A,Al (A), or anti-lgM-beads ( V , + ) (B) in the continued absence or presence of 5 pmollL Wortmannin. Cultures were incubated for72 hours, with 'H-thymidine added for the last 18 hours. The results are representative of two separate experiments. AL Fig 5. Wortmannin does not interfere with anti-lgM-induced tyrosine phosphorylation. RL cells were pretreated overnight in the absence (-1 or presence (+) of5pmol/L Wortmannin. Cells were then stimulatedwith media only (MI, Ryy-beads (CB), or RnlgM-beads (pB) for 10 minutes at 37"C, and lysed with icecold lysing buffer. Lysates were run directly on mini-gels before being transferred to nitrocellulose (A), or were immunoprecipitatedovernight with anti-PI3K p85 antisera before blotting (B). Blots were probedwith an antiPTyrMoAb, followedby HPOGaMlg and ECL detection. Each panel is representative of three separate experiments. induced PKC activation (Fig 6). even at S pmol/L concentrations. Although these results clearly show that P13K activity is decreased by Wortmannin in our system, itis possible that this is not a direct effect, and that Wortmannin is working through another, as yet unidentified, substrate. Taken together, these studies point to PI3K as a critical component of signaling pathways through a diverse array of receptors, in different cell types. Furthermore, it seems to play a role in a variety of cellular responses ranging from cytoskeletal changes to receptor internalization and trafficking to inhibition or stimulation of cellular proliferation. The precise mechanism bywhich PI3K exerts these effects is unclear. It has been shown thatone of the major endproducts of PI3K activity, PI-3,4,P2, can activate the zeta isoform of PKC."It has also been suggested that PI3K may exert its cytoskeletal function through activation of a small GTPbinding protein, R ~ c . There ~' is also homology between the catalytic subunit ofP13Kand a yeast protein, Vps34p, involved in protein delivery to the yeast vacuole,5' againimplicating PI3K in receptor traffickingandprotein sorting. [L - - 8 40 - 0.0 0.1 1 .o [PMA]ng/ml 10.0 0.0 5.0 50.0 [Beads]pg/ml 500.0 From www.bloodjournal.org by guest on November 7, 2014. For personal use only. P13K ACTIVITY IN HUMAN B LYMPHOMA CELLS Therefore, in our system, PI3K could be interacting with a downstream signaling component that leads to growth inhibition, or alternatively, it could be involved in the internalization of surface Ig after ligation, which may be required for growth arrest to occur. Experiments are underway to examine these possibilities, and preliminary results suggest that PI3K may be linked to stimulation of G, in our system (data not shown). In summary, we have shown here that ligation of either IgM or IgD on the surface of a human B-lymphoma cell line results in tyrosine phosphorylation of PDK p85, enzyme activation, and growth arrest. The PI3K inhibitor Wortmannin specifically interferes with PI3K activity, and abolishes anti-Ig-induced growth arrest, thus showing that PDK plays a critical role in Ig-mediated signaling. Further studies are needed to define the precise function of PI3K in B-cell responses. ACKNOWLEDGMENT We thank Dr David Kaplan for help with the P13 kinase assays, Gitte Jorgensen for excellent technical assistance, and Laura Martinez for help with the manuscript. REFERENCES 1. Hombach J, Leclercq L, Radbruch A, Rajewsky K, Reth M: A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J 7:3451, 1988 2. Chen J, Stall AM, Herzenberg LA, Herzenberg LA: Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J 9:2117, 1990 3. Campbell KS, Cambier JC: B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J 9:441, 1990 4. Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M: Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343:760, 1990 5. Campbell KS, Hager EJ, Friedrich RI, Cambier JC: IgM antigen receptor complex contains phosphoprotein products of B29 and mb-I genes. Proc Natl Acad Sci USA 88:3982, 1991 6. Van Noesel JM, Van Lier RAW, Cordell JL, Tse AGD, Van Schijndel GMW, De Vries EFR, Mason DY, Borst J: The membrane IgM-associated heterodimer on human B cells is a newly defined B cell antigen that contains the protein product of the mb-I gene. J Immunol 146:3881, 1991 7. Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC: The B cell antigen receptor complex: Association of Ig-a and Ig-p with distinct cytoplasmic effectors. Science 258:123, 1992 8. Lin J, Justement LB: The MB"329 heterodimer couples the B cell antigen receptor to multiple src family protein tyrosine kinases. J Immunol 149:1548, 1992 9. Hutchcroft JE, Harrison ML, Geahlen RL: B lymphocyte activation is accompanied by phosphorylation of a 72-kDa protein-tyrosine kinase. J Biol Chem 266:14846, 1991 10. Burkhardt AL, Brunswick M, Bolen JB, Mond JJ:Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA 88:7410, 1991 11. Campbell MA, Sefton BM: Association between B-lymphocyte membrane immunoglobulin and multiple members of the src family of protein tyrosine kinases. Mol Cell Biol 12:2315, 1992 209 12. Gold MR, Law DA, DeFranco AL: Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature 345:810, 1990 13. Campbell MA, Sefton BM: Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J 9:2125, 1990 14. Gold MR, Crowley MT, Martin CA, McCormick F, DeFranco AL: Targets of B lymphocyte antigen receptor signal transduction include the p21" GTPase-activating protein (GAP) and two GAPassociated proteins. J Immunol 150:377, 1993 15. Carter RH, Park DJ, Rhee SG, Fearon DT: Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci USA 88:2745, 1991 16. Hempel WM, Schatzman RC, DeFranco AL: Tyrosine phosphorylation of phospholipase C-72 upon cross-linking of membrane Ig on murine B lymphocytes. J Immunol 148:3021, 1992 17. Coggeshall KM, McHugh JC, AltmanA: Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-72 in B lymphocytes. Proc Natl Acad Sci USA 89:5660, 1992 18. Bustelo XR, Barbacid M: Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science 256: 1196, 1992 19. Yamanashi Y, Fukui Y, Wongsasant B, Kinoshita Y, Ichimori Y, Toyoshima K, Yamamoto T: Activation of src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Roc Natl Acad Sci USA 89:1118, 1992 20. Gold MR, Matsuuchi L, Kelly RB, DeFranco AL: Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc NatlAcad Sci USA 88:3436, 1991 21. Hata D, Kawakami T, Ishigami T, Kim KM, Heike T, Katamura K, Mayumi M, Mikawa H: Tyrosine phosphorylation of IgMand IgD-associated molecules of a human B lymphoma cell line B104. Int Immunol 4:797, 1992 22. Matsuo T, Nomura J, Kuwahara K, Igarashi H, Inui S, Hamaguchi M, Kimoto M, Sakaguchi N: Cross-linking of B cell receptorrelated MB-1 molecule induces protein tyrosine phosphorylation in early B lineage cells. J Immunol 150:3766, 1993 23. Pur6' E, Tardelli L: Tyrosine phosphorylation is required for ligand-induced internalization of the antigen receptor on B lymphocytes. Proc Natl Acad Sci USA 89:114, 1992 24. Lane PJL, Ledbetter JA, McConnell FM, Draves K, Deans J, Schieven CL, Clark EA: The role of tyrosine phosphorylation in signal transduction through surface Ig in human B cells. J Immunol 146:715, 1991 25. Mizuguchi J, Yamanashi Y, Ehara K, Tamura T, Nariuchi H, Gyotoku Y, Fukazawa H, Uehara Y, Yamamoto T: Tyrosine protein kinase is involved in anti-IgM-mediated signaling in BAL17 B lymphoma cells. J Immunol 148:689, 1992 26. Panayotou G, Waterfield MD: Phosphatidylinositol 3-kinase: A key enzyme in diverse signalling processes. Trends Cell Biol 2:358, 1992 27. Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC: Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem 265:19704, 1990 28. Auger KR, Cantley LC: Novel polyphosphoinositides in cell growth and activation. Cancer Cells 3263, 1991 Turck CW, Wil29. Escobedo JA, Kaplan DR, Kavanaugh W, liams LT: A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol 11:11 25, 1991 30. Lev S , Givol D, Yarden Y: Interkinase domain of kit contains From www.bloodjournal.org by guest on November 7, 2014. For personal use only. 210 the binding site for phosphatidylinositol 3' kinase. Proc Natl Acad Sci USA 89:678, 1992 3 I . Backer JM, Myers MG Jr, Shoelson SE, Chin DJ, Sun X-J, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, White MF: Phosphatidylinositol 3"kinase is activated by association with IRS-l during insulin stimulation. EMBO J 11:3469, 1992 32. Raffioni S, Bradshaw RA: Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor, andnerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA 89:9121, 1992 33. Thompson PA, Gutkind JS, Robbins KC, Ledbetter JA, Bolen JB: Identification of distinct populations of PI-3 kinase activity following T-cell activation. Oncogene 7:719, 1992 34. Prasad KVS, Janssen 0, Kapeller R, Raab M, Cantley LC, Rudd CE: Src-homology 3 domain of protein kinase ~59""mediates binding to phosphatidylinositol 3-kinase in T cells. Proc Natl Acad Sci USA 90:7366, 1993 35. Gold MR, Chan VWF, Turck CW, DeFranco AL: Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J Immunol 148:2012, 1992 36. Pleiman CM, Clark MR, Timson Gauen LK, Winitz S, Coggeshall KM, Johnson GL, Shaw AS, Cambier JC: Mapping of sites on the src family protein tyrosine kinases ~ 5 5 ~p59Iy", ' ~ . and ~56''" which interact with the effector molecules phospholipase C-y2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol 13:5877, 1993 37. Tuveson DA, Carter RH, Soltoff SP, Fearon DT: CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science 260:986, 1993 38. Prasad KVS, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE: T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-MetXaa-Met motif. Proc Natl Acad Sci USA 91:2834, 1994 39. Truitt KE, Hicks CM, Imboden JB: Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3kinase in Jurkat T cells. J Exp Med 179:1071, 1994 40. Fantl WJ, Escobedo JA, Martin CA, Turck CW, del Rosario M, McCormick F, Williams LT: Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell 69:413, 1992 41. Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L: Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol 4:385, 1994 BECKWITH ET AL 42. JolyM, Kazlauskas A,Fay FS, Corvera S: Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science 263:684, 1994 43. Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y: Inhibition of histamine secretion by Wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem 268:25846, 1993 44. Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindley G, Vlahos CJ: Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res 54:2419, 1994 45. BeckwithM, Urba WJ, Fenis DK, Freter CE, KuhnsDB, Moratz CM, Longo DL: Anti-IgM-mediated growth inhibition of a human B lymphoma cell line is independent of phosphatidylinositol turnover and protein kinase C activation and involves tyrosine phosphorylation. J Immunol 147:2411, 1991 46. Beckwith M, Longo DL, O'Connell CK, Moratz CM, Urba WJ: Phorbol ester-induced, cell-cycle-specific, growth inhibition of human B-lymphoma cell lines. J Natl Cancer Inst 82:501, 1990 47. Rehe GT, Katona IM, BrunswickM,WahlLW, June CH, Mond JJ: Activation of human B lymphocytes by nanogram concentrations of anti-IgM-dextran conjugates. Eur J Immunol 20: 1837, 1990 48. Kaplan DR, Whitman M, Schaffhausen B, Dallas DC, White M, Cantley L, Roberts TM: Phosphotidylinositol metabolism and polyomavirus-mediated transformation. ProcNatlAcadSci USA 83:3624, 1986 49. Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D: Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature 369:327, 1994 50. Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J: PDGF- and insulin-dependent pp70ShKactivation mediated by phosphatidylinositol-3-OH kinase. Nature 370:7 1, 1994 51. Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR: Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol 14:4902, 1994 52. Nakanishi H, Brewer KA,Exton JH: Activation of the z isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 208:13, 1993 53. Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD: Phosphotidylinositol 3-kinase encoded by yeastVPS34 gene essential for protein sorting. Science 260:88, 1993
© Copyright 2026