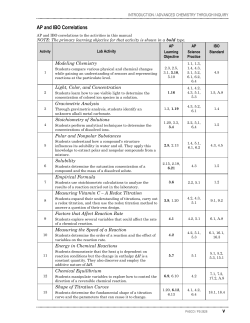
1999 AP Chemistry Free
1999
CHEMISTRY
SECTION II
(Total time—90 minutes)
Part A
Time—40 minutes
YOU MAY USE YOUR CALCULATOR FOR PART A.
CLEARLY SHOW THE METHOD USED AND STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is
to your advantage to do this, because you may earn partial credit if you do and you will receive little or no credit if
you do not. Attention should be paid to significant figures. Be sure to write all your answers to the questions on the
lined pages following each question in this booklet.
Answer Question 1 below. The Section II score weighting for this question is 20 percent.
→ NH +(aq) + OH -(aq)
NH3(aq) + H2O(l) ←
4
1. In aqueous solution, ammonia reacts as represented above. In 0.0180 M NH3(aq) at 25°C, the hydroxide ion
concentration, [OH -] , is 5.60 ¥ 10-4 M. In answering the following, assume that temperature is constant at
25°C and that volumes are additive.
(a) Write the equilibrium-constant expression for the reaction represented above.
(b) Determine the pH of 0.0180 M NH3(aq).
(c) Determine the value of the base ionization constant, Kb , for NH3(aq).
(d) Determine the percent ionization of NH3 in 0.0180 M NH3(aq).
(e) In an experiment, a 20.0 mL sample of 0.0180 M NH3(aq) was placed in a flask and titrated to the
equivalence point and beyond using 0.0120 M HCl(aq).
(i) Determine the volume of 0.0120 M HCl(aq) that was added to reach the equivalence point.
(ii) Determine the pH of the solution in the flask after a total of 15.0 mL of 0.0120 M HCl(aq) was added.
(iii) Determine the pH of the solution in the flask after a total of 40.0 mL of 0.0120 M HCl(aq) was added.
AP® CHEMISTRY
1999 SCORING GUIDELINES
Question 1
9 Points
One point deduction for mathematical error (maximum once per question)
One point deduction for error in significant figures* (maximum once per
question)
*number of significant figures must be correct within +/- one digit.
(except for pH: +/- two digits)
+
[ NH 4 ][OH − ]
[NH 3 ]
(a)
K=
(b)
[OH−]
(c)
Kb =
= 5.60 ×
10−4
1 pt
Þ {
(5.60 × 10 −4 ) 2
0.0180 − 5.60 × 10
−4
pOH = 3.252
or
} Þ pH = 10.748
[H+] = 1.79 × 10−11
= 1.74 × 10 −5 (or 1.80 × 10 −5 )
1 pt
2 pts
Note: 1st point for [NH4+] = [OH−] = 5.60 × 10−4; 2nd point for correct answer
5.60 × 10 −4
× 100 % = 3.11% (or 0.0311)
0.0180
(d)
% ionization =
(e)
NH3 + H+ → NH4+
(i)
mol NH3 = 0.0180 M × 0.0200 L = 3.60 × 10−4 mol = mol H+ needed
3.60 × 10 −4 mol
= 0.0300 L = 30.0 mL
vol HCl solution =
0.0120 M
(ii)
1 pt
1 pt
mol H+ added = mol 0.0120 M × 0.0150 L = 1.80 × 10−4 mol H+ added
= 1.80 × 10−4 mol NH4+ produced
[NH4+] =
1.80 × 10 −4 mol
= 0.00514 M = [NH3]
0.0350 L
1 pt
Note: Point earned for 1.80 × 10−4 mol, or 0.00514 M [NH3] or [NH4+],
or statement “halfway to equivalence point”.
Copyright © 2001 by College Entrance Examination Board. All rights reserved. College Board, Advanced Placement Program, AP, and the acorn logo
are registered trademarks of the College Entrance Examination Board.
2
AP® CHEMISTRY
1999 SCORING GUIDELINES
Question 1 (cont.)
Kb = 1.80 ×
10−5
+
[ NH 4 ][OH − ]
=
= [OH − ] Þ pOH = 4.745 Þ pH = 9.255
[ NH 3 ]
(= 1.74 × 10−5)
(iii)
(= 4.759)
1 pt
(= 9.241)
10.0 mL past equivalence point
0.0100 L × 0.0120 M = 1.20 × 10−4 mol H+ in 60.0 mL
[H + ] =
0.000120 mol
= 0.00200 M
0.0600 L
pH = − log (2.00 × 10−3) = 2.700
1 pt
______________________________________________________________________________________
One point deduction for mathematical error (maximum once per question)
One point deduction for error in significant figures* (maximum once per question)
*number of significant figures must be correct within +/− one digit (except for pH: +/− two digits)
Copyright © 2001 by College Entrance Examination Board. All rights reserved. College Board, Advanced Placement Program, AP, and the acorn logo
are registered trademarks of the College Entrance Examination Board.
3
2004 AP® CHEMISTRY FREE-RESPONSE QUESTIONS (Form B)
Your responses to the rest of the questions in this part of the examination will be graded on the basis of the accuracy
and relevance of the information cited. Explanations should be clear and well organized. Examples and equations
may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses.
Answer BOTH Question 5 below AND Question 6 printed on page 11. Both of these questions will be graded. The
Section II score weighting for these questions is 30 percent (15 percent each).
5. An experiment is performed to determine the molar mass of an unknown solid monoprotic acid, HA, by titration
with a standardized NaOH solution.
(a) What measurement(s) must be made to determine the number of moles of NaOH used in the titration?
(b) Write a mathematical expression that can be used to determine the number of moles of NaOH used to reach
the endpoint of the titration.
(c) How can the number of moles of HA consumed in the titration be determined?
(d) In addition to the measurement(s) made in part (a), what other measurement(s) must be made to determine
the molar mass of the acid, HA ?
(e) Write the mathematical expression that is used to determine the molar mass of HA.
(f) The following diagram represents the setup for the titration. In the appropriate boxes below, list the
chemical(s) needed to perform the titration.
(g) Explain what effect each of the following would have on the calculated molar mass of HA. Justify your
answers.
(i) The original solid acid, HA, was not completely dry at the beginning of the experiment.
(ii) The procedure called for 25 mL of H2O in the Erlenmeyer flask, but a student used 35 mL of H2O.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
GO ON TO THE NEXT PAGE.
10
AP® CHEMISTRY
2004 SCORING GUIDELINES (Form B)
Question 5
5. An experiment is performed to determine the molar mass of an unknown solid monoprotic acid, HA, by titration
with a standardized NaOH solution.
(a) What measurement(s) must be made to determine the number of moles of NaOH used in the titration?
Initial volume of standardized NaOH solution and
final volume of standardized NaOH solution
(volume at the endpoint of the titration)
1 point for identifying both initial and final volume
of base
(b) Write a mathematical expression that can be used to determine the number of moles of NaOH used to reach
the endpoint of the titration.
MNaOH × VNaOH
(Molarity of NaOH solution) times (volume (in L) of NaOH added)
1 point for mathematical expression
(c) How can the number of moles of HA consumed in the titration be determined?
HA + NaOH → NaA + H2O
moles HA = moles NaOH
moles monoprotic acid = moles NaOH
1 point for showing conversion based on stoichiometry of
the neutralization reaction
1 mol HA
nHA = moles NaOH
1 mol NaOH
(d) In addition to the measurement(s) made in part (a), what other measurement(s) must be made to determine the
molar mass of the acid, HA ?
mass of HA
1 point for measurement
(e) Write the mathematical expression that is used to determine the molar mass of HA.
mass HA
mol HA
1 point for quotient
mass of HA measured in part (d) divided by the moles
of HA determined in part (c)
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
10
AP® CHEMISTRY
2004 SCORING GUIDELINES (Form B)
Question 5 (cont’d.)
(f) The following diagram represents the setup for the titration. In the appropriate boxes below, list the
chemical(s) needed to perform the titration.
Chemicals needed in flask: solid weak monoprotic acid
(HA) and an indicator to detect endpoint of titration
1 point for either one of two chemicals in flask,
2 points for both
Chemical in buret: standardized NaOH solution
1 point for NaOH in the buret
(g) Explain what effect each of the following would have on the calculated molar mass of HA. Justify your
answers.
(i) The original solid acid, HA, was not completely dry at the beginning of the experiment.
Measured mass of HA is larger; so, according to expression in
part (e), calculated molar mass will be higher than it should.
1 point for the effect on molar mass and
explanation.
(ii) The procedure called for 25 mL of H2O in the Erlenmeyer flask, but a student used 35 mL
of H2O.
No effect on calculated molar mass, because mathematical
expression for molar mass does not include amount of
water used to dissolve solid HA. Both mass and number
of moles of HA are unaffected by the addition of water.
1 point for effect on molar mass and explanation.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for AP students and parents).
11
AP® CHEMISTRY
2004 SCORING COMMENTARY (Form B)
Question 5
Sample: 5A
Score: 10
This response earns a perfect score of 10 points: 1 point for part (a), 1 point for part (b), 1 point for part (c),
1 point for part (d), 1 point for part (e), 3 points for part (f), 1 point for part (g)(i), and 1 point for part (g)(ii).
Sample: 5B
Score: 8
The point is not earned in part (b). The point is not earned in part (g)(i) because the calculated molar mass
will be higher than the actual molar mass.
Sample: 5C
Score: 6
The point is not earned in part (a) because there is no mention of initial and final volumes or change in
volume, just “volume”. Only 2 out of 3 points are earned in part (f) because the indicator is not included. The
point is not earned in part (g)(i) because the justification given does not provide any additional information:
there is no explanation that the mass corresponding to a given n seems larger than it actually is, so the
calculated molar mass is higher than the actual molar mass. The point is not earned in part (g)(ii) because the
additional water has no effect.
Copyright © 2004 by College Entrance Examination Board. All rights reserved.
Visit apcentral.collegeboard.com (for AP professionals) and www.collegeboard.com/apstudents (for students and parents).
6
2002 AP® CHEMISTRY FREE-RESPONSE QUESTIONS (Form B)
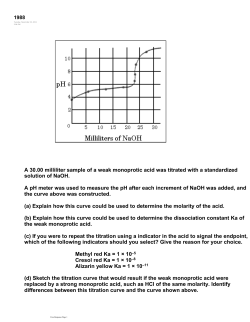
8. The graph below shows the result of the titration of a 25 mL sample of a 0.10 M solution of a weak acid, HA ,
with a strong base, 0.10 M NaOH.
(a) Describe two features of the graph above that identify HA as a weak acid.
(b) Describe one method by which the value of the acid-dissociation constant for HA can be determined using
the graph above.
(c) On the graph above, sketch the titration curve that would result if 25 mL of 0.10 M HCl were used instead
of 0.10 M HA.
(d) A 25 mL sample of 0.10 M HA is titrated with 0.20 M NaOH.
(i) What volume of base must be added to reach the equivalence point?
(ii) The pH at the equivalence point for this titration is slightly higher than the pH at the equivalence point
in the titration using 0.10 M NaOH. Explain.
END OF EXAMINATION
Copyright © 2002 by College Entrance Examination Board. All rights reserved.
Advanced Placement Program and AP are registered trademarks of the College Entrance Examination Board.
13
AP® CHEMISTRY
2002 SCORING GUIDELINES (Form B)
Question 8
8 points
The graph below shows the result of the titration of a 25 mL sample of a 0.10 M solution of a weak acid, HA ,
with a strong base, 0.10 M NaOH.
(a) Describe two features of the graph above that identify HA as a weak acid.
The initial pH of the solution before base has been added is
greater than 1 (the pH expected for a 0.1 M strong acid).
The pH at the equivalence point is greater than 7.
AND/OR
There is a rapid increase in the pH after adding a small amount
of base at the beginning of the titration. The increase quickly
diminishes on continued addition of base (buffer region).
1 point earned for initial pH > 1
1 point earned for pH > 7 at
equivalence point
Copyright © 2002 by College Entrance Examination Board. All rights reserved.
Advanced Placement Program and AP are registered trademarks of the College Entrance Examination Board.
18
AP® CHEMISTRY
2002 SCORING GUIDELINES (Form B)
Question 8 (cont’d.)
(b) Describe one method by which the value of the acid-dissociation constant for HA can be determined using
the graph above.
The Ka for the weak acid can be obtained by determining
the pH at the:
1. half-equivalence point in the titration where
Ka = 10–pH
2. zero volume of base
3. equivalence point
(Any point on the titration curve is acceptable with
justification.)
1 point earned for indicating any one
of the first three points (at left) identified
on the titration curve.
1 point earned for describing the
determination of Ka from that point.
(For any other point on the curve,
2 points for correct justification.)
(c) On the graph above, sketch the titration curve that would result if 25 mL of 0.10 M HCl were used
instead of 0.10 M HA.
The graph should have the
following features:
1. pH before adding any base
is 1
2. the equivalence point pH
is 7 at 25 mL
3. the titration curve beyond
the equivalence point is
nearly identical to the
original curve
1 point earned for any two features and 2 points for all three.
Beginning the pH at 1, the equivalence point at pH = 7 (when the
volume is equal to the volume of the base required to neutralize the
strong acid), and the ending pH of the solution is nearly the same as
the original curve
Copyright © 2002 by College Entrance Examination Board. All rights reserved.
Advanced Placement Program and AP are registered trademarks of the College Entrance Examination Board.
19
AP® CHEMISTRY
2002 SCORING GUIDELINES (Form B)
Question 8 (cont’d.)
(d) A 25 mL sample of 0.10 M HA is titrated with 0.20 M NaOH.
(i) What volume of base must be added to reach the equivalence point?
2.5 mmol HA = 2.5 mmol OH–
1 point earned for the correct volume
2.5 mmol OH = 13 mL
0.20 mmol/mL
(ii) The pH at the equivalence point for this titration is slightly higher than the pH at the equivalence point
in the titration using 0.10 M NaOH. Explain.
In the titration with 0.1 M NaOH, the total volume at
the equivalence point is 50 mL. In the titration with
0.20 M NaOH the total volume at the equivalence
point is 37.5 mL.
The smaller volume in the titration with 0.2 M NaOH
means the [A–], the molar concentration of the conjugate
base of HA, is larger compared to the [A–] at the
equivalence point with 0.1 M NaOH.
1 point earned for correct explanation
Therefore, the pH is slightly higher.
Copyright © 2002 by College Entrance Examination Board. All rights reserved.
Advanced Placement Program and AP are registered trademarks of the College Entrance Examination Board.
20
AP® CHEMISTRY
2002 SCORING COMMENTARY (Form B)
Question 8
Sample 8A (Score 8)
This response earns all 8 points — 2 points for part (a), 2 points for part (b), 2 points for part (c), 1 point for
part (d)(i), and 1 point for part (d)(ii).
Sample 8B (Score 6)
This response earned a total of 6 points — 2 points for part (a), 2 points for part (b), 1 point for part (c), 1 point
for part (d)(i), and 0 points for part (d)(ii).
In this very good response, the graph in part (c) does not start at a pH of 1, thus only one of the two available
points is earned. The response in part (d)(ii) does not earn the point.
Copyright © 2002 by College Entrance Examination Board. All rights reserved.
Advanced Placement Program and AP are registered trademarks of the College Entrance Examination Board.
5
© Copyright 2026