Fever of Unknown Origin in Childhood: Diagnostic Challenges
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Annals of the Rheumatic Diseases 1994; 53: 429-433
429
MASTERCLASS
Fever of unknown origin in childhood: difficulties
in
diagnosis
Katherine Martin, E Graham Davies, John S Axford
Case report
A twelve year old white boy presented to
another hospital with a two month history of
intermittent fever with night sweats, general
malaise, arthralgia and myalgia. He had
marked cervical lymphadenopathy. Latex
agglutination for toxoplasma antibodies was
positive at a dilution of 1/128. A diagnosis of
acquired toxoplasmosis was made and sulphadiazine 1 g four times a day, trimethoprim 300
mg twice a day and folinic acid 15 mg
alternative days, were started. Over the next
week he developed a generalised urticarial rash,
peripheral oedema and profuse bloody
diarrhoea and was referred to our unit.
On examination he was delirious with a
persistent fever of up to 42°C and he was
bleeding from his nose and mouth. He was
generally oedematous with marked ascites and
bilateral pleural effusions. His skin was
erythematous with a petechial rash and after a
few days began to desquamate. His liver was
palpable 5 cm below the costal margin. Full
blood count and film revealed a normochromic
normocytic anaemia (haemoglobin 6-8 g/dl),
neutrophilia with left shift (white cell count
41 6 X 109/1 with 93% polymorphonuclear
cells) and thrombocytopaenia (platelet count
66 x 109/1). The circulating lymphocyte count
was normal (1-2 X 109/1). Coagulation studies
revealed evidence of disseminated intravascular coagulation (prothrombin time 16 s
(control 11-15 s), kaolin partial thromboplastin time 68 s (control 34-48 s), thrombin
time 18 s (control 11-15 s), plasma fibrinogen
1-2 g/l (normal 2-0-4-0 g/l), fibrinogen
degradation products 64 to 128 mg/l (normal
< 8 mg/1)). Erythrocyte sedimentation rate
(ESR) was 30 mm/hour, C-reactive protein
Academic
Rheumatology Group
and Department of
Child Health, St.
George's Hospital
Medical School,
London, United
Kingdom
K Martin
E G Davies
J S Axford
Correspondence to:
Dr Katherine Martin,
Department of Child Health,
St. George's Hospital
Medical School, Cranmer
Terrace, London SW17 ORE,
United Kingdom.
.I
I2IMAJa26I32d12i2,41S722B2a 4iUi"=2 sho4m62I-cmI
A2
lOUj,s,q2lOUIWZ,
-
416
....
3"
3"
1
_
.
I
.
4.-.
- .
|
. I . .
.
...I l.\
. tt t /
7ii[=20
lfif4 *
270
nI
r
o
.\ w
J210
., ..-. s aI
.
2001
37.0 -4-6--I
.
3vL
p
300
37.0
I 37S.1
.I
I.
..
.
,4
as
.
.
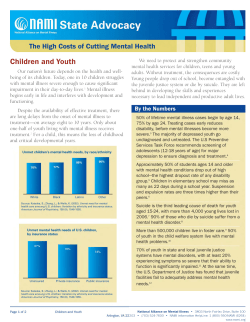
Figure I Temperature chart.
1:
A
4-.
0---
(CRP) 208 mg/I. Liver function tests were
abnormal: alanine transaminase 91 IUAL
(normal range 1-40), gamma glutamyl
transferase 134 IUAL (normal range 0-60),
bilirubin 18 micromolVL (0-17), alkaline
phosphatase 217 IU/L (30-100) and albumin
18 g/l (35-45). Renal impairment was apparent
with a raised serum creatinine (224 micromol/
L (60-110)). Chest radiograph showed right
middle lobe consolidation. Abdominal
ultrasound scan (USS) confirmed hepatosplenomegaly and ascites. Echocardiogram
showed a small pericardial effusion.
A diagnosis of Stevens-Johnson syndrome
with acute renal failure and disseminated
intravascular coagulation (DIC) was made.
Supportive therapy, broad spectrum antibiotics and discontinuation of sulphonamides
resulted in marked improvement. Cultures of
blood, cerebrospinal fluid, urine and stool grew
no pathogenic organisms. Repeat toxoplasma
serology showed a positive dye test (125 units)
and positive latex agglutination at a dilution of
1/128, but negative IgM by enzyme linked
immunosorbent assay and immunosorbent
agglutination assay, indicating probable past
infection. Serology for Epstein-Barr virus,
cytomegalovirus, viral hepatitis, mycoplasma,
brucella, leptospira and legionella was
negative. Mantoux at a dilution of 1/1000 was
anergic. Anti-streptolysin 'O' titre was less than
200 IU/ml, anti-deoxyribonuclease B titre was
less than 100 U/ml. Anti-nuclear antibody was
negative. Plasma immunoglobulin levels and
immunoglobulin G subclass levels were within
the normal range and complement studies
were normal.
Two weeks after admission the patient was
much improved but had persistent intermittent
fevers and marked hepatomegaly. Liver
function tests returned to normal. Repeat
abdominal USS showed a diffusely abnormal
liver texture. Liver biopsy showed minimal
focal fatty change with no signs of vasculitis.
Bone marrow trephine showed hypercellularity
and reactive changes only. Both were sterile on
culture.
The patient continued to experience daily
fevers (fig 1) and a fine evanescent rose
coloured maculopapular rash which exhibited
Koebner's phenomenon (fig 2) was noted for
the first time at the peak of the fever. He
developed swelling and stiffness of the
proximal interphalangeal joints of the hands
and effusions in the left knee and right ankle
joints. A diagnosis of systemic onset juvenile
chronic arthritis (S-JCA) was made and the
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Martin, Davies, Axford
430
a*
/
Figure 2 Appearance of rash.
patient was treated with diclofenac sodium 50
mg three times a day. Slit lamp examination of
the eyes was normal. Two weeks later there was
no improvement in systemic symptoms
although the arthritis had largely resolved. The
haemoglobin had fallen to 6&4 g/dl
necessitating transfusion. Acute phase
reactants remained raised (ESR = 1 5 mm/hr,
CRP = 189 mg/l, ferritin 5148 microg/l). As
the patient remained systemically unwell and
in view of the rapidly falling haemoglobin,
prednisolone 40 mg/day was added with
resultant slow improvement. After resolution
of disease activity steroids were weaned and the
patient currently remains well and off all
medication.
Table 1 Causes of FUO
Infections
Post-infectious inflammatory disease, for example, rheumatic
fever
Non infectious inflammatory diseases
S-JCA
For example
systemic lupus erythematosus
polyarteritis nodosa
Kawasaki's disease
inflammatory bowel disease
sarcoidosis
familial mediterranean fever
Malignancies
leukaemia
For example
lymphoma
neuroblastoma
Wilm's tumour
Miscellaneous
For example
factitious fever
drug fever
diabetes insipidus
hypothalamic dysfunction
ectodermal dysplasia
familial dysautonomia
Discussion
Differential diagnosis of fever of unknown
origin in childhood
This case illustrates well some of the pitfalls in
the diagnosis of S-JCA. The child's initial
presentation was with fever of unknown origin
(FUO) which has been defined as "the
presence of fever for eight or more days in a
child in whom a careful and thorough history
and physical examination and preliminary
laboratory data fail to reveal the probable cause
of fever".' The differential diagnosis of FUO is
wide and includes infectious diseases (systemic
and localised), autoimmune rheumatic
diseases, and neoplasia (table 1). The
commonest cause in childhood is infectious
disease (22-55%)2 5 (see table 2).
Table 2 Infections presenting as FUO
Systemic
Viral, for example
Localised
infectious mononucleosis*
cytomegalovirus*
hepatitis A, B*
human immunodeficiency virus*
tuberculosis*
salmonellosis*
brucellosis*
legionellosis
cat scratch fever
Rickettsial disease (for example, Q fever)
Chlamydial diseases (for example, psittacosis)
Spirochaete infections (for example, Lyme disease*, leptospirosis)
Parasitic infections (for example, malaria, toxoplasmosis)
Fungal infections (for example, histoplasmosis)
Bacterial, for example
Urinary tract infection
Osteomyelitis*
Sinusitis
Endocarditits
Occult abscesses (for example, hepatic, pelvic)
*may also cause arthritis
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Fever of unknown origin in childhood: difficulties in diagnosis
Retrospective analyses of admissions in the
United States of America with FUO show that
a diagnosis of 'juvenile rheumatoid arthritis'
was made in three to thirteen per cent of
cases.2-5 Other autoimmune rheumatic
diseases (systemic lupus erythematosus,
Henoch-Schonlein purpura, polyarteritis
nodosa, undefined vasculitis) account for up to
a further 5% of cases. Autoimmune rheumatic
diseases were commoner in the older age group
(JCA was diagnosed in seven of 48 cases of
FUO aged six years or older).2
An infectious aetiology was initially
considered in the above case. Marked cervical
lymphadenopathy together with a positive latex
agglutination test for toxoplasma misled the
referring clinicians into a diagnosis of acquired
toxoplasmosis. Latex agglutination for
toxoplasma measures IgG and detectable titres
of IgG usually persist for life after acute
infection. Serological diagnosis of acute
toxoplasmosis requires either the detection of
IgM or a rise in IgG titre.
Systemic onset juvenile chronic arthritis.
Diagnostic criteria and epidemiology
Diagnostic criteria for systemic onset juvenile
chronic arthritis have recently been suggested.6
The criteria for the diagnosis of 'definite'
S-JCA are arthritis, characteristic rash and
quotidian fever lasting for more than two
weeks. A 'probable' diagnosis of S-JCA can be
made if there is typical rash and fever,
organomegaly and/or lymphadenopathy and/or
serositis in the absence of arthritis.
Juvenile chronic arthritis (JCA) has an
annual incidence of approximately one in five
to ten thousand children.7 8 Four to thirty per
cent of these present with systemic onset
disease.7'- S-JCA may occur at any age
throughout childhood. The reported sex incidence varies showing an excess of males in
some senes - 1113-14 and an excess of females
in others.'5"-7
The diagnosis of S-JCA was delayed in this
case because the clinical picture was obscured
by the development of a persistent high fever,
desquamating erythematous rash, mucositis,
encephalopathy, renal failure and DIC. This
was attributed to an acute hypersensitivity
reaction to sulphadiazine. Intolerance to
suphonamides appears to occur with increased
frequency in patients with S-JCA. Sustained
high fever and rash were reported in three of
four patients with S-JCA treated with sulphasalazine.'8 In one case there were associated
liver function abnormalities. Two children out
of five with S-JCA were withdrawn from a
second study of suphasalazine'9 because of
severe side effects (fever, rash and leucopaenia
in one and nausea, vomiting, headache and
abnormal liver function tests in the other).
DIC has also been described in S-JCA both as
a direct manifestation of disease activity in
association with renal and hepatic damage,2021
in association with presumed infection22 and in
association with non-steroidal anti-inflammatory drug and intramuscular gold
therapy.2'
431
As the acute hypersensitivity reaction
resolved the clinical picture returned to that at
presentation with intermittent spiking fevers,
although marked hepatomegaly persisted. In
addition to the high spiking quotidian fever (fig
1), the appearance of the characteristic rash
(fig 2) and arthritis led to the diagnosis ofJCA.
Our patient demonstrates the need for
thorough and repeated examination which
other authors have stressed.4 13
Clinical features of systemic onset
juvenile chronic arthritis
(A) TYPICAL FEATURES
Daily or twice daily temperature elevations to
39°C or above with rapid return to baseline are
characteristic of S-JCA and often accompanied
by sweating, chills, myalgia and arthralgia.
Typically the fever spike occurs in the evening.
However, intermittent fevers are also seen in
pyogenic infections, tuberculosis and lymphomal and several authors2 5 have found that
the pattern of fever was not useful in
differentiating the cause of FUO.
A rash occurs in up to 94% of children with
S-JCA9 13-14 but may not be present or
characteristic on initial evaluation. Typically it
is erythematous, fine and mascular. It is
evanescent, usually appearing at the height of
the fever and it is therefore essential to reexamine the child when febrile. The
commonest sites are the trunk and limbs23 but
the face and neck are affected in more than
50% of cases and the rash may also occur on
the palms and soles. Occasionally, the rash may
be confined to the axillae and might therefore
be missed unless specifically searched for. The
rash is rarely pruritic, but Koebner's
phenomenon is common at sites of friction
from clothing and may sometimes be
demonstrated by rubbing the skin.
Children with S-JCA may develop oligo- or
poly-arthritis. The commonest sites of arthritis
in S-JCA are the wrists, knees, ankles, elbows,
hips, metacarpo-phalangeal joints and proximal inter-phalageal joints.'2 17 Involvement of
the temporo-mandibular joints also occurs
frequently and involvement of the cervical
spine is present at onset of disease in about a
quarter of patients.'2 Although arthralgia and
myalgia are frequent but non-specific
symptoms at the onset of S-JCA, arthritis may
be transient or absent at presentation. In
Schaller and Wedgwood's series'4 29 of 32
patients with S-JCA had overt arthritis during
the first six months of disease but Calabro
describes 18 of 40 children with systemic onset
disease in whom arthritis was initially absent.9
These children were originally diagnosed as
having FUO, the diagnosis of S-JCA being
made after the development of arthritis four
months to nine years (mean 2-2 years, median
8-5 months) after the onset of fever.
(B) OTHER FEATURES
Reticuloendothelial hyperplasia, most frequently lymphadenopathy, occurs in approximately 80-90% of children with active
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Martin, Davies, Axford
432
S-JCA,9 11 14 but is also common in other
causes of FUO.2 Lymphadenopathy may
occasionally be so prominent as to suggest a
lymphoma."3 Hepatomegaly (occurring in one
third of cases) is less common than splenomegaly (found in approximately 75% of
cases),9 although massive hepatomegaly has
been reported.24
Serositis is common in S-JCA. Pericardial
effusions are detectable in more than 80% of
patients during active disease25 although
symptomatic pericarditis is much less frequent.
Constrictive pericarditis and cardiac tamponade are extremely rare.26 Pleural effusions
are less common than pericardial effusions and
usually small. Subclinical myocarditis is
reported to occur in up to 10% of children with
S-JCA25 although clinically significant
myocardial involvement is rare. Myocarditis
may present with chest pain or heart failure but
tachycardia out of proportion to the degree of
pyrexia or anaemia may be the only clinical
sign. Differentiation from viral myocarditis
may be difficult. Both pericarditis and
myocarditis have been reported as the
presenting manifestations of S-JCA.27
Differentiation of S-JCA from acute rheumatic fever may cause problems especially in
the presence of S-JCA associated carditis.
Although functional cardiac murmurs are
common, valvular disease is extremely rare in
JCA. Rheumatic fever is rare in children under
the age of five years and the fever pattern in
rheumatic fever is typically more sustained and
lower grade than that of S-JCA.28 Associated
arthritis is typically migratory, asymetric and
painful. There may be evidence of a previous
group A P-haemolytic streptococcal infection,
although the ASOT may be moderately and
chronically elevated in approximately one third
of children with JCA.29 30
Abdominal pain is occasionally a prominent
feature of S-JCA and may increase the difficulties in distinguishing S-JCA from inflammatory bowel disease (which may also cause
FUO and arthritis). Abdominal pain or
distension may occasionally be so severe as to
suggest an acute abdomen.9 Pneumonitis has
rarely been reported in S-JCA, but is usually
mild and transient.3' Cerebral manifestations
(marked irritability, drowsiness, seizures and
meningismus) have been reported in 25% of
one series of children with S-JCA,'3 but these
are rare in other series except in relation to
complicating factors such as metabolic
derangements or salicylate toxicity. Iridocyclitis
should be sought in all patients with JCA but
is uncommon in systemic onset disease.'5-16 32
the consideration of alternative diagnoses such
as malignancies or systemic lupus erythematosus. Mildly raised transaminases are
common35 and hypoalbuminaemia may be
marked. The erythrocyte sedimentation
rate9 12-13 33 and C-reactive protein36 are often
considerably raised and hypergammaglobulinaemia" is common. Serum ferritin levels
are characteristically extremely high during
active phases of S-JCA.37 Fassbender et al38
reported a decreased proportion of concanavalin
A reactive alpha,-acid glycoprotein (AGP)
variants in patients with S-JCA compared with
healthy controls and patients with acute
bacterial infections. Children with S-JCA have
an increased prevalence of agalactosyl oligosaccharides associated with immunoglobulin G
compared with healthy controls.39 The
prevalence of agalactosyl-IgG is not increased,
however, in patients with infectious diseases.40
The investigation of glycosylation of immune
molecules may prove useful in discriminating
between acute infection and active JCA.
Malignancy is the cause of 2-13% of cases of
FUO in children in the USA,2 is sometimes
associated with arthralgia or arthritis and has
been misdiagnosed as S-JCA.1A2 The pattern
of fever is usually remittent4' (that is, the
temperature fluctuates but does not return to
normal), in contrast to JCA and bone or joint
pain may be more pronounced than in S-JCA.43
Careful examination of full blood count and
film for any abnormalities is therefore essential.
Severe anaemia, leucopaenia, thrombocytopaenia or abnormal white blood cell
appearance on the blood film are indications for
bone marrow examination. In addition to its
role of diagnosis of haematological
malignancies, bone marrow examination may
rarely be of use in the diagnosis of infection.44
A predominance of plasma cells has been
reported in the bone marrow of patients with
JCA but also occurs in patients with FUO.45
Bone marrow examination may not always be
diagnostic of a leukaemia at presentation and
may need to be repeated.43 46
Radiographic features
Careful examination of radiographs may reveal
lesions characteristic of leukaemia (osteopaenia, lytic lesions, metaphyseal rarefaction
and periosteal lesions).47 Chest radiographs
may reveal asymptomatic pleuritis14 or an
increased cardiac shadow indicating possible
pericarditis or myocarditis. Scanning procedures such as abdominal and pelvic USS,
abdominal computed tomography, radioisotope bone scanning, indium and gallium
scanning may be helpful to exclude alternative
diagnoses but rarely lead to an unsuspected
Laboratory investigations
Laboratory investigations are frequently unhelp- diagnosis in cases of FUO.3 Rheumatoid
ful in the diagnosis of S-JCA. Autoimmune arthritis has been reported to be a cause of false
serology is characteristically negative. '13 Severe positive indium-labelled polymorphonuclear
anaemia (commonly hypochromic and leucocyte scans.48
microcytic)33 and thrombocytosis'2 are usual in
S-JCA but non-specific. Neutrophilia is often
pronounced9 "-" but may also be a false pointer Summary
to bacterial infection. Leucopaenia34 and We have described a child with systemic onset
thrombocytopaenia are rare and should lead to juvenile chronic arthritis who presented
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Fever of unknown origin in childhood: difficulties in diagnosis
initially with fever of unknown origin. Treatment of a presumed infection led to a severe
allergic response with Stevens-Johnson
syndrome, renal failure and DIC. This reaction
obscured the features of the underlying disease
and delayed the diagnosis.
Key points
* Systemic onset juvenile chronic arthritis
often presents with fever of unknown
origin.
* Arthritis may be absent at presentation.
* Thorough and repeated examination for
the typical rash and careful temperature
charting may aid diagnosis.
* A wide variety of alternative diagnoses
may need to be considered in the absence
of typical features.
1 Lorin M I, Feigin R D. Fever without localising signs and
fever of unknown origin. In: Feigin R D, Cherry J D, eds.
Textbook of pediatric infectious disease. Philadelphia: W B
Saunders, 1992: 1012-22.
2 Pizzo P A, Lovejoy F H, Smith D H. Prolonged fever in
children: Review of 100 cases. Pediatrics 1975; 55:
468-73.
3 Steele R W, Jones S M, Lowe B A, Glasier C M. Usefulness
of scanning procedures for diagnosis of fever of unknown
origin in children. Pediatr 1991; 119: 526-30.
4 McClung H J. Prolonged fever of unknown origin in
children. Am Dis Child 1972; 124: 544-50.
5 Lohr J A, Hendley J 0. Prolonged fever of unknown origin.
Clin Pediatr (Phila) 1977; 16: 768-73.
6 Southwood T R, Woo P. Childhood arthritis: the name
game. BrJ Rheumatol 1993; 32: 421-3.
7 Kunnamo I, Kallio P, Pelkonen P. Incidence of arthritis in
urban Finnish children. A prospective study. Arthritis
Rheum 1986; 29: 1232-8.
8 Andersson Gare B, Fasth A. Epidemiology of juvenile
chronic arthritis in Southwestem Sweden: A 5-year
prospective population study. Pediatrics 1992; 90: 950-8.
9 Calabro J J, Bumstein S L, Staley H L. JRA posing as fever
of unknown origin. Arthritis Rheum 1977; 20s: 178-80.
10 Ansell B M. Juvenile chronic polyarthritis: Series 3. Arthritis
Rheum 1977; 20s: 176-8.
11 Schaller J G. The diversity of JRA. A 1976 look at the
subgroups of chronic childhood arthritis. Arthritis Rheum
1977;20s: 52-61.
12 Brewer E J, Giannini E H, Person D A. Manifestations of
disease. In: Juvenile Rheumatoid Arthritis. Philadelphia: W
B Saunders Co, 1982: 1-53.
13 Calabro J J, Holgerson W B, Sonpal G M, Khoury M I.
Juvenile rheumatoid arthritis: a general review and report
of 100 patients observed for 15 years. Semin Arthritis
Rheum 1976; 5: 257-98.
14 Schaller J, Wedgwood R J. Juvenile rheumatoid arthritis: a
review. Pediatrics 1972; 50: 940-53.
15 Stillman J S, Barry P E. Juvenile rheumatoid arthritis: Series
2. Arthritis Rheum 1977; 20s: 171-5.
16 Brewer E J, Giannini E H. A comparative study of the
epidemiologic and clinical natural histories of juvenile
rheumatoid arthritis subtypes. Arthritis Rheum 1980; 23:
656-7.
17 Stillman J S, Barry P E, Bell C L, et al. Clinical characteristics and classification of juvenile rheumatoid arthritis. In:
Jayson M I V, ed. Still's disease: juvenile chronic polyarthritis.
London: Academic Press, 1976: 47-58.
18 Hertzberger-ten Cate R, Cats A. Toxicity of sulfasalazine in
systemic juvenile chronic arthritis. Clin Exp Rheumatol
1991; 9: 85-8.
19 Ansell B M, Hall M A, Loftus J K, et al. A multicentre pilot
study of sulphasalazine in juvenile chronic arthritis. Clin
Exp Rheumatol 1991; 9: 201-3.
20 Schwartz D, Averbuch M, Pines A, Kornovsky R, Levo Y.
Disseminated intravascular coagulation with renal and
liver damage as the predominant manifestations of
433
recurrent relapses in systemic juvenile rheumatoid
arthritis. Ann Rheum Dis 1992; 51: 347-9.
21 Silverman E D, Miller J J, Bernstein B, Shafai T.
Consumption coagulopathy associated with systemic
juvenile rheumatoid arthritis. Jf Pediatr 1983; 103: 872-6.
22 De Vere-Tyndall A, Macauley D, Ansell B M. Disseminated
intravascular coagulation complicating systemic juvenile
chronic arthritis ("Still's disease"). Clin Rheumatol 1983;
2: 415-8.
23 Calabro J J, Marchesano J M. Rash associated with juvenile
rheumatoid arthritis. Jf Pediatr 1968; 72: 611-9.
24 Schaller J, Beckwith B, Wedgwood R J. Hepatic involvement
in juvenile rheumatoid arthritis. Jf Pediatr 1970; 77:
203-10.
25 Bernstein B, Takahashi M, Hanson V. Cardiac involvement
Pediatr 1974; 85:
in juvenile rheumatoid arthritis.
313-7.
26 Yancey C L, Doughty R A, Cohlan B A, Athreya B H.
Pericarditis and cardiac tamponade in juvenile
rheumatoid arthritis. Pediatrics 1981; 68: 369-73.
27 Marin-Garcia J, Sheridan R, Hanissian A S. Echocardiographic detection of early cardiac involvement in
juvenile rheumatoid arthritis. Pediatrics 1984; 73: 394-7.
28 McMinn F J, Bywaters E G L. Differences between the fever
of Still's disease and that of rheumatic fever. Ann Rheum
Dis 1959; 18: 293-7.
29 Calabro J J. Clinical features of Still's disease: a general
review and report of 100 patients observed for 15 years.
In: Jayson M I V, ed. Still's Disease: Juvenile Chronic
Polyarthritis. London: Academic Press, 1976: 1-47.
30 Sievers K, Ahvonen P, Aho K, Wager 0. Serological
patterns in juvenile rheumatoid arthritis. Rheumatism
1963; 19: 88-93.
31 Athreya B, Doughty R A, Bookspan M, Schumacher H R,
Sewell E M, Chatten J. Pulmonary manifestations of
juvenile rheumatoid arthritis: a report of eight cases and
review. Clin Chest Med 1980; 1: 361-74.
32 Cassidy J T, Sullivan D B, Petty R E. Clinical patterns of
chronic iridocyclitis in children with juvenile rheumatoid
arthritis. Arthritis Rheum 1977; 20s: 224-7.
33 Harvey A R, Pippard M J, Ansell B M. Microcytic Anaemia
in juvenile chronic arthritis. ScandJ7 Rheumatol 1987; 16:
53-9.
34 Scopelitis E, Perez M, Blundo J J. Leukopenia in Still's
disease. JAMA 1984; 252: 2450-52.
35 Rachelefsky G S, Kar N C, Coulson A, Sarkissian E, Stiehm
E R, Paulus H E. Serum enzyme abnormalities in juvenile
rheumatoid arthritis. Pediatrics 1976; 58: 730-6.
36 Gwyther M, Schwarz H, Howard A, Ansell B M. C-reactive
protein in juvenile chronic arthritis: an indicator of disease
activity and possibly amyloidosis. Ann Rheum Dis 1982;
41: 259-62.
37 Pelkonen P, Swanljung K, Siimes M A. Ferritinemia as an
indicator of systemic disease activity in children with
systemic juvenile rheumatoid arthritis. Acta Paediatr
Scand 1986; 75: 64-8.
38 Fassbender K, Michels H, Zepp F, et al. Glycosylation of
alpha,-acid glycoprotein in systemic onset juvenile
rheumatoid arthritis and acute bacterial infection: value
in differential diagnosis. Rheumatol 1993; 20: 123-7.
39 Parekh R B, Isenberg D A, Ansell B M, Roitt I M, Dwek
R A, Rademacher T W. Galactosylation of IgG associated
oligosaccharides: reduction in patients with adult and
juvenile onset rheumatoid arthritis and relation to disease
activity. Lancet 1988; L: 966-9.
40 Parekh R, Isenberg D, Rook G, Roitt I, Dwek R,
Rademacher T. A comparative analysis of diseaseassociated changes in the galactosylation of serum IgG.
JAutoimmun 1989; 2:101-14.
41 Brewer E J. Pitfalls in the diagnosis of juvenile rheumatoid
arthritis. Pediatr Clin North Am 1986; 33: 1015-32.
42 Bradlow A, Barton C. Arthritic presentation of childhood
leukaemia. Postgrad Med 1991; 67: 562-4.
43 Schaller J. Arthritis as a presenting manifestation of
malignancy in children. Pediatr 1972; 81: 793-7.
44 Hayani A, Mahoney D H, Fernbach D J. Role of bone
marrow examination in the child with prolonged fever. Jf
Pediatr 1990; 116: 919-20.
45 Starling K A, Brewer E J. Plasmacytosis of the bone marrow
in juvenile rheumatoid arthritis. Clin Res 1973; 21: 115.
46 Ansell B M. Case report 40. Skeletal Radiol 1977; 2: 113-5.
47 Rogalsky R J, Black G B, Reed M H. Orthopaedic
manifestations of leukemia in children. J7 Bone Joint Surg
[Am] 1986; 68-A: 494-501.
48 Rooney P J, Jenkins R T, Smith K M, Coates G. "'Indiumlabelled polymorphonuclear leucocyte scans in
rheumatoid arthritis an important clinical cause of false
positive results. BrJ Rheumatol 1986; 25: 167-70.
Downloaded from ard.bmj.com on August 22, 2014 - Published by group.bmj.com
Fever of unknown origin in childhood:
difficulties in diagnosis.
K Martin, E G Davies and J S Axford
Ann Rheum Dis 1994 53: 429-433
doi: 10.1136/ard.53.7.429
Updated information and services can be found at:
http://ard.bmj.com/content/53/7/429
These include:
Email alerting
service
Receive free email alerts when new articles cite this article. Sign up in
the box at the top right corner of the online article.
Notes
To request permissions go to:
http://group.bmj.com/group/rights-licensing/permissions
To order reprints go to:
http://journals.bmj.com/cgi/reprintform
To subscribe to BMJ go to:
http://group.bmj.com/subscribe/
© Copyright 2026