Worksheet buffers 2014
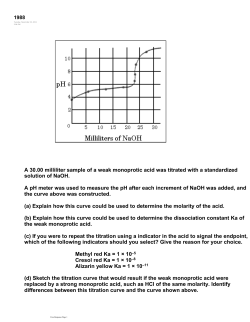
AP Chem worksheet:Buffers, The common ion effect Page 1 1. Calculate the pH of a buffer solution that is 0.060 M formic acid and 0.030 M potassium formate.! (3.44) 2. Calculate the pH of a buffer solution composed of 0.12 M benzoic acid and 0.20 M sodium benzoate.! (4.42) 3. Calculate the pH of a solution made of 0.10 M Pyridine (a weak base) and 0.15 M pyridine hydrochloride (its salt). (Hint: calculate Ka from Kb for the base).! (5.05) 4. Explain why a mixture of HCl and KCl does not function as a buffer whereas a mixture of Benzoic acid and sodium benzoate does. 5. Which of the following solutions has the greatest buffer capacity and which has the least: Explain your answer.! (a) ! (a) 0.10 M HC2H3O2 and 0.10 M NaC2H3O2, pH = 4.74 ! (b) 1.8 x 10–5 M HCl, pH= 4.74 ! (c) 0.01 M HC2H3O2 and 0.010 M NaC2H3O2, pH = 4.74 6. Which of these weak acids (and its salt) would be the best choice for making a buffer at pH = 5.0: acetic acid, hydrocyanic acid or hypochlorous acid. Explain your answer.! (acetic) ! How to make a buffer: Method 1! 7. Calculate the concentration of sodium formate that must be present in a 0.10 M solution of formic acid to produce a buffer with a pH of 3.80.! (0.114 M) 8. You have 250 mL of 0.10 M NH3 solution. How many grams of NH4Cl do you need to add to make a buffer with a pH of 9.00?! (need 0.18 M NH4+, 0.045 mol, 2.38 g) 9. You have 150 mL of a 0.100 M acetic acid solution, HC2H3O2. How many grams of! Page 2 ! sodium acetate, NaC2H3O2, should you add to make a buffer with a pH of 4.60? (0.0724 M, 0.0109 ! mol, 0.89 g) Adding strong bases, acids to weak acid and base solutions 10. Calculate the pH of a solution formed by adding 0.0050 moles of NaOH to 1.0 Liter of a 0.015 M solution of benzoic acid.! (3.90) 11. Calculate the pH of a solution made when 0.060 mol of HNO3 is added to 1.0 L of a 0.15 M solution of NH3.! (9.43) How to make a buffer: Method 2 12. How many mol of NaOH would you have to add to 0.15 liter of a 0.100 M HClO solution to make a buffer with a pH of 7.00?! (0.023 M, 0.0035 mol) 13. How many mol of HCl would you have to add to 0.25 liter of 0.200 M methlyamine (a weak base) solution to make a buffer with a pH of 11.00?! (0.061 M, 0.015 mol) Adding acids, bases to Buffer solutions 14. Consider a liter of a buffered solution that is 0.110 M in formic acid and 0.100 M in sodium formate. a. Calculate the pH of the buffer.! (3.70) ! b. Calculate the pH of the buffer after the addition of 0.015 mol HNO3.! (3.57) ! c. Calculate the pH of the original buffer after the addition of 0.015 mol NaOH.! (3.83) AP Chem Worksheet: Indicators, Titration curves ! Indicators: 1. Calculate the pKa and Ka for the following indicators: ! a. Bromocresol green: yellow at pH 4, green at pH 4.6, blue at pH 5.2 ! (2.5x10–5) ! (5 x10–4) b. Methyl orange : red at pH 2.8, orange at pH 3.8 (and higher pH’s)! Page 3 2. Alizarin yellow R (acidic form yellow, basic form red) has a Ka of 6 x 10–12. At what pH does this indicator change color? ! (11.2) Indicators and Titration curves: 3. For what kind of titrations (Strong/weak base, strong/ weak acid) are these likely to be good indicators? The pH range for their color change is given. (See ans. below) a. Bromocresol purple : pH 5.2 to 6.8 b. Bromocresol green: pH 3.8 to 5.4 c. Thymolphthalein : pH 9.4 to 10.6 d. Bromothymol blue: pH 6.0 to 7.6 4. Determine the pH at the equivalence point of the following Titrations. It’s the pH of the salt made by combining the acid and base. *** From dilution, the Molarity of the salt is half the original acid or base. a. 0.200 M NaOH with 0.200 M HCl! (7) b. 0.100 M HClO with 0.100 M NaOH! (10.11) c. 0.200 M hydroxylamine with 0.200 M HCl! (3.52) (Ans to 3. a. WB&SA, SB&SA; b. WB&SA (Very WB), SB&SA; c. SB&WA, SB&SA d. SB & SA) AP Chem Worksheet Titration Curves Page 4 Curve II Curve I Curve II Curve I Consider the above titration curves. ! Curve I ( initial pH = 1)! Curve II (initial pH = 3.5) 1. a. The initial pH, final pH ! _1.0__, _____! _3.5_, _____ b. At the equivalence point: volume of base added is _____ mL ! ______ mL ! ! and the pH is! ______! _______ c. The vertical region is (Circle)! Short, medium, long ! Short, medium, long d. Therefore this is a titration of a: ______ acid & ______ base ! ______ acid & ______ base 2. Circle the buffer region(s), if any. Is it (are they) acidic or basic? 3. a. What is the approximate Ka for each acid (if weak)? How did you figure this out? I or II b. What is the approximate Ka for each base (if weak)? How did you figure this out? I or II 4. Which of the following would be good indicators for each of these titrations? Why? Methyl orange : pH 2.8 to pH 3.8 ! Bromothymol blue: pH 6.0 to 7.6 Bromocresol purple : pH 5.2 to 6.5! phenolphthalein : pH 8.0 to 9.5 I: 1. 1.0, 9.0; b. 5.0 mL, pH = 5.0, c. med, d. SA &WB ; 2. around pH 8.5, 3a. not, b. 3 x 10–9; 4. Br purple II: 1. 3.5, 12; b. 12 mL, pH =9.5; c. med, d. WA & SB; 2. around pH 6; 3a. 10–6, b. not; 4. phenolphthalein AP Chem: Titration curve of a strong acid/strong base titration:! Page 5 A 20.0 ml sample of 0.200 M HCl solution is titrated with 0.200 M NaOH solution. Calculate the pH of the solution after the following volumes of base have been added. a. initial pH ! (0.7) b. 15.0 ml! (1.54) c. 19.9 ml! (3.30) d. 20.0 ml! (7.0) e. 20.1 ml! (10.7) f. 25.0 ml ! (12.35) ! 5. A hypothetical weak acid, HA, was combined with NaOH in the following proportions: 0.20 mol HA, 0.08 mol NaOH. The mixture was diluted to a total volume of 1.0 L and the pH was found to be 4.80. a. What is the pKa of the acid?! (4.98) ! b. How many additional mol of NaOH would need to be added to the solution to increase the pH to 5.00?! (0.022 mol) Titration curve of a Weak acid/strong base titration:! Page 6 A 20.0 ml sample of 0.200 M acetic acid is titrated with 0.200 M NaOH. Calculate the pH of the solution after the following volumes of base have been added. a. Initial pH (original weak acid solution)! (2.72) Before equivalence point: Use the Henderson Hasselbach equation b. 9.0 ml! (4.65) ! c. 10.0 ml! (4.74) d. 11.0 ml! (4.83) e. 19.0 ml! (6.02) f. 19.9 ml ! (7.04) At the equivalence point: pOH from the diluted salt solution! g. 20.0 ml! ! (8.87) After equivalence point : same as strong acid/strong base: pOH from excess base. h. 20.1 ml! (10.70) i. 21.0 ml! (11.69) j. 25.0 ml! (12.35)
© Copyright 2026