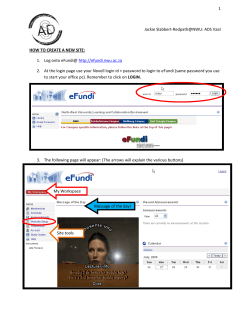
2012 – Physician Services Lab Sheet
2012 – Physician Services Lab Sheet Cardiac Rhythm Disease Management Patient Name Date Physician IP or OP ICD Registry? YES PACEMAKER SYSTEM IMPLANTS DEVICE FOLLOW-UP PACEMAKERS IN PERSON ❏ 33206 Insert new or replace perm PM w/atrial lead ❏ 33207 Insert new or replace perm PM w/ventricular lead ❏ 33208 Insert new or replace perm PM w/atrial and ventricular leads PACEMAKER GENERATOR PROCEDURES ❏ 33212 Insert PM generator only; w/existing single lead ❏ 33213 Insert PM generator only; w/existing dual leads ❏ 93279** Programming device evaluation; single lead pacemaker system ❏ 93280** Programming device evaluation; dual lead pacemaker system ❏ 93281** Programming device evaluation; multiple lead pacemaker system ❏ 93288** Interrogation device evaluation; single, dual, or multiple lead pacemaker system ❏ 33221 Insert PM generator only; w/existing multiple leads ❏ 33227 Remove perm PM generator w/replacement of PM generator; single lead system ❏ 33228 Remove perm PM generator w/replacement of PM generator; dual lead system ❏ 93294 Interrogation device evaluation(s); single, dual, or multiple lead pacemaker system – PC ❏ 33229 Remove perm PM generator w/replacement of PM generator; multiple lead system ❏ 93296 Interrogation device evaluation(s); single, dual, or multiple lead pacemaker/ICD system – TC ❏ 33233 Remove perm PM generator only REMOTE PACEMAKER (UP TO 90 DAYS; Do not report if the monitoring period is less than 30 days) OTHER PACEMAKER DEVICE EVALUATIONS OR ELECTRONIC ANALYSIS PACEMAKER LEADS ❏ 33210 Insert/replace temp lead/catheter – single chamber lead ❏ 33211 Insert/replace temp lead/catheter – dual chamber leads ❏ 33215 Reposition previously implanted transvenous right atrial or right ventricular lead ❏ 33216 Insert single transvenous lead, perm PM or ICD ❏ 33217 Insert 2 transvenous leads, perm PM or ICD ❏ 93293** TTM rhythm strip pacemaker evaluation(s), up to 90 days ❏ 93286** Peri-procedural device evaluation (in person) and programming device system parameters before or after a surgery, procedure, or test; pacemaker system ❏ 93724** Electronic analysis of antitachycardia pacemaker system COMPREHENSIVE EP EVALUATIONS ❏ 93619** Comp EP eval; RA and RV pacing/recording/His ❏ 93620** Comp EP eval w/arrhythmia; RA and RV pacing/recording/His ❏ +93621** Comp EP eval w/arrhythmia; LA pacing/recording from CS or LA ❏ 33218 Repair single transvenous lead, perm PM or ICD ❏ 33220 Repair 2 transvenous leads, perm PM or ICD ❏ 33224 Insert LV lead, attach to previously placed generator ❏ +93622** Comp EP eval w/arrhythmia; LV pacing/recording ❏ +33225 Insert LV lead at time of generator implant ❏ +93623** Programmed stimulation and pacing after IV drug infusion ❏ 33226 Reposition previously implanted LV lead RECORDING, MAPPING, AND PROCEDURES ❏ 33234 Remove transvenous pacemaker single lead system ❏ 93600** Bundle of His recording ❏ 33235 Remove transvenous pacemaker dual lead system ❏ 93602** Intra-atrial recording ❏ 93603** Right ventricular recording Upgrade single PM to dual PM (includes removal of PM generator, test existing lead, insert new lead and insert new PM generator) ❏ +93609** Intraventricular and/or intra-atrial mapping of tachycardia ❏ 93610** Intra-atrial pacing Revision/relocation of skin pocket for pacemaker ❏ 93612** Intraventricular pacing ❏ +93613 Intracardiac electrophysiologic 3-D mapping Insert new or replace perm PM w/atrial lead ❏ 93615** Esophageal recording of atrial electrogram Insert new or replace perm PM w/ventricular lead ❏ 93616** Esophageal recording of atrial electrogram; w/pacing Insert new or replace perm PM w/atrial and ventricular leads ❏ 93618** Induction of arrhythmia by electrical pacing 33212 Insert PM generator only; w/existing single lead ❏ 93624** EP follow-up w/pacing/recording w/induction or attempted induction of arrhythmia ❏ 93631** Intra-operative epicardial/endocardial pacing/mapping ❏ 93660** Tilt table evaluation PACEMAKER OTHER ❏ ❏ 33214 33222 CRT-P IMPLANT* ❏ ❏ ❏ ❏ 33206 33207 33208 ❏ 33213 Insert PM generator only; w/existing dual leads ❏ 33214 Upgrade single PM to dual PM (includes removal of PM generator, test existing lead, insert new lead and insert new PM generator) ❏ 33221 Insert PM generator only; w/existing multiple leads ❏ +33225 Insert LV lead at time of generator implant OTHER COMMENTS ❏ Page 1 of 2 2012 – Physician Services Lab Sheet ICD (IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR) SYSTEM IMPLANT 33249 Insert or replace perm pacing ICD system with transvenous lead(s), single or dual chamber ❏ 93641** Test device/leads at initial implant or replacement ICD GENERATOR PROCEDURES ❏ 33230 Insert ICD generator only; w/existing dual leads ❏ 33231 Insert ICD generator only; w/existing multiple leads ❏ 33240 Insert ICD generator only; w/existing single lead ❏ 33241 Remove ICD generator only ❏ 33262 Remove ICD generator w/replacement of ICD generator; single lead system ❏ 33263 Remove ICD generator w/replacement of ICD generator; dual lead system ❏ 33264 Remove ICD generator w/replacement of ICD generator; multiple lead system 33215 Reposition previously implanted transvenous right atrial or right ventricular lead ❏ 93298 Interrogation device evaluation(s); ILR – PC ❏ 93299 Interrogation device evaluation(s); ICM or ILR – TC Device Follow-Up: ICM (Implantable Cardiovascular Monitor) In Person ❏ 93290** Interrogation device evaluation; ICM REMOTE ICM (Up to 30 days; Do not report if the monitoring period is less than 10 days) ❏ 93297 Interrogation device evaluation(s); ICM – PC ❏ 93299 Interrogation device evaluation(s); ICM or ILR – TC CATHETER ABLATIONS ICD LEADS ❏ REMOTE ILR (Up to 30 days; Do not report if the monitoring period is less than 10 days) ❏ 93650 Catheter ablation AV node w or w/o temp pacemaker ❏ 93651 Catheter ablation for SVT ❏ 93652 Catheter ablation for VT ❏ +93462 Left heart cath by transseptal puncture ❏ +93662** Intracardiac echo during intervention/includes imaging CARDIOVERSION ❏ 33216 Insert single transvenous lead, perm PM or ICD ❏ 33217 Insert 2 transvenous leads, perm PM or ICD ❏ 92960 Cardioversion, elective, conversion of arrhythmia; external ❏ 33218 Repair single transvenous lead, perm PM or ICD ❏ 92961 Cardioversion, elective, conversion of arrhythmia; internal ❏ 33220 Repair 2 transvenous leads, perm PM or ICD ❏ 33224 Insert LV lead, attach to previously placed generator ❏ +33225 Insert LV lead at time of generator implant ❏ 33226 Reposition previously implanted LV lead ❏ 93303** Echo transthoracic, congenital; complete Remove single or dual ICD leads; by thoracotomy ❏ 93304** Echo transthoracic, congenital; follow-up/limited study Remove single or dual ICD lead(s); by transvenous extraction ❏ 93306** Echo transthoracic 2-D, complete w/spectral and color flow Doppler ❏ 93307** Echo transthoracic 2-D, complete w/o spectral and color flow Doppler CRT-D IMPLANT* ❏ 93308** Echo transthoracic 2-D, follow-up/limited study ❏ +33225 Insert LV lead at time of generator implant ❏ +93320** Doppler pulsed/continuous wave; complete ❏ 33230 Insert ICD pulse generator only; with existing dual leads ❏ +93321** Doppler pulsed/continuous wave; follow-up/limited study ❏ 33231 Insert ICD pulse generator only; with existing multiple leads ❏ +93325** Doppler echo color flow velocity mapping ❏ 33240 Insert ICD pulse generator only; with existing single lead ❏ 33249 Insert or replace perm pacing ICD system with transvenous lead(s), single or dual chamber ❏ ❏ 33243 33244 EP ICD DEVICE FOLLOW-UP ❏ ICD OTHER ❏ ❏ 33223 93641** Revision of skin pocket for ICD DEVICE FOLLOW-UP: ICDs IN PERSON ❏ 93282** Programming device evaluation; single lead ICD system ❏ 93283** Programming device evaluation; dual lead ICD system ❏ 93284** Programming device evaluation; multiple lead ICD system ❏ 93289** Interrogation device evaluation; single, dual, or multiple lead ICD system ❏ 93295 Interrogation device evaluation(s); single, dual, or multiple lead ICD system – PC ❏ 93296 Interrogation device evaluation(s); single, dual, or multiple lead pacemaker/ICD system – TC OTHER ICD DEVICE EVALUATIONS ❏ 93287** Peri-procedural device evaluation (in person) and programming device system parameters before or after a surgery, procedure, or test; ICD system ILR (IMPLANTABLE LOOP RECORDER) ❏ 33282 Implant patient-activated cardiac event recorder ❏ 33284 Remove implantable patient-activated cardiac event recorder DEVICE FOLLOW-UP: ILR IN PERSON ❏ 93285** Programming device evaluation; ILR ❏ 93291** Interrogation device evaluation; ILR EP evaluation single/dual ICD WEARABLE ICD VEST IN PERSON Test device/leads at initial implant or replacement REMOTE ICD (UP TO 90 DAYS; Do not report if the monitoring period is less than 30 days) 93642** TRANSTHORACIC ECHOCARDIOGRAPHY ❏ 93292** Interrogation device evaluation; wearable defibrillator system ❏ 93745** Initial set-up and programming, wearable ICD Commonly Used Modifiers: Q0 (zero): Denotes primary prevention patient for Medicare ICD device implants -22: Increased procedural services (work required to provide a service is substantially greater)* -25: Significant, separately identifiable E & M service by the same physician on the same day of the procedure or other service* -26: Professional component* -51: Multiple procedures rendered at the same operative session by the same provider (payer dependent)* -52: Reduced services* -53: Discontinued procedure (physician elects to terminate a surgical or diagnostic procedure)* -76: Repeat procedure or service by same physician or other qualified health care professional* -78: Unplanned return to the OR/procedure room by the same physician or other qualified health care professional following initial procedure for a related procedure during the postoperative period* -79: Unrelated procedure or service by the same physician during the postoperative period* NOTES: Codes indicated with a + symbol in front of them are “add-on” codes; bill in addition to the primary service/procedure. TC: Technical Component; PC: Professional Component * CPT® copyright 2011 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. ** Service performed in a facility setting (i.e., hospital) may require a -26 modifier that represents professional component only. These coding suggestions do not replace seeking coding advice from the payer and/or your own coding staff. The ultimate responsibility for correct coding lies with the provider of services. Please contact your local payer for interpretation of the appropriate codes to use for specific procedures. Medtronic makes no guarantee that the use of this information will prevent differences of opinion or disputes with Medicare or other third party payers as to the correct form of billing or the amount that will be paid to providers of service. Page 2 of 2 UC200804760d EN © Medtronic, Inc. 2011. Minneapolis, MN. All Rights Reserved. Printed in USA. 12/2011 ❏
© Copyright 2026