Accelerated Chemistry Week 27 1415
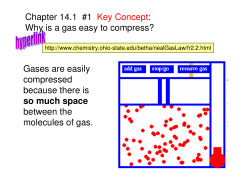
Mr. Morrow’s Accelerated Chemistry Week 27 Syllabus: Stoichiometry/KMT/Gas Laws Monday, March 9: Topic: “Stoichiometry and KMT Introduction” Objectives 1. See the objectives on the next page Q and A Session (10 minutes) ______ Reaction Stoichiometry Web Quest-Q and A (DUE TODAY) Lecture (30 minutes) ______ KMT Demo Quizzes #3: Students writing “essays” after watching a demonstration ______ KMT Worksheet: Student answering questions with teacher assistance, lecture videos and demonstrations Homework (30+ minutes) ______ Sakai Stoichiometry Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Monday, March 9th @10pm) ______ Sakai KMT Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thursday, March 11th) ______ Sakai STATES OF MATTER Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thurs., March 11th) ______ KMT Demo Quizzes #1-3: DUE Monday, March 16th ______ Pre Lab 18 Boyle’s Law Tuesday-Wednesday, March 10-11: Topic: “Gas Laws/Lab 18 Boyle’s Law” Objectives 1. See the objectives on the next page Lab (50 minutes) Lab 18: Non-Dual Enrollment students doing Lab 18 on Tuesday then worksheet on Wednesday. Lecture (50 minutes) ______ Gas Law Problem Worksheet: Dual enrollment student answering questions with teacher assistance, lecture videos and demonstrations. Dual enrollment students doing worksheet on Tuesday then Lab on Wednesday. Homework (30+ minutes) ______ Sakai KMT Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thursday, March 11th) ______ Sakai STATES OF MATTER Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thurs., March 11th) ______ UNIT 6 HOMEWORK-(DUAL ENROLLMENTS-DUE Sunday, March 15th) ______ KMT Demo Quizzes #1-3 (DUE Tuesday, March 17th) ______ Pre Lab 16 Dry Ice ______ Post Lab 18 Boyle’s Law (Due Monday, March 16th) Thursday, March 12: Topic: “Lab 16 Dry Ice” Objectives 1. See the objectives on the next page Lab (50 minutes) Lab 16 Dry Ice: Student lab teams experiment with dry ice to form liquid carbon dioxide KMT Demo Quiz # 4: Students writing “essays” after watching a demonstration Homework ______ Lab 16 Dry Ice (Due Monday, March 16th) ______ Lab 18 Boyle’s Law (Due Monday, March 16th) ______ Sakai KMT Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thursday, March 11th) ______ Sakai STATES OF MATTER Quiz-(ONLY FOR NON-DUAL ENROLLMENTS-DUE Thurs., March 11th) ______ UNIT 6 HOMEWORK-(DUAL ENROLLMENTS-DUE Sunday, March 15th) ______ KMT Demo Quizzes #1-4 (DUE Tuesday, March 17th) Friday, March 13: Topic: “Gas Laws” Objectives 1. See the objectives on the next page Lab (20 minutes) Lab 16 Dry Ice: Post Lab Q and A Lab 17 Molar Mass: Pre Lab Q and A Lab 18 Boyles Law: Post Lab Q and A Seat Work (30 minutes) ______ Gas Law Problem Worksheet: Student answering questions Homework ______ Lab 16 Dry Ice (Due Monday, March 16th) ______ Lab 18 Boyle’s Law (Due Monday, March 16th) ______ UNIT 6 HOMEWORK-(DUAL ENROLLMENTS-DUE Sunday, March 15th) ______ KMT Demo Quizzes #1-4: Students writing “essays” after watching a demo (DUE Tuesday, March 17th) OBJECTIVES FOR STOICHIOMETRY, KMT, STATES OF MATTER AND GAS LAWS 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Determine empirical formula of a compound from experimental data Explain the mole concept, and convert between mass, moles, and # of particles. Determine mass percent composition of a sample from experimental data. Determine mass percent composition of a compound from its formula. Determine empirical formula of a compound from its mass percent composition. Determine molecular formula of a compound from its mass percent composition and molecular mass Complete and balance chemical equations, including ionic equations. Use a periodic table to calculate the molar mass of a compound. Use a balanced equation to calculate mass yield and percentage yield for a chemical reaction. Apply limiting reactant data, when provided, to these calculations. Use a balanced equation to calculate the mole, number of particles, volume or mass of reactant required for a chemical reaction or amount of product made. Calculate the molarity of a solution. Calculate the molarity of an ion in solution from the molarity of the compound supplying that ion. Solve calculation problems involving titration. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. List the major postulates of the kinetic molecular theory and use them to explain states of matter. Compare and contrast real and ideal gases based on degrees of intermolecular attraction. Describe the different types of intermolecular attractive forces based on intramolecular forces. Relate the strength of London dispersion forces to molecular mass. Describe surface tension and viscosity. List and explain the unusual properties of water. Predict whether a substance is ionic, covalent, molecular, or metallic and predict a substance’s state of matter. Explain the phase diagrams of water and carbon dioxide. Define and explain triple point, sublimation point, boiling point, melting point, and critical temperature and pressure. Explain the relationship between temperature, average kinetic energy and kinds of molecular motion. Explain gas behavior in terms of KMT. 24. Distinguish between ideal and real gases. 25. Explain the phase diagrams of water and carbon dioxide. 26. Define and explain triple point, sublimation point, boiling point, melting point, and critical temperature and pressure. 27. Explain the relationship between temperature, average kinetic energy and kinds of molecular motion 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. Be able to convert one unit of pressure into another. Describe the relationship between the volume and pressure of a gas at constant temperature and number of moles: Boyle's law. Describe the relationship between the volume and temperature of a gas at constant pressure and number of moles: Charles' law. Describe the relationship between the pressure and temperature of a gas at constant volume and number of moles: Gay-Lussac’s law. Describe the relationship between volume and the number of moles of a gas at constant temperature and pressure: Avogadro's law. Apply Dalton’s Law of partial pressure to calculate the pressure of combined gases and to calculate the partial pressures of gases in mixtures. Calculate pressure of gas collected over water. Explain why real gases deviate from ideal gas behavior. Quantitatively apply PV = nRT or PV = mRT/M to ideal gases by solving for one variable, given all the others. Relate molar mass of a gas particle and speeds of gas particles using Graham’s law. Explain why real gases deviate from ideal gas behavior. Distinguish between ideal and real gases.
© Copyright 2026