Pre-Lab 8: Acid/Base Chemistry
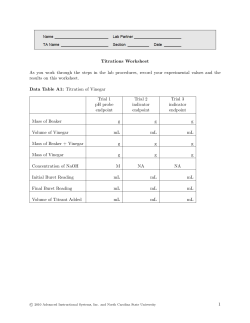
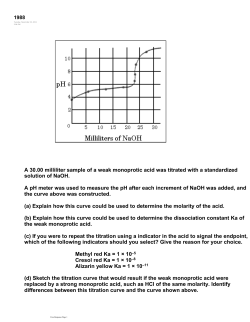
Name:_____________ Section:____________ Pre-Lab 8: Acid/Base Chemistry Answer the following questions after reading the background information at the beginning of the lab. This should be completed before coming to lab. 1. Write the equations for the dissociation of the following acids and bases in water: a) HNO3 b) HF c)Mg(OH)2 2. Give the balanced equation for the neutralization of the following acid/base combinations: a) HNO3 + KOH b) H3PO4 + Ca(OH)2 3. Convert the following pH to [H3O+], or [OH-]: a) pH = 3.25 b) pH = 8.45 ~ 92 ~ This page was left blank intentionally! ~ 93 ~ Lab 8: Acid/Base Chemistry Objectives: Understand the Acid/Base Scale. Calculate Hydronium ion concentration from pH. Titrate household vinegar and calculate the concentration of acetic acid. Background Information: Acids and Bases are used in many different products. We measure the strength of an acid or base using the pH scale from 1-14. When an acid dissociates in water it forms a hydronium, H3O+, ion. The pH scale measure the concentration of the hydronium ion free in solution, where: pH = -log([H3O+]) The reaction that occurs between an acid and a base is called neutralization. The products of a neutralization reaction are a salt and water, such as the reaction between HCl and NaOH below: HCl(aq) + NaOH(aq) → H2O(l) +NaCl(aq) In this experiment, you will measure the pH of several common products, calculate the hydronium ion concentration, as well as perform an acid/base titration, to calculate the concentration of a store bought vinegar solution. The chemical name for vinegar is acetic acid. Part 1. Measurement of pH This portion of the experiment will look at solutions of common products. We will measure the pH of the solution, followed by a calculation of the hydrogen ion, H+, concentration. Equipment List: 12 well spotting plate pH paper strips Procedure: 1. Take one of the test solutions and put 2-3 drops in a well on the spotting plate. 2. Take a piece of the pH paper, about 1/4 inch long, and set it on a watch glass. 3. Dip the end of a glass rod into the solution and then onto the pH paper. 4. Write down the color of the paper on the chart. 5. Find the color on the color scale and record the pH. 6. Calculate the H3O+ concentration. 7. Repeat this procedure for each solution. ~ 94 ~ Chart of pHs Solution Color pH ~ 95 ~ [H3O+] Part II. Titration of Household Vinegar This portion of the experiment involves the titration of a solution of household vinegar. Vinegar is a dilute solution of acetic acid, HC2H3O2, which can be neutralized with a solution of NaOH. By measuring the amount of NaOH needed, we can calculate the concentration of the acetic acid in the vinegar. In order to see when the solution is neutral, an indicator is used, which changes colors when the solution pH goes from acidic to basic. Equipment List: Ring Stand Burette Clamp Burette Tube w/ Stopcock Standardized NaOH solution 250 mL beaker 100 mL Graduated Cylinder Funnel 250mL Erlenmeyer Flask Procedure: 1. Assemble the ring stand with the burette clamp. 2. Clamp the burette in the clamp. 3. Using a beaker, get ~50 mL of the NaOH solution. 4. Using the funnel, fill the burette up to where the solution level is in the scale near the top of the burette. (Make sure the stopcock on the burette is closed) 5. Measure 15mL of the vinegar solution in the graduate cylinder. 6. Put the solution in a clean 250mL Erlenmeyer flask. 7. Add 10mL of distilled water to the solution. 8. Add a few drops of the indicator solution. 9. Titrating: Record the starting volume on the burette in the chart that follows. 10. To begin titration, use one hand to hold the Erlenmeyer with the vinegar solution and the other to turn the stopcock on the Burette. 11. Continue to swirl the solution during the titration. 12. Add small amount of the NaOH to the vinegar and wait until the solution returns to the original color. As the solution gets closer to neutral, this will take longer. 13. Add NaOH until the solution stays the indicator color. 14. Record the final volume of the solution in the burette in the chart below. 15. Repeat the procedure for a second vinegar solution. ~ 96 ~ Titration Data Table Trial #1 Trial #2 What is the [OH-] of the Standard solution: What is the initial volume of the burette What is the final volume of the burette: What is the amount of NaOH solution used: What was the final color of the indicator after the titration: Data Analysis – Calculation of the Acetic Acid Concentration Calculate the molarity and %(m/m) of the acetic acid in the vinegar solution and report the values in the table below. Trial #1 Molarity %(m/m) ~ 97 ~ Trial #2 Name:_________________ Acid/Base Chemistry Report Sheet Partner:________________ Section:________________ Part I. Questions: Which solution had the lowest pH? Which solution had the highest pH? What is the balanced equation for carbonic acid’s dissociation in water? Part II. Titrating Vinegar: Fill in the Chart with Your Results 1. Average Volume needed 2. Average number of moles of NaOH used. 3. [H3O+] in the Vinegar Bottle Additional Questions: 1. What is the balanced reaction for the neutralization of acetic acid by NaOH? 2. What would the concentration be if the original volume of vinegar we titrated was 45.0 mL instead of 25.0mL, but the titration still required the same volume of NaOH to neutralize it? ~ 98 ~ This page was intentionally left blank!! ~ 99 ~
© Copyright 2026