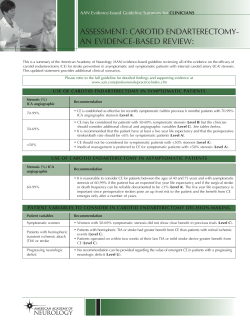
Assessment of the Patient with Hyperacute Stroke: Imaging and Therapy 1
Radiology Review James M. Provenzale, MD Reza Jahan, MD Thomas P. Naidich, MD Allan J. Fox, MD Index terms: Brain, CT, 10.1211 Brain, MR, 10.1214, 10.12144 Brain, infarction, 10.78, 17.78 Cerebral blood vessels, thrombosis, 173.78, 174.78 Review Thrombolysis, 10.1265, 17.1265 Published online 10.1148/radiol.2292020402 Radiology 2003; 229:347–359 Abbreviations: ACA ⫽ anterior cerebral artery ECASS ⫽ European Cooperative Acute Stroke Study MCA ⫽ middle cerebral artery NIHSS ⫽ National Institutes of Health Stroke Scale NINDS ⫽ National Institute of Neurological Disorders and Stroke PCA ⫽ posterior cerebral artery tPA ⫽ tissue plasminogen activator 1 From the Department of Radiology, Duke University Medical Center, Box 3808, Durham, NC 27710-3808 (J.M.P.); Department of Radiology, University of California, Los Angeles (R.J.); Department of Radiology, Mount Sinai Medical Center, New York, NY (T.P.N.); and Department of Medical Imaging, University of Toronto, Sunnybrook & Women’s College Health Sciences Centre, Toronto, Ontario, Canada (A.J.F.). From the 2000 RSNA scientific assembly. Received April 3, 2002; revision requested June 6; revision received October 17; accepted December 10. Address correspondence to J.M.P. © RSNA, 2003 Assessment of the Patient with Hyperacute Stroke: Imaging and Therapy1 Neuroimaging is an important part of the assessment of patients with hyperacute stroke. As new treatments that may reverse cerebral ischemia have been developed, the role of neuroimaging has changed from simply anatomic depiction of early infarction to identification, by means of physiologic (rather than simply anatomic) information, of regions that are at risk for infarction. The goal of such imaging techniques is to monitor successes and complications of recently developed treatments such as thrombolysis. © RSNA, 2003 Computed tomography (CT) remains the imaging modality that is most commonly used for initial evaluation of the patient with hyperacute stroke. It is important that radiologists be adept at recognizing the subtle findings of cerebral infarction in the first few hours after symptom onset. However, advanced magnetic resonance (MR) imaging techniques are very valuable in better defining the extent of initial infarct and in showing the region that is at risk to proceed to infarction if no therapy is provided (ie, the so-called ischemic penumbra). The U.S. Food and Drug Administration has approved intravenous recombinant tissue plasminogen activator (tPA) for the treatment of acute ischemic stroke within 3 hours of symptom onset. In this review, we will (a) explain the difficulties that are encountered with commonly used neuroimaging techniques for hyperacute stroke assessment, (b) explore the issues involved in determining mechanisms of stroke and the arterial territory involved in an infarct, and (c) critically assess the methods available to reduce the size of cerebral infarctions in the hyperacute setting. INTRODUCTION The proportion of the elderly in the general population has substantially increased in the past few decades. The number of people with acute ischemic stroke has also increased. At the same time, the past decade has seen the verification of new treatments for acute stroke, which represents a true advance for stroke patients (1,2). Neuroimaging is an important part of the assessment of patients for these new treatments, as well as for monitoring of successes and complications. For decades, the role of neuroimaging for acute stroke has been one of exclusion of lesions that mimic ischemic stroke (eg, intracerebral hemorrhage, subdural hematoma, cerebritis, hemiplegic or hemisensory migraine, and causes of focal seizures such as tumors and arteriovenous malformations). Before the introduction of the new treatments, imaging could reasonably be performed within a rather wide time window after symptom onset, because imaging findings did not typically alter stroke therapy. The introduction of intravenous thrombolysis with tPA has radically changed the role of neuroimaging for stroke evaluation. The notable randomized studies of intravenous thrombolysis treatment have been the European Cooperative Acute Stroke Study (ECASS) trial and the American National Institute of Neurological Disorders and Stroke (NINDS) trial (1,2). Neuroimaging played an important (although different) role in each of these studies. The ECASS trial prescribed that patients with stroke symptoms of less than 6 hours in duration and who did not have identifiable infarction of greater than one-third of the middle cerebral artery (MCA) territory on CT images be considered for randomization for possible treatment with intravenous tPA (1). The ECASS results showed that many cases were admitted to the trial despite large infarctions because of nonrecognition of the subtle 347 Radiology findings of hyperacute infarction. These findings, which had been described years earlier, include decrease in gray matter attenuation relative to that of white matter, sulcal compression, and high attenuation in the affected arteries (3). When an expert ECASS panel classified the CT images as showing less or more than onethird of the MCA territory, the large majority of the most serious hemorrhagic complications were associated with infarcts greater than one-third of the MCA territory and had a poorer outcome (4). For those with small or no visible infarction, the outcome was significantly improved and the treatment was validated. The importance of quick and accurate evaluation of acute stroke patients to determine eligibility for thrombolytic therapy was firmly planted. The NINDS trial established that intravenous tPA treatment is efficacious if administered less than 3 hours after symptom onset (2,5). Because multiple clinical parameters must be verified before treatment, little latitude in time exists for imaging studies to be performed. The clinical catchphrase “time is brain” is used by stroke associations, stroke teams, and stroke neurologists to emphasize that time-consuming complex imaging studies and analyses decrease the therapeutic options available to patients and the likelihood of a successful intervention. The field of stroke imaging has greatly advanced in the past few years, and the clinical utility of new techniques is under investigation. However, the time limitations for imaging mandated by the brief therapeutic window has allowed emphasis on easily accessible rapid imaging techniques (such as CT) to be maintained, rather than less accessible longer techniques (such as MR imaging). Unenhanced brain CT remains the most commonly performed neuroimaging technique prior to the decision to deploy intravenous tPA (6). A well-trained radiologist will often be able to discern the extent of early ischemia if appropriate attention is paid to the unenhanced CT study. IMAGING FINDINGS IN HYPERACUTE STROKE The accuracy of interpretation of early stroke on CT images is greatly improved if each case is used as a self-learning project (7). Critical comparison of initial CT studies (obtained during the hyperacute stroke stage) to follow-up CT or diffusion-weighted MR imaging studies is 348 䡠 Radiology 䡠 November 2003 Figure 1. Hypoattenuating basal ganglia sign in a 62-year-old man with ischemic symptoms referable to the right MCA territory. (a) Transverse unenhanced CT image obtained 6 hours after onset of symptoms shows subtle hypoattenuating appearance of the right lentiform nucleus (arrows) relative to appearance of the left lentiform nucleus, consistent with early infarction. (b) Transverse unenhanced CT image obtained 28 hours after onset of symptoms shows that the right lentiform nucleus (arrows) now has a markedly hypoattenuating appearance. an important method for increasing the sensitivity and specificity of individual image interpreters. To use images to help diagnose the presence and extent of cerebral infarction within the time window allowed for innovative stroke therapies, it is important that the radiologist be familiar with the features of cerebral ischemia evident on CT and MR images. These findings are outlined in the following sections. CT Findings CT findings obtained within the first 3– 6 hours of cerebral ischemia, when present, are often subtle. Nonetheless, at most institutions CT remains the initial imaging study for evaluation of acute stroke because it is widely accessible, convenient, has a short imaging time, and is sensitive for detection of hemorrhage. However, advances in CT imaging technology and innovations in image assessment have allowed CT findings to be documented earlier and earlier. For instance, the conspicuity of infarcts on unenhanced CT images can be increased by use of variable window width and center level settings at a workstation (as opposed to printed film with standard CT window settings) to accentuate the gray matter–white matter contrast (8). The distinction between normal brain and edematous tissue is thereby increased. The important CT findings during the early stages of cerebral ischemia can be classified as (a) mass effect, (b) hypoattenuating appearance of gray matter structures, and (c) presence of one or more hyperattenuating arteries. Any combination of these findings may be present, or all may be absent. The finding of hypoattenuating gray matter structures is typically seen as a gray matter structure becoming isoattenuating to adjacent white matter structures or, in essence, blurring of the gray matter–white matter junction. One example is the so-called insular ribbon sign, in which the insula (which is composed of gray matter) becomes isoattenuating to adjoining white matter (9). Another example is hypoattenuation of the basal ganglia, which then becomes isoattenuating to adjacent white matter structures such as the internal capsule and the external capsule (Fig 1). Examples of early mass effect include narrowing of the sylvian fissure (in MCA infarcts) or loss of cortical sulci (Fig 2). The hyperattenuating artery sign is thought to represent stasis of flow due to arterial thrombus; this sign is most frequently seen in MCA thrombosis but can be seen in any cerebral vessel (3,10). Unfortunately, normal arteries relatively often appear hyperattenuating, and this sign should be careProvenzale et al Radiology Figure 2. Subtle mass effect as an indication of early infarction in a 72-year-old man with symptoms referable to the left MCA. (a) Transverse contrast material– enhanced CT image obtained 4 hours after onset of symptoms shows effacement of sulci in left temporal lobe (arrows), as compared with the right temporal lobe, consistent with early infarction. (b) Transverse unenhanced CT image obtained 32 hours after onset of symptoms shows hypoattenuation and increased mass effect in the left temporal lobe (straight arrows) and insula (curved arrow). fully interpreted in light of clinical history and other CT findings. MR Imaging Conventional spin-echo MR imaging is more sensitive and specific than CT for detection of cerebral ischemia during the 1st few hours after symptom onset (Fig 3). The findings seen at this stage are hyperintense signal on T2-weighted images, mass effect, loss of arterial flow voids, and stasis of contrast material within vessels in affected territories after contrast material administration; in addition but less commonly, hypointense signal is seen on T1-weighted images (11). Many early findings are analogous to those seen on CT images. For instance, the distinction between gray matter structures and adjacent white matter structures can be lost on T2-weighted MR images (owing to increased signal intensity in white matter structures) in a manner similar to the loss of the gray matter– white matter distinction seen on CT images. On the other hand, loss of MR imaging flow voids and stasis of contrast material within arteries subserving an infarcted territory does not directly reflect the presence of thrombus itself (as is the case with the hyperattenuating artery CT sign) but instead reflects stasis of flow distal to a thrombus. Despite the greater Volume 229 䡠 Number 2 sensitivity of conventional MR images compared with CT images in the 1st few hours, false-negative MR studies can be seen within the 1st few hours if diffusionweighted or perfusion-weighted sequences are not performed. Diffusion-weighted MR imaging is a technique that is even more sensitive than conventional MR imaging for detection of hyperacute cerebral ischemia (12). For this reason, it is routinely used at centers designed for treatment of early stroke. Diffusion-weighted MR imaging is based on the principle that the random (brownian) motion of water molecules in living tissues can be quantitatively measured by using specific gradient pulses in conjunction with a 90°–180° pulse sequence. After application of such a pulse sequence, signal is lost in tissues due to a variety of factors. Tissues with a higher rate of water diffusion undergo greater signal loss in a given period of time than do tissues with a lower rate of water diffusion. As a result, tissues with a lower rate of water diffusion appear brighter. After the onset of cell death (cytotoxic edema), mechanisms for maintaining steady-state proportions of intracellular and extracellular water are altered, and the proportion of water in the intracellular space increases. The overall rate of microscopic water motion within such tissue is diminished. Therefore, tissues containing cells undergoing cytotoxic edema appear bright on diffusion-weighted images. High signal intensity on diffusion-weighted images can be seen in the 1st few hours after stroke onset (ie, within the generally accepted time frame for thrombolysis), at a time when T2-weighted images still show a normal appearance (Fig 4) (12). Results of animal studies have shown that abnormal signal intensity on diffusion-weighted images can be seen within minutes after the onset of cerebral ischemia (12). However, because cerebral ischemia does not occur under controlled conditions in humans (such as is the case in animal experiments), the precise time of onset of abnormal signal intensity in humans is not known with certainty. In addition, in many cases of hyperacute stroke in which hyperintense signal is already present on T2-weighted images, diffusion-weighted imaging better defines the size of the ischemic region. Although diffusion-weighted ischemic changes are generally considered to be permanent (and therefore reflect infarction) in clinical studies, recent evidence has shown that in some cases such changes can be reversed with prompt treatment. In one study in which large artery recanalization was achieved by using tPA within 6 hours of symptom onset (13), diffusion-weighted image abnormalities were seen to substantially decrease in a number of patients within 9 hours after thrombolysis. In about half of patients, however, a secondary increase in the size of the diffusion-weighted imaging abnormality was seen within 1 week. Although diffusion-weighted imaging has proved to be a valuable tool for evaluation of hyperacute stroke, in most cases diffusion-weighted images do, in fact, show regions of irreversible ischemia. However, it is also important for the stroke neurologist to define the area that is at risk of proceeding to infarction if no therapy is administered. MR perfusion imaging is a technique that allows depiction of both areas of irreversible ischemia and areas of reversible ischemia (Fig 5). MR perfusion imaging can be performed by using various techniques, including exogenous techniques (eg, use of infusion MR contrast agents) and endogenous techniques (eg, arterial spin tagging) (14). In addition, exogenous methods can be performed by using T2*weighted techniques or T1-weighted techniques. However, dynamic susceptibility-contrast (T2*-weighted) MR imaging is probably the most commonly used technique for stroke imaging, with many Assessment of the Patient with Hyperacute Stroke 䡠 349 Radiology Figure 3. Discrepancy between size of infarct seen on CT and MR images in a 59-year-old man with ischemic symptoms referable to the right hemisphere. (a) Transverse unenhanced CT image obtained 16 hours after symptom onset shows right frontal lobe infarct (straight arrow) containing hemorrhagic regions. Note a more subtle area of hypoattenuation (curved arrow) posterior to hemorrhagic focus, consistent with another region of early infarction. (b) Transverse diffusion-weighted MR image (b ⫽ 1,000 sec/mm2) obtained 4 hours after a shows area of high signal intensity consistent with infarction in a region larger than that seen in a. Apparent diffusion coefficient map (not shown) showed decrease of approximately 40% in apparent diffusion coefficient, indicating restricted water diffusion (consistent with early infarction) in this region compared with that of normal brain. (c) Dynamic susceptibility-contrast (T2*-weighted) cerebral blood volume map (repetition time msec/echo time msec, 1,500/80) obtained a few minutes after b shows region of decreased cerebral blood volume (arrows) that conforms closely to region of restricted water diffusion shown in b. Therefore, a matched diffusion-perfusion abnormality is seen in this patient. of the other techniques reserved for tumor imaging or other research topics. In the dynamic susceptibility-contrast technique, a bolus of MR contrast material is rapidly infused intravenously and a hemodynamic map is generated that is based on the degree of signal intensity decrease produced by the contrast material. From such maps, various parameters, such as relative cerebral blood volume, mean transit time, and bolus arrival time, can be calculated. The exact role of each of the major MR perfusion parameters in evaluation of the hyperacute stroke patient is a matter of active debate. However, it appears that mean transit time maps generally show the largest area of abnormality and often overestimate final infarct size; relative cerebral blood volume maps tend to underestimate final infarct size. In one study in which investigators compared blood volume maps, blood flow maps, and mean transit time maps with change in size from initial infarct size to final infarct size(15), a mismatch between initial blood flow maps and a diffusion abnormality more often predicted growth of infarct than did a mismatch between initial blood volume maps and a diffusion abnormality. In that study, however, blood volume maps best correlated with change in infarct size from initial imag350 䡠 Radiology 䡠 November 2003 Figure 4. Discordant findings between T2-weighted and diffusion-weighted MR images in a 63-year-old man with ischemic symptoms of 3 hours duration referable to the right hemisphere. (a) Transverse T2-weighted MR image (2,700/80) shows no abnormalities in the right hemisphere. (b) Diffusion-weighted MR image (b ⫽ 1,000 sec/mm2) obtained a few minutes after a shows two foci of high signal intensity in the right centrum semiovale. Apparent diffusion coefficient map (not shown) confirmed that these regions had restricted water diffusion, consistent with early infarction. ing to follow-up imaging. The authors noted that this finding may have reflected the result of threshold effects. Another MR perfusion imaging parameter that shows preliminary promise is flow heterogeneity (16). The capacity to Provenzale et al Radiology Figure 5. Discordant findings between diffusion-weighted and hemodynamic MR images in a 60-year-old man with ischemic symptoms of 5 hours duration referable to the left hemisphere. (a) Transverse contrast-enhanced T1-weighted MR image (500/20) shows stasis of contrast material in many arteries within the left MCA distribution (arrows). (b) Transverse diffusion-weighted MR image (b ⫽ 1,000 sec/mm2) obtained during the same MR examination as a shows focal regions of high signal intensity within the anterior portion of the left MCA territory. On apparent diffusion coefficient map (not shown), these regions were seen to have restricted water diffusion, consistent with early infarction. Posterior portion of MCA territory has normal signal intensity and normal apparent diffusion coefficient values. Therefore, the area containing arteries showing stasis of contrast material was larger than that of restricted diffusion. (c) Mean transit time map obtained a few minutes after b. Prolonged mean transit time (consistent with ischemia) is shown as red and orange areas; normal transit time is yellow. Large region of prolonged mean transit time (arrows) is seen. Note that area of ischemia on this map is much larger than area of restricted water diffusion seen in b; however, it conforms relatively well to the area of stasis of contrast material seen in a. The portion of tissue with prolonged mean transit time that has normal signal intensity on b (diffusion-perfusion mismatch) may represent the so-called ischemic penumbra. alter the heterogeneity of blood transit times (also referred to as flow heterogeneity) is believed to be a major function of the cerebrovascular autoregulatory system. In normal brain tissue, probability density functions of relative flows show a distribution of values that are skewed toward high capillary flow velocities. In animals, decreases in cerebral perfusion pressure are associated with loss of high-flow components (17). Recently, investigators have shown that increases in mean transit time are associated with loss of the high-flow-velocity components and produce a resultant homogenization of capillary flows, which was predictive of final infarct size (16). It is generally accepted that tissue that is seen to have abnormalities on both perfusion and diffusion-weighted images has already undergone infarction (ie, irreversible ischemia). However, tissue that is seen to have perfusion abnormalities but normal diffusion imaging properties is thought by many investigators to represent reversible ischemia. In particular, tissue showing such a “diffusion-perfusion mismatch” is thought by many investigators to represent the so-called ischemia penumbra—that is, the region of Volume 229 䡠 Number 2 decreased perfusion that is potentially reversible because it is above the level critical for maintenance of the Na⫹ K⫹ ⫺ ATPase pump (18). This penumbra has long been sought as a potential therapeutic target; whether this mismatch represents a therapeutic target is a matter of active investigation. However, this information is potentially valuable to the treating physician because it helps determine the risk-benefit ratio in a particular patient. For example, a patient with no (or a very small) degree of mismatch is considered unlikely to benefit from thrombolytic therapy. In such a patient, the ratio of benefit (clinical recovery) to cost (ie, complications of therapy) would generally be considered to be low. Recently, hemodynamic CT imaging has become available, which may provide the hemodynamic information needed for assessment of hyperacute stroke with the use of CT, rather than MR, imaging. Early results with this technique have shown that it is sensitive for detection of early cerebral ischemia (19). Postprocessing of CT perfusion imaging data, like that for MR perfusion imaging data, is short (ie, can be performed in less than 2 minutes) and does not substan- tially delay decision making in the hyperacute stroke setting. Results of early investigations of CT perfusion imaging (20) suggest that cerebral blood volume deficits may indicate regions of irreversible hemodynamic deficit (in a manner similar to that provided by diffusionweighted MR images). Furthermore, cerebral blood volume deficits may predict minimal final infarct size (20). On the other hand, some investigators (21) believe cerebral blood volume and mean transit time deficits on CT perfusion images may represent both infarcted tissue and surrounding tissue that is likely to proceed to infarction if no therapy is provided (ie, the so-called ischemic penumbra). VARIABILITY OF VASCULAR DISTRIBUTIONS AND ETIOLOGY OF BORDER-ZONE INFARCTIONS Physicians considering whether to treat acute stroke by means of intraarterial thrombolysis or conservative management often use anatomic CT or MR images to assess affected vascular territories and determine whether infarction is em- Assessment of the Patient with Hyperacute Stroke 䡠 351 Radiology bolic or hypotensive in nature. Unfortunately, increasing evidence indicates that neither the territories affected nor the nature of the stroke can be accurately diagnosed on the basis of such anatomic studies. An evaluation of patterns of cerebral watershed territories illustrates the extent of this problem. Schneider (22) and Zulch (23) each defined border-zone infarctions as ischemic lesions situated in the border zone between two neighboring vascular territories (22–26). The incidence of such border-zone infarcts ranges from 0.7% to 3.2% of cerebral infarctions (27,28). Early work suggested that lesions restricted to the border zones would more likely represent hypotensive events, whereas those situated within the arterial territories (and branches) would more likely be embolic (29–32). Continuing research has now led to major reassessment of previous concepts of normal vascular distributions, border zones, and hypotensive infarctions. Difficulties in Determination of Arterial Territories Affected by Infarction To determine whether an individual has sustained a border-zone infarction, one must be able to determine the territories supplied by the major intracerebral arteries. However, initial indications that one could reliably use the anatomic locus of the infarction to predict the involved vessel are now open to question. It is apparent that identification of specific arterial territories in an individual patient is more difficult than was initially realized. For instance, marked variation has been found in the laterality of the anterior cerebral artery (ACA) supply. One ACA supplies portions of the contralateral cerebral hemisphere in 12%–25% of brains (33–35). The area of contralateral supply is usually small, but in 4%–7% of brains there is a major contralateral supply (33–35). In addition, great variability is seen in the volume of brain supplied by the major cerebral arteries. In one study of vascular distributions of major cerebral arteries, van der Zwan et al (26) showed that no groups of hemispheres exhibited the same combinations of arterial territorial volumes. The ACA could supply 18%–35% of any one hemisphere; the MCA, 34%– 64% of the same hemisphere; and the posterior cerebral artery (PCA), 9%– 40% of that hemisphere (26). Thus, van der Zwan et al concluded that “the location of a cortical infarct in or at the border of the area of variation, visualized with CT or MR imaging, gives no 352 䡠 Radiology 䡠 November 2003 certainty about the pathogenesis of the disease. Although the occurrence of bilateral infarcts may suggest a hemodynamic pathogenesis, the interpretation of the CT or MR images based exclusively on the location of the infarct may lead to false diagnosis” (25, p 936). Identification of Specific Border Zones in Individual Patients Another problem encountered in determining whether an infarct is a borderzone infarct is that of definition of specific border zones in a particular patient. van der Zwan et al (24 –26) studied the vascular territories in 25 unfixed human brains obtained at postmortem examination. After ligation of the posterior medial choroidal and anterior choroidal arteries, these authors individually cannulated the two ACAs distal to the anterior communicating artery, the two PCAs distal to the posterior communicating artery, and the two MCAs. They then simultaneously infused colored medium into all arteries, taking care to maintain the perfusion pressure in each vessel at a constant 93 mm Hg until the distributions of the perfusate stabilized at welldefined borders. They designated these borders the “equal pressure boundaries.” van der Zwan et al (24 –26) found that the locations of the equal pressure boundaries (EPBs) varied greatly in both the superficial and the deep arterial distributions of each vessel. Not one brain showed a symmetric pattern of intracerebral perfusion. So many variations existed that the authors simply described the results in terms of minimal and maximal regions of distribution for each vessel. Notably, the MCA territory on the convexity extended far upward to reach the interhemispheric fissure, separating the convexity distributions of the ACA from those of the PCA in 26% of cases (25). The EPB for the ACA and PCA lay anywhere along the length of the brain from the superior frontal gyrus to the occipital lobe on both the superior and the medial surfaces of the hemisphere (25). The EPB for the ACA and MCA varied equally widely over the convexity from the superior frontal gyrus to the inferior frontal sulcus, while the EPB for the PCA and MCA varied anywhere from the superior temporal sulcus on the convexity to the occipitotemporal sulcus on the inferior surface of the brain (25). Thus, the superior portion of the precentral gyrus (Brodmann area 4) was supplied by the ACA in 94% of cases, the MCA in 4% of cases, and the PCA in 2% of cases. Furthermore, the superior portion of the postcentral gyrus (Brodmann areas 1–3 and 5) was supplied by the ACA in 78% of cases, the MCA in 6% of cases, and the PCA in 16% of cases (25). Curiously, these authors did not address the problem of bihemispheric ACA supply. They acknowledged that ligation of the choroidal vessels led to distortion of the EPB along the hippocampus but did not elaborate on any possible distortion introduced into the other territories assessed (25). Because of the great variability outlined above, it is now common to describe cortical infarctions as “territorial” if they fall completely within the maximum possible “van der Zwan territory” of a cerebral artery, and as potentially “border zone” if the infarction falls outside these maxima. Reassessment of Stroke Mechanism in Border-Zone Infarctions Another difficulty encountered by the radiologist assessing imaging studies in a patient suspected of having a borderzone infarction is that the mechanism of infarction in border-zone infarcts is less clear than was previously thought. Border-zone (watershed) infarctions were originally conceived of as the common consequence of severe large vessel disease complicated by acute (or acute plus later sustained) hypotension (29 –31). However, border-zone infarctions are uncommon in unselected patients with acute stroke and do not occur more often in patients with hemodynamic compromise than in those with cardiac sources of stroke (36). No evidence has been found for a selective increase in cerebral oxygen extraction fraction in patients with carotid artery occlusion (37,38). In one study of 110 patients with carotid artery occlusion (39), no statistically significant difference was found in the incidence of cortical border-zone infarctions between patients with and those without evidence of hemodynamic compromise (measured as increased oxygen extraction fraction at positron emission tomography). Cortical border-zone infarcts have been noted as often in patients with cardiac sources of embolism (3.2%) as in those with severe carotid artery obstruction (⬎70% stenosis or occlusion) (3.6%) (36). Moreover, border-zone infarctions are rarely (5.2%) the initial manifestation of carotid occlusion, as might be expected for acute lowflow states (40). Instead, border-zone infarction accounts for 72% of delayed strokes in patients with occluded internal Provenzale et al Radiology carotid arteries (40). Thus, the specific hemodynamic basis of border-zone infarctions is not established. Considerations of the causes of borderzone infarction now emphasize the known phenomenon of “directed embolization” (41– 44). The majority of cerebral artery bifurcations are of unequal size, with the smaller vessel exiting at an acute angle to the larger parent vessel. As a consequence, particulate matter may be directed with the flow toward the end territories of the major vessels at the border zone (44). It has been shown (41) that experimental embolic infarctions involved the ACA in 8% of trials if only one embolus was released into the circulation but involved the ACA in 50% of trials if several emboli were released (in which case the first embolus never lodged in the ACA). Therefore, an initial occlusion of MCA branches may redirect flow (and flow-directed emboli) into the ACA (41). These observations have subsequently been confirmed and the concept extended to the entire cerebrovascular tree (42,43). Cerebral watershed infarction as a result of emboli has been documented in patients at postmortem examination, resulting in the conclusion that the infarctions were caused by directed embolization (44). These data indicate the high likelihood that border-zone infarctions result from directed cardiac or arterial emboli. Hennerici et al concluded that “the cortical wedge-type of borderzone infarction, said to result from hemodynamic compromise in low-flow perfusion territories, is an ambiguous observation and may be seen in patients with cerebral embolism and hemodynamic compromise due to severe carotid disease” (36). Microemboli in relation to border-zone infarctions are now well recognized (29,45). Recently, attempts have been made to synthesize these concepts by suggesting that reduced cerebral perfusion limits the ability of blood flow to clear emboli from the vessels, allowing them to pass to the border zones (46). Emerging Concepts The vascular resistance of arteries in the white matter has been shown to be about 3.8 times the vascular resistance in the gray matter (26). Allowing for that difference, the caliber (diameter) of the parent cerebral artery correlates well (r ⫽ 0.73) with the volume of the gray and white matter irrigated by each vessel (26). The mean diameters of the postcommunicating ACA (2.24 mm), the postcommunicating PCA (2.04 mm), and the Volume 229 䡠 Number 2 MCA (2.70 mm) lie in direct relative proportion as the supplied territory (26). Measurement of the diameters of these vessels in healthy individuals would provide one measure of the anatomic variations present in each hemisphere and perhaps serve as a guide to later analysis of any strokes. Deep border-zone infarctions appear to correlate well with hemodynamic compromise (47). It has been shown that in a group of patients with internal carotid occlusion, a “rosary” pattern of deep white matter infarcts was present only in patients with increased cerebral oxygen extraction fraction in the involved hemisphere (sensitivity, 22%; specificity, 100%) (39). This pattern was described as consisting of three or more lesions 3 mm in diameter or larger arranged in a linear fashion parallel to the lateral ventricle in the centrum semiovale or corona radiata. Results of other studies (48 –51) also support the validity of deep watershed infarctions being related to hemodynamic compromise. Thus, at least one imaging sign appears to correlate with hemodynamic compromise. Analysis of Infarct Frequency Distribution Another approach to infarct analysis is to determine the frequency with which infarctions affect each of a large number of defined anatomic zones. Specific analysis of the distribution of any infarct may then be compared with the zonal frequency distributions of diverse infarcts as a guide to the nature of the lesion. Application of such analysis to 30 border-zone infarctions studied among 150 supraventricular infarctions showed that parasagittal border-zone cortical infarctions exhibit a bimodal frontal and parietal distribution (52). The anterior corticalsubcortical border zone (ACA/MCA) infarcts show peak frequency at the junction of the superior frontal sulcus and precentral sulcus and involve the adjacent portions of the superior frontal gyrus, middle frontal gyrus, precentral gyrus, and paracentral lobule. The posterior cortical-subcortical (distal ACA/MCA or PCA/MCA) border-zone infarctions most frequently involve the superior parietal lobule and the precuneus. As a consequence, detection of small foci of abnormal signal intensity specifically at these two sites should increase the index of suspicion for a possible source of emboli or other risk factors for stoke. However, until the individual vascular territories can be displayed easily in vivo at the time of stroke assessment, imaging display of infarct topography can provide only inferences with regard to the specific arteries affected and the mechanisms involved. THROMBOLYTIC THERAPY The majority of ischemic strokes are due to thromboembolic arterial occlusions (53,54). Angiographic studies obtained in stroke patients within 8 hours of symptom onset show arterial occlusions corresponding to the symptoms in more than 80% of cases (6,55). An acute arterial occlusion rapidly produces a core of infarcted brain tissue surrounded by hypoxic but potentially salvageable tissue— in other words, the ischemic penumbra (56 –59). The important role of thrombosis in stroke combined with the success of thrombolysis in acute myocardial infarction has generated great interest in cerebral fibrinolysis. The goal of thrombolytic therapy is rapid restoration of blood flow and preservation of the ischemic penumbra. The U.S. Food and Drug Administration has approved intravenous recombinant tPA for the treatment of acute ischemic stroke within 3 hours of symptom onset (2). In addition, intraarterial thrombolysis has been shown to improve neurologic outcome in patients with acute ischemic stroke (6). The following sections highlight the results of various clinical trials. Intravenous Thrombolysis Two thrombolytic agents have been utilized in trials of acute stroke treatment with intravenous medication: streptokinase and tPA (1,2,60 – 64). Intravenous streptokinase.—Three trials of intravenous streptokinase for treatment of acute stroke have been reported: the Multicenter Acute Stroke Trial–Europe, the Multicenter Acute Stroke Trial– Italy, and the Australian Streptokinase Trial (60 – 62). The dose of streptokinase given in these trials was the same as that given in the acute myocardial infarction trials, namely, 1.5 million IU. Treatment was initiated within 4 hours in the Australian trial and within 6 hours in the other two trials (60 – 62). All of the streptokinase trials were halted prematurely due to poor outcome or an excess rate of mortality in the treated group, making it unlikely that streptokinase will be used in subsequent randomized trials for acute ischemic stroke. Several reasons can be cited for the failure of streptokinase in these trials. First, Assessment of the Patient with Hyperacute Stroke 䡠 353 Radiology the dose of streptokinase was likely high because it was equivalent to the dose used in the coronary thrombolysis trials. A lower dose might have been effective but with lower mortality. For instance, approximately two-thirds of the cardiac dose was used in the successful NINDS intravenous tPA trial (2). Second, in all three trials the patients reporting negative results were treated up to 4 hours and 6 hours after onset of symptoms. The investigators in the Australian trial (62) suggested that streptokinase may be effective if given within 3 hours of symptom onset. However, the number of patients treated within this time was not large enough to show statistical significance. The third reason for failure of streptokinase may have been the use of antiplatelet and antithrombotic agents within the first 24 hours after treatment, resulting in a higher risk of hemorrhage. In the NINDS tPA trial, use of antiplatelet and antithrombotic agents was avoided in the first 24 hours after administration of tPA, perhaps leading to safer use of the thrombolytic drug. On the basis of results from the abovementioned studies, the American Academy of Neurology recommendation regarding streptokinase in treatment of acute stroke (65), published in 1996, states that outside the setting of a clinical trial, streptokinase administration is not indicated for the management of acute ischemic stroke. Intravenous tPA.—Four phase 3 trials of intravenous tPA for acute ischemic stroke have been published (1,2,63,64). Food and Drug Administration approval of tPA was based on data from the NINDS tPA trial (2). The study was composed of two clinical trials, part I and part II, with both trials conducted in identical fashion by using the same inclusion and exclusion criteria (2). Altogether, 624 patients were treated within 3 hours of symptom onset with a dose of 0.9 mg of tPA per kilogram of body weight and a maximum dose of 90 mg. In part I of the study, early outcome was evaluated. The primary hypothesis tested in part I was that at 24 hours, a greater proportion of patients treated with tPA would improve by 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), as compared with scores of patients receiving a placebo. Indeed, 47% (67 of 144) of patients treated with tPA improved by 4 or more points on the NIHSS, compared with 39% (57 of 147) in the placebo group (P ⫽ .21). In part II of the study, the long-term functional outcome of patients at 3 354 䡠 Radiology 䡠 November 2003 months was evaluated. The primary hypothesis tested in part II was that there would be a significant difference between the tPA group and the placebo group in terms of the proportion of patients with minimal or no deficit. Minimal or no deficit was defined according to one of the following four outcome scales: NIHSS score of 0 or 1, Barthel Index greater than 95, modified Rankin Scale score of 0 or 1, and Glasgow Outcome Scale score of 1. For all four outcome measures, the tPA treated group fared better than the placebo group. The tPA patients were 32% (NIHSS), 38% (Barthel Index), 50% (modified Rankin Scale), and 55% (Glasgow Outcome Scale) more likely to have a good outcome as defined above. The absolute percentage difference was 11%– 13%, depending on the outcome measure being used—that is, for every 100 patients treated with tPA, there would be an additional 11–13 patients with minimal or no deficit, compared with 100 patients not treated with tPA. The NINDS investigators have also reported follow-up in these patients at 12 months (66,67). The patients treated with tPA were at least 30% more likely to have minimal or no deficit at 1-year follow-up. There was no significant difference in mortality at 12 months between the two groups (24% vs 28%, P ⫽ .29). The results indicate a sustained benefit of tPA at 12 months. Overall, 6% of the patients receiving tPA had symptomatic intracranial hemorrhage, compared with 0.6% in the placebo group. Despite the higher rate of symptomatic intracranial hemorrhage in the tPA group, there was no significant difference in mortality between the two groups. Several explanations have been proposed to account for these apparently contradictory findings (68). On the one hand, tPA may have decreased mortality by reducing the size of infarction and, hence, reducing the likelihood of death. Alternatively, the patients with symptomatic intracranial hemorrhage had large infarction that, even without hemorrhage, would have had high associated mortality. In other words, in the tPA group, patients with large infarctions died with hemorrhagic transformation, while in the placebo group these same patients died but without hemorrhagic transformation. This would result in a higher rate of symptomatic intracranial hemorrhage in the tPA group but no significant difference in mortality between the two groups. The NINDS investigators sought to identify variables associated with hemor- rhage in patients who received tPA (5). The only variables independently associated with a risk of symptomatic intracranial hemorrhage were severity of neurologic deficit as measured with the NIHSS and presence of brain edema or mass effect on CT images obtained prior to treatment. Despite this fact, however, patients with a severe neurologic deficit were more likely to have a favorable outcome if treated with tPA than were those who received the placebo. The results were similar in the analysis of patients with edema or mass effect seen on pretreatment CT images. The investigators thus concluded that despite a higher rate of symptomatic intracranial hemorrhage, patients with severe stroke or edema or mass effect on pretreatment CT images are reasonable candidates for tPA if it is administered within 3 hours of symptom onset (5). The NINDS investigators also performed a subgroup analysis to identify stroke patients in whom treatment with tPA was particularly hazardous or efficacious (69). They concluded that no pretreatment information significantly affected outcome. Three additional phase 3 trials with intravenous tPA have been reported: ECASS, ECASS II, and Alteplase Thrombolysis for Acute Non-interventional Therapy in Ischemic Stroke (ATLANTIS) (1,63,64). All had negative results. The ECASS was a prospective, multicenter, double-blind, placebo-controlled study and differed from the NINDS study in several important aspects (1). First, the dose of tPA in ECASS was 1.1 mg/kg with a maximum dose of 100 mg; the NINDS study used a dose of 0.9 mg/kg and a maximum dose of 90 mg. Second, patients were treated up to 6 hours after onset of symptoms. Third, patients with a major early sign of ischemia on CT images (defined as hypoattenuation involving more than one-third of the MCA territory) were excluded. The primary treatment end point included Barthel Index and modified Rankin Scale scoring at 90 days. In the intent-to-treat analysis, no significant difference in either of the two primary end points was seen between the two groups of patients, which was thought to be due to the fact that there were protocol violations in a substantial number of patients included in the analysis. To further address this issue, an analysis of the target population was also performed by excluding 109 patients that were included in the trial but were later found to have been associated with protocol violations. Analysis of the target population revealed no significant differProvenzale et al Radiology ence in Barthel Index scores between the two groups but a significant difference in the modified Rankin Scale score in favor of the tPA treated patients. Sixty-six of the excluded 109 patients were excluded because of abnormalities on CT images (52 had major early infarction signs, two had primary hemorrhage, 12 had unavailable or uninterpretable CT images). The investigators concluded that tPA is beneficial in improving some functional outcomes in a subgroup of patients with moderate to severe neurologic deficits and without extended signs of ischemia on initial CT image. Identification of this subgroup of patients is difficult, however, and largely depends on identification of early signs of infarct on CT images. Furthermore, treatment of ineligible patients is associated with an unacceptable risk of hemorrhage and death. Therefore, the investigators concluded that intravenous tPA administration within 6 hours of symptom onset could not be recommended. In the ECASS, no significant difference in the frequency of parenchymal hemorrhage in general was found between the tPA and placebo groups in either the intent-to-treat or the target population analyses. However, large parenchymal hemorrhages were more frequent in the tPAtreated group. For that reason, the ECASS II trial was designed with a lower dose of intravenous tPA (0.9 mg/kg, chosen to match NINDS criteria) given within 6 hours of symptom onset. The investigators found no significant difference in the primary end point (modified Rankin Scale score at 90 days) between the tPA and placebo groups. The results did not confirm a significant benefit for tPA. The approved use of tPA remained restricted to patients presenting within 3 hours of symptom onset. However, this severely restricted the use of the drug, as evidenced by data showing that, since approval of the drug, fewer than 5% of all stroke patients were receiving tPA (64,70,71). Therefore, the ATLANTIS trial set out to assess the safety and efficacy of tPA (0.9 mg/kg, with maximum dose of 90 mg as in NINDS trial) in patients 3–5 hours after symptom onset. This trial was a phase 3, placebo-controlled, doubleblind, randomized study. The primary end point was an excellent neurologic recovery at day 90 (NIHSS score ⱕ 1). In the target population, 32% of the patients in the placebo group and 34% of the patients in the tPA group had an excellent recovery at 90 days (P ⫽ .65). There was a significant increase in the occurrence of symptomatic intracranial Volume 229 䡠 Number 2 hemorrhage in the tPA group (1.1% vs 7.0%; P ⬍ .001). Therefore, this study did not show a significant benefit of tPA in patients treated 3–5 hours after ictus. On the basis of these results, the recommendation of the American Academy of Neurology is that tPA administered intravenously within 3 hours of symptom onset is indicated in patients meeting the inclusion and exclusion criteria as set forth on the basis of the NINDS tPA trial data (65). However, intravenous administration of tPA more than 3 hours after stroke is not recommended. Despite the approval of tPA for treatment of acute ischemic stroke, outlined above, there has been much trepidation about its use, and several criticisms have been advanced. It has been pointed out that many patients with symptoms of acute ischemic stroke may not have occlusive thromboemboli (72). The evaluation of patients with stroke is typically performed rapidly, with no pathophysiologic assessment to document the presence of an occlusive clot. It has been argued, then, that many patients will receive a potentially dangerous drug although they do not have the problem for which the drug is intended. Additionally, concern has been raised about whether use of tPA would be less efficacious in general community practice, as compared with use in the idealized tertiarycare university hospital conditions under which the trial was carried out. The Standard Treatment with Alteplase to Reverse Stroke, or STARS, study was designed to address concerns over the use of tPA in the community setting (73). In this prospective multicenter study, the results of intravenous tPA treatment of patients with acute ischemic stroke in 57 medical centers (24 academic, 33 community) in the United States were reported. The results confirmed the beneficial effects of tPA administered within 3 hours of symptom onset, with findings similar to those obtained in the NINDS trial. However, conflicting data were found in another study in which results were reported for stroke patients treated with intravenous tPA in essentially all the hospitals in Cleveland, Ohio (74). The results of that study showed a significantly higher rate of symptomatic intracranial hemorrhage and mortality in patients receiving the drug. However, a large percentage of patients in the study had deviations from national treatment guidelines. As in other studies, evidence of a learning curve for use of tPA, which was reflected in a decrease in the occurrence of guideline deviations and an in- crease in the rate of intravenous tPA use over time, was seen in the Cleveland experience (74). Intraarterial Thrombolysis In order for thrombolytic drugs to induce lysis of acute thromboemboli, a therapeutic dose of the drug must reach the target. However, if major arteries are blocked, intravenous administration may result in insufficient drug delivery. Therefore, much interest has developed in intraarterial delivery of thrombolytic agents. Compared with intravenous therapy, localized intraarterial thrombolysis has the theoretical advantage of achieving faster and more complete recanalization with use of a lower dose. Because the agent is administered during cerebral angiography, clot lysis can be directly assessed, allowing drug infusion to be stopped when clot lysis is achieved (Fig 6). This feature allows an optimal amount to drug to be administered and may diminish the risk of adverse effects. In addition, intraarterial treatment can be initiated up to 6 hours after symptom onset, and the therapeutic window is wider than for that for intravenous therapy. The first report of intraarterial thrombolysis, in which five patients with vertebrobasilar occlusion were treated, was published in 1983 (75). Three patients had successful recanalization, and all three had subsequent neurologic improvement. One year later, the same investigators reported treating two patients with distal internal carotid artery occlusions by using urokinase (76). Both patients showed clinical improvement. Since then, a large number of case series have been published (77–90). Neurologic improvement has been reported to be variable in these studies: Minimal or no neurologic deficit was reported in 15%– 75% of patients. Differences in multiple factors across studies likely contributed to this wide variation in outcome, including differences in (a) grading system used for assessment of outcome, (b) dose of thrombolytic agent used, (c) baseline patient demographics (eg, age and baseline neurologic status), and (d) sites of arterial occlusion. Complete recanalization was seen, on the average, in approximately 40% of patients, and partial recanalization was seen in 35% (77–90). These rates of recanalization are higher than those reported for intravenous thrombolysis (96 –98). Thus far, however, no randomized trials have been performed to compare intraarterial and intravenous thrombolysis. Assessment of the Patient with Hyperacute Stroke 䡠 355 Radiology Figure 6. Reversal of diffusion abnormalities after intraarterial thrombolysis in a 75-year-old woman with acute onset of left hemiplegia. (a) Right common carotid angiogram, anteroposterior view, obtained before therapy shows occlusion of proximal portion of right MCA. (b) Right common carotid angiogram, anteroposterior view, obtained after intraarterial thrombolysis with 20 mg of tPA infused during 2 hours shows complete recanalization of the artery. (c) Top left: Diffusion-weighted MR image (DWI; b ⫽ 1,000 mm/sec2) obtained before therapy shows high signal intensity consistent with restricted diffusion, indicative of ischemia. Top right: Apparent diffusion coefficient (ADC) map obtained before therapy confirms that restricted diffusion is present within area of high signal intensity on diffusion-weighted image. Bottom left: Diffusion-weighted image obtained within a few hours after treatment shows reversal of abnormal signal intensity seen on pretreatment diffusion-weighted image. Bottom right: Apparent diffusion coefficient map obtained after therapy shows that area of restricted diffusion has resolved. In this patient, it was thought that ischemic penumbra included the region of restricted diffusion; therefore, thrombolysis and resumption of normal flow allowed rescue of penumbral tissue. As with intravenous thrombolysis, only results from randomized trials can be used to answer questions regarding the safety and efficacy of intraarterial therapy. Two such randomized trials have been performed for intraarterial thrombolysis: Prolyse in Acute Cerebral Thromboembolism Trial (PROACT) and PROACT II (6,56). The larger of the two trials, PROACT II, included patients treated within 6 hours of symptom onset who had angiographically demonstrated occlusion of the MCA (M1 or M2 occlusion) (6). The primary outcome of the trial was the ability to live independently at 3 months after stroke. Of the 474 patients who underwent angiography, 180 were enrolled, with 121 receiving intraarterial prourokinase and low-dose intravenous heparin and 59 receiving low-dose intravenous heparin alone. At 2 hours, 67% of patients receiving prourokinase had complete or partial recanalization, compared with 18% in the heparin-only group (P ⬍ .001). The primary outcome of the study was attained by 40% of the patients treated with prourokinase, compared with 25% in the heparin-only group (P ⫽ .04). However, symptomatic intracranial hemorrhage was seen in 10% of patients undergoing thrombolysis, compared with 2% in the heparin-only group (P ⫽ .06). Nonetheless, the authors of the study determined that intraarterial thrombolysis within 6 hours of symptom onset was 356 䡠 Radiology 䡠 November 2003 shown to have a benefit in patients with MCA occlusion. The U.S. Food an Drug Administration did not approve prourokinase after the PROACT II study and instead asked that an additional study be performed, requiring two positive phase 3 trials prior to approval of the drug. Ab- bott Laboratories is currently considering reopening the PROACT trial. Intraarterial Thrombolysis Trials in Posterior Circulation A stroke in the posterior circulation stroke differs in several respects from Provenzale et al Radiology ischemic stroke in the anterior circulation. First, clinical outcome in patients with vertebrobasilar occlusion is less favorable; death occurs in the majority of patients, and severe deficit occurs in most survivors (83,94,95). In addition, patients with posterior circulation ischemic events often have coexistent severe intracranial large-artery atherosclerotic disease (96). This feature, which presents a unique problem because of the high risk of rethrombosis after treatment, is uncommon in anterior circulation ischemic events (75,83,87). Study results have suggested that thrombolysis beyond 6 hours in the anterior circulation can be associated with high rates of hemorrhagic transformation and poor outcome (102). No conclusive data exist to indicate increased risk beyond 6 hours in posterior circulation strokes; however, to our knowledge, no randomized trials of intraarterial thrombolysis in patients with vertebrobasilar stroke have been performed. In addition, although thrombolysis has been attempted up to 24 hours after symptom onset in patients with posterior circulation ischemic events, the issue of how long a delay after symptom onset can be tolerated before the start of treatment has not been specifically examined (75,77,87). In one pilot study in which the safety and efficacy of intraarterial urokinase were evaluated in patients with severe brainstem stroke and vertebrobasilar occlusion, 16 patients with vertebrobasilar occlusion were treated within 24 hours of symptom onset (98). Incremental doses of urokinase were administered until clot lysis was achieved or a maximal dose of 1 million U was given. Complete or partial recanalization was initially achieved in 13 of 16 (81%) patients. Of these, reocclusion occurred within 24 hours in two, giving a final recanalization rate of 69%. The 6-month functional status was assessed with the Barthel Index, with a good outcome defined as a score 60 or greater. Eleven (69%) patients survived, nine (56%) with a good outcome and two (12%) with severe deficit. Recanalization correlated with survival (P ⫽ .02), but the time between symptom onset and thrombolysis was not predictive of outcome. The authors concluded that intraarterial thrombolysis in the posterior circulation is safe, feasible, and capable of achieving recanalization in the majority of patients. Combined Intravenous and Intraarterial Treatment One major disadvantage of intraarterial thrombolysis is treatment delay due Volume 229 䡠 Number 2 to the need to assemble the neurointerventional team and prepare the angiography suite. A combined approach of intravenous and intraarterial thrombolysis allows the advantages of both treatment strategies. Intravenous therapy is initiated without delay but at a lower dose than is used in standard intravenous therapy. This technique allows some tPA to be given intraarterially, which provides a higher recanalization rate than can potentially be achieved than with intravenous treatment alone. The Emergency Management of Stroke study is the only reported trial of combined intravenous and intraarterial thrombolysis (99). This study was a double-blind randomized trial with a total of 35 patients enrolled within 3 hours of symptom onset. Seventeen patients were randomized to receive intravenous tPA at a dose of 0.6 mg/kg (maximum dose, 60 mg), and 18 patients in the second arm of the study received an intravenous placebo. These patients then underwent immediate angiography. If no clot was visualized, angiography was stopped. If a clot was visualized, intraarterial treatment with tPA was initiated with 20 mg of the drug given over 2 hours or until recanalization was achieved. The study showed higher recanalization rates in the combined intravenous-intraarterial group (55% full recanalization) than in the intravenous-only group (10% full recanalization). The numbers of patients with symptomatic hemorrhage in the two groups were similar, and no differences in 7-day or 3-month outcomes were seen, although more deaths were recorded in the combined intravenous-intraarterial group. The investigators concluded that the combined approach was feasible, reasonably safe, and worthy of further study. Currently, the combined intravenousintraarterial strategy is being evaluated in a larger pilot trial, the Interventional Management of Stroke trial, which is supported by the National Institutes of Health. The trial involves 14 centers in North America and will include 80 patients. Patients with an NIHSS score of greater than 10 within 3 hours of symptom onset will be offered treatment with intravenous tPA at 0.6 mg/kg, followed by angiography. If an arterial occlusive lesion persists, up to 22 mg tPA will be injected intraarterially during 2 hours. Outcomes will be compared with those of the placebo group of the NINDS trial. Mechanical Therapies The experience of interventional cardiologists in treating acute myocardial infarction may predict the future of interventional neuroradiologists in treating ischemic stroke. Randomized studies in the setting of acute myocardial infarction have shown the superiority of mechanical strategies such as angioplasty and mechanical clot lysis in improving outcome, compared with outcomes associated with thrombolytic agents alone (100). The use of angioplasty in reopening cerebral vessels in patients with ischemic symptoms has been reported (101). A potential disadvantage of this strategy is the possibility of forcing the clot into the deep penetrating arteries, with worsening ischemia and the potential risk of arterial rupture. The Angiojet catheter (Possis Medical, Minneapolis, Minn), which is able to fragment and vacuum extract clot, has also been used to treat patients with acute cerebral ischemia (102). Methods of delivery of energy to fragment a clot, including the use of ultrasound and laser devices, are also being developed. However, a current limitation of mechanical devices is the relatively larger catheter size and limited flexibility, which impede access to the tortuous vessels of the intracranial circulation. CONCLUSION In the past decade, substantial improvements have been made in imaging of hyperacute cerebral infarction. Nonetheless, the simplest and most available technique—namely, CT—remains the imaging method that is most relied on for evaluation of hyperacute stroke patients. The NINDS tPA trial has shown the safety and efficacy of intravenous tPA in treatment within 3 hours of symptom onset in patients with ischemic stroke. The effectiveness and safety of intravenous tPA beyond 3 hours is yet to be shown. Intraarterial delivery of tPA appears to lyse clots more effectively than does intravenous delivery and has extended the time window for intervention to 6 hours. Nonetheless, intraarterial delivery takes longer, and, thus far, no direct comparison of the two methods of treatment has been reported. The approach of combined intraarterial and intravenous thrombolysis has the theoretical advantage of combining the benefits of both methods; the results of the Interventional Management of Stroke trial are eagerly awaited. Mechanical approaches to ischemic stroke may eliminate the Assessment of the Patient with Hyperacute Stroke 䡠 357 Radiology need for thrombolytic drugs that increase the risk of reperfusion hemorrhage. However, further advances in catheter technology are required to meet the special requirements of clot lysis in the cerebral circulation. The future treatment of acute ischemic stroke will likely include a combination of mechanical strategies and thrombolytic agents that minimize the risk of intracranial hemorrhage and maximize recanalization rates. 16. 17. 18. References 1. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274:1017–1025. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581– 1587. Gacs G, Fox AJ, Barnett HJM, Vinuela F. CT visualization of intracranial arterial thromboembolism. Stroke 1983; 14:756 –762. Von Kumer R, Allen KL, Holle R, et al. Acute stroke usefulness of early CT findings before thrombolytic therapy. Radiology 1997; 205: 327–333. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke: the NINDS t-PA Stroke Study Group. Stroke 1997; 28: 2109 –2118. Furlan AJ, Higashida R, Weschler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study—a randomized controlled trial. JAMA 1999; 282: 2003–2011. Marks MP, Holmgren EB, Fox AJ, Patel S, von Kummar R, Froehlich J. Evaluation of early computed tomographic findings in acute ischemic stroke. Stroke 1999; 30:389 – 392. Lev MH, Farkas J, Gemmete JJ, et al. Acute stroke: improved nonenhanced CT detection— benefits of soft-copy interpretation by using variable window width and center level settings. Radiology 1999; 213:150 – 155. Truwit CL, Barkovich AJ, Gean-Marton A, Hibri, Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology 1990; 176: 801–806. Tomsick TA, Brott TG, Chambers AA, et al. Hyperdense middle cerebral artery sign on CT: efficacy in detecting middle cerebral artery thrombosis. AJNR Am J Neuroradiol 1990; 11:473–477. Elster AD, Moody DM. Early cerebral infarction:gadopentetate dimeglumine enhancement. Radiology 1990; 177:627–632. Provenzale JM, Sorensen AG. Diffusionweighted MR imaging in acute stroke: theoretical considerations and clinical applications. AJR Am J Roentgenol 1999; 173:1459 – 1468. Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/ perfusion magnetic resonance imaging. Ann Neurol 2000; 47:462–469. Petrella JR, Provenzale JM. MR perfusion imaging of the brain: techniques and applications. AJR Am J Roentgenol 2000; 175: 207–220. Sorensen AG, Copen WA, Ostergaard L, et 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 358 䡠 Radiology 䡠 November 2003 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 1999; 210:519 – 527. Ostergaard L, Sorensen AG, Chesler DA, et al. Combined diffusion-weighted and perfusion-weighted flow heterogeneity magnetic resonance imaging in acute stroke. Stroke 2000; 31:1097–1103. Hudetz AG, Feher G, Kampine JP. Heterogeneous autoregulation of cerebrocortical capillary flow: evidence for functional thoroughfare channels? Microvasc Res 1996; 51:131– 136. Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke 1981; 12:723–725. Eastwood JD, Lev MH, Azhari T, et al. CT perfusion imaging with deconvolution analysis:A pilot study of patients with acute middle cerebral artery stroke. Radiology 2002; 222:227–237. Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 2001; 32:2021–2028. Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab 2000; 20:1276 –1293. Schneider M. Durchblutung und sauerstoffversorgung des gehirns. Verh Dtsch Tes Kreislaufforsch 1953; 19:3–12. Zulch KJ. The cerebral infarct: pathology, pathogenesis, and computed tomography. Berlin,Germany:Springer-Verlag,1985;123– 145. van der Zwan A, Hillen B. Review of the variability of the territories of the major cerebral arteries. Stroke 1991; 22:1078 – 1084. van der Zwan A, Hillen B, Tulleken CAF, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg 1992; 77:927–940. van der Zwan A, Hillen B, Tulleken CAF, Dujovny M. A quantitative investigation of the variability of major cerebral arterial territories. Stroke 1993; 24:1951–1959. Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: analysis of 1, 000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092. Bories J, Derhy S, Chiras J. Second part: roles of scanning; CT, PET and MRI—CT in hemispheric ischaemic attacks. In Bories J, ed. Cerebral ischaemia: a neuroradiological study. Berlin, Germany: Springer-Verlag, 1985; 18 –33. Adams JH, Brierley JB, Connor RCJ, Treip C. The effects of systemic hypotension upon the human brain: clinical and neuropathologic observations in 11 cases. Brain 1966; 89:235–268. Brierley JB, Excell BJ. The effects of profound systemic hypotension upon the brain of M. rhesus: physiological and pathological observations. Brain 1966; 89: 269 –298. Torvik A. The pathogenesis of watershed infarcts in the brain (editorial). Stroke 1984; 15:221–223. Romanul FCA, Abramowicz A. Changes in brain and pial vessels in arterial borer zones. Arch Neurol 1964; 11:40 –65. Baptista AG. Studies on the arteries of the brain. II. the anterior cerebral artery: some anatomic features and their clinical implications. Neurology 1963; 13:825–835. Moniz E. Die cerebral arteriographie und 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. phlebographie. Berlin, Germany: SpringerVerlag, 1949; 413. Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg 1978; 49:204 –228. Hennerici M, Daffertschofer M, Jakobs L. Failure to identify cerebral infarct mechanisms from topography of vascular territory lesions. AJNR Am J Neuroradiol 1998; 19: 1067–1074. Carpenter DA, Grubb RL Jr, Powers WJ. Borderzone hemodynamics in cerebrovascular disease. Neurology 1990; 40:1587–1592. Derdeyn CP, Simmons NR, Videen TO, et al. Absence of selective deep white matter ischemia in chronic carotid disease:a positron emission tomographic study of regional oxygen extraction. AJNR Am J Neuroradiol 2000; 21:631–638. Derdeyn CP, Khosla A, Videen TO, et al. Severe hemodynamic impairment and border zone–region infarction. Radiology 2001; 220:195–201. Bogousslavsky J, Regli F. Borderzone infarctionsdistaltointernalcarotidarteryocclusion: prognostic implications. Ann Neurol 1986; 20:346 –350. Einsiedel-Lechtape H. Neuroradiology of vascular and atrophic lesions in the geriatric age: secondary emboli—a frequent sequela of complete extracranial internal carotid artery occlusion. Neuroradiology 1978; 16:96 –100. Gacs GY, Merei FT, Bodosi M. Balloon catheter as a model of cerebral emboli in humans. Stroke 1982; 13:39 –42. Gacs GY, Fox AJ, Barnett HJM, Vinuela F. Occurrence and mechanisms of occlusion of the anterior cerebral artery. Stroke 1983; 14:952–959. Pollanen MS, Deck JHN. Directed embolization is an alternate cause of cerebral watershed infarction. Arch Pathol Lab Med 1989; 113:1139 –1141. Torvik A, Skullerud K. Watershed infarcts in the brain caused by micro emboli. Clin Neuropathol 1982; 1:99 –105. Caplan LR, Hennerici M. Hypothesis: impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke? Arch Neurol 1998; 55:1475–1482. Waterston JA, Brown MM, Butler P, Swash M. Small deep cerebral infarcts associated with occlusive internal carotid artery disease: a hemodynamic phenomenon? Arch Neurol 1990; 47:953–957. Yamauchi H, Fukuyama H, Yamaguchi S, Miyoshi T, Kimura J, Konishi J. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol 1991; 48:1067–1071. Isaka Y, Nagano K, Narita M, Ashida K, Iamizumi M. High signal intensity on T2weighted magnetic resonance imaging and cerebral hemodynamic reserve in carotid occlusive disease. Stroke 1997; 28:354 –357. Mull M, Schwarz M, Thron A. Cerebral hemispheric low-flow infarcts in arterial occlusive disease. Lesion patterns and angiomorphological conditions. Stroke 1997; 28: 118 –123. Krapf H, Widder B, Skalej M. Small rosarylike infarctions in the centrum ovale suggest hemodynamic failure. AJNR Am J Neuroradiol 1998; 19:1479 –1484. Naidich TP, Firestone MI, Blum JT, Abrams KJ. Vascular territories and watersheds:a zonal frequency analysis of the gyral-sulcal extent of cerebral infarctions. III. MCA and watershed infarctions. Neuroradiology (in press). Provenzale et al 53. Radiology 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. Feussner JR, Matchar DB. When and how to study the carotids. Ann Intern Med 1988; 109:805–818. Zeumer H, Freitag HJ, Knospe V. Intravascular thrombolysis in central nervous system cerebrovascular disease. Neuroimaging Clin N Am 1992; 2:359 –369. del Zoppo GJ, Poeck K, Pessin M, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992; 32:78 –86. del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT:a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998; 29:4 –11. Baron J. Mapping the ischemic penumbra with PET: implications for acute stroke treatment. Cerebrovascular Diseases 1999; 9:193–201. Heiss WD, Thiel A, Grond M, Graf R. Which targets are relevant for therapy of acute ischemic stroke? Stroke 1999; 30:1486 – 1489. Nagesh V, Welch KM, Windham JP, et al. Time course of ADCw changes in ischemic stroke: beyond the human eye! Stroke 1998; 29:1778 –1782. Thrombolytic therapy with streptokinase in acute ischemic stroke: the Multicenter Acute Stroke Trial–Europe Study Group. N Engl J Med 1996; 335:145–150. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Multicentre Acute Stroke Trial–Italy (MAST-I) Group. Lancet 1995; 346:1509 –1514. Donnan GA, Davis SM, Chambers BR, et al. Streptokinase for acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996; 276:961–966. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset: The ATLANTIS study—a randomized controlled trial. JAMA 1999; 282:2019 –2026. Practice advisory: thrombolytic therapy for acute ischemic stroke–summary statement—report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1996; 47:835–839. Kwiatkowski TG, Libman R, Frankel M, et al. The NINDS rt-PA Stroke Study: sustained benefit at 1 year. Stroke 1998; 29:288. Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at 1 year: National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med 1999; 340:1781–1787. Broderick JP. Recanalization therapies for acute ischemic stroke. Semin Neurol 1998; 18:471–484. The NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke: subgroup analysis of the NINDS t-PA stroke trial. Stroke 1997; 28:2119 –2125. Volume 229 䡠 Number 2 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. Chiu D, Krieger D, Villar-Cordova C, et al. Intravenous tissue plasminogen activator for acute ischemic stroke: feasibility, safety, and efficacy in the first year of clinical practice. Stroke 1998; 29:18 –22. Tanne D, Bates VE, Verro P, et al. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey—the t-PA Stroke Survey Group. Neurology 1999; 53:424 –427. Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol 1995; 52:1119 – 1122. Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissuetype plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplases to Reverse Stroke (STARS) study. JAMA 2000; 283:1145–1150. Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA 2000; 283:1151–1158. Zeumer H, Hacke W, Ringelstein EF. Local intraarterial thrombolysis in vertebrobasilar thromboembolic disease. AJNR Am J Neuroradiol 1983; 4:401–404. Zeumer H, Hundgen R, Ferbert A, Ringelstein EB. Local intraarterial fibrinolytic therapy in inaccessible internal carotid occlusion. Neuroradiology 1984; 26:315–317. Zeumer H, Freitag HJ, Zanella F, Thie A, Arning C. Local intra-arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r-TPA). Neuroradiology 1993; 35: 159 –162. Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol 1995; 16:1977–1986. Theron J, Courtheoux P, Casasco A, et al. Local intraarterial fibrinolysis in the carotid territory. AJNR Am J Neuroradiol 1989; 10: 753–765. Barr JD, Mathis JM, Wildenhain SL, Wechsler L, Jungreis CA, Horton JA. Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol 1994; 5:705– 713. del Zoppo GJ, Ferbert A, Otis S, et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke: a pilot study. Stroke 1988; 19:307–313. Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988; 19:802–812. Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intraarterial therapy improves outcome in patients with acute vertebrobasilar disease. Stroke 1988; 19:1216 – 1222. Ezura M, Kagawa S. Selective and superselective infusion of urokinase for embolic stroke. Surg Neurol 1992; 38:353–358. Barnwell SL, Clark WM, Nguyen TT, O’Neill OR, Wynn ML, Coull BM. Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. AJNR Am J Neuroradiol 1994; 15:1817–1822. Brandt T, von Kummer R, Müller-Küppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion: variables affecting 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. recanalization and outcome. Stroke 1996; 27:875–881. Becker KJ, Monsein LH, Ulatowski J, Mirski M, Williams M, Hanley DF. Intraarterial thrombolysis in vertebrobasilar occlusion. AJNR Am J Neuroradiol 1996; 17:255–262. Ueda T, Sakaki S, Kumon Y, Ohta S. Multivariable analysis of predictive factors related to outcome at 6 months after intraarterial thrombolysis for acute ischemic stroke. Stroke 1999; 30:2360 –2365. Jahan R, Duckwiler GR, Kidwell CS, et al. Intraarterial thrombolysis for treatment of acute stroke: experience in 26 patients with long-term follow-up. AJNR Am J Neuroradiol 1999; 20:1291–1299. Suarez JI, Sunshine JL, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999; 30:2094 – 2100. Yamaguchi T, Hayakawa T, Kiuchi H. Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis 1993; 3:269 –272. Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993; 14:3–13. Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992; 42:976 –982. Bruckmann H, Ferbert A, del Zoppo GJ, Hacke W, Zeumer H. Acute basilar thrombosis: angiologic-clinical comparison and therapeutic implications. Acta Radiol 1987; 369:38 –42. Archer CR, Horenstein S. Basilar artery occlusion:clinical and radiographic correlation. Stroke 1977; 8:383–391. Bogousslavsky J, Regli F, Maeder P, Meuli R, Nader J. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurology 1993; 43: 1528 –1533. del Zoppo GJ, Zeumer H, Harker LA. Thrombolytic therapy in stroke: possibilities and hazards. Stroke 1986; 17:595–607. Mitchell PJ, Gerraty RP, Donnan GA, et al. Thrombolysis in the vertebrobasilar circulation: The Australian urokinase stroke trial—a pilot study. Cerebrovasc Dis 1997; 7:94 –99. Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999; 30:2598 –2605. Weaver WD, Simes RJ, Betriu A, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA 1997; 278:2093–2098. Balousek PA, Knowles HJ, Higashida RT, del Zoppo GJ. New interventions in cerebrovascular disease: the role of thrombolytic therapy and balloon angioplasty. Curr Opin Cardiol 1996; 11:550 –557. Bücker A, Schmitz-Rode T, Vorwerk D, Günther RW. Comparative in vitro study of two percutaneous hydrodynamic thrombectomy systems. J Vasc Interv Radiol 1996; 7:445–449. Assessment of the Patient with Hyperacute Stroke 䡠 359
© Copyright 2026