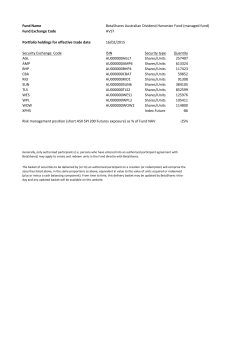
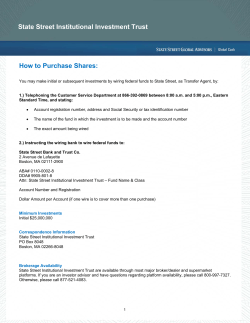
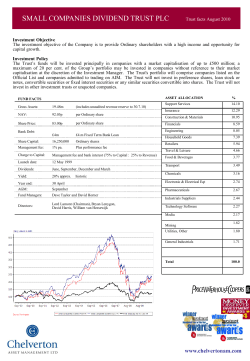
15.05.29.Notice of Meeting & Proxy Form
SUN BIOMEDICAL LIMITED ACN 001 285 230 NOTICE OF GENERAL MEETING A General Meeting of the Company will be held at Level 2, 1 Walker Avenue, West Perth on Tuesday, 30 June 2015 at 10.30 am (WST). This Notice of General Meeting should be read in its entirety. If Shareholders are in doubt as to how they should vote, they should seek advice from their accountant, solicitor or other professional adviser prior to voting. Should you wish to discuss any matter please do not hesitate to contact the Company Secretary by telephone on (08) 9481 3860. 1 SUN BIOMEDICAL LIMITED ACN 001 285 230 NOTICE OF GENERAL MEETING Notice is hereby given that a general meeting of Shareholders of Sun Biomedical Limited (Company) will be held at Level 2, 1 Walker Avenue, West Perth on Tuesday, 30 June 2015 at 10.30 am (WST) (Meeting). The Explanatory Memorandum to this Notice provides additional information on matters to be considered at the Meeting. The Explanatory Memorandum and the Proxy Form forms part of this Notice. The Directors have determined pursuant to regulation 7.11.37 of the Corporations Regulations 2001 (Cth) that the persons eligible to vote at the Meeting are those who are registered as Shareholders on 28 June 2015 at 10.30am (WST). Terms and abbreviations used in this Notice and Explanatory Memorandum are defined in Section 11. AGENDA 1. Resolution 1 – Approval of Acquisition of Dimerix Bioscience Limited To consider, and if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: “That, subject to each of the other Acquisition Resolutions being passed, for the purposes of Listing Rules 11.1.2 and 7.1, and for all other purposes, Shareholders approve the issue of up to 750,000,041 Shares, 75,000,040 Class A Performance Shares, 75,000,040 Class B Performance Shares and 75,000,040 Class C Performance Shares (Vendor Shares) to the Vendors (or their nominees) as consideration for the Acquisition, and the significant change in the scale of the Company's activities resulting from the Acquisition, on the terms and conditions set out in the Explanatory Memorandum." Voting Exclusion The Company will disregard any votes cast on this Resolution by the Vendors and their nominees and a person who might obtain a benefit, except a benefit solely in the capacity of a holder of ordinary securities, if the Resolution is passed, and any associates of those persons. However, the Company will not disregard a vote if: (a) it is cast by the person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the person chairing the Meeting as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. 2 2. Resolution 2 – Approval of new class of Securities - Performance Shares To consider and, if thought fit, to pass with or without amendment, the following resolution as a special resolution: "That, subject to each of the other Acquisition Resolutions being passed, for the purposes of section 246B(1) of the Corporations Act and Article 2.3 of the Constitution of the Company and for all other purposes, the Company be authorised to create a new class of share on the terms and conditions in Schedule 4 in the Explanatory Memorandum accompanying this Notice (Performance Shares).” 3. Resolution 3 – Approval of issue of Vendor Shares to Dr Anton Uvarov To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: “That, subject to each of the other Acquisition Resolutions being passed, for the purposes of Listing Rule 10.11 and for all other purposes, Shareholders approve and authorise the Directors to allot and issue Vendor Shares comprising 2,549,810 Shares, 254,981 Class A Performance Shares, 254,981 Class B Performance Shares and 254,981 Class C Performance Shares (the Related Party Shares) to Dr Anton Uvarov (or his nominee) as consideration for the sale and purchase of Dimerix Shares in which he has a beneficial interest, on the terms and conditions in the Explanatory Memorandum.” Voting Exclusion The Company will disregard any votes cast on this resolution by Dr Anton Uvarov and any of his associates. However, the Company will not disregard a vote if: 4. (a) it is cast by the person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the person chairing the Meeting as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. Resolution 4 – Ratification of Tranche 1 Placement To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: "That, for the purposes of Listing Rule 7.4, and for all other purposes, Shareholders approve and ratify the prior issue by the Company of 60,000,000 Shares (Tranche 1 Placement Shares) each at an issue price of $0.01 (Tranche 1 Placement) on the terms and conditions set out in the Explanatory Memorandum." 3 Voting Exclusion The Company will disregard any votes cast on this Resolution by a Tranche 1 Placement Participant and any of their associates. However, the Company will not disregard a vote if: 5. (a) it is cast by the person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the person chairing the Meeting as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. Resolution 5 – Authority to issue Tranche 2 Placement Shares To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: "That, subject to each of the other Acquisition Resolutions being passed, for the purpose of Listing Rule 7.1 and for all other purposes, Shareholders approve and authorise the Directors to issue up to 100,000,000 Shares (Tranche 2 Placement Shares) each at an issue price of $0.01 (Tranche 2 Placement) on the terms and conditions set out in the Explanatory Memorandum.” Voting Exclusion The Company will disregard any votes cast on this Resolution by a person who may participate in the Tranche 2 Placement and a person who might obtain a benefit (except a benefit solely in their capacity as holder of ordinary securities) if the Resolution is passed and any associates of those persons. However, the Company will not disregard a vote if: 6. (a) it is cast by the person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the person chairing the Meeting as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. Resolution 6 – Appointment of Dr James Williams as a Director To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: "That, subject to each of the other Acquisition Resolutions being passed, in accordance with Article 6.2(c) of the Constitution, and with effect from completion of the Acquisition, Dr James Williams be appointed as a Director." 4 7. Resolution 7 – Appointment of Dr Sonia Poli as a Director To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: "That, subject to each of the other Acquisition Resolutions being passed, in accordance with Article 6.2(c) of the Constitution, and with effect from completion of the Acquisition, Dr Sonia Poli be appointed as a Director." 8. Resolution 8 – Authority to grant Adviser Options To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: "That, subject to each of the other Acquisition Resolutions being passed, for the purpose of Listing Rule 7.1 and for all other purposes, Shareholders approve and authorise the Directors to grant up to 60,000,000 Adviser Options each exercisable at $0.01 on or before 30 June 2017 to Forrest Capital (or its nominees) to raise a total of $6,000 on the terms and conditions set out in the Explanatory Memorandum.” Voting Exclusion The Company will disregard any votes cast on this Resolution by Forrest Capital and its nominees and a person who might obtain a benefit (except a benefit solely in their capacity as holder of ordinary securities) if the Resolution is passed and any associates of those persons. However, the Company will not disregard a vote if: 9. (a) it is cast by the person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the person chairing the Meeting as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. Resolution 9 – Authority to grant Transaction Options To consider and, if thought fit, to pass with or without amendment, the following resolution as an ordinary resolution: “That, subject to each of the other Acquisition Resolutions being passed, for the purposes of Listing Rule 7.1 and for all other purposes, Shareholders approve and authorise the Directors to grant up to 30,851,594 Transaction Options, each exercisable at $0.02 on or before 30 June 2017, on the terms and conditions set out in the Explanatory Memorandum.” Voting Exclusion The Company will disregard any votes cast on this Resolution by any person who may participate in the issue of the Transaction Options and a person who might obtain a benefit (except a benefit solely in the capacity of a holder of ordinary securities) if the Resolution is passed and any associates of those persons. 5 However, the Company will not disregard a vote if: (a) it is cast by a person as proxy for a person who is entitled to vote, in accordance with directions on the Proxy Form; or (b) it is cast by the Chairman as proxy for a person who is entitled to vote, in accordance with a direction on the Proxy Form to vote as the proxy decides. Dated 26 May 2015 BY ORDER OF THE BOARD Howard Digby Executive Chairman 6 SUN BIOMEDICAL LIMITED ACN 001 285 230 EXPLANATORY MEMORANDUM 1. Introduction This Explanatory Memorandum has been prepared for the information of Shareholders in connection with the business to be conducted at the Meeting to be held at Level 2, 1 Walker Avenue, West Perth on Tuesday, 30 June 2015 at 10.30 am (WST). This Explanatory Memorandum should be read in conjunction with, and forms part of, the accompanying Notice. The purpose of this Explanatory Memorandum is to provide information to Shareholders in deciding whether or not to pass the Resolutions set out in the Notice. A Proxy Form is located at the end of the Explanatory Memorandum. 2. Action to be taken by Shareholders Shareholders should read the Notice and this Explanatory Memorandum carefully before deciding how to vote on the Resolutions. 2.1 Proxies A Proxy Form is attached to the Notice. This is to be used by Shareholders if they wish to appoint a representative (a 'proxy') to vote in their place. All Shareholders are invited and encouraged to attend the Meeting or, if they are unable to attend in person, sign and return the Proxy Form to the Company in accordance with the instructions thereon. Lodgment of a Proxy Form will not preclude a Shareholder from attending and voting at the Meeting in person. Please note that: (a) a member of the Company entitled to attend and vote at the Meeting is entitled to appoint a proxy; (b) a proxy need not be a member of the Company; and (c) a member of the Company entitled to cast two or more votes may appoint two proxies and may specify the proportion or number of votes each proxy is appointed to exercise, but where the proportion or number is not specified, each proxy may exercise half of the votes. The enclosed Proxy Form provides further details on appointing proxies and lodging Proxy Forms. 7 3. Resolution 1 – Approval of Acquisition of Dimerix Bioscience Limited 3.1 Background The Company announced on 13 May 2015 that it had entered into an implementation agreement with Dimerix (Implementation Agreement) to acquire 100% of Dimerix (Acquisition). Dimerix Bioscience Limited (Dimerix) is a public unlisted clinical stage drug discovery and development company, based in Melbourne. Dimerix’s lead clinical program is a Phase II study in patients with Chronic Kidney Disease, using its novel combination therapy, DMX-200. The acquisition will transform Sun Biomedical into an advanced clinical stage company with an asset that has the potential to make a considerable impact in the treatment of Chronic Kidney Disease (CKD). Upon successful results from the Phase II study, Dimerix intends to pursue the pathway of registration of a product for an orphan indication subgroup of CKD patients, such as Nephrotic Syndrome, and partner development of the product in the larger CKD indications. Dimerix's team has applied its patented GPCR drug discovery technology for its own internal research identifying and developing therapeutic treatments. Dimerix leverages its knowledge of drug target interaction and develops new combination therapies using already marketed compounds for new medical indications. This positions Dimerix's therapies with a fast route to market due to extensive safety data for the selected compounds removing the requirement for Phase I studies and allowing to proceed directly to Phase Il efficacy studies. Further information on Dimerix and its activities is set out in Schedule 1. Dimerix is subject to a range of risks which apply to the biotechnology sector including the risk of failure in the development and commercialisation of its products (for example, through adverse clinical outcomes, failure to achieve necessary regulatory approvals, the product being found to be uneconomic or unable to compete with products marketed by third parties), challenge to its intellectual property rights and obtaining sufficient funding to implement its overall business strategy. These risks and others are discussed in more detail in Schedule 3. Under the terms of the Implementation Agreement, the Company has agreed to issue the Vendor Shares as consideration to the Vendors. This Meeting has been called by the Board to seek the necessary approvals required to effect the Acquisition. 3.2 Commercial Terms Under the terms of the Implementation Agreement, the Company will enter into acquisition agreements with each of the Vendors (Acquisition Agreements) to acquire 100% ownership of Dimerix for total consideration of: (a) 750,000,041 Shares; (b) 75,000,040 Class A Performance Shares (convertible into 75,000,040 Shares upon receipt by the Company or Dimerix of a notice of allowance from the United States Patent and Trademark Office in relation to the US patent application number 13/979,127 (or any divisional or continuation thereof) within 24 months of completion of the Acquisition) (Class A Performance Shares); 8 (c) 75,000,040 Class B Performance Shares (convertible into 75,000,040 Shares upon the Board making an investment decision to proceed to file an application to the US Food and Drug Administration for a pre-Investigational New Drug (“pre-IND”) meeting to progress development of DMX200 following the receipt of data generated under the current clinical trial for chronic kidney disease supporting further progression of the technology within 48 months of completion of the Acquisition) (Class B Performance Shares); and (d) 75,000,040 Class C Performance Shares (convertible into 75,000,040 Shares upon receipt of ethics approval allowing commencement of a second clinical trial derived from the Dimerix platform and in relation to an indication that is not covered under the existing Austin Human Research Ethics Committee approval within 48 months of completion of the Acquisition) (Class C Performance Shares), (the Vendor Shares). Resolution 1 seeks Shareholder approval for the issue of the Vendor Shares (refer to Section 3.10 for further details). One of the Directors, Dr Anton Uvarov, has a beneficial interest in 231,000 shares in Dimerix. Approval of the issue of Vendor Shares to Dr Uvarov as a related party for the purposes of Listing Rule 10.11 is being sought in Resolution 3 (see Section 5 for further details). Under the terms of the Implementation Agreement, the Acquisition Agreements will be subject to certain conditions which must be satisfied or waived by 8 July 2015 (unless extended by agreement between the parties). These conditions have been satisfied with the exception of the following conditions which remain outstanding at the date of this Notice: (a) the Company obtaining all necessary shareholder approvals as are required to give effect to the Acquisition; (b) all of the Vendors entering into an Acquisition Agreement with the Company in respect of their shares in Dimerix; (c) the Company receiving firm commitments and cleared funds for the full amount of the Tranche 2 Placement; and (d) no material breach of the warranties given in the Implementation Agreement having occurred. If completion has not occurred under the Acquisition Agreements by 8 July 2015, then the Implementation Agreement and the Acquisition Agreements will be at an end (unless extended by agreement between the parties). The Implementation Agreement requires the parties to comply with certain obligations prior to completion including: (a) an obligation on Dimerix to ensure that (other than as agreed between the parties): (i) Dimerix conducts its business in the ordinary course and does not incur any material liabilities (other than in the usual conduct of business), encumber or dispose of any assets, issue any securities or declare or pay any dividend; (ii) Dimerix complies with the terms of specified material contracts; 9 (iii) (b) Dimerix takes: (A) all steps necessary to maintain in good standing the intellectual property owned by Dimerix; and (B) all reasonable steps to prevent any infringement of that intellectual property by any third parties; (iv) Dimerix uses its reasonable endeavours to preserve its assets and goodwill, including preserving its current business relationships; and (v) nothing is knowingly done by Dimerix which is likely to have a material adverse impact on Dimerix or its assets; and an obligation on the Company to ensure that (other than as agreed between the parties): (i) it conducts its business in the ordinary course and will not incur expenditure materially different from its expected expenditure as at the date of the Implementation Agreement, materially change any intellectual property it currently owns or licenses or any current projects it is undertaking or commence any new projects, nor agree to do any of those things; (ii) it conducts its business in the ordinary course and does not incur any material liabilities (other than in the usual conduct of business), encumber or dispose of any assets, issue any securities or pay any dividend; (iii) nothing is done by the Company which is likely to have a material adverse impact on the Company and its assets; and (iv) it complies with the terms of the specified material contracts; and (v) it uses its reasonable endeavours to preserve its assets and goodwill, including preserving its current business relationships. Prior to Completion the Company and Dimerix will consult with each other in relation to any material expenditure or unusual items that may arise. The Company will also keep Dimerix informed about the Company’s expenditure and cash position. The Implementation Agreement contains standard commercial warranties about Dimerix and its assets and limits of vendor liability that are usual for a transaction of this type. If a breach of any of these warranties come to light prior to completion of the Acquisition, the Company will have the right to terminate the Implementation Agreement. Under the terms of the Implementation Agreement and the relevant Acquisition Agreements, Vendors holding approximately 74% of the shares in Dimerix will agree that the Shares they will receive as consideration for the sale and purchase of their Dimerix shares will be subject to between 6 and 12 months voluntary escrow from the date of issue. The Vendors have the right to nominate two directors to the Board of the Company, and current Directors, Mr Evan Cross and Mr Peter Webse, will resign with effect from completion of the Acquisition. Mr Webse will continue as Company Secretary. 10 Under the Implementation Agreement: 3.3 (a) the Company will grant up to 60,000,000 Adviser Options to Forrest Capital at an issue price of 0.0001 cents per Option (see Section 9); and (b) the Company will grant of up to 30,851,594 Transaction Options to past and present employees and consultants of Dimerix for nil consideration (see Section 10). Capital Raising and Use of Funds The Company intends to undertake a placement of up to 160,000,000 Shares each at an issue price of $0.01 to raise up to $1,600,000 (before costs) (Capital Raising) in two tranches with: (a) the first tranche of 60,000,000 Shares to be issued under the Company's existing placement capacity following the execution of Acquisition Agreements by all of the Vendors prior to the date of the Meeting (Tranche 1 Placement); and (b) the second tranche of 100,000,000 Shares to be issued subject to shareholder approval (Tranche 2 Placement), to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital. Resolution 5 seeks Shareholder approval for the Tranche 2 Placement (refer to Section 7 for further details). Forrest Capital has assisted the Company with the Capital Raising. 3.4 Effect of the Acquisition on the Company (a) Capital Structure Below is a table showing the Company’s current capital structure and the possible capital structure following completion of the Acquisition and the Tranche 2 Placement. Balance at the date of this Notice Number of Shares Number of Options 473,640,008 (1) 20,857,143 (2) Shares to be issued pursuant to the 750,000,041 Acquisition - Shares to be issued on conversion of 75,000,040 (3) the Class A Performance Shares - Shares to be issued on conversion of 75,000,040 (3) the Class B Performance Shares - Shares to be issued on conversion of 75,000,040 (3) the Class C Performance Shares - Shares to be issued pursuant to the 100,000,000 Tranche 2 Placement - Adviser Options to be issued to Forrest Capital 60,000,000 (4) 11 Number of Shares Number of Options Transaction Options to be issued pursuant to the Implementation Agreement 30,851,594 (5) Balance following completion of the 1,548,640,169 Acquisition 111,708,737 (1) Assumes that the Tranche 1 Placement Shares have been issued. It is intended that these will be issued prior to the date of the Meeting. (2) Comprises 20,857,143 unlisted options each exercisable at $0.007 on or before 31 December 2017. (3) Refer to Section 3.2 for further details. (4) Each exercisable at $0.01 on or before 30 June 2017. Refer to Section 9 for further details. (5) Each exercisable at $0.02 on or before 30 June 2017. Refer to Section 10 for further details. (b) Pro Forma Balance Sheet following the Acquisition A pro-forma balance sheet of the Company on completion of the Acquisition and the Capital Raising is set out in Schedule 2. 3.5 Risk Factors While the Company has undertaken a due diligence process (including financial, legal, technical and other risks) in relation to Dimerix and its assets, it should be noted that the usual risks associated with biotechnology companies with a small market capitalisation are expected to remain despite completion of due diligence. Shareholders and investors should also be aware that the Acquisition Agreements to acquire Dimerix will be conditional on a number of events (refer to Section 3.2 above). Accordingly there is a risk that the Acquisition may not be completed. Investing in a company involves risks of various kinds, some of which are within the realms of influence of the Company and some, arising from external factors, which may be beyond the control of the Company. A summary of the risks associated with the Acquisition and ongoing research and development activities of Dimerix are outlined in Schedule 3. 3.6 Advantages of the Acquisition The Directors are of the view that the following non-exhaustive list of advantages may be relevant to a Shareholder's decision on how to vote on Resolution 1: (a) the Acquisition represents an opportunity for the Company to diversify its interests to include drug discovery and developing new therapeutic compounds; (b) the Acquisition creates an increase in the size of the Company’s existing operations and assets of the Company. This may enhance the prospects of the Company that currently has been operating without significant success, and is small in scale; (c) in the current market environment there is a greater likelihood of creating Shareholder value by expanding the Company’s business to include drug discovery and developing new therapeutic compounds; 12 3.7 (d) the proposed changes to the Board will provide an experienced and balanced set of skills to guide the growth of the Company; and (e) the potential increase in market capitalisation of the Company following completion of the Acquisition and the associated Capital Raising may lead to increased coverage from investment analysts, access to improved equity capital market opportunities and increased liquidity. Disadvantages of the Acquisition The Directors are of the view that the following non-exhaustive list of disadvantages may be relevant to a Shareholder's decision on how to vote of Resolution 1: 3.8 (a) the Company will be changing its activities to include drug discovery and developing new therapeutic compounds, which may not be consistent with the objectives of all Shareholders; (b) the acquisition of Dimerix will result in the issue of the Vendor Shares and the Tranche 2 Placement Shares which will have a dilutionary effect on the holdings of Shareholders; (c) significant future outlays of funds from the Company will be required for Dimerix; and (d) risk factors associated with the expansion of the Company’s activities some of which are outlined in Schedule 3. Plans for the Company if the Acquisition is not completed If the Company does not complete the Acquisition, the Company will continue with its current activities and continue to seek, and undertake due diligence on, new opportunities for growth. 3.9 Directors’ Recommendation in relation to Acquisition Based on the information available, including the information contained in this Explanatory Memorandum the Directors (other than Dr Anton Uvarov, who has excluded himself in light of his beneficial interest in Dimerix shares to be acquired by the Company) recommend that Shareholders vote in favour of the Acquisition Resolutions. 3.10 Shareholder Approvals Resolution 1 seeks Shareholder approval pursuant to Listing Rule 7.1 for the issue of the Vendor Shares to the Vendors and pursuant to Listing Rule 11.1.2 for the significant change in the scale of the Company's activities resulting from the Acquisition. Resolution 1 is an ordinary Resolution and is subject to each of the other Acquisition Resolutions being passed. 3.11 Listing Rule 7.1 Listing Rule 7.1 provides that a company must not (subject to specified exceptions), without the approval of shareholders, issue or agree to issue during any 12 month period any equity securities, or other securities with rights to conversion to equity (such as an option), if the number of those securities exceeds 15% of the number of fully paid ordinary securities on issue at the commencement of that 12 month period. 13 Given the Vendor Shares to be issued under Resolution 1 will exceed the Company’s 15% threshold and none of the exceptions contained in Listing Rule 7.2 apply, Shareholder approval is required in accordance with Listing Rule 7.1. 3.12 Listing Rule 11.1.2 Listing Rule 11.1 provides that where an entity proposes to make a significant change, either directly or indirectly, to the nature or scale of its activities, it must provide full details to ASX as soon as practicable and comply with the following: (a) provide to ASX information regarding the change and its effect on future potential earnings, and any information that ASX asks for; (b) if ASX requires, obtain the approval of holders of its shares and any requirements of ASX in relation to the notice of meeting (the notice of meeting must include a voting exclusion statement); and (c) if ASX requires, meet the requirements of Chapters 1 and 2 of the Listing Rules as if the Company were applying for admission to the official list of ASX. ASX has indicated to the Company that given the change in the scale of the Company’s activities resulting from the Acquisition it requires the Company to obtain Shareholder approval. The Company is not required to re-comply with the admission requirements set out in Chapters 1 and 2 of the Listing Rules. A voting exclusion is included in the Notice. 3.13 Information required by Listing Rule 7.3 For the purposes of Listing Rule 7.3 information regarding the issue of the Vendor Shares is provided as follows: (a) The maximum number of Shares the Company will issue to the Vendors is: (i) 750,000,041 Shares on completion of the Acquisition; (ii) 75,000,040 Class A Performance Shares on completion of the Acquisition (and 75,000,040 Shares on conversion of the Class A Performance Shares); (iii) 75,000,040 Class B Performance Shares on completion of the Acquisition (and 75,000,040 Shares on conversion of the Class B Performance Shares); and (iv) 75,000,040 Class C Performance Shares on completion of the Acquisition (and 75,000,040 Shares on conversion of the Class C Performance Shares). (b) The Vendor Shares may be issued no later than three months after the date of the Meeting (or such later date to the extent permitted by an ASX waiver or modification of the Listing Rules). (c) The Vendor Shares will be issued as consideration for the Acquisition and accordingly no funds will be raised from the issue of the Vendor Shares. (d) The Vendor Shares will be issued to the Vendors, who are not related parties of the Company (other than Dr Anton Uvarov – see Section 5). No Vendor will hold more than 20% of the Shares in the Company following completion of the Acquisition and none of the Vendors are associates. 14 4. (e) The Shares to be issued are ordinary shares and rank equally with the Company's existing Shares. (f) The Performance Shares to be issued are converting performance shares which will convert into Shares on a one for one basis on achievement of the relevant milestone and with the terms and conditions in Schedule 4. (g) Subject to paragraph (b), the Vendor Shares may be issued progressively. (h) A voting exclusion statement is included in the Notice. Resolution 2 – Approval of new class of Securities - Performance Shares The Company seeks Shareholder approval to create the Performance Shares as a new class of Shares on the terms and conditions in Schedule 4. Resolution 2 is a special resolution. Resolution 2 is subject to the passing of each of the other Acquisition Resolutions. Under Article 2.1 of the Constitution and subject to the Corporations Act, the Listing Rules and the Constitution, the Directors may at any time issue such number of shares either as ordinary shares or shares of a named class or classes (being either an existing class or a new class) at the issue price that the Directors determine. Section 246C(5) of the Corporations Act provides that if a company has one class of share and seeks to issue a new class of share, such issue is taken to vary the rights attached to the shares already issued. Under section 246B(1) of the Corporations Act, if a company has a constitution which sets out the procedure for varying or cancelling (in the case of a company with share capital) rights attached to shares in a class of shares, those rights may be varied or cancelled only in accordance with the procedure. In accordance with Article 2.3(a)(i) and (iii) of the Constitution, the Company may vary rights attached to shares in a class by a special resolution of the Company and a special resolution passed at a meeting of the members holding shares in that class. Accordingly, the Company seeks approval from Shareholders for the issue of the Performance Shares as a new class of shares on the terms set out in Schedule 4. The Company will also seek approval in Resolutions 1 and 4 from Shareholders to issue Performance Shares to the Vendors. The Company has requested the ASX to consider whether the terms are appropriate and equitable for the purposes of Listing Rule 6.1. Accordingly the terms of the Performance Shares remain subject to approval by the ASX. 15 5. Resolution 3 – Approval of issue of Vendor Shares to Dr Anton Uvarov 5.1 Background Resolution 3 seeks the approval of Shareholders pursuant to Listing Rule 10.11 for the Directors to issue Vendor Shares comprising 2,549,810 Shares, 254,981 Class A Performance Shares, 254,981 Class B Performance Shares and 254,981 Class C Performance (the Related Party Shares) to Dr Anton Uvarov (or a trust of which he is a beneficiary). Shareholder approval is required under Listing Rule 10.13 for the proposed issue of the Related Party Shares to Dr Uvarov because, as a Director, Dr Uvarov is a related party of the Company. The Related Party Shares will be issued to Dr Uvarov (or a trust of which he is a beneficiary) as consideration for the sale and purchase of 231,000 Dimerix shares (representing less than 1% of Dimerix shares on issue) in which he has a beneficial interest. As Dr Uvarov is a related party of the Company, the Related Party Shares will be subject to 12 months ASX escrow from issue under item 5 of Appendix 9B to the Listing Rules. Shareholder approval of the issue of the Related Party Shares to Dr Uvarov means that this issue will not reduce the Company's 15% placement capacity under Listing Rule 7.1. In any event, the Related Party Shares form part of the Vendor Shares for which approval is sought under Resolution 1. The Directors (other than Dr Uvarov) have determined that shareholder approval is not required under Listing Rule 10.1 or Chapter 2E of the Corporations Act. Resolution 3 is an ordinary resolution. Resolution 3 is subject to the approval of each of the other Acquisition Resolutions. 5.2 Specific information required by Listing Rule 10.13 For the purposes of Listing Rule 10.13, information is provided as follows: (a) The Related Party Shares will be issued to Dr Anton Uvarov (or his nominee). Dr Uvarov is a Director of the Company. (b) The maximum number of Securities to be issued to Dr Uvarov is: (i) 2,549,810 Shares on completion of the Acquisition; (ii) 254,981 Class A Performance Shares on completion of the Acquisition (and 254,981 Shares on conversion of the Class A Performance Shares in accordance with their terms); (iii) 254,981 Class B Performance Shares on completion of the Acquisition (and 254,981 Shares on conversion of the Class B Performance Shares in accordance with their terms); and (iv) 254,981 Class C Performance Shares on completion of the Acquisition (and 254,981 Shares on conversion of the Class C Performance Shares in accordance with their terms). 16 (c) The Company will issue the Related Party Shares no later than 1 month after the date of the General Meeting (or such later date to the extent permitted by an ASX waiver or modification of the Listing Rules). (d) The Related Party Shares will be issued as consideration for the acquisition of Dimerix Shares from Dr Uvarov and accordingly no funds will be raised from the issue of the Related Party Shares. (e) The Related Party Shares are ordinary shares and rank equally with the Company's existing Shares. As noted above, the Related Party Shares will be subject to 12 months ASX escrow from issue. (f) The Performance Shares to be issued are converting performance shares which will convert into Shares on a one for one basis on achievement of the relevant milestone and with the terms and conditions in Schedule 4. (g) Subject to paragraph (c), the Vendor Shares may be issued progressively. (h) A voting exclusion statement is included in this Notice. 6. Resolution 4 – Ratification of Tranche 1 Placement 6.1 General It is intended that prior to the date of the Meeting, once Acquisition Agreements have been executed by all of the Dimerix shareholders, the Company will issue 60,000,000 Shares at an issue price of $0.01 each to the Tranche 1 Placement Participants to raise $600,000 (before costs). The funds raised from the issue of the Tranche 1 Placement Shares will be used by the Company to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital. The Tranche 1 Placement Shares will be issued within the Company’s 15% annual limit permitted under Listing Rule 7.1 without the need for Shareholder approval. A summary of Listing Rule 7.1 is provided in Section 3.11. Listing Rule 7.4 provides that where a company in general meeting ratifies a previous issue of securities made pursuant to Listing Rules 7.1 (and provided that the previous issue did not breach Listing Rule 7.1) the issue of those securities will be deemed to have been with shareholder approval for the purpose of Listing Rule 7.1. Resolution 4 seeks Shareholder approval for the ratification of the issue of the Tranche 1 Placement Shares pursuant to Listing Rule 7.4. The effect of Shareholders passing Resolution 4 will be to restore the Company's ability to issue securities within the 15% placement capacity under Listing Rule 7.1 during the next 12 months and within the additional 10% placement capacity under Listing Rule 7.1A during the 12 months from the date of the Company’s 2014 Annual General Meeting approved by Shareholders at that meeting, without obtaining prior Shareholder approval. Resolution 4 is an ordinary resolution. 17 6.2 Information required by Listing Rule 7.5 For the purposes of Listing Rule 7.5 information regarding the issue of the Tranche 1 Placement Shares is provided as follows: (a) It is intended that 60,000,000 Shares will be issued prior to the date of the Meeting pursuant to the Tranche 1 Placement. (b) The Tranche 1 Placement Shares will be issued at $0.01 each. (c) The Tranche 1 Placement Shares comprise fully paid ordinary shares of the Company ranking equally with all other fully paid ordinary shares of the Company. (d) The Tranche 1 Placement Shares will be issued to the Tranche 1 Placement Participants, none of who are related parties of the Company. (e) The funds raised from the issue of the Tranche 1 Placement Shares will be used by the Company to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital. (f) A voting exclusion statement is included in the Notice. 7. Resolution 5 - Authority to issue Tranche 2 Placement Shares 7.1 General As announced to the ASX on 13 May 2015, the Company intends to undertake a placement of 100,000,000 Shares each at an issue price of $0.01, to raise $1,000,000 (before costs) as the second tranche of the Placement. The funds raised from the issue of the Tranche 2 Placement Shares will be used by the Company to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital. Given the Tranche 2 Placement Shares to be issued under Resolution 5 will exceed the 15% threshold set out in Listing Rule 7.1 and none of the exceptions contained in Listing Rule 7.2 apply, Shareholder approval is required under Listing Rule 7.1. A summary of Listing Rule 7.1 is provided in Section 3.11. Resolution 5 is an ordinary resolution and is subject to each of the other Acquisition Resolutions being passed. 7.2 Information required by Listing Rule 7.3 For the purposes of Listing Rule 7.3, information regarding the issue of the Tranche 2 Placement Shares is provided as follows: (a) The maximum number of Shares that the Company may issue under the Tranche 2 Placement is 100,000,000. (b) The Tranche 2 Placement Shares may be issued no later than three months after the date of the Meeting (or such later date to the extent permitted by an ASX waiver or modification of the Listing Rules). (c) The Tranche 2 Placement Shares will be issued at an issue price of $0.01 per Share. 18 (d) The Tranche 2 Placement Shares will be issued to sophisticated and professional investor clients of Forrest Capital, none of whom will be related parties of the Company. (e) The Tranche 2 Placement Shares will comprise fully paid ordinary shares of the Company ranking equally with all other fully paid ordinary shares of the Company. (f) The funds raised from the issue of the Tranche 2 Placement Shares will be used by the Company to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital. (g) Subject to paragraph (b), the issue of the Tranche 2 Placement Shares may occur progressively. (h) A voting exclusion statement is included in the Notice. 8. Resolutions 6 and 7 – Appointment of Directors 8.1 General Pursuant to the Implementation Agreement, Dimerix has the right to appoint two directors to the Board of the Company with effect from completion of the Acquisition (refer to Section 3.2 for further details). Dimerix has nominated Dr James Williams and Dr Sonia Poli as its nominees to be appointed as executive Chairman and a non-executive Director, respectively. Article 6.2(c) of the Constitution provides that the Company in general meeting may by ordinary resolution appoint any person as a Director. Accordingly, Dr James Williams and Dr Sonia Poli seek approval to be appointed as Directors with effect from completion of the Acquisition. Resolutions 6 and 7 are ordinary resolutions and are subject to each of the other Acquisition Resolutions being passed. 8.2 Candidate Director’s Profile – Dr James Williams PhD, MBA, GAICD (Resolution 6) Dr Williams is a co-founder of Dimerix as well as co-founder and Investment Director of Yuuwa Capital LP, a venture capital firm based in Western Australia. He was the CEO of Dimerix between 2007 and 2009, and executive Director from 2011 to the present time. Prior to establishing Yuuwa Capital, he was Managing Director of two medical device companies, ASXlisted Resonance Health Ltd and Argus Biomedical Pty Ltd, both of which secured regulatory approvals under his leadership. He conceived, co-founded and is a former CTO and Director of iCeutica, Inc., a clinical stage nano drug reformulation company. iCeutica was acquired by Philadelphia-based Iroko Pharmaceuticals in 2011. Iroko received FDA approval for the first two iCeutica formulations in late 2013 and early 2014. Dr Williams is a Director of Yuuwa investee companies Adalta Pty Ltd, Eriden Technologies Pty Ltd, PolyActiva Pty Ltd and Nexgen Plants Pty Ltd. He is also a Director of Linear Clinical Research Ltd, a specialist early phase clinical trial unit and a member of the “Panel of Experts” for the University of Western Australia’s Pathfinder Fund. Dr Williams is co-author of numerous scientific papers and patents, including those related to Dimerix’s lead program. 19 8.3 Candidate Director’s Profile – Dr Sonia Poli PhD (Resolution 7) Dr Poli has worked within Swiss Stock Exchange listed companies Hoffman la Roche and Addex Therapeutics, where she has held leadership and executive positions across various disciplines in drug discovery, pre-clinical development and translational science. Dr Poli has interacted with regulatory authorities, investors and public funding institutions. She has promoted academic collaborations and supported R&D collaborations with external partners, and she has participated in successful fund raising activities. Dr Poli is currently acting as CSO for Addex Therapeutics, and has been on the Board of Directors of Dimerix since May 2014. Dr Poli is an accomplished R&D professional with 20 years international experience in large and small pharmaceutical companies. She has broad knowledge of small molecule drug design, optimisation and early clinical development, with expertise which encompasses multiple therapeutic areas. She is the co-inventor of a new anti-emetic medicine, recently included in the National Comprehensive Cancer Network Antiemesis Guidelines as a recommended option. Dr Poli is co-author of more than 40 scientific papers and several patents. Dr Poli holds a Masters degree and a PhD in industrial chemistry from Milan University (Italy). She currently resides in Geneva, Switzerland. 9. Resolution 8 - Authority to grant Adviser Options 9.1 General The Company has agreed, subject to Shareholder approval, to grant up to 60,000,000 Adviser Options to Forrest Capital (or its nominees) for a total issue price of $6,000. The Adviser Options will each be exercisable at $0.01, with an expiry date of 30 June 2017. Further terms and conditions of the Adviser Options are set out in Schedule 5. Shareholder approval of the grant of the Adviser Options means that this grant will not reduce the Company's 15% placement capacity under Listing Rule 7.1. A summary of Listing Rule 7.1 is provided in Section 3.1. Resolution 8 is an ordinary resolution and is subject to each of the other Acquisition Resolutions being passed. 9.2 Information required by Listing Rule 7.3 For the purposes of Listing Rule 7.3, information regarding the grant of the Adviser Options is provided as follows: (a) The maximum number of Adviser Options that the Company may grant under Resolution 8 is 60,000,000. (b) The Adviser Options may be granted no later than three months after the date of the Meeting (or such later date to the extent permitted by an ASX waiver or modification of the Listing Rules). (c) The Adviser Options will be granted at an issue price of 0.0001 cents per Option. The funds raised from the grant of the Adviser Options will be used by the Company to fund the Acquisition and expenditure on the assets owned by Dimerix and the existing business of the Company and to provide on-going working capital 20 (d) The Adviser Options will be granted to Forrest Capital (or its nominees) who is not a related party of the Company. (e) The Adviser Options are each exercisable at $0.01 on or before 30 June 2017. Further terms and conditions of the Adviser Options are set out in Schedule 5. (f) Subject to paragraph (b), the grant of the Adviser Options may occur progressively. (g) A voting exclusion statement is included in the Notice. 10. Resolution 9 – Authority to grant Transaction Options 10.1 General Resolution 9 seeks Shareholder approval pursuant to Listing Rule 7.1 for the grant of up to 30,851,594 Transaction Options to past and present employees and consultants of Dimerix pursuant to the Implementation Agreement. The Transaction Options will each be exercisable at $0.02, with an expiry date of 30 June 2017. Further terms and conditions of the Transaction Options are set out in Schedule 6. The Transaction Options will be granted to parties who had previously provided operational and management services to Dimerix and were to be remunerated for the provision of those services (including by way of option issue), including the Dimerix board and management (including the proposed Directors, Dr James Williams and Dr Sonia Poli). As Dimerix had not yet issued those options at the time of signing the Implementation Agreement, the Company and Dimerix agreed that it would be more efficient for the Company to grant the Transaction Options to those parties directly. The proposed Directors, Dr James Williams and Dr Sonia Poli, will receive 10,762,183 and 2,152,437 Transaction Options, respectively. Shareholder approval for the grant of these Transaction Options to Dr Williams and Dr Poli is not required under Listing Rule 10.11 because Exception 6 under Listing Rule 10.12 applies (i.e. Dr Williams and Dr Poli will become related parties of the Company by reason only of the transaction which is the reason for the issue of the Transaction Options). Shareholder approval of the grant of the Transaction Options means that this grant will not reduce the Company's 15% placement capacity under Listing Rule 7.1. A summary of Listing Rule 7.1 is provided in Section 3.1. Resolution 9 is an ordinary resolution and is subject to the approval of each of the other Acquisition Resolutions. 10.2 Specific information required by Listing Rule 7.3 Pursuant to and in accordance with Listing Rule 7.3, the following information is provided in relation to Resolution 9: (a) The maximum number of Transaction Options to be granted under Resolution 9 is 30,851,594. (b) The Company will issue the Transaction Options no later than three months after the date of the Meeting (or such later date to the extent permitted by any ASX waiver or modification of the Listing Rules). 21 11. (c) The Transaction Options will be issued for nil cash consideration. Accordingly no funds will be raised from the issue of the Transaction Options. (d) The Transaction Options will be issued to past and present employees and consultants of Dimerix in accordance with the Implementation Agreement. As noted above, 10,762,183 will be issued to Dr James Williams, and 2,152,437 Transaction Options will be issued to Dr Sonia Poli, who are proposed Directors of the Company. Otherwise, none of the recipients are related parties of the Company. (e) The Transaction Options will each be exercisable at $0.02 on or before 30 June 2017 and otherwise have the terms and conditions as set out in Schedule 6. (f) The grant of the Transaction Options may occur progressively, subject to paragraph (b) above. (g) A voting exclusion statement is included in the Notice. Definitions $ means Australian Dollars. Acquisition has the meaning in Section 3.1. Acquisition Agreements has the meaning in Section 3.2. Acquisition Resolutions means the Resolutions other than Resolution 4. Adviser Option means an Option exercisable at $0.01 on or before 30 June 2017 and otherwise with the terms and conditions in Schedule 5. ASX means ASX Limited (ACN 008 624 691) and, where the context permits, the Australian Securities Exchange operated by ASX. Board means the board of Directors. Capital Raising means the Tranche 1 Placement and the Tranche 2 Placement. Chairman means the chairman of this Meeting. Class A Performance Shares has the meaning in Section 3.2. Class B Performance Shares has the meaning in Section 3.2. Class C Performance Shares has the meaning in Section 3.2. Closely Related Party means has the meaning in section 9 of the Corporations Act. Company or Sun Biomedical means Sun Biomedical Limited ACN 001 285 230. Corporations Act means the Corporations Act 2001 (Cth). Dimerix means Dimerix Bioscience Limited ACN 112 223 417. Director means a director of the Company. Explanatory Memorandum means the explanatory memorandum attached to the Notice. 22 Forrest Capital means Forrest Capital Pty Ltd ACN 118 115 834. Listing Rules means the listing rules of ASX. Meeting has the meaning in the introductory paragraph of the Notice. Notice means this notice of meeting. Option means an option to acquire a Share. Performance Shares means the Class A Performance Shares, the Class B Performance Shares and the Class C Performance Shares. Proxy Form means the proxy form attached to this Notice. Resolution means a resolution contained in this Notice. Section means a section contained in this Explanatory Memorandum. Share means a fully paid ordinary share in the capital of the Company. Shareholder means a shareholder of the Company. Tranche 1 Placement has the meaning in Resolution 4. Tranche 1 Placement Participants means the participants in the Tranche 1 Placement being professional and sophisticated investor clients of Forrest Capital. Tranche 1 Placement Shares has the meaning in Resolution 4. Tranche 2 Placement has the meaning in Resolution 5. Tranche 2 Placement Shares has the meaning in Resolution 5. Transaction Option means an Option exercisable at $0.02 on or before 30 June 2017 and otherwise with the terms and conditions in Schedule 6. Vendors means the shareholders of Dimerix. Vendor Shares has the meaning in Resolution 1. WST means Western Standard Time, being the time in Perth, Western Australia. In this Notice, words importing the singular include the plural and vice versa. 23 Schedule 1 Overview of Dimerix and its activities 1. Review of Clinical Assets 1.1. Dimerix – DMX200 Clinical Trial & Pipeline Opportunities Dimerix lead therapy known as DMX200 recently commenced a Phase II clinical trial in Australia under the Clinical Trial Notification (CTN) pathway for the treatment of patients with chronic kidney disease (CKD). Ethics was granted in September 2014 and patient screening commenced shortly after. Patients are required to be stabilized on the standard of care therapy prior to dosing with DMX200. Safety and reduction of the amount of protein leaking into the urine, proteinuria, are the endpoints for this study. Data from the clinical trial is expected to be available during CY 1H 2016. Dimerix has additional opportunities that the Company intends to progress through animal studies in the next 0 – 12 months; these include: i. application of its lead therapy in other diseases including diabetic retinopathy and non-alcoholic steatohepatitis (NASH); ii. application of alternative drug combinations in multiple sclerosis and cancer fatigue. 1.2. DMX200 — A new approach for CKD DMX200 Figure 1. DMX200 significantly reduces proteinuria in animal model. Using its proprietary technology Dimerix has identified a combination of two drugs that, when administered together in the appropriate ratio, improves the outcome of chronic kidney disease in the animal models of the disease. This combination known as DMX200, involves drugs targeting the angiotensin Il Type 1 (AT1R) and the chemokine 2 receptors (CCR2). The studies in the 5/6 sub-total nephrectomy (STNx) rat model of chronic kidney disease, compared the efficacy of the DMX200 combination of irbesartan (IRB) and propagermanium (PPG), acting on the angiotensin Il and chemokine receptors respectively, to treatment with either drug alone. DMX200, when compared to either drug alone: i. Significantly reduced the amount of proteinuria (see Figure 1); ii. Significantly reduced the amount of macrophage infiltration into the kidney, and; iii. Significantly reduced the loss of podocytes in the kidney. 24 These data, in combination with the laboratory pharmacology produced using Dimerix's core technology, provide a compelling case to progress the DMX200 formulation into Phase Il clinical trials for chronic kidney disease. The data was recently published in one of the top peer-reviewed journals (Ayoub et.al., PLoS One 2015, Mar 25, 10(3)) 1.3. Clinical Rationale 1.3.1. Rationale for Development of Propagermanium - Irbesartan Combination Therapy Dimerix Bioscience has data suggesting a combination approach involving the simultaneous antagonism of both the angiotensin receptor (AT1R) and the chemokine receptor (CCR2) may provide improved antiproteinuria efficacy. The combination proposed by Dimerix consists of the AT1R blocker irbesartan together with the CCR2 pathway inhibitor – propagermanium. Dimerix have completed three pre-clinical studies examining this hypothesis in the STNx rat model of chronic kidney disease and proteinuria. In these three studies AT1R and CCR2 blockade have been shown to act synergistically to reduce proteinuria and podocyte loss to a greater level than when treated with blockade of either AT1 or CCR2 alone. In the current Phase II clinical study it is planned to use the same agents as studied in the pre-clinical studies. IRB is approved for human use and is well established in the treatment of patients with renal disease. There are currently no approved selective CCR2 receptor blockers, although a number of pharmaceutical companies are testing various investigational CCR2 inhibitors as monotherapies for a range of indications. Propagermanium has been selected for clinical development because it has activity as a CCR2 inhibitor (Reference: Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004 Jul;165(1):237-46), has a long history of human use and is the only commercially available CCR2 product. Irbesartan is an off-patent angiotensin receptor blocker and is a standard of care. 2. Review of Marketing and Development Strategy Rationale 2.1. Lead Indication - Chronic Kidney Disease Chronic kidney disease (CKD) can result from diabetes, high blood pressure and diseases that cause inflammation in the kidneys. As CKD progresses it can lead to end-stage renal disease (ESRD), where the kidneys fail completely. A person with ESRD must receive a kidney transplant or regular blood-cleansing treatments called dialysis. CKD is a large problem with over 26 million people affected in the United States alone, with 8.5 million having Stage 3 (of 5 stages) or worse disease progression. US Sales of an estimated US$2.3 billion are mainly derived from Stage 5 CKD with Stages 3 and 4 representing largely untapped markets. 2.2. CKD -Medical Need Chronic kidney disease (CKD) is a major public health problem in Australia and throughout the world. It is estimated that 10% of all adults presenting to a general practice in Australia have CKD. One in ten Australian adults show at least one indicator of kidney damage. Progression of CKD ultimately results in kidney failure requiring dialysis. The cost of treating end stage kidney failure is estimated to be around $12 billion to the Australian Government from 2009 to 2020. Kidney disease contributes to approximately 15% of all hospitalisations in Australia. Individuals with CKD have a 2 to 3-fold greater risk of cardiac death than individuals without CKD. Recent studies have confirmed that even early CKD constitutes a significant risk factor for cardiovascular events and death. For people with CKD, the risk of dying from cardiovascular events is up to 20 times greater than requiring dialysis or transplantation. As such there is a large unmet need for therapies that may stop or reduce the progression of CKD. It is for this reason that Dimerix are developing DMX200 and based on encouraging animal data are now recruiting patients into a Phase II clinical study. 25 However CKD is comprised of a number of sub indications such as Nephrotic Syndrome, which has a small patient population enabling the orphan drug pathway to be initiated, and Diabetic Nephropathy which is increasingly common due to the increasing prevalence of Type II diabetes. 2.3. Rational for Clinical Development Approach Kidney disease can arise from diabetic and non-diabetic disorders. Several large interventional studies have uniformly found that proteinuria is a major risk factor for the progression of renal disease. Furthermore, a reduction of proteinuria regardless of whether kidney disease is from diabetic or nondiabetic disorders has been associated with a decreased risk of adverse renal end-points. Proteinuria can be measured from urine samples provided in a clinical study with reduction monitored over a twelve week time period for proposed dosing levels. As a result, for some indications, in the United States, the Food and Drug Administration (FDA) will consider a reduction in proteinuria as a primary endpoint to approve treatments. The DMX200 Phase II clinical program in chronic kidney disease will measure the reduction in proteinuria from patients with both diabetic and non-diabetic disorders. Phase I is not required due to the extensive clinical history of the components of DMX200. Dimerix intends to initially target development of the treatment for Nephrotic Syndrome, as an orphan indication, to leverage the reduced statistical burden imposed by regulatory bodies for such diseases. Nephrotic syndrome affects approximately 20,000 – 25,000 patients in the United States and there is currently a lack of approved treatments for this condition. In the United States, an orphan-drug designation is granted for novel drugs that treat a rare disease or condition affecting fewer than 200,000 patients in the U.S. Orphan drugs have faster development time lines and lower R&D expenses, due to the reduced size of the trials required by the FDA. Once on the market, they tend to have less competition, lower marketing costs, and a longer life-cycle with less risk of generic erosion. The DMX200 trial is planned to include up to 60 patients (in two parts) and will be conducted initially at three sites in Melbourne. Proof of concept of reduction in proteinuria, is expected to enable outlicensing of DMX-200 to a large partner to develop for prevalent indications such as Diabetic Nephropathy 2.4. DMX200 – Additional Indications DMX200 could also be used for non-renal complications such as such as diabetic retinopathy and nonalcoholic steatohepatitis (NASH). There is a large market for treatment of diabetic retinopathy (over 8 million in the US alone as per National Eye Institute); however approvals for new treatments in large medical indications like this will require more complex trials measuring multiple end points over longer periods. Dimerix strategy is thus to initially target orphan indications and use this data to support partnering with a major pharmaceutical company to address the significantly larger, and more complex, market segments including diabetic nephropathy, diabetic retinopathy, and NASH. 26 Schedule 2 – Pro-forma Balance Sheet SUN BIOMEDICAL LIMITED Consolidated Pro-forma Balance Sheet As at 31 December 2014 Sun Biomedical Dimerix Bioscience Audit Reviewed Unaudited Dec-14 Dec-14 $ $ Proforma Adjustments Consolidated $ $ Current Assets Cash and cash equivalents 2 1,693,385 682,642 1,481,750 3,857,777 22,089 140,414 - 162,503 0 2,919 - 2,919 1,715,474 825,975 1,481,750 4,023,199 5,447 2,887 - 8,334 - - 3,321,112 3,321,112 5,447 2,887 3,321,112 3,329,446 1,720,921 828,862 4,802,862 7,352,645 Trade and other payables (26,117) (21,659) - (47,776) Provisions (11,266) (7,499) - (18,765) Total Current Liabilities (37,383) (29,158) - (66,541) Total Liabilities (37,383) (29,158) - (66,541) 1,683,538 799,704 4,802,862 7,286,104 31,057,535 4,378,510 (25,321,135) 10,114,910 Trade and other receivables Other assets Total Current Assets Non-Current Assets Property, plant & equipment Intangibles - Intellectual Property 1 Total Non-Current Assets Total Assets Current Liabilities Net Assets Equity Issued capital 3(i) Performance share reserve 3(ii) Option reserve Accumulated (profit)/losses Net Equity - - 750,000 750,000 157,979 - (67,979) 90,000 (29,531,976) (3,578,806) 29,441,976 3,668,806 1,683,538 799,704 4,802,862 7,286,104 27 Notes to the Proforma Consolidated Balance Sheet Note 1 – Business Combinations Business combinations occur when an entity, referred to as the acquirer, obtains control over one or more businesses (the acquiree). This is typically achieved through the acquisition of in excess of 50% of the voting rights of the acquiree. For the purpose of this pro-forma, the acquirer for accounting purposes is deemed to be Dimerix Bioscience Limited, and Sun Biomedical Limited is the acquiree. As a result, reverse acquisition principles have been applied. The pro-forma adjustments reflect the fair value of the consideration paid to acquire Sun Biomedical. The deemed fair value of Sun Biomedical is based on the share price of Sun Biomedical at the deemed acquisition date (573,640,008 shares at $0.01 per share) and adjusted for the performance shares to be issued ($750,000). The difference between the deemed purchase price ($6,486,400) and the fair value of the pro-forma recognised net assets of Sun Biomedical ($3,165,288) amounting to $3,321,112 has been allocated to Intangible Assets – Intellectual Property. The cost of acquisition has been determined as follows: $ 537,640,008 Shares at $0.01 225,000,120 Performance Shares at a deemed value of 5,736,400 750,000 6,486,400 Note 2 – Cash and Cash Equivalents Current Assets Cash and cash equivalents Adjustments to the pro-forma cash balance are summarised as follows: Proceeds from issue of 160,000,000 shares at $0.01 Capital raising costs and other expenses Other cash expenses Sun Biomedical $ 1,693,385 Dimerix Bioscience $ 682,642 Combined $ 2,376,027 1,600,000 (96,000) (22,250) 3,857,777 28 Note 3 – Contributed Equity (i) Issued Capital Consolidated Adjusted 31 December 2014 $ 30 June 2014 10,114,910 30,286,353 Number of Shares 31 December 2014 Consolidated 2014 $ Balance at 31 December 2014 413,640,008 31,057,535 Shares to be issued prior to the Dimerix Transaction pursuant to a capital raising 160,000,000 1,600,000 Issued capital Capital raising costs - cash (96,000) Capital raising costs – Advisor share options (222,000) 573,640,008 Merger of Sun Biomedical Limited and Dimerix Bioscience Limited Elimination of existing Sun Biomedical shares Total shares outstanding at Balance Date (ii) 32,339,535 (32,339,535) Existing Dimerix shares on acquisition Issue of Sun Biomedical shares on acquisition $ 4,378,510 750,000,000 5,736,400 1,323,640,008 10,114,910 Performance Shares Reserve Under the terms of the proposed acquisition agreement, Sun Biomedical will issue performance shares as noted in this Notice of Meeting. These have been valued taking into account the estimated probability that the performance conditions will be achieved, resulting in a value of $750,000, which has been credited to the Performance Shares Reserve. 29 Schedule 3 – Risk Factors 1. Introduction There are a number of risks associated with the Acquisition that may have an impact on the financial returns received by Shareholders. These risks are important for Shareholders to understand. Shareholders are already exposed to a number of risks through their existing shareholding in the Company. A number of these risks are inherent in investing in securities generally and also inherent in any biotechnology company such as that of the Company and Dimerix. The risk factors facing Dimerix, and consequently the Company, include, but are not limited to, those detailed below. The below list of risk factors ought not to be taken as exhaustive of the risks faced by the Dimerix, and consequently, the Company. Additional risks not presently known to the Company, or if known, not considered material, may also have an adverse impact. The Directors believe that the advantages of the Acquisition outweigh the associated extent of the risks particularly as the Company has activities other than the activities of Dimerix. 2. Specific risk factors (a) Future Product Development and Commercialisation Dimerix is developing DMX200, its lead therapy, and continues to invest in further development in order to find new treatments for diseases and stay ahead of any emerging competition. The development and commercialisation of pharmaceutical products is subject to the inherent risk of failure, including the possibility that the products proposed to be developed by Dimerix may: • be found to be unsafe or ineffective; • fail to demonstrate any material benefit or advancement in safety and/or efficacy of an existing product; • fail to receive necessary regulatory approvals; • be difficult or impossible to manufacture on the necessary scale; • be uneconomical to market or otherwise not commercially exploitable; • fail to be developed prior to the successful marketing of a similar product by competitors; • compete with products marketed by third parties that are superior; and • fail to achieve the support or acceptance of physicians, patients or the medical community. In the case of DMX200, Dimerix intends to continue a Phase II Clinical Trial in the form described in Schedule 1. To do so, the clinical trial sites will need to recruit sufficient patients to obtain results from the study. Dimerix is in the process of opening 3 sites to minimise any risk of not recruiting sufficient patients and neither the Company nor Dimerix has reason to believe patients will not be able to be recruited in the ordinary course. The Company gives no guarantee as to timing of recruitment and numbers of patients recruited. 30 The Company gives no guarantee that further development of the Dimerix intellectual property will be successful, that development milestones will be achieved, or that the intellectual property will be developed into further products that are commercially exploitable. There are many risks inherent in the development of pharmaceutical products, particularly where the products are in the early stages of development. Projects can be delayed or fail to demonstrate any benefit, or may cease to be viable for a range of scientific and commercial reasons. (b) Intellectual property rights Dimerix relies on its ability to develop and commercialise intellectual property. A failure to develop and commercialise its intellectual property successfully would lead to a loss of opportunities and adversely impact on the operating results and financial position of Dimerix. Dimerix's success depends, in part, on its ability to obtain patents, maintain trade secret protection and operate without infringing the proprietary rights of third parties. Although Dimerix will implement all reasonable endeavours to protect its intellectual property, there can be no assurance that these measures will be sufficient. There is always a risk of third parties claiming involvement in technological and medical discoveries, and if any disputes arise, they can adversely affect Dimerix. Further, competition in retaining and sustaining protection of intellectual property and the complex nature of intellectual property can lead to expensive and lengthy patent disputes for which there can be no guaranteed outcome. Some competitors may be able to sustain the costs of litigation or proceedings more effectively than Dimerix because of greater financial resources. Securing rights to intellectual property, and in particular patents, is an integral part of securing potential product value in the outcomes of pharmaceutical research and development. The granting of a patent does not guarantee that the rights of others are not infringed or that competitors will not develop competing intellectual property that circumvents such patents. The patent position of pharmaceutical companies can be highly uncertain and frequently involve complex legal and scientific evaluation. The breadth of claims allowed in pharmaceutical patents and their enforceability cannot be predicted. There can be no assurance that any patents Dimerix may own or control or licence now and in the future will afford Dimerix a competitive advantage, commercially significant protection of the intellectual property, or that any of the projects that may arise from the intellectual property will have commercial application. Further, any patents protecting the intellectual property of Dimerix will have a finite life. There are no guarantees that revenues from a successful commercialisation would extend past patent expiry dates. Dimerix's lead therapy, DMX200, is protected by a number of pending applications for patents. Dimerix's proposed second therapy, referred to as DMX300, will also be developed within the protection of these patent applications. Dimerix's assay technology, Receptor HIT, is protected by a number of granted patents. The ability of Dimerix to successfully develop and commercialise DMX200 and DMX300 may be materially affected by an application for a patent in that patent family not being successful. Even if the patents are granted, there is still a risk that disputes may arise in relation to the patents (including those in place for Receptor HIT) resulting in the type of consequences referred to above and other parties may develop competing or superior technology impacting on the commercialisation of those patents. (c) Regulatory risks Dimerix’s operations are subject to laws, regulatory restrictions and certain government directives, recommendations and guidelines relating to, amongst other things, occupational 31 health and safety, laboratory practice, use and handling of hazardous materials, prevention of illness and injury and environmental protection. Any changes may increase the cost of compliance in the future. The pharmaceutical regulatory regime, which includes pre-clinical studies and clinical trials of each product in order to establish its safety and efficacy, is uncertain, can take significant periods of time and require the expenditure of significant resources. This includes securing clinical investigators and medical institutions to enrol patients in Dimerix's clinical trials and other third parties to perform data collection and analysis. As a result, Dimerix may face costs and delays outside of its control. Data obtained from pre-clinical and clinical activities are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval or clearance. Before Dimerix can market and sell its products, it must demonstrate that the products are safe and effective and must obtain necessary approvals from market regulators (for example, the Australian Therapeutic Goods Administration and the United States Food and Drug Administration). (d) Clinical validation risk The process of securing marketing approval of a new product is both costly and time consuming. Moving from discovery to development and subsequent commercialisation typically involves multiple and progressively larger clinical trials. Such trials can be expensive, time consuming, may be delayed or may fail. Clinical trial success can be impacted by a number of factors including incomplete or slower than expected recruitment of a sufficient number of patients, failure to meet trial end points, lack of product effectiveness during the trial, safety issues and modifications to trial protocols or changes to regulatory requirements for trials. There is no guarantee that any future trials will demonstrate that Dimerix's products are successful. This may delay the market adoption rate and impact Dimerix's future performance. (e) Sufficiency of funding Dimerix's ability to implement its overall business strategy may depend in part on its ability to continue to raise additional funds. Following the acquisition, the Company's ability to raise further capital (equity or debt) within an acceptable time, or a sufficient amount and on terms acceptable to it will vary according to a number of factors, including the success of current projects, the result of research and development and other cyclical factors affecting the Company, Dimerix and financial and share markets generally. No assurance can be given that future funding will be available, or that it will be available on terms acceptable to the Company. The Company will not have sufficient capital from the Capital Raising to progress through to marketing approval. Thus, the Company will either have to raise additional capital through further offers, or rely on securing a commercial transaction to further this program internally. (f) Manufacturing and product quality risks Dimerix currently uses third party manufacturers to produce its products. There is no guarantee that its manufacturing partners will be able to meet Dimerix's cost, quality and volume requirements which are needed to be competitive. Dimerix's products must also meet the regulatory requirements which are subject to continual review including inspection by regulatory authorities. Failure by Dimerix or its suppliers to continuously comply with applicable regulatory requirements or failure to take satisfactory corrective action in response to adverse inspection, could result in enforcement actions, including a public warning letter, a shutdown of, or restrictions on, Dimerix's outsourced 32 manufacturing operations, delays in approving or clearing products, refusal to permit the import or export of Dimerix's products, product recalls or other enforcement action. (g) Product liability The future sale of its products will expose Dimerix to product liability risks which are inherent in the research and development, manufacturing, marketing and use of its products. Following the Acquisition, the Company will seek to obtain and maintain adequate levels of insurance to cover product liability risks. Despite this, there can be no guarantee that adequate insurance coverage will be available at an acceptable cost (or in adequate amounts), if at all, or that product liability or other claims will not materially and adversely affect the operations and condition of the Company following the Acquisition. A product liability claim may give rise to significant liabilities as well as damage the Company’s and Dimerix's reputation. (h) Industry and competition Dimerix's potential competitors may include companies with substantially greater resources and access to more markets. Therefore, competitors may succeed in developing products that are safe, more effective or otherwise commercially superior than those being developed by Dimerix or which could render Dimerix's products obsolete and/or otherwise uncompetitive. In addition, Dimerix may not be able to compete successfully against current or future competitors where aggressive pricing policies are employed to capture market share. Such competition could result in price reductions, reduced gross margins and loss of market share, any of which could materially and adversely impact the Company’s future business, operating results and financial position. Technological change presents Dimerix with significant opportunities for growth. However, the risk remains that any competitor may introduce a new technology enabling it to gain a significant competitive advantage over Dimerix. (i) Reputational risk Dimerix's reputation and brand and its products are important to Dimerix's standing in the pharmaceutical and biotechnology industries and also important in attracting and retaining high calibre medical professionals. Any reputation damage or negative publicity around Dimerix or its products could adversely impact Dimerix’s business. Reputational damage could arise due to a number of circumstances including: • inadequate services or unsatisfactory clinical outcomes for patients; • error, malpractice or negligence of Dimerix’s employees; or • error, malpractice or negligence of the licensed medical specialists performing the treatments. (j) Reliance of key personnel Due to the specialised nature of Dimerix's business, its ability to commercialise its products and maintain its research program will depend in part on its ability to attract and retain suitably qualified management, scientists and research personnel. 33 There can be no assurance that Dimerix will be able to attract or retain sufficiently qualified scientific and management personnel, or maintain its relationship with key scientific organisations. The loss of key scientific and management personnel could have a detrimental impact on Dimerix and following the Acquisition this may adversely affect the Company and may impede the achievement of its research, product development and commercialisation objectives. Dimerix also faces competition to employ and retain the services of such individuals. (k) Health care insurers and reimbursement In both domestic and foreign markets, treatment volumes are likely to be influenced by the availability and amounts of reimbursement of patients’ medical expenses by third party payer organisations including government agencies, private health care insurers and other health care payers. There is no assurance that reimbursement of any products or services developed and commercialised by Dimerix will be available to patients at all or without substantial delay. Even if such reimbursement is provided, the approved reimbursement amounts may not be sufficient to enable Dimerix to sell products development on a profitable basis. (l) Uncertainty of future profitability Dimerix is not expected to be profitable in the near future and is therefore reliant on external funding. The Company’s ability to operate profitably in the future will depend in part on its ability to successfully commercialise Dimerix's products and grow sales of its assay business and/or develop an international distribution network on appropriate terms. Other factors that will determine the Company’s profitability following the Acquisition are its ability to manage its costs, its ability to execute its development and growth strategies, economic conditions in the markets in which it operates, competitive factors and regulatory developments. Accordingly, the extent of future profits, if any, and the time required to achieve a sustained profitability are uncertain. (m) Limited operating history Dimerix was incorporated in December 2004 and accordingly has a limited operating history. Dimerix is not currently making an operating profit and the Acquisition should be considered in light of the risks, expenses and difficulties frequently encountered by companies in their early stage of development. (n) No independent valuation No independent valuation has been carried out on Dimerix or its products. Valuations of technology and intellectual property operating in new markets are imprecise and subjective. The Directors do not believe that an independent valuation would be meaningful given the likely qualifications to, and limitations of, such a valuation and the difficulties in predicting the future commercial success of Dimerix and its products. 34 3. General Risks (a) Additional Requirements for Capital The Company's capital requirements depend on numerous factors. Depending on the Company's ability to generate income from its operations, the Company may require further financing in the future. Any additional equity financing will dilute shareholdings, and debt financing, if available, may involve restrictions on financing and operating activities. If the Company is unable to obtain additional financing as needed, it may be required to reduce the scope of its operations and scale back its development programmes as the case may be. The Company is raising funds to undertake clinical trials. There is a risk that funds raised may be insufficient to undertake the trial as planned. The Company may therefore need to raise additional funds to achieve its clinical and commercial objectives. The Company may also need to raise additional funds to obtain regulatory approvals if the clinical trials are successful and to commercialize its intellectual property if regulatory approvals are obtained. Unfavourable trial results may adversely affect the Company's ability to raise funding. The Company may also incur additional expenses in pursuing other indications for DMX200. Even if Dimerix's products receive regulatory approval for commercialisation, it may not achieve commercial success. Commercial revenues, if any, will be derived from the sales of a treatment which may not become commercially available for several years, if at all. In addition to the expenses of developing DMX200, the Company will incur substantial additional expenses to operate as a public company, to protect its intellectual property rights and to defend against intellectual property‐related claims. Accordingly, the Company may require additional financing subsequent to the Capital Raising to achieve its business objectives. Additional financing may not be available on acceptable terms, or at all. Further issues of securities may dilute the holdings of existing shareholders. If funding is not available, the Company may have to slow or otherwise limit the testing and development of DMX200, limit the number of other products it attempts to develop, delay commercialisation or reduce the scope of any sales and marketing activities. (b) Potential Acquisitions As part of its business strategy, the Company may make acquisitions of, or significant investments in, complementary companies or assets. Any such transactions will be accompanied by risks commonly encountered in making such acquisitions. (c) Market Conditions Share market conditions may affect the value of the Company’s quoted securities regardless of the Company’s operating performance. Share market conditions are affected by many factors such as: (i) general economic outlook; (ii) interest rates and inflation rates; (iii) currency fluctuations; (iv) changes in investor sentiment towards particular market sectors; 35 (v) the demand for, and supply of, capital; and (vi) terrorism or other hostilities. The market price of securities can fall as well as rise and may be subject to varied and unpredictable influences on the market for equities in general and pharmaceutical stocks in particular. Neither the Company nor the Directors warrant the future performance of the Company or any return on an investment in the Company. (d) Research and Development The Company can make no representation that any of its proposed research into or development of its intellectual property (including the Dimerix intellectual property) necessary to achieve commercial production will be successful, that development milestones will be achieved, or that the intellectual property will be developed into products that are commercially exploitable. There are many risks inherent in the development of pharmaceutical products, particularly where the products are in the early stages of development. Projects can be delayed or fail to demonstrate sufficient benefit, or research may cease to be viable for a range of scientific and commercial reasons. (e) Insurance The Company will, where possible and economically practicable, endeavor to mitigate some risks by procuring relevant insurance cover. However, such insurance cover may not always be available or economically justifiable. The policy provisions and exclusions may render a particular claim by the Company outside the scope of the insurance cover. While the Company will undertake all reasonable due diligence in assessing the creditworthiness of its insurance providers, there will remain the risk that an insurer defaults in payment of a legitimate claim by the Company under an insurance policy. (f) Regulatory Risk The introduction of new legislation or amendments to existing legislation by governments, developments in existing common law, or the respective interpretation of the legal requirements in any of the legal jurisdictions which govern the Company's (including Dimerix's) operations or contractual obligations, could impact adversely on the assets, operations and, ultimately, the financial performance of the Company and its Shares. In addition, there is a commercial risk that legal action may be taken against the Company (including Dimerix) in relation to commercial matters. (g) Forward Looking Information Certain information in this Notice constitutes forward looking information that is subject to risks and uncertainties and a number of assumptions, which may cause actual future events to be materially different from the expectations expressed or implied in this Notice. (h) Growth There is a risk that the Company may be unable to manage its future growth successfully. The ability to hire and retain skilled personnel and third party personnel may also be a significant obstacle to growth. 36 (i) Investment Highly Speculative The above list of risk factors ought not to be taken as exhaustive of the risks faced by the Company or by investors in the Company. The above factors, and others not specifically referred to above may, in the future, materially affect the financial performance of the Company and the value of the Company's securities. Therefore, the Shares carry no guarantee with respect to the payment of dividends, returns of capital or the market value of the Shares. 37 Schedule 4 - Terms and Conditions of Performance Shares For the purpose of these terms and conditions: ASX means ASX Limited ACN 008 624 691 or, as the context permits, the securities exchange operated by that entity. Change of Control Event means: (a) (b) the occurrence of: (i) the offeror under a takeover offer in respect of all Shares announcing that it has achieved acceptances in respect of 50.1% or more of the Shares; and (ii) that takeover bid has become unconditional; or the announcement by the Company that: (i) (ii) shareholders of the Company have at a Court convened meeting of shareholders voted in favour, by the necessary majority, of a proposed scheme of arrangement under which all Shares are to be either: (A) cancelled; or (B) transferred to a third party; and the Court, by order, approves the proposed scheme of arrangement. Company means Sun Biomedical Limited ACN 001 285 230. Completion means completion of the sale and purchase of all of the issued capital in Dimerix pursuant to the sale agreements to be entered into by the Company and each of the shareholders of Dimerix pursuant to the terms of the Implementation Agreement between the Company and Dimerix dated 8 May 2015. Dimerix means Dimerix Bioscience Limited ACN 112 223 417. DMX-200 means the combination therapy treatments covered under US patent application number 13/979,127 and any related patents, patent applications, continuations or divisionals thereof. Expiry Date means the A Expiry Date, the B Expiry Date and the C Expiry Date (as applicable). Holder means a holder of a Performance Share. Listing Rules means the Listing Rules of the ASX. Performance Share means a Class A Performance Share, a Class B Performance Share and/or a Class B Performance Share (as applicable). Share means a fully paid ordinary share in the Company. 1. Conversion and expiry of Performance Shares (a) (Conversion on achievement of A Milestone) Upon receipt by the Company or Dimerix of a notice of allowance from the United States Patent and Trademark Office in relation to the US patent application number 13/979,127 (or any divisional or continuation thereof) (A Milestone), each Class A Performance Share will convert into a Share on a one for one basis. (b) (A Expiry) The A Milestone must be achieved on or before 5.00pm (WST) on the later of 30 June 2017 or the date which is 24 months after the date of Completion (A Expiry Date). (c) (Conversion on achievement of B Milestone) Upon the Company’s board of directors making an investment decision to proceed to file an application to the US Food and 38 Drug Administration for a pre-Investigational New Drug (“pre-IND”) meeting to progress development of DMX200 following the receipt of data generated under the current clinical trial for chronic kidney disease supporting further progression of the technology (B Milestone), each Class B Performance Share will convert into a Share on a one for one basis. (d) (B Expiry) The B Milestone must be achieved on or before 5.00pm (WST) on the later of 30 June 2019 or the date which is 48 months after the date of Completion (B Expiry Date). (e) (Conversion on achievement of C Milestone) Upon receipt of ethics approval allowing commencement of a second clinical trial derived from the Dimerix platform and in relation to an indication that is not covered under the existing Austin Human Research Ethics Committee approval (C Milestone), each Class C Performance Share will convert into a Share on a one for one basis. (f) (C Expiry) The C Milestone must be achieved on or before 5.00pm (WST) on the later of 30 June 2019 or the date which is 48 months after the date of Completion (C Expiry Date). (g) (No conversion) To the extent that: (i) the Class A Performance Shares have not converted into Shares on or before the A Expiry Date, then all such unconverted Class A Performance Shares held by each Holder will automatically consolidate into one Class A Performance Share and will then convert into one Share; (ii) the Class B Performance Shares have not converted into Shares on or before the B Expiry Date, then all such unconverted Class B Performance Shares held by each Holder will automatically consolidate into one Class B Performance Share and will then convert into one Share; and. (iii) the Class C Performance Shares have not converted into Shares on or before the C Expiry Date, then all such unconverted Class C Performance Shares held by each Holder will automatically consolidate into one Class C Performance Share and will then convert into one Share. (h) (Conversion procedure) The Share or Shares issued on conversion of a Performance Share will be issued to the Holder and the Company will issue the Holder with a new holding statement for the Share or Shares as soon as practicable following the conversion of each Performance Share. (i) (Ranking of shares) Each Share into which the Performance Share will convert will upon issue: (i) rank equally in all respects (including, without limitation, rights relating to dividends) with other issued Shares; (ii) be issued credited as fully paid; (iii) be duly authorised and issued by all necessary corporate action; and (iv) be issued free from all liens, charges and encumbrances whether known about or not including statutory and other pre-emptive rights and any transfer restrictions. 39 2. Conversion on change of control (a) (b) 3. Subject to paragraph (b), if there is a Change of Control Event in relation to the Company prior to the conversion of the Performance Shares, then: (i) each of the A Milestone, the B Milestone and the C Milestone will be deemed to have been achieved; and (ii) each Performance Share will automatically and immediately convert into a Share. If the number of Shares to be issued as a result of the conversion of all Performance Shares due to a Change in Control Event in relation to the Company is in excess of 10% of the total fully diluted share capital of the Company at the time of the conversion, then the number of Class A Performance Shares, Class B Performance Shares and Class C Performance Shares to be converted will be prorated so that the aggregate number of Shares issued upon conversion of the Performance Shares is equal to 10% of the entire fully diluted share capital of the Company. Rights attaching to Performance Shares (a) (Share capital) Each Performance Share is a share in the capital of the Company. (b) (General meetings) Each Performance Share confers on a Holder the right to receive notices of general meetings and financial reports and accounts of the Company that are circulated to shareholders. A Holder has the right to attend general meetings of shareholders of the Company. (c) (No voting rights) A Performance Share does not entitle a Holder to vote on any resolutions proposed at a general meeting of shareholders of the Company. (d) (No dividend rights) A Performance Share does not entitle a Holder to any dividends. (e) (Rights on winding up) Each Performance Share entitles a Holder to participate in the surplus profits or assets of the Company upon winding up of the Company, but only to the extent of $0.0001 per Performance Share. (f) (Not transferable) A Performance Share is not transferable. (g) (Reorganisation of capital) If the Company undertakes a bonus issue or there is a reorganisation (including, without limitation, consolidation, sub-division, reduction or return) of the issued capital of the Company, the number of Shares to which the Performance Shares of a Holder can convert will be increased or decreased (as applicable) to the number of Shares which the Holder would hold if the Performance Shares had been converted to Shares before the record date for the bonus issue or reorganisation. (h) (Quotation of shares on conversion) An application will be made by the Company to ASX for official quotation of the Shares issued upon the conversion of each Performance Share within the time period required by the Listing Rules. (i) (Participation in entitlements and bonus issues) A Performance Share does not entitle a Holder to participate in new issues of capital offered to holders of Shares, such as bonus issues and entitlement issues. (j) (No other rights) A Performance Share does not give a Holder any rights other than those expressly provided by these terms and those provided at law where such rights at law cannot be excluded by these terms. 40 Schedule 5 - Terms and Conditions of Adviser Options Entitlement Each Option gives the holder (Optionholder) the right to subscribe for one fully paid ordinary share in the Company (Share) upon exercise of the Option. Exercise Price The exercise price payable upon exercise of each Option is $0.01. Vesting Conditions None. Expiry Date 5.00pm (WST) on 30 June 2017. Lapse/Expiry Unless the Company’s board of directors determines otherwise in its absolute discretion, an unexercised Option will lapse upon the earliest to occur of: Notice of Exercise (a) where an Optionholder purports to transfer, assign, mortgage, charge or otherwise dispose of or encumber (in whole or in part) an Option; or (b) the Expiry Date. An Optionholder may exercise their Options by lodging with the Company, on or prior to the Expiry Date: (a) in whole or in part, and if exercised in part, multiples of 1,000 must be exercised on each occasion; (b) a written notice of exercise of Options specifying the number of Options being exercised (Exercise Notice); and (c) a cheque or electronic funds transfer for the Exercise Price for the number of Options being exercised. Cheques shall be in Australian currency made payable to the Company and crossed "Not Negotiable". An Exercise Notice is only effective when the Company has received the full amount of the Exercise Price in cleared funds. Timing of issue of Shares Within 10 Business Days of receipt of the Exercise Notice accompanied by the Exercise Price, the Company will issue the number of Shares required under these terms and conditions in respect of the number of Options specified in the Exercise Notice. Shares issued on exercise All Shares issued upon the exercise of Options will upon issue rank equally in all respects with the then issued Shares. Quotation of Shares on exercise The Company will apply for official quotation on ASX of all Shares issued upon exercise of Options within 10 Business Days after the date of issue of those Shares. Quotation of Options The Options will be unlisted upon grant. No application for quotation of the Options will be made. Options not transferable The Options will not be transferable. Participation in new issues There are no participation rights or entitlements inherent in the Options and Optionholders will not be entitled to participate in new issues of capital offered to shareholders during the currency of the Options. However, the Company will 41 give the holders of Options notice of the proposed issue prior to the date for determining entitlements to participate in any such issue. Adjustment for bonus issues of Shares Adjustment for entitlement issue If the Company makes a bonus issue of Shares or other securities to existing shareholders (other than an issue in lieu or in satisfaction of dividends or by way of dividend reinvestment): (a) the number of Shares which must be issued on the exercise of an Option will be increased by the number of Shares which the Optionholder would have received if the Optionholder had exercised the Option before the record date for the bonus issue; and (b) no change will be made to the Exercise Price. If the Company makes an issue of Shares pro rata to existing shareholders (other than an issue in lieu or in satisfaction of dividends or by way of dividend reinvestment) the Exercise Price of an Option will be reduced according to the following formula: New exercise price = Adjustments for reorganisation O - E[P-(S+D)] N+1 O = the old Exercise Price of the Option. E = the number of underlying Shares into which one Option is exercisable. P = average market price per Share weighted by reference to volume of the underlying Shares during the 5 trading days ending on the day before the ex rights date or ex entitlements date. S = the subscription price of a Share under the pro rata issue. D = the dividend due but not yet paid on the existing underlying Shares (except those to be issued under the pro rata issue). N = the number of Shares with rights or entitlements that must be held to receive a right to one Share. If there is any reorganisation of the issued share capital of the Company, the rights of the Optionholder may be varied to comply with the ASX Listing Rules which apply to a reorganisation of capital at the time of the reorganisation. 42 Schedule 6 - Terms and Conditions of Transaction Options Entitlement Each Option gives the holder (Optionholder) the right to subscribe for one fully paid ordinary share in the Company (Share) upon exercise of the Option. Issue price of Options Options are issued for no consideration. Exercise Price The exercise price payable upon exercise of each Option is $0.02. Vesting Conditions For each Optionholder (other than Kevin Pfleger): 1/3 Options will automatically vest on issue (Tranche 1). 1/3 Options will automatically vest six months after issue (Tranche 2). 1/3 Options will automatically vest 12 months after issue (Tranche 3). For Options granted to Kevin Pfleger: All Options will automatically vest on completion of any transaction which generates $50,000 or more in revenue in Dimerix’s assay business. If any takeover, merger, trade sale or change of control event occurs, any unvested Options will immediately vest. Expiry Date 5.00pm (WST) on 30 June 2017. Lapse/Expiry Unless the Company’s board of directors determines otherwise in its absolute discretion, an unexercised Option will lapse upon the earliest to occur of: Notice of Exercise (c) where an Optionholder purports to transfer, assign, mortgage, charge or otherwise dispose of or encumber (in whole or in part) an Option; or (d) the Expiry Date. An Optionholder may exercise their Options by lodging with the Company, on or prior to the Expiry Date: (d) in whole or in part, and if exercised in part, multiples of 1,000 must be exercised on each occasion; (e) a written notice of exercise of Options specifying the number of Options being exercised (Exercise Notice); and (f) a cheque or electronic funds transfer for the Exercise Price for the number of Options being exercised. Cheques shall be in Australian currency made payable to the Company and crossed "Not Negotiable". An Exercise Notice is only effective when the Company has received the full amount of the Exercise Price in cleared funds. Timing of issue of Shares Within 10 Business Days of receipt of the Exercise Notice accompanied by the Exercise Price, the Company will issue the number of Shares required under these terms and conditions in respect of the number of Options specified in the Exercise Notice. Shares issued on exercise All Shares issued upon the exercise of Options will upon issue rank equally in all respects with the then issued Shares. 43 Quotation of Shares on exercise The Company will apply for official quotation on ASX of all Shares issued upon exercise of Options within 10 Business Days after the date of issue of those Shares. Quotation of Options The Options will be unlisted upon grant. No application for quotation of the Options will be made. Options not transferable The Options will not be transferable. Participation in new issues There are no participation rights or entitlements inherent in the Options and Optionholders will not be entitled to participate in new issues of capital offered to shareholders during the currency of the Options. However, the Company will give the holders of Options notice of the proposed issue prior to the date for determining entitlements to participate in any such issue. Adjustment for bonus issues of Shares If the Company makes a bonus issue of Shares or other securities to existing shareholders (other than an issue in lieu or in satisfaction of dividends or by way of dividend reinvestment): Adjustment for entitlement issue (c) the number of Shares which must be issued on the exercise of an Option will be increased by the number of Shares which the Optionholder would have received if the Optionholder had exercised the Option before the record date for the bonus issue; and (d) no change will be made to the Exercise Price. If the Company makes an issue of Shares pro rata to existing shareholders (other than an issue in lieu or in satisfaction of dividends or by way of dividend reinvestment) the Exercise Price of an Option will be reduced according to the following formula: New exercise price = Adjustments for reorganisation O - E[P-(S+D)] N+1 O = the old Exercise Price of the Option. E = the number of underlying Shares into which one Option is exercisable. P = average market price per Share weighted by reference to volume of the underlying Shares during the 5 trading days ending on the day before the ex rights date or ex entitlements date. S = the subscription price of a Share under the pro rata issue. D = the dividend due but not yet paid on the existing underlying Shares (except those to be issued under the pro rata issue). N = the number of Shares with rights or entitlements that must be held to receive a right to one Share. If there is any reorganisation of the issued share capital of the Company, the rights of the Optionholder may be varied to comply with the ASX Listing Rules which apply to a reorganisation of capital at the time of the reorganisation. 44 All registry communications to: Automic Registry Services PO Box 223 West Perth WA 6872 ACN 001 285 230 Holder Number Security Holder Appointment of Proxy – General Meeting I/We being a Shareholder entitled to attend and vote at the Meeting, hereby appoint OR The Chair as my/our proxy (Name of Proxy) or failing the person/body corporate named, or if no person/body corporate is named, the Chairman of the Meeting, as my/our proxy to act generally at the meeting on my/our behalf, including to vote in accordance with the following directions (or, if no directions have been given, and to the extent permitted by law, as the proxy sees fit), at the Meeting of the Company to be held at Level 2, 1 Walker Avenue, West Perth on Tuesday, 30 June 2015 at 10.30 am (WST) and at any adjournment or postponement of the Meeting and to vote in accordance with the following directions (or if no directions have been given, and to the extent permitted by law as the proxy sees fit). Proxy appointments will only be valid and accepted by the Company if they are made and received no later than 48 hours before the meeting. Important – If the Chairman of the Meeting is your proxy or is appointed your proxy by default If the Chairman of the Meeting is your proxy or is appointed your proxy by default, unless you indicate otherwise by ticking either the ‘for’, ‘against’ or ‘abstain’ box in relation to a Resolution, you will be authorising the Chairman to vote in accordance with the Chairman’s voting intentions on each Resolution even if the Resolution is connected directly or indirectly with the remuneration of a member of the Key Management Personnel. The Chairman of the Meeting intends to vote all available proxies in favour of all Resolutions. Unless indicated otherwise by ticking the “for”,” against” or “abstain” box you will be authorising the Chair to vote in accordance with the Chair’s voting intention. VOTING ON BUSINESS OF THE MEETING Resolutions 1 Approval of Acquisition of Dimerix Bioscience Limited 2 Approval of new class of Securities Performance Shares 3 Approval of issue of Vendor Shares to Dr Anton Uvarov 4 Ratification of Tranche 1 Placement 5 For Against Abstain Resolutions 6 Appointment of Dr James Williams as a Director 7 Appointment of Dr Sonia Poli as a Director 8 Authority to grant Adviser Options 9 For Against Abstain Authority to grant Transaction Options Authority to issue Tranche 2 Placement Shares Please note: If you mark the abstain box for a particular Resolution, you are directing your proxy not to vote on that Resolution on a show of hands or on a poll and your votes will not be counted in computing the required majority on a poll. SIGNATURE OF SHAREHOLDER(S): Individual or Shareholder 1 Shareholder 2 Shareholder 3 Sole Director / Company Secretary Director Director / Company Secretary INSTRUCTIONS FOR COMPLETING ‘APPOINTMENT OF PROXY’ FORM APPOINTING A PROXY A Shareholder entitled to attend and cast a vote at the Meeting is entitled to appoint a proxy to attend and vote on their behalf at the Meeting. The appointed proxy may be an individual or body corporate. If a Body Corporate is appointed to act as your proxy then a representative of that Body Corporate must be appointed to act as its representative. When attending the meeting, the representative must bring a formal notice of appointment as per section 250D of the Corporations Act. Such notice must be signed as required by section 127 of the Corporations Act or the Body Corporate’s Constitution. If a Shareholder is entitled to cast 2 or more votes at the Meeting, the Shareholder may appoint a second proxy to attend and vote on their behalf at the Meeting. However, where both proxies attend the Meeting, voting may only be exercised on a poll. The appointment of a second proxy must be done on a separate copy of the Proxy Form. A Shareholder who appoints 2 proxies may specify the proportion or number of votes each proxy is appointed to exercise. If a Shareholder appoints 2 proxies and the appointments do not specify the proportion or number of the Shareholder’s votes each proxy is appointed to exercise, each proxy may exercise one-half of the votes. Any fractions of votes resulting from the application of these principles will be disregarded. A duly appointed proxy need not be a Shareholder. Note: If you wish to appoint a second proxy, you may copy this form but you must return both forms together. VOTING ON BUSINESS OF MEETING A Shareholder may direct a proxy how to vote by marking one of the boxes opposite each item of business. The direction may specify the number of votes that the proxy may exercise by writing the number of Shares next to the box marked for the relevant item of business. Where a box is not marked the proxy may vote as they choose subject to the relevant laws. Where more than one box is marked on an item the vote will be invalid on that item. SIGNING INSTRUCTIONS Individual: Where the holding is in one name, the Shareholder must sign. Joint holding: Where the holding is in more than one name, all of the Shareholders should sign. Power of attorney: If you have not already lodged the power of attorney with the registry, please attach a certified photocopy of the power of attorney to this Proxy Form when you return it. Companies: Where the company has a sole director who is also the sole company secretary, that person must sign. Where the company (pursuant to Section 204A of the Corporations Act) does not have a company secretary, a sole director can also sign alone. Otherwise, a director jointly with either another director or a company secretary must sign. Please sign in the appropriate place to indicate the office held. ATTENDING THE MEETING Completion of a Proxy Form will not prevent individual Shareholders from attending the Meeting in person if they wish. Where a Shareholder completes and lodges a valid Proxy Form and attends the Meeting in person, then the proxy’s authority to speak and vote for that Shareholder is suspended while the Shareholder is present at the Meeting. LODGEMENT OF VOTES To be effective, a validly appointed proxy must be received by the Company not less than 48 hours prior to commencement of the Meeting. Proxy appointments can be lodged by: a) Hand Delivery – Level 2, 1 Walker Ave, West Perth WA 6005; or b) Post - to PO Box 271, West Perth WA 6892; or c) Facsimile - (08) 9321 1204 if faxed from within Australia or +61 8 9321 1204 if faxed from outside Australia. Proxy Forms received later than this time will be invalid
© Copyright 2026