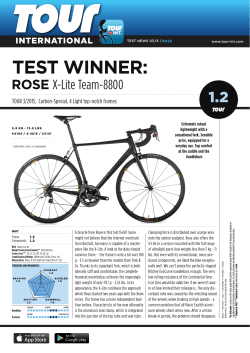
In vitro propagation of âSarraâ rose cv. Rosa sp. using axillary buds
In vitro propagation of “Sarra” rose cv. Rosa sp. using axillary buds. By Sarra Ali Saad Hamed B.Sc. (Honours) Faculty of Agriculture Kharoum University 1999 A Thesis submitted in Partial Fulfillment of The Requirement of the Degree of Master of Science in Agriculture Thesis superviser: Prof.Abd El Gaffar El Hag Saeed . Thesis co- superviser: Dr.Sayda Omer El Howayris . Department of Agricultural Biotechnology and Botany University of Khartoum Sudan(2003) Dedication To all my dignified teachers and virtuous people who encouraged me. To my great dearest teachers father and mother who tought me loving knowledge and Perseverance . To my dearest husband who was very helpfull and patient with me . To my dearest sister Sawsan and all my relatives and friends who helped me so much . To my dearest sun Ali who tought me the meaning of life . I give them this research as a present with all my great love and respect . Sarra Acknowledgement Praise and thanks to Allah who enabled me to finish this research . I would like to thank all those who helped me to carry out this research and bring it to its last form . My great thanks to my helpfull supervisor prof.Abd El Gaffar El Hag Said for all his great help in all this research stages. I am greatfull for all his time , knowledge and experience which he shared with me generously. Also I would like to express my gratitude to Dr. Sayda omer El Howayris my co-supervisor who spared no effort to supervise and guide this study. Iam great full for her encouragement, advice, and patience . I would like to express my gratitude to all Leena company staff who were very helpful and patient with me until I finished my practical work . All my thanks for all my friends, relatives, and all virtuous people who helped me to finish this study and bring it in this form . Contents 1- Introduction 1 2- Literature Review: 2-1.Botany of Roses 2-2.Taxonomy 2-3.Uses of Roses 2-4.Propagation of Roses 2-4.1.Traditional Techniques 2-4.2. Tissue Culture Techniques 2-4.2.1.Media Used 2-4.2.2.Plant Material and Explantation 2-4.2.3.Preparation of Media 2-4.2.4.Preparation of Explants 2-4.2.5.Tissue Culture of higher Species 2 2 2 4 6 6 7 7 8 8 8 9 3- Materials and Methods: 3-1.Plant Material 3-2.Sterilization 3-3.Culture Media 3-4.Culture of Explants 3-5.Experimentations 3-5.1.The Effect of Different Concentrations of Murashige and Skoog (1962)Salt Mixture on In Vitro Growth and Development of rose Axillary Buds. 3-5.2. The effect of Different Concentrations of Sodium Hypophosphate Salt(NaH2PO4) on In Vitro Growth and Development of rose Axillary Buds. 3-5.3.Energy Source: (Carbohydrates): 3-5.3.1.Sucrose 3-5.3.2.Glucose 3-5.4. The Effect of Different Concentrations of ThiamineHCl (Vitamin B1) on In Vitro Cultured Rose Axillary Buds 3-5.5. The Effect of Different Concentrations of Myo-Inositol on In Vitro Cultured Rose Axillary Buds 3-5.6. The Effect of Different Concentrations of Adenine Sulphate (A/S) on In Vitro Cultured Rose Axillary Buds 3-5.7. The Effect of Different Concentrations of Naphthalene Acetic Acid (NAA) and Benzyle Adenine(BA) on In Vitro Growth Rose Axillary Buds 18 18 18 18 18 18 18 20 20 20 20 20 20 20 20 3-5.8. The Effect of the Physical state of the Nutient Medium on the growth and development of In Vitro Cultured Rose Axillary Buds 3-5.9. The Effect of Darkness on growth and development of In Vitro Cultured Rose 3-6.Statistical Analysis 20 20 20 4-Results : 22 4-1.Effect of different concentrations ofMurashige and Skoog (MS,1962) salt mix on growth and development of rose axillary buds 22 4-2. Effect of different concentrations of sodium hypophosphate NaH2PO4 salt on growth and development of rose axillary buds 22 4-3.Effect of energy sources(carbohydrates): 4-3.1. Effect of different concentrations of sucrose on growth and development of rose axillary buds 4-3.2. Effect of different concentrations of glucose on growth and development of rose axillary buds 22 22 4-4.Effect of different concentrations of Thiamine –HCl (Vitamin B1 )on growth and development of rose axillary buds 37 4-5. Effect of different concentrations of Adenine Sulphate (A/S) (Vitamin B1 )on growth and development of rose axillary buds 37 4-6. Effect of different concentrations of myo-inositole on growth and development of rose axillary buds 37 4-7. Effect of different concentrations of growth regulators : 4-7.1.Naphthaline acetic acid (NAA) 4-7.2.Benzyl adenine(BA) 4-7.3. Benzyladenine(BA) + Naphthaline acetic acid (NAA) 37 37 37 57 4-8. Effect of the physical state of the medium 57 4-9.The effect of darkness 57 5-Discussion : 69 5-1. Effect of different concentrations of (MS) salt mix on growth and development of rose axillary buds 69 22 5-2. Effect of different concentrations of sodium hypophosphate NaH2PO4 salt on growth and development of rose axillary buds 5-3. .Effect of energy sources(carbohydrates) 5-3.1. Effect of different concentrations of sucrose on growth and development of rose axillary buds 5-3.2. Effect of different concentrations of glucose on growth and development of rose axillary buds 69 69 69 70 5-4. Effect of different concentrations of Thiamine –HCl (Vitamin B1 )on growth and development of rose axillary buds 70 5-5. Effect of different concentrations of Adenine Sulphate (A/S) (Vitamin B1 )on growth and development of rose axillary buds 70 5-6. Effect of different concentrations of myo-inositole on growth and development of rose axillary buds 71 5-7. Effect of different concentrations of growth regulators on growth and development of axillary buds 5-7.1. Naphthaline acetic acid (NAA) 5-7.2. Benzyl adenine(BA) 5-7.3. Benzyl adenine(BA) + Naphthaline acetic acid (NAA) 71 71 71 71 5-8. Effect of the physical state of the medium on growth and development of rose axillary buds 72 5-9.Effect of darkness 72 6-References 7- Appendix (1) 8- Appendix (2) 74 86 87 List of Tables Table(1): Effect of different concentrations of MS salts on in vitro growth and development of rose axillary buds. 23 Table(2): Effect of different concentrations of NaH2PO4 salts on in vitro growth and development of rose axillary buds. 26 Table(3): Effect of different concentrations of sucrose on in vitro growth and development of rose axillary buds. 29 Table(4): Effect of different concentrations of glucose on in vitro growth and development of rose axillary buds. 33 Table(5): Effect of different concentrations of thiamine -HCl on in vitro growth and development of rose axillary buds. 38 Table(6): Effect of different concentrations of adenine sulphate on in vitro growth and development of rose axillary buds. 42 Table(7): Effect of different concentrations of myo-inositol on in vitro growth and development of rose axillary buds. 46 Table(8): Effect of different concentrations of NAA on in vitro growth and development of rose axillary buds. 49 Table(9): Effect of different concentrations of BA on in vitro growth and development of rose axillary buds. 53 Table(10): Effect of different concentrations of BA+NAA on in vitro growth and development of rose axillary buds. 58 Table(11): Effect of the physical state of the medium on the in vitro growth and development of rose axillary buds. 62 Table(12): Effect of darkness on in vitro growth and development of rose axillary buds. 66 List of Figures Fig.(1): Effect of MS saltsmix concentration on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 24 Fig.(2): Effect of different concentrations of NaH2PO4 salt on plant height(cm) of in vitro cultured plantlets of “Sarra” rose cultivar 27 Fig.(3): Effect of different concentrations of NaH2PO4 salt on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 28 Fig.(4): Effect of different concentrations of sucrose on plant height(cm) of in vitro cultured plantlets of “Sarra” rose cultivar 30 Fig.(5): Effect of different concentrations of sucrose on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 31 Fig.(6): Effect of different concentrations of glucose on number of shoots of in vitro cultured plantlets of “Sarra” rose cultivar 34 Fig.(7): Effect of different concentrations of glucose on number of nods of in vitro cultured plantlets of “Sarra” rose cultivar 35 Fig.(8): Effect of different concentrations of thiamine-HCl on plant height(cm) of in vitro cultured plantlets of “Sarra” rose cultivar 39 Fig.(9): Effect of different concentrations of thiamine-HCl on number of shoots of in vitro cultured plantlets of “Sarra” rose cultivar 40 Fig.(10):Effect of different concentrations of adenine sulphate on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 43 Fig.(11):Effect of different concentrations of adenine sulphate on number of shoots of in vitro cultured plantlets of “Sarra” rose cultivar 44 Fig.(12): Effect of different concentrations of myo-inositol on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar Fig.(13): Effect of different concentrations of myo-inositol on plant height(cm)of in vitro cultured plantlets of “Sarra” rose cultivar Fig.(14): Effect of different concentrations of NAA on plant height(cm)of in vitro cultured plantlets of “Sarra” rose cultivar 47 48 50 Fig.(15): Effect of different concentrations of NAA on number of roots of in vitro cultured plantlets of “Sarra” rose cultivar 51 Fig.(16): Effect of different concentrations of BA on plant height(cm)of in vitro cultured plantlets of “Sarra” rose cultivar 54 Fig.(17):Effect of different concentrations of BA on number of shoots of in vitro cultured plantlets of “Sarra” rose cultivar 55 Fig.(18): Effect of different concentrations of BA+NAA on plant height(cm)of in vitro cultured plantlets of “Sarra” rose cultivar 59 Fig.(19): Effect of different concentrations of BA+NAA on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 60 Fig.(20): Effect of the physical state of the medium on plant height (cm)of in vitro cultured plantlets of “Sarra” rose cultivar 63 Fig.(21): Effect of the physical state of the medium on number of leaves of in vitro cultured plantlets of “Sarra” rose cultivar 64 Fig.(22): Effect of darkness on plant height (cm)of in vitro cultured plantlets of “Sarra” rose cultivar 67 Fig.(23): Effect of darkness on number of nods of in vitro cultured plantlets of “Sarra” rose cultivar 68 List of Plates Plate(1): Effect of the three multiplication media (A ,B ,and C) on the growth of the explants of “Sarra” rose. 19 Plate(2): “Sarra” rose plantlets growing on different MS salt mix strengths . 25 Plate(3): “Sarra” rose plantlets growing on different concentrations of sucrose . 32 Plate(4): “Sarra” rose plantlets growing on different concentrations of glucose . 36 Plate(5): “Sarra” rose plantlets growing on different concentrations of thiamine –HCl . 41 Plate(6): “Sarra” rose plantlets growing on different concentrations of adenine sulphate. 45 Plate(7): “Sarra” rose plantlets growing on different concentrations of NAA . 52 Plate(8): “Sarra” rose plantlets growing on different concentrations of BA. 56 Plate(9): “Sarra” rose plantlets growing on different concentrations of BA +NAA . Plate(10): “Sarra” rose plantlets cultured on agar or cotton . 61 65 Abstract This study was carried out at Lena Plant Tissue Culture Laboratory (Um al-kora) to determine the optimum concentration of Murashige and Skoog(MS, 1962) medium components for the growth of “Sarra” rose cultivar. Axillary buds were grown on Murashige and skoog multiplication medium (A). several trails were then conducted to modify his medium to fit “Sarra” rose cultivar’s clonal propagation. Full strength MS salt mix containing 30 gm/l sucrose, full strength sodium phosphate(Na H2 PO4) ,2.0 X Thiamine-HCL),and (0.3 mg/l) benzyl adenine (BA) for shoot growth and development . best rooting was obtained when using the above medium components plus 0.3 mg/l naphthalene acetic acid (NAA) instead of BA. Adding each growth regulator alone gives better results than adding them in combination. Adding adenine sulphate and myo-inositol to the medium doesn’t make great differences in plant growth but they improve it to some extent. Glucose can be used instead of sucrose and it gives better results than sucrose but the problem is that, glucose is more expensive than sucrose so it is not economically feasible. Using cotton instead of agar gave excellent results which encourage using it to avoid the expensive cost of agar. Incubation of plantlets under darkness did not give good results except plant height so there is no need for this treatment except if there is an urgent need for a quick increase in plant height. ﻣﻠﺨﺺ ﺍﻻﻃﺮﻭﺣﺔ ﺃﺟﺮﻯ ﻫﺬﺍ ﺍﻟﺒﺤﺚ ﺑﻤﻌﻤﻞ ﺷﺮﻛﺔ ﻟﻴﻨﺔ ﻟﺰﺭﺍﻋﺔ ﺍﻻﻧﺴﺠﺔ ﺍﻟﻨﺒﺎﺗﻴﺔ ﺍﻟﻤﺤﺪﻭﺩﺓ ﺑﺄﻡ ﺍﻟﻘﺮﻯ ،ﻻﻳﺠﺎﺩ ﺍﻟﺘﺮﺍﻛﻴﺰ ﺍﻟﻤﺜﺎﻟﻴﺔ ﻟﻮﺳﻂ ﻣﻮﺭﺍﺷﻴﺠﻰ ﻭﺳﻜﻮﻕ 1962ﻡ ﺍﻟﺼﺎﻟﺤﺔ ﻟﻨﻤﻮ ﺻﻨﻒ ﺍﻟﻮﺭﺩ ))ﺳﺎﺭﺓ((. ﺯﺭﻋﺖ ﺍﻟﺒﺮﺍﻋﻢ ﺍﻟﺠﺎﻧﺒﻴﺔ ﻓﻰ ﻭﺳﻂ ﻣﻮﺭﺍﺷﻴﺠﻰ ﻭﺳﻜﻮﺝ )ﺃ( ﻭﺍﻋﻘﺐ ﺫﻟﻚ ﻋﺪﺓ ﺗﺠﺎﺭﺏ ﻟﺘﺤﻮﻳﺮ ﻫﺬﺍ ﺍﻟﻮﺳﻂ ﻟﻴﻼﺋﻢ ﺍﻟﺘﻜﺎﺛﺮ ﺍﻟﺴﻼﻟﻰ ﻟﺼﻨﻒ ﺍﻟﻮﺭﺩ )ﺳﺎﺭﺓ(. ﺃﻓﻀﻞ ﺑﻴﺌﺔ ﻟﻨﻤﻮ ﻭﺗﻜﺸﻒ ﺍﻷﻓﺮﻉ ﻫﻰ ﺃﻣﻼﺡ ﻣﻮﺭﺍﺷﻴﺠﻰ ﻭﺳﻜﻮﻕ ﺍﻟﺨﻤﺴﺔ ﻋﻨﺪ ﺍﻟﺘﺮﻛﻴﺰ ﺍﻟﻜﺎﻣﻞ ﻷﻣﻼﺡ ﻫﺬﺍ ﺍﻟﻮﺳﻂ ﻣﻊ30،ﻣﻠﺠﻢ/ﻟﺘﺮ ،ﺳﻜﺮﻭﺯ ( ( ﺿﻌﻒ ﺗﺮﻛﻴﺰ Na H2 PO4ﻭﺍﻟﺘﺮﻛﻴﺰ ﺍﻟﻜﺎﻣﻞ ﻟﻤﻠﺢ ﻓﻮﺳﻔﺎﺕ ﺍﻟﺼﻮﺩﻳﻮﻡ (( ﺗﻢ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ BAﻓﻴﺘﺎﻣﻴﻦ ﺍﻟﺜﻴﺎﻣﻴﻦ ﻣﻊ 0.3ﻣﻠﺠﻢ/ﻟﺘﺮ ﺑﻨﺰﻳﻞ ﺍﺩﻳﻨﻴﻦ ﻟﻠﻮﺳﻂ NAAﺍﻓﻀﻞ ﺗﺠﺬﻳﺮ ﺑﺎﺿﺎﻓﺔ 0.3ﻣﻠﺠﻢ/ﻟﺘﺮ ﻣﻦ ﺣﻤﺾ ﻧﺎﻓﺘﺎﻟﻴﻦ ﺍﻟﺨﻠﻴﻚ ﺍﻟﻐﺬﺍﺋﻰ ﺍﻋﻼﻩ ﺑﺪﻻﹰ ﻣﻦ ﺍﻟﺒﻨﺰﺍﻳﻞ ﺍﺩﻳﻨﻴﻦ ﻭﻛﺎﻧﺖ ﺍﺿﺎﻓﺔ ﻛﻞ ﻫﺮﻣﻮﻥ ﻋﺎﻯ ﺣﺪﺓ ﺗﻌﻄﻰ ﻧﺘﺎﺋﺞ ﺃﻓﻀﻞ ﻣﻦ ﺍﺿﺎﻓﻨﻬﺎ ﻣﻊ ﺑﻌﻀﻬﺎ ﻟﻠﻮﺳﻂ . ﺍﺩﺕ ﺍﺿﺎﻓﺔ ﻛﺒﺮﻳﺘﺎﺕ ﺍﻟﺪﻳﻨﻴﻦ ﻭﻣﻴﻮﺃﻧﻮﺳﻴﺘﻮﻝ ﻟﺘﺤﺴﻦ ﺍﻟﻨﻤﻮ ﺑﻌﺾ ﺍﻟﺸﻰ ﻭﻟﻜﻦ ﻟﻢ ﺗﻄﻔﻰ ﻓﺮﻭﻗﺎﺕ ﻣﻌﻨﻮﻳﺔ ﻋﻠﻴﻪ. ﻳﻤﻜﻦ ﺍﺳﺘﺨﺪﺍﻡ ﺳﻜﺮ ﺍﻟﺠﻠﻜﻮﺯ ﻛﺒﺪﻳﻞ ﻟﺴﻜﺮ ﺍﻟﺴﻜﺮﻭﺯ ﺣﻴﺚ ﺗﻌﻄﻰ ﻧﺘﺎﺋﺞ ﺍﻓﻀﻞ ﻣﻨﻪ ﻟﻜﻦ ﻳﻌﺎﺏ ﻋﻠﻴﻪ ﺗﻜﻠﻔﺘﺔ ﺍﻟﻌﺎﻟﻴﺔ ﻣﻘﺎﺭﻧﺔ ﻣﻊ ﺍﻟﺴﻜﺮﻭﺯ ﺣﻴﺚ ﻳﺼﺒﺢ ﺍﺳﺘﻌﻤﺎﻟﻪ ﻏﻴﺮ ﺍﻗﺘﺼﺎﺩﻯ . ﺍﺳﺘﺨﺪﺍﻡ ﺍﻟﻘﻄﻦ ﺑﺪﻝ ﺍﻷﺟﺎﺭﺍﻋﻄﻰ ﻧﺘﺎﺋﺞ ﻣﻤﺘﺎﺯﺓ ﻣﻤﺎ ﻳﺸﺠﻊ ﺍﻣﻜﺎﻧﻴﺔ ﺍﺳﺘﻌﻤﺎﻟﻪ ﻛﺒﺪﻳﻞ ﻣﺜﺎﻟﻰ ﻟﻸﺟﺎﺭ ﻟﺘﺤﺎﺷﻰ ﺗﻜﻠﻔﺔ ﺳﻌﺮ ﺃﻻﺟﺎﺭ ﺍﻟﺒﺎﻫﻈﺔ. ﺣﻀﺎﻧﺔ ﺍﻟﻨﺒﻴﺘﺎﺕ ﻓﻰ ﺍﻟﻈﻼﻡ ﻟﻢ ﺗﻌﻄﻰ ﻧﺘﺎﺋﺞ ﺟﻴﺪﺓ ﻋﺪﺍ ﺍﻟﻄﻮﻝ ﻟﺬﺍ ﻻﻳﻨﺼﺢ ﺑﻬﺬﻩ ﺍﻟﻤﻌﺎﻣﻠﺔ ﺍﻻ ﻓﻰ ﺣﺎﻟﺔ ﺍﻟﺤﻮﺟﺔ ﺍﻟﻤﻠﺤﺔ ﻻﻋﻄﺎﺀ ﺍﻻﺳﺘﻄﺎﻟﺔ ﺍﻟﺴﺮﻳﻌﺔ ﻟﻠﻨﺒﺎﺕ . Abreviations NAA: αnaphthalene acetic acid. Kin/kn:Kinetin. BA/BAP: Benyl adenine/Benzyl aminopurine IBA:Indol butyric acid. IAA:Indole acetic acid. 2iP:2-Isopentyl adenine. 2, 4-D:2,4-Dichorophenoxy acetic acid. GA3:Gibberellic acid. BPA:N-benzyl-9(2 tetrahydro Pyranl) adenin TDZ:N-Phynyl-N-1,2,3-thidiazol-5 ylurea. MS:Murashige and skoog(1962). B5:Gamborg, et al.(1968). GD:Gresshoff and Doyl. SH:Schenk and Hild Brandt WS:Wolter and Skoog. WPM:Lloyd and Mc Cown Woody Plant Medium. NaH2PO4:Sodium hypophosphate. expt.:experiment. h/hr:hour. Min:minute. Conc.:concentration. 1/ Introduction Roses are grown for their profusion of flowers (often fragrant),and sometimes for their fruits. Roses have been cultivated as garden plants since very early times. They are the most widely grown and loved of the flowering woody perennials. Commercially , they are probably the most important flower crop in the world. But till now very little is known about their culture. In Sudan Roses are the most popular ornamental plants . They are grown in garden beds or in pots for general landscape purposes. There has been a real consiousness about the importance of landscaping and ornamental plants in varios towns of the country the last few years indicating high rate of urbanization. On the other hand , roses can be considered as a cash by crop exporting cut rose flowers during the winter time especially to Europe. Very few researchs has been done on rose culture in Sudan in general and tissue culture in particular . One of the most important roses in Sudan is a variety called “Sarra” . This cultivar bears high temperature and it flowers all the year round even during the summer while the other varieties flower only during the winter months. Sarra like other rose cultivar is traditionally propagated by budding .This method of clonal propagation is tedious laborious and is very slow. Trials to use tissue culture in “Lena Company” for the clonal propagation of “Sarra” were not successful. This research was carried out as an attempt to tailor the medium components that will best suit the clonal propagation of “Sarra”, the most expensive rose among all other rose cultivars 2/ Literature Review 2-1 Botany of roses: Roses belong to the family Rosaceae. Plants of the genus Rosa are deciduos or sometimes evergreen shrubs,with an up right, climbing or trailing stems, usually prickly, rarely un- armed .Leaves are divided into usually 5to7 oval leaflets,with rounded or pointed tips, that are sometime toothed. Stems usually bear thorns , or prickles fully and hardy. Roses are polycarpic self induuctive plants , which initiate flowers irrespective of photoperiod or temperature. They initiate flowers autonomously on every growing shoot after a certain size is attained. Every shoot blooms unless the flower bud dies early in its development. Roses have a determinant inflorescence that may assume a corymbose , paniculate , or solitary form. When flowers are borne singly, as in many hybrid tea cultivars, there are still undeveloped flower buds in the axils of the leaves immediately below the terminal flower. These buds can develop into short flowering shoots under favorable environmental conditions. Flowers are borne on an upright , prickly stems (the end of short branchlets). Sepals and petals are 5 , rarely 4 , stamens and pistils are numerous , enclosed in a usually urnshaped receptacle. Flower colours range from, white , pink , yellow , orange , to lavender with many shades , hues and tints in between. The doubling of a flower is simply the replacement of the stamens and styles by petals Fruits formed from fertilized flowers are called hips which become fleshy and berrylike at maturity , enclosing several or many bone achenes. They are reputed to be high in vitamin C content and consequently are in high demand by health food advocates (Mohamed,1994;Larson,1980; Brickell,1989). 2-2 Taxonomy: Present day commercial rose cultivars are all hybrids of rose species that are extinct. Even the earlier representations are probably a long way from the wild species. (Larson,1980);Gault and Synge,1971) Wild roses and hybrid species share most of the characteristics of the parent species including shrubs and climbing roses. Today varieties of Roses are divided into old garden roses and modern garden roses. 2.2.1Old garden roses : 2.2.1.1.Alba: large freely branching shrubs with clusters of usually 5-7 semi.double to double flowers. 2.2.1.2.Bourbon: Open remontant shrubs that may be trained to climb produces usually fully double flowers. 2.2.1.3.China: Spindly remontant shrubs that produce single to double flowers. 2.2.1.4.Damask: Open shrubs with usually very fragrant semi-to fully double flowers. 2.2.1.5.Gallica: Shrubs of fairly dense free-branching growth. 2.2.1.6. Hybrid Perpetual: Vigorous, free branching , remontant shrubs that bear fully double flowers. 2.2.1.7.Moss: Ofen lax shrubs with a fury moss-like growth on stems and calyex. 2.2.1.8.Noisette: Remontant climbing roses that bear clusters of up to 9 usually double flowers with a slight spicy fragrance. 2.2.1.9.Portland: Upright remontant shrubs with simi-double to double flowers. 2.2.1.10.Provence: Thorny shrubs that produce scented , usually double to fully double flowers. 2.2.1.11.Sempervirens: Semi ever green climbing roses with numerous semi to fully double flowers. 2.2.1.12.Tea: Remontant shrubs and climbing roses that produce spicy scented , stander – stemmed , pointed , semi- to fully double flowers. 2.2.2. Modern roses: 2.2.2.1.Shrub: A diverse group of modern roses, most of which are remontant , that grow larger (mostly 1-2 meters height) than most bush roses. Has single to fully double flowers , held singly or in sprays , in summer and / or autumn. It is suitable for beds and borders and for growing as specimen plants. 2.2.2.2.Large flowered bush (Hybrid Tea): Remontant shrubs with mostly pointed , double flowers , (8cm) or more across , borne singly or in groups of 3 ,it flowers in summer- autumn and is excellent for beds, borders, hedges and for cutting. 2.2.2.3.Cluster flowered bush (Floribunda): Remontant shrubs that produce sprays of usually 3-25 single to fully double flowers in summer – autumn. It is excellent for beds, borders, hedges and for cutting. “Sarra” roses belong to this group. 2.2.2.4. Dwarf cluster flowered bush (Patio): Neat, remontant shrubs , 38-60 cm high, that – bear sprays of generally 3-11 single to double flowers in summer autumn. It is ideal for beds, borders, hedges and for growing in containers. 2.2.2.5. Miniature bush: Remontant shrubs , 45 cm high , with sprays of usually 3-11 tiny , single to fully double flowers in summer-autumn. It has tiny leaves . It is suitable for rock gardens , small spaces and for growing in containers. 2.2.2.6. Polyanthe: Tough, compact, remontant shrubs with sprays of usually 7-15 small 5 petalled , single to double flowers.It flower in summer – autumn. Its suitable for beds. 2.2.2.7. Ground cover: Trailing and spreading roses , many of them are remontant with single to fully double flowers , borne mostly in clusters of 3 – 11 and flower in summer and / or autumn. They are suitable for beds , banks and walls. 2.2.2.8. Climbing : Vigrous climbing roses , some of which are remontant , with stiff stems and borne single to fully double flowers . They are borne singly or in clusters from late spring to autumn. They are suitable for training over walls fences and pergolas. 2.2.2.9. Rambler: Vigorous climbing roses with lax stems. They have clusters of 3-21 single to fully double flowers .Flowering mainly in summer and are suitable for training over walls , fences , pergolas and trees. 2.3. Uses of Roses : 2.3.1. Wild life: Roses hips are eaten by migration bird and used as a winter food for song birds . Rabbits feed on the bark and the shoots . Wild roses form thickets used as nesting sites for small mammals and birds. 2.3.2. Horticulture: Roses are used in landscaping cover and hedges. They are also used as cut flowers. Roses are available in almost any color imaginable and are suited to a number of sites. 2.3.3. Medicinal: Fruits are eaten as a source of vitamin C and are used to treat diarrhea. Bark tea is also used for dysentery. Flowers are used in China by some people as a stimulant and as a tonic .They promote and Improve blood circulation,and reduce stomachache. Decoration of the bark is used to treat worms. The bark is brewed to make medicinal tea used to reduce fever and as an antiinflammatory drug. 2.3.4. Aromatic: Roses flowers scent perfumes sachets , potpourris. A recipe used by Scottish chemist for fifty years. It was purely a liquid one , the essences consisting of Musk , Vanilla , Sandal wood, Oatchouli , Verbena , Neroli and Otto of Roses. Roses water is used in cosmatic industry. 2.3.5. Food : Rose water may be extracted from rose petals . Rose water may be used to flavor desserts , pie crusts , chicken dishes and is also used as a wash to protect the skin. Roses are rich in vitamin C , A and E . Wild rose hips can be eaten . The flowers can be used for potopourri , eaten raw , made into tea , or jam . Rose petals and fruit are used as preservatives and rose hips may be used in soups, and as dried fruits . They are an excellent source of vitamin C (three rose hips contain as much Vitamin C as a whole orange). 2.3.6. Historical uses: Romans crowned bridal couples with roses and placed them as banquet centerpieces . The American Indians used roses medicinally to treat sores , blisters , and as an eye wash. People have some beliefs about magical properties of roses .Red roses has long been used for love spells , yellow for luck and rose plants has been grown in garden to attract fairies who protect their surroundings. 2.3.7. Other uses: seed are used as decorations , particularly strung together as neck lace. Stems are used to make baskets. Burnet rose gives a violet dye used in dyeing silk. Silk and moslin may be dyed with a peach colour taken from the juice of Scotch rose hips . Anonymous 2.4. Propagation of Roses: 2.4.1. Traditional techniques: 2.4.1.1. Propagation by seeds: Natures own method to renew or increase the rose population is by seed. Seed propagation is used by rose breeders for the development of new cultivars. Rose seeds do not germinate readily after harvest. A period of after ripening is necessary before the seeds are ready to germinate. The fruits or “hips” should be harvested when the color changes from green to red , yellow or variations of these two colours. Seeds are removed from the “hips ” sown in flats and stored at a refrigerator 4oC for at least 3-4 weeks. Seed flats are transferred to room a temperature of 18o-21oC and then germinated . Final germination takes about 2-3 weeks. Seedlings should be transplanted to a good growing medium for growth untit first bloom. 2.4.1.2. Cuttings: Roses should if possible be grown on their own roots, that is from cuttings. Commercially it is hardly a viable proposition for various reasons: amount of material required; time taken to produce good saleable plant, particularly Hibrid Teas with pithy growth , are just not suitable. The percentage of rooted plants is much lower than that produced by budding. Possible exceptions are some Ramblers and Miniatures which root readily, but these do not produce better plants than those budded in the equivalent time. Cuttings can be taken at any time between October and March depending upon the intended planting date Cutting wood should be selected from flowering shoots that have been allowed to develop to full bloom. In this way the propagator is certain that the shoot producing flowers is true to type. 2.4.1.3. Grafting : Grafted plants are seldom used for commercial cut flower production. Rosa menetti under stock is used in the production of grafted plants. It is practiced on the West Coast of America and Europe. Stocks are field grown for 1 year before being ready to be grafted .When the under stock are ready for grafting, one eye from the known cultivar rose shoots should be prepared by making a slanting cut approximately 2-3 cm long at its base. A similar cut is made in the under stock just above the soil line. “The grafts are tied with a budding rubber”. The graft unions occure in approximately 10 days.Grafted plants shoud be acclimatized for about one month before plantig in their perinant places. 2.4.1.4. Budding: Budded plants are the most popular type used by commercial rose flowers growers. It has been used for a long time recognized as the commercial method of propagating roses in all parts of the world. The most common under stock for budded plants is Rosa manetti with occasional use of Rosa adorata in cold climates. stock are areas.The budding produced on especially maintained stock blocks normally segregated from regular growing procedure consists of making a vertical and horizontal cut in the under stock to from a “T”shaped cut. The “T” is placed well below the shoots that arise from the under stock An eye is removed Under from a previously prepared shoot of a named cultivar, inserted into the “T” shaped cut and wrapped with a budding rubber. Three to 4 weeks after budding , Rosa manetti under stock is cut back. In warm climates like Sudan Rosa banksiae is used as an under stock. One of the great advantages of budding is that only one “eye”is required to produce a plant, a matter of great importance with new or scarce cultivars. It has to be borne in mind that rose cultivars start off as one plant; when this is a new and very distinct rose, any early build up of stock is of considerable significance to the grower. But in this method we need a long time to produce new plants. (Larson,1980 ; Gault and synge,1971) 2.4.2 Tissue Culture techniques: Plant cell and tissue culture techniques have served as methodologies of physiology and biochemistry in a quest to increase our knowledge of cell biology for half a century .Today tissue culture has largely been integrated in biotechnology and permits the regeneration of plants as clones and as transgenics (Vasil and Thorpe 1994) .In vitro culture of higher plants is the culture of embryos ,seeds ,plants ,organs, tissues , cells or protoplasts on nutrient media under sterile conditions . (Pierik 1984) . 2.4.2.1.Media used: Nutrient media require double distilled water .Inorganic and organic compounds should be research grade .All are soluble in water ,except hormones which must be dissolved in organic solvents or acid before being added to the medium .several chemical companies offer formulations of varying degree of completeness so as to allow for modifications .Catalogues of manufactures of fine chemicals offer Murashige and Skoog (1962) based salt mixture each without sucrose ,agar ,vitamins and hormones .they also present a variety of gelling agents ,hormones and other biochemicals for best choice and preparation .Success in plant cell culture is largely determined by the quality of nutrient media .Formulations designed by Linsmaier and Skoog (1965) ,Gamborg ,et al (1972) can be regarded as standard tissue culture media MS medium was designed to test the effects of organic supplements on growth and development of tissue cultures . It was standardized with regard to inorganic nutrients and formulated for tobacco pith tissue culture.It became widely accepted because it supports the growth and development of cells,tissues,and organs of a wide variety of plant species ranging from herbaceous species to woody species(astonitiong alike in size and external appearance the reproducibility was more satisfactory and optimal yield of new growth were obtained) (Vasel andThorp,1994). Plant material and explantation : 2.4.2.2 The scope of plant species employed in tissue culture has been broadened , particularly due to perceived or actual needs to extend micro propagation to plants which are of commercial value or are rare and threatened by extinction . The process of dissection and culture of small organs or tissue sections is referred to as explanation .Explant choice ,the timing of excision ,and pretreatment are important determinants of culture success .Healthy ,vigorously growing plants will render suitable explants .Origin and size of explanted tissue determine the development of the established culture Number and physiological status of paranchyma cells subtending the cut surface of the explant will give rise to a proportional amount of callus . Explanted cells, tissue and organs as well as their environment must be sterile (Vasil and Thorpe, 1994). 2.4.2.3 Preparation of media: Nutritional and hormonal requirements of plant tissue and organ cultures consists of two essential substances: organic compounds like sugars , amino acids , vitamins ,growth regulators ,undefined mixtures of substances like coco- nut milk and east extract ; Inorganic compounds (macro and micro mineral elements like N ,P ,K ,Ca ,Mg , S ,Fe ,Zn ,B ,Mn ,Cu ,Co ,Ni ,Al ,Mo ,I). To determine the right composition of a nutrient medium for a particular explant it may take at least a year especially when no information is available on the plant you are working with .When no agar (a solidifier) is added to a nutrient medium it is called a liquid medium with agar it is a solid nutrient medium .There are different ways to express the concentration of a particular substance the most common terms are: volume percent ,weight percent ,Molar,Microgram /l g/ml and PPm .The medium should be sterilized before culturing .Sterilization is mostly done in autoclaves ,sometimes by way of filtering and occasionally chemically or by irradiation .(Pierk 1984). 2.4.2.4 Preparation of explants: Before an explant is put on a nutrient medium the piece of the plant from which the explants is cut has to be disinfected .This disinfection is usually done by submerging the plant piece for a few seconds in 70% alcohol and subsequently in 1.0%-1.5 Na OCL clorex or commercial bleach) for 10-30 minutes .It is then washed (several times with sterilized water to remove all traces of the bleach,obviously ,disinfection is an important condition for successful culture in vitro .Nutrient media will fast become overgrown with molds and bacteria if disinfection has been imperfect .When sterilizing and preparing tissues it will be necessary to use tweezers ,scalpels , etc which have been sterilized in (96% alcohol) and flamed before hand (Pierik 1984) . . . . 2.4.25 Tissue culture of higher species: Propagation of herbaceous plants and trees, whether ornamentals, fruit crops or forest trees by tissue culture techniques has been described as having potential merit to rapidly increase clones with specific growth characteristics; in large number necessary for plantation conditions. The application of these techniques to roses may provide a viable solution to mass production of elite clones with desirable ornamental traits. In vitro propagation of woody species has, however lagged behind as compared to herbaceous species. Until 1977 woody species have been categorized intractable in culture and thus much less attention has been given to there tissue culturing. Many reasons have contributed to this state of tissue culture in woody species. Woody species have long generation time due to a slow growth rate which in naturally reflected under in vitro conditions where low propagation rates have been observed (Bonga and Durzan, 1987) besides, growth in woody species occurs in flushes the number of these flushes depend on environmental conditions. This is accompanied by the formation of shoots of different ontogenetic age and different developmental phases greatly influencing the regeneration potential of explants obtained from them(Murashige, 1977). Difficulty in disinfestations of explants obtained from woody species has also played a role in impeding tissue culture of woody species trees and shrubs are usually grown in open fields under natural conditions for years harbouring various kinds of contaminants.Another problem associated with woody species tissue culture is the excessive callus formation at the bases of explant when cultured on nutrient media (Mogranahan et al.,1987). Nevertheless, the number of woody plants being propagated successfully by tissue culture methods has significantly increased in the last decade through out the world both in terms of crops being propagating or in total number of plants produced (George and Shrington, 1984, Thorpe et al., 1991 ). Emphasis is now placed on micropropagation for clonal multiplication of economically valuable plants, because it can provide greater rates of propagation and plants that are disease-free. “Sarra” rose cultivar is newly introduced to Sudan. Mother plants are expensive. Vegetative plant propagules are thus limited. In addition to that conventional vegetative propagation method have proved difficult, slow, laborious and are seasonal. Tissue culture techniques have been realized by Jordan (1987) as highly suitable for the rapid clonal propagation under such cases . Establishment of an in vitro propagation scheme would enhance cloning of “Sarra” rose cultivar since propagules for further propagation can be derived from plantlets growing in vitro circumventing the requirements, for explants from limited mother plants available. In this way stock-independent in vitro procedure based on recycling of contaminants-free propagules could be be developed on one hand. On the other hand in vitro propagation of “Sarra” rose cultivar could be carried out all-yearround deturing the seasonal restriction of mother plant growth. “Sarra” rose cultivar flowers almost around the year affecting greatly the availability of propagules for conventional propagation methods. Such a problem does not usually arise and propagation can be done at any time of the year. By the development of tissue culture propagation system. Two tissue culture techniques to propagate plants exist: the first of these is the proliferation and induction of axillary and apical shoot tips. Here shoot apices or nodal segments each containing at least an axillary bud are excised and cultured on a nutrient medium. This is the most widely used technique for the clonal propagation of desired genotype. Plantlet produce are clonal (Murshage, 1974). These plantlets grow and develop from pre-existing lateral buds. The technique is thus called direct propagation and the number of plants produced depends on the size of shoot tips cultured and is genotype-dependent and reflects the spontaneous or induced ability of geno-type to produce branches. Generally speaking any plant species that produce branches or respond to that if treated with growth regulators can easily be propagated by this technique (Murshage 1974, Hussey, 1978). The second technique developed for in vitro plant propagation involves an initial step of callus formation prior to induction of adventitious organs (organogenesis) or embryo formation (embryogenesis). Various reports are available on organogenesis of woody species (Goyal and Arya, 1981, Barlass and Skene, 1982; Mhatre, et al., 1985; Barbieri and Marini, 1987; Omura et al., 1987; Amin and Jaiswal, 1087). Embroyogenesis or asexual embryo formation however has been reviewed by Tisserat, et al., (1979), and by Durzan (1988). A wide degree of genetic variability among plantlet produced via callusmediated organ-or embryo-genesis has been observed (Mc Comb and Newton,1981; Navaro, et al., 1985). The principal of these in vitro propagation technique (called indirect propagation method ) for plant multiplication has been considered elsewhere (Evans et al., 1981) ehnce do not warrant elaboration here where a high level of fidelity in in vitro regenerated plantlets is desired. The most widely used tissue culture technique in any true-to-type clonal propagation is the culture of shoot-apices or-segments. (Murashige, 1974; Lawrence, 1981) here both axillary and apical meristems are induced to proliferate and grow into shoots which can then be rooted and grown into whole plantlet in vitro or ex- vitro or transferred to fresh medium for further proliferation. The process can be continued indefinitely provided optimal media and cultural conditions for normal promotion of vegetative growth in vitro are satisfied. The method of axillary shoot enhancement has since been applied to a wide range herbaceous and woody species (Murashige, 1974). It has been described as having potential merit to rapidly increase desired cultivars up to a million-fold in a year (Whitehead and Giles, 1977). It provides genetic stability as plantlets produced originate directly from performed or newly formed buds. (D’Amato, 1977). The procedure of this technique typically involves excision of shoot apices or stem segments with a single node (nodal cutting), surface disinfestations and culture in a suitable medium to induce shoot proliferation. Multiple shoots or only a single shoot may develop depending upon many factors; among which the genotype of plant under consideration and the culture media are most important. In vitro developed shoots, in turn, produce axillary and apical buds which can be induced to proliferate and grow into whole plantlets. Through serial subcultures the process can be repeated over and over as needed. Nevertheless difficulties still remain concerned vegetative propagation of woody species by tissue culture methods. These difficulties greatly affect the rapidity with which plant propagation can be achieved by using the technique of proliferation of axillary shoots of in vitro cultured shoot apices. The selection of a suitable source of ex-plant for culture initiation, its conditions, the juvenile/adult phases, contamination, the slow rate of growth, browning and vitrification are all but some of the factorsthat are of great importance and can make the difference between the success and failure of a culture. Roses have been grown vegitatively by conventional methods such as rooting of cuttings and grafting. Not more than 10 cuttings generally can be made from a large rose plant. In addition to seasonality and limited number of mother plants these methods of propagation has led to disease and nematode infestation during field culture that can be traced back to the stock material used for propagation. Emphasis is now placed on micopropagation of economically valuable plants, because it can provide greater rates of propagation and plants that are disease-free. There are numerous reports of Rosa sp. tissue cultures including embryo culture (Lammerts, 1946; Asen and Larsen, 1951; Von Abrams and Hand,1956; Semeniuk, et al., 1963; Graifenberg, 1973), callus culture (Jacobs, et al., 1968; Nesius, et al., 1972) and shoot apices culture (Hill, 1976; Jacobs et al., 1969; Jacobs et al., 1970 a,b; Elliott 1970; Graifenberg et al., 1975; Skirvin and Chu, 1979; Hasegawa, 1979; Ara et al., 1997; Singh and Syamal, 2000) and protoplast culture (Kim et al., 2003). Though Rosa sp. tissue culture has been established in the early fifties no report on the development of a system for in vitro rose propagation existed until the late seventies when Skirvin and Chu (1979) achieved shoot proliferation by culturing shoot tips of “Forever Yours” rose on a modified Murashige and Skoog medium (MS 1962) supplemented with BA (2.0 mg/l) and NAA (0.1 mg/l). shoots were successfully rooted in vitro on half strength MS medium with out hormones . Rooted shoots were transferred to green house for acclimatization and they grew well thereafter . Hasegawa,(1979) also cultured shoot-tips and lateral buds from green house grown rose plants and obtained multiple shoots with a 6-fold increase in 8 weeks by reculturing in vitro derived shootlets on the the same medium.3.0 mg/liter BA and 0.3 mg/liter IAA were found to be optimum for the proliferation of multiple shoots. Rooting was low on a medium containing 0.3 mg /l IAA and devoid of BA. Rooting of in vitro produced plantlets has been the subject of a number of studies where the importance of auxin type and amount, salt content of the nutrient medium or a combination of both influence the rooting process in vitro. Successful achievement of rooting on media devoid of growth regulators has been reported by Skirvin and Chu, (1979) and by Hasegawa, (1980).The type of auxin incorporated in the rooting medium is of vital importance. The auxin IAA and NAA were found to be effective while IBA was not (Kosh-Khui and Sink,1982), contrary to the finding of Alderson et al.,(1995) and Ara et al., (1979) where IBA was the rooting hormone of in vitro produced plantlet. IAA was reported to induce good rooting but at 10 times the NAA concentration needed (Hasegawa, 1980). The combination of two auxins were found to increase rooting more any of the auxins alone (Khosh. Khui and Sink, 1982). Differences in hormonal requirements for rooting rose cultivars has been reported by Valles and Boxus (1987). As far as salt concentration is concerned sevrl reports confirmed the superiority of reduced salt concentration in rooting media for rose plantlets (Skirvia and Chu, 1979; Davies, 1980; Arnold et al.,1995) and for plants in general (Murashige, 1979). Low salt concentration in rooting media significantly increased root initiation for “Improved Blaze” roses (Hyndman et al., 1982) and for “Rosmanini” dwarf rose cultivar (Scotti-Campos and Pais, 1990). On the other hand, reduced salt concentration coupled with an auxin (NAA) (Skirvin et al., 1984) or a combination of auxins (Khosh-Khiu and Sink, 1982) greatly enhanced rooting of “ Improved Blaze” plantlets. The interactive effect of low salt concentrations and auxin have been extensively studied by Arnold, et al., ()1995 the authers were unsuccessful in determining the relationship between these two factors. (Sultanbawa and Phatak, 1991)carried out an experiment on sterile ornamental peper to propagate it by using two ways, cuttings and in vitro shoot tip culture .Cuttings were rooted in the green house divided into two treatments one treated with Rootone and a control treatment. The second way was by using in vitro shoot tip culture using (MS) medium with 84 mM sucrose ,1% agar, two concentrations of IBA 4.9 or 9.8 µM or 8.8 µM BA in half strength of MS medium and a control without growth regulators .They found that the best of cuttings was these treated with Rootone, but when comparing it with in vitro cultured plants after 8 weeks just 40% of the cuttings have rooted compared with 60%rooted plants after 8 weeks in half strength Ms medium without growth regulators (Gawel, et al. 1990)worked on in vitro propagation of Miscanthus sinensis (ornamental plant) by using immature inflorescences of this plant. Three varieties were cultured on modified MS medium with 9.0 µM 2,4-D,20 g/l sucrose,2.0 g/l Gel rite, and 0.75 g/l MgCl² .Orango genesis was observed 8-12 weeks after callus initiation, shoots were rooted on half strength MS medium without growth regulators .After rooting ,tillers were initiated . Propagation through in vitro tillering is suggested as a successfull method for propagating this plant . Auxins , medium salt concentrations , and their interactive effects on rooting of two winter hardy roses : ( Rosa kordesii Wulf ' John Franklin ' & ' ChamPlain ') and two hybrid teas: ( Rosa hybrida ' John Paul II ' & ' Landora ' ) were studied . The auxins ( in mg . liter-1 ) IAA ( 0 , 0.3 , 1.5 , 3.0 , 6.0 , or 15.0 ) , IBA (0 , 0.1 , 0.2 , 0.5 , 1.0 , or 3.0 ) and NAA (0 , 0.1 , 0.2 , 0.5 , 1.0 , or 3.0 ). Each were combine factorially with modified MS medium ( 1/4 , 1/2 , 1/3 and full MS concentration. ) and were tested for optimal rooting response . ' ' John Franklin ' , ' John Paul II ' , and ' Landara ' rooted well with low or no auxin medium to high salt concentration . Optimum rooting for ' Champlain ' was achieved with high IAA and low salts or with intermediate IBA and NAA concentrations. and low medium salts . The interactive effects of auxin and medium salts for 'Champlain' showed that as salt concentration. increased , the amount of IBA or NAA required for optimal rooting also increased . The effects of auxins and medium concentration. on root counts per shoot were similar to those for percent rooting . Adding Auxin to the medium reduced root length for all cultivars , but salt concentration had a minimal effect . Roots generally were shortest at the highest IBA and NAA conc. salt concentration had little effect on root length . (Arnold , et al . 1995 ) . Effect of seven basal media , three carbon sources and four cytokinins on shoot organogenesis in the chrismas tree Scots pine Pinus sylvestris . was investigated by ( Ill-Whansul and korban ,1998 )as following : Seven basal media formulations were selected , they in- cluded MS medium , 1/2 MS , Gresshoff and Doy (GD) , Schenk and Hild brandt (SH ) , Wolter and Skoog ( WS ) Lloyd and Mc Cown ( Woody plant medium : WPM ) , and Gamborg , et al. (Gamborg's B5 ) along with three carbon sources including sucrose , glucose and fructose ( each at a concentration of 58.4 m M ) each medium contained 5 u M BA . Embryos grown on media lacking any growth regulators were used as control . Effect of different concentraion of the folowing cytokines BA ,BPA , Zeatin , and TDZ Provided at four concentrations ( 5 , 10 , 15 , 20 u M ) was investigated . Hyperhydricity of explants and shoot regenerantes was observed on basal media containing fructose , especially with half strength MS , MS , and WPM . Explants grown on a GD medium with sucrose produced the highest frequency of regeneration (81%) and with no hyperhydricity observed of developing adventitious shoots . Among three cytokines tested including BA , BPA , and TDZ ( at four concentrations for each ) . 5 u MBA resulted in the highest regeneration frequency and mean number of adventitious shoots per embryo . Shoot regenerantes were elongated after transfer to a GD medium containing 2g-1 activated charcoal and no growth regulators . After one month rooting was induced on 10% of explants . Pear Pyrus Communis in vitro propagation experiment using a double phase culture system was done by ( Rodriguez and Diaz- Sala ,1991 ) using two MS media : M S 1 ( MS salts plus 100 mg thiamine / Liter ) and M S 2 ( MS salts with half strength nitrates but double strength calcium chloride and magnesium sulphate plus ( mg / liter ) : 100 myo – inositol , 1 nicotinic acid , 1 pyridoxine . HCL , and ascorbic acid . Rooting was accomplished by two methods : a) Culture on solid MS 2 medium plus IBA ( 30, 10 , 5 µM ) or NAA ( 30, 10 .5 u M) in darkness flowed by 10 to 15 days of culture on a half strength solid hormone free medium ( MS 2 ) under 16 hr photo period . Two)1 min immersion in an IBA solution ( 5 , 2.5 , 0.5 , 0.05 µM ) followed by 20 days of culture on a half strength solid hormone free medium ( MS2 ) with a 16 hr photo period . After 40 days there was a clear difference between the shoot number per explants obtained according to the culture system used . Shoot multiplication was always higher when a liquid medium was used as an overlay proliferation of the plant was accomplished with a yield of 10 – 5 new shoots per explant . Rooting without callus formation was achieved by immersing the basal end in 5µ M IBA solution for 1 min . ( Balch and Alejo, 1997 ) studied the in vitro plant regeneration of Mexican Lime and Mandarin by Direct organogenesis starting from seedlings of Mexican Lime ( Citrus aurantifolia Christem . Swing ) and mandarin ( Citrus reticulate Blanco cv. Monica ). An other experiment was conducted to investigate the influence of incubation in several light conditions . The last experiment was to test the rooting of in vitro generated shoots, adventitious shoots were cultured in a half strength basal medium amended with 2% sucrose , 0.2% activated charcoal and supplemented with the following auxins : 2.7 µ M NAA , 5.4 µM NAA , 2.5 µM IBA , and 4.9 µM IBA . The optimal culture medium from both species was MS medium with vitamins from B5 medium , 5% sucrose , 33.3 µ M BA and 5.4 µM NAA , The best response was obtained when the segments were incubated at 25o (+/-) 2oc for 21days in darkness followed by 29 days on a 16/8 h light / dark cycle ( fluorescent light , 54 µM mol . m-2 s-1 ) . The best regeneration system tested allowed the attainment of adventitious shoots from 96% and 88% of the explants in Mexican Lime and Mandarin respectively . In Mexican Lime an average of 7-8 well differentiated shoots per explants was obtained , and in mandarin the yield was5-1 rooting of 70% . of the shoots was achieved in culture medium with NAA ( 2.7 5.4 u M ) or IBA ( 2.5-4.9 u M ). An efficient system for in vitro shoot formation and elongation of Dwarf pomegranate was done by ( Zhang and Stoltz, 1991 ) ; the explants were placed vertically in culture tubes each containing 10 ml modified MS medium . For shoot formation and elongation the medium was supplemented with the flowing combination of plant growth regulators : With the NAA level fixed at 2.0 µ M ; BA level were ( 0.0,0.5,1.0,2.0,4.0,0.8 or 16.0 u M) ; with BA fixed at 2 µ M NAA levels were ( 0,0.5,1.0,2.0,4.0,8.0, or 16 µ M ) .The experiment consisted of 14 treatments : two factors ( BA and NAA ) each with seven levels . Each culture produced a mean of 5.2 shoots with 1 µ M BA with NAA fixed at 2.0 µ M . BA at other concentrations resulted in fewer shoots , and at higher levels callus was produced , also found BA at 1.0 µ M to result in high shoot production from leaf callus of tree pomegranate . There were 6.6 shoots produced per culture with 1.0 µ M NAA with BA fixed at 2.0 µ M NAA at other concentrations resulted in lower shoot production , and at higher levels callus was produced . Shoots were > 35 mm long at BA levels <= 1.0 µ M with NAA fixed at 2.0 µ M . At BA levels >= 2.0 µ M , shoots length was< 20 mm . At 8.0 µ M NAA shoot length was 8.0 mm . Therefore , shoot elongation decreased with BA and NAA conc. > 1.0 and 3.0 µM respectively. The work showed that auxin NAA at 1.0 µ M is optimal for in vitro shoot proliferation of dwarf pomegranate and that BA levels > 2.0 µ M inhibit shoot formation . A method has been developed for producing putative adventitous shoots form proliferating shoots of chimeral Rosa multiflora rootstock . Axillary buds with an internodal section ( 1 cm ) were explanted onto the modified MS medium developed for roses ( Skirvin and Chu 1979) containing BA 2.0 mg-1 . When single shoot micro cuttings were subcultured onto the same medium at 3-4 week intervals the shoots fail to elongate more than 1-1.5 cm , new leaflets were small and the basal levels began to senesce . Because the leaf senesce observed in the cultures resembled classic ethylene injury . The problem might be overcome by reducing ethylene levels in vitro by use of an inhibitor of ethylene synthesis like silver nitrate . shoot proliferation was studied by studying the effect of TDZ at three concentrations ( 0.5 , 1.0 , and 1.5 µ M ) alone or in combination with three levels of NAA ( 0.0, 0.05,and 1.0 mg/L ) . Rooting was studied after harvesting shoots ( 2-3cm tall ) from proliferating cultures ; cultures were moved to several rooting media that contained various auxins , sugars , as well as activated charcoal , silver nitrate and GA3 . There were 6 media M ( MS 0.0 , growth regulators 0.0 , fructose 20 , sucrose 20 ) , CH ( 1/3 MS , NAA 0.1 , sucrose 30 ) , R5 ( ½ MS , NAA 0.1 , IAA 0.5 , glucose 20 , fructose 20 ) , R9 ( MS , GA3 0.1 , NAA 0.05 , IAA 0.5 , IBA 0.05 , glucose 20 , fructose 20 ) , R10 ( MS , GA3 0.5 , NAA 0.5 , IAA 1.0 , IBA 0.5 , AgNO3 3.4 , sucrose 40 , active charcoal 0.2 gm/1 ) , all sugars in g/1 and all growth regulators in mg/1 . The best rooting ( 50% was observed on R10 medium which produced healthy shoots and 50% rooting . Subculture on medium with 1µM TDZ development compact nodular callus that later after one or two subcultures onto the same medium , formed putative adventitious shoots . About half of these shoots rooted on MS medium supplemented with three auxins ( NAA 0.5 mg L/1 , IAA 1.0 mg /1 , IBA 0.5 mg /1 , GA3 0.5 mg /1 , silver nitrate 3.4 mg /1 , activated charcoal 200 mg /1 , and sucrose 40 g /1 ) ( Rosu , et al. , 1995 ) . ( Nayak , et al., 1997 ) studied in vitro propagation of three epiphytic orchids through TDZ to induce high frequency shoot proliferation ; The medium of MS containing 100 mg /1 ( w/v ) myoinositol and 3% (w/v ) sucrose was used . This medium was further supplemented whith 4.4 - 44 µ M BA or 0.045 – 9.0 µ M TDZ , either individually or in combination with 5.4 – 27 µ M NAA , with 0.8% (w/v) agar . For rooting of shoots MS medium was used with 0.2% phytagel and 3% sucrose . The medium was further supplemented with IBA or NAA 5.4 – 10.8 µ M . MS medium containing BA or TDZ with the latter begin more effective at 2.2 – 4.5 µ M . Shoots which developed on a TDZ containing medium elongated following transfer to a medium containing 2.2 µ M BA and 10.8 µ M NAA conc . of TDZ above the optimal level had an inhibitory effect on shoot regeneration . In both Dendrobium species the number of shoot bud formation was greatly influenced by explant orientation . Regenerated s hoots were rooted on MS containing 10.8 µ M IBA . A study was conducted to develop a thornless Black berry ( Rubus spp ) cultivar called Navaho , The initial medium was one half strength MS with 30 g sucrose and 0.3 g acid washed activated charcoal/l . Nodal segments were then transferred to jars for 3 week of proliferation . The jars contained 2.5 ml of one of the media : full strength MS plus 0.3 g acid washed activated charcoal /l (A) ; full strength MS ( B) and one half strength MS(C) . All three media were supplemented with 30.0 g sucrose / l , 7.0 g agar / liter , 8.9 µ M BA , 0.5 µ M IBA , 0.29 µ M GA . Nodal segments cultured in medium (A) produced vigorous non proliferating shoots with several long roots . Nodal segments cultured on (B) and(C )were very similar and formed three to five adventitious shoots from (B) and (C) were divided and subcultured on the same respective media for continued proliferation , although micro shoots on (B) developed slight chlorosis . Single micro shoots , removed from proliferating clumps from (B) and (C) and transferred to (A) grew vigorous with numeric roots with out shoot proliferation . About 90% of all micro shoots transferred to a produced roots with in 2 week , with the only losses due to contamination of the vessel ( Fernandes and Clark 1991 ) . Easter lily ( Lilium longiflorum Tunb.) was in vitro propagated from pedicels by ( Liu and Burger ,1986) ,pedicel sections from the flowers were cultured on a modified MS medium containing various concentration of cytokinins and auxins , two cytokinins kinetin and BAP and two auxins IAA and NAA were used in several combinations . These growth regulators were added to a basal medium consisting of MS salts, 87.6mM (3%) sucrose , 555 µ M (100 mg /1) myo-inositol . 1.2 u M(0.4 mg /1) thiamin and HCL , and 0.6% phytagar . The medium tested for root initiation consisted of an MS medium containing no auxin ( control ) or 10 µ M of either IAA , IBA , or NAA . A fifthmedium contained 20 µ M NAA . A combination of BAP (5 µ M ) and NAA ( 2 µ M ) resulted in the greatest number of adventitious buds on pedicel section . A gradient in the formation of buds in the pedicel was observed , with the section nearest the receptacle forming the greatest number , especially when the section was placed upside-down on the culture medium . IAA ( 10 µ M ) and IBA ( 10 µ M ) were most effective in stimulating adventitious roots in vitro derived shoots . This vegetative propagation technique provides a way of amplifying floral mutants of Easter lily . Cotyledons from developing 6-8 week old embryos of Liatris Spicata (L) Wild . ( blazing star ) were cultured on MS medium containing 0.0 , 0.4 ,4.4 , or 44.4 µ M BA or 0.0 , 0.2 , 2.2 , or 22.2 µ M TDZ to induce adventitious shoot formation . the highest percentage of cotyledons forming the most shoots was on medium containing 2.2 µ M TDZ . Cotyledon derived callus cultured on medium containing 4.4 µM BA formed = 16 times more adventitious shoots than on 2.2 µ M TDZ . Adventitious shoots derived from cotyledons or callus produced roots when placed on MS medium containing 5.0 µ M IBA . Regenerated plants that flowered in the field appeared homogeneous. ( Stim- art and Mather ,1996 ) . Four muscandine grape ( Vitus rotundifolia Michx ) cultivars ( Carlos , Noble , Regale , and Tarheel ) were evaluated for their ability to be cultured in vitro . Axillary buds were placed on MS medium as modified by Chee . Different levels of BA 0.5 to 10.0 µ M , Kinetin 0.5 to 5.0 µ M , and TDZ 0.5 to 11.3 µ M , and different explant position were evaluated for their effect on in vitro explant establishment and shoot production . TDZ ( 2.3 to 4.5 µ M ) alone or in combination with BA ( 1.0 to 5.0 µ M ) or kin ( 1.0 or 5.0 µ M ) was defective for establishing axillary buds . Similar levels were also effective for promoting shoot proliferation . Explants originating from the 10 basal nodes of a shoot with at least 25 nodes gave better shoot proliferation than explants originating from the 10 distal nodes . ( Sudarsono and Goldy ,1991 ) . Cotton fibers were tested as a substitute for agar in tissue culture by ( Moraes – Cerdeira ,1995 ) . The cost of agar has prompted the researcher to search for an alternative more economical medium support . Effectiveness as a medium support was evaluated in terms of callus maintenance and shoot organogenesis using Artemisia , Agrostis and Taxus plants . Taxus and Agrostis calli cultivated on liquid media with cotton fiber as medium support ( 25 mL of medium per gram of cotton ) grew better than calli on agar ( 0.8% w/v ) . There were no significant differences in shoot organogenesis of Artimisia and Agrostis grown in 25 ml of medium per gram of cotton from those grown in agar medium . A modified culture medium is presented that promotes in vitro rooting of grapevine rootstocks and (Vitis vinifera L.)growth regulators .Study of 15 Vitis genotypes indicated a strong genotype dependent response to culture medium and growth regulators with respect to formation of roots in one node shoot segments .The media used were MS ,PH 5.7 ,and a medium which they had designated Roubelakis ,Containing (in mg.l ) NH4NO3 500,KNO3 1000 ,CaCl2.2H2O 200, MgSO4.7H2O1.0 ,KI 0.5 ,CuSO4.5H2O 0.01,CoCl2.6H2O 0.01 ,ETDA 40 ,biotin 0.1 ,nicotinic acid 5, pyridoxine 5,thiamin 5,panthothenic acid 5 ,myo- inositol 100 ,Sucrose 2%(W/V) and agar 0.7% (W/V) pH 6.4 .IBA was added at 1,2,3,5,8, µM .The affect of the antioxidant citric acid at µM And of activated charcoal at 3% (W/V) were also tested . In preliminary experiments on rhzoegenesis ,IBA gave superior results to IAA,NAA ,2,4-D and was further used in this work .The number of roots forming on one node segments in the rootstock and Vitis vinifera cultivars was determined .Of the 15 genotypes that were studied seven did not form roots in MS medium with out IBA ,whereas the remaining eight developed poor root systems .In Roublelakis medium without IBA ,almost all genotypes developed strong root systems ,with the exception of (Liatiko) and (SO4)which perform poorly in both media in the absence (Liatiko) or in the presence (SO4) of IBA. (Roubelakis – Angelakis and Zivanovitc ,1991) 3- Materials & Methods 3.1 Plant Material: The plant material for this study was selected from vigorously growing one year old plants of roses “Sarra cultivar”. Branches 1.5cm in length containing axillary buds were taken from these plants for explant prepration. 3.2 Sterilization: The branches were first washed under running tap water to remove dust and reduce the contamination. The axillary buds were placed in an antioxidant solution (100 mg/l citric acid + 150 mg/l ascorbic acid) for one hour. They were then disinfested under a laminar-air-flow cabinet by dipping in 75% ethanol (V/V) for few seconds, rinsed by sterilized distilled water followed by immersion in 10% commercial bleach (Clorox) containing two drops of Tween-20 per 100 ml as a wetting agent shaken for 15 minutes on a shaker. The axillary buds were then rinsed three times with sterilized distilled water to remove all of the sterilant. 3.3 Culture media: The salt formulation used through out this study was that of Murshage and Skoog, (1962). The components of each stock solution this salt mix is given in appendix (1). In the beginning three basal media were tested for there efficiency in supporting the growth and the development of rose explants. These three media are Murashuge Multiplication Medium A (MMMA), Murashuge Multiplication Medium B (MMMB), and Murashuge Multiplication Medium C (MMMC). The chemical composition of each of these media is shown in appendix (2). Medium preparation and sterilization were carried out as usual. A one liter medium was prepared of each three media. The chemical components of each medium was mixed and the pH was adjusted to 5.7±1 with 0.1 N(HCL) or 0.1N (NaOH) before the addition of agar. The medium was then heated to dissolve the agar and then distributed into 15 × 150ml culture tubes at a rate 25ml each tube. The tubes were closed with Bellco Kaputs closures. The tubes containing the medium were sterilized at 121˚C for 15 minute at 15 psi in an outoclave. The medium was cooled slanted and stored until use. 3.4 Culture of explant: Sterile axillary buds were established, one axillary bud per test tube a total of 10 axillary buds for each of three Murshage multiplication media. Cultured tubes were incubated at 20±/1˚C and under 8 hour photoperiod in the growth room. Data were recorded after 6 weeks incubation period. Data recorded included plant height, number of shoots, number of leaves, and number of roots produced. Growth vigor was also recorded visually. The result of this initial experiment showed that Murashuge Multiplication Medium (A) (MMMA)was superior to the other two multiplication media in all parameters measured (plate 1). It was thus decided to use Murashige multiplication medium (A) (MMMA) as basal medium through out this study. 3.5 Experimentations: 3.5.1 The effect of different concentrations of Murashige and Skoog (1962) salt mixture on in vitro growth and development of rose axillary buds: MS salt concentrations were tested as follows: 0.25 x, 0.5 x, 1.0 x (full MS salt concentration), 2.0 x, and 4.0 x. Plate (1) 3.5.2 The effect of different concentrations of NaH2PO4.H2O salt on in vitro growth and development of rose axillary buds: The following concentrations of sodium hypophosphate were tested: 0.25 x, 0.5 x, 1.0 x, 2.0 x, and 4.0 x. 3.5.3 Energy source (Carbohydrates): 3.5.3.1 Sucrose: It’s the most used of the carbohydrates as an energy source in plant tissue culture media. It was tested at: 0.75%, 1.5%, 3.0%, 6.0% and12%. 3.5.3.2 Glucose: It’s a monosaccharide has been found to be superior or equal to sucrose as an essential medium component especially for in vitro culture of monocots. A concentration test of glucose was conducted using the following concentrations: 0.75%, 1.5%, 3.0%, 6.0%, and 12.0%. 3.5.4 the effect of different concentrations of Thiamine – HCL (Vit. B1) on in vitro culture rose axillary buds: Thiamine –HCL is one of the (B) complex group of vitamins has been sited as the most important vitamin in plant tissue culture the essentiality of which has been proven. A test has been conduced to determine the best concentration for sore axillary buds growth and development. The following concentrations (in mg/l) has been tested: 0.1, 0.2, 0.4, 0.8, and 1.6. 3.5.5 The effect of different concentrations of myo-inositol on in vitro cultured rose axillary buds: the hexitol myo-inositol is a pentose suger. It has been used in tissue culture medium as a vitamin. The following concentrations (in mg/l) have been tested: 25,50,100,200, and 400. 3.5.6 The effect of different concentrations of adenine sulphate (A/S) on in vitro cultured rose axillary buds: Concentrations of A/S tested included (in mg/l): 20, 40, 80, 160, and 320. 3.5.7 The effect of different concentrations of Naphthaline acetic acid (NAA) and benzyle adenine (BA) alone and in combination on in vitro growth of rose axillary buds: A factorial experiment was conducted to determine the optimum concentration(s) most suitable for rose axillary buds growth are proliferation. The following concentrations of (NAA) (in mg/l) were tested: 0.0, 0.01, 0.03, 0.1, and 0.3. The concentrations of BA tested in (in mg/l) were: 0.0, 0.1, 0.3, 1.0, and 3.0. 3.5.8The effect of physical state of the nutrient medium on the growth and development of in vitro cultured rose axillary buds: A comparison of the growth rose of explants on a agar solidified nutrient medium (7.000 mg/l) with there growth on a plate form made of cotton fibber was conducted. 3.5.9 The effect of darkness on growth and development of in vitro cultured rose axillary buds: The experimental layout was as follows: Cultured plantlets were incubated under the normal incubation photoperiod (8 hours dark/16 hours light) as control. The dark treatments consist of growing cultured plantlets under continuous darkness before being exposed to the normal incubation photoperiod. Cultured planets were incubated under continuous darkness for one week; two weeks; three weeks and four weeks before being exposed to normal incubation photoperiod. The dark condition under incubation was imposed by surrounding a shelf in the incubation room with black cloths. 3.6 Statistical analysis: The experimental layout was a completely randomized design. Data were recorded every two weeks for a total of six weeks for each test. Parameters measured included: Plant height, number of leaves, number of nodes, number of shoots, number of roots if any, callus formation, and overall plant vigar. A computer program (SAS) was use in the analysis of data. 4 - Results 4.1 Effect of different concentrations of Murashige and Skoog (MS 1962) salt mix on growth and development of rose axillary buds: Table (1) shows that there were significant differences between the five concentrations of MS medium salt mix tested in all parameters. The concentration 1.0 x was the best of all in plant height, number of leaves (Fig.1) number of nodes, and number of shoots. Very high or low concentrations (4.0x, 0.25x) resulted in adverse effects in all growth parameter measured (Plate 2). 4.2 Effect of different concentrations of NaH2PO4 salt on growth and development of rose axillary buds: Generally there was significant reduction in growth and development when using 0.25x and 4.0x after two and four weeks from culture but differences became more significant after 6 weeks form culture for all parameters recorded. Table (2) indicates that the highest growth rates in plant height (Fig. 2), number of leaves (Fig.3), number of nodes, and number of shoots were obtained at 1.0x concentration, the normal concentration usually used in tissue culture media. 4.3 Effect of energy sources (carbohydrates): 4.3.1 Effect of different concentrations of sucrose on growth and development of rose axillary buds: Table (3) shows that increase of sucrose concentration from 0.75% to 3.0% resulted in significant increase in all parameters measured, namely plant height (Fig. 4), number of leaves (Fig. 5), number of nodes, and number of shoots per plant. The concentration 3.0% was the best one while lower and higher concentrations significantly reduced growth rates (Plate 3). 4.3.2 Effect of different concentrations of glucose on growth and development of rose axillary buds: Table (4) shows that all parameters measured increased with increasing glucose concentraton until the concentration of 3.0% where best results were reached. This increase was not significant in the first two weeks but significant reduction was obtained at 12.0%. as for weeks 4 and 6 there is generally significant increase up to 3.0% followed by decrease at 12.0% concentration. Fig. (6) and (7) show effect of different glucose concentrations on number of shoots and nodes. Plate (4) shows rose plantlets growing on different glucose concentrations. Table 1:Effect of different concentrations of MS salts on in vitro growth and development of rose axillary buds. Weeks After culture trea t. A B plant hieght (cm) 1.3 b 1.24 bc No.of leaves 5.8 c 6.8 c No.of nodes No.of shoots No.of roots Root length (cm) 5.8 c 1.4 bc 0.0 0.0 6.8 c 1c 0.0 0.0 0.0 0.0 C 1.68 a 10.8 a 10.8 a 2.4 a D 1.34 b 8.4 b 8.4 b 1.8 ab 0.0 0.0 E 1.1 c 3.2 d 1c 0.0 0.0 A 1.28 c 7.6 bc 7.6bc 1.4 c 0.0 0.0 1.4 c 9.2 b 9.2 b 1.2 c 0.0 0.0 C 2.4 a 19.2 a 19.2 a 3.2 a 0.0 0.0 D 1.82 b 16.4 a 16.4 a 2.2 b 0.0 0.0 B 3.2d E 1.16 c 4.8 c 4.8 c 1c 0.0 0.0 A 1.24 d 8.4 b 8.4 b 1.2 c 0.0 0.0 1.66 c 9.6 b 9.6 b 1c 0.0 0.0 2.96 a 23.2 a 23.2 a 3.6 a 0.0 0.0 2.46 b 20 a 20 a 2.8 b 0.0 0.0 1.18 d 4.6 c 4.6 c 1c 0.0 0.0 B C D E A= 0.25x B = 0.50x C = 1.0x D = 2.0x E = 4.0x Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . Fig. 1: Effect of MS salts mix concentrationon number of leaves of in vitro cultured plantlets of "Sarra" rose cultivar. A = 0.25X B = 0.50X C = 1.00X D = 2.00X E = 4.00X Plate 2 Table 2:Effect of different concentrations of Na H2 PO4 salt on in vitro growth and development of rose axillary buds. Weeks After culture plant trea t. A B C No.of leaves hieght (cm) 1.22 b 1.58 a 8c 12.6 ab 1.54 a 12.6 ab D 1.6 a E 1.26 b 9.2 bc A B 14.0 a No.of nodes No.of shoots no.of roots Root length (cm) 8c 1.6 ab 0.0 0.0 12.6 ab 2.2 a 0.0 0.0 12.6 2.2 a ab 14.0 a 2.0 ab 0.0 0.0 0.0 0.0 9.2 bc 1.2 b 0.0 0.0 1.26 b 10.4 b 10.4 b 1.4 b 0.0 0.0 1.64 a 14.6 a 0.0 0.0 14.6 a 2 ab C 1.72 a 16.8 a 16.8 a 2.8 a 0.0 0.0 D 1.68 a 17 a 17 a 0.0 0.0 E 1.24 b 14.4 b 10.4 b 1.4 b 0.0 0.0 A 1.3 c 11.4 b 11.4 b 1.8 b 0.0 0.0 2.06 b 19.2 a 19.2 a 2.8 ab 0.0 0.0 2.58 a 21 a 21. a 3.6 a 0.0 0.0 B C 2.2 ab D E 2.28 ab 20.6 a 20.6 a 2.8 ab 0.0 0.0 1.3 c 12.6 b 12.6 b 1.4 b 0.0 0.0 A= 0.25x B = 0.50x C = 1.0x D = 2.0x E = 4.0x Means with the same letter(s) are not significantly different using Duncan Multiple Range Test. Fig. 2: Effect of different concentrations of NaH2PO4 salt on plant height (cm)of in vitro cultured plantlets "Sarra"cultivar A = 0.25X B = 0.50X C = 1.00X D = 2.00X E = 4.00X Fig. 3: Effect of different concentrations of NaH2PO4 salt on number of leaves of in vitro cultured plantlets of "Sarra"rose cultivar A = 0.25X B = 0.50X C = 1.00X D = 2.00X E = 4.00X Table 3:Effect of different concentrations of sucrose on in vitro growth and development of rose axillary buds. Weeks After culture plant trea t. hieght (cm) 1.34 bc No.of leaves No.of nodes No.of shoots No.of roots Root length (cm) 6 bc 1b 0.0 0.0 8.6 ab 1.4 ab 0.0 0.0 10.4 a 2a 0.0 0.0 D 1.52 10.4 a ab 1.22 c 10.2 a 10.2 a 1.8 a 0.0 0.0 E 1.08 c 5.4 c 5.4 c 1b 0.0 0.0 A 1.3 b 15.4 c 15.4 c 2.8 ab 0.0 0.0 1.8 a 20.6 ab 20.6 ab 2.6 ab 0.0 0.0 C 1.9 a 21.4 a 21.4 a 3.8 a 0.0 0.0 D 1.2 b 16.4 bc 16.4 bc 2.2 b 0.0 0.0 E 1.02 b 6.8 b 6.8 d 1c 0.0 0.0 A 1.44 c 23.4 b 23.4 b 3.2 bc 0.0 0.0 1.74 b 25.6 ab 25.6 ab 4.2 a 0.0 0.0 2.6 a 28 a 28 a 3.8 ab 0.0 0.0 1.3 cd 23.4 b 23.4 b 2.8 c 0.0 0.0 1.06 d 7.4 c 7.4 c 1.2 d 0.0 0.0 A 1.64 a 6 bc 8.6 ab B C B B C D E A= 0.75% B = 1.5% C = 3.0% D =6.0% E =12.0% Means with the same letters are not significantly different using Duncan Multiple Range Test . Fig. 4: Effect of different concentrations of sucrose on plant height (cm) of in vitro cultured plantlets of "Sara" rose cultivar. A = 0.75% B =1.5% C = 3.0% D = 6.0% E = 12.0% Fig. 5: Effect of different concentration of sucrose on number of leaves of in vitro cultured plantlets of"Sarra" rose cultivar A = 0.75% B =1.5% C = 3.0% D = 6.0% E = 12.0% Plate (3) Table 4:Effects of different concentrations of glucose on in viro growth and development of rose axillary buds. Weeks After culture plant trea t. A B hieght (cm) 1.36 a 1.28 a No.of leaves 7.6 a 9.8 a No.of nods No.of shoots No.of roots Root length (cm) 7.6 a 1.2 b 0.0 0.0 9.8 a 2.6 a 0.0 0.0 C 1.44 a 9.8 a 9.8 a 2.6 a 0.0 0.0 D 1.24 a 9.0a 9.0 a 3.2 a 0.0 0.0 E 1.18 a 4.2 b 4.2 b 1.2 b 0.0 0.0 A 1.8 b 13 c 13 c 2 bc 0.0 0.0 1.78b 14.6 bc 14.6 bc 2.8 ab 0.0 0.0 18.8 a 3.2 a 0.0 0.0 17.4 ab 17.4 ab 3.4 a 0.0 0.0 B C 2.16 a 18.8 a D 1.7 b E 1.14 c 5.4 d 1.2 c 0.0 0.0 A 1.14 c 13.54 c 13.54 c 2.4 b 0.0 0.0 2.22 c 22 b 22 b 3.8 a 0.0 0.0 3.72 a 30.2 a 30.2 a 4.4 a 0.0 0.0 2.86 b 29 a 29 a 4.6 a 0.0 0.0 1.20 d 6.4 d 6.4 d 1.4 c 0.0 0.0 B C D E 5.4 d A= 0.75% B = 1.5 %C = 3.0% D = 6.0% E = 12.0% Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . Fig. 6: Effect of different concentrations of glucose on number of shoots of in vitro cultured plantlets of "Sarra"rose cultivar A = 0.75% B =1.5% C = 3.0% D = 6.0% E = 12.0% Fig. 7: Effect of different concentrations of glucose on number of nodes of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.75% B =1.5% C = 3.0% D = 6.0% E = 12.0% Plate (4) 4.4 Effect of different concentrations of Thiamine HCL (Vitamin B1) on growth and development of rose axillary buds: Table (5) shows that there was a significant increase in all parameters measured with an increase in thiamin-HCL concentration until 0.8 mg/l was reached which gave the best results in plant height (Fig. 8) and number of shoots (Fig. 9) after 6 weeks from cultures while the concentration 0.4mg/l was the best on the number of leaves and nodes. Growth rate decrease at 1.6mg/l plate (5) shows rose plantlets growing on different thiamine-HCL concentrations. 4.5 Effect of different concentrations of Adenine Sulphate (A/S) on growth and development of rose axillary buds: Table (6) shows that there was a little difference on growth rates: (Plant height, number of leaves (Fig. 10), number of nodes, and number of shoots (Fig. 11) between the five concentrations tested. Plate (6) shows rose plantlets growing on different A/S concentrations. 4.6 Effect of different concentrations of myo-inositol on growth and development of rose axillary buds: Table (7) shows that increasing the concentration of myo-inositol has no effect on plant growth rates after six weeks from culture where no significant differences between the different concentrations was observed, with only slight difference after two or four weeks on number of leaves (Fig. 12) and nodes but there were no significant differences on plant height (Fig. 13) and number of shoots. 4.7 Effect of different concentrations of growth regulators: 4.7.1 Naphthaline acetic acid (NAA): Table (8) shows that changes of NAA concentrations has no effect on plant height, number of leaves, number of nodes, and number of shoots after two or four weeks form culture but after six weeks from cluture higher concentrations significantly increased plant height (Fig. 14), number of roots (Fig. 15) and root length. Number of roots was also increased after four weeks from culture. Plate (7) shows rose plantlets growing on different NAA concentrations. 4.7.2 Benzyle adenine (BA): Table (9) shows that there was significant increase in plant height (Fig. 16),number of leaves, number of nodes, and number of shoots (Fig. 17) with increase in BA concentrations. The concentration (0.3mg/l) was the best for all parameters measured except number of shoots where (1.0mg/l) was the best. Increasing the concentrations to 3.0mg/l growth rates of all parameters measured decreased. Roots were induced on plantlets grown on medium free of growth regulators and on lowest concentrations (0.1mg/l). plate (8) shows rose plantlets growing on different BA concentrations. Table 5:Effect of different concentrations of thiamine – HCl on in vitro growth and development of rose axillary buds. Weeks After culture plant trea t. mg/l A B C D hight (cm) 1.16 b 1.3 ab No.of leaves 7.4bc 8.8 b No.of nodes No.of shoots No.of roots Root length (cm) 7.4 bc 1.16 b 0.0 0.0 8.8 b 2 ab 0.0 0.0 1.36 b 20.2 20.2 3.2 a 0.0 a a 1.44 a 9.2 b 9.2 b 2 ab 0.0 0.0 0.0 E A B C D E A B C D E 1.28 5.6 c 5.6 c 1.6 0.0 ab ab 1.24 b 11.8 11.8 2.0 a 0.0 b b 0.0 1.42 b 0.0 14 b 0.0 0.0 14 b 2.2 a 1.36 b 20.2 a 2.2 a 19.4 a 1.32 b 12.2 b 1.3 b 14.8 b 20.2 a 19.4 a 12.2 b 14.8 b 3.2 a 0.0 0.0 2.4 a 0.0 0.0 2.2 a 0.0 0.0 2.2 c 0.0 0.0 1.46 b 19 b 19 b 0.0 0.0 1.6 b 27.0 a 27.0a 2.6 abc 3.6 a 0.0 0.0 2.36 a 25.8 a 25.8 a 3.8 a 0.0 0.0 1.32 b 15.4 b 15.4 b 2.4 bc 0.0 0.0 A= 0.1 B = 0.2 C = 0.4 D = 0.8 E = 1.6 all in mg /l Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . Fig. 8: Effect of different concentrations of Thiamin-HCl on plant height (cm) of in vitro cultured plantlets of "Sarra"rose cultivar A =0.1 B =0.2 C =0.4 D = 0.8 E = 1.6 all in mg/l Fig. 9: Effect of different concentrations of Thiamin-HCl on number of shoots of in vitro cultured plantlets of "Sarra"rose cultivar A =0.1 B =0.2 C =0.4 D = 0.8 E = 1.6 all in mg/l Plate (5) Table 6:Effect of different concentrations of adenine sulphate on in vitro Weeks After culture growth and development of rose axillary buds . Treat A Plant Hieght (cm) 1.14 a No.of leaves 6.8 ab No.of nodes No.of shoots No.of roots Root length (cm) 6.8 ab 1a 0.0 0.0 1.2 a 0.0 0.0 1.24 a 7.6 a 7.6 a C 1.16 a 6.6 ab 6.6 ab 1 a 0.0 0.0 D 1.14 a 8a 8a 1a 0.0 0.0 E 1.14 a 6b 6b 1a 0.0 0.0 A 1.36 a 20.4 a 20.4 a 3.6 a 0.0 0.0 1.76 a 19.4 a 19.4 a 2.6 ab 0.0 0.0 1.46 a 17 ab 17 ab 2.4 b 0.0 0.0 1.44 a 17.6 ab 17.6 ab 2.8 a 0.0 0.0 B B C D E 1.52 a 14.2 b 14.2 b 2.8 a 0.0 0.0 A 1.6 ab 26.4 a 26.4 a 4.2 a 0.0 0.0 1.86 a 25.2 a 25.2 a 3.6 b 0.0 0.0 1.58 b 23 ab 23 ab 3.2 b 0.0 0.0 1.54 b 22.8 ab 22.8 ab 3.4 b 0.0 0.0 1.52 b 19.8 b 19.8 b 3.2 b 0.0 0.0 B C D E A =20 B =40 C = 80 D = 160 E = 320 ( all in mg /l ) Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . Fig. 10: Effect of different concentrations of Adenine sulphate on number of of in vitro cultured plantlets of "Sarra"rose cultivar leaves A =20 B =40 C =80 D =160 E =320 all in mg/l Fig. 11: Effect of different cocentrations of adenine sulphate on number of shoots of in vitro cultured plantlets of"Sarra" rose cultivar A =20 B =40 C =80 D =160 E =320 all in mg/l Plate(6) Table7:Effect of different concentrations of myo –inositol on in vitro growth and development of rose axillary buds. plant Weeks After culture trea t. A B hight (cm) 1.08 a 1.12 a No.of leaves 5.4 a 3.4 b No.of nodes No.of shoots No.of roots Root length (cm) 5.4 a 1a 0.0 0.0 3.4 b 1.0 a 0.0 0.0 C 1.12 a 4.8 ab 4.8 ab 1.0 a 0.0 0.0 D 1.16 a 5.8 a 1.0 a 0.0 0.0 E 1.14 a 4.8 ab 4.8 ab 1.0a 0.0 0.0 A 1.26 a 19.4 a 19.4 a 3 .0a 0.0 0.0 1.26 a 0.0 0.0 1.34 a 17.8 a 17.8 a 2.8 a 0.0 0.0 3.0a 0.0 0.0 E 1.34 a 17.4 ab 1.34 a 14 b 2.8 a 0.0 0.0 A 1.44 a 24.4 a 24.4 a 3.2 a 0.0 0.0 1.54 a 25.2 a 25.2 a 3.8 a 0.0 0.0 1.5 a 24.2 a 24.2 a 3.6 a 0.0 0.0 1.44 a 23.6 a 23.6 a 4.4 a 0.0 0.0 B C D 15.4 b 5.8 a 15.4 b 17.4 ab 14 b 2.8 a B C D E 1.5 a 22.6 a 22.6 a 3.6 a 0.0 0.0 A= 25 B = 50 C = 100 D = 200 E = 400 ( all in mg /l) Means with the same letter(s)are not significantly different using Duncan Multiple Range Test . Fig. 12: Effect of different concentrations of myo-inositol on number of leaves of in vitro cultured plantlets of "Sarra" rose cultivar A =25 B =50 C =100 D =200 E = 400 all in mg/l Fig. 13: Effect of different concentrations of myo-inositol on plant height (cm) of in vitro cultured plantlets of "Sarra" rose cultivar A =25 B =50 C =100 D =200 E = 400 all in mg/l Table 8:Effect of different concentrations of NAA on in vitro growth and development of rose axillary buds. Weeks After culture plant trea t. 0.0 0.01 hight (cm) 1.9 a 1.7 a No.of leaves 5.4 a 3.6 a No.of nodes No.of shoots No.of roots Root length (cm) 5.4 a 1a 0.0 0.0 3.6 a 1a 0.0 0.0 0.03 1.84 a 4.6 a 4.6 a 1.4 a 0.0 0.0 0.1 1.94 a 3.6 a 3.6 a 1a 0.0 0.0 0.3 1.96 a 4 a 4a 1a 0.0 0.0 2.2 a 6.4 a 6.4 a 1.2 a 1.333 b 6.9 a 1.7 b 5.8 a 5.8 a 1a 1b 1.6 a 0.0 0.01 0.03 2.04 a 6.8 a 6.8 a 1.2 a 2.5 ab 1.058 a 0.1 2.4 a 6a 1.2 a 3.4 a 0.3 2.18 a 6.6 a 6.6 a 1.5 a 3.67 a 0.613 a 2.2bc 6.4 a 6.4 a 1.2 b 1.333 b 2.1 a 1.86 c 6.8 a 6.8 a 1b 1.67 b 1.333ab 2.18 bc 8.6 a 8.6 a 2a 2.6 b 0.676 b 2.72 a 8.4 a 8.4 a 1.2 b 4.6 a 1.626 ab 2.34 b 8.8 a 8.8 a 1.2 b 4.75 a 1.005 b 0.0 0.01 0.03 0.1 6a 0.3 Means with the same letter(s) are not significantly different using Duncan Multiple Range Test. 1.556 a Fig. 14: Effect of different cocentrations of NAA on plant height (cm) of in vitro cultured plantlets of "Sarra"rose cultivar A = 0.00mg/l B = 0.01mg/l C = 0.03mg/l D = 0.10mg/l E = 0.30mg/l Fig. 15: Effect of different concentrations of NAA on number of roots of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.00mg/l B = 0.01mg/l C = 0.03mg/l D = 0.10mg/l E = 0.30mg/l Plate(7) Table9:Effect of different concentrations of BA on in vitro growth and development of rose axillary buds Weeks After culture plant trea t. mg/l 0.0 hight (cm) 2.02 a No.of leaves 5.4 bc No.of nods No.of shoots No.of roots Root length (cm) 5.4 b 1b 0.0 0.0 0.0 0.0 1.4 c 4.4 c 4.4 b 1b 0.3 2a 7a 7a 2.2 a 0.0 0.0 1.0 1.72 b 7.8 a 7.8 a 2.2 a 0.0 0.0 1.7 b 0.0 2.1 b 6.08 6.8 1.8 0.0 b ab ab 5.8 d 5.8 d 1.2 c 0.0 1.7 c 6.4 d 0.1 3.0 0.0 0.1 0.3 1.0 3.0 0.0 0.1 0.3 1.0 3.0 2.78 a 23.6 a 2.06 b 18 b 0.0 6.4 d 1.4 c 0.0 0.0 23.6 a 18 b 3.2 ab 4a 0.0 0.0 0.0 0.0 1.88 bc 2.2 b 11.8 11.8 2.8 b 0.0 c c 6.4 d 6.4 d 1.2 c 1.33 b 0.0 1.92 c 10 c 10 c 1.2 c 5a 1.84 a 3.3 a 30 a 30.6 a 4b 0.0 0.0 2.4 ab 27 ab 27 ab 5.2 a 0.0 0.0 2.02 b 18.2 b 18.2 b 3.6 b 0.0 0.0 Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . 2.1 a Fig. 16: Effect of different concentrations of BA on plant height (cm) of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.00mg/l B = 0.10mg/l C = 0.30mg/l D = 1.00mg/l E = 3.00mg/l Fig. 17: Effect of differrent concentrations of BA on number of shoots of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.00mg/l B = 0.10mg/l C = 0.30mg/l D = 1.00mg/l E = 3.00mg/l Plate (8) 4.7.3 BA + NAA: Table (10) shows that there were significant differences between the different concentrations of the two growth regulators on their effects on growth rates. The lowest concentrations of both growth regulators (0.1 BA + 0.01 NAAmg/l) was the best concentration for plant height (Fig.18) but when concentrations of both growth regulators was increased plant height decreased. On the other hand number of leaves (Fig. 19), number of nodes and number of shoots increased in highest concentration (BA 3.0 + NAA 0.3 mg/l) of both growth regulators, while low concentrations and medium free of growth regulators induced rooting. Plate (9) shows rose plantlet growing on different combinations of BA + NAA concentrations. 4.8 The physical state of the medium: As shown in table (11) there were no significant differences between all parameters measured. (Fig. 20) shows the effect of physical state of the medium on plant height, while (Fig. 21) shows the effect of the physical state of the medium on number of leaves using agar or cotton. Plate (10) shows rose plantlets growing on the two different physical states, cotton or agar. 4.9 The effect of darkness: Four weeks of treatment with darkness resulted in a more significant increase of plant height (Fig. 22) and significant decreases on number of leaves, number of nodes (Fig.23), and number of shoots (Table 12). Table 10:Effect of different combinations of BA+NAA concentrations on in vitro growth and development Of rose axillary buds. plant Weeks After culture BA+NAA mg/l Hight (cm) No.of nodes No.of shoots No.of roots Root length (cm) 5.4 c 1b 0.0 0.0 10.6 a 1.8 ab 0.0 0.0 1.58 b 7 bc 7 bc 1.6 ab 0.0 0.0 1.38 b 7.2 abc 0.0 0.0 1.38 b 9.2 ab 7.2 1.2 b abc 9.2 ab 2.2 a 0.0 0.0 2.1a 5.8 b 5.8 b 1.2 b 2a 1.17 a 2.02 ab 11.8 a 11.8 a 1.8 ab 1a 0.5 a 9 ab 1.8 ab 0.0 0.0 1.0+0.1 1.74 9 ab bc 1.54 c 10 a 10 a 1.8 ab 0.0 0.0 3.0+0.3 1.42 c 12 a 12 a 2.4 a 0.0 0.0 0.0 0.1+0.01 0.3+0.03 1.0+0.1 3.0+0.3 0.0 0.1+0.01 0.3+0.03 2.02 a No.of leaves 1.92 a 5.4 c 10.6 a 0.0 0.1+0.01 2.2 a 6.4 b 6.4 b 1.2 b 1.33 a 2.1 a 2.16 a 12.6 a 12.6 a 1.6 ab 1a 0.8 a 10.6 ab 10.6 ab 1.8ab 0.0 0.0 0.3+0.03 1.9 ab 1.0+0.1 1.68 bc 12.6 a 12.6 a 2.2 a 0.0 0.0 3.0+0.3 1.5 c 15 a 15a 2.4 a 0.0 0.0 Means with the same letter(s) are not significantly different using Duncan Multiple Range Test . Fig. 18: Effect of different concentrations of BA+NAA on plant height (cm) of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.00+0.00mg/l B = 0.10+0.01mg/l C = 0.30+0.03mg/l D = 1.00+0.10mg/l E = 3.00+0.30mg/l Fig. 19: Effect of different concentrations of BA+NAA on number of leaves of in vitro cultured plantlets of "Sarra" rose cultivar A = 0.00+0.00mg/l B = 0.10+0.01mg/l C = 0.30+0.03mg/l D = 1.00+0.10mg/l E = 3.00+0.30mg/l Plate(9) Table 11:Effects of the physical stat e of the medium on in vitro growth and development of rose axillary buds Weeks After cultur e trea t. plant hight (cm) 1.24 a A No.of leaves 4.6 a No.of nodes No.of shoots No.of roots Root length (cm) 4.6 a 1a 0.0 0.0 1.38 a 4.8 a 4.8 a 1a 0.0 0.0 1.46 a 10 a 10 a 2.2 a 0.0 0.0 1.54 a 10.6 a 10.6 a 2.4 a 0.0 0.0 2.3 a 15.8 a 15.8 a 2.8 a 0.0 0.0 2.45 a 16 a 16 a 2.75 a 0.0 0.0 B A B A B A= control (agar) B = cotton Means with the same letter(s) are not significantly different using Duncan Multiple Range Test Fig. 20: Effects of the physical state of the medium on plant height (cm) of in vitro cultured plantlets of "Sarra" rose cultivar A = Agar B = Cotton Fig. 21: Effects of the physical stateof the medium on number of leaves of in vitro cultured plantlets of "Sarra" rose cultivar A = Agar B = Cotton Plate(10) Weeks After culture plant trea t. A B C hight (cm) 1.32 c 1.54 bc 1.68 abc No.of leaves No.of nodes No.of shoots No.of roots Root length (cm) 12.4 a 2.4 a 0.0 0.0 13 a 13 a 2.2 ab 0.0 0.0 9.8 ab 9.8 ab 1.67 ab 0.0 0.0 12.4 a 1.82 7.2 ab ab 2.04 a 6 b D E 7.2 ab 1.4 ab 0.0 0.0 6b 0.0 0.0 1b Table 12:Effect of darkness on in vitro growth and development of rose axillary buds A= control B = 1 week after culture C= 2weeks after culture D= 3 weeks after culture E =4 weeks after culture Means with the same letter(s) are not significantly different using Duncan Multiple Range Test Fig. 22: Effect of darkness on plant height (cm) of in vitro cultured plantlets of "Sarra"rose cultivar Fig. 23: Effect of darkness on number of nodes of in vitro cultured plantlets of "Sarra" rose cultivar 5- Discussion 5.1 Effect of different concentrations of MS salt mix on growth and development of rose axillary buds: there are various salt formulations in plant tissue culture developed for various purposes, but the most popular in most tissue culture laboratories is Murshige and Skoog salt, (1962). It is balanced ionically and contains high level of mineral elements especially nitrogen (Masaad 1999). MS salt concentrations have a high effect on plant growth. 1.0x MS concentration generally used gave the best results in all parameters measured. The plantlets had the best appearance with the biggest and greenest leaves, strong and longest shoots with out any deformity in all organs. The highest concentration 4.0x had the poorest growth. Leaves and shoots were dwarf and pale with a lot of burnt plant parts and callus formation. This poor growth may be attributed to the toxic effect of salts at this concentration where the plants were stressed. The lowest concentration 0.25x also resulted in weak growth and this may be due to the lack of some essential mineral elements important for plant growth. This result is similar to the findings of (Abd El Hameed 1999) and (Fernandez and Clark 1991) but does not agree with the results reported by (Masaad 1999). The differences between the two species of plants may be the cause. 5.2 Effect of different concentration of NaH2PO4 salt on growth and development of rose axillary buds: Best growth and development of the plants was observed in the medium containing the concentration 1.0x. leaves were big and dark green, shoots were strong and healthy, and all other growth parameters were excellent, but the growth and development decreased when very low or high concentrations (0.25x or 4.0x) were used. This may be attributed to the lack of this salt at the low concentration and its toxic effect at the high concentration. The plants were weak and pale with small and burnt leaves and dwarf shoots. This result shows the importance of the addition and direct effect of this salt on “Sarra” rose in vitro culture conferming previous reports (Murashige, 1974). 5.3 Effect of energy sources (carbohydrates): Plant tissues, organs, or cells cultured in vitro can not synthesize all essential growth factors, and thus depend totally on what is supplied in the nutrient medium. A source of energy and carbon in the form of sugars is usually added to the nutrient media. The classical MS medium contained 3% sucrose as a source of energy and carbon. However the optimum concentration and type of sugar varies with plant species (Masaad 1999). 5.3.1 Effect of different concentrations of sucrose on growth and development of rose axillary buds: The results obtained showed that when increasing the concentration from (0.75% to 3.0%) all growth rates increased, but they then started to decrease at high concentrations. At 3.0% concentration plants were vigorously growing with dark green leaves and strong shoots while at high concentrations growth was poor and weak specially at (12.0%) concentration where callus formation was induced. These results showed that sugar is very essential for plant growth but may be toxic at high concentrations and adversely affect plants growth. These results agree with (Abd El Hameed 1999) working with banana and (Hussein 2002) working with cidir who found that 3.0% gave best shoot length and number of nodes but disagree with (Masaad 1999) working with grapes. The differences in results may be attributed to the variation in plant species. 5.3.2 Effect of different concentrations of glucose on growth and development of rose axillary buds: Sucrose can be substituted by glucose specially in monocots tissue culture. Excellent results were obtained at 3.0% concentration where growth rates increased from the lowest concentration used (0.75%) until the 3.0% concentration then decreased to its lowest rate at the concentration (12.0%). These results are similar to that of (Abd El Hameed 1999) but contradict the findings of (Ill – Whansul and Korban 1998) who found that sucrose was better. This may be the result of different media used in addition to differences in plants species. Masaad (1999) on the other hand found the best results with glucose at low concentrations. This is referred to the differences between plant species. Comparing all concentrations of glucose with there similar sucrose ones we find that glucose concentrations were the best even at the highest most toxic concentration (12.0%). This may be because glucose is a simple sugar produced naturally by plants so it is easily absorbed and quickly assimilated while sucrose need some time to hydroly to simple sugar before being absorbed by plants.That is why glucose gives better results than sucrose. But the problem is that glucose is more expensive than sucrose and it is not readily available as is sucrose. It can be used for research purposes and as needed. 5.4 Effect of different concentrations of thiamie-HCL (Vitamin B1) on growth and development of rose axillary buds: The result of the experiment proved that thimaimine-HCL is an essential element for plant growth and development at all concentrations tested. It gave good results and increased plant growth up to the highest concentration (1.6 mg/l) where plant growth decreased meaning that very high concentrations may be toxic. 5.5 Effect of different concentrations of adenine sulphate (A/S) on growth and development of rose axillary buds: Adenine is one of the organic bases in nucleic acid of plant cell. It has been used for the first time in plant tissue culture by Skoog and Tsue (1948). It is usually added in the form of adenine sulphate – 2H2O because it is more soluble in water in this form. It is categorized as growth regulator because its effects are similar to those of cytokinins especially Kinetin (Masaad 1999). The results of this study proved that A/S had no significant differences between the different concentrations tested. However the lowest concentration tested (20mg/l) significantly increased the number of leaves, nodes, and shoots while the concentration in vitro cultured need lower concentrations of A/S for optimum growth. Poor results were recorded at the highest concentration (320 mg/l) meaning that higher concentrations of A/S were inhibitory. (40mg/l) gave the best plant height. These results indicate that plants 5.6 Effect of different concentrations of myo-inositol on growth and development of rose axillary buds: Myo-inositol a sugar alcohol, has been considered in plant tissue culture as a vitamin and it was never used as a source of energy or carbon source like other sugars (Masaad 1999). In this study there were no significant differences between all concentrations tested, however lower concentrations (50 mg/l) gave best plant height, number of leaves, and nodes, while number of shoots was the best at the concentration (200mg/l). this last result was similar to (Masaad 1999) who assured that high concentration improved growth and development. This means that the responses to may-inositol inclusion in culture media varied greatly from essential to beneficial and can be added in low concentrations for “Sarra” rose cultivar to improve growth and development of cultured axillary buds. 5.7 Effect of different concentrations of growth regulators on growth and development of axillary buds: Exogenously added auxin, and or cytokinins to in vitro cultures explants result in a variety of responses which depend on type and concentration of hormone added, plant species, type of explants and the purpose of culture initiation. Auxin stimulate cell expansion while cytokinins promote cell division (Masaad 1999). 5.7.1 Naphthaline acetic acid (NAA): Results showed that highest concentration (0.3 mg/l) was the optimum concentration for number of leaves, nodes, and roots, but for plant height 0.1 mg/l was better though differences were not significant compared to 0.3 mg/l.Best number of shoots was obtained at (0.03 mg/l). Lower concentrations of NAA increased root length while higher concentrations induce shorter roots, being shortest at (0.3 mg/l). Best results for root length was recorded with media without growth regulators. These results are similar to the reports of Arnold et al., 1995 who found that adding auxin to the medium reduced roots length for all cultivars tested in his own experiment. All culture with NAA rooted even at low concentrations proving that this growth regulator is important for rooting of roses. 5.7.2 Benzyl adenine (BA): Best results for plant height, number of leaves and nodes were obtained at the concentration 0.3 mg/l but the highest number of shoots was obtained at 1.0 mg/l with no significant differences when compared with 0.3 mg/l which follows it in its positive effects. These results mean that cytokinins with moderate concentration give best shoot growth because of its suppressive action on apical dominance of main shoot meristem (Masaad 1999). BA is thus very important for rose shoot growth and development. 5.7.3 Benzyl adenine + naphthalene acetic acid (BA + NAA): Growth and development in plants is controlled by the internal interaction of growth regulators. Auxins and cytokinin are the most important of these substances. The interaction of both with the environment and the genetic mape up of the plant control growth and developments in plants (Masaad 1999). Best results for number of leaves, nodes and shoots were obtained at the highest concentration (3.0 BA + 0.3 NAA) of both growth regulators. Plant height was however was best on medium free of both regulators. The experiment showed that the addition of growth regulators inhibits plant height specially at high concentrations. In this experiment number of leaves and plant height results are similar to the results of Masaad 1999 who found that increasing BA + NAA concentrations increases number of leaves but decreases plant height. The addition of BA +NAA inhibits rooting which were induced on media free of or contains very low concentrations of these growth regulators. When comparing BA + NAA results with BA or NAA added alone to the media we found the following differences: BA + NAA compared with BA added alone we found the best concentration of BA which scored best results had better shoot growth and development than BA + NAA combination best concentration . When comparing NAA adding alone with BA + NAA combination in there best concentration that scored best results we found that BA + NAA combination failed to produce roots except at their lowest concentrations (BA 0.1 + NAA 0.01). These results showed that adding growth regulators singly gave better results than adding them in combination for “Sarra” rose cultivar taking the purpose of culture (rooting or shoot growth in consideration). 5.8 Effect of the physical state of the medium on growth and development of rose axillary buds: Cotton fibers tested as an economical substitute for agar in tissue culture because the cost of agar. Results showed that there were no significant differences in all growth rates when using cotton compared with plants grown on agar solidified medium. This means that agar has no direct effect on plant growth so it can be substituted for by cotton or other substitutes. This result is similar to the previous reports (Moreas – Cerdira 1995); (Rodriguez and Diaz – Sala 1991). 5.9 Effect of darkness: Incubating culture plants in darkness did not improve plant growth except in plant height which was affected by etiolation , but all other growth parameters measured were reduced which means that there is no need for this treatment except when there is an urgent need for a quick increase in plant height for other tissue purposes. References * Abd El Hameed, H.A.F .1999. In vitro clonal propagation of the dwarf cavindish banana (Musa spp.) using shoot tips. M. Sc. Department of Horticulture Faculty of Agriculture Khartoum University. * Alderson, P.G., Mc Kinless, J., Rice, R.D. 1988. Rooting of cultured rose shoots. Acta Horticulturae. 226: 175-179. * Amin, M.N. and Jaiswall, V.S.1987. Rapid clonal propagation of guava through in vitro shoot proliferation on nodal explants of mature trees. Plant, Cell, Tissue and Organ Culture 9: 235243. * Ara, K.A., Hossain, M.M., Quasem, M.A., Ali, M., and Ahmed, J.U. 1979. Micropropagation of Rose: Rosa sp.cv. Peace. Plant Tissue culture 7: 135-142. * Arnold, N.P., Binns, M.R, Cloutier, D.C, Barthakur N.N., and Pellerin, R. 1995. Auxins, Salt concentrations, and their interactions during in vitro rooting of winter hardy and hybrid tea roses. Hort Science 30 (7): 1436-1440. * Asen, S. and Larsen, R.E. 1951. Artificial culturing of rose embryos. Penn. State College Progress Rpt. 40. * Balch, E.P.M and Alejo, N.O. 1997. In vitro plant regeneration of Mexican lime and Mandarin by direct organogenesis. HortScience 32(5): 931-934. * Barbieri, C. and Marini, S.1987. Plant regeneration from Actinidia sp. callus cultures. J. Hort. Sci. 62: 107-109. * Barlass, M. and Skene, K.G. M. 1982. In vitro plantlet formation from Citrus sp. and hybrids. Scientia Hort. 17:333-341. * Bonga , J.M and Durzan, D.J. 1987. Cell and Tissue Culture in Forestry. Vol.3. Martinus Nijhoff Publishers, Lancaster, pp. 261-271. * Brickell, C. .1989. Plant and Flowers Dorling Kindersly LTD., London. * Butcher, D.N and Ingram, D.S. 1976. Plant tissue culture . Edward Arnold. * D'Amato, F. 1977. Cytogenetics of differentiation in tissue and cell culture. In: Reinert ,J. and Bajaj, Y.P.S. (eds). Applied and fundamental aspects of plant cell, tissue and organ culture, Springer- Verlag, Berlin. pp. 343-357. * Davies, D.L. 1980. Rapid propagation of roses in vitro. Scientia Hort. 13: 385-389. * Durzan, D.J. 1988. Somatic polyembryogenesis for the multiplication of tree crop's. Biotech. Genet. Eng. Rev. 6:339376. * Elliot, R.F. 1970. Axenic culture of meristem tips of Rosa multiflora .Planta . 95:183 – 186 . * Evans, D.A. ,Sharp W.R. ,and Flick, C.E. 1981. Growth and behaviour of cell cultures : Embryogenesis and organogenesis . In: Thorpe, T.A.(ed) .Plant tissue culture :Methods and Applications in Agriculture . Academic Press ,New York. pp 45-113. * Fernandez , G.E. and Clark, J.R .1991. In vitro propagation of the erect thornless Navaho Black berry. HortScience 26(9):1219 * Gamborge,O.L., MillerR.A. and Ojima,K. 1968 . Nutrient requirements of suspension cultures of soyabean root cells. Exp. Cell. Res. 50:151-158. * Gault, S.M. and Synge, P.M. .1971. The Dictionary of Roses in colours. Rainbird Reference Books limited. * Gawel, N.J Robacker, C.D, and Corley, W.L. 1990. In vitro propagation of Miscanthus sinensis. Hort Science 25 (10): 1291-1293. * George, E.F. and Sherrington, P.D. 1984. Plant Propagation by Tissue Culture Exegenetics Ltd. Eversely, England. * Goyal, Y. and Arya, H.C. 1981. Differentiation in cultures of Prosopis cineraria Linn. Curr. Sci. 50: 468-469. * Graifenberg, A. 1973. Culture in vitro of embryos and parts of achenes in Rosa canina. Rev. Hort. 57: 374-380. * Graifenberg, A., Giustiniani, L. and Papandreou, A. 1975. In vitro culture of shoot apices of Rosa multiflora Thumb. and Rosa indica Mayor. Agric. Ital 1/3: 119-128. * Hasegawa, P.M. 1979. In vitro propagation of rose. HortScience 14:610-612. * Hasegawa, P.M. 1980. Factors affecting shoot and root initiations from cultured rose shoot tips. J.A mer. Soc. Hort. Sci. 105:216-220. * Hill, G.P. 1967. Morphogenesis of shoot primordia in cutlured stem tissue of garden rose. Nature 216: 596-597. * Hussein, N.M.A. .2002. Propagation of Ziziphus spina Christi L. through in vitro culture. PhD. Faculty of Agriculture Gezira University. * Hussey, G. 1978. The Application of Tissue Culture to the Vegetative Propagation of Plants. Sci. Progress. Oxford 65: 183-208. * Hyndman, S.E., Hasegawa, P.M. and Bressan, R.A. 1982. Stimulation of root initiation from cultured rose shoots through the use of reduced concentrations of mineral salts. HortSiernce 17: 82-83. * Ill-Whansul and Korban, S.S 1998. Effect of media, carbon sources and cytokinins on shoot organogenesis in the christmas tree scots pine Binus sylvestrisL. Journal of Horticultural Science and Biotechnology 73(6)822-827. * Jacobs, G. Allen, P. and Bornman, C.H. 1969. Tissue culture studies on rose. Use of shoot tip explants. I. Auxin: cytokinin effects. Agroplantae 1:79-87. * Jacobs, G., Allen, P. and Bornman, C.H. 1970 a. Tissue culture studies on rose.II. cytokinin: gibberellin effects. Agroplantae. 2: 25-27. * Jacobs, G., P. Allen, P. and Bornman,C.H. 1970b. Tissue culture studies on rose. III. Auxin: gibberellin effects. Agroplantae. 2: 45-49. * Jacobs, G.P., Bornman, C.H., and Allen, P.1968. Tissue culture studies on rose. Use of pith explants. South African J.Agr. Sci. 11: 673-678. * Jordan, M. 1987. In vitro culture of Prosopis Sp. In: Bonga, J.M. and Durzan, D.J. (eds). Cell and tissue culture in forestry. Vol. 3., Martinus Nijhoff Publishers, Lancaster pp. 370-384. * Khosh- khui, M. and Sink, K.C. 1982. Rooting - enhancement of Rosa hybrida for tissue culture propagations. Scientia Hort. 17: 371-376. * Kim, S.W., Oh, S.C., In, D.S., Liu, J.R. 2003. Plant regeneration of rose (Rosa hybrida) from embryogenic cell derived protoplasts. Plant Cell, Tissue and Organ culture 73:15-19. * Lammerts, W.E. 1946. The use of embryo culture in rose breeding. Plants and Gardens 2: 111. * Larson, R.A. .1980. Introduction to Floriculture . Academic Press. * Lawrence, R. H.Jr. 1981. In vitro plant cloning system. Env. Expt. Bot. 21: 289-300. * Linsmair,E.M. and Skoog,F. 1965 . Physiol. Plantarum 18, 100 . * Liu, L. and Burger, D.W. .1986. In vitro propagation of easter lily from pedicle HortScience 21(6):1437-1438. * Masaad, M.M. .1999. Clonal Propagation of a local variety of grape (Vitis sp.) via shoot tip culture. M.Sc. Department of Biology Faculty of Education University of Khartoum. * Mc Comb, J.A. and Newton, S.1981. Propagation of Kangaroo paws using tissue culture . J. Hort. Sci. 56:181-183. * Mhatre, M., Bapat, V.A. and Rao, P.S. 1985. Regeneration of plants from the cultures of leaves and axillary buds of mulberry ( Morus indica L.) Plant Cell Rep.4:78-80. * Mogranahan, G.H., Driver, J.A, and Tulecke, M.1987. Tissue Culture of Juglan sp. In : Bonga, J.M. and Durzan, D.J. (eds). Cell and tissue culture in forestry. Vol.3, Martinus Nijhoff Publisher Lancaster, pp. 261-271. * Mohamed, S.A. .1994. A study of some aspects of Rose growing. M.Sc. Department of Horticulture, Faculty of Agriculture, Khartoum University. * Moraes - Cedeira, R.M. . 1995. Cotton Fiber as a substitute for agar support in tissue culture. Hort -Science 30 (5): 10831083. * Murashige, T. 1974. Plant propagation through tissue cultures. Ann. Rev. of Plant Physiol. 25:135-166. * Murashige, T. 1977. Manipulation of organ culture in plant tissue culture Bot. Bull. Acad. Sin. 18: 1-24. * Murashige, T. 1979. Principles of rapid propagation . In: Huges, K.W., Hanks, R., and Constantin, M.(eds). Propagation of higher plants through tissue culture application. National Tech. Info. Serv., U.S.Dept. of commerce, Springfield, Va. PP. 14-24. * Murashige,T. and Skoog,F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures.Physiol. Plant. 15:473-497. * Navarro, L., Ortiz, J.M., and Juarez ,J.1985. Aberrant citrus plants obtained by somatic embryogenesis of nucelli cultured in vitro. Hortscience 20: 214-215. * Nayak, N.R., Rath, S.P., Patnaik, S. .1997. In vitro propagation of three epiphytic orchids, Cymbidium aloifolium (L.) Sw., Dendrobium aphyllum (Rox6.) Fisch. and Dendrobium moschatum (Buch- Ham Sw.) through thidiazuron induced high frequency shoot proliferation. Scientia Horticulturae 71: 243-250. * Nesius, K.K. Uchytil, L.E., and Fletcher, J.S. 1972. Minimal organic medium for suspension cultures of Pauls Scarlet rose. Planta 106: 173: 176. * Omura, M., Matsuta, N., Moriguchis, T., and Kozaki, I. 1987. Adventitions shoot and plantlets formation from cultured pomegranate leaf explants. HortScience 22:133-134. * Pierik, R.L.M. .1984. In vitro Culture of Higher Plants. Department of Horticulture, Wageningen- Netherland. Agricultural University, * Rodriguez, R.and Diaz- Sala, C. 1991 Pear in vitro propagation using a double phase culture system. HortScience 26(1): 6264. * Rosu, A., Skirvin, R. M., Bein, A., Norton, M.A., Kushad, M., and Otterbacher, A.G. 1995. The development of putative adventitious shoots from chimeral thornelss rose (Rosa multiflora Thunb. Exj. Murr) in vitro. Journal of Horticultural Science 70(6)901-907. * Roubelkis- Angelakis , K.A., Zivanovitc, S.B.1991. A new culture media for in vitro rhizogenesis of grapevine ( Vitis Spp.) genotypes. HortScience 26 (12): 1551-1553. * Scotti- Campos, P. and Pais, M.S.S. 1990. Mass propagation of the dwarf rose cultivar "Rosamini". Scientia Hort. 43: 321330. * Semeniuk, P., Stewart, R.N., and Uhring, J. 1963. Induced secondary dormancy of rose embryos. Proc. Amer. Soc. Hort. Sci. 83: 825-828. * Singh, S.K. and Syamal, M.M. 2000. Antiauxin enhance Rosa hybrida L. micropropagaton. Biologia Plantarum 43:279-281. * Skirvin, R.M.; Chu, M.C., and Walter, J.C. 1984. Tissue culture of the rose. Amer. Rose Ann. 69: 91-97. * Skirvin, R.M. and Chu, M.C. 1979. In vitro propagation of “Forever Yours” Rose .HortScience . 14:608 – 610. * Skoog, F. and Tsui. 1948 . Am. J. Bot. 35: 782-787. * Smith,R..1992. Plant tissue culture techiques and experiments. Academic Press, INC. * Stimart, D.P. and Mather, J.C .1996. Regenerating adventitious shoots from in vitro culture of Liatris spicata (L.) Willd. cotyledons. HortScience 31(1): 154-155. * Sudarsona and Goldy, R.G. .1991. Growth regulators and axillary bud position effects on in vitro establishment of Vitis rotundifolia. HortScience 26(3): 304-307. * Sultanbawa, F. and Phatak, S.C 1991. Propagation of sterile ornamental peper by cuttings and in vitro shoot tip culture. HortScience 26 [8]:1078 * Thorpe, T.A.;Harry, I.S. and Kumar,P.P. 1991. Application of micropropagation to forestry In: Debergh, P.C and Zimmerman, R.H. (eds), Micropropagation technology and application. Kluwer Academic Dordrecht/Boston/London, pp311-336b. Publishers, * Tisserat, B., Esan, E.B., and Murashige, T. Somatic embryogenesis in angiosperms. Hort. Rev. 1: 1-78. * Valles, M. and Boxus, Ph. 1987. Micropropagation of several Rosa hybrida L. cultivars. Acta Hort. 212:611-617. * Vasil, I.K., Scowcroft, W.R., and Frey, K.J. Plant improvement and somatic cell genetics. Academic press. * Von Abrams, G.J. and Hand, M.E. 1956. Seed dormancy in Rosa Sp. As a function of climate. Amer, J.Bot. 43: 7-12. * Whitehead, H.C.M. and Giles, K.L. 1977. Rapid propagation of Populars by tissue culture methods. N.Z.J. for Sci. 7: 40-43. * Zhang , B.and Stoltz, L.P. 1991. In vitro shoot formation and elongation of dwarf pomegranate . HortScience 26(8):1084. Appendix (1) Appendix (1):Inorganic salt stocks of Murashige and Skoog(1962) medium. Chemical Concentration(g/l) Nitrate stock: Ammonium nitrate,NH4NO3 Potassium nitrate,KNO3 165.0 190.0 Sulphate stock: Magnesium sulfate,MgSO4.7H2O Manganous sulfate,MnSO4.H2O Zinc sulfate,ZnSO4.7H2O Cupric sulfate,CuSO4.5H2O 37.0 1.69 0.86 0.0025 Halide stock: Calcium chloride,CaCl2.2H2O Potasium iodide,KI Cobalt chloride,CoCl2.6H2O PBMo stock: Potasium phosphate,KH2PO4 Boric acid,H3BO3 Sodium molybdate,Na2MoO4.2H2O 44.0 0.083 0.0025 17.0 0.620 0.025 NaFe EDTA stock: Ferrous sulfate,FeSO2.7H2O Ethylenediamineteraacetic acid,disodium salt, Na2EDTA (Smith ,R. 1992). (Smith ,R. 1992). 2.784 3.724 Appendix (2) Medium A 1.Sucrose 2.Thiamine- HCl 3.Myo-inositol 4.NAA 5.Cytokinins 6.Adenin sulphate 7.Agar 30.0 0.4 100 0.3 (BA)3.0 80 7000 B 30.0 0.4 100 0.2 Kinetin 2.0 80 7000 C 30.0 0.4 100 0.1 Kinetin 1.0 80 7000 Appendix (2) Cemical composition of Murashige and Skoog Multiplication media A, B, and C. All three media contain MS salt mix +NaH2PO4.2H2O
© Copyright 2026