Sylllabus - Harvard Program in Therapeutic Science
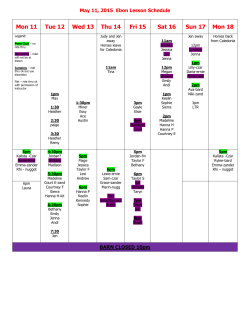
BCMP 236. Modern Drug discovery: from principles to patients Tim Mitchison and Nathanael Gray (Medical School) and members of the Department Half course (spring term). Tu., Th., 3:30-‐5. This course will familiarize students with central concepts in drug action and therapeutics at the level of molecules, cells, tissues and patients. These concepts and methods are central to modern drug development and regulatory evaluation. In the course we will cover drug-‐target interactions, Pharmacokinetics and Pharmacodynamics at a quantitative level, the clinical trials process, biomarkers and new frontiers in Therapeutic development. This course will focus on modern approaches to therapeutic discovery and development, for small molecules, protein based therapeutics, nucleic acid based drugs, antibacterial compounds and nano particles. This course will follow the current paradigms and pipeline doe drug development from target identification to first in human studies. Examples are drawn from numerous unmet medical needs including cancer, HIV, neurodegenerative and infectious diseases. The course will include computational and quantitative exercises. Note: This course is a reworking of the BCMP 309qc and 307qc quarter courses. Spring 2014 Meeting Dates: January 29, February 3, 5, 10, 12, 17, 19, 24, 26, M arch 3, 5, 10, 12, 24, 26, 31 April 2, 7, 9, 14, 16, 21, 23, 28, 30, M ay 5,7,12 Meeting Time: 3h30pm to 5pm First Meeting: 1/29/2015 Final Meeting: 5/12/2015 Location: TMEC 126 Class size: May be limited Course Directors: Tim Mitchison: [email protected] ; Nathanael Gray: [email protected] TAs: Nieneke Moret and Adam Brown Course faculty: Catherine Dubreuil, [email protected] COURSE INFORMATION: (1) Detailed course goals and objectives: • Understand and analyze drug response pathways • Understand and analyze drug development pipelines • Know modern drug design paradigms • Quantitative analysis of Pharmacokinetics • Drug-‐receptor interactions • Understand differences in development, including PK, of small molecules, biologics, anti-‐bacterial agents. (2) Course outline: This course will have four main modules: 1. Module 1: Target Selection 2. Module 2: Target to hit (include small molecules, Biologics, nucleic acids and anti-‐-‐-‐bacterial) 3. Module 3: Preclinical drug development 4. Module 4: First in human (phase I and II trials) (3) Workload and assessments This course will have 2 separate types of assessments: in class discussions/presentations and problem sets. There will be a total of 5 quantitative problems sets. Students are NOT required to use MATLAB to do these and may use any software they choose if applicable. Each student must hand individual work. All Problem set will be due BEFORE the start of class on the due day. You must hand in your own individual assignments. Working with others on your is permitted, HOWEVER, YOU MUST NOTE WHO YOU WORKED WITH AND YOUR ANSWERS MUST BE YOUR OWN! Late papers will be accepted with a legitimate excuse, but may not get full credit. (4) Course policy and Student expectations Students are expected to come to lecture having read the materials in advance. Attendance to every discussion section is mandatory. Failure to attend tutorial due to an unexcused absence will severely impact the overall course grade. • Only excused absences will be considered: an example of an excused absence is a medical emergency and must be accompanied by a doctor's note OR other situations that will be determined by the course instructors. If you know you will not be able to attend a tutorial session, you must notify the course director in advance OR within 24 hours following the end of missed class. • • • Classroom Courtesy: As a courtesy to all the members of our classroom community, please turn off your cell phones and keep your side conversations to a strict minimum. • Students are expected to be punctual and arrive on time to lecture. Lectures will start on time • Academic Honesty: You are expected to be familiar with and to follow the University’s policies on academic integrity and plagiarism: http://webdocs.registrar.fas.harvard.edu/ugrad_handbook/2009_2010/cha pter2/academic_dishonesty.html and http://isites.harvard.edu/icb/icb.do?keyword=k70847&pageid=icb.page355 322 All references and sources used in your assignments MUST be properly cited in accordance with Harvard guidelines. Instances of academic dishonesty may result in sanctions including but not limited to, failing grades being issued, and other consequences. BCMP 236 Schedule 2015 Date/time Location Lecturer Topic Modules: 01/29/2015: 3-‐5pm TMEC 126 Tim Mitchison Intoduction to the class, current drugs, difficult targets Module 1: Kinetics, target and binding Psets 02/03/2015: 3-‐5pm TMEC 126 Tim Mitchison Receptor theory, enzyme inhibition Module 1: Kinetics, target and binding 02/05/2015: 3-‐5pm TMEC 126 Discussion sections Receptor theory, enzyme inhibition discussion Module 1: Kinetics, target and binding 02/10/2015: 3-‐5pm TMEC 126 Steve Haggarty Target selection Module 1: Target selection 02/12/2015: 3-‐5pm TMEC 126 Steve Haggarty Target selection cont. 45 mins Discussion: Schizophrenia paper/targets 45 mins IN CLASS Module 1: Target selection 02/17/2015: 3-‐5pm TMEC 126 Steve Haggarty Assay development for drug discovery Module 2: Target to Hit 02/19/2015: 3-‐5pm TMEC 126 Sara Buhrlage Small Molecules 2: Screening and structure based design Module 2: Target to Hit Small molcules 02/24/2015: 3-‐5pm TMEC 126 Sara Buhrlage Small Molecules 3: Optimization, what makes a good molecule Module 2: Target to Hit Small molcules 02/26/2015: 3-‐5pm TMEC 126 Tim Springer Biologics 1: Therapeutics Antibody development Module 2: Target to Hit Biologics 03/03/2015: 3-‐5pm TMEC 126 Tim Springer Biologics 2: Protein Therapeutic development Module 2: Target to Hit Biologics 03/05/2015: 3-‐5pm TMEC 126 Dinah Sah Nucleic acid 1: siRNA Module 2: Target to Hit Nucleic acid 03/10/2015: 3-‐5pm TMEC 126 Discusion sections Nucleic acid 3: paper discussion or case study? AAV gene therapy for ALS-‐SOD1 Module using 2: TRarget NAi to Hit Nucleic acid 03/12/2015: 3-‐5pm TMEC 126 Tim Mitchison Pharmacodymanics Module 3: Preclinical 03/24/2015: 3-‐5pm TMEC 126 Tim Mitchison Pharmacokinetics 1 Module 3: Preclinical 03/26/2015: 3-‐5pm TMEC 126 Tim Mitchison Pharmacokinetics 2 Module 3: Preclinical 03/31/2015: 3-‐5pm TMEC 126 Natalie Agar Pharmacokinetics 3 Module 3: Preclinical 04/02/2015: 3-‐5pm TMEC 126 Suzanne Walker Anti-‐bacterials 1: Antibiotic development, PK, TOX, Clinic Module 2: Target to Hit Anti bacterials 04/07/2015: 3-‐5pm TMEC 126 Suzanne Walker Anti-‐bacterials 2: Antibiotics, target selection and resistance Module 2: Target to Hit Anti bacterials 04/09/2015: 3-‐5pm TMEC 126 Suzanne Walker or discussion? Anti-‐bacterials 3: Anti virals or discussion? Compare contract AB with viral? Module 2: Target to Hit Anti bacterials 04/14/2015: 3-‐5pm TMEC 126 Ofer Levy Vaccine development Module 2: Target to Hit Vaccines 04/16/2015: 3-‐5pm TMEC 126 Omid Farokhzad Pharmacokinetics 4: Nano particles 04/21/2015: 3-‐5pm TMEC 126 Omid Farokhzad Pharmacokinects 5: Nano particles 04/23/2015: 3-‐5pm TMEC 126 Vishal Vaidya Toxicology Module 3: Preclinical 04/28/2015: 3-‐5pm TMEC 126 Vishal Vaidya Advanced topics in biomarkers Module 3: Preclinical 04/30/2015: 3-‐5pm TMEC 126 Natalie Agar Imaging Biomarkers Module 3: Preclinical 05/05/2015: 3-‐5pm TMEC 126 Discussion section Discussion section Phase 1 and 2 trials 05/07/2015: 3-‐5pm TMEC 126 Tim Mitchison Failure, endpoints Phase 1 and 2 trials 05/12/2015: 3-‐5pm TMEC 126 Jerry Avorn Trial design Jerry Avorn Regulatory Science Phase 1 and 2 trials Pset 1 Pset 2: Target selection and assays Pset 3: small molecules Biologics Nucleic acid Anti-‐bacterials Pset 4: Quantitative PK Module 3: Preclinical discussion? Module 3: Preclinical Pset 5: Biomarker, Tox first in man
© Copyright 2026