Reimbursement Guide
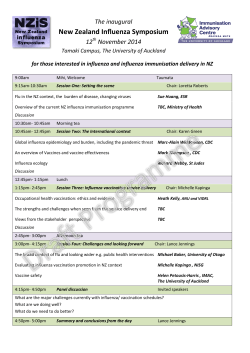
CODING AND BILLING Reference Guide For more information call the Rapivab™ Reimbursement Hotline 1-844-RAPIVAB (1-844-727-4822) Program Specialists are available from 9:00 AM to 5:00 PM EST, Monday through Friday. Separate Reimbursement for Rapivab™ (peramivir injection) Setting of Care Medicare Medicaid Private Payers Yes Varies Varies Yes Varies Varies Hospital Outpatient Departments include: • Emergency Department (ED) • Observation Setting • Hospital provider-based clinics Physician offices include: • Urgent Care Clinics • Private practices • Free-standing Infusion Centers Hospital Outpatient: Medicare reimburses for Rapivab™ separately when used in the outpatient setting of care. Reimbursement will be processed through correct reporting of an unclassified drug code using the corresponding units delineated in the instructions below. Private insurers and state Medicaid agencies have varying reimbursement policies; many will provide separate reimbursement for Rapivab™ in the outpatient setting. Physician Offices: Medicare reimburses for Rapivab™ separately when used in physician offices. Reimbursement will be processed through correct reporting of an unclassified drug code using the corresponding units delineated in the instructions below. Private insurers and state Medicaid agencies have varying reimbursement policies; many will provide separate reimbursement for Rapivab™ in the physician office. Please see Important Safety Information | Please see Full Prescribing Information In addition to the list of relevant codes below, please refer to sample forms in this document for guidance on completing these forms accurately. What are the relevant codes for Rapivab™ in my setting of care? Code Set Setting of Care Code and Description HCPCS Codes •H ospital outpatient At launch, report Rapivab™ with a nonspecific, unclassified HCPCS code. Used to report RAPIVAB department •H ospital outpatient provider-based clinic C9451 - Unclassified drugs or biologicals (Required by Medicare; other payers also may use it) In addition, enter the drug-identifying information as required by the payer. Generally the following information is required: the drug name (brand and generic), NDC, dose, and method of administration. Example: Rapivab™ (peramivir injection) NDC 61364-181-01, 600 mg via IV infusion • Urgent care clinic • Physician office At launch, report Rapivab™ with a non-specific, unclassified HCPCS code. J3490 - Unclassified drugs In addition, enter the drug-identifying information as required by the payer. Generally the following is required: the drug name (brand and generic), NDC, dose, and method of administration. Example: Rapivab™ (peramivir injection) NDC 61364-181-01, 600 mg via IV infusion NDC • Hospital outpatient department Used to report RAPIVAB •H ospital outpatient provider-based clinic Most payers will require the NDC to identify Rapivab™, especially while it is billed with an unclassified code. • Urgent care clinic Vial (one): NDC 61364-181-01 Carton (three): NDC 61364-181-03 • Physician office 200 mg, peramivir, single-use vial Claim form requirements for NDCs vary by payer. See example claim forms that follow for more detail. Please see Important Safety Information | Please see Full Prescribing Information What are the relevant codes for Rapivab™ in my setting of care? Code Set Setting of Care Code and Description Revenue Codes • Hospital outpatient department Used to attribute hospital charges to specific cost centers •H ospital outpatient Revenue codes vary by service provided and also vary depending on patient status. provider-based clinic 0636 - Drugs Requiring Detailed Coding Required by Medicare to obtain pass-through payment for drugs in the outpatient department. 0250 - Pharmacy, General 0260 - Intravenous Therapy, General Some revenue codes are required to obtain appropriate reimbursement for a specific service performed in a specific setting of care; for other services, revenue codes may vary. ICD-9-CM Diagnosis†# Codes used to report diagnoses • Hospital outpatient department • Hospital outpatient provider-based clinic Rapivab™ is indicated for the treatment of acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than 2 days. • Urgent care clinic • 487.0 – Influenza, with pneumonia • Physician office • 487.1 – I nfluenza, with other respiratory manifestations • 487.8 – Influenza, with other manifestations •4 88.XX – Influenza due to identified flu virus (avian, 2009 H1N1, novel) CPT Codes# • Hospital outpatient department Infusion Service Codes used to report IV infusion • Hospital outpatient 96365 Intravenous infusion, for therapy, prophylaxis, or diagnosis; initial, up to 1 hour provider-based clinic • Urgent care clinic • Physician office † ICD-10-CM (diagnosis codes) and ICD-10-PCS (procedure codes) will replace ICD-9-CM effective October 1, 2015. # This may not be an all-inclusive list; see payer-specific coverage policies for covered indication. Please see Important Safety Information | Please see Full Prescribing Information SAMPLE CMS - 1500 CLAIM FORM For services provided in the physician office This document is provided for your guidance only. Please call Rapivab™ Reimbursement Hotline at 1-844-RAPIVAB to verify coding and claim information for specific payers SAMPLE Box 21 – Diagnosis Codes Enter the appropriate ICD-9-CM diagnosis codes (see example) Box 19 – Appropriate area for drug name, NDC code, total units billed and any additional information the payer requires. Example: 487.0 – Influenza, with pneumonia The example shown includes the 11 digit NDC code required by payers. 487.1 – Influenza, with other respiratory manifestations 487.8 – Influenza, with other manifestations 488.XX – Influenza due to identified flu virus (avian, 2009 H1N1, novel) Box 24 – Procedures, Services, or Supplies Enter the NDC code beginning with “N4” and the appropriate HCPCS code Example: J3490 – Unclassified Drug Code with N4 61364-181-01 Example Procedure Code: 96365 – Intravenous Infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); initial, up to 1 hour Please see Important Safety Information | Please see Full Prescribing Information SAMPLE CMS-1450 CLAIM FORM For services provided in hospital setting This document is provided for your guidance only. Please call Rapivab™ Reimbursement Hotline at 1-844-RAPIVAB to verify coding and claim information for specific payers SAMPLE Revenue Code, Description, HCPCS (Fields 42-44) Enter the appropriate revenue code and description corresponding to the HCPCS code in field 44 Example Revenue Codes: Medicare: Please use revenue code 636 - drugs requiring detailed coding Field 66 - Diagnosis Code Enter the appropriate ICD-9-CM diagnosis code (see example) Example: 487.0 – Influenza, with pneumonia Non-Medicare: May require revenue code 250 - general pharmacy 487.1 Example HCPCS: Medicare: C9399 – Unclassified drugs or biologicals 487.8 – Influenza, with other manifestations Non-Medicare: J3490 – Unclassified Drug Code - Please indicate the number of units in Box 80 488.XX – Influenza due to identified flu virus (avian, 2009 H1N1, novel) Example Procedure Codes: 96365 - Intravenous Infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); initial, up to 1 hour – Influenza, with other respiratory manifestations Note: E nter the appropriate diagnosis as reflected in the patient’s medical record Box 80 Comments: Rapivab™ (peramivir injection) NDC 61364-181-01 200 mg single use vial, total 600 mgs Administered IV Please see Important Safety Information | Please see Full Prescribing Information GLOSSARY CMS=Centers for Medicare & Medicaid Services; CPT=Current Procedural Terminology; HCPCS=Healthcare Common Procedure Coding System; ICD-9-CM=International Classification of Diseases, Ninth Revision, Clinical Modification; NDC=National Drug Code. DISCLAIMER The use of this guide is strictly for reimbursement purposes. This guide provides a summary of coding, coverage, and payment of Rapivab™ (peramivir injection) for its FDA (Food and Drug Administration) approved uses as indicated in the prescribing information. The information contained in this document is not intended for purposes of providing clinical practice guidelines for use of Rapivab™. Please see the package insert for more information. BioCryst Pharmaceuticals specifically disclaims liability or responsibility for the results or consequences of any actions taken in reliance on information in this guide. BioCryst Pharmaceuticals cannot guarantee nor is responsible for, the payment of any claim. The coding, coverage, and payment for Rapivab™ may vary by payer, plan, patient, and setting of care. For more information, healthcare professionals should check with individual payers for specific coding, coverage and payment requirements in the use of Rapivab™. It is the sole responsibility of the healthcare professional to properly code and ensure the accuracy of all claims used in seeking reimbursement. All services must be medically appropriate and properly supported in the patient’s medical records. INDICATION Rapivab is indicated for the treatment of acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than 2 days. • E fficacy of Rapivab was based on clinical trials in which the predominant influenza virus type was influenza A; a limited number of subjects infected with influenza B virus were enrolled. • Influenza viruses change over time. Emergence of resistance substitutions could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use Rapivab. • Efficacy could not be established in patients with serious influenza requiring hospitalization. IMPORTANT SAFETY INFORMATION Contraindications None Warnings and Precautions • R are cases of serious skin reactions, including Stevens-Johnson syndrome and erythema multiforme have occurred with Rapivab. Appropriate treatment should be instituted if a serious skin reaction occurs or is suspected. • Patients with influenza may be at an increased risk of hallucinations, delirium and abnormal behavior early in their illness. There have been postmarketing reports (from Japan) of delirium and abnormal behavior leading to injury in patients with influenza who were receiving neuramini dase inhibitors, including Rapivab. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made, but they appear to be uncommon. Patients with influenza should be closely monitored for signs of abnormal behavior. • S erious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. Rapivab has not been shown to prevent such complications. Adverse Reactions • The most common adverse reaction (incidence >2%) is diarrhea (8% Rapivab vs. 7% placebo). • Lab abnormalities (incidence >2%) occurring more commonly than placebo were elevated ALT 2.5 times the upper limit of normal (3% vs. 2%), elevated serum glucose greater than 160 mg/dL (5% vs 3%), elevated CPK more than 6 times the upper limit of normal (4% vs. 2%) and neutrophils less than 1.0 x 109/L (8% vs. 6%). Concurrent use with Live Attenuated Influenza Vaccine Antiviral drugs may inhibit viral replication of a live attenuated influenza vaccine (LAIV). The concurrent use of Rapivab with LAIV intranasal has not been evaluated. Because of the potential for interference between these two products, avoid use of LAIV within 2 weeks before or 48 hours after administration of Rapivab unless medically indicated. # This may not be an all-inclusive list; see payer-specific coverage policies for covered indication. Please see Important Safety Information | Please see Full Prescribing Information Rapivab™ is a registered trademark of BioCryst Pharmaceuticals, Inc. © Copyright 2014 BioCryst Pharmaceuticals, Inc.
© Copyright 2026