Detection of Mycotoxins in Corn Meal Extract with LC-MS/MS Application
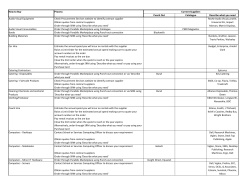
Application Note: 523 Detection of Mycotoxins in Corn Meal Extract Using Automated Online Sample Preparation with LC-MS/MS Yang Shi, Catherine Lafontaine, François Espourteille Thermo Fisher Scientific, Franklin, MA Introduction Key Words s4RANSCEND4,8 s4URBO&LOW 4ECHNOLOGY s4316ANTAGE s&OOD3AFETY Since the discovery of aflatoxin in 1960, mycotoxin research has received considerable attention. Mycotoxins are a group of naturally occurring toxic substances produced by certain molds, which can contaminate food and feed. The inhalation or absorption of mycotoxins into the body may cause harm, including kidney or liver damage, cancer, or even death in man or animals.1 From a food safety perspective, the aflatoxins, ochratoxin A, patulin, fumonisins, trichothecenes, and zearalenone are the mycotoxins of major concern. Many countries now monitor mycotoxin levels in food and feed products. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is currently a common analytical approach for the quantification of mycotoxin contamination.2 Sample preparation for LC-MS/MS analysis can be time and labor intensive, often involving pH modification, solid phase or immunoaffinity column clean-up extraction, multi-step extract clean-up, and pre-concentration.3 The strict regulation published by the European Union in 1999 asking for lower detection limits and higher method reliability presented a new analytical challenge.4 In this study we describe an easy, comprehensive, LC-MS/MS method using a Thermo Scientific Transcend TLX-1 system powered by Thermo Scientific TurboFlow technology to analyze multiple mycotoxin residues in corn meal extract. Figure 1 illustrates a typical Transcend™ TLX-1 system with the Thermo Scientific TSQ Vantage triple stage quadrupole mass spectrometer. Goal Develop a rapid and sensitive automated, online sample preparation LC-MS/MS method to detect and quantify multiple mycotoxins in corn meal extract resulting in a shorter assay time and increased throughput. Experimental 4HEMATRIXSTANDARDCURVE Five grams of corn meal purchased from a local grocery store were extracted using 25 mL of 70% methanol in water followed by 60 minutes of ultra-sonication. The extract sat overnight at room temperature. The resulting solution was then centrifuged at 6000 RPM for 20 minutes. The supernatant was used to prepare the matrix calibrators and QC samples. Each milliliter of supernatant corresponds to 0.2 g solid corn meal powder as the unit of conversion. Figure 1. Thermo Scientific Transcend TLX system with TSQ Vantage triple quadrupole mass spectrometer The stock mix solution of analytes was prepared in methanol. Table 1 lists selected reaction monitoring (SRM) transitions and stock concentrations for individual analytes. Eight mycotoxins were analyzed under positive electrospray ionization (ESI) mode. The remaining three compounds, deoxynivalenol (DON), nivalenol (NIV), and 3-acetyl-DON (3-AcDON), were analyzed under negative electrospray ionization (ESI) mode. ,#-3-ETHODSUSINGNEGATIVE%3)MODE-ETHOD" Table 1. Analytes list #OMPOUNDS 3TOCK 0ARENT 0RIMARY 3ECONDARY CONCENTRATION (m/z) (m/z) (m/z) «GM, 4URBO&LOW-ETHOD0ARAMETER Column: Research column A 0.5 x 50 mm Injection Volume: 10 µL Solvent A: water Solvent B: methanol Solvent C: 0.1% ammonium hydroxide Solvent C: 45:45:10 ACN: isopropanol: acetone (v:v:v) (0,#-ETHOD0ARAMETERS Aflatoxins B1 313 241 285 0.050 Aflatoxins B2 315 259 287 0.015 Aflatoxins G1 329 243 283 0.050 Aflatoxins G2 331 245 275 0.015 Zearalenone (ZEA) 319 187 185 10.000 Ochratoxin A (OTA) 404 239 221 1.000 -ASS3PECTROMETER0ARAMETERS Fumonisins B1 (FB1) 722 334 352 2.500 MS: Fumonisins B2 (FB2) 706 336 318 2.500 TSQ Vantage triple stage quadrupole mass spectrometer Deoxynivalenol (DON) 295 138 265 20.000 MS Ionization Source: H-ESI Nivalenol (NIV) 311 281 205 20.000 Spray Voltage: 4.5 kV 3-Acetyl-DON (3-AcDON) 337 307 173 20.000 Sheath Gas Pressure (N2): 50 arbitrary units ,#-3-ETHODSUSINGPOSITIVE%3)MODE-ETHOD! 4URBO&LOW©-ETHOD0ARAMETERS Column: TurboFlow Cyclone-P 0.5 x 50 mm Injection Volume: 10 µL Solvent A: 10 mM ammonium acetate in water Solvent B: 0.1% formic acid in acetonitrile (ACN) Solvent C: 1:1:1 ACN: isopropanol: acetone (v:v:v) with 0.3% formic acid Analytical Column: Hypersil GOLD™ 2.1 x 50 mm, 1.9 µm Solvent A: 0.1% formic acid in water Solvent B: 0.1% formic acid in ACN Auxiliary Gas Pressure (N2): 20 arbitrary units Vaporizer Temperature: 250 °C Capillary Temperature: 270 °C Collision Gas Pressure: 1.5 mTorr The LC method views from Thermo Scientific Aria Operating Software are shown in Figures 2 and 3. (0,#-ETHOD0ARAMETERS Analytical Column: Thermo Scientific Hypersil GOLD 2.1 x 100 mm, 1.9 µm Solvent A: 0.1% formic acid in water Solvent B: 0.1% formic acid in ACN Figure 2. Method A view in Aria OS software -ASS3PECTROMETER0ARAMETERS MS: TSQ Vantage™ triple stage quadrupole mass spectrometer MS Ionization Source: Heated Electrospray Ionization (H-ESI) Spray Voltage: 5 KV Sheath Gas Pressure (N2): 50 arbitrary units Auxiliary Gas Pressure (N2): 20 arbitrary units Vaporizer Temperature: 209 °C Capillary Temperature: 270 °C Collision Gas Pressure: 1.5 mTorr Figure 3. Method B view in Aria OS software Results and Discussion Figure 6 presents the linear fit calibration curves for DON and NIV, indicating excellent linear fits over the dynamic range. Table 3 summarizes detection, quantitation limits, and standard curve linearity for three analytes analyzed in negative ion mode. For all analytes, the quantitation limits obtained using the present methodology comply with the maximum levels in foods defined by European Union.6 To the best of our knowledge, this is the first application of its type to detect these three compounds using an automated online sample preparation technique coupled to tandem mass spectrometry. In addition, a lower limit of quantitation (LOQ) could be achieved by increasing sample injection volume since TurboFlow columns can handle larger injections (up to a few hundred microliters) while regular HPLC or UHPLC columns can not. Figure 4 shows the comparison of chromatograms of eight analytes at 1:100 dilutions in methanol and corn meal extract, indicating excellent chromatographic separation in both solvent standard and matrix. Matrix-matched calibration standards showed linear response of two orders of magnitude (r2 > 0.99) for six of them (Table 2). Significant signal enhancement was observed for FB1 and FB2 due to matrix-induced ionization variability, which was previously reported by other researchers.5 In future work, the isotope-labeled internal standard might be used to compensate for the matrix interference. Because DON, NIV, and 3-AcDON have a better signal response under negative ionization mode, a separate LC-MS/MS method was developed. Figure 5 shows the chromatograms of DON, NIV, and 3-AcDON identified at 100 ng/mL fortified in the corn meal extract. RT: 4.50 - 10.00 RT: 4.50 - 10.00 SM: 13G NL: 5.78E5 TIC F: + p ESI SRM ms2 313.109 [241.138-241.148, 285.172-285.182] MS ICIS 1_100_std_B_002 RT: 7.12 AA: 15634265 100 SM: 13G 50 NL: 5.22E5 TIC F: + p ESI SRM ms2 313.109 [241.138-241.148, 285.172-285.182] MS ICIS 1_100_std_B_corn_002 RT: 7.12 AA: 11351226 100 50 NL: 9.61E4 TIC F: + p ESI SRM ms2 315.123 [259.150-259.160, 287.194-287.204] MS ICIS 1_100_std_B_002 RT: 6.95 AA: 2970358 100 RT: 8.54 AA: 420771 50 50 NL: 3.35E5 TIC F: + p ESI SRM ms2 329.000 [242.995-243.005, 282.995-283.005] MS ICIS 1_100_std_B_002 RT: 6.91 AA: 10343546 100 NL: 1.25E5 TIC F: + p ESI SRM ms2 315.123 [259.150-259.160, 287.194-287.204] MS ICIS 1_100_std_B_corn_002 RT: 6.91 AA: 4028649 100 RT: 8.54 AA: 313113 NL: 3.60E5 TIC F: + p ESI SRM ms2 329.000 [242.995-243.005, 282.995-283.005] MS ICIS 1_100_std_B_corn_002 RT: 6.91 AA: 8571712 100 50 50 NL: 3.88E5 TIC F: + p ESI SRM ms2 331.132 [245.127-245.137, 274.995-275.005] MS ICIS 1_100_std_B_002 RT: 8.55 AA: 8499060 100 50 50 RT: 6.74 AA: 880570 RT: 8.66 AA: 7959175 100 50 RT: 8.24 AA: 24390610 100 RT: 8.55 AA: 5872986 100 NL: 4.19E5 TIC F: + p ESI SRM ms2 319.160 [115.089-115.099, 185.067-185.077, 187.136-187.146, 283.188-283.198] MS ICIS 1_100_std_B_002 100 NL: 1.15E6 TIC F: + p ESI SRM ms2 404.151 [220.912-220.922, 239.079-239.089] MS ICIS 1_100_std_B_002 100 RT: 6.69 AA: 781042 RT: 8.71 AA: 11177335 50 50 NL: 3.19E5 TIC F: + p ESI SRM ms2 331.132 [245.127-245.137, 274.995-275.005] MS ICIS 1_100_std_B_corn_002 RT: 6.45 AA: 4886413 NL: 5.88E5 TIC F: + p ESI SRM ms2 319.160 [115.089-115.099, 185.067-185.077, 187.136-187.146, 283.188-283.198] MS ICIS 1_100_std_B_corn_002 NL: 2.04E6 TIC F: + p ESI SRM ms2 404.151 [220.912-220.922, 239.079-239.089] MS ICIS 1_100_std_B_corn_002 RT: 8.29 AA: 40293551 50 NL: 5.70E5 TIC F: + p ESI SRM ms2 706.140 [318.387-318.397, 336.336-336.346] MS ICIS 1_100_std_B_002 RT: 7.54 AA: 14738742 100 50 50 RT: 8.87 AA: 3101293 NL: 3.22E5 TIC F: + p ESI SRM ms2 722.116 [334.341-334.351, 352.377-352.387] MS ICIS 1_100_std_B_002 RT: 6.79 AA: 7724904 100 NL: 2.92E6 TIC F: + p ESI SRM ms2 706.140 [318.387-318.397, 336.336-336.346] MS ICIS 1_100_std_B_corn_002 RT: 7.49 AA: 63767620 100 50 NL: 2.42E6 TIC F: + p ESI SRM ms2 722.116 [334.341-334.351, 352.377-352.387] MS ICIS 1_100_std_B_corn_002 RT: 6.79 AA: 49577376 100 50 RT: 8.65 AA: 1441806 6 5 7 Time (min) 8 9 10 5 6 7 Time (min) 8 9 10 Figure 4. Comparison of chromatograms of 8 SRM analytes in methanol and corn flour extract (1:100 dilution of stock mixture) NL: 1.25E5 m/z= 137.63-138.63 F: - p ESI SRM ms2 295.140 [138.123-138.133, 247.161-247.171, 265.169-265.179] MS ICIS 100ng_ml_002 RT: 3.71 AA: 916274 SN: 15540RMS 100 80 DON 60 40 20 3.43 4.18 0 NL: 1.89E5 m/z= 280.67-281.67 F: - p ESI SRM ms2 311.148 [119.108-119.118, 183.072-183.082, 191.115-191.125, 197.078-197.088, 203.087-203.097, 281.170-281.180] MS ICIS 100ng_ml_002 NIV Relative Abundance 100 80 RT: 3.51 AA: 1020801 SN: 618RMS 60 4.00 40 20 0 NL: 1.70E5 m/z= 306.63-307.63 F: - p ESI SRM ms2 337.148 [173.126-173.136, 217.155-217.165, 307.125-307.135] MS ICIS 100ng_ml_002 RT: 3.97 AA: 1142785 SN: 4961RMS 100 80 3-AcDON 60 40 20 0 3.0 3.61 3.14 3.2 3.4 3.6 Time (min) 3.8 4.0 4.2 Figure 5 Selected chromatograms of DON, NIV, and 3-AcDON detected at 100 ng/mL fortified in the corn meal extract Table 2. Limit quantitation (LOQ) and standard curve linearity (r2) for analytes detected in positive ion mode #OMPOUNDS ,/1 NGG 0.50 0.9956 G1 0.50 0.9910 OTA 5.00 0.9937 ZEA 50.00 0.9955 FB1 12.50 0.9984 FB2 12.50 0.9965 Scientific maintains ,/1 NGG Deoxynivalenol (DON) 25.00 0.9934 tative organizations Nivalenol (NIV) 25.00 0.9933 throughout the world. 3-Acetyl-DON (3-AcDON) 25.00 0.9925 Deoxynivalenol Y = 71516.1+10580*X R^2 = 0.9934 W: 1/X a network of represen- R2 Nivalenol Y = 219026+8122.82*X R^2 = 0.9933 W: 1/X 12000000 9500000 11500000 NIV 9000000 11000000 DON 10500000 10000000 8500000 8000000 9500000 7500000 9000000 7000000 8500000 8000000 6500000 7500000 6000000 7000000 5500000 6500000 Area Area offices, Thermo Fisher #OMPOUNDS R2 B1 In addition to these Table 3. LOQ and standard curve linearity for analytes detected in negative ion mode 6000000 5500000 5000000 4500000 5000000 4000000 4500000 3500000 4000000 3000000 3500000 3000000 2500000 2500000 2000000 2000000 1500000 1500000 1000000 1000000 500000 500000 0 0 0 100 200 300 400 500 600 700 800 900 1000 0 1100 100 200 300 400 500 600 700 800 900 1000 1100 ng/ml ng/ml Figure 6. Calibration curves for DON and NIV Conclusion Developing a rapid and sensitive quantitative method is always a major goal for mycotoxins analysis.7 Two quick, automated online sample preparation LC-MS/MS methods have been developed that are sensitive enough to detect mycotoxins in corn meal extract. By eliminating manual sample preparation, the reliability of this methodology was improved significantly. The sample throughput could be improved by multiplexing the two methods on different LC channels using a Transcend TLX-2 (or TLX-4) system. Future work will focus on the application of this methodology on various food matrices and references. References 1 Pitt, J.I. What are mycotoxins? Australian Mycotoxin Newsletter 1996, 7(4), 1. 2 Spanjer, M.C., Rensen, P.M., Scholten, J.M. LC-MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2008, 25, 472-89. 3 Shephard, G.S., Determination of mycotixins in human foods, Chem. Soc. Rev., 2008, 37, 2468-77. 4 Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. http://eur-lex. europa.eu/, accessed on Apr. 17, 2011. 5 Li, W., Herrman, T.J., Dai, S. Y., Rapid Determination of Fumonisins in Corn-Based Products by Liquid Chromatography/Tandem Mass Spectrometry, J. AOAC Int., 2010, 93, 1472-81. 6 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R18 81:20100701:EN:PDF. Accessed on Mar. 15, 2011. 7 Rahmani, A., Jinap, S., Soleimany, F., Quantitative and qualitative analysis of mycotoxins, Compr. Rev. Food Sci. Food Safety, 2009, 8, 202-251. !FRICA/THER +27 11 570 1840 !USTRALIA +61 3 9757 4300 !USTRIA +43 1 333 50 34 0 "ELGIUM +32 53 73 42 41 #ANADA +1 800 530 8447 #HINA +86 10 8419 3588 $ENMARK +45 70 23 62 60 %UROPE/THER +43 1 333 50 34 0 &INLAND.ORWAY 3WEDEN +46 8 556 468 00 &RANCE +33 1 60 92 48 00 'ERMANY +49 6103 408 1014 )NDIA +91 22 6742 9434 )TALY +39 02 950 591 Japan +81 45 453 9100 ,ATIN!MERICA +1 561 688 8700 -IDDLE%AST +43 1 333 50 34 0 .ETHERLANDS +31 76 579 55 55 .EW:EALAND +64 9 980 6700 2USSIA#)3 +43 1 333 50 34 0 3OUTH!FRICA +27 11 570 1840 3PAIN +34 914 845 965 3WITZERLAND +41 61 716 77 00 UK +44 1442 233555 53! +1 800 532 4752 WWWTHERMOSCIENTIFICCOM Legal Notices: ©2011 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. This information is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Not all products are available in all countries. Please consult your local sales representative for details. Part of Thermo Fisher Scientific Thermo Fisher Scientific, San Jose, CA USA is ISO Certified. AN63403_E 04/11S
© Copyright 2026