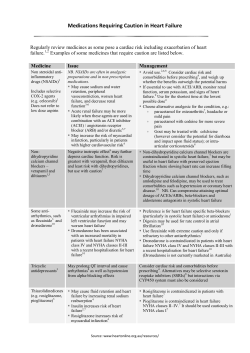
Sample CMS 1450 (UB-04) Form:
Sample CMS 1450 (UB-04) Form: How to bill for a drug with an assigned HCPCS for Medicare hospital outpatient departments. Check with your local payers for appropriate coding requirements. __ __ __ 4 3a PAT. CNTL # b. MED. REC. # 2 __ 1 6 5 FED. TAX NO. 8 PATIENT NAME 9 PATIENT ADDRESS a 10 BIRTHDATE 31 11 SEX OCCURRENCE 12 32 DATE a c ADMISSION 13 HR 14 TYPE 15 SRC 16 DHR 17 STAT 33 OCCURRENCE DATE CODE OCCURRENCE DATE DATE Optional CODE - Column 43: CODE Kyprolis® (carfilzomib) for Injection 18 7 STATEMENT COVERS PERIOD FROM THROUGH b b TYPE OF BILL 19 20 34 OCCURRENCE CODE DATE 35 CODE 38 Indicate name of medication administered. Example: Kyprolis (carfilzomib) for Injection CONDITION CODES 24 22 23 21 a 25 26 36 CODE OCCURRENCE SPAN FROM THROUGH 39 CODE 40 CODE VALUE CODES AMOUNT 27 d 28 e 29 ACDT 30 STATE 37 OCCURRENCE SPAN FROM THROUGH VALUE CODES AMOUNT b Column 47: Total Charges 41 CODE VALUE CODES AMOUNT Indicate the facility’s actual charges for products and procedures. c d 42 REV. CD. 44 HCPCS / RATE / HIPPS CODE 43 DESCRIPTION 45 SERV. DATE 46 SERV. UNITS 47 TOTAL CHARGES 48 NON-COVERED CHARGES 49 1 1 2 2 3 3 4 4 Column 46: Service Units 5 5 6 6 Report the appropriate unit of 7 service. Carfilzomib is billed on a 8 “per milligram” basis. 9 Example: One vial of Kyprolis 10 is equal to 60 units of J9047 11 Column 42: Revenue Code(s) 7 8 Enter appropriate product/procedure 9 revenue codes. 10 Product11Revenue Codes: 12 0636 “Drugs that require Example: 13 detail coding” 14 Please note: Revenue code requirements may vary15by payer. Check with payer to 16 determine the appropriate revenue code for billing.17 18 Procedure Revenue Codes: 19 Enter appropriate procedure revenue code. 20 Use the most appropriate revenue code for 21 the service performed. 22 Example: 0335 “Radiology-Therapeutic23 PAGE OF Chemotherapy-IV” 50 PAYER NAME Column 44: Product/Procedure Code(s) Product Code: Indicate the appropriate HCPCS code for the drug administered. Example: J9047 Injection, carfilzomib, 1 mg Procedure Code: Example: 96409 Chemotherapy administration; intravenous, push technique, single or initial substance/drug 12 13 14 15 16 17 18 19 20 21 22 TOTALS CREATION DATE 52 REL. INFO 51 HEALTH PLAN ID 53 ASG. BEN. 23 55 EST. AMOUNT DUE 54 PRIOR PAYMENTS 56 NPI A 57 A B OTHER B PRV ID C C 58 INSURED’S NAME 62 INSURANCE GROUP NO. 61 GROUP NAME 59 P. REL 60 INSURED’S UNIQUE ID A A B B C C 63 TREATMENT AUTHORIZATION CODES Box 67: Diagnosis Code A B C 66 DX 67 I A Enter appropriate ICD-9-CM diagnosis code. A Example: B C D 203.02 Multiple myeloma J K L M71 PPS 70 PATIENT in relapse 69 ADMIT DX REASON DX PRINCIPAL PROCEDURE a. 74 CODE DATE a b OTHER PROCEDURE CODE DATE b. OTHER PROCEDURE CODE DATE e. 65 EMPLOYER NAME 64 DOCUMENT CONTROL NUMBER c CODE OTHER PROCEDURE CODE DATE B E N 75 72 ECI F O 76 ATTENDING G P NPI LAST c. OTHER PROCEDURE CODE DATE d. OTHER PROCEDURE CODE DATE 77 OPERATING 81CC a UB-04 CMS-1450 APPROVED OMB NO. 78 OTHER b LAST c 79 OTHER d LAST C 68 73 QUAL FIRST NPI LAST 80 REMARKS H Q QUAL FIRST NPI QUAL FIRST NPI QUAL FIRST THE CERTIFICATIONS ON THE REVERSE APPLY TO THIS BILL AND ARE MADE A PART HEREOF. NUBC ™ National Uniform Billing Committee LIC9213257 For reimbursement assistance and personalized patient support services, contact Onyx Pharmaceuticals 360™ at 1-855-ONYX-360 (1-855-669-9360). Please note: This document is an abbreviated reference and the information described herein is subject to continual change and interpretation. Providers must assure themselves that this information is accurate and applicable to the claim being filed and should contact the insurer to determine the appropriate codes for the services provided. Onyx Pharmaceuticals, Inc. does not guarantee that use of any code(s) will assure coverage or payment at any level. The information presented is for informational purposes only and is not intended to provide reimbursement or legal advice. The appropriateness of filing any particular claim is and remains the responsibility of the provider based on the provider’s own judgment. Please see Important Safety Information and full Prescribing Information. Indication and Important Safety Information INDICATION Kyprolis® (carfilzomib) for Injection is indicated for the treatment of patients with multiple myeloma who have received at least 2 prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. Clinical benefit, such as improvement in survival or symptoms, has not been verified. CONTRAINDICATIONS None. WARNINGS AND PRECAUTIONS The safety of KYPROLIS was evaluated in clinical studies of 526 patients with relapsed and/or refractory multiple myeloma. Cardiac Arrest, Congestive Heart Failure, Myocardial Ischemia: Death due to cardiac arrest has occurred within a day of KYPROLIS administration. New onset or worsening of pre-existing congestive heart failure with decreased left ventricular function or myocardial ischemia have occurred following administration of KYPROLIS. Cardiac failure events (e.g., cardiac failure congestive, pulmonary edema, ejection fraction decreased) were reported in 7% of patients. Monitor for cardiac complications and manage promptly. Withhold KYPROLIS for Grade 3 or 4 cardiac events until recovery and consider whether to restart KYPROLIS based on a benefit/risk assessment. Patients with New York Heart Association Class III and IV heart failure, myocardial infarction in the preceding 6 months, and conduction abnormalities uncontrolled by medications may be at greater risk for cardiac complications. Pulmonary Hypertension: Pulmonary arterial hypertension (PAH) was reported in 2% of patients treated with KYPROLIS and was Grade 3 or greater in less than 1% of patients. Evaluate with cardiac imaging and/or other tests as indicated. Withhold KYPROLIS for pulmonary hypertension until resolved or returned to baseline and consider whether to restart KYPROLIS based on a benefit/risk assessment. Pulmonary Complications: Dyspnea was reported in 35% of patients enrolled in clinical trials. Grade 3 dyspnea occurred in 5%; no Grade 4 events, and 1 death (Grade 5) was reported. Monitor and manage dyspnea immediately; interrupt KYPROLIS until symptoms have resolved or returned to baseline. Infusion Reactions: Infusion reactions were characterized by a spectrum of systemic symptoms including fever, chills, arthralgia, myalgia, facial flushing, facial edema, vomiting, weakness, shortness of breath, hypotension, syncope, chest tightness, or angina. These reactions can occur immediately following infusion or up to 24 hours after administration of KYPROLIS. Administer dexamethasone prior to KYPROLIS to reduce the incidence and severity of reactions. Inform patients of the risk and symptoms, and to contact physician if symptoms of an infusion reaction occur. Tumor Lysis Syndrome: Tumor lysis syndrome (TLS) occurred following KYPROLIS administration in < 1% of patients. Patients with multiple myeloma and a high tumor burden should be considered to be at greater risk for TLS. Prior to receiving KYPROLIS, ensure that patients are well hydrated. Monitor for evidence of TLS during treatment, and manage promptly. Interrupt KYPROLIS until TLS is resolved. Thrombocytopenia: KYPROLIS causes thrombocytopenia with platelet nadirs occurring around Day 8 of each 28-day cycle and recovery to baseline by the start of the next 28-day cycle. In patients with multiple myeloma, 36% of patients experienced thrombocytopenia, including Grade 4 in 10%. Thrombocytopenia following KYPROLIS administration resulted in a dose reduction in 1% of patients and discontinuation of treatment with KYPROLIS in < 1% of patients. Monitor platelet counts frequently during treatment with KYPROLIS. Reduce or interrupt dose as clinically indicated. Hepatic Toxicity and Hepatic Failure: Cases of hepatic failure, including fatal cases, have been reported (< 1%). KYPROLIS can cause elevations of serum transaminases and bilirubin. Withhold KYPROLIS in patients experiencing Grade 3 or greater elevations of transaminases, bilirubin, or other liver enzyme abnormalities until resolved or returned to baseline. After resolution, consider if restarting KYPROLIS is appropriate. Monitor liver enzymes frequently. Embryo-fetal Toxicity: KYPROLIS can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals. There are no adequate and well-controlled studies in pregnant women using KYPROLIS. Carfilzomib caused embryo-fetal toxicity in pregnant rabbits at doses that were lower than in patients receiving the recommended dose. Females of reproductive potential should be advised to avoid becoming pregnant while being treated with KYPROLIS. ADVERSE REACTIONS Serious adverse reactions were reported in 45% of patients. The most common serious adverse reactions were pneumonia (10%), acute renal failure (4%), pyrexia (3%), and congestive heart failure (3%). Adverse reactions leading to discontinuation of KYPROLIS occurred in 15% of patients and included congestive heart failure (2%), cardiac arrest, dyspnea, increased blood creatinine, and acute renal failure (1% each). The most common adverse reactions (incidence ≥ 30%) were fatigue (56%), anemia (47%), nausea (45%), thrombocytopenia (36%), dyspnea (35%), diarrhea (33%), and pyrexia (30%). USE IN SPECIFIC POPULATIONS Since dialysis clearance of KYPROLIS concentrations has not been studied, the drug should be administered after the dialysis procedure. More Information: For more information about KYPROLIS, visit www.KYPROLIS.com/hcp. Onyx, Onyx Pharmaceuticals, Onyx Pharmaceuticals logo, Onyx Pharmaceuticals 360, Kyprolis and Kyprolis logo are all trademarks of Onyx Pharmaceuticals, Inc. ©2013 Onyx Pharmaceuticals, Inc., South San Francisco, CA Please see full Prescribing Information. 1112-CARF-447bR1 December 2013 Printed in USA
© Copyright 2026