Frequent expression of interleukin-10 by Epstein-Barr virus-harboring
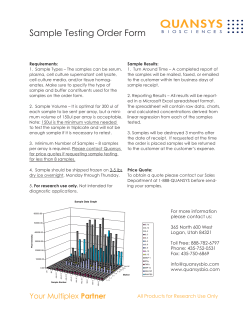
From www.bloodjournal.org by guest on November 14, 2014. For personal use only. 1996 87: 2918-2929 Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin's disease H Herbst, HD Foss, J Samol, I Araujo, H Klotzbach, H Krause, A Agathanggelou, G Niedobitek and H Stein Updated information and services can be found at: http://www.bloodjournal.org/content/87/7/2918.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From www.bloodjournal.org by guest on November 14, 2014. For personal use only. Frequent Expression of Interleukin-l0 by Epstein-Barr Virus-Harboring Tumor Cells of Hodgkin’s Disease By Hermann Herbst, Hans-Dieter Foss, Jens Samol, lguaracyra Araujo, Heike Klotzbach, Hans Krause, Angelo Agathanggelou, Gerald Niedobitek. and Harald Stein Tumor cells of Epstein-Barr virus(EBV)-associated Hodgkin‘s disease (HD) express the viral protein,latent infection membrane protein-l (LMPl), but evade cytotoxic responses normally directed at this antigen. We tested whether local production of the immunoregulatoryinterleukins (IL1-4 and -10 may have arole in this process. IL4 RNA was not detectable in any ofthe HD cases. Bycontrast, isotopicin situ hybridiration and correlation with the presence of EBVgene products showed significantly higher proportions of cases with 11-10 expressing tumor cells in LMP1-positive (17 of 26, 66%) as compared with LMP1-negative HD cases(sixof 37, 16%). Absence of EBV BCRFl RNA indicated that the transcripts originated from the cellular IL-10 gene. Similarly, an associa- tion between IL-10 expression and EBV-infection of tumor cells was found in AIDS-related malignant non-Hodgkin lymphomas (ARL). Very small proportions of EBV-infected cells, mainly blasts, expressed IL-10 in infectious mononucleosis tonsils. Thus, although not entirely exclusive to EBVpositivecases, IL-10 expression is frequently associated with EBV-infectionin HD andARL and appearsto be upregulated by EBV, most likelythrough LMP1. In view of the established inhibitory effects of 11-10 on cell mediated immunity, it is suggested that IL-10 expression may contribute to evasion of LMP1-positive cellsfrom cytotoxicity directedat viral antigens. 0 1996 by The American Societyof Hematology. M factor (CSIF), is a pleiotropic cytokine produced mainly by activated T cells and T-cell clones, activated monocytes, and activated B lymphocyte^.""^ IL-10 inhibits expression of interferon-y and IL-2 production by the TH1 subset of Thelper cellsI5 and can directly inhibit T-cell growth.16In macrophages, L 1 0 suppresses synthesis of certain cytokines at the transcriptional level and downregulates major histocompatibility complex (MHC) class I1 molecules resulting in reduced antigen-specific T-cell IL10 is also a growth factor for mast cells” and supports immune responses by inducing proliferation and differentiation of activated B cells into antibody secreting cells and by promoting immunoglobulin isotype switch.” IL-10 shows structural and functional homology to the gene product of the EBV BCRFl open reading frame that is expressed during the lytic cycle of infection2’ and often referred to as viral IL-10 (vIL-10).13Moreover, cellular IL-10 (cIL-10) shows autocrine growth supporting activity for EBV-infected B cells in vitro.21cIL-l0 was also found in various EBV-infected and noninfected B-cell lines” as well as inEBV’ ARL cases?’ Likewise, IL-4 has B-cell growth and differentiation promoting functions, induces isotype switch to IgE, and upregulates CD23 expression.= Produced by TH2cells, IL-4 acts as an autocrine growth factor on these cells. Similar to IL10 and augmenting its effects, IL-4 promotes mast cell growth and inhibits THl cell activity in antiparasitic and other immune reaction^.^^.^" We report on expression of cIL-10 and vIL-10, as well as IL-4, in a large series of HD including LMP1’ and LMPlcases and in six HD-derived cell lines. Controls consisted of 17 ARL, six normal tonsils, and tonsils from eight patients with infectious mononucleosis (IM), the clinically apparent form of primary EBV infection.” Though nonneoplastic, the atypical cells occurring in IM share a number of morphological and phenotypical properties with HRS cells, such as the expression of cytokines and activation antigens.” Discrimination between EBV’ and EBV- cytokine expressing cells was achieved by applying a simultaneous in situ hybridization procedure with nonisotopic detection of the abundant EBV-encoded small nuclear transcripts, EBER-1 and -2, and isotopic detection of cIL- 10 transcript^.^,^^ ANY CASES OF Hodgkin’s disease (HD) are associated with Epstein-Barr virus (EBV) infection of the neoplastic cells, Hodgkin and Reed-Stemberg (HRS) cells.’” EBV’ HRS cells frequently express the EBV-encoded latent infection membrane protein-l (LMP1),’-4which in healthy individuals is a target of cell mediated c y t o t o ~ i c i t y re,~~~ sulting in rapid elimination of LMPl expressing cells. This may explain why EBV’ lymphoid cells of immunocompetent persistently EBV-infected individual^^,^ but also tumor cells of endemic Burkitt’s lymphoma cases: almost never express LMP1. Lymphomas arising in a setting of overt immunodeficiency, such as posttransplant lymphoproliferative disease (PTLD) and AIDS-related non-Hodgkin lymphomas (ARL), on the other hand, frequently display LMP1.I’ Whereas tolerance of EBV-encoded antigens in PTLDandARLmaybereadily explained by numerical changes among T cells, the mechanisms that protect LMPl+ HRS cells from elimination by cytotoxic cells are not understood. It is conceivable that local factors such as immunoregulatory cytokines may contribute to these reactions, with interleukins (IL)-4 and -10 representing likely candidates for such effects. IL-10, previously known as cytokine synthesis inhibitory From Konsultations- und Referenuentrum furLymphknoten- und Hamatopathologie amInstitut fur Pathologie and Institutfur Immunologie, Klinikum Benjamin Franklin, Freie Universitat Berlin, Berlin, Germany; and the Department of Pathology, University of Birmingham, Birmingham, United Kingdom. Submitted March 27,1995; accepted November 17, 1995. Supported by Deutsche Krebshilfe, Mildred-Scheel-St@ng, Grant No. W81/92/Hel and Deutsche Forschungsgemei~scha~ Gram No. Ste318/5-l. Address reprint requests to Hermann Herbst,MD, Institut fur Pathologie, Universitatskrankenhaus Eppendolj; Martinistr 52, 20246 Hamburg, Germany. The publication costsof this article were defrayedin part by page chargepayment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 0 1996 by The American Society of Hematology. 0006-4971/96/8707-0014$3.00/0 2918 Blood, Vol 87, No 7 (April l), 1996:pp 2918-2929 From www.bloodjournal.org by guest on November 14, 2014. For personal use only. DISEASE INTERLEUKIN-l0 IN HODGKIN‘S 2919 Table 1. 11-10 Transcripts in HD and ARL ~ ~~~~~ ~~~~~~~~~~~~ ~ IL-lO+ EBER’ HD, Id 26/63 HD, all 013 ARL, BL ARL, cb 616 ARL, ib ARL, pb 10117all ARL, LMP1+ CaseOotal No. of Cases Diagnosis HD. Ip 5/26 HD, ns 19/32 HD, mc ~ 2/4 5/26 19/32 o/ 1 26/63 213 214 2/6 616 212 12/17 216 011 212 IL-10’ CasedEBER’ Cases 214 (50%) 3/26 (12%) 18/32 156%) 011 23/63 (41%) 2/3 316 516 112 11/17 (65%) 212 1/51(20%) 14/19 (74%)* 010 17/26 (66%)t 112 212 516 112 9/12 (75%) EBERIL-10’ Cases Cases 012 2/21 (10%) 4/13 (31%)* 011 6/37 (16%)t 111 1/4 010 010 215 (40%) Figures refer to gene expression in HRS cells and ARL cells. Abbreviations: HD, Hodgkin’s disease; Ip, lymphocyte predominance; ns, nodular sclerosis; mc, mixed cellularity; Id, lymphocyte depletion; ARL, AIDS-related non-Hodgkinlymphoma; BL, Burkitt’s and Burkitt-like lymphoma; cb, centroblastic lymphoma; ib, immunoblastic lymphoma; pb, immunoblastic lymphoma with plasmoblastic differentiation. Differencessignificant at P .05level. t Differences significant at P < ,001 level. MATERIALS AND METHODS Tissues. Formalin-fixed and paraffin-embedded lymph nodes from 63 HD cases typed according to the Rye Conference Classification, 17 AIDS-related lymphomas (Table l), and tonsillar tissue from eight patients with the clinical diagnosis of IM were drawn from the files of the Institute of Pathology, University Hospital Benjamin Franklin, Berlin, Germany. Six paraffin-embedded normal tonsils with slight to moderate follicular hyperplasia without necrosis served as further controls. The morphological and immunophenotypical details of these tonsillar IM tissues were recently described.” Cell lines, phytohemagglutinin-blasrs. The Hodgkin’s diseasederived cell lines (HDDCL) L428, L540, and L59lZ8were obtained from Dr V. Diehl, Cologne, Germany. COand HoZ9were kindly provided by Dr D.B. Jones, Southampton, UK, and K“Hz3’ was a gift from Dr H. Kamesaki, Kyoto, Japan. Paraffin-embedded cell pellets of the EBV-producer cell line B95-8, donated by Dr N. Muller-Lantzsch, Homburg, Germany, served as a control for the detection of lytic cycle gene products. Supernatants for enzymelinked immunosorbent assay (ELISA) determinations were harvested 48 hours after seeding of the cells at an initial density of 5 X IOs/ mL. Peripheral blood leukocytes were purified over a Hypaque (Pharmacia, Uppsala, Sweden) gradient and cultivated for 72 hours in the presence of 10 mg/mL PHA (phytohemagglutinin-M; GIBCO-BRL, Eggenstein, Germany). Immunohistology. Four micrometer sections of paraffin-embedded tissue blocks were stained by the immunoalkaline phosphatase (APAAP) method using newfuchsin as chromogen.” The primary monoclonal reagents were CSL-4, a cocktail of four antibodies specific for LMPI, antibody PE2 against EBNA2, antibody BZ.1 specific for BZLFl (ZEBRA) protein, and the antibodies Ber-H2 (CD30), L26 (CD20), PFI (specific for the T-cell antigen receptor P-chain), PG-M1 (CD68). and C3D1 (CD15). With the exception of PFI, which was from T-cell Sciences, Cambridge, MA, all primary antibodies, as well as rabbit antimouse immunoglobulin and APAAP-complex, were purchased from DAKO, Glostrup, Denmark. Both PE2 and BZ.l required microwave irradiation (10 minutes in 10 mrnoVL citrate buffer at 650 W) to obtain staining in paraffin sections. Probes. cRNA probes were prepared by subcloning of cytokine gene fragments in the run-off transcription vector pGEMl (Promega Biotec, Heidelberg, Germany). The CL-l0 probe was the 1.0-kb EcoRYHindIII fragment of pH5C” (%8191, ATCC, American Type Culture Collection, Rockville, MD). The IL-4 probe was subcloned as a 310-bp Nhe ZIEcoRI fragment of pcD-hIL-4” (#57592, ATCC). The L - 6 cDNA probe wasthe 0.6-kb EcoRYPst I fragment of ~ X M 3 0 9 ; generously ~ provided by Genetics Institute, Boston, MA. The nucleic acid sequences of all cytokine probes were determined on the DNA sequencer 373 (Applied Biosystems, Foster City, CA) and proved to conform to published data. After linearization of the run-off vector constructs with appropriate restriction enzymes, T7, T3, or SP6 RNA-polymerases (GIBCO-BRL, Eggenstein, Germany), respectively, were employed to obtain transcripts of either the antisense (complementary to mRNA), or sense (anticomplementary, negative control) strands. The 640-bp Apu I-Acc I BCRFIIvIL-IO probe (71% sequence identity with the human IL-l0 cDNA), as well as the pBluescript-based plasmid with the BCRFl sequence outside the IL-IO homology, were kindly provided by Dr D. Emilie, Villejuif, France. For the generation of vIL-IO specific RNA probes, Kpn I and Acc I restriction endonucleases were used for antisense and sense probes, re~pectively?~ obtain To the BCFWl probe outside the L - l 0 homology region, Stu I endonuclease was used for antisense, and AccI for sense probes.23 Transcription and labeling ofRNA probes were performed as outlined previously?” Briefly, for in situ hybridizations 60 pCi of [35S]-uridine-5’-(~-thio)-triphosphate (1,250 CUmmol; New England Nuclear, Dreieich, Germany) were added to a IO-pL reaction mixture (0.5 mmoVL each adenosine-, cytosine-, and guanosine-5’-triphosphate/l mol/L dithiothreitoVl0 U human placental RNase inhibitor16 m o l / L MgClJlO mmoVL Tris-HCl pH 7.512 mmoVL spermidindl0 mmol/L NaCl, including 1 pg of linearized plasmid and 16 U of either SP6 or T7 RNA polymerase). The reaction was allowed to proceed for 60 minutes at 40°C. The plasmid DNA was digested with 25 mg/mL RNase-free DNase I in a mixture containing 2.5 mg/mL of yeast tRNA and 10 U of RNase inhibitor for I O minutes at 37°C. Free ribonucleotides were removed by phenol-chloroform extraction followed by ethanol precipitation. To increase the penetration into tissue, the size of the [95S]-labeledRNA probes wasadjusted to 50 to 200 bases length by a controlled alkaline hydrolysis in 80 mmoVL NaHCOJ120 mmoVL Na2C03pH 10.2/10 mmoVL dithio- From www.bloodjournal.org by guest on November 14, 2014. For personal use only. HERBST ET AL 2920 threitol at 60°C. After neutralization in 0.2 mol/L sodium acetate/ I % acetic acid pH 6.0/10 mmol/L dithiothreitol, and ethanol precipitation,RNAprobeswerestoredat -80°C. The averagespecific activity was 1.3 X 10' c p d p g . EBER 1 and EBER2 specific pBluescript-basedvectors: prepared fromplasmidspJJJlandpJJJ2 kindly provided by Dr J. Amand, Manchester, UK, wereused for the generationof digoxigenin-labeled RNA probes by substituting [35S]-labeledby digoxigenated uridinetriphosphate(Boehringer-Mannheim,Mannheim,Germany)ata concentrationof 0.5 mmollL in theabove reaction.' EBERIand EBER2 probes were used in combination.' In siru hpbridizntion. The procedure, including prehybridization, hybridization, removal of nonspecifically bound probe by RNase A digestion,andfurtherwashingprocedures, was performed as described.'.74 Inbrief,sectionsweredeparaffinized in xylene,rehydrated in graded ethanol baths, treated in 0.2 N HCl for 20 minutes and then washed in diethylpyrocarbonate (DEPC)-treated H 2 0 for 5 minutes.After 10 minutesofdigestion with 0.5 mg/mL pronase (Boehringer Mannheim) in phosphate buffered saline (PBS) and a quick rinse in 0. I mol/L glycinePBS, slides were fixed for 20 minutes in ice-cold 4% paraformaldehyde/PBS.Sections were then washed in PBS for S minutes and acetylated in a freshly prepared solution of acetic anhydride diluted 1:400 in 0.1 m o l n triethanolamine, pH 8.0, for 10 minutes. After a washing step in PBS for S minutes, sections were dehydrated in graded ethanols ( 1 minute per step)andairdriedbeforehybridization.A total of 0.025mLof (SO% formamide/lO% dextransulfate/l0 hybridization mixture mmol/L dithiothreitol/O.l mol/L Tris-HCI pH 7.5/0. I mol/L NaPOJ 0.3 mol/L NaCI/SO m m o l k EDTA/l X Denhardt's solution/0.2 mg/ mLyeasttRNA)containing2 X IO' cpmof ["S]-labeled RNA probe were applied to each section and covered with a siliconized cover slip. For combined detection of L 1 0 and EBER, both digoxigenin-labeledEBERRNA probes and ["S]-labeled RNAprobes were added to the hybridization mixture. Hybridization was continued in a sealed humid chamber for 18 hours at 50°C. Cover slips and excess of probe were removed by washing for 4 hours at 52°C in 0.1 mol/L Tris-HC1 pH 7.Y0.1 mol/LNa2POJ0.3mol/LNaCl/ SO mmol/LEDTA/IOmmol/Ldithiothreitol.Thesolutionwas changed after 1 hour. To decrease background, slides were rinsed in Tris-buffered saline (TBS) and subjected to digestion with 20 pg/ mL RNase A in 0 . 1 mol/L Tris-HC1 pH 7 3 1 m m o l n EDTAMI.5 mol/L NaCl for 30 minutes at 37°C. After a 30-minute wash at 37°C in the same buffer without the enzyme, sections were furtherrinsed (SSC)/O.l% sodiumdodecylsulfate in 2Xstandardsalinecitrate (SDS) and 0.1 X SSC/O.l% SDS for IS minutes each, dehydrated in graded ethanols containing0.3 mol/L ammonium acetate( I 0 seconds per step), and air dried before autoradiography. l n case of combined in situ hybridization with EBER probes, autoradiography was preceded by detection of bound digoxigenin by immunohistology using alkaline phosphatase-coupled Fahfragments of digoxigenin-specific antibodies(BoehringerMannheim)andthechromogennewfuchsin." Immunostainedslidesweredehydrated by quickrinses in graded ethanols before coating with the autoradiographic emulsion. Autoradiography was performed by dipping the dehydrated slides into Ilford G5 nuclear emulsion (Ilford, Mobberley Cheshire, UK) melted at 42°C anddiluted1:lin 0.6 mol/Lammoniumacetate. After 2 hours of drying, the slides were stored in light-proof boxes containingdesiccantandexposed at 4°C for 3 to I O days.The exposedslidesweredeveloped in KodakD19developer(Kodak, Hemel Hampstead, UK) for 2.5 minutes, rinsed in l % acetic acid, and fixed in Kodak Fixer for 3 minutes. After extensive washing in tap water, the slides were finally counterstained i n hematoxylin and mounted in Kaiser's glycerol gelatin. With respect to both the EBER probes and [%]-labeled probes, nodifferencesinsensitivitywerenoticeable when noniaotopic in situ hybridization for the detection of EBER or radioactive in situ hybridization for the detection of cytokine gene transcriptswere run in parallel instead of being used in combination. Furthcr controls in consisted of hybridizations with senseRNAprobesforEBER combination with antisense probes for cytokine genesand vice versa in a simultaneous in situ hybridization procedure. These experiments showed only a weak homogeneouslydistributedautoradiographic background signal for sense probes in the presence of specific signals fortheantisenseprobes. In situ hybridizationusingsenseprobes for EBER or cytokine genes alone showed only weak nonspecific background. Prolonged exposure times up to 4 months ensured that negative results were not due to premature termination of the exposure. The incubation of sections with Micrococcus nuclease (Boehringer Mannheim) before in situ hybridization resulted in the extinction of the specific autoradiographic signal, confirming that RNA sequences were the targets of the hybridization procedure." Cells presenting with graincountsfourtimesabovebackgroundsignal as defined by hybridization with sense-strand control probes were considered positive Polprnerusc, chuin reaction. Total RNA was extracted from cell lines and peripheral blood lymphocytes (PBL), digested with DNase I andtranscribed to cDNAusingrandomhexamereprimersand reversetranscriptase (RT)fromMoloneymurineleukemia virus (Perkin-Elmer Cetus, Weiterstadt, Germany). In brief, l pg of total 25 minutes at 42°C with S0 U of cellular RNA was incubated for RT and20 U placental RNase inhibitorin a 20-pL volume containing 2.5 pmol/L random primers, S mmol/L MgCIZ, SO mmol/L KCI, I O mmol/L Tris-HCI, and each 1 mmol/L of deoxynucleoside-tliphosphates, heated to 99°C and subsequently cooled at 5°C for each S minutes.Oligonucleotideprimersweresynthesizedcorresponding to positions 88-108 (5'-CCCCCTCTGTTCTrCCTG) and 436-41 h (S'-ACTCTGGTTGGCTTCCTTCAC) of the human 1L-4 cDNA." to positions 49-69 (5"CTCTGTTGCCTGGTCCTCCTG) and 544524 (S'-GTITCGTATCTTCATTGTCAT) of thehuman 1L- I O cDNA,"as well as to positions 103-122 (S'-GTGGGGCGCCCCAGGCACCA) and 642-619 (5"CTCCTTAATGTCACGCACGATTTC) of the human &actin gene.36 The primers wereused to obtain amplification products of 348 bp for IL-4, 495 bp for IL-IO, and S39 bpfor0-actin.respectively.Thepolymerasechain reaction (PCR) reaction mixture consisted of 1.5 mmol/L MgCI:, SO mmol/ L KCI, I O mmol/L Tris-HCI, each 0.2 pmol/L of both primers. 2.5 U of Taq DNA polymerase (Perkin-Elmer Cetus)in a 0 . 1 mL volume containing20 pL of the above mentioned RTreactionmixture. Reaction conditionswereeach I minuteat 96°C and 60°C. and 2 minutes at 72°C for 30 cycles followed by a final extension for 10 minutes at 72°C. ELISA. Culturesupernatants from HD-derived cell lines were assayed for immunoreactive human IL-4, as well as human clL-IO and vIL-IO, using E L B A kits, the latter being based on antibodies reacting with both cIL-l0 and vIL-IO, according to the suppliers' recommendations (Cytoscreen, BioSource International. Camarillo, CA). The ELlSA kit for the detection of human IL-4 was supplied by H. Biermann Diagnostica, Bad Nauheim, Germany. The lower detection limits were 18 pg/mL and 15 pg/mL for human cIL-IO'' and human IL-4, respectively. Sturisrics. Statistical evaluation was performed using the x' test with correction for continuity as devised by Yates. RESULTS Hodgkin 's disease. cIL- 10 expression by HRS cells was detectable in 23 of 63 HD cases (Table I ) . Expression of this cytokine was not distributed homogeneously among the From www.bloodjournal.org by guest on November 14, 2014. For personal use only. DISEASE INTERLEUKIN-l0 IN HODGKIN'S 2921 Fig 1. Detection of cellular IL-10 transcripts in HRS cells of two cases of HDmc (A-C and D-F, respectively) and one case of HDns (G, H) by in situ hybridization using a [35S1-labeled antisenseand sense RNA probes. Specific hybridization signal is largely restricted to HRS cells (A, D, E, G). In situ hybridizations with IL-10 sense (control) RNA probe display few homogeneously distributed silver grains (B, F, H). CD30 staining shows a larger number of tumor cells, indicating that only a proportion of HRS cells display detectable IL-10 transcript levels (Cl. Autoradiographic exposure time 21 days (A, B), 28 days (D, E), and 32 days (F-H), original magnification x 250 (A-C, E-H), and x 50 (D). different histotypes of HD. Among 32 HD mixed cellularity (mc) cases, 18 cases displayed IL-IO'HRS cells, whereas IL-10 expression by tumor cells was notable in only 3 of 26 HD nodular sclerosis (ns) cases. The autoradiographic signal was restricted to 10% to 70% of tumor cells as identified by comparison with CD30-staining of adiacent sections (Fig I ) or combined in situ hybridization for the demonstration of IL-IO and EBER (Fig 2A). The signal intensity varied, in some cases requiring up to 6 weeks of autoradiography to be clearly visible above background signal. EBV infection ofHRS cells wasfound in 26 cases of our series, with all tumor cells expressing EBER transcripts andvariable proportions of tumor cells stained with antibodies against LMPI, confirming previously reported labeling patterns.'"' Unlike LMPI, EBER transcripts were not entirely restricted to HRS cells, but also found in small numbers of reactive lymphocytes. HRS cells with cIL-l0 transcripts were found in 17 of 26 (66%) HD cases withEBER' tumor cells. In contrast, only 6 (16%) among those 37 HD cases with EBER- HRS cells showed cIL-IOexpression by a proportion of tumor cells. IL-IO expression was not restricted to HRS cells, but was also observed in scattered reactive cells morphologically compatible with lymphocytes and macrophages in S1 of 63. ie, 80%. of the HD cases. In nine HD cases with EBER- tumor cells investigated by simultaneous ILIOEBER-specific in situ hybridization autoradiographic and nonisotopic signals were not colocalized, indicating that ILI O expression was not linked to EBV infection in small reactive cells. Eight HD cases with EBER' tumor cells and high IL-IO transcript levels in some of these cells were also hybridized withboth BCRFI probes. Whereas the 640 baseBCRFI probe comprising the region of homology to the clL- I O gene showed the same pattern as the cIL-IO probe, no hybridization signal was observed withthe entirely EBV-specific BCRFI probe. even after extended periods of autoradiography. BZLFI-specific immunostaining was not noticeable in any of the 23 EBV-positive HD cases. The IL-4 probe did not generate any specific autoradiographic signal when applied to S2 HD cases. The IL-6 probe, included as a control, From www.bloodjournal.org by guest on November 14, 2014. For personal use only. 2922 hybridized to HRS cells in 43 of 63 HD cases, and in all 63 cases to scattered reactive cells morphologically compatible with lymphocytes, endothelial cells, and macrophages, thus confirming the presence of sufficient levels of RNA tranAn average of 2 to 3 weeks of autoradiographic exposure was required to obtain signal intensities with the IL-6 probe similar to those seen with the cIL-l0 probe after 4 to 6 weeks of exposure, indicating lower transcript copy numbers for cIL-IO relative to IL-6. HD-derived cell lines. The EBV- cell line Ho showed IL-l0 protein in culture supernatant at a concentration of 4.15 ng/mL by ELISA and specific RNA transcripts in cytocentrifuge preparations by in situ hybridization, respectively, thus further confirming the specificity of the IL-l0 probes. All other HD-derived cell lines, CO, KM-H2, L428, L540, and the EBV' line L591 (EBER', LMPl ', EBNA2') did not display IL-IO expression in both assays (Fig 3, Table 2). IL-4 RNA and protein were not detectable in cells or supernatants by in situ hybridization or ELISA, respectively, of any of the six HD-derived cell lines. To exclude IL-4 and IL-l0 gene expression below the sensitivity threshold of both assays, sequences of the cIL-IO,IL-4,and &actin genes were amplified by PCR after RT of cellular RNA (Fig 4). HERBST ET AL P-Actin-specific amplification products were obtained in similar amounts from all cell lines, as well as from mitogenactivated PBL. Large amounts of IL-10-specific PCR product were amplifiable from Ho RNA. The cell line, CO, produced a faint band indicating thepresence of only small amounts of gene transcripts. Faint IL-4-specific bands were found after amplification of L428 and Ho RNA. Infectious mononucleosis. Application ofin situ hybridization with the [3SS]-labeled IL-IO-specific probes to sections ofIM tonsils showed autoradiographic signals over scattered interfollicular and subepithelial cells with lymphoid morphology. Applying simultaneous in situ hybridization for EBER and IL-IO RNA, IL-10-specific transcripts were found in less than 1% of all EBER+ cells (Fig 5). most of which were blasts (Fig 2B through D). Expression of BCRFl-specific sequences wasnot detectable, although few BZLFIpositive cells were found, often in association withcrypt epithelium (Fig 2E). Expression of IL-4 was not detectable in the IM tonsils, neither in the expanded interfollicular areas nor within follicles. AIDS-related lymphomas. 17ARLwiththe diagnoses listed in Table 1 were tested for the presence of cellular and viral L 1 0 transcripts. All cases displayed a B-cell pheno- From www.bloodjournal.org by guest on November 14, 2014. For personal use only. INTERLEUKIN-10 IN HODGKIN'SDISEASE 2923 Fig 2. Simultaneous hybridization for the detection of IL-10 transcripts (autoradiographic signal) and EBER (digoxigenin-labeled probe, red signal) on a case of mixed cellularity HD (A) andin t w o cases of IM (B, C). A proportion of EBER' tumor cells (A) or blasts (B, C), respectively, display coexpression of both transcripts (arrows). (D) Control hybridization with EBER and 11-10 sense probes, same case as in 2C. BZLFlspecific immunostaining of an IM tonsil (E) and anAIDS-related lymphoma (F) display scattered cells that may have entered the lyticcycle of EBV infection. Autoradiographic exposure time 28 days, original magnification x 132 (A), and x 250 (B-F). type. The EBERs were detected in 12 of17 cases, all of which also expressed LMPl with the exception of the Burkitt and Burkitt-like lymphomas. IL-10 was found in 9 of 12 EBV+ ARL, and in 2 of 5 EBV- ARL with both the C L 10 and vIL-10 probes (Fig 6). In three cases, few neoplastic cells displayed BZLF1-specific staining (Fig 2F), two of these cases also contained very few cells hybridizing with the BCRFl probe detecting sequences outside of the IL-10 homology region. Controls. Few small EBER+ lymphoid cells were found in 4 of 6 normal tonsils, double labeling with the IL-10 probe showed the absence of IL-10 transcripts from those cells. IL-10 was restricted to occasional scattered interfollicular lymphoid cells, as well as a few cells in germinal centers. The number of these cells was considerably smaller as compared with TM tonsils. Few IL-4-expressing lymphoid cells, most likely representing T cells:' were found within germinal centers of two tonsillar specimens with follicular From www.bloodjournal.org by guest on November 14, 2014. For personal use only. HERBSTET AL 2924 hyperplasia (Fig 7). Cytospin preparations of PHA-stimulated peripheral blood cells served as positive controls, displaying transcripts in up to 2% and 7% of the cells for IL-4 and IL-l0 probes, respectively. On RT-PCR, mitogenactivated PBL produced clear bands with IL-4-, IL-IO-, and P-actin-specific primers (Fig 4). Paraffin-embedded cell pellets from the EBV-producer cell line B95-8 showed a small proportion of labeled cells with both BCRFI-probes (Fig 8). DISCUSSION Fig 3. In situ hybridization of HD-derived cell lines Ho (AI and L591 (B) with the ell-10 antisense probe; 11-10 is expressed in Ho (EBVnegative), but notin L591 cells (EBV-positivel. With theexception of IL-10 expression in Ho, 11-4,as well as L 1 0 antisense and sense probes, displayed background signals as shown in (B). Autoradiographic exposure time 20 days, original magnification x 100. We have demonstrated IL-l0 expression by variable proportions of HRS cells in 40% of a series of 63 HD cases, as well as in IM blasts and mitogen-induced peripheral blood cells. Allof these cells share the phenotype of activated cells. Thus, in this context IL-IO expression may be considered another marker of cellular activation. The findings further extend the panoply of cytokines demonstrated in HRS cells, HD-derived cell lines, and IM blasts. Underlining its suggested importance as a pleiotropic immunomodulatory cytokine, IL-IORNA was also found to be regularly expressed by scattered small cells, mainly with lymphoid and macrophage morphology, innormal lymphoid tissues, in EBV-negative cells of IM tonsils, and in reactive nonmalignant, EBV-negative cells of HD. The role of these cells and the mechanism triggering their L 1 0 expression remain to be determined. L 1 0 and L 4 expression could be induced by mitogen in peripheral blood mononuclear cells. A role for IL-4 as an autocrine growth factor has previously been suggested for the cell line I ~ t 2 8 . ~IL-4, ' however, which shows a spectrum of activities similar to &l0 and which may augment L 1 0 effects, was not detectable by in situ hybridization in HD and IM in vivo. Despite its inhibitory potential in cell-mediated immune reactions, IL-4 is thus less likely to be relevant in the context of HDandEBV infection. Unlike previously investigated ~ytokines,3*-~~.~'~' L10 expression by HRS cells showed a close correlation with EBV infection of these cells and with the mixed cellularity histotype ofHD. With respect to IL-IO and EBV, similar figures were found in our control group of ARL confirming Table 2. 11-10 and IL-4 Expression in HD-Derived Cell Lines Hybridization Cell Line HD, HD, L428 L540 L591 CO Ho KM-HZ TVW ns HD, ns ns HD, ns HD, ns HD, rnc EBER - In Situ BCRFlt11-4 IL-10 IL-10 - - - - - - + RT-PCR ELISA. + IL-4 - - - - - 4150 - - - IL-10 11-4 - (+)* - - (+) + - (+) - Mean of three independent experiments with standard deviation <lo%, dimension ispg/mL. Threshold of ELISA 18 pg/rnL for IL-10 and 15 pglrnL for IL-4, respectively. t Probe-outside of IL-10 homology region. (+) faint band in RT-PCR. * From www.bloodjournal.org by guest on November 14, 2014. For personal use only. INTERLEUKIN-l0 IN HODGKIN'SDISEASE C m 1 2 3 4 5 6 7 m Fig 4. Amplification of IL-4, IL-10. and pactin cDNA sequences by PCR subsequent to RT of total cellular RNA. Amplification products of 495 bp representing clL-l0 sequences were obtained from CO and Ho cells (Al. lanes 4 and 5. L428 and Ho produced a 348-bp product with IL-4-specific primers (B), lanes 1 and 5. The 539-bp p-actinspecific amplification products were obtained from all cell lines indicating the presence of sufficient RNA in HD-derived cell line extracts (C), lanes 1 through 6. PHA-activated peripheral blood leukocytes contained RNA for IL-4, IL-10. and p-actin (lane 7, A, B, C). m = 1 kb ladder as molecular size marker, bar indicates 500 bp position. Lanes 1 (L428). 2 (L5401, 3 (L591). 4 (CO), 5 (Ho), 6 (KM-HZ), 7 (mitogenactivated PBL). previously reported findings'.' and supporting the interpretation that IL-IO may have a role for virus-associated tumors including HD. IL-IO is produced by most B-lymphoblastoid and many other EBV-infected cell lines and has been suggested to support growth through autocrine regulatory I O O ~ S . ' However, ~."~ IL-IO is obviously not required byall EBV-infected cell lines, as evidenced by the lack of IL-IO expression in the HD-derived cell line L59 I . Lack of BCRFl RNA also indicates a strictly latent type of EBV infection in this cell line. Similarly, IL-IO transcripts were not found in EBER'LMPI - small reactive cells that occur in variable, usually small numbers along with or without EBV-infection of HRS cells in many cases of HD..' Such EBER'LMPI cells are found in addition to EBER'/LMP' cells under the 2925 condition of acute infection and may be observed in small numbers in persistently EBV-infected individuakx Therefore, at variance with a previous report, there is no evidence for a mandatory function of IL-IO in the maintenance of the immortalized state in vivo."."' What may be the biological significance of the close association of IL-IO expression with EBV infection in HD and ARL? Autocrine or paracrine growth supporting functions maybe discussed for IL-IO in HDandARL,anissueto beclarified by analysis of1L-IO receptors on tumor and neighboring cells and functional studies on HD-derived cell lines. Autocrine activities have already been suggested for several other cytokines in HDZX.4Z and in EBV-transformed B cells?x.51so that autocrine functions cannot be considered a unique feature ofIL-IO expression. Promotion of B-cell differentiation and isotype switching by IL-IO" may play a role for B cells of the reactive infiltrate in HD and for some ARL, such as immunoblastic lymphomas with partial plasmoblastic differentiation, but are unlikely to be of importance for HRS cells. which do not further differentiate in vivo." More critical than growth supporting autocrine functions of IL-IO in HD may be its suggested inhibitory activities on macrophages and THI cells. In leishmaniosis and leprosy, eg, IL-4 and IL-IO may act synergistically by suppressing a delayed type hypersensitivity immune Individuals with prominent IL-IO and IL-4 responses to these infections remain able to mount a humoral immune response, but do not react with the formation of THI cells and subsequent activation of macrophages. Some retroviruses also appear to induce an overproduction in downregulatory cytokines. which is closely associated with the onset of immunodeficiency. In human immunodeficiency virus(HIV) infection.IL-IO and/or IL-4 synthesis by T112cells is increased. while interferon-y and/or IL-2 production by TH1 02 0 -1 -2 -3 -4-5 -6 -7 -8 c a m no. Fig 5. Relative distribution of IL-lO+ cells among EBV-infected cells in IM tonsils (eight cases) showing an average of approximately 1% of IL10' cells among EBER' cells, mainly blasts. From www.bloodjournal.org by guest on November 14, 2014. For personal use only. 2926 HEABST ET AL Fig 8. In situ hybridization ofparaffin-embedded cells 895-8 cells with the small BCRF1-specific antisense probe outside of the IL-10 homology. A small proportionof cells (so-called "virus factories'') in this EBV-producer cell line displays specific signals with both antisense and sense RNA probes due t o the detection of not only RNA, but also linear EBV DNA. Autoradiographic exposure time 6 days, original magnification x 50. Fig 6. Detection ofIL-10 transcripts in a Proportion of cells of an EBV' immunoblastic B-cell lymphoma relatedt o HIV infection using the clL-10 probe (A) and the vlL-10 probe (B). Autoradiographic exposure time 24 (A)and 42 (B) days, originalmagnification x 250. Fig 7 . Detectionof IL-4 transcripts in scattered cells within a germina1 center of a tonsil with unspecific follicular hyperplasia. Autoradiographic exposure time 34 days, original magnification x 160. cells is ~uppressed.'~Mechanisms of cytokine-mediated cross-regulation were therefore suggested to play a role in the pathogenesis of AIDS, contributing both to the progression ofHIVinfectionandthe increase in susceptibility to opportunistic infections and malignancies."." A similar role, although without significant involvement of IL-4, maybe anticipated for IL-l0 in the context of HD with or without EBV infection. IL-IOinduced suppression of T celland macrophage functions in the microenvironment of a virus infected cell may explain tolerance to vira[ neo-antigens. EBV-specificcytotoxicityOf derived from fresh HD tissues, Frisan et foundEBVspecific cytotoxicity only in HDwith EBV- tumor cells, but not in EBV' cases. The authors concluded that tumorassociated suppression of EBV-specific T-cell responses mayplayan important role inthe pathogenesis ofEBV' HD." Interestingly, the cells forming rosettes around HRS cells seem to consist ofTHI rather than TH2 lymphocytes because they display a CD45RO'/IL-2' phen~type.~".~' The number of virus-encoded gene products expressed during EBV latency is limited. In type I latencyonlythe EBNAI antigen, as well as EBER transcripts, are expressed. This type of latency istypicallyfound in smallEBER' lymphoid cells described above, in EBV' Burkitt lymphomas,and in some EBV' carcinomas. Type I1 latencyis characterized by the additional expression of LMPl and, in some cases, LMP2. This type of EBV latent gene expression is typical for EBV+ HRS cells and undifferentiated nasopharyngeal carcinoma." The full spectrum of EBV latent gene products includes five additional EBNA proteins and is realizedin lymphoblastoid cell lines, frequently in PTLDand also in cases of ARL.'" With the exception of EBNAI, which appears to be nonimmunogenic,-59 LMP and EBNA Proteins are targets OfcelI-mediatedcytotoxicity. LMP1-specific T cells, eg, may be reactivated from the peripheral blood of From www.bloodjournal.org by guest on November 14, 2014. For personal use only. 2927 INTERLEUKIN-l0 IN HODGKIN'S DISEASE healthy, EBV-infected individual^.^.^ HD patients with EBV' HRS cells, on the other hand, develop high titers of LMP1-specific antibodies indicating a largely intact humoral immunity,6'but obviously fail to develop cytotoxic responses against the viral neo-antigen LMP1. Induction of IL-10 expression may therefore confer some survival advantage to virus-infected cells expressing these viral antigens. This view is supported by the restriction of IL-10 expression among EBV-infected cells mainly to blasts in IM tonsils, to HRS cells in HD, and to lymphoma cells in HIV-infected patients. All of these cell types display type I1 or 111 latency with expression ofat least LMPl as a potential target to cytotoxicity. BCRFI-specific hybridization was absent in HD tissues and limited to few cells in some EBV' ARL. Also, expression of BZLFI, which transactivates lytic cycle genes, was not found in our EBV+ HD cases. This indicates that the transcripts detected with the IL-l0 probes originated exclusively or predominantly from the cIL-10 gene and not from its EBV-encoded homolog. This leads to the question as to the EBV gene product responsible for cIL-10 gene induction in EBV' cells with types I1 and 111 latency. Many EBV latent gene products directly or indirectly transactivate cellular genes. A role for EBNA2, -3a, -3b, -3c and -LP gene products is, however, unlikely because of their absence from HRS cells. This leaves LMPl as a likely candidate protein mediating EBV-induced IL-IO expression in HRS cells and ARL. LMPl upregulates IL- 10 mRNA levels in LMP1transfected keratinocytes 23-fold as compared with parental cells." In B cells, LMPl interacts with LAPl (LMP1-associated protein-]) and EBI6 (EBV-induced protein-6) and, by imitating activated tumor necrosis factor- (TNF)-receptors, forms a constitutive signal resulting in pleiotropic effects6' including upregulation of bcl-2 following activation of members of the NF-KB family of nuclear transcription factom6' Itis conceivable that the upregulatory effect of LMPl on IL- 10 expression is not merely restricted to keratinocytes. While LAPl, EBI6, and NF-KB may be essential components of LMPl signal other, as yet, undefined factors seem to be required to mediate the cell-specific functions of LMPl . The absence of such factors may explain the lack of IL-IO expression in HRS cells of some EBV+ HD and ARL cases, as well as L591 cells. Alternatively, IL10 RNA levels may have been below the threshold of detection in some of these cases. The presence of IL-10 transcripts in some tumors with type I latency such as Burkitt's lymphomas in AIDS patients is not in contradiction with the proposed upregulatory role of LMP1, because IL-10 expression may merely coincide with EBV infection in these cases. In support of this interpretation, IL- 10 expression, independent of EBV-infection, was found in six of our LMP- HD cases, as well as in the HD-derived cell lines Ho and Co. In conclusion, IL-10 expression by HRS cells and ARL is more frequently detectable in EBV' cases, most of which are LMPI', than in cases not associated with EBV. Upregulated IL- 10 expression may be among the factors contributing to the immunological dysfunctions observed in HD, in particular thelack of cell-mediated cytotoxicity towards LMPl expressing cells. However, IL-l0 is not consistently expressed in all HRS and ARL cells of all cases and is therefore likely to be only one of many factors contributing to the complex pattern of clinical and histological presentation of these tumors. ACKNOWLEDGMENT This work contains parts of the MD theses of J. Samol, I. Araujo, andH. Klotzbach. The authors are grateful to U. Tank and M. Thiel for excellent technical and to L. Oehring for phototechnical assistance. We thank Dr M. Masucci for sharing information before publication. The authors dedicate this worktothememoryofProf Tibor Diamantstein, Head of the Institute of Immunology at the Klinikum Benjamin Franklin of the Free University Berlin, who died on December 5 , 1995. REFERENCES 1. Herbst H, Dallenbach F, Hummel M, Niedobitek G, Pileri S, Muller-Lantzsch N, Stein H: Epstein-Barr virus latent membrane antigen expression in Hodgkin and Reed-Stemberg cells. Proc Natl Acad Sci USA 88:4766, 1991 2. Pallesen G, Hamilton-Dutoit SJ, Rowe M, Young LS: Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet 337:320, 1991 3. Herbst H, Niedobitek G: Epstein-Barr virus in Hodgkin's disease. EBV Report 1:31, 1994 4. Herbst H, Steinbrecher E, Niedobitek G, Young LS, Brooks L, Muller-Lantzsch N, Stein H: Distribution and phenotype of Epstein-Barr virus-harboring cells in Hodgkin's disease. Blood 80:484, 1992 5. Thorley-Lawson DA, Israelsohn ES: Generation of specific cytotoxic T cells with a fragment of the Epstein-Barr virus-encoded p63/latent membrane protein. Proc Natl Acad Sci USA 845384, 1987 6. Murray RJ, Wang D, Young LS, Wang F, Rowe M, Kieff E, Rickinson AB: Epstein-Barr virus-specific cytotoxic T-cell recognition of transfectants expressing the virus-encoded latent membrane protein LMP. J Virol 62:3747, 1988 7. Murray RJ, Kurilla MG, Griffin HM, Brooks JM, Mackett M, Arrand JR.Rowe M, Burrows SR, Moss DJ, Kieff E, Rickinson AB:Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia virus. Proc Natl Acad Sci USA 87:2906, 1990 8. Miyashita EM,YangB, Lam KM, Crawford DH, ThorleyLawson DA: A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell 80593, 1995 9. Rowe M, Rowe DT, Gregory CD, Young LS, Farrell PJ, RupaniH, Rickinson AB: Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J 6:2743, 1987 IO. Hamilton-Dutoit SJ, Rea D, Raphael M, Sandvej K, Delecluse HJ, Gisselbrecht C, Marelle L, van Krieken HJ, Pallesen G: EpsteinBarr virus latent gene expression and tumor cell phenotype in acquiredimmunodeficiencysyndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am J Pathol 143:1072, 1993 11. Mosmann TR: Properties and functions of interleukin-10. Adv Immunol 56: I , 1994 12. Miyazaki I, Cheung KK, Dosch HM: The role of the IL-l0 and other EBV latency genes early during B cell growth transformation. Semin Virol 5:405-414, 1994 From www.bloodjournal.org by guest on November 14, 2014. For personal use only. 2928 13. Vieira P, De Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, de Vries JE, Roncarolo MG, Mosmann TR, Moore KW: Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: Homology toEpstein Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA 88:1172, 1991 14. De Wad-Malefyt R, Abrams I, Bennett B, Figdor CG, de Vries JE: Interleukin-l0 (IL-IO) inhibits cytokine synthesis by hu10 produced by monoman monocytes: An autoregulatory role of Lcytes. J Exp Med 174:1209, 1991 15. Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A: IL-IO acts on the antigen presenting cell to inhibit cytokine production by Th I cells. J Immunol 146:3444, 1991 16. Taga K, Mostowski H, Tosato G : Human interleukin-l0 can directly inhibit T cell growth. Blood 81:2964, 1993 17.De Waal-Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE: Interleukin-l0 (IL-IO) and viral IL-I0 strongly reduce antigen specific humanT cell proliferation by diminishing the antigen presenting capacity of monocytes via downregulation of class I1 major histocompatibility complex expression. J Exp Med 174:915, 1991 18. Thompson-Snipes LA, Dhar V,BondMW, Mosmann TR, Moore KW, Rennick DM: Interleukin 10: A novel stimulatory factor for mast cells and their progenitors. J Exp Med 173507, 1991 19. Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J: Interleukin-IO is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA 89:1890, 1992 20. Ryon JJ, Hayward SD, MacMahon EME, Mann RB, Ling Y, Charache P, Andersen Phelan J, Miller G , Ambinder RF: In situ detection of lytic Epstein-Barr virus infection: Expression ofthe Not1 early gene and viral interleukin-IO late gene in clinical specimens. J Infect Dis 168:345, 1993 21. Burdin N, Peronne C, Banchereau J, Rousset F: Epstein Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med 177:295, 1993 22.Benjamin D, Knobloch TJ, DaytonMA: Human B cell interleukin- IO: B cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt’s lymphoma constitutively secrete large quantities of interleukin-IO. Blood 80: 1289, 1992 23. Emilie D, Touitou R, Raphael M, Peuchmaur M, Devergnee 0, Rea D, Coumbraras J, Crevon MC, Edelman L, Joab 1, Galanaud P: In vivo production of interleukin-IO by malignant cells in AIDS lymphomas. Eur J Immunol 22:2937, 1992 24. Paul WE: Interleukin-4: A prototypic immunoregulatory lymphokine. Blood 77:1859, 1991 25. Sieling PA, Abrams JS, Yamamura M, Salgame P, Bloom BR, Rea TH, Modlin RL: Immunosuppressive roles for IL-IO and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol 150:5501, 1993 26. Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, Berzofsky JA, Mosmann TR, James SL, Morse HC: Role of T cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev 127:183, 1992 27. Foss HD, Herbst H, Hurnmel M, Araujo I, Latza U, RanscZ, C, Dallenbach F, Stein H: Patterns of cytokine gene expression in infectious mononucleosis. Blood 83~707,1994 28.Diehl V, Kirchner HH, Burrichter H, Stein H, Fonatsch C, Gerdes J, Schaadt M, Heit W, Uchanska-Ziegler B, Heintz F, Sueno K: Characteristics of Hodgkin’s disease-derived cell lines. Cancer Treat Rep 66:615, 1982 29. Jones DB,FurleyAJW, Gerdes J, Greaves MF, Stein H, Wright DH: Phenotypic and genotypic analyses of two cell lines HERBST ET AL derived from Hodgkin’s disease tissue biopsies. Recent Results Cancer Res I 17:62, 1988 30. KamesakiH, Fukuhwa S, Tatsumi E, Uchino H, Yamabe H, Miwa H, Shirakawa S, Hatanaka M, Honjo T: Cytochemical, immunologic, chromosomal and molecular genetic analysis of a novel cell line derived from Hodgkin’s disease. Blood 68:285, 1986 31. Stein H, Gatter K, Asbahr H, Mason DY. Useof freezedried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest 52:676, 1985 32. Yokota T, Otsuka T, Mosmann T, Banchereau J, DeFrance T, Blanchard D, De Vries JE, Lee F, Arai K: Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor I, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci USA 835894. 1986 33. Sutherland GR, Baker E, Callen DF,Hyland VJ, Wong G, Clark S, Jones SS, Eglinton LK, Shannon MF, Lopez AF: Interleukin-4 isat5q31and interleukin-6 is at 7pIS. HumGenet 79:335, I988 34. Milani S, Herbst H, Schuppan D, Hahn EG, Stein H: In situ hybridization for procollagen types I, 111, and IV mRNA in normal and fibrotic rat liver: Evidence for predominant expression in nonparenchymal liver cells. Hepatology 10:84, 1989 35. Williamson DJ: Specificity of riboprobes for intracellular RNA in hybridization histochemistry. J Histochem Cytochem 26:81 I , 1988 36. Kunzendorf U, Kruger-Krasagakes S, Notter M, HockH, Walz G, Diamantstein T: A sialyl-Le(x)-negative melanoma cell line binds to E-selectin but not to P-selectin. Cancer Res 54: 1 109, 1994 37. Kriiger-Krasagakes S, Krasagakis K, Garbe C, Schmitt E, Huls C, Blankenstein T. Diamantstein T: Expression of interleukin10 in human melanomas. Br J Cancer 70: I 182, 1994 38. Jucker M, Abts H, Li W, Schindler R, Merz H, Gunther A, von Kalle C, Schaadt M, Diamantstein T, Feller AC, Krueger GRF, Diehl V, Blankenstein T, Tesch H: Expression of interleukin-6 and interleukin-6 receptor in Hodgkin’s disease. Blood 77:2413, 1991 39. Foss HD, Herbst H, Oelmann E,Samol J, Grebe M. Blankenstein T, Matthes J, Qin ZH, Falini B, Pileri S, Diamantstein T, Stein H: Lymphotoxin, tumor necrosis factor and interleukin-6 gene transcripts are present in Hodgkin and Reed-Stemberg cells of most Hodgkin’s disease cases. Br J Haematol 84:627, 1993 40. Butch AW, Chung CH, Hoffmann JW, Nahm MH: Cytokine expression by germinal center cells. J Immunol 150:39, 1993 41. Newcom SR, Ansari AA, Gu L: Interleukin-4 is an autocrine growth factor secreted by the L-428 Reed-Sternberg cell. Blood 79:191, 1992 42. Kretschmer C, Jones DB, Morrison K, Schlueter C, Feist W, Ulmer AJ, Arnoldi J, Matthes J, Diamantstein T, Flad HJ, Gerdes J: Tumor necrosis factor-a and lymphotoxin production in Hodgkm’s disease. Am J Pathol I37:341, 1990 43. Merz H, Houssiau FA, Orscheschek K, Renauld JC, Fliedner A, Herin M, Noel H, Kadin M, Muller-Hermelink HK, Van Snick J, Feller AC: Interleukin-9 expression in human malignant lymphomas: Unique association with Hodgkin’s disease and large cell anaplastic lymphoma. Blood 78: I3 1 1, 1991 44. Xeni L, Birg F, Guigou V, Bouabdallah R, Poizot-Martin I , Hassoun J: In situ expression of the IL-la and the TNF-a genes by Reed-Stemberg cells in Hodgkin’s disease. Int J Cancer 50:689, I992 45. Foss HD, Hummel M, Gottstein S, Ziemann K, Falini B, Herbst H, Stein H: Frequent expression of IL-7 gene transcripts in tumor cells of classical Hodgkin’s disease. Am J Pathol 146:l , I995 46. Samoszuk M, Nansen L: Detection of interleukin-S messenger RNA in Reed-Stemberg cells of Hodgkin’s disease with eosinophilia. Blood 75:13, 1990 From www.bloodjournal.org by guest on November 14, 2014. For personal use only. INTERLEUKIN-l0 IN HODGKIN‘S DISEASE 47. Gruss HJ, Brach MA, Drexler HG, Bonifer R, Mertelsmann RH, Herrmann F: Expression of cytokine genes, cytokine receptor genes, and transcription factors in cultured Hodgkin and Reed-Sternberg cells. Cancer Res 52:3353, 1992 48. Miyazaki I, Cheung RK, Dosch HM: Viral interleukin-IO is critical for the induction of B cell growth transformation by EpsteinBarr virus. J Exp Med 178:439, 1993 49. Tosato G , Tanner J, Jones KD, Revel M, Pike SE: Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol 64:3033, 1990 50. Baumann MA, Paul CC: Interleukin-5 is an autocrine growth factor for Epstein-Barr virus transformed B lymphocytes. Blood 79: 1763, 1992 5 1. Paul NL, Ruddle NH: Lymphotoxin. AnnuRev Immunol 6:407, 1988 52. Herbst H, Stein H, Niedobitek G : Epstein-Barr virusand CD30’ malignant lymphomas. Crit Rev Oncog 4:191, 1993 53. Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T: Production ofIL-IO by CD4+ T lymphocytes correlates withdown regulation of Thl cytokine synthesis in helminth infection. J Immuno1 147:2713, 1991 54. Clerici M, Shearer GM: A THI TH2switch is a critical step in the etiology of HIV infection. Immunol Today 14:107, 1993 55. Frisan T, Sjoberg J, Dolcetti R, Boiocchi M, De Re V, Carbone C, Brautbar C, Battat S, Biberfeld P, Eckman M, Ost A, Christensson B, Sundstrom C, Bjorkholm M, Pisa P, Masucci MG: Local suppression of Epstein-Barr virus (EBV)-specific cytotoxicity in biopsies of EBV-positive Hodgkin’s disease. Blood 86:1493, 1995 56. Poppema S: The nature of the lymphocytes surrounding ReedStemberg cells in nodular lymphocyte predominance and in other types of Hodgkin’s disease. Am J Pathol 135:351, 1989 + 2929 57. Peuchmaur M, Emilie D, Crevon MC, Solal-Celigny P, Maillot MC, Legmaire G, Galanaud P: IL-2 mRNA expression in Tacpositive malignant lymphomas. Am J Pathol 136:383, 1990 58. Deacon EM, Pallesen G , Niedobitek G , Crocker J, Brooks L, Rickinson AB, Young LS: Epstein-Barr virus and Hodgkin’s disease: Transcriptional analysis of virus latency in the malignant cells. J Exp Med 177:339, 1993 59. Levitskaya J, Coram M, Levitsky V, Imreh S, SteigenvaldMullern PM, Klein G , Kurilla MG, Masucci MG: Inhibition of antigen processing by the internal repeat of the Epstein-Barr virus nuclear antigen-l. Nature 375:685, 1995 60. Chen HS, Kevan-Jah S, Siinzenich KO, Gresser FA, MiillerLantzsch N: Expression of the Epstein-Barr virus latent membrane protein (LMP) in insect cells and detection of antibodies in human sera against this protein. Virology 190:106, 1992 61. Rochford R, Dawson CW, Mosier DE, Young LS: Epstein Barr virus latent proteins activated cytokine gene expression in epithelial cells, in: Epstein-Barr Virus and Associated Diseases. Cold Spring Harbor Meeting on Cancer Cells 1994 Bookof Abstracts. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory, 1994, p 70 (abstr) 62. Mosialos G , Birkenbacb M, Yalamanchili R, VanArsdale T, Ware C, Kieff E: The Epstein-Barr virus transforming protein LMPl engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389, 1995 63. Rowe M, Peng-Pilon M, Huen DS, Hardy R, Croom-Carter D, Lundgren E, Rickinson AB: Upregulation of bcl-2 by the EpsteinBarr virus latent membrane protein LMP-I: A B-cell-specific response that is delayed relative to NF-KB activation and to induction of cell surface markers. J Virol 685602, 1994
© Copyright 2026