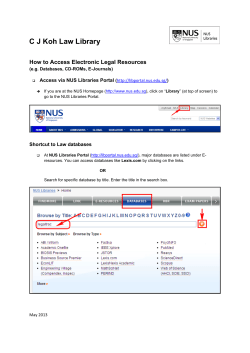
List of Laboratory Rotation projects
List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Department of Anatomy Details of PI Project Title with a brief description Prof Bay Boon Huat Peroxiredoxin III as a prognostic marker and therapeutic target in breast cancer PI’s email id [email protected] Office Address Department of Anatomy, NUS Telephone Number 6516 3200 The Peroxiredoxin (Prx) family comprise of six thiol-specific antioxidant enzymes which catalyze the reduction of hydrogen peroxide (H2O2). Prxs confer protection against oxidative stress and regulate cell signaling associated with H2O2 as a secondary messenger, thereby influencing cell differentiation, proliferation and apoptosis. Prx III (encoded by the PRDX3 gene), is found to be localized mainly in the mitochondria. Our previous studies have shown that silencing the Prx III gene enhanced cell proliferation and metastatic potential in breast cancer in vitro. The hypothesis in this study is that Prx III is involved in breast carcinogenesis and could be a potential prognostic biomarker and molecular target for cancer therapy. Hence, the specific aims of this study are: • Aim 1: Perform functional studies of Prx III in breast cancer progression and chemosensitivity to therapeutic drugs. Silencing and overexpression of the PRDX3 gene will be performed followed by: (a) evaluating the effects on functional processes, viz., cell proliferation, cell cycle, adhesion, migration, invasion and cancer cell-endothelial interaction. (b) establishing mechanistic pathways of Prx III by gene arrays and proteome analysis to establish which potential signal transduction pathway(s) are activated following siRNA treatment. Advanced quantitative mass-spectrometry-based SILAC (Stable isotope labeling Amino Acids in Culture) will be used for pathway and network analysis (c) determining the chemosensitivity of breast cancer cells to a panel of drugs after silencing the target PRDX3 genes. • Aim 2: Conduct an in vivo study to verify the effects and deduce putative effectors of Prx III in tumour metastasis using a metastatic mouse model for human breast cancer. • Aim 3: Determine the utility of Prx III as clinical predictors of tumour aggressiveness in clinical samples. This study will lead to new knowledge on the mechanistic pathways involved in Prx III-mediated breast cancer progression. A/Prof George Yip PI’s email id [email protected] Office Address Department of Anatomy, National University of Singapore, 4 Medical Drive, Block MD10, Singapore 117594 Expression and Functional Analysis of Glycosaminoglycans and Proteoglycans in Breast Cancer Glycosaminoglycans are highly negatively charged molecules made up of repeating disaccharide subunits consisting of an amino sugar and an uronic acid. They are covalently linked to core protein backbones to form proteoglycans. Besides structural roles, glycosaminoglycans and proteoglycans have important biological functions in regulating cell behaviour through their interactions with growth factors and signalling molecules. They are also involved in microRNA- and exosome-mediated regulation of cancer. In this study, we aim to elucidate the effects of these molecules on cancer cell List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Telephone Number 6516 3206 activities, to investigate their potential clinical use as biomarkers and prognostic indicators, and to utilise them for the development of novel therapeutic targets. A variety of cell and molecular biology techniques will be employed in these studies, including cell culture, immunohistochemistry, electron microscopy, ion exchange chromatography, proteoglycan quantification assays, real-time PCR, in situ hybridisation, gene cloning, and microarray analysis. References: 1. Kumar AV et al (2014) HS3ST2 modulates breast cancer cell invasiveness via MAP kinase- and Tcf4 (Tcf7l2)-dependent regulation of protease and cadherin expression. Int J Cancer (in press). 2. Ibrahim SA et al (2012) Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPaseand E-cadherin-dependent mechanism. Int J Cancer, 131, E884-96. 3. Yip GW (2011) Breast cancer: Novel therapeutic targets. Recent Pat Anticancer Drug Discov 6:164-165. 4. Koo CY et al (2008) Targeting heparan sulphate proteoglycans in breast cancer treatment. Recent Pat Anticancer Drug Discov 3:151-158. 5. Yip GW et al (2006) Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther 5:2139-2148. A/Prof S Thameem Dheen PI’s email id [email protected] Office Address Department of Anatomy, NUS Telephone Number 6516 3217 Analysis of miRNAs in mouse embryonic neural stem cells derived from diabetic pregnancy Diabetes during pregnancy is of serious concern as it causes spontaneous abortions and congenital malformations. About 10% of the fetuses from diabetic pregnancies display congenital malformations in various organ systems, including neurological systems, leading to perturbations in memory performance. Recently, several signaling molecules and epigenetic factors such as microRNAs (miRNAs) have been shown to be critical for brain development and function. Neural stem cells (NSCs) are self- renewing multipotent cells, which give rise to the neuronal and glial cells of the brain. Proper neural tube development and closure depends on several genes and morphogens, which are involved in NSC proliferation, migration, adhesion, differentiation and apoptosis. Disturbances or alterations in gene expression or cellular events that regulate neural tube closure results in failure of closure of the neural tube (neural tube defect, NTD). It has been well established that epigenetic modifications are involved in cell fate specification of NSCs. In recent years, the role of microRNAs (miRNAs) in regulating a number of cellular processes including development, proliferation, differentiation and plasticity has gained attention. miRNAs have been shown to regulate brain development by altering the expression of various regulatory genes involved in neurogenesis and gliogenesis during neurodevelopment. Given that gene expression profiling in cranial neural tubes of embryos from diabetic pregnancy has revealed altered expression of several genes in the developing brains exposed to maternal diabetes (Jiang et al 2008), and that miRNAs fine tune gene expression, it is hypothesized that maternal diabetes alters expression of miRNAs that regulate genes critical for neural tube closure and patterning. In order to address this, we performed List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake miRNA expression profiling using mouse embryonic NSCs isolated from forebrain of embryos from control and diabetic pregnancy. Preliminary results of miRNA expression profiling in NSCs from embryos of diabetic pregnancy has revealed differential expression of several miRNAs that are known to be associated with neural tube development. Thus, in the proposed study, the student will explore the role of specific miRNAs in regulation of cell fate specification of NSCs derived from mouse embryos of normal and diabetic pregnancy. We believe that these findings may explain the basis for patterning defects observed in the developing brain exposed to maternal diabetes. References: Sukanya et al. (2013) Hyperglycemia modulates the expression of miRNAs and cell fate specification of mouse embryonic neural stem cells. PLoS One. 11;8(6):e65945. Jiang et al. (2008) Global gene expression analysis of cranial neural tubes in embryos of diabetic mice. Journal of neuroscience research 86: 3481-3493. Fu et al., (2007). Aldose reductase is implicated in high glucose-induced oxidative stress in mouse embryonic neural stem cells. J Neurochem. 103(4):1654-65. Department of Biochemistry Details of PI Project Title with a brief description Dr Chen Ee Sin Anti-cancer drug resistance mechanism through the regulation of chromatin structure PI’s email id [email protected] Office Address MD6, Level 14, North Telephone Number 6516 5616 The genomic DNA of all eukaryotic cells are intricately organized around structural proteins called histones into a higher order chromatin. The most fundamental subunits of the chromatin is called nucleosome that contains 8 histone molecules organized around about 146 base pairs of DNA. The nucleosome is the scaffold on which all DNA-dependent processes including transcription, DNA repair and recombination occur and the integrity of nucleosomal organization bears direct impact on important cell cycle processes such as chromosome segregation and DNA replication. These two processes are commonly targeted by many anti-cancer drugs to induce genomic instability in cancer cells. However often, the effects of the drugs also affect normal cells resulting in profuse side effects associated with chemotherapeutic treatments. This project is aim at finding mechanisms that protect chromatin against the damage caused by chemotherapeutic drugs. We will use fission yeast as a model organism to uncover universal mechanism in order to facilitate targeted testing in human breast and gastric cancer cells. A/Prof Caroline Lee Project 1: PI’s email id [email protected] Colorectal cancer (CRC) is the most common cancer in Singapore with around 2000 new cases each year. About a third of these cases are metastatic (stage IV) at diagnosis and around one third of curatively resected cases (stages I-III) will relapse suggesting that a substantial proportion of patients may need treatment for metastatic/relapsed CRC. The two commonest combination drug regimes for this cancer in Office Address MD6, Level 14 or Level 6, Lab 5, National Cancer Centre List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Telephone Number 6516 3251 or 6436 8353 Singapore are CAPOX (capecitabine and platinum-based oxaliplatin) or CAPIRI (capecitabine and topoisomerase-I inhibitor) but the response rate to these regimes is only ~40-45%. Developing a reliable early predictive biomarker of response to these drugs in metastatic CRC can lead to appropriate tailoring of treatment for individual patients and help move us closer to a truly personalized care. It will not only be cost effective but will also reduce unnecessary side-effects in patients who will not benefit from the drug treatment and novel treatments can be explored for these patients. However, there are currently no reliable tests for early prediction of response to chemotherapy in these patients. Genetic single-nucleotide-polymorphisms (SNPs) have strongly been implicated in the determination of differences in drug response and hence can serve as a useful early predictive biomarker for response. Here, we propose a novel pathway-based, potentially-functional SNP (pfSNP) approach to identify SNPs that may serve as predictive biomarker of response to these drugs in 300 treatment-naïve CRC patients who receive either CAPOX or CAPIRI. Response that will be assessed include tumor shrinkage according to standard RECIST criteria, toxicity according to standard NCI-CTC criteria, progression-free survival and overall survival. A simple, robust, cost-effective point-of-care genotyping assay will then be developed for polymorphisms that can predict drug response. We will also automate this point-of-care test so that it will be capable of translating genetic information to clinically relevant information about drug response. Project 2: Hepatocellular carcinoma (HCC), amongst the top 5 cancers in Singapore is the third leading global cause of cancer death with five-year survival of ~5%. Although molecular advances have led to an increased understanding of the genetic changes that occur in HCC, there is still inadequate knowledge about the full spectrum of molecular mechanisms and epigenetic events in hepatocarcinogenesis. Current diagnosis of HCC relies on routine screening of at-risk patients, including those with cirrhosis due to viral hepatitis, by screening serum alpha-fetoprotein (sAFP) levels in conjunction with hepatic ultrasonography but this combination has limitations. Although cost-effective, ultrasonography has only 60% sensitivity and 97% specificity. sAFP is only 40-60% sensitive as many tumors do not produce AFP or do so at very advanced stage. Hence, there is an urgent need to identify better, more reliable non-invasive biomarkers with higher sensitivity and specificity for early detection of HCC. Here, we propose to interrogate miRNAs/small RNAs, by themselves or in combination with current available AFP/Ultrasound biomarkers, as emerging novel biomarkers for predicting individuals who are likely to develop cirrhosis or at the early stage of cirrhosis as well as those likely to develop HCC or at early stages of HCC. Retrospective, as well as prospectively collected samples with yearly follow-up and sample collection from 4 groups of individuals, namely, Normal Non-cirrhotic (NNC), HBV chronic carriers (HBCC), Cirrhotic and HCC will be examined. Next-Generation Deep Sequencing on prospective samples at zero time point will be employed to identify novel miRNAs/small RNAs List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake that are that are associated with cirrhosis and/or HCC. Real- time RT-PCR will be used on the retrospective samples to validate promising miRNAs while prospective samples collected at 24 month time point will be used to confirm the robustness of these validated biomarkers. These novel biomarkers either alone or in combination with current biomarkers may be clinically useful. Dr Deng Lih Wen PI’s email id [email protected] Office Address Department of Biochemistry, MD6-14-02S Telephone Number 6516 1239 Targeting MLL5β mediated transcriptional control of the E6/E7 oncogenes as a basis for novel therapeutic strategies for HPV16/18 associated cancers. Our lab has previously identified a new MLL5 isoform, MLL5β, which expression was correlated to high-risk HPV16/18 cervical cancers. Our initial data suggest that MLL5β is involved in the regulation and expression of key oncogenes E6 and E7 in cervical cancer and targeted silencing of MLL5β could inhibit HPV16/18 cervical cancer development. We have recently established that cisplatin, the current gold standard in cervical cancer care modulates its anti-tumour effects through MLL5β. Our findings have also suggested that targeted gene silencing of MLL5β has potential applications as a mode of therapeutic intervention for HPV16/18-related cancers with less cytotoxic effects compared to cisplatin. Currently we have identified a key post-translation modification (PTM) on MLL5β, which is essential for the assembly of the transcriptional activation complex required to initiate E6/E7 transcription. Based on this finding we have moved on to identify potential small molecular inhibitors of this PTM and are investigating their potential applications in cervical cancer therapy. However, much remains to be done to better understand the full role of MLL5β in E6/E7 expression regulation such as what are other components of this MLL5β-associated transcriptional complex as well as the exact role of MLL5β in the complex. A better understanding of these molecular events will lead to the potential identification of better therapeutic avenues for cervical cancer therapy. We are looking for an enthusiastic and motivated candidate to focus on further understanding these molecular events. The candidate will work on identifying the other components of the MLL5β transcription activation complex as well as further understand the histone methyltransferase role of MLL5β in the complex. The outcome of the project will be to harness this knowledge and translate it to the development of novel molecular targeted therapeutic strategies for cervical cancer therapy. Targeting Cancer Metabolism In Inhibiting Tumor Progression Metabolic switch is an important event during tumor progression. It is believed that cancer cells reprogram their metabolic processes so as to exhibit high glycolysis to feed their rapid proliferation, a phenomenon known as Warburg effect. The adaptation to glucose metabolism in cancer cells produces high metabolic acid that in turn confers tumor survival and invasion. The invasion of in situ tumor cells to distant sites, also known as cancer metastasis, accounts for more than 80% of cancer-associated death. Studies have also found that cancer cells which acquire high invasiveness are usually highly resistant to chemotherapy. Conceptually, intertwining cancer metabolism, particularly the glycolysis process would serve as an effective way to target cancer progression. However, the inter-link between cancer metabolism and List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake cancer invasiveness is unclear at the moment. Our preliminary data demonstrated that our compound of interest interfered with cancer glycolysis and potentially affected cancer metastasis process. In this project, we are interested in finding the underlying cellular metabolic changes in cancer cells across different invasive stages and understand how these metabolic switches affect cancer hostile behavior. We also intend to screen for pharmacological compounds that exhibit synergistic effects to achieve anti-cancer survival and anti-metastasis. Findings from this project will provide further knowledge on cancer progression and shed light in developing innovative cancer therapy strategy. Dr Lim Yoon Pin PI’s email id [email protected] Office Address MD4, level 1. 5 Science Drive 2, Singapore 119545 Telephone Number 6601 1891 Function and mechanism of a novel WBP2 oncogene in breast cancer and metastasis Through cutting-edge phosphoproteomics technology, we have identified a novel breast cancer associated gene (1). Our lab is the first to demonstrate that WBP2 transcription co-activator is a new breast oncogene that upon phosphorylation is able to transform normal mammary cells into cancer cells and make mild cancer cells aggressive by activating multiple other oncogenic pathways (2). We have also identified a new interacting partner of WBP2 that modulates the expression of WBP2 and its Wnt-promoting activities in breast cancer cells (3). The aim of the new project is to elucidate how WBP2 turns on other oncogenes and contributes to breast oncogenesis and metastasis using a wide array of in vitro and animal model assays associated with signal transduction, transcription and cancer biology. The proposed study is likely to result in the mapping of signaling cascades and transcriptional networks associated with the WBP2 thus providing new insights into the molecular etiology of breast cancer. Our lab also has a strong emphasis on translational research – for example in the exploitation of WBP2 as a biomarker and drug target. A patent has been filed for the use of WBP2 in cancer detection and therapy. Students will have a chance to participate in commercialization of intellectual properties associated with WBP2. 1. 2. 3. Dr Long Yun Chau PI’s email id [email protected] Office Address 8 Medical Drive, MD7, #03-13 Chen, Y., et.al and Lim, Y. P. (2007) Differential Expression of Novel Tyrosine Kinase Substrates during Breast Cancer Development. Mol Cell Proteomics 6, 2072-2087 (5 year impact factor: 9.4) Lim, S. K., et.al. and Lim, Y. P. (2011) Tyrosine phosphorylation of transcriptional coactivator WW-domain binding protein 2 regulates estrogen receptor alpha function in breast cancer via the Wnt pathway. FASEB J 25, 3004-3018 (5 year impact factor: 7.2) Lim S.K., et.al. and Lim YP (2014) WIP negatively regulates WBP2 expression and WBP2-mediated Wnt activation in breast cancer cells (manuscript in preparation). The role of energy sensing network in exercise-induced skeletal muscle adaptation The beneficial effects of exercise on health are widely recognized. Exercise could improve and prevent chronic metabolic disorders such as diabetes and obesity. However, the cellular mechanism behind these effects is largely unknown. Optimization of exercise regimen or pharmacological recapitulation List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Singapore 117597 Telephone Number 6601 2084 of exercise effects requires close examination of intramuscular events that triggers the exercise adaptations. Our previous and current work has aimed to define the mechanism that drives exercise-induced metabolic adaptation in skeletal muscle. Exercise induces a multitude of cellular disturbances in muscle including decreased intramuscular energy levels. Exercise-induced energy deficit is considered an important stimulus for the beneficial metabolic outcomes. AMP-activated protein kinase (AMPK) is an important signal transducer which is activated in response to energy stress during exercise. Our previous work in mouse models provided evidence that activation of this protein kinase is sufficient to increase skeletal muscle glycogen store, fatty acid utilization and muscle endurance – benefits that are derived from exercise training. Conversely, disruption of muscle AMPK signaling accelerated the progression of muscle fatigue and impaired exercise-mediated metabolic gene expression. These results provided important evidence that AMPK signaling mediates some of the exercise-mediated metabolic responses in skeletal muscle. In the current study, we hypothesize that AMPK plays a role in autophagy and amino acids metabolism in skeletal muscle, and this effect is critical for metabolic benefits of exercise and endurance. Role of insulin-induced glycolysis in the regulation of PGC1α-mediated myotube lipid metabolism Our body is subjected to irregular nutrient supply, including the transition between fasting and feeding. Therefore, the ability of our body to selectively store and utilize different energy substrates is critical for energy balance. Given the substantial mass and energy consumption, skeletal muscle plays a critical role in the regulation of energy balance, and impaired skeletal muscle metabolism is closely associated with metabolic diseases such as type 2 diabetes and obesity. Thus, investigation of skeletal muscle energy substrate metabolism is critical for the understanding of pathophysiology of metabolic diseases. Under postprandial conditions, insulin stimulates skeletal muscle glucose uptake and utilization via Akt, a protein kinase that activates downstream effectors of insulin. Conversely, skeletal muscle increases lipid oxidation under fasting conditions, which coincident with the induction of PGC1α - a transcription coactivator that has been implicated in the induction of lipid metabolic gene program. Nonetheless, the interaction between the disparate insulin and PGC1α pathways which are activated under contrasting nutritional states remains largely unknown. This research project aims to establish the role of nutrient (glucose) and hormone (insulin) in the regulation of PGC1α cultured myotubes, and the impact of this regulation on lipid metabolic gene program in myotubes. Role of insulin-like signaling in the regulation of SIRT1 deacetylase The loss of skeletal muscle mass and metabolic function is a common feature of muscle pathologies associated with inactivity (atrophy), aging (sarcopenia) and diseases (cachexia). The loss of muscle function is mainly attributed to elevated protein degradation and resistance to anabolic effects of growth hormones such as insulin and insulin-like growth factor 1 (IGF1). These anabolic hormones are critical signals that stimulate skeletal muscle glycogen and protein synthesis (anabolic metabolism), predominantly via the Akt kinase pathway. Conversely, nutrient deprivation activates Silent Information Regulator T1 (SIRT1) deacetylase and forkhead box protein O1 (FOXO1) proteins that induce catabolic metabolism in skeletal muscle. The increase of catabolic over anabolic pathway may be a molecular basis for the loss of List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake muscle mass and function. Nonetheless, the mechanism by which such delicate balance is regulated remains largely unknown. This proposal aims to evaluate the novel role of insulin-like signals in the regulation of SIRT1, and the unknown function of SIRT1 in the regulation of glucose and amino acid metabolism in cultured myotubes. The specific aims are (1) to determine if insulin-like signals regulate SIRT1 via nutrient metabolism, (2) to establish the role Akt in the regulation of SIRT1-induced FOXO1 pathways, and (3) to examine the impact of AKT-SIRT1 pathways on energy substrate metabolism. Cultured myotubes will be treated with insulin or IGF1 in the absence of presence of metabolic inhibitors to determine the function of these hormones on SIRT1 function. The role of Akt in the regulation of SIRT1-dependent FOXO1 activity will be evaluated via pharmacological and genetic alteration of the kinase and deacetylase. The metabolic effects of Akt-SIRT1 pathway will be determined by direct measurements of glucose and amino acids metabolism in myotubes to provide direct readout for changes in metabolic fluxes. The research will provide important insight into the pathophysiology of skeletal muscle metabolism, via the identification of insulin-like hormones as regulators of SIRT1. It will also provide critical evidence for SIRT1 and IGF1 as potential drug targets for muscle wasting diseases. Dr Yeong Foong May PI’s email id [email protected] Office Address MD4, Level 1 Telephone Number 6516 8866 We are interested in how cells regulate cytokinesis such that it occurs only after chromosome segregation so as to produce two viable daughter cells. We plan to study the links between cell cycle regulation and cytokinesis, with particular focus on how the cell cycle machinery affects endocytosis at the cytokinesis site. Endocytosis is a key cellular process that leads to the internalization of proteins and membranes from the plasma membrane. During cytokinesis, endocytosis occurs at the cytokinesis site and perturbations to endocytosis can cause a failure in cytokinesis Our preliminary data suggest that the key cell cycle, the cyclin-dependent kinase (Cdk1) activity in mitosis has an apparent role in preventing endocytosis and cytokinesis. Indeed, upon the destruction of the mitotic Cdk1 activity, the both cytokinesis and endocytosis at the neck are triggered. This was evident from our initial data showing that key endocytic components accumulate at the neck only during cytokinesis. We plan to make use of budding yeast as our model for the proposed study, given that both processes are conserved and that the budding yeast serves as a good system for complex genetic manipulations, we plan to make use of budding yeast to understand the regulatory relationship among Cdk1 activity and endocytosis during cytokinesis. Our lab has the necessary tools and techniques to execute experiments including genetic and physical screens. Moreover, we also conduct time-lapsed imaging experiments in addition to physical interaction assays. We aim to provide insights into how cells normally prevent cytokinesis until after nuclear division by studying how cells regulate endocytosis during cytokinesis. The proper execution of cytokinesis is important, given that premature cytokinesis can cause a loss of cell viability while failure to execute cytokinesis can lead to tetraploidy in human cells, which is a precursor to tumourous growths. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Department of Medicine Details of PI Project Title with a brief description Dr Chester Lee Drum A Protein Dynamic Approach to Engineered Translational Therapeutics PI’s email id [email protected] Capitalizing on the prior training of the professor, the lab leverages a structural biology approach (i.e., Drum et al. Nature 2003) to create clinically relevant engineered proteins for both diagnostic and therapeutic purposes. “The protein folding problem” is one of the most interesting in biology --how does a linear peptide with more conformations than there are atoms in universe, know to fold into essentially a single functional conformation? Can biochemical engineering fundamentally effect this process in a way to make it amenable to oncological or cardiovascular treatments? In this project you will use a novel form of protein expression invented by our lab to study protein folding dynamics and novel cellular uptake mechanisms for drug delivery. The project is a continuation of research begun at Massachusetts Institute of Technology and will create nanometer scaled uptake vesicles for the delivery of active protein substrates. Our lab has a full time synthetic chemist, biochemist and materials engineer in addition to full molecular biology and protein biosynthesis support which is an excellent setting for a graduate student who wants an efficient approach to a high impact problem, not bounded by a single technique. Although Prof. Drum was clinically trained as a cardiologist (BWH, Harvard Med School) he is also an award winning structural biologist who is interested in developing talented students into future scientific leaders. Please enquire if interested. Office Address Yong Loo Lin Sch of Medicine, 14 Medical Drive, 08-01, Singapore 117599 Telephone Number 6601 3001 Prof Daniel G Tenen PI’s email id [email protected] Office Address 14 Medical Drive, Centre for Translational Medicine, MD6, Level 12, Singapore 117599 Telephone Number 6516 8239 Investigation of mutation on TERT promoter in giloblastoma for potential therapy target. Glioblastoma is the most common and most aggressive malignant primary brain tumor in humans. Recently studies demonstrated that a transcriptional role of mutations on telomerase reverse transcriptase (TERT) gene promoter in it. Although evidence showed that these mutations correlated with higher transcription activation of TERT and a more aggressive phenotype, the deep mechanism is still unclear. Collaborated with Dr. Vinay in IMCB, here we found a novel transcriptional factor binding site on the mutation site by using biostatistical analysis. We propose that this novel binding site will increase the transcriptional activity of TERT promoter and therefore upregulates TERT protein level. To test our hypothesis, we plan to do 1) biochemical analysis to investigate the deep mechanism to see whether this transcriptional factor indeed binds to TERT promoter and how it regulates TERT level, 2) in vitro and in vivo assay to confirm whether with this mutation, this novel transcriptional factor promotes a more aggressive phenotype, 3) multiple analysis in clinical samples to confirm whether this transcriptional factor and related pathway are indeed highly activated and caused more aggressive primary human tumor. Our study will suggest a novel therapy target for giloblastoma. Prof Edward H Koo PI’s email id The Laboratory for Molecular Neurodegeneration at YLL School of Medicine was established by Dr. Ling Shuo-Chien and Prof. Edward Koo in late 2013 when we first joined the NUS faculty. Our research focus is on the List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake [email protected] Office Address Centre for Translational Medicine, MD6-10-01Q Telephone Number 6601 3706 mechanisms of neurodegenerative diseases, a group of brain disorders that is often age-associated and includes Alzheimer and Parkinson diseases, amyotrophic lateral sclerosis, frontotemporal dementia, among others. We take a cell and molecular biology approach to our studies with an emphasis on animal models and translational research. My laboratory’s philosophy is to study both the “normal and abnormal biology” of genes and proteins that are implicated in disease pathogenesis. In many instances, mutations are found in genes with unknown function and which require thorough investigations of the basic functions of these genes and proteins before we can appreciate how their dysfunction contributes to the disease phenotype. My laboratory has a particular emphasis is on the pathophysiology of Alzheimer disease where we have concentrated on both basic and translational studies as well as experimental therapeutics. Ongoing and future projects include: Mechanisms of synaptic injury in Alzheimer disease Role of caspase cleavage of the amyloid precursor protein (APP) in neurodegeneration Mechanisms of tau mediated synaptic dysfunction Investigations into mechanisms of neuronal vulnerability in Alzheimer disease Contribution of blood-brain barrier to neurodegeneration Neurobiological basis of cognitive loss due to chemotherapy treatment in cancer patients (“chemobrain”) Prof Lee Kok Onn Generation of functional insulin-producing cells for diabetes therapy PI’s email id [email protected] Diabetes has become the pandemic metabolic disease affecting over 382 million populations worldwide. Insulin-dependent diabetic patients rely on multiple injections of insulin daily while still developing various cardiovascular complications. Transplantation of pancreas or isolated pancreatic islets to replace the lost insulin-secreting beta-cells is the only therapy to cure the disease. But the lack of organ donors has severely limited such promising treatment. To circumvent this obstacle, our research aims to differentiate stem cells or reprogram somatic cells such as fibroblasts and adipocytes into functional insulin-producing cells using the knowledge and technologies of molecular biology, stem cell biology, biochemistry, diabetes and animal models. These surrogate beta-cells may not only be applied for the replacement therapy of diabetes but also can be used to define the molecular mechanism for pathogenesis of diabetes. Our work has been awarded by American Society for Cell Biology (http://www.ascb.org/files/2005pressbook.pdf), widely covered by media (http://www.news.gov.sg/public/sgpc/en/media_releases/agencies/astar/press_ release/P-20081120-1.print.html?AuthKey; http://www.sciencedaily.com/releases/2008/11/081120130539.htm), featured in the NUS research gallery (http://www.nus.edu.sg/research/rg155.php), and supported by competitive national research grants. Example publications: Li G.D, Luo R, Zhang J, Yeo KS, Lian Q, Xie F, Tan EKW, Caille D, Kon O.L, Salto-Tellez M, Meda P, and Lim SK. (2009) Generating mESC-derived insulin-producing cell lines through an intermediate lineage-restricted progenitor line. Stem Cell Res, 2:41-55, E-pub http://dx.doi.org/10.1016/j.scr.2008.07.006 Li G.D, Luo R, Zhang J, Yeo KS, Xie F, Tan EKW, Caille D, Que J, Kon O.L, Salto-Tellez M, Meda P, and Lim SK. (2009) Derivation of functional insulin- Office Address Endocrinology, National University of Singapore 1E Kent Ridge Road NUHS Tower Block Level 10 Singapore 119228 Telephone Number 6772 4341 Co supervisor: A/Prof Li Guodong [email protected] Telephone Number 83111022 List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake producing cell lines from primary mouse embryo culture. Stem Cell Res, 2:2940, E-pub http://dx.doi.org/10.1016/j.scr.2008.07.004 Shao S, Gao Y, Xie B, Xie F, Lim SK, LI GD. (2011) Correction of hyperglycemia in type 1 diabetic models by transplantation of encapsulated insulin-producing cells derived from mouse embryo progenitor. J Endocrinol, 208:245-255, e-pub http://dx.doi.org/10.1530/JOE-10-0378 Prof Nicholas Paton PI’s email id [email protected] Office Address Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Rm W10009D, NUHS Tower Block, 1E Kent Ridge Road, Singapore 119228 Telephone Number 6772 6988 Development of Whole Blood Bactericidal Activity Assay for Tuberculosis Therapeutics Tuberculosis (TB) is one of the world's deadliest infectious diseases with an estimated 9 million new cases and 1.4 million deaths per year. It is caused by Mycobacterium tuberculosis, which is becoming increasingly resistant to current drugs, thereby posing a major public health problem in Asia and worldwide. Our group’s research is focused on investigating new approaches for delivering improved, more efficient treatments for TB. The whole blood bactericidal activity assay (WBA) is an ex vivo model for measuring effects of administered drugs, host factors and strain factors on mycobacterial sterilization. Mycobacteria added to human whole blood cultures undergo phagocytosis and remain intracellular in 72-h cultures. During TB treatment regimens, drug levels in the whole blood cultures mirror those in the circulation at the time of phlebotomy. If performed in parallel with pharmacokinetics measurements, the method can be used to evaluate the effect of drugs throughout the dosing cycle. To date, the method has not been utilized to investigate the synergy between the immune system and TB drugs on mycobacterial activity, nor has it been used to look at the effects of drugs on different TB strains. Both of these are potentially very useful aspects of this experimental paradigm which deserve to be explored. The aim of this research project therefore is to establish WBA as a method in clinical trials investigating new drug regimens against tuberculosis. The PhD candidate in charge of this project will develop the method and extend it to measuring host immune responses and effects on different mycobacterial strains. He/she will have an opportunity to learn the principles of multinational clinical trials, will be involved in clinical pharmacokinetics studies, will acquire specialized in-demand skills to work in BSL-3 laboratory environment, and will learn to transform data into knowledge for improving human lives. Prof Nobuhiro Yuki PI’s email id [email protected] Office Address #09-01 Neuroscience Research Centre, Centre for Translational Medicine, MD6, 14 Medical Drive, Singapore 117599 Telephone Number Comparison of IVIG with the different immunoglobulin effects on cell viability in neuromyelitis optica Neuromyelitis optica (NMO) is a variant of multiple sclerosis, identified by a spectrum of autoimmune astrocytopathies, with phenotypic presentation of optical neuritis and relapsing inflammatory myopathies. Detection of IgG antibodies against aquaporin 4 (AQP4; water pore proteins present in the cell membrane), in the sera distinguishes it from other inflammatory demyelinating neuropathies. It has been recently established that the AQP4-IgG have a deleterious effect on cell viability and this event is reversible in absence of human complement. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake 6516 1092 In support to this data, a good response to intravenous immunoglobulin (IVIg) therapy has been observed among the NMO patients cohort. Therefore, the main aim of this project is to understand whether this observation is due to the effect of IVIg therapy on cell viability and how the different components contribute to it. The effect of different formulations of IVIg and its components - IgA, IgM and F(ab)’ fragment, in restoring cell viability in presence of NMO plasma is to be investigated. In addition to this hypothesis, there is an underlying dispute on the pathomechanism based on the expression of the two AQP4 variants - M1 and M23 which is to be investigated in this study. The human astrocytoma cell line (CRL1718) is the in vitro model of choice along with HEK293, a human embryonic kidney cell line, both known to express AQP4. The validation of the cell lines for suitability of AQP4 study, reactivity of NMO patient sera and expression profile of AQP4 variants are the prime requisites in this study. Following which the cell viability of these cells in the presence of IVIg and NMO patients’ sera will be tested via cell-based assays and flow cytometry. Identification of true target of antibodies from Lambert-Eaton Myasthenic Syndrome (LEMS) LEMS is a rare autoimmune neuromuscular disorder in which patients manifest both muscle weakness and autonomic nervous system disruption. It is believed to be due to autoantibodies targeting presynaptic voltage-gated calcium channels (VGCCs) extracellularly, specifically P/Q- and N- types VGCCs. However, the distribution of VGCCs and the manifestation of symptoms suggested that VGCCs may not be the true target. We hypothesise that there could be an unidentified protein X that is closely associated with VGCCs which results in the false identification of the true target. The aim of the study is to prove that VGCCs is not the true target and identify this protein X as the true target in which antibodies from LEMS patients bind to. We will use techniques of immunocytochemistry, immunohistochemistry, immunoprecipitation for the study. Structure/function analyses of bacterial sialyltransferase Cst-II with site directed mutation Sialyltransferase Cst-II exist in either mono-functional (α2,3-sialyltransferase) or bi-functional (α2,3-/α2,8-sialyltransferase) forms. Three amino acid residues, Asn51, Leu54, and Ile269, are specific for the bi-functional Cst-II, but a single mutation (Asn51 → Thr51) is sufficient to induce the monofunctional Cst-II. However, little is known about the correlation between structural and such functional differences. The aim of this study is to demonstrate the structure-activity relationship of mono-functional and bi-functional Cst-II. Molecular biological approach will be able to create bi-functional mammalian sialyltransferases, no cases are known in which with bi-function, such as α2,3-/α2,8-sialyltransferase. The structural differences will be determined by in silico analysis. The data obtained from in silico will provide helpful information about structural changes of sialyltransferase following their functional changes by site direct mutation. Active site mutants of mammalian sialyltransferase will List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake cause a change in enzymatic reaction and structure. Such data will be helpful to indicate the structure-activity relationship of sialyltransferases. Identification of the novel antibodies in demyelinating diseases. Multiple sclerosis (MS) and neuromyelitis optica (NMO) are demyelinating diseases of the central nervous system. A specific serum autoantibody to the astrocytic water channel aquaporin-4 plays an important role in the pathogenesis of NMO. In contrast, antibodies to neurofascin 186 and KIR4.1 have been reported in patients with MS, but these findings have not been replicated by independent groups. Guillain–Barré syndrome and chronic inflammatory demyelinating polyneuropathy (CIDP) are immune-mediated neuropathies of the peripheral nervous system. Guillain-Barré syndrome is classified into acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN). IgG autoantibodies bind to gangliosides such as GM1 and GD1a at the nodes of Ranvier in the peripheral motor nerves and subsequent complement activation cause the development of AMAN. In contrast, pathogenic autoantibodies remain to be identified in patients with AIDP or CIDP, although some antigens have been proposed as autoantibody targets. Autoantibody binding to myelin and subsequent complement activation may be involved in the pathogenesis of central and peripheral demyelinating diseases. Our collaborator kindly gives us the purified central and peripheral nerve myelin proteins. Moreover, we have already prepared sera from patients with MS, CIDP and AIDP. Our preliminary results have suggested the probable presence of novel autoantibody reacting with extracted proteins from mouse tissues (e.g. central nerve and peripheral nerve) in patients sera. We will identify the targets of autoantibodies in MS, CIDP and AIDP. Non-radioactive serological diagnosis of myasthenia gravis Myasthenia gravis (MG) is the most common autoimmune disease of the neuromuscular junction (NMJ) with a prevalence of 200-300 per million populations. Most MG patients (~85%) have autoantibodies against the muscle acetylcholine receptor (AChR), and about 6% of MG patients have autoantibodies against the muscle specific kinase (MuSK) and low-density lipoprotein receptor-related protein 4 (Lrp4). Currently, the most sensitive assays for the detection of the autoantibodies in MG sera have been the radioimmunoprecipitation assays. However, the use of radioactivity limits availability of these tests in many diagnostic laboratories. To improve nonradioactive assays for detection of antibodies to the AChR, MuSK and Lrp4, we plan to perform cell-based ELISA assay using a cell line transfected with the target auto-antigens. This assay has very high sensitivity and has been reported to detect auto-antibodies that can not be detected by RIPA. We hope that this study may possible to develop the assays as a routine practice in future. Identification of novel glycolipid X project Fisher syndrome is characterised by ophthalmoplegia, ataxia, and areflexia. The anti-GQ1b IgG antibodies often detected in patients with Fisher syndrome may have a role in the pathophysiology of this ophthalmoplegia. The List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake ganglioside GQ1b is expressed in the paranodal portion of human ocular motor nerves and is the possible target molecule in this disease. Previously, Mori et al. reported a patient with acute ataxia and areflexia, but no ophthalmoplegia, associated with anti-GQ1b IgG antibodies. Clinical findings, electrophysiological studies, and postural body sway analysis suggested that this patient’s ataxia was very similar to that seen in patients with Fisher syndrome. This patient did not present with ophthalmoplegia. The clinical and laboratory findings suggest that this case is a variant form of Fisher syndrome. ELISA showed the presence of IgG antibodies to GQ1b and GT1a, a situation commonly described in Fisher syndrome, Guillain-Barré syndrome (GBS) with ophthalmoplegia, GBS without ophthalmoplegia and acuteoropharyngeal palsy. TLC immunostaining using the patient’s serum reacted with GT1a and more weakly with GQ1b and unidentified glycolipid. We hypothesise that this unidentified glycolipid X is novel disease-related antigen. Identification of glycolipid X using anti-ganglioside antibody assays and mass spectroscopy is useful for elucidation of the pathophysiology. Identification of ophthalmopathy the pathogenic autoantibodies in Graves’ Graves’ ophthalmopathy (GO) is a potentially debilitating and disfiguring orbital inflammatory disease. Its association with autoimmune thyroid disorders is believed to be caused by a putative cross-reactive antigen connecting the thyroid gland and the extrathyroidal tissues, the nature of which remains contentious. A number of orbital auto-antigens have been suggested: thyroglobulin, extraocular muscle antigens, thyroid stimulating hormone receptor (TSHR), insulin-like growth factor 1 receptor (IGF-1R). Although TSHR is considered as one of the major antigens in the orbit, TSHR antibodies cannot explain the development of ophthalmopathy in patients in some patients with Graves’ hyperthyroidism, Hashimoto’s thyroiditis or in newborns with neonatal thyrotoxicosis. Ninety percent of patients with GO have Graves’ hyperthyroidism; the remaining 10% have hypothyroidism or are euthyroid. Thyroid stimulating antibodies recognize the extracellular domain of TSHR. We hypothesize that patients with GO have autoantibodies against a protein with similar structure to the TSHR receptor and we aim to identify these autoantibodies responsible for the development of ophthalmopathy. These autoantibodies when bound to the TSHR-like receptor expressed by fibroblasts or adipocytes in eye muscle are able to induce differentiation of adipocytes. Hence, fibroblasts when exposed to sera from patients with GO would proliferate and develop GO. We aim to identify the autoantibodies responsible for the immunopathologic mechanism in GO. Using bioinformatics analysis of the epitope region of TSHR, we identified a possible antigen with similar structure to the extracellular domain of TSHR. Now we aim to target this TSHR mimic molecule and produce an overexpression plasmid of this molecule. We plan to transfect with the candidate TSHR mimic molecule overexpression plasmid and treat with normal serum or GO patient serum. This will be followed by autoantibody recognition of TSHR and the TSHR mimic molecule evaluated with immunocytochemistry. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Dr Swaine Chen PI’s email id [email protected] Office Address 60 Biopolis Street, Genome #02-01, Singapore 138672 Telephone Number 6808 8074 Towards curing severe recurrent urinary tract infection by understanding mechanisms of intracellular infection Urinary tract infection (UTI), particularly of the bladder (cystitis), is an extremely common infectious disease, affecting more than half of all women at some point in their life. Recurrent UTI is especially problematic; patients often suffer for years with this condition, altering their sexual, dietary, and social habits. The only treatment currently is long term antibiotic therapy, but this often does not cure the disease. UTI research in a mouse model of infection has identified an intracellular population of bacteria that can persist within the epithelial tissue of the bladder. These bacteria are protected from antibiotic treatment as well as from clearance by the host immune response. They can reactivate weeks to months after the initial infection to cause a subsequent recurrence. A primary focus of the lab is understanding how these intracellular bacteria persist and cause recurrent infection; we do this using animal models and are developing tools to directly study urine samples from human UTI patients. We have established the mouse model of cystitis in the lab. We have very fine genetic control of uropathogenic Escherichia coli, which we use to determine the genetic basis for disease. We have recently also developed single cell genomics tools that allow us to profile expression of intracellular bacteria from infected mouse samples. Finally, we have clinical collaborators that can facilitate collection of samples from human UTI patients. The project is to apply these tools to gain an understanding of the gene expression during intracellular expression using these bacterial, animal, genomics, and clinical tools. The end goal is to identify pathways or genes that may eventually lead to diagnostic tools and therapeutic targets for improving our current therapy for recurrent UTI. There is a strong translational aspect to this project, as all animal studies will be validated in primary infected human samples. Students will have an opportunity to learn skills in animal models of disease; bacterial genomics and pathogenesis; next generation sequencing; and rigorous experimental design and data analysis. A strong background in bacterial genetics and molecular cloning is preferred. Experience with computer programming, mathematics, statistics, and/or genomics will also be important for sequencing data analysis. Highly motivated and creative graduate students are encouraged to apply. Department of Microbiology Details of PI Project Title with a brief description Dr Justin Chu Jang Hann Drug discovery and target validation for medically important mosquitoborne viruses PI’s email id [email protected] The re-emerging infections caused by mosquito-borne viruses such as dengue and chikungunya are on the raise at an explosively rate in many parts of the List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Office Address MD4, Level 5, Dept of Microbiology, 5 Science Drive 2 Telephone Number 6516 3278 world. In the view of the absence of an effective vaccine and antiviral drug against these viral pathogens, the developments of effective antiviral strategies against these viruses are in high demand. The intent of this study is to perform an established high content cell-based screening platform that could identify compounds that prevent virus replication by (i) inducing antiviral gene expression pathways in host cells, (ii) interfering with viral proteins and their functions (iii) interfering essential host-viral protein interactions. Although the assay can identify compounds that target distinct steps of the virus replication cycle, we are interested in those that stimulate cellular responses necessary to confer initial resistance to virus infection. Bioactive compound libraries will be screened on the high content cell-based assay in triplicate. All of the compounds that meet the designated criteria of a “hit” in the primary high content screening platform will be further confirmed with secondary assays (infectious plaque assays, protein over-expression and RNA interference studies). The efficacy of the selected compounds will be further validated in murine models. In additional, the antiviral targets or mechanisms will be dissected by a combination of reverse genetics, proteomics and bioimaging approaches. The research data generated from this study are essential for the rapid identification of potential prophylactic or therapeutic leads that will be helpful in better preventive management of individuals from suffering viral infections. In this proposal, re-emerging flavivirus dengue and chikungunya viruses will be used as the model for the “proof of concept” development. Prof Nicholas Gascoigne Reversing age-related thymic involution PI’s email id [email protected] As we age, the output of new T cells from the thymus is greatly reduced. It has recently been shown that this process can be reversed by expression of a single transcription factor, Foxn1, in the epithelial cells of the thymus. The cortical thymic epithelial cells give signals for positive selection of developing thymocytes, and mice lacking Foxn1, so-called “nude” mice, lack T cells (as well as hair). Foxn1 expression decreases with age, and re-expression of this gene in “middle-aged” mice reversed the involution of the thymus and restored production of naïve T cells [1]. We aim to develop vectors to allow Foxn1 to be re-expressed in order to reverse this process, eventually in humans. This project is to develop expression systems for Foxn1, and to test them in tissue cultured epithelial cells and then in mice. Office Address 5 Science Drive 2, Block MD4, YLLSOM Telephone Number 6516 3275 1. Bredenkamp, N., Nowell, C.S., Blackburn, C.C. (2014) Regeneration of aged thymus by a single transcription factor. Development 141: 1627 Control of T cell activation by Themis in response to weak antigens Themis is a protein discovered by this lab that is expressed in the T-cell lineage [1]. It controls thymocyte development by controlling the strength of signalling via its interaction with the phosphatase SHP1 (PTPN6) which turns off the signalling cascade [2]. This project will study how Themis works in mature T cells, and whether Themis inhibition can be used to enhance responses to weak antigens such as tumour antigen. 1. 2. Fu, G. et al., Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol 2009. 10: 848 Fu, G. et al., Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013. 504: 441 List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake T-cell control of Type 2 diabetes Themis is a protein discovered by this lab that is expressed only in the T-cell lineage, controlling selection of naïve T cells in the thymus [1]. It works through controlling the selection threshold for T cell receptor signalling at a particular point in T cell development [2]. We found that Themis-deficient mice develop obesity and metabolic syndrome, leading to type 2 diabetes (T2D). This indicates a T-cell mediated role in this disease, which is normally considered to be inflammatory in nature. The aim of this project is to identify the type of T-cells that cause the disease, and the mechanism by which they induce T2D, to use T-cells from Themis-sufficient mice to try to control or cure the disease, and to investigate a role for Themis in human T2D. 1. 2. Fu, G. et al., Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol 2009. 10: 848 Fu, G. et al., Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013. 504: 441 Dr Stephan Gasser I have 2 different projects: PI’s email id [email protected] 1) Current clinical biomarkers for cancer are often specific for certain types of cancer and many biomarkers are not detectable in the serum of patients. Serum-based markers offer key advantages including taking of samples with minimal inconvenience for the patients, the possibility to perform longitudinal studies of cancer patients, and early detection of tumors with a sensitive assay. We recently discovered the presence of genome-derived DNA with specific properties, including the presence of distinct retroelements and the potential to form triplex structures, in the cytosol and exosomes of many mouse and human tumor cells (Lam et al., Cancer Research 2014). Preliminary experiments show that the generation of such DNA is linked to replication stress, which is thought to be prevalent in most cancers. Hence, we plan to characterize the double-stranded (ds) DNA in serum exosomes of cancer patients and exosomes secreted by tumor cells. We propose to 1) deepsequence DNA present in serum exosomes of tumor patients and exosomes secreted by human tumors and tumor cell lines, 2) analyze the DNA sequences for conserved DNA sequences or properties among the different cancer samples. 3) develop an assay to detect exosomal dsDNA in the serum of cancer patients. A diagnostic test for a serum-based general tumor marker will be of interest to various companies in the diagnostic field. Office Address Department of Microbiology, NUS Telephone Number 6516 7209 2) Anti-viral defense mechanisms partially depend on sensors that recognize DNA associated with virus infections in the cytosol. The same defense mechanisms have also been implicated in tumor surveillance in several settings and the understanding of the molecular basis of tumor recognition is a topic of intense research interest. We recently demonstrated that B cell lymphomas and many tumor cell lines of various origins constitutively express cytosolic DNA as a consequence of oncogene-induced DNA damage and repair. Our analysis showed that the expression of the cytosolic DNA sensor ZBP1 is highly upregulated in human B cell chronic lymphocytic leukemia, one of the most common types of lymphoma in Singapore and the United States. To investigate the role of Zbp1 in tumorigenesis, we crossed Zbp1- List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake deficient mice to Eμ-Myc mice, a model for non-Hodgkin's B cell lymphoma. Strikingly, Zbp1-deficient Eμ-Myc mice showed an increased survival and a lower B cell tumor load. Moreover, around one in five Zbp1-deficient Eμ-Myc mice showed no signs of lymphoma at death while all Zbp1+/+Eμ-Myc mice died of lymphoma. Preliminary data indicate that tumor cells in Zbp1-deficient Eμ-Myc mice accumulate higher levels of DNA damage, upregulate the DNA damage response and are more prone to apoptosis. Co-immunoprecipitation experiments suggest that ZBP1 interacts with DNA repair proteins and nuclear RNA export proteins. Our hypothesis is that ZBP1 prevents the accumulation of damaged nuclear DNA and contributes to the transport of DNA to the cytosol for recognition by innate immune sensors. We plan to 1) study the role of Zbp1 in the tumorigenesis of non-Hodgkin's B cell lymphoma, 2) characterize the role of Zbp1 in DNA repair and DNA damage 3) identify and characterize proteins and DNA that interact with ZBP1. In summary, our proposal aims to show that the DNA sensor ZBP1 plays a critical role in tumorigenesis. A/Prof Tan Yee Joo Understanding cell death regulation in hepatitis C virus infection PI’s email id [email protected] Hepatitis C virus (HCV), a positive-stranded RNA virus of the family Flaviviridae, infects an estimated 3% of people worldwide and is one of the major causes of liver diseases. The HCV genome encodes a precursor polyprotein of ~3,000 amino acids that is processed to give rise to three structural (core, E1 and E2) and seven non-structural (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) viral proteins. Unlike the approximately 24,000 genes in the human genome, the number of HCV proteins is very limited. Thus, HCV interacts extensively with the cellular machineries in the human host and uses them to its advantage. Apoptosis and other forms of cell death have been observed in hepatitis C virus (HCV) infection in vitro and in vivo but the detailed understanding of this intricate viral-host interplay is still lacking. Massive apoptosis in the liver leads to acute liver failure, while the dysregulation of cell death pathways is a major factor in the development of hepatocellular carcinoma (HCC). Apoptosis of hepatocytes during HCV infection has been proposed to be a direct cause of liver fibrosis, which can further lead to cirrhosis and liver failure. Hence, it is important to understand the cellular mechanisms underlying HCV-mediated apoptosis as well as other cell death pathways. Our hypothesis is that HCV proteins are engaged in a network of interaction with a group of interconnected host cell death regulating proteins to yield a tightly regulated system that ensures optimal HCV replication and survival in the host cell. In this project, we will use transcriptomic analysis as well as siRNA library screening to identify host factors that are regulated by HCV infection and/or interact with the HCV viral proteins. The functional significance of novel viral-host interactions identified will be analyzed by using cell culture as well as mouse models of infection. Office Address Dept of Microbiology, MD4, Level 3, 5 Science Drive 2, Singapore 117597 Telephone Number 6516 3541 Generating antiviral neutralizing antibodies and characterizing their mechanism of inhibition Seasonal influenza A virus causes significant morbidity and mortality yearly while newly emerged strains continue to pose pandemic threats. Current strategies against influenza include vaccination and antiviral drug treatment. However, predicting the major strain that may cause the next pandemic is the main obstacle in current vaccine development. Moreover, some viruses have acquired resistance to approved antiviral drugs. Passive immunotherapy is now increasingly being used to treat human infectious diseases and there is a List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake demand for the development of neutralizing mAbs for passive immunotherapy in the event of a highly pathogenic flu pandemic as this could be particularly useful for protecting certain groups of people, such as immuno-compromised patients or the elderly, who may not respond well to vaccines. As the hemagglutinin (HA) protein mediates viral entry, it has been the main target for the preclinical studies on antibody-based immunotherapy and these studies suggest that it may be a viable option to administer neutralizing HA mAbs as a form of passive immunotherapy for influenza A infection. However, viral escape mutants were observed when anti-HA mAbs were used individually. Combination therapy, where multiple steps in the virus life cycle are inhibited simultaneously, is highly recommended to minimize the development of escape viruses. Hence, this study uses a multi-disciplinary approach to determine if other viral proteins of influenza A virus can stimulate neutralizing antibodies that prevent viral infection and/or replication. If we can develop combination therapy by using a mixture of mAbs that bind to different components of the virus, this will minimize the chance of drug resistant virus developing and this will help in the establishment of a novel class of antiviral therapeutic drugs. Department of Obstetrics & Gynaecology Prof Ganesan Adaikan PI’s email id [email protected] Office Address Department of OBGYN, NUHS Tower Block Level 12, 1E Kent Ridge Road, Singapore 119228 Telephone Number 6775 9240/9049 8636 Endothelial progenitor cells as a novel therapeutic option in vasculogenic erectile dysfunction Erectile dysfunction (ED) is an increasingly common disease, afflicting both young and old men. The problem is exacerbated by aging population, as well as changes in diet and lifestyle. Metabolic diseases, a major risk factor in ED, are also on the rise. Pathophysiological studies demonstrate that ED in these patients stem from compromised vascular function leading to ischaemic cavernosa, and resultant loss of erectile function. Consequently, existing palliative drugs, such as sildenafil, that are unable to restore vascular function are also ineffective for reversing or curing ischaemic ED found in patients with metabolic disease. Based on our interesting pilot study, it was hypothesised that the injection of endothelial progenitor cells (EPC) are expected to facilitate the formation of de novo vasculature in the ischaemic penile tissue in animal models of ED and improve vascular networks and restore erectile function through increased perfusion, as well as re-innervation. The project involves validation of penile ischemia and vasculogenic erectile dysfunction (ED) in rabbit and rodent models of metabolic diseases: (1) characterization of sub-populations of EPC derived from umbilical cord blood to stimulate angiogenesis and support nervous networks. (2) evaluation of EPC therapy in reversing endothelial and erectile dysfunction in animal models of vasculogenic ED. Department of Orthopaedic Surgery Dr Angelo H All Induced Human Oligodendrocyte Precursor Cell Improved Functionality after Spinal Cord Injury Transplants for List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake PI’s email id [email protected] Office Address NUHS - Dep. of Orthopedic Surgery, 1E Kent Ridge Road, NUHS Tower Block, Level 11 and NUS – SINAPSE Institute: 28 Medical Drive, CeLS Building, 5th floor Telephone Number 6772 3322 (NUHS) 6601 3198 (NUS) We propose to directly convert adult human fibroblasts into "induced human oligodendrocyte precursor” cells and to study the remyelination capability of these cells both in vitro and in vivo. We will generate patientspecific cells in a shorter time frame than iPS cells as direct conversion does not require reprogramming to base cells. These newly generated cells would also have significantly reduced risk of immune rejection even when compared to properly differentiated OPs from currently available human iPS cell lines. The goal of this project is to study whether these cells remyelinate demyelinated axons in the hostile postinjury spinal cord micro-environment in rats. We will also test the cells’ myelin production capabilities in a micro-chamber in vitro. Hypothermia after Spinal Cord Injury: Early Markers of Recovery Spinal cord injury (SCI) is a devastating condition that can lead to paralysis of the limbs below the injury level. Beyond the initial trauma of injury, the secondary phase of injury comprises inflammation, demyelination and apoptosis, which are all major pathological factors that exacerbates the progression of injury. Since mitigating this secondary phase of injury greatly improves patient outcomes, it has motivated the search for an early neuroprotective therapy. In animal models, primary focus has been on the effects of post-SCI hypothermia on motor behavioral and histological outcomes with only a few studies of the electrophysiological function of descending spinal cord pathways during cooling. Thus, there is a critical need for establishing the full benefits of hypothermic neuroprotection after SCI. Unlike other studies, our unique focus is on the hypothermia-induced enhancements of the afferent sensory conduction, a vital function of the sensory-motor system, as assessed by multi-limb somatosensory evoked potentials. The cooling will be followed by a single recording session to acquire local field potentials (LFPs) and multi-unit activity (MUA) from the dorsal pathways in the vicinity of the injury, followed later by somatosensory evoked potentials (SSEPs), which are cortical response waveforms drawn out by peripheral stimulation. The acute microelectrode recordings will be used to develop an early statistical marker to identify animals with maximal long-term recovery of SSEPs. The standard motor behavioral scoring (BBB) and histopathology will be used to provide complementary measures of recovery. The goal of this project is to use the contusive rat model of SCI, followed by an early, local cooling of the injured region, to optimize a hypothermia treatment process that can adequately mitigate the pathological factors in the second phase of injury. Transplantation of Conjugated Nanoparticles to Limit Spinal Cord Injury We propose to synthesize and administer conjugated nanoparticles and study their capability to limit spinal cord injury (SCI) in a rat model. This treatment approach will enable us to spare more healthy axons and neuropathways within the spinal cord parenchyma and slow down progress of injury after trauma. This is important as SCI is a time-sensitive pathology with a very short window of treatment. The goal of this proposal is to generate a new generation of nanoparticles, which will be safe and effective in preventing progress of trauma in our in vivo model of spinal cord injury. Crosstalk among Neural Pathways after Incomplete Spinal Cord Injury List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Spinal Cord Injury (SCI) in the pediatric population is fairly rare but bears huge socioeconomic consequences. Patients with SCI before their adolescence have different mechanisms of injury and have a better neurological recovery potential for incomplete and mild to severe injuries when compared to adults. Although the SCI is usually diagnosed with the use of MRI, often pediatric SCI are without radiological anomaly. There is also no evidence for the use of neuroprotective approaches for the treatment of SCI in children. There is the need for a better determination of the mechanism of SCI at the epicenter of injury. An understanding of the mechanisms that help promote the improved neurological recovery observed in pediatric patients would also enhance future therapies for all SCI patients. This project deals with incomplete SCI and the study of post-contusion electrophysiological changes in the vicinity of the injury for pediatric demographics. The goal of this proposal is to characterize functional reorganization of neuropathways on the neuronal reconnection formation at the epicenter of contusion induced SCI. Behavioral Assessment after Spinal Cord Injury Spinal Cord Injury (SCI) is a serious condition that severely impairs mobility and quality of life with no treatment options. After injury, some axons are anatomically continued but not functional. These neurons are electrically excitable cells and they are the focus of our treatment in the acute phase. One approach to treat SCI is through limiting and preventing the destruction of healthy neurons in the spinal cord parenchyma, thus aiding functional recovery. A set of motor behavioral assessments, such as BBB open field locomotion test, thermal sensation, rotorod, tactile allodynia and balance beam will be proposed to thoroughly investigate the onset of injury as well as longterm progress of injury. We will study phenotypic outcomes and the possible limitations during the progress of injury. These assessments will be statistically analyzed and compared with the histological examination as well as our previous electrophysiological assessments. Prof Lee Eng Hin PI’s email id [email protected] Office address NUHS Tower Block, Level 11, 1E Kent Ridge Road, Singapore 119288 Telephone Number 6516 6576 Effect of substrate patterning on the chondrogenic differentiation of mesenchymal stem cells. The main focus of our lab is the optimization of chondrogenesis towards a normal cartilage phenotype for cartilage tissue engineering. Mesenchymal stem cell (MSC) differentiation is influenced by its microenvironment. Manipulation of extracellular biophysical and/or biochemical microenvironment of stem cell can improve the efficiency of tissue engineering approaches using MSCs. We have identified specific biochemical and topographical cues for the directed differentiation of MSCs to specific cartilage phenotype. However, the mechanisms induced by these microenvironmental cues have not yet been fully understood. The project will investigate the effect of incorporating specific biochemical and/or physical cues in the substrate to achieve the desired lineage and phenotype specification of the differentiated cells. The study will aim to elucidate the mechanotransduction mechanisms that affect specific functional outcomes, including the optimisation of the manipulation conditions, and validation of the approach in in vivo animal model. Primary stem cell culture techniques and molecular biology analysis will be employed in these studies, including immunohistochemistry, confocal microscopy, scanning electron microscopy, real-time PCR and Western Blot. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake References: Wu Y, Law JBK, He AY, Low HY, Hui JHP, Lim CT, Yang Z, Lee EH. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine: NBM 2014 (in press). Raghothaman D, Leong MF, Lim TC, Toh JKC, Wan ACA, Yang Z, Lee EH. Engineering cell matrix interactions in assembled polyelectrolyte fiber hydrogels for mesenchymal stem cell chondrogenesis. Biomaterials 2014 Mar;35(9):2607-16. Department of Otolaryngology Details of PI Project Title with a brief description A/Prof Wang De Yun Quantitative assessment of the virulence of rhinoviruses, influenza viruses and respiratory syncytial viruses and host defense mechanisms of human nasal epithelium in vitro PI’s email id [email protected] Office Address NUHS Tower Block, Level 7, Department of Otolaryngology Telephone Number 6772 5373 The viral upper respiratory infections (URIs), including common cold, influenza and acute rhinosinusitis, are very common disease, affecting millions of people annually. They can be caused by any of the more than 200 different strains of viruses, such as rhinovirus, coronavirus, respiratory syncytial viruses (RSV), influenza, parainfluenza and adenoviruses. With the SARS pandemic of 2003 and that of H1N1 in 2009, global concerns was raised, stemming from virulent new strains of viruses such as corona virus (or SARS virus), H1N1, H5N1, which appear to be more promiscuous and circulate in several species and are endemic in humans, birds and pigs. In addition to continuous efforts in surveillance of naturally occurring viruses in humans and domestic animal hosts, experimental studies elucidating susceptibilities to respiratory viruses and response to infection by human nasal epithelium, which is the primary target site for common cold and influenza viruses, are required. In this study, we aim to develop an in vitro model for screening and quantitative assessment of the virulence of common cold and influenza viruses, and the host defense mechanisms of human nasal epithelium using our newly developed in vitro experimental models of human nasal epithelial stem/progenitor cells (hNESPCs) and differentiated epithelial cells derived from hNESPCs in an airliquid interface (ALI) culture. In addition, we will investigate the host defense functions including viral fusion and uncoating, transport of viral ribonucleoprotein (RNP) complexes from nucleus, replication, transcription and translation of the viral genome, export of viral RNP complexes from the nucleus, viral assembly and budding, and potential drug targets, which will provide us with a useful reference for public health risk assessment, interventions, disease control and prognosis for viral upper respiratory infections. This information is particularly useful in preparation for eventual pandemics or outbreak of viral infection. Department of Paediatrics Details of PI Project Title with a brief description List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake A/Prof Heng Chew Kiat PI’s email id [email protected] Office Address Department of Paediatrics, NUHS Tower Block, Level 12, 1E Kent Ridge Road, Singapore 119228 Telephone Number 6772 3354 Investigation of a newly identified protein, androgen-dependent tissue factor pathway inhibitor regulating protein (ADTRP), for its role in coronary artery disease Recently, a GWAS in the Chinese population has identified rs6903956 within the C6orf105 gene (now known as androgen-dependent tissue factor pathway inhibitor “TFPI” regulating protein or ADTRP) on chromosome 6p24.1 as a novel susceptibility locus for CAD. ADTRP is a protein encoded by C6orf105 that regulates TFPI expression. TFPI is the major inhibitor of tissue factor-factor VIIa–dependent FXa generation. Hence, ADTRP can be considered a protective factor of CAD. As it is a newly discovered protein, very little is known about its characteristics. This provides ample scope for investigation. Co supervisor: The specific aims of this study are: Prof Yechiel Friedlander Email: [email protected] Please contact A/Prof Heng Chew Kiat if you are interested in this project 1. 2. 3. 4. To determine which of the two known isoforms of ADTRP is the predominant form through the use of Human Coronary Artery Endothelial Cell (HCAEC) line. To determine the changes in ADTRP in response to androgen in HCAEC. To select meaningful single nucleotide polymorphisms for genotyping to determine if any are significantly associated with CAD in the Singaporean population. To conduct genetic variant screening in ADTRP gene The methods employed in this study include cell culture, PCR, qRT-PCR, genotyping, bioinformatics and statistical analyses. Identification of rare variants with strong effects on early onset myocardial infarction There is an increasing trend of early onset of myocardial infarction (MI) in patients who are below 40 years. A proportion of these do not have strong conventional risk factors such as smoking, hypertension and diabetes. In fact some of the patients with early onset of MI lead physically active lives and do not appear to have any conventional risk factors except for being male. It is therefore of interest to investigate the genetic factors underlying this early onset phenotype. Genome-wide association studies (GWAS) have thus far identified genetic variants that may predispose individuals to MI. However, these are mostly variants that are common in the population and are hence unlikely to account for the small subset of patients who had MI at a young age. We aim to carry out exome sequencing using next generation sequencing (NGS) technology for MI patients <40 years old) who have been identified from the National University Heart Centre in NUH. The number of variants identified from these patients is expected to be very large. We will employ filtering strategies to obtain meaningful number of target genes that we can conduct experiments to verify their functions. We believe be insightful information could be gleaned from such an approach. A/Prof Lee Yung Seng PI’s email id [email protected] Prenatal, perinatal, and postnatal determinants of adiposity and metabolic phenotype in childhood This research is part of the birth cohort study “Growing Up in Singapore Towards healthy Outcome” (GUSTO) of the TCR flagship programme List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Office Address Pediatrics, NUHS Tower Block, Level 12, 1E Kent Ridge Road, S(119228) Telephone Number 6772 4112 “Developmental Origins: Singapore” (DevOS). Prenatal, perinatal, and possibly early postnatal factors determine the subsequent growth of the child. This project aims to uncover the factors which determine the size and body composition of the newborns, and predictors of the growth of young children in the first few years of life, including maternal factors (diet and nutrition, habitus, pregnancy complications), birth events, and the baby’s growth pattern in the first few months’ of life. Specifically the candidate will determine the factors which predict catch up growth in the group of children with lower birth weights, as well as those with growth failure. The student will get opportunities to study epigenetic changes of candidate genes which predict the subsequent growth pattern of the children, as well as predictors of failed catch up growth. Determining body composition of infants and young children This research is part of the birth cohort study “Growing Up in Singapore Towards healthy Outcome” (GUSTO) of the TCR flagship programme “Developmental Origins: Singapore” (DevOS). The candidate will study the various modalities of body composition assessment of infants and young children, including MRI of abdominal fat compartments and whole body fat, bioelectrical impedance, and air displacement plethysmography. There will also be opportunity to study correlation of the body composition parameters with developmental factors and epigenetic biomarkers. A/Prof Lynette Shek Pei-Chi PI’s email id [email protected] Office Address Department of Pediatrics NUHS Tower Block, Level 12, 1E Kent Ridge Road, Singapore 119228 Telephone Number 6772 4420 Upper airway nasal microbiota and its relationship with persistent rhinitis and wheezing in early childhood There is little data on the pattern of nasal microbiome colonization in early life, and its influence on disease development. A Singapore birth cohort GUSTO) is followed up for allergic disorders including rhinitis in early childhood. We have shown that the prevalence of rhinitis for at least 4 weeks is common (18.8%) at the age of 18 months, and is associated with wheeze and eczema. We aim to analyze the diversity and abundance of upper respiratory microbiota from archived nasal swabs taken at regular intervals in the first 18 months of life and correlate the microbiota data with the clinical outcomes of early onset rhinitis, and subsequent development of allergic rhinitis and wheeze/asthma at age of 5 years. Co supervisor: Prof Lee Bee Wah Email: [email protected] Prof Yap Hui Kim Role of angiomotin in the pathogenesis of membranous nephropathy PI’s email id [email protected] Idiopathic membranous nephropathy (IMN) is the commonest cause for nephrotic syndrome in adults, and half of the patients progress to renal failure. Despite its prevalence, the molecular mechanisms behind IMN are poorly understood. Current treatment options are therefore empirical and often ineffective. IMN is a result of immune complex deposition in the glomerular basement membrane. The triggers for immune complex formation are unknown. Office Address Pediatrics, NUHS Tower Block, Level 12, 1E Kent Ridge Road, S(119228) List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Telephone Number 6772 4411 In our preliminary study, we have identified a local Chinese family with Xlinked recessive membranous nephropathy, Fanconi syndrome and anti-tubular basement membrane (anti-TBM) antibodies. Using exome sequencing, we have found angiomotin (AMOT) as a novel candidate gene, with the mutation p.S50G in the p130-AMOT isoform which is critical in maintaining tight junctions between cells. In our preliminary work, we have demonstrated that AMOT is expressed in human podocytes and renal tubular cells, both of which depend highly on tight junctions for their cellular function. Our hypothesis is that the mutation p.S50G in the AMOT gene is functional and the anti-TBM antibodies present in the family are anti-AMOT antibodies. Our specific aims are to: 1) Study the expression of angiomotin and p130-AMOT isoform in human podocytes and renal tubular cell cultures, and in animal and human kidney tissues by immunostaining 2) Study the identity of anti-TBM antibodies present in the family with p.S50G AMOT mutation. 3) Elucidate the function of the angiomotin mutation p.S50G by its transfection into HEK293, human podocyte and renal tubular cell cultures, observing its effect on cell morphology, angiomotin subcellular distribution and its interactions with known and novel binding partners. 4) Introduce the p.S50G mutation in rats using Transcription activator-like effector nucleases (TALENs) and studying its phenotypic effects. Our study will improve the understanding of the mechanisms of IMN, which can result in more targeted therapy with more efficiency and less side effects. Role of Soluble Urokinase Plasminogen Activator Receptor (suPAR) in Pathogenicity of Focal Segmental Glomerulosclerosis (FSGS) Immune-mediated focal segmental glomerulosclerosis (FSGS) is the most common cause of acquired end-stage renal disease in children worldwide. Studies on the pathogenetic mechanism suggested a plausible role for induction of urokinase plasminogen activator receptor (uPAR) signaling with increase in circulating soluble uPAR. Several questions, however, remained unanswered: i) High suPAR levels are also found in non-proteinuric diseases (eg. sepsis); ii) Is the whole suPAR molecule or one of its subunits, the elusive proteinuric-inducing circulating factor; iii) Are immune cells the only possible source of suPAR in FSGS? Our preliminary studies demonstrated increased suPAR production in monocyte supernatant and injured podocytes. We therefore hypothesized that: 1. Secreted suPAR from podocytes promotes podocyte migration, contributing to elevated circulating suPAR levels in FSGS patients; 2. A specific isoform of suPAR may be responsible for podocye foot process effacement; 3. Filtration of suPAR into urine in the presence of podocyte damage may explain the discrepant suPAR levels observed in FSGS patients, and the elevated suPAR levels in the presence of decreased glomerular filtration rate. Thus, the primary aims are to: 1. Demonstrate suPAR production by injured podocytes and its role in promoting podocyte migration, 2. Identify the suPAR isoform responsible for foot process effacement, 3. Identify the upstream mediators leading to increased uPAR expression in podocytes and monocytes/immune cells. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake The secondary aim is to validate the above findings in both wild type and Plaur-/- (CD87-/-) mice via introduction of these upstream mediators, to determine if uPAR is increased on the mouse podocytes resulting in FSGS. The tertiary aim is to assess the clinical utility of suPAR (plasma+urine) as an early biomarker of podocyte injury. Understanding the role of suPAR and its isoforms in podocyte injury will facilitate the design of novel targeted therapies for this disease, as well as provide a potential biomarker. Department of Pharmacology Details of PI Project Title with a brief description Dr Alan Prem Kumar To Evaluate the Potential Utility of Annexin A1 as a Biomarker of Response to PPARγ Agonist, Efatutazone, in Breast, Colon and Lung Carcinoma PI’s email id [email protected] Office Address MD6, 14 Medical Drive, #11-01M Telephone Number 6516 5456 NUS-Daiichi Sankyo Material Transfer Agreement L146737 (NUS Ref. TL2013-077) Despite the evidence of PPARγ ligands as potential new anti-tumor therapeutic option, clinical trials with the use of PPARγ ligands for cancer treatment were not consistent. In addition, the glitazone family of drugs has shown toxicity in the clinic and has been either withdrawn or no longer prescribed to patients. CS-7017 (efatutazone), with an in vitro IC50 of 10nM, is the newest and potent PPARγ investigational drug on clinical trials. In patients, it’s given orally 0.50mg BID as determined from Phase I studies. Recently, Daiichi Sankyo, Inc (USA) has completed two Phase II studies on CS-7017 in advanced colorectal cancer. Our preliminary findings established identification of a novel biomarker, Annexin A1 (ANXA1) predictive of response to PPARγ ligands in vitro and in vivo. The objective of this study (a NUS-Daiichi Sankyo Project) is to further characterize the suitability of ANXA1 as a predictive and companion marker for CS-7017 therapy in breast, colon and lung cancers. Identification and Characterization of Novel Protein Tyrosine Kinase-6 Inhibitors for the Treatment of Breast Cancer RCA - NUS and GenoMed Inc, USA (NUS Ref. RL2013-007) Breast cancer is a diverse malignancy; understanding the molecular mechanisms driving tumor progression will facilitate development of targeted therapies. For example, tamoxifen, an anti-estrogen agent, used for treating estrogen receptor (ER)-positive breast tumors, and trastuzumab, a monoclonal antibody targeting HER2-overexpressing tumors, have benefitted specific subsets of breast cancer patients. However, the enormous variation in breast cancer initiation and growth complicate biological approaches to this malignancy and challenge the effectiveness of current therapies. Elucidation of novel, targetable signaling pathways in breast cancers would provide means of addressing current deficiencies in the treatment of the disease. We've found a SNP in the upstream region of the breast tumor kinase (Brk), also known as protein tyrosine kinase 6 (PTK6), about 5 kb from the gene's transcription start site, to be highly associated with ovarian (OR 10.36), breast (OR 5.93), and List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake prostate (OR 5.42) cancers, but not colon, lung, or pancreatic cancers (OR 1). Although PTK6 is undetectable in the normal mammary gland, it is overexpressed in more than 60% of human breast tumors and breast cancer cell lines, with the highest levels in advanced tumors. In multivariate analysis, the disease-free survival of patients of >240 months was directly associated with the protein expression level of PTK6 (P=0.001), but was also inversely associated with nodal status (P=0.001) and tumor size (P=0.01). However, to date there is no inhibitor available for PTK6. Using molecular docking, we have identified a series of novel PTK6 inhibitors. The project is to first test the inhibitory potential of these new drugs in breast cancer models, possibly extending to ovarian and prostate cancers. The project will then be extended to decipher the molecular mechanism/targets in vitro and in vivo by which PTK6 inhibition mediates its anticancer property and the underlying events that “switch on” expression of PTK6 in breast cancer. A/Prof Bian Jinsong PI’s email id [email protected] Office Address MD11, Room #05-30A Telephone Number 6516 5502 Activation of Na+/K+ ATPase (NKA) is a new strategy to treat ischemic heart diseases Na+/K+ ATPase (NKA) is responsible for maintaining the electrochemical gradient, and hence the membrane potential, of the cell membrane. The subunit of NKA is important in maintaining the function of α-subunit. In oxidative stress, glutathionylation of 1-subunit impairs this interaction and decreases NKA activity. In chronic heart injury, NKA loss contributes to the development of CHF. Our preliminary data showed disruption of this interaction may induce heart dysfunction and injury. In this project, we will express the soluble extracellular β ectodomains, which lack glutathionylation site but are still able to interact with and activate α1-NKA, and study the cardioprotective effects of these ectodomains. Inhibition of abnormal protein aggregation by hydrogen sulfide: a potential approach to treat neurodegenerative diseases Neurodegenerative disorders represent a major cause of disability and death, with an unmet need for therapies that alter disease progression. Hydrogen sulfide (H2S) has recently been hypothesized to be an important neuromodulator in the brain. Abnormal generation and metabolism of H2S may be actively involved in the pathogenesis of central nervous system (CNS) diseases. We recently reported that both endogenous and exogenous application of H2S produces therapeutic effects on Parkinson’s disease and Alzheimer’s disease. However, the molecular mechanisms are still unclear. We hypothesize that H2S may have the potentials to retard pathological process by prevention of abnormal protein aggregation. In this project, we will examine the effects of H2S on Tau phosphorylation, α-synuclein nitration and amyloid β aggregation. H2S-induced S-sulfhydration, autophagy preservation, anti-oxidative stress and mitochondrial protection will be studied. Dr Pieter Eichhorn Identification and roles of Dubiquitinating enzymes in the TGFB pathway PI’s email id [email protected] TGF-β is essential for embryogenesis and tissue homoeostasis in multicellular organisms. Furthermore, in advanced cancers TGF-β can act as an oncogenic factor and according to growing clinical evidence, TGF-β can be considered a therapeutic target in cancer. Ubiquitin modification of the TGF-β signaling pathway is emerging as a key mechanism of TGF-β pathway control. However, the role of deubiquitinating enzymes (DUBs), which mediate the Office address MD6 Level 11 CSI List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Telephone Number 6516 5475 removal and processing of ubiquitin, is less well understood. I am interested in studying and elucidating the roles of DUBs in the TGF-β pathway. Department of Physiology Details of PI Project Title with a brief description A/Prof Celestial TSM Yap To investigate the roles of cytoskeletal proteins in oncogenic signaling PI’s email id [email protected] Gelsolin (GSN) is an actin-associated cytoskeletal protein involved in binding and severing actin filaments, thus controlling cytoskeletal turnover in response to cellular signals (eg. migration, apoptosis). Gelsolin has also emerged as a new player in pathways regulating signaling and gene transcription. We previously uncovered a novel role of gelsolin in promoting invasion of colorectal tumour cells by enhancing extracellular matrix breakdown, through the upregulation of urokinase plasminogen activator (uPa). Office Address Department of Physiology, Block MD 9 Level 4, 2 Medical Drive, Singapore 117597 Telephone Number 6516 3294 We aim to ascertain if specific oncogenic signaling pathways interact with gelsolin to influence cancer cell behaviour. Our preliminary analysis of microarray data from human breast cancer tissues and in vitro work on cell lines showed a correlation between gelsolin expression with various oncogenic signaling pathway modulators, suggesting possible involvement of gelsolin in regulating oncogenic signaling. The specific signaling pathways identified to have potential interactions with gelsolin are known to induce aggressive tumour cell behavior, including invasion and epithelial-mesenchymal transformation. Gelsolin knockdown by siRNA decreases the expression of target genes controlled by oncogenic signaling, while gelsolin overexpression increases signaling activity. In addition, we found that induction of specific oncogenic signaling in breast cancer cells also increased the expression of gelsolin, suggesting that the activities of gelsolin may be recruited by certain oncogenic signaling pathways in a feed-forward loop to enhance their target effects. Collectively, these data suggest novel regulatory roles of gelsolin in signaling pathways that promote cancer progression. We will confirm the interactions between gelsolin and the specific signaling pathways identified by our laboratory, as well as examine the significance of these interactions on cancer cell behavior and clinical outcomes. A/Prof Herbert Schwarz How does the cytokine receptor CD137 contribute to the pathogenesis of Hodgkin lymphoma? PI’s email id [email protected] Office Address CeLS, level 3, Immunology Programme Telephone Number 6516 7773 Hodgkin’s lymphoma (HL) is a cancer of the lymphatic system, and one of the most common cancers among the young adults. No effective therapies exist for HL. HL is characterized by an extensive tumor stroma which is essential for the persistence and pathogenesis of HL. This tumor stroma is induced by the malignant cells in HL, the Hodgkin Reed-Sternberg (HRS) cells, which are a small minority among the cells of the tumor stroma that consists mainly of infiltrating leukocytes. It is largely unknown by which mechanisms HRS cells cause HL. Recently we found that the tumor necrosis factor receptor family member CD137 is ectopically expressed by HRS cells, but not by corresponding healthy cells (Cancer Research. 73(2):652-61, 2013). We have identified several changes induced by CD137 in HRS cells that may cause or enhance List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake the malignancy of HL. These chances involve the recruitment of leukocytes that form the tumor stroma, the secretion of growth factors for HRS cells, and escape from immune surveillance. The project will characterize the contribution of CD137 to the pathogenesis of HL, and aim at identifying novel immunotherpeutic approaches for therapy. Dr Lee Chi Wai PI’s email id [email protected] Office Address Block MD9, 2 Medical Drive, Singapore 117597 Telephone Number 65165955 Cellular and molecular mechanisms underlying the neuronal development and diseases Synapses are specialized cell membrane domains that facilitate neuronal communication in the intricate nervous system. These synaptic specializations develop in response to molecular interactions between pre- and postsynaptic cells. A major goal of current research in developmental neuroscience is to elucidate the mechanisms underlying how synapses are assembled. The nervemuscle synapse, neuromuscular junction (NMJ), which controls all muscle movements, has been considered as the best model for the study of synaptogenesis due to its large size, simplicity and accessibility. When neurons and muscle cells are cultured together, functional NMJs are formed spontaneously. The structure and physiology of mature vertebrate NMJs are well understood. Currently, our laboratory specifically focuses on the signal transduction and cytoskeletal mechanisms underlying synapse development, disease, and regeneration. Three major areas are being pursued in our lab: (1) postsynaptic receptor trafficking in the pathogenesis of muscular dystrophy; (2) cytoskeletal dynamics in neuronal growth cones during axonal outgrowth and pathfinding; (3) axonal trafficking of mitochondria in synaptic formation, function, and elimination. Using the simple and elegant Xenopus primary culture system, a variety of techniques, including live-cell time-lapse fluorescence microscopy, superresolution microscopy, molecular biology, immunocytochemistry, and Western blotting will be applied to these experimental systems to gain understanding to the cellular and molecular mechanism of synaptic development. Our goal is to not only gain a mechanistic understanding of the molecular and cellular aspects of neuronal structure and function, but also provide insights into the cellular basis for neurological disorders A/Prof Lina Lim Hsiu Kim PI’s email id [email protected] Office Address Centre for Life Sciences, 28 Medical Drive, S117456 Telephone Number 6516 5515 Control of PPARγ and NFκB by Annexin-A1 in inflammatory macrophages and breast cancer Inflammation occurs through dynamically varying levels of pro- and antiinflammatory cytokines competing for an upper hand either by activation of signalling cascades or inhibition of downstream signals. NFKB is a master transcription factor involved in the transcription of a number of proinflammatory as well as anti-inflammatory genes which could control inflammation and resolution of inflammation. Natural NF-κB inhibitors exist that would assist to regulate the production of cytokines during inflammation. One such natural inhibitor of NF-κB is PPAR-γ, which has been shown to be anti-inflammatory in a number of models of inflammation. In the following investigation, we will define the regulation of PPAR-γ by another anti- List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake inflammatory molecule we have been working on for more than 10 years, Annexin-1. This project will focus on the regulation of PPAR-γ by ANXA1 in the context of inflammatory macrophage function and cancer and will answer important questions related to PPAR-γ agonist use clinically in the treatment of inflammatory diseases. Annexin-A1: a host factor modulating influenza virus replication The influenza virus infects millions of people each year and can result in severe or even fatal complications. Understanding host responses to influenza infection will enable the development of more effective anti-viral therapies. The host immune system recognizes viral RNA via specific receptors and intracellular sensors that directly activate anti-viral immune responses. Previous research has revealed diverse yet important roles for Annexin family proteins in modulating the course of influenza infection. However, the role of Annexin-A1 (ANXA1) in influenza virus infection has not been addressed. ANXA1 is increased in nasal swab samples obtained from influenza infected patients. In addition, presence of ANXA1 increases virus replication and results in more weight loss after virus infection. We therefore hypothesize that ANXA1 may play a critical role in host anti-viral responses. These data will enhance our understanding of disease pathogenesis and may lead to the identification of novel targets for anti-viral immunotherapies. Dr Ling Shuo-Chien PI’s email id [email protected] Office Address MD6, #10-01R, Centre for Translational Medicine (CeTM) Telephone Number 6601 3645 Mechanisms of Neuronal and Synaptic Dysfunctions in Ageing and Neurodegeneration Aging poses both a fascinatingly biological question and a growing medical problem as aging is the leading risk factor for age-associated diseases, many of which are late adult-onset neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Frontotemporal dementia (FTD), and Amyotrophic Lateral Sclerosis (ALS, also known as motor neuron disease). The current goal of my lab is to define and understand the processes underlying normal and pathological brain aging by elucidating the underlying molecular and cellular mechanisms and how perturbation of the at-risk systems cause premature failing that leads to neurodegeneration. The overall strategy is to use disease-related genes as molecular handles to probe the gene-phenotype relationship systematically in mice that are genetic mimics of human diseases during aging and neurodegeneration with top-down and bottom-up approaches. Specifically, we focus on two RNA-binding proteins, TDP-43 (TAR DNA-binding protein 43 KDa) and FUS/TLS (fused in sarcoma/translocated in liposarcoma), whose mutations are causal for ALS and FTD and inclusions of both proteins are the defining pathological hallmarks in the majority of ALS and FTD patients. On the top-down approach, we will interrogate molecular and synaptic changes in selective brain regions and in neuronal populations as well as the contribution of nonneuronal neighboring cells by combining mouse genetics with genome-wide quantitative methodologies. On the bottom-up approach, we will reconstruct an in vitro neural network by building a mechanically- and chemically-defined microfluidic system to analyze at a single cell resolution as well as to manipulate with temporal and spatial precision. By integrating neuroscience with leading edge technology, we expect that we will identify quantitative changes in gene expression that are critical for initiating and/or accelerating age-dependent neurodegeneration. List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake The long-term goal of our laboratory is to revert the molecular and cellular alternations to rescue neuronal damages and ultimately to slow down aging and subsequent neurodegeneration (300 words). A/Prof Reshma Taneja Identifying therapeutic targets for skeletal muscle disorders PI’s email id [email protected] De-regulation of epigenetic control is increasingly apparent in many human pathologies including cancer. Small molecule inhibitors to chromatin modifying proteins have shown great promise in preclinical trials validating druggability of epigenetic modulators. In muscle cells, proliferation and differentiation are mutually exclusive events that are regulated by chromatinassociated proteins. Among these, lysine methyltransferases that mediate methylation of histone and non-histone proteins play a central role in maintaining this equilibrium. Office Address MD9, Level 4 Telephone Number 6516 3236 We have recently shown that G9a, a lysine methyltransferase inhibits differentiation of skeletal muscle cells. G9 mediates histone H3 lysine 9 dimethylation (H3K9me2), a post-translational modification associated with transcriptional repression on myogenic promoters that are expressed during differentiation. Moreover, G9a also methylates MyoD, a key transcription factor that is essential for muscle differentiation. Using muscle cells from control and G9a conditional knockout mice, we will profile the G9a methylome. These studies allow us to obtain an integrated view by which G9a-mediated methylation of histone and non-histone proteins balance growth and differentiation of muscle cells. Our results will help design therapies for muscle disorders that are characterized by a differentiation defect. Prof Shazib Pervaiz Redox Regulation of protein phosphatase PP2A in carcinogenesis PI’s email id [email protected] Over the years, our work has highlighted the critical role of an altered redox metabolism on cell survival and death signaling in cancer cells. Using a variety of model systems such as drug-induced apoptosis, receptor mediated death signaling, and oncogene-induced cell survival, we demonstrated that the intracellular ratio between the two main reactive oxygen species (ROS), superoxide and hydrogen peroxide (O2-:H2O2), determines cancer cell response to death signals; a tilt in favor of superoxide promotes cell survival whereas an increase in hydrogen peroxide favors death execution via activation of the death promoting protein Bax. Of note, we have highlighted a novel biological activity of Bcl-2 by providing experimental evidence linking Bcl-2-induced increase in mitochondrial superoxide levels to the anti-apoptotic activity of Bcl-2. Interestingly, an elevated O2-:H2O2 ratio induced by either pharmacological inhibition (DDC) or gene knockdown of Cu/Zn SOD resulted in an increase in phosphorylation of Bcl-2, specifically at Ser70 (S70), and this site specific phosphorylation of Bcl-2 enhanced the anti-apoptotic activity of Bcl-2, thereby rendering cancer cells resistant to chemotherapy-induced apoptosis. To that end, we have uncovered a novel mechanism in which an increase in intracellular O2- endows cancer cells with a survival advantage via tyrosine nitration-mediated detachment of B56δ from PP2A catalytic core, and the eventual accumulation of S70 phosphorylated Bcl-2 with potent anti-apoptotic activity. The mechanism of redox modulation of PP2A in the context of carcinogenesis is under Office Address 2 Medical Drive, Building MD9, Level 4 Telephone Number 6516 6602 List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake investigation. Specific targeting of mutant K-Ras expressing cancers via Aktdependent ROS production This project involves investigating the molecualr mechanism(s) underlying the activity of a novel small molecule compound against mutant Kras expressing human cancers. Identification of a novel protein TMTC2 in death receptor sensitization of human cancers Our preliminary studies indicate that ligation of the death receptors DR4 and DR5 (TRAIL R1 and TRAIL R2) upregulates the expression of a novel transmembrane protein TMTC2. Upregulation of TMTC2 increases death receptor sensitivity while its gene knockdown inhibited TRAIL-mediated cell death in human nasopharyngeal carcinoma cell lines. The sub-cellular localization of TMTC2 appears to place it at the ER, however this has not been validated in more than one cell lines. Notably, TMTC2 induction appears to be under the influence of intracellular reactive nitrogen species (RNS). The mechanism of induction, protein structure and localization as well as the death sensitizing activity of TMTC2 is under study. Identification of surrogate death signaling pathways in cisplatinresistant human cancer cells Platinum-based compounds are the main line of treatment for a number of clinical cancers. However, development of drug resistance remains a therapeutic challenge. We have generated cisplatin-resistant clones from human lung and ovarian carcinoma cell lines. These cells become resistant to most chemotherapeutic drugs, thus suggesting a MDR phenotype. Although, the mechanism of this drug resistance is not clearly understood, we have made a remarkable observation during the course of these studies, i.e that cells that become resistant to cisplatin become highly sensitive to death receptor-mediated apoptosis. The mechanism of activation of this surrogate death signaling in the context of cisplatin resistance is under investigation Mechanism of statin-induced apoptosis in cancer cells We recently demonstrated that the cholesterol lowering drug, simvastatin, induces apoptosis in human colorectal and breast carcinoma cells. We identified a critical role for intracellular ROS and downstream JNK activation in statin-induced apoptosis. Interestingly, we showed a massive induction and activation of Rho family of proteins, Rac1, Rho and cdc42 in statin treated cells, upstream of ROS production. Of note, the Rho family of proteins is prenylated despite their localization in the cytoplasm as opposed to the plasma membrane. The mechanism of this non-membrane dependent prenylation as well as ROS production in this model is under investigation. Dr Thai Tran PI’s email id [email protected] Effects of anti-malarial drug, Artesunate, in steroid-resistant model of asthma Airway smooth muscle (ASM) cell hyperplasia contributes to airway wall remodeling (AWR) in asthma. Glucocorticoids, which are used as first-line List of Potential Supervisors and Projects available for Laboratory Rotation – January 2015 intake Office Address MD9, Department of Physiology Telephone Number 6516 3663 therapy for the treatment of inflammation in asthma, have limited impact on AWR and protracted usage of high doses of glucocorticoids are associated with an increased risk of side effects. Moreover, patients with the severe asthma often show reduced sensitivity to glucocorticoids/steroids. Artesunate, a semi-synthetic artemisinin derivative used to treat malaria with minimal toxicity, attenuates allergic airway inflammation in mice but its impact on AWR in patients with asthma and especially in those that are resistant to current steroid treatment is not known. a) Steroid-Resistant Airway Hyperresponsiveness In Vivo Mouse Models To develop steroid-resistant airway hyperreponsiveness, mice will be given intratracheal administration of 40 ml IFN-g (1.5 mg) + LPS (50 ng). Dexamethasone (1 mg/kg) and/or test compound will be given intraperitoneally for 3 consecutive days, commencing 3 d before IFN-g/LPS treatment. Airway hyperreponsiveness will be measured 12 h after the combined cytokine treatment. At the same time point, BAL fluid and lung tissues will be collected for analysis. For each complete set of experiment, mice will be divided into 6 treatment groups: (1) PBS control, (2) IFN-g/LPS treatment (steroid-resistant), (3) IFN-g/LPS + DMSO vehicle control, (4) IFNg/LPS + drug-optimum dose (test compound), (5) IFN-g/LPS + dexamethasone (1 mg/kg), and (6) IFN-g/LPS + dexamethasone 1 mg/kg plus test compound (restore steroid sensitivity). Optimum drug dose will be determined in preliminary studies. (b) Steroid-Resistant Alveolar Macrophage In Vitro Model Mouse alveolar macrophages will be isolated and stimulated with PBS, IFN-g (1.5 mg/ml), LPS (50 ng/ml), or IFN-g (1.5 mg/ml) + LPS (50 ng/ml) for 24 h and followed by treatment with vehicle, dexamethasone (1 mM), test compound (10 mM), or dexamethasone (1 mM) + test compound (10 mM for restoration of steroid sensitivity) for another 24 h. Supernatants and cell lysates will be collected for analysis. To detect impairment of glucocorticoid receptor (GR) translocation by IFN-g/LPS, and restoration of GR translocation by test compound, macrophages will be fixed, permeabilized, probed with polyclonal antibody to GR and stained with Cy3-conjugated secondary antibody. The nucleus will be stained with DAPI. GR translocation in macrophages will be visualized using a fluorescence microscope.
© Copyright 2026