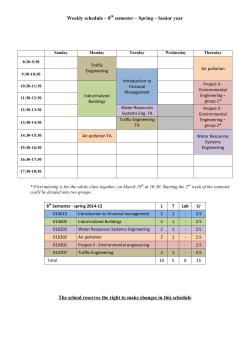
Faculty of Chemical Technology and Engineering 2015/2016
FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING COURSES OFFER ACADEMIC YEAR 2015/2016 Course title Person responsible for the course CH_1A_S_C06 BIOCHEMISTRY Elwira K. Wróblewska, PhD CH_1A_S_CO2 FUNDAMENTALS OF CRYSTALLOGRAPHY AND DIFFRACTION METHODS Piotr Tabero, PhD,DSc INSTRUMENTAL ANALYSIS Monika Gąsiorowska, PhD METHODS OF ORGANIC COMPOUNDS IDENTIFICATION Jacek A. Soroka, professor SPECTROSCOPIC METHODS Marta Sawicka, PhD Course code (if applicable) Semester (winter/ summer) ECTS points CHEMISTRY CH_1A_S_D01_ 15 winter/ summer 4 winter 2 winter/ summer winter/ summer winter/ summer 4 3 4 CHEMICAL ENGINEERING WTICH_ICHP_1A _S_1 ADSORPTION ENGINEERING Bogdan Ambrożek, PhD,DSc WTICH_ICHP_2A _S_2 AGITATION AND AGITATED VESSELS Joanna Karcz, professor AN INTRODUCTION TO NUMERICAL ANALYSIS WITH PROCESS ENGINEERING APPLICATIONS USING MATHCAD AND MATLAB APPLIED MATHEMATICS AND MODELING FOR CHEMICAL ENGINEERS APPLIED PETROLEUM RESERVOIR ENGINEERING BASIC PRINCIPLES AND CALCULATIONS IN CHEMICAL ENGINEERING BIOENVIRONMENTAL HEAT AND MASS TRANSFER WTICH_ICHP_1A _S_3 WTICH_ICHP_1A _S_4 WTICH_ICHP_1A _S_5 WTICH_ICHP_1A _S_6 4 4 Józef Nastaj, professor Konrad Witkiewicz, PhD winter or summer 4 Józef Nastaj, professor Konrad Witkiewicz, PhD winter or summer 4 Józef Nastaj, professor winter or summer 4 Józef Nastaj, professor Konrad Witkiewicz, PhD winter or summer 4 Józef Nastaj, professor BIOPROCESS ENGINEERING Joanna Karcz, professor CHEMICAL AND MOLECULR THERMODYNAMICS CHEMICAL AND PROCESS THERMODYNAMICS Józef Nastaj, professor Konrad Witkiewicz, PhD Józef Nastaj, professor Konrad Witkiewicz, PhD CHEMICAL ENGINEERING DESIGN Bogdan Ambrożek, PhD,DSc CHEMICAL ENGINEERING FUNDAMENTALS CHEMICAL ENGINEERING PROCESS SIMULATION USING ASPEN PLUS winter or summer winter or summer Joanna Karcz, professor Bogdan Ambrożek, PhD,DSc CHEMICAL PROCESS EQUIPMENT Bogdan Ambrożek, PhD,DSc CHEMICAL REACTION ENGINEERING Bogdan Ambrożek, PhD,DSc winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer 4 4 4 4 4 4 5 4 4 1 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ CHEMICAL REACTORS ENGINEERING Paulina Pianko-Oprych, PhD summer 5 COMPUTER AIDED PROBLEMS IN CHEMICAL ENGINEERING Józef Nastaj, professor Konrad Witkiewicz, PhD winter or summer 4 ENERGY AND ENVIRONMENT Paulina Pianko-Oprych, PhD summer 4 WTICH_ICHP_1A _S_7 ENVIRONMENTAL POLLUTION CONTROL Bogdan Ambrożek, PhD,DSc WTICH_ICHP_2A _S_8 FLUIZIDATION ENGINEERING Bogdan Ambrożek, PhD,DSc FUNDAMENTALS OF MATLAB IN CHEMICAL AND PROCESS ENGINEERING FUNDAMENTALS OF RESERVOIR FLUID BEHAVIOR AND ITS PROPERTIES 4 Józef Nastaj, professor winter or summer 4 Bogdan Ambrożek, PhD,DSc WTICH_ICHP_1A _S_10 HETEROGENEOUS CATALYSIS Bogdan Ambrożek, PhD,DSc HYBRID SOURCES OF ENERGY Paulina Pianko-Oprych, PhD WTICH_ICHP_1A _S_12 WTICH_ICHP_1A _S_13 WTICH_ICHP_1A _S_14 MASS TRANSFER MATHEMATICAL METHODS IN CHEMICAL ENGINEERING MODELING AND SIMULATION IN CHEMICAL ENGINEERING MODERN DRYING TECHNIQUES – THEORY AND PRACTICE Józef Nastaj, professor Henryk Łącki, PhD Bogdan Ambrożek, PhD,DSc Józef Nastaj, professor Bogdan Ambrożek, PhD,DSc Bogdan Ambrożek, PhD,DSc Bogdan Ambrożek, PhD,DSc Józef Nastaj, professor Konrad Witkiewicz, PhD WTICH_ICHP_2A _S_15 MULTIPHASE FLOWS Joanna Karcz, professor WTICH_ICHP_1A _S_16 NATURAL GAS ENGINEERING Bogdan Ambrożek, PhD,DSc NUMERICAL AND ANALYTICAL METHODS WTH MATLAB Józef Nastaj, professor Konrad Witkiewicz, PhD NUMERICAL METHODS Bogdan Ambrożek, PhD,DSc WTICH_ICHP_1A _S_17 WTICH_ICHP_2A _S_18 WTICH_ICHP_1A _S_19 WTICH_ICHP_1A _S_20 NUMERICAL METHODS IN CHEMICAL ENGINEERING OPTIMIZATION IN CHEMICAL ENGINEERING 4 winter or summer HEAT TRANSFER WTICH_ICHP_1A _S_11 5 Józef Nastaj, professor Konrad Witkiewicz, PhD WTICH_ICHP_1A _S_9 HYDROGEN AS A FUTURE ENERGY CARRIER INTRODUCTION TO CHEMICAL ENGINEERING INTRODUCTION TO THERMODYNAMICS OF IRREVERSIBLE PROCESSES winter or summer winter or summer Bogdan Ambrożek, PhD,DSc Bogdan Ambrożek, PhD,DSc PARTICULATE TECHNOLOGY Bogdan Ambrożek, PhD,DSc PETROLEUM PRODUCTION SYSTEMS Józef Nastaj, professor POLYMATH, MATHAD AND MATLAB FOR CHEMICAL ENGINEERS Józef Nastaj, professor Konrad Witkiewicz, PhD PROCESS DESIGN Paulina Pianko-Oprych, PhD PROCESS DYNAMICS AND CONTROL Bogdan Ambrożek, PhD,DSc PROCESS KINETICS Józef Nastaj, professor Konrad Witkiewicz, PhD QUALITY ENGINEERING Jolanta Szoplik, PhD SEPARATION PROCESSES Bogdan Ambrożek, PhD,DSc winter or summer winter or summer summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer winter or summer summer winter or summer winter or summer winter or summer winter or summer 4 5 2 4 4 4 4 5 5 4 4 4 4 4 4 4 4 4 4 9 4 4 2 5 2 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ SIMULATION OF CHEMICAL ENGINEERING PROCESSES USING MATHAD AND MATLAB Józef Nastaj, professor Konrad Witkiewicz, PhD winter or summer 4 SPECIAL METHODS OF SEPARATION Anna Kiełbus-Rąpała, PhD winter or summer 2 TECHNICAL THERMODYNAMICS Paulina Pianko-Oprych, PhD summer 3 THE PREDICTION OF PROPERTIES OF GASES AND LIQUIDS THERMODYNAMICS WITH CHEMICAL ENGINEERING APPLICATIONS TRANSPORT AND DISTRIBUTION OF NATURAL GAS Józef Nastaj, professor Konrad Witkiewicz, PhD Józef Nastaj, professor Konrad Witkiewicz, PhD TRANSPORT PHENOMENA Bogdan Ambrożek, PhD,DSc winter or summer winter or summer winter or summer winter or summer WTiICh/IISt/OSr /C-7 ANALYSIS OF AIR POLLUTION Elżbieta Huzar, PhD WTiICh/IISt/OSr /D-3b ANALYSIS OF FOOD CONTAMINANTS Alicja Wodnicka, PhD WTICH_ICHP_1A _S_21 Jolanta Szoplik, PhD 4 4 4 4 TECHNOLOGY ANALYSIS OF WATER AND EFFLUENTS APPLIED METROLOGY AND MEASUREMENTS FOR CHEMISTS BIODEGRADABLE POLYMERS TCH_2A_S_D01 _09 Sylwia Mozia, PhD,DSc Dariusz Moszyński, assistant professor Katarzyna Wilpiszewska, PhD winter or summer winter or summer 4 2 summer 4 winter 3 winter/ summer winter/ summer 2 BIOMATERIALS Piotr Sobolewski, PhD BIOMATERIALS AND IMPLANTS Mirosława El Fray, professor summer 4 BIOMIMETICS Mirosława El Fray, professor summer 3 BIOPOLYMERS Piotr Sobolewski, PhD BIOPROCESS ENGINEERING Piotr Sobolewski, PhD CHARACTERIZATION METHODS AND PROPERTIES OF POLYMERIC MATERIALS CHEMICAL PROCESSES IN INORGANIC INDUSTRY AND ENVIRONMENTAL ENGINEERING Agnieszka Piegat, PhD winter/ summer winter/ summer 4 4 4 winter 3 Sylwia Mozia, professor summer 4 CHEMICAL REACTORS Beata Michalkiewicz, professor summer 3 WTiICh/IISt/TCh /D4-8 CHEMISTRY AND TECHNOLOGY OF MEDICINES Halina Kwiecień, professor WTiICh/IISt/OSr /C-1 CHROMATOGRAPHIC METHODS Małgorzata Dzięcioł, PhD COMPUTER-AIDED DESIGN OF CHEMICAL INDUSTRIAL PLANTS ELECTRICAL ENGINEERING FOR CHEMISTS Ryszard J. Kaleńczuk, professor winter 3 Dariusz Moszyński, PhD,DSc winter 3 Agata Markowska-Szczupak, PhD,DSc winter 3 Krzysztof Lubkowski, PhD winter 2 Dariusz Moszyński, PhD,DSc winter 4 Dariusz Moszyński, PhD,DSc winter 2 Krzysztof Lubkowski, PhD winter 2 Dariusz Moszyński, PhD,DSc winter 5 Mirosława El Fray, professor Summer 3 WTiICh/IISt/TCh /D12-7 WTiICh/IISt/TCh /D12-5 ELEMENTS OF BIOTECHNOLOGY WTiICh/IISt/TCh /D12-4 FUNDAMENTALS OF INORGANIC CHEMICALS COMMODITY SCIENCE HETEROGENEOUS CATALYSIS IN INDUSTRY INDUSTRIAL AUTOMATION AND PROCESS CONTROL FOR CHEMISTS INDUSTRIAL CHEMISTRY INSTRUMENTAL ANALYSIS OF NANOMATERIALS INTRODUCTION TO MATERIALS ENGINEERING winter or summer winter or summer 4 4 3 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ WTiICh/IISt/TCh /D12-1 WTiICh/IISt/TCh /D12-10 WTiICh/IISt/TCh /D12-11 WTiICh/ISt/TCh/ D2-3 WTiICh/ISt/TCh/ D2-4 WTiICh/ISt/TCh/ D2-5 WTiICh/IISt/TCh /D12-9 IT TECHNOLOGIES FOR CHEMICAL APPLICATIONS Rafał Wróbel, PhD,DSc winter 2 MEMBRAN SEPARATION PROCESSES Maria Tomaszewska, professor winter 4 NANOLAYERS AND THIN FILMS Dariusz Moszyński, PhD,DSc summer 3 winter 2 summer 2 winter/ summer 2 winter 3 Winter/ summer 3 NANOPARTICLES AND ENVIRONMENT NANOTECHNOLOGY AND CRYSTALLINE NANOMATERIALS PAINTS AND ADHESIVES TECHNOLOGY Beata Tryba, professor Ewa Borowiak-Paleń, professor Krzysztof Kowalczyk, PhD PHYSICAL CHEMISTRY OF SURFACES Dariusz Moszyński, PhD,DSc POLYMER COMPOSITES Ryszard Pilawka, PhD Krzysztof Gorący, PhD POLYMER CHEMISTRY Mirosława El Fray, professor winter 2 POLYMERS IN MEDICINE Mirosława El Fray, professor summer 3 POWER ENGINEERING IN CHEMICAL INDUSTRY Jacek Przepiórski, professor summer 2 PRINCIPLES OF BIOTECHNOLOGY Piotr Sobolewski, PhD winter/ summer 4 QUALITY AND RISK MANAGEMENT IN CHEMICAL INDUSTRY Krzysztof Karakulski, PhD,DSc summer 2 RESEARCH PROJECT Halina Kwiecień, professor winter or summer 12 Krzysztof Lubkowski, PhD summer 2 Rafał Wróbel, PhD,DSc winter 3 winter/ summer 2 winter 2 WTiICh/IISt/TCh /C01 SMALL SCALE PRODUCTS IN INORGANIC INDUSTRY SURFACE PHENOMENA AND INDUSTRIAL CATALYTIC PROCESSES WTiICh/IISt/TCh /D12-6 TECHNOLOGICAL PROJECT Marek Gryta, professor WTiICh/IISt/TCh /D12-2 TECHNOLOGIES FOR WASTE AND POLLUTANTS MINIMIZATION IN CHEMICAL INDUSTRY Joanna Grzechulska – Damszel, PhD,DSc TECHNOLOGIES IN ENVIRONMENTAL PROTECTION I AND II Elżbieta Huzar, PhD Winter or summer 2 (I) 2 (II) TECHNOLOGY OF DYES AND INTERMEDIATES I AND II Halina Kwiecień, professor winter or summer 2 (I) 2 (II) Mirosława El Fray, professor summer 2 Dariusz Moszyński, PhD,DSc winter 5 winter/ summer 3 WTiICh/ISt/OSr/ B-6-1 WTiICh/ISt/OSr/ B-6-2 WTiICh/IISt/TCh /D4-5 WTiICh/IISt/TCh /D4-11 WTiICh/IISt/TCh /D12-3 WTiICh/IISt/O Sr/C-10 TESTING METHODS OF BIO- AND NANOMATERIALS TESTING METHODS OF INORGANIC PRODUCTS THERMAL ANALYSIS OF PLASTICS Ryszard Pilawka, PhD Krzysztof Gorący, PhD TISSUE ENGINEERING Mirosława El Fray, professor summer 3 TOXICOLOGICAL ASSESSMENT OF MATERIALS AND PRODUCTS Małgorzata Dzięcioł, PhD winter or summer 4 VACUUM TECHNOLOGY Dariusz Moszyński, PhD,DSc summer 3 4 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ CHEMISTRY Course title BIOCHEMISTRY Teaching method Lecture , Classes, Laboratory Person responsible for the course Elwira Wróblewska, PhD E-mail address to the person responsible for the course elwira.wroblewska@ zut.edu.pl Course code (if applicable) CH_1A_S_C06 ECTS points 4 Type of course obligatory Level of course bachelor Semester winter/summer Language of instruction English Hours per week Lecture - 2, Classes - 1, Laboratory - 1 Hours per semester Lecture - 30, Classes 15, Laboratory - 15 Objectives of the course The course aims to give a general knowledge about the biochemistry. Entry requirements Fundamentals of inorganic and organic chemistry, biology, chemical calculations. Course contents Lecture: Proteins – compositions, structure and function / ligand binding, enzymes, enzyme kinetics, membrane proteins/. Metabolism – bioenergetics, glycolysis and gluconeogenesis. The Citric Acid Cycle. Metabolic regulation. Oxidative phosphorylation. Classes: Kinetics of reaction, chemical equilibrium, acid-base equilibrium, acid-base behavior of amino acid, osmotic pressure, kinetics of enzyme reactions, spectroscopy in biochemistry. Laboratory: Characteristic chemical reaction of amino acid and proteins, colors reactions of carbohydrates, physical chemical properties of lipids. Assessment methods Learning outcomes Recommended readings grade Fundamental scientific knowledge about processes in living organisms. Application of mathematics and computational methods to the field of biochemistry. Laboratory result analysis and their interpretation. 1. 2. Biochemistry, Donald Voet, Judith G.Voet; NJ : John Wiley & Sons, cop. 2011. Biochemistry: International Edition, Jeremy M. Berg, John L. Tymoczko, Lubert Stryer; W. H. Freeman. Harper's Illustrated Biochemistry by Robert K. Murray, Darryl K. Granner, Peter A. Mayes; 3. Additional information Course title FUNDAMENTALS METHODS Teaching method Lecture OF CRYSTALLOGRAPHY AND DIFFRACTION 5 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Person responsible for the course Piotr Tabero, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) CH_1A_S_CO2 ECTS points 2 Type of course Optional Level of course Bachelor Semester Winter Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course To acquaint students with the fundamentals of crystallography, principles, possibilities and practical application of diffraction methods for clarifying the structures and the identification and characterization of inorganic and organic substances in solid state. To teach students to use structural and structural-chemical information from diffraction methods and the available literature and structural databases, to solution of chemical problems. Entry requirements Fundamentals of mathematics and chemistry Course contents Basic definitions in crystallography; solid; crystal; physical properties of solids; Bravais lattices and crystal systems; Symmetry in crystals; morphology of crystals,; point groups and space groups; International Tables for X-Ray Crystallography; radii of atoms and ions; coordination polyhedral; simple structures of elements and compounds; crystal structures and defects; solid solutions; X- rays and their properties; reciprocal lattices; diffraction by crystals; Intensity of diffraction reflection; powder diffraction; qualitative and quantitative phase analysis; investigation of single crystals; indexation of powder diffraction patterns; lattice parameter determination; effects of point defects, line defects, planar defects, grain size , stacking faults, anti-phase interfaces, textures and internal stresses on diffraction pattern; high –temperature and low-temperature measurements; high-pressure measurements; investigations of polymorphic transitions; investigation of semi-crystalline and amorphous materials; investigation of liquid crystals; solution of crystal structure; Rietveld methodsnt; ab initio methods; diffraction of electrons and neutrons. Assessment methods Class test Learning outcomes Student knows characteristic physical properties and terminology associated with solids and crystallography Student knows construction and operation techniques of advanced equipment for investigation of solids with the help of diffraction methods Student can choose proper research method to obtain a certain research goal Student can analyse structure and crystallography by means of advanced research techniques Student knows popular crystallography software and crystallography databases and is able to use it effectively Student knows how to explain the data obtained and the phenomena exhibited in the materials analysis Student knows how to design studies and elaborate results 6 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. 2. 3. 4. Recommended readings 5. 6. 7. 8. 9. C. Giacovazzo, H. Z. Monaco, D. Biterbo, F. Scordari, G. Gilli, G. Zanotti, M. Catti, Fundamentals of Crystallography, IUCR, Oxford University Press, 2000 David B. Williams, C. Barry Carter: Transmission Electron Microscopy, Plenum Press, New York and London, 1996 Olaf Engler, Valerie Randle: Introduction to Texture Analysis. Macrotexture, Microtexture and Orientation Mapping, CRC Press, Taylor & Francis Group, Boca Raton London New York, 2010 Cullity B.D.: Elements of X-ray Diffraction, Addison-Wesley Publishing Company, Inc., London , 1978 P. Luger, Modern X-ray Analysis on Single Crystals, Walter de Gruyter and Co., Berlin 1980. Glusker, J. P.; Lewis, M.; Rossi, M. “Crystal Structure Analysis for Chemists and Biologists” VCH, New York, 1994 W.I.F. David, K. Shankland, L.B. McCusker and Ch. Baerlocher, Edt. Structure determination form powder diffraction data. IUCr Monographs on crystallography, 2002. Oxford Science publications A. Gaunier, X-ray Diffraction in Crystals, Imperfect Crystals, and Amorphous Bodies, Courier Corporation, New York, 1994 WEB Links/References (Indicated by tutor) Additional information Course title INSTRUMENTAL ANALYSIS Teaching method lecture, laboratory, classes Person responsible for the course Monika Gąsiorowska, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course Obligatory Level of course bachelor Semester Winter/summer Language of instruction English Hours per week 1- lecture, 3 -laboratory, 1classes Hours per semester 75 (15-lecture, 15classes, 45-laboratory) Objectives of the course Theoretical and practical learning about instrumental methods applied in quantitative and qualitative analysis; theoretical studies about the phenomena used in the particular method as well as practical interpretation of the results given. Entry requirements Basis of physical chemistry, organic chemistry, general chemistry, analytical methods 7 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Classification of the methods of instrumental analysis, particularly spectroscopic and chromatographic ones. Explanation of wave-particle duality of electromagnetic radiation and influence of its absorption/emission by atom or molecule on their properties. Theoretical studies of phenomena proceeding in the molecule/atom under the irradiation and their application in particular methods i.e. ultraviolet-visual spectroscopy (UV-VIS), infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR), mass spectroscopy (MS), atomic absorption spectroscopy (AAS), X-ray absorption, atomic emission spectroscopy (AES), flame photometry, inductively coupled plasma spectrometry (ICP), Xray fluorescence (XRF), atomic fluorescence. Explanation of phenomena, concepts, and definitions used in chromatographic methods. The ways of separation of a mixture components. The way of the interpretation of the results obtained by above methods. Examples of the applications of the methods in qualitative and quantitative analysis. Measurements and interpretation of obtained by selected methods spectra/chromatograms (spectroscopy UV-VIS, IR, NMR, XRF, AAS, ICP, and gas chromatography) and their use in qualitative and/or quantitative analysis. Assessment methods grade Learning outcomes Student knows and understands the phenomena applied in the instrumental analysis. He has a knowledge about the fundamentals of the selected spectroscopic and chromatographic methods. Student is able to choose the appropriate method in order to solve particular problem concerning qualitative and/or quantitative analysis and is able to plan and carry out the experiment with the interpretation of obtained results. Recommended readings Obligatory 1. J. M. Hollas, Modern spectroscopy, John Wiley, 2004. 2. L.D. Field, S. Sternhall, J.R. Kalman, Organic structures from spectra, 3 rd ed., Chichester, John Wiley and Son, 2002. 3. J.R. Chapman, Practical Organic Mass Spectrometry, 2nd ed., ., Chichester, John Wiley and Son, 1993. 4. Ira N. Levin, Molecular spectroscopy, New York : Wiley-Interscience, 1975. 5. C. N. R. Rao, Ultra-violet and visible spectroscopy: chemical applications, 3rd ed., London, Butterworths, 1975. 6. Spectroscopy, ed. D. A. Ramsay, London: Butterworths; Baltimore, University Park Press, 1976. 7. Stefan Hüfner, Photoelectron spectroscopy: principles and applications, 2nd ed., Berlin, Springer, 1996. Additional/optional 1. Ch. Reichardt, Solvents and solvent effects in organic chemistry, 2 nd rev. and enl. ed., Weinheim, VCH, 1990. 2. Pradip K. Ghosh, Introduction to photoelectron spectroscopy, New York [etc.], John Wiley and Sons, 1983. 3. M. Slavin, Atomic absorption spectroscopy, New York [etc.], John Wiley & Sons, 1978. 4. J. Mika, T. Török, Analytical emission spectroscopy : fundamentals, Budapest, Akadémiai Kiadó, 1973. 5. Yoshito Takeuchi and Alan P. Marchand, Applications of NMR spectroscopy to problems in stereochemistry and conformational analysis, Deerfield Beach, Florida, Verlag Chemie International, 1986. Additional information Course title METHODS OF ORGANIC COMPOUNDS IDENTIFICATION Teaching method Lecture, Laboratory Person responsible for the course Jacek A. Soroka, professor E-mail address to the person responsible for the course [email protected] 8 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course code (if applicable) ECTS points 3 Type of course Obligatory Level of course bachelor Semester winter / summer Language of instruction English Hours per week Lecture- 1 Laboratory-2 Hours per semester Lecture – 15, Laboratory - 30 Objectives of the course To gain the knowledge about the methods of organic compounds identification. Entry requirements Fundamentals of physical and organic chemistry Course contents Classification of the methods of quantitative analysis of organic compounds, especially spectroscopic and chromatographic ones. Explanation of theoretical fundamentals of the interaction of electromagnetic radiation with an atom or molecule. Explanation of phenomena, concepts, and definitions used in chromatographic methods. Application of selected methods i.e. ultraviolet-visual spectroscopy (UV-VIS), infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR), mass spectroscopy (MS), atomic absorption (AAS), gas chromatography (GC) in quantitative analysis of organic compounds. The way of the interpretation of the results obtained by above methods. Assessment methods grade Learning outcomes Student has a knowledge about the fundamentals of the selected method of organic compounds identyfication. Student is able to choose the appropriate method in order to solve particular problem concerning quantitative analysis and can plane and carry the experiment with the interpretation of obtained results. 1. 2. 3. Recommended readings 4. 5. 6. 7. 8. 9. Field, L. D, Strnhell, S, Kalman, J.R, Organic structures from spectra, Chichester: John Wiley and Sons, 2002. Bartecki, A. , Lang, L. Absorption spectra in the ultraviolet and visible region. Budapest : House of the Hungarian. Academy of Sciences, 1982. Láng, L., Holly, S, Sohár, P, Absorption spectra in the infrared region. Budapest: Akadémiai Kiadó, 1980. Strobel, Howard A., Chemical instrumentation : a systematic approach to instrumental analysis, Reading, Mass. : Addison-Wesley, 1960. Perkampus, Heinz-Helmut, Encyclopedia of spectroscopy, Weinheim : VCH, 1995. Hollas, J. Michael , Modern spectroscopy, Chichester : John Wiley, 1992. Evans, Myron Wyn, The photon’s magnetic field : optical NMR spectroscopy, Singapore : World Scientific, 1992 Rahman, Atta-ur, One and two dimensional NMR spectroscopy, Amsterdam : Elsevier, 1989. Parker, Sybil P. Red, Spectroscopy source book, New York : McGraw Hill, 1988. Additional information Course title SPECTROSCOPIC METHODS Teaching method Lecture, Classes, Laboratory Person responsible for the course Marta Sawicka, PhD E-mail address to the person responsible for the course [email protected] 9 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course code (if applicable) CH_1A_S_D01_15 ECTS points 4 Type of course Obligatory Level of course bachelor Semester winter / summer Language of instruction English Hours per week Lecture- 1, Classes- 1, Laboratory -3 Hours per semester Lecture - 15, Classes 15, Laboratory - 45 Objectives of the course To gain the knowledge about the theory of spectroscopic methods and their application in qualitative and quantitative analysis. Entry requirements Fundamentals of physical and organic chemistry Course contents Explanation of wave-particle duality of electromagnetic radiation and influence of its absorption/emission by atom or molecule on their properties. Theoretical studies of phenomena proceeding in the molecule/atom under the irradiation and their application in particular methods i.e. ultraviolet-visual spectroscopy (UV-VIS), infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR), mass spectroscopy (MS), atomic absorption. Measurements and interpretation of spectra obtained by selected methods. The selection and the use of particular methods in qualitative and/or quantitative analysis. Assessment methods grade Learning outcomes Student knows how the electromagnetic radiation can interact with the matter. He has a knowledge about the fundamentals of the selected spectroscopic method. Student is able to choose the appropriate method in order to solve particular problem concerning qualitative and/or quantitative analysis and can plane and carry the experiment with the interpretation of obtained results. 1. Recommended readings Field, L. D, Strnhell, S, Kalman, J.R, Organic structures from spectra, Chichester: John Wiley and Sons, 2002. 2. Reichardt, Christian, Solvents and solvent effects in organic chemistry, Weinheim : VCH, 1990. 3. Bartecki, A. , Lang, L. Absorption spectra in the ultraviolet and visible region. Budapest : House of the Hungarian. Academy of Sciences, 1982. 4. Láng, L., Holly, S, Sohár, P, Absorption spectra in the infrared region. Budapest: Akadémiai Kiadó, 1980. 5. Strobel, Howard A., Chemical instrumentation : a systematic approach to instrumental analysis, Reading, Mass. : Addison-Wesley, 1960. 6. Perkampus, Heinz-Helmut, Encyclopedia of spectroscopy, Weinheim : VCH, 1995. 7. Hollas, J. Michael , Modern spectroscopy, Chichester : John Wiley, 1992. 8. Evans, Myron Wyn, The photon’s magnetic field : optical NMR spectroscopy, Singapore : World Scientific, 1992 9. Rahman, Atta-ur, One and two dimensional NMR spectroscopy, Amsterdam : Elsevier, 1989. 10. Parker, Sybil P. Red, Spectroscopy source book, New York : McGraw Hill, 1988. 11. Schulman, Stephen G. Red, Molecular luminescence spectroscopy : methods and applications., New York : John Wiley, 1988. Additional information 10 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ CHEMICAL ENGINEERING Course title ADSORPTION ENGINEERING Teaching method Lecture; Project. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_1 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Project - 1 Hours per semester Lecture – 30 Project - 15 Objectives of the course The course aims to familiarize students with the basic of adsorption processes and methods of their design. Entry requirements Physical chemistry, Chemical engineering fundamentals. Course contents Introduction. History of adsorption engineering. Adsorbents. Adsorption equilibria. Thermodynamic of adsorption. Adsorption of gaseous mixtures. Rates of adsorption and transport effects. Adsorption processes and adsorption cycles. Batch processes. Fixed and moving bed processes. Fluidized bed. processes. Simulated moving bed processes. Regeneration of adsorbents. Pressure swing adsorption. Thermal swing adsorption. Design of adsorption systems. Short-cut and scoping methods. Modeling and simulation of adsorption systems. Aspen Adsorption. Scale-up and pilot-plant studies of adsorption processes. Selected commercial adsorption processes. Assessment methods Lecture – oral exam Project – project work Learning outcomes The student will be able to: Demonstrate basic knowledge of adsorption phenomena. Demonstrate basic knowledge of production and properties of adsorbents. Demonstrate basic knowledge of adsorption equilibrium, kinetics and dynamics. Identify the various types of cyclic adsorption systems. Demonstrate basic knowledge of modeling and simulation of adsorption systems. Describe the scientific principles associated with adsorption equipments. Demonstrate basic knowledge of adsorption processes design and operation. Apply Aspen Adsorption for design of adsorption systems Recommended readings 1. 2. 3. 4. 5. 6. 7. 8. Ruthven D.M., Principles of adsorption and adsorption processes, Wiley, 1984. Suzuki M., Adsorption engineering, Kodansha 1990. Ruthven D.M., Knaebel K.S., Pressure swing adsorption, VCH 1994. Thomas W. J., Crittenden B., Adsorption Technology and Design, Elsevier 1998. Yang R.T., Gas Separation by Adsorption Processes, Imperial College Press, 1997. Do D.D., Adsorption analysis: equilibria and kinetics, Imperial College 1998. Yang R.T., Adsorbents : fundamentals and applications, Wiley 2003. Valenzuela D.P., Myers A. L., Adsorption Equilibrium Data Handbook, Prentice Hall 1989. Additional information 11 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title AGITATION AND AGITATED VESSELS Teaching method Lecture/Laboratory/Project. Person responsible for the course Joanna Karcz, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_2A_S_2 ECTS points 4 Type of course Obligatory Level of course Bachelor / Master Semester Winter / Summer Language of instruction English Hours per week Lecture - 1, Laboratory - 1 Project - 1 Hours per semester Lecture - 15, Laboratory - 15 Project - 15 Objectives of the course The course aims to give a general introduction to the theory and practice of agitation and agitated vessels. Entry requirements Chemical engineering fundamentals. Course contents Introductory remarks; Agitation and agitated vessels; Types of the agitated vessels. Types of the impellers; Hydrodynamics in agitated vessels; Mixing time; Power consumption; Heat transfer; Mass transfer; Blending of miscible liquids; Solid-liquid mixing; Gas-liquid mixing; Liquid-liquid mixing; Gas-solid-liquid mixing. Mixing with chemical reactions; Mixing of particulate solids. Assessment methods Lecture – exam; Laboratory - continuous assessment, reports; Project - project work. Learning outcomes The student will be able to: Identify the various types of mixing equipment used in the chemical processing industry. Understand the hydrodynamics of mixing. Understand the engineering principles of mixing Formulate basic equation for heat and mass transfer problems occurring in agitated vessels. Apply engineering principles of mixing to design of agitated vessels. 1. 2. Recommended readings 3. 4. 5. Harnby N., Edwards M.F., Nienow A.W., Mixing in the Process Industries, ButterworthHeinemann, Oxford, 1997. Mixing Equipment (Impeller Type), AIChE Equipment Testing Procedure, 3rd Edition, New York, 2001, ISBN 0-8169-0836-2. Nagata S., Mixing. Principles and Applications, Halsted Press, New York, 1975. Paul E.L., Atiemo-Obeng V.A, Kresta S.M; (Ed.). Handbook of Industrial Mixing, John Wiley & Sons, Inc., New York, 2004. Tatterson G.B.: Fluid Mixing and Gas Dispersion in Agitated Tanks, McGraw-Hill, Inc., New York, 1991. Additional information 12 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title AN INTRODUCTION TO NUMERICAL ANALYSIS WITH PROCESS ENGINEERING APPLICATIONS USING MATHCAD AND MATLAB Teaching method Lecture / computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the numerical methods analysis to chemical and process engineering problems solution using Mathcad and Matlab. Entry requirements Chemical engineering, mathematics, numerical methods Course contents Mathematical Mathcad and Matlab functions useful in engineering computations. Mathcad and Matlab instructions. Matrix operations. Analysis of experimental data: data import and export, approximation, data: interpolation, data smoothing. Solving of equations: linear equations and linear equations systems, nonlinear equations and nonlinear equation systems, ordinary differential equations (ODE), types of equations and boundary conditions, Matlab numerical integrators, stiff ordinary differential equations, unsteady-state processes, nonlinear dynamics. Solution of partial differential equations: first and second order equations, initial value and boundary value problems, steady-state and unsteadystate. Numerical solution method (Initial value problem). Approximate methods for boundary value problems: weighted residuals. Solution of the selected problems in chemical engineering: basic principles and calculations, problems of regression and correlation of data, advanced solution methods in problem solving. Thermodynamics. Heat transfer. Mass transfer. Problems of fluid mechanics. Examples of selected problems: variation of reaction rate with temperature, shooting method for solving two-point boundary value problems, fugacity coefficients for ammonia – experimental and predicted, optimal pipe length for draining a cylindrical tank in turbulent flow, unsteady-state conduction in two dimensions, simultaneous heat and mass transfer in catalyst particles, etc. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of Mathcad and Matlab functions. Identify the various types of numerical techniques. Understand methods of data analysis. Solve selected problems associated with chemical and process engineering using Mathcad and Matlab 13 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. 2. Recommended readings 3. 4. 5. A. Gilat, V. Subramanian, Numerical methods: An introduction with applications using Matlab, John Wiley & Sons, Inc., New York, 2011. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. L. Fausett, Numerical methods using Mathcad, Prentice Hall, Pearson Education Ltd., London, 2002. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007 Additional information Course title APPLIED MATHEMATICS ENGINEERS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) AND MODELING FOR CHEMICAL E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the modeling of selected chemical and process engineering problems and their solution using Mathcad and Matlab. Entry requirements Chemical engineering, mathematics, numerical methods Course contents Formulation of physicochemical problems. Combining rate and equilibrium concepts. Boundary conditions and sign conventions. Model hierarchy and its importance in analysis. Solution techniques for models yielding ordinary differential equations (ODE). Staged process models: the calculus of finite differences. Numerical solution method (Initial value problem). Approximate methods for boundary value problems: weighted residuals. Solution of the selected problems in chemical engineering: basic principles and calculations, problems of regression and correlation of data, advanced solution methods in problem solving. Thermodynamics. Heat transfer. Mass transfer. Problems of fluid mechanics. Examples of selected problems: variation of reaction rate with temperature, shooting method for solving two-point boundary value problems, fugacity coefficients for ammonia – experimental and predicted, optimal pipe length for draining a cylindrical tank in turbulent flow, unsteady-state conduction in two dimensions, simultaneous heat and mass transfer in catalyst particles, etc. Assessment methods Lecture: oral exam Computer laboratory: grade 14 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Demonstrate basic knowledge of Mathcad and Matlab functions and instructions. Identify the various types of numerical methods (computational techniques). Demonstrate ability of using Mathcad and Matlab to solve basic and advanced problems associated with chemical and process engineering. 1. 2. 3. Recommended readings 4. 5. 6. R.G. Rice, D.D. Do, Applied mathematics and modeling for chemical engineers, John Wiley & Sons, Inc., New York, 1995. O.T. Hanna, O.C. Sandall, Computational methods in chemical engineering, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1995. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. L. Fausett, Numerical methods using Mathcad, Prentice Hall, Pearson Education Ltd., London, 2002. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007 Additional information Course title APPLIED PETROLEUM RESERVOIR ENGINEERING Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Introduction to the reservoir engineering of oil and natural gas. Entry requirements Chemical engineering, thermodynamics Course contents Introduction to reservoir engineering: history, reservoirs types defined with reference to phase diagrams. Review of: rock properties, gas properties, crude oil properties, reservoir water properties. Derivation of material balance equation. Single-phase gas reservoirs. Gas reservoirs as storage reservoirs. Gas-condensate reservoirs. Calculation of initial gas and oil. Undersaturated oil reservoirs (material balances in). Saturated oil reservoirs. Mass balance in saturated reservoirs. Volatile oil reservoirs. Maximum efficient rate (MER). Single-phase fluid flow in reservoirs. Productivity index. Water influx. The displacement of oil and gas. Introduction to enhanced oil recovery processes. Job functions of the reservoir engineer. Prediction of future production rates from a given reservoir or specific well. 15 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of applied petroleum reservoir engineering. Identify the various types of reservoirs and reservoirs fluids. Calculate reservoir mass balances and natural gases and oils properties. Describe the scientific principles associated with oil recovery processes. Recommended readings 1. 2. 3. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. B.C. Craft, M.F. Hawkins, Applied Petroleum Reservoir Engineering, Prentice Hall PTR, New Jersey 1991. B.E. Poling, J.M. Prausnitz, J.P. O’Connel, The Properties of Gases and Liquids, McGrawHill, New York 2001. Additional information Course title BASIC PRINCIPLES ENGINEERING Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) AND CALCULATIONS IN CHEMICAL E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Developing of systematic problem solving skills. Learning what material balances are, how to formulate and apply them, and how to solve them. Learning what energy balances are and how to apply them. Learning how to deal with the complexity of big problems. Entry requirements Mathematics, physics, chemical engineering 16 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction to chemical engineering calculations: units and dimensions, conventions in methods of analysis and measurement, chemical equation and stoichiometry. Problem solving: techniques of problem solving, computer-based tools, sources of data. Material balances: the material balance, program of analysis of material balance problems, solving material balance problems that do not involve chemical reactions, solving material balance problems that involve chemical reactions, solving material balance problems involving multiple subsystems, recycle, bypass, and purge calculations. Gases, vapors, liquids, and solids: ideal gas law calculations, real gas relationships, vapor pressure and liquids, vaporliquid equilibria for multicomponent systems, partial saturation and humidity, material balances involving condensation and vaporization. Energy balances: concepts and units, calculation of enthalpy changes, application of the general energy balance without reactions occurring, energy balances that account for chemical reaction, reversible processes and the mechanical energy balance, heats of solution and mixing, humidity charts and their use. Solving simultaneous material and energy balances: analyzing the degree of freedom in a steady-state process. Unsteady-state material and energy balances. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Explain the basic elements of engineering calculations. Demonstrate basic knowledge of material and energy balances. Solve typical problems associated with simplified process modeling in chemical engineering. 1. 2. Recommended readings 3. 4. 5. D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, Prentice Hall International (UK) Limited, London, 1996 W.L. Luyben, L.A. Wenzel, Chemical Process Analysis: Mass and Energy Balances, Int. Ser. in Phys. & Chem. Eng. Sci., Englewood Cliffs, NJ, Prentice Hall, 1988 E.I., Shaheen, Basic Practice of Chemical Engineering, 2nd ed. Boston, Houghton Mifflin, 1984 B.E. Poling, J.M. Prausnitz, J.P. O’Connel, The Properties of Gases and Liquids, 5-th ed., McGraw-Hill, New York, 2001 J.B. Riggs, An Introduction to Numerical Methods, 2nd ed., Lubbock, TX,Texas Tech. University Press, 1994 Additional information Course title BIOENVIRONMENTAL HEAT AND MASS TRANSFER Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) 17 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course Presentation of the basic energy and mass transport mechanisms to many biological and environmental processes. Entry requirements Chemical engineering, physical chemistry Course contents Problem formulation in the transport processes. Transport in the mammalian system. Transport in plant systems. Transport in industrial food and biological processing. Transport in the bioenvironmental system. Energy transfer: equilibrium, energy conservation, and temperature. Modes of heat transfer. Governing equation and boundary conditions of heat transfer. Conduction heat transfer: steady-state. Conduction heat transfer: unsteady-state. Convective heat transfer. Heat transfer with change of phase: freezing and thawing, freezing of pure water, freezing of solutions and biomaterials (solutions, cellular tissues, cooling rates and success of freezing), temperature profiles and freezing time, evaporation. Radiative energy transfer. Equilibrium, mass conservation, and kinetics. Modes of mass transfer. Governing equations and boundary conditions of mass transfer. Diffusion mass transfer: steady state. Diffusion mass transfer: unsteady state. Convection mass transfer. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of heat and mass transfer processes in biological systems. Identify the various types of heat and mass transport mechanisms in natural environment. Describe the scientific principles associated with bioenvironmental heat and mass balances. Solve typical calculational problems associated with heat and mass transfer in natural environment. 1. Recommended readings 2. 3. 4. A.K. Datta, Biological and bioenvironmental heat and mass transfer, Marcel Dekker Inc., New York 2002. J.C. Slattery, Advanced transport phenomena, Cambridge University Press, Cambridge, 1999. S.A. Berger, W. Goldsmith, E.R. Lewis, Introduction to bioengineering, Oxford University Press, Oxford, 1999. C.J. Geankoplis, Transport processes and unit operations, Prentice-Hall International Ltd, New Jersey, 1993. Additional information Course title BIOPROCESS ENGINEERING Teaching method Lecture/Project Person responsible for the course Joanna Karcz, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_3 ECTS points 4 Type of course Obligatory Level of course Bachelor / Master Semester winter / summer Language of instruction English 18 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Hours per week Lecture - 2, Project - 1 Objectives of the course The course aims to give a general introduction to the theory of bioprocess engineering. Entry requirements Chemical engineering fundamentals Course contents Introductory remarks; Biotechnology and bioprocess engineering; Regulatory constraints. An overview of biological basics of bioprocess engineering: Enzymes; Cells; Major metabolic pathways; The grow of cells. Engineering principles for bioprocesses: Operating considerations for bioreactors; Selection, scale-up, operation, and control of bioreactors; Recovery and purification of products; Instrumentation and control; Sterilization of process fluids; Finishing steps for purification; Integration of reaction and separation. Traditional industrial bioprocesses. Nonconventional bioprocesses. Assessment methods Lecture – exam; Project - project work. Learning outcomes The student will be able to: Understand the principles, stoichiometry, and kinetics of biological processes. Explain the basic elements of bioreactors. Understand the engineering principles for bioprocesses. Describe quantitatively the dynamic behavior of bioreactors. 1. 2. 3. Recommended readings 4. 5. 6. Hours per semester Lecture - 30, Project - 15 Doran P.M., Bioprocess Engineering Principles, Academic Press, London 1995. Dutta R., Fundamentals of Biochemical Engineering, Springer, Berlin 2008. Lydersen B.K., D’Elia N.A., Nelson K.L., Bioprocess Engineering, John Wiley & Sons, Inc., New York, 1994. Shuler M.L., Kargi F., Bioprocess Engineering: Basic Concepts, Prentice Hall, New Jersey 2002. Van’t Riet K., Tramper J., Basic Bioreactor Design, Marcel Dekker Inc., New York, 1991. Flickinger M.C., Drew S.W., Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation, Wiley, New York 1999. Additional information Course title CHEMICAL AND MOLECULAR THERMODYNAMICS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) 19 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course Presentation of difficult subjects like thermodynamics in a logical but, at the same time, quite thorough way. Thermodynamics and the necessary mathematics are embodied as a series of a dozen ‘frames’. Entry requirements Chemical engineering, physical chemistry Course contents Introduction: the terminology of thermodynamics, the variables and quantities. Thermodynamics principles for open systems. Work in open system. The First Law of thermodynamics for open systems. The Second Law of thermodynamics. The PVT behavior of fluids. Generalized equation of state. Heat effects due to change of temperature, pressure or change of phase. Mixing heat effects. Chemical heat effects. Principles of phase equilibrium and applied phase equilibrium. Additional topics in phase equilibrium: Activity coefficients based on Henry’s law, the solubility of gases in liquids, solid-liquid equilibria. Chemical equilibrium. Gibbs-Helmholtz equation. Qualitative interpretation of Van’t Hoff equation. Coupled reaction. Intermolecular forces, Corresponding states, and Osmotic systems. Electrolyte solutions. High-pressure phase equilibria. A brief introduction to statistical thermodynamics. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of chemical thermodynamics and molecular thermodynamics of fluid phase equilibria. Describe the scientific principles associated with solving thermodynamic problems. Solve engineering problems associated with chemical and molecular thermodynamics. Recommended readings 1. 2. 3. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. J.M. Prausnitz, R.N. Lichtenthaler, E.G. de Azevedo, Molecular Thermodynamics of Fluid Phase Equilibria, Prentice Hall PTR, New Jersey 1999. Additional information Course title CHEMICAL AND PROCESS THERMODYNAMICS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) 20 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course An attempt to produce thermodynamics lecture and laboratory suitable for the age of the personal computers. Computer-aided calculations, using a general purpose numerical analysis program - Polymath Entry requirements Chemical engineering, physical chemistry Course contents Introduction: the terminology of thermodynamics, the variables and quantities. Thermodynamics principles for open systems. Work in open system. The First Law of thermodynamics for open systems. The Second Law of thermodynamics. The PVT behavior of fluids. Generalized equation of state. Heat effects due to change of temperature, pressure or change of phase. Mixing heat effects. Chemical heat effects. Principles of phase equilibrium and applied phase equilibrium. Additional topics in phase equilibrium: Activity coefficients based on Henry’s law, the solubility of gases in liquids, solid-liquid equilibria. Chemical equilibrium. Principles of phase equilibrium. Thermodynamic analysis of processes. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of chemical and process thermodynamics. Describe the scientific principles associated with solving thermodynamic problems. Solve typical and complex problems associated with philosophy and practice of modeling thermodynamic systems. 1. 2. 3. Recommended readings 4. 5. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2008. H.S. Fogler, Elements of chemical reaction engineering, 4th ed., Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2006. D. Kondepudi, Introduction to modern thermodynamics, John Wiley & Sons Inc., Chichester, UK, 2008. Additional information Course title CHEMICAL ENGINEERING DESIGN Teaching method Lecture; Project. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_4 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English 21 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Lecture - 30, project - 15 Hours per week Lecture - 2, project - 1 Objectives of the course The course aims to give a general introduction to the chemical engineering design. Entry requirements Chemical engineering fundamentals Course contents Introduction to design. Design information. Physical properties of chemical compounds. Materials of Construction. Costing. Mechanical design of process equipment. Flow-sheeting. Material and energy balances. Energy utilization. Piping and instrumentation. Equipment selection, specification and design: separation columns, heat-transfer equipment. Aspen simulation. Plant location and site selection. Environmental considerations. Safety and loss prevention. Assessment methods Lecture – oral exam Project – project work Learning outcomes The student will be able to: Apply knowledge of chemical engineering fundamentals to identify and solve chemical engineering design problems. Perform step-by-step design of chemical engineering processes. Use of Aspen Plus for chemical engineering design. Recommended readings 1. 2. Hours per semester Sinnott R.K., Coulson & Richardson’s Chemical Engineering, Vol. 6: Chemical Engineering Design, Butterworth-Heinemann, Oxford 2003. Luyben W.L., Distillation design and control using Aspen simulation, Wiley, New York 2006. Additional information Course title CHEMICAL ENGINEERING FUNDAMENTALS Teaching method Lecture/Classes/Laboratory Person responsible for the course Joanna Karcz, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_5 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes – 1 Laboratory - 1 Hours per semester Lecture - 30 Classes - 15 Laboratory - 15 Objectives of the course The course aims to give a general introduction to the chemical engineering. Entry requirements Physics; Mathematics. 22 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction. Units and dimensions. Flow of fluids. Energy and momentum balance. Flow in pipes and channels. Flow of compressible fluids. Flow of multiphase mixtures. Flow measurement. Pressure measurement. Mixing of Liquids. Pumping of fluids. Heat transfer. Mass transfer. The boundary layer theory. Simultaneous momentum, heat and mass transfer. Humidification and water cooling. Particulate solids. Motion of Particles in a Fluid. Sedimentation. Fluidization. Flow of Fluids through Granular Beds and Packed Columns. Liquid Filtration. Leaching. Distillation. Absorption of gases. Liquid - liquid Extraction. Evaporation. Assessment methods Lecture - exam, Classes - grade, Laboratory - continuous assessment, reports. Learning outcomes The student will be able to: Formulate governing equation for momentum, heat and energy transfer. Understand the principles of dimensional analysis and empirical methods. Apply general governing equation for momentum and energy to analysis of fluid flow problems. Describe pressure and flow measurement techniques. Formulate basic equation for heat and mass transfer problems. Describe the basic physical operations of chemical engineering. 1. 2. 3. Recommended readings 4. 5. 6. 7. Coulson J.M., Richardson J.F., Backhurst J. R., Harker J. H., Coulson & Richardson’s Chemical Engineering, Vol. 1: Fluid Flow, Heat Transfer and Mass Transfer., Butterworth-Heinemann, Oxford 1999. Coulson J.M., Richardson J.F., Backhurst J. R., Harker J. H., Coulson & Richardson’s Chemical Engineering, Vol. 2: Particle Technology and Separation Processes, Butterworth-Heinemann, Oxford 2002. Richardson J.F., Peacock D.G., Coulson & Richardson’s Chemical Engineering, Vol. 3: Chemical & Biochemical Reactors & Process Control, Butterworth-Heinemann, Oxford 2007. Backhurst J.R., Harker J.H., Richardson J.F., Coulson & Richardson’s Chemical Engineering, Vol. 4: Solutions to the Problems in Vol. 1, Butterworth-Heinemann, Oxford 2001. Backhurst J.R., Harker J.H., Coulson & Richardson’s Chemical Engineering, Vol. 5: Solutions to the Problems in Volumes 2 and 3, Butterworth-Heinemann, Oxford 2002. Sinnott R.K., Coulson & Richardson’s Chemical Engineering, Vol. 6: Chemical Engineering Design, Butterworth-Heinemann, Oxford 2003. Denn M.M., Chemical Engineering. An introduction, Cambridge University Press,New York 2012. Additional information Course title CHEMICAL ENGINEERING PROCESS SIMULATION USING ASPEN PLUS Teaching method Lecture; Laboratory. Person responsible for the course Bogdan Ambrożek, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 5 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English 23 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Lecture -2, Laboratory - 2 Objectives of the course The course aims to familiarize students with the basic of modeling and simulation in chemical engineering using Aspen Plus. Entry requirements Mathematics. Chemical engineering fundamentals. Course contents Introduction to chemical engineering process simulation. Introduction to the Aspen Plus interface. Simulation file creation. Basic process options and simulation tools in Aspen Plus. Selecting physical property models. The data regression system. Unit operation models. Reaction and reactors. Separation columns. Processes with recycle. Sensitivity analysis. Optimization. Assessment methods Lecture – exam Laboratory - continuous assessment, report. Learning outcomes The student will be able to: Develop the process models based on conservation principles. Use Aspen Plus to model chemical engineering processes. 1. 2. Recommended readings 3. 4. 5. 6. Hours per semester Lecture -30, Laboratory - 30 Hours per week Hangos K.M., Cameron L.T., Process modelling and model analysis, Academic Press 2001. Dhurjati P., Shiflett M., Modeling and simulation in chemical engineering using Aspen and Matlab, CRC Press 2014. Rice R.G., Do D.D., Applied mathematics and modeling for chemical engineers, Wiley 2012. Finlayson B.A., Introduction to chemical engineering computing, Wiley 2005. Schefflan R., Teach Yourself the Basics of Aspen Plus, Wiley 2011. Luyben W.L., Chemical Reactor Design and Control, Wiley 2007. Additional information Course title CHEMICAL PROCESS EQUIPMENT Teaching method Lecture, Classes Person responsible for the course Bogdan Ambrożek, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course Obligatory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week Lecture - 2, Classes - 1 Hours per semester Lecture - 30, Classes - 15 Objectives of the course The course aims to familiarize students with the basic of process technology equipment and systems. 24 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Chemical engineering fundamentals Course contents Basic terms. Introduction to process equipment. Flowsheets. Drivers for moving equipment. Flow of fluids. Fluid transport equipment. Pumps, compressors, turbines and motors. Valves: applications and theory of operation. Tanks, piping, and vessels. Heat transfer and heat exchangers. Dryers and cooling towers. Mixing and agitation. Boilers. Furnaces. Instruments. Process control diagrams. Utility systems. Reactor Systems. Distillation and absorption systems. Adsorption and ion exchange. Crystallization from solutions and melts. Extraction. Other separation systems. Plastics Systems. Costs of individual equipment. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Identify the various types of equipment used in the chemical-processing industry. Explain the basic elements of chemical process equipment. Describe the scientific principles associated with chemical process equipment. Describe the operation and maintenance of chemical process equipment. Troubleshoot typical problems associated with the operation of chemical process equipment. Describe the basic instruments used in the process industry. Identify and draw standard instrument symbols. Describe temperature, pressure, flow, and level-measurement techniques. Identify the elements of a control loop. Describe the various concepts associated with utility systems 1. Recommended readings 2. 3. 4. Thomas Ch. E., Process technology equipment and systems, Cengage Learning, Stamford 2015. Walas S. M., Chemical Process Equipment, Butterworth-Heinemann, Newton 1990. Cheremisinoff N. P., Handbook of Chemical Processing Equipment, ButterworthHeinemann, Boston 2000. Elizabeth T. Lieberman E. T.,, Norman P. Lieberman Lieberman N., A Working Guide to Process Equipment, McGraw-Hill 2008. Additional information Course title CHEMICAL REACTION ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_6 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture - 2, Classes - 2 Hours per semester Lecture - 30, Classes - 30 25 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course The course aims to familiarize students with the basic of chemical reaction engineering Entry requirements Physical chemistry Course contents Introduction. Fundamental concepts. The General Mass Balance Equation. Reactor sizing. Stoichiometry. Conversion. The Reaction Order. The Rate Law. Collection and analysis of rate data. Multiple reactions. Reaction mechanisms. Catalytic reactors. Three-phase reactors. Isothermal and nonisothermal reactor design. Biochemical reactors. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Describe and define the rate of reaction. Derive the mass balance equation. Apply the mass balance equation to the most common types of industrial reactors. Write the rate law in terms of concentrations, and temperature. Use nonlinear regression to determine the rate law parameters. Apply the differential and integral methods for analysis of reactor data. Define a catalyst and describe its properties. Describe the steps in a catalytic reaction. Suggest a mechanism and apply the concept of a rate-limiting step to derive a rate law. Recommended readings 1. 2. 3. Fogler H.S., Elements of chemical reaction engineering, Prentice-Hall, New Jersey 1999. Levenspiel O., Chemical reaction engineering, Wiley, New York 1999. Luyben W.L., Chemical reactor design and control, Wiley, New York 2007. Additional information Course title CHEMICAL REACTORS ENGINEERING Teaching method Lecture, workshop Person responsible for the course Paulina Pianko-Oprych, PhD Course code (if applicable) E-mail address to the person responsible for the course paulina.pianko @zut.edu.pl ECTS points 5 Type of course obligatory Level of course bachelor Semester summer Language of instruction English Hours per week Lecture: 2h workshop: 1h Hours per semester Lecture: 30h workshop: 15h Objectives of the course Designing chemical process in reactors, use of physicochemical and mathematical knowledge. Rational design and analysis of performance of multiphase reactors. Entry requirements Mathematics, Chemistry, Physical chemistry, Transfer of momentum, heat and mass 26 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Lecture: Chemical kinetics and rate equations mechanism, reaction rate, rate constant, influence of temperature, approximation in kinetics; macrokinetic equation, classification of reactors and choice of reactor type: homogeneous and heterogeneous reactors, batch reactor and continuous reactors, adiabatic and reactors with heat transfer, general material, species and thermal balances, batch reactor: reaction time, isothermal and non-isothermal operation, batch reactor: choice of volume, continuous stirred tank reactors: design equations, residence time, steady – state, tubular reactor: design equations, residence time, steady-state, continuous stirred tank and tubular reactors; heat transfer, ideal reactors; comparison for a single reaction and for multiple reactions, ideal reactors; dynamic characteristic, cascade of reactors; cell model, dispersion model, reactors for complete kinetic model. Workshop: Solution of the reaction engineering projects for homogeneous tank and tubular reactors. Assessment methods Lecture: class test Workshop: class test Learning outcomes Students gain knowledge in the formulation and solution of equations of mathematical models of various types of chemical reactors. 1. Recommended readings 2. 3. DOE fundamentals Handbook Thermodynamics, heat transfer and fluid flow, Washington, 1992. D.H.F. Liu, B.G. Liptak Environmental Engineers’ Handbook, Lewis Publishers, New York, 1997. R. W. Missen, C. A. Mims, B. A. Saville, Introduction to Chemical Reaction Engineering and Kinetics, Wiley, New York 1999. Additional information Course title COMPUTER AIDED PROBLEMS IN CHEMICAL ENGINEERING Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Solving of the selected problems in chemical engineering using computer programs Entry requirements Chemical engineering, chemical and process thermodynamics, numerical methods 27 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Fundamental logic and the definition of engineering task. Complexity. Data structures. Object representation and reasoning. Fitting polynomials and correlation equations to vapor pressure data. Bubble point and dew point for a non-ideal multi-component mixture. Regression of heterogeneous catalytic rate data. Solution of stiff ordinary differential equations. Shooting method for solving two-point boundary value problems. Laminar flow of non-Newtonian fluids in a horizontal pipe. Unsteady-state conduction in two dimensions. Multicomponent diffusion in a porous layer covering a catalyst. Semibatch reactor with reversible liquid phase reaction. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of data analysis. Identify the various types of numerical methods, select and use the proper method to solve calculation problem. Solve typical problems associated with chemical and process engineering using Simulink (Matlab) 1. 2. Recommended readings 3. 4. B. Raphael, I.A.C. Smith, Fundamentals of computer-aided engineering, John Wiley & Sons Ltd., Chichester, 2003. M.B. Cutlib, M. Shacham, Problem Solving in Chemical and Biochemical Engineering with POLYMATH, Excel, and MATLAB, Prentice Hall, Boston 2008. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. Additional information Course title ENERGY AND ENVIRONMENT Teaching method Lecture, workshop Person responsible for the course Paulina Pianko-Oprych, PhD Course code (if applicable) E-mail address to the person responsible for the course paulina.pianko @zut.edu.pl ECTS points 4 Type of course elective Level of course bachelor Semester summer Language of instruction English Hours per week Lecture: 2h workshop: 1h Hours per semester Lecture: 30h workshop: 15h Objectives of the course Familiarize students with the basic concepts of integrated ways of using available renewable energy sources. As a result of completion of the course the student should understand and know the principles of operation of various types of non-conventional energy sources. Student acquire skills for calculating energy systems such as solar collectors, heat pumps, biomass boilers. 28 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Mathematics, Physics, Technical Thermodynamics Course contents Lecture: Basic concepts related to the use of energy. Environmental aspects of energy production and use: pollution of the atmosphere, hydrosphere and lithosphere, the greenhouse effect, changes in the stratospheric ozone layer, acid rain. Basic concepts and principles of thermodynamics necessary for the understanding of energy conservation. Motor cycles, refrigerators and heat pumps, the irreversibility of the process, exergy, exergy efficiency (type II). Thermodynamic analysis of thermal processes. Global energy balances. Analysis of energy utilization. Economical use of energy and how its recovery. Heat pumps, energy accumulation, isolation. Waste heat recovery. Complete heating and cooling systems in manufacturing plants. Heat exchanger network design. Combined heat and power. Renewable energy sources and assess the possibility of their use. Renewable energy technologies for the production of electricity, heat and hydrogen. Workshop: Open system energy balance. Analysis of the rendering of heat: heat and mass balance in terms of concurrent heat exchanger and counter (heat exchange with the phase transition, and without it), driving the temperature differential, transfer coefficients and heat transfer, heat transfer surface. Basic concepts and principles of thermodynamics. Law of thermodynamics for open and closed system. Enthalpy calculations. Circuits: Clausius Rankine, heat pumps, refrigeration applied using low temperature waste energy. Thermodynamic analysis of thermal processes. Energy: global energy balance, the calculation of energy losses and efficiency. Assessment methods Lecture: class test Workshop: class test Learning outcomes The student has knowledge of the energy with respect to the protection of the environment. 1. Recommended readings 2. 3. 4. DOE fundamentals Handbook Thermodynamics, heat transfer and fluid flow, Washington, 1992. D.H.F. Liu, B.G. Liptak, Environmental Engineers’ Handbook, Lewis Publishers, New York, 1997. W. P. Cunningham, B.W. Saigo, Environmental science: a global concern, 5 Edition, McGraw-Hill, Boston, 1999. G. Miller, G. Tyler, Living in the environment: Principles, connections and solutions., Brooks Cole Publishing Company, Pacific Grove, CA, 2002. Additional information Course title ENVIRONMENTAL POLLUTION CONTROL Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_7 ECTS points 5 Type of course Obligatory Level of course Bachelor/master 29 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 2 Hours per semester Lecture – 30 Classes - 30 Objectives of the course The course aims to familiarize students with the basic of air, water, control. Entry requirements Physical chemistry. Chemical engineering fundamentals Course contents Introduction. Basic concepts. Air Pollution. Smog in troposphere. Ozone depletion in stratosphere. Acid Rain. Aerosols: deposition and nucleation. Control of air Pollution: absorption; adsorption, biofiltration, catalytic destruction. Particles capture. Water Pollution: organic, inorganic, biological. Waste Water Treatment: aerobic and anaerobic digesters, activated sludge process. Soil pollution: types of soil pollution, sources of soil pollution, effects of soil pollution. Monitoring and control of soil pollution. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Identify the various types of air, water, and soil pollutants. Explain the effects of pollutants on human beings and environment. Describe the sources of air, water, and soil pollutants. Demonstrate basic knowledge of control technologies preventing air, water, and soil pollution. 1. Recommended readings 2. 3. 4. and soil pollution Peirce J.J., Vesilind P.A., Weiner R.F., Environmental Pollution and Control, Elsevier, Amsterdam 1997. Hill M.K., Understanding Environmental Pollution. A Primer, Cambridge University Press, Cambridge 2004. Flagan R.C., Fundamentals of air pollution engineering, Prentice-Hall, New Jersey 1988. Mirsal I.A., Soil Pollution: Origin, Monitoring and Remediation, Springer, Berlin 2004. Additional information Course title FLUIZIDATION ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_8 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture - 2, Classes - 1 Hours per semester Lecture - 30, Classes - 15 30 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course The course aims to familiarize students with the basic of fluizidation engineering.. Entry requirements Chemical engineering fundamentals. Course contents Introduction. Mapping of fluidization regimes. Distributors. Gas jets. Pumping power. Bubbles in dense beds. Bubbling fluidized beds. High-velocity fluidization. Mixing, segregation, and Staging. Particle-to-gas mass and heat transfer. Catalytic Reactions in Fluidized Beds. Heat transfer between fluidized beds and surfaces. Size distribution of solids in fluidized beds. Industrial applications of fluidized beds. Design of fluidized beds operations. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Explain the basic elements of fluidized bed equipments. Demonstrate basic knowledge of fluidized bed phenomena. Identify the various types of fluidization regimes. Understand bubble mechanics, and quality of fluidization. Describe the scientific principles associated with fluidized bed equipments. Demonstrate basic knowledge of applications and design of fluidized bed equipments. Describe the operation and maintenance of fluidized bed equipments. Troubleshoot typical problems associated with the fluidized bed equipments. 1. Recommended readings 2. 3. 4. Kunii D., Levenspiel O., Fluidization Engineering, Butterworth-Heinemann, Boston 1991. Gupta C.K., Sathiyamoorthy D., Fluid Bed Technology in Materials Processing, CRC, Boca Raton 1999. Smith P.G., Applications of Fluidization to Food Processing, Blackwell Science, Oxford 2007. Gibilaro L.G., Fluidization dynamics, Butterworth-Heinemann, Boston 2001. Additional information Course title FUNDAMENTALS ENGINEERING Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) OF MATLAB IN CHEMICAL AND PROCESS E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the modeling of selected chemical and process engineering problems and their solution using Matlab. 31 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Chemical engineering, mathematics, numerical methods Course contents Matlab environment. Mathematical Matlab functions useful in engineering computations. Matlab instructions. Matrix operations. Graphs and graphics: 2D and 3D graphs, visualization of statistical data, surfaces in the space, space curves, animations. Analysis of experimental data: data import and export, approximation, data: interpolation, data smoothing. Solution of equations: linear equations, nonlinear equations and nonlinear equation systems, ordinary differential equations (ODE), types of equations and boundary conditions, Matlab numerical integrators, stiff ordinary differential equations, unsteadystate processes, nonlinear dynamics. Solution of partial differential equations: first and second order equations, initial value and boundary value problems, steady-state and unsteady-state. Identification of the differential equations parameters - solving of the reverse problems. Numerical solution method (Initial value problem). Approximate methods for boundary value problems: weighted residuals. Solution of the selected problems in chemical engineering: basic principles and calculations, problems of regression and correlation of data, advanced solution methods in problem solving. Thermodynamics. Heat transfer. Mass transfer. Problems of fluid mechanics. Examples of selected problems: variation of reaction rate with temperature, shooting method for solving two-point boundary value problems, fugacity coefficients for ammonia – experimental and predicted, optimal pipe length for draining a cylindrical tank in turbulent flow, unsteady-state conduction in two dimensions, simultaneous heat and mass transfer in catalyst particles, etc. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of Matlab functions and instructions. Demonstrate ability of programming using Matlab. Identify the various types of applied numerical methods. Solve typical and more advanced problems associated with chemical and process engineering using Matlab techniques. 1. Recommended readings 2. 3. 4. O.T. Hanna, O.C. Sandall, Computational methods in chemical engineering, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1995. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007. Additional information Course title FUNDAMENTALS OF RESERVOIR FLUID BEHAVIOR AND ITS PROPERTIES Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) Type of course compulsory E-mail address to the person responsible for the course [email protected] ECTS points 4 Level of course Bachelor 32 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Introduction to the reservoir engineering of oil and natural gas. Entry requirements Chemical engineering, thermodynamics Course contents Fundamentals of reservoir fluid behavior: classification of reservoir and reservoir fluids, pressure-temperature diagram, oil reservoir, gas reservoir, undefined petroleum fractions. Reservoir-fluid properties: properties of natural gases, behavior of ideal gases, behavior of real gases, effect of non-hydrocarbon components on the Z-factor, non-hydrocarbon adjustment methods, correction for high-molecular-weight gases, gas formation volume factor, properties of crude oil systems, crude oil gravity, specific gravity of the solution gas, gas solubility, bubble-point pressure, oil formation volume factor, crude oil density, crude oil viscosity. Laboratory analysis of reservoir fluids. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of reservoir fluids and their properties. Identify the various types of methods in fluid properties estimation. Solve typical calculation problems associated with analysis of reservoir fluids. 1. Recommended readings 2. 3. T. Ahmed, Reservoir engineering, 2nd ed.,Gulf Professional Publishing (ButterworthHeinemann), Boston, 2001. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. B.E. Poling, J.M. Prausnitz, J.P. O’Connel, The Properties of Gases and Liquids, McGrawHill, New York 2001. Additional information Course title HEAT TRANSFER Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_9 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 33 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course The course aims to familiarize students with the basic of heat transfer Entry requirements Mathematics, Physics. Course contents Introduction. Heat conduction. Convective heat transfer: laminar and turbulent. Simultaneous heat and mass transfer. Boiling. Condensation. Radiation. Heat exchanger: type of equipment. Heat exchanger calculations. Using Aspen to design of heat exchanger. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Identify the different modes of heat transfer. Formulate basic equation for heat transfer problems. Solve differential and algebraic equations associated with heat transfer using analytical and numerical methods. Apply heat transfer principles to design heat exchanger. Apply Aspen Plus to design of heat exchanger. Recommended readings 1. 2. Incropera F.P., Lavine A.S., DeWitt D.P., Fundamentals of Heat and Mass Transfer, Wiley, New York 2011. Rathore M.M., Kapuno R.R., Engineering Heat Transfer, Jones & Bartlett Learning, Sudbury 2011. Additional information Course title HETEROGENEOUS CATALYSIS Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_10 ECTS points 5 Level of course Bachelor/master Type of course Obligatory Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 2 Hours per semester Lecture – 30 Classes - 30 Objectives of the course The course aims to familiarize students with the basic of heterogeneous catalysis. Entry requirements Physical chemistry. 34 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction. A brief history of catalysis. Surfaces and adsorption: Energetics; Pore structure and surface area; Isotherms and rates; Experimental aspects of adsorption. The catalytic process. The catalyst and the catalytic site. Catalyst preparation. Catalyst Characterization. Catalytic Reactors: Static reactors; Stirred and recirculation reactors; Flow reactors; Pulse Reactors. Measurement of catalytic kinetics. Exemplary catalytic reactions. Catalysis in environmental protection. Assessment methods Lecture – exam Classes – grade Learning outcomes Upon completion of this course, the students should be familiar with the basic knowledge about structures, preparation methods and reactivity of catalysts, such as zeolites, monoliths, supported metals, carbon catalysts, etc. Demonstrate basic knowledge of the use of catalysts in catalytic processes in chemical, pharmaceutical and food industry and in environmental protection. 1. Recommended readings 2. 3. Ross J.R.H., Heterogeneous catalysis. Fundamentals and applications, Elsevier, Amsterdam 2012. Satterfield, Charles N., Mass transfer in heterogeneous catalysis, R. E. Krieger Pub. Co., Huntington, N.Y. 1981. J. M. Thomas, W. J. Thomas, Principles and Practice of Heterogeneous Catalysis, VCH, Weinheim 1997. Additional information Course title HYBRID SOURCES OF ENERGY Teaching method Lecture, workshop Person responsible for the course Paulina Pianko-Oprych, PhD Course code (if applicable) E-mail address to the person responsible for the course paulina.pianko @zut.edu.pl ECTS points 2 Type of course elective Level of course master Semester summer Language of instruction English Hours per week Lecture: 1h Workshop: 1h Hours per semester Lecture: 15h Workshop: 15h Objectives of the course Familiarize students with the basic concepts of integrated ways of using available renewable energy sources. Entry requirements Mathematics, Physics, Technical Thermodynamics 35 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Lecture: Basic definitions of hybrid systems. Integrated ways of using renewable energy sources: water - sun, water - wind, solar - wind, wind - the sun - the water, the integration of hydro and geothermal energy. Classifications and application of hybrid systems: the original source - solar and secondary sources: chemical battery, wind turbines, diesel generator, fuel cells. Hybrid photovoltaic systems. Construction and operation of the fuel cell. Types and performance of fuel cells. Hybrid heating systems: heat pump assisted biomass-fired boilers, solar collectors connected to a conventional heat source, heat recovery units, thermo fireplace with gas or oil boiler. Batteries and power applications. Hydrogen economy. Alternative vehicles - hybrid car with power from the fuel cell, hybrid car with a combustion engine. The efficiency of a fuel cell car. Impact on the environment. Hybrid systems in nuclear power - energy production and processing of radioactive waste. Advantages and disadvantages of hybrid energy sources. Workshop: Solutions of problems connected with energy conversion technologies in industrial energy systems. Optimization of industrial energy systems considering future costs associated with greenhouse gas emissions. Overview of energy policy instruments and their impact on industrial energy system decision-making. Assessment methods Lecture: class test workshop: class test Learning outcomes The student has knowledge of the energy with respect to the protection of the environment. 1. Recommended readings 2. 3. 4. DOE fundamentals Handbook Thermodynamics, heat transfer and fluid flow, Washington, 1992. D.H.F. Liu, B.G. Liptak, Environmental Engineers’ Handbook, Lewis Publishers, New York, 1997. W. P. Cunningham, B.W. Saigo, Environmental science: a global concern, 5 Edition, McGraw-Hill, Boston, 1999. G. Miller, G. Tyler, Living in the environment: Principles, connections and solutions., Brooks Cole Publishing Company, Pacific Grove, CA, 2002. Additional information Course title HYDROGEN AS A FUTURE ENERGY CARRIER Teaching method Lecture, laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) 36 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course Presentation of the future possibility of a hydrogen application as a future energy carrier. Entry requirements Chemical engineering, physical chemistry, thermodynamics Course contents Introduction: history of hydrogen. Hydrogen as a fuel: fossil fuels, the carbon cycle and biomass energy, the hydrogen cycle. Properties of hydrogen: hydrogen gas, interaction of hydrogen with solid surfaces, the four states of hydrogen and their characteristics and properties, surface engineering of hydrides. Hydrogen production: electrolysis – hydrogen production using electricity. Hydrogen storage. Applications: internal combustion engines, hydrogen in space applications, fuel cells using hydrogen. Hydrogen from the sun energy. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of hydrogen and its properties. Describe the scientific principles associated with hydrogen production, especially from natural source (sun energy), storage, transport and application (Fuel Cells). Solve typical problems associated with process modeling concerning hydrogen applications. 1. Recommended readings 2. 3. 4. 5. A. Züttel, A. Borgschulte, L. Schlapbach eds., Hydrogen as a future energy carrier, Viley-VCH, Weincheim, 2008. B. Elvers ed., Handbook of fuels. Energy sources fortransportation, 2007. G.A. Olah, A. Goeppert, G.K.S. Prakash, Beyond oil and gas: The methanol economy, Viley-VCH, Weincheim, 2006. K. Sundmacher, A. Kienle, H.J. Pesch, J.F. Berndt, G. Huppmannn eds., Molten carbonate fuel cells, Viley-VCH, Weincheim, 2007. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. Additional information Course title INTRODUCTION TO CHEMICAL ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Henryk Łącki, PhD Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_11 ECTS points 4 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes -1 Hours per semester Lecture – 30 Classes -15 Objectives of the course The course aims to familiarize students with the history and basic of chemical engineering. 37 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Physics, Mathematics. Course contents Introduction. Chemical engineering definition: What is Chemical Engineering ?; How chemical engineering differs from pure chemistry or other types of engineering? What do Chemical Engineers do?; Diversity of employment. History and perspectives of chemical engineering. Concerns of chemical engineering. Concept of unit operations. Case studies and examples. Basic tools of chemical engineering: physical, chemical, mathematical and biological sciences, transport phenomena, thermodynamics, kinetics and reactors design. Scaleup, modeling and simulation. Multiscale modeling. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Identify and understand the chemical engineering profession. Understand the basic chemical engineering principles. Identify the basic tools of chemical engineering. Develop problem solving skills. Use software (POLYMATH, MATLAB) to solve chemical engineering problems. Recommended readings 1. 2. Denn M.M., Chemical Engineering. An introduction, Cambridge University Press, New York 2012. Furter W.F.(Ed.), History of Chemical Engineering, Advances in Chemistry Series (190), American Chemical Society 1980. Additional information Course title INTRODUCTION PROCESSES Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) TO THERMODYNAMICS OF IRREVERSIBLE E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L) 2 (Lab) Hours per semester 30 (L) 30 (Lab) Objectives of the course Modern thermodynamics is a theory of irreversible processes. Modern thermodynamics, formulated in twentieth century by Lars Onsager, Theophile De Donder, Ilya Prigogine and others is a theory of irreversible processes that very much include time: it relates entropy, the central concept of thermodynamics, to irreversible processes. Entry requirements Chemical engineering, physical chemistry 38 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction: the terminology of thermodynamics, the variables and quantities. Basic concepts and the laws of gases. The First Law of thermodynamics. The Second Law of thermodynamics and the arrow of time. Entropy in the realm of chemical reactions. Premium principles and general thermodynamic relations. Applications: equilibrium and equilibrium systems. Thermodynamics of phase change. Thermodynamics of solutions. Thermodynamics of chemical transformations. Introduction to non-equilibrium systems. Thermodynamics of radiation. Biological systems. Classical stability theory. Critical phenomena and configurational heat capacity. Elements of statistical thermodynamics. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of irreversible thermodynamics. Identify the various methods of thermodynamic problems solving. Solve typical problems associated with modern thermodynamics. 1. 2. Recommended readings 3. 4. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. D. Kondepudi, Introduction to modern thermodynamics, John Wiley & Sons Inc., Chichester, UK, New York 2008. J.M. Prausnitz, R.N. Lichtenthaler, E.G. de Azevedo, Molecular Thermodynamics of Fluid Phase Equilibria, Prentice Hall PTR, New Jersey 1999. Additional information Course title MASS TRANSFER Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc. E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_12 ECTS points 4 Type of course Obligatory. Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 Objectives of the course The course aims to familiarize students with the basic of mass transfer. Entry requirements Mathematics, Physics. Course contents Introduction. Molecular diffusion. Convective mass transfer: laminar and turbulent. Simultaneous heat and mass transfer. Interface mass transfer. Mass exchanger: type of equipment. Mass exchanger calculations. Design of mass exchanger using Aspen. 39 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Identify and understand the various mechanisms of mass transfer. Formulate basic equation for mass transfer problems. Use of experimentally derived correlations for estimating mass transfer coefficient for a variety of flow situations. Apply mass transfer principles to design mass transfer equipment. 1. Recommended readings 2. 3. Incropera F.P., Lavine A.S., DeWitt D.P., Fundamentals of Heat and Mass Transfer, Wiley, New York 2011. Hines A.L., Maddox R.N., Mass transfer: fundamentals and applications, Prentice-Hall, New Jersey 1985. Cussler E.L., Diffusion: mass transfer in fluid systems, Cambridge University Press, New York 1997. Additional information Course title MATHEMATICAL METHODS IN CHEMICAL ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_13 ECTS points 5 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture -2 Classes - 2 Hours per semester Lecture -30 Classes - 30 Objectives of the course The objective of the course is to develop the mathematical and modeling skills needed to solve chemical engineering problems. Entry requirements Fundamentals of mathematics. Course contents Formulation of physicochemical problems. Modelling: model building process. Model hierarchy. Models with many variables. Boundary conditions. Vector spaces. Matrices. Matrix algebra: row operations, direct elimination methods, iterative methods. Special functions. Ordinary differential equations. First-order equations. Solution methods for second-order nonlinear equations. Linear equations of higher order. Coupled Simultaneous ODE. Series solution methods. Integral functions. Staged-process models. The calculus of finite differences. Approximate methods for ODE solution. Perturbation methods. Initial value problems. Boundary value problems: weighted residuals. Elements of complex variables. Laplace transforms. Solution techniques for solving PDEs. Assessment methods Lecture – exam Classes – grade 40 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes Recommended readings The student will be able to: Describe chemical engineering processes in mathematical form. Identify analytical solution to the differential equations. Interpret the solution to differential equations. 1. 2. 3. Rice R.G., Do D.D., Applied mathematics and modeling for chemical engineers, Wiley, New York 2012. Finlayson B.A., Introduction to chemical engineering computing, Wiley, New York 2005. Basmadjian D., The art of modeling in science and engineering, CRC, Boca Raton 2000. Additional information Course title MODELING AND SIMULATION IN CHEMICAL ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_14 ECTS points 5 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture -2, Classes - 2 Hours per semester Lecture -30, Classes - 30 Objectives of the course The course aims to familiarize students with the basic of modeling and simulation in chemical engineering. Entry requirements Mathematics. Fundamentals of chemical engineering. Course contents Analysis of experimental results. Nonlinear parameter estimation. Dimensional analysis. Scaling. Mathematical model development. Synthesis of sub-models. Classification of models: deterministic, stochastic, lumped and distributed parameter. Modelling and simulation techniques. Population balance models. Microbial population. Monte Carlo methods. Nonlinear dynamics and chaos. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Develop of process models based on conservation laws and process data. Use computational techniques to solve the process models. Use simulation tools such as MATLAB, POLYMATH, and ASPEN PLUS. 41 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. 2. Recommended readings 3. 4. 5. Hangos K.M., Cameron L.T., Process modelling and model analysis, Academic Press, San Diego 2001. Ingham J., Dunn I.J., Heinzle E., Prenosil J.E., Snape J.B., Chemical engineering dynamics, Wiley, Weinheim 2007. Dobre T.G., Marcano J.G.S., Chemical engineering. Modelling, simulation and similitude, Wiley, Weinheim 2007. Rice R.G., Do D.D., Applied mathematics and modeling for chemical engineers, Wiley, New York 2012. Finlayson B.A., Introduction to chemical engineering computing, Wiley, New York 2005. Additional information Course title MODERN DRYING TECHNIQUES – THEORY AND PRACTICE Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the theory and practice of drying process. Presentation of novel techniques and apparatuses for drying of various materials Entry requirements Chemical engineering, thermodynamics Course contents Moisture in gases and solids: thermodynamic of moist gas, thermodynamic of moist solids. Heat and mass transfer in drying processes. Drying kinetics. Experimental methods in drying. General principles of dryer design. Mathematical modeling of drying processes. Drying in energy fields. Performance of modern industrial dryers. Miscellaneous drying problems: selection of dryer, energy aspects. Procedures for choosing of a dryer. Selection schemes. Batch dryers (e.g. Vacuum dryers, Fluid-bed batch dryers, Tray dryers, Agitated pan dryers etc.). Continuous dryers – selection tree (e.g. Conduction dryer with inert stripping gas, e.g. plate dryer, Milling/flash drying, Band (Belt) dryer, Flash dryer, possibly with product recirculation, Convection/conduction dryer with rotating shell or agitation, e.g. disc or rotary dryer, Fluid-bed dryer, circular stirred tank rectangular, spray dryer, Miscellaneous continuous dryers, etc.). Processing liquids, slurries, and pastes (Spray dryers, Film drum dryers, Continuous Fluid-bed dryers/Granulators, Cylindrical scrapedsurface evaporator/Crystallizer/Dryer, Agitated pan or vacuum dryers). Special drying techniques (Infrared drying, Dielectric drying, Freeze-drying, Steam drying). Qualitative comparison of Convective, Conduction, and Dielectric dryer types. Testing on Small-scale dryers. Example of dryer selection procedure. Assessment methods Lecture: oral exam Computer laboratory: grade 42 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Demonstrate basic knowledge of thermodynamics of moist gas and solid. Explain the basic elements of drying kinetics. Identify the various types of drying methods. Demonstrate basic knowledge of applications and design of dryers. Solve typical problems associated with dryers design and modeling. 1. Recommended readings 2. 3. 4. C. Strumiłło, T. Kudra, Drying: Principles, Applications and Design, Gordon and Breach Sci. Publ., New York 1986. C.M. Van ’t Land, Drying in the Process Industry, John Wiley & Sons, Inc., New York 2012. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. Additional information Course title MULTIPHASE FLOWS Teaching method Lecture; Project. Person responsible for the course Joanna Karcz, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_2A_S_15 ECTS points 4 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture - 2, Project - 1 Hours per semester Lecture - 30, Project - 15 Objectives of the course The course aims to give a general introduction to the theory of multiphase flow and to provide the necessary theoretical basis for design of multiphase pipelines. Entry requirements Chemical engineering fundamentals. Course contents Introduction to multiphase flow: basic definitions, equations of motion, interaction with turbulence. Gas–liquid and liquid-liquid flow systems. Cavitation. Boiling and condensation. Fluid–solid flow systems. Fluidized beds. Aerosol flows. Particle separation systems. Spray systems. Dry powder flows. Granular flows. Microscale and microgravity flows. Multiphase interactions. Multiphase flows in pipes: flow regime maps, concentration distributions and pressure drop. Assessment methods Lecture - exam, Project - project work. Learning outcomes The student will be able to: Analyze and characterize the multiphase systems. Solve a variety of engineering problems involving multiphase flows. Apply chemical engineering principles to design of multiphase flow systems. 43 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. Recommended readings 2. 3. 4. Brennen Ch.E., Fundamentals of Multiphase Flow, Cambridge University Press, Cambridge 2005. Crowe C.T. (Ed.), Multiphase flow handbook, CRC Press, Boca Raton 2006. Faghri A., Zhang Y., Transport Phenomena in Multiphase Systems, Elsevier Academic, Boston, 2006 Perry's Chemical Engineers' Handbook, McGraw-Hill, New York 2007. Additional information Course title NATURAL GAS ENGINEERING Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_16 ECTS points 4 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes –15 Objectives of the course The course aims to familiarize students with the basic of natural gas engineering. Entry requirements Fundamentals of chemical engineering Course contents Introduction. Origin and production of natural gas. Composition and properties of natural gas. Gas processing. Recovery, storage, and transportation. Water removal. Liquids removal. Nitrogen removal. Acid gas removal. Fractionation. Hydrogen sulfide conversion. Processes. Compression and cooling. Volumetric measurement. Pipeline design. Transportation of LNG. Hydrate control. Pipeline cleaning. Emissions control and environmental aspects. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Estimate natural gas properties and to predict the performance of the natural gas. Understand the formation of hydrates. Demonstrate basic knowledge of separation of natural gas and liquid. Demonstrate basic knowledge of hydrates removal from natural gas. Demonstrate basic knowledge of miscellaneous gas removal from natural gas. Demonstrate basic knowledge of dehydration of natural gas. Identify the most common type of equipment used in the processing of natural gas. Identify various types of instruments and controls used in the processing of natural gas. 44 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. Recommended readings 2. 3. 4. Guo B., Ghalambor A., Natural Gas Engineering Handbook, Gulf Publishing Company, Houston 2005. Younger A.H., Natural Gas Processing Principles and Technology - Part I, II, University of Calgary 2004. Abdel-Aal H.K., Aggour M., Fahim M. A., Petroleum and gas field processing, Marcel Dekker New York 2003. Speight J.G., Natural Gas. A Basic Handbook, Gulf Publishing Company, Houston 2007. Additional information Course title NUMERICAL AND ANALYTICAL METHODS WTH MATLAB Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the numerical and analytical methods analysis to chemical and process engineering problems solution using Matlab. Entry requirements Chemical engineering, mathematics, numerical methods Course contents Numerical modeling for engineering. Matlab fundamentals. Matrices. Roots of algebraic and transcendental equations. Numerical integration. Numerical integration of ordinary differential equations (ODE). Curve fitting. Optimization. Partial differential equations. Iteration method. Laplace transforms. Solution of equations: linear equations, nonlinear equations and nonlinear equation systems, ordinary differential equations (ODE), types of equations and boundary conditions, Matlab numerical integrators, stiff ordinary differential equations, unsteady-state processes, nonlinear dynamics. Solution of partial differential equations: first and second order equations, initial value and boundary value problems, steady-state and unsteady-state. Numerical solution method (Initial value problem). Approximate methods for boundary value problems: weighted residuals. Solution of the selected problems in chemical engineering: basic principles and calculations, problems of regression and correlation of data, advanced solution methods in problem solving. Thermodynamics. Heat transfer. Mass transfer. Problems of fluid mechanics. Examples of selected problems: variation of reaction rate with temperature, shooting method for solving two-point boundary value problems, fugacity coefficients for ammonia – experimental and predicted, optimal pipe length for draining a cylindrical tank in turbulent flow, unsteadystate conduction in two dimensions, simultaneous heat and mass transfer in catalyst particles, etc. Assessment methods Lecture: oral exam Computer laboratory: grade 45 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Demonstrate basic knowledge Matlab functions and instructions. Identify the various types of numerical and analytical methods of problem solution. Solve typical problems associated with chemical and process engineering using Matlab with Simulink. 1. 2. Recommended readings 3. 4. 5. A. Gilat, V. Subramanian, Numerical methods: An introduction with applications using Matlab, John Wiley & Sons, Inc., New York, 2011. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. W. Bober, C-T Tsai, O. Masory, Numerical and analytical methods with Matlab, CRC Press – Taylor & Francis Group, London,2009. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007. Additional information Course title NUMERICAL METHODS Teaching method Lecture; Classes Person responsible for the course Bogdan Ambrożek, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course Obligatory Level of course Bachelor/Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 Objectives of the course The course aims to familiarize students with the basic of numerical methods and their application in science and engineering. Entry requirements Mathematics. Course contents Systems of linear algebraic equations. Systems of non-linear algebraic equations. Interpolation and curve fitting. Numerical differentiation. Numerical integration. Eigenvalues and eigenvectors of matrices. Solutions of ODEs: Runge Kutta, multistep methods, Gear’s algorithm, stiffness and stability of algorithms. Solutions of PDEs: finite difference, finite elements, method of lines, shooting methods. Introduction to optimization. Discrete transform methods: Fourier series, applications of discrete Fourier series, discrete Chebyshev transform and applications. Assessment methods Lecture – exam Classes – grade 46 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Use of modern computational and numerical techniques in engineering. Understand how the algorithms work and why numerical algorithms sometimes give unexpected results. 1. 2. Recommended readings 3. 4. Chapra S.C., Canale R.P., Numerical Methods for Engineers, McGraw-Hill, Boston 1998. Rao S.S., Applied Numerical Methods for Engineers and Scientists, Prentice Hall, New Jersey 2002. Kiusalaas J., Numerical Methods in Engineering with Python 3, Cambridge University Press, Cambridge 2013. Moin P., Fundamentals of engineering numerical analysis, Cambridge University Press, New York 2010. Additional information Course title NUMERICAL METHODS IN CHEMICAL ENGINEERING Teaching method Lecture; Classes Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_17 ECTS points 4 Type of course Obligatory Level of course Bachelor / Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 Objectives of the course The course aims to familiarize students with the basic of numerical methods and their application in chemical engineering. Entry requirements Mathematics. Course contents Systems of linear algebraic equations. Systems of non-linear algebraic equations. Interpolation and curve fitting. Numerical differentiation. Numerical integration. Eigenvalues and eigenvectors of matrices. Solutions of ODEs: Runge Kutta, multistep methods, Gear’s algorithm, stiffness and stability of algorithms. Solutions of PDEs: finite difference, finite elements, method of lines, shooting methods. Introduction to optimization. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Use of modern computational and numerical techniques in chemical engineering. Understand how the algorithms work and why numerical algorithms sometimes give unexpected results. 47 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. 2. 3. 4. Recommended readings 5. 6. 7. 8. 9. Beers K.J., Numerical methods for chemical engineering. Applications in MATLAB, Cambridge University Press, Cambridge 2007. Elnashaie S., Uhlig F., Numerical techniques for chemical and biological engineers using MATLAB, Springer, New York 2007. Warnecke G., Analysis and numerics for conservation laws, Springer, Berlin 2005. Rice R.G., Do D.D., Applied mathematics and modeling for chemical engineers, Wiley, New York 1995. Chapra S.C., Canale R.P., Numerical Methods for Engineers, McGraw-Hill, Boston 1998. Rao S.S., Applied Numerical Methods for Engineers and Scientists, Prentice Hall, New Jersey 2002. Cutlib M.B., Shacham M., Problem Solving in Chemical Engineering with Numerical Methods, Prentice Hall, New Jersey 2009. Hanna O.T., Sandall O.C., Computational Methods In Chemical Engineering, Prentice Hall, New Jersey 1995. Constantinides A., Mostoufi N., Numerical Methods for Chemical Engineers with Matlab Applications, Prentice Hall, New Jersey 1999. Additional information Course title OPTIMIZATION IN CHEMICAL ENGINEERING Teaching method Lecture; Classes Person responsible for the course Bogdan Ambrożek, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course Obligatory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 Objectives of the course The course aim is to teach students how to use optimization algorithms to improve the design and operation of chemical processes. Entry requirements Mathematics, Chemical engineering fundamentals Course contents Formulation of optimization problems. Classification of optimization problems. Techniques of optimization. One-dimensional and n-dimensional search techniques. Optimization modeling platforms: MATLAB, ASPEN,and HYSYS. Application of optimization methods in Chemical Engineering. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Understand the concepts of the different optimization methods. Apply the knowledge of optimization to solve chemical engineering problems. Apply MATLAB, ASPEN,and HYSYS to solve optimization problems. 48 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. Recommended readings 2. 3. Edgar T.F., Himmelblau D.M., Lasdon L.S., Optimization of Chemical Processes, McGraw Hill 2001. Corsano G., Montagna J.M., Iribarren O.A., Aguirre P. A., Mathematical Modeling Approaches for Optimization of Chemical Processes, Nova Science Publishers 2009. Rangaiah G.P., Bonilla-Petriciolet A. (Eds), Multi-Objective Optimization in Chemical Engineering: Developments and Applications, Wiley 2013. Additional information Course title PARTICULATE TECHNOLOGY Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_18 ECTS points 4 Type of course Obligatory Level of course Bachelor / Master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 1 Hours per semester Lecture – 30 Classes - 15 Objectives of the course The course aims to familiarize students with the basic of particulate technology. Entry requirements Fundamentals of chemical engineering. Course contents Particle characterization. Particle size analysis. Motion of solid particles in a fluid. Multiple particle systems. Colloids and fine particles. Fluid flow through a packed bed. Filtration. Fluidization. Pneumatic transport. Separation of particles from a gas. Mixing and segregation of particles. Particles size reduction. Particles mechanics. Discharge of particulate bulk solids. Storage and flow of powders. Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Understand and apply the theoretical fundamentals of particle technology in chemical engineering. Understand the experimental methods necessary to characterize the properties of particles and powders. Understand the hydrodynamics of gas-solid systems. Recommended readings 1. 2. 3. 4. Rhodes M., Introduction to Particle Technology, Wiley, Chichester 2008. Particles, bubbles and drops-their motion, heat and mass transfer, World Scientific Publishing, London 2006. Aste T., Tordesillas A., Di Matteo T. (Editors), Granular and complex materials, World Scientific Publishing, London 2007. Gregory J., Particles in Water. Properties and Processes, CRC, Boca Raton 2006. 49 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Additional information Course title PETROLEUM PRODUCTION SYSTEMS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Introduction to the reservoir engineering of oil and natural gas. Entry requirements Chemical engineering, thermodynamics Course contents Introduction to reservoir engineering: history, reservoirs types defined with reference to phase diagrams; review of: rock properties, gas properties, crude oil properties, reservoir water properties. Derivation of material balance equation. Single-phase gas reservoirs. Gascondensate reservoirs. Undersaturated gas reservoirs. Saturated gas reservoirs. Single fluid flow in reservoirs. The role of petroleum production engineering. Production from undersaturated oil reservioirs. Production from two-phase reservoirs. Production from natural gas reservoirs. The near wellbore condition and damage characterization; skin effects. Wellbore flow performance. Well deliverability. Forecast of well production. Well test design and data acquisition. Well diagnosis with production logging. Design of hydraulic fracture treatments. Gas lift (maximum production rate). Pomp-assisted lift. Systems analysis. Environmental concerns in petroleum production engineering. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of petroleum production systems. Calculate basic reservoir fluids properties. Formulate reservoir fluids mass balance. Describe the scientific principles associated with petroleum and natural gas production engineering. Recommended readings 1. 2. 3. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. M.J. Economides, A.D. Hill, C. Ehling-Economides, Petroleum Production Systems, Prentice Hall PTR, New Jersey 1994. B.E. Poling, J.M. Prausnitz, J.P. O’Connel, The Properties of Gases and Liquids, McGrawHill, New York 2001. 50 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Additional information Course title POLYMATH, MATHAD AND MATLAB FOR CHEMICAL ENGINEERS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Practical use of the Polymath, Mathcad and Matlab to solving of the various computational problems appearing in chemical and process engineering Entry requirements Chemical engineering, mathematics, numerical methods Course contents Problem solving with mathematical software packages. Basic principles and calculations. Regression and correlation of data. Problem solving with Polymath. Problem solving with Mathcad. Problem solving with Matlab. Advanced techniques in problem solving. Thermodynamic problems. Selected fluid mechanics problems. Selected heat transfer problems. Selected mass transfer problems. Problems of chemical reaction engineering. Phase equilibria and distillation. Process dynamic and control. Biochemical engineering. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of Polymath, Mathcad and Matlab functions and instructions. Identify the various types of numerical methods. Demonstrate ability of using Polymath, Mathcad and Matlab basic and advanced techniques in problem solving. Solve typical problems associated with chemical and process engineering using Polymath, Mathcad and Matlab. 51 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ 1. 2. 3. Recommended readings 4. 5. 6. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2008. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. O.T. Hanna, O.C. Sandall, Computational methods in chemical engineering, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1995. L. Fausett, Numerical methods using Mathcad, Prentice Hall, Pearson Education Ltd., London, 2002. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007. H.S. Fogler, Elements of chemical reaction engineering, 4th ed., Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2006. Additional information Course title PROCESS DESIGN Teaching method Lecture, workshop Person responsible for the course Paulina Pianko-Oprych, PhD Course code (if applicable) E-mail address to the person responsible for the course paulina.pianko @zut.edu.pl ECTS points 9 Type of course obligatory Level of course master Semester summer Language of instruction English Hours per week Lecture: 3h Project: 4h Hours per semester Lecture: 45h workshop: 60h Objectives of the course Design procedures. Raw materials and processes chemical technology. Solutions of the process line. Selection, design and operation of large – scale industrial apparatus. Economic analysis. Solving of problems in momentum, heat and mass transfer processes. Design of selected apparatus. Balance of materials and energy. CAD for design of chemical processes. Entry requirements Mathematics, Chemical engineering, Chemical technology Course contents Lecture: Procedure of elaboration new technologies; rules of preparing documentation of process design, feasibility study; raw materials and product, process description; selection of processes and operations; selection and calculation procedure of apparatus and installations; balance of materials and energy; technological scheme of an industrial installation; CAD for design of chemical processes; economic analysis of an investment enterprise. Project: Formulation of plant design problem, scope and objectives; construction of flow sheet; plant location selection; construction of process description, process flow diagram, mass and energy balance; selection and sizing of major process equipment; construction materials selection; equipment layout plot plan; construction cost estimation and plant economic analysis; piping and instrumentation diagram; plant design report preparation. 52 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods Learning outcomes Lecture: class test workshop: written report The student can explain the industrial system design methodology in accordance with current legislation and based on modern computer-aided design tools 1. 2. 3. 4. Recommended readings 5. 6. 7. 8. Himmelblau, Basic principles and calculation in chemical engineering, New York, 1986. G.I. Wells, L.M. Rose, The art of chemical process design, Elsevier, 1986. W. D. Seider, Process design principles, J.W.& S., 1999. J. B. Riggs, An Introduction to Numerical Methods for Chemical Engineers, Texas Tech University Press, Lubbock, Texas, 1982. O. T. Hanna, O.C. Sandal, Computation Methods in Chemical Engineering, Prenti-Hall, Englewood Cliff, New Jersey, 1997. W. D. Baasel, Preliminary Chemical Engineering Plant Design, 2 Edition, van Nostrand, New York, 1990. M. S. Peters, K.D. Timmerhaus, Plant Design and Economics for Chemical Engineers, McGraw-Hill Book Co., Inc., New York, 1991. R. K. Sinnott, Coulson-Richardson’s Chemical Engineering, vol. 6, An Introduction to Chemical Engineering Design, Pergamon Press, Oxford, 1985. Additional information Course title PROCESS DYNAMICS AND CONTROL Teaching method Lecture; Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_19 ECTS points 4 Type of course Obligatory Level of course Bachelor / Master Semester winter / summer Language of instruction English Hours per week Lecture – 2, Classes. - 2 Hours per semester Lecture – 30, Classes. - 30 Objectives of the course The course aims to familiarize students with the basic of process dynamics and control with emphasis on chemical engineering applications. Entry requirements Mathematics, Fundamentals of chemical engineering. Course contents Introduction. Process modeling fundamentals. Modeling for process operation. Transformation techniques. Linearization of model equations. Operating points of a systems. Process simulation in Matlab Simulink. Frequency response analysis. The dynamic behavior of systems. Detailed analysis of selected processes: mixing process, chemical stirred tank reactors, tubular reactors, heat exchangers, evaporators and separators, distillation columns, fermentation reactors. Black box modeling. Time-series identification. Neural networks. Fuzzy modeling. Process control and instrumentation. Behavior of controlled processes. Control of selected processes. 53 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods Lecture – exam Classes – grade Learning outcomes The student will be able to: Analyze the transient behavior of chemical engineering processes. Understand the behavior of control systems. 1. Recommended readings 2. 3. Roffel B., Betlem B., Process Dynamics and Control. Modeling for Control and Prediction, Wiley, Chichester 2006. Ingham J., Dunn I.J., Heinzle E., Pfenosi1 J.E., Chemical Engineering Dynamics, VCH, Weinheim 1994. Luyben M.L., Luyben W.L., Essentials of Process Control, MCGraw-Hill 1997. Additional information Course title PROCESS KINETICS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the various kinetic problems in chemical and process engineering. Entry requirements Mathematics, physics, chemical engineering Course contents Introduction to chemical engineering calculations: units and dimensions, conventions in methods of analysis and measurement, chemical reaction equation and stoichiometry. Basic concepts and definitions. Chemical engineering kinetics and thermodynamics. Conductive, convective and radiative heat transfer. Mass transfer in gases and liquids. A study of the design of chemical engineering systems. Kinetics of homogeneous systems and the interpretation of kinetic data. Heterogeneous systems. Two fluid-phase systems. Fixed bed adsorption. Fluid bed systems. The film model. Surface renewal models. Adsorption and chemical reaction. Introduction to chemical reaction engineering. The design of single and multiple reactors for simple, simultaneous and consecutive reactions. The influence of temperature, pressure and flow on chemical engineering systems. Assessment methods Lecture: oral exam Computer laboratory: grade 54 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Demonstrate basic knowledge of chemical engineering kinetics and thermodynamics. Identify and describe mathematically the chemical and physical processes associated with chemical and process engineering. Solve typical problems associated with process design. 1. 2. Recommended readings 3. 4. R.B. Bird, W.E. Stewart, E.N. Lightfoot, Transport phenomena, John Wiley & Sons, Inc., New York, 2007. H.S. Fogler, Elements of chemical reaction engineering, 4th ed., Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2006. D.M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, Prentice Hall International (UK) Limited, London, 1996 E.I., Shaheen, Basic Practice of Chemical Engineering, 2nd ed. Boston, Houghton Mifflin, 1984 Additional information Course title QUALITY ENGINEERING Teaching method Lecture, workshop Person responsible for the course Jolanta Szoplik, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course Compulsory Level of course Bachelor Semester summer / winter Language of instruction English Hours per week Lecture (1h), workshop (1h) Hours per semester L (15h), W (15h) Objectives of the course The course aim is to give a general introduction to the theory and practice of quality management and to know methods useful in quality control and improvement. Entry requirements Mathematics, statistics – basic courses Course contents The meaning of quality and quality improvement, quality engineering terminology, statistical methods for quality control and improvement. Probability distribution: binomial distribution, Poisson distribution, normal distribution. Calculate the probability of finding (z) scraps in the sample. Calculate the probability that product will meet or exceed the specification, calculate the fraction of produced conform to specification. Statistical process control SPC – principles, methods and tools: Pareto Chart, Cause and effect diagram, scatter diagram, Shewhart control charts: (variables control charts x -R or x-s; attributes control charts p, np, c or u). Acceptance sampling plans for attributes, single and double plans for attributes. Find the normal, tightened and reduced single and double sampling plans. Discuss the differences in the various sampling plans. Acceptance sampling plans for variables. Method s and . Find the normal, tightened and reduced plans using two methods. Discuss the differences in the various plans. 55 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods grade Learning outcomes Student has the knowledge about the methods and tools used to control the process and product quality. Student has the ability to choose the methods and the calculation of the parameters characterizing the quality of process and product. 1. Recommended readings 2. 3. Doty L.A.: Statistical Process Control. Second edition. Industrial Press Inc. New York, 1996 Montgomery D.C.: Introduction to Statistical Quality Control. Fifth edition. John Wiley & Sons, Inc. 2005. Montgomery D.C.: Statistical Quality Control.. A modern introduction. Six edition. John Wiley & Sons, Inc. 2009. Additional information Course title SEPARATION PROCESSES Teaching method Lecture;Classes. Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_20 ECTS points 5 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture -2 Classes - 2 Hours per semester Lecture -30 Classes - 30 Objectives of the course The course aims to familiarize students with the basic of separation processes Entry requirements Physical chemistry. Fundamentals of chemical engineering. Course contents Introduction. Fundamental concepts. Thermodynamics of separation processes. Mass transfer and diffusion. Single equilibrium stages calculations. Flash calculations. Cascades systems. Hybrid systems. Absorption. Stripping of dilute mixtures. Distillation. Liquid–liquid Extraction. Multicomponent, multistage separations. Supercritical extraction. Membrane separations. Adsorption. Ion exchange. Chromatography. Electrophoresis. Mechanical phase separations. Assessment methods Lecture – oral exam Project – project work 56 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes Recommended readings The student will be able to: Demonstrate basic knowledge of separation of chemical mixtures by industrial processes, including bioprocesses. Describe the scientific principles associated with separation equipments. Demonstrate basic knowledge of making mass balances and specifying component recovery and product purity. Demonstrate basic knowledge of modeling and simulation of separation processes using POLYMATH, ASPEN PLUS and HYSYS. 1. 2. 3. 4. Seader J.D., Henley E.J., Separation process principles, Wiley, New York 2006. Seader J. D., Henley E.J., Roper D.K., Martin R.E., Separation process principles. Chemical and biochemical operations, Wiley, New York 2011. Wankat P.C., Separation Process Engineering, Prentice Hall, New Jersey 2012. Noble R.D., Terry P.A., Principles of chemical separations with environmental applications, Cambridge University Press, New York 2004. Additional information Course title SIMULATION OF CHEMICAL ENGINEERING PROCESSES USING MATHAD AND MATLAB Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course Presentation of the selected chemical and process engineering problems and their solution using Mathcad or Matlab. Entry requirements Chemical engineering, mathematics, numerical methods Course contents Solution of the selected problems in chemical engineering: basic principles and calculations, problems of regression and correlation of data, advanced solution methods in problem solving. Thermodynamics. Heat transfer. Mass transfer. Problems of fluid mechanics. Examples of selected problems: dew point calculation for an ideal binary mixture, variation of reaction rate with temperature, shooting method for solving two-point boundary value problems, fugacity coefficients for ammonia – experimental and predicted, optimal pipe length for draining a cylindrical tank in turbulent flow, heat transfer from a triangular fin, unsteady-state conduction in two dimensions, simultaneous heat and mass transfer in catalyst particles. Assessment methods Lecture: oral exam Computer laboratory: grade 57 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes The student will be able to: Demonstrate basic knowledge of Mathcad and Matlab functions and instructions. Identify the various types of numerical methods. Demonstrate ability of using Mathcad and Matlab to solve basic calculation problems. Solve typical fundamental problems associated with chemical and process engineering using Mathcad and Matlab. 1. 2. Recommended readings 3. 4. 5. L. Fausett, Numerical methods using Mathcad, Prentice Hall, Pearson Education Ltd., London, 2002. L. Fausett, Numerical methods using Matlab, Prentice Hall, Pearson Education Ltd., 2nd ed., London, 2007. O.T. Hanna, O.C. Sandall, Computational methods in chemical engineering, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1995. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 1999. H. Moore, Matlab for engineers, 2nd ed., Pearson Education International, New York, 2007. Additional information Course title SPECIAL METHODS OF SEPARATION Teaching method Lecture, workshop Person responsible for the course Anna Kiełbus-Rąpała, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course Compulsory Level of course Master Semester winter / summer Language of instruction English Hours per week Lecture (1h) Workshop (1h) Hours per semester L(15h) W(15h) Objectives of the course The course aim is to give the information about special techniques used to separation of substances: the principle of separation, physical basis, equipment, advantages and disadvantages of particular method; the examples of use. Entry requirements Basis of Chemical Engineering Course contents Introduction. Classification and characteristics of permeation methods (membrane separation processes: micro-, ultra- and nanofiltration, reverse osmosis, electrolysis, dialysis, electrodialysis, gas and vapour permeation, pervaporation, membrane distillation; liquid membrane). Membrane separation in nuclear technology. Separation in ultracentrifuges. Thermal diffusion. Surface sorption methods (bubble or foam separation, flotation). Zone refining (zone melting), recrystallization. Coprecipitation. Electroforetic separation methods. Chromatographic separation. Chemical methods, ion exchange. Magnetic separation. 58 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods grade Learning outcomes Student has the knowledge about the different special methods used to separation of mixtures. Student has ability to explain the physical basis, principle of operation of particular method and equipment wanted for it. Student has ability to choose the appropriate method of separation for a given mixture, and to explain away the choice. Recommended readings 1. 2. 3. Patnaik, P. Dean's Analytical Chemistry Handbook, 2nd ed. McGraw-Hill, 2004. Reiner Westermeier, Electrophoresis in practice, 3rd ed., Wiley, 2005. Rickwood D., Ford T., Steensgaard J., Centrifugation: Essential Data, John Wiley & Son Ltd., 1994. Additional information Course title TECHNICAL THERMODYNAMICS Teaching method Lecture, workshop Person responsible for the course Paulina Pianko-Oprych, PhD Course code (if applicable) E-mail address to the person responsible for the course paulina.pianko @zut.edu.pl ECTS points 3 Type of course obligatory Level of course bachelor Semester summer Language of instruction English Hours per week Lecture: 1h Workshop: 1h Hours per semester Lecture: 15h workshop: 15h Objectives of the course Acquiring of technical and thermodynamic knowledge needed to study other engineering courses. The base knowledge of heat technique and engineering thermodynamics connected with thermodynamic processes and properties of pure substances and mixtures will be presented and practically tested. Entry requirements Mathematics 59 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Lecture: Definition of system, units, fundamentals of equations of state: ideal gas, virial and cubic equations; First Law of Thermodynamics: energy balance, definition of internal energy, heat, work, enthalpy and heat capacity. Ideal gas energy balance: closed and open system, isothermal, isobaric, isometric and adiabatic processes. Second Law of Thermodynamics: Carnot cycle, entropy, process spontaneity criteria. Entropy change of ideal gases: isothermal, isobaric, isometric, adiabatic and mixing processes. Free energy: Gibbs and Helmholtz, lost work and exergy. Properties of real fluids: calculation of ΔU, ΔH and ΔS using thermodynamic diagrams and tables, introduction to calculations using equations of state. Estimation of density, relation between saturated vapour pressure and boiling point, variables at critical condition. Ideal gas flow systems: expansion, compression and throttling. Thermodynamic cycles: Otto, Diesel, Brayton, Rankine, refrigeration, and liquefaction. Workshop: Solutions of problems connected with thermodynamic changes of ideal gases. Problems with equations of state. Determination of thermodynamic properties of pure substances. Problems about solutions. Problems concerning of phase equilibria in multicomponent systems. Assessment methods Lecture: class test workshop: class test Learning outcomes The student has general knowledge in the field of technical thermodynamics; should be able to define the basic concepts of thermodynamics and to identify and describe the thermodynamic processes. 1. Recommended readings 2. 3. 4. J.M. Smith, H.C. Van Ness, M.M. Abbott: Introduction to Chemical Engineering Thermodynamics. McGraw Hill, Boston, 2001. J. Gmehling, B. Kolbe: Thermodynamik. Georg Thieme, Stuttgart, 1988. D.P. Tassios: Applied Chemical Engineering Thermodynamics. Springer, Berlin, 1993. S. I. Sandler, Chemical and Engineering Thermodynamics, 2 Edition, John Wiley& Sons, New York, 1989. Additional information Course title THE PREDICTION OF PROPERTIES OF GASES AND LIQUIDS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) Objectives of the course The prediction methods presentation of various gases and liquids properties data. 60 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Chemical engineering, thermodynamics Course contents The estimation of physical properties. Pure component constants. Thermodynamic properties of ideal gases. Pressure-Volume-Temperature relationships of pure gases and liquids. Pressure-Volume-Temperature relationships of mixtures. Thermodynamic properties of pure components and mixtures. Fluid phase equilibria in multicomponent systems. Solubilities of solids in liquids. Viscosity evaluation methods of gases and liquids. Viscosities of gas mixtures at low and high pressures. Effect of high pressure and temperature on liquid viscosity. Thermal conductivity evaluation methods of gases, liquids and their mixtures. Effect of temperature on the low-pressure thermal conductivity of gases. Effect of pressure on the thermal conductivity of gases. Diffusion coefficient evaluation methods of gases and liquids. Diffusion in multicomponent gas and liquid mixtures. Pureliquid surface tension evaluation methods. Variation of pure-liquid surface tension with temperature. Surface tension of mixtures. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of gasses and liquids properties. Identify the various types of methods in physical properties estimation and data analysis. Solve typical problems associated with fluids physical properties calculations. 1. Recommended readings 2. 3. 4. B.E. Poling, J.M. Prausnitz, J.P. O’Connel, The Properties of Gases and Liquids, McGrawHill, New York 2001. C.L. Yaws, Chemical Properties Handbook, McGraw Hill, New York 1999. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. Additional information Course title THERMODYNAMICS APPLICATIONS Teaching method Lecture, computer laboratory Person responsible for the course Józef Nastaj, professor Konrad Witkiewicz, PhD Course code (if applicable) WITH CHEMICAL ENGINEERING E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course Bachelor Semester winter / summer Language of instruction English Hours per week 2 (L), 2 (Lab) Hours per semester 30 (L), 30 (Lab) 61 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course The principles of thermodynamics, carefully developed to provide students of chemical engineering with a deep and intuitive understanding of the practical applications of these fundamental ideas and principles. Logical explanations introduce core thermodynamic concepts in the context of their measurement and experimental origin, giving students a thorough understanding of how theoretical concepts apply to practical situations. An attempt to produce thermodynamics lecture and laboratory suitable for the age of the personal computers. Computer-aided calculations, using a general purpose numerical analysis program - Polymath Entry requirements Chemical engineering, physical chemistry Course contents Macroscopic, microscopic, and molecular aspects of thermodynamics. Problems and concepts at the interface of mechanics and thermodynamics. Phases, interfaces, dispersions, and the first three principles of thermodynamics. Internal energy, the First Law, heat, conservation of total energy, mass and energy balances, enthalpy, and heat capacities. Equations of state for one-component and multicomponent systems. Applications of the mass and energy balances and the equations of state to several classes of thermodynamic problems. The Second Law, absolute temperature, entropy definition and calculation, and entropy inequality. Further implications of the Second Law. Introduction of the Helmholtz free energy, Gibbs free energy, chemical potential, and applications to phase equilibria, heat transfer, and mass transfer. Thermodynamic fugacity, thermodynamic activity, and other thermodynamic functions (U, H, S, A, G, μi) of ideal and nonideal solutions. Vapor–liquid equilibria with applications to distillation. Gas–liquid equilibria and applications to gas absorption or desorption. Applications to liquid–liquid equilibria and liquid–liquid extraction. Osmosis, osmotic pressure, osmotic equilibrium, and reverse osmosis. The Third Law and the molecular basis of the Second and Third Laws. Chemical reaction equilibria. One reaction. Chemical reaction equilibria. Two or more reactions occurring simultaneously. Applications of thermodynamics to energy engineering and environmental engineering. Assessment methods Lecture: oral exam Computer laboratory: grade Learning outcomes The student will be able to: Demonstrate basic knowledge of thermodynamics. Identify the various types of thermodynamic equilibria. Understand mass and energy balances. Describe the scientific principles associated with solving thermodynamic problems. Solve typical calculation problems associated with thermodynamics. 1. 2. 3. Recommended readings 4. 5. B.G. Kyle, Chemical and Process Thermodynamics, Prentice Hall PTR, New Jersey 1999. H.D.B. Jenkins, Chemical Thermodynamics at Glance, Blackwell Publishing Ltd, Oxford 2008. M.B. Cutlip, M. Shacham, Problem solving in chemical engineering with numerical methods, Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2008. H.S. Fogler, Elements of chemical reaction engineering, 4th ed., Prentice Hall International Series in the Physical and Chemical Engineering Sciences, New Jersey, 2006. E.I. Franses, Thermodynamics with Chemical Engineering Applications, Cambridge University Press, Cambridge, 2014. Additional information Course title TRANSPORT AND DISTRIBUTION OF NATURAL GAS Teaching method Lecture, workshop, laboratory 62 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Person responsible for the course Jolanta Szoplik, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course Compulsory Level of course Master Semester Winter/Summer Language of instruction English Hours per week L (1h), W (1h), Lab (1h) Hours per semester L(15h), W(15h), Lab(15h) Objectives of the course The course aim is to know the methods of the calculations of parameters characterizing gas flow in high, middle and low pressure pipeline networks, such as: diameter of the pipeline, the volumetric gas flow streams, speed of gas in pipelines and the pressure drop of gas in each pipeline of the network. Entry requirements Process Engineering – general information Course contents The property of the natural gas – the requirements concerning the quality and the classification of natural gas distributed by pipeline network. Construction and main types of gas pipeline networks and the characteristics of the basic elements of the network. High pressure pipelines – construction, materials. The structure and characteristics of gas pipeline network. The compressor station (construction, compression of the gas, the cooling of the gas). High pressure reduction station (construction, the reduction pressure of gas, the heating of the gas). Middle pressure gas reduction station (construction, maintenance). Gas terminal and gas reduction point. Middle and low pressure gas pipeline network – construction, materials. The calculations of the gas pressure drops during gas transportation, based on the equations and nomographs and the calculations of the pipeline diameter. The underground natural gas storages. The variety of the gas consumption in time. The forecasting of gas demand and workloads of gas pipeline network. Equipment for measuring the flow and quality of natural gas – the types and characteristics of the equipment. The gas flow simulation (steady-state or dynamic). The methods of perform the structure of gas pipeline network. The computer programs used to simulate of gas flow in a pipeline network. The simulation of gas flow in selected part of gas networks with different structures of pipelines or different overpressure of a gas stream. Assessment methods written exam, project work Learning outcomes Student has the ability of the calculations of basic parameters characterizing gas flow in pipeline networks. Student has the skill of the calculations and choice main equipment including in the device which support the transport and distribution of the natural gas. Recommended readings 1. 2. Osiadacz A.J.: Simulation and analysis of gas network. E&FN Spon., London, 1987. Kralik J., Stiegler P., Vostry Z., Zavorka J.: Dynamic Modeling of Large-Scale Networks with Application to Gas Distribution. Elsevier, Amsterdam, 1988. Additional information Course title TRANSPORT PHENOMENA Teaching method Lecture; Classes 63 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Person responsible for the course Bogdan Ambrożek, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTICH_ICHP_1A_S_21 ECTS points 4 Type of course Obligatory Level of course Bachelor/master Semester winter / summer Language of instruction English Hours per week Lecture – 2 Classes - 2 Hours per semester Lecture – 30 Classes - 30 Objectives of the course The course aims to familiarize students with the basic of transport phenomena with emphasis on chemical engineering applications. Entry requirements Mathematics; Physics; Course contents Momentum Transport: Viscosity; Mechanisms of momentum transport; Momentum balances; Velocity distributions in laminar and turbulent flow; Interphase transport of momentum in isothermal systems; Macroscopic balances for isothermal flow systems. Energy Transport: Mechanisms of energy transport; Thermal conductivity; Energy balances; Temperature distributions in solids; The equations of change for nonisothermal systems; Temperature distributions in turbulent flow; Interphase transport in nonisothermal systems; Macroscopic balances for nonisothermal systems. Mass transport: Mechanisms of mass transport; Diffusivity; Mass balances; Concentration distributions in solids. Equations of change for multicomponent systems; Concentration distributions in turbulent flow, Interphase transport; Macroscopic mass balances for multicomponent systems. Assessment methods Lecture – oral exam Classes – grade Learning outcomes The student will be able to: Formulate governing equation for momentum, mass, and heat transfer. Identify the terms describing storage, convection, diffusion, dispersion, and generation in the general governing equation for momentum, mass, and heat transfer. Understand the various components needed for setting up conservation equations. Utilize information obtained from solutions of the balance equations to solve chemical engineering problems. Appreciate relevance of transport phenomena in chemical engineering. Recommended readings 1. 2. 3. Bird R.B., Stewart W.E., Lightfoot E.N., Transport Phenomena, Wiley, New York 2007. Brodkey R.S., Hershey H.C., Transport phenomena. A unified approach, McGraw-Hill, New York 1988. Kessler, David P. Greenkorn. Kessler D.P., Greenkorn R.A., Momentum, heat, and mass transfer fundamentals, Marcel Dekker, Basel 1999. Additional information 64 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ TECHNOLOGY Course title ANALYSIS OF AIR POLLUTION Teaching method Lecture and laboratory Person responsible for the course Elżbieta Huzar, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/OSr/C-7 ECTS points 4 Type of course Optional Level of course Master or bachelor Semester Winter or summer Language of instruction English Hours per week 5 (L-1, Lab-4) Hours per semester 75 (L-15, Lab-60) Objectives of the course Methods of samples collection and analysis of common air pollutants Entry requirements Basic knowledge of organic and inorganic chemistry Course contents Lecture: Problems in trace analysis. Collection and pretreatment of samples. Isolation and aspiration techniques. Enrichment of analytes. Passive, dynamic and denuder methods. Use of glass scrubbers, solid adsorbents and cryogenic traps. Preparation of gas standard mixtures. Sampling of aerosols. Air monitoring. Laboratory: Collection of air samples by isolation and aspiration techniques. Determination of organic and inorganic air pollutants (acetone, diethyl ether, phenol, aromatic hydrocarbons, hydrochloride, carbon disulfide) by spectrophotometric and chromatographic methods. Validation of applied methods. Assessment methods Lecture: written exam Laboratory: written reports, grade Learning outcomes Student will be able to describe and apply different techniques of air sampling. Student will be able to perform analysis of popular air pollutants by Spectrophotometric and chromatographic methods. Student will be able to perform validation of analytical procedure. 1. Recommended readings 2. 3. 4. Mudakavi J.R., Principles and Practices of Air Pollution Control and Analysis, I.K. International Publishing House Pvt. Ltd., New Delhi 2010. Berezkin V.G., Drugov Y.S., Gas chromatography in air pollution analysis, Journal of Chromatography Library, Volume 49, Elsevier 1991. Mullins E., Statistics for the quality control chemistry laboratory, RSC, Cambridge 2003. New horizons and challenges in environmental analysis and monitoring, ed. J. Namieśnik, W. Chrzanowski, P. Żmijewska, CEEAM, Gdańsk 2003. (http://www.pg.gda.pl/chem/CEEAM/Dokumenty/CEEAM_ksiazka/New_ANG.htm Additional information 65 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title ANALYSIS OF FOOD CONTAMINANTS Teaching method Laboratory Person responsible for the course Alicja Wodnicka, PhD E-mail address to the person responsible for the course alicja.wodnicka@ zut.edu.pl Course code (if applicable) WTiICh/IISt/OSr/D-3b ECTS points 2 Type of course optional Level of course Master of bachelor Semester winter or summer Language of instruction English Hours per week 3 (Lab-3) Hours per semester 45 (Lab-45) Objectives of the course Analysis of typical contaminants naturally generated in food and brought from environment Entry requirements Basics of analytical chemistry Course contents Natural contaminations present in foods. Natural toxicants generated in food during spoilage processes. Examination of ethanol content in beverages. Changes in plant oils at high temperature. Products of fats oxidation. Environmental toxicants (pesticides, pharmaceuticals, industrial contaminants). Pesticide residues in food. Examination of adulterated food. Assessment methods Laboratory written reports, essay and test (grade) Learning outcomes Student will be able to collect and organize data from literature. Student will be able to explain sources of different food contaminants. Student will be able to perform analysis of selected food contaminants and examine adulteration of food. Recommended readings 1. 2. 3. Food Safety: Contaminants and Toxins, ed. J.P.F. D'Mello, CABI, Trowbrige 2003. Toxins in Food, ed. W.M. Dąbrowski, Z.E. Sikorski, CRC Press, Boca Raton, Florida 2005. Coultate T.P., Food: the Chemistry of its Components, RSC, Cambridge 2009. Additional information Course title ANALYSIS OF WATER AND EFFLUENTS Teaching method Lecture (L), workshop (theoretical exercises, W) and laboratory (Lab) Person responsible for the course Sylwia Mozia, professor Course code (if applicable) Type of course Optional E-mail address to the person responsible for the course [email protected] ECTS points 4 Level of course Bachelor 66 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester summer Language of instruction English Hours per week L: 2h W: 1h Lab: 4h Hours per semester L: 30h W: 15h Lab: 60h Objectives of the course Student will get theoretical knowledge on chemical composition of natural waters, water and wastewater treatment processes, drinking water quality standards and wastewater quality standards, methods of preservation and analysis of water and wastewater samples. Student will get practical skills in the area of analysis of water and wastewater parameters. Entry requirements Water and wastewater treatment, analytical chemistry Course contents Lecture: Characteristics of surface water and groundwater. Classification of waters. Regulations concerning drinking water quality. Characteristics of municipal wastewater and selected industrial effluents. Wastewater quality standards. Aims and ranges of water and wastewater analysis. Fundamentals of analysis of water and wastewater. Background of sampling. Sample stabilization and safe keeping. Physical and chemical indicators of water and wastewater contamination. Indicators of bacteriological contamination of water. Methods of analysis of water and wastewater. Laboratory: Determination of PO43-, N-NO3-, N-NH4+ and dissolved oxygen concentrations, determination of COD-Cr, COD-Mn, TOC, alkalinity, acidity, hardness, color, turbidity and pH of water, evaluation of water corrosivity. Workshop: Calculation of solutions concentrations, pH, hardness, alkalinity and acidity of natural waters, corrosivity, BOD. Regulations concerning drinking water quality. Assessment methods Lecture: written exam Workshop: class test/grade Laboratory: class test/grade Learning outcomes At the completion of this course, students will be able to: Understand fundamental water chemistry. Learn the parameters that characterize the constituents found in potable water and wastewater. Comprehend water/wastewater quality data. Characterize water and wastewater. Plan and carry out experiments for analysis of water and wastewater quality, collect experimental data, analyze and interpret results, write technical reports and give presentations. 1. 2. Recommended readings 3. 4. 5. 6. 7. Handbook of Water Analysis, Second Edition, Ed. Leo M.L. Nollet, CRC Press LLC, USA, 2007. K. Kaur, Handbook of water and wastewater analysis, Atlantic Publishers & Distributors (P) Ltd., 2007. Kirk-Othmer, Chemical Technology and the Environment, Vol. 1 and 2, 2007 Handbook of Environmental Chemistry, ed. O. Hutzinger, Vol.5, part A, Water Pollution, Springer-Verlag 1991 B.J. Alloway, D.C. Ayres Chemical Principles of Environmental pollution, Blackie Academic & Professional 1993 Water treatment, Plant Design, 3th Edition, American Water Works Association, McGraw 1998 W.J. Masschelein, Unit Processes in Drinking Water Treatment, Marcel Dekker Inc. 1992 Additional information 67 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title APPLIED METROLOGY AND MEASUREMENTS FOR CHEMISTS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course compulsory Level of course bachelor Semester winter Language of instruction English Hours per week 2 (L) + 2 (Lab) Hours per semester 30 (L) + 30 (Lab) Objectives of the course Come to know the theory of metrology, the techniques of measurement applied in chemistry and chemical engineering. Entry requirements Mathematics, physics, electrical engineering, analytical chemistry Course contents The basics of metrology, techniques of measurements: mass, temperature, pressure, flow, electrical properties, chemical composition of gas mixtures. Sample preparation, experiment planning and data assessment. Practical rules for experiment performance. Assessment methods written exam Learning outcomes Recommended readings Student knows the principles of experimental data assessment. Student knows the fundamental methods of measurement applied in chemical engineering. Student is able to choose and perform the basic measurement experiments. 1. 2. D.M. Anthony: Engineering metrology. Pergamon, Oxford 1986. James Ronald Leigh: Temperature measurement and control. P. Peregrinus, London 1988. Additional information Course title BIODEGRADABLE POLYMERS Teaching method lecture and laboratory Person responsible for the course Katarzyna Wilpiszewska, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course optional Level of course Bachelor Semester winter/summer Language of instruction English 68 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Hours per week 1L 2Lab Objectives of the course To gain the knowledge on biodegradable polymers: natural and biotechnological polymers, their structure, properties, modification and (novel) application. Entry requirements Chemical technology, organic chemistry Course contents Lectures: Definition of biodegradability and methods of its determination. The most important groups of biopolymers: polysaccharides (cellulose, starch, chitosan, alginates), proteins, and latex – structure, properties, modification, and application (including nanostructures). Polymers prepared via biochemical synthesis – properties, and application. Lab classes: Modification of polysaccharides (i.e. starch, cellulose) and using titration as well as spectrometric methods for polysaccharide characterisation, thermal properties of biopolymers, biopolymers as fillers. Applying biopolymers (microcapsules, paper glues, flocculants, etc). Assessment methods Written examination, an laboratory exams Learning outcomes At the completion of this course, the successful student will be able to: classify polymer according to their origin and chemical structure, determine the physicochemical properties as well as biodegradability of biodegradable polymers, predict and explain material behavior in various conditions, indicate common, conventional and novel application of biodegradable polymers. Recommended readings 1. 2. 3. Hours per semester 45 M. Stevens, Polymer chemistry, 1999, Oxford University Press C. Bastioli (Ed.), Handbook of biodegradable polymers, 2005, Rapra Technology Ltd. R. Smith, Biodegradable polymers for industrial applications, 2005, Woodhead Publishing Ltd. Additional information Course title BIOMATERIALS Teaching method Lecture plus literature project Person responsible for the course Piotr Sobolewski, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 4 Type of course Level of course Bachelor/master Semester winter/summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course Provide an understanding of the principles of biomaterials science, including a background in human physiology and cell biology Entry requirements none 69 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction and definitions; biomaterial-blood modification; selected topics/case studies contact; host response; surface Assessment methods Oral presentation Learning outcomes After completion of this course, the successful student will be able to 1) define important keywords, 2) describe the interactions between materials and blood, 3) describe the steps of the host response to a material, and 4) discuss important material-related design considerations for medical devices/implants Recommended readings 1. Ratner et al. Biomaterials Science Additional information Course title BIOMATERIALS AND IMPLANTS Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 4 Type of course Level of course master Semester summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course This course is aimed at giving an introduction to polymeric biomaterials and implants used in medicine. Student will be able to define basic terms related with biomaterials and implants, will be able to select materials for particular application according the application requirements, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on chemistry or polymer chemistry Course contents Polymeric biomaterials: basic concepts of biocompatibility; synthetic polymers and composites as implants; biodegradable polymers for tissue engineering; stimuli responsive polymers for drug delivery; metals and ceramic in biomedical applications; environmental management of biodegradable polymers. Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: compare and contrast different polymeric biomaterials, predict and explain material behavior in living environment, classify implants according time of implantation and their function 70 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Recommended readings 1. 2. 3. Black J., Bilogical Performance of Materials, Marcel Dekker, New York, 1999 Wise D.L., Biomaterials and Bioengineering Handbook, Marcel Dekker, New York, 2000 Ratner B.D., Biomaterials Science, Elsevier, New York 2004 Additional information Course title BIOMIMETICS Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 3 Type of course Level of course master Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course This course is aimed at giving an introduction to the field of designing of modern material using inspirations from nature. Student will be able to define basic terms related biomimetics, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on chemistry or polymer chemistry, materials science Course contents Inspirations from the nature towards developments of functional surfaces; hybrid and tough material inspired by bone structure; self-healing materials Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: compare and contrast different biological structures, predict and explain material behavior when mimicking natural environment, formulate and test hypothesis Recommended readings 1. 2. Y. Bar-Cohen, Biomimetics Biologically Inspired Technologies, CRC Taylor&Francis, New York, 2006 Ratner B.D., Biomaterials Science, Elsevier, New York 2004 Additional information 71 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title BIOPOLYMERS Teaching method Lecture plus literature project Person responsible for the course Piotr Sobolewski, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 4 Type of course Level of course Bachelor/master Semester winter/summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course Provide an introduction to biopolymers, as a class of materials, including a background in microbiology and cell biology. Entry requirements none Course contents Introduction and definitions; proteins; polysaccharides; polyhydroxyalkanoates; latex; select topics/case studies Assessment methods Oral presentation Learning outcomes After completion of this course, the successful student will be able to 1) explain the difference between biopolymers and bio-based polymers, 2) describe the chemistry of each of the main classes of biopolymers, 3) discuss example applications of biopolymers, as possible replacements for petroleum-based polymers Recommended readings 1. Kaplan Biopolymers from Renewable Resources Additional information Course title BIOPROCESS ENGINEERING Teaching method Lecture plus literature project Person responsible for the course Piotr Sobolewski, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 4 Type of course Level of course Bechelor/master 72 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester winter/summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course Provide an understanding of the principles of bioprocess engineering, Introduce bioreactor and separations theory, as well as practical considerations, including GMP Entry requirements Principles of Biotechnology at the undergraduate level Course contents bioreactors; immobilization; enzymes; separations theory; GMP; select topics and practical considerations Assessment methods Oral presentation Learning outcomes After completion of this course, the successful student will be able to: 1) describe the necessary steps involved in developing a bioprocess in order to obtain a bioproduct 2) describe the pros and cons of various bioreactor types 3) describe the pros and cons of immobilization Recommended readings 1. Shuler & Kargi, Bioprocess Engineering: Basic Concepts. Additional information Course title CHARACTERIZATION METHODS AND PROPERTIES OF POLYMERIC MATERIALS Teaching method lectures and laboratory Person responsible for the course Agnieszka Piegat, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) TCH_2A_S_D01_09 ECTS points 3 Type of course obligatory Level of course bachelor Semester winter Language of instruction English Hours per week 1 lecture, 1 laboratory Hours per semester 15 lecture, 15 laboratory Objectives of the course This course is aimed at giving knowledge on fundamental methods for characterization polymeric materials Entry requirements Polymer chemistry, chemical technology, instrumental analysis Course contents Lectures: Classification of polymers properties ; microscopic techniques in evaluation of polymers morphology (transmission electron microscopy, scanning electron microscopy, light microscopy); mechanical properties; thermal analysis of polymers (DSC, DMTA, TGA); liquid crystal polymers; influence of different additives on selected properties. 73 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods written exam and grade Learning outcomes Recommended readings 1. 2. 3. Students will be able to develop a knowledge about the techniques used for polymers characterization. Students will be able to use presented techniques effectively in the delivery of instruction, assessment, and professional development. Students will be able to use to identify, formulate, and solve problems at the area of polymer characterization. Z. Guo, L. Tan, Fundamentals and Applications of Nanomaterials, Artech House, 2009 David D.J., Misra A., Relating materials properties to structure. Handbook and software for polymer calculations and materials properties, Technomic Publishing Co., 1999 Available research papers and other sources Additional information Course title CHEMICAL PROCESSES IN INORGANIC ENVIRONMENTAL ENGINEERING INDUSTRY AND Teaching method Lecture (L) Person responsible for the course Sylwia Mozia, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/TCh/D12-7 ECTS points 4 Type of course Obligatory Level of course Master Semester Summer Language of instruction English Hours per week L: 3h Hours per semester L: 45h Objectives of the course Student will get theoretical knowledge on chemical processes in inorganic industry and environmental engineering, including technologies of flue gas desulfurization and NOx removal, purification of air, production of building and construction materials, as well as electrochemical methods of synthesis of inorganic compounds and treatment of metal surfaces. Entry requirements Fundamentals of chemistry and chemical technology 74 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Part I: Technologies of flue gas desulfurization and NOx removal, purification of air: general information concerning pollution with SOx and NOx, EU regulations, sources of sulfur and formation of SOx, wet and dry methods applied for desulfurization of flue gases, modern regenerative methods, formation of NOx during combustion of fuels, removal of NOx from flue gases including catalytic methods, preparation of pure air. Part II: Building materials. Lime, gypsum, cement, concrete, prefabricated products. Ceramics: ceramic building materials, electroceramics, metal ceramics, ceramic whiteware. Glass and glassware. Different sorts of glass, glass wool, ceramic and glass fibres, frits. Part III: Industrial electrochemistry: electrolysis of aqueous solutions; electrolysers; factors influencing electrolysis; electrolysis of aqueous solutions of NaCl; electrolysis of spent HCl; electrochemical treatment of metal surfaces – electroplating; hydroelectrometallurgy; electrochemical synthesis of inorganic compounds Assessment methods Exam Learning outcomes At the completion of this course, students will be able to: Understand fundamentals of chemical processes applied in industry, including processes of flue gas desulfurization, NOx removal, and purification of air, processes and methods applied in building and construction industry and well as electrochemical processes utilized for production of organic and inorganic compounds, in electroplating and hydroelectrometallurgy. Analyze and propose methods of manufacturing of numerous products using chemical processes. Analyze and propose methods of purification of flue gases emitted by chemical industry. Describe the properties of materials and the engineering aspects for various chemical processes applied in inorganic industry. 1. 2. 3. Recommended readings Ullmann's Encyclopedia of Industrial Chemistry, 6th edition (2002) Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition Ron Zevenhoven, Pia Kilpinen, Control of pollutants in flue gases and fuel gases, ISBN 951-22-5527-8 (available on line) 4. Boynton R.S., Chemistry and technology of lime and limestone, John Wiley, New York 1980. 5. Cement, Concrete, and Aggregates (ed. R.D. Hooton), ASTM International, West Consh., PA 2003. 6. Volf M.B., Chemical approach to glass, Elsevier, Amsterdam 1984. 7. Loewenstein K.L., The manufacturing technology of continuous glass fibres, Elsevier Scientific Publ.Co., Amsterdam 1973. 8. Hocking M.B., Modern Chemical Technology and Emission Control, Springer-Verlag, Berlin 1985 9. Pletcher D., Walsh F. C., Industrial Electrochemistry, Springer-Verlag GmbH, 2007 10. Wendt H., Kreysa G., Electrochemical Engineering: Science and Technology in Chemical and Other Industries, Springer Science & Business Media, 1999 11. Landau U., Electrochemistry in Industry New Directions. Springer Verlag 2013 Additional information Course title CHEMICAL REACTORS Teaching method lecture/laboratory/classes Person responsible for the course Beata Michalkiewicz, professor E-mail address to the person responsible for the course beata.michalkiewicz@ zut.edu.pl 75 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course code (if applicable) ECTS points 3 Type of course compulsory Level of course bachelor Semester summer Language of instruction English Hours per week 1 lecture, 1 laboratory, 1 classes Hours per semester 15 lecture, 15 laboratory, 15 classes Objectives of the course chemical reactors design Entry requirements Mathematics, Inorganic Chemistry, Physical Chemistry Course contents Definition of the reaction rate. Independence of the reactions. Kinetics and thermochemistry of the reactions. Rate of homogenous and heterogeneous reactions. Variables affecting the rate of reaction. Reaction in series and in parallel. Collection and analysis of rate data. Differential and integral method of analysis. Testing kinetic models. Design of continuous stirred tank reactor, tubular flow reactor, batch reactor, packed bed reactor and reactors in series. Assessment methods written exam Learning outcomes Recommended readings 1. H. Scott Fogler, Elements of chemical Reaction Engineering, Pearson Education International, 2006 2. R. Aris, Introduction to the analysis of chemical reactors, Prentice-Hall Inc. 1969 3. R. Aris, Elementary chemical reactor analysis, Prentice-Hall Inc. 1965 4. K. R. Westerterp at al. Chemical reactor design and operation, John Wiley & Sons, 1984P.N. Cheremisinoff, L.M. Ferrante, Waste Reduction for Pollution Prevention, Butterworth-Heinemenn Ltd, Linacre House, Jordan Hil, Oxford OX2 8DP, 1992. 5. Publications from the internet site: www.envirowise.gov.uk Additional information Course title CHEMISTRY AND TECHNOLOGY OF MEDICINES Teaching method Lecture and laboratory Person responsible for the course Halina Kwiecień, professor E-mail address to the person responsible for the course halina.kwiecien@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh/D4-8 ECTS points 4 Type of course Optional Level of course Master or bachelor Semester Winter or summer Language of instruction English 76 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Hours per week 5 (L-2, Lab-3) Objectives of the course Come to know about chemistry and technology of medicines, methods of drug discovery and development of pharmaceutical industry Entry requirements Basics of organic chemistry, biochemistry, chemical technology, chemical engineering Course contents Lectures: Nomenclature and classification of medicines. Biologics, derived from natural product source, semisynthetics and synthetics drugs. Mechanism of action and biotransformation. Methods of new drug discovery, combinatorial chemistry. Processes and apparatus in pharmaceutical industry. Wastes and “green” pharmaceutical technology. Chemistry and technology of following groups of drugs: analgesic, sulfonamides, cardiovascular, anticancer antihistaminic, psychotropic, antifungal. Biotechnology, natural and synthetic antibiotics. Laboratory: synthesis of 1-2 products by standard processes in pharmaceutical chemistry. Purification, chromatographic and spectral analyses of the products. Assessment methods Lectures: written exam Laboratory: written report, grade Learning outcomes Student will be able to describe the basic aspects of the chemistry and technology of medicines Student will be able to characterize the main unit operation and process applied in pharmaceutical industry Student will be able to indicate the environmental pharmaceutical persistent pollutants from pharmaceutical industry Student will be able to apply the acquired knowledge in the synthesis and analysis of selected medicines Recommended readings 1. 2. 3. Hours per semester 75 (L-30, Lab-45) Lednicer D., „The Organic Chemistry of Drug Synthesis”, Willey, New York, 1995 Kleemann A., Engel J „Pharmaceutical Substances. Syntheses, Patents, Applications”, Thieme, Stuttgard, 4th Edition, 2001 Furniss B.S., Hannaford A.J., Smith, P.W.G., Tatchell A.R. “Vogel’s Textbook of Practical Organic chemistry”. Fifth Ed., The School of Chemistry, Thames Polytechnic, London, 1989. Additional information Course title CHROMATOGRAPHIC METHODS Teaching method Lecture and laboratory Person responsible for the course Małgorzata Dzięcioł, PhD E-mail address to the person responsible for the course malgorzata.dzieciol@ zut.edu.pl Course code (if applicable) WTiICh/IISt/OSr/C-1 ECTS points 4 Type of course Optional Level of course Master or bachelor Semester winter or summer Language of instruction English 77 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Hours per week 6 (L-2, Lab-4) Objectives of the course Theoretical and practical aspects of chromatographic methods Entry requirements Basic knowledge of organic chemistry Course contents Lecture: General theory of chromatography. Classification of chromatographic methods. Retention parameters. Resolution. Separation efficiency of column. Identification and quantification methods in chromatography. Gas chromatography (GC) – principles, instrumentation, carrier gas, columns and stationary phases, sampling, detectors, applications. High performance liquid chromatography (HPLC) – instrumentation, eluents, stationary phases, normal and reversed-phase chromatography, isocratic and gradient elution, detectors, applications. Thin layer chromatography (TLC) – principles, adsorbents and plates, chambers, development techniques, densitometry. Laboratory: Maintenance and method development in gas chromatography. Evaluation of separation efficiency. Qualitative and quantitative analysis in gas chromatography. Application of GC/MS method in identification of compounds. Qualitative and quantitative analysis in HPLC method. Assessment methods Lecture - written exam Laboratory – written reports, grade Learning outcomes Student will be able to classify chromatographic methods and describe different chromatographic separation processes. Student will be able to describe basic instrumentation used in chromatography. Student will be able to apply chromatographic methods in order to perform qualitative and quantitative analysis of organic compounds. Recommended readings 1. 2. 3. Hours per semester 90 (L-30, Lab-60) Braithwaite A., Smith F.J., Chromatographic Methods, Springer 1996 McNair H.M., Miller J.M., Basic Gas Chromatography (Second Edition), Wiley 2009 Snyder L.R., Kirkland J.L., Dolan J.W., Introduction to Modern Liquid Chromatography, Wiley 2010 Additional information Course title COMPUTER-AIDED DESIGN OF CHEMICAL INDUSTRIAL PLANTS Teaching method lecture, practice Person responsible for the course Ryszard J. Kaleńczuk, professor E-mail address to the person responsible for the course ryszard.kalenczuk@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh/D12-5 ECTS points 3 Level of course Master Type of course obligatory Semester winter Language of instruction English Hours per week 1 lecture, 3 practice Hours per semester 15 lecture, 45 practice 78 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course Knowledge of the using of modern computer tools for industrial plant simulation and optimization Entry requirements Mathematics I, Mathematics II, Physics, Computer Science, Physical Chemistry I, Physical Chemistry II, Basis of Chemical Engineering I, Basis of Chemical Engineering II, Modelling of chemical processes, Chemical engineering- industrial processes of synthetic chemistry Course contents Description of the computer program for the modelling and simulation of the chemical process e.g. industrial production of sulphuric acid. Structure of the program, modes of the program, Presentation of the process simulation basing on the chosen example. Laboratory exercise with the program which simulates the industrial production of the chemical compounds. Modelling of its own industrial process. Optimization of the process parameters to get the highest product yield. Assessment methods written exam Learning outcomes The student has broad knowlegde in the field of investigation of computer-aided design tools in chemical industry. The student can used knowlegde in the field of investigation of computer-aided design tools in chemical industry. Happy accede to supplement their knowledge related to the thesis Recommended readings Description of computer programme for processes simulation Additional information Limit of number of students>=5 Course title ELECTRICAL ENGINEERING FOR CHEMISTS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course compulsory Level of course Bachelor Semester winter Language of instruction english Hours per week 2 (L) + 2 (Lab) Hours per semester 30 (L) + 30 (Lab) Objectives of the course Come to know the basic laws of electrical engineering, electrical circuits and appliances Entry requirements Mathematics, physics 79 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Basic concepts of electricity. Ohm’s law. Electrical safety. Series and parallel circuits. Kirchhoff’s law. DC network analysis. Batteries and power systems. Conductors and insulators. Capacitors. Magnetism and electromagnetism. Basic AC Theory. Reactance and impedance. Transformers, Generators, Motors. Polyphase AC circuits. DC and AC metering circuits. Basic semiconductor theory Assessment methods written exam Learning outcomes Student knows the principal laws of electrical engineering. Student knows basic electrical appliances and is able to apply them properly. Student is able to build simple electric circuits and to measure electrical properties. Recommended readings 1. 2. William H. Roadstrum, Dan H. Wolaver: Electrical engineering for all engineers. Wiley, New York 1987. D.F. Warne: Newnes Electrical Engineer’s Handbook. Newnes, Oxford 2000. Additional information Course title ELEMENTS OF BIOTECHNOLOGY Teaching method Lecture, laboratory Person responsible for the course Agata Markowska-Szczupak, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course obligatory Level of course Bachelor Semester winter Language of instruction English Hours per week lectures (L - 1), laboratory (Lab-1) Hours per semester 15 (L) +15 (Lab.) Objectives of the course The course will offer a methods of biotechnology, application of microorganisms and enzymes in bioremediation and technology including examples of real-world applications. Entry requirements Biology (college or higher) taken within the last 5 years. Course contents Different approaches to the term of biotechnology (white, green, red, etc.). GMOs plants and animals. Production of microbial biomass. Applications of fermentation techniques in biotechnology. Types of bioreactors. An introduction to biocatalysis. Kinetics and characteristics of enzymes. Assessment methods Written exam (Lecture) Project work (Laboratory) 80 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes On 1. 2. 3. successful completion of this module, students should be able to: Provide a detailed overview of biotechnology method and technologies. Outline and explain current advances biotechnology. Identify and consider the methodologies and ethical considerations of engineered (transgenic) plants and animals. genetically 1. Recommended readings Alexander M., Biodegradation and Bioremediation (2nd edition), Academic Press, Cornell University, Ithaca, New York, 1999. 2. Evans G. M., Furlong J. C., Environmental Biotechnology: Theory and Application, Wiley, New York, 2003. 3. Vogel H. C., Todaro C. L., Fermentation and Biochemical Engineering Handbook: Principles, Process Design, and Equipment (2nd edition), William Andrew Pub, New York, 1996. Additional/optional 4. Ratledge C., Kristiansen B., Basic Biotechnology (2nd edition), Cambridge University Press, Cambridge, 2006. 5. Volmar B., Götz F., Microbial Fundamentals of Biotechnology, Wiley-VCH , New York, 2001. Additional information Course title FUNDAMENTALS SCIENCE OF INORGANIC CHEMICALS COMMODITY Teaching method lecture Person responsible for the course Krzysztof Lubkowski, PhD E-mail address to the person responsible for the course krzysztof.lubkowski@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh-D12-4 ECTS points 2 Type of course compulsory Level of course master Semester winter Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Come to know about the inorganic chemicals commodity science Entry requirements Bases of economy, management and marketing, Management of quality and chemical products, Production management, Unit processes in chemical technology, Chemical technology – raw materials, Chemical technology – chemical industry processes, Nitrogen industry, Mineral fertilizers. Course contents Basic concepts in commodity science. Characteristics of raw materials and products of inorganic chemistry with regard to their physicochemical and commercial properties, obtaining and processing technology. Quality evaluation of raw materials and inorganic products in terms of their compliance with the law. Standards and laws governing the quality of inorganic products and their designation. Packing and its influence on the quality of inorganic products. Storage and transport conditions of inorganic products. Inorganic product market. 81 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods written exam Learning outcomes After the course, students will be able to understand fundamentals of commodity science related to chemical industry products. 1. 2. Recommended readings 3. 4. 5. Hocking M.B., Modern Chemical Technology and Emission Control, SpringerVerlag, Berlin 1985. Budde F., Farha G.A., Frankemolle H., Value Creation: Strategies for the Chemical Industry, Wiley-VCH, New York 2001. The Chemical Industry at the Millennium: Maturity, Restructuring, and Globalization, Peter H. Spitz (ed.), Chemical Heritage Foundation, New York 2003. Industrial Minerals & Rocks: Commodities, Markets, and Uses, J.E. Kogel, N.C. Trivedi, J.M. Barker, S.T. Krukowski (Eds), Society of Mining Metallurgy and Exploration, New York 2006. Feingenbaum A.V., Total Quality Control – Engineering and Management, Mc Graw-Hill Book, New York 1961. Additional information Course title HETEROGENEOUS CATALYSIS IN INDUSTRY Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 4 Type of course compulsory Level of course bachelor Semester winter Language of instruction English Hours per week 2 (L) + 1 (W) + 1 (Lab) Hours per semester 30 (L) + 15 (W) + 15 (Lab) Objectives of the course Come to know the catalytic action of solids, physicochemical background of these processes and their applications in chemical industry Entry requirements Inorganic and organic chemistry, Physical chemistry, Physical chemistry of surfaces Course contents Catalyst and catalysis in heterogeneous systems. Catalytic action. Catalyst preparation, deactivation, regeneration. The experimental methods for catalysts’ examination. Most frequently used catalytic materials. Industrial catalytic processes in inorganic, organic and polymer industries. Assessment methods written exam 82 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes Student knows the principles of heterogeneous catalysis. Student knows the fundamental structure and composition of catalysts as well as the processes leading to the preparation of industrial catalysts. Student knows the most important industrial processes where heterogeneous catalysis play the major role. Student is able to prepare samples of catalysts and evaluate their properties. Recommended readings 1. 2. 3. G.A. Somorjai: Introduction to surface chemistry and catalysis. Wiley, New York 1994. Catalysis: an integrated approach. Elsevier, Amsterdam 2000. Encyclopedia of catalysis. Wiley, Hoboken 2003. Additional information Course title INDUSTRIAL CHEMISTS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc. Course code (if applicable) AUTOMATION AND PROCESS CONTROL FOR E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course compulsory Level of course bachelor Semester winter Language of instruction English Hours per week 1 (L) + 2 (Lab) Hours per semester 15 (L) + 30 (Lab) Objectives of the course Come to know the theory of automation and process control, the techniques of regulation as well as equipment used for these purposes. Entry requirements Mathematics, physics, electrical engineering, analytical chemistry Course contents Theory of regulation. Regulators, actuators. The techniques of regulation: temperature, pressure, flow, concentration. Industrial equipment of automation. Assessment methods written exam Learning outcomes Student knows the principles of regulation and automation. Student knows the fundamental methods of process control applied in chemical engineering. Student is able to chose a basic process control equipment and set proper parameters of its work. Recommended readings 1. 2. Peter G. Martin and Gregory Hale : Automation made easy : everything you wanted to know about automation and need to ask, International Society of Automation, 2010. Encyclopedia of chemical processing and design. Ed. John J. Mcketta. -- Vol. 43, Process control, feedback simulation to Process optimization, Marcel Dekker, 1993 Additional information 83 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title INDUSTRIAL CHEMISTRY Teaching method lecture Person responsible for the course Krzysztof Lubkowski, PhD Course code (if applicable) E-mail address to the person responsible for the course krzysztof.lubkowski@ zut.edu.pl ECTS points 2 Type of course optional Level of course bachelor Semester winter Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Come to know about the production methods of industrial chemicals Entry requirements Unit processes and operations in chemical technology, Chemical technology – raw materials, Chemical engineering. Course contents Natural and derived sodium and potassium salt, industrial bases (calcium and sodium carbonate, calcium oxide), sulfur and sulfuric acid, phosphorus and phosphoric acid, ammonia, nitric acid, mineral fertilizers, glass, construction materials – lime, cement, gypsum, pigments, aluminium and its compounds. Assessment methods written exam Learning outcomes After the course, students will be able to understand fundamentals of chemical processes applied in industry. 1. 2. 3. Recommended readings 4. 5. 6. 7. Büchner W., Schliebs R., Winter G., Büchel K.H., Industrial Inorganic Chemistry, VCH, Weinheim 1989. White H.L., Introduction to Industrial Chemistry, John Wiley and Sons, New York 1986. Hocking M.B., Modern Chemical Technology and Emission Control, SpringerVerlag, Berlin 1985. The Chemical Industry, Edited by C.A. Heaton, Blackie, London 1982. R. Norris Shrere, J.A. Brink, Chemical Process Industries, McGraw-Hill Book Company, New York 1977. Riegel’s Handbook of Industrial Chemistry, 7th edition, Edited by James K. Kent, Van Nostrand Reinhold Company, New York 1974. K.K. Kobe, Inorganic Process Industries, The Macmillan Company, New York 1948. Additional information Course title INSTRUMENTAL ANALYSIS OF NANOMATERIALS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc. E-mail address to the person responsible for the course [email protected] 84 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course code (if applicable) ECTS points 5 Type of course compulsory Level of course master Semester winter Language of instruction English Hours per week 3 (L) + 4 (Lab) Hours per semester 45 (L) + 60 (Lab) Objectives of the course Come to know the theory and techniques of instrumental analytical methods applied to the characterization of nanomaterials Entry requirements Inorganic chemistry, Physical chemistry, Physics Course contents Instrumental methods of chemical composition analysis. Selecting of a proper analytical methods. Theoretical basics of atomic spectroscopy. Inductively Coupled Plasma, ICP. Infrared Spectroscopy, Raman Spectroscopy, X-Ray Fluorescence, X-Ray Microanalysis. Chemical analysis of the surface structures and properties. Fundamentals of Electrospectroscopy. X-ray Photoelectron Spectroscopy, Ultraviolet Photoemission Spectroscopy, Auger Electron Spectroscopy, Electron Energy Loss Spectroscopy. Adsorption/desorption methods and temperature programmed techniques. Thermogravimetry, Temperature Programmed Desorption, Temperature Programmed Oxidation, Temperature Programmed Reduction, Temperature Programmed Surface Reaction. Analysis of phase composition, structure and topography. X-Ray Diffraction, Reflection High Energy Electron Diffraction, Low Energy Electron Diffraction, Scanning Electron Microscopy, Transmission Electron Microscopy, Atomic Force Microscopy, AFM. Assessment methods written exam Learning outcomes Student knows the most important analytical methods utilized for testing nanomaterials. Student is able to choose a proper group of analytical methods to assess given set of properties. Student knows how to prepare samples for analytical methods and is able to carry out simple analysis. Recommended readings 1. 2. 3. John A. Dean, Analytical Chemistry Handbook, McGraw-Hill Companies, 2000 Helmut Günzler, Alex Williams, Handbook of Analytical Techniques, Wiley-VCH, 2001. Encyclopedia of nanoscience and nanotechnology. editor Hari Singh Nalwa, American Scientific Publishers, 2004. Additional information Course title INTRODUCTION TO MATERIALS ENGINEERING Teaching method lecture Person responsible for the course Mirosława El Fray, professor Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 85 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Type of course Level of course master Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course This course is aimed at giving an introduction to materials engineering, including polymers and (nano)composites. Student will be able to define basic terms related to polymers and (nano)composites, will be able to select materials for particular application according the application requirements, will be able to work in a group, and will be able to broaden her/his knowledge in the field Entry requirements Passed examination on chemistry or polymer chemistry, materials science Course contents Basic material structures with elements of crystallography, production methods of metals, ceramics and polymers; modern engineering materials: light weight composites, nanocomposites; characterization methods of materials Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: compare and contrast different materials, predict and explain structure=properties relationship, determine material properties for engineering applications Recommended readings 1. 2. N. P. Cheremisinoff, Polymer characterization, Noyes Pub., 1996 I.M. Ward, J. Sweeney, The mechanical properties of solid polymers, Wiley, 2004 Additional information Course title IT TECHNOLOGIES FOR CHEMICAL APPLICATIONS Teaching method lecture Person responsible for the course Rafał J. Wróbel, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course obligatory Level of course master Semester winter Language of instruction English Hours per week 1 lecture Hours per semester 15 Objectives of the course Ability of solving physical and chemical problems by numerical methods. Ability of publishing data in web. Ability of creation of online tools for chemical applications. 86 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Basics of Mathematics, Physics, Chemistry Course contents Short introduction to MS Windows system and file managers. Numerical methods in MS Excel. Basic of programming in c++, js, python for solving physical and chemical problems. Basics of html, css and php MySQL for web applications Assessment methods written exam Learning outcomes Student will be able to apply popular IT tool for solving various chemical engineering problems Recommended readings 1. 2. http://html.net/ www.php.net/tut.php Additional information Course title MEMBRAN SEPARATION PROCESSES Teaching method lecture and laboratory Person responsible for the course Maria Tomaszewska, professor E-mail address to the person responsible for the course maria.tomaszewska@ zut.edu.pl ECTS points 4 Course code (if applicable) WTiICh/IISt/TCh/D12-1 Type of course obligatory Level of course Bachelor Semester winter Language of instruction English Hours per week 2 lecture 3 laboratory Hours per semester 30 lecture 45 laboratory Objectives of the course Come to know about techniques of membrane separation, methods of membrane preparation, and application of membrane techniques in chemical engineering and biotechnology Entry requirements Chemical technology, chemical engineering, environmental engineering Course contents Lectures: Introduction to membrane processes. Definition of a membrane. Membrane processes. Preparation of polymeric and inorganic membranes. Characteristics of membranes. Driving forces of mass transfer. Polarisation phenomena and membrane fouling. Membrane modules and their characteristics. Pressure driven membrane processes – microfiltration, ultrafiltration, nanofiltration, reverse osmosis. Techniques with a concentration difference as a driven force – gas and vapour separation, pervaporation, dialysis, membrane distillation. Electrically driven membrane processes – electrodialysis. Bi-polar membranes. Liquid membranes. Contactors. Membrane reactors and bioreactors. Examples of membrane processes application in chemical engineering, biotechnology and environment engineering Laboratory: water and wastewater treatment using membrane processes: RO, NF, UF and MD 87 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods written exam and grade Learning outcomes Student should have a knowledge on membrane and membrane processes and membrane processes application in chemical engineering, inorganic industry and environment engineering 1. Recommended readings 2. 3. 4. M.Mulder, Basic Principles of Membranes Technology, Kluwer Academic Publishers, 1991, 2003 N.N.Li, A.G.Fane, W.S.Winston Ho, T.Matsuura, Advanced Membrane Technology and Application, Wiley 2008 M.K.Turner, Effective Industrial Membrane Processes: Benefits and Opportunities, Elsevier Applied Science, 1991 Handbook of Industrial Membranes, ed. K.Scott, Elsevier Advanced Technology, 1997 Additional information Course title NANOLAYERS AND THIN FILMS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course compulsory Level of course master Semester summer Language of instruction English Hours per week 2 (L) + 1 (Lab) Hours per semester 30 (L) + 15 (Lab) Objectives of the course Come to know the structure, composition and properties of nanolayers and thin films; their preparation techniques and testing methods. Entry requirements Inorganic chemistry, Physical chemistry, Physics Course contents Common examples of nanolayers and thin films. Preparation techniques: Vacuum evaporation, electron beam evaporation, magnetron sputtering, reactive sputtering, chemical vapor deposition, electroplating, spray-on techniques, liquid phase epitaxy. Principles of industrial processes utilizing thin film deposition, e.g. photolithography. Applications of nanolayers and thin films in science and technology. Principal analytical techniques for nanolayers and thin films testing: X-ray Photoelectron Spectroscopy, Auger Electron Spectroscopy, Reflection High Energy Electron Diffraction, Low Energy Electron Diffraction, Scanning Electron Microscopy, Transmission Electron Microscopy, Atomic Force Microscopy. Assessment methods written exam 88 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes Recommended readings Student knows the structure and composition of commonly used nanolayers and thin films. Student knows most important preparation techniques used to the formation of these structures. Student knows most important analytical methods utilized for testing these structures. Student is able to prepare and test simple examples of nanolayers and thin films. 1. Encyclopedia of nanoscience and nanotechnology. editor Hari Singh Nalwa, American Scientific Publishers, 2004. Additional information Course title NANOPARTICLES AND ENVIRONMENT Teaching method Lecture Person responsible for the course Beata Tryba, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 2 Type of course compulsory Level of course master Semester winter Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Come to know about the influence of nanotechnology and nanoparticles on the human life and environment; regulations about management of the nanomaterials; risk assesment of the nanoparticles effect on the human body. Entry requirements Fundamentals of nanotechnology, Characterization techniques of nanomaterials. Course contents Introduction to nanotechnology and nanoparticles. Characteristics of nanoparticles in the environment. Risk assesment of the nanoparticles effect on the human body and environment – regulations, law, ethics. Assesment of nanoparticles toxicology - in vitro and in vivo as well as simulation computer methods - QSAR. Assessment methods class test Learning outcomes Participant of this course will get knowledge and wide awarness on the presence of nanoparticles in the commercial products and their distribution pathway to environment. This knowledge will involve also the impact of the nanoparticles on the animals and humans health, their toxicity and way of safety handling. The student would be able to easy recognize products obtained though the nanotechnology and will be aware the risk of using it and deposition in the environment according to the estimation of whole cycle of life. 1. Recommended readings 2. 3. J.C. Miller, R. Serrato, J. M. Represas-Cardenas, G. Kundahl “The Handbook of Nanotechnology, Business, Policy, and Intellectual property Law”, John Wiley & Sons, Inc. G. Hunt, M. Mehta, „Nanotechnology, Risk, Ethics and Law”. Ecotoxicology, 17(1-8) (2008), Springer 89 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Additional information Course title NANOTECHNOLOGY AND CRYSTALLINE NANOMATERIALS Teaching method lecture Person responsible for the course Ewa Mijowska, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/TCh/D12-10 ECTS points 2 Type of course obligatory Level of course Master Semester summer Language of instruction English Hours per week 1 lecture Hours per semester 15 lectures Objectives of the course Come to know about the fundamental knowledge about nanotechnology and nanosize effect in nanocrystalline materials Entry requirements Fundamentals of chemical engineering, Characterization techniques of materials Course contents Introduction to nanotechnology. Morphology of different carbon nanostructures and crystalline nanomaterials. Preperation techniques of nano—sized materials. Size effect in properties of materials. Characterization of nanomaterials. Examples of application of nanomaterials in industry Assessment methods oral exam Learning outcomes The student has broad knowledge in the field of nanotechnology of nanocrystalline materials, can specific knowledge in the field of inorgannic nanomaterials, theoretically based knowledge in technology and characterisation of the crystalline nanomaterials and has knowledge about the current trend of developments on the field of nanotechnology. Used the knowledge to analyse the data and is able to estimate the methods of carbon and inorganic nanomaterials preparation. Recommended readings 1. 2. M.D. Ventra, S. Evoy, J.R. Heflin, “Introduction to nanoscale science and technology”, Springer 2004. W.A. Goddard, D.W. Brenner, S.E. Lyshevski, G. J. Lafrate, „Handbook of nanoscience, engineering and technology”, CRC Press LLC 2003. Additional information 90 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title PAINTS AND ADHESIVES TECHNOLOGY Teaching method Lecture, laboratory Person responsible for the course Krzysztof Kowalczyk, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 2 Type of course Level of course Bachelor, master Semester winter/summer Language of instruction English Hours per week 1 (L), 2 (Lab) Hours per semester 15 (L), 30 (Lab) Objectives of the course To gain the knowledge about technology and application of organic varnishes (decorative, protective) paints as well as adhesives Entry requirements Chemical technology, chemical engineering, polymer technology Course contents Lectures: Definitions of a varnish, paint, adhesive, binder, film forming substance, pigment, filler, solvent, diluent. Characterization of the most popular binders, fillers, pigments (decorative, anticorrosive), solvents, additives. Preparation of solventless, solventborne, powder as well as waterborne coating compositions. Preparation of liquid and solid adhesives. Application of coating compositions. Testing methods of liquid and dry/cured coating compositions. Testing methods of adhesives and joints. Laboratory: Preparation of coating compositions and adhesives, application and testing of coating compositions and adhesives. Assessment methods written exam and grade Learning outcomes Skills of preparation, characterization and application of coating compositions and adhesives Recommended readings 1. 2. 3. 4. Z. Wicks, F. Jones: Organic, John Wiley&Sons, Hoboken 2007; M. Xanthos: Functional fillers for plastics, Wiley-VCH, Weinheim 2005; J. Bieleman: Additives for coatings, Wiley-VCH, Weinheim 2000; J. Koleske: Paint and coating testing manual, ASTM, Philadelphia, 1995. Additional information Course title PHYSICAL CHEMISTRY OF SURFACES Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc E-mail address to the person responsible for the course [email protected] 91 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course code (if applicable) ECTS points 3 Type of course compulsory Level of course master Semester winter Language of instruction English Hours per week 2 (L) + 1 (Lab) Hours per semester 30 (L) + 15 (Lab) Objectives of the course Come to know the processes taking place on the surface of solids, the mechanisms and laws ruling them Entry requirements Inorganic and organic chemistry, Physical chemistry Course contents Materials of developed surface. Surfaces and interfaces. The techniques of surface science. Electrical, mechanical and optical properties of surfaces. Thermodynamics on surfaces. Surface phenomena. Sorption processes. Adsorption and desorption. Lubrication, wetting, adhesion. Macromolecular surface films. Chemical reactions on surfaces. Solid – gas reactions. Oxidation, passivation and structure of thin films. Assessment methods written exam Learning outcomes Student knows the structure of surfaces and interfaces. Student knows fundamental laws applicable to the processes performed on the surfaces of solids. Student knows the basic experimental methods applied to evaluate the properties of solid surfaces and is able to perform respective experiments. Recommended readings 1. 2. G.A. Somorjai: Introduction to surface chemistry and catalysis. Wiley, New York 1994. John C. Vickerman, Ian S. Gilmore: Surface analysis: the principal techniques. Wiley, New York 2009. Additional information Course title POLYMER COMPOSITES Teaching method Lecture/laboratory Person responsible for the course Ryszard Pilawka, PhD Krzysztof Gorący, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course optional Level of course Semester winter/summer Language of instruction English, German Hours per week 1 Lecture, 3 Lab Hours per semester 60 92 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Objectives of the course Properties of Polymer Composites, Technologies of Manufacturing Entry requirements Basics of plastics Polymer properties Course contents Materials used in Polymer Composites (Fibers and Polymers), Properties of Polymer Composites, Methods of Investigations, Technologies for Manufacturing of Composites. Practical meaning of Composites Assessment methods Grade Learning outcomes Student should know and explain of polymer technology and polymer chemistry. Student is able to describe and explain the differences between the various resins and their properties, and indicate the method processing and areas of applications. Student is able to interpret and describe physicochemical, mechanical and thermal properties of polymers depending on thermosets kind. Student is able to top up the information obtained by the content of the lectures in literature object. Recommended readings 1. Ronald F. Gibson – Principles of Composite Material Mechanics Additional information Course title POLYMER CHEMISTRY Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 2 Type of course Level of course MSc Semester winter Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course This course is aimed at giving an introduction to polymer chemistry. Student will be able to define basic terms related with polymers synthesis and properties, will be able to select materials for particular application according the application requirements, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on organic chemistry, materials science Course contents Basic definitions: monomer, polymer, molecular mass; conformation of macromolecules; basic mechanisms of polymer reactions, synthesis methods of polymers 93 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: predict and explain molecular structure of polymeric materials, classify polymers according their synthesis methods and intrinsic properties Recommended readings 1. 2. F.J. Davis, Polymer chemistry, Oxford University Press, 2004 N. P. Cheremisinoff, Polymer characterization, Noyes Pub., 1996 Additional information Course title POLYMERS IN MEDICINE Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 3 Type of course Level of course master Semester summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course This course is aimed at giving an introduction to polymeric biomaterials used in medicine. Student will be able to define basic terms related to polymers used in medicine, will be able to select suitable polymers for particular application according the application requirements, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on chemistry or polymer chemistry, materials science Course contents Polymeric biomaterials: basic concepts of biocompatibility; synthetic polymers and composites; biodegradable polymers for tissue engineering; stimuli responsive polymers for drug delivery; metals and ceramic in biomedical applications; environmental management of biodegradable polymers. Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: compare and contrast different polymeric biomaterials, predict and explain material behavior in living environment, classify implants according time of implantation and their function Recommended readings 1. 2. 3. Black J., Biological Performance of Materials, Marcel Dekker, New York, 1999 Wise D.L., Biomaterials and Bioengineering Handbook, Marcel Dekker, New York, 2000 Ratner B.D., Biomaterials Science, Elsevier, New York 2004 94 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Additional information Course title POWER ENGINEERING IN CHEMICAL INDUSTRY Teaching method lecture Person responsible for the course Jacek Przepiórski, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course jacek.przepiorski@ zut.edu.pl ECTS points 2 Type of course Compulsory Level of course Bachelor Semester summer Language of instruction English Hours per week L:1 Hours per semester L:15 Objectives of the course Student will get theoretical knowledge on elements of power engineering in chemical industry, will understand how the power is generated, managed and distributed. Entry requirements Fundamentals of chemical technology and engineering Course contents Natural resources of raw materials used by chemical industry. Elements of fuel combustion. Characterization of the types of energy used in chemical industry. Characteristics of basic methods of heat generation and energy transfer. Power demand of the major unit operation. Heat transfer media in chemical industry: low and high pressure steam, organic liquids, silicone oils, air, water, brines. Water for steam boilers and coolant circuit. Solid and liquid wastes, pollution emission. Heat exchangers. Heat of reactions. Heat exchange in an exemplary technological process. Search for new sources of energy. Non-conventional sources of energy. Assessment methods Oral exam, continuous assessment Learning outcomes At the completion of this course, students will be able to: Understand fundamentals power and heat generation applied in chemical industry and use of energy in sense of distribution and consumption in specific processes. Recommended readings 1. 2. 3. Ullmann's Encyclopedia of Industrial Chemistry, 6th edition (2002) Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition Ron Zevenhoven, Pia Kilpinen, Control of pollutants in flue gases and fuel gases, ISBN 951-22-5527-8, Additional information 95 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title PRINCIPLES OF BIOTECHNOLOGY Teaching method Lecture plus literature project Person responsible for the course Piotr Sobolewski, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 4 Type of course Level of course Bachelor/master Semester Winter/summer Language of instruction English Hours per week 2 Hours per semester 30 Objectives of the course Provide an understanding of the principles of biotechnology. Establish a background in microbiology, genetics, cellular metabolism, and enzyme kinetics. Entry requirements none Course contents Principles of microbiology; overview of genetics; fermentation; GMO; enzymes; practical applications Assessment methods Oral presentation Learning outcomes After completion of this course, the successful student will be able to: 1) compare and contrast eukaryotic and prokaryotic organisms, 2) describe the steps involved in cellular protein synthesis, 3) explain the function of enzymes in biology and biotechnology, 4) discuss the pros and cons of GMO Recommended readings 1. Ratledge & Kristiansen, Basic Biotechnology Additional information Course title QUALITY AND RISK MANAGEMENT IN CHEMICAL INDUSTRY Teaching method lecture Person responsible for the course Krzysztof Karakulski, PhD,DSc E-mail address to the person responsible for the course krzysztof.karakulski@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh/D12-11 ECTS points 2 Type of course compulsory/optional Level of course master 96 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Presentation of procedures of product control in chemical industry in compliance with ISO standards and risk assessment associated with utilization of installations in chemical industry Entry requirements Chemical technology, Unit operations in chemical technology, ISO standards, European regulations on industrial safety Course contents European regulations concerning quality management according to ISO standards. Techniques of products control. Systems of environment management and industry safety. Operation with dangerous liquids, internal transport, electric energy versus industrial safety. Problems of ventilation. Storage and transport of chemicals and dangerous substances. Protection of machines and devices, explosive limits of gaseous mixtures, evaluation of fire hazard of constructional materials, self-igniting substances. Case studies – examples from industry. Assessment methods written exam Learning outcomes The students will be able to identify high-risk industrial sites, will be familiar with the general obligations of the operator, details contained in notification, the requirements of the operator to produce a safety report and the methods of classification of the chemical plants in accordance with risk associated with major-accident hazards involving dangerous substances 1. 2. Recommended readings 3. 4. 5. R.L. Hoover, R.L. Hancock, Health, Safety and Environment Control, Van Nostrand, New York, 1989. N.I. Sax, Dangerous Properties of Industrial Materials, 7 th ed., Van Nostrand, New York, 1989. L.N. Moses, D. Lindstrom, Transportation of Hazardous Materials, Kluwer Academic Publishers, Boston, 1993. Publications from the internet site: www.envirowise.gov.uk Council Directive 96/82/EC of 9 December 1996 on the control of major-accident hazards involving dangerous substances. Additional information Course title RESEARCH PROJECT Teaching method Laboratory and seminar Person responsible for the course Halina Kwiecień, professor E-mail address to the person responsible for the course halina.kwiecien@ zut.edu.pl Course code (if applicable) WTiICh/ISt/TCh/D2-3 WTiICh/ISt/TCh/D2-4 WTiICh/ISt/TCh/D2-5 ECTS points 12 Type of course Optional Level of course Master or bachelor 97 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester Winter or summer Language of instruction English Hours per week 9 (L-7, S-2) Hours per semester 135 (L-105, S-30) Objectives of the course Applying of knowledge and skills learned during studies to solving a practical research problem Entry requirements Fundamentals of chemistry, mathematics and analytical methods Course contents The students accomplish the research project concerning a given subject. It consist of literature studies, concept of project realization, selection of used materials, performing the selected process, characteristic of obtained products, control measurements using proper methods and instruments, calculations, discussion of the results, conclusions. Description of all this aspects should be given in the written project report. Assessment methods Learning outcomes Student will be able to analyze new research problems and to propose strategies to solve them. Student will be able to elaborate and to execute research project under the supervision of the tutor. Student will be able to perform evaluation and interpretation of data from the literature and from the experimental work. Student will be able to prepare of written scientific report and to prepare oral presentation using audiovisual ways. Recommended readings Literature connected with the research subject, including books, articles and patents assessment of progress of the work (presentations during seminar) assessment of the quality of written project report oral exam, including final presentation Additional information Course title SMALL SCALE PRODUCTS IN INORGANIC INDUSTRY Teaching method lecture Person responsible for the course Krzysztof Lubkowski, Ph.D. E-mail address to the person responsible for the course krzysztof.lubkowski@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh-D12-9 ECTS points 2 Type of course compulsory Level of course master Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Come to know about the production methods of small scale inorganic chemicals 98 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Entry requirements Unit processes and operations in chemical technology, Chemical technology – raw materials, Chemical technology – chemical industry processes, Chemical engineering. Course contents Inorganic pigments, sorbents, fillers, coagulants, silicon emulsions, silicon pastes, inorganic phosphorous compounds - characteristics, properties, methods of production, application. Assessment methods written exam Learning outcomes After the course, students will be able to understand fundamentals of small scale products production in chemical industry. 1. Recommended readings Hocking M.B., Modern Chemical Technology and Emission Control, SpringerVerlag, Berlin 1985. 2. The Chemistry of synthetic dyes and pigments, H.E. Lubs (ed), Reinhold, New York 1955. 3. Pigment Handbook, P.A. Lewis (ed.), John Wiley & Sons, New York 1988. 4. Winkler, J., Titanium Dioxide, Vincentz Network, Hannover, 2003. 5. Industrial Inorganic Pigments, G. Buxbaum, G. Pfaff (eds.), Wiley-VCH, Weinheim 2005. 6. High performance pigments, H.M. Smith (ed), Wiley-VCH, Weinheim 2001. 7. Wypych G., Handbook of Fillers, The Definitive User's Guide and Databook of Properties, Effects and Uses, Plastics Design Library, 1998. 8. Jancar J., Mineral fillers in thermoplastics: raw materials and processing, SpringerVerlag, Berlin - Heidelberg 1999. 9. Corbridge D.E.C., Phosphorus: an outline of its chemistry, biochemistry and technology, Elsevier Scientific Publ. Co., Amsterdam 1978. 10. Yang R.T., Adsorbents: fundamentals and applications, John Wiley and Sons, Hoboken, 2003. Additional information Course title SURFACE PHENOMENA AND INDUSTRIAL CATALYTIC PROCESSES Teaching method lecture/laboratory Person responsible for the course Rafał J. Wróbel, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/TCh/C01 ECTS points 3 Type of course obligatory Level of course master Semester winter Language of instruction English Hours per week 1 lecture 2 laboratory 1 classes Hours per semester 15 lecture 30 laboratory 15 classes Objectives of the course Understanding of the catalytic processes and surface methods required for investigation of catalytic phenomena Entry requirements Mathematics, Physics, Inorganic Chemistry, Physical Chemistry 99 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course contents Introduction to surface phenomena. Elementary steps in heterogeneous reactions. Surface reactions. Structure and production of catalysts. Industrial application of catalysts. Basics of XPS, AES, MS, EDX, SEM, TEM, STM, AFM, FIM, FEM etc. techniques. Assessment methods written exam Learning outcomes Student will learn principles of surface science methods, catalytic processes and basics of vacuum technologies. Recommended readings 1. 2. Handbook of heterogeneous Catalysis, John Wiley and Sons, 2014, ISBN: 9783527610044 J. C. Riviere, S. Myhra, Handbook of Surface and Interface Analysis, CRC Press, 2009 Additional information Course title TECHNOLOGICAL PROJECT Teaching method Lecture Person responsible for the course Marek Gryta, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/TCh/D12-6 ECTS points 2 Type of course compulsory / obligatory Level of course master Semester winter / summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Capacity of evaluation, interpretation and synthesis of the chemical data and information; elaborate and execute projects in the ambit of chemistry; recognize and analyze new problems and to plan strategies to solve them. Analyzing an influence that has impact on the project. Entry requirements Chemical technology, Unit operations in chemical engineering. Course contents The students accomplish the technological project concerning a given subject: a description of technological concept, a block diagram of assumed manner of its realization, selection and description of used raw materials, characteristic of obtained products, description of wastes and a proposal of their management, flow diagram with description of control measurement instruments, fundamental project calculations, mass balance calculations and Sankey’s diagram. Assessment methods oral exam and/or project work Learning outcomes Strategically and operative decisions that are taken in the matrix of the company to reach the project objectives. Taking decisions about the best way to manage a project assuming prefixed data and costs. 100 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Recommended readings Obligatory 1. C.A. Heaton, Industrial Chemistry, Blackie and Sons, Glasgow 1991 2. Lees’ Loss Prevention in the Process Industries, Vol.1-3 (3rd Ed.)ed. By Mannau S., Elsevier 2005 3. D.L. Wise, D. Trantdo, Process engineering for pollution control and waste minimization, Marcel Dekker, New York 1994 Additional/optional 1. CRC Handbook of Chemistry and Physics, 87 th ed., 2006-2007, Taylor & Francis 2006 2. KIRK-OTHMER Encyclopedia of Chemical Technology, 5th ed., John Wiley & Sons, 2004 3. Hewitt G.F., Handbook of Heat Exchanger Design, Hemisphere Pub., Washington DC 1990 Additional information Course title TECHNOLOGIES FOR WASTE AND POLLUTANTS MINIMIZATION IN CHEMICAL INDUSTRY Teaching method Lecture Person responsible for the course Joanna Grzechulska – Damszel, PhD,DSc E-mail address to the person responsible for the course joanna.grzechulska@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh/D12-2 ECTS points 2 Type of course Obligatory Level of course Master Semester Winter Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course Come to know about the legal regulations and technologies concerning waste and pollutants minimization in chemical industry Entry requirements Chemical technology, Unit operations in water and wastewater treatment, Technology of water and wastewater Course contents European regulations concerning waste management. Environmental impact assessment. Life cycle analysis. Responsible Care Program. The concept of cleaner production. Techniques of waste and pollutants minimization. Case studies – examples from industry. Assessment methods Exam Learning outcomes Student knows the present state of environment. Student knows the law regulations concerning the waste management. Student knows the methods of wastes and pollutants minimization. Student knows technologies applied to wastes and pollutants minimization. Student can assess the negative effect of wastes to the environment and is aware of possibilities to minimize it. Student can assess the negative effect of wastes introduced to the environment and can select adequate methods and technologies to minimize it. 101 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Recommended readings Obligatory 1. N. P. Cheremisinoff, Handbook of Solid Waste Management and Waste Minimization Technologies, Elsevier, 2003. 2. B. Crittenden, S. Kolaczkowski, Waste minimization guide, Institute of Chemical Engineers, UK, 1995. 3. Process engineering for pollution control and waste minimization / edited by Donald L. Wise, Debra J. Trantolo, Marcel Dekker, New York, 1994. Additional/optional 1. P.N. Cheremisinoff, L.M. Ferrante, Waste Reduction for Pollution Prevention, Butterworth-Heinemenn Ltd, Linacre House, Jordan Hil, Oxford OX2 8DP, 1992. 2. Publications from the internet site: www.envirowise.gov.uk Additional information Course title TECHNOLOGIES IN ENVIRONMENTAL PROTECTION I AND II Teaching method Lecture, seminar, laboratory Person responsible for the course Elżbieta Huzar, PhD E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/ISt/OSr/B-6-1 WTiICh/ISt/OSr/B-6-2 ECTS points I: 2 II: 2 Type of course Optional Level of course Bachelor or master Semester Winter or summer Language of instruction English Hours per week I: 2 (L-1, S-1) II: 2 (Lab-2) Hours per semester I: 30 (L-15, S-15) II: 30 (Lab-30) Objectives of the course Knowledge about contaminations in air and contaminations from air, water and wastewater. Entry requirements Inorganic and organic chemistry Course contents Lectures: Contaminants in air and water. Sources of emission of air pollutants. Global problems of air protection. Methods of dust extraction and types of dust collectors: inertial separators, fabric filters, wet scrubbers, electrostatic precipitators, unit collectors. Systems of monitoring of air pollutants. Alternative sources of energy. Sources of water pollutants. Characteristic, classification, composition and specificity of effluents. Technologies for removing of contaminants from water. Conventional treatment systems: primary treatment, secondary treatment. Advanced treatment processes: filtration systems, oxidation processes, ultraviolet treatment, electrolysis. Seminar: methods of emission control, methods of desulphuration of combustion gases, methods of clean-up of municipal and industrial effluents. Laboratory: elimination of iron from water, the use of activated carbon for the removal of oxidizable compounds from water, elimination of phosphorus from water by precipitation method, determination of nitrogen dioxide in air by spectrophotometric method, adsorption of toluene on granular activated carbon, study of paracetamol adsorption – verification of Freundlich adsorption isotherm water. Technologies for removing 102 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Assessment methods Lecture - written exam Seminar – essays and presentations, grade laboratory – written reports, grade Learning outcomes Student will be able to characterize popular environmental pollutants and indicate sources of its emission. Student will be able to explain principles of operation of devices and technologies used in environment protection. Student will be able to collect, organize and present data from literature. Student will be able to perform analysis of selected pollutants and evaluate the treatment processes efficiency. 1. 2. Recommended readings 3. 4. Wilhelm Batel, Dust Extraction Technology: Principles, Methods, Measurement Technique, John Wiley & Sons Ltd, 1973 Horan, N. J., Biological wastewater treatment systems: theory and operation. John Wiley & Sons Ltd, 1989 Matthew A. Tarr, Chemical Degradation Methods for Wastes and Pollutants Environmental and Industrial Applications, Marcel Dekker, 2003 A.T. Gireczycki, Ł. Kurowski, J. Thullie, Gas clearing and wastewater treatment fo industrial and engineering chemistry students, Politechnika Śląska, Gliwice 2011. Additional information Course title TECHNOLOGY OF DYES AND INTERMEDIATES I AND II Teaching method Lecture and laboratory Person responsible for the course Halina Kwiecień, professor E-mail address to the person responsible for the course halina.kwiecien@ zut.edu.pl Course code (if applicable) WTiICh/IISt/TCh/D4-5 WTiICh/IISt/TCh/D4-11 ECTS points I: 2 II: 2 Type of course Optional Level of course Master or bachelor Semester winter or summer Language of instruction English Hours per week 4 (L-2, Lab-2) Hours per semester 60 (L-30, Lab-30) Objectives of the course Come to know about chemistry and applications of dyes, technology of dyes and development in dyes industry Entry requirements Basics of organic chemistry and technology, chemical engineering Course contents Lectures: Introduction: historical development of synthetic dyes, development of colour and constitution theory. Classification of colorants by chemical structures and by application. Azo dyes, structure, synthesis and properties. Carbocyclic and heterocyclic mono-and poly- azo dyes. Basic structure, synthesis and properties following dyes: anthraquinone, triarylmethane and their heterocyclic analogues, polycyclic aromatic carbonyl, indigoid, dyes, polimethine and phthalocyanine. Production of dyes, “green processes” in dyes industry. Application of colorants. Dyeing of wool, cellulosic, acetate polyester, polyamide and acrylic fibres. Dyes for nontextile applications. Dyes in the new technology industries: for displays, for optical data storage, laser dyes. 103 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Laboratory: synthesis of dyes (acid, basic, direct or reactive dyes), purification, chromatographic and UV-VIS analysis of the products. Dyeing of wool or cellulosic fibres. Assessment methods Lecture: essay, grade Laboratory: written report, grade Learning outcomes Student will be able to characterize the main groups of dyes. Student will be able to describe technology of the main groups of dyes. Student will be able to indicate the environmental pollutants of dyes industry and ways of waste disposal. Student will be able to apply the acquired knowledge in the synthesis, analysis and application of selected dyes. 1. Recommended readings 2. 3. Waring D.R., Hallas G., „The Chemistry and Application of Dyes”, Plenum Press, New York, 1994 2. Hunger K., “Industrial Dyes. Chemistry Properties , Applications”, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003. Furniss B.S., Hannaford A.J., Smith, P.W.G., Tatchell A.R. “Vogel’s Textbook of Practical Organic chemistry”. Fifth Ed., The School of Chemistry, Thames Polytechnic, London, 1989. Additional information Course title TESTING METHODS OF BIO- AND NANOMATERIALS Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 2 Type of course Level of course MSc Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course This course is aimed at giving an introduction to basic testing methods of bio- and nanomaterials. Student will be able to define basic terms related testing methods and equipment, will be able to select materials for particular application according their properties, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on chemistry, materials science, physics Course contents Interphase phenomena (contact angle, surface energy); microscopic techniques (optical microscopy, scanning electron microscopy); thermal analysis of bio- and nanomaterials; chemical structure (IR, NMR, UV-VIS), mechanical properties of bio- and nanomaterials Assessment methods examination 104 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes At the completion of this course, the successful student will be able to: predict and explain material chemical, thermal and surface properties, classify materials according their structure and properties Recommended readings 1. 2. N. P. Cheremisinoff, Polymer characterization, Noyes Pub., 1996 Koo J.H., Polymer nanocomposites, The McGraw-Hill Comp., 2006 Additional information Course title TESTING METHODS OF INORGANIC PRODUCTS Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc E-mail address to the person responsible for the course [email protected] Course code (if applicable) WTiICh/IISt/TCh/D12-3 ECTS points 5 Type of course compulsory Level of course master Semester winter Language of instruction English Hours per week 3 (L) + 4 (Lab) Hours per semester 45 (L) + 60 (Lab) Objectives of the course Come to know the theory and techniques of instrumental methods for materials characterization Entry requirements Inorganic chemistry, Physical chemistry, Physics Course contents Instrumental methods of chemical composition analysis. Selecting of a proper analytical methods. Theoretical basics of atomic spectroscopy. Inductively Coupled Plasma, ICP. Atomic absorption spectroscopy, AAS. Molecular spectra method. Infrared Spectroscopy, IR, Raman Spectroscopy RS. X-ray methods. X-Ray Fluorescence, XRF. X-Ray Microanalysis). Chemical analysis of the surface of solid state. Physicochemical basics of Electrospectroscopy methods. Methods: Electron Spectroscopy for Chemical Analysis, ESCA, including X-ray Photoelectron Spectroscopy, XPS, and Ultraviolet Photoemission Spectroscopy, UPS; Auger Electron Spectroscopy, AES, Electron Energy Loss Spectroscopy. Adsorption/desorption methods and temperature programmed techniques. Thermogravimetry, TG, Temperature Programmed Desorption, TPD, Temperature Programmed Oxidation, TPO, Temperature Programmed Reduction, TPR, Temperature Programmed Surface Reaction, TPSR. Mass spectrometry. Analysis of phase composition, structure and topography. X-Ray Diffraction, XRD, Reflection High Energy Electron Diffraction, RHEED, Low Energy Electron Diffraction, LEED. Mössbauer Spectroscopy. Scanning Electron Microscopy, SEM, and Transmission Electron Microscopy, TEM, Atomic Force Microscopy, AFM. Assessment methods written exam 105 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Learning outcomes Student knows the most important analytical methods utilized for testing inorganic samples. Student is able to choose a proper group of analytical methods to assess given set of properties. Student knows how to prepare samples for analytical methods and is able to carry out simple analysis. Recommended readings 1. 2. John A. Dean, Analytical Chemistry Handbook, McGraw-Hill Companies, 2000 Helmut Günzler, Alex Williams, Handbook of Analytical Techniques, Wiley-VCH, 2001. Additional information Course title THERMAL ANALYSIS OF PLASTICS Teaching method Lecture/laboratory Person responsible for the course Ryszard Pilawka, PhD Krzysztof Gorący, PhD Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 Type of course compulsory/obligatory Level of course Bachelor/master Semester winter/summer Language of instruction English, German Hours per week 1 Lecture, 3 Lab Hours per semester 60 Objectives of the course The aim of thermal analysis, equipment, practice of thermal analysis Entry requirements Basics of plastics Polymer properties Course contents Methods of thermal analysis, physical principles of thermal analysis, instrument operation. Practical evaluation of results. Assessment methods Grade Learning outcomes Student should know and explain of polymer technology and polymer chemistry. Student is able to describe and explain the dependence between construction of polymers and their properties, and indicate the method processing and areas of applications. Student is able to interpret and describe physicochemical properties of polymers depending on their construction chemical and molecular. Student is able to top up the information obtained by the content of the lectures in literature object. Recommended readings 1. G.W Ehrenstein, G. Riedel, P. Trawiel; Thermal Analysis Of Plastics: Theory and Practice Additional information 106 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Course title TISSUE ENGINEERING Teaching method lecture Person responsible for the course Mirosława El Fray, professor E-mail address to the person responsible for the course [email protected] Course code (if applicable) ECTS points 3 Type of course Level of course master Semester summer Language of instruction English Hours per week 1 Hours per semester 15 Objectives of the course This course is aimed at giving an introduction to tissue engineering concepts. Student will be able to define basic terms related to tissue engineering, will be able to select materials for particular tissue reconstruction/regeneration, will be able to work in a group, and will be able to broaden her/his knowledge in the field. Entry requirements Passed examination on chemistry or polymer chemistry, materials science Course contents Natural tissue structure and function, cell growth and proliferation, biodegradable materials, including polymers and ceramic for tissue engineering, preparation methods of 3D scaffolds; regulatory aspects Assessment methods examination Learning outcomes At the completion of this course, the successful student will be able to: compare and contrast different biodegradable materials, predict and explain cell behavior in scaffolding system, determine material properties for tissue engineering approach Recommended readings 1. 2. R.L. Reis, J. San Roman, Biodegradable Systems in Tissue Engineering and Regenerative Medicine, CRC Press, 2004 Ratner B.D., Biomaterials Science, Elsevier, New York 2004 Additional information Course title TOXICOLOGICAL ASSESSMENT OF MATERIALS AND PRODUCTS Teaching method Lecture, seminar and laboratory Person responsible for the course Małgorzata Dzięcioł, PhD E-mail address to the person responsible for the course malgorzata.dzieciol@ zut.edu.pl Course code (if applicable) WTiICh/IISt/OSr/C-10 ECTS points 4 Type of course Optional Level of course Master or bachelor 107 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Semester winter or summer Language of instruction English Hours per week 5 (L-1, S-1, Lab-3) Hours per semester 75 (L-15, S-15, Lab45) Objectives of the course Toxicological aspects of raw materials and industrial products daily use. Methods of studies in quality control of products. Entry requirements Basic knowledge of organic and inorganic chemistry Course contents Lecture and seminar: Sources of toxic substances in environment. Toxic effects of chemical substances. Factors influencing toxicity. Health risk assessment of daily use products. Toxicological aspects connected with polymeric materials. Emission of toxic compounds from plastics during production, processing and fire. Methods of studies of emission from plastics. Techniques of sample preparation: solvent extraction, static and dynamic headspace technique. Migration testing of plastic materials intended to come into contact with food. Overall and specific migration. Food simulants. Toxic substances in food. Food contamination from environment. Toxic products of food processing. Food additives. Natural harmful food components. Toxic ingredients of cosmetics. Laboratory: Analysis of toxic components of products. Studies of emission of volatile compounds from daily use products. Analysis of preservatives and toxic compounds in food and cosmetics. Assessment methods Lecture - written test, grade Seminar - essays and presentations, grade Laboratory – written reports, grade Learning outcomes Student will products. Student will Student will Student will 1. 2. Recommended readings 3. 4. 5. be able to explain sources of different toxic compounds in materials and be able to collect, organize and present data from literature. be able to describe and apply different techniques of sample preparation. be able to perform analysis of selected toxic components of products. Fundamental Toxicology, ed. Duffus J.H., Worth H.G.J., RSC Publishing 2006 Henneuse-Boxus C., Pacary T., Emissions from Plastics, Report 161, Rapra Review Reports, 2003 Crompton T.R., Additive Migration from Plastics into Foods – a Guide for Analytical Chemists, Smithers Rapra Technology Limited, 2007 Food Safety and Food Quality, ed. Hester R.E., Harrison R.M., The Royal Society of Chemistry, 2001 Food Safety: Contaminants and Toxins, ed. D’Mello J.P.F., CABI Publishing, 2003 Additional information Course title VACUUM TECHNOLOGY Teaching method lecture, laboratory Person responsible for the course Dariusz Moszyński, PhD,DSc Course code (if applicable) E-mail address to the person responsible for the course [email protected] ECTS points 3 108 FACULTY OF CHEMICAL TECHNOLOGY AND ENGINEERING ______________________________________________________________________________________________ Type of course compulsory Level of course master Semester summer Language of instruction English Hours per week 1 (L) + 2 (Lab) Hours per semester 15 (L) + 30 (Lab) Objectives of the course Come to know the principles of low pressure conditions and vacuum, techniques to produce and measure vacuum as well as the most common applications of vacuum. Entry requirements Physical chemistry, Physics Course contents Fundamentals of vacuum generation. Designing of vacuum systems. Vacuum pumps. Outgassing. Phenomena Induced by Electron Irradiation. Vacuum Gauges. Emitters for Electron Probes. Assessment methods written exam Learning outcomes Student knows the physical laws applied to calculate properties concerned in vacuum equipment. Student knows the most important vacuum equipment, vacuum pumps and gauges. Student is able to design a simple vacuum system and maintain its operation. Recommended readings 1. Handbook of vacuum technology , ed. by Karl Jousten, Wiley-VCH Verlag, 2008 Additional information 109
© Copyright 2026